

New Autism Study Reveals 'Tantalizing Clues' About Its Development
S cientists have made a breakthrough in our understanding of the neuroscience behind autism spectrum disorders that promises to "revolutionize" the way we approach treatment, scientists say. The discovery revolves around an important chemical messenger that we tend to associate with pleasure and reward: dopamine.
Autism spectrum disorders are a diverse group of conditions characterized by some degree of difficulty with social interaction and communication. They affect roughly 1 in 100 children worldwide, according to data from the World Health Organization.
There are many potential causes of autism spectrum disorders, and both environmental and genetic factors are thought to play a role. A lot of questions still exist about the biochemical mechanisms that underlie these conditions, but recent evidence suggests that dopamine, the famous "feel-good" hormone, might play a role.
"While dopamine is commonly recognized as a neurotransmitter, its significance in the developmental aspects of autism is largely unexplored," said lead investigators Lingyan Xing and Gang Chen of China's Nantong University in a statement.
"Recent studies have highlighted the crucial roles of dopamine and serotonin in [neurotypical brain] development and their importance in the construction of neural circuits," they continued. "In addition, studies have indicated that the use of dopamine-related drugs during pregnancy is associated with an increased risk of autism in children.
"Armed with these tantalizing clues, we embarked on a mission to bridge the gap between dopamine's known functions and its potential impact on neurodevelopmental disorders, particularly autism," Lingyan and Gang said.
In a study published in The American Journal of Pathology , Lingyan, Gang and their colleagues investigated the role of dopamine signaling in autism development. "Our quest was to uncover a novel therapeutic target that could revolutionize the way we approach autism treatment," Lingyan and Gang said.
The study consisted of two parts. The first involved analyzing changes in gene expression in the brains of people with autism. The second used zebra fish models to explore how perturbations in dopamine signaling could produce autism-like behaviors.
In the first part of the study, the team found that patients with autism showed changes in the expression of genes involved in dopamine-signaling pathways and brain development. The authors say this indicated a potential link between dopamine disruption and autism development.
To explore this link further, the team re-created these disrupted dopamine pathways in the brains of zebra fish larvae and found that the larvae with signal disruption developed brain circuit abnormalities and behaviors reminiscent of human autism.
"We were surprised by the extent of the impact that dopaminergic signaling has on neuronal specification in zebrafish, potentially laying the groundwork for circuit disruption in autism-related phenotype," Gang wrote.
Lingyan added: "This research sheds light on the role of dopamine in neural circuit formation during early development, specifically in the context of autism. Understanding these mechanisms could lead to novel therapeutic interventions targeting dopaminergic signaling pathways to improve outcomes in individuals with autism and other neurodevelopmental disorders."
Do you have a tip on a health story that Newsweek should be covering? Do you have a question about autism? Let us know via [email protected].
Start your unlimited Newsweek trial

- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Brain cells, interrupted: How some genes may cause autism, epilepsy and schizophrenia

Jon Hamilton

New research probes the relationship between certain genes and brain disorders like autism and schizophrenia. Jill George / NIH hide caption
New research probes the relationship between certain genes and brain disorders like autism and schizophrenia.
A team of researchers has developed a new way to study how genes may cause autism and other neurodevelopmental disorders: by growing tiny brain-like structures in the lab and tweaking their DNA.
These "assembloids," described in the journal Nature , could one day help researchers develop targeted treatments for autism spectrum disorder, intellectual disability, schizophrenia, and epilepsy.
"This really accelerates our effort to try to understand the biology of psychiatric disorders," says Dr. Sergiu Pașca , a professor of psychiatry and behavioral sciences at Stanford University and an author of the study.
The research suggests that someday "we'll be able to predict which pathways we can target to intervene" and prevent these disorders, adds Kristen Brennand , a professor of psychiatry at Yale who was not involved in the work.

Shots - Health News
Researchers link autism to a system that insulates brain wiring.
The study comes after decades of work identifying hundreds of genes that are associated with autism and other neurodevelopmental disorders. But scientists still don't know how problems with these genes alter the brain.
"The challenge now is to figure out what they're actually doing, how disruptions in these genes are actually causing disease," Pașca says. "And that has been really difficult."
For ethical reasons, scientists can't just edit a person's genes to see what happens. They can experiment on animal brains, but lab animals like rodents don't really develop anything that looks like autism or schizophrenia.
So Pașca and a team of scientists tried a different approach, which they detailed in their new paper .
The team did a series of experiments using tiny clumps of human brain cells called brain organoids . These clumps will grow for a year or more in the lab, gradually organizing their cells much the way a developing brain would. And by exposing an organoid to certain growth factors, scientists can coax it into resembling tissue found in brain areas including the cortex and hippocampus.
"We can actually make different parts of the nervous system in a dish from stem cells ," Pașca says. When these parts are placed in the same dish, they will even form connections, much like an actual brain. The resulting structure is called an assembloid .
Pașca's team thought they could use assembloids to study how developmental disorder genes affect special brain cells called interneurons, which are thought to play a role in several psychiatric disorders.
Research News
The first wiring map of an insect's brain hints at incredible complexity.
During pregnancy and the first two years of life, these special cells must complete a remarkable journey.
"Interneurons are born in deep regions of the brain, and then they have to migrate all the way to the cortex," Pașca says. "So you can imagine that during that migration a lot of things could go awry."
Pașca's team simulated the migration of interneurons by creating assembloids containing two types of organoids. One resembled an area deep in the brain called the subpallium, where most interneurons are generated. The other organoid resembled the cerebral cortex, where interneurons are supposed to end up.
"And then we've put them together, allowing these interneurons to move towards the cerebral cortex," he says.
The process worked just the way it's supposed to in assembloids containing typical organoids. So next, the team used a gene-editing technique called CRISPR to alter the organoids.
This approach allowed the team to study the effect of more than 400 genes associated with neurodevelopmental disorders. And they found that 46 of those genes were involved in either the generation of interneurons, or with their migration. Knock out a part of those genes and interneurons no longer arrived where they were supposed to.
In the cerebral cortex, interneurons serve as inhibitory neurons, which means they act a bit like the brake in a car. The interneurons can release a neurotransmitter that tells other neurons to reduce their activity.
Meanwhile, excitatory neurons act as the accelerator, telling other cells to become more active.
Brain networks rely on a delicate balance between excitatory and inhibitory neurons. Too much acceleration and the result can be an epileptic seizure. Too much brake and vital information may get lost or delayed.
Want to understand your adolescent? Get to know their brain
The study is important because it offers a way for scientists to study the effect of many genes at the same time, and identify the ones that affect a particular type of cell or cell function during brain development, says Dr. Guo-li Ming , a professor of neuroscience at the University of Pennsylvania's Perelman School of Medicine.
The research also shows clearly how gene variants could lead to autism or some other neurodevelopmental disorder by disturbing interneurons.
"That would be a disaster" in a developing brain, Ming says. "The circuitry would be wrong and the signaling would be wrong, and ultimately the brain functioning would be wrong."
Ming, who was not connected with study, says her lab would like to use the combination of assembloids and CRISPR in their own research on schizophrenia, another psychiatric disorder with a neurodevelopmental origin.
Pașca's study could help brain scientists make the sort of advances that cancer researchers have in the past few decades, says Brennand.
"Thirty years ago, we might have thought all intestinal cancers should be treated the same way and all lung cancers should be treated the same way," she says. "Now we know a lot better."
Instead of choosing treatments according to the location of a cancer, doctors study a tumor's genes to determine which therapy is most likely to work. A similar approach could eventually help people with autism spectrum disorder, epilepsy, and schizophrenia, Brennand says.
"This improved genetic understanding will let us do better," she says, "because we'll know which pathways we can target to intervene."
- neuroscience
- schizophrenia
- genetic research
- neurobiology
- autism spectrum disorder
- brain disorders
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New study links autism spectrum disorder to disrupted developmental dopamine
by Eileen Leahy, Chhavi Chauhan, PhD, Elsevier
Recent evidence suggests that dopamine plays a crucial role in neural development. In a novel study, investigators demonstrated the link between disrupted developmental dopamine signaling and autism spectrum disorder (ASD).
Their findings underscore the importance of studying developmental signaling pathways to understand the etiology of ASD, paving the way for potential targeted interventions. Their findings appear in The American Journal of Pathology .
Lead investigators Lingyan Xing, Ph.D., and Gang Chen, Ph.D., Key Laboratory of Neuroregeneration of Jiangsu and the Ministry of Education, Co-innovation Center of Neuroregeneration, NMPA Key Laboratory for Research and Evaluation of Tissue Engineering Technology Products, Nantong University, explain, "While dopamine is commonly recognized as a neurotransmitter, its significance in the developmental aspects of autism is largely unexplored. Recent studies have highlighted the crucial roles of dopamine and serotonin in development and their importance in the construction of neural circuits.
"In addition, studies have indicated that the use of dopamine-related drugs during pregnancy is associated with an increased risk of autism in children. Armed with these tantalizing clues, we embarked on a mission to bridge the gap between dopamine's known functions and its potential impact on neurodevelopmental disorders, particularly autism. Our quest was to uncover a novel therapeutic target that could revolutionize the way we approach autism treatment."
Investigators studied the role of disrupted dopaminergic signaling in the etiology of ASD by integrating human brain RNA sequencing transcriptome analysis and a zebrafish model, recognized for its high degree of conservation with humans.
To analyze the developmental deficits in ASD systematically, two large publicly available data sets were retrieved from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database and RNA sequencing data from Arkinglab. Transcriptome analysis of human brains revealed significant correlations between changes in dopaminergic signaling pathways and neural developmental signaling in patients with autism. This suggests a potential link between disrupted developmental dopamine signaling and autism pathology.
To explore this link further researchers used the zebrafish model to study the effects of disrupted dopaminergic signaling on neural circuit development. They found that perturbations in developmental dopaminergic signaling led to neural circuit abnormalities and behavioral phenotypes reminiscent of autism in zebrafish larvae. The study also uncovered a potential mechanism by which dopamine impacts neuronal specification through the modulation of integrins.
Dr. Chen comments, "We were surprised by the extent of the impact that dopaminergic signaling has on neuronal specification in zebrafish, potentially laying the groundwork for circuit disruption in autism-related phenotype. Furthermore, the unexpected involvement of integrins as downstream targets of dopaminergic signaling provides new insights into the mechanisms underlying neurodevelopmental disorders."
Dr. Xing concludes, "This research sheds light on the role of dopamine in neural circuit formation during early development, specifically in the context of autism. Understanding these mechanisms could lead to novel therapeutic interventions targeting dopaminergic signaling pathways to improve outcomes in individuals with autism and other neurodevelopmental disorders."
ASD is a developmental disorder that usually manifests itself in early childhood. Although clinical outcomes vary greatly from case to case, autism is characterized by both a restricted interest in social interaction and repetitive behavior. This coincides with disruptions in brain connectivity shown by diffusion tension imaging.
Studies have shown that several neurodevelopmental processes may be affected in ASD, including neurogenesis, neural migration, axon pathfinding, and synaptic formation, all of which can lead to neural circuit disruption.
Explore further
Feedback to editors
New analysis estimates the effects of race-neutral lung function testing on patients, hospitals, and beyond

World-first trial shows benefits of finding and treating undiagnosed asthma and COPD
4 hours ago

Acetaminophen shows promise in warding off acute respiratory distress syndrome, organ injury in patients with sepsis
8 hours ago

Modular communicative leadless ICD found to be safe and exceeds performance expectations
May 18, 2024

Creativity and humor shown to promote well-being in older adults via similar mechanisms

Sweet taste receptor affects how glucose is handled metabolically by humans

Better medical record-keeping needed to fight antibiotic overuse, studies suggest

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses

A long-term ketogenic diet accumulates aged cells in normal tissues, new study shows
May 17, 2024

Gut bacteria enhance cancer immunotherapy in mouse study
Related stories.

Metabolism of autism reveals developmental origins
May 10, 2024
The hunt for disrupted brain signals behind autism
Nov 14, 2022
AI could help in the early diagnosis of autism, study finds
Dec 20, 2023
Understanding the role of a new enzyme in the development of autism spectrum disorder
Jan 4, 2024
Vitamin D alters developing neurons in the brain's dopamine circuit, finds study
May 25, 2023
Unlocking the secrets of the brain's dopaminergic system
Dec 5, 2023
Recommended for you
Study opens the door to designing therapies to improve lung development in growth-restricted fetuses

Primary health coverage found to have prevented more than 300,000 child deaths in four Latin American countries
May 16, 2024

Simple learning test may be used to diagnose autism at just six months of age
Genetics, environment and health disparities linked to increased stress and mental health challenges during adolescence

Likelihood of kids and young people smoking and vaping linked to social media use

Study finds brain wiring predicted adolescents' emotional health during COVID-19 pandemic
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Study identifies new metric for diagnosing autism
Autism spectrum disorder has yet to be linked to a single cause, due to the wide range of its symptoms and severity. However, a study by University of Virginia researchers suggests a promising new approach to finding answers, one that could lead to advances in the study of other neurological conditions.
Current approaches to autism research involve observing and understanding the disorder through the study of its behavioral consequences, using techniques like functional magnetic resonance imaging that map the brain's responses to input and activity, but little work has been done to understand what's causing those responses.
However, researchers with UVA's College and Graduate School of Arts & Sciences have been able to better understand the physiological differences between the brain structures of autistic and non-autistic individuals through the use of Diffusion MRI, a technique that measures molecular diffusion in biological tissue, to observe how water moves throughout the brain and interacts with cellular membranes. The approach has helped the UVA team develop mathematical models of brain microstructures that have helped identify structural differences in the brains of those with autism and those without.
"It hasn't been well understood what those differences might be," said Benjamin Newman, a postdoctoral researcher with UVA's Department of Psychology, recent graduate of UVA School of Medicine's neuroscience graduate program and lead author of a paper published this month in PLOS: One . "This new approach looks at the neuronal differences contributing to the etiology of autism spectrum disorder."
Building on the work of Alan Hodgkin and Andrew Huxley, who won the 1963 Nobel Prize in Medicine for describing the electrochemical conductivity characteristics of neurons, Newman and his co-authors applied those concepts to understand how that conductivity differs in those with autism and those without, using the latest neuroimaging data and computational methodologies. The result is a first-of-its-kind approach to calculating the conductivity of neural axons and their capacity to carry information through the brain. The study also offers evidence that those microstructural differences are directly related to participants' scores on the Social Communication Questionnaire, a common clinical tool for diagnosing autism.
"What we're seeing is that there's a difference in the diameter of the microstructural components in the brains of autistic people that can cause them to conduct electricity slower," Newman said. "It's the structure that constrains how the function of the brain works."
One of Newman's co-authors, John Darrell Van Horn, a professor of psychology and data science at UVA, said, that so often we try to understand autism through a collection of behavioral patterns which might be unusual or seem different.
"But understanding those behaviors can be a bit subjective, depending on who's doing the observing," Van Horn said. "We need greater fidelity in terms of the physiological metrics that we have so that we can better understand where those behaviors coming from. This is the first time this kind of metric has been applied in a clinical population, and it sheds some interesting light on the origins of ASD."
Van Horn said there's been a lot of work done with functional magnetic resonance imaging, looking at blood oxygen related signal changes in autistic individuals, but this research, he said "Goes a little bit deeper."
"It's asking not if there's a particular cognitive functional activation difference; it's asking how the brain actually conducts information around itself through these dynamic networks," Van Horn said. "And I think that we've been successful showing that there's something that's uniquely different about autistic-spectrum-disorder-diagnosed individuals relative to otherwise typically developing control subjects."
Newman and Van Horn, along with co-authors Jason Druzgal and Kevin Pelphrey from the UVA School of Medicine, are affiliated with the National Institute of Health's Autism Center of Excellence (ACE), an initiative that supports large-scale multidisciplinary and multi-institutional studies on ASD with the aim of determining the disorder's causes and potential treatments.
According to Pelphrey, a neuroscientist and expert on brain development and the study's principal investigator, the overarching aim of the ACE project is to lead the way in developing a precision medicine approach to autism.
"This study provides the foundation for a biological target to measure treatment response and allows us to identify avenues for future treatments to be developed," he said.
Van Horn added that study may also have implications for the examination, diagnosis, and treatment of other neurological disorders like Parkinson's and Alzheimer's.
"This is a new tool for measuring the properties of neurons which we are particularly excited about. We are still exploring what we might be able to detect with it," Van Horn said.
- Birth Defects
- Medical Devices
- Nervous System
- Brain Tumor
- Learning Disorders
- Disorders and Syndromes
- Autistic spectrum
- Brain damage
- Social cognition
- Rett syndrome
- Attention-deficit hyperactivity disorder
- Learning disability
Story Source:
Materials provided by University of Virginia College and Graduate School of Arts & Sciences . Original written by Russ Bahorsky. Note: Content may be edited for style and length.
Journal Reference :
- Benjamin T. Newman, Zachary Jacokes, Siva Venkadesh, Sara J. Webb, Natalia M. Kleinhans, James C. McPartland, T. Jason Druzgal, Kevin A. Pelphrey, John Darrell Van Horn. Conduction velocity, G-ratio, and extracellular water as microstructural characteristics of autism spectrum disorder . PLOS ONE , 2024; 19 (4): e0301964 DOI: 10.1371/journal.pone.0301964
Cite This Page :
Explore More
- High-Efficiency Photonic Integrated Circuit
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
- Toward Human Brain Gene Therapy
- Whale Families Learn Each Other's Vocal Style
- AI Can Answer Complex Physics Questions
- Otters Use Tools to Survive a Changing World
- Monogamy in Mice: Newly Evolved Type of Cell
- Sustainable Electronics, Doped With Air
Trending Topics
Strange & offbeat.
An Update on Psychopharmacological Treatment of Autism Spectrum Disorder
- Current Perspectives
- Open access
- Published: 14 January 2022
- Volume 19 , pages 248–262, ( 2022 )
Cite this article
You have full access to this open access article

- Ramkumar Aishworiya 1 , 2 , 3 ,
- Tatiana Valica 1 , 4 ,
- Randi Hagerman ORCID: orcid.org/0000-0001-5029-8448 1 , 5 &
- Bibiana Restrepo 1 , 5
45k Accesses
52 Citations
3 Altmetric
Explore all metrics
While behavioral interventions remain the mainstay of treatment of autism spectrum disorder (ASD), several potential targeted treatments addressing the underlying neurophysiology of ASD have emerged in the last few years. These are promising for the potential to, in future, become part of the mainstay treatment in addressing the core symptoms of ASD. Although it is likely that the development of future targeted treatments will be influenced by the underlying heterogeneity in etiology, associated genetic mechanisms influencing ASD are likely to be the first targets of treatments and even gene therapy in the future for ASD. In this article, we provide a review of current psychopharmacological treatment in ASD including those used to address common comorbidities of the condition and upcoming new targeted approaches in autism management. Medications including metformin, arbaclofen, cannabidiol, oxytocin, bumetanide, lovastatin, trofinetide, and dietary supplements including sulforophane and N-acetylcysteine are discussed. Commonly used medications to address the comorbidities associated with ASD including atypical antipsychotics, serotoninergic agents, alpha-2 agonists, and stimulant medications are also reviewed. Targeted treatments in Fragile X syndrome (FXS), the most common genetic disorder leading to ASD, provide a model for new treatments that may be helpful for other forms of ASD.
Similar content being viewed by others

An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder

Concerns About ABA-Based Intervention: An Evaluation and Recommendations

Autism Spectrum Disorders and ADHD: Overlapping Phenomenology, Diagnostic Issues, and Treatment Considerations
Avoid common mistakes on your manuscript.
Introduction
ASD is a complex neurodevelopmental, biologically based condition with an estimated prevalence of 1 in 44 people [ 1 ] that impacts all areas of child development — from behavior, problem solving abilities and self-care skills, to complex social communication ability, language, and executive functioning skills. The range of symptoms and severity of ASD vary greatly from child to child, and clinical manifestations depend on the individual’s age, cognitive and language abilities, and co-occurring conditions. The last revision of the Diagnostic and Statistical Manual (DSM-5) defines ASD as impairments in two main domains: (1) social communication and interaction, which comprises challenges in social-emotional reciprocity, challenges in using nonverbal strategies during social interaction, and challenges developing, maintaining and understanding relationships, and (2) restricted, repetitive, and stereotyped patterns of behavior, manifested by unusual repetitive movements or behaviors, restricted interests, insistence on sameness and inflexible adherence to routines, as well as sensory challenges ranging from seeking to avoiding certain sensory stimuli [ 2 , 3 , 4 ]. However, a range of behavioral, cognitive, and emotional disturbances in ASD can also be attributed to a high rate of co-occurring mental health and medical conditions such as attention deficit hyperactivity disorder (ADHD), anxiety, depression, phobias, intellectual disability, speech/language impairment, restrictive/avoidant food intake, sleep issues, sensory processing issues, and genetic conditions. This often makes the recognition, diagnosis, and clinical management of ASD even more complex and difficult [ 5 , 6 , 7 , 8 ].
Classic medical management of medical conditions has largely revolved around pharmacological treatment. However, despite decades of research in ASD, current evidence has only established behavioral (non-pharmacological) treatments as the mainstay of management to address the core symptoms of ASD. Part of the reason for a lack of efficacy in many treatment studies stems from the heterogeneous etiology underlying the overall term of ASD. Some studies have subdivided enrolled patients either by their genetic etiology or phenotypic features to address this. The aim of this paper is to provide a current update on the pharmacological treatments available for ASD and therapeutic subtypes of ASD, covering both the established ones and upcoming/emerging treatments which have potential based on scientific evidence to become standard treatments in the next few years. A systematic literature search was completed on Medline, Scopus, and Embase with key search terms of “autism,” “autism spectrum disorder,” “targeted treatments,” “pharmacological therapy,” and “management” to identify relevant articles. Here we have highlighted the psychopharmacological treatments that have the most efficacy and are also, in most cases, available now or in the near future to clinicians. However, we also recognize the current mainstay of behavioral intervention in the management of ASD and will briefly review those which are supported by strong empirical evidence.
Non-pharmacological (Behavioral) Interventions
In 1987, Lovaas published an article which introduced a new treatment approach describing a significant improvement of IQ scores and educational functioning in almost 50% of children with ASD [ 9 ]. Also known as The Lovaas Method of Applied Behavior Analysis, and subsequently as discrete trial training (DTT), it is an intensive, highly structured, long-term, one-on-one behavior intervention designed for young children, which has a strong empirical support and has become the foundation for many of the evidence-based behavioral interventions in use today [ 10 ]. Subsequently, through decades of extensive research, a number of modifications and adaptations of the Lovaas method have since been developed. These can be used in different settings, environments, and procedures, and have been shown to be effective in addressing the core impairments of ASD in social communication, speech, behaviors, play, and learning [ 11 , 12 , 13 , 14 ].
Odom et al. [ 13 ] and Wong et al. [ 15 ] have classified behavioral evidence–based interventions into two groups: comprehensive treatment models (CTMs) and focused interventions.
Comprehensive treatment models focused on core ASD symptoms have been found to improve language, cognitive, and functional language skills in young children, using intensive and long-term multi-disciplinary strategies in naturalistic environments. Instructions can be provided at home or in a classroom setting, individually or in a group, provided by instructors or by parents. Examples of well-established CTMs include Early Behavior Intervention (EIBI) [ 16 ], Early Start Denver model (ESDM) [ 17 ], Developmental, Individual difference, relationship-based model (DIR/Floortime, or Greenspan model) [ 18 ], Pivotal Response Training (PRT) [ 19 ], and Treatment and education of autistic and related communication handicapped children (TEACCH) [ 20 ].
Focused interventions address a single skill or a specific area of developmental domain and are provided for a short time, until the skill is mastered. They can also be effective to address life-threatening or socially inappropriate behaviors that require rapid addressing. Examples include social skill training, toilet training, modeling, cognitive behavioral intervention, and behavioral strategies like prompting, ignoring, time delay, reinforcement, discrete trial teaching, and extinction. These can be implemented as a structured session or in a naturalistic setting at home, school, clinic, or community settings, with peers or parents, and have behavioral, developmental, or educational purposes. Peer-mediated Instruction and Intervention (PMII), also known as “Peer Modeling,” “Peer Initiation Training,” “Peer support” [ 21 , 22 ], and Picture Exchange Communication System (PECS) [ 23 ], are also other examples of focused interventions.
Behavioral interventions work most effectively when started at an early age and the majority cater to young children to optimize their development and learning skills. The sociocultural beliefs and economic capability of the family also moderate treatment impact and outcome [ 24 ]. However, behavioral interventions do have a role in older children, adolescents, and adults as well; the targets of these interventions change in older individuals to include social, vocational, leisure skills, and independent living. Research in behavioral interventions for adults with ASD is still limited and will need to be expanded in future.
Established Psychopharmacological Treatments
The use of psychotropic medications has markedly increased over the last decades; approximately two-thirds of autistic adolescents have been treated with psychotropic medications, especially those with challenging behaviors and co-occurring conditions like intellectual disability (ID), medical, and mental health diagnoses. Co-occurring mental health conditions have been reported in approximately 70% of autistic individuals ranging from attention deficit and hyperactivity disorder (ADHD), irritability, aggression, mood, and anxiety issues [ 8 , 25 , 26 ]. Mandell et al. reported that 56% were prescribed at least one psychotropic medication and 20% were prescribed three or more [ 27 ]. Individuals with ASD frequently are treated with multiple medications, including off-label use (e.g., use of antipsychotic medications in younger children). Studies have reported high rates of polypharmacy ranging from 12 to 35% based on the type of studies [ 28 , 29 ]. The increasing prescription rates for individuals with ASD is not completely understood. For instance, some authors have postulated that this may be influenced by improvements in diagnostic and clinician awareness of co-occurring mental health issues [ 25 ]. However, other researchers have reported demographic factors influencing pharmacological treatment. For instance, in a large study, those who were uninsured or exclusively privately insured were less likely to use more than 3 medications than were those insured by Medicaid [ 30 ]. Prescription medications may also be affected by demographic factors including race, ethnicity, and geography. Studies have reported that challenging behaviors and mental health diagnoses are influencing factors [ 29 ]. For example, polypharmacy is often necessary since one treatment for anxiety may not be helpful for another comorbid condition such as ADHD. Such polypharmacy will be more common as specific treatments for dysfunctional pathways are utilized which go hand in hand with other treatments for common comorbidities of ASD. An example is metformin which can downregulate the mTOR pathway, and this treatment works well with stimulants for ADHD and also Selective Serotonin Reuptake Inhibitors (SSRIs) for anxiety.
Prescribers must consider medications not only for symptoms of associated psychopathology but also as targeted treatments that have the potential to reverse the neurobiological abnormalities and should be considered as a part of an individualized therapeutic program with behavioral and educational interventions.
General Principles in Using Pharmacological Treatment in ASD
Frequently, identification and management of psychiatric issues can be complex, especially for those with limited language repertoire, low cognitive function, and those experiencing uncertain symptoms. Diagnostic overshadowing is common (failure to identify other conditions in the presence of a certain diagnosis). A high level of clinical suspicion for co-occurring mental health conditions is required for children and adolescents with communication challenges. Managing clinicians should obtain information from the child, when possible, family and other providers including teachers and therapists. Environmental changes and lack of skills can be the source of undesired behaviors and should be considered in the plan of care.
Pharmacological interventions are sometimes indicated and may facilitate their participation in therapy and enhance their daily functioning. The principles used for psychopharmacological management are the same for children with ASD as for those with typical development. However, prescribers should keep in mind that children with ASD tend to be more sensitive to medication effects and more likely to have adverse effects than children without ASD. Therefore, pharmacological treatment should be started at lower doses, and adjusted more slowly than in neurotypical children. Obtaining objective symptom measures from different sources before and after the intervention is key to objectively evaluate the response of treatment in different settings.
Serotoninergic Medications
Serotoninergic medications regulate the levels of serotonin which is a key messenger specially involved in the gastrointestinal, cardiovascular, and the central nervous system (CNS). The serotonin level has been reported to be elevated in the autistic population, and it has been theorized that serotonin dysregulation is associated with symptoms frequently seen in autistic individuals ranging from repetitive behaviors to anxiety. PET studies have demonstrated that young children (under 5 years old) with ASD have lower levels of serotonin in the CSF [ 31 ]. Studies of lymphoblastoid cell lines in patients with ASD compared to controls have demonstrated a deficit of enzymes that convert tryptophan to serotonin [ 32 ]. These studies suggested that those with ASD would benefit from treatment with an SSRI to stimulate neurogenesis and neuroprotection [ 33 ]. There are three different groups of medications that influence the serotonin levels: the SSRIs, SNRIs (serotonin-norepinephrine reuptake inhibitors), and tricyclic antidepressants. The SSRIs are one of the most commonly prescribed medications for autistic individuals to treat anxiety, mood issues, and irritability. However, results of available clinical trials have been inconsistent in the benefits of SSRI’s for improving aggression and the core symptoms of ASD [ 34 ].
A retrospective study of children with FXS (ages 12 to 50 months) demonstrated improvement in the trajectory of both receptive and expressive language measures on the Mullen Scales of Early Learning (MSEL) in those treated with low-dose sertraline vs those who did not receive sertraline [ 35 ]. These results led to a controlled trial for 6 months of sertraline in children ages 2 to 6 with FXS (60% also had ASD) treated clinically with low dose sertraline (2.5 to 5.0 mg/day) [ 36 ]. Those treated with sertraline demonstrated greater improvement in motor and visual subtests and the Cognitive T score on the MSEL compared to those on placebo. In the children with both FXS and ASD, there was also significant improvement on the Expressive Language subscale compared to placebo [ 36 ]. In the same controlled trial, a passive visual eye tracking measure of receptive vocabulary was also significantly improved in those treated with sertraline compared to placebo [ 37 ]. These studies suggest that young children with FXS both with and without ASD benefit from low-dose sertraline treatment. However, a similar study in young children ages 2 to 6 with idiopathic ASD (without FXS) treated with low-dose sertraline did not demonstrate a benefit of sertraline compared to placebo [ 38 ]. Therefore, the genetic subtype of ASD makes a difference in response to treatment and all children diagnosed with ASD must have genetic testing including Fragile X DNA testing and a CGH array for starters and subsequent whole exome sequencing (WES) or whole genome sequencing (WGS) if the initial studies are negative [ 39 ].
Atypical Antipsychotics
There are two medications approved by the FDA for the treatment of irritability associated with ASD: risperidone, approved for children older than 5 years of age [ 40 ], and aripiprazole, approved for children 6 to 17 years of age [ 41 ]; clinical trials found them to be effective in reducing irritability and, to a lesser degree, repetitive behaviors. These two atypical antipsychotic medications have affinity for dopamine, 5-HT, alpha-adrenergic, and histaminergic receptors in the brain. They also share similar safety profiles; the most common side effects include fatigue, increased appetite, GI symptoms, hyperprolactinemia, weight gain, and sedation, and less commonly activation including restlessness and akathisia. They are also linked to more serious side effects including dyslipidemia, hyperglycemia, metabolic syndrome, and extrapyramidal symptoms or drug-induced movement disorders. Therefore, close clinical and laboratory monitoring is recommended. Given that the efficacy and safety of these medications have not been established for the long-term treatment of irritability in autistic individuals, it is important to periodically re-evaluate the need for continuation of treatment. Since the development of atypical antipsychotics, the use of the conventional antipsychotics has been reserved for more severe cases refractory to the newer generation medications, due to the narrower safety profile and greater incidence of adverse reactions including extrapyramidal symptoms such as tardive dyskinesia with conventional antipsychotics.
Stimulant Medications
Stimulants are usually the first line of treatment to treat co-occurring attention deficit and hyperactivity disorder (ADHD) as they present with a rapid clinical effect and there is enough data supporting their use and safety. Approximately half of autistic children also meet criteria for ADHD [ 42 ], but prevalence widely varies based on samples [ 8 , 43 , 44 , 45 , 46 , 47 ]. Treating co-occurring ADHD symptoms in autistic individuals should focus on improvement in enhancing their daily function in multiple settings, including learning, and hopefully long-term functional outcomes improving associated symptoms causing impairment in the academic setting, peer relationships, and emotional regulation, which are also key predictors and mediators of functional difficulties in adulthood. Before starting a patient on a regimen, the prescribing clinician should assess the potential risks for pharmacotherapy by obtaining a complete past medical history, family history, and a physical examination with a specific focus on the cardiovascular system. It is important to obtain pretreatment baseline information and a close follow up to objectively evaluate the impact of common side effects associated with pharmacotherapy for ADHD (i.e., appetite changes, hypertension, weight loss, sleep disturbances, headaches, abdominal pain). Baseline sleep problems do not appear to predict stimulant-related sleep problems and may improve with stimulant therapy [ 48 ]. Adolescent patients should be assessed for substance use or abuse prior to starting treatment.
There are two main stimulant families: the amphetamines are usually slightly more efficacious than the methylphenidate derivates which are usually better tolerated [ 49 ]. In a systematic review and network meta-analysis that included 81 published and unpublished randomized trials in > 10,000 neurotypical children, amphetamines were slightly more efficacious than methylphenidate in reducing clinician-rated core symptoms of ADHD at approximately 12 weeks; however, amphetamines were less tolerable than placebo and methylphenidate was better tolerated than amphetamines [ 49 ]. Specific systematic review of four-crossover trials in autistic children (113 participants) age 5 to 13 years found low-quality evidence that short-term treatment with methylphenidate may improve hyperactivity and inattention in children with ASD, and the only significant adverse side effect was reduced appetite as rated by parents; however, there was no evidence of impact on core ASD symptoms or improvement in social interaction [ 50 ]. In the largest crossover trial, approximately 50% of children with ASD responded to methylphenidate based on the hyperactivity subscale of the Aberrant Behavior Checklist (ABC); the effect size ranged from 0.20 to 0.54, depending upon dose and rater, with greater improvement at higher doses; then, this modest effect supports that Methylphenidate exerts a lower effect on primary ADHD symptoms in individuals with ASD compared to those in the neurotypical population. Six of 66 children in the double-blind phase (9.1%;) discontinued treatment due to adverse effects, including irritability, repetitive behaviors, tics, insomnia, and reduced appetite [ 51 ].
Treatment failure is defined by lack of satisfactory improvement in core symptoms of ADHD at the maximum dose or the occurrence of intolerable adverse effects. At least half of the children who presented with an inadequate response or side effects to a certain medication may respond well to another one. For those children failing to respond to two different medications, the prescriber should evaluate other causes for the limited therapeutic response including (1) the presence of comorbid psychiatric diagnosis, (2) unrealistic expectations about the expected clinical response, (3) misuse or medication diversion, and (4) lack of adherence to the regimen. Children on stable maintenance dose should be followed every 6 months to monitor side effects and evaluate clinical response.
Alpha-2-adrenergic Agonists
There is also evidence about the use of alpha 2 agonists to improve core ADHD symptoms, but alpha-2-adrenergic agonists (i.e., guanfacine and clonidine) are frequently used in children under 5 year old with ADHD or hyperarousal, cases with poor response to a trial of stimulants, or selective norepinephrine reuptake inhibitors, have unacceptable side effects, or have significant co-occurring conditions (i.e., sleep issues). However, studies of alpha-2-agonists in ASD are limited and have small sample sizes. Guanfacine has been reported to be safe and effective in the treatment of hyperactivity and impulsiveness in children with ASD [ 52 , 53 ]. The most common side effects of guanfacine include sedation, constipation, irritability, and aggression. A small crossover study has also suggested positive effects of clonidine in ASD including decreased irritability, stereotypy, hyperactivity, inappropriate speech, and hyperarousal behaviors [ 54 ].
Data from randomized trials, systematic reviews, and meta-analyses show that atomoxetine and alpha-2-adrenergic agonists are more effective than placebo in reducing the core symptoms of ADHD, but as a class, they are less effective than stimulants [ 49 , 55 , 56 ]. Similarly, it is key to obtain objective targeted symptom measures at baseline and during treatment to objectively evaluate the response to treatment in different settings.
In a recent review of nine controlled trials of 430 children with ASD comparing the response between methylphenidate, atomoxetine, and guanfacine, methylphenidate and atomoxetine had superior effects than placebo in addressing ADHD symptoms; however, the response for hyperactivity symptoms was less than observed in neurotypical populations with both medications [ 57 ]. Worse treatment outcomes were associated with individuals with lower cognitive functioning.
Sleep issues are frequently reported in children with ASD potentially affecting their behavior, daily functioning, and family life. There is some evidence suggesting that low melatonin levels affect the circadian rhythm in autistic children [ 58 ]. In cases where behavioral and environmental sleep interventions have been implemented with limited response, clinicians may recommend the use of melatonin which is usually well tolerated and has a low incidence of side effects [ 59 ]. There is increasing evidence for the use of prolonged-release melatonin in autistic individuals with limited response to regular release formulations [ 60 ]. Melatonin is an over-the-counter product that is not regulated by the FDA. When parents/caregivers purchase melatonin, they should seek a formulation that contains melatonin as the only active ingredient.
N-acetylcysteine
N-acetylcysteine (NAC) is another antioxidant that can be purchased over the counter (OTC), and it can improve the imbalance of excitation: inhibition (E:I) that is seen in some forms of ASD [ 61 ]. NAC works by two mechanisms to lower the E:I imbalance; it lowers glutamatergic neurotransmission, and the cysteine leads to an increase in glutathione synthesis which is an important antioxidant. Cysteine is also oxidized to cystine, which further helps to reduce glutamatergic neurotransmission [ 62 ]. Hardan and colleagues carried out a controlled trial of escalating doses of NAC from 900 mg once a day for 4 weeks, increasing to bi-daily dosing for 4 weeks and then tri-daily dosing for the last 4 weeks compared to placebo. They randomized 33 subjects with ASD ages 3.2 to 10.7 years and after 12 weeks of treatment they found significant improvement on their primary outcome measure, irritability subscale on the ABC ( p < 0.001) for patients treated with NAC compared to placebo. Additional improvements were seen in stereotypic behaviors with significance reached on the RBS-S Stereotypies subscale ( p < 0.014) and the SRS Autism Mannerisms subscale ( p < 0.045) for those treated with NAC vs placebo [ 62 ]. NAC was well tolerated although an occasional patient did not like the taste or had minimal gastrointestinal side-effects.
Dietary Supplements
Sulforaphane is a naturally occurring isothiocyanate (found in broccoli and other cruciferous vegetables) [ 63 , 64 , 65 ]. Sulforaphane is an antioxidant, anti-inflammatory, and mitochondrial protective agent that has been studied in several animal models and humans with neurodegenerative and neurodevelopmental disorders [ 66 ]. Sulforaphane is a sulfur-rich dietary phytochemical which can penetrate the blood brain barrier, and it subsequently induces the nuclear factor erythroid 2 related factor 2 ( Nrf2 ) signaling cascade that stimulates the expression of more than 200 genes that are antioxidants and involved in detoxification and neuroprotection in the CNS [ 67 ]. The effect leads to reduction of superoxide and other reactive oxygen species (ROS), upregulation of the proteozome system to digest unfolded or misfolded proteins, enhancement of autophagy, inhibition of pro-inflammatory cytokines, protection from heme toxicity, and defense of neuronal cells from Aβ 42 -mediated cytotoxicity.
There have been a few studies in patients with ASD [ 68 , 69 ] including a controlled trial of young men ages 13 to 27 with moderate to severe ASD treated with sulforaphane ( n = 29) compared to placebo ( n = 15) for 18 weeks. Significant improvements were seen on the Aberrant Behavior Checklist (ABC), the Clinical Global Improvement Scale (CGI-I), and the Social Responsiveness Scale 2 (SRS) [ 69 ]. This positive trial lead to a more detailed study in children with ASD, a randomized controlled trial of sulforaphane lasting 15 weeks followed by an open label trial for another 15 weeks in 57 children ages 3 to 12 years [ 70 ]. Although the primary outcome measure, the Ohio Autism Clinical Impressions Scale, did not improve significantly in those on sulforaphane, a secondary measure, the ABC, did significantly improve on sulforaphane vs placebo but the SRS did not. In addition, there were significant improvements in the biomarkers including the glutathione redox status, mitochondrial respiration, inflammatory markers, and heat shock proteins on sulforaphane vs placebo, and these improvements correlated with improvements on the ABC. They utilized a commercial product of sulforaphane called Avmacol made by Nutrimax with a tablet dose of 2 to 8 tablets per day depending on the weight of the child (equivalent to 2.2 μmol/kg/day). There were no significant adverse events and the supplement was well tolerated.
Other antioxidants have been studied in ASD including omega-3 fatty acids [ 71 , 72 ] with mixed results, and these antioxidants promote glutathione recycling by facilitating the conversion of oxidized glutathione into reduced glutathione. A more recent study was carried out by Mazahery et al. [ 73 ] in 111 children with ASD ages 2.5 to 8 years, and they were randomized to placebo, Vitamin D 2000 IU/day, or omega-3 722 mg/day or both interventions for 1 year of treatment. Seventy-three patients completed a year of therapy, and those on both treatments had a significant reduction in their primary outcome measure, irritability on the ABC subscale ( p < 0.001) compared to placebo, and those treated with vitamin D alone also had a reduction in irritability also compared to placebo ( p < 0.45) [ 73 ]. These studies suggest that antioxidants may be a helpful ancillary treatment in some patients with ASD, although biomarkers of oxidative stress would be helpful to assess in further studies to better identify those who would benefit from this treatment.
Emerging Targeted Treatments with a Possible Role in ASD
Oxytocin (OXT) is a neuropeptide synthesized in the hypothalamus that plays a critical role in social functioning. Extant literature has shown that OXT enhances social processing in typically developing adults (enhanced eye contact, better emotion recognition in faces) immediately after its administration [ 74 ]. There have been generally positive results of OXT in adults with ASD, with trials showing improvements in repetitive behaviors, social reciprocity, and emotion recognition [ 75 , 76 , 77 ]. However, all these trials studied only short-term benefits (within a few weeks) of OXT administration. A recent randomised, placebo-controlled, double-blind study in adults with ASD showed improvements in self-reported repetitive behaviors and positive mood at 1 year post treatment after an initial 4 weeks of oxytocin treatment [ 78 ]. However, in this same study, there were no significant treatment benefits for social responsiveness with OXT [ 78 ]. Another recent study in young adults with ASD also did not demonstrate any immediate benefits of OXT on empathy and social perception [ 79 ].
Results of OXT studies in children have overall been more equivocal with mixed results. Although 4 studies showed positive short-term results of OXT administration on social responsiveness (following 4 or 5 weeks of OXT administration) [ 80 , 81 , 82 , 83 ], another 2 studies did not demonstrate any OXT specific improvements in social responsiveness or repetitive behavior in children with ASD [ 84 , 85 ]. A recent randomized controlled trial (RCT) however did not show any significant effects between the OXT and placebo group in aberrant behavior, social communication, or cognition [ 86 ]. At the neural networks level, it has been shown that intranasal OXT leads to increased activation in the brain regions known to be involved in perceiving and thinking about social-emotional information and enhances effective connectivity between nodes of the brain’s reward and socioemotional processing systems [ 87 , 88 ]. There were no noted side effects in these studies on children with ASD, thus far, although animal studies have raised the possibility of increased basal OXT levels with long-term OXT administration; the clinical effects of this being unclear. Of pertinence, there remains a lack of conclusive evidence for the long-term beneficial effects of OXT in addressing the core symptoms of autism [ 89 ]. Given that the vast majority of studies in children utilize parent-reported outcome measures of social and behavioral symptoms, inherent limitations of bias in reporting even in placebo-controlled trials are likely to come into play. Another important consideration is whether the gains that are seen with OXT administration in the experimental setting translate to real life and this is also unclear. The role of OXT thus far has been limited to its immediate effect after administration and hence is not a single treatment option for ASD. Nonetheless, as illustrated by a recent meta-analysis, there does seem to be overall beneficial effects of OXT on social symptoms of ASD, although this review included both children and adults [ 90 ]. There is also some promising research looking at the role of OXT in combination with other treatment modalities including behavior therapy and probiotics, with clinical trials in this area ongoing [ 91 , 92 ]. It is also likely that the effects of OXT in ASD are modulated by age, gender, and possibly genetic factors [ 77 , 79 , 90 ]. As such, although it holds much promise, the use of OXT in individuals with ASD is currently not a mainstream treatment.
Bumetanide is a well-established loop diuretic that works by inhibiting sodium–potassium-chloride co-transporters, namely, NKCC1 and NKCC2. Bumetanide has been purported as a potential treatment in autism due to its inherent chloride-related antagonist effects which is linked to GABA-ergic inhibition [ 93 ]. Bumetanide has been shown to reduce broad ASD symptomatology in children following a 3-month treatment course in 2 placebo-controlled randomized controlled trials [ 94 , 95 ]. Both of these trials used outcomes that are screening tools for ASD, namely, the SRS and the Childhood Autism Rating Scale (CARS). Another open-label trial of 6 children with severe ASD and intellectual disability showed parent-reported improvement in communicative abilities of all children after 3 months of bumetanide [ 96 ]. However, a recent double-blind, placebo-controlled, phase 2 superiority trial in children with ASD without severe intellectual disability did not show any treatment benefits on the core symptoms of ASD as measured on the SRS-2 [ 97 ]. It did show treatment benefits on the repetitive behavior scale, with no major adverse effects. Another study has suggested possible combined effects of bumetanide with ABA therapy in improving ASD symptoms on the CARS, although this was not a randomized controlled trial [ 98 ]. There are 2 phase 3 clinical studies ongoing now, which may shed further information on the potential benefits of bumetanide in ASD [ 99 ]. There is some functional-MRI-based evidence suggesting that bumetanide reduces the exaggerated amygdala activation to eye contact in individuals with ASD and contributes to increased eye-gaze time with biological stimuli and better emotional face perception [ 100 , 101 ]. Regardless, based on current literature, there is inconclusive evidence for the role of bumetanide in addressing the core symptoms of ASD [ 102 , 103 ].
Targeted treatments that reverse known neurobiological abnormalities in subgroups of ASD where there is also animal data to demonstrate benefit have emerged in the last decade for subgroups of ASD. The subgroup of ASD that is leading the way in targeted treatments is FXS, the most common single gene cause of ASD. In addition, post mortem studies have shown that FMRP, the protein that is missing or deficient in FXS, is also deficient in the brain in patients with idiopathic ASD without a fragile X mutation [ 104 , 105 ]. Therefore, FXS is a model for targeted treatments in other subtypes of ASD and treatments that work well in FXS may also be beneficial for other forms of ASD. So we will describe some of the targeted treatment studies with compounds that are available currently, although not FDA approved for FXS nor ASD.
Animal studies in FXS have demonstrated a hyperactive insulin receptor and up-regulation of the mammalian target of rapamycin complex 1 (mTORC1) and mitogen-activated protein kinase/extracellular signal-related kinases (MAPK/ERK) signaling pathways, as well as elevation of MMP-9 levels in the absence of FMRP, the protein produced by the FMR1 gene [ 106 , 107 , 108 ]. Metformin is a bi-guanide that is a primary treatment for type 2 diabetes, but it can also reduce the appetite in individuals with obesity. Therefore, studies of metformin were first carried out in patients with FXS who demonstrated obesity, often with the Prader-Willi-phenotype of FXS [ 109 ]. In a handful of patients with FXS treated clinically with metformin between the ages of 4 and 60 years old, there was improvement in overeating but also on the ABC subscales of irritability, aggression, and social avoidance [ 109 ]. Parents also stated that they saw improvement in the expressive language abilities in conversation. The potential language improvements are currently being studied in a controlled trial of metformin occurring over 3 sites, 2 in Canada (Edmonton and Montreal), and one site in the USA at the MIND Institute funded by the Azrieli Foundation (NCT03479476, NCT03862950). Patients ages 6 to 45 are recruited into a randomized controlled trial lasting 4 months with outcome measures including the Expressive Language Sampling as the primary outcome but also event related potentials, eye tracking, NIH toolbox, and other behavioral measures are assessed. The results will be available in 2022. Additional open label studies have been carried out with metformin including one in children ages 2 to 7 years old with FXS, and improvements were seen in behavior and development on the MSEL [ 110 ]. Individual case studies have shown that macroorchidism did not develop in boy who started metformin clinically before puberty [ 111 ] and two adults with FXS improved their IQ when using metformin for over one year [ 112 ].
Lovastatin is a commonly used statin that lowers cholesterol levels, but it does this by inhibiting 3-hydroxy-3methylglutaryl coenzyme A (3HMG-CoA) reductase, and it is FDA approved for lowering hypercholesterolemia or hyperlipidemia in children and adults. This action lowers the excessive protein production of the MEK-ERK pathway which are elevated in FXS. Studies of lovastatin treatment in the FXS knock out (KO) mouse rescued excess protein synthesis and also epilepsy [ 113 ]. These animal studies stimulated FXS patient trials. The one controlled trial included 32 children with FXS between 10 to 17 years treated in a RCT for 20 weeks with a dose of 10 to 40 mg a day as tolerated [ 114 ]. In addition, the patients all received Parent Implemented Language Intervention (PILI) [ 115 ] delivered by distance video teleconferencing over 12 weeks by a speech and language therapist with 4 sessions per week. Parents were taught a set of language facilitation techniques that were utilized with shared story telling sessions with their child. The main outcome measures were the number of utterances and new words utilized in addition to additional language scales, behavioral measures (ABC), and the CGI-I. So this study compared the combined effects of lovastatin plus PILI to PILI alone with placebo. Remarkably, there was significant improvements from baseline in both groups, but the outcomes were the same in both groups; that is, PILI alone had as much improvement as lovastatin plus PILI demonstrating the power of intensive language intervention [ 114 ].
Cannabidiol
Cannabidiol (CBD) is a phytocannabinoid found in Cannabis sativa , marijuana. Although there are hundreds of phytocannabinoids in marijuana, CBD is the second most common one after delta-9-tetrahydrocannabinol (THC) which has psychotropic properties. Marijuana has been used for 8000 years in India, China, and Middle East for fiber and medicinal properties; then introduced to Europe in early nineteenth century by Napoleon’s army returning from Egypt and then to Britain for medical use by a surgeon who served in India. CBD is the non-psychotropic component of marijuana, and there are numerous therapeutic effects of this drug including treatment of anxiety, pain, nausea, and motor deficits including the tremor in Parkinson’s disease [ 116 ]. CBD has both neuromodulatory and neuroprotective effects through a number of mechanisms including blocking neuroinflammation and potentiating anti-inflammatory pathways, improving mitochondrial function, GABA A agonist potentiation, stimulation of 5HT 1A receptors, and enhancing levels of anandamide (AEA) [ 116 , 117 , 118 , 119 ].
The endocannabinoid system has two receptors CB 1 found primarily in the CNS and CB 2 found throughout the body and the immune system. The primary endogenous ligands for CB 1 and CB 2 receptors are called endocannabinoids (ECs) and include anandamide AEA and 2-arachidonoylglycerol (2-AG). The ECs modulate synaptic transmission throughout the CNS, yielding widespread influence on cognition and behavior. The ECs are synthesized and released from post-synaptic membrane-bound phospholipids in response to neuronal signaling and act as retrograde signaling molecules across the synaptic cleft to stimulate CB 1 receptors on the presynaptic terminal, and they can inhibit neurotransmitter release from the presynaptic terminal. Enzymes that function in synthesizing 2-AG include phospholipase C and diacylglycerol lipase (DAGL).
CBD has also been shown to act as a positive allosteric modulator at GABA A receptors, and controlled trials have shown that CBD in the form of Epidiolex is an effective anticonvulsant in Dravet syndrome and Lennox-Gastaut syndrome [ 120 ]. CBD’s ability to enhance endocannabinoid levels and facilitate GABAergic transmission may serve to improve the balance in inhibitory and excitatory transmission and help restore neuronal function and synaptic plasticity in patients with ASD and FXS even when there is no epilepsy. Animal models of both FXS and ASD have shown benefits when treated with CBD [ 121 , 122 ]. Studies of individuals with ASD treated with CBD and open label trials of CBD are reviewed by Nezgovorva et al. [ 123 ]; however, the preparations studied have both CBD and variable levels of THC, although in general, benefits were seen in irritability, sleep disorders, tantrums, and anxiety. Currently, studies of cannabidavarin (CBDV) are taking place in ASD and CBDV has also been helpful in animal models of ASD [ 123 ].
Recently, the development of a topical CBD that is manufactured so that there is no THC has facilitated studies in both ASD and in FXS. The BRIGHT study was an open label study of children ages 3 to 17 with ASD lasting 14 weeks, and benefits were seen in most outcome measures including the ABC and measures of anxiety [ 124 ]. Currently, a controlled trial of this topical CBD called Zyn002 is taking place in children with ASD. Another recent randomized controlled trial (RCT) looking at an oral preparation of CBD in children and young adults with ASD demonstrated positive improvements in behavior and social communication with CBD [ 125 ].
Huessler et al. [ 126 ] carried out an open label trial of Zyn002 in Australia for children with FXS of ages 3–17 years old with doses of the transdermal CBD at doses 250 mg bi-daily for 12 weeks (ACTRN12617000150347). Both the primary outcome, the Anxiety Mood and Depression (ADAMS) scale and the secondary measures including the ABC, demonstrated efficacy. Subsequently, a multicenter controlled trial of over 200 children with FXS was carried out and efficacy was seen in only those children with > 90% methylation with FXS on the primary outcome measure of the Social Avoidance subscale of the ABC FX , a scale that has been developed for FXS modified from the ABC (Berry-Kravis et al. 2022 under review Sci Trans Medicine). Currently, the FDA has not approved Zyn002 for general use, but an additional multicenter controlled trial is now taking place to win this approval. It is very likely that the current controlled trials taking place for ASD and FXS will show efficacy for subgroups for both disorders, and subsequently, CBD will be more broadly utilized.
Arbaclofen, also called STX209, is a selective ɣ-aminobutyric acid type B receptor agonist that is the R-enantiomer of racemic baclofen. For many subtypes of ASD, there is a GABA B deficit and arbaclofen has rescued the behavioral deficits including social deficits in the mouse models of idiopathic ASD [ 127 ], deletion of 16p11.2 [ 128 ], and FXS [ 129 ]. There are 3 pathways that are improved with arbaclofen: Stimulation of presynaptic GABA B receptors inhibits glutamate release thereby lowering the mGluR5 pathway. Stimulation of GABA receptors also improves inhibition that is down-regulated in many forms of ASD and arbaclofen also enhances K channel activation which can also be down-regulated in many forms of ASD [ 130 ]. The promising mouse studies led to human trials in FXS [ 131 ] that initially showed improvements in those with ASD plus FXS leading to phase 3 trials in FXS [ 132 ]. However, the adult studies of FXS did not demonstrate efficacy and the pediatric trials did not reach significance for the primary outcome measure, but did show limited improvements in secondary measures including the Parenting Stress Index because of lowered irritability in the children [ 132 ].
Both open label studies in idiopathic ASD and in those with a 16p11.2 deletion have been carried out with positive behavioral benefits. A controlled trial with idiopathic ASD has also been started but not yet reported. Arbaclofen has been well tolerated even at higher doses up to 15 mg tri-daily so it is likely that further studies will be carried out both in ASD and in subtypes of ASD including FXS.
Trofinetide
Insulin like growth factor 1 (IGF1) is considered an emerging treatment for ASD in animal and cellular models in ASD [ 133 , 134 , 135 , 136 ]. Trofinetide is an analogue of the amino-terminal tripeptide of IGF1, and it has been studied in patient groups of ASD subtypes. Trofinetide is glycyl-L-2-methylprolyl-L-glutamic acid, and it was studied in a controlled phase 2 trial in 82 children with Rett syndrome ages 5 to 15 years, and significant benefit was found in the high-dose group (200 mg/kg/day) compared to placebo [ 137 ]. Significant benefits were seen in several measures including the Rett Syndrome Behavior Scale, the Rett Syndrome Clinician Rating Scale, and a visual analogue scale. This report led to a multicenter phase 3 controlled trial in Rett syndrome which is ongoing currently.
Trofinetide has also been studied in a 28-day controlled trial in adolescent and adult patients with FXS [ 138 ]. Patients were randomized to trofinetide 35 mg/kg/day, 70 mg/kg/day, or placebo. Results demonstrated that the 70 mg/kg/day was significantly beneficial compared to placebo with a permutation test utilizing the primary components of the Fragile X Syndrome Rating Scale, a Fragile X Specific Domain Scale on a visual analogue format, and the ABC FX.
In the fragile X knockout (KO), mouse studies trofinetide had several positive effects at a dose of 100 mg/kg/day yielding insight as to why it is beneficial in FXS [ 139 ]. The KO mouse was deficient in IGF1 in the brain, and this was normalized with trofinetide treatment for 28 days. Improvements in dendritic spine abnormalities, astrogliosis, neuroinflammation, glial activation, and downregulation of the MEK-ERK and PI3K-mTOR pathways were seen with trofinetide treatment leading to improvements in behavior and morphology of FXS [ 139 ]. Clearly, trofinetide is a treatment that improves multiple pathways that are dysregulated in more than one subtype of ASD and further studies at optimal doses will be carried out and some are currently taking place.
Phosphordiasterase 4D Inhibitors
It has been known for many years that cAMP, an important energy compound for improving synaptic connections, is down regulated in FXS [ 140 ]. Recent animal studies have shown that an inhibitor of cAMP breakdown called a phosphordiasterase 4DE inhibitor can rescue features of FXS in the KO mouse model and Drosophila model and can raise the cAMP levels to normal [ 141 , 142 ]. These studies led to patient trials of a PDE4D inhibitor called BPN14770, and an exciting study was recently published, a randomized controlled trial in 30 adult males with FXS that demonstrated improvements not only in behavior but also in the primary outcome measure, the NIH toolbox, and secondary measures after only 12 weeks of treatment [ 143 ]. This is the first treatment of FXS that demonstrated improvements in cognition, specifically in Oral Reading Recognition, Picture Vocabulary and Cognition Crystallized Composite Score in the NIH toolbox that has been modified for use in those with ID. The caregivers also used the Visual Analog Caregiver Rating Scales and demonstrated improvement in language and daily functioning. Families are excited that this is the first of hopefully many new medications that can reverse cognitive deficits and further controlled trials are in the planning stages.
Anavex 2–73
Anavex 2–73 (AV 2–73; Blarcamesine) is a sigma 1 receptor agonist that works between the endoplasmic reticulum and the mitochondrial membrane to normalize calcium dysregulation, oxidative stress, and mitochondrial dysfunction which is seen in many forms of ASD. It has demonstrated significant benefits in the KO mouse model of FXS where multiple behaviors were improved and deficient brain derived neurotropic factor (BDNF) levels were normalized [ 144 ]. In addition, Kaufman et al. [ 145 ] also reported significant benefits in the Rett syndrome mouse model with a rescue of behavior and BDNF levels and this work lead to patient studies in Rett syndrome that have demonstrated efficacy in a controlled trial (Anavex Life Sciences press release 2021). AV2-73 also has beneficial effects in neurodegenerative disorders because of improvement in proteostasis, autophagy, oxidative stress, prevention of protein aggregates, and improvement in mitochondrial function leading to benefits in Alzheimer’ disease and Parkinson’s disease dementia [ 146 , 147 , 148 ]. Significant potential exists for AV2-73 to improve symptoms in Fragile X-associated Tremor Ataxia (FXTAS), a neurodegenerative disease seen in approximately 40% of older carriers of the fragile X premutation, because calcium dysregulation, mitochondrial dysfunction, proteostasis, and aggregations of proteins causing inclusions occur in FXTAS [ 149 ].
Gene Therapy
Although this therapy is not available for clinicians to utilize in their patients, exciting research studies particularly after the advances in CRISPR/Cas9 technology have become available. The possibility of introducing a normal gene or protein into the CNS to treat ASD or other neurodevelopmental disorders where the mutation is known is exciting. Another example of gene therapy is the introduction of antisense oligonucleotides (ASOs) to silence RNA or gene products that are deleterious. In Angelman syndrome, where the maternal copy of UBE3A is mutated or absent, ASOs have been utilized to activate the paternal copy of UBE3A in the CNS to compensate for the missing maternal copy. Recently, a controlled trial of GTX-102, an ASO, was tried in 5 individuals with Angelman syndrome ages 5 to 15 years old. The protocol involved the intrathecal injection of GTX-102 at increasing doses once monthly for 4 months. However, an adverse effect of leg weakness was seen at the higher doses leading to an inability to walk in two patients. This was found to be related to inflammation at the level where the LP was carried out so these patients were treated with anti-inflammatories with resolution of this side effect. The future is bright for further gene therapy interventions in ASD and other neurodevelopmental disorders.
The current evidence-based management of ASD in children relies primarily on behavioral interventions to address the core symptoms of the condition. The role of pharmacological treatments currently is primarily to address co-morbid conditions associated with ASD and increases with age. These medications including anti-psychotic agents and stimulant medications are important in the clinical management of patients with ASD. However, the emergence of targeted treatments for subgroups of ASD where the genes responsible for the ASD are known and the neurobiology and potential targeted treatments have been studied to reverse the neurobiological abnormalities at least in the animal models has led to several recent achievements in patients as described here. Of note is that there are commonalities among disorders causing ASD that suggest that a targeted treatment for one disorder will be beneficial for other disorders. For instance, GABA deficits are seen in many forms of ASD and medications that are agonists for the GABA system such as CBD are likely to be helpful for many subtypes of ASD as described above. Mitochondrial dysfunction is associated with many forms of ASD, so medications that will improve mitochondrial dysfunction are likely to be helpful for many subtypes of ASD [ 150 ]. The promise of gene therapy is becoming a reality for many disorders such as Duchene Muscular Dystrophy, Spinal Muscular Atrophy, and even Angelman Syndrome because of CRISPR/Cas 9 technology so in the next few years, many additional forms of ASD will be treated with gene therapy. Until then, some of the treatments outlined here can be tried and more will become available in the near future.
Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2018. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2021;70(11):1–16.
Johnson J, Spitzer R, Williams J. Diagnostic and Statistical Manual of Mental Disorders-IV TR. Washington, DC: American Psychiatric Association; 2000.
Google Scholar
Hyman SL, Levy SE, Myers SM. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1).
Association D-AP. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing. 2013.
Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133(1):e54–63.
Article PubMed PubMed Central Google Scholar
Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36(7):849–61.
Article PubMed Google Scholar
Mannion A, Leader G. Comorbidity in autism spectrum disorder: a literature review. Research in Autism Spectrum Disorders. 2013;7(12):1595–616.
Article Google Scholar
Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–9.
Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J Consult Clin Psychol. 1987;55(1):3.
Article CAS PubMed Google Scholar
Slocum TA, Detrich R, Wilczynski SM, Spencer TD, Lewis T, Wolfe K. The evidence-based practice of applied behavior analysis. The Behavior Analyst. 2014;37(1):41–56.
Leaf JB, Leaf R, McEachin J, Taubman M, Ala’i-Rosales S, Ross RK, et al. Applied behavior analysis is a science and, therefore, progressive. Journal of autism and developmental disorders. 2016;46(2):720–31.
Granpeesheh D, Tarbox J, Dixon DR. Applied behavior analytic interventions for children with autism: a description and review of treatment research. Ann Clin Psychiatry. 2009;21(3):162–73.
PubMed Google Scholar
Odom SL, Boyd BA, Hall LJ, Hume K. Evaluation of comprehensive treatment models for individuals with autism spectrum disorders. J Autism Dev Disord. 2010;40(4):425–36.
Smith T. What is evidence-based behavior analysis? The Behavior Analyst. 2013;36(1):7–33.
Wong C, Odom SL, Hume KA, Cox AW, Fettig A, Kucharczyk S, et al. Evidence-based practices for children, youth, and young adults with autism spectrum disorder: a comprehensive review. J Autism Dev Disord. 2015;45(7):1951–66.
Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). The Cochrane database of systematic reviews. 2012;10:Cd009260.
Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17-23.
Wieder S, Greenspan SI. Climbing the symbolic ladder in the DIR model through floor time/interactive play. Autism. 2003;7(4):425–35.
Koegel RL, Koegel LK. Pivotal response treatments for autism: communication, social, & academic development: Paul H Brookes Publishing; 2006.
Mesibov GB, Shea V. The TEACCH program in the era of evidence-based practice. J Autism Dev Disord. 2010;40(5):570–9.
Hume K, Steinbrenner JR, Odom SL, Morin KL, Nowell SW, Tomaszewski B, et al. Evidence-based practices for children, youth, and young adults with autism: third generation review. Journal of Autism and Developmental Disorders. 2021:1–20.
Hall T, Stegila A. Peer mediated instruction and intervention. Wakefield, MA: National Center on Accessing the General Curriculum Retrieved February. 2003;8:2007.
Bondy AS, Frost LA. The picture exchange communication system. Focus on autistic behavior. 1994;9(3):1–19.
Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, et al. Early intervention for children with autism spectrum disorder under 3 years of age: recommendations for practice and research. Pediatrics. 2015;136(Supplement 1):S60–81.
Feroe AG, Uppal N, Gutiérrez-Sacristán A, Mousavi S, Greenspun P, Surati R, et al. Medication use in the management of comorbidities among individuals with autism spectrum disorder from a large nationwide insurance database. JAMA Pediatr. 2021;175(9):957–65.
Levy SE, Giarelli E, Lee LC, Schieve LA, Kirby RS, Cunniff C, et al. Autism spectrum disorder and co-occurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. Journal of developmental and behavioral pediatrics : JDBP. 2010;31(4):267–75.
Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121(3):e441–8.
Coury DL, Anagnostou E, Manning-Courtney P, Reynolds A, Cole L, McCoy R, et al. Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(Supplement 2):S69–76.
Spencer D, Marshall J, Post B, Kulakodlu M, Newschaffer C, Dennen T, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833–40.
Rosenberg RE, Mandell DS, Farmer JE, Law JK, Marvin AR, Law PA. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007–2008. J Autism Dev Disord. 2010;40(3):342–51.
Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel RD, Mangner TJ, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23(2–3):171–82.
Boccuto L, Chen C-F, Pittman AR, Skinner CD, McCartney HJ, Jones K, et al. Decreased tryptophan metabolism in patients with autism spectrum disorders. Molecular autism. 2013;4(1):1–10.
Article CAS Google Scholar
Jansson L, Louhivuori L, Wigren H-K, Nordström T, Louhivuori V, Castrén M, et al. Brain-derived neurotrophic factor increases the motility of a particular N-methyl-D-aspartate/GABA-responsive subset of neural progenitor cells. Neuroscience. 2012;224:223–34.
Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews. 2013(8).
Indah Winarni T, Chonchaiya W, Adams E, Au J, Mu Y, Rivera SM, et al. Sertraline may improve language developmental trajectory in young children with fragile x syndrome: a retrospective chart review. Autism research and treatment. 2012;2012.
Hess LG, Fitzpatrick SE, Nguyen DV, Chen Y, Gaul KN, Schneider A, et al. A randomized, double-blind, placebo-controlled trial of low-dose sertraline in young children with fragile X syndrome. Journal of developmental and behavioral pediatrics: JDBP. 2016;37(8):619.
Yoo K, Burris J, Gaul K, Hagerman R, Rivera S. Low-dose sertraline improves receptive language in children with fragile X syndrome when eye tracking methodology is used to measure treatment outcome. J Psychol Clin Psychiatry. 2017;7(6):00465.
Potter LA, Scholze DA, Biag HMB, Schneider A, Chen Y, Nguyen DV, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psych. 2019;10:810.
Hagerman Ba. Pediatric and Neurological assessments In: Hollander HaF, editor. Textbook of autism spectrum disorders 2nd edition: Am Psychiatric Assoc Publishing Washington DC; 2022. p. 87 - 100.
McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347(5):314–21.
Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–40.
Salazar F, Baird G, Chandler S, Tseng E, O’sullivan T, Howlin P, et al. Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2283–94.
Mansour R, Ward AR, Lane DM, Loveland KA, Aman MG, Jerger S, et al. ADHD severity as a predictor of cognitive task performance in children with autism spectrum disorder (ASD). Research in Developmental Disabilities. 2021;111:103882.
Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? J Atten Disord. 2009;13(2):117–26.
Ames CS, White SJ. Brief report: Are ADHD traits dissociable from the autistic profile? Links between cognition and behaviour. J Autism Dev Disord. 2011;41(3):357–63.
Pondé MP, Novaes CM, Losapio MF. Frequency of symptoms of attention deficit and hyperactivity disorder in autistic children. Arq Neuropsiquiatr. 2010;68:103–6.
Rong Y, Yang C-J, Jin Y, Wang Y. Prevalence of attention-deficit/hyperactivity disorder in individuals with autism spectrum disorder: a meta-analysis. Research in Autism Spectrum Disorders. 2021;83:101759.
Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. Journal of developmental and behavioral pediatrics: JDBP. 2016;37(5):395.
Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. The Lancet Psychiatry. 2018;5(9):727–38.
Sturman N, Deckx L, van Driel ML. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database of Systematic Reviews. 2017(11).
Posey DJ, Aman MG, McCracken JT, Scahill L, Tierney E, Arnold LE, et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: an analysis of secondary measures. Biol Psychiat. 2007;61(4):538–44.
Scahill L, Aman MG, McDougle CJ, McCracken JT, Tierney E, Dziura J, et al. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589–98.
Posey DJ, Puntney JI, Sasher TM, Kem DL, McDougle CJ. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J Child Adolesc Psychopharmacol. 2004;14(2):233–41.
Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J Clin Psychiatry. 1992;53(3):77–82.
CAS PubMed Google Scholar
Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1551–9.
Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53(2):153–73.
Rodrigues R, Lai MC, Beswick A, Gorman DA, Anagnostou E, Szatmari P, et al. Practitioner Review: Pharmacological treatment of attention-deficit/hyperactivity disorder symptoms in children and youth with autism spectrum disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2021;62(6):680–700.
Carmassi C, Palagini L, Caruso D, Masci I, Nobili L, Vita A, et al. Systematic review of sleep disturbances and circadian sleep desynchronization in autism spectrum disorder: toward an integrative model of a self-reinforcing loop. Front Psych. 2019;10:366.
Buckley AW, Hirtz D, Oskoui M, Armstrong MJ, Batra A, Bridgemohan C, et al. Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2020;94(9):392–404.
Gringras P, Nir T, Breddy J, Frydman-Marom A, Findling RL. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2017;56(11):948–57. e4.
Rubenstein J, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–67.
Article CAS PubMed PubMed Central Google Scholar
Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiat. 2012;71(11):956–61.
Sestili P, Fimognari C. Cytotoxic and antitumor activity of sulforaphane: the role of reactive oxygen species. Biomed Res Int. 2015;2015:402386.
Liang J, Hänsch GM, Hübner K, Samstag Y. Sulforaphane as anticancer agent: a double-edged sword? Tricky balance between effects on tumor cells and immune cells. Adv Biol Regul. 2019;71:79–87.
Kamal MM, Akter S, Lin CN, Nazzal S. Sulforaphane as an anticancer molecule: mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch Pharm Res. 2020;43(4):371–84.
Jardim FR, Almeida FJS, Luckachaki MD, Oliveira MR. Effects of sulforaphane on brain mitochondria: mechanistic view and future directions. J Zhejiang Univ Sci B. 2020;21(4):263–79.
Uddin MS, Al Mamun A, Jakaria M, Thangapandiyan S, Ahmad J, Rahman MA, et al. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Science of the Total Environment. 2020;707:135624.
Singh K, W Zimmerman A. Sulforaphane treatment of young men with autism spectrum disorder. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2016;15(5):597–601.
Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci. 2014;111(43):15550–5.
Zimmerman AW, Singh K, Connors SL, Liu H, Panjwani AA, Lee L-C, et al. Randomized controlled trial of sulforaphane and metabolite discovery in children with Autism Spectrum Disorder. Molecular autism. 2021;12(1):1–22.
CAS Google Scholar
Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL. A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev Disord. 2011;41(5):545–54.
Dolske MC, Spollen J, McKay S, Lancashire E, Tolbert L. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17(5):765–74.
Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9–16.
Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426–50.
Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Molecular autism. 2012;3(1):16.
Egawa J, Watanabe Y, Shibuya M, Endo T, Sugimoto A, Igeta H, et al. Resequencing and association analysis of OXTR with autism spectrum disorder in a Japanese population. Psychiatry Clin Neurosci. 2015;69(3):131–5.
Yamasue H, Okada T, Munesue T, Kuroda M, Fujioka T, Uno Y, et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry. 2020;25(8):1849–58.
Bernaerts S, Boets B, Bosmans G, Steyaert J, Alaerts K. Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up. Molecular autism. 2020;11(1):1–14.
Mayer AV, Wermter A-K, Stroth S, Alter P, Haberhausen M, Stehr T, et al. Randomized clinical trial shows no substantial modulation of empathy-related neural activation by intranasal oxytocin in autism. Sci Rep. 2021;11(1):1–13.
Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiat. 2010;67(7):692–4.
Yatawara C, Einfeld S, Hickie I, Davenport T, Guastella A. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2016;21(9):1225–31.
Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci. 2017;114(30):8119–24.
Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188–98.
Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord. 2014;44(3):521–31.
Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56(4):444–52.
Sikich L, Kolevzon A, King BH, McDougle CJ, Sanders KB, Kim S-J, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med. 2021;385(16):1462–73.
Gordon I, Jack A, Pretzsch CM, Vander Wyk B, Leckman JF, Feldman R, et al. Intranasal oxytocin enhances connectivity in the neural circuitry supporting social motivation and social perception in children with autism. Sci Rep. 2016;6:35054.
Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci. 2013;110(52):20953–8.
Yamasue H, Domes G. Oxytocin and autism spectrum disorders. Behavioral Pharmacology of Neuropeptides: Oxytocin. 2017:449–65.
Huang Y, Huang X, Ebstein RP, Yu R. Intranasal oxytocin in the treatment of autism spectrum disorders: a multilevel meta-analysis. Neuroscience & Biobehavioral Reviews. 2021.
Kong X-J, Liu J, Liu K, Koh M, Sherman H, Liu S, et al. Probiotic and oxytocin combination therapy in patients with autism spectrum disorder: a randomized, double-blinded, placebo-controlled pilot trial. Nutrients. 2021;13(5):1552.
Spanos M, Chandrasekhar T, Kim S-J, Hamer RM, King BH, McDougle CJ, et al. Rationale, design, and methods of the Autism Centers of Excellence (ACE) network Study of Oxytocin in Autism to improve Reciprocal Social Behaviors (SOARS-B). Contemporary Clinical Trials. 2020;98:106103.
Lemonnier E, Ben-Ari Y. The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr. 2010;99(12):1885–8.
Lemonnier E, Degrez C, Phelep M, Tyzio R, Josse F, Grandgeorge M, et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Translational psychiatry. 2012;2(12):e202-e.
Lemonnier E, Villeneuve N, Sonie S, Serret S, Rosier A, Roue M, et al. Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Translational psychiatry. 2017;7(3):e1056-e.
Fernell E, Gustafsson P, Gillberg C. Bumetanide for autism: open-label trial in six children. Acta Paediatr. 2021;110(5):1548–53.
Sprengers JJ, Van Andel DM, Zuithoff NP, Keijzer-Veen MG, Schulp AJ, Scheepers FE, et al. Bumetanide for core symptoms of autism spectrum disorder (BAMBI): a single center, double-blinded, participant-randomized, placebo-controlled, phase-2 superiority trial. J Am Acad Child Adolesc Psychiatry. 2021;60(7):865–76.
Du L, Shan L, Wang B, Li H, Xu Z, Staal WG, et al. A pilot study on the combination of applied behavior analysis and bumetanide treatment for children with autism. J Child Adolesc Psychopharmacol. 2015;25(7):585–8.
Crutel V, Lambert E, Penelaud P-F, Albarrán Severo C, Fuentes J, Rosier A, et al. Bumetanide oral liquid formulation for the treatment of children and adolescents with autism spectrum disorder: Design of two phase III studies (SIGN Trials). J Autism Dev Disord. 2021;51(8):2959–72.
Hadjikhani N, Johnels JÅ, Lassalle A, Zürcher NR, Hippolyte L, Gillberg C, et al. Bumetanide for autism: more eye contact, less amygdala activation. Sci Rep. 2018;8(1):1–8.
Hadjikhani N, Zürcher NR, Rogier O, Ruest T, Hippolyte L, Ben-Ari Y, et al. Improving emotional face perception in autism with diuretic bumetanide: a proof-of-concept behavioral and functional brain imaging pilot study. Autism. 2015;19(2):149–57.
Ben-Ari Y, Lemonnier E. Using bumetanide to treat autism appears promising but further clinical trials are needed to confirm this approach. Wiley Online Library; 2021.
James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537–44.
Fatemi SH, Kneeland RE, Liesch SB, Folsom TD. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr Res. 2010;124(1–3):246–7.
Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. 2011;60(7–8):1221–6.
Gantois I, Popic J, Khoutorsky A, Sonenberg N. Metformin for treatment of fragile X syndrome and other neurological disorders. Annu Rev Med. 2019;70:167–81.
Monyak RE, Emerson D, Schoenfeld BP, Zheng X, Chambers DB, Rosenfelt C, et al. Insulin signaling misregulation underlies circadian and cognitive deficits in a Drosophila fragile X model. Mol Psychiatry. 2017;22(8):1140–8.
Esfahanian N, Shakiba Y, Nikbin B, Soraya H, Maleki-Dizaji N, Ghazi-Khansari M, et al. Effect of metformin on the proliferation, migration, and MMP-2 and-9 expression of human umbilical vein endothelial cells. Mol Med Rep. 2012;5(4):1068–74.
Dy ABC, Tassone F, Eldeeb M, Salcedo-Arellano MJ, Tartaglia N, Hagerman R. Metformin as targeted treatment in fragile X syndrome. Clin Genet. 2018;93(2):216–22.
Biag HMB, Potter LA, Wilkins V, Afzal S, Rosvall A, Salcedo‐Arellano MJ, et al. Metformin treatment in young children with fragile X syndrome. Molecular genetics & genomic medicine. 2019;7(11):e956.
Protic D, Kaluzhny P, Tassone F, Hagerman RJ. Prepubertal metformin treatment in fragile X syndrome alleviated macroorchidism: a case study. Advances in Clinical and Translational Research. 2019;3(1):1–5.
Protic D, Aydin EY, Tassone F, Tan MM, Hagerman RJ, Schneider A. Cognitive and behavioral improvement in adults with fragile X syndrome treated with metformin‐two cases. Molecular genetics & genomic medicine. 2019;7(7):e00745.
Osterweil EK, Chuang S-C, Chubykin AA, Sidorov M, Bianchi R, Wong RK, et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77(2):243–50.
Thurman AJ, Potter LA, Kim K, Tassone F, Banasik A, Potter SN, et al. Controlled trial of lovastatin combined with an open-label treatment of a parent-implemented language intervention in youth with fragile X syndrome. J Neurodev Disord. 2020;12(1):1–17.
Nelson S, McDuffie A, Banasik A, Feigles RT, Thurman AJ, Abbeduto L. Inferential language use by school-aged boys with fragile X syndrome: effects of a parent-implemented spoken language intervention. J Commun Disord. 2018;72:64–76.
Patricio F, Morales-Andrade AA, Patricio-Martínez A, Limón ID. Cannabidiol as a therapeutic target: Evidence of its neuroprotective and neuromodulatory function in parkinson’s disease. Frontiers in Pharmacology. 2020;11.
Bakas T, Van Nieuwenhuijzen P, Devenish S, McGregor I, Arnold J, Chebib M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res. 2017;119:358–70.
Leweke F, Piomelli D, Pahlisch F, Muhl D, Gerth C, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational psychiatry. 2012;2(3):e94-e.
Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–43.
Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2018;391(10125):1085–96.
Qin M, Zeidler Z, Moulton K, Krych L, Xia Z, Smith CB. Endocannabinoid-mediated improvement on a test of aversive memory in a mouse model of fragile X syndrome. Behav Brain Res. 2015;291:164–71.
Wei D, Dinh D, Lee D, Li D, Anguren A, Moreno-Sanz G, et al. Enhancement of anandamide-mediated endocannabinoid signaling corrects autism-related social impairment. Cannabis and cannabinoid research. 2016;1(1):81–9.
Nezgovorva F. Taylor and Hollander Cannabis, Cannabionids and Immunomodulary agents. Textbook of Autism Spectrum Disorders: Am Psychiatric Assoc Publishing Washington DC; 2022. p. 586–603.
Heussler H, Michael Duhig TH, Carol O’Neill, Donna Gutterman, Joseph M. Palumbo, Terri Sebree. Longer term tolerability and efficacy of ZYN002 cannabidiol transdermal gel in children and adolescents with autism spectrum disorder (ASD): an open label phase 2 study (BRIGHT [ZYN2 CL 030]). Society for Developmental and Behavioral Pediatrics Annual Meeting USA2021.
Aran A, Harel M, Cassuto H, Polyansky L, Schnapp A, Wattad N, et al. Cannabinoid treatment for autism: a proof-of-concept randomized trial. Molecular Autism. 2021;12(1):6.
Heussler H, Cohen J, Silove N, Tich N, Bonn-Miller MO, Du W, et al. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J Neurodev Disord. 2019;11(1):1–9.
Silverman JL, Pride M, Hayes J, Puhger K, Butler-Struben H, Baker S, et al. GABA B receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in Two mouse models of autism. Neuropsychopharmacology. 2015;40(9):2228–39.
Stoppel LJ, Kazdoba TM, Schaffler MD, Preza AR, Heynen A, Crawley JN, et al. R-baclofen reverses cognitive deficits and improves social interactions in two lines of 16p11. 2 deletion mice. Neuropsychopharmacology. 2018;43(3):513–24.
Henderson C, Wijetunge L, Kinoshita MN, Shumway M, Hammond RS, Postma FR, et al. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Science translational medicine. 2012;4(152):152ra28-ra28.
Guglielmi L, Servettini I, Caramia M, Catacuzzeno L, Franciolini F, D’Adamo MC, et al. Update on the implication of potassium channels in autism: K+ channelautism spectrum disorder. Front Cell Neurosci. 2015;9:34.
Article PubMed PubMed Central CAS Google Scholar
Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Science translational medicine. 2012;4(152):152ra27-ra27.
Berry-Kravis E, Hagerman R, Visootsak J, Budimirovic D, Kaufmann WE, Cherubini M, et al. Arbaclofen in fragile X syndrome: results of phase 3 trials. J Neurodev Disord. 2017;9(1):1–18.
Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, et al. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci. 2009;106(6):2029–34.
Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013;503(7475):267–71.
Linker SB, Mendes AP, Marchetto MC. IGF-1 treatment causes unique transcriptional response in neurons from individuals with idiopathic autism. Molecular Autism. 2020;11(1):1–13.
Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Molecular autism. 2013;4(1):1–4.
Glaze DG, Neul JL, Kaufmann WE, Berry-Kravis E, Condon S, Stoms G, et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019;92(16):e1912–25.
Berry-Kravis E, Horrigan JP, Tartaglia N, Hagerman R, Kolevzon A, Erickson CA, et al. A double-blind, randomized, placebo-controlled clinical study of trofinetide in the treatment of fragile X syndrome. Pediatr Neurol. 2020;110:30–41.
Deacon RM, Glass L, Snape M, Hurley MJ, Altimiras FJ, Biekofsky RR, et al. NNZ-2566, a novel analog of (1–3) IGF-1, as a potential therapeutic agent for fragile X syndrome. NeuroMol Med. 2015;17(1):71–82.
Berry-Kravis E, Huttenlocher PR. Cyclic AMP metabolism in fragile X syndrome. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1992;31(1):22–6.
Choi CH, Schoenfeld BP, Bell AJ, Hinchey J, Rosenfelt C, Gertner MJ, et al. Multiple drug treatments that increase cAMP signaling restore long-term memory and aberrant signaling in fragile X syndrome models. Front Behav Neurosci. 2016;10:136.
Gurney ME, Cogram P, Deacon RM, Rex C, Tranfaglia M. Multiple behavior phenotypes of the fragile-X syndrome mouse model respond to chronic inhibition of phosphodiesterase-4D (PDE4D). Sci Rep. 2017;7(1):1–11.
Berry-Kravis EM, Harnett MD, Reines SA, Reese MA, Ethridge LE, Outterson AH, et al. Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: a randomized, placebo-controlled, phase 2 clinical trial. Nat Med. 2021;27(5):862–70.
Reyes ST, Deacon RM, Guo SG, Altimiras FJ, Castillo JB, van der Wildt B, et al. Effects of the sigma-1 receptor agonist blarcamesine in a murine model of fragile X syndrome: neurobehavioral phenotypes and receptor occupancy. Sci Rep. 2021;11(1):1–14.
Kaufmann WE, Sprouse J, Rebowe N, Hanania T, Klamer D, Missling CU. ANAVEX® 2–73 (blarcamesine), a Sigma-1 receptor agonist, ameliorates neurologic impairments in a mouse model of Rett syndrome. Pharmacology Biochemistry and Behavior. 2019;187:172796.
Hampel H, Williams C, Etcheto A, Goodsaid F, Parmentier F, Sallantin J, et al. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: analysis of the blarcamesine (ANAVEX2‐73) Phase 2a clinical study. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2020;6(1):e12013.
Macfarlane S, Maruff P, Cecchi M, Moore D, Zografidis T, Missling C. P1–046: New exploratory Alzheimer’sdDrug Anavex 2–73: dose dependent clinical cognitive improvement observed in mini mental state examination (MMSE) and other cognitive markers in a phase 2A study in mild-to-moderate Alzheimer’s patients. Alzheimer's & Dementia. 2016;12:P419-P.
Christ MG, Clement AM, Behl C. The sigma-1 receptor at the crossroad of proteostasis, neurodegeneration, and autophagy. Trends Neurosci. 2020;43(2):79–81.
Hagerman R, Hagerman P. Fragile X-associated tremor/ataxia syndrome: Pathophysiology and management. Curr Opin Neurol. 2021;34(4):541–6.
Frye RE, editor Mitochondrial dysfunction in autism spectrum disorder: Unique abnormalities and targeted treatments. Seminars in pediatric neurology; 2020: Elsevier.
Download references
This research was supported by grants from the Azrieli Foundation, clinical trial funding to the MIND Institute from Zynerba for the CBD study, and the MIND Institute Intellectual and Developmental Disabilities Research Center P50 HD103526.
Author information
Authors and affiliations.
Medical Investigation of Neurodevelopmental Disorders (MIND) Institute, University of California Davis, 2825 50th Street, Sacramento, CA, 95817, USA
Ramkumar Aishworiya, Tatiana Valica, Randi Hagerman & Bibiana Restrepo
Khoo Teck Puat-National University Children’s Medical Institute, National University Health System, 5 Lower Kent Ridge Road, Singapore, 119074, Singapore
Ramkumar Aishworiya
Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, 1E Kent Ridge Road, Singapore, 119228, Singapore
Association for Children With Autism, Chisinau, Moldova
Tatiana Valica
Department of Pediatrics, University of California Davis School of Medicine, 4610 X St, Sacramento, CA, 95817, USA
Randi Hagerman & Bibiana Restrepo
You can also search for this author in PubMed Google Scholar
Contributions
Disclosure forms provided by the authors are available with the online version of this article.
Corresponding author
Correspondence to Randi Hagerman .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 436 kb)
Supplementary file2 (pdf 435 kb), supplementary file3 (pdf 508 kb), supplementary file4 (pdf 433 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Aishworiya, R., Valica, T., Hagerman, R. et al. An Update on Psychopharmacological Treatment of Autism Spectrum Disorder. Neurotherapeutics 19 , 248–262 (2022). https://doi.org/10.1007/s13311-022-01183-1
Download citation
Accepted : 03 January 2022
Published : 14 January 2022
Issue Date : January 2022
DOI : https://doi.org/10.1007/s13311-022-01183-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Autism spectrum disorder
- Pharmacology
- Medications
- Find a journal
- Publish with us
- Track your research

New Research May Change How We Think About the Autism Spectrum
Insar keynote suggests brain differences correlate with cognition—not diagnosis..
Posted May 16, 2022 | Reviewed by Davia Sills
- What Is Autism?
- Find a therapist to help with autism
- Dr. Evdokia Anagnostou presented the results of neuroimaging studies at the International Society for Autism Research 2022 annual meeting.
- Of note, brain differences clustered along dimensions of cognition and hyperactivity, not diagnosis.
- These findings suggest we need to reconsider how we classify neurodivergence.
University of Toronto child neurologist Evdokia Anagnostou dropped a bombshell in her keynote Saturday at the annual meeting of the International Society of Autism Research (INSAR) in Austin, Texas, which may call into question the validity of the autism spectrum disorder (ASD) diagnosis.
What Brain Scans Tell Us About Autism Spectrum Disorder
Anagnostou and her colleagues had set out to use neuroimaging to identify brain differences unique to ASD, as compared to other neurodevelopmental differences like ADHD , OCD , and intellectual disability. And they did find that brain differences clustered into different groups—but not by diagnosis. In fact, brain scans could not distinguish children who had been diagnosed with ASD from those who had been diagnosed with ADHD or OCD.
“Dr. Anagnostou reported data from multiple papers that looked at over 3,500 children,” Dr. Alycia Halladay, Chief Science Officer at the Autism Science Foundation, explained to me. “These studies looked at multiple structural and functional features of the brain—including cortical gyrification (the way the brain folds in the cortex), connectivity of different brain regions, and the thickness of the cortical area—and found no differences based on diagnosis.”
Groupings did emerge, but they were along totally different axes. Added Halladay, “The brains themselves were more similar based on cognitive ability, hyperactivity, and adaptive behavior.” In other words, the brains of mildly affected autistic children looked much more like the brains of kids with ADHD than they did like those of severely autistic children.
Validity of the Autism Spectrum Diagnosis May Be at Stake
If replicated, these findings could have tremendous implications for our current diagnostic framework. During the question and answer period following her talk, Anagnostou described two children who both carried the diagnosis of autism; one was very mildly affected, while the other had such disordered behavior that “even their bus driver knows” he is autistic. “Should these kids have the same diagnosis?” she asked.
Right now, they do—but there has been a growing dissatisfaction among many stakeholders in the autism community with the American Psychiatric Association’s introduction of the all-encompassing ASD diagnosis in the 2013 revision of the Diagnostic and Statistical Manual (DSM-5) to replace more narrowly defined categories, including Asperger syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS), and childhood disintegrative disorder.
In 2021, the Lancet Commission —a group of 32 researchers, clinicians, autistic individuals, and family members—called for the creation of a new label, “profound autism,” that would carve out those autistic individuals who also suffer from cognitive and language impairments and require round-the-clock supervision. “Anagnostou’s data converge nicely with the Lancet Commission’s proposal,” Halladay observed. “They provide biological evidence for a category that was originally defined solely by external criteria.”
At the very least. The real question is whether this work demands an even more radical re-imagining of our classification of neurodevelopmental differences. If, as Anagnostou’s data demonstrates, cognition and hyperactivity are much more correlated with brain difference than variables like social deficit that have been considered core symptoms of autism, then perhaps it’s time to scrap our current model and introduce new diagnoses based on these more salient dimensions. Aligning our diagnostic system with underlying biology is the first step in the development of targeted interventions for some of the most intractable and dangerous behaviors exhibited by the developmentally disabled, such as aggression , elopement, self-injury , and pica (the compulsion to eat inedible objects).
As Anagnostou opened her talk, “Nature doesn’t read the DSM.” But, as our understanding of the brain advances, shouldn’t the DSM reflect these divisions in nature?

Amy S.F. Lutz, Ph.D. , is a historian of medicine at the University of Pennsylvania. She is the author of We Walk: Life with Severe Autism (2020) and Each Day I Like It Better: Autism, ECT, and the Treatment of Our Most Impaired Children (2014) . She is also the Vice-President of the National Council on Severe Autism (NCSA).
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
ORIGINAL RESEARCH article
Autism spectrum disorder research: knowledge mapping of progress and focus between 2011 and 2022.

- 1 National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), NHC Key Laboratory of Mental Health (Peking University), Peking University Sixth Hospital, Peking University Institute of Mental Health, Beijing, China
- 2 Translational Medicine Center of Chinese Institute for Brain Research, Beijing, China
- 3 Guangdong Key Laboratory of Mental Health and Cognitive Science, Institute for Brain Research and Rehabilitation (IBRR), South China Normal University, Guangzhou, China
Background: In recent years, a large number of studies have focused on autism spectrum disorder (ASD). The present study used bibliometric analysis to describe the state of ASD research over the past decade and identify its trends and research fronts.
Methods: Studies on ASD published from 2011 to 2022 were obtained from the Web of Science Core Collection (WoSCC). Bibliometrix, CiteSpace, and VOSviewer were used for bibliometric analysis.
Results: A total of 57,108 studies were included in the systematic search, and articles were published in more than 6,000 journals. The number of publications increased by 181.7% (2,623 in 2011 and 7,390 in 2021). The articles in the field of genetics are widely cited in immunology, clinical research, and psychological research. Keywords co-occurrence analysis revealed that “causative mechanisms,” “clinical features,” and “intervention features” were the three main clusters of ASD research. Over the past decade, genetic variants associated with ASD have gained increasing attention, and immune dysbiosis and gut microbiota are the new development frontiers after 2015.
Conclusion: This study uses a bibliometric approach to visualize and quantitatively describe autism research over the last decade. Neuroscience, genetics, brain imaging studies, and gut microbiome studies improve our understanding of autism. In addition, the microbe-gut-brain axis may be an exciting research direction for ASD in the future. Therefore, through visual analysis of autism literature, this paper shows the development process, research hotspots, and cutting-edge trends in this field to provide theoretical reference for the development of autism in the future.
Introduction
Autism spectrum disorder (ASD) refers to a group of early-onset, lifelong, heterogeneous neurodevelopmental conditions with complex mechanisms of emergence ( 1 ). The prevalence of ASD has increased from 1 in 69 by 2012 to 1 in 44 by 2018, as reported by the Centers for Disease Control and Prevention for 2012–2018 ( 2 , 3 ). Recent research estimates the male-to-female ratio is closer to 2:1 or 3:1, indicating a higher diagnostic prevalence of autism in males compared to females ( 4 – 6 ). Some studies have shown a high heritability of 80–93% in ASD and reported hundreds of risk gene loci ( 7 ).
Specific autistic characteristics usually appear before the age of 3 years, and some children on the spectrum may have limited nonverbal and verbal communication by the age of 18–24 months ( 8 , 9 ). The diagnosis of ASD is based on the core features of social communication impairment and unusual and repetitive sensory-motor behavior ( 10 ). Some autistic individuals can be definitively diagnosed with autism as early as 2–3 years of age and the mean age of diagnosis for autistic children is still 4–5 years ( 1 , 11 ). It is important to stress that more adults are getting assessed for possible autism ( 5 ). As autism is increasingly diagnosed, multidisciplinary involvement can help have a positive impact on the well-being and quality of life for both children and adults on the spectrum ( 12 ). Several mental diseases also affect autistic individuals, increasing the diagnosis complexity ( 13 ).
Over the past decade, researchers have struggled to explain the neurological etiology, and great progress has been made in the genetics, epigenetics, neuropathology, and neuroimaging of ASD ( 9 ). However, there is a lack of systematic review of field research and discussion of future research hotspots. Bibliometrics ( 14 ) belongs to interdisciplinary research, which has been widely used in science by analyzing highly cited papers, field keyword clustering, and the internal cooperation links of countries, thus providing a comprehensive interpretation of the development process of autism research field ( 15 ).
In some of the previous bibliometrics studies on ASD, a single software was used to focus on a specific field or research aspect of the autism ( 16 – 18 ), and the trend in the past decade has not yet been displayed. The present study comprehensively combines Bibliometrix package, CiteSpace, and VOSviewer to (1) dynamically assess quantitative indicators of ASD research publications and use different index indicators to measure the quality of research; (2) further identify the most contributing countries, institutions, journals, and authors; (3) analyze the citation network architecture; (4) determine the top 100 most cited papers; (5) conduct keyword analysis. Subsequently, bibliometrics was used to understand the current hotspots and trends in the field of ASD research for further in-depth investigation.
Materials and methods
Data collection and search strategies.
We comprehensively searched the Web of Science Core Collection (WoSCC) database from 2011 to 2022. WoSCC is a daily updated database covering an abstract index of multidisciplinary literature that exports complete citation data, maintained by Thomson Reuters (New York, NY, USA) ( 19 ). The articles’ data were independently searched by two researchers on May 29, 2022, to avoid bias caused by database updates. The scientometric retrieval process is illustrated in Figure 1 . A total of 68,769 original articles in English language were retrieved, excluding 11,661 irrelevant articles, such as meeting abstracts, editorial materials, corrections, and letters. A total of 57,108 documents were exported, and the retrieved documents would be exported in the form of all records and references.
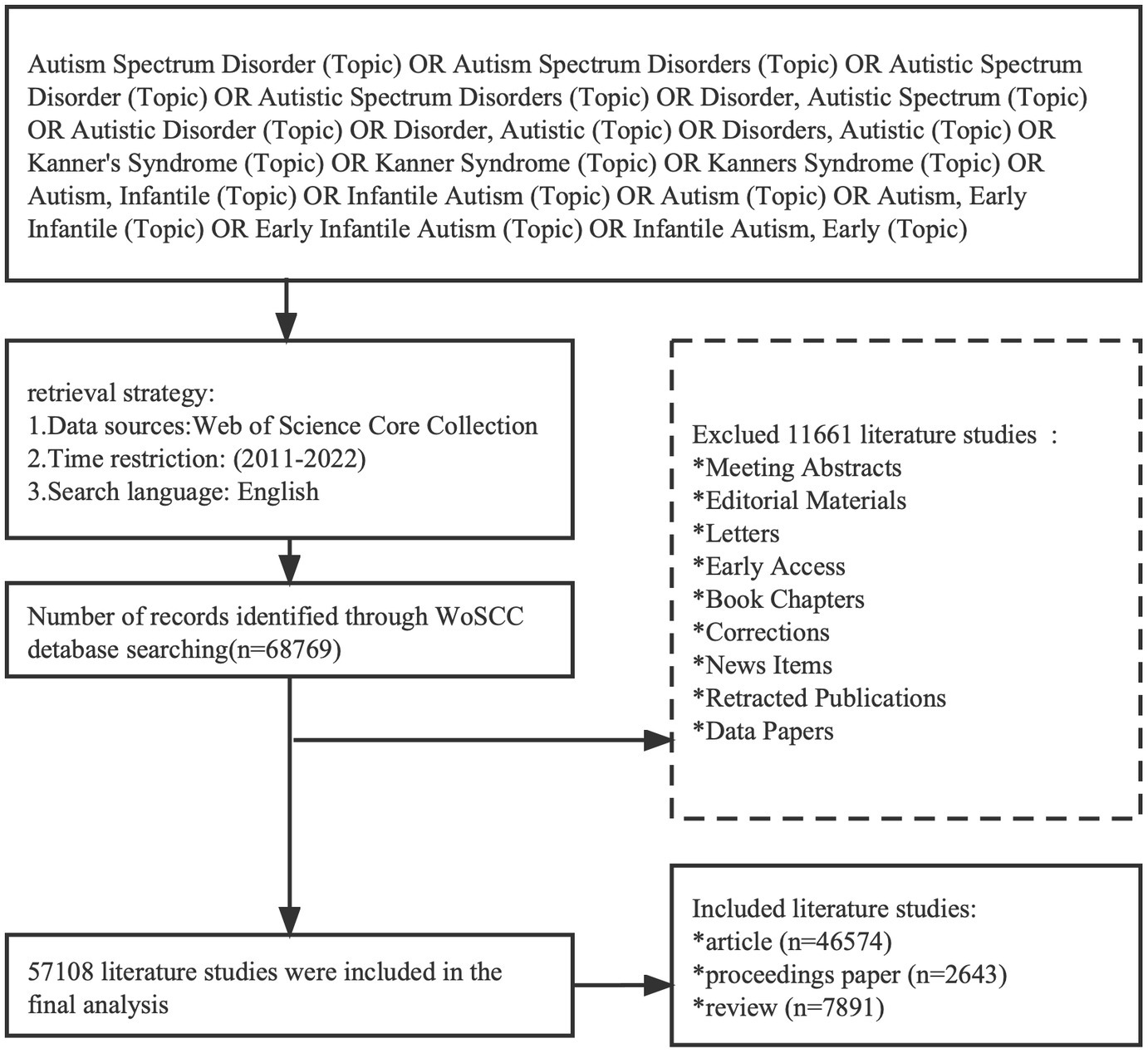
Figure 1 . Flowchart of the screening process.
Grey prediction model
Grey models (GM) are used to construct differential prediction models with limited and incomplete data ( 20 ). The GM (1,1) model, with high accuracy and convenient calculations, is extensively utilized in the energy and medical industries ( 21 ). We used the standard GM (1,1) model to forecast the annual publication volume over the next 5 years. The operation of GM (1,1) model was done by using Python software.
Bibliometric analysis and visualization
The records of the retrieved publications were exported to Bibliometrix, CiteSpace, and VOSviewer for further bibliometric analysis.
Bibliometrix package (running on R4.0.3) was utilized to capture and extract the bibliographic information on selected publications, including topic, author, keywords, and country distribution ( 22 ). The productivity of authors/journals in the field was measured by the number of publications (Np) and assessing metrics, such as the number of citations, publication h-index value, and m-index value. The h-index is used to quantify the scientific output and measure the citation impact, and two people with similar h-index may have a similar impact in the scientific field, even if the total number of papers or total citations are different ( 23 ). The m-index can be used to compare the influence of scholars with different academic career years. The number of citations of a document is a measure of its scientific impact to a certain extent ( 24 ). Bibliometrix package was also used to screen the top 100 articles and explore research trends and hotspots.
VOSviewer is a free computer program to visualize bibliometric maps ( 25 ). The keyword co-occurrence network was constructed using VOSviewer. CiteSpace is based on the Java environment and uses methods, such as co-occurrence analysis and cluster analysis, for the visualization of scientific literature research data in specific disciplines. The visual knowledge maps were constructed using the procedural steps of CiteSpace ( 26 ), including time slicing, threshold, pruning, merging, and mapping; then, the contribution of countries and institutions of ASD over the past decade was assessed based on centrality scores. The co-citation network and dual-map of references were constructed by CiteSpace. A dual-map ( 27 ) overlay is a bipartite overlay analysis method by CiteSspace, which uses the distribution map cited journals in the WoS database as the base map, and the map generated by the cited literature data as the overlay map.
Annual publications
A total of 57,108 articles were included in this study, consisting of 46,574 articles, 2,643 conference papers, and 7,891 reviews. From 2011 to 2022, the number of publications maintained a steady growth rate ( Figure 2A ), and the grey prediction model predicted the trend of increasing publication volume in the next 5 years ( Figure 2B ). The main information for all publications is shown in Supplementary Table S1 .
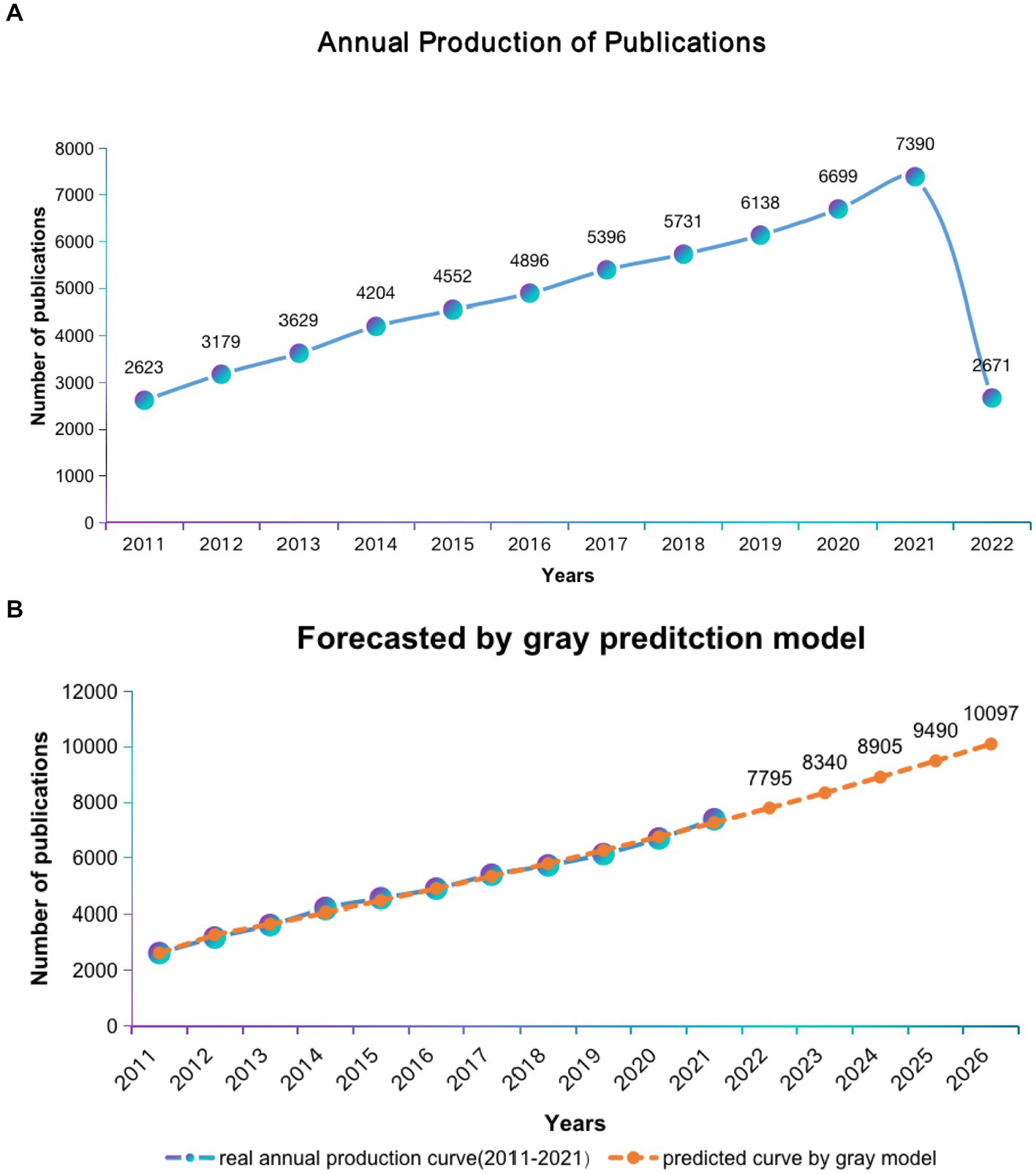
Figure 2 . Global trends in publications of ASD research. (A) Single-year publication output over the past decade. (B) Model forecast curves for publication growth trends.

Distribution of countries and institutions
Autism-related research has been conducted by researchers from a variety of countries and institutions, and articles in this field have been cited 1,231,588 times ( Tables 1 , 2 ). CiteSpace visualizes collaborative networks between institutions and countries ( Figures 3A , B ). As shown in the international collaborations network of autism research ( Figure 3C ), the USA and UK are the leading countries working closely with other countries.
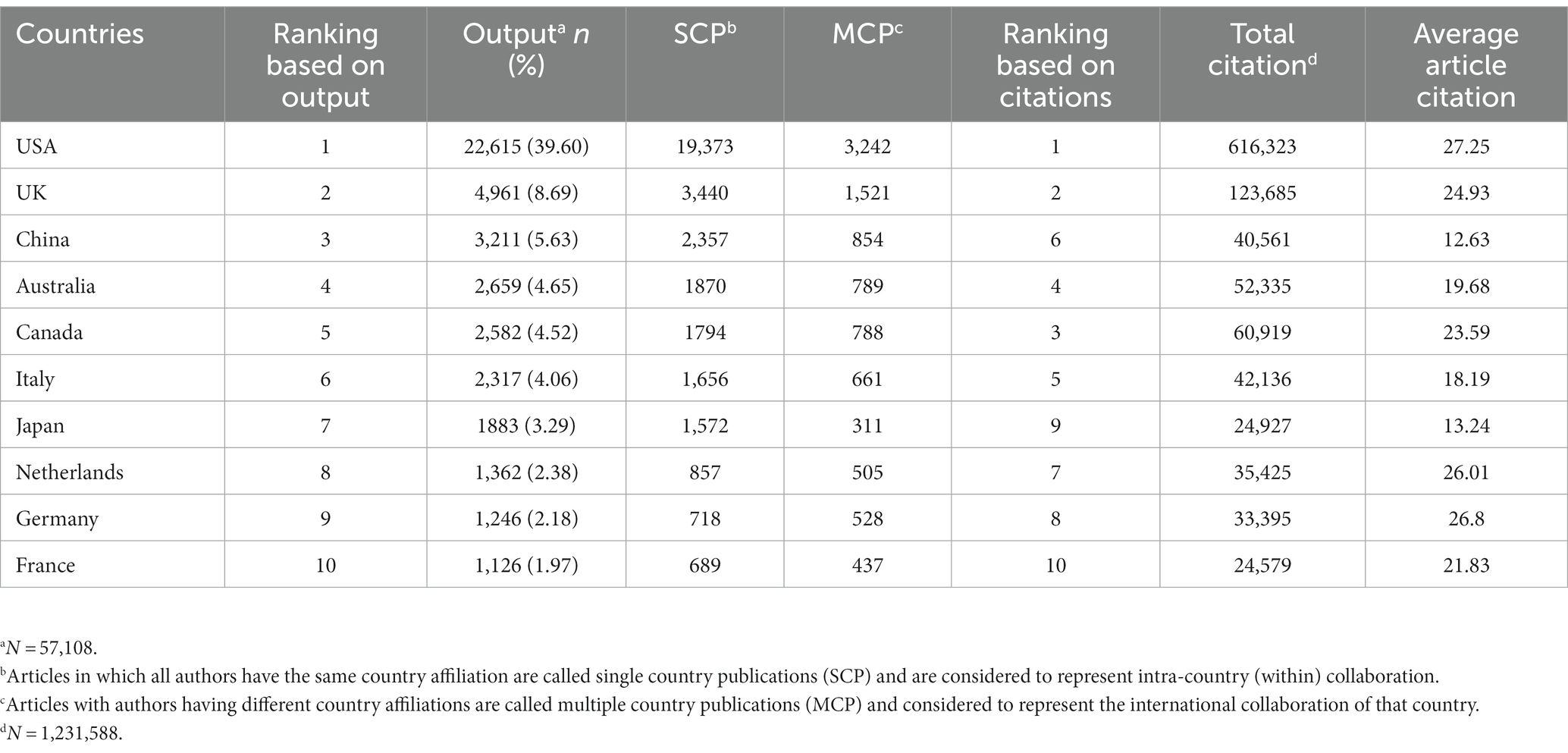
Table 1 . Publications in top 10 most productive countries.
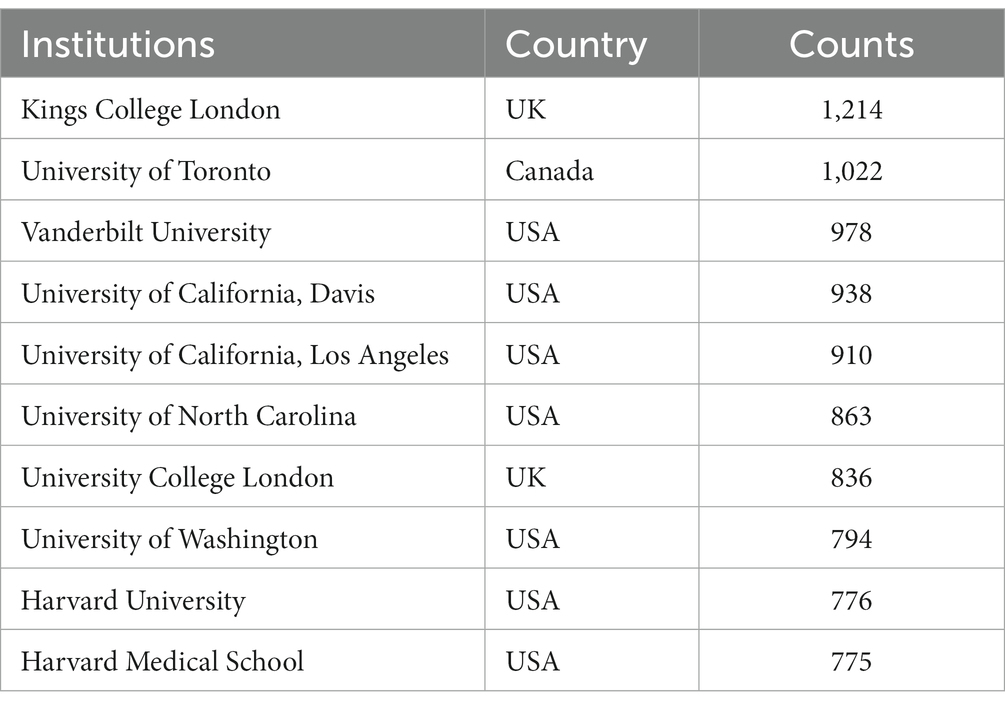
Table 2 . Publications in top 10 most productive Institutions.
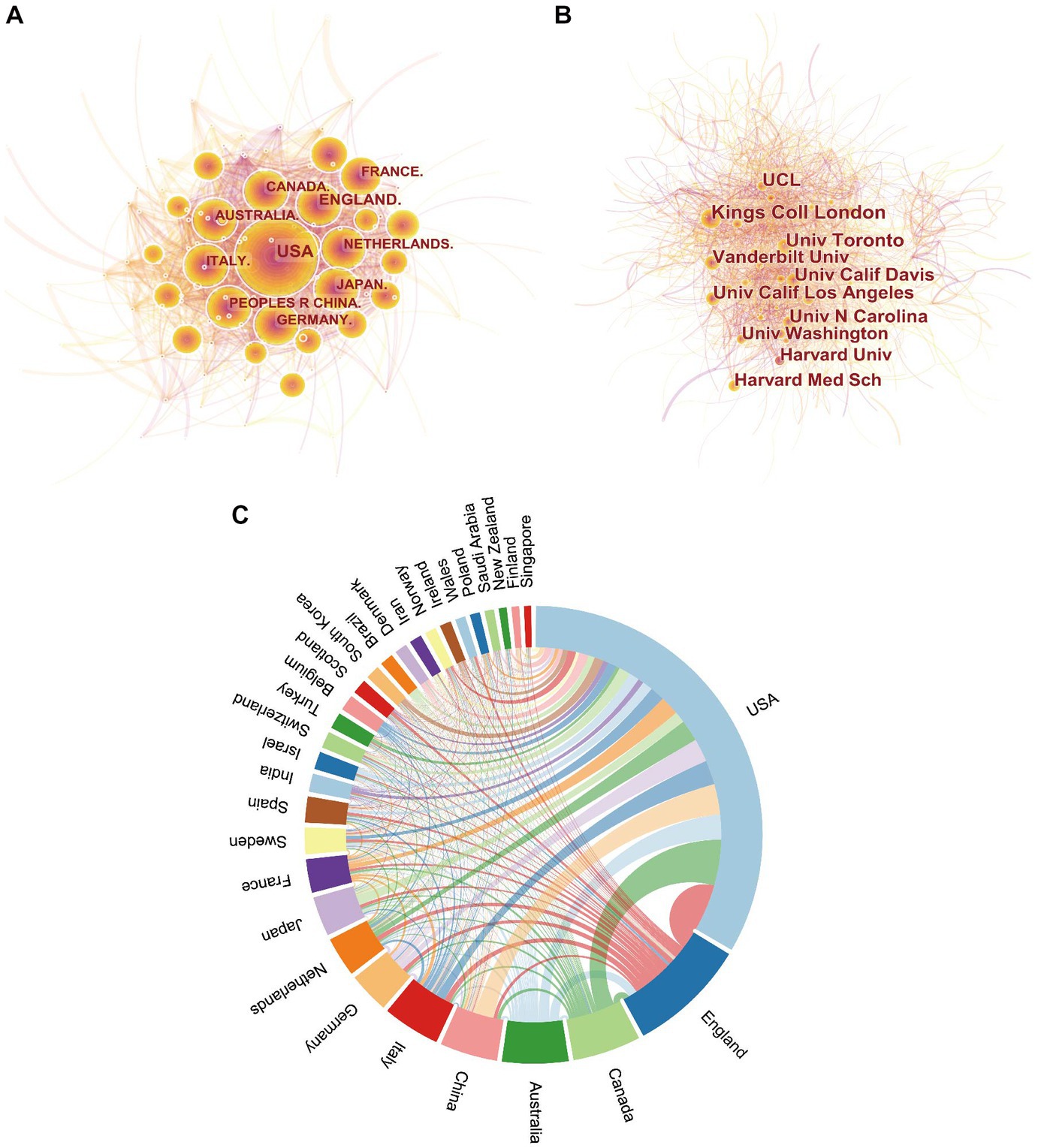
Figure 3 . The distribution of countries and institutions. Map of countries (A) and institutions (B) contributed to publications related to ASD research. (C) Network diagram showing international collaborations involved in ASD research. The nodes represent the countries and institutions; the color depth and size of the circle are positively correlated to the number of posts. The thickness of the curved connecting lines represents the strength of collaboration in the countries and institutions.
Analysis of journals
The h-index combines productivity and impact; typically, a high h-index means a high recognition. As presented in Table 3 , the Journal of Autism and Developmental Disorders, PLOS One, and Molecular Psychiatry were among the top three of the 20 journals with the highest h-index. The Journal of Autism and Developmental Disorders has the highest number of articles (3478) and cited number of publications (90308). Among the top 20, four journals with impact factors >10 include Molecular Psychiatry (IF: 13.437), Biological Psychiatry (IF: 12.810), Proceedings of the National Academy of Sciences of the United States of America (IF: 12.779), Journal of the American Academy of Child and Adolescent Psychiatry (IF: 13.113), which have been cited more than 10,000 times. In addition, 75% of journals belong to Q1 ( Table 3 ). The cited journals provided the knowledge base of the citing journals. The yellow paths illustrate that studies published in “molecular, biology, immunology” journals tended to cite journals primarily in the domains of “molecular, biology, genetics,” and “psychology, education, social.” The paths colored with grass-green paths illustrate that studies published in “medicine, medical, clinical” journals tended to cite journals primarily in the domains of “molecular, biology, and genetics.” The pale blue paths showcase that research published in “psychology, education, health” journals preferred to quote journals mostly in the domains of “molecular, biology, genetics,” “health, nursing, medicine,” and “psychology, education, social ( Figure 4 ).”
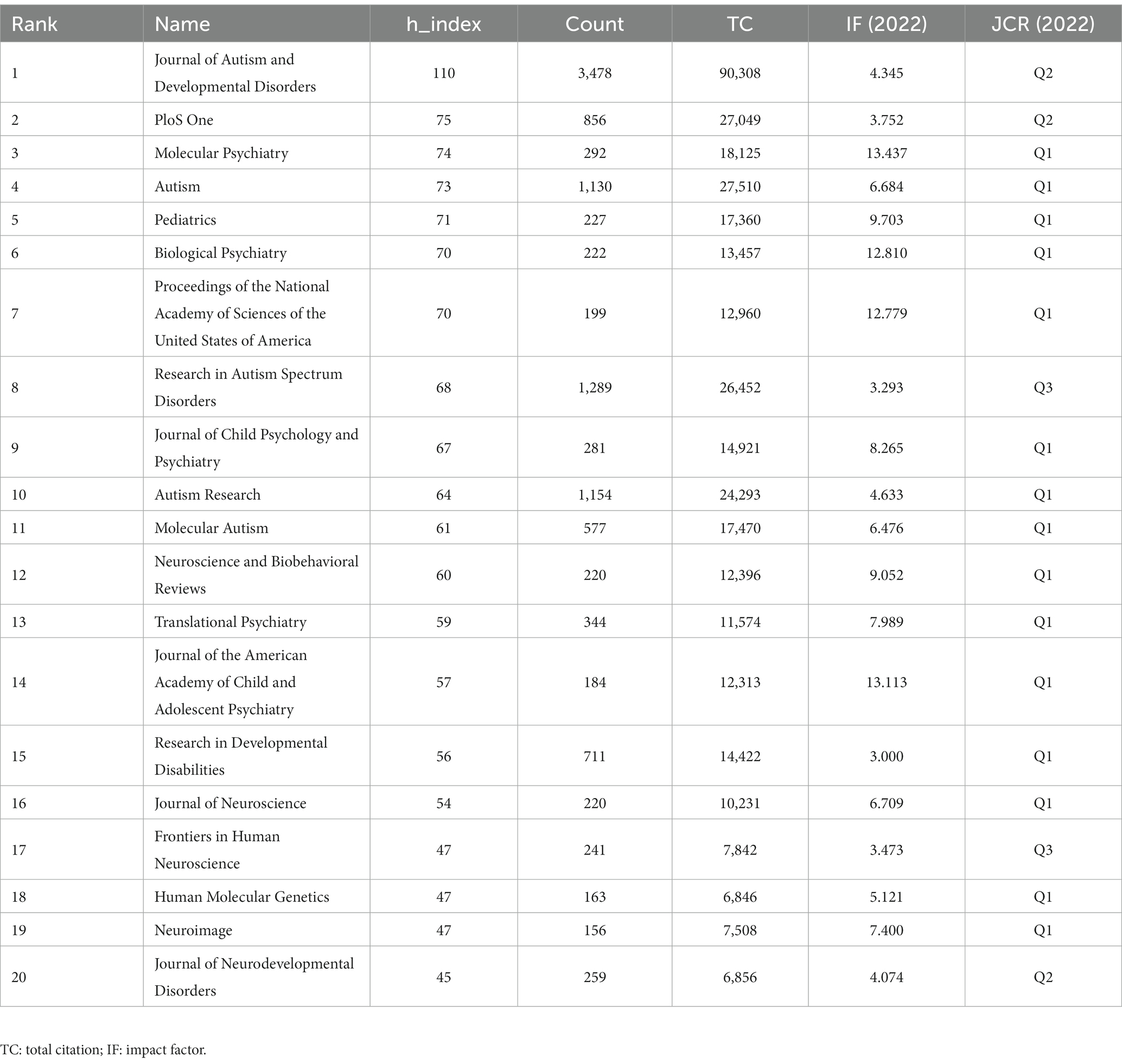
Table 3 . Top 20 journals ranked by h_index.
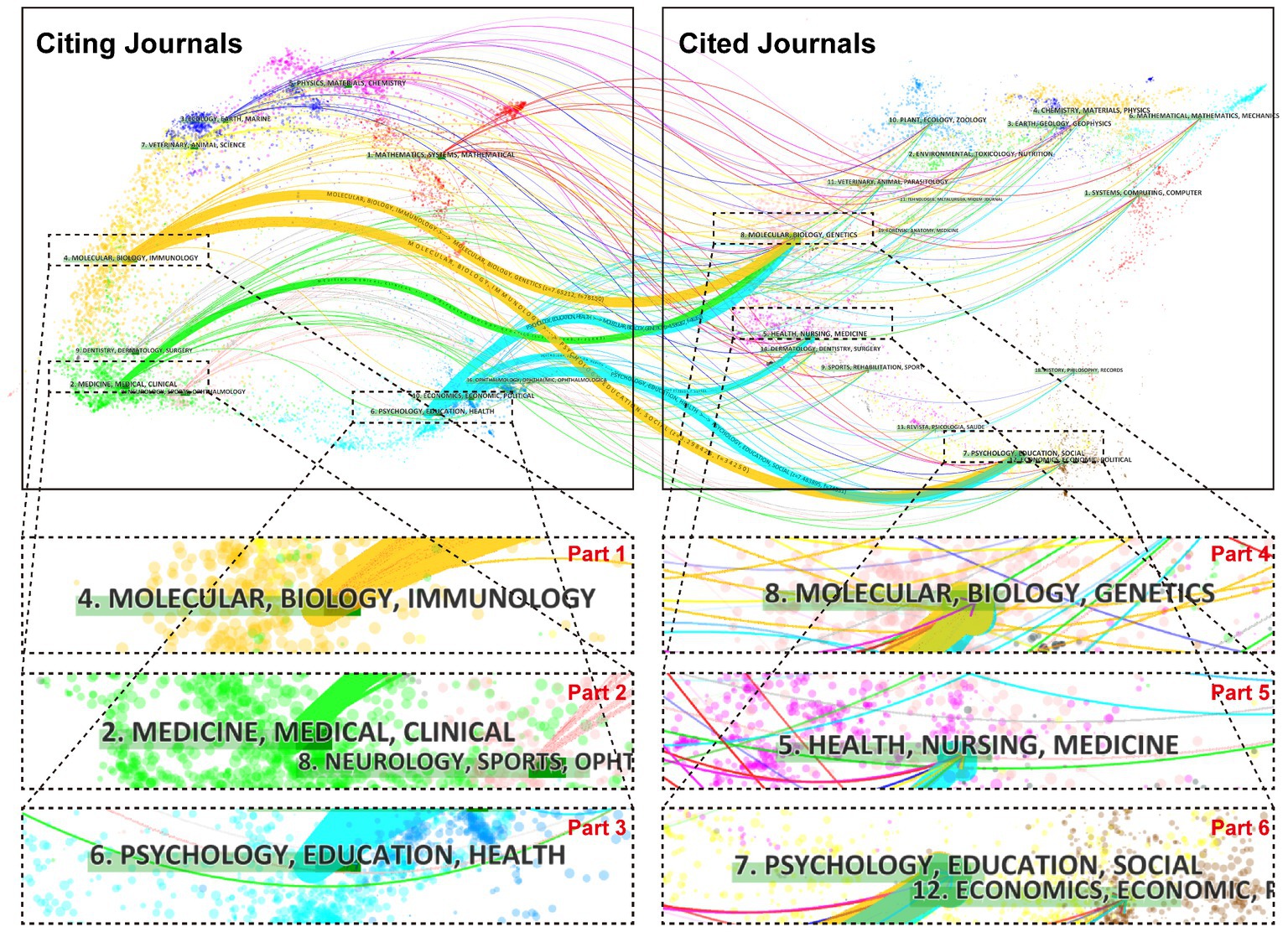
Figure 4 . A dual-map overlay of journals that published work related to ASD. A presentation of citation paths at a disciplinary level on a dual-map overlay. The width of the paths is proportional to the z-score-scale citation frequency. The labels on the map represent the research subjects covered by the journals, and the wavy curve connects the citing articles on the left side of the map and the cited articles on the right side of the map.
Analysis of authors
The top 10 most effective authors who have contributed to autism research are listed in Table 4 . The g-index and m-index are derivatives of the h-index, and if scientists publish at least 10 articles, of which 7 papers have been cited cumulatively 51 (>49), the g-index is 7; the m-index is related to the academic age of the scientists. The large g-index, h-index, and m-index indicate a great influence on the scholar’s academic influence and high academic achievement. Professor Catherine Lord from the USA is ranked first and has made outstanding contributions to autism research over the past 10 years. In terms of the number of publications, Simon Baron-Cohen was the most productive author ( n = 278), followed by Tony Charman ( n = 212) and Christopher Gillberg ( n = 206). In terms of citations in this field, Daniel H. Geschwind was ranked first (18,127 citations), followed by Catherine Lord (14,830 citations) and Joseph D. Buxbaum (14,528 citations).
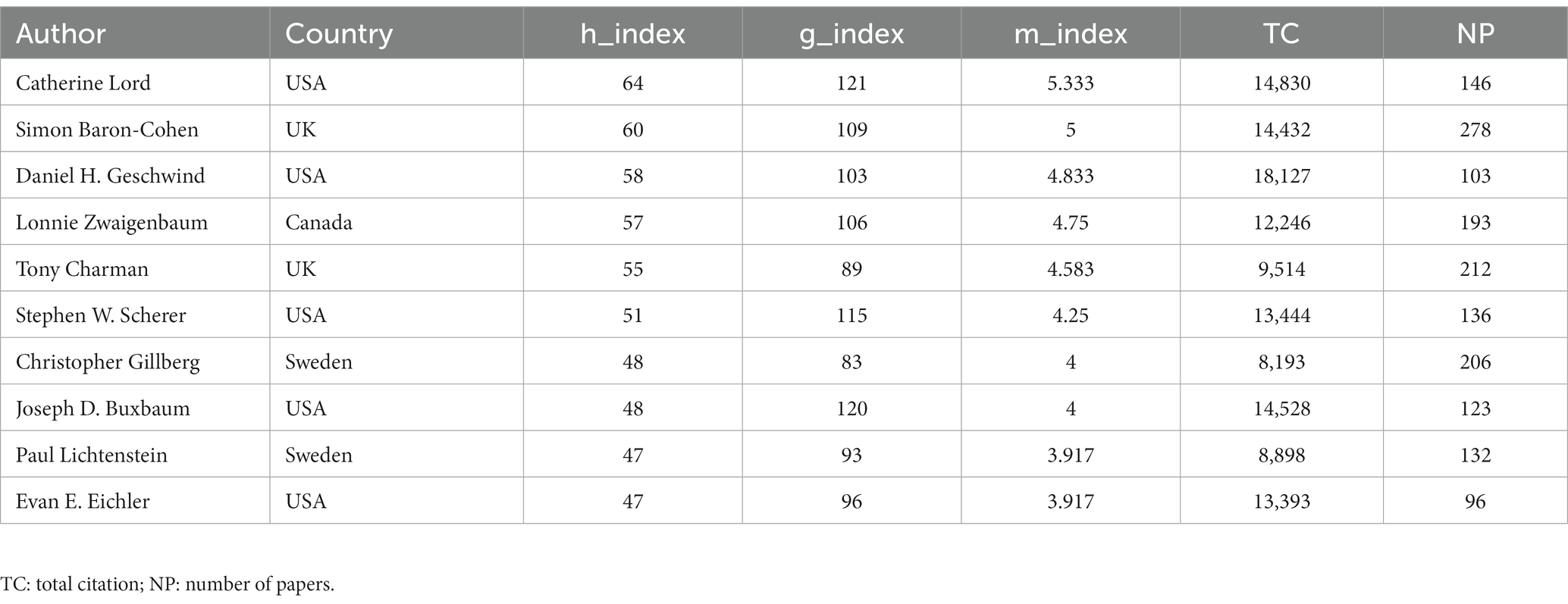
Table 4 . Top 10 most effective authors contributing to autism research.
Analysis of reference
The co-citation analysis network of 1,056,125 references ( Figure 5A ) showed that two articles appear simultaneously in the bibliography of the third cited document. The top 20 co-cited references (over the past decade) summarized in ASD studies are listed in Supplementary Table S2 . Most of this highly cited literature focuses on the genetic field, discovering genetic risk loci and associated mutations, constructing mutation networks highly associated with autism, and identifying genes associated with autism synaptic destruction. Some studies indicated that de novo mutations in ASD might partially explain the etiology. Multiple studies have revealed genetic variants associated with ASD, such as rare copy number variants (CNVs), de novo likely gene-disrupting (LGD) mutations, missense or nonsense de novo variants, and de novo duplications. In the cluster network graph, different colors represent varied clusters, and each node represents a cited paper, displaying the distribution of topics in the field ( Figure 5B ). The network is divided into 25 co-citation clusters ( Figure 5B ), primarily related to the diagnosis, etiology, and intervention of autism. The etiological studies include five clusters, de novo mutation, inflammation, gut microbiota, mitochondrial dysfunction, and mouse model. Intervention literature focuses on early intensive behavioral intervention, intranasal oxytocin, video modeling, and multisensory integration. The diagnostic aspects of ASD include neuroimaging functional connectivity and Diagnostic and Statistical Manual of Mental Disorders (DSM-5). In addition, some of the references focus on gender/sex differences and sleep problems. Coronavirus disease 2019 (COVID-19) is a new cluster for autism research.
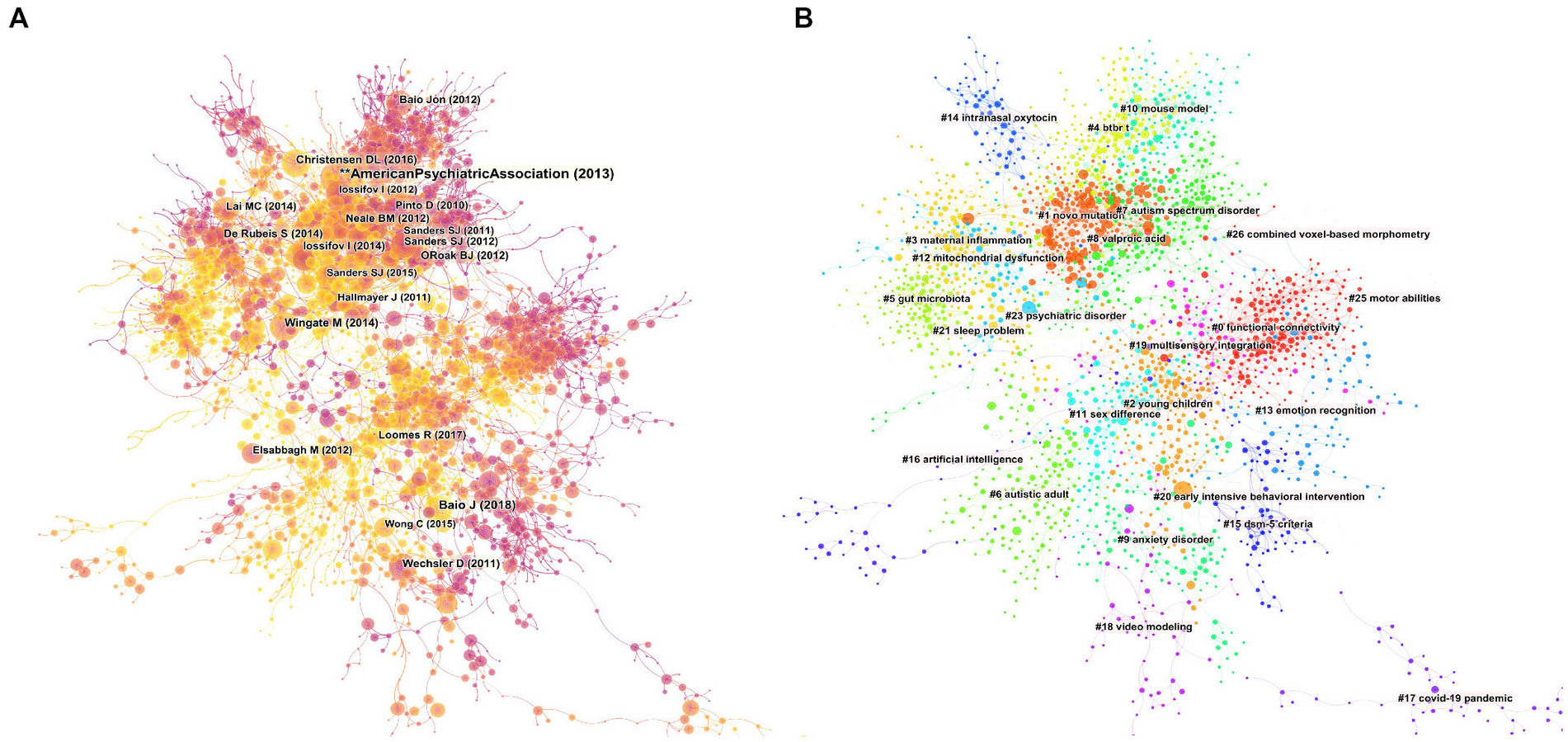
Figure 5 . Mapping on co-cited references. (A) A network map showing the co-cited references. (B) Co-cited clusters with cluster labels.
Co-occurrence analysis of keywords
The co-occurrence analysis of keywords in ASD research articles was performed using VOSviewer software; the keywords that occurred ≥200 times were analyzed after being grouped into four clusters of different colors ( Figure 6A ); the temporal distribution of keywords is summarized in Figure 6B . This map identifies various categories of research: Etiological mechanisms (red), Clinical features (green), Intervention features (blue), and the Asperger cluster (yellow). In the “Etiological mechanisms” cluster, the research includes brain structure and function, genetics, and neuropathology. In the “Clinical features” cluster, the common keywords were “symptoms,” “diagnosis,” “prevalence,” and its comorbidities, including “anxiety” and “sleep.” In the “Intervention features” cluster, the research population of ASD is concentrated in “young children,” “intervention,” and “communication.” These interventions improve the learning and social skills through the involvement of parents and schools.
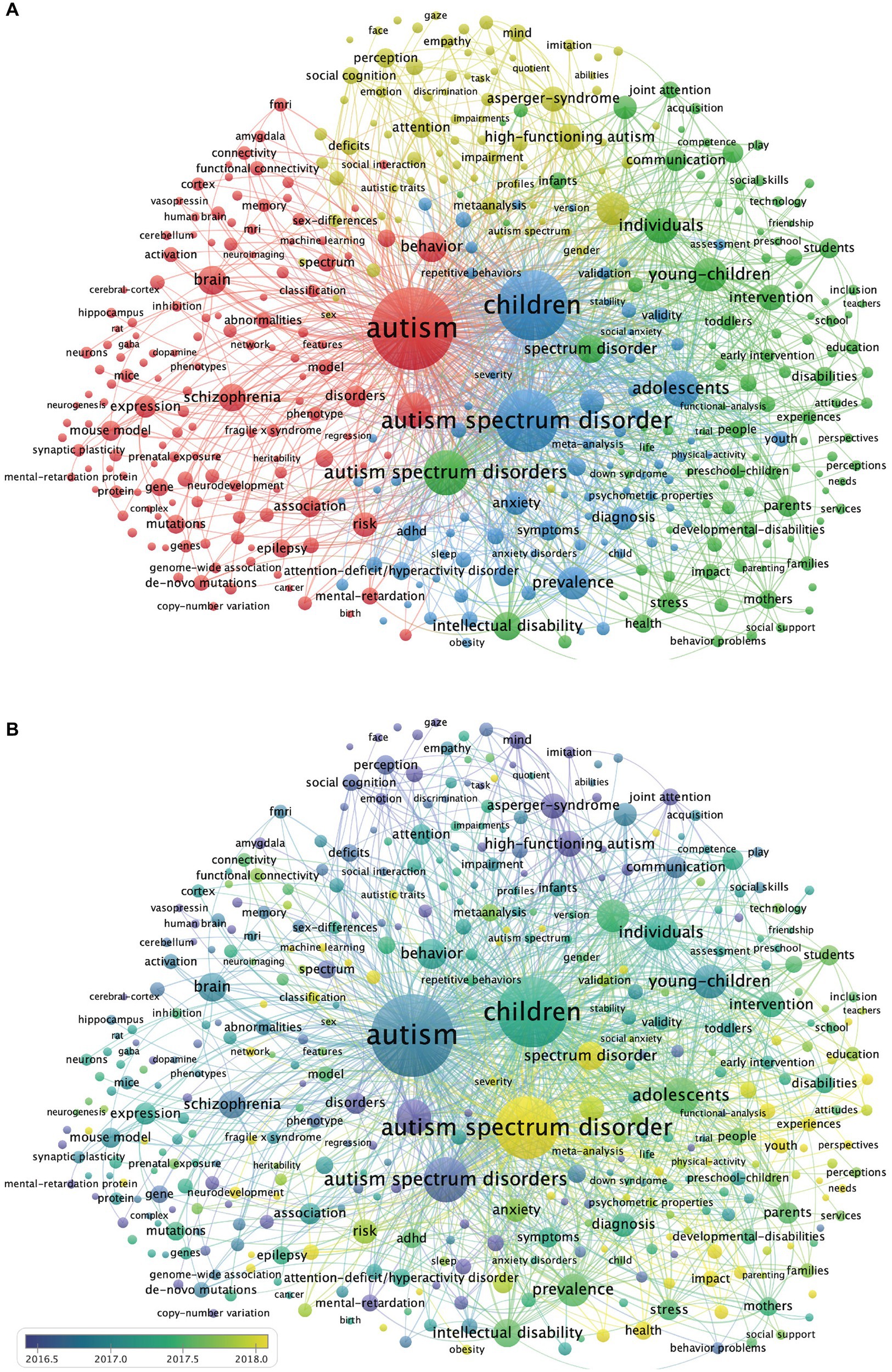
Figure 6 . Keywords co-occurrence network. (A) Cluster analysis of keywords. There are four clusters of keywords: red indicates Cluster 1 ( n = 145), green indicates Cluster 2 ( n = 104), blue indicates Cluster 3 ( n = 78), yellow indicates Cluster 4 ( n = 80). (B) Evolution of keyword frequency. A minimum number of occurrences of a keyword = 200. Overall, 407 keywords met the threshold criteria. The yellow keywords appear later than purple keywords.
The 100 top-cited publications
The screening of the 100 most cited publications on ASD between 2011 and 2022 by Bibliometrix software package, each with >500 citations. The detailed evaluation index information for countries, institutions, journals, and authors ( Supplementary Tables S3 – S6 ).
Taken together, the results indicated that the United States is the country that publishes the most highly cited articles ( n = 64), including single-country publications ( n = 37) and multiple-country publications ( n = 27); most articles are from academic institutions within the USA ( Figures 7A , B ).
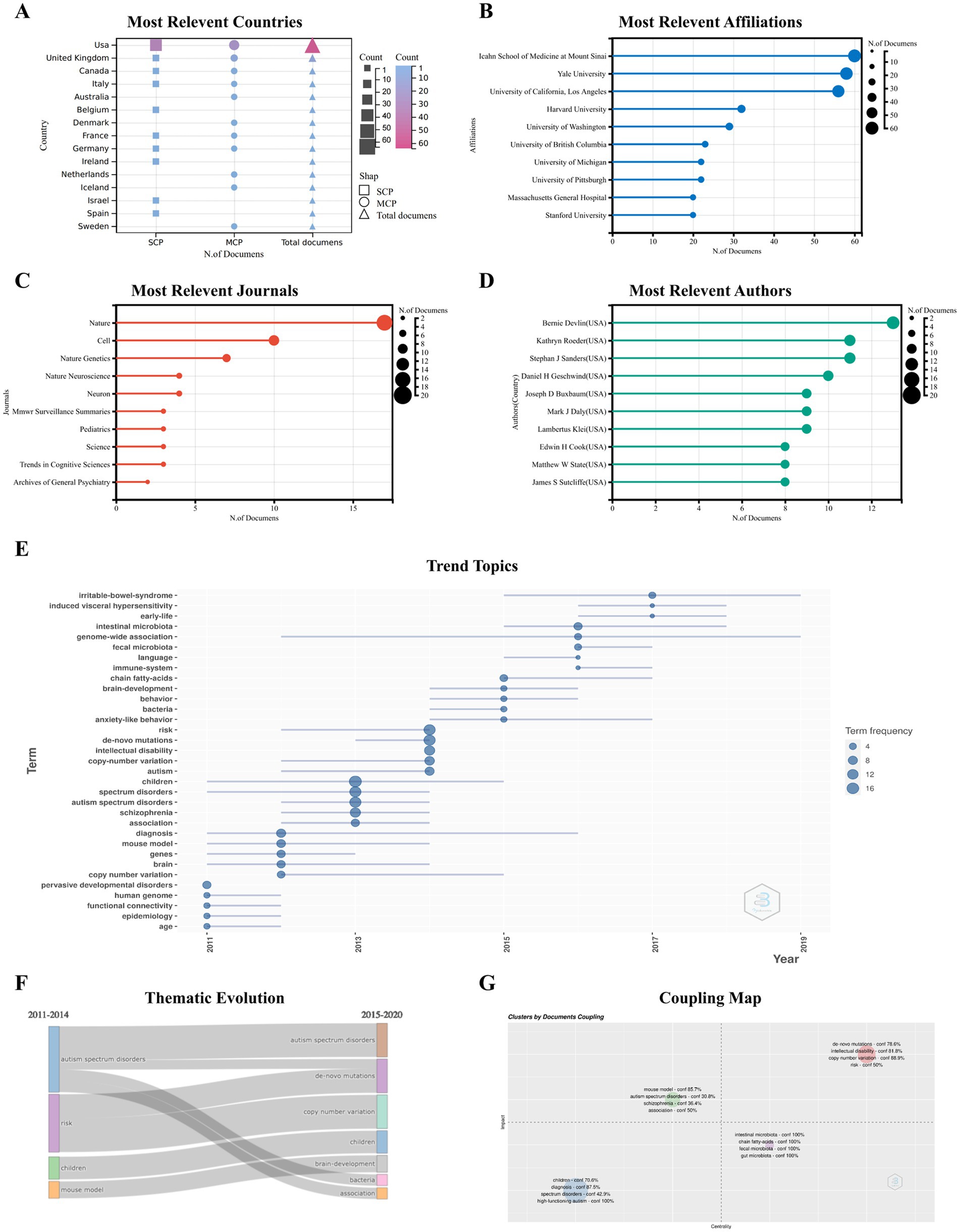
Figure 7 . Analysis of the 100 top-cited publications Characteristics of 100 top-cited publications. The most relevant countries (A) , affiliations (B) , journals (C) and authors (D) . Trend topics (E) and thematic evolution (F) of 100 top-cited publication. Coupling Map (G) : the coupled analysis of the article, references and keywords is carried out, the centrality of the x -axis is displayed, the y -axis is the impact, and the confidence (conf%) is calculated.
The 100 top-cited ASD publications were published in 48 journals; 17 articles were published in Nature ( n = 17), making it the highest h-index journal in this list ( Supplementary Table S5 ). In addition, 10 articles were published in Cell, and 7 articles were published in Nature Genetics ( Figure 7C ). When considering the individual authors’ academic contributions, Bernie Devlin provided 13 publications, followed by Kathryn Roeder and Stephan J Sanders, with 11 publications each ( Figure 7D ). The details of the top 10 top-cited papers are summarized in Table 5 . An article titled “A general framework for estimating the relative pathogenicity of human genetic variants” published by Martin Kircher in Nature Genetics, received the highest number of citations ( n = 3,353).
Table 5 . Detail of top 10 citation paper.
The 100 top-cited ASD articles encompassed a range of keywords ( Figure 7E ) and displayed the main cluster of themes through specific periods (2011–2022) by analyzing those in the selected literature. The Sankey diagrams of thematic evolution explain the topics that evolved throughout the years ( Figure 7F ). In summary, the core topics of the ASD field in 2011–2014 consisted of the risk of childhood ASD and further developed into the field of human genetic variants, such as CNV and de novo mutations. In the subperiod 2015–2020, the further expansion of studies in this field leads to new clusters, such as “immune system,” “brain development,” and “fecal microbiota.” Genome research in the upper right quadrant, including mutations and risk, is a major and evolving theme. The coupled map showing the brain-gut axis field, including intestinal microbiota and chain fatty acids, located in the lower right corner is crucial for autism research but is not yet well-developed ( Figure 7G ). The research on autism, including animal models, schizophrenia, is a well-developed field, but that on high-functioning autism and diagnosis is a marginal field.
This study used various bibliometric tools and software to analyze the published articles on ASD based on the WoSCC database from 2011 to 2022. By 2022, the annual number of publications and citations of ASD-related research showed an overall upward trend, reflecting the sustained interest and the diversity of areas.
General information
In terms of regional distribution, researchers from different countries and regions have participated in autism research, and international cooperation has been relatively close over the past decade. The scientific research is supported by several countries and institutions, as well as by large-scale international cooperation ( 28 , 29 ). The USA has the highest collaboration performance, especially with UK, Canada, Australia and China. In addition to the limitations of financial aid, ethical, cultural, and racial issues are complex constraints that should be overcome for more diversity in autism research ( 30 , 31 ). We speculated that further collaboration between institutions and countries could promote autism research.
Among the top 20 academic journals, most of the papers were in the Journal of Autism and Developmental Disorders. The frequent publishing of ASD-related papers indicates the interest of readers and journal editors in Autism. Also, substantial studies have been carried out on ASDs, autism, and molecular autism. These journals are ascribed to the field of ASD, focusing on autism research and communication ASD science. However, the analysis of the 10 most cited publications revealed that they were published in such as Nature, Cell, Lancet; these ASD studies were all from high-impact journals.
From the perspective of authors, some of them have made outstanding contributions to global ASD research. Professor Catherine Lord, the top rank for h-index, m-index analysis conducted by the author, and who developed the two gold standards for autism diagnosis ( 32 , 33 ), are the most influencing factors in the field. ASD is a disease with complex genetic roots. Dr. Catherine Lord has conducted multiple studies using genome-wide association study (GWAS) and gene set analysis to identify variant signatures in autism ( 34 ). A recent meta-analysis showed that 74–93% of ASD risk is heritable, with an analysis of CNVs that highlights the key role of rare and de novo mutations in the etiology of ASD ( 35 ). Variation-affected gene clusters on networks associated with synaptic transmission, neuronal development, and chromatin regulation ( 36 , 37 ). The identification of the cross-disorder genetic risk factors found by assessing SNP heritability in five psychiatric disorders ( 38 ). Five of the top 10 cited papers in Table 5 focus on genetic variation, suggesting that over the past decade, research has shifted from a general concept of genetic risk to the different types of genetic variations associated with autism.
Simon Baron-Cohen of the Autism Research Center at the University of Cambridge was the most published author between 2011 and 2021. He contributed to the mind-blindness hypothesis of autism, developed the autism spectrum quotient (AQ) screening tool for autism, and focused on gender differences in autism ( 39 – 41 ). There are gender/sex differences in the volume and tissue density of brain regions, including the amygdala, hippocampus, and insula, and the heart-blind hypothesis links emotional recognition in individuals with autism to deficits in the amygdala ( 41 – 43 ). Then, Simon et al. backed up the “extreme male brain” theory of autism in a study of 36,000 autistic individuals aged 16–89 ( 44 ). Recently, an increasing number of studies from different perspectives have focused on how sex/gender differences are related to autism ( 4 , 5 , 45 ). In the future, studies of neural dimorphism in brain development in autism need to be conducted across the lifespan to reduce age-induced biases ( 41 ).
Hotspots and Frontiers
Keyword analysis was a major indicator for research trends and hotspot analysis. This study shows that keywords for autism research include etiological mechanism, clinical characteristics, and intervention characteristics. Genetic, environmental, epigenetic, brain structure, neuropathological, and immunological factors have contributed to studying its etiological mechanism ( 46 , 47 ). The studies on the abnormal cortical development in ASD have reported early brain overgrowth ( 48 ), reduced resting cerebral blood flow in the medial PFC and anterior cingulate ( 49 ), focal disruption of neuronal migration ( 50 ), and transcriptomic alterations in the cerebral cortex of autism ( 51 ). Genomics studies have identified several variants and genes that increase susceptibility to autism, affecting biological pathways related to chromatin remodeling, regulation of neuronal function, and synaptic development ( 51 – 54 ). In addition, many autism-related genes are enriched in cortical glutamatergic neurons, and mutations in the genes encoding these proteins result in neuronal excitation-inhibitory balance ( 51 , 55 ). A recent study using single-cell sequencing of the developing human cerebral cortex found strong cell-type-specific enrichment of noncoding mutations in ASD ( 56 ). Interestingly, genes interact with the environment; some studies have shown that environmental exposure during pregnancy is a risk factor for brain development ( 57 ), and there are changes in DNA methylation in the brains of ASD patients, reflecting an underlying epigenetic dysregulation.
Presently, the diagnosis of ASD is mainly based on symptoms and behaviors, but the disease has a high clinical heterogeneity, and the individual differences between patients are obvious ( 58 ). In this study, the keywords of the intervention cluster show the importance of early individualized intervention. Patient data are multidimensional, and individualized diagnoses could be made at multiple levels, such as age, gender, clinical characteristics, and genetic characteristics ( 59 ). Early individual genetic diagnosis aids clinical evaluation, ranging from chromosomal microarray (CMA) to fragile X genetic testing ( 60 ). However, the results of genetic research cannot guide the treatment. Notably, the treatment of autism is dominated by educational practices and behavioral interventions ( 61 ). Medication may address other co-occurring conditions, such as sleep disturbances, epilepsy, and gastrointestinal dysfunction ( 9 ). Professor Catherine Lord pointed out that the future of autism requires coordinated, large-scale research to develop affordable, individualized, staged assessments and interventions for people with ASD ( 62 ). Professor Baron-Cohen noted that increasing the sample size and collecting data from the same individual multiple times could reduce heterogeneity ( 58 ). In addition, screening for objective and valid biomarkers in the future would help to stratify diagnosis and reduce heterogeneity.
According to the keyword trend analysis of 100 highly cited documents, the genetic risk of autism was determined as the hot focus of research, and immune dysregulation and gut microbiome are the new development frontiers after 2015. Patients with ASD have altered immune function, microglia activation was observed in postmortem brain samples, and increased production of inflammatory cytokines and chemokines was observed in cerebrospinal fluid. The microglia are involved in synaptic pruning, and cytokines also affect neuronal migration and axonal projections ( 63 – 65 ). In addition, abnormal peripheral immune responses during pregnancy might affect the developing brain, increasing likelihood of autism ( 66 ). Several studies have pointed to abnormalities in immune-related genes in the brain and peripheral blood of autistic patients ( 51 , 67 , 68 ). Immune dysfunction is involved in the etiology of ASD and mediates the accompanying symptoms of autism. The patients have multiple immune-related diseases, asthma, allergic rhinitis, Crohn’s disease, and gastrointestinal dysfunction ( 69 – 71 ). Children with frequent gastrointestinal symptoms, such as abdominal pain, gas, constipation, or diarrhea, had pronounced social withdrawal and stereotyped behavior ( 70 – 72 ). Several studies suggested that these autism-related gastrointestinal problems might be related to intestinal microbiota composition ( 72 – 74 ). Accumulating evidence suggested that the microbiota-gut-brain axis influences human neurodevelopment, a complex system involving immune, metabolic, and vagal pathways in which bacterial metabolites directly affect the brain by disrupting the gut and blood–brain barrier ( 75 – 78 ). Fecal samples from children with autism contained high Clostridium species and low Bifidobacterium species ( 79 , 80 ). Probiotics can modulate gut microbiota structure and increase the relative abundance of Bifidobacteria , and clinical studies have shown that supplementation with probiotic strains improves attention problems in children with autism ( 81 , 82 ). Recent clinical trials have shown that microbiota transfer therapy improves gastrointestinal symptoms and autism-like behaviors in children with ASD ( 83 , 84 ).
This scientometric study comprehensively analyzes about a decade of global autism research. Research in the field of autism is increasing, with the United States making outstanding contributions, while neuroscience, genetics, brain imaging studies, or studies of the gut microbiome deepen our understanding of the disorder. The study of the brain-gut axis elucidates the mechanism of immunology in autism, and immunological research may be in the renaissance. The current data serve as a valuable resource for studying ASD. However, the future of autism needs further development. In the future, relevant research should be included for a complete representation of the entire autism population, and further collaboration between individuals, institutions, and countries is expected to accelerate the development of autism research.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary material , further inquiries can be directed to the corresponding authors.
Author contributions
MJ, DZ, JL, and LW conceived and designed the study. MJ, TL, XL, KY, and LZ contributed to data collection and data analysis. MJ wrote the original manuscript. DZ, JL, and LW revised the article and contributed to the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Key-Area Research and Development Program of Guangdong Province (2019B030335001) and the National Natural Science Foundation of China (grant numbers 82171537, 81971283, 82071541, and 81730037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1096769/full#supplementary-material
1. Newschaffer, CJ, Croen, LA, Daniels, J, Giarelli, E, Grether, JK, Levy, SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health . (2007) 28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007
CrossRef Full Text | Google Scholar
2. Christensen, DL, Braun, KVN, Baio, J, Bilder, D, Charles, J, Constantino, JN, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ . (2018) 65:1–23. doi: 10.15585/mmwr.ss6513a1
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Maenner, MJ, Shaw, KA, Bakian, AV, Bilder, DA, Durkin, MS, Esler, A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ . (2021) 70:1–16. doi: 10.15585/mmwr.ss7011a1
4. Green, RM, Travers, AM, Howe, Y, and McDougle, CJ. Women and autism Spectrum disorder: diagnosis and implications for treatment of adolescents and adults. Curr Psychiatry Rep . (2019) 21:22. doi: 10.1007/s11920-019-1006-3
5. Huang, Y, Arnold, SR, Foley, KR, and Trollor, JN. Diagnosis of autism in adulthood: a scoping review. Autism . (2020) 24:1311–27. doi: 10.1177/1362361320903128
6. Loomes, R, Hull, L, and Mandy, WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry . (2017) 56:466–74. doi: 10.1016/j.jaac.2017.03.013
7. Geschwind, DH. Genetics of autism spectrum disorders. Trends Cogn Sci . (2011) 15:409–16. doi: 10.1016/j.tics.2011.07.003
8. Landa, R, and Garrett-Mayer, E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry . (2006) 47:629–38. doi: 10.1111/j.1469-7610.2006.01531.x
9. Hyman, SL, Levy, SE, and Myers, SM. Council on children with disabilities SOD, behavioral P. identification, evaluation, and management of children with autism spectrum disorder. Pediatrics . (2020) 145:e20193447. doi: 10.1542/peds.2019-3447
10. Lord, C, Elsabbagh, M, Baird, G, and Veenstra-Vanderweele, J. Autism Spectrum disorder. Lancet . (2018) 392:508–20. doi: 10.1016/S0140-6736(18)31129-2
11. Zwaigenbaum, L, and Penner, M. Autism spectrum disorder: advances in diagnosis and evaluation. BMJ . (2018) 361:k1674. doi: 10.1136/bmj.k1674
12. Bottema-Beutel, K, Kapp, SK, Lester, JN, Sasson, NJ, and Hand, BN. Avoiding ableist language: suggestions for autism researchers. Autism Adulthood . (2021) 3:18–29. doi: 10.1089/aut.2020.0014
13. Simonoff, E, Pickles, A, Charman, T, Chandler, S, Loucas, T, and Baird, G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry . (2008) 47:921–9. doi: 10.1097/CHI.0b013e318179964f
14. Cooper, ID. Bibliometrics basics. J Med Libr Assoc . (2015) 103:217–8. doi: 10.3163/1536-5050.103.4.013
15. Chen, C, and Song, M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One . (2019) 14:e0223994. doi: 10.1371/journal.pone.0223994
16. Carmona-Serrano, N, Moreno-Guerrero, AJ, Marin-Marin, JA, and Lopez-Belmonte, J. Evolution of the autism literature and the influence of parents: a scientific mapping in web of science. Brain Sci . (2021) 11:74. doi: 10.3390/brainsci11010074
17. Rong, P, Fu, Q, Zhang, X, Liu, H, Zhao, S, Song, X, et al. A bibliometrics analysis and visualization of autism spectrum disorder. Front Psychol . (2022) 13:884600. doi: 10.3389/fpsyt.2022.884600
18. Wang, K, Duan, W, Duan, Y, Yu, Y, Chen, X, Xu, Y, et al. A bibliometric insight of genetic factors in Asd: emerging trends and new developments. Brain Sci . (2020) 11:33. doi: 10.3390/brainsci11010033
19. Wang, YX, Arora, R, Choi, Y, Chung, HW, Egorov, VI, Frahm, J, et al. Implications of web of science journal impact factor for scientific output evaluation in 16 institutions and investigators’ opinion. Quant Imaging Med Surg . (2014) 4:453–61. doi: 10.3978/j.issn.2223-4292.2014.11.16
20. Luo, X, Duan, H, and He, L. A novel riccati equation grey model and its application in forecasting clean energy. Energy . (2020) 205:118085. doi: 10.1016/j.energy.2020.118085
21. Yan, J, Li, Y, and Zhou, P. Impact of Covid-19 pandemic on the epidemiology of stds in China: based on the Gm (1,1) model. BMC Infect Dis . (2022) 22:519. doi: 10.1186/s12879-022-07496-y
22. Ahmad, T. Global research trends in Mers-Cov: a comprehensive bibliometric analysis from 2012 to 2021. Front Public Health . (2022) 10:933333. doi: 10.3389/fpubh.2022.933333
23. Hirsch, JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A . (2005) 102:16569–72. doi: 10.1073/pnas.0507655102
24. Eyre-Walker, A, and Stoletzki, N. The assessment of science: the relative merits of post-publication review, the impact factor, and the number of citations. PLoS Biol . (2013) 11:e1001675. doi: 10.1371/journal.pbio.1001675
25. van Eck, NJ, and Waltman, L. Software survey: Vosviewer, a computer program for bibliometric mapping. Scientometrics . (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
26. Chen, C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A . (2004) 101:5303–10. doi: 10.1073/pnas.0307513100
27. Xu, X, and Feng, C. Mapping the knowledge domain of the evolution of emergy theory: a bibliometric approach. Environ Sci Pollut Res Int . (2021) 28:43114–42. doi: 10.1007/s11356-021-14959-3
28. Flotte, TR. The science policy implications of a trump presidency. Hum Gene Ther . (2017) 28:1–2. doi: 10.1089/hum.2016.29037.trf
29. Gostin, LO. Government and science: the unitary executive versus freedom of scientific inquiry. Hast Cent Rep . (2009) 39:11–2. doi: 10.1353/hcr.0.0114
30. Butrous, G. International cooperation to promote advances in medicine. Ann Thorac Med . (2008) 3:79–81. doi: 10.4103/1817-1737.41913
31. Watson, R. Developing countries need stronger ethical guidelines on research. BMJ . (2007) 334:1076. doi: 10.1136/bmj.39220.615127.DB
32. Luyster, R, Gotham, K, Guthrie, W, Coffing, M, Petrak, R, Pierce, K, et al. The autism diagnostic observation schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord . (2009) 39:1305–20. doi: 10.1007/s10803-009-0746-z
33. Zheng, S, Kaat, A, Farmer, C, Kanne, S, Georgiades, S, Lord, C, et al. Extracting latent subdimensions of social communication: a cross-measure factor analysis. J Am Acad Child Adolesc Psychiatry . (2021) 60:768–782.e6. doi: 10.1016/j.jaac.2020.08.444
34. Autism Spectrum Disorders Working Group of The Psychiatric Genomics C. Meta-analysis of Gwas of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism . (2017) 8:21. doi: 10.1186/s13229-017-0137-9
35. Tick, B, Bolton, P, Happe, F, Rutter, M, and Rijsdijk, F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry . (2016) 57:585–95. Epub 2015/12/29. doi: 10.1111/jcpp.12499
36. Sanders, SJ, He, X, Willsey, AJ, Ercan-Sencicek, AG, Samocha, KE, Cicek, AE, et al. Insights into autism Spectrum disorder genomic architecture and biology from 71 risk loci. Neuron . (2015) 87:1215–33. doi: 10.1016/j.neuron.2015.09.016
37. Pinto, D, Delaby, E, Merico, D, Barbosa, M, Merikangas, A, Klei, L, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet . (2014) 94:677–94. doi: 10.1016/j.ajhg.2014.03.018
38. Cross-Disorder Group of the Psychiatric Genomics C Lee, SH, Ripke, S, Neale, BM, Faraone, SV, Purcell, SM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide Snps. Nat Genet . (2013) 45:984–94. doi: 10.1038/ng.2711
39. Baron-Cohen, S, Wheelwright, S, Skinner, R, Martin, J, and Clubley, E. The autism-spectrum quotient (Aq): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord . (2001) 31:5–17. doi: 10.1023/a:1005653411471
40. Grove, R, Baillie, A, Allison, C, Baron-Cohen, S, and Hoekstra, RA. The latent structure of cognitive and emotional empathy in individuals with autism, first-degree relatives and typical individuals. Mol Autism . (2014) 5:42. doi: 10.1186/2040-2392-5-42
41. Lai, MC, Lerch, JP, Floris, DL, Ruigrok, AN, Pohl, A, Lombardo, MV, et al. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res . (2017) 95:380–97. doi: 10.1002/jnr.23948
42. Ruigrok, AN, Salimi-Khorshidi, G, Lai, MC, Baron-Cohen, S, Lombardo, MV, Tait, RJ, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev . (2014) 39:34–50. doi: 10.1016/j.neubiorev.2013.12.004
43. Baron-Cohen, S, Ring, HA, Bullmore, ET, Wheelwright, S, Ashwin, C, and Williams, SC. The amygdala theory of autism. Neurosci Biobehav Rev . (2000) 24:355–64. doi: 10.1016/s0149-7634(00)00011-7
44. Greenberg, DM, Warrier, V, Allison, C, and Baron-Cohen, S. Testing the empathizing-systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proc Natl Acad Sci U S A . (2018) 115:12152–7. doi: 10.1073/pnas.1811032115
45. Baron-Cohen, S, Cassidy, S, Auyeung, B, Allison, C, Achoukhi, M, Robertson, S, et al. Attenuation of typical sex differences in 800 adults with autism Vs. 3,900 controls. PLoS One . (2014) 9:e102251. doi: 10.1371/journal.pone.0102251
46. Neuhaus, E, Beauchaine, TP, and Bernier, R. Neurobiological correlates of social functioning in autism. Clin Psychol Rev . (2010) 30:733–48. doi: 10.1016/j.cpr.2010.05.007
47. Young, AM, Chakrabarti, B, Roberts, D, Lai, MC, Suckling, J, and Baron-Cohen, S. From molecules to neural morphology: understanding neuroinflammation in autism spectrum condition. Mol Autism . (2016) 7:9. doi: 10.1186/s13229-016-0068-x
48. Courchesne, E, Campbell, K, and Solso, S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res . (2011) 1380:138–45. doi: 10.1016/j.brainres.2010.09.101
49. Ohnishi, T, Matsuda, H, Hashimoto, T, Kunihiro, T, Nishikawa, M, Uema, T, et al. Abnormal regional cerebral blood flow in childhood autism. Brain . (2000) 123:1838–44. doi: 10.1093/brain/123.9.1838
50. Wegiel, J, Kuchna, I, Nowicki, K, Imaki, H, Wegiel, J, Marchi, E, et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol . (2010) 119:755–70. doi: 10.1007/s00401-010-0655-4
51. Voineagu, I, Wang, X, Johnston, P, Lowe, JK, Tian, Y, Horvath, S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature . (2011) 474:380–4. doi: 10.1038/nature10110
52. Bernier, R, Golzio, C, Xiong, B, Stessman, HA, Coe, BP, Penn, O, et al. Disruptive Chd8 mutations define a subtype of autism early in development. Cells . (2014) 158:263–76. doi: 10.1016/j.cell.2014.06.017
53. Krumm, N, Turner, TN, Baker, C, Vives, L, Mohajeri, K, Witherspoon, K, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet . (2015) 47:582–8. doi: 10.1038/ng.3303
54. Gilman, SR, Iossifov, I, Levy, D, Ronemus, M, Wigler, M, and Vitkup, D. Rare De novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron . (2011) 70:898–907. doi: 10.1016/j.neuron.2011.05.021
55. Naaijen, J, Bralten, J, Poelmans, G, consortium I, Glennon, JC, Franke, B, et al. Glutamatergic and gabaergic gene sets in attention-deficit/hyperactivity disorder: association to overlapping traits in Adhd and autism. Transl Psychiatry . (2017) 7:e999. doi: 10.1038/tp.2016.273
56. Trevino, AE, Muller, F, Andersen, J, Sundaram, L, Kathiria, A, Shcherbina, A, et al. Chromatin and gene-regulatory dynamics of the developing human cerebral cortex at single-cell resolution. Cells . (2021) 184:5053–5069.e23. doi: 10.1016/j.cell.2021.07.039
57. Guinchat, V, Thorsen, P, Laurent, C, Cans, C, Bodeau, N, and Cohen, D. Pre-, Peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand . (2012) 91:287–300. doi: 10.1111/j.1600-0412.2011.01325.x
58. Lombardo, MV, Lai, MC, and Baron-Cohen, S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry . (2019) 24:1435–50. doi: 10.1038/s41380-018-0321-0
59. Mottron, L. A radical change in our autism research strategy is needed: back to prototypes. Autism Res . (2021) 14:2213–20. doi: 10.1002/aur.2494
60. Jeste, SS, and Geschwind, DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol . (2014) 10:74–81. doi: 10.1038/nrneurol.2013.278
61. Reichow, B, Hume, K, Barton, EE, and Boyd, BA. Early intensive behavioral intervention (Eibi) for young children with autism spectrum disorders (Asd). Cochrane Database Syst Rev . (2018) 5:CD009260. doi: 10.1002/14651858.CD009260.pub3
62. Lord, C, Charman, T, Havdahl, A, Carbone, P, Anagnostou, E, Boyd, B, et al. The lancet commission on the future of care and clinical research in autism. Lancet . (2022) 399:271–334. doi: 10.1016/S0140-6736(21)01541-5
63. Vargas, DL, Nascimbene, C, Krishnan, C, Zimmerman, AW, and Pardo, CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol . (2005) 57:67–81. doi: 10.1002/ana.20315
64. Bessis, A, Bechade, C, Bernard, D, and Roumier, A. Microglial control of neuronal death and synaptic properties. Glia . (2007) 55:233–8. doi: 10.1002/glia.20459
65. Onore, C, Careaga, M, and Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun . (2012) 26:383–92. doi: 10.1016/j.bbi.2011.08.007
66. Patterson, PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res . (2009) 204:313–21. doi: 10.1016/j.bbr.2008.12.016
67. Filosi, M, Kam-Thong, T, Essioux, L, Muglia, P, Trabetti, E, Spooren, W, et al. Transcriptome signatures from discordant sibling pairs reveal changes in peripheral blood immune cell composition in autism spectrum disorder. Transl Psychiatry . (2020) 10:106. doi: 10.1038/s41398-020-0778-x
68. Glatt, SJ, Tsuang, MT, Winn, M, Chandler, SD, Collins, M, Lopez, L, et al. Blood-based gene expression signatures of infants and toddlers with autism. J Am Acad Child Adolesc Psychiatry . (2012) 51:934–944.e2. doi: 10.1016/j.jaac.2012.07.007
69. Kohane, IS, McMurry, A, Weber, G, MacFadden, D, Rappaport, L, Kunkel, L, et al. The co-morbidity burden of children and Young adults with autism spectrum disorders. PLoS One . (2012) 7:e33224. doi: 10.1371/journal.pone.0033224
70. Needham, BD, Adame, MD, Serena, G, Rose, DR, Preston, GM, Conrad, MC, et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry . (2021) 89:451–62. doi: 10.1016/j.biopsych.2020.09.025
71. Robinson-Agramonte, MLA, Noris Garcia, E, Fraga Guerra, J, Vega Hurtado, Y, Antonucci, N, Semprun-Hernandez, N, et al. Immune dysregulation in autism spectrum disorder: what do we know about it? Int J Mol Sci . (2022) 23:3033. doi: 10.3390/ijms23063033
72. Chaidez, V, Hansen, RL, and Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord . (2014) 44:1117–27. doi: 10.1007/s10803-013-1973-x
73. Strati, F, Cavalieri, D, Albanese, D, De Felice, C, Donati, C, Hayek, J, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome . (2017) 5:24. doi: 10.1186/s40168-017-0242-1
74. Hsiao, EY, McBride, SW, Hsien, S, Sharon, G, Hyde, ER, McCue, T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cells . (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
75. Cryan, JF, O’Riordan, KJ, Cowan, CSM, Sandhu, KV, Bastiaanssen, TFS, Boehme, M, et al. The microbiota-gut-brain Axis. Physiol Rev . (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
76. Dinan, TG, and Cryan, JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol . (2017) 595:489–503. doi: 10.1113/JP273106
77. Fiorentino, M, Sapone, A, Senger, S, Camhi, SS, Kadzielski, SM, Buie, TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism . (2016) 7:49. doi: 10.1186/s13229-016-0110-z
78. Srikantha, P, and Mohajeri, MH. The possible role of the microbiota-gut-brain-Axis in autism Spectrum disorder. Int J Mol Sci . (2019) 20:2115. doi: 10.3390/ijms20092115
79. De Angelis, M, Piccolo, M, Vannini, L, Siragusa, S, De Giacomo, A, Serrazzanetti, DI, et al. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One . (2013) 8:e76993. doi: 10.1371/journal.pone.0076993
80. Finegold, SM, Dowd, SE, Gontcharova, V, Liu, C, Henley, KE, Wolcott, RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe . (2010) 16:444–53. doi: 10.1016/j.anaerobe.2010.06.008
81. Duque, A, Demarqui, FM, Santoni, MM, Zanelli, CF, Adorno, MAT, Milenkovic, D, et al. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food Res Int . (2021) 149:110657. doi: 10.1016/j.foodres.2021.110657
82. Grimaldi, R, Cela, D, Swann, JR, Vulevic, J, Gibson, GR, Tzortzis, G, et al. In vitro fermentation of B-Gos: impact on Faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol Ecol . (2017) 93:fiw233. doi: 10.1093/femsec/fiw233
83. Kang, DW, Adams, JB, Gregory, AC, Borody, T, Chittick, L, Fasano, A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome . (2017) 5:10. doi: 10.1186/s40168-016-0225-7
84. Li, N, Chen, H, Cheng, Y, Xu, F, Ruan, G, Ying, S, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol . (2021) 11:759435. doi: 10.3389/fcimb.2021.759435
Keywords: autism spectrum disorder, bibliometric study, CiteSpace, VOSviewer, research frontiers
Citation: Jiang M, Lu T, Yang K, Li X, Zhao L, Zhang D, Li J and Wang L (2023) Autism spectrum disorder research: knowledge mapping of progress and focus between 2011 and 2022. Front. Psychiatry . 14:1096769. doi: 10.3389/fpsyt.2023.1096769
Received: 16 November 2022; Accepted: 10 April 2023; Published: 25 April 2023.
Reviewed by:
Copyright © 2023 Jiang, Lu, Yang, Li, Zhao, Zhang, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, [email protected] ; Lifang Wang, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- May 18, 2024 | Discover How MIT’s SuperLimbs Help Astronauts Stand Tall on the Moon
- May 18, 2024 | Toxic Downpour: “Forever Chemicals” Rain on All Five Great Lakes
- May 18, 2024 | New UCLA Research Reveals That Practice Significantly Rewires the Brain
- May 18, 2024 | Scientists Discover Potential Opioid Replacement for Back Pain
- May 18, 2024 | Termites on Tour: How Climate Change Is Bringing Pests to Your Doorstep
What Causes Autism? New Research Uncovers a Key Factor in Brain Development
By Texas A&M University College of Medicine July 31, 2022
The findings of this research reveal a significant component in the underlying causes of neural tube birth defects, intellectual disabilities, and autism risk.
Researchers from Texas A&M College of Medicine have provided answers to important questions concerning how the neocortex develops, providing new information about the root causes of intellectual disabilities.
A significant advancement in our understanding of how the brain develops has been accomplished by researchers at Texas A&M University College of Medicine . This new research advances our understanding of how the region of the brain that distinguishes humans from other animals develops and sheds light on what causes intellectual disabilities, such as autism spectrum disorders.
For many years, scientists have recognized a significant relationship between mammalian intelligence and a thin layer of cells in the neocortex, the region of the brain that governs higher-order processes like cognition, perception, and language. The neocortex’s surface area reflects how highly developed an organism’s mental ability is. For instance, the human neocortex is only around three times thicker than the mouse equivalent. However, the human neocortex has a 1,000-fold larger surface area than that of mice. Autism spectrum disorders and intellectual impairments are among the developmental deficiencies caused by malformations in this region of the brain.
What is unknown is how evolutionary expansion of this section of the brain happens selectively in favor of growing the neocortex’s surface area at the cost of increasing its thickness. An important aspect of this process is how the initial populations of neural stem cells, which serve as the brain’s building blocks, distribute themselves.
“There are many, what we’ll call, individual processing units that are horizontally arranged in the neocortex. The more surface area you have, the more of these processing units you can accommodate,” said Vytas A. Bankaitis, Distinguished Professor at the College of Medicine, E.L. Wehner-Welch Foundation Chair in Chemistry, and co-author of this study, which was published in Cell Reports . “The question is, why is the neocortical surface area so much greater relative to its thickness as one climbs up the mammalian evolutionary tree? Why do neural stem cells spread themselves in a lateral direction as they proliferate and not pile on top of each other?”
This question is key because when the cells do not spread out, but instead pile up, it creates a thicker neocortex with a smaller surface area — a characteristic that has been observed in cases of intellectual disability and even autism.
“One of the most studied genetic causes of intellectual disability is a mutation in a gene that was originally called LIS1,” said Zhigang Xie, assistant professor at the College of Medicine and co-author of the study. “This genetic mutation will cause a smooth brain, which is associated with intellectual disability. And one typical observation is that the neocortex of the patient is thicker than normal. There are also very recent studies that identify common differences in the brain of autism that include abnormally thickened regions of the neocortex in those individuals.”
Scientists have known for some time that as neural stem cells divide, their nuclei move up and down within their anatomical space as a function of the cell cycle, a process called interkinetic nuclear migration. They do so by employing a cytoskeletal network that acts like train tracks with engines that move the nuclei up or down in a closely regulated manner. Although several ideas have been proposed, it remains an enigma why the nuclei move in this way, how this network of train tracks is controlled, and what role interkinetic nuclear migration plays in development of the neocortex.
In their study, Xie and Bankaitis provide answers to these questions.
As for why, Bankaitis explains that when there are so many cells so close together in the embryonic stage of neocortical development, the movement of their nuclei up and down causes opposing upward and downward forces that spreads the dividing neural stem cells out.
“Think about a tube of toothpaste,” Bankaitis said. “If you were to take that toothpaste tube, put it between your hands, push up from the bottom and push down from the top, what would happen? It would flatten and spread out. That’s essentially how this works. You have an upward force and a downward force caused by the movement of the nuclei that spreads these cells out.”
Xie and Bankaitis also demonstrate how the cells do this by linking together several distinct pathways that cooperate to “tell” the newborn neural stem cells where to go.
“I think for the first time, this really puts together molecules and signaling pathways that indicate how this process is controlled and why it would be linked or associated with neurodevelopmental deficiencies,” Bankaitis said. “We have taken a biochemical pathway, linked it to a cell biological pathway, and linked it to a signaling pathway that talks to the nucleus to promote the nuclear behavior that generates a force that develops a complicated brain. It’s now a complete circuit.”
The results of this study uncover an important factor in the underlying causes of autism risk, intellectual disabilities and neural tube birth defects. The new knowledge on the basic principles regulating the shape of the neocortex will also help the design of in vitro brain culture systems that more accurately reflect the developmental processes of interest and improve the prospects for neurological drug development.
“While there might prove to be many reasons why a neocortex thickens instead of spreads, our work provides a new perspective on why patients with autism and intellectual disabilities often display a thicker cortex,” Xie said. “The fact that the LIS1 gene product is a core regulator of nuclear migration, including the interkinetic nuclear migration that we study in this work, supports the conclusions we reach in this paper.”
Reference: “Phosphatidylinositol transfer protein/planar cell polarity axis regulates neocortical morphogenesis by supporting interkinetic nuclear migration” by Zhigang Xie and Vytas A. Bankaitis, 31 May 2022, Cell Reports. DOI: 10.1016/j.celrep.2022.110869
The study was funded by the NIH/ National Institutes of Health and the Robert A Welch Foundation.
More on SciTechDaily

UC Davis Researchers Identify a Biomarker for Autism

Using Blood To Uncover the Secrets of One Type of Autism

This Key Protein Is Essential for Brain Cell Longevity and Growth
Does Autism Begin in the Womb? Research Breakthrough May Lead to New Treatment Strategies
Overgrowth of Key Brain Structure Identified in Babies Who Later Develop Autism
Researchers Discover Human-Specific Gene for Building a Bigger Brain
New Stanford Research Shows Differences Between Brains of Girls and Boys With Autism

Neuroscientists Built an Ultra Detailed Map of the Brain Motor Cortex, From Mice to Monkeys to Humans
17 comments on "what causes autism new research uncovers a key factor in brain development".
I just wonder how does the thicker neucortex in Humans and other mammals collate to the Âûtistic and Co-morbidity genes that we Neurodiverse persons have and explain brighter hundreds of percent more almost hyperactivity in Right hemisphere, Tempora, Frontal lobes and Amygdala, compared to greyzones and work around in reduced activity left hemisphere and the thousands of Autism Genome Project Autistic, Co-morbidity and mutations in a few million Autistic and Co-morbidity confirmed participants recorded as 1 in 5.8 and 1 in 5.9 in Denmark and Sweden by cottaging of many multiple traits, Co-morbidities and coping mechanisms.
Autism isn’t a disability. If autism is a disability, thing so is womanhood.
Ok, Peter. Once you figure out what causes the disability in those identified autistic then you can make that declaration. Until then, and within the household I exist in, there are strengths in the identity, but in the same vein, there are also several disabling aspects as well. Hence it IS a disability. When people make bold statements like this, they are slowly creating a reality where supports could be taken away to those most profoundly affected since they are trying to sell the idea that “it’s not a disability.”
Tylenol is currently being called out for knowing that their drug causes autism and not releasing those findings.
While plenty of autistic people are disabled I’m not sure we can really say autism is a disability. It’s certainly not an intellectual disability. There is a high co-morbidity with other disorders, some of which are more of a disability than others but there are plenty of autistic people who are no less capable than any autistic person. Sometimes the same traits that appear to be a disability in some situations can be an advantage in others. My son is very sensitive to sound, he gets overwhelmed easily and I can see how and why that’s perceived as a disability. However, I have the same sensitivity and, while it was stressful and difficult in childhood, as an adult I have a great ear for subtle differences in sound. That’s an advantage in music and vocal impressions, it also allows me to identify what my children are doing without looking at them. I know the sound of pretty much every toy and surface. So perhaps suitability to environment varies and it’s not so clear cut as disability/not a disability. This can apply to traits that no one calls disabilities too. Some people love cold weather, I can’t function in cold weather at all but I’m not uncomfortable at 90 degrees. My lack of tolerance to the cold isn’t a disability but it does make me unsuited to a cold environment. In the same way we could say that autistic children who have meltdowns in chaotic environments are simply unsuited to noisier, more urban environments. Without a significant improvement of our understanding of what exactly autism is, how it works, where to draw the line between autism and its comorbidities, I suppose it’s open to debate. As a side note, when someone says “autism isn’t a disability”, it might be worth considering that they could be autistic and not see themselves as disabled, or the parent of an autistic child who doesn’t find the experience disabling. I am autistic and don’t consider myself disabled. I have two children who have been diagnosed with autism, one of whom is non-verbal. Those diagnoses don’t guarantee disability payments in and of themselves in my corner of the world anyway.
“plenty of autistic people who are no less capable than any allistic person.” Autocorrect doesn’t know the word allistic aparently.
How can we trust any research coming out of Texas a&m when they allow such egregious conflicts of interest in their programs?
Doing beef research and taking donations from organizations connected to the beef industry, and then not reporting it in their research?
They better get their house in order or might as well shut down the school.
Reading this make more questions, why does it affect more boys? and why is it that we are seeing so many cases? This finding is great but we need to find out the cause enviro, consumption foods products? We have 2-3% of Canadians are affected by this diagnosis.
My IQ is 135. I have hyperlexia with full reading comprehension. I have read a lot of credible medical literature which states that Autism/ASD is a neurodevelopmental disorder, not an intellectual disorder.
Autism isn’t an intellectual disability. The first paragraph says “… and sheds light on what causes intellectual disabilities, such as autism spectrum disorders”. The rest of the article uses “intellectual disabilities and autism” which accurately reflects the research findings. This needs to be edited as makes the authors appear ignorant to anyone who knows anything about autism.
Try tô find reasons of autism in vaccination.
As someone who can or at least say I am not autistic not that I have been told by a Dr so please read this as a possibly dumb by honest question anyway but my best friend has a son that is autistic or has a form of it possibly we are close and watched him as he grew up and I noticed some of the things about him seems like habits ,disliking change and not understanding sarcasm so I guess my question is was he born autistic because his sister and his brother are not was it in his genes or d.n.a.? Or can children develop autism threw outside factors?
While there may indeed be some truth to this, as I think there is, there is still much about it I am questioning. Yes, this research supposedly provides some possible answers, but there are also quite a few unanswered questions and vital missing pieces of this complicated “intellectual disability” puzzle. Which for the record, is incorrectly titled since Autism is in fact NOT technically considered an intellectual disability, but instead a neurological one. And not even a disability. It’s labeled a disorder. And many people would argue with that. But for time, sanity, and technicality sake, it’s a disorder. Regardless, I wish I had more hope in this but if the people doing this work are comparing and calling Autism the same as intellectual disabilities…well, let’s just say i have my doubts.
This was a great article. Hats off to the team finding these correlations and observations. These are the findings that lead us in directions that hold answers.
It’s so sad to read these comments. Questioning these scientific finding because of the funding channels uses as an o stitution..situation… arbitrarily saying that autism shouldn’t be considered a disability because of personal observations of qualitative factors is mind blowing. It’s as though there is an entire wave of people who are not being taught how anything on the world actually works. Or why. Clearly discernment has left the building.
If one wants to protect the status quo of autism, I just can’t begin to fathom why but thats not important, which is something alot of you could learn, but back to the issue, if you convince the world over that autism should not be looked at as a disabilty then you better take a long hard look at yourself in the mirror and ask yourself a few questions: 1. Do you have any business voicing opinions like that on platforms that are far reaching? Let me translate for the younger crowd… do you have any scientific/educational/vocational credentials that support your words as anything more than an opinion or thought? If so, pipe down. 2. If your message hits and the world over decides it is not a disabilityany more, research will cease. Do I understand the power of change? Have I performed efficacy studies that support making this change or am I only doing this for the fluffy feelings Inside me?
Honestly, I read about all these school shootings and I’m always surprised when I find out the shooter WASNT a teacher.
Good luck to those of you who are unknowingly barking for research, similar to that performed in this article, to stop. You’re the real disability in the world. You’ve convinced us all.
My son was born in 2009. Extra genetic material on line 16, tourette’s syndrome followed at age 3, ADHD, OCD, and lastly behavioral problems. No doctor out of the 20+ doctors we seen knew how to treat or help him. I am sad daily as I had to place him in his father’s care. I have 6 children under 12yo. He would look for any opportunity to get too one of his sister’s and beat them until he seen blood. He would laugh and think it was so cool. I wish I knew what went so wrong.
I am a 73 year old recently retired physician, very successful in my 45 year career. As my brain has gradually reaclimated to “normal” life after 45 years on constant “high alert”, I have noticed a reemergence of intellectual, cognitive and social patterns of function which are very suggestive of ASD, and which were evidently unlearned during my decades of acquired “normal” behavior. Throughout my life I have dealt with often awkward and cringy social abilities. Many professional, personal and romantic relationships suffered from my unique ability to sabotage them. Since I come from a family in which nearly all males of one lineage (my paternal grandfather’s) are clearly on the spectrum, I’ve realized, for the first time that I am as well. A few of these males are/were severely disabled, but much more frequently they are like me — reasonably social but with an awkwardness which is counterbalanced by savant abilities which allow us to reach high levels of mastery in fields like medicine and teaching. All of us seem to have high levels of social justice awareness, which only seems to add to our perceived level of occupational success. It is becoming clear to me, looking back on my family’s four generations of approximately 90% incidence of ASD among the men, that this persistence of a high incidence of ASD would not occur unless the autistic spectrum disorders are a normal variant of human intellectual and social development. In other words, ASD must be normal-ish, must have served some sort of social purpose during the Ice Age, for them to persist this long. It’s not hard to imagine there being an advantage for a tribe to include a member who thinks somewhat like game animals, a la Temple Grandin; or a member like myself who appears to have an ability to see connections between seemingly unrelated abstractions; or a member who sees off-kilter dream-like narrative explanations for disturbing events in the environment — proto- myths and stories. It may be that our abilities are not needed since the evolution of culture, or perhaps these functions have been assumed and performed by new agents within the culture. Whatever, it’s clear to me, at least, that the ASD are not disorders, developmental abberrations or illnesses. Our oddball abilities should be cultivated, while we are taught how to work around our cringiness.
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Transl Pediatr
- v.9(Suppl 1); 2020 Feb
Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation
Holly hodges.
1 Department of Pediatrics, Baylor College of Medicine and Meyer Center for Developmental Pediatrics, Texas Children’s Hospital, Houston, TX, USA;
Casey Fealko
2 Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, MI, USA;
Neelkamal Soares
3 Department of Pediatric and Adolescent Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, MI, USA
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors. There have been recent concerns about increased prevalence, and this article seeks to elaborate on factors that may influence prevalence rates, including recent changes to the diagnostic criteria. The authors review evidence that ASD is a neurobiological disorder influenced by both genetic and environmental factors affecting the developing brain, and enumerate factors that correlate with ASD risk. Finally, the article describes how clinical evaluation begins with developmental screening, followed by referral for a definitive diagnosis, and provides guidance on screening for comorbid conditions.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors ( 1 ). In 2013, the Diagnostic and Statistical Manual of Mental Disorders —5 th edition (DSM-5) was published, updating the diagnostic criteria for ASD from the previous 4 th edition (DSM-IV) ( Table 1 ) ( 1 , 2 ).
ASD, autism spectrum disorder; SPCD, social (pragmatic) communication disorder.
In DSM-5, the concept of a “spectrum” ASD diagnosis was created, combining the DSM-IV’s separate pervasive developmental disorder (PDD) diagnoses: autistic disorder, Asperger’s disorder, childhood disintegrative disorder, and pervasive developmental disorder not otherwise specified (PDD-NOS), into one. Rett syndrome is no longer included under ASD in DSM-5 as it is considered a discrete neurological disorder. A separate social (pragmatic) communication disorder (SPCD) was established for those with disabilities in social communication, but lacking repetitive, restricted behaviors. Additionally, severity level descriptors were added to help categorize the level of support needed by an individual with ASD.
This new definition is intended to be more accurate and works toward diagnosing ASD at an earlier age ( 3 ). However, studies estimating the potential impact of moving from the DSM-IV to the DSM-5 have predicted a decrease in ASD prevalence ( 4 , 5 ) and there has been concern that children with a previous PDD-NOS diagnosis would not meet criteria for ASD diagnosis ( 5 - 7 ). There are varying reports estimating the extent of and effects of this change. One study found that with parental report of ASD symptoms alone, the DSM-5 criteria identified 91% of children with clinical DSM-IV PDD diagnoses ( 8 ). However, a systematic review suggests only 50% to 75% of individuals maintain diagnoses ( 9 ) and other studies have also suggested a decreased rate of diagnosis of individuals with ASD under the DSM-5 criteria ( 10 ). Often those who did not meet the requirements were previously classified as high functioning Asperger’s syndrome and PDD-NOS ( 11 , 12 ). Overall, most studies suggest that the DSM-5 provides increased specificity and decreased sensitivity compared to the DSM-IV ( 5 , 13 ); so while those diagnosed with ASD are more likely to have the condition, there is a higher number of children whose ASD diagnosis is missed, particularly older children, adolescents, adults, or those with a former diagnosis of Asperger’s disorder or PDD-NOS ( 14 ). Nevertheless, the number of people who would be diagnosed under the DSM-IV, but not under the new DSM-5 appears to be declining over time, likely due to increased awareness and better documentation of behaviors ( 4 ).
It has yet to be determined how the new diagnosis of SPCD will impact the prevalence of ASD. One study found the new SPCD diagnosis encompasses those individuals who possess subthreshold autistic traits and do not qualify for a diagnosis of ASD, but who still have substantial needs ( 15 ). Furthermore, children who previously met criteria for PDD-NOS under the DSM-IV might now be diagnosed with SPCD.
Epidemiology
The World Health Organization (WHO) estimates the international prevalence of ASD at 0.76%; however, this only accounts for approximately 16% of the global child population ( 16 ). The Centers for Disease Control and Prevention (CDC) estimates about 1.68% of United States (US) children aged 8 years (or 1 in 59 children) are diagnosed with ASD ( 6 , 17 ). In the US, parent-reported ASD diagnoses in 2016 averaged slightly higher at 2.5% ( 18 ). The prevalence of ASD in the US more than doubled between 2000–2002 and 2010–2012 according to Autism and Developmental Disabilities Monitoring Network (ADDM) estimates ( 6 ). Although it may be too early to comment on trends, in the US, the prevalence of ASD has appeared to stabilize with no statistically significant increase from 2014 to 2016 ( 19 ). Changing diagnostic criteria may impact prevalence and the full impact of the DSM-5 diagnostic criteria has yet to be seen ( 17 ).
Insurance mandates requiring commercial plans to cover services for ASD along with improved awareness have likely contributed to the increase in ASD prevalence estimates as well as the increased diagnosis of milder cases of ASD in the US ( 6 , 20 , 21 ). While there was only a modest increase in prevalence immediately after the mandates, there have been additional increases later as health care professionals better understood the regulatory and reimbursement process. The increase in prevalence may also be due to changes in reporting practices. One study in Denmark found the majority of increase in ASD prevalence from 1980–1991 was based on changes of diagnostic criteria and inclusion of outpatient data, rather than a true increase in ASD prevalence ( 21 ).
ASD occurs in all racial, ethnic, and socioeconomic groups, but its diagnosis is far from uniform across these groups. Caucasian children are consistently identified with ASD more often than black or Hispanic children ( 6 ). While the differences appear to be decreasing, the continued discrepancy may be due to stigma, lack of access to healthcare services, and a patient’s primary language being one other than English.
ASD is more common in males ( 22 , 23 ) but in a recent meta-analysis ( 24 ), true male-to-female ratio is closer to 3:1 than the previously reported 4:1, though this study was not done using the DSM-5 criteria. This study also suggested that girls who meet criteria for ASD are at higher risk of not receiving a clinical diagnosis. The female autism phenotype may play a role in girls being misdiagnosed, diagnosed later, or overlooked. Not only are females less likely to present with overt symptoms, they are more likely to mask their social deficits through a process called “camouflaging”, further hindering a timely diagnosis ( 25 ). Likewise, gender biases and stereotypes of ASD as a male disorder could also hamper diagnoses in girls ( 26 ).
Several genetic diagnoses have an increased rate of co-occurring ASD compared to the average population, including fragile X, tuberous sclerosis, Down syndrome, Rett syndrome, among others; however, these known genetic disorders account for a very small amount of overall ASD cases ( 27 - 30 ). Studies of children with sex chromosome aneuploidy describe a specific social functioning profile in males that suggests more vulnerability to autism ( 22 , 23 , 31 , 32 ). With the increased use of chromosomal microarray, several sites (chromosome X, 2, 3, 7, 15, 16, 17, and 22 in particular) have proven to be associated with increased ASD risk ( 28 ).
Other risk factors for ASD include increased parental age and prematurity ( 33 - 35 ). This could be due to the theory that older gametes have a higher probability of carrying mutations which could result in additional obstetrical complications, including prematurity ( 36 ).
ASD is a neurobiological disorder influenced by both genetic and environmental factors affecting the developing brain. Ongoing research continues to deepen our understanding of potential etiologic mechanisms in ASD, but currently no single unifying cause has been elucidated.
Neuropathologic studies are limited, but have revealed differences in cerebellar architecture and connectivity, limbic system abnormalities, and frontal and temporal lobe cortical alterations, along with other subtle malformations ( 28 , 37 , 38 ). A small explorative study of neocortical architecture from young children revealed focal disruption of cortical laminar architecture in the majority of subjects, suggesting problems with cortical layer formation and neuronal differentiation ( 39 ). Brain overgrowth both in terms of cortical size and additionally in terms of increased extra-axial fluid have been described in children with ASD and are areas of ongoing study both in terms of furthering our understanding of its etiology, but also as a potential biomarker ( 40 , 41 ).
Genetic factors play a role in ASD susceptibility, with siblings of patients with ASD carrying an increased risk of diagnosis when compared to population norms, and a much higher, although not absolute, concordance of autism diagnosis in monozygotic twins ( 42 - 44 ).
Genome wide association studies and whole exome sequencing methods have broadened our understanding of ASD susceptibility genes, and learning more regarding the function of these genes can shed light on potential biologic mechanisms ( 45 ). For example candidate genes in ASD include those that play a role in brain development or neurotransmitter function, or genes that affect neuronal excitability ( 46 , 47 ). Many of the genetic defects associated with ASD encode proteins that are relevant at the neuronal synapse or that are involved in activity-dependent changes in neurons, including regulatory proteins such as transcription factors ( 42 , 48 ). Potential “networks” of ASD genetic risk convergence include pathways involved in neurotransmission and neuroinflammation ( 49 ). Transcriptional and splicing dysregulation or alterations in epigenetic mechanisms such as DNA methylation or histone acetylation and modification may play a role ( 42 , 49 - 51 ). A recent study describes 16 newly identified genes associated with ASD that raise new potential mechanisms including cellular cytoskeletal structure and ion transport ( 52 ). Ultimately, ASD remains one of the most genetically heterogeneous neuropsychiatric disorders with rarer de novo and inherited variants in over 700 genes ( 53 ).
While genetics clearly play a role in ASD’s etiology, phenotypic expression of genetic susceptibility remains extremely variable within ASD ( 54 ). Genetic risk may be modulated by prenatal, perinatal, and postnatal environmental factors in some patients ( 35 ). Prenatal exposure to thalidomide and valproic acid have been reported to increase risk, while studies suggest that prenatal supplements of folic acid in patients exposed to antiepileptic drugs may reduce risk ( 55 - 57 ). Research has not confirmed if a small positive trial of folinic acid in autism can be used to recommend supplementation more broadly ( 58 ). Advanced maternal and paternal age have both been shown to have an increased risk of having a child with ASD ( 59 ). Maternal history of autoimmune disease, such as diabetes, thyroid disease, or psoriasis has been postulated, but study results remain mixed ( 60 , 61 ). Maternal infection or immune activation during pregnancy is another area of interest and may be a potential risk factor according to recent investigations ( 62 - 65 ). Both shorter and longer inter-pregnancy intervals have also been reported to increase ASD risk ( 66 ). Infants born prematurely have been demonstrated to carry a higher risk for ASD in addition to other neurodevelopmental disorders ( 34 ). In a prior epidemiologic review, obstetric factors including uterine bleeding, caesarian delivery, low birthweight, preterm delivery, and low Apgar scores were reported to be the few factors more consistently associated with autism ( 67 ). A recent meta-analysis reported several pre, peri and postnatal risk factors that resulted in an elevated relative risk of ASD in offspring ( 35 ), but also revealed significant heterogeneity, resulting in an inability to make true determination regarding the importance of these factors.
Despite the hysteria surrounding the now retracted Lancet article first published in 1998, there is no evidence that vaccines, thimerosal, or mercury is associated with ASD ( 68 - 70 ). In the largest single study to date, there was not an increased risk after measles/mumps/rubella (MMR) vaccination in a nationwide cohort study of Danish children ( 70 ).
Ultimately, research continues to reveal factors that correlate with ASD risk, but no causal determinations have been made. This leaves much room for discovery with investigators continuing to elucidate new variants conveying genetic risk, or new environmental correlates that require further study ( 52 ).
Evaluation in ASD begins with screening of the general pediatric population to identify children at-risk or demonstrating signs suggestive of ASD, following which a diagnostic evaluation is recommended. The American Academy of Pediatrics (AAP) guidelines recommend developmental surveillance at 9, 15 and 30 months well child visits and autism specific screening at 18 months and again at 24 or 30 months ( 28 , 71 ). Early red flags for ASD include poor eye contact, poor response to name, lack of showing and sharing, no gesturing by 12 months, and loss of language or social skills. Screening tools for ASD in this population include the Modified Checklist for Autism in Toddlers, Revised, with Follow-up (M-CHAT-R/F) and Survey of Wellbeing of Young Children (SWYC) ( 72 , 73 ). Red flags in preschoolers may include limited pretend play, odd or intensely focused interests, and rigidity. School age children may demonstrate concrete or literal thinking, have trouble understanding emotions, and may even show an interest in peers but lack conversational skills or appropriate social approach. If there is suspicion of ASD in these groups, screening tools available include the Social Communication Questionnaire (SCQ), Social Responsiveness Scale (SRS), and Autism Spectrum Screening Questionnaire (ASSQ) ( 74 - 76 ).
If concerns are raised at screening, primary care clinicians are recommended to refer the child to early intervention if less than 3 years of age or to the public school system for psychoeducational evaluation in order to establish an individual education program (IEP) if the child is three years of age or older. Clinicians should additionally refer the child to a specialist (pediatric neurologist, developmental-behavioral pediatrician, child psychiatrist, licensed child psychologist) for a definitive diagnosis and comprehensive assessment ( 71 ). A comprehensive assessment should include a complete physical exam, including assessment for dysmorphic features, a full neurologic examination with head circumference, and a Wood’s lamp examination of the skin. A parent interview, collection of any outside informant observations, and a direct clinician observation of the child’s current cognitive, language, and adaptive functioning by a clinician experienced with ASD should be components of this comprehensive assessment. ( 28 , 71 , 77 , 78 ).
Additionally, primary care clinicians need to be aware of (and evaluate for) potential co-occurring conditions in children with ASD. According to a surveillance study of over 2,000 children with ASD, 83% had an additional developmental diagnosis, 10% had at least one psychiatric diagnosis, and 16% at least one neurologic diagnosis ( 79 ). In the past, rates of co-morbid intellectual disability (ID) in patients with ASD were reported from 50% to 70%, with the most recent CDC estimate reported at 31.0% (26.7% to 39.4%) with ID defined as intelligence quotient (IQ) ≤70 ( 6 , 80 ). Other common co-occurring medical conditions include gastrointestinal (GI) disorders, including dietary restrictions and food selectivity, sleep disorders, obesity, and seizures ( 81 - 84 ). Studies using electronic health record (EHR) analysis revealed prevalence of epilepsy ~20% and GI disorders [without inflammatory bowel disease (IBD)] at 10–12% ( 82 ). Epilepsy has been shown to have higher prevalence rates in ASD with comorbid ID and medical disorders of increased risk such as tuberous sclerosis complex (TSC) ( 85 - 87 ). GI disorders or GI symptomatology, including diarrhea, constipation, restrictive eating, or reflux, have been shown to be prominent in ASD across multiple studies ( 81 , 82 , 88 , 89 ). Sleep problems have been reported to occur in anywhere from 50% to 73% of patients with ASD with variation in prevalence dependent on the definition of sleep symptoms or the measurement tool used ( 90 - 92 ). Rates of overweight and obesity in ASD are reported to be roughly 33% and 18% respectively, higher than rates in typically developing children ( 81 - 84 , 93 ).
Other behavioral or psychiatric co-occurring conditions in ASD include anxiety, attention deficit/hyperactivity disorder (ADHD), obsessive compulsive disorder, and mood disorders or other disruptive behavior disorders ( 81 ). Rates of co-occurring ADHD are reported anywhere from 25% to 81% ( 81 , 94 ). A recent meta-analysis of 30 studies measuring rates of anxiety and 29 studies measuring rates of depression reported a high degree of heterogeneity from the current literature, but stated pooled lifetime prevalence for adults with ASD to be 42% for any anxiety disorder and 37% for any depressive disorder, though the use of self-report measures and the presence of ID could influence estimates ( 95 ). In children with ASD seeking treatment, the rate of any anxiety disorder was found to be similar at 42% and in addition this study reported co-morbid oppositional defiant disorder at a rate of 46% and mood disorders at 8%, with 66% of the sample of over 600 patients having more than one co-occurring condition ( 94 ).
Currently no clear ASD biomarkers or diagnostic measures exist, and the diagnosis is made based on fulfillment of descriptive criteria. In light of a relatively high yield in patients with ASD, clinical genetic testing is recommended and can provide information regarding medical interventions or work up that might be necessary and help with family planning ( 96 ). The American College of Medical Genetics and Genomics (ACMGG) guidelines currently recommend chromosomal microarray for all children, fragile X testing in males, and additional gene sequencing, including PTEN and MECP2 , in certain patients as first tier genetic testing in the work up of ASD ( 97 ). High resolution G-banded karyotype, once recommended for all patients with ASD, is no longer routinely indicated based on recent consensus recommendations, but might still be performed in patients with a family or reproductive history suggestive of chromosomal rearrangements or specific syndromes such as sex chromosome anomalies or Trisomy 21 ( 96 - 98 ). Several professional societies recommend genetic testing for ASD, including the American Academy of Neurology, the AAP, ACMGG, and the American Academy of Child and Adolescent Psychiatry, and a child may require further referral to a geneticist and/or genetic counselor, depending on results of testing ( 25 , 28 , 97 , 99 ). As the field of genetics continues to advance rapidly, recent publications suggest whole exome sequencing may become the preferred method for clinical genetic testing in individuals with ASD ( 100 , 101 ).
Aside from genetic testing, no other laboratory work up is routinely recommended for every patient with a diagnosis of ASD. However, further evaluation may be appropriate for patients with particular findings or risk factors. Metabolic work-up should be considered in patients with any of the following concerning symptoms or signs: a history of clear developmental regression including loss or plateau of motor skills; hypotonia; recurrent episodes of vomiting, lethargy or hypoglycemia; microcephaly or poor growth; concern for other organ involvement; coarse features; or concern for seizures or ataxia. Based on the patient’s history and presentation, components of a metabolic laboratory evaluation could include complete blood count (CBC), liver and renal function tests, lactate, pyruvate, carnitine, amino acids, an acylcarnitine profile, urine organic acids and/or urine glycosaminoglycans ( 97 , 102 ). Children with a history of pica should have a lead level measured ( 28 , 103 ). In a child with significantly restricted food intake, one should consider a laboratory evaluation of nutritional status. Sleep symptoms may warrant a referral for a possible sleep study, and if restless sleep symptoms are present, an evaluation for iron deficiency is not unreasonable, particularly if dietary rigidity limits iron intake ( 104 ).
Neuroimaging is not routinely recommended for every patient with ASD ( 28 , 99 ), but may be appropriate in patients with a suspicion for TSC or other neurocutaneous disorders, microcephaly, or an abnormal neurologic exam (spasticity, severe hypotonia, unilateral findings). Patients with suspected seizures should have an electroencephalography (EEG) obtained ( 102 ). If accessible, it might be appropriate to immediately refer children with concern for further genetic, metabolic or neurologic conditions to a specialist who can then obtain and interpret the aforementioned testing. At this time there is inadequate evidence to recommend routine testing for celiac disease, immunologic or neurochemical markers, mitochondrial disorders, allergy testing, hair analysis, intestinal permeability studies, erythrocyte glutathione peroxidase studies, stool analysis, urinary peptides or vitamin and mineral deficiencies without a history of severe food selectivity.
ASD is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors. Recent changes to the diagnostic criteria occurred with the transition to the new diagnostic manual (DSM-5) and will likely impact prevalence, which currently stands at 1 in 59 children in the US. ASD is a neurobiological disorder influenced by both genetic and environmental factors affecting the developing brain. Research continues to reveal factors that correlate with ASD risk and these findings may guide further etiologic investigation, but no final causal pathway has been elucidated. Clinical evaluation begins with developmental screening of the general pediatric population to identify at-risk children, followed by referral to a specialist for a definitive diagnosis and comprehensive neuropsychological assessment. Children with ASD should also be screened for common co-morbid diagnoses. While no clear biomarkers or diagnostic measures exist, clinical genetic testing is recommended as part of the initial medical evaluation. Further medical work up or subspecialist referrals may be pursued based on specific patient characteristics.
Acknowledgments
Funding: None.
Ethical Statement : The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest : The authors have no conflicts of interest to declare.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 16 January 2020
Autism spectrum disorder
- Catherine Lord 1 ,
- Traolach S. Brugha 2 ,
- Tony Charman 3 ,
- James Cusack 4 ,
- Guillaume Dumas 5 ,
- Thomas Frazier 6 ,
- Emily J. H. Jones 7 ,
- Rebecca M. Jones 8 , 9 ,
- Andrew Pickles 3 ,
- Matthew W. State 10 ,
- Julie Lounds Taylor 11 &
- Jeremy Veenstra-VanderWeele 12
Nature Reviews Disease Primers volume 6 , Article number: 5 ( 2020 ) Cite this article
45k Accesses
681 Citations
428 Altmetric
Metrics details
- Autism spectrum disorders
- Cognitive neuroscience
- Paediatrics
Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted interests and/or sensory behaviours beginning early in life. The worldwide prevalence of autism is just under 1%, but estimates are higher in high-income countries. Although gross brain pathology is not characteristic of autism, subtle anatomical and functional differences have been observed in post-mortem, neuroimaging and electrophysiological studies. Initially, it was hoped that accurate measurement of behavioural phenotypes would lead to specific genetic subtypes, but genetic findings have mainly applied to heterogeneous groups that are not specific to autism. Psychosocial interventions in children can improve specific behaviours, such as joint attention, language and social engagement, that may affect further development and could reduce symptom severity. However, further research is necessary to identify the long-term needs of people with autism, and treatments and the mechanisms behind them that could result in improved independence and quality of life over time. Families are often the major source of support for people with autism throughout much of life and need to be considered, along with the perspectives of autistic individuals, in both research and practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
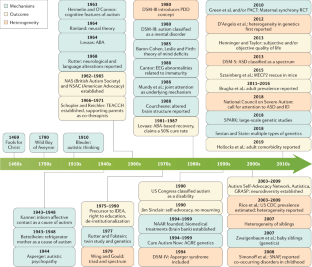
Similar content being viewed by others

Neurogenetic disorders across the lifespan: from aberrant development to degeneration
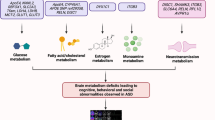
Neuroimaging genetics approaches to identify new biomarkers for the early diagnosis of autism spectrum disorder
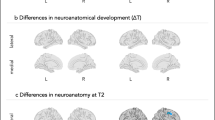
Cross-sectional and longitudinal neuroanatomical profiles of distinct clinical (adaptive) outcomes in autism
Lord, C. et al. Autism from 2 to 9 years of age. Arch. Gen. Psychiatry 63 , 694–701 (2006). This paper establishes that autism is a stable diagnosis (as a spectrum) beginning at least by 2 years of age. The paper also establishes parent interview and clinician observation as predictive of autism at 9 years of age. Finally, it is the first paper that shows that the specific DSM-IV-TR diagnoses is unstable across childhood but that the instability is almost all shifting across categories not outside the spectrum.
Article PubMed Google Scholar
Risi, S. et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 45 , 1094–1103 (2006).
Loomes, R., Hull, L. & Mandy, W. P. L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56 , 466–474 (2017).
Brugha, T. S. et al. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry 209 , 498–503 (2016). This paper uses active case-finding to provide representative estimates of the prevalence of autism and demonstrated that rates of autism in men and women are equivalent in adults with moderate-to-profound intellectual disability.
Brugha, T., Bankart, J., McManus, S. & Gullon-Scott, F. CDC autism rate: misplaced reliance on passive sampling? Lancet 392 , 732–733 (2018).
Baxter, A. J. et al. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 45 , 601–613 (2015).
Article CAS PubMed Google Scholar
Elsabbagh, M. et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 5 , 160–179 (2012).
Article PubMed PubMed Central Google Scholar
Magnusson, C. et al. Migration and autism spectrum disorder: population-based study. Br. J. Psychiatry 201 , 109–115 (2012).
Goodman, R. & Richards, H. Child and adolescent psychiatric presentations of second-generation Afro-Caribbeans in Britain. Br. J. Psychiatry 167 , 362–369 (1995).
Dyches, T. T., Wilder, L. K., Sudweeks, R. R., Obiakor, F. E. & Algozzine, B. Multicultural issues in autism. J. Autism Dev. Disord. 34 , 211–222 (2004).
Keen, D. V., Reid, F. D. & Arnone, D. Autism, ethnicity and maternal immigration. Br. J. Psychiatry 196 , 274–281 (2010).
McManus, S., Bebbington, P., Jenkins, R. & Brugha, T. Adult Psychiatric Morbidity Survey: mental health and wellbeing in England, 2014. NHS https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014 (2016).
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1789–1858 (2018).
Article Google Scholar
Marcheselli, F. et al. Mental health of children and young people in England, 2017. NHS https://digital.nhs.uk/data-and-information/publications/statistical/mental-health-of-children-and-young-people-in-england/2017/2017 (2018).
Brugha, T. C. et al. Autism Spectrum Disorder, Adult Psychiatric Morbidity Survey 2014. (2014).
Lundstrom, S., Reichenberg, A., Anckarsater, H., Lichtenstein, P. & Gillberg, C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 350 , h1961 (2015).
Tromans, S., Chester, V., Kiani, R., Alexander, R. & Brugha, T. The prevalence of autism spectrum disorders in adult psychiatric inpatients: a systematic review. Clin. Pract. Epidemiol. Ment. Health 14 , 177–187 (2018).
Modabbernia, A., Velthorst, E. & Reichenberg, A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol. Autism 8 , 13 (2017).
Article PubMed PubMed Central CAS Google Scholar
Wu, S. et al. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr. Scand. 135 , 29–41 (2017).
Taylor, L. E., Swerdfeger, A. L. & Eslick, G. D. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine 32 , 3623–3629 (2014).
Lai, M.-C., Lombardo, M. V. & Baron-Cohen, S. Autism. Lancet 383 , 896–910 (2014).
Velikonja, T., Fett, A.-K. & Velthorst, E. Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry 76 , 135–151 (2019).
McNally Keehn, R. H., Lincoln, A. J., Brown, M. Z. & Chavira, D. A. The coping cat program for children with anxiety and autism spectrum disorder: a pilot randomized controlled trial. J. Autism Dev. Disord. 43 , 57–67 (2013).
Jones, E. J. H., Gliga, T., Bedford, R., Charman, T. & Johnson, M. H. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 39 , 1–33 (2014).
Ozonoff, S. et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics 128 , e488–e495 (2011).
PubMed PubMed Central Google Scholar
Jones, R. M. & Lord, C. Diagnosing autism in neurobiological research studies. Behav. Brain Res. 251 , 113–124 (2013).
Johnson, M. H. Autism: demise of the innate social orienting hypothesis. Curr. Biol. 24 , R30–R31 (2014).
Johnson, M. H., Jones, E. J. H. & Gliga, T. Brain adaptation and alternative developmental trajectories. Dev. Psychopathol. 27 , 425–442 (2015).
The Lancet Psychiatry. Of mice and mental health. Lancet Psychiatry 6 , 877 (2019).
Nelson, C. A. et al. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev. Psychopathol. 14 , 499–520 (2002).
Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 45 , 984–994 (2013).
Article CAS Google Scholar
Gaugler, T. et al. Most genetic risk for autism resides with common variation. Nat. Genet. 46 , 881–885 (2014).
Article CAS PubMed PubMed Central Google Scholar
Wang, K., Gaitsch, H., Poon, H., Cox, N. J. & Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet. 49 , 1319–1325 (2017).
Sanders, S. J. et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87 , 1215–1233 (2015).
Satterstrom, F. K. et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Preprint at https://doi.org/10.1101/484113 (2019).
Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485 , 237–241 (2012).
Neale, B. M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485 , 242–245 (2012).
O’Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485 , 246–250 (2012).
Sanders, S. J. et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70 , 863–885 (2011).
Levy, D. et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70 , 886–897 (2011).
Sebat, J. et al. Strong association of de novo copy number mutations with autism. Science 316 , 445–449 (2007). This paper is the first to focus explicitly on simplex autism and show the importance of de novo CNVs in simplex cases, versus familial cases, versus controls.
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51 , 431–444 (2019).
Willsey, J. et al. De novo coding variants are strongly associated with Tourette syndrome. Eur. Neuropsychopharmacol. 29 , S737 (2019).
Epi4K Consortium. Epi4K: gene discovery in 4,000 genomes. Epilepsia 53 , 1457–1467 (2012).
Jamain, S. et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 34 , 27–29 (2003). This is the first paper to show a de novo loss-of-function mutation in a synaptic gene associated with non-syndromic autism and was a harbinger for many of the findings that came after.
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515 , 216–221 (2014).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515 , 209–215 (2014).
Sestan, N. & State, M. W. Lost in translation: traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron 100 , 406–423 (2018).
State, M. W. & Sestan, N. The emerging biology of autism spectrum disorders. Science 337 , 1301–1303 (2012).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511 , 421–427 (2014).
Article PubMed Central CAS Google Scholar
Devlin, B. & Scherer, S. W. Genetic architecture in autism spectrum disorder. Curr. Opin. Genet. Dev. 22 , 229–237 (2012).
de la Torre-Ubieta, L., Won, H., Stein, J. L. & Geschwind, D. H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 22 , 345–361 (2016).
SFARI Gene Website. https://gene.sfari.org/ (2019).
Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155 , 1008–1021 (2013).
Ben-David, E. & Shifman, S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol. Psychiatry 18 , 1054–1056 (2013).
Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155 , 997–1007 (2013).
Pinto, D. et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466 , 368–372 (2010).
Gilman, S. R. et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70 , 898–907 (2011).
Fuccillo, M. V. Striatal circuits as a common node for autism pathophysiology. Front. Neurosci. 10 , 27 (2016).
Velmeshev, D. et al. Single-cell genomics identifies cell type-specific molecular changes in autism. Science 364 , 685–689 (2019).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377 , 1713–1722 (2017).
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378 , 625–635 (2018).
Matharu, N. et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363 , eaau0629 (2019).
Abudayyeh, O. O. et al. RNA targeting with CRISPR–cas13. Nature 550 , 280–284 (2017).
Power, J. D. et al. Customized head molds reduce motion during resting state fMRI scans. NeuroImage 189 , 141–149 (2019).
Solso, S. et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol. Psychiatry 79 , 676–684 (2016).
Clements, C. C. et al. Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry 75 , 797–808 (2018).
Ecker, C. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch. Gen. Psychiatry 69 , 195–209 (2012).
Langen, M. et al. Changes in the development of striatum are involved in repetitive behavior in autism. Biol. Psychiatry 76 , 405–411 (2014).
Elsabbagh, M. & Johnson, M. H. Autism and the social brain: the first-year puzzle. Biol. Psychiatry 80 , 94–99 (2016).
Courchesne, E. et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57 , 245–254 (2001).
Hazlett, H. C. et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry 62 , 1366–1376 (2005).
Wolff, J. J. et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169 , 589–600 (2012).
Hazlett, H. C. et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 542 , 348–351 (2017). This seminal paper, through careful recruitment and methodology, was the first to show significant early differences that may contribute to our understanding of developmental features in neural structure and circuits .
Wolff, J. J. et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism 8 , 8 (2017).
Emerson, R. W. et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med. 9 , eaag2882 (2017).
Smith, E. et al. Cortical thickness change in autism during early childhood: CT in early childhood ASD. Hum. Brain Mapp. 37 , 2616–2629 (2016).
Uddin, L. Q., Dajani, D. R., Voorhies, W., Bednarz, H. & Kana, R. K. Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl. Psychiatry 7 , e1218 (2017).
Herringshaw, A. J., Ammons, C. J., DeRamus, T. P. & Kana, R. K. Hemispheric differences in language processing in autism spectrum disorders: a meta-analysis of neuroimaging studies. Autism Res. 9 , 1046–1057 (2016).
He, Y., Byrge, L. & Kennedy, D. P. Non-replication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Preprint at https://doi.org/10.1101/640797 (2019).
Lawrence, K. E., Hernandez, L. M., Bookheimer, S. Y. & Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 12 , 53–65 (2019).
Plitt, M., Barnes, K. A., Wallace, G. L., Kenworthy, L. & Martin, A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc. Natl Acad. Sci. USA 112 , E6699–E6706 (2015).
Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19 , 659–667 (2014).
Doyle-Thomas, K. A. R. et al. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann. Neurol. 77 , 866–876 (2015).
Supekar, K. et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 5 , 738–747 (2013).
Dajani, D. R. & Uddin, L. Q. Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res. 9 , 43–54 (2016).
Hull, J. V. et al. Resting-state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry 7 , 205 (2017).
Lombardo, M. V. et al. Different functional neural substrates for good and poor language outcome in autism. Neuron 86 , 567–577 (2015).
Carlisi, C. O. et al. Disorder-specific and shared brain abnormalities during vigilance in autism and obsessive-compulsive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 644–654 (2017).
Alaerts, K., Swinnen, S. P. & Wenderoth, N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci. 11 , 1002–1016 (2016).
Kirkovski, M., Enticott, P. G., Hughes, M. E., Rossell, S. L. & Fitzgerald, P. B. Atypical neural activity in males but not females with autism spectrum disorder. J. Autism Dev. Disord. 46 , 954–963 (2016).
Venkataraman, A. et al. Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. NeuroReport 27 , 1081–1085 (2016).
Levisohn, P. M. The autism-epilepsy connection. Epilepsia 48 , 33–35 (2007).
Cantor, D. S., Thatcher, R. W., Hrybyk, M. & Kaye, H. Computerized EEG analyses of autistic children. J. Autism Dev. Disord. 16 , 169–187 (1986).
Lefebvre, A. et al. Alpha waves as a neuromarker of autism spectrum disorder: the challenge of reproducibility and heterogeneity. Front. Neurosci. 12 , 662 (2018).
Tierney, A. L., Gabard-Durnam, L., Vogel-Farley, V., Tager-Flusberg, H. & Nelson, C. A. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLOS ONE 7 , e39127 (2012).
Oberman, L. M. et al. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res. 24 , 190–198 (2005).
Fan, Y.-T., Decety, J., Yang, C.-Y., Liu, J.-L. & Cheng, Y. Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry 51 , 981–988 (2010).
Southgate, V. & Hamilton, A. F. Unbroken mirrors: challenging a theory of autism. Trends Cogn. Sci. 12 , 225–229 (2008).
Bernier, R., Aaronson, B. & McPartland, J. The role of imitation in the observed heterogeneity in EEG mu rhythm in autism and typical development. Brain Cogn. 82 , 69–75 (2013).
Raymaekers, R., Wiersema, J. R. & Roeyers, H. EEG study of the mirror neuron system in children with high functioning autism. Brain Res. 1304 , 113–121 (2009).
Dumas, G., Soussignan, R., Hugueville, L., Martinerie, J. & Nadel, J. Revisiting mu suppression in autism spectrum disorder. Brain Res. 1585 , 108–119 (2014). This paper replicates the mu suppression deficits in autism during action observation but questions, through high-density spectral analyses and source reconstruction, its previously drawn relation to the mirror neuron system.
Marco, E. J., Hinkley, L. B. N., Hill, S. S. & Nagarajan, S. S. Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res. 69 , 48R–54R (2011).
Schwartz, S., Shinn-Cunningham, B. & Tager-Flusberg, H. Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev. 87 , 106–117 (2018).
Kang, E. et al. Atypicality of the N170 event-related potential in autism spectrum disorder: a meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 , 657–666 (2018).
Bonnet-Brilhault, F. et al. GABA/glutamate synaptic pathways targeted by integrative genomic and electrophysiological explorations distinguish autism from intellectual disability. Mol. Psychiatry 21 , 411–418 (2016).
Schilbach, L. Towards a second-person neuropsychiatry. Phil. Trans. R. Soc. B 371 , 20150081 (2016). This review supports that psychiatric disorders are more commonly characterized by impairments of social interaction rather than social observation, and advocates for an interactive turn in neuropsychiatry .
Barraza, P. et al. Implementing EEG hyperscanning setups. MethodsX 6 , 428–436 (2019).
Dumas, G., de Guzman, G. C., Tognoli, E. & Kelso, J. A. The human dynamic clamp as a paradigm for social interaction. Proc. Natl Acad. Sci. USA 111 , E3726–E3734 (2014).
Jones, E. J. H. et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk. J. Neurodev. Disord. 8 , 7 (2016).
Ciarrusta, J. et al. Social brain functional maturation in newborn infants with and without a family history of autism spectrum disorder. JAMA Netw. Open 2 , e191868 (2019).
Levin, A. R., Varcin, K. J., O’Leary, H. M., Tager-Flusberg, H. & Nelson, C. A. EEG power at 3 months in infants at high familial risk for autism. J. Neurodev. Disord. 9 , 34 (2017).
Kolesnik, A. et al. Increased cortical reactivity to repeated tones at 8 months in infants with later ASD. Transl. Psychiatry 9 , 46 (2019).
Rippon, G., Brock, J., Brown, C. & Boucher, J. Disordered connectivity in the autistic brain: challenges for the ‘new psychophysiology’. Int. J. Psychophysiol. 63 , 164–172 (2007).
Rosenberg, A., Patterson, J. S. & Angelaki, D. E. A computational perspective on autism. Proc. Natl Acad. Sci. USA 112 , 9158–9165 (2015).
Masuda, F. et al. Motor cortex excitability and inhibitory imbalance in autism spectrum disorder assessed with transcranial magnetic stimulation: a systematic review. Transl. Psychiatry 9 , 110 (2019).
O’Reilly, C., Lewis, J. D. & Elsabbagh, M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLOS ONE 12 , e0175870 (2017).
Khan, S. et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 138 , 1394–1409 (2015).
Chen, H., Nomi, J. S., Uddin, L. Q., Duan, X. & Chen, H. Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum. Brain Mapp. 38 , 5740–5755 (2017).
Catarino, A., Churches, O., Baron-Cohen, S., Andrade, A. & Ring, H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin. Neurophysiol. 122 , 2375–2383 (2011).
Engemann, D. A. et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 141 , 3179–3192 (2018).
Open Science Collaboration. Psychology. Estimating the reproducibility of psychological science. Science 349 , aac4716 (2015).
Lord, C. et al. Autism diagnostic observation schedule: ADOS-2 (Western Psychological Services, 2012).
Regier, D. A. et al. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry 170 , 59–70 (2013).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders , 5th Edn (American Psychiatric Association, 2013).
World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). https://icd.who.int/browse11/l-m/en (WHO, 2018).
Constantino, J. N. & Charman, T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol. 15 , 279–291 (2016).
Lord, C. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch. Gen. Psychiatry 69 , 306–313 (2012).
Miller, J. N. & Ozonoff, S. The external validity of Asperger disorder: lack of evidence from the domain of neuropsychology. J. Abnorm. Psychol. 109 , 227–238 (2000).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders , Fourth Edn (American Psychiatric Association, 1994).
Green, D., Chandler, S., Charman, T., Simonoff, E. & Baird, G. Brief report: DSM-5 sensory behaviours in children with and without an autism spectrum disorder. J. Autism Dev. Disord. 46 , 3597–3606 (2016).
Ozonoff, S. et al. Diagnosis of autism spectrum disorder after age 5 in children evaluated longitudinally since infancy. J. Am. Acad. Child Adolesc. Psychiatry 57 , 849–857.e2 (2018).
Russell, G., Steer, C. & Golding, J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc. Psychiatry Psychiatr. Epidemiol. 46 , 1283–1293 (2011).
Charman, T. & Gotham, K. Measurement issues: screening and diagnostic instruments for autism spectrum disorders—lessons from research and practice. Child Adolesc. Ment. Health 18 , 52–63 (2013).
Ashwood, K. L., Buitelaar, J., Murphy, D., Spooren, W. & Charman, T. European clinical network: autism spectrum disorder assessments and patient characterisation. Eur. Child Adolesc. Psychiatry 24 , 985–995 (2015).
Rutter, M., LeCouteur, A. & Lord, C. Autism Diagnostic Interview-Revised (ADI-R). (Western Psychological Services, 2003).
Durkin, M. S. et al. Autism screening and diagnosis in low resource settings: challenges and opportunities to enhance research and services worldwide. Autism Res. 8 , 473–476 (2015). This position paper highlights the challenges to translating knowledge on better awareness, understanding, identification and diagnosis (and then treatments) from the past two decades of clinical research in high-income countries into low-income and middle-income countries.
Baird, G. et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 368 , 210–215 (2006).
Luyster, R. et al. The autism diagnostic observation schedule — toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord. 39 , 1305–1320 (2009).
de Vries, P. J. Thinking globally to meet local needs: autism spectrum disorders in Africa and other low-resource environments. Curr. Opin. Neurol. 29 , 130–136 (2016).
Article PubMed CAS Google Scholar
Georgiades, S., Bishop, S. L. & Frazier, T. Editorial perspective: longitudinal research in autism—introducing the concept of ‘chronogeneity’. J. Child Psychol. Psychiatry 58 , 634–636 (2017).
Fountain, C., Winter, A. S. & Bearman, P. S. Six developmental trajectories characterize children with autism. Pediatrics 129 , e1112–e1120 (2012).
Kim, S. H. et al. Variability in autism symptom trajectories using repeated observations from 14 to 36 months of age. J. Am. Acad. Child Adolesc. Psychiatry 57 , 837–848.e2 (2018).
Bussu, G. et al. Latent trajectories of adaptive behaviour in infants at high and low familial risk for autism spectrum disorder. Mol. Autism 10 , 13 (2019).
Zerbi, V. et al. Dysfunctional autism risk genes cause circuit-specific connectivity deficits with distinct developmental trajectories. Cereb. Cortex 28 , 2495–2506 (2018).
Fein, D. et al. Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry 54 , 195–205 (2013).
Anderson, D. K., Liang, J. W. & Lord, C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry 55 , 485–494 (2014).
Chlebowski, C., Robins, D. L., Barton, M. L. & Fein, D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics 131 , e1121–e1127 (2013).
Stenberg, N. et al. Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatr. Perinat. Epidemiol. 28 , 255–262 (2014).
Pierce, K., Courchesne, E. & Bacon, E. To screen or not to screen universally for autism is not the question: why the task force got it wrong. J. Pediatr. 176 , 182–194 (2016).
Siu, A. L. et al. Screening for autism spectrum disorder in young children: US Preventive Services Task Force recommendation statement. JAMA 315 , 691–696 (2016).
Øien, R. A. et al. Clinical features of children with autism who passed 18-month screening. Pediatrics 141 , e20173596 (2018).
Sánchez-García, A. B., Galindo-Villardón, P., Nieto-Librero, A. B., Martín-Rodero, H. & Robins, D. L. Toddler screening for autism spectrum disorder: a meta-analysis of diagnostic accuracy. J. Autism Dev. Disord. 49 , 1837–1852 (2019).
Marlow, M., Servili, C. & Tomlinson, M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: recommendations for use in low- and middle-income countries. Autism Res. 12 , 176–199 (2019).
Raza, S. et al. Brief report: evaluation of the short quantitative checklist for autism in toddlers (Q-CHAT-10) as a brief screen for autism spectrum disorder in a high-risk sibling cohort. J. Autism Dev. Disord. 49 , 2210–2218 (2019).
Charman, T. et al. Testing two screening instruments for autism spectrum disorder in UK community child health services. Dev. Med. Child Neurol. 58 , 369–375 (2016).
Brett, D., Warnell, F., McConachie, H. & Parr, J. R. Factors affecting age at ASD diagnosis in UK: no evidence that diagnosis age has decreased between 2004 and 2014. J. Autism Dev. Disord. 46 , 1974–1984 (2016).
Zuckerman, K. E., Lindly, O. J. & Sinche, B. K. Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. J. Pediatr. 166 , 1431–1439.e1 (2015).
Boterberg, S., Charman, T., Marschik, P. B., Bölte, S. & Roeyers, H. Regression in autism spectrum disorder: a critical overview of retrospective findings and recommendations for future research. Neurosci. Biobehav. Rev. 102 , 24–55 (2019).
Pearson, N., Charman, T., Happé, F., Bolton, P. F. & McEwen, F. S. Regression in autism spectrum disorder: reconciling findings from retrospective and prospective research. Autism Res. 11 , 1602–1620 (2018).
Ozonoff, S. & Iosif, A.-M. Changing conceptualizations of regression: what prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev. 100 , 296–304 (2019). Despite its potential importance as a biological marker and/or subgroup of ASD, developmental regression has remained very poorly understood. This paper outlines recent data and reconceptualization about patterns of onset (and loss) that chime with a more contemporaneous understanding of ASD as a heterogeneous condition in terms of its manifestation both within and across individuals .
Brugha, T. S. et al. Validating two survey methods for identifying cases of autism spectrum disorder among adults in the community. Psychol. Med. 42 , 647–656 (2012).
Brugha, T. S. The Psychiatry of Adult Autism and Asperger Syndrome: a Practical Guide (Oxford Univ. Press, 2018).
Epstein, J., Johnson, D. E. & Conners, C. K. Conners Adult ADHD Diagnostic Interview for DSM-IV (CAADID) (MHS, 2001).
Lai, M.-C. et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6 , 819–829 (2019).
Havdahl, A. & Bishop, S. Heterogeneity in prevalence of co-occurring psychiatric conditions in autism. Lancet Psychiatry 6 , 794–795 (2019).
Croen, L. A. et al. The health status of adults on the autism spectrum. Autism 19 , 814–823 (2015).
Mannion, A., Leader, G. & Healy, O. An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with autism spectrum disorder. Res. Autism Spectr. Disord. 7 , 35–42 (2013).
Soke, G. N., Maenner, M. J., Christensen, D., Kurzius-Spencer, M. & Schieve, L. A. Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. J. Autism Dev. Disord. 48 , 2663–2676 (2018).
Chandler, S. et al. Emotional and behavioural problems in young children with autism spectrum disorder. Dev. Med. Child Neurol. 58 , 202–208 (2016).
Pezzimenti, F., Han, G. T., Vasa, R. A. & Gotham, K. Depression in youth with autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am. 28 , 397–409 (2019).
Hwang, Y. I. J., Srasuebkul, P., Foley, K. R., Arnold, S. & Trollor, J. N. Mortality and cause of death of Australians on the autism spectrum. Autism Res. 12 , 806–815 (2019).
Hirvikoski, T. et al. Premature mortality in autism spectrum disorder. Br. J. Psychiatry 208 , 232–238 (2016).
Havdahl, K. A. et al. Multidimensional influences on autism symptom measures: implications for use in etiological research. J. Am. Acad. Child Adolesc. Psychiatry 55 , 1054–1063.e3 (2016).
Nicolaidis, C. et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic-community partnership. J. Gen. Intern. Med. 28 , 761–769 (2013).
Schreibman, L. et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J. Autism Dev. Disord. 45 , 2411–2428 (2015).
Tomlinson, M. et al. Setting global research priorities for developmental disabilities, including intellectual disabilities and autism: setting research priorities for developmental disabilities. J. Intellect. Disabil. Res. 58 , 1121–1130 (2014).
Rahman, A. et al. Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in South Asia in India and Pakistan (PASS): a randomised controlled trial. Lancet Psychiatry 3 , 128–136 (2016).
Lovaas, O. I. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J. Consult. Clin. Psychol. 55 , 3–9 (1987).
Nevill, R. E., Lecavalier, L. & Stratis, E. A. Meta-analysis of parent-mediated interventions for young children with autism spectrum disorder. Autism 22 , 84–98 (2018).
Kasari, C. et al. Randomized controlled trial of parental responsiveness intervention for toddlers at high risk for autism. Infant Behav. Dev. 37 , 711–721 (2014).
Shire, S. Y. et al. Hybrid implementation model of community-partnered early intervention for toddlers with autism: a randomized trial. J. Child Psychol. Psychiatry 58 , 612–622 (2017).
Siller, M., Hutman, T. & Sigman, M. A parent-mediated intervention to increase responsive parental behaviors and child communication in children with ASD: a randomized clinical trial. J. Autism Dev. Disord. 43 , 540–555 (2013).
Rogers, S. J. et al. Effects of a brief early start denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 51 , 1052–1065 (2012).
Green, J. et al. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet 375 , 2152–2160 (2010).
Pickles, A. et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet 388 , 2501–2509 (2016).
Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 125 , e17–e23 (2010).
Charman, T. Editorial: trials and tribulations in early autism intervention research. J. Am. Acad. Child Adolesc. Psychiatry 58 , 846–848 (2019).
Rogers, S. J. et al. A multisite randomized controlled two-phase trial of the early start denver model compared to treatment as usual. J. Am. Acad. Child Adolesc. Psychiatry 58 , 853–865 (2019).
Dawson, G. et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry 51 , 1150–1159 (2012).
Myers, S. M., Johnson, C. P. & The Council on Children With Disabilities. Management of children with autism spectrum disorders. Pediatrics 120 , 1162–1182 (2007).
Laugeson, E. A., Frankel, F., Gantman, A., Dillon, A. R. & Mogil, C. Evidence-based social skills training for adolescents with autism spectrum disorders: the UCLA PEERS program. J. Autism Dev. Disord. 42 , 1025–1036 (2012).
Reichow, B., Servili, C., Yasamy, M. T., Barbui, C. & Saxena, S. Non-specialist psychosocial interventions for children and adolescents with intellectual disability or lower-functioning autism spectrum disorders: a systematic review. PLOS Med. 10 , e1001572 (2013).
Brignell, A. et al. Communication interventions for autism spectrum disorder in minimally verbal children. Cochrane Database Syst. Rev. 11 , CD012324 (2018).
PubMed Google Scholar
Tarver, J. et al. Child and parent outcomes following parent interventions for child emotional and behavioral problems in autism spectrum disorders: a systematic review and meta-analysis. Autism 23 , 1630–1644 (2019).
Keefer, A. et al. Exploring relationships between negative cognitions and anxiety symptoms in youth with autism spectrum disorder. Behav. Ther. 49 , 730–740 (2018).
Bearss, K. et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. JAMA 313 , 1524–1533 (2015).
Da Paz, N. S. & Wallander, J. L. Interventions that target improvements in mental health for parents of children with autism spectrum disorders: a narrative review. Clin. Psychol. Rev. 51 , 1–14 (2017).
Kasari, C. et al. Children with autism spectrum disorder and social skills groups at school: a randomized trial comparing intervention approach and peer composition. J. Child Psychol. Psychiatry 57 , 171–179 (2016).
Marshall, D. et al. Social stories in mainstream schools for children with autism spectrum disorder: a feasibility randomised controlled trial. BMJ Open 6 , e011748 (2016).
Taylor, J. L. et al. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 130 , 531–538 (2012).
Pallathra, A. A., Cordero, L., Wong, K. & Brodkin, E. S. Psychosocial interventions targeting social functioning in adults on the autism spectrum: a literature review. Curr. Psychiatry Rep. 21 , 5 (2019).
White, S. W. et al. Psychosocial treatments targeting anxiety and depression in adolescents and adults on the autism spectrum: review of the latest research and recommended future directions. Curr. Psychiatry Rep. 20 , 82 (2018).
Shattuck, P. T., Wagner, M., Narendorf, S., Sterzing, P. & Hensley, M. Post-high school service use among young adults with an autism spectrum disorder. Arch. Pediatr. Adolesc. Med. 165 , 141–146 (2011).
Wehman, P. et al. Effects of an employer-based intervention on employment outcomes for youth with significant support needs due to autism. Autism 21 , 276–290 (2017).
McCracken, J. T. et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 347 , 314–321 (2002).
Owen, R. et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 124 , 1533–1540 (2009).
McPheeters, M. L. et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics 127 , e1312–e1321 (2011).
Anagnostou, E. et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry 73 , 928–937 (2016).
Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 62 , 1266–1274 (2005).
Handen, B. L. et al. Atomoxetine, parent training, and their combination in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 54 , 905–915 (2015).
Scahill, L. et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry 172 , 1197–1206 (2015).
Hollander, E. et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am. J. Psychiatry 169 , 292–299 (2012).
King, B. H. et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch. Gen. Psychiatry 66 , 583–590 (2009).
Anagnostou, E. et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 1580 , 188–198 (2014).
Guastella, A. J. et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry 56 , 444–452 (2015).
Parker, K. J. et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 11 , eaau7356 (2019).
Bolognani, F. et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 11 , eaat7838 (2019).
Rubenstein, J. L. R. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2 , 255–267 (2003).
Veenstra-VanderWeele, J. et al. Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology 42 , 1390–1398 (2017).
Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 8 , 321ra5 (2016).
Krueger, D. A. et al. Everolimus for treatment of tuberous sclerosis complex-associated neuropsychiatric disorders. Ann. Clin. Transl. Neurol. 4 , 877–887 (2017).
Georgiades, S. & Kasari, C. Reframing optimal outcomes in autism. JAMA Pediatr. 172 , 716–717 (2018).
Bishop-Fitzpatrick, L. et al. Characterizing objective quality of life and normative outcomes in adults with autism spectrum disorder: an exploratory latent class analysis. J. Autism Dev. Disord. 46 , 2707–2719 (2016).
The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 28 , 551–558 (1998).
Gotham, K. et al. Characterizing the daily life, needs, and priorities of adults with autism spectrum disorder from interactive autism network data. Autism 19 , 794–804 (2015).
Taylor, J. L. & Seltzer, M. M. Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. J. Autism Dev. Disord. 41 , 566–574 (2011).
Orsmond, G. I., Shattuck, P. T., Cooper, B. P., Sterzing, P. R. & Anderson, K. A. Social participation among young adults with an autism spectrum disorder. J. Autism Dev. Disord. 43 , 2710–2719 (2013).
Henninger, N. A. & Taylor, J. L. Outcomes in adults with autism spectrum disorders: a historical perspective. Autism 17 , 103–116 (2013).
Howlin, P. & Moss, P. Adults with autism spectrum disorders. Can. J. Psychiatry 57 , 275–283 (2012).
Farley, M. A. et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Res. 2 , 109–118 (2009).
Taylor, J. L., Henninger, N. A. & Mailick, M. R. Longitudinal patterns of employment and postsecondary education for adults with autism and average-range IQ. Autism 19 , 785–793 (2015).
Lai, M.-C. et al. Quantifying and exploring camouflaging in men and women with autism. Autism 21 , 690–702 (2016).
van Heijst, B. F. & Geurts, H. M. Quality of life in autism across the lifespan: a meta-analysis. Autism 19 , 158–167 (2015).
Moss, P., Mandy, W. & Howlin, P. Child and adult factors related to quality of life in adults with autism. J. Autism Dev. Disord. 47 , 1830–1837 (2017).
Bishop-Fitzpatrick, L., Mazefsky, C. A. & Eack, S. M. The combined impact of social support and perceived stress on quality of life in adults with autism spectrum disorder and without intellectual disability. Autism 22 , 703–711 (2017).
Kamio, Y., Inada, N. & Koyama, T. A nationwide survey on quality of life and associated factors of adults with high-functioning autism spectrum disorders. Autism 17 , 15–26 (2013).
Mason, D. et al. Predictors of quality of life for autistic adults. Autism Res. 11 , 1138–1147 (2018).
Autistica. Your questions shaping future autism research. https://www.autistica.org.uk/downloads/files/Autism-Top-10-Your-Priorities-for-Autism-Research.pdf (2016).
Ontario Brain Institute. Community priorities for research on neurodevelopmental disorders. http://braininstitute.ca/img/JLA-NDD-Final-Report.pdf (2018).
den Houting, J. Neurodiversity: an insider’s perspective. Autism 23 , 271–273 (2018).
Szatmari, P. Risk and resilience in autism spectrum disorder: a missed translational opportunity? Dev. Med. Child Neurol. 60 , 225–229 (2018).
Markowitz, L. A. et al. Development and psychometric evaluation of a psychosocial quality-of-life questionnaire for individuals with autism and related developmental disorders. Autism 20 , 832–844 (2016).
Ryan, S. & Cole, K. R. From advocate to activist? Mapping the experiences of mothers of children on the autism spectrum. J. Appl. Res. Intellect. Disabil. 22 , 43–53 (2009).
McCann, D., Bull, R. & Winzenberg, T. The daily patterns of time use for parents of children with complex needs: a systematic review. J. Child Health Care 16 , 26–52 (2012).
Karst, J. S. & Van Hecke, A. V. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev. 15 , 247–277 (2012).
Lounds, J., Seltzer, M. M., Greenberg, J. S. & Shattuck, P. T. Transition and change in adolescents and young adults with autism: longitudinal effects on maternal well-being. Am. J. Ment. Retard. 112 , 401–417 (2007).
Burke, M. & Heller, T. Individual, parent and social-environmental correlates of caregiving experiences among parents of adults with autism spectrum disorder. J. Intellect. Disabil. Res. 60 , 401–411 (2016).
Kim, S. H., Bal, V. H. & Lord, C. Longitudinal follow-up of academic achievement in children with autism from age 2 to 18. J. Child Psychol. Psychiatry 59 , 258–267 (2017).
Lord, C., Bishop, S. & Anderson, D. Developmental trajectories as autism phenotypes. Am. J. Med. Genet. C Semin. Med. Genet. 169 , 198–208 (2015).
Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancel Glob. Health 6 , e1100–e1121 (2018).
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Primers 1 , 15067 (2015).
Patel, V. et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 387 , 1672–1685 (2016).
Franz, L., Chambers, N., von Isenburg, M. & de Vries, P. J. Autism spectrum disorder in sub-Saharan Africa: a comprehensive scoping review. Autism Res. 10 , 723–749 (2017).
World Health Organization. Training parents to transform children’s lives. https://www.who.int/mental_health/maternal-child/PST/en/ (WHO, 2019).
Naslund, J. A. et al. Digital innovations for global mental health: opportunities for data science, task sharing, and early intervention. Curr. Treat. Options Psychiatry https://doi.org/10.1007/s40501-019-00186-8 (2019).
Sadowsky, J., Donvan, J. & Zucker, C. In a different key: the story of autism. J. Hist. Behav. Sci. 54 , 66–67 (2018). This paper presents a different, broad overview of the changes in perspective about autism and ASD over the years .
Rutter, M., Greenfeld, D. & Lockyer, L. A five to fifteen year follow-up study of infantile psychosis. II. Social and behavioural outcome. Br. J. Psychiatry 113 , 1183–1199 (1967).
Hermelin, B. & O’Connor, N. Psychological Experiments with Autistic Children (Pergamon Press, 1970).
Rimland, B. Infantile Autism: the Syndrome and its Implications for a Neural Theory of Behaviour (Meredith Publishing Company, 1964).
Frith, U. Studies in pattern detection in normal and autistic children: I. Immediate recall of auditory sequences. J. Abnorm. Psychol. 76 , 413–420 (1970).
Folstein, S. & Rutter, M. in Autism (eds. Rutter M. & Schopler E.) 219–241 (Springer, 1978).
Mundy, P., Sigman, M. & Kasari, C. A longitudinal study of joint attention and language development in autistic children. J. Autism Dev. Disord. 20 , 115–128 (1990).
Schopler, E. & Reichler, R. J. Parents as cotherapists in the treatment of psychotic children. J. Autism Child. Schizophr. 1 , 87–102 (1971).
Sinclair, J. Don’t mourn for us. Autism Network International http://www.autreat.com/dont_mourn.html (1993).
Wing, L. & Gould, J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 9 , 11–29 (1979).
Chawner, S. et al. A genetic first approach to dissecting the heterogeneity of autism: phenotypic comparison of autism risk copy number variants. Eur. Neuropsychopharmacol. 29 (Suppl. 3), S783–S784 (2019).
Modabbernia, A., Mollon, J., Boffetta, P. & Reichenberg, A. Impaired gas exchange at birth and risk of intellectual disability and autism: a meta-analysis. J. Autism Dev. Disord. 46 , 1847–1859 (2016).
Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309 , 1696–1703 (2013).
Xie, F., Peltier, M. & Getahun, D. Is the risk of autism in younger siblings of affected children moderated by sex, race/ethnicity, or gestational age? J. Dev. Behav. Pediatr. 37 , 603–609 (2016).
Guy, A. et al. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J. Pediatr. 166 , 269–275.e3 (2015).
Schendel, D. & Bhasin, T. K. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 121 , 1155–1164 (2008).
Windham, G. C. et al. Maternal pre-pregnancy body mass index and gestational weight gain in relation to autism spectrum disorder and other developmental disorders in offspring. Autism Res. 12 , 316–327 (2019).
Schmidt, R. J. et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr. 96 , 80–89 (2012).
Conde-Agudelo, A., Rosas-Bermudez, A. & Norton, M. H. Birth spacing and risk of autism and other neurodevelopmental disabilities: a systematic review. Pediatrics 137 , e20153482 (2016).
Lyall, K. et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 38 , 81–102 (2017).
Cheslack-Postava, K., Liu, K. & Bearman, P. S. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 127 , 246–253 (2011).
Conti, E., Mazzotti, S., Calderoni, S., Saviozzi, I. & Guzzetta, A. Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum. Reprod. 28 , 3316–3327 (2013).
Lehti, V. et al. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum. Reprod. 28 , 812–818 (2013).
Rossignol, D. A., Genuis, S. J. & Frye, R. E. Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry 4 , e360 (2014).
Curran, E. A. et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child Psychol. Psychiatry 56 , 500–508 (2015).
Chandler, S., Howlin, P., Simonoff, E., Kennedy, J. & Baird, G. Comparison of parental estimate of developmental age with measured IQ in children with neurodevelopmental disorders. Child Care Health Dev. 42 , 486–493 (2016).
Charman, T. et al. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol. Med. 41 , 619–627 (2011).
Sparrow, S. S., Cicchetti, D. & Balla, D. A. Vineland Adaptive Behavior Scales, 2nd Edn. https://doi.org/10.1037/t15164-000 (AGS, 2005).
Jones, R. M., Pickles, A. & Lord, C. Evaluating the quality of peer interactions in children and adolescents with autism with the Penn Interactive Peer Play Scale (PIPPS). Mol. Autism 8 , 28 (2017).
Lord, C., Elsabbagh, M., Baird, G. & Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 392 , 508–520 (2018).
Duncan, A. W. & Bishop, S. L. Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism 19 , 64–72 (2015).
Download references
Acknowledgements
The authors thank J. McCauley, S. Gaspar, K. Byrne and A. Holbrook from UCLA for help with manuscript preparation. S. Tromans is thanked for his updated review of the epidemiology literature. We recognize the many investigators who contributed research that we cannot cite due to space limitations. C.L. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHHD; R01 HD081199), the National Institute of Mental Health (NIMH; R01MH081873-01A1) and the Simons Foundation. T.S.B. is supported by grants from the Health and Social Care Information Centre, Leeds, and the National Institute for Health Research (NIHR HTA; grant ref. NIHR127337). T.C. is supported by grants from Innovative Medicines Initiative 2 (no. 777394), the Medical Research Council (MRC; grants MR/K021389/1) and the NIHR (grant 13/119/18). J.C. is funded by Autistica. G.D. is supported by the Institut Pasteur. T.F. is supported by the Autism Speaks Foundation. E.J.H.J. is supported by grants from the Economic and Social Research Council (ESRC; ES/R009368/1), the Innovative Medicines Initiative 2 (no. 777394), the MRC (MR/K021389/1) and the Simons Foundation (609081). R.M.J. acknowledges the Mortimer D. Sackler Family and the NIMH (R01MH114999). J.L.T. is supported by grants from the FAR fund and the NIMH (R34 MH104428, R03 MH 112783 and R01 MH116058). A.P. is partially supported by the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR (NF-SI-0617-10120). M.W.S. is supported by the National Institutes of Health (NIH; MH106934, MH109901, MH110928, MH116487 MH102342, MH111662, MH105575 and MH115747), the Overlook International Foundation and the Simons Foundation. J.V.-V. is supported by the NIH (MH016434 and MH094604), the Simons Foundation and the New York State Psychiatric Institute. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Author information
Authors and affiliations.
Departments of Psychiatry and School of Education, University of California, Los Angeles, Los Angeles, CA, USA
Catherine Lord
Department of Health Sciences, University of Leicester, Leicester, UK
Traolach S. Brugha
Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK
Tony Charman & Andrew Pickles
Autistica, London, UK
James Cusack
Institut Pasteur, UMR3571 CNRS, Université de Paris, Paris, France
Guillaume Dumas
Autism Speaks, New York, NY, USA
Thomas Frazier
Centre for Brain & Cognitive Development, University of London, London, UK
Emily J. H. Jones
The Sackler Institute for Developmental Psychobiology, New York, NY, USA
Rebecca M. Jones
The Center for Autism and the Developing Brain, White Plains, NY, USA
Department of Psychiatry, Langley Porter Psychiatric Institute and Weill Institute for Neurosciences, University of California, San Francisco, CA, USA
Matthew W. State
Department of Pediatrics and Vanderbilt Kennedy Center, Vanderbilt University Medical Center, Nashville, TN, USA
Julie Lounds Taylor
Department of Psychiatry, Columbia University, New York, NY, USA
Jeremy Veenstra-VanderWeele
You can also search for this author in PubMed Google Scholar
Contributions
All authors read and edited the full document. Introduction (C.L.), Epidemiology (T.S.B.), Mechanisms/pathophysiology (M.W.S., G.D., R.M.J., T.C. and E.J.H.J.), Diagnosis, screening and prevention (T.C., E.J.H.J. and T.S.B.), Management (T.S.B., T.C., E.J.H.J., J.L.T. and J.V.-V.), Quality of life (J.L.T., J.C. and T.F.), Outlook (C.L. and A.P.), Overview of Primer (C.L.).
Corresponding author
Correspondence to Catherine Lord .
Ethics declarations
Competing interests.
C.L. acknowledges the receipt of royalties from Western Psychological Services for the sale of the Autism Diagnostic Interview-Revised (ADIR), the Autism Diagnostic Observation Schedule (ADOS) and the Social Communication Questionnaire (SCQ). T.S.B. has received royalties from Cambridge University Press and Oxford University Press. T.C. has served as a consultant to F. Hoffmann-La Roche. and has received royalties from Guilford Publications and Sage Publications. T.F. has received federal funding research support from, acted as a consultant to, received travel support from, and/or received a speaker’s honorarium from the Brain and Behaviour Research Foundation, Bristol-Myers Squibb, the Cole Family Research Fund, EcoEos, Forest Laboratories, Ingalls Foundation, IntegraGen, Kugona LLC, the National Institutes of Health, Roche Pharma, Shire Development and the Simons Foundation. J.L.T. receives compensation from Sage Publishers for editorial work. A.P. receives royalties from Imperial College Press, Oxford University Press and Western Psychological Services. M.W.S. serves on the scientific advisory boards and has stock or stock options for Arett Pharmaceuticals and BlackThorn Therapeutics. J.V.-V. has consulted or served on an advisory board for Novartis, Roche Pharmaceuticals and SynapDx, has received research funding from Forest, Novartis, Roche Pharmaceuticals, Seaside Therapeutics, SynapDx, and has received an editorial stipend from Springer and Wiley. All other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks S. Spence, P. Szatmari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lord, C., Brugha, T.S., Charman, T. et al. Autism spectrum disorder. Nat Rev Dis Primers 6 , 5 (2020). https://doi.org/10.1038/s41572-019-0138-4
Download citation
Accepted : 26 November 2019
Published : 16 January 2020
DOI : https://doi.org/10.1038/s41572-019-0138-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Pharmacological and non-pharmacological interventions for irritability in autism spectrum disorder: a systematic review and meta-analysis with the grade assessment.
- Hangnyoung Choi
- Jae Han Kim
- Marco Solmi
Molecular Autism (2024)
The gut metabolite indole-3-propionic acid activates ERK1 to restore social function and hippocampal inhibitory synaptic transmission in a 16p11.2 microdeletion mouse model
- Dilong Wang
- Ningning Li
Microbiome (2024)
Effects of mini-basketball training program on social communication impairments and regional homogeneity of brain functions in preschool children with autism spectrum disorder
- Dandan Chen
BMC Sports Science, Medicine and Rehabilitation (2024)
Dietary intake and gastrointestinal symptoms are altered in children with Autism Spectrum Disorder: the relative contribution of autism-linked traits
- Saijun Huang
Nutrition Journal (2024)
Sex, hormones and cerebrovascular function: from development to disorder
- Adeline Collignon
- Laurence Dion-Albert
- Vanessa Coelho-Santos
Fluids and Barriers of the CNS (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Signs and Symptoms
- Living with Autism Spectrum Disorder
- Frequently Asked Questions (FAQs)
- Data and Statistics on Autism Spectrum Disorder
- Autism Materials and Resources
- Diagnosis ASD
- Information on ASD for Healthcare Providers
- Acceptance Month Partner Toolkit
- 2023 Community Report on Autism
- Autism Data Visualization Tool
At a glance
The latest data on autism spectrum disorder (ASD) from CDC's Autism and Developmental Disabilities Monitoring (ADDM) Network.

Prevalence of ASD
- About 1 in 36 children has been identified with autism spectrum disorder (ASD) according to estimates from CDC's Autism and Developmental Disabilities Monitoring (ADDM) Network. [Read Article]
- ASD is reported to occur in all racial, ethnic, and socioeconomic groups. [Read Article]
- ASD is nearly 4 times more common among boys than among girls. [Read Article]
- About 1 in 6 (17%) children aged 3–17 years were diagnosed with a developmental disability, as reported by parents, during a study period of 2009–2017. These included autism, attention-deficit/hyperactivity disorder, blindness, and cerebral palsy, among others. [Read Summary]
What Is Prevalence?
Identified prevalence of asd, addm network 2000-2020: combining data from all sites, resource.
Search through a collection of information from peer-reviewed autism prevalence studies.
Autism Prevalence Studies Data Table
CDC's 2023 Community Report on Autism
Cdc's 2023 community report on autism provides summaries of the latest addm data:.
- A Snapshot of Autism Spectrum Disorder in 2020
- Progress in Early Identification Disrupted during the COVID-19 Pandemic among 4-year-old Children
- A New Pattern in Racial and Ethnic Differences Emerges in ASD Identification among 8-year-old Children
- Site Snapshots Overview
Autism Spectrum Disorder (ASD)
Autism spectrum disorder (ASD) is a developmental disability that can cause significant social, communication and behavioral challenges. CDC is committed to continuing to provide essential data on ASD and develop resources that help identify children with ASD as early as possible.
For Everyone
Health care providers, public health.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
Autism Spectrum Disorder
What is asd.
Autism spectrum disorder (ASD) is a neurological and developmental disorder that affects how people interact with others, communicate, learn, and behave. Although autism can be diagnosed at any age, it is described as a “developmental disorder” because symptoms generally appear in the first 2 years of life.
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) , a guide created by the American Psychiatric Association that health care providers use to diagnose mental disorders, people with ASD often have:
- Difficulty with communication and interaction with other people
- Restricted interests and repetitive behaviors
- Symptoms that affect their ability to function in school, work, and other areas of life
Autism is known as a “spectrum” disorder because there is wide variation in the type and severity of symptoms people experience.
People of all genders, races, ethnicities, and economic backgrounds can be diagnosed with ASD. Although ASD can be a lifelong disorder, treatments and services can improve a person’s symptoms and daily functioning. The American Academy of Pediatrics recommends that all children receive screening for autism. Caregivers should talk to their child’s health care provider about ASD screening or evaluation.
What are the signs and symptoms of ASD?
The list below gives some examples of common types of behaviors in people diagnosed with ASD. Not all people with ASD will have all behaviors, but most will have several of the behaviors listed below.
Social communication / interaction behaviors may include:
- Making little or inconsistent eye contact
- Appearing not to look at or listen to people who are talking
- Infrequently sharing interest, emotion, or enjoyment of objects or activities (including infrequent pointing at or showing things to others)
- Not responding or being slow to respond to one’s name or to other verbal bids for attention
- Having difficulties with the back and forth of conversation
- Often talking at length about a favorite subject without noticing that others are not interested or without giving others a chance to respond
- Displaying facial expressions, movements, and gestures that do not match what is being said
- Having an unusual tone of voice that may sound sing-song or flat and robot-like
- Having trouble understanding another person’s point of view or being unable to predict or understand other people’s actions
- Difficulties adjusting behaviors to social situations
- Difficulties sharing in imaginative play or in making friends
Restrictive / repetitive behaviors may include:
- Repeating certain behaviors or having unusual behaviors, such as repeating words or phrases (a behavior called echolalia)
- Having a lasting intense interest in specific topics, such as numbers, details, or facts
- Showing overly focused interests, such as with moving objects or parts of objects
- Becoming upset by slight changes in a routine and having difficulty with transitions
- Being more sensitive or less sensitive than other people to sensory input, such as light, sound, clothing, or temperature
People with ASD may also experience sleep problems and irritability.
People on the autism spectrum also may have many strengths, including:
- Being able to learn things in detail and remember information for long periods of time
- Being strong visual and auditory learners
- Excelling in math, science, music, or art
What are the causes and risk factors for ASD?
Researchers don’t know the primary causes of ASD, but studies suggest that a person’s genes can act together with aspects of their environment to affect development in ways that lead to ASD. Some factors that are associated with an increased likelihood of developing ASD include:
- Having a sibling with ASD
- Having older parents
- Having certain genetic conditions (such as Down syndrome or Fragile X syndrome)
- Having a very low birth weight
How is ASD diagnosed?
Health care providers diagnose ASD by evaluating a person’s behavior and development. ASD can usually be reliably diagnosed by age 2. It is important to seek an evaluation as soon as possible. The earlier ASD is diagnosed, the sooner treatments and services can begin.
Diagnosis in young children
Diagnosis in young children is often a two-stage process.
Stage 1: General developmental screening during well-child checkups
Every child should receive well-child check-ups with a pediatrician or an early childhood health care provider. The American Academy of Pediatrics recommends that all children receive screening for developmental delays at their 9-, 18-, and 24- or 30-month well-child visits, with specific autism screenings at their 18- and 24-month well-child visits. A child may receive additional screening if they have a higher likelihood of ASD or developmental problems. Children with a higher likelihood of ASD include those who have a family member with ASD, show some behaviors that are typical of ASD, have older parents, have certain genetic conditions, or who had a very low birth weight.
Considering caregivers’ experiences and concerns is an important part of the screening process for young children. The health care provider may ask questions about the child’s behaviors and evaluate those answers in combination with information from ASD screening tools and clinical observations of the child. Read more about screening instruments on the Centers for Disease Control and Prevention (CDC) website.
If a child shows developmental differences in behavior or functioning during this screening process, the health care provider may refer the child for additional evaluation.
Stage 2: Additional diagnostic evaluation
It is important to accurately detect and diagnose children with ASD as early as possible, as this will shed light on their unique strengths and challenges. Early detection also can help caregivers determine which services, educational programs, and behavioral therapies are most likely to be helpful for their child.
A team of health care providers who have experience diagnosing ASD will conduct the diagnostic evaluation. This team may include child neurologists, developmental pediatricians, speech-language pathologists, child psychologists and psychiatrists, educational specialists, and occupational therapists.
The diagnostic evaluation is likely to include:
- Medical and neurological examinations
- Assessment of the child’s cognitive abilities
- Assessment of the child’s language abilities
- Observation of the child’s behavior
- An in-depth conversation with the child’s caregivers about the child’s behavior and development
- Assessment of age-appropriate skills needed to complete daily activities independently, such as eating, dressing, and toileting
Because ASD is a complex disorder that sometimes occurs with other illnesses or learning disorders, the comprehensive evaluation may include:
- Blood tests
- Hearing test
The evaluation may lead to a formal diagnosis and recommendations for treatment.
Diagnosis in older children and adolescents
Caregivers and teachers are often the first to recognize ASD symptoms in older children and adolescents who attend school. The school’s special education team may perform an initial evaluation and then recommend that a child undergo additional evaluation with their primary health care provider or a health care provider who specialize in ASD.
A child’s caregivers may talk with these health care providers about their child’s social difficulties, including problems with subtle communication. For example, some children may have problems understanding tone of voice, facial expressions, or body language. Older children and adolescents may have trouble understanding figures of speech, humor, or sarcasm. They also may have trouble forming friendships with peers.
Diagnosis in adults
Diagnosing ASD in adults is often more difficult than diagnosing ASD in children. In adults, some ASD symptoms can overlap with symptoms of other mental health disorders, such as anxiety disorder or attention-deficit/hyperactivity disorder (ADHD).
Adults who notice signs of ASD should talk with a health care provider and ask for a referral for an ASD evaluation. Although evaluation for ASD in adults is still being refined, adults may be referred to a neuropsychologist, psychologist, or psychiatrist who has experience with ASD. The expert will ask about:
- Social interaction and communication challenges
- Sensory issues
- Repetitive behaviors
- Restricted interests
The evaluation also may include a conversation with caregivers or other family members to learn about the person’s early developmental history, which can help ensure an accurate diagnosis.
Receiving a correct diagnosis of ASD as an adult can help a person understand past challenges, identify personal strengths, and find the right kind of help. Studies are underway to determine the types of services and supports that are most helpful for improving the functioning and community integration of autistic transition-age youth and adults.
What treatment options are available for ASD?
Treatment for ASD should begin as soon as possible after diagnosis. Early treatment for ASD is important as proper care and services can reduce individuals’ difficulties while helping them build on their strengths and learn new skills.
People with ASD may face a wide range of issues, which means that there is no single best treatment for ASD. Working closely with a health care provider is an important part of finding the right combination of treatment and services.
A health care provider may prescribe medication to treat specific symptoms. With medication, a person with ASD may have fewer problems with:
- Irritability
- Repetitive behavior
- Hyperactivity
- Attention problems
- Anxiety and depression
Read more about the latest medication warnings, patient medication guides, and information on newly approved medications at the Food and Drug Administration (FDA) website .
Behavioral, psychological, and educational interventions
People with ASD may be referred to a health care provider who specializes in providing behavioral, psychological, educational, or skill-building interventions. These programs are often highly structured and intensive, and they may involve caregivers, siblings, and other family members. These programs may help people with ASD:
- Learn social, communication, and language skills
- Reduce behaviors that interfere with daily functioning
- Increase or build upon strengths
- Learn life skills for living independently
Other resources
Many services, programs, and other resources are available to help people with ASD. Here are some tips for finding these additional services:
- Contact your health care provider, local health department, school, or autism advocacy group to learn about special programs or local resources.
- Find an autism support group. Sharing information and experiences can help people with ASD and their caregivers learn about treatment options and ASD-related programs.
- Record conversations and meetings with health care providers and teachers. This information may help when it’s time to decide which programs and services are appropriate.
- Keep copies of health care reports and evaluations. This information may help people with ASD qualify for special programs.
How can I find a clinical trial for ASD?
Clinical trials are research studies that look at new ways to prevent, detect, or treat diseases and conditions. The goal of clinical trials is to determine if a new test or treatment works and is safe. Although individuals may benefit from being part of a clinical trial, participants should be aware that the primary purpose of a clinical trial is to gain new scientific knowledge so that others may be better helped in the future.
Researchers at NIMH and around the country conduct many studies with patients and healthy volunteers. We have new and better treatment options today because of what clinical trials uncovered years ago. Be part of tomorrow’s medical breakthroughs. Talk to your health care provider about clinical trials, their benefits and risks, and whether one is right for you.
To learn more or find a study, visit:
- NIMH’s Clinical Trials webpage : Information about participating in clinical trials
- Clinicaltrials.gov: Current Studies on ASD : List of clinical trials funded by the National Institutes of Health (NIH) being conducted across the country
Where can I learn more about ASD?
Free brochures and shareable resources.
- Autism Spectrum Disorder : This brochure provides information about the symptoms, diagnosis, and treatment of ASD. Also available en español .
- Digital Shareables on Autism Spectrum Disorder : Help support ASD awareness and education in your community. Use these digital resources, including graphics and messages, to spread the word about ASD.
Federal resources
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Neurological Disorders and Stroke
- National Institute on Deafness and Other Communication Disorders
- Centers for Disease Control and Prevention (CDC)
- Interagency Autism Coordinating Committee
- MedlinePlus (also available en español )
Research and statistics
- Science News About Autism Spectrum Disorder : This NIMH webpage provides press releases and announcements about ASD.
- Research Program on Autism Spectrum Disorders : This NIMH program supports research focused on the characterization, pathophysiology, treatment, and outcomes of ASD and related disorders.
- Statistics: Autism Spectrum Disorder : This NIMH webpage provides information on the prevalence of ASD in the U.S.
- Data & Statistics on Autism Spectrum Disorder : This CDC webpage provides data, statistics, and tools about prevalence and demographic characteristics of ASD.
- Autism and Developmental Disabilities Monitoring (ADDM) Network : This CDC-funded program collects data to better understand the population of children with ASD.
- Biomarkers Consortium - The Autism Biomarkers Consortium for Clinical Trials (ABC-CT) : This Foundation for the National Institutes of Health project seeks to establish biomarkers to improve treatments for children with ASD.
Last Reviewed: February 2024
Unless otherwise specified, the information on our website and in our publications is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.

- Copy/Paste Link Link Copied
What are the treatments for autism?
There is currently no one standard treatment for autism spectrum disorder (ASD).
But there are many ways to help minimize the symptoms and maximize abilities. People who have ASD have the best chance of using all of their abilities and skills if they receive appropriate therapies and interventions.
The most effective therapies and interventions are often different for each person. However, most people with ASD respond best to highly structured and specialized programs. 1 In some cases, treatment can greatly reduce symptoms and help people with autism with daily activities.
Research shows that early diagnosis and interventions, such as during preschool or before, are more likely to have major positive effects on symptoms and later skills. Read more about early interventions for autism.
Because there can be overlap in symptoms between ASD and other disorders, such as attention deficit hyperactivity disorder (ADHD), 2 it's important that treatment focus on a person's specific needs, rather than the diagnostic label.
Select the links for more information on each type of treatment for ASD.
- Behavioral management therapy
- Cognitive behavior therapy
- Early intervention
- Educational and school-based therapies
- Joint attention therapy
- Medication treatment
- Nutritional therapy
- Occupational therapy
- Parent-mediated therapy
- Physical therapy
- Social skills training
- Speech-language therapy
If you have a question about treatment, talk to a health care provider who specializes in caring for people with ASD. These resources have more information about treatments for autism:
- The Centers for Disease Control and Prevention describes some treatment options. http://www.cdc.gov/ncbddd/autism/treatment.html
- National Institute of Mental Health. (2011). A parent's guide to autism spectrum disorder. Retrieved March 8, 2012, from http://www.nimh.nih.gov/health/publications/a-parents-guide-to-autism-spectrum-disorder/index.shtml
- Kotte, A., Joshi, G., Fried, R., Uchida, M., Spencer, A., Woodworth, K. Y., et al. (2013). Autistic traits in children with and without ADHD. Pediatrics, 132 (3), e612–e622.

IMAGES
COMMENTS
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition that has been intensely investigated since the mid-20th century. ... Daily science news on research developments and the ...
S cientists have made a breakthrough in our understanding of the neuroscience behind autism spectrum disorders that promises to "revolutionize" the way we approach treatment, scientists say. The ...
Autism spectrum disorders are a group of neurodevelopmental disorders that are characterized by impaired social interaction and communication skills, and are often accompanied by other behavioural ...
Breakthrough Treatment for Autism Spectrum Disorder Within Reach: Scientists Discover Key Clue. By DGIST (Daegu Gyeongbuk Institute of Science and Technology) December 25, 2022. In research that could lead to a fundamental treatment of autism spectrum disorder, scientists have identified the cell-specific molecular network of the developmental ...
Researchers have identified 46 genes that can disrupt a process that is critical to early brain development. The finding could help scientists find new treatments for disorders including autism.
In late 2001-early 2002 we received four exciting papers with findings on the genetics of autism that were published together in our March 2002 issue, with an accompanying editorial [2,3,4,5,6 ...
Researchers have shed new light on the changes in metabolism that occur between birth and the presentation of autism spectrum disorder (ASD) later in childhood. The researchers discovered that a ...
Autism spectrum disorder (ASD) is a class of neurodevelopmental conditions with a large epidemiological and societal impact worldwide. To date, numerous studies have investigated the associations ...
Simons Foundation Autism Research Initiative, Simons Foundation, New York, NY, USA. Department of Pediatrics, Yale School of Medicine, New Haven, CT, USA. ... Autism—or autism spectrum disorder, the formal term used in the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) and WHO's ...
June 25, 2019 — A new study that sheds light on how the brain processes language could lead to a better understanding of autism spectrum disorder, schizophrenia and other neurodevelopmental ...
Understanding the role of a new enzyme in the development of autism spectrum disorder Jan 4, 2024 Vitamin D alters developing neurons in the brain's dopamine circuit, finds study
Nov. 11, 2021 — Research suggested that autism spectrum disorder (ASD) may be at least partly caused by differences in the composition of the gut microbiota, based on the observation that ...
Epidemiology and Etiopathogenesis of Autism Spectrum Disorder. According to the most recent epidemiological studies carried out in the United States, ASD recurs in 1 in 36 children at age 8, and it is about 4 times more frequent among males than females. 9 The prevalence of this condition has increased enormously over the last few decades; This increase would be to some extent apparent as ...
Abstract. While behavioral interventions remain the mainstay of treatment of autism spectrum disorder (ASD), several potential targeted treatments addressing the underlying neurophysiology of ASD have emerged in the last few years. These are promising for the potential to, in future, become part of the mainstay treatment in addressing the core ...
About the journal. Research in Autism Spectrum Disorders (RASD) publishes high quality empirical articles and reviews that contribute to a better understanding of Autism Spectrum Disorders (ASD) at all levels of description; genetic, neurobiological, cognitive, and behavioral. The primary focus of the journal is to …. View full aims & scope.
Dr. Evdokia Anagnostou presented the results of neuroimaging studies at the International Society for Autism Research 2022 annual meeting. Of note, brain differences clustered along dimensions of ...
Introduction. Autism spectrum disorder (ASD) refers to a group of early-onset, lifelong, heterogeneous neurodevelopmental conditions with complex mechanisms of emergence ().The prevalence of ASD has increased from 1 in 69 by 2012 to 1 in 44 by 2018, as reported by the Centers for Disease Control and Prevention for 2012-2018 (2, 3).Recent research estimates the male-to-female ratio is closer ...
Advances in autism research, 2021: continuing to decipher the secrets of autism 1427 reveals a module of co-expressed genes consistently associated with autism spectrum disorder.
Prevalence figures that referred to 4.5 per 10,000 in the 1960s have been replaced by newer estimates suggesting that 1 in 59 children (16 per 1,000) present with an autism spectrum disorder (ASD) in 2014 . The widening of the definition of autism has undoubtedly contributed to the significant increase in the numbers of people being diagnosed.
A recent study analyzed the DNA of more than 35,584 people worldwide, including 11,986 autistic individuals. The scientists identified variants in 102 genes linked with an increased probability of ...
The first paragraph says "… and sheds light on what causes intellectual disabilities, such as autism spectrum disorders". The rest of the article uses "intellectual disabilities and autism" which accurately reflects the research findings. This needs to be edited as makes the authors appear ignorant to anyone who knows anything about ...
ARI works to advance the understanding of autism spectrum disorder by funding research and education on its causes and treatments. ... Autism Research Review International ARI's award-winning quarterly journal is now available for free online Learn More Check out our Winter, 2024 issue and browse past publications ... Get News & Updates from ...
Definition. Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors ( 1 ). In 2013, the Diagnostic and Statistical Manual of Mental Disorders —5 th edition (DSM-5) was published, updating the diagnostic criteria for ASD ...
A new study demonstrates the profound influence of gut microbes on behavior, particularly in the context of autism spectrum disorders (ASD). The study reveals that gut discomfort in mice reduces social behaviors, a phenomenon reversible by specific bacterial introductions.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder affecting individuals worldwide and characterized by deficits in social interaction along with the presence of restricted interest and repetitive behaviors. Despite decades of behavioral research, little is known about the brain mechanisms that influence social behaviors among children with ASD. This, in part, is due to ...
Abstract. Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted ...
A New Pattern in Racial and Ethnic Differences Emerges in ASD Identification among 8-year-old Children; Site Snapshots Overview; On This Page ... Autism spectrum disorder (ASD) is a developmental disability that can cause significant social, communication and behavioral challenges. CDC is committed to continuing to provide essential data on ASD ...
Learn about autism spectrum disorder, including signs and symptoms, causes, how it is diagnosed, and potential treatments. ... Clinical trials are research studies that look at new ways to prevent, detect, or treat diseases and conditions. The goal of clinical trials is to determine if a new test or treatment works and is safe.
There is currently no one standard treatment for autism spectrum disorder (ASD). There is currently no one standard treatment for autism spectrum disorder (ASD). ... treatment can greatly reduce symptoms and help people with autism with daily activities. Research shows that early diagnosis and interventions, such as during preschool or before ...