Publications
Our research.
- Huang Y, Rodgers WJ, Middleton RM, et al. Willingness to receive a COVID-19 vaccine in people with multiple sclerosis – UK MS Register survey. Multiple Sclerosis and Related Disorders 2021;55:103175. doi:10.1016/j.msard.2021.103175 PENDING
- Middleton RM, Pearson OR, Ingram G, et al. A Rapid Electronic Cognitive Assessment Measure for Multiple Sclerosis: Validation of Cognitive Reaction, an Electronic Version of the Symbol Digit Modalities Test. J Med Internet Res 2020;22:e18234. doi:10.2196/18234
- Nicholas RS, Heaven ML, Middleton RM, et al. Personal and Societal Costs of Multiple Sclerosis in the UK: A Population-Based MS Registry Study. Multiple Sclerosis Journal - Experimental, Translational and Clinical 2020;6:205521732090172. doi:10.1177/2055217320901727
- Middleton RM, Rodgers WJ, Chataway J, et al. Validating the portal population of the United Kingdom Multiple Sclerosis Register. Multiple Sclerosis and Related Disorders 2018;24:3–10. doi:10.1016/j.msard.2018.05.015
- Balbuena LD, Middleton RM, Tuite-Dalton K, et al. Sunshine, Sea, and Season of Birth: MS Incidence in Wales. PLOS ONE 2016;11:e0155181. doi:10.1371/journal.pone.0155181
- Jones KH, Jones PA, Middleton RM, et al. Physical Disability, Anxiety and Depression in People with MS: An Internet-Based Survey via the UK MS Register. PLoS ONE 2014;9:e104604. doi:10.1371/journal.pone.0104604
- Osborne LA, Gareth Noble J, Maramba IDC, et al. Outcome Measures for Multiple Sclerosis. Physical Therapy Reviews 2014;19:24–38. doi:10.1179/1743288X13Y.0000000094
- Osborne LA, Middleton RM, Jones KH, et al. Desirability and Expectations of the UK MS Register: Views of People with MS. International Journal of Medical Informatics 2013;82:1104–10. doi:10.1016/j.ijmedinf.2013.07.005
- Jones KH, Ford DV, Jones PA, et al. How People with Multiple Sclerosis Rate Their Quality of Life: An EQ-5D Survey via the UK MS Register. PLoS ONE 2013;8:e65640. doi:10.1371/journal.pone.0065640
- Jones KH, Ford DV, Jones PA, et al. The Physical and Psychological Impact of Multiple Sclerosis Using the MSIS-29 via the Web Portal of the UK MS Register. PLoS ONE 2013;8:e55422. doi:10.1371/journal.pone.0055422
- Noble JG, Osborne LA, Jones KH, et al. Commentary on ‘Disability Outcome Measures in Multiple Sclerosis Clinical Trials’. Multiple Sclerosis Journal 2012;18:1718–20. doi:10.1177/1352458512457847
- Jones KH, Ford DV, Jones PA, et al. A Large-Scale Study of Anxiety and Depression in People with Multiple Sclerosis: A Survey via the Web Portal of the UK MS Register. PLoS ONE 2012;7:e41910. doi:10.1371/journal.pone.0041910
- Ford DV, Jones KH, Middleton RM, et al. The Feasibility of Collecting Information from People with Multiple Sclerosis for the UK MS Register via a Web Portal: Characterising a Cohort of People with MS. BMC Medical Informatics and Decision Making 2012;12:73. doi:10.1186/1472-6947-12-73
- Osborne LA, Noble JG, Lockhart-Jones HM, et al. Sources of Discovery, Reasons for Registration, and Expectations of an Internet-Based Register for Multiple Sclerosis: Visualisations and Explorations of Word Uses and Contexts. International Journal of Healthcare Information Systems and Informatics 33AD;7:27–43. doi:10.4018/jhisi.2012070103
- Osborne LA, Lockhart-Jones HM, Middleton RM, et al. Identifying and Addressing the Barriers to the Use of an Internet-Register for Multiple Sclerosis: International Journal of Healthcare Information Systems and Informatics 31AD;8:1–16. doi:10.4018/jhisi.2013010101

COVID-19 Related Publications
- Garjani A, Middleton RM, Nicholas R, et al. Recovery From COVID-19 in Multiple Sclerosis: A Prospective and Longitudinal Cohort Study of the United Kingdom Multiple Sclerosis Register. _Neurol Neuroimmunol Neuroinflamm_2022;9:e1118. doi:10.1212/NXI.0000000000001118
- Middleton R, Craig E, Rodgers W, et al. COVID-19 in Multiple Sclerosis: Clinically reported outcomes from the UK Multiple Sclerosis Register. Multiple Sclerosis and Related Disorders 2021;56:103317. doi:10.1016/j.msard.2021.103317
- Huang Y, Rodgers WJ, Middleton RM, et al. Willingness to receive a COVID-19 vaccine in people with multiple sclerosis – UK MS Register survey. Multiple Sclerosis and Related Disorders 2021;55:103175. doi:10.1016/j.msard.2021.103175
- Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology 2021;97:e1870–85. doi:10.1212/WNL.0000000000012753
- Garjani A, Hunter R, Law GR, et al. Mental health of people with multiple sclerosis during the COVID-19 outbreak: A prospective cohort and cross-sectional case–control study of the UK MS Register. _Mult Scler_2021;:13524585211020436. doi:10.1177/13524585211020435
- Nair R das, Hunter R, Garjani A, et al. Challenges of developing, conducting, analysing and reporting a COVID-19 study as the COVID-19 pandemic unfolds: an online co-autoethnographic study. BMJ Open 2021;11:e048788. doi:10.1136/bmjopen-2021-048788
- Garjani A, Middleton RM, Hunter R, et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Multiple Sclerosis and Related Disorders 2021;:102939. doi:10.1016/j.msard.2021.102939
- Evangelou N, Garjani A, dasNair R, et al. Self-diagnosed COVID-19 in people with multiple sclerosis: a community-based cohort of the UK MS Register. J Neurol Neurosurg Psychiatry 2020;:jnnp-2020-324449. doi:10.1136/jnnp-2020-324449
- Peeters LM, Parciak T, Walton C, et al. COVID-19 in people with multiple sclerosis: A global data sharing initiative. Mult Scler 2020;:135245852094148. doi:10.1177/1352458520941485
Collaborators' Research
- Garjani A, Hunter R, Law GR, et al. Mental health of people with multiple sclerosis during the COVID-19 outbreak: A prospective cohort and cross-sectional case–control study of the UK MS Register. Mult Scler 2021;:13524585211020436. doi:10.1177/13524585211020435
- Kamudoni P, Johns J, Cook KF, et al. Standardizing fatigue measurement in multiple sclerosis: the validity, responsiveness and score interpretation of the PROMIS SF v1.0 – Fatigue (MS) 8a. Multiple Sclerosis and Related Disorders 2021;:103117. doi:10.1016/j.msard.2021.103117
- Goodwin E, Hawton A, Whitty JA, et al. Exploring the Factors that Influence Workforce Participation for People with Multiple Sclerosis: A Discrete Choice Experiment. J Occup Rehabil Published Online First: 27 January 2021. doi:10.1007/s10926-020-09952-5
- Veldhuijzen van Zanten J, Douglas MR, Ntoumanis N. Fatigue and fluctuations in physical and psychological wellbeing in people with multiple sclerosis: A longitudinal study. Multiple Sclerosis and Related Disorders 2021;47:102602. doi:10.1016/j.msard.2020.102602
- Vickaryous N, Jitlal M, Jacobs BM, et al. Remote testing of vitamin D levels across the UK MS population—A case control study. PLoS ONE 2020;15:e0241459. doi:10.1371/journal.pone.0241459
- Coe S, Tektonidis T, Coverdale C, et al. A cross sectional assessment of nutrient intake and the association of the inflammatory properties of nutrients and foods with symptom severity, in a large cohort from the UK Multiple Sclerosis Registry. Nutrition Research 2020;:S0271531720305716. doi:10.1016/j.nutres.2020.11.006
- Allen-Philbey K, Middleton R, Tuite-Dalton K, et al. Can We Improve the Monitoring of People With Multiple Sclerosis Using Simple Tools, Data Sharing, and Patient Engagement? Front Neurol 2020;11:464. doi:10.3389/fneur.2020.00464
- Salter A, Stahmann A, Ellenberger D, et al. Data Harmonization for Collaborative Research among MS Registries: A Case Study in Employment. Mult Scler 2020;:135245852091049. doi:10.1177/1352458520910499
- Lincoln NB, Bradshaw LE, Constantinescu CS, et al. Cognitive Rehabilitation for Attention and Memory in People with Multiple Sclerosis: A Randomized Controlled Trial (CRAMMS). Clin Rehabil 2019;:026921551989037. doi:10.1177/0269215519890378
- Glaser A, Stahmann A, Meissner T, et al. Multiple Sclerosis Registries in Europe – an Updated Mapping Survey. Multiple Sclerosis and Related Disorders Published Online First: October 2018. doi:10.1016/j.msard.2018.09.032
- Goodwin E, Green C, Hawton A. Health State Values Derived from People with Multiple Sclerosis for a Condition-Specific Preference-Based Measure: Multiple Sclerosis Impact Scale–Eight Dimensions–Patient Version (MSIS-8D-P). Value in Health Published Online First: June 2018. doi:10.1016/j.jval.2018.03.019
- Dennison L, Brown M, Kirby S, et al. Do People with Multiple Sclerosis Want to Know Their Prognosis? A UK Nationwide Study. PLOS ONE 2018;13:e0193407. doi:10.1371/journal.pone.0193407
- Baker D, Anandhakrishnan A, Tuite-Dalton KA, et al. How to Refer to People with Disease in Research Outputs: The Disconnection between Academic Practise and That Preferred by People with Multiple Sclerosis. Multiple Sclerosis and Related Disorders 2016;10:127–33. doi:10.1016/j.msard.2016.09.007
- Flachenecker P, Buckow K, Pugliatti M, et al. Multiple Sclerosis Registries in Europe - Results of a Systematic Survey. Multiple Sclerosis Journal 2014;20:1523–32. doi:10.1177/1352458514528760
To read about the research that has been done on the UK MS Register in more layman’s terms, have a look at the MS Register news pages !
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 85, Issue 1
- Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- I S Mackenzie 1 ,
- S V Morant 1 ,
- G A Bloomfield 2 ,
- T M MacDonald 1 ,
- J O'Riordan 3
- 1 Medicines Monitoring Unit (MEMO) , University of Dundee, Dundee , UK
- 2 Multiple Sclerosis National Therapy Centres, Whitchurch , UK
- 3 Tayside Multiple Sclerosis Research Unit, Department of Neurology , Ninewells Hospital and Medical School, Dundee , UK
- Correspondence to Dr Isla S Mackenzie, Medicines Monitoring Unit (MEMO), University of Dundee, Dundee DD1 9SY, UK; i.s.mackenzie{at}dundee.ac.uk
Objectives To estimate the incidence and prevalence of multiple sclerosis (MS) by age and describe secular trends and geographic variations within the UK over the 20-year period between 1990 and 2010 and hence to provide updated information on the impact of MS throughout the UK.
Design A descriptive study.
Setting The study was carried out in the General Practice Research Database (GPRD), a primary care database representative of the UK population.
Main outcome measures Incidence and prevalence of MS per 100 000 population. Secular and geographical trends in incidence and prevalence of MS.
Results The prevalence of MS recorded in GPRD increased by about 2.4% per year (95% CI 2.3% to 2.6%) reaching 285.8 per 100 000 in women (95% CI 278.7 to 293.1) and 113.1 per 100 000 in men (95% CI 108.6 to 117.7) by 2010. There was a consistent downward trend in incidence of MS reaching 11.52 per 100 000/year (95% CI 10.96 to 12.11) in women and 4.84 per 100 000/year (95% CI 4.54 to 5.16) in men by 2010. Peak incidence occurred between ages 40 and 50 years and maximum prevalence between ages 55 and 60 years. Women accounted for 72% of prevalent and 71% of incident cases. Scotland had the highest incidence and prevalence rates in the UK.
Conclusions We estimate that 126 669 people were living with MS in the UK in 2010 (203.4 per 100 000 population) and that 6003 new cases were diagnosed that year (9.64 per 100 000/year). There is an increasing population living longer with MS, which has important implications for resource allocation for MS in the UK.
- Multiple Sclerosis
- Epidemiology
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
https://doi.org/10.1136/jnnp-2013-305450
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Individuals with multiple sclerosis (MS) can experience high levels of disability and impaired quality of life for prolonged periods. The costs of the disease in the UK, including health and social care and productivity losses, are high and correlate with disease severity. 1 , 2 It is important to have accurate and up to date information on the prevalence of MS in the UK in order to understand the impact of this disease and to ensure that adequate resources are provided nationally and regionally for people affected by MS. National studies have been carried out in the past, but recent data are lacking. 3–6 To address this need, work on compiling an online national MS register began in 2011 ( http://www.ukmsregister.org ). A dedicated Scottish National MS Register for incident MS cases was established in 2010. 7
The General Practice Research Database (GPRD) is a longitudinal database containing details of patients’ demographics, medical diagnoses, referrals to consultants and hospitals, and primary care prescriptions from a representative sample of general practices in the UK. 8 Two previous studies have used the GPRD to study the epidemiology of MS in the UK, the first reporting for the period 1993–2000. 6 A more recent study investigated the prevalence of MS between 2000 and 2008 stratified by age, sex, geographical region and calendar year. 7
Study design
This was a population-based study using the GPRD. The study protocol was reviewed and approved by the Independent Scientific Advisory Committee (ISAC) of GPRD. No further ethical approval is required for studies using GPRD that do not involve patient contact.
This was a descriptive study. Its aim was to estimate the incidence and prevalence of MS by age in men and women and to describe secular trends and geographic variations within the UK between 1990 and 2010.
Study population
The study population included all patients with acceptable data who contributed follow-up time to the database after 1990. GPRD defines a patient's data as unacceptable if there is evidence of poor data recording, non-contiguous follow-up or if their registration with the practice is temporary. Eligible follow-up time for each patient started with their practice's ‘up-to-standard’ (UTS) date or the patient's date of registration with the practice if this was later. GPRD applies standard criteria to define the date at which any individual practice's data become ‘UTS’ to ensure quality of data.
The first 2 years of follow-up time for each patient were treated as a screening period, and incidence and prevalence rates were calculated for follow-up time after the screening period. We chose this screening period because preliminary analyses showed that incidence rates were high in the first 2 years of follow-up and prevalence rates were low, particularly in the first year. This is probably due to inclusion of patients with prevalent disease whose initial diagnosis pre-dated the computerisation of their practice's records.
The follow-up period ended with the earlier of either their transfer-out date or their practice's last data collection date.
For GPRD, Read codes for confirmed diagnoses of MS (ie, codes beginning F20) were used. For Hospital Episode Statistics (HES) the International Classification of Diseases (ICD10) code for MS (G35) was used. Incident cases were defined as the first occurrence of a code for MS if it occurred after the 2-year screening period.
Statistical analysis
The analysis plan is shown in figure 1 .
- Download figure
- Open in new tab
- Download powerpoint
Analysis plan. GPRD, General Practice Research Database; HES, Hospital Episode Statistics; ONS, Office of National Statistics; MS, multiple sclerosis.
For every patient, the number of days of follow-up available on the GPRD was calculated for each year from 1990 to 2010. We determined whether patients had any prior diagnosis of MS in the GPRD on the 1st January each year and, if not, whether any incident diagnosis occurred during the year.
Incidence rates were estimated from Poisson regression models with log(time at risk) as an offset variable. Prevalence rates were estimated from logistic regression models. The explanatory variables in the models were age, year and region. Geographical regions were defined as Scotland, Wales, Northern Ireland and the 10 Strategic Health Authorities of England. Data for men and women were analysed separately.
Mortality rates were analysed using logistic regression models.
Hospital episode statistics
HES data were available for about 44% of patients in the GPRD from 1997 to 2010. We estimated the prevalence and incidence of MS in these patients over this period of time using GPRD data only, as described above. We compared these rates with those calculated for the same patients using the additional diagnoses obtained from HES. Age-specific rates of under-recording of MS in the GPRD were estimated from inverse polynomials fitted to the ratios of cases identified from HES and the GPRD together versus the GPRD alone. These rates were used to adjust estimates of incidence and prevalence rates for the whole GPRD population.
Office for National Statistics
We applied these adjusted age-specific and gender-specific incidence and prevalence rates to population statistics obtained from the Office for National Statistics (ONS) for the UK population to estimate the absolute numbers of new and prevalent cases of MS in the UK population in 2010. 9 We obtained sex-specific and age-specific mortality rates for England and Wales in 2000 and 2010 from the ONS 10 and used them to calculate period life expectancy at birth in those years. To estimate the numbers of incident and prevalent cases of MS in the UK population in 2010 for men and women in each decade of life, we calculated incidence and prevalence rates in the entire GPRD population and applied age-specific correction factors to account for under-reporting in GP records alone. We applied the corrected rates in 2010 to the total national UK population based on ONS figures. 9
The numbers of patients with UTS follow-up time on the GPRD increased from 1.1 million in 1990 to at least 4.0 million between 2006 and 2010. The GPRD population included about 8% of the UK population in 2010, and their age and sex distributions were similar to those of the whole population ( table 1 ).
- View inline
Age and sex distributions of the UK population (ONS) and the GPRD population in 2010
Secular trends
The prevalence of MS increased by about 2.4% per year (95% CI 2.3% to 2.6%) in men and women over the study period ( figure 2 A) and reached 285.8 per 100 000 in women (95% CI 278.7 to 293.1) and 113.1 per 100 000 in men (95% CI 108.6 to 117.7) in 2010. The prevalence rates that are below the trend line in the early 1990s may be an artefact due to patients being first diagnosed before their entry to the database, despite the 2-year screening period. There was no change in MS prevalence in patients below the age of 50, but annual rates of increase were over 4% in patients aged ≥60 years ( figure 2 B).
Secular trends in the prevalence of multiple sclerosis (General Practice Research Database 1990–2010). (A) Prevalence (per 10 5 (per 100 000) patients) in women and men (all age groups). (B) Variation in prevalence by age group (% change per year, both sexes).
There was a consistent downward trend in the incidence of MS in the whole study population over the 20-year study period ( figure 3 A). In 2010, MS incidence in women fell to 11.52 per 100 000/year (95% CI 10.96 to 12.11) and in men to 4.84 per 100 000/year (95% CI 4.54 to 5.16). The rate of decline between 1990 and 2010 was 1.51% per year (95% CI 0.99% to 2.07%) and did not differ between men and women (p=0.682) or with age (p=0.494) ( figure 3 B). This implies that the female-to-male ratio among incident cases, approximately 2.4, did not change significantly over the study period.
Secular trends in the incidence of multiple sclerosis (General Practice Research Database 1990–2010). (A) Incidence (per 10 5 patient years) in women and men (all age groups). (B) Variation in incidence by age group (% change per year, both sexes).
Mortality rates fell in the GPRD population over the study period. In the 70–79-year age group, for example, they fell from 5.41% per year (95% CI 5.25% to 5.58%) in 1990 to 2.82% per year (95% CI 2.76% to 2.87%) in 2010 in men and from 3.15% per year (95% CI 3.04% to 3.26%) to 1.88% per year (95% CI 1.84% to 1.92%) in women over the same time period. Among other age groups, the proportional decline was similar. The mortality rate among patients with MS was more than twice that of other patients in all age groups and in both sexes, but also declined at a similar proportional rate.
Life expectancy rose from 75.6 to 78.3 years in men and from 79.9 to 81.8 years in women. We applied the age-specific mortality ratios for people with and without MS observed in the present study to estimate changes in life expectancies in people with MS over the same decade. They increased from 61.4 to 65.4 years in men and from 68.7 to 71.6 years in women.
The peak incidence of MS occurred at the age of 40 years in women and 45 years in men ( figure 4 A), while peak prevalence rates occurred at the ages of 56 years and 59 years, respectively, ( figure 4 B).
Incidence and prevalence of multiple sclerosis in women and men by age (General Practice Research Database 1990–2010). (A) Incidence (per 10 5 patient years). (B) Prevalence (per 10 5 patients).
Regional variation
There was significant variation in the incidence and the prevalence of MS between regions of the UK (p<0.001) (see online supplementary Figures 5a and 5b). The highest prevalence and incidence rates were observed in Scotland. Among the other 12 regions of the UK, latitude accounted for 13.8% (men) and 4.0% (women) of the variation in incidence rates, and 2.0% (men) and 0.2% (women) of the variation in prevalence rates, none of which was statistically significant.
Between 1997 and 2010 GPRD and HES data were available for a subset of patients (approximately 44%, table 2 ). Tables 3 and 4 show the age-specific and sex-specific prevalence and incidence rates of MS in this subgroup of patients based on the GPRD alone, and the rates when the additional diagnoses recorded in HES are included. HES identified an additional 744 prevalent cases and 121 incident cases in men and 1521 prevalent cases and 227 incident cases in women. GPRD alone underestimated the prevalence of MS by 7.0% in men and 5.5% in women and incidence by 21.3% in men and 17.2% in women over the period 1997–2010. Age-specific correction factors were estimated.
GPRD study population by year and the subset with HES data available
Age-specific and gender-specific prevalence of MS using GPRD alone and GPRD with HES (1997–2010)
Age-specific and gender-specific incidence of MS using GPRD alone and GPRD with HES (1997–2010)
Overall estimates of the UK MS population in 2010
Table 5 shows overall estimates of the numbers of incident and prevalent cases of MS in the UK population in 2010 for men and women in each decade of life. We estimate that 126 669 people were living with MS in the UK at the beginning of 2010 (203.4 per 100 000 population) and that 6003 new cases were diagnosed during that year (9.64 per 100 000/year). Women accounted for 72% of prevalent and 71% of incident cases. We also estimated the numbers of incident and prevalent cases of MS in the four countries which comprise the UK ( table 5 ).
Estimated numbers of incident and prevalent cases of MS in the UK population in 2010
Principal findings of the study
We estimate that the prevalence of MS in the UK in 2010, including diagnoses obtained from HES, was 289.0 per 100 000 in women and 115.0 per 100 000 in men. The overall prevalence of MS increased by approximately 2.4% per year between 1990 and 2010 in women and men. This increase in prevalence was due to a convergence of absolute mortality rates in patients with and without MS, the result of mortality rates falling by about 3% per year in both groups. There was no change in MS prevalence in patients below the age of 50, but annual rates of increase were over 4% in patients aged ≥60 years. We observed a decline in the rate at which new cases of MS were diagnosed, and the rising prevalence rate can likely be accounted for by trends in mortality rates. There was a consistent downward trend in overall incidence of MS in the whole study population over the 20-year study period, and the rate of decline did not differ between men and women or with age. It is possible that this is due to new diagnostic techniques which reduced the risk of false positive diagnoses over the study period. The maximum incidence of MS occurred at age 40 years (women) to 45 years (men). We were not able to analyse the effects of prior pregnancy on the age of onset of MS in women in this study, although it has previously been reported that pregnancy reduces the risk of onset of MS. 11 We found significant regional variation in incidence and prevalence rates in the UK. We found the highest incidence and prevalence rates among the 13 regions of the UK in Scotland, but no trend with latitude among the other 12 regions. This suggests that the difference between Scotland and other regions of the UK is probably not the result of a consistent trend with latitude, but may involve factors not associated with latitude. We were not able to analyse the different regions of Scotland separately using the GPRD.
Strengths and weaknesses of the study
A major strength of this study is that it covers a representative sample of GPs spread geographically throughout the UK, and a patient population with age and sex distributions similar to those of the general UK population. The study population of some 4 million patients provides greater statistical precision than earlier regional surveys. Our analyses depend upon the accuracy of diagnosis and recording of MS by GPs: there may have been miscoding of tentative MS diagnoses as definite MS cases, leading to an overestimate in the number of MS cases, or under-recording may have led to an underestimate in the number of cases. In a systematic review of 212 publications using the GPRD, Herrett et al reported that the median proportion of cases with a confirmed diagnosis based on additional internal or external validation was 89% across all disease groups and 81% for nervous system diseases 12 but there has not yet been a validation of MS diagnoses specifically within GPRD. We addressed some of the limitations of the GPRD records by also using HES, which allowed us to estimate the extent of under-recording of MS in the GPRD.
Relation to other studies
The prevalence rates we found are slightly higher than the rates reported by Thomas et al in 2007, also using the GPRD: 281.0 per 100 000 (95% CI 273.0 to 289.0) among women and 108.0 per 100 000 (95% CI 103.0 to 113.0) among men, with the highest prevalence in those aged 55–64 years. 13 This study and our study found maximum prevalence for MS in patients around the age of 60.
Alonso and colleagues reported incidence rates of 7.2 (95% CI 6.5 to 7.7) per 100 000 person-years in women and 3.1 (95% CI 2.6 to 3.5) in men in the UK between 1993 and 2000 in their GPRD study, which are somewhat lower than our findings. 6 The UK has a relatively high incidence of MS compared to other countries. An overall incidence rate of MS of 3.6 per 100 000 person-years in women and 2.0 in men was reported in a review of studies of the incidence of MS published between 1966 and 2007. 14
The downward trend in incidence that we found is in contrast to studies in Denmark, where the female incidence of MS has almost doubled since the 1970s while male incidence has remained constant. 15 These authors found a general, but not ubiquitous, increase in MS incidence in Western Europe and North America. 15 However, they point out that many of the studies included only small numbers of cases and random variations may have contributed to the irregular patterns observed. Moreover, separate surveys carried out and analysed at different times may be subject to methodological differences. It is not clear why our study has detected a decreasing incidence while others have suggested increasing incidence. Changes in awareness of MS and the challenges of diagnosing MS may account for changes incidence over time. However, we could identify no specific reason why the methodology or data source we used should have had an impact on our finding of decreasing incidence of MS over the period of the study.
Sex ratio in MS
In the current study, the mean female-to-male ratio for MS was 2.4 and there was no trend with time over the 20-year study period. In their 2008 review of published studies on the incidence of MS, Alonso and Hernán reported that the female-to-male ratio increased from 1.4 in 1955 to 2.3 in 2000. 14 This increase in the sex ratio for MS is not ubiquitous, however, and there are striking geographic variations. For example, a recent analysis of trends in the sex ratio in MS for individuals born between 1930 and 1989 found a marked increase in Northern Europe (not including the UK) (from 2.09 to 3.77), but only a moderate increase in Southern Europe (from 1.46 to 2.31). 16 In contrast, a study in Sweden found a mean female-to-male ratio for MS of 2.62, with no clear trend with year of birth for individuals born between 1931 and 1985. 17 A recent review reported a significant increase in the MS prevalence female-to-male sex ratio in the UK between 1949 and 2009—a much longer time period than our study. 18 It is possible that this historical trend in female-to-male sex ratio for MS has now stabilised. This may be partly accounted for by changing health-related behaviours of men in recent years, perhaps having more contact with medical services than was the case historically. We are not able to identify any particular reason why the study methodology or data source could have confounded our findings regarding sex-ratio.
Regional variations in MS
A recent study using HES data for the period 1999–2005 showed regional variations in hospital admission rates for MS in England. 19 This study found significantly higher MS admissions in more northern regions of England even after adjusting for social deprivation and UK birthplace. Early studies on MS suggested a trend with latitude with increasing prevalence in more temperate climates in Northern and Southern hemispheres. 3 , 20–26 However, the idea that there is a relationship between latitude and MS incidence or prevalence in Western Europe has been dismissed recently by some authors. 15 In contrast, Simpson and colleagues reported that there was a statistically significant positive association between MS prevalence and latitude globally, although there were some exceptions to the latitudinal gradient in some parts of Europe. 18
Regional variation in MS epidemiology may be due to genetic or environmental factors and interactions between them. A study in Ireland found that the HLA DRB1*15 allele associated with MS susceptibility is more common in areas of higher prevalence. 25 The exact role of such factors in the epidemiology of MS remains to be ascertained. One recent study found that the distribution of HLA DRB1 accounted for 52% of the variation in MS prevalence by latitude in Europe, 27 whereas another study suggested that non-HLA DRB1 factors play an important role in regional MS variations in Europe. 18 For example, it has been suggested that lack of vitamin D may increase susceptibility to MS. This is supported by studies on the effect of month of birth on subsequent risk of MS in Northern and Southern hemispheres. 28–29 Vitamin D also reversibly blocks the progression of experimental autoimmune encephalomyelitis, a mouse model of MS. 30 Further evidence comes from a recent genetic study which demonstrated a causative role for the CYP27B1 gene, which encodes the vitamin D-activating 1-α hydroxylase enzyme. 31 Such factors may have a role in increasing the incidence of MS in Scotland relative to other parts of the UK. Further studies are needed to investigate the causative factors of MS, particularly the role of Vitamin D, genetic susceptibility factors and infective agents.
Conclusions
This study provides a comprehensive picture of the prevalence and incidence of MS throughout the UK over two decades. It shows that more than 6000 people in the UK were newly diagnosed with MS in 2010 and that patients with MS are living longer, leading to a rising population living with the disease. This has important implications for resource provision in the UK.
- McCrone P ,
- Lindgren P ,
- Swingler RJ ,
- Williams ES ,
- Forbes RB ,
- Swingler RJ
- ↵ ISD Scotland . Scottish Multiple Sclerosis Register . http://www.isdscotland.org/Health-Topics/Quality-Improvement/The-MS-Register (accessed 29 Jan 2013 ).
- ↵ Office for National Statistics . Population estimates for UK, England and Wales, Scotland and Northern Ireland, Population Estimates Timeseries 1971 to Current Year . http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk—england-and-wales—scotland-and-northern-ireland/population-estimates-timeseries-1971-to-current-year/index.html (accessed 27 Nov 2012 ).
- ↵ Office for National Statistics . Death registrations summary tables, England and Wales, 2010 . http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-227638 (accessed 24 June 2013 ).
- Runmarker B ,
- Herrett E ,
- Thomas SL ,
- Schoonen WM ,
- Williams R ,
- Williams T ,
- Koch-Henriksen N ,
- Sorensen PS
- Trojano M ,
- Lucchese G ,
- Graziano G ,
- Boström I ,
- Stawiarz L ,
- Landtblom AM
- Simpson S Jr . ,
- Blizzard L ,
- Ramagopalan SV ,
- Seagroatt V ,
- Sutherland JM
- Acheson ED ,
- Bachrach CA ,
- Poskanzer DC ,
- Walker AM ,
- Yonkondy J ,
- Van der Mei IA ,
- Ponsonby AL ,
- Lonergan R ,
- Kinsella K ,
- Fitzpatrick P ,
- Wilson SV ,
- Handel AE ,
- Handunnethi L ,
- Giovannnoni G ,
- Willer CJ ,
- Dyment DA ,
- Sadovnick AD ,
- Giovannoni G ,
- Ramagopalan S
- Cantorna MT ,
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online figures
Contributors All authors were involved in drafting and reviewing the manuscript. Statistical analysis was carried out by SVM. The guarantor for the study is ISM.
Funding This study was funded by a grant from the Multiple Sclerosis National Therapy Centres (MSNTC), Registered Charity No.1 031 690. This grant supported study meetings but MSNTC had no input into the design of the study, collection, analysis or interpretation of the data or in the decision to submit the paper for publication.
Competing interests All authors have completed the Unified Competing Interests form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) the authors have no support from any company for the submitted work; (2) JOR has been involved as principal investigator in clinical trials, a consultant on advisory boards and invited guest speaker for Biogen Idec, Merc Serono, Bayer Schering, Teva Pharmaceuticals and Novartis; TM has been a consultant on advisory boards for Novartis in the area of multiple sclerosis and has other potential competing interests but not in this therapeutic area; IM holds research grants from Novartis and Menarini in different therapeutic areas; GB and SM have no specified relationships with companies that might have an interest in the submitted work in the previous 3 years; (3) the authors’ spouses, partners or children have no financial relationships that may be relevant to the submitted work; (4) GB was a trustee of MSNTC from June 2008 to June 2012 and now acts as a consultant for MSNTC (both positions were unpaid). The other authors have no non-financial interests that may be relevant to the submitted work.
Ethics approval ISAC approval.
Provenance and peer review Not commissioned; externally peer reviewed.
Open Access This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Read the full text or download the PDF:
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
Bring photo ID to vote Check what photo ID you'll need to vote in person in the General Election on 4 July.
- Health and social care
- Public health
- Health conditions
- Multiple sclerosis: prevalence, incidence and smoking status
- Public Health England
Multiple sclerosis: prevalence, incidence and smoking status - data briefing
Published 4 February 2020

© Crown copyright 2020
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/multiple-sclerosis-prevalence-incidence-and-smoking-status/multiple-sclerosis-prevalence-incidence-and-smoking-status-data-briefing
This briefing shows findings from a study using a sample of anonymised primary care records in relation to the prevalence, incidence and demographic characteristics of patients with a diagnosis of multiple sclerosis ( MS ) in England.
The target audiences for this briefing are health commissioners and providers of services supporting patients with MS . These findings are available and presented at a national level with the intention that they are then interpreted to inform a local assessment of the needs of patients with MS and the provision of health and care services.
2. Main findings
MS estimated prevalence is 190 cases per 100,000 population, with 105,800 individuals in England
MS is more than twice as common in females than males, 272 versus 106 per 100,000 population
females in the 50 to 59 years age group are 3 times more likely than males of a similar age to have MS (578 and 184 per 100,000 population respectively)
highest prevalence for MS occurs in the 60 to 69 years age group for both sexes (females 598 and males 228 per 100,000 population)
75% of males and females with MS are aged between 40 and 74 years of age
MS estimated incidence of between 8 and 11 new cases diagnosed each year in England per 100,000 population
on average 4,950 new cases of MS are diagnosed each year in England
smoking rates among males with MS are likely to be higher than those in the general population
males and females with MS are more likely to be ex-smokers than males and females in the general population
3. Background
There is a lack of robust routine statistics on the prevalence, incidence and demography characteristics of individuals with MS in England. Knowledge of the frequency of diseases is an important requirement for understanding population health, commissioning and planning services, and understanding variation in health and care.
The Multiple Sclerosis Society estimate that around 110,000 people are living with MS in the UK, with 5,200 new cases diagnosed each year [footnote 1] . However, these estimates are based on data up to 2010 and so there is uncertainty as to the true size of the current MS population.
4. Methodology
4.1 data source and cohort definition, data source.
This study uses a sample of anonymised primary care records provided by The Health Improvement Network ( THIN ) [footnote 2] dataset (version January 2018) to investigate cases of MS . The THIN population relates to all patients in the THIN dataset which:
- are permanently registered in the practice
- have no previous change of practice registration
- have acceptable medical data records
- are registered to a practice in England
This version of THIN dataset contains data covering 385 primary care practices in the UK, with over 3 million active patients. In England, 2% (129) of primary care practices participate in the network, accounting for 2% (1.2 million) of registered patients. In other parts of the UK participation by primary care practices in the network is higher. In Wales 19% of practices are part of THIN , while in Scotland the proportion is 14% and Northern Ireland is 12%.
Cohort definitions
The study cohort (patients with MS ) are those patients of all ages that have primary care record in the THIN dataset with a diagnosis of MS recorded in the care record. MS diagnosis is defined as one of the 12 F20 Read code classifications recommended by the expert advisory group.
- F20..00 Multiple sclerosis
- F20..11 Disseminated sclerosis
- F200.00 Multiple sclerosis of the brain stem
- F201.00 Multiple sclerosis of the spinal cord
- F202.00 Generalised multiple sclerosis
- F203.00 Exacerbation of multiple sclerosis
- F204.00 Benign multiple sclerosis
- F205.00 Malignant multiple sclerosis
- F206.00 Primary progressive multiple sclerosis
- F207.00 Relapsing and remitting multiple sclerosis
- F208.00 Secondary progressive multiple sclerosis
- F20z.00 Multiple sclerosis NOS
In addition to the above clinical and age criteria, for a case to be eligible for inclusion, they also need to comply with operation criteria and have valid and complete activity dates associated with care and treatment of MS in the study period.
4.2 Algorithms for data calculations
The prevalence of MS in this study is calculated using the point prevalence method which is the number of eligible cases in the study cohort as a proportion of the eligible THIN population. The census date for the calculation is 31 January 2018, as this is the latest time point in the version of THIN dataset used.
The calculated rate is an estimate of the point prevalence of MS recorded in primary care records. For the remainder of this briefing the term prevalence will be used to describe the above recorded point prevalence calculation.
The incidence of MS in this study is calculated using the one-year cumulative incidence rate methodology which is the number of new cases of MS diagnosed during a financial year (new cases of MS in the cohort data) divided by the number of people at risk in the population in that year. The population at risk per financial year is defined as THIN population at the beginning of that financial year that are not already diagnosed with MS . For this study the cumulative incidence was calculated for nine individual financial years up to March 2017.
The cumulative incidence methodology [footnote 3] is selected for this study due to limitations in calculating the MS -free periods precisely using THIN dataset. For the remainder of this briefing the term incidence will be used to describe the above recorded cumulative incidence calculation.
Estimates of MS for England
The number of diagnosed cases of MS in England is estimated by applying age and sex standardisation factors using an estimate of the England resident population [footnote 4] to the calculated prevalence. The derived value is an estimate of the number of people in England with a recorded diagnosis of MS on their primary care record.
The number of new diagnosed cases of MS in a financial year in England is estimated by applying standardisation factors using estimates of the England resident population [footnote 4] to the calculated incidence. The derived value is an estimate of the number of people in England with a new diagnosis of MS in the financial year, recorded in their primary care record.
4.3 Limitations of the study
The findings of this study are limited to analysis of the primary care records of those practices using the Vision [footnote 5] software and actively subscribing to the THIN data sharing protocols. In addition, the study relies on the practice of good quality and timely record keeping by primary care staff.
Known data issues exist around the format of activity dates and the use of default system values for month/day. An additional 187 MS cases were identified in the dataset that were excluded from the calculations due to incomplete data records. The inclusion of these cases increased the estimated prevalence to 205 per 100,000 population, equating to an estimated 114,200 cases in England.
In terms of age and sex of patients, THIN is a good representative of the population in England. However, the dataset is not a random sample and as such the dataset contains sampling bias in terms of geographical location and socioeconomic characteristics. This dataset does not include individuals that are in the prison system during the study period.
5. Epidemiology of Multiple Sclerosis
5.1 estimated prevalence of ms in england.
The findings of this study show that the prevalence of MS in England is estimated to be 190 people per 100,000 population. This estimate equates to 105,780 individuals as at the end of January 2018. MS in females is more than double that of the prevalence males, with 272 females per 100,000 population compared to 106 for males. Females accounted for over 72% of the recorded cases of MS in the cohort (Figure 1).
Figure 1: Multiple Sclerosis - estimated prevalence and estimated cases in England (January 2018, all ages, age and sex standardised prevalence)
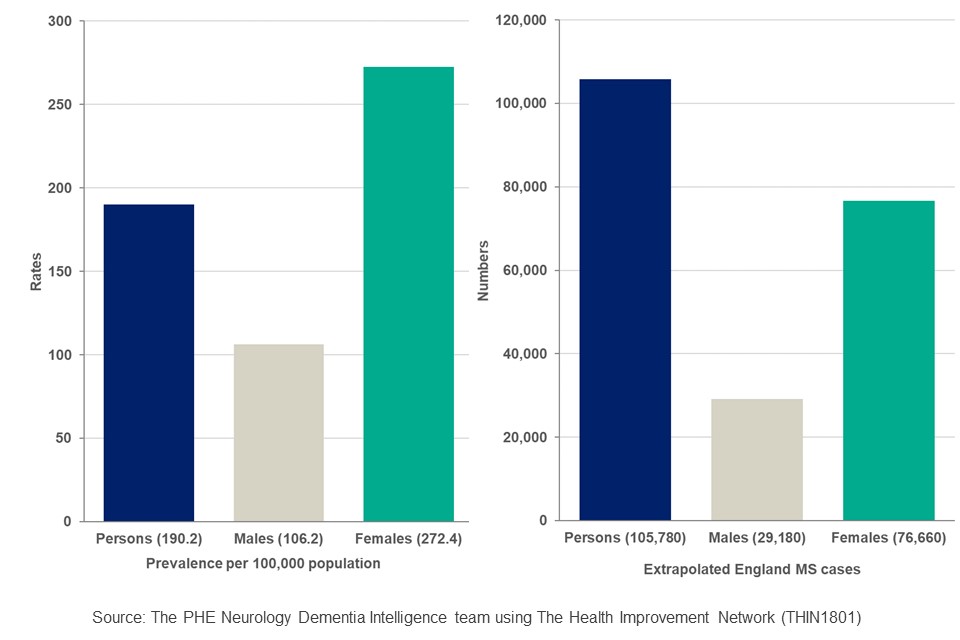
The estimated prevalence of MS in females by age group is higher than for males in equivalent age groups, with the biggest variation of 3 times as many being in the 50 to 59-year age group. The rates being 578 and 184 per 100,000 population respectively. The variation remains more than 2 times the rate with all ages over 30 years, as shown in Figure 2.
Figure 2: Multiple Sclerosis - estimated prevalence by age group (age and sex standardised, per 100,000 population, England)
| Age band | Male | Female | Persons |
|---|---|---|---|
| 20 to 29 years | 42 | 65 | 54 |
| 30 to 39 years | 82 | 190 | 137 |
| 40 to 49 years | 143 | 358 | 249 |
| 50 to 59 years | 184 | 578 | 378 |
| 60 to 69 years | 228 | 598 | 414 |
| 70 to 79 years | 209 | 483 | 353 |
| 80 to 89 years | 89 | 203 | 155 |
Source: The PHE Neurology Dementia Intelligence team using The Health Improvement Network ( THIN )
5.2 Estimated incidence of MS in England
The estimated incidence of MS was calculated for the 9 financial years 2008 to 2009 through to 2016 to 2017. Figure 3 shows that the incidence varies from 8 cases to 11 cases per 100,000 population each year in England during the period. The mean number of new cases of MS diagnosed each year, over the time period is estimated at 4,950.
Figure 3: Multiple Sclerosis – estimated incidence by financial year (England, all ages, crude rates)
| Financial year based on the first event date recorded | Incidence per 100,000 (95% Confidence Interval) | England extrapolated incidence |
|---|---|---|
| 2008 to 2009 | 9.5 (7.9 to 11.4) | 4,900 |
| 2009 to 2010 | 8.1 (6.6 to 9.9) | 4,300 |
| 2010 to 2011 | 8.3 (6.8 to 10.1) | 4,400 |
| 2011 to 2012 | 9.6 (8.0 to 11.5) | 5,100 |
| 2012 to 2013 | 9.7 (8.1 to 11.6) | 5,200 |
| 2013 to 2014 | 9.0 (7.5 to 10.9) | 4,900 |
| 2014 to 2015 | 8.7 (7.1 to 10.5) | 4,700 |
| 2015 to 2016 | 8.6 (7.1 to 10.4) | 4,800 |
| 2016 to 2017 | 11.0 (9.3 to 11.0) | 6,100 |
Although there is a variation between the incidence for each financial year, the overlapping nature of the confidence intervals ( CI ) associated with rates suggests that there is no overall statistical change in the period.
5.3 Comparability of the MS findings of this study with other studies
The findings of this study show in 2018 that an estimated 105,800 people in England have a diagnosis of MS , with the prevalence estimated as 190 per 100,000 population. This study shows that on average 4,950 new cases of MS was diagnosed in England each year during the financial years ending 2009 to 2017, with a mean incidence rate of 9 per 100,000 population per year.
The only recent previous study published for England into MS was the IS Mackenzie study [footnote 6] , published in 2013. This study, using data from 2010 and earlier, used an alternative sample dataset and methodology, by linking primary care records to hospital activity records. Mackenzie estimated that for England there were 104,450 people with MS , with a prevalence of 200 per 100,000 and 4,750 new cases diagnosed at a rate of 9 per 100,000 population.
Although the methodologies used in the 2 studies are different, the findings are very similar for the prevalence and are the same for the incidence. The numerical difference in the number of cases and new cases of MS in England between the studies will also be accounted for in part by the 6% increase in the England population between the study period of 2010 and 2018.
6. Living with Multiple Sclerosis
This section illustrates the findings of the analysis of the unadjusted data contained in the THIN database in relation to people with MS . The purpose is to describe the characteristics of people with MS in THIN dataset, however this may not be representative of all people with MS in England.
Annually there are around 100 new cases of MS diagnosed and reported in THIN dataset. For the presentation of the characteristics of new cases, data has been summed for the financial years April 2012 to March 2017.
6.1 People with MS recorded in THIN dataset
In the UK around 7,000 patients in THIN dataset have a diagnosis of MS . The largest proportion of people with MS , as shown in Figure 4, is 38% which have a registration in a primary care practice based in Scotland, 34% in England, 18% in Wales and 10% are registered in Northern Ireland. The number of MS cases reported by country in the UK reflects the coverage of the participating primary care practices in the THIN database. In THIN 40% of active patients are registered with practices in England, 29% in Scotland, 22% in Wales and 8% in Northern Ireland.
Figure 4: Multiple Sclerosis – number of patients in THIN dataset (UK and by country 2018)
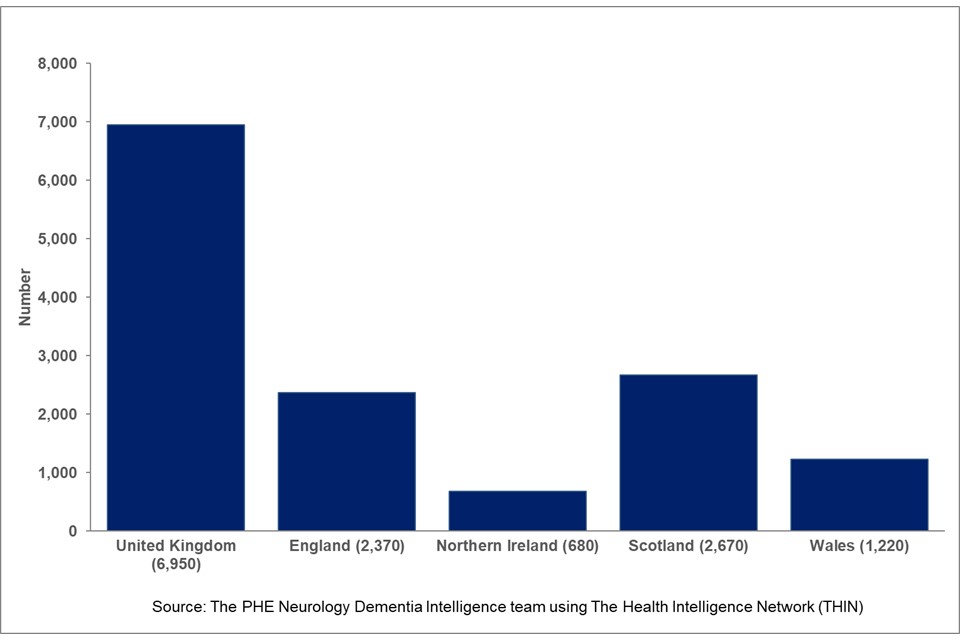
Figure 5 shows that most of the cases of MS in the UK are in female patients, 72% with the number being approximately 1.5 times higher than that for male patients. The proportions of male and female cases are similar across the other countries of the UK.
Figure 5: Multiple Sclerosis – number of patients with MS in dataset (UK and country, sex, 2018)
| Males | Females | Total | |
|---|---|---|---|
| United Kingdom | 28% | 72% | 6,946 |
| England | 28% | 72% | 2,370 |
| Northern Ireland | 30% | 70% | 684 |
| Scotland | 27% | 73% | 2,668 |
| Wales | 30% | 70% | 1,224 |
Age and sex characteristics of patients with MS
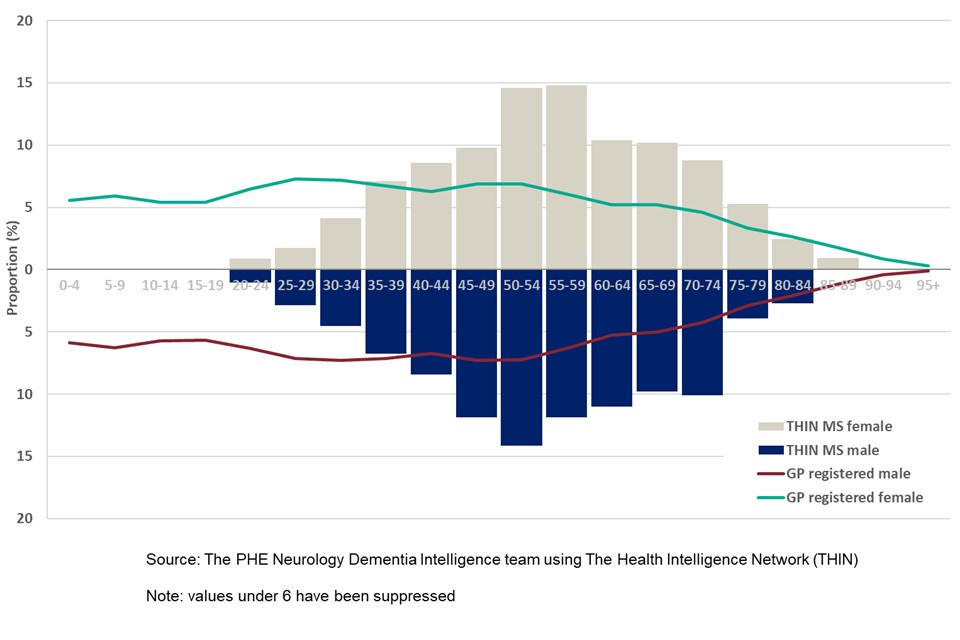
In England there are 1,705 females with a diagnosis of MS recorded in THIN dataset. In total 72% of females with MS are under 65 years of age, with 3% being under 30 years of age. 28% of females with MS are aged 65 years and older, with 9% being aged 75 years and older. The largest proportion of cases, as shown in Figure 6, occur in the 50 to 54 years and 55 to 59 years age groups, accounting for 29% of all females with MS . The median age of a female with MS is 55 to 59 years.
There are 665 males with a diagnosis of MS recorded in THIN dataset in England. 73% of males with MS are aged under 65 years, with 5% being under 30 years of age. In total 27% of males with MS are aged 65 years and older, 7% being 75 years and older. The largest proportion of the males with MS , 14%, occurs in the 50 to 54 years age group. The median age of a male with MS is 55 to 59 years.
Figure 6: Multiple Sclerosis - age distribution of patients with MS and GP registered population by sex (financial year April 2016 to March 2017 for MS cases, 2017 for registered population, England)
Age and sex characteristics of new cases of MS
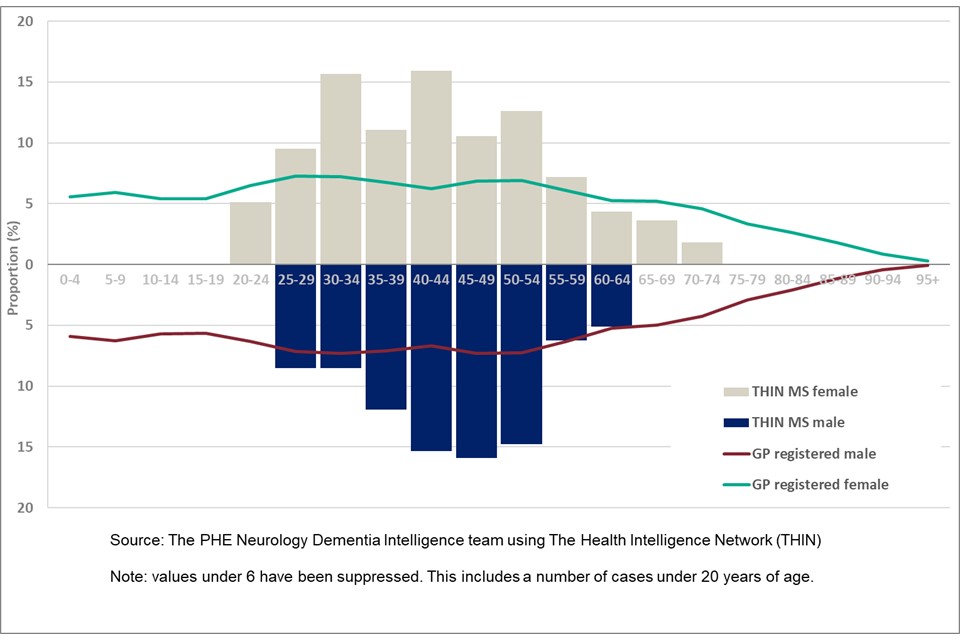
Females accounted for 69% of all new cases of MS in THIN dataset in the period April 2012 to March 2017. In total 93% of new female cases are of working age (under 65 years of age), with 16% under 30 years of age. The highest proportion of new female cases occur in the 30 to 34 years and 40 to 44 years age groups, with 16% of new cases in each group, shown in Figure 7. The median age of females with a new diagnosis of MS is 40 to 44 years.
New cases of MS among males accounted for 31% of all new MS cases in the period. 93% of new male cases were of working age, 15% aged under 30 years. The highest proportion of new recorded diagnoses occurred in the 45 to 49 years age group (16%). The median age of males with a new diagnosis of MS is also 45 to 49 years of age.
Figure 7: Multiple Sclerosis – age distribution of new cases of MS and GP registered population by sex (financial years April 2012 to March 2017 for new cases, 2017 for registered population, England)
6.2 Smoking status of patients with MS
Clinical Guidance 189 [footnote 7] from The National Institute for Health and Care Excellence (NICE) recommends that following a diagnosis of MS , clinical staff should advise patients not to smoke as it may increase the progression of disability due to the condition. A view supported by the MS Society in their factsheet on MS and smoking [footnote 8] . Data on the smoking status of patients with MS and those without a diagnosis of MS was analysed using the latest recorded smoking status for each case, for those 18 years and older from the THIN dataset.
Figure 8: Smoking status of patients with MS and without MS by sex (2018, aged 18 years and over, England)
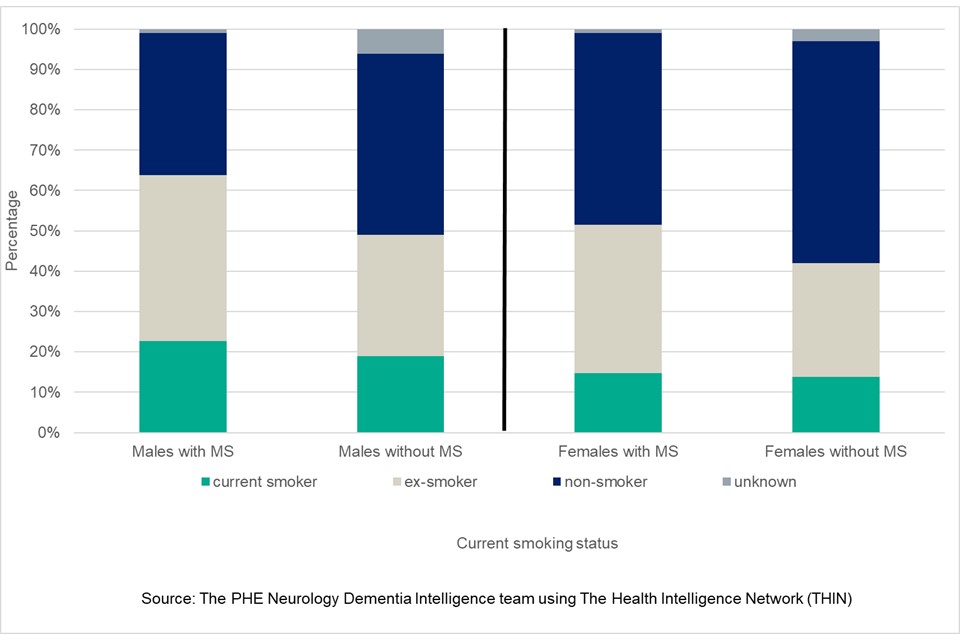
Ex-smokers constitute the largest proportion of males with a diagnosis of MS , 41% ( CI 37% to 45%), with this proportion larger than male ex-smokers without MS 30% ( CI 30% to 30%), shown in Figure 8. The proportion of males with MS that are current smokers is also higher than those without a diagnosis, 23%( CI 20% to 26%) compared to 19% (19% to 19%).
In terms of non-smoker status, a smaller proportion of males with MS are classed as non-smokers 35% ( CI 32% to 39%) compared to those without MS 45% ( CI 45% to 45%).
For females with MS , the smoking status of the largest proportion of cases are non-smokers 47% ( CI 45% to 50%), with the same status being the largest group without a diagnosis of MS , 55% ( CI 55% to 55%). The proportions of females with MS that are current smokers is 15% ( CI 13% to 17%), which is a similar proportion to those without diagnosis 14% ( CI 14% to 14%). A larger proportion of females with MS are now ex-smokers 37% ( CI 35% to 39%) compared to those without a diagnosis 28% ( CI 28% to 28%).
It is noteworthy that the proportions of ex-smokers, among both males and females with MS , are larger than those without a diagnosis. Whether this is due to individuals listening to clinical advice and whether this behavioural change is being supported by PHE initiatives on stop smoking is unclear from the study findings. However, there are still 23% of males and 15% of females with MS that are classed as smokers, so there still remains opportunity to improve the public health messaging and support to individuals and clinical staff to achieve the published recommendations for the management of MS in adults.
7. Conclusions
The purpose of the current study was to provide insight into the characteristics of people with MS , working in collaboration with the MS Society and using the THIN dataset. The methodologies and approach used in this study reflect the capabilities and capacities within the Neurology Dementia Intelligence team at PHE.
The estimate for the prevalence of MS in England is 190 cases per 100,000 population, accounting for 105,800 cases. The prevalence rate for females is 2.5 times higher than for males, 272 and 106 cases per 100,000 population respectively. The largest variation in rates between the sexes is in the age group 50 to 59 years, with the rate for females in excess of 3 times the rate for males (578 and 184 respectively). The highest prevalence rates for MS in both sexes occurs in the 60 to 69 years age group (males 228 and females 598).
The incidence of MS in England has remained consistent across the study period, financial years ending 2009 through to 2017. Although the annual rates have varied between 8 and 11 new cases per 100,000 population each year, the changes are not statistically significant. Each year there are on average 4,950 new case of MS diagnosed and recorded in primary care records.
The clinical advice for those with a diagnosis of MS is to cease smoking. The study findings show that larger proportions of both males and females with MS were classed as ex-smokers, than those without MS . However, 23% of males and 15% females with MS were still classed as smokers.
Further research is required to better understand the smoking status of those with MS . Clearly there are smoking cession successes within this population group that should be evaluated. However, opportunities still exist to improve the public health messaging relating to smoking and the on-going management of MS . This could include improvements in communication between specialist neurological staff who support people with MS and the providers of local smoking cessation services.
8. Acknowledgements
The authors of this briefing are members of the neurology and dementia intelligence team at PHE – Marta Szczepaniak, Katie Dowden, Michael Jackson, Julia Verne and supported by Sue Foster and Simran Sandhu.
The authors would like to thank members of the Multiple Sclerosis Society for their support in this project and the access provided to the specialist clinical and academic expertise.
SRC Reference Number: 18THIN087. THIN is a registered trademark of Cegedim SA in the United Kingdom and other countries. Reference made to the THIN database is intended to be descriptive of the data asset licensed by IQVIA.
9. References
Multiple Sclerosis Society. MS in the UK 2018 (viewed August 2019) ↩
Vision Health. The Health Improvement Network (viewed August 2019) ↩
World Health Organisation. Basic Epidemiology (viewed March 2018) ↩
Office for National Statistics. Population estimates 2018 (viewed August 2019) ↩ ↩ 2
Vision Health. Vision software (viewed December 2019) ↩
Mackenzie IS and others. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. Journal of Neurology, Neurosurgery and Psychiatry Online First, published on September 19, 2013 Volume 85 Issue 1 (viewed August 2019) ↩
National Institute for Health and Care Excellence: Multiple sclerosis in adults: management; Clinical guideline (CG186; 1.47) (viewed December 2019) ↩
Multiple Sclerosis Society: Smoking and MS (viewed August 2019) ↩
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
How Many People Have MS? A Case Study of the UK
Cite this chapter.

- Paul J. Bull 2
219 Accesses
This chapter is concerned primarily with establishing an estimate of the number of people with Multiple Sclerosis (PwMS) in the United Kingdom (UK) and the ways in which that number may change through time, the ‘components of change’. It begins by placing the UK in an international context, demonstrating it is a country with one of the highest MS prevalence rates in the world; a finding that of itself helps to justify the geographical emphasis of this book. It then reviews the various estimates for the number of PwMS in the UK before considering first how MS varies within and between its four constituent countries and, second how the separate components of change have influenced these numbers.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Get 10 units per month
- Download Article/Chapter or Ebook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Unable to display preview. Download preview PDF.
Similar content being viewed by others

Increasing age of multiple sclerosis onset from 1920 to 2022: a population-based study
High incidence and increasing prevalence of multiple sclerosis in british columbia, canada: findings from over two decades (1991–2010), epidemiology of ms in russia, a historical review, author information, authors and affiliations.
Independent Researcher, UK
Paul J. Bull
You can also search for this author in PubMed Google Scholar
Copyright information
© 2015 Paul J. Bull
About this chapter
Bull, P.J. (2015). How Many People Have MS? A Case Study of the UK. In: People with Multiple Sclerosis. Palgrave Macmillan, London. https://doi.org/10.1057/9781137457066_5
Download citation
DOI : https://doi.org/10.1057/9781137457066_5
Publisher Name : Palgrave Macmillan, London
Print ISBN : 978-1-349-56851-2
Online ISBN : 978-1-137-45706-6
eBook Packages : Palgrave Social Sciences Collection Social Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Case Studies in Multiple Sclerosis
- January 2016
- ISBN: 978-3-319-31188-3
- This person is not on ResearchGate, or hasn't claimed this research yet.
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
Chapters (19)
- 1 Recommendation

- CLIN NEUROPHARMACOL

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

Progressive MS Patients with Considerable Disability Ably Treated with Cladribine, UK Case Study Reports
by Alice Melão, MSc | July 17, 2018
Share this article:
Cladridine may be effective in preventing disability progression and reducing damage to nerve cells in people with progressive forms of multiple sclerosis (MS), researchers suggest based on a case study of two such patients given the injectable treatment.
MS is characterized by progressive degeneration of cells in the central nervous system, mostly due to an impaired immune response and inflammation. The disease can be progressive from onset, identified as primary progressive MS or PPMS . But most patients are diagnosed with RRMS and — after a period of relapses and remissions that can last about 10 years — transition to secondary progressive MS or SPMS .
Eleven different classes of disease-modifying therapies (DMTs) are currently approved in Europe and the U.S. to treat MS, but only Ocrevus ( ocrelizumab ) has been approved to treat progressive MS , with its use largely restricted to PPMS patients.
This highlights the urgent need for DMTs that can effectively treat progressive disease forms.
In the study “ Disease activity in progressive multiple sclerosis can be effectively reduced by cladribine, ” researchers at the Blizard Institute , part of Queen Mary University of London , detailed two progressive MS patients who they treated off-label with cladribine injections. The study was published in Multiple Sclerosis and Related Disorders .
Cladribine, marketed under the name Mavenclad by EMD Serono (Merck KGaA outside the U.S. and Canada), is approved as an oral treatment for relapsing MS in Argentina , Canada , Australia , Israel, Europe , and United Arab Emirates .
The first case involved a man of Asian ascent who had been diagnosed with relapsing MS at age 21, with unilateral inflammation of the optic nerve as the initial disease manifestation.
He was treated with Rebif ( interferon beta-1a ), but stopped that treatment after two years because it failed to prevent his relapses. For the next nine years, he went without treatment despite a high relapse rate. His condition progressed significantly, showing moderate disability ( EDSS 4.0) with a significant brain lesion burden.
At this point he started using Tecfidera ( dimethyl fumarate ), but decided to stop after three weeks due to side effects that included fatigue, flushing, and abdominal discomfort.
A detailed analysis of his disease course now showed chronic deterioration had started around six months ago, indicating the man had entered the SPMS stage. In 18 months, his EDSS scores — which quantify disability and its progression over time— rose from 4.0 to 6.0 (severe disability), and had a nearly 4.5-fold higher levels of neurofilament light chain (NfL) determined from cerebral spinal fluid (CSF) sample. Levels of NfL, a protein, is being investigated as a biomarker of nerve cell damage in MS.
One month later, doctors decided to start treating him off-label with subcutaneous (under-the-skin) cladribine, sold under the brand name Litak (approved to treat hairy cell leukemia). He was treated on a schedule of cladribine 10 mg injections for three days on weeks one and five.
The second case concerned a woman experiencing progressive weakness in left her leg beginning at age 43. Medical evaluation revealed brain lesions and brain volume consistent with demyelinating disease.
Two year later, her disability had worsened, and her EDSS score was 5.5 (a level at which walking aids are needed for any distance). Laboratory analysis found such high levels of NfL in her CFS sample that were “off the scale” — more than 26 fold — and beyond accurate quantification).
Her medical team began treating her as well with cladribine injections on a similar treatment schedule.
Cladribine was well-tolerated, with no adverse reactions being reported on follow-up visits. Both patients experienced significant reduction in inflammation and disease activity, as determined by brain magnetic resonance imaging (MRI) scans and by a drop in NfL levels of 73% in the man, and 80% in the woman.
The man showed mild disability progression while on cladribine, with his EDSS moving from 6.0 to 6.5 in one year; disability in the woman remained stable.
Supported by these positive outcomes, the researchers suggest that progressive MS patients “with detectable disease activity (MRI, elevated NfL) should be considered for DMTs.”
And, they added: “Over and above its licensed indication (relapsing MS), cladribine may be an effective treatment option” for these patients.
The team also suggested that NfL levels can be “a sensitive index of treatment effect” in progressive MS, and “may be a useful outcome in clinical trials targeting this patient group.”

Recent Posts
- Immune cells can take on healing abilities to repair nerve fibers: Study
My routine for dealing with MS and heat sensitivity while traveling
Ms complicates everything, even recovering from shoulder surgery, recommended reading.

Immune cell behavior could inform MS treatment strategies: Study
Subscribe to our newsletter.
Get regular updates to your inbox.
Clinical Presentation: Case History # 1 Ms. C is a 35 year old white female. She came to Neurology Clinic for evaluation of her long-term neurologic complaints. The patient relates that for many years she had noticed some significant changes in neurologic functions, particularly heat intolerance precipitating a stumbling gait and a tendency to fall. Her visual acuity also seemed to change periodically during several years. Two months ago the patient was working very hard and was under a lot of stress. She got sick with a flu and her neurologic condition worsened. At that time, she could not hold objects in her hands, had significant tremors and severe exhaustion. She also had several bad falls. Since that time she had noticed arthralgia on the right and subsequently on the left side of her body. Then, the patient abruptly developed a right hemisensory deficit after several days of work. The MRI scan was performed at that time and revealed a multifocal white matter disease - areas of increased T2 signal in both cerebral hemispheres. Spinal tap was also done which revealed the presence of oligoclonal bands in CSF. Visual evoked response testing was abnormal with slowed conduction in optic nerves. (Q.1) (Q. 2) (Q.3) Today, the patient has multiple problems related to her disease: she remains weak and numb on the right side; she has impaired urinary bladder function which requires multiple voids in the mornings, and nocturia times 3. She became incontinent and now has to wear a pad during the day. (Q.4) She also has persistent balance problems with some sensation of spinning, and she is extremely fatigued. REVIEW OF SYSTEMS is also significant for a number of problems related to her suspected MS. The patient has a tendency to aspirate liquids and also solids. (Q.5) (Q.6) She complains of tinnitus which is continuous and associated with hearing loss, more prominent on the left. She has decreased finger dexterity and weakness of the hands bilaterally. She also complains of impaired short-term memory and irritability. FAMILY HISTORY is significant for high blood pressure, cancer and heart disease in the immediate family. PERSONAL HISTORY is significant for mumps and chicken pox as a child, and anemia and allergies with hives later in life. She also had a tubal ligation. NEUROLOGIC EXAMINATION: Cranial Nerve II - disks are sharp and of normal color. Funduscopic examination is normal. Cranial Nerves III, IV, VI - no extraocular motor palsy or difficulties with smooth pursuit or saccades are seen. Remainder of the cranial nerve exam is normal except for decreased hearing on the left, and numbness in the right face, which extends down into the entire right side. The Weber test reveals greater conductance to the right. Rinne's test reveals air greater than bone bilaterally. (Q.7) The palate elevates well. Swallow appears to be intact. Tongue movements are slowed, but tongue power appears to be intact. Motor examination reveals relatively normal strength in the upper extremities throughout. However, rapid alternating movements are decreased in both upper extremities and the patient has dysdiadochokinesia in the left hand. (Q.8) Mild paraparesis is noted in both legs without severe spasticity. Deep tendon reflexes are +2 and symmetrical in the arms, +3 at the ankles and at the knees. Bilateral extensor toe sign are present. Sensory exam reveals paresthesia on the right to touch and decreased pin sensation on the right diffusely. The patient has mild vibratory sense loss in the distal lower extremities. Romberg's is negative. (Q.9) Tandem gait is mildly unstable. Ambulation index is 7.0 seconds for 25 feet. (The patient takes 7.0 seconds to walk 25 feet.) Diagnosis: Multiple Sclerosis with laboratory support. © John W.Rose, M.D., Maria Houtchens, MSIII, Sharon G. Lynch, M.D.

- < Previous
Home > UTHealth > McGovern Medical School > Journal Articles > 1593

Journal Articles
Polygenic liability for anxiety in association with comorbid anxiety in multiple sclerosis.
Kaarina Kowalec Arvid Harder Casandra Dolovich Kathryn C Fitzgerald Amber Salter Yi Lu Charles N Bernstein James M Bolton Gary Cutter John D Fisk Joel Gelernter Lesley A Graff Sara Hägg Carol A Hitchon Daniel F Levey Fred D Lublin Kyla A McKay Scott Patten Amit Patki Murray B Stein Hemant K Tiwari Jerry S Wolinsky Ruth A Marrie
Publication Date
Annals of Clinical and Translational Neurology
OBJECTIVE: Comorbid anxiety occurs often in MS and is associated with disability progression. Polygenic scores offer a possible means of anxiety risk prediction but often have not been validated outside the original discovery population. We aimed to investigate the association between the Generalized Anxiety Disorder 2-item scale polygenic score with anxiety in MS.
METHODS: Using a case-control design, participants from Canadian, UK Biobank, and United States cohorts were grouped into cases (MS/comorbid anxiety) or controls (MS/no anxiety, anxiety/no immune disease or healthy). We used multiple anxiety measures: current symptoms, lifetime interview-diagnosed, and lifetime self-report physician-diagnosed. The polygenic score was computed for current anxiety symptoms using summary statistics from a previous genome-wide association study and was tested using regression.
RESULTS: A total of 71,343 individuals of European genetic ancestry were used: Canada (n = 334; 212 MS), UK Biobank (n = 70,431; 1,390 MS), and the USA (n = 578 MS). Meta-analyses identified that in MS, each 1-SD increase in the polygenic score was associated with ~50% increased odds of comorbid moderate anxious symptoms compared to those with less than moderate anxious symptoms (OR: 1.47, 95% CI: 1.09-1.99). We found a similar direction of effects in the other measures. MS had a similar anxiety genetic burden compared to people with anxiety as the index disease.
INTERPRETATION: Higher genetic burden for anxiety was associated with significantly increased odds of moderate anxious symptoms in MS of European genetic ancestry which did not differ from those with anxiety and no comorbid immune disease. This study suggests a genetic basis for anxiety in MS.
Humans, Multiple Sclerosis, Male, Female, Adult, Middle Aged, Multifactorial Inheritance, Comorbidity, Case-Control Studies, Anxiety Disorders, Anxiety, Canada, United States, United Kingdom, Aged, Genome-Wide Association Study, Genetic Predisposition to Disease
Since June 27, 2024
Included in
Neurology Commons
To view the content in your browser, please download Adobe Reader or, alternately, you may Download the file to your hard drive.
NOTE: The latest versions of Adobe Reader do not support viewing PDF files within Firefox on Mac OS and if you are using a modern (Intel) Mac, there is no official plugin for viewing PDF files within the browser window.
Advanced Search
- Notify me via email or RSS
- All Collections
- Monthly Spotlight
- Open Journals
- Dissertations and Thesis
- Library Collections
- Disciplines
Author Corner
- Get Started Guide
- Submit Research
- NIH Public Access Policy
- McGovern Medical School
- Texas Medical Center Library
- McGovern Historical Center
- 713-795-4200
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
- Open access
- Published: 25 June 2024
Intracerebral haemorrhage in multiple sclerosis: assessing the impact of disease-modifying medications
- Brian M. Ou Yong 1 ,
- Wireko Andrew Awuah 2 ,
- Muhammad Hamza Shah 3 ,
- Vivek Sanker 4 ,
- Jonathan Kong Sing Huk 1 ,
- Sujashree Yadala Venkata 1 ,
- Diti H. Patel 5 ,
- Joecelyn Kirani Tan 6 ,
- Noor Ayman Khan 7 ,
- Ajitha Kulasekaran 1 ,
- Manali Sarkar 8 ,
- Toufik Abdul-Rahman 2 &
- Oday Atallah 9
European Journal of Medical Research volume 29 , Article number: 344 ( 2024 ) Cite this article
242 Accesses
Metrics details
Multiple Sclerosis (MS) is a complex autoimmune disorder that significantly impacts the central nervous system, leading to a range of complications. While intracranial haemorrhage (ICH) is a rare but highly morbid complication, more common CNS complications include progressive multifocal leukoencephalopathy (PML) and other CNS infections. This severe form of stroke, known for its high morbidity and mortality rates, presents a critical challenge in the management of MS. The use of disease-modifying drugs (DMDs) in treating MS introduces a nuanced aspect to patient care, with certain medications like Dimethyl Fumarate and Fingolimod showing potential in reducing the risk of ICH, while others such as Alemtuzumab and Mitoxantrone are associated with an increased risk. Understanding the intricate relationship between these DMDs, the pathophysiological mechanisms of ICH, and the individualised aspects of each patient's condition is paramount. Factors such as genetic predispositions, existing comorbidities, and lifestyle choices play a crucial role in tailoring treatment approaches, emphasising the importance of a personalised, vigilant therapeutic strategy. The necessity for ongoing and detailed research cannot be overstated. It is crucial to explore the long-term effects of DMDs on ICH occurrence and prognosis in MS patients, aiming to refine clinical practices and promote patient-centric, informed therapeutic decisions. This approach ensures that the management of MS is not only comprehensive but also adaptable to the evolving understanding of the disease and its treatments.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune inflammatory disorder of the central nervous system (CNS) that can lead to neurological defects and severe incapacitation [ 1 ]. With a global prevalence of 2.8 million, it is the most common debilitating neurological disease among young adults, with symptoms beginning around the ages of 20–40 [ 2 , 3 ]. Notably, MS is a challenging diagnosis, as symptoms may vary depending on the severity of the inflammatory reaction as well as the location of CNS lesions. Possible neurological symptoms include vision impairment, focal weakness, paraesthesia, incontinence, and cognitive dysfunction. In addition to the diversity of symptoms, it is common for MS patients to have other comorbid conditions, such as autoimmune conditions like inflammatory bowel disease and rheumatoid arthritis, and less commonly, strokes [ 4 ].
Intracerebral haemorrhage (ICH), characterised by bleeding into the brain parenchyma, is the second most common cause of strokes [ 5 ]. Spontaneous cerebrovascular haemorrhage has a poor prognosis, with approximately 50% of patients dying within 1 year, and cerebral amyloid angiopathy being the most common cause [ 6 ]. The most common cause of ICH is hypertension, but secondary causes include vascular malformations, aneurysms, chronic alcohol use, and medications that increase the risk of bleeding, such as warfarin and apixaban [ 7 ]. Risk factors such as hypertension (HTN), hyperlipidemia, and tobacco exposure are suggested to accelerate the progression of MS. However, these vascular risk factors are also tied to cerebrovascular diseases such as ICH. Compared to the general population, MS patients are at an increased risk of developing strokes [ 8 ]. A recent retrospective cohort study showed that 0.19% of MS patients experienced ICH. To put this in perspective, the general population experiences ICH at a rate of 24.6 per 100,000 people each year, and it often leads to death [ 9 , 10 ]. It is worth noting that MS patients face a higher risk of hemorrhagic strokes [ 8 ], which adds to the overall severity of their condition. This underscores the importance of prevention strategies, especially since there are limited treatment options available.
Recent MS treatments consist of DMD therapies, which are medications targeted to prevent relapses and progression to disability. This narrative review aims to synthesise the available evidence in the literature, highlighting the influence of various DMDs on the occurrence of ICH in MS patients and exploring their potential role in preventing such occurrences. The research delves into the anti-inflammatory properties of commonly prescribed DMDs in MS management, examining how they may either contribute to or deter ICH. Additionally, this review discusses current practices and future directions to address the research gap, proposing strategies for incorporation into upcoming clinical practices.
Methodology
This narrative review focused on the occurrence of ICH associated with DMDs and their potential protective effect of DMDs against ICH in MS patients. A comprehensive literature search was systematically conducted using PubMed, EMBASE, Google Scholar, and the Cochrane Library, focusing on English-language studies with no timeline applied, encompassing randomised clinical trials (RCTs), meta-analyses, systematic reviews, observational studies, and case–control. Search terms like "disease-modifying drugs," "multiple sclerosis," and “intracranial haemorrhage” were used. A total of 25 articles were included out of 86 articles identified. Furthermore, a manual review of selected articles, reviews, meta-analyses, and practice guidelines was conducted. Abstracts and unpublished studies were excluded from the review. A summary of the methodology employed is presented in Fig. 1 .

Prisma flow diagram
Pathophysiology of ICH in MS
The integrity of the blood–brain barrier (BBB) is crucial for maintaining CNS equilibrium. However, in MS, inflammatory autoimmune responses break down the BBB, marked by the recruitment of lymphocytes, microglia, and macrophages to lesion sites [ 11 ]. This exacerbates BBB permeability and facilitates further immune cell infiltration, fuelling the inflammatory cascade. Consequently, leukocyte infiltration into the CNS modifies BBB permeability and induces inflammation by expressing inflammatory cytokines, reactive oxygen species (ROS), and enzymes [ 11 ]. Inflammatory mediators significantly affect BBB integrity and the immune response. For instance, ICAM-1, stimulated by cytokines like TNF-α, is an early marker of immune activation, correlating with BBB damage, cerebrospinal fluid (CSF) pleocytosis, and TNF-α levels in active MS [ 12 ]. Moreover, viruses and environmental pollutants can diminish immunity in genetically susceptible individuals and trigger the release of proinflammatory mediators such as IL-6 and NF-κB [ 13 ]. These factors accelerate changes in endothelial tight junctions, increasing BBB permeability and allowing leukocyte migration into the brain parenchyma. This disruption of the BBB plays a critical role in the pathophysiology of ICH in MS patients.
Chronic inflammation in MS can also induce angiogenesis. While angiogenesis is a natural response to tissue injury and inflammation, in the context of MS, it could form abnormal and fragile blood vessels [ 14 ]. These newly formed vessels often lack the structural integrity of normal vasculature, making them more prone to rupture. Additionally, the process of vascular remodelling in MS includes the thickening and stiffening of existing vessel walls due to fibrosis [ 15 ]. This, combined with the disruption of the BBB, could result in increased vascular resistance and hypertension within the CNS. Both conditions are recognised risk factors for haemorrhage, as the heightened pressure and structural weakness make the vessels more susceptible to rupture under stress.
Furthermore, vasculitis could also occur in the context of MS [ 15 ]. This condition further exacerbates the risk of ICH by weakening the structural integrity of the blood vessels. Inflammatory vasculitis involves immune-mediated damage to the blood vessel walls, which could lead to their thinning and increased fragility [ 16 ]. The compromised vessels are at a higher risk of rupture, especially in the dynamic environment of the CNS where blood flow and pressure can fluctuate significantly. As a result, vasculitis in MS patients can create a direct pathway to haemorrhagic events, compounding the already elevated risks due to other inflammatory and structural changes in the vasculature. Figure 2 summarises the complex pathophysiology of ICH in patients with MS.

Pathophysiology of Intracerebral Haemorrhage in Multiple Sclerosis Patients. ICH Intracerebral haemorrhage, BBB Blood–brain barrier, CSF Cerebrospinal fluid, ICAM-1 Intercellular adhesion molecule 1, TNF-α Tumour necrosis factor alpha, T cells: T Lymphocytes, B cells: B Lymphocytes, ROS Reactive oxygen species, IL-6 Interleukin 6, NF-κB Nuclear factor kappa-light-chain-enhancer of activated B cells
Role of DMDs on ICH in MS patients
Various DMDs have been used in the treatment of MS, including Interferon (IFN) beta, Fingolimod, Alemtuzumab, Mitoxantrone (MX), Natalizumab, Siponimod, Dimethyl fumarate, and Ozanimod. Research indicates that certain DMDs, such as dimethyl fumarate, ozanimod, fingolimod, and siponimod, have been associated with a lower risk of ICH in MS patients. Conversely, DMDs like Alemtuzumab, Mitoxantrone, Natalizumab, and IFN-beta have been associated with increased risk of ICH in individuals with MS.
Other alternative DMDs used to treat MS, such as Glatiramer acetate, Daclizumab, Teriflunomide, Fingolimod, Rituximab, Siponimod, Dimethyl Fumarate, and Ocrelizumab, have adverse effects, but there have been no recorded incidences of ICH in MS patients. Some of these drugs have been associated with bleeding issues, but notably, no intracranial bleeding has been reported. For example, Glatiramer acetate is an immunomodulatory drug for treating RRMS. One report describes a case where a patient developed refractory immune thrombocytopenic purpura (ITP) 2 months after starting Glatiramer acetate for MS [ 17 ]. During her hospital stay, the patient experienced transient episodes of vaginal, oral, skin, and gastrointestinal bleeding.
DMDs with potential protective effect against ICH in MS patients
The anti-inflammatory properties of DMDs have been shown to reduce the occurrence of certain diseases, particularly the occurrence of ICH in MS patients, through various mechanisms. A retrospective cohort study done by Zulfiqar et al. showed that DMD could have a protective effect against ICH in MS patients. This effect was shown to persist even after adjusted analyses for potential confounders like lifestyle factors and comorbidities [ 9 ]. An in-depth review of the mechanisms of action of the various DMDs will allow us to understand the neuroprotective and neuro-damaging properties of these drugs.
Dimethyl fumarate
In a mouse model study, Dimethyl fumarate (DMF) has shown potential in treating ICH in MS through mechanisms involving the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Central to this mechanism is the nuclear factor erythroid-2-related factor 2 (Nrf2), a transcription factor that oversees the expression of antioxidant response element (ARE) genes [ 18 , 19 ]. In the human brain, Nrf2 is mainly found in non-neuronal cells like microglia and macrophages. The activation of Nrf2 in these cells, particularly through agents like sulforaphane, is noted for enhancing erythrocyte phagocytosis, contributing to the clearance of hematomas [ 20 ]. A study involving a group of 26 individuals with ICH indicated the presence of Nrf2 activation in the brain. Although the levels of Nrf2 activation in ICH patients were lower compared to those in the control group, this points to a potential therapeutic avenue for ICH treatment [ 21 ].
Further experimental research on ICH in mouse models has connected the anti-inflammatory and neurological improvement properties of DMF to the activation of Casein kinase 2 and the upregulation of Nrf2 signalling pathways [ 22 ]. This pathway includes the upregulation of antioxidant genes that protect cells from oxidative damage, which is crucial for neuroprotection in MS patients. Additional studies reinforce DMF's therapeutic potential in ICH scenarios. It has been observed in rat and mouse models that DMF, even when administered 24 h after the onset of ICH, can effectively promote hematoma resolution, reduce neurological deficits, and decrease brain edema, primarily through the activation of Nrf2 genes [ 23 ]. Especially notable is the finding that high doses of DMF (100 mg/kg) led to a significant reduction in the brain's fluid content, particularly affecting the ganglia and cortex [ 23 ]. These insights highlight the prospective role of DMF in managing ICH for MS patients, largely via mechanisms involving the Nrf2 pathway.
Fingolimod is a sphingosine 1-phosphate receptor (S1PR) modulator that binds to S1PR1, 3, 4, and 5. S1PR1 is expressed in lymphocytes, neurons, glia, and vascular endothelia [ 24 ]. It also hinders the regression of lymphocytes from lymph nodes and their recirculation, thereby reducing the migration of pathogenic cells in the CNS. Fingolimod can cross the BBB and enter the CNS to have direct effects on the neural and glial cells. Therefore, through modulation of S1PR in the CNS and immune system, fingolimod has anti-inflammatory and neuroprotective effects in ICH [ 25 ]. A study using mouse and rat models to investigate ICH demonstrated that the administration of Fingolimod yielded numerous advantages in ICH management. These benefits encompassed the reduction of brain oedema, short-term enhancements in sensorimotor functions, improved long-term motor coordination and cognitive function, decreased circulating lymphocytes, diminished migration of T lymphocytes into the brain, lowered expression of pro-inflammatory mediators in the brain, and mitigated risk of brain atrophy [ 26 ]. In a separate study, it was observed that Fingolimod was well-tolerated by patients with small and moderate-sized ICH in the basal ganglia, leading to improved outcomes and reduced perihaematomal oedema. The compound was found to be beneficial in minimising short-term neurological deficits and promoting enhanced neurologic recovery in the long term [ 25 ]. Given that this study was conducted in patients without MS, further investigations are needed to assess its applicability and efficacy in MS patients.
It is evident that Fingolimod holds the potential to exert significant therapeutic effects and offer holistic management of ICH in MS patients. It also has the potential to mitigate the damage caused by hemorrhagic events in the brain. Its ability to inhibit the circulation and migration of pathogenic cells into the CNS could lead to a reduction in the inflammatory response associated with ICH, which may help in limiting secondary damage caused by immune reactions and potentially reduce the risk of recurrent ICH episodes in MS patients. This might contribute to a more stable long-term prognosis [ 27 ]. It is worth noting that while S1PR1 plays a pivotal role in mediating the effects of fingolimod, it remains uncertain whether solely targeting S1PR1 is sufficient and indispensable for fingolimod to confer its beneficial effects [ 27 ]. Considering the widespread distribution of S1PR1 across various cells involved in the process of ICH, coupled with the preferential localization of RP101075 in the brain, there exists the possibility of other potential cellular targets beyond immune cells and their specific anatomical locations for immune interventions, which necessitates further investigation [ 27 ].
Additionally, the activation of S1PR3 by fingolimod is partially responsible for its undesirable effects on the cardiovascular system and organ fibrosis, potentially posing notable safety concerns. A study has shown that fingolimod can increase blood pressure and predispose patients to stroke [ 28 ]. Moreover, there has been a case where an MS patient treated with fingolimod developed posterior reversible encephalopathy syndrome (PRES), which has also been associated with ICH [ 29 , 30 ] . As a result, in January 2012, the European Medicines Agency (EMA) updated its recommendations regarding the use of fingolimod, particularly in MS patients with a prior history of cerebrovascular issues [ 31 ]. They also advised that if a particular patient necessitates fingolimod treatment despite their medical history, it is crucial to conduct comprehensive monitoring. Specifically, cardiac activity should be monitored for a minimum of 6 h following the administration of the initial dose via regular ECG tracings and blood pressure measurements [ 32 ]. This guidance was further corroborated and adopted by the Central and East European (CEE) MS expert group.
Siponimod (BAF312) functions as an S1P analogue, selectively targeting S1PR types 1 and 5, similar to Fingolimod [ 33 ]. Studies in a mouse model of ICH have demonstrated Siponimod’s multiple benefits, including reduced lymphocyte counts leading to lymphopenia, decreased brain and perihaematomal edema, improved survival rates, better neurological outcomes up to 72 h post-ICH, and reduced weight loss [ 33 ]. These effects are thought to stem from siponimod’s capacity to modulate brain tissue inflammation through S1PR1, thereby limiting secondary brain damage [ 33 ]. Siponimod operates by making S1PR1 receptors unresponsive to normal exit signals from lymph nodes [ 34 ] and exerts other actions such as affecting glial cell function, reducing demyelination, and lowering circulating monocyte levels, independently of S1PR3 [ 35 ]. However, siponimod also activates G-protein-coupled inwardly rectifying potassium channels in human atrial myocytes, potentially explaining the observed rapid yet temporary bradycardia onset in some studies [ 34 ]. Additionally, its interaction with 5-G protein-coupled S1P receptors, located in crucial organs like the lungs, heart, and kidneys, suggests a broader impact on various physiological processes during treatment [ 34 ].
The aforementioned studies suggest that the multifaceted approach to siponimod makes it potentially beneficial for treating ICH in MS patients. However, given its impact on various physiological processes and the potential cardiovascular effect of the medication, it is crucial to exercise caution when used in patients, especially those with predisposing factors. There is an imperative need for further research and clinical trials that investigate the use of siponimod in patients with ICH, given the promising results observed in preclinical studies.
Ozanimod, an oral sphingosine-1-phosphate (S1P) receptor modulator, has been found to be effective in reducing the annualised relapse rate, new or enlarging T2 lesions, and gadolinium-enhancing lesions in MS patients through selective modulation of S1P1 and S1P5 receptors [ 36 , 37 ]. In mice with ICH, Ozanimod was found to decrease hematoma volume and subsequent brain water content, leading to enhanced neurological function and reduced body weight loss post-ICH [ 36 ]. This effect is attributed to the reduction of activated microglia and infiltrated neutrophils surrounding the hematoma. Additionally, the study also highlighted Ozanimod’s ability to diminish brain cell death and preserve the integrity of the BBB, further emphasising its neuroprotective effects [ 36 ]. Ozanimod exhibits a strong preference for the S1PR1 subtype over S1PR5. Its selectivity for S1PR1 is over 10,000 times greater than for S1PR2, 3, and 4 [ 37 ]. This high specificity of Ozanimod for S1PR1 helps reduce potential safety concerns related to the activation of S1PR3, which has been associated with various adverse events including hypertension, macular oedema, pulmonary toxicity, and liver toxicity [ 38 ]. Given these findings, the neuroprotective effects observed in mouse models of ICH can be considered relevant for MS patients, where Ozanimod’s mechanism of reducing activated microglia and infiltrated neutrophils, decreasing hematoma volume, and preserving the integrity of the blood–brain barrier could potentially translate into protective effects against CNS injuries, including ICH. DMDs with the potential to prevent ICH in MS patients are illustrated in Fig. 3 .

Disease-modifying drugs with protective effect against intracerebral haemorrhage in multiple sclerosis patients. DMD Disease-modifying drugs, ICH Intracerebral haemorrhage, MS Multiple sclerosis, Nrf2 Nuclear factor erythroid-2-related factor 2, S1P Sphingosine 1-1-phosphate, S1PR Sphingosine 1-1-phosphate receptor
DMDs associated with increased ICH risk in MS patients
DMDs play a crucial role in managing MS by altering the disease course and reducing relapse rates. However, the broad effects of DMDs, especially concerning ICH, necessitate a detailed understanding of their impacts.
Alemtuzumab
Alemtuzumab is a monoclonal antibody that targets the CD52 protein on T and B lymphocytes, effectively reducing inflammation. Notably, there have been reports of ICH in patients treated with alemtuzumab. For instance, a study documented five instances of ICH in patients with relapsing–remitting multiple sclerosis (RRMS) after they received only three to five doses of alemtuzumab [ 39 ]. These cases of ICH occurred within hours of the infusion, and notably, these patients had no previous history of bleeding. Symptoms like headaches and chest pain were reported during the drug infusion [ 39 ]. In another case, a patient developed ICH and subsequently passed away 6 days after starting an intravenous infusion of alemtuzumab, displaying symptoms such as headache, vomiting, and bradycardia [ 40 ]. Furthermore, a significant decrease in the patient's platelet count was noted on the fifth day after starting the treatment [ 40 ]. These cases highlight the potential risk of ICH in patients undergoing treatment with alemtuzumab, underscoring the need for careful monitoring, especially in the initial stages of treatment.
The exact mechanism through which alemtuzumab leads to ICH remains uncertain, but one theory points to the drug's potential effect on blood pressure. Research has shown that there's an average increase in mean systolic and diastolic blood pressure (SBP and DBP) of about 20 mmHg and 6 mmHg, respectively, after the first alemtuzumab infusion [ 41 ]. Moreover, studies have highlighted a correlation between alemtuzumab administration and blood pressure changes or increases, occurring even beyond the typical monitoring period after administration [ 39 ]. This suggests a potential link between elevated SBP and DBP and an increased risk of ICH. Contrarily, another study found no significant difference in mean SBP following alemtuzumab infusion [ 42 ]. Consequently, revised recommendations during alemtuzumab administration include considering inpatient admission for MS patients on alemtuzumab whose mean systolic blood pressure increases significantly during infusion or those who have a notable rise above their baseline due to the ICH risk [ 43 ]. In such cases, thorough monitoring of vital signs, frequent neurological assessments, and strict blood pressure control are advised during the hospital stay [ 39 ]. If the patient can tolerate a different DMD, considering a switch from alemtuzumab is recommended, and these guidelines have been incorporated into the American MS DMD guidelines. Before administering alemtuzumab, a detailed review of the patient's medical history, including any bleeding disorders or strokes, current medication, blood pressure readings, platelet counts, and risk factors for developing ICH, is crucial for personalised treatment decisions and risk assessment.
In addition to the potential blood pressure-related mechanisms, alemtuzumab may also induce ICH through secondary immune thrombocytopenic purpura (ITP). Reports indicate that 1.54% of patients with ITP developed ICH as a complication [ 44 ]. A patient was documented as developing drug-induced ITP after alemtuzumab treatment. Further supporting this, an analysis of alemtuzumab patients revealed that 2.3% developed ITP [ 45 ]. While the exact mechanism is unknown, the association between alemtuzumab and ICH is underscored by the connection between alemtuzumab-induced ITP and the occurrence of ICH.
Mitoxantrone
Mitoxantrone (MX) is recognized for its immunosuppressive properties, particularly in the context of MS, where it plays a crucial role in inhibiting the proliferation of T cells, B cells, and macrophages, reducing antigen presentation, and diminishing the release of proinflammatory cytokines [ 46 ]. While incidents of ICH related to MX usage are rare, a case was reported where a patient developed ICH after being diagnosed with acute myeloid leukaemia (AML) after being administered a single MX dose. Notably, a blood test performed two days prior to the ICH diagnosis showed a significantly reduced platelet count in the patient [ 47 ]. The mechanism by which MX might lead to an increased risk of ICH in MS patients can be traced back to its known side effects, which include the potential to induce TRAL. A study reviewing 12511 patients on MX found a minor percentage developing TRAL, with a significant portion of these cases being acute promyelocytic leukaemia (APL) and acute myelocytic leukaemia (AML), both of which can lead to thrombocytopenia [ 48 ]. Additionally, there was a report of a patient who developed therapy-related pre-B cell acute lymphoblastic leukaemia and pancytopenia only 6 months after starting MX treatment [ 49 ]. The precise mechanism through which MX induces ICH is not well defined. However, it is recognised that ICH is a common complication in leukaemia cases, especially when accompanied by thrombocytopenia—a condition frequently observed in 40–60% of leukaemia patients [ 50 , 51 ]. The connection between thrombocytopenia and the incidence of ICH in patients with leukaemia has been well documented [ 50 ]. Therefore, it is plausible to consider that MX might lead to ICH by first inducing TRAL, accompanied by thrombocytopenia, which in turn could lead to the development of ICH.
Natalizumab
Natalizumab, a monoclonal antibody, functions by hindering leukocyte adherence to endothelial cells through the blockade of the α4-integrin subunit, which is found in lymphocytes, monocytes, and eosinophils [ 52 ]. By interrupting this interaction, Natalizumab prevents leukocytes from migrating into the target organ, thereby effectively reducing inflammation [ 52 ]. A case was reported suggesting a possible association between Natalizumab and ICH. In this case, a patient developed ICH 22 days following the third dose of Natalizumab, despite having no prior history of bleeding disorders, vascular complications, or hypertension [ 53 ]. Given that only one case has been reported, it is crucial to exercise caution in suggesting that Natalizumab can predispose to ICH. One proposed mechanism for Natalizumab-induced ICH is its potential effect on angiogenesis inhibition. Research has shown that α4β1-integrin plays a role in angiogenesis by facilitating the adhesion of large-vessel endothelial cells to the extracellular matrix proteins thrombospondin 1 (TSP1) and thrombospondin 2 (TSP2). Inhibition of α4β1 can disrupt angiogenic processes [ 54 ]. TSP1 and TSP2 are also known to support endothelial cell survival and proliferation via α4β1-integrins [ 54 ]. It has been observed that neutralising antibodies against endothelial α4-integrin significantly hamper angiogenesis triggered by factors like tumour necrosis factor-α and soluble VCAM-1 [ 55 ]. Additionally, α4β1-integrin is instrumental in the homing of CD34 + progenitor cells to the vascular endothelium during the process of neovascularization, which is critical for tissue repair [ 56 ]. Consequently, the inhibition of α4-integrin-mediated angiogenesis presents a plausible hypothesis for the occurrence of haemorrhage.
IFN-β has a multifaceted mechanism of action that is not completely understood. It seems to augment the levels and expression of anti-inflammatory substances while concurrently decreasing the expression of proinflammatory cytokines [ 57 ]. IFN-β engages with specific receptors on human cell surfaces, setting off a cascade of events leading to the expression of various interferon-stimulated genes and markers such as MHC Class I, Mx protein, OAS, β2-microglobulin, and neopterin [ 57 ]. Different forms of IFN-β, including IFN-β-1a, IFN-β-1b, and peginterferon beta-1a, are commonly used to manage MS, each having similar mechanisms of action and effectiveness but varying in terms of administration routes and tolerability [ 58 ]. Despite its prevalent use and typically mild to moderate side effects, the potential of IFN-β to cause ICH has been underexplored [ 59 ]. Cases have been reported where patients with prolonged IFN-β treatment, specifically those with secondary progressive MS (SPMS) and relapsing–remitting MS (RRMS), experienced sudden symptoms leading to the discovery of substantial ICH, even in the absence of prior conditions like hypertension, headache, or thrombocytopenia [ 60 , 61 ]. While IFN-β is generally perceived as safe, there have been occurrences of unforeseen adverse effects, including unreported instances of ICH. The exact mechanism connecting IFN-β to ICH is still speculative, but it is proposed that extended use may induce vascular changes, subsequently increasing the ICH risk [ 60 ]. Investigations, such as a pilot study examining IFN-β-1a's impact on intracranial vascular tone regulation in RRMS patients, have shown significant changes, like an increase in mean blood flow velocity in several cerebral arteries 10 h after IFN-β administration [ 62 ]. These findings suggest a potential association between IFN-β administration and unrecognised vascular modifications that might predispose individuals to ICH. DMDs associated with increased ICH risk in MS patients are illustrated in Fig. 4 .

Disease-modifying drugs associated with increased ICH risk in MS patients. Abbreviations: AML Acute myelocytic leukaemia, APL Acute promyelocytic leukaemia, CD-52 Cluster of differentiation-52, DBP Diastolic blood pressure, DMD Disease-modifying drugs, ICH Intracerebral haemorrhage, ITP Immune thrombocytopenic purpura, MS Multiple sclerosis, RRMS Relapsing–remitting multiple sclerosis, SBP Systolic blood pressure, SPMS Secondary progressive multiple sclerosis, TRAL Therapy-related acute leukaemia, TSP Thrombospondin
Discussion and future prospects
Recent research into the impact of DMDs on ICH in MS has highlighted the multifaceted nature of these medications. Studies have reinforced the role of DMDs in reducing disability progression in MS, showcasing their significant role in managing the disease. However, their broader impacts, particularly concerning ICH, require careful consideration due to the varying effects on the immune system and other physiological processes [ 63 ]. The differential impact of DMDs on peripheral blood B cell subsets has been noted, which may influence their overall effect on the immune system. This differentiation is crucial in understanding the comprehensive effects of DMDs, including their potential implications for ICH risk and severity [ 64 ].
Moreover, the possibility of repurposing drugs initially developed for MS might offer new therapeutic pathways for ICH management. However, rigorous preclinical and clinical validation is paramount before these drugs can be considered for clinical use in the context of ICH [ 65 ]. The safety profiles of DMDs are under constant scrutiny, with ongoing pharmacovigilance studies being pivotal in understanding the adverse drug reactions (ADRs) associated with these therapies. This knowledge is crucial for optimising the use of DMDs and minimising potential risks, including ICH [ 66 ].
Future prospects involve a comprehensive understanding of the molecular pathways influenced by DMDs, the identification of patient-specific factors influencing drug efficacy and safety, and the development of rigorous monitoring protocols to mitigate risks. Large-scale, multicentric longitudinal studies are crucial to gathering robust data on the long-term effects of DMDs on the incidence and prognosis of ICH in MS patients. This includes understanding the molecular pathways influenced by these drugs, identifying patient-specific factors influencing drug efficacy and safety, and developing rigorous monitoring protocols to mitigate risks [ 67 ]. Emerging therapies that modulate neuroinflammation hold promise for improving the prognosis of ICH. Understanding the role of drugs like minocycline, sphingosine-1-phosphate receptor modulators, and statins in controlling neuroinflammation could revolutionise the treatment landscape for ICH [ 68 ]. For instance, CAA, which is one of the common causes of lobar haemorrhage, currently has limited therapeutic options. However, a recent study has shown that minocycline was associated with a reduction in ICH recurrence [ 69 ].
Recent studies have voiced the need for therapies that can arrest and reverse the persistent accumulation of disabilities associated with progressive forms of MS. Neural stem cell (NSC) therapies have shown unexpected neurotrophic support and the ability to inhibit detrimental host immune responses following transplantation into the chronically inflamed CNS [ 70 ]. Understanding the underlying mechanisms of these therapies and validating their efficacy through clinical trials could open new avenues for MS treatment, especially in its progressive stages. The protracted nature of neuroinflammation in ICH provides a window of opportunity for innovative therapies to subdue the undesired consequences. Investigating the potential of histaminergic drugs in MS and their influence on the differentiation of oligodendrocyte precursors, demyelination, and the remyelination process presents a novel approach to addressing neuroinflammation and fostering repair mechanisms in the CNS [ 71 ].
Moreover, new research also highlights the prognostic utility of serum biomarkers such as S100 calcium-binding protein B, white blood cell count, and copeptin, which could potentially guide the selection of DMDs for individual patients, tailoring treatments to mitigate risks while maximising efficacy [ 72 ]. Similarly, the differentiation in the impact of short-term versus long-term use of DMDs on the incidence and severity of ICH presents a critical area for future investigation. Understanding the optimal duration of DMD therapy is essential for balancing the therapeutic benefits against potential adverse effects. Moreover, considering demographic factors such as age and gender in the context of DMD treatment can lead to more personalised approaches, as these factors may influence the risk profile for ICH in MS patients [ 72 ].
Study limitations
While this review provides a comprehensive synthesis of existing literature on the impact of DMDs on ICH in MS patients, it is essential to acknowledge certain limitations that may impact the generalisability and depth of the findings. Firstly, the inclusion of studies was limited to those available in the selected databases and relevant literature, potentially leading to a bias in the reviewed evidence. The exclusion of studies published in languages other than English may have resulted in the oversight of relevant contributions from non-English literature. Additionally, the retrospective nature of narrative reviews inherently introduces a risk of selection bias, as the studies included were based on the author's judgement and interpretation of the role of DMDs on ICH in MS patients. Moreover, ICH represents a rare complication in MS. Consequently, the infrequent occurrence of ICH in MS patients results in a scarcity of data, limiting the basis from which findings can be extrapolated. Most of the data currently available are derived from mouse models, with limited data from human studies involving MS patients. This disparity underscores the need for further research specifically focused on human subjects to better understand the potential link between DMDs and ICH in the MS population. Currently, there is inadequate research dedicated to investigating the severity of ICH in MS patients undergoing DMD therapies. It is crucial to explore this aspect, considering the potential influence of lifestyle, socioeconomic factors, and addiction determinants on both the occurrence and severity of ICH in this specific population. Finally, variations in study methodologies and outcome measures across the included studies may contribute to heterogeneity and limit the ability to draw definitive conclusions.
The review outlines the impact of various DMDs on ICH occurrence and prevention. Noteworthy findings indicate that certain DMDs, such as Dimethyl Fumarate, Fingolimod, Siponimod, and Ozanimod, exhibit potential protective effects against ICH, while others like Alemtuzumab, Mitoxantrone, Natalizumab, and Interferon beta may pose risks. However, given the complexity surrounding MS, DMDs, and ICH, caution is warranted in asserting that these medications definitively prevent ICH in MS patients. Considering the complexity surrounding MS, DMDs, and ICH, this review calls for ongoing research to address the identified limitations. A thorough exploration of the mechanisms underlying DMD effects, coupled with well-designed comparative studies, will contribute to a more nuanced understanding of ICH in the context of MS treatment. These insights can inform clinical practices, enhance patient care, and guide the development of tailored therapeutic approaches in the ever-evolving landscape of MS management.
Availability of data and materials
No additional data available.
Abbreviations
- Multiple sclerosis
Central nervous system
- Intracerebral haemorrhage
Hypertension
Disease-modifying drug
Randomised clinical trial
- Blood–brain barrier
Cerebrospinal fluid
Intercellular adhesion molecule 1
Tumour necrosis factor alpha
Reactive oxygen species
Interleukin 6
Nuclear factor kappa-light-chain-enhancer of activated B cells
Subarachnoid haemorrhage
Nuclear factor erythroid-2-related factor 2
Antioxidant response element
Sphingosine 1-phosphate receptor
Relapsing–remitting multiple sclerosis
European Medicines Agency
Central and East European
Progressive multifocal leukoencephalopathy
Acute myeloid leukaemia
Therapy-related acute leukaemia
Acute promyelocytic leukaemia
Secondary progressive multiple sclerosis
Systolic blood pressure
Diastolic blood pressure
Immune thrombocytopenic purpura
Thrombospondin 1
Thrombospondin 2
Vascular cell adhesion molecule 1
Major histocompatibility complex
Clinically isolated syndrome
Neural stem cell
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Article CAS PubMed Google Scholar
Tafti D, Ehsan M, Xixis KL. Multiple sclerosis. Treasure Island: StatPearls Publishing; 2024.
Google Scholar
Walton C, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–21.
Article PubMed PubMed Central Google Scholar
Benjaminsen E, et al. Comorbidity in multiple sclerosis patients from Nordland County, Norway—validated data from the Norwegian Patient Registry. Mult Scler Relat Disord. 2021;48:102691.
Article PubMed Google Scholar
Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14(4):300–6.
Witsch J, et al. Prognostication after intracerebral hemorrhage: a review. Neurol Res Pract. 2021;3(1):22.
Badjatia N, Rosand J. Intracerebral hemorrhage. Neurologist. 2005;11(6):311–24.
Hong Y, et al. Multiple sclerosis and stroke: a systematic review and meta-analysis. BMC Neurol. 2019;19(1):139.
Zulfiqar M, et al. Intracerebral haemorrhage in multiple sclerosis: a retrospective cohort study. J Stroke Cerebrovasc Dis. 2019;28(2):267–75.
Poon MT, Bell SM, Al-Shahi SR. Epidemiology of intracerebral haemorrhage. Front Neurol Neurosci. 2015;37:1–12.
PubMed Google Scholar
Ortiz GG, et al. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659.
Sharief MK, et al. Increased levels of circulating ICAM-1 in serum and cerebrospinal fluid of patients with active multiple sclerosis. Correlation with TNF-alpha and blood-brain barrier damage. J Neuroimmunol. 1993;43(1–2):15–21.
Li Y, et al. Underlying mechanisms and potential therapeutic molecular targets in blood-brain barrier disruption after subarachnoid hemorrhage. Curr Neuropharmacol. 2020;18(12):1168–79.
Article CAS PubMed PubMed Central Google Scholar
Girolamo F, Coppola C, Ribatti D, Trojano M. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun. 2014;2:84. https://doi.org/10.1186/s40478-014-0084-z .
Wakefield AJ, More LJ, Difford J, McLaughlin JE. Immunohistochemical study of vascular injury in acute multiple sclerosis. J Clin Pathol. 1994;47(2):129–33. https://doi.org/10.1136/jcp.47.2.129 .
Adams CW, Poston RN, Buk SJ, Sidhu YS, Vipond H. Inflammatory vasculitis in multiple sclerosis. J Neurol Sci. 1985;69(3):269–83. https://doi.org/10.1016/0022-510x(85)90139-x .
Sagy I, Shalev L, Levi I, Shleyfer E, Valdman S, Barski L. Glatiramer Acetate-associated refractory immune thrombocytopenic purpura. EJCRIM. 2016. https://doi.org/10.12890/2016_000399 .
Alfieri A, et al. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589(17):4125–36.
Qiu J, Dando O, Febery JA, Fowler JH, Chandran S, Hardingham GE. Neuronal activity and its role in controlling antioxidant genes. Int J Mol Sci. 2020;21(6):1933.
Zhao X, et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2015;133(1):144–52.
Christopher E, et al. Nrf2 activation in the human brain after stroke due to supratentorial intracerebral haemorrhage: a case–control study. BMJ Neurol Open. 2022;4:e000238.
Iniaghe LO, et al. Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis. 2015;82:349–58.
Zhao X, et al. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke. 2015;46(7):1923–8.
Aktas O, et al. Fingolimod is a potential novel therapy for multiple sclerosis. Nat Rev Neurol. 2010;6:373–82.
Fu Y, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71(9):1092–101.
Rolland WB, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55.
Sun N, et al. Selective sphingosine-1-phosphate receptor 1 modulation attenuates experimental intracerebral hemorrhage. Stroke. 2016;47(7):1899–906.
Framke E, Thygesen LC, Malmborg M, Schou M, Sellebjerg F, Magyari M. Risk of cardiovascular disease in patients with multiple sclerosis treated with fingolimod compared to natalizumab: a nationwide cohort study of 2095 patients in Denmark. Mult Scler. 2024;30(2):184–91. https://doi.org/10.1177/13524585231221415 .
Lindå H, von Heijne A. A case of posterior reversible encephalopathy syndrome associated with gilenya(®) (fingolimod) treatment for multiple sclerosis. Front Neurol. 2015;6:39. https://doi.org/10.3389/fneur.2015.00039 .
Aranas RM, Prabhakaran S, Lee VH. Posterior reversible encephalopathy syndrome associated with hemorrhage. Neurocrit Care. 2009;10(3):306–12. https://doi.org/10.1007/s12028-009-9200-5 .
Boiko AN, Gusev EI. Contemporary algorithms for the diagnosis and treatment of multiple sclerosis based on individual assessment of patients’ status. Neurosci Behav Physiol. 2018;48:870–82.
Article Google Scholar
Fazekas F. Fingolimod in the treatment algorithm of relapsing remitting multiple sclerosis: a statement of the Central and East European (CEE) MS Expert Group. Wien Med Wochenschr. 2012;162(15–16):354–66.
Bobinger T, et al. Siponimod (BAF-312) attenuates perihemorrhagic Edema and improves survival in experimental intracerebral hemorrhage. Stroke. 2019;50(11):3246–54.
Gergely P, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specifc efects on heart rate. Br J Pharmacol. 2012;167(5):1035–47.
O’Sullivan C, et al. The dual S1PR1/S1PR5 drug BAF312 (siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinfamm. 2016;13:31.
Wang F, et al. Neuroprotection by Ozanimod Following Intracerebral Hemorrhage in Mice. Front Mol Neurosci. 2022;15:927150.
Scott FL, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173(11):1778–92.
Cohen JA, et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):373–81.
Azevedo CJ, et al. Intracerebral haemorrhage during alemtuzumab administration. Lancet Neurol. 2019;18(4):329–31.
Alemtuzumab: petechiae and epistaxis. Reactions Weekly 1988. https://doi.org/10.1007/s40278-023-51993-1
Shosha E, et al. Blood pressure changes during alemtuzumab infusion for multiple sclerosis patients. Eur J Neurol. 2021;28(4):1396–400.
Bachmann H, et al. Alemtuzumab in multiple sclerosis: a retrospective analysis of occult hemorrhagic magnetic resonance imaging lesions and risk factors. Eur J Neurol. 2021;28:4209–13.
Ozanimod. MS Society 2023. https://www.mssociety.org.uk/about-ms/treatments-and-therapies/disease-modifying-therapies/ozanimod-zeposia .
Hallan DR, et al. Immune thrombocytopenic purpura and intracerebral hemorrhage, incidence, and mortality. Cureus. 2022;14(4):e24447.
PubMed PubMed Central Google Scholar
Cuker A, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management. Mult Scler. 2020;26(1):48–56.
Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004;63(12 Suppl 6):S15–8.
CAS PubMed Google Scholar
Arruda WO, et al. Acute myeloid leukaemia induced by mitoxantrone: case report. Arq Neuropsiquiatr. 2005;63(2A):327–9.
Ellis R, Boggild M. Therapy-related acute leukaemia with Mitoxantrone: what is the risk and can we minimise it? Mult Scler. 2009;15(4):505–8.
Cartwright MS, et al. Mitoxantrone for multiple sclerosis causing acute lymphoblastic leukemia. Neurology. 2007;68(19):1630–1.
Chern JJ, et al. Clinical outcome of leukemia patients with intracranial hemorrhage. Clinical article J Neurosurg. 2011;115(2):268–72.
Kantarjian H, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109(9):1705–14.
Selewski DT, et al. Natalizumab (Tysabri). AJNR Am J Neuroradiol. 2010;31(9):1588–90.
Shah R, et al. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol. 2010;65(6):431–9.
Calzada MJ, et al. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ Res. 2004;94:462–70.
Nakao S, et al. Synergistic effect of TNF-alpha in soluble VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol. 2003;170(11):5704–11.
Jin H, et al. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116(3):652–62.
Madsen C. The innovative development in interferon beta treatments of relapsing-remitting multiple sclerosis. Brain Behav. 2017;7(6):e00696.
Filipi M, Jack S. Interferons in the treatment of multiple sclerosis: a clinical efficacy, safety, and tolerability update. Int J MS Care. 2020;22(4):165–72.
Kolb-Mäurer A, et al. An update on peginterferon beta-1a management in multiple sclerosis: results from an interdisciplinary board of German and Austrian neurologists and dermatologists. BMC Neurol. 2019;19(1):130.
Shahmohammadi S, et al. Intracerebral hemorrhage in a patient with multiple sclerosis receiving interferon beta-1α. Arch Neurosci. 2017;4(2):e42758.
Niederwieser G, et al. Intracerebral haemorrhage under interferon-beta therapy. Eur J Neurol. 2001;8(4):363–4.
Dattola V, et al. Relationship between Interferon Beta-1A administration and intracranial vascular tone regulation in patients with relapsing-remitting multiple sclerosis: a pilot study. Biomed Res Int. 2017;2017:5421416.
Amato MP, et al. Disease-modifying drugs can reduce disability progression in relapsing multiple sclerosis. Brain. 2020;143(10):3013–24.
Kemmerer CL, et al. Differential effects of disease modifying drugs on peripheral blood B cell subsets: a cross sectional study in multiple sclerosis patients treated with interferon-β, glatiramer acetate, dimethyl fumarate, fingolimod or natalizumab. PLoS ONE. 2020;15(7):e0235449.
Crilly S, et al. Revisiting promising preclinical intracerebral haemorrhage studies to highlight repurposable drugs for translation. Int J Stroke. 2021;16(2):123–36.
Maniscalco GT, et al. Preliminary results of the FASM study, an on-going Italian active pharmacovigilance project. Pharmaceuticals (Basel). 2020;13(12):466.
Xue M, Yong VW. Neuroinflammation in intracerebral haemorrhage: immunotherapies with potential for translation. Lancet Neurol. 2020;19(12):1023–32.
Pluchino S, Smith JA, Peruzzotti-Jametti L. Promises and limitations of neural stem cell therapies for progressive multiple sclerosis. Trends Mol Med. 2020;26(10):898–912.
Bax F, Warren A, Fouks AA, et al. Minocycline in severe cerebral amyloid angiopathy: a single-center cohort study. J Am Heart Assoc. 2024;13(4):e033464. https://doi.org/10.1161/JAHA.123.033464 .
Alghamdi BS, AboTaleb HA. Melatonin improves memory defects in a mouse model of multiple sclerosis by up-regulating cAMP-response element-binding protein and synapse-associated proteins in the prefrontal cortex. J Integr Neurosci. 2020;19(2):229–37.
Troiani Z, et al. Prognostic utility of serum biomarkers in intracerebral hemorrhage: a systematic review. Neurorehabil Neural Repair. 2021;35(11):946–59.
Huang J, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(23):12952–60.
Download references
Acknowledgements
We acknowledge Icormed Research Collaborative for helping with this project.
Not applicable.
Author information
Authors and affiliations.
School of Medicine, University of Glasgow, Glasgow, UK
Brian M. Ou Yong, Jonathan Kong Sing Huk, Sujashree Yadala Venkata & Ajitha Kulasekaran
Faculty of Medicine, Sumy State University, Sumy, 40007, Ukraine
Wireko Andrew Awuah & Toufik Abdul-Rahman
School of Medicine, Queen’s University Belfast, Belfast, UK
Muhammad Hamza Shah
Department of Neurosurgery, Trivandrum Medical College, Thiruvananthapuram, India
Vivek Sanker
Nova Southeastern University Dr. Kiran C Patel College of Allopathic Medicine, Davie, FL, USA
Diti H. Patel
Faculty of Medicine, University of St Andrews, St. Andrews, Scotland, UK
Joecelyn Kirani Tan
DOW Medical College, DOW University of Health Sciences (DUHS), Baba-E-Urdu Road, Karachi, Pakistan
Noor Ayman Khan
MGM Medical College Navi, Mumbai, Maharashtra, India
Manali Sarkar
Department of Neurosurgery, Hannover Medical School, Carl-Neuberg-Strasse 1, 30625, Hannover, Germany
Oday Atallah
You can also search for this author in PubMed Google Scholar
Contributions
B.M.O.Y.: conceptualization, data curation, formal analysis, writing—original draft, writing—review and editing. W.A.A., M.H.S., J.K.S.H., S.Y.V., D.H.P., J.K.T., N.A.K., A.K., M.S., T.A.R.: data curation, formal analysis, methodology, writing—original draft, writing—review and editing. V.S.: data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing. O.A.: data curation, formal analysis, methodology, writing—original draft, writing—review and editing, supervision. All authors: Approval of final draft.
Corresponding author
Correspondence to Wireko Andrew Awuah .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ou Yong, B.M., Awuah, W.A., Shah, M.H. et al. Intracerebral haemorrhage in multiple sclerosis: assessing the impact of disease-modifying medications. Eur J Med Res 29 , 344 (2024). https://doi.org/10.1186/s40001-024-01945-x
Download citation
Received : 13 February 2024
Accepted : 19 June 2024
Published : 25 June 2024
DOI : https://doi.org/10.1186/s40001-024-01945-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Disease-modifying drugs
- Neurological complications
- Neuroinflammation
- Pharmacotherapy in MS
- CNS immunity
European Journal of Medical Research
ISSN: 2047-783X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Albumin and multiple sclerosis: a prospective study from UK Biobank
Affiliations.
- 1 Department of Neurology, Laboratory of Neurodegenerative Disorders, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
- 2 Department of Neurology, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China.
- PMID: 38915402
- PMCID: PMC11194376
- DOI: 10.3389/fimmu.2024.1415160
Background: Multiple sclerosis (MS) is a chronic inflammatory disease affecting the central nervous system. While previous studies have indicated that albumin, the primary protein in human plasma, may exert influence on the inflammatory process and confer beneficial effects in neurodegenerative disorders, its role in the context of MS has been underexplored. Here, we aimed to explore the link between albumin and the risk of MS.
Methods: Employing data from the UK Biobank, we investigated the association between baseline levels of serum and urine albumin and the risk of MS using Cox proportional hazards regression analysis.
Results: A higher baseline level of serum albumin was associated with a lower risk of incident MS (HR=0.94, 95% CI: 0.91-0.98, P=7.66E-04). Subgroup analysis revealed a more pronounced effect in females, as well as participants with younger ages, less smoking and deficient levels of vitamin D. Conversely, no association was identified between baseline microalbuminuria level and risk of incident MS.
Conclusion: Higher serum albumin level at baseline is linked to a reduced risk of MS. These results contribute to an enhanced understanding of albumin's role in MS, propose the potential use of albumin as a biomarker for MS, and have implications for the design of therapeutic interventions targeting albumin in clinical trials.
Keywords: UK Biobank; albumin; microalbuminuria; multiple sclerosis; protective effect.
Copyright © 2024 Chen, Li, Zhao and Shang.
PubMed Disclaimer
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Schematic overview of the study…
Schematic overview of the study design.
Forest plot showing the association…
Forest plot showing the association between albumin and multiple sclerosis. (A) Results from…
Similar articles
- The Role of Diet in Multiple Sclerosis Onset: A Prospective Study Using UK Biobank. Barbero Mazzucca C, Scotti L, Comi C, Vecchio D, Chiocchetti A, Cappellano G. Barbero Mazzucca C, et al. Nutrients. 2024 Jun 2;16(11):1746. doi: 10.3390/nu16111746. Nutrients. 2024. PMID: 38892680 Free PMC article.
- Ratio of Red Blood Cell Distribution Width to Albumin Level and Risk of Mortality. Hao M, Jiang S, Tang J, Li X, Wang S, Li Y, Wu J, Hu Z, Zhang H. Hao M, et al. JAMA Netw Open. 2024 May 1;7(5):e2413213. doi: 10.1001/jamanetworkopen.2024.13213. JAMA Netw Open. 2024. PMID: 38805227 Free PMC article.
- Association between combined exposure to ambient air pollutants, genetic risk, and incident gout risk: A prospective cohort study in the UK Biobank. Liu W, Ye L, Hua B, Yang Y, Dong Z, Jiang Y, Li J, Sun X, Ye D, Wen C, Mao Y, He Z. Liu W, et al. Semin Arthritis Rheum. 2024 Jun;66:152445. doi: 10.1016/j.semarthrit.2024.152445. Epub 2024 Mar 30. Semin Arthritis Rheum. 2024. PMID: 38579592
- Serum albumin and risk of incident diabetes and diabetic microvascular complications in the UK Biobank cohort. Cai YW, Zhang HF, Gao JW, Cai ZX, Cai JW, Gao QY, Chen ZT, Liao GH, Zeng CR, Chen N, Liu PM, Wang JF, Chen YX. Cai YW, et al. Diabetes Metab. 2023 Sep;49(5):101472. doi: 10.1016/j.diabet.2023.101472. Epub 2023 Sep 6. Diabetes Metab. 2023. PMID: 37678759
- The Norwegian Multiple Sclerosis Registry and Biobank. Myhr KM, Grytten N, Torkildsen Ø, Wergeland S, Bø L, Aarseth JH. Myhr KM, et al. Acta Neurol Scand. 2015;132(199):24-8. doi: 10.1111/ane.12427. Acta Neurol Scand. 2015. PMID: 26046555 Review.
- Marcus R. What is multiple sclerosis? Jama. (2022) 328:2078. doi: 10.1001/jama.2022.14236 - DOI - PubMed
- Olek MJ. Multiple sclerosis. Ann Internal Med. (2021) 174:Itc81–itc96. doi: 10.7326/AITC202106150 - DOI - PubMed
- Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. (2017) 13:25–36. doi: 10.1038/nrneurol.2016.187 - DOI - PubMed
- Paul A, Comabella M, Gandhi R. Biomarkers in multiple sclerosis. Cold Spring Harbor Perspect Med. (2019) 161(1):51–8. doi: 10.1101/cshperspect.a029058 - DOI - PMC - PubMed
- Ziemssen T, Akgün K, Brück W. Molecular biomarkers in multiple sclerosis. J neuroinflamm. (2019) 16:272. doi: 10.1186/s12974-019-1674-2 - DOI - PMC - PubMed
- Search in MeSH
Related information
Grants and funding, linkout - more resources, full text sources.
- Frontiers Media SA
- PubMed Central
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 25 June 2024
A scoping review assessing the usability of digital health technologies targeting people with multiple sclerosis
- Fiona Tea 1 na1 ,
- Adam M. R. Groh 2 na1 ,
- Colleen Lacey 3 &
- Afolasade Fakolade ORCID: orcid.org/0000-0003-3405-5782 4
npj Digital Medicine volume 7 , Article number: 168 ( 2024 ) Cite this article
186 Accesses
5 Altmetric
Metrics details
- Neuroimmunology
- Therapeutics
- Translational research
Digital health technologies (DHTs) have become progressively more integrated into the healthcare of people with multiple sclerosis (MS). To ensure that DHTs meet end-users’ needs, it is essential to assess their usability. The objective of this study was to determine how DHTs targeting people with MS incorporate usability characteristics into their design and/or evaluation. We conducted a scoping review of DHT studies in MS published from 2010 to the present using PubMed, Web of Science, OVID Medline, CINAHL, Embase, and medRxiv. Covidence was used to facilitate the review. We included articles that focused on people with MS and/or their caregivers, studied DHTs (including mhealth, telehealth, and wearables), and employed quantitative, qualitative, or mixed methods designs. Thirty-two studies that assessed usability were included, which represents a minority of studies (26%) that assessed DHTs in MS. The most common DHT was mobile applications ( n = 23, 70%). Overall, studies were highly heterogeneous with respect to what usability principles were considered and how usability was assessed. These findings suggest that there is a major gap in the application of standardized usability assessments to DHTs in MS. Improvements in the standardization of usability assessments will have implications for the future of digital health care for people with MS.
Similar content being viewed by others

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

Short- and long-term neuropsychiatric outcomes in long COVID in South Korea and Japan

Principal component-based clinical aging clocks identify signatures of healthy aging and targets for clinical intervention
Introduction.
Digital health technologies (DHTs) offer complementary methods to track and manage symptoms, improve treatment adherence, and increase access to healthcare for diverse patient populations 1 , 2 . In the context of a heterogeneous and prognostically challenging neurodegenerative disorder such as multiple sclerosis (MS), DHTs have significant potential to promote disease management and personalized patient care 3 , 4 . The potential impact of DHTs in MS is even more apparent when one considers the nature of clinical assessments in this population. Specifically, evaluations typically occur at 6-12-month intervals and require clinical visits for a comprehensive examination. This low frequency of patient consultation is reported to contribute to un/under-reported disease progression 5 . A potential solution is increasing the frequency of clinical consultations, but this is constrained by time, cost, and geography, leading to inequity in healthcare access. To address some of these gaps in care provision, DHTs have become more integrated into the long-term care of people with MS 6 , 7 , 8 .
While the need for innovative digital solutions in MS care is clear, a recent review of 30 unique mobile health applications found that they did not meet the needs of people with MS 9 . Several factors may potentially thwart the successful implementation of DHTs, including but not limited to, the level of digital and health literacy of end users and the perceived usefulness of, or satisfaction with, a DHT 10 . Indeed, it is well established that the incorporation of usability principles into the design and evaluation of DHTs is fundamental to ensuring they are appropriately targeted to the end users’ needs and adopted long-term 11 , 12 , 13 . Usability describes the extent to which DHTs can be “used by specified users to achieve specified goals with effectiveness, efficiency, and satisfaction in a specified context of use” 14 , and typically considers core principles such as effectiveness, learnability, physical comfort/acceptance, ease of maintenance/repairability, and operability 15 . Indeed, the evaluation of usability is important in the process of development and commercialization of DHTs. Government authorities such as the Federal Drug Administration recommend that medical devices (including DHTs) not yet on the market provide a report summarizing potential target users, training necessary for the operation of the product, usability testing, and any problems encountered during technology evaluation 16 . Furthermore, the National Health Service in the UK requires consideration of usability and accessibility principles when approving health apps solicited from industry 17 . Despite such a clear need for usability evaluation of DHTs, there has yet to be a comprehensive assessment of DHT usability in the context of MS. Other groups have assessed the usability of mobile health applications 1 , 18 , but there is a need to conduct a comprehensive usability evaluation of all DHTs employed by people with MS, especially wearable technologies. The aim of this scoping review was, therefore, to examine the extent to which usability principles have been considered in DHTs for people with MS and to summarize the methods of usability evaluation. A preliminary search of the Joanna Briggs Institute Database of Systematic Reviews and Implementation Reports was conducted to confirm that there are no current or in-progress scoping or systematic reviews on the same topic.
By following a Population Concept Context mnemonic, we generated a primary review question: how has usability been considered in studies of DHTs targeting people with MS? This primary review question was then divided into four sub-questions:
What are the participant characteristics (e.g., age, gender, disease severity) included in studies of DHTs in the context of MS?
What are the components (e.g., type of technology and delivery platform, development stage) of DHTs targeting people with MS?
What assessment methods (e.g., questionnaires, interviews) of usability are incorporated into the design and/or evaluation of DHTs for people with MS?
What usability outcomes (e.g., accessibility, flexibility) are reported from the evaluation of DHTs for people with MS?
Selection of sources of evidence
A total of 5990 studies were identified in the search process (see Fig. 1 ). Following duplicate removal ( n = 1432), the titles and abstracts of 4558 studies were screened, and 4262 studies were deemed irrelevant. A total of 279 studies moved to full-text review, where 247 articles were excluded; of note, 89 studies did not assess usability. Inter-rater agreement for title and abstract screening was 0.45, and between 0.57 and 0.73 for full-text review (given there were three raters for this stage). These values indicate moderate to substantial agreement between reviewers, respectively 19 . A total of 32 studies were included in the final narrative synthesis.

Data extracted from Covidence. Exclusion Criteria: 1. Review papers, animal studies, unpublished trial data, conference abstracts, opinion pieces, case studies, and letters.; 2. No reported usability outcome measure; 3. Studies with data in MS patients that is not accessible, or that is presented in conjunction with co-morbidities; 4. DHT not for patient populations with MS (primary diagnosis), healthcare practitioners, formal care providers, and researchers; 5. Machine learning and AI studies to assess healthcare data for MS. κ = Cohen’s kappa value.
Description of Included Studies and Participants
The characteristics of the included studies and participants are summarized in Table 1 . Most studies were conducted in Europe ( n = 18, 56%), followed by North America ( n = 9, 28%). Four studies (13%) involved multi-country investigations. More than half of the studies were published between 2020-2023 (n = 19, 59%). Most studies used a mixed-methods ( n = 16, 50%) or quantitative ( n = 15, 47%) design. One study (0.3%) used a qualitative design.
Across the studies, the total number of participants was 1213, with the sample sizes ranging between 4 and 126. Most participants were female (71%), with RRMS (73%). Two studies (6.3%) included people with CIS. Of the 28 (86%) studies that reported mean/median age of participants, the age ranged between 36.8 and 56.8 years old. Across all participants, the mean EDSS scores ranged between 1 and 6.5, while the mean disease duration ranged between 6 and 24 years. The education level of participants was reported in 11 (34%) studies and ranged between 8 th grade and doctorate level.
Description of Usability Components of Digital Health Technologies (DHTs)
The characteristics of the DHTs are summarized in Table 2 . The majority of DHTs were application-based (apps) ( n = 23, 70%), followed by wearables ( n = 7, 21%), Website/Internet ( n = 6, 18%), and others (n = 2, 6%): a game console and a virtual reality system. Five studies used a combination of apps and wearables ( n = 4) or apps and Internet/Website ( n = 1). Most DHTs were implemented in the patient’s home or community ( n = 30, 94%). The remaining two DHTs (6%) were used in a hospital/clinic setting. Of the 30 DHTs implemented in a patient’s home, eight were additionally evaluated within a research facility ( n = 7) or hospital ( n = 1). All DHTs were evaluated by people with MS ( n = 32), with seven DHTs evaluated by two users (22%): people with MS and formal health/social care providers ( n = 6) or family caregivers ( n = 1). Over half the DHTs were the final versions ( n = 17, 53%), while the remainder were in various stages of iterative development ( n = 15, 47%). The DHTs were intended for various uses, including remote self-management, education, symptom assessment and monitoring, cognitive and physical rehabilitation, and supporting therapeutic interventions.
Methods of Usability Evaluation
The methods of usability evaluations within each study are detailed in Table 2 . When considering the number of methods used to evaluate DHTs across the studies, there was an even split, wherein half of the studies implemented a single method of evaluation ( n = 16, 50%), while the remaining studies utilized two ( n = 9, 28%) or more methods ( n = 7, 22%). Usability of the DHTs was most commonly evaluated using questionnaires (n = 26, 81%), followed by interviews ( n = 12, 37%), task completion tests ( n = 9, 28%), think aloud protocols ( n = 4, 12%), focus groups ( n = 3, 9%), and others ( n = 4, 12%) which included patient feedback. Within the 26 studies that utilized questionnaires, most included scales developed by the research team ( n = 15, 58%). Of the studies that used a standardized questionnaire, the most common scale was the System Usability Scale (SUS) 20 , used in seven studies (27%).
Description of Usability Outcomes
Table 2 summarizes the usability characteristics across the included studies. There was a wide variety and number of usability characteristics reported across studies, with 20 unique usability characteristics reported in over a third of studies ( n = 12, 37.5%). Three studies (9%) reported usability as a general term. Across all studies, the most assessed usability characteristic was user satisfaction ( n = 17, 53%). Other usability characteristics assessed were adherence ( n = 6, 19%), acceptability ( n = 5, 16%), feasibility ( n = 5, 16%), usefulness ( n = 4, 12.5%), and efficiency ( n = 3, 9%). There were seven usability characteristics only reported by two independent studies. Most studies reported two ( n = 13, 40.5%) or more ( n = 13, 40.5%) usability characteristics, while six studies (19%) reported a single usability characteristic. While the overall conclusions from usability assessment varied, studies most often reported a combination of positive feedback and suggestions for improving future iterations of the DHTs. Two studies explicitly mentioned that participant feedback from usability assessment was incorporated into DHT development 21 , 22 .
The current scoping review is the first to examine usability characteristics and testing methods in DHTs with a specific focus on people with MS. Usability was evaluated in less than a third of relevant studies, indicating limited consideration of this important topic within the context of DHTs in MS. Evaluation of usability was highly heterogeneous across studies, both in terms of the number of reported characteristics and assessment methods. The most evaluated DHTs were mobile applications and most studies used different types of questionnaires to assess usability. DHTs were evaluated by people with MS, with limited inclusion of health/social care providers or family caregivers in the process. Below, we first summarize key findings and knowledge gaps, and subsequently make recommendations for future work to advance the design and implementation of DHTs in MS.
DHTs have become a rapidly evolving modern health intervention tool, especially amidst the COVID pandemic, and likely beyond 23 . The importance of evaluating the usability of DHTs has been recently highlighted in the context of other chronic diseases, such as Parkinson’s disease, and in elderly individuals 24 , 25 , 26 , 27 . A recent review of mobile applications in MS reported only six of 14 studies had evaluated usability 18 . In the current review, which encompassed the broad spectrum of DHTs, the 32 studies only represented 26% of relevant DHT studies in MS. There is a clear lack of usability assessment in over half of DHT studies in MS. Furthermore, we reported minimal involvement of caregivers and health/social care professionals. The inclusion of usability assessments by formal care providers and family caregivers should be considered given the importance of MS caregivers in patient care 28 , 29 . Evaluation of usability is a critical part of the development and effective use of DHTs, and therefore should be considered in emerging DHTs targeting people with MS.
Our findings show that the evaluation methods and usability outcomes assessed were very heterogeneous across studies. Indeed, this heterogeneity in usability assessment and lack of consistency across studies have been reported across other neurodegenerative and chronic diseases, such as dementia, diabetes, and cardiovascular disease 24 , 30 . Usability assessment approaches similarly varied more broadly in studies investigating medical devices across several populations depending on the DHT and its intended use 31 . In the current study, the most frequently used method to evaluate usability was questionnaires, and most studies implemented independent, non-validated, questionnaires developed by the research team. The most commonly used standardized questionnaire was the SUS. Although a valid and reliable measure, the SUS is a self-reported measure with inherent bias and was not intended to be comprehensive in its approach to evaluate usability 32 , 33 . Further, the SUS is not specific nor adapted to a particular DHT type, limiting its ability to describe specific aspects of usability.
The usability of a DHT can encompass a wide range of characteristics and these outcomes will vary based on the intended use of the DHT. We reported a large number of different usability outcome characteristics across studies, with the most common usability characteristic evaluated being user satisfaction. Indeed, the SUS questionnaire, the most used usability assessment method, primarily captures user satisfaction, with additional sub-scales for learnability and usability. The usability characteristics reported varied across studies but were somewhat consistent within DHT type. For example, DHT studies that incorporated a wearable component assessed tolerance or wear-time, whereas self-management DHTs used terms such as engagement and acceptability. Furthermore, there were a large number of studies that reported unique descriptors of usability. It is important to capture multiple characteristics of usability, however consistent terminology and descriptions of usability outcomes is important for cross-study comparisons and validation. There is a clear need to develop more standardized and comprehensive approaches to assess the usability of DHTs for people with MS.
The dramatic rise in remote patient management necessitates a framework to effectively evaluate the intended use and quality of DHTs to enhance and optimize user experience. Herein, we provide recommendations to advance research on DHTs in MS. Usability assessments and outcomes should be tailored for the intended use of the DHT and the target user. Usability testing does not require an enormous time investment, in fact, the likelihood of acquiring novel information after six to nine users is minimal 34 . We found an average of 35 participants assessed usability across the studies included in this review, which is indeed sufficient to reliably and effectively assess DHT usability. Half the evaluated studies were still undergoing development of their DHT, and only two studies explicitly reported that participant feedback from usability testing was incorporated into subsequent DHT development 21 . Future studies should also consider usability in the context of people with MS who experience additional barriers when accessing DHTs, such as low socio-economic status, rurality, older age, and more severe disability 35 .
The future of DHTs in MS requires an updated standardized usability framework, and more targeted usability outcomes specific to the type and application of DHT in patient care. Implementation of both qualitative and quantitative measures is important 36 , 37 . To update currently used standardized methods, like the SUS, objective and comprehensive measures of usability assessments are needed that do not rely only on self-report. Future work should therefore apply a mixed methods approach to assess usability and implement user feedback during stages of DHT development. Incorporating these considerations, we recommend the following to improve the assessment of DHT usability:
Common and clearly defined usability characteristics of DHTs should be evaluated. For example, user satisfaction and acceptability characteristics could be rated on a numeric scale.
Additional criterion specific to the type of DHT, and user (patient or caregiver) should be assessed separately. For example, wearables should include wear-time, and apps that require tests should include task completion time.
Qualitative measures, such as interviews or focus groups, should be conducted in conjunction with quantitative measures. These should assess user feedback and aim to report the subtle challenges with usability not captured by quantitative measures.
These usability metrics, if combined in the form of a summative score, could be useful to compare across studies of various DHT types. Usability results from these metrics should be integrated into the development of the DHT. Finally, the current scoping review highlights a major gap in the application of standardized usability evaluations and outcomes of current DHTs implemented in MS care. Importantly, our results highlight the opportunity to implement improved methods of usability assessment which will have major implications in the future of mobile care for people living with MS.
This review has several limitations that warrant consideration. First, we have included only English-language articles due to a lack of resources for translation. It is possible that articles published in other languages may have included additional information on usability evaluations of DHTs designed for people with MS. Telehealth and virtual telerehabilitation studies were also excluded in the current review. Furthermore, given the heterogeneity in usability principles and methods used to evaluate usability, it was not possible to synthesize the quantitative and qualitative data reported accurately. Nonetheless, we extracted and included these data in Supplementary Fig. 4 for interested readers. The usability of most DHTs was reported to be good, with satisfaction ranging from ~80-90%. The lowest usability scores were typically associated with wearable technologies. Few studies further evaluated the 10-20% of pwMS who struggled with DHT usage. Future research should focus on this sub-group of the MS population to understand how to develop more targeted and usable DHTs.
We followed the Joanna Briggs Institute guidance for scoping reviews 38 , 39 . The review is reported in accordance with the PRISMA Extension for Scoping Reviews 40 (Supplementary Fig. 1 ). We have registered our protocol prospectively in the Open Science Framework: https://osf.io/y7gqp/ .
Eligibility Criteria
Participants.
Studies focusing on adults (≥18 years old) with MS and/or their caregivers were considered for inclusion. Any subtype of MS, including Clinically/Radiologically Isolated Syndrome (CIS/RIS), Relapsing-Remitting MS (RRMS), Primary Progressive MS (PPMS), and Secondary Progressive MS (SPMS) was eligible for inclusion. We excluded animal studies and studies involving mixed populations (i.e., MS and other conditions) where data from people with MS could not be separated from other conditions. We further excluded studies focusing on formal health and/or social care professionals.
Studies that described usability characteristics (e.g., comfort, ease of use, accessibility, flexibility, etc.) and/or usability testing methods (e.g., questionnaires, task completion, “Think-Aloud” protocols, interviews, heuristic testing, and focus groups, etc.) of DHTs were included. We excluded multicomponent studies in which data on the DHT component could not be extracted. Studies on electronic medical records, medical monitoring devices, machine learning, artificial intelligence, biomedical applications, systems for intelligent processing of genetic data, and assistive devices, were excluded.
We considered studies conducted in any geographic location and setting (e.g., hospital settings, primary care, community care, or at home) and published in English. Our pre-screening results found limited studies on DHTs in the context of MS prior to 2010. Therefore, to focus on the most recent and relevant DHT studies, we considered studies published from 2010 until the present.
Types of Sources
We included peer-reviewed quantitative, qualitative, and mixed-methods studies. We recognize that computer-based digital technology development may be conducted outside of academia and published in non-traditional or non-peer-reviewed outlets – but in the context of providing evidence-based information for health and social care providers and researchers, peer-reviewed evidence is considered the gold standard. We excluded systematic and non-systematic reviews, dissertations, conference abstracts and proceedings, observational studies, case reports, opinion pieces, commentaries, and protocols.
Search Strategy
We implemented a systematic, peer-reviewed three-step search strategy in line with the framework developed by the Joanna Briggs Institute and in consultation with a health sciences librarian. The process began with a preliminary search in PubMed to find key articles relevant to the three components of the research question: usability, DHTs, and MS. Using these articles, a list of keywords was developed for the search strategy, and the syntax was modified such that it could be applied to the other databases. The systematic search was then run in five databases: Web of Science, OVID Medline, CINAHL, Embase, and medRxiv. Specific to the medRxiv search, only the MS search component was used. An example of the search terms used in Medline can be found in Supplementary Fig. 2 .
Selection of Sources of Evidence
All results were uploaded to Covidence (Veritas Health Innovation, Melbourne, Australia) to facilitate de-duplication, screening of titles and abstracts, full-text review, and extraction. Titles and abstract screening were pilot-tested by two reviewers on a random sample of 10 studies before screening. Following pilot testing, all authors were involved in both screening phases, with two independent reviewers examining each article. When reviewers disagreed about the inclusion status of a citation, another reviewer examined the citation, and a three-way discussion was held to reach a consensus. Full texts for all potentially relevant articles were uploaded to Covidence for further screening by three independent reviewers. Discrepancies in the inclusion/exclusion of full-text studies were resolved during a consensus meeting. A manual search of reference lists from the full-text articles included was then performed to identify additional studies that met the inclusion criteria.
Data Charting Process
Data extraction was completed using the Covidence 2.0 customizable template with categories adapted from the Joanna Briggs Institute 38 . Extracted variables included study characteristics (authors, year of publication, country of origin, study design), participant characteristics (age, sex, disease duration, Expanded Disability Status Scale (EDSS), and MS phenotype), DHT information (name, type, purpose, implementation setting, stage of DHT development) and usability considerations (evaluation method, questionnaire type, and DHT evaluator). We piloted the template, which led to the inclusion of “not reported” options for several items and the addition of examples to some item definitions to enhance consistency and ease of use. Three independent reviewers performed data extraction. When reviewers disagreed about the inclusion status of a citation, another reviewer examined the citation, and a three-way discussion was held to reach a consensus.
Data analysis
Data synthesis was performed in Microsoft Excel (Microsoft Office, 2019). We calculated inter-rater agreement during title and abstract and full-text screening (before the consensus meeting) using Cohen’s Kappa. No formal measures of agreement were used during the data extraction because differences in capitalization and punctuation generated messages of inconsistency, even if the critical content was the same between reviewers. Our focus was on the synthesis of descriptive features of the studies relative to usability, not on a synthesis of actual study results. We used descriptive statistics (frequencies, median and ranges), with data presented graphically and in tabular format as appropriate. We generated descriptive summaries of study characteristics (i.e., frequency/distribution of publication year, country in which the study was conducted, study design), participant characteristics (mean/median age, disease duration, and Expanded Disability Status Scale (EDSS), and frequency/distribution of sex, and MS phenotype), DHTs included in the literature (frequency/distribution of type, implementation setting, stage of DHT development), and usability considerations (frequency/distribution of usability characteristics, testing methods, and usability evaluator).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
No data sets were generated or analyzed during the current study. The aggregated data analyzed in this study are available from the corresponding author upon reasonable request. This scoping review uses peer-reviewed articles and therefore does not require ethical approval.
Code availability
No code was generated during the current study.
Spreadbury, J. H., Young, A. & Kipps, C. M. A comprehensive literature search of digital health technology use in neurological conditions: review of digital tools to promote self-management and support. J Med Internet Res 24 , e31929 (2022).
Article PubMed PubMed Central Google Scholar
Dillenseger, A. et al. Digital biomarkers in multiple sclerosis. Brain Sci 11 , 1519 (2021).
Ziemssen, T. & Haase, R. Digital innovation in multiple sclerosis management. Brain Sciences 12 , 40 (2022).
Article Google Scholar
Gromisch, E. S., Turner, A. P., Haselkorn, J. K., Lo, A. C. & Agresta, T. Mobile health (mHealth) usage, barriers, and technological considerations in persons with multiple sclerosis: a literature review. JAMIA Open 4 , ooaa067 (2020).
Marziniak, M. et al. The use of digital and remote communication technologies as a tool for multiple sclerosis management: narrative review. JMIR Rehabil Assist Technol 5 , e5 (2018).
Marrie, R. A. et al. Use of eHealth and mHealth technology by persons with multiple sclerosis. Multiple Sclerosis and Related Disorders 27 , 13–19 (2019).
Article PubMed Google Scholar
Scholz, M., Haase, R., Schriefer, D., Voigt, I. & Ziemssen, T. Electronic health interventions in the case of multiple sclerosis: from theory to practice. Brain Sciences 11 , 180 (2021).
Specht, B. et al. Multiple Sclerosis in the Digital Health Age: Challenges and Opportunities - A Systematic Review. medRxiv 2023-11 (2023).
Giunti, G., Guisado Fernández, E., Dorronzoro Zubiete, E. & Rivera Romero, O. Supply and demand in mhealth apps for persons with multiple sclerosis: systematic search in app stores and scoping literature review. JMIR Mhealth Uhealth 6 , e10512 (2018).
Cummins, N. & Schuller, B. W. Five crucial challenges in digital health. Frontiers in Digital Health 2 , 536203 (2020).
Aiyegbusi, O. L. Key methodological considerations for usability testing of electronic patient-reported outcome (ePRO) systems. Qual Life Res 29 , 325–333 (2020).
Maramba, I., Chatterjee, A. & Newman, C. Methods of usability testing in the development of eHealth applications: A scoping review. Int J Med Inform 126 , 95–104 (2019).
Yen, P.-Y. & Bakken, S. Review of health information technology usability study methodologies. J Am Med Inform Assoc 19 , 413–422 (2012).
Grassi, P. A., Garcia, M. E. & Fenton, J. L. Digital Identity Guidelines: Revision 3 . NIST SP 800-63-3 https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.800-63-3.pdf , https://doi.org/10.6028/NIST.SP.800-63-3 (2017)
Story, M. F. Maximizing usability: the principles of universal design. Assist Technol 10 , 4–12 (1998).
Article CAS PubMed Google Scholar
Ranzani, F. & Parlangeli, O. In Textbook of Patient Safety and Clinical Risk Management (eds Donaldson, L., Ricciardi, W., Sheridan, S. et al.) Ch. 32 , Springer, Cham, https://doi.org/10.1007/978-3-030-59403-9_32 (2021)
Mathews, S. C. et al. Digital health: a path to validation. npj Digit. Med. 2 , 1–9 (2019).
Google Scholar
Howard, Z., Win, K. T. & Guan, V. Mobile apps used for people living with multiple sclerosis: A scoping review. Multiple Sclerosis and Related Disorders 73 , 104628 (2023).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33 , 159–174 (1977).
Brooke, J. SUS. A quick and dirty usability scale. Usability Eval Indus 189–194 , 4–7 (1996).
Thomas, S. et al. Creating a digital toolkit to reduce fatigue and promote quality of life in multiple sclerosis: participatory design and usability study. Jmir Formative Research 5 , e19230 (2021).
Hsieh, K., Fanning, J., Frechette, M. & Sosnoff, J. Usability of a fall risk mHealth app for people with multiple sclerosis: mixed methods study. Jmir Human Factors 8 , e25604 (2021).
Abernethy, A. et al. The Promise of Digital Health: Then, Now, and the Future. NAM Perspect , https://doi.org/10.31478/202206e (2022)
Agarwal, P. et al. Assessing the quality of mobile applications in chronic disease management: a scoping review. NPJ Digit Med 4 , 46 (2021).
Wang, Q. et al. Usability evaluation of mHealth apps for elderly individuals: a scoping review. BMC Medical Informatics and Decision Making 22 , 317 (2022).
Article CAS PubMed PubMed Central Google Scholar
Deb, R., Bhat, G., An, S., Shill, H. & Ogras, U. Y. Trends in Technology Usage for Parkinson’s Disease Assessment: A Systematic Review. MedRxiv 2021-02 (2021).
Debelle, H. et al. Feasibility and usability of a digital health technology system to monitor mobility and assess medication adherence in mild-to-moderate Parkinson’s disease. Frontiers in Neurology 14 , 1111260 (2023).
Dunn, J. Impact of mobility impairment on the burden of caregiving in individuals with multiple sclerosis. Expert Rev Pharmacoecon Outcomes Res 10 , 433–440 (2010).
Lorefice, L. et al. What do multiple sclerosis patients and their caregivers perceive as unmet needs? BMC Neurology 13 , 177 (2013).
McKay, F. H. et al. Evaluating mobile phone applications for health behaviour change: A systematic review. J Telemed Telecare 24 , 22–30 (2018).
Bitkina, O. V. L., Kim, H. K. & Park, J. Usability and user experience of medical devices: An overview of the current state, analysis methodologies, and future challenges. International Journal of Industrial Ergonomics 76 , 102932 (2020).
Lewis, J. R. & Sauro, J. The Factor Structure of the System Usability Scale. In Human Centered Design (ed. Kurosu, M.) 94–103, Springer, Berlin, Heidelberg, https://doi.org/10.1007/978-3-642-02806-9_12 (2009).
Peres, S. C., Pham, T. & Phillips, R. Validation of the System Usability Scale (SUS): SUS in the Wild. Proceedings of the Human Factors and Ergonomics Society Annual Meeting 57 , 192–196 (2013).
Nielsen, J. & Landauer, T. K. A mathematical model of the finding of usability problems. In Proceedings of the INTERACT ’93 and CHI ’93 Conference on Human Factors in Computing Systems 206–213, Association for Computing Machinery, New York, NY, USA, https://doi.org/10.1145/169059.169166 (1993).
Marrie, R., Kosowan, L., Cutter, G., Fox, R. & Salter, A. Disparities in telehealth care in multiple sclerosis. Neurology-Clinical Practice 12 , 223–233 (2022).
Johnson, S. G., Potrebny, T., Larun, L., Ciliska, D. & Olsen, N. R. Usability methods and attributes reported in usability studies of mobile apps for health care education: scoping review. JMIR Medical Education 8 , e38259 (2022).
Wohlgemut, J. M. et al. Methods used to evaluate usability of mobile clinical decision support systems for healthcare emergencies: a systematic review and qualitative synthesis. JAMIA Open 6 , ooad051 (2023).
Peters, M. D. J. et al. Guidance for conducting systematic scoping reviews. JBI Evidence Implementation 13 , 141–146 (2015).
Peters, M. D. J. et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 18 , 2119–2126 (2020).
Tricco, A. C. et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. https://doi.org/10.7326/M18-0850 (2018).
Download references
Acknowledgements
This work was completed as part of an endMS Scholar Program for Researchers IN Training (SPRINT) interdisciplinary learning project while Adam Groh, Colleen Lacey, and Fiona Tea were enrolled in the program. The SPRINT is part of the endMS National Training Program funded by MS Canada.
Author information
These authors contributed equally: Fiona Tea, Adam M. R. Groh.
Authors and Affiliations
Department of Neuroscience, Université de Montréal, Montreal, QC, Canada
Department of Neurology and Neurosurgery, Montreal Neurological Institute-Hospital, McGill University, Montreal, QC, Canada
Adam M. R. Groh
Department of Psychology, University of Victoria, Victoria, BC, Canada
Colleen Lacey
School of Rehabilitation Therapy, Queen’s University, Kingston, ON, Canada
Afolasade Fakolade
You can also search for this author in PubMed Google Scholar
Contributions
AG: Conceptualization, Data curation, Writing – original draft. CL: Conceptualization, Data curation, Writing – original draft. FT: Conceptualization, Data curation, Writing – original draft. AF: Conceptualization, Data curation, Supervision, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Afolasade Fakolade .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tea, F., Groh, A.M.R., Lacey, C. et al. A scoping review assessing the usability of digital health technologies targeting people with multiple sclerosis. npj Digit. Med. 7 , 168 (2024). https://doi.org/10.1038/s41746-024-01162-0
Download citation
Received : 04 October 2023
Accepted : 12 June 2024
Published : 25 June 2024
DOI : https://doi.org/10.1038/s41746-024-01162-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Acad Pathol
- v.9(1); 2022
Educational Case: Multiple sclerosis
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see https://www.journals.elsevier.com/academic-pathology/news/pathology-competencies-for-medical-education-pcme . 1
Primary objective
Objective NSC3.3: Multiple sclerosis. Describe the pathogenesis, clinical presentation, and gross and microscopic pathologic features of multiple sclerosis.
Competency 2: Organ System Pathology; Topic NSC: Nervous System – Central Nervous System; Learning Goal 3: Spinal Cord Disorders.
Secondary objective
Objective NSC6.1: Autoimmune mechanisms in multiple sclerosis. Describe the autoimmune mechanism mediated by CD4 + T cells that react against self-myelin antigens in multiple sclerosis and outline the clinicopathologic features of the disease.
Competency 2: Organ System Pathology; Topic NSC: Nervous System – Central Nervous System; Learning Goal 6: Demyelinating Disorders.
Patient presentation
A 32-year-old woman with no past medical history presents to the emergency room with a 6-month history of waxing and waning unilateral visual impairment and facial numbness. She was well until 6 months ago when she noticed the onset of right-sided facial numbness and blurred vision lasting several weeks. She states that three episodes have occurred during the past 6-month time period. There was no associated muscle weakness of the facial muscles. Earlier today, upon waking up, the patient noted a sudden onset of blurry vision in her right eye and numbness on the right side of her face. She states she has not observed any muscle weakness, gait disturbance, fever, or urinary incontinence.
Diagnostic findings, Part 1
Physical examination reveals a well appearing, anxious woman. Vital signs are temperature: 98.6 °F, heart rate: 82 beats per minute, blood pressure: 116/84 mmHg, respiratory rate: 16 breaths per minute. Neurologic exam reveals 20/20 vision in the left eye and 20/100 vision in the right eye. Muscle strength is 5/5 in all extremities. There is unilateral loss of sensation on the entire right half of the face; otherwise, all other cranial nerves are intact. Romberg sign is negative, and no gait disturbances are noted. Cardiac, pulmonary, and abdominal examinations are unremarkable.
Questions/discussion points, Part 1
What is the differential diagnosis based on the clinical findings.
Relapsing-remitting visual deficits are suggestive of optic neuritis which, along with new-onset facial neuropathy manifesting as numbness, are most suggestive of a central nervous system (CNS) demyelinating disease. Demyelinating disorders that affect the CNS can be grouped by their etiologies, which includes inflammatory, infectious, and toxic-metabolic-nutritional ( Table 1 ). Among inflammatory disease processes, the relapsing-remitting nature of vision deficits in a woman in her 30s raises multiple sclerosis (MS) highest in the differential diagnosis, discussed below. In addition to MS, other demyelinating disorders in the differential include neuromyelitis optica spectrum disorder (NMOSD) and acute disseminated encephalomyelitis (ADEM). NMOSD present with relapsing-remitting neurological symptoms and lesions on magnetic resonance imaging (MRI) studies are similar to those in MS. However, lesions in NMOSD are characteristically limited to the spinal cord and optic nerves, whereas MS characteristically has cranial involvement in addition to the spinal cord and optic nerves. ADEM is rarely confused with MS as it is usually a monophasic, self-limiting, post-viral, or rarely post-vaccination disease of childhood. It typically presents with acutely evolving, multifocal CNS disease, whereas in MS, the neurological deficits during initial presentation or a relapse are usually limited to a single site or a few sites. ADEM can rarely manifest with relapses, although, in this setting, MRI lesions are typically more extensive and symmetric than MS.
Table 1
Disorders that may present with myelin loss in the central nervous system, peripheral nervous system, or both.
| CNS myelin affected | PNS myelin affected | |
|---|---|---|
| Multiple sclerosis (MS) | + | – |
| Acute disseminated encephalomyelitis (ADEM) | + | – |
| Neuromyelitis optica spectrum disorder (NMSOD) | + | – |
| Guillain-Barré syndrome | – | + |
| Chronic inflammatory demyelinating polyradiculoneuropathy | – | + |
| Progressive multifocal leukoencephalopathy (PML) | + | – |
| Lysosomal storage diseases | ||
| Krabbe disease (β-galactosidase deficiency) | + | + |
| Metachromatic leukodystrophy (arylsulfatase deficiency) | + | + |
| Peroxisomal disorders | ||
| Adrenoleukodystrophy | + | + |
Abbreviations: CNS, central nervous system; PNS, peripheral nervous system.
Infectious etiologies of CNS demyelination include progressive multifocal leukoencephalopathy, Lyme disease, and neurosyphilis. Progressive multifocal leukoencephalopathy is an infection of oligodendroglial cells by the JC virus leading to demyelination in the setting of immunodeficiency (e.g., acquired immunodeficiency disease or iatrogenic immunosuppression). The optic nerve is myelinated by oligodendroglial cells; therefore, the optic nerve is affected in progressive multifocal leukoencephalopathy and not in peripheral nervous system (PNS) demyelinating diseases like Guillain-Barré syndrome or chronic inflammatory demyelinating polyradiculoneuropathy. Early disseminated stage 2 lyme disease can present with recurrent cranial neuropathies in the context of meningitis.
Inherited toxic-metabolic-nutritional disorders that lead to loss of myelin (leukodystrophy) include the lysosomal storage diseases Krabbe disease and metachromic leukodystrophy, as well as the peroxisomal disease adrenoleukodystrophy. These disorders typically present in childhood with a slow, progressive course, eventually leading to symptoms in both the CNS and PNS due to loss of myelin. Adrenoleukodystrophy also leads to adrenal cortex dysfunction due to steroid hormone production deficits, manifesting clinically as Addison disease. The leukodystrophies are inherited, with an autosomal recessive inheritance in Krabbe disease and metachromic leukodystrophy and an X-linked pattern of inheritance in adrenoleukodystrophy.
Inflammatory disorders that can mimic MS include cerebral vasculitis, systemic lupus erythematosus, Sjogren syndrome, and neurosarcoidosis. These disorders only rarely present initially with neurological symptoms, and systemic signs and symptom characteristics of these disorders are usually present. MRI studies and laboratory testing performed on blood and cerebrospinal fluid (CSF) can help differentiate between an inflammatory demyelinating disorder and infectious and inflammatory disease processes. Arteriovenous malformations can result in relapsing-remitting, single-site neurological symptoms similar to MS, but MRI and computed tomography angiography can distinguish vascular malformation from other disorders. Similarly, tumors in certain locations can mimic MS symptoms. Pituitary adenomas, craniopharyngiomas, and meningiomas can occur in the sella turcica region and compress on the optic chiasm and optic nerves resulting in visual deficits, although characteristically with a progressive loss of vision rather than with relapsing and remitting symptoms. 2 , 3
Define the different clinical subtypes (phenotypes) of multiple sclerosis
In 1996, the US National MS Society defined three phenotypes of MS, which were later refined by Lublin et al., in 2013: relapsing-remitting (RRMS), secondary-progressive (SPMS), and primary-progressive (PPMS). 4 RRMS is defined as having relapses that last at least 24h and have complete or partial remission of symptoms between attacks. RRMS can transform into SPMS, which is where symptoms are no longer stable between relapses and instead there is progressive accumulation of disability. PPMS is when a patient initially presents with a progressive accumulation of disability, without a period of RRMS beforehand.
In addition to refining the definitions of the MS phenotypes, Lublin et al. introduced a new category: the clinically isolated syndrome (CIS). A CIS is defined as the first clinical presentation of a disease that could be MS but has yet to fulfill the dissemination in time (DIT) criteria required to diagnose MS. DIT will be described in more detail below, but as it requires at least two attacks to have occurred, MS cannot be diagnosed at the initial presentation. The inclusion of CIS as a subgroup of MS allows patients with probable MS to begin treatment earlier than before its inclusion. Another concept the Lublin group added was active vs. not active MS. Active MS is defined as a patient with clinical evidence of a relapse or a new gadolinium-enhancing lesion on a current MRI. Conversely, not active MS is a patient without clinical evidence of a relapse or a new lesion on MRI. “Active” and “not active” are used as modifiers to the MS phenotype; thus, a patient can have RRMS – active, or SPMS – not active. Lublin et al. used 1 year as the minimum time frame to assess for activity; thus, if the annual MRI for MS activity showed no new lesions, and there were no clinical relapses in the past year, the patient would have “not active” MS. However, no recommendation for what time frame to use was given in this article, and in 2020, Lublin et al. published an article to clarify the necessity of defining a time frame in which to define activity or else this modifier would have little meaning. 5
What are the diagnostic criteria for multiple sclerosis?
The diagnosis of MS incorporates a combination of clinical, imaging, and laboratory criteria, which are compiled by an expert panel and then revised periodically, most recently in 2010 and 2017. 6 , 7 These criteria are termed the McDonald criteria, after the lead author on the paper detailing the criteria that were originally composed in 2001. 8 Due to the reliance on the combination of information, as there is no single laboratory test that can diagnose MS, consideration and exclusion of alternative disease processes is critical to the diagnostic workup. To diagnose MS, you must demonstrate dissemination of lesions in the CNS in space and time (DIS/DIT). DIS and DIT are defined as either clinical or radiologic evidence of greater than one lesion at different anatomical locations, separated in time by a period of complete or partial remission. The McDonald criteria define different ways DIS and DIT can be demonstrated to make the diagnosis. In a patient with a relapsing-remitting presentation of MS, DIS can be demonstrated through either:
- - Objective, clinical evidence of ≥2 lesions or
- - ≥ 1 symptomatic or asymptomatic MS-typical T2 lesions in 2 or more areas of the CNS: periventricular, juxtacortical/cortical, infratentorial, or the spinal cord.
DIT can be demonstrated through either:
- - ≥ 2 typical MS attacks separated by a period of remission,
- - The simultaneous presence of both enhancing and non-enhancing symptomatic or asymptomatic MS-typical MRI lesions,
- - A new T2-enhancing MRI lesion compared to a baseline scan, or
- - The presence of CSF-specific oligoclonal bands.
If, however, a patient initially presents with a continual progression of disability, MS can still be diagnosed if they have had at least 1 year of disability progression and two of the following:
- - ≥ 1 symptomatic or asymptomatic MS typical T2 lesions (periventricular, juxtacortical/cortical, or infratentorial),
- - ≥ 2 T2 spinal cord lesions, or
- - The presence of CSF-specific oligoclonal bands. 6
What imaging is indicated and what results would support a diagnosis of MS?
An MRI of the brain and spinal cord is extremely important in the diagnosis of MS as it is very sensitive in detecting white matter abnormalities. To diagnose MS there should be at least one typical MS lesion in at least two areas that are characteristic of MS. A typical MS lesion is a focal hyperintensity on a T2 weighted sequence, round/ovoid in shape, ranges from a few millimeters to 1–2 cm in size, and is at least 3 mm in its long axis. Characteristic locations include periventricular (in direct contact with the lateral ventricles, without intervening normal white matter), juxtacortical/cortical (in direct contact with the cortex, without intervening normal white matter), infratentorial (in the brainstem, cerebellar peduncles, or cerebellum), or anywhere in the spinal cord (the cervical cord is the most frequently involved). Another feature characteristic of MS lesions is gadolinium enhancement. Gadolinium enhancement is seen in acute MS lesions and is transient, usually lasting 4 weeks or less. This feature can help support the DIT criteria of diagnosis, as the presence of gadolinium-enhancing and nonenhancing lesions confirms the presence of new and chronic lesions. 9
What laboratory testing is indicated and what results would support a diagnosis of MS?
CSF analysis and serum antibody testing can be useful, especially when the clinical picture is not “classic” for MS to support or cast doubt on the diagnosis of MS. In the workup of MS, CSF analysis should include white blood cell count, red blood cell count, protein concentration, glucose level, immunoglobulin G (IgG) index, and oligoclonal band testing. The white blood cell count, protein concentration, and glucose levels are helpful in ruling out MS; white blood cell counts can be mildly elevated in MS, but very high counts (>50/mm 3 ), low glucose level, and high total protein are more indicative of infection than MS. A high red blood cell count likely indicates a traumatic tap, which may make the other tests uninterpretable, so a CSF analysis with high red blood cells should be interpreted with caution. An IgG index (IgG CSF /IgG Serum )/(Albumin CSF /Albumin Serum ) is indicative of how much IgG is being produced in the CSF and is used instead of just measuring the level of IgG in the CSF because peripherally produced IgG can cross the blood–brain barrier and be measured in the CSF. Another method to detect CSF-specific IgG is oligoclonal bands. Through isoelectric focusing and immunoblotting, antibodies can be visualized as dark bands. Oligoclonal bands are antibodies seen only in the CSF and not in the patient's serum. Two or more oligoclonal bands in the CSF suggest intrathecal production of IgG (as seen in MS) rather than a systemic production of IgG that is being leaked into the CSF. In the latter case, the bands of IgG antibodies being detected in the serum would be observed in the CSF as well. 10 Another laboratory test that is sometimes used in the workup of MS is testing the serum for the presence of antibodies. There is no specific antibody associated with MS, but detection of specific antibodies can help rule out MS. Antibodies against an aquaporin-4 water channel in astrocytes is seen in NMOSD and can help rule out MS if present. Anti-myelin oligodendrocyte glycoprotein (anti-MOG) targets one of the proteins found in myelin, and though once thought to be indicative of MS, it has been discovered to be a separate entity, termed anti-MOG syndrome. The clinical course of anti-MOG syndrome is like ADEM in pediatric patients, whereas adults typically show optic neuritis and brainstem encephalitis. Importantly though, pediatric and adult patients with seropositive anti-MOG titers don't ever fulfill diagnostic criteria for MS, further solidifying anti-MOG syndrome as a separate entity from MS. 11
What electrophysiologic testing could be performed and what results would support a diagnosis of MS?
Evoked potentials (EPs) are used to measure electrical activity in areas of the brain and spinal cord. There are different types of EPs, and the ones most used in MS are visual (testing the optic nerve) and motor EPs. There are certain situations EPs can be helpful: when the MRI is equivocal or to predict the aggressiveness of the disease. MRI is more sensitive than an EP and is better at diagnosing MS, but if the MRI is equivocal, an EP can be used to help support or rule out the diagnosis. Second, EPs are better at predicting the clinical course of MS as it can detect early or even subclinical demyelination prior to its visualization on MRI. EPs can be used to monitor a patient, and if an EP is positive, more aggressive treatment can be initiated. 12
Diagnostic findings, Part 2
Lumbar puncture and blood draw are performed, and CSF and serum obtained for additional studies. The results are listed in Table 2 , Table 3 . T2 FLAIR MRI images of the brain, optic nerves, and spinal cord are also obtained ( Fig. 1 ). Focal hyperintensities are seen in the brain, right optic nerve, and spinal cord. The clinical presentation, imaging, and lab data are consistent with MS as the diagnosis.
Table 2
Cerebrospinal fluid (CSF) values.
| Test | Reference range | Patient's results |
|---|---|---|
| Color | Colorless | Colorless |
| Turbidity | Clear | Clear |
| Clot | Negative | Negative |
| RBC (cells/mm ) | <1 | 0 |
| WBC (cells/mm ) | 0–5 | 3 |
| Neutrophils (%) | 0–6 | 0 |
| Lymphocytes (%) | 40–60 | 95 |
| Monocytes (%) | 15–45 | 5 |
| Glucose (mg/dL) | 40–70 | 61 |
| Protein (mg/dL) | 15–45 | 40 |
Table 3
Additional results.
| Test | Reference range | Patient's results |
|---|---|---|
| IgG, CSF (mg/dL) | 0–4.5 | 7.2 |
| Albumin, CSF (mg/dL) | 5–34 | 31 |
| IgG, serum (mg/dL) | 620–1520 | 1129 |
| Albumin, serum (g/dL) | 3.5–4.9 | 3.8 |
| IgG index | 0.32–0.60 | 0.78 |
| Oligoclonal bands, CSF | No Bands | 10 bands identified in CSF; absent in serum |
| Myelin basic protein, CSF (mcg/L) | 2.0–4.0 | 2.1 |
| Anti-aquaporin 4 antibodies (U/mL) | <1.6 | <1.6 |
| Anti-myelin oligodendrocyte antigen antibodies (titer) | <1:10 | <1:10 |
Abbreviation: CSF, cerebrospinal fluid.

Parasagittal MRI image demonstrates several periventricular demyelinating plaques (red arrowheads) referred to as Dawson fingers in multiple sclerosis. Reproduced with permission from Harrison Klause, MD, EVMS, Norfolk, VA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Questions/discussion points, Part 2
Describe the epidemiologic features of ms.
MS is a disorder that leads to disability in young adults. Patients are usually between 15 and 45 years of age when symptoms present. The mean age of onset is from 28 to 31 years. The age of onset varies among the clinical subtypes (phenotypes). RRMS has an earlier onset, averaging between 25 and 29 years, with SPMS presenting at a mean age between 40 and 49 years of age. The estimated male to female ratio is 1.4–2.3 to 1. Geographic variation exists with MS more common in northern latitudes. In the US, the estimated prevalence is 1–1.5 per 1000 individuals. 2 , 13
How does autoimmunity play a role in the mechanism of MS?
Normally, when a dendritic cell detects a foreign antigen, it presents the antigen to CD4 + T cells and releases cytokines that induce inflammation and helps shape the adaptive immune system. In MS, dendritic cells are overactivated and migrate through the blood–brain barrier to induce Th1 and Th17 differentiation in the CNS. The proportion of Th17 to Th1 cells is also increased in the peripheral blood of MS patients during acute relapses. Th17 releases matrix metalloproteinase and granulocyte macrophage colony-stimulating factor, which increases blood–brain barrier permeability and recruits bone marrow-derived monocytes, respectively. Th1 and Th17 are both involved in ectopic lymphoid follicle formation and play a role in activating B-cells. In MS patients, B-cells produce autoantibodies that mediate demyelination and axonal disruption. Also, memory B-cells differentiate into CSF plasma cells, which produce antibodies that manifest as oligoclonal bands on protein electrophoresis. B-cells are important regulators of the immune system, and this regulatory function is defective in MS patients, leading to autoreactive B-cells and an overactive immune system. In addition to B-cells and T-cells, astrocytes, the gut microbiome, and dieting patterns are also thought to play a role in the immune response in MS patients. Astrocytes play an important role in maintaining the blood–brain barrier and regulate the activity of microglia and oligodendrocytes. Dysfunction in these processes is thought to contribute to demyelination, axonal damage, and infiltration of pro-inflammatory leukocytes into the CNS. 2 , 14
Describe the gross and histological findings observed in the brain from a patient with multiple sclerosis
Fig. 2 is a picture from an autopsy patient with MS who died of an unrelated cause. There are several well-circumscribed, gray-tan, irregularly shaped paraventricular and juxtacortical plaques (arrows), representing chronic MS plaques that are demyelinated. On histology, active plaques can be recognized by the presence of foamy macrophages, which are stripping myelin from axons and digesting it in lysosomes ( Fig. 3 , Fig. 4 ). In chronic plaques, there is little to no myelin left, which is highlighted with the Luxol fast blue stain which stains myelin blue ( Fig. 5 , Fig. 6 , Fig. 7 ). 2

Multiple sclerosis. In a coronal section of brain, multiple sharply defined, tan-gray plaques are identified in the white matter, adjacent to the right ventricle (paraventricular), involving the cortex at the gray matter-white interface (juxtacortical), and in other locations (arrows).

Active MS plaque shows abundant foamy macrophages, which are ingesting the myelin breakdown products, accompanied by an intense lymphocytic perivascular infiltrate (perivascular cuffing) (H&E stain, intermediate magnification).

Foamy macrophages (arrowheads) are distended with myelin breakdown product (Luxol fast blue stain, high magnification). Reproduced with permission from Suzanne Zein-Powell, MD, Methodist Hospital, Houston, TX. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)

A c oronal section of midbrain at the interface between the pons and midbrain shows several areas of demyelination, most notably centrally within the cerebral peduncle, within the corticospinal fiber tract (arrowhead) (Luxol fast blue stain, no magnification). Reproduced with permission from the College of American Pathologists. AUB, 1996 Education Programs. Northfield, IL: College of American Pathologists; 1996. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)

On histological examination, the brain shows an area of demyelination with axonal preservation (arrowhead) seen as the tan-gray plaque on gross examination. (Luxol fast blue stain, intermediate magnification). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)

Chronic plaque at interface with normal white matter. The axons are retained within the plaque; however, many have not been remyelinated. In addition, macrophages and lymphocytes are decreased in number in a chronic plaque, so the cellularity within a chronic plaque is less than in an active/acute plaque ( Fig. 3 , Fig. 4 ). (Luxol fast blue stain, intermediate magnification). Courtesy of Philip Boyer, MD, PhD, Brody School of Medicine, Greenville, NC. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Do a patient's acute exacerbation symptoms correlate with pathologic findings?
Clinical symptoms of acute exacerbations are correlated histopathologically with focal inflammatory demyelinating white matter lesions. Inflammatory cells recruited from the circulation, mostly T-cells and macrophages, accumulate in the lesions and eventually lead to partial/complete demyelination. Overall, white matter demyelination and peripheral immune cell accumulation are pathological hallmarks of an acute plaque and correlate with clinical symptoms. Additionally, edema associated with the inflammatory lesion likely contributes to the observed functional deficits, especially in regions with low swelling capacity, such as the spinal cord. Both demyelination and compression of nerve fibers lead to reduced conduction velocity and sometimes complete conduction block. 15
Describe the pathological hallmarks of progressive disease
Although there is likely some crossover, disease progression is characterized more by neurodegeneration than focal, autoimmune-driven inflammation like that of acute relapses. In the later chronic stages of MS, all aspects of the neuron undergo degenerative changes including axons, cell bodies, dendrites, spines, and neurotransmitter metabolism. The direct mechanism leading to neurodegeneration is unknown, but possible mechanisms include microglia activation, reactive oxygen species, and mitochondrial dysfunction. The triggers of neurodegeneration seen in chronic, progressive MS are the “normal appearing” white matter (NAWM) lesions and tissue damage in the gray matter. NAWM appears normal in routine stains and imaging; however, detailed histological studies reveal diffuse gliosis, microglial activation, vascular fibrosis, perivascular cuffing by inflammatory cells, perivascular lipofuscin, abnormal endothelial tight junctions, blood–brain barrier breakdown, and/or vessels containing proliferating endothelial cells. Axonal loss has also been observed in NAWM. Notably, NAWM lesions correlate better with clinical disability than focal inflammatory white matter lesions. In addition to white matter, gray matter is damaged in progressive MS. Damage can extend throughout the cortex and subcortical regions. An important element of gray matter damage is meningeal inflammation. Lymphoid structures resembling B-cell follicles form in the meninges. They are found extensively in patients with primary progressive MS who exhibit a more severe clinical course. 16 , 17
Describe remyelination in MS
Remyelination in the CNS is accomplished by oligodendrocytes, and in MS patients, they contribute to the complete or partial resolution of clinical symptoms in RRMS. Remyelination is dependent on adult oligodendrocyte progenitor cells (OPC) as preexisting, mature oligodendrocytes cannot add to the pool of myelinogenic oligodendrocytes. It is thought that the main reason remyelination fails in MS is because OPC become quiescent and unable to differentiate, but there are likely other factors that contribute to the failure to remyelinate. For example, reactive astrocytes secrete inhibitors of remyelination at the site of demyelination. Similarly, clearance of myelin debris is an important step in remyelination since it contains remyelination inhibitors. The macrophages and activated microglia that are responsible for phagocytosis of debris also secrete various neutrophilic factors. There is also an age dependent decline in remyelination, and this is more clearly due to decreased differentiation of OPC. Mechanistically, it is thought that aged OPC become less responsive to factors that induce differentiation through dysfunction of the mTOR pathway. Finally, remyelination also depends on the location in the CNS. For example, periventricular lesions are less amenable to remyelination than subcortical lesions. Overall, as patients age and the disease progresses, there is less remyelination of lesions, correlating with progressive clinical dysfunction. 18
How is MS treated?
Treatment is multifactorial including counseling, physical therapy, exercise and pharmacotherapy. Pharmacotherapy consists of medications directed at immunosuppression or immunomodulation. 2 Although not curative, pharmacotherapy may ameliorate symptoms. Disease modifying therapeutic agents depends on which clinical subtype (phenotype) (CIS, RRMS, SPMS, and PPMS) the patient presents with. Monoclonal antibodies (natalizumab, ocrelizumab, rituximab, ofatumumab, and alemtuzumab) may be indicated for active disease. Fumarates (e.g. dimethyl fumarate) and sphingosine 1-phosphate receptor modulators (e.g. fingolimod) are other considerations along with injectable agents, such as recombinant human interferon beta-1b, recombinant human interferon beta-1a, and glatiramer acetate. Healthcare workers need to consider the risk benefit of selected agents, given the potential adverse effects including infection. 2 , 19 , 20 , 21
Teaching Points
- • Multiple sclerosis (MS) is a chronic demyelinating disorder of autoimmune etiology in which the clinical findings are separated in both time and space.
- • MS presents with symptoms usually between 15 and 45 years of age. It is twice as common in women and has a prevalence between 1/1000 persons in the US.
- • The diagnosis of MS incorporates a combination of clinical, imaging, and laboratory data to show dissemination of lesions in space and time, along with the consideration and exclusion of alternative diagnoses.
- • Laboratory testing contributes to the diagnostic workup of a patient with MS in the differential diagnosis; however, no single laboratory test, in isolation, is diagnostic of MS.
- • The most characteristic finding seen on MRI are T2-hyperintense and/or gadolinium contrast-enhancing T1 cerebral hemisphere periventricular, juxtacortical, infratentorial, and spinal cord white matter lesions.
- • The presence of CSF-specific oligoclonal bands can substitute for MRI data demonstrating dissemination in time.
- • Autoimmunity is thought to play an important role in the pathogenesis of MS, involving T and B cell dysfunction.
- • Acute exacerbations are characterized by demyelination, inflammation, and edema.
- • Chronic, progressive disease is characterized by neurodegeneration, axonal damage, normal appearing white matter lesions, and gray matter abnormalities.
- • Remyelination is thought to play a role in the partial or complete recovery of neurological function in relapsing-remitting MS.
- • As MS progresses and patients age, remyelination lessens and neurological dysfunction becomes permanent.
- • Treatment of MS is multifactorial including counseling, physical therapy, exercise, and pharmacotherapy.
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Acknowledgments
Fig. 2 , Fig. 3 , Fig. 6 were obtained during the scope of US government employment for Dr. Conran.
- Search Menu
- Sign in through your institution
- Editor's Choice
- Advance articles
- Review Series
- Virtual Issues
- Clinical & Experimental Treatment of…
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Call for Papers
- About Clinical & Experimental Immunology
- About the British Society for Immunology
- Editorial Board
- Early Career Researcher Editorial Board
- Advertising & Corporate Services
- Publishing with us
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, approaches to overcome rituximab resistance in aid, car t-cell therapy, tce: clinical trial experience and technical aspects, employing t cells to disrupt the b–t collaboration: car t and tce, developing personalized b cell targeting regimens, conclusions, acknowledgements, ethical approval, conflict of interests, author contributions.
- < Previous
Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?
- Article contents
- Figures & tables
- Supplementary Data
Kavina Shah, Maria Leandro, Mark Cragg, Florian Kollert, Franz Schuler, Christian Klein, Venkat Reddy, Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?, Clinical and Experimental Immunology , Volume 217, Issue 1, July 2024, Pages 15–30, https://doi.org/10.1093/cei/uxae031
- Permissions Icon Permissions
B and T cells collaborate to drive autoimmune disease (AID). Historically, B- and T-cell (B–T cell) co-interaction was targeted through different pathways such as alemtuzumab, abatacept, and dapirolizumab with variable impact on B-cell depletion (BCD), whereas the majority of patients with AID including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and organ transplantation benefit from targeted BCD with anti-CD20 monoclonal antibodies such as rituximab, ocrelizumab, or ofatumumab. Refractory AID is a significant problem for patients with incomplete BCD with a greater frequency of IgD − CD27 + switched memory B cells, CD19 + CD20 − B cells, and plasma cells that are not directly targeted by anti-CD20 antibodies, whereas most lymphoid tissue plasma cells express CD19. Furthermore, B–T-cell collaboration is predominant in lymphoid tissues and at sites of inflammation such as the joint and kidney, where BCD may be inefficient, due to limited access to key effector cells. In the treatment of cancer, chimeric antigen receptor (CAR) T-cell therapy and T-cell engagers (TCE) that recruit T cells to induce B-cell cytotoxicity have delivered promising results for anti-CD19 CAR T-cell therapies, the CD19 TCE blinatumomab and CD20 TCE such as mosunetuzumab, glofitamab, or epcoritamab. Limited evidence suggests that anti-CD19 CAR T-cell therapy may be effective in managing refractory AID whereas we await evaluation of TCE for use in non-oncological indications. Therefore, here, we discuss the potential mechanistic advantages of novel therapies that rely on T cells as effector cells to disrupt B–T-cell collaboration toward overcoming rituximab-resistant AID.

B–T-cell collaboration in the pathogenesis of autoimmune disease
B- and T-cell (B–T-cell) collaboration perpetuates chronic inflammation in a range of autoimmune diseases (AID) including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS) [ 1 , 2 ]. This cellular collaboration may occur through contact-dependent or -independent pathways through cytokines and other immune stimuli. Within lymphoid aggregates and the germinal center, B–T-cell interactions involve an array of molecular pairings [ 3 ], summarized in Fig. 1 and Table 1 . These signals stimulate T-cell secretion of cytokines and promote differentiation of naïve to memory B cells and plasma cells (PCs), Fig. 1 . Some of these pathways have been targeted, as discussed later, whereas others are the subject of novel therapeutic strategies.
Overview of CD antigens and other molecules involved in B- and T-cell collaboration along with their function/utility
| Marker (± ligand/receptor) . | Meaning/function/application . |
|---|---|
| CD3 (TCR) | T-cell activation signaling and regulation of TCR expression |
| CD4 (MHC II) | T-helper cell |
| CD8 (MHC I) | Cytotoxic T cell |
| CD19 (co-receptor for BCR) | Pan B cell marker. Regulates B-cell development, activation, and differentiation |
| CD20 | B-cell activation and proliferation. Also present on a minority of T cells |
| CD27 (CD70) | Marker of B- and T-cell memory |
| CD28 (CD80/86) | Co-stimulation between B and T cells |
| CD40 (CD40L) | Co-stimulation between B and T cells |
| BAFF-R (BAFF) or BLyS | B-cell activating factor enhances B-cell survival |
| PD-1 (PD-L1 and PD-L2) | Programmed cell death, down-regulates the immune response |
| CXCL-10 (CXCR3) | Recruitment of monocytes, T cells, NK cells |
| CXCL-13 (CXCR5) | B-cell chemoattractant |
| CCR2 (CCL-2 also known as MCP-1) | Trafficking of monocytes to inflammatory sites |
| ICOS-ICOSL | ICOS part of the CD28 superfamily, provides co-stimulatory signal to activated T cells upon binding to ICOS-L |
| IL21-IL21R | Promotes proliferation and function of T and B cells, enhances cytotoxicity of CD8 T cells and NK cells |
| TCR-MHCII | MHC displays peptides to the TCR, and TCR can discriminate foreign from self-peptides |
| Marker (± ligand/receptor) . | Meaning/function/application . |
|---|---|
| CD3 (TCR) | T-cell activation signaling and regulation of TCR expression |
| CD4 (MHC II) | T-helper cell |
| CD8 (MHC I) | Cytotoxic T cell |
| CD19 (co-receptor for BCR) | Pan B cell marker. Regulates B-cell development, activation, and differentiation |
| CD20 | B-cell activation and proliferation. Also present on a minority of T cells |
| CD27 (CD70) | Marker of B- and T-cell memory |
| CD28 (CD80/86) | Co-stimulation between B and T cells |
| CD40 (CD40L) | Co-stimulation between B and T cells |
| BAFF-R (BAFF) or BLyS | B-cell activating factor enhances B-cell survival |
| PD-1 (PD-L1 and PD-L2) | Programmed cell death, down-regulates the immune response |
| CXCL-10 (CXCR3) | Recruitment of monocytes, T cells, NK cells |
| CXCL-13 (CXCR5) | B-cell chemoattractant |
| CCR2 (CCL-2 also known as MCP-1) | Trafficking of monocytes to inflammatory sites |
| ICOS-ICOSL | ICOS part of the CD28 superfamily, provides co-stimulatory signal to activated T cells upon binding to ICOS-L |
| IL21-IL21R | Promotes proliferation and function of T and B cells, enhances cytotoxicity of CD8 T cells and NK cells |
| TCR-MHCII | MHC displays peptides to the TCR, and TCR can discriminate foreign from self-peptides |
CXCL: CXC chemokine ligand; CCR: C-C motif chemokine receptor; ICOS, MCP: monocyte chemoattractant protein; MHC: major histocompatibility complex; TCR: T-cell receptor.
![multiple sclerosis case study uk Pathways of B–T-cell co-stimulation and trials of therapeutic agents. Molecular pairings are explained in Table 1. Drugs that target co-stimulation are outlined here. Dapirolizumab is an anti-CD40L mAb, currently in phase III study in SLE (NCT04294667). Bleslumab is an IgG4 mAb that targets CD40 which underwent phase II trial in plaque psoriasis with no clinical improvement compared to placebo [4], and demonstrated non-inferiority compared with standard of care for acute rejection in renal transplant recipients [5]. Iscalimab is another anti-CD40 mAb which is undergoing phase II trial in SLE and Sjogren’s Syndrome (NCT03656562, NCT04541589). Abatacept inhibits CD80/86 to prevent engagement with CD28 and is approved for use in RA but failed to meet the primary endpoint in the lupus nephritis phase III trial. AMG 557, anti-ICOSL antibody, underwent phase II trial in SLE and a newer therapy inhibiting ICOSL and BAFF is undergoing phase II trial (NCT04058028). PD-1 agonist, Peresolimab demonstrated modest improvement in disease activity in a phase II trial for patients with RA. Image created using Biorender.com](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cei/217/1/10.1093_cei_uxae031/1/m_uxae031_fig1.jpeg?Expires=1722697693&Signature=S8S1bUnlFbOsBlfAm6NtZyFq-1-M4LnUeM7XhVqgltjw9A6FY5wdRk7~kH69HQw7V5wT0PvlTt1QEr4Jv4C4c2EHvG05HMhoIBON4E5LYFRlOtIUr7NASYOY5sMmXERmBT5p-NvEGl3GfWb2WWa7AoyOtx36Yobu4U-Y0~tsfehI7ma-akanpSlSGAp0nMW03zqwpcsc5d0JRS-3FbDVXpZI~JJV0bFa8SIMOQdYqIaQbAqAEK4VkhZ3aDe-2GBHG~UG9A0JsckPthk87OQsgu~f46f-8C2Sp~ZrAJQOVAUXjv4VwAFrrGKW6YQFWRYO6hVYYoBM2NzSKy0igUuC6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pathways of B–T-cell co-stimulation and trials of therapeutic agents. Molecular pairings are explained in Table 1 . Drugs that target co-stimulation are outlined here. Dapirolizumab is an anti-CD40L mAb, currently in phase III study in SLE (NCT04294667). Bleslumab is an IgG4 mAb that targets CD40 which underwent phase II trial in plaque psoriasis with no clinical improvement compared to placebo [ 4 ], and demonstrated non-inferiority compared with standard of care for acute rejection in renal transplant recipients [ 5 ]. Iscalimab is another anti-CD40 mAb which is undergoing phase II trial in SLE and Sjogren’s Syndrome (NCT03656562, NCT04541589). Abatacept inhibits CD80/86 to prevent engagement with CD28 and is approved for use in RA but failed to meet the primary endpoint in the lupus nephritis phase III trial. AMG 557, anti-ICOSL antibody, underwent phase II trial in SLE and a newer therapy inhibiting ICOSL and BAFF is undergoing phase II trial (NCT04058028). PD-1 agonist, Peresolimab demonstrated modest improvement in disease activity in a phase II trial for patients with RA. Image created using Biorender.com
In this context of an ongoing immune response, an appreciation of B-cell biology is helpful. B cells originate from hematopoietic stem cells in the bone marrow and undergo differentiation in secondary lymphoid organs [ 6 ]. Differential expression of various cell surface markers, including cluster of differentiation (CD) molecules and immunoglobulin isotypes help to define classical subpopulations including naïve B cells (IgD+CD27−), unswitched memory B cells (IgD+CD27+), switched memory B cells (IgD−CD27+) and double negative memory B cells (IgD−CD27−) [ 6 ]. Naïve B cells have not yet encountered antigen, whereas switched memory B cells are primed to respond to antigen and double negative memory B cells increase with aging, autoimmunity, and chronic infectious diseases [ 7 ]. Until recently, the focus of B-cell depletion therapy has been on rituximab, an anti-CD20 monoclonal antibody that is widely used in hematological malignancies and AID (discussed in more detail below). The first FDA approved targeted biologic therapy for SLE was Belimumab, a mAb directed at B-cell activating factor (BAFF, also known as BLyS) [ 8 ], however, real-world data demonstrates variable success [ 9 , 10 ]. BAFF is a B-cell survival and differentiation factor and is elevated in the serum of patients with SLE [ 11 ].
B–T-cell interactions in the peripheral inflammatory sites of various AID including RA SLE, type I diabetes mellitus, and celiac disease exhibit a population of T cells which are termed T-peripheral helper cells [ 1 , 12 , 13 ]. Rao et al . identified these cells, adjacent to B cells in lymphoid aggregates of the synovium in patients with RA as PD-1hiCXCR5 − CD4 + which lack Bcl6 but produce IL-21 and CXCL13, resulting in B-cell differentiation into plasmablasts (PBs) [ 14 ]. This perpetuates B–T-cell networking in inflamed tissues, where ectopic lymphoid structures [ 15 ] are formed. Thus, B–T-cell collaboration occurs in both lymphoid tissues and at sites of inflammation.
Disrupting the B–T-cell networking in AID, historical perspectives
B–T-cell collaboration is a dominant source of chronic inflammation in AID. Hence, disrupting this network is an appealing therapeutic strategy. Over the past four decades, B–T-cell co-stimulation was targeted through different pathways such as alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H), abatacept (cytotoxic T-lymphocyte antigen 4 immunoglobulin), and dapirolizumab (anti-CD40L) with variable impact on B-cell depletion (BCD), Fig. 2 . In the 1980s, alemtuzumab was used to deplete CD52 expressing cells including B and T cells, providing the first insights into disrupting B–T-cell networking. The 1990s trials of alemtuzumab in RA were terminated due to suboptimal therapeutic index probably owing to prolonged depletion of regulatory T cells [ 16 ], although it continues to be used to treat MS (albeit at lower doses). Abatacept inhibits the co-stimulatory CD28-CD80/86 pathway and is approved for RA [ 17 ] although the ALLURE trial of abatacept in lupus nephritis (LN) did not meet its primary endpoint [ 18 ]. Attempts have been made to block other key co-stimulatory signaling pathways including the CD40-CD40L axis. Second-generation agents have been developed including dapirolizumab-pegol which had favorable biomarker and safety response in SLE [ 19 ]; phase III results are awaited (NCT04294667). Therefore, despite these advances, there remains a great unmet need for disrupting B–T-cell collaboration in refractory patients with AID.

Historical timeline of therapies that target B–T-cell collaboration in autoimmune disease. These agents were designed either to deplete B cells and/or disrupt the B–T-cell collaboration. The top row denotes the target antigen, the second row demonstrates the drugs that have undergone clinical trial (later two, t are yet to undergo clinical trial in AID). The third row represents therapies that interrupt B–T-cell networking and the fourth row represents treatments that employ T cells as effector cells. Text in italics under CD20 represents other approved anti-CD20 mAbs, *denotes pending approval
BCD with rituximab in RA and SLE; why is it suboptimal?
In the past three decades, BCD therapy with the CD20 monoclonal antibody rituximab, has revolutionized the treatment of severe or refractory AID and has been approved for use in RA [ 20 ], ANCA vasculitis [ 21 ], and pemphigus vulgaris (PV) [ 22 ] and is prescribed widely “off-licence” in SLE [ 23 ] and in immune thrombocytopenic purpura (ITP) [ 24 ]. Data from the Lupus Nephritis Assessment with Rituximab (LUNAR) study reported complete BCD with complete response, as defined in the study [ 25 ]. However, there remains a significant proportion of patients, up to 30%, who have disease refractory to rituximab, particularly in the context of incomplete BCD [ 23 ] and/or repopulation with PB and switched memory B cells (IgD − CD27 + , SwMBC) [ 26 ].
How do memory B cells and CD19 + CD20 − PBs evade rituximab?
B cells can evade rituximab’s effects either through intrinsic mechanisms (lacking CD20 expression and antigenic modulation) or extrinsic mechanisms such as restricted vascular access to effector cells as discussed previously [ 27 ]. Upon activation, naïve B cells solicit T-cell co-stimulation in lymphoid tissues and at sites of inflammation such as the joint and the kidney to differentiate into memory B cells and antibody-secreting cells including short-lived CD19 + CD20 − PBs and long-lived CD20 − PCs [ 14 , 28 ]. In RA, rituximab fails to completely deplete SwMBC and CD19 + CD20 − PCs in lymphoid tissues [ 29 ], joints, and bone marrow [ 30–32 ] contributing to poor response. In patients with ITP with poor response to rituximab, autoreactive splenic memory B cells down-regulate their BCR and up-regulate anti-apoptotic proteins and evades rituximab while retaining the capacity to reactivate and differentiate into autoantibody secreting CD19 + CD20 − PBs [ 24 ]. In muscle-specific kinase myasthenia gravis, autoreactive SwMBC evades rituximab and differentiate into autoantibody secreting CD19 + CD20 − PBs contributing to relapse [ 33 ]. Further, rituximab has no direct effect on CD19 + CD20 − PBs and PCs, as they do not express CD20 [ 34 , 35 ]. Thus, SwMBCs, CD19 + CD20 − PBs and CD19 + CD20 − PCs evade rituximab through distinct mechanisms, Fig. 3 .

Life cycle of B lineage cells. B cells originate in the bone marrow and migrate through peripheral circulation into lymphoid tissues such as lymph nodes and the spleen. Naïve B cells mature into memory B cells which then differentiate into switched memory B cells, SwMBC (IgD − ,CD27+), or double negative memory B cells (DN MBC; IgD − , CD27 − ) entering the peripheral circulation or plasma blasts (PBs) and plasma cells (PCs) a majority of which reside in the bone marrow, tissues, and inflammatory sites. Proportions of CD19 + CD20 + versus CD19 + CD20 − B cells are demonstrated pictorially within each subpopulation. Anti-CD20 monoclonal antibodies such as rituximab may not completely deplete CD19 + CD20 + B cells in tissue and do not target CD19 + CD20 − B cells, therefore, alternative strategies of depletion including CD19 targeting approaches may help to overcome rituximab resistance in autoimmunity
Broadly, anti-CD20 mAbs can be grouped into types I and II, where type I mAbs such as rituximab, are more efficient at clustering CD20 compared to type II anti-CD20 mAbs [ 36 ]. This enables efficient complement activation and therefore enhanced complement-dependent cytotoxicity (CDC), however, it also increases the propensity for internalization of CD20:CD20 mAb complexes by B cells [ 37 ]. In addition, incomplete BCD with rituximab may be related to its internalization of rituximab [ 38 ]. Type II anti-CD20 mAbs such as obinutuzumab may, at least in part, overcome this resistance mechanism [ 27 ]. In a pivotal phase II study, obinutuzumab was shown to improve clinical response in LN [ 39 ] and phase III studies are ongoing. However, CD19 + CD20 − PBs and CD19 + CD20 − PCs are still not directly targeted. Furthermore, disease-associated macrophage phagocytic defects [ 40 ] and vascular access limitations may compromise the ability of anti-CD20 mAbs (and other B-cell depleting mAbs, such as those directed to CD19) to evoke antibody-dependent cellular phagocytosis (ADCP) [ 27 , 41 ] as they rely on FcγR-bearing effector cells. In addition, NK cells are also scarce in tissues, limiting antibody dependent cellular cytotoxicity (ADCC). For example, we have previously reported that incomplete depletion and/ or persistent infiltration of B cells in the kidneys was associated with active LN refractory to rituximab [ 42 ].
Through histological analysis of kidney [ 43 ] and skin [ 44 ] of patients with AID, and the synovium in patients with RA [ 14 ], we know that B cells interact with T cells in lymphoid tissues and at sites of inflammation, to differentiate into autoantibody secreting PBs and PCs. At these sites, limited access to rituximab’s key effector cells, macrophages, and NK cells, may compromise depletion. Thus, antigen expression, modulation, and access to effector cells influence the efficiency of rituximab-mediated BCD. Therefore, it is important to consider both alternative target antigens and therapies that recruit other effector cells to improve BCD.
Is CD19 an ideal target?
CD19 regulates the threshold for B-cell activation as a co-receptor of the BCR complex [ 45 ] with consequent implications for influencing autoimmunity [ 46 ]. CD19 deficiency impairs humoral immunity, at least in part, due to an increased threshold for B-cell activation [ 47 ] whereas overexpression is associated with AID such as SLE [ 28 ]. When compared with CD19 − CD20 − PCs, CD19 + CD20 − PCs accumulate more mutations and retain greater proliferative capacity, at least in vitro [ 34 ]. These observations implicate a significant role for CD19 in B-cell differentiation and activation.
When compared with CD20, B lineage cells express CD19 at an earlier stage in development and retain expression through all stages of differentiation into CD19 + CD20 − PBs and some CD19 + CD20 − PCs [ 28 ]. CD19 hi CD11c + memory B cells in humans were shown to respond robustly to antigen challenge, in vitro [ 48 ]. More recent evidence suggests that double negative (IgD − CD27 − ) DN B cells which express the transcription factor T-box expressed in T cells (T-bet) encoded by Tbx21 , termed DN-T-bet + B cells are expanded in aging, are associated with higher mortality from COVID-19 infection and disease activity in SLE as well as disease pathogenesis in RA. Therefore they are of great interest in the field of B-cell research [ 49 ].
Further, they demonstrate increased expression of CD19 which strengthens the argument to target CD19 in AID (Shah et al ., in preparation). Considering the availability of newer therapies that target CD19, particularly in the field of oncology, we reappraise the concept of targeting CD19, put forward over a decade ago, to treat AID [ 28 ]. In addition, evidence from oncology highlights that cancers refractory to monoclonal antibodies have been effectively treated with CD19-targeted chimeric antigen receptor (CAR) T cells, probably owing to the deeper depletion of B cells which provides promise for patients with AID resistant to current mAb therapy, highlighted by the published case series in SLE [ 50 ]. These mechanistic considerations indicate that targeting CD19, particularly in AID, may overcome anti-CD20 mAb resistance.
How to target CD19-T-cell engagement as a mechanism of action?
Therapeutic options to target CD19 + B cells and PCs include (i) anti-CD19 mAbs; (ii) CD19-targeted CAR T cells; and (iii) CD19-directed T-cell engagers (TCE). The anti-CD19 mAb inebilizuzmab is approved for the treatment of neuromyelitis optica spectrum disorder [ 51 ] and showed initial promising results in a clinical trial in systemic sclerosis [ 52 ]. BCD with inebilizumab was greater in transgenic mice blood and spleen as well as in an in vitro ADCC assay using human PBMCs when compared to rituximab [ 53 ]. However, similar to rituximab, anti-CD19 mAbs are also disposed to internalization [ 54 ] and would be limited by disease-associated macrophage phagocytic defects [ 40 ] and vascular access limitations. Therefore, CD19-directed CAR T cells and CD19 TCE may be of greater utility in AID and will be discussed in the following sections.
The introduction of CAR T cells to treat cancer has been instrumental in providing individualized, targeted treatment through genetically engineered T cells that express a CAR specific to a tumor-associated antigen, such as CD19 in B cell [ 55 ] malignancies. Recognition of the target antigen-bearing B cells activates CAR T cells to proliferate and selectively eliminate the target B cells. The basic structure of a CAR includes an extracellular surface domain for antigen recognition (typically derived from an antibody fragment), a transmembrane domain, and an intracellular signaling domain that activates T cells (typically derived from CD3z chain). The evolution of CAR from first to fourth generation includes the addition of co-stimulatory domains (one in second generation and two in third generation CARs) as well as co-expression of additional transgenes for cytokine secretion (fourth generation) [ 56 ], Fig. 4 .

Evolution of CARs across the generations. All CARs have a single chain variable region of a mAb. ( A ) first-generation CARs contain an intracellular signaling domain of CD3 zeta chain alone; ( B ) second-generation includes a single co-stimulatory domain (CD28 or 4-1BB); ( C ) third-generation CARs combine two of the above co-stimulatory domains; and ( D ) fourth-generation CARs are diversified in that they can express cytokines. Image created using BioRender.com
Once administered, CAR T cells can also expand and establish immune memory, thus providing long-term surveillance of disease as described in malignancy [ 57 ]. CAR T-cell therapy has been approved for the treatment of B-cell acute lymphoblastic leukemia (ALL), lymphoma, and multiple myeloma [ 55 ]. Factors such as antigen overload are considered to contribute to undesirable effects including cytokine release syndrome (CRS) and neurotoxicity, leading to newer generation therapies with fewer toxicities being developed [ 58 ]. Complete remission for at least 3 years, of various relapsed B-cell malignancies was demonstrated in 51% of patients treated with CAR T-cell therapies, with few late-onset side effects [ 59 ]. This success led to CAR T cells being explored for treating refractory AID.
CAR T-cell therapy in AID
The success of using CAR T-cell therapy for the management of B-cell malignancies inspired its research in a range of AID including SLE, myasthenia gravis, and type 1 diabetes mellitus, as outlined in Table 1 . In animal models of SLE, anti-CD19 CAR T-cell treatment resulted in profound and sustained BCD with low circulating PCs and increased survival rates [ 60 ]. This data provided the basis for the use of anti-CD19 CAR T-cell therapy in the treatment of five patients with refractory multiorgan lupus which was well tolerated leading to serological and clinical remission at relatively short follow-up [ 50 ]. Probably owing to lower antigen load, the first cohort of patients with SLE-treated with anti-CD19 CAR T-cell therapy experienced only low-grade CRS [ 61 ], of which tocilizumab (anti-IL-6 receptor mAb) was used (successfully) in only one patient owing to persistent fevers for 3 days [ 50 ]. Thus, current preliminary evidence suggests that CD19 targeting CAR T-cell therapy seems a safe and effective therapeutic strategy in AID such as SLE. Anti-CD19 CAR T-cell therapy was associated with a reduction in autoantibodies and pro-inflammatory cytokines including IL-6 and TNF-α [ 62 ]. Intriguingly, despite excellent clinical responses, the authors demonstrated an increase in serum BAFF levels.
With regard to other autoimmune diseases, single case studies of anti-CD19 CAR T-cell therapy indicate a potential use of the approach also in anti-synthetase syndrome [ 63 ] and systemic sclerosis [ 64 ]. To note, an important potential confounder when appraising the mechanisms of response to CAR T-cell therapy is the use of lymphocyte depletion with fludarabine that may have contributed to the response. Several studies exploring the safety, tolerability, and preliminary efficacy of anti-CD19 CAR T-cell therapy in AID have been initiated (NCT05938725, NCT05869955, NCT03030976, NCT05798117, and NCT05930314).
Limitations of CAR T-cell therapy
Although the case examples of anti-CAR T cells in AID are promising, it is also important to understand the limitations. Two of the five patients treated with anti-CD19 CAR T-cell therapy had persistence of clonotypic IgG in follow-up samples, demonstrating suboptimal depletion and/or rapid repopulation of memory B cells [ 50 ]. Remarkably, despite lower antigen overload, three of five SLE patients treated with anti-CD19 CAR T-cell therapy repopulated their B cells by day 50 after treatment [ 50 ] when compared with prolonged BCD achieved in B-cell malignancies up to several years post-infusion [ 55 ]. Potential explanations for incomplete depletion and/or relatively early repopulation of B cells include (i) complete depletion of target cells removing the sustained stimulus needed to maintain an optimal pool of CAR T cells, as CAR T cells had disappeared at week 4 after treatment; (ii) higher proportion of senescent and/or exhausted SLE CAR T cells; and (iii) potential inhibition of CAR T-cell expansion due to the persistent effects of immunosuppression such as mycophenolate mofetil beyond cessation of therapy [ 65 ].
Implications of lymphodepletion in AID
Patients with AID, particularly SLE, are often lymphopenic owing to the underlying disease process and the effects of immunosuppression, which may impact the process of leukapheresis required to generate the CAR T cells. Nevertheless, patients with active SLE in the previously discussed case series [ 50 ] were successfully leukapheresed before CAR T-cell therapy and concurrent treatment with steroids and immunosuppressive agents [ 66 ]. The process of lymphodepletion itself increases the likelihood of infections and is an additional step preceding CAR T-cell therapy, compared to “off the shelf” TCE therapy.
Risks of hypogammaglobulinemia
A major consideration with CAR T-cell therapy is the risk of hypogammaglobulinemia; this may be observed with TCE but likely to a lesser extent. In the treatment of cancer, approximately a third of patients develop hypogammaglobulinemia following CAR T-cell infusion [ 67 ], owing to potent and persistent depletion of normal CD19 + B cells. Very low IgG levels can arise from 9 weeks after treatment and continue beyond 4 years [ 67 ]. This poses a risk of serious life-threatening infections, necessitating intravenous immunoglobulin infusions as a prevention strategy, as per the majority of trials [ 68 ], however, this can be expensive and not readily accessible for all patients.
Importantly, B-cell aplasia and hypogammaglobulinemia result in suboptimal vaccine responses, which is also a significant concern especially in the current era of SARS-CoV-2 infection with only 29% of patients who receive CAR T-cell therapy for lymphoma/myeloma mounting a clinically relevant antibody response to vaccination [ 69 ]. Reassuringly, vaccine responses were stable following CAR T-cell therapy in the SLE case series [ 50 ], likely related to the remaining pool of CD19 − plasma cells which are able to secrete antibodies 2 years post-treatment [ 70 ]. These aspects also need to be accounted for during TCE trial design in AID.
Logistical limitations of CAR T cell therapy
Logistical limitations are also considerable. For example, in patients with rapidly progressing cancer or AID, the practical feasibility of CAR T-cell therapy may be limited as there is typically a protracted vein-to-vein time of approximately 6–8 weeks, due to the time required for producing, transporting, and ensuring quality control of the personalized cell therapy, as illustrated in the graphical abstract. This process is typical for most CAR T-cell therapies, although the novel YTB323 omits the ex vivo expansion stage (NCT05798117).
Further disadvantages of CAR T-cell therapy include the high cost involved with engineering and storage of CAR T cells and the specialist training required to administer treatment as detailed in Table 2 . Therefore, readily available and effective novel treatments are required while awaiting CAR T-cell therapy [ 79 ]. One approach to obviate the limiting factor of individual custom-made CAR T cells is the generation of “universal CAR T cells” as reviewed by Zhao et al . [ 56 ]. These can serve as “off the shelf” therapies to treat a wide range of clinical indications as they are engineered to target multiple antigens. Further gene editing work is underway to ensure universal CAR T cells are not depleted by the recipient’s immune system and are able to expand without causing harmful effects [ 80 ].
Evidence for the use of CAR T-cell therapies in non-malignant settings
| Specialty . | Indication . | Study phase/type . | Outcome . | Ref . |
|---|---|---|---|---|
| Neurology | Multiple sclerosis (murine model = experimental autoimmune encephalomyelitis) | Murine model | Depleted B cells in peripheral blood and CNS Improved clinical scores of EAE | [ ] |
| Myasthenia Gravis (using anti-B-cell maturation antigen CAR T cells) | Phase 1b/2a (human) | Safe, well-tolerated, and clinical improvement Phase IIb ongoing (NCT04146051) | [ ] | |
| Transplant medicine | Post-transplant lymphoproliferative disorder (PTLD) post-renal transplant | Case series ( = 3) (human) | Demonstrated safety and feasibility (with regard to stopping immunosuppression) however only one of three patients maintained in remission at 3 months follow-up | [ ] |
| Case series of three patients with refractory PTLD post solid organ transplants (cardiac transplant, kidney transplant, and pancreas transplant) | Case series ( = 3) (human) | Poor outcomes, multiple complications including CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), acute kidney injury, lack of response to CAR T-cell therapy, and mortality | [ ] | |
| Refractory PTLD post heart and kidney transplant | Case report (human) | Six months post CAR T-cell infusion, clinically well, and normal ejection fraction on echocardiography | [ ] | |
| Rheumatology | Systemic lupus erythematosus | Case series ( = 5) (human) | Deep depletion of B cells, clinical improvement, normalization of anti-ds-DNA antibodies and all achieved remission after 3 months. Three patients repopulated B cells less than 50 days post CAR T-cell therapy (although mainly naïve B cells) | [ ] |
| Systemic sclerosis (diffuse cutaneous) | Case report (human) | Extensive fibrosis (skin, heart, and lung)—all showing improvement post treatment Well tolerated, mild CRS (Grade 1), no signs of ICANS. | [ ] | |
| Anti-synthetase syndrome (myositis and interstitial lung disease) | Case report ( = 2) (human) | Treated with CD19-targeting CAR T cells. Excellent outcome with biochemical, serological, and radiological resolution of myositis and improvement in pulmonary function tests/CT chest. | [ , ] | |
| Dermatology | Pemphigus vulgaris—target antigen desmoglein 3 | Preclinical study, (human) | Depletion of Dsg3 cells and antibodies in human pemphigus vulgaris model | [ ] |
| Endocrinology | Type I diabetes Mellitus—target antigen Insulin | Murine model | Delayed onset of diabetes but no long-term protection | [ ] |
| Specialty . | Indication . | Study phase/type . | Outcome . | Ref . |
|---|---|---|---|---|
| Neurology | Multiple sclerosis (murine model = experimental autoimmune encephalomyelitis) | Murine model | Depleted B cells in peripheral blood and CNS Improved clinical scores of EAE | [ ] |
| Myasthenia Gravis (using anti-B-cell maturation antigen CAR T cells) | Phase 1b/2a (human) | Safe, well-tolerated, and clinical improvement Phase IIb ongoing (NCT04146051) | [ ] | |
| Transplant medicine | Post-transplant lymphoproliferative disorder (PTLD) post-renal transplant | Case series ( = 3) (human) | Demonstrated safety and feasibility (with regard to stopping immunosuppression) however only one of three patients maintained in remission at 3 months follow-up | [ ] |
| Case series of three patients with refractory PTLD post solid organ transplants (cardiac transplant, kidney transplant, and pancreas transplant) | Case series ( = 3) (human) | Poor outcomes, multiple complications including CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), acute kidney injury, lack of response to CAR T-cell therapy, and mortality | [ ] | |
| Refractory PTLD post heart and kidney transplant | Case report (human) | Six months post CAR T-cell infusion, clinically well, and normal ejection fraction on echocardiography | [ ] | |
| Rheumatology | Systemic lupus erythematosus | Case series ( = 5) (human) | Deep depletion of B cells, clinical improvement, normalization of anti-ds-DNA antibodies and all achieved remission after 3 months. Three patients repopulated B cells less than 50 days post CAR T-cell therapy (although mainly naïve B cells) | [ ] |
| Systemic sclerosis (diffuse cutaneous) | Case report (human) | Extensive fibrosis (skin, heart, and lung)—all showing improvement post treatment Well tolerated, mild CRS (Grade 1), no signs of ICANS. | [ ] | |
| Anti-synthetase syndrome (myositis and interstitial lung disease) | Case report ( = 2) (human) | Treated with CD19-targeting CAR T cells. Excellent outcome with biochemical, serological, and radiological resolution of myositis and improvement in pulmonary function tests/CT chest. | [ , ] | |
| Dermatology | Pemphigus vulgaris—target antigen desmoglein 3 | Preclinical study, (human) | Depletion of Dsg3 cells and antibodies in human pemphigus vulgaris model | [ ] |
| Endocrinology | Type I diabetes Mellitus—target antigen Insulin | Murine model | Delayed onset of diabetes but no long-term protection | [ ] |
To this end, we consider alternative strategies, with the potential of TCE bispecific antibodies as a novel therapeutic option to disrupt B-T cell collaboration in AID. Table 2 outlines the major differences and similarities of using CAR T-cell therapy and TCEs.
TCE represents a novel class of targeted therapeutics that recruit T cells [ 81 ]. From a clinical perspective, in the late 1990s, the potential for bispecific antibodies as therapeutic interventions became clearer for cancers such as breast, leukemia, and lung [ 82 ], which led to a surge of interest in their use and FDA approval of catumaxomab for malignant ascites [ 83 ] and blinatumomab for refractory B-ALL [ 84 ] More recently, three CD20 T-cell engagers, mosunetuzumab, glofitamab, and epcoritamab have been approved for treatment of refractory/relapsed follicular lymphoma and refractory/relapsed diffuse large B-cell lymphoma [ 85 ]. Technological advancements over time have enabled a range of modifications to enhance the flexibility and number of binding sites, half-life, production yield, and potency of these therapeutics [ 86 ].
TCE technologies
TCEs can be broadly categorized into (i) small, short half-life bispecific antibody fragments (single chain variable fragments) such as bispecific T-cell engagers (BiTE ® s) which require repeated administration ( Fig. 5A ); and (ii) larger IgG-based T-cell bispecific antibodies (TCBs) with extended half-lives ( Fig. 5B and C ). The development of TCBs has evolved from single chain variable fragments in the early 1990s [ 87 ], to the development of “knobs into holes” (KiH) technology in the late 1990s [ 88 ] to the more advanced technologies including CrossMab to engineer bispecific antibodies [ 89 , 90 ], Fig. 5 .

Selected TCE formats in a schematic representation used for T-cell redirecting therapies. ( A ) Blinatumomab, tandem scFv (single chain variable fragment) (BiTE) format. ( B ) Mosunetuzumab, IgG-based-TCE with monovalent binding using a native antibody structure with 1 Fab arm to bind CD20 (target antigen) and 1 Fab arm to bind CD3 on T cells, combined with the KiH technology as demonstrated in the CH3 domain to achieve heavy chain heterodimerization. ( C ) Epcoritamab, IgG-based TCE with point mutations in each Fc region (CH3 domain) to allow controlled Fab-arm exchange, termed DuoBody®. ( D ) Glofitamab, bivalent binding to increase the avidity of TCE binding to the target antigen, CD20, with additional KiH and CrossMab VH-VL with charge interactions using variable regions. Image created using Biorender.com
Blinatumomab, a BiTE ® composed of two single-chain antibodies targeting CD19 on B cells and CD3ε on T cells fused via a flexible linker ( Fig. 5A ), is approved for B-cell ALL [ 85 ]. It is engineered to have a short half-life of 2 h to enable tight control of serum levels in case of adverse events. Blinatumomab relies on the presence of CD19 + target cells to activate T cells, with sensitive response from CD8 + T cells to induce lysis of tumor cells as demonstrated in video-assisted microscopy studies [ 91 ]. In vitro studies of human B-lymphoma cells demonstrated a higher degree of tumor cell elimination with blinatumomab compared to rituximab [ 92 ]. Interestingly, the combination of blinatumomab and rituximab was synergistically more efficient, especially at low effector-to-target cell ratios and low Blinatumomab concentrations [ 92 ]. This combined effect was found to be due to potent activation of pro-caspases 3 and 7 in target cells, which is instrumental in triggering granzyme-mediated apoptosis. The BiTE subtype is potent with regard to target cell killing. Regardless, the requirement for repeat dosing of Blinatumomab may limit its routine use in clinical practice.
Three CD20 TCE have been approved for refractory B cell lymphomas: mosunetuzumab, glofitamab, and epcoritamab [ 85 ], Fig. 5 . Mosunetuzumab is an IgG-based TCE with 1:1 binding to CD20 and CD3; it uses KiH technology and in vitro assembly to overcome incorrect light chain association [ 93 ]. Epcoritamab is also IgG-based, although employs the unique DuoBody® technology with point mutations in each Fc region (CH3 domain) to allow controlled Fab-arm exchange [ 94 ]. Recent IgG-based TCEs have been developed for increased avidity. Glofitamab has two Fab regions which bind CD20, one Fab region which binds CD3 (so-called 2:1 format), and a longer half-life of 10 days, owing to its Fc region and interaction with FcRn [ 90 ]. The Fc also includes the P329G LALA mutations [ 81 ], which abolish conventional effector functions and therefore it employ a different mechanism of action compared to rituximab. The 2:1 format ( Fig. 5C ) enables greater potency with regard to B-cell cytotoxicity compared to 1:1 antibodies, thought to be due to the close proximity of the CD20 binder and CD3 binder, resulting in a more stable T cell to target B-cell synapse induced by the head-to-tail fusion design [ 95 ].
Effector mechanisms of TCEs: lessons learnt from treating malignant disease
Bispecific antibodies can redirect the effector function of various immune cells. T cells are promising as effector cells as they are abundant, able to expand rapidly, and have potent cytotoxic capacity. TCE are designed to by-pass the normal major histocompatibility complex–T-cell receptor (MHC–TCR) interaction usually required between antigen presenting cells and T cells, and instead co-engage the CD3 molecules on the T cell and form an immunological synapse via the target antigen such as CD19 or CD20 on the surface of B cells that helps redirect co-stimulation to cytotoxicity [ 96 , 97 ], Fig. 6 . This synapse is similar to that formed during cytotoxicity with CAR T cells. The CD20-TCE recruitment of T cells is evident in in vitro culture assays demonstrating that tumor lysis is dependent on T-cell recruitment, activation, and expansion of CD4 + and more profoundly CD8 + subsets [ 81 ]. Importantly, CD20-TCE depleted B cells in the spleen and lymph nodes, efficiently [ 81 ]. These findings may be of relevance to AID where inefficient BCD in lymphoid tissues and inflammatory sites, as discussed earlier, contributes to refractory disease.

The potential effect of immunosuppressive treatments on T-cell effector function. Mycophenolate mofetil (MMF) as per the bottom panel, results in fewer T cells to serve as effector cells for therapies such as CD19 TCE and CD19 CAR T cells. MMF can directly reduce the number of T cells and impair their activation and reduce their cytotoxicity against target B cells with lower release of perforin and granzyme molecules. Image created using Biorender.com
As discussed above, in AID, B and T cells colocalize in lymphoid tissues and at inflammatory sites. Therefore, using CAR T cells or TCE that employ T cells as effector cells to deplete B cells may provide a distinct advantage over rituximab-mediated BCD that relies on macrophages and/or NK cells as the dominant effector mechanism. The key differences and similarities between CAR T-cell therapy and TCE therapy are described in Table 3 .
Mechanistic differences and similarities between CAR T and TCE: experience in oncology
| . | CAR T-cell therapy . | TCE . |
|---|---|---|
| Side effect profile | Variable between CAR T regimens. In some oncological indications, about 80% suffer CRS, longer lasting and at a higher grade Neurotoxicity: immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in approximately 13–21% of patients, lasting 4–5 times longer than with TCE. | Variable between different TCE and indications. Approx. 50% suffer CRS, earlier onset but shorter duration. Obinutuzumab (anti-CD20mAb) pre-treatment limits CRS Neurologic side effects e.g. headache but less severe than ICANS, much less frequent than CAR T cells. |
| Efficacy | Higher rates of complete response in hematological malignancies | Dose-dependent response, but can be up to 30% less effective than CAR T cell therapy |
| Pre-conditioning | Leukodepletion so higher rates of infection and risk of rejection in transplant patients. | No preconditioning, but pre-medication with dexamethasone to reduce cytokine production and with obinutuzumab for glofitamab |
| Hypogammaglobulinemia | Persistence of engineered T cells resulting in sustained B-cell aplasia and hypogammaglobulinemia may require IVIg | TCB can deplete normal B cells and plasma precursor cells leading to a higher risk of hypogammaglobulinemia, but therapeutic regimen could be personalized according to clinical need |
| Effector cell type | Engineered T cells Less differentiated T cells (naïve and memory) show better efficacy than effector T cells | Endogenous T cells Antigen-experienced T cells mediate TCE-induced cell death, whereas naïve T cells are not activated |
| Cost | +++ (~£300 000 in the UK) [ ] | ++ (~£56,000 per cycle UK) [ ] |
| Production | Personalized therapy requiring individual engineering of patient’s T cells—labor intensive, time-consuming (resulting in disease progression), and higher risk of a production error. Also requires the patient to have sufficient peripheral T-cell counts for successful isolation of T cells from leukapheresis. | “Off the shelf” medication, so technically less delay to administration than CAR T-cell therapy. Can be manufactured in large quantities. Can be used independently of peripheral lymphocyte counts |
| Administration | Single intravenous administration, however, from decision to treat to administering therapy can be 6–8 weeks when disease may progress. Specialist training of staff required to administer CAR T-cell therapy and monitor for complications during infusion | Shorter half-life so may need repeat dosing. Quick to administer so can treat patient promptly and halt progression of disease. No additional specialist training required, similar administration to routine mAbs used such as rituximab. |
| Approval for use | ALL, large B-cell lymphoma, mantle cell lymphoma, multiple myeloma (FDA approval) | Blinatumomab (CD3-CD19) for ALL, epcoritamab-bysp and glofitamab (CD3-CD20) for DLBCL (FDA approval), mosunetuzumab (CD3-CD20) for follicular lymphoma |
| Repeat treatment | Complicated due to maintenance of T-cell pool, patient factors (risk of infection). | More convenient and standardized |
| . | CAR T-cell therapy . | TCE . |
|---|---|---|
| Side effect profile | Variable between CAR T regimens. In some oncological indications, about 80% suffer CRS, longer lasting and at a higher grade Neurotoxicity: immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in approximately 13–21% of patients, lasting 4–5 times longer than with TCE. | Variable between different TCE and indications. Approx. 50% suffer CRS, earlier onset but shorter duration. Obinutuzumab (anti-CD20mAb) pre-treatment limits CRS Neurologic side effects e.g. headache but less severe than ICANS, much less frequent than CAR T cells. |
| Efficacy | Higher rates of complete response in hematological malignancies | Dose-dependent response, but can be up to 30% less effective than CAR T cell therapy |
| Pre-conditioning | Leukodepletion so higher rates of infection and risk of rejection in transplant patients. | No preconditioning, but pre-medication with dexamethasone to reduce cytokine production and with obinutuzumab for glofitamab |
| Hypogammaglobulinemia | Persistence of engineered T cells resulting in sustained B-cell aplasia and hypogammaglobulinemia may require IVIg | TCB can deplete normal B cells and plasma precursor cells leading to a higher risk of hypogammaglobulinemia, but therapeutic regimen could be personalized according to clinical need |
| Effector cell type | Engineered T cells Less differentiated T cells (naïve and memory) show better efficacy than effector T cells | Endogenous T cells Antigen-experienced T cells mediate TCE-induced cell death, whereas naïve T cells are not activated |
| Cost | +++ (~£300 000 in the UK) [ ] | ++ (~£56,000 per cycle UK) [ ] |
| Production | Personalized therapy requiring individual engineering of patient’s T cells—labor intensive, time-consuming (resulting in disease progression), and higher risk of a production error. Also requires the patient to have sufficient peripheral T-cell counts for successful isolation of T cells from leukapheresis. | “Off the shelf” medication, so technically less delay to administration than CAR T-cell therapy. Can be manufactured in large quantities. Can be used independently of peripheral lymphocyte counts |
| Administration | Single intravenous administration, however, from decision to treat to administering therapy can be 6–8 weeks when disease may progress. Specialist training of staff required to administer CAR T-cell therapy and monitor for complications during infusion | Shorter half-life so may need repeat dosing. Quick to administer so can treat patient promptly and halt progression of disease. No additional specialist training required, similar administration to routine mAbs used such as rituximab. |
| Approval for use | ALL, large B-cell lymphoma, mantle cell lymphoma, multiple myeloma (FDA approval) | Blinatumomab (CD3-CD19) for ALL, epcoritamab-bysp and glofitamab (CD3-CD20) for DLBCL (FDA approval), mosunetuzumab (CD3-CD20) for follicular lymphoma |
| Repeat treatment | Complicated due to maintenance of T-cell pool, patient factors (risk of infection). | More convenient and standardized |
Aside from requiring lymphodepletion, an important aspect to highlight is that the expansion of CARs in vivo cannot be controlled, demonstrated by the rapid rise in circulating CARs, reaching up to 59% by day nine post-infusion [ 50 ].
In addition, the expansion and duration of CAR T-cell action is not easily controlled, whereas a TCE can be given at a specific dose and the half-life of the molecule is expected to determine its duration of action. Overall, treatment with TCE may potentially overcome some of these limitations of CAR T-cell therapy such as a lag time from decision to treatment to allow for engineering of CAR T cells, prior leukapheresis, and requirement for specialist centers with experience of cell-based immunotherapies.
Immunological/biological pitfalls in recruiting T cells as effector cells
Despite the undoubted promise of CAR T cells and TCE, there remain potential hurdles. Both CAR T cells and TCE may evoke “bystander killing” of antigen-negative cells directly in contact with antigen-positive cells [ 100 ]. While this local bystander effect is desirable in the treatment of solid tumors to prevent the escape of antigen-negative cancer cells, the potential implications of this in AID are unknown.
More recently, there are an increasing number of reports of macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH) as a complication of CAR T-cell therapy given for hematological malignancies, possibly as a distinct variant of CRS [ 101 ]. MAS/HLH is a serious condition of hyperinflammation, fevers, and cytopenias, and can be life-threatening. Patients with autoimmune disease such as SLE are already predisposed to developing secondary MAS/HLH [ 102 ], therefore initiation of CAR T-cell therapy in this cohort needs careful consideration.
Another potential pitfall with recruiting T cells as effector cells is a possible reduction in T-cell counts, which may increase the risk of infection, due to apoptosis noted with first-generation CAR T-cell treatments [ 103 ]. Reassuringly, in studies with CD20-TCB, peripheral T-cell counts decreased in the first 24 h of drug administration before returning to baseline by 72 h [ 81 ], considered to reflect an activation-induced marginalization. Therefore, the risk in the short term with these agents seems low but will need monitoring in the long term.
Impact of the tissue microenvironment
An additional consideration is the tissue microenvironment, which is known to influence T-cell cytotoxicity. AID-related T-cell subpopulations with features of anergy, exhaustion, and senescence may compromise the efficiency of TCE [ 104 ]. In addition, resistance to TCEs may arise from immune escape, through the expression of immune checkpoint molecules such as PD-1. In this context, combination treatment with checkpoint inhibitors, already explored in cancer immunotherapy may be limited by the potential activation of autoreactive T cells [ 105 ]. Alternatively, next generation trispecific TCEs to additionally provide co-stimulation may be beneficial [ 106 ]. As CD3 is a pan T-cell marker, TCEs can recruit all T-cell populations including naïve, regulatory T cells, and exhausted T cells as effector cells. In AID, regulatory and exhausted T cells are associated with disease remission and improved prognosis [ 107 ]. Mechanistic clinical studies will help us understand the clinical relevance of these potential limitations.
Clinical adverse effects of recruiting T cells as effector cells
The main adverse effect associated with both types of T-cell therapy is CRS, which is the rapid systemic release of pro-inflammatory cytokines including IL-6, IL-10, TNF-α, and IFN-γ, upon activation of the T cells [ 108 ]. CRS manifests as fever, fatigue, and vasodilation, and can lead to multi-organ failure. Pre-treatment with corticosteroids such as dexamethasone may reduce the risk of CRS. Anti-IL-6 receptor antibody, tocilizumab, has been approved for use prior to CAR T-cell therapy to attenuate CRS [ 109 ]. In murine models, combination treatment with Janus Kinase (JAK) inhibitors or mammalian target of rapamycin (mTOR) inhibitor, restricted CD19-TCB-related CRS while retaining their efficacy [ 110 ].
Immune effector cell-associated neurotoxicity syndrome (ICANS) is another dose-dependent unwanted side effect unique to patients receiving T-cell engaging treatments, through adherence of T cells to cerebral microvascular endothelium and migration across the blood-brain barrier [ 111 ]. In ALL, ICANS, characterized by headache, dizziness, tremor, confusion, and encephalopathy, was associated with high-dose blinatumomab given in the first treatment cycle, probably owing to the higher tumor burden. As the target cell load is much lower in AID, the required dose of TCEs will be lower, consequently, the risk of CRS and ICANS should be lower than that reported for cancer immunotherapy.
What is the impact of immunosuppressive therapy on T-cell cytotoxicity in the context of TCE and CAR T cells?
Other important considerations include AID-specific concurrent drug regimens. For example, transplant recipients and patients with AID and transplant recipients receive immunosuppressants to regulate immune response. In the context of T-cell-based therapy, concurrent use of immunosuppressants may inhibit the effector function of the T cells, thereby, compromising the efficiency of CAR T cells and TCEs. For example, mycophenolate mofetil (MMF) can induce apoptosis in activated human T cells [ 112 ]; and in a murine model, mycophenolic acid, the active form of MMF has shown dose-dependent reduction in the generation of cytotoxic T cells [ 113 ]. Fig. 6 illustrates the potential impact of immunosuppressants on T-cell cytotoxicity in the context of TCE and CAR T-cell therapies. Therefore withholding immunosuppressants for a period of time to allow for T-cell recovery to enhance performance may be considered in prospective trial design [ 114 ].
In a case series of renal transplant recipients requiring CAR T-cell therapy for post-transplant lymphoproliferative disorders (PTLD), MMF was discontinued at the time of PTLD diagnosis (with DLBCL), and tacrolimus was stopped 2 weeks prior to leukapheresis for production of CAR T cells [ 73 ]. Similarly, a report of CAR T-cell infusion for anti-synthetase syndrome involved tapering azathioprine and steroids 7 days before leukapheresis and starting MMF 35 days after CAR T-cell infusion [ 76 ], which allowed for harvesting of fully functional T cells. This aligns with our proposition of correct sequencing of immunosuppressive treatments including the use of corticosteroids to allow full efficacy of TCE and/or CAR T therapies.
Where pathogenic B-cell identity is well described, CAR T therapy can potentially enhance the prospects for personalized therapy. For example, desmoglein 3 targeting CAR T cells were engineered to selectively eliminate Dsg3 specific B cells, in vitro and in vivo in animal models [ 115 ] toward developing therapies for PV. Currently, a phase I study of BCMA CAR T therapy (NCT04561557) is ongoing for the treatment of neurological disorders including Aquaporin-related neuromyelitis optica spectrum disorder (NMOSD). However, the identity of pathogenic B cells remains elusive for the majority of AID, where non-selective BCD therapy remains the current standard strategy.
In routine practice of managing AID, rituximab induction therapy incorporates two doses of 1 g, given 2 weeks apart. Retreatment with the same or lower dose of rituximab, is usually at 6 months or longer for optimal management of disease activity [ 17 ]. Current evidence highlights that response can be improved with better depletion with a lower frequency of memory B cells and PB in RA and SLE [ 27 ]. As discussed previously, presumably due to more efficient BCD, obinutuzumab treatment seems to be effective in LN [ 39 ]. To this end, targeting CD19 and disrupting the B–T-cell networking in AID, with CD19/CD3 TCEs or CAR T cells would be expected to provide mechanistic advantages. For example, targeting CD19, expressed on memory B cells, CD19 + CD20 − PBs, and CD19 + CD20 − PCs should help deplete these “rituximab-resistant cells” whereas the use of TCEs would help direct T cells from B-cell “co-stimulation to cytotoxicity” to disrupt B–T networking. Key lessons from previous SLE rituximab trials include (i) patient selection with regard to disease manifestations, severity of disease activity, serological parameters, and previous treatment are important to consider so as not to exclude the most active patients, (ii) defining standard concomitant therapy in the comparator and placebo arms as variable usage of glucocorticoid and immunosuppressants such as MMF can impact outcomes, (iii) defining endpoints in particular the steroid sparing effect, (iv) selecting the right disease activity index, and (v) defining follow-up duration and side effects. These serve as a reminder of the importance of optimal trial design to evaluate the “real” potential of TCE [ 25 , 116 ].
Optimizing co-therapies with immunosuppressants, and sequential therapy with rituximab
Co-therapy with immunosuppressants and/or rituximab therapy may influence the efficacy and safety of TCEs. As demonstrated in Fig. 6 , patients with AID are often being treated with immunosuppression such as MMF and corticosteroids. Therefore, considering discontinuation of MMF for 3–6 weeks [ 50 ] may optimize the effector function of T cells to disrupt the B–T-cell network in AID. Thereafter, a delayed introduction of MMF may be considered as needed for optimal control of disease activity.
Sequential therapy with rituximab, which is already competitively priced as a biosimilar, followed by CD19-TCE will enable targeting of B–T-cell networks in ectopic lymphoid tissue within peripherally inflamed tissues in AID, Fig. 3 . A potential limitation of this sequence is that rituximab therapy may result in lower expression of CD19 [ 24 ], probably through internalization as shown in vitro [ 38 ], thus, compromising the efficiency of CD19-TCE or CD19-CAR T therapy. Therefore, treatment with CD19-TCE first followed by rituximab, as needed, could be considered as an alternative strategy for those with poor depletion with CD19-TCE alone. In this context, it would be important to have strategies to detect B cells using novel antibodies that bind an alternative epitope to the therapeutic mAbs, less challenging for CD19 as it is a bigger antigen than CD20.
CD19 CAR T-cell or CD19-TCE therapy to convert B- and T-cell co-stimulation into conflict and disrupt their networking could prove to be a paradigm shift in treating AID. TCE, designed and developed through advanced antibody engineering methods, offers a mechanistically sound, logistically convenient, and favorable alternative therapeutic strategy in the management of refractory AID. To this end, mechanistic studies of TCE in AID, particularly during early-phase clinical trials, are of critical importance to optimize the use of TCE in combination with standard-of-care therapy as an alternative strategy to deplete B-lineage cells to improve outcomes for people with refractory AID.
Not applicable.
None declared.
Funders include Cancer Research UK (DRCRPG-May23/100001), VR's work is funded by MRC-CARP Fellowship from the UK Research and Innovation, Medical Research Council (MR/T024968/1), research grant from UCLH Biomedical Research Centre, National Institute for Health and Care Research, and Roche Innovation Center, Zurich.
Florian Kollert, Roche: Employment and Stock Ownership; Franz Schuler, Roche: Employment, patents, stock ownership; Christian Klein, Roche: Employment, patents, stock ownership; Venkat R Reddy, Research grants from Roche.
Rao DA. The rise of peripheral T helper cells in autoimmune disease . Nat Rev Rheumatol 2019 , 15 , 453 – 4 . doi: 10.1038/s41584-019-0241-7
Google Scholar
van Langelaar J , Rijvers L , Smolders J , van Luijn MM. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers . Front Immunol 2020 , 11 , 760 . doi: 10.3389/fimmu.2020.00760
Petersone L , Edner NM , Ovcinnikovs V , Heuts F , Ross EM , Ntavli E , et al. . T cell/B cell collaboration and autoimmunity: an intimate relationship . Front Immunol 2018 , 9 , 1941 . doi: 10.3389/fimmu.2018.01941
Anil Kumar MS , Papp K , Tainaka R , Valluri U , Wang X , Zhu T , et al. . Randomized, controlled study of bleselumab (ASKP1240) pharmacokinetics and safety in patients with moderate-to-severe plaque psoriasis . Biopharm Drug Dispos 2018 , 39 , 245 – 55 . doi: 10.1002/bdd.2130
Harland RC , Klintmalm G , Jensik S , Yang H , Bromberg J , Holman J , et al. . Efficacy and safety of bleselumab in kidney transplant recipients: a phase 2, randomized, open-label, noninferiority study . Am J Transplant 2020 , 20 , 159 – 71 . doi: 10.1111/ajt.15591
Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies . Arthritis Res Therapy 2013 , 15 , S3 . doi: 10.1186/ar3908
Sachinidis A , Garyfallos A. Double negative (DN) B cells: a connecting bridge between rheumatic diseases and COVID-19 ? Mediterr J Rheumatol 2021 , 32 , 192 – 9 . doi: 10.31138/mjr.32.3.192
Horowitz DL , Furie R. Belimumab is approved by the FDA: what more do we need to know to optimize decision making ? Curr Rheumatol Rep 2012 , 14 , 318 – 23 . doi: 10.1007/s11926-012-0256-4
Venturelli V , Isenberg DA. Targeted therapy for SLE-what works, what doesn’t, what’s next . J Clin Med 2023 , 12 , 3198 . doi: 10.3390/jcm12093198
Parodis I , Vital EM , Hassan S-U , Jönsen A , Bengtsson AA , Eriksson P , et al. . De novo lupus nephritis during treatment with belimumab . Rheumatology 2020 , 60 , 4348 – 54 . doi: 10.1093/rheumatology/keaa796
Guerreiro Castro S , Isenberg DA. Belimumab in systemic lupus erythematosus (SLE): evidence-to-date and clinical usefulness . Ther Adv Musculoskelet Dis 2017 , 9 , 75 – 85 . doi: 10.1177/1759720X17690474
Christophersen A , Lund EG , Snir O , Sola E , Kanduri C , Dahal-Koirala S , et al. . Distinct phenotype of CD4(+) T cells driving celiac disease identified in multiple autoimmune conditions . Nat Med 2019 , 25 , 734 – 7 . doi: 10.1038/s41591-019-0403-9
Ekman I , Ihantola E-L , Viisanen T , Rao DA , Näntö-Salonen K , Knip M , et al. . Circulating CXCR5(-)PD-1(hi) peripheral T helper cells are associated with progression to type 1 diabetes . Diabetologia 2019 , 62 , 1681 – 8 . doi: 10.1007/s00125-019-4936-8
Rao DA , Gurish MF , Marshall JL , Slowikowski K , Fonseka CY , Liu Y , et al. . Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis . Nature 2017 , 542 , 110 – 4 . doi: 10.1038/nature20810
Pitzalis C , Jones GW , Bombardieri M , Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity . Nat Rev Immunol 2014 , 14 , 447 – 62 . doi: 10.1038/nri3700
Cooles FAH , Anderson AE , Drayton T , Harry RA , Diboll J , Munro L , et al. . Immune reconstitution 20 years after treatment with alemtuzumab in a rheumatoid arthritis cohort: implications for lymphocyte depleting therapies . Arthritis Res Ther 2016 , 18 , 302 . doi: 10.1186/s13075-016-1188-6
NICE . Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor . Technology Appraisal Guidance [TA195] 2010 .
Dooley MA , Appel G , Furie R , Wofsy D , Takeuchi T , Malvar A , Doria A , et al. . A phase III randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of abatacept or placebo on standard of care in patients with active class III or IV lupus nephritis . Arthritis Rheumatol . 2018 , 70 , 1 – 3584 .
Furie RA , Bruce IN , Dörner T , Leon MG , Leszczyński P , Urowitz M , et al. . Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus . Rheumatology (Oxford, England) 2021 , 60 , 5397 – 407 . doi: 10.1093/rheumatology/keab381
Norris-Grey C , Cambridge G , Moore S , Reddy V , Leandro M. Long-term persistence of rituximab in patients with rheumatoid arthritis: an evaluation of the UCL cohort from 1998 to 2020 . Rheumatology 2021 , 61 , 591 – 6 . doi: 10.1093/rheumatology/keab248
Smith KGC , Jones RB , Burns SM , Jayne DRW. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment . Arthritis Rheumatism 2006 , 54 , 2970 – 82 . doi: 10.1002/art.22046
Werth VP , Joly P , Mimouni D , Maverakis E , Caux F , Lehane P , et al. .; PEMPHIX Study Group . Rituximab versus mycophenolate mofetil in patients with Pemphigus vulgaris . N Engl J Med 2021 , 384 , 2295 – 305 . doi: 10.1056/NEJMoa2028564
Shah K , Cragg M , Leandro M , Reddy V. Anti-CD20 monoclonal antibodies in systemic lupus erythematosus . Biologicals 2021 , 69 , 1 – 14 . doi: 10.1016/j.biologicals.2020.11.002
Crickx E , Chappert P , Sokal A , Weller S , Azzaoui I , Vandenberghe A , et al. . Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia . Sci Transl Med 2021 , 13 , eabc3961 . doi: 10.1126/scitranslmed.abc3961
Gomez Mendez LM , Cascino MD , Garg J , Katsumoto TR , Brakeman P , Dall’Era M , et al. . Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis . Clin J Am Soc Nephrol 2018 , 13 , 1502 – 9 . doi: 10.2215/CJN.01070118
Vital EM , Dass S , Buch MH , Henshaw K , Pease CT , Martin MF , et al. . B cell biomarkers of rituximab responses in systemic lupus erythematosus . Arthritis Rheum 2011 , 63 , 3038 – 47 . doi: 10.1002/art.30466
Reddy V , Dahal LN , Cragg MS , Leandro M. Optimising B-cell depletion in autoimmune disease: is obinutuzumab the answer ? Drug Discov Today 2016 , 21 , 1330 – 8 . doi: 10.1016/j.drudis.2016.06.009
Mei HE , Schmidt S , Dorner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity . Arthritis Res Ther 2012 , 14 , S1 . doi: 10.1186/ar3909
Ramwadhdoebe TH , van Baarsen LGM , Boumans MJH , Bruijnen STG , Safy M , Berger FH , et al. . Effect of rituximab treatment on T and B cell subsets in lymph node biopsies of patients with rheumatoid arthritis . Rheumatology (Oxford) 2019 , 58 , 1075 – 85 . doi: 10.1093/rheumatology/key428
Thurlings RM , Vos K , Wijbrandts CA , Zwinderman AH , Gerlag DM , Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response . Ann Rheum Dis 2008 , 67 , 917 – 25 . doi: 10.1136/ard.2007.080960
Teng YK , Levarht EW , Toes RE , Huizinga TW , van Laar JM. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation . Ann Rheum Dis 2009 , 68 , 1011 – 6 . doi: 10.1136/ard.2008.092791
Nakou M , Katsikas G , Sidiropoulos P , Bertsias G , Papadimitraki E , Raptopoulou A , et al. . Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response . Arthritis Res Ther 2009 , 11 , R131 . doi: 10.1186/ar2798
Stathopoulos P , Kumar A , Nowak RJ , O’Connor KC. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis . JCI Insight 2017 , 2 , e94263 . doi: 10.1172/jci.insight.94263
Mei HE , Wirries I , Frolich D , Brisslert M , Giesecke C , Grun JR , et al. . A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow . Blood 2015 , 125 , 1739 – 48 . doi: 10.1182/blood-2014-02-555169
Halliley JL , Tipton CM , Liesveld J , Rosenberg AF , Darce J , Gregoretti IV , et al. . Long-lived plasma cells are contained within the CD19(−)CD38(hi)CD138(+) subset in human bone marrow . Immunity 2015 , 43 , 132 – 45 . doi: 10.1016/j.immuni.2015.06.016
Cragg MS , Morgan SM , Chan HTC , Morgan BP , Filatov AV , Johnson PWM , et al. . Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts . Blood 2003 , 101 , 1045 – 52 . doi: 10.1182/blood-2002-06-1761
Lim SH , Vaughan AT , Ashton-Key M , Williams EL , Dixon SV , Chan HTC , et al. . Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy . Blood 2011 , 118 , 2530 – 40 . doi: 10.1182/blood-2011-01-330357
Reddy V , Cambridge G , Isenberg DA , Glennie MJ , Cragg MS , Leandro M. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus . Arthritis Rheumatol 2015 , 67 , 2046 – 55 . doi: 10.1002/art.39167
Furie RA , Aroca G , Cascino MD , Garg JP , Rovin BH , Alvarez A , et al. . B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial . Ann Rheum Dis 2022 , 81 , 100 – 7 . doi: 10.1136/annrheumdis-2021-220920
Mok CC , Lau CS. Pathogenesis of systemic lupus erythematosus . J Clin Pathol 2003 , 56 , 481 – 90 . doi: 10.1136/jcp.56.7.481
Gong Q , Ou Q , Ye S , Lee WP , Cornelius J , Diehl L , et al. . Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy . J Immunol 2005 , 174 , 817 – 26 . doi: 10.4049/jimmunol.174.2.817
Reddy VR , Pepper RJ , Shah K , Cambridge G , Henderson SR , Klein C , et al. . Disparity in peripheral and renal B-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab ? Rheumatology 2021 , 61 , 2894 – 904 . doi: 10.1093/rheumatology/keab827
Chang A , Henderson SG , Brandt D , Liu N , Guttikonda R , Hsieh C , et al. . In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis . J Immunol (Baltimore, Md. : 1950) 2011 , 186 , 1849 – 60 . doi: 10.4049/jimmunol.1001983
Fetter T , Niebel D , Braegelmann C , Wenzel J. Skin-associated B cells in the pathogenesis of cutaneous autoimmune diseases—implications for therapeutic approaches . Cells 2020 , 9 , 2627 . doi: 10.3390/cells9122627
Carter RH , Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes . Science 1992 , 256 , 105 – 7 . doi: 10.1126/science.1373518
Tedder TF , Inaoki M , Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity . Immunity 1997 , 6 , 107 – 18 . doi: 10.1016/s1074-7613(00)80418-5
van Zelm MC , Reisli I , van der Burg M , Castaño D , van Noesel CJM , van Tol MJD , et al. . An antibody-deficiency syndrome due to mutations in the CD19 gene . N Engl J Med 2006 , 354 , 1901 – 12 . doi: 10.1056/NEJMoa051568
Glass DR , Tsai AG , Oliveria JP , Hartmann FJ , Kimmey SC , Calderon AA , et al. . An integrated multi-omic single-cell atlas of human B cell identity . Immunity 2020 , 53 , 217 – 32.e5 . doi: 10.1016/j.immuni.2020.06.013
Wing E , Sutherland C , Miles K , Gray D , Goodyear CS , Otto TD , et al. . Double-negative-2 B cells are the major synovial plasma cell precursor in rheumatoid arthritis . Front Immunol 2023 , 14 , 1241474 . doi: 10.3389/fimmu.2023.1241474
Mackensen A , Müller F , Mougiakakos D , Böltz S , Wilhelm A , Aigner M , et al. . Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus . Nat Med 2022 , 28 , 2124 – 32 . doi: 10.1038/s41591-022-02017-5
Cree BAC , Bennett JL , Kim HJ , Weinshenker BG , Pittock SJ , Wingerchuk DM , et al. .; N-MOmentum study investigators . Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial . Lancet 2019 , 394 , 1352 – 63 . doi: 10.1016/S0140-6736(19)31817-3
Schiopu E , Chatterjee S , Hsu V , Flor A , Cimbora D , Patra K , et al. . Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study . Arthritis Res Ther 2016 , 18 , 131 . doi: 10.1186/s13075-016-1021-2
Herbst R , Wang Y , Gallagher S , Mittereder N , Kuta E , Damschroder M , et al. . B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody . J Pharmacol Exp Ther 2010 , 335 , 213 – 22 . doi: 10.1124/jpet.110.168062
Reddy V , Cambridge G , Isenberg DA , Glennie MJ , Cragg MS , Leandro M. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus . Arthritis Rheumatol 2015 , 67 , 2046 – 55 .
Cappell KM , Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far . Nat Rev Clin Oncol 2023 , 20 , 359 – 71 . doi: 10.1038/s41571-023-00754-1
Zhao J , Lin Q , Song Y , Liu D. Universal CARs, universal T cells, and universal CAR T cells . J Hematol Oncol 2018 , 11 , 132 . doi: 10.1186/s13045-018-0677-2
Feins S , Kong W , Williams EF , Milone MC , Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer . Am J Hematol 2019 , 94 , S3 – 9 . doi: 10.1002/ajh.25418
Mullard A. FDA approves fourth CAR-T cell therapy . Nat Rev Drug Discov 2021 , 20 , 166 . doi: 10.1038/d41573-021-00030-w
Cappell KM , Sherry RM , Yang JC , Goff SL , Vanasse DA , McIntyre L , et al. . Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy . J Clin Oncol: Off J Am Soc Clin Oncol 2020 , 38 , 3805 – 15 . doi: 10.1200/JCO.20.01467
Kansal R , Richardson N , Neeli I , Khawaja S , Chamberlain D , Ghani M , et al. . Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus . Sci Transl Med 2019 , 11 , eaav1648 . doi: 10.1126/scitranslmed.aav1648
Taubmann J , Müller F , Boeltz S , Völkl S , Aigner M , Kleyer A , et al. . OP0141 Long term safety and efficacy of CAR-T cell treatment in refractory systemic lupus erythematosus—data from the first seven patients . Ann Rheum Dis 2023 , 82 , 93 – 4 .
Nunez D , Patel D , Volkov J , Wong S , Vorndran Z , Müller F , et al. . Cytokine and reactivity profiles in SLE patients following anti-CD19 CART therapy . Mol Ther Methods Clin Dev 2023 , 31 , 101104 . doi: 10.1016/j.omtm.2023.08.023
Müller F , Boeltz S , Knitza J , Aigner M , Völkl S , Kharboutli S , et al. . CD19-targeted CAR T cells in refractory antisynthetase syndrome . Lancet (London, England) 2023 , 401 , 815 – 8 . doi: 10.1016/S0140-6736(23)00023-5
Bergmann C , Müller F , Distler JHW , Györfi A-H , Völkl S , Aigner M , et al. . Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells . Ann Rheum Dis 2023 , 82 , 1117 – 20 . doi: 10.1136/ard-2023-223952
Griffin J , Xue S , Carpenter B , Velica P , Holler A , Nicholson E , et al. . T cells engineered for resistance to mycophenolate mofetil demonstrate enhanced expansion and improved tumour control in immunosuppressed hosts . Mol Ther 2014 , 22 , S58 .
Kretschmann S , Völkl S , Reimann H , Krönke G , Schett G , Achenbach S , et al. . Successful generation of CD19 chimeric antigen receptor T cells from patients with advanced systemic lupus erythematosus . Transplant Cell Ther 2023 , 29 , 27 – 33 . doi: 10.1016/j.jtct.2022.10.004
Wat J , Barmettler S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions . J Allergy Clin Immunol Pract 2022 , 10 , 460 – 6 . doi: 10.1016/j.jaip.2021.10.037
Hill JA , Giralt S , Torgerson TR , Lazarus HM. CAR-T—and a side order of IgG, to go? Immunoglobulin replacement in patients receiving CAR-T cell therapy . Blood Rev 2019 , 38 , 100596 . doi: 10.1016/j.blre.2019.100596
Wiedmeier-Nutor JE , Iqbal M , Rosenthal AC , Bezerra ED , Garcia-Robledo JE , Bansal R , et al. . Response to COVID-19 vaccination post-CAR T therapy in patients with non-hodgkin lymphoma and multiple myeloma . Clin Lymphoma Myeloma Leuk 2023 , 23 , 456 – 62 . doi: 10.1016/j.clml.2023.03.002
Bhoj VG , Arhontoulis D , Wertheim G , Capobianchi J , Callahan CA , Ellebrecht CT , et al. . Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy . Blood 2016 , 128 , 360 – 70 . doi: 10.1182/blood-2016-01-694356
Gupta S , Simic M , Sagan SA , Shepherd C , Duecker J , Sobel RA , et al. . CAR-T cell–mediated B-cell depletion in central nervous system autoimmunity . Neurology - Neuroimmunology Neuroinflammation 2023 , 10 , e200080 .
Granit V , Benatar M , Kurtoglu M , Miljković MD , Chahin N , Sahagian G , et al. .; MG-001 Study Team . Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study . Lancet Neurol 2023 , 22 , 578 – 90 . doi: 10.1016/S1474-4422(23)00194-1
Mamlouk O , Nair R , Iyer SP , Edwards A , Neelapu SS , Steiner RE , et al. . Safety of CAR T-cell therapy in kidney transplant recipients . Blood 2021 , 137 , 2558 – 62 . doi: 10.1182/blood.2020008759
Krishnamoorthy S , Ghobadi A , Santos RD , Schilling JD , Malone AF , Murad H , et al. . CAR-T therapy in solid organ transplant recipients with treatment refractory posttransplant lymphoproliferative disorder . Am J Transplant 2021 , 21 , 809 – 14 . doi: 10.1111/ajt.16367
Oren D , DeFilippis EM , Lotan D , Clerkin KJ , Fried J , Reshef R , et al. . Successful CAR T cell therapy in a heart and kidney transplant recipient with refractory PTLD . JACC CardioOncol 2022 , 4 , 713 – 6 . doi: 10.1016/j.jaccao.2022.09.002
Pecher AC , Hensen L , Klein R , Schairer R , Lutz K , Atar D , et al. . CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome . JAMA 2023 , 329 , 2154 – 62 . doi: 10.1001/jama.2023.8753
Lee J , Lundgren DK , Mao X , Manfredo-Vieira S , Nunez-Cruz S , Williams EF , et al. . Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris . J Clin Invest 2020 , 130 , 6317 – 24 . doi: 10.1172/JCI138416
Zhang L , Sosinowski T , Cox AR , Cepeda JR , Sekhar NS , Hartig SM , et al. . Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes . J Autoimmun 2019 , 96 , 50 – 8 . doi: 10.1016/j.jaut.2018.08.004
Guidetti A , Perrone G , Coluccia P , Fumagalli L , Dodero A , Farina L , et al. . The real life accessibility to CAR T-cell therapy: current experience in the only active center in Italy . Blood 2019 , 134 , 5619 – 5619 . doi: 10.1182/blood-2019-125286
Jo S , Das S , Williams A , Chretien A-S , Pagliardini T , Le Roy A , et al. . Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing . Nat Commun 2022 , 13 , 3453 . doi: 10.1038/s41467-022-30896-2
Bacac M , Colombetti S , Herter S , Sam J , Perro M , Chen S , et al. . CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies . Clin Cancer Res 2018 , 24 , 4785 – 97 . doi: 10.1158/1078-0432.CCR-18-0455
Ball ED , Guyre PM , Mills L , Fisher J , Dinces NB , Fanger MW. Initial trial of bispecific antibody-mediated immunotherapy of CD15-bearing tumors: cytotoxicity of human tumor cells using a bispecific antibody comprised of anti-CD15 (MoAb PM81) and anti-CD64/Fc gamma RI (MoAb 32) . J Hematother 1992 , 1 , 85 – 94 . doi: 10.1089/scd.1.1992.1.85
Seimetz D , Lindhofer H , Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy . Cancer Treat Rev 2010 , 36 , 458 – 67 . doi: 10.1016/j.ctrv.2010.03.001
Przepiorka D , Ko CW , Deisseroth A , Yancey CL , Candau-Chacon R , Chiu HJ , et al. . FDA approval: blinatumomab . Clin Cancer Res 2015 , 21 , 4035 – 9 . doi: 10.1158/1078-0432.CCR-15-0612
Tapia-Galisteo A , Álvarez-Vallina L , Sanz L. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies . J Hematol Oncol 2023 , 16 , 83 . doi: 10.1186/s13045-023-01482-w
Kontermann RE , Brinkmann U. Bispecific antibodies . Drug Discov Today 2015 , 20 , 838 – 47 . doi: 10.1016/j.drudis.2015.02.008
Gruber M , Schodin BA , Wilson ER , Kranz DM. Efficient tumor cell lysis mediated by a bispecific single chain antibody expressed in Escherichia coli . J Immunol 1994 , 152 , 5368 – 74 .
Ridgway JB , Presta LG , Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization . Protein Eng 1996 , 9 , 617 – 21 . doi: 10.1093/protein/9.7.617
Klein C , Sustmann C , Thomas M , Stubenrauch K , Croasdale R , Schanzer J , et al. . Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies . MAbs 2012 , 4 , 653 – 63 . doi: 10.4161/mabs.21379
Surowka M , Schaefer W , Klein C. Ten years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins . mAbs 2021 , 13 , 1967714 . doi: 10.1080/19420862.2021.1967714
Hoffmann P , Hofmeister R , Brischwein K , Brandl C , Crommer S , Bargou R , et al. . Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct . Int J Cancer 2005 , 115 , 98 – 104 . doi: 10.1002/ijc.20908
d’Argouges S , Wissing S , Brandl C , Prang N , Lutterbuese R , Kozhich A , et al. . Combination of rituximab with blinatumomab (MT103/MEDI-538), a T cell-engaging CD19-/CD3-bispecific antibody, for highly efficient lysis of human B lymphoma cells . Leuk Res 2009 , 33 , 465 – 73 . doi: 10.1016/j.leukres.2008.08.025
Sun LL , Ellerman D , Mathieu M , Hristopoulos M , Chen X , Li Y , et al. . Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies . Sci Transl Med 2015 , 7 , 287ra70 – ra70 . doi: 10.1126/scitranslmed.aaa4802
Engelberts PJ , Hiemstra IH , de Jong B , Schuurhuis DH , Meesters J , Beltran Hernandez I , et al. . DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing . EBioMedicine 2020 , 52 , 102625 . doi: 10.1016/j.ebiom.2019.102625
Klein C , Neumann C , Fauti T , Weinzierl T , Freimoser-Grundschober A , Waldhauer I , et al. . Abstract 3629: Engineering a novel asymmetric head-to-tail 2 + 1 T-cell bispecific (2 + 1 TCB) IgG antibody platform with superior T-cell killing compared to 1 + 1 asymmetric TCBs . Cancer Res 2017 , 77 , 3629 – 3629 . doi: 10.1158/1538-7445.am2017-3629
Grakoui A , Bromley SK , Sumen C , Davis MM , Shaw AS , Allen PM , et al. . The immunological synapse: a molecular machine controlling T cell activation . Science 1999 , 285 , 221 – 7 . doi: 10.1126/science.285.5425.221
Martz E. Multiple target cell killing by the cytolytic T lymphocyte and the mechanism of cytotoxicity . Transplantation 1976 , 21 , 5 – 11 . doi: 10.1097/00007890-197601000-00002
NICE. Axicabtagene ciloleucel for treating relapsed or refractory diffuse large B-cell lymphoma after first-line chemoimmunotherapy . Technology appraisal guidance [TA895]. 2023 , 1 – 24 .
NICE . Blinatumomab for treating acute lymphoblastic leukaemia in remission with minimal residual disease activity . Technology appraisal guidance [TA589] . 2019 , 1 – 24 .
Ross SL , Sherman M , McElroy PL , Lofgren JA , Moody G , Baeuerle PA , et al. . Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing . PLoS One 2017 , 12 , e0183390 . doi: 10.1371/journal.pone.0183390
Rainone M , Ngo D , Baird JH , Budde LE , Htut M , Aldoss I , et al. . Interferon-γ blockade in CAR T-cell therapy-associated macrophage activation syndrome/hemophagocytic lymphohistiocytosis . Blood Adv 2023 , 7 , 533 – 6 . doi: 10.1182/bloodadvances.2022008256
Lerkvaleekul B , Vilaiyuk S. Macrophage activation syndrome: early diagnosis is key . Open Access Rheumatol 2018 , 10 , 117 – 28 . doi: 10.2147/OARRR.S151013
Zhang C , Liu J , Zhong JF , Zhang X. Engineering CAR-T cells . Biomarker Res 2017 , 5 , 1 – 6 .
Crespo J , Sun H , Welling TH , Tian Z , Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment . Curr Opin Immunol 2013 , 25 , 214 – 21 . doi: 10.1016/j.coi.2012.12.003
Zhang K , Kong X , Li Y , Wang Z , Zhang L , Xuan L. PD-1/PD-L1 Inhibitors in patients with preexisting autoimmune diseases . Front Pharmacol 2022 , 13 , 854967 . doi: 10.3389/fphar.2022.854967
Wu L , Seung E , Xu L , Rao E , Lord DM , Wei RR , et al. . Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation . Nat Cancer 2020 , 1 , 86 – 98 . doi: 10.1038/s43018-019-0004-z
McKinney EF , Lee JC , Jayne DRW , Lyons PA , Smith KGC. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection . Nature 2015 , 523 , 612 – 6 . doi: 10.1038/nature14468
Singh A , Dees S , Grewal IS. Overcoming the challenges associated with CD3+ T-cell redirection in cancer . Br J Cancer 2021 , 124 , 1037 – 48 . doi: 10.1038/s41416-020-01225-5
Le RQ , Li L , Yuan W , Shord SS , Nie L , Habtemariam BA , et al. . FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome . Oncologist 2018 , 23 , 943 – 7 . doi: 10.1634/theoncologist.2018-0028
Leclercq G , Haegel H , Toso A , Zimmermann T , Green L , Steinhoff N , et al. . JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy . J ImmunoTher Cancer 2022 , 10 , e003766 . doi: 10.1136/jitc-2021-003766
Zhou S , Liu M , Ren F , Meng X , Yu J. The landscape of bispecific T cell engager in cancer treatment . Biomarker Res 2021 , 9 , 1 – 23 .
Nakamura M , Ogawa N , Shalabi A , Maley WR , Longo D , Burdick JF. Positive effect on T-cell regulatory apoptosis by mycophenolate mofetil . Clin Transplant 2001 , 15 , 36 – 40 . doi: 10.1034/j.1399-0012.2001.00006.x
Eugui EM , Mirkovich A , Allison AC. Lymphocyte-selective antiproliferative and immunosuppressive effects of mycophenolic acid in mice . Scand J Immunol 1991 , 33 , 175 – 83 . doi: 10.1111/j.1365-3083.1991.tb03747.x
Prémaud A , Rousseau A , Johnson G , Canivet C , Gandia P , Muscari F , et al. . Inhibition of T-cell activation and proliferation by mycophenolic acid in patients awaiting liver transplantation: PK/PD relationships . Pharmacol Res 2011 , 63 , 432 – 8 . doi: 10.1016/j.phrs.2011.01.005
Ellebrecht CT , Bhoj VG , Nace A , Choi EJ , Mao X , Cho MJ , et al. . Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease . Science 2016 , 353 , 179 – 84 . doi: 10.1126/science.aaf6756
Reddy V , Jayne D , Close D , Isenberg D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design . Arthritis Res Ther 2013 , 15 , S2 – 16 . doi: 10.1186/ar3910
| Month: | Total Views: |
|---|---|
| April 2024 | 561 |
| May 2024 | 1,003 |
| June 2024 | 1,015 |
Email alerts
Citing articles via.
- About Clinical & Experimental Immunology
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1365-2249
- Copyright © 2024 British Society for Immunology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

COMMENTS
We calculated £509,003 in non-medical costs over a year and £435,488 in medical costs generated over 3 months. People with multiple sclerosis reported self-funding 75% of non-medical costs with non-medical interventions having long-term potential benefits.
Day-case appointments formed the largest medical cost group, closely followed by consultations. ... MSO901727 Supplemental material for Personal and societal costs of multiple sclerosis in the UK: A population-based MS Registry study by Richard S Nicholas, Martin L Heaven, Rodden M Middleton, Manoj Chevli, Ruth Pulikottil-Jacob, Kerina H Jones ...
We estimate that there are over 130,000 people with MS in the UK, and that each year nearly 7,000 people are newly diagnosed. This means around 1 in every 500 people in the UK lives with MS, and each week over 130 people are diagnosed with MS. Table 1. Estimates for the prevalence of MS in men and women in the UK. Table 2.
Mental health of people with multiple sclerosis during the COVID-19 outbreak: A prospective cohort and cross-sectional case-control study of the UK MS Register Mult Scler. 2022 Jun;28 ... prospective longitudinal cohort and cross-sectional case-control online questionnaire study. It includes 2010 pwMS from the UK MS Register and 380 people ...
Results . 728 participants with MS were matched with healthy controls (n = 2,912) using a propensity score approach.In a multivariable analysis, compared to those who scored low in the composite lifestyle score (0-1 healthy lifestyle factors), people who adopted all four low risk lifestyle factors showed a 71% lower odds of having MS (OR = 0.29; 95% CI: 0.15-0.56).
Garjani A, Hunter R, Law GR, et al. Mental health of people with multiple sclerosis during the COVID-19 outbreak: A prospective cohort and cross-sectional case-control study of the UK MS Register. Mult Scler 2021;:13524585211020436.
Objectives To estimate the incidence and prevalence of multiple sclerosis (MS) by age and describe secular trends and geographic variations within the UK over the 20-year period between 1990 and 2010 and hence to provide updated information on the impact of MS throughout the UK. Design A descriptive study. Setting The study was carried out in the General Practice Research Database (GPRD), a ...
highest prevalence for MS occurs in the 60 to 69 years age group for both sexes (females 598 and males 228 per 100,000 population) 75% of males and females with MS are aged between 40 and 74 years ...
A study of more than 22,000 people with multiple sclerosis has discovered the first genetic variant associated with faster disease progression, which... Read more Cambridge scientists reverse ageing process in rat brain stem cells
This chapter is concerned primarily with establishing an estimate of the number of people with Multiple Sclerosis (PwMS) in the United Kingdom (UK) and the ways in which that number may change through time, the 'components of change'. ... Bull, P.J. (2015). How Many People Have MS? A Case Study of the UK. In: People with Multiple Sclerosis ...
We conducted a nested case-control study using data from the UK Clinical Practice Research Datalink. Multiple sclerosis cases diagnosed from 2001 until 2022 were identified from electronic healthcare records and matched to unaffected controls based on year of birth. ... Case-control study of multiple sclerosis risk in CPRD Aurum ...
Oct 2017. Case Studies in Multiple Sclerosis. pp.27-32. A 28-year-old woman with known relapsing-remitting multiple sclerosis (RRMS) for 2 years was seen in the emergency room for a subacute onset ...
Adherence to a healthy lifestyle and multiple sclerosis: a case-control study from the UK Biobank. Nicola Veronese, Lin Yang, Laura Piccio, Lee Smith, Joseph Firth, Wolfgang Marx, Gianluigi Giannelli, Maria Gabriella Caruso, Anna Maria Cisternino, Maria Notarnicola, Rossella Donghia, Mario Barbagallo, Luigi Fontana ... Lin ; Piccio, Laura et ...
by Alice Melão, MSc July 17, 2018. Cladridine may be effective in preventing disability progression and reducing damage to nerve cells in people with progressive forms of multiple sclerosis (MS ...
Background: Multiple sclerosis (MS) is a common and disabling condition. The importance of healthy lifestyle for this disease is poorly explored. Objective: To test whether adherence to healthier lifestyle patterns is associated with a lower presence of multiple sclerosis (MS). Methods: By using a case-control design, we investigated the combined association of four healthy lifestyle-related ...
multiple sclerosis case, with an increase in the MDS. Certain food groups and individual foods may also be associated with an increased or decreased association of being a multiple sclerosis case. This study may have significant effects on health planning in The Republic of Cyprus.
Clinical Presentation: Case History # 1. Ms. C is a 35 year old white female. She came to Neurology Clinic for evaluation of her long-term neurologic complaints. The patient relates that for many years she had noticed some significant changes in neurologic functions, particularly heat intolerance precipitating a stumbling gait and a tendency to ...
Introduction. Multiple sclerosis (MS) is a chronic, progressive neurological disorder, and is the major cause of non-traumatic disability in young adults [].Mortality rates are significantly higher in people with MS compared with the general population [2-4], yet causes of death and factors influencing survival in MS patients are not well understood, and further data addressing these ...
A total of 4455 females were included: 891 cases and 3564 controls. No association was seen between oral contraceptive exposure and subsequent MS, or between any contraceptive, combined oral contraceptive pill (COCP) or progesterone-only pill (POP) use 0-2, 2-5 or >5 years prior to MS. Conclusions: In the largest population-based study to date, we find no evidence of an association between ...
METHODS: Using a case-control design, participants from Canadian, UK Biobank, and United States cohorts were grouped into cases (MS/comorbid anxiety) or controls (MS/no anxiety, anxiety/no immune disease or healthy). We used multiple anxiety measures: current symptoms, lifetime interview-diagnosed, and lifetime self-report physician-diagnosed.
Multiple Sclerosis (MS) is a complex autoimmune disorder that significantly impacts the central nervous system, leading to a range of complications. While intracranial haemorrhage (ICH) is a rare but highly morbid complication, more common CNS complications include progressive multifocal leukoencephalopathy (PML) and other CNS infections. This severe form of stroke, known for its high ...
Background: Multiple sclerosis (MS) is a chronic inflammatory disease affecting the central nervous system. While previous studies have indicated that albumin, the primary protein in human plasma, may exert influence on the inflammatory process and confer beneficial effects in neurodegenerative disorders, its role in the context of MS has been underexplored.
Digital health technologies (DHTs) have become progressively more integrated into the healthcare of people with multiple sclerosis (MS). To ensure that DHTs meet end-users' needs, it is ...
Incidence and prevalence of multiple sclerosis in the UK 1990-2010: a descriptive study in the General Practice Research Database ... Van der Mei IA, Ponsonby AL, Dwyer T, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 2003; 327:316. [PMC free article] [Google Scholar] 25.
People who start taking medication soon after the first signs of multiple sclerosis (MS) may have a lower risk of disability later, a study suggests. Symptoms of MS may include fatigue, numbness ...
Multiple sclerosis (MS) is a chronic inflammatory disease affecting the central nervous system. While previous studies have indicated that albumin, the primary protein in human plasma, may exert influence on the inflammatory process and confer beneficial effects in neurodegenerative disorders, its role in the context of MS has been ...
Case Study. Bob is 65 and has had multiple sclerosis for 15 years. He has a wheelchair and drives a specially adapted car. He lives with his wife, Jean, in a cottage in the country and they have always been involved in several community and church activates. Jean is Bob's main carer and although Bob is quite independent, Jean tends to do ...
Researchers at the University of Cambridge say their discovery of "new rules of the immune system" could improve the treatment of inflammatory diseases such as multiple sclerosis (MS).
The mean age of onset is from 28 to 31 years. The age of onset varies among the clinical subtypes (phenotypes). RRMS has an earlier onset, averaging between 25 and 29 years, with SPMS presenting at a mean age between 40 and 49 years of age. The estimated male to female ratio is 1.4-2.3 to 1.
With regard to other autoimmune diseases, single case studies of anti-CD19 CAR T-cell therapy indicate a potential use of the approach also in anti-synthetase syndrome and systemic sclerosis . To note, an important potential confounder when appraising the mechanisms of response to CAR T-cell therapy is the use of lymphocyte depletion with ...