- The Open University
- Guest user / Sign out
- Study with The Open University

My OpenLearn Profile
Personalise your OpenLearn profile, save your favourite content and get recognition for your learning

Addressing ethical issues in your research proposal
This article explores the ethical issues that may arise in your proposed study during your doctoral research degree.
What ethical principles apply when planning and conducting research?
Research ethics are the moral principles that govern how researchers conduct their studies (Wellcome Trust, 2014). As there are elements of uncertainty and risk involved in any study, every researcher has to consider how they can uphold these ethical principles and conduct the research in a way that protects the interests and welfare of participants and other stakeholders (such as organisations).
You will need to consider the ethical issues that might arise in your proposed study. Consideration of the fundamental ethical principles that underpin all research will help you to identify the key issues and how these could be addressed. As you are probably a practitioner who wants to undertake research within your workplace, consider how your role as an ‘insider’ influences how you will conduct your study. Think about the ethical issues that might arise when you become an insider researcher (for example, relating to trust, confidentiality and anonymity).
What key ethical principles do you think will be important when planning or conducting your research, particularly as an insider? Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination.
Key ethical issues that you will address as an insider researcher include:
- Gaining trust
- Avoiding coercion when recruiting colleagues or other participants (such as students or service users)
- Practical challenges relating to ensuring the confidentiality and anonymity of organisations and staff or other participants.
(Heslop et al, 2018)
A fuller discussion of ethical principles is available from the British Psychological Society’s Code of Human Research Ethics (BPS, 2021).
You can also refer to guidance from the British Educational Research Association and the British Association for Applied Linguistics .

Ethical principles are essential for protecting the interests of research participants, including maximising the benefits and minimising any risks associated with taking part in a study. These principles describe ethical conduct which reflects the integrity of the researcher, promotes the wellbeing of participants and ensures high-quality research is conducted (Health Research Authority, 2022).
Research ethics is therefore not simply about gaining ethical approval for your study to be conducted. Research ethics relates to your moral conduct as a doctoral researcher and will apply throughout your study from design to dissemination (British Psychological Society, 2021). When you apply to undertake a doctorate, you will need to clearly indicate in your proposal that you understand these ethical principles and are committed to upholding them.
Where can I find ethical guidance and resources?
Professional bodies, learned societies, health and social care authorities, academic publications, Research Ethics Committees and research organisations provide a range of ethical guidance and resources. International codes such as the Universal Declaration of Human Rights underpin ethical frameworks (United Nations, 1948).
You may be aware of key legislation in your own country or the country where you plan to undertake the research, including laws relating to consent, data protection and decision-making capacity, for example, the Data Protection Act, 2018 (UK). If you want to find out more about becoming an ethical researcher, check out this Open University short course: Becoming an ethical researcher: Introduction and guidance: What is a badged course? - OpenLearn - Open University
You should be able to justify the research decisions you make. Utilising these resources will guide your ethical judgements when writing your proposal and ultimately when designing and conducting your research study. The Ethical Guidelines for Educational Research (British Educational Research Association, 2018) identifies the key responsibilities you will have when you conduct your research, including the range of stakeholders that you will have responsibilities to, as follows:
- to your participants (e.g. to appropriately inform them, facilitate their participation and support them)
- clients, stakeholders and sponsors
- the community of educational or health and social care researchers
- for publication and dissemination
- your wellbeing and development
The National Institute for Health and Care Research (no date) has emphasised the need to promote equality, diversity and inclusion when undertaking research, particularly to address long-standing social and health inequalities. Research should be informed by the diversity of people’s experiences and insights, so that it will lead to the development of practice that addresses genuine need. A commitment to equality, diversity and inclusion aims to eradicate prejudice and discrimination on the basis of an individual or group of individuals' protected characteristics such as sex (gender), disability, race, sexual orientation, in line with the Equality Act 2010.
The NIHR has produced guidance for enhancing the inclusion of ‘under-served groups’ when designing a research study (2020). Although the guidance refers to clinical research it is relevant to research more broadly.
You should consider how you will promote equality and diversity in your planned study, including through aspects such as your research topic or question, the methodology you will use, the participants you plan to recruit and how you will analyse and interpret your data.
What ethical issues do I need to consider when writing my research proposal?

You might be planning to undertake research in a health, social care, educational or other setting, including observations and interviews. The following prompts should help you to identify key ethical issues that you need to bear in mind when undertaking research in such settings.
1. Imagine you are a potential participant. Think about the questions and concerns that you might have:
- How would you feel if a researcher sat in your space and took notes, completed a checklist, or made an audio or film recording?
- What harm might a researcher cause by observing or interviewing you and others?
- What would you want to know about the researcher and ask them about the study before giving consent?
- When imagining you are the participant, how could the researcher make you feel more comfortable to be observed or interviewed?
2. Having considered the perspective of your potential participant, how would you take account of concerns such as privacy, consent, wellbeing and power in your research proposal?
[Adapted from OpenLearn course: Becoming an ethical researcher, Week 2 Activity 3: Becoming an ethical researcher - OpenLearn - Open University ]
The ethical issues to be considered will vary depending on your organisational context/role, the types of participants you plan to recruit (for example, children, adults with mental health problems), the research methods you will use, and the types of data you will collect. You will need to decide how to recruit your participants so you do not inappropriately exclude anyone. Consider what methods may be necessary to facilitate their voice and how you can obtain their consent to taking part or ensure that consent is obtained from someone else as necessary, for example, a parent in the case of a child.
You should also think about how to avoid imposing an unnecessary burden or costs on your participants. For example, by minimising the length of time they will have to commit to the study and by providing travel or other expenses. Identify the measures that you will take to store your participants’ data safely and maintain their confidentiality and anonymity when you report your findings. You could do this by storing interview and video recordings in a secure server and anonymising their names and those of their organisations using pseudonyms.
Professional codes such as the Code of Human Research Ethics (BPS, 2021) provide guidance on undertaking research with children. Being an ‘insider’ researching within your own organisation has advantages. However, you should also consider how this might impact on your research, such as power dynamics, consent, potential bias and any conflict of interest between your professional and researcher roles (Sapiro and Matthews, 2020).
How have other researchers addressed any ethical challenges?
The literature provides researchers’ accounts explaining how they addressed ethical challenges when undertaking studies. For example, Turcotte-Tremblay and McSween-Cadieux (2018) discuss strategies for protecting participants’ confidentiality when disseminating findings locally, such as undertaking fieldwork in multiple sites and providing findings in a generalised form. In addition, professional guidance includes case studies illustrating how ethical issues can be addressed, including when researching online forums (British Sociological Association, no date).
Watch the videos below and consider what insights the postgraduate researcher and supervisor provide regarding issues such as being an ‘insider researcher’, power relations, avoiding intrusion, maintaining participant anonymity and complying with research ethics and professional standards. How might their experiences inform the design and conduct of your own study?
Postgraduate researcher and supervisor talk about ethical considerations
Your thoughtful consideration of the ethical issues that might arise and how you would address these should enable you to propose an ethically informed study and conduct it in a responsible, fair and sensitive manner.
British Educational Research Association (2018) Ethical Guidelines for Educational Research. Available at: https://www.bera.ac.uk/publication/ethical-guidelines-for-educational-research-2018 (Accessed: 9 June 2023).
British Psychological Society (2021) Code of Human Research Ethics . Available at: https://cms.bps.org.uk/sites/default/files/2022-06/BPS%20Code%20of%20Human%20Research%20Ethics%20%281%29.pdf (Accessed: 9 June 2023).
British Sociological Association (2016) Researching online forums . Available at: https://www.britsoc.co.uk/media/24834/j000208_researching_online_forums_-cs1-_v3.pdf (Accessed: 9 June 2023).
Health Research Authority (2022) UK Policy Framework for Health and Social Care Research . Available at: https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/uk-policy-framework-health-social-care-research/uk-policy-framework-health-and-social-care-research/#chiefinvestigators (Accessed: 9 June 2023).
Heslop, C., Burns, S., Lobo, R. (2018) ‘Managing qualitative research as insider-research in small rural communities’, Rural and Remote Health , 18: pp. 4576.
Equality Act 2010, c. 15. Available at: https://www.legislation.gov.uk/ukpga/2010/15/introduction (Accessed: 9 June 2023).
National Institute for Health and Care Research (no date) Equality, Diversity and Inclusion (EDI) . Available at: https://arc-kss.nihr.ac.uk/public-and-community-involvement/pcie-guide/how-to-do-pcie/equality-diversity-and-inclusion-edi (Accessed: 9 June 2023).
National Institute for Health and Care Research (2020) Improving inclusion of under-served groups in clinical research: Guidance from INCLUDE project. Available at: https://www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 (Accessed: 9 June 2023).
Sapiro, B. and Matthews, E. (2020) ‘Both Insider and Outsider. On Conducting Social Work Research in Mental Health Settings’, Advances in Social Work , 20(3). Available at: https://doi.org/10.18060/23926
Turcotte-Tremblay, A. and McSween-Cadieux, E. (2018) ‘A reflection on the challenge of protecting confidentiality of participants when disseminating research results locally’, BMC Medical Ethics, 19(supplement 1), no. 45. Available at: https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-018-0279-0
United Nations General Assembly (1948) The Universal Declaration of Human Rights . Resolution A/RES/217/A. Available at: https://www.un.org/en/about-us/universal-declaration-of-human-rights#:~:text=Drafted%20by%20representatives%20with%20different,all%20peoples%20and%20all%20nations . (Accessed: 9 June 2023).
Wellcome Trust (2014) Ensuring your research is ethical: A guide for Extended Project Qualification students . Available at: https://wellcome.org/sites/default/files/wtp057673_0.pdf (Accessed: 9 June 2023).
More articles from the research proposal collection

Writing your research proposal
A doctoral research degree is the highest academic qualification that a student can achieve. The guidance provided in these articles will help you apply for one of the two main types of research degree offered by The Open University.
Level: 1 Introductory
Defining your research methodology
Your research methodology is the approach you will take to guide your research process and explain why you use particular methods. This article explains more.

Writing your proposal and preparing for your interview
The final article looks at writing your research proposal - from the introduction through to citations and referencing - as well as preparing for your interview.
Free courses on postgraduate study

Are you ready for postgraduate study?
This free course, Are you ready for postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and ensure you are ready to develop the skills and confidence to pursue your learning further.

Succeeding in postgraduate study
This free course, Succeeding in postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and to develop the skills and confidence to pursue your learning further.

Applying to study for a PhD in psychology
This free OpenLearn course is for psychology students and graduates who are interested in PhD study at some future point. Even if you have met PhD students and heard about their projects, it is likely that you have only a vague idea of what PhD study entails. This course is intended to give you more information.
Become an OU student
Ratings & comments, share this free course, copyright information, publication details.
- Originally published: Tuesday, 27 June 2023
- Body text - Creative Commons BY-NC-SA 4.0 : The Open University
- Image 'Pebbles balance on a stone see-saw' - Copyright: Photo 51106733 / Balance © Anatoli Styf | Dreamstime.com
- Image 'Camera equipment set up filming a man talking' - Copyright: Photo 42631221 © Gabriel Robledo | Dreamstime.com
- Image 'Applying to study for a PhD in psychology' - Copyright free
- Image 'Succeeding in postgraduate study' - Copyright: © Everste/Getty Images
- Image 'Addressing ethical issues in your research proposal' - Copyright: Photo 50384175 / Children Playing © Lenutaidi | Dreamstime.com
- Image 'Writing your proposal and preparing for your interview' - Copyright: Photo 133038259 / Black Student © Fizkes | Dreamstime.com
- Image 'Defining your research methodology' - Copyright free
- Image 'Writing your research proposal' - Copyright free
- Image 'Are you ready for postgraduate study?' - Copyright free
Rate and Review
Rate this article, review this article.
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews
For further information, take a look at our frequently asked questions which may give you the support you need.
Research Ethics & Ethical Considerations
A Plain-Language Explainer With Examples
By: Derek Jansen (MBA) | Reviewers: Dr Eunice Rautenbach | May 2024

Research ethics are one of those “ unsexy but essential ” subjects that you need to fully understand (and apply) to conquer your dissertation, thesis or research paper. In this post, we’ll unpack research ethics using plain language and loads of examples .
Overview: Research Ethics 101
- What are research ethics?
- Why should you care?
- Research ethics principles
- Respect for persons
- Beneficence
- Objectivity
- Key takeaways
What (exactly) are research ethics?
At the simplest level, research ethics are a set of principles that ensure that your study is conducted responsibly, safely, and with integrity. More specifically, research ethics help protect the rights and welfare of your research participants, while also ensuring the credibility of your research findings.
Research ethics are critically important for a number of reasons:
Firstly, they’re a complete non-negotiable when it comes to getting your research proposal approved. Pretty much all universities will have a set of ethical criteria that student projects need to adhere to – and these are typically very strictly enforced. So, if your proposed study doesn’t tick the necessary ethical boxes, it won’t be approved .
Beyond the practical aspect of approval, research ethics are essential as they ensure that your study’s participants (whether human or animal) are properly protected . In turn, this fosters trust between you and your participants – as well as trust between researchers and the public more generally. As you can probably imagine, it wouldn’t be good if the general public had a negative perception of researchers!
Last but not least, research ethics help ensure that your study’s results are valid and reliable . In other words, that you measured the thing you intended to measure – and that other researchers can repeat your study. If you’re not familiar with the concepts of reliability and validity , we’ve got a straightforward explainer video covering that below.
The Core Principles
In practical terms, each university or institution will have its own ethics policy – so, what exactly constitutes “ethical research” will vary somewhat between institutions and countries. Nevertheless, there are a handful of core principles that shape ethics policies. These principles include:
Let’s unpack each of these to make them a little more tangible.
Ethics Principle 1: Respect for persons
As the name suggests, this principle is all about ensuring that your participants are treated fairly and respectfully . In practical terms, this means informed consent – in other words, participants should be fully informed about the nature of the research, as well as any potential risks. Additionally, they should be able to withdraw from the study at any time. This is especially important when you’re dealing with vulnerable populations – for example, children, the elderly or people with cognitive disabilities.
Another dimension of the “respect for persons” principle is confidentiality and data protection . In other words, your participants’ personal information should be kept strictly confidential and secure at all times. Depending on the specifics of your project, this might also involve anonymising or masking people’s identities. As mentioned earlier, the exact requirements will vary between universities, so be sure to thoroughly review your institution’s ethics policy before you start designing your project.
Need a helping hand?
Ethics Principle 2: Beneficence
This principle is a little more opaque, but in simple terms beneficence means that you, as the researcher, should aim to maximise the benefits of your work, while minimising any potential harm to your participants.
In practical terms, benefits could include advancing knowledge, improving health outcomes, or providing educational value. Conversely, potential harms could include:
- Physical harm from accidents or injuries
- Psychological harm, such as stress or embarrassment
- Social harm, such as stigmatisation or loss of reputation
- Economic harm – in other words, financial costs or lost income
Simply put, the beneficence principle means that researchers must always try to identify potential risks and take suitable measures to reduce or eliminate them.

Ethics Principle 3: Objectivity
As you can probably guess, this principle is all about attempting to minimise research bias to the greatest degree possible. In other words, you’ll need to reduce subjectivity and increase objectivity wherever possible.
In practical terms, this principle has the largest impact on the methodology of your study – specifically the data collection and data analysis aspects. For example, you’ll need to ensure that the selection of your participants (in other words, your sampling strategy ) is aligned with your research aims – and that your sample isn’t skewed in a way that supports your presuppositions.
If you’re keen to learn more about research bias and the various ways in which you could unintentionally skew your results, check out the video below.
Ethics Principle 4: Integrity
Again, no surprises here; this principle is all about producing “honest work” . It goes without saying that researchers should always conduct their work honestly and transparently, report their findings accurately, and disclose any potential conflicts of interest upfront.
This is all pretty obvious, but another aspect of the integrity principle that’s sometimes overlooked is respect for intellectual property . In practical terms, this means you need to honour any patents, copyrights, or other forms of intellectual property that you utilise while undertaking your research. Along the same vein, you shouldn’t use any unpublished data, methods, or results without explicit, written permission from the respective owner.
Linked to all of this is the broader issue of plagiarism . Needless to say, if you’re drawing on someone else’s published work, be sure to cite your sources, in the correct format. To make life easier, use a reference manager such as Mendeley or Zotero to ensure that your citations and reference list are perfectly polished.
FAQs: Research Ethics
Research ethics & ethical considertation, what is informed consent.
Informed consent simply means providing your potential participants with all necessary information about the study. This should include information regarding the study’s purpose, procedures, risks, and benefits. This information allows your potential participants to make a voluntary and informed decision about whether to participate.
How should I obtain consent from non-English speaking participants?
What about animals.
When conducting research with animals, ensure you adhere to ethical guidelines for the humane treatment of animals. Again, the exact requirements here will vary between institutions, but typically include minimising pain and distress, using alternatives where possible, and obtaining approval from an animal care and use committee.
What is the role of the ERB or IRB?
An ethics review board (ERB) or institutional review board (IRB) evaluates research proposals to ensure they meet ethical standards. The board reviews study designs, consent forms, and data handling procedures, to protect participants’ welfare and rights.
How can I obtain ethical approval for my project?
This varies between universities, but you will typically need to submit a detailed research proposal to your institution’s ethics committee. This proposal should include your research objectives, methods, and how you plan to address ethical considerations like informed consent, confidentiality, and risk minimisation. You can learn more about how to write a proposal here .
How do I ensure ethical collaboration when working with colleagues?
Collaborative research should be conducted with mutual respect and clear agreements on roles, contributions, and publication credits. Open communication is key to preventing conflicts and misunderstandings. Also, be sure to check whether your university has any specific requirements with regards to collaborative efforts and division of labour.
How should I address ethical concerns relating to my funding source?
Key takeaways: research ethics 101.
Here’s a quick recap of the key points we’ve covered:
- Research ethics are a set of principles that ensure that your study is conducted responsibly.
- It’s essential that you design your study around these principles, or it simply won’t get approved.
- The four ethics principles we looked at are: respect for persons, beneficence, objectivity and integrity
As mentioned, the exact requirements will vary slightly depending on the institution and country, so be sure to thoroughly review your university’s research ethics policy before you start developing your study.

Psst... there’s more!
This post was based on one of our popular Research Bootcamps . If you're working on a research project, you'll definitely want to check this out ...
You Might Also Like:

Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Ethical considerations in research: Best practices and examples

To conduct responsible research, you’ve got to think about ethics. They protect participants’ rights and their well-being - and they ensure your findings are valid and reliable. This isn’t just a box for you to tick. It’s a crucial consideration that can make all the difference to the outcome of your research.
In this article, we'll explore the meaning and importance of research ethics in today's research landscape. You'll learn best practices to conduct ethical and impactful research.
Examples of ethical considerations in research
As a researcher, you're responsible for ethical research alongside your organization. Fulfilling ethical guidelines is critical. Organizations must ensure employees follow best practices to protect participants' rights and well-being.
Keep these things in mind when it comes to ethical considerations in research:
Voluntary participation
Voluntary participation is key. Nobody should feel like they're being forced to participate or pressured into doing anything they don't want to. That means giving people a choice and the ability to opt out at any time, even if they've already agreed to take part in the study.
Informed consent
Informed consent isn't just an ethical consideration. It's a legal requirement as well. Participants must fully understand what they're agreeing to, including potential risks and benefits.
The best way to go about this is by using a consent form. Make sure you include:
- A brief description of the study and research methods.
- The potential benefits and risks of participating.
- The length of the study.
- Contact information for the researcher and/or sponsor.
- Reiteration of the participant’s right to withdraw from the research project at any time without penalty.
Anonymity means that participants aren't identifiable in any way. This includes:
- Email address
- Photographs
- Video footage
You need a way to anonymize research data so that it can't be traced back to individual participants. This may involve creating a new digital ID for participants that can’t be linked back to their original identity using numerical codes.
Confidentiality
Information gathered during a study must be kept confidential. Confidentiality helps to protect the privacy of research participants. It also ensures that their information isn't disclosed to unauthorized individuals.
Some ways to ensure confidentiality include:
- Using a secure server to store data.
- Removing identifying information from databases that contain sensitive data.
- Using a third-party company to process and manage research participant data.
- Not keeping participant records for longer than necessary.
- Avoiding discussion of research findings in public forums.
Potential for harm
The potential for harm is a crucial factor in deciding whether a research study should proceed. It can manifest in various forms, such as:
- Psychological harm
- Social harm
- Physical harm
Conduct an ethical review to identify possible harms. Be prepared to explain how you’ll minimize these harms and what support is available in case they do happen.
Fair payment
One of the most crucial aspects of setting up a research study is deciding on fair compensation for your participants. Underpayment is a common ethical issue that shouldn't be overlooked. Properly rewarding participants' time is critical for boosting engagement and obtaining high-quality data. While Prolific requires a minimum payment of £6.00 / $8.00 per hour, there are other factors you need to consider when deciding on a fair payment.
First, check your institution's reimbursement guidelines to see if they already have a minimum or maximum hourly rate. You can also use the national minimum wage as a reference point.
Next, think about the amount of work you're asking participants to do. The level of effort required for a task, such as producing a video recording versus a short survey, should correspond with the reward offered.
You also need to consider the population you're targeting. To attract research subjects with specific characteristics or high-paying jobs, you may need to offer more as an incentive.
We recommend a minimum payment of £9.00 / $12.00 per hour, but we understand that payment rates can vary depending on a range of factors. Whatever payment you choose should reflect the amount of effort participants are required to put in and be fair to everyone involved.
Ethical research made easy with Prolific
At Prolific, we believe in making ethical research easy and accessible. The findings from the Fairwork Cloudwork report speak for themselves. Prolific was given the top score out of all competitors for minimum standards of fair work.
With over 25,000 researchers in our community, we're leading the way in revolutionizing the research industry. If you're interested in learning more about how we can support your research journey, sign up to get started now.
You might also like

High-quality human data to deliver world-leading research and AIs.

Follow us on
All Rights Reserved Prolific 2024

What Are the Ethical Considerations in Research Design?
When I began my work on the thesis I was always focused on my research. However, once I began to make my way through research, I realized that research ethics is a core aspect of the research work and the foundation of research design.
Research ethics play a crucial role in ensuring the responsible conduct of research. Here are some key reasons why research ethics matter:

Let us look into some of the major ethical considerations in research design.
Ethical Issues in Research
There are many organizations, like the Committee on Publication Ethics , dedicated to promoting ethics in scientific research. These organizations agree that ethics is not an afterthought or side note to the research study. It is an integral aspect of research that needs to remain at the forefront of our work.
The research design must address specific research questions. Hence, the conclusions of the study must correlate to the questions posed and the results. Also, research ethics demands that the methods used must relate specifically to the research questions.
Voluntary Participation and Consent
An individual should at no point feel any coercion to participate in a study. This includes any type of persuasion or deception in attempting to gain an individual’s trust.
Informed consent states that an individual must give their explicit consent to participate in the study. You can think of consent form as an agreement of trust between the researcher and the participants.
Sampling is the first step in research design . You will need to explain why you want a particular group of participants. You will have to explain why you left out certain people or groups. In addition, if your sample includes children or special needs individuals, you will have additional requirements to address like parental permission.
Confidentiality
The third ethics principle of the Economic and Social Research Council (ESRC) states that: “The confidentiality of the information supplied by research subjects and the anonymity of respondents must be respected.” However, sometimes confidentiality is limited. For example, if a participant is at risk of harm, we must protect them. This might require releasing confidential information.
Risk of Harm
We should do everything in our power to protect study participants. For this, we should focus on the risk to benefit ratio. If possible risks outweigh the benefits, then we should abandon or redesign the study. Risk of harm also requires us to measure the risk to benefit ratio as the study progresses.
Research Methods
We know there are numerous research methods. However, when it comes to ethical considerations, some key questions can help us find the right approach for our studies.
i. Which methods most effectively fit the aims of your research?
ii. What are the strengths and restrictions of a particular method?
iii. Are there potential risks when using a particular research method?
For more guidance, you can refer to the ESRC Framework for Research Ethics .
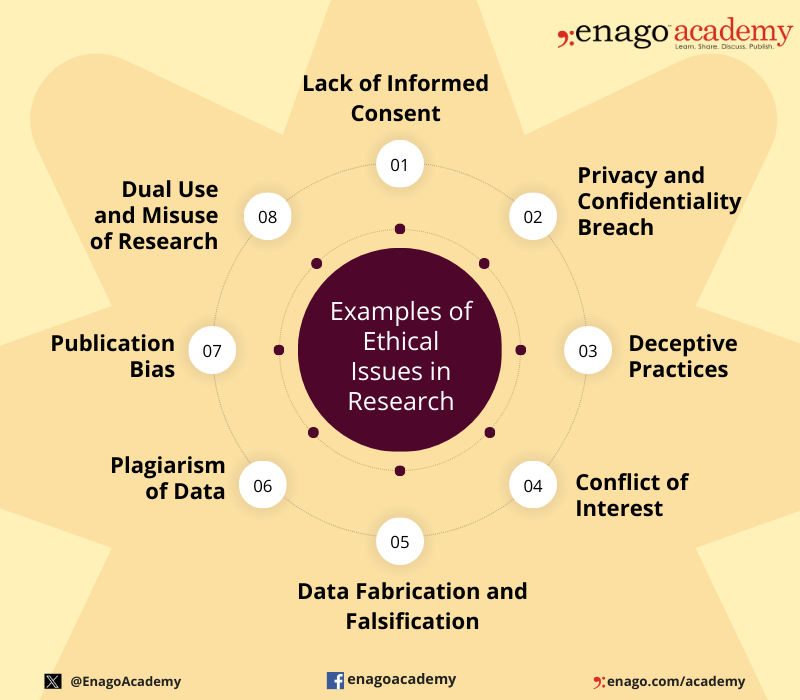
Ethical issues in research can arise at various stages of the research process and involve different aspects of the study. Here are some common examples of ethical issues in research:
Institutional Review Boards
The importance of ethics in research cannot be understated. Following ethical guidelines will ensure your study’s validity and promote its contribution to scientific study. On a personal level, you will strengthen your research and increase your opportunities to gain funding.
To address the need for ethical considerations, most institutions have their own Institutional Review Board (IRB). An IRB secures the safety of human participants and prevents violation of human rights. It reviews the research aims and methodologies to ensure ethical practices are followed. If a research design does not follow the set ethical guidelines, then the researcher will have to amend their study.
Applying for Ethical Approval
Applications for ethical approval will differ across institutions. Regardless, they focus on the benefits of your research and the risk to benefit ratio concerning participants. Therefore, you need to effectively address both in order to get ethical clearence.
Participants
It is vital that you make it clear that individuals are provided with sufficient information in order to make an informed decision on their participation. In addition, you need to demonstrate that the ethical issues of consent, risk of harm, and confidentiality are clearly defined.
Benefits of the Study
You need to prove to the panel that your work is essential and will yield results that contribute to the scientific community. For this, you should demonstrate the following:
i. The conduct of research guarantees the quality and integrity of results.
ii. The research will be properly distributed.
iii. The aims of the research are clear and the methodology is appropriate.
Integrity and transparency are vital in the research. Ethics committees expect you to share any actual or potential conflicts of interest that could affect your work. In addition, you have to be honest and transparent throughout the approval process and the research process.
The Dangers of Unethical Practices
There is a reason to follow ethical guidelines. Without these guidelines, our research will suffer. Moreover, more importantly, people could suffer.
The following are just two examples of infamous cases of unethical research practices that demonstrate the importance of adhering to ethical standards:
- The Stanford Prison Experiment (1971) aimed to investigate the psychological effects of power using the relationship between prisoners and prison officers. Those assigned the role of “prison officers” embraced measures that exposed “prisoners” to psychological and physical harm. In this case, there was voluntary participation. However, there was disregard for welfare of the participants.
- Recently, Chinese scientist He Jiankui announced his work on genetically edited babies . Over 100 Chinese scientists denounced this research, calling it “crazy” and “shocking and unacceptable.” This research shows a troubling attitude of “do first, debate later” and a disregard for the ethical concerns of manipulating the human body Wang Yuedan, a professor of immunology at Peking University, calls this “an ethics disaster for the world” and demands strict punishments for this type of ethics violation.
What are your experiences with research ethics? How have you developed an ethical approach to research design? Please share your thoughts with us in the comments section below.
I love the articulation of reasoning and practical examples of unethical research
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- AI in Academia
- Trending Now
6 Leading AI Detection Tools for Academic Writing — A comparative analysis
The advent of AI content generators, exemplified by advanced models like ChatGPT, Claude AI, and…

- Reporting Research
Choosing the Right Analytical Approach: Thematic analysis vs. content analysis for data interpretation
In research, choosing the right approach to understand data is crucial for deriving meaningful insights.…

- Industry News
China’s Ministry of Education Spearheads Efforts to Uphold Academic Integrity
In response to the increase in retractions of research papers submitted by Chinese scholars to…


Comparing Cross Sectional and Longitudinal Studies: 5 steps for choosing the right approach
The process of choosing the right research design can put ourselves at the crossroads of…

- Publishing Research
- Understanding Ethics
Understanding the Difference Between Research Ethics and Compliance
Ethics refers to the principles, values, and moral guidelines that guide individual or group behavior…
Unlocking the Power of Networking in Academic Conferences
Intersectionality in Academia: Dealing with diverse perspectives
Meritocracy and Diversity in Science: Increasing inclusivity in STEM education
Avoiding the AI Trap: Pitfalls of relying on ChatGPT for PhD applications

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

As a researcher, what do you consider most when choosing an image manipulation detector?
Ethical Issues in Research
- Living reference work entry
- First Online: 05 March 2021
- Cite this living reference work entry

- Juwel Rana 2 , 3 , 4 ,
- Segufta Dilshad 2 &
- Md. Ali Ahsan 5
375 Accesses
2 Citations
The most important human endeavor is the striving for morality in our actions. Our inner balance and even our very existence depend on it. Only morality in our actions can give beauty and dignity to life – Albert Einstein.
Ethics ; Methodology ; Mixed-method research ; Observation ; Qualitative research ; Quantitative research ; Research ; Research design ; Research ethics
Ethics is a set of standards, a code, or value system, worked out from human reason and experience, by which free human actions are determined as ultimately right or wrong, good, or evil. If acting agrees with these standards, it is ethical, otherwise unethical.
Scientific research refers to a persistent exercise towards producing new knowledge to unveil a new stream of ideas in academia for humankind.
Research ethics refer to some of the genres that researchers follow to protect the rights in developing research strategies and building a trusted relationship between the...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Bulmer M (1982) Social Research Ethics: An Examination of the Merits of Covert Participant Observation. Holmes & Meier Publishers
Google Scholar
Butler I (2002) A Code of Ethics for Social Work and Social Care Research. Br J Soc Work [Internet]. 32(2):239–48. Available from: https://doi.org/10.1093/bjsw/32.2.239
Fisher CB, Anushko AE (2008) The SAGE Handbook of Social Research Methods [Internet]. London: SAGE Publications Ltd; p. 95–109. Available from: https://methods.sagepub.com/book/the-sage-handbook-of-socialresearch-methods
Hill J, Wright LT (2001) A qualitative research agenda for small to medium-sized enterprises. Mark Intell Plan 19(6):432–443
Homan R (1991) The ethics of social research. Addison-Wesley Longman Limited
Israel M, Hay I (2006) Research ethics for social scientists. Sage
Kimmel AJ (1988) Ethics and values in applied social research. 1st ed. SAGE Publications Inc
Orb A, Eisenhauer L, Wynaden D (2001) Ethics in qualitative research. J Nurs Scholarsh 33(1):93–96
Principles of research ethics [Internet]. Lund Research Ltd. 2012 [cited 2020 Dec 15]. Available from: https://dissertation.laerd.com/principles-of-research-ethics.php
Robley LR (1995) The ethics of qualitative nursing research. J Prof Nurs 11(1):45–48
Wiles R, Charles V, Crow G, Heath S (2006) Researching researchers: lessons for research ethics. Qual Res. 6(3):283–99
Download references
Author information
Authors and affiliations.
Department of Public Health, School of Health and Life Sciences, North South University, Dhaka, Bangladesh
Juwel Rana & Segufta Dilshad
Department of Biostatistics and Epidemiology, School of Health and Health Sciences, University of Massachusetts Amherst, Amherst, MA, USA
Department of Research and Innovation, South Asia Institute for Social Transformation (SAIST), Dhaka, Bangladesh
Space and Environment Research Center (SERC), Rajshahi, Bangladesh
Md. Ali Ahsan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Juwel Rana .
Editor information
Editors and affiliations.
Florida Atlantic University, Boca Raton, FL, USA
Ali Farazmand
Rights and permissions
Reprints and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry.
Rana, J., Dilshad, S., Ahsan, M.A. (2021). Ethical Issues in Research. In: Farazmand, A. (eds) Global Encyclopedia of Public Administration, Public Policy, and Governance. Springer, Cham. https://doi.org/10.1007/978-3-319-31816-5_462-1
Download citation
DOI : https://doi.org/10.1007/978-3-319-31816-5_462-1
Received : 01 February 2021
Accepted : 14 February 2021
Published : 05 March 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-31816-5
Online ISBN : 978-3-319-31816-5
eBook Packages : Springer Reference Economics and Finance Reference Module Humanities and Social Sciences Reference Module Business, Economics and Social Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Ethical Considerations
Ethical Considerations can be specified as one of the most important parts of the research. Dissertations may even be doomed to failure if this part is missing.
According to Bryman and Bell (2007) [1] the following ten points represent the most important principles related to ethical considerations in dissertations:
- Research participants should not be subjected to harm in any ways whatsoever.
- Respect for the dignity of research participants should be prioritised.
- Full consent should be obtained from the participants prior to the study.
- The protection of the privacy of research participants has to be ensured.
- Adequate level of confidentiality of the research data should be ensured.
- Anonymity of individuals and organisations participating in the research has to be ensured.
- Any deception or exaggeration about the aims and objectives of the research must be avoided.
- Affiliations in any forms, sources of funding, as well as any possible conflicts of interests have to be declared.
- Any type of communication in relation to the research should be done with honesty and transparency.
- Any type of misleading information, as well as representation of primary data findings in a biased way must be avoided.
In order to address ethical considerations aspect of your dissertation in an effective manner, you will need to expand discussions of each of the following points to at least one paragraph:
1. Voluntary participation of respondents in the research is important. Moreover, participants have rights to withdraw from the study at any stage if they wish to do so.
2. Respondents should participate on the basis of informed consent. The principle of informed consent involves researchers providing sufficient information and assurances about taking part to allow individuals to understand the implications of participation and to reach a fully informed, considered and freely given decision about whether or not to do so, without the exercise of any pressure or coercion. [2]
3. The use of offensive, discriminatory, or other unacceptable language needs to be avoided in the formulation of Questionnaire/Interview/Focus group questions.
4. Privacy and anonymity or respondents is of a paramount importance.
5. Acknowledgement of works of other authors used in any part of the dissertation with the use of Harvard/APA/Vancouver referencing system according to the Dissertation Handbook
6. Maintenance of the highest level of objectivity in discussions and analyses throughout the research
7. Adherence to Data Protection Act (1998) if you are studying in the UK
In studies that do not involve primary data collection, on the other hand, ethical issues are going to be limited to the points d) and e) above.
Most universities have their own Code of Ethical Practice. It is critically important for you to thoroughly adhere to this code in every aspect of your research and declare your adherence in ethical considerations part of your dissertation.
My e-book, The Ultimate Guide to Writing a Dissertation in Business Studies: a step by step assistance offers practical assistance to complete a dissertation with minimum or no stress. The e-book covers all stages of writing a dissertation starting from the selection to the research area to submitting the completed version of the work within the deadline. John Dudovskiy

[1] Bryman, A. & Bell, E. (2007) “Business Research Methods”, 2nd edition. Oxford University Press.
[2] Saunders, M., Lewis, P. & Thornhill, A. (2012) “Research Methods for Business Students” 6th edition, Pearson Education Limited.
Ethical consideration of the research proposal and the informed-consent process: An online survey of researchers and ethics committee members in Thailand
Affiliations.
- 1 a Department of Tropical Hygiene, Faculty of Tropical Medicine , Mahidol University , Bangkok , Thailand.
- 2 b Office of Research Services, Faculty of Tropical Medicine , Mahidol University , Bangkok , Thailand.
- PMID: 30987450
- DOI: 10.1080/08989621.2019.1608190
Researchers designing and conducting studies using human data should consider the values and principles of ethical conduct. Research ethics committees (RECs) typically evaluate the ethical acceptability of research proposals. Sometimes, differences arise between how researchers and RECs interpret ethical principles, and how they decide what constitutes ethical conduct. This study aimed to explore the opinions of these two groups about the importance of core ethical issues in the proposal and in the informed-consent process. An anonymous online questionnaire was distributed to a target population in health-related academic/research institutes across Thailand; 219 researchers and 72 REC members participated. Significantly, more REC members than researchers attributed the highest importance to three core ethical considerations - risk/benefit, vulnerability, and confidentiality/privacy. For the informed-consent process, significant differences were found for communication of risks, decision-making authority for consent, process for approaching study participants, and availability of a contact for study deviations/violations. The different ratings indicate differences in the groups' perspectives on ethical principles, which may affect focal congruence on ethical issues in the proposal. Communication of these findings should help close gaps between REC and researcher perceptions. Further study should investigate how RECs and researchers perceive equivocal ethics terms.
Keywords: Ethical consideration; ethics committee; informed-consent form; informed-consent process; perception.
- Committee Membership*
- Ethics Committees, Research*
- Informed Consent / ethics*
- Research Design
- Research Personnel / psychology*
- Surveys and Questionnaires
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Ethical Considerations in Research | Types & Examples
Ethical Considerations in Research | Types & Examples
Published on 7 May 2022 by Pritha Bhandari .
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviours, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to:
- Protect the rights of research participants
- Enhance research validity
- Maintain scientific integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research aims with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Prevent plagiarism, run a free check.
Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process, so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
- What the study is about
- The risks and benefits of taking part
- How long the study will take
- Your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymise data collection. For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymisation is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants, but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study, as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources, counselling, or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine scientific integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Bhandari, P. (2022, May 07). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved 27 May 2024, from https://www.scribbr.co.uk/research-methods/ethical-considerations/
Is this article helpful?

Pritha Bhandari
Other students also liked, a quick guide to experimental design | 5 steps & examples, data collection methods | step-by-step guide & examples, how to avoid plagiarism | tips on citing sources.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Anaesth
- v.60(9); 2016 Sep
How to write a research proposal?
Department of Anaesthesiology, Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India
Devika Rani Duggappa
Writing the proposal of a research work in the present era is a challenging task due to the constantly evolving trends in the qualitative research design and the need to incorporate medical advances into the methodology. The proposal is a detailed plan or ‘blueprint’ for the intended study, and once it is completed, the research project should flow smoothly. Even today, many of the proposals at post-graduate evaluation committees and application proposals for funding are substandard. A search was conducted with keywords such as research proposal, writing proposal and qualitative using search engines, namely, PubMed and Google Scholar, and an attempt has been made to provide broad guidelines for writing a scientifically appropriate research proposal.
INTRODUCTION
A clean, well-thought-out proposal forms the backbone for the research itself and hence becomes the most important step in the process of conduct of research.[ 1 ] The objective of preparing a research proposal would be to obtain approvals from various committees including ethics committee [details under ‘Research methodology II’ section [ Table 1 ] in this issue of IJA) and to request for grants. However, there are very few universally accepted guidelines for preparation of a good quality research proposal. A search was performed with keywords such as research proposal, funding, qualitative and writing proposals using search engines, namely, PubMed, Google Scholar and Scopus.
Five ‘C’s while writing a literature review

BASIC REQUIREMENTS OF A RESEARCH PROPOSAL
A proposal needs to show how your work fits into what is already known about the topic and what new paradigm will it add to the literature, while specifying the question that the research will answer, establishing its significance, and the implications of the answer.[ 2 ] The proposal must be capable of convincing the evaluation committee about the credibility, achievability, practicality and reproducibility (repeatability) of the research design.[ 3 ] Four categories of audience with different expectations may be present in the evaluation committees, namely academic colleagues, policy-makers, practitioners and lay audiences who evaluate the research proposal. Tips for preparation of a good research proposal include; ‘be practical, be persuasive, make broader links, aim for crystal clarity and plan before you write’. A researcher must be balanced, with a realistic understanding of what can be achieved. Being persuasive implies that researcher must be able to convince other researchers, research funding agencies, educational institutions and supervisors that the research is worth getting approval. The aim of the researcher should be clearly stated in simple language that describes the research in a way that non-specialists can comprehend, without use of jargons. The proposal must not only demonstrate that it is based on an intelligent understanding of the existing literature but also show that the writer has thought about the time needed to conduct each stage of the research.[ 4 , 5 ]
CONTENTS OF A RESEARCH PROPOSAL
The contents or formats of a research proposal vary depending on the requirements of evaluation committee and are generally provided by the evaluation committee or the institution.
In general, a cover page should contain the (i) title of the proposal, (ii) name and affiliation of the researcher (principal investigator) and co-investigators, (iii) institutional affiliation (degree of the investigator and the name of institution where the study will be performed), details of contact such as phone numbers, E-mail id's and lines for signatures of investigators.
The main contents of the proposal may be presented under the following headings: (i) introduction, (ii) review of literature, (iii) aims and objectives, (iv) research design and methods, (v) ethical considerations, (vi) budget, (vii) appendices and (viii) citations.[ 4 ]
Introduction
It is also sometimes termed as ‘need for study’ or ‘abstract’. Introduction is an initial pitch of an idea; it sets the scene and puts the research in context.[ 6 ] The introduction should be designed to create interest in the reader about the topic and proposal. It should convey to the reader, what you want to do, what necessitates the study and your passion for the topic.[ 7 ] Some questions that can be used to assess the significance of the study are: (i) Who has an interest in the domain of inquiry? (ii) What do we already know about the topic? (iii) What has not been answered adequately in previous research and practice? (iv) How will this research add to knowledge, practice and policy in this area? Some of the evaluation committees, expect the last two questions, elaborated under a separate heading of ‘background and significance’.[ 8 ] Introduction should also contain the hypothesis behind the research design. If hypothesis cannot be constructed, the line of inquiry to be used in the research must be indicated.
Review of literature
It refers to all sources of scientific evidence pertaining to the topic in interest. In the present era of digitalisation and easy accessibility, there is an enormous amount of relevant data available, making it a challenge for the researcher to include all of it in his/her review.[ 9 ] It is crucial to structure this section intelligently so that the reader can grasp the argument related to your study in relation to that of other researchers, while still demonstrating to your readers that your work is original and innovative. It is preferable to summarise each article in a paragraph, highlighting the details pertinent to the topic of interest. The progression of review can move from the more general to the more focused studies, or a historical progression can be used to develop the story, without making it exhaustive.[ 1 ] Literature should include supporting data, disagreements and controversies. Five ‘C's may be kept in mind while writing a literature review[ 10 ] [ Table 1 ].
Aims and objectives
The research purpose (or goal or aim) gives a broad indication of what the researcher wishes to achieve in the research. The hypothesis to be tested can be the aim of the study. The objectives related to parameters or tools used to achieve the aim are generally categorised as primary and secondary objectives.
Research design and method
The objective here is to convince the reader that the overall research design and methods of analysis will correctly address the research problem and to impress upon the reader that the methodology/sources chosen are appropriate for the specific topic. It should be unmistakably tied to the specific aims of your study.
In this section, the methods and sources used to conduct the research must be discussed, including specific references to sites, databases, key texts or authors that will be indispensable to the project. There should be specific mention about the methodological approaches to be undertaken to gather information, about the techniques to be used to analyse it and about the tests of external validity to which researcher is committed.[ 10 , 11 ]
The components of this section include the following:[ 4 ]
Population and sample
Population refers to all the elements (individuals, objects or substances) that meet certain criteria for inclusion in a given universe,[ 12 ] and sample refers to subset of population which meets the inclusion criteria for enrolment into the study. The inclusion and exclusion criteria should be clearly defined. The details pertaining to sample size are discussed in the article “Sample size calculation: Basic priniciples” published in this issue of IJA.
Data collection
The researcher is expected to give a detailed account of the methodology adopted for collection of data, which include the time frame required for the research. The methodology should be tested for its validity and ensure that, in pursuit of achieving the results, the participant's life is not jeopardised. The author should anticipate and acknowledge any potential barrier and pitfall in carrying out the research design and explain plans to address them, thereby avoiding lacunae due to incomplete data collection. If the researcher is planning to acquire data through interviews or questionnaires, copy of the questions used for the same should be attached as an annexure with the proposal.
Rigor (soundness of the research)
This addresses the strength of the research with respect to its neutrality, consistency and applicability. Rigor must be reflected throughout the proposal.
It refers to the robustness of a research method against bias. The author should convey the measures taken to avoid bias, viz. blinding and randomisation, in an elaborate way, thus ensuring that the result obtained from the adopted method is purely as chance and not influenced by other confounding variables.
Consistency
Consistency considers whether the findings will be consistent if the inquiry was replicated with the same participants and in a similar context. This can be achieved by adopting standard and universally accepted methods and scales.
Applicability
Applicability refers to the degree to which the findings can be applied to different contexts and groups.[ 13 ]
Data analysis
This section deals with the reduction and reconstruction of data and its analysis including sample size calculation. The researcher is expected to explain the steps adopted for coding and sorting the data obtained. Various tests to be used to analyse the data for its robustness, significance should be clearly stated. Author should also mention the names of statistician and suitable software which will be used in due course of data analysis and their contribution to data analysis and sample calculation.[ 9 ]
Ethical considerations
Medical research introduces special moral and ethical problems that are not usually encountered by other researchers during data collection, and hence, the researcher should take special care in ensuring that ethical standards are met. Ethical considerations refer to the protection of the participants' rights (right to self-determination, right to privacy, right to autonomy and confidentiality, right to fair treatment and right to protection from discomfort and harm), obtaining informed consent and the institutional review process (ethical approval). The researcher needs to provide adequate information on each of these aspects.
Informed consent needs to be obtained from the participants (details discussed in further chapters), as well as the research site and the relevant authorities.
When the researcher prepares a research budget, he/she should predict and cost all aspects of the research and then add an additional allowance for unpredictable disasters, delays and rising costs. All items in the budget should be justified.
Appendices are documents that support the proposal and application. The appendices will be specific for each proposal but documents that are usually required include informed consent form, supporting documents, questionnaires, measurement tools and patient information of the study in layman's language.
As with any scholarly research paper, you must cite the sources you used in composing your proposal. Although the words ‘references and bibliography’ are different, they are used interchangeably. It refers to all references cited in the research proposal.
Successful, qualitative research proposals should communicate the researcher's knowledge of the field and method and convey the emergent nature of the qualitative design. The proposal should follow a discernible logic from the introduction to presentation of the appendices.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS 2024 Datasets and Benchmarks Track
If you'd like to become a reviewer for the track, or recommend someone, please use this form .
The Datasets and Benchmarks track serves as a venue for high-quality publications, talks, and posters on highly valuable machine learning datasets and benchmarks, as well as a forum for discussions on how to improve dataset development. Datasets and benchmarks are crucial for the development of machine learning methods, but also require their own publishing and reviewing guidelines. For instance, datasets can often not be reviewed in a double-blind fashion, and hence full anonymization will not be required. On the other hand, they do require additional specific checks, such as a proper description of how the data was collected, whether they show intrinsic bias, and whether they will remain accessible. The Datasets and Benchmarks track is proud to support the open source movement by encouraging submissions of open-source libraries and tools that enable or accelerate ML research.
The previous editions of the Datasets and Benchmarks track were highly successful; you can view the accepted papers from 2021 , 2002 , and 2023 , and the winners of the best paper awards 2021 , 2022 and 2023
CRITERIA. W e are aiming for an equally stringent review as the main conference, yet better suited to datasets and benchmarks. Submissions to this track will be reviewed according to a set of criteria and best practices specifically designed for datasets and benchmarks , as described below. A key criterion is accessibility: datasets should be available and accessible , i.e. the data can be found and obtained without a personal request to the PI, and any required code should be open source. We encourage the authors to use Croissant format ( https://mlcommons.org/working-groups/data/croissant/ ) to document their datasets in machine readable way. Next to a scientific paper, authors should also submit supplementary materials such as detail on how the data was collected and organised, what kind of information it contains, how it should be used ethically and responsibly, as well as how it will be made available and maintained.
RELATIONSHIP TO NeurIPS. Submissions to the track will be part of the main NeurIPS conference , presented alongside the main conference papers. Accepted papers will be officially published in the NeurIPS proceedings .
SUBMISSIONS. There will be one deadline this year. It is also still possible to submit datasets and benchmarks to the main conference (under the usual review process), but dual submission to both is not allowed (unless you retracted your paper from the main conference). We also cannot transfer papers from the main track to the D&B track. Authors can choose to submit either single-blind or double-blind . If it is possible to properly review the submission double-blind, i.e., reviewers do not need access to non-anonymous repositories to review the work, then authors can also choose to submit the work anonymously. Papers will not be publicly visible during the review process. Only accepted papers will become visible afterward. The reviews themselves are not visible during the review phase but will be published after decisions have been made. The datasets themselves should be accessible to reviewers but can be publicly released at a later date (see below). New authors cannot be added after the abstract deadline and they should have an OpenReview profile by the paper deadline. NeurIPS does not tolerate any collusion whereby authors secretly cooperate with reviewers, ACs or SACs to obtain favourable reviews.
SCOPE. This track welcomes all work on data-centric machine learning research (DMLR) and open-source libraries and tools that enable or accelerate ML research, covering ML datasets and benchmarks as well as algorithms, tools, methods, and analyses for working with ML data. This includes but is not limited to:
- New datasets, or carefully and thoughtfully designed (collections of) datasets based on previously available data.
- Data generators and reinforcement learning environments.
- Data-centric AI methods and tools, e.g. to measure and improve data quality or utility, or studies in data-centric AI that bring important new insight.
- Advanced practices in data collection and curation that are of general interest even if the data itself cannot be shared.
- Frameworks for responsible dataset development, audits of existing datasets, identifying significant problems with existing datasets and their use
- Benchmarks on new or existing datasets, as well as benchmarking tools.
- In-depth analyses of machine learning challenges and competitions (by organisers and/or participants) that yield important new insight.
- Systematic analyses of existing systems on novel datasets yielding important new insight.
Read our original blog post for more about why we started this track.
Important dates
- Abstract submission deadline: May 29, 2024
- Full paper submission and co-author registration deadline: Jun 5, 2024
- Supplementary materials submission deadline: Jun 12, 2024
- Review deadline - Jul 24, 2024
- Release of reviews and start of Author discussions on OpenReview: Aug 07, 2024
- End of author/reviewer discussions on OpenReview: Aug 31, 2024
- Author notification: Sep 26, 2024
- Camera-ready deadline: Oct 30, 2024 AOE
Note: The site will start accepting submissions on April 1 5 , 2024.
FREQUENTLY ASKED QUESTIONS
Q: My work is in scope for this track but possibly also for the main conference. Where should I submit it?
A: This is ultimately your choice. Consider the main contribution of the submission and how it should be reviewed. If the main contribution is a new dataset, benchmark, or other work that falls into the scope of the track (see above), then it is ideally reviewed accordingly. As discussed in our blog post, the reviewing procedures of the main conference are focused on algorithmic advances, analysis, and applications, while the reviewing in this track is equally stringent but designed to properly assess datasets and benchmarks. Other, more practical considerations are that this track allows single-blind reviewing (since anonymization is often impossible for hosted datasets) and intended audience, i.e., make your work more visible for people looking for datasets and benchmarks.
Q: How will paper accepted to this track be cited?
A: Accepted papers will appear as part of the official NeurIPS proceedings.
Q: Do I need to submit an abstract beforehand?
A: Yes, please check the important dates section for more information.
Q: My dataset requires open credentialized access. Can I submit to this track?
A: This will be possible on the condition that a credentialization is necessary for the public good (e.g. because of ethically sensitive medical data), and that an established credentialization procedure is in place that is 1) open to a large section of the public, 2) provides rapid response and access to the data, and 3) is guaranteed to be maintained for many years. A good example here is PhysioNet Credentialing, where users must first understand how to handle data with human subjects, yet is open to anyone who has learned and agrees with the rules. This should be seen as an exceptional measure, and NOT as a way to limit access to data for other reasons (e.g. to shield data behind a Data Transfer Agreement). Misuse would be grounds for desk rejection. During submission, you can indicate that your dataset involves open credentialized access, in which case the necessity, openness, and efficiency of the credentialization process itself will also be checked.
SUBMISSION INSTRUCTIONS
A submission consists of:
- Please carefully follow the Latex template for this track when preparing proposals. We follow the NeurIPS format, but with the appropriate headings, and without hiding the names of the authors. Download the template as a bundle here .
- Papers should be submitted via OpenReview
- Reviewing is in principle single-blind, hence the paper should not be anonymized. In cases where the work can be reviewed equally well anonymously, anonymous submission is also allowed.
- During submission, you can add a public link to the dataset or benchmark data. If the dataset can only be released later, you must include instructions for reviewers on how to access the dataset. This can only be done after the first submission by sending an official note to the reviewers in OpenReview. We highly recommend making the dataset publicly available immediately or before the start of the NeurIPS conference. In select cases, requiring solid motivation, the release date can be stretched up to a year after the submission deadline.
- Dataset documentation and intended uses. Recommended documentation frameworks include datasheets for datasets , dataset nutrition labels , data statements for NLP , data cards , and accountability frameworks .
- URL to website/platform where the dataset/benchmark can be viewed and downloaded by the reviewers.
- URL to Croissant metadata record documenting the dataset/benchmark available for viewing and downloading by the reviewers. You can create your Croissant metadata using e.g. the Python library available here: https://github.com/mlcommons/croissant
- Author statement that they bear all responsibility in case of violation of rights, etc., and confirmation of the data license.
- Hosting, licensing, and maintenance plan. The choice of hosting platform is yours, as long as you ensure access to the data (possibly through a curated interface) and will provide the necessary maintenance.
- Links to access the dataset and its metadata. This can be hidden upon submission if the dataset is not yet publicly available but must be added in the camera-ready version. In select cases, e.g when the data can only be released at a later date, this can be added afterward (up to a year after the submission deadline). Simulation environments should link to open source code repositories
- The dataset itself should ideally use an open and widely used data format. Provide a detailed explanation on how the dataset can be read. For simulation environments, use existing frameworks or explain how they can be used.
- Long-term preservation: It must be clear that the dataset will be available for a long time, either by uploading to a data repository or by explaining how the authors themselves will ensure this
- Explicit license: Authors must choose a license, ideally a CC license for datasets, or an open source license for code (e.g. RL environments). An overview of licenses can be found here: https://paperswithcode.com/datasets/license
- Add structured metadata to a dataset's meta-data page using Web standards (like schema.org and DCAT ): This allows it to be discovered and organized by anyone. A guide can be found here: https://developers.google.com/search/docs/data-types/dataset . If you use an existing data repository, this is often done automatically.
- Highly recommended: a persistent dereferenceable identifier (e.g. a DOI minted by a data repository or a prefix on identifiers.org ) for datasets, or a code repository (e.g. GitHub, GitLab,...) for code. If this is not possible or useful, please explain why.
- For benchmarks, the supplementary materials must ensure that all results are easily reproducible. Where possible, use a reproducibility framework such as the ML reproducibility checklist , or otherwise guarantee that all results can be easily reproduced, i.e. all necessary datasets, code, and evaluation procedures must be accessible and documented.
- For papers introducing best practices in creating or curating datasets and benchmarks, the above supplementary materials are not required.
- For papers resubmitted after being retracted from another venue: a brief discussion on the main concerns raised by previous reviewers and how you addressed them. You do not need to share the original reviews.
- For the dual submission and archiving, the policy follows the NeurIPS main track paper guideline .
Use of Large Language Models (LLMs): We welcome authors to use any tool that is suitable for preparing high-quality papers and research. However, we ask authors to keep in mind two important criteria. First, we expect papers to fully describe their methodology, and any tool that is important to that methodology, including the use of LLMs, should be described also. For example, authors should mention tools (including LLMs) that were used for data processing or filtering, visualization, facilitating or running experiments, and proving theorems. It may also be advisable to describe the use of LLMs in implementing the method (if this corresponds to an important, original, or non-standard component of the approach). Second, authors are responsible for the entire content of the paper, including all text and figures, so while authors are welcome to use any tool they wish for writing the paper, they must ensure that all text is correct and original.
REVIEWING AND SELECTION PROCESS
Reviewing will be single-blind, although authors can also submit anonymously if the submission allows that. A datasets and benchmarks program committee will be formed, consisting of experts on machine learning, dataset curation, and ethics. We will ensure diversity in the program committee, both in terms of background as well as technical expertise (e.g., data, ML, data ethics, social science expertise). Each paper will be reviewed by the members of the committee. In select cases where ethical concerns are flagged by reviewers, an ethics review may be performed as well.
Papers will not be publicly visible during the review process. Only accepted papers will become visible afterward. The reviews themselves are also not visible during the review phase but will be published after decisions have been made. Authors can choose to keep the datasets themselves hidden until a later release date, as long as reviewers have access.
The factors that will be considered when evaluating papers include:
- Utility and quality of the submission: Impact, originality, novelty, relevance to the NeurIPS community will all be considered.
- Reproducibility: All submissions should be accompanied by sufficient information to reproduce the results described i.e. all necessary datasets, code, and evaluation procedures must be accessible and documented. We encourage the use of a reproducibility framework such as the ML reproducibility checklist to guarantee that all results can be easily reproduced. Benchmark submissions in particular should take care to ensure sufficient details are provided to ensure reproducibility. If submissions include code, please refer to the NeurIPS code submission guidelines .
- Was code provided (e.g. in the supplementary material)? If provided, did you look at the code? Did you consider it useful in guiding your review? If not provided, did you wish code had been available?
- Ethics: Any ethical implications of the work should be addressed. Authors should rely on NeurIPS ethics guidelines as guidance for understanding ethical concerns.
- Completeness of the relevant documentation: Per NeurIPS ethics guidelines , datasets must be accompanied by documentation communicating the details of the dataset as part of their submissions via structured templates (e.g. TODO). Sufficient detail must be provided on how the data was collected and organized, what kind of information it contains, ethically and responsibly, and how it will be made available and maintained.
- Licensing and access: Per NeurIPS ethics guidelines , authors should provide licenses for any datasets released. These should consider the intended use and limitations of the dataset, and develop licenses and terms of use to prevent misuse or inappropriate use.
- Consent and privacy: Per NeurIPS ethics guidelines , datasets should minimize the exposure of any personally identifiable information, unless informed consent from those individuals is provided to do so. Any paper that chooses to create a dataset with real data of real people should ask for the explicit consent of participants, or explain why they were unable to do so.
- Ethics and responsible use: Any ethical implications of new datasets should be addressed and guidelines for responsible use should be provided where appropriate. Note that, if your submission includes publicly available datasets (e.g. as part of a larger benchmark), you should also check these datasets for ethical issues. You remain responsible for the ethical implications of including existing datasets or other data sources in your work.
- Legal compliance: For datasets, authors should ensure awareness and compliance with regional legal requirements.
ADVISORY COMMITTEE
The following committee will provide advice on the organization of the track over the coming years: Sergio Escalera, Isabelle Guyon, Neil Lawrence, Dina Machuve, Olga Russakovsky, Joaquin Vanschoren, Serena Yeung.
DATASETS AND BENCHMARKS CHAIRS
Lora Aroyo, Google Francesco Locatello, Institute of Science and Technology Austria Lingjuan Lyu, Sony AI
Contact: [email protected]
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Funding news for global health researchers: May 27, 2024
Subscribe to Funding News for Global Health Researchers
NIH global health news: View and subscribe to Fogarty's Global Health Matters newsletter
Featured Fogarty News and Information
- Collaborating to fortify Kinshasa University’s research ethics system Global Health Matters , Fogarty International Center, March/April 2024
- High H5N1 influenza levels found in mice given raw milk from infected dairy cows NIAID News, May 24, 2024
- Scientists map networks regulating gene function in the human brain NIMH News, May 24, 2024
On behalf of the Fogarty International Center at the U.S. National Institutes of Health (NIH), the following funding opportunities, notices and announcements may be of interest to those working in the field of global health research. Updates are typically distributed once a week.
Special Announcements
NINDS Requests Information on Global Neurological Diseases
The National Institute Neurological Diseases and Stroke (NINDS) has released a Request for Information from all stakeholders to identify global health research priorities, capacity building and training needs, and best practices or strategies that could facilitate equitable global health research in neurological disorders.
- Request for Information (NOT-NS-24-018)
- Response date: July 1, 2024
NIH Office of Women’s Health Research Releases Notice of Special Interest
The NIH Office of Research on Women’s Health (ORWH) and Office of AIDS Research (OAR), in partnership with Institute, Center, and Office (ICO) partners across NIH are issuing this notice to highlight interest in receiving HIV research and training grant applications that explicitly and intersectionally center the health needs of cisgender women and girls, and gender-diverse people.
- Notice of Special Interest (NOT-OD-24-119)
- Expiration date: January 8, 2026
Featured Upcoming Event
The Barmes Global Health Lecture returns June 5
After a four-year hiatus, the David E. Barmes Global Health Lecture series is back!
- Topic : Global HIV/AIDS Response: Then, Now, Future
- Speaker : Ambassador Dr. John Nkengasong, U.S. Global AIDS Coordinator and Senior Bureau Official for Global Health Security and Diplomacy.
- Date/Time : Wednesday, June 5 at 1 p.m. ET (USA)
- Location : NIH campus, Bethsda, MD (USA) and Virtual
- Registration & information
US Government Jobs in Global Health
These vacancies are only open to U.S. Citizens and U.S. Nationals. Foreign nationals or legal permanent residents are not eligible for consideration.
Physician - Public Health
The Centers for Disease Control (CDC) is seeking two Physicians, (Physician - Public Health - GP-15) (Physician - Public Health/Bilingual – GP-14) in locations around the globe.
- Physician (Public Health)
- Physician (Public Health/Bilingual)
- Application deadline: May 31, 2024
Upcoming Deadlines for Fogarty Funding Opportunities
- Bioethics Application deadline: June 6, 2024
- Chronic, Noncommunicable Diseases and Disorders Research Training Application deadline: July 15, 2024
- Global Infectious Disease Research Training Application due date: August 6, 2024
- Fogarty HIV Research Training Application deadline: August 22, 2024
Upcoming deadlines for all Fogarty funding opportunities
Funding opportunities on which Fogarty is a partner:
- Dissemination and Implementation Research in Health (R03 Clinical Trial Not Allowed) (PAR-22-106) Application due date: Multiple dates, see announcement.
- Dissemination and Implementation Research in Health (R21 Clinical Trial Optional) (PAR-22-109) Application due date: Multiple dates, see announcement.
Administrative supplements for current grantees:
Current Fogarty grantees can apply for supplementary funds via the notices below:
- Administrative Supplements to Promote Research Continuity and Retention of NIH Mentored Career Development (K) Award Recipients and Scholars Application due date: June 30, 2024
- Administrative Supplement for Continuity of Biomedical and Behavioral Research Among First-Time Recipients of NIH Research Project Grant Awards Application due date: June 30, 2024
All administrative supplements
Funding Opportunities
NIH funding opportunities focusing on global health and foreign collaboration:
- Centers for Research in Emerging Infectious Diseases (CREID) Network (U01 Clinical Trial Not Allowed) (RFA-AI-24-006) Application due date: June 21, 2024
- Centers for Research in Emerging Infectious Diseases (CREID) Network Coordination Center (U01 Clinical Trial Not Allowed) (RFA-AI-24-016) Application due date: June 21, 2024
- Feasibility of Novel Diagnostics for TB in Endemic Countries (FEND for TB) (R01 Clinical Trial Not Allowed) (RFA-AI-24-010) Application due date: June 28, 2024
- Limited Interaction Targeted Epidemiology: Epidemiology of Transmission and Treatment of HIV Among People Who Are at Increased Risk for HIV Infection in Latin America (LITE-LA) (UG3/UH3 Clinical Trial Optional) (RFA-AI-24-009) Application due date: July 30, 2024
NIH funding opportunities for which foreign components may apply:
- NIAMS Clinical Trial Implementation Cooperative Agreement (UG3/UH3 Clinical Trial Required) (PAR-24-208) Application due date: Multiple dates, see announcement.
- Single Source: NINDS Exploratory Clinical Trials (U01 Clinical Trial Required) (PAR-24-215) Application due date: Multiple dates, see announcement.
- Safety and Efficacy of Amyloid-Beta Directed Antibody Therapy in Mild Cognitive Impairment and Dementia with Evidence of Lewy Body Dementia and Amyloid-Beta Pathology (U01 - Clinical Trial Required) (RFA-NS-25-010) Application due date: Multiple dates, see announcement.
Other Funding News
Other funding updates that may be of interest to global health researchers.
General NIH notices:
- Notice to Extend the Response Date of NOT-AG-24-013, "Request for Information (RFI) on Data Management, Sharing, and Secondary Data Use Challenges and Opportunities" (NOT-AG-24-020)
- Notice of Information: Additional Priorities for NCCIH Natural Product Clinical Trial Funding Opportunities (NOT-AT-24-042)
- Notice of Pre-Application and Technical Assistance Webinar for RFA-MH-25-185 and RFA-MH-25-186 (NOT-MH-24-330)
- Update to HeLa Cell Whole Genome Sequence Data Submission and Access Under the NIH Lacks-Family Agreement (NOT-OD-24-098)
- Notice of Availability: Draft NIH Intramural Research Program Policy: Promoting Equity Through Access Planning (NOT-OD-24-125)
- Request for Information (RFI): Critical Challenges and Opportunities For Lymphatic Scientific, Clinical, and Disease Communities (NOT-HL-24-014) Response date: June 28, 2024
- Request for Information (RFI): National Institute of Mental Health (NIMH) Strategic Plan Evaluation (NOT-MH-24-135) Response date: July 24, 2024
Notices of changes to NIH funding opportunities:
- Notice of NCCIH Participation in “Notice of Special Interest (NOSI): Climate Change and Health" (NOT-AT-24-044)
NIH Notices of Special Interest (NOSIs):
- Administrative Supplement for Natural History and Longitudinal Dynamics of Lung Nodules using the NLST dataset (NOT-CA-24-058) Expiration date: July 9, 2024
- Interpersonal Communication Research to Advance Dental, Oral, and Craniofacial Health over the Lifecourse (NOT-DE-24-027) Expiration date: September 8, 2027
Non-NIH funding opportunities:
- The 2024-2025 HGHI Burke Climate and Health Fellowship applications are now open! The fellowship award includes a salary of $75,000 per year for a total of up to two years to support scholarship related to climate and health. During the fellowship period, Climate and Health Fellows will be an integral member of the Harvard Global Health Institute and Salata Institute and will participate in cross-University engagements on climate change. Applications deadline: May 31, 2024
- CRDF Global is accepting proposals from joint U.S., Japan, and other regional Asia-Pacific (APac) based investigators working in the field of infectious disease and immunology research for the U.S.-Japan Cooperative Medical Sciences Program (USJCMSP) Collaborative Awards . Applications deadline: June 3, 2024
- The WHO Health Ethics and Governance Unit invites proposals designed to help facilitate the promotion of ethically sound and equitable climate and health research. Proposals should clearly identify intended outputs and outline how these can support the development of ethical research into health and climate change. Applications deadline: June 17, 2024
- Wellcome is providing funding for multidisciplinary teams to generate evidence on where dengue and Zika viruses co-circulate and investigate the implications this has on host immune responses and clinical outcomes. Applications deadline: June 25, 2024
- The three-year NOMIS–STRI Postdoctoral Research Fellowship in Animal Behavior in the Tropics is seeking outstanding postdoc biologists to study animal behavior in Panama. Applications deadline: July 15, 2024
- The American Association for Cancer Research and AstraZeneca Endometrial Cancer Research Fellowship is accepting applications from postdocs to conduct endometrial cancer research. Application deadline: July 18, 2024
- Climate Change and Human Health Seed Grants from the Burroughs Wellcome Fund supporting small, early stage grants. Application deadline: July 25, 2024
- The L’Oréal UNESCO For Women in Science Egypt Young Talent Program identifies and rewards talented young female scientists from Egypt working in life or physical sciences. Application deadline: July 31, 2024
- More non-NIH funding opportunities
Manuscript, Abstract & Poster Submission Opportunities
- Submissions due: June 28, 2024
- Submissions due: June 30, 2024
- Submissions due: July 18, 2024
- Submissions due: November 30, 2024
Events for global health researchers:
Sapporo Exposome Symposium
- Date: May 27–29
- Location: Sapporo, Japan
- Organized by the Mount Sinai Institute for Climate Change, Environmental Health, and Exposomics
World Health Assembly
- Date: May 27–June 1
- Location: Geneva, Switzerland
GIS & UTHealth Houston Global Conference 2024
- Date: May 29
- Location: Houston, TX (USA)
- Hosted by the Global Implementation Society
Global Social Determinants of Health
- Location: Geneva, Switzerland and Virtually
- Hosted by Boston University School of Public Health
Leveraging Partnerships to Improve the Health of Young People Living with HIV in Peru
- Date: May 31
- Location: Virtual
- Part of the Harvard Global Health Institute’s Coffee Sessions virtual series
Disaster Research Response (DR2) Network in Asia Workshop
- Date: May 31–June 1
- Location: Kawasaki, Kanagawa, Japan
- Sponsored by the National Institute of Environmental Health Sciences (NIEHS), USA and the National Institute for Environmental Studies, Japan
World Conference on Research Integrity
- Date: June 2–4
- Location: Athens, Greece
Annual Conference For Advancing Participatory Science
- Date: June 3-6
- Hosted by the Association for Advancing Participatory Sciences
Fogarty Advisory Board Meeting
- Date: June 4
- Location: Bethesda, MD (USA) and Virtual
Global HIV/AIDS Response: Then, Now, Future
- Date: June 5
- Speaker: Ambassador Dr. John N. Nkengasong
- Part of the David E. Barmes Global Health Lecture series
- Sponsored by Fogarty International Center and the National Institute of Dental and Craniofacial Research (NIDCR)
Webinar on Updates to NIH Training Grant Applications
- Hosted by the NIH Office of Extramural Research
Fogarty calendar of events
- Português Br
- Journalist Pass
Participate in Mayo Clinic’s upcoming AI Summit
Kris Schanilec
Share this:

Mayo Clinic will hold its 2024 Artificial Intelligence (AI) Summit , "Generative Multimodal AI — Potentials and Challenges," on July 8–9 in Rochester and virtually. The event will bring together AI experts and the healthcare community to discuss advances in large multimodal models and their use in healthcare.

"The role of artificial intelligence is evolving rapidly in areas from preventing and diagnosing diseases to developing new cures," says Cui Tao, Ph.D ., chair of the Department of Artificial Intelligence and Informatics. "The summit is an exciting opportunity to explore the possibilities while navigating the challenges of developing and using transformative technology in healthcare." Dr. Tao is the Nancy Peretsman and Robert Scully Chair of AI and Informatics.
The event will be hosted by the Department of Artificial Intelligence and Analytics, in collaboration with the Mayo Clinic AI community.
Event details
The AI Summit will offer opportunities to hear from experts in AI, share knowledge and network through lightning talks, panel discussions and a poster session.
The keynote speakers will be:
- John Halamka, M.D. , president, Mayo Clinic Platform "The Next Milestones for Predictive and Generative AI in Healthcare"
- Thomas Fuchs, DrSc , dean of Artificial Intelligence and Human Health, Mount Sinai "Beyond 1 Million Slides: How the Era of Foundation Models is Supercharging Precision Medicine"
- Anant Madabhushi, Ph.D. , professor of biomedical engineering, Emory University "Walking the Talk: Validating AI for Precision Medicine with Clinical Trials"
- Shauna Overgaard, co-director of AI Validation and Stewardship, Mayo Clinic "Implementing Quality Management Systems to Close the AI Translation Gap and Facilitate Safe, Ethical and Effective Health AI Solutions"
- Hoifung Poon , general manager at Health Futures, Microsoft Research "The Emergence of Multimodal Generative AI in Biomedicine"
Hamid Tizhoosh, Ph.D. , Artificial Intelligence and Informatics, will chair the event.
View the complete agenda .
Register to attend, submit an abstract
Registration for both Mayo Clinic and external participants is $350 for in-person or virtual attendance, with a discounted rate of $250 for students.
Register today to attend.
You can submit your work in an AI-related area for consideration to present at the summit. The conference will cover an array of topics — from generative AI and discovery science to AI bias and data security.
Three presentation formats are available:
- Lightning talk (10 minutes)
- Poster presentation
- Workshop/tutorial
Learn more and submit an abstract proposal on the conference website . Proposals will be accepted through June 1.
For more details, visit the AI Summit website .
- Mayo Clinic Minute: Common types of skin cancer 2024 hurricane preparedness: Be ready for storm season
Related Articles


IMAGES
VIDEO
COMMENTS
Revised on May 9, 2024. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments ...
Ethical Considerations. Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination. Key ethical issues that you will address as an insider researcher include: Gaining trust.
Ethics Principle 1: Respect for persons. As the name suggests, this principle is all about ensuring that your participants are treated fairly and respectfully.In practical terms, this means informed consent - in other words, participants should be fully informed about the nature of the research, as well as any potential risks. Additionally, they should be able to withdraw from the study at ...
The inclusion of validating findings, ethical considerations (to be addressed shortly), the need for preliminary results, and early evidence of practical significance focus a reader's attention on key elements often overlooked in discussions about proposed projects. Format for a Qualitative Proposal
Underpayment is a common ethical issue that shouldn't be overlooked. Properly rewarding participants' time is critical for boosting engagement and obtaining high-quality data. While Prolific requires a minimum payment of £6.00 / $8.00 per hour, there are other factors you need to consider when deciding on a fair payment.
of power and authority are all 'ethical considerations inherent in and raised. by ESL research' (p. . 1) . Koulouriotis further reiterates the point that a great. proportion of research in ESL ...
In the sections to follow, we map out the various ethical dimensions of designing a research project step by step: addressing the fundamental question of why and for whom we do research (Sect. 10.2); an exploration of the ethical considerations of the research design itself, including the recruitment of study participants (Sects. 10.3 and 10.4 ...
The third ethics principle of the Economic and Social Research Council (ESRC) states that: "The confidentiality of the information supplied by research subjects and the anonymity of respondents must be respected.". However, sometimes confidentiality is limited. For example, if a participant is at risk of harm, we must protect them.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication. Scientists and researchers must always adhere to a certain code of conduct when collecting data ...
Ethical considerations in research have always been challenging, including ethical concerns vis-a-vis time, funding, accessibility, and proper implementation of these concerns. ... In experimental research, especially involving human subjects, the researcher needs to submit an appropriate ethics proposal, although that process can slow down the ...
NIH Clinical Center researchers published seven main principles to guide the conduct of ethical research: Social and clinical value. Scientific validity. Fair subject selection. Favorable risk-benefit ratio. Independent review. Informed consent. Respect for potential and enrolled subjects.
Ethical considerations in research framework for evaluating research is outlined by Emanuel et al.7 Steps suggested in the process of eval-uating ethical research include: 1.Value in terms of the knowledge extracted and applied from the research 2.Scientific validity reflecting the methodology 3.Selection of subjects 4.Risk-benefit ratio
Factors contributing to proposal rejections included weak research questions or hypotheses, poor questionnaires/interview schedule design and inadequate research ethics consideration in the proposal.
In order to address ethical considerations aspect of your dissertation in an effective manner, you will need to expand discussions of each of the following points to at least one paragraph: 1. Voluntary participation of respondents in the research is important. Moreover, participants have rights to withdraw from the study at any stage if they ...
Kathleen Haynes. W eek 10, Discussion-Initial Post. Ethical Considerations in Research. Research is an important component of expanding knowledge and understanding the. world, and the people that ...
In the conduct of research, several key principles and actions must be observed and preserved. These include freedom from harm, right to self-determination, right to privacy, and right to anonymity and confidentiality (See Figure 2). Freedom from physical or mental harm or discomfort should be of utmost concern to the researcher.
Research ethics committees (RECs) typically evaluate the ethical acceptability of research proposals. Sometimes, differences arise between how researchers and RECs interpret et … Researchers designing and conducting studies using human data should consider the values and principles of ethical conduct.
Getting ethical approval for your study. Before you start any study involving data collection with people, you'll submit your research proposal to an institutional review board (IRB).. An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution's code of conduct. They check that your research materials and procedures ...
Ethical Considerations T he consideration of ethics in research, and in general business for that matter, is of growing importance. It is, therefore, critical that you understand the basics of ethical research and how this might affect your research project. This is especially important if your research involves inter-
Ethical consideration of the research proposal and the informed-consent process: An online survey of researchers and ethics committee members in Thailand ... This study aimed to explore the opinions of these two groups about the importance of core ethical issues in the proposal and in the informed-consent process. An anonymous online ...
INTRODUCTION. A clean, well-thought-out proposal forms the backbone for the research itself and hence becomes the most important step in the process of conduct of research.[] The objective of preparing a research proposal would be to obtain approvals from various committees including ethics committee [details under 'Research methodology II' section [Table 1] in this issue of IJA) and to ...
Ethics and responsible use: Any ethical implications of new datasets should be addressed and guidelines for responsible use should be provided where appropriate. Note that, if your submission includes publicly available datasets (e.g. as part of a larger benchmark), you should also check these datasets for ethical issues.
The WHO Health Ethics and Governance Unit invites proposals designed to help facilitate the promotion of ethically sound and equitable climate and health research. Proposals should clearly identify intended outputs and outline how these can support the development of ethical research into health and climate change.
The protectio n of human subjects through the. application of appropriat e ethical princi ples is. important in any research study (1). In a. qualitative study, et hical considerations have a ...
You can submit your work in an AI-related area for consideration to present at the summit. The conference will cover an array of topics — from generative AI and discovery science to AI bias and data security. Three presentation formats are available: Lightning talk (10 minutes) Poster presentation; Workshop/tutorial
Provide guidance and counsel to the research administration team and research faculty on the most effective way to achieve an effective, quality proposal and coordinate a timely submission process, ensuring appropriate approvals. Approve proposal submission requests and action requests, and unfunded research requests.