WAEC Chemistry Questions and Answers 2024 Objectives and Essay
- Post author: Study Admin
- Post published: November 19, 2023
- Post category: School News
- Post comments: 0 Comments
WAEC chemistry 2024 answers are now available. WAEC chemistry questions and answers 2024/2025 objective and essay and other exam details for WASSCE 2024 are on this page. See the 2024 WAEC chemistry answers for both objective and theory below. Get the WAEC chemistry objective and essay answers here.
The 2024 chemistry WAEC OBJ and theory questions and answers are provided here for free. All you have to do is to go through the questions and take note of the WAEC chemistry answers 2024. Read on to find out.

WAEC Chemistry Questions and Answers 2024 Objective and Essay
Have you been searching on Google in order to get the WASSCE chemistry questions and answers 2024? If so, we have got you covered!
We have the 2024 WAEC chemistry questions and our team of experts will soon upload the WAEC chemistry questions and their accurate answers to help you pass the 2024 WAEC chemistry examination.
The 2024 WAEC chemistry theory questions and OBJ will be uploaded any moment from now. So if you are searching for the WAEC chemistry answers 2024 for objective and theory, then you are on the right page. See WAEC chemistry objective and essay questions and answers below.
WAEC Chemistry Answers 2024 Objective and Theory
The West Africa Examinations Council (WAEC) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in May/June and November/December respectively.
The 2024 WAEC chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year’s examination are in the syllabus, and nearly 90% of the questions are repeated.
You don’t have to worry about the 2024 WAEC chemistry questions and answers PDF (essay and objective). The WAEC chemistry answers 2024 will be uploaded any moment from now. All you need to do is to keep refreshing this page so as not to miss out.
Once again, keep refreshing this page because we will upload the original WAEC chemistry questions and answers for this year’s exams on this page at any moment from now. Also, to download the past questions and answers, click on this link WAEC chemistry past questions .
If you have any questions about the WAEC chemistry questions 2024 and answers, feel free to use the comment box below or use the Chat With Us button and we will respond immediately.
The 2024 WAEC chemistry answers will be posted here. Be patient. Keep checking and reloading this page for the correct answers. WAEC 2024 chemistry answers loading…….
There is nothing like WAEC chemistry expo 2024 online. All students are advised to avoid all patronizing online fraudsters/vendors who claim to provide such services.
You Might Also Like
Ahmadu bello university post utme form 2023/2024, list of courses offered at the federal university gashua, jamb subject combination for agriculture, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

- Username Password Remember me Sign in New here ? Join Us

Chemistry 2023 WAEC Past Questions
The vapour density of an organic compound with the molecular formula \(C_2H_4O_2\), is [H=1.0, C=12.0, O= 16.0]
The following statements are correct except
- A. energy is released when liquids change to solids.
- B. carbon atoms in gaseous methane are further apart than those in solid diamond.
- C. there is large decrease in the volume of a solid metal when pressure is applied to it.
- D. particles move faster in the gaseous state than in the liquid state.
Which of the following reactions represents the hydrolysis of an alkanoate?
- A. \(CH_3COOH + OH^- ⇌H^+⇌ CH_3COO^- + H_2O\)
- B. \(CH_3COOH + C_2H_5OH ⇌H^+⇌ CH_3COOC_2H_5 + H_2O\)
- C. \(CH_3COOC_2H_5 + H_2O ⇌H^+⇌ CH_3COO^- + CH_3CH_2OH\)
- D. \(CH_3COOCH_2CH_3 + H_2O ⇌H^+⇌ CH_3COOH + CH_3CH_2OH\)
The following steps are scientific methods except
- A. experimentation
- B. problem identification
- C. Analysis
- D. Open-mindedness
Alkenes can be manufactured by
- A. the combustion of alkanes.
- B. the cracking of hydrocarbons.
- C. polymerization reactions.
- D. addition of hydrogen to unsaturated vegetable oils.
- Mathematics
- English Language
- Animal Husbandry
- Literature in English
- Accounts - Principles of Accounts
- Christian Religious Knowledge (CRK)
- Agricultural Science
- Islamic Religious Knowledge (IRK)
- Civic Education
- Further Mathematics
- Home Economics
- Book Keeping
- Data Processing
- Catering Craft Practice
- Computer Studies
- Physical Education
- Office Practice
- Technical Drawing
- Food and Nutrition
- Home Management

Home » EXAM NEWS » WAEC Chemistry Objective And Theory Answers 2023/2024
WAEC Chemistry Objective And Theory Answers 2023/2024

Waec Chemistry Questions and Answers for free to all Weac candidates In Ghana, Liberia, Nigeria, Sierra Leone, and The Gambia. On this page, all the Waec Chemistry questions and answers for 2023 and the most common questions and answers are released here.
Waec Candidates that applied for the West African Examination Council (WAEC) SSCE Examination will write their Waec Chemistry For Science students. All details you need for you to be successful and pass this 2023 Waec Exam will also be given and make sure you read all through.
2023 Waec Chemistry Exam Papers
2023 Waec Chemistry Exam Papers Are
- Waec Chemistry Essay Questions
- Waec Chemistry Objectives Questions,
You are writing the 2 papers in only one day. In this post, the previous Year’s Waec questions and answers for Chemistry are released and the 2023 Waec Chemistry Exam Questions will also be released for those participating in the 2023 Waec examination.
2023 Waec Chemistry Questions and Answers Objective (paper 1)
The 2023 Waec Chemistry questions and answers loading! 2023 Chemistry objective answers Loading!! 2023 Waec Chemistry Theory Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped.
Previous Year WAEC Chemistry OBJ ANSWERS
CHEM-OBJ 1caacabcdbc 11babaadbdca 21bccaabcdcb 31abcbcccbbc 41abdbbbaabb Chem-Theory-Answers
==================================
2023 Waec Chemistry Questions and Answers THEORY (paper 2)
The 2023 Waec Chemistry Theory questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry Theory Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped.
3d) VIEW IMAGE HERE – CLICK
3ai) structural isomerism is the existence of two or more compounds ( known as isomers) with the same molecular formula but different molecular structures
3ai) i) tertiary alkanol ii) secondary alkanol iii) primary alkanol
3bi) i) 2[CH3 CH2 COOH ] 2K(s)—-> ii) [CH3 CH2 COO ] K + H2 i) 2[CH3 CH2 COOH ] +2 K(s) ——> 2[ CH3 CH2 COO ] K + H2
ii) CH3 CH2 COOH + C4 H9OH HEAT—->CH3 CH2 COO C4 H9 + H20 H2SO4
iii)CH3 CH2 CH2 CH20H + H^+ KMnO4——> CH3 CH2 CH2 CH2 – 0H EXCESS
3bii) i) potassium ethanoate,hydrogen ii) butyl propanoate and water iii) enthanal
3c) The percentage composition of the hydrocarbon = 100 H + C = 100 14.3 + C =100 C= 100 – 14.3 C= 85.7% ================
1a) nucleons is the collective name for two important sub-atomic particles:neutrons and protons
1b) Graham’s law of diffusion states that at a constant temperature and presure,the rate of diffusion of a gas is inversely proportional to the square root of it density
1c) because when aluminum is exposed to moist air,a thin continuous coating of aluminum oxide is formed, which prevents futher attack of the aluminium by atmospheric oxygen and water or steam under normal conditions
1d) i) electron – affinity ii) electron – negativity iii) ionization – energy
1e) i) the molecule of real gases occupies space and there are forces of the attraction between them ii) real gases do liquefy when their temperature droops.
1f) i)used in seperation of different component of crude oil. ii)it is used for separating acetonic from water iii) it is used for obtaining different gases from air for industrial use.
1g) i)concentration of ions in electrolyte ii)the nature of the electrode iii)the position of ions in the electrochemical series
1h) i)substitution reaction ii)addition reaction
1i) i)carbon dioxide ii)water vapour iii) carbon monoxide
1j) i)centrifugation ii)sieving iii)evaporation to dryness ==================================
2a) i) isotopy ii) they have the same number of proton (atomic number) but different mass number iii) oxygen,carbon iv)1s^2,2s^2,2p^6,3s^2,3p^5
2bi) PHYSICAL PROPERTIES METAL i) high melting and boiling point ii) they are good conductor of heat and electricity
NON-MATEL i)they have lower melting and boiling point ii)they are poor conductors of heat and electricity.
CHEMICAL PROPERTIES METAL i) they form basic and ampheric oxides ii)the react by electron lose or donation of electron NON-METAL i)the form acidic oxides ii)the react by sharing and accepting electrons
2bii) i) Al2 O3(Aluminum oxide) ii) sodium hydride (NaH) iii)zinc trioxocarbonate(iv)ZnCo3 iv)silicon tetrachloride (SiCl4)
2ci) i) variable oxidation states ii) complex ion formation iii) they possess strong metallic bonding
2cii) 1s^2,2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2
2ciii) Zinc is not considered as a typical transition element bacause it has only one oxidation state of +2, it metallic ion are not coloured and it is not used as a catalyst.
2d) Na2Co3 + MgCl2 —->2NaCl + MgCo3 1mole 1mole 2mole 1mole molar mass of Na2 Co3 = 2 * 23 + 12 * 1 + 16 * 3 = 46+12+48 = 106g ==================================
5ai) Na2S2O3 ===>2 + 2x + 6 = 0 2x = 6 – 2 = 4 X = 4/2 X = 2 The oxidation number of sulphur is 2
5aii) Rhombic&monoclinic
5aiii) – Both are tetravalent – both are allotrope of carbon
5bi) CO2&Chloroflorocarbon
5bii) There is increase in sun radiation reaching the earths surface ie Global warming
5biii) ThunderStorm
5iv) I2KNo3. ——–>2KNO3 + O2 2AgNo3 ———>. 2Ag + 2No
5ci) Calcium chloride in a solution can give rise to crystal using filtration and evaporation to dryness. The sol is filtered into filtrate and residue b4 evaporation to dryness takes place.
5cii) – Because of presence of hydrogen bonding in NH3 – Because Iodine as higher molecular mass than chlorine
5di) Mol of Nacl = Mass / MM = 5.85/ 58.01 = 0.1mol From the equation 2mol of Nacl gives 2mol of HCL, 0.1mol of Nacl gives 0.1mol of Hcl Vol of Hcl = 0.1 x 22.4 = 2.24mol/dm^3 ================
Practice Waec Chemistry Questions On Objective
If you can be able to access these questions with the appropriate answers without using textbooks or materials you are 80% ready for the 2023 Chemistry Exam. Kindly submit the answer in the comment section below and if you are interested in the 2023 Waec Chemistry answers kindly show interest.
The components of universal indicator solution can best be separated by
A. Chromatography B. Filtration C. Evaporation D. Crystallization E. Fractional distillation
The number of replaceable hydrogen atoms in one acid indicates its
A. Basicity B. Acidity C. Alkalinity D. Reactivity E. PH value
Catalytic hydrogenation of alkenes produces compounds with the general formula
A. CnH2n+1 OH B. CnH2n+1 C. CnH2n+2 D. CnH2n-2 E. Cx(H2O)y
A measure of the degree of disorder in a chemical system is known as the
A. Enthalpy B. Free energy C. Activation energy D. Entrophy E. Equilibrium
Which of the following occurs when an aqueous solution of sodium hydroxide is electrolysed using graphite electrodes?
A. Sodium metal is produced at the anode B. Sodium amalgam is formed at the cathode C. Oxygen gas is produced at the anode D. The grahite anode dissolves E. The resulting solution becomes acidic
If the volume of a given mass of gas at 0oC is 27.3cm3, what will be the volume of the gas at 10oC, pressure remaining constant?
A. 2.73cm3 B. 28.3cm3 C. 37.3cm3 D. 273cm3 E. 283cm3
Glucose can be obtained from starch by
A. Hydrogenation B. Dissoiation C. Hydrolysis D. Dialysis E. Dehydration
How many faradays of electricity are required to liberate 9g aluminium? (AI = 27)
A. 0.1 B. 0.3 C. 1.0 D. 2.7 E. 3.0
When water is dropped on calcium carbide, the gaseous product is an
A. Alkane B. Alkene C. Alkyne D. Alkanol E. Alkanal
Practice Waec Chemistry Questions On Theory
a) Define each of the following terms and indicate one use of each: (i) Nuclear fission; (ii) Nuclear fusion. (b) Alpha particle emission by 29325� proceduces an element A. Beta particle emission by the particle A produces another element B. Element B also undergoes alpha particle emission to produce 22789��. Write balanced equations to represent the above statement. (c) The models below represent the filling of orbitals in an atom.
State which rule(s) is/are violated or obeyed by each model. (d) Explain why the boiling point of H2S with relative molecular mass of 34 is lower than that of H2O with relative molecular mass of 18.
(e) HCI is passed into each of the following solvents: (i) water; (ii) methylbenzene. I. State the effect of each solution on blue litmus paper II. Compare the electrical conductivities of the two solutions.
(f) Zinc dust is added to copper (II) tetraoxosulphate (VI) solution. State; (i) what is observed; (ii) the type of reaction that occurs.
Question 2 :
(a)(i) State two differences betwecii the properties of solids and gases (ii) What process does each of X, Y and Z represent in the changes shown below?

Waec Chemistry Question and Answers
(b)(i) State Charles’ Law (ii) Draw a sketch to graphically illustrate Charles’ Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory. (ii) Consider the reaction represented by the Solid or Liquid following equation: H2(�) + Cl2(�) → 2HCI(�) Use the kinetic theory to explain how the rate of formation of HCI(�) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case that produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.
The 2023 Waec Chemistry Theory and objective questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry objective Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped.
Before you leave this page kindly make sure you understand and know how WASSCE grades your WAEC Subject. The reason why we are doing this, is not to frighten or scare you but to put you in check and see reasons why you need to be serious with your Government study for the 2023 Government Exam
Waec Grading System For all Waec Candidates
Do you know that the West African Examination Council (WAEC) Board has published the Waec grading system of results? Kindly check below to see the meaning of the Waec Grading Result .
The table below shows candidates’ positioning of the Waec grading of results ranging from A1, B2, B3, C4, C5, C6, D7, E8, F9 are the complete list of Waec Grading Result. All Waec candidates must fall into one of the Waec Grading Systems.
| A1 | 1 | EXCELLENT |
| B2 | 2 | VERY GOOD |
| B3 | 3 | GOOD |
| C4 | 4 | CREDIT |
| C5 | 5 | CREDIT |
| C6 | 6 | CREDIT |
| D7 | 7 | PASS |
| E8 | 8 | PASS |
| F9 | 9 | FAIL |
WAEC Gradi n g Percentage Scores
- A1 Excellent 75% – 100%
- B2 Very good 70% – 74%
- B3 Good 65% – 69%
- C4 Credit 60% – 64%
- C5 Credit 55% – 59%
- C6 Credit 50% – 54%
- D7 Pass 45% – 49%
- E8 Pass 40% – 45%
- F9 Failure 0% – 44%
If you have been wondering where to get the best and latest Waec news updates and guide about Waec 2023, how to pass Waec, a timetable for 2022/2023 Waec, free online and hardcopy Waec past questions, and hot topics to read for Waec, latest news updates and Waec CBT practice platform, then subscribe to the newsletter or join our Forum
If actually, this information is awesome and useful to you please kindly share using via Facebook, WhatsApp, Twitter, and Google+
You may also like

MAY/JUNE 2024 WAEC Chemistry Practical Specimen For...

May/June WAEC Agricultural Practical Specimen For 2024

2024 WAEC EXAM TIMETABLE FOR ALL WAEC CANDIDATES PDF...

2024 JAMB DAY1 EXAM QUESTIONS AND ANSWERS FOR JAMB...

Approved Jamb Cut-off Mark For Law Into Nigeria Law...

How Many Candidates Registered For 2024 UTME JAMB Exam...
About the author.
38 Comments
Please answer some of these questions for me
If are are interested in Waec Chemistry Practical Answers
WhatsApp us now
09035742503
TO GET THE 2023 WAEC CHEMISTRY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…….
NO TIME TO WASTE …..
We are recommending all our users to WAEC Worker who helped people on physics
Chat him for help
All that to him
Am interested
Those of you that want to receive chemistry Practical Answers fast
WhatsApp us now not free
TO GET THE 2023 WAEC CHEMISTRY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
please I need it
Check your gmail
Please, kindly notify me when the correct questions and answers for chemistry WAEC 2023 have dropped
Please help me send chemistry answers for 2023waec
How can i get the question and answers for chemistry
WhatsApp me You’ll get WAEC 2023 theory Questions and Answers immediately. WhatsApp 07085160362
TO GET THE 2023 WAEC CHEMISTRY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW.
NO TIME TO WASTE
2023 WAEC theory Questions and Answers WhatsApp 07085160362 Get the answers an hour before the exam.
Do you have for chemistry theory too??
Chemistry theory WhatsApp me on 07085160362 You’ll receive answers 1hr before the Tim for the exams.
I just WhatsApp you sir
TO GET THE 2023 WAEC CHEMISTRY OBJ & THEORY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
Is the question real But I cannot do it because it my mummy phone please help
I am interested in the chemistry essay and objective for 2023…
I just send you a message And the question should be send to me before tomorrow so I can do some practice
And the question should be send to me before tomorrow so I can do some
Pls how can i get chemistry Question before the exam
2023 WAEC CHEMISTRY OBJ & THEORY QUESTIONS AND ANSWERS ARE NOW AVAILABLE.. TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
NO TIME TO WASTE….
2023 WAEC CHEMISTRY OBJ & THEORY QUESTIONS AND ANSWERS ARE NOW AVAILABLE.. TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW……
NO TIME TO WASTE…….
Please i need in now
Please what if I don’t have WhatsApp account
Like aw many minutes before the exam and is it free
Please if there is waec group or your group add me up 08052263004
what about the ones above are they not correct ?
I enjoyed the site…and want to be participating in this site…..
1A,B,CDCBCDB
Leave a Comment X
Save my name, email, and website in this browser for the next time I comment.
Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here
WAEC GCE Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
WAEC GCE Chemistry Questions and Answers 2023 . Welcome to 2023 WAEC Chemistry Questions and Answers. You will find WAEC GCE Chemistry Objective Answers, WAEC Chemistry Essay 2023, WAEC GCE 2023 Chemistry, and the tips you need to pass your WAEC GCE Chemistry examination with ease.

Table of Contents
WAEC GCE Chemistry Questions and Answers 2023 (Expo)
The 2023 WAEC GCE Chemistry expo will be posted here during the WAEC GCE Chemistry examination. Keep checking and reloading this page for the answers.
WAEC GCE 2023 Chemistry Answers Loading.. .
Today’s WAEC GCE Chemistry OBJ Answers:
———————————————————————————–
Note: The answers below are the 2020 Nov/Dec answers.
NOTE: Pls Trace It From Your Objective. [If you see any options here pick it from your objectives]
1 Involves the loss and gain of electrons 2 Polymerisation 3 Global warming 4 3 5 Zinc ions 6 +1.56V 7 Ethene 8 NH3 9 Propanol 10 28 11 Hydrolysis 12 d-orbital 13 Lowering the activation energy 14 Closeness between reactant particles 15 Remaining the same with time 16 Reaction vessel Fels cool during the reaction 17 Faster 18 Solvent extraction 19 Saturated Solution 20 2.75mol/dm³ 21 Partially dissociates in aqueous solution 22 138g 23 HI 24 2.00cm³ 25 hydrogen chloride 26 strong electrovalent bond between ions 27 is not ductile 28 Electrons 29 C2H4 30 Aluminium 31 0.010mol/dm³ 32 PbCO3 33 Linear 34 HCL and HOCL 35 +1 36 Ionic bond 37 have relatively low ionization energy 38 sour to taste 39 I,III and IV only 40 chrometography 41 2.00 dm 42 mole of solvent in 1dm³ of solution 43 does not contain neutron 44 1s²2s²2p⁶ 45 Ammonium chloride 46 IV 47 I,II and IV only 48 Quantum numbers of Electrons 49 -273⁰C 50 Mass number
1-10: DDDADBBABA
11-20: CDABDCCDDC
21-30: ACDBDCCDAB
31-40: BCADABDBAC
More Answers loading…
WAEC GCE Chemistry Theory Answers:
(i) Sodium trioxonitrate (v) decahydrate
NaNO₃ . 10H₂O
(ii) Sodium Oxide –> Na₂O
(iii) Potassium tetraoxophospate (v) –> K₃PO₄
Products formed are:
Hydrogen gas (a+ cathode)
Chlorine gas (a+ anode)
it decrease down the group
As the atomic radius increases down the group the attraction of the positive nucleus of the electron outer most electron, thus the ionization energy decreases
Nitrogen and carbon (ii) oxide
it is used to heat furnace
it is a source of nitrogen for the manufacture of ammonia
Sodium hydrogen – used in qualitative analysis
– Purification of bauxite
Sodium trioxocarbonate (iv)
-Manufacture of glass
-As a water soften
2-amino propane
(2ai) Percentage C5H12 of mass m = 7.2g Volume of O2 = 20.0dm³ (i) from the general combustion equation CxHy(g) + (x+y/4)O2 –> XCO2 + y/2H2O C5H12(l) + 802(g) –> 5CO2(g) + 6H2O(l)
(2aii) 1 mole of C5H12(72g) = 5 moles of CO2 At stop 7.2gC5H12 = x volume of CO2 X = 7.2g×5×22.4dm³/72g X = 5×2.224 = 11.2dm³ of CO2
(2aiii) Volume of oxygen left after the reaction from the equation of reaction 1 mole of C5H12(72g) = 8(22.4)dm³ 7.2g = x X = 7.2×8×22.4/72 = 17.92dm³ Volume of O2 left after the reaction = 20.0dm³ – 17.92dm³ = 2.08dm³
(2b) When molecules collide with one another they possess kinetic energy. As most energetic molecules (those with greater kinetic energy) try to escape. Their escape may be facilitated by heat or by passing a wave of air over the container or by increasing the surface area of the container. As they try to do this, some molecules will loose energy on collision and fall back to the container; as such the average kinetic of the molecules of the liquid in the container reduces which results to cooling effect.
(2ci) Avogadro’s Law states that the total number of atoms/molecules of a gas (i.e. the amount of gaseous substance) is directly proportional to the volume occupied by the gas at constant temperature and pressure.
(2cii) N2(g) + 3H2—> 2NH3 Where 1 mole = 30cm³ of gas
At constant temperature, the volume of a fixed mass of gas. When the volume of a cylinder or a container is increased, the gases have more space to travel and collide hence the pressure is reduced but as the volume is decreased or compressed the gases have less space to travel therefore more pressure is built up.
No (3ai) ¹³R, ⁸Q ¹³R=1s²,2s²,2p⁶,3s²,3p¹ ⁸Q=1s²,2s²,2p⁴
(3aii) ¹³R=2,8,3 Valency of ¹³R is 3 ⁸Q= 2,6 Valency of ⁸Q=2
(3di) 2H² SO4(aq)+4NaOH(aq)—>2Na² SO4(aq)+4H²O(s)
(3dii) Sodium teraoxosulphate (iv) salt and water
(3diii) The resulting solution NaSO4 is basic and will have no effect on litmus paper
(3div) When heated to dryness it can be used as a dehydrating agent
Typing….
—————————————————————————————————————–
The questions below are strictly for practice.
1. Which of the following statements best explains the difference between a gas and a vapour?
(a) Unlike gases, vapours are liquids at room temperature
(b) Unlike gases, vapour can easily be condensed into liquids
(c) Unlike gases, vapour is readily converted into solids
(d) Vapours are generally denser than gases
2. Consider the following reaction equation: 2HCI + Ca(OH) 2 → CaCI 2 + H 2 O. what is the volume of 0.1 moldm -3 , HCI that would completely neutralize 25 cm 3 or 0.3 moldm -3 Ca(OH) 2 ?
(a) 150 cm 3
(b) 75 cm 3
(c) 30 cm 3
(d) 25 cm 3
3. Cu and HNO 3 are not suitable for preparing hydrogen gas because of their
(a) Reactivity and oxidation respectively
(b) conductivity and corrosiveness respectively
(c) melting point and reduction respectively
(d) electronegativity and solubility respectively
4. Which of the following formulae cannot be an empirical formula?
(c) P 2 O 5
(d) N 2 O 4
5. One of the criteria for confirming the purity of benzene is to determine its
(a) Heat capacity
(b) boiling point
6. When chlorine is passed through a sample of water, the pH of the water sample would be (a) <7
(a) 1.20 x 10 23
(b) 2.41 x 10 23
(c) 3.62 x 10 23
(d) 4.82 x 10 23
8. The strength of metallic bonds depends on the
(a) charge density of the atoms
(b) ductility of the metal
(c) number of valence electrons
(d) total number of electrons in the atoms
9. When zinc is added to AgNO 3 solution, crystals of silver forms on the zinc surface. This indicates that zinc is
(a) oxidized
(b) reduced
(c) decomposed
(d) dissociated
(d) Cu 2 O 2
- WAEC GCE Mathematics Questions and Answers
- WAEC GCE Physics Questions and Answers
11. The change in the oxidation state of iron in the reaction represented by the equation below is 2FeCI 3 + H 2 S →2FeCI 2 + 2HCI + S
(a) +2 to +3
(b) +3 to +2
(c) 0 to +2
(d) +3 to 0
12. Which of the following methods can be used to separate blood cells from plasma?
(a) Centrifugation
(b) Filtration
(c) Chromatography
(d) Distillation
13. Which of the following statements about ionic radius is correct? ‘Ionic radius
(a) Increases as nuclear charge increases
(b) decreases as nuclear charge increases
(c) decreases as nuclear charges decreases.
(d) remains constant as nuclear charge increases
14. Analysis of a hydrocarbon shows that it contains 0.93 g of carbon per gram of the compound. The mole ratio of carbon to hydrogen in the compound is [H=1.0, C=12.0]
15. The law of definite proportions states that
(a) pure samples of same compound contain the same elements combined in the same proportion by mass
(b) pure samples of substances are in the same proportion by mass
(c) chemical compounds are pure because they contain the same elements
(d) matter can neither be created nor destroyed
17. Atoms are electrically neutral because they
(a) don not conduct electricity
(b) contain equal number of protons and electrons
(c) are composed of neutrons and electrons
(d) cannot be attracted by electromagnetic field
18. Common salt (NaC1) is used for preserving foods. Which of the following properties could be used to determine its purity before use?
(a) Solubility in water
(b) melting point
(c) Relative density
(d) Crystalline nature
19. Which of the following electron configurations represents the transition element chromium ( 24 Cr)?
(a) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4
(b) 1s 2 2 2s 2 2p 6 3s 2 3p 6 3d 6
(c) 1s 2 2s 2 2p 6 2s 2 3d 4 4s 1 (d) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5
20. The atomic number of an isotope of hydrogen is equal to its mass number because it
(a) has a totally filled valence shell
(b) has a high charge to mass ratio
(c) does not contain neutrons (d) exhibits isotopy
21. The total number of shared pair of electrons in the compound below is
22. The bonding pair of electrons in a hydrogen chloride molecule is pulled towards the chlorine atom because
(a) Chlorine has a larger atomic size
(b) chlorine has a large atomic mass
(c) chlorine is more electronegative
(d) there is no bonding orbitals within the hydrogen atom
23. The solubility of CO 2 in water can be accounted for by
(a) van der waal’s forces
(b) ionic attraction
(c) dipole attraction
(d) covalent bonding
24. Which of the following properties would not influence electrovalent bond information?
(a) Electronegativity
(b) Electron affinity
(c) Ionization potential
(d) Catalytic ability
25. Particles in a solid exhibit
(a) Vibrational motion only
(b) vibrational and translational motion
(c) vibrational and random motion
(d) random and translational motion
WAEC GCE Chemistry Essay 2023
The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Physics repeated questions and answers.
These questions are for practice. The 2023 WAEC GCE Chemistry expo will be posted on this page 30 minutes before the WAEC GCE Chemistry examination starts. Keep checking and refreshing this page for the answers.
If you have any questions about the WAEC GCE Chemistry questions and answers, kindly drop your questions in the comment box.
Last Updated on October 2, 2023 by Admin
Related posts:

8 thoughts on “WAEC GCE Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
How can I slove hard question in question
Pls i’m a candidate for gce advance level and i need chemistry papers 2023
Please I need mathematics, chemistry, physics and biology questions
Pls always post me from 2021till 2023 in 9 subject which is Econ, maths Igbo chemistry physics, Agric civic bio, Eng
I need waec English for today
I need past questions on all science subjects please
Pls I need the question for chemistry, physics, biology, agricultural science, english and mathematics
Okay heard you
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

WAEC Chemistry Questions and Answers 2023 | Theory and OBJ- Download PDF
WAEC Chemistry Questions and Answers 2023 | Theory and OBJ- Download PDF. WAEC Chemistry Questions and Answers 2023… If you are about to write the West African Senior Certificate Exams (WASSCE), you will realize that you need the WAEC Past Questions and Answers.
This article provides you with WAEC Chemistry Questions and Answers for the WASSCE 2023. It contains information regarding the WAEC Chemistry Theory and Objective Questions and Answers.
WAEC Chemistry Objective Questions
1. Which of the statements below is correct about Isotopes of the same element?
- WAEC CASS 2023 | Offline Download, Portal and Registration Software.
- WAEC GCE Result 2023/2024 is Out- (August/September 2nd Series Exam)
- WAEC Result 2023/2024 is Out- WAEC Result Checker 2023
- Have the Same number of Protons, neutrons, and electrons.
- Same Protons and neutrons but a different number of electrons.
- Same number Protons and electrons but a different number of neutrons.
- The number of Neutrons and electrons is the same but the number of protons differs.
2. The chemical bond that is formed by the transfer of electrons is known as?
- Covalent Bond
- Dative Bond
- Metallic bond
3. Two electrons can fill the same orbital if only?
- They have different Angular momentum quantum numbers.
- Magnetic quantum numbers differ
- Principal quantum numbers are different.
- Spin quantum numbers not the same
4. Which of the substances listed below is not a hydrocarbon?
Related Posts:
- WAEC Chemistry Practical Questions and Answers…
- NECO Chemistry Past Questions and Answers | Theory…
- WAEC Biology Questions and Answers 2023 | Theory and…
- WAEC Commerce Questions and Answers 2023 | Theory…
- WAEC Economics Questions and Answers 2023 | Theory…
- WAEC English Questions and Answers 2023 | Theory and…
5. The complete ionization of A substance into hydroxonium ions indicates:
- Strong acid.
- Strong base.
6. One of the solutions below that can resist changes in pH when a small quantity of a base or acid is added is?
- Buffer solution
- Neutral solution
- Saturated solution
- Supersaturated solution
7. Write a balanced chemical equation for a 2.47g dry pure copper (II) oxide which is completely reduced copper using a laboratory gas. If the mass of the residue left is found to be 1.97g.
- Cu + O → CuO.
- 2Cu+O 2 → 2CuO
The correct answer is B. 2Cu+O 2 → 2CuO. See how this question is solved in the number one (No.1) question in the theory section.
Use this question to answer question 2 & 3
8. Calculate the mass of anhydrous sodium-trioxocarbonate (IV) present in a 300cm 3 of 0.1M; (Na =23, C=12, O=16).
The correct answer is A. 3.18g. See solving in No.2 ques. in the theory section.
9. The number of Na 2 CO 3 particles present in the solution
- 6.02 X 10 23
- 0.1 X 6.02 10 23
- 1.81 X 10 22
- 1.81 X 10 23
- 6.02 X 10 22
The correct answer is C. 1.81 X 10 22.
WAEC Chemistry Theory Questions
These are possible WAEC Chemistry theory questions and answers 2023 that may appear in this year’s exam.
There are not actual questions and answers but, sample questions for practices.
2.47g of dry pure copper (II) oxide was completely reduced to a copper using laboratory gas. The mass of the residue left was found to be 1.97g. Write a chemical equation for this reaction.
Molar mass of copper atoms =63.5
No. of moles of copper atoms =1.97
=63.5 = 0.03 mole
Molar mass of oxygen atoms = 16
No. of moles Oxygen atom = (2.47 – 1.97) = 0.5
Mole ratio of Cu to O = 0.03 : 0.03
= 1 : 1
Therefore, the equation is Cu + O → CuO.
But Oxygen is diatomic, so we have 2Cu+O 2 → 2CuO
2. Calculate the;
- mass of anhydrous sodiumtrioxocarbonate (IV) present in a 300cm 3 of 0.1M;
- Number of Na 2 CO 3 particles present in the solution (Na =23, C=12, O=16)
a. Molar concentration of Na 2 CO 3 =0.1M
Molar mass of Na 2 CO 3 = 106gmol -1
Mass concentration = Molar concentration X Molar mass
=10.6gdm -3
i.e 1000cm3 of 0.1M solution contain 10.6g of Na 2 CO 3
Therfore,300cm 3 of 0.1M solution will contain
= 300 X 10.6/1000
=3.18g of Na 2 CO 3
b. Number of Na 2 CO 3 particles
=Molar concentration X 6.02 X 10 23
=0.1 X 6.02 10 23
=6.02 X 10 22
Now, 100cm 3 of 0.1M solution contains 6.02X10 22 Na 2 CO 3 particles
Therefore, 300cm 3 of 0.1M solution will contain 300X6.02 X10 22 /1000
= 1.81 X 10 22 particular of Na 2 CO 3 .
How to Download WAEC Past Questions and Answers
WAEC Past Questions and Answers are available for download for all subjects in PDF. To download WAEC Past Questions and Answers for Chemistry, you only need a phone, a whatsapp account and a Gmail address.
Then call 07063986527 and drop and WhatsApp message.
Click the Link Below to download WAEC Past Questions and Answers for Chemistry.
Download WAEC Past Questions and Answers for all Subjects Including practical.
Disclaimer: Please note that we are not in any way affiliated to any of the organization’s mention here. Some of the organizations here do not release their past questions, so we can only compile likely questions and answers very related to the organization.
If You Want More Information, please subscribe to our email list and leave a comment with your number below, also Follow us on Facebook , Twitter , and Linkedin
Disclaimer: Please note that we are not in any way affiliated with any of the organizations or institutions here. All articles here are for the sole purpose of providing information. All Past questions are gotten from previous years’ examinations and likely questions from the Internet and related exams. We are not in any way promising that what you find in the past questions is what you will find in your examination. We are not in any way responsible for how the reader uses the information here. Please do consult an expert or professional in your field should the need arise. Copyrights Infringement: No article from this website should be copied without a proper reference and link to the page picked from. Anything otherwise will lead to legal action of copyright infringement. All articles on this website are products of painstaking research from our writers and journalist. Should you find any material bearing semblance here to any material on your page, please quickly notify us by sending a mail to [email protected] and we will immediately commence the process of taking it down.
WAEC Building Construction Questions and Answers 2023 | Theory and OBJ- Download PDF
Waec gce timetable 2023/2024: january/february first series, related articles, waec gce 2023/2024 registration form and instruction guide, waec biology questions and answers 2023 | theory and obj- download pdf, waec commerce questions and answers 2023 | theory and obj- download pdf, waec applied electricity/ basic electricity questions and answers 2023 | theory and obj- download pdf, how to buy waec scratch card online in 2023- the definitive guide, waec english questions and answers 2023 | theory and obj- download pdf.
- WAEC Biology Practical Questions and Answers 2023/2024 PDF Download
St Charles Edu Services
Genuine Exam Past Questions and Answers Online Bookshop – PDF and MS Word Download
WAEC Chemistry Past Questions and Answers in 2023 PDF Download Objective & Theory
Are you writing the West Africa Examination Council WAEC Internal or External examination, if yes you need the WAEC Past Questions on Chemistry
we at stcharlesedu.com has compiled a good number of Chemistry WAEC Past Questions and Answers in Pdf Chemistry 2 – Theory/Essay Questions. Chemistry 1 – Objective Test Questions.
Our research has confirm that candidate that uses WASSCE Chemistry past questions to prepare is ten times better than those who do not.
Table of Contents
- 1.1 Chemistry WAEC Objective Questions
- 2 SSCE WAEC Chemistry Theory Questions
- 3 Chemistry WAEC Essay Questions
- 4 Free WAEC Chemistry Exam Past Questions Download
- 5 How to Get WASSCE Chemistry Exam Past Questions and Answers
SSCE WAEC Chemistry Objective Questions and Answers
CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout.
Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen. Give only one answer to each question. An example is given below
What others are downloading WAEC Past Questions for all Subjects
Chemistry WAEC Objective Questions
Which of the following elements reacts with water? A. Carbon B. Iodine C. Sodium D. Sulphur
The correct answer is Sodium, which is lettered C and therefore answer space C would be shaded. [A] [ B ] [C] [ D ]
Think carefully before you shade the answer spaces; erase completely any answer you wish to change.
Which of the following raw materials is used in the plastic industry? A. Ethene B. Methane C. Sulphur D. Hydrogen
Which of the following organic compounds can undergo both addition and substitution reactions? A. Petane B. Benzene C. Propane D. Hexane
Which of the following equations represents a redox reaction? A. AgNO 3 (aq) + KCl(ag)->AgCl(s)+ KNO 3 (aq) B. HNO 3 (aq)+ NaOH(aq) -> NaNO 3 (aq) + H 2 O(l) C. CaCO 3 (s) -> CaO(s) + CO 2 (g) D. 2H 2 S(g) + SO 2 (g) -> 2H 2 O(I) + 3S(g)
T he process of extraction of iron from its ore is A. decomposition. B. oxidation. C. reduction. D. sublimation.
What is the solubility of a salt if 0.4 g of it is obtained on evaporating 200 cm3 of its saturated solution to dryness? A. 0.08 gdm -3 B. 2.00 gdm -3 C. 8.00 gdm -3 D. 80.00 gdm -3
An acidic salt has A. double anions in its aqueous solution. B. a single cation in its aqueous solution. C. hydrogen ions in its aqueous solution. D. hydrogen atoms in its aqueous solution.
A reaction is endothermic if the A. reaction vessel feels cool during the reaction. B. enthalpy change is negative. C. bond forming energy exceeds bond breaking energy. D. heat of formation of reactants exceeds heat of formation of products.
In which of the following compounds does hydrogen form ionic compounds? A. CH 4 B. HCl C. NH 3 D. NaH
Consider the following reaction equation: Br 2 + 2KI -> 2KBr + I 2 . Bromine is acting as A. an oxidizing agent. B. a reducing agent. C. an acid. D. a base.
An organic compound has the empirical formula CH 2 . If its molar mass is 42 gmol-1 what is its molecular formula? [H = 1.0, C = 12.0] A. C 2 H 4 B. C 3 H 4 C. C 3 H 6 D. C 4 H 8
Ethene is produced from ethanol by A. decomposition. B. hydrolysis. C. ozonolysis. D. dehydration.
Consider the following equilibrium reaction: 2 AB(g) + B 2 (g) -><- 2AB 3 (g) AH = -XkJmol -1 The backward reaction will be favored by A. a decrease in pressure. B. an increase in pressure. C. a decrease in temperature. D. an introduction of a positive catalyst.
What is the mass of solute in 500 cm 3 of 0.005 moldm -3 H 2 SO 4 ? [H =1.0, O = 16.0, S = 32.0] A. 0.490 g B. 0.049 g C. 0.245 g D. 0.0245 g
Pure water can be made to boil at a temperature lower than 100 °C by A. reducing its quantity. B. decreasing the external pressure. C. distilling it. D. increasing the external pressure.
Consider the following sketch of the solubility curve of some substances. Note: scroll down to download the free chemistry waec questions in pdf copy to view the sketch
At what temperature does the solubility of KNO 3, equal that of NaNO 3 ? A. 0°C B. 20 °C C. 30 °C D. 40 °C
When a salt is added to its saturated solution, the salt A. dissolves and the solution becomes super saturated. B. dissolves and the solution becomes unsaturated. C. precipitates and the solution remains unchanged. D. dissolves and crystals are formed.
When substance X was added to a solution of bromine water, the solution became colorless. X is likely to be A. propane. B. propanoic acid. C. propyne. D. propanol.
The preferential discharge of ions during electrolysis is influenced by the A. mechanism of electrolysis. B. electrolytic reactions. C. nature of the electrode. D. type of electrolytic cell.
The valence electrons of 12 Mg are in the A. 3s orbital. B. 2px orbital. C. 2s orbital. D. 1s orbital.
Stainless Steel is an alloy comprising of A. Fe and C. B. Fe and Ni. C. Fe, C and Ni. D. Fe, C and Al.
The number of hydrogen ions in 1.0 dm 3 of 0.02 moldm -3 tetraoxosulphate(VI) acid is [NA = 6.02 x 1023] A. 1.2 x 10 22 B. 1.2 x 10 23 . C. 2.4 x 10 22 . D. 2.4 x 10 23 .
The most suitable substance for putting out petrol fire is A. water. B. carbon(IV)oxide. C. fire blanket. D. sand.
The following factors would contribute to environmental pollution except A. production of ammonia. B. manufacture of cement. C. photosynthesis. D. combustion.
The position of equilibrium in a reversible reaction is affected by A. particle size of the reactants. B. vigorous stirring of the reaction mixture. C. presence of a catalyst. D. change in concentration of the reactants.
The diagram below illustrates a conical flask containing water and ice.
NOTE: scroll down and download the free chemistry pdf past questions to see the diagram
Which of the following statements about the diagram is correct? A. The water is at a lower temperature than the ice B. Energy is absorbed when the ice changes to water C. Energy is released when the ice changes to water D. The water molecules vibrate about a fixed point
Which of the following statements best explains the differences between a gas and a vapor? A. Unlike gases, vapors are liquids at room temperature B. Unlike gases, vapor can easily be condensed into liquids C. Unlike gases, vapour is readily converted into solids D. Vapours are generally denser than gases
Consider the following reaction equation: 2HCl + Ca(OH) 2 –> CaCl 2 + H 2 O. What is the volume of 0.1 moldrn -3 HCl that would completely neutralize 25cm 3 of 0.3 moldm -3 Ca(OH) 2 ? A. 150 cm 3 B. 75 cm 3 C. 30 cm 3 D. 25 cm 3
Cu and HNO 3 are not suitable for preparing hydrogen gas because of their A. reactivity and oxidation respectively. B. conductivity and corrosiveness respectively. C. melting point and reduction respectively. D. electro negativity and solubility respectively.
Which of the following formulae cannot be an empirical formula? A. CH B. CH2 C. P2O5 D. N204
One of the criteria for confirming the purity of benzene is to determine its A. heat capacity. B. boiling point. C. mass. D. colour.
Want more Chemistry Objective Test Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answers (Obj and Essay) in PDF Format from us.
SSCE WAEC Chemistry Theory Questions
Chemistry Paper 2 Paper 2 will last for 2 hours This paper consists of two sections A and B. Answer one questions from Section A and three questions from Section B.
Credit will be given for clarity of expression and orderly presentation of material.
SECTION A (1ai) Define the term fermentation. (1aii) Name the catalyst that can be used for this process.
(b) Name two factors which determines the choice of an indicator for an acid-base titration. (c) Consider the following reaction equation: [Fe + H2S04 ] FeS04 + H2. Calculate the mass of unreacted iron when 5.0g of iron reacts with 10cm3 of 1.0 moldrrv3 H SO [Fe = 56.0] (d) Name one: (di) Heavy chemical used in electrolytic cells; (dii) Fine chemical used in textile industries.
(e) Explain briefly how a catalyst increases the rate of a chemical reaction. (f) (i) Write the chemical formula for the product formed when ethanoic acid reacts with ammonia. (ii) Give the name of the product formed in 1 (f) (i)..
(g) List three properties of aluminum that makes it suitable for the manufacture of drink can. (h) State two industrial uses of alkylalkanoates. (i) List two effects of global warming. (j) Name two steps involved in the crystallization of a salt from its solution.
Chemistry WAEC Essay Questions
SECTION B. 2ai. State the collision theory of reaction rates. 2aii.Using the collision theory, explain briefly how temperature can affect the rate of a chemical reaction.
bi. Sketch a graphical representation of Charles’s law. bii. Calculate the volume of oxygen that would be required for the complete combustion of 2.5moles of ethanol at s.t.p. [ molar volume at s.t.p = 22.4dm3]
ci. Define esterification. cii. Give two uses of alkanoates. ciii. Give the products of the alkaline hydrolysis of ethyl ethanoate.
d. A tin coated plate and a galvanized plate were exposed for the same length of time. di. Which of the two plates corrodes faster? dii. Explain briefly your answer in 2 (d) (i).
Want more Chemistry Theory Questions like this? Get the Complete WAEC Chemistry Exam Past Questions and Answer (Obj and Essay) in PDF Format from us.
Free WAEC Chemistry Exam Past Questions Download
Click to Download your free NECO Past Question on Painting and Decorating Paper 2 and 3
Link 1: WASSCE Chemistry Questions Booklet Link 2: WASSCE Chemistry Questions Booklet
How to Get WASSCE Chemistry Exam Past Questions and Answers
To get the complete and more recent copy of the West Africa Examination Council WAEC Past Questions and answer
Take Note of the following step
Make a Call Call or whatsapp us on 08051311885 for the account number to make payment and how to received your complete copy of the past questions to be sent directly to your email address or whatsapp number.
Mode of Payment. Mobile Transfer or Direct Bank Deposit.
After Payment send us the following Depositor Name: Name of Product Paid for: Valid email address.
DELIVERY ASSURANCE We will deliver the past question to you 10 mins after confirmation of payment to the email you will send to us.
Related Posts:
- WAEC Technical Drawing Past Questions PDF Download – Objective, Essay, Building Plan/Practical Drawing
- WAEC Government Past Questions and Answers in 2023 PDF Download Objective & Theory
- WAEC Financial Accounting Past Questions and Answer 2023 – Objective & Essay
- WAEC Visual Art Past Questions and Answers – Objective, Theory in 2023
- WASSCE/WAEC Electrical Installation & Maintenance Past Questions PDF – Objective/Essay
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
WAEC chemistry past questions and answers (PDF) free
Free download waec chemistry past questions, join waec whatsapp group (sciences) 2021.
February 02 2021
WhatsApp Group for WAEC Arts students 2021
February 03 2021
May/June 2023 WAEC expo/runs (also, join WhatsApp Group)
February 10 2021
Add a Comment
Notice: Posting irresponsibily can get your account banned!
Comments, Page 1/1
I can click on answer
i need 2023 physics
I need past question for 2023 wace
I really love the past questions and answer because it help to no how to answer the weac questions
I need 2003 past questions and answers
Past question and answer on chemistry 2022
i need 2021 question on chemistry
I need an answers
I need past paers for 2021
I need 2019 past question
I really like past questions andanswers good done
nice one this is good
Featured Posts
Latest posts.
2024 WAEC CHEMISTRY: Chemistry (Chems) WAEC Authentic Questions and Answer 2024 (1531)
Notice board, with examplaza a1 is sure in 2024 neco.
Account Number: 7040209000
Bank Name: Wema
Account Name: Monify Onuwa
Note: After payment upload your proof of payment to prnt.sc and send the link and subject(s) name to 07035334615 as TEXT MESSAGE to get your pin and whatsapp group link. Pos, Transfer, Airtime are allowed. If you want to pay using recharge card, send it to the number as text message. Do not subscribe on Whatsapp, we reply faster via text message.
OBJ & NOTICE SECTION
Chemistry (chems) waec authentic questions and answer 2023 password/pin/code: 1531 ..
CHEMISTRY OBJ
1- 10: ACADABBDAC
11-20: BCACBBCCBA
21-30: ACBABDCCCB
31-40: CBBDAABDAD
41-50: ACCDABDDCD
Welcome to official 2024 Chemistry WAEC answer page. We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo. WAEC Chemistry Questions and Answers 2024. WAEC Chems Expo for Theory & Objective (OBJ) PDF: verified & correct expo Solved Solutions, Chemistry (Chems) WAEC Authentic Questions and Answer 2024. 2024 WAEC EXAM Chemistry Questions and Answers
CLICK HERE TO VIEW ANSWER No. 1 (V1) on Chemistry

CLICK HERE TO VIEW ANSWER No. 1 (V2) on Chemistry

CLICK HERE TO VIEW ANSWER No. 2 (V2) on Chemistry

CLICK HERE TO VIEW ANSWER No. 2 (V1) on Chemistry

CLICK HERE TO VIEW ANSWER No. 3 on Chemistry

CLICK HERE TO VIEW ANSWER No. 5 on Chemistry


GENERAL & QUESTIONS SECTION

Welcome to official 2024 Chemistry WAEC answer page. We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo
Name: examplaza.com
Founded: 2010 (14 years)
Founder: Mr. Onuwa
Headquarters: Borno, Nigeria
Official Website: https://examplaza.com/
Official Contact: +2348108515604
READ THIS. CLICK HERE NOW

RELATED ANSWERS
Education News and Academic Guides
- WAEC Questions and Answers
WAEC Chemistry Questions And Answers For 2023 | Objectives And Theories

Join one of the lucky candidates who will have access to the May/June WAEC Chemistry Questions and Answers for 2023 | Objectives and Theories . It is not everyone who is writing the 2023 WAEC examination will be privileged to see the information that I am going to reveal to you here.
Are you a candidate who is sitting for the May/June WAEC examination for 2023? This article is very important for you especially if you are a science student.
In this article, I am going to be revealing to you those questions that you are going to see in chemistry on the examination date. If you are interested in becoming one of the early birds who will have access to the exact questions and the answers, kindly read this article to the end.
First of all, let me start by answering some of the frequently asked questions about WAEC chemistry questions every year. These questions are going to give you more guides about the 2023 WAEC questions and how you would be able to answer them.
How Many Questions are in WAEC Chemistry
How to answer waec chemistry questions, reasons for poor performance in chemistry, what you should read for waec chemistry 2023, waec chemistry objective questions for 2023, waec chemistry objective answers for 2023, waec chemistry essay questions for 2023, related links, related posts.
The WAEC chemistry examination is usually made up of not less than three subsections with each subsection containing a specified number of questions.
This section presents to you the number of questions that you are going to see in the WAEC Chemistry Examination for 2023.
This part is usually the objective part of the Chemistry examination. It is usually made up of 50 questions in all and candidates are expected to answer all the questions contained therein.
These are usually compulsory questions that you are expected to finish. They carry 1 mark respectively, making a total of 50 marks.
In this section, you will be given about 7 questions and would be required to answer just 5 questions in all. While entering this part, it is necessary to check for the question(s) that have been made compulsory by WAEC.
The paper 3 is made up of 3 questions which are usually practical questions. You would be expected to answer all the questions compulsorily.
They are quite good number of students who have concluded that chemistry is one of the hardest subjects to write in WAEC examination. This could be as a result of how they were thought in their respective secondary schools. Some of them do not even have a good knowledge of chemistry.
For one to pass chemistry examination at one sitting in WAEC , they are some principles that must be put in practice. These are the rules that are applicable during the time of writing the examination.
If you follow the guidelines that I am going to give you in this section, then you will make an excellent result in chemistry
The principles for answering WAEC chemistry examination include the following:
- Read Instructions
Once you have settled to take your examination in the examination hall, the next thing that you must do first is to read through the instructions on top of the page before you can proceed with reading the questions.
Most times, some instructions are specific to some questions, maybe one or two questions. Here, you still have to take those instructions very serious.
2. Read Carefully
Before you can proceed to answering WAEC chemistry questions, it is highly recommended that you read carefully to understand any question before you can start to answer the questions.
It is possible to see questions that would be similar to what you have been seeing before, probably in past questions, but they are not the same.
This is the point where it is very necessary that you meticulously go through the question before you answer, to avoid choosing the wrong option for objectives and giving wrong explanations for theory questions.
3. Take Note of Compulsory Questions
In every WAEC examination, there is/are usually some questions that are specially made compulsory by the West Africa Examination Council. These questions are always seen in the paper 2 which is theory aspect of it.
You must ensure that you are able to answer those compulsory questions before you can think of answering any other ones. The compulsory questions do carry special mark.
4. Start with the Simplest
The complexity of every question in WAEC chemistry varies. After you have gone through the questions, you would be able to tell which of the questions are simple and the ones that are difficult.
The best approach in such case requires that you answer the questions from the simplest to the most complex ones. This is important because it will help to cushion you against examination tension.
Not only that, it also helps in the management of your time. If you are finding any question difficult to answer, you have to leave it and go to the next. Thereafter, you can re-visit those questions that you have left unanswered.
5. Attempt all the Question
Though it is advisable that you start answering your question from the simplest, leaving the more difficult ones at the first attempts, it does not imply that you should submit you examination without answering those ones you skipped.
It quite understandable that you may not know all the asked questions, you are still required choose your answers even the ones that you do not really know very well.
Sometime, your guess can fall in place and become the right answer. So always ensure that you attempt all you question before you submit.
6. Review your Answers
After you have attempted the entire given questions, you still have to go through all the answers to check for any errors and possible corrections.
By going through the questions, you would be able to see any skipped question(s), if any and the ones you mistakenly clicked the wrong options.
It is an error to assume that WAEC chemistry examination is hard. Failure to perform excellently in WAEC chemistry examination is a question of whether the candidate knows the right things to do and if he/she is doing it right.
The poor performance that have been seen in some candidates’ results over the years are as a result of poor application of the basic principles for writing WAEC chemistry examination and some other factors that I am going to show you here.
1. Inadequate Preparation
This is the most important aspect of the reasons for poor performance in WAEC Chemistry that should be considered.
It is obtainable in all areas that when there is poor preparation for any examination they will be poor result. As a good student, you are required to prepare for the WAEC chemistry examination to the point that you would be sure of scoring high.
2. Poor Time Management
Time management is another important factor that can lead to poor performance in every examination. You should learn how to manage your time especially when you are in the examination hall.
The practice of time management starts from the period of the candidate’s preparation for the examination. Once you can answer your past questions comfortably with the limited time that will be provided, you will still manage your time very well on the examination date.
3. Lack of Adherence to Given Instructions
There are some students who are fond of ignoring instructions when come for examination. Jumping into answering of questions without reading the given instructions is very wrong. This contributes in a big way to poor performance in the examination.
If you want to have good performance in WAEC chemistry, you have to strictly pay close attention to any given instructions.
4. Submitting Incomplete Answers
When a candidate submits the examination without attempting all the questions, it will automatically affect his/her score. It is important you answer all the questions before your submission.
In WAEC chemistry examination, they are some topics that are considered very important. These topics are where most of the questions that are repeatedly asked by the WAEC for every year.
As you continue to read this section, I am going to reveal those topics to you. If you are able to read the chemistry topics that I am going to show you here very well before the examination, it means that you can attempt up to 90% of WAEC chemistry questions correctly.
These topics include the following:
- Nature of Matter
- Separation Techniques
- Atomic Structure
- Chemical Combination
- Kinetic Theory of Matter
- Acids, Bases and Salts
- Periodic Table
- Non-metals and their Compounds
- Metals and their Compounds
- Electrolysis
- Energy and Chemical Reactions
- Chemical Equilibrium
- Rate of Reaction
- Air and Air Pollution
- Water, Solution and Solubility
- Shapes of Molecules and Solids
- Radioactivity
- Hydrocarbon
- Organic chemistry
However, you should note that reading with the WAEC syllables is the best practice. The topics listed above are just suggestion of areas that you can concentrate so as to get a good result in the WAEC chemistry examination.
The following are what you are likely going to see in the 2023 WAEC chemistry examination for objective section.
1. A mixture of sugar and sulphur can be separated by
A. dissolution in water, evaporation and filtration
B. filtration, evaporation and dissolution in water
C. dissolution in water, filtration and evaporation
D evaporation dissolution in water and filtration
2. Which of the following is a physical change?
A. Freezing ice-cream
B. Dissolving calcium in water
C. Burning kerosene
D. Exposing white phosphorus to air.
3. The percentage of water of crystallization in ZnSO.7H 2 O is
[Zn = 65, S = 32, O = 16 , H=1]
4. 0.0075 mole of calcium trioxocarbonate(IV) is added to 0.015 mole of a solution of hydrochloric acid. The volume of gas evolved at s.t p. is
[Molar volume of a gas at s t.p = 22.4dm 3 ]
5. A gas exerts pressure on its container because
A. the molecules of a gas collide with the walls of the container.
B some of the molecules are moving faster than others.
C. of the collisions of the molecules with each other
D. of the mass of the molecules of the gas
6. The basic assumption in the kinetic theory of gases that the collisions of the gaseous molecules are perfectly elastic implies that the
A. forces of attraction and repulsion are in equilibrium
B. gaseous molecules can occupy any available space
C. gaseous molecules will continue their motion indefinitely
D gases can be compressed
7. If an atom is represented as 11 23 X, which of the following deductions is correct?
A. It contains 12 protons.
B. It forms a covalent chloride.
C. Its atomic number is 23
D. It is an alkali metal.
8. If the relative molecular mass of an element is not a whole number, it can be deduced that the element is
A naturally radioactive
B. abundant in nature
C. a transition metal
D. an isotopic mixture
9. Cathode rays cause an object placed behind a perforated anode to cast a shadow on the screen. This observation shows that the rays
A. are positively charged
B. are negatively charged
C. have mass
D. travel in straight lines
10. Which quantum number divides shells into orbitals?
A. Principal
B. Azimuthal
C. Magnetic
11. The type of bonding in [Cu(NH 3 ) 4 ] 2+ is
A. coordinate B. electrovalent C. metallic D. covalent.
12. The mixture of gases used in a photographer’s flash tube is
A. Argon and krypton
B. Krypton and Xenon
C helium and argon
D. argon and xenon
13. When sodium trioxocarbonate(IV) decahydrate loses its water of crystallization to the atmosphere, the process is
A deliquescence
B. efflorescence
C. hygroscopic
D. effervescence
14. Water can be obtained as the only product during the
A. combustion of hydrocarbons
B. neutralization of an acid by a base
C. combustion of hydrogen
D. Electrolysis of brine.
15. If 10.5g of lead (II) trioxonitrate (V) is dissolved in 20cm³ of distilled water at 18°C, the solubility of the solute in moldm -3 is
[Pb = 207 N = 14, O = 16]
16. For a given solute, the concentrations of its saturated solution in different solvents are
A. the same at the same temperature
B. different at the same temperature
C. the same at different temperatures
D. constant
17. The major source of oxides of nitrogen is from the burning of
D. chlorofluorocarbons
18. The acid used in electrolysis of water is dilute
C. H 2 SO 4
19. What volume of 1.5 M solution of KOH would contain 0 045 moles? A 67.50cm³
B 30.00cm 3
C. 6.75cm 3
20. The salt formed from a reaction between citric acid and sodium hydroxide in solution will be
21. The colour change observed when testing for reducing agents using acidified potassium heptaoxodichromate(VI) solution is
A. yellow to purple
B. orange to green
C. green to orange
D. purple to yellow
22. The oxidation state of Cr in K 2 Cr 2 O 7 is
23. Which of the following metals is purified commercially by electrolysis?
24. What current will deposit 3.25g of zinc in 2 hrs?
See: Updated May/June WAEC Timetable for the 2023
[Zn = 65, F = 96500C mol – ¹]
25. C (s) +H 2 O (q) → H 2(q) +CO (q) ∆G for the reaction above at 1300K is – 43kJ At this temperature, the reaction is
A not feasible
B. at equilibrium
C. feasible
D. exothermic.
26. Two equal bulbs, one containing ammonia and the other nitrogen, are opened mouth-to-mouth to each other at room temperature. The entropy in the mixture of gases is likely to
A. remain unchanged
C. decrease

27. In the diagram above, which of the curves represents the evolution of oxygen with time in the equation 2KCIO 3(s) → 2KCl (s) + 3O 2(g) ?
28. A catalyst increases the rate of a chemical reaction by providing a path that
A. raises the activation energy
B. increases the temperature
C. lowers the activation energy
D. increases the concentration.
29. CuO (s) + H 2(g) ↔ Cu (s) + H 2 O (l) .
What is the effect of increasing the pressure on the equilibrium reaction above?
A. The equilibrium is shifted to the left.
B. The equilibrium is shifted to the right.
C. There is no effect.
D. More H 2(g) is produced.
30. The equilibrium of an endothermic reaction which proceeds with an increase in volume can be shifted in the reverse direction by
A. increasing the temperature and decreasing the pressure
B. increasing the pressure and decreasing the temperature
C. decreasing the temperature and increasing the pressure
D. decreasing the pressure and decreasing the temperature
31. The oxidation of ammonia in excess air produces
32. Hydrogen sulphide gas can act as
A. an oxidizing agent
B. a dehydrating agent
C. a bleaching agent
D. a precipitating agent
33. The gasification of coke is used for the manufacture of
A. producer gas
B. natural gas
C. synthetic gas
D. industrial gas
34. The gas that is most useful in protecting humans against solar radiations is
A. chlorine
C. carbon (IV) oxide
D. hydrogen sulphide.
35. Which of the allotrope of carbon is a constituent of a lead pencil?
A. Graphite
C. Lampblack
36. Which of the following metals will show the highest metallic character?
37. Copper metal dissolves in concentrated trioxonitrate(V) acid with the resultant evolution of
38. The type of iron that is best suited for welding, making nails, chains and iron rods is
A. pig iron
B. wrought iron
C. cast iron
D. iron pyrites.
39. In the electrolytic extraction of aluminium, the function of the molten cryolite is to
A. precipitate aluminium hydroxide
B. lower the melting point of aluminium oxide
C. act as a raw material
D. act as a solvent
40. One of the products of the thermal decomposition of sodium trioxonitrate(V) is
B. nitrogen
C. sodium dioxonitrate (III)
D. sodium oxide.
41. A cracking process in petroleum refining can be represented by
A. heptane to heptene
B. heptane to 3-methylhexame
C. heptane to propene and butane
D. heptane to 2,3,3-trimethybutane.
42. Which of the following molecule has two other positional isomers?
A. CH 3 CH₂Br
B. CH 3 CHBr
C. CH 3 CBr₂F 2
D. CH₂CHBrCH₂Br
43. Which of the following class of compounds can exist as dipolar ions in solution?
A. Alkanoic acids.
B. Fatty acids.
C. Dialkanoic acids.
D. Amino acids.
44 Lucas reagent is used to test for
B. alkanoic acids.
C. alkanols
45. A compound commonly used for sterilization and preservation of specimens and food is
D. ammonia.
46. An organic compound reacted with bromine water to give a colourless solution. The compound is probably an
D. alkanone.
47. Which of the following hydrocarbons is mainly used as fuel?
A. Methylene.
B. Ethylene.
48. The molecular formula of a common organic laboratory anaesthetic is
49. The simplest branched-chain hydrocarbon is
50. Organic molecules that have the suffix-ene are unsaturated hydrocarbons that have
A. a single bond
B. a double bond
C. a triple bond
D. an ionic bond
See also: How to Check WAEC Result Online – 2023
ZnSO 4 .7H 2 O = 65 + 64 + 7(18) = 287
% water of crystallization = 126/287 x 100/1 = 44% (A)
4. CaCO 3 + 2HCl → CaCl 2 + CO 2 + H 2 O
1 mole of CaCO 3 produce 1 mole CO 2
∴ 0.0075 mole CaCO 3 will produce 0.0075 mole CO 2
1 mole of CO 2 occupy 22.4dm 3 = 22400cm 3
∴0.0075 CO 2 will occupy 0.0075 x 22400cm 3
5. A 6. B 7. D 8. D 9. A 10. B 11. A 12. B 13. B 14. C
15. Pb(NO 3 ) 2 = 331
20 cm 3 of distilled water is saturated by 10.5g Pb(NO 3 ) 2
1000cm 3 will be saturated by
= 10.5/20 x 1000/1 = 525gdm -3
In moldmol -3 = 525/331 = 1.59 = 1.60moldm -3 (A)
16. A 17. C 18. C
19. 1.0M of 1000cm 3 contains 56g of KOH
1.5M of 1000cm 3 will contain 84g of KOH
∴1.5M contain 84/56 = 1.5M
i.e 1.5M = 1000cm 3
∴0.045 moles 1000/1 x 0.045 = 30.00cm 3 Ans = B
20. D 21. B
More answers loading…>>
See also: 2023 WAEC Biology Practical Specimen, Questions and Answers
These are the kinds of theory questions that you are likely going to see in WAEC Chemistry for 2023.
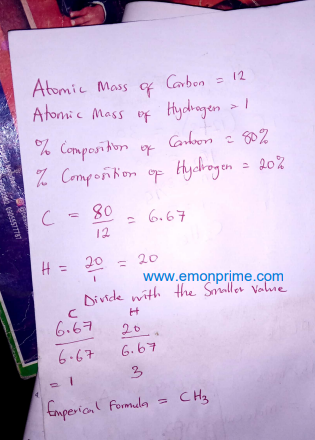
1. Compound A consisting of carbon and hydrogen only. The compound was found to contain 80% carbon by mass. (a) Calculate the empirical formula of compound A using the data above. (b) The relative molecular mass of compound A was found to be 30. Use this information to deduce the molecular formula of compound A. [H = 1.00 C = 12.00]
2. (a) When calcium oxide and coke are heated in an electric furnace, the products are carbon (ii) oxide and calcium carbide (CaC2), write the equation for this reaction.
(b) The addition of water to calcium carbide leads to the formation of calcium hydroxide and ethyne. Write the equation for the production of ethyne.
3. Calculate the percentage by mass of silicon tetrachloride. [2 marks] 4. Ammonia, NH3, and phosphine, Ph3, are the hydrides of the first two elements in group 5. (a) Draw a dot and cross diagram for the ammonia molecule. [2 marks] (b) Sketch and explain the shape of the ammonia molecule. [3 marks]
5. The first ionization energy of chlorine is +1260KJmol-1. (a) Define the term first ionization energy. (b) State and explain the general trend in the values of the first ionization energy for the elements across the period, sodium to argon in the periodic table.
6. An aqueous solution has a pH of 4.0. (a) (i) What is the hydrogen ion concentration of the solution? (ii) What effect will it have on litmus paper? (iii) Which of the following salt solutions would have the same effect on litmus? Give a reason for your answer. NH4Cl(aq); NaCl(aq) ; CH3OON(aq). (b) (i) Differentiate between a fine chemical and a heavy chemical. (ii) Name two sources of air pollution. (iii) Suggest one way of reducing air pollution in cities
WAEC Chemistry Theory Answers for 2023
Keep refreshing this page if you wish to get all solutions to the chemistry theory question for 2023.
WAEC English Questions And Answers 2023 | Objectives, Test Of Orals and Essay
WAEC Economics Questions And Answers 2023 | Theories And Objectives
WAEC French Questions And Answers 2023 | Theories And Objectives
WAEC Mathematics Questions And Answers 2023 | Theory And Objectives
WAEC Physics Practical For 2023 | Specimens/Apparatus, Questions And Answers
2023 WAEC Biology Practical Specimen | Questions And Answers
Complete WAEC Physics Questions And Answers For 2023 (Objectives & Theory)
I believe that you have found this article very useful. For any other questions about the WAEC Chemistry Questions and Answers for 2023 , kindly make use of the comment section.
Please do well to share this information with others
WAEC Literature Questions and Answers 2024/2025 | Novels, Essay & Objective
Waec civic education questions and answers 2024 | essay & objective, leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.
My Scholarship Baze
Waec chemistry questions and answers 2023.
Here is the waec chemistry question and Answers for 2023.

(1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
(1b) loading.
(1c) The increase in the first ionization energies across a period is mainly due to the increasing effective nuclear charge and decreasing atomic radius. The stronger pull from the increasing nuclear charge and the closer proximity of the electrons to the nucleus make it harder to remove an electron, leading to higher ionization energies. (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
(1bi) A, B (1bii) C
(1b) (i) Element B (2:8:2) (ii) Element C (2:8:1)
(1c) The increase in the first ionization energies across a period is mainly due to the increasing effective nuclear charge and decreasing atomic radius. The stronger pull from the increasing nuclear charge and the closer proximity of the electrons to the nucleus make it harder to remove an electron, leading to higher ionization energies. _*Number 5*_
*ai)*…
*Reaction between Iron (Fe) and Dilute H2SO4:*
Iron reacts with dilute sulfuric acid to form iron sulfate (FeSO4) and hydrogen gas (H2). The balanced chemical equation for this reaction is:
3Fe(s) + 4H2SO4(aq) -> 3FeSO4(aq) + 2H2(g) + 2H2O(l)
In this reaction, iron displaces hydrogen from the acid, resulting in the production of iron sulfate and hydrogen gas. The iron sulfate is soluble in water and remains in solution, while the hydrogen gas is released as a gas.
*Reaction between Aluminum (Al) and Dilute H2SO4:*
Aluminum reacts differently with dilute sulfuric acid due to the protective oxide layer that forms on its surface. The oxide layer prevents further reaction by acting as a barrier between the aluminum metal and the acid. However, the reaction can occur if the protective layer is disrupted or removed
2Al(s) + 3H2SO4(aq) -> Al2(SO4)3(aq) + 3H2(g)
In this reaction, aluminum sulfate (Al2(SO4)3) and hydrogen gas are produced. The aluminum sulfate formed is soluble in water and remains in solution, while the hydrogen gas is liberated as a gas. Number 1di)
Two examples of aliphatic compounds are:
A. Ethane (C2H6): Ethane is a saturated hydrocarbon belonging to the alkane family. It consists of a straight chain of two carbon atoms bonded to each other, with six hydrogen atoms attached to the carbons.
B. Butanol (C4H10O): Butanol is an alcohol with a four-carbon straight chain. It has the chemical formula C4H10O and exists in four isomeric forms: n-butanol (normal butanol), sec-butanol (secondary butanol), isobutanol (isopropyl alcohol), and tert-butanol (tert-butyl alcohol).
If you want early answers, Join the whatsapp group from top of the site and chat the admin for VIP group
Also on notifications so you get notified each time we update the site.
Related posts:
- WAEC chemistry practical Questions and Answers 2023
- WAEC Chemistry questions and answers 2020
- June 23rd JAMB CHEMISTRY QUESTIONS AND ANSWERS 2021
- JAMB CHEMISTRY 2023 LIKELY QUESTION
- Jamb Chemistry past questions and answers PDF downoad
- Waec Chemistry Answers 2022
- WAEC CHEMISTRY ANSWERS 2021
- Waec gce chemistry answers 2021
- NECO CHEMISTRY ANSWERS 2020
Related Posts

WAEC PHYSICS ANSWERS 2021

Waec physics answers 2022

WAEC FINANCIAL ACCOUNTING ANSWERS 2020
About the author.
MyScholarshipBaze
I love surfing the web and providing great information for my readers. I am an Editor At Myscholarshipbaze.com
One Response
Your qstns aren’t coming on time and it’s affecting our plans for the revision
Leave a Reply Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
Privacy Overview

Home » WAEC Chemistry Answers 2024 Questions [Essay/OBJ] is Out
2024 WAEC Chemistry Questions & Answers for Essay & Objectives.
The Waec Chemistry Answers 2024 essay and objective questions for the West African Examination Council (WAEC) Chemistry SSCE exam paper scheduled to be written on Wednesday, 22nd May 2024 can now be studied from here.
The 2024 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective exam will commence at 11:30 pm and will last for 1hr.
In this post, we will be posting the West African Senior School Certificate Examinations (WASSCE) Chemistry questions for candidates who will participate in the examination from past questions.

Continue reading below.
WAEC Chemistry Answers 2024.
PAPER 2 [Essay] Answer any FOUR questions. Write your answers on the answer booklet provided.
1. (a) (i) What is the common name given to the group VII elements? (ii) Name the hydrides of the first two elements in group VII. (iii) State three chemical properties of group VII elements.
(b) Copy and complete the following table
| Particles | Number of neutrons | Number of electrons | Number of Protons | Mass Number |
| W2+ | 12 |
|
| 24 |
| X2- |
|
| 8 | 16 |
| Y |
| 13 |
| 27 |
| Z | 12 | 11 |
|
|
(c) (i) Define each of the following processes: nuclear fission; nuclear fusion. (ii) Give one use of each process in 1(c)(i)
(d) (i) List three types of radiation that are produced during radioactivity. (ii) Arrange the radiations listed in 1(d)(i) in order of increasing: penetrating power; ionizing power.
ANS: (a) (i) Halogens (ii) Hydrogen fluoride; Hydrogen chloride (iii) high electron affinity/strong oxidizing agents/electron acceptor – highly electronegative; – react with hydrogen to form acid; – form salts with metals; – react with alkalis to form salts; – react with water to form acids; – displacement of lower halogens from their acids/salts; – react with hydrocarbon to form alkyl halides. (b)
| Particles | Number of neutrons | Number of electrons | Number of Protons | Mass Number |
| W2+ | 12 | 10 | 12 | 24 |
| X2- | 8 | 10 | 8 | 16 |
| Y | 14 | 13 | 13 | 27 |
| Z | 12 | 11 | 11 | 23 |
(c)(i) I. Nuclear fission – splitting of a heavy nucleus into two smaller nuclei of similar mass with the release of a large amount of energy and radiation. II. Nuclear fusion – a combination of smaller nuclei to form a large nucleus with the release of large amounts of energy and radiation. (ii) I. Used to generate electricity/nuclear bomb/production of new elements/production of radioisotopes. II. Used to produce nuclear weapons/atomic bombs/production of new elements/production radioisotopes. (d) (i) alpha, beta, gamma OR α , β, (ii) I. α < β < increasing penetrating power II. <β < α increasing ionizing power.
2. (a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
(b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca. Explain briefly why the elements in each pair have similar chemical properties.
(c) Explain briefly the following terms using an appropriate example in each case (i) homologous series; (ii) heterolytic fission.
(d) State the indicator(s) which could be used to determine the end-point of the following titrations: (i) dilute hydrochloric acid against sodium hydroxide solution; (ii) dilute hydrochloric acid against ammonium hydroxide solution; (iii) ethanoic acid against sodium hydroxide solution.
(e) A solid chloride E which sublimed on heating reacted with an alkali F to give a choking gas G. G turned moist red litmus paper blue. Identify E, F and G.
ANS: (a) (i) The atom has a small/ tiny positively charged centre /nucleus with electrons surrounding the space around the centre. (ii) Atomic number of an element is the number of protons/electrons in an atom of the element while the mass number is the sum of the protons and neutrons in the atom of the element. (iii) Chlorine atom is made up of a mixture of isotopes and the relative atomic mass of chlorine is the average of its isotopic masses.
(b) (i) Is the (minimum) energy required to remove one mole of an electron from one mole of gaseous atom (to form one mole gaseous charged ion (ii) High melting /boiling point; Ability to conduct electricity in the molten state or in solution; Solid at room temperature; Soluble in water or polar solvents /insoluble in non-polar solvents. (iii) Atoms of the elements in each pair have the same number of electrons in their outer-most shell therefore similar chemical properties.
(c) (i) Is a family of organic compounds: – where successive members differ by –CH2 of the molar mass of 14; – with similar chemical properties; – which conform to the same general formula; – which show a gradation of physical properties; – which have the same general method of preparation. e.g alkanes, alkenes , alkanols. (ii) Is a process in which a (covalent) bond is broken in such a way that the electron pair is completely transferred to one of the atoms (resulting in the formation of ions) H ÷ CI → H+ + Cl-/ HCl ® H+ + Cl- (d)(i) Methyl orange/ methyl red/ phenolphthalein; (ii) Methyl orange/ methyl red; (iii) Phenolphthalein. (e) E – NH4Cl F – NaOH, KOH, or Ca (OH)2, Li OH, CsOH, Ba(oH)2, Mg(OH)2 G – NH3.
3. (a) (i) Define saturated solution. (ii) The solubility of KN03 at 20°C was 3.00 mol dm-3 If 67.0g of KN03 was added to 250cm3 of water and stirred at 20°C, determine whether the solution formed was saturated or not at that temperature.
(b) (i) Distinguish between the dative bond and covalent bond. (ii) Explain why sugar and common salt do not conduct electricity in the solid state. (iii) State the type of intermolecular forces present in I. hydrogen fluoride; II. argon. (iv) Consider the compounds with the following structures: S – H —-N and 0 – H —–N In which of the compounds is the hydrogen bond stronger? Give a reason for your answer.
(c) (i) State Dalton’s Law of Partial Pressure. (ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p.[ standard vapour pressure of water at 18°C = 15 mmHg]
ANS: (a) (i) Is a solution that contains the maximum amount of solute it can dissolve at a given temperature (in the presence of undissolved solute). (ii) Solubility of KN03 in in g dm-3 = 3.00 x 101 = 303 .. 1000cm3 of saturated solution = 303g 250cm3 of the solution = 303 x 250 1000 = 75.8 g Since the quantity of KN03 added (67.0) to 250 cnr’ of water is less than the maximum amount required to form a saturated solution, then the solution is unsaturated.
(b)(i) In a dative bond, only one of the participating atoms/ species donated electrons to be shared by both atoms while in a covalent bond both participating atoms/ species contribute equally to the electrons being shared. (ii) Sugar is covalent while common salt (NaCl) is electrovalent/ ionic. Electrical conductivity (in compounds) depends on the presence of mobile ions. (iii) The intermolecular forces present in hydrogen fluoride and argon were hydrogen bond and van der Waal’s forces respectively. (iv) 0 – H —- N has a stronger hydrogen bond because oxygen is more electronegative and smaller in size than sulphur.
(c)(i) the total pressure exerted by a mixture of gases that do not react chemically is equal to the sum of the individual partial pressures of the gases in the mixture. (ii) pressure of the dry gas (P 1) = 700 – 15 = 685 mmHg VI = 200cm 3 , TI = 18°C = 273 + 18 = 291K, P2 = 760 mmHg, T2 = 273 P1V1 = P2V2 T1 T2 V2 = P1V1T 2 = P2T1
= 685 x 200 x 273 760 x 291
= 169.1cm 3
4. (a) (i) Define nuclear fission (ii) A certain natural decay series starts with and ends with. Each step involves the loss of an alpha or a beta particle. Using the given information, deduce how many alpha and beta particles were emitted. (b) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1 Explain briefly the effect of each of the following changes on the equilibrium composition; (i) increase in the concentration of B; (ii) decrease in pressure of the system; (iii) addition of catalyst. (c) The lattice energies of three sodium halides are as follows:
| Compound | NaF | NaBr | NaI |
| Lattice energy/kJmol-1 | 890 | 719 | 670 |
Explain briefly the trend.
(d) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2
(e) Iron is manufactured in a blast furnace using iron ore (Fe2O3), coke and limestone. Write the equation for the reaction(s) at the: (i) top of the furnace; (ii) middle of the furnace; (iii) bottom of the furnace. (f) (i) Name two products of destructive distillation of coal. (ii) Give one use of each product in 3(f)(i).
5. Copy and complete the following table:
| Oxide of nitrogen | Oxidation state of nitrogen | Colour of gas | Solubility in water | Action on damp blue litmus paper |
| N2O | + 1 | Insoluble | ||
| NO | Colourless | |||
| NO2 |
(i) Mention one compound that makes water I. temporarily hard; II. permanently hard. (ii) State one method that could be used to remove I. only temporary hardness; II. permanent hardness. (iii) Write an equation to show the removal of: I. temporary hardness; II. permanent hardness. (c) (i) List three sources of water pollution. (ii) Mention two ways by which water pollution can be controlled. (d) state the function of each of the following substances in the purification of water for town supply: I. sodium aluminate (III) (NaAIO2); II. lime (calcium hydroxide); III. calculated mount of iodine; IV. sand bed.
6. (a) (i) What is meant by atomicity? (ii) Mention one element in each case which is I. monatomic, II. diatomic, III. tetratomic. (iii) Write the orbital electron configuration of I. 20Ca, II. 9F. a. In which group does each of the elements belong? b. How many unpaired electrons are present in 9F? c. How many electrons are present in 20Ca2+?
(b) (i) Write a balanced equation for the thermal decomposition of KCƖO3. (ii) Mention the catalyst that could be used to increase the rate of reaction in 6(b)(i). (ii) If 5.0 g of KCƖO3 was decomposed by heat, determine the volume of oxygen produced at s.t.p. [Molar gas volume at s.t.p. = 22.4dm3, K = 39, Cl = 35.5, O = 16]
(c) (i) Mention the products formed when each of the following substances is heated strongly: I. ZnCO3; II. CuSO4.5H2O. (ii) State the colour change observed when each of the residues in 1(d)(i) above is allowed to cool.
7. (a) Describe briefly how each of the following aqueous solutions could be identified in the laboratory: (i) Ammonium trioxocarbonate (IV); (ii)Ammonium chloride. (b) Arrange the following compounds in order of increasing boiling point and give reasons for your answer: CS2, NaF and CO2. (c) List two gases each that are: (i) acidic; (ii) highly soluble in water; (iii) oxidized by acidified KMnO4(aq). (d) In a tabular form, compare the elements silicon and sulphur under the following properties: (i) metallic character; (ii) physical state; (iii) conduction of electricity. (e) A cuboid piece of sodium metal measures 3 cm x 4 cm x 10 cm. If the density of sodium is 0.971 g cm-3, calculate the number of atoms in the sodium metal. [ Na = 23; Avogadro constant = 6.02 x 1023 mol_1 ]
8. (a) (i) Define standard electrode potential. (ii) State two factors that affect the value of standard electrode potential. (iii) Give two uses of the values of standard electrode potential. (iv) Draw and label a diagram for an electrochemical cell made up of Cu2+/Cu; = + 0.34 Zn2+/Zn; = – 0.76 (v) Calculate the e.m.f of the cell in 8(a)(iv) above (b) (i) In terms of electron transfer, define I. oxidation; II. oxidizing agent. (ii) Balance the following redox reaction: MnO4- + I- H+ I2Mn2+
(c) Classify each of the following oxides as basic, amphoteric, acidic or neutral: (i) Carbon (II) oxide; (ii) Sulphur(IV) oxide; (iii) Aluminium oxide; (iv) Lithium oxide.
(d) What is hydrogen bonding?
WAEC Chem Objective Questions 202 4 .
PAPER 1 [Essay] Answer All questions in this section. Write your answers on the answer sheet provided.
1. Two immiscible liquids with different boiling points can be separated by _____ A. The use of separating funnel B. Evaporation C. Distillation D. Decantation.
2. A mixture of CaCl2 and CaCO3 in water can be separated by ______ A. Evaporation B. Sublimation C. Distillation D. Decantation.
3. What is responsible for metallic bonding? A. sharing of electrons between the metal atoms B. attraction between the atomic nuclei and the cloud of electrons C. Transfer of electrons from one atom to another D. attraction between positive and negative ions.
4. 25cm3 of 1.5M solution of NaCl are added to 50cm3 of 3M NaCl. The molar concentration of the resulting solution is ________ A. 2.5M B. 3M C. 2.25M D. 4.5M
5. A solution of salt formed from HCl and NH3 solutions is _____ A. Acidic B. Basic C. complex D. Neutral
6. Which of the following elements will burn in excess oxygen to form a product that is neutral to litmus? A. carbon B. Hydrogen C. Sulphur D. Sodium
7. A current was passed for 10 mins and 0.2mole of Cu was deposited. How many grammes of Ag will it deposit? (Cu = 64, Ag = 108) A. 43.2g B. 21.6g C. 10.8g D. 5.4g
8. Pollution of underground water by metal ions is very likely in a soil that has high ________ A. Acidity B. Alkalinity C. Chloride content D. Nitrate content
9. Producer gas is a gas with low caloric value because it contains more ____ A. CO2 than O2 B. N2 than CO C. CO2 than N2 D. N2 than CO2
10. Silver chloride turns grey when exposed to sunlight because _____ A. The silver ion is reduced to silver B. The silver ion is oxidized to silver C. Silver is a transition metal D. The silver chloride forms complexes in the sun.
11. Which of these compounds exhibits resonance? A. Benzene B. Ethanol C. Propene D. Butyne
15. Hydrolysis of CH3COOCH2CH3 in dilute HCl produces ______ A. CH3COOH + CH3CH3 B. CH3CH2OH + CH3COCl C. CH3COOH + CH3CH2OH D. CH3COOH + CH3CH3
16. Calculate the volume of CO2 measured at s.t.p produced on heating 250g of potassium hydrogen trioxocarbonate (IV) strongly. (K = 39, H = 1, C = 12, O = 16) A. 28dm3 B. 2.8dm3 C.5.6dm3 D. 11.2dm3
17. The boiling points of water, ethanol, methylbenzene and butan-2-ol are 373.0K, 351.3K, 383.6K and 372.5K respectively. Which liquid has the highest vapour pressure at 323.0K? A. Water B. Methylbenzene C. Ethanol D. Butan-2-ol
18. The conclusion from Rutherford’s alpha scattering experiment is that ______ A. Atoms are mostly empty space with a small nucleus B. Emissions from radioactive substances consist of three main components C. There is a nuclear pull on orbital electrons D. Electrons are deflected by both magnetic and electric fields.
19. Elements P, Q and R have atomic numbers 9, 16 and 20 respectively. Which of them would gain electron(s) during ionic bonding A. Q and R B. P and R C. P and Q D. P, Q and R.
20. Which of the following has the lowest PH? A. 5cm3 of M/10 HCl B. 10cm3 of M/10 HCl C. 20cm3 of M/8 HCl D. 15cm3 of M/2 HCl
21. Which of the following is an acid salt? A. (NH4)2CO3 B. CHCOONa C. KHSO4 D. MgSO4.7H2O
22. Cr2O2-7 + 14H+ + 6I → 2Cr3+ + 3I2 + 7H2O. The change in the oxidation number of oxygen in the equation above is _______ A. 0 B. 1 C. 2 D. 7
23. During electrolysis of CuSO4 solution using platinum electrodes, which of the following occurs ______ A. Acidity increases at the cathode B. Oxygen is liberated at the cathode C. PH decreases at the cathode D. PH of solution increases.
24. Which of the following ions is a pollutant in drinking water even in trace quantities? A. Ca2+ B. Pb2+ C. Mg2+ D. Fe2+
25. The solubility of a salt of molar mass 100g at 20oC is 0.34mol/dm3. If 3.4g of that salt dissolved completely 250cm3 of water at that temperature, the resulting solution is ________ A. A suspension B. Saturated C. Unsaturated D. Supersaturated
26. Catalyst is important in in chemical industry in that ______ A. it affects the purity of the products B. it affects the quantity of the products C. it increases the time for reaching equilibrium D. Bond breaking is slowed down.
27. An alkanioc acid has a molecular mass of 88. Name the acid. (C = 12, O =16, H = 1) A. Propanioc acid B. Botanioc acid C. Pentanioc acid D. But-2-ionic acid
28. Ethyne undergoes the following reactions EXCEPT A. Polymerization B. Addition C. Combustion D. Etherification
Keep following, more questions and answers will be added soon.
PS: Once again, there is nothing like waec chemistry expo. Do not fall victim to scammers online trying to obtain money from you with fake promises of having access to live question paper before the exam. What we have on this page are likely exam questions from waec past questions and answers to serve as a revision guide.
- Privacy Policy
- ADMISSION UPDATES
- SCHOOL GISTS

- Recruitment
WAEC GCE Chemistry 2023 Questions & Answers Obj and Essay Free Expo

Recommended: WAEC Result 2023 is out | Check WAEC Result Now For School Candidates Free
What You'll Learn in this Post
WAEC GCE Chemistry Questions 2023 Objective and Essay
To subscribe for the Verified questions and answers for Chemistry , click here or the following link via https://paystack.com/pay/WAEC GCEpaymentgate .
Answers loading……………
WAEC GCE Chemistry 2023 Answers Objective Essay
Finally, click here to subscribe for Verified Expo for WAEC GCE Chemistry Drama & Poetry questions and answers.
Or check: https://paystack.com/pay/WAEC GCEpaymentgate
Thanks for reading.

RELATED ARTICLES MORE FROM AUTHOR
Npf recruitment exam result 2024 is out check now, nigeria police shortlisted candidates 2024 is out download pdf, edo state teachers recruitment exam past questions and answers pdf 2024 download, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
Study Materials
Best eductional blog in nigeria, waec chemistry questions and answers 2024 objectives and essay.
May 4, 2024 Agunwa Franklin Post UTME 0
WAEC Chemistry Questions | For those seeking the WAEC Chemistry 2024 exam answers, your search ends here. Our team has meticulously compiled the questions and accurate answers for both the objective and theory sections of the exam.
WAEC Chemistry Questions and Answers 2024: Have you been all over the internet for the WASSCE Chemistry questions and answers for 2024? Look no further.
WAEC Chemistry Questions and Answers 2024 Objective and Essay
We’re ready with the 2024 WAEC Chemistry questions, and our expert team will soon upload the questions along with their precise answers to aid you in acing the 2024 WAEC Chemistry examination.
Stay tuned as we’ll be uploading the 2024 WAEC Chemistry theory questions and objectives shortly. Whether you’re seeking the WAEC Chemistry answers for 2024’s objective or theory sections, you’re in the right place. Find the WAEC Chemistry objective and essay questions and answers below.
WAEC Chemistry Answers 2024 Objective and Theory
The West Africa Examinations Council (WAEC) administers the Senior Secondary Certificate Examination and the General Certificate in Education in Nigeria during May/June and November/December respectively.
The 2024 WAEC Chemistry questions are sourced from the SS1 to SS3 Chemistry syllabus. Thus, all questions in this year’s examination are syllabus-based, with nearly 90% being repetitions.
No need to fret over obtaining the 2024 WAEC Chemistry questions and answers PDF (essay and objective). The WAEC Chemistry answers for 2024 will be available shortly. Simply refresh this page to stay updated and avoid missing out.
Refresh this page periodically as we’ll soon upload the authentic WAEC Chemistry questions and answers for this year’s exams. Additionally, you can access past questions and answers by clicking this link: WAEC Chemistry Past Questions
Should you have any inquiries regarding the 2024 WAEC Chemistry questions and answers, feel free to utilize the comment box below or click the Chat With Us button, and we’ll promptly respond.
Stay tuned as we’re gearing up to post the 2024 WAEC Chemistry answers here. Keep checking back and refreshing this page for the correct answers. The WAEC 2024 Chemistry answers are on their way.
Beware : There’s no such thing as WAEC Chemistry expo 2024 online. All students are advised to steer clear of online fraudsters/vendors claiming to offer such services.
Related Posts:

Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright @ 2021 | Sitemap
WAEC Chemistry Questions and Answers 2023 (100% Sure) Theory & Obj Solution
Get free Verified WAEC Chemistry Questions and Answers 2023. WAEC May/June Free Chemistry EXPO answers. West African Examinations Council Chemistry Theory and Objective Answers for you to have good WAEC result. You will also understand how WAEC Chemistry questions are set and how to answer them. The West African Examinations Council is an examination board saddled with the responsibility of setting, testing and conducting WASSCE in all West African country.
Click Here Now To Chat Us on WhatsApp For Subscription Details
Please Note that the WAEC 2023 Chemistry Questions and Answers and any other WAEC expo is provided by us for free. We understand that a lot of website charge of collect money from student to provide WAEC expo Chemistry Answers to them. WAEC questions and answers are provided for free. We will do same during Other Exam like WAEC GCE.
WAEC 2023 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once the verification process complete, we will go ahead to upload it here.
Be at alert! Keep refreshing this page for WAEC Chemistry Theory Questions and Answers
2023 Chemistry Essay Questions Loading 93.8%
Also Read: How To Check Your JAMB Result
WAEC Chemistry Questions and Answers 2023 OBJECTIVES (OBJ) ANSWERS Loading
2023 VERIFIED Chemistry OBJ:…Loading
WAEC Chemistry Questions and Answers 2023 loading…
Please do not panic and fall into the right hand. 2023 Chemistry Answers will be posted for free here once ready.
We are right now getting things ready for you. The WAEC 2023 Chemistry theory questions and answers will be posted any moment from now. All you need do is to keep refreshing this page until you see the answers.
WAEC Chemistry -ESSAY-ANSWERS-joberplanet.com
Answers Loading….
Go to joberplanet.com and keep refreshing it. It will load all the answers
keep refreshing it. It will load all the answers
STILL SOLVING
If you have any questions about the WAEC Chemistry theory & Obj 2023, kindly let us know in the comment box.
We are happy you are here for the 2023 Chemistry answers. The complete solution will be made free in some minutes before the Chemistry examination.
WAEC Chemistry questions 2023, WAEC 2023 Chemistry questions and answers, Chemistry WAEC 2023, WAEC questions 2023/2023, History 2023,
Now Loading…94.5%
We want to hear from you concerning the WAEC Chemistry Questions and Answers 2023 , kindly use the comment section below to get to us.
Related Posts
WAEC Marketing Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC Visual Art Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC Physical Education Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC Music Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC IRK/IRS Questions and Answers 2023 (100% Sure) Theory & Obj Solution
WAEC CRK/CRS Questions and Answers 2023 (100% Sure) Theory & Obj Solution
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
Say Goodbye to JAMB, Enter 200-Level Directly! Gain University Admission via JUPEB/IJMB . Enjoy Low Fees! Call 08033006849 Now!
HOME » Past Questions » Free WAEC Past Questions and Answers for All Subjects
Free WAEC Past Questions and Answers for All Subjects
Free WAEC past questions and answers are available here for download!

Are you in your last stage of Secondary School Education (May/June) or not in the School system (GCE)? If yes, you can now download West African Senior School Certificate Examination (WASSCE) past papers to assist you with your studies.
The importance of using past questions in preparing for your West African Senior School Certificate Examination (WASSCE), cannot be over emphasised. By using past exam papers as part of your preparation, you can find out what you already know and at the same time also find out what you do not know well enough or don’t know at all.
See: WAEC Timetable for May/June Candidates and WAEC Timetable for GCE Candidates .
WAEC Past Questions
- WAEC Agricultural Science Past Questions
- WAEC Biology Past Questions
- WAEC Chemistry Past Questions
- WAEC Commerce Past Questions
- WAEC CRK Past Questions
- WAEC Economics Past Questions
- WAEC English Past Questions
- WAEC Financial Accounting Past Questions
- WAEC Further Maths Past Questions
- WAEC Geography Past Questions
- WAEC Literature in English Past Questions
- WAEC Mathematics Past Questions
- WAEC Physics Past Questions
- WAEC Technical Drawing Past Questions
- WAEC Visual Arts Past Questions
- WAEC Yoruba Past Questions
See also: WAEC Latest Syllabus for all subjects and WAEC Sample Questions and Scheme for All Subjects .
Do you have any other past question(s) other than the ones listed here? If yes, don’t hesitate to share them with others by sending it to [email protected] .
Practice WAEC Past Questions and Answers Online – All Subjects.
WAEC recently launched a portal called WAEC e-learning to curb the number of failures in the WAEC May/June SSCE by creating a portal that contains the resources for all WAEC approved subjects that will students understand the standards required for success in respective examinations.
WAEC e-learning contains past questions and solutions of all subjects.
WAEC e-learning portal is accessible from http://waeconline.org.ng/e-learning/index.htm.
Don’t forget to share this with your friends if you want their success.
Similar Posts:
- West African Examinations Council (WAEC) Job Recruitment
- West Africa Examinations Council (WAEC) Expo/Runz
- New Exam Options for WAEC GCE Candidates: Computer or Paper-Based Tests
- West African Examinations Council (WAEC) Timetable
- Joint Universities Preliminary Examinations Board (JUPEB) Past Questions
- WAEC Economics Past Questions | FREE DOWNLOAD
- WAEC English Language Past Questions | FREE DOWNLOAD
- WAEC Financial Accounting Past Questions | FREE DOWNLOAD
- UNDERGRADUATE
- POSTGRADUATE
- Submit Job for Free
- Get A Professional CV Today
- Advertise with us

WAEC English Essay Questions And Answers 2023
Waec english answers is out – waec english language essay, objective & test of oral questions released..
The Waec English answers 2023 to waec English questions can now be seen here. The West African Examination Council (WAEC) English Language SSCE paper will be written on Wednesday, 17th May 2023.
There will be three papers in Waec English 2023 – Papers 1, 2 (Objectives and Essay) to be written from 9:30 am to 12.30 pm and Paper 3 (Test of Orals) which will start by 2:00 pm and end by 2.45 pm. The WAEC English question 2023 comes in the following question papers.
- PAPER 1: This Will consist of eighty multiple-choice questions, all of which should be answered within 1 hour for 40 marks.
- PAPER 2: Will consist of five essay topics and a passage each to test candidates’ comprehension and summary skills. Candidates will be expected to write an essay on one of the topics and answer all the questions on Comprehension and Summary passages. The paper will last 2 hours and carry 100 marks.
- PAPER 3: Will consist of sixty multiple-choice items on Test of Orals for candidates in Nigeria and Liberia, and on Listening Comprehension for candidates in Ghana, The Gambia, and Sierra Leone. All the questions will be answered in 45 minutes for 30 marks.
There is nothing like Waec English expo online . In this post, we will be posting sample questions from Waec English past questions and answers that we feel are likely questions for preparation.

WAEC English Essay Questions 2023.
PAPER 2 SECTION A ESSAY [50 marks]
Answer ONE question only from this section. All questions carry equal marks. Your answer should not be less than 450 words . You are advised to spend about 50 minutes on this section.
1. A friend of yours who has been living in the US for some years has written to invite you to join him. Write a reply to his letter giving, at least, three reasons why you would rather remain in your country.
2. Write a letter to the Minister of Works in your country complaining about the deplorable condition of the roads in your area and the effects this has on the lives of your people.
3. Write an article suitable for publication in one of your national dailies discussing the vandalization of public facilities in your country and its effects.
4. You are the Chief Speaker in a debate on the topic: Parents should allow their children to choose their own careers. Write your speech for or against the topic.
5. Write a story to illustrate the saying: The devil makes work for idle hands .

SECTION B COMPREHENSION [20 marks] You are advised to spend about 30 minutes on this section.
6. Read the following passage carefully and answer the questions on it.

Our planet is at risk. Our environment is under threat. The air we breathe, the water we drink, the seas we fish in, and soils we farm, the forests, animals, and plants that surround us are in danger. New terms and words describe these problems: acid rain, the greenhouse effect, global warming, holes in the ozone layer, desertification, and industrial pollution. We are changing our environment. More and more gases and wastes escape from our factories. Rubbish, oil silages and detergents damage our rivers and seas. Forests give us timber and paper, but their loss results in soil erosion and also endangers wildlife.
SECTION C SUMMARY [30 marks] You are advised to spend about 40 minutes on this section.
7. Read the following passage carefully and answer the questions on it.

Waec English Objective Questions 2023.
PAPER 1 (Objectives) SECTION 1
In each of the following sentences, there is one underlined word and one gap. From the list of words lettered A to D, choose the one that is most nearly opposite in meaning to the underlined word and that will, at the same time, correctly fill the gap in the sentence.
1. Most African countries face poverty while few enjoy _______ A. influence B. money C. affluence D. power.
2. Last year our farmers cultivated more crops than they _______ A. destroyed B. uprooted C. harvested D. yielded.
From the words lettered A to D, choose the word that best completes each of the following sentences.
Waec English Objective Questions 2023
11. There would have been a riot in our school but for the timely _____ of our staff. A. intervention B. interruption C. interference D. invasion.
12. The armed robbers ______ every room in the bank to look for money. A. explored B. ransacked C. raked D. swept.
13. The discontented men _______ up trouble among the workers. A. Starred B. Steered C. Stirred D. Started.
WAEC Oral English Objectives Answers 2023
ORAL ENGLISH 01-10: CACDAABCAD 11-20: DBACADDBCC 21-30: ACBDADBBBC 31-40: ADDDDACCCB 41-50: CDCABADCDA 51-60: BABBDBABDD
After each of the following sentences, a list of possible interpretations is given. Choose the interpretation that you consider most appropriate for each sentence.
21. Ade is too clever by half. This means that Ade is A. far cleverer than others B. actually very stupid in his behaviour C. annoyingly clever D. behaving to be clever but is not.
22. Ameh is really being economical with the truth. This means that Ameh A. is being praised for being honest B. does not know enough C. knows more than he is prepared to say D. is not telling the truth.
From the words lettered A to D below each of the following sentences, chose the word or group of words that is nearest in meaning to the underlined word as it is used in the sentence.
31. By failing to attend the interview, Idoko has lost a golden opportunity. A. blessed B. bright C. good D. delightful.
32. I hope the principal would be gracious enough to forgive us. A. cordial B. polite C. merciful D. gentle.
33. The man’s story gave us an inkling of what we went through during the war. A. a taste B. a possible idea C. a wrong notion D. a suggestion.
34. Our aunt has expressed deep appreciation for Onyinye’s invaluable assistance during the party. A. Immeasurable B. Praiseworthy C. Selfless D. Worthless.
35. Many world leaders have continued to condemn the South African Prime Minister for his truculent posture. A. Impetuous B. Impertinent C. Aggressive D. Impervious.
36. It is usually hard to change the course of action when one crosses the Rubicon. The underlined expression is used in this sentence means to. A. Pass through a place called Rubicon B. Cross a river called Rubicon C. Cross a bridge called Rubicon D. Be irrevocably committed.
From the words or group of words lettered A to D, choose the word or group of words that best completes each of the following sentences.
41. A good citizen abides _______ the rules of the land. A. with B. in C. at D. by
42. Since his swearing-in, the governor _______ his hometown. A. had not been visiting B. has not visited C. did not visit D. had not visited.
Waec English Test of Orals Answers 2023.
PAPER 3 (Test of Oral) SECTION 1
From the words lettered A to D, choose the word that has the same vowel sound as the one represented by the letters underlined.
1. look A. glue B. you C. cup D. curious.
2. wit A. fright B. wheat C. tree D. market.
From the words lettered A to D, choose the word that has the same consonant sound(s) as the one represented by the letter(s) underlined.
4. plucked A. smiled B. slammed C. luck D. table.
From the words lettered A to D, choose the word that rhymes with the given word.
6. carrier A. area B. barrier C. serious D. ravine. 7. drought A. crowd B. nought C. shout D. taught.
In each of the following questions, the main /primary stress is indicated by writing the syllable on which it occurs in capital letters. From the words lettered A to D, choose the one that has the correct stress .
9. acrimony A. A-cri-mo-ny B. a-CRI-mo-ny C. a-cri-MO-ny D. a-cri-mo-NY.
10. Information A. inforMAtion B. INformation C. inFORmation D. InformaTION.
PS: Once again, there is nothing like Waec English expo. Do not fall victim to scammers online trying to obtain money from you with fake promises of having access to a live question paper before the exam. What we have on this page are likely exam questions from Waec English past questions and answers to serve as a revision guide.
Keep following this page and make sure you bookmark this site for reference purposes. If you have any questions, endeavour to use the comment section below.

Adeyinka is the founder and content creator at Career Acada. He’s a technology expert and web developer. He holds a degree in Genetics and loves impacting life for a better society.
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)

RELATED ARTICLES MORE FROM AUTHOR

Seplat Energy Graduate Trainee 2024

UBA Graduate Management Trainee 2024

Dufil Graduate Engineering Trainee 2024
Kindly drop your comment here cancel reply, even more news.

POPULAR CATEGORY
- EDUCATION 133
- SCHOLARSIHP 122
- POSTGRADUATE 70
- COLLEGES 63
- COMPANIES 48
- Privacy Policy
- Write For Us Nigeria

Waec 2024 Chemistry Practical Obj & Essay Questions And Answers Now Available
May 15, 2024 Wakagist Admin Waec 0

Join Our Telegram Channel By Clicking The Link… to get the free Complete 2024 WAEC CHEMISTRY PRACTICAL ANSWERS FREE HURRY UP ….. Hurry Up
(1) ATTENTION :- PLEASE DONT COME HERE FOR FREE ANSWER… BE WARNED
(2) attention:- you are advise to send your subscription details on whatsapp… add us on whatsapp with => 09054348225, if you need the answer on whatsapp is available, it cost #500… add us on whatsapp with => 09054348225.
*2024 WAEC CHEMISTRY PRACTICAL ANSWERS*
*NUMBER THREE*
(i ) Carbon dioxide (CO2) (ii) Ammonia (NH3) (iii) Oxygen (O2) (iv)Hydrogen chloride (HCl)
(3b) (PICK TWO) (ii) Burette (iii) Volumetric flask (iv) Micropipette
(3c) Safely contain and remove harmful fumes, gases, or vapors generated during experiments, preventing their exposure to lab personnel.
(PICK THREE) (i) Safety goggles/glasses (ii) Lab coat/apron (iii) Gloves (iv)Face mask/respirator (v) Closed-toe shoes/footwear (vi) Ear protection (if needed for specific experiments or environments)
===================================
(2a) When all of C is added to a test tube with about 10cm³ of distilled water and shaken, the mixture is filtered. The residue and the filtrate are obtained.
Observations: The residue: This will contain the insoluble salt(s) present in C.
The filtrate: This will contain the soluble salt(s) present in C.
(2bi) Heating about 2cm³ of the filtrate in a boiling tube strongly.
Observations: If there is effervescence (bubbling), it indicates the presence of a carbonate salt in the filtrate.
The gas evolved is likely carbon dioxide (CO₂).
(2bii) Adding dilute HCl to half of the residue in a test tube.
Observations: If there is effervescence (bubbling), it indicates the presence of a carbonate salt in the residue. The gas evolved is likely carbon dioxide (CO₂).
(2biii) Adding aqueous NaOH in drops, then in excess, to about 2cm³
Observations: If a white precipitate forms upon adding NaOH drops, and it dissolves upon excess addition of NaOH, it indicates the presence of a metal hydroxide in the solution.
(2biv) Adding aqueous ammonia in drops, then in excess, to another 2cm³ of the clear solution from (ii).
Observations: If a colored precipitate forms upon adding ammonia drops, and it dissolves upon excess ammonia addition, it indicates the presence of a metal hydroxide in the solution.
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
ADVERTISE | CONTACT US | PRIVACY POLICY | ABOUT US | DISCLAIMER

COMMENTS
May/June (OBJ & Essay) 2023 for WAEC Chemistry QUESTION AND ANSWER ROOM FREE [School Candidates] Wednesday, 24th May, 2023 Chemistry 2 (Essay) - 9:30am - 11:30am
The 2023 answers will be posted here on 24th May during the exam. WAEC Chemistry OBJ Answers Loading…. 1-10: ACADABBDAC. 11-20: BCAABBCCBB. 21-30: ACBABDACBB. 31-40: ABBDAADCDD. 41-50: BDCDDCDDCD. (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table.
The 2024 WAEC chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year's examination are in the syllabus, and nearly 90% of the questions are repeated. You don't have to worry about the 2024 WAEC chemistry questions and answers PDF (essay and objective).
Chemistry 2023 WAEC Past Questions. A. energy is released when liquids change to solids. B. carbon atoms in gaseous methane are further apart than those in solid diamond. C. there is large decrease in the volume of a solid metal when pressure is applied to it. D. particles move faster in the gaseous state than in the liquid state.
The 2023 Waec Chemistry Theory questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry Theory Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped. 3d) VIEW ...
WAEC GCE Chemistry Essay 2023. The above questions are not exactly 2023 WAEC Chemistry questions and answers but likely WAEC Physics repeated questions and answers. These questions are for practice. The 2023 WAEC GCE Chemistry expo will be posted on this page 30 minutes before the WAEC GCE Chemistry examination starts.
These are possible WAEC Chemistry theory questions and answers 2023 that may appear in this year's exam. There are not actual questions and answers but, sample questions for practices. 2.47g of dry pure copper (II) oxide was completely reduced to a copper using laboratory gas. The mass of the residue left was found to be 1.97g.
Welcome to official 2024 Chemistry WAEC answer page. We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo. WAEC Chemistry Questions and Answers 2024.
A. 2023 WAEC GCE CHEMISTRY ESSAY (THEORY) ANSWERS | 12TH DECEMBER, 2023. (1d) (i) Ensure that both copper (II) oxide and dilute trioxonitrate (V) acid used in the reaction are of high purity. (ii) Ensure that the beakers and stirring rods used are clean and dry. OR.
SSCE WAEC Chemistry Objective Questions and Answers. CHEMISTRY Paper 1 (Objective Test Questions) Paper 1 will last for 1 hours Use HB pencil throughout. Answer All Questions Each question is followed by four options lettered A to D. Find out the correct options for each question and shade in pencil on your answer sheet, the answer space which bears the same letter as the option you Chosen.
February 10 2021. We've made available some WAEC chemistry past questions and answers to help the students that will take chemistry during their West African Examinations Council. The free copies of the chemistry past questions can be downloaded from the link provided here. We hope to.
2024 WAEC CHEMISTRY (CHEM) THEORY (ESSAY) ANSWERS: (2a) (I)Deliquescence is the process by which a substance absorbs moisture from the atmosphere until it dissolves in the absorbed water and forms a solution. This typically occurs with hygroscopic substances that have a high affinity for water. (II)Efflorescence is the process by which a ...
We provide 2024 Chemistry WAEC Questions and Answers on Essay, Theory, OBJ midnight before the exam, this is verified & correct WAEC Chems Expo ... Chemistry (Chems) WAEC Authentic Questions and Answer 2023 Password/Pin/Code: 1531. CHEMISTRY OBJ. 1- 10: ACADABBDAC. 11-20: BCACBBCCBA. 21-30: ACBABDCCCB. 31-40: CBBDAABDAD. 41-50: ACCDABDDCD ...
See also: 2023 WAEC Biology Practical Specimen, Questions and Answers. WAEC Chemistry Essay Questions for 2023. These are the kinds of theory questions that you are likely going to see in WAEC Chemistry for 2023. SECTION A. 1. Compound A consisting of carbon and hydrogen only. The compound was found to contain 80% carbon by mass.
This is a video of our top 10 commonly repeated topics in WAEC Chemistry to aid students who are preparing to write the 2023 WASSCE Chemistry. It is a summar...
waec Chemistry Questions and answers 2023 Here is the waec chemistry question and Answers for 2023. ANSWERS (1a) A transition element, also known as a transition metal, is an element that belongs to the d-block of the periodic table. (1b) loading. (1c) The increase in the first ionization energies across a period is mainly due to the increasing effective nuclear charge and decreasing atomic ...
The Waec Chemistry Answers 2024 essay and objective questions for the West African Examination Council (WAEC) Chemistry SSCE exam paper scheduled to be written on Wednesday, 22nd May 2024 can now be studied from here. The 2024 Chemistry Essay answer paper will start at 9:30 am and will last for 2hrs while the Objective exam will commence at 11: ...
WAEC GCE Chemistry 2023 Question and Answers: Get WAEC GCE Chemistry Objective & essay questions and answers for Tuesday, 12th December, 2023, Paper III & II: Objective & Essay -Chemistry - 9:30am - 11:00am, Objective - 11:00am - 12:15pm. Recommended: WAEC Result 2023 is out | Check WAEC Result Now For School Candidates Free
The 2024 WAEC Chemistry questions are sourced from the SS1 to SS3 Chemistry syllabus. Thus, all questions in this year's examination are syllabus-based, with nearly 90% being repetitions. No need to fret over obtaining the 2024 WAEC Chemistry questions and answers PDF (essay and objective). The WAEC Chemistry answers for 2024 will be ...
WAEC questions and answers are provided for free. We will do same during Other Exam like WAEC GCE. WAEC 2023 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once the verification process complete, we will go ahead to upload it here.
If yes, don't hesitate to share them with others by sending it to [email protected]. Practice WAEC Past Questions and Answers Online - All Subjects. WAEC recently launched a portal called WAEC e-learning to curb the number of failures in the WAEC May/June SSCE by creating a portal that contains the resources for all WAEC approved ...
The Waec English answers 2023 to waec English questions can now be seen here. The West African Examination Council (WAEC) English Language SSCE paper will be written on Wednesday, 17th May 2023. There will be three papers in Waec English 2023 - Papers 1, 2 (Objectives and Essay) to be written from 9:30 am to 12.30 pm and Paper 3 (Test of ...
2023 WAEC GCE (1st Series) 2023 WAEC GCE (2nd Series) 2023 NECO GCE EXPO ... *2024 WAEC CHEMISTRY PRACTICAL ANSWERS* *NUMBER THREE* (3a) (PICK TWO) (i ) Carbon dioxide (CO2) (ii) Ammonia (NH3) ... Waec 2024 Agricultural Science Obj & Essay Questions And Answers Now Available. Next. WAEC GCE candidates (2nd series) can choose CBT or PPT ...