Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Clinical Study
- Open access
- Published: 27 May 2008

The impact of cancer research: how publications influence UK cancer clinical guidelines
- G Lewison 1 &
- R Sullivan 2 , 3 , 4
British Journal of Cancer volume 98 , pages 1944–1950 ( 2008 ) Cite this article
1241 Accesses
35 Citations
Metrics details
This article has been updated
There has been a substantially increased interest in biomedical research impact assessment over the past 5 years. This can be studied by a number of methods, but its influence on clinical guidelines must rank as one of the most important. In cancer, there are 43 UK guidelines (and associated Health Technology Assessments) published (up to October 2006) across three series, each of which has an evidence base in the form of references, many of which are papers in peer-reviewed journals. These have all been identified and analysed to determine their geographical provenance and type of research, in comparison with overall oncology research published in the peak years of guideline references (1999–2001). The UK papers were cited nearly three times as frequently as would have been expected from their presence in world oncology research (6.5%). Within the United Kingdom, Edinburgh and Glasgow stood out for their unexpectedly high contributions to the guidelines' scientific base. The cited papers from the United Kingdom acknowledged much more explicit funding from all sectors than did the UK cancer research papers at the same research level.
Similar content being viewed by others

The future of clinical trials in urological oncology

A taxonomy of early diagnosis research to guide study design and funding prioritisation
Interactions with the pharmaceutical industry and the practice, knowledge and beliefs of medical oncologists and clinical haematologists: a systematic review
It is increasingly being recognised that the quantitative evaluation of biomedical research cannot depend only on the counting of citations in the serial literature. They may measure academic influence, but the funders of such research are usually more concerned to see if it has had a practical benefit, especially to patients. One of the ways in which research can influence practice is through its contribution to the evidence base supporting clinical guidelines ( Heffner, 1998 ; Gralla et al, 1999 ; Connis et al, 2000 ; Van Wersch and Eccles, 2001 ; Aldrich et al, 2003 ). These are increasingly being used across many countries in the routine clinical care of cancer patients. Most of them are published by national professional medical associations (e.g., Rizzo et al, 2002 ; Atwood et al, 2004 ; Makuuchi and Kokudo, 2006 ), but some are developed by governmental bodies (e.g., Pogach et al, 2004 ).
It is normal for such guidelines to have lists of references that comprise their evidence base. However, the quality of the evidence is sometimes doubtful ( Ackman et al, 2000 ; Watine, 2002 ; Burgers and van Everdingen, 2004 ), and schemes have been devised to grade the quality of the clinical trials, which form a large part of the evidence base (e.g., Psaty et al, 2000 ; Liberati et al, 2001 ; Michaels and Booth, 2001 ; Hess, 2003 ; Guyatt et al, 2006 ). Even when the guidelines have been published, they are sometimes criticised as inadequate ( Jacobson, 1998 ; Norheim, 1999 ; Walker, 2001 ), insufficient ( Toman et al, 2001 ) or they may become outdated ( Shekelle et al, 2001 ). There is also the question of whether the guidelines will actually be followed in clinical practice ( Grol, 2001 ; Butzlaff et al, 2002 ; Bonetti et al, 2003 ; Bloom et al, 2004 ). The breadth of oncology practice (both patients and treatment modalities), the rapid evolution of new treatments and the often diverse interpretation of ‘evidence’ by health-care professionals mean many patients are treated with hospital-specific protocols rather than national guidelines. This situation is particularly acute in certain site-specific cancers, for example, lung ( Sambrook and Girling, 2001 ).
A further cause of disagreement is the question of cost: a new drug may be clinically effective and better than existing drugs or a placebo, but so costly that an equivalent or greater health gain may be achievable by other means, for example, better screening to detect the disease at an early stage. This can cause considerable dissension and lead to lawsuits to make the drug available for particularly articulate patients ( Dyer, 2006a ), or from companies and patients' advocacy groups, which sometimes receive their subsidies ( Dyer, 2006b ). Lobbying of the UK National Institute for Health and Clinical Excellence (NICE) by pharmaceutical firms is now rife ( Ferner and McDowell, 2006 ), and a US politician has adopted bully-boy tactics in his efforts to subvert evidence-based medicine ( Kmietowicz, 2006 ). The cost basis of NICE's recommendations has also been criticised: the figure of £30 000 (€40 000, $60 000) per quality-adjusted life year appears not to have a scientific basis or to take account of the social costs of disease ( Collier, 2008 ).
Despite all these criticisms, clinical guidelines are nevertheless gaining increasing recognition as the way forward. It does, therefore, seem worthwhile to treat them as an outcome indicator, even though a partial one, of the clinical impact of the research they cite. Several studies have analysed the evidence base of selected clinical guidelines ( Grant, 1999 ; Grant et al, 2000 ; Lewison and Wilcox-Jay, 2003 ). They have established that the papers cited are very clinical (when positioned on a scale from clinical observation to basic research); that the UK guidelines overcite the UK research papers; and that the cited papers are quite recent, with a temporal distribution comparable to that of the papers cited on biomedical research papers. Research from other European countries seems to be cited about as much as would be expected on the UK clinical guidelines, but that from Japan and from most developing countries is almost totally ignored.
In this study, we examined three sets of the UK guidelines on a single subject, cancer, and the references on 43 different guidelines, almost all concerned with treatment rather than with prevention. The bibliographic details of the references were assembled in a file and compared with those of cancer research publications in the three peak years (1999–2001). The objective was to answer several policy-related questions:
how do countries' relative presences among the cited references compare with their presences in cancer research?
how many of the cited references are actually classifiable as cancer research?
what is the research level (RL) distribution of these cited references compared with that of cancer research papers?
are the cited references published in journals of high citation impact?
how does the funding of the cited papers compare with that of cancer research overall?
The latter two questions need to take account of the finding that the references on clinical guidelines are much more clinical than other biomedical research.
Materials and methods
Uk cancer guidelines and the analysis of their references.
There are three sets of clinical guidelines commonly used in the United Kingdom:
Published by the British Medical Association in Clinical Evidence . This takes the form of a book that is revised and extended every 6 months, but is also accessible on the Web (to people in the United Kingdom);
Developed by the National Institute for Health and Clinical Excellence (NICE) for the National Health Service (NHS) in England and Wales, based on Health Technology Assessments (HTAs). Most of these last are available on the Web, but not all (although it is intended by NICE that they should be). They were used in the present study, because the references on the actual guidelines were usually not visible;
Developed by the Scottish Intercollegiate Guidelines Network (SIGN) for use by the NHS in Scotland. All these are freely available on the Web
Only a minority of these guidelines and HTAs are applicable to cancer. The numbers are, respectively, 15, 18 and 10. Each of these 43 documents has a set of references, most of which are articles in peer-reviewed journals. A total of 3217 references were found and their details downloaded to file. Their addresses were parsed by means of a special macro so that the integer and fractional counts of each country were listed for each paper (a paper with two addresses in the United Kingdom and one in France would count unity for each on an integer count basis, but 0.67 for the United Kingdom and 0.33 for France using fractional counting). The RL of each paper was determined using the new system developed by Lewison and Paraje (2004) , in which each journal is assigned an RL based on the presence of ‘clinical’ and ‘basic’ words in the titles of papers it has published on a scale from 1=clinical to 4=basic. In addition, the RL of groups of individual cited papers could be calculated with reference to their individual titles, and the presence of ‘clinical’ or ‘basic’ words within them. The potential citation impact (PCI) of each cited paper was also determined with reference to a file of Journal Expected Citation Rates provided by Thomson Scientific (London, UK). This gave the mean number of citations for papers published in a journal in a given year and cited in the year of publication and the 4 subsequent years.
Funding data for virtually all the UK papers (790 out of 796) were obtained from inspection of the acknowledgements to their funding sources in the British Library. Many of the papers had previously been looked up for the Research Outputs Database ( Webster et al, 2003 ) or for other projects, and only 151 needed to be sought anew. The main comparator used to normalise the results of the analysis of the cited references was a file of world oncology research papers ( Cambrosio et al, 2006 ). For the years 1999–2001, there were over 100 000 such papers, and their characteristics were used to see how the cited references compared with them, with due account being taken of the differences expected in mean RLs (the cited references being more clinical than oncology papers overall).
Time and research level distributions
Figure 1 shows the distribution of the 3217 cited references by publication date. There is a clear peak in the year 2000, and 31% of all the references were published in the 3 years, 1999–2001, so this was the time period used for many of the comparisons with world oncology research.
Time distribution of the 3217 references on the UK cancer clinical guidelines.
Of the references classed as ‘articles’ or ‘reviews’, 88% were within the subfield of oncology as defined by Cancer Research UK ( Cambrosio et al, 2006 ). This percentage remained sensibly constant over the period, 1994–2004. However, the references were in much more clinical journals than world oncology papers for the year 2000, the peak year for the numbers of references, see Figure 2 . This result was obtained earlier ( Grant et al, 2000 ; Lewison and Wilcox-Jay, 2003 ) but with a much simplified (and less accurate) method of categorisation of journals by RL. Of the 3217 papers, 2747 titles (86%) had either a ‘clinical’ or a ‘basic’ keyword, and the mean RL was 1.07, which is very close to the lower end of the scale (RL=1.0), and much below the mean RL based on all the papers in the individual journals (RL=1.43). This shows that the references were being published in journals that were relatively more basic than the papers themselves, and reinforces the message that the papers were, therefore, almost entirely clinical observation.
RL distributions (cumulative percentages) for references on cancer clinical guidelines (solid squares) and for oncology research in 2000 (open triangles).
Geographical analysis
The presence of 20 leading countries in oncology research for 2000 and in the references from the clinical guidelines is shown in Table 1 , where the data have been shown on a fractional count basis. Figure 3 presents the ratio between a country's presence in the guideline references and its presence in oncology research, that is, the values shown in the last column of Table 1 . As would be expected, the UK oncology research is cited more than expected from its presence in world oncology by a factor of almost 3, but several other European countries' work is also relatively overcited, notably that of Denmark, Ireland and Sweden. Although Italy, which is strong in clinical trials, shows to advantage, Germany is relatively much undercited compared with its presence in cancer research in recent years. Japanese work is almost ignored, but it is likely that the Science Citation Index, where most of the references were found, does not cover Japanese clinical journals. This, however, is only a small part of the reason for the paucity of Japanese references.
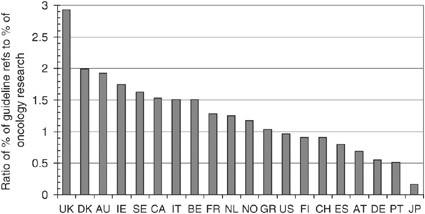
Ratio of countries' presence among the UK cancer clinical guideline references and their presence in world oncology research, 2000: fractional counts. Country codes as listed in Table 1 .
Within the United Kingdom, certain cities showed relatively to advantage in terms of their percentage presence within the fractional UK total of 605 papers cited by the guidelines, compared with that in the 2332 UK oncology papers published in 2000. The analysis is conveniently carried out on the basis of postcode area, the first one or two letters of the UK postcode system, for example, B=Birmingham, CB=Cambridge. Figure 4 shows a scatter plot for the 26 leading areas (out of 124), accounting for about two-thirds of both totals. The spots above the diagonal line represent areas that are more frequently cited than expected, and vice versa. Among the former, EH=Edinburgh and G=Glasgow are prominent, in part because the SIGN guidelines overcite Scottish research papers, together with SM=Sutton and Cheam (the location of the Institute of Cancer Research) and OX=Oxford.
Scatter plot of the fractional count percentage presence of the leading 26 UK postcode areas within the UK papers cited on the UK cancer clinical guidelines plotted against their percentage presence in the UK oncology research outputs in 2000. Codes: AB=Aberdeen, B=Birmingham, BS=Bristol, BT=Belfast, CB=Cambridge, CF=Cardiff, DD=Dundee, EC=London EC (St Bart's), EH=Edinburgh, G=Glasgow, HA=Harrow, L=Liverpool, LE=Leicester, LS=Leeds, M=Manchester, NE=Newcastle upon Tyne, NG=Nottingham, NW=London NW (Royal Free), OX=Oxford, S=Sheffield, SE=London SE (Guys, Kings and St Thomas'), SM=Sutton and Cheam (Institute of Cancer Research), SO=Southampton, SW=London SW (St George's), W=London W (Imperial), WC=London WC (UCL).
Table1 and Figure 3 show overall values, but an analysis can also be made of subsets of papers for groups of 2 or 3 years, chosen so that the four periods each have about 20% of the total cited references, see Table 2 . For nearly all the countries, there are close similarities between the time trends, which suggest that the guidelines are rather consistent in the geography of their citing behaviour. Thus, Australia, Canada, Sweden, the United Kingdom and the United States have all shown a reducing presence in oncology research, and a reducing presence in the guideline references; Germany, on the other hand, has increased its presence in both (but is still much undercited). France and Japan increased their presence in both sets of papers, but it went down slightly during the latest period.
Journal citation impact scores
The references cited tend to be published in high-impact journals. Table 3 shows that in each RL grouping, the guideline references are published in journals with a higher mean citation score (the PCI, of the papers) than world oncology papers from the year 2000.
The overall mean is higher, too, at 19.9 cites in 5 years compared with 13.4. The ‘superior performance’ of the guideline references occurs because a large number of them are published in the high-impact general journals, The Lancet (138 of them), New England Journal of Medicine (133), British Medical Journal (78) and Journal of the American Medical Association (50).
The funding of the UK cited references
Of the 796 UK papers, all but 6 were found and inspected to determine their funding sources. These were taken both from the addresses (as for some organisations this is an indication of funding) and from the formal acknowledgements. For the purposes of this analysis, funding sources were grouped into five main sectors:
UK government, both departments and agencies;
UK private nonprofit, including collecting charities, endowed foundations, hospital trustees, mixed (academic) and other nonprofit. A subset of this sector is Cancer Research UK, and its two predecessors, the Cancer Research Campaign and the Imperial Cancer Research Fund;
pharmaceutical industry, both domestic and foreign (it is often difficult to distinguish as some subsidiaries have considerable autonomy in the use of research funds), and including biotech companies;
nonpharma industry;
no funding acknowledged.
The remaining funding organisations are foreign governmental and private nonprofit sources, and international organisations, such as the European Commission (EC) and the World Health Organization (WHO).
The funding sources vary with the RL of the papers: the more clinical papers have fewer sources and the more basic papers have more. Table 4 shows the analysis for the UK papers in oncology in 1999–2001, and Table 5 shows the results for the UK papers cited on cancer clinical guidelines. For each RL group, an estimate has been made of the funding that would have been expected had they been typical of the UK cancer research, and in the last row there are given the ratios of observed-to-expected numbers of papers (integer counts) on the assumption that the cancer clinical guideline citations are typical of oncology, but with due allowance for the different RL distributions.
For example, the UK oncology papers in the first group (RL from 1.0 to 1.5) have the UK government funding on 11.1% of them, so it might be expected that there would be 0.111 × 544=60.4 government-funded papers among the corresponding group cited on cancer clinical guidelines. In fact, there were 149 such papers, showing that many more are government funded than might have been expected. When the totals for each of the six groups are added, it can be seen that the observed number of the UK government-funded papers is almost twice the predicted number. The observed total is still higher ( × 2.5) for the pharma industry-funded papers, and a little lower for Cancer Research UK papers ( × 1.8), for nonpharma industry papers ( × 1.6) and the UK private nonprofit papers ( × 1.3). Not surprisingly, there are many fewer ‘unfunded’ papers, the ratio of observed-to-expected numbers of papers being only just over half.
The UK cancer clinical guidelines are sufficient in number and variety to provide a fair window on the impact of cancer research on clinical practice, not only in the United Kingdom, but in other leading countries, particularly in western Europe. We have seen that almost all the references (88%) are to papers that are within the subfield of cancer research. Because about one-third of the research supported by Cancer Research UK, in common with that of other medical research charities working in a particular disease area, is out with this subfield (most of this would comprise basic biology), it follows that little of this work can be expected to influence clinical guidelines – hardly a surprising conclusion, but nevertheless one that is worth stating.
Many of the guideline references are to papers in the US and the UK general medical journals – The Journal of the American Medical Association , New England Journal Medical , British Medical Journal and The Lancet . This is one reason, but by no means the only one, for the guideline references as a whole to be in high impact, and therefore well known, journals. It appears that if researchers want their work, particularly clinical trials, to be part of the evidence base for clinical guidelines, then it is desirable for them to publish in highly cited journals. Disproportionately, many of these papers will have been funded by government or the pharmaceutical industry, with charities also playing an enhanced role compared with cancer research overall. This highlights one pitfall of national guidelines in the context of research impact assessments; many important, high quality clinical trials – either because they are early phase or negative – will not make it into guidelines. The impact of research on national clinical guidelines is just one parameter that can describe the utility of health research ( Kuruvilla et al, 2006 ).
When account is taken of the clinical nature of the work cited on guidelines, the big increase in the percentage of the papers that acknowledge funding – whether from government, charities or industry – is striking ( Table 5 ). Many (37%) of these clinical papers with RLs greater than 1.5 are reports of clinical trials, and 85% of the latter acknowledge funding compared with 71% of the others. Cancer Research UK plays the biggest role, and supports over one-third of these trials, more even than the pharmaceutical industry as a whole, or the UK government.
The geographical analysis of the cited papers reveals that the UK papers have a threefold higher presence among them than in world cancer research. In part, this reflects the differences in cancer management between countries. Such overcitation also occurs on other scientific papers, so it is hardly surprising that it was found here. It might be expected that the UK guidelines, which aim to show which treatments are cost-effective, would reflect in particular the different financial basis of health-care provision in this country compared with that elsewhere, and so papers concerned with economics and costs would be even more overcited if they were from the United Kingdom. In fact, this does occur, but to a very minor extent (22% from the United Kingdom compared with 19% overall; the difference not being significant).
The distribution of the cited papers within the United Kingdom differs from what might have been expected based purely on overall numbers and on the extent to which the cities carry out clinical observation rather than basic research. The simple comparison of Figure 4 needs also to take account of the mean RL of papers from each area, and, when this is done ( Figure 5 ), a different pattern emerges, with EH=Edinburgh, OX=Oxford and CB=Cambridge forming an axis of excellence (on this indicator) and other areas' output being less cited on guidelines. The distance of the spots from this axis gives one indicator of the performance of the different centres, an imperfect one to be sure, as there will be other confounding factors not considered here, but nevertheless a useful complement to the traditional bibliometric criterion based purely on citation counts in the scientific literature.
Comparison of the fractional count percentage presence of the 19 leading UK postcode areas with >50 cited papers cited by the UK cancer clinical guidelines divided by their presence in the UK oncology research in 2000 with the mean RL of their cited papers (scale: 1=clinical observation, 4=basic research). Area codes as listed in the legend to Figure 4 .
There are in the database enough cited papers from a few other countries to enable a similar evaluation to be carried out for them. However, these data are inevitably skewed by being viewed through the prism of the UK clinical recommendations. It would be highly desirable to complement them with the results of similar exercises carried out in other countries with extensive sets of clinical guidelines, or at a European or international level. Then, provided the data were collected in exactly the same way, they could be pooled and a more international perspective on the utility of cancer research would emerge that research evaluators could employ. Such an activity could appropriately be coordinated by the European Cancer Managers' Research Forum, with all data contributors having also the right to gain access to the data provided by workers in other countries.
Change history
16 november 2011.
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
Ackman ML, Druteika D, Tsuyuki RT (2000) Levels of evidence in cardiovascular clinical-practice guidelines. Can J Cardiol 16 (10): 1249–1254
CAS PubMed Google Scholar
Aldrich R, Kemp L, Williams JS, Harris E, Simpson S, Wilson A, McGill K, Byles J, Lowe J, Jackson T (2003) Using socioeconomic evidence in clinical-practice guidelines. BMJ 327 : 1283–1285
Article Google Scholar
Atwood CW, McCrory D, Garcia JGN, Abman SH, Ahearn GS (2004) Pulmonary-artery hypertension and sleep-disordered breathing – ACCP evidence-based clinical-practice guidelines. Chest 126 (1): S72–S77
Bloom BS, de Pouvourville N, Chhatre S, Jayadevappa R, Weinberg D (2004) Breast cancer treatment in clinical practice compared to best evidence and practice guidelines. Br J Cancer 90 (1): 26–30
Article CAS Google Scholar
Bonetti D, Johnston M, Pitts NB, Deery C, Ricketts I, Bahrami M, Ramsay C, Johnston J (2003) Can psychological models bridge the gap between clinical guidelines and clinician behaviour – a randomized controlled-trial of an intervention to influence dentists' intention to implement evidence-based practice. Br Dent J 195 (7): 403–407
Burgers JS, van Everdingen JJE (2004) Beyond the evidence in clinical guidelines. Lancet 364 : 392–393
Butzlaff M, Floer B, Koneczny N, Vollmar HC, Lange S, Isfort J, Kunstmann W (2002) Assessment and utilization of clinical guidelines by primary care physicians and internists. Zeitschrift für artzliche Fortbildung und Qualitatssicherung 96 (2): 127–133
Google Scholar
Cambrosio A, Keating P, Mercier S, Lewison G, Mogoutov A (2006) Mapping the emergence and development of translational cancer research. Eur J Cancer 42 : 3140–3148
Collier J (2008) Parliamentary review asks NICE to do better still. BMJ 336 : 56–57
Connis RT, Nickinovich DG, Caplan RA, Arens JF (2000) The development of evidence-based clinical practice guidelines – integrating medical science and practice. Int J Technol Assess Health Care 16 (4): 1003–1012
Dyer C (2006a) Patient is to appeal High court ruling on breast cancer drug. BMJ 332 : 443
PubMed PubMed Central Google Scholar
Dyer C (2006b) NICE faces legal challenge over restriction on dementia drug. BMJ 333 : 1085
Ferner RE, McDowell SE (2006) How NICE may be outflanked. BMJ 332 : 1268–1271
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clarksnow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG (1999) Recommendations for the use of anti-emetics: evidence-based, clinical practice guidelines. J Clin Oncol 17 (9): 2971–2994
Grant J (1999) Evaluating the outcomes of biomedical research on healthcare. Research Evaluation 8 (1): 33–38
Grant J, Cottrell R, Cluzeau F, Fawcett G (2000) Evaluating ‘payback’ on biomedical research from papers cited in clinical guidelines – applied bibliometric study. BMJ 320 : 1107–1111
Grol R (2001) Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care 39 (8): 1146–1154
Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schünemann H (2006) Grading strength of recommendations and quality of evidence in clinical guidelines. Chest 129 (1): 174–181
Heffner JE (1998) Does evidence-based medicine help the development of clinical practice guidelines? Chest 113 (3): S172–S178
Hess DR (2003) Evidence-based clinical practice guidelines: where's the evidence and what do I do with it? Respir Care 48 (9): 838–839
PubMed Google Scholar
Jacobson JJ (1998) Evidence-based clinical practice guidelines: friend or foe? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86 (2): 137
Kmietowicz Z (2006) Experts defend NICE against attack by US politician. BMJ 333 : 1087
PubMed Central Google Scholar
Kuruvilla S, Mays N, Pleasant A, Walt G (2006) Describing the impact of health research: a research impact framework. BMC Health Serv Res 6 : 134
Lewison G, Paraje G (2004) The classification of biomedical journals by research level. Scientometrics 60 (2): 145–157
Lewison G, Wilcox-Jay K (2003) Getting biomedical research into practice: the citations from UK clinical guidelines. Proceedings of the 9th International Conference on Scientometrics and Informetrics . Beijing, China. 152–160
Liberati A, Buzzetti R, Grilli R, Magrini N, Minozzi S (2001) Which guidelines can we trust: assessing strength of evidence behind recommendations for clinical practice. West J Med 174 (4): 262–265
Makuuchi M, Kokudo N (2006) Clinical practice guidelines for hepatocellular carcinoma: the first evidence-based guidelines from Japan. World J Gastroenterol 12 (5): 828–829. See English translation of guidelines at http://www.jsh.or.jp
Michaels J, Booth A (2001) Pragmatic system for the grading of evidence and recommendations in clinical guidelines. J Clin Excellence 3 (3): 139–145
Norheim OF (1999) Health-care rationing: are additional criteria needed for assessing evidence-based clinical practice guidelines? BMJ 319 : 1426–1429
Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder D, Sawin CT (2004) Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 27 : B82–B89
Psaty BM, Furberg CD, Pahor M, Alderman M, Kuller LH (2000) National guidelines, clinical trials and quality of evidence. Arch Int Med 160 (19): 2577–2580
Rizzo JD, Lichtin AE, Woolf SH, Seidenfeld J, Bennett CL, Cella D, Djulbegovic B, Goode MJ, Jakubowski AA, Lee SJ, Miller CB, Rarick MU, Regan DH, Browman GP, Gordon MS (2002) Use of Epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. J Clin Oncol 20 (19): 4083–4107
Sambrook RJ, Girling DJ (2001) A national survey of the chemotherapy regimens used to treat small cell lung cancer (SCLC) in the United Kingdom. Br J Cancer 84 (11): 1447–1452
Shekelle PG, Ortiz E, Rhodes S, Morton SC, Eccles MP, Grimshaw JM, Woolf SH (2001) Validity of the agency for health-care research and quality clinical practice guidelines: how quickly do guidelines become outdated? J Am Med Assoc 286 (12): 1461–1467
Toman C, Harrison MB, Logan J (2001) Clinical practice guidelines: necessary but not sufficient for evidence-based patient education and counseling. Patient Educ Counsel 43 (3): 279–287
Van Wersch A, Eccles M (2001) Involvement of consumers in the development of evidence-based clinical guidelines: practical experiences from the North of England Evidence-Based Guideline Development Programme. Qual Health Care 10 (1): 10–16
Walker T (2001) Evidence-based clinical guidelines: are they effective? N Z Med J 114 : 20
Watine J (2002) Are laboratory investigations recommended in current medical practice guidelines supported by available evidence? Clin Chem Lab Med 40 (3): 252–255
Webster BM, Lewison G, Rowlands I (2003) Mapping the Landscape II: Biomedical Research in the UK, 1989–2002 . Department of Information Science, The City University: Available at http://www.ucl.ac.uk/ciber/MappingtheLandscape.php
Download references
Acknowledgements
This work was supported by European Cancer Research Managers Forum, Cancer Research UK; the Chief Scientist Office, Scottish Government; the Medical Research Council; and the Wellcome Trust. The MS Excel macros used to perform the geographical analysis of the papers were written by Dr Philip Roe. The identification of the individual papers cited on the clinical guidelines was carried out by Isla Rippon and Vicky Friedlander.
Author information
Authors and affiliations.
School of Library, Archive & Information Studies, University College London, Gower Street, London, WC1E 6BT, UK
Cancer Research UK, 61 Lincolns' Inn Fields, London, WC2A 3PX, UK
Department of Social Policy (Population, Health & Society), London School of Economics & Political Science, Houghton Street, London, WC2A 2AE, UK
European Cancer Research Managers Forum, London, UK,
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to R Sullivan .
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
Reprints and permissions
About this article
Cite this article.
Lewison, G., Sullivan, R. The impact of cancer research: how publications influence UK cancer clinical guidelines. Br J Cancer 98 , 1944–1950 (2008). https://doi.org/10.1038/sj.bjc.6604405
Download citation
Received : 13 October 2007
Revised : 04 April 2008
Accepted : 13 April 2008
Published : 27 May 2008
Issue Date : 17 June 2008
DOI : https://doi.org/10.1038/sj.bjc.6604405
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Exploring the clinical translation intensity of papers published by the world’s top scientists in basic medicine.
- Dongyu Zang
Scientometrics (2023)
Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment
- Catherine R. Hanna
- Kathleen A. Boyd
- Robert J. Jones
Health Research Policy and Systems (2021)
A new database of the references on international clinical practice guidelines: a facility for the evaluation of clinical research
- Magnus Eriksson
- Annika Billhult
- Grant Lewison
Scientometrics (2020)
A characterization of professional media and its links to research
- Diana Hicks
- Julia Melkers
- Kimberley R. Isett
Scientometrics (2019)
Does citation matter? Research citation in policy documents as an indicator of research impact – an Australian obesity policy case-study
- Robyn Newson
- Lucie Rychetnik
- Adrian Bauman
Health Research Policy and Systems (2018)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
The Institute of Cancer Research
http://instituteofcancerresearch.org.uk/

Together we will beat cancer

We do not charge for any of our publications
Please allow 3 to 5 days for delivery

Updated early diagnosis leaflets - available now
Spot cancer early
Updated leaflets giving information about possible cancer symptoms

Reducing cancer risk
Cut your cancer risk leaflets, healthy living resources, infographics.

Services for cancer patients
Materials promoting our services, including clinical trials.

Get the latest health professional news
Sign up to cancer insight for evidence-based cancer information and tools to support your practice. Sent straight to your inbox.

Up-to-date cancer data and statistics
Includes national and local statistics, GP practice data and planning tools for commissioners.
Featured publications

Easy read leaflet - How to try to not get cancer

You can be smoke free - A3 poster
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancer Control
- v.28; Jan-Dec 2021
Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges From a Biomedical Perspective
Patricia piña-sánchez.
1 Oncology Research Unit, Oncology Hospital, Mexican Institute of Social Security, Mexico
Antonieta Chávez-González
Martha ruiz-tachiquín, eduardo vadillo, alberto monroy-garcía, juan josé montesinos, rocío grajales.
2 Department of Medical Oncology, Oncology Hospital, Mexican Institute of Social Security, Mexico
Marcos Gutiérrez de la Barrera
3 Clinical Research Division, Oncology Hospital, Mexican Institute of Social Security, Mexico
Hector Mayani
Since the second half of the 20th century, our knowledge about the biology of cancer has made extraordinary progress. Today, we understand cancer at the genomic and epigenomic levels, and we have identified the cell that starts neoplastic transformation and characterized the mechanisms for the invasion of other tissues. This knowledge has allowed novel drugs to be designed that act on specific molecular targets, the immune system to be trained and manipulated to increase its efficiency, and ever more effective therapeutic strategies to be developed. Nevertheless, we are still far from winning the war against cancer, and thus biomedical research in oncology must continue to be a global priority. Likewise, there is a need to reduce unequal access to medical services and improve prevention programs, especially in countries with a low human development index.
Introduction
During the last one hundred years, our understanding of the biology of cancer increased in an extraordinary way. 1 - 4 Such a progress has been particularly prompted during the last few decades because of technological and conceptual progress in a variety of fields, including massive next-generation sequencing, inclusion of “omic” sciences, high-resolution microscopy, molecular immunology, flow cytometry, analysis and sequencing of individual cells, new cell culture techniques, and the development of animal models, among others. Nevertheless, there are many questions yet to be answered and many problems to be solved regarding this disease. As a consequence, oncological research must be considered imperative.
Currently, cancer is one of the illnesses that causes more deaths worldwide. 5 According to data reported in 2020 by the World Health Organization (WHO), cancer is the second cause of death throughout the world, with 10 million deaths. 6 Clearly, cancer is still a leading problem worldwide. With this in mind, the objective of this article is to present a multidisciplinary and comprehensive overview of the disease. We will begin by analyzing cancer as a process, focusing on the current state of our knowledge on 4 specific aspects of its biology. Then, we will look at cancer as a global health problem, considering some epidemiological aspects, and discussing treatment, with a special focus on novel therapies. Finally, we present our vision on some of the challenges and perspectives of cancer in the 21 st century.
The Biology of Cancer
Cancer is a disease that begins with genetic and epigenetic alterations occurring in specific cells, some of which can spread and migrate to other tissues. 4 Although the biological processes affected in carcinogenesis and the evolution of neoplasms are many and widely different, we will focus on 4 aspects that are particularly relevant in tumor biology: genomic and epigenomic alterations that lead to cell transformation, the cells where these changes occur, and the processes of invasion and metastasis that, to an important degree, determine tumor aggressiveness.
Cancer Genomics
The genomics of cancer can be defined as the study of the complete sequence of DNA and its expression in tumor cells. Evidently, this study only becomes meaningful when compared to normal cells. The sequencing of the human genome, completed in 2003, was not only groundbreaking with respect to the knowledge of our gene pool, but also changed the way we study cancer. In the post-genomic era, various worldwide endeavors, such as the Human Cancer Genome Project , the Cancer Genome ATLAS (TCGA), the International Cancer Genome Consortium, and the Pan-Cancer Analysis Working Group (PCAWG), have contributed to the characterization of thousands of primary tumors from different neoplasias, generating more than 2.5 petabytes (10 15 ) of genomic, epigenomic, and proteomic information. This has led to the building of databases and analytical tools that are available for the study of cancer from an “omic” perspective, 7 , 8 and it has helped to modify classification and treatment of various neoplasms.
Studies in the past decade, including the work by the PCAWG, have shown that cancer generally begins with a small number of driving mutations (4 or 5 mutations) in particular genes, including oncogenes and tumor-suppressor genes. Mutations in TP53, a tumor-suppressor gene, for example, are found in more than half of all cancer types as an early event, and they are a hallmark of precancerous lesions. 9 - 12 From that point on, the evolution of tumors may take decades, throughout which the mutational spectrum of tumor cells changes significantly. Mutational analysis of more than 19 000 exomes revealed a collection of genomic signatures, some associated with defects in the mechanism of DNA repair. These studies also revealed the importance of alterations in non-coding regions of DNA. Thus, for example, it has been observed that various pathways of cell proliferation and chromatin remodeling are altered by mutations in coding regions, while pathways, such as WNT and NOTCH, can be disrupted by coding and non-coding mutations. To the present date, 19 955 genes that codify for proteins and 25 511 genes for non-coding RNAs have been identified ( https://www.gencodegenes.org/human/stats.html ). Based on this genomic catalogue, the COSMIC (Catalogue Of Somatic Mutations In Cancer) repository, the most robust database to date, has registered 37 288 077 coding mutations, 19 396 fusions, 1 207 190 copy number variants, and 15 642 672 non-coding variants reported up to August 2020 (v92) ( https://cosmic-blog.sanger.ac.uk/cosmic-release-v92/ ).
The genomic approach has accelerated the development of new cancer drugs. Indeed, two of the most relevant initiatives in recent years are ATOM (Accelerating Therapeutics for Opportunities in Medicine), which groups industry, government and academia, with the objective of accelerating the identification of drugs, 13 and the Connectivity Map (CMAP), a collection of transcriptional data obtained from cell lines treated with drugs for the discovery of functional connections between genes, diseases, and drugs. The CMAP 1.0 covered 1300 small molecules and more than 6000 signatures; meanwhile, the CMAP 2.0 with L1000 assay profiled more than 1.3 million samples and approximately 400 000 signatures. 14
The genomic study of tumors has had 2 fundamental contributions. On the one hand, it has allowed the confirmation and expansion of the concept of intratumor heterogeneity 15 , 16 ; and on the other, it has given rise to new classification systems for cancer. Based on the molecular classification developed by expression profiles, together with mutational and epigenomic profiles, a variety of molecular signatures have been identified, leading to the production of various commercial multigene panels. In breast cancer, for example, different panels have been developed, such as Pam50/Prosigna , Blue Print , OncotypeDX , MammaPrint , Prosigna , Endopredict , Breast Cancer Index , Mammostrat, and IHC4 . 17
Currently, the genomic/molecular study of cancer is more closely integrated with clinical practice, from the classification of neoplasms, as in tumors of the nervous system, 18 to its use in prediction, as in breast cancer. 17 Improvement in molecular methods and techniques has allowed the use of smaller amounts of biological material, as well as paraffin-embedded samples for genomic studies, both of which provide a wealth of information. 19 In addition, non-invasive methods, such as liquid biopsies, represent a great opportunity not only for the diagnosis of cancer, but also for follow-up, especially for unresectable tumors. 20
Research for the production of genomic information on cancer is presently dominated by several consortia, which has allowed the generation of a great quantity of data. However, most of these consortia and studies are performed in countries with a high human development index (HDI), and countries with a low HDI are not well represented in these large genomic studies. This is why initiatives such as Human Heredity and Health in Africa (H3Africa) for genomic research in Africa are essential. 21 Generation of new information and technological developments, such as third-generation sequencing, will undoubtedly continue to move forward in a multidisciplinary and complex systems context. However, the existing disparities in access to genomic tools for diagnosis, prognosis, and treatment of cancer will continue to be a pressing challenge at regional and social levels.
Cancer Epigenetics
Epigenetics studies the molecular mechanisms that produce hereditable changes in gene expression, without causing alterations in the DNA sequence. Epigenetic events are of 3 types: methylation of DNA and RNA, histone modification (acetylation, methylation, and phosphorylation), and the expression of non-coding RNA. Epigenetic aberrations can drive carcinogenesis when they alter chromosome conformation and the access to transcriptional machinery and to various regulatory elements (promoters, enhancers, and anchors for interaction with chromatin, for example). These changes may activate oncogenesis and silence tumor-suppressor mechanisms when they modulate coding and non-coding sequences (such as micro-RNAs and long-RNAs). This can then lead to uncontrolled growth, as well as the invasion and metastasis of cancer cells.
While genetic mutations are stable and irreversible, epigenetic alterations are dynamic and reversible; that is, there are several epigenomes, determined by space and time, which cause heterogeneity of the “epigenetic status” of tumors during their development and make them susceptible to environmental stimuli or chemotherapeutic treatment. 22 Epigenomic variability creates differences between cells, and this creates the need to analyze cells at the individual level. In the past, epigenetic analyses measured “average states” of cell populations. These studies revealed general mechanisms, such as the role of epigenetic marks on active or repressed transcriptional states, and established maps of epigenetic composition in a variety of cell types in normal and cancerous tissue. However, these approaches are difficult to use to examine events occurring in heterogeneous cell populations or in uncommon cell types. This has led to the development of new techniques that permit marking of a sequence on the epigenome and improvement in the recovery yield of epigenetic material from individual cells. This has helped to determine changes in DNA, RNA, and histones, chromatin accessibility, and chromosome conformation in a variety of neoplasms. 23 , 24
In cancer, DNA hypomethylation occurs on a global scale, while hypermethylation occurs in specific genomic loci, associated with abnormal nucleosome positioning and chromatin modifications. This information has allowed epigenomic profiles to be established in different types of neoplasms. In turn, these profiles have served as the basis to identify new neoplasm subgroups. For example, in triple negative breast cancer (TNBC), 25 and in hepatocellular carcinoma, 26 DNA methylation profiles have helped to the identification of distinct subgroups with clinical relevance. Epigenetic approaches have also helped to the development of prognostic tests to assess the sensitivity of cancer cells to specific drugs. 27
Epigenetic traits could be used to characterize intratumoral heterogeneity and determine the relevance of such a heterogeneity in clonal evolution and sensitivity to drugs. However, it is clear that heterogeneity is not only determined by genetic and epigenetic diversity resulting from clonal evolution of tumor cells, but also by the various cell populations that form the tumor microenvironment (TME). 28 Consequently, the epigenome of cancer cells is continually remodeled throughout tumorigenesis, during resistance to the activity of drugs, and in metastasis. 29 This makes therapeutic action based on epigenomic profiles difficult, although significant advances in this area have been reported. 30
During carcinogenesis and tumor progression, epigenetic modifications are categorized by their mechanisms of regulation ( Figure 1A ) and the various levels of structural complexity ( Figure 1B ). In addition, the epigenome can be modified by environmental stimuli, stochastic events, and genetic variations that impact the phenotype ( Figure 1C ). 31 , 32 The molecules that take part in these mechanisms/events/variations are therapeutic targets of interest with potential impact on clinical practice. There are studies on a wide variety of epidrugs, either alone or in combination, which improve antitumor efficacy. 33 However, the problems with these drugs must not be underestimated. For a considerable number of epigenetic compounds still being under study, the main challenge is to translate in vitro efficacy of nanomolar (nM) concentrations into well-tolerated and efficient clinical use. 34 The mechanisms of action of epidrugs may not be sufficiently controlled and could lead to diversion of the therapeutic target. 35 It is known that certain epidrugs, such as valproic acid, produce unwanted epigenetic changes 36 ; thus the need for a well-established safety profile before these drugs can be used in clinical therapy. Finally, resistance to certain epidrugs is another relevant problem. 37 , 38

Epigenetics of cancer. (A) Molecular mechanisms. (B) Structural hierarchy of epigenomics. (C) Factors affecting the epigenome. Modified from Refs. 31 and 32 .
As we learn about the epigenome of specific cell populations in cancer patients, a door opens to the evaluation of sensitivity tests and the search for new molecular markers for detection, prognosis, follow-up, and/or response to treatment at various levels of molecular regulation. Likewise, the horizon expands for therapeutic alternatives in oncology with the use of epidrugs, such as pharmacoepigenomic modulators for genes and key pathways, including methylation of promoters and regulation of micro-RNAs involved in chemoresponse and immune response in cancer. 39 There is no doubt that integrated approaches identifying stable pharmagenomic and epigenomic patterns and their relation with expression profiles and genetic functions will be more and more valuable in our fight against cancer.
Cancer Stem Cells
Tumors consist of different populations of neoplastic cells and a variety of elements that form part of the TME, including stromal cells and molecules of the extracellular matrix. 40 Such intratumoral heterogeneity becomes even more complex during clonal variation of transformed cells, as well as influence the elements of the TME have on these cells throughout specific times and places. 41 To explain the origin of cancer cell heterogeneity, 2 models have been put forward. The first proposes that mutations occur at random during development of the tumor in individual neoplastic cells, and this promotes the production of various tumor populations, which acquire specific growth and survival traits that lead them to evolve according to intratumor mechanisms of natural selection. 42 The second model proposes that each tumor begins as a single cell that possess 2 functional properties: it can self-renew and it can produce several types of terminal cells. As these 2 properties are characteristics of somatic stem cells, 43 the cells have been called cancer stem cells (CSCs). 44 According to this model, tumors must have a hierarchical organization, where self-renewing stem cells produce highly proliferating progenitor cells, unable to self-renew but with a high proliferation potential. The latter, in turn, give rise to terminal cells. 45 Current evidence indicates that both models may coexist in tumor progression. In agreement with this idea, new subclones could be produced as a result of a lack of genetic stability and mutational changes, in addition to the heterogeneity derived from the initial CSC and its descendants. Thus, in each tumor, a set of neoplastic cells with different genetic and epigenetic traits may be found, which would provide different phenotypic properties. 46
The CSC concept was originally presented in a model of acute myeloid leukemia. 47 The presence of CSCs was later proved in chronic myeloid leukemia, breast cancer, tumors of the central nervous system, lung cancer, colon cancer, liver cancer, prostate cancer, pancreatic cancer, melanoma, and cancer of the head and neck, amongst others. In all of these cases, detection of CSCs was based on separation of several cell populations according to expression of specific surface markers, such as CD133, CD44, CD24, CD117, and CD15. 48 It is noteworthy that in some solid tumors, and even in some hematopoietic ones, a combination of specific markers that allow the isolation of CSCs has not been found. Interestingly, in such tumors, a high percentage of cells with the capacity to start secondary tumors has been observed; thus, the terms Tumor Initiating Cells (TIC) or Leukemia Initiating Cells (LIC) have been adopted. 46
A relevant aspect of the biology of CSCs is that, just like normal stem cells, they can self-renew. Such self-renewal guarantees the maintenance or expansion of the tumor stem cell population. Another trait CSCs share with normal stem cells is their quiescence, first described in chronic myeloid leukemia. 49 The persistence of quiescent CSCs in solid tumors has been recently described in colorectal cancer, where quiescent clones can become dominant after therapy with oxaliplatin. 50 In non-hierarchical tumors, such as melanoma, the existence of slow-cycling cells that are resistant to antimitogenic agents has also been proved. 51 Such experimental evidence supports the idea that quiescent CSCs or TICs are responsible for both tumor resistance to antineoplastic drugs and clinical relapse after initial therapeutic success.
In addition to quiescence, CSCs use other mechanisms to resist the action of chemotherapeutic drugs. One of these is their increased numbers: upon diagnosis, a high number of CSCs are observed in most analyzed tumors, making treatment unable to destroy all of them. On the other hand, CSCs have a high number of molecular pumps that expulse drugs, as well as high numbers of antiapoptotic molecules. In addition, they have very efficient mechanisms to repair DNA damage. In general, these cells show changes in a variety of signaling pathways involved in proliferation, survival, differentiation, and self-renewal. It is worth highlighting that in recent years, many of these pathways have become potential therapeutic targets in the elimination of CSCs. 52 Another aspect that is highly relevant in understanding the biological behavior of CSCs is that they require a specific site for their development within the tissue where they are found that can provide whatever is needed for their survival and growth. These sites, known as niches, are made of various cells, both tumor and non-tumor, as well as a variety of non-cellular elements (extracellular matrix [ECM], soluble cytokines, ion concentration gradients, etc.), capable of regulating the physiology of CSCs in order to promote their expansion, the invasion of adjacent tissues, and metastasis. 53
It is important to consider that although a large number of surface markers have been identified that allow us to enrich and prospectively follow tumor stem cell populations, to this day there is no combination of markers that allows us to find these populations in all tumors, and it is yet unclear if all tumors present them. In this regard, it is necessary to develop new purification strategies based on the gene expression profiles of these cells, so that tumor heterogeneity is taken into account, as it is evident that a tumor can include multiple clones of CSCs that, in spite of being functional, are genetically different, and that these clones can vary throughout space (occupying different microenvironments and niches) and time (during the progression of a range of tumor stages). Such strategies, in addition to new in vitro and in vivo assays, will allow the development of new and improved CSC elimination strategies. This will certainly have an impact on the development of more efficient therapeutic alternatives.
Invasion and Metastasis
Nearly 90% of the mortality associated with cancer is related to metastasis. 54 This consists of a cascade of events ( Figure 2 ) that begins with the local invasion of a tumor into surrounding tissues, followed by intravasation of tumor cells into the blood stream or lymphatic circulation. Extravasation of neoplastic cells in areas distant from the primary tumor then leads to the formation of one or more micrometastatic lesions which subsequently proliferate to form clinically detectable lesions. 4 The cells that are able to produce metastasis must acquire migratory characteristics, which occur by a process known as epithelial–mesenchymal transition (EMT), that is, the partial loss of epithelial characteristics and the acquirement of mesenchymal traits. 55

Invasion and metastasis cascade. Invasion and metastasis can occur early or late during tumor progression. In either case, invasion to adjacent tissues is driven by stem-like cells (cancer stem cells) that acquire the epithelial–mesenchymal transition (EMT) (1). Once they reach sites adjacent to blood vessels, tumor cells (individually or in clusters) enter the blood (2). Tumor cells in circulation can adhere to endothelium and extravasation takes place (3). Other mechanisms alternative to extravasation can exist, such as angiopelosis, in which clusters of tumor cells are internalized by the endothelium. Furthermore, at certain sites, tumor cells can obstruct microvasculature and initiate a metastatic lesion right there. Sometimes, a tumor cells that has just exit circulation goes into an MET in order to become quiescent (4). Inflammatory signals can activate quiescent metastatic cells that will proliferate and generate a clinically detectable lesion (5).
Although several of the factors involved in this process are currently known, many issues are still unsolved. For instance, it has not yet been possible to monitor in vivo the specific moment when it occurs 54 ; the microenvironmental factors of the primary tumor that promote such a transition are not known with precision; and the exact moment during tumor evolution in which one cell or a cluster of cells begin to migrate to distant areas, is also unknown. The wide range of possibilities offered by intra- and inter-tumoral heterogeneity 56 stands in the way of suggesting a generalized strategy that could resolve this complication.
It was previously believed that metastasis was only produced in late stages of tumor progression; however, recent studies indicate that EMT and metastasis can occur during the early course of the disease. In pancreatic cancer, for example, cells going through EMT are able to colonize and form metastatic lesions in the liver in the first stages of the disease. 52 , 57 Metastatic cell clusters circulating in peripheral blood (PB) are prone to generate a metastatic site, compared to individual tumor cells. 58 , 59 In this regard, novel strategies, such as the use of micro-RNAs, are being assessed in order to diminish induction of EMT. 60 It must be mentioned, however, that the metastatic process seems to be even more complex, with alternative pathways that do not involve EMT. 61 , 62
A crucial stage in the process of metastasis is the intravasation of tumor cells (alone or in clusters) towards the blood stream and/or lymphatic circulation. 63 These mechanisms are also under intensive research because blocking them could allow the control of spreading of the primary tumor. In PB or lymphatic circulation, tumor cells travel to distant parts for the potential formation of a metastatic lesion. During their journey, these cells must stand the pressure of blood flow and escape interaction with natural killer (NK) cells . 64 To avoid them, tumor cells often cover themselves with thrombocytes and also produce factors such as VEGF, angiopoietin-2, angiopoietin-4, and CCL2 that are involved in the induction of vascular permeability. 54 , 65 Neutrophils also contribute to lung metastasis in the bloodstream by secreting IL-1β and metalloproteases to facilitate extravasation of tumor cells. 64
The next step in the process of metastasis is extravasation, for which tumor cells, alone or in clusters, can use various mechanisms, including a recently described process known as angiopellosis that involves restructuring the endothelial barrier to internalize one or several cells into a tissue. 66 The study of leukocyte extravasation has contributed to a more detailed knowledge of this process, in such a way that some of the proposed strategies to avoid extravasation include the use of integrin inhibitors, molecules that are vital for rolling, adhesion, and extravasation of tumor cells. 67 , 68 Another strategy that has therapeutic potential is the use of antibodies that strengthen vascular integrity to obstruct transendothelial migration of tumor cells and aid in their destruction in PB. 69
Following extravasation, tumor cells can return to an epithelial phenotype, a process known as mesenchymal–epithelial transition and may remain inactive for several years. They do this by competing for specialized niches, like those in the bone marrow, brain, and intestinal mucosa, which provide signals through the Notch and Wnt pathways. 70 Through the action of the Wnt pathway, tumor cells enter a slow state of the cell cycle and induce the expression of molecules that inhibit the cytotoxic function of NK cells. 71 The extravasated tumor cell that is in a quiescent state must comply with 2 traits typical of stem cells: they must have the capacity to self-renew and to generate all of the cells that form the secondary tumor.
There are still several questions regarding the metastatic process. One of the persisting debates at present is if EMT is essential for metastasis or if it plays a more important role in chemoresistance. 61 , 62 It is equally important to know if there is a pattern in each tumor for the production of cells with the capacity to carry out EMT. In order to control metastasis, it is fundamental to know what triggers acquisition of the migratory phenotype and the intrinsic factors determining this transition. Furthermore, it is essential to know if mutations associated with the primary tumor or the variety of epigenetic changes are involved in this process. 55 It is clear that metastatic cells have affinity for certain tissues, depending on the nature of the primary tumor (seed and soil hypothesis). This may be caused by factors such as the location and the direction of the bloodstream or lymphatic fluid, but also by conditioning of premetastatic niches at a distance (due to the large number of soluble factors secreted by the tumor and the recruitment of cells of the immune system to those sites). 72 We have yet to identify and characterize all of the elements that participate in this process. Deciphering them will be of upmost importance from a therapeutic point of view.
Epidemiology of Cancer
Cancer is the second cause of death worldwide; today one of every 6 deaths is due to a type of cancer. According to the International Agency for Research on Cancer (IARC), in 2020 there were approximately 19.3 million new cases of cancer, and 10 million deaths by this disease, 6 while 23.8 million cases and 13.0 million deaths are projected to occur by 2030. 73 In this regard, it is clear the increasing role that environmental factors—including environmental pollutants and processed food—play as cancer inducers and promoters. 74 The types of cancer that produce the greatest numbers of cases and deaths worldwide are indicated in Table 1 . 6
Total Numbers of Cancer Cases and Deaths Worldwide in 2020 by Cancer Type (According to the Global Cancer Observatory, IARC).
Data presented on this table were obtained from Ref. 6.
As shown in Figure 3 , lung, breast, prostate, and colorectal cancer are the most common throughout the world, and they are mostly concentrated in countries of high to very high human development index (HDI). Although breast, prostate, and colorectal cancer have a high incidence, the number of deaths they cause is proportionally low, mostly reflecting the great progress made in their control. However, these data also reveal the types of cancer that require further effort in prevention, precise early detection avoiding overdiagnosis, and efficient treatment. This is the case of liver, lung, esophageal, and pancreatic cancer, where the difference between the number of cases and deaths is smaller ( Figure 3B ). Social and economic transition in several countries has had an impact on reducing the incidence of neoplasms associated with infection and simultaneously produced an increase in the types related to reproductive, dietary, and hormonal factors. 75

Incidence and mortality for some types of cancer in the world. (A) Estimated number of cases and deaths in 2020 for the most frequent cancer types worldwide. (B) Incidence and mortality rates, normalized according to age, for the most frequent cancer types in countries with very high/& high (VH&H; blue) and/low and middle (L&M; red) Human Development Index (HDI). Data include both genders and all ages. Data according to https://gco.iarc.fr/today , as of June 10, 2021.
In the past 3 decades, cancer mortality rates have fallen in high HDI countries, with the exception of pancreatic cancer, and lung cancer in women. Nevertheless, changes in the incidence of cancer do not show the same consistency, possibly due to variables such as the possibility of early detection, exposure to risk factors, or genetic predisposition. 76 , 77 Countries such as Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the United Kingdom have reported a reduction in incidence and mortality in cancer of the stomach, colon, lung, and ovary, as well as an increase in survival. 78 Changes in modifiable risk factors, such as the use of tobacco, have played an important role in prevention. In this respect, it has been estimated that decline in tobacco use can explain between 35% and 45% of the reduction in cancer mortality rates, 79 while the fall in incidence and mortality due to stomach cancer can be attributed partly to the control of Helicobacter pylori infection. 80 Another key factor in the fall of mortality rates in developed countries has been an increase in early detection as a result of screening programs, as in breast and prostate cancer, which have had their mortality rates decreased dramatically in spite of an increase in their incidence. 76
Another important improvement observed in recent decades is the increase in survival rates, particularly in high HDI countries. In the USA, for example, survival rates for patients with prostate cancer at 5 years after initial diagnosis was 28% during 1947–1951; 69% during 1975–1977, and 100% during 2003–2009. Something similar occurred with breast cancer, with a 5-year survival rate of 54% in 1947–1951, 75% in 1975–1977, and 90% in 2003–2009. 81 In the CONCORD 3 version, age-standardize 5-year survival for patients with breast cancer in the USA during 2010–2014 was 90%, and 97% for prostate cancer patients. 82 Importantly, even among high HDI countries, significant differences have been identified in survival rates, being stage of disease at diagnosis, time for access to effective treatment, and comorbidities, the main factors influencing survival in these nations. 78 Unfortunately, survival rates in low HDI countries are significantly lower due to several factors, including lack of information, deficient screening and early detection programs, limited access to treatment, and suboptimal cancer registration. 82 It should be noted that in countries with low to middle HDI, neoplasms with the greatest incidence are those affecting women (breast and cervical cancer), which reflects not only a problem with access to health services, but also a serious inequality issue that involves social, cultural, and even religious obstacles. 83
Up to 42% of incident cases and 47% of deaths by cancer in the USA are due to potentially modifiable risk factors such as use of tobacco, physical activity, diet, and infection. 84 It has been calculated that 2.4 million deaths by cancer, mostly of the lung, can be attributed to tobacco. 73 In 2020, the incidence rate of lung cancer in Western Africa was 2.2, whereas in Polynesia and Eastern Asia was 37.3 and 34.4, respectively. 6 In contrast, the global burden of cancer associated with infection was 15.4%, but in Sub-Saharan Africa it was 30%. 85 Likewise, the incidence of cervical cancer in Eastern Africa was 40.1, in contrast with the USA and Canada that have a rate of 6.2. This makes it clear that one of the challenges we face is the reduction of the risk factors that are potentially modifiable and associated with specific types of cancer.
Improvement of survival rates and its disparities worldwide are also important challenges. Five-year survival for breast cancer—diagnosed during 2010-2014— in the USA, for example, was 90%, whereas in countries like South Africa it was 40%. 82 Childhood leukemia in the USA and several European countries shows a 5-year survival of 90%, while in Latin-American countries it is 50–76%. 86 Interestingly, there are neoplasms, such as pancreatic cancer, for which there has been no significant increase in survival, which remains low (5–15%) both in developed and developing countries. 82
Although data reported on global incidence and mortality gives a general overview on the epidemiology of cancer, it is important to note that there are great differences in coverage of cancer registries worldwide. To date, only 1 out of every 3 countries reports high quality data on the incidence of cancer. 87 For the past 50 years, the IARC has supported population-based cancer registries; however, more than one-third of the countries belonging to the WHO, mainly countries of low and middle income (LMIC), have no data on more than half of the 18 indicators of sustainable development goals. 88 High quality cancer registries only cover 4% of the population in Africa, 8% in Asia, and 7% in Latin America, contrasting with 83% in the USA and Canada, and 33% in Europe. 89 In response to this situation, the Global Initiative for Cancer Registry Development was created in 2012 to generate improved infrastructure to permit greater coverage and better quality registries, especially in countries with low and middle HDI. 88 It is expected that initiatives of this sort in the coming years will allow more and better information to guide strategies for the control of cancer worldwide, especially in developing regions. This will enable survival to be measured over longer periods of time (10, 15, or 20 years), as an effective measure in the control of cancer. The WHO has established as a target for 2025 to reduce deaths by cancer and other non-transmissible diseases by 25% in the population between the ages of 30–69; such an effort requires not only effective prevention measures to reduce incidence, but also more efficient health systems to diminish mortality and increase survival. At the moment, it is an even greater challenge because of the effects of the COVID-19 pandemic which has negatively impacted cancer prevention and health services. 90
Oncologic Treatments
A general perspective.
At the beginning of the 20th century, cancer treatment, specifically treatment of solid tumors, was based fundamentally on surgical resection of tumors, which together with other methods for local control, such as cauterization, had been used since ancient times. 91 At that time, there was an ongoing burst of clinical observations along with interventions sustained on fundamental knowledge about physics, chemistry, and biology. In the final years of the 19 th century and the first half of the 20th, these technological developments gave rise to radiotherapy, hormone therapy, and chemotherapy. 92 - 94 Simultaneously, immunotherapy was also developed, although usually on a smaller scale, in light of the overwhelming progress of chemotherapy and radiotherapy. 95
Thus began the development and expansion of disciplines based on these approaches (surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy), with their application evolving ever more rapidly up to their current uses. Today, there is a wide range of therapeutic tools for the care of cancer patients. These include elements that emerged empirically, arising from observations of their effects in various medical fields, as well as drugs that were designed to block processes and pathways that form part of the physiopathology of one or more neoplasms according to knowledge of specific molecular alterations. A classic example of the first sort of tool is mustard gas, originally used as a weapon in war, 96 but when applied for medical purposes, marked the beginning of the use of chemicals in the treatment of malignant neoplasms, that is, chemotherapy. 94 A clear example of the second case is imatinib, designed specifically to selectively inhibit a molecular alteration in chronic myeloid leukemia: the Bcr-Abl oncoprotein. 97
It is on this foundation that today the 5 areas mentioned previously coexist and complement one another. The general framework that motivates this amalgam and guides its development is precision medicine, founded on the interaction of basic and clinical science. In the forecasts for development in each of these fields, surgery is expected to continue to be the fundamental approach for primary tumors in the foreseeable future, as well as when neoplastic disease in the patient is limited, or can be limited by applying systemic or regional elements, before and/or after surgical resection, and it can be reasonably anticipated for the patient to have a significant period free from disease or even to be cured. With regards to technology, intensive exploration of robotic surgery is contemplated. 98
The technological possibilities for radiotherapy have progressed in such a way that it is now possible to radiate neoplastic tissue with an extraordinary level of precision, and therefore avoid damage to healthy tissue. 99 This allows administration of large doses of ionizing radiation in one or a few fractions, what is known as “radiosurgery.” The greatest challenges to the efficacy of this approach are related to radio-resistance in certain neoplasms. Most efforts regarding research in this field are concentrated on understanding the underlying biological mechanisms of the phenomenon and their potential control through radiosensitizers. 100
“Traditional” chemotherapy, based on the use of compounds obtained from plants and other natural products, acting in a non-specific manner on both neoplastic and healthy tissues with a high proliferation rate, continues to prevail. 101 The family of chemotherapeutic drugs currently includes alkylating agents, antimetabolites, anti-topoisomerase agents, and anti-microtubules. Within the pharmacologic perspective, the objective is to attain a high concentration or activity of such molecules in specific tissues while avoiding their accumulation in others, in order to achieve an increase in effectiveness and a reduction in toxicity. This has been possible with the use of viral vectors, for example, that are able to limit their replication in neoplastic tissues, and activate prodrugs of normally nonspecific agents, like cyclophosphamide, exclusively in those specific areas. 102 More broadly, chemotherapy also includes a subgroup of substances, known as molecular targeted therapy, that affect processes in a more direct and specific manner, which will be mentioned later.
There is no doubt that immunotherapy—to be explored next—is one of the therapeutic fields where development has been greatest in recent decades and one that has produced enormous expectation in cancer treatment. 103 Likewise, cell therapy, based on the use of immune cells or stem cells, has come to complement the oncologic therapeutic arsenal. 43 Each and every one of the therapeutic fields that have arisen in oncology to this day continue to prevail and evolve. Interestingly, the foreseeable future for the development of cancer treatment contemplates these approaches in a joint and complementary manner, within the general framework of precision medicine, 104 and sustained by knowledge of the biological mechanisms involved in the appearance and progression of neoplasms. 105 , 106
Immunotherapy
Stimulating the immune system to treat cancer patients has been a historical objective in the field of oncology. Since the early work of William Coley 107 to the achievements reached at the end of the 20 th century, scientific findings and technological developments paved the way to searching for new immunotherapeutic strategies. Recombinant DNA technology allowed the synthesis of cytokines, such as interferon-alpha (IFN-α) and interleukin 2 (IL-2), which were authorized by the US Food and Drug Administration (FDA) for the treatment of hairy cell leukemia in 1986, 108 as well as kidney cancer and metastatic melanoma in 1992 and 1998, respectively. 109
The first therapeutic vaccine against cancer, based on the use of autologous dendritic cells (DCs), was approved by the FDA against prostate cancer in 2010. However, progress in the field of immunotherapy against cancer was stalled in the first decade of the present century, mostly due to failure of several vaccines in clinical trials. In many cases, application of these vaccines was detained by the complexity and cost involved in their production. Nevertheless, with the coming of the concept of immune checkpoint control, and the demonstration of the relevance of molecules such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed cell death molecule-1 (PD-1), immunotherapy against cancer recovered its global relevance. In 2011, the monoclonal antibody (mAb) ipilimumab, specific to the CTLA-4 molecule, was the first checkpoint inhibitor (CPI) approved for the treatment of advanced melanoma. 110 Later, inhibitory mAbs for PD-1, or for the PD-1 ligand (PD-L1), 111 as well as the production of T cells with chimeric receptors for antigen recognition (CAR-T), 112 which have been approved to treat various types of cancer, including melanoma, non-small cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), and hepatocellular carcinoma, among others, have changed the paradigm of cancer treatment.
In spite of the current use of anti-CTLA-4 and anti-PD-L1 mAbs, only a subgroup of patients has responded favorably to these CPIs, and the number of patients achieving clinical benefit is still small. It has been estimated that more than 70% of patients with solid tumors do not respond to CPI immunotherapy because either they show primary resistance, or after responding favorably, develop resistance to treatment. 113 In this regard, it is important to mention that in recent years very important steps have been taken to identify the intrinsic and extrinsic mechanisms that mediate resistance to CPI immunotherapy. 114 Intrinsic mechanisms include changes in the antitumor immune response pathways, such as faulty processing and presentation of antigens by APCs, activation of T cells for tumor cell destruction, and changes in tumor cells that lead to an immunosuppressive TME. Extrinsic factors include the presence of immunosuppressive cells in the local TME, such as regulatory T cells, myeloid-derived suppressor cells (MDSC), mesenchymal stem/stromal cells (MSCs), and type 2 macrophages (M2), in addition to immunosuppressive cytokines.
On the other hand, classification of solid tumors as “hot,” “cold,” or “excluded,” depending on T cell infiltrates and the contact of such infiltrates with tumor cells, as well as those that present high tumor mutation burden (TMB), have redirected immunotherapy towards 3 main strategies 115 ( Table 2 ): (1) Making T-cell antitumor response more effective, using checkpoint inhibitors complementary to anti-CTLA-4 and anti-PD-L1, such as LAG3, Tim-3, and TIGT, as well as using CAR-T cells against tumor antigens. (2) Activating tumor-associated myeloid cells including monocytes, granulocytes, macrophages, and DC lineages, found at several frequencies within human solid tumors. (3) Regulating the biochemical pathways in TME that produce high concentrations of immunosuppressive molecules, such as kynurenine, a product of tryptophan metabolism, through the activity of indoleamine 2,3 dioxygenase; or adenosine, a product of ATP hydrolysis by the activity of the enzyme 5’nucleotidase (CD73). 116
Current Strategies to Stimulate the Immune Response for Antitumor Immunotherapy.
Abbreviations: TME, tumor microenvironment; IL, interleukin; TNF, Tumor Necrosis Factor; TNFR, TNF-receptor; CD137, receptor–co-stimulator of the TNFR family; OX40, member number 4 of the TNFR superfamily; CD27/CD70, member of the TNFR superfamily; CD40/CD40L, antigen-presenting cells (APC) co-stimulator and its ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; STING, IFN genes-stimulator; RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation-associated protein 5; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high mobility group B1 protein; TLR, Toll-like receptor; HVEM, Herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T lymphocyte antigen 4; PD-L1, programmed death ligand-1; TIGIT, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibition motives; CSF1/CSF1R, colony-stimulating factor-1 and its receptor; CCR2, Type 2 chemokine receptor; PI3Kγ, Phosphoinositide 3-Kinase γ; CXCL/CCL, chemokine ligands; LFA1, lymphocyte function-associated antigen 1; ICAM1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indolamine 2,3-dioxigenase; TGF, transforming growth factor; LAG-3, lymphocyte-activation gene 3 protein; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CD73, 5´nucleotidase; ARs, adenosine receptors; Selectins, cell adhesion molecules; CAR-T, chimeric antigen receptor T cell; TCR-T, T-cell receptor engineered T cell.
Apart from the problems associated with its efficacy (only a small group of patients respond to it), immunotherapy faces several challenges related to its safety. In other words, immunotherapy can induce adverse events in patients, such as autoimmunity, where healthy tissues are attacked, or cytokine release syndrome and vascular leak syndrome, as observed with the use of IL-2, both of which lead to serious hypotension, fever, renal failure, and other adverse events that are potentially lethal. The main challenges to be faced by immunotherapy in the future will require the combined efforts of basic and clinical scientists, with the objective of accelerating the understanding of the complex interactions between cancer and the immune system, and improve treatment options for patients. Better comprehension of immune phenotypes in tumors, beyond the state of PD-L1 and TME, will be relevant to increase immunotherapy efficacy. In this context, the identification of precise tumor antigenicity biomarkers by means of new technologies, such as complete genome sequencing, single cell sequencing, and epigenetic analysis to identify sites or subclones typical in drug resistance, as well as activation, traffic and infiltration of effector cells of the immune response, and regulation of TME mechanisms, may help define patient populations that are good candidates for specific therapies and therapeutic combinations. 117 , 118 Likewise, the use of agents that can induce specific activation and modulation of the response of T cells in tumor tissue, will help improve efficacy and safety profiles that can lead to better clinical results.
Molecular Targeted Therapy
For over 30 years, and based on the progress in our knowledge of tumor biology and its mechanisms, there has been a search for therapeutic alternatives that would allow spread and growth of tumors to be slowed down by blocking specific molecules. This approach is known as molecular targeted therapy. 119 Among the elements generally used as molecular targets there are transcription factors, cytokines, membrane receptors, molecules involved in a variety of signaling pathways, apoptosis modulators, promoters of angiogenesis, and cell cycle regulators. 120
Imatinib, a tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia, became the first targeted therapy in the final years of the 1990s. 97 From then on, new drugs have been developed by design, and today more than 60 targeted therapies have been approved by the FDA for the treatment of a variety of cancers ( Table 3 ). 121 This has had a significant impact on progression-free survival and global survival in neoplasms such as non-small cell lung cancer, breast cancer, renal cancer, and melanoma.
FDA Approved Molecular Targeted Therapies for the Treatment of Solid Tumors.
Abbreviations: mAb, monoclonal antibody; ALK, anaplastic lymphoma kinase; CDK, cyclin-dependent kinase; CTLA-4, cytotoxic lymphocyte antigen-4; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stroma tumor; mTOR, target of rapamycine in mammal cells; NSCLC, non-small cell lung carcinoma; PARP, poli (ADP-ribose) polimerase; PD-1, programmed death protein-1; PDGFR, platelet-derived growth factor receptor; PD-L1, programmed death ligand-1; ER, estrogen receptor; PR, progesterone receptor; TKR, tyrosine kinase receptors; SERM, selective estrogen receptor modulator; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Modified from Ref. [ 127 ].
Most drugs classified as targeted therapies form part of 2 large groups: small molecules and mAbs. The former are defined as compounds of low molecular weight (<900 Daltons) that act upon entering the cell. 120 Targets of these compounds are cell cycle regulatory proteins, proapoptotic proteins, or DNA repair proteins. These drugs are indicated based on histological diagnosis, as well as molecular tests. In this group there are multi-kinase inhibitors (RTKs) and tyrosine kinase inhibitors (TKIs), like sunitinib, sorafenib, and imatinib; cyclin-dependent kinase (CDK) inhibitors, such as palbociclib, ribociclib and abemaciclib; poli (ADP-ribose) polimerase inhibitors (PARPs), like olaparib and talazoparib; and selective small-molecule inhibitors, like ALK and ROS1. 122
As for mAbs, they are protein molecules that act on membrane receptors or extracellular proteins by interrupting the interaction between ligands and receptors, in such a way that they reduce cell replication and induce cytostasis. Among the most widely used mAbs in oncology we have: trastuzumab, a drug directed against the receptor for human epidermal growth factor-2 (HER2), which is overexpressed in a subgroup of patients with breast and gastric cancer; and bevacizumab, that blocks vascular endothelial growth factor and is used in patients with colorectal cancer, cervical cancer, and ovarian cancer. Other mAbs approved by the FDA include pembolizumab, atezolizumab, nivolumab, avelumab, ipilimumab, durvalumab, and cemiplimab. These drugs require expression of response biomarkers, such as PD-1 and PD-L1, and must also have several resistance biomarkers, such as the expression of EGFR, the loss of PTEN, and alterations in beta-catenin. 123
Because cancer is such a diverse disease, it is fundamental to have precise diagnostic methods that allow us to identify the most adequate therapy. Currently, basic immunohistochemistry is complemented with neoplastic molecular profiles to determine a more accurate diagnosis, and it is probable that in the near future cancer treatments will be based exclusively on molecular profiles. In this regard, it is worth mentioning that the use of targeted therapy depends on the existence of specific biomarkers that indicate if the patient will be susceptible to the effects of the drug or not. Thus, the importance of underlining that not all patients are susceptible to receive targeted therapy. In certain neoplasms, therapeutic targets are expressed in less than 5% of the diagnosed population, hindering a more extended use of certain drugs.
The identification of biomarkers and the use of new generation sequencing on tumor cells has shown predictive and prognostic relevance. Likewise, mutation analysis has allowed monitoring of tumor clone evolution, providing information on changes in canonic gene sequences, such as TP53, GATA3, PIK3CA, AKT1, and ERBB2; infrequent somatic mutations developed after primary treatments, like SWI-SNF and JAK2-STAT3; or acquired drug resistance mutations such as ESR1. 124 The study of mutations is vital; in fact, many of them already have specific therapeutic indications, which have helped select adequate treatments. 125
There is no doubt that molecular targeted therapy is one of the main pillars of precision medicine. However, it faces significant problems that often hinder obtaining better results. Among these, there is intratumor heterogeneity and differences between the primary tumor and metastatic sites, as well as intrinsic and acquired resistance to these therapies, the mechanisms of which include the presence of heterogeneous subclones, DNA hypermethylation, histone acetylation, and interruption of mRNA degradation and translation processes. 126 Nonetheless, beyond the obstacles facing molecular targeted therapy from a biological and methodological point of view, in the real world, access to genomic testing and specific drugs continues to be an enormous limitation, in such a way that strategies must be designed in the future for precision medicine to be possible on a global scale.
Cell Therapy
Another improvement in cancer treatment is the use of cell therapy, that is, the use of specific cells as therapeutic agents. This clinical procedure has 2 modalities: the first consists of replacing and regenerating functional cells in a specific tissue by means of stem/progenitor cells of a certain kind, 43 while the second uses immune cells as effectors to eliminate malignant cells. 127
Regarding the first type, we must emphasize the development of cell therapy based on hematopoietic stem and progenitor cells. 128 For over 50 years, hematopoietic cell transplants have been used to treat a variety of hematologic neoplasms (different forms of leukemia and lymphoma). Today, it is one of the most successful examples of cell therapy, including innovative modalities, such as haploidentical transplants, 129 as well as application of stem cells expanded ex vivo . 130 There are also therapies that have used immature cells that form part of the TME, such as MSCs. The replication potential and cytokine secretion capacity of these cells make them an excellent option for this type of treatment. 131 Neural stem cells can also be manipulated to produce and secrete apoptotic factors, and when these cells are incorporated into primary neural tumors, they cause a certain degree of regression. They can even be transfected with genes that encode for oncolytic enzymes capable of inducing regression of glioblastomas. 132
With respect to cell therapy using immune cells, several research groups have manipulated cells associated with tumors to make them effector cells and thus improve the efficacy and specificity of the antitumor treatment. PB leckocytes cultured in the presence of IL-2 to obtain activated lymphocytes, in combination with IL-2 administration, have been used in antitumor clinical protocols. Similarly, infiltrating lymphocytes from tumors with antitumor activity have been used and can be expanded ex vivo with IL-2. These lymphocyte populations have been used in immunomodulatory therapies in melanoma, and pancreatic and kidney tumors, producing a favorable response in treated patients. 133 NK cells and macrophages have also been used in immunotherapy, although with limited results. 134 , 135
One of the cell therapies with better projection today is the use of CAR-T cells. This strategy combines 2 forms of advanced therapy: cell therapy and gene therapy. It involves the extraction of T cells from the cancer patient, which are genetically modified in vitro to express cell surface receptors that will recognize antigens on the surface of tumor cells. The modified T cells are then reintroduced in the patient to aid in an exacerbated immune response that leads to eradication of the tumor cells ( Figure 4 ). Therapy with CAR-T cells has been used successfully in the treatment of some types of leukemia, lymphoma, and myeloma, producing complete responses in patients. 136

CAR-T cell therapy. (A) T lymphocytes obtained from cancer patients are genetically manipulated to produce CAR-T cells that recognize tumor cells in a very specific manner. (B) Interaction between CAR molecule and tumor antigen. CAR molecule is a receptor that results from the fusion between single-chain variable fragments (scFv) from a monoclonal antibody and one or more intracellular signaling domains from the T-cell receptor. CD3ζ, CD28 and 4-1BB correspond to signaling domains on the CAR molecule.
Undoubtedly, CAR-T cell therapy has been truly efficient in the treatment of various types of neoplasms. However, this therapeutic strategy can also have serious side effects, such as release of cytokines into the bloodstream, which can cause different symptoms, from high fever to multiorgan failure, and even neurotoxicity, leading to cerebral edema in many cases. 137 Adequate control of these side effects is an important medical challenge. Several research groups are trying to improve CAR-T cell therapy through various approaches, including production of CAR-T cells directed against a wider variety of tumor cell-specific antigens that are able to attack different types of tumors, and the identification of more efficient types of T lymphocytes. Furthermore, producing CAR-T cells from a single donor that may be used in the treatment of several patients would reduce the cost of this sort of personalized cell therapy. 136
Achieving wider use of cell therapy in oncologic diseases is an important challenge that requires solving various issues. 138 One is intratumor cell heterogeneity, including malignant subclones and the various components of the TME, which results in a wide profile of membrane protein expression that complicates finding an ideal tumor antigen that allows specific identification (and elimination) of malignant cells. Likewise, structural organization of the TME challenges the use of cell therapy, as administration of cell vehicles capable of recognizing malignant cells might not be able to infiltrate the tumor. This results from low expression of chemokines in tumors and the presence of a dense fibrotic matrix that compacts the inner tumor mass and avoids antitumor cells from infiltrating and finding malignant target cells.
Further Challenges in the 21st Century
Beyond the challenges regarding oncologic biomedical research, the 21 st century is facing important issues that must be solved as soon as possible if we truly wish to gain significant ground in our fight against cancer. Three of the most important have to do with prevention, early diagnosis, and access to oncologic medication and treatment.
Prevention and Early Diagnosis
Prevention is the most cost-effective strategy in the long term, both in low and high HDI nations. Data from countries like the USA indicate that between 40-50% of all types of cancer are preventable through potentially modifiable factors (primary prevention), such as use of tobacco and alcohol, diet, physical activity, exposure to ionizing radiation, as well as prevention of infection through access to vaccination, and by reducing exposure to environmental pollutants, such as pesticides, diesel exhaust particles, solvents, etc. 74 , 84 Screening, on the other hand, has shown great effectiveness as secondary prevention. Once population-based screening programs are implemented, there is generally an initial increase in incidence; however, in the long term, a significant reduction occurs not only in incidence rates, but also in mortality rates due to detection of early lesions and timely and adequate treatment.
A good example is colon cancer. There are several options for colon cancer screening, such as detection of fecal occult blood, fecal immunohistochemistry, flexible sigmoidoscopy, and colonoscopy, 139 , 140 which identify precursor lesions (polyp adenomas) and allow their removal. Such screening has allowed us to observe 3 patterns of incidence and mortality for colon cancer between the years 2000 and 2010: on one hand, an increase in incidence and mortality in countries with low to middle HDI, mainly countries in Asia, South America, and Eastern Europe; on the other hand, an increase in incidence and a fall in mortality in countries with very high HDI, such as Canada, the United Kingdom, Denmark, and Singapore; and finally a fall in incidence and mortality in countries like the USA, Japan, and France. The situation in South America and Asia seems to reflect limitations in medical infrastructure and a lack of access to early detection, 141 while the patterns observed in developed countries reveal the success, even if it may be partial, of that which can be achieved by well-structured prevention programs.
Another example of success, but also of strong contrast, is cervical cancer. The discovery of the human papilloma virus (HPV) as the causal agent of cervical cancer brought about the development of vaccines and tests to detect oncogenic genotypes, which modified screening recommendations and guidelines, and allowed several developed countries to include the HPV vaccine in their national vaccination programs. Nevertheless, the outlook is quite different in other areas of the world. Eighty percent of the deaths by cervical cancer reported in 2018 occurred in low-income nations. This reveals the urgency of guaranteeing access to primary and secondary prevention (vaccination and screening, respectively) in these countries, or else it will continue to be a serious public health problem in spite of its preventability.
Screening programs for other neoplasms, such as breast, prostate, lung, and thyroid cancer have shown outlooks that differ from those just described, because, among other reasons, these neoplasms are highly diverse both biologically and clinically. Another relevant issue is the overdiagnosis of these neoplasms, that is, the diagnosis of disease that would not cause symptoms or death in the patient. 142 It has been calculated that 25% of breast cancer (determined by mammogram), 50–60% of prostate cancer (determined by PSA), and 13–25% of lung cancer (determined by CT) are overdiagnosed. 142 Thus, it is necessary to improve the sensitivity and specificity of screening tests. In this respect, knowledge provided by the biology of cancer and “omic” sciences offers a great opportunity to improve screening and prevention strategies. All of the above shows that prevention and early diagnosis are the foundations in the fight against cancer, and it is essential to continue to implement broader screening programs and better detection methods.
Global Equity in Oncologic Treatment
Progress in cancer treatment has considerably increased the number of cancer survivors. Nevertheless, this tendency is evident only in countries with a very solid economy. Indeed, during the past 30 years, cancer mortality rates have increased 30% worldwide. 143 Global studies indicate that close to 70% of cancer deaths in the world occur in nations of low to middle income. But even in high-income countries, there are sectors of society that are more vulnerable and have less access to cancer treatments. 144 Cancer continues to be a disease of great social inequality.
In Europe, the differences in access to cancer treatment are highly marked. These treatments are more accessible in Western Europe than in its Eastern counterpart. 145 Furthermore, highly noticeable differences between high-income countries have been detected in the cost of cancer drugs. 146 It is interesting to note that in many of these cases, treatment is too costly and the clinical benefit only marginal. Thus, the importance of these problems being approached by competent national, regional, and global authorities, because if these new drugs and therapeutic programs are not accessible to the majority, progress in biomedical, clinical and epidemiological research will have a limited impact in our fight against cancer. We must not forget that health is a universal right, from which low HDI countries must not be excluded, nor vulnerable populations in nations with high HDI. The participation of a well-informed society will also be fundamental to achieve a global impact, as today we must fight not only against the disease, but also against movements and ideas (such as the anti-vaccine movement and the so-called miracle therapies) that can block the medical battle against cancer.
Final Comments
From the second half of the 20th century to the present day, progress in our knowledge about the origin and development of cancer has been extraordinary. We now understand cancer in detail in genomic, molecular, cellular, and physiological terms, and this knowledge has had a significant impact in the clinic. There is no doubt that a patient who is diagnosed today with a type of cancer has a better prospect than a patient diagnosed 20 or 50 years ago. However, we are still far from winning the war against cancer. The challenges are still numerous. For this reason, oncologic biomedical research must be a worldwide priority. Likewise, one of the fundamental challenges for the coming decades must be to reduce unequal access to health services in areas of low- to middle income, and in populations that are especially vulnerable, as well as continue improving prevention programs, including public health programs to reduce exposure to environmental chemicals and improve diet and physical activity in the general population. 74 , 84 Fostering research and incorporation of new technological resources, particularly in less privileged nations, will play a key role in our global fight against cancer.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Hector Mayani https://orcid.org/0000-0002-2483-3782
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- JME Commentaries
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 47, Issue 11
- Cancer Research UK’S obesity campaign in 2018 and 2019: effective health promotion or perpetuating the stigmatisation of obesity?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Natasha Varshney
- Liverpool School of Medicine , University of Liverpool , Liverpool , UK
- Correspondence to Natasha Varshney, University of Liverpool, Liverpool L69 3BX, UK; hlnvarsh{at}liv.ac.uk
In 2018 and 2019 Cancer Research UK (CRUK) launched a controversial advertising campaign to inform the British public of obesity being a preventable cause of cancer. On each occasion the advertisements used were emotive and provoked frustration among the British public which was widely vocalised on social media. As well serving to educate the public of this association, the advertisements also had the secondary effect of acting as health promotion through social marketing, a form of advertising designed to influence behavioural changes. As CRUK delivered a public health message through its campaign, the advertisements should be held according to the ethical principles which underpin healthcare in the UK. This article evaluates whether the advertisements used by CRUK in 2018 and 2019 fulfilled the ethical principles of beneficence, autonomy, non-maleficence and justice. It is found that while providing an important message, the oversimplification of obesity as being the result of personal decisions ignored the complex aetiology and served to stigmatise the target demographic, potentially disengaging them from the message. Additionally, posting cancer as the consequence of obesity invokes feelings of fear due to its connotations of suffering and premature death. Based on available evidence, the use of fear in social marketing does not create sustained behavioural change. This essay recommends that CRUK discontinue its use of such strategies in its future social marketing endeavours.
- health promotion
- political philosophy
Data availability statement
Data sharing is not applicable as no data sets were generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information. All data are available via PubMed. The health intervention ladder produced by the Nuffield Council of Bioethics is available for use via the publications section at www.nuffieldbioethics.org.
https://doi.org/10.1136/medethics-2020-106192
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Obesity, defined as a body mass index (BMI) of over 30, 1 continues to present an increasing challenge to individuals affected by it, health providers and governing bodies. Data published by the King’s Fund 1 found that in 2010, 67.8% of men and 57.8% of women were categorised as either overweight or obese. The health consequences of obesity include cardiovascular disease, diabetes and cancer, all of which result in increased mortality. While obesity is the complex product of genes and socioeconomic factors, lifestyle behaviours such as reduced physical activity and increased consumption of high-fat foods contribute to the disease state. 1
Cancer Research UK (CRUK), founded in 2002, has transformed the way in which cancer is diagnosed and treated, through its financial support of innovative research projects. Its website also describes its purpose as providing ‘cancer information to the public’. 2 As part of this mission, realising that obesity is the second leading preventable cause of cancer in the UK, CRUK launched a national advertising campaign in 2018 to raise awareness of this fact among the general public, and repeated it in 2019. The ethical shortcomings of these advertisements were identified through the fierce public debate triggered by them, with many members of the British public vocalising on social media their anger and frustration at CRUK’s propagation of obesity stigmatisation through the advertisements. 3
In both 2018 and 2019, CRUK advertisements were part of a national campaign, appearing on billboards and at bus stops. For both years, the adverts were minimalistic and two out of the three adverts used images of cigarettes and cigarette packaging. In 2018 ( figure 1 ), two adverts were launched to promote awareness of obesity as a risk factor for cancer. The first depicts a hangman game with letters missing in the word obesity ( figure 1A ). The second features potato chips enclosed in an open cigarette packet ( figure 1B ). In 2019 ( figure 2 ), images of popular cigarette packets were used with the word obesity replacing the brand name. In both years, a short sentence stated the risk between obesity and cancer. In an official statement released by CRUK in July 2019, after the release of the second advertising campaign, CRUK explained its choice of using cigarette packaging in the advertisements by describing how ‘smoking is the nation’s biggest preventable cause of cancer’ 4 followed by obesity.
- Download figure
- Open in new tab
- Download powerpoint
Parts 1A and 1B depict Cancer Research UK advertisements displayed from February 2018 to March 2018 as part of a national campaign to increase awareness of obesity as a causative factor for cancer.
Cancer Research UK advertisements displayed nationally in July 2019 to increase awareness on the association between cancer and obesity.
CRUK’s advertisements serve to inform the public but also have the secondary effect of acting as a public health intervention through social marketing. Social marketing can be defined as the use of commercial marketing methods to promote behavioural changes in a population. 5 Because CRUK’s advertisements carry this public health message, CRUK must be expected to honour the four ethical principles which underpin any health intervention, that is, of beneficence, autonomy, non-maleficence and justice. This review aims to evaluate whether the advertisements produced by CRUK respected these principles and whether CRUK should repeat such advertisements in future.
Beneficence
Successful conveyance of an important health message.
Tackling the rising prevalence of obesity has been a priority for health services worldwide. Consequences of obesity include premature mortality, hypertension, type 2 diabetes mellitus and cancer. Arnold and colleagues estimated that an elevated BMI is responsible for 3.6% of the incidence of cancer globally and found obesity to be the second most common preventable cause of cancer after smoking. 6 According to CRUK, when the initial campaign was launched in 2018, awareness of obesity as a cause of cancer was low at 15%. As a result of the campaign, awareness of this association was found to be 43%. It should be noted that CRUK does not describe the method used to assess this outcome. If these self-reported data are correct, then the educational value of CRUK’s campaign cannot be questioned. Based on this CRUK acted responsibly in relaying this information to, and in the best interests of, the British public.

Advertisements’ use as antiobesity social marketing through fear appeals
It can be argued that the advertisements, through their secondary guise as social marketing, were designed to inform and empower individuals to make lifestyle decisions which are beneficial to their health such as increased exercise and reduced consumption of high-calorie foods. Cancer connotes premature death and suffering and this fear may have acted as a trigger for individuals to lose weight to achieve improved health outcomes. This well-intentioned motivation provided by the advertisements is another example of how CRUK attempted to act for the good of the British public.
The form of health promotion that CRUK advertisements represent has been identified as communicative persuasion. According to Buchanan, 7 there are four underlying principles used in health promotion which progressively see a reduction in an individual’s freedom to choose. Applying Buchanan’s principles, it can be argued that CRUK’s adverts are a form of the third most restrictive practice of communicative persuasion via fear appeals. Fear appeals can be described as emotive messages which elicit negative emotions, such as anxiety, to persuade the audience to change their behaviour.
The idea of using fear to stimulate behavioural change is not novel in UK health promotion strategies. In 1987, in response to the AIDS epidemic, the Department of Health released a graphic television campaign consisting of a short video featuring apocalyptic imagery of a monolith and an iceberg with the narrator urging viewers not to ignore signs of AIDS and that everyone was vulnerable to it. This television campaign can be perceived as strikingly more intimidating than CRUK’s campaign; in comparison, the message was shocking and left no room for misinterpretation. However, the long-term effects of fear appeals remain limited. Vidanapathirana et al 8 conducted a systematic review evaluating mass media interventions in HIV prevention and found that media interventions designed to induce fear are effective in the short term but do not facilitate long-term behavioural changes. An explanation for this is provided by the seminal work carried out by Janis and Feshbach in 1953 9 who specifically investigated the effects of fear appeals and found that they produced three common behaviours, these being inattentiveness, aggressiveness and defensive avoidance. Similar to the obvious consequences of death portrayed in the 1987 media campaign, the connotations of cancer are premature morality and suffering. The potential fear of these consequences stimulated by CRUK’s campaign undermines their efforts at encouraging long-term changes. Rather than produce a positive behavioural change where obese individuals adopt healthy lifestyle behaviours of exercising more and eating a balanced diet, a maladaptive response to the campaign could occur with the message being ignored and dismissed, due to the way it had been presented. There are numerous other studies which agree that fear is an ineffective way to induce adoption of healthy lifestyle behaviours 10 11 and based on this evidence it is surprising that CRUK chose fear as the crux of its advertising campaign. Evidence shows that while fear appeals may be effective at inducing desired behavioural changes in the short term, the effect is not sustained and ultimately provides no lasting benefit to the individual.
Autonomy: CRUK advertisements: a criticism of individual choice?
The increasing numbers of people termed medically obese are creating a large financial and social burden on the UK government. Kent et al found that an increase in BMI led to a corresponding increase in the proportion of annual health costs associated with excess weight. 12 Kent performed a cohort study in England using data from the Million Women Study and found that the proportion of annual costs attributed to excess weight increased from 13% among women with a BMI between 25 and 30 kg/m 2 to 52% among women with a BMI in excess of 40kg/m 2 . These costs were associated with an increased prevalence of circulatory and gastrointestinal disease as well as neoplasms. Kent’s findings provide an insight into the financial implications associated with obesity for one demographic group and emphasise the importance of finding a sustainable solution to this.
UK governments have implemented numerous corporate and public health policies to reduce obesity prevalence which include the 2003 Travel to School initiative, the 2004 National Fruit and Vegetable initiative and the 2007 television advertisement restrictions which prevent advertising of high-fat, sugar and salt foods in or around the showing of children’s television programmes. All were important policies in reducing the prevalence of childhood obesity. A government-commissioned report in 2007 set the basis for the direction of future antiobesity policies. 13 This report highlighted that interventions across all levels, including individual and national, were needed and that these interventions should occur across all age groups. This formed the basis of the 2009 social marketing campaign ‘Change4Life’ (C4L). 14 C4L is the main campaign to reduce childhood obesity and targets families to encourage them to adopt healthier lifestyles by increasing their uptake of physical activity and promoting healthy dietary habits. It has since broadened its scope to target adults whose unhealthy behaviours put them at increased risk of chronic disease. Created by Public Health England (PHE), C4L was founded on the principles of ‘Libertarian Paternalism’ set out in the Nuffield Council on Bioethics 2007 report, 15 a concept which seeks to encourage healthy behaviour by providing information and enabling individual choice. This concept is low down on the public health intervention ladder endorsed by the Nuffield Trust, 16 to facilitate acceptance by and engagement from the public. C4L has strengthened over the years with each new government and new corporate partners including Disney and Sport England.
By encouraging individuals to reassess their lifestyle behaviours in a supportive and optional way without criticising their existing unhealthy behaviours, C4L does not alienate any individual and respects their autonomy. The British public have the choice to not to look at the advertisements and read about the interventions, but on the chance they do, by presenting obesity as analogous to smoking, which carries the connotation that smoking is subject to an individual’s free will, CRUK could be accused of criticising an individual’s personal choice and not respecting their autonomy.
In contrast to CRUK, the libertarian paternalism approach adopted by PHE is judged to be the best way in which to respect an individual’s right to make their own decisions. By excluding the term obesity in its materials, C4L is able to engage with motivated users while at the same time respecting their right to live life according to their own decisions. It can be argued that the state has a duty to oversee the general welfare of the population on utilitarian grounds, for example, through legislation banning smoking indoor public spaces. It is widely accepted that when an individual’s actions have the potential to directly harm other members of the public, the government has a legitimate basis to intervene. If no such legitimacy exists, the government could be accused of attempting to curtail an individual’s freedoms: this is the complete antithesis of a fully democratic state. While CRUK is not going so far as to curtail an individual’s decisions, it is certainly not respecting an individual’s decision to smoke and completely ignoring the fact that for some individuals due to biochemical neurological changes, addiction has taken hold. It could be argued that when this physiological state of dependence occurs there is very little an individual can do to easily counter it.
A study conducted by Croker in 2011 17 evaluated the impact of the C4L campaign 2 years after its launch. The study design encompassed an intervention group exposed to C4L resources, to learn whether the campaign facilitated the adoption of healthy behaviours. The results were disappointing, with participants stating that the C4L campaign had little effect on their dietary behaviours and physical activity levels, although there was a significant increase in acknowledgement of the importance of the latter. It is important to note that this study was conducted soon after the initial launch in 2009 and is the only available data assessing the impact of C4L. The brand has increased in terms of its scope and resources; therefore, the data Croker found in 2011 are no longer valid given the evolution of the campaign. To assess the impact of using the liberal paternalism approach on modifying lifestyle behaviours, it is recommended that a randomised longitudinal cohort study be conducted. Including a study group exposed to stand-alone advertisements comprising fear appeals, such as those used by CRUK, would serve to definitely establish what the best form of social marketing is for reducing obesity prevalence.
Non-maleficence
A promotion of obesity stigmatisation.
The aetiology of smoking has been judged to be psychosocial. 18 Other environmental factors influence individuals choosing to smoke. Nonetheless, independent choice can be considered to be a significant contributing factor. On this basis, through the use of cigarette packet imagery, CRUK could be suggesting that obesity is also subject to independent choice. Additionally, smoking carries connotations of addiction, a behaviour which can be oversimplified to be the result of a lack of self-control. Using this interpreted context as the background to an antiobesity message implies that obesity too is due to the lack of self-regulatory behaviour. These inferences are harmful to those who are obese and also fail to acknowledge the complex, multifactorial aetiology of obesity. Brandkvist et al conducted a longitudinal cohort study and provided genetic evidence that single nucleotide polymorphisms can affect the obesogenic physiological pathways in the body, which in conjunction with individual health behaviours can lead to obesity. 19 These behaviours include a sedentary lifestyle, increasing use of cars and labour-saving machines and decisions to consume cheap, high-energy fast food in excessive amounts, which in turn is facilitated by the environmental factor of increased fast-food outlets and higher costs associated with fruits and vegetables. As Mitchell et al 20 state, the obesity crisis is due to the environment and culture which facilitates it, rather than individual agency. Mitchell even goes as far as to argue that weight loss, as promoted by CRUK advertisements, is futile in aiming to reduce obesity, given that most obese individuals are unable to sustain weight loss. By focusing on obesity as the product of a lack of self-control and independent choice, CRUK fails to acknowledge this complex aetiology and places the responsibility entirely on the obese individual. This stigmatisation could result in doing more harm than good by deterring the obese from engaging with the important health promotion message that losing weight will lead to better health outcomes for them.
Weiner et al assessed the relationship between perceptions of personal responsibility and stigmatising conditions 21 and found that individuals with high stigmatising conditions were seen as having correspondingly high rates of personal responsibility and were less likely to evoke feelings of pity and support. Weight-based stigmatisation is, unfortunately, a very prevalent form of discrimination in society and has wholly negative effects on efforts to reduce weight. Stigmatisation is not a useful tool to discourage unhealthy behaviours. Indeed, Amy et al 22 found that obese women delayed seeking healthcare due to disrespectful behaviours exhibited towards them by health professionals. The adverse effects of weight-based stigmatisation include an increased stress response which increases circulating levels of the hormone cortisol, which itself increases appetite, as well as negatively impacting an individual’s mental health leading to depression and anxiety. These mental states can also lead to increased appetite, perpetuating the cycle of increased caloric consumption.
A corresponding argument should also be discussed regarding the stigmatisation of cancer. It has already been suggested that the connotations of cancer include premature mortality and suffering; CRUK’s use of cancer as a motivating factor for viewers to adopt healthy lifestyle behaviours further reinforces the negative associations of cancer in that there is nothing worse than this disease. While this assumption may be true for some patients, the reinforcing of the message by having it displayed directly and publicly may be detrimental to their well-being, challenging their mental resilience. Additionally, by suggesting cancer could be the result of a self-regulatory action, it calls into question an individual’s shortcomings. This is plainly physiologically false and fails to appreciate the complex aetiology of cancer being the product of genetic and environmental factors. To a layperson, such assumptions run the risk of reducing empathy and support for patients with cancer and ultimately could work against CRUK.
False inference on lung cancer causation
Viewing the imagery of cigarette packages, a lay member of the public could wrongly associate obesity as attributable to lung cancer. Neither the 2018 nor the 2019 advertisements which used cigarette imagery elaborated on which specific cancers is obesity a risk factor for. Lauby-Secretan and colleagues found significant evidence of obesity as a risk factor for 13 different types of cancer—lung cancer is not in this group 23 (see box 1 ). CRUK’s decision to use images of cigarette packaging could be a source of confusion for the target audience and also acts as misinformation
A list of 13 cancers identified by Lauby-Secretan et al in 2016 which have sufficient evidence to show that excess fat absence reduces the risk of development of that particular cancer 23
Cancer site or type where there is sufficient evidence in humans that obesity absence is preventative from developing the cancer.
Oesophagus: adenocarcinoma.
Gastric cardia.
Colon and rectum.
Gall bladder.
Breast: postmenopausal.
Corpus uteri.
Kidney: renal cell.
Meningioma.
Multiple myeloma.
Justice: a well-intended but feeble attempt to reduce health inequalities
CRUK’s campaign was nationwide, ensuring that the message of obesity contributing to cancer was received by everyone across the UK, creating equity in population knowledge. Additionally, as stated above by Mitchell et al , 20 a larger group of factors contributing to obesity are environmental, which includes low family income and low parental education status. 24 Due to this there is an increased obesity prevalence among individuals from a low socioeconomic background, leading to the consequences of increased morbidity and mortality, including cancer. CRUK’s campaign can be seen ameliorating these health inequalities because if the fear appeal aspect of the advertisements stimulates individuals to adopt healthy behaviours including increased exercise and consuming a balanced and nutritious diet, obesity prevalence could reduce. However, the advertisements lack useful signposting to potential resources individuals can use to reduce their weight, dampening their potential effectiveness. In an Australian study by Thomas et al , 25 the impact on behavioural changes through an antiobesity social marketing television campaigns was measured. Similar to the CRUK, the first advert ‘Measure Up’ informed individuals of the health risks associated with obesity, however it was followed up by a ‘Swap it’ advert which provided suggestions on how to reduce weight. While the campaign was effective at increasing awareness and encouraging personal responsibility for reducing obesogenic factors, a major finding from the study was that participants identified a lack of practical tools as a barrier to implementing behavioural change. CRUK’s adverts mirror this. While effective at increasing awareness, their role at producing meaningful behavioural changes is limited by the lack of signposting. In comparison to this, C4L produces practical resources to help users develop healthy behavioural changes.
The effects of CRUK’s advertisements have not been independently assessed previously. Based on CRUK’s self-reported data, the campaign has a strong impact at increasing awareness but the more important effect of motivating individuals to change their unhealthy behaviours has not been assessed. Therefore, effectiveness as an antiobesity social marketing tool remains unproven. The status of the campaign in 2020 is currently unknown; however, in light of this essay, it would be recommended that CRUK discontinue its use of fear appeals in future social marketing campaigns. While it is important to convey to the public, the association between obesity and cancer, the nature of the advertisements fails to respect the British public’s autonomy and has been judged to cause more harm than good through stigmatising its target audience. Additionally, it is suggested that for CRUK to facilitate acceptance, engagement and increase autonomy, a small qualitative study should be conducted with the target population to learn about cognitions and motivations to adopt healthier behaviours, making the public health message conveyed effective. CRUK should also focus its efforts on promoting educational and supportive messages, potentially in tandem with PHE’S C4L campaign which has a higher chance of facilitating sustained behavioural changes.
Ethics statements
Patient consent for publication.
Not required.
Acknowledgments
With sincere thanks to Dr Dan Cuthbertson, Mr Ashok Sharma and Dr Senthan Rudrakumar for their continual support and guidance in the publication of this essay.
- ↵ Obese people outnumber smokers two to one [press release] Cancer Research UK, 3rd , 2019 . Available: https://www.cancerresearchuk.org
- Pandeya N ,
- Byrnes G , et al
- Buchanan DR
- Vidanapathirana J ,
- Abramson MJ ,
- Forbes A , et al
- Richard S ,
- Yom-Tov E ,
- Muennig P ,
- El-Sayed AM
- Gray A , et al
- Cross- Government obesity Unit DoHaDoC, School and Families
- Aveyard PN ,
- Griffiths PE ,
- Brandkvist M ,
- Bjørngaard JH ,
- Ødegård RA , et al
- Mitchell NS ,
- Catenacci VA ,
- Wyatt HR , et al
- Magnusson J
- Aalborg A ,
- Lyons P , et al
- Lauby-Secretan B ,
- Scoccianti C ,
- Loomis D , et al
- Braithwaite D ,
- Akinyemiju TF
- Thomas SL ,
- Pettigrew S , et al
Contributors The primary author is the sole contributor to this work.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Other content recommended for you.
- Margaret McCartney: Cancer patients should not be shamed Margaret McCartney, BMJ, 2018
- Industry must cut calories in savoury food products by 20%, says Public Health England Abi Rimmer, BMJ, 2018
- Means, ends and the ethics of fear-based public health campaigns Ronald Bayer et al., Journal of Medical Ethics, 2016
- Area deprivation, screen time and consumption of food and drink high in fat salt and sugar (HFSS) in young people: results from a cross-sectional study in the UK Fiona Thomas et al., BMJ Open, 2019
- How social marketing works in health care W Douglas Evans, BMJ, 2006
- Seven days in medicine: 19-25 September 2018 , BMJ, 2018
- Why we should never do it: stigma as a behaviour change tool in global health Alexandra Brewis et al., BMJ Global Health, 2019
- Potential social marketing applications for knowledge translation in healthcare: a scoping review protocol Heather Colquhoun et al., BMJ Open, 2023
- Descriptive epidemiology of changes in weight and weight-related behaviours of Australian children aged 5 years: two population-based cross-sectional studies in 2010 and 2015 Louise L Hardy et al., BMJ Open, 2018
- The potential of shame as a message appeal in antismoking television advertisements Claudia Amonini et al., Tobacco Control, 2015
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
- Science & Technology
Proteins in blood could give cancer warning seven years earlier

15 May 2024
Proteins linked to cancer can start appearing in people’s blood more than seven years before they’re diagnosed, our funded researchers have found. In the future, it’s possible doctors could use these early warning signs to find and treat cancer much earlier than they’re able to today.
Across two studies, researchers at Oxford Population Health identified 618 proteins linked to 19 different types of cancer, including 107 proteins in a group of people whose blood was collected at least seven years before they were diagnosed.
The findings suggest that these proteins could be involved at the very earliest stages of cancer. Intercepting them could give us a way to stop the disease developing altogether.
“This research brings us closer to being able to prevent cancer with targeted drugs – once thought impossible but now much more attainable,” explained Dr Karl Smith-Byrne, Senior Molecular Epidemiologist at Oxford Population Health, who worked on both papers.
For now, though, we need to do further research. The team want to find out more about the roles these proteins play in cancer development, how we can use tests to spot the most important ones, and which drugs we can use to stop them driving cancer.
Comparing blood samples with proteomics
B oth studies, published today in Nature Communications , used a powerful technique called proteomics to find important differences in blood samples between people who did and did not go on to develop cancer.
To be able to prevent cancer, we need to understand the factors driving the earliest stages of its development. These studies are important because they provide many new clues about the causes and biology of multiple cancers, including insights into what’s happening years before a cancer is diagnosed. We now have technology that can look at thousands of proteins across thousands of cancer cases, identifying which proteins have a role in the development of specific cancers, and which might have effects that are common to multiple cancer types.
In the first study , scientists analysed 44,000 blood samples collected and stored by UK Biobank, including over 4,900 samples from people who were later diagnosed with cancer.
Their analysis of 1,463 proteins in each sample revealed 107 that changed at least seven years before a cancer diagnosis and 182 that changed at least three years before a cancer diagnosis.
In the second study , the scientists looked at genetic data from over 300,000 cancer cases to do a deep dive into which blood proteins were involved in cancer development and could be targeted by new treatments.
This time, they found 40 proteins in the blood that influence someone’s risk of getting nine different types of cancer. While altering these proteins may increase or decrease the chances of someone developing cancer, more research is needed to make sure targeting them with drugs doesn’t cause unintended side effects.
Preventing cancer means looking out for the earliest warning signs of the disease. That means intensive, painstaking research to find the molecular signals we should pay closest attention to. Discoveries from this research are the crucial first step towards offering preventive therapies, which is the ultimate route for giving people longer, better lives, free from the fear of cancer.
I am a cancer survivor and routinely surprised (disappointed?) by the contingent nature of identifying and treating the disease. By way of this article I’m so glad to see the science of early detection take center stage! Congrats and thanks for the work you do.
Tell us what you think
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Read our comment policy .
Highlighted content
An animal's guide to staying safe in the sun, hpv vaccine slashes cervical cancer rates across society, the ‘mystery’ culprit causing kidney cancer worldwide, are ultra-processed foods linked to cancer, following cupid’s arrow: a new blood test to find cancer of unknown primary, making cancer screening work for you, can vaping cause changes in our cells, that cancer conversation podcast - one to one with dr anisha patel, related topics.
- Cancer Research UK-funded research
- Early detection
Home / Essay Samples / Health / Cancer / An Overview of Cancer Research Program in the UK
An Overview of Cancer Research Program in the UK
- Category: Health , Economics , Business
- Topic: Cancer , Non-Profit Organization , Organizational Structure
Pages: 2 (910 words)
Views: 14797
- Downloads: -->
Aims and Objectives
Ownership and liability, stakeholders, organisational structure.
--> ⚠️ Remember: This essay was written and uploaded by an--> click here.
Found a great essay sample but want a unique one?
are ready to help you with your essay
You won’t be charged yet!
Walmart Essays
Nestle Essays
Pepsi Essays
Google Essays
Enron Essays
Related Essays
We are glad that you like it, but you cannot copy from our website. Just insert your email and this sample will be sent to you.
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Your essay sample has been sent.
In fact, there is a way to get an original essay! Turn to our writers and order a plagiarism-free paper.
samplius.com uses cookies to offer you the best service possible.By continuing we’ll assume you board with our cookie policy .--> -->