An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Membranes (Basel)


A Review on the Use of Membrane Technology Systems in Developing Countries
Nur hidayati othman.
1 School of Chemical Engineering, College of Engineering, Universiti Teknologi MARA, Shah Alam 40450, Selangor, Malaysia; ym.ude.mtiu@hamihsahrun (N.H.A.); moc.liamg@lizufanazays (N.S.F.); ym.ude.mtiu@671haizuaf (F.M.); ym.ude.mtiu@namaz_rawanum (M.Z.S.)
Nur Hashimah Alias
Nurul syazana fuzil, fauziah marpani, munawar zaman shahruddin, chun ming chew.
2 Taman Industri Meranti Perdana, Pusat Teknologi Sinar Meranti, Techkem Group, No. 6, Jalan IMP 1/3, Puchong 47120, Selangor, Malaysia; ym.moc.mekhcet@gndivad
Kam Meng David Ng
Woei jye lau.
3 Advanced Membrane Technology Research Centre (AMTEC), Universiti Teknologi Malaysia, Skudai 81310, Johor, Malaysia; ym.mtu@eyjieowl (W.J.L.); ym.mtu@izuafa (A.F.I.)
Ahmad Fauzi Ismail
Associated data.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Fulfilling the demand of clean potable water to the general public has long been a challenging task in most developing countries due to various reasons. Large-scale membrane water treatment systems have proven to be successful in many advanced countries in the past two decades. This paves the way for developing countries to study the feasibility and adopt the utilization of membrane technology in water treatment. There are still many challenges to overcome, particularly on the much higher capital and operational cost of membrane technology compared to the conventional water treatment system. This review aims to delve into the progress of membrane technology for water treatment systems, particularly in developing countries. It first concentrates on membrane classification and its application in water treatment, including membrane technology progress for large-scale water treatment systems. Then, the fouling issue and ways to mitigate the fouling will be discussed. The feasibility of membrane technologies in developing countries was then evaluated, followed by a discussion on the challenges and opportunities of the membrane technology implementation. Finally, the current trend of membrane research was highlighted to address future perspectives of the membrane technologies for clean water production.
1. Introduction
The demand to increase clean water supply due to the proliferation of global population has prompted significant attention from world leaders [ 1 ]. In most urbanized areas of the world, clean water supply is produced from large-scale water treatment plants and is channelled through multiple networks of piping systems as tap water to the consumers [ 2 ]. These water treatment plants utilize various unit operations to purify raw water into safe and clean water before distribution [ 3 ]. The conventional water treatment process has been the dominant method to produce clean water on a large scale throughout the decades, especially in developing countries [ 4 ]. Some of the main advantages of the conventional process are the low capital and operational costs for the water treatment systems [ 5 ]. The main objectives of the treatment process are to remove the suspended solids and harmful bacteria through disinfection from the raw water.
Developing countries are generally defined as poor or middle-income nations based on the average income per person [ 6 ]. The water infrastructures of these countries are usually less developed due to financial constraints. There are various challenges faced by developing countries all over the world in providing a clean water supply to the people. In the Africa continent, a quantitative assessment conducted in Tanzania concluded the temporal variability model in drinking water faecal contamination against climate changes over 20 months [ 7 ]. Researchers from India, the second most populated country in Asia, have developed a cost-effective chlorine disinfection method for potable water [ 8 ]. These examples have indicated the emphasis on ensuring bacteria-free safe drinking water treatment processes in developing countries. The lack of water infrastructure has motivated many researchers to explore innovative, cost-effective water treatment methods.
Another major socio-economic obstacle faced by developing countries is the significant population living in rural or remote areas [ 9 ]. Due to the vast distance of certain rural villages and the low-density population, it is not economically feasible to connect water and electricity supply from the nearest city to these isolated locations. A study has indicated that an unregulated, privately financed or self-supply groundwater supply system in rural villages of Bangladesh pose a high risk of untested water quality [ 10 ]. Another analysis conducted in rural Kenya highlighted the failure risk for groundwater supply sources sustainability [ 11 ]. All these studies share similarities, pointing to the challenges of water security issues in rural areas in developing countries.
One of the suggestions to alleviate the clean water shortages in rural villages is to install regulated decentralized water treatment systems to cater to a small community. An economic feasibility study conducted in various developing countries such as Egypt, Nepal and Tanzania has highlighted some interesting and significant findings [ 12 ]. All three investigation sites have shown no realistic chance of recovering the initial or capital investment for the rural water treatment systems. In another study conducted on rural water treatment systems in Zimbabwe, similar economic drawbacks were also observed. The lack of technical support and expertise was also highlighted as one of the significant challenges to ensure proper operation and maintenance of these facilities [ 13 ]. Although decentralized small-scale water treatment systems have been proposed to solve rural clean water supply, the financial and technical aspects remain the most significant obstacles. Conventional water treatment systems offer economies of scale in urban areas, but they might not be suitable for rural areas with a small population and community lacking in various fields.
There are many challenges faced in the operation of these conventional systems which have prompted the emergence of other alternatives systems. In recent years, membrane filtration systems have emerged as one of the most widely used alternatives for large-scale water treatment plants and decentralized small-scale systems [ 14 ]. It has its advantages and drawbacks, for instance, the higher overall cost that hinders its adoption in developing countries [ 15 ]. For the last few decades, the thriving global economies have propelled many developing countries towards middle-income nations, enabling higher expenditure allocated for water infrastructures. These current developments present challenges and opportunities for researchers, scientists, policymakers, investors and stakeholders to explore mutually beneficial solutions.
The industrial-scale conventional water treatment system is widely used in developing countries due to its significantly lower cost versus other high-end systems. Surface water is commonly the primary source of raw water to these conventional water treatment plants to supply clean or tap water to the public [ 16 ]. In the conventional system, inorganic based coagulant (such as aluminium sulphate and ferric chloride) is added into the raw surface water for the coagulation/flocculation process. Prior to the coagulant dosing, the raw water passes through a cascading aerator for the natural aeration process. The raw water with the coagulated particles and then passed through the clarification or sedimentation tanks for the solid–liquid separation. Final polishing is carried out with media sand filters before the chlorine disinfection process takes place. The chlorinated water is then ready to be supplied as tap water to the consumer. Figure 1 shows the summarized block diagram of the conventional water treatment system.

Block diagram comparison between conventional and membrane-based water treatment systems.
The main operational cost for the conventional system is on the purchasing of chemicals (coagulant, chlorine, etc.) as well as electricity. The production cost can go as low as USD 0.01/m 3 of clean water produced in developing countries [ 5 ]. One of the most critical aspects of this system is the precise control of the coagulant dosage, pH and mixing to ensure the fine suspended solids are coagulated for the clarification and filtration processes [ 17 ]. Without a proper coagulation process, the system might fail to produce the desired quality of the treated water with turbidity below 5 NTU to comply with the World Health Organization (WHO) recommended drinking water standard. This is the major drawback of the conventional system, which relies heavily on the chemical coagulation/flocculation process to ensure an efficient solid–liquid separation in the system [ 18 ].
Conventional water treatment systems have been utilised extensively worldwide, including in high-income European countries [ 19 ]. In recent years, membrane-based filtration for large-scale water treatment systems has gained significant acceptance, particularly in developed countries. The membrane-based system’s higher capital and operational expenditure have caused the water production cost to be significantly higher for these countries. It has been suggested by the United Nations Development Programme (UNDP) that the water cost should not exceed 3% of household income [ 20 ]. In general, most household incomes in developed countries are significantly much higher than in developing countries, and thus higher water tariffs are imposed as well. The higher water tariff is necessary to sustain the membrane-based water treatment system, which usually provides a much more consistent and better water quality. Typically, the industrial-scale direct filtration membrane-based water treatment system consists of a pre-treatment process before the membrane filtration. After the filtration process, chlorine disinfection of the filtrate is carried out before supplying to consumers. Figure 1 shows the summarized block diagram of the membrane-based water treatment system.
The low footprint required and the consistent water quality produced by membrane systems have made it attractive, particularly in urban cities. The water tariff of these membrane systems can go as high as $ 1.52/m 3 of water consumed [ 21 ]. The affordability of the consumers incurred a significant impact on the sustainability of these advanced systems. One of the most common membrane filtration systems for surface raw water treatment is ultrafiltration (UF) [ 22 ]. UF membranes with a pore size of 0.002–0.1 µm are commonly used to segregate fine solids from the filtrate. It is a low-pressure membrane filtration system that is suitable to replace conventional media sand filters. This low-pressure membrane filtration system with a much smaller footprint has made it attractive for large-scale applications [ 23 ]. As with other membrane filtration systems, the UF system comes with its own challenges and higher maintenance cost. Two of the most commonly used large-scale membrane water treatment systems are UF and reverse osmosis (RO) [ 24 ].
2. Membrane Technology for Water Treatment System
Typical municipal water treatment plant utilizing the conventional system heavily relies on the chemical-based coagulation/flocculation processes. The chemical dosage needs to be regulated periodically as they depend highly on the raw water source quality. Therefore, it is challenging to control the treated water’s quality without appropriate knowledge and monitoring techniques. In contrast to chemical-based treatments, membrane filtration systems produce microbiologically safe drinking water with no chemicals added, making it appropriate for drinking and sanitation requirements. The modular nature of membrane systems will allow various treatment capacities suited for the requirements.
2.1. Membrane Classifications
A membrane is a permeable or semi-permeable barrier that allows certain substances in the source waters to pass through the membrane while selectively restricting others. The separation of contaminants depends on the properties such as size and charge ( Figure 2 ). The movement across the membrane requires a driving force that includes pressure difference, concentration gradient and a potential field to initiates ions movement. The pressure-driven membrane systems are categorized based on the operating pressure. Low-pressure membrane systems such as microfiltration (MF) and ultrafiltration (UF) are typically operated in the range between 10 to 30 psi. In contrast, high-pressure membrane systems such as nanofiltration (NF) and reverse osmosis (RO) require high operating pressures, varying between 75 to 250 psi. RO utilises a dense membrane where the pore size is less than 1 nm. Therefore, it is capable of removing almost all inorganic contaminants and the smallest organic molecules. In addition to being widely accepted as seawater desalination technology, RO has been observed to be efficient for the removal of synthetic organic contaminants (SOCs) such as herbicides and pesticides from polluted groundwaters.

Membrane processes and application.
Besides the pressure-driven membrane, an electrical potential can also be used to initiate dissolved ions to transfer across a water-impermeable membrane and has been regularly used for desalination purposes. This electrically driven membrane process is known as electrodialysis (ED) or reverse electrodialysis (RED). The induced direct current (DC) causes cation (+ve charged ions) to transfer across the cationic membrane to the cathode (-ve charged electrode) while restrained at the anionic membrane’s surface. Conversely, anions (-ve charge ions) move across the anionic membrane to the anode (+ve charged electrode) but are hindered at the cationic membrane. Consequently, a “dilute” stream-containing minimal salt concentration and a “concentrated” stream with higher salinity than the feed water can be obtained. The ion-exchange membranes (IEMs) used in this ED are typically expensive, contributing to the high cost of the membrane system.
The membrane is the “heart” of every membrane separation process as it plays a vital role in controlling the permeation of specific components. The selection of membrane process is subjected to the membrane properties. Membrane materials can substantially influence the properties and characteristics of the membrane, such as hydrophobicity and surface charge, thus, altering the separation characteristic of the membrane. Most of the membranes are fabricated from cellulose or non-cellulose organic polymers such as polyethersulfone (PES), polysulfone (PSF), polyvinyl difluoride (PVDF), and polypropylene. However, these polymeric membranes are not suitable for harsh conditions. As a result, inorganic ceramic membranes have been proposed to treat highly contaminated wastewaters such as oily water and highly turbidity water. The inorganic membrane is preferable to the polymeric organic membrane due to its excellent chemical stability, improved mechanical strength and can be used for high-temperature operation.
Membrane morphology can be categorized into symmetric or asymmetric structures. The symmetric structure is where the membrane has a uniform pore size or consistent morphology throughout the membrane’s cross-section. Conversely, an asymmetric structure comprises two main layers (a thin dense layer supported on a porous substrate) with diverse morphology and permeability properties. In asymmetric design, the pressure drop typically occurs in the thin dense separation layer, while the porous support layer aids in minimizing the transport resistance of the permeate across the membrane. The asymmetric membrane can be made either from one single material or a combination of different materials for separation and support layers.
Membrane performances are typically measured in terms of permeability rate or flux and selectivity. Membrane flux measures the diffusion’s rate of molecules across the membrane. It is the underlying step in membrane characterization and is highly reliant on the operating conditions of the membrane systems, such as pressure, temperature, and velocity at the membrane surface. Selectivity is typically based on membrane properties, particularly pore size or molecular weight cut-off (MWCO). MWCO signifies the lowest molecular weight solute (Daltons) in which 90% of the solute is retained by the membrane and commonly used to characterise UF. The significance of pore size towards membrane performance is constrained by the formation of a fouling layer on a membrane surface, which can assess rejection capability. This rejection characteristic is used to measure the performance of membranes.
Membranes for water treatment can be fabricated either in flat sheet or hollow fibre (capillary) form. A flat sheet membrane supported onto woven or non-woven support is typically assembled into a spiral-wound module for RO and NF systems. The spiral wound module is formed by wounding a membrane across a centre core tube for permeate, and a flow spacer material is then placed between the membrane. Hollow fibre membrane is typically utilized for drinking water MF and UF systems. The hollow-fibre membranes are bundled, and both ends are potted using resin before being housed in a cylindrical module.
2.2. Membrane Applications in Water Treatment
There are four types of raw water sources: groundwater, surface water, seawater, and rainwater that can be used for drinking and potable water supply. As these waters might contain various pollutants, appropriate treatment to remove disease-causing agents is required to ensure its suitability for drinking purposes. Groundwater is sourced from large underground aquifers, and a deep well must be drilled to take out the water. Surface water is water located above the ground, such as lakes, rivers, and streams. Groundwater commonly had minimal suspended solids, organics matters, and other potential foulants compared to surface water. Therefore, most of the membrane system configurations can be utilized for groundwater.
Surface water typically has higher suspended solids, dissolved organics, and microorganisms that require further treatment and filtration. In addition, the quality of surface water is directly affected by the use of land and human activities. In a more populated area, surface water quality might be low due to contamination from various sources. As the conventional surface water sources are drying up, seawater might be the best option as it is the most abundant source of aqueous solution in the world [ 25 ]. However, due to the high salinity of seawater, where the total dissolved solids (TDS) is around 35,000 ppm, it is vital to treat seawater and convert it into potable quality water with a TDS of between 200–500 ppm [ 26 ]. Rainwater is considered a high-quality source of water, but it might be acidic due to air contamination. Besides, rainwater could be exposed to zinc due to the rainwater collection system that is commonly collected through zinc roofing [ 27 ].
2.2.1. Removal of Organic Compound
Natural organic matter (NOM) is a complex organic material discovered in groundwater and surface waters [ 28 ]. While it is not toxic, the presence of NOM can decrease the quality of the potable water by modifying its colour, odour and taste. It can function as a transporter of the poisonous organic and inorganic compounds such as pesticides and radionuclides in aqueous ecosystems [ 29 ]. Fulvic acids (FA, MW of 500–2000 Da) and hydrophobic humic acids (HA, MW ≤ 2000–5000 Da) are among the main components of NOM. These compounds may form strong complexes with heavy metals, causing the formation of organometallic complexes. Consequently, the transportation ability, bioavailability and toxicity will be increased, thus causing many health hazards [ 30 ]. As a result, it is essential to remove NOM during the water treatment processes.
Chlorination is utilized as one of the conventional disinfection treatments, but the interaction of chlorine with NOM forms a series of human carcinogenic constituents, including adsorbable organic halides (AOX), trihalomethanes (THM), halogenated acetic acids, halogenated aldehydes, ketones, halogenated acetonitryles, amines and other disinfection by-products (DBP) [ 31 ]. A membrane offers the possibility not only in removing DBP, but also NOM [ 32 ]. However, as the molecular size distribution of NOM is greatly varied from 1 nm to 0.45 μm, the removal efficiency depends on the properties of the membrane used [ 33 ]. NF and tight UF membranes were found to provide adequate initial disinfection (i.e., >4-log removal of all pathogens) and extensive removal of NOM. NF has been utilized in drinking water production in small communities (populations of 25–500) and able to remove pathogen and form potential DBPs precursors, which makes it suitable for NOM removal [ 34 ].
The elimination of NOM through RO also reduces the chlorine dosage needed for maintaining the residual chlorine concentration in the water distribution system due to biological activity reduction in the water [ 35 ]. This however is restricted by the high concentration of colloids and suspensions in the surface water [ 36 ]. UF and MF are capable of removing colloids and ionic and non-ionic organic compounds, which are suitable for NOM and high molecular weight DBP precursors removal [ 37 ]. However, an integrated system should be used mainly for medium- and low-molecular weight compounds [ 38 ]. For example, a UF module consists of a dense membrane (e.g., ca. 1000 Da) or a hybrid UF/MF system with coagulation, adsorption or oxidation processes have been proposed for the removal of humic substances from water [ 39 , 40 , 41 , 42 ].
Pharmaceutical active compounds (PhACs) and endocrine disrupting compounds (EDCs) are anthropogenic micropollutants frequently found in natural waters. EDCs can also enter the environment by industrial and municipal wastewater discharge. Activated carbon adsorption or advanced oxidation processes (AOPs) are typically utilised for this type of micropollutants [ 43 ]. However, when high content of NOM is observed in the feedwater, the technology proposed earlier is not attractive due to the increment of operating cost. Besides, by-products of undefined biological activity could be formed through AOPs. As a result, a pressure-driven membrane system seems to be more appropriate to be used. As EDCs are low MW pollutants, NF or integrated MF/NF systems can be employed where more than 90% removal rate of EDCs was observed [ 44 ]. Yoon et al. [ 45 ] showed that EDC removal using UF and NF membrane is based on hydrophobic adsorption and size exclusion mechanisms. A hybrid NF-AOPs system has been proposed for the removal of various PhACs from wastewater treatment plants. Complete removal of these pollutants was observed due to the synergistic effects of NF and AOPS [ 46 ].
Membrane bioreactors (MBRs) have also been investigated for removing pharmaceutical products and medicines from wastewater. It was observed that a longer retention time offered by MBR could improve the biological degradation and removal of PhAC and EDC. The production of plastics, typically PVC, has caused the presence of phthalates (a plastic agent) in the environment. A series of RO, NF and UF systems has been evaluated for phthalate removal in water, and the efficiency of removal was found to be in the range of between 97.6% to almost 99.9% [ 47 ]. Most of the contaminants discussed above can cause a direct impact on aquatic organisms even at a trace concentration, which has raised public concern, especially for water reuse purposes [ 48 ].
2.2.2. Removal of Inorganic Compound
Desalination is a process of mineral components removal and is regularly used to obtain potable water from seawater (35,000 mg/L) and brackish underground waters (2000–5000 mg/L). RO has been seen as a competitive system to conventional distillation techniques. A pre-treatment system is required before RO to ensure that the permeate water meets the quality stipulated in the quality of drinking water regulation. The pre-treatment of raw water is vital to avoid membrane pollution such as fouling and scaling, extending the membrane’s life. A simpler membrane filtration system can be utilized for groundwater, as it is typically cleaner than surface water. The surface water needs to be treated extensively before it can be used, as it can contains various types and high concentrations of pollutants. Thus, a more complicated RO water treatment system including coagulation and adsorption might be required for surface waters. UF and MF have been seen as the most appropriate pre-treatment system prior to desalination. It removes suspended substances, some organic compounds and microbiological pollution, making it less contaminated feedwater for the RO system.
NF has been widely used as an alternative solution for water softening compared to chemical softening and ion-exchange methods due to its lower labour and operation costs. Hard water refers to a source of water with high mineral content, which can cause scaling [ 49 ]. The removal rate of hardness using NF is around 90%, and it is highly dependent on the type of membrane used and its operating conditions (water salinity and hardness). Recently, an integrated biological contact oxidation precipitation-UF-NF system was investigated, and it was observed that besides enhancement in removal rate, the life of the membrane could be prolonged [ 50 ].
Contamination of nitrate in water resources has become significant, and it can be from the discharge of industrial wastewater or nitrogen fertilizers used in agriculture. The maximum allowable concentration of nitrates in drinking water has been set up at around 50 mg NO 3 − /L as higher than that, will be toxic to humans [ 51 ]. RO has been used to treat borehole waters in rural areas in South Africa, and it was found to be effectively suitable for water denitrification where the nitrate-nitrogen was reduced from 42.5 mg/L to only 0.9 mg/L in the permeate [ 52 ]. In addition, the treatment cost of RO is comparable with the cost of ion exchange and electrodialysis systems.
Heavy metals pollutants such as iron, mercury, arsenic, chromium, copper, and lead might be present in the water source as a result of industrialization and urbanization. In many cases, a hybrid pressure-driven membrane and conventional water treatment systems is considered an attractive alternative towards environmental protection and the economy of the process. A hybrid pressure-driven combined air oxidation and MF system was used to remove iron from underground waters, especially when the amount of iron is high [ 53 ]. The system is more compact, and most importantly, high-quality clean water can be attained irrespective of the raw water quality. In order to reduce the amount of arsenic in drinking water, RO/UF along with hybrid coagulation-MF/UF can be utilized. RO TFC-ULP Koch membrane removes 99% of As (from 60 to 0.9 μg/L) from groundwater while DK2540F Desal membrane is able to remove 88–96% of As. NF membranes are also applied to As removal, and 97% removal of As (V) was obtained for membrane NF-70 (by FilmTec). The mechanism of arsenic removal using NF was found depending on the sieving separation and electrostatic repulsion between ions present in the treated solution and charged membrane.
2.3. Progress of Membrane Technology for Large-Scale Water Treatment Systems
The significant drop in surface raw water quality due to human activities has caused a lot of difficulties for the conventional water treatment system to produce the desired treated water quality [ 54 ]. As an alternative, membrane-based water filtration systems can offer a more robust and consistent solid–liquid separation process to produce good quality potable water. Membrane filtration has been utilized in commercial water treatment systems for decades. Its global market has reached up to an estimated USD 26.3 billion in the year 2017 and an expected 8.5% yearly growth [ 55 ]. Large-scale membrane water treatment systems are mostly commissioned in developed countries with high per capita income due to various reasons. First, the small footprints of membrane systems compared to the conventional system made it feasible to construct on land-scarce urban cities [ 56 ]. In addition, the modular type membrane system could be upgraded easily within a short time to suit the increase of water demand accordingly [ 57 ]. The quality of membrane has also improved significantly with distinctive advantages such as low fouling properties, higher chemical resistance tolerance and good mechanical strength, to name a few. Within the past few decades, commercial-scale membrane water treatment plants have significantly increased the total production of treated water.
The current shift of technology from conventional to membrane-based water treatment systems in developed countries is driven by the robustness of the latter. In developed countries, wide implementation of water treatment technology, including membrane filtration, has minimized the contamination risk of potable water. European countries such as Spain have upgraded their existing water treatment facilities in Barcelona with UF and RO systems to improve the water supply quality [ 58 ]. One of the push-and-pull factors for the proliferation of these membrane systems is the increasing demand for high water quality due to global population increase and rapid industrialization [ 59 ]. Water reclamation using membrane technology has been widely applied in Singapore to produce NEWater to cater for 40% of the country water demand [ 60 ]. In Singapore, a submerged hollow-fibre membrane was first installed in Chestnut Avenue Water Works (CAWW) in 2003 to produce drinking water. Then, the system was enhanced by adding submerged ceramic-based membrane system leading to a design capacity of 36,400 m 3 /d. This allow higher flux system to fulfil the demand [ 61 ]. In India, the desalination technology is essential for meeting the freshwater requirements. However due to high fossil fuel cost and remote area, it is expensive to establish large water treatment facilities. Therefore, the integration of renewable energy can helps to minimize the energy usage and cost [ 62 ]. All these positive developments are pointing towards the high feasibility of large-scale membrane water treatment systems.
2.4. Advantages and Disadvantages of Membrane Technologies for Water Treatment
One of the more prominent advantages of membrane filtration compared with the conventional water treatment system is the more consistent quality of the treated water [ 63 ]. Conventional systems rely heavily on the coagulation–flocculation process for the fine, suspended particles to form dense flocs. These flocs shall subsequently be separated in the clarifier and media filter through depth filtration. Failure to observe an effective coagulation–flocculation process will render poor solid–liquid separation in these units operation. Unlike the conventional system, membrane filtration (e.g., UF) is based on a cake filtration mechanism which enables effective solid–liquid separation even without the coagulation-flocculation process [ 64 ]. Consistent water quality is a critical aspect, especially in manufacturing, whereby it can significantly impact the end product outcome. Many membrane filtration systems are installed in these factory premises to ensure high control of the treated water consistency and quality for their production or manufacturing process. It has been reported that membranes are more effective in removing contaminants such as bacteria and dyes than adsorption technology [ 65 ]. All these findings have consolidated that, with a proper design of the membrane water filtration systems, high quality of filtrate can be produced.
Another distinctive feature of membrane-based water treatment systems is the relatively small footprint required compared to the conventional sand/media filtration system [ 5 ]. The modular concept of most pressurized membranes enables fast and easy upgrades to increase the treated water output capacity. Population growth of high-density urban cities are expected to be 1.9% from the year 2020 until 2030 [ 66 ]. This translates to roughly about 10% increase in water demand every five years for domestic usage in the urban areas. Under limited or scarce land and the urgency for municipal water treatment plant upgrades, a membrane-based system seems to be a feasible option to be considered.
Overall capital and operational cost of industrial-scale membrane water treatment systems are generally higher than the conventional freshwater system [ 5 ]. This is a significant drawback, especially for many developing countries whereby the per capita income of the population is much lower than the global average. Besides the higher overall cost incurred, one of the most prominent disadvantages of membrane systems is the fouling issue [ 67 ]. Membrane fouling issues have been widely reported in many industrial-scale membrane water treatment plants [ 68 , 69 , 70 ]. Membrane fouling causes higher operational cost due to increase trans-membrane pressure to maintain the desired filtration flux. In order to mitigate membrane fouling, chemical cleaning and periodic monitoring of the system are often carried out.
Membrane filtration systems, especially UF and MF, automatically require backwashes sequence as frequent as 2 to 4 times per hour, depending on feed water quality and flux [ 71 ]. This is essential to ensure minimal fouling before the next sequence of filtration begins. It is common for large-scale membrane water treatment plants to be equipped with computerized process control hardware and software to ensure uninterrupted operation with minimal human intervention [ 72 ]. All these facilities require periodic maintenance, which necessitates additional costs to the system operators [ 73 ]. Although production cost of the membrane has dropped due to economy of scale, another relevant expenditure such as electricity, labour, spare parts cost has increased due to inflation. The issue of affordability remains one of the biggest challenges, especially to most developing countries towards the adoption of large-scale membrane water treatment systems.
2.5. Fouling in Membrane Systems
Membrane systems need to be adequately maintained to prolong their life cycle. The main problems faced by the membrane system are fouling and scaling. Fouling, including cake layer formation or pore blocking by organics is the result of concentration polarization (CP). CP is a phenomenon that take places when there is an increase of rejected component at the boundary layer near to the membrane surface [ 74 ]. This can cause damage to the membrane, leading to the decrease of permeate flux and product water quality. In contrast, membrane scaling occurs when dissolved substances precipitate from the solution and accumulate on the membrane surface or lodge in its pores. Organic molecules with a bigger size than the membrane’s pores can cause adsorption on the surface, which can cause blockage at the membrane entrance. This blockage forms a cake layer after a period when the membrane system is put into operation. This will reduce the cross-sectional area of the membrane and cause resistance in the membrane process. Consequently, a reduction of flux could occur. Figure 3 shows an illustration of blockage in the membrane system. Four models are used to describe the blocking phenomena, including complete blocking, standard blocking, intermediate blocking and cake filtration. Complete blocking assumes that all the molecules that reach the membrane surface completely block the membrane pores’ entrance. The standard blocking occurs when the molecules enter through the membrane’s pores and deposit over the pore walls [ 75 ]. Therefore, the volume of membrane pores is assumed to decrease proportionally with permeate volume. As for cake filtration, the molecules are considered bigger than the membrane pores and thus only deposit on the membrane surface. The intermediate blocking is typically less restrictive and occurs due to the simultaneous pore blocking and surface deposit phenomenon [ 76 ].

Illustration on membrane blockage by retained molecule: ( a ) complete blocking, ( b ) standard blocking, ( c ) intermediate blocking, and ( d ) cake filtration. Reprinted from [ 76 ] with permission from Taylor & Francis Ltd. 2013.
Humic acid (HA) or fulvic acid (FA) is a common compound in surface water and seawater, thus introducing a cake layer on the membrane surface [ 77 ]. The compound can lead to a severe fouling phenomenon. Besides organic matter, inorganic compounds such as mineral salt (CaCO 3 , CaSO 4 , BaSO 4 , SrSO 4 ), metal ions may also introduce fouling and scaling. Generally, the severity of foulant is depended on the feed water of the membrane system. Usually, their proportion is 50% organics, 30% colloidal substance and 20% of mineral. Although minimal chemical component is applied in a membrane system, the concentrated retentate stream typically has higher concentrations of contaminants. Depending on the type of contaminants, the concentrate solution can either be disposed of through dilution, deep well injection, spray irrigation, or disposal in the municipal sewer.
2.6. Mitigation of Fouling in Membrane
Promising research efforts on membrane manufacturing focuses on finding suitable membrane materials with high chemical stability in an aggressive environment and solving the fouling problems. These can yield significant improvements in membrane performance, which are commonly constrained by the permeability–selectivity trade-off. Current research trends aiming to minimise fouling are moving in three directions: (a) modification of membrane using antifouling materials; (b) installation of pre-treatment system, which highly depends on feed content in which the pre-treatment system is mainly used to prolong the membrane’s life; (c) use of physical and chemical techniques in which backwashing and flushing procedures are used to recover the flux loss. In some cases, the loss is irreversible, and the only option is to replace the membrane with a new one. This leads to an escalation in the operating cost of the membrane system.
2.6.1. Membrane Modification
Membrane properties are generally described as pore size, hydrophilicity, surface charge, chemical stability, thickness, mechanical strength, and thermal resistance. These properties vary based on membrane materials, structure, form, and application. With the recent progressions in nanomaterials and advanced membrane fabrication methods, simpler and reproducible surface modification techniques such as coatings, grafting and polymerization have been proposed. This membrane modification aims to increase fouling resistance, improve selectivity, and enhance the lifetime of the membrane. Nanomaterials are single materials sized between 1 and 100 nm and can be in the form of nanoparticles, nanofibers, two-dimensional layer materials, and other nanostructured nanomaterials ( Figure 4 ). They exhibit excellent chemical and physical stabilities and have an enormous surface area, allowing extraordinary permeation properties. These nanomaterials might also provide additional antibacterial, antifouling and photocatalytic activity, which opens a new path to ultra-fast and highly selective membranes for water purification [ 78 ].

Nanomaterial-based membrane. Reprinted from [ 78 ] with permission from Elsevier, 2017.
A number of well-studied antimicrobial nanomaterials include titanium oxide [ 79 ], gold (Au), zinc oxide (ZnO), silver (Ag), carbon nanotube (CNT), graphene, and graphene oxide [ 80 ], have been utilized in various type of membrane-based water treatment system. The advances in nanotechnology and engineered nanomaterials presented a leapfrogging prospect to the next-generation membrane-based water treatment systems with a more affordable price and better purification efficiency. The nanomaterials can be integrated into the membrane to boost the efficiency and physicochemical characteristics by depositing them on the membrane surfaces or embedded into the matrices. Generally, there are five different nanocomposite membranes, including typical mix-matrix nanocomposite, thin-film nanocomposite, thin-film composite with nanocomposite substrate, and surface located nanocomposite [ 81 ] ( Figure 5 ).

Design of nanocomposite membrane. ( a ) conventional nanocomposite, ( b ) surface coated membrane, ( c ) TFC with nanocomposite substrate, ( d ) thin film nanocomposite, ( e ) surface coated TFC. Reprinted from [ 81 ] with permission from Springer Nature, 2019.
Despite significant progress on the development of nanocomposite membranes, several challenges need to be overcome for future scale-up manufacturing and application. Typically, the cost of most of the nanoparticles is high and requires a multi-step synthesis procedure, which results in poor reproducibility. Besides, it is challenging to ensure the homogenous distribution of nanoparticles in the polymeric matrix due to its tendency to highly agglomerate and incompatibility with the polymer matrix [ 82 ]. The agglomeration is caused by strong interactions between the nanoparticles and weak polymer–nanoparticles interfacial interaction, which cause the membranes to be vulnerable to defects and have poor separation efficiency. Therefore, it is vital to optimize nanofiller stability in the host polymer by establishing adhesive interface compatibility between the nanomaterials and polymer matrix [ 83 ]. Detailed investigation of the long-term stability of nanocomposite membranes is vital as poor adhesion of nanomaterial inside the polymer matrix or on the surface of the membranes could lead to the loss or leaching of nanomaterials during the filtration process. This will not only alter the membrane performance but can cause secondary contamination of the water [ 84 ]. Until today, the environmental and health side effects of nanomaterials for water treatment have not been systematically concluded [ 85 ].
2.6.2. Pre-Treatment
The appropriate way to control irreversible fouling is through preliminary treatment of raw water prior to entering the membrane filtration system. A number of hybrid- and integrated-membrane systems such as coagulation, adsorption, biological filtration, oxidation and membrane bioreactors have been proposed [ 86 ]. The pre-treatment system’s choice is highly dependent on feed water characteristics, whereby every foulant has its unique method to selectively remove the foulant. Commonly, a pre-treatment system is beneficial for surface water filtration compared to underground water due to large amounts of contaminations. Several MF or UF systems have been utilized as a pre-treatment system to maintain the consistent and reliable operation of a seawater reverse osmosis plant ( Figure 6 ) [ 87 ].

Pre-treatment processes: ( a ) Conventional pre-treatment process and ( b ) membrane-based pre-treatment process. Reprinted from [ 87 ] with permission from Elsevier, 2019.
Pre-treatment by coagulation can selectively eliminate charged particles such as colloidal particles and metal ions. Alum is used as a coagulant to coagulate charged particles and settled down the foulant, while an activated carbon filter can be used to remove coagulated substances to produce feed that has the lowest colloids content [ 88 ]. A more advanced pre-treatment process using hydrogen peroxide (H 2 O 2 ), ozone, UV radiation, and photocatalysts allows the molecular structure and properties alteration of the foulant due to the decomposition of organic pollutants. Consequently, low organic loading and biological fouling can be observed. Combined membrane systems are particularly useful for the treatment of surface water, which in contrast to underground water, is often described by the existence of large amounts of contaminations.
The application of the hybrid system combining adsorption on powdered activated carbon (PAC) with UF/MF to treat natural waters is more efficient than the process of unit membrane filtration [ 89 ]. The addition of carbon increases the efficiency of the membranes and the effectiveness of contaminations removal. The membrane acts as a physical barrier, inhibiting the passage of PAC, and thus the organic compounds which have been adsorbed on PAC are retained. This means the substances that lead to fouling are entirely retained by the PAC and do not deposit on the surface of the membrane.
Besides coagulation and adsorption on activated carbon, ozonation is also applied for the treatment of potable water. The purpose of ozone is to decrease the fouling phenomenon and quality of produced water as well as to increase the membrane lifetime [ 90 ]. The performance of an integrated coagulation–ozonation–ceramic UF-activated carbon filtration was evaluated for drinking water treatment from the micro-polluted surface water in southern China. It was discovered that the in situ ozonation in the membrane tank improves the removal efficiency for multiple contaminants, thus reducing the membrane fouling.
A higher degree of eliminating organic substances in potable water production can also be achieved by combining the filtration on a biologically active bed with membrane filtration. A 20 m 3 /day pilot-scale ozonation, ceramic membrane filtration (CMF) and biologically active carbon (BAC) filtration was utilized for indirect potable reuse, aiming for wastewater reclamation ( Figure 7 ). The degradation rate of trace organic compounds was found to be more than 96%, and in situ ozonation was observed to be more efficient to degrade the organic pollutants and fouling due to higher residual ozone concentration in the tank [ 91 ]. Besides, the system has a better removal rate of ammonia and N-nitrosodimethylamine from the ozonated water. For denitrification of nitrate (NO 3 − )-polluted drinking water, membrane bioreactors to denitrify the water have been proposed as an alternative to biodegradation and filtration on sand beds or adsorption on activated carbon as it can provide complete retention of the biomass [ 92 ].

Schematic representation of the pilot-scale hybrid process for drinking water treatment. Reprinted from [ 91 ] with permission from Elsevier, 2014.
2.6.3. Post-Treatment
Membrane systems can be operated in “dead-end” or “direct” mode, in which it has one feed stream and one filtrate stream [ 93 ]. In a dead-end manner, contaminants in the feed stream accumulate on the membrane surface and are held in place by hydraulic forces acting perpendicular to the membrane, producing a cake layer. The cake layer is typically removed from MF/UF systems through backwashing. The membrane will be replaced once the system faces a significant flow or transmembrane pressure (TMP) drop due to irreversible fouling. “Cross-flow” or “tangential flow” is operated by utilizing a high-pressure feedwater flow across the membrane. The solution is divided into two parts: (i) permeate, where a stream passes across the membrane, and (ii) retentate, where the remaining fluid flow on the membrane surface without separation or filtration. The retentate is usually concentrated with all rejected contaminants. The crossflow helps in maintaining a constant permeate flowrate and prolongs the membrane life by reducing irreversible membrane fouling ( Figure 8 ).

Membrane flow configurations and fouling formation [ 93 ].
A backwash process is typically carried out to eliminate contaminants collected on the membrane. It is conducted when there is an increase in TMP and/or a decline in permeate flux [ 94 ]. The direction and flow from the backwash will dislocate the contaminants from the membrane surface and washed out through the discharge line. A 5–10% reduction of system productivity can be expected after the backwash due to the volume of filtrate applied during the backwash. This indicates that although backwashing could enhance the fluxes, the complete removal of foulants could not be obtained alone through the backwashing process [ 95 ]. As a result, a chemical cleaning process needs to be utilised for inorganic scaling or foulants that cannot be removed by backwash [ 96 ]. Chemical cleaning is performed separately and in staggered manner for each membrane unit to minimize the number of units undergoing cleaning at one time.
The foulants that the backwash or chemical cleaning process can eliminate is identified as reversible fouling. After a particular time, the membrane system will face irreversible fouling, where the foulants cannot be eliminated through backwash and chemical cleaning [ 97 ]. Chemical cleaning agents are categorized into alkaline, acids, metal chelating agents, surfactants and enzyme [ 98 ]. Each chemical is targeted explicitly for a specific form of fouling. Strong caustic bases are typically utilized for dissolving organic material [ 99 ], while citric acid can be used for inorganic scaling [ 100 ]. Detergents and surfactants might be the best option for organic foulants that are difficult to dissolve [ 99 ]. Frequently, a combination of various chemicals might be utilized owing to the presence of numerous types of foulants in the water source. Table 1 summarizes the general chemical cleaning used for various type of fouling.
Chemical cleaning for various types of fouling.
| Fouling Type | Chemical Cleaning and Findings | Ref |
|---|---|---|
| Municipal wastewater: Organic, inorganic and biofouling and microbial | NaOH-EDTA-SDS alkaline treatment and citric acid (pH 2) treatment. 70% of membrane foulants were removed by cleaning. Bacteria with excessive extracellular polymeric substance (EPS) such as Pseudomonas and Zoogloea were more resistant to chemical cleaning | [ ] |
| Surface water: Organic, inorganic and biofouling | 2% HCl and caustic 2% NaClO. Alkaline cleaning removed most of the microorganisms and organic foulants on both membrane’s external and inner surfaces. Acidic cleaning effectively removed the inorganic scales. | [ ] |
| (pomegranate) juice | 1%w/w P3 Ultrasil 53 solution (a neutral enzymatic powder containing organic and inorganic surfactants). 90–95% of the initial water permeability was recovered | [ ] |
| Humic acid and Sodium alginate mixture: Organic fouling | sodium hypochlorite (NaClO). Concentration as low as 1 mg/L and backwash time 30 s leads to flux recovery of 92.1%. | [ ] |
| Sugarcane juice: biofouling | Acidic, alkaline, protease (i.e., trypsin), dextranase and lysozyme solutions. The use of enzymatic dextranase cleaning to degrade dextran foulant layer prior alkaline cleaning leads better removal rate. | [ ] |
| Surface water and ground water with NOM: Organic fouling | 0.1 M Citric acid, 0.1 M caustic NaOH, and 0.001 M surfactant SDS. Surfactant was not effective to remove high NOM content. High cross-flow velocity and longer cleaning time influenced the efficiency of caustic cleaning. | [ ] |
| Domestic wastewater: Organic and biofouling | NaOCl and citric acid as the order. The organic foulants such as FA and HA and microbes (proteobacteria, Firmucutes, Epsilon bacteria and Bacteroides) were effectively removed by NaOCl | [ ] |
| Boiler water: Inorganic fouling | HCl, H SO , H PO , nitric acid, citric acid, NaOH, potassium, EDTA, SDS and commercial dish washing detergents | [ ] |
Polymeric-based membranes are known to be less tolerant to chemical cleaning than the inorganic ceramic membrane [ 109 ]. Thus, the chemical used for the cleaning and the regularity of the cleaning should be decided appropriately. For potable reuse application, the cleaning can be scheduled as frequently as once per day to once per month, depending on the quality of wastewater that need to be treated. In general, a clean-in-place (CIP) practice means the cleaning was carried out when the membrane modules remained within the membrane unit (in situ) [ 110 ]. A high velocity of cleaning solution is re-circulated through the membrane system at elevated temperature to create scouring action and to enhance the foulant’s solubility. A soak and flushing cycle was then conducted to eradicate residual traces of the cleaning solution. The processes may be undertaken several times using different cleaning solutions for multiple types of foulants. As compared to regular backwashing, chemical cleaning is performed only when necessary. As for MF and UF systems, the chemical cleaning will only be performed when the productivity rate cannot be restored using a backwash process. However, for NF and RO systems, chemical cleaning is performed when the flux decreases in the range of 10–15% or when the differential pressure increases more than 50%.
2.7. From Laboratory to Commercialization of Membrane Technology
One of the main criterium for consideration of new technology is its significant advantages over the conventional system. Membrane technology has increasingly emerged as a sustainable solution for water treatment, and considerable efforts have been made to enhance efficiency to attract investments. This could include lowering capital, operations and maintenance costs, simpler operation, better water quality produced and reduction in waste production. Until today, the main hurdles to large-scale implementation of membrane systems are its capital and operational costs. Constant innovations in the design of membrane systems aim to reduce the capital and operational cost to make it competitive compared to conventional treatment processes.
Scaling up the lab-scale membrane manufacturing and membrane operation for field performance testing remains challenging, particularly in ensuring the consistency in membrane quality for large volume processes, effective membrane module design and fabrication techniques. Besides, it is not easy to predict harsh operating conditions with possible field contaminants fluctuation during the field operation. As a result, all these challenges need to be addressed appropriately to ensure the membrane can be effectively applied as per the intended field specification. The challenges are divided into three sections: (1) Membrane manufacturing scale-up, (2) Module development and (3) Commercial-scale demonstration challenge. As for membrane manufacturing scale-up, many iterations and detailed characterization are required to evaluate the membrane’s formulation that yields the best performances. It is also vital that the manufacturing of membranes should be reproducible without significant changes to the membrane structure, and the formulations should be scalable for manufacturing lines. The repetition of formulation development and characterization will add more cost, besides the cost of raw materials. Issues related to the environment, particularly the selection of solvents and chemicals involved in membrane manufacturing, should be considered. Long-term mechanical and chemical stability in process environments should also be carried out. As for module development at the pilot scale, leaks and sealing issues should be avoided to ensure reproducible QA/QC. During the assembly of the module before the performance investigation, the issue related to membrane integrity should be considered. Verification at the demonstration-scale level, especially for different water sources, should be carried out to validate the potential of the membrane system. During testing of a commercial membrane system, pressure drop and mass transfer issues should be investigated thoroughly.
3. Feasible Membrane Technologies for Water Treatment in Developing Countries
Many developing countries in Asia and Africa face shortages of clean water supply to meet the demand [ 111 ]. Most of these countries are still using conventional water treatment systems extensively due to the low capital and operational cost. The consumers in these developing countries often pay much lower water tariffs than those living in developed countries, as the production cost of treated water with the conventional system is much lower. The affordability of the consumers becomes one of the biggest obstacles for the country to move forward with the more advance membrane-based filtration systems.
The increase of human activities such as industrialization and land development causes pollution levels to ascend drastically [ 112 ]. This poses a challenging situation for the conventional water treatment system to produce the desired treated water quality. Higher loading of contaminants and inconsistent raw water qualities will affect the treatment efficiency of the conventional system. Some of the operators of these systems would have no choice but to stop the water treatment plant operation if the desired treated water quality is not achieved due to the fluctuating quality of the raw water. Under these circumstances, the membrane filtration system becomes an alternative option to consistently produce excellent treated water quality.
3.1. Membrane System for Clean Drinking Water Production
Some of the most commonly used large-scale membrane water treatment systems are ultrafiltration (UF) and reverse osmosis (RO) [ 113 ]. Seawater reverse osmosis (SWRO) plants commonly utilized UF as pre-treatment and RO for desalination [ 114 ]. As for surface freshwater, typically, only UF is used for the solid–liquid separation process. Compared to the RO process, treating freshwater is much more economical than sea water due to the lower pressure required for the UF [ 115 ]. Most developing countries are more inclined to use freshwater as their raw water source in large-scale water treatment plants for economic reasons. Rural villagers in low-income countries such as Kenya have resorted to various rain harvesting methods to fulfil their daily freshwater needs [ 116 ]. In the rural area of South Africa, borehole water was found to have high nitrate–nitrogen and salinity, which is not safe for human consumption. Therefore, the potential of the RO system to produce clean water was investigated ( Figure 9 ). It was found that the RO process is effective for water denitrification and water desalination. The capital cost for 50 m 3 /d output RO plant was found to be around the USD 29,900, while the operational cost was approximately USD 0.50/m 3 [ 52 ]. This cost was considered high, and thus chemical dosing or blending borehole water with RO product water are needed.

Flow diagram of the RO plant in South Africa. Reprinted from [ 52 ] with permission from Elsevier, 2003.
Kaya et al. [ 117 ] proposed the use of NF as a pre-treatment stage of reverse osmosis (RO) process for seawater desalination system located at Urla Bay, Izmir ( Figure 10 ). It was found that the scaling issue on SWRO membrane decreased significantly, which led to the reduction of desalination cost. Although permeate flux and recovery enhanced, the rate was greatly affected by the type of NF membranes due to the difference in pore size.

Flow diagram of the NF + SWRO integrated desalination process. Reprinted from [ 117 ] with permission from Elsevier, 2015.
Although large-scale membrane water treatment systems are still at the infant stage in many developing countries, some have achieved quite impressive results [ 68 , 116 , 117 ]. These membrane filtration systems usually complement or support the existing conventional system to increase the treated water capacity to meet growing demand. Large-scale membrane systems such as RO is impractical for economically challenged developing countries, particularly for populations living in rural and remote villages [ 118 ]. Such systems would require substantial advanced infrastructures and electricity supply for operations. A small-scale solar-powered membrane water treatment system is much more feasible for these rural villages [ 119 ]. It is still an uphill task for many developing countries to develop the capital and operational budget to maintain these facilities for the rural areas.
There is a vast potential for membrane-based desalination systems such as RO to replace the energy-intensive thermal/distillation technology [ 120 ]. This is mainly due to the lower energy consumption and higher efficiency of the RO systems. Recent technological developments in polymeric and ceramic type membranes have further propelled the potential for large-scale desalination systems with much lower operational costs [ 121 ]. Growing global population and rapid industrialization have pushed many countries to desalination and wastewater reclamation technology to fulfil the water demand. Some of the critical issues of RO membrane fouling have been addressed with membrane surface modifications to mitigate the problem [ 122 ]. One of the significant challenges of the desalination system is to ensure the affordability of the treated water to the consumers or end-users. More than 50% of the installed desalination RO plants are currently located in the United States of America and the Middle East, which is home to less than 5% of the global population [ 123 ].
Natural surface water remains one of the most widely used raw water sources for large-scale water treatment plants [ 124 ]. Many UF membrane systems have been utilized to process raw surface water into safe drinking water for public use [ 125 ]. Rapid industrialization in China for the past two decades has propelled the emergence of numerous research and development (R&D) on large-scale UF membrane water treatment plants [ 23 , 70 ]. The applications of UF systems are particularly suitable for highly urbanized cities that are facing the scarcity of land for the treatment infrastructures. It has been reported that UF systems take up almost 70% less footprint compares to the conventional sand/media filtration system [ 5 ]. With the distinct advantages of being more compact and having higher filtrate quality, UF membrane systems have become another feasible alternative worth considering in many metropolitan cities.
Table 2 lists out several membrane systems that have been used in developing countries. To date, small-scale membrane systems are typically used to produce potable water from brackish or seawater. Most studies indicate that a pre-treatment is critical to ensure that the feed water is compatible with the membrane system. Not only that, the use of a pre-treatment system before the membrane system enhances the efficiency and life expectancy of the membrane by decreasing the fouling and scaling issue. Some of these membrane-based systems treat contaminated water, which further substantiate the improved water quality obtained using this technology. As cost is a huge concern in most developing countries, surface water or freshwater will still be the priority, as it costs much less for treatment compared to seawater.
Membrane system used in developing countries.
| Country | Water Source | Membrane System | Pre-Treatment (Capacity) | Conclusions | Ref |
|---|---|---|---|---|---|
| Malaysia | Surface water and Groundwater | UF | Nil (15,536 MLD) | Effective at removing heavy metals (Cr, Cd, Zn, Cu, Ni, and Pb from 92% to 100%) but expensive. | [ ] |
| Turkey | Seawater | RO and NF-Desalination | NF (Not available) | NF could be an ideal pre-treatment step for the SWRO desalination to improve permeate flux and recovery by eliminating the scaling problem and reducing the cost of the desalination process | [ ] |
| South Africa | Groundwater | Gravity driven UF | -Nil (5000 L/d) | The microbiological quality of the permeate was acceptable, and the integrity of the filtration membrane was still maintained after ten months. Total coliform removal (2419.2 to 7 cfu/100 mL) and and Enterococc: Complete removal | [ ] |
| South Africa | Borehole Water | RO Denitrification and Desalination | 3 dual media sand filters using 2.5-μm cartridge filter (50 m /d) | RO effectively for water denitrification in a rural setting. Nitrate–nitrogen (reduced from 42.5 to 0.9 mg/L) and TDS of RO (reduced from 1292 to 24 mg/L) | [ ] |
| India | Pesticide contaminated surface water | NF and RO | Coagulation and Adsorption (Not available) | Needs a pre-treatment to produce drinking water. NF reduced hardness, COD, TOC, and completely removed microbial content. | [ ] |
| India | Arsenic contaminated water | NF | Nil (Not available) | NF remove arsenic (99.80%) following World Health Organization (WHO) level | [ ] |
| Mozambique | Freshwater | UF | Sand filter of 150 µm and 25µm (Not available) | Permeate flux remained constant and post-chlorination is required at the permeate tank prior to the distribution point to ensure suitable microbiological criteria. | [ ] |
| Brazil | Brackish Water | RO-Desalination | Nil (Not available) | The desalinated water showed rejections ~ 94% for SO , 97% for TDS and 100% for F . | [ ] |
| Indonesia | Brackish Water | RO-Desalination | Degasifier, coagulation and dual-media filter (Not available) | The groundwater can be treated by RO powered using renewable energy or a simple desalination plant using solar still. Both technologies are efficient and cheap. Modularity allows for upgrades and minimizes operational interruptions when membrane under maintenance. | [ ] |
| Vietnam | Seawater | Air gap membrane distillation (AGMD) | MF HF (46 L/h) | The seawater AGMD desalination proved feasible for both technical and economic. Produce 46 L h of high-quality distillate with specific energy consumption of 87 kWh·m without any issue of membrane fouling and wetting when dealing with real seawater. | [ ] |
| Vietnam | Wastewater and Seawater | MF, UF, NF, RO, FO and MD | Filtration (Not available) | FO and MD can be used in small-scale systems at low expenses. A membrane offers compactness, system modularization, and lower energy consumption | [ ] |
| Vietnam | Surface Water | NF and ED hybrid process | Electrodialysis (Not available) | ED–NF is an effective alternative for small surface water treatment plants in rural Vietnam. The water quality generated was according VN guideline. | [ ] |
| Southern India | Membrane filtered water and household container water samples | Decentralized membrane filtration | Filtration (Not available) | Membrane filters helped reduce faecal coliform bacteria and decentralized water filtration infrastructure may be effective in places where the microbiological quality of water is not addressed correctly. Initial costs for installation and maintenance are affordable. | [ ] |
| Thailand | Freshwater | Ozonation (Submerged Ceramic MD and UF | AC filter with 50µm (5 m /h) | This multi-stage process ensures efficient drinking water production free from viruses and pathogens. Due to low space requirements, compact treatment units for decentralised units are needed. | [ ] |
| Sri Lanka | Groundwater | Nanofiltration (NF) | Sand and AC filters, cation exchange resin, precision filter (20 m /d) | The NF plant’s permeate water reduce hardness, fluoride, and DOC. Fulfils Sri Lankan drinking water requirements and is well approved by society’s stakeholders. | [ ] |
| China | Reservoir | Hollow fibre UF | Filtration (100,000 m /d) | During the 7-year operation, the UF membrane was effective to avoid breakthrough of organic substance from microorganism metabolic activity. | [ ] |
| China | Raw Water | UF | Coagulation (Not available) | Effective turbidity and other metals removals, including total removal of coliform bacteria. Coagulation process is needed before UF for surface water with high turbidity and varying quality. | [ ] |
| South Africa | Surface Water | Low Pressure UF | Sand Filter (Not available) | UF produce quality potable water at low operating pressures ranging from 100 to 150 kPa hydrostatic pressure. Excellent removal of turbidity and no coliforms or faecal coliforms. | [ ] |
3.2. Cost Analysis of Membrane Systems
It has been widely accepted that the membrane system incurred much higher capital and operational expenditure compared to the conventional system [ 63 ]. The most significant saving of the membrane system is on the smaller footprint required [ 141 ]. In high-dense population urban cities, land acquisition to build a water treatment plant is substantial and incurs a significant cost. Developed countries have taken full advantage of the small footprint to build compact large-scale membrane water treatment systems to fulfil the demand for highly populated urban cities [ 142 ]. Unfortunately, the smaller footprint might be the only significant cost saving for these systems compared to the conventional water treatment systems. It is widely documented that capital and operational expenses for large-scale traditional water treatment systems is much lower and thus often become the most preferred system in developing countries [ 143 ]. One of the most significant operational expenses for large-scale water treatment systems is the electricity consumption. Industrial-scale UF membrane water treatment systems could cost more than 20 times in electricity consumption compared to the conventional system using the same source of raw water as feed. It has been anticipated that due to the mass production and competition among membrane manufacturer, more affordable and higher quality of membrane will be made available in the near future. In developing countries, small to medium scale membrane-based water treatment systems are confined to privately owned factories to cater to production needs. This is primarily due to the insufficient clean water supply from government-owned facilities for these factories.
Over the last few years, the cost of manufacturing polymeric membranes has reduced substantially due to better production techniques and economies of scale. It has been reported that an industrial-scale UF membrane plant capital cost is only about 6% more than the conventional system. However, the estimated electricity cost for the UF system is more than 20 times higher [ 5 ]. One of the hidden costs of the membrane system is the periodic maintenance required. Due to the more complicated automation, highly trained technicians and engineers are often stationed at these water treatment plants, which incurred significant maintenance costs. In addition, many mechanical equipment (e.g., pumps, valve actuators, electrical relays, etc.) are installed to enable the complete automation for periodical cleaning of the membrane. Unlike the membrane system, which requires backwash or cleaning every few hours of filtration, the conventional system could operate for days before a backwash is initiated [ 144 ]. This allows less costly automation installation, and the conventional system could be operated under manual mode to reduce the overall capital and operational expenditures.
A detailed analysis between industrial-scale UF and conventional water treatment systems for raw surface water has indicated that the overall cost of the UF system is still much higher [ 5 ]. Table 3 summarizes the various cost incurred in general between the two water treatment systems.
General costs comparison between UF membrane and conventional sand/media water treatment systems.
| UF Membrane System | Conventional Sand/Media System | |
|---|---|---|
| Construction/Capital Cost | Higher | Lower |
| Operational Cost | Higher | Lower |
| Maintenance Cost | Higher | Lower |
| Land Requirement | Lower | Higher |
Table 3 implies that as land scarcity becomes more apparent, especially in high-density urban cities, the UF membrane system would become a more attractive solution. A land-scarce developed nation such as Singapore has adopted many large-scale membranes water treatment plants to fulfil their water needs [ 21 ]. It is estimated that more and more developed countries shall face similar land scarcity issues due to the mass migration to cities or urbanization.
3.3. Affordability, Supply and Demand for Clean Water
Most developing countries indicate a much lower per capita income compared to developed countries [ 145 ]. This shows the less spending power on basic necessities such as water and electricity utilities. The governments in these countries have little choice but to continue large-scale conventional water treatment systems to ensure the affordability of the consumers. The overall operational cost of these water facilities are mainly derived from revenue collected from the consumers based on the stipulated water tariff imposed [ 146 ]. Affordable water tariff is generally defined as less than 5% of household income spent on water bills [ 147 ]. In Southeast Asia (SEA), in developing countries such as Malaysia and Indonesia, water tariffs are much lower compared to developed nations such as Singapore, although these countries are close neighbours. Large-scale membrane systems are used extensively in Singapore for clean water production, requiring much higher costs than conventional systems.
It is estimated that the water demand of most developing countries shall keep on increasing as they move forward with more industrialization activities and population growth [ 148 ]. This has posed a strain on the existing raw water resources and water treatment plants to produce sufficient treated water for the country needs. When the relatively unpolluted raw water sources are scarce, the next step is to look for raw water with much higher contaminant loading but which is still abundantly available. Due to the limitations of the depth filtration mechanism in the conventional media sand filters, it is incapable of handling some contaminant removal to ensure an effective solid–liquid separation process [ 149 ]. A membrane system such as UF offers a feasible alternative to handle high suspended loading and yet provides a good quality filtrate through the surface filtration mechanism [ 150 ]. This enables raw water with much higher contaminant loading to be processed with less retention time in the system.
The correlation between demand, supply and affordability has posed a serious issue to many developing countries. The demand can be met by constructing more feasible water treatment systems to produce the supply. Nevertheless, due to limited “unpolluted” raw water sources, a lower grade of raw water sources has to be used. That shall increase the overall production cost and inevitably increase the water tariff. Raising water tariffs significantly will have a chain of economic repercussions, especially for the developing countries.
4. Challenges and Opportunities of Membrane Technology Implementation in Developing Countries
Improving the standard of living for the population in developing countries remains an uphill task without sufficient clean potable water supply for all, especially in rural areas. This research paper intends to highlight the feasibility of various membrane technologies for water treatment in these countries. There are many limitations in developing countries to seek more reliable alternative water treatment processes such as membrane systems. One of the main challenges for large-scale membrane systems in these countries is the overall cost incurred against the affordability of consumers. Many stakeholders (e.g., government and private sectors) are keen on adopting large-scale membrane water treatment systems in developing countries, looking at the positive economic, rapid industrialization, and population growth. Based on the current consumer’s affordability in most developing countries, the water tariff needs to remain low to accommodate the population income. Adopting a large-scale membrane water treatment system will undoubtedly increase the water tariff significantly. A balance needs to be reached to ensure that clean water supply is sufficient and water tariff remains affordable to most people in these countries. One option is to allow the treated water from these large-scale membrane water treatment plants to be supplied to only the industry or factory for their manufacturing process [ 151 ]. Higher water tariffs could be imposed on these profit-making industries to encourage these factories to install their own water treatment facilities in order to fulfil their manufacturing demand. This would free up more resources from the municipal water treatment plants to cater for domestic users. Government intervention is required as a proper guideline has to be drawn up to ensure compliance [ 152 ].
Decentralized small-scale water treatment systems have been a feasible solution for many rural villages with a small population in developing countries. Small-scale UF systems have been utilized as a direct filtration process without using any coagulant or chemicals for Malaysia’s rural river water source [ 119 ]. In another project, a membrane-based water treatment system was set up and monitored in Tanzania’s rural community for over 9 months [ 153 ]. This low-cost system has shown promising results as a decentralized water treatment pilot plant for other similar rural villages. The major challenge of these decentralized systems is the long term operational and maintenance cost. Local governments have to formulate a workable solution to ensure the sustainability of rural water supply schemes.
Direct filtration using a low-pressure membrane such as UF is suitable for raw water with low turbidity and suspended solids without any coagulant required [ 68 ]. Unfortunately, most raw water source fluctuates in quality and a more robust pre-treatment need to be in place to prevent severe membrane fouling. A hybrid membrane process has been vigorously studied to ensure higher contaminant removal and mitigate membrane fouling issues [ 154 ]. In this process, activated carbon, which is commonly used as filtration media in the conventional water treatment system, is added prior to the membrane filtration process. This process has the advantage of maintaining a relatively small footprint and yet provides an extra precaution to reduce membrane fouling even with relatively polluted raw water. An additional operational cost will be incurred for the continuous addition of the activated carbon, which requires a feasibility study for the long run.
One of the highest operational costs for membrane water treatment systems is electricity utilization [ 155 ]. Renewable solar power has been considered a viable energy source to reduce the carbon release in producing electricity [ 156 ]. The notion of having “free” electricity for the pressure-driven membrane filtration process would be of interest to many large-scale facilities stakeholders. In most cases, a significant amount of initial capital expenditure will have to be disbursed for all the necessary solar panels and ancillary equipment before converting solar energy to electricity could be achieved [ 157 ]. Instead of paying electricity as an operational cost, solar energy harnessing facilities have become a capital cost. The feasibility of using renewable energy in large-scale membrane water treatment systems will be determined by various factors and differs for each developing country.
It is an uphill task in most developing countries to provide basic utilities such as electricity and clean water, especially to the vast rural populations [ 158 ]. Infrastructure developments in these countries are concentrated in urban cities whereby all the major economic activities thrive. Both government and private sectors rely on each other to ensure a conducive business environment in the towns for mutual benefits. As for the rural villages, infrastructure developments are hampered due to the lack of business activities. Governments in developing countries would need to build up their financial coffers to upgrade the basic utility supply for rural villagers. Most developing countries have plans to provide these utilities, but financial constraints are still prevalent in many regions.
4.1. Current Scenario of Water Treatment Facilities in Developing Countries
Many developing countries are still struggling to provide clean water to some rural areas lacking water and electricity supply [ 158 ]. Due to the low population density and per capita income, supplying these utilities in rural areas is challenging. Many rural villagers can only obtain raw untreated water from ponds or rivers for daily usage because of the lack of piped water supply in these remote areas [ 159 ]. It is a challenging task to ensure that all areas in these developing countries have access to clean piped water with limited resources from the government.
A conventional water treatment system is still the most cost-effective process, provided a relatively “unpolluted” raw water source is available [ 160 ]. Due to the constant increase of water demand caused by thriving industrialization in developing countries, these raw water sources are slowly depleting. Many cases of raw water contamination have caused these large-scale conventional water treatment plants to be shut down as these systems are not designed to removed other harmful dissolved pollutants [ 161 ]. Membrane filtration systems offer more robust removal efficiency, especially with the combination of UF and RO membranes [ 162 ]. Developed countries such as Singapore have even used membrane systems to recycle sewage water into high grade processed water for various industry applications [ 60 ]. The dwindling “unpolluted” raw water sources in developing countries are slowly pushing them to adopt a more robust system to ensure the sustainability of their clean water supply. Due to the relatively high cost incurred in developing countries, small and medium-sized membrane plants are mostly installed at manufacturing factories. Most of these factories rely heavily on clean water supply for their manufacturing process but piped water supply from local authorities might not guarantee both quality and quantity to fulfil their demand. Thus, it is quite common for these factories to source raw water (e.g., groundwater or nearby stream) and carry out the water treatment process themselves at their premises with the authority’s approval.
Consumption power of the people in developing countries is usually much lower due to their below-average per capita income. The basic utilities such as water and electricity tariffs are usually kept low through government intervention (such as subsidy) to ensure the people’s affordability [ 163 ]. In general, the conventional water treatment system is the most affordable process to provide clean water according to World Health Organization (WHO) standards. Many of these developing countries are still allocating a vast sum to build large-scale conventional water treatment plants to fulfil the country water demand. The water tariffs in these countries are much lower than in developed countries in the same region. The sustainability of these water treatment plants depends on the revenue collection based on the approved water tariffs.
4.2. Technology Transfer from Developed/Advanced Countries
Developing countries face two main challenges in adopting large-scale membrane water treatment systems. The first is the relatively low water tariff as well as the affordability of the people. It is commonly accepted that capital and operational expenditure for a membrane system is much higher than the conventional system. Thus, it will incur a higher water tariff to ensure economic sustainability [ 164 ]. The second obstacle is the lack of infrastructure and experts to support the operation of these advanced systems. Membrane systems require highly automated operations with competent personnel. Most of these membranes and supporting equipment are unavailable in developing countries and are imported from more advanced or developed countries overseas. The willingness and co-operation between these countries (developed and developing countries) is paramount to ensure a successful technology transfer of these advanced water treatment systems.
One of the possible solutions is to promote the privatization of water supply to prospective investors. The build, operate and transfer (BOT) business model allows minimal financial burden from local government to develop water infrastructure for the public [ 165 ]. The conventional and membrane-based water treatment systems both have their distinctive advantages and weaknesses. A detailed feasibility study of BOT model for developing countries is always necessary because it involves long-term investment. Investors have to ensure the water tariffs have to be affordable for the public and yet the revenue collected will enable them to make a continuous profit. Striking such balance is another uphill task for all the stakeholders involved. Both technology transfer and investment from foreign investors might be a possible solution to ensure the sustainability of large-scale membrane water treatment systems in developing countries.
5. Current Trends and Future Outlook
5.1. improvements in membrane modules and membrane system configurations.
Researchers are looking into other strategies to overcome fouling challenges and enhance membrane performances, including improved membrane module design and novel hydrodynamics. Typically, the design of membrane modules is plate and frame, hollow-fibre, tubular and spiral wound [ 166 ]. A hollow fibre module typically comprises a number of hollow fibres bundled together into an element forming a pressure vessel. The HF module presents high packing density and consistent permeate flow with minimal pressure drop, allowing 10 times more flux than spiral wound modules. In a spiral wound module, two membranes are separated by a feed channel spacer and placed where their active side facing each other is centrally connected to a perforated permeate collection coil. The feed channel spacer promotes turbulence conditions and permits the homogenous flow of feed water across the membrane [ 167 ]. Generally, two standard techniques to enhance module performances include design effective module with optimized flow geometry, known as passive enhancement and utilization of external energy such as bubbling, vibrations and ultrasound to prompt a high shear regime to minimize the fouling and concentration polarization phenomena, which is known as active enhancement [ 168 ].
In a recent development, a rotating disc module was fitted with a UF membrane to control the thickness of the cake layer and ease membrane fouling through flow velocity improvement and shearing force in the presence of flocs [ 169 ]. The system revealed that floc-based cake layers could be efficiently controlled with module rotation, making them suitable for drinking water treatment systems. Another industrial dynamic filtration unit consists of disks or rotors rotating near the fixed membranes or rotating organic/ceramic disk membranes and vibrating systems [ 170 ]. A high shear rate can be produced with no significant feed flow rates and pressure drops, a feasible substitute for crossflow filtration. A novel magnetically induced membrane vibration (MMV) system was examined in a lab-scale MBR to replace the conventional submerged membrane module ( Figure 11 ). Instead of using coarse bubbles aeration for the sheer production, an intermittent vibration technique was utilized, leading to energy saving [ 171 ].

Schematic diagram of novel magnetic vibrating module (MVM) that offers high flux and lower degree of fouling. Reprinted from [ 171 ] with permission from Elsevier, 2012.
A helical membrane module unit comprises an inside helical spacer and an outside cover to reduce filtration resistance, reduce fouling, and enhance permeate flux [ 172 ]. The helical membrane consists of two sheets of terylene filter cloth, with a pore size of around 22 µm and a total effective area of 0.022 m 2 . The membrane was supported on a plastic spacer resembling DNA helix that acts as a “stirrer” and “rotating ladder” ( Figure 12 ) for mass transfer improvement through vortex mixing and turbulence enhancement. The new module was found to decrease filtration resistance because of vortex mixing and intensified turbulence at the membrane surface. As a result, the expense per amount of mass transferred can be minimised, which can be directly translated into energy consumption and module manufacturing cost.

( a-1 , a-2 ) the fish-bone or broom-like structure of spacer ( b ) cross view of helical membrane and its mounting in filtration chamber. Adapted from [ 172 ] with permission from Elsevier, 2010.
Recently, surface patterning using a 3D printer was utilized to modify the membrane surface topography as a way to mitigate membrane fouling [ 173 ]. Besides the hydrodynamic effect, surface patterning can inhibit the deposition of foulants on the valleys when the particle size is bigger than the valley size or by modifying the particle crystallization entropy when the size is comparable. In addition, novel and innovative membrane module components can be developed quickly and at a low cost using 3D printing technology. The fabrication and optimization of membrane module elements could be promptly prototyped, which is unreachable using traditional manufacturing methods ( Figure 13 ). The patterned surface permits the generation of eddies, and with the cross-flow velocity combination, the back-diffusion of foulant to the bulk liquid can be facilitated [ 174 ].

( a ) Solid-based, ( b ) liquid-based and ( c ) powder-based 3D printing technologies. Reprinted from [ 174 ] with permission from Elsevier, 2016.
5.2. Development of Renewable Energy-Driven Membrane System
As for the development of system infrastructure for clean water production, energy use and the carbon footprint of water consumption have emerged as critical issues. As a result, the correlation between water and energy consumption, known as water-energy nexus implications, is integral to developing a sustainable and low-cost water purification system. The growth of water resources should not come with high-cost energy consumption. Due to the high energy and separation efficiency, membrane-based technologies have earned pervasive implementation in various water treatment processes. Ghaffour et al. [ 175 ] reviewed the challenges and potential applications of using renewable energy-driven desalination technologies. They indicated that solar-driven RO plants allow for the elimination of the fossil fuel dependency for producing adequate freshwater. However, the efficiency of the solar energy-driven system is highly linked with the design and arrangement of PV arrays, tilt angle and cleaning methods. An efficient system can ensure an uninterrupted system, increasing the distillate production and cut down the overall water production cost [ 176 ]. Elmaadawy et al. [ 177 ] investigated several types of the renewable energy system for large-scale RO desalination plants (1500 m 3 /d) and compared two off-grid scenarios with various combinations of hybrid power systems and diesel systems. The results demonstrate that the recommended system significantly reduces from 60–81.5% compared to existing diesel with respect to the net present cost, renewable fraction, cost of energy, and carbon dioxide emission, respectively.
Chew and Ng [ 119 ] investigated the feasibility of a solar-powered UF system at a rural village in Perak, Malaysia and the performances was compared with a sand/media filtration system. A solar-powered UF system can obtain a higher quality of treated water with less than 0.4 NTU turbidity and lower operating cost and carbon release. The use of cross-flow filtration operation mode eliminates a daily intermittent backwash sequence, which further simplifies the daily operational routine suitable for rural areas. However, one of the main challenges of a solar-driven system is related to energy storage capacities. The prospective for lithium-ion (Li-ion) batteries and supercapacitors (SCs) on a photo voltaic-powered RO membrane (PV-membrane) system was evaluated, and it was observed that average specific energy consumption (SEC) of 4 kWh/m 3 with fully charged batteries is used to produce clean water [ 178 ].
5.3. Development of Alternative Pressure-Driven Membrane System for Desalination
In addition to using renewable energy with a traditional membrane filtration system, advanced membrane operations can be attractive for the production of renewable energy in the future and might shift the conventional perception of renewable energy sources. Seawater desalination is seen as one of the solutions to clean water scarcity. It allows a climate-independent source of drinking water and is increasingly being used to provide drinking water around the globe. More desalination plants worldwide are using RO technology due to its simplicity and low energy cost compared to flash distillation thermal processes. Besides RO, forward osmosis (FO) and membrane distillation (MD) are among the emerging membrane-based system appealing for desalination [ 179 ]. FO and MD have received significant attention from researchers in the membrane field and industry, as shown by the increased publications. Among the essential features of this third-generation desalination process is the ability of FO and MD to minimize the energy required, and the operating cost is much lower than RO [ 180 ].
During the RO process, a high operating pressure (up to 1000 psi) drives the saltwater through a membrane. As a result, the energy use in RO is typically higher. Meanwhile, FO operates at low pressure, thus requiring lesser energy and low fouling compared to the RO process. In FO, the water in the feed solution (low osmotic pressure) freely flows through a selectively semipermeable membrane to the draw solution (high osmotic pressure) under the osmotic pressure difference. FO has been utilized in desalination and complex industrial streams [ 181 ]. When FO was used for high salinity brines (TDS > 70,000 mg/L), the recovery of feed water was found to be more than 60%, and the quality of treated water could meet the discharge quality criteria of surface water [ 182 ]. RO and FO typically use similar types and pore size membranes. In comparison [ 183 ], MD is a thermally driven membrane system using a microporous, hydrophobic, vapour-filled membrane. The MD is not entirely viable at a commercial level because of several reasons: (1) vague water production cost (WPC) due to high specific energy consumption (SEC), (2) lack of suitable membranes and modules, (3) membrane pore wetting phenomenon due to the use of unsuitable membranes, and (4) undefined long-term operation because of membrane fouling and/or scaling [ 184 ]. Figure 14 compares the working principles of RO, FO and MD.

Working principles of reverse osmosis, forward osmosis and membrane distillation Reprinted from [ 185 ] with permission from Elsevier, 2018.
5.4. Zero Discharge Liquid (ZLD)
Besides the water and energy shortage, lawful obligations for the discharge of waste and wastewater have forced the industry to change its liquid waste management approach to be more sustainable through integrated concepts. As a result, verifying the prospective applicability of zero liquid discharge (ZLD) for water treatment is essential. ZLD is a water treatment approach where all wastewater is purified and recycled, resulting in zero discharge at the end of the treatment cycle. ZLD relates residual output in terms of waste, wastewater and energy loss to the process input based on materials and energy, allowing the prospect of using a process cycle where wastewater treatment is envisioned for water recycling, considering the mass balance of materials other than water and the energy balance.
The environmental impacts of brine dumping and greenhouse gas (GHG) emissions from seawater desalination plants and wastewater treatment plants have been a rising concern due to water scarcity. An innovative and energy-autonomous (through solar energy) pilot SOL-BRINE system with a capacity to treat 2 m 3 /day of brine has been installed in Greece to achieve ZLD in a desalination plant. It was found that the recovery of water (> 90%) and dry salt (full recovery) can be achieved [ 186 ]. Recently, China and India have been among the countries that have implemented strict regulations that make ZLD as a necessary strategy for sustainable wastewater management and to protect freshwater resources. In China, the ZLD approach has been widely adopted in developing new power and chemical plants due to public protest. The government of India has established a draft policy where the textile plants need to install ZLD facilities if they are producing more than 25 m 3 of wastewater effluent/day [ 187 ].
Although ZLD could minimize contamination and enhance clean water supply, one of the significant concerns of ZLD at the industry level is the high capital cost and intensive energy consumption [ 188 ]. As a result, membrane-based technology is theoretically an attractive approach that can be utilized to reach this aim. Several recent works have emphasized the use of a series of membrane processes as a possible way to accomplish ZLD at the industry. In Tamil Nadu, the Perundurai Common Effluent Treatment Plant is among the first plant in India that implemented ZLD to treat and manage effluents from several textile processing industries using RO and evaporator [ 189 ]. Although the cost of ZLD is slightly higher, the expenditure of the system is expected to be reduced through salt recovery.
One of the examples is the FO-based ZLD system in Changxing coal-fired power plant that is capable of producing high-quality permeate water for reuse in any industrial process and concentrating brines up to a high TDS concentration for further studies processing in a crystallizer [ 190 ]. However, not many works have discussed in detail the challenges encountered with ZLD approaches.
6. Outlook on the Adaptation of Membrane Filtration Technology by Developing Countries
Rapid industrialization in many developing countries is swiftly transforming these countries’ economic landscape. More factories will require a higher volume of clean water for product manufacturing to increase productivity. The increase in industrial activities would bring forth more income to the government in terms of tax revenue to improve public utilities such as clean water supply to the public and industry. Stakeholders or government officials would need to devise future plans to ensure the sustainability of their country water resources in the coming years. The depletion of good quality raw water sources from reservoirs such as rivers and lakes are prevalent due to pollution caused by human activities, particularly in rapidly industrialized developing countries. The conventional water treatment system utilizing sand or media filtration which most developing countries heavily rely on might not be able to handle the more challenging task of treating polluted raw water sources.
Membrane technology will be one of the most feasible alternatives to carry out the more challenging task of water purification due to its many advantages over the conventional system. Membrane filtration has a compact design and high degree of flexibility, and therefore the use of membrane systems is expected to rise in future. It has been a game changer utilized to convert seawater into potable water in desalination plants around the world. Nevertheless, the cost factor remains one of the biggest obstacles for its adaptation in developing countries. With the increased income of rapid industrialized developing countries and the more affordable cost due to technological advancement in membrane technology, it is envisaged that membrane systems will become highly feasible in the near future. The successful implementation of many large-scale membrane water treatment plants in advanced countries further substantiates the maturity of membrane technology for water purification.
From the global industrial complex perspective, the most significant issue is how to make membrane technology affordable in low-income countries with restricted access to RO technology. Perhaps, membrane systems’ capital investment and operating costs must be reduced significantly to make this viable. The utilization of solar energy might be able to mitigate some of the high operational cost for membrane-based desalination, but the high capital investment for the solar PV system remains a challenge for most developing countries. Freshwater sources will still remain the main sources of raw water for treatment in these countries, and desalination would be an alternative when freshwater resources have been depleted. This review intends to provide stakeholders and researchers with comprehensive information to evaluate membrane technology for water treatment, particularly in developing countries.
Acknowledgments
The authors would like to acknowledge the Universiti Teknologi MARA, Techkem Group and Universiti Teknologi Malaysia for providing all the supports in this research project.
Author Contributions
N.H.O. contributed to writing the manuscript. N.H.A. contributed to gathering related journals for reviewing. N.S.F. contributed to check the content and manuscript formatting. F.M. contributed to writing on the membrane modification. M.Z.S. contributed to finding the related journals for reviewing. C.M.C. contributed to writing the manuscript. K.M.D.N. contributed to reviewing the manuscript. W.J.L. contributed to reviewing the manuscript. A.F.I. contributed to reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
This research and APC were funded by College of Engineering and Universiti Teknologi MARA (Grant no: 600-RMC/YTR/5/3 (002/2020). This research was also funded by Techkem Group Research and Novel Technology (TechGRANT) fund (Project No.: MEMD-WES2535).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no competing interests.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 05 November 2020
A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment
- Md. Nahid Pervez ORCID: orcid.org/0000-0001-6187-5351 1 ,
- Malini Balakrishnan 2 ,
- Shadi Wajih Hasan 3 ,
- Kwang-Ho Choo ORCID: orcid.org/0000-0002-4773-5886 4 ,
- Yaping Zhao 5 ,
- Yingjie Cai 6 ,
- Tiziano Zarra 1 ,
- Vincenzo Belgiorno 1 &
- Vincenzo Naddeo ORCID: orcid.org/0000-0002-3395-3276 1
npj Clean Water volume 3 , Article number: 43 ( 2020 ) Cite this article
14k Accesses
76 Citations
26 Altmetric
Metrics details
- Pollution remediation
- Sustainability
The concept of nanomaterials membranes (NMs) promises to be a sustainable route to improve the membrane characteristics and enhance the performance of membrane bioreactors (MBRs) treating wastewater. This paper provided a critical review of recent studies on the use of membranes incorporating nanomaterials in membrane bioreactor (NMs-MBR) applications for wastewater treatment. Novel types of nanomaterials membranes were identified and discussed based on their structural morphologies. For each type, their design and fabrication, advances and potentialities were presented. The performance of NMs-MBR system has been summarized in terms of removal efficiencies of common pollutants and membrane fouling. The review also highlighted the sustainability and cost viability aspects of NMs-MBR technology that can enhance their widespread use in wastewater treatment applications.
Similar content being viewed by others
Strategies for mitigating challenges associated with trace organic compound removal by high-retention membrane bioreactors (HR-MBRs)
Advanced wastewater treatment and membrane fouling control by electro-encapsulated self-forming dynamic membrane bioreactor
Effect of organic loading rates on the performance of membrane bioreactor for wastewater treatment behaviours, fouling, and economic cost
Introduction.
Membrane bioreactors (MBRs) have proven to be one of the most effective technologies globally for treating wastewater from different sources 1 , 2 , 3 . In MBRs operation, wastewater treatment is carried out via a combination of biological unit (for the biodegradation of waste streams) and membrane filtration unit (for the separation of treated water from biosolids using membrane module). The two major categories of MBRs, based on their configuration and hydrodynamic control of membrane fouling, are submerged (SMBRs) and side stream MBRs. This technology was introduced >30 years ago and offers the advantages of a smaller footprint, high-quality treated water, less sludge production, low energy demand, and a higher removal rate for pollutants. Therefore, for obtaining high-quality treated wastewater, MBRs are recommended over other techniques, such as activated carbon adsorption, filtration, coagulation etc.; they can also be integrated with oxidation processes, such as photolysis, sonolysis and chemical/electrochemical oxidation for removal of micropollutants 4 , 5 , 6 , 7 . Thus, MBRs are well suited for on-site reuse of treated wastewater. As a consequence, driven by growing water scarcity, ageing infrastructure and increasingly stringent discharge norms, the MBR market is growing rapidly and has successfully contributed to the broader wastewater treatment market; furthermore, MBRs represent a significant market source for other membrane systems, especially microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and forward osmosis (FO) 8 , 9 , 10 .
Based on the literature, it is evident that in recent years, remarkable progress has been made in wastewater treatment via MBR technology. Wastewater reuse has been targeted 11 , 12 and a range of industrial wastewaters have been tested 13 , 14 . Of particular interest are highly polluting effluents from sectors, such as tanneries 15 and textiles 16 , 17 , as well as wastewaters with high salinity 18 . For example, textile wastewater containing azo dyes has been treated in a bioaugmented MBR coupled with a granular activated carbon (GAC) packed anaerobic zone resulting in over 95% dye removal in a short time 19 . Removal of micropollutants is also a focus area. For example, Trinh et al. 20 investigated a full-scale MBR treating municipal wastewater for the removal of 48 trace organic chemical contaminants and found that removal percentage was over 90%. Katsou et al. 21 illustrated removal of heavy metals from wastewater with removal efficiencies of Cu (II), Pb (II), Ni (II), and Zn (II) of 80%, 98%, 50%, and 77%, respectively. Mannina et al. 22 reported an MBR pilot plant experimental study designed to treat saline wastewater contaminated with hydrocarbons, which resulted in a high total chemical oxygen demand (COD) removal of ~90%.
A plethora of studies have been done to enhance the performance of MBRs by integrating them with other systems 10 , 23 , 24 . In general, objectives of the integrated MBR systems are to improve permeate rates, decrease membrane fouling, increase process stability and to obtain treated wastewater of requisite quality. Laera et al. 25 integrated an MBR with oxidation (either ozonation or a UV/H 2 O 2 process) for the removal of organics and degradation of components in pharmaceutical wastewater. This integrated system was able to remove COD in the range of 85 − 95%, with complete removal of degraded products. De Jager et al. 26 investigated color removal from textile wastewater using a pilot-scale, dual-stage MBR-Reverse osmosis (RO) system. The performance analysis revealed that total organic carbon (TOC) removal exceeded 80%, and in excess of 90% color removal efficiencies were recorded, potentially meeting the required discharge and reuse standards for wastewater. Phattaranawik et al. 27 reported on a novel wastewater treatment process using membrane distillation bioreactor (MDBR) technology. This system has the potential to produce high-quality treated wastewater in one-step with capital and operation cost comparable to MBR coupled with RO.
In all MBR studies, membrane fouling is recognized as a major obstacle to the smooth operation and widespread application of this technology 28 , 29 , 30 , 31 . Fouling, in turn, requires membrane cleaning leading to higher operating costs due to additional energy, chemicals and system downtime. A variety of measures has been investigated for fouling control. To enhance performance, additives such as activated carbon have been used 32 . Deng et al. 33 reported membrane fouling reduction using a sponge-submerged MBR (SSMBR); due to its higher zeta potential, particle size and relative hydrophobicity of the sludge flocs was increased. For economic feasibility, natural minerals have been used to obtain significant fouling reduction in terms of increased membrane permeability 34 . Variations in MBR operation such as moving bed MBR (MB-MBR) have been studied but are characterized by higher cake layer deposition and correspondingly higher irreversible fouling than in conventional MBR 35 , 36 . Izadi et al. 37 reported reduced fouling with an integrated fixed bed MBR (FB-MBR). Membrane fouling control has been achieved with other strategies such as microbial fuel cell (MFC) MBR hybrids 38 , enzymatic quorum quenching for biofouling control 39 and electro moving bed MBR (eMB-MBR) technology 40 . In particular, several studies have reported electrically-induced MBR technology for highly efficient wastewater treatment with significant membrane fouling reduction 41 , 42 , 43 , 44 , 45 .
To reduce fouling in MBR operation, the betterment of operational techniques in parallel with the design and development of improved membranes is imperative. Novel membranes for MBRs are especially promising in view of recent advances in nanomaterial-based membranes 46 , 47 , 48 , 49 , 50 that offer leapfrogging opportunities to develop next-generation nanomaterials MBR (NMs-MBR) wastewater treatment technology. Many efforts have been made to embed various types of nanomaterials into membrane structures as doping materials to enhance membrane properties, such as hydrophilicity and antifouling characteristics and this approach has been used successfully in MBR technology 51 , 52 , 53 . A UK-based company located near Beacon Hill, Poole in Dorset, UK, is having contaminants removed via Werle’s MBR-coupled nanotechnology with improves landfill leachate quality and reduces COD, ammonia and solids significantly 54 . It is hoped that nanotechnology-enabled wastewater treatment promises to not only improve performance and offer affordable MBR wastewater treatment solutions but also to provide new treatment capabilities that could allow economic utilization of MBR facilities.
At the time of writing this manuscript, to the best of our knowledge, there are no reports documenting the influence of nanomaterials-based membranes in MBR technology for wastewater treatment. Hence, we present a critical review of the recent developments on the nanomaterials membrane bioreactor (NMs-MBR) technology for wastewater treatment in the current work and all existing papers have been covered. Based on structural characteristics and suitability for wastewater treatment, different types of nanomaterials membrane bioreactors can be classified as follows: (1) nanofibers membrane bioreactor (NFs-MBR) 55 , 56 , (2) nanoparticles membrane bioreactor (NPs-MBR) 57 , 58 , (3) nanotubes membrane bioreactor (NTs-MBR) 59 , 60 , (4) nanocrystals membrane bioreactor (NCs-MBR) 61 , 62 , (5) nanowires membrane bioreactor (NWs-MBR) 63 , and (6) nanosheets membrane bioreactor (NSs-MBR) 64 , 65 (Fig. 1a ). Growing recent publications related to the application of novel nanomaterials-based membrane in MBRs is evidence of increasing interest in this field (Fig. 1b ) and historical development of nanomaterials membrane bioreactor technology has been depicted in Fig. 1c . The sustainability and cost-benefit analysis of NMs-MBR technology are also discussed in this work for broader application. Finally, the challenges and future perspectives of this new technology are provided.
a Examples of commonly used nanomaterials membrane bioreactor (NMs-MBR) technology (left to right) nanofibers membrane bioreactor (NFs-MBR), nanoparticles membrane bioreactor (NPs-MBR), nanotubes membrane bioreactor (NTs-MBR), nanocrystals membrane bioreactor (NCs-MBR), nanowires membrane bioreactor (NWs-MBR), nanosheets membrane bioreactor (NSs-MBR), and the advantages of using NMs-MBR technology are fouling control, high efficiency and sustainability. b Diagram of the total number of publications related to different types of nanomaterials membrane bioreactor (NMs-MBR) technology. Until 3rd August 2020, which were collected from the web of science scientific database, c Historical development of nanomaterials membrane bioreactor (NMs-MBR) technology for wastewater treatment. In 2005, Tae-Hyun Bae investigated the ability of TiO 2 -embedded nanocomposite membrane for membrane bioreactor (NPs-MBR), In 2009, Decostere Bjorge evaluated the electrospun nanofiber membrane for membrane bioreactor (NFs-MBR), In 2014, Chuanqi Zhao prepared nanosheets membrane and tested for membrane bioreactor (NSs-MBR), In 2015, Zahra Rahimi applied nanotubes membrane for membrane bioreactor (NTs-MBR), In 2018, Jinling Lv synthesized nanocrystal membrane and used for membrane bioreactor (NCs-MBR) and In 2019, Xiafei Yin established nanowires membrane and used for membrane bioreactor (NWs-MBR).
Fundamentals of NMs-MBR technology
Despite the advantages of MBRs with respect to a smaller footprint and better treated water quality, the technology is limited by membrane fouling, which results in flux deterioration and downtime for membrane cleaning. The conventional MBR market is dominated by polymeric membranes such as polyvinylidene difluoride (PVDF) and polyethersulfone (PES) 66 , 67 , 68 . Among the various strategies investigated to reduce membrane fouling, improvement in membrane properties is one method. Examples include the implementation of NMs-MBR technology and other NM-based membranes 69 . The design and development of NMs-MBR systems represent a breakthrough technology as NMs-MBR are expected to comprehensively address the fouling issue while maintaining high flux and treated effluent quality. The set-up would be similar to conventional MBRs coupling aerobic or anaerobic biological treatment and membrane filtration, as shown in a typical schematic representation of submerged (a) aerobic NMs-MBR (ANMs-MBR) 70 and (b) anaerobic NMs-MBR (AnNMs-MBR) 71 are shown in Fig. 2 . NMs-based membranes are more effective than conventional membranes with respect to hydrophilicity, surface roughness, thermal stability, hydraulic stability, fouling control, higher water permeability, and higher selectivity due to their small surface pore size 72 , 73 . Because of the improved properties of NMs-based membranes, the system footprint can be further reduced and overall performance enhanced. The fabrication methods used for NM membranes focus mainly on the addition of NMs into the polymer support, but deposition of NMs on the surface of the membrane is also increasingly used 50 . To date, a variety of NM-based membranes, including nanofibres 74 , nanoparticles 75 , nanotubes 76 , nanocrystals 77 , nanowires 78 and nanosheets 79 have been used for water treatment applications.
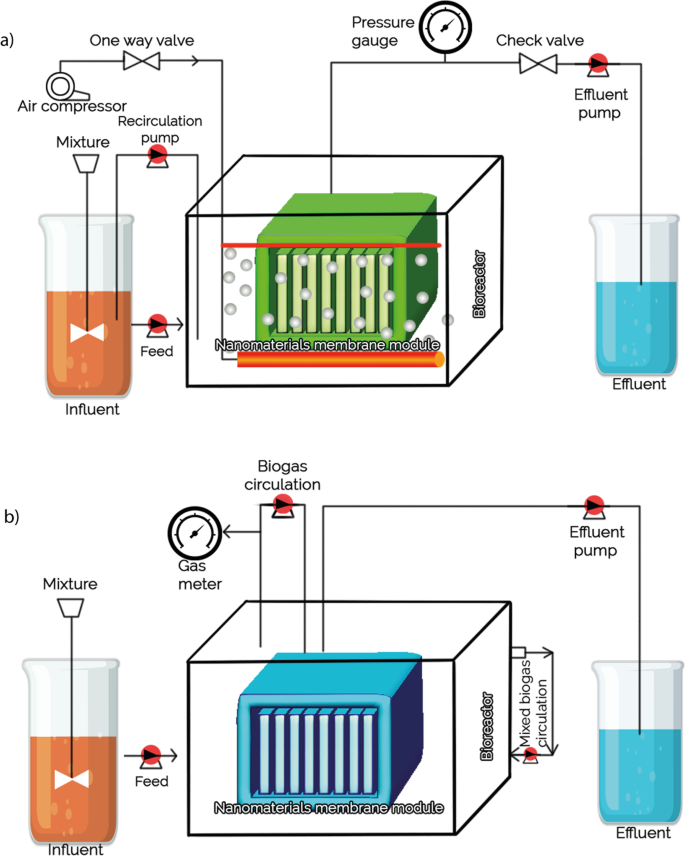
A typical schematic representation of submerged ( a ) aerobic nanomaterials membrane bioreactor (ANMs-MBR). The feed tank was loaded with wastewater influent and the mixture was ensured for continuous flow. Then feed tank was connected into the bioreactor tank through a pump and a membrane module (where different types of nanomaterials membrane can be used) was placed in the bioreactor tank and aeration medium was created by an air compressor in order to obtained simultaneous aeration/filtration system. In addition, a pressure gauge was activated to close the suction pump flow and open the backwash channel when the range of transmembrane pressure out of standard and consequently fouling on the membrane surface appeared. Finally, the effluent tank collected clean water for further application. b Anaerobic nanomaterials membrane bioreactor (AnNMs-MBR). The feed tank loaded wastewater influent was pumped into the bioreactor with a membrane module (where different types of nanomaterials membrane can be used). In this case, the reactor was fully air locked, and the production of biogas amount was detected by a portable gas meter. Subsequently, clean water was storage at the effluent tank for further use.
NMs-MBR technology: performances and progress
The unprecedented success of NMs membrane is recognized as an alternative route to enhance the performance, including mitigation of fouling issues. Here, we summarize briefly the common types of NMs-MBR that have been used in wastewater treatment.
Nanofibers membrane bioreactor (NFs-MBR)
Generally, nanofibers (NFs) membranes contain fibers with diameters that are typically less than 100 nm 80 , 81 . NFs-based membranes offer a suitable platform for a variety of applications due to their extremely high aspect ratio, which helps them to interlock with each other in a subtle form. Of all the methods available for the fabrication of NFs membranes, electrospinning is the most common approach and is followed by several researchers and industries worldwide 82 , 83 . Electrospinning offers several advantages such as ease of operation, material selectivity, and low cost. Additionally, the fiber characteristics in terms of high porosity, surface-to-volume ratio and variable arrangements can be controlled 84 . The physical and chemical properties of electrospun NFs membranes can be easily adjusted for multi-purpose applications. In particular, several studies presented in the literature have shown that NFs membranes are highly effective adsorbents and catalysts 85 , 86 . For example, Sundaran et al. 87 synthesized a novel polyurethane (PU)/graphene oxide (GO)-based electrospun NFs membrane for dye adsorption that could remove 95% methylene blue and 92% rhodamine B, respectively. Hosseini et al. 88 prepared a novel montmorillonite (MMT) clay-chitosan/poly(vinyl alcohol) (PVA)-based electrospun NFs affinity membrane that removed 95% Basic Blue 41 (BB41) and was characterized by high flux and antifouling properties.
Motivated by the above mentioned potential of NFs membranes, researchers have coupled them with MBRs to create NFs-MBRs (Fig. 3 ). The use of electrospun NFs in MBRs was initiated by Bjorge et al. 89 and different applications examined. First, the membrane was functionalized using silver nanoparticles for pathogen removal; the functionalized membrane showed significantly higher pathogen removal (63%) than the non-functionalized membrane (10%). Second, the flat-sheet electrospun NFs membrane was used for wastewater treatment in a lab-scale submerged MBR. The results showed high removal of turbidity (99%), total suspended solids (TSS) (99%), COD (94%) and ammonium (NH 4 + ) (93%). Last, wastewater generated in a music festival was treated in the NFs-MBR and the removal of suspended solids (SS), COD, total nitrogen (TN), and total phosphorus (TP) were within discharge limits.
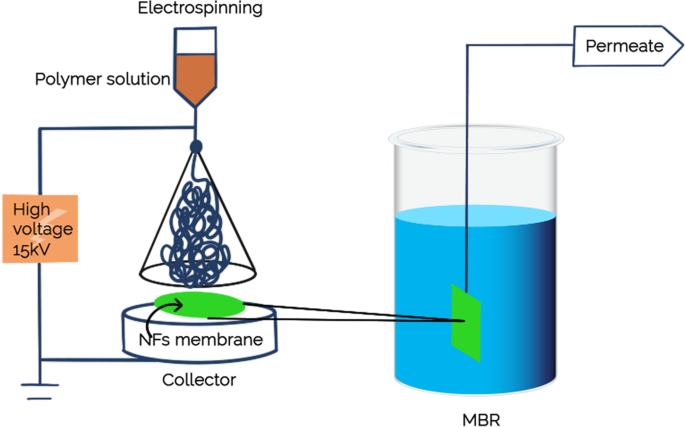
The synthesis of nanofibers membrane was typically carried out by using an electrospinning machine, whereby a selected amount of polymeric solution was injected on a syringe connected by a plastic tube and passed to a needle. Then, a high voltage generator was supplied and acquires a Taylor cone shape, leading to the formation of nanofibers membrane and collected on an alumina disk plate. Furthermore, the as-prepared membrane can be used in an MBR plant for improved permeate effluent quality.
Daels et al. 90 reported a comparative study of electrospun NFs membranes in three MBR set-ups viz., an activated sludge MBR, an activated sludge MBR with a cationic polymer (MPE50) flux enhancer, and a trickling filter (TF)-MBR. In the presence of the trickling filter, the NFs-MBR possessed better performance in terms of reduced irreversible fouling due to simultaneous turbidity removal of 75% by the membrane and trickling filter. Bilad et al. 55 prepared electrospun NFs membrane and compared them with conventional commercial membranes in a lab-scale MBR. Heat treatment of the NFs membranes prevented layered fouling on the membrane surface and the overall performance was comparable to commercial membranes. Kim et al. 91 fabricated and explored the performance of flat-sheet electrospun NFs membrane using PVDF blended with polymethyl methacrylate (PMMA) for wastewater treatment in the laboratory and pilot-scale MBR systems. The prepared membrane surface was much smoother and had higher levels of porosity and permeability than a conventional cast membrane, indicating lower fouling. In pilot tests with secondary effluent from the wastewater treatment plant of the local zoo, COD removal (48%) was low but suspended solids removal was complete.
Nanoparticles (NPs) addition in NFs membranes has also been reported. Zhao et al. 92 fabricated three-dimensional (3D) woven fabric filters decorated with silver nanoparticles doped polyacrylonitrile (PAN) NFs for wastewater treatment in an MBR. Microscopy of the used membrane showed that NFs-embedded fabric filter surface had few clusters of proteins, bacteria cells and polysaccharides. The silver nanoparticles doped NFs membranes showed 40–50% higher flux and substantially greater flux recovery than the undoped membranes. The results were attributed to the excellent antimicrobial property of the silver nanoparticles. Moradi et al. 56 investigated the performance of PAN electrospun NFs membrane deposited with fumarate-alumoxane (FumA) nanoparticles for use in an MBR application. Incorporating low amounts of FumA nanoparticles improved surface hydrophilicity and reduced the irreversible fouling in terms of the highest flux recovery ratio of 96% and the lowest irreversible fouling rate of 4% for 2 wt% Fum-A addition.
NFs-based membranes have also been used in extractive MBRs (EMBR), which is based on combining an aqueous-aqueous extractive membrane process and biodegradation. For example, Wang’s group first applied polydimethylsiloxane (PDMS)-coated PVDF nanofibrous composite membrane for phenol removal in EMBR 93 . Results showed that after two weeks of operation, the mass transfer coefficient for phenol removal was stable at 4.1 ± 0.3 × 10 −7 m.s −1 ; this was four times higher than with commercial silicone rubber. Subsequently, they developed a novel PDMS-coated NFs composite membrane with superhydrophobic or superhydrophilic surfaces with the better performance 94 . Among them, a nanofibrous membrane with superhydrophobic surface exhibited higher stability over 14 days for industrial wastewater treatment and demonstrated 10-times higher phenol extraction efficiency than that of the conventional PDMS membrane. On the other hand, superhydrophilic surface was covered by more polysaccharides and led to lower stability.
In another work, Shao’s group prepared PDMS/PMMA-based electrospun nanofiber membrane and used it in an EMBR system for phenol saline wastewater treatment 95 . High-mass transfer coefficient of phenol (8.8 × 10 −7 m s −1 ) and high salt rejection (>99.96%) indicated high selectivity of salt/phenol. The same membrane was used in treating phenol-laden saline wastewaters in a novel external EMBR system 96 . High simultaneous removal of phenol (14.1–290.7 mg L −1 ) and ammonium (0.5–43.5 mg L −1 ) were achieved with a decrease in toxicity (6.3–70.5%).
The role of electrospun nanofiber membranes in anaerobic membrane bioreactors has been examined recently 97 . Results showed that during short-term filtration test, the as-prepared PVDF nanofiber membrane performed better than the commercial membrane in terms of low transmembrane pressure (TMP) with excellent flux retention. Additionally, suspended solids removal was over 99%, which was comparable to the commercial membrane. The membrane is currently being investigated on a full-scale anaerobic membrane bioreactor system. Table 1 summarizes NFs-MBR technology used in wastewater treatment.
Nanoparticles membrane bioreactor (NPs-MBR)
The use of NPs represents a promising direction for enhancing the performance of NMs-based media in wastewater treatment due to their large surface area and size- and shape-dependent properties. Additionally, they are suitable for functionalization with several chemical groups to augment their catalytic properties 98 , 99 , 100 . However, there are still some limitations to the widespread use of NPs in wastewater treatment applications. These include the separation of exhausted NPs from the treated water for reusability, their tendencies to aggregate in the system and the need for an in-depth understanding of the behavior and fate of NPs in the wastewater treatment systems 101 , 102 . Hence, it is necessary to develop supporting material that could help to maintain their performance. Membrane-based materials are playing a key role in the development of novel NPs-based membranes for effective wastewater treatment.
Recent attempts have shown that NPs-embedded membrane can be used to improve the performance of MBR systems (Fig. 4 ). Bae and Tak 51 published a pioneering work on self-assembled titanium dioxide (TiO 2 ) nanocomposite membrane that reduced fouling significantly in MBR wastewater treatment system. This work inspired many researchers to investigate NPs-MBR systems for wastewater treatment. For instance, Su et al. 103 explored a similar approach using a TiO 2 composite membrane in the MBR system. The presence of TiO 2 NPs coated on the membranes improved the surface hydrophilicity and reduced membrane fouling than the virgin membrane. Liu et al. 104 developed nano-TiO 2 /PVA polyester composite membrane with 10 μm pore size and tested in anoxic/oxic MBR systems. Incorporation of nano-TiO 2 played a significant role in improving membrane performance in terms of lower fouling rate with higher pure water flux, as well as higher removal of contaminants compared to commercial PVDF membrane. Hu et al. 105 applied nano-TiO 2 on the PVDF membrane surface and tested in algal MBRs for wastewater treatment. The modified membrane showed the best removal efficiencies of P (78%) and N (34%); it had enhanced hydrophilicity and only 50% of the total resistance of the pristine membrane. Tavakolmoghadam et al. 106 modified PVDF membrane surface by sputtered nano-TiO 2 and applied in MBRs. The modified membrane had higher hydrophilicity and two-fold improvement in the filtration index compared to the pristine membrane. In addition, minimal leaching of nano-TiO 2 particles occurred after washing, as confirmed by EDX analysis. Compared to pristine polypropylene membranes, polypropylene/TiO 2 nanocomposite membranes used in MBR for treatment of oil refinery wastewater showed better antifouling characteristics 107 , 108 . The results demonstrated that presence of small amount of nano-TiO 2 (0.75 wt%) enhanced the thermal and mechanical properties of the modified membrane besides increasing the critical flux (64 L m -2 h −1 for the modified membrane compared to 34.5 L m -2 h −1 for the pristine membrane).
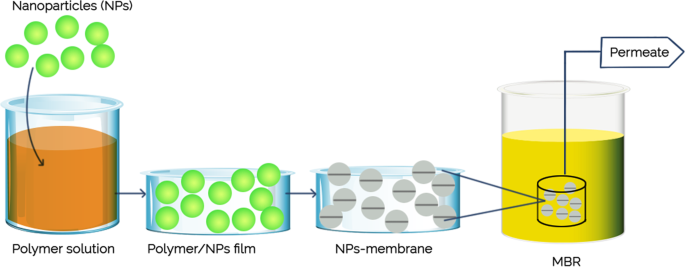
The nanoparticles membrane was prepared according to the wet spinning technique. Typically, a certain amount of nanoparticles was gently added to the polymer solution bath and keep overnight for degas. Then, a mixed bath kept in the water bath another 24 h and dried of the as-prepared membrane to be loaded in the MBR tank for wastewater treatment.
Homayoonfal et al. 109 examined polysulfone (PSf)/alumina nanocomposite membranes with the principal aim of reducing biofouling in the MBR system. Their filtration experiments demonstrated that the addition of alumina NPs increased the membrane hydrophilicity and resulted in 83% reduction in membrane fouling. In addition, 91% of dye rejection (DR) could be achieved, indicating that nanoparticles blended membranes enhanced MBR performance. A magnetic nanocomposite membrane influences MBR treatment performance, as reported by Mehrnia et al. 53 . They prepared a magnetic membrane by blending Fe 3 O 4 NPs of size 60–70 nm into a PSf ultrafiltration membrane. The results demonstrate that nanocomposite membranes have 27% lower filtration resistance (Rf), 30% higher flux and led to 41% higher COD removal than a commercial membrane. Amini et al. 110 introduced silica (SiO 2 ) NPs-based high-density polyethylene (HDPE) membranes for the MBR system. They produced a flat-sheet type of membrane via a thermally induced phase separation (TIPS) approach. An increased amount of SiO 2 NPs (0.5 wt.% and above) could enhance the membrane porosity. NPs addition controlled fouling with over 70% reduction in irreversible fouling ratio. Liang et al. 111 tethered superhydrophilic silica NPs to poly(methacrylic acid) grafted PVDF membrane for fouling control in an MBR system. While the bare membrane showed rapid flux decline to ~40% of the initial flux, the functionalized membrane maintained ~56% of the initial flux and flux recovery upon cleaning was ~100%.
NPs incorporated membranes have also been investigated in the electric field attached MBRs. Li et al. 112 synthesized and tested graphene (Gr)/PANi-phytic acid membrane in such a system. Even with a small amount of graphene (0.02 mg L −1 ), the membrane displayed good conductivity, antifouling and filtration properties. Liu et al. 113 modified polyester filter cloth with Gr/polypyrrole (PPy) or GO/PPy and successfully investigated the antifouling property with yeast suspension. Application of electric field enhanced flux in the Gr/PPy modified membrane by 20% compared to 10% with the membrane modified by PPy alone.
Alsalhy et al. 114 studied the effect of zinc oxide (ZnO) NPs on polyvinyl chloride (PVC) membrane performance for the treatment of hospital wastewater through MBR technology. The benefit of using ZnO NPs was that they acted as an antibiofouling material, thus overcoming the formation of a cake layer on the membrane surface. Addition of 0.1 g of ZnO NPs improved pure water permeability by 315% and a maximum flux recovery efficiency of 87% was obtained with the incorporation of 0.3 g of ZnO NPs. Functionalized NPs have also been incorporated in MBR membranes. Etemadi et al. 115 prepared a dual functionalized nanodiamond (ND) using an amino group as well as polyethylene glycol (PEG) grafted onto a cellulose acetate (CA) nanocomposite membrane to improve its surface hydrophilicity and efficiency within the MBR system. Fouling recovery ratio of 95 % was obtained. Tizchang et al. 116 prepared silanized nanodiamond (SND) NPs intercalated PSf membrane that was tested in an MBR system for wastewater treatment. The neat PSf membrane exhibited a higher contact angle (83.10 o ) and lower flux recovery ratio (FRR) (28.89%), while the functionalized membrane revealed higher FRR (58.93%) and improved hydrophilicity (contact angle 76.44 o ). In sequence, they reported improved FRR in their other study carried out by Kivi et al. 117 wherein nanodiamond nanoparticles were modified using two approaches viz. thermal carboxylation (ND-COOH) and grafting with polyethylene glycol (ND-PEG) and then used in preparing HDPE composite membrane for enhanced MBR system performance. Membranes with 0.75 wt% ND-PEG were the best in terms of high flux and anti-fouling properties exhibiting FRR of 77.9% compared to 61.7% for the bare membrane.
Pirsaheb et al. 118 fabricated a novel nanocomposite PES membrane using hydrophilic polycitrate-Alumoxane (PC-A) NPs and achieved better MBR performance. The addition of these hydrophilic PC-A NPs increased the surface hydrophilicity of the membrane resulting in a high antifouling ability; complete turbidity removal was also obtained. Mahmoudi et al. 119 prepared silver-decorated GO (Ag-GO)/PES membrane for MBR application. For the modified membrane, hydrophilicity was significantly improved (39 ± 2.9˚) compared to the bare membrane (67 ± 3.59˚). Moreover, the pristine membrane operation stability was limited to 20 h during MBR run, while the modified membrane continued to operate for a longer period with effective resistance against fouling. Ahsani et al. 57 fabricated PVDF nanocomposite membrane with immobilized Ag-SiO 2 nanoparticles and tested it in a submerged MBR system treating real pharmaceutical wastewater. The nanocomposite membrane showed improved hydrophilicity and considerable antibiofouling ability with FRR of 76%, while the neat membrane surface exhibited more extracellular polymeric substance with a lower FRR of 58%. Behboudi et al. 58 developed PVC/modified Ag NPs membrane; studies in an MBR system showed higher COD removal of 94% compared to 66% with the pristine membrane. Subsequently, they prepared and tested polyvinyl chloride/polycarbonate/modified silver NPs-based nanocomposite membrane 120 . The presence of nanoparticles enhanced the membrane performance in MBR system in terms of higher removal percentage of COD (98.1%) and increased flux recovery (97.2%). Table 2 summarizes NPs-MBR technology used for wastewater treatment.
Nanotubes membrane bioreactor (NTs-MBR)
Nanotubes (NTs), which are primarily carbon-based materials, have received significant attention for removal of contaminants from wastewater 121 , 122 . Their tunable properties, namely, high aspect ratios, large surface areas, easy functionalization and water transport, make them particularly attractive 123 , 124 . Compared to activated carbon, carbon NTs offer the advantages of excellent self-assembly on supporting materials via chemical vapor deposition and can be immobilized in membrane filters 125 . NTs-based membranes have been employed in MBR systems (NTs-MBR) to enhance the system performance (Fig. 5 ).
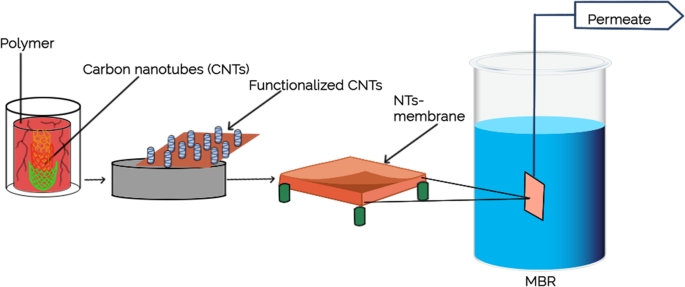
The successful preparation of nanotubes membrane ideally obtained through the use of polymer and carbon nanotubes mixed solution. In addition, carbon nanotubes could be functionalized and formed a casting solution with polymer, which was transferred to a glass plate by a sliding blade and generated a flat surface of the membrane. After that, the as-prepared membrane placed in the MBR module for treating wastewater.
Rahimi and co-workers 52 designed a novel membrane using multi-walled carbon nanotubes (MWCNTs) blended PES for an MBR system. The carbon NTs were initially functionalized with amino groups before embedding in the PES substrate using the phase inversion method. Different concentrations of MWCNTs (0.05, 0.1 and 1 wt.%) were used and the modified MWCNTs exhibited excellent stability (up to 5 h). Among the different membranes, 0.1 wt.% loaded sample showed high porosity (89.3%) and low contact angle (52 o ), leading to the most permeable membrane with pure water flux of 106.75 kg/m 2 h −1 . This membrane showed higher bovine serum albumin (BSA) rejection (~60%) compared to the control (bare) membrane (~25%). The antifouling property was also improved with a FRR of 89.7% against 70% for the control; this was attributed to ionized amine groups on the modified MWCNTs surface bonding with a water layer thereby averting protein adsorption and consequent fouling.
In another study, Ayyaru et al. 59 fabricated a novel PVDF membrane blended with CNTs, with and without sulfonation; these membranes were studied for sludge retention in a wastewater treatment plant via an MBR system. Though incorporation of CNTs improved the membrane properties compared to the pristine PVDF membrane, sulphonated CNTs were more effective compared to CNTs without functionalization. Incorporation of sulphonated CNTs increased membrane porosity (84%), enhanced hydrophilicity (contact angle of 51 o ) and improved protein (BSA) rejection (90%). The FRR (83.52%) was considerably higher than for the pristine PVDF membrane (50% FRR). Finally, the authors demonstrated that unlike the CNTs, the prepared modified membranes were non-toxic to bacteria.
Carbon nanotubes hollow fiber membranes (CNTs-HFMs) have also been developed and used in anaerobic electro-assisted membrane bioreactor 60 . The performance of this novel system was quantified at low temperature (15 − 20 °C) over an operation period of around 100-days. Good TMP recovery with a COD removal of over 95% was reported with the application of the electric field. Their next studies in anaerobic MBR with CNTs membrane in the presence of electric field 126 showed a similar trend. It was noticed that after 120 min filtration, a relatively high membrane flux rate (412.2 L bar −1 m − 2 h −1 ) was achieved for electric-assisted MBR while flux rate was halved (200.6 L bar −1 m – 2 h −1 ) in the absence of electric field. The quality of treated effluent was good (COD < 50 mg L −1 , NH 4+ -N < 2 mg L −1 ). Overall, the results strongly demonstrated that the presence of electric field could significantly alleviate the fouling plus enhance pollutants removal during MBR operation. Mulopo 127 prepared CNTs-PSf nanocomposite membranes for use in an anaerobic MBR designed to treat the bleach effluent from pulp and paper industries. Permeability of the CNTs nanocomposite membrane was higher (0.6–0.7 L m − 2 h −1 ) compared to the neat PSf membrane (0.15–0.25 L m −2 h −1 ); this is due to the O–H bonds that modifies membrane properties such as contact angle and roughness. However, COD and SS removal were similar with both membranes.
In another study by Khalid et al. 128 , CNTs were functionalized with PEG and used as nanofillers in the preparation of PSf nanocomposite membrane for wastewater treatment in MBR. They followed a non-solvent induced phase separation (NIPS) method. The prepared CNTs-PEG showed excellent dispersion stability even after 30 days without any agglomeration while the pure CNTs had poor dispersion stability of 1 day. The addition of varying amounts of CNTs-PEG (0.1–1.0 wt%) improved membrane hydrophilicity and water permeability. Best results were obtained with 0.25 wt% CNTs-PEG addition with increased hydrophilicity evinced by lower contact angle (57°) compared to 65° for pristine PSf membrane. The membrane porosity was higher—54% for CNTs-PEG PSf compared to 44% for pristine PSf. The permeability increased four-fold (from 4.41 L m – 2 h −1 bar −1 for pristine PSf to 16.84 L m – 2 h −1 bar −1 for CNTs-PEG PSf) and the FRR was enhanced as well (57% for pristine PSf against 80% for CNTs-PEG PSf). Table 3 presents the data of NTs-MBR technology used for wastewater treatment.
Nanocrystals membrane bioreactor (NCs-MBR)
Nanocrystals (NCs) are yet another form of NMs that have been considered for the development of nanocomposite membranes for wastewater treatment 129 . Among NCs, cellulose NCs (CNCs) are prime candidates as they are environmentally friendly, possess excellent thermal properties and are biodegradable 130 , 131 . Their superior mechanical properties, such as high tensile strength (~7 GPa) and high Young’s modulus (~130 GPa) allows them to be used widely as fillers 132 , 133 . Moreover, CNC surface is easily functionalized through different chemical moieties, which can improve the removal efficiency of specific pollutants. Addition of NCs to a membrane surface reportedly imbues the membrane with characteristics such as hydrophilicity, charge density and surface roughness, resulting in improved membrane performance 134 , 135 . Figure 6 shows the concept of NCs-based membranes in MBR systems (NCs-MBR).
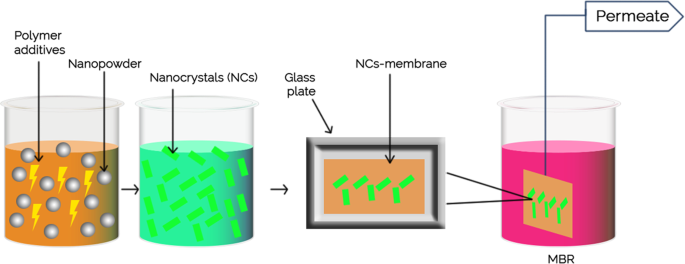
The synthesis of nanocrystals was possibly composed by a mixed solution of polymer and nanopowder and acid hydrolysis step was followed. Then, it was added to the glass plate containing a polymeric support and phase inversion method acquires a nanocrystal membrane. Accordingly, the as-prepared membrane was fixed in the MBR membrane module in order to have a better quality of effluent.
In a recent study, a graphene oxide-cellulose nanocrystal (GO-CNC) composite/PVDF-based membrane was developed and tested for long-term MBR operation 61 . Compared to the pristine PVDF membrane (55% porosity and 0.3 µm pore size), the prepared GO-CNC/PVDF membrane showed higher porosity (80%) and larger mean pore size (1.2 µm); this translates to higher permeability and lower resistance to filtration. The pure water flux of GO-CNC/PVDF membrane was 3.2 times higher than the pristine PVDF membrane. The contact angle of the pristine PVDF membrane was 65.3°, whereas GO-CNC/PVDF membrane showed a much lower contact angle of 39.3° due to the strong H-bond interactions between the CNCs’ –OH groups and the GO sheets’ oxygen groups, thereby imparting better antifouling property to the membrane 136 . Surface charge (zeta potential) is another important membrane characteristic 137 . The zeta potential of GO-CNC/PVDF membrane (−17 mV) is twice that of the pristine PVDF membrane (−8 mV), suggesting that the foulants deposition on the membrane surface will be reduced because of the presence of more negatively charged GO-CNC composites. This was confirmed in MBR testing. For GO-CNC/PVDF membrane, one-time chemical cleaning was needed in 73 days in contrast to three chemical cleaning cycles required for the pristine PVDF membrane over this period. The cake layer thickness of 104.9 μm in the pristine PVDF membrane reduced to 41.38 μm for the GO-CNC/PVDF membrane indicating less foulants were deposited on the functionalized membrane surface significantly decreasing the fouling rate. Membrane fouling rate determined by measuring the relative flux after the cleaning process indicated that substantially irreversible fouling occurred on the unmodified membrane surface 138 while the flux could be successfully recovered with the GO-CNC/PVDF membrane proving that the modified membrane had better antifouling property.
In another study, Li et al. 62 developed Pd– reduced graphene oxide (rGO)/PVDF-carbon fiber cloth membrane and tested it for wastewater treatment in an MBR/MFC-coupled system. Here, Pd nanocrystal-rGO composite was synthesized and deposited on the surface of PVDF/carbon fiber through the electrodeposition process. The anti-fouling flux of the membranes was measured under two conditions viz. with and without application of electric field; the developed membrane functioned as a cathode. The presence of electric field enhanced the flux rate nearly two-fold 128–130 L m −2 h −1 compared to 66 L m −2 h −1 in the absence of electric field), indicating that membrane fouling could be controlled through the application of electric field during MBR operation. Removal of contaminants after 30 days of operation was stable with a high removal efficiency of COD (90%) and NH 4 + -N (81%) being achieved. The details are presented in Table 4 .
Nanowires membrane bioreactor (NWs-MBR)
Within the family of NMs membranes, nanowire (NW) membranes have also emerged as materials with excellent mechanical, chemical, and thermal stabilities for use in wastewater treatment 139 , 140 . Nanowires offer higher aspect ratio (length to diameter/width ratio) of over 1000 compared to other one-dimensional nanomaterials such as nanofibers with an aspect ratio of 3–5; this makes nanowires useful in environmental applications 141 . The presence of NWs on a membrane surface enhanced pollutant removal and compared to conventional membranes, NW membranes exhibit flexible, uniform and multifunctional activity and could be used to remove other foulants such as microorganisms and trace organics 142 , 143 .
NWs-based membranes can be used in MBRs (NWs-MBR) to control membrane fouling and to achieve high treatment efficiency (Fig. 7 ). The only scientific article on NWs-MBR was published recently by Yin et al. 63 where they prepared an innovative Cu-NW conductive microfiltration membrane and used it successfully in MBR application. Long-term operation was conducted at the presence of spontaneous electric field (SEF-MBR) to enhance the reduction in membrane fouling. In the initial 40 days, the membrane flux decreased and then became stable for both the Cu-NW membrane and commercial PVDF membrane. The SEF-MBR flux was 2.1 times higher than the control MBR with the commercial PVDF membrane; the fouling layer of around 80 μm on the Cu-NW membrane surface was thinner than on the PVDF membrane (179 μm). Monitoring the extracellular polymeric substances (EPS) on the membrane surface as an indicator of the extent of fouling showed that the total EPS amount was 62.0 mg/g VSS for the commercial PVDF membrane, while Cu-NW membrane surface had a lower total EPS content of 42.4 mg/g VSS. COD, total nitrogen and total phosphorous removal (94.5%, 78.5%, and 86.6%, respectively) were marginally better in the SEF-MBR when compared to the conventional MBR (92.7% COD, 70.5% total nitrogen and 80.4% total phosphorous removal). Overall, these results suggested that novel NMs-based membranes combined with an electric field could considerably enhance MBR performance. The details are presented in Table 4 .
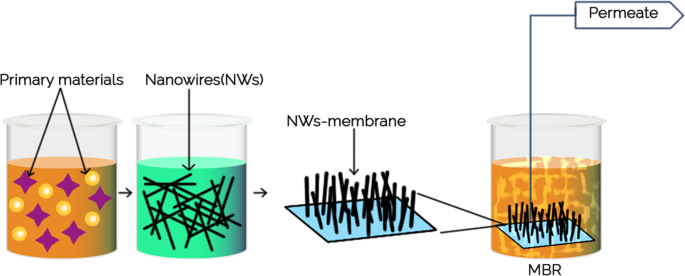
Nanowires membrane was also prepared by following the phase inversion method. A variety of polymeric materials and nanowires were gradually mixed to obtain a homogeneous solution and cast on non-woven support, which is termed as nanowires membrane. Finally, the as-prepared nanowires membrane was used in the MBR configurations for purifying water.
Nanosheets membrane bioreactor (NSs-MBR)
Nanosheets, two-dimensional (2D) NMs with atomic or molecular ratios, have received significant consideration as next-generation membrane materials for wastewater treatment 144 . Various kinds of nanosheet membranes, such as boron nitride (BN) nanosheet membranes 145 , GO nanosheet membranes 146 , titania nanosheet membranes 147 , molybdenum disulfide (MoS 2 ) nanosheet membranes 148 and graphitic carbon nitride (g-C 3 N 4 ) nanosheet membranes 149 , have been used in water purification. Among these, the GO-based nanosheet membranes have demonstrated high efficiency due to their high charge density, different oxidation states and high mechanical strength.
Figure 8 displays the schematic for NSs-MBR system for wastewater treatment. In a recent study, Fathizadeh et al. 64 prepared novel PES hollow fiber (HF) membranes coated with single-layer GO nanosheet (SLGO) and UV-treated SLGO and successfully tested these in MBR applications.The pure PES membrane surface roughness was 44 nm and after loading of SLGO, it decreased to 33 nm due to the UV-irradiation process. Also, the modified membrane exhibited excellent permeability (65 L m −2 h −1 bar −1 ) and the low fouling (<15% permeance reduction) compared to the pure PES membrane. A small amount of SLGO coating (6.2 mg m −2 ) was adequate to obtain 99% TOC removal in long-term MBR operation.The reason could be explained that the GO flakes were grafted by extra functional groups such as carboxyl or hydroxyl groups during UV-irradiation, which promoted the performances.
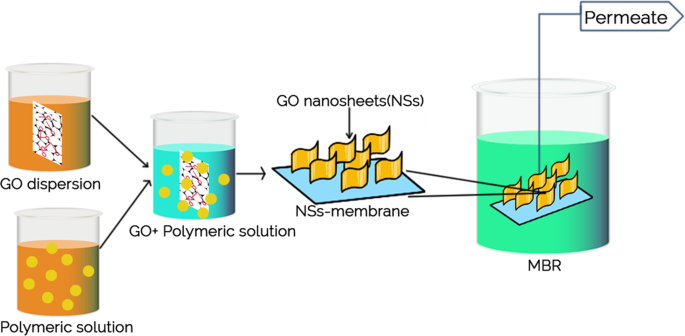
At first, GO nanosheet was prepared by adding a known amount of graphite powder and mixed with a polymeric solution. After sonication, a homogeneous solution was observed and placed on a non-woven cloth by a casting knife. Then, the formed membrane was placed in the water bath for 24 h for using in the MBR module. Moreover, clean water was collected for further use.
In 2014, Zhao et al. 65 developed a novel composite microfiltration membrane by blending PVDF and GO nanosheets for MBR system. The GO-modified membrane showed higher hydrophilicity (contact angle of 60.50 ± 1.80 o ) than pristine PVDF membrane (contact angle of 78.30 ± 2.40 o ) because of the hydrophilic nature of GO, which actively increased the surface charge ratio of oxygen-containing groups on the membrane surface. Larger pore size (0.089 μm) and higher water permeability (552 L m − 2 h −1 bar −1 ) was obtained compared to the pristine PVDF membrane (0.041 μm pore size and 171 L m −2 h −1 bar −1 water permeability). The critical flux (the flux above which deposition of particles or colloids occurs rapidly on the membrane surface forming cake or gel layer) was higher for the modified membrane (48–50 L m − 2 h −1 ) compared to the pristine PVDF (30–33 L· L m − 2 h −1 ). Concentration of EPS (17.87 g/m 2 ) deposited on the pristine PVDF membrane surface was over three-time higher than with the modified membrane (5.97 g/m 2 ). As a consequence of this lower fouling, cleaning frequency was reduced with the modified membrane exhibiting three-times longer filtration time than the PVDF membrane.
Ghalamchi et al. 150 prepared a novel PES microfiltration membrane containing g-C 3 N 4 nanosheets/Ag 3 PO 4 NPs through a phase inversion process for use in an MBR system. The addition of nanosheet improved the hydrophilicity of the prepared membrane compared to the bare PES membrane. Water flux was enhanced from 262 L m − 2 h −1 to 360 L m − 2 h −1 with a loading of 0.5 wt% nanosheet on the membrane surface. Moreover, the antifouling ability (determined with BSA filtration) was improved with an FRR of 57.5% for the nanosheet membrane; this was higher than the FRR of the bare PES membrane (39.1%).
Zinadini et al. 151 synthesized GO nanosheet intercalated PES membranes with varying amounts of PES and GO for the treatment of milk process wastewater in an MBR system. Addition of GO decreased the contact angle and increased the water flux. A combination of 15 wt% PES loaded with 0.5 wt% GO was the most optimal in terms of high water flux and anti-fouling ability (FRR of 92.8%). The MBR performance improved at high mixed liquor suspended solids (MLSS) concentration of 14,000 mg/L with high flux (~30 kg m −2 h −1 ) and high removal of COD (95.4%), TN (66.8%) and TP (76.1%).
Beygmohammdi et al. 152 studied the influence of different amounts (0–2 wt%) of GO and polyvinylpyrrolidone (PVP) grafted GO (PVP-GO) on the properties of pristine PVDF membrane in MBR application. Herein, PVP was successfully immobilized onto GO nanosheet through in-situ polymerization technique. The PVP-GO NPs so obtained had smaller particle size than the pristine GO nanosheets. Incorporation of 1.5 wt% GO or PVP-GO was optimal to obtain high hydrophilicity and pure water permeation. It is well known that the addition of GO improves membrane hydrophilicity 153 . Similar trend is observed in this study as well with the contact angle decreasing to 62.7° (PVP-GO/PVDF) and 74.8° (GO/PVDF)_from 105° for the neat PVDF membrane. The pure water flux, critical flux and FRR respectively are also higher (311.9 L m −2 h −1 , 72.3 L m − 2 h −1 and 80.81% for PVP-GO/PVDF; 205.2 L m −2 h −1 , 41.5 L m −2 h −1 , and 74.62% for GO/PVDF) compared to the neat PVDF membrane (85.2 L m − 2 h −1 , 19 L m − 2 h −1 , and 60.55%). In MBR operation, after 360 min filtration, 70% of the initial flux was maintained with PVP-GO/PVDF membrane compared to 49% with the neat PVDF membrane.
In another work, Wu et al. 154 prepared PVP-GO/PVDF membrane through the chemical grafting approach and applied the membranes in algae-membrane photo-bioreactor (MPBR) systems for treating organic-rich wastewater. The PVP-GO/PVDF membrane demonstrated higher hydrophilicity (contact angle 62°) compared to the pristine PVDF membrane (contact angle 97°) and a remarkable improvement in pure water flux from 380 L m − 2 h −1 (pristine membrane) to 614 L m − 2 h −1 (PVP-GO/PVDF membrane). Moreover, contaminants removal efficiency in PVP-GO/PVDF–MBR (COD 97.8%, NH 4 -N + 96.8%, NO 3 -N 76.9%) was higher than that in conventional PVDF-MBR (COD 93%, NH 4 -N + 93.1%, NO 3 -N 70.6%). The details are presented in Table 4 .
Membrane fouling
It is well recognized that membrane fouling in MBRs continues to be a major challenge 136 . Fouling in MBRs resulting from an interaction between the sludge components and the membrane material, can be either reversible or irreversible. While reversible fouling can be removed by applying physical treatments like air sparging and backflushing, chemical cleaning is needed to eradicate irreversible fouling. Reversible fouling always has a higher fouling rate than irreversible fouling 155 . From the foulant materials perspective, fouling can be classified under biological, organic and inorganic fouling. Biofouling caused by growth and deposition of biomass on the membrane surface is one of the most critical fouling problems in the long-term operation of MBRs. Organic fouling occurs due to deposition of organic matter, polysaccharides, lipids, proteins etc. while inorganic fouling refers to the accumulation of inorganic matter such as salts on the membrane surface 156 .
Specifically, several mechanisms are considered for membrane fouling phenomena in the MBRs operation 29 , such as solutes or colloids adsorption occurred within/on the membrane surface, cake layer formation and sludge flocs deposition on the membrane surface, foulants detachment due to shear forces and the temporal and spatial changes of foulants, i.e., the differences of biopolymer components and bacterial community in the cake layer appeared under the long-term MBRs operation. Typically, long-term MBRs operation carried out at constant flux with a variable TMP ranges. During the MBRs operation, an initial rise in TMP with a long-term weak increase TMP continued and third stage process formulated based on sudden TMP jump. It is known that sudden TMP increased observation called a severe membrane fouling condition in MBRs operation that causes higher critical flux than local flux; this necessitates membrane cleaning thereby disrupting MBR operation 157 , 158 . As the flux determines the membrane area required for the application, it has a strong influence on capital cost. Frequent membrane cleaning that might also reduce the membrane operational stability and lifespan thereby needing membrane replacement, adds up to the operational cost. Thus, approaches to control and minimize membrane fouling in MBRs continue to be researched extensively. Furthermore, contributing to membrane fouling are several factors such as membrane properties, operational conditions, feed components etc. so these have to be addressed comprehensively for fouling prevention in MBRs.
Accordingly, the preceding sections of this review paper have outlined how a variety of nanomaterials impregnated membranes that consistently exhibit enhanced hydrophilicity, can be utilized in controlling the fouling rate in NMs-MBRs operation. Figure 9 depicts how the integration of NMs membranes into the MBR process has been shown to be effective in improving antifouling behavior 61 . The strong interaction between the hydroxyl groups of CNC and hydroxyl, epoxide, carbonyl and carboxyl of GO facilitates the solution homogeneity and successful deposition on PVDF surface. At the initial stage of filtration, the commercial PVDF membrane can easily retain the suspended solids via the sieving mechanism due to the small pore size/porosity, resulting in irreversible fouling. With the more hydrophilic CNC/PVDF and GO-CNC/PVDF membranes, EPS, sludge flocs or microbes have less opportunity to attach with the membrane surface. Because of their hydrophilicity, which assists to adsorb water molecules on a large scale, the rate of adsorbents deposition is slower and results in a relatively thin cake layer on the membrane surface, Consequently, less membrane fouling occurs by using these membranes in long-term MBR operation 61 .
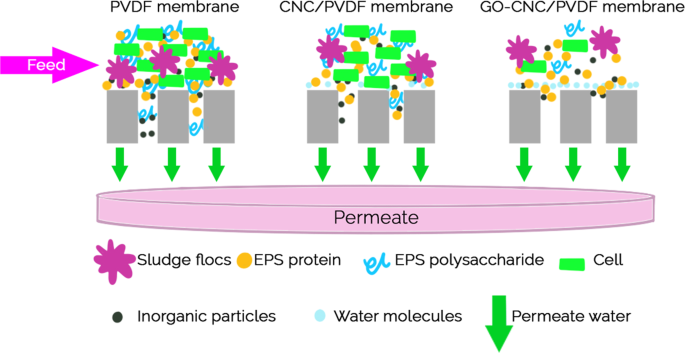
There are three types of membranes were chosen for understanding the fouling mechanism. Pristine PVDF membrane, the attachment of EPS, sludge flocs and suspended particles are higher than CNC/PVDF membrane, while a very few opportunities were observed for GO-CNC/PVDF membrane. More water molecules pass through the GO-CNC/PVDF membrane and less fouling occurred sequentially.
Sustainability and cost viability
The concept of sustainability in wastewater treatment has been of concern in the scientific community in recent years. In the literature, there are many studies available on the development of membranes incorporating NMs using green chemistry principles, but the application of such membranes in MBRs for wastewater treatment are not yet reported on a lab-scale/commercial scale. For example, Lv et al. 159 fabricated electrospun nanofibrous PVA and konjacglucomannan (KGM)-based membranes loaded with ZnO nanoparticles with ecofriendly thermal cross-linking. These membranes were tested for environmental applications such as air filtration, photocatalytic degradation of dyes and bacteria. In another study, Ren et al. 160 reported poly(lactic acid) (PLA) stereocomplex crystallite (SC)-based electrospun nanofibers loaded with an adsorbent obtained by the polymerization of tannic acid and hexamethylenediamine for \({\mathrm{Cr}}\left( {{\mathrm{VI}}} \right)\) removal. Environment-friendly plant-based components have also been examined. Copper oxide nanoparticles, produced by plant mediated green synthesis, have been incorporated in PES-CA nanocomposite membranes and plant extracts like curcumin in the nanomaterial form have been incorporated in PES membrane matrix 136 , 137 . The importance of nanomaterial choice has been emphasized with low-cost and non-toxic materials such as chitosan and iron-based nanomaterials being recommended based on sustainability aspects 161 .
From the literature, it is evident that while membranes incorporating NPs and to a lesser extent, NFs, have been investigated extensively for MBR applications, there are limited studies on membranes based on other NMs (NCs, NTs, NSs). This is possibly because NFs can be readily manufactured since electrospinning is a well-established process; also nanoparticles and CNTs are widely available commercially. Nanoparticles anchored on polymeric membranes and electrospun nanofiber membrane surface has demonstrated long-term permeability and better antifouling ability. Moreover, though NMs such as nanosponges, nanorods, and quantum dots-based membranes have been applied for wastewater treatment 162 , 163 , they have not yet been used in MBR applications. Among these, quantum dots-based membrane could be one of the most effective membranes for MBR applications due to better hydrophilicity, permeability and fouling resistance properties 164 .
The cost of NMs-based membranes needs to be established. Bjorge et al. 89 estimated NFs membranes cost to be 20€/m 2 that is cheaper than commercial membranes (50€/m 2 ). Fatarella et al. 165 estimated the production cost of NFs membranes to be 5€/m 2 , with the major cost component (75%) coming from the non-woven support. Indeed, the use of cheaper supports can reduce the total cost. This cost is much lower than the cost of traditional cast polymeric membranes, which run to 14–50€/m 2 (refs. 9 , 90 ). However, the cost of raw materials for the development of NMs membranes can be quite varied. For example, reports suggest that clay-based NMs membranes enhance the pollutants removal capacity from wastewater in a sustainable way 166 , 167 but there is a huge inequality in price between clays and some polymers (Suplementary Table S1 ). However, more detailed cost-benefit analyses are required for the future development of NMs-MBR systems.
Emerging applications of NMs-MBR technology
This review focuses on wastewater treatment applications of novel NMs-MBR technology. However, based on the previous success of conventional MBRs, NMs-MBR technology can also be employed in other emerging areas. The treatment of landfill leachates is one of the critical issues in environmental pollution control. A high concentration of organic and inorganic compounds, including micropollutants can be found in landfill leachates thereby threatening the quality of surface and groundwater sources. There have been a number of successful studies on the treatment of landfill leachates using MBR technology; 168 , 169 thus, NMs-MBRs can be readily employed in this application 54 . Dabaghian et al. 170 reported a comprehensive review of the potentiality of nanostructured membranes for landfill leachate treatment, and suggested that NMs membrane could lead to greater benefits than commercial membranes. Yet another prospect is in the area of anaerobic digestion (biomethanation) of organic wastes (including excreta) that includes the steps of hydrolysis, acidogenesis, acetogenesis and methanogenesis. Addition of nanomaterials helps to accelerate the digestion process, resulting in a high amount of volatile fatty acids (VFAs) and biogas being produced 171 , 172 , 173 . Instead of biogas production, alternatives such as hydrogen generation, recovery of VFAs for chemicals production etc. are being increasingly explored 174 , 175 . MBRs are being employed in this application 176 , 177 and thus NMs-MBRs also have potential. With the increasing shift from waste treatment to resource recovery, nutrients (nitrogen and phosphorus) recovery is another key area; in this context, hybrid systems including MBR-based hybrids have been recommended 178 . With an emphasis on reducing energy use and recovering resources, anaerobic MBRs are being investigated extensively 179 . NMs-MBRs especially have a role in this sector considering the more serious membrane fouling that is encountered in anaerobic MBR systems 158 .
Challenges and future perspective
In spite of the progress in the application of nanomaterials-based MBR systems for wastewater treatment, there are challenges to be addressed for accelerating the practical application of NMs-MBR technology. It is necessary to justify the possible environmental threats that may be associated with this technology. Leaching of nanomaterials into aquatic environments can occur during long-term operation of such NMs-MBR, during the membrane synthesis process itself as well as due to inappropriate disposal of used membranes. The nanomaterials so released can undergo an environmental transformation, be taken-up by various aquatic organisms and could potentially pose a risk to both human health and environmental systems 180 , 181 . Assessment of nanomaterials membrane stability and understanding the dynamics of the release of nanomaterials from the membrane matrix is, therefore, a crucial part of the long-term NMs-MBR operation; besides the environmental implications, leaching of nanomaterials from the membrane surface could adversely impact membrane performance and its lifespan. For instance, the antimicrobial properties of the membrane could be improved by the addition of silver nanoparticles as biocidal agents but if continuously leached into water bodies, the desirable properties would not be achieved 48 , 182 , 183 .
Yet another challenge is the large-scale manufacture of the NMs membranes. Various techniques such as roll-to-roll, phase inversion, interfacial polymerization, stretching, track-etching and electrospinning process are in use. The roll-to-roll technique is still preferable for manufacturing of membranes, but it is a time-consuming process with low selectivity and difficulty in membrane pore size tuning 184 . Phase inversion and interfacial polymerization are widely used membrane fabrication techniques to obtain low-surface roughness, which is of primary interest to develop low-fouling membranes. However, phase inversion and interfacial polymerization induced membranes have low water permeability that would have to be enhanced through functionalization approaches 185 . The stretching method is useful to fabricate hydrophobic membranes but organic and biofouling propensity restricts its application. The track-etched method-based membrane offers some unique features such as low-surface roughness, but the low flux due to small porosity and high cost makes them less attractive 186 . Electrospinning technique is increasingly becoming popular as it offers a simple preparation method and different sizes of membranes can be produced; the challenge lies in jet instability, use of toxic solvents and adjustment of various operation parameters that are required to obtain membranes of adequate quality 89 , 187 .
According to our knowledge, many companies have started large-scale production of membranes/filters incorporating nanomaterials targeted at various applications (Table 5 ). Though this is not exactly what we proposed (use of such membranes in MBRs), considering the widespread applications of nanomaterials-based membranes/filters in water and wastewater segment, it can be expected that the MBR segment will subsequently be covered comprehensively by such products.
In short, it is imperative to find out the best possible solutions for the NMs-MBRs technology by mitigating the challenges as described previously. Efforts to prevent leaching of nanomaterials would involve more robust methods of fixing the nanomaterials on the membrane matrix such as chemical grafting. Advanced fabrication options that are both scalable and cost-effective need to be investigated; in this context additive (3D) manufacturing holds promise 188 . Additionally, operation parameters, feed characteristics and reactor configurations need to be suitably adjusted with effective protocols for NMs-MBR systems. In particular, maintenance practices including cleaning procedures have to be evolved and implemented regularly to control the operating cost and output. Consequently, the assessment of techno-economic analysis is one of the essential parameters that must be attempted in NMs-MBRs technology. This process could be interpreted by three indicators viz. economic viability, technical feasibility and environmental sustainability since it is very important for bioenergy and biobased products 189 . There is no techno-economic analysis data available specifically on NMs-MBRs technology, but it can follow models for conventional MBRs, which have been published in recent years 190 , 191 , 192 . It is of importance to control fouling by adopting different strategies. Lab-scale to real-scale transition must be started by maintaining the potential benefits such as sustainability and energy consumption. These aspects would, therefore, be key in promoting NMs-MBR technology for wastewater treatment.
This paper has critically reviewed the progress in the development of NMs-MBR applications for wastewater treatment. The possibility of synthesizing nanomaterials incorporated novel membranes with specific properties opens up opportunities for their use in MBR systems for wastewater treatment. While the use of NMs-MBR technology demonstrates good performance in terms of lower fouling and enhanced removal efficiency of pollutants, there are several aspects that are poorly understood presently. These include the cost of large-scale manufacture of such membranes, their lifespan in full-scale applications, the possibility of leaching of the nanomaterials in the wastewater/sludge etc. These issues should be further investigated before integration of nanomaterials membranes with MBRs, which will assist in designing next-generation MBRs technologies.

Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request (Prof. Vincenzo Naddeo, V.N.).
Code availability
No code was attempted or used during the current manuscript.
Judd, S. J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 305 , 37–45 (2016).
Article CAS Google Scholar
Shin, C. & Bae, J. Current status of the pilot-scale anaerobic membrane bioreactor treatments of domestic wastewaters: a critical review. Bioresour. Technol. 247 , 1038–1046 (2018).
Hamedi, H., Ehteshami, M., Mirbagheri, S. A., Rasouli, S. A. & Zendehboudi, S. Current status and future prospects of membrane bioreactors (MBRs) and fouling phenomena: a systematic review. Can. J. Chem. Eng. 97 , 32–58 (2019).
Li, Y.-Z., He, Y.-L., Liu, Y.-H., Yang, S.-C. & Zhang, G.-J. Comparison of the filtration characteristics between biological powdered activated carbon sludge and activated sludge in submerged membrane bioreactors. Desalination 174 , 305–314 (2005).
Gurung, K., Ncibi, M. C., Shestakova, M. & Sillanpää, M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl. Catal. B-Environ. 221 , 329–338 (2018).
Nguyen, L. N., Hai, F. I., Kang, J., Price, W. E. & Nghiem, L. D. Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int. Biodeter. Biodegr. 85 , 474–482 (2013).
Sathya, U., Nithya, M. & Balasubramanian, N. Evaluation of advanced oxidation processes (AOPs) integrated membrane bioreactor (MBR) for the real textile wastewater treatment. J. Environ. Manag. 246 , 768–775 (2019).
Arévalo, J. et al. Wastewater reuse after treatment by tertiary ultrafiltration and a membrane bioreactor (MBR): a comparative study. Desalination 243 , 32–41 (2009).
Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment (Elsevier, Oxford, 2011).
Neoh, C. H., Noor, Z. Z., Mutamim, N. S. A. & Lim, C. K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 283 , 582–594 (2016).
Hai, F. I., Yamamoto, K. & Lee, C.-H. Membrane Biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse . Ch. 1 (IWA Publishing, London, 2019).
Falizi, N. J. et al. Evaluation of MBR treated industrial wastewater quality before and after desalination by NF and RO processes for agricultural reuse. J. Water Process. Eng. 22 , 103–108 (2018).
Article Google Scholar
Lin, H. et al. Membrane bioreactors for industrial wastewater treatment: a critical review. Crit. Rev. Env. Sci. Tec. 42 , 677–740 (2012).
Mutamim, N. S. A., Noor, Z. Z., Hassan, M. A. A. & Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: a performance review. Desalination 305 , 1–11 (2012).
Wang, Z. Encyclopedia of Membranes (Springer, Berlin, 2015).
Jegatheesan, V. et al. Treatment of textile wastewater with membrane bioreactor: a critical review. Bioresour. Technol. 204 , 202–212 (2016).
Sun, F., Sun, B., Hu, J., He, Y. & Wu, W. Organics and nitrogen removal from textile auxiliaries wastewater with A2O-MBR in a pilot-scale. J. Hazard. Mater. 286 , 416–424 (2015).
Tan, X., Acquah, I., Liu, H., Li, W. & Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 220 , 1150–1162 (2019).
Hai, F. I., Yamamoto, K., Nakajima, F. & Fukushi, K. Bioaugmented membrane bioreactor (MBR) with a GAC-packed zone for high rate textile wastewater treatment. Water Res. 45 , 2199–2206 (2011).
Trinh, T. et al. Removal of trace organic chemical contaminants by a membrane bioreactor. Water Sci. Technol. 66 , 1856–1863 (2012).
Katsou, E., Malamis, S. & Loizidou, M. Performance of a membrane bioreactor used for the treatment of wastewater contaminated with heavy metals. Bioresour. Technol. 102 , 4325–4332 (2011).
Mannina, G., Cosenza, A., Di Trapani, D., Capodici, M. & Viviani, G. Membrane bioreactors for treatment of saline wastewater contaminated by hydrocarbons (diesel fuel): an experimental pilot plant case study. Chem. Eng. J. 291 , 269–278 (2016).
Goswami, L. et al. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: a review. J. Water Process. Eng. 26 , 314–328 (2018).
Sert, G. et al. Investigation of mini pilot scale mbr-nf and mbr-ro integrated systems performance—preliminary field tests. J. Water Process. Eng. 12 , 72–77 (2016).
Laera, G. et al. Removal of organics and degradation products from industrial wastewater by a membrane bioreactor integrated with ozone or UV/H2O2 treatment. Environ. Sci. Technol. 46 , 1010–1018 (2011).
De Jager, D., Sheldon, M. & Edwards, W. Colour removal from textile wastewater using a pilot-scale dual-stage MBR and subsequent RO system. Sep. Purif. Technol. 135 , 135–144 (2014).
Phattaranawik, J., Fane, A. G., Pasquier, A. C. & Bing, W. A novel membrane bioreactor based on membrane distillation. Desalination 223 , 386–395 (2008).
Le-Clech, P., Chen, V. & Fane, T. A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 284 , 17–53 (2006).
Meng, F. et al. Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res. 43 , 1489–1512 (2009).
Iorhemen, O. T., Hamza, R. A. & Tay, J. H. Membrane fouling control in membrane bioreactors (MBRs) using granular materials. Bioresour. Technol. 240 , 9–24 (2017).
Iorhemen, O., Hamza, R. & Tay, J. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: membrane fouling. Membranes 6 , 33 (2016).
Alighardashi, A., Pakan, M., Jamshidi, S. & Shariati, F. P. Performance evaluation of membrane bioreactor (MBR) coupled with activated carbon on tannery wastewater treatment. Membr. Water Treat. 8 , 517–528 (2017).
Google Scholar
Deng, L. et al. A comparison study on membrane fouling in a sponge-submerged membrane bioreactor and a conventional membrane bioreactor. Bioresour. Technol. 165 , 69–74 (2014).
Malamis, S., Andreadakis, A., Mamais, D. & Noutsopoulos, C. Comparison of alternative additives used for the mitigation of membrane fouling in membrane bioreactors. Desalin. Water Treat. 52 , 5740–5747 (2014).
Di Trapani, D., Di Bella, G., Mannina, G., Torregrossa, M. & Viviani, G. Comparison between moving bed-membrane bioreactor (MB-MBR) and membrane bioreactor (MBR) systems: influence of wastewater salinity variation. Bioresour. Technol. 162 , 60–69 (2014).
Yang, S., Yang, F., Fu, Z. & Lei, R. Comparison between a moving bed membrane bioreactor and a conventional membrane bioreactor on organic carbon and nitrogen removal. Bioresour. Technol. 100 , 2369–2374 (2009).
Izadi, A., Hosseini, M., Darzi, G. N., Bidhendi, G. N. & Shariati, F. P. Performance of an integrated fixed bed membrane bioreactor (FBMBR) applied to pollutant removal from paper-recycling wastewater. Water Resour. Ind. 21 , 100111 (2019).
Wang, J. et al. Evaluation of energy-distribution of a hybrid microbial fuel cell–membrane bioreactor (MFC–MBR) for cost-effective wastewater treatment. Bioresour. Technol. 200 , 420–425 (2016).
Oh, H.-S. et al. Control of membrane biofouling in MBR for wastewater treatment by quorum quenching bacteria encapsulated in microporous membrane. Environ. Sci. Technol. 46 , 4877–4884 (2012).
Millanar-Marfa, J. et al. Fouling mitigation and wastewater treatment enhancement through the application of an electro moving bed membrane bioreactor (eMB-MBR). Membranes 8 , 116 (2018).
Giwa, A. & Hasan, S. Theoretical investigation of the influence of operating conditions on the treatment performance of an electrically-induced membrane bioreactor. J. Water Process. Eng. 6 , 72–82 (2015).
Giwa, A. & Hasan, S. W. Numerical modeling of an electrically enhanced membrane bioreactor (MBER) treating medium-strength wastewater. J. Environ. Manag. 164 , 1–9 (2015).
Ahmed, M., Elektorowicz, M. & Hasan, S. W. GO, SiO2, and SnO2 nanomaterials as highly efficient adsorbents for Zn2+ from industrial wastewater—a second stage treatment to electrically enhanced membrane bioreactor. J. Water Process. Eng. 31 , 100815 (2019).
Garcia, D. T. et al. Photocatalytic ozonation under visible light for the remediation of water effluents and its integration with an electro-membrane bioreactor. Chemosphere 209 , 534–541 (2018).
Ahmed, M. A. & Hasan, S. W. Fe and Zn removal from steel making industrial wastewater by electrically enhanced membrane bioreactor. Desalin. Water Treat. 93 , 9–21 (2017).
Daer, S., Kharraz, J., Giwa, A. & Hasan, S. W. Recent applications of nanomaterials in water desalination: a critical review and future opportunities. Desalination 367 , 37–48 (2015).
Ibrahim, Y., Abdulkarem, E., Naddeo, V., Banat, F. & Hasan, S. W. Synthesis of super hydrophilic cellulose-alpha zirconium phosphate ion exchange membrane via surface coating for the removal of heavy metals from wastewater. Sci. Total. Environ. 690 , 167–180 (2019).
Akther, N., Phuntsho, S., Chen, Y., Ghaffour, N. & Shon, H. K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 584 , 20–45 (2019).
Giwa, A. et al. Selectivity of nanoporous MnO2 and TiO2 membranes for residual contaminants in treated wastewater. Chem. Eng. Technol. 41 , 413–420 (2018).
Pendergast, M. M. & Hoek, E. M. A review of water treatment membrane nanotechnologies. Energ. Environ. Sci. 4 , 1946–1971 (2011).
Bae, T.-H. & Tak, T.-M. Preparation of TiO2 self-assembled polymeric nanocomposite membranes and examination of their fouling mitigation effects in a membrane bioreactor system. J. Membr. Sci. 266 , 1–5 (2005).
Rahimi, Z., Zinatizadeh, A. & Zinadini, S. Preparation of high antibiofouling amino functionalized MWCNTs/PES nanocomposite ultrafiltration membrane for application in membrane bioreactor. J. Ind. Eng. Chem. 29 , 366–374 (2015).
Mehrnia, M. R. & Homayoonfal, M. Fouling mitigation behavior of magnetic responsive nanocomposite membranes in a magnetic membrane bioreactor. J. Membr. Sci. 520 , 881–894 (2016).
Robinson, T. Membrane bioreactors: nanotechnology improves landfill leachate quality. Filtr. Separat. 44 , 38–39 (2007).
Bilad, M. R., Westbroek, P. & Vankelecom, I. F. Assessment and optimization of electrospun nanofiber-membranes in a membrane bioreactor (MBR). J. Membr. Sci. 380 , 181–191 (2011).
Moradi, G., Zinadini, S., Rajabi, L. & Dadari, S. Fabrication of high flux and antifouling mixed matrix fumarate-alumoxane/PAN membranes via electrospinning for application in membrane bioreactors. Appl. Surf. Sci. 427 , 830–842 (2018).
Ahsani, M., Hazrati, H., Javadi, M., Ulbricht, M. & Yegani, R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep. Purif. Technol. 249 , 116938 (2020).
Behboudi, A., Jafarzadeh, Y. & Yegani, R. Enhancement of antifouling and antibacterial properties of PVC hollow fiber ultrafiltration membranes using pristine and modified silver nanoparticles. J. Environ. Chem. Eng. 6 , 1764–1773 (2018).
Ayyaru, S., Pandiyan, R. & Ahn, Y.-H. Fabrication and characterization of anti-fouling and non-toxic polyvinylidene fluoride-sulphonated carbon nanotube ultrafiltration membranes for membrane bioreactors applications. Chem. Eng. Res. Des. 142 , 176–188 (2019).
Yang, Y., Qiao, S., Jin, R., Zhou, J. & Quan, X. Novel anaerobic electrochemical membrane bioreactor with a CNTs hollow fiber membrane cathode to mitigate membrane fouling and enhance energy recovery. Environ. Sci. Technol. 53 , 1014–1021 (2019).
Lv, J., Zhang, G., Zhang, H. & Yang, F. Graphene oxide-cellulose nanocrystal (GO-CNC) composite functionalized PVDF membrane with improved antifouling performance in MBR: Behavior and mechanism. Chem. Eng. J. 352 , 765–773 (2018).
Li, Y. et al. The performance of Pd-rGO electro-deposited PVDF/carbon fiber cloth composite membrane in MBR/MFC coupled system. Chem. Eng. J. 365 , 317–324 (2019).
Yin, X., Li, X., Wang, X., Ren, Y. & Hua, Z. A spontaneous electric field membrane bioreactor with the innovative Cu-nanowires conductive microfiltration membrane for membrane fouling mitigation and pollutant removal. Water Environ. Res. 91 , 780–787 (2019).
Fathizadeh, M. et al. Antifouling UV-treated GO/PES hollow fiber membrane in membrane bioreactor (MBR). Environ. Sci.: Water Res. Technol. 5 , 1244–1252 (2019).
CAS Google Scholar
Zhao, C., Xu, X., Chen, J., Wang, G. & Yang, F. Highly effective antifouling performance of PVDF/graphene oxide composite membrane in membrane bioreactor (MBR) system. Desalination 340 , 59–66 (2014).
Santos, A. & Judd, S. The commercial status of membrane bioreactors for municipal wastewater. Sep. Sci. Technol. 45 , 850–857 (2010).
Krzeminski, P., Leverette, L., Malamis, S. & Katsou, E. Membrane bioreactors–a review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 527 , 207–227 (2017).
Li, P. et al. Identify driving forces of MBR applications in China. Sci. Total. Environ. 647 , 627–638 (2019).
Saikia, J., Gogoi, A. & Baruah, S. Environmental Nanotechnology. Ch. 7 (Springer, Cham, 2019).
Mirbagheri, S. A., Bagheri, M., Bagheri, Z. & Kamarkhani, A. M. Evaluation and prediction of membrane fouling in a submerged membrane bioreactor with simultaneous upward and downward aeration using artificial neural network-genetic algorithm. Process. Saf. Environ. 96 , 111–124 (2015).
Amouamouha, M. & Badalians Gholikandi, G. Assessment of anaerobic nanocomposite membrane bioreactor efficiency intensified by biogas backwash. Chem. Eng. Process. Process. Intensif. 131 , 51–58 (2018).
Kim, J. & Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 158 , 2335–2349 (2010).
Qu, X., Brame, J., Li, Q. & Alvarez, P. J. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc. Chem. Res. 46 , 834–843 (2012).
Liao, Y., Loh, C.-H., Tian, M., Wang, R. & Fane, A. G. Progress in electrospun polymeric nanofibrous membranes for water treatment: fabrication, modification and applications. Prog. Polym. Sci. 77 , 69–94 (2018).
Lakhotia, S. R., Mukhopadhyay, M. & Kumari, P. Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment. Sci. Rep. 8 , 4976 (2018).
Dong, Y. et al. Stable superhydrophobic ceramic-based carbon nanotube composite desalination membranes. Nano. Lett. 18 , 5514–5521 (2018).
Rafieian, F., Jonoobi, M. & Yu, Q. A novel nanocomposite membrane containing modified cellulose nanocrystals for copper ion removal and dye adsorption from water. Cellulose 26 , 3359–3373 (2019).
Tan, X. et al. Novel AgNWs-PAN/TPU membrane for point-of-use drinking water electrochemical disinfection. Sci. Total. Environ. 637 , 408–417 (2018).
Karkooti, A. et al. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Membr. Sci. 560 , 97–107 (2018).
Su, Z., Ding, J. & Wei, G. Electrospinning: a facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. Rsc. Adv. 4 , 52598–52610 (2014).
Pervez, M. N. & Stylios, G. K. Investigating the synthesis and characterization of a novel “green” H2O2-assisted, water-soluble chitosan/polyvinyl alcohol nanofiber for environmental end uses. Nanomaterials 8 , 395 (2018).
Pervez, M. N. & Stylios, G. K. An experimental approach to the synthesis and optimisation of a ‘green’nanofibre. Nanomaterials 8 , 383 (2018).
Pervez, M. N., Stylios, G. K., Liang, Y., Ouyang, F. & Cai, Y. Low-temperature synthesis of novel polyvinylalcohol (PVA) nanofibrous membranes for catalytic dye degradation. J. Clean. Prod. 262 , 121301 (2020).
Xue, J., Wu, T., Dai, Y. & Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 119 , 5298–5415 (2019).
Alias, N. H. et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 360 , 1437–1446 (2019).
Cheng, C., Li, X., Yu, X., Wang, M. & Wang, X. Electrospinning: Nanofabrication and Applications. Ch. 14 (Elsevier, Norwich, 2019).
Sundaran, S. P., Reshmi, C. R., Sagitha, P., Manaf, O. & Sujith, A. Multifunctional graphene oxide loaded nanofibrous membrane for removal of dyes and coliform from water. J. Environ. Manag. 240 , 494–503 (2019).
Hosseini, S. A., Vossoughi, M., Mahmoodi, N. M. & Sadrzadeh, M. Clay-based electrospun nanofibrous membranes for colored wastewater treatment. Appl. Clay. Sci. 168 , 77–86 (2019).
Bjorge, D. et al. Performance assessment of electrospun nanofibers for filter applications. Desalination 249 , 942–948 (2009).
Daels, N. et al. The use of electrospun flat sheet nanofibre membranes in MBR applications. Desalination 257 , 170–176 (2010).
Kim, H.-C. et al. Electrospun nanofibrous PVDF–PMMA MF membrane in laboratory and pilot-scale study treating wastewater from seoul zoo. Desalination 346 , 107–114 (2014).
Zhao, F. et al. Antimicrobial three dimensional woven filters containing silver nanoparticle doped nanofibers in a membrane bioreactor for wastewater treatment. Sep. Purif. Technol. 175 , 130–139 (2017).
Jin, M.-Y., Liao, Y., Loh, C. H., Tan, C.-H. & Wang, R. Preparation of polydimethylsiloxane–polyvinylidene fluoride composite membranes for phenol removal in extractive membrane bioreactor. Ind. Eng. Chem. Res. 56 , 3436–3445 (2017).
Liao, Y., Goh, S., Tian, M., Wang, R. & Fane, A. G. Design, development and evaluation of nanofibrous composite membranes with opposing membrane wetting properties for extractive membrane bioreactors. J. Membr. Sci. 551 , 55–65 (2018).
Ren, L.-F. et al. High-performance electrospinning-phase inversion composite PDMS membrane for extractive membrane bioreactor: fabrication, characterization, optimization and application. J. Membr. Sci. 597 , 117624 (2020).
Ren, L.-F. et al. Novel external extractive membrane bioreactor (EMBR) using electrospun polydimethylsiloxane/polymethyl methacrylate membrane for phenol-laden saline wastewater. Chem. Eng. J. 383 , 123179 (2020).
Gee, S. & Smith, A. L. Development of electrospun nanofiber membranes for anaerobic membrane bioreactors, 16th World Conference on Anaerobic Digestion Conference AD16 . (Delft, The Netherlands, 2019).
Mahmoodi, N. M. & Saffar-Dastgerdi, M. H. Zeolite nanoparticle as a superior adsorbent with high capacity: synthesis, surface modification and pollutant adsorption ability from wastewater. Microchem. J. 145 , 74–83 (2019).
Nasseh, N., Taghavi, L., Barikbin, B. & Nasseri, M. A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 179 , 42–54 (2018).
Ahmad, A. et al. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. Rsc. Adv. 5 , 30801–30818 (2015).
Al-Hamadani, Y. A. et al. Stabilization and dispersion of carbon nanomaterials in aqueous solutions: a review. Sep. Purif. Technol. 156 , 861–874 (2015).
Qu, X., Alvarez, P. J. & Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 47 , 3931–3946 (2013).
Su, Y.-C., Huang, C., Pan, J. R., Hsieh, W.-P. & Chu, M.-C. Fouling mitigation by TiO2 composite membrane in membrane bioreactors. J. Environ. Eng. 138 , 344–350 (2011).
Liu, L., Zhao, C. & Yang, F. TiO2 and polyvinyl alcohol (PVA) coated polyester filter in bioreactor for wastewater treatment. Water Res. 46 , 1969–1978 (2012).
Hu, W., Yin, J., Deng, B. & Hu, Z. Application of nano TiO2 modified hollow fiber membranes in algal membrane bioreactors for high-density algae cultivation and wastewater polishing. Bioresour. Technol. 193 , 135–141 (2015).
Tavakolmoghadam, M., Mohammadi, T., Hemmati, M. & Naeimpour, F. Surface modification of PVDF membranes by sputtered TiO2: fouling reduction potential in membrane bioreactors. Desalin. Water Treat. 57 , 3328–3338 (2016).
Fonouni, M., Etemadi, H., Yegani, R. & Zarin, S. Fouling characterization of TiO2 nanoparticle embedded polypropylene membrane in oil refinery wastewater treatment using membrane bioreactor (MBR). Desalin. Water Treat. 90 , 99–109 (2017).
Etemadi, H., Milad, F. & Yegani, R. Investigation of antifouling properties of polypropylene/TiO2 nanocomposite membrane under different aeration rate in membrane bioreactor system. Biotechnol. Rep. 25 , e00414 (2020).
Homayoonfal, M., Mehrnia, M. R., Rahmani, S. & Mojtahedi, Y. M. Fabrication of alumina/polysulfone nanocomposite membranes with biofouling mitigation approach in membrane bioreactors. J. Ind. Eng. Chem. 22 , 357–367 (2015).
Amini, M., Etemadi, H., Akbarzadeh, A. & Yegani, R. Preparation and performance evaluation of high-density polyethylene/silica nanocomposite membranes in membrane bioreactor system. Biochem. Eng. J. 127 , 196–205 (2017).
Liang, S. et al. Organic fouling behavior of superhydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes functionalized with surface-tailored nanoparticles: implications for organic fouling in membrane bioreactors. J. Membr. Sci. 463 , 94–101 (2014).
Li, N., Liu, L. & Yang, F. Highly conductive graphene/PANi-phytic acid modified cathodic filter membrane and its antifouling property in EMBR in neutral conditions. Desalination 338 , 10–16 (2014).
Liu, L., Zhao, F., Liu, J. & Yang, F. Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating yeast suspensions in EMBR. J. Membr. Sci. 437 , 99–107 (2013).
Alsalhy, Q. F., Al-Ani, F. H., Al-Najar, A. E. & Jabuk, S. I. A study of the effect of embedding ZnO-NPs on PVC membrane performance use in actual hospital wastewater treatment by membrane bioreactor. Chem. Eng. Process. Process. Intensif. 130 , 262–274 (2018).
Etemadi, H., Yegani, R. & Seyfollahi, M. The effect of amino functionalized and polyethylene glycol grafted nanodiamond on anti-biofouling properties of cellulose acetate membrane in membrane bioreactor systems. Sep. Purif. Technol. 177 , 350–362 (2017).
Tizchang, A., Jafarzadeh, Y., Yegani, R. & Khakpour, S. The effects of pristine and silanized nanodiamond on the performance of polysulfone membranes for wastewater treatment by MBR system. J. Environ. Chem. Eng. 7 , 103447 (2019).
Kivi, M. A., Alinia, H., Jafarzadeh, Y. & Yegani, R. High-density polyethylene membranes embedded with carboxylated and polyethylene glycol-grafted nanodiamond to be used in membrane bioreactors. J. Appl. Polym. Sci. 136 , 47914 (2019).
Pirsaheb, M. et al. Fabrication of high-performance antibiofouling ultrafiltration membranes with potential application in membrane bioreactors (MBRs) comprising polyethersulfone (PES) and polycitrate-Alumoxane (PC-A). Sep. Purif. Technol. 211 , 618–627 (2019).
Mahmoudi, E., Ng, L. Y., Ang, W. L., Teow, Y. H. & Mohammad, A. W. Improving membrane bioreactor performance through the synergistic effect of silver-decorated graphene oxide in composite membranes. J. Water Process. Eng. 34 , 101169 (2020).
Behboudi, A., Jafarzadeh, Y. & Yegani, R. Incorporation of silica grafted silver nanoparticles into polyvinyl chloride/polycarbonate hollow fiber membranes for pharmaceutical wastewater treatment. Chem. Eng. Res. Des. 135 , 153–165 (2018).
Ahmad, J., Naeem, S., Ahmad, M., Usman, A. R. & Al-Wabel, M. I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 246 , 214–228 (2019).
Chen, H. et al. Enhanced adsorption of U (VI) and 241Am (III) from wastewater using Ca/Al layered double hydroxide@ carbon nanotube composites. J. Hazard. Mater. 347 , 67–77 (2018).
De Volder, M. F., Tawfick, S. H., Baughman, R. H. & Hart, A. J. Carbon nanotubes: present and future commercial applications. Science 339 , 535–539 (2013).
Lee, K.-J. & Park, H.-D. Effect of transmembrane pressure, linear velocity, and temperature on permeate water flux of high-density vertically aligned carbon nanotube membranes. Desalin. Water Treat. 57 , 26706–26717 (2016).
Lee, B. et al. A carbon nanotube wall membrane for water treatment. Nat. Commun. 6 , 7109 (2015).
Yang, Y., Qiao, S., Jin, R., Zhou, J. & Quan, X. A novel aerobic electrochemical membrane bioreactor with CNTs hollow fiber membrane by electrochemical oxidation to improve water quality and mitigate membrane fouling. Water Res. 151 , 54–63 (2019).
Mulopo, J. Bleach plant effluent treatment in anaerobic membrane bioreactor (AMBR) using carbon nanotube/polysulfone nanocomposite membranes. J. Environ. Chem. Eng. 5 , 4381–4387 (2017).
Khalid, A., Abdel-Karim, A., Atieh, M. A., Javed, S. & McKay, G. PEG-CNTs nanocomposite PSU membranes for wastewater treatment by membrane bioreactor. Sep. Purif. Technol. 190 , 165–176 (2018).
Ling, S. et al. Design and function of biomimetic multilayer water purification membranes. Sci. Adv. 3 , e1601939 (2017).
Abouzeid, R. E., Khiari, R., El-Wakil, N. & Dufresne, A. Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromolecules 20 , 573–597 (2018).
Carpenter, A. W., de Lannoy, C.-F. & Wiesner, M. R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 49 , 5277–5287 (2015).
Ni, X., Cheng, W., Huan, S., Wang, D. & Han, G. Electrospun cellulose nanocrystals/poly (methyl methacrylate) composite nanofibers: morphology, thermal and mechanical properties. Carbohyd. Polym. 206 , 29–37 (2019).
Zhou, C., Chu, R., Wu, R. & Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 12 , 2617–2625 (2011).
He, J. et al. Diffusion and filtration properties of self-assembled gold nanocrystal membranes. Nano. Lett. 11 , 2430–2435 (2011).
Goetz, L. A., Jalvo, B., Rosal, R. & Mathew, A. P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 510 , 238–248 (2016).
Meng, F. et al. Fouling in membrane bioreactors: an updated review. Water Res. 114 , 151–180 (2017).
Bellona, C. & Drewes, J. E. The role of membrane surface charge and solute physico-chemical properties in the rejection of organic acids by NF membranes. J. Membr. Sci. 249 , 227–234 (2005).
Lin, H. et al. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 460 , 110–125 (2014).
Wang, X. et al. Fabrication of PbO2 tipped Co3O4 nanowires for efficient photoelectrochemical decolorization of dye (reactive brilliant blue KN-R) wastewater. Sol. Energ. Mat. Sol. C. 191 , 381–388 (2019).
Wang, S. et al. Lead-free sodium niobate nanowires with strong piezo-catalysis for dye wastewater degradation. Ceram. Int. 45 , 11703–11708 (2019).
Jeevanandam, J. et al. Sustainable Nanoscale Engineering . Ch. 4 (Elsevier, Amsterdam, 2020).
Jiang, R., Wen, W. & Wu, J.-M. Titania nanowires coated PEI/P25 membranes for photocatalytic and ultrafiltration applications. New. J. Chem. 42 , 3020–3027 (2018).
Zhang, X., Du, A. J., Pan, J., Wang, Y. & Sun, D. D. New Membranes and Advanced Materials for Wastewater Treatment. Ch. 14 (American Chemical Society, Washington DC, 2009).
Zhou, J. et al. Two-dimensional superstructures filled into polysulfone membranes for highly improved ultrafiltration: the case of cuprous iodide nanosheets. J. Membr. Sci. 576 , 142–149 (2019).
Yang, G. et al. A novel nanocomposite membrane combining BN nanosheets and GO for effective removal of antibiotic in water. Nanomaterials 9 , 386 (2019).
Kashefi, S., Borghei, S. M. & Mahmoodi, N. M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 276 , 153–162 (2019).
Zhou, T., Ma, L., Gan, M., Wang, H. & Hao, C. Sandwich-structured hybrids: a facile electrostatic self-assembly of exfoliated titania nanosheets and polyaniline nanoparticles and its high visible-light photocatalytic performance. J. Phys. Chem. Solids 125 , 123–130 (2019).
Liang, X. et al. Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J. Membr. Sci. 573 , 270–279 (2019).
Xiao, G. et al. Superior adsorption performance of graphitic carbon nitride nanosheets for both cationic and anionic heavy metals from wastewater. Chin. J. Chem. Eng. 27 , 305–313 (2019).
Ghalamchi, L., Aber, S., Vatanpour, V. & Kian, M. A novel antibacterial mixed matrixed PES membrane fabricated from embedding aminated Ag3PO4/g-C3N4 nanocomposite for use in the membrane bioreactor. J. Ind. Eng. Chem. 70 , 412–426 (2019).
Zinadini, S. et al. Preparation and characterization of antifouling graphene oxide/polyethersulfone ultrafiltration membrane: application in MBR for dairy wastewater treatment. J. Water Process. Eng. 7 , 280–294 (2015).
Beygmohammdi, F., Kazerouni, H. N., Jafarzadeh, Y., Hazrati, H. & Yegani, R. Preparation and characterization of PVDF/PVP-GO membranes to be used in MBR system. Chem. Eng. Res. Des. 154 , 232–240 (2020).
Ghaffar, A., Zhang, L., Zhu, X. & Chen, B. Porous PVdF/GO nanofibrous membranes for selective separation and recycling of charged organic dyes from water. Environ. Sci. Technol. 52 , 4265–4274 (2018).
Wu, W. et al. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 379 , 122368 (2020).
Field, R. Membrane Technology: Membranes for Water Treatment . Ch. 1 (Wiley-VCH, Weinheim, 2010).
Mutamim, N. S. A., Noor, Z. Z., Hassan, M. A. A., Yuniarto, A. & Olsson, G. Membrane bioreactor: applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 225 , 109–119 (2013).
Cho, B. D. & Fane, A. G. Fouling transients in nominally sub-critical flux operation of a membrane bioreactor. J. Membr. Sci. 209 , 391–403 (2002).
Lin, H. et al. A review on anaerobic membrane bioreactors: applications, membrane fouling and future perspectives. Desalination 314 , 169–188 (2013).
Lv, D. et al. Ecofriendly electrospun membranes loaded with visible-light-responding nanoparticles for multifunctional usages: highly efficient air filtration, dye scavenging, and bactericidal activity. Acs. Appl. Mater. Inter. 11 , 12880–12889 (2019).
Ren, Y. et al. Stereocomplex crystallites-based eco-friendly nanofiber membranes for removal of Cr (VI) and antibacterial effect. Acs. Sustain. Chem. Eng. 7 , 16072–16083 (2019).
Kamali, M., Persson, K. M., Costa, M. E. & Capela, I. Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: current status and future outlook. Environ. Int. 125 , 261–276 (2019).
Wu, M. et al. Fabrication of composite nanofiltration membrane by incorporating attapulgite nanorods during interfacial polymerization for high water flux and antifouling property. J. Membr. Sci. 544 , 79–87 (2017).
Arkas, M., Allabashi, R., Tsiourvas, D., Mattausch, E.-M. & Perfler, R. Organic/inorganic hybrid filters based on dendritic and cyclodextrin “nanosponges” for the removal of organic pollutants from water. Environ. Sci. Technol. 40 , 2771–2777 (2006).
Zhao, G., Hu, R., Li, J. & Zhu, H. Graphene oxide quantum dots embedded polysulfone membranes with enhanced hydrophilicity, permeability and antifouling performance. Sci. China Mater. 62 , 1177–1187 (2019).
Fatarella, E., Iversen, V., Grinwis, S. & Paulussen, S. Textile material for membrane filtration, AMADEUS final report (2009).
Bojnourd, F. M. & Pakizeh, M. Preparation and characterization of a nanoclay/PVA/PSf nanocomposite membrane for removal of pharmaceuticals from water. Appl. Clay. Sci. 162 , 326–338 (2018).
Ahmad, T., Guria, C. & Mandal, A. Optimal synthesis and operation of low-cost polyvinyl chloride/bentonite ultrafiltration membranes for the purification of oilfield produced water. J. Membr. Sci. 564 , 859–877 (2018).
Bohdziewicz, J., Neczaj, E. & Kwarciak, A. Landfill leachate treatment by means of anaerobic membrane bioreactor. Desalination 221 , 559–565 (2008).
Tarnacki, K., Lyko, S., Wintgens, T., Melin, T. & Natau, F. Impact of extra-cellular polymeric substances on the filterability of activated sludge in membrane bioreactors for landfill leachate treatment. Desalination 179 , 181–190 (2005).
Dabaghian, Z., Peyravi, M., Jahanshahi, M. & Rad, A. S. Potential of advanced nano-structured membranes for landfill leachate treatment: a review. ChemBioEng. Rev. 5 , 119–138 (2018).
Casals, E. et al. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 10 , 2801–2808 (2014).
Zhang, L. et al. Evaluating the influences of ZnO engineering nanomaterials on VFA accumulation in sludge anaerobic digestion. Biochem. Eng. J. 125 , 206–211 (2017).
Baniamerian, H. et al. Application of nano-structured materials in anaerobic digestion: current status and perspectives. Chemosphere 229 , 188–199 (2019).
Atasoy, M., Owusu-Agyeman, I., Plaza, E. & Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: current status and future challenges. Bioresour. Technol. 268 , 773–786 (2018).
Parchami, M., Wainaina, S., Mahboubi, A., I’Ons, D. & Taherzadeh, M. J. MBR-assisted VFAs production from excess sewage sludge and food waste slurry for sustainable wastewater treatment. Appl. Sci. 10 , 2921 (2020).
Paudel, S. et al. Anaerobic hydrogen fermentation and membrane bioreactor (MBR) for decentralized sanitation and reuse-organic removal and resource recovery. Environ. Eng. Res. 19 , 387–393 (2014).
Nguyen, A. Q. et al. Derivation of volatile fatty acid from crop residues digestion using a rumen membrane bioreactor: a feasibility study. Bioresour. Technol. 312 , 123571 (2020).
Yan, T. et al. A critical review on membrane hybrid system for nutrient recovery from wastewater. Chem. Eng. J. 348 , 143–156 (2018).
Song, X. et al. Resource recovery from wastewater by anaerobic membrane bioreactors: opportunities and challenges. Bioresour. Technol. 270 , 669–677 (2018).
Valsami-Jones, E. & Lynch, I. How safe are nanomaterials? Science 350 , 388–389 (2015).
Wen, Y., Yuan, J., Ma, X., Wang, S. & Liu, Y. Polymeric nanocomposite membranes for water treatment: a review. Environ. Chem. Lett. 17 , 1539–1551 (2019).
Goh, P. S., Ismail, A. F. & Hilal, N. Nano-enabled membranes technology: sustainable and revolutionary solutions for membrane desalination? Desalination 380 , 100–104 (2016).
Westerhoff, P. et al. Low risk posed by engineered and incidental nanoparticles in drinking water. Nat. Nanotechnol. 13 , 661–669 (2018).
Kellenberger, C. R. et al. Roll-to-roll preparation of mesoporous membranes by nanoparticle template removal. Ind. Eng. Chem. Res. 53 , 9214–9220 (2014).
Liu, Y. et al. A facile and scalable fabrication procedure for thin-film composite membranes: Integration of phase inversion and interfacial polymerization. Environ. Sci. Technol. 54 , 1946–1954 (2020).
Lalia, B. S., Kochkodan, V., Hashaikeh, R. & Hilal, N. A review on membrane fabrication: structure, properties and performance relationship. Desalination 326 , 77–95 (2013).
Bhardwaj, N. & Kundu, S. C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 28 , 325–347 (2010).
Singh, M. et al. Additive manufacturing of mechanically isotropic thin films and membranes via microextrusion 3D printing of polymer solutions. Acs. Appl. Mater. Inter. 11 , 6652–6661 (2019).
Murthy, G. S. Bioenergy: Principles and Applications . Ch. 27 (Wiley Blackwell, Hoboken, 2016).
Roccaro, P. & Vagliasindi, F. G. A. Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability . Ch. 67 (Springer, Cham, 2020).
Aydiner, C. et al. Techno-economic viability of innovative membrane systems in water and mass recovery from dairy wastewater. J. Membr. Sci. 458 , 66–75 (2014).
Bertanza, G. et al. A comparison between two full-scale MBR and CAS municipal wastewater treatment plants: techno-economic-environmental assessment. Environ. Sci. Pollut. R. 24 , 17383–17393 (2017).
Download references
Acknowledgements
We would like to express their sincere gratitude to the support from (i) Sanitary Environmental Engineering Division (SEED) and grants (FARB projects) from the University of Salerno coordinated by prof. V. Naddeo; (ii) Inter-University Consortium of Relevant Hazardous (Consorzio inter-Universitario per la previsione e la prevenzionedei Grandi Rischi, C.U.G.RI.); (iii) grant INT/Italy/P-17/2016 (SP) from Department of Science and Technology, Ministry of Science and Technology, Government of India; (iv) grant (No. 2019R1H1A2080148) from the National Research Foundation of Korea, funded by the Korean government; and (v) the Center for Membranes and Advanced Water technology (CMAT) at Khalifa University of Science and Technology (Abu Dhabi, UAE).
Author information
Authors and affiliations.
Sanitary Environmental Engineering Division (SEED), Department of Civil Engineering, University of Salerno, via Giovanni Paolo II 132, 84084, Fisciano, SA, Italy
Md. Nahid Pervez, Tiziano Zarra, Vincenzo Belgiorno & Vincenzo Naddeo
The Energy and Resources Institute (TERI), Darbari Seth Block, IHC Complex, Lodhi Road, New Delhi, 110003, India
Malini Balakrishnan
Center for Membranes and Advanced Water Technology (CMAT), Department of Chemical Engineering, Khalifa University of Science and Technology, P.O. Box, 127788, Abu Dhabi, UAE
Shadi Wajih Hasan
Department of Environmental Engineering, Kyungpook National University, 80 Daehak-ro, Buk-gu, Daegu, 41566, Republic of Korea
Kwang-Ho Choo
School of Ecological and Environmental Sciences, Shanghai Key Laboratory for Urban Ecological Process and Eco-Restoration in East China Normal University, and Institute of Eco-Chongming, 200062, Shanghai, China
Yaping Zhao
Hubei Provincial Engineering Laboratory for Clean Production and High Value Utilization of Bio-Based Textile Materials, Wuhan Textile University, 430200, Wuhan, China
Yingjie Cai
You can also search for this author in PubMed Google Scholar
Contributions
M.N.P., T.Z., V.N.—concept and original draft, M.B.—reviewing & editing, S.W.H.—reviewing & editing, K.H.C.—reviewing & editing, Y.Z.— reviewing & editing, Y.C.—reviewing & editing, V.B.—reviewing & editing, T.Z.—reviewing & editing, V.N.—reviewing, editing, & supervision. Finally, all authors have read and approved the final manuscript.
Corresponding author
Correspondence to Vincenzo Naddeo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Pervez, M.N., Balakrishnan, M., Hasan, S.W. et al. A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment. npj Clean Water 3 , 43 (2020). https://doi.org/10.1038/s41545-020-00090-2
Download citation
Received : 14 May 2020
Accepted : 12 October 2020
Published : 05 November 2020
DOI : https://doi.org/10.1038/s41545-020-00090-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Sustainable wastewater reuse for agriculture.
- Anastasis Christou
- Vasiliki G. Beretsou
- Despo Fatta-Kassinos
Nature Reviews Earth & Environment (2024)
Polyamidoamine-based graphene oxide nanoparticles as adsorbent for mitigation of fouling in electrochemical membrane bioreactor
- M. Salami-Kalajahi
International Journal of Environmental Science and Technology (2024)
Tuning the surface functionality of polyethylene glycol-modified graphene oxide/chitosan composite for efficient removal of dye
- Md. Nahid Pervez
- Md Anwar Jahid
- Vincenzo Naddeo
Scientific Reports (2023)
Effect of packing media type of moving and fixed on performance and membrane fouling in biofilm-based MBR processes
- Yousef Rahimi
- Kazem Godini
- Serveh Fathi
Biomass Conversion and Biorefinery (2023)
A Comprehensive Review on Application of Lignocellulose Derived Nanomaterial in Heavy Metals Removal from Wastewater
- Vineet Kumar
Chemistry Africa (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Journal Menu
- Membranes Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
- arrow_forward_ios Forthcoming issue arrow_forward_ios Current issue
- Vol. 14 (2024)
- Vol. 13 (2023)
- Vol. 12 (2022)
- Vol. 11 (2021)
- Vol. 10 (2020)
- Vol. 9 (2019)
- Vol. 8 (2018)
- Vol. 7 (2017)
- Vol. 6 (2016)
- Vol. 5 (2015)
- Vol. 4 (2014)
- Vol. 3 (2013)
- Vol. 2 (2012)
- Vol. 1 (2011)
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
Membrane Technologies for Water and Wastewater Treatment: Advances, Challenges, and Future Avenues
- Print Special Issue Flyer
- Special Issue Editors
Special Issue Information
- Published Papers
A special issue of Membranes (ISSN 2077-0375). This special issue belongs to the section " Membrane Processing and Engineering ".
Deadline for manuscript submissions: closed (20 December 2022) | Viewed by 21543
Share This Special Issue
Special issue editor.

Dear Colleagues,
Membranes play a significant role in providing innovative, profitable, eco-friendly, and sustainable separation technologies for water and wastewater treatment. The quality of pre-treated water gives rise to opportunities for continuous advancements in terms of system design and configuration, in-process challenges, and productivity, with respect to environmental and economic perspectives. Scientists and engineers are also working on integrated approaches, containing a combination of two or more membrane-based systems, and/or other technologies for water and wastewater treatment.
This Special Issue aims to attract submissions of research and review articles emphasizing the scientific and engineering aspects of membrane-based water treatment, including, but not limited to, the following themes:
- Advances in membrane material and system configurations;
- Advances in membrane technologies for water and wastewater treatment;
- Membrane transport phenomena;
- Pressure-driven membrane process;
- Membrane bioreactors;
- Integrated water and wastewater treatment systems;
- Desalination;
- Membrane technologies and energy perspectives;
- Fouling issues and antifouling strategies;
- Green antiscalants.
Dr. Muhammad Kashif Shahid Guest Editor
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website . Once you are registered, click here to go to the submission form . Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Membranes is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
- water reuse, recycling, and reclamation
- membrane bioreactors
- environmental remediation
- membrane fouling
- membrane modification
- integrated membrane water treatment
- renewable energy-driven membrane technologies
Published Papers (7 papers)
Jump to: Review

Graphical abstract

Jump to: Research
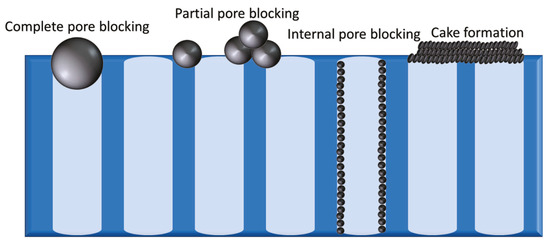
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Desalination and Water Treatment
Research Trend of Membranes for Water Treatment by Analysis of Patents and Papers’ Publications
Submitted: 07 December 2017 Reviewed: 22 March 2018 Published: 19 September 2018
DOI: 10.5772/intechopen.76694
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Desalination and Water Treatment
Edited by Murat Eyvaz and Ebubekir Yüksel
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
2,146 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Since the beginning of water shortage by disasters such as global warming, environmental pollution, and drought, development of original technology and studies have been undertaken to increase the availability of water resources. Among the technologies, water treatment technology using membranes has a better water quality improvement than existing physicochemical and biological processes. Moreover, it is environmental-friendly technology that does not use chemicals. Water treatment membranes are applied to various fields such as wastewater treatment, water purification, seawater desalination, ion exchange process, ultra-pure water production, and separation of organic solvents. Furthermore, water treatment technologies using membranes will increasingly expand. The core technology of the water treatment membrane is to control the size of pores for membrane performance and is being researched to improve performance. In this chapter, the frequencies of presentation are filed by country, institution, and company through technology competitiveness and evaluation of patents and papers. In addition, evaluation of technologies for wastewater treatment, water purification, seawater desalination, and ion exchange process was carried out in the same way as before. Finally, future research directions were suggested by using evaluation results.
- water treatment
- desalination
- ion exchange
Author Information
Chang hwa woo *.
- Gyeongsang National University Academy and Industry Collaboration, Jinjusi, Republic of Korea
*Address all correspondence to: [email protected]
1. Introduction
The world is now expected to become water scarce as a result of global warming, and by 2025, it is estimated that water-scarce countries will increase by more than 30% compared to 1995 [ 1 ]. The twentieth century was the era of black gold, represented by oil, but the age of water, or blue gold, is expected to emerge in the twenty-first century. Due to the global problems faced by the world, such as population growth, industrialization, and climate change, a steady increase in water demand and a disparity in regional water supply are urgently needed to be resolved. Population Action International (PAI) currently has 550 million people living in water-pressure or water-starved countries, and from 2.4 billion to 3.4 billion people will live in water-starved or water-deprived countries by 2025. According to the World Meteorological Organization (WMO) report, 653 million people in 2025 and 2.43 billion in 2050 will suffer direct water shortages.
Various water treatment techniques have been studied to secure water resources in order to solve the water shortage phenomenon. In the water treatment field, there are water treatment processes such as wastewater and wastewater treatment to remove pollutants, water treatment for drinking water, and seawater desalination for seawater reuse ( Figure 1 ).

Various applications of water treatment membranes. (a) MBR process (Toray Industries, Inc.), (b) water treatment process (Yeongdeungpo water purification center), (c) desalination process (Doosan heavy industries & construction), (d) ion exchange membrane (Tokuyama America, Inc.).
There are also a number of related technologies, among which water treatment technologies using membranes have shown very high growth rates of 10–20% per year [ 2 , 3 ]. Frost and Sullivan estimate that the world’s membrane-based water treatment market will grow from $ 5.54 billion in 2012 to $ 1.27 billion by 2020 (CAGR of 10.2%). Major growth factors include increased demand for drinking water, reuse of sewage, increased desalination facilities based on membranes, and strengthening of environmental standards. In particular, it is expected that there will be a significant increase in the market in the Asia-Pacific region based on rapid industrialization, population growth, and demand for advanced technologies [ 4 ].
The separation membrane has a selective filtration function that selectively passes a specific component, as well as selective permeability capable of separating dissolved substances or mixed gases dissolved in a liquid [ 5 , 6 , 7 ]. Membrane separation technology comprehensively means various separation processes using such selective permeability of the membrane. As shown in Figure 2 , the separation membrane used for water treatment produces clean water by allowing the water (B) to pass but not allowing the suspended material (A) to pass through. Membranes can be divided into microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) depending on the pore size [ 8 , 9 , 10 ]. Figure 3 shows the separation performance according to the pore size of the membrane, and Table 1 shows the membrane characteristics of various process parameters [ 11 ].

Schematic of membrane filtration process.

A scheme of the membrane for water purification processes.
| Microfiltration | Ultrafiltration | Nanofiltration | Reverse osmosis | |
|---|---|---|---|---|
| Mechanism or separation | Sieving | Sieving | Sieving + solution/diffusion + exclusion | Solution/diffusion + exclusion |
| Materials | CA, CE, PAN, PC, PE, POF, PP, PS, PTFE, PVDF | CA, CE, PA, PAN, TFC, PS, PVDF | CA, PA, TFC | CA, PA, PS, TFC |
| MWCO (Da) | >100,000 | >2000–100,000 | 300–1000 | 100–200 |
| Structure | Porous isotropic | Porous asymmetric | Finely porous asymmetric/composite | Nonporous asymmetric/composite |
| Law governing transfer | Darcy’s law | Darcy’s law | Fick’s law | Fick’s law |
| Pore size range (μm) | 0.1–10 | 0.01–0.1 | 0.001–0.01 | <0.001 |
| Rejects | Particulates, clay, bacteria | Macromolecules, proteins, polysaccharides, viruses | HMWC, mono-, di-, and oligosaccharides, polyvalent anions | HMWC, LMWC, sodium chloride, glucose, amino acids, proteins |
| Operating pressure (psi) | 1–30 | 3–80 | 70–220 | 800–1200 |
| Fluxes (L/m h) | 500–10,000 | 100–2000 | 20–200 | 10–100 |
Correlation of membrane features with ranges of separation [ 6 ].
Microfiltration is a membrane separation process for separating a solute having a solute size of about 0.1–10 μm. It is preferable that the membrane used at this time is about 0.01–10 μm in pore size and the pore accounts for about 80% Do. As for the material of the membrane, cellulose-type, nylon, PVC, polytetrafluoroethylene (PTFE), and various other polymer materials are suitable. In a microfiltration process, propulsion is represented by a pressure difference, where the pressure difference is typically 1–30 psig. The separation effect of this membrane is fundamentally dependent on the pore size of the membrane and the size of the substance to be separated. If the size of the substance to be separated is smaller than the pore size, it does not pass through the entire membrane but the substance to be separated is adsorbed on the membrane or is not transmitted by steric hindrance near the pore. The biggest problem of the microfiltration process is the deposition of colloidal material on the membrane surface, which reduces the flow rate by blocking the pores, which can be replaced or regenerated to restore the original state [ 12 , 13 , 14 , 15 ].
Ultrafiltration is a membrane separation process that separates macromolecules or colloidal particles with molecular sizes ranging from 10 to 1000 Å. The pore size ranges from 20 to 500 Å. This method uses a differential pressure as a thrust for the separation operation similar to the reverse osmosis method. The pressure differential used in ultrafiltration is usually in the range of 10–100 psig because particles with a high molecular weight have relatively low osmotic pressure and thus do not require high pressure to apply pressure above osmotic pressure. Ultrafiltration is the same as reverse osmosis in mathematical modeling but fundamentally different from reverse osmosis. The reverse osmosis is largely governed by the correlation between the membrane and the dissolved salt, whereas ultrafiltration is dominated by the solute and pore size. In other words, ultrafiltration has a separation effect by the steric hindrance at the micropore inlet and the frictional resistance between the solute and the pore wall in the pore. The molecular weight cut off (MWCO) in the ultrafiltration method is an important item. The closer the slope is to infinity, the narrower the fractional molecular weight distribution which can be regarded as an excellent filter membrane. Ultrafiltration has a wide range of industrial applications in the middle of reverse osmosis and microfiltration in terms of the size of the separation object. The membrane material is the same as the material of the reverse osmosis membrane and has only a large pore size in terms of being hydrophilic [ 16 , 17 , 18 ].
Nanofiltration is the process of treating hundreds to thousands of molecules with medium molecular weight, the range of treatment of reverse osmosis membranes and ultrafiltration membranes. Nanofiltration is used for separation of small solvent molecules due to deformation of reverse osmosis membrane, but even large molecules of polysaccharides such as sugar can be separated. Nanofiltration membranes usually have a fractional molecular weight of 20–70% NaCl and organic solvents of 200–500. This fractional range corresponds to a diameter of about 10 Å, or 1 nm, of the molecule. This membrane is used for seawater treatment in the pressure range of 0.4–0.7 MPa which is 1/4–1/2 of the reverse osmosis pressure. The exclusion mechanism is similar to reverse osmosis and is widely applied to the separation of salt and organic matter of appropriate molecular weight. The nanofiltration membranes can be used at a rate of 50–97.5% at the same time and are used to replace the ion exchange method in the water softening process [ 19 , 20 , 21 ].
Reverse osmosis is a membrane separation process that separates solutes smaller than 10 Å in size of ions and molecules and was industrialized in seawater desalination and wastewater treatment in the 1970s. The membranes are composed of asymmetric cellulose acetate or aromatic polyamide which is formed as an active layer for a separating effect on the supporting layer. Recently, a composite membrane capable of removing up to 99% of dissolved salts has been developed. The composite membrane is formed of a polymer thin film having a high salt removal effect on the support layer. The support membrane is mainly composed of polysulfone having high mechanical strength and chemical resistance, and cellulose triacetate and cross-linked polyether are mainly used as the separation layer. Since the reverse osmosis membrane has almost no pores, it can be regarded as a nonporous membrane, which is permeated through the gap between micelles forming organic polymers or micelles. In the reverse osmosis method, since the dielectric constant of the organic polymer is low, the dissolved salt is not adsorbed to the membrane. In addition, in high pressure (800–1500 psig), water, which is a solvent, permeates in proportion to the osmotic pressure difference. The separation effect is increased. Since the reverse osmosis is not a separation operation according to the molecular size, deposition of organic substances such as microfiltration and ultrafiltration is less and consequently, the lifetime of the membrane is increased. Reverse osmosis membranes are being used not only for separation and removal of dissolved salts but also for separation of organic and aromatic hydrocarbons with low molecular weight [ 22 , 23 , 24 ].
Treatment and the reusing of wastewater are mainly based on the activated sludge process. In the activated sludge process, the amount of generated sludge is large, the treatment cost is high, and it is vulnerable to impacts such as biological oxygen demand (BOD) overload and toxicity, and problems such as sludge bulking occur. However, the membrane bioreactor (MBR) does not need to regulate the amount of microorganisms in the reactor and does not cause the sludge expansion phenomenon. In addition, it has excellent durability against load generated in the operation such as impact, toxicity, and organic load. It is expected that the technology of the MBR process will increase gradually because of the advantages of this separation membrane process [ 25 , 26 ].
In most of the domestic large and medium-sized water purification facilities, using river water or lake water as a water source, problems occur periodically. However, the conventional water treatment methods such as coagulation, sedimentation, filtration, and disinfection processes are inferior in taste and odor. There is a limit in effectively controlling harmful organic substances and the like. In order to overcome the limitations of this conventional treatment method, microfiltration or ultrafiltration using a membrane has been shown to be a breakthrough technology that can replace the existing rapid sand filtration system because it completely removes turbidity and pathogenic microorganisms. In addition, it can easily combine with the existing unit-altitude water treatment process such as ozone-activated carbon to optimize the process configuration suitable for the characteristics of raw water and water quality, and it is more compact and easier to maintain than the existing water treatment method [ 27 , 28 ].
Techniques for converting seawater to fresh water include conventional coagulation, coagulation, sedimentation, single- or two-phase granular filtration, dissolved air flotation, and low-pressure membrane filtration techniques using microfiltration or ultrafiltration membranes. Conventional pretreatment processes are generally used in seawater desalination, but it is difficult to completely remove float or colloidal particles, which makes it difficult to supply water in a stable manner. However, when the membrane is pretreated, it has the advantage of reducing the cost required in the desalination process, such as increasing the permeation flow rate, reducing the washing cycle, reducing the use of washing chemicals, reducing energy consumption, and reducing maintenance costs [ 29 , 30 ].
A membrane electrode assembly (MEA), which is one of the most important components among the separation membranes used in the ion exchange process, includes a proton exchange membrane fuel cell (PEMFC). The membrane consists of two electrodes, cathode and anode, which determine the performance of the fuel cell [ 31 , 32 , 33 ]. Currently, the most widely used hydrogen ion exchange membranes are produced by DuPont’s nafion® as well as Dow Chemical, 3 M, and others. The perfluorinated proton exchange membrane has been applied to most commercial fuel cell devices due to its high chemical/mechanical stability and excellent hydrogen ion transfer ability. However, since the production process requires high temperature/high pressure conditions, the production cost has increased. The limitation is that it has a pollution problem, and there is a problem that performance is reduced at high temperature due to low glass transition temperature [ 34 , 35 ]. Therefore, research on the production of a hydrocarbon-based proton exchange membrane having a relatively high manufacturing cost and high thermal stability has been actively pursued as an alternative thereto [ 36 , 37 , 38 , 39 ].
In this review, the water treatment membranes are divided into wastewater treatment membranes, water treatment membranes, seawater desalination membranes, and ion exchange membrane separators. Through analysis of domestic and foreign patent information and publication of papers, technical trends and recent technology trends, And to analyze the trend of technology development of water treatment membranes by summarized graphs.
2. Analysis of patents and articles on water treatment membranes
In this chapter, we show all patents and papers for water treatment membranes. In the past 20 years (1995–2014), we have divided membranes for wastewater treatment, separation membranes for water treatment, and seawater desalination membrane trends. In evaluating the competitiveness of patent technology, patents filed after 2015 are analyzed only as valid patents before 2015 except for the fact that they were undisclosed. The patent database of WIPS was used for the analysis of the patent. The patent data were searched through the keyword search and the secondary classification was performed using the library method for noise and pattern removal. Finally, a final classification was carried out by experts in each field. The patent activity index (PAI), the patent intensity index (PII), the patent market-power index (PII), the patent market power index (PMI), and patent citation index (PCI) were the categories. These terms can be defined as follows. Patent activity is defined as the absolute number of patent applications based on the number of public/patent publications issued by the Patent Office. Patent concentration refers to providing information about the technological innovation activities that a country concentrates on relative to other countries. The patent market power refers to the use of patents as an indicator of the patentability of patents when the number of family patents is large, because patents are applied only when they are in commercial profit or for technology competition in the relevant country. Finally, patent impact is the measure of the impact of the patent on future patents, and the US patent with patent information for the patents is targeted [ 40 , 41 , 42 , 43 ].
The number of patent applications has been evaluated in the 10 countries, including Korea, the USA, Japan, China, and Europe, which are the major developing countries, for patent technology competitiveness. A total of 4629 patent applications were searched. 1144 patents were related to separation membranes for wastewater treatment, 734 patents related to water treatment membranes, 668 patents related to seawater desalination membranes, and 2083 patents related to membranes for ion exchange processes. Figure 4 shows the patent application trends by technology in the last 20 years.

Patent applications 1996–2016 in membrane related technology.
The thesis has been published in the past 20 years (1997–2016), and it has been evaluated for technical competitiveness. 13,506 papers are related to the separation membrane for wastewater treatment, 7958 papers are related to the separation membrane for water treatment, 9524 papers related to separation membrane for desalination, and 16,254 papers related to separation membrane for an ion exchange process. Figure 5 shows the trend of publications by technology in the past 20 years. The database used to analyze the technological competitiveness of the paper is the Scopus paper retrieval system, which collects information by category and country and uses the Bibliometric Activity Index (BAI), Bibliometric Citation Index (BCI), and Bibliometric Intensity Index (BII). These terms can be defined as follows. The thesis activity is the number of absolute dissertations, which shows the corresponding country for the technology divided by the total number of countries. The impact of a paper is defined as an index that provides information that can be compared with other countries in terms of the quality of the paper. Finally, the thesis density can be defined as an index that provides information on the relative concentration of a technological innovation activity in a given technology sector relative to other countries in terms of the number of relative papers published. Major countries participating in the analysis of information are the top 13 countries such as Korea, the USA, Japan, China, and Europe ( Figure 6 ).

Papers publications 1996–2016 in membrane related technology.

Top countries with the highest number of patent application.
2.1. Evaluation of patent technology competitiveness of water treatment membranes
As a result of analyzing the number of applications for separation membrane-related patents, the following results were obtained: Japan (2093 cases), Korea (1134 cases), the USA (585 cases), Canada (135 cases), Germany (97 cases), France (49 cases), Italy (35 cases), the Netherlands (33 cases), and China (31 cases). As for the patent activities, Japan was overwhelmingly ranked high (0.49), followed by Korea (0.27), the USA (0.14), Canada (0.03), and Germany (0.02). Table 2 and Figure 7 show the patents’ utilization rate, patent concentration, patent market power, and patent influence by country.
| Index of evaluation | KR | JP | US | CA | DE | FR | GB | IT | NL | CN |
|---|---|---|---|---|---|---|---|---|---|---|
| PAI | 0.27 | 0.49 | 0.14 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| PII | 4.46 | 3.92 | 3.76 | 3.42 | 3.13 | 3.59 | 3.91 | 2.76 | 3.85 | 3.98 |
| PMI | 0.46 | 0.81 | 1.71 | 2.02 | 2.64 | 2.66 | 3.12 | 3.49 | 2.06 | 1.23 |
| PCI | 0.51 | 0.50 | 1.18 | 1.87 | 0.67 | 0.51 | 1.02 | 2.40 | 0.66 | 0.14 |
An analysis table about PAI, PII, PMI, and PCI by each country.
ISO code: KR, Korea; JP, Japan; US, United States of America; CA, Canada; DE, Germany; FR, France; GB, United Kingdom; IT, Italy; NL, Netherlands; CN, China.
PAI: Patent Activity Index, PII: Patent Intensity Index, PMI: Patent Market-power Index, PCI: Patent Citation Index.

An analysis graph about PAI, PII, PMI, and PCI by each country.
In terms of the degree of patent concentration in each country, Japan tends to concentrate most on water treatment membranes (1.20), Korea on wastewater treatment membranes (1.72), and the USA on ion exchange membranes (1.13)—1.37, 1.39, 1.32, and 1.86 for Canada, Germany, the United Kingdom, and Italy, respectively, for the ion exchange membrane, 1.25 for the wastewater treatment membrane for France and 1.77 and 1.25 for the water treatment membranes.
As a result of analyzing the number of family patents of separation membrane-related patents, the following results were obtained: Japan (5651 cases), the USA (3328 cases), Korea (1743 cases), Canada (910 cases), Germany (852 cases), France (426 cases), Italy (407 cases), the Netherlands (226 cases), and China (127 cases). (11.63), Britain (10.39), France (8.86), Germany (8.78), Netherlands (6.85), Canada (6.74), the United States (5.69) and China (4.10), Japan (2.70), and Korea (1.54). The results of this study are as follows: Italy (3.49), Britain (3.12), France (2.66), Germany (2.64), the Netherlands (2.06), Canada (2.02), the USA (1.71) (0.81), followed by Korea (0.46).
As a result, the total number of patents for separator-related patents was derived from the USA (4238 cases), Canada (1537 cases), Japan (979 cases), Germany (267 cases), Italy (163 cases), France (101 cases), the Netherlands (96 cases), and China (11 cases). The number of registered patents related to membranes was 271 in the USA, 147 in Japan, 62 in Canada, 30 in Germany, 26 in Korea, 15 in France, 12 in the UK, (31.86), Canada (24.79), the United States (15.64), and the United States (15.64), followed by the United States), Britain (13.58), Germany (8.90), Netherlands (8.73), Korea and France (6.73), Japan (6.66) and China (1.83). The results of this study are as follows: Italy (2.40), Canada (1.87), the USA (1.18), the UK (1.02), Germany (0.67), the Netherlands (0.66), Korea and France (0.51), and China (0.14). Among them, Korea ranked 2nd place in patent activity, 10th place in patent market power, and 7th place in patent efficacy, and patent concentration tended to be concentrated on wastewater treatment membrane (1.72) ( Figure 8 ).

Top countries with the highest number of paper publications.
2.2. Evaluation of technology competitiveness of water treatment membranes
As a result of analyzing the number of published papers related to membranes, the results obtained were as follows: the USA (11,435), China (9235), Japan (3183), Korea (3013), Germany (3005), Canada (2370), the United Kingdom (2312), Spain (2296), Australia (2260), Italy (1710), and the Netherlands (1422). Therefore, in the activity of the thesis, the USA (0.24) was the highest, followed by China (0.20), Japan (0.07), Korea (0.06), and Germany (0.06). Table 3 and Figure 9 show the activities of each country, the influence of the thesis, and the concentration of the thesis.
| Index of evaluation | KR | JP | US | CA | DE | FR | GB | IT | NL | CN | IN | CA | ES |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAI | 0.06 | 0.07 | 0.24 | 0.05 | 0.06 | 0.05 | 0.05 | 0.04 | 0.03 | 0.2 | 0.05 | 0.05 | 0.05 |
| BCI | 4.03 | 3.8 | 4.02 | 3.91 | 4.04 | 3.99 | 3.98 | 3.95 | 4.21 | 3.94 | 4.02 | 3.91 | 4.06 |
| BII | 0.05 | 0.06 | 0.31 | 0.06 | 0.08 | 0.06 | 0.06 | 0.04 | 0.04 | 0.11 | 0.04 | 0.06 | 0.04 |
An analysis table about BAI, BII, and BCI by each country.
ISO code: KR, Korea; JP, Japan; US, United States of America; CA, Canada; DE, Germany; FR, France; GB, United Kingdom; IT, Italy; NL, Netherlands; CN, China; IN, India; CA, Canada; ES, Spain.
BAI: Bibliometric Activity Index, BCI: Bibliometric Citation Index, BII: Bibliometric Intensity Index.

An analysis graph about BAI, BII, and BCI by each country.
As a result, the total number of citations for the membrane-related papers was found to be 351,996 in the US, 125,276 in China, 84,777 in Japan, 69,354 in Japan, 66,727 in the UK, 66,492 in Canada, (66,239), Australia (61,057), Korea (60,156), Netherlands (43,128), Spain (41,663), Italy (41,281) and India (40,121), Followed by the United States (0.31), China (0.11), Germany (0.08), Japan / France / Canada / Britain (0.6).
According to the concentration of each country, the USA tends to concentrate the most in the ion exchange process membrane (1.15), China in the wastewater treatment membrane (1.36), and Korea in the seawater desalination membrane (1.19). China, Spain, and Italy are the most important for separating membranes for wastewater treatment, Germany for water treatment membranes, Korea, India, the UK, Australia, and the Netherlands for seawater desalination membranes, USA, Japan, And the researcher.
3. Analysis of patent applicants and papers published by technology
In this chapter, we analyzed the top applicants and papers published by each technology and each classification (separation membranes for wastewater treatment, purification membranes for water treatment, separators for seawater desalination, and separation membranes for ion exchange processes). In the case of patents, applicants from all countries are categorized as applicants according to the section. The applicants are divided into three sections (1995–1999), two sections (2000–2004), three sections (2005–2009) (1997 ~ 2001), two sections (2002 ~ 2006), three sections (2007 ~ 2001), and three sections (2011 ~ 2016), and the changes in the top 13 countries were confirmed.
Looking at the top applicants by overall technology, although the overall strength of Japan can be seen, the number of Korean applicants for technology is gradually increasing (Korea Institute of Energy Research, Woongjin Chemical, LG Chem).
Toray Industries, Japan, ranked second place (31), second place (43), second place (43), and third place (48) (53 cases).
In the case of the thesis, if we look at the top publishing countries by overall technology, the overall strength of the USA is strong, but China and Korea are showing a sharp rise, and Japan is steadily declining. In Korea, it is ranked 13th place (130 cases) in one section, 7th place (538 cases) in two sections, 5th place (890 cases) in three sections, and 3rd place (1455 cases) in four sections.
Figures 10 and 11 and Tables 4 and 5 show the top patent applicants and top papers published by technology in the last 10 years.

Comparison of total number of patent applications of global company in field of different membrane technology between the periods 2006 and 2016.

Comparison of total number of papers publications of global company in field of different membrane technology between the periods 2006 and 2016.
| Ranking | MBR | Water treatment | Desalination | Ion exchange | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Company | Country | Number | Company | Country | Number | Company | Country | Number | Company | Country | Number | |
| 1 | KIST | KR | 9 | Toray Industries | JP | 15 | Toray Industries | JP | 29 | Kuraray | JP | 24 |
| 2 | Sumitomo Electric | JP | 9 | Mitsubishi Rayon | JP | 8 | Toray chemical | JP | 21 | KIER | KR | 22 |
| 3 | General Electric | US | 9 | Toshiba | JP | 8 | LG Chemical | KR | 12 | Asahi Glass | JP | 22 |
| 4 | Toray Industries | JP | 9 | Woongjin Coway | KR | 5 | Sumitomo Electric | JP | 12 | Chlorine Engineers | JP | 21 |
| 5 | Philos | KR | 7 | Toray Chemical | JP | 4 | Woongjin Chemical | KR | 12 | Asahi Kasei Chemicals | JP | 17 |
| 6 | Synopex | KR | 7 | Woongjin Chemical | KR | 4 | KRICT | KR | 8 | Asahi Kasei E-Materials | JP | 17 |
| 7 | Kurita Water | JP | 6 | Aquaporin | DK | 3 | KIER | KR | 8 | Fuji Film | JP | 14 |
| 8 | Palo Alto Research | US | 6 | Kobelco Eco-Solution | JP | 3 | Korea University | KR | 8 | Nitto Denko | JP | 11 |
| 9 | Samsung C&T | KR | 5 | Celgard | US | 3 | Mitsubishi Heavy Ind | JP | 7 | Fuji Film Manufacturing | NL | 11 |
| 10 | Aqua Ecos | JP | 5 | General Electric | US | 3 | KIST | KR | 6 | General Electric | US | 11 |
Patent application company by each technology (past 10 years).
ISO code: KR, Korea; JP, Japan; US, United States of America; DK, Denmark; NL, Netherlands.
| Ranking | MBR | Water treatment | Desalination | Ion exchange | ||||
|---|---|---|---|---|---|---|---|---|
| Country | Number | Country | Number | Country | Number | Country | Number | |
| 1 | China | 1998 | China | 812 | China | 1015 | United States | 1032 |
| 2 | United States | 921 | United States | 751 | United States | 970 | China | 1255 |
| 3 | Spain | 481 | South Korea | 223 | South Korea | 453 | South Korea | 429 |
| 4 | Australia | 405 | India | 219 | Australia | 358 | India | 360 |
| 5 | India | 364 | Australia | 205 | India | 266 | Japan | 294 |
| 6 | South Korea | 350 | Spain | 166 | Spain | 263 | Germany | 249 |
| 7 | Italy | 288 | Canada | 149 | United Kingdom | 199 | Canada | 236 |
| 8 | Canada | 273 | Germany | 136 | Germany | 191 | France | 225 |
| 9 | United Kingdom | 217 | Japan | 134 | Japan | 184 | United Kingdom | 208 |
| 10 | Germany | 210 | United Kingdom | 130 | Netherlands | 157 | Spain | 180 |
| 11 | Japan | 203 | France | 120 | France | 151 | Italy | 141 |
| 12 | France | 185 | Netherlands | 112 | Canada | 143 | Australia | 121 |
| 13 | Netherlands | 154 | Italy | 107 | Italy | 140 | Netherlands | 109 |
Papers application country by each technology (past 10 years).
3.1. Top applicant and posting office of separation membranes for wastewater treatment
Japan is the main applicant of the separation membranes for wastewater treatment. In Korea, the rankings are gradually increasing over time. (Korea Institute of Science and Technology, Korea Advanced Institute of Science and Technology, Woongjin Chemical), 4 (Korea Institute of Science and Technology), 2 (Korea Institute of Science and Technology, Phylos, Sinopec, and Samsung C & T). Among them, Korea Institute of Science and Technology (KIST) ranked eighth place (4 cases) in two sections, third place (7 cases) in three sections, and first place (9 cases) in four sections.
In the case of the top publishing countries, the USA is strong overall, but China has steadily climbed to the top of the four sections, and Japan is steadily declining. In Korea, Korea ranked eighth place (46 cases) in the first section, fourth place (179 cases) in the second section, sixth place (236 cases) in the third section, and sixth place (350 cases) in the fourth section.
3.2. Top applicants and posting authorities for separation membrane for water treatment
The top applicants of water treatment membranes are generally Japan, but in Canada and the UK in the second section, in Korea in the third section and in Korea and the United States and Denmark in the fourth section, It can be seen that patent applications are increasingly being made in various countries. Toray Industries in Japan has filed more than 10 patents in all segments and continues to apply for patents related to separation membranes for water treatment. The Korean applicant has applied for Woongjin Coway (fourth place, 6 cases) in three sections, Woongjin Coway (fourth place, 5 cases) and Woongjin Chemical (sixth place, 4 cases) Can be.
In the case of the top publishing countries, the overall strength of the USA is strong, but China and Korea have shown a sharp rise, and Japan is showing a steady decline. In Korea, there are 12 (17 cases), 1 (5 cases), 3 (7 cases), and 3 (223 cases).
3.3. Top applicant and posting office of seawater desalination membrane
The top applicant for seawater desalination membranes is Toray Industries, Japan, which has filed more than 10 applications in all segments, showing continuous applications for membrane-related desalination membranes. There are four applicants from Korea (Saehan), two from two sections (Saehan and KEPCO), four from three sections (Gwangju Institute of Science and Technology, Woongjin Chemical, Siontech and Hyosung) (LG Chem, Woongjin Chemical, Korea Research Institute of Chemical Technology, Korea Institute of Energy Research, Korea University, Korea Institute of Science and Technology).
In the case of the top publishing countries, the overall strength of the USA is strong but China and Korea have shown a sharp rise and Japan is showing a steady decline. Korea ranked 13th place (14 cases) in the first section, 13th place (53 cases) in the second section, 3rd place (203 cases) in the third section, and 3rd place (453 cases) in the fourth section.
3.4. Top applicant and posting office for separation membranes for ion exchange processes
Asahi Glass is ranked first place in the first section (28 cases), first place in the second section (41 cases), first place in the third section (76 cases) (22 cases), showing a slight decline. In addition, Chlorine Engineers ranked sixth (8 cases) in one section, fourth place (19 cases) in two sections, fifth place (19 cases) in three sections, and fourth place (21 cases) in four sections. The application rose steadily. 1 in the three sections (Samsung SDI) and one in four sections (Korea Institute of Energy Research) in the ion exchange process.
In the case of top publishing countries, the USA has shown a decline but China and Korea have shown a sharp rise. Korea ranked 13th place (53 cases) in the first section, 7th place (207 cases) in the second section, 4th place (325 cases) in the third section, and 4th place (429 cases) in the fourth section.
4. Conclusion
The purpose of this review is to examine the progress of research on the water treatment membranes of each country by evaluating the technical competitiveness of the patent and thesis of the water treatment membranes. In order to evaluate the competitiveness of patent technology, we analyzed the technology using four evaluation items: patent activity, patent concentration, patent market power, and patent influence. Of the 4433 valid patents searched for in relation to water treatment membrane technology, Korea, which belongs to the top 10 countries, ranked second place in patent activity, 10th place in patent market power, and 7th place in patent efficacy, indicating a concentration tendency. As a result of analyzing the top applicants by technology and section, it is found that Japan is much stronger overall, among which Toray Industries, Nitto Denko, and Asahi Glass are among the top applicants. In recent years, however, applications have been actively being made in Korea, such as Korea Institute of Energy Research, Korea Institute of Science and Technology, and Saehan. In addition, some applications related to the technology have appeared in other countries such as the USA and Canada. The competitiveness of patent technology in Korea can be relatively low compared to Japan in relation to technology, but if the trend is steadily rising in the recent years, the competitiveness of patent technology in Korea will sufficiently increase in future.
In case of publishing the thesis, we analyzed the technical competitiveness of the thesis on the related technology by using three evaluation items, thesis activity, thesis influence, and thesis concentration. Among the 47,242 validated papers related to water treatment membrane technologies, Korea ranked among the top 13 countries in terms of thesis activities and ninth in terms of thesis influence, and the concentration of thesis was mostly on seawater desalination membranes (1.19). As a result of analyzing the top ranking countries by technology and sector, overall, the USA has shown stronger strength; China and Korea are rising sharply, and Japan has shown a declining trend. The technology competitiveness in Korea can be relatively low compared to the developed countries in the related technology, but if the trend is steadily rising in the recent years, the competitiveness of paper technology in Korea will sufficiently increase in future.
When the patent application and the publication of the papers are comprehensively judged, the activities of patents and theses are actively carried out, but the qualitative level (market power, influence, concentration) is lower than those of the competitors. Among the four water treatment membrane technologies, Ion Exchange Membrane has the highest concentration of patents, of 0.57, the lowest among the top 10 countries, and its market power and influence remain low. The results of the papers showed a similar tendency as the patent applications. Though the activities of the thesis were recorded in the top 13 of the top applications, the influence of the quality of the thesis was recorded at the lowest level (ninth place). Particularly, in the case of the Republic of Korea, the separation membrane for ion exchange process among the four water treatment membrane technologies has the highest activity rate but the least influence. This is considered to be a result of the high price formation, low durability, commercialization technology, and the formation of a supply system in the ion exchange membrane for the ion exchange process in the domestic market. In order to solve this problem (solar, wind, geothermal, etc.), technological internalization through technological advancement, as well as a system that can secure economic efficiency, is important. In addition, it is necessary to develop pro-market-type products suitable for demand sites such as urban areas and industrial complexes that can respond to the increase in social demand for greenhouse gas reduction and stability of the metropolitan area system. It is necessary to utilize it as a strategy to cope with climate change and power plants and to utilize renewable energy in the medium and long term. Will gradually increase..
Acknowledgments
This study was carried out through the analysis of the data of WIPS, a patent analysis agency, and with the help of Kim Se-Jong, Ph.D.
- 1. Peter H. Global freshwater resources: Soft-path solutions for the 21st century. Science. 2003; 22 :1524-1528
- 2. Hong S, Lee S, Kim J, Kim J, Ju YG. Evolution of RO process for green future. KIC News. 2011; 14 :9-20
- 3. Kim S, Lee J, Nam S. Characterization of PVDF-DBP materials for thermally induced phase separation. Membrane Journal. 2016; 26 :449-457
- 4. Mallevialle J, Odeldaal PE, Wiesner MR. Water Treatment Membrane Processes, 11-11. NY, USA: McGraw-Hill; 1996
- 5. Hwang H, Nam S, Koh H, Ha S, Barbieri G, Drioli E. The effect of operating conditions on the performance of hollow fiber membrane modules for CO 2 /N 2 separation. Journal of Industrial and Engineering Chemistry. 2012; 18 :205-211
- 6. Park J, Kim D, Nam S. Characterization and preparation of PEG-polyimide copolymer asymmetric flat sheet membranes for carbon dioxide separation. Membrane Journal. 2015; 25 :547-557
- 7. Kim D, Nam S. Research and development trends of polyimide based material for gas separation. Membrane Journal. 2013; 23 :393-408
- 8. Strathmann H. Membrane separation process. Journal of Membrane Science. 1981; 9 :121-189
- 9. Hong S, Park J. Hybrid water treatment of tubular ceramic MF and photocatalyst loaded polyethersulfone beads: Effect of organic matters, adsorption and photo-oxidation at nitrogen back-flushing. Membrane Journal. 2013; 23 :61-69
- 10. Park Y, Nam S. Characterization of water treatment membrane using various hydrophilic coating materials. Membrane Journal. 2017; 27 :60-67
- 11. Zhang TC, Surampalli RY, Vigneswaran S, Tyagi RD, Ong SL. Membrane Technology and Environmental Applications, 41-74. VA, USA: American Society of Civil Engineers; 2012
- 12. Choi H, Park H. Preparation of higher reinforced PVdF hollow fiber microfiltration. Membrane Journal. 2010; 20 :320-325
- 13. Laninovoc V. Relationship between type of non solvent additive and properties of polyethersulfone membranes. Desalination. 2005; 186 :39-46
- 14. van de Witte P, Dijkstra P, van den Berg JWA, Feijen J. Phase separation processes in polymer solutions relation to membrane formation. Journal of Membrane Science. 1996; 117 :1-31
- 15. Shim H, Lee Y, Nam S, Choi Y. Depth cartridge filter for industrial liquid filtration. Membrane Journal. 2009; 19 :173-182
- 16. Kim M, Park J. Membrane fouling control effect of periodic water-back-flushing in the tubular carbon ceramic ultrafiltration system for recycling paper wastewater. Membrane Journal. 2001; 11 :190-203
- 17. Fiksdal L, Leiknes T. The effect of coagulation with MF/UF membrane filtration for removal of virus in drinking water. Journal of Membrane Science. 2006; 279 :364-371
- 18. Jang J, Chung Y, Lee Y, Nam S. Preparation and properties of membranes for the application of desalting, refining and concentrating for dye processing. Membrane Journal. 2006; 16 :213-220
- 19. Costa A, Pinho M. Performance and cost estimation of nanofiltration for surface water treatment in drinking water production. Desalination. 2006; 196 :55-65
- 20. Hilal N, Al-Zoubi H, Mohammad A, Darwish N. Nanofiltration of highly concentrated salt solutions up to seawater salinity. Desalination. 2005; 184 :315-326
- 21. Courfia K, Saidou N, Mouhamadou A, Michel F, André D. Performance of nanofiltration (NF) and low pressure reverse osmosis (LPRO) membranes in the removal of fluorine and salinity from brackish drinking water. Journal of Water Resource and Protection. 2011; 3 :912-917
- 22. Dalvi A, Al-Rasheed R, Javeed M. Studies on organic foulants in the seawater feed of reverse osmosis plants of SWCC. Desalination. 2000; 132 :217-232
- 23. Kim S, Woo S, Hwang H, Koh H, Ha S, Choi H, Nam S. Preparation and properties of chlorine-resistance loose reverse osmosis hollow-fiber membrane. Membrane Journal. 2010; 20 :304-311
- 24. Hwang H, Koh H, Nam S. Preparation and properties of cellulose triacetate membranes for reverse osmosis. Membrane Journal. 2007; 17 :277-286
- 25. Won I, Kim D, Chung K. Transmembrane pressure of the sinusoidal flux continuous operation mode for the submerged flat-sheet membrane bioreactor in coagulant dosage. Membrane Journal. 2015; 25 :7-14
- 26. Mayhew M, Stephenson T. Low biomass yield activated sludge: A review. Environmental Technology. 1997; 18 :883-892
- 27. Marcel M. Basic Principles of Membrane Technology. 2nd ed. Dordrecht/Boston/London, USA: Kluwer Academic Publishers; 1996. pp. 157-209
- 28. Watanabe Y, Kimura K, Suzuki T. Membrane application to water purification process in Japan—Development of hybrid membrane system. Water Science and Technology. 2000; 41 :9-16
- 29. Knops F, van Hoof S, Futselaar H, Broens L. Economic evaluation of a new ultrafiltration membrane for pretreatment of seawater reverse osmosis. Desalination. 2007; 203 , 300-306
- 30. Jang H, Kwon D, Kim J. Seawater desalination pretreatments and future challenges. Membrane Journal. 2015; 25 :301-309
- 31. Kim D, Jo M, Nam S. A review of polymer-nanocomposite electrolyte membranes for fuel cell application. Journal of Industrial and Engineering Chemistry. 2015; 21 :36-52
- 32. Kim D, Jeong M, Nam S. Research trends in ion exchange membrane processes and practical applications. Applied Chemical Engineering. 2015; 26 :1-16
- 33. Hwang H, Kim Y, Nam S, Rhim J. Preparation of PVA/PAM/zirconium phosphate membrane for proton exchange membranes. Membrane Journal. 2004; 14 :117-125
- 34. Yu D, Nam S, Yoon S, Kim T, Lee J, Nam S, Hong Y. Edge protection using polyacrylonitrile thin-films for hydrocarbon-based membrane electrode assemblies. Journal of Industrial and Engineering Chemistry. 2015; 28 :190-196
- 35. Jeong M, Nam S. Reviews on preparation and membrane applications of polybenzimidazole polymers. Membrane Journal. 2016; 26 :253-265
- 36. Kim D, Hwang H, Jung S, Nam S. Sulfonated poly(arylene ether sulfone)/laponite-SO 3 H composite membrane for direct methanol fuel cell. Journal of Industrial and Engineering Chemistry. 2012; 18 :556-562
- 37. Lee S, Kim H, Nam S, Park C. Synthetic strategies for high performance hydrocarbon polymer electrolyte membranes (PEMs) for fuel cells. Membrane Journal. 2016; 26 :1-13
- 38. Lee D, Kim S, Nam S, Kim H. Synthesis and ion conducting properties of anion exchange membranes based on PBI copolymers for alkaline fuel cells. Membrane Journal. 2010; 20 :217-221
- 39. Park C, Nam S, Hong Y. Molecular dynamics (MD) study of proton exchange membranes for fuel cells. Membrane Journal. 2016; 26 :329-336
- 40. Woo CH. Study on Matrix Module for Predicting of Emerging ICT Technology [master degree dissertation]. Asan, Korea: Hoseo University; 2014
- 41. No SY, Jang GY, Kim MJ, Lee JW. 2009 National R&D Patent Performance Survey, Analysis Report. Korea: KIPO; 2009. pp. 109-110
- 42. Development of Core Components and Element Technology for Wearable Smart Device. Korea: KISPEP; 2016. pp. 232-233
- 43. Analysis of ICT Technology Competitiveness using Quantitative Information for 2015. Korea: IITP; 2015. pp. 11-15
© 2018 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Edited by Murat Eyvaz
Published: 19 September 2018
By Saad Alami Younssi, Majda Breida and Brahim Achiou
2277 downloads
By Jesús Álvarez-Sánchez, Griselda Evelia Romero-Lópe...
1240 downloads
By Magdi Osman Ali Hamed
1224 downloads
Reverse Osmosis Technology, its Applications and Nano-Enabled Membrane

- Abia State University

- Michael Okpara University of Agriculture, Umudike

- Akanu Ibiam Federal Polytechnic, Unwana
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- CHEMOSPHERE

- Lourdes Castro Mieles

- Ifeanyi Emmanuel Chukwunyere
- J Coast Conservat

- Deny Juniwati

- Trioso Purnawarman
- Zhang Shuqi

- A Prasanna Gracy
- K Berlin Asha
- Magori Jackson Nyangi

- John H. Lienhard V

- Kulkarni G. S
- J MEMBRANE SCI

- A. Pozderović

- Robert J. Petersen

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Novel membrane separation technologies and membrane processes
- Published: 24 April 2021
- Volume 15 , pages 717–719, ( 2021 )
Cite this article

- Yanying Wei 1 ,
- Gongping Liu 2 ,
- Jianquan Luo 3 ,
- Libo Li 1 &
1368 Accesses
18 Citations
Explore all metrics
Article PDF
Download to read the full article text
Avoid common mistakes on your manuscript.
Wang W, Wei Y, Fan J, Cai J, Lu Z, Ding L, Wang H. Recent progress of two-dimensional nanosheet membranes and composite membranes for separation applications. Frontiers of Chemical Science and Engineering, 2021, 15(4): 793–819
Google Scholar
Qu K, Huang K, Xu Z. Recent progress in the design and fabrication of MXene-based membranes. Frontiers of Chemical Science and Engineering, 2021, 15(4): 820–836
Liu Q, Chen M, Mao Y, Liu G. Theoretical study on Janus graphene oxide membrane for water transport. Frontiers of Chemical Science and Engineering, 2021, 15(4): 913–921
Zhao D, Chen H, Wang Y, Li B, Duan C, Li Z, Li L. Molecular dynamics simulation on DNA translocating through MoS 2 nanopores with various structures. Frontiers of Chemical Science and Engineering, 2021, 15(4): 922–934
Li X, Tan S, Luo J, Pinelo M. Nanofiltration for separation and purification of saccharides from biomass. Frontiers of Chemical Science and Engineering, 2021, 15(4): 837–853
Download references
Author information
Authors and affiliations.
School of Chemistry & Chemical Engineering, South China University of Technology, Guangzhou, 510640, China
Yanying Wei & Libo Li
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing, 211816, China
Gongping Liu
State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100190, China
Jianquan Luo
State Key Laboratory of Chemical Engineering, School of Chemical Engineering, East China University of Science and Technology, Shanghai, 200237, China
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Yanying Wei , Gongping Liu , Jianquan Luo , Libo Li or Zhi Xu .
Additional information
We sincerely thank the Editors-in-Chief and editorial team to assemble this Special Issue and hope the readers enjoy the articles.
Prof. Yanying Wei received her Ph.D. degree under the supervision of Prof. Haihui Wang at South China University of Technology in 2013. During 2013–2015, she worked as a Humboldt research fellow in Prof. Juergen Caro’s group at Leibniz University of Hannover. She is currently a professor in the School of Chemistry & Chemical Engineering at South China University of Technology. Her research focuses on novel 2D nanosheets membranes, MOF membranes for various separation and catalytic membrane reactors. She has published over 70 peer-reviewed journal papers at Nat. Sustain., Nat. Commun., Sci. Adv., JACS, Angew. Chem. Int. Ed. , etc., 2 co-authored monographs and held 39 patents.
Prof. Gongping Liu received his Ph.D. degree under the supervision of Prof. Wanqin Jin at Nanjing University of Technology in 2013, and then joined Nanjing Tech University as an assistant professor. During 2015–2017, he worked as a postdoctoral fellow in Professor William J. Koros group at Georgia Institute of Technology. He is currently a professor in the College of Chemical Engineering at Nanjing Tech University. His research focuses on advanced membranes for molecular separations based on mixed-matrix and 2D materials.
Prof. Jianquan Luo received his Ph.D. degrees respectively in biochemical engineering from Institute of Process Engineering, CAS in 2010 and in chemical engineering from University of Technology of Compiegne (UTC), France in 2012. Then, he moved to Technical University of Denmark (DTU) as a postdoctoral fellow (funded by Hans Christian Ørsted postdoc fellowship), and worked in the Center for BioProcess Engineering, DTU Chemical Engineering. At the end of 2014, he returned to Institute of Process Engineering, CAS as a professor. His research interests mainly focus on the design and application of advanced membranes for biomanufacturing and food processing. He has published over 130 SCI tracked publications with 4000 total citations (Google Scholar), 2 co-authored monographs and held 31 patents. His H-index is 32 in Web of Science.
Prof. Libo Li received his B.S. (2001) and M.S. (2005) degrees from Tsinghua University, and Ph.D. (2011) from University of California at Davis (Advisor: Prof. Toby Allen). After working with Prof. Ken Dill at Stonybrook University as a postdoc (2011–2014), he joined South China University of Technology (China) as an Associate Professor, and is currently Full Professor. His research interests focus on simulating ion/molecule transportation in nanochannels, with implications for separation, sensing and energy storage/conversion. He has published over 70 peer-reviewed journal papers at Sci. Adv., Angew. Chem. Int. Ed. , etc.
Prof. Zhi Xu joined East China University of Science and Technology (ECUST) in 2019 as a full professor. He obtained his Ph.D. degree from University of Cincinnati in 2015. Prior to joining ECUST, he has joined University of Cincinnati and University of Oxford as a postdoctoral fellow from 2015 to 2019. His current research interests focus on the synthesis of 2D materials and membrane separation.
Rights and permissions
Reprints and permissions
About this article
Wei, Y., Liu, G., Luo, J. et al. Novel membrane separation technologies and membrane processes. Front. Chem. Sci. Eng. 15 , 717–719 (2021). https://doi.org/10.1007/s11705-021-2053-y
Download citation
Received : 05 January 2021
Published : 24 April 2021
Issue Date : August 2021
DOI : https://doi.org/10.1007/s11705-021-2053-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
2. Membrane Technology for Wastewater Treatment. Basically, a membrane is a barrier which separates two phases from each other by restricting movement of components through it in a selective style [].Membranes have been in existence since the 18 th century. Since then, a lot of improvements have taken place to make membranes better suited for many different applications [].
Authors Scope; Roy and Ragunath (2018) The use of membrane technologies to tackle the sustainability of water and energy. Le and Nunes (2016) Opportunities for application of membrane technologies in sustainable water and energy production, with a focus on the potential application area, present status and challenges faced in research and development of membrane materials.
This research paper intends to highlight the feasibility of various membrane technologies for water treatment in these countries. There are many limitations in developing countries to seek more reliable alternative water treatment processes such as membrane systems. ... Membrane technology will be one of the most feasible alternatives to carry ...
The inclusion criteria cover: (a) paper focus on membrane technology, (b) studies that address how membrane technology contributes in sustainable water resources management, (c) peer-reviewed research articles, (d) review papers, (e) paper published in English language. The exclusion criteria involve: (a) narrative reviews, (b) papers not ...
Membrane technology has emerged as a favorite choice for reclaiming water from different wastewater streams for re-use. This review looks at the trending membrane technologies in wastewater treatment, their advantages and disadvantages. ... Feature papers represent the most advanced research with significant potential for high impact in the ...
Membrane technology encompasses a wide spectrum of separation methods that rely on ... underlining the significance of membrane technology in the energy sector and proposing future research directions. 2 Membrane Technologies for Energy-Saving ... The double-layer photothermal paper-based composite film collects incident light and converts it ...
Since its inception in the 19 th century, microfiltration (MF) has evolved as a membrane-based separation technology for treating various effluents and wastewaters. This review aims to familiarize its readers with general and specific research trends on various topics in MF. The level of research interest has been measured by the number of publications in that area for each year.
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Advanced Membrane Technology ...
Membrane bioreactors (MBRs) have proven to be one of the most effective technologies globally for treating wastewater from different sources 1,2,3.In MBRs operation, wastewater treatment is ...
Membrane Technology and Research, Inc, CA, USA. Search for more papers by this author. Book Author(s): Richard W. Baker, Richard W. Baker. ... This fourth edition of Membrane Technology and Applications provides a fully updated and comprehensive introduction to the science and technology of separation membranes. Chapter 1 is a general ...
Overview. Membranes and Membrane Technologies is a peer-reviewed journal focusing on man-made, engineered membranes and their technologies. Publishes articles on new membrane materials, characterization, and testing of membrane materials. Covers a wide range of areas, from filtration processes to environmental protection.
Membrane technology utilizes this selective permeation to regulate the flow of particles, molecules, and ions for containment, concentration, separation, and/or purification. This technology can improve the process and energy efficiency in the fields of drug delivery, tissue engineering, water filtration, chemical recovery, greenhouse gas ...
This paper is a review on the possibility of using an expanded granular sludge bed reactor (EGSB) coupled with a membrane bioreactor (MBR) and an ultraviolet (UV) system to treat poultry ...
Building on the success of the previous edition, Membrane Technology and Applications Third Edition provides a comprehensive overview of separation membranes, their manufacture and their applications. Beginning with a series of general chapters on membrane preparation, transport theory and concentration polarization, the book then surveys ...
the relevant research fields. In this Special Issue, we included seven review articles and six featured research papers from research groups from China and overseas, including 2D nanosheets membranes separation (MXene membranes, graphene oxide (GO) membranes, MoS 2 membranes) and imprinted membranes, as well as several
The membrane technology has been applied to enhance the food quality and quantity owing to the ease of separating different kinds of juices and milk from water. ... This product is of significant interest in the paper, pulp, and pharmaceutical industries ... Microfiltration membrane processes: a review of research trends over the past decade. J ...
There is growing interest in the food industry to develop approaches for large-scale production of bioactive molecules through continuous downstream processing, especially from sustainable sources. Membrane-based separation technologies have the potential to reduce production costs while incorporating versatile multiproduct processing capabilities. This review describes advances in membrane ...
A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the ...
The core technology of the water treatment membrane is to control the size of pores for membrane performance and is being researched to improve performance. In this chapter, the frequencies of presentation are filed by country, institution, and company through technology competitiveness and evaluation of patents and papers.
On the research scale, > 50,000 research articles related to membrane technology have been published since the 1960s with about two-thirds published between 2008 and 2017, which indicates that membrane technology is growing research area [92]. This book chapter gives an overview of membrane technologies, including the common fabrication methods ...
Osmosis membrane technology. Reverse Osmo sis (RO) i s a method of obtaining pure water from water co ntaining a salt, as in. desalination [1]. It is a water purification technology that uses a ...
We sincerely thank the Editors-in-Chief and editorial team to assemble this Special Issue and hope the readers enjoy the articles. Prof. Yanying Wei received her Ph.D. degree under the supervision of Prof. Haihui Wang at South China University of Technology in 2013. During 2013-2015, she worked as a Humboldt research fellow in Prof. Juergen Caro's group at Leibniz University of Hannover.
Ion-exchange resin upgraded for use in the electronics sector. December 2021 View PDF. More opportunities to publish your research: Browse open Calls for Papers. Read the latest articles of Membrane Technology at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Richard W. Baker. Membrane Technology and Research, Inc., Menlo Park, California, USA. Search for more papers by this author