
Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download

Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
- Last modified on: 1 month ago
- Reading Time: 11 Minutes
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Here, we have provided case based/passage based questions for Class 10 Science Chapter 1 Chemical Reactions and Equations.

| CBSE | |
| U | Class 10 Students |
| Science | |
| Chapter 1 Chemical Reactions and Equations | |
| Case Study Questions | |
| 3 | |
| Yes |
Table of Contents
Case Study/Passage Based Questions on Chemical Reactions and Equations
Case Study/Passage Based Questions
Question 1:
Corrosion is the phenomenon of deterioration of surface of metal in presence of air and moisture. It is a natural process and in the presence of a moist atmosphere, chemically active metals get corroded. This is an oxidation reaction. Rusting is the process where iron corrodes due to exposure to the atmosphere. The main circumstance of corrosion occurs with iron because it is a structural material in construction, bridges, buildings, rail transport, ships, etc. Aluminium is also an important structural metal, but even aluminium undergoes oxidation reactions. However, aluminium doesn’t corrode or oxidize as rapidly as its reactivity suggests. Copper (Cu) corrodes and forms a basic green carbonate.
(i) What is rusting?
(ii) Which two metals do not corrode easily?
(iii) Write the chemical name of the compound formed on corrosion of silver.
(iv) Corrosion is (a) a redox reaction (b) a reduction reaction (c) a displacement reaction (d) an oxidation reaction
Also read: Assertion Reason Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question 2:
Oxidation is the process of gaining of oxygen, or losing of hydrogen. Reduction is the process of losing of oxygen or gaining of hydrogen. The substance which undergoes oxidation is the reducing agent while the substance which undergoes reduction is known as the oxidising agent. Oxidation and reduction always take place together and these type of reactions are known as redox reactions. Some of the examples of redox reactions are given below:

(i) Give two examples of oxidation reaction from your everyday life.
(ii) Write the oxidising agent in the reaction III and VI.
(iii) Which of the following is an oxidising agent? (a) LiAlH 4 (b) Alkaline KMnO 4 (c) Acidified K 2 Cr 2 O 7 (d) Both (b) and (c)
(iv) Out of oxidation and reduction, which reaction takes place at anode?
Also read: Extra Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question 3:
A chemical reaction is a representation of chemical change in terms of symbols and formulae of reactants and products. There are various types of chemical reactions like combination, decomposition, displacement, double displacement, oxidation and reduction reactions. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. All combustion reactions are exothermic reactions.
(i) The chemical reaction in which a single substance breaks down into two or more simpler substances upon heating is known as (a) thermal decomposition reaction (b) photo decomposition reaction (c) electric decomposition reaction (d) both (a) and (c)
(ii) The massive force that pushes the rocket forward through space is generated due to the (a) combination reaction (b) decomposition reaction (c) displacement reaction (d) double displacement reaction
(iii) A white salt on heating decomposes to give brown fumes and yellow residue is left behind. The yellow residue left is of (a) lead nitrate (b) nitrogen oxide (c) lead oxide (d) oxygen gas
(iv) Which of the following reactions represents a combination reaction? (a) CaO (s) + H 2 O (l) → Ca(OH) 2 (aq) (b) CaCO 3 (s) → CaO (s) + CO 2 (g) (c) Zn(s) + CuSO 4 (aq) → ZnSO 4 (aq) + Cu(s) (d) 2FeSO 4 (s) → Fe 2 O 3 (s) +SO 2 (g) + SO 3 (g)
(v) Complete the following statements by choosing correct type of reaction for X and Y. Statement 1: The heating of lead nitrate is an example of ‘X’ reaction. Statement 2: The burning of magnesium is an example of ‘Y’ reaction. (a) X- Combination, Y- Decomposition (b) X- Decomposition, Y-Combination (c) X- Combination, Y-Displacement (d) X- Displacement, Y-Decomposition
Related Posts
Download cbse books.
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
3 thoughts on “ Case Study and Passage Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations ”
Good examples! But can you please available practical types and equations type of case based questions which we can read and learn an then they help us to solve the Boards examm. Pleaseeww🙂🙂🙂
would love to see more equation based questions. nevertheless, it proved quite useful in my revision!
after going through the above content child should develops ideas to answer based on knowledge acquired.
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
myCBSEguide
- Case Study Questions Class...
Case Study Questions Class 10 Science
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Download Case study questions for CBSE class 10 Science in PDF format from the myCBSEguide App . We have the new pattern case study-based questions for free download. Class 10 Science case study questions
This article will guide you through:
What are case study questions?
- Sample Papers with Case Study questions
- Class 10 Science Case Study question examples
- How to get case-based questions for free?
- How to attempt the case-based questions in Science?
Questions based on case studies are some real-life examples. The questions are asked based on a given paragraph i.e. Case Study. Usually, 4-5 questions are asked on the basis of the given passage. In most cases, these are either MCQs or assertion & reason type questions. Let’s take an example to understand. There is one paragraph on how nitrogen is generated in the atmosphere. On the basis of this paragraph, the board asks a few objective-type questions. In other words, it is very similar to the unseen passages given in language papers. But the real cases may be different. So, read this article till the end to understand it thoroughly.
What is CBE?
CBSE stands for competency-based education. The case study questions are part of this CBE. The purpose of CBE is to demonstrate the learning outcomes and attain proficiency in particular competencies.
Questions on Real-life Situations
As discussed the case study questions are based on real-life situations. Especially for grade 10 science, it is very essential to have the practical knowledge to solve such questions. Here on the myCBSEguide app, we have given many such case study paragraphs that are directly related to real-life implications of the knowledge.
Sample Papers with Case Study Questions
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App . There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions. In some cases, you will find that the question is not asked directly from the passage but is based on the concept that is discussed there. That’s why it is very much important to understand the background of the case study paragraph.
CBSE Case Study Sample Papers
You can download CBSE case study sample papers from the myCBSEguide App or Student Dashboard. Here is the direct link to access it.
Case Study Question Bank
As we mentioned that case study questions are coming in your exams for the last few years. You can get them in all previous year question papers issued by CBSE for class 1o Science. Here is the direct link to get them too.
Class 10 Science Case Study Question Examples
As you have already gone through the four questions provided in the CBSE model question paper , we are proving you with other examples of the case-based questions in the CBSE class 10 Science. If you wish to get similar questions, you can download the myCBSEguide App and access the Sample question papers with case study-type questions.
Case-based Question -1
Read the following and answer any four questions: Salt of a strong acid and strong base is neutral with a pH value of 7. NaCl common salt is formed by a combination of hydrochloride and sodium hydroxide solution. This is the salt that is used in food. Some salt is called rock salt bed of rack salt was formed when seas of bygone ages dried up. The common salt thus obtained is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, and bleaching powder.
- Phosphoric acid
- Carbonic acid
- Hydrochloric acid
- Sulphuric acid
- Blue vitriol
- Washing soda
- Baking soda
- Bleaching powder
Case-based Question -2
- V 1 + V 2 + V 3
- V 1 – V 2 +V 2
- None of these
- same at every point of the circuit
- different at every point of the circuit
- can not be determined
- 20 3 Ω 203Ω
- 15 2 Ω 152Ω
Case-based Question -3
- pure strips
- impure copper
- refined copper
- none of these
- insoluble impurities
- soluble impurities
- impure metal
- bottom of cathode
- bottom of anode
How to Attempt the Case-Based Questions in Science?
Before answering this question, let’s read the text given in question number 17 of the CBSE Model Question Paper.
All living cells require energy for various activities. This energy is available by the breakdown of simple carbohydrates either using oxygen or without using oxygen.
See, there are only two sentences and CBSE is asking you 5 questions based on these two sentences. Now let’s check the first questions given there.
Energy in the case of higher plants and animals is obtained by a) Breathing b) Tissue respiration c) Organ respiration d) Digestion of food
Now let us know if you can relate the question to the paragraph directly. The two sentences are about energy and how it is obtained. But neither the question nor the options have any similar text in the paragraph.
So the conclusion is, in most cases, you will not get direct answers from the passage. You will get only an idea about the concept. If you know it, you can answer it but reading the paragraph even 100 times is not going to help you.
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- CBSE Practice Papers 2023
- Class 10 Science Sample Papers 2024
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
3 thoughts on “Case Study Questions Class 10 Science”
Where is the answer
Class 10 Science MCQ
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 1st Standard - CVBHSS Materials
- 2nd Standard - CVBHSS Materials
- 3rd Standard - CVBHSS Materials
- 4th Standard - CVBHSS Materials
- 5th Standard - CVBHSS Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships

CBSE 10th Standard Science Subject Chemical Reactions and Equations Chapter Case Study Questions With Solution 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 10 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
Cbse 10th standard science subject chemical reactions and equations case study questions with solution 2021.
10th Standard CBSE
Final Semester - June 2015
Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it. In a chemical equation, the substances which combine or react are called reactants and new substances produced are called products. A chemical equation is a short hand method of representing a chemical reaction. A balanced chemical equation has equal number of atoms of different elements in the reactants and products side. An unbalanced chemical equation has unequal number of atoms of one or more elements in reactants and products. Formulae of elements and compounds are not changed to balance an equation. (i) Consider the following reaction: pMg 3 N 2 + qH 2 O ⇾ rMg(OH) 2 + sNH 3 When the equation is balanced, the coefficients p, q, r, s respectively are
(ii) Which of the following information is not conveyed by a balanced chemical equation?
(iii) The balancing of chemical equations is in accordance with
(iv) Which of the following chemical equations is an unbalanced one?
(v) Which of the following statements is/are correct?
In decomposition reactions, a single reactant breaks down to form two or more products. A decomposition reaction is opposite to combination reaction. Thermal decomposition reactions use the energy in form of heat for the decomposition of reactants. Electrolytic decomposition reactions involve the use of electrical energy for the decomposition of reactant molecules. Photolysis or photochemical decomposition involves the use of light energy for the purpose of decomposition. (i) Which of the following reactions is a decomposition reaction?
\({ (ii) \ } 2 \mathrm{~Pb}\left(\mathrm{NO}_{3}\right)_{2} \longrightarrow 2 \mathrm{PbO}+n A+\mathrm{O}_{2}\) What is nA in the given reaction?
(iii) Amino acid is formed by the decomposition of which component of our diet?
(iv) Silver chloride on exposure to sunlight for a long duration turns grey due to (I) the formation of silver by decomposition of silver chloride (II) sublimation of silver chloride (III) decomposition of chlorine gas from silver chloride (IV) oxidation of silver chloride The correct statement(s) is/are
(v) What type of chemical reaction takes place when electricity is passed through water?
Redox reactions are those reactions in which oxidation and reduction occur Simultaneously. A redox reaction is made up of two half reactions. In the first half reaction, oxidation takes place and in second half reaction, reduction occurs. Oxidation is a process in which a substance loses electrons and in reduction, a substance gains electrons. The substance which gains electrons is reduced and acts as an oxidising agent. On the other hand, a substance which loses electrons is oxidised and acts as a reducing agent. (i) Which of the following is a redox reaction?
| \({ (a) \ } \mathrm{CaCO}_{3} \rightarrow \mathrm{CaO}+\mathrm{CO}_{2}\) | \(\text { (b) } \mathrm{H}_{2}+\mathrm{Cl}_{2} \rightarrow 2 \mathrm{HCl}\) |
| \({ (c) \ } \mathrm{CaO}+2 \mathrm{HCl} \rightarrow \mathrm{CaCl}_{2}+\mathrm{H}_{2} \mathrm{O}\) | \(\text { (d) } \mathrm{NaOH}+\mathrm{HCl} \rightarrow \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}\) |
(ii) Identify the reaction in which H2 02 is acting as a reducing agent.
| \(\text { (a) } \mathrm{H}_{2} \mathrm{SO}_{3}+\mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow \mathrm{H}_{2} \mathrm{SO}_{4}+\mathrm{H}_{2} \mathrm{O}\) | \(\text { (b) } 2 \mathrm{Hl}+\mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow 2 \mathrm{H}_{2} \mathrm{O}+\mathrm{I}_{2}\) |
| \(\text { (c) } \mathrm{Cl}_{2}+\mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow 2 \mathrm{HCl}+\mathrm{O}_{2}\) | \(\text { (d) } 2 \mathrm{FeCl}_{2}+2 \mathrm{HCl}+\mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow 2 \mathrm{FeCl}_{3}+2 \mathrm{H}_{2} \mathrm{O}\) |
(iii) For the following reactions, identify the one in which H 2 S acts as a reducing agent.
| \(\text { (a) } \mathrm{CuSO}_{4}+\mathrm{H}_{2} \mathrm{~S} \longrightarrow \mathrm{CuS}+\mathrm{H}_{2} \mathrm{SO}_{4}\) | \(\text { (b) } \mathrm{Cd}\left(\mathrm{NO}_{3}\right)_{2}+\mathrm{H}_{2} \mathrm{~S} \longrightarrow \mathrm{CdS}+2 \mathrm{HNO}_{3}\) |
| \(\text { (c) } 2 \mathrm{FeCl}_{3}+\mathrm{H}_{2} \mathrm{~S} \longrightarrow 2 \mathrm{FeCl}_{2}+2 \mathrm{HCl}+\mathrm{S}\) | (d) None of these |
(iv) For the following reaction, identify the correct statement. \(\mathrm{ZnO}+\mathrm{CO} \longrightarrow \mathrm{Zn}+\mathrm{CO}_{2}\)
| is being oxidised | |
(v) In the following reaction, which substance is reduced? \(\mathrm{PbS}+4 \mathrm{H}_{2} \mathrm{O}_{2} \longrightarrow \mathrm{PbSO}_{4}+4 \mathrm{H}_{2} \mathrm{O}\)
| O | O |
In a balanced chemical reaction, equal number of atoms are present on both sides of reaction. A balanced chemical reaction is based on law of conservation of mass which means that total mass of reactants and products participating in a reaction must be equal. For example, a balanced chemical equation of burning of magnesium in oxygen to form magnesium oxide is written as : \(2 \mathrm{Mg}+\mathrm{O}_{2} \longrightarrow 2 \mathrm{MgO}\) The mass of reactants (2 x 24 + 32 = 80) is equal to the mass of products [2 x (24 + 16) = 80] (i) In a reaction, 35 g of reactant, PQ breaks down into 20 g of product, P and an unknown amount of product, Q. Using the law of conservation of mass, weight of products, Q will be
| ( |
(ii) When solid mercury (II) oxide is heated, liquid mercury and oxygen gas are produced. Which of the following statements is true regarding the balanced chemical equation for this process? (a) 1 mole of mercury (II) oxide produces two moles of mercury and one mole of oxygen gas (b) 2 moles of mercury (II) oxide produce one mole of mercury and one mole of oxygen gas (c) 1 mole of mercury (II) oxide produces half mole of mercury and half mole of oxygen gas (d) 2 moles of mercury (II) oxide produce 2 moles of mercury and one mole of oxygen gas (iii) Which of the following laws is satisfied by a balanced chemical equation?
(iv) In the given chemical reaction \(\mathrm{C}_{6} \mathrm{H}_{6(l)}+15 \mathrm{O}_{2(g)} \longrightarrow m \mathrm{CO}_{2(g)}+n \mathrm{H}_{2} \mathrm{O}_{(l)}\) The values of m and n are respectively
| ( |
(v) Sulphur dioxide reacts with oxygen to form sulphur trioxide. What would be the molar ratio of sulphur dioxide to sulphur trioxide?
In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be observed easily. These easily observed features which take place as a result of chemical reaction are known as characteristics of chemicals reactions. Some important characteristics of chemical reactions are: (I) Evolution of heat (II) Formation of precipitate (III) Change in colour (IV) Change in temperature (V) Change in state Anyone of these general characteristics can tell us whether a chemical reaction has taken place or not. (i) Reaction of magnesium with air is a/an
(ii) In the following reaction \(\mathrm{Ca}_{(a q)}^{2+}+2 \mathrm{OH}_{(a q)}^{-} \longrightarrow \mathrm{Ca}(\mathrm{OH})_{2(s)}\) precipitate of calcium hydroxide will be of
(iii) In the given reaction, \(\mathrm{S}_{(s)}+\mathrm{O}_{2(g)} \longrightarrow \mathrm{SO}_{2}\) the physical state of SO 2 is
(iv) Which one of the following processes involve chemical reactions?
(v) In which of the following reactions, high amount of heat energy will be evolved?
*****************************************
Cbse 10th standard science subject chemical reactions and equations case study questions with solution 2021 answer keys.
\({ (i) }(\mathrm{b}): \mathrm{Mg}_{3} \mathrm{~N}_{2}+6 \mathrm{H}_{2} \mathrm{O} \longrightarrow 3 \mathrm{Mg}(\mathrm{OH})_{2}+2 \mathrm{NH}_{3}\) (ii) (d) (iii) (c): In a balanced chemical equation, total mass of reactants must be equal to the total mass of products. This is the statement of law of conservation of mass. (iv) (b) (v) (d)
(i) (c) \({ (ii) \ }(b): \ 2 \mathrm{~Pb}\left(\mathrm{NO}_{3}\right)_{2} \longrightarrow 2 \mathrm{PbO}+4 \mathrm{NO}_{2}+\mathrm{O}_{2}\) (iii) (c) : Proteins in our diet get broken down into amino acids. \({ (iv) }(\mathrm{a}): 2 \mathrm{AgCl}_{(\mathbf{s})} \stackrel{\text { Sunlight }}{\longrightarrow} 2 \mathrm{Ag}_{(s)}+\mathrm{Cl}_{2(g)}\) (v) (b): Electrolysis of water is electrolytic decomposition. \(\mathrm{H}_{2} \mathrm{O} \stackrel{\text { Current }}{\longrightarrow} 2 \mathrm{H}_{2}+\mathrm{O}_{2}\)
(I) (b) : H 2 is oxidised to HCI while Cl 2 is reduced to HCl. (ii) (c) \((iii) (c): 2 \mathrm{Fe} \mathrm{Cl}_{3}+\mathrm{H}_{2} \mathrm{~S} \longrightarrow 2 \mathrm{FeCl}_{2}+2 \mathrm{HCl}+\mathrm{s}\) H 2 Sitself gets oxidised to Sand reduces FeCl 3 to FeCI 2 (iv) (a ): ZnO is reduced to Zn and CO is oxidised to CO 2 (v) (b) : H 2 O 2 is reduced to water by removal of oxygen.
\(\text { (i) }(\mathbf{d}): P Q \longrightarrow P+Q\) \(35 \mathrm{~g} \quad \ \ \ 20 \mathrm{~g}+\text { ? }\) According to law of conservation of mass, Mass of PQ = Mass of P + Mass of Q \(\therefore\) Mass of Q = (35 - 20)g = 15 g \({ (ii) \ }(\mathbf{d}): 2 \mathrm{HgO}_{(s)} \longrightarrow 2 \mathrm{Hg}_{(l)}+\mathrm{O}_{2(g)}\) (iii) (b) (iv) (b) (v) (b)
(i) (a) (ii) (d) : Calcium hydroxide is a white colour solid. (iii) (c) : SO 2 is gaseous in nature. (iv) (d) : When copper is heated in the presence of air in a very high temperature, a chemical reaction takes place. Copper reacts with oxygen of the air to form a thin layer of copper oxide on the surface of metallic copper (v) (c) : On burning ofL.P.G., heat is evolved.
Related 10th Standard CBSE Science Materials
10th standard cbse syllabus & materials, cbse 10th maths probability chapter case study question with answers, cbse 10th maths statistics chapter case study question with answers, cbse 10th maths surface areas and volumes chapter case study question with answers, cbse 10th maths areas related to circles chapter case study question with answers, cbse 10th maths circles chapter case study question with answers, cbse 10th maths some applications of trigonometry chapter case study question with answers, cbse 10th maths introduction to trigonometry chapter case study question with answers, cbse 10th maths coordinate geometry chapter case study question with answers, cbse 10th maths triangles chapter case study question with answers, cbse 10th maths arithmetic progressions chapter case study questions with answers, cbse 10th maths quadratic equations chapter case study questions with answers, cbse 10th social science the making of a global world chapter case study question with answers, cbse 10th social science nationalism in india chapter case study question with answers, cbse 10th social science the rise of nationalism in europe chapter case study question with answers, cbse 10th maths pair of linear equation in two variables chapter case study question with answers.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

10th Standard CBSE Study Materials

10th Standard CBSE Subjects
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Case Study Chapter 1 Chemical Reactions and Equations
Please refer to Chapter 1 Chemical Reactions and Equations Case Study Questions with answers provided below. We have provided Case Study Questions for Class 10 Science for all chapters as per CBSE, NCERT and KVS examination guidelines. These case based questions are expected to come in your exams this year. Please practise these case study based Class 10 Science Questions and answers to get more marks in examinations.
Case Study Questions Chapter 1 Chemical Reactions and Equations
Case/Passage – 1 The reaction between MnO 2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released .

Question: Identify the correct statement from the following: (a) MnO2 is getting reduced whereas HCl is getting oxidized (b) MnO2 is getting oxidized whereas HCl is getting reduced. (c) MnO2 and HCl both are getting reduced. (d) MnO2 and HCl both are getting oxidized.
Question: Chlorine gas reacts with _____ to form bleaching powder. (a) dry Ca(OH) 2 (b) dil. solution of Ca(OH) 2 (c) conc. solution of Ca(OH) 2 (d) dry CaO
Question: In the above discussed reaction, what is the nature of MnO2? (a) Acidic oxide (b) Basic oxide (c) Neutral oxide (d) Amphoteric oxide
Question: The chemical reaction between MnO2 and HCl is an example of: (a) displacement reaction (b) combination reaction (c) redox reaction (d) decomposition reaction.
Question: What will happen if we take dry HCl gas instead of aqueous solution of HCl? (a) Reaction will occur faster. (b) Reaction will not occur. (c) Reaction rate will be slow. (d) Reaction rate will remain the same.
Case/Passage – 2
Chemistry in Automobiles: For an internal combustion engine to move a vehicle down the road, it must convert the energy stored in the fuel into mechanical energy to drive the wheels. In your car, the distributor and battery provide this starting energy by creating an electrical “spark”, which helps in combustion of fuels like gasoline. Below is the reaction depicting complete combustionof gasoline in full supply of air: 2C 8 H 18 (I) + 25O 2 (g) → 16 ‘X’ + Y Question: Which of the following are the products obtained from the reaction mentioned in the above case? Product ‘ X’ Product ‘Y’ (a) CO 2 H 2 O 2 (b) H 2 O CO (c) CH 3 OH H 2 O (d) CO 2 H 2 O
Question: On the basis of evolution/absorption of energy, which of the following processes are similar to combustion of fuel? (i) Photosynthesis in plants (ii) Respiration in the human body (iii) Decomposition of vegetable matter (iv) Decomposition of ferrous sulphate. (a) (ii) & (iii) (b) (i) & (ii) (c) (iii) & (iv) (d) (ii) & (i)
Question: ‘Although nitrogen is the most abundant gas in the atmosphere, it does not take part in combustion’. Identify the correct reason for this statement. (a) Nitrogen is a reactive gas (b) Nitrogen is an inert gas (c) Nitrogen is an explosive gas (d) Only hydrocarbons can take part in combustion
Question: Identify the types of chemical reaction occurring during the combustion of fuel: (a) Oxidation & Endothermic reaction (b) Decomposition & Exothermic reaction (c) Oxidation & Exothermic reaction (d) Combination & Endothermic reaction
Question: ‘A student while walking on the road observed that a cloud of black smoke belched out from the exhaust stack of moving trucks on the road.’ Choose the correct reason for the production of black smoke: (a) Limited supply of air leads to incomplete combustion of fuel. (b) Rich supply of air leads to complete combustion of fuel. (c) Rich supply of air leads to a combination reaction. (d) Limited supply of air leads to complete combustion of fuel.
Related Posts

Case Study Chapter 13 Magnetic Effect of Electric Current

Life Lines of National Economy Class 10 Social Science Notes and Questions

CBSE Class 10 English Fog Summary
- Bihar Board
CFA Institute
Srm university.
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
- CBSE Class 10 Study Material
Important Case Study Questions for CBSE Class 10 Science Exam 2024 with Answers
Download case study questions for class 10 science to prepare for the cbse board exam 2024. these multiple choice type questions with answers are published by the cbse board to provide sample questions to students..

CBSE Class 10 Science Case Study Questions 2024: Get here the questions based on case studies to practise for the CBSE Class 10 Science exam 2024. The CBSE Class 10 Science Question Bank on Case Studies, provided in this article, can be very helpful for understanding how the source based or case based questions are asked in the board exam. This question bank is published by the CBSE Board itself which makes it a very reliable source for the board exam preparations. Each question has five sub-questions with each followed by four options and a correct answer. Students can easily download these sample questions in PDF format and refer to the same for their exam preparations.
Note: Check the reduced CBSE Syllabus for Class 10 Science for 2024 Exam and then practise the case study questions accordingly for the CBSE Class 10 Board Exam 2024.
Important* Important Last Minute Tips and Resources for CBSE Class 10 Science Exam 2024
SCIENCE- Class X
Sample Case Studies
1. Read the following and answer any four questions from 1.1 to 1.5:
Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

1.1 The substance not likely to contain CaCO 3 is
a) Dolomite
b) A marble statue
c) Calcined gypsum
d) Sea shells.
Answer: c) Calcined gypsum
1.2 A student added 10g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition took place?
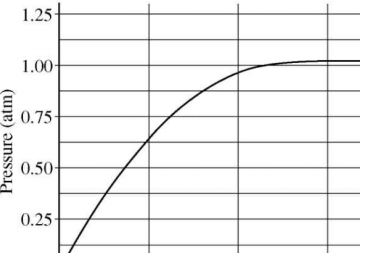
a) 15-20 min
b) 10-15 min
c) 5-10 min
Answer: d) 0-5 min
1.3 Gas A, obtained above is a reactant for a very important biochemical process which occurs in the presence of sunlight. Identify the name of the process -
a) Respiration
b) Photosynthesis
c) Transpiration
d) sphotolysis
Answer: b) Photosynthesis
1.4 Marble statues are corroded or stained when they repeatedly come into contact with polluted rain water. Identify the main reason.

a) decomposition of calcium carbonate to calcium oxide
b) polluted water is basic in nature hence it reacts with calcium carbonate
c) polluted water is acidic in nature hence it reacts with calcium carbonate
d) calcium carbonate dissolves in water to give calcium hydroxide.
Answer: c) polluted water is acidic in nature hence it reacts with calcium carbonate
1.5 Calcium oxide can be reduced to calcium, by heating with sodium metal. Which compound would act as an oxidizing agent in the above process?
b) sodium oxide
d) calcium oxide
Answer: d) calcium oxide
2. Read the following and answer any four questions from 2.1 to 2.5:
The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released.
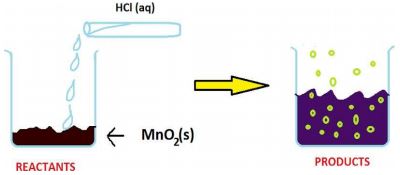
2.1 The chemical reaction between MnO 2 and HCl is an example of:
a) displacement reaction
b) combination reaction
c) redox reaction
d) decomposition reaction
Answer: c) redox reaction
2.2 Chlorine gas reacts with _______ to form bleaching powder.
a) dry Ca(OH) 2
b) dil. solution of Ca(OH) 2
c) conc. solution of Ca(OH) 2
Answer: a) dry Ca(OH) 2
2.3 Identify the correct statement from the following:
a) MnO 2 is getting reduced whereas HCl is getting oxidized
b) MnO 2 is getting oxidized whereas HCl is getting reduced.
c) MnO 2 and HCl both are getting reduced.
d) MnO 2 and HCl both are getting oxidized.
Answer: a) MnO 2 is getting reduced whereas HCl is getting oxidized
2.4 In the above discussed reaction, what is the nature of MnO 2 ?
a) Acidic oxide
b) Basic oxide
c) Neutral oxide
d) Amphoteric oxide
Answer: b) Basic oxide
2.5 What will happen if we take dry HCl gas instead of aqueous solution of HCl?
a) Reaction will occur faster.
b) Reaction will not occur.
c) Reaction rate will be slow.
d) Reaction rate will remain the same.
Answer: b) Reaction will not occur.
Also, check below other important study material released by the CBSE Board:
CBSE Class Maths Case Study Questions for All Chapters (Published by CBSE)
MCQs for Class 10 English Footprints without Feet (Published by CBSE)
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- KTET Hall Ticket 2024
- UKSSSC Excise Inspector Result 2024
- OSSC Skill Test Admit Card 2024
- UPSC CSE Admit Card 2024
- UP Lok Sabha Election Result 2024
- Varanasi Lok Sabha Election Result 2024
- Asaduddin Owaisi vs Madhavi Latha
- Purnia Lok Sabha Election Result 2024
- Vidisha Lok Sabha Election Result 2024
- CBSE Study Material
- CBSE Class 10
Latest Education News
KTET Hall Ticket 2024 Live: Admit Card Link to Active at ktet.kerala.gov.in
MHT CET Result 2024 Date and Time: Maharashtra CET Results to be Released on June 19 at cetcell.mahacet.org, Direct Link to Check Scores
Bernoulli's Principle: Definition, Formula, Equation And FAQ’s
राजस्थान पीटीईटी अपेक्षित कट ऑफ मार्क्स 2024: जानें क्या होगी न्यूनतम योग्यता अंक
NEET PG 2024 Final Edit Window Closes Today, Make Changes At nbe.edu.in
UP B.Ed Answer Key 2024: यूपी बीएड प्रवेश परीक्षा की उत्तर कुंजी यहाँ से करें डाउनलोड
JoSAA 2024 Counselling: Category-wise Top 20 Percentile Cut-off Marks Out of 500 Released, Check Details Here
PSEB Class 9th Home Science Syllabus 2024-25: Download New Syllabus PDF
JEE Advanced Cut-Off 2024 Released: Check IIT Madras JEE Advanced Category-Wise Cut-Off, Rank, and Qualifying Marks
Only eagle eyes can spot O among D’s in 5 seconds!
JEE Advanced Toppers List With Marks 2024 OUT, Check IIT Toppers Name, CRL, and Zone Here
Rajasthan PTET Question Paper 2024: यहां से डाउनलोड करें राजस्थान पीटीईटी पेपर पीडीएफ
UP Board Class 9 Moral Sports and Physical Education Syllabus 2024-25 OUT: Download Now!
(Updated) SA vs BAN Head to Head in T20 World Cup: Check Stats, Records and Results
उत्तर प्रदेश के किस जिले में हैं सबसे अधिक तहसील, जानें
Rajasthan PTET Question Paper 2024: Download B.Ed Paper PDF
Kerala PSC Staff Nurse Answer Key 2024 Out at keralapsc.gov.in: Here's Download Link
PM Modi Cabinet 2024: मोदी का राजतिलक, नई कैबिनेट में किसे मिली जगह और कौन हुआ बाहर? देखें पूरी लिस्ट
Rajasthan PTET Answer Key 2024: राजस्थान पीटीईटी परीक्षा की उत्तर कुंजी यहाँ देखें
Meet the 2024 Modi Cabinet: मोदी मंत्रिमंडल के सबसे अमीर मंत्री कौन है?
CBSE Expert
CBSE Class 10 Science Case Study Questions Download Free PDF
If you are looking for the CBSE Class 10 Science Case Study Questions in PDF, then you are in the right place. CBSE 10th Class Case Study for the Science Subject is available here. These Case studies can help the students to solve the different types of questions that are based on the case study.

CBSE Board will be asking case study questions based on Science subjects in the upcoming board exams. Thus, it becomes an essential resource to study.
The Science Subject case study for class 10th covers a wide range of chapters from the Science. Students willing to score good marks in their board exams can use it. The questions are highly interactive and it allows students to use their thoughts and skills to solve such kinds of questions.

Case Study Questions Class 10 Science
In board exams, students will find the questions based on assertion and reasoning . Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
- Case Study Questions for Chapter 1 Chemical Reactions and Equations
- Case Study Questions for Chapter 2 Acids, Bases, and Salts
- Case Study Questions for Chapter 3 Metals and Non-Metals
- Case Study Questions for Chapter 4 Carbon and Its Compounds
- Case Study Questions for Chapter 5 Periodic Classification of elements
- Case Study Questions for Chapter 6 Life Processes
- Case Study Questions for Chapter 7 Control and Coordination
- Case Study Questions for Chapter 8 How do organisms reproduce?
- Case Study Questions for Chapter 9 Heredity and Evolution
- Case Study Questions for Chapter 10 Light reflection and refraction
- Case Study Questions for Chapter 11 Human eye and colorful world
- Case Study Questions for Chapter 12 Electricity
- Case Study Questions for Chapter 13 Magnetic effects of current
- Case Study Questions for Chapter 15 Our Environment
The above Case studies for CBSE Class 10 Science will help you to score good marks in the Case Study questions that have been coming in your examinations. These CBSE Class 10 Science Case Study have been developed by experts of cbseexperts.com for benefit of Class 10 students.
Class 10 Science Assertion and Reason Questions
Case Study Type Questions in Science Class 10
Case Study Type Questions in Science Class 10 include the information or data. Students willing to solve them are required to read the passage carefully and then solve them. While solving the paragraph the ideal way is to highlight the key information or given data.
Because later it will ease them to write the final answers. Science Case study type questions consist of 4 to 5 questions that should be answered in an MCQ manner.
While reading the paragraph students will get the clue in between about the possible answer of the question. They should definitely highlight those questions. This is the best way to solve such kind of Case study Type Questions.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
Case Study Questions Class 10 Science Metals and Nonmetals
Case study questions class 10 science chapter 3 metals and nonmetals.
CBSE Class 10 Case Study Questions Science Metals and Nonmetals. Important Case Study Questions for Class 10 Board Exam Students. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Metals and Nonmetals.
CBSE Case Based Questions Class 10 Science Chemistry Chapter 3
Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue. The impurities must be removed from the ore prior to the extraction of the metal. The processes Several steps are involved in the extraction of pure metal from ores.Metals and Non-metalsused for removing the gangue from the ore are based on the differences between the physical or chemical properties of the gangue and the ore. Different separation techniques are accordingly employed.
Ans: Oxides of these metals are reduced to metal by simply heating.
Ans: 2Cu2O + 3O2+ heat 👉 2Cu2O(s) + 2SO2(g)
CASE STUDY 2 Metals and Nonmetals
Ans: The metal oxides are reduced to corresponding metal by simply using suitable reducing agents such as coke(carbon).
iv) Name some other reducing agent which can be used to extract metal from metal oxide?
CASE STUDY : 3 on Metals and Nonmetals
Iron when exposed to moist air for a long time acquires a coating of a brown flaky substance called rust. Let us find out the conditions under which iron rusts.
iv) What is meant by Galvanization?
Ans:It is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc.
Ans: Mg(s) + HCl(aq) 👉 MgCl2 + H2(g)
Ans: It is a freshly prepared mixture of concentrated HCl and Conc. Nitric acid in the ratio of 3:1. It is highly corrosive and fuming liquid.
Ans- Al2O3 + 6HCl 👉 2AlCl3 + 3H2O
Ans: Sodium Oxide and potassium oxide.
Leave a Reply Cancel reply
We have a strong team of experienced teachers who are here to solve all your exam preparation doubts, case study questions class 7 maths unitary method, in the bazaars of hyderabad class 11 mcq questions answers for wbchse semester 1, to autumn class 11 mcq question answers, assam scert class 8 geography and economics chapter 8 solutions.
- Class 6 Maths
- Class 6 Science
- Class 6 Social Science
- Class 6 English
- Class 7 Maths
- Class 7 Science
- Class 7 Social Science
- Class 7 English
- Class 8 Maths
- Class 8 Science
- Class 8 Social Science
- Class 8 English
- Class 9 Maths
- Class 9 Science
- Class 9 Social Science
- Class 9 English
- Class 10 Maths
- Class 10 Science
- Class 10 Social Science
- Class 10 English
- Class 11 Maths
- Class 11 Computer Science (Python)
- Class 11 English
- Class 12 Maths
- Class 12 English
- Class 12 Economics
- Class 12 Accountancy
- Class 12 Physics
- Class 12 Chemistry
- Class 12 Biology
- Class 12 Computer Science (Python)
- Class 12 Physical Education
- GST and Accounting Course
- Excel Course
- Tally Course
- Finance and CMA Data Course
- Payroll Course
Interesting
- Learn English
- Learn Excel
- Learn Tally
- Learn GST (Goods and Services Tax)
- Learn Accounting and Finance
- GST Tax Invoice Format
- Accounts Tax Practical
- Tally Ledger List
- GSTR 2A - JSON to Excel
Are you in school ? Do you love Teachoo?
We would love to talk to you! Please fill this form so that we can contact you
Case Based Questions (MCQ)
- NCERT Questions
- Questions from inside the book
- Teachoo Questions
- Assertion Reasoning Questions (MCQ)
- MCQ from Past Year Papers
- MCQs from NCERT Exemplar
Question 5 - Case Based Questions (MCQ) - Chapter 1 Class 10 - Chemical Reactions and Equations
Last updated at April 16, 2024 by Teachoo
In the following chemical reaction zinc oxide reacts with carbon to produce zinc metal and carbon monoxide.’’
Zno + c → zn + co , (a) name the substance getting oxidised and reduced in the above reaction: , (i) c and zno , (ii) zn and c , (iii) zno and co , (iv) co and zno .
ZnO + C → Zn + CO

- Addition of oxygen is known as oxidation
- Removal of oxygen is known as reduction.
Since in the equation
- Oxygen is being removed from ZnO
- and is added to C.
Therefore, C is undergoing oxidation and ZnO reduction.
So, the correct answer is (i) C and ZnO
(b) Name the type of reaction:
(i) oxidation reaction , (ii) reduction reaction , (iii) redox reaction , (iv) decomposition reaction .

Since both oxidation and reduction is taking place in this reaction, it is a redox reaction.
So, the correct answer is (iii) redox reaction
(c) The reduction reaction involves:
(i) gain of electrons , (ii) loss of electrons , (iii) increase in oxidation state , (iv) addition of oxygen .
So, the correct answer is (i) gain of electrons
(d) Which of the following is the effect of oxidation reaction in everyday life:
(i) precipitation , (ii) fermentation , (iii) corrosion , (iv) hydrogenation of oil .
Let’s look at each of the reaction , one by one
- Precipitation reaction reaction is not an oxidation reaction because this simply involves the formation of an insoluble substance (precipitate).
- Fermentation breaking down of food in the absence of oxygen
- Corrosion involves the oxidation of iron to form rust
- Hydrogenation of oil is not an oxidation reaction because in this, hydrogen atoms are added over the double bonds of hydrocarbons.
So, the correct answer is (iii) Corrosion
(e) The reactions used in black and white photography:
(i) decomposition of silver bromide , (ii) decomposition of silver chloride , (iii) both , (iv) none of the above .
Black and white photography is looks like
- The photographic papers are prepared by using silver chloride and silver bromide which are initially white in color.
- On exposure to air, AgCl and AgBr decompose and lose their color and black and white images are obtained.
So, the correct answer is (iii) Both

Maninder Singh
CA Maninder Singh is a Chartered Accountant for the past 14 years and a teacher from the past 18 years. He teaches Science, Economics, Accounting and English at Teachoo
Hi, it looks like you're using AdBlock :(
Please login to view more pages. it's free :), solve all your doubts with teachoo black.

Case Study Questions Class 10 Science Chapter 4 Carbon and Its Compounds
- Post author: studyrate
- Post published:
- Post category: class 10th
- Post comments: 0 Comments
CBSE Board Exam is on the way, so you must practice some good Case Study Questions Class 10 Science to boost your preparation to score 95+% on Boards. In this post, you will get Case Study and Passage Based Questions that will come in CBSE Class 10 Science Board Exams.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason . There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Carbon and its Compounds Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 10 Science Chapter 4 Carbon and Its Compounds .
Case Study/Passage Based Questions
Question 1:
Read the following and answer any four questions from (i) to (v).
The compounds which have the same molecular formula but differ from each other in physical or chemical properties are called isomers and the phenomenon is called isomerism. When the isomerism is due to differences in the arrangement of atoms within the molecule, without any reference to space, the phenomenon is called structural isomerism . In other words. structural isomers are compounds that have the same molecular formula but different structural formulas, i.e., they are different in the order in which different atoms are linked. In these compounds, carbon atoms can be linked together in the form of straight chains, branched chains or even rings.
(i) Which of the following sets of compounds have the same molecular formula? (a) Butane and iso-butane (b) Cyclohexane and hexene (C) Propanal and propanone (d) All of these
Answer: (d) All of these
(ii) In order to form branching, an organic compound must have a minimum of (a) four carbon atoms (b) three carbon atoms (c) five carbon atoms (d) any number of carbon atoms.
Answer: (a) four carbon atoms
(iii) Which of the following is an isomeric pair? (a) Ethane and propane (b) Ethane and ethene (c) Propane and butane (d) Butane and 2-methylpropane
Answer: (d) Butane and 2-methylpropane
(iv) Among the following the one having longest chain is (a) neo-pentane (b) iso-pentane (C) 2-methylpentane (d) 2,2-dimethylbutane.
Answer: (C) 2-methylpentane
(v) The number of isomers of pentane is (a) 2 (b) 3 (c) 4 (d) 5
Answer: (b) 3
Question 2:
Food, clothes, medicines, books, or any of the things are all based on this versatile element carbon. In addition, all living structures are carbon-based. The earth’s crust has only 0.02% carbon in the form of minerals. The element carbon occurs in different forms in nature with widely varying physical properties. Both diamond and graphite are formed by carbon atoms, the difference lies in the manner in which the carbon atoms are bonded to one another. Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation.
(i) From the given alternatives, whose chemical and physical properties are not the same? (a) Graphite and Diamond (b) Phosphorous and Sulphur (c) Carbon and Hydrogen (d) Methyl alcohol and Acetic acid
Answer: (d) Due to presence of different functional groups methyl alcohol and acetic acid. Possess different physical and chemical properties.
(ii) Which of the following statements is not correct? (a) Graphite is much less dense than diamond (b) Graphite is black and soft (c) Graphite has low melting point (d) Graphite feels smooth and slippery
Answer: (c) Graphite has low melting point
(iii) Which of the following are isomers? (a) Butane and isobutene (b) Ethane and ethene (c) Propane and propyne (d) Butane and isobutane
Answer: (d) Butane and isobutane have same chemical formula but different arrangement of atoms and have different structure.
(iv) Which one of the following is not an allotrope of carbon? (a) Soot (b) Graphite (c) Diamond (d) Carborundum
Answer: (d) Carborundum is SiC (silicon carbide).
(v) Pentane has the molecular formula C 5 H 12 . It has (a) 5 covalent bonds (b) 12 covalent bonds (c) 16 covalent bonds (d) 17 covalent bonds
Answer: (c) 16 covalent bonds
Question 3:
A homologous series is a series of organic compounds which belong to the same family (i.e. possess same functional group) and show similar chemical properties. The members of this series are called homologous and differ from each other by the number of CH 2 units in the main carbon chain.
3.1) Which of the following is not the property of a homologous series ? (a) They all contain double bond. (b) They differ by 14 units by mass. (c) They show similar chemical properties. (d) They can be represented by a general formula.
Answer: (a) They all contain double bond
3.2) Which of the following represent the name and formula of the 2nd member of homologous series having general formula C n H 2n + 2 ? (a) Methane CH 4 (b) Ethane C 2 H 6 (c) Ethyne C 2 H 6 (d) Ethene C 2 H 4
Answer: (b) Ethane C2H6
3.3) The chemical properties of which of the following compounds is similar to the butane ? (a) Propyne (b) Pentane (c) Butyne (d) Propene
Answer: (b) Pentane
3.4) The difference between two consecutive members in a homologous series in alkanes in terms of molecular mass and number of atoms of elements is : (a) 14 a.m.u and CH respectively (b) 12 a.m.u and CH respectively (c) 14 a.m.u and CH2 respectively (d) 12 a.m.u and CH3 respectively
Answer: (c) 14 a.m.u and CH2 respectively
3.5) Which of the following does not belong to the same homologous series ? (a) CH 4 (b) C 2 H 6 (c) C 3 H 8 (d) C 4 H 8
Answer: (d) C4H8
Question 4:
Some elements exist in various forms. These forms have different physical properties but have the same chemical properties. They are called allotropes of the element. Carbon, Phosphorous and sulphur have allotropes. Diamond and graphite are the allotropes of carbon. The differences in the physical properties of diamond and graphite are because of the manner in which the carbon atoms are arranged.
4.1) The hardest substance known which doesn’t conduct electricity is : (a) graphite (b) fullerence (c) diamond (d) methane
Answer:(c) diamond
4.2) Diamond is a : (a) three dimensional structure (b) one dimensional structure (c) two dimensional structure (d) none of these
Answer:(a) three dimensional structure
4.3) Graphite is used as a crucible to melt metals because : (a) it has low melting point (b) it has high boiling point (c) it has low boiling point (d) it has high melting point
Answer:(d) it has high melting point
4.4) Which of the following is correct the structure of diamond ? (a) Carbon atoms are held together by single covalent bonds. (b) Carbon atoms conduct electricity in the molten state. (c) Layers of atoms slide easily over each other. (d) Electrons move freely through the structure.
Answer:(a) Carbon atoms are held together by single covalent bonds.
4.5) Diamond is not a good conductor of electricity because : (a) it is very hard. (b) it has no free electrons. (c) it is not water soluble. (d) its structure is very compact.
Answer:(b) it has no free electrons..
Hope the information shed above regarding Case Study and Passage Based Questions for Class 10 Science Chapter 4 Carbon and Its Compounds with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 10 Science Carbon and Its Compounds Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible. By Team Study Rate
You Might Also Like
Class 10 maths case study questions pdf download, class 10 maths case study questions chapter 3 pair of linear equations in two variables.
![case study class 10 chemistry Read more about the article [PDF]CBSE Class 10th Previous Year Question Papers PDF with Solutions](https://schools.studyrate.in/wp-content/uploads/2022/10/WhatsApp-Image-2022-10-27-at-5.37.18-PM-300x169.jpeg)
[PDF]CBSE Class 10th Previous Year Question Papers PDF with Solutions
Leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
- Important Questions
- Important Questions Class 10 Chemistry
- Important Questions Class 10 Chemistry Chapter 1 Chemical Reactions and Equations

Class 10 Chemistry Chapter 1 - Chemical Reactions and Equations Important Questions with Answers
Class 10 chemistry important questions with answers are provided here for Chapter 1 Chemical Reactions and Equations. These important questions are based on CBSE board curriculum and correspond to the most recent Class 10 chemistry syllabus. By practising these Class 10 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Annual examinations.
Download Class 10 Chemistry Chapter 1 Chemical Reactions and Equations Important Questions with Answers PDF by clicking on the button below. Download PDF
Recommended Videos
Chemical reactions and equations class 10 in one-shot.

Class 10 Chapter 1 Chemical Reactions and Equations Important Questions with Answers
Multiple choice type questions.
Q1. Which of the following gases is used to store fat and oil-containing foods for a long time?
- Carbon dioxide
(3) Nitrogen gas is used to store fat and oil-containing foods for a long time.
Q2. The chemical reaction between Hydrogen sulphide and iodine to give Hydrogen iodide and sulphur is given below:
H 2 S + I 2 → 2HI + S.
The reducing and oxidising agents involved in this redox reaction are:
- Iodine and sulphur, respectively
- Iodine and hydrogen sulphide, respectively
- Sulphur and iodine, respectively
- Hydrogen sulphide and sulphur, respectively
(2) Iodine is an oxidising agent, and hydrogen sulphide is the reducing agent in the reaction mentioned above.
Short Answer Type Questions
Q1. Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a )Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773K to form ammonia gas.
(b )Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water.
(c ) Ethanol is warmed with ethanoic acid to form ethyl acetate in the presence of concentrated H 2 SO 4 .
(d) Ethene is burnt in the presence of oxygen to form carbon dioxide and water and releases heat and light.
It is an addition reaction.
(b ) NaOH (aq) + CH 3 COOH (aq) → CH 3 COONa (aq) + H 2 O (l)
It is a double displacement or a neutralisation reaction.
It is a double displacement or an esterification reaction.
(d ) C 2 H 4 (g) + 3 O 2 (g) → 2 CO 2 (g) + 2 H 2 O (g) + Heat + light
It is a redox or a combustion reaction.
Q2. Write the balanced chemical equations for the following reactions and identify the type of
reaction in each case.
(a ) In the thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
(b ) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
(c ) Chlorine gas is passed in an aqueous potassium iodide solution to form potassium
chloride solution and solid iodine.
(d ) Ethanol is burnt in the air to form carbon dioxide and water and releases heat.
(a ) Fe 2 O 3 (s) + 2 Al (s) → Al 2 O 3 (s) + 2 Fe (l) + Heat
It is a displacement or redox reaction.
(b ) 3 Mg (s) + N 2 (g) → Mg 3 N 2 (s)
It is a combination reaction.
(c ) 2 KI (aq) + Cl 2 (g) → 2 KCl (aq) + I 2 (s)
Q3. Complete the missing components / variables given as x and y in the following reactions
(a) Pb(NO 3 ) 2 (aq) + 2 KI (aq) → Pbl 2 (x) + 2 KNO 3 (y)
(b) Cu (s) + 2 AgNO 3 (aq) → Cu(NO 3 ) 2 (aq) + x(s)
(c) Zn (s) + H 2 SO 4 (aq) → ZnSO 4 (x) + H 2 (y)
(a) Pb(NO 3 ) 2 (aq) + 2 KI (aq) → Pbl 2 (s) + 2 KNO 3 (aq)
(b) Cu (s) + 2 AgNO 3 (aq) → Cu(NO 3 ) 2 (aq) + 2 Ag(s)
(c) Zn (s) + H 2 SO 4 (aq) → ZnSO 4 (aq) + H 2 (g)
Q4. Which among the following changes are exothermic or endothermic in nature?
(a) Decomposition of ferrous sulphate
(b) Dilution of sulphuric acid
(c) Dissolution of sodium hydroxide in water
(d) Dissolution of ammonium chloride in water
(a ) The decomposition of ferrous sulphate is an example of an endothermic reaction because heat is absorbed during this reaction.
(b ) The dilution of sulphuric acid is an example of an exothermic reaction because heat is released during this reaction.
(c ) The dissolution of sodium hydroxide in water is an example of an exothermic reaction because heat is released during this reaction.
(d ) The dissolution of ammonium chloride in water is an example of an endothermic reaction because heat is absorbed during this reaction.
Q5. Identify the reducing agent in the following reactions
(a ) 4 NH 3 + 5 O 2 → 4 NO + 6 H 2 O
(b ) H 2 O + F 2 → HF + HOF
(c ) Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
(d ) 2 H 2 + O 2 → 2 H 2 O
(a ) Here, ammonia (NH 3 ) is the reducing agent.
(b ) Here, water (H 2 O) is the reducing agent.
(c ) Here, carbon monoxide (CO) is the reducing agent.
(d ) Here, hydrogen (H 2 ) is the reducing agent.
Q6. Identify the oxidising agent (oxidant) in the following reactions
(a ) Pb 3 O 4 + 8 HCI → 3 PbCl 2 + Cl 2 + 4 H 2 O
(b ) 2 Mg + O 2 → 2 MgO
(c ) CuSO 4 + Zn → Cu + ZnSO 4
(d ) V 2 O 5 + 5 Ca → 2 V + 5 CaO
(e ) 3 Fe + 4 H 2 O → Fe 3 O 4 + 4 H 2
(f ) CuO + H 2 → Cu + H 2 O
(a ) Pb 3 O 4 is the oxidising agent here. The oxidation state of Pb in Pb 3 O 4 reduces from + 6 to + 2 in PbCl 2. Thus it acts as an oxidising agent.
(b ) O 2 is the oxidising agent here. The oxidation state of oxygen in elemental form O 2 reduces from 0 to – 2 in MgO . Thus it acts as an oxidising agent.
(c ) CuSO 4 is the oxidising agent here. The oxidation state of Cu in CuSO 4 reduces from + 2 to 0 in Cu . Thus it acts as an oxidising agent.
(d ) V 2 O 5 is the oxidising agent here. The oxidation state of V in V 2 O 5 reduces from + 5 to 0 in V . Thus, it acts as an oxidising agent.
(e ) H 2 O is the oxidising agent here. The oxidation state of oxygen in H 2 O reduces from – 2 to – 3 in H 2 O . Thus it acts as an oxidising agent.
(f ) CuO is the oxidising agent here. The oxidation state of Cu in CuO reduces from + 2 to 0 in Cu . Thus, it acts as an oxidising agent.
Q7. Write the balanced chemical equations for the following reactions
(a ) Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate.
(b ) Sodium hydrogen carbonate on reaction with hydrochloric acid gives sodium chloride, water and liberates carbon dioxide.
(c ) On treatment with potassium iodide, copper sulphate precipitates cuprous iodide (Cu 2 I 2 ), liberates iodine gas and forms potassium sulphate.
(a ) Na 2 CO 3 + HCl → NaCl + NaHCO 3
(b ) NaHCO 3 + HCl → NaCl + H 2 O + CO 2
(c ) 2 CuSO 4 + 4 KI → Cu 2 I 2 + 2 K 2 SO 4 + I 2
Q8. A solution of potassium chloride, when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction?
Chemical reaction: KCl + AgNO 3 → KNO 3 + AgCl
It is a double displacement reaction.
Q9. Ferrous sulphate decomposes with the evolution of a gas having a characteristic dour of burning sulphur. Write the chemical reaction involved and identify the type of reaction.
FeSO 4 (s) + Heat → Fe 2 O 3 (s) + SO 2 (g) + SO 3 (g)
It is a thermal decomposition reaction.
Q10. Why do fireflies glow at night?
Fireflies glow at night because of a chemical reaction involving light’s emission. Fireflies store a protein (luciferin) that combines with oxygen in the air to form a new substance (oxyluciferin) and the evolution of energy in light.
Q11. Grapes hanging on the plant do not ferment, but after being plucked from the plant can be
fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical
When attached to the plants, Grapes are living, and therefore, their immune system prevents fermentation. The microbes can grow in the plucked grapes, which can be fermented under anaerobic conditions. This is a chemical change.
Q12. Which among the following are physical or chemical changes?
(a ) Evaporation of petrol
(b ) Burning of Liquefied Petroleum Gas (LPG)
(c ) Heating of an iron rod to red hot.
(d ) Curdling of milk
(e ) Sublimation of solid ammonium chloride
(a ) Evaporation of petrol is a physical change as it only gets converted from one physical state to another.
(b ) Burning of Liquefied Petroleum Gas (LPG) is a chemical change as heating produces carbon dioxide and water.
(c ) The heating of an iron rod to red hot is a physical change as heating involves only temperature change.
(d ) The curdling of milk is a chemical change as it affects the chemical composition of the milk.
Q13. We made the following observations during the reaction of some metals with dilute hydrochloric acid.
(a) Silver metal does not show any change
(b) The temperature of the reaction mixture rises when aluminium (Al) is added.
(c) The sodium metal reaction is highly explosive.
(d) Some gas bubbles are seen when lead (Pb) is reacted with the acid.
Explain these observations giving suitable reasons.
(a ) Silver does not show any characteristics change because silver is less reactive than hydrogen. Thus, it cannot displace hydrogen from dilute hydrochloric acid.
(b ) The reaction between aluminium (Al) and hydrochloric acid is highly exothermic. Thus, the temperature of the reaction mixture rises.
(c ) Sodium is a highly reactive metal. It reacts with hydrochloric acid, vigorously forming hydrogen gas and a large amount of heat.
(d ) When lead reacts with hydrochloric acid, the gas bubbles observed are hydrogen gas.
Pb (s) + 2 HCl (aq) → PbCl 2 (s) + H 2 (g)
Q14. A substance X, an oxide of a group 2 element, is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution which turns red litmus blue. Identify X and also write the chemical reactions involved.
Here, X is calcium oxide.
- Calcium oxide is used intensively in the cement industry.
- The element present in it (in bones also) is calcium.
- On treatment with water, calcium oxide forms a solution of calcium hydroxide [Ca(OH) 2 ], which is an alkali. Hence, it turns red litmus blue.
CaO (s) + H 2 O (l) → Ca(OH) 2 (aq) + Heat
Q15. Write a balanced chemical equation for each following reaction and classify
(a ) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(b ) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
(c ) Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
(d ) Hydrogen sulphide gas reacts with oxygen gas to form solid sulphur and liquid water
(a ) Pb(CH 3 COO) 2 + 2 HCl → PbCl 2 + 2 CH 3 COOH
(b ) 2 Na + 2 C 2 H 5 OH → 2 C 2 H 5 ONa+ H 2
It is a displacement or a redox reaction.
It is a redox reaction.
(d ) 2 H 2 S + O 2 → 2 S + 2 H 2 O
Q16. Why do we store silver chloride in dark coloured bottles?
We store silver chloride in the dark-coloured bottles because silver chloride decomposes into silver and chlorine gas in sunlight.
Q17. Balance the following chemical equations and identify the type of chemical reaction.
(a ) Mg (s) + Cl 2 (g) → MgCI 2 (s)
(b ) HgO (s) + Heat → Hg (l) + O 2 (g)
(c ) Na (s) + S (s) → Na 2 S (s)
(d ) TlCl 4 (l) + Mg (s) → Tl (s) + MgCl 2 (s)
(e ) CaO (s) + SiO 2 (s) → CaSiO 3 (s)
(f ) H 2 O 2 (l) + UV → H 2 O (l) + O 2 (g)
(b ) 2 HgO (s) + Heat → 2 Hg (l) + O 2 (g)
(c ) 2 Na (s) + S (s) → Na 2 S (s)
(d ) TlCl 4 (l) + 2 Mg (s) → Tl (s) + 2 MgCl 2 (s)
It is a displacement reaction.
(f ) 2 H 2 O 2 (l) + UV → 2 H 2 O (l) + O 2 (g)
It is a decomposition reaction.
Q18. A magnesium ribbon is burnt in oxygen to give a white compound X accompanied by light emission. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(a) Write the chemical formulae of X and Y.
(b) Write a balanced chemical equation when X is dissolved in water.
Here, X is magnesium oxide, and Y is magnesium nitride.
(a ) The chemical formulae of X are MgO and Y is Mg 3 N 2 .
(b ) When X is dissolved in water following reaction occurs.
MgO + H 2 O → Mg(OH) 2
Q19. Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why?
Zinc is more reactive than copper as Zinc is placed above hydrogen, and copper is placed below hydrogen in the activity series of metals. Thus, zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not.
Q20. A silver article generally turns black when kept in the open for a few days. The article, when rubbed with toothpaste again, starts shining.
(a ) Why do silver articles turn black when kept in the open for a few days? Name the phenomenon involved.
(b ) Name the black substance formed and give its chemical formula.
(a ) The silver article turns black when kept in the air because the silver article reacts with sulphur compounds such as hydrogen sulphide (H 2 S) present in the air to form silver sulphide Ag 2 S. This phenomenon is called corrosion. It is also known as tarnishing of silver.
(b ) The black substance is silver sulphide. Its chemical formula is Ag 2 S.
Related Videos
Balancing of chemical equations.

Long Answer Type Questions
Q1. On heating blue coloured powder of copper (I) nitrate in a boiling tube, copper oxide
(black), oxygen gas, and a brown gas X is formed
(a) Write a balanced chemical equation of the reaction.
(b) Identity the brown gas X evolved.
(c) Identify the type of reaction.
(d) What could be the pH range of the aqueous solution of the gas X?
(a ) 2 CuNO 3 (s) + Heat → 2 CuO (s) + 4 NO 2 (g) + O 2 (g)
(b ) The brown gas is of nitrogen dioxide.
(c ) It is a thermal decomposition reaction.
(d ) NO 2 gas reacts with water to produce nitric acid. Thus, its pH range will be less than 7.
Q2. Give the characteristic tests for the following gases
The characteristics test for
(a ) CO 2 : CO 2 turns lime water milky due to the formation of insoluble calcium carbonate.
CO 2 + Ca(OH) 2 → CaCO 3 + H 2 O
(b ) SO 2 : SO 2 turns purple coloured acidic potassium permanganate solution colourless.
5 SO 2 + 2 KMnO 4 + 2 H 2 O → K 2 SO 4 + 2 MnSO 4 + 2 H 2 SO 4
(c ) O 2 : We can confirm the evolution of oxygen gas by bringing a burning candle near the mouth of the test tube containing the reaction mixture. The intensity of the flame increases because oxygen supports burning.
(d ) H 2 : Hydrogen (H 2 ) gas burns with a pop sound when a burning candle is brought near it.
Q3. What happens when a piece of
(a) Zinc metal is added to copper sulphate solution?
(b) Aluminium metal is added to dilute hydrochloric acid?
(c) Silver metal is added to copper sulphate solution?
Also, write the balanced chemical equation if the reaction occurs
(a ) Zinc metal reacts with copper sulphate solution and forms colourless zinc sulphate and reddish-brown copper metal.
Zn (s) + CuSO 4 (aq) → ZnSO 4 (aq) + Cu (s)
(b ) Aluminium metal reacts with dilute hydrochloric acid to form aluminium chloride and hydrogen gas.
2 Al (s) + 6 HCl (aq) → 2 AlCl 3 (aq) + 3 H 2 (g)
(c ) Silver is less reactive than copper. Hence, no reaction will occur.
Q4. When zinc granules are treated with a dilute solution of H 2 SO 4 , HCI, HNO 3 , NaCI and NaOH. Write the chemical equations if a reaction occurs.
- Zinc granules react with dilute sulphuric acid to form zinc sulphate and hydrogen gas.
Zn (s) + H 2 SO 4 (aq) → ZnSO 4 (aq) + H 2 (g)
- Zinc granules react with dilute hydrochloric acid to form zinc chloride and hydrogen gas.
Zn (s) + H 2 SO 4 (aq) → ZnCl 2 (aq) + H 2 (g)
- Zinc granules react with dilute nitric acid to form zinc nitrate, water and dinitrogen gas.
Zn (s) + H 2 SO 4 (aq) → Zn(NO 3 ) 2 (aq) + H 2 O (l) + N 2 O (g)
- Zinc does not react with sodium chloride
Zn (s) + NaCl → No Reaction.
- Zinc granules react with dilute sodium hydroxide to form zinc hydroxide and hydrogen gas.
Zn (s) + NaOH (aq) → Zn(OH) 2 (aq) + Na (g)
Q5. A white precipitate is obtained when adding a drop of barium chloride solution to an aqueous sodium sulphite solution.
(a ) Write a balanced chemical equation of the reaction involved
(b ) What other name can be given to this precipitation reaction?
(c ) On adding dilute hydrochloric acid to the reaction mixture, white residue disappears. Why?
(a ) BaCl 2 + Na 2 SO 3 ⟶ BaSO 3 + 2 NaCl
(b ) It can be assigned as a double displacement reaction.
(c ) On adding dilute hydrochloric acid to the reaction mixture, white residue disappears due to the formation of barium chloride.
BaSO 3 + 2 HCl ⟶ BaCl 2 + SO 2 + H 2 O
Q6. You are provided with two containers made up of copper and aluminium. You are also
provided with dilute HCI, HNO 3 , ZnCl 2 and H 2 O solutions. In which of the above
containers we can keep these solutions?
The solution of dilute HCI, HNO 3 , ZnCl 2 and H 2 O can be kept in a container made of copper since copper is a less reactive metal and is placed below the hydrogen in the reactivity series. Hence it does not react with HCI, HNO 3 , ZnCl 2 and H 2 O. At the same time, aluminium is a highly reactive metal and can react with these solutions. Thus container made of copper is suitable to keep the given solutions.
Chemical Reactions and Equations – Previous Year Questions

Oxidation & Reduction – Concept + Quiz

| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
- STATE WISE BOOKS
- ENGINEERING EXAM
- SCHOLARSHIP OLYMPIAD
- STATE BOOKS
CBSE Class 10 Science Case Based MCQ Questions
CBSE Term 1 exam is not so far and surely you have begun the preparation for the board exam. Therefore we have dedicated this page to help you out in your preparation. This year for the first time the board has introduced the Case based Questions which will be asked in the Term 1 exam that is likely to be held in November-December.
Therefore, we have brought you the CBSE Class 10 Science Case Based MCQ on this page. The questions are given in objective types. Such types of questions are solved by reading the given scenario in the paragraph.
All these science case study questions are developed as per the new CBSE Pattern. The team of subject matter experts have crafted the given MCQs. The Case Based Questions that we are providing here are worthy to solve and practice because class 10th syllabus has been taken into consideration while preparing the problems.
CBSE Class 10 Science Case Study, Assertion & Reasoning, MCQ
The Class tenth CBSE Science Case Study, Assertion & Reasoning, MCQs are very helpful in practicing and getting a deep understanding in the topics of science. However, a comprehensible knowledge of NCERT Science Book is a must to be able to solve these types of problems.
The PDF that is available here to download contains the problems in three different variants; One is general objective types of questions that is MCQ (Multiple Choice Questions), second one is Assertion and Reasoning and the last one is Case-Based Questions.
| Chapter 1 Chemical Reactions and Equations | |
| Chapter 2 Acids, Bases and Salts ( | |
| Chapter 3 Metals and non – metals | |
| Chapter 6 Life processes | |
| Chapter 10 Light – Reflection and Refraction | |
| Chapter 11 Human Eye and Colourful World | |
| Chapter 4 Carbon & Its Compounds (MCQ) | |
| Chapter 5 Periodic Classification Of Elements (MCQ) | |
| Chapter 7 Control and Coordination (MCQ) | |
| Chapter 8 How Do Organisms Reproduce (MCQ) | |
| Chapter 9 Heredity & Evolution (MCQ) | |
| Chapter 12 Electricity (MCQ) | |
| Chapter 13 Magnetic Effects Of Electric Current (MCQ) | |
| Chapter 14 Sources of Energy (MCQ) | |
| Chapter 15 Our Environment (MCQ) | |
| Chapter 16 Management and Natural Resources |
If you Download PDF CBSE Class 10 Science Case Study from the given links, then you will be able to get the quick revision for each chapter that will help you to recall your learnings and give you information about some important Chemicals Formulas and reactions.
What are Assertion & Reasoning Questions?
Assertion & Reasoning questions are basically a type of multiple-choice question that is solved by reading the given statement and the reason. The question typically consists of one statement followed by its reason. Students' duty is to verify both statements and reason whether they are correct or not and if they are correct then it is time to look at whether the given statement truly satisfies the reason or not.
These questions should not be difficult to solve but you have to have rigorous and extensive practice. The Assertion & Reasoning questions along with the solutions are given in the CBSE Class 10 Science case study 2021-2022 PDF that is available here.
Class 10th has very basic and important chapters that are necessary to solve. A few chapters that are available in the beginning of the science books are Chemical Reactions and Equations, Acids, Bases and Salts, Metals and Non-metals, Carbon and Its Compounds, Periodic Classification of Elements, Life Processes, etc.
Since you are here to find out the CBSE Class 10 Science MCQ Based Questions, the possibilities are you need CBSE Class 10 Maths Case Study Questions too.
FAQs on CBSE Class 10th Science Case Study Questions

Case Study Questions are based on the data which are given in the form of passage. These types of questions generally consist of real life examples. Usually it contains upto 4 or 5 questions.
To prepare for Class 10 Science MCQ Be thorough with the concepts, Practice the questions regularly, Attempt online tests as much as you can. To do all these things visit the Selfstudys.com. They are providing everything for free of cost.
In class 10 Science Based Questions you will be asked to answer the questions that are explained in the standard Xth Science Syllabus. However, the problems will be related to real world examples.
To find Class 10 Science Chapter wise Assertion and Reason Questions you simply need to reach at the Selfstudys website. It provides all the study resources for free of cost. You will be able to download the assertion reason with solutions as well.
No, CBSE Class 10 Science Case Based MCQ Question is not difficult, if you pay a good attention to the given paragraph. It is important to be able to find the tiny details in the passage to answer the Case Based questions.

CBSE Class 10 Results 2024 : CBSE Class 10 Answer Book Photocopy Applications Open

CBSE 10th 2024-25 : Social Science Official Competency Focused Practice Questions released by CBSE

CBSE Class 10 Revaluation Application 2024 Process Begins : How to Apply, Fees, Direct Link & Step-by-Step Guide

CBSE Class 10 Result 2024 Latest Update : Verification of Marks, Revaluation & Photocopy of Answer Sheet; Check Complete Process

CBSE Class 10 Result 2024 Out: 93.60% Pass Percentage, JNV and Kendriya Vidyalaya shine with 99.09% score
CBSE Class 10th Result 2024 Out : Supplementary, Re-verification & Re-evaluation Date Released; Check Details Here

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.


COMMENTS
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Here, we have provided … Continue reading Case Study and Passage Based Questions for Class 10 Science Chapter 1 ...
CBSE Case Study Questions Class 10 Science Acids, Bases and Salts CASE STUDY : 1. The reaction between carbon dioxide and calcium hydroxide (lime water), Calcium hydroxide, which is a base, reacts with carbon dioxide to produce a salt and water. Since this is similar to the reaction between a base and an acid, we can conclude that nonmetallic ...
In CBSE Class 10 Science Paper, Students will have to answer some questions based on Assertion and Re a son. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Acids, Bases, and Salts Case Study Questions With Answers
CBSE Case Study Questions Class 10 Science Chemical Reactions and Equations Case Study 1. A solution of slaked lime produced by the reaction is used for white washing walls. Calcium hydroxide reacts slowly with the carbon dioxide in air to form a thin layer of calcium carbonate on the walls. Calcium carbonate is formed after two to three days ...
As mentioned above, Case Based Questions will carry a total of 12 marks i.e about 15 percent of the total CBSE Class 10 Science marks will come from the Case Study Based Question.
These Case Study Questions Class 10 Science are written by experts. Download Books for Boards. Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th. ... Case Study 3: Chemical reactions and equations are fundamental concepts in chemistry that help us understand the ...
Class 10 Science Sample Papers with case study questions are available in the myCBSEguide App. There are 4 such questions (Q.No.17 to 20) in the CBSE model question paper. If you analyze the format, you will find that the MCQs are very easy to answer. So, we suggest you, read the given paragraph carefully and then start answering the questions.
CBSE 10th Standard Science Subject Chemical Reactions and Equations Case Study Questions With Solution 2021. 10th Standard CBSE. Reg.No. : Science. Time : 00:30:00 Hrs. Total Marks : 20. Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it.
Statement 1: The heating of lead nitrate is an example of 'X' reaction. Statement 2: The burning of magnesium is an example of 'Y' reaction. Question 2: In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be ...
We have provided Case Study Questions for Class 10 Science for all chapters as per CBSE, NCERT and KVS examination guidelines. These case based questions are expected to come in your exams this year. Please practise these case study based Class 10 Science Questions and answers to get more marks in examinations. ... Case/Passage - 2. Chemistry ...
To support your preparation for Class 10 Science examinations, we have created a comprehensive PDF resource containing a collection of case study questions designed specifically for this subject. This PDF includes a variety of case studies covering different topics in Physics, Chemistry, and Biology. It will provide you with ample practice ...
The CBSE Class 10 Science Question Bank on Case Studies, provided in this article, can be very helpful for understanding how the source based or case based questions are asked in the board exam.
Case Study Questions Class 10 Science. In board exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. Case Study Questions for Chapter 1 Chemical Reactions and Equations.
Chemistry Chapters for Case Study Questions. Chemical Reactions and Equations. Acids, Bases and Salts. Metals and Non Metals. Carbon and Its Compounds. Periodic Classification of Elements. TopperLearning provides a complete collection of case studies for CBSE Class 10 Chemistry students. Improve your understanding of biological concepts and ...
CBSE Case Based Questions Class 10 Science Chemistry Chapter 3. CASE STUDY :1. Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue. The impurities must be removed from the ore prior to the extraction of the metal. The processes Several steps are involved in the extraction ...
Accurate answers of all the Case-based questions given in the PDF. Case Study class 10 Science solutions are prepared by subject experts referring to the CBSE Syllabus of class 10. Free to download in Portable Document Format (PDF) so that students can study without having access to the internet.
Question 5 - Case Based Questions (MCQ) - Chapter 1 Class 10 - Chemical Reactions and Equations. Last updated at April 16, 2024 by Teachoo. In the following chemical reaction zinc oxide reacts with carbon to produce zinc metal and carbon monoxide.''. ZnO + C → Zn + CO. (a) Name the substance getting oxidised and reduced in the above ...
CBSE Class 10th - SCIENCE : Chapterwise Case Study Question & Solution. In board exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
4.5) Diamond is not a good conductor of electricity because : (a) it is very hard. (b) it has no free electrons. (c) it is not water soluble. (d) its structure is very compact. Show Answer. Hope the information shed above regarding Case Study and Passage Based Questions for Class 10 Science Chapter 4 Carbon and Its Compounds with Answers Pdf ...
By practising these Class 10 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 10 Annual examinations. Download Class 10 Chemistry Chapter 1 Chemical Reactions and Equations Important Questions with Answers PDF by clicking on the button below. Download PDF. Recommended ...
CBSE Class 10 Science exam 2022-23 will have a set of questions based on case studies in the form of MCQs. CBSE Class 10 Science Question Bank on Case Studies given in this article can be very helpful in understanding the new format of questions. Each question has five sub-questions, each followed by four options and one correct answer.
Such types of questions are solved by reading the given scenario in the paragraph. CBSE Class 10 Science Chapter Wise Case Study. Science Chapter 1 Chemical Reactions & Equations Case Study. Science Chapter 2 Acids, Bases & Salts Case Study. Science Chapter 3 Metals & Non-Metals Case Study. Science Chapter 4 Carbon & Its Compounds Case Study.