- Research article
- Open access
- Published: 09 October 2021

To control floating drug delivery system in a simulated gastric environment by adjusting the Shell layer formulation
- Yu-Tung Hsu 1 ,
- Chen-Yu Kao 2 , 3 ,
- Ming-Hua Ho ORCID: orcid.org/0000-0001-6620-4207 1 , 4 &
- Shiao-Pieng Lee 5 , 6
Biomaterials Research volume 25 , Article number: 31 ( 2021 ) Cite this article
5499 Accesses
7 Citations
Metrics details
Gastroretentive drug delivery system (GDDS) are novel systems that have been recently developed for treating stomach diseases. The key function of all GDDS systems is to control the retention time in the stomach. However, research into the bulk density or entanglement of polymers, especially regarding their effects on drug float and release times, is scarce.
In this research, we prepared the floating core-shell beads carrying tetracycline. The ratio of chitosan and xanthan gum in the shell layer was changed to modify polymer compactness. Tetracycline was encapsulated in the alginate core.
Using scanning electron microscopy (SEM) techniques, we observed that the shell formulation did not change the bead morphology. The cross-sectional images showed that the beads were highly porous. The interaction between anionic xanthan gum and cationic chitosan made the shell layer dense, resisting to the mass transfer in the shell layer. Due to the high mass transfer resistance to water penetration, the longer float and delivery time were caused by the dense surface of the beads. The cell culture demonstrated that floating core-shell beads were biocompatible. Importantly, the beads with tetracycline showed a significant prolonged anti-bacterial effect.
Research results proved that the floating and releasing progress of core-shell beads can be well controlled by adjusting the shell layer formulation that could promote the function of gastroretentive drugs.
Introduction
Oral administration is the most common drug delivery method as it is multifunctional and convenient [ 1 ]. The size of drug carriers is usually adjusted to 1–2 mm, allowing the medicine in the stomach to pass through the pylorus and enter into the small intestine [ 2 ]. Advances in pharmaceutical techniques have enabled to release drugs in a specific position in vivo to lower toxicity, decrease side effects, and promote efficiency. Thus, the gastroretentive drug delivery system (GDDS) has been developed for treating stomach cancer, ulcer and infection. GDDS could keep drugs in stomach for a prolonged period to achieve a specific release, including several types: floating, mucoadhesive, expandable and rafting forming drug delivery systems.
The floating drug delivery system (FDDS) is also called a hydrodynamically balanced system (HBS). In FDDS, the drugs carriers float in gastric juice to ensure that drugs do not leave stomach shortly. It efficiently increases drug bioavailability by prolonging the release period. The variation of drug concentrations in blood is also decreased [ 3 ]. FDDS is a potential treatment for stomach and duodenum diseases. The density of FDDS carriers must be lower than those of the gastric juice and chymus. Therefore, medicines float and are slowly released in the stomach, compared with the convectional drug delivery methods. Most antibacterial agents have low minimum inhibitory concentration (MIC) to Helicobacter Pylori in vitro, but are not very effective for the eradication of infection caused by Helicobacter Pylori in vivo . The short residence time is the key problem [ 4 ]. Better stability and prolonged residence time allow more effective antibiotic penetration through the gastric mucus layer to suppress or eradicate Helicobacter Pylori in stomach [ 5 , 6 ], which would be achieved by FDDS.
Currently, most FDDS would not float for 2–8 h; however, the drug float and release periods need to be prolonged. To obtain improved drug float and release times, previous research studies have widely investigated control of the drug carrier materials. Kawashima, Sato, Thanoo [ 7 , 8 , 9 , 10 ] et al. prepared hollow spheres for FDDS by using the emulsion-solvent diffusion method. These studies focused on controlling the carrier density by solvent diffusion. In particular, polymer porosity dominates the solvent diffusion rates that determining the carrier floating behaviors. Xu, Choi and El-Kamel et al. added gas-forming/generating agents in polymers to increase the porosity, and therefore, the floating properties [ 11 , 12 , 13 ]. Indeed, the porosity was influenced by the amount of gas-generating agents. According to prior researches, the gas generating agents and solvent diffusion would significantly affect the porosity in FDDS. In contrast, research into the bulk density or entanglement of polymers, especially regarding their effects on drug float and release times, is scarce.
In this research, we prepared the core-shell floating particles for GDDS. We used two polymers, chitosan and xanthan gum, as the shell layer, which are cationic and anionic, respectively. Chitosan and xanthan gum were widely used in previous researches for the drug delivery, so their biocompatibility and biodegradability have been well approved. Manca et al. controlled the ratios of chitosan and xanthan gum to adjust the surface potential of liposome with coating layer, which influenced the rheological properties of microparticles in aerosol performance [ 14 ]. In the study of Kulkarni et al., chitosan and xanthan gum were blended to produce dense particles. Their results supported that the chitosan/xanthan gum ratio influenced the mucoadhesive properties [ 15 ]. Fareez et al. prepared chitosan-coated alginate/xanthan gum beads. The surface properties of beads were determined by chitosan shell layer [ 16 ]. Although chitosan and xanthan gum were used in these studies, the core-shell and porous particles with shell layer controlled by chitosan and xanthan gum interactions have been never applied for the FDDS and GDDS system.
Adjusting the chitosan/xanthan gum (C/X) ratio enabled us to adjust the polymeric entanglement, and allowed us to control the shell layer properties and structures in floating beads. We studied how the shell layer affects the float, release and biocompatibility properties. Moreover, the efficient drug carriers with better duration in GDDS would be developed by controlling the shell layer properties.
Materials and methods
Alginic acid sodium salt (medium viscosity), xanthan gum (from Xanthomonas campestris ), NaHCO 3 (sodium bicarbonate powder), chitosan (low molecular weight), tetracycline (> 98.0%) were purchased from Sigma-Aldrich (St. Louis, MO). CaCl 2 (calcium chloride) and HCl and acetic acid were purchased from J.T. Baker, Japan. Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), penicillin, trypsin was purchased from Gibco Life Technologies (Thermo Fisher Scientific - TW). Kaighn’s modification of Ham’s F-12 medium was purchased from Manassas, VA, USA. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) kit was purchased from Carlsbad, CA, USA. Distilled deionized (DI) water was used throughout the experiment.
Preparation of floating beads containing tetracycline
Two solutions were prepared respectively for core-shell beads. One was the tetracycline-alginate aqueous solution for core, and the other was the chitosan/xanthan-gum (C/X) solution for shell. First, 50 mg tetracycline and 200 mg alginate were mixed in 10 ml DI water, and stirred for 2 h to obtain the tetracycline-alginate solution. 200 mg NaHCO3 was then added into alginate solution, and stirred at 500 rpm for 1 h.
On the other hand, the C/X solution was prepared by mixing chitosan solution and xanthan gum solution. The chitosan solution was fabricated by dissolving chitosan powders in 1 v/v% acetic acid aqueous solution, and xanthan gum solution was prepared by dissolving xanthan gum in DI water. After that, chitosan and xanthan gum solutions were mixed with different C/X ratios of 4:1, 4:2, 4:3, 4:4 and 1:4, where CaCl2 was also added as the concentration of 1 w/v%.
After finishing tetracycline-alginate solution for core and C/X solution for shell respectively, we extruded the tetracycline-alginate solution through a 26-gauge needle into the continuously stirred C/X solution. The C/X shell layer was then formed outside the alginate core, and the solution with floating beads was stabilized after continuously stirred for 15 min. After the beads were collected and washed with DI water, they were vacuum dried for 48 h and then stored in 4 °C until used. The exact formulations of core and shell layer were described in Table 1 .
FE-SEM (field emission scanning electron microscope) analysis
SEM (JEOL JSM-6390LV, Japan) images were taken to analyze the morphology of floating beads. The beads were dried at 4 °C. After that, the bead samples were spread and fixed on the metal plate with double-sided carbon tape, and a gold layer was then coated on the sample surface under vacuum using an auto-sputter coater for 1 min under argon atmosphere. The beads morphologies were then observed with SEM. With SEM images, the bead diameters were measured by Image J software.
Swelling and floating test
The swelling percentage of beads was determined by measuring the extent of swelling of the polymer matrix in pH 2 aqueous solution. The weight of dried beads was recorded. After the immersion in pH 2 buffer for 1, 2, 4, 6, 8, 12 and 24 h, water on bead surfaces was removed by filter paper and the weight beads were measured again. The swelling percentage was calculated by using the following equation.
For the floating analysis, twenty dried beads were kept in pH 2 buffer for 24 h with continuous shaking. After 1, 2, 4, 6, 8, 12 and 24 h, the floating percentage was calculated according to following equation:
N f : number of floating beads; Ns: number of settled beads
In vitro release and encapsulation efficiency of tetracycline
The dry beads were immersed in pH 2 buffer in the dynamic gastric simulator [ 17 ]. After 2, 4, 6, 8, 12 and 24 h, 10 ml solution was taken out and analyzed by UV/VIS spectrophotometer at 266 nm, followed by refilling fresh 10 ml buffer.
In the analysis of encapsulation efficiency, beads were suspended in pH 2 buffer with continuous shaking at 37 °C for 24 h, where a part of encapsulated tetracycline was released. Then, the beads were completely broken by using ultrasonic shaker for 30 min, and the residual tetracycline would be completely released. After the solution was filtrated, UV/VIS spectrophotometer was applied to quantify tetracycline in buffer by deducting the absorbance at 266 nm, allowing the determination of encapsulation efficiency as following equation.
Cytotoxicity test
MTT assay was applied to evaluate the cytotoxicity of core-shell beads. In this experiment, a stomach cell line, AGS, was seeded on a 24-well plate with the density of 5 × 10 3 cells per well. AGS has been applied in the cytotoxicity of biomaterials for stomach in previous researches [ 18 ]. As the cell monolayer were cultured to confluence, they were exposed to fluid extracts. The extracts were obtained by placing the core-shell beads in culture medium (0.2 g core-shell particles in 1 ml medium) for 24 h at 37 °C. The C/X = 4:3 core-shell beads were applied in this research. Each fluid extract obtained was then applied to AGS monolayer, replacing the medium that had nourished the cells. The cells were then cultured with extracts for 1 day. After the cell culture, the metabolic activity of AGS was determined by MTT assay. The cells were incubated with 1 mg/mL of MTT for 4 h. Then, the MTT was removed and the formazan crystals were dissolved with dimethyl sulfoxide for 30 min. Finally, absorbance values were read at 570 nm by using an automatic microplate reader (ELx800; Bio-Tek Instruments, Winooski, VT, USA).
Antibacterial testing
LB medium (from Creative Life Science Co., Ltd.) was applied for the culture of E. coli which was from Bioresources Conservation and Research Center (BCRC), Taiwan. The medium with cultured bacterial was added onto agar plate evenly, followed by an overnight culture. After that, core-shell beads were added onto the agar plate, and the antibacterial effects were observed at various time points.
Statistical analysis
The one-way ANOVA was used to analyze the statistical significance of particle diameters, encapsulation efficiency, floating percentage, and cell viability. The two-way ANOVA was used to analyze the statistical significance of swell percentage.
Table 1 demonstrates the floating bead formulations. In this research, the mass ratio of chitosan to xanthan gum (C/X) in the shell layer was adjusted to 4:1, 4:2, 4:3, 4:4 and 1:4, where the amounts of salts (CaCl 2 and NaHCO 3 ) and alginate in core were fixed.
The morphologies of core-shell particles were analyzed by using SEM as shown in Fig. 1 (a). The beads were roughly spherical, with a diameter ranging from 1.5 to 2 mm. The particle diameters were analyzed using Image J and presented in Fig. 1 (b). The formulation of the shell layer had a weak impact on the particle morphologies and diameters. The ANOVA analysis indicated p > 0.1 for the formulation effects on particle diameters, revealing the ratios of chitosan and xanthan gum did not change particle size significantly. The cross-sectional images of core-shell beads are presented in Fig. 2 . Results show that the particles prepared in this research were highly porous. On the other hand, the surfaces of floating beads were highly dense. The whole particle was highly dense when only alginate was applied to prepare dense bead.
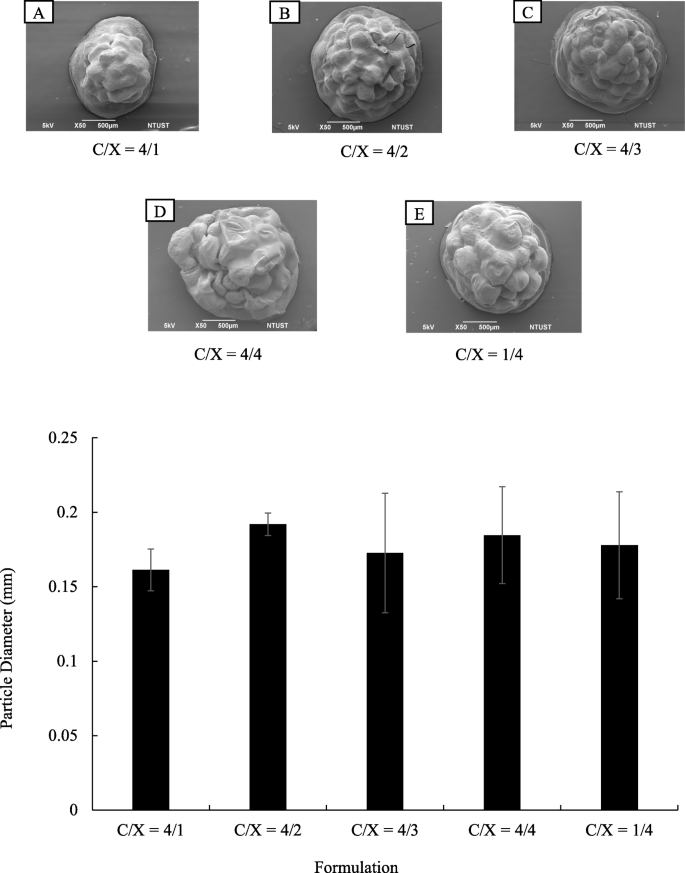
Morphologies of core-shell floating beads with various formulation in chitosan/xanthan-gum shell layer ( n = 20). (a) SEM images of floating beads. The C/X ratios are 4:1 (A), 4:2 (B), 4:3 (C), 4:4. (D) and 1:4 (E). (b) Diameters of beads with various formulations in chitosan/xanthan-gum (C/X) shell layer ( p > 0.1 form ANOVA test, n ≥ 10)

The cross-sectional SEM images of floating beads with various formulation in chitosan and xanthan gum shell layer. The C/X ratios are 4:1 (A), 4:2 (B), 4:3 (C), 4:4 (D) and 1:4 (E). The magnified images of alginate dense bead (F) and floating beads with C/X = 4:3 (G) were presented to identify the dense and porous structures in beads
Figure 3 revealed the swelling ratios of core-shell particles with different immersion periods at pH 2. As shown in Fig. 3 (a), all kinds of particles were gradually swelled during first 6 h ( p < 0.05 from ANOVA test) and reached a steady state over the next 2 h. The highest swelling ratios at steady state were 150–250%.
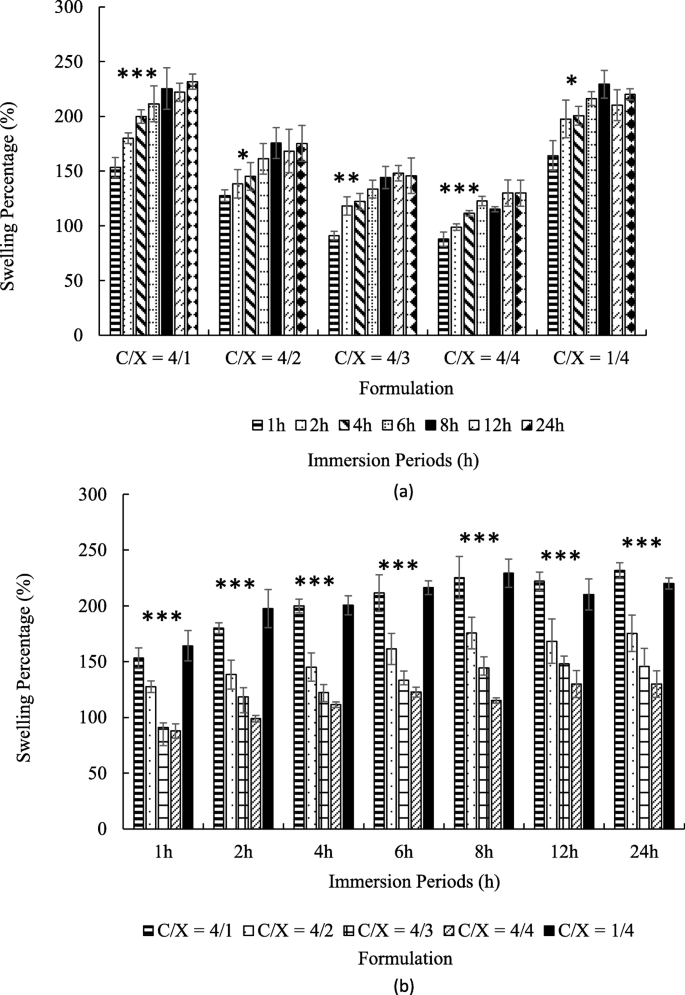
The swelling ratios of core-shell beads with various in chitosan/xanthan-gum (C/X) formulation in pH = 2 buffer ( n ≥ 4). The times for immersion were 1, 2, 4, 6, 8, 12 and 24 h. The results were pooled in accordance with formulation (a) and with immersion period (b). In (a), the significance from ANOVA test from 1 h to 6 h with fixed formulation was marked by * ( p < 0.05), ** ( p < 0.01) and *** ( p < 0.005) for indicated groups. In (b), the significance from ANOVA test for C/X ratio with fixed immersion time was marked by *** ( p < 0.005)
Figure 3 (b) shows that the particles are swelled faster and steady-state swelling ratios increased when the amounts of chitosan were much higher or much lower than the amounts of xanthan gum, such as C/X = 4:1 and 1:4. In contrast, the swelling ratios were relatively low with C/X = 4:3 and 4:4. At all the given time point, C/X = 4/1 and 1/4 showed the highest swelling ratio, and the second high were C/X = 4/2. The lowest swelling ratio appeared in C/X = 4/3 and C/X = 4/4. All differences mentioned in this paragraph were significant according to t-test ( p < 0.05).
Figure 4 presents the floating percentages of core-shell beads in the aqueous solution with pH 2 after immersion for different periods. Figure 4 shows that the floating percentage of chitosan and alginate beads respectively decreased to 55 and 11.6% after 24 h when there is no core-shell structure. Results in Fig. 4 also show that about 90 and 88% of core-shell particles would still keep their floating conditions after 8 and 24 h. The differences between core-shell beads and non-core-shell beads (dense chitosan and alginate beads) were statistically significant after the immersion for 6 h. On the contrary, the ANOVA test indicated that there is no significant difference caused by the ratios of chitosan and xanthan gum ( p > 0.15).
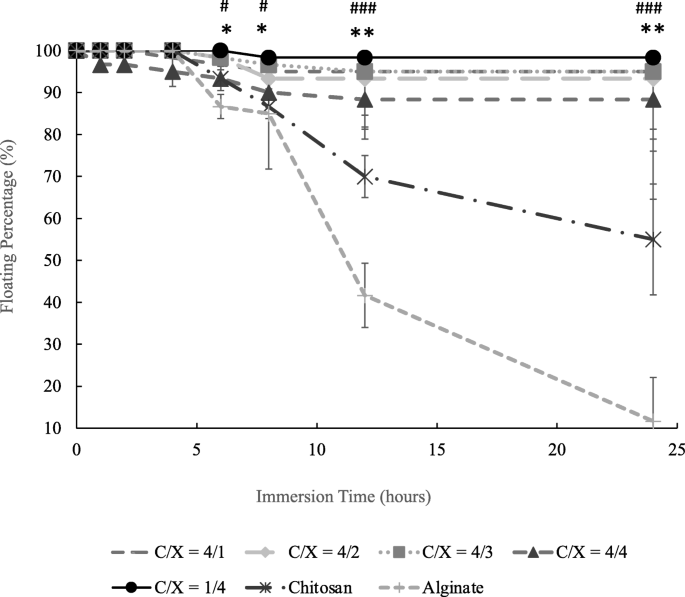
The floating percentage of core-shell beads with various chitosan/xanthan gum ratios, chitosan beads and alginate beads ( n ≥ 3). The significant difference between core-shell beads with all the formulations and dense chitosan beads was indicated by * ( p < 0.05) and ** ( p < 0.01) from t-test at the same immersion time. The significant difference between core-shell beads with all the formulations and dense alginate beads was indicated by # ( p < 0.05), ## ( p < 0.01) and ### ( p < 0.005) from t-test at the same immersion time ( n ≥ 3). Chitosan and alginate beads were dense particles without core-shell structure. There is not significant differences between floating beads with different C/X ( p > 0.15) from ANOVA test
The encapsulation efficiency and releasing rate of tetracycline of floating beads are described in Fig. 5 . There was significant difference in the formulation C/X = 4/1 and C/X = 4/4, as revealed in Fig. 5 (a). The release profile in Fig. 5 (b) was evaluated in dynamic conditions. The results support that the formulation of shell layer actually influence the releases of tetracycline ( p < 0.1 and p < 0.05 from ANOVA test) at 2nd, 4th and 6th hr. That is, the particles released tetracycline faster when the amounts of chitosan are much higher or much lower than the amounts of xanthan gum, such as C/X = 4:1 and 1:4.
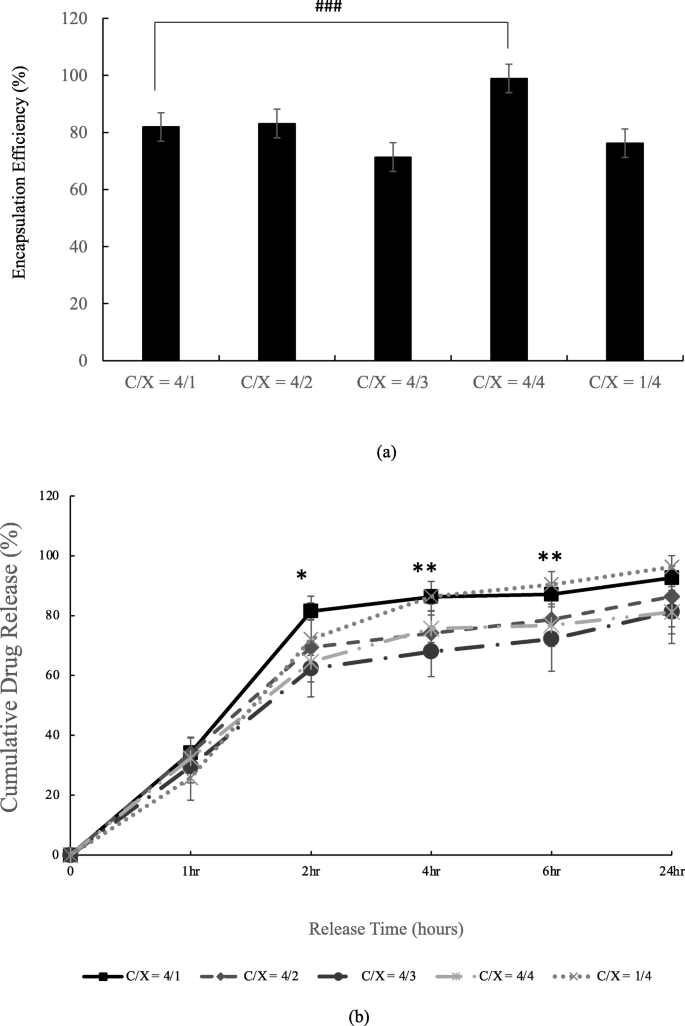
Encapsulation efficiency (a) and releasing profile (b) of tetracycline-alginate floating beads in pH = 2 buffer. In (a), the significant differences were indicated by ### ( p < 0.005) from t-test ( n ≥ 3). In (b), the significant differences were indicated by * ( p < 0.1) and ** ( p < 0.05) from ANOVA test with the same release time ( n ≥ 4)
The biocompatibility of floating particles was analyzed by culturing AGS cell line. The C/X = 4:3 core-shell beads were applied because they showed the best floating and long-term release properties in this research. The results in Fig. 6 identify the good biocompatibility of floating beads when the bead concentration was as high as 1.5 mg/ml, which contained 149.03 μg/ml tetracycline, 112.9 times higher than the effective concentration of tetracycline for a 60-kg person [ 17 ].
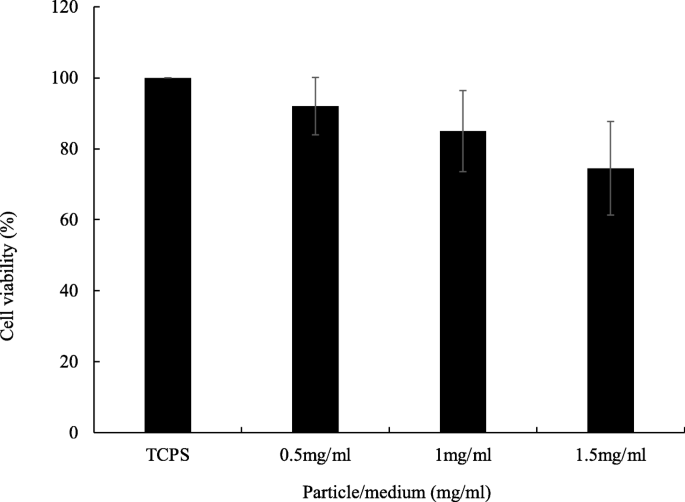
Biocompatibility of floating beads. The bead amounts in culture medium were 0.5, 1 and 1.5 mg/ml, respectively. Floating beads are core-shell beads with C/X = 4/3. TCPS was tissue culture polystyrene which was used as the controlled group. The culture period was 24 h. From ANOVA test, there was no statistical difference ( p > 0.05 and n ≥ 4). From t test, all the core-shell bead groups were not significantly different from TCPS ( p > 0.05 and n ≥ 4)
The anti-bacterial effects of floating beads are proved in Fig. 7 . The obvious anti-bacterial rings are formed by applying beads with encapsulated tetracycline onto cultured E. coli. It was caused by the released tetracycline, and the core-shell beads without tetracycline did not result in any anti-bacterial ring (Fig. 7 (a), (b), and (c)). To identify the duration of the floating particles, we immersed the particles in a pH 2-buffer for 2 and 4 h before conducting the anti-bacterial experiments. The results in Fig. 7 (e) and (f) proved that the beads can efficiently suppress E. coli though there was a pre-release for 2 and 4 h. Compared with the beads without prerelease in Fig. 7 (d), the anti-bacterial effect did not decay, as shown in in Fig. 7 (e) and (f). Figure 7 (g) presented the anti-bacterial effects of alginate beads without tetracycline. The alginate beads were immersed in a pH 2-buffer for 4 h before the test. The result shows that the alginate bead is not effective on E. coli .
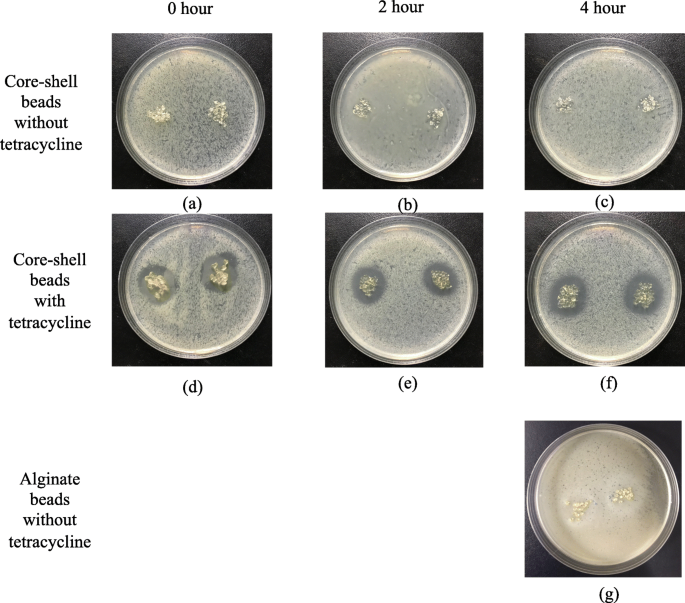
Antibacterial effects of floating beads with/without tetracycline for different immersion periods. (a), (b) and (c) are core-shell beads (C/X=4/3) without tetracycline, and (d), (e) and (f) are core-shell beads (C/X=4/3) with tetracycline. (g) is non-core-shell alginate beads without tetracycline. The immersion time before antibacterial test is 0 hour for (a) (d), 2 hours for (b) (e), and 4 hours for (c) (f) and (g). The beads in (d), (e) and (f) encapsulate 69.9 μg tetracycline in total
The densities of chitosan and xanthan gum used in this research were approximately 0.3 and 1.5 g/cm 3 , respectively. The bulk densities of blended chitosan and xanthan gum were approximately 1.47 g/cm 3 when the ratio was 1:1. After get mixed, chitosan and xanthan gum demonstrated high density compared with their original values. Thus, we assume that interactions between chitosan and xanthan gum increase the density of the two components. The anionic polymer chains of xanthan gum and protonated chitosan exert high intermolecular forces because of the electro-affinity. Polymer interactions make the shell layer dense. In previous researches, polyelectrolyte complexes (PECs) were prepared by blending chitosan and polyanions, such as carrageenan [ 19 ], alginate [ 20 ], poly (acrylic acid) (PAA) and poly (vinylpyrrolidone) (PVP) [ 21 ]. By varying the amounts of positive chitosan and negative polymers, the charge density would be changed, causing differences in the diffusion through PECs. The mass transfer properties were highly related to the polymeric electrostatic interactions, corresponded to our finding in this research.
According to SEM images, the bead surfaces are dense, which would prolong floating periods due to the resistance of mass transfer in water penetration. The dense skin layer could be help prolong the release time of encapsulated drugs. The C/X ratios did not significantly affect the porosity of core-shell beads.
The positive charges of chitosan and negative charges of xanthan gum would result in a strong polymer interaction. These interactions make the shell layer dense, forming a resistance of mass transfer in the shell layer. This hinders water penetration into particles, and therefore, the swelling ratio is low. This result in Fig. 3 revealed that the particle swelling can be tuned by controlling the electro-statistical properties of the shell layer. Besides, the high swelling ratios of core-shell beads show that all the beads developed in this research are very hydrophilic. The hydrophilic particles can provide good encapsulation efficiency and release profile due to the high affinity between the drugs and particles.
The retention periods of FDDS in the stomach reported in previous studies were about 2–4 h [ 22 ]. The floating time of core-shell particles was about 24 h, which was much longer than the above residual periods. It indicates that the dense shell layer developed in this research can prolong the floating time of drugs in the stomach.
When the amounts of chitosan are much higher or much lower than xanthan gum (C/X = 4:1 and 1:4), the release of tetracycline is also higher than those from C/X = 4:3 and 4:4. This is due to the strong interactions between positive chitosan and negative xanthan gum, which results in a dense shell layer. With the dense shell layer, the particle swelling is slow, and the tetracycline release is delayed due to the high resistance in mass transfer. The results supported that the prolonged release can be achieved by controlling the formulation of the dense layer in the core-shell floating beads.
Many researches supported that the ionic crosslinking, which was achieved by mixing chitosan and polyanions, was effective on the control release [ 23 , 24 , 25 ]. In this research, we adjusted the ratio between positive chitosan and negative xanthan gum, and the diffusion properties were thus controlled. The differences between this study and previous researches lies in the components diffusing through polycation/polyanion layer. The water diffusion through shell layer was influenced in this work to prolong the swelling and floating periods of core-shell porous beads. On the other hand, most previous researches focused on the controlled release of encapsulated drugs but not on the floating behaviors.
The floating beads are proved to be biocompatible, and can carry effective antibiotics when they were applied. The released tetracycline from core-shell beads present clear antibacterial effects. According to the statistical analysis, the C/X ratio significantly prolonged the swelling ( p < 0.005 from ANOVA test) and drug release ( p < 0.1 and p < 0.05 from ANOVA test). This supports that the core-shell beads developed in this research can continuously delivery antibiotics for a long period for a certain period.
In this research, we developed core-shell floating beads for GDDS with porous alginate core and a dense chitosan/xanthan-gum shell layer. The compactness of the shell layer in floating beads was controlled by adjusting the ratios of anionic xanthan gum and cationic chitosan. When the C/X ratio was 4:3 and 4:4, the shell layer would be dense and would cause high resistance of mass transfer under the water penetration. Thus, a low swelling rate and a prolonged release was achieved. The experimental results proved the high biocompatibility of the floating beads, and the anti-bacterial effects of beads were also significant after the release for 4 h. This study proposed the method to modify the properties of shell layer, allowing the control of the swelling and release behaviors of floating beads.
Availability of data and materials
All data generated or analyzed in this study are included in this published article.
Abbreviations
Gastroretentive drug delivery system
Scanning electron microscopy
Floating drug delivery system
Hydrodynamically balanced system
Minimum inhibitory concentration
Chitosan/xanthan-gum ratio
Dulbecco’s modified eagle medium
Fetal bovine serum
Analysis of variance
Patel SS, Ray S, Thakur RS. Formualtion and evaluation of floating drug delivery system containing clarithromycin for helicobacter pylori. Acta Pol Pharm. 2006;63:53–61.
CAS Google Scholar
Nayak RMAK, Biswarup D. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res. 2010;3:2–10.
Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: a review. AAPS Pharm Sci Tech. 2005;6:E372–90.
Article Google Scholar
Shah S, Qaqish R, Patel V, Amiji M. Evaluation of the factors influencing stomach-specific delivery of antibacterial agents for helicobacter pylori infection. J Pharm Pharmacol. 1999;51:667–72.
Article CAS Google Scholar
Yokel RA, Dickey KM, Goldberg AH. Selective adherence of a sucralfate-tetracycline complex to gastric ulcers: implications for the treatment of Helicobacter pylori . Biopharm Drug Dispos. 1005;16:475–9.
Umamaheshwari RB, Suman R, Jain NK. Anti helicobacter pylori effect of mucoadhesive nanoparticle bearing amoxicillin in experimental gerbils. APPS Pharm Sci Tech. 2004;325:60–8.
Kawashima Y, Niwa T, Takeuchi H, Hino T, Ito Y. Preparation of multiple unit hollow microspheres (microballoons) with acrylic resin containing tranilast and their drug release characteristics ( in vitro ) and floating behavior ( in vivo ). J Control Release. 1991;16:279–90.
Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. In vitro evaluation of floating and drug releasing behaviors of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm. 2004;57:235–43.
Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm. 2003;55:297–304.
Thanoo BC, Sunny MC, Jayakrishnan A. Oral sustained-release drug delivery systems using polycarb onate microspheres capable of floating on the gastric fluid. J Pharm Pharmacol. 1993;45:21–4.
Xu X, Sun M, Zhi F, Hu Y. Floating matrix dosage form for phenoporlamine hydrochloride based on gas forming agent: in vitro and in vivo evaluation in healthy volunteers. Int J Pharm. 2006;310:139–45.
Choi B, Park HJ, Hwang S, Park J. Preparation of alginate beads for floating drug delivery system: effects of CO2 gas-forming agents. Int J Pharm. 2002;239:81–91.
El-Kamel A, Sokar M, Al Gamal S, Naggar V. Preparation and evaluation of ketoprofen floating oral delivery system. Int J Pharm. 2001;220:13–21.
Manca ML, Manconi M, Lai F, Loy G, Matricardi P, Fadda AM. Liposomes coated with chitosan-xanthan gum (chitosomes) as a potential carriers for pulmonary delivery of rifampicin. J Pharm Sci. 2012;101:566–75.
Kulkarni N, Wakte P, Naik J. Development of floating chitosan-xanthan beads for oral controlled release of glipizide. Int J Pharm Investig. 2015;5:73–80.
Fareez IM, Lim SM, Mishra RK, Ramasamy K. Chitosan coated alginate-xanthan gum bead enhanced pH and thermotelerance of lactobacillus plantarum LAB12. Int J Biol Macromol. 2015;72:1419–28.
Ferrua MJ, Singh RP. Human gastric simulator (Riddet model), The impact of food bioactives on health; 2015. p. 61–71.
Google Scholar
Al-Dhafri K, Ching CL, Philip K. Phyto-synthesis of silver nanoparticles and its bioactivity response towards nosocomial bacterial pathogens. Biocatal Agric Biotechnol. 2019;18:1–7.
Briones AV, Sato T. Encapsulation of glucose oxidase (GOD) in polyelectrolyte complexes of chitosan–carrageenan. React Funct Polym. 2010;70:19–27.
Cárdenas A, Argüelles-Monal W, Goycoolea F, Higuera-Ciapara I, Peniche C. Diffusion through membranes of polyelectrolyte complex of chitosan and alginate. Macromol Biosci. 2003;3:535–9.
Jin S, Liu M, Chen S, Gao C. A drug-loaded gel based on polyelectrolyte complexes of poly (acrylic acid) with poly (vinylpyrrolidone) and chitosan. Mater Chem Phys. 2010;123:463–70.
Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235–59.
Mi FL, Shyu SS, Wong TB, Jang SF, Lee ST, Lu KT. Chitosan polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. Effect of pH- dependent ionic crosslinking or interpolymer complex using tripolyphosphate or polyphosphate as reagent. J Appl Polym Sci. 1999;74:1093–107.
Mi FL, Chen CT, Tseng YC, Kuan CY, Shyu SS. Iron (III)- carboxymethylchitin microsphere for the pH-sensitive release of 6- mercaptopurine. J Control Release. 1997;44:19–32.
Shu X, Zhu KJ. A. novel, approach to prepare tripolyphosphate/ chitosan complex beads for controlled release drug delivery. Int J Pharm. 2000;201:51–8.
Download references
Acknowledgements
The authors would like to thank Precious Instrumentation Center of NTUST for the assistances in SEM set up.
This work was financially supported by National Science Council, Taiwan (NSC, No. 108–2221-E-011-107-) and by National Taiwan University of Science and Technology (NTUST), Tri-Service General Hospital and National Defense Medical Center.
Author information
Authors and affiliations.
Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei, 10617, Taiwan
Yu-Tung Hsu & Ming-Hua Ho
Graduate Institute of Biomedical Engineering, National Taiwan University of Science and Technology, Taipei, 10607, Taiwan
Chen-Yu Kao
Biomedical Engineering Research Center, National Defense Medical Center, Taipei, 11490, Taiwan
R&D Center for Membrane Technology, National Taiwan University of Science and Technology, Taipei, 10617, Taiwan
Ming-Hua Ho
Division of Oral and Maxillofacial Surgery, Department of Dentistry, Tri-Service General Hospital, Taipei, 11490, Taiwan
Shiao-Pieng Lee
Department of Biomedical Engineering, National Defense Medical Center, Taipei, 11490, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
Ming-Hua Ho, Shiao-Pieng Lee and Chen-Yu Kao designed this research. Yu-Tung Hsu performed the experiments. Shiao-Pieng Lee supported the culture and analysis of AGS cells. Ming-Hua Ho, Yu-Tung Hsu, Shiao-Pieng Lee and Chen-Yu Kao wrote the manuscript. Ming-Hua Ho supervised the project and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Correspondence to Chen-Yu Kao , Ming-Hua Ho or Shiao-Pieng Lee .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hsu, YT., Kao, CY., Ho, MH. et al. To control floating drug delivery system in a simulated gastric environment by adjusting the Shell layer formulation. Biomater Res 25 , 31 (2021). https://doi.org/10.1186/s40824-021-00234-6
Download citation
Received : 30 March 2021
Accepted : 15 September 2021
Published : 09 October 2021
DOI : https://doi.org/10.1186/s40824-021-00234-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gastroretentive drug delivery
- Core-shell particles
- Floating beads
- Xanthan gum
- Anti-bacterial effect
Biomaterials Research
ISSN: 2055-7124
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Floating drug delivery systems: a review
Affiliation.
- 1 Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, New Delhi 110062, India. [email protected]
- PMID: 16353995
- PMCID: PMC2750381
- DOI: 10.1208/pt060347
The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with special focus on the principal mechanism of floatation to achieve gastric retention. The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to design single-unit and multiple-unit floating systems, and their classification and formulation aspects are covered in detail. This review also summarizes the in vitro techniques, in vivo studies to evaluate the performance and application of floating systems, and applications of these systems. These systems are useful to several problems encountered during the development of a pharmaceutical dosage form.
PubMed Disclaimer
Similar articles
- Floating Drug Delivery Systems: An Emerging Trend for the Treatment of Peptic Ulcer. Namdev A, Jain D. Namdev A, et al. Curr Drug Deliv. 2019;16(10):874-886. doi: 10.2174/1567201816666191018163519. Curr Drug Deliv. 2019. PMID: 31894738 Review.
- Drug release kinetics and physicochemical characteristics of floating drug delivery systems. Adibkia K, Hamedeyazdan S, Javadzadeh Y. Adibkia K, et al. Expert Opin Drug Deliv. 2011 Jul;8(7):891-903. doi: 10.1517/17425247.2011.574124. Epub 2011 Apr 21. Expert Opin Drug Deliv. 2011. PMID: 21506906 Review.
- Gastroretentive floating drug-delivery systems: a critical review. Kotreka UK, Adeyeye MC. Kotreka UK, et al. Crit Rev Ther Drug Carrier Syst. 2011;28(1):47-99. doi: 10.1615/critrevtherdrugcarriersyst.v28.i1.20. Crit Rev Ther Drug Carrier Syst. 2011. PMID: 21395515 Review.
- Optimization studies on floating multiparticulate gastroretentive drug delivery system of famotidine. Gupta R, Pathak K. Gupta R, et al. Drug Dev Ind Pharm. 2008 Nov;34(11):1201-8. doi: 10.1080/03639040802005016. Drug Dev Ind Pharm. 2008. PMID: 18686085
- Microspheres as floating drug-delivery systems to increase gastric retention of drugs. Soppimath KS, Kulkarni AR, Rudzinski WE, Aminabhavi TM. Soppimath KS, et al. Drug Metab Rev. 2001 May;33(2):149-60. doi: 10.1081/dmr-100104401. Drug Metab Rev. 2001. PMID: 11495501 Review.
- In Vitro and In Vivo Evaluation of Magnetic Floating Dosage Form by Alternating Current Biosusceptometry. Rodrigues GS, Barboza JM, Buranello LP, Brandão VM, Ferrari PC, Soares GA, Miranda JRA. Rodrigues GS, et al. Pharmaceutics. 2024 Mar 2;16(3):351. doi: 10.3390/pharmaceutics16030351. Pharmaceutics. 2024. PMID: 38543245 Free PMC article.
- Towards multifunctional robotic pills. Mundaca-Uribe R, Askarinam N, Fang RH, Zhang L, Wang J. Mundaca-Uribe R, et al. Nat Biomed Eng. 2023 Sep 18. doi: 10.1038/s41551-023-01090-6. Online ahead of print. Nat Biomed Eng. 2023. PMID: 37723325 Review.
- Personalised 3D-Printed Mucoadhesive Gastroretentive Hydrophilic Matrices for Managing Overactive Bladder (OAB). Khizer Z, Akram MR, Tahir MA, Liu W, Lou S, Conway BR, Ghori MU. Khizer Z, et al. Pharmaceuticals (Basel). 2023 Feb 28;16(3):372. doi: 10.3390/ph16030372. Pharmaceuticals (Basel). 2023. PMID: 36986471 Free PMC article.
- SeDeM expert system with I-optimal mixture design for oral multiparticulate drug delivery: An encapsulated floating minitablets of loxoprofen Na and its in silico physiologically based pharmacokinetic modeling. Rauf-Ur-Rehman, Shoaib MH, Ahmed FR, Yousuf RI, Siddiqui F, Saleem MT, Qazi F, Khan MZ, Irshad A, Bashir L, Naz S, Farooq M, Mahmood ZA. Rauf-Ur-Rehman, et al. Front Pharmacol. 2023 Mar 3;14:1066018. doi: 10.3389/fphar.2023.1066018. eCollection 2023. Front Pharmacol. 2023. PMID: 36937845 Free PMC article.
- Optimization and In Vitro Characterization of Telmisartan Loaded Sodium Alginate Beads and Its In Vivo Efficacy Investigation in Hypertensive Induced Animal Model. Uthumansha U, Prabahar K, Gajapathy DB, El-Sherbiny M, Elsherbiny N, Qushawy M. Uthumansha U, et al. Pharmaceutics. 2023 Feb 20;15(2):709. doi: 10.3390/pharmaceutics15020709. Pharmaceutics. 2023. PMID: 36840031 Free PMC article.
- Hirtz J. The git absorption of drugs in man: a review of current concepts and methods of investigation. Br J Clin Pharmacol. 1985;19:77S–83S. - PMC - PubMed
- Ponchel G, Irache JM. Specific and non-specific bioadhesive particulate system for oral delivery to the gastrointestinal tract. Adv Drug Del Rev. 1998;34:191–219. doi: 10.1016/S0169-409X(98)00040-4. - DOI - PubMed
- Lenaerts VM, Gurny R. Gastrointestinal Tract-Physiological variables affecting the performance of oral sustained release dosage forms. In: Lenaerts V, Gurny R, editors. Bioadhesive Drug Delivery System. Boca Raton, FL: CRC Press; 1990.
- Deshpande AA, Shah NH, Rhodes CT, Malick W. Development of a novel controlled-release system for gastric retention. Pharm Res. 1997;14:815–819. doi: 10.1023/A:1012171010492. - DOI - PubMed
- Rednick AB, Tucker SJ. Sustained release bolus for animal husbandry. US patent 3 507 952. April 22, 1970.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement
Modern Approaches to Obtaining Floating Drug Dosage Forms (A Review)
- Published: 08 December 2022
- Volume 56 , pages 1277–1284, ( 2022 )
Cite this article

- E. V. Blynskaya 1 , 2 ,
- V. P. Vinogradov 1 ,
- S. V. Tishkov 2 ,
- S. N. Suslina 1 &
- K. V. Alekseev 2
117 Accesses
Explore all metrics
Floating dosage forms (FDFs) represent widely used types of gastroretentive drug delivery systems that provide targeted delivery to the upper gastrointestinal tract. This article proposes a classification of FDFs according to which approaches to achieving drug flotation are reviewed based on published works. FDFs present on the market and the concepts used to create them are described. Various parameters and factors affecting the efficacy of this type of dosage form that must be taken into account in the drug development process and the problems that this approach to gastroretention solves are described. FDF development directions utilizing existing progress and promising for the future are highlighted.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Physicochemical Basic Principles for Solid Dosage Forms

Risk-based approach for systematic development of gastroretentive drug delivery system
N. N. Vrettos, C. J. Roberts, and Z. Zhu, Pharmaceutics , 13 (10), 1591 (2021).
Article CAS Google Scholar
C. M. Lopes, C. Bettencourt, A. Rossi, et al., Int. J. Pharm. , 510 (1), 144 – 158 (2016).
J. Tripathi, P. Thapa, R. Maharjan, and S. H. Jeong, Pharmaceutics , 11 (4), 193 (2019).
P. K. Sadhu, A. A. Baji, N. V. Shah, et al., Int. J. Pharm. Res., 12 (2), 2047 – 2059 (2020).
Google Scholar
M. V. Leonova, Lech. Delo , No. 1, 21 – 31 (2009).
K. V. Alekseev, E. V. Blynskaya, E. Yu. Karbusheva, et al., Farmatsiya , No. 6, 35 – 38 (2012).
P. Suradkar, R. Mishra, and T. Nandgude, Res. J. Pharm. Technol. , 12 (11), 5633 – 5640 (2019).
Article Google Scholar
S.-J. Hwang, H. Park, and K. Park, Crit. Rev. Ther. Drug Carrier Syst. , 15 (3), 243 – 284 (1998).
J. Timmermans and A. J. Moes, J. Pharm. Sci. , 82 (8), 854 – 854 (1993).
J. Timmermans and A. J. Moes, Int. J. Pharm. , 62( 2–3), 207 – 216 (1990).
M. Kamba, Y. Seta, A. Kusai, et al., Int. J. Pharm. , 208 (1–2), 61 – 70 (2000).
V. K. Pawar, S. Kansal, G. Garg, et al., Drug Delivery , 18 (2), 97 – 110 (2011).
P. Mojaverian, P. H. Vlasses, P. E. Kellner, and M. L. Rocci, Pharm. Res. , 5 (10), 639 – 644 (1988).
V. A. Eberle, A. Haring, and J. Schoelkopf, Drug Dev. Ind. Pharm. , 42 (5), 808 – 817 (2016).
M. Saab, M. Issa, W. Samy, and H. El-Maradny, Pharmazie , 71 (12), 701 – 708 (2016).
CAS Google Scholar
C. Pi, T. Feng, J. Liang, et al., Int. J. Biol. Macromol. , 112 , 1038 – 1047 (2018).
K. Huanbutta and T. Sangnim, J. Drug Delivery Sci. Technol. , 52 , 831 – 837 (2019).
B. R. Giri, E. S. Song, J. Kwon, et al., Pharmaceutics , 12 (1), 77 (2020).
A. Haimhoffer, F. Fenyvesi, I. Lekli, et al., Pharmaceutics , 13 (10), 1571 (2021).
A. J. Svagan, A. Mullertz, and K. Lobmann, J. Pharm. Pharmacol. , 69 (11), 1477 – 1484 (2017).
N. A. Alhakamy, S. M. Badr-Eldin, O. A. A. Ahmed, et al., Biomolecules , 10 (7), 1 – 17 (2020).
K. Huanbutta, S. Limmatvapirat, S. Sungthongjeen, and P. Sriamornsak, AAPS PharmSciTech , 17 (3), 693 – 699 (2016).
B. A. Daihom, E. R. Bendas, M. I. Mohamed, and A. A. Badawi, J. Drug Delivery Sci. Technol. , 59 , 101941 (2020).
R. K. Jadi, R. Bomma, and V. Sellappan, J. Appl. Pharm. Sci. , 6 (5), 112 – 118 (2016).
M. Panda, M. E. B. Rao, J. Panda, et al., Int. J. Appl. Pharm. , 13 (3), 130 – 136 (2021).
M. Rahamathulla, S. Saisivam, and H. V. Gangadharappa, AAPS PharmSciTech , 20 (1), 35 (2019).
S. Treesinchai, S. Puttipipatkhachorn, T. Pitaksuteepong, and S. Sungthongjeen, Asian J. Pharm. , 11 (1), 130 – 131 (2016).
T. U. Wani, K. B. Mir, A. A. Fazli, et al., J. Drug Delivery Sci. Technol. , 60 , 102006 (2020).
S. Kim, K.-M. Hwang, Y. S. Park, et al., Int. J. Pharm. , 550 (1 – 2), 160 – 169 (2018).
S. Chittam and A. Bhosale, Int. J. Appl. Pharm. , 13 (5), 223 – 229 (2021).
A. K. J. Shinde, N. S. Patil, T. S. Jadhav, and H. N. More, Int. J. Appl. Pharm. , 12 (4), 218 – 227 (2020).
S. R. Kamble, B. Poul, P. Udapurkar, and K. Biyani, Asian J. Pharm. , 10 (3), 290 – 299 (2016).
F. Sonvico, C. Conti, G. Colombo, et al., J. Controlled Release , 262 , 296 – 304 (2017).
Poornima and S. Priya, Indian J. Pharm. Educ. Res. , 55 (2), 100 – 111 (2021).
J. A. Avbunudiogba, C. A. Alalor, and Q. D. Okolocha, Turkish J. Pharm. Sci. , 17 (6), 645 – 652 (2020).
S. Cvijic, S. Ibric, J. Parojcic, and J. Djuris, J. Drug Delivery Sci. Technol. , 45 , 1 – 10 (2018).
S. M. Ahmed, A. A. Ali, A. M. Ali, and O. A. Hassan, Drug Des., Dev. Ther. , 10 , 4061 – 4071 (2016).
D. M. Mehta, P. B. Parejiya, H. K. Patel, et al., J. Pharm. Invest. , 46 (5), 453 – 465 (2016).
S. Shirisha, S. K. Sahoo, and M. R. Yamsani, Int. J. Drug Delivery Technol. , 7 (4), 244 – 254 (2017).
V. D. Gulkari, S. S. Bakhle, and L. S. Yelane, Int. J. Pharm. Pharm. Sci ., 8 (5), 356 – 360 (2016).
A. C. Ovenseri, O. Clifford, and U. M. Uwumagbe, Pak . J. Pharm. Sci. , 31 (4), 1243 – 1249 (2018).
K. Venkateswarlu and K. B. Chandrasekhar, Marmara Pharm. J. , 20 (2), 172 – 183 (2016).
U. D. Budaya and S. Surini, Int. J. Appl. Pharm. , 12 (1), 192 – 196 (2020).
S. S. Nawaj, M. K. Iqbal, and G. J. Khan, Asian J. Pharm. , 11 (2), 135 – 146 (2017).
K. K. Majumder, M. Kumar, R. Pahwa, et al., Int. J. Appl. Pharm. , 13 (5), 306 – 310 (2021).
S. Alexandar, M. Kumar, M. V. Kumudhavalli, et al, J. Pharm. Sci. Res., 9 (2), 145 – 149 (2017).
S. R. Baratam and J. Vijayaratna, Asian J. Pharm. Clin. Res. , 11 (6), 148 – 151 (2018).
S. V. Reddy, A. V. Badarinath, and K. Gnana Prakash, Asian J. Pharm. , 12 (2), 106 – 114 (2018).
S. Wang, H. Wen, and P. Li, J. Drug Delivery Sci. Technol. , 51 , 7 – 17 (2019).
L. Rajesh Patro, J. Geethanjali, K. Harish, et al., Pharm. Lett. , 8 (2), 17 – 25 (2016).
B. Ramu and P. Shanmunga Pandiyan, Asian J. Pharm. Clin. Res. , 10 (5), 150 – 155 (2017).
R. S. Kharwade, S. M. More, and U. N. Mahajan, Asian J. Pharm. Clin. Res. , 10 (3), 444 – 448 (2017).
Y. Dange, D. Randive, S. Bhinge, et al., Indian Drugs , 54 (3), 23 – 27 (2017).
M. H. Ali, M. A. Bhuiyan, M. S. Reza, and S. Karim, Dhaka Univ. J. Pharm. Sci. , 15 (2), 203 – 208 (2016).
I. Singh and V. Saini, Pak. J. Pharm. Sci. , 29 (2), 511 – 519 (2016).
S. Anepu, L. Duppala, and M. Soma Sundari, Asian J. Pharm. Clin. Res. , 10 (2), 281 – 290 (2017).
M. A. El Nabarawi, M. H. Teaima, R. A. A. El-Monem, et al., Drug Des., Dev. Ther. , 11 , 1081 – 1093 (2017).
R. Bahri-Najafi, A. Mostafavi, N. Tavakoli, et al., Res. Pharm. Sci. , 12 (2), 128 – 136 (2017).
D. Narendar, N. Arjun, K. Someshwar, and Y. Madhusudan Rao, J. Pharm. Invest. , 46 (3), 253 – 263 (2016).
R. K. Gunda and A. Vijayalakshmi, Thai J. Pharm. Sci. , 43 (3), 138 – 148 (2019).
A. K. Abbas and A. T. Alhamdany, Turk. J. Pharm. Sci. , 17 (2), 159 – 171 (2020).
S. Kumar and N. Goyal, Indian J. Pharm. Educ. Res. , 55 (2), 354 – 362 (2021).
Y. Hendrika, J. Reveny, and S. Sumaiyah, Asian J. Pharm. Clin. Res. , 11 (4), 72 – 77 (2018).
S. Z. Chemate, G. R. Godge, K. K. Pawa, and K. A. Rupnar, Turk. J. Pharm. Sci. , 13 (1), 91 – 102 (2016).
R. Talukder and R. Fassihi, Drug Dev. Ind. Pharm. , 30 (10), 1019 – 1028 (2004).
S. Das, S. Kaur, and V. K. Rai, Drug Delivery Transl. Res ., 11 (5), 1849 – 1877 (2021).
Download references
Author information
Authors and affiliations.
People’s Friendship University of Russia (RUDN University), 6 Miklukho-Maklaya St, Moscow, 117198, Russia
E. V. Blynskaya, V. P. Vinogradov & S. N. Suslina
Research Zakusov Institute of Pharmacology, 8 Baltiiskaya St, Moscow, 125315, Russia
E. V. Blynskaya, S. V. Tishkov & K. V. Alekseev
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to V. P. Vinogradov .
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 9, pp. 51 – 58, September, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Blynskaya, E.V., Vinogradov, V.P., Tishkov, S.V. et al. Modern Approaches to Obtaining Floating Drug Dosage Forms (A Review). Pharm Chem J 56 , 1277–1284 (2022). https://doi.org/10.1007/s11094-022-02786-w
Download citation
Received : 15 April 2022
Published : 08 December 2022
Issue Date : December 2022
DOI : https://doi.org/10.1007/s11094-022-02786-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- gastroretentive drug delivery systems
- floating dosage forms
- peroral dosage forms
- bioavailability
- modified release
- Find a journal
- Publish with us
- Track your research

Floating Drug Delivery Systems: A Comprehensive Review of Formulation Strategies and Applications
- K. Jaganathan Department of Pharmaceutics, JKKMMRF’S Annai JKK Sampoorani Ammal College of Pharmacy, Ethirmedu, Komarapalayam – 638183, Namakkal (DT), Tamil Nadu, India
- D. Devanandh Department of Pharmaceutics, JKKMMRF’S Annai JKK Sampoorani Ammal College of Pharmacy, Ethirmedu, Komarapalayam – 638183, Namakkal (DT), Tamil Nadu, India
- S. Chandra Department of Pharmaceutics, JKKMMRF’S Annai JKK Sampoorani Ammal College of Pharmacy, Ethirmedu, Komarapalayam – 638183, Namakkal (DT), Tamil Nadu, India
- N. Senthilkumar Department of Pharmaceutics, JKKMMRF’S Annai JKK Sampoorani Ammal College of Pharmacy, Ethirmedu, Komarapalayam – 638183, Namakkal (DT), Tamil Nadu, India
Floating drug delivery systems (FDDS) have garnered significant attention in pharmaceutical research due to their ability to improve drug bioavailability and therapeutic efficacy. This comprehensive review aims to provide an in-depth analysis of the formulation strategies and applications of floating drug delivery systems.The review commences by discussing the physiological basis of gastric retention, highlighting the importance of FDDS in achieving prolonged residence time within the stomach. It explores the factors affecting gastric emptying and their impact on FDDS performance. Various approaches for formulating buoyant drug delivery systems, including single-unit and multiple-unit systems, are elucidated along with their respective advantages and limitations.Furthermore, the review delves into the diverse range of polymers, gelling agents, and gas-generating agents employed in FDDS formulation. Special emphasis is placed on recent advancements in material science and their contribution to enhancing the floating properties, drug release kinetics, and overall performance of these systems. Additionally, the integration of innovative technologies such as microbubbles, magnetic particles, and mucoadhesive polymers is explored for their potential to further optimize FDDS functionality.The applications of FDDS go beyond improving drug delivery to include therapeutic areas such as gastroesophageal reflux disease, peptic ulcers, motion sickness, and local gastric treatment. The review highlights the clinical significance of FDDS in these contexts, shedding light on recent clinical trials and outcomes.In conclusion, this review underscores the profound impact of floating drug delivery systems on pharmaceutical research and patient care. It provides a comprehensive understanding of the formulation strategies, materials, and applications associated with FDDS, paving the way for continued innovation in drug delivery and therapeutic effectiveness.
How to Cite
- Endnote/Zotero/Mendeley (RIS)
SidebarMenu
Indexing & Abstracting Publication charges Payment option Model Manuscript Authors Details Form Copy Right Form Announcements Aim & Scope Statistics
EditorialTeam

ArticleTemplate

Current Issue
Information.
- For Readers
- For Authors
- For Librarians
HITS College of Pharmacy, Ghatkesar, Hyderabad, India.
Contact Info:
Information :.
Terms of Use
Privacy Policy

Floating drug delivery system: an outlook
- Ananta Choudhury Department of Pharmacy, Assam Down Town University, Panikhaiti, Guwahati, Assam, India Pin- 781026
- Lalmalsawmi Renthlei Department of Pharmacy, Assam Down Town University, Panikhaiti, Guwahati, Assam, India Pin- 781026
- Manjima Dewan Department of Pharmacy, Assam Down Town University, Panikhaiti, Guwahati, Assam, India Pin- 781026
- Raju Ahmed Department of Pharmacy, Assam Down Town University, Panikhaiti, Guwahati, Assam, India Pin- 781026
- Himal Barakoti Department of Pharmacy, Assam Down Town University, Panikhaiti, Guwahati, Assam, India Pin- 781026
- Biplab Kumar Dey Department of Pharmacy, Assam Down Town University, Panikhaiti, Guwahati, Assam, India Pin- 781026
Floating drug delivery is considered as the most effective amongst the several approaches of gastro retentive drug delivery systems. The short gastric residence times (GRT) and unpredictable gastric emptying times (GET) are the two most important parameters that play a vital role in improving the bioavailability of drugs those are having an absorption window at the stomach. The floating drug delivery approach is a low-density system that may be effervescent or Non-Effervescent type with sufficient buoyancy to flow over the gastric contents and remain buoyant in the stomach without affecting the stomachic emptying rate for a prolonged duration. Floating dosage forms include tablets, granules, capsules, microspheres, microparticle, etc. are few formulations available commercially. A comprehensive summary of different floating drug delivery and its present status has been highlighted in this review.
Chanda R, Roy A, Bahadur S, Saha S, Das S, Choudhury A. Floating drug delivery: A potential alternative to conventional therapy. International Journal of PharmTech Research., 2(1): 49-59, (2010).
Sujoy Das, S. Bahadur, A. Choudhury, S. Saha. Development and characterization of extended-release gastro retentive drug delivery. Journal of Pharmacy Research. 02(9):24-29, (2009).
Chaudhari V, Rathod H and Modasia M., Formulation and evaluation of floating drug delivery system containing theophylline as a model drug. International Journal of Pharmacy & Life Sciences, 2(4): 695-703,(2011)
Dash TR, Verma P., Matrix Tablets: An Approach towards Oral Extended Release Drug Delivery. International Journal of Pharma Research & Review, 2(2):12-24,(2013)
Pandey N, Sah NA, Mahara K., Formulation and Evaluation of Floating Microsphere of Nateglinide. International Journal of Pharma Sciences and Research, 7(11): 453-464,(2016)
Gaikwad VD, Yadav VD, Jadhav PD., Formulation and evaluation of floating matrix tablets of diltiazem hydrochloride. Asian Journal of Pharmaceutics, 245-251,(2012)
Singh BS, Chaurasia H, Varshney S, Reena and Kotiyal D., Formulation and Evaluation of Fast Dissolving Tablets of Sumatriptan Succinate. International Journal of Pharmaceutical Sciences and Research,4(5): 1912-1917,(2013)
Balata G. Design and Evaluation of Gastroretentive Floating Tablet of Nizatidine: A Trial to Improve its Efficacy. International Journal of Pharmacy and Pharmaceutical Sciences, 6(5): 423-429,(2014)
Choudhury A, Dash S K, Roy A, Bahadur S, Saha, Das S., Design and Evaluation of RanitidineHydrochloride for Oral Controlled Release. Research Journal of Pharmaceutical Dosage Forms and technology, 01(2):167-170, 2009.
Malviya S, Singh S, Pandey J, Kondalkar AK and Tagde P., Formulation and evaluation of floating microbeads of ciprofloxacin HCl by emulsion gelation method. Der Pharmacia Lettre, 5(2):63-68,(2013)
Chowdhury MEH, Pathan MSI., Preparation and evaluation of floating matrix tablets of Ranitidine Hydrochloride. The Pharma Innovation,1(7): 43-50,(2012)
Dev. J, Ghosh. A, kalayan. S K, Paul. P, Choudhury .A, Formulation and evaluation of metformine HCL floating tablet using pectin as a natural polymer. International research journal of pharmaceutical sciences. 01(01):0022-0026, (2010)
Patial K, Dua JS, Menra M and Prasad DN., A review: Floating drug delivery system. World Journal of Pharmaceutical Research,6: 614-633,(2016)
Gharti KP, Thapa P, Budhathoki U, Bhargava A., Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug. Journal of Young Pharmacists ,4:201-207,
Vijayakumar A, Senthilnathan B, Ravichandiran V., A Review Article on Different Types of Floating Drug Delivery Systems. International Journal of Pharmacy and Pharmaceutical Sciences, 4(1): 45-50.(2012)
Sahil K, Akanksha M, Sudeep B, Premjeet S., Floating Drug Delivery System: A Review. International Research Journal of Pharmacy, 2(9): 18-24,(2011)
Chanchal, Kumar S, Kakar S., A Review on Floating Tablet. Indian Journal of Pharmaceutical and Biological Research, 6(1): 22-29,(2018)
Arunachalam A, Karthikeyan M, Konam K, Prasad HP, Sethuraman S, Ashutoshkumar S, Manidipa S., Floating drug delivery systems: A review. International Journal of Research in Pharmaceutical Sciences, 2(1):76-83,(2012)
Sabale V, Sakarkar SN, Pund S, Sabale PM., Formulation and evaluation of floating dosage form: An overview. Systemic reviews in Pharmacy, 1(1):33-38,(2010)
Kaur B, Sharma S, Sharma G, Saini R, Singh S, Nagpal M, Jain UK, Sharma M., A review of floating drug delivery system. Asian journal of biomedical and pharmaceutical science, 3(24): 1-6,(2013)
Gupta P, Gnanarajan, Kothiyal P., Floating drug delivery system: a review. International Journal of Pharma Research & Review, 4(8):37-44,(2015)
Tripathi P, Ubaidulla U, Khar RK, Vishwavibhuti., Floating drug delivery system. International Journal of Research and Development in Pharmacy and Life Sciences, 1: 1-10,(2012)
Jha P, Prajapati V, Solanki H, Jani G, Kotak U., Pharmaceutical aspects of various floating drug delivery system. World journal of pharmacy and pharmaceutical sciences, 4(4):569-589,(2015)
Iqubal MK, Singh PK, Shuaib M, Iqubal A, Singh M., Recent advances in direct compression technique for pharmaceutical tablet formulation. International journal of pharmaceutical research and development, 6(1): 49-57,(2014)
Kumar SA, Vivek D, Vandana A., Role of natural polymers used in floating drug delivery system. Journal of pharmaceutical and scientific innovation, 1(3):11-15,(2012)
Kulkarni DP and Saboo SS., Polymers used in floating drug delivery system: A review. European Journal of Pharmaceutical and Medical Research,4(8):611-616,(2017)
Koushik AK, Tiwari AK, Gaur A., Role of excipients and polymeric advancements in preparation of floating drug delivery system. International journal of pharmaceutical investigation,5: 1-11,(2015)
Bonthagarala B, Nama S, Kothamasu S, Vadamuddala M, Donthiboina S., Formulation and evaluation of sumatriptan succinate floating bilayered tablets. International daily journal, 17(49):23-35,(2014)
Haranath C, Reddy JR, Devanna N., Formulation and evaluation of non-effervescent floating tablets of cimetidine employing ozokerite wax. International journal of research in pharmacy and chemistry, 7(2):171-180,(2017)
Saritha D, Sathish D and Rao YM., Formulation and evaluation of gastroretentive floating tablets of domperidone maleate. Journal of Applied Pharmaceutical Science, 3: 68-73,(2012)
Nagamani V, Jhansi C., Formulation and Evaluation of Floating Tablets Using Nimesulide as a Model Drug. International Research Journal of Engineering and Technology,4:1245-1250,(2017)
Rathore J, Parmar HK., Formulation and evaluation of floating tablet norfloxacin. International Journal of Pharma Sciences and Research, 6(1): 23-27,(2015)
Kumari SD, Vengatesh S, Elango K, Damayanthi RD, Deattu N, Christina P., Formulation and Evaluation of Floating tablets of Ondansetron Hydrochloride. International Journal of Drug Development & Research, 4:265-273,(2012)
Sarfaraz Md, LingaReddy B, Doddayya H, Rajagopal UH., Design and in-Vitro Evaluation of Gastro Retentive Floating Tablets of Repaglinide. International journal of drug delivery and research, 5(3):322-332,(2013)
Patil H, Prashar B, Chandel A, Thakur V., Formulation and Evaluation of Floating Tablet of Pantoprazole Sodium Sequihydrate. Journal of Pharmacy Research, 5(9):4659-4662,(2012)
Reddy VS, Badarinath AV, Prakash KG., Formulation and evaluation of floating tablets of ciprofloxacin hydrochloride. Asian Journal of Pharmaceutics , 12(2): 106-113,(2018)
Pierce MW., Transdermal delivery of sumatriptan for the treatment of acute migraine. The Journal of the American Society for Experimental NeuroTherapeutics, 7: 159-163,(2010)
Davoudi ET, Noordin MD, Kadivar A, Dehghan BK, Farjam AS, Javar HA., Preparation and Characterization of a Gastric Floating Dosage Form of Capecitabine. BioMedResearchInternational, 1-8,(2013)
Pawar HK, Gharat PR, Dhavale RV, Joshi PR, Rakshit PP., Development and evaluation of gastroretentive floating tablets of an antihypertensive drug using hydrogenated cottonseed oil. ISRNPharmaceutics,1-9,(2013)
Mukund JY, Kantilal BR, Sudhakar RN., Floating microspheres: a review. Brazilian Journal of Pharmaceutical Sciences, 18: 18-30,(2012)
Nanjwade BK, Adichwal SK, Nanjwade VK, Gaikwad KR, Thakare SA and Manvi FV., Development and Evaluation of Gastroretentive Floating Tablets of Glipizide Based on Effervescent Technology. Drug metabolism and toxicology, 3:2-5,(2012)
Rahim SA, Carter P, Elkordy A., Design and evaluation of effervescent floating tablets based on hydroxyethyl cellulose and sodium alginate using pentoxifylline as a model drug. Drug Design, Development and Therapy, 9:1843-1857,(2015)
Patil JM, Hirlekar RS, Gide BS, Kadam VJ., Trends on floating drug delivery system. Journal of scientific and industrial research, 65: 11-21,(2006)
Avachat MK. and Dhamne AG., Gastric floating system, WO 02/102415 A1, 2002.
Watts PJ, Smith A, Bond JR and Lafferty WCI. Floating drug delivery composition, WO 01/58424 A1, 2001.
Hassan M. Gastro Retentive Drug Delivery System Comprising An Extruded Hydratable Polymer, WO 03/105812 A1, 2003.
Meijerink HJC, Changoer L, Blom W, Visser MR, Frijlink HW and Eissens AC. Gastro-retentive Drug Delivery System, WO 2014/014348 A1, 2014.
Gerard DE., Schoelkopf J, Gane PA.C, Eberle VA, Alles R, Puchkov M and Huwyler J. Gastroretentive drug formulation and delivery systems and their method of preparation using functionalized calcium carbonate, WO 2014/057026 A1, 2014.
Pragya Baghel, Amit Roy, Shashikant Chandrakar, Sanjib Bahadur,Pulsatile Drug Delivery System: A Promising Delivery System, Research Journal of Pharmaceutical Dosage Forms and Technology, 5(3), 111-114, 2013
How to Cite
- Endnote/Zotero/Mendeley (RIS)
Copyright (c) 2020 Ananta Choudhury, Lalmalsawmi Renthlei, Manjima Dewan, Manjima Dewan, Raju Ahmed, Raju Ahmed, Himal Barakoti, Himal Barakoti, Biplab Kumar Dey

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License .
Make a Submission
It has been brought to our notice that some organizations claim to have an association with us for publication. This is to inform all the stakeholders that the Journal of Applied Pharmaceutical Research (or any associated person) does not have any association/ affiliation/ agreement/ contract with such organizations or third-party consultancy. It has come to our attention that there might be communication from unauthorized email addresses claiming to represent us. Please be aware that any communication received from email addresses not officially recognized by us may not be legitimate and should be treated cautiously. We do not endorse such representation or disclose responsibility for actions outside our official communication channels. If you have any doubts or concerns about the authenticity of a communication received, please contact us directly through the official channels provided on our website.
"Embrace the Momentum: Now Bimonthly!"
Big News! We Hear You, Authors & Readers!
✨With immense gratitude for the overwhelming response from our cherished authors and readers, we are thrilled to unveil our latest transformation, JOAPR will be published BIMONTHLY from August 2023. ✨
Journal Policies and Information
Focus & Scope
Open Access Policy
Publication Ethics
Competing Interest
Human, Animal rights, Informed Consent
Review Policy
Privacy Policy
Crossmark Policy
Publication fees & waivers
Reviewers Guidelines
License agreements
Plagiarism Policy
Archive and Preservation Policy
Advertisement Policy
About Publisher (CPA)
COPE Guidelines
Everyone is advised to follow these COPE Guidelines for reference and strict compliance
Visit Count
- For Readers
- For Authors
- For Librarians
ISSN No. 2348-0335
Journal of Applied Pharmaceutical Research is indexed by number of agencies/ organization/ databases like SCOPUS , Directory of Open Access Journal (DOAJ), Index Copernicus, Crossref, OLCC WorldCat, Garuda, Dimensions, Chemical Abstract Services (CAS), OpenAIRE, Google Scholar, J-Gate, Scilit, International Committee of Medical Journal Editors (ICMJE), Indonesia one search, Indian Citation Index, CNKI, Bielefeld Academic Search Engine (BASE), PKP-Index, Neliti
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 25 June 2024
Precision drug delivery to the central nervous system using engineered nanoparticles
- Jingjing Gao 1 ,
- Ziting (Judy) Xia 2 ,
- Swetharajan Gunasekar 2 ,
- Christopher Jiang 2 ,
- Jeffrey M. Karp 2 , 3 , 4 , 5 , 6 &
- Nitin Joshi ORCID: orcid.org/0000-0001-8138-7611 2 , 3
Nature Reviews Materials ( 2024 ) Cite this article
7 Altmetric
Metrics details
- Drug delivery
- Neuroscience
Development of novel therapies for central nervous system (CNS) disorders has experienced a high failure rate in clinical trials owing to unsatisfactory efficacy and adverse effects. One of the major reasons for limited therapeutic efficacy is the poor penetration of drugs across the blood–brain barrier. Despite the development of multiple drug delivery platforms, the overall drug accumulation in the brain remains sub-optimal. Another critical but overlooked factor is achieving precision delivery to a specific region and cell type in the brain. This specificity is crucial because most neurological disorders exhibit region-specific vulnerabilities. Multiple trials have failed owing to adverse CNS effects induced by nonspecific drug targeting. In this Review, we highlight the key regions and cell types that should be targeted in different CNS diseases. We discuss how physiological barriers and disease-mediated changes in the blood–brain barrier and the overall brain can impact the precision delivery of therapeutics via the systemic route. We then perform a systematic analysis of the current state-of-the-art approaches developed to overcome these barriers and achieve precision targeting at different levels. Finally, we discuss potential approaches to accelerate the development of precision delivery systems and outline the challenges and future research directions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
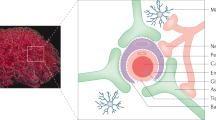
Strategies for delivering therapeutics across the blood–brain barrier
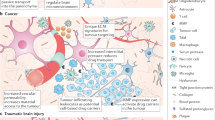
Drug delivery to the central nervous system
Nanomedicine-based immunotherapy for central nervous system disorders
Valori, C. F., Possenti, A., Brambilla, L. & Rossi, D. Challenges and opportunities of targeting astrocytes to halt neurodegenerative disorders. Cells 10 , 2019 (2021).
Article CAS PubMed PubMed Central Google Scholar
Prabakaran, A. et al. Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: current advancements and challenges. Expert Opin. Drug Deliv. 19 , 87–102 (2022).
Article Google Scholar
Markowicz-Piasecka, M. et al. Current approaches to facilitate improved drug delivery to the central nervous system. Eur. J. Pharm. Biopharm. 181 , 249–262 (2022).
Article CAS PubMed Google Scholar
Doody, R. S. et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 369 , 341–350 (2013).
Imbimbo, B. P. & Giardina, G. A. M. γ-Secretase inhibitors and modulators for the treatment of Alzheimer’s disease: disappointments and hopes. Curr. Top. Med. Chem. 11 , 1555–1570 (2011).
Kouhi, A. et al. Brain disposition of antibody-based therapeutics: dogma, approaches and perspectives. Int. J. Mol. Sci. 22 , 6442 (2021).
Chang, H.-Y. et al. Brain pharmacokinetics of anti-transferrin receptor antibody affinity variants in rats determined using microdialysis. mAbs 13 , 1874121 (2021).
Article PubMed PubMed Central Google Scholar
Keiser, M. S. et al. Toxicity after AAV delivery of RNAi expression constructs into nonhuman primate brain. Nat. Med. 27 , 1982–1989 (2021).
Ling, Q., Herstine, J. A., Bradbury, A. & Gray, S. J. AAV-based in vivo gene therapy for neurological disorders. Nat. Rev. Drug Discov. 22 , 789–806 (2023).
Lee, H. et al. Multi-omic analysis of selectively vulnerable motor neuron subtypes implicates altered lipid metabolism in ALS. Nat. Neurosci. 24 , 1673–1685 (2021).
Herb, B. R. et al. Single-cell genomics reveals region-specific developmental trajectories underlying neuronal diversity in the human hypothalamus. Sci. Adv. 9 , eadf6251 (2023).
Vialle, R. A., de Paiva Lopes, K., Bennett, D. A., Crary, J. F. & Raj, T. Integrating whole-genome sequencing with multi-omic data reveals the impact of structural variants on gene regulation in the human brain. Nat. Neurosci. 25 , 504–514 (2022).
Yao, Z. et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624 , 317–332 (2023). This article introduces a collaborative effort from the BRAIN Initiative Cell Census Network, presenting cellular maps of the mouse brain with high spatial resolution.
Chini, M. & Hanganu-Opatz, I. L. Prefrontal cortex development in health and disease: lessons from rodents and humans. Trends Neurosci. 44 , 227–240 (2021).
Fu, H., Hardy, J. & Duff, K. E. Selective vulnerability in neurodegenerative diseases. Nat. Neurosci. 21 , 1350–1358 (2018). This paper provides a comprehensive overview of the current understanding of biological mechanisms underpinning selective neuronal and regional vulnerability in neurological disorders.
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72 , 305–315 (2015).
Del Tredici, K., Rüb, U., De Vos, R. A. I., Bohl, J. R. E. & Braak, H. Where does Parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61 , 413–426 (2002).
Article PubMed Google Scholar
De Marchi, F. et al. Cognitive dysfunction in amyotrophic lateral sclerosis: can we predict it? Neurol. Sci. 42 , 2211–2222 (2021).
Crockford, C. et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 91 , e1370–e1380 (2018).
Lassmann, H. Multiple sclerosis pathology. Cold Spring Harb. Perspect. Med. 8 , a028936 (2018).
Haider, L. et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139 , 807–815 (2016).
Knopman, D. S. et al. Alzheimer disease. Nat. Rev. Dis. Primers 7 , 33 (2021).
Schöll, M. et al. Biomarkers for tau pathology. Mol. Cell. Neurosci. 97 , 18–33 (2019).
Musolino, P. L. et al. Brain endothelial dysfunction in cerebral adrenoleukodystrophy. Brain 138 , 3206–3220 (2015).
Pong, S., Karmacharya, R., Sofman, M., Bishop, J. R. & Lizano, P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry 6 , 30–46 (2020).
Estudillo, E. et al. Thinking outside the black box: are the brain endothelial cells the new main target in Alzheimer’s disease? Neural Regen. Res. 18 , 2592 (2023).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603 , 885–892 (2022).
Mrdjen, D. et al. The basis of cellular and regional vulnerability in Alzheimer’s disease. Acta Neuropathol. 138 , 729–749 (2019).
Bennett, H. C. & Kim, Y. Pericytes across the lifetime in the central nervous system. Front. Cell. Neurosci. 15 , 627291 (2021).
Giguère, N., Burke Nanni, S. & Trudeau, L.-E. On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front. Neurol. 9 , 455 (2018).
Mamelak, M. Parkinson’s disease, the dopaminergic neuron and gammahydroxybutyrate. Neurol. Ther. 7 , 5–11 (2018).
Reiner, A. & Deng, Y.-P. Disrupted striatal neuron inputs and outputs in Huntington’s disease. CNS Neurosci. Ther. 24 , 250–280 (2018).
Spoleti, E. et al. Dopamine neuron degeneration in the ventral tegmental area causes hippocampal hyperexcitability in experimental Alzheimer’s disease. Mol. Psychiatry 29 , 1265–1280 (2024).
Nobili, A. et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 8 , 14727 (2017).
Chen, X.-Q. & Mobley, W. C. Exploring the pathogenesis of Alzheimer disease in basal forebrain cholinergic neurons: converging insights from alternative hypotheses. Front. Neurosci. 13 , 446 (2019).
Baker-Nigh, A. et al. Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer’s disease. Brain 138 , 1722–1737 (2015).
Vana, L. et al. Progression of tau pathology in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. Am. J. Pathol. 179 , 2533–2550 (2011).
Tan, R. H. et al. Cerebellar neuronal loss in amyotrophic lateral sclerosis cases with ATXN2 intermediate repeat expansions. Ann. Neurol. 79 , 295–305 (2016).
Fukutani, Y., Cairns, N. J., Rossor, M. N. & Lantos, P. L. Purkinje cell loss and astrocytosis in the cerebellum in familial and sporadic Alzheimer’s disease. Neurosci. Lett. 214 , 33–36 (1996).
Singh-Bains, M. K. et al. Cerebellar degeneration correlates with motor symptoms in Huntington disease. Ann. Neurol. 85 , 396–405 (2019).
Louis, E. D. et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov. Disord. 24 , 1600–1605 (2009).
Crabé, R., Aimond, F., Gosset, P., Scamps, F. & Raoul, C. How degeneration of cells surrounding motoneurons contributes to amyotrophic lateral sclerosis. Cells 9 , 2550 (2020).
Elgayar, S. A. M., Abdel-Hafez, A. A. M., Gomaa, A. M. S. & Elsherif, R. Vulnerability of glia and vessels of rat substantia nigra in rotenone Parkinson model. Ultrastruct. Pathol. 42 , 181–192 (2018).
Cragnolini, A. B. et al. Regional brain susceptibility to neurodegeneration: what is the role of glial cells? Neural Regen. Res. 15 , 838–842 (2019).
PubMed Central Google Scholar
Matute, C., Alberdi, E., Ibarretxe, G. & Sánchez-Gómez, M. V. Excitotoxicity in glial cells. Eur. J. Pharmacol. 447 , 239–246 (2002).
Petito, C. K., Olarte, J. P., Roberts, B., Nowak, T. S. & Pulsinelli, W. A. Selective glial vulnerability following transient global ischemia in rat brain. J. Neuropathol. Exp. Neurol. 57 , 231–238 (1998).
Kim, Y.-K. & Na, K.-S. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 70 , 117–126 (2016).
Giuliani, F., Goodyer, C. G., Antel, J. P. & Yong, V. W. Vulnerability of human neurons to T cell-mediated cytotoxicity. J. Immunol. 171 , 368–379 (2003).
Paß, T., Wiesner, R. J. & Pla-Martín, D. Selective neuron vulnerability in common and rare diseases — mitochondria in the focus. Front. Mol. Biosci. 8 , 676187 (2021).
Penzes, P., Buonanno, A., Passafarro, M., Sala, C. & Sweet, R. A. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J. Neurochem. 126 , 165–182 (2013).
Purves, D. et al. in Neuroscience 2nd edn Ch. 1 (Sinauer Associates, 2001).
Eipel, C., Abshagen, K. & Vollmar, B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J. Gastroenterol. 16 , 6046–6057 (2010).
Dalal, R., Bruss, Z. S. & Sehdev, J. S. Physiology, Renal Blood Flow and Filtration (StatPearls, 2023).
Cabral, H., Li, J., Miyata, K. & Kataoka, K. Controlling the biodistribution and clearance of nanomedicines. Nat. Rev. Bioeng. 2 , 214–232 (2024). This comprehensive review details consequence of different NP designs on their pharmacokinetics, with abundant clinical data included.
Du, B., Yu, M. & Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 3 , 358–374 (2018).
Wang, J. & Liu, G. Imaging nano–bio interactions in the kidney: toward a better understanding of nanoparticle clearance. Angew. Chem. Int. Ed. 57 , 3008–3010 (2018). This review details quantitative NP–kidney interactions along with coverage on strategies to modulate NP size, shape and surface chemistry to minimize glomerular clearance.
Article CAS Google Scholar
Rawal, M., Singh, A. & Amiji, M. M. Quality-by-design concepts to improve nanotechnology-based drug development. Pharm. Res. 36 , 153 (2019).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33 , 941–951 (2015). This seminal review discusses the design principles of NPs to avoid delivery challenges, with focus on opsonization, blood vessel fluid dynamics and site-directed entry.
Park, J.-K. et al. Cellular distribution of injected PLGA-nanoparticles in the liver. Nanomedicine 12 , 1365–1374 (2016).
Poon, W. et al. Elimination pathways of nanoparticles. ACS Nano 13 , 5785–5798 (2019).
Lazarovits, J. et al. Supervised learning and mass spectrometry predicts the in vivo fate of nanomaterials. ACS Nano 13 , 8023–8034 (2019).
Anraku, Y., Kishimura, A., Kobayashi, A., Oba, M. & Kataoka, K. Size-controlled long-circulating PICsome as a ruler to measure critical cut-off disposition size into normal and tumor tissues. Chem. Commun. 47 , 6054–6056 (2011).
Lundqvist, M. et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano 5 , 7503–7509 (2011).
Cai, R. & Chen, C. The crown and the scepter: roles of the protein corona in nanomedicine. Adv. Mater. 31 , e1805740 (2019).
Monopoli, M. P., Åberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotech 7 , 779–786 (2012).
Lundqvist, M. et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105 , 14265–14270 (2008).
García-Álvarez, R., Hadjidemetriou, M., Sánchez-Iglesias, A., Liz-Marzán, L. M. & Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 10 , 1256–1264 (2018).
Palchetti, S. et al. The protein corona of circulating PEGylated liposomes. Biochim. Biophys. Acta 1858 , 189–196 (2016).
Wang, H. et al. The nature of a hard protein corona forming on quantum dots exposed to human blood serum. Small 12 , 5836–5844 (2016).
Dobrovolskaia, M. A. et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 5 , 106–117 (2009).
Sakulkhu, U., Mahmoudi, M., Maurizi, L., Salaklang, J. & Hofmann, H. Protein corona composition of superparamagnetic iron oxide nanoparticles with various physico-chemical properties and coatings. Sci. Rep. 4 , 5020 (2014).
Lesniak, A. et al. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 6 , 5845–5857 (2012).
Liu, K. et al. Multiomics analysis of naturally efficacious lipid nanoparticle coronas reveals high-density lipoprotein is necessary for their function. Nat. Commun. 14 , 4007 (2023).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20 , 101–124 (2021).
Salvati, A. et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotech 8 , 137–143 (2013).
Zhao, Z., Ukidve, A., Kim, J. & Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 181 , 151–167 (2020).
Chen, F. et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotech 12 , 387–393 (2017).
Hoshyar, N., Gray, S., Han, H. & Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 11 , 673–692 (2016).
Moghimi, S. M., Simberg, D., Skotland, T., Yaghmur, A. & Hunter, A. C. The interplay between blood proteins, complement, and macrophages on nanomedicine performance and responses. J. Pharmacol. Exp. Ther. 370 , 581–592 (2019).
Huang, W., Xiao, G., Zhang, Y. & Min, W. Research progress and application opportunities of nanoparticle–protein corona complexes. Biomed. Pharmacother. 139 , 111541 (2021).
Klepac, D. et al. Interaction of spin-labeled HPMA-based nanoparticles with human blood plasma proteins — the introduction of protein-corona-free polymer nanomedicine. Nanoscale 10 , 6194–6204 (2018).
Müller, K., Fedosov, D. A. & Gompper, G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci. Rep. 4 , 4871 (2014).
Lane, L. A. Physics in nanomedicine: phenomena governing the in vivo performance of nanoparticles. Appl. Phys. Rev. 7 , 011316 (2020).
Cooley, M. et al. Influence of particle size and shape on their margination and wall-adhesion: implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 10 , 15350–15364 (2018).
Kumar, A., Rivera, R. G. H. & Graham, M. D. Flow-induced segregation in confined multicomponent suspensions: effects of particle size and rigidity. J. Fluid Mech. 738 , 423–462 (2014).
Tosi, G., Duskey, J. T. & Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 17 , 23–32 (2020).
Pandit, R., Chen, L. & Götz, J. The blood-brain barrier: physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 165 – 166 , 1–14 (2020).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20 , 26–41 (2020).
Kucharz, K. et al. Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles. Nat. Commun. 12 , 4121 (2021).
Uchida, Y. et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 117 , 333–345 (2011).
Yeo, N. J. Y., Chan, E. J. J. & Cheung, C. Choroidal neovascularization: mechanisms of endothelial dysfunction. Front. Pharmacol. 10 , 1363 (2019).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 , 133–150 (2018). This comprehensive review discusses the BBB dysfunction in multiple neurological disorders using neuroimaging studies in living human and post-mortem brain tissues.
Malek, N. et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov. Disord. 31 , 1518–1526 (2016).
Drouin-Ouellet, J. et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: potential implications for its pathophysiology. Ann. Neurol. 78 , 160–177 (2015).
Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. 217 , e20190062 (2020).
Nguyen, B., Bix, G. & Yao, Y. Basal lamina changes in neurodegenerative disorders. Mol. Neurodegener. 16 , 81 (2021).
Prakash, R. & Carmichael, S. T. Blood–brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 28 , 556–564 (2015).
Price, L., Wilson, C. & Grant, G. in Translational Research in Traumatic Brain Injury Ch. 4 (eds Laskowitz, D. & Grant, G.) (CRC, 2016).
Clond, M. A. et al. Reactive oxygen species-activated nanoprodrug of ibuprofen for targeting traumatic brain injury in mice. PLoS ONE 8 , e61819 (2013).
Xu, J. et al. Theranostic oxygen reactive polymers for treatment of traumatic brain injury. Adv. Funct. Mater. 26 , 4124–4133 (2016).
Ruozi, B. et al. PLGA nanoparticles loaded cerebrolysin: studies on their preparation and investigation of the effect of storage and serum stability with reference to traumatic brain injury. Mol. Neurobiol. 52 , 899–912 (2015).
Gaudin, A. et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat. Nanotechnol. 9 , 1054–1062 (2014).
Chen, H. et al. Nanoerythropoietin is 10-times more effective than regular erythropoietin in neuroprotection in a neonatal rat model of hypoxia and ischemia. Stroke 43 , 884–887 (2012).
D’Ambrosi, N. & Apolloni, S. Fibrotic scar in neurodegenerative diseases. Front. Immunol. 11 , 1394 (2020).
Fernández-Klett, F. & Priller, J. The fibrotic scar in neurological disorders. Brain Pathol. 24 , 404–413 (2014).
Sun, N. et al. Single-nucleus multiregion transcriptomic analysis of brain vasculature in Alzheimer’s disease. Nat. Neurosci. 26 , 970–982 (2023). This transcriptomic study across 6 brain regions from 220 individuals with AD and 208 age-matched controls demonstrates a large number of differentially expressed genes in neurovascular units, including endothelium, astrocytes and pericytes.
Zhang, W. et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 17 , 47 (2020).
Yang, A. C. et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 583 , 425–430 (2020).
Winkler, E. A. et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18 , 521–530 (2015).
Zhao, L. et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat. Commun. 11 , 4413 (2020).
Liu, M., Fang, X., Yang, Y. & Wang, C. Peptide-enabled targeted delivery systems for therapeutic applications. Front. Bioeng. Biotechnol. 9 , 701504 (2021).
Deane, R., Sagare, A. & Zlokovic, B. The role of the cell surface LRP and soluble LRP in blood-brain barrier Aβ clearance in Alzheimer’s disease. Curr. Pharm. Des. 14 , 1601–1605 (2008).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99 , 21–78 (2019).
Li, W. et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci. Adv. 7 , eabd6889 (2021).
Hladky, S. B. & Barrand, M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11 , 26 (2014).
Shetty, A. K. & Zanirati, G. The interstitial system of the brain in health and disease. Aging Dis. 11 , 200–211 (2020).
Nakada, T. & Kwee, I. L. Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 25 , 155–166 (2019).
Gao, Y. et al. Simulation study of the effects of interstitial fluid pressure and blood flow velocity on transvascular transport of nanoparticles in tumor microenvironment. Comput. Methods Prog. Biomed. 193 , 105493 (2020).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140 , 918–934 (2010).
Whiteford, J. R., De Rossi, G. & Woodfin, A. Mutually supportive mechanisms of inflammation and vascular remodeling. Int. Rev. Cell Mol. Biol. 326 , 201–278 (2016).
Zhang, X. et al. High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice. Natl Sci. Rev. 6 , 1223–1238 (2019).
Wolak, D. J. & Thorne, R. G. Diffusion of macromolecules in the brain: implications for drug delivery. Mol. Pharm. 10 , 1492–1504 (2013).
Hammarlund-Udenaes, M., Fridén, M., Syvänen, S. & Gupta, A. On the rate and extent of drug delivery to the brain. Pharm. Res. 25 , 1737–1750 (2008).
Barua, S. & Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today 9 , 223–243 (2014).
Engin, A. B. et al. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: the cell and immune system. Part. Fibre Toxicol. 14 , 22 (2017).
Chen, K. L. & Bothun, G. D. Nanoparticles meet cell membranes: probing nonspecific interactions using model membranes. Environ. Sci. Technol. 48 , 873–880 (2014).
Mahmoudi, M. et al. Cell ‘vision’: complementary factor of protein corona in nanotoxicology. Nanoscale 4 , 5461–5468 (2012).
Behzadi, S. et al. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 46 , 4218–4244 (2017).
Smith, S. A., Selby, L. I., Johnston, A. P. R. & Such, G. K. The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjug. Chem. 30 , 263–272 (2019).
Foroozandeh, P. & Aziz, A. A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 13 , 339 (2018).
Treuel, L., Jiang, X. & Nienhaus, G. U. New views on cellular uptake and trafficking of manufactured nanoparticles. J. R. Soc. Interface 10 , 20120939 (2013).
Biber, K. et al. Microglial drug targets in AD: opportunities and challenges in drug discovery and development. Front. Pharmacol. 10 , 840 (2019).
Fatoba, O., Itokazu, T. & Yamashita, T. Microglia as therapeutic target in central nervous system disorders. J. Pharmacol. Sci. 144 , 102–118 (2020).
Mahmood, A. & Miron, V. E. Microglia as therapeutic targets for central nervous system remyelination. Curr. Opin. Pharmacol. 63 , 102188 (2022).
Kimelberg, H. K. & Nedergaard, M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7 , 338–353 (2010).
Leng, F. & Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17 , 157–172 (2021).
Zhang, G., Wang, Z., Hu, H., Zhao, M. & Sun, L. Microglia in Alzheimer’s disease: a target for therapeutic intervention. Front. Cell Neurosci. 15 , 749587 (2021).
Donat, C. K., Scott, G., Gentleman, S. M. & Sastre, M. Microglial activation in traumatic brain injury. Front. Aging Neurosci. 9 , 208 (2017).
Kandell, R. M., Kudryashev, J. A. & Kwon, E. J. Targeting the extracellular matrix in traumatic brain injury increases signal generation from an activity-based nanosensor. ACS Nano 15 , 20504–20516 (2021).
Lutton, E. M. et al. Endothelial targeted strategies to combat oxidative stress: improving outcomes in traumatic brain injury. Front. Neurol. 10 , 582 (2019).
Wang, R. et al. Strategies for the design of nanoparticles: starting with long-circulating nanoparticles, from lab to clinic. Biomater. Sci. 9 , 3621–3637 (2021).
Zhu, G. H., Gray, A. B. C. & Patra, H. K. Nanomedicine: controlling nanoparticle clearance for translational success. Trends Pharmacol. Sci. 43 , 709–711 (2022).
Soo Choi, H. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25 , 1165–1170 (2007).
Weiss, M. et al. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 19 , 5 (2021).
Dilliard, S. A. & Siegwart, D. J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 8 , 282–300 (2023).
Bertrand, N. et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 8 , 777 (2017).
Wang, J.-L. et al. The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials 182 , 104–113 (2018).
Ben-Akiva, E. et al. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci. Adv. 6 , eaay9035 (2020).
Hu, C.-M. J. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108 , 10980–10985 (2011).
Gurnani, P. et al. Probing the effect of rigidity on the cellular uptake of core-shell nanoparticles: stiffness effects are size dependent. Small 18 , 2203070 (2022).
Madathiparambil Visalakshan, R. et al. The influence of nanoparticle shape on protein corona formation. Small 16 , 2000285 (2020).
Cheng, J. et al. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 136 , 507–523 (2018).
Geranmayeh, M. H., Rahbarghazi, R. & Farhoudi, M. Targeting pericytes for neurovascular regeneration. Cell Commun. Signal. 17 , 26 (2019).
Porro, G. M. et al. Identifying molecular tags selectively retained on the surface of brain endothelial cells to generate artificial targets for therapy delivery. Fluids Barriers CNS 20 , 88 (2023).
Lasagna-Reeves, C. et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 393 , 649–655 (2010).
Liu, D.-F. et al. Magnetic resonance imaging of post-ischemic blood-brain barrier damage with PEGylated iron oxide nanoparticles. Nanoscale 6 , 15161–15167 (2014).
Shankar, R., Joshi, M. & Pathak, K. Lipid nanoparticles: a novel approach for brain targeting. Pharm. Nanotechnol. 6 , 81–93 (2018).
Dehouck, B. et al. A new function for the LDL receptor: transcytosis of LDL across the blood–brain barrier. J. Cell Biol. 138 , 877–889 (1997).
Jose, S. et al. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 474 , 6–13 (2014).
Cox, A. et al. Evolution of nanoparticle protein corona across the blood-brain barrier. ACS Nano 12 , 7292–7300 (2018). This study reveals drastic changes in protein corona composition around NPs as they pass form the blood to the brain side, which can alter their final distribution inside the brain parenchyma.
Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 20 , 362–383 (2021).
Ciofani, G. et al. Roadmap on nanomedicine for the central nervous system. J. Phys. Mater. 6 , 022501 (2023).
Jefferies, W. A. et al. Transferrin receptor on endothelium of brain capillaries. Nature 312 , 162–163 (1984).
Sheridan, C. Drugs catch a ride through the blood–brain barrier. Nat. Biotechnol. 41 , 1182–1184 (2023).
Hultqvist, G., Syvänen, S., Fang, X. T., Lannfelt, L. & Sehlin, D. Bivalent brain shuttle increases antibody uptake by monovalent binding to the transferrin receptor. Theranostics 7 , 308–318 (2017).
Zhou, Q.-H. et al. Receptor-mediated abeta amyloid antibody targeting to Alzheimer’s disease mouse brain. Mol. Pharm. 8 , 280–285 (2011).
Weber, F. et al. Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 22 , 149–162 (2018).
Niewoehner, J. et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81 , 49–60 (2014).
Li, X. et al. Enhanced in vivo blood–brain barrier penetration by circular tau–transferrin receptor bifunctional aptamer for tauopathy therapy. J. Am. Chem. Soc. 142 , 3862–3872 (2020).
Kang, T. et al. Enhancing glioblastoma-specific penetration by functionalization of nanoparticles with an iron-mimic peptide targeting transferrin/transferrin receptor complex. Mol. Pharm. 12 , 2947–2961 (2015).
Lane-Donovan, C. E., Philips, G. T. & Herz, J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron 83 , 771–787 (2014).
Kreuter, J. et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 10 , 317–325 (2002).
Hartl, N., Adams, F. & Merkel, O. M. From adsorption to covalent bonding: apolipoprotein E functionalization of polymeric nanoparticles for drug delivery across the blood-brain barrier. Adv. Ther. 4 , 2000092 (2021).
Neves, A. R., Queiroz, J. F., Lima, S. A. C., Reis, S. & Apo, E. Functionalization of solid lipid nanoparticles enhances brain drug delivery: uptake mechanism and transport pathways. Bioconjug. Chem. 28 , 995–1004 (2017).
Zhang, D. et al. Near infrared-activatable biomimetic nanogels enabling deep tumor drug penetration inhibit orthotopic glioblastoma. Nat. Commun. 13 , 6835 (2022).
Molino, Y. et al. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. 31 , 1807–1827 (2017).
Duro-Castano, A. et al. Targeting Alzheimer’s disease with multimodal polypeptide-based nanoconjugates. Sci. Adv. 7 , eabf9180 (2021).
Zhang, Z. et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 10 , 3561 (2019).
Wiley, D. T., Webster, P., Gale, A. & Davis, M. E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl Acad. Sci. USA 110 , 8662–8667 (2013).
Banks, W. A. & Erickson, M. A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 37 , 26–32 (2010).
Klyachko, N. L. et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials 140 , 79–87 (2017). This article presents one of the early successful demonstrations of cellular backpacks as a means to transport NPs across the BBB.
Wu, J. R., Hernandez, Y., Miyasaki, K. F. & Kwon, E. J. Engineered nanomaterials that exploit blood-brain barrier dysfunction for delivery to the brain. Adv. Drug Deliv. Rev. 197 , 114820 (2023).
Brenner, J. S. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 9 , 2684 (2018).
Knox, E. G., Aburto, M. R., Clarke, G., Cryan, J. F. & O’Driscoll, C. M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 27 , 2659–2673 (2022).
Nian, K., Harding, I. C., Herman, I. M. & Ebong, E. E. Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front. Physiol. 11 , 605398 (2020).
Ogawa, K. et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J. Control. Release 348 , 34–41 (2022).
Abrahao, A. et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 10 , 4373 (2019).
Tønnesen, J., Hrabĕtová, S. & Soria, F. N. Local diffusion in the extracellular space of the brain. Neurobiol. Dis. 177 , 105981 (2023).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4 , 147ra111 (2012).
Abbott, N. J. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 45 , 545–552 (2004).
Jin, B.-J., Smith, A. J. & Verkman, A. S. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol. 148 , 489–501 (2016).
Ray, L., Iliff, J. J. & Heys, J. J. Analysis of convective and diffusive transport in the brain interstitium. Fluids Barriers CNS 16 , 6 (2019).
Kreuter, J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J. Nanosci. Nanotechnol. 4 , 484–488 (2004).
Surfactants influence polymer nanoparticle fate within the brain. Biomaterials 277 , 121086 (2021).
McKenna, M., Shackelford, D., Ferreira Pontes, H., Ball, B. & Nance, E. Multiple particle tracking detects changes in brain extracellular matrix and predicts neurodevelopmental age. ACS Nano 15 , 8559–8573 (2021).
Negron, K., Khalasawi, N. & Suk, J. S. in Nanotherapy for Brain Tumor Drug Delivery (eds Agrahari, V. et al.) 179–204 (Springer, 2021).
Gu, X. et al. Clearance of two organic nanoparticles from the brain via the paravascular pathway. J. Control. Release 322 , 31–41 (2020).
Waggoner, L. E. et al. Porous silicon nanoparticles targeted to the extracellular matrix for therapeutic protein delivery in traumatic brain injury. Bioconjug Chem. 33 , 1685–1697 (2022).
Mann, A. P. et al. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat. Commun. 7 , 11980 (2016).
Zhao, Y. et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J. Control. Release 233 , 64–71 (2016).
Han, Z. et al. A novel targeted nanoparticle for traumatic brain injury treatment: combined effect of ROS depletion and calcium overload inhibition. Adv. Healthc. Mater. 11 , 2102256 (2022).
Carron, S. F., Alwis, D. S. & Rajan, R. Traumatic brain injury and neuronal functionality changes in sensory cortex. Front. Syst. Neurosci. 10 , 47 (2016).
Praça, C. et al. A nanoformulation for the preferential accumulation in adult neurogenic niches. J. Control. Release 284 , 57–72 (2018).
Hour, F. Q. et al. Magnetic targeted delivery of the SPIONs-labeled mesenchymal stem cells derived from human Wharton’s jelly in Alzheimer’s rat models. J. Control. Release 321 , 430–441 (2020).
Merienne, N. et al. Cell-type-specific gene expression profiling in adult mouse brain reveals normal and disease-state signatures. Cell Rep. 26 , 2477–2493.e9 (2019).
Lake, B. B. et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36 , 70–80 (2018).
Skene, N. G. & Grant, S. G. N. Identification of vulnerable cell types in major brain disorders using single cell transcriptomes and expression weighted cell type enrichment. Front. Neurosci. 10 , 16 (2016).
Pak, V. et al. Distinctive whole-brain cell-types strongly predict tissue damage patterns in eleven neurodegenerative disorders. eLife 12 , RP89368 (2023).
Lee, H.-G., Wheeler, M. A. & Quintana, F. J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 21 , 339–358 (2022).
Kwon, H. S. & Koh, S.-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegen. 9 , 42 (2020).
Maragakis, N. J. & Rothstein, J. D. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2 , 679–689 (2006).
Joshi, C. R. et al. Reaching for the stars in the brain: polymer-mediated gene delivery to human astrocytes. Mol. Ther. Nucleic Acids 12 , 645–657 (2018).
Sabourian, P. et al. Targeting reactive astrocytes by pH-responsive ligand-bonded polymeric nanoparticles in spinal cord injury. Drug Deliv. Transl. Res. 13 , 1842–1855 (2023).
Surnar, B. et al. Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection. Proc. Natl Acad. Sci. USA 115 , E12333–E12342 (2018).
Zhu, J. et al. Reactive A1 astrocyte-targeted nucleic acid nanoantiepileptic drug downregulating adenosine kinase to rescue endogenous antiepileptic pathway. ACS Appl. Mater. Interfaces 15 , 29876–29888 (2023).
Colonna, M. & Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35 , 441–468 (2017).
Ortiz, C. et al. Molecular atlas of the adult mouse brain. Sci. Adv. 6 , eabb3446 (2020).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554 , 475–480 (2018).
Zhao, N., Francis, N. L., Calvelli, H. R. & Moghe, P. V. Microglia-targeting nanotherapeutics for neurodegenerative diseases. APL Bioeng. 4 , 030902 (2020).
Albanese, A., Sykes, E. A. & Chan, W. C. W. Rough around the edges: the inflammatory response of microglial cells to spiky nanoparticles. ACS Nano 4 , 2490–2493 (2010).
Hutter, E. et al. Microglial response to gold nanoparticles. ACS Nano 4 , 2595–2606 (2010).
Choi, B. et al. Highly selective microglial uptake of ceria–zirconia nanoparticles for enhanced analgesic treatment of neuropathic pain. Nanoscale 11 , 19437–19447 (2019).
Ralvenius, W. T. et al. Nanoparticle-mediated delivery of anti-PU.1 siRNA via localized intracisternal administration reduces neuroinflammation. Adv. Mater. 36 , e2309225 (2023).
Roussarie, J.-P. et al. Selective neuronal vulnerability in Alzheimer’s disease: a network-based analysis. Neuron 107 , 821–835.e12 (2020).
Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573 , 75–82 (2019).
Surmeier, D. J., Obeso, J. A. & Halliday, G. M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 18 , 101–113 (2017).
Khan, F. A., Almohazey, D., Alomari, M. & Almofty, S. A. Impact of nanoparticles on neuron biology: current research trends. Int. J. Nanomed. 13 , 2767–2776 (2018).
Stojiljković, A. et al. High-content analysis of factors affecting gold nanoparticle uptake by neuronal and microglial cells in culture. Nanoscale 8 , 16650–16661 (2016).
Orlando, A. et al. Mesoporous silica nanoparticles trigger mitophagy in endothelial cells and perturb neuronal network activity in a size- and time-dependent manner. Int. J. Nanomed. 12 , 3547–3559 (2017).
Prabhu, B. M., Ali, S. F., Murdock, R. C., Hussain, S. M. & Srivatsan, M. Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology 4 , 150–160 (2010).
Dante, S. et al. Selective targeting of neurons with inorganic nanoparticles: revealing the crucial role of nanoparticle surface charge. ACS Nano 11 , 6630–6640 (2017).
Walters, R. et al. Nanoparticle targeting to neurons in a rat hippocampal slice culture model. ASN Neuro 4 , 383–392 (2012).
Zapukhliak, O. S., Kachanovska, V. O., Isaeva, E. V., Netsyk, O. V. & Isaev, D. S. Surface charge impact in nonsynaptic model of epilepsy in rat hippocampus. Fiziol. Zhurnal 62 , 35–40 (2016).
Gao, Y. et al. RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain. Biomacromolecules 15 , 1010–1018 (2014).
Kwon, E. J., Skalak, M., Lo Bu, R. & Bhatia, S. N. Neuron-targeted nanoparticle for siRNA delivery to traumatic brain injuries. ACS Nano 10 , 7926–7933 (2016).
Khongkow, M. et al. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 9 , 8278 (2019).
Ren, M. et al. Functionalized nanoparticles in prevention and targeted therapy of viral diseases with neurotropism properties, special insight on COVID-19. Front. Microbiol. 12 , 767104 (2021).
Zhou, R. et al. Targeted brain delivery of RVG29‐modified rifampicin‐loaded nanoparticles for Alzheimer’s disease treatment and diagnosis. Bioeng. Transl. Med. 7 , e10395 (2022).
Chung, E. P. et al. Targeting small molecule delivery to the brain and spinal cord via intranasal administration of rabies virus glycoprotein (RVG29)-modified PLGA nanoparticles. Pharmaceutics 12 , 93 (2020).
Dos Santos Rodrigues, B., Arora, S., Kanekiyo, T. & Singh, J. Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res. 1734 , 146738 (2020).
Lian, M., Hueffer, K. & Weltzin, M. M. Interactions between the rabies virus and nicotinic acetylcholine receptors: a potential role in rabies virus induced behavior modifications. Heliyon 8 , e10434 (2022).
Fontana, I. C., Kumar, A. & Nordberg, A. The role of astrocytic α7 nicotinic acetylcholine receptors in Alzheimer disease. Nat. Rev. Neurol. 19 , 278–288 (2023).
Hoogland, I. C. M. et al. Microglial cell response in α7 nicotinic acetylcholine receptor-deficient mice after systemic infection with Escherichia coli . J. Neuroinflamm. 19 , 94 (2022).
Jurado-Coronel, J. C. et al. Targeting the nicotinic acetylcholine receptors (nAChRs) in astrocytes as a potential therapeutic target in Parkinson’s disease. Curr. Pharm. Des. 22 , 1305–1311 (2016).
Moon, J. H., Kim, S. Y., Lee, H. G., Kim, S. U. & Lee, Y. B. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar β amyloid peptide (1-42)-stimulated microglia. Exp. Mol. Med. 40 , 11–18 (2008).
Park, I.-K., Lasiene, J., Chou, S.-H., Horner, P. J. & Pun, S. H. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine). J. Gene Med. 9 , 691–702 (2007).
Liu, J. K. et al. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiol. Dis. 19 , 407–418 (2005).
Wang, P. et al. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J. Control. Release 279 , 220–233 (2018).
Zhang, Y. et al. Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. Int. J. Nanomed. 7 , 1015–1022 (2012).
Mathew, A. et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 7 , e32616 (2012).
Guo, Q. et al. A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer’s disease mice. J. Control. Release 320 , 347–362 (2020).
Guo, Q. et al. Brain-neuron targeted nanoparticles for peptide synergy therapy at dual-target of Alzheimer’s disease. J. Control. Release 355 , 604–621 (2023).
Hou, Q. et al. Dual targeting nanoparticles for epilepsy therapy. Chem. Sci. 13 , 12913–12920 (2022).
Garcia-Chica, J. et al. An overview of nanomedicines for neuron targeting. Nanomedicine 15 , 1617–1636 (2020).
Spencer, A. P. et al. Breaking barriers: bioinspired strategies for targeted neuronal delivery to the central nervous system. Pharmaceutics 12 , 192 (2020).
Zhang, F., Lin, Y.-A., Kannan, S. & Kannan, R. M. Targeting specific cells in the brain with nanomedicines for CNS therapies. J. Control. Release 240 , 212–226 (2016).
Zhang, H. et al. Selective neuronal targeting, protection and signaling network analysis via dopamine-mediated mesoporous silica nanoparticles. Med. Chem. Commun. 6 , 1117–1129 (2015).
Ferreira-Vieira, T. H., Guimaraes, I. M., Silva, F. R. & Ribeiro, F. M. Alzheimer’s disease: targeting the cholinergic system. Curr. Neuropharmacol. 14 , 101–115 (2016).
Qian, K. et al. Cholinergic neuron targeting nanosystem delivering hybrid peptide for combinatorial mitochondrial therapy in Alzheimer’s disease. ACS Nano 16 , 11455–11472 (2022).
Luthi-Carter, R. et al. Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Hum. Mol. Genet. 9 , 1259–1271 (2000).
Dencker, D. et al. Muscarinic acetylcholine receptor subtypes as potential drug targets for the treatment of schizophrenia, drug abuse, and Parkinson’s disease. ACS Chem. Neurosci. 3 , 80–89 (2011).
Article PubMed Central Google Scholar
Piggott, M. A. et al. Muscarinic receptors in basal ganglia in dementia with Lewy bodies, Parkinson’s disease and Alzheimer’s disease. J. Chem. Neuroanat. 25 , 161–173 (2003).
Rinne, J. O., Lo¨nnberg, P., Marjama¨ki, P. & Rinne, U. K. Brain muscarinic receptor subtypes are differently affected in Alzheimer’s disease and Parkinson’s disease. Brain Res. 483 , 402–406 (1989).
Liu, W. et al. Applications of machine learning in computational nanotechnology. Nanotechnology 33 , 16 (2022).
Reker, D. et al. Computationally guided high-throughput design of self-assembling drug nanoparticles. Nat. Nanotechnol. 16 , 725–733 (2021).
Lv, H. & Chen, X. Intelligent control of nanoparticle synthesis through machine learning. Nanoscale 14 , 6688–6708 (2022).
Tao, H. et al. Nanoparticle synthesis assisted by machine learning. Nat. Rev. Mater. 6 , 701–716 (2021).
Pasqualini, R. & Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 380 , 364–366 (1996).
Pleiko, K. et al. In vivo phage display: identification of organ-specific peptides using deep sequencing and differential profiling across tissues. Nucleic Acids Res. 49 , e38 (2021).
Bakhshinejad, B., Karimi, M. & Khalaj-Kondori, M. Phage display: development of nanocarriers for targeted drug delivery to the brain. Neural Regen. Res. 10 , 862–865 (2015).
Li, J. et al. Identification of peptide sequences that target to the brain using in vivo phage display. Amino Acids 42 , 2373–2381 (2012).
Li, J. et al. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 32 , 4943–4950 (2011).
Ruta, A., Krishnan, K. & Elisseeff, J. H. Single-cell transcriptomics in tissue engineering and regenerative medicine. Nat. Rev. Bioeng . 2 , 101–119 (2024).
Jung, N. & Kim, T.-K. Spatial transcriptomics in neuroscience. Exp. Mol. Med. 55 , 2105–2115 (2023).
Garland, E. F., Hartnell, I. J. & Boche, D. Microglia and astrocyte function and communication: what do we know in humans? Front. Neurosci. 16 , 824888 (2022).
Matejuk, A. & Ransohoff, R. M. Crosstalk between astrocytes and microglia: an overview. Front. Immunol. 11 , 1416 (2020).
Szepesi, Z., Manouchehrian, O., Bachiller, S. & Deierborg, T. Bidirectional microglia–neuron communication in health and disease. Front. Cell. Neurosci. 12 , 323 (2018).
Jha, M. K., Jo, M., Kim, J.-H. & Suk, K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist 25 , 227–240 (2019).
Linville, R. M. & Searson, P. C. Next-generation in vitro blood–brain barrier models: benchmarking and improving model accuracy. Fluids Barriers CNS 18 , 56 (2021).
Bagchi, S. et al. In-vitro blood-brain barrier models for drug screening and permeation studies: an overview. Drug Des. Dev. Ther. 13 , 3591–3605 (2019).
Pérez-López, A., Torres-Suárez, A. I., Martín-Sabroso, C. & Aparicio-Blanco, J. An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv. Drug Deliv. Rev. 196 , 114816 (2023).
Rouleau, N., Murugan, N. J. & Kaplan, D. L. Functional bioengineered models of the central nervous system. Nat. Rev. Bioeng. 1 , 252–270 (2023).
Dara, S., Dhamercherla, S., Jadav, S. S., Babu, C. M. & Ahsan, M. J. Machine learning in drug discovery: a review. Artif. Intell. Rev. 55 , 1947–1999 (2022).
Vora, L. K. et al. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 15 , 1916 (2023).
Dawson, T. M., Golde, T. E. & Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 21 , 1370–1379 (2018).
Kodamullil, A. T. et al. Of mice and men: comparative analysis of neuro-inflammatory mechanisms in human and mouse using cause-and-effect models. J. Alzheimers Dis. 59 , 1045–1055 (2017).
Xie, F. et al. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int. J. Nanomed. 7 , 163–175 (2012).
Anraku, Y. et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 8 , 1001 (2017).
Zhou, Y. et al. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 6 , eabc7031 (2020).
Arora, S. & Singh, J. In vitro and in vivo optimization of liposomal nanoparticles based brain targeted Vgf gene therapy. Int. J. Pharm. 608 , 121095 (2021).
Boado, R. J. et al. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol. Bioeng. 99 , 475–484 (2008).
Hou, J. et al. Accessing neuroinflammation sites: monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci. Adv. 5 , eaau8301 (2019).
Zensi, A. et al. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 137 , 78–86 (2009).
Sorrentino, N. C. et al. A highly secreted sulphamidase engineered to cross the blood-brain barrier corrects brain lesions of mice with mucopolysaccharidoses type IIIA. EMBO Mol. Med. 5 , 675–690 (2013).
Spencer, B. et al. A neuroprotective brain-penetrating endopeptidase fusion protein ameliorates Alzheimer disease pathology and restores neurogenesis. J. Biol. Chem. 289 , 17917–17931 (2014).
Song, Q. et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol. Pharm. 13 , 3976–3987 (2016).
Meng, F. et al. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer’s disease. Colloids Surf. B Biointerfaces 134 , 88–97 (2015).
Bana, L. et al. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood-brain-barrier: implications for therapy of Alzheimer disease. Nanomedicine 10 , 1583–1590 (2014).
Liu, Y. et al. A leptin derived 30-amino-acid peptide modified pegylated poly- l -lysine dendrigraft for brain targeted gene delivery. Biomaterials 31 , 5246–5257 (2010).
Thom, G. et al. A peptide derived from melanotransferrin delivers a protein-based interleukin 1 receptor antagonist across the BBB and ameliorates neuropathic pain in a preclinical model. J. Cereb. Blood Flow Metab. 39 , 2074–2088 (2019).
Liu, L. et al. Targeted exosome coating gene-chem nanocomplex as ‘nanoscavenger’ for clearing α-synuclein and immune activation of Parkinson’s disease. Sci. Adv. 6 , eaba3967 (2020).
Broadwell, R. D., Baker-Cairns, B. J., Friden, P. M., Oliver, C. & Villegas, J. C. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. III. Receptor-mediated transcytosis through the blood-brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp. Neurol. 142 , 47–65 (1996).
Ulbrich, K., Hekmatara, T., Herbert, E. & Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 71 , 251–256 (2009).
Yu, Y. J. et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl Med. 3 , 84ra44 (2011).
Papademetriou, I. T., Garnacho, C., Schuchman, E. H. & Muro, S. In vivo performance of polymer nanocarriers dually-targeted to epitopes of the same or different receptors. Biomaterials 34 , 3459–3466 (2013).
Valiante, S. et al. Peptide gH625 enters into neuron and astrocyte cell lines and crosses the blood–brain barrier in rats. Int. J. Nanomed. 10 , 1885–1898 (2015).
CAS Google Scholar
Minami, S. S. et al. Selective targeting of microglia by quantum dots. J. Neuroinflamm. 9 , 22 (2012).
Liu, H. et al. Targeting microglia for therapy of Parkinson’s disease by using biomimetic ultrasmall nanoparticles. J. Am. Chem. Soc. 142 , 21730–21742 (2020).
Ren, M. et al. RVG peptide-functionalized favipiravir nanoparticle delivery system facilitates antiviral therapy of neurotropic virus infection in a mouse model. Int. J. Mol. Sci. 24 , 5851 (2023).
Download references
Acknowledgements
The authors thank all authors whose work in CNS drug delivery and related areas contributed to this Review. The authors also thank the reviewers for their constructive suggestions, which helped them to improve this Review.
Author information
Authors and affiliations.
Department of Biomedical Engineering, Center for Bioactive Delivery, Institute for Applied Life Sciences, University of Massachusetts, Amherst, MA, USA
Jingjing Gao
Center for Nanomedicine, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA, USA
Ziting (Judy) Xia, Swetharajan Gunasekar, Christopher Jiang, Jeffrey M. Karp & Nitin Joshi
Harvard Medical School, Boston, MA, USA
Jeffrey M. Karp & Nitin Joshi
Harvard-MIT Program in Health Sciences and Technology, MIT, Cambridge, MA, USA
Jeffrey M. Karp
Harvard Stem Cell Institute, Cambridge, MA, USA
Broad Institute of MIT and Harvard, Cambridge, MA, USA
You can also search for this author in PubMed Google Scholar

Contributions
All authors researched data for the article. J.G., Z.J.X., S.G., J.M.K. and N.J. contributed substantially to the discussion of the content. J.G., Z.J.X., S.G. and C.J. wrote the article. C.J. crafted all the figures. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Correspondence to Jingjing Gao , Jeffrey M. Karp or Nitin Joshi .
Ethics declarations
Competing interests.
N.J. and J.M.K. have one pending patent on nanoparticles for gene delivery in the brain. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Materials thanks Horacio Cabral and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Basal Ganglia: https://my.clevelandclinic.org/health/body/23962-basal-ganglia
Cognitive functioning: https://mayfieldclinic.com/pe-pd.htm
(BBB). A selective barrier formed by endothelial cells, astrocytes and pericytes that regulates the passage of substances from the bloodstream into the central nervous system.
A classification system that describes the progression of abnormal protein deposits, particularly tau proteins, within specific brain regions, providing insights in pathology progression in neurodegenerative diseases such as Alzheimer disease.
Produced by the choroid plexus in brain ventricles; it surrounds the brain and spinal cord, providing mechanical support, nutrient delivery, waste removal and regulation of intracranial pressure within the central nervous system.
A local delivery method that utilize pressure to drive the flow of therapeutic agents through the brain parenchyma.
A dynamic network of proteins and carbohydrates in the brain that surround neurons and glial cells, influencing synaptic plasticity, cell adhesion and neuronal migration.
A waste clearance system unique to the brain that relies on glial cells (especially astrocytes) to facilitate the cerebrospinal fluid–interstitial fluid exchange in the perivascular space.
A fluid that fills the brain interstitial space and directly surrounds neurons and glial cells for nutrient delivery, waste removal and cell signalling.
The main functional tissue of the brain, consisting of neurons, glial cells and other acellular supporting structures to maintain the cognitive and physiological function of the brain.
A layer of proteins that adsorb onto the surface of nanoparticles upon exposure to biological fluids, influencing their behaviour, interactions and biological responses.
(RES). A network of phagocytic cells, mainly macrophages, that are primarily located in the liver and spleen and actively remove foreign substances via engulfment.
Specialized intercellular junctions between endothelial cells that create a barrier to control the passage of ions, molecules and cells across epithelial and endothelial cell layers.
The process by which macromolecules or particles are transported across a cell, involving their uptake on one side through endocytosis, intracellular transport, and release on the opposite side through exocytosis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Gao, J., Xia, Z.(., Gunasekar, S. et al. Precision drug delivery to the central nervous system using engineered nanoparticles. Nat Rev Mater (2024). https://doi.org/10.1038/s41578-024-00695-w
Download citation
Accepted : 28 May 2024
Published : 25 June 2024
DOI : https://doi.org/10.1038/s41578-024-00695-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Nature Reviews Materials ( Nat Rev Mater ) ISSN 2058-8437 (online)
nature.com sitemap
About nature portfolio.
- Press releases
- Press office
Discover content
- Journals A-Z
- Articles by subject
- protocols.io
- Nature Index
Publishing policies
- Nature portfolio policies
- Open access
Author & Researcher services
- Reprints & permissions
- Research data
- Language editing
- Scientific editing
- Nature Masterclasses
- Research Solutions
Libraries & institutions
- Librarian service & tools
- Librarian portal
- Open research
- Recommend to library
Advertising & partnerships
- Advertising
- Partnerships & Services
- Branded content
Professional development
- Nature Careers
- Nature Conferences
Regional websites
- Nature Africa
- Nature China
- Nature India
- Nature Italy
- Nature Japan
- Nature Middle East
- Privacy Policy
- Use of cookies
- Your privacy choices/Manage cookies
- Legal notice
- Accessibility statement
- Terms & Conditions
- Your US state privacy rights
© 2024 Springer Nature Limited
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Turk J Pharm Sci
- v.19(4); 2022 Aug

Features and Facts of a Gastroretentive Drug Delivery System-A Review
Kuldeep vinchurkar.
1 Indore Institute of Pharmacy, Department of Pharmaceutics, Indore, India
2 Devi Ahilya Vishwavidyalaya University, School of Pharmacy, Department of Pharmaceutics, Indore, India
Jitendra SAINY
Masheer ahmed khan, sheetal mane, dinesh k mishra, pankaj dixit.
English oral delivery of drug was the commonly used modality because of patient compliance and ease of administration. After oral administration of any drug, its bioavailability is affected by its residence time in stomach. Recently, gastroretentive drug delivery systems (GRDDS) have gained wide acceptance for drugs with a narrow absorption window, decreased stability at high alkaline pH, and increased solubility at low pH. This approach develops a drug delivery system, which gets retained within gastric fluid, thereby releasing its active principles in the stomach. Some methods used to achieve gastric retention of drugs include the use of effervescence agents, mucoadhesive polymers, magnetic material, bouncy enhancing excipient, and techniques that form plug-like devices that resist gastric emptying. This review provides a concise account of various attributes of recently developed approaches for GRDDS.
INTRODUCTION
Oral administration is popular despite continuous improvement in drug delivery approaches owing to patient comfort and ease of administration. Controlled release drug delivery systems are designed for oral administration. These drug delivery systems release the medication in a predetermined, predictable, and controlled way. They are not suitable for drugs with low bioavailability due to stability or absorption issues. 1 These problems can get better through modern approaches, which are designed to increase the residence of such drugs in the stomach for an extended time. Such drug delivery systems are called gastroretentive drug delivery systems (GRDDS). GRDDS are suitable for those drugs, which are absorbed from the stomach ( e.g. albuterol), 2 labile at alkaline pH ( e.g. ranitidine and metformin), 3 poorly soluble at alkaline pH ( e.g. furosemide and diazepam), 4 and having a narrow window of absorption ( e.g. riboflavin and levodopa). 5
Some of the common advantages associated with use of GRDDS include improved patient compliance by reducing the frequency of dosing; improved therapeutic efficacy of drugs with a short half-life; site-specific delivery of medications; sustained and controlled release of drugs in the stomach; enhanced residence time of drugs at the absorption site; improved bioavailability from the gastrointestinal tract; avoiding dose dumping of medicines. 6
To develop GRDDS, different materials like ion-exchange resins, mucoadhesives, high-density materials, raft forming substances, magnetic substances, and super porous hydrogels are used. 7 , 8
This review provides a concise account of various attributes of recently developed approaches for GRDDS.
Anatomy and physiology of the stomach
Knowledge about the anatomy and physiology of the stomach is essential for the successful formulation of gastroretentive dosage forms. Anatomically, the stomach is divided into three areas: the proximal portion toward the esophagus is fundus, followed by the body, which serves as a storage site for engulfed food, and the antrum, last part that connects the body to the small intestine. Antrum helps in churning action and in gastric emptying. 9 In fasting state, a sequence of contractions occurs cyclically through the stomach and intestine every 120-180 min, called the migrating myoelectric cycle. It is further divided into four phases. The pattern of contraction changes in a fed state is termed as the digestive motility pattern. 10 This pattern comprises phase 1- (basal phase); phase 2- (preburst phase); phase 3- (burst phase); and phase 4. 11 Figure 1 depicts the motility pattern in the gastrointestinal tract.

Motility pattern in gastrointestinal tract
Physicochemical properties of GRDDS
Physicochemical properties of GRDDS include density, size, and shape of the dosage form, which play major roles in the formulation of GRDDS. The dosage forms having a density lower than the gastric contents can float to the surface, while high-density systems sink to the bottom of the stomach. For an ideal formulation, the density should be in the range of 1.0-2.5 g/cm 3 . Dosage forms having a diameter of more than 7.5 mm show better gastric residence time (GRT). Circular, spherical or tetrahedron-shaped devices show excellent gastroretentive properties. 12
Physiological factors affecting retention of GRDDS in the stomach
The most important factors controlling the gastric retention time of dosage forms include fed or unfed state, nature of the meal, caloric content, and frequency of feeding. In the case of a fasting environment, gastric retention time is less due to the increase in GI motility. Emptying of gastric content occurs due to peristalsis. If peristalsis coincides with dosage form administration, the gastric residence is short. However, after meals, peristalsis is delayed and may help increase the gastric residence of the formulation. A high-calorie meal containing proteins, fats, and fibrous compounds increases gastric retention time. In the case of multiple meals, the gastric retention is more than a single meal due to persistent inhibition of peristalsis.
Also, some other factors, such as sex and age, affect gastric retention. Compared with males, females have a slower gastric emptying time irrespective of height, weight, and body surface. A person at the age of more than 70 exhibits longer GRT. In comparison, neonates show less GRT compared with geriatric patients. 13 , 14 , 15
Gastroretentive dosage form approaches
Continuous research and advancements in various approaches to gastroretentive dosage forms over the last few years are as presented in Figure 2 . These approaches to GRDDS help in delivering the medicament in a sustained and restrained way through the gastrointestinal tract.

Approaches of gastroretentive drug delivery system
Classification of GRDDS
GRDDS are classified into mainly two types: floating and non-floating systems. Floating systems are further classified into effervescent system and non-effervescent systems based on the mechanism of floating, while non-floating systems classified into four different classes based on the mechanism used for gastroretention. Figure 3 depicts the classification of the GRDDS.

Classification of gastroretentive drug delivery system
I- High-density system
The density of dosage form plays an important factor in the formulation of the GRDDS. A high-density system uses its weight as a retention mechanism. To enhance the gastric residence of a drug in the stomach, its density must exceed the normal stomach content (1.004 g/mL). 16 Figure 4A depicts the principle of a high-density system. Clarke et al. 17 compared gastrointestinal transit of placebo pellet systems of varying densities using gamma scintigraphy. They reported that GRT of such a formulation can be extended from an average of 5.8 h to 25 h, depending more on density than on the diameter of the pellets.

Different types of gastroretentive drug delivery system. A) High density system, B) floating/low density system, C) inflatable system, D) mucoadhesive system, E) magnetic system (i- stomach, ii- gastric fluid, iii- dosage form)
II- Floating or low-density system
Another approach to increase gastric residence is to lower the density of dosage form than the normal gastric content. These systems remain buoyant due to lower density and provide continuous drug release. In this way, they increase GRT of the drug and improve its bioavailability. 18 Figure 4B depicts the principle of floating or low-density systems.
(A) Effervescent system
This system uses carbonates ( e.g. sodium bicarbonate) to generate in situ carbon dioxide (CO 2 ). 19 , 20 Organic acids ( e.g. citric and tartaric acids) are added to speed up the reaction, thus reducing the density of dosage form and remaining buoyant in the stomach. 20 It is categorized into two classes:
a) Volatile liquid/vacuum type: These are further classified into three types.
i) Inflatable system
It consists of a pullout system having a space filled with volatile liquids that evaporate at body temperature. Thus, when these systems are introduced into the stomach, the chamber inflates, and the system floats. The inflatable chamber comprises a bioerodible polymer filament that is made from polymers like polyvinyl alcohol and polyethylene. When the inflatable chamber floats in the gastrointestinal fluid, the polymer gradually dissolves and releases the drug. After some time, due to polymer dissolution, the inflatable section collapses. 19 , 20 Figure 4C depicts a floating effervescent type of inflatable system.
ii) Intragastric floating system
It contains a chamber filled with a vacuum and includes a microporous compartment serving as a drug reservoir. 20 Figure 5 depicts a floating type of intragastric system. Patel et al. 21 developed intragastric floating tablets of verapamil HCl using hydroxypropyl methylcellulose (HPMC), carbopol, and xanthan gum as gel-forming agents. Buoyancy was achieved by adding an effervescent mixture of sodium bicarbonate and anhydrous citric acid. Optimized formulation exhibited satisfactory results with a short buoyancy lag time of 36 sec, a total buoyancy time of more than 24 h, and controlled drug release for up to 24 h.

Intragastric floating gastrointestinal drug delivery system.
iii) Intragastric-osmotically controlled system
Osmotic control can be achieved using a biodegradable capsule comprising inflatable floating support congestion with an osmotic pressure-controlled drug delivery device. 22 , 23 Zhao et al. 24 used fenofibrate-loaded mesoporous silica nanoparticles to prepare an oral push-pull osmotic pump. Polyethylene oxide (100,000) and polyethylene oxide (6,000,000) were selected as suspending agents and expanding agents, respectively. Cellulose acetate was used as a semipermeable membrane along with polyethylene glycol 6,000 to increase flexibility and control the membrane permeability. The prepared system is reported to stay in the stomach for a period of 21.72 h rather than 12.48 h of the reference tablet and delivers the drug in an approximately zero-order manner for 24 h.
b) Matrix tablets: They are of two types, i.e. single-layer and bilayer matrix tablets. The single-layer matrix tablets are prepared using a drug and a hydrocolloid forming gel, while the bilayer matrix tablet contains one immediate-release layer and another sustained release layer. Saisivam et al. 25 developed single-layer floating matrix tablets of losartan potassium using different proportions of HPMC-K4M and karaya gum as retarding polymer and sodium bicarbonate as an effervescent agent by direct compression method. Results of an in vivo study of optimized formulation displayed the floatability of tablet in gastric content and prolonged the GRT to approximately 12 h. X-ray imaging study in albino rabbits indicated the residence of tablet in the stomach even after a period of 12 h.
c) Gas generating systems: Gas-generating systems are prepared using effervescent compounds along with hydrophilic polymers.
i) Floating capsules
These dosage forms involve encapsulation of drugs in hydrophilic polymers like ethyl cellulose and eudragit RS-100 with effervescent agents such as sodium bicarbonate, calcium carbonate, etc. Moursy et al. 26 developed a hydrodynamically balanced capsule containing a mixture of nicardipine hydrochloride and hydrocolloids. Upon contact with gastric fluid, the capsule shell dissolves with subsequent swelling, forming a gelatinous barrier, which remains buoyant in the gastric juice for an extended period.
ii) Floating pills
Multiple unit types of oral floating dosage forms have been developed using a hydrophilic polymer in the outer layer and an effervescent agent in the inner layer. When it comes in contact with the gastric fluid, the outer layer of hydrophilic polymer starts to swell and then sinks, but as the effervescent agent meets gastric content, it releases CO 2 , and the system starts to float. 27 , 28 Meka et al. 29 prepared multiple-unit minitab of captopril based on a gas formation technique to prolong the GRT and to increase the overall bioavailability of the drug. They developed drug-containing core units using the direct compression process, which were coated with three successive layers of an inner seal coat, effervescent layer (sodium bicarbonate), and an outer gas-entrapped polymeric membrane of polymethacrylates (eudragit RL30D, RS30D, and combinations of them). They found that increasing the coating level of gas-entrapped polymeric membrane decreased the drug release.
iii) Floating systems with ion exchange resins
These floating systems are mainly developed to prolong the GRT of dosage forms using ion exchange resin. They consist of drug resin complex beads loaded with bicarbonate ions, and they are coated with hydrophilic polymers. 30 It results in the generation of CO 2 gas when it comes in contact with the gastric fluid and causes the beads to float. Atyabi et al. 31 developed a floating system based on an ion exchange resin, which consists of resin beads, loaded with bicarbonate and a negatively charged drug that was bound to the resin. Two resins, i.e. Amberlite IRA-400 and Dowex 2 x 10, were investigated and both exhibited in vitro floating times of over 24 h using a standardized procedure. The coated dosage form remained for over 3 h in the stomach with the non-coated system and demonstrated a marked increase in retention over conventional formulation.
(B) Non-effervescent systems
In non-effervescent floating systems, the drug comes in contact with gastric fluid and it swells. It maintains its shape, and its density remains less than one, hence it floats in gastric juice. 32 Matrix forming polymer, gel-forming, or swellable type hydrocolloids are used for these types of floating systems. They are further classified as follows:
i. Hydrodynamically balanced systems (HBS)
These systems mainly consist of a mixture of drugs and hydrocolloids that forms a gelatinous barrier, when it comes in contact with gastric fluid due to swelling of the combination. It remains buoyant in the stomach for an extended period as its bulk density is less than one in gastric fluid. Nayak and Malakar 33 developed gastroretentive theophylline HBS capsules using HPMC, polyethylene oxide, polyvinylpyrrolidone, ethylcellulose, liquid paraffin, and lactose to control the delivery of theophylline for a longer period in the stomach with a minimum floating time of 6 h.
ii. Microballoons
Microballoons are described by the gradual addition of drug-containing emulsion into a volatile solvent. On evaporation of the solvent, gas is generated in a dispersed polymer droplet, which results in the formation of an interior orifice in the microsphere of the drug with polymer. It is also called emulsion solvent diffusion method. 22 The floating time of microspheres depends upon the type and amount of polymer used in the formulation. Gupta et al. 34 developed pantoprazole sodium-loaded microspheres using eudragit L100 by adopting an emulsion solvent diffusion method with a non-effervescent approach. The results of in vitro and in vivo studies exhibited a suitable drug-release pattern in terms of increased bioavailability and efficient ulcer healing effect. Figure 6 depicts the steps involved in the preparation of microballoons by solvent diffusion method.

Preparation technique and mechanism of microballoons formation
iii. Alginate beads
These systems are prepared using a hydrocolloid gel-forming agent and sodium alginate as the interlocking agents. In the presence of gastric fluid, the hydrocolloid absorbs water and forms a barrier that results in entrapment of air in the polymer, which causes swelling of the polymer, and hence the dosage form starts to float, and results in releasing the drug for a prolonged period. Ghareeb and Radhi 35 developed trimetazidine calcium alginate floating beads using sodium alginate solution (2, 3, and 4% w/v), HPMC, and peppermint oil (15, 20, and 25% v/v) using emulsion gelation method. They found that oil entrapped floating beads gave promising results for sustaining the release of the drug over 10 h.
iv. Layered tablets
Layered tablets are more popular due to ease of their preparation, low cost, and high stability.
a. Single-layered floating tablets: These tablets were developed by mixing drug and gas generating agents within the matrix tablet. These formulations have lower bulk density than gastric fluid, and thus they remain buoyant in the stomach by increasing GRT. 36 Kim et al. 37 developed non-effervescent gastroretentive tablets of pregabalin once a day using wet granulation and compaction. They found that the amounts of HPMC and crospovidone were found be critical factors affecting in vitro dissolution and floating properties of the prepared tablets. Figure 7 depicts a schematic of single-layered floating tablets.

Mechanism of single layer tablet
b. Double-layered floating tablets: It comprises of two formulations separated by layering, one on top of the other, having two different release profiles. 3 , 38 Kuldeep et al. 39 developed a bilayer floating tablet of metoprolol succinate (sustained-release layer) and rosuvastatin calcium (immediate-release layer) by direct compression method. HPMC K100, K4M, and K15M were used as gel-forming agents, while cross carmellose sodium, sodium starch glycolate, and crospovidone were used as super disintegrant. Sodium bicarbonate is used as an effervescent agent. From the in vitro buoyancy study, it was observed that as the concentration of gas-generating agents increases, floating lag time decreases. Also, the polymer gas generating agent ratio was found to influence the floating lag time and the total duration of floating.
III- Mucoadhesive and bioadhesive systems
A mucoadhesive and bioadhesive system uses its adhesive properties to target a drug to a specific region of the body for an extended period. Figure 4D displays a mucoadhesive system of GRDDS. For this, bioadhesive or mucoadhesive polymers are mainly used. 40 Natural polymers such as sodium alginate, gelatin, guar gum, etc. , and semisynthetic polymers such as HPMC, lectins, carbopol, and sodium carboxymethyl cellulose are widely used for mucoadhesion. The adhesion is mediated by hydration, bonding, or receptor interactions. 41 , 42 Madgulkar et al. 43 developed sustained-release tablets of itraconazole using mucoadhesive polymer carbopol 934P and HPMC K4M. They confirmed sustained drug release and gastric retention for six hours in albino rats. Figure 8 depicts the principle of mucoadhesive drug delivery systems.

Principle of mucoadhesive drug delivery system
IV- Swelling system
These systems, when come in contact with gastric fluid, their size increases significantly than that of the pyloric sphincter and thus, after swelling, remain logged in the stomach. These are also called a “plug type system”. 44 Controlled and sustained drug release is achieved using an appropriate excipient. The swelling ability of polymer mainly depends upon the degree of cross-linking of hydrophilic polymer network. The high degree of cross-linking maintains the integrity of the system, while a low degree of cross-linking causes extensive swelling resulting in rapid dissolution of the polymer. 45
V- Superporous hydrogels
Superporous hydrogels are a three-dimensional network of hydrophilic polymers that have numerous super-size pores inside them. The swelling of superporous hydrogels occurs by the mechanism of capillary wetting through interconnected open pores. To develop superporous hydrogels, certain ingredients like initiators and cross-linkers are used to initiate the cross-linking. 46 Other ingredients were foam stabilizers, foaming aids, and foaming agents. Desu et al. 47 developed a superporous hydrogel system using N’ , N’ -methylene bisacrylamide as the cross-linking operator and polyvinyl alcohol as a composite specialist, ammonium persulfate and N , N -tetramethylenediamine as an initiator pair and Span 80 as a surfactant. They are used as a froth stabilizer to make a permeable structure using the gas-forming method.
VI- Magnetic system
In this system, by using a strong magnet with a powerful magnetic field onto the body surface, the movement of gastroretentive formulation with a small internal magnet is controlled. Several reports tell about the positive results of this system, but the success of this system depends upon the selection of the magnet position with very high precision. 48 Gröning et al. 49 developed peroral acyclovir depot tablets with an internal magnet. An extracorporeal magnet was used to prolong the GRT of the dosage form and to influence the duration of absorption of acyclovir. They performed an in vivo study with five healthy male subjects and determined the plasma concentration-time profiles of acyclovir. Computer simulations were carried out to display the influence of GRT of acyclovir depot preparations on the plasma concentration-time profiles of acyclovir. Figure 4E displays a magnetic system of GRDDS.
In vitro assessment
For GRDDS, in vitro assessment is very essential to predict gastric transit behavior. Following are the parameters, which should be considered for designing novel gastroretentive formulations.
i. Buoyancy lag time
It is the time taken for gastroretentive formulations to move onto the surface of the dissolution medium. It is determined using a USP dissolution apparatus containing 900 mL of 0.1 N HCl solution as a testing medium maintained at 37°C. The time required to float different dosage forms noted as floating lag time. 50
ii. Floating time
This determines the buoyancy of dosage form. In this test, a specific dissolution apparatus is used depending upon the type of dosage form with 900 mL of dissolution medium kept at 37°C. The floating time or floating duration of the dosage form is determined by visual observation. 51 , 52
iii. Specific gravity/density
Specific gravity estimates are essential for both low-density and high-density GRDDS. Specific gravity is determined using the displacement method. 53
iv. Swelling index
Swelling index is determined by immersing the tablets in 0.1 N HCl at 37°C and their periodic removal at regular intervals. 54
v. Water uptake
In this study, the dosage form is removed from the dissolution medium after the regular interval and a weight change is determined. 55
Water uptake (WU) = (W t - W o ) * 100/W o
where W t = weight of the dosage form at time t, W o = initial weight of the dosage form
vi. Weight variation
Various official methods are recommended by pharmacopeias to calculate the weight variation. Usually, the individual and average weight of 20 tablets are recorded. From these data, average weight and weight variation is calculated. 56 , 57
iii. Hardness and friability
Hardness or crushing strength is determined using a Monsanto tester, Strong cobb tester, Pfizer tester, etc. Friability (strength) of tablets is determined using a Roche friabilator. 58 , 59
viii. In vitro dissolution tests
This test is performed to determine drug release from GRDDS in gastric fluid and intestinal fluid maintained at 37°C at a definite time using USP dissolution type II apparatus (paddle). 59 , 60
Here, after in vitro assesment, Table 1 represents the recent trends in GRDDS, while Table 2 represents the names of drug candidates for GRDDS.

Evaluation of microsphere and beads
An optical microscope was used to measure the particle size of beads and microspheres. Surface morphology and cross-sectional morphology are evaluated with the help of a scanning electron microscope.
In vivo assessment
A. radiology.
This technique is mainly used to determine the position of gastroretentive dosage form filled with barium sulfate (radio-opaque marker) inside the body system concerning time using X-ray. X-ray pictures are taken at different intervals to record the correct position of the dosage form. 61 , 62
b. Scintigraphy
Similar to radiology, it is used to determine in vivo floating behavior of the gastroretentive dosage form. In scintigraphy, 99mTc pertechnetate is used as an emitting material instead of an X-ray to engulf the formulation to record the image. 63 , 64
c. Gastroscopy
Gastroscopy is widely used for visual examinations of gastroretentive dosage forms. In this technique, an illuminate optical, tubular, and slender instrument called “endoscope” is used to look deep inside the body parts such as the stomach, esophagus, and small intestine. 65 , 66
d. Ultrasonography
It is a diagnostic imaging technique, in which ultrasound is used for imaging internal body structures. The main disadvantage of this test is non-detectability at entrails. 1 , 66 , 67
e. 13 C octanoic acid breath test
Radioactive 13 C octanoic acid is used to assess the extent of absorption of drugs from GRDDS. This compound gets absorbed from the duodenum, and, when it is radiolabelled, then after its metabolism, the CO 2 exhaled in breath can be correlated with the amount of octanoic acid absorbed. The radiolabelled CO 2 was measured by isotope ratio mass spectroscopy. 65 , 66
f. Magnetic marker monitoring
Compared with radiology and scintigraphy, this method is radiation-less, and thus is non-hazardous. 67 , 68 It involves real-time tracking of the dosage form in the gastrointestinal tract. 69 , 70 This technique is mainly used for the determination of the gastrointestinal motility and dissolution behavior of pharmaceuticals. In this technique, the dosage form is labeled as a magnetic dipole by incorporating a trace of ferromagnetic particles and recording the magnetic dipole field by an apparatus responsive to bio-magnetic measurement. 71 , 72 , 73
Advantages and applications of gastroretentive delivery systems
Gastroretentive dosage forms release the drug in a controlled manner to their specific site of action. 74 These systems help increase the bioavailability of drugs that get metabolized in the upper part of the gastrointestinal tract, such as riboflavin and levodopa, etc . 75 , 76 For drugs that have a short half-life, gastroretentive dosage forms help reduce the dosing frequency and improve patient compliance by enhancing GRT. Also, they provide a sustained and prolonged release of drugs in the stomach and intestine, which are helpful in local therapy. 77 , 78 , 79 Lastly, Table 3 depicts the gastroretentive technologies adopted by various pharmaceutical companies, and Table 4 represents the list of commonly used drugs for various floating systems.

GRDDS are unique systems and have become important in the last three decades. It offers various advantages, viz ., site-specific, slow, and controlled release of drugs from different types of gastroretentive dosage forms, thus improving patient compliance and reducing the side effects by minimizing dosing frequency. Therefore, it is expected that in the future, various pharmaceutical companies will come forward to initialize gastroretentive drug delivery technology to create excellent advantages, prolonging patents, and a better outcome for their marketed formulations.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.

IMAGES
VIDEO
COMMENTS
A floating dosage type is a commonly accepted method especially for drugs with. restricted absorption sites in the small upper intestine. Disadvantages of the delivery mechanism for floati ng ...
A multiple unit oral floating drug delivery system of famotidine was developed to prolong gastric residence time, target stomach mucosa and increase drug bioavailability. Drug and polymer compatibility was studied by subjecting physical mixtures of drug and polymers to differential scanning calorimetry. Cod liver oil entrapped calcium alginate ...
This review summarizes the design of the FDDS systems, factors that affect floating system, advantages, limitations, evaluation parameters and applications. Available via license: CC BY 4.0 ...
Abstract. The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with special focus on the principal mechanism of floatation to achieve gastric retention. The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to ...
Background Gastroretentive drug delivery system (GDDS) are novel systems that have been recently developed for treating stomach diseases. The key function of all GDDS systems is to control the retention time in the stomach. However, research into the bulk density or entanglement of polymers, especially regarding their effects on drug float and release times, is scarce. Methods In this research ...
Floating drug delivery system (FDDS) is the main approach to prolonging the gastric residence time in the stomach in which the bilayer floating tablet has the main role. It is more suitable for the treatment of local infections such as peptic ulcer, gastritis, Zollinger-Ellision syndrome, indigestion, and other local infections related to the ...
The floating drug delivery system (FDDS) is also called a hydrodynamically balanced system (HBS). In FDDS, the drugs carriers float in gastric juice to ensure that drugs do not leave stomach shortly. ... It indicates that the dense shell layer developed in this research can prolong the floating time of drugs in the stomach. When the amounts of ...
The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with special focus on the principal mechanism of floatation to achieve gastric retention. The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to design single-unit and multiple-unit floating systems, and their ...
Floating. Drug Delivery Systems (FDDS) is one of the gastro-retentive dosage for ms used to achieve. extended duration of gastric residency. The aim of writing this review on floating drug ...
Abstract. The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with special focus on the principal mechanism of floatation to achieve gastric retention. The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to ...
Floating dosage forms (FDFs) represent widely used types of gastroretentive drug delivery systems that provide targeted delivery to the upper gastrointestinal tract. This article proposes a classification of FDFs according to which approaches to achieving drug flotation are reviewed based on published works. FDFs present on the market and the concepts used to create them are described. Various ...
Finally, with an increasing understanding of polymer behavior and the role of the biological factors mentioned above, it is suggested that future research work in the floating drug delivery systems should be aimed at discovering means to accurately control the drug input rate into the GI tract for the optimization of the pharmacokinetic and ...
The floating drug delivery approach is a low-density system that may be effervescent or. Non-Effervescent type with sufficient buoyancy to flow over the gastric contents and remain. buoyant in the stomach without affecting the stomachic emptying rate for a prolonged duration.
INTRODUCTION. The oral route plays an important role in therapy as it is the most preferred and convenient route for drug delivery systems. 1 Gastroretentive drug delivery systems (GRDDSs) are an advanced approach for the novel drug-delivery systems in which the drug is retained in the stomach for a prolonged period. 2,3 GRDDSs are particularly suitable for drugs having a narrow absorption ...
Abstract and Figures. The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with special focus on the principal mechanism of floatation ...
Floating drug delivery systems (FDDS) have garnered significant attention in pharmaceutical research due to their ability to improve drug bioavailability and therapeutic efficacy. This comprehensive review aims to provide an in-depth analysis of the formulation strategies and applications of floating drug delivery systems.The review commences by discussing the physiological basis of gastric ...
A review of floating drug delivery system. Asian journal of biomedical and pharmaceutical science, 3(24): 1-6,(2013) Gupta P, Gnanarajan, Kothiyal P., Floating drug delivery system: a review. International Journal of Pharma Research & Review, 4(8):37-44,(2015) Tripathi P, Ubaidulla U, Khar RK, Vishwavibhuti., Floating drug delivery system.
FLOATING DRUG DELIVERY SYSTEM: A REVIEW ¹Rohit D. Jondhale, ²*Pallavi S. Gadhave, ²Ashish A. Mahajan, ²Mohini S. Bodhane ... Nasik. ABSTRACT In recent years scientific and technological advancements have been made in the research and development of rate-controlled oral drug delivery systems by overcoming physiological adversities, such as ...
2. Floating Drug Delivery Systems. Floating drug delivery systems, DF, the value of the bulk density of which is initially lower than the density of gastric juice (1.004 g/cm 3) or decreases to the required value after ingestion for a certain period of time [1,2,3,6,7].This property allows the drug to remain on the surface of the contents of the stomach, thereby avoiding evacuation to the ...
Nivas et al. European Journal of Pharmaceutical and Medical Research www.ejpmr.com │ 321Vol 9, Issue 3, 2022.│ ISO 9001:2015 Certified Journal │ FLOATING DRUG DELIVERY SYSTEM (FDDS): TYPES, ADVANTAGES, DISADVANTAGES & APPROACHES Ram Nivas*1, Suraj Mandal2, Himani Gururani3, Pragati Saxena4, Sanjeev Kumar5 and Dr. Navneet Verma6
Abstract. Floating drug delivery system (FDDS) helps to improve the buoyancy property of the drug over the gastric fluids and hence maintain the longer duration of action. It is helpful in ...
The development of therapeutics for central nervous system disorders suffers from high failure rates owing to poor blood-brain barrier penetration and lack of targeted delivery. This Review ...
Terray is developing new drugs for inflammatory diseases including lupus, psoriasis and rheumatoid arthritis. The company, Dr. Berlin said, expects to have drugs in clinical trials by early 2026 ...
INTRODUCTION. Gastroretentive drug delivery systems are designed to be retained in the stomach for a prolonged time and release their active ingredients and thereby enable sustained and prolonged input of the drug to the upper part of the gastrointestinal tract.[] A modified release drug delivery system with prolonged residence time in the stomach is of particular interest for drugs- acting ...
After oral administration of any drug, its bioavailability is affected by its residence time in stomach. Recently, gastroretentive drug delivery systems (GRDDS) have gained wide acceptance for drugs with a narrow absorption window, decreased stability at high alkaline pH, and increased solubility at low pH.