- Skip to secondary menu
- Skip to main content
- Skip to primary sidebar
Statistics By Jim
Making statistics intuitive

Retrospective Study: Definition & Examples
By Jim Frost 1 Comment
What is a Retrospective Study?
A retrospective study an experimental design that looks back in time and assesses events that have already occurred. The researchers already know the outcome for each subject when the project starts. Instead of recording data going forward as events happen, these studies use participant recollection and data that were previously recorded for reasons not relating to the project. These studies typically don’t follow patients into the future.
In retrospective designs, the researchers collect their data using existing records. Consequently, they can complete their assessment more quickly and inexpensively than a prospective study that must follow subjects over time and record the data under carefully controlled conditions. However, the data that a retrospective study uses might not have been measured consistently or accurately because they weren’t explicitly designed to be part of a study.

The statistical analysis for a retrospective study is frequently the same as for prospective designs (looking forward). The main difference is that the project occurs after the outcomes are known rather than how researchers analyze the data.
Statisticians consider retrospective designs to be inferior to prospective methods because they tend to introduce more bias and confounding. Retrospective studies are observational studies by necessity because they assess past events and it is impossible to perform a randomized, controlled experiment with them. However, they can be quicker and cheaper to complete, making them a good choice for preliminary research. Findings from a retrospective study can help inform a prospective experimental design. Learn more about Experimental Designs .
Retrospective Study Designs
Retrospective studies use various designs. While these designs differ in detail, they all tend to compare subjects with and without a condition and determine how they differ. Using the usual hypothesis tests, researchers can determine whether there are statistically significant relationships between subject variables (risk factors , personal characteristics, etc.) and the outcome of interest.
Cohort and case-control studies are standard retrospective designs. Let’s learn more about them!
Retrospective Cohort Study
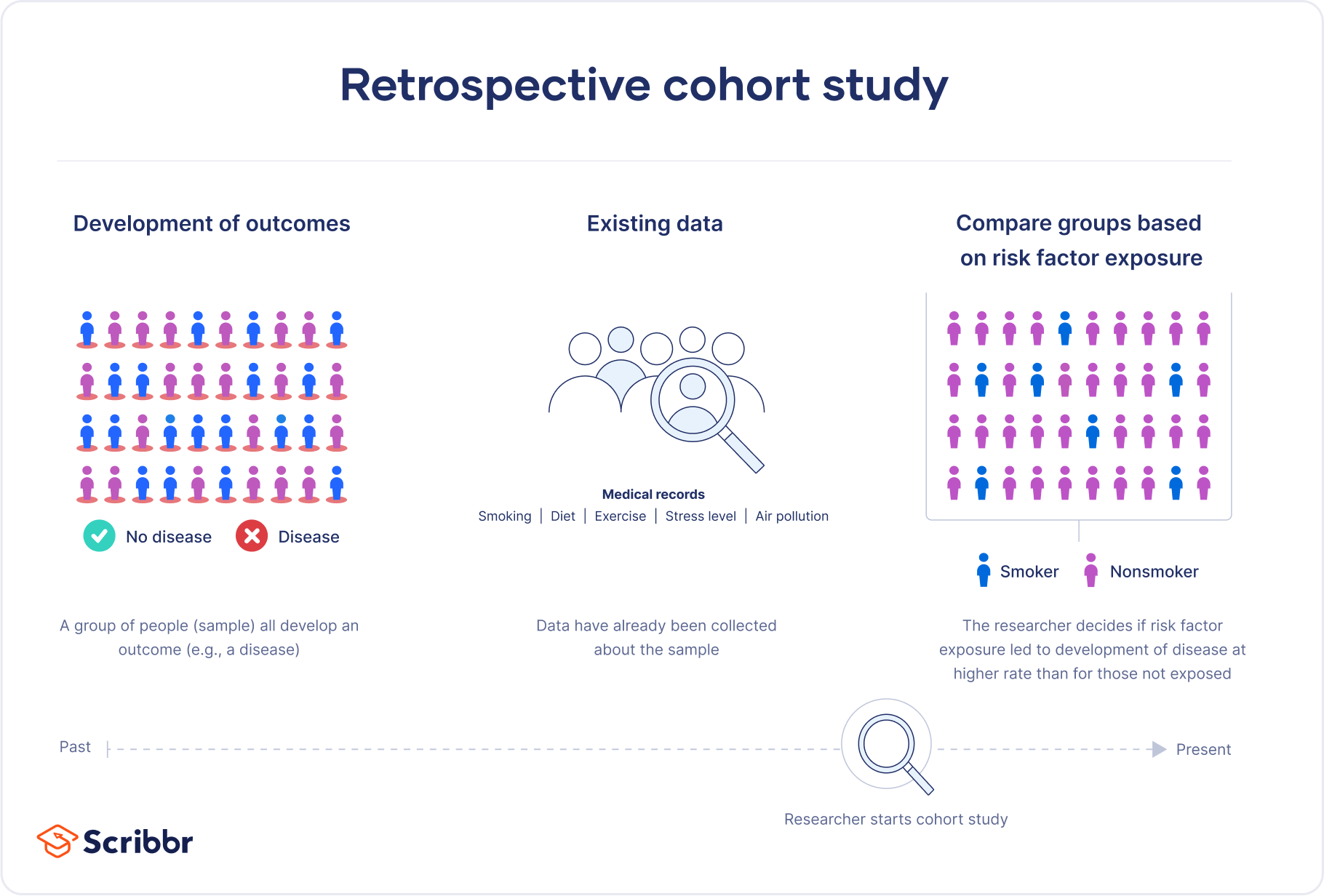
This study design compares groups of subjects who are similar overall but differ in a particular characteristic, such as exposure to a risk factor. Because it is a retrospective study, the researchers find individuals where the outcomes are known when the project starts. Retrospective cohort studies frequently determine whether exposure to risk and protective factors affects an outcome. These are longitudinal studies that use existing datasets to look back at events that have already occurred. Learn more about Longitudinal Studies: Overview, Examples & Benefits .
In these projects, researchers use databases and medical records to identify patients and gather information about them. They can also ask subjects to recall their exposure over time. Then the researchers analyze the data to determine whether the risk factor correlates with the outcome of interest.
Suppose researchers hypothesize that exposure to a chemical increases skin cancer and conduct a retrospective cohort study. In that case, they can form a cohort based on a group commonly exposed to that chemical (e.g., a particular job). Then they access medical databases and records to collect their data. After identifying their subjects and obtaining the medical information, they can immediately analyze the data, comparing the outcomes for those with and without exposure.
Learn more about Cohort Studies .
Case-Control Studies
Case-control designs are generally retrospective studies. Like their cohort counterparts, case-control studies compare two groups of people, those with and without a condition. These designs both assess risk and protective factors.
Retrospective cohort and case-control studies are similar but generally have differing goals. Cohort designs typically assess known risk factors and how they affect outcomes at different times. Case-control studies evaluate a particular incident, and it is an exploratory design to identify potential risk factors.
For example, a case-control assessment might evaluate an episode of severe illness occurring after a company picnic to identify potential food culprits.
Learn more about Case-Control Studies .
Advantages of a Retrospective Study
A retrospective study tends to have the following advantages compared to a prospective design:
Cheaper : You don’t need a lab or equipment to measure information. Others did that for you!
Faster : The events have already occurred in a retrospective study—no need to wait for them to happen and then look for the differences between the groups.
Great for rare diseases : You can specifically look through a database for individuals with a rare disease or condition. In a prospective experiment, you need an immense sample size and hope enough of the rare outcomes occur for you to analyze.
Disadvantages of a Retrospective Study
Unfortunately, they tend to have the following disadvantages relating to a greater propensity for inaccuracies, inconsistencies, lack of controlled conditions, and bias:
- A retrospective study uses data measured for other purposes.
- Different people, procedures, and equipment might have recorded the data, leading to inconsistencies.
- Measurements might have occurred under differing conditions.
- Control variables might not be measured, leading to confounding.
- Recall bias.
Dean R Hess, Retrospective Studies and Chart Reviews , Respiratory Care , October 2004, 49 (10) 1171-1174.
Share this:

Reader Interactions
November 7, 2022 at 8:26 am
Coincidentally, I just read this Israeli retrospective cohort study regarding the incidence of myocarditis and pericarditis in unvaxxed post-COVID-19 patients: https://pubmed.ncbi.nlm.nih.gov/35456309/
Good news for a change.
Comments and Questions Cancel reply
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Retrospective Cohort Study? | Definition & Examples
What Is a Retrospective Cohort Study? | Definition & Examples
Published on February 10, 2023 by Tegan George . Revised on June 22, 2023.
A retrospective cohort study is a type of observational study that focuses on individuals who have an exposure to a disease or risk factor in common. Retrospective cohort studies analyze the health outcomes over a period of time to form connections and assess the risk of a given outcome associated with a given exposure.
It is crucial to note that in order to be considered a retrospective cohort study, your participants must already possess the disease or health outcome being studied.
Table of contents
When to use a retrospective cohort study, examples of retrospective cohort studies, advantages and disadvantages of retrospective cohort studies, other interesting articles, frequently asked questions.
Retrospective cohort studies are a type of observational study . They are often used in fields related to medicine to study the effect of exposures on health outcomes. While most observational studies are qualitative in nature, retrospective cohort studies are often quantitative , as they use preexisting secondary research data. They can be used to conduct both exploratory research and explanatory research .
Retrospective cohort studies are often used as an intermediate step between a weaker preliminary study and a prospective cohort study , as the results gleaned from a retrospective cohort study strengthen assumptions behind a future prospective cohort study.
A retrospective cohort study could be a good fit for your research if:
- A prospective cohort study is not (yet) feasible for the variables you are investigating.
- You need to quickly examine the effect of an exposure, outbreak, or treatment on an outcome.
- You are seeking to investigate an early-stage or potential association between your variables of interest.
Retrospective cohort studies use secondary research data, such as existing medical records or databases, to identify a group of people with an exposure or risk factor in common. They then look back in time to observe how the health outcomes developed. Case-control studies rely on primary research , comparing a group of participants with a condition of interest to a group lacking that condition in real time.
Prevent plagiarism. Run a free check.
Retrospective cohort studies are common in fields like medicine, epidemiology, and healthcare.
You collect data from participants’ exposure to organophosphates, focusing on variables like the timing and duration of exposure, and analyze the health effects of the exposure. Example: Healthcare retrospective cohort study You are examining the relationship between tanning bed use and the incidence of skin cancer diagnoses.
Retrospective cohort studies can be a good fit for many research projects, but they have their share of advantages and disadvantages.
Advantages of retrospective cohort studies
- Retrospective cohort studies are a great choice if you have any ethical considerations or concerns about your participants that prevent you from pursuing a traditional experimental design .
- Retrospective cohort studies are quite efficient in terms of time and budget. They require fewer subjects than other research methods and use preexisting secondary research data to analyze them.
- Retrospective cohort studies are particularly useful when studying rare or unusual exposures, as well as diseases with a long latency or incubation period where prospective cohort studies cannot yet form conclusions.
Disadvantages of retrospective cohort studies
- Like many observational studies, retrospective cohort studies are at high risk for many research biases . They are particularly at risk for recall bias and observer bias due to their reliance on memory and self-reported data.
- Retrospective cohort studies are not a particularly strong standalone method, as they can never establish causality . This leads to low internal validity and external validity .
- As most patients will have had a range of healthcare professionals involved in their care over their lifetime, there is significant variability in the measurement of risk factors and outcomes. This leads to issues with reliability and credibility of data collected.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
The primary difference between a retrospective cohort study and a prospective cohort study is the timing of the data collection and the direction of the study.
A retrospective cohort study looks back in time. It uses preexisting secondary research data to examine the relationship between an exposure and an outcome. Data is collected after the outcome you’re studying has already occurred.
Alternatively, a prospective cohort study follows a group of individuals over time. It collects data on both the exposure and the outcome of interest as they are occurring. Data is collected before the outcome of interest has occurred.
Retrospective cohort studies are at high risk for research biases like recall bias . Whenever individuals are asked to recall past events or exposures, recall bias can occur. This is because individuals with a certain disease or health outcome of interest are more likely to remember and/or report past exposures differently to individuals without that outcome. This can result in an overestimation or underestimation of the true relationship between variables and affect your research.
No, retrospective cohort studies cannot establish causality on their own.
Like other types of observational studies , retrospective cohort studies can suggest associations between an exposure and a health outcome. They cannot prove without a doubt, however, that the exposure studied causes the health outcome.
In particular, retrospective cohort studies suffer from challenges arising from the timing of data collection , research biases like recall bias , and how variables are selected. These lead to low internal validity and the inability to determine causality.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
George, T. (2023, June 22). What Is a Retrospective Cohort Study? | Definition & Examples. Scribbr. Retrieved June 24, 2024, from https://www.scribbr.com/methodology/retrospective-cohort-study/
Is this article helpful?
Tegan George
Other students also liked, what is a case-control study | definition & examples, what is an observational study | guide & examples, what is recall bias | definition & examples, "i thought ai proofreading was useless but..".
I've been using Scribbr for years now and I know it's a service that won't disappoint. It does a good job spotting mistakes”
About Stanford GSB
- The Leadership
- Dean’s Updates
- School News & History
- Commencement
- Business, Government & Society
- Centers & Institutes
- Center for Entrepreneurial Studies
- Center for Social Innovation
- Stanford Seed
About the Experience
- Learning at Stanford GSB
- Experiential Learning
- Guest Speakers
- Entrepreneurship
- Social Innovation
- Communication
- Life at Stanford GSB
- Collaborative Environment
- Activities & Organizations
- Student Services
- Housing Options
- International Students
Full-Time Degree Programs
- Why Stanford MBA
- Academic Experience
- Financial Aid
- Why Stanford MSx
- Research Fellows Program
- See All Programs
Non-Degree & Certificate Programs
- Executive Education
- Stanford Executive Program
- Programs for Organizations
- The Difference
- Online Programs
- Stanford LEAD
- Seed Transformation Program
- Aspire Program
- Seed Spark Program
- Faculty Profiles
- Academic Areas
- Awards & Honors
- Conferences
Faculty Research
- Publications
- Working Papers
- Case Studies
Research Hub
- Research Labs & Initiatives
- Business Library
- Data, Analytics & Research Computing
- Behavioral Lab
Research Labs
- Cities, Housing & Society Lab
- Golub Capital Social Impact Lab
Research Initiatives
- Corporate Governance Research Initiative
- Corporations and Society Initiative
- Policy and Innovation Initiative
- Rapid Decarbonization Initiative
- Stanford Latino Entrepreneurship Initiative
- Value Chain Innovation Initiative
- Venture Capital Initiative
- Career & Success
- Climate & Sustainability
- Corporate Governance
- Culture & Society
- Finance & Investing
- Government & Politics
- Leadership & Management
- Markets and Trade
- Operations & Logistics
- Opportunity & Access
- Technology & AI
- Opinion & Analysis
- Email Newsletter
Welcome, Alumni
- Communities
- Digital Communities & Tools
- Regional Chapters
- Women’s Programs
- Identity Chapters
- Find Your Reunion
- Career Resources
- Job Search Resources
- Career & Life Transitions
- Programs & Webinars
- Career Video Library
- Alumni Education
- Research Resources
- Volunteering
- Alumni News
- Class Notes
- Alumni Voices
- Contact Alumni Relations
- Upcoming Events
Admission Events & Information Sessions
- MBA Program
- MSx Program
- PhD Program
- Alumni Events
- All Other Events
- Operations, Information & Technology
- Organizational Behavior
- Political Economy
- Classical Liberalism
- The Eddie Lunch
- Accounting Summer Camp
- Videos, Code & Data
- California Econometrics Conference
- California Quantitative Marketing PhD Conference
- California School Conference
- China India Insights Conference
- Homo economicus, Evolving
- Political Economics (2023–24)
- Scaling Geologic Storage of CO2 (2023–24)
- A Resilient Pacific: Building Connections, Envisioning Solutions
- Adaptation and Innovation
- Changing Climate
- Civil Society
- Climate Impact Summit
- Climate Science
- Corporate Carbon Disclosures
- Earth’s Seafloor
- Environmental Justice
- Operations and Information Technology
- Organizations
- Sustainability Reporting and Control
- Taking the Pulse of the Planet
- Urban Infrastructure
- Watershed Restoration
- Junior Faculty Workshop on Financial Regulation and Banking
- Ken Singleton Celebration
- Marketing Camp
- Quantitative Marketing PhD Alumni Conference
- Presentations
- Theory and Inference in Accounting Research
- Stanford Closer Look Series
- Quick Guides
- Core Concepts
- Journal Articles
- Glossary of Terms
- Faculty & Staff
- Researchers & Students
- Research Approach
- Charitable Giving
- Financial Health
- Government Services
- Workers & Careers
- Short Course
- Adaptive & Iterative Experimentation
- Incentive Design
- Social Sciences & Behavioral Nudges
- Bandit Experiment Application
- Conferences & Events
- Get Involved
- Reading Materials
- Teaching & Curriculum
- Energy Entrepreneurship
- Faculty & Affiliates
- SOLE Report
- Responsible Supply Chains
- Current Study Usage
- Pre-Registration Information
- Participate in a Study
A How-To Guide for Conducting Retrospective Analyses: Example COVID-19 Study
In the urgent setting of the COVID-19 pandemic, treatment hypotheses abound, each of which requires careful evaluation. A randomized controlled trial generally provides the strongest possible evaluation of a treatment, but the efficiency and effectiveness of the trial depend on the existing evidence supporting the treatment. The researcher must therefore compile a body of evidence justifying the use of time and resources to further investigate a treatment hypothesis in a trial. An observational study can help provide this evidence, but the lack of randomized exposure and the researcher’s inability to control treatment administration and data collection introduce significant challenges for non-experimental studies. A proper analysis of observational health care data thus requires an extensive background in a diverse set of topics ranging from epidemiology and causal analysis to relevant medical specialties and data sources. Here we provide 10 rules that serve as an end-to-end introduction to retrospective analyses of observational health care data. A running example of a COVID-19 study presents a practical implementation of each rule in the context of a specific treatment hypothesis. When carefully designed and properly executed, a retrospective analysis framed around these rules will inform the decisions of whether and how to investigate a treatment hypothesis in a randomized controlled trial.
- Priorities for the GSB's Future
- See the Current DEI Report
- Supporting Data
- Research & Insights
- Share Your Thoughts
- Search Fund Primer
- Affiliated Faculty
- Faculty Advisors
- Louis W. Foster Resource Center
- Defining Social Innovation
- Impact Compass
- Global Health Innovation Insights
- Faculty Affiliates
- Student Awards & Certificates
- Changemakers
- Dean Jonathan Levin
- Dean Garth Saloner
- Dean Robert Joss
- Dean Michael Spence
- Dean Robert Jaedicke
- Dean Rene McPherson
- Dean Arjay Miller
- Dean Ernest Arbuckle
- Dean Jacob Hugh Jackson
- Dean Willard Hotchkiss
- Faculty in Memoriam
- Stanford GSB Firsts
- Class of 2024 Candidates
- Certificate & Award Recipients
- Dean’s Remarks
- Keynote Address
- Teaching Approach
- Analysis and Measurement of Impact
- The Corporate Entrepreneur: Startup in a Grown-Up Enterprise
- Data-Driven Impact
- Designing Experiments for Impact
- Digital Marketing
- The Founder’s Right Hand
- Marketing for Measurable Change
- Product Management
- Public Policy Lab: Financial Challenges Facing US Cities
- Public Policy Lab: Homelessness in California
- Lab Features
- Curricular Integration
- View From The Top
- Formation of New Ventures
- Managing Growing Enterprises
- Startup Garage
- Explore Beyond the Classroom
- Stanford Venture Studio
- Summer Program
- Workshops & Events
- The Five Lenses of Entrepreneurship
- Leadership Labs
- Executive Challenge
- Arbuckle Leadership Fellows Program
- Selection Process
- Training Schedule
- Time Commitment
- Learning Expectations
- Post-Training Opportunities
- Who Should Apply
- Introductory T-Groups
- Leadership for Society Program
- Certificate
- 2024 Awardees
- 2023 Awardees
- 2022 Awardees
- 2021 Awardees
- 2020 Awardees
- 2019 Awardees
- 2018 Awardees
- Social Management Immersion Fund
- Stanford Impact Founder Fellowships and Prizes
- Stanford Impact Leader Prizes
- Social Entrepreneurship
- Stanford GSB Impact Fund
- Economic Development
- Energy & Environment
- Stanford GSB Residences
- Environmental Leadership
- Stanford GSB Artwork
- A Closer Look
- California & the Bay Area
- Voices of Stanford GSB
- Business & Beneficial Technology
- Business & Sustainability
- Business & Free Markets
- Business, Government, and Society Forum
- Second Year
- Global Experiences
- JD/MBA Joint Degree
- MA Education/MBA Joint Degree
- MD/MBA Dual Degree
- MPP/MBA Joint Degree
- MS Computer Science/MBA Joint Degree
- MS Electrical Engineering/MBA Joint Degree
- MS Environment and Resources (E-IPER)/MBA Joint Degree
- Academic Calendar
- Clubs & Activities
- LGBTQ+ Students
- Military Veterans
- Minorities & People of Color
- Partners & Families
- Students with Disabilities
- Student Support
- Residential Life
- Student Voices
- MBA Alumni Voices
- A Week in the Life
- Career Support
- Employment Outcomes
- Cost of Attendance
- Knight-Hennessy Scholars Program
- Yellow Ribbon Program
- BOLD Fellows Fund
- Application Process
- Loan Forgiveness
- Contact the Financial Aid Office
- Evaluation Criteria
- GMAT & GRE
- English Language Proficiency
- Personal Information, Activities & Awards
- Professional Experience
- Letters of Recommendation
- Optional Short Answer Questions
- Application Fee
- Reapplication
- Deferred Enrollment
- Joint & Dual Degrees
- Entering Class Profile
- Event Schedule
- Ambassadors
- New & Noteworthy
- Ask a Question
- See Why Stanford MSx
- Is MSx Right for You?
- MSx Stories
- Leadership Development
- How You Will Learn
- Admission Events
- Personal Information
- GMAT, GRE & EA
- English Proficiency Tests
- Career Change
- Career Advancement
- Daycare, Schools & Camps
- U.S. Citizens and Permanent Residents
- Requirements
- Requirements: Behavioral
- Requirements: Quantitative
- Requirements: Macro
- Requirements: Micro
- Annual Evaluations
- Field Examination
- Research Activities
- Research Papers
- Dissertation
- Oral Examination
- Current Students
- Education & CV
- International Applicants
- Statement of Purpose
- Reapplicants
- Application Fee Waiver
- Deadline & Decisions
- Job Market Candidates
- Academic Placements
- Stay in Touch
- Faculty Mentors
- Current Fellows
- Standard Track
- Fellowship & Benefits
- Group Enrollment
- Program Formats
- Developing a Program
- Diversity & Inclusion
- Strategic Transformation
- Program Experience
- Contact Client Services
- Campus Experience
- Live Online Experience
- Silicon Valley & Bay Area
- Digital Credentials
- Faculty Spotlights
- Participant Spotlights
- Eligibility
- International Participants
- Stanford Ignite
- Frequently Asked Questions
- Founding Donors
- Location Information
- Participant Profile
- Network Membership
- Program Impact
- Collaborators
- Entrepreneur Profiles
- Company Spotlights
- Seed Transformation Network
- Responsibilities
- Current Coaches
- How to Apply
- Meet the Consultants
- Meet the Interns
- Intern Profiles
- Collaborate
- Research Library
- News & Insights
- Program Contacts
- Databases & Datasets
- Research Guides
- Consultations
- Research Workshops
- Career Research
- Research Data Services
- Course Reserves
- Course Research Guides
- Material Loan Periods
- Fines & Other Charges
- Document Delivery
- Interlibrary Loan
- Equipment Checkout
- Print & Scan
- MBA & MSx Students
- PhD Students
- Other Stanford Students
- Faculty Assistants
- Research Assistants
- Stanford GSB Alumni
- Telling Our Story
- Staff Directory
- Site Registration
- Alumni Directory
- Alumni Email
- Privacy Settings & My Profile
- Success Stories
- The Story of Circles
- Support Women’s Circles
- Stanford Women on Boards Initiative
- Alumnae Spotlights
- Insights & Research
- Industry & Professional
- Entrepreneurial Commitment Group
- Recent Alumni
- Half-Century Club
- Fall Reunions
- Spring Reunions
- MBA 25th Reunion
- Half-Century Club Reunion
- Faculty Lectures
- Ernest C. Arbuckle Award
- Alison Elliott Exceptional Achievement Award
- ENCORE Award
- Excellence in Leadership Award
- John W. Gardner Volunteer Leadership Award
- Robert K. Jaedicke Faculty Award
- Jack McDonald Military Service Appreciation Award
- Jerry I. Porras Latino Leadership Award
- Tapestry Award
- Student & Alumni Events
- Executive Recruiters
- Interviewing
- Land the Perfect Job with LinkedIn
- Negotiating
- Elevator Pitch
- Email Best Practices
- Resumes & Cover Letters
- Self-Assessment
- Whitney Birdwell Ball
- Margaret Brooks
- Bryn Panee Burkhart
- Margaret Chan
- Ricki Frankel
- Peter Gandolfo
- Cindy W. Greig
- Natalie Guillen
- Carly Janson
- Sloan Klein
- Sherri Appel Lassila
- Stuart Meyer
- Tanisha Parrish
- Virginia Roberson
- Philippe Taieb
- Michael Takagawa
- Terra Winston
- Johanna Wise
- Debbie Wolter
- Rebecca Zucker
- Complimentary Coaching
- Changing Careers
- Work-Life Integration
- Career Breaks
- Flexible Work
- Encore Careers
- Join a Board
- D&B Hoovers
- Data Axle (ReferenceUSA)
- EBSCO Business Source
- Global Newsstream
- Market Share Reporter
- ProQuest One Business
- Student Clubs
- Entrepreneurial Students
- Stanford GSB Trust
- Alumni Community
- How to Volunteer
- Springboard Sessions
- Consulting Projects
- 2020 – 2029
- 2010 – 2019
- 2000 – 2009
- 1990 – 1999
- 1980 – 1989
- 1970 – 1979
- 1960 – 1969
- 1950 – 1959
- 1940 – 1949
- Service Areas
- ACT History
- ACT Awards Celebration
- ACT Governance Structure
- Building Leadership for ACT
- Individual Leadership Positions
- Leadership Role Overview
- Purpose of the ACT Management Board
- Contact ACT
- Business & Nonprofit Communities
- Reunion Volunteers
- Ways to Give
- Fiscal Year Report
- Business School Fund Leadership Council
- Planned Giving Options
- Planned Giving Benefits
- Planned Gifts and Reunions
- Legacy Partners
- Giving News & Stories
- Giving Deadlines
- Development Staff
- Submit Class Notes
- Class Secretaries
- Board of Directors
- Health Care
- Sustainability
- Class Takeaways
- All Else Equal: Making Better Decisions
- If/Then: Business, Leadership, Society
- Grit & Growth
- Think Fast, Talk Smart
- Spring 2022
- Spring 2021
- Autumn 2020
- Summer 2020
- Winter 2020
- In the Media
- For Journalists
- DCI Fellows
- Other Auditors
- Academic Calendar & Deadlines
- Course Materials
- Entrepreneurial Resources
- Campus Drive Grove
- Campus Drive Lawn
- CEMEX Auditorium
- King Community Court
- Seawell Family Boardroom
- Stanford GSB Bowl
- Stanford Investors Common
- Town Square
- Vidalakis Courtyard
- Vidalakis Dining Hall
- Catering Services
- Policies & Guidelines
- Reservations
- Contact Faculty Recruiting
- Lecturer Positions
- Postdoctoral Positions
- Accommodations
- CMC-Managed Interviews
- Recruiter-Managed Interviews
- Virtual Interviews
- Campus & Virtual
- Search for Candidates
- Think Globally
- Recruiting Calendar
- Recruiting Policies
- Full-Time Employment
- Summer Employment
- Entrepreneurial Summer Program
- Global Management Immersion Experience
- Social-Purpose Summer Internships
- Process Overview
- Project Types
- Client Eligibility Criteria
- Client Screening
- ACT Leadership
- Social Innovation & Nonprofit Management Resources
- Develop Your Organization’s Talent
- Centers & Initiatives
- Student Fellowships
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
The Case-Control Study : A Practical Review for the Clinician
From the Department of Pediatrics, University of Virginia Medical Center, Charlottesville (Dr Hayden), the Department of Pediatrics and Epidemiology, McGill University, Montreal (Dr Kramer), and the Department of Medicine, Yale University, New Haven, Conn (Dr Horwitz).
The retrospective case-control study is an important research strategy commonly encountered in the medical literature. A thoughtfully designed, carefully executed case-control study can be an invaluable source of clinical information, and physicians must often base important decisions about patient counseling and management on their interpretation of such studies. Unfortunately, the retrospective direction of case-control studies—looking "backwards" from an outcome event to an antecedent exposure—is accompanied by numerous methodological hazards. Careful attention must be paid to selection of appropriate study groups; definition and detection of the outcome event; definition and ascertainment of the exposure; assurance that the compared groups were equally susceptible to the outcome event at baseline; and careful statistical analysis. If systematic bias enters the research at any of these points, erroneous conclusions can result. Greater familiarity with the case-control method should enable clinicians to be more critically insightful when interpreting the results of published studies using this design format.
( JAMA 1982;247:326-331)
Hayden GF , Kramer MS , Horwitz RI. The Case-Control Study : A Practical Review for the Clinician . JAMA. 1982;247(3):326–331. doi:10.1001/jama.1982.03320280046028
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Retrospective Studies and Chart Reviews
Chris nickson.
- Nov 3, 2020
- Retrospective studies are designed to analyse pre-existing data, and are subject to numerous biases as a result
- Retrospective studies may be based on chart reviews (data collection from the medical records of patients)
- case series
- retrospective cohort studies (current or historical cohorts)
- case-control studies
STATISTICAL ANALYSIS USED IN RETROSPECTIVE STUDIES
- Compare outcomes between treatment and control group
- Used if treatment and control group are selected by a chance mechanism
- Divide all patients into subgroups according to a risk factor, then perform comparison within these subgroups
- Used if only one key confounding variable exists
- Find pairs of patients that have specific characteristics in common, but received different treatments; compares outcome only in these pairs
- Used if only a few confounders exist and if the size of one of the comparison groups is much larger than the other
- More than one confounder is controlled simultaneously, if a larger number of confounders needs to be adjusted for computer software and statistical advice is necessary
- Used if sample size is large
- Simple description of data
- Used if sample size is low and other options failed
ADVANTAGES OF RETROSPECTIVE STUDIES
- quicker, cheaper and easier than prospective cohort studies
- can address rare diseases and identify potential risk factors (e.g. case-control studies)
- not prone to loss of follow up
- may be used as the initial study generating hypotheses to be studied further by larger, more expensive prospective studies
DISADVANTAGES OF RETROSPECTIVE STUDIES
- inferior level of evidence compared with prospective studies
- controls are often recruited by convenience sampling, and are thus not representative of the general population and prone to selection bias
- prone to recall bias or misclassification bias
- subject to confounding (other risk factors may be present that were not measured)
- cannot determine causation, only association
- some key statistics cannot be measured
- temporal relationships are often difficult to assess
- retrospective cohort studies need large sample sizes if outcomes are rare
SOURCES OF ERROR IN CHART REVIEWS AND THEIR SOLUTIONS
From Kaji et al (2014) and Gilbert et al (1996):
- establish whether necessary information is available in the charts
- establish if there are sufficient charts to perform the analysis with adequate precision
- perform a sample size calculation
- Declare any conflict of interest Provide evidence of institutional review board approval
- Submit the data collection form, as well as the coding rules and definitions, as an online appendix
- Case selection or exclusion using explicit protocols and well described the criteria
- Ensure all available charts have an equal chance of selection
- Provide a flow diagram showing how the study sample was derive from the source population
- define the predictor and outcome variables to be collected a priori
- Develop a coding manual and publish as an online appendix
- Use standardized abstraction forms to guide data collection
- Provide precise definitions of variables
- Pilot test the abstraction form
- Ensure uniform handling of data that is conflicting, ambiguous, missing, or unknown
- Perform a sensitivity analysis if needed
- Blind chart reviewers to the etiologic relation being studied or the hypotheses being tested. If groups of patients are to be compared, the abstractor should be blinded to the patient’s group assignment
- Describe how blinding was maintained in the article
- Train chart abstractors to perform their jobs.
- Describe the qualifications and training of the chart abstracters.
- Ideally, train abstractors before the study starts, using a set of “practice” medical records.
- Ensure uniform training, especially in multi-center studies
- Monitor the performance of the chart abstractors
- Hold periodic meetings with chart abstractors and study coordinators to resolve disputes and review coding rules.
- A second reviewer should re-abstract a sample of charts, blinded to the information obtained by the first correlation reviewer.
- Report a kappa-statistic, intraclass coefficient, or other measure of agreement to assess inter-rater reliability of the data
- Provide justification for the criteria for each variable
SOURCES OF ERROR FROM THE USE OF ELECTRONIC MEDICAL RECORDS
Potential biases introduced from:
- use of boilerplates (a unit of writing that can be reused over and over without change)
- items copied and pasted
- default tick boxes
- delays in time stamps relative to actual care
References and Links
- CCC — Case-control studies
Journal articles
- Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996 Mar;27(3):305-8. PMID: 8599488 .
- Kaji AH, Schriger D, Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014 Sep;64(3):292-8. PMID: 24746846 .
- Sauerland S, Lefering R, Neugebauer EA. Retrospective clinical studies in surgery: potentials and pitfalls. J Hand Surg Br. 2002 Apr;27(2):117-21. PMID: 12027483 .
- Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005 Apr;45(4):448-51. PMID: 15795729 .

Critical Care
Chris is an Intensivist and ECMO specialist at the Alfred ICU in Melbourne. He is also a Clinical Adjunct Associate Professor at Monash University . He is a co-founder of the Australia and New Zealand Clinician Educator Network (ANZCEN) and is the Lead for the ANZCEN Clinician Educator Incubator programme. He is on the Board of Directors for the Intensive Care Foundation and is a First Part Examiner for the College of Intensive Care Medicine . He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives.
After finishing his medical degree at the University of Auckland, he continued post-graduate training in New Zealand as well as Australia’s Northern Territory, Perth and Melbourne. He has completed fellowship training in both intensive care medicine and emergency medicine, as well as post-graduate training in biochemistry, clinical toxicology, clinical epidemiology, and health professional education.
He is actively involved in in using translational simulation to improve patient care and the design of processes and systems at Alfred Health. He coordinates the Alfred ICU’s education and simulation programmes and runs the unit’s education website, INTENSIVE . He created the ‘Critically Ill Airway’ course and teaches on numerous courses around the world. He is one of the founders of the FOAM movement (Free Open-Access Medical education) and is co-creator of litfl.com , the RAGE podcast , the Resuscitology course, and the SMACC conference.
His one great achievement is being the father of three amazing children.
On Twitter, he is @precordialthump .
| INTENSIVE | RAGE | Resuscitology | SMACC
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- En español – ExME
- Em português – EME
Case-control and Cohort studies: A brief overview
Posted on 6th December 2017 by Saul Crandon

Introduction
Case-control and cohort studies are observational studies that lie near the middle of the hierarchy of evidence . These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately.
Case-control studies
Case-control studies are retrospective. They clearly define two groups at the start: one with the outcome/disease and one without the outcome/disease. They look back to assess whether there is a statistically significant difference in the rates of exposure to a defined risk factor between the groups. See Figure 1 for a pictorial representation of a case-control study design. This can suggest associations between the risk factor and development of the disease in question, although no definitive causality can be drawn. The main outcome measure in case-control studies is odds ratio (OR) .
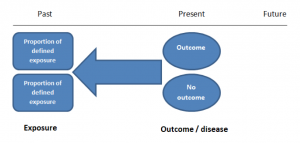
Figure 1. Case-control study design.
Cases should be selected based on objective inclusion and exclusion criteria from a reliable source such as a disease registry. An inherent issue with selecting cases is that a certain proportion of those with the disease would not have a formal diagnosis, may not present for medical care, may be misdiagnosed or may have died before getting a diagnosis. Regardless of how the cases are selected, they should be representative of the broader disease population that you are investigating to ensure generalisability.
Case-control studies should include two groups that are identical EXCEPT for their outcome / disease status.
As such, controls should also be selected carefully. It is possible to match controls to the cases selected on the basis of various factors (e.g. age, sex) to ensure these do not confound the study results. It may even increase statistical power and study precision by choosing up to three or four controls per case (2).
Case-controls can provide fast results and they are cheaper to perform than most other studies. The fact that the analysis is retrospective, allows rare diseases or diseases with long latency periods to be investigated. Furthermore, you can assess multiple exposures to get a better understanding of possible risk factors for the defined outcome / disease.
Nevertheless, as case-controls are retrospective, they are more prone to bias. One of the main examples is recall bias. Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing factors a lot more vividly than a mother who had a healthy birth.
A summary of the pros and cons of case-control studies are provided in Table 1.
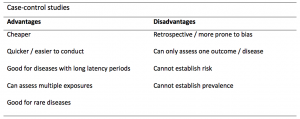
Table 1. Advantages and disadvantages of case-control studies.
Cohort studies
Cohort studies can be retrospective or prospective. Retrospective cohort studies are NOT the same as case-control studies.
In retrospective cohort studies, the exposure and outcomes have already happened. They are usually conducted on data that already exists (from prospective studies) and the exposures are defined before looking at the existing outcome data to see whether exposure to a risk factor is associated with a statistically significant difference in the outcome development rate.
Prospective cohort studies are more common. People are recruited into cohort studies regardless of their exposure or outcome status. This is one of their important strengths. People are often recruited because of their geographical area or occupation, for example, and researchers can then measure and analyse a range of exposures and outcomes.
The study then follows these participants for a defined period to assess the proportion that develop the outcome/disease of interest. See Figure 2 for a pictorial representation of a cohort study design. Therefore, cohort studies are good for assessing prognosis, risk factors and harm. The outcome measure in cohort studies is usually a risk ratio / relative risk (RR).
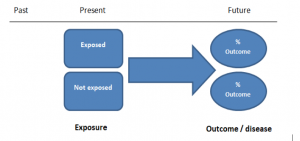
Figure 2. Cohort study design.
Cohort studies should include two groups that are identical EXCEPT for their exposure status.
As a result, both exposed and unexposed groups should be recruited from the same source population. Another important consideration is attrition. If a significant number of participants are not followed up (lost, death, dropped out) then this may impact the validity of the study. Not only does it decrease the study’s power, but there may be attrition bias – a significant difference between the groups of those that did not complete the study.
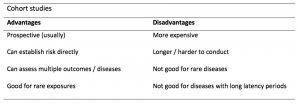
Cohort studies can assess a range of outcomes allowing an exposure to be rigorously assessed for its impact in developing disease. Additionally, they are good for rare exposures, e.g. contact with a chemical radiation blast.
Whilst cohort studies are useful, they can be expensive and time-consuming, especially if a long follow-up period is chosen or the disease itself is rare or has a long latency.
A summary of the pros and cons of cohort studies are provided in Table 2.
The Strengthening of Reporting of Observational Studies in Epidemiology Statement (STROBE)
STROBE provides a checklist of important steps for conducting these types of studies, as well as acting as best-practice reporting guidelines (3). Both case-control and cohort studies are observational, with varying advantages and disadvantages. However, the most important factor to the quality of evidence these studies provide, is their methodological quality.
- Song, J. and Chung, K. Observational Studies: Cohort and Case-Control Studies . Plastic and Reconstructive Surgery.  2010 Dec;126(6):2234-2242.
- Ury HK. Efficiency of case-control studies with multiple controls per case: Continuous or dichotomous data . Biometrics . 1975 Sep;31(3):643–649.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.  Lancet 2007 Oct;370(9596):1453-14577. PMID: 18064739.
Saul Crandon
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Case-control and Cohort studies: A brief overview
Very well presented, excellent clarifications. Has put me right back into class, literally!
Very clear and informative! Thank you.
very informative article.
Thank you for the easy to understand blog in cohort studies. I want to follow a group of people with and without a disease to see what health outcomes occurs to them in future such as hospitalisations, diagnoses, procedures etc, as I have many health outcomes to consider, my questions is how to make sure these outcomes has not occurred before the “exposure disease”. As, in cohort studies we are looking at incidence (new) cases, so if an outcome have occurred before the exposure, I can leave them out of the analysis. But because I am not looking at a single outcome which can be checked easily and if happened before exposure can be left out. I have EHR data, so all the exposure and outcome have occurred. my aim is to check the rates of different health outcomes between the exposed)dementia) and unexposed(non-dementia) individuals.
Very helpful information
Thanks for making this subject student friendly and easier to understand. A great help.
Thanks a lot. It really helped me to understand the topic. I am taking epidemiology class this winter, and your paper really saved me.
Happy new year.
Wow its amazing n simple way of briefing ,which i was enjoyed to learn this.its very easy n quick to pick ideas .. Thanks n stay connected
Saul you absolute melt! Really good work man
am a student of public health. This information is simple and well presented to the point. Thank you so much.
very helpful information provided here
really thanks for wonderful information because i doing my bachelor degree research by survival model
Quite informative thank you so much for the info please continue posting. An mph student with Africa university Zimbabwe.
Thank you this was so helpful amazing
Apreciated the information provided above.
So clear and perfect. The language is simple and superb.I am recommending this to all budding epidemiology students. Thanks a lot.
Great to hear, thank you AJ!
I have recently completed an investigational study where evidence of phlebitis was determined in a control cohort by data mining from electronic medical records. We then introduced an intervention in an attempt to reduce incidence of phlebitis in a second cohort. Again, results were determined by data mining. This was an expedited study, so there subjects were enrolled in a specific cohort based on date(s) of the drug infused. How do I define this study? Thanks so much.
thanks for the information and knowledge about observational studies. am a masters student in public health/epidemilogy of the faculty of medicines and pharmaceutical sciences , University of Dschang. this information is very explicit and straight to the point
Very much helpful
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

An introduction to different types of study design
Conducting successful research requires choosing the appropriate study design. This article describes the most common types of designs conducted by researchers.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Critical Analysis of Retrospective Study Designs: Cohort and Case Series
Affiliations.
- 1 Department of Orthopedics, Division of Foot and Ankle Surgery, West Penn Hospital, 4800 Friendship Avenue, N1, Pittsburgh, PA 15224, USA.
- 2 Department of Orthopedics, West Penn Hospital Foot & Ankle Surgery, Allegheny Health Network, 4800 Friendship Avenue, N1, Pittsburgh, PA 15224, USA. Electronic address: [email protected].
- 3 Department of Orthopedics, West Penn Hospital Foot & Ankle Surgery, Allegheny Health Network, 4800 Friendship Avenue, N1, Pittsburgh, PA 15224, USA.
- PMID: 38388124
- DOI: 10.1016/j.cpm.2023.09.002
Retrospective studies represent an often used research methodology in the podiatric scientific literature, with cohort studies and case series being two of the most prevalent designs. Choosing a retrospective method is often dependent on multiple factors, two of the most important being details of the research question to be explored and the sample size that can be acquired. When analyzing literature, a reader must understand how retrospective studies work to critically examine the methods, results, and discussions to determine if the conclusion is reasonable and might be applied to clinical practice.
Keywords: Case series; Cohort study; Retrospective study.
Copyright © 2023 Elsevier Inc. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Disclosure The authors have no financial disclosures.
Similar articles
- Study Designs in Clinical Research. Mendoza AE, Yeh DD. Mendoza AE, et al. Surg Infect (Larchmt). 2021 Aug;22(6):640-645. doi: 10.1089/sur.2020.469. Surg Infect (Larchmt). 2021. PMID: 34270360
- Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Munn Z, et al. JBI Evid Synth. 2020 Oct;18(10):2127-2133. doi: 10.11124/JBISRIR-D-19-00099. JBI Evid Synth. 2020. PMID: 33038125
- The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
- Observational designs in clinical multiple sclerosis research: Particulars, practices and potentialities. Jongen PJ. Jongen PJ. Mult Scler Relat Disord. 2019 Oct;35:142-149. doi: 10.1016/j.msard.2019.07.006. Epub 2019 Jul 20. Mult Scler Relat Disord. 2019. PMID: 31394404 Review.
- Study designs in dermatology: Practical applications of study designs and their statistics in dermatology. Silverberg JI. Silverberg JI. J Am Acad Dermatol. 2015 Nov;73(5):733-40; quiz 741-2. doi: 10.1016/j.jaad.2014.07.062. J Am Acad Dermatol. 2015. PMID: 26475533 Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
- W.B. Saunders
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

- Get Started
- Determine Protocol Type
- Not Human Subjects Research and Not Research
Case Study Types
Is my case study considered human subjects research, retrospective case study review/report.
- Generally completed by a retrospective review of medical records that highlights a unique treatment, case, or outcome
- Often clinical in nature
- A report about five or fewer clinical experiences or observations identified during clinical care
- Does not involve biospecimens or FDA-regulated products (e.g., drugs, devices, biologics) that have not been approved for use in humans
- Does not include articles requiring exemption from FDA oversight
- Does not include articles under an IND/IDE
Prospective Single Subject Case Study Review/Report
- Often social/behavioral in nature
- In-depth prospective analysis and report involving unique or exceptional observations or experiences about one, or a few, individual human subjects
- Is intended to contribute to generalizable knowledge

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
Guide to retrospective case study data reports
Section 1 – what is being analyzed.
- Case Studies Home
- Raton Basin, Colorado
- Killdeer, North Dakota
- Northeastern Pennsylvania
- Southwestern Pennsylvania
- Wise County, Texas
For each case study site, EPA researchers took samples from a variety of sources and tested them for a broad range of substances and chemicals, including components of hydraulic fracturing fluids. Water conditions, such as temperature and dissolved oxygen referred to as “parameters” were also monitored and recorded. These are listed in the first table labeled, “Analytes and Parameters.” (Analytes tab)
Although each case study site has different geology and hydrology, EPA generally uses the same water quality test methods for all the sites to assess conditions in the area.

Section 2 – Understanding the Data
Many of the data have notes and labels that represent important descriptions for understanding what the values listed mean. All of the possible data notes and abbreviations are listed in the following reference tables:
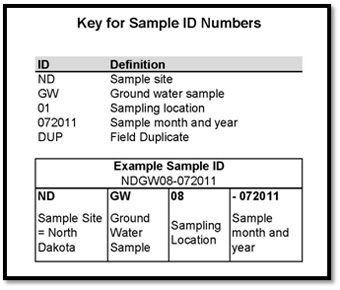
Section 3 – Key for Sample ID Numbers
Each sample taken has a unique identification number. The Key explains the numbering system for the samples.
Section 4 – Data Tables
Data for the samples are grouped together in tables for Parameters, Metals, Volatile Organic Compounds, etc. The sample ID’s will appear in more than one table depending on the analytes included in each table.
- Hydraulic Fracturing Study Home
- Final Assessment
- EPA Published Research
- Fact Sheets
- Questions & Answers about the Final Assessment
- EPA Hydraulic Fracturing - Agency Main Page
- Open access
- Published: 27 June 2024
Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review
- Hyunjoo Kim 1 , 2 ,
- Bomi Hong 3 ,
- Sanghee Kim 4 ,
- Seok-Min Kang 5 &
- Jeongok Park ORCID: orcid.org/0000-0003-4978-817X 4
Systematic Reviews volume 13 , Article number: 167 ( 2024 ) Cite this article
Metrics details
Chemotherapy-related cardiotoxicity is a significant concern because it is a major cause of morbidity. This study aimed to provide in-depth information on the symptoms of chemotherapy-related cardiotoxicity (CRCT) by exploring literature that concurrently reports the types and symptoms of CRCT in patients with breast cancer.
A scoping review was performed according to an a priori protocol using the Joanna Briggs Institute’s guidelines. The participants were patients with breast cancer. The concept was the literature of specifically reported symptoms directly matched with CRCT and the literature, in English, from 2010, and the context was open. The search strategy included four keywords: “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” All types of research designs were included; however, studies involving patients with other cancer types, animal subjects, and symptoms not directly related to CRCT were excluded. Data were extracted and presented including tables and figures.
A total of 29 articles were included in the study, consisting of 23 case reports, 4 retrospective studies, and 2 prospective studies. There were no restrictions on the participants’ sex; however, all of them were women, except for one case report. The most used chemotherapy regimens were trastuzumab, capecitabine, and doxorubicin or epirubicin. The primary CRCT identified were myocardial dysfunction and heart failure, followed by coronary artery disease, pulmonary hypertension, and other conditions. Major tests used to diagnose CRCT include echocardiography, electrocardiography, serum cardiac enzymes, coronary angiography, computed tomography, and magnetic resonance imaging. In all case reports, CRCT was diagnosed through an incidental checkup according to the patient’s symptom presentation; however, only 10 of these studies showed a baseline checkup before chemotherapy. The five most common CRCT symptoms were dyspnea, chest pain, peripheral edema, fatigue, and palpitations, which were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Dyspnea with trastuzumab treatment and chest pain with capecitabine treatment were particularly characteristic. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days.
Conclusions
This scoping review allowed data mapping according to the study design and chemotherapy regimens. Cardiac assessments for CRCT diagnosis were performed according to the patient’s symptoms. There were approximately five types of typical CRCT symptoms, and the timing of symptom occurrence varied. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Peer Review reports
Breast cancer is currently the most common cancer worldwide. Its incidence and mortality rates in East Asia in 2020 accounted for 24% and 20% of the global rates, respectively, and these rates are expected to continue increasing until 2040 [ 1 ]. In the USA, since the mid-2000s, the incidence rate of breast cancer has been increasing by 0.5% annually, while the mortality rate has been decreasing by 1% per year from 2011 to 2020 [ 2 ]. Despite the improved long-term survival rate in patients with breast cancer due to the development of chemotherapy, the literature has highlighted that cardiotoxicity, a cardiac problem caused by chemotherapy, could be a significant cause of death among these patients [ 3 ]. Chemotherapy-related cardiotoxicity (CRCT) can interfere with cancer treatment and progress to congestive heart failure during or after chemotherapy [ 4 ], potentially lowering the survival rate and quality of life of patients with cancer [ 5 ].
The term cardiotoxicity was first used in the 1970s to describe cardiac complications resulting from chemotherapy regimens, such as anthracyclines and 5-fluorouracil. The early definition of cardiotoxicity centered around heart failure, but the current definition is broad and still imprecise [ 6 ]. The 2022 guidelines on cardio-oncology from the European Society of Cardiology (ESC) define cardiotoxicity as including cardiac dysfunction, myocarditis, vascular toxicity, arterial hypertension, and cardiac arrhythmias. Some of these definitions reflect the symptoms. For example, cardiac dysfunction, which accounts for 48% of cardiotoxicity in patients with cancer, is divided into asymptomatic and symptomatic cardiac dysfunction. Asymptomatic cardiac dysfunction is defined based on left ventricular ejection fraction (LVEF), myocardial global longitudinal strain, and cardiac biomarkers. Symptomatic cardiac dysfunction indicates heart failure and presents with ankle swelling, breathlessness, and fatigue [ 7 ]. The ESC guidelines for heart failure present more than 20 types of symptoms [ 8 ]; however, to the best of our knowledge, few studies have been conducted to determine which heart failure symptoms and their characteristics are associated with CRCT in patients with breast cancer. Similarly, there is a lack of information related to vascular toxicity such as myocardial infarction [ 7 ].
Professional societies in cardiology and oncology have proposed guidelines for the prevention and management of cardiotoxicity in patients with cancer. According to the American Society of Clinical Oncology and the ESC, it is recommended to identify high-risk patients, comprehensively evaluate clinical signs and symptoms associated with CRCT, and conduct cardiac evaluations before, during, and after chemotherapy [ 7 , 9 , 10 ]. In addition, guidelines for patients with cancer, including those for breast cancer survivorship care, emphasize that patients should be aware of the potential risk of CRCT and report symptoms, such as fatigue or shortness of breath to their healthcare providers [ 7 , 11 , 12 ]. Although these guidelines encompass cardiac monitoring as well as symptom observation, many studies have focused solely on objective diagnostic tests, such as echocardiography, cardiac magnetic resonance, and cardiac biomarkers [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ], which means that there is little interest in CRCT symptoms in patients under breast cancer care.
This lack of interest in CRCT symptoms may be related to the absence of a specific symptom assessment tool for CRCT. Symptom monitoring of CRCT in patients with breast cancer was conducted through patient interviews and reported using the appropriate terminology [ 23 ]. In terms of interviews, patients with cancer experienced the burden of expressing symptoms between cardiovascular problems and cancer treatment. Qualitative research on patients with cancer indicates that these patients experience a daily battle to distinguish the symptoms they experience during chemotherapy [ 24 ]. To reduce the burden of identifying CRCT symptoms, it is crucial to educate patients with breast cancer undergoing chemotherapy about these symptoms. To report cardiotoxicity, healthcare providers in oncology can use a dictionary of terms called the Common Terminology Criteria for Adverse Events (CTCAE) for reporting adverse events in patients with cancer [ 25 ]. Patients can also use Patient-Reported Outcome (PRO), which allows unfiltered reporting of symptoms directly to the clinical database [ 26 ]. PRO consists of 78 symptomatic adverse events out of approximately 1,000 types of CTCAE [ 27 ]. Basch et al. suggested that PRO could enable healthcare providers to identify patient symptoms before they worsen, thereby improving the overall survival rate of patients with metastatic cancer [ 28 ]. This finding implies that symptoms can provide valuable clues for enhancing the timeliness and accuracy of clinical assessments of CRCT [ 29 ]. Therefore, it is necessary to explore the scope of research focusing on CRCT symptoms for prevention and early detection of CRCT in patients with breast cancer. The detailed research questions are as follows:
What are the general characteristics of the studies related to CRCT in patients with breast cancer?
What diagnostic tools and monitoring practices are used to detect CRCT?
What are the characteristics and progression of symptoms associated with CRCT?
A scoping review is a research method for synthesizing evidence that involves mapping the scope of evidence on a particular topic [ 30 ]. It aims to clarify key concepts and definitions, identify key characteristics of factors related to a concept, and highlight gaps or areas for further research [ 30 ]. This study used a scoping review methodology based on the Joanna Briggs Institute (JBI) framework. The JBI methodology, refined from the framework initially developed by Arksey and O’Malley [ 31 ], involves developing a research question, establishing detailed inclusion and exclusion criteria, and selecting and analyzing literature accordingly [ 32 ]. In contrast to systematic reviews, scoping reviews can encompass a variety of study designs and are particularly suitable when the topic has not been extensively studied [ 33 ]; hence, the decision was made to conduct a scoping review.
Development of a scoping review protocol
To conduct this review, an a priori scoping review protocol was developed to enhance transparency and increase the usefulness and reliability of the results. The protocol included the title, objective, review questions, introduction, eligibility criteria, participants, concept, context, types of evidence source, methods, search strategy, source of evidence selection, data extraction, data analysis and presentation, and deviation from the protocol [ 34 ] (Supplementary File 1).
Eligibility criteria
A participant-concept-context (PCC) framework was constructed based on the following research criteria. The participants were patients with breast cancer. The concept was that studies that specifically reported symptoms directly matched to CRCT in patients with breast cancer and the literature, published in English since 2010, in line with the year the CRCT guidelines were announced by the Cardio-Oncology Society. The context was open. We included all types of research designs. The exclusion criteria were studies that included patients with other types of cancer, involved animal subjects, and reported symptoms not directly related to CRCT.
Search strategy
The keywords consisted of “breast cancer,” “chemotherapy,” “cardiotoxicity,” and “symptoms.” The keywords for “cardiotoxicity” were constructed according to the clinical cardiotoxicity report and ESC guidelines [ 7 , 35 ]. The keywords for “symptoms” included 40 specific symptoms of arrhythmia, heart failure, and cardiac problems [ 36 , 37 ] (Supplementary Table 1). We used PubMed, Embase, and CINAHL.
Source of evidence selection
Duplicate studies were removed using EndNote 21. The titles and abstracts were then reviewed according to the inclusion criteria, the primary literature was selected, and the final literature was selected through a full-text review. Any disagreements were resolved through discussions between the investigators.
Data extraction
The data from the literature included the general characteristics of the study, as well as information on the patients, chemotherapy, cardiotoxicity, and symptoms. The general characteristics of the study included author, publication year, country of origin, study design; patient information including sample size, sex, age, cancer type, and cancer stage; chemotherapy information including chemotherapy regimen; cardiotoxicity information including type of cardiotoxicity, diagnostic tests, and times of assessment; and symptom information including type of symptom, characteristics of symptom worsening or improvement, onset time, progression time, and time to symptom improvement. Information on whether to receive chemotherapy after the diagnosis of cardiotoxicity was explored.
Data analysis and presentation
The contents of the included studies were divided into three categories: (1) general characteristics, which encompassed study designs, patients, and medications; (2) type of CRCT and cardiac assessment for CRCT; and (3) characteristics and progression of the symptoms associated with CRCT. CRCT symptom-related data are presented in tables and figures.
In total, 487 studies were identified through database searches, and 116 duplicates were subsequently removed. After reviewing the titles and abstracts, we excluded 197 studies in which participants had cancers other than breast cancer, no symptoms, or symptom-related expressions. Of the remaining 174 studies, 146 were excluded after full-text review. Among the excluded studies, 79 were mainly clinical trials that the symptoms were not directly related to CRCT, 62 did not report specific symptoms, four were in the wrong population, and one was unavailable for full-text review. An additional study was included after a review of references, bringing the final count to 29 studies included in the analysis (Fig. 1 ).

Preferred reporting items for systematic reviews flowchart
General characteristics of studies including designs, sex and age, chemotherapy regimen, and CRCT criteria
Table 1 presents the general characteristics of the studies included in this review. The majority of these studies were published in the USA ( n =14), with Japan ( n =3), and Romania ( n =2) following. The study designs primarily consisted of case reports ( n =23), retrospective studies ( n =4), and prospective studies ( n =2).
All case reports involved female patients, except for one involving a male patient. Five quantitative studies did not specify or limit the sex of the participants, and one retrospective study included only female patients. In terms of cancer stage, the majority of studies involved patients with advanced breast cancer ( n =13), while a smaller number involved patients with early-stage breast cancer ( n =4). Twelve studies did not specify the cancer stage. Approximately 20 types of chemotherapy regimens are currently in use. Trastuzumab, which is a human epidermal growth factor receptor 2 (HER2) blocker, was mentioned in the majority of studies ( n =8), followed by capecitabine (an antimetabolite) ( n =7), and doxorubicin or epirubicin (anthracycline-based chemotherapy) ( n =6). Current chemotherapy and previous treatment methods were described together, with the exception of eight studies. Six quantitative studies defined the CRCT criteria, five of which were based on decreased LVEF and one of which was based on significant cardiac symptoms and/or electrocardiogram changes. Twenty-three case reports described the cardiovascular diagnosis as CRCT.
Diagnostic tools and monitoring practice for CRCT
Table 2 displays the types of CRCT, diagnostic tools, and times of cardiac assessment according to chemotherapy regimens. The most prevalent CRCT were myocardial dysfunction and heart failure, identified in 12 case studies, respectively. This was followed by coronary artery disease, represented in 8 case studies, pulmonary hypertension in 2 case studies, and a single case study of periaortitis. The most used test for diagnosing CRCT was echocardiography ( n =22), followed by EKG ( n =20), various types of cardiac enzymes ( n =16), coronary angiography (CAG, n =12), computed tomography ( n =6), and magnetic resonance imaging (MRI, n =4). Regarding the CRCT symptom assessment tools, the CTCAE was used in two studies, the New York Heart Association classification for heart failure in two studies, the dyspnea assessment scale in one study, and symptoms of cardiac origin, which consisted of chest pain, dyspnea, and palpitations in one study.
Regarding the times of cardiac evaluation, two studies performed regular cardiac checkups including before, during, and after chemotherapy. There were 10 case studies and six quantitative studies describing cardiac function testing before chemotherapy, of which seven studies performed regular cardiac screening tests and two studies mentioned cardiac screening even after the completion of chemotherapy. The frequency of regular checkups varied from every 3 months to every two to four cycles. In all case reports ( n =23), CRCT were diagnosed through incidental checkups based on patients’ symptom presentation, and in most cases, several tests were performed subsequentially for CRCT diagnosis. In one case study, cardiac evaluation was conducted 3 days after the patient’s initial symptom presentation, when the symptoms became more severe.
Characteristics and progression of symptoms associated with CRCT
Table 3 shows the descriptive scope of the CRCT-related symptoms according to the chemotherapy regimens used in the included studies. The mapping factors included initial symptoms, symptom onset or severity, symptom progression, medical management, and CRCT results. One of the most frequent symptoms associated with CRCT was dyspnea, which was discussed in 19 studies and described as difficulty in breathing, shortness of breath, or New York Heart Association (NYHA) class II or III. When dyspnea appeared as the initial symptom of CRCT, the symptom progression was worsening in eight case studies and persistent in two cases. Chest pain was described in 12 studies as a symptom characterized by a squeezing, tingling, burning, tightened, or atypical feeling that was relieved by rest and exacerbated by exertion. Other symptoms included peripheral edema ( n =6), fatigue ( n =5), and palpitation ( n =2). The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool.
The symptoms could be categorized based on the type of chemotherapy regimens used. In the case studies involving anthracycline-based regimen and HER2 blockers, dyspnea was the most frequently observed symptom ( n =7), followed by peripheral edema ( n =2), and chest pain or discomfort ( n =2). In case studies where antimetabolites were used, specifically capecitabine, chest pain was a common and prominent symptom. This chest pain typically manifested between 1 and 7 days after drug administration and persisted until treatment. Notably, four out of seven patients reported this symptom on the first day of chemotherapy, according to the case reports. The time for first symptom onset after chemotherapy ranged from 1 hour to 300 days, with anthracycline-based regimens requiring 3–55 days, trastuzumab requiring 60–300 days, and capecitabine requiring 1–7 days. Figure 2 shows the progression of symptoms in case studies, detailing the time of symptom onset, the date of symptom reporting, and the date of treatment completion following the use of chemotherapy. The studies that did not specify any of the dates of symptom onset, reporting, and completion of treatment were excluded from the figure.

Figure 3 shows symptoms according to the main types of chemotherapy regimens reported in case studies. Dyspnea with trastuzumab and chest pain with capecitabine are particularly characteristic. A retrospective study included in this scoping review reported that chest pain was the most common symptom associated with capecitabine, followed by dyspnea and palpitation [ 40 ]. Furthermore, peripheral edema was primarily observed with anthracycline, alkylating, and HER2 blockers, while fatigue was noted with various anticancer drugs, irrespective of the type of chemotherapy regimen.

Ongoing chemotherapy was discontinued after CRCT was detected in 20 case studies. When patients presented symptoms indicative of CRCT, the majority were promptly hospitalized for further evaluation, medication, or interventional treatment. The majority of studies indicated the initiation of cardiac medication ( n =21), with three case studies involving coronary intervention and two involving treatment with wearable devices. Most management procedures were conducted in a general ward or an intensive care unit.
In most case studies, symptoms improved following cardiac treatment, with either complete or partial recovery of LVEF observed in 19 instances. However, a few studies reported a poor prognosis, including two instances of death. LVEF recovered in most patients within 6 months when treated with an anthracycline-based regimen and HER2 blockers (Fig. 2 ). A retrospective study reported that the rates of complete or partial recovery of CRCT following treatment with doxorubicin-based chemotherapy and trastuzumab were 42.9% and 86.1%, respectively [ 39 ]. Another retrospective study noted that the recovery time of CRCT when treated with HER2 blockers increased in correlation with the severity of the NYHA class, ranging from 8 to 80 weeks [ 38 ]. In the case of the antimetabolite capecitabine, all patients recovered within a day to a week, except one patient who did not recover.
This scoping review was conducted to explore the scope of studies focusing on CRCT symptoms, including the general characteristics of the studies, diagnostic tools, monitoring practices related to detecting CRCT, and the characteristics and progression of symptoms associated with CRCT. The primary findings of this review were as follows: (1) common symptoms related to CRCT and differences in symptoms according to the chemotherapy regimens used were identified; (2) the symptoms reported by the patient served as clues to suspect a specific type of CRCT; and (3) regular monitoring practices for CRCT prevention and detection were insufficient.
First, the current study identified common symptoms such as dyspnea, chest pain, peripheral edema, fatigue, and palpitation associated with CRCT, as well as variations in symptoms depending on the chemotherapy regimen used in patients with breast cancer. Among these symptoms, dyspnea, edema, and chest pain were frequently observed in patients receiving anthracycline-based and/or HER2 blocker drugs. These symptoms, which are associated with heart failure, appeared later compared to those observed with capecitabine, as depicted in Fig. 2 . This may be due to the known impact of anthracycline-based and/or HER2 blocker regimens on cardiomyocytes and other cells, leading to myocardial damage [ 42 ]. Therefore, the symptoms are related to heart failure, potentially resulting from the impairment of ventricular filling or ejection in patients undergoing treatment with these regimens [ 43 ].
In a similar vein, Attin et al. (2022) documented the occurrence of symptoms such as lower extremity edema, chest pain, difficulty breathing, and fatigue before the diagnosis of CRCT in women undergoing breast cancer treatment. They conducted a retrospective and longitudinal investigation of the symptoms, signs, and cardiac tests of 15 patients who experienced CRCT, using their electronic medical records. In their study, cardiotoxicity was defined by an echocardiogram or MRI showing a decrease in LVEF of 5 to 10%, with a specialist’s confirmation note. They compared the number of symptom occurrences during the first half of the year with those during the second half of the year prior to the diagnosis of cardiotoxicity. Specifically, the frequency of lower-extremity edema significantly increased from three occurrences in the first half of the year to 17 occurrences in the second half of the year. The frequency of symptoms for dyspnea and chest pain also increased from 10 and 8 times, respectively, to 16 and 14 times in the second half of the year. While there was limited information on the doses or timing of chemotherapy, 87% of the patients received the same chemotherapy regimens, namely anthracyclines and/or HER2 blockers [ 44 ]. This suggests that the increase in symptom occurrence over time may be related to the accumulation of anthracycline and the duration of anti-HER2 therapy [ 45 ].
Salyer et al. (2019) conducted a study on the prevalent symptoms of heart failure and their clustering. They identified three symptom clusters: sickness behavior, gastrointestinal disturbance, and discomfort of illness. Notably, dyspnea, edema, and pain were grouped into the discomfort of illness cluster, which aligns with the symptoms we observed in patients treated with anthracyclines and/or HER2 blockers [ 46 ]. Therefore, it is crucial for patients undergoing treatment with anthracyclines and/or HER2 blockers to be vigilant for symptoms such as dyspnea, edema, or chest pain, as these are indicative of heart failure.
Chest pain caused by vasospasm was a predominant symptom in patients taking antimetabolite regimens such as oral capecitabine, and it manifested as the following types of cardiotoxicities: vasospasm-related arrhythmia, myocardial disease, and ischemia [ 47 ]. Vasospasm can be triggered by endothelial dysfunction, hypersensitive vascular smooth muscle, reactive oxidative stress, or chemotherapy regimens [ 48 , 49 ]. According to previous studies, in patients using antimetabolite drugs such as 5-fluorouracil or capecitabine, chest pain was usually reported to occur from several hours to 72 hours after the first administration [ 47 , 50 , 51 , 52 , 53 ]. To detect chemotherapy-related coronary vasospasm in the early stage, it is recommended to carefully monitor typical or atypical symptoms of chest pain and EKG monitoring during drug infusion [ 54 ]. Muco et al. (2022) reported severe outcomes resulting from delayed management of vasospastic angina symptoms. The patient’s cardiac evaluation was performed 3 days after the onset of symptoms, and unfortunately, she did not recover from brain damage caused by coronary vasospastic sequelae. The authors stressed the importance of medical teams recognizing the symptoms of CRCT through vigilant monitoring and patient education [ 55 ].
As seen in the symptoms of CRCT caused by heart failure and vasospasm, careful observation of symptoms and conducting appropriate tests are crucial to prevent cardiotoxicity and minimize damage. These characteristics of CRCT and the associated symptoms related to chemotherapy regimens can provide crucial educational content for healthcare providers and patients preparing for chemotherapy. In addition, CRCT and symptom progression according to chemotherapy regimens could be used to formulate research questions for future systematic reviews.
Second, the preventive management of CRCT necessitates adherence to recommended guidelines. The 2022 ESC guidelines on cardio-oncology have updated the classification of CRCT and the monitoring protocols based on the chemotherapy regimens used [ 7 ]. The CRCT identified in the current study aligns with the drug toxicity outlined in the 2022 ESC guidelines. These guidelines advocate for regular cardiac monitoring before, during, and after chemotherapy to prevent and manage CRCT induced by anthracycline and HER2 blockers [ 7 , 12 ]. In this scoping review, two of 23 records described cardiac monitoring before, during, and after chemotherapy. An Australian multicenter study revealed that 59% of patients were referred to a cardiologist before CRCT occurred, but only 15% of patients diagnosed with CRCT had consulted a cardiologist before chemotherapy [ 41 ]. Given the declining mortality rates among cancer patients, managing CRCT requires a collaborative approach between oncology and cardiology to minimize mortality and morbidity in patients with breast cancer undergoing chemotherapy [ 7 ]. Therefore, it remains crucial to emphasize adherence to cardiac monitoring guidelines and foster cooperation between oncology and cardiology.
Additionally, symptom assessment is important for the early detection of patients with CRCT. The studies included in the current scoping review assessed whether patients’ symptoms could detect CRCT using interviews with patients, the New York Heart Association classification, a dyspnea assessment scale, and CTCAE tools. The United States National Cancer Institute recommends that healthcare providers use CTCAE and patients with cancer use PRO to report adverse events, including symptoms. CTCAE is a broad and comprehensive terminology that encompasses adverse events related to cancer treatment, has been used since the 1980s [ 25 ], and is not specialized in cardiotoxicity. Additionally, a discrepancy between CTCAE and PRO discovered that healthcare providers often underestimate both the incidence and duration of symptoms compared to the patients [ 56 , 57 , 58 ]. Specifically, healthcare providers tend to report symptom severity as lower than that reported by patients. For instance, there are notable discrepancies between healthcare providers and patients when reporting severe or very severe symptoms of fatigue, dyspnea, and limb edema in patients with early-stage breast cancer undergoing chemotherapy. The reported rates were 8% and 22% for fatigue, 0% and 4% for dyspnea, and 0% and 5% for limb edema, from healthcare providers and patients, respectively. Therefore, it is necessary to develop a user-friendly questionnaire to assess the various symptoms of CRCT.
Finally, we found that once CRCT was confirmed, cardiac treatment was promptly initiated and chemotherapy was frequently halted until CRCT resolution. A Delphi study on the use of anthracycline and trastuzumab proposed altering the treatment schedule or discontinuing treatment until there was an improvement in LVEF [ 59 ]. However, the professional societies did not provide definitive recommendations regarding continuing or ceasing ongoing chemotherapy. Instead, they suggested that the decision to continue or discontinue ongoing chemotherapy should be made based on the patient’s potential risks and benefits [ 60 ]. For example, Polk et al. (2016) reported that out of 22 patients with CRCT resulting from capecitabine, six continued medications with or without cardiac treatment; some of these patients experienced the same symptoms, while others did not exhibit significant symptoms [ 40 ]. Further research is required to explore the continuation or discontinuation of chemotherapy when CRCT is confirmed.
This study has some limitations. First, although we did not restrict the patients’ sex when reviewing the literature, most patients, except for one, were female. This may be related to the lower incidence of breast cancer in men. Second, although this scoping review mapped CRCT symptoms according to chemotherapy regimens, including anthracycline-based drugs, HER2 blockers, and antimetabolites, it did not cover cardiotoxicity related to other types of chemotherapy regimens. Thus, exploring the symptoms by focusing on expanded chemotherapy regimens and cardiovascular toxic diseases will assist in overcoming this limitation. Third, of the 29 studies, 23 were case reports with some grey literature, which may be justified by the nature of scoping reviews that allow for inclusion irrespective of the data source [ 61 ] and the study type. Experimental or observational clinical studies use objective criteria, such as diagnostic tests to generate primary evidence. However, case reports have led to new medical discoveries regarding the prevention and treatment of diseases [ 62 ]. Given the nature of case reports, specific symptoms that could provide clues for evaluating CRCT in patients with breast cancer are most often found in these reports. We incorporated grey literature to gather more comprehensive information on CRCT-related symptoms. However, to mitigate the potential issue of unverified quality in grey literature, we initially organized 16 studies from peer-reviewed literature and subsequently incorporated the grey literature into our findings. This approach helped to clarify the results of the peer-reviewed literature, particularly the types of chemotherapy regimens [ 63 ]. Finally, regarding the literature selection criteria, we examined articles written in English and published since 2010, the year the cardio-oncology guidelines were announced, thereby excluding articles published before 2010.
This scoping review allowed data mapping according to the study design and chemotherapy regimens. The key messages included a type of CRCT, cardiac assessment, and in-depth information regarding the CRCT symptoms. There were approximately five typical CRCT symptoms, including dyspnea, chest pain, peripheral edema, fatigue, and palpitations, and the timing of symptom occurrence varied. The symptoms were assessed by patient-reported symptom presentation rather than using a symptom assessment tool. Therefore, developing and applying a CRCT-specific and user-friendly symptom assessment tool are expected to help healthcare providers and patients manage CRCT symptoms effectively.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I. Current and future burden of breast cancer: global statistics for 2020 and 2040. The Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Article PubMed Google Scholar
Agha A, Wang X, Wang M, Lehrer EJ, Horn SR, Rosenberg JC, Trifiletti DM, Diaz R, Louie AV, Zaorsky NG. Long-term risk of death from heart disease among breast cancer patients. Front Cardiovasc Med. 2022;9: 784409.
Oikawa M, Ishida T, Takeishi Y. Cancer therapeutics-related cardiovascular dysfunction: Basic mechanisms and clinical manifestation. J Cardiol. 2023;81(3):253–9.
Piepoli MF, Adamo M, Barison A, Bestetti RB, Biegus J, Böhm M, Butler J, Carapetis J, Ceconi C, Chioncel O, et al. Preventing heart failure: a position paper of the Heart Failure Association in collaboration with the European Association of Preventive Cardiology. Eur J Heart Fail. 2022;24(1):143–68.
Chung R, Ghosh AK, Banerjee A: Cardiotoxicity: precision medicine with imprecise definitions. In., vol. 5: Archives of Disease in childhood; 2018: e000774.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). European Heart Journal - Cardiovascular Imaging. 2022;23(10):e333–465.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O et al: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022, 24(1):4-131.
Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2017;13(4):270–5.
Lanza O, Ferrera A, Reale S, Solfanelli G, Petrungaro M, Tini Melato G, Volpe M, Battistoni A: New insights on the toxicity on heart and vessels of breast cancer therapies. Med Sci (Basel) 2022, 10(2).
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73.
Lee GA, Aktaa S, Baker E, Gale CP, Yaseen IF, Gulati G, Asteggiano R, Szmit S, Cohen-Solal A, Abdin A, et al. European Society of Cardiology quality indicators for the prevention and management of cancer therapy-related cardiovascular toxicity in cancer treatment. Eur Heart J Qual Care Clin Outcomes. 2022;9(1):1–7.
Alexandraki A, Papageorgiou E, Zacharia M, Keramida K, Papakonstantinou A, Cipolla CM, Tsekoura D, Naka K, Mazzocco K, Mauri D et al: New insights in the era of clinical biomarkers as potential predictors of systemic therapy-induced cardiotoxicity in women with breast cancer: a systematic review. Cancers (Basel) 2023, 15(13).
Di Lisi D, Manno G, Madaudo C, Filorizzo C, Intravaia RCM, Galassi AR, Incorvaia L, Russo A, Novo G: Chemotherapy-related cardiac dysfunction: the usefulness of myocardial work indices. Int J Cardiovasc Imaging 2023.
Kar J, Cohen MV, McQuiston SA, Malozzi CM. Can global longitudinal strain (GLS) with magnetic resonance prognosticate early cancer therapy-related cardiac dysfunction (CTRCD) in breast cancer patients, a prospective study? Magn Reson Imaging. 2023;97:68–81.
Article CAS PubMed Google Scholar
Lim A, Jang H, Jeon M, Fadol AP, Kim S. Cancer treatment-related cardiac dysfunction in breast cancer survivors: a retrospective descriptive study using electronic health records from a Korean tertiary hospital. Eur J Oncol Nurs. 2022;59: 102163.
Liu W, Li W, Li H, Li Z, Zhao P, Guo Z, Liu C, Sun L, Wang Z. Two-dimensional speckle tracking echocardiography help identify breast cancer therapeutics-related cardiac dysfunction. BMC Cardiovasc Disord. 2022;22(1):548.
Article CAS PubMed PubMed Central Google Scholar
Mauro C, Capone V, Cocchia R, Cademartiri F, Riccardi F, Arcopinto M, Alshahid M, Anwar K, Carafa M, Carbone A et al: Cardiovascular side effects of anthracyclines and HER2 inhibitors among patients with breast cancer: a multidisciplinary stepwise approach for prevention, early detection, and treatment. J Clin Med 2023, 12(6).
Okushi Y, Saijo Y, Yamada H, Toba H, Zheng R, Seno H, Takahashi T, Ise T, Yamaguchi K, Yagi S et al: Effectiveness of surveillance by echocardiography for cancer therapeutics-related cardiac dysfunction of patients with breast cancer. J Cardiol 2023.
Ositelu K, Trevino A, Tong A, Chen MH, Akhter N: Challenges in cardiovascular imaging in women with breast cancer. Curr Cardiol Rep 2023.
Terui Y, Sugimura K, Ota H, Tada H, Nochioka K, Sato H, Katsuta Y, Fujiwara J, Harada-Shoji N, Sato-Tadano A, et al. Usefulness of cardiac magnetic resonance for early detection of cancer therapeutics-related cardiac dysfunction in breast cancer patients. Int J Cardiol. 2023;371:472–9.
Thavendiranathan P, Shalmon T, Fan CS, Houbois C, Amir E, Thevakumaran Y, Somerset E, Malowany JM, Urzua-Fresno C, Yip P, et al. Comprehensive cardiovascular magnetic resonance tissue characterization and cardiotoxicity in women with breast cancer. JAMA Cardiol. 2023;8(6):524–34.
Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–7.
White J, Byles J, Williams T, Untaru R, Ngo DTM, Sverdlov AL. Early access to a cardio-oncology clinic in an Australian context: a qualitative exploration of patient experiences. Cardiooncology. 2022;8(1):14.
PubMed PubMed Central Google Scholar
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P: CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003, 13(3):176-181.
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP et al: Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014, 106(9).
Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Am Soc Clin Oncol Educ Book. 2016;36:67–73.
Article Google Scholar
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–8.
Liu L, Suo T, Shen Y, Geng C, Song Z, Liu F, Wang J, Xie Y, Zhang Y, Tang T, et al. Clinicians versus patients subjective adverse events assessment: based on patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res. 2020;29(11):3009–15.
Munn Z, Pollock D, Khalil H, Alexander L, Mclnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evidence Synthesis. 2022;20(4):950–2.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32.
Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H: Chapter 11: scoping reviews (2020 version). In: JBI Manual for Evidence Synthesis. edn. Edited by Aromataris E MZ: JBI; 2020.
Munn Z, Peters MD, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC medical research methodology. 2018;18:1–7.
Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, Pollock D, Tricco AC, Munn Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–68.
Bohdan M, Kowalczys A, Mickiewicz A, Gruchala M, Lewicka E: Cancer therapy-related cardiovascular complications in clinical practice: current perspectives. J Clin Med 2021, 10(8).
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63.
Bozkurt B, Coats A, Tsutsui H: Universal definition and classification of heart failure. J Card Fail 2021.
Aldiab A. Cardiotoxicity with adjuvant trastuzumab use in breast cancer: a single institution»s experience. J Saudi Heart Assoc. 2010;22(3):133–6.
Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–21.
Polk A, Shahmarvand N, Vistisen K, Vaage-Nilsen M, Larsen FO, Schou M, Nielsen DL: Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 2016, 6(10).
Clark RA, Marin TS, McCarthy AL, Bradley J, Grover S, Peters R, Karapetis CS, Atherton JJ, Koczwara B. Cardiotoxicity after cancer treatment: a process map of the patient treatment journey. Cardiooncology. 2019;5:14.
Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021;280: 119760.
Malik A, Brito D, Vaqar S, Chhabra L: Congestive heart failure. In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2023.
Attin M, Reifenstein K, Mehta S, Arcoleo K, Lin CD, Storozynsky E. Reported signs, symptoms, and diagnostic tests before cardiotoxicity among women with breast cancer: a pilot study. J Cardiovasc Nurs. 2022;37(2):104–11.
Huang P, Dai S, Ye Z, Liu Y, Chen Z, Zheng Y, Shao X, Lei L, Wang X. Long-term tolerance and cardiac function in breast cancer patients receiving trastuzumab therapy. Oncotarget. 2017;8(2):2069–75.
Salyer J, Flattery M, Lyon DE. Heart failure symptom clusters and quality of life. Heart Lung. 2019;48(5):366–72.
Padegimas A, Carver JR. How to diagnose and manage patients with fluoropyrimidine-induced chest pain: a single center approach. JACC CardioOncol. 2020;2(4):650–4.
Sheth MA, Widmer RJ, Dandapantula HK. Pathobiology and evolving therapies of coronary artery vasospasm. Proc (Bayl Univ Med Cent). 2021;34(3):352–60.
PubMed Google Scholar
Hokimoto S, Kaikita K, Yasuda S, Tsujita K, Ishihara M, Matoba T, Matsuzawa Y, Mitsutake Y, Mitani Y, Murohara T, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. J Cardiol. 2023;82(4):293–341.
Kanduri J, More LA, Godishala A, Asnani A. Fluoropyrimidine-associated cardiotoxicity. Cardiol Clin. 2019;37(4):399–405.
Garbis K, Rafiee MJ, Luu J. 5-fluorouracil-induced coronary vasospasm: a cardiovascular magnetic resonance imaging case report. Glob Cardiol Sci Pract. 2023;2023(3): e202316.
Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, Appel JM, Nielsen DL. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020;59(4):475–83.
Becker K, Erckenbrecht JF, Häussinger D, Fueling T. Cardiotoxicity of the antiprolif erative compound fluorouracil. Drugs. 1999;57(4):475–84.
Lestuzzi C, Vaccher E, Talamini R, Lleshi A, Meneguzzo N, Viel E, Scalone S, Tartuferi L, Buonadonna A, Ejiofor L, Schmoll HJ. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25(5):1059–64.
Muco E, Patail H, Shaik A, McMahon S. Capecitabine-associated coronary vasospasm and cardiac arrest. Cureus. 2022;14(8): e28184.
Montemurro F, Mittica G, Cagnazzo C, Longo V, Berchialla P, Solinas G, Culotta P, Martinello R, Foresto M, Gallizioli S, et al. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol. 2016;2(4):445–52.
Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y, Basch E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–76.
Galizia D, Milani A, Geuna E, Martinello R, Cagnazzo C, Foresto M, Longo V, Berchialla P, Solinas G, Calori A, et al. Self-evaluation of duration of adjuvant chemotherapy side effects in breast cancer patients: a prospective study. Cancer Med. 2018;7(9):4339–44.
Gavila J, Seguí M, Calvo L, López T, Alonso JJ, Farto M. Sánchez-de la Rosa R: Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol. 2017;19(1):91–104.
Leong DP, Lenihan DJ. Clinical practice guidelines in cardio-oncology. Heart Fail Clin. 2022;18(3):489–501.
Munn Z, Pollock D, Khalil H, Alexander L, McLnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evid Synth. 2022;20(4):950–2.
Li YR, Jia Z, Zhu H. Understanding the value of case reports and studies in the context of clinical research, research design and evidence-based practice. J Case Reports and Studies. 2013;1(2):1–4.
Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52(4):256–61.
Download references
Acknowledgements
The authors thank Nawon Kim, a librarian at the Yonsei University Medical Library, for building search terms and guiding the database searches.
This research is supported by the Brain Korea 21 FOUR Project founded by the National Research Foundation (NRF) of Korea, Yonsei University College of Nursing.
Author information
Authors and affiliations.
Graduate School, College of Nursing, Yonsei University, 50 Yonsei-ro, Seoul, South Korea
Hyunjoo Kim
Severance Cardiovascular Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Brain Korea 21 FOUR Project, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
College of Nursing and Mo-Im Kim Nursing Research Institute, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Sanghee Kim & Jeongok Park
Department of Internal Medicine, Division of Cardiology, Heart Failure Center, Severance Hospital, Yonsei University, 50-1 Yonsei-ro, Seoul, South Korea
Seok-Min Kang
You can also search for this author in PubMed Google Scholar
Contributions
HK, BH, SK, and JP contributed to the study conception and design. The literature search and record screening were performed by HK and BH under the supervision of JP. Material preparation, data collection, and analysis were performed by HK, BH, and JP. The first draft of the manuscript was written by HK and JP commented on each updated version of the manuscript. The tables and figures were prepared by BH under the instruction of JP. SK helped to interpret the data and provided critical feedback on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jeongok Park .
Ethics declarations
Ethics approval and consent to participate.
This study was exempted from ethical approval by the Yonsei University Institute Review Board, and consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, H., Hong, B., Kim, S. et al. Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: a scoping review. Syst Rev 13 , 167 (2024). https://doi.org/10.1186/s13643-024-02588-z
Download citation
Received : 25 December 2023
Accepted : 14 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s13643-024-02588-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Chemotherapy
- Cardiotoxicity
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 22 June 2024
Multicenter and multimodal imaging study reveals rare fundus lesions in patients after SARS-CoV-2 infection
- Guangqi An 1 , 2 ,
- Bo Lei 3 , 8 ,
- Zhili Wang 3 ,
- Kaizhuan Yang 4 ,
- Dongsheng Fan 5 ,
- Bing Li 6 ,
- Haixin Fang 1 ,
- Min Zhang 1 , 2 ,
- Yu Zhao 1 ,
- Xuemin Jin 1 , 2 &
- Liping Du 1 , 2
Scientific Reports volume 14 , Article number: 14369 ( 2024 ) Cite this article
689 Accesses
66 Altmetric
Metrics details
- Eye abnormalities
- Retinal diseases
- Vision disorders
To define the characteristics of fundus manifestations in patients after SARS-CoV-2 infection with multimodal imaging techniques. This is a retrospective multicenter and multimodal imaging study including 90 patients. All patients with a visual complaint occurring immediately after SARS-CoV-2 infection were referred to six clinics between December 2022 and February 2023. Demographic information and the temporal relationship between SARS-CoV-2 infection and visual symptoms were documented. The characteristics of the fundus lesions were evaluated using multimodal imaging. Ninety patients from six hospitals were included in this study, including 24 males (26.67%) and 66 (73.33%) females. Seventy-eight patients (86.66%) (146 eyes) were diagnosed with Acute Macular Neuroretinopathy (AMN). The AMN patients were primarily young women (67.95%). Sixty-eight patients (87.18%) had AMN in both eyes. Thirty-eight eyes (24.36%) included Purtscher or Purtscher-like lesions. optical coherence tomography and infrared retinal photographs can show AMN lesions well. Eleven cases were diagnosed with simple Purtscher or Purtscher-like retinopathy (2 cases, 2.22%), Vogt‒Koyanagi‒Harada (VKH) syndrome or VKH-like uveitis (3 cases, 3.33%), multiple evanescent white-dot syndrome (MEWDS) (2 cases, 2.22%), and rhino-orbital-cerebral mucormycosis (ROCM) (5 cases, 5.56%). After SARS-CoV-2 infection, diversified fundus lesions were evident in patients with visual complaints. In this report, AMN was the dominant manifestation, followed by Purtscher or Purtscher-like retinopathy, MEWDS, VKH-like uveitis, and ROCM.
Similar content being viewed by others
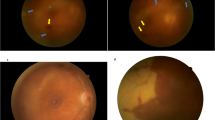

Diagnostic and therapeutic challenges in acute retinal necrosis; an update
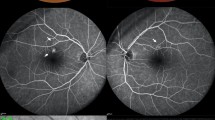
Ocular findings among patients surviving COVID-19
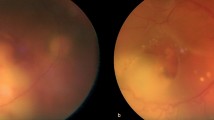
Retinal involvement and ocular findings in COVID-19 pneumonia patients
Introduction.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a life-threatening disease with a serious respiratory infection and multiorgan involvement 1 . SARS-CoV-2 primarily affects the anterior segment of the eye. The most frequently reported ocular conditions include conjunctival hyperemia, chemosis, epiphora, and even frank conjunctivitis 2 , 3 . It is controversial whether SARS-CoV-2 affected the retina in the early stages of the COVID-19 pandemic 4 .
Since 2019, the SARS-COV-2 virus has undergone adaptive mutation that could lead to an increase in transmissibility and virulence or a change in the presentation of clinical disease 5 . The Delta variant was identified in December 2020, and the Omicron variant was identified in November 2021, both of which caused surges in the number of infections 6 .
With the increasing number of COVID-19 patients, a growing number of changes have been observed in the retina and choroid 7 . In 2020, a cross-sectional study revealed retinal lesions associated with SARS-CoV-2, including dilated veins (27.7%), tortuous vessels (12.9%), retinal hemorrhages (9.25%), and cotton-wool spots (7.4%) 8 . A review in 2022 suggested the potential involvement of the posterior segment in SARS-CoV-2, either in the initial or later stage 9 . However, few studies have systematically described the manifestations of the posterior segment of the eye by multimodal imaging study 10 .
Due to the lockdown policy was suddenly lifted, there was a surge in the number of Omicron infections in Chinese people from late December 2022 to late January 2023 in China 11 . Based on the large number of SARS-CoV-2 infected patients, it was possible for us to observe a series of patients with rare fundus Lesions after SARS-CoV-2 infection. These rare fundus lesions with a large increase over the same period of the previous year may be related to SARS-CoV-2 infection. Because these lesions are rare and often lead to misdiagnosis, reasonable examination methods can help us quickly identify lesions and diagnose them.
In the present case series, multimodality imaging was used to describe retinal and/or choroidal conditions that could be associated with SARS-CoV-2 infections. We outlined the features of retinal and choroidal manifestations following SARS-CoV-2 infections and clarified the best diagnostic methods to improve our understanding of their pathogenesis and diagnosis.
Materials and methods
Participants.
This was a cross-sectional multicenter and multimodal imaging study. From December 2022 to February 2023, 90 patients were identified with retinal and/or choroidal conditions associated with COVID‑19 infection in six clinics, including the First Affiliated Hospital of Zhengzhou University and/or Zhengzhou University People’s Hospital and/or The Second People's Hospital of Zhengzhou and/or Luoyang Central Hospital Affiliated to Zhengzhou University and/or Nanyang Eye Hospital, Nanyang and/or The First Affiliated Hospital of Nanyang Medical College. The incidence of these diseases in the same period of the previous year was collected from the outpatient information databases of those hospitals.
All patients tested positive by real‑time reverse transcription‑polymerase chain reaction (RT‑PCR) and/or antigen detection for SARS‑CoV‑2 from pharyngeal or nasopharyngeal swabs during the active phase of SARS‑CoV‑2 infection and eye symptoms occurred whitin one month after SARS‑CoV‑2 infection. Patients who were not infected with SARS‑CoV‑2 and whose ocular symptoms occurred more than one month after SARS‑CoV‑2 infection were excluded.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University [2023-KY-0637]. Based on the Declaration of Helsinki , we collected demographic information, SARS‑CoV‑2 infection symptoms, chief complaints and other clinical examination results after informed consent was signed by the patients or their guardians. The clinical examination results included slit‑lamp examination and indirect ophthalmoscopy results, best corrected visual acuity (BCVA), spherical equivalent (SE), fundus photo images, visual field analysis (VF), infrared retinal photographs (IR), optical coherence tomography (OCT), optical coherence tomography angiography (OCTA), fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), multifocal electrophysiology (mf ERG), fundus autofluorescence (FAF) and adaptive optics (AO). All patients were diagnosed and recorded by 2 doctors individually. In cases of disagreement, the senior ophthalmologist made the final decision.
Method of eye examination
All outpatients were examined by slit‑lamp and ophthalmoscopy. BCVA and SE were tested by optometrists. Fundus photos and FAF were taken using an ultrawide field fundus camera (Daytona P200T, Optomap, UK) and fundus camera (CLARUS 500 v1.1, Carl Zeiss Co. Ltd., Germany).
mf ERG (RETI-scan multifocal ERG (Roland Consult, Germany) was performed on the patients. VF examination (Humphrey Field Analyzer 3, Carl Zeiss Co. Ltd., Germany) or micro examination (MP3-microperimerter Ver.1.2.1, NIDEK Co. Ltd., Japan) was selected according to the patient's symptoms.
Skilled ophthalmologists performed swept source OCT (SS-OCT) (VG200 or VG100, SVision, China) or spectral domain OCT (SD-OCT) (Spectralis OCT, Heidelberg Engineering GmbH, Germany), which included stars in 16 or 32 lines and multiline scanning with a scan line length of 10–16 mm. SS-OCTA scanning was performed with a scan square of 6 mm × 6 mm or 12 mm × 12 mm. The depth of the scanning was 3–6 mm. The scanning laser wavelength was 1050 nm or 870 nm. IR images were taken by a 40° × 40° confocal scanning superluminescent (diode) ophthalmoscope (cSSO) with a wavelength of 820 nm or confocal scanning laser ophthalmoscope (cSLO) with a wavelength of 810 nm.
FFA and ICGA (Spectralis HRA, Heidelberg Engineering GmbH, Germany) were performed to observe vascular lesions. AO (rtx1, Imagine Eyes, Orsay, France) was performed to clarify the status of the optic cone optic rod cells.
Statistical analysis
SPSS statistics 26.0 software (IBM Corp.; USA) were used to analyze the data. Categorical data are described using frequencies and percentages. The Kolmogorov‒Smirnov test was performed to verify whether all the data sets were distributed normally. Continuous variables with a normal distribution are presented as the mean ± standard deviation (SD), and a t test was used for comparisons. Nonnormal variables are reported as medians (interquartile ranges). A P value less than or equal to 0.05 was considered statistically significant.
COVID-19 infection, vaccination history and demographics of patients
Ninety patients were included in this study, including 24 males (26.67%) and 66 (73.33%) females. Their age was 31 ± 15 years old with a range of 10–85 years (Table 1 ).
All patients had a history of COVID-19 infection. Visual symptoms occurred 0–30 days after the onset of fever, dry cough, malaise, etc. The mean interval between COVID-19 infection and visual symptom onset was median 2 days, IQR [2, 3]. Five patients had diabetes, six patients had kidney disease, four patients had hypertension.
We reviewed the detailed COVID-19 infection history, medication history and vaccination history in 33 of the 90 patients. All patients had a fever of 37.5–40 °C. Among them, 24 patients (71.88%) took ibuprofen, acetaminophen or Tylenol to reduce fever, whereas 9 (28.125%) did not. Twelve patients (36.37%) were dehydrated (sweating profusely and unable to drink) prior to the onset of symptoms, while 20 patients (63.63%) were dehydrated. Seven patients (21.21%) received 2 doses of inactivated COVID-19 vaccine, 19 patients (57.58%) received 3 doses of inactivated COVID-19 vaccine, and 7 patients (21.21%) did not receive inactivated COVID-19 vaccine.
Clinical characteristics of our case series and multimodal imaging
Among all patients, 78 (86.66%) were diagnosed with acute macular neuroretinopathy (AMN). The remaining 12 patients were diagnosed with simple Purtscher-like retinopathy (2 patients, 2.22%), Vogt‒Koyanagi‒Harada-like (VKH-like) uveitis (3 patients, 3.33%), multiple evanescent white-dot syndrome (MEWDS) (2 patients, 2.22%), and rhino-orbital-cerebral mucormycosis (ROCM) (5 patients, 5.56%).
However, in December 2021 to February 2022 year, there were only one Purtscher retinopathy patient with chest trauma, one ROCM patient and two AMN patients. Although there are as many as 21 patients first diagnosed as VKH, their clinical symptoms are different from those after SARS-CoV-2 infection. The number of MEWDS patient cannot obtain because MEWDS does not have an ICD diagnostic code.
Acute macular neuroretinopathy (AMN) and Purtscher or Purtscher-like retinopathy
A total of 78 patients (146 eyes) were diagnosed with AMN, including 22 males (28.21%) and 56 (71.79%) females. Their age was 29 ± 11 years old with a range of 10 to 64 years old. The majority of patients were young women (67.95%). There was no statistically significant difference in age between the sexes ( t = − 0.22, P = 0.830). Their complaints were "black shadows or dark spots in front of the eyes or visual field defects" (38 cases, 48.72%) and "blurred vision. "(40 cases, 51.28%).
Ten patients (12.82%) had visual problems in one eye. Sixty-eight patients (87.18%) had visual problems in both eyes. The BCVA of the 146 diseased eyes was median 0.13, IQR [0.00–0.36] logMAR with a range of 0.00 to 2.00 Log MAR. In a total of 146 eyes with AMN, the BCVAs at presentation were generally well documented to be 0.30 LogMAR or better (96 eyes, 65.75%). 1.00 LogMAR or worse in 18 eyes (12.33%).
At the initial visit, nineteen of the seventy-eight cases (38 of 146 eyes; 24.36% of cases, 26.03% of eyes) presented with either cotton-wool spots or Purtscher-like retinopathy. Ten of these patients (52.63%) had kidney disease or hypertension.
OCT with IR were used in 146 eyes (100.00%), and in each IR image, AMN lesions were dark or gray with well-demarcated margins with oval (Fig. 1 B1), petal-shaped (Fig. 1 C1), multifocal dark spots (Fig. 1 D1). AMN lesions on OCT exhibited one or more abnormal characteristics, including outer retinal hyperreflectivity (Fig. 1 B2,B3 yellow arrowhead) which was relation to photoreceptors and bipolar cells (Fig. 1 A1,A2), ellipsoid zone loss (Fig. 1 C2,C3 yellow arrows), small cavity in macula (Fig. 2 B1 yellow arrowhead) and thinning of the outer nuclear layer (ONL) (Fig. 1 D2,D3 yellow arrowhead).
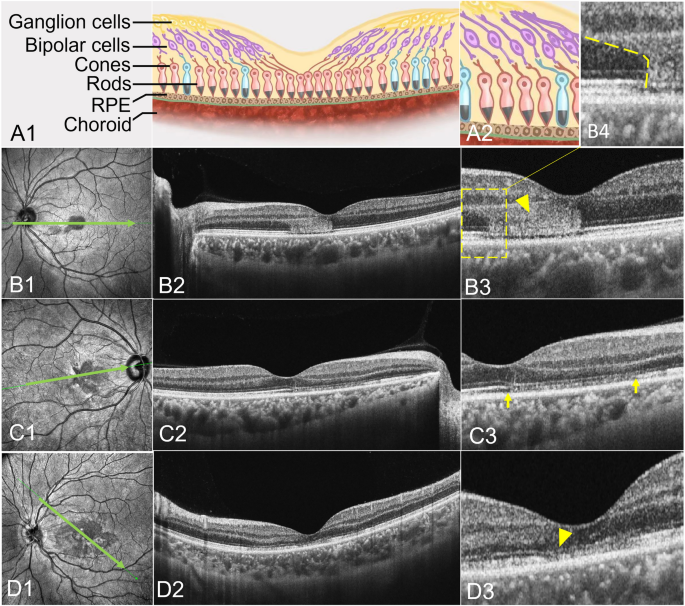
The cSSO images (a type of IR image) ( B1 ; C1 ; D1 ) and the SS-OCT B-scan images ( B2 , 3,4; C2 ,3; D2 ,3) of AMN lesions. AMN lesions were dark or gray with well-demarcated margins with oval ( B1 ), petal-shaped ( C1 ), multifocal dark spots ( D1 ). The acute phase of AMN lesions exhibited one or more abnormal characteristics, including outer retinal hyperreflectivity in SS-OCT B-scan images ( B2 , B3 , B4 ). The shape of the lesion was consistent with the nerve fiber direction of the cone and rod cells ( A2 , B4 yellow dotted line). The later period of AMN lesions exhibited ellipsoid zone loss ( C2 , C3 ) and thinning of the outer nuclear layer ( D2 , D3 ).
Multimodal fundus images of a 40 year-old female with AMN.Fundus photos ( A ), SS-OCT B-scan images ( B ), IR images ( C ), AO images ( D ), mf ERG ( F ) and micro-VF images ( F ). The BCVAs of her right and left eyes were 0.52 and 0.30 LogMAR (A; B1,2; F). After a month, the BCVAs of her right and left eyes increased to 0.1 and 0.00 LogMAR (B3,4; C; D; E). Compared with B1 and B2, the ellipsoid zone loss in B3 and B4 was largely restored, and the macular edema in B1 disappeared in B3.
Fundus photos were taken in 57 of 146 eyes. The lesions showed wedge-shaped petal-shaped, oval slightly dark areas in 16 eyes (28.07%) (Fig. 2 A1,A2). The contrast sensitivity of the fundus photo was not as good as that of IR and OCT (Fig. 2 B1,B2,B3,B4,C1,C2, Fig. 3 C). Four eyes underwent AO and showed small patches of cone rod cell loss in the area corresponding to the lesions (Fig. 2 D1,D2 yellow boxes). Ten eyes underwent mf ERG and all showed one to several abnormal findings, including diminished amplitudes and diminished implicit time (Fig. 2 E1,E2,E3,E4,E5,E6, Fig. 3 B1,B2). Thirty-six eyes with AMN experienced one to several paracentral scotomas by Amsler grid, VF or micro-VF testing. The mf ERG and VF specialty corresponded closely to the shape and location of the clinical lesion (Fig. 3 A,B1). The shape of VF abnormalities was wedge-shaped, boot-shaped, and round-shaped (Fig. 2 F1,F2). The en face OCT exhibits the same hypo reflectivity as the OCT B-scan (Fig. 3 D).
Multimodal fundus images of an 11 year-old female with AMN. Micro VF and fundus photos ( A ), mf ERG ( B1 , B2 ), IR images ( C ) and en face images analyzed based on SS-OCTA and SS-OCT B-scan images ( D ). The BCVAs of her right and left eyes were 0.80 and 0.70 LogMAR. The area enclosed by the yellow line shows the extent of the lesion. The dark area on the en face image ( D ) was consistent with the range of ellipsoid loss in the B-scan (green dashed line), which was smaller than those areas on Micro VF, mf ERG and IR images.
VKH-like uveitis and multiple evanescent white-dot syndrome (MEWDS)
A 49-year-old female patient presented with complaints of visual distortion in both eyes, without accompanying headache or tinnitus (Fig. 4 A,B,C). Initial BCVAs were 0.52 Log MAR for the right eye and 1.3 Log MAR for the left eye. Following the onset of fever, she was diagnosed with SARS-CoV-2 via an antigen test. Five days post-diagnosis, the patient reported progressive hazy vision in her left eye, prompting a hospital visit. A month later, similar symptoms developed in her right eye. Ophthalmic examination revealed conjunctival congestion and peripheral anterior synechia in both eyes. The vitreous was cloudy, obscuring the fundus, indicative of significant intraocular inflammation with both anterior chamber and vitreous haze initially graded at 3 + based on the Standardization of Uveitis Nomenclature (SUN) Working Group 12 criteria with mutton-fat keratic precipitates (KPs). Therapeutic intervention was initiated with prednisone (60 mg daily), tobramycin-dexamethasone eye drops (administered every four hours), and 0.1% atropine eye ointment (applied twice daily). Significant reduction in anterior segment inflammation was observed within three days, inflammation in the right eye's anterior chamber and vitreous haze decreased to 0.5 + , while the left eye improved to 1 + , and the fundus became visible (Fig. 4 D1,D2). OCT performed at this time revealed multifocal serous neurosensory retinal detachment (Fig. 4 E1,E2). Subsequent improvement in BCVAs was noted, with values of 0.22 Log MAR in the right eye and 0.30 Log MAR in the left eye, alongside the resolution of neurosensory retinal detachment. The rapid amelioration of inflammation across both the anterior chamber and vitreous highlights the efficacy of the prescribed treatment regimen.
Multimodal fundus images from a 49-year-old female ( A , B , C , D , E ) and a 43 year-old female ( F , G ). Initially, the vitreous was opaque in the 49-year-old female ( A ). FFA ( B ) and ICGA ( C ) showed optic disc leakage and fluorescence accumulation in the advanced macula. After 3 days of treatment, the vitreous opacity was reduced ( D ), and neurosensory retinal detachment decreased ( E ). In the 43 year-old female, FFA ( F ) initially showed optic disc leakage. After treatment, neurosensory retinal detachment decreased, and choroidal thickness was reduced over time ( G ).
Another 43-year-old female presented with complaints of blurred vision in both eyes, without accompanying headache or tinnitus. Four days after experiencing a fever due to SARS-CoV-2 infection, her BCVAs were 0.10 LogMAR in both eyes. FFA and OCT were performed (Fig. 4 F1, F2,F3,F4,G1,G2), leading to a diagnosis of VKH syndrome. Initial examination revealed anterior chamber and vitreous cells graded at 1 + with mutton-fat KPs, and vitreous haze also graded at 1 + . Treatment was initiated with prednisone (60 mg once daily) and 0.1% atropine eye ointment (one drop twice daily). The patient experienced rapid improvement; after seven days of treatment, her BCVAs improved to 0.00 LogMAR in both eyes. Subsequent FFA and OCT imaging showed a reduction and eventual resolution of neurosensory retinal detachment over time (Fig. 4 G1,G2). The patient underwent a one-month course of systemic corticosteroid treatment and did not experience any relapse over the following six months.
Following SARS-CoV-2 infection, these two patients developed blurred vision and exhibited inflammation in both the anterior chamber and the vitreous body. Their symptoms are consistent with the typical manifestations of Vogt-Koyanagi-Harada (VKH) disease, characterized by pan-uveitis and multifocal serous retinal detachment, specifically granulomatous uveitis. Notably, this included mutton-fat KPs, a hallmark of VKH disease. However, unlike typical VKH cases, they did not show systemic symptoms such as tinnitus and skin changes. However, they responded well to corticosteroid treatment without any relapse.
A 21-year-old male patient was diagnosed with MEWDS and complained of "blurred vision on the left eye" five days after a fever caused by SARS-CoV-2. The BCVAs were 0.00 and 0.10 LogMAR in his right and left eyes. Some yellow-white punctate lesions were faintly seen on fundus photography (Fig. 5 A1,A2), OCT showed that there were structural abnormalities in the outer retina (Fig. 5 C1,C2). The mf ERG showed that the visual sensitivity of the patient's left eye was reduced (Fig. 5 D1,D2). There were some highly fluorescent lesions on the FAF (Fig. 5 B1,B2) and scattered high fluorescence spots on FFA (Fig. 5 E). However, the patient did not undergo ICGA due to drug allergies. We suggested that the patient should return to the clinic two weeks later, but he did not return. Then, we performed a telephone follow-up and were informed that his symptoms had been eliminated. Another 37-year-old female had similar symptoms.
Multimodal fundus images from a 21 year-old maleFundus photos ( A ), FAF ( B ), OCT ( C ), mf ERG ( D ), and FFA ( E ). The area enclosed by the yellow line and indicated by a yellow arrowhead in A2 shows the extent of the lesion. The range of ellipsoid loss in C2 was shown (yellow arrow).
Rhino-orbital-cerebral mucormycosis (ROCM)
A 49 year-old male patient with SARS-CoV-2-related pneumonia had recurrent fever for 20 days and underwent "intracranial hematoma drainage" because of "diabetic ketoacidosis and cerebral hemorrhage." Then, he developed swelling on the right side of the face 3 days later. Sooner afterward, he suddenly lost vision in his right eye. This patient had a history of high blood pressure and diabetes but did not take medications regularly. Due to fever and infection, his vital signs were unstable, and his right eye was not treated (Fig. 6 B). Pale retinal and “cherry red spot” suggested central retinal artery occlusion (CRAO). Fungal cultures of nasal tissue in patients showed wide septum hyphae, which was characteristic of Mucormyces. This confirmed that he had ROCM. The patient's right eyeball was fixed in the upper right position and could only be slightly moved up and down instead of left and right (Fig. 6 A). Unfortunately, the optic disc of the right eye was pale (Fig. 6 B1). Interestingly, we observed cotton-wool spots around the optic disc of the left eye (Fig. 6 B2.B3). OCT showed retinal-choroidal atrophy and interlaminar edema in his right eye (Fig. 6 C1). The cotton-wool patch showed thickening of the neuroepithelium (Fig. 6 C2,C3 yellow arrow head). The patient's magnetic resonance imaging (MRI) showed abnormal signals in the right orbital tip (Fig. 6 D1,D2,D3).
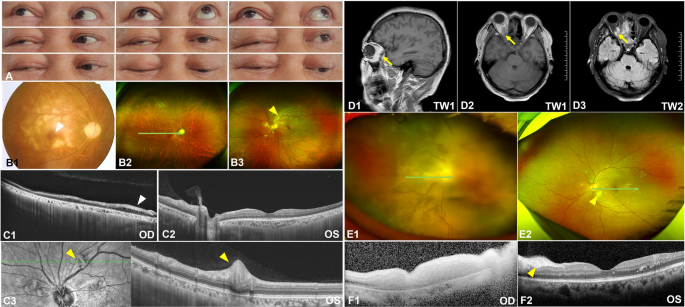
Multimodal fundus images from a 49 year-old male patient ( A , B , C , D ) and a 49-year-old female patient ( E , F ). White arrowhead showed the cherry erythema in B1, while yellow arrowhead showed the cotton-wool spots in B3 where the nerve fiber layer was swelling (C3). F1 showed the acute phase of arterial occlusion with extensive retinal edema.
Another 49-year-old female patient was also diagnosed with ROCM. She also had diabetic ketoacidosis, which suggested poor glycemic control. Similarly, she lost her vision in her right eye. Fundus photographs showed cotton-wool spots and hemorrhages on her left eye as well (Fig. 6 E1,E2). OCT images showed the acute phase of arterial occlusion with extensive retinal edema (Fig. 6 F1) and Purtscher-like retinopathy (Fig. 6 F2 yellow arrow head). Blood tests showed that her white blood cells were 13.68 (reference 3.5–9.5) 10^9/L, neutrophil percentage was 83.5% (reference 40–75%), DD-dimer was as high as 10.79 (reference 0–0.3) mg/L (DDU), fibrinogen degradation products (FDP) were as high as 10.79 (reference 0–5) mg/L, and C-reactive protein (CRP) and procalcitonin (PCT) and interleukin 6 (IL-6) were more than 5 times the normal value.
Both patients did not receive ocular treatment because their vision was no light perception (NLP). In addition, three other female patients aged 77, 81, and 85 years who had ROCM and diabetes underwent eye removal.
There have been more than 757 million confirmed cases and 6.8 million deaths being reported worldwide since the outbreak of COVID-19 in November 2019 13 . By targeting the ACE2 receptor in human endothelial cells, SARS-CoV-2 attacks host cells through the transmembrane spike protein (S protein). Therefore, tissues with ACE2 receptors are susceptible to SARS-CoV-2 infection 14 . ACE2 is widely expressed in endothelial cells of the lung, blood vessels, heart, kidney, small intestine and other tissues and organs, with low expression in the liver and nose. These organs are vulnerable to damage following SARS-CoV-2 infection 15 , 16 . Moreover, ACE2 is a major converting enzyme in the vascular protective axis of the renin-angiotensin system in the retina, and its downregulation may lead to retinal ischemia, which is related to microangiopathy, retinitis, and retinal degeneration 16 , 17 , 18 .
The meta-analysis conducted by Sen et al 3 in 2021 revealed that conjunctivitis is the principal ocular manifestation following SARS-CoV-2 infection, with previous research largely focusing on individual case reports. In contrast, our research expands the scope by presenting a substantial series of cases from six medical centers, utilizing multimodal imaging to document the experiences of 90 patients. This approach provides robust evidence of ocular involvement after SARS-CoV-2 infection. Our findings underscore that in the real world, AMN is a significant and closely associated retinal lesion with after SARS-CoV-2 infection, highlighting its prevalence and clinical importance in affected patients.
Previous literature has reported retinal and choroidal manifestations that may be associated with SARS-CoV-2, as shown in Table 2 . Based on the pathological mechanism, we classify these manifestations as noninfectious or infectious. Noninfectious manifestations may be related to ischemia or inflammation. RVO and RAO are common diseases in ophthalmology, and patients often have underlying diseases such as diabetes and hypertension 19 , 20 . Therefore, these diseases were not included in our case series as fundus lesions related to SARS-CoV-2. However, rare eye diseases such as AMN and ROCM have increased in incidence alongside the rise in SARS-CoV-2 infection rates 21 , 22 .
Acute macular neuroretinopathy and Purtscher or Purtscher-like retinopathy
We observed this case series with SARS-CoV-2-related retinal and choroidal manifestations. Typically, the patient was a young woman who developed visual impairment two days following the onset of infection symptoms. As the standard, with the relationship of cause, trigger and disease, our results suggested a close relationship between the emergence of AMN or Purtscher or Purtscher-like retinopathy and SARS-CoV-2 infection.
AMN is a relatively rare disorder involving transient or permanent central or paracentral scotomas 39 , 40 . It is characterized by dark, reddish-brown macular lesions and corresponds precisely to visual field abnormalities 41 . AMN has been reported to occur in several different clinical settings. The majority of patients are women in their reproductive years who develop symptoms in association with oral contraceptives, hypotension, viral illness, intravitreous injection, vaccination and sympathomimetic agents (epinephrine, caffeine). 3 , 42 , 43 , 44 , 45 , 46 , 47 . Since the syndrome was initially characterized by Bos and Deutmann 49 in 1975, the pathophysiology of AMN has been the subject of intense discussion, especially in light of the disorder's diverse causes.
Acute retinal lesions are characterized by faint retinal translucency on bio microscopy and hyperreflectivity in the outer plexiform and outer nuclear layers on OCT. FFA and ICGA do not reveal any retinal or choroidal vascular leakage, perfusion deficits or transmission defects. mfERG testing shows reduced amplitudes within the scotomatous areas 50 . Evolution of macular lesions is characterized by resolution within several days of the initial retinal translucency and hyperreflectivity, followed by the development of reddish-brown lesions that appear dark on IR and show thinning of the outer nuclear layer and attenuation or loss of the ellipsoid and interdigitation zones on OCT. Compared to FFA, ICGA and FAF, OCT and IR images display the lesions of AMN more effectively. The cSSO fundus photography used in this study is a laser with a wavelength of 820 nm; it is also an IR imaging approach in essence. Due to the destruction of the elliptical zone, the laser is absorbed by the deeper and stronger retinal epithelium, demonstrating the essence of shadow and OCT is optical coherence imaging, and the areas with mixed or dense tissue structure will show high reflection 51 . From the perspective of imaging alone, the morphology of hyper reflex in the early stage of the onset of AMN was consistent with that of the fiber of the cone and rod cells, which indicates the affected site (Fig. 1 A1,A2) 41 , 48 . At present, the generation of AMN is mainly dominated by two theories: the inflammation-related immune theory and the vascular-related ischemia and hypoxia theory 50 , 52 . This needs to be discussed in combination with vascular parameters, and our research team will explore in future research.
VKH disease is an immune-mediated disorder characterized by bilateral uveitis frequently associated with neurological (meningeal), auditory, and integumentary symptoms. Auditory manifestations (tinnitus, hearing loss and vertigo) and others (including headache, neck and back stiffness) usually occur before or concurrently with ocular involvement 53 . A previous study linked VKH to SARS-CoV-2 Vaccines 54 . The VKH-like patients in this series responded favorably to corticosteroid therapy. The cases of VKH-like uveitis were particularly notable for their lack of typical systemic symptoms like tinnitus and skin changes, suggesting a unique, non-granulomatous panuveitis potentially triggered by COVID-19. This aligns with observations that COVID-19 may induce a distinct immune or inflammatory pathway, leading to VKH-like manifestations. Both patients showed excellent response to corticosteroid treatment without recurrence, highlighting the effectiveness of standard anti-inflammatory treatments in these atypical presentations. MWDES is related to colds and viral infections. Both patients in this study developed symptoms five days after experiencing SARS-CoV-2-related fever, which is considered a cause.
ROCM can be a serious complication of severe SARS-CoV-2 infection, particularly in patients with uncontrolled diabetes. The risk factors predisposing patients to ROCM are uncontrolled diabetes, neutropenia, hematological malignancies, organ transplantation, trauma and burn, and use of immunosuppressants such as corticosteroids 38 , 39 . Patients were often blinded by mucormycosis invasion of the orbital apex leading to orbital apex syndrome forming retinal artery obstruction. This disease is easily misdiagnosed due to its reputation as a difficult-to-treat mold infection and its high mortality in patients with SARS-CoV-2 infection, particularly those with pulmonary disease. A careful management plan can be successful for rhino-orbital cerebral disease if there is early diagnosis of infection and control of infection 55 .
In conclusion, the retinal and choroidal conditions after SARS-CoV-2 infection are diverse, including AMN, MEWDS, VKH-like uveitis, and ROCM. Multimodal imaging may be used to evaluate the lesions from the anatomical and functional levels, and an appropriate examination with multimodal imaging is beneficial for patient management and follow-up.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Sharma, A., Tiwari, S., Deb, M. K. & Marty, J. L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 56 , 106054. https://doi.org/10.1016/j.ijantimicag.2020.106054 (2020).
Article CAS PubMed PubMed Central Google Scholar
Costa, I. F. et al. Ocular findings among patients surviving COVID-19. Sci. Rep. 11 , 11085. https://doi.org/10.1038/s41598-021-90482-2 (2021).
Article CAS PubMed PubMed Central ADS Google Scholar
Sen, M. et al. COVID-19 and eye: A review of ophthalmic manifestations of COVID-19 Indian. J. Ophthalmol. 69 , 488–509. https://doi.org/10.4103/ijo.IJO_297_21 (2021).
Article Google Scholar
Karampelas, M., Dalamaga, M. & Karampela, I. Does COVID-19 involve the retina?. Ophthalmol. Ther. 9 , 1–3. https://doi.org/10.1007/s40123-020-00299-x (2020).
Harvey, W. T. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19 , 409–424. https://doi.org/10.1038/s41579-021-00573-0 (2021).
Aleem, A., Akbar Samad, A. B. & Slenker, A. K. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). StatPearls (2022).
Jevnikar, K. et al. An update on COVID-19 related ophthalmic manifestations. Ocul. Immunol. Inflamm. 29 , 684–689. https://doi.org/10.1080/09273948.2021.1896008 (2021).
Article CAS PubMed Google Scholar
Invernizzi, A. et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 27 , 100550. https://doi.org/10.1016/j.eclinm.2020.100550 (2020).
Article PubMed PubMed Central Google Scholar
Zhang, Y. & Stewart, J. M. Retinal and choroidal manifestations of COVID-19. Curr. Opin. Ophthalmol. 32 , 536–540. https://doi.org/10.1097/ICU.0000000000000801 (2021).
Article PubMed Google Scholar
McGrath, O. E. & Aslam, T. M. Use of imaging technology to assess the effect of COVID-19 on retinal tissues: A systematic review. Ophthalmol. Ther. 11 , 1017–1030. https://doi.org/10.1007/s40123-022-00509-8 (2022).
Pan, Y. et al. Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis. Lancet 401 , 664–672. https://doi.org/10.1016/S0140-6736(23)00129-0 (2023).
Read, R. W. et al. Classification criteria for vogt-koyanagi-harada disease. Am. J. Ophthalmol. 228 , 205–211. https://doi.org/10.1016/j.ajo.2021.03.036 (2021).
World Health Organization. https://www.who.int/europe/emergencies/situations/covid-19 (Updated 2023.02.05).
Jackson, C. B. et al. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23 , 3–20. https://doi.org/10.1038/s41580-021-00418-x (2022).
Guney, C. & Akar, F. Epithelial and endothelial expressions of ACE2: SARS-CoV-2 entry routes. J. Pharm. Pharm. Sci. 24 , 84–93. https://doi.org/10.18433/jpps31455 (2021).
Pagliaro, P. et al. Angiotensin-converting enzyme 2: a key enzyme in key organs. J. Cardiovasc. Med. (Hagerstown) 23 , 1–11. https://doi.org/10.2459/JCM.0000000000001218 (2022).
Yener, A. U. COVID-19 and the eye: Ocular manifestations, treatment and protection measures. Ocul. Immunol. Inflamm. 29 , 1225–1233. https://doi.org/10.1080/09273948.2021.1977829 (2021).
Lumbers, E. R. et al. The interacting physiology of COVID-19 and the renin-angiotensin-aldosterone system: Key agents for treatment. Pharmacol. Res. Perspect. 10 , e00917. https://doi.org/10.1002/prp2.917 (2022).
Hayreh, S. S., Podhajsky, P. A. & Zimmerman, M. B. Retinal artery occlusion: Associated systemic and ophthalmic abnormalities. Ophthalmology 116 , 1928–1936. https://doi.org/10.1016/j.ophtha.2009.03.006 (2009).
Modjtahedi, B. S., Do, D. & Shaw, J. Correspondence regarding changes in the incidence of retinal vascular occlusions after COVID-19 diagnosis-reply. JAMA Ophthalmol. 140 , 1143–1144. https://doi.org/10.1001/jamaophthalmol.2022.3479 (2022).
Azar, G. et al. Did the COVID-19 pandemic increase the incidence of acute macular neuroretinopathy?. J. Clin. Med. 10 , 5038. https://doi.org/10.3390/jcm10215038 (2021).
Nehara, H. R. et al. Coronavirus disease, diabetes and glucocorticoid a terrible trio for invasive mucormycosis: An observational study from Northwest Rajasthan. J. Assoc. Phys. India 69 , 11–12 (2022).
Google Scholar
Nourinia, R. et al. Branch retinal vein occlusion after COVID-19. J. Fr. Ophtalmol. 44 , e441–e443. https://doi.org/10.1016/j.jfo.2021.06.003 (2021).
Cuadros Sanchez, C. et al. Central retinal vein occlusion presumably associated with lupus anticoagulant induced by SARSCoV-2. Ocul. Immunol. Inflamm. 30 , 2010–2013. https://doi.org/10.1080/09273948.2021.1933077 (2022).
Au, S. C. L. & Ko, C. K. L. Central retinal artery occlusion in patients with COVID-19: Imaging for underlying causes. Radiology 300 , E315. https://doi.org/10.1148/radiol.2021210479 (2021).
Thatcher, M. D., Wu, L. Z. & Varma, R. Bilateral purtscher-like retinopathy associated with COVID-19 infection. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2022.6255 (2023).
Chan, A. X., Ritter, M. & Bakhoum, M. F. Bilateral cotton wool spots after ambulatory COVID-19. Int. J. Infect. Dis. 105 , 414–415. https://doi.org/10.1016/j.ijid.2021.02.119 (2021).
Pereira, L. A. et al. Retinal findings in hospitalised patients with severe COVID-19. Br. J. Ophthalmol. 106 , 102–105. https://doi.org/10.1136/bjophthalmol-2020-317576 (2022).
Giacuzzo, C., Eandi, C. M. & Kawasaki, A. Bilateral acute macular neuroretinopathy following COVID-19 infection. Acta Ophthalmol. 100 , e611–e612. https://doi.org/10.1111/aos.14913 (2022).
Castro, C. S. et al. Paracentral acute middle maculopathy after COVID-19 disease: Multimodal evaluation. Retin. Cases Brief Rep. https://doi.org/10.1097/ICB.0000000000001301 (2022).
Fischer, N. A., Wann, R. C. & Crosson, J. N. Acute posterior multifocal placoid pigment epitheliopathy following COVID-19 infection. Am. J. Ophthalmol. Case Rep. 29 , 101790. https://doi.org/10.1016/j.ajoc.2022.101790 (2023).
Providencia, J. et al. Serpiginous choroiditis presenting after SARS-CoV-2infection: A new immunological trigger?. Eur. J. Ophthalmol. https://doi.org/10.1177/1120672120977817 (2022).
Landecho, M. F. et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease?. J. Intern. Med. 289 , 116–120. https://doi.org/10.1111/joim.13156 (2021).
Yepez, J. B. et al. Vogt-Koyanagi-Harada disease following COVID-19 infection. Case Rep. Ophthalmol. 12 , 804–808. https://doi.org/10.1159/000518834 (2021).
Mohd-Alif, W. M. et al. Bilateral and multiple central serous chorioretinopathy following COVID-19 infection: A case report and literature review. Cureus 14 , e23246. https://doi.org/10.7759/cureus.23246 (2022).
Agarwal, M. et al. Endogenous endophthalmitis a complication of COVID-19 pandemic: A case series. Ocul. Immunol. Inflamm. 29 , 726–729. https://doi.org/10.1080/09273948.2021.1945111 (2021).
Nishiyama, T. et al. Acute retinal necrosis in a patient on immunosuppressive treatment for COVID-19 pneumonia: A case report. BMC Ophthalmol. 22 , 462. https://doi.org/10.1186/s12886-022-02692-5 (2022).
Kaur, R., Khan, B. & Sharma, A. Optical coherence tomography of retinal artery occlusion associated with mucormycosis and COVID-19. JAMA Ophthalmol. 139 , e214064. https://doi.org/10.1001/jamaophthalmol.2021.4064 (2021).
Dubey, S. et al. COVID-19 associated rhino-orbital-cerebral mucormycosis: An observational study from Eastern India, with special emphasis on neurological spectrum. Diabetes Metab. Syndr. 15 , 102267. https://doi.org/10.1016/j.dsx.2021.102267 (2021).
Hufendiek, K. et al. Classification and characterization of acute macular neuroretinopathy with spectral domain optical coherence tomography. Int. Ophthalmol. 38 , 2403–2416. https://doi.org/10.1007/s10792-017-0742-9 (2018).
Fawzi, A. A. et al. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina 32 , 1500–1513. https://doi.org/10.1097/IAE.0b013e318263d0c3 (2012).
Fekri, S. et al. Acute macular neuroretinopathy and COVID-19 vaccination: Case report and literature review. J. Fr. Ophtalmol. 46 , 72–82. https://doi.org/10.1016/j.jfo.2022.09.008 (2023).
Powers, J. H. et al. Multimodal imaging of type 2 acute macular neuroretinopathy in a young woman. Digit. J. Ophthalmol. 27 , 44–47. https://doi.org/10.5693/djo.02.2021.06.004 (2021).
Gupta, N. et al. Acute macular neuroretinopathy (AMN) related to energy drink consumption. BMJ Case Rep. 12 , e232144. https://doi.org/10.1136/bcr-2019-232144 (2019).
Ashfaq, I. et al. Acute macular neuroretinopathy associated with acute influenza virus infection. Ocul. Immunol. Inflamm. 29 , 333–339. https://doi.org/10.1080/09273948.2019.1681470 (2021).
Dutta Majumder, P. & Agarwal, A. Acute macular neuroretinopathy and paracentral acute middle maculopathy during SARS-CoV-2 infection and vaccination. Vaccines (Basel) 11 , 474. https://doi.org/10.3390/vaccines11020474 (2023).
Radwan, L. M. et al. Acute macular neuroretinopathy associated with intravitreal anti-VEGF injection: A case report. Am. J. Ophthalmol. Case Rep. 28 , 101687. https://doi.org/10.1016/j.ajoc.2022.101687 (2022).
Guardiola, G. A. et al. Acute macular neuroretinopathy in dengue virus serotype 1. Am. J. Ophthalmol. Case Rep. 25 , 101250. https://doi.org/10.1016/j.ajoc.2021.101250 (2022).
Bos, P. J. & Deutman, A. F. Acute macular neuroretinopathy. Am. J. Ophthalmol. 80 , 573–584. https://doi.org/10.1016/0002-9394(75)90387-6 (1975).
Bhavsar, K. V. et al. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv. Ophthalmol. 61 , 538–565. https://doi.org/10.1016/j.survophthal.2016.03.003 (2016).
Lains, I. et al. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 84 , 100951. https://doi.org/10.1016/j.preteyeres.2021.100951 (2021).
Kulikov, A. N. et al. Retinal microvasculature alteration in paracentral acute middle maculopathy and acute macular neuroretinopathy: A quantitative optical coherence tomography angiography study. Retin. Cases Brief Rep. 14 , 343–351. https://doi.org/10.1097/ICB.0000000000000709 (2020).
Du, L., Kijlstra, A. & Yang, P. Vogt-Koyanagi-Harada disease: Novel insights into pathophysiology, diagnosis and treatment. Prog. Retin. Eye Res. 52 , 84–111. https://doi.org/10.1016/j.preteyeres.2016.02.002 (2016).
Chen, X. et al. Ocular adverse events after inactivated COVID-19 vaccination in Xiamen. Vaccines (Basel) 10 , 408. https://doi.org/10.3390/vaccines10030482 (2022).
Article CAS Google Scholar
Hoenigl, M. et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe 3 , e543–e552. https://doi.org/10.1016/S2666-5247(21)00237-8 (2022).
Download references
Acknowledgements
This work was supported by National Natural Science Foundation of China [81970792 and 82171040] and Medical Science and Technology Project of Health Commission of Henan Province [YXKC2020026]. The authors would like to thank Dr. Kang Chen for providing us free mf ERG test and thank Dr. Yubao Zheng in Nanyang Eye Hospital, Dr. Min Hou in Luoyang Central Hospital Affiliated to Zhengzhou University, Dr. Zhenzhen Liu in the Third People’s Hospital of Henan Povince, Dr. Jing Wang in The First Affiliated Hospital of Zhengzhou Universityand Dr. Shiqing Li, Dr. Qingge Guo, Dr. Xiao Chen, Dr. Xiaohong Guo, Dr. Changgeng Liu in Henan Eye Hospital for providing us fundus photos, OCT and FFA images.
Author information
Authors and affiliations.
Department of Ophthalmology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Guangqi An, Haixin Fang, Min Zhang, Lin Li, Yu Zhao, Xuemin Jin & Liping Du
Institute of Fundus Diseases, Zhengzhou University, Zhengzhou, Henan, China
Guangqi An, Min Zhang, Xuemin Jin & Liping Du
Henan Eye Hospital, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, Henan, China
Bo Lei & Zhili Wang
The Second People’s Hospital of Zhengzhou, Zhengzhou, Henan, China
Kaizhuan Yang
Department of Ophthalmology, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang, Henan, China
Dongsheng Fan
Nanyang Municipal Eye Hospital, Nanyang, Henan, China
Department of Ophthalmology, The First Affiliated Hospital of Nanyang Medical College, Nanyang, Henan, China
Eye institute, Henan Academy of Innovations in Medical Science, Zhengzhou, Henan, China
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design: G.A., L.D., X.J. Data collection: G.A., Z.W., K.Y., D.F., B.L, K.F., H.F., M.Z., Y.Z., H.C. Analysis and interpretation: G.A. Obtained funding: B.L., L.D., X.J. Overall responsibility: G.A., Z.W., L.L., B.L., L.D., X.J.
Corresponding authors
Correspondence to Xuemin Jin or Liping Du .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
An, G., Lei, B., Wang, Z. et al. Multicenter and multimodal imaging study reveals rare fundus lesions in patients after SARS-CoV-2 infection. Sci Rep 14 , 14369 (2024). https://doi.org/10.1038/s41598-024-65216-9
Download citation
Received : 02 March 2024
Accepted : 18 June 2024
Published : 22 June 2024
DOI : https://doi.org/10.1038/s41598-024-65216-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute macular neuroretinopathy
- Multimodal Imaging
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Comparison between surgical and non-surgical management of primary hyperparathyroidism during pregnancy: a systematic review
- Meta-Analysis
- Open access
- Published: 25 June 2024
Cite this article
You have full access to this open access article

- Shezifi Eli 1 , 2 ,
- Shlomo Gozlan Gal 3 &
- Zaina Adnan ORCID: orcid.org/0000-0001-7482-3104 1 , 4
Explore all metrics
The management of primary hyperparathyroidism (PHPT) during pregnancy may be surgical or conservative. This study compared adverse outcomes between surgical and non-surgical treatments. Additionally, the study investigated the correlation between serum calcium values and complication rates.
A systematic review of retrospective studies, case series, and case reports. Biochemical parameters, interventions, and outcomes of each pregnancy were recorded. The study population comprised two groups: the non-surgical and surgical groups. Adverse outcomes were categorized as maternal, obstetric, or neonatal.
The surgical and non-surgical groups consisted of 163 and 185 patients, respectively. A positive correlation was observed between the mean maternal gestational calcium value and both maternal and obstetric complication. Neonatal complications were more prevalent in patients treated conservatively across all maternal calcium values (p < 0.001). No significant differences were observed in maternal outcomes and overall obstetric outcomes between the study groups, albeit a higher mean serum calcium value in the surgical group (12.3 mg/dL) compared with the non-surgical group (11.1 mg/dL).
Conclusions
Given the significantly lower neonatal adverse outcomes in the non-surgical group compared to the surgical group, along with non-inferior maternal and obstetric outcomes in the surgical group, the overall data of this study suggest that parathyroidectomy is favorable to non-surgical management even in cases of mild hypercalcemia.
Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) is a relatively rare condition to encounter in women of reproductive age, with an estimated rate of 7.7–50 cases per 100,000 women of this population [ 1 , 2 ].
Pregnancy is characterized by physiological changes in calcium metabolism which must be considered: calcium absorption increases twofold by third trimester, driven by vitamin D and parathyroid hormone-related protein (PTHrP). The latter is synthesized during pregnancy from the amnion, as well as breasts to lesser extent, and other reproductive tissues [ 3 ]. PTHrP acts similarly to parathyroid hormone (PTH), promoting epithelial growth and tissue differentiation in the fetus and acting as the primary stimulus for the active placental calcium pump [ 3 ]. Total serum calcium values decrease during pregnancy due to the increased intravascular volume, calcium placental efflux, and increased urinary output. Finally, ionized calcium levels remain unchanged, and PTH falls to the low-normal range [ 4 , 5 , 6 , 7 ].
The etiology of gestational PHPT is most often a parathyroid adenoma, and less commonly parathyroid hyperplasia (5–10%) or parathyroid carcinoma (<1%) [ 8 , 9 ]. PHPT may also occur as part of an inherited syndrome, and genetic counseling has been recommended for patients younger than 40 carrying the disease [ 10 , 11 ].
PHPT may be managed conservatively or surgically. Conservative treatment is typically recommended for asymptomatic patients, or those with mild hypercalcemia, or as a bridge to surgery. It includes close follow-up, electrolyte, and hormonal monitoring, along with hydration alone in mild to moderate patients or additional pharmacological therapy in cases of refractory hypercalcemia [ 7 , 12 , 13 ]. The definitive treatment for this condition is parathyroidectomy, which can be performed via cervical exploration or minimally invasive parathyroidectomy (MIP). [ 9 , 14 ] Reports of successful ablation of the gland are also documented [ 15 , 16 , 17 ].
Data regarding pregnancy complication rates from gestational PHPT are variable. Norman et al. reported a significant difference in pregnancy loss in gestational PHPT [ 18 ], and citations across the literature claim complications of PHPT during pregnancy may reach as high as 67% of mothers and 80% of offspring [ 19 , 20 , 21 ]. However, more recent large cohorts have reported no increased risk of obstetric complications [ 22 , 23 ]. Although several studies have shown increased risk of complications in non-surgical treatment compared to surgical treatment, current guidelines suggest surgery only when the patient is either symptomatic or with hypercalcemia exceeding 1 mg/dL above the normal limits [ 7 , 24 ]. Similarly, surgical safety is controversial. While some studies conclude that surgery during pregnancy causes more adverse events than surgery on non-pregnant patients [ 25 , 26 ], others conclude that the risks of parathyroidectomy are minimal, hence surgery should not be delayed when necessary [ 27 , 28 , 29 , 30 ]. A recent Chinese expert consensus concluded surgery should be considered regardless of the stage of pregnancy when hypercalcemia appears hazardous to the patient or fetus [ 7 ]. In mind of the above controversies, the current study compared the complication rates between surgical and non-surgical managements, utilizing all the relevant published data available.
Study design
A literature search on PubMed, ScienceDirect (Elsevier), and Google Scholar was performed using the terms: “pregnancy” or “gestation”, “hyperparathyroidism”, “PHPT”, and “parathyroidectomy”. Database included published studies between 1980 and 2023. The study population comprised of women who experienced primary hyperparathyroidism (PHPT) during pregnancy and either underwent parathyroidectomy during pregnancy (surgical group) or were managed conservatively (non-surgical group). Recorded data included the patient’s age, biochemical values, obstetric and medical history, time of diagnosis, etiology, presenting symptoms, management, and complications, which were subsequently classified as maternal, obstetric, or neonatal. We excluded reports involving significant comorbidities, such as metastatic non-parathyroid cancer or any condition unrelated to PHPT severe enough to require management in the intensive care unit (ICU) during pregnancy, reports with missing data, and patients treated with alternative management not addressed in this article’s scope. We excluded cases with calcium values exceeding 15 mg/dl as these were deemed rare and complex, leading to higher complication rates and outcome bias. Additionally, we excluded records involving ectopic parathyroid adenomas, parathyroid carcinomas, and patients positive for multiple endocrine neoplasia type 1 (MEN1) as these rare cases were correlated with extended hospital stays, alternative management strategies, and complicated outcomes.
Data collection
The initial search yielded 777 articles, manually filtered to identify 259 relevant articles for screening. Following the screening process for duplications and exclusion criteria, 168 publications were selected, comprising 348 cases for data analysis (as depicted in the PRISMA flow diagram in Fig. 1 ). Each pregnancy was analyzed as a single case. Maternal, obstetric, and neonatal complications were considered as adverse outcomes that occurred solely during pregnancy or shortly after delivery. Hypercalcemia was defined as total serum calcium exceeding 10.5 mg/dL. Normal range for PTH were defined as 10–65 pg/mL. Calcium values utilized for analysis represented the average of total serum calcium values measured during pregnancy, as ionized calcium was not consistently reported in a significant number of articles. In cases where albumin levels were provided or corrected calcium values were available, the corrected values were utilized for analysis.

PRISMA flowchart indicating the process for identification and selection of the included studies. *Other reasons included records in which patients did not have primary hyperparathyroidism during pregnancy
Statistical analysis
Analysis was performed by the Beer-Sheva Faculty of Health Sciences in Israel using Windows GraphPad Prism version 10.2.2. Demographic data is expressed as either raw values or medians and interquartile ranges (IQR) when data did not follow a normal distribution. Statistical significance was assumed as p < 0.05. Differences in complication rates were calculated using the t-test was for quantitative data, and chi-square test for categorical data. Correlation between calcium rates and complication rates was calculated using Pearson’s correlation.
Out of the 348 cases analyzed, 163 (47%) underwent parathyroidectomy (the surgical group), while 185 (53%) were managed conservatively (the non-surgical group). The mean ages were 31 ± 3 and 31.5 ± 3.5 years, respectively. Average total calcium levels for the surgical group were higher, with a median of 12.3 mg/dL (range:11.5–13.3), compared to 11.1 mg/dL (range: 10.7–11.6) in the non-surgical group. Median PTH levels were 137 pg/mL (range: 94–237) and 123 pg/mL (range:71–224) for the respective groups. 61% of the surgical group underwent surgery during the second trimester, 25% during the third trimester, and 7% during the first trimester. (see Table 1 )
In the non-surgical group, most patients were treated using IV fluids. Data regarding specific therapeutic agents and their outcomes were missing for most cases; 18 patients received furosemide, an additional 18 received calcitonin and 15 patients received cinacalcet. Positive results to treatment were described in only 17 patients. Thus, statistically significant results regarding the efficacy of different pharmacological regimens could not be achieved.
Maternal, obstetric, and neonatal complication rates comparing the study arms are presented in Table 2 with the following results: Maternal complications occurred in 19.5% of the entire study population. No significant differences in maternal complication rates were observed between the study arms. The overall obstetric complications were not significantly different between study groups as well, however, subcategory analysis revealed higher rates of preeclampsia/eclampsia and preterm labor in the non-surgical group and higher rates of hyperemesis gravidarum in the surgical group. Rates of pregnancy loss were higher in the non-surgical group, with 15.3% compared to 1.3% in the surgical group. From a total of 22 cases of pregnancy loss, 10 cases described 1st-trimester miscarriage, and the rest did not specify further details. Numbers of neonatal complications were significantly higher in the non-surgical group compared to the surgical group. This difference was evident in transient neonatal hypocalcemia (24.4% vs. 2.7%), hypocalcemic tetany (10.7% vs. none), hypocalcemic convulsions (6.9% vs. none), and ICU admissions (9.9% vs. 3.3%), but not neonatal demise.
A statistically significant positive correlation was observed between serum calcium values and both maternal and obstetric complication rates (p < 0.05), but not neonatal complications (Table 3 ). Nevertheless, complication rates in the non-surgical group were significantly higher across all calcium levels compared with the surgical group (with p < 0.001, as illustrated in Fig. 2 ).

Comparison of neonatal complication rates according to calcium stratification between surgical and non-surgical groups. *p-value for the overall data shown is <0.001
Maternal complications
Maternal complications affected 19.5% of the study population in the current study, with no significant difference in complication rates between the surgical and non-surgical groups. Interestingly, maternal complications in the surgical group were not elevated despite the higher mean serum calcium levels. This outcome could potentially be attributed to parathyroidectomy. The variations in calcium levels between the study groups most likely stem from the selection of patients in the higher spectrum of mean serum calcium towards surgical intervention, rather than non-surgical management. Schnatz et al. hypothesized that adverse outcomes in patients selected for surgical intervention could stem from underlying long-term untreated disease rather than from surgery itself [ 31 , 32 ]. The risk of surgery has been reported to be minimal [ 28 , 33 , 34 , 35 ], with curative results in 95–98% of cases [ 9 , 14 , 36 , 37 ]. In the present study, the operation was curative in 98% of cases, with postoperative complications occurring in 4.9%. Specifically, three patients experienced hungry bone syndrome, three suffered from hypocalcemic tetany, one patient had permanent hypoparathyroidism, and one patient experienced transient vocal cord palsy.
Obstetric complications
Obstetric complications resulted in significant differences on a few parameters, namely, preterm delivery, preeclampsia, and pregnancy loss, which occurred at higher rates in the non-surgical group. Additionally, hyperemesis gravidarum occurred at higher rates in the surgical group, most likely due to the early diagnosis of their symptomatic disease and subsequent selection for surgical intervention (Table 2 ). Analysis of the data presented in this article suggests that the increase in pregnancy loss arises from losses that occurred in the first trimester and early second trimester before potential surgical intervention. Consequently, these instances were categorized under the non-surgical group. The pregnancy loss rate for the entire study population was 7.8%, lower than the documented 15% rate for women in the general population aged 30–34 [ 38 , 39 ]. Overall, the current study findings suggest that parathyroidectomy did not significantly alter the overall rates of obstetric complications when compared to non-surgical management, aligning with the conclusions reported previously by Hirsch et al. [ 23 ] and Abood and Vestergaard [ 22 ], however, it may potentially be associated to a reduced risk of preterm delivery and preeclampsia.
Neonatal complications
Neonatal adverse outcomes were significantly more prevalent in the non-surgical group than in the surgical group. The significant difference was evident across all maternal mean calcium values (Fig. 2 ). These results are supported by previously reported data [ 30 , 40 , 41 ]. Sandler et al. that revealed that even in asymptomatic PHPT, infant complications were less prevalent in the surgical group [ 41 ]. The variation in adverse outcomes between the groups primarily consisted of transient postpartum hypocalcemia, hypocalcemic tetany, convulsions, and subsequently, a higher number of ICU admissions. Neonatal hypocalcemia can be severe and prolonged, often necessitating long-term calcium supplementation [ 42 ], with median onset of clinical manifestations at the 11th day postpartum [ 7 ]. Although a significant correlation was observed between calcium values and maternal and obstetric adverse outcomes, no correlation was seen between neonatal complications and mean maternal gestational calcium values, indicating that neonatal adverse outcomes result from complex interactions beyond mean calcium values alone. Neonatal hypocalcemia is attributed to the suppression of parathyroid glands in utero. After birth, the neonate relies on kidney reabsorption and intestinal absorption, facilitated by an active PTH and calcitriol-dependent mechanism [ 3 ]. However, the suppressed parathyroid glands are unable to meet the increased demand, leading to hypocalcemia within the first days to weeks after delivery. During gestation, most calcium is actively transported through the placenta, regulated by PTHrP. 80% of mineral requirements reaches the fetus during the third trimester of pregnancy [ 7 , 43 ]. Notably, PTH itself does not cross the placenta, and it remains uncertain whether the hormone affects the transfer of calcium through the placenta based on animal models [ 3 ]. In this study, most patients in the surgical group underwent parathyroidectomy during the second trimester, indicating their mean gestational calcium during the third trimester remained within normal values. This observation could potentially account for the reduced risk of postpartum neonatal hypocalcemia and its sequelae in the surgical group. However, the limited number of cases specifying post-operative and subsequent gestational serum calcium values made it challenging to draw statistically significant conclusions. Similarly, due to data unavailability, it was impossible to compare the efficacy and outcomes between different non-surgical treatment regimens. Future prospective studies may investigate whether reduced third-trimester calcium values correlate with lower rates of neonatal complications, preferably utilizing ionized calcium, as this marker is more reliable during pregnancy, and compare it with the outcomes of non-surgical management.
Adverse neonatal outcomes were significantly fewer in the surgical group than in the non-surgical group. This difference was evident across all mean maternal gestational calcium values, suggesting that surgical intervention may yield superior neonatal outcomes, regardless of maternal calcium values. Maternal and overall obstetric complication rates did not significantly differ between the study groups. Additionally, the present study identified a positive correlation between maternal and obstetric outcomes and mean maternal gestational calcium levels, consistent with previously published findings. Given the superior neonatal outcomes alongside non-inferior maternal and obstetric outcomes, the overall data presented in this study suggest that parathyroidectomy is favorable over conservative treatment, even in cases of mild primary hyperparathyroidism.
Limitations
The primary limitation of this study was the compilation of a patient database from published sources comprising retrospective studies, case series, and case reports. This method introduced a selection bias toward more severe cases, typically reported in the literature. However, this bias is partially mitigated by including 249 cases identified through retrospective reviews. Nevertheless, it cannot be ruled out that asymptomatic patients and those with PHPT within the normocalcemic range may exhibit lower complication rates than those documented in this study.
Data availability
This article has utilized data from widely available, previously published data. All data supporting the findings of this study are available within the paper and its supplementary information which is available in an online repository ( https://doi.org/10.17632/6vjfskyvzc.2 ). The Search algorithm is depicted in Table 1 , and anonymous case data is in Table 2 of the online repository.
M.W. Yeh, P.H.G. Ituarte, H.C. Zhou, S. Nishimoto, I.-L. Amy Liu, A. Harari et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J. Clin. Endocrinol. Metab. 98 , 1122–1129 (2013). https://doi.org/10.1210/jc.2012-4022
Article CAS PubMed PubMed Central Google Scholar
S.H. Golden, K.A. Robinson, I. Saldanha, B. Anton, P.W. Ladenson, Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J. Clin. Endocrinol. Metab. 94 , 1853–1878 (2009). https://doi.org/10.1210/jc.2008-2291
C.S. Kovacs, The role of PTHrP in regulating mineral metabolism during pregnancy, lactation, and fetal/neonatal development. Clin. Rev. Bone Miner. Metab. 12 , 142–164 (2014). https://doi.org/10.1007/s12018-014-9157-6
Article CAS Google Scholar
J. Bollerslev, L. Rejnmark, A. Zahn, A. Heck, N.M. Appelman-Dijkstra, L. Cardoso et al. European expert consensus on practical management of specific aspects of parathyroid disorders in adults and in pregnancy: recommendations of the ESE Educational Program of Parathyroid Disorders (PARAT 2021). Eur. J. Endocrinol. 186 , R33–R63 (2021). https://doi.org/10.1530/EJE-21-1044
Article PubMed Central Google Scholar
H.L. Barrett, A. McElduff, Vitamin D and pregnancy: an old problem revisited. Best Pract. Res. Clin. Endocrinol. Metab. 24 , 527–539 (2010). https://doi.org/10.1016/j.beem.2010.05.010
Article CAS PubMed Google Scholar
C.S. Kovacs, H.M. Kronenberg, Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 18 , 832–872 (1997). https://doi.org/10.1210/edrv.18.6.0319
H. Zhong, Q. Liao, J. Liu, X. Chen, Y. Hu, S. Jian et al. Expert consensus on multidisciplinary approach to the diagnosis and treatment of primary hyperparathyroidism in pregnancy in China. Endocrine 82 , 282–295 (2023). https://doi.org/10.1007/s12020-023-03392-w
J.P. Bilezikian, M.L. Brandi, R. Eastell, S.J. Silverberg, R. Udelsman, C. Marcocci et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J. Clin. Endocrinol. Metab. 99 , 3561–3569 (2014). https://doi.org/10.1210/jc.2014-1413
R. Udelsman, G. Åkerström, C. Biagini, Q.-Y. Duh, P. Miccoli, B. Niederle et al. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 99 , 3595–3606 (2014). https://doi.org/10.1210/jc.2014-2000
S.M. Wilhelm, T.S. Wang, D.T. Ruan, J.A. Lee, S.L. Asa, Q.-Y. Duh et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 151 , 959–968 (2016). https://doi.org/10.1001/jamasurg.2016.2310
Article PubMed Google Scholar
A. McCarthy, S. Howarth, S. Khoo, J. Hale, S. Oddy, D. Halsall et al. Management of primary hyperparathyroidism in pregnancy: a case series. Endocrinol. Diabetes Metab. Case Rep. 2019 , 19–0039 (2019). https://doi.org/10.1530/EDM-19-0039
Article PubMed PubMed Central Google Scholar
A.N. DiMarco, K. Meeran, I. Christakis, V. Sodhi, C. Nelson-Piercy, N.S. Tolley et al. Seventeen cases of primary hyperparathyroidism in pregnancy: a call for management guidelines. J. Endocr. Soc. 3 , 1009–1021 (2019). https://doi.org/10.1210/js.2018-00340
F. Cetani, F. Saponaro, C. Marcocci, Non-surgical management of primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 32 , 821–835 (2018). https://doi.org/10.1016/j.beem.2018.09.006
C. Nastos, A. Paspala, I. Mavroeidi, F. Stavratis, V. Lampadiari, S. Kalantaridou et al. Surgical management of primary hyperparathyroidism during pregnancy: a systematic review of the literature. Gynecol. Endocrinol. 37 , 1086–1095 (2021). https://doi.org/10.1080/09513590.2021.1932801
S. Bansal, R.M. Kaushik, R. Kaushik, S. Modi, S. Raghuvanshi, A. Kusum, Primary hyperparathyroidism presenting as severe hypercalcemia with acute pancreatitis in pregnancy. Gynecol. Endocrinol. 36 , 469–472 (2020). https://doi.org/10.1080/09513590.2019.1698028
R. Pal, S.K. Bhadada, N. Gupta, A. Behera, N. Aggarwal, A. Aggarwal et al. Primary hyperparathyroidism in pregnancy: observations from the Indian PHPT registry. J. Endocrinol. Invest 44 , 1425–1435 (2021). https://doi.org/10.1007/s40618-020-01441-z
L. Zhang, Y.R. Luo, Y. Hu, Y. Zhai, H. Gao, Z. Cao, Primary hyperparathyroidism in pregnancy: insights from a case of a 28-year-old woman with miscarriages and hyperemesis gravidarum. Ann. Lab. Med. 41 , 336–338 (2021). https://doi.org/10.3343/alm.2021.41.3.336
J. Norman, D. Politz, L. Politz, Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin. Endocrinol. 71 , 104–109 (2009). https://doi.org/10.1111/j.1365-2265.2008.03495.x
L. Kohlmeier, R. Marcus, Calcium disorders of pregnancy. Endocrinol. Metab. Clin. North Am. 24 , 15–39 (1995). https://doi.org/10.1016/S0889-8529(18)30052-5
M. Som, J.S. Stroup, Primary hyperparathyroidism and pregnancy. Bayl Univ. Med. Cent. Proc. 24 , 220–223 (2011). https://doi.org/10.1080/08998280.2011.11928719
Article Google Scholar
F.L. Delmonico, R.M. Neer, A.B. Cosimi, A.B. Barnes, P.S. Russell, Hyperparathyroidism during pregnancy. Am. J. Surg. 131 , 328–337 (1976). https://doi.org/10.1016/0002-9610(76)90127-6
A. Abood, P. Vestergaard, Pregnancy outcomes in women with primary hyperparathyroidism. Eur. J. Endocrinol. 171 , 69–76 (2014). https://doi.org/10.1530/EJE-13-0966
D. Hirsch, V. Kopel, V. Nadler, S. Levy, Y. Toledano, G. Tsvetov, Pregnancy outcomes in women with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 100 , 2115–2122 (2015). https://doi.org/10.1210/jc.2015-1110
A.A. Khan, D.A. Hanley, R. Rizzoli, J. Bollerslev, J.E.M. Young, L. Rejnmark et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos. Int. 28 , 1–19 (2016). https://doi.org/10.1007/s00198-016-3716-2
S.Y. Huang, P.-H. Lo, W.-M. Liu, Y.-G. Cherng, C.-C. Yeh, T.-L. Chen et al. Outcomes after nonobstetric surgery in pregnant patients: a nationwide study. Mayo Clin. Proc. 91 , 1166–1172 (2016). https://doi.org/10.1016/j.mayocp.2016.06.021
S. Kuy, Outcomes following thyroid and parathyroid surgery in pregnant women. Arch. Surg. 144 , 399–406 (2009). https://doi.org/10.1001/archsurg.2009.48
M.C. Tolcher, W.E. Fisher, S.L. Clark, Nonobstetric surgery during pregnancy. Obstet. Gynecol. 132 , 395–403 (2018). https://doi.org/10.1097/AOG.0000000000002748
R. Cohen-Kerem, C. Railton, D. Oren, M. Lishner, G. Koren, Pregnancy outcome following non-obstetric surgical intervention. Am. J. Surg. 190 , 467–473 (2005). https://doi.org/10.1016/j.amjsurg.2005.03.033
V. Balinskaite, A. Bottle, V. Sodhi, A. Rivers, P.R. Bennett, S.J. Brett et al. The risk of adverse pregnancy outcomes following nonobstetric surgery during pregnancy: estimates from a retrospective cohort study of 6.5 million pregnancies. Ann. Surg. 266 , 260–266 (2017). https://doi.org/10.1097/SLA.0000000000001976
H. Jiao, L. Sun, Y. Liu, J. Zhou, X. Chen, J. Liu et al. Multidisciplinary team efforts to improve the pregnancy outcome of pregnancy complicated with primary hyperparathyroidism: case series from a single hospital. BMC Pregnancy Childbirth 21 , 576 (2021). https://doi.org/10.1186/s12884-021-04042-7
P.F. Schnatz, S.L. Curry, Primary hyperparathyroidism in pregnancy: evidence-based management. Obstet. Gynecol. Surv. 57 , 365–376 (2002). https://doi.org/10.1097/00006254-200206000-00022
P.F. Schnatz, S. Thaxton, Parathyroidectomy in the third trimester of pregnancy. Obstet. Gynecol. Surv. 60 , 672–682 (2005). https://doi.org/10.1097/01.ogx.0000180889.23678.fb
K.C. Kort, H.J. Schiller, P.J. Numann, Hyperparathyroidism and pregnancy. Am. J. Surg. 177 , 66–68 (1999). https://doi.org/10.1016/S0002-9610(98)00302-X
M. Nejdlova, T. Johnson, Anaesthesia for non-obstetric procedures during pregnancy. Contin. Educ. Anaesth. Crit. Care Pain 12 , 203–206 (2012). https://doi.org/10.1093/bjaceaccp/mks022
B.C. Visser, R.E. Glasgow, K.K. Mulvihill, S.J. Mulvihill, Safety and timing of nonobstetric abdominal surgery in pregnancy. Dig. Surg. 18 , 409–417 (2001). https://doi.org/10.1159/000050183
R. Udelsman, Z. Lin, P. Donovan, The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann. Surg. 253 , 585–591 (2011). https://doi.org/10.1097/SLA.0b013e318208fed9
J.M. Ruda, C.S. Hollenbeak, B.C. Stack, A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol. Neck Surg. 132 , 359–372 (2005). https://doi.org/10.1016/j.otohns.2004.10.005
R. Rai, L. Regan, Recurrent miscarriage. The Lancet 368 , 601–611 (2006). https://doi.org/10.1016/S0140-6736(06)69204-0
R.M. Silver, Fetal death. Obstet Gynecol 109 , 153–167 (2007). https://doi.org/10.1097/01.AOG.0000248537.89739.96
V. Dochez, G. Ducarme, Primary hyperparathyroidism during pregnancy. Arch. Gynecol. Obstet. 291 , 259–263 (2015). https://doi.org/10.1007/s00404-014-3526-8
M.L. Sandler, R. Ho, M.H. Xing, S. Gidumal, H. Spitzer, J.C. Levy et al. Primary hyperparathyroidism during pregnancy treated with parathyroidectomy: a systematic review. The Laryngoscope 131 , 1–7 (2021). https://doi.org/10.1002/lary.29489
H.A. Korkmaz, B. Özkan, D. Terek, C. Dizdarer, S. Arslanoğlu, Neonatal seizure as a manifestation of unrecognized maternal hyperparathyroidism. J. Clin. Res. Pediatr. Endocrinol. 5 , 206–208 (2013). https://doi.org/10.4274/Jcrpe.1037
P. Pothiwala, S.N. Levine, Parathyroid surgery in pregnancy: review of the literature and localization by aspiration for parathyroid hormone levels. J. Perinatol. 29 , 779–784 (2009). https://doi.org/10.1038/jp.2009.84
Download references
Open access funding provided by Bar-Ilan University.
Author information
Authors and affiliations.
Bar-Ilan University, The Azrieli Faculty of Medicine, Safed, Israel
Shezifi Eli & Zaina Adnan
Laniado Hospital, Netanya, Israel
Shezifi Eli
Department of Physiology and Cell Biology, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Shlomo Gozlan Gal
Division of Endocrinology and Metabolism, Clalit Medical Health Care Services, Haifa and Western Galilee District, Zvulon Medical Center, Haifa, Israel
Zaina Adnan
You can also search for this author in PubMed Google Scholar
Contributions
The authors E.S. and A.Z. formulated the study conception and design. The author E.S. screened and collected the data for analysis. The author S.G.G. performed the statistical analysis and prepared the tables and figures. All authors reviewed the results and formulated the conclusions for the draft manuscript. The author E.S. wrote the manuscripts. The author A.Z. commented on previous versions and reviewed the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Zaina Adnan .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental table 1, supplemental table 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Eli, S., Gal, S.G. & Adnan, Z. Comparison between surgical and non-surgical management of primary hyperparathyroidism during pregnancy: a systematic review. Endocrine (2024). https://doi.org/10.1007/s12020-024-03930-0
Download citation
Received : 27 March 2024
Accepted : 11 June 2024
Published : 25 June 2024
DOI : https://doi.org/10.1007/s12020-024-03930-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Primary hyperparathyroidism
- hypercalcemia
- Parathyroidectomy
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 19 June 2024
The experıance of tertıary center for adult granulosa cell tumor: whıch factors predıct survival?
- Mustafa Şahin 1 ,
- Tufan Arslanca 1 ,
- Yeşim Özkaya Uçar 1 ,
- Gülşah Tiryaki Güner 1 ,
- İlker Selçuk 1 &
- Hakan Raşit Yalçın 1
Journal of Ovarian Research volume 17 , Article number: 127 ( 2024 ) Cite this article
159 Accesses
Metrics details
This retrospective study aims to evaluate the clinical course and long-term outcomes of patients diagnosed with adult granulosa cell tumors (AGCT).
The study analyzed a cohort of 112 AGCT patients with a median follow-up of 87 months. Data regarding disease-free survival (DFS), overall survival (OS), recurrence rates, and prognostic factors were collected and analyzed. Surgical interventions, including lymphadenectomy and cytoreductive surgery, were assessed for their impact on outcomes.
The study revealed favorable long-term outcomes, with a 5-year DFS of 85% and a 10-year DFS of 83%. Additionally, a 5-year OS of 100% and a 10-year OS of 96% were observed. Recurrence occurred in 13.4% of cases, with advanced stage and positive peritoneal cytology identified as independent poor prognostic factors for DFS. Lymph node involvement was rare, and routine lymphadenectomy did not improve outcomes. Conservative surgery showed comparable DFS rates to definitive surgery in early-stage disease. However, cytoreductive surgery was crucial for advanced and recurrent tumors, with complete tumor resection enhancing survival outcomes.
The study underscores the importance of vigilant follow-up and individualized treatment strategies for AGCT patients. Despite the retrospective nature of the analysis, the substantial patient cohort and meticulous surgical interventions contribute valuable insights into AGCT management. Prospective multicenter studies are warranted to further elucidate prognostic factors and optimize treatment approaches for this rare malignancy.
Granulosa cell tumors are the most common malignant sex cord stromal tumors (SCST), accounting for approximately 2–5% of all ovarian malignancies with an incidence rate of approximately 0.4–1.7/100.000 [ 1 ]. Granulosa cell tumors are divided into two histological types; adult granulosa cell tumors (AGCT) and juvenile granulosa cell tumors. Approximately 95% of all patiens possess the adult variant. Although, AGCT is usually diagnosed in the premenopausal or early postmenopausal period, it can be seen at younger ages as well. AGCT has a favorable prognosis and shows a slow clinical course. The 5-year overall survival rate is 75–95% in stage I, 55–75% in stage II, and 22–50% in stage III/IV. The tumor stage is the most significant factor associated with oncologic outcome [ 1 , 2 ]. Recurrences in AGCT are mostly multifocal and the most common site of recurrence is the pelvis. Recurrence rates range from 6 to 48% and 50–80% of patients who have a mortal course. Recurrences are most common within 5 years following surgery, while late recurrences can be observed after 30–40 years [ 3 , 4 ]. Due to the slow growth of the tumor and hormonal symptoms, most patients are diagnosed in the early stages [ 5 ].
The primary treatment for AGCT is surgery, which can be curative in the early stages. In the postmenopausal period, the standard treatment is a hysterectomy and a bilateral salpingo-oophorectomy. In early-stage patients who wish to preserve their fertility, conservative surgery that preserves the other ovary and uterus can be performed [ 6 ]. The role of surgical staging in the treatment of AGCT is still unclear. The benefit of lymphadenectomy is controversial and only the removal of suspicious lymph nodes is recommended [ 7 ].
The National Comprehensive Cancer Network (NCCN) guideline recommends adjuvant chemotherapy in advanced stages. However, there is no evident consensus on adjuvant treatment in stage 1C [ 8 ]. The role of adjuvant chemotherapy in the treatment of primary or recurrent disease in AGCT is still unclear [ 2 ].
The rarity of AGCT makes it difficult to recognize the prognostic factors, predict the oncologic outcomes and determine the appropriate treatment. In this study, we aimed to investigate the clinicopathologic prognostic factors affecting the recurrence and survival in AGCT patients.
Materials and methods
The data of 112 patients who have been diagnosed and treated for AGCT between the years of January 2004 and August 2019 in the gynecologic oncology clinic were retrospectively evaluated. Data was obtained from the electronic database system, patient files, pathology reports and operative notes. Ethics committee approval has been obtained for the study (decision number 14 dated April 27, 2021).
The International Federation of Gynecology and Obstetrics (FIGO) 2014 staging system was used for staging [ 9 ]. Those operated on before 2014 were re-staged according to 2014 FIGO criteria, by re-evaluating the pathology reports. The extent of the first operation was evaluated according to the extent of the disease and the desire for fertility. The surgical procedure, in which at least part of the one ovary and uterus were preserved, was defined as “conservative surgery”. In our center, conservative treatment is applied to patients with stage IA, 1B and stage IC1 according to the 2014 FIGO staging system, and to patients with fertility potential and close follow-up opportunity. Patients with fertility desires underwent unilateral salpingo-oophorectomy without hysterectomy. “Definitive surgery” was defined as hysterectomy and bilateral salpingo-oophorectomy. The inclusion of lymphadenectomy and omentectomy in the surgical procedure was determined by the senior surgeon. The upper limit of para-aortic lymphadenectomy was the left renal vein. When evidence of a more extensive disease existed, cytoreductive surgical techniques were used, as well as, staging surgery.
The adjuvant treatment decision was made by the gynecologic oncology council. In our hospital, bleomycin, etoposide and cisplatin (BEP; 3 or 4 cycles) are most commonly preferred in the adjuvant treatment of AGCT, and platinum-based chemotherapy regimens such as carboplatin/ paclitaxel (CP; 6 cycles) or etoposide/cisplatin (EP; 6 cycles) are also used. Chemotherapy response in patients was evaluated according to RECIST 1.1 criteria [ 10 ]. Clinical responses were defined as follows: (a) Complete clinical response (CCR): Complete disappearance of lesions and absence of new lesions; (b) Partial clinical response (PCR): A reduction in the size of lesions by at least 30%; (c) Progressive disease (PD): A ≥ 20% increase in the maximum diameter of the lesion, the appearance of a new lesion ≥ 1 cm, or progression of a non-target lesion; (d) Stable disease (SD): Lesions that were neither in the partial clinical response group, nor in the progressive disease group, based on the smallest overall diameters at the time of the study. The clinical response of patients was evaluated 1 month after the first treatment (surgery + adjuvant treatment) using clinical, laboratory parameters and imaging methods.
After treatment, patients were followed up every 3 months for the first 2 years, every 6 months until the fifth year and annually thereafter. We defined recurrences distal to the pelvic inlet as pelvic recurrence, recurrences between the pelvic inlet and diaphragm as upper-abdominal recurrence, and other recurrences as extra-abdominal recurrence. Cytologically defined ascites and peritonitis carcinomatosa were considered as upper-abdominal recurrence, and recurrence in the liver parenchyma was evaluated as extra-abdominal recurrence.
Statistical analysis
Statistical analysis was performed using SPSS version 22 (IBM, Chicago, USA). Disease-free survival (DFS) was defined as the time from operation until recurrence/progression of disease or last contact in those who did not develop recurrence. Overal survival (OS), was defined as the time from disease to death or last contact. The Kaplan–Meier method was used for survival analysis and differences were analyzed by the log-rank test. Factors with a p value less than 0.05 in the univariate analysis were included in the multivariate analysis. The Cox regression model was used in the multivariate analysis. The cut-off point for statistical significance was set as p value less than 0.05.
Clinical, surgical, and pathological features
The mean age of the 112 patients that constituted the study group was 50.3 ± 12.57 years and ranged between 17 and 81 years of age. The mean age of the 13 patients (11.6%) that underwent conservative surgery was 30.6 ± 7.6 years and ranged between 17 and 43 years of age.
Abdominal or pelvic pain (24.1%) and palpable adnexal mass (21.4%) were the most frequently reported presenting symptoms, followed by abdominal distention (18.8%) and vaginal bleeding (17.9%). Other less common symptoms accounted for 7.1% of cases. Only 12 patients (10.7%) were asymptomatic and were diagnosed incidentally during investigations conducted for non-gynecologic reasons. The mean tumor size was 89.6 ± 55.76 mm and ranged between 10 and 300 mm. Lymphadenectomy was performed in 94 of the patients (83.9%). Pelvic and para-aortic pelvic lymph node dissection was performed in 91 patients (81.3%), and only pelvic lymph node dissection was performed in 3 patients (2.6%). The mean number of lymph nodes removed in these patients was 51 ± 26.27 and ranged between 7 and 132. Three (3.2%) of the patients who underwent lymphadenectomy had positive lymph node metastasis and the metastases were in the pelvic lymph nodes. One hundred-four (92.8%) patients were stage I, 3 (2.7%) were stage II, 4 (3.6%) were stage III, and 1 (0.9%) was stage IV. Preoperative cyst rupture was detected in 2 (1.8%) patients, and 17 (15.2%) patients had intraoperative cyst rupture. Peritoneal cytology revealed malignancy in 12 (10.7%) of the patients and 4 (4%) had metastases in the omentum. The average time from the diagnosis of the disease to the first surgery of 112 patients was 15.2 ± 10.3 days and ranged from 1 to 54 days. Complementary surgery was performed in 35 (31.2%) patients after the first surgery. The average time from diagnosis of the disease to completion surgery is 45.7 ± 11.5 days and varies between 23 and 67 days. No residue tumor was observed in all patients during initial surgery (Table 1. ).
Adjuvant treatment and survival analysis
Adjuvant chemotherapy was administered to 30 patients. Of these, 22 were stage IC and 8 were stage 2–4. Of these, 17 patients (64.9%) received BEP, and 13 (35.3%) received others as adjuvant therapy (12 patients CP and 1 patient EP). While the 5-year DFS was 68% in the group receiving BEP, it was 59% in the other group ( p = 0.773). It was observed that adjuvant treatment types did not determine DFS. Thirty patients received adjuvant therapy, with a 5-year disease-free survival (DFS) rate of 64%. Conversely, 82 patients did not receive adjuvant therapy, and their 5-year DFS rate was 94%. The 5-year DFS rate significantly decreased in patients receiving adjuvant therapy compared to those who did not (64% vs. 94%; p < 0.001) (Table 2 ).
The median follow-up period of the patients was 87 months and ranged between 4 and 215 months. During this period, it was observed that 15 (13.4%) patients developed recurrence and 3 (2.7%) died becouse of the disease. Of the patients included in the study, the 5-year DFS was 85%, 10-year DFS was 83%. 5-year OS was 100% and the 10-year OS was 96%.
In the univariate analysis, positive peritoneal cytology, advanced stage and receiving of adjuvant treatment were associated with poor DFS. The 5-year DFS decreased from 93 to 56% in patients with positive peritoneal cytology ( p = 0.001) (Fig. 1 ). The 5-year DFS which was 91% in stage 1, was 13% in stages 2–4 ( p < 0.001) (Fig. 2 ). 5-year DFS significantly reduced in those receiving adjuvant therapy (respectively, 64% vs . 94%; p < 0.001) (Table 2. ). However, this relationship was thought to be related to the stage of the disease, as treatment was mostly given to those experiencing stage IC-IV disease. In stage 1, 73.3% ( n = 22/104) of patients received adjuvant treatment and all of them were in stage IC, whereas this rate was 100% ( n = 8/8) in stages 2–4 ( p < 0.001). Statistical analysis could not be performed for OS, as death due to disease occurred in only 3 patients.

Cancer-specific survival of patients with granulosa cell tumors by peritoneal cytology

Cancer-specific survival of patients with granulosa cell tumors by FIGO stage
Since adjuvant treatment was significantly correlated with stage, a model was created using peritoneal cytology and stage for multivariate analysis. Accordingly, stage 2–4 and positive peritoneal cytology were found to be independently poor prognostic factors for recurrence (respectively, odds ratio (OR) = 114.042, 95% confidence interval (CI) = 19.415–669.883, p < 0.001 and OR = 4.251, 95% CI = 1.125–16.072, p = 0.033) (Table 2. ).
Recurrence pattern
Of the 15 patients with recurrence, 7 (46.7%) had recurrence only in the pelvic region, 3 (20%) only in the upper abdominal region, 4 (26.6%) in the pelvic and upper abdominal region, and 1 (6.6%) in the upper abdominal and extra-abdominal region. Recurrences were observed as focal in 10 (66.6%) and as multifocal in 5 (33.3%) patients. The mean time for recurrence was 29 months and ranged between 9 and 86 months. Eight of the patients were in stage 1 and 7 were in stages 2–4.
Conservative surgery was applied in 1 patient (patient no: 1) at initial surgery. In addition adjuvant chemotherapy was given to 11 patients after initial surgery. Lymph node metastasis was present in 3 patients, omental metastasis in 4 patients and peritoneal cytology showed malignancy in 5 patients. Tumor-cyst rupture was present in 3 of the patients (Table 3 ).
It has been determined that after the first recurrence, 14 (93.3%) of these patients underwent secondary cytoreductive surgery, followed by salvage chemotherapy. No residue tumor was observed in these patients after secondary cytoreduction. CCR was obtained with salvage treatment in all of these 14 patients. One patient, who developed extra-abdominal recurrence, received salvage chemotherapy and external radiotherapy ( patient no: 9 ). After 18 months of follow-up, no recurrence has been observed yet in this patient. In finally, all patients with first recurrence CCR was achieved with salvage treatments after the first recurrence (Table 3 ).
It has been determined, that 6 of the patients developed a second recurrence and 5 patients with the secondary recurrence underwent tertiary cytoreductive surgery, followed by salvage chemotherapy (Table 4 ). No residue tumor was observed in these 5 patients after tertiary cytoreduction. One patient with extra-abdominal recurrence received salvage chemotherapy and external radiotherapy ( patient no 10 ). CCR was achieved in 4 patients following salvage treatment after the second recurrence. Two patients with omental and extra-abdominal recurrence died due to progressive disease; the follow-up periods of these patients were 52 and 154 months after initial surgery, respectively ( patient no 2, and 10, respectively ).
Furthermore, 3 of the 4 patients, with whom CCR was obtained after the second recurrence, developed a third recurrence and 2 of the patients underwent quaternary cytoreductive surgery, followed by salvage chemotherapy. No residue tumor was observed in these patients after quartenary cytoreduction. CCR was achieved in these two patients. The patient who were given salvage chemotherapy and external radiotherapy for extra-abdominal recurrence died due to progressive disease ( patient no 7 ) (Table 4 ).
The clinical course of AGCT progresses slowly and the prognosis is good. Mangili et al. have reported 5-year DFS as 91.5%, 10-year DFS as 71.6% and 5-year OS as 97%, 10-year OS as 95% [ 11 ]. In our study with a median follow-up period of 87 months, 5-year DFS was 85%, 10-year DFS was 83% and 5-year OS was 100%, 10-year OS was 96%. Recurrence was observed in 13.4% of the patients. In multivariate analysis, advanced stage and positive peritoneal cytology were independent poor prognostic factors for DFS. Recurrence was 114 times higher in patients with stage 2–4 compared to stage I and 4.2 times higher in patients with positive peritoneal cytology compared to those with negative peritoneal cytology.
Stage is a well-defined prognostic factor associated with recurrence and survival in ACGT. Schumer et al. have reported 5-year OS to be 75–95% in stage I, 55–75% in stage II, 50% in stage III and 22% in stage IV [ 1 ]. Karalok et al. have reported that 5-year DFS was 96% in stage I, 70% in stage III and 50% in stage IV [ 6 ]. In our study, the 5-year DFS, which was 91% in stage 1, decreased to 13% in stages 2–4.
Guidelines from ESGO, SIOPE, and ESMO currently recommend the BEP regimen as the most commonly used regimen for advanced and recurrent AGCTs [ 12 ]. However, response rates for the conventional combination of bleomycin in recent studies are only between 22 and 35% [ 13 ]. The carboplatin/paclitaxel combination is emerging as a less toxic alternative to BEP.
Adjuvant chemotherapy is advocated especially in advanced stages and macroscopic residual disease [ 2 , 10 , 11 ]. Adjuvant chemotherapy may also be considered for extensive inoperable disease or recurrent disease. However, despite the high survival rate in AGCT, the role of adjuvant chemotherapy in the early stages is unclear. According to a recent meta-analysis, the administration of adjuvant chemotherapy did not improve the oncological and prognostic outcomes of AGCT , regardless of whether the patients had early or advanced/recurrent disease [ 13 ].
The development of new targeted drugs in conjunction with molecular studies in adjuvant treatment may increase the survival rates. Among the targeted drugs investigated for AGCT, antiangiogenic drugs have garnered attention. In a study by Tsoi et al., Bevacizumab (a monoclonal anti-VEGF-A antibody) treatment demonstrated reduced tumor growth and prolonged survival in AGCT [ 14 ]. But, in an randomized clinical trial of patients with relapsed SCST, adding bevacizumab to paclitaxel did not benefit [ 15 ]. New targeted approaches, such as tumor necrosis factor-related apoptosis-inducing ligand, FOXL2'nin (Forkhead box L2), nuclear factor kappa B (NF-kB), phosphatidyl inositol-3-kinase serine/threonine kinase pathway, and mammalian target of rapamycin (mTOR), may prove effective in treating AGCT [ 16 ].
Another adjuvant treatment option is radiotherapy. Evans et al.'s study found that radiotherapy had no significant effect on the relapse rate, with relapse occurring in 20% of patients receiving radiotherapy [ 17 ]. Similarly, Ohel et al. were unable to demonstrate any advantage in the use of radiotherapy for AGCT [ 18 ]. However, contrasting these findings, more recent and comprehensive studies have indicated that adjuvant radiotherapy (RT) can prolong survival in patients with advanced or recurrent AGCT disease. In the study by Hauspy et al., adjuvant RT resulted in a significantly longer disease-free survival (DFS) [ 19 ]. Moreover, in a recent comprehensive review by Barcellini et al., RT has shown promise and feasibility for unresectable AGCT and recurrent diseases [ 20 ]. The efficacy of radiotherapy in AGCT is not well defined due to limited data.
Positive peritoneal cytology is a controversial prognostic factor in AGCT. Especially in stage I (IC), it makes receiving adjuvant chemotherapy controversial. Lee et al. have found the positive peritoneal cytology rate of 11.8% in AGCT [ 21 ]. This rate was 10.7% in our study. In the studies presented by Lee et al. and Björkholm et al. peritoneal cytology positivity was found to be significant in terms of recurrence [ 9 , 22 ]. In our study, the probability of recurrence was increased 4.2-fold in patients with positive peritoneal cytology and 5-year DFS decreased from 93 to 56%. On the contrary, in the studies of Park et al. and Ertas et al. no correlation has been demonstrated between peritoneal cytology positivity and recurrence [ 2 , 23 ].
The incidence of lymph node metastasis at primary surgery in AGCT is low. Wang et al. have reported the incidence of lymph node metastasis as a 3.9% [ 24 ]. In our study, the rate of lymph node involvement was 3.2%. The addition of lymphadenectomy to the surgical procedure did not improve oncological outcomes. Similarly, Erkılınç et al. have also reported that lymphadenectomy did not lead to improvement in DFS and OS and, on the contrary, increased postoperative morbidity [ 25 ]. Abu-Rustum et al. have reported an isolated nodal recurrence rate of 5.9% and suggested that recurrences may be due to occult nodal metastases that were not detected at the time of the initial diagnosis [ 26 ]. Nevertheless, in the study presented by ourselves, no lymphatic recurrence was detected in any of the 15 patients with recurrence. In conclusion, removal of only suspicious lymph nodes rather than routine lymphadenectomy is the preferred surgical approach in AGCT.
Surgery is the primary treatment option for newly diagnosed or recurrent AGCT. However, the limits of primary surgery are not clear. Definitive surgery for early-stage primary tumors has been demonstrated to provide no survival or recurrence advantage compared with conservative surgery. The indications and the prognosis of the conservative approach are controversial [ 27 ]. In our study, conservative surgery did not worsen DFS rates when compared to definitive surgery. As for the advanced stage and recurrent tumors on the other hand, cytoreductive surgery is the most effective treatment method [ 8 ]. Sun et al. have stated that 85% of the patients with residual tumors developed recurrence [ 27 ]. In both primary and recurrent disease surgery, cytoreduction, having the goal of leaving no residual tumor, is important in terms of recurrence and DFS.
Due to the rarity of the disease, surgical experience data for AGCT recurrence is limited and there exists no consensus on how to choose the treatment. In the study by Lee et al. and Abu Rustum et al. most of the recurrences were intraperitoneal and 70% were pelvic [ 19 , 21 ]. In our study, 93.3% of the primary recurrences were pelvic and intraabdominal and 6.6% were extra-abdominal. Recurrences in AGCT are usually focal and localized in one region [ 16 ]. In the study we presented, 66.6% of recurrences were focal and 33.3% were multifocal. This facilitates to avoid leaving residual tumor in salvage cytoreduction. Mangili et al. have reported that optimal debulking surgery is an effective treatment in case of recurrence [ 11 ]. However, recurring recurrences may develop during follow-up. In our study, surgeries have been performed on 14 out of 15 patients with recurrence, 4 out of 6 patients with second recurrence and 2 out of 3 patients with third recurrence without leaving residual tumor and complete clinical response has been obtained in all patients with the treatments offered. Whereas, 2 patients who could not undergo surgery due to extensive widespread disease died due to progressive disease in the second and third recurrence. In recurrent AGCTs, complete resection of the tumor determines survival outcomes [ 13 ]. If complete resection of the tumor can be achieved with salvage cytoreductions in recurrences, complete clinical response can be obtained in such patients.
Maximal cytoreductive surgery forms the cornerstone of treatment for primary and recurrent GCT. In cases of suboptimal surgical outcomes or unresectable metastatic disease, chemotherapy is commonly employed. However, there is limited data available on the use of adjuvant chemotherapy following complete cytoreductive surgery at recurrence [ 28 , 29 ]. Surgery is recommended for patients with relapse according to ESGO guidelines. If the patient who has undergone complete debulking has not received chemotherapy afterward, follow-up or chemotherapy may be recommended. If one has received chemotherapy, the first option after surgery is follow-up, and the second is chemotherapy [ 30 ]. Yumru-Celiksoy et al. found that no benefit was derived from adjuvant systemic treatment, of any type, following complete cytoreductive surgery in patients with GCT-relapse [ 31 ]. A study by Memorial Sloan Kettering showed that chemotherapy did not improve the recurrence-free interval of patients with GCTs, even though also non-tumor-free operated patients were included [ 32 ]. In the multicenter retrospective MITO-9 study, further relapses were observed in 33% of patients who underwent surgery alone versus 37.5% of patients who underwent secondary cytoreductive surgery followed by chemotherapy. Mangili et al. noted that postoperative residual tumor was the only risk factor for decreased survival [ 11 ]. If surgery is performed without a tumor with repeated cytoreductive surgeries, the toxicity of unnecessary subsequent chemotherapy should be avoided [ 33 ].
İnhibin is secreted by granulosa cell tumors and is a useful tumor marker that falls after tumor removal and is also a marker for tumor recurrence. CA125 is not increased in GCTs, but sometimes it is useful in detecting relapse in those with values of Alfa-fetoprotein (AFP) / Beta-Human Chorionic Gonadotropin (β-hCG) within the normal range [ 25 ]. The production of estradiol by AGCT varies widely, and its value as a tumor marker is limited [ 34 ]. In the study by Haltia et al., HE4 and CA125 levels in AGCT patients were generally found to be below normal reference limits [ 35 ]. Rey et al. demonstrated that serum AMH can be considered as a marker for the diagnosis of ovarian AGCT [ 36 ]. Robertson et al. showed that inhibin levels were not elevated in all patients with AGCT and that serum inhibin was not specific for the diagnosis of AGCT [ 37 ]. Although the hormonal activity of ovarian AGCT suggests that the synthesized hormones may serve as tumor markers, the use of these tumor markers they have limited use in diagnosis and follow-up.
The retrospective nature of the study is the most important disadvantage. The relative high number of patients, a follow-up period of approximately 90 months, lymphadenectomy in 83.9% of the patients and the fact that the surgeries were performed without leaving residual tumors constitute the strengths of the study. In the present study, all the procedures have been performed by gynecooncologists and the pathology specimens have been evaluated by gynecopathologists as well.
Advanced stage and peritoneal cytology are factors associated with survival and recurrence in AGCT. For appropriate eligible patients, offering of fertility-sparing approach at an early stage is a safe choice. Removal of suspicious lymph nodes should be preferred over systematic lymph node dissection. Most recurrences are curable with surgery and completion of surgery without leaving any residuals is the most important factor for survival. Since AGCT is rare and recurrence can occur at any stage; prospective, randomized, well-controlled and multicenter studies are required to clarify the prognostic factors.
Availability of data and materials
Data is provided within the manuscript or supplementary information files.
Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21(6):1180–9.
Article PubMed Google Scholar
Park JP, Jin KL, Kim DY, Kim JH, Kim YM, Kim KR, et al. Surgical staging and adjuvant chemotherapy in the management of patients with adult granulosa cell tumors of the ovary. Gynecol Oncol. 2012;125(1):80–6.
McConechy MK, Färkkilä A, Horlings HM, Talhouk A, Unkila-Kallio L, Van Meurs HS, et al. Molecularly defined adult granulosa cell tumor of the ovary: the clinical phenotype. J Natl Cancer Inst. 2016;108:11.
Article Google Scholar
East N, Alobaid A, Goffin F, Ouallouche K, Gauthier P. Granulosa cell tumour: a recurrence 40 years after initial diagnosi. J Obstet Gynaecol Can. 2005;27:363–4.
Färkkilä A, Haltia UM, Tapper J, McConechy MK, Huntsman DG, Heikinheimo M, et al. Pathogenesis and treatment of adult-type granulosa cell tumor of the ovary. Ann Med. 2017;49(5):435–47.
Karalok A, Turan T, Ureyen I, Tasci T, Basaran D, Koc S, et al. Prognostic factors in adult granulosa cell tumor: a long follow-up at a single center. Int J Gynecol Cancer. 2016;26(4):619–25.
Seagle BLL, Ann P, Butler S, Shahabi S. Ovarian granulosa cell tumor: a National Cancer Database study. Gynecol Oncol. 2017;146(2):285–91.
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020. NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:191–226.
Article CAS PubMed Google Scholar
Prat J. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26(2):87.
Article CAS PubMed PubMed Central Google Scholar
Schwartz LH, Litiere S, De Viries E, Ford R, Gwyther S, Mandrekar S, Shankar L, et al. RECIST 1.1—update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
Article PubMed PubMed Central Google Scholar
Mangili G, Ottolina J, Gadducci A, Giorda G, Breda E, Savarese A. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer. 2013;109:29–34.
Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:1–18.
Van Meurs HS, Buist MR, Westermann AM, Sonke GS, Kenter GG, Van Der Velden J. Effectiveness of chemotherapy in measurable granulosa cell tumors. A retrospective study and review of literature. Int J Gynecol Cancer. 2014;24:496–505.
Tsoi M, Lague MN, Boyer A, et al. Anti-VEGFA therapy reduces tumor growth and extends survival in a murine model of ovarian granulosa cell tumor. Transl Oncol. 2013;6:226–33.
Ray-Coquard I, Harter P, Lorusso D, Dalban C, Vergote I, Fujiwara K, et al. Efect of weekly paclitaxel with or without bevacizumab on progression-free rate among patients with relapsed ovarian sex cord-stromal tumors: the ALIENOR/ENGOT-ov7 randomized clinical trial. JAMA Oncol. 2020;6(12):1923–30.
Zhuang Y, Zhang S, Liu Y, Yang H. Can adjuvant chemotherapy improve the prognosis of adult ovarian granulosa cell tumors?: A narrative review. Medicine. 2022;101:11.
Evans AT 3rd, Gaffey TA, Malkasian GD Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55(2):231–8.
PubMed Google Scholar
Ohel G, Kaneti H, Schenker JG. Granulosa cell tumors in Israel: a study of 172 cases. Gynecol Oncol. 1983;15(2):278–86.
Hauspy J, Beiner ME, Harley I, Rosen B, Murphy J, Chapman W, et al. Role of adjuvant radiotherapy in granulosa cell tumors of the ovary. Int J Radiat Oncol Biol Phys. 2011;79(3):770–4.
Barcellini A, Mangili G, Fodor A, Secondino S, Zerbetto F, Charalampopoulou A, et al. Granulosa cell tumors (GCTs) of the ovary: what is the role of radiotherapy? Crit Rev Oncol Hematol. 2023;181:103889.
Lee IH, Choi CH, Hong DG, Song JY, Kim YJ, Kim KT, et al. Clinicopathologic characteristics of granulosa cell tumors of the ovary: a multicenter retrospective study. J Gynecol Oncol. 2011;22(3): 188.
Björkholm E, Silfverswärd C. Prognostic factors in granulosa-cell tumors. Gynecol Oncol. 1981;11(3):261–74.
Ertas IE, Gungorduk K, Taskin S, Akman L, Özdemir A, Goklu R, et al. Prognostic predictors and spread patterns in adult ovarian granulosa cell tumors: a multicenter long-term follow-up study of 108 patients. Int J Clin Oncol. 2014;19(5):912–20.
Wang D, Cao D, Jia C, Huang H, Yang J, Wu M, et al. Analysis of oncologic and reproductive outcomes after fertility-sparing surgery in apparent stage I adult ovarian granulosa cell tumors. Gynecol Oncol. 2018;151(2):275–81.
Erkılınç S, Taylan E, Karataşlı V, Uzaldı İ, Karadeniz T, Gökçü M, et al. Does lymphadenectomy effect postoperative surgical morbidity and survival in patients with adult granulosa cell tumor of ovary? J Obstet and Gynaecol. 2019;45(5):1019–25.
Abu-Rustum NR, Restivo A, Soslow R, Sabbatini P, Sonoda Y, Barakat RR, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. 2006;103:31–4.
Sun HD, Lin H, Jao MS, Wang KL, Liou WS, Hung YC, et al. A long-term follow-up study of 176 cases with adult-type ovarian granulosa cell tumors. Gynecol Oncol. 2012;124(2):244–9.
Brink GJ, Groeneweg JW, Hoof L, Zweemer RP, Witteveen PO. Response to systemic therapies in ovarian adult granulosa cell tumors: a literature review. Cancers (Basel). 2022;14(12):2998.
Gurumurth M, Bryant A, Shanbhag S. Efectiveness of diferent treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent). Cochrane Database Syst Rev. 2014;2014:CD006912.
Google Scholar
ESGO Rare Cancers guideline. Available online: https://guidelines.esgo.org/rare-cancers-algorithms/ .
Yumru-Celiksoy H, Dickie C, Seckl MJ, Aydın E, Sozen H, Topuz S, et al. Efectiveness of adjuvant systemic therapy following complete cytoreductive surgery in patients with recurrent granulosa cell tumours of the ovary. Scientifc Reports. 2024;14:993.
Article CAS Google Scholar
Meisel JL, Hyman DM, Jotwani A, Zhou Q, Abu-Rustum NR, Iasonos A, et al. The role of systemic chemotherapy in the management of granulosa cell tumors. Gynecol Oncol. 2015;136(3):505–11.
Mangili G, Sigismondi C, Frigerio L, Candiani M, Savarese A, Giorda G, et al. Recurrent granulosa cell tumors (GCTs) of the ovary: a MITO-9 retrospective study. Gynecol Oncol. 2013;130(1):38–42.
Kaye SB, Davies E. Cyclophosphamide, adriamycin, and cisplatinum for the treatment of advanced granulosa cell tumor, using serum estradiol as a tumor marker. Gynecol Oncol. 1986;24:261–4.
Haltia UM, Hallamaa M, Tapper J, Hynninen J, Alfthan H, Kalra B, et al. Roles of human epididymis protein 4, carbohydrate antigen 125, inhibin B and anti-Müllerian hormone in the differential diagnosis and follow-up of ovarian granulosa cell tumors. Gynecol Oncol. 2017;144(1):83–9.
Rey RA, Lhomme C, Marcillac I, Lahlou N, Duvillard P, Josso N, Bidart JM. Antimullerian hormone as a serum marker of granulosa cell tumorsof the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol. 1996;174:958–65.
Robertson DM, Pruysers E, Burger HG, Jobling T, McNeilage J, Healy D, et al. Inhibins and ovarian cancer. Mol Cell Endocrinol. 2004;225:65–71.
Download references
Author information
Authors and affiliations.
Department of Gynecological Oncology Surgery, Ankara Bilkent City Hospital, Universiteler Mahallesi, 1604. Cadde No: 9. Çankaya, Ankara, Türkiye
Mustafa Şahin, Tufan Arslanca, Yeşim Özkaya Uçar, Gülşah Tiryaki Güner, İlker Selçuk & Hakan Raşit Yalçın
You can also search for this author in PubMed Google Scholar
Contributions
MŞ: data analysis, writing (wrote the main text). TA: writing, designing. YÖU: desing, statistical analysis (made statistics). GTG: rewievs, prepared the figures. iS: rewievs. HRY: rewievs,data analysis.
Corresponding author
Correspondence to Mustafa Şahin .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Şahin, M., Arslanca, T., Uçar, Y.Ö. et al. The experıance of tertıary center for adult granulosa cell tumor: whıch factors predıct survival?. J Ovarian Res 17 , 127 (2024). https://doi.org/10.1186/s13048-024-01453-w
Download citation
Received : 03 April 2024
Accepted : 11 June 2024
Published : 19 June 2024
DOI : https://doi.org/10.1186/s13048-024-01453-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adult granulosa cell tumors
- Prognostic factors
- Cytoreductive surgery
- Recurrent tumors
Journal of Ovarian Research
ISSN: 1757-2215
- General enquiries: [email protected]

- Our Latest News
Retrospective study based on electronic health records finds popular diabetes and weight-loss drugs associated with reduction in incidence and recurrence of alcohol-use disorder by at least half

A new study by researchers at the Case Western Reserve University School of Medicine reveals that the popular diabetes and weight-loss drugs Wegovy and Ozempic are linked to reduced incidence and recurrence of alcohol abuse or dependence.
The team’s findings, recently published in the journal Nature Communications, may suggest a possible new treatment for excessive alcohol use—including alcohol-use disorder (AUD), a health condition that causes about 178,000 deaths in the United States each year, according to the Centers for Disease Control.
To date, the U.S. Food and Drug Administration (FDA) has approved only three medications to treat AUD.
The active ingredient in Wegovy and Ozempic is semaglutide, which belongs to a class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1). GLP-1 helps regulate blood sugar in type 2 diabetes and reduces appetite.
The researchers examined electronic health records of nearly 84,000 patients with obesity. They found those treated with semaglutide, compared to those treated with other anti-obesity medications, showed a 50% to 56% decrease for both the initiation and re-occurrence of alcohol-use disorder in the year following.

“This is very promising news in that we may have a new therapeutic method to treat AUD,” said Rong Xu, a professor of biomedical informatics at the School of Medicine and the study’s lead researcher.
Xu, also director of the medical school’s Center for AI in Drug Discovery , was joined by medical school co-authors Nathan Berger , the Hanna-Payne Professor of Experimental Medicine, and Pamela Davis , the Arline H. and Curtis F. Garvin Research Professor. Nora D. Volkow , director of the National Institute on Drug Abuse , also co-authored the study.
“We collected real-world evidence in a manner similar to our previous two studies reported earlier this year,” Berger said. “In January we showed that semaglutide is associated with a decrease in suicidal thoughts, and in March , we demonstrated that semaglutide is also associated with a reduction in both new diagnoses and recurrence of cannabis-use disorder.”
Similar findings were replicated when the team examined electronic health records for about 600,000 patients with type 2 diabetes. Again, they found consistent reductions in alcohol-use disorder diagnoses among those treated with semaglutide.
“While the findings are promising and provide preliminary evidence of the potential benefit of semaglutide in AUD in real-world populations,” Davis said, “further randomized clinical trials are needed to support its use clinically for AUD.”
Research reported in this press release was supported by the National Institute on Alcohol Abuse and Alcoholism, National Institute on Aging, and National Cancer Institute, all of the National Institutes of Health, under award numbers AA029831, AG057557, AG061388, AG062272, AG07664, CA221718, CA043703, and CA2332216. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
For more information please contact Patty Zamora at [email protected] .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Res Notes

The clinical case report: a review of its merits and limitations
Trygve nissen.
1 Department of Clinical Medicine, University of Tromsø, N-9038 Tromsø, Norway
2 Division of General Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
3 Division of Addictions and Specialized Psychiatry, University Hospital of North Norway, N-9291 Tromsø, Norway
The clinical case report has a long-standing tradition in the medical literature. While its scientific significance has become smaller as more advanced research methods have gained ground, case reports are still presented in many medical journals. Some scholars point to its limited value for medical progress, while others assert that the genre is undervalued. We aimed to present the various points of view regarding the merits and limitations of the case report genre. We searched Google Scholar, PubMed and select textbooks on epidemiology and medical research for articles and book-chapters discussing the merits and limitations of clinical case reports and case series.
The major merits of case reporting were these: Detecting novelties, generating hypotheses, pharmacovigilance, high applicability when other research designs are not possible to carry out, allowing emphasis on the narrative aspect (in-depth understanding), and educational value. The major limitations were: Lack of ability to generalize, no possibility to establish cause-effect relationship, danger of over-interpretation, publication bias, retrospective design, and distraction of reader when focusing on the unusual.
Conclusions
Despite having lost its central role in medical literature in the 20th century, the genre still appears popular. It is a valuable part of the various research methods, especially since it complements other approaches. Furthermore, it also contributes in areas of medicine that are not specifically research-related, e.g. as an educational tool. Revision of the case report genre has been attempted in order to integrate the biomedical model with the narrative approach, but without significant success. The future prospects of the case report could possibly be in new applications of the genre, i.e. exclusive case report databases available online, and open access for clinicians and researchers.
Throughout history the clinical case report and case report series have been integral components of medical literature [ 1 ]. The case report genre held a strong position until it was sidelined in the second half of the 20 th century [ 2 , 3 ]. New methodologies for research articles paved the way for evidence-based medicine. Editors had to make space for these research articles and at the same time signaled less enthusiasm for publishing case reports [ 4 ]. This spurred some heated debates in medical journals as readers were worried that the traditional case report was in jeopardy [ 5 , 6 ]. Those who welcomed the new trend with fewer case reports being published pointed mainly to their low quality and inclination to emphasize mere curiosa [ 7 - 9 ]. Some of the proponents of the genre claimed that the case report had been and still was indispensible for furthering medical knowledge and that it was unique in taking care of the detailed study of the individual patient as opposed to the new research methods with their “…nomothetic approach [taking] precedence…” [ 5 ]. Still, the case report got a low ranking on the evidence hierarchy. After a decline in popularity a new interest for the case report emerged, probably beginning in the late 1990s [ 2 ]. A peer-reviewed ‘Case reports’ section was introduced in the Lancet in 1995 [ 10 ]. In 2007, the first international, Pubmed-listed medical journal publishing only case reports was established [ 11 , 12 ]. In the following years, several similar journals, for the most part online and open-access, have been launched.
The present debate is not so much focused on whether case reporting is obsolete or not. Some of the discussions after the turn of the century have been about adapting the case report genre to new challenges. One example is the suggestion of incorporating the narrative, i.e. “… stressing the patient’s story”, in the case report [ 13 ]. The authors termed their initiative “The storied case report”. Their endeavor was not met with success. In analyzing the causes for this, they wondered if “… junior trainees find it too hard to determine what is relevant and senior trainees find it too hard to change their habits” [ 13 ]. A similar attempt was done when the editors of the Journal of Medical Case Reports in 2012 encouraged authors to include the patients’ perspectives by letting patients describe their own experiences [ 14 ].
Notwithstanding, we feel there is much to be gained from having an ongoing discussion highlighting the indications and contraindications for producing case reports. This can to some degree be facilitated by getting an understanding of the merits and limitations of the genre. The objective of this article is to present the merits and limitations of case reports and case series reports.
We adopted Taber’s Cyclopedic Medical Dictionary’s definition of the case report : “A formal summary of a unique patient and his or her illness, including the presenting signs and symptoms, diagnostic studies, treatment course and outcome” [ 15 ]. A case report consists of one or two cases, most often only one. The case series or case series report usually consists of three to ten cases [ 16 ]. (In the following we use the term case report to denote both case reports and case series report). Case reports are most often naturalistic and descriptive. Sometimes, however, they can be prospective and experimental.
As literature specifically dealing with the case report genre seemed harder to elicit from the databases than the vast amount of particular case reports, we performed iterative searches. We searched Google Scholar and PubMed using the search terms ‘case report(s)’, ‘case series’, ‘case series report(s)’, ‘case reporting’ in various combinations with ‘clinical’, ‘medical’, ‘anecdotal’, ‘methodology’, ‘review’, ‘overview’, ‘strengths’, ‘weaknesses’, ‘merits’, and ‘limitations’. Further references were identified by examining the literature found in the electronic searches. We also consulted major textbooks on epidemiology [ 17 , 18 ], some scholars of medical genres [ 19 , 20 ] and a monograph on case reporting by the epidemiologist M. Jenicek [ 16 ]. We delimited our review to the retrospective, naturalistic, and descriptive case report, also labeled the “traditional” or “classic” case report, and case series including such reports. Thus we excluded other types, such as the planned, qualitative case study approach [ 21 ] and simulated cases [ 22 - 24 ]. Finally, we extracted the relevant data and grouped the merits and limitations items in rank order with the items we judged to be the most important first.
New observations
The major advantage of case reporting is probably its ability to detect novelties [ 16 ]. It is the only way to present unusual, uncontrolled observations regarding symptoms, clinical findings, course of illness, complications of interventions, associations of diseases, side effects of drugs, etc. In short, anything that is rare or has never been observed previously might be important for the medical community and ought to be published. A case report might sensitize readers and thus facilitate detection of similar or identical cases.
Generating hypotheses
From a single, or preferably several single case reports or a case series, new hypotheses could be formulated. These could then be tested with formal research methods that are designed to refute or confirm the hypotheses, i.e. comparative (observational and experimental) studies.
There are numerous examples of new discoveries or major advancements in medicine that started with a case report or, in some cases, as humbly as a letter to the editor. The first concern from the medical community about the devastating side effect of thalidomide, i.e. the congenital abnormalities, appeared as a letter to the editor in the Lancet in 1961 [ 25 ]. Soon thereafter, several case reports and case series reports were published in various journals. Case reporting is thus indispensable in drug safety surveillance (pharmacovigilance) [ 26 ].
Sometimes significant advancements in knowledge have come not from what researchers were pursuing, but from “accidental discoveries”, i.e. by serendipity. The story of Alexander Fleming’s discovery of penicillin in 1928 is well known in the medical field [ 27 ]. Psychiatry has profited to a large degree from this mode of advancing medical science as many of the drugs used for mental disorders have been discovered serendipitously [ 27 ]. One notable example is the discovery of the effect of lithium on manic episodes in patients with manic-depressive disorder [ 28 ]. A more recent discovery is the successful treatment of infantile hemangiomas with systemic propranolol. This discovery was published, as a case series report, in the correspondence section in New England Journal of Medicine [ 29 ]. However, the evidence for the effect of this treatment is still preliminary, and several randomized trials are under way [ 30 , 31 ].
Clear and operational entities are prerequisites for doing medical research. Descriptions must come before understanding. Clinical observations that lead to new disorders being described are well suited for case reporting. The medical literature is replete with case-based articles describing new diseases and syndromes. One notable example is the first description of neurasthenia by G. Beard in Boston Medical and Surgical Journal in 1869 [ 32 ].
Researching rare disorders
For rare disorders randomized controlled trials (RCTs) can be impossible to run due to lack of patients to be enrolled. Research on drug treatment and other kinds of interventions must therefore be based on less rigorous methodologies, among them case series and case reports. This would be in accordance with the European Commission’s recommendation to its members to improve health care for those with rare disorders [ 33 ].
Solving ethical constraints
Case reporting can be valuable when ethical constraints prohibit experimental research. Take as an example the challenge of how to manage the side effects of accidental extravasation of cytotoxic drugs. As RCTs on humans seem unethical in this clinical situation the current guidelines rest on small observational studies, case reports and animal studies [ 34 ]. Or another example: Physical restraint is sometimes associated with sudden, unexpected death. The cause or causes for this are to some degree enigmatic, and it is hard to conceive of a controlled study that could be ethical [ 35 , 36 ]. Case reports and case series being “natural experiments” might be the only evidence available for guiding clinical practice.
In-depth narrative case studies
Case reporting can be a way of presenting research with an idiographic emphasis. As contrasted to nomothetic research, an idiographic approach aims at in-depth understanding of human phenomena, especially in the field of psychology and psychiatry. The objective is not generalizable knowledge, but an understanding of meaning and intentionality for an individual or individuals. Sigmund Freud’s case studies are relevant examples. This usage of case reports borders on qualitative research. Qualitative studies, although developed in the social sciences, have become a welcome contribution within health sciences in the last two decades.
Educational value
Clinical medical learning is to a large degree case-based. Typical case histories and vignettes are often presented in textbooks, in lectures, etc. Unusual observations presented as published case reports are important as part of doctors’ continuing medical education, especially as they demonstrate the diversity of manifestations both within and between medical diseases and syndromes [ 37 , 38 ]. Among the various medical texts, the case report is the only one that presents day-to-day clinical practice, clinicians’ diagnostic reasoning, disease management, and follow-up. We believe that some case reports that are written with the aim of contributing to medical knowledge turn out to be of most value educationally because the phenomena have already been described elsewhere. Other case reports are clearly primarily written for educational value [ 37 ]. Some journals have regular sections dedicated to educational case reports, e.g. The Case Records of the Massachusetts General Hospital in the New England Journal of Medicine and the Clinical Case Conference found in the American Journal of Psychiatry.
The cost of doing a case report is low compared to planned, formal studies. Most often the necessary work is probably done in the clinical setting without specific funding. Larger studies, for instance RCTs, will usually need an academic setting.
Fast publication
The time span from observation to publication can be much shorter than for other kinds of studies. This is obviously a great advantage as a case report can be an important alert to the medical community about a serious event. The unexpected side effects of the sedative-antinauseant thalidomide on newborn babies is a telling story. The drug had been prescribed during pregnancy to the babies’ mothers. After the first published observation of severe abnormalities in babies appeared as a letter to the editor of the Lancet in December 16 th , 1961 [ 25 ], several case reports and series followed [ 39 , 40 ]. It should be mentioned though that the drug company had announced on December 2 nd , 1961, i.e. two weeks before the letter from McBride [ 25 ], that it would withdraw the drug form the market immediately [ 41 ].
Flexible structure
Riaz Agha, editor of the International Journal of Surgery Case Reports suggests that the case report, with its less rigid structure is useful as it “… allows the surgeon(s) to discuss their diagnostic approach, the context, background, decision-making, reasoning and outcomes” [ 42 ]. Although the editor is commenting on the surgical case report, the argument can be applied for the whole field of clinical medicine. It should be mentioned though, that other commentators have argued for a more standardized, in effect more rigid, structure [ 43 ].
Clinical practice can be changed
Case reporting can lead to or contribute to a change in clinical practice. A drug might be withdrawn from the market. Or a relabeling might change the attitude to and treatment of a condition. During Word War I the shell shock syndrome was labeled and described thoroughly in several articles in the Lancet , the first of them appearing in February 1915 [ 44 ]. The author was the British captain and military doctor Charles S. Myers. Before his efforts to bring good care and treatment to afflicted soldiers there had been a common misconception that many of these dysfunctional soldiers were malingerers or cowards.
Exercise for novice researchers
The case report format is well suited for young doctors not yet trained as researchers. It can be an opportunity for a first exercise in authoring an article and a preparation for a scientific career [ 37 , 45 , 46 ].
Communication between the clinical and academic fields
Articles authored by clinicians can promote communication between practicing clinicians and academic researchers. Observations published can generate ideas and be a trigger for further studies. For instance, a case series consisting of several similar cases in a short period can make up the case-group for a case–control study [ 47 ]. Clinicians could do the observation and publish the case series while the case–control study could be left to the academics.
Entertainment
Some commentators find reading case reports fun. Although a rather weak argument in favor of case reporting, the value of being entertained should not be dismissed altogether. It might inspire physicians to spend more time browsing and reading scientific literature [ 48 ].
Studying the history of medicine
Finally, we present a note on a different and unintended aspect of the genre. The accumulated case reports from past eras are a rich resource for researching and understanding medical history [ 49 , 50 ]. A close study of old case reports can provide valuable information about how medicine has been practiced through the centuries [ 50 , 51 ].
Limitations
No epidemiological quantities.
As case reports are not chosen from representative population samples they cannot generate information on rates, ratios, incidences or prevalences. The case or cases being the numerator in the equation, has no denominator. However, if a case series report consists of a cluster of cases, it can signal an important and possibly causal association, e.g. an epidemic or a side effect of a newly marketed drug.
Causal inference not possible
Causality cannot be inferred from an uncontrolled observation. An association does not imply a cause-effect relationship. The observation or event in question could be a mere coincidence. This is a limitation shared by all the descriptive studies [ 47 ]. Take the thalidomide tragedy already mentioned as an example; Unusual events such as congenital malformations in some of the children born to mothers having taken a specific drug during pregnancy does not prove that the drug is the culprit. It is a mere hypothesis until further studies have either rejected or confirmed it. Cause-effect relationships require planned studies including control groups that to the extent possible control for chance, bias and confounders [ 52 ].
Generalization not possible
From the argument above, it follows that findings from case reports cannot be generalized. In order to generalize we need both a cause-effect relationship and a representative population for which the findings are valid. A single case report has neither. A case series, on the other hand, e.g. many “thalidomide babies” in a short time period, could strengthen the suspicion of a causal relationship, demanding further surveillance and research.
Publication bias could be a limiting factor. Journals in general favor positive-outcome findings [ 53 ]. One group of investigators studying case reports published in the Lancet found that only 5% of case reports and 10% of case series reported treatment failures [ 54 ]. A study of 435 case reports from the field of dentistry found that in 99.1%, the reports “…clearly [had] a positive outcome and the intervention was considered and described as successful by the authors” [ 55 ].
Overinterpretation
Overinterpretation or misinterpretation is the tendency or temptation to generalize when there is no justification for it. It has also been labeled “the anecdotal fallacy” [ 56 ]. This is not a shortcoming intrinsic to the method itself. Overinterpretation may be due to the phenomenon of case reports often having an emotional appeal on readers. The story implicitly makes a claim to truth. The reader might conclude prematurely that there is a causal connection. The phenomenon might be more clearly illustrated by the impact of the clinician’s load of personal cases on his or her practice. Here exemplified by a young doctor’s confession: “I often tell residents and medical students, ‘The only thing that actually changes practice is adverse anecdote.’” [ 57 ].
Emphasis on the rare
As case reporting often deals with the rare and atypical, it might divert the readers’ attention from common diseases and problems [ 58 ].
Confidentiality
Journals today require written informed consent from patients before publishing case reports. Both authors and publishers are responsible for securing confidentiality. A guarantee for full confidentiality is not always possible. Despite all possible measures taken to preserve confidentiality, sometimes the patient will be recognized by someone. This information should be given to the patient. An adequately informed patient might not consent to publication. In 1995 in an Editorial in the British Journal of Psychiatry one commentator, Isaac Marks, feared that written consent would discourage case reports being written [ 59 ]. Fortunately, judged form the large number of reports being published today, it seems unlikely that the demand for consent has impeded their publication.
Other methodological limitations
Case reports and series are written after the relevant event, i.e. the observation. Thus, the reports are produced retrospectively. The medical record might not contain all relevant data. Recall bias might prevent us from getting the necessary information from the patient or other informants such as family members and health professionals.
It has also been held against case reporting that it is subjective. The observer’s subjectivity might bias the quality and interpretation of the observation (i.e. information bias).
Finally, the falsification criterion within science, which is tested by repeating an experiment, cannot be applied for case reports. We cannot design another identical and uncontrolled observation. However, unplanned similar “experiments” of nature can be repeated. Several such observations can constitute a case series that represents stronger indicative evidence than the single case report.
The major advantages of case reporting are the ability to make new observations, generate hypotheses, accumulate scientific data about rare disorders, do in-depth narrative studies, and serve as a major educational tool. The method is deficient mainly in being unable to deliver quantitative data. Nor can it prove cause-effect relationship or allow generalizations. Furthermore, there is a risk of overinterpretation and publication bias.
The traditional case report does not fit easily into the qualitative-quantitative dichotomy of research methods. It certainly shares some characteristics with qualitative research [ 16 ], especially with regard to the idiographic, narrative perspective – the patient’s “interior world” [ 60 ] – that sometimes is attended to. Apart from “The storied case report” mentioned in the Background-section, other innovative modifications of the traditional case report have been tried: the “evidence-based case report” [ 61 ], the “interactive case report” [ 62 ] and the “integrated narrative and evidence based case report” [ 63 ]. These modifications of the format have not made a lasting impact on the way case reports in general are written today.
The method of case reporting is briefly dealt with in some textbooks on epidemiology [ 17 , 18 ]. Journals that welcome case reports often put more emphasis on style and design than on content in their ‘instruction to authors’ section [ 64 ]. As a consequence, Sorinola and coworkers argue for more consensus and more consistent guidance on writing case reports [ 64 ]. We feel that a satisfactory amount of guidance concerning both style and content now exists [ 12 , 16 , 65 , 66 ]. The latest contribution, “The CARE guidelines”, is an ambitious endeavor to improve completeness and transparency of reports [ 66 ]. These guidelines have included the “Patient perspective” as an item, apparently a bit half-heartedly as this item is placed after the Discussion section, thus not allowing this perspective to influence the Discussion and/or Conclusion section. We assume this is symptomatic of medicine’s problem with integrating the biomedical model with “narrative-based medicine”.
In recent years the medical community has taken an increased interest in case reports [ 2 ], especially after the surge of online, exclusive case report journals started in 2007 with the Journal of Medical Case Reports (which was the first international, Pubmed-listed medical journal publishing only case reports) as the first of this new brand. The climate of skepticism has been replaced by enthusiasm and demand for more case reports. A registry for case reports, Cases Database, was founded in 2012 [ 67 ]. On the condition that it succeeds in becoming a large, international database it could serve as a register being useful for clinicians at work as well as for medical research on various clinical issues. Assuming Pamela P. Powell’s assertion that “[a]lmost all practicing physicians eventually will encounter a case worthy of being reported” [ 60 ] is valid, there should be no shortage of potential cases waiting to be reported and filed in various databases, preferably online and open access.
Limitations of this review
There are several limitations to this study. It is a weakness that we have not been able to review all the relevant literature. The number of publications in some way related to case reports and case report series is enormous, and although we have attempted to identify those publications relevant for our purpose (i.e. those that describe the merits and limitations of the case report genre), we might have missed some. It was difficult to find good search terms for our objective. Still, after repeated electronic searches supplemented with manual searches in reference lists, we had a corpus of literature where essentially no new merits or limitations emerged.
As we point out above, the ranking of merits and limitations represents our subjective opinion and we acknowledge that others might rank the importance of the items differently.
The perspective on merits and limitations of case reporting has been strictly medical. As a consequence we have not analyzed or discussed the various non-medical factors affecting the publication of case reports in different medical journals [ 2 ]. For instance, case reports are cited less often than other kinds of medical research articles [ 68 ]. Thus they can lower a journal’s impact factor, potentially making the journal less attractive. This might lead some high-impact journals to publish few or no case reports, while other journals have chosen to specialize in this genre.
Before deciding on producing a case report or case series based on a particular patient or patients at hand, the observant clinician has to determine if the case report method is the appropriate article type. This review could hopefully assist in that judgment and perhaps be a stimulus to the continuing debate in the medical community on the value of case reporting.
Competing interests
The authors declare that there are no competing interests.
Authors’ contributions
TN contributed to the conception, drafting, and revision of the article. RW contributed to the conception, drafting, and revision of the article. Both authors approved the final manuscript.
Acknowledgements
There was no specific funding for this study.
- Cabán-Martinez AJ, Beltrán WF. Advancing medicine one note at a time: the educational value in clinical case reports. BMC Res Notes. 2012; 5 :293. doi: 10.1186/1756-0500-5-293. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The recent history of the clinical case report: a narrative review. JRSM Sh Rep. 2012; 3 :87. doi: 10.1258/shorts.2012.012046. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nissen T, Wynn R. The history of the case report: a selective review. JRSM Open. 2014; 5 :4. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G. Fare the well – the Editor’s last words. Br J Psychiatry. 2003; 182 :465–466. doi: 10.1192/bjp.182.6.465. [ CrossRef ] [ Google Scholar ]
- Williams DDR. In defence of case of the case report (Letter) Br J Psychiatry. 2004; 184 :84. doi: 10.1192/bjp.184.1.84. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Enoch MD. Case reports (Letter) Br J Psychiatry. 2005; 185 :169. [ PubMed ] [ Google Scholar ]
- Nahum AM. The clinical case report: “Pot boiler” or scientific literature? Head Neck Surg. 1979; 1 :291–292. doi: 10.1002/hed.2890010402. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doherty M. What value case reports? (Editorial) Ann Rheum Dis. 1994; 53 :1–2. doi: 10.1136/ard.53.1.1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Procopio M. Publication of case reports. Br J Psychiatry. 2005; 187 :91. [ PubMed ] [ Google Scholar ]
- Bignall J, Horton R. Learning from stories – Lancet’s case reports. Lancet. 1995; 346 :1256. [ PubMed ] [ Google Scholar ]
- Kidd M, Hubbard C. Introducing Journal of Medical Case Reports. J Med Case Rep. 2007; 1 :1. doi: 10.1186/1752-1947-1-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rison RA. A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes. J Med Case Reports. 2013; 7 :239. doi: 10.1186/1752-1947-7-239. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bayoumi AM, Kopplin PA. The storied case report. CMAJ. 2004; 171 :569–570. doi: 10.1503/cmaj.1031503. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kidd MR, Saltman D. Case reports at the vanguard of the 21 st century medicine (Editorial) J Med Case Rep. 2012; 6 :156. doi: 10.1186/1752-1947-6-156. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Venes D. Taber’s Cyclopedic Medical Dictionary. 21. Philadelphia: F.A. Davis Company; 2009. [ Google Scholar ]
- Jenicek M. Clinical case reporting in evidence-based medicine. Oxford: Butterworth Heinemann; 1999. [ Google Scholar ]
- Fletcher RH, Fletcher SW. Clinical epidemiology: The essentials. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. [ Google Scholar ]
- Sackett DL, Brian Haynes R, Tugwell P. Clinical epidemiology. Boston: Little, Brown and Company; 1985. [ Google Scholar ]
- Berkencotter C. Patient tales. Case histories and the uses of narrative in psychiatry. Columbia: University of South Carolina Press; 2008. [ Google Scholar ]
- Hunter KM. Doctors’ stories. The narrative structure of medical knowledge. Princeton: Princeton University Press; 1991. [ Google Scholar ]
- Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach. BMC Med Res Methodol. 2011; 11 :100. doi: 10.1186/1471-2288-11-100. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Kvalvik AM, Hynnekleiv T. Attitudes to coercion at two Norwegian psychiatric units. Nord J Psychiatry. 2011; 65 :133–137. doi: 10.3109/08039488.2010.513068. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R, Myklebust LH, Bratlid T. Psychologists and coercion: decisions regarding involuntary psychiatric admission and treatment in a group of Norwegian psychologists. Nord J Psychiatry. 2007; 61 :433–437. doi: 10.1080/08039480701773139. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aaker E, Knudsen A, Wynn R, Lund A. General practitioners’ reactions to non-compliant patients. Scand J Prim Health Care. 2001; 19 :103–106. doi: 10.1080/028134301750235330. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McBride WG. Thalidomide and congenital abnormalities. (Letter) Lancet. 1961; 278 :1358. [ Google Scholar ]
- Chakra CAN, Pariente A, Pinet M, Nkeng L, Moore N, Moride Y. Case series in drug safety. A review to determine characteristics and quality. Drug Saf. 2010; 33 :1081–1088. doi: 10.2165/11539300-000000000-00000. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci. 2006; 8 :335–344. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949; 2 :349–352. [ PubMed ] [ Google Scholar ]
- Léauté-Labrèze C, de la Roque Dumas E, Hubiche T, Boralevi F. Propranolol for severe hemangiomas of infancy. (Letter to the editor) N Engl J Med. 2008; 358 :2649–2651. doi: 10.1056/NEJMc0708819. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fretheim A. Godt nok dokumentert? (Sufficiently documented?) (Editorial) Tidsskr Nor Legeforen. 2010; 130 :1806. [ PubMed ] [ Google Scholar ]
- U.S. National Institutes of Health. Studies found for the search 'propanolol and hemangiomas' in the ClinicalTrials.gov database. Accessed on 22 April, 2014 at: http://www.clinicaltrials.gov/ct2/results?term=propanolol+and+hemangiomas&Search=Search .
- Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869; 80 :217–221. doi: 10.1056/NEJM186904290801301. [ CrossRef ] [ Google Scholar ]
- Taylor DM. Developing a strategy for the management of rare disorders. BMJ. 2012; 344 :e2417. doi: 10.1136/bmj.e2417. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bjånes TK. Lokale bivirkninger ved parenteral administrering av legemidler. (Localized side effects caused by parenteral administration of drugs) Tidsskr Nor Legeforen. 2011; 131 :472–474. [ PubMed ] [ Google Scholar ]
- Nissen T, Rørvik P, Haugslett L, Wynn R. Physical restraint and near death of a psychiatric patient. J Forensic Sci. 2013; 58 :259–262. doi: 10.1111/j.1556-4029.2012.02290.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynn R. Coercion in psychiatric care: clinical, legal, and ethical controversies. Int J Psychiatry Clin Pract. 2006; 10 :247–251. doi: 10.1080/13651500600650026. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lundh A, Christensen M, Jørgensen AW. International or national publication of case reports. Dan Med Bul. 2011; 58 :A4242. [ PubMed ] [ Google Scholar ]
- Grønli O, Wynn R. Normocalcemic hyperparathyroidism and treatment resistant depression. Psychosomatics. 2013; 54 :493–497. doi: 10.1016/j.psym.2012.10.008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward SP. Thalidomide and congenital abnormalities. BMJ. 1962; 2 (5305):646–647. doi: 10.1136/bmj.2.5305.646. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Coodin FJ, Uchida CM, Murphy CH. Phocomelia: report of three cases. CMAJ. 1962; 87 :735–739. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Heyman DJ. Managing director of the Destillers Company Ltd. (Letter) Lancet. 1961; 278 :1262. [ Google Scholar ]
- Agha R, Rosin RD. Time for a new approach to case reports. (Editorial) Int J Surg Case Rep. 2010; 1 :1–3. doi: 10.1016/j.ijscr.2010.04.001. doi:10.1016/j.ijscr.2010.04.001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aronson JA. Anecdotes as evidence. (Editorial) BMJ. 2003; 326 :1346. doi: 10.1136/bmj.326.7403.1346. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Myers CS. A contribution to the study of the shell shock. Being an account of three cases of loss of memory, vision, smell and taste, admitted into the Duchess of Westminster’s War Hospital, Le Touquet. Lancet. 1915; 185 :316–320. doi: 10.1016/S0140-6736(00)52916-X. [ CrossRef ] [ Google Scholar ]
- Morris BA. The importance of case reports. (Letter to the Editor) CMAJ. 1989; 141 :875–876. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rison RA. Neurology case reports: a call for all. J Med Case Rep. 2011; 5 :113. doi: 10.1186/1752-1947-5-113. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet. 2002; 359 :145–149. doi: 10.1016/S0140-6736(02)07373-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang S. Anecdotes in medicine - 15 years of Lancet case reports. Lancet. 2010; 376 :1448–1449. doi: 10.1016/S0140-6736(10)60624-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rose JC, Corn M. Dr. E. and other patients: new lessons from old case reports. J Hist Med Allied Sci. 1984; 39 :3–32. doi: 10.1093/jhmas/39.1.3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. Practice versus theory: tenth-century case histories from Islamic Middle East. Soc Hist Med. 2000; 13 :293–306. doi: 10.1093/shm/13.2.293. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez Millán C. The case history in Medieval Islamic medical literature: Tajarib and Mujarrabat as source. Med Hist. 2010; 54 :195–214. doi: 10.1017/S0025727300006712. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. [ Google Scholar ]
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991; 337 :867–872. doi: 10.1016/0140-6736(91)90201-Y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Albrecht J, Meves A, Bigby M. Case reports and case series from the Lancet had significant impact on medical literature. J Clin Epidemiol. 2005; 58 :1227–1232. doi: 10.1016/j.jclinepi.2005.04.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oliveira GJ, Leles CR. Critical appraisal and positive outcome bias in case reports published in Brazilian dental journals. J Dent Educ. 2006; 70 :869–874. [ PubMed ] [ Google Scholar ]
- Charlton BG, Walston F. Individual case studies in clinical research. J Eval Clin Practice. 1998; 4 :147–155. doi: 10.1111/j.1365-2753.1998.tb00081.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stuebe AM. Level IV evidence – adverse anecdote and clinical practice. N Engl J Med. 2011; 365 :8–9. doi: 10.1056/NEJMp1102632. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoffman JR. Rethinking case reports. West J Med. 1999; 170 :253–254. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wilkinson G, Fahy T, Russell G, Healy D, Marks I, Tantam D, Dimond B. Case reports and confidentiality. (Editorial) Br J Psychiatry. 1995; 166 :555–558. doi: 10.1192/bjp.166.5.555. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Powell PP. The single-case report in medical literature: the ‘Elephant man’ serves as an exellent example. J Am Med Writers Assoc. 1990; 5 :9–12. [ Google Scholar ]
- Glasziou P. Evidence based case report: twenty year cough in a non-smoker. BMJ. 1998; 316 :1660–1661. doi: 10.1136/bmj.316.7145.1660. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harker N, Montgomery A, Fahey T. Interactive case report. Treating nausea and vomiting during pregnancy: case outcome. BMJ. 2004; 328 :276. doi: 10.1136/bmj.328.7434.276. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reis S, Hermoni D, Livingstone P, Borkan J. Integrated narrative and evidence based case report: case report of paroxysmal atrial fibrillation and anticoagulation. BMJ. 2002; 325 :1018–1020. doi: 10.1136/bmj.325.7371.1018. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sorinola O, Olufowobi O, Coomarasamy A, Khan KS. Instructions to authors for case reporting are limited: a review of a core journal list. BMC Med Educ. 2004; 4 :4. doi: 10.1186/1472-6920-4-4. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rossor MN. In: How to write a paper. 4. Hall GM, editor. London: Blackwell Publishing; 2008. How to write a case report; pp. 71–75. [ Google Scholar ]
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. CARE group. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 2013; 7 :223. doi: 10.1186/1752-1947-7-223. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BioMed Central. Cases Database. Accessed on 22 April, 2014 at: http://www.casesdatabase.com .
- Mason RA. The case report – an endangered species? (Editorial) Anesthesia. 2001; 56 :99–102. doi: 10.1046/j.1365-2044.2001.01919.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
Purdue University - Extension - Forestry and Natural Resources
Forestry & natural resources, got nature blog, case study: maple tree pests – purdue landscape report.
Purdue Landscape Report: Recently a homeowner in Hamilton County posted on the Indiana Native Plant Society Facebook page with concerns about aphids, mites, and apple scab in her maple and oak trees. She asked for a second opinion and treatment options for these pests. I reached out to the homeowner and requested permission to collect samples from the trees. Let’s look at what I found (or didn’t find) and discuss when and if these issues should be treated.
Apple Scab on Maples? First, we can eliminate the concern about apple scab because maple and oak trees are not hosts for this pathogen. Apple scab is caused by the fungus Venturia inaequalis , and hosts include apples, crabapples, hawthorn, mountain ash, firethorn, and loquat. There are other fungal diseases which cause leaf spotting in maples, such as Anthracnose, tar spot, and Phyllosticta leaf spot. To diagnosis these diseases, homeowners can submit a sample to the Purdue Plant & Pest Diagnostic Lab , or hire a certified arborist to assess the tree. However, all these diseases are primarily aesthetic issues. A healthy tree will not die from these pathogens and does not require treatment. You can find more information about maple diseases in this publication: Diseases in Hardwood Tree Plantings . You can also find a previous article on Tar Spot in Maple in the Purdue Landscape Report (Issue 18-12).

Fig. 1. Severe spider mite damage on maple leaves. (Photo: S. D. Frank, North Carolina State University)
Spider Mites The next pest concern on these trees is spider mites. Out of the dozens of leaves I collected, I found only two immature mites on a couple of maple leaves I examined. This is a very small mite presence, and it is not recommended to treat for spider mites unless the populations threaten the health or appearance of the tree. Limiting pesticide usage will conserve the natural enemies, which are vital to keeping mite populations in check. In fact, improper pesticide applications can kill these important natural enemies and worsen mite infestations.
When do you know if the mite population is large enough to merit intervention? Check your trees for signs of heavy feeding damage, such as leaf stippling (Fig. 1), or dense webbing on the leaves. Mites can also be monitored by placing a sheet of paper (8.5×11”) beneath a branch and striking the limb. Chemical treatments should be considered when you count ≥24 mites per strike. You can find detailed management recommendations and a full list of pesticide options in this Purdue Extension publication: Spider Mites on Ornamentals .

Fig. 2: Adult painted maple aphids found on maple in Hamilton Co, Indiana. (Photo: Andrew Johnston, Purdue University).
Aphids The aphids I found on the homeowner’s maple trees are Drepanaphis acerifoliae , or the painted maple aphid (Fig. 2). This species only feeds on maple trees, and is not a threat to the oak tree on this homeowner’s property. Painted maple aphid is a very common aphid in our region. I found only a few aphids on one of the maple trees I sampled, which is not enough to require treatment. In addition, one of the aphids was a “mummy”, or a carcass left behind from a parasitoid wasp. This indicates that natural enemies are already at work managing the aphid population. Aphids may rarely require chemical control if their numbers grow large enough to produce significant amounts of honeydew, which can result in sooty mold outbreaks.

Fig. 3: White-marked tussock moth caterpillar. (Photo: John Obermeyer, Purdue University).
Tussock Moth The only insect I found on the oak tree was a white-marked tussock moth caterpillar, Orgyia leucostigma (Fig. 3). This is the likely culprit for the minor feeding damage I noticed. These are not significant pests and do not require treatment. Don’t touch them, though! The setae of this caterpillar are irritating and may cause allergic reactions.
Overall, the pests I found were minimal and non-threatening to the trees. It’s normal to find some insect pressure in the landscape. Knowing when and if to treat requires accurate diagnosis and monitoring of pest levels. Check out PurduePlantDoctor.com for an easy-to-use diagnostic aid and treatment recommendations.
Original article posted: Purdue Landscape Report .
Subscribe and receive the newsletter: Purdue Landscape Report Newsletter .
Resources: Large Spots on Maple Leaves that Look Like Tar , Purdue Extension News Find an Arborist , International Society of Arboriculture Diseases in Hardwood Tree Plantings , The Education Store, Purdue Extension’s resource center Fifty Common Trees of Indiana An Introduction to Trees of Indiana Shrubs and Woody Vines of Indiana and the Midwest , The Education Store Tree Installation: Process and Practices , The Education Store Forest Improvement Handbook , The Education Store Invasive Species , Playlist, Purdue Extension – FNR YouTube Channel Report Invasive Species , Purdue Invasive Species What are invasive species and why should I care? , Got Nature? Blog, Purdue Extension – Forestry and Natural Resources Indiana Department of Natural Resources: Invasive Species Indiana Invasive Species Council ID That Tree , Purdue Extension-FNR YouTube playlist Tree Defect Identification , The Education Store Tree Wound and Healing , Got Nature? Blog, Purdue Extension – Forestry and Natural Resources
Alicia Kelley , Cooperative Agricultural Pest Survey (CAPS) Coordinator Purdue Extension – Entomology
RELATED POSTS:

Recent Posts
- Case Study: Maple Tree Pests – Purdue Landscape Report Posted: June 26, 2024 in Disease , Forests and Street Trees , Plants , Spiders , Urban Forestry , Wildlife , Woodlands
- Woodland Management Moment: Oak Regeneration – Protecting Seedlings Posted: June 24, 2024 in Forestry , Urban Forestry , Wildlife , Woodland Management Moment , Woodlands
- Stories in the Bark – Patterns and Growth Posted: June 22, 2024 in Forestry , Forests and Street Trees , Wildlife , Woodlands
- Indiana’s Surprise Onion Plants Emerge Posted: June 20, 2024 in Forestry , Forests and Street Trees , Plants , Urban Forestry , Wildlife , Woodlands
- Celebrate Pollinator Week With Flowers of June Tour Posted: in Forestry , Gardening , Wildlife
- Leaf Curl and Blister Due to Wet Weather – Purdue Landscape Report Posted: June 7, 2024 in Forestry , Forests and Street Trees , Urban Forestry , Wildlife
- Learn How to Support Oak-Hickory Ecosystems Posted: June 4, 2024 in Forestry , How To , Urban Forestry , Wildlife
- Turtle Traffic: Save Turtles From Roads – My DNR Posted: June 3, 2024 in How To , Wildlife
- Spongy Moth in Spring Time – Purdue Landscape Report Posted: in Alert , Forestry , Forests and Street Trees , How To , Invasive Insects , Urban Forestry
- Cottonwood Seed Storm Posted: May 31, 2024 in Forestry , Urban Forestry
- Aquaculture/Fish
- Aquatic/Aquaculture Resources
- Ask the Expert
- Christmas Trees
- Community Development
- Forests and Street Trees
- Got Nature for Kids
- Great Lakes
- Invasive Animal Species
- Invasive Insects
- Invasive Plant Species
- Natural Resource Planning
- Nature of Teaching
- Publication
- Timber Marketing
- Uncategorized
- Urban Forestry
- Wood Products/Manufacturing
- Woodland Management Moment

IMAGES
VIDEO
COMMENTS
Retrospective cohort and case-control studies are similar but generally have differing goals. Cohort designs typically assess known risk factors and how they affect outcomes at different times. Case-control studies evaluate a particular incident, and it is an exploratory design to identify potential risk factors.
Revised on June 22, 2023. A retrospective cohort study is a type of observational study that focuses on individuals who have an exposure to a disease or risk factor in common. Retrospective cohort studies analyze the health outcomes over a period of time to form connections and assess the risk of a given outcome associated with a given exposure.
The retrospective chart review (RCR), also known as a medical record review, is a type of research design in which pre-recorded, patient-centered data are used to answer one or more research questions [ 1 ]. The data used in such reviews exist in many forms: electronic databases, results from diagnostic tests, and notes from health service ...
Although there are significant benefits to big retrospective datasets obtained from EHR systems, designing studies that overcome the challenges associated with retrospective cohort and case-control design remain an issue that undermine the generalizability, validity and reliability of results of these otherwise meaningful studies.
Key points • A case series is a retrospective, noncomparative investigation that evaluates a group of patients with a known medical condition, disease state, exposure, or who have undergone a similar procedure. 1 The primary outcome of a case series is to identify a testable hypothesis that may be further evaluated with a more rigorous study design; secondary outcomes include commentary on ...
Introduction. retrospective study uses existing data that have been recorded for reasons other than research. In health care these are often called "chart reviews" because the data source is the medical record. Figure 1 contrasts retrospec-tive and prospective studies. There are 3 general types of retrospective study: case report, case ...
Here we provide 10 rules that serve as an end-to-end introduction to retrospective analyses of observational health care data. A running example of a COVID-19 study presents a practical implementation of each rule in the context of a specific treatment hypothesis.
A retrospective study uses existing data that have been recorded for reasons other than research. A retrospective case series is the description of a group of cases with a new or unusual disease or treatment. With a case-control study, cases with and without the condition of interest are identified, and the degree of exposure to a possible risk ...
retrospective study: case report, case series, and case-con-trol study. A retrospective study contains many of the. same study-design elements as a prospective study (Table. 1).
Failure to report on informed consent and approval by an ethics review board has been described to be frequent in clinical research, even in prestigious journals. ... In conclusion, the decision on whether to proceed to ethics review in case of retrospective studies depends on individual IRB, journal guidelines and editor's discretion, in ...
The retrospective case-control study is an important research strategy commonly encountered in the medical literature. A thoughtfully designed, carefully executed case-control study can be an invaluable source of clinical information, and physicians must often base important decisions about patient counseling and management on their interpretation of such studies.
The case report is an observational study in which one to four clinical cases are reviewed; however, there is no unified criteria. 4, 5, 6, ... The author reviewed retrospective clinical cases in chronological order and highlighted key aspects of the patient's clinical signs and symptoms.
Retrospective studies are designed to analyse pre-existing data, and are subject to numerous biases as a result. Retrospective studies may be based on chart reviews (data collection from the medical records of patients) Types of retrospective studies include: case series. retrospective cohort studies (current or historical cohorts)
Case-control studies are retrospective. ... Often case-control studies require the participants to self-report their exposure to a certain factor. Recall bias is the systematic difference in how the two groups may recall past events e.g. in a study investigating stillbirth, a mother who experienced this may recall the possible contributing ...
Abstract. Retrospective studies represent an often used research methodology in the podiatric scientific literature, with cohort studies and case series being two of the most prevalent designs. Choosing a retrospective method is often dependent on multiple factors, two of the most important being details of the research question to be explored ...
According to Hess (2004) there are three kinds of retrospective studies (a) case series, (b) case control, and (c) matched case-control. A retrospective case series is used to report information on researcher identified self-selected similar cases. A case control study is comprised of subjects with and without the condition of interest. The ...
Retrospective studies are Dr Mohit Goyal Abstract an important tool to study rare diseases, manifestations and outcomes. CARE Pain & Arthritis Findings of these studies can form the basis on which prospective studies Centre are planned. Retrospective studies however have several limitations owing Udaipur 313002 to their design.
Level 1: Systematic Reviews & Meta-analysis of RCTs; Evidence-based Clinical Practice Guidelines. Level 2: One or more RCTs. Level 3: Controlled Trials (no randomization) Level 4: Case-control or Cohort study. Level 5: Systematic Review of Descriptive and Qualitative studies. Level 6: Single Descriptive or Qualitative Study.
This retrospective study reviewed the records of adult patients (≥20 years) with persistent symptoms following COVID-19 infection who underwent IOS testing at the Changhua Christian Hospital Pulmonary Function Laboratory between October 1, 2019, and December 31, 2022. ... although statistical significance was not observed in this case either ...
Retrospective Case Study Review/Report. Generally completed by a retrospective review of medical records that highlights a unique treatment, case, or outcome. Often clinical in nature. A report about five or fewer clinical experiences or observations identified during clinical care. Does not involve biospecimens or FDA-regulated products (e.g ...
Retrospective comparative study; case-control study; or systematic review of these studies: IV: Case-series: V: ... The common scenario is when a subject with disease (case) will unconsciously recall and report an exposure with better clarity due to the disease experience. Interviewer bias occurs when the interviewer asks leading questions or ...
Section 1 - What is being analyzed. For each case study site, EPA researchers took samples from a variety of sources and tested them for a broad range of substances and chemicals, including components of hydraulic fracturing fluids. Water conditions, such as temperature and dissolved oxygen referred to as "parameters" were also monitored ...
The study designs primarily consisted of case reports (n=23), retrospective studies (n=4), and prospective studies (n=2). Table 1 General characteristics of the studies (n=29) Full size table. All case reports involved female patients, except for one involving a male patient. Five quantitative studies did not specify or limit the sex of the ...
To define the characteristics of fundus manifestations in patients after SARS-CoV-2 infection with multimodal imaging techniques. This is a retrospective multicenter and multimodal imaging study ...
Purpose The management of primary hyperparathyroidism (PHPT) during pregnancy may be surgical or conservative. This study compared adverse outcomes between surgical and non-surgical treatments. Additionally, the study investigated the correlation between serum calcium values and complication rates. Methods A systematic review of retrospective studies, case series, and case reports. Biochemical ...
In this study, the correlation between marital status and the prognosis in critically ill patients aged ≥65 years with CeVD was explored. 2 MATERIALS AND METHODS 2.1 Study design, area and period. It is a retrospective cohort study, which data were extracted from the Medical Information Mart for Intensive Care IV (MIMIC-IV).
This retrospective study aims to evaluate the clinical course and long-term outcomes of patients diagnosed with adult granulosa cell tumors (AGCT). The study analyzed a cohort of 112 AGCT patients with a median follow-up of 87 months. Data regarding disease-free survival (DFS), overall survival (OS), recurrence rates, and prognostic factors were collected and analyzed.
A new study by researchers at the Case Western Reserve University School of Medicine reveals that the popular diabetes and weight-loss drugs Wegovy and Ozempic are linked to reduced incidence and recurrence of alcohol abuse or dependence. The team's findings, recently published in the journal Nature Communications, may suggest a possible new treatment for excessive alcohol use—including ...
We delimited our review to the retrospective, naturalistic, and descriptive case report, also labeled the "traditional" or "classic" case report, and case series including such reports. Thus we excluded other types, such as the planned, qualitative case study approach [ 21 ] and simulated cases [ 22 - 24 ].
Purdue Landscape Report: Recently a homeowner in Hamilton County posted on the Indiana Native Plant Society Facebook page with concerns about aphids, mites, and apple scab in her maple and oak trees. She asked for a second opinion and treatment options for these pests. I reached out to the homeowner and requested permission to collect samples from the trees.