

Clinical Research Assistant Job Description [Updated for 2024]
In the evolving landscape of healthcare, the importance of Clinical Research Assistants is more pronounced than ever.
As we advance in medical science, there is an escalating demand for diligent individuals who can aid, progress and protect our research endeavors in the clinical field.
But let’s delve deeper: What’s truly expected from a Clinical Research Assistant?
Whether you are:
- A job seeker trying to grasp the essence of this role,
- A hiring manager outlining the ideal candidate,
- Or simply curious about the intricacies of clinical research,
You’re in the right place.
Today, we introduce a customizable Clinical Research Assistant job description template, crafted for easy posting on job boards or career sites.
Let’s dive right into it.
Clinical Research Assistant Duties and Responsibilities
Clinical Research Assistants play a crucial role in clinical trials, undertaking tasks that contribute to the overall execution and success of the research.
They work closely with the clinical research coordinators and investigators in conducting studies.
Their duties and responsibilities include:
- Assist in the design, preparation and coordination of research projects and clinical trials
- Collect, process, and maintain research data and patient information, ensuring confidentiality and compliance with ethical standards
- Perform administrative tasks such as preparing and maintaining forms and documents including consent forms, case report forms, and regulatory documents
- Conduct literature reviews and assist in the preparation of research protocols, manuscripts, and presentations
- Assist in patient recruitment and follow-ups, schedule appointments and coordinate with clinical staff
- Perform basic medical tests under supervision, such as taking blood samples, and ensure that all samples are properly labeled and stored
- Ensure all research is conducted in accordance with the protocol, standard operating procedures, good clinical practice and the applicable regulatory requirements
- Help prepare for audits and respond to audit findings
- Maintain research-related inventory and order supplies as needed
Clinical Research Assistant Job Description Template
We are seeking a dedicated Clinical Research Assistant to join our team.
The ideal candidate will be responsible for assisting with the planning and execution of clinical trials, collecting and analyzing data, and maintaining accurate records.
A strong understanding of medical terminology, good organizational skills, and the ability to work both independently and as part of a team are key to succeeding in this role.
Responsibilities
- Assist in the design, administration, and monitoring of clinical trials.
- Analyze and evaluate clinical data gathered during research.
- Ensure compliance with protocol and overall clinical objectives.
- Prepare and present detailed reports on the progress of ongoing research.
- Assist in the preparation of manuscripts for publication.
- Participate in the design of experiments or field work.
- Ensure all research is compliant with necessary protocols and regulations.
- Assist in the creation and maintenance of databases for data entry.
- Collaborate with team members to ensure trials remain on schedule.
Qualifications
- Proven work experience as a Clinical Research Assistant or similar role.
- Knowledge of the principles and procedures of clinical research.
- Ability to interpret, analyze, and communicate scientific data.
- Strong computer skills, including Microsoft Office Suite (Word, PowerPoint, and Excel).
- Strong critical and analytical thinking skills.
- Excellent written and verbal communication skills.
- BSc degree in Life Sciences or related field.
- Health insurance
- Dental insurance
- Retirement plan
- Paid time off
- Continuing education opportunities
Additional Information
- Job Title: Clinical Research Assistant
- Work Environment: This position is primarily office-based but may require some travel for meetings and site visits.
- Reporting Structure: Reports to the Clinical Research Coordinator or Clinical Research Manager.
- Salary: Salary is based upon candidate experience and qualifications, as well as market and business considerations.
- Pay Range: $45,000 minimum to $65,000 maximum
- Location: [City, State] (specify the location or indicate if remote)
- Employment Type: Full-time
- Equal Opportunity Statement: We are an equal opportunity employer and value diversity at our company. We do not discriminate on the basis of race, religion, color, national origin, gender, sexual orientation, age, marital status, veteran status, or disability status.
- Application Instructions: Please submit your resume and a cover letter outlining your qualifications and experience to [email address or application portal].
What Does a Clinical Research Assistant Do?
Clinical Research Assistants work in a variety of settings, including hospitals, laboratories, pharmaceutical companies, and universities.
Their main responsibility is to assist with conducting clinical trials, research studies, and experiments.
They work under the supervision of Clinical Research Coordinators or Principal Investigators and are responsible for tasks such as data collection, patient recruitment, and maintaining research databases.
Their job involves preparing and organizing study materials, such as consent forms, questionnaires, and instructions.
They may also be involved in the informed consent process, explaining study objectives, risks, and benefits to potential participants.
Clinical Research Assistants also play a crucial role in collecting and processing samples, such as blood or tissue, and recording data accurately and efficiently.
They must also ensure adherence to good clinical practices and regulatory guidelines to ensure the integrity of the study and the safety of the study participants.
In some instances, they may also assist with data analysis and contribute to the preparation of research reports or scientific publications.
Clinical Research Assistant Qualifications and Skills
A proficient Clinical Research Assistant should have qualifications and skills that align with your job description, such as:
- Strong knowledge of clinical research principles, guidelines, and regulations to ensure compliance in all research activities.
- Excellent attention to detail and organizational skills for managing multiple projects, maintaining accurate documentation, and ensuring data integrity.
- Proficiency in using medical and research software for data entry, analysis and report generation.
- Strong written and verbal communication skills for interaction with research participants, healthcare professionals, and members of the research team.
- Interpersonal skills to establish rapport with patients and research subjects, ensuring their comfort and cooperation during research studies.
- Problem-solving skills to identify and address issues that may impact research outcomes or violate research protocols.
- Physical stamina for long hours of work, often on their feet, and the ability to handle potentially stressful situations.
- Experience or training in medical terminology, patient care, or a related field to understand and interpret clinical data and interact effectively with healthcare professionals.
Clinical Research Assistant Experience Requirements
Candidates for a Clinical Research Assistant position are typically expected to have at least 1 to 2 years of relevant experience, often acquired through internships, part-time roles, or volunteer work in clinical research settings.
These roles could include Clinical Research Intern, Data Entry Clerk in a medical setting, Medical Scribe, or Clinical Laboratory Assistant.
In these positions, aspiring Clinical Research Assistants can gain hands-on experience with tasks like data collection, patient interactions, research protocol adherence, and administrative duties.
Candidates with more than 2 to 3 years of experience in these or similar roles may have developed specialized skills or knowledge in certain areas of clinical research.
They may be accustomed to handling more responsibilities, including managing smaller projects or assisting with the coordination of research studies.
Candidates with over 5 years of experience may have had the opportunity to lead or supervise a small research team, manage multiple projects simultaneously, or collaborate closely with senior researchers or clinicians.
These individuals may be ready for a more senior or managerial role within the field of clinical research.
Advanced degrees, such as a Master’s or PhD, while not always a requirement, can often compensate for the lack of work experience, especially in roles that involve more sophisticated research procedures or data analysis tasks.
Clinical Research Assistant Education and Training Requirements
Clinical Research Assistants typically hold a bachelor’s degree in a life science, nursing, or a related field.
They must have knowledge of clinical research protocols and principles along with an understanding of medical terminology.
Some positions may prefer candidates with a master’s degree in a relevant field such as clinical research, biotechnology, or public health.
Clinical Research Assistants are expected to be familiar with data collection methods, regulatory guidelines and patient care procedures.
There are also certain certifications like Certified Clinical Research Professional (CCRP) or Certified Clinical Research Coordinator (CCRC) that can enhance a candidate’s profile.
Continuing education is crucial in this field to stay updated with the latest research methodologies and regulatory changes.
Some employers may also require Clinical Research Assistants to have basic CPR or ACLS certification for emergency situations.
Experience in a clinical or research setting, while not always a requirement, can also be beneficial.
Overall, they should possess strong organizational, communication, and analytical skills, along with a keen eye for detail.
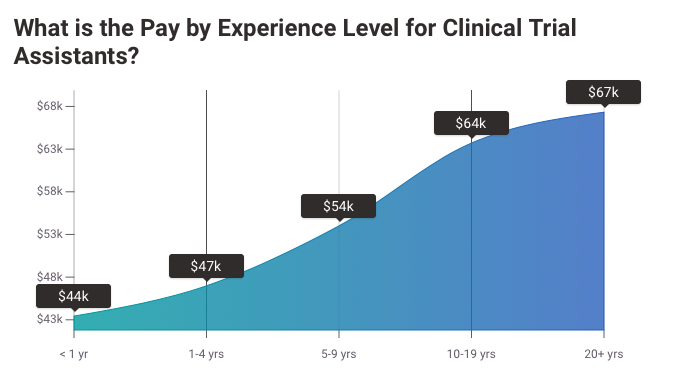
Clinical Research Assistant Salary Expectations
The average salary for a Clinical Research Assistant is $44,905 (USD) per year.
However, the actual salary can vary widely based on the individual’s experience, educational qualifications, location, and the organization they work for.
Additionally, certain specialized skills may also contribute to higher pay in this role.
Clinical Research Assistant Job Description FAQs
What skills does a clinical research assistant need.
Clinical Research Assistants should have excellent attention to detail, as their work often involves collecting and analyzing complex data.
They should possess strong communication and interpersonal skills to work efficiently with other research staff and interact with participants.
They must also have good organizational skills to manage multiple tasks and deadlines effectively.
Knowledge of clinical practices, data management and basic statistical analysis is also beneficial.
Do Clinical Research Assistants need a degree?
Most Clinical Research Assistant roles require a bachelor’s degree in a life science or health-related field.
Some positions may prefer candidates with a master’s degree or significant work experience.
It’s also beneficial to have knowledge of Good Clinical Practice (GCP) guidelines and the regulations that govern clinical research.
What should you look for in a Clinical Research Assistant resume?
A Clinical Research Assistant resume should demonstrate a strong background in life sciences or health care.
Look for experience in clinical research, data management, and compliance with research regulations.
Skills in data analysis, clinical protocol development, and patient interaction are also beneficial.
Certifications, such as a Certified Clinical Research Professional (CCRP) or similar, can also be a plus.
What qualities make a good Clinical Research Assistant?
A good Clinical Research Assistant is detail-oriented and has a strong understanding of scientific research methods.
They should be able to multitask, manage their time effectively, and work independently.
Good communication and interpersonal skills are essential for coordinating with other team members and interacting with study participants.
Integrity and a commitment to ethical practices are also vital in this role.
What are the daily duties of a Clinical Research Assistant?
A Clinical Research Assistant typically starts their day by reviewing the tasks and deadlines for ongoing studies.
They may spend a significant part of their day collecting, processing, and managing patient data.
They may also prepare materials for research studies, interact with study participants, and assist in the development of research protocols.
They often work closely with other team members to ensure the smooth running of clinical trials.
So there you have it.
Today, we pulled back the veil on what it truly means to be a clinical research assistant .
And guess what?
It’s not just about collecting data.
It’s about paving the way to medical breakthroughs, one research study at a time.
With our handy clinical research assistant job description template and real-world examples, you’re ready to take the next step.
But why stop there?
Go even further with our job description generator . It’s your ultimate guide to creating pinpoint-accurate listings or meticulously fine-tuning your resume to perfection.
Every research data point contributes to a larger discovery.
Let’s make those breakthroughs. Together.
How to Become a Clinical Research Assistant (Complete Guide)
Prestige and Paychecks: The Jobs That Define Success
AI’s Employment Expedition: The Jobs It’s Exploring Next
Wave Goodbye to Worry: The Most Stress-Free Jobs Out There
Oddball Occupations: The Weirdest Jobs in Existence
The Editorial Team at InterviewGuy.com is composed of certified interview coaches, seasoned HR professionals, and industry insiders. With decades of collective expertise and access to an unparalleled database of interview questions, we are dedicated to empowering job seekers. Our content meets real-time industry demands, ensuring readers receive timely, accurate, and actionable advice. We value our readers' insights and encourage feedback, corrections, and questions to maintain the highest level of accuracy and relevance.
Similar Posts
![clinical research study assistant job description Certified Eating Disorder Specialist Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/05/certified-eating-disorder-specialist-job-description-768x512.webp)
Certified Eating Disorder Specialist Job Description [Updated for 2024]
![clinical research study assistant job description Production Supervisor Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/02/production-supervisor-job-description-768x512.webp)
Production Supervisor Job Description [Updated for 2024]
![clinical research study assistant job description Car Transport Broker Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/car-transport-broker-job-description-768x512.png)
Car Transport Broker Job Description [Updated for 2024]
![clinical research study assistant job description Candidate Experience Manager Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/candidate-experience-manager-job-description-768x512.png)
Candidate Experience Manager Job Description [Updated for 2024]
![clinical research study assistant job description Child Psychologist Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/02/child-psychologist-job-description-768x512.webp)
Child Psychologist Job Description [Updated for 2024]
![clinical research study assistant job description Avionics Quality Assurance Technician Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/avionics-quality-assurance-technician-job-description-768x512.png)
Avionics Quality Assurance Technician Job Description [Updated for 2024]
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- On-demand Video Interviews
- Live Interviews and Scheduling
- Applicant Tracking Software
- Interview Questions & Job Descriptions
- Careers Page Builder
- Assessments, evaluation, testing
- AI Assistant & Virtual Recruiter
- Plagiarism Checker
- Integrations
- Mobile Applications
- Collaboration Tools
- Tutorials & Help Center
- Quick Product Tour (2 min video)
- Free Job Description Templates
- ROI Calculator
- Developer Resources
- API Reference
- System Status
- Interview Prep
- Search for jobs
- Resume Builder
- Resume Templates
- Resume Examples
Clinical Research Assistant Job Description Template
The Clinical Research Assistant job description template provides a general overview of the responsibilities and requirements associated with this role. This template is designed to be customized by organizations seeking to hire a Clinical Research Assistant, with specific criteria and qualifications tailored according to their needs. The role of a Clinical Research Assistant involves supporting clinical research studies and other related projects, with the end goal of improving patient outcomes and advancing medical knowledge.
Job Summary:
A Clinical Research Assistant provides support to clinical trials and research studies conducted by pharmaceutical and biotechnological companies, medical research institutions, and government agencies.
Key Responsibilities:
- Assist with protocol and informed consent form development
- Coordinate efforts for clinical trial activities and data management
- Conduct patient screenings, enrollment, and follow-ups per protocol
- Collect and organize study data and samples as per protocol
- Manage study and patient calendars, schedules, and activities
- Communicate with clinical trial site personnel, investigators, and study monitors
- Ensure compliance with study protocols, regulatory requirements, and internal policies
- Assist with writing and editing of study-related documents
Qualifications:
- Bachelor's degree in life sciences or relevant field
- Experience in clinical research or healthcare settings is preferred
- Knowledge of Good Clinical Practice (GCP) guidelines and regulations
- Strong organizational and communication skills
- Attention to detail, accuracy, and timeliness
- Proficiency in Microsoft Office and data analysis software
- Able to work independently and in a team environment
Job Title and Overview
Qualifications and requirements, responsibilities and duties.
- Conduct patient interviews to gather data for clinical studies
- Assist in the preparation of regulatory submissions, including the maintenance of regulatory files
- Monitor study sites to ensure compliance with protocol and regulatory requirements
- Assist in the management of study databases, ensuring complete and accurate data entry
- Coordinate and schedule research participant visits and procedures
- Assist with the preparation of study documents and presentations
Salary and Benefits
Frequently asked questions on creating clinical research assistant job posting, what are the qualifications required for a clinical research assistant.
A Clinical Research Assistant should have completed a Bachelor’s degree from a medical or science discipline. Candidates should be adept in communication, coordination, and data management. Strong analytical and writing skills are essential. Experience in medical research or clinical research is preferred.
What are the responsibilities of a Clinical Research Assistant?
- Assist in the conduct of clinical trials/ medical research studies
- Collect and manage data and samples
- Patient recruitment and screening
- Assist in regulatory submissions, study progress reports, and other study documents
- Ensure that studies are conducted according to Good Clinical Practice and other regulatory requirements
What should be included in a Clinical Research Assistant job posting?
- Qualifications required
- Job Description and responsibilities
- Work experience required, if any
- Skills required
- Compensation package and benefits
- Location of job
- Date of posting
- Last date for application submission
How long should a Clinical Research Assistant job posting be?
The job posting should be brief and focused. It should not be too long as it may not get read completely. The posting should convincingly convey the key qualifications, job requirements, responsibilities, and compensation package. It is suggested to limit the posting to one to two pages.
In what format should the job posting be posted?
Job posting should be posted in the “job” section of the website in a downloadable text or PDF format. HTML format can also be used for posting the job description. The posting should not include any images, graphics, or multimedia files.
Whom to contact in case of queries?
Queries can be made via email or phone. The contact details should be provided in the job posting. Queries related to the job posting can be directed to the HR team of the organization.
Related Job Descriptions
Start saving time and money on recruiting.
Start today for free to discover how we can help you hire the best talents.

This site uses cookies to make it work properly, help us to understand how it’s used and to display content that is more relevant to you. For more information, see our Privacy Policy
Protect your data
This site uses cookies and related technologies for site operation, and analytics as described in our Privacy Policy . You may choose to consent to our use of these technologies, reject non-essential technologies, or further manage your preferences.
- Resume and Cover Letter
- Research Assistant Job...
Research Assistant Job Description
4 min read · Updated on September 03, 2019
In order to ensure your professional resume will support your goals, use this research assistant job description to inform what you should highlight on your resume.
By reviewing job description examples, you'll be able to identify what technical and soft skills , credentials and work experience matter most to an employer in your target field.
Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives.
May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related area. Knowledge of FDA regulatory requirements is required. Has knowledge of commonly-used concepts, practices and procedures within a particular field. Rely on instructions and pre-established guidelines to perform the functions of the job. Work under immediate supervision. Primary job functions do not typically require exercising independent judgment. Typically reports to a supervisor or manager.
Responsibilities:
Conduct literature reviews
Collect and analyze data
Prepare materials for submission to granting agencies and foundations
Prepare interview questions
Recruit and/or interview subjects
Maintain accurate records of interviews, safeguarding the confidentiality of subjects, as necessary
Summarize interviews
Provide ready access to all experimental data for the faculty researcher and/or supervisor
Request or acquire equipment or supplies necessary for the project
Manage and respond to project related email
Prepare, maintain and update website materials
Supervise undergraduate students working on the research project (maintaining records on assignment completion, acting as liaison/mediator between the undergraduate students and the faculty researcher)
Attend project meetings
Attend area seminars and other meetings as necessary
Summarize project results
Prepare progress reports
Prepare other articles, reports and presentations
Monitor the project budget
Travel to field sites to collect and record data and/or samples as appropriate to the specific objectives of the study
As appropriate to the specified position, code and verify data in accordance with specified research protocol and coding procedures and enter data into a computer database and/or spreadsheet application for subsequent analysis
Develop or assist in the development of interview schedules; contact potential subjects to introduce and explain study objectives and protocol and to arrange interviews, either in person or by telephone
Identify and compile lists of potential research subjects in accordance with study objectives and parameters, as appropriate to the individual position
Conduct and record face-to-face and/or telephone interviews with subjects, in accordance with predetermined interview protocol, data collection procedures and documentation standards
Review and edit data to ensure completeness and accuracy of information; follow up with subjects to resolve problems or clarify data collected
May set up, calibrate and maintain laboratory and/or field research equipment, as specified by the requirements of the study
May lead or guide the work of student employees
Perform miscellaneous job-related duties as assigned
Prepare findings for publication and assist in laboratory analysis, quality control, or data management
Write and contribute to publications
Develop research protocols
Track progress over time
Assist with preparation of all educational and training workshops and evaluation strategies
Engage clinical and community partners in research
Market training and technical assistance resources to clinical partners and academic investigators
Develop assessment and evaluation tools
Compile data for progress reports
Requirements:
Completed degree(s) from an accredited institution that are above the minimum education requirement may be substituted for experience on a year for year basis
High school diploma or equivalent; college degree preferred
Research Assistant top skills & proficiencies:
Communication
Attention to detail
Critical thinking
Technical skills
Statistical and Graphical Analysis of Data
Ability to maintain quality, safety and/or infection control standards
Planning and scheduling
Interviewing
- Data Collection
Related Articles:
7 Signs Your Resume is Making You Look Old
Why a Simple Resume Layout is a Successful Resume
Software Developer Top Needed Skills
See how your resume stacks up.
Career Advice Newsletter
Our experts gather the best career & resume tips weekly. Delivered weekly, always free.
Thanks! Career advice is on its way.
Share this article:
Let's stay in touch.
Subscribe today to get job tips and career advice that will come in handy.
Your information is secure. Please read our privacy policy for more information.
Job Description
Clinical Research Assistant job description
Example clinical research assistant requirements on a job description.
- Bachelor's degree in a related field
- Previous experience in clinical research
- Familiarity with research protocols
- Proficiency in data analysis
- Knowledge of HIPAA regulations
- Ability to work independently
- Strong communication skills
- Excellent attention to detail
- Ability to multitask
- Demonstrated problem-solving skills
Clinical Research Assistant job description example 1
Children's hospital los angeles clinical research assistant job description, clinical research assistant job description example 2, kelly services clinical research assistant job description, clinical research assistant job description example 3, massachusetts general hospital clinical research assistant job description, resources for employers posting clinical research assistant jobs.

Clinical Research Assistant job description FAQs
What are the most common skills on a job description for a clinical research assistant, what does a clinical research assistant do.
Updated March 14, 2024
Editorial Staff
The Zippia Research Team has spent countless hours reviewing resumes, job postings, and government data to determine what goes into getting a job in each phase of life. Professional writers and data scientists comprise the Zippia Research Team.
Related Job Descriptions
- Clinical Assistant Description
- Clinical Associate Description
- Clinical Coordinator Description
- Clinical Project Manager Description
- Clinical Research Associate Description
- Clinical Research Coordinator Description
- Clinical Research Manager Description
- Clinical Research Nurse Description
- Clinical Trial Manager Description
- Coordinator And Research Assistant Description
- Research Administrator Description
- Research Assistant Description
- Research Coordinator Description
- Research Nurse Description
- Research Project Coordinator Description
Clinical Research Assistant Related Hirings
- Hiring A Clinical Assistant
- Hiring A Clinical Associate
- Hiring A Clinical Coordinator
- Hiring A Clinical Project Manager
- Hiring A Clinical Research Associate
- Hiring A Clinical Research Coordinator
- Hiring A Clinical Research Manager
- Hiring A Clinical Research Nurse
- Hiring A Clinical Trial Manager
- Hiring A Coordinator And Research Assistant
- Hiring A Research Administrator
- Hiring A Research Assistant
- Hiring A Research Coordinator
- Hiring A Research Nurse
- Hiring A Research Project Coordinator
Clinical Research Assistant Related Jobs
- Clinical Assistant
- Clinical Associate
- Clinical Coordinator
- Clinical Project Manager
- Clinical Research Associate
- Clinical Research Coordinator
- Clinical Research Manager
- Clinical Research Nurse
- Clinical Trial Manager
- Coordinator And Research Assistant
- Research Administrator
- Research Assistant
- Research Coordinator
- Research Nurse
- Research Project Coordinator
What Similar Roles Do
- Clinical Assistant Responsibilities
- Clinical Associate Responsibilities
- Clinical Coordinator Responsibilities
- Clinical Project Manager Responsibilities
- Clinical Research Associate Responsibilities
- Clinical Research Coordinator Responsibilities
- Clinical Research Manager Responsibilities
- Clinical Research Nurse Responsibilities
- Clinical Trial Manager Responsibilities
- Coordinator And Research Assistant Responsibilities
- Research Administrator Responsibilities
- Research Assistant Responsibilities
- Research Coordinator Responsibilities
- Research Nurse Responsibilities
- Research Project Coordinator Responsibilities
- Zippia Careers
- Executive Management Industry
- Clinical Research Assistant
- Clinical Research Assistant Job Description
Browse executive management jobs

How to Become a Clinical Research Assistant: A Complete Guide to Becoming A CTA with No Experience on Resume

How To Become A Clinical Research Assistant
A complete guide to becoming a clinical trial assistant with no experience on resume.
Clinical Research Assistant
The work of a clinical trial/research assistant (CTA) in clinical research can never be overstated. It is an important career that requires a lot of interest and dedication in order to be successful. If you have developed interest in becoming a CTA, there are certain questions that you must ask yourself. Are you really cut out for this career path? Are you eager to take up more responsibilities in a work place? Can you monitor the trial subject and ensure that the trial is conducted in a safe and ethical manner? If your answers to this questions are yes, then you might just be cut out for the job of a clinical trial assistant.
CCRPS offers the only accredited 5-day, on-demand advanced clinical research assistant certification (ACRAC) course available to help your learn and apply knowledge and increase your chances of 1) getting a job 2) being efficient and successful in your career.
Responsibilities of a Clinical Research Assistant
A clinical trial assistant have a lot of responsibilities and roles to fulfill within a clinical research institute to ensure the success of a project. Some of these responsibilities include:
Maintaining the standard operating procedures (SOP).
Provide regular report updates of the progress of clinical studies to the appropriate personnel.
Planning and conducting of pre-study site evaluation.
Conduct clinical site feasibility and are as well involved in study visibility.
Assess the study subjects to ensure that the appropriate clinical protocols are observed and the trial is in sync with laid down regulations.
Research Assistant Job Description
Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives.
May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related area. Knowledge of FDA regulatory requirements is required. Has knowledge of commonly-used concepts, practices and procedures within a particular field. Rely on instructions and pre-established guidelines to perform the functions of the job. Work under immediate supervision. Primary job functions do not typically require exercising independent judgment. Typically reports to a supervisor or manager.
Minimum Education Requirements For Clinical Trial Assistant
Requirements:
Completed degree(s) from an accredited institution that are above the minimum education requirement may be substituted for experience on a year for year basis
High school diploma or equivalent; college degree preferred
The educational requirement for a clinical research assistant is at the very least a high school diploma or associate degree in a health science. That's the least requirement, although more employers now prefer a B.Sc degree. Even if you don’t have a health science degree. if you took sciences related courses like nursing, life sciences, medical science, biotechnology, you should absolutely let the companies you are applying to know.
Another avenue you can become a clinical trial assistant is through certification. This is possible and is most common for people without formal education in the fields mentioned. Certification can be very demanding as it requires a lot of administrative knowledge in the area of clinical research. Many CTAs move on to become CRCs, CRAs, and administrators.
Skills You Need To Show On Your Research Assistant Resume
To be successful as a clinical research assistant, there are certain skill sets that are required.
A knowledge of the challenges and restrictions involved in the implementation and retention of databases.
A complete understanding of the responsibilities and liabilities involved in the use of humans for trial tests.
An ability to make excellent clinical development plan.
Must be able to ensure that data gotten from clinical trials are accurate and reliable and the legal rights and privacy of the subjects are protected.
Having these above listed skills and being efficient in them make the job of a clinical trial assistant easier and more interesting. Responsibilities:
Conduct literature reviews
Collect and analyze data
Prepare materials for submission to granting agencies and foundations
Prepare interview questions
Recruit and/or interview subjects
Maintain accurate records of interviews, safeguarding the confidentiality of subjects, as necessary
Summarize interviews
Provide ready access to all experimental data for the faculty researcher and/or supervisor
Request or acquire equipment or supplies necessary for the project
Manage and respond to project related email
Prepare, maintain and update website materials
Supervise undergraduate students working on the research project (maintaining records on assignment completion, acting as liaison/mediator between the undergraduate students and the faculty researcher)
Attend project meetings
Attend area seminars and other meetings as necessary
Summarize project results
Prepare progress reports
Prepare other articles, reports and presentations
Monitor the project budget
Travel to field sites to collect and record data and/or samples as appropriate to the specific objectives of the study
As appropriate to the specified position, code and verify data in accordance with specified research protocol and coding procedures and enter data into a computer database and/or spreadsheet application for subsequent analysis
Develop or assist in the development of interview schedules; contact potential subjects to introduce and explain study objectives and protocol and to arrange interviews, either in person or by telephone
Identify and compile lists of potential research subjects in accordance with study objectives and parameters, as appropriate to the individual position
Conduct and record face-to-face and/or telephone interviews with subjects, in accordance with predetermined interview protocol, data collection procedures and documentation standards
Review and edit data to ensure completeness and accuracy of information; follow up with subjects to resolve problems or clarify data collected
May set up, calibrate and maintain laboratory and/or field research equipment, as specified by the requirements of the study
May lead or guide the work of student employees
Perform miscellaneous job-related duties as assigned
Prepare findings for publication and assist in laboratory analysis, quality control, or data management
Write and contribute to publications
Develop research protocols
Track progress over time
Assist with preparation of all educational and training workshops and evaluation strategies
Engage clinical and community partners in research
Market training and technical assistance resources to clinical partners and academic investigators
Develop assessment and evaluation tools
Compile data for progress reports
Where To Reach Out For Trial Assistant Experiences And Internships
Landing that first trial assistant experience or internship can be a stepping stone to a rewarding career in clinical research. In this blog, we'll explore various avenues to find these valuable opportunities and launch your journey in this dynamic field.
Education and Certification:
While not always mandatory, a degree in a life sciences field like biology, health sciences, or nursing can be beneficial. However, even without a degree, you can break into the field. Consider pursuing a certification program offered by organizations like the Association of Clinical Research Professionals (ACRP) to demonstrate your knowledge and commitment.
Finding Trial Assistant Opportunities:
Industry-Specific Platforms: Leverage job boards frequented by the clinical research community. Look for platforms like Society for Clinical Research Associates (SOCRA) or ACRP job boards. These boards often list internship and entry-level positions specifically for Clinical Trial Assistants (CTAs).
General Job Boards: Don't neglect popular job boards like Indeed or LinkedIn. Utilize relevant keywords like "clinical trial assistant internship" or "research assistant" to filter your search and uncover a wider range of opportunities.
University Resources:
Career Services Departments: Many universities have dedicated career centers that assist students in finding internships. Connect with your career advisor to discuss your interest in clinical research and explore internship opportunities within the university or with partnering institutions.
Research Departments: Universities often conduct their own clinical trials. Reach out to professors or research departments to inquire about potential research assistant positions. This can provide valuable hands-on experience.
Government Websites:
Regulatory Agencies: The US Food and Drug Administration (FDA) offers student volunteer programs ([resources for getting experience in clinical research]).
Networking:
Professional Associations: Join associations like ACRP or SOCRA. Attend industry conferences or webinars to connect with professionals, learn about the field, and discover potential internship or job openings.
LinkedIn: Build your professional profile on LinkedIn and connect with individuals working in clinical research. Reach out to them politely and express your interest in gaining experience. Show genuine curiosity and highlight your transferable skills.
Local Directories:
CTAs can leverage online directories to target their search. After obtaining your certification, reach out to request experiences or internships at:
Clinical research organizations (CROs)
Pharmaceutical companies
Biotechnology companies
These directories can be found through professional association websites or a simple online search using terms like "USA clinical trial directory" or "USA CRO directory". Here are some examples:
ClinicalTrials.gov (a comprehensive listing of clinical trials registered in the US)
CRO Directory (searchable directory of contract research organizations)
BioPharmCatalyst (industry resource with listings of clinical trials and CROs)
Volunteering:
Hospitals and Research Institutions: Volunteer at hospitals or research institutions involved in clinical trials. This can provide valuable firsthand experience and build connections with professionals in the field.
Additional Tips:
Tailor Your Resume and Cover Letter: Highlight relevant coursework, volunteer experiences, and any transferable skills that demonstrate your aptitude for the role. Research the specific company or institution you're applying to and tailor your application to their needs.
Be Proactive: Don't wait for opportunities to come to you. Research companies conducting trials in your area and directly contact their clinical research departments. Express your enthusiasm and willingness to learn.
By exploring these avenues and demonstrating your enthusiasm, you'll increase your chances of landing a trial assistant experience or internship and taking that crucial first step towards a fulfilling career in clinical research.
Clinical Trial Assistant Training
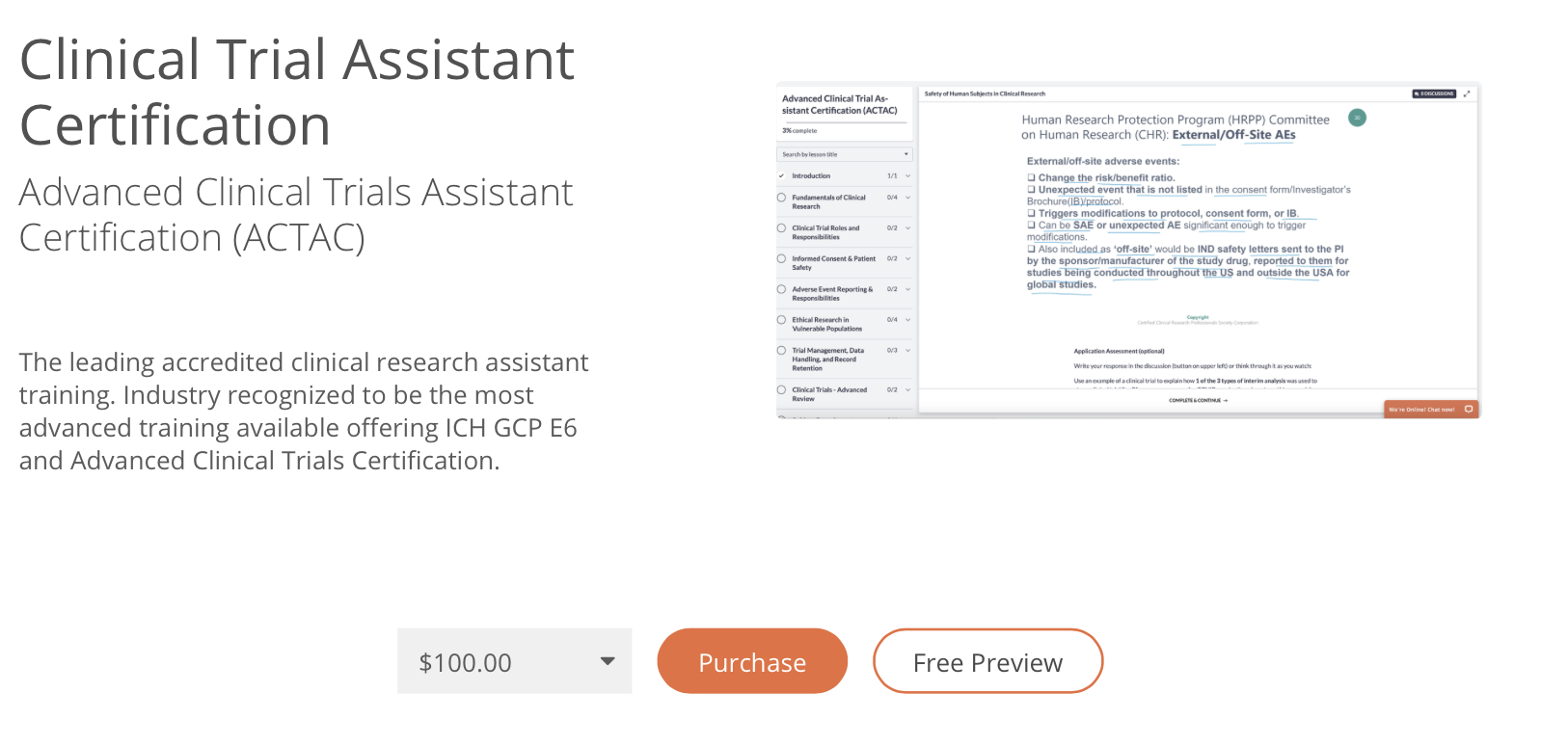
Unlike the hundreds of CTAs who apply to a position, you can give your resume and interview a huge advantage by having certification. CCRPS' offers complete clinical trial assistant training and certification by the ACCRE through our clinical trial assistant training course. Certification as a CTA can help you show competency to work and apply for roles; many students use the course as a way to update their resume and land the interview at the site they desire. If you plan to continue a career in clinical research, ask our 24/7 chat and phone advisors for partial scholarships. We also offer up to 4 month payment plans ($100 per month).
Advanced Clinical Trial Assistant Training: CTA Syllabus CCRPS
DEMO COURSE
Introduction
Accreditation Council For Clinical Research & Education for CCRPS
Fundamentals Of Clinical Research
An Introduction to Clinical Research
An Overview of ICH GCP
Code of Federal Regulations
CFR 21 Part 11
Clinical Trial Roles And Responsibilities
Sponsor/CRO Responsibilities
13 Principles, IRB, & Investigator Roles
Informed Consent & Patient Safety
Informed Consent FREE PREVIEW
Safety of Human Subjects in Clinical Research FREE PREVIEW

Adverse Event Reporting & Responsibilities
Reporting Responsibilities of the Investigators
Adverse Events
Ethical Research In Vulnerable Populations
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated FREE PREVIEW
Ethics of Research Involving Pregnant Women and Fetuses
Ethics of Research Involving Prisoners
Trial Management, Data Handling, And Record Retention
Trial Management – Data Handling and Record Retention
a) Common Terminology Used In Clinical Research
b) Commonly Used Abbreviations and Terms in Clinical Research
Clinical Trials - Advanced Review
Advanced Designs of Clinical Trials
Advanced Review of Phases of Clinical Trials (Preclinical & Phase 0-4)
FREE PREVIEW
Subject Recruitment, Retention, And Compliance
Patient Recruitment in Clinical Trials
Patient Engagement and Retention in Clinical Trials
Patient Adherence and Compliance in Clinical Trials
Misconduct And Fraud
Scientific Misconduct and Fraud
Detecting Falsification
Clinical Trial Assistant Certification Exam
ICH GCP Clinical Trials Assistant Exam (30 Questions)
What To Know For Clinical Trial Assistant Interview Questions
The work of a clinical research assistant is one of extreme importance to the clinical research institute, and employers will to testing to see if you understand what position entails.
Clinical research assistants is to test new medications, therapies and types of treatment and new medical devices to be sure of the safety of their use and the efficacy or efficiency of their work. These clinical trials are very much regulated and seriously monitored to ensure that they comply with the laid down regulations. The need to keep various records, in order to meet up with compliance requirements can be a burden. That is where clinical research assistants come in.
Clinical research assistant are responsible for performing the different safety and quality checks within their unit. Some of these checks are routinely carried out daily, weekly or monthly. The daily checks are usually the first thing they carry out on resuming to work everyday. This is to ensure the safety of all the staffs and volunteers within the clinical research institute and as well improve the quality of the data collected and the results.
The job of a clinical research assistant is to help in finding subjects that can be used for clinical trials, they are responsible for collecting and analyzing the data gotten from clinical tests and trials and they also evaluate the result. They are the ones that keep all the record of activities in the clinical research institute for the purpose of references. They practically ensure that the clinical trial activities are in line with laid down regulations. The amount of data to be collected, evaluated and stored form the trials make this job an important one. That means it is a job in high demand.
Their importance means that there are a variety of places where they can work. Clinical research assistants can work at clinical research institutes, medical centers, pharmaceutical companies, biotech companies and a whole host of other medically and clinically inclined organizations.
The standard equipment like freezers and fridges are checked at least twice daily. This is important because they are used for storing specific research samples and other medications that needs to be kept in controlled temperature and a slight deviation from that can affect the validity of the result and the research. The emergency equipment are also checked regularly on a daily basis.
Part of a clinical research assistant's job is to assist all members of the team and deal with different queries from members of the public. It is also their duty to control all medical stock used in their unit, prepare materials for screening visits, prepare consent forms, questionnaires and information sheets and keeping study files while archiving the files for completed studies.
In the midst of these many duties, it is very important that the clinical research assistant is very capable of multitasking. A good communication skill (both written and verbal) is very important to do this work successfully. One thing that is a must for anyone aspiring to take up this job is to have a keen eye for details. It is also important to be able to ask the right question and develop your knowledge base as much as possible. If you can demonstrate that you have these skills in your interview, you should be all set to go.
Clinical Research Assistant Salary

Per Payscale
The salary of a clinical research assistant can vary depending on different factors like location, institution or employer etc. However, the average yearly salary is $41,000 at an hourly average of $17. It can rise as high as $55,000 or as low as $32,000.
If you'll like to apply for the post of a clinical research assistant, it makes it easier for you if you have B.Sc in life science or social science related courses. If you don't, go enroll for a bachelor's degree and get experiences by volunteering in clinical trials
The purpose of clinical research is to test new medications, therapies, and new medical devices to be sure of the safety of their use and their efficiency. These clinical trials are very much regulated and seriously monitored to ensure that they comply with the laid down regulations. The need to keep various records, in order to meet up with compliance requirements can be a burden.
That is where clinical research assistants come in….
CTA Salary Prediction Provided by Payscale
CCRPS offers the only clinical research assistant certification (ACRAC) course available
The job of a clinical research assistant is to help in finding subjects that can be used for clinical trials, collecting and analyzing the data from clinical tests, and they also evaluate the result.
They are the ones who keep all the record of activities in the clinical research institute for future references. They practically ensure that the clinical trial activities are in line with laid down regulations. The amount of data to be collected, evaluated and stored form the trials make this job an important one. That means it is a job in high demand.
Their importance means that there are a variety of places where they can work. Clinical research assistants can work at clinical research institutes, medical centers, pharmaceutical companies, biotech companies and a whole host of other medically and clinically inclined organizations.
The educational requirements required to work as a clinical research assistant includes a bachelor’s degree, master’s degree or a doctorate degree in life sciences or other medical related sciences.
These are just basic educational requirements, if you are interested in getting into clinical research, you need more than just degrees in life science. Not because they are not important but because they do not offer you the core knowledge and experience needed to be successful in this career. Based on your chosen discipline in clinical research, you can choose to offer courses related to your discipline and you will be taught by seasoned and experience lecturers in the industry keen to pass on their knowledge and experience. You can also register to be a member of clinical research based associations at CCRPS and find more expert information on the clinical research field. All you need to have a rapid career is right here.
CCRPS offers the only accredited 5-day, on-demand clinical research assistant certification (ACRAC) course available to help your learn and apply knowledge and increase your chances of 1) getting a job 2) being efficient and successful in your career.
Principal Investigator Training - Role of Principal Investigator in Clinical Research
Pharmacovigilance: a complete guide to pharmacovigilance and drug safety training.
- MSKCC.org external-link
- Employee login
- Talent Community
Career Areas
- Saved Jobs (0)
- Jobs Search
- Equality, Diversity & Inclusion
- Innovation & Growth
- Our Culture
- Our Locations
- Experienced Professionals
- Administrative
- Advanced Practice Providers
- Allied Health
- Data Science & Engineering
- Digital Informatics & Technology Solutions
- Environmental Health & Safety
- Facilities Management
- Nurse Anesthetists
- Professionals
- Rehabillitation
- Support Services
- Other Career Areas
- Students & Graduates
- Ready To Work
- Anesthesiology & Critical Care
- Pathology & Laboratory Medicine
- Medical Physics
- Neurosurgery
- Psychiatry & Behavioral Sciences

Shape the future of cancer care
Our incredible researchers work tirelessly to discover knowledge and help us shape the future of cancer care. Your discoveries can help change the way the world understands cancer. The knowledge you uncover will result in new strategies to control cancer and could also mean scientific progress in treatment for other diseases and conditions.
Research Opportunities
Discover a new path to conquering cancer.
Whether it’s low-dose radiation or flipping off the ENPP1 switch as a new treatment approach, our researchers are always building on what the world knows about cancer. With your expertise and passion, you can help us advance science and change people’s lives.
Clinical Research
As a pathology data coordinator, research study assistant, specialist, or manager, you could contribute to research ranging from analyzing genetic changes in tumor samples to developing new imaging techniques.
Clinical Research Administration
Clinical Research Administration provides clinical research compliance and operations support for MSK. We are responsible for protocol activation, data management, quality assurance, and information technology.
Laboratory Research
As one of our research technicians or assistants, you'll work on bench research in one of 100+ laboratories.
Research Support
MSK has several opportunities to help drive our research efforts. From animal care technicians to analytical chemistry specialists to bioinformatics experts, this team contributes to innovative science and our mission to cure cancer.
Research & Technology Management
Research and Technology Management (RTM) focuses on three major components research funding and project administration, technology development, compliance review, systems. • Research Funding & Project Administration handles the management of institutional and external research funds and funding proposals. • Technology Development protects and licenses inventions developed by MSK investigators, obtaining industrial funding for our laboratories, and negotiating and reviewing private consulting agreements. • Compliance Review and Oversight is charged with ensuring compliance with federal, state, institutional, and sponsor regulations regarding research grants management. • The RTM systems team creates and manages several electronic systems that support various components of the MSK research enterprise.
Bioinformatics
The Bioinformatics team provides bioinformatics consultation, training, and resource development to Memorial Sloan Kettering investigators and supports the high-performance computing resources of the Computational Biology Center. Bioinformatics jobs include bioinformatics engineer, bioinformatics analyst or scientist, and Unix system administrator.

HEAR FROM OUR EMPLOYEES
” Research fund managers are valuable to MSK because we provide administrative and financial guidance to the research community. ”

” Working in the Early Drug Development Service has been incredibly interesting, and I’ve learned and grown so much since joining the service. ”
A look at life inside
See what it’s like in our Research Department. And perhaps picture yourself there.

Your next opportunity could be right here.
Search Jobs and Apply Now

Clinical Trial Assistant Job Description, Key Duties and Responsibilities
This post provides detailed information on the clinical trial assistant job description, including the key duties, tasks, and responsibilities they commonly perform.
It also highlights the major requirements you may be asked to meet to be hired for the clinical trial assistant role by most recruiters/employers.
Clinical trials play a role in the field of research and drug development.
They serve as a means to assess the safety and effectiveness of treatments for use.
Conducting these studies involves the efforts of individuals with diverse skills and backgrounds. Among these roles clinical trial assistants hold importance.
Clinical trial assistants provide support to research teams by coordinating and managing aspects of clinical trials.
Their responsibilities may vary depending on the phase of the study, therapeutic area and size of the site. However, their overarching role remains integral in ensuring efficient operations throughout the process – from recruiting participants to collecting data.
What Does a Clinical Trial Assistant Do?
Clinical trial assistants play a role that involves supporting research teams throughout the process of executing and managing clinical trials.
Common job titles for individuals in this position include clinical research assistant and clinical study assistant.
Typically, clinical trial assistants report to either a clinical trial manager or a clinical research coordinator.
They work across settings such as pharmaceutical companies, contract research organizations (CROs) academic medical centers, hospitals, and clinics.
A Bachelor’s degree in a science or healthcare field is typically required to qualify for this role.
Moreover, obtaining the Certified Clinical Research Professional (CCRP) certification is highly beneficial.
The clinical trial assistant job description involves participant recruitment, data collection, coordinating study visits, processing samples, and maintaining regulatory binders.
It also entails performing duties such as supply management, budget assistance, ethics submissions, site initiation, and quality control.
The clinical trial assistant role is essential for ensuring clinical trials adhere to protocols and good clinical practice guidelines.
Strong organization, attention to detail, communication, and computer skills are preferred by employers.
Regulatory bodies like the FDA, EMA, MHRA, OHRP, and GCP provide oversight for clinical trials.
Clinical trial assistants are the backbone of research teams, enabling complex clinical studies through their diligent coordination and support.
Clinical Trial Assistant Job Description Example/Sample/Template
The clinical trial assistant job description consists of the following duties, tasks, and responsibilities:
- Assist with participant recruitment activities such as prescreening, informed consent, and enrollment
- Schedule participant study visits and coordinate travel if needed
- Collect clinical trial data and specimens according to protocol
- Process, label, and ship specimens to central labs. Maintain shipment logs
- Administer study drug to participants following dispensing procedures
- Monitor and record adverse events and side effects reported by participants
- Maintain regulatory documents, including ethics submissions, site contracts, and essential documents binders
- Assist with preparing ethics amendment submissions and renewals
- Coordinate investigational product management including inventory, accountability logs, and destruction
- Perform randomization and unblinding procedures per protocol specifications
- Assist with data entry, cleaning, and queries in the EDC system
- Organize site initiation visits and coordinate staff training as needed
- Create study calendars and reminders for participant visits and follow-up
- Manage study supplies and track inventory and reorders
- Submit serious adverse event reports to sponsors and regulatory bodies
- Coordinate shipments of monitoring reports and other study documents
- Assist with preparation of site payments and study expense tracking
- Participate in internal and external audits to ensure GCP compliance
- Perform quality control on case report forms and study documents
- Communicate with study sponsors, CROs, and vendors regarding trial needs
- Maintain study databases, files, and archiving systems
- Participate in study team meetings and trainings
- Provide general administrative support for clinical research staff
- Coordinate translation services for non-English speaking participants
- Provide guidance and assistance to participants throughout trial completion.
Clinical Trial Assistant Job Description for Resume
If you have worked before as a clinical trial assistant or are presently working in that role and are making a new resume or CV, then you can create an effective Professional Experience section by applying the sample clinical trial assistant job description provided above.
You can highlight the duties and responsibilities you have carried out or are currently performing as a clinical trial assistant in your resume’s Professional Experience by utilizing the ones in the clinical trial assistant job description example above.
This will assure the recruiter/employer that you have been successful performing the clinical trial assistant duties and responsibilities and enhance your chances of being hired, especially if the new job that you are seeking requires some work experience as a clinical trial assistant.
Clinical Trial Assistants Requirements: Skills, Knowledge, and Abilities for Career Success
Clinical trial assistants require a specific set of skills, knowledge, and abilities to effectively coordinate and manage clinical trials. These key competencies, which are usually required by recruiters/employers when hiring for the position include:
- Organization and attention to detail: Ability to track and manage large amounts of information and juggle multiple tasks
- Communication skills: Communicate clearly with research participants, staff, sponsors, and vendors
- Problem-solving skills: Identify issues proactively and develop solutions
- Teamwork and collaboration: Work cooperatively with all study team members
- Computer literacy: Proficiency with MS Office, database management, EDC systems
- Analytical thinking: Interpret protocols, collect accurate data, identify errors
- Time management and prioritization: Meet deadlines and manage workload efficiently
- Stress management: Perform well under pressure and manage fluctuating workloads
- Regulatory knowledge: Understand and apply regulations, such as GCP, HIPAA, ISO, etc.
- Clinical research processes: Understand all aspects of clinical research coordination and execution
- Healthcare and scientific knowledge: It is important for clinical trial assistants to have an understanding of the field they are studying
- Participant care skills: Clinical trial assistants should possess compassion and show care when interacting with participants
- Training abilities: They need to be able to provide instructions and training to staff members.
By acquiring these competencies clinical trial assistants can offer support to their research teams and contribute to the success of clinical trials.
The role requires a combination of organizational, communication, regulatory, and interpersonal skills.
Clinical Trial Assistant Salary and Top Paying Locations
Based on data from the U.S. Bureau of Labor Statistics the average annual salary for clinical trial assistants was $58,103 as of May 2022.
Here are some states that offer the best salaries for clinical trial assistants:
- San Francisco, CA ($113,063)
- Seattle, WA ($100,612)
- Boston, MA ($97,756)
- New York, NY ($94,738)
- Elizabeth, NJ ($94,475).
Clinical trial assistants play a role in the execution of research studies. Their coordination and operational support are crucial in making complex trials possible.
Key responsibilities include recruitment, data collection, supply management and regulatory documentation.
Strong organization and communication skills along with an understanding of research contribute greatly to the success of these professionals.
With the growth of the pharmaceutical industry and medical research, there is increasing need for skilled clinical trial assistants.
Their attention to detail, compassion for participants, and grasp of regulations enable the delivery of rigorous, ethical, and successful clinical trials.
Their contributions ultimately help advance healthcare through the development of new treatments.
For those interested in entering this field, a science degree combined with clinical research certificate programs provides ideal preparation.
Hands-on internships and entry-level assistant roles build the experience to advance.
With dedication and the right competencies, clinical trial assistants can find rewarding careers that pave the way for medical discoveries.
This post is helpful to individuals interested in the clinical trial assistant career. They will be able to learn all they need to know about what clinical trial assistants do to decide if that’s the job they want to do.
It is also beneficial to recruiters/employers in making detailed job description for the clinical trial assistant position in their organizations for use in hiring for the role.
Recommended:

This Site Uses Cookies
Privacy overview.
- Job Descriptions
- Healthcare and Medical Job Descriptions
Clinical Research Associate Job Description
Clinical research associates manage clinical trials and studies pertaining to biotechnological and pharmaceutical products, drugs, and procedures. A clinical research associate, also known as a CRA, conducts research to ensure these products are safe to allow on the market.
Try Betterteam
Post your jobs to 100+ job boards
- Reach over 250 million candidates.
- Get candidates in hours, not days.
Clinical Research Associate Job Description Template
We are looking for an organized, flexible clinical research associate to oversee clinical trials. The clinical research associate will develop and outline trial protocols, establish trial sites, train site staff, and manage Investigational Product (IP) and trial materials. The clinical research associate will manage multiple aspects of the subjects' welfare. You will conduct regular site visits, generate and distribute internal and external newsletters, prepare final reports, and liaise with interested parties regarding all trial aspects. You will play a leading role in generating and overseeing documentation and records.
To be successful in this role, you should be able to recognize logistical problems and initiate appropriate solutions. Ideal candidates will be detail-oriented, have the ability to multitask, and be able to collaborate with various role players.
Clinical Research Associate Responsibilities:
- Creating and writing trial protocols, and presenting these to the steering committee.
- Identifying, evaluating, and establishing trial sites, and closing sites down on completion of the trial.
- Training site staff on therapeutic areas, protocol requirements, proper source documentation, and case report form completion.
- Liaise with the ethics committee regarding the rights, safety, and well-being of trial subjects.
- Ordering, tracking, and managing IP and trial materials.
- Overseeing and documenting IP dispensing inventory, and reconciliation.
- Protecting subjects’ confidentiality, updating their information, and verifying IP have been dispensed and administered according to protocol.
- Conducting regular site visits, coordinating project meetings, and writing visit reports.
- Implementing action plans for sites not meeting expectations.
- Liaising with regulatory authorities.
- Ensuring compliance with SOPs and local regulations, and ICH and GCP guidelines.
- Other tasks and responsibilities as needed.
Clinical Research Associate Requirements:
- Bachelor’s degree in biological science or a related field.
- 2+ years of experience as a clinical research associate.
- Knowledge of the pharmaceutical industry, terminology, and practices.
- Knowledge of FDA regulations and their practical implementation.
- Strong verbal and written communication skills.
- Proficient computer skills.
- Proficient with Microsoft Office Word, Excel, and PowerPoint.
- Ability to manage and prioritize workload effectively.
- Available to travel extensively and on short notice, and ability to manage travel schedules, such as flight schedules.
- Valid driver’s license, proficient driving skills, own reliable transport, and up-to-date car insurance.
Related Articles:
Biochemist job description, chemical engineer job description, clinical research associate interview questions, biochemist interview questions, chemical engineer interview questions, clinical research associate resume.
- Skip to main menu
- Skip to user menu
Clinical Research Assistant

- Bachelors of Science degree or equivalent in Psychology, Neuroscience or related field.
- Proficient in computer skills including word processing, data entry, spreadsheet, database management, and entry level statistical/programming skills required.
- Strong clinical skills, including fundamental clinical skills such as establishing rapport, maintaining participant motivation, empathic responding, ability to work effectively as part of a multidisciplinary team, and ability to use clinical judgment in (rare) emergency situations.
- Strong organizational skills, including understanding of HIPAA regulations.
- Ability to organize meetings, and present reports.
- Demonstrated experience working in and fostering a diverse faculty, staff and student environment or commitment to do so as a staff member at VCU.
- Masters degree or equivalent in Psychology, Neuroscience or related field
- Writing experience with first author and co-author peer reviewed manuscripts
- Knowledge of statistical analysis and software applications
- Experience recruiting, consenting, and carrying out study procedures with opioid use disorder (OUD) treatment participants, including during pregnancy and postpartum
- Qualitative research experience, including data collection, coding, analysis
- Ability to set up projects and surveys in REDCap, perform sample size calculations using GPower 3.1, analyze qualitative data (such as with ATLAS.ti.8) and quantitative statistical analysis (such as with SAS 9.4 and GraphPad Prism).
- Experience with preparing and maintaining Institutional Review Board (IRB) and regulatory paperwork to comply with institutional regulatory requirements
- Experience in mentoring and training student research trainees, incorporating them into research teams
Share this job
Get job alerts
Create a job alert and receive personalized job recommendations straight to your inbox.

Assistant Clinical Research Coordinator
🔍 stanford, california, united states.
Stanford University School of Medicine and the Heart Center Clinical and Translational Research Program (CTRP) is seeking an Assistant Clinical Research Coordinator (ACRC) to p erform administrative support duties related to the collection of clinical data and/or the coordination of clinical studies in pediatric cardiology. Work under supervision of the principal investigator and/or study coordinator/supervisor.
Duties include:
· Schedule and/or call subjects for appointments; contact participants with reminders or other requirements.
· Prepare, distribute, and process questionnaires.
· Perform clerical duties in the preparation of regulatory documents. Maintain all forms and documents, including consent forms and master subject logs. File all appropriate correspondence.
· Assist with the screening, recruiting, and obtaining consent of study participants. Review medical records and/or perform telephone or in-person interviews to gather data, as needed.
· Administer standard study questionnaires and tests, score test measurements and questionnaires, and code data for computer entry. Perform quantitative review of forms, tests, and other measurements for completeness and accuracy.
· Extract data from source documents for research studies as directed. Collect data and complete case report forms.
· Prepare, process, and ship specimens/samples accurately under well-defined requirements.
· Order and maintain equipment and supplies.
· Process study compensation payments and thank you letters to subjects upon completion of trial activities. Assist with post-study activities, as needed.
- - Other duties may also be assigned
~ All members of the Department of Pediatrics are engaged in continuous learning and improvement to foster a culture where diversity, equity, inclusion, and justice are central to all aspects of our work. The Department collectively and publicly commits to continuously promoting anti-racism and equity through its policies, programs, and practices at all levels. ~
DESIRED QUALIFICATIONS:
- Excellent oral and written communication skills
- Proficiency in using computers, software, and web-based applications
- Proficiency in English and Spanish (verbal and written) required.
EDUCATION & EXPERIENCE (REQUIRED):
Two-year college degree and one year of relevant experience or an equivalent combination of experience, education, and training.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
General knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
PHYSICAL REQUIREMENTS*:
· Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
· Occasionally sit, reach above shoulders, perform desk based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
· Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
* - Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
· Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious diseases and infections.
· May require extended or unusual work hours, including weeknights and weekends, based on research requirements and business needs.
Position may be required to support studies that are conducted at off-site clinics (within a 50-mile radius of the main campus). Incumbent will need to provide own transportation with the ability to get to/from these off-site clinics.
Stanford University provides pay ranges representing its good faith estimate of what the University reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location, and external market pay for comparable jobs. The pay range for this position working in the California Bay area is $25.48-$31.25.
- Schedule: Full-time
- Job Code: 1012
- Employee Status: Regular
- Department URL: http://pediatrics.stanford.edu/
- Requisition ID: 103246
- Work Arrangement : Hybrid Eligible
My Submissions
Track your opportunities.
Similar Listings
Stanford, California, United States
📁 Research
Post Date: Apr 03, 2024
Post Date: May 02, 2024
Post Date: Mar 19, 2024
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs
Secondary Menu
Clinical research assistant position @ bradley hospital, east providence ri.
Job Descriptions:
Work with the Principal Investigator and collaborators to advance our understanding of the brain and behavior mechanisms underlying psychiatric disorders. In particular there is a focus on autism spectrum disorder mood disorders and sleep difficulties. Under the general supervision of the Principal Investigator assist in the acquisition and analysis of participant information including assessments and magnetic resonance imaging (MRI) scanning. Interview participants to gather information. Prepare and maintain study records. Enter data and participate in qualitative/quantitative scoring and analysis of data. Opportunities to prepare and participate in presentations and posters may be available. Assist with coordination of research activities. This research experience would be helpful for candidates interested in applying for graduate and/or medical school in the future.
Responsibilities:
- Complete research assessments with children and families.
- Assist in identification and follow-up of participants meeting criteria for inclusion in clinical research studies. Ensure protocol eligibility requirements are met.
- Establish and/or maintain study record for each participant. Interview participant and/or family to explain nature of study; conduct telephone interviews. Elicit cooperation and gather information to complete study. Facilitate obtaining signed consent forms. Schedule participant interview to complete documentation. According to established protocol administer standardized and non-standardized research observations and assessments such as intelligence tests. Assist with Institutional Review Board (IRB) applications.
- Review medical records to abstract information per study protocols.
- Monitor adherence to protocol. Follow-up with participant to correct or complete documentation.
- Work within the team to gather MRI neuroimaging data.
- Process data by using Microsoft Excel SPSS and MRI image analysis software. Prepare data for presentation.
- May assist in planning research protocols. May assist in manuscript preparation.
- In collaboration with the Data Specialist and/or Principal Investigator collect and organize participant data into an appropriate format to facilitate data entry and analysis. Enter study information into database.
- May coordinate activities and participate in the training of volunteers or others assigned to research projects to interview participants to complete forms or to perform data abstraction or data entry duties.
- Review literature pertaining to research being conducted in order to better understand project and to gather relevant information.
Requirements:
- Bachelors Degree in Psychology Neuroscience Biological or Behavioral Sciences or related area including courses in research methodologies and statistics.
- Excellent computer and communication (verbal and written) skills.
- Strong organizational & interpersonal skills.
- The ability to work well with children adolescents and parents.
- Availability to regularly work some evenings and Saturdays is required.
- Prior research experience in human subjects (including with children and/or adolescents with neurodevelopmental and/or mental health conditions) psychology neuroscience neuroimaging or a data-driven behavioral science is strongly preferred.
- Minimum of six months of undergraduate or post-graduate research experience. Prior neuroimaging experience is a plus but not required.
Click here for more information and to apply
- Professional development
- Post-graduation
- Diversity, Equity & Inclusion
- Climate Handbook
- P&N Team Resources
- Degree Requirements
- Practicum and Ongoing Research Projects in Psychology
- Research Participation Requirements for Psychology Courses
- Summer Vertical Integration Program (VIP)
- Psychology Courses
- Graduate School Advice
- Career Options
- Forms & Resources
- Global Education
- Trinity Ambassadors
- Co-requisite Requirement
- Neuroscience Courses
- Neuroscience: Undergraduate Research Opportunities
- Neuroscience Research Practicum & Laboratories
- Summer Neuroscience Program
- Research Independent Study in Neuroscience
- Graduation with Distinction
- Frequently Asked Questions
- Neuroscience Teaching Lab
- Student Spotlights
- Neuroscience Graduation 2024 Program
- Other Job Boards
- Student Organizations
- Clinical Psychology
- Cognition & the Brain
- Developmental Psychology
- Social Psychology
- Systems and Integrative Neuroscience
- Admitting Faculty
- Application FAQ
- Financial Support
- Teaching Opportunities
- Departmental Graduate Requirements
- MAP/Dissertation Committee Guidelines
- MAP/Oral Exam Guidelines/Timeline
- Dissertation and Final Examination Guidelines
- Awards for Current Students
- Teaching Resources
- Instructor/TA Guidelines
- Faculty Mentorship Vision Statement
- All Courses
- Psychology: Course Sequence
- Psychology: Methods Courses
- Neuroscience: Course Clusters
- Neuroscience: Courses By Category
- Primary Faculty
- Joint Graduate Training Faculty
- Instructional Faculty
- Secondary Faculty
- Graduate Students
- Postdocs, Affiliates, and Research Scientists
- Faculty Research Labs
- Research News Stories
- Child Studies
- Community Volunteers
- Charles Lafitte Foundation: Funding Support
- Meet Our Alumni
- For Current Students
- Assisting Duke Students
- Neuroscience Graduation 2023 Program
- Psychology Graduation 2023 Program
- Giving to the Department
Clinical Research Lab Assistant - 128814
Job description, #128814 clinical research lab assistant.
UCSD Layoff from Career Appointment : Apply by 3/25/2024 for consideration with preference for rehire. All layoff applicants should contact their Employment Advisor.
Special Selection Applicants : Apply by 4/04/2024. Eligible Special Selection clients should contact their Disability Counselor for assistance.
DESCRIPTION
The Department of Pediatrics is one of the largest departments within the School of Medicine with approximately 250 Faculty, 127 postdoctoral fellows (both MDs and PhDs) along with over 320 support staff (not including hospital staff). In addition, the Department has 68 clinical residents distributed across the Divisions. The missions of research, education and patient care are intertwined, and are integral to the goals of the department.
The Department manages a university-affiliated children's health system with the physicians and leadership of the University of California, San Diego (UCSD), Rady Children's Hospital, and Rady Children's Specialists of San Diego, a Medical Practice Foundation, unifying pediatric patient care, research, education and community service programs.
Under the supervision of the PI, the incumbent will support the clinical research and quality improvement efforts of the study team. Support the clinical research operation; communicate with research participants, schedule visits, assist in the handling and processing of biological samples; shipping of samples for general lab assays and data entry. May be involved in clinical and quality improvement projects that also involve recruitment, and ensuring performance of protocols according to schedule, including data entry and reporting on data results back to participants. Incumbent will assist in the day-to-day operation of the research team. Responsible for assisting with and ensuring the quality of data collected, maintaining databases, ensuring adequate lab supplies for the function of the lab. Under supervision maintain a sample flowchart of active clinical studies and or quality improvement projects and how participants flow through these project protocols as well as monitoring study/project samples/data collections. Will assist clinical team with participant home study visits to collect questionnaire data and clinical specimens. Performs other duties as assigned.
MINIMUM QUALIFICATIONS
Graduation from high school or a General Education Diploma and one year of laboratory or field experience or two years of college including courses in the natural, physical or social sciences; or an equivalent combination of education and experience.
Experience in processing and shipping laboratory specimens-ambient and frozen. Demonstrated experience with guidelines of biohazardous handling. Knowledge and strict utilization of universal precautions when handling laboratory specimens.
Experience managing biological samples, storage, and inventory.
Attention to detail and strong organizational skills.
Proven effective interpersonal, oral, and written communication skills.
Ability to prioritize workload and work independently.
Ability to maintain and protect confidentiality of participant information.
Excellent problem-solving skills with ability to propose alternative strategies to resolve issues.
Must be IATA-certified to process and ship human specimens.
Certified in hazardous materials handling.
PREFERRED QUALIFICATIONS
- Graduation from college including courses in the natural, physical or social sciences and Clinical Research experience.
SPECIAL CONDITIONS
Employment is subject to a criminal background check and pre-employment physical.
Must be willing to work flexible hours to include early morning and weekends.
Must be willing and able to work with biohazardous materials.
Must be IATA certified to process and ship human specimens.
Position will be based in San Diego and may require travel for home visits.
Must be willing to be vaccinated against common diseases encountered in health care.
Must be willing to carry and promptly respond to a cell during working hours.
Pay Transparency Act
Annual Full Pay Range: $40,340 - $46,771 (will be prorated if the appointment percentage is less than 100%)
Hourly Equivalent: $19.32 - $22.40
Factors in determining the appropriate compensation for a role include experience, skills, knowledge, abilities, education, licensure and certifications, and other business and organizational needs. The Hiring Pay Scale referenced in the job posting is the budgeted salary or hourly range that the University reasonably expects to pay for this position. The Annual Full Pay Range may be broader than what the University anticipates to pay for this position, based on internal equity, budget, and collective bargaining agreements (when applicable).
If employed by the University of California, you will be required to comply with our Policy on Vaccination Programs, which may be amended or revised from time to time. Federal, state, or local public health directives may impose additional requirements. If applicable, life-support certifications (BLS, NRP, ACLS, etc.) must include hands-on practice and in-person skills assessment; online-only certification is not acceptable.
UC San Diego Health Sciences is comprised of our School of Medicine, Skaggs School of Pharmacy and Pharmaceutical Sciences, The Herbert Wertheim School of Public Health and Human Longevity Science, and our Student Health and Well-Being Department. We have long been at the forefront of translational - or "bench-to-bedside" - research, transforming patient care through discovery and innovation leading to new drugs and technologies. Translational research is carried out every day in the hundreds of clinical trials of promising new therapies offered through UC San Diego Health, and in the drive of our researchers and clinician-scientists who are committed to having a significant impact on patient care. We invite you to join our team!
Applications/Resumes are accepted for current job openings only. For full consideration on any job, applications must be received prior to the initial closing date. If a job has an extended deadline, applications/resumes will be considered during the extension period; however, a job may be filled before the extended date is reached.
To foster the best possible working and learning environment, UC San Diego strives to cultivate a rich and diverse environment, inclusive and supportive of all students, faculty, staff and visitors. For more information, please visit UC San Diego Principles of Community .
UC San Diego is an Equal Opportunity/Affirmative Action Employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, age or protected veteran status.
For the University of California’s Affirmative Action Policy please visit: https://policy.ucop.edu/doc/4010393/PPSM-20 For the University of California’s Anti-Discrimination Policy, please visit: https://policy.ucop.edu/doc/1001004/Anti-Discrimination
UC San Diego is a smoke and tobacco free environment. Please visit smokefree.ucsd.edu for more information.
UC San Diego Health maintains a marijuana and drug free environment. Employees may be subject to drug screening.
Application Instructions
Please click on the link below to apply for this position. A new window will open and direct you to apply at our corporate careers page. We look forward to hearing from you!
Share This Page
Posted : 5/16/2024
Job Reference # : 128814
JOIN OUR TALENT COMMUNITY
Interested in working at UC San Diego and UC San Diego Health but can't find a position that's right for you? Submit your resume to our Talent Community to be considered for future opportunities that may align with your expertise. Please note, by joining our Talent Community, you are not applying for a position with UC San Diego Campus and Health. Rather, this is an additional way for our Talent Acquisition team to find candidates with specific credentials, if an opportunity arises. You are still encouraged to regularly check back on our career site or sign up for Job Alerts to apply for openings that are a match for your background.
- Career Sites by Recruiting.com
- Undergraduate Students
- Masters Students
- PhD/Doctoral Students
- Postdoctoral Scholars
- Faculty & Staff
- Families & Supporters
- Prospective Students
- Explore Your Interests / Self-Assessment
- Build your Network / LinkedIn
- Search for a Job / Internship
- Create a Resume / Cover Letter
- Prepare for an Interview
- Negotiate an Offer
- Prepare for Graduate School
- Find Funding Opportunities
- Prepare for the Academic Job Market
- Search for a Job or Internship
- Advertising, Marketing, and Public Relations
- Arts & Entertainment
- Consulting & Financial Services
- Engineering & Technology
- Government, Law & Policy
- Hospitality
- Management & Human Resources
- Non-Profit, Social Justice & Education
- Retail & Consumer Services
- BIPOC Students & Scholars
- Current & Former Foster Youth
- Disabled Students & Scholars
- First-Generation Students & Scholars
- Formerly Incarcerated Students & Scholars
- International Students & Scholars
- LGBTQ+ Students & Scholars
- Students & Scholars with Dependents
- Transfer Students
- Undocumented Students & Scholars
- Women-Identifying Students & Scholars
University of Colorado Anschutz Medical Campus
Undergraduate Student Research Assistant
- Share This: Share Undergraduate Student Research Assistant on Facebook Share Undergraduate Student Research Assistant on LinkedIn Share Undergraduate Student Research Assistant on X
Student Assistant V
REPRODUCTIVE HEALTH AND JUSTICE RESEARCH
Employer/Dept: CU College of Nursing
Supervisor: Kate Coleman-Minahan PhD, RN, FNP-BC – Assistant Professor, Tenure Track
Job Title: Undergraduate Student Research Assistant (Student Assistant V)
Job Location: Education II North, 13120 East 19th Avenue, Aurora, CO 80045 / The position is eligible for remote work
Job Description: This position is for an undergraduate student research assistant for a longitudinal study on how restrictive immigration and health policy shape immigrant young adult’s reproductive goals using in-depth interviews and surveys at the CU College of Nursing. The preferred candidate had previous experience working on research projects Work will be performed under the supervision of CU Nursing faculty member Dr. Kate Coleman-Minahan.
The student research assistant’s duties will include transcribing and editing qualitative interviews, assists with participant recruitment, including designing flyers and outreach to organizations in Colorado and Arizona, monitors and records national and state-level reproductive health and immigration policy changes and media attention, assists with qualitative coding and analysis, depending on research experience and helps organize community advisory boards, depending on project timing. The total work commitment for the student research assistant is expected to be 10 hours per week.
Eligibility Requirements:
- Currently enrolled at least half-time as an undergraduate student in public health, sociology, anthropology, psychology or other health or social science at an accredited higher education institution.
- Must be in good academic standing and cannot be in violation with any University policies or standards
Competencies:
- Passionate about reproductive health and justice and immigrants rights
- Dependable and accountable
- Excellent time management and organizational skills
- Interested in research and gaining research skills
Length of Employment: 10 hours per week for Summer 2024. There is an opportunity to continue during Fall 2024 for 5 hours per week depending on student interest and project needs
Pay Range: $19-20 per hour depending on level of research experience
Application Process: Submit a cover letter and resume through the posting on the CU Anschutz Handshake
JOB ANNOUNCEMENT FOR A SENIOR GRADUATE POSITION AS RESEARCH ASSISTANT ON THE CLINICAL TRIALS PLATFORM OF THE FUNDACIÓN DE INVESTIGACIÓN BIOMÉDICA DEL HOSPITAL UNIVERSITARIO DE LA PRINCESA.
Job information, offer description.
La Fundación de Investigación Biomédica del Hospital Universitario de la Princesa is seeking applications for a Senior Graduate position as a Research Assistant on the Clinical Trials Platform with a permanent contract under the Replacement Rate policy.
Requirements
- Experience in clinical trial monitoring.
- Experience providing administrative and documentary support in managing contracts with centers as well as liaising with Ethics Committees and the Spanish Agency of Medicines and Medical Devices (AEMPS).
- Knowledge of Good Clinical Practice guidelines.
- Experience in clinical research within the framework of biomedical projects.
- Previous experience working with the Spanish Clinical Research Network (SCReN) will be valued.
- Proficiency in Office, database management, and statistical analysis packages.
- Medium level of spoken and written English.
Additional Information
The evaluation criteria for this offer are as follows, up to a maximum of 15 points: • Demonstrable experience in clinical research and monitoring of clinical trials within the framework of biomedical projects: 0.25 points per month up to a maximum of 4 points. • Proficiency in computer programs related to clinical research: 0.5 points for each program up to a maximum of 2 points. • Additional training in courses related to clinical trials or clinical research, scholarships obtained, or publications: 0.5 point for each up to a maximum of 2 points. • Linguistic competence - English B1 = 1 point, Advanced = 2 points. • Personal interview: up to a maximum of 5 points.
Applications that meet the requirements and are submitted within the timeframe will be reviewed by a jury composed of three members (a president and two members)
TASKS TO BE PERFORMED • Preparation of protocols and necessary documentation. • Implementation of clinical trials: procedures with Ethics Committees and AEMPS. • Contract management. • Monitoring of clinical trials. • Support in conducting clinical trials from various hospital services.
Work Location(s)
Where to apply.

IMAGES
VIDEO
COMMENTS
Responsibilities. Assist in the design, administration, and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives. Prepare and present detailed reports on the progress of ongoing research. Assist in the preparation of manuscripts for publication.
Clinical research assistants work in hospitals, laboratories, and other institutions that conduct scientific studies. They identify subjects or clinical trials, collect data, evaluate results, monitor clinical trials, and take notes on activities. They audit research trials and ensure all clinical trial protocols are in compliance.
A clinical research assistant, CRA, is responsible for providing quality patient care and service to the clinical research clients and the healthcare providers. The clinical research assistant job description involves working closely with doctors, nurses, and other technicians in performing clinical and laboratory procedures.
Clinical research assistants work in hospitals, laboratories, and other institutions that conduct scientific studies. They identify subjects or clinical trials, collect data, evaluate results, monitor clinical trials, and take notes on activities. They audit research trials and ensure all clinical trial protocols are in compliance.
To write an effective clinical research assistant job description, begin by listing detailed duties, responsibilities and expectations. ... Serves as a primary contact for clinical centers in multi-center research studies; Develop study materials - serving to train imaging sites; Assure study protocol compliance of CT data receipt by the ...
As a clinical research assistant, your responsibilities include preparing the laboratory, processing volunteers, taking biological samples or vital signs, and organizing data. You may also be required to set up and clean work areas. Your job is to assist the researchers in any way possible, helping them conduct sound, ethical, and ...
The Clinical Research Assistant will also be responsible for data entry, scheduling appointments and calibrating laboratory equipment. This individual will coordinate and participate in clinical research studies conducted by investigators at Center For Sight using Good Clinical Practice. Travel to multiple locations is required. Benefits:
A competent Clinical Research Associate should be able to perform various duties and responsibilities. Proper fulfillment of a Clinical Research Associate's duties and responsibilities brings success to your company. Clinical Research Associates should assist in organizing and monitoring the different stages of clinical trials.
The Clinical Research Assistant job description template provides a general overview of the responsibilities and requirements associated with this role. This template is designed to be customized by organizations seeking to hire a Clinical Research Assistant, with specific criteria and qualifications tailored according to their needs.
Clinical Research Assistant (Clinical) Job Description ... procedures and collection of information for study patients according to protocols, and for protecting the health, safety, and welfare of research participants. ... B.S. or B.A. with at least (1) year of clinical and/or clinical research experience • At minimum, must have a proficient ...
Clinical Trials Research Assistant. Eastern Carolina Women's Center. New Bern, NC 28562. $37,000 - $44,000 a year. Full-time. 8 hour shift. Easily apply. Job Summary: We are seeking a highly motivated and detail-oriented Clinical Research Associate to join our team. The Clinical Research Associate will be….
Research Assistant Job Description. Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives. May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related ...
Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure study protocols are being followed.
Kelly Services clinical research assistant job description. + Uses phlebotomy skills to collect lab specimens from patients, only if trained. + Collects vital signs, weight, height, body measurements, EKG, etc. according to study requirements and documents appropriately in source document.
The Clinical Research Assistant will have the opportunity to work on scholarly projects (e.g., scientific publications, conference submissions) and receive mentorship (e.g., graduate school applications) from the Principal Investigator who is a licensed clinical psychologist and faculty member in the Department of Psychiatry and Human Behavior.
Research Assistant Job Description. Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives. May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related ...
communities and advancing health equity research and are seeking likeminded professionals to join our team. Location: Winston-Salem, NC or Charlotte, NC, with travel between sites As a Clinical Research Assistant, you will play an important role on the team. Responsibilities: • Assist with the coordination and implementation of research studies.
Discover a new path to conquering cancer. Whether it's low-dose radiation or flipping off the ENPP1 switch as a new treatment approach, our researchers are always building on what the world knows about cancer. With your expertise and passion, you can help us advance science and change people's lives. Clinical Research.
The clinical trial assistant job description involves participant recruitment, data collection, coordinating study visits, processing samples, and maintaining regulatory binders. ... Clinical trial assistants play a role in the execution of research studies. Their coordination and operational support are crucial in making complex trials possible.
Candidate with 2-4 yrs. Provide day-to-day departmental/project support activities, such as managing study supply inventory and orders, shipping, filing, faxing. Maintain databases/spreadsheets and compile reports. Sets up files related to a variety of clinical trials. Qualifications for clinical trial assistant.
Clinical Research Associate Responsibilities: Creating and writing trial protocols, and presenting these to the steering committee. Identifying, evaluating, and establishing trial sites, and closing sites down on completion of the trial. Training site staff on therapeutic areas, protocol requirements, proper source documentation, and case ...
Research Assistants perform a variety of routine, non-technical duties in assisting technical and professional personnel in a research laboratory setting. The Research Assistant will be expected to perform most components of data collection from human-subject volunteers required for a biomedical behavioral research study. Minimum Hiring Standards:
Job Summary. Stanford University School of Medicine and the Heart Center Clinical and Translational Research Program (CTRP) is seeking an Assistant Clinical Research Coordinator (ACRC) to perform administrative support duties related to the collection of clinical data and/or the coordination of clinical studies in pediatric cardiology.
This research experience would be helpful for candidates interested in applying for graduate and/or medical school in the future. Responsibilities: Complete research assessments with children and families. Recruit participants. Assist in identification and follow-up of participants meeting criteria for inclusion in clinical research studies.
Under the supervision of the PI, the incumbent will support the clinical research and quality improvement efforts of the study team. Support the clinical research operation; communicate with research participants, schedule visits, assist in the handling and processing of biological samples; shipping of samples for general lab assays and data entry.
Clinical research assistants work in hospitals, laboratories, and other institutions that conduct scientific studies. They identify subjects or clinical trials, collect data, evaluate results, monitor clinical trials, and take notes on activities. They audit research trials and ensure all clinical trial protocols are in compliance.
Soc/Clin Research Assistant. University of North Carolina at Chapel Hill. Chapel Hill, NC 27599. $46,000 - $65,000 a year. Full-time + 1. Monday to Friday. Experience working in clinical research environment. The Associate Clinical Research Coordinator is responsible for the execution of clinical research protocols….
Job Description: This position is for an undergraduate student research assistant for a longitudinal study on how restrictive immigration and health policy shape immigrant young adult's reproductive goals using in-depth interviews and surveys at the CU College of Nursing. The preferred candidate had previous experience working on research ...
Prior to submitting your application, please review and update (if necessary) the information in your candidate profile as it will transfer to your application. Job Title:Clinical Research Assistant Department:Medicine | IM Cardiovascular Medicine. To provide assistance in support of clinical research studies; assist with implementation and ...
• Demonstrable experience in clinical research and monitoring of clinical trials within the framework of biomedical projects: 0.25 points per month up to a maximum of 4 points. • Proficiency in computer programs related to clinical research: 0.5 points for each program up to a maximum of 2 points.