- Share full article
Advertisement
Supported by

A ‘Game Changer’ for Patients With Esophageal Cancer
A drug that unleashes the immune system offers a rare glimmer of hope for those with a cancer that resists most treatments.

By Gina Kolata
For decades, esophageal cancer has defied scientific attempts to discover a therapy that extends patients’ survival, year after year claiming the lives of such illustrious people as Humphrey Bogart, Christopher Hitchens and Ann Richards, the former governor of Texas.
Now a large clinical trial offers hope, finding that a drug that unleashes the immune system to attack cancer cells can double the disease-free survival times in patients from 11 months to 22 months. The study was published on Wednesday in the New England Journal of Medicine.
“It is a game changer,” said Dr. David Ilson, an esophageal cancer expert at Memorial Sloan Kettering Cancer Center in New York, who wrote an editorial accompanying the research. “We’ve waited a long time for this.”
In the trial, sponsored by Bristol-Myers Squibb, 794 patients in 29 countries were randomly assigned to receive infusions of the drug, nivolumab, or a placebo.
The patients had all had chemotherapy and radiation followed by surgery to remove their cancers. As usually happens, pathology reports showed that the surgery did not remove all of the cancer cells, which still lurked in lymph nodes and elsewhere, setting the stage in these patients for their cancers to return as incurable metastases.
Nivolumab is approved for some patients with other cancers, like Hodgkin’s lymphoma, melanoma and colorectal cancer. With the new study, experts expect the drug will readily win approval for treatment of early-stage esophageal cancer.
Dr. Ronan Kelly, director of the Charles A. Sammons Cancer Center at Baylor University Medical Center and lead author of the new study, said he and the other researchers urgently wanted to help the 75 percent of patients who go through extraordinarily difficult sequences of radiation, chemotherapy and surgery that disfigures the digestive system, only to learn that cancer is still present or has a high likelihood of recurring.
Without some other form of treatment, “we knew many would recur quickly,” Dr. Kelly said. Additional chemotherapy not only was difficult for patients to tolerate, but it also did not seem to help. Nivolumab has few side effects and seemed worth a try.
Esophageal cancer is rare in the United States, accounting for 1 percent of all cancers; about 15,000 patients die each year. But it is the seventh most common cancer globally, and frequently seen in East Asia, although it is not clear why, Dr. Ilson said.
Smoking is a risk factor, but researchers do not think the high smoking rates in China, for example, explain the high incidence. “We don’t think it’s environmental,” Dr. Ilson said.
Other risk factors include alcohol consumption and acid reflux disease.
Because the cancer is so rare in the United States, it has not gotten much research attention. While new treatments have revolutionized prospects for other cancer patients, those with esophageal cancer could only look on longingly.
That has weighed heavily on people with the disease, said Mindy Mordecai. Her husband, John, died of esophageal cancer in March 2008. She started an advocacy group called Esophageal Cancer Action Network.
“You can’t even imagine how demoralizing it is to see all the progress around you. ‘Please sir, may I have some more gruel?’” she said, quoting Oliver Twist, the Charles Dickens character in the eponymous novel, asking for a pittance.
The new findings must be seen in the context of what patients go through when they develop esophageal cancer, experts said. Most learn they have the cancer after it has progressed to a point where they are unlikely to survive.
But every patient hopes to be one of the lucky ones. “Our patients are always waiting for the other shoe to drop,” Ms. Mordecai said. “You have to understand what it’s like to live with that every day.”
The first step for most is chemotherapy and radiation. The treatment is so harsh that an oncology nurse told Mr. Mordecai it “brings Navy Seals to their knees,” Ms. Mordecai recalled.
The chemotherapy has difficult side effects, and the radiation causes a burning sensation that makes it difficult to swallow. “Food won’t go down,” Ms. Mordecai said. “You just feel rotten.”
The next step is major surgery. A doctor takes out most of the patient’s esophagus, the tract leading from the mouth to the stomach, and then grabs the stomach and pulls it up, attaching it to a stump of esophagus left behind.
The result is a stomach that is vertical, not horizontal, and lacks the sphincter muscle that normally keeps stomach acid from spilling out. For the rest of their lives, patients can never lie flat — if they do, the contents of their stomach, including acid, pours into their throats. They can choke, cough and aspirate.
Recovery is difficult, and morbidity and mortality are high. But most patients go through with the operation once they weigh their options. To refuse the treatment means giving up and letting the cancer close off the esophagus to the point where some cannot even swallow their own saliva, said Dr. Paul Helft, a professor of surgery and an ethicist at Indiana University School of Medicine.
The treatment is so long and harrowing that Dr. Helft often uses it to teach medical students and other trainees about informed consent — about how patients must be fully informed before they start any given treatment. Esophageal cancer patients in particular must be told that they are likely to have a recurrence within the first year.
Ms. Mordecai said her husband had his surgery at the end of September 2007. By Dec. 6, he had untreatable metastases in his liver. Now, she said, patients may have a glimmer of hope.
Dr. Ilson, who has spent his career trying to develop therapies to help patients with esophageal cancer, said that he did not expect this treatment to succeed: “We all get nihilistic when faced with years of negative studies.”
“This is really a landmark paper,” he added, and the drug “will become a new standard of care.”
An earlier version of this article misstated the year of Mr. Mordecai's surgery. It was 2007, not 2008.
How we handle corrections
Gina Kolata writes about science and medicine. She has twice been a Pulitzer Prize finalist and is the author of six books, including “Mercies in Disguise: A Story of Hope, a Family's Genetic Destiny, and The Science That Saved Them.” More about Gina Kolata
The Fight Against Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention. Here’s how to avoid one of the deadliest forms of skin cancer.
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why .
Should alcoholic beverages have cancer warning labels? Ireland will require them starting in 2026, and there are nascent efforts elsewhere .
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot .
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.
Esophageal Cancer Research
A treatment regimen that combines the immunotherapy drug nivolumab (Opdivo) with either another immunotherapy drug or chemotherapy may be a new initial treatment option for people with advanced esophageal cancer, a large clinical trial finds.
For some people with advanced esophageal cancer, the immunotherapy drugs pembrolizumab (Keytruda) and nivolumab (Opdivo) may become part of early treatment for the disease, according to results from two large clinical trials.
FDA has approved the immunotherapy drug pembrolizumab (Keytruda) to treat some patients with advanced esophageal cancer. Patients must have certain levels of the protein PD-L1 on their tumors, as determined by an FDA-approved test.
A new study by The Cancer Genome Atlas Research Network could help classify esophageal cancers according to their genetic and molecular alterations and identify potential new treatment options.
Patients with esophageal cancer who received chemotherapy and radiation before surgery survived, on average, nearly twice as long as patients treated with surgery alone, according to results of a randomized clinical trial published May 31, 2012, in the New England Journal of Medicine.

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Esophageal cancer is one of the deadliest cancers
Share this:.
By Dana Sparks
The sixth most common cause of cancer deaths world-wide, esophageal cancer occurs in the esophagus — a long, hollow tube that runs from the throat to the stomach — and can occur anywhere along the esophagus. Men are more likely to develop esophageal cancer than women. While treatable, esophageal cancer is rarely curable.
"It's an uncommon cancer," says Dr. Shanda Blackmon , a Mayo Clinic general thoracic surgeon. "But it's one of the deadliest cancers we know."
Dr. Blackmon says survival rates are improving, but many people don't realize they have esophageal cancer until it's in the advanced stages.
In this "Mayo Clinic Q&A" podcast video , Dr. Blackmon discusses the risks, causes, symptoms and advances in treatments for esophageal cancer. She also explains what patients can expect with a diagnostic endoscopy and describes a new technique at Mayo Clinic that involves dropping a sponge down the patient's esophagus:
____________________________________________
For the safety of its patients, staff and visitors, Mayo Clinic has strict masking policies in place. Anyone shown without a mask was either recorded prior to COVID-19 or recorded in a nonpatient care area where social distancing and other safety protocols were followed.
A version of this article was originally published on the Mayo Clinic News Network .
Learn more about esophageal cancer and find an esophageal cancer clinical trial at Mayo Clinic.
Join the Esophageal Cancer Group on Mayo Clinic Connect or a virtual Esophageal Cancer Support Meeting .
Related Posts

Researchers are studying a risk-prediction tool that could help healthcare professionals better assess which patients need screening.

Dr. James East explains that there can be potentially severe complications if GERD is left untreated, including esophageal cancer.

Thoracic surgeon Dr. Shanda Blackmon explains why it's critical to detect and treat esophageal cancer early in its development.
Advertisement
Esophageal Cancer: Overview, Risk Factors, and Reasons for the Rise
- Published: 09 October 2023
- Volume 25 , pages 275–279, ( 2023 )
Cite this article

- Steve Lander 1 ,
- Eric Lander 2 &
- Michael K. Gibson 2
1495 Accesses
5 Citations
8 Altmetric
Explore all metrics
Purpose of Review
Esophageal cancer (EC) is a common cancer affecting many regions of the world and carries significant morbidity and mortality. In this article, we review the key risk factors and their associated impact on the changing incidence and prevalence of EC subtypes within different global regions. We also highlight potential reasons for the ever-changing epidemiology of this prevalent cancer type.
Recent Findings
There has been a shift in incidence of Esophageal Adenocarcinoma (AC) and Squamous Cell Carcinoma (SCC) within certain populations primarily due to an increase prevalence of primary risk factors. In Western nations, more often the United States, there has been a shift from SCC predominance to the majority of new cases of EC being adenocarcinoma. This shift within the United States has largely correlated with a rise in obesity. The prevalence of AC in Asia is also starting to rise as more countries adopt a western diet.
The pathophysiology, associated risk factors, and presentation of ESCC and AC are different. This difference is seen in varying lifestyles, population health, and certain genetic risks. With further development closer analysis of primary risk factors and implementation of policies and programs that promote public health literacy, there is a potential to decrease esophageal cancer’s global disease burden.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Epidemiology and Risk Factors for Esophageal Cancer

Esophageal Cancer

Pathogenesis of Esophageal Cancer
Data availability.
Not applicable
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Morgan E, Soerjomataram I, Rumgay H, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and Projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649-58 e2. https://doi.org/10.1053/j.gastro.2022.05.054 . The paper presents the latest data from the GLOBOCAN data base. This is important as this data set provides valuble information regarding the change esophogeal cancer epidemiology.
Article PubMed Google Scholar
Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310(6):627–36. https://doi.org/10.1001/jama.2013.226450 .
Article CAS PubMed Google Scholar
Businello G, Parente P, Mastracci L, et al. The pathologic and molecular landscape of esophageal squamous cell carcinogenesis. Cancers (Basel). 2020;12(8). https://doi.org/10.3390/cancers12082160 .
Yokoyama A, Kakiuchi N, Yoshizato T, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565(7739):312–7. https://doi.org/10.1038/s41586-018-0811-x .
• Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–21. https://doi.org/10.1007/s12328-020-01237-x . This paper highlights the global trends in esophogeal cancer up to 2020, which is some of the most up date data on the subject.
Xie SH, Rabbani S, Petrick JL, Cook MB, Lagergren J. Racial and ethnic disparities in the incidence of esophageal cancer in the United States, 1992–2013. Am J Epidemiol. 2017;186(12):1341–51. https://doi.org/10.1093/aje/kwx221 .
Article PubMed PubMed Central Google Scholar
Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. https://doi.org/10.2105/AJPH.2010.191668 .
Howlader N NA, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. based on November 2018 SEER data submission, posted to the SEER web site ed.
Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19(6):1468–70. https://doi.org/10.1158/1055-9965.EPI-10-0012 .
Lu CL, Lang HC, Luo JC, et al. Increasing trend of the incidence of esophageal squamous cell carcinoma, but not adenocarcinoma. Taiwan Cancer Causes Control. 2010;21(2):269–74. https://doi.org/10.1007/s10552-009-9458-0 .
Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol. 2017;112(8):1247–55. https://doi.org/10.1038/ajg.2017.155 .
Eslick GD. Epidemiology of esophageal cancer. Gastroenterol Clin North Am. 2009;38(1):17–25. https://doi.org/10.1016/j.gtc.2009.01.008 . ( vii ).
• Grille VJ, Campbell S, Gibbs JF, Bauer TL. Esophageal cancer: the rise of adenocarcinoma over squamous cell carcinoma in the Asian belt. J Gastrointest Oncol. 2021;12(Suppl 2):S339–49. https://doi.org/10.21037/jgo-2019-gi-08 . The paper discusses the change in tide of esophogeal cancer within the Asian Belt, a region of northern china into mongolia. This is important as this region has some of the highest concentration of new cases of ESCC. This dicusses change in increase incidence of adenocarcioma within the region. This is an important epidemiologic shift that must be discussed.
Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(6):822–7. https://doi.org/10.1038/ajg.2014.71 .
Tuyns AJ, Pequignot G, Abbatucci JS. Oesophageal cancer and alcohol consumption; importance of type of beverage. Int J Cancer. 1979;23(4):443–7. https://doi.org/10.1002/ijc.2910230402 .
Islami F, Poustchi H, Pourshams A, et al. A prospective study of tea drinking temperature and risk of esophageal squamous cell carcinoma. Int J Cancer. 2020;146(1):18–25. https://doi.org/10.1002/ijc.32220 .
Yu C, Tang H, Guo Y, et al. Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: a Population-Based Cohort Study. Ann Intern Med. 2018;168(7):489–97. https://doi.org/10.7326/M17-2000 .
Wang L, Zhu D, Zhang C, et al. Mutations of O6-methylguanine-DNA methyltransferase gene in esophageal cancer tissues from Northern China. Int J Cancer. 1997;71(5):719–23. https://doi.org/10.1002/(sici)1097-0215(19970529)71:5%3c719::aid-ijc5%3e3.0.co;2-u .
Cheng SJ, Sala M, Li MH, Chouroulinkov I. Esophageal cancer in Linxian county, China: a possible etiology and mechanism (initiation and promotion). Carcinog Compr Surv. 1982;7:167–74.
CAS PubMed Google Scholar
Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97(4):301–6. https://doi.org/10.1093/jnci/dji042 .
Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer. 2013;133(2):473–85. https://doi.org/10.1002/ijc.28024 .
Yang CS, Sun Y, Yang QU, et al. Vitamin A and other deficiencies in Linxian, a high esophageal cancer incidence area in northern China. J Natl Cancer Inst. 1984;73(6):1449–53.
Tachibana M, Abe S, Yoshimura H, et al. Squamous cell carcinoma of the esophagus after partial gastrectomy. Dysphagia. 1995;10(1):49–52. https://doi.org/10.1007/BF00261281 .
Keeney S, Bauer TL. Epidemiology of adenocarcinoma of the esophagogastric junction. Surg Oncol Clin N Am. 2006;15(4):687–96. https://doi.org/10.1016/j.soc.2006.07.014 .
Alexandre L, Broughton T, Loke Y, Beales IL. Meta-analysis: risk of esophageal adenocarcinoma with medications which relax the lower esophageal sphincter. Dis Esophagus. 2012;25(6):535–44. https://doi.org/10.1111/j.1442-2050.2011.01285.x .
Lagergren J, Bergstrom R, Adami HO, Nyren O. Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med. 2000;133(3):165–75. https://doi.org/10.7326/0003-4819-133-3-200008010-00007 .
Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23(1):3–9. https://doi.org/10.1016/j.semradonc.2012.09.008 .
Chen R, Ma S, Guan C, et al. The National Cohort of Esophageal Cancer-Prospective Cohort Study of esophageal cancer and precancerous lesions based on high-risk population in China (NCEC-HRP): study protocol. BMJ Open. 2019;9(4):e027360. https://doi.org/10.1136/bmjopen-2018-027360 .
Liu M, He Z, Guo C, et al. Effectiveness of intensive endoscopic screening for esophageal cancer in China: a community-based study. Am J Epidemiol. 2019;188(4):776–84. https://doi.org/10.1093/aje/kwy291 .
Download references
Author information
Authors and affiliations.
Department of Medicine, The University of Tennessee Health Sciences Center, Memphis, TN, USA
Steve Lander
Department of Medicine, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA
Eric Lander & Michael K. Gibson
You can also search for this author in PubMed Google Scholar
Contributions
Steve Lander MD – Primary Author
Eric Lander MD – Contributor and Editor
Michael K Gibson MD PhD FACP – Author, Editor, and Principal Investigator
Corresponding author
Correspondence to Steve Lander .
Ethics declarations
Ethical approval.
Not applicable.
Disclosures
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Lander, S., Lander, E. & Gibson, M.K. Esophageal Cancer: Overview, Risk Factors, and Reasons for the Rise. Curr Gastroenterol Rep 25 , 275–279 (2023). https://doi.org/10.1007/s11894-023-00899-0
Download citation
Accepted : 31 August 2023
Published : 09 October 2023
Issue Date : November 2023
DOI : https://doi.org/10.1007/s11894-023-00899-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Esophageal cancer
- Epidemiology
- Squamous cell carcinoma
- Adenocarcinoma
- Risk factors
- Surveillance
- Public health
- Find a journal
- Publish with us
- Track your research
- Introduction
- Conclusions
- Article Information
APC indicates annual percentage change; SEER, Surveillance, Epidemiology, and End Results.
a P <.05.
APC indicates annual percentage change.
eFigure. Heatmap of Joinpoint Analysis of Esophageal Cancer (EC), Adenocarcinoma of Esophagus (ACE), and Squamous Carcinoma of Esophagus (SCE) Incidence by Age Group, Sex, and Race (2000-2018; SEER 21)
Data sharing statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Rodriguez GM , DePuy D , Aljehani M, et al. Trends in Epidemiology of Esophageal Cancer in the US, 1975-2018. JAMA Netw Open. 2023;6(8):e2329497. doi:10.1001/jamanetworkopen.2023.29497
Manage citations:
© 2024
- Permissions
Trends in Epidemiology of Esophageal Cancer in the US, 1975-2018
- 1 Divisions of Hematology and Medical Oncology, Stanford University School of Medicine, Stanford, California
- 2 Lawrence J. Ellison Institute for Transformative Medicine, Los Angeles, California
- 3 Permanente Medical Group, Santa Clara, California
- 4 Departments of Medicine, Chemical, and Material Sciences and Quantitative and Computational Biology, University of Southern California, Los Angeles
- 5 Esophageal Diseases Center, Hamon Center for Therapeutic Oncology Research, Division of Hematology and Oncology, Simmons Cancer Center, UT Southwestern Medical Center, Dallas, Texas
- 6 VA North Texas Health Care System, Dallas
- 7 VA Palo Alto Health Care System, Palo Alto, California
- 8 Stanford University School of Medicine, Stanford, California
Question How did the incidence patterns of esophageal cancer (EC) and its 2 primary histologic subtypes, squamous cell carcinoma and adenocarcinoma (ACE), change from 1975 to 2018?
Findings In this population-based cross-sectional study of 47 648 patients with EC, the overall annual percentage change (APC) in incidence of EC increased significantly from 1975 to 2004 by 0.53 and then modestly declined from 2004 to 2018 with an APC of −1.25. From 2000 to 2018, squamous cell carcinoma incidence significantly declined, with an APC of −2.80, while ACE incidence increased from 2000 to 2006 with an APC of 2.51 before stabilizing from 2006 to 2018.
Meaning The results of this cross-sectional study suggest that understanding factors associated with plateaued rates of ACE may help inform public health interventions.
Importance Esophageal cancer (EC) is the 7th most common cancer worldwide and 14th in the US. More data are needed to study the changing incidence patterns of its 2 primary histologic subtypes, squamous cell carcinoma of the esophagus (SCE) and adenocarcinoma of the esophagus (ACE).
Objective To examine temporal trends in incidence rates of EC, ACE, and SCE from 1975 through 2018.
Design, Setting, and Participants In this population-based cross-sectional study, data were derived from 9 Surveillance, Epidemiology, and End Results (SEER) registries from January 1975 through December 2018 and from all 21 registries for January 2000 through December 2018 for patients with a diagnosis of EC from 1975 through 2018 ( International Classification of Disease—Oncology, Third Edition codes). Age-adjusted incidence rates (AAIRs) of EC, ACE, and SCE were calculated. The timing and magnitude of the annual percentage change (APC) in incidence were examined using Joinpoint regression analyses. Data analysis was started in 2021 and updated and completed in 2023.
Main Outcome and Measures The APC for age-adjusted EC incidence rates as stratified by histology, anatomical location, stage, sex, age, race and ethnicity, and geographic region.
Results A total of 47 648 patients with a diagnosis of EC were retained for analysis. These included 22 419 (47.1%) with a diagnosis of SCE, 22 217 (46.6%) with ACE, and 3012 (6.3%) with other subtypes. The AAIR for EC changed from 4.14 per 100 000 population in 1975 to 4.18 in 2018, AAIRs of SCE declined from 3.06 in 1975 to 1.15 in 2018 as well as for ACE, and AAIRs increased from 0.42 in 1975 to 2.78 in 2018. From 1975 through 2004, EC incidence significantly increased (APC, 0.53; 95% CI, 0.4 to 0.7) but significantly decreased (APC, −1.03; 95% CI, −1.3 to −0.7) from then until 2018. The APC of SCE significantly continued to decline (−2.80, 95% CI, −3.0 to −2.6), and ACE increased from 2000 to 2006 (APC, 2.51; 95% CI, 1.0 to 4.0) but has since stabilized from 2006 to 2018.
Conclusions and Relevance The results of this cross-sectional study suggest that the incidence of EC modestly declined since 2004 and that the incidence of SCE continued to decline while the incidence rate of ACE plateaued for more than a decade. Understanding factors associated with plateaued rates of ACE may help inform public health interventions.
Esophageal cancer (EC) is the seventh most common cancer and the sixth leading cause of cancer mortality worldwide, with about 544 000 deaths reported in 2020. 1 The 5-year survival for all stages of EC combined is around 20%. 2 In the US, EC is the 14th most common cancer, with an estimated 21 560 new diagnoses and 16 120 expected deaths in 2023. 2 Men are at higher risk of developing EC than women. 2 Management of EC has evolved to include immunotherapy as part of the standard treatment for early and advanced stages. 3 Squamous cell carcinoma of the esophagus (SCE) and adenocarcinoma of the esophagus (ACE) are the 2 most common histologic subtypes of EC. Risk factors for EC include gastroesophageal reflux disease (GERD), Barrett esophagus, obesity, metabolic syndrome, alcohol use, and tobacco smoking. 4 Studies have shown that obesity is likely to be associated with ACE through an independent and a GERD-dependent and Barrett esophagus–dependent mechanism. 5
The incidence of SCE has steadily declined during the last few decades. 6 In contrast, the incidence of ACE increased from the 1970s to 2006 from 3.6 cases per million to 25.6 cases per million, a 7-fold increase. 7 A study spanning from 1997 to 2014 suggested that ACE incidence decreased or stabilized. 6 Longer follow-up is needed to study the overall incidence patterns of EC, ACE, and SCE and provide a better delineation of the changes in trends in recent years. More data are also needed to investigate incidence patterns among subgroup populations in the US. The objective of this study was to conduct a retrospective population-based analysis to examine temporal trends in incidence rates of EC, ACE, and SCE from 1975 through 2018 using Surveillance, Epidemiology, and End Results (SEER) 9 (the registry with the longest follow-up duration) and SEER 21 (the registry that covers the largest US population registry) and provide an update on recent changes in incidence.
Data used in this study were derived from SEER of the National Cancer Institute, a population-based cancer database. For the period between 1975 through 2018, we used data from 9 SEER registries that covered 9.4% of the US population. From 2000 through 2018, data from all 21 SEER registries, which covered 36.7% of the US population, were used. 8
Records for patients with a diagnosis of EC from 1975 through 2018 were retrieved from the SEER databases using the International Classification of Disease—Oncology, Third Edition ( ICD-O-3 ). 9 Cases with ICD-O-3 morphology codes 8050 to 8082 were classified as SCE, those with codes 8140-8573 as ACE, and those with codes other than 8050 to 8082 and 8140-8573 as other subtypes. This study used deidentified SEER data; thus, institutional review board was not required and informed consent was waived.
Patient demographic characteristics included age group at diagnosis (<65 years, 65-75 years, and >75 years), race and ethnicity (Black, Hispanic, and non-Hispanic White), and sex. Race and ethnicity classification was based on electronic health records and is abstracted by SEER. Census regions were used to assign states for regional analysis. 10 For analysis of stage variation, cases were classified by SEER stage (localized, regional, or distant) at diagnosis. 11 Anatomical distribution was across 4 locations as defined by ICD-O-3 topography codes: cervical esophagus (C15.0), upper thoracic portion (C15.3), middle thoracic portion (C15.4), and lower thoracic portion (C15.5).
Data analyses were conducted in a 2-step approach. In the initial step, age-adjusted incidence rates (AAIRs) for EC, ACE, and SCE were calculated using SEER proprietary statistical analysis software (SEER*Stat, version 8.3.9.2; National Cancer Institute). Incidence rates were computed per 100 000 individuals and age-adjusted to the 2000 US standard population. During the subsequent step, and using incidence rates obtained in the first step, the timing and magnitude of the annual percentage change (APC) in incidence trends over time were examined using the National Cancer Institute’s Joinpoint Trend Analysis Software, version 4.9.0.0. The software tests the statistical significance of a change in trend using a Monte Carlo permutation method (in this study, the overall significance level was set to .05 and number of permutations to 4499). A minimum and maximum of 0 and 3 joinpoints, respectively, were selected. Incidence was considered to be rising when the APC’s 95% CI was greater than 0 and falling when the APC’s 95% CI was less than 0. If the APC’s 95% CI included 0, the incidence trend was considered stable. All figures (except Figure 1 B and the eFigure in Supplement 1 , which do not depict rates) were prepared such that a 10° slope reflects a 1% annual rate of change. 12 The reporting of study results followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) checklist for cross-sectional studies.
A total of 47 648 patients with a diagnosis of EC were retained for analysis. These included 22 419 (47.1%) with a diagnosis of SCE, 22 217 (46.6%) with ACE, and 3012 (6.3%) with other subtypes.
The AAIR for EC overall changed modestly from 4.14 per 100 000 population in 1975 to 4.18 in 2018 despite large changes in the AAIRs of SCE (3.06 in 1975, 1.15 in 2018) and ACE (0.42 in 1975, 2.78 in 2018) ( Figure 1 A). As such, the histologic subtype responsible for most EC incidence has gradually shifted from SCE (73.91% of all EC incidence in 1975) to ACE (66.51% of all EC incidence in 2018) ( Figure 1 B).
Joinpoint models of EC, ACE, and SCE overall incidence from 1975 to 2018 (SEER 9) and 2000 to 2018 (SEER 21) are shown in Figure 2 . SEER 9 suggests that EC incidence was increasing significantly from 1975 to 2004 (APC, 0.53; 95% CI, 0.4 to 0.7) and then declined significantly by 1.0% annually (95% CI, −1.3 to −0.7) until 2018. SEER 21 also points to EC incidence beginning to decline in 2004, although at a slightly faster rate (APC, −1.25; 95% CI, −1.5 to −1.0). In SEER 9, SCE incidence appeared stable from 1975 to 1986, declined from 1986 to 2011 (APC, −3.28; 95% CI, −3.5 to −3.0), and then stabilized after 2011. However, in SEER 21, SCE incidence continued to decline until 2018 (2000-2018: APC, −2.80; 95% CI, −3.0 to −2.6). A rapid rise in ACE incidence from 1975 to 1999 (APC, 7.61; 95% CI, 7.0 to 8.2) was observed in SEER 9, which slowed after 1999 (1999-2018: APC, 0.56; 95% CI, 0.1 to 1.0). Incidence of ACE stabilized in 2006 according to SEER 21 (2000-2006: APC, 2.51; 95% CI, 1.0 to 4.0) ( Figure 2 ).
Joinpoint models of EC, SCE, and ACE incidence from 2000 to 2018 (SEER 21) as stratified by age group, sex, and race and ethnicity are shown in the eFigure in Supplement 1 . For EC overall, 4 of 48 groups exhibited increasing incidence for any period (non-Hispanic White male individuals overall, non-Hispanic White older than 75 years overall, male individuals older than 75 years overall, and non-Hispanic White male individuals older than 75 years). The fastest rate of increase was observed among both non-Hispanic White male individuals older than 75 years and non-Hispanic White individuals older than 75 years overall (APC, 3.3; 95% CI, 0.4 to 6.3) from 2000 to 2004. Among the older than 75 years group, 4 subgroups exhibited a joinpoint in incidence in 2004 that was followed by declining incidence: older than 75 years overall, non-Hispanic White overall, male individuals overall, and non-Hispanic White males overall. A similar trend was observed among non-Hispanic White male individuals younger than 65 years, non-Hispanic White male individuals overall, male individuals overall, and non-Hispanic White individuals overall (eFigure in Supplement 1 ).
For SCE, 42 of 48 groups saw declining incidence during the entire period. Groups that demonstrated statistically significant rapid rates of decline included all Hispanic male individuals (2015 to 2018: APC, −9.0 95% CI, −15.5 to −2.0), Hispanic male individuals aged 65 to 75 years (2010 to 2018: APC, −8.5; 95% CI, −11.9 to −4.9), and Black male individuals younger than 65 years (2000 to 2018: APC, −6.2; 95% CI, −6.9 to −5.5). However, the subgroup of Hispanic male individuals older than 75 years exhibited the fastest rates of decline, although it was not statistically significant (2000 to 2004: APC, −12.3; 95% CI, −24 to 1.2; 2016 to 2018: APC, −24.5; 95% CI, −51 to 16.5). This lack of significance may be attributed to the limited number of patients within that age group (eFigure in Supplement 1 ).
Incidence of ACE was stable among 32 of 48 groups during the entire period. The largest rate of increase was observed among non-Hispanic White individuals older than 75 years overall (APC, 7.2; 95% CI, 2.8 to 11.7) and non-Hispanic White male individuals older than 75 years (APC, 7.2; 95% CI, 2.7 to 11.9) from 2000 to 2004. The same 4 groups from the older than 75 years group that exhibited a shift in EC incidence overall in 2004 also did so for ACE incidence, after which incidence plateaued. Other groups exhibited similar trends, including all those of White male individuals, with segments beginning in 2000 and ending between 2004 and 2007 characterized by increases in incidence that were followed by segments until 2018 with relatively little change in or stabilized incidence (eFigure in Supplement 1 ).
Data for EC, SCE, and ACE AAIRs by stage at diagnosis were available from 2004 to 2018 (SEER 21), and corresponding joinpoint models are shown in Figure 3 . Incidence of localized EC was lower than regional and distant and declined from 2004 to 2016 by −3.26% per year (95% CI, −3.9 to −2.6). Incidence of ACE was stable. Localized SCE incidence declined from 2004 to 2013 at an APC of −6.46 (95% CI, −7.6 to −5.3) and then stabilized. Regional EC incidence was stable, SCE incidence fell by 1.42% annually (95% CI, −2.2 to −0.6) from 2004 to 2018, and ACE incidence rose from 2004 to 2016 (APC, 1.24; 95% CI, 0.5 to 1.9). Distant EC and ACE incidence were steady, although SCE incidence fell at an APC of −2.73 (95% CI, −3.7 to −1.7) from 2004 to 2018 ( Figure 3 ).
Incidence trends of EC, ACE, and SCE by anatomical site of origin from 2000 to 2018 are shown in Figure 4 . Incidence of EC originating in the cervical esophagus declined from 2000 to 2018 by 3.62% annually (95% CI, −4.61 to −2.63). This change was largely associated with the declining SCE incidence (2000 to 2018: APC, −3.35; 95% CI, −4.39 to −2.3), the predominant histology in the cervical esophagus. From 2000 to 2018, incidence of EC originating in the upper thoracic portion of the esophagus declined at an APC of −0.84% (95% CI, −1.42 to −0.25), SCE incidence declined at an APC of −0.73 (95% CI, −1.38 to −0.06), and ACE incidence was stable. Incidence of EC originating in the midthoracic portion of the esophagus declined by 2.27% annually from 2000 to 2018 (95% CI, −2.64 to −1.9). Midthoracic SCE incidence declined at an APC of 2.57 (95% CI, −3.01 to −2.13), and ACE incidence declined at an APC of −0.90 (95% CI, −1.57 to −0.22) from 2000 to 2018. Incidence of EC was highest for cases originating in the lower thoracic portion of the esophagus and was stable from 2000 to 2018. Lower thoracic ACE incidence was also stable, and SCE incidence declined by 3.22% annually (95% CI, −3.66 to −2.78) ( Figure 4 ).
Figure 5 depicts joinpoint models of EC, ACE, and SCE incidence patterns by region. Incidence was lowest in the West, where EC incidence declined by −0.99% annually (95% CI, −1.3 to −0.7), ACE incidence was stable, and SCE incidence declined at an APC of −2.53 (95% CI, −2.9 to −2.2) from 2000 to 2018. In the Northeast, EC incidence was stable, while ACE incidence first climbed at an APC of 3.14 (95% CI, 2.0 to 4.2) from 2000 to 2008 and then subsequently stabilized. Incidence of SCE declined by 2.79% annually (95% CI, −3.0 to −2.5). In the South, EC and SCE incidence declined from 2000 to 2018 (EC: APC, −0.89; 95% CI, −1.1 to −0.6; SCE: APC, −3.20; 95% CI, −3.7 to −2.7), while ACE incidence climbed by 2.10% annually (95% CI, 0.8 to 3.4) from 2000 to 2007 before decelerating to APC of 0.54 (95% CI, 0.0 to 1.1) from 2007 to 2018. Lastly, in the Midwest, EC incidence did not change significantly, SCE incidence declined by −4.20% annually (95% CI, −5.9 to −2.5) from 2000 to 2011 and stabilized thereafter, and ACE incidence rose from 2000 to 2006 (APC, 4.2; 95% CI, 2.0 to 6.4) and from 2006 to 2018 (APC, 0.78; 95% CI, 0.1 to 1.4) ( Figure 5 ).
In this retrospective population-based cross-sectional study, we examined temporal trends in incidence rates of EC and its 2 most common histologic subtypes, ACE and SCE, from 1975 through 2018 using the SEER 9 and SEER 21 registries. Based on the SEER 9, EC incidence increased from 1975 to 2004 at an APC of 0.53 (95% CI, 0.4 to 0.7) and then declined significantly by 1.0% annually (95% CI, −1.3 to −0.7) until 2018. In SEER 21, we found that despite age, sex, racial and ethnic, and geographic variation, the overall incidence of EC modestly declined since 2004, with an APC of −1.25 (95% CI, −1.5 to −1.0). Specifically, SEER 9 suggested that SCE declined from 1986 to 2011 (APC, −3.28; 95% CI, −3.5 to −3.0) and then stabilized after 2011. However, in SEER 21, from 2000 to 2018, SCE incidence significantly continued to decline (APC of −2.80, 95% CI, −3.0 to −2.6). Conversely, ACE incidence increased from 2000 to 2006 (APC of 2.51, 95% CI, 1.0 to 4.0) but then stabilized.
In this study, similar to other studies, we observed a sharp increase in the incidence rate of ACE from 1975 to the early 2000s 6 , 7 prior to plateauing. The surge in ACE has been attributed to the marked rise in obesity and metabolic syndrome, risk factors that are more associated with ACE than SCE. 13 , 14 However, while obesity rates in the US continue to increase, 15 ACE incidence has plateaued during the last decade. The factors associated with this stable trend of ACE are not well understood. We speculate that a possible explanation for this pattern could be a counteracting association of the declining smoking rates 16 with the increasing obesity rates. However, tobacco use is a risk factor more closely associated with SCE than ACE and may have a limited association with ACE incidence. 17 The role of proton pump inhibitors (PPIs) in ACE incidence remains controversial. While one may speculate that the increased use of PPIs 18 may be associated with reduced transition of Barrett esophagus to ACE, studies show that ACE incidence continued to increase 6 , 7 following the US Food and Drug Administration approval of PPIs in 1989. 19 Some studies have shown that long-term use of PPIs is associated with increased risk of ACE. 20 - 24 One possible explanation is that patients have fewer symptoms and are less aware of their carcinogenic bile reflux with PPI use. More studies are needed to understand their role in ACE incidence.
The rates of upper endoscopies have increased over the years 25 in the US, which could potentially be associated with an increase in the identification of premalignant lesions. Surprisingly, we did not observe an increase in the incidence of localized disease in EC, which would be expected if more premalignant lesions were identified through upper endoscopies. Instead, the incidence of localized disease in EC decreased or stabilized since the early 2000s. Further, we did not find evidence of stage migration. In the subgroup analysis by stage, we found that the incidence of regional and distant EC generally decreased or stabilized since the early 2000s, a change from the previously observed upward trend in SEER-based analysis from 1975 to 2006. 7
In a subgroup analysis by race and ethnicity, age group, and sex, we found that non-Hispanic White male individuals overall exhibited the fastest rate of increase in EC and ACE at any point compared with other racial and ethnic groups. This finding has been reported in other studies that found that non-Hispanic White male individuals showed higher rates of ACE than other racial and ethnic groups. 6 Studies to understand the reason for the higher incidence in this group are needed, although the higher incidence of GERD and Barrett esophagus, which predisposes to ACE, in this population likely plays a role.
Treatment options with the addition of immunotherapy are improving overall survival for EC, 3 but the prognosis for EC remains poor. 2 Therefore, we hope these findings will motivate public health interventions to reduce exposure to modifiable risk factors for EC. Interventions targeting obesity, metabolic syndrome, and smoking may decrease the incidence of EC.
This study had several strengths and limitations. First, to our knowledge, this study is the most updated, nationwide, population-based analysis of the incidence of EC, ACE, and SCE in the US. Second, the study contains the largest cohort and reports on 44 years of data, the longest period studied. Third, 1 limitation is that we did not include the Asian and Other Pacific Islander individuals in the subgroup analysis because of the small sample size in the registries, so we were unable to explore incidence trends among this racial group. Fourth, we selected the 3 age group categories (<65 years, 65-75 years, and >75 years) to divide the cohort evenly for the subgroup analysis. Therefore, we may have obtained different subgroup incidence trends if we had divided the age groups differently. Also, given the limited number of patients younger than 50 years, we were unable to create a subgroup to analyze young-onset EC, as it would have resulted in insufficient statistical power to have drawn conclusive results. Fifth, the SEER 9 and SEER 21 registries do not have data from all the states; however, the registries cover 9.4% and 36.7% of the US population, respectively, and provide one of the best representative US cancer data registries. Sixth, the SEER database did not include information on GERD, Barret esophagus, or obesity. Therefore, we were unable to describe the prevalence of these factors during different periods. Finally, the SEER database has limited individual level data and does not include detailed clinical characteristics (ie, performance status) or specific treatment information (ie, chemotherapy regimens) to conduct individualized or causal inference analyses.
In this population-based cross-sectional analysis from 1975 to 2018, we found that the incidence of EC and SCE continue to decrease while the incidence of ACE stabilized. Further studies are needed to identify the factors contributing to these patterns in incidence, particularly why the ACE incidence has plateaued for more than a decade and has not continued to decrease like SCE.
Accepted for Publication: July 9, 2023.
Published: August 22, 2023. doi:10.1001/jamanetworkopen.2023.29497
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Rodriguez GM et al. JAMA Network Open .
Corresponding Author: Albert Y. Lin, MD, Department of Oncology, 3801 Miranda Avenue, Palo Alto, CA 94304-1207 ( [email protected] ).
Author Contributions: Drs Lin and Aljehani had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Aljehani, Bien, Lee, Lin.
Acquisition, analysis, or interpretation of data: Rodriguez, DePuy, Aljehani, Bien, Wang, Lin.
Drafting of the manuscript: Rodriguez, DePuy, Aljehani, Lin.
Critical review of the manuscript for important intellectual content: Rodriguez, Aljehani, Bien, Lee, Wang, Lin.
Statistical analysis: DePuy, Aljehani, Lin.
Obtained funding: Aljehani.
Administrative, technical, or material support: DePuy, Aljehani, Lin.
Supervision: Rodriguez, Aljehani, Lee, Wang, Lin.
Conflict of Interest Disclosures: Dr Lin reported grants from US Department of Defense during the conduct of the study. No other disclosures were reported.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We thank Susan Devesa, PhD, National Cancer Institute, for her helpful discussion in data analysis. No compensation was given for her contribution.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Explore CRI’s 2023 Cancer Research Impact
Immunotherapy For Esophageal Cancer
How is immunotherapy for esophageal cancer changing the outlook for patients.
Reviewed by:
Samuel J. Klempner, MD Dana-Farber / Harvard Cancer Center
Immunotherapy for esophageal cancer is being explored to reduce recurrence, as a first-line treatment, and in novel combinations for advanced stage cancer.
Two main types of cancer can affect the esophagus, a muscular tube through which food passes from the mouth to the stomach, as well as the gastroesophageal junction (GEJ):
- squamous cell carcinoma: cancer that begins in flat cells lining the esophagus
- adenocarcinoma: cancer that begins in cells that make and release mucus, which are usually associated with ectopic gastric mucosa
Esophageal cancer is three to four times more common in men than in women. Risk factors for esophageal cancer include smoking tobacco and heavy alcohol use as well as having acid reflux, which can inflame the cells of the esophagus and GEJ. Esophageal cancer is estimated to affect approximately 500,000 people globally each year. In the U.S. alone, there will be an estimated 22,000 new cases of esophageal cancer and 16,000 deaths in 2023. The five-year relative survival rate for patients with esophageal cancer is 43% for patients with localized disease found only in the esophagus; 23% for regional disease that has spread to nearby lymph nodes and organs; and 5% for metastatic disease that has spread to distant parts of the body.
Esophageal Cancer Treatment Options
When esophageal cancer is caught early, there are several effective therapies available, including endoscopic therapies, surgery, chemotherapy, and radiotherapy. Surgery remains the most common treatment for esophageal cancer, and surgery, chemotherapy, and radiation work well against localized cancers. Chemotherapy forms the backbone of therapy in patients with advanced or metastatic tumors.
Immunotherapy is class of treatments that take advantage of a person’s own immune system to help kill cancer cells. There are six FDA-approved immunotherapy options for esophageal cancer.
Targeted Antibodies
- Ramucirumab (Cyramza ®): a monoclonal antibody that targets the VEGF/VEGFR2 pathway and inhibits tumor blood vessel growth; approved for subsets of patients with advanced gastroesophageal cancer
- Trastuzumab (Herceptin®): a monoclonal antibody that targets the HER2 pathway; approved for subsets of patients with advanced, HER2-positive gastroesophageal cancer, including as a first-line therapy
- Trastuzumab deruxtecan (Enhertu®): an antibody-drug conjugate that targets the HER2 pathway; approved for subsets of patients with advanced gastroesophageal cancer
Immunomodulators
- Dostarlimab (Jemperli): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced esophageal or gastroesophageal cancer that has DNA mismatch repair deficiency (dMMR)
- Nivolumab (Opdivo®): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced esophageal or gastroesophageal cancer
- Pembrolizumab (Keytruda®) : a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced esophageal or gastroesophageal cancer
Immunotherapy research on esophageal cancer is ongoing and holds the promise of new treatment options. Several other immunotherapy approaches for esophageal cancer have shown promise in early clinical trials.
CRI’s Impact in Esophageal Cancer
Immunotherapy has the potential to improve the outlook for patients and families affected by the disease and bring us ever closer to effective, lasting cures for esophageal cancer.
- CRI researchers analyzed NY-ESO-1 cancer-testis (CT) antigen expression in esophageal cancer and have sought to correlate this expression with disease stage and clinical outcome (high expression frequency indicates a feasible vaccine target).
- Clinical investigator Eiichi Nakayama, MD, and colleagues at Okayama University Graduate School of Medicine and Dentistry in Japan reported in the International Journal of Cancer that a vaccine composed of the NY-ESO-1f long peptide administered with the immune stimulants Montanide ISA-51 and Picibanil OK-432 could elicit integrated immune responses including antibodies, CD4+ helper T cells, and CD8+ killer T cells in nine out of the ten patients enrolled in a phase I clinical trial.
Explore CRI’s current funding for esophageal cancer research in our funding directory.
78% of cases occur in men
500K Newly diagnosed patients each year globally
Esophageal Cancer Clinical Trial Targets
Discover the different proteins, pathways, and platforms that scientists and physicians are pursuing to develop new cancer treatments. Use this information to consider your clinical trial options.
Targeted antibodies are proteins produced by the immune system that can be customized to target specific markers on cancer cells in order to disrupt cancerous activity, especially unrestrained growth. Antibody-drug conjugates (ADCs) are equipped with anti-cancer drugs that they can deliver to tumors. Bi-specific T cell-engaging antibodies (BiTEs) bind both cancer cells and T cells in order to help the immune system respond more quickly and effectively. Antibody targets under evaluation in esophageal cancer clinical trials include:
- cMET: a growth-related pathway that is often abnormally activated in cancer
- Claudin 18.2: a surface protein overexpressed in some esophageal cancers and involved in invasion and survival
- DKK1: a secreted protein involved in migration, self-renewal, and blood vessel formation
- EGFR: a pathway that controls cell growth and is often mutated in cancer
- FGF/FGF-R: a pathway that controls cell growth, death, and migration
- HER2: a pathway that controls cell growth and is commonly overexpressed in cancer and associated with metastasis
- TROP2: a protein that is commonly overexpressed in cancer and appears to aid cancer cell self-renewal, proliferation, invasion, and survival
- VEGF/VEGF-R: a pathway that can promote blood vessel formation in tumors
Cancer vaccines are designed to elicit an immune response against tumor-specific or tumor-associated antigens, encouraging the immune system to attack cancer cells bearing these antigens. Cancer vaccines can be made from a variety of components, including cells, proteins, DNA, viruses, bacteria, and small molecules. Cancer vaccine targets under evaluation in esophageal cancer clinical trials include:
- Human Papilloma Virus (HPV)-related antigens: foreign viral proteins expressed by HPV-infected cancer cells
- Mesothelin: a protein that is commonly overexpressed in cancer and may aid metastasis
- Telomerase: an enzyme that helps maintain the health of cellular DNA; exploited by cancer cells to achieve immortality
- Tumor-associated antigens (TAAs): proteins often expressed at abnormally high levels on tumor cells that can be used to target them; also found on normal cells at lower levels
Adoptive cell therapy takes a patient’s own immune cells, expands or otherwise modifies them, and then reintroduces them to the patient, where they can seek out and eliminate cancer cells. In CAR T cell therapy, T cells are modified and equipped with chimeric antigen receptors (CARs) that enable superior anti-cancer activity. Natural killer cells (NKs) and tumor infiltrating lymphocytes (TILs) can also be enhanced and reinfused in patients. Cell-based immunotherapy targets under evaluation in esophageal cancer clinical trials include:
- Claudin 18.2: a surface protein overexpressed in some esophageal cancers and involved in tumor invasion and survival
- Epstein-Barr Virus (EBV)-related antigens: foreign viral proteins expressed by EBV-infected cancer cells
- MAGE antigens: the genes that produce these proteins are normally turned off in adult cells, but can become reactivated in cancer cells, flagging them as abnormal to the immune system
Immunomodulators manipulate the “brakes” and “gas pedals” of the immune system. Checkpoint inhibitors target molecules on immune cells to unleash new or enhance existing immune responses against cancer. Cytokines regulate immune cell maturation, growth, and responsiveness. Adjuvants can stimulate pathways to provide longer protection or produce more antibodies. Immunomodulator targets under evaluation in esophageal cancer clinical trials include:
- CD40: activating this co-stimulatory pathway can kick-start adaptive immune responses
- CD137 (also known as 4-1BB): activating this co-stimulatory pathway can help promote the growth, survival, and activity of cancer-fighting T cells
- CTLA-4: blocking this pathway can help promote expansion and diversification of cancer-fighting T cells
- CXCR4: blocking this pathway can promote the migration and recruitment of immune cells
- GITR: activating this pathway can help prevent immunosuppression and increase the survival of cancer-fighting T cells
- ICOS: activating this co-stimulatory pathway on T cells can help enhance immune responses against cancer
- IDO: blocking this enzyme’s activity can help prevent cancer-fighting T cells from being suppressed
- IL-2/IL-2R: activating this cytokine pathway can help promote the growth and expansion of cancer-fighting T cells
- LAG3: blocking this pathway may be able to help prevent suppression of cancer-fighting T cells
- OX40: activating this co-stimulatory pathway can help promote T cell survival after activation
- PD-1/PD-L1: blocking this pathway can help prevent cancer-fighting T cells from becoming “exhausted,” and can restore the activity of already-exhausted T cells
- STAT3: activating this intracellular signaling protein can help stimulate adaptive immune responses
- TIGIT: blocking this pathway may be able to help prevent suppression of cancer-fighting T cells
- TIM-3: blocking this pathway may be able to help prevent suppression of cancer-fighting T cells
Oncolytic virus therapy uses viruses that are often, but not always, modified in order to infect tumor cells and cause them to self-destruct. This can attract the attention of immune cells to eliminate the main tumor and potentially other tumors throughout the body. Viral platforms under evaluation in esophageal cancer clinical trials include:
- Adenovirus: a family of common viruses that can cause a wide range of typically mild effects including sore throat, fatigue, and cold-like symptoms
- Maraba virus: a virus found exclusively in insects
Find an Immunotherapy Clinical Trial
Create a profile and fill out a questionnaire to identify immunotherapy clinical trials for which you may be eligible.
Need more information? Learn more about clinical trials .

News & Events

AACR 2022 Recap: T Cells Still On Top, But Make Room for Myeloid Cells

#Immune2Cancer Day 2022
On Friday, June 10, 2022, we invite you to raise awareness of the lifesaving potential of immunotherapy.

Giving Tuesday 2022
Be a part of the global generosity movement and celebrate all acts of giving. #GivingTuesday
This website uses tracking technologies, such as cookies, to provide a better user experience. If you continue to use this site, then you acknowledge our use of tracking technologies. For additional information, review our Privacy Policy .
Clinical Trials
Esophageal cancer.
Displaying 70 studies
The purpose of this study is to evaluate circulating tumor DNA as a potential biomarker for pre-surgery treatment response in patients who have esophageal cancer.
The purpose of this study is to learn about why esophageal cancer develops. We would like to test the tissue to find out which way of saving esophageal tissue gives us the best information (DNA and RNA) to use for future studies and collect a blood sample to use as a comparison for normal and tumor DNA.
The purpose of this study is to see if different depths of submucosal tumor invasion in esophageal cancer can predict lymph node involvement and survival.
To prospectively collect blood and tumor tissue from esophageal cancer patients to identify specific esophageal cancer mutations that can be measured in the blood (cell free DNA) during the course of treatment as a marker of response and recurrence.
The purpose of this research study is to find out what effects, good and/or bad, the drug regorafenib has on stomach or esophagus cancer after completion standard chemotherapy, radiation therapy and surgery prescribed by your doctor. In this study the participant will either get regorafenib or a "placebo".
The purpose of this study is to get definite evidence for the effectiveness of a short preoperative inspiratory muscle training protocol on the level of sickness and recovery of patients who are having the surgical removal of a portion of the esophagus.
This is a prospective observational study designed to observe the toxicity and efficacy of PBS proton RT for patients with esophageal cancer undergoing trimodality therapy. The investigators hypothesize that PBS proton RT will be associated with a favorable adverse event profile and quality of life, with similar disease control outcomes, relative to historical comparisons of patients treated with photon RT.
The aims of this study are to:
Study the history of esophageal leiomyomas in patients followed with periodic surveillance with endoscopic ultrasound.
Determine baseline characteristics and symptoms of patients diagnosed with esophageal leiomyomas.
Determine the transformation rate of malignancy or symptoms (if previously asymptomatic) in patients who undergo interval surveillance EUS examinations for esophageal leiomyomas.
Determine risk factors for progression of symptoms or malignancy in patients followed with interval surveillance EUS exams for esophageal leiomyomas.
Assess morbidity and mortality in patients followed with interval surveillance EUS examinations for esophageal leiomyomas.
Identify malignant sub-epithelial tumors, including leiomyosarcomas, granular cell tumors, and gastrointestinal stromal tumors (GIST) to see ...
The purpose of this study is to assess how well paclitaxel, with or without cixutumumab, works for treating patients who have esophageal cancer or gastroesophageal junction cancer that has spread to other places in the body. Drugs used in chemotherapy, such as paclitaxel, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Cixutumumab may kill cancer cells by blocking the action of a protein needed for cancer cell growth. Giving paclitaxel with or without cixutumumab may kill more tumor cells.
Using the analysis of group velocity for a screening application and then higher order analysis based on the elastic and viscious components of the shear modulus may allow discrimination between extent of tumor invasion through the esophageal wall if appropriately correlated with pathological findings.
Surgery has been historically the mainstay treatment for advanced pre-malignant lesions and early esophageal cancers. However, esophagectomy is associated with significant morbidity and mortality. With the advance of therapeutic endoscopy, there has been a growing interest and application of endoscopic resection and mucosal ablative techniques for the treatment of these diseases. Esophageal stricture(ES) formation has become an increasingly recognized complication of extensive endoscopic mucosal ablation and/or resection. The resultant symptomatic stricture development can significantly impair a patient's quality of life. Endoscopic therapy of esophageal strictures with balloon dilation and/or local steroid injection is invasive, costly, and associated with the potential ...
THe purpose of this international study is to identify the incidence of oesophago-gastric leaks, identify when they are diagnosed and how they are specifically managed.
The purpose of this study is to collect blood and tumor tissue from esophageal cancer patients in order to identify specific esophageal cancer mutations that can be measured in the blood (cell free DNA) during the course of treatment as a marker of response and recurrence.
The purpose of this study is to evaluate how well proton beam radiation therapy compared with intensity modulated photon radiotherapy works in treating patients with stage I-IVA esophageal cancer. Proton beam radiation therapy uses a beam of protons (rather than x-rays) to send radiation inside the body to the tumor without damaging much of the healthy tissue around it. Intensity modulated photon radiotherapy uses high-energy x-rays to deliver radiation directly to the tumor without damaging much of the healthy tissue around it. It is not yet known whether proton beam therapy or intensity modulated photon radiotherapy will work better in ...
RATIONALE: PET scans done during chemotherapy may help doctors assess a patient's response to treatment and help plan the best treatment.
PURPOSE: This randomized phase II trial is studying PET scan imaging in assessing response in patients with esophageal cancer receiving combination chemotherapy.
This randomized phase III trial studies how well radiation therapy, paclitaxel, and carboplatin with or without trastuzumab work in treating patients with esophageal cancer. Radiation therapy uses high-energy x-rays to kill tumor cells. Drugs used in chemotherapy, such as paclitaxel and carboplatin, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Monoclonal antibodies, such as trastuzumab, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry tumor-killing substances to ...
This Phase 2, open-label, parallel, 3-cohort, multicenter study will evaluate the safety and efficacy of various combinations of the anti-T-cell immunoglobulin and ITM domain (TIGIT) monoclonal antibody domvanalimab, the anti-programmed cell death protein 1 (PD-1) monoclonal antibody zimberelimab, and multiagent chemotherapy in the first--line setting, and of various combinations of domvanalimab, zimberelimab, the cluster of differentiation 73 (CD73) inhibitor quemliclustat, and chemotherapy in the second-line (2L) or greater setting in participants with locally advanced unresectable or metastatic gastric, gastroesophageal junction (GEJ), and esophageal adenocarcinoma.
The purpose of this study is to evaluate the treatment of patients with HER2-positive Gastric cancer (GC) or Gastroesophageal Junction (GEJ) cancer to determine the effectiveness of margetuximab combined with INCMGA00012 (also known as MGA012) (Cohort A) and margetuximab combined with INCMGA00012 or MGD013 and chemotherapy compared to trastuzumab combined with chemotherapy (Cohort B).
The purpose of this study is to confirm the performance of the Captivator™ endoscopic mucosal resection device for resection of early tumor growth in Barrett's Esophagus.
The purpose of this study is to find out whether it is better to receive a new drug, BBI608, in addition to paclitaxel chemotherapy or better to receive paclitaxel chemotherapy alone as second line treatment for gastric and gastroesophageal junction cancer after prior first line platinum and fluoropyrimidine based chemotherapy.
The purpose of this study is to anayze the usefulness of treatment with nivolumab and ipilimumab in addition to standard of care chemotherapy and radiation therapy in patients with esophageal and gastroesophageal junction adenocarcinoma who are undergoing surgery. Immunotherapy with antibodies, such as nivolumab and ipilimumab, may remove the brake on the body's immune system and may interfere with the ability of tumor cells to grow and spread. Chemotherapy and radiation therapy may reduce the tumor size and the amount of normal tissue that needs to be removed during surgery. A combined treatment with nivolumab and ipilimumab, chemotherapy, and radiation therapy ...
The purpose of this study is to determine if Regorafenib improves overall survival in refractory AGEC A randomized phase III, double-blind, placebo-controlled trial with 2:1 (regorafenib : placebo) randomization and stratification by:
- Location of tumor (GEJ vs. gastric);
- Geographic region (Asia vs. Rest of World);
- Prior VEGF inhibitors (Yes vs No).
This phase Ib/II trial studies the side effects and best way to give pembrolizumab with combination chemotherapy and radiation therapy before surgery and to see how well it works in treating adult patients with gastroesophageal junction or gastric cardia cancer that has spread from where it started to nearby tissue and can be removed by surgery. Monoclonal antibodies, such as pembrolizumab, may block tumor growth in different ways by targeting certain cells. Drugs used in chemotherapy work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping ...
The purpose of this study is to explore various biomarkers in the post-treatment tissue of patients who have responded to cancer therapy and compare it to the biopsy performed prior to cancer treatment.
The purpose of this study is to characterize the safety and tolerability of DKN-01 in combination with tislelizumab ± CAPOX (capecitabine + oxaliplatin) in patients with inoperable, locally advanced or metastatic G/GEJ adenocarcinoma.
This is an international, multicenter Phase 2/3 study in subjects with locally-advanced unresectable or metastatic HER2+ GEC who have received prior treatment with a HER2-directed antibody, and have received 1 prior line of therapy in the advanced disease setting.
This is a randomized Phase 2 study of novel SEQUEnced immunotherapy (pembrolizumab) with anti-angiogenesis and chemotherapy in advanced gastric and gastroesophageaL junction (GEJ) adenocarcinoma (SEQUEL) designed to to evaluate the best overall response rate (BORR) of combined ramucirumab (RAM) plus paclitaxel (+/- pembrolizumab) following induction pembrolizumab (PEM) in patients with advanced gastric and GEJ adenocarcinoma.
The purpose of this study is to compare, in a non-inferiority fashion, the progression-free survival (PFS) in patients with metastatic refractory gastric/Gastroesophageal Junction (GEJ) adenocarcinoma receiving the combination of ramucirumab with TAS-102 vs. paclitaxel and ramucirumab.
This study is being done to see if the NvisionVLE Imaging System can accurately determine the diagnostic performance of staging of T1 esophageal adenocarcinoma.
This study aims to elucidate the relationship between the microbiome, inflammation, and the microenvironment in Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC), with the end goal of developing a non-endoscopic testing strategy based on pathogenic factors to identify patients at highest risk for EAC. To accomplish this the investigators will enroll 100 patients with known BE (50 with dysplasia or EAC) and 50 subjects without BE undergoing upper endoscopy. Prior to endoscopy each subject will undergo three minimally invasive potential screening and surveillance tests: saliva (oral microbiome), breath test (exhaled volatile organic compounds), and tethered capsule sponge sampling (methylated DNA ...
The purpose of this study is to determine whether cryotherapy is effective in the treatment of persistent high grade dysplasia (HGD) or early esophageal adenocarcinoma (IMCA) in patients who have not responded to radiofrequency ablation (RFA).
The goal of this research is to determine the natural history of Barrett's esophagus (BE) using tethered capsule endomicroscopy (TCE) in patients undergoing surveillance endoscopy.
The purpose of this study is:
- To assess the efficacy of treatment with checkpoint inhibitors (Pembrolizumab or Nivolumab) in metastatic gastric and esophageal carcinoma through retrospective chart review.
- To explore if response to checkpoint inhibitors is dependent on biomarkers on tumor tissue.
To determine whether the combination of MM-111 plus paclitaxel and trastuzumab is more effective than paclitaxel and trastuzumab alone
Inclusion Criteria:
- Patients with Barrett’s Esophagus (BE) or Esophageal Adenocarcinoma (EAC) that are planned for Endoscopic Submucosal Dissection (ESD) treatment.
Exclusion Criteria:
- Patients without BE or EAC or that are not planned for ESD treatment.
The purpose of this study is to evaluate if the capsule sponge device can detect the presence of Barrett's Esophagus.
This is a study of pembrolizumab for advanced gastric or gastroesophageal junction adenocarcinoma; pembrolizumab will be given as monotherapy to participants who have had previous treatment or who are treatment-naïve; pembrolizumab will also be evaluated as combination therapy with cisplatin and 5-Fluorouracil (5-FU) in treatment-naïve participants. The primary study hypothesis is that pembrolizumab will provide a clinically meaningful overall response rate.
The purpose of this study is to determine serum, saliva, and tissue levels of nitrates in patients with normal squamous epithelium, erosive esophagitis, non-dysplastic BE (NDBE), and BE with high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC); to compare serum and tissue levels of IL-8 with varying levels of BE dysplasia and EAC; and to determine whether there is an association between nitrate levels and IL-8.
A Phase 1b/2, open label, multi-center, clinical study of Chimeric Antigen Receptor T Cells (CAR-T) targeting claudin18.2 in patients with advanced gastric, pancreatic or other specified digestive system cancers.
The purpose of this study is to determine the effect of endoscopic cryoablation for lessening the effects of dysphagia on the quality of life (QOL) of patients with unresectable esophageal or gastroesophageal junctional cancer.
The purpose of this study is to determine the 12-month cardiac event rate after radiation or chemo-radiation for the treatment of lung or esophageal cancer.
The purpose of the study is to see if the plasma assay of MDMs using optimized markers and analytically sensitive assays will detect early-stage esophageal cancer at high specificity.
The purpose of this study is to determine the safety and preliminary effectiveness of APR-246 in combination with pembrolizumab in subjects with solid tumor malignancies. The study will include a safety lead-in portion followed by a phase 2 expansion portion in specific disease groups.
This study will evaluate if the sponge capsule device can accurately detect the presence of Barrett's Esophagus and prevalent dysplasia/adenocarcinoma detection, in a screening population, with and without chronic gastroesophageal reflux disease.
The purpose of this study is to determine the pharmacodynamically-active dose of gevokizumab and the tolerable dose and preliminary effectiveness of gevokizumab in combination with the standard of care anti-cancer therapy in patients with metastatic colorectal cancer, metastatic gastroesophageal cancer and metastatic renal cell carcinoma.
This randomized phase III trial studies how well early palliative care integrated with standard care works compared with standard care alone in improving the quality of life of patients and their family caregivers. Palliative care focuses on improving the quality of life for patients with advanced diseases and their family members by providing support for relief of physical symptoms, emotional and psychological support, and counseling. Patients who receive palliative care along with their regular care at an earlier time in their disease may experience fewer emotional and physical issues from their cancer. This may also improve the quality of life ...
The primary purpose of this study is to assess the safety and tolerability of RP-6306 with FOLFIRI in patients with eligible advanced solid tumors, determine the maximum tolerated dose (MTD), identify a recommended phase 2 dose (RP2D) and preferred schedule, and assess preliminary anti-tumor activity.
Patients in the study will be treated with Melphalan/HDS and will receive up to 6 total treatments. This study will evaluate the safety and effects of the treatment.
The purpose of this study is to evaluate the safety and tolerability, and determine the maximum tolerated dose of INCB062079 in subjects with advanced hepatocellular carcinoma and other malignancies.
The purpose of this study is to evaluate the safety, dose, immunogenicity and early clinical activity of GRT-C901 and GRT-R902, a personalized neoantigen cancer vaccine, in combination with nivolumab and ipilimumab, in patients with metastatic non-small cell lung cancer, microsatellite stable colorectal cancer, gastroesophageal adenocarcinoma, and metastatic urothelial cancer.
The primary purpose of this study is to assess the safety and tolerability of TAB001 in subjects with various advanced malignancies and to evaluate the recommended Phase 2 dose. The secondary objectives are to: 1) describe the pharmacokinetic (PK) profile of TAB001, 2) evaluate antitumor activity of TAB001; 3) determine the immunogenicity of TAB001 , and 4) evaluate pharmacodynamic effects of TAB001 on its target receptor, programmed cell death 1 (PD-1), as well as effects on the immune system. The exploratory objectives are to: 1) evaluate biomarkers that may correlate with activity of TAB001, 2) evaluate the utility of PD-L1 & ...
The purpose of Part A of this study is to characterize the safety, tolerability, and biological effects of CUE-102. The goal of Part B is to expand the safety and immune activity data at the RP2D identified in Part A, and to evaluate antitumor activity at this dose.
The purpose of this study is to evaluate dose escalation and expansion of MT-5111 (a recombinant fusion protein) in subjects with HER2-positive solid tumors.
The purpose of these phase I/II trial studies is to analyze the side effects and best dose of liposomal irinotecan and rucaparib when given together with fluorouracil and leucovorin calcium, and to see how well they work in treating patients with pancreatic, colorectal, gastroesophageal, or biliary cancer that has spread to other places in the body. Drugs used in chemotherapy, such as liposomal irinotecan, fluorouracil, leucovorin calcium, and rucaparib, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading.
This is an open label, multi-center, Phase Ib dose escalation study of BBI608 administered in combination with either FOLFOX6 with and without bevacizumab, or CAPOX, or FOLFIRI with and without bevacizumab, or regorafenib. A study cycle will consist of daily and continuous oral administration of BBI608 for four weeks (28 days) in combination with FOLFOX6 with and without bevacizumab, or CAPOX or FOLFIRI with and without bevacizumab, or regorafenib.
The purpose of this study is to determine the recommended dose of tucatinib when combined with trastuzumab and modified FOLFOX7 (mFOLFOX7) or CAPOX in subjects with human epidermal growth factor receptor 2 (HER2)+ gastrointestinal cancers.
The primary purpose of this study is to obtain de-identified, clinically characterized, whole blood specimens to evaluate biomarkers associated with cancer for diagnostic assay development.
The purpose of this study is to investigate the antitumor activity of navicixizumab monotherapy or in combination with paclitaxel or irinotecan in patients with advanced solid tumors including:
- Cohort A: Colorectal cancer (CRC);
- Cohort B: Gastric and gastroesophageal junction (GEJ) cancer;
- Cohort C: Triple-negative breast cancer (TNBC);
- Cohort D: Platinum-resistant/refractory epithelial ovarian, primary peritoneal, or fallopian tube cancer (ovarian cancer).
The primary objectives for this study are:
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 6 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 12 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 18 months of documentation of HLA-A LOH status
- The percentage of subjects who can enroll on an A2 CAR T-cell therapy study within approximately 24 months of HLA-A LOH status
- Percentage of screened subjects experiencing loss ...
The purpose of this trial is to evaluate the safety of GEN1046 in patients with malignant solid tumors.
The primary objective of this proposal is to develop a Thoracic Specimen Registry at Mayo Clinic. The purpose of the registry will be to support ongoing research in the etiology, early diagnosis, clinical management, and prognosis of lung cancer and other cancers and diseases of the thorax by developing a complete repository of specimens from patients with thoracic disease including but not limited to suspected lung cancer, mediastinal and pleural tumors and from patients at a very high risk of developing other thoracic cancers or other thoracic diseases.
The purpose of this study is to demonstrate the safety and tolerability of JAB-3312 in combination with anti-PD-1 mAb or MEKi or KRASi or EGFR-TKI in patients with advanced solid tumors.
This is a Phase I, open label study to evaluate the safety, tolerability, and immunogenicity of INO-1400 alone or in combination with INO-9012, delivered by electroporation in subjects with high-risk solid tumor cancer with no evidence of disease after surgery and standard therapy. Subjects will be enrolled into one of six treatment arms. Subjects will be assessed according to standard of care. Restaging and imaging studies will be performed to assess disease relapse per NCCN guidelines. RECIST will be used to validate the findings in cases of relapse.
The purpose of this multicenter prospective observational case-control study is to train and validate Adela’s cfMeDIP-seq based methylome profiling platform to detect and differentiate multiple cancer subtypes. In addition, this study includes longitudinal follow-up for a subset of participants to train and validate the methylome profiling platform to detect minimal residual disease and recurrence.
The purpose of this study is to determine the prevalence of genetic mutations in cancer patients from various ethnic populations seeking care at Mayo Clinic cancer clinics.
The purpose of this study is to evaluate the safety, tolerability, and preliminary anti-tumor activity of SAR444881 alone and in combination with pembrolizumab or with cetuximab. The study will enroll advanced cancer patients with unresectable or metastatic disease who are refractory to or are not candidates for standard approved therapy and will be comprised of two parts - an initial "3 + 3" dose escalation phase (Part 1) with Sub-Parts 1A (monotherapy SAR444881), 1B (SAR444881 in combination with pembrolizumab) and 1C (SAR444881 in combination with cetuximab) followed by a dose optimization/expansion phase (Part 2), including Sub-Part 2A (Dose Optimization) with Cohorts A1 (SAR444881 in ...
The purpose of this study is to evaluate the challenges, behavioral patterns, and preferences of minority patient participation in clinical trials. Also, to develop and validate a personalized clinical trial educational platform to boost participation among underserved cancer patients.
The purpose of this study is to collect blood and tissue samples from patients with and without cancer to evaluate laboratory tests for early cancer detection which may help researchers develop tests for the early detection of cancers.
GRAIL is using deep sequencing of circulating cell-free nucleic acids (cfNAs) to develop assays to detect cancer early in blood. The purpose of this study is to collect biological samples from donors with a new diagnosis of cancer (blood and tumor tissue) and from donors who do not have a diagnosis of cancer (blood) in order to characterize the population heterogeneity in cancer and non-cancer subjects and to develop models for distinguishing cancer from non-cancer.
Falls are common and catastrophic in cancer patients. Cancer patients are vulnerable to falls due to muscle loss. In prescribing exercise in a data driven manner to cancer patients, our hypothesis is this "prescription" for exercise will eventually be demonstrated to reduce the occurrence of injurious falls.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Skip to Content
- Conquer Cancer
- ASCO Journals
- f Cancer.net on Facebook
- t Cancer.net on Twitter
- q Cancer.net on YouTube
- g Cancer.net on Google
Types of Cancer
- Navigating Cancer Care
- Coping With Cancer
- Research and Advocacy
- Survivorship
Esophageal Cancer: Latest Research
ON THIS PAGE: You will read about the scientific research being done to learn more about this type of cancer and how to treat it. Use the menu to see other pages. Doctors are working to learn more about esophageal cancer, ways to prevent it, how to best treat it, and how to provide the best care to people diagnosed with this disease. The following areas of research may include new options for patients through clinical trials. Always talk with your doctor about the best diagnostic and treatment options for you.
Chemoprevention. Researchers are looking at using aspirin and acid-reducing medication to prevent esophageal adenocarcinoma in people with Barrett’s esophagus. Research is still ongoing, and people are encouraged to talk with their doctor before taking any medications or dietary supplements for this reason. Learn about the basics of chemoprevention .
Use of PET scan. In addition to helping find out the cancer’s stage (see Diagnosis ), PET scans may be used to find out how well treatment is working to shrink a tumor before surgery. Researchers are studying the use of PET scan to evaluate and possibly change treatment before surgery .
Chemotherapy advances. Doctors are studying combinations of different drugs, such as capecitabine (Xeloda), cisplatin (available as a generic drug), docetaxel (Docefrez, Taxotere), fluorouracil (5-FU, Efudex), irinotecan (Camptosar), oxaliplatin (Eloxatin), paclitaxel, and trifluridine-tipiracil combination (Lonsurf). Research is ongoing to find new drugs that are effective for esophageal cancer.
Targeted therapy. Several types of targeted therapies are currently being studied for esophageal cancer. For example, researchers are looking at new drugs that block vascular endothelial growth factor (VEGF). Learn more about advances in molecular profiling of tumors to identify targets in GI cancers, including esophageal cancer.
Immunotherapy. New drugs and combinations that include immunotherapy continue to be studied.
Palliative care/supportive care . Clinical trials are underway to find better ways of reducing symptoms and side effects of current esophageal cancer treatments to improve comfort and quality of life for patients.
Looking for More About the Latest Research?
If you would like more information about the latest areas of research in esophageal cancer, explore these related items that take you outside of this guide:
To find clinical trials specific to your diagnosis, talk with your doctor or search online clinical trial databases now .
Visit the Cancer.Net Blog to review research and topics related to esophageal cancers.
Visit the website of Conquer Cancer, the ASCO Foundation , to find out how to help support cancer research. Please note that this link takes you to a different ASCO website.
The next section in this guide is Coping with Treatment . It offers some guidance on how to cope with the physical, emotional, social, and financial changes that cancer and its treatment can bring. Use the menu to choose a different section to read in this guide.
Esophageal Cancer Guide
Cancer.Net Guide Esophageal Cancer
- Introduction
- Medical Illustrations
- Risk Factors
- Symptoms and Signs
- Stages and Grades
- Types of Treatment
- About Clinical Trials
- Latest Research
- Coping with Treatment
- Follow-Up Care
- Questions to Ask the Health Care Team
- Additional Resources
View All Pages
Timely. Trusted. Compassionate.
Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology (ASCO), the voice of the world's oncology professionals.
Find a Cancer Doctor
- Open access
- Published: 14 May 2024
Long-term prognosis after endoscopic submucosal dissection for esophageal cancer in older adult patients
- Hirona Konishi 1 ,
- Yuji Urabe 2 ,
- Takeo Nakamura 1 ,
- Kazuki Ishibashi 1 ,
- Junichi Mizuno 1 ,
- Motomitsu Fukuhara 1 ,
- Takeshi Takasago 1 ,
- Hidenori Tanaka 1 ,
- Akiyoshi Tsuboi 1 ,
- Ken Yamashita 1 ,
- Yuichi Hiyama 3 ,
- Hidehiko Takigawa 1 ,
- Takahiro Kotachi 1 ,
- Ryo Yuge 1 ,
- Akira Ishikawa 4 &
- Shiro Oka 1
BMC Gastroenterology volume 24 , Article number: 164 ( 2024 ) Cite this article
123 Accesses
2 Altmetric
Metrics details
The validity of endoscopic submucosal dissection (ESD) for esophageal squamous cell carcinoma (ESCC) in older individuals with comorbidities remains unclear. Therefore, this study evaluated the safety and efficacy of ESD and additional treatment for ESCC in older adult patients.
The clinicopathological characteristics and clinical outcomes of 398 consecutive older adult patients (≥ 65 years) with 505 lesions who underwent ESD for ESCC at the Hiroshima University Hospital between September 2007 and December 2019 were retrospectively evaluated. Additionally, the prognoses of 381 patients who were followed up for > 3 years were assessed.
The mean patient age and procedure time were 73.1 ± 5.8 years and 77.1 ± 43.5 min, respectively. The histological en bloc resection rate was 98% (496/505). Postoperative stenosis, perforation, pneumonia, and delayed bleeding were conservatively treated in 82 (16%), 19 (4%), 15 (3%), and 5 (1%) patients, respectively. The 5-year overall and disease-specific survival rates were 78.9% and 98.0%, respectively (mean follow-up time: 71.1 ± 37.3 months). Multivariate analysis showed that age and the American Society of Anesthesiologists classification of physical status class ≥III (hazard ratio: 1.27; 95% confidence interval: 1.01–1.59, p = 0.0392) were independently associated with overall survival. A significantly lower overall survival rate was observed in the high-risk follow-up group than in the low-risk follow-up and high-risk additional treatment groups ( p < 0.01). However, no significant difference in disease-specific survival was observed among the three groups.
Conclusions
ESD is safe for ESCC treatment in patients aged ≥ 65 years. However, additional treatments should be considered based on the patient’s general condition.
Peer Review reports
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors in East Asia [ 1 ]. In Japan, the peak age for ESCC onset is the 60s, with most cases occurring in individuals in their 50s and 70s [ 2 ]. Recently, Japan has had an increasingly aging population, resulting in an increasing incidence of ESCC among older individuals [ 3 ].
Generally, physiological functions and activities of daily living (ADL) decline as patients age, usually limiting their treatment options for cancer [ 4 ]. Physiological functions, ADL, and basic diseases in older adult patients vary widely among older individuals, and treatments should be considered based on the expected life prognosis, living environment, and social background. Furthermore, many patients with ESCC have underlying diseases, such as chronic obstructive pulmonary and cardiovascular diseases, because ESCC occurs mostly in regular smokers and drinkers [ 5 ].
Chemoradiotherapy (CRT) and surgical resection are usually used to treat ESCC. However, CRT frequently causes lung damage, and cardiac and pulmonary functions affect the ability to tolerate surgery. Surgery-related mortality is reportedly 2% in thoracic ESCC surgery [ 6 ] and is expected to be even higher in older adult patients and those with underlying comorbidities. Therefore, some cases of ESCC are difficult to treat using surgery or CRT [ 7 , 8 ].
Endoscopic submucosal dissection (ESD) has been reported to be useful in treating ESCC, and it is considered a treatment option for patients with ESCC. However, the indication for ESD in ESCC is T1 cancer without lymph node metastasis. Additional treatment with surgery or CRT is required in cases with positive vertical margins or the risk of lymph node metastasis after ESD [ 9 ]. However, additional treatment may be difficult in patients with superficial ESCCs who require additional treatment with CRT or surgery after ESD owing to their age or underlying disease. Furthermore, determining the safety and prognosis after ESD for these patients is crucial for developing treatment strategies for superficial ESCCs in older individuals and patients with underlying diseases. Therefore, this study aimed to investigate the safety and prognosis of ESD in older adult patients with ESCC, considering their age and physical condition.
Study design and population
Consecutive patients with superficial ESCCs aged ≥ 65 years who underwent ESD at Hiroshima University Hospital between September 2007 and December 2019 were retrospectively enrolled. Prognoses and recurrence among patients who did not regularly visit our hospital were surveyed via telephone.
The following variables were investigated to determine the clinical outcomes of ESD: (i) complete en bloc resection, (ii) mean procedure time, and (iii) complications (perforation, postoperative bleeding, pneumonia, and postoperative stenosis). Older individuals were defined as those aged ≥ 65 years [ 10 ]. Individuals aged 65–74 and ≥ 75 years were defined as early- and late-term older adult persons, respectively, since physical function is believed to be deteriorating in late-term older adults [ 11 ]. Therefore, we divided the age group by 75 years to investigate the prognosis.
ESD procedure
The number of Lugol-voiding lesions per endoscopic view was counted, and the grading was classified into the following categories: (i) grade A, no lesions; (ii) grade B, 1–9 lesions; and (iii) grade C, ≥ 10 lesions [ 12 ].
Esophageal ESD was performed by gastrointestinal endoscopists affiliated with the Japanese Society of Gastroenterological Endoscopy who had performed > 100 ESD procedures. High-frequency electrocautery was performed using ESG100 (Olympus, Tokyo, Japan) or VIO300D (ERBE Elektromedizin GmbH, Tubingen, Germany). An upper gastrointestinal endoscope with a water-delivery function (GIF-Q260J or GIF-H290T; Olympus Optical Industries Corporation, Tokyo, Japan) and a transparent hood attachment (TOP Corporation, Tokyo, Japan) were used. ESD was performed using DualKnife/DualKnife J and SB knife Jr after marked dots were placed outside the lesion margins using iodine staining. The injection solution was a 10% glycerin solution containing a small amount of indigo carmine for full incisions. A solution of 0.4% sodium hyaluronate (Muco Up; Boston Scientific, Tokyo, Japan) diluted twice with a 10% glycerin solution containing a small amount of indigo carmine was used for submucosal injection. The procedure time was defined as the time from the initial mucosal injection to the completion of resection. Postoperative bleeding was defined as bleeding that required transfusion or resulted in decreased hemoglobin levels by 2 g/dL within > 24 h after the procedure. Perforation was diagnosed if endoscopically confirmed during the procedure or if mediastinal emphysema or a small amount of free air was observed during chest computed tomography (CT). Stenosis was defined as the failure of a conventional single-channel endoscope to pass through the stenosis. Bleeding was endoscopically stopped using hemostatic forceps (Coagrasper, Olympus, Tokyo, Japan), and transfusion was performed as required. In cases with perforation, the mucosal defect was completely closed using EZ clips (Olympus) endoscopically, if possible. The patients were allowed to skip meals and take antibiotics until their fever and abdominal pain resolved and inflammatory findings improved. Patients with pneumonia were allowed to skip meals and take antibiotics until their fever resolved and the inflammatory findings improved. For patients with more than three-quarters circumference resection of the esophagus, a localized steroid injection was administered after ESD to prevent stenosis.
Repeated endoscopic balloon dilation was performed in patients with postoperative stenosis.
Histopathologic evaluation
The specimens resected using ESD were stretched, pinned, and fixed in a 10% formalin solution. They were sliced at 2-mm-thick intervals and evaluated microscopically. The depth of the submucosa was determined following the General Rules for Clinical and Pathological Studies on Cancer of the Esophagus, outlined by the Japanese Society for the Esophagus [ 13 ]. Lesions were classified as T1a (epithelial [EP]/lamina propria mucosal [LPM]/muscularis mucosal [MM]) or T1b (submucosal [SM]) carcinomas. Lymphovascular invasion was assessed using only hematoxylin and eosin staining until October 2013 and subsequently with Elastica van Gieson and D2-40, in addition to hematoxylin and eosin. Curative resection is not clearly defined in the Japanese Esophageal Association guidelines [ 13 , 14 ]; therefore, it was defined as T1a carcinoma with negative lymphovascular status in this study. Moreover, curative and noncurative resection cases were classified as the low-risk follow-up and high-risk groups, respectively. The complete en bloc resection rate was defined as a one-piece resection of the entire lesion with endoscopically and pathologically negative margins. Furthermore, the degree of submucosal fibrosis was classified into the none, mild, and severe groups based on a previous report [ 15 ].
Follow-up schedule after ESD
Patients underwent annual upper gastrointestinal endoscopy after curative resection. Those who underwent additional surgery after noncurative resection also had medical examinations every 3 months postoperatively. CT examinations and upper gastrointestinal endoscopy were performed every 6 months and annually, respectively. Moreover, patients who received follow-up treatment or CRT after noncurative resection also underwent upper gastrointestinal endoscopy 1–2 months after ESD, and ulcer scars were confirmed after excision. Subsequently, upper gastrointestinal endoscopy was performed every 4–6 months, while CT was conducted every 4–6 months to evaluate lymph node metastasis, distant metastasis, and recurrence. However, the surveillance period was flexible depending on each patient’s physical condition. Recurrence was confirmed based on imaging or pathological findings. Local residual recurrence was defined as the scar recurrence after ESD. Death due to ESCC was defined as primary cancer death, and death from other causes as deaths due to other diseases.
Variables investigated
Variables for clinical outcomes of ESD were investigated as follows: complete en bloc resection, average procedure time, and adverse events (postoperative stenosis, perforation, pneumonia, and delayed bleeding). We analyzed the risk factors for poor prognosis and compared overall survival (OS) and disease-specific survival (DSS) according to the risk factors for poor prognosis among the low-risk follow-up, high-risk additional treatment, and high-risk follow-up groups.
The American Society of Anesthesiologists classification of physical status (ASA-PS) [ 16 ] was used for categorizing the preoperative physical status of patients as follows: (i) ASA-PS class I, normal healthy patients; (ii) ASA-PS class II, patients with mild systemic disease; (iii) ASA-PS class III, patients with a severe systemic disease that is not life-threatening; (iv) ASA-PS class IV, patients with extreme systemic disorders that have become an imminent threat to life regardless of the type of treatment; (v) ASA-PS class V, moribund patients who are not expected to survive; and (vi) ASA-PS class VI, patients declared brain-dead whose organs are being removed for donor purposes. Prognostic nutritional indexes were also evaluated, including the Onodera Prognostic Nutritional Index (PNI = 10 albumin (Alb) [g/dL] + 0.005 total lymphocyte count [/mm 3 peripheral blood]) [ 17 ], neutrophil-to-lymphocyte ratio (NLR = total lymphocyte count [/mm 3 peripheral blood]/total neutrophil count [/mm 3 peripheral blood]) [ 18 ], Geriatric Nutritional Risk Index (GNRI = 14.89 Alb [g/dL] + 41.7 weight [kg]/22 height 2 [m 2 ]) [ 19 ], Alb, and body mass index (= weight [kg]/height 2 [m 2 ]).
Ethical statement
This study protocol was conducted in accordance with the principle of the Declaration of Helsinki and was approved by the Institutional Review Board of Hiroshima University (approval number: E2023-0195). All patients were informed of the risks and benefits of ESD and provided written informed consent.
Statistical analysis
Quantitative data are presented as mean ± standard deviation or percentage. Differences in categorical variables were analyzed using the chi-square test with the Yates correction or Fisher’s exact test. The risk factors for poor prognosis were analyzed using univariate and multivariate analyses. Continuous and qualitative variables were analyzed using Student’s t -test or the Mann–Whitney U test and Pearson’s chi-squared test, respectively. Statistical significance was set at p < 0.05. Logistic regression analysis was performed to examine the risk factors for poor OS. OS and DSS rates were calculated using the Kaplan–Meier method. All statistical analyses were performed using JMP statistical software version 16.0.0 (SAS Institute, Cary, North Carolina, USA).
Patient and lesion characteristics
Among the consecutive patients with superficial ESCC who underwent ESD, 398 older adult patients with 505 superficial lesions were enrolled. Prognoses and recurrences were evaluated in 381 older adult patients (96%). Supplementary Tables 1 and 2 present the clinicopathological characteristics of the patients.
ESD outcomes
Table 1 presents the short-term outcomes. The mean procedure time, en bloc resection rate, and complete en bloc resection rate were 77.1 min, 98%, and 95%, respectively. Severe fibrosis was observed in 86 (17%) lesions. Endoscopic balloon dilatation was performed in 82 (16%) patients because of postoperative stenosis. Additionally, perforation, pneumonia, and delayed bleeding were observed in 19 (4%), 15 (3%), and 5 (1%) patients, respectively. All cases were resolved with conservative treatment, and no ESD-related deaths occurred. Pathological diagnoses included 380 (75%), 61 (12%), and 64 (13%) EP/LPM, MM, and SM lesions, respectively. In total, 44 (9%) and 22 (4%) lesions had positive lymphatic invasion and venous invasion, respectively. Noncurative resections using ESD (lymphovascular invasion positive and/or pT1b-SM) were observed with 76 (15%) lesions. Supplementary Table 3 presents the clinical characteristics of lesions and short-term outcomes by ASA-PS. Tumor size, procedure time, pathological diagnosis, and lymphovascular involvement were significantly different between the ASA-PS classes I/II and III.
Prognoses after ESD
The prognoses of 381 (96%) patients were investigated (mean follow-up period of 71.1 ± 37.3 months). Among the 76 patients diagnosed with noncurative resection based on the pathological findings from ESD specimens, 40, 7, 1, and 6 were additionally treated with CRT, radiotherapy (RT), chemotherapy, and surgery, respectively, and 22 were followed up without additional treatment.
Supplementary Table 4 shows the causes of death in these patients. In total, 104 patients died during the observation period; seven of them died because of ESCC. Four of the seven patients were diagnosed with an invasive depth of pT1a without lymphovascular invasion based on the pathological findings after ESD and were followed up without additional treatment. The diagnoses were pT1a-EP, pT1a-LPM, and pT1a-MM in one, one, and two cases, respectively (the pT1a-EP and pT1a-LPM in one case each were performed before October 2013). Lymph node metastasis from the metachronous carcinoma, which was 0–IIa in the cervix and diagnosed as cT1b-SM, recurred in the patient diagnosed with pT1a-EP, while lymph node metastasis from the primary carcinoma recurred in the other patients. Contrastingly, three of the seven patients who died because of ESCC were diagnosed with invasive depth pT1a with lymphovascular invasion or pT1b based on the pathological findings after ESD. Two of the three patients were treated with CRT after ESD, and the other patient was followed up without additional treatment.
The most common cause of death from other diseases was cancer, excluding esophageal cancer, pneumonia, and cardiac disease. The breakdown of deaths due to other cancers was as follows: lung cancer, n = 7 (19%); oral cancer, n = 6 (16%); liver cancer, n = 4 (11%); colorectal cancer, n = 4 (11%); pancreatic cancer, n = 4 (11%); bladder cancer, n = 3 (8%); gastric cancer, n = 2 (5%); malignant lymphoma cancer, n = 2 (5%); myelodysplastic syndromes, n = 2 (5%); duodenal cancer, n = 1 (3%); renal cancer, n = 1 (3%); and occult primary cancer, n = 1 (3%). Overall, 24 of the 37 patients had a history of other organ cancers, and we performed ESD because their cancers were believed to be under control. However, 15 patients died because of the recurrence of their cancers. Two of the 37 patients were diagnosed with other organ cancers for which they were planned to undergo curative surgery simultaneously with ESCC. Although both patients underwent surgery for other cancers after ESD for ESCC, they died owing to uncontrolled cancer of other organs. Subsequently, 11 of the 37 patients died of newly found cancer of other organs > 2 years after ESD for ESCC.
Table 2 presents the OS according to the prognostic factors. Univariate analysis with log-rank tests showed that age, ASA-PS, Alb, PNI, GNRI, NLR, tumor location, and history of advanced cancer, excluding esophageal cancer, were significantly associated with impaired survival. However, multivariate analysis showed that age (hazard ratio: 1.02; 95% confidence interval (CI): 1.00–1.04, p = 0.0458) and ASA-PS class III (hazard ratio: 1.27; 95% CI 1.01–1.59, p = 0.0392) were independently associated with OS.
Figure 1 shows the OS and DSS rates of older adult patients with ESCC after ESD. No difference in OS and DSS rates was observed between the < 75-year-old and ≥ 75-year-old groups (Fig. 1 a and b). The OS rate in the ASA-PS class III was significantly lower than that in the ASA-PS class I/II ( p < 0.0001), although no difference in DSS rate was observed between the two groups (Fig. 1 c and d). A significantly lower OS rate was observed in the high-risk follow-up group than in the low-risk follow-up and high-risk additional treatment groups ( p < 0.01). However, no significant difference was observed in DSS among the three groups (Fig. 1 e and f). A significantly lower DSS rate was observed in the high-risk group of patients in the ASA-PS class III than in the low-risk group of patients in the ASA-PS class III ( p < 0.05). Furthermore, no difference was observed in DSS between the high-risk additional treatment and high-risk follow-up groups (Fig. 1 g and h).

Overall and disease-specific survival (OS and DSS) rates in 381 older adult patients with superficial ESCC. ( a , b ) OS and DSS according to age. No difference in OS and DSS was observed between the < 75-year-old and ≥ 75-year-old groups. ( c ) OS according to the ASA-PS classification. A significantly lower OS rate was observed in patients in the ASA-PS class III than in those in the ASA-PS class I/II ( p < 0.0001). ( d ) DSS according to the ASA-PS classification. No difference was observed between patients in the ASA-PS classes I/II and III. ( e ) OS according to the presence or absence of additional treatments after ESD. A significantly lower OS rate was observed in the high-risk follow-up group than in the low-risk follow-up and high-risk additional treatment groups ( p < 0.01). ( f ) DSS according to the presence or absence of additional treatments after ESD. No significant difference was observed between the high-risk follow-up, low-risk follow-up, and high-risk additional treatment groups. ( g , h ) DSS according to the presence or absence of additional treatments among patients in the ASA-PS class III. A significantly lower DSS rate was observed in the high-risk group than in the low-risk group ( p < 0.05), and no difference in DSS rate was observed between the high-risk additional treatment and the high-risk follow-up group
This study revealed that no ESD-related deaths occurred among patients with ESCC aged ≥ 65 years, and age and ASA-PS were significantly associated with poor prognostic factors. Additionally, no significant difference was observed in the prognoses between the presence or absence of additional treatment in the high-risk groups of patients in the ASA-PS class III, which is a poor prognostic factor. Previous studies reported no differences in short-term ESD outcomes between older and younger patients [ 12 , 20 , 21 ]. Meanwhile, the postoperative 30- and 90-day mortality rates were significantly higher in older adult patients (≥ 70 years) than in younger patients (< 70 years) [ 22 ]. Previous studies [ 23 , 24 ] reported significantly more postoperative adverse events in older adult patients than in younger patients. Additionally, comparing the short- and long-term outcomes of endoscopic versus surgical therapy for early ESCC in older adult patients showed that the 2-year survival rate after endoscopy was significantly higher than that after surgery [ 25 ]. Therefore, ESD for ESCC is considered appropriate and a safe and minimally invasive treatment for older adult patients.
Our study’s data showed that age and the ASA-PS were poor prognostic factors in older adult patients with ESCC after ESD. Previous studies [ 26 , 27 , 28 , 29 , 30 ] have examined the association between nutritional and general health statuses and prognosis in older adult patients with cancer. However, only a few studies have investigated the relationship between nutritional status and prognosis after ESD for ESCC [ 31 ]. Therefore, we hypothesized that patients’ nutritional and general health statuses, such as their ASA-PS and tumor grade, are associated with prognoses in older adult patients who underwent ESD for ESCC. Consequently, this study’s findings show that nutritional status correlated with prognosis in the univariate analysis and not independently in the multivariate analysis, indicating that the ASA-PS and age were associated with prognosis. The findings of previous studies and our study differed because ESD is a minimally invasive treatment compared to surgery, and ESD treatment is relatively unlikely to contribute to the worsening of nutritional status or decline in ADL compared to surgery. Therefore, the ASA-PS can be considered a simple and useful index to indicate poor prognosis in patients after ESD for ESCC.
In this study, treatment methods were selected based on the physicians’ decision, considering the patient’s general condition and aspirations. Therefore, the results in Supplementary Table 3 indicate that patients in poor general condition tended to be selected for ESD, a minimally invasive treatment, even in cases of cT1b-SM. Additionally, the procedure time might have been significantly longer in the ASA-PS class III cases than in the ASA-PS class I/II cases because they had more lesions that were larger and deeper in tumor size and depth, respectively.
The most common cause of death from other diseases was cancer, excluding esophageal cancer, pneumonia, and cardiac disease. This suggests that patients who could not undergo additional surgery based on the physicians’ decision may have had poorer prognoses than other patient groups. Moreover, in patients in the ASA-PS class III, which is a poor prognostic factor, the presence or absence of additional treatment after ESD in the high-risk group did not result in differences in the prognoses. This may be because (i) surgical resection was avoided and CRT, RT, or chemotherapy was opted for in ASA-PS class III cases, considering the patient’s general condition; (ii) treatments were performed with less than the usual dose at the physicians’ discretion; (iii) patients lacked sufficient survival time to benefit from additional treatments; and (iv) from previous reports, patients with poor general health and numerous comorbidities, such as those in the ASA-PS class III, particularly older adults, were more prone to treatment-related adverse events [ 7 , 8 ]. In addition, although ASA-PS class III cases had significantly larger tumor diameters, longer procedure times, a higher proportion of pT1b-SM cases, and a higher proportion of lymphatic cases than ASA-PS class I/II cases (Supplementary Table 3 ), there was no significant difference in DSS between ASA-PS class I/II and III cases (Fig. 1 d). Therefore, ESD can be safely performed in older adult patients. Moreover, if the pathological diagnosis after ESD is curative, the death of patients with ESCC may be prevented, regardless of their general condition. However, follow-up without additional treatment after ESD may be considered acceptable for patients in the high-risk group with poor general conditions.
This study had some limitations. First, this was a single-center, retrospective study rather than a multicenter study. The concept of sarcopenia related to muscle mass is reportedly useful as a prognostic factor in older individuals [ 32 ]. However, because this is a retrospective study, we did not measure grip strength, tests related to physical function, and skeletal muscle mass before ESD to assess the presence of sarcopenia. Therefore, we could not evaluate its relevance to the results of this study. Second, no comparison was made between older and younger patients. Third, the common criteria for deciding which treatment is better, as an additional treatment or follow-up, are unclear, and the decision is left to the attending physician’s discretion. Fourth, central pathological reviews were not performed. Although experienced pathologists evaluated the patients according to the guidelines, variations in the histologic diagnoses may exist. Finally, no exact method exists for the surveillance of ESCC after ESD in older adults, and it varies slightly based on the attending physician’s discretion.
In conclusion, our study showed that ESD was a safe and effective treatment and prevented ESCC-related death in older adult patients regardless of age and ASA-PS class ≤ III. Furthermore, to limit additional treatment after ESD to only older high-risk cases, a list of high-risk cases with rapid progression to additional treatment after ESD should be narrowed down using pathological and genetic analyses.
Data availability
All data generated during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- Endoscopic submucosal dissection
American Society of Anesthesiologists classification of physical status
Esophageal squamous cell carcinoma
Activities of daily living
Chemoradiotherapy
Computed tomography
Lamina propria mucosal
Muscularis mucosal
Onodera Prognostic Nutritional Index
Neutrophil-to-lymphocyte ratio
Geriatric Nutritional Risk Index
Radiotherapy
Confidence interval
Overall survival
Disease-specific survival
Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–7.
Article PubMed Google Scholar
Watanabe M, Toh Y, Ishihara R, et al. Comprehensive registry of esophageal cancer in Japan, 2015. Esophagus. 2023;20(1):1–28.
Fitzmaurice C, Dicker D, Pain A, et al. The global burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–27.
Van Deudekom FJ, Klop HG, Hartgrink HH, et al. Functional and cognitive impairment, social functioning, frailty and adverse health outcomes in elderly patients with esophageal cancer, a systematic review. J Geriatr Oncol. 2018;9(6):560–8.
Oze I, Charvat H, Matsuo K, et al. Revisit of an unanswered question by pooled analysis of eight cohort studies in Japan: does cigarette smoking and alcohol drinking have interaction for the risk of esophageal cancer? Cancer Med. 2019;8(14):6414–25.
Article CAS PubMed PubMed Central Google Scholar
Motoyama S, Yamamoto H, Miyata H, et al. Impact of certification status of the institute and surgeon on short-term outcomes after surgery for thoracic esophageal cancer: evaluation using data on 16,752 patients from the National Clinical Database in Japan. Esophagus. 2020;17(1):41–9.
Schweigert M, Solymosi N, Dubecz A, et al. Current outcome of esophagectomy in the very elderly: experience of a German high-volume center. Am Surg. 2013;79(8):754–63.
Lu X, Wu H, Wang J, et al. Short- and long-term outcomes of definitive chemoradiotherapy in patients with esophageal carcinoma aged ≥ 75 years. Mol Clin Oncol. 2014;2(2):297–301.
Japan Esophageal Society. Japanese classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14(1):1–36.
Article Google Scholar
OECD: Elderly population (indicator). 2023. https://data.oecd.org/pop/elderly-population.htm . Accessed 29 November 2023.
Ouchi Y, Rakugi H, Arai H, et al. Redefining the elderly as aged 75 years and older: proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int. 2017;17(7):1045–7.
Katada C, Yokoyama T, Yano T, et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology. 2016;151(5):860–e97.
Article CAS PubMed Google Scholar
Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20(3):343–72.
Article PubMed PubMed Central Google Scholar
Mizumoto T, Hiyama T, Oka S, et al. Curative criteria after endoscopic resection for superficial esophageal squamous cell carcinomas. Dig Dis Sci. 2018;63(6):1605–12.
Matsumoto A, Tanaka S, Oba S, et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010;45(11):1329–37.
Keats AS. The ASA classification of physical status—a recapitulation. Anesthesiology. 1978;49(4):233–6.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5.
CAS PubMed Google Scholar
Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73(3–4):215–20.
Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–83.
Song BG, Min YW, Lee JH, et al. Efficacy and safety of endoscopic submucosal dissection in elderly patients with esophageal squamous cell carcinoma. Surg Endosc. 2017;31(10):3905–11.
Matsumoto Y, Kimura K, Zhou Q, et al. Treatments and outcomes of elderly patients with esophageal cancer: comparison with younger patients. Mol Clin Oncol. 2019;11(4):383–9.
PubMed PubMed Central Google Scholar
Laurent A, Marechal R, Farinella E, et al. Esophageal cancer: outcome and potential benefit of esophagectomy in elderly patients. Thorac Cancer. 2022;13(19):2699–710.
Morita M, Egashira A, Yoshida R, et al. Esophagectomy in patients 80 years of age and elderly with carcinoma of the thoracic esophagus. J Gastroenterol. 2008;43(5):345–51.
Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2010;90(3):900–7.
Cummings LC, Kou TD, Schluchter MD, et al. Outcomes after endoscopic versus surgical therapy for early esophageal cancers in an elderly population. Gastrointest Endosc. 2016;84(2):232–e401.
Kakiuchi Y, Kuroda S, Kikuchi S, et al. Prognostic risk factors for postoperative long-term outcomes in elderly stage IA gastric cancer patients. J Gastrointest Oncol. 2022;13(5):2178–85.
Zhang B, Li Y, Chen Y. Prognosis-related nutritional score for Cancer patients (PRNS): a clinical nutritional score derived from a retrospective cohort study. J Transl Med. 2022;20(1):477.
Yoshifuku Y, Oka S, Tanaka S, et al. Long-term prognosis after endoscopic submucosal dissection for early gastric cancer in super-elderly patients. Surg Endosc. 2016;30(10):4321–9.
Nishimura T, Oka S, Tanaka S, et al. Long-term prognosis after endoscopic submucosal dissection for colorectal tumors in patients aged over 80 years. BMC Gastroenterol. 2021;21(1):324.
Dang C, Wang M, Qin T, et al. How can we better predict the prognosis of patients with pancreatic cancer undergoing surgery using an immune-nutritional scoring system? Surgery. 2022;172(1):291–302.
Iwai N, Dohi O, Yamada S, et al. Prognostic risk factors associated with esophageal squamous cell carcinoma patients undergoing endoscopic submucosal dissection: a multi-center cohort study. Surg Endosc. 2022;36(4):2279–89.
Fang P, Zhou J, Xiao X, et al. The prognostic value of Sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(1):3–16.
Download references
Acknowledgements
Author information, authors and affiliations.
Department of Gastroenterology, Hiroshima University Hospital, Hiroshima, Japan
Hirona Konishi, Takeo Nakamura, Kazuki Ishibashi, Junichi Mizuno, Motomitsu Fukuhara, Takeshi Takasago, Hidenori Tanaka, Akiyoshi Tsuboi, Ken Yamashita, Hidehiko Takigawa, Takahiro Kotachi, Ryo Yuge & Shiro Oka
Department of Gastrointestinal Endoscopy and Medicine, Hiroshima University Hospital, 1-2-3, Kasumi, Minamiku, Hiroshima, 734-8551, Japan
Department of Clinical Research Center, Hiroshima University Hospital, Hiroshima, Japan
Yuichi Hiyama
Department of Molecular Pathology, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
Akira Ishikawa
You can also search for this author in PubMed Google Scholar
Contributions
YU contributed to the conception. HK, TN, KI, JM, and MF acquired data. HK and YU analyzed and interpreted data. HK and YU drafted the article. TT, HiT, AT, KY, YH, HoT, TK, RY, AI, and SO revised the manuscript. All authors have approved the submitted manuscript.
Corresponding author
Correspondence to Yuji Urabe .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted in accordance with the principles of the Declaration of Helsinki, and its protocol was approved by the Institutional Review Board of Hiroshima University (approval number: E2023-0195). All patients were informed of the risks and benefits of ESD and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Konishi, H., Urabe, Y., Nakamura, T. et al. Long-term prognosis after endoscopic submucosal dissection for esophageal cancer in older adult patients. BMC Gastroenterol 24 , 164 (2024). https://doi.org/10.1186/s12876-024-03234-7
Download citation
Received : 31 January 2024
Accepted : 19 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12876-024-03234-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Esophageal cancer
- Older individual
- American Society of anesthesiologists classification of physical status class
- Long-term prognosis
BMC Gastroenterology
ISSN: 1471-230X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Medicine (Baltimore)
- v.102(34); 2023 Aug 25
- PMC10470670
Real-world treatment patterns and survival for locally advanced esophageal squamous cell carcinoma
Hua-chun luo.
a Department of Tumor Integrated Therapy, The Fuzhou First General Hospital Affiliated with Fujian Medical University, Fuzhou, Fujian, China
Jing-Jing Wu
b Department of Radiotherapy, The 900th Hospital of the Joint Logistics Team, Fujian Medical University, Fuzhou, Fujian, China
c Department of Radiotherapy, Dongfang Hospital of Xiamen University, Xiamen, China.
Lv-Juan Cai
Zhi-yong shen, meng-jing wu, fei-fan chen, zhi-chao fu, fang-wei xie.
The “real world” treatment mode and clinical efficacy of locally advanced esophageal squamous cell carcinoma (LAESCC) are unclear. Meanwhile, the role of immunotherapy in the clinical practice is also puzzling. We conducted the research to investigate the statue of “real world” LAESCC. The clinical data of patients with locally advanced esophageal squamous cell carcinoma which met the criteria from January 2010 to December 2019 have been retrospectively analyzed, and the distribution of clinical treatment patterns has been analyzed. They cover such aspects as dfferences in survival time and further analysis of the differences in overall survival (OS) and progression-free survival (PFS) between patients who received immunotherapy and those who did not receive immunotherapy. What is more, Cox risk regression model has also been used to evaluate the risk factors affecting the prognosis of LAESCC. The cases of a total of 5328 newly diagnosed patients with esophageal cancer were collected, and a total of 363 patients were included in the study, with a median age of (46.2 ± 7.8) years old; 84 (23.1%) and 279 (76.9%) patients received 1L and ≥ 2L, respectively; Concurrent chemoradiotherapy (74.1%) and paclitaxel combined with platinum-based chemotherapy (14.3%) were the main first-line treatment options; fluorouracil combined with cisplatin regimen-based chemotherapy (63.8%) was the main treatment option for ≥ 2L, of which 69 patients (25.3%) received immunization treatment; OS of patients with 1 line of therapy and ≥ 2L were (22.4 ± 7.2) months and (38.7 ± 8.5) months, respectively, and the comparison between groups was statistically significant ( P < .05); among 69 patients with ≥ 2L who received immunotherapy, PFS and The OS was (14.6 ± 6.9) and (45.3 ± 9.7) respectively, and the comparison between the groups was statistically significant (all P < .05). Cox multivariate analysis has shown that clinical stage, immunotherapy, concurrent chemoradiotherapy, and ≥ 2L are the main factors affecting OS. and immunotherapy, concurrent chemoradiotherapy, and ≥ 2L are independent factors affecting PFS. Concurrent chemoradiotherapy is currently one of the standard treatments for LAESCC, and most patients are still willing to receive second-line or above treatments. Adding immunotherapy to standard treatment modalities may further optimize clinical treatment modalities and improve patient outcomes.
1. Introduction
Esophageal cancer is one of the most common digestive system tumors with high morbidity and mortality worldwide. [ 1 ] Due to differences in region, skin color, and pathogenic factors, squamous cell carcinoma is the predominant form of esophageal cancer in the Chinese population. [ 2 ] The existing diagnosis and treatment guidelines or norms are mainly based on clinical staging, and the treatment plan is selected after multidisciplinary consultation. There is no obvious difference in the treatment mode of esophageal squamous cell carcinoma or adenocarcinoma. [ 3 ] Affected by the anatomical structure, about 70% of newly diagnosed esophageal cancer patients in my country have lost the indications for radical surgery when they go to the clinic, especially for patients with locally advanced esophageal cancer. How to choose a more effective treatment mode is a difficulty in current research. [ 4 ] RTOG85-01 trial established the status of concurrent chemoradiotherapy for inoperable esophageal cancer. Compared with traditional radiotherapy, chemotherapy and other methods, immunotherapy, as an innovative treatment method, had its clinical efficacy confirmed in many clinical experiments, [ 5 ] but as for when to intervene and how to choose the best treatment, the plan is not yet clear. After the failure of the first-line treatment, how to choose a clinical plan for second- or even third-line treatment, and whether there is a survival benefit are all blind spots in current research. This study retrospectively analyzed the clinical data of 363 patients with locally advanced esophageal squamous cell carcinoma, trying to explore the “real world” treatment mode and clinical efficacy of locally advanced esophageal squamous cell carcinoma, and further evaluate the role of immunotherapy to provide data support for clinical practice.
2. Materials and methods
2.1. general data of patients.
The clinical data of patients diagnosed with esophageal cancer from January 2010 to December 2019 in the 900th Hospital of the Joint Logistics Support Force of the Chinese People’s Liberation Army were retrospectively analyzed. Inclusion criteria: The diagnosis of esophageal squamous cell carcinoma was confirmed by histopathological examination; Bone whole body imaging, upper abdominal MRI, abdominal color Doppler ultrasound or PET-CT examination, no distant organ metastasis; Through chest CT and gastric Intestinal barium meal examination, no indication of radical surgery after multidisciplinary consultation; Eastern cooperative oncology group score of 0 to 2; Age ≤ 75 years old; Complete clinical data. Exclusion criteria: Patients with severe and uncontrollable underlying diseases; Patients with a history of malignant tumors; Patients who are unwilling to receive long-term follow-up; Patients with psychiatric diseases; Incomplete clinical data. This study was approved by the hospital ethics committee, and all patients signed informed consent.
2.2. Determination of treatment mode
First-line treatment is defined as the treatment mode that patients receive after diagnosis; second-line treatment is defined as a new treatment mode after patients receiving first-line treatment due to disease progression or intolerance of the side effects of first-line treatment. The time interval is >21 days; the third-line treatment is defined as receiving a new treatment mode after receiving the second-line treatment due to disease progression or intolerance of the side effects of the first-line treatment, and the time interval between the second-line treatment and the second-line treatment is >21 days. All treatment modes can be single or single drug treatment methods, and are not limited to 2 or more drugs or treatment methods.
2.3. Treatments
2.3.1. chemotherapy alone paclitaxel combined with cisplatin..
Paclitaxel 135 mg/m2 intravenous drip d1, cisplatin 75 mg/m2 intravenous drip d1, q3w; fluorouracil combined with cisplatin regimen (PF): 750 mg/m2 CIV d1-5, cisplatin 75 mg/m2 intravenous drip d1, q3w.
2.3.2. The concurrent radio chemotherapy and radiotherapy plan is as follows.
Thermoplastic body membrane combined with vacuum pad for body position fixation, the patient is placed in the supine position, hands placed in front of the forehead, Philips large aperture 4.0 CT scanning is positioned, and the scanning range is from the level of the hyoid bone to the lower border of the right kidney; the slice thickness is 5 mm, and the scanned image is transmitted to the Eclips treatment planning system. Target area delineation principle: delineate the target area in combination with baseline evaluation of gastrointestinal barium meal examination, chest CT and electronic gastroscopy. GTV is the esophageal tumor lesion, which is 1 cm in the upper and lower directions, and 0.6 cm in the other directions as PGTV. The dose of 95% PGTV is 1.8 to 2.0 Gy/time, 1 time/day, 5 times/week, a total of 25 to 30 times, and the total amount is 50 to 60 Gy; GTVnd is The enlarged lymph nodes can be seen on CT, and the PGTVnd is 0.5 cm from each side. The dose of 95% PGTVnd is 1.8 to 2.0 Gy/time, 1 time/day, 5 times/week, a total of 25 to 30 times, and the total amount is 50 to 60Gy; CTV is the mediastinal prevention lymph node. The drainage area varies according to the location of the primary tumor in the esophagus. The lymph node drainage area of upper thoracic esophageal cancer includes supraclavicular lymph node drainage area, paraesophageal lymph node drainage area, group 2, group 4, group 5, and group 7 lymph node drainage area; lymph node drainage area of middle thoracic esophageal cancer includes paraesophageal lymph node drainage area, 7 groups of lymph node drainage areas; the lower thoracic lymph node drainage areas include paraesophageal, 4, 5, 7, and left gastric and paracardial lymph node drainage areas; CTV externally 0.3 cm is PCTV, and the dose of 95% PCTV is 1.8 to 2Gy/ times, 1 time/day, 5 times/week, a total of 30 times, with a total amount of 50 to 54Gy. Normal organ dose limits are as follows: V20 ≤ 20% for both lungs, V45 ≤ 0% for spinal cord, and V30 ≤ 5% for heart.
2.3.3. Concurrent chemotherapy regimen.
Cisplatin 30 mg/m2 intravenous infusion, d1 to 2 combined with albumin paclitaxel injection 260 mg/m2 intravenous infusion, d1, a total of 2 cycles of concurrent chemotherapy during the concurrent chemoradiotherapy.
2.3.4. Immunotherapy.
ICI includes pembrolizumab, toripalimab, camrelizumab, sintilizumab, and tilelizumab. The doses of pembrolizumab, carrelizumab, sintilizumab, and tilelizumab received by the patients were fixed doses of 200 mg every 3 weeks. The therapeutic dose of toripalimab is a fixed dose of 240 mg every 3 weeks. All patients included in the study received at least 1 cycle of the above immunization regimen until tumor progression.
2.4. Observation indicators
Tumor staging was based on the 2017 TNM staging standard of the American Joint Committee on Cancer. To compare the difference in general information and tumor objective response rate (ORR) between only one line of therapy (IL) and at least 2 lines of therapy, Among them, ORR = [number of complete response (CR) + number of partial response (PR)]/total number*100%; overall survival (OS) between patients with IL and ≥ 2L; difference in OS and progression-free survival (PFS) between patients who received immunotherapy and those who did not receive immunotherapy; and further The prognostic factors affecting OS were analyzed.
The overall survival time was defined as the time from the day of diagnosis to death or loss to follow-up; the time of disease progression-free survival was defined as the time from the day of diagnosis to the imaging-proven tumor progression (PD) and/or the appearance of new metastatic lesions. The efficacy evaluation criteria were based on the WHO RECISIT criteria. CR was defined as the complete disappearance of esophageal lesions and the disappearance or reduction of metastatic lymph nodes to normal (short diameter < 1 cm); PR was defined as the sum of the diameters of esophageal lesions and mediastinal lymph node lesions. Shrinkage ≥ 30%; PD is defined as a ≥ 20% increase in the sum of diameters of esophageal and mediastinal lymph node lesions compared to baseline assessment or the appearance of any 1 new lesion; esophageal and mediastinal lymph node lesions in the SD definition, The criterion for the sum of diameters is between PR and PD. The CR, PR, PD, and SD were finally confirmed through the joint confirmation of an associate chief imaging physician and associate chief clinical physician.
2.5. Follow-up
The follow-up time was up to December 31, 2020, and the median follow-up time was (42.4 ± 7.8) months. Follow-up methods included telephone, inpatient and outpatient follow-up. Review every 3 months for the first 2 years after treatment, and every 6 months for the 3rd to 5th years. Review items include chest CT, abdominal color Doppler ultrasound, gastrointestinal barium meal, blood routine, liver and kidney function, etc.
During the follow-up process of all patients, if the disease progresses, the treatment plan needs to be changed. That is, the increase in the number of treatment lines needs to be confirmed by 2 deputy chief physicians; if the 2 deputy chief physicians disagree, the third chief physician will carry out Decision; if the time of disease progression is ≥ 6 months from the last treatment, the chemotherapy regimen remains unchanged, and it is still included in the category of ≥ 2L.
2.6. Statistical methods
Statistical analysis was performed using SPSS software (version 26.0, IBM Software, Armonk, NY). Descriptive test was used to count the general data of patients with IL and ≥ 2L, and chi-square test or t test was used for comparison between groups; Square test; OS and PFS analysis using Kaplan–Meier method, parallel Log-rank test; Cox hazard regression model was used to evaluate the prognostic risk factors affecting OS in patients with esophageal cancer. P < .05 was considered to be statistically significant.
3.1. General information of research subjects
Casees of a total of 5328 newly diagnosed patients with esophageal cancer were collected, and a total of 363 patients met the inclusion and exclusion criteria, 136 (37.5%) females, 84 (23.1%) patients received IL therapy only, 279 (76.9%) patients received ≥ 2L regimen treatment, and only 69 patients received immunotherapy in ≥ 2L regimen treatment, See Figure Figure1 1 .

All patients screening flow chart (esophage cancer, EPC; esophageal squamous cell carcinoma, ESCC).
Among the first-line treatment regimens, the largest proportion was concurrent chemoradiotherapy in 269 cases (74.1%), followed by paclitaxel combined with platinum-based chemotherapy in 52 cases (14.3%); among ≥ 2L regimens, the largest proportion was PF-based chemotherapy regimen 147 cases (52.7%), followed by paclitaxel combined with platinum-based chemotherapy in 36 cases (12.9%), and 36 patients (12.9%) participating in clinical trials. In the ≥ 2L regimen, 69 patients (25.3%) received immunotherapy. See Figure Figure2 2 .

(A) Breakdown of treatments received in the first-line setting, (B) breakdown of treatments received in the ≥ 2-line setting.
3.2. Comparison of general conditions of patients in IL group and patients in ≥ 2L group.
The general conditions of the 2 groups of patients, such as age, gender, Eastern cooperative oncology group score, TNM stage, tumor location, length were comparable ( P > .05). After first-line treatment, the ORR of the 2 groups of patients was comparable. The rates were 90.5% and 87.8%, respectively, and there was no statistical difference between the groups ( P > .05). See Table Table1 1 .
Baseline characteristics of patients with advanced esophacacinoma cancer who received at least one line of systemic therapy (n, %).
≥2L = at least two lines of therapy, CR = complete response, ECOG = Eastern cooperative oncology group, IL = one line of therapy, PR = partial response.
3.3. Comparison of OS between IL and ≥ 2L patients
The OS of IL patients was (22.4 ± 7.2) months, and the OS of ≥ 2L patients was (38.7 ± 8.5) months, and there was a statistically significant difference between the groups (2 = 322.9, P < .05)., (95% CI: −0.18 to 1.38). See Figure Figure3 3 .

(A) Overall survival curve between IL and ≥ 2L, (B) free-progression survival between immunotherapy and nonimmunotherapy, and (C) overall survival curve between immunotherapy and nonimmunotherapy. IL = one line of therapy.
3.4. Comparison of PFS and OS with and nonimmunotherapy
Among them, 279 patients with ≥ 2L, 69 (24.7%) received immunotherapy, PFS and OS were (14.6 ± 6.9) months and (45.3 ± 9.7) months, respectively, 210 patients (75.3%) who did not receive immunotherapy, PFS and OS were (10.2 ± 3.5) months and (33.8 ± 8.6) months, respectively, and there were significant differences in PFS and OS between those who received and those who did not receive immunotherapy ( P < .05). See Figure Figure3 3 .
3.5. Multivariate analysis affecting the prognosis of patients with locally advanced esophageal cancer
The univariate analysis showed the patients who received immunotherapy, concurrent chemoradiotherapy and ≥ 2L therapy had better prognosis. The result was the same for the patients whose TNM stage was III stage. See Table Table2. 2 . The clinical stage, immunotherapy, concurrent chemoradiotherapy, and ≥ 2L were included in the Cox proportional hazards regression model. Independent factors; while immunotherapy, concurrent chemoradiotherapy, and ≥ 2L were independent factors affecting PFS. See Table Table3 3 .
The univariate analysis of prognostic factors for locally advanced esophageal cancer (%).
≥2L = at least two lines of therapy.
Cox multivariate analysis of influencing prognosis of local advanced esophageal cancer.
≥2L = at least two lines of therapy, OS = overall survival, PFS = progression-free survival.
4. Discussion
Esophageal cancer cells proliferate actively and have poor sensitivity to radiotherapy and chemotherapy. Locally advanced esophageal cancer has a large tumor burden and a high risk of recurrence, metastasis, esophageal perforation, bleeding, and other complications. [ 6 ] The development of radiotherapy technology, the optimization of chemotherapy drugs, targeted drugs, anti-vascular drugs and other treatment methods, especially the emergence of immunotherapy in many clinical trials of esophageal cancer, have changed the traditional treatment mode of locally advanced esophageal cancer. Whether patients with locally advanced esophageal cancer can receive the best regimen in the “real world” treatment, and whether immunotherapy can improve the prognosis of patients with esophageal cancer after failure of first-line therapy, remain controversial.
Among the 5328 patients with esophageal squamous cell carcinoma in the past 10 years, there were 363 patients with locally advanced unresectable patients, accounting for 6.8%. The occurrence of esophageal squamous cell carcinoma is related to smoking, drinking, poor dietary habits, genetic factors, and lack of micronutrients. Due to the lack of screening guidelines for esophageal squamous cell carcinoma, patients may delay the best time for treatment. [ 7 ] Although the development of imaging technology has improved the diagnosis rate of esophageal squamous cell carcinoma, if the screening of early gastroscope is actively carried out, the diagnosis rate of early esophageal squamous cell carcinoma can be effectively improved. [ 8 ] Whether the proportion in the total population shows a decreasing trend year by year needs further research. In European and American countries, the incidence of esophageal squamous cell carcinoma has shown a downward trend, but the incidence of males has increased to varying degrees. [ 9 ] In this study, the proportion of male patients was significantly higher than that of female patients (62.5% vs 37.5%), which was significantly related to regional ethnic distribution and incidence factors. The age group of 40 to 60 years is the high incidence age of esophageal cancer, but the clinical treatment costs vary greatly depending on the age of onset and histological stage. [ 10 , 11 ] The median age of locally advanced esophageal squamous cell carcinoma in this study was (47.4 ± 6.8) years, which was basically consistent with previous reports. Among people over 40 years old, popularizing the early screening of electronic gastroscope can improve the detection rate of early esophageal squamous cell carcinoma and minimize the expenditure of medical insurance.
Concurrent chemoradiotherapy is one of the standard treatment options for patients with locally advanced esophageal cancer, but the overall effect is poor. The treatment mode of different chemotherapy regimens combined with radiotherapy has gradually become a research hotspot. [ 12 ] The modality of concurrent radiotherapy with paclitaxel, cisplatin, and fluorouracil (DCF-RT) has a better prognosis than concurrent radiotherapy with cisplatin and fluorouracil (CF-RT), and there is no significant increase in clinical adverse effects. [ 13 ] In the real-world, nearly 269 patients (74.1%) received concurrent radiotherapy with paclitaxel combined with cisplatin, which also indirectly reflects the concurrent radio chemotherapy regimen of 2 or more drugs, which is the first choice for first-line treatment. It can inhibit the proliferation of tumor cells to the greatest extent and achieve the purpose of improving the efficacy, [ 14 ] but there is no consensus on the optimal concurrent chemoradiotherapy regimen. With the deepening of immunotherapy research, in many large clinical studies, the addition of immunotherapy has significantly improved the prognosis compared with chemotherapy alone. [ 15 , 16 ] However, affected by my country’s economic factors and medical insurance system, chemotherapy alone in the first-line regimen is mainly paclitaxel combined with platinum (14.3%). With the approval of immunotherapy drugs for esophageal squamous cell carcinoma indications, the medical treatment strategy for patients with unresectable locally advanced esophageal squamous cell carcinoma will also be completely changed. Radiation therapy has the effect of destroying the DNA double-strand of tumor cells, improving antigen exposure and presentation, and has a synergistic effect with immunotherapy. [ 17 ] Adding immunotherapy on the basis of the 3-drug combination can theoretically overcome the limitation of cross-resistance and improve the control rate of esophageal lesions, but there is no prospective study to confirm the side effects and safety. Therefore, in the first-line treatment regimen, concurrent chemoradiotherapy is still the current mainstream mode, and concurrent chemoradiotherapy with chemotherapy drugs combined with immunotherapy may be the best choice.
Studies have confirmed that although the palliative treatment mode can reduce the side effects of treatment to a certain extent, it does not improve the overall survival rate. For patients with esophageal squamous cell carcinoma who have received multiple lines of treatment, more attention should be paid to comprehensive treatment based on nutritional support. [ 18 ] In the real-world, esophageal squamous cell carcinoma has a high risk of recurrence or metastasis after first-line therapy due to its poor sensitivity to chemoradiotherapy. [ 19 ] However, most of the patients did not have advanced tumor manifestations such as cachexia, and the overall physical condition was still in the stage of tolerable treatment. Therefore, 279 patients (76.9%) received ≥ 2L regimen. There were 147 cases (52.7%) who received PF-based chemotherapy regimen. After first-line paclitaxel combined with platinum regimen, PF was used as a continuous chemotherapy strategy in second-line regimen, which indirectly confirmed that paclitaxel, cisplatin, and fluorouracil are the main consolidation of esophageal squamous cell carcinoma. Chemotherapy, either at baseline or second-line. [ 20 ] In a multi-center study program, the above 3 chemotherapy drugs were used as the research objects, and they were combined with each other. In the progress of the initial diagnosis program, other programs were used as continuous treatment. [ 21 ] This study is basically consistent with this treatment model. As a necessary stage of drug research and development, clinical trials have gradually put the interests of patients in the most important position, and ensured the safety and efficacy of patients through blind review, supervision and other measures. [ 22 ] Thirty-six patients (12.9%) in the ≥ 2L regimen participated in the clinical trial. Compared with the treatment mode of IL, there was a greater improvement, mainly because there was no effective second-line treatment regimen for esophageal cancer. The research sponsor urgently needs This situation is changed through clinical trials; it also indirectly shows that the concept of clinical trials is more and more accepted by the Chinese community. Currently, the approved immunotherapy drugs for esophageal cancer are mainly programmed death-1 and programmed death-ligand 1 (PD-L1). In 2017, the US Food and Drug Administration approved pembrolizumab for the treatment of recurrent localized tumors that express PD-L1 (CPS ≥ 1) and progress after receiving 2 or more chemotherapy regimens After advanced gastroesophageal junction adenocarcinoma; pembrolizumab was approved in 2019 for recurrent, locally advanced, or metastatic esophageal cancer after first- or multiple-line systemic therapy. In recent years, domestic drugs such as carelizumab and sintilimab have gradually been written into the first-line treatment guidelines from the third-line. Limited by objective factors such as study time, in the past 10 years, although only 69 patients (25.3%) received immunotherapy, and all of them were used after first-line treatment, this also indirectly reflects the rapid progress of drug research and development in China in recent years. the China Food and Drug Administration has also accelerated the approval process for clinical oncology drugs.
After further stratified analysis, the overall survival time of patients receiving ≥ 2L regimen was significantly better than that of IL patients, and clinical stage, immunotherapy, concurrent chemoradiotherapy, at least 2 lines of therapy and other factors were closely related to prognosis. For patients with esophageal squamous cell carcinoma, consistent with other solid tumors, the tumor burden is positively related to the prognosis of the patients, and the local control rate of the tumor should be emphasized. [ 23 ] The OS of patients with IL and ≥ 2L in this study were (22.4 ± 7.2) months and (38.7 ± 8.5) months, respectively, which were significantly higher than the 13.1 months reported by Janjigian, [ 24 ] who only used chemotherapy combined with immunotherapy, confirming that Radiation therapy plays a very important role in locally advanced unresectable esophageal squamous cell carcinoma. Differences in pathology types and study populations may cause bias in study results. Immunotherapy significantly improved PFS and OS in patients with ≥ 2L, which was consistent with the findings of Zhang et al, [ 25 ] further confirming that immunotherapy may have synergistic effects with radiotherapy and chemotherapy. [ 26 ] After concurrent chemoradiotherapy, neutrophils were significantly increased, and tumor mutation load and neoantigen load were significantly reduced, which may be the mechanism of action of potential immunotherapy. [ 27 ]
However, limited by the actual sample size included, our conclusions may be biased. Due to the large deviation in sample size at each stage, there would be bias in the research results. In addition, the choice of different immunological drugs and combination regimens may affect the final survival data. Due to the retrospective design, we did not pay attention to the expression of esophageal cancer-related molecules such as HER-2 and PD-L1 in patients. Furthermore, in real-world studies, we may not be able to focus on some concomitant diseases in which patients show no symptoms other than the tumor.
5. Conclusion
In the era of immunotherapy, the prognosis of unresectable locally advanced esophageal squamous cell carcinoma is expected to be further improved. In China’s unique medical environment, concurrent chemoradiotherapy as a standard treatment mode is being more closely integrated with immunotherapy. According to the biological characteristics of esophageal squamous cell carcinoma in China, the development of effective and reasonable first- and second-line treatment strategies can further improve the survival time of patients.
Author contributions
Conceptualization: Huachun Luo, Fang-Wei Xie.
Data curation: Jing-Jing Wu, Jing Feng, Meng-Jing Wu.
Formal analysis: Zhi-Chao Fu, Fang-Wei Xie.
Funding acquisition: Huachun Luo.
Investigation: Li-Jun Zhu, Fei-Fan Chen.
Methodology: Lv-Juan Cai, Zhi-Yong Shen.
Writing – original draft: Huachun Luo.
Abbreviation:
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Luo H-C, Wu J-J, Zhu L-J, Cai L-J, Feng J, Shen Z-Y, Wu M-J, Chen F-F, Fu Z-C, Xie F-W. Real-world treatment patterns and survival for locally advanced esophageal squamous cell carcinoma. Medicine 2023;102:34(e34647).
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 17, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New guideline: Barrett's esophagus precedes esophageal cancer, but not all patients need abnormal cell removal
by American Gastroenterological Association
The American Gastroenterological Association's (AGA) new evidence-based Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett's Esophagus and Related Neoplasia, published today in Gastroenterology , establishes updated guidance for Barrett's esophagus patients.
A precursor to esophageal cancer , Barrett's esophagus is a condition in which the cells in the esophagus have been replaced with non-cancerous abnormal cells. These cells can progress to a condition called dysplasia , which may in turn become cancer. Dysplasia is considered low-grade or high-grade, depending on the degree of cellular change.
"While the benefit is clear for patients with high-grade dysplasia, we suggest considering endoscopic eradication therapy for patients with low-grade dysplasia after clearly discussing the risks and benefits of endoscopic therapy," said guideline author Dr. Tarek Sawas, assistant professor in the department of internal medicine at UT Southwestern.
"A patient-centered approach ensures that treatment decision is made collaboratively, taking into account both the medical evidence and the patient's preferences and values. Surveillance is a reasonable option for patients who place a higher value on harms and a lower value on the uncertain benefits regarding reduction of esophageal cancer mortality"
Endoscopic eradication therapy consists of minimally invasive procedures such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), followed by ablation (burning or freezing) techniques.
Key guideline takeaways:
- For patients with low-grade dysplasia, it may be appropriate to either remove or monitor the cells. This is a decision doctors and patients should make together after discussing the risks and benefits of treatment.
- For patients with high-grade dysplasia, AGA recommends endoscopic therapy to remove the abnormal pre-cancerous cells.
- Most patients undergoing endoscopic eradication can be safely treated with EMR, which has a lower risk of adverse events. Patients who undergo ESD can face an increased risk of strictures and perforation. AGA recommends reserving ESD primarily for lesions suspected of harboring cancers invading more deeply into the wall of the esophagus or those who have failed EMR.
- Patients with Barrett's esophagus (dysplasia or early cancer) should be treated and monitored by expert endoscopists and pathologists who have experience in Barrett's neoplasia.
"We need to have a conversation with patients in clinic prior to when they show up in the endoscopy unit on a gurney. Patients need to be fully aware of the risks and benefits, both in the short term but also in the long run, to decide which treatment approach is best for them. This decision often comes down to personal factors and values," added guideline author Dr. Joel Rubenstein, who is the director of the Barrett's Esophagus Program at the University of Michigan.
The guideline provides the following general implementation considerations:
- Tobacco use and obesity are risk factors for esophageal adenocarcinoma, so counseling patients to abstain from tobacco use and to lose weight can help improve outcomes.
- In patients with Barrett's esophagus, reflux control should be optimized with both medication and lifestyle modifications.
Guideline: https://www.gastrojournal.org/article/S0016-5085(24)00302-0/fulltext
Clinical decision support tool: https://www.gastrojournal.org/article/S0016-5085(24)00432-3/fulltext
Spotlight (infographic): https://www.gastrojournal.org/article/S0016-5085(24)00433-5/fulltext
Explore further
Feedback to editors
New analysis estimates the effects of race-neutral lung function testing on patients, hospitals, and beyond
7 hours ago

World-first trial shows benefits of finding and treating undiagnosed asthma and COPD
10 hours ago

Acetaminophen shows promise in warding off acute respiratory distress syndrome, organ injury in patients with sepsis
13 hours ago

Modular communicative leadless ICD found to be safe and exceeds performance expectations
May 18, 2024

Creativity and humor shown to promote well-being in older adults via similar mechanisms

Sweet taste receptor affects how glucose is handled metabolically by humans

Better medical record-keeping needed to fight antibiotic overuse, studies suggest

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses

A long-term ketogenic diet accumulates aged cells in normal tissues, new study shows

Gut bacteria enhance cancer immunotherapy in mouse study
Related stories, new recommendations for endoscopic eradication therapy in barrett's esophagus.
Apr 6, 2018
Results of early endoscopic exam critical for assessment of Barrett's patients
Feb 14, 2019
Updated Barrett's Guideline aims to improve patient outcomes
Sep 5, 2019
New studies add to understanding of treatments for Barrett's esophagus
Oct 6, 2017

Adenocarcinoma, high-grade dysplasia up with barrett esophagus
Mar 18, 2019
Guidelines updated for Barrett esophagus diagnosis and management
Apr 22, 2022
Recommended for you
Cell types and molecules usually associated with autoimmune diseases found to be normal components of gut immunity

Combining two tumor markers can help track down colorectal cancer subtypes
May 16, 2024

Study identifies genetic link between inflammatory bowel disease and Parkinson's disease
May 14, 2024

Autism's missing microbes may influence social behavior by protecting the gut
May 10, 2024

ERR-gamma 'trains' stomach stem cells to become acid-producing cells

Study shows damaging impact of heat waves on vital organs
May 8, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Oesophageal cancer articles from across Nature Portfolio
Oesophageal cancer is a malignancy of the oesophagus or food pipe. It is a rare malignancy. Nearly all oesophageal cancer are squamous cell carcinomas (cancer arising from the flat cells of the oesophagus mucosa) or adenocarcinomas (cancer arising from the glandular cells of the oesophagus submucosa).
Latest Research and Reviews

HLA-A + tertiary lymphoid structures with reactivated tumor infiltrating lymphocytes are associated with a positive immunotherapy response in esophageal squamous cell carcinoma
- Dandan Zhang
- Dongxian Jiang
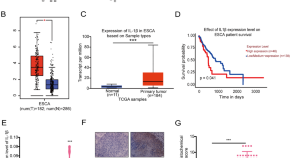
IL-1β promotes esophageal squamous cell carcinoma growth and metastasis through FOXO3A by activating the PI3K/AKT pathway
- Shuangshuang Chen
- Hongchun Liu
The pluripotency factor NANOG contributes to mesenchymal plasticity and is predictive for outcome in esophageal adenocarcinoma
Van der Zalm et al. apply Ridge regression analysis to RNA-seq data from esophageal cancer samples to predict mesenchymal transitions occurring in patients. Expression of NANOG in pre-treatment biopsies is associated with poor response to neoadjuvant chemoradiation, recurrence, and median overall survival in patients with esophageal adenocarcinoma.
- Amber P. van der Zalm
- Mark P. G. Dings
- Maarten F. Bijlsma
Jag1/2 maintain esophageal homeostasis and suppress foregut tumorigenesis by restricting the basal progenitor cell pool
Dysregulation of basal progenitor cells induces esophageal tumorigenesis but the underlying mechanism is less explored. Here, the authors show that Jag1/2 deficiency promotes expansion of basal progenitor cells, leading to reduced squamous epithelial differentiation and enhanced formation of squamous cell carcinoma in the foregut.
- Haidi Huang
- Yongchun Zhang
Disentangling oncogenic amplicons in esophageal adenocarcinoma
Esophageal adenocarcinoma is characterised by frequent amplifications in oncogenes. Here, the authors use short- and long-read sequencing approaches to analyze primary tumor samples and tumour-derived organoids and to investigate the mechanisms underlying complex amplifications.
- Alvin Wei Tian Ng
- Dylan Peter McClurg
- Rebecca C. Fitzgerald
Multimodal analysis of cfDNA methylomes for early detecting esophageal squamous cell carcinoma and precancerous lesions
Esophageal squamous cell carcinoma is most commonly detected at a late stage, which limits survival and treatment options. Here, the authors utilise whole genome bisulfite sequencing to create a cfDNA framework to detect cfDNA methylation, copy number variants and fragmentation.
News and Comment
Reply to l. celio et al..
- Hiroko Minatogawa
- Naoki Izawa
- Takako Eguchi Nakajima
Sugemalimab with chemotherapy for advanced ESCC
- Katrina Ray
Salivary miRNA signature for ESCC
- Jordan Hindson

Pan-cancer analysis of TRPV2 identifies its potential role as a prognostic and immunologic biomarker in oesophageal cancer
- Yousheng Mao
Air pollution and oesophageal cancer risk
- Eleni Kotsiliti
Large-scale genomic analyses reveal alterations and mechanisms underlying clonal evolution and immune evasion in esophageal cancer
Esophageal cancers feature distinct manifestations between and within patients which complicate precision diagnosis, prognosis, and patient care. New genomic and epigenomic research uncovers novel mechanisms underlying both inter- and intra-tumoral heterogeneity in esophageal cancer, with significant biological and translational implications.
- De-Chen Lin
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
To develop a prognostic model for neoadjuvant immunochemotherapy efficacy in esophageal squamous cell carcinoma by analyzing the immune microenvironment
Affiliations.
- 1 Department of Pathology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
- 2 Department of Endoscopy Center, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
- 3 Department of Radiotherapy, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
- 4 Department of Thoracic Surgery, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China.
- 5 Genecast Biotechnology Co., Ltd, Wuxi, China.
- PMID: 38726002
- PMCID: PMC11079241
- DOI: 10.3389/fimmu.2024.1312380
Objective: The choice of neoadjuvant therapy for esophageal squamous cell carcinoma (ESCC) is controversial. This study aims to provide a basis for clinical treatment selection by establishing a predictive model for the efficacy of neoadjuvant immunochemotherapy (NICT).
Methods: A retrospective analysis of 30 patients was conducted, divided into Response and Non-response groups based on whether they achieved major pathological remission (MPR). Differences in genes and immune microenvironment between the two groups were analyzed through next-generation sequencing (NGS) and multiplex immunofluorescence (mIF). Variables most closely related to therapeutic efficacy were selected through LASSO regression and ROC curves to establish a predictive model. An additional 48 patients were prospectively collected as a validation set to verify the model's effectiveness.
Results: NGS suggested seven differential genes (ATM, ATR, BIVM-ERCC5, MAP3K1, PRG, RBM10, and TSHR) between the two groups (P < 0.05). mIF indicated significant differences in the quantity and location of CD3+, PD-L1+, CD3+PD-L1+, CD4+PD-1+, CD4+LAG-3+, CD8+LAG-3+, LAG-3+ between the two groups before treatment (P < 0.05). Dynamic mIF analysis also indicated that CD3+, CD8+, and CD20+ all increased after treatment in both groups, with a more significant increase in CD8+ and CD20+ in the Response group (P < 0.05), and a more significant decrease in PD-L1+ (P < 0.05). The three variables most closely related to therapeutic efficacy were selected through LASSO regression and ROC curves: Tumor area PD-L1+ (AUC= 0.881), CD3+PD-L1+ (AUC= 0.833), and CD3+ (AUC= 0.826), and a predictive model was established. The model showed high performance in both the training set (AUC= 0.938) and the validation set (AUC= 0.832). Compared to the traditional CPS scoring criteria, the model showed significant improvements in accuracy (83.3% vs 70.8%), sensitivity (0.625 vs 0.312), and specificity (0.937 vs 0.906).
Conclusion: NICT treatment may exert anti-tumor effects by enriching immune cells and activating exhausted T cells. Tumor area CD3+, PD-L1+, and CD3+PD-L1+ are closely related to therapeutic efficacy. The model containing these three variables can accurately predict treatment outcomes, providing a reliable basis for the selection of neoadjuvant treatment plans.
Keywords: esophageal squamous cell carcinoma (ESCC); neoadjuvant immunochemotherapy (NICT); predictive model; response; tumor immune microenvironment (TIME).
Copyright © 2024 Yehan, Sheng, Hong, Jiayu, Jun, Juan, Min, Jiaxin, Shangzhi, Yi, Qifeng, Xuefeng, Wenwu, Xueyan, Yang and Zongyao.
Publication types
- Research Support, Non-U.S. Gov't
- Biomarkers, Tumor
- Esophageal Neoplasms* / drug therapy
- Esophageal Neoplasms* / immunology
- Esophageal Neoplasms* / therapy
- Esophageal Squamous Cell Carcinoma* / drug therapy
- Esophageal Squamous Cell Carcinoma* / immunology
- Esophageal Squamous Cell Carcinoma* / therapy
- Immunotherapy / methods
- Middle Aged
- Neoadjuvant Therapy* / methods
- Retrospective Studies
- Treatment Outcome
- Tumor Microenvironment* / immunology
Associated data
- figshare/10.6084/m9.figshare.23951592
Grants and funding
Are Much Needed Advances in Early Esophageal Cancer Detection on the Horizon?

Esophageal cancer remains plagued by a lack of effective techniques to detect early malignancies. Due to this major clinical challenge, esophageal cancer cases are often diagnosed at an advanced stage, with limited effective treatment options. (Our recent article covered esophageal cancer’s symptoms, treatments, and challenges in detail.)
The poor efficacy of esophageal cancer treatment methods begs the need for new techniques and approaches to screen for esophageal cancer comprehensively. A recent publication in BME Frontiers describes one such new technique that could potentially speed up our ability to detect, visualize, and diagnose esophageal cancer.
Researchers have developed a technology aptly named MAGIC (multifunctional ablative gastrointestinal imaging capsule), and their data imply a potential groundbreaking innovation to address the clinical needs surrounding esophageal cancer surveillance. MAGIC combines two necessary modalities: optical coherence tomography ( OCT ), a noninvasive technique for examining tissue, and tethered capsule endomicroscopy ( TCE ), a technology that quickly collects microscopic pictures of the esophagus in patients without sedation.
OCT uses near-infrared light to penetrate several hundred microns into a biological sample. This technology measures the amount of light which scatters off the sample and constructs a profile of the depth of the tissue at each location. Using a scanning beam of light, OCT can collect data for an entire cross-section of tissue and identify the structure. While OCT TCE technologies have shown advances in surveillance, suboptimal resolution prevents the detection of early esophageal lesions.
In developing MAGIC, researchers utilized OCT imaging at two distinct wavelengths and an ultracompact camera for TCE. The research team also added an ablation laser, which provided the capability to extract tissue for highly detailed biopsies.
The study demonstrated the ability of MAGIC to image the esophagus in pig models. When operating at a wavelength of 800 nanometers, the researchers obtained high resolution and contrast of the surface layers of the esophagus. The images allowed the detection of early changes and abnormal growths in the esophagus. Utilizing a 1300 nanometer wavelength, the researchers could penetrate the esophageal tissue further, allowing assessment of the invasion of cancer cells between layers of tissue.
The ultracompact camera built into MAGIC helped the researchers visualize the esophagus during the procedure and provided the potential to guide laser ablation when warranted. Notably, the feasibility of MAGIC without sedation suggests a possibility of developing this technology for quick and efficient treatment of the esophagus in an outpatient setting.
MAGIC has paired complementary technologies to improve endoscopic screening and diagnosis of esophageal lesions in animal models. The authors indicate ongoing studies will continue to validate this technology and develop its clinical potential.
Sources: BME Front , J Biomed Opt , Dig Endosc

- Sponsored Content
- Infographics
- Health & Medicine
- Clinical & Molecular DX
- Cell & Molecular Biology
- Genetics & Genomics
- Microbiology
- Neuroscience
- Drug Discovery & Development
- Plants & Animals
- Cannabis Sciences
- Earth & The Environment
- Space & Astronomy
- Chemistry & Physics


COMMENTS
The study was published on Wednesday in the New England Journal of Medicine. "It is a game changer," said Dr. David Ilson, an esophageal cancer expert at Memorial Sloan Kettering Cancer Center ...
The new findings build on recent studies testing immunotherapy drugs in esophageal cancer. In 2019, the drug pembrolizumab (Keytruda) was approved for the treatment of some patients with advanced esophageal cancer. The following year, researchers reported that using nivolumab plus chemotherapy as an initial treatment for advanced stomach cancer ...
Simple Summary. Over 500,000 individuals died due to esophageal cancer (EC) worldwide in 2020. More than four decades of etiological research have predominantly focused on the esophageal squamous cell carcinoma (ESCC) subtype, which accounts for 80% of EC cases, while less etiologic research has been done on the esophageal adenocarcinoma (EAC) subtype whose incidence rates are rapidly ...
Most of the esophageal cancer (EC) is esophageal squamous cell carcinoma (ESCC) or esophageal adenocarcinoma (EAC), which is a malignant tumor originating from the esophageal epithelium. Global epidemiological data in 2020 showed that EC ranks seventh in terms of incidence (604,100 new cases) and sixth in the overall mortality (544,076 deaths ...
Cancer is an international interdisciplinary journal publishing articles on the latest clinical cancer research findings, spanning the breadth of oncology disciplines. A review of 2021's advances in the treatment of esophageal/gastroesophageal junction cancers, particularly in locally advanced disease (CheckMate-577), metastatic HER2-negative ...
Esophageal cancer is the seventh leading cause of cancer mortality worldwide. Esophageal squamous-cell carcinoma (ESCC) is the most common histological subtype of esophageal cancer, accounting for approximately 90% of all cases worldwide [1, 2].Many ESCCs are unresectable at diagnosis, and over half of patients treated with curative intent eventually have a relapse [3,4,5,6,7,8,9,10,11].
Immunotherapy for esophageal cancer. Anti-PD-1 after surgery improves the survival of patients with esophageal cancers in a clinical trial. The standard of care for patients with esophageal cancer ...
In this review, we have summarized the latest research on targeted therapy drugs for esophageal cancer, including ESCC and EAC, and also focused on several drugs that are most likely to be ...
A 10-15% response rate to immune therapies has been reported in patients with recurrent or metastatic oesophageal cancer [ 82 ]. Advances in the use of immune checkpoint inhibitors for ...
A new study by The Cancer Genome Atlas Research Network could help classify esophageal cancers according to their genetic and molecular alterations and identify potential new treatment options. Patients with esophageal cancer who received chemotherapy and radiation before surgery survived, on average, nearly twice as long as patients treated ...
Esophageal cancer (EC) represents the 6th most frequent cancer-related cause of death worldwide, with 500,000 estimated new cases per year with an overall survival rate of 20% in five years [ 1, 2 ]. The two predominant histological types are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC).
By Dana Sparks. The sixth most common cause of cancer deaths world-wide, esophageal cancer occurs in the esophagus — a long, hollow tube that runs from the throat to the stomach — and can occur anywhere along the esophagus. Men are more likely to develop esophageal cancer than women. While treatable, esophageal cancer is rarely curable.
JAMA Network Open. Research. August 22, 2023. This cross-sectional study examines temporal trends in incidence rates of esophageal cancer, adenocarcinoma of the esophagus, and squamous cell carcinoma of the esophagus from 1975 through 2018. Oncology Cancer Epidemiology Gastroenterology and Hepatology Esophageal Disease Gastroenterology.
Regarding specific high-risk populations like China, where half of all new cases are identified, there are new research and public health initiatives underway. The National Cohort of Esophageal Cancer- Prospective Cohort Study of Esophageal Cancer and Precancerous Lesions Based on High-Risk Populations (NCEC-HRP), has been developed.
New Trends in Esophageal Cancer Management. Esophageal cancer (EC) is a condition with a five-year survival rate of around 15% for all stages considered. It is the eighth most common cancer and the sixth worst prognosis because of its poor survival rate and aggressiveness [ 1 ]. It is important to distinguish the two main histological subtypes ...
Over 500,000 individuals died due to esophageal cancer (EC) worldwide in 2020. More than four decades of etiological research have predominantly focused on the esophageal squamous cell carcinoma (ESCC) subtype, which accounts for 80% of EC cases, while less etiologic research has been done on the esophageal adenocarcinoma (EAC) subtype whose incidence rates are rapidly increasing in some high ...
This April for Esophageal Cancer Awareness Month, discover the new research, new treatments, and progress for a future immune to esophageal cancer. Cancer Research and Treatment Update. Cancer patients and leading oncologists joined a lively discussion across 14 informative sessions at the 2021 Virtual Immunotherapy Patient Summit last October ...
This cross-sectional study examines temporal trends in incidence rates of esophageal cancer, adenocarcinoma of the esophagus, and squamous cell carcinoma ... Get the latest research based on your areas of interest. ... EC is the 14th most common cancer, with an estimated 21 560 new diagnoses and 16 120 expected deaths in 2023. 2 Men are at ...
The Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium was created in 2009 as a collaboration across the UK to better understand esophageal adenocarcinoma (OAC).
In the U.S. alone, there will be an estimated 22,000 new cases of esophageal cancer and 16,000 deaths in 2023. The five-year relative survival rate for patients with esophageal cancer is 43% for patients with localized disease found only in the esophagus; 23% for regional disease that has spread to nearby lymph nodes and organs; and 5% for ...
PURPOSE: This randomized phase II trial is studying PET scan imaging in assessing response in patients with esophageal cancer receiving combination chemotherapy. Radiation Therapy, Paclitaxel, and Carboplatin with or without Trastuzumab in Treating Patients with Esophageal Cancer Scottsdale/Phoenix, AZ; Rochester, MN.
Current research is testing esophageal cancer DNA from liquid biopsies to find specific mutations. Researchers are hoping to find out if the gene changes could help doctors choose the best drugs for patients. Studies are also looking at whether the liquid biopsy tumor DNA can help predict how the tumor might respond to certain drugs, or how ...
Researchers are looking at using aspirin and acid-reducing medication to prevent esophageal adenocarcinoma in people with Barrett's esophagus. Research is still ongoing, and people are encouraged to talk with their doctor before taking any medications or dietary supplements for this reason. Learn about the basics of chemoprevention.
The validity of endoscopic submucosal dissection (ESD) for esophageal squamous cell carcinoma (ESCC) in older individuals with comorbidities remains unclear. Therefore, this study evaluated the safety and efficacy of ESD and additional treatment for ESCC in older adult patients. The clinicopathological characteristics and clinical outcomes of 398 consecutive older adult patients (≥ 65 years ...
1. Introduction. Esophageal cancer is one of the most common digestive system tumors with high morbidity and mortality worldwide. [] Due to differences in region, skin color, and pathogenic factors, squamous cell carcinoma is the predominant form of esophageal cancer in the Chinese population. [] The existing diagnosis and treatment guidelines or norms are mainly based on clinical staging, and ...
A precursor to esophageal cancer, Barrett's esophagus is a condition in which the cells in the esophagus have been replaced with non-cancerous abnormal cells.These cells can progress to a ...
A comprehensive review (DOI: 10.20892/j.issn.2095-3941.2023.0177), conducted by researchers at Zhengzhou University, Henan Cancer Hospital, and the Marshall Medical Research Center, marks a ...
New genomic and epigenomic research uncovers novel mechanisms underlying both inter- and intra-tumoral heterogeneity in esophageal cancer, with significant biological and translational ...
1 Department of Pathology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, ... The choice of neoadjuvant therapy for esophageal squamous cell carcinoma (ESCC) is controversial. This study aims to provide a basis for clinical treatment selection by establishing a predictive model for the efficacy of neoadjuvant immunochemotherapy ...
The poor efficacy of esophageal cancer treatment methods begs the need for new techniques and approaches to screen for esophageal cancer comprehensively. A recent publication in BME Frontiers describes one such new technique that could potentially speed up our ability to detect, visualize, and diagnose esophageal cancer.