Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character

Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Spring 2024 | VOL. 36, NO. 2 CURRENT ISSUE pp.A4-174
- Winter 2024 | VOL. 36, NO. 1 pp.A5-81
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Case Study 1: A 55-Year-Old Woman With Progressive Cognitive, Perceptual, and Motor Impairments
- Scott M. McGinnis , M.D. ,
- Andrew M. Stern , M.D., Ph.D. ,
- Jared K. Woods , M.D., Ph.D. ,
- Matthew Torre , M.D. ,
- Mel B. Feany , M.D., Ph.D. ,
- Michael B. Miller , M.D., Ph.D. ,
- David A. Silbersweig , M.D. ,
- Seth A. Gale , M.D. ,
- Kirk R. Daffner , M.D.
Search for more papers by this author
CASE PRESENTATION
A 55-year-old right-handed woman presented with a 3-year history of cognitive changes. Early symptoms included mild forgetfulness—for example, forgetting where she left her purse or failing to remember to retrieve a take-out order her family placed—and word-finding difficulties. Problems with depth perception affected her ability to back her car out of the driveway. When descending stairs, she had to locate her feet visually in order to place them correctly, such that when she carried her dog and her view was obscured, she had difficulty managing this activity. She struggled to execute relatively simple tasks, such as inserting a plug into an outlet. She lost the ability to type on a keyboard, despite being able to move her fingers quickly. Her symptoms worsened progressively for 3 years, over which time she developed a sad mood and anxiety. She was laid off from work as a nurse administrator. Her family members assumed responsibility for paying her bills, and she ceased driving.
Her past medical history included high blood pressure, Hashimoto’s thyroiditis with thyroid peroxidase antibodies, remote history of migraine, and anxiety. Medications included mirtazapine, levothyroxine, calcium, and vitamin D. She had no history of smoking, drinking alcohol, or recreational drug use. There was no known family history of neurologic diseases.
What Are Diagnostic Considerations Based on the History? How Might a Clinical Examination Help to Narrow the Differential Diagnosis?
Insidious onset and gradual progression of cognitive symptoms over the course of several years raise concern for a neurodegenerative disorder. It is helpful to consider whether or not the presentation fits with a recognized neurodegenerative clinical syndrome, a judgment based principally on familiarity with syndromes and pattern recognition. Onset of symptoms before age 65 should prompt consideration of syndromes in the spectrum of frontotemporal dementia (FTD) and atypical (nonamnesic) presentations of Alzheimer’s disease (AD) ( 1 , 2 ). This patient’s symptoms reflect relatively prominent early dysfunction in visual-spatial processing and body schema, as might be observed in posterior cortical atrophy (PCA), although the history also includes mention of forgetfulness and word-retrieval difficulties. A chief goal of the cognitive examination would be to survey major domains of cognition—attention, executive functioning, memory, language, visual-spatial functioning, and higher somatosensory and motor functioning—to determine whether any domains stand out as more prominently affected. In addition to screening for evidence of focal signs, a neurological examination in this context should assess for evidence of parkinsonism or motor neuron disease, which can coexist with cognitive changes in neurodegenerative presentations.
The patient’s young age and history of Hashimoto’s thyroiditis might also prompt consideration of Hashimoto’s encephalopathy (HE; also known as steroid-responsive encephalopathy), associated with autoimmune thyroiditis. This syndrome is most likely attributable to an autoimmune or inflammatory process affecting the central nervous system. The time course of HE is usually more subacute and rapidly progressive or relapsing-remitting, as opposed to the gradual progression over months to years observed in the present case ( 3 ).
The patient’s mental status examination included the Montreal Cognitive Assessment (MoCA), a brief global screen of cognition ( 4 ), on which she scored 12/30. There was evidence of dysfunction across multiple cognitive domains ( Figure 1 ). She was fully oriented to location, day, month, year, and exact date. When asked to describe a complex scene from a picture in a magazine, she had great difficulty doing so, focusing on different details but having trouble directing her saccades to pertinent visual information. She likewise had problems directing her gaze to specified objects in the room and problems reaching in front of her to touch target objects in either visual field. In terms of other symptoms of higher order motor and somatosensory functioning, she had difficulty demonstrating previously learned actions—for example, positioning her hand correctly to pantomime holding a brush and combing her hair. She was confused about which side of her body was the left and which was the right. She had difficulty with mental calculations, even relatively simple ones such as “18 minus 12.” In addition, she had problems writing a sentence in terms of both grammar and the appropriate spacing of words and letters on the page.
FIGURE 1. Selected elements of a 55-year-old patient’s cognitive examination at presentation a
a BNT-15=Boston Naming Test (15-Item); MoCA=Montreal Cognitive Assessment.
On elementary neurologic examination she had symmetrically brisk reflexes, with spread. She walked steadily with a narrow base, but when asked to pass through a doorway she had difficulty finding her way through it and bumped into the door jamb. Her elemental neurological examination was otherwise normal, including but not limited to brisk, full-amplitude vertical eye movements, normal visual fields, no evidence of peripheral neuropathy, and no parkinsonian signs such as slowness of movement, tremor, or rigidity.
How Does the Examination Contribute to Our Understanding of Diagnostic Considerations? What Additional Tests or Studies Are Indicated?
The most prominent early symptoms and signs localize predominantly to the parietal association cortex: The patient has impairments in visual construction, ability to judge spatial relationships, ability to synthesize component parts of a visual scene into a coherent whole (simultanagnosia or asimultagnosia), impaired visually guided reaching for objects (optic ataxia), and most likely impaired ability to shift her visual attention so as to direct saccades to targets in her field of view (oculomotor apraxia or ocular apraxia). The last three signs constitute Bálint syndrome, which localizes to disruption of dorsal visual networks (i.e., dorsal stream) with key nodes in the posterior parietal and prefrontal cortices bilaterally ( 5 ). She has additional salient symptoms and signs suggesting left inferior parietal dysfunction, including ideomotor limb apraxia and elements of Gerstmann syndrome, which comprises dysgraphia, acalculia, left-right confusion, and finger agnosia ( 6 ). Information was not included about whether she was explicitly examined for finger agnosia, but elements of her presentation suggested a more generalized disruption of body schema (i.e., her representation of the position and configuration of her body in space). Her less prominent impairment in lexical-semantic retrieval evidenced by impaired confrontation naming and category fluency likely localizes to the language network in the left hemisphere. Her impairments in attention and executive functions have less localizing value but would plausibly arise in the context of frontoparietal network dysfunction. At this point, it is unclear whether her impairment in episodic memory mostly reflects encoding and activation versus a rapid rate of forgetting (storage), as occurs in temporolimbic amnesia. Regardless, it does not appear to be the most salient feature of her presentation.
This localization, presenting with insidious onset and gradual progression, is characteristic of a PCA syndrome. If we apply consensus clinical diagnostic criteria proposed by a working group of experts, we find that our patient has many of the representative features of early disturbance of visual functions plus or minus other cognitive functions mediated by the posterior cerebral cortex ( Table 1 ) ( 7 ). Some functions such as limb apraxia also occur in corticobasal syndrome (CBS), a clinical syndrome defined initially in association with corticobasal degeneration (CBD) neuropathology, a 4-repeat tauopathy characterized by achromatic ballooned neurons, neuropil threads, and astrocytic plaques. However, our patient lacks other suggestive features of CBS, including extrapyramidal motor dysfunction (e.g., limb rigidity, bradykinesia, dystonia), myoclonus, and alien limb phenomenon ( Table 1 ) ( 8 ).
| Feature | Posterior cortical atrophy | Corticobasal syndrome |
|---|---|---|
| Cognitive and motor features | Visual-perceptual: space perception deficit, simultanagnosia, object perception deficit, environmental agnosia, alexia, apperceptive prosopagnosia, and homonymous visual field defect | Motor: limb rigidity or akinesia, limb dystonia, and limb myoclonus |
| Visual-motor: constructional dyspraxia, oculomotor apraxia, optic ataxia, and dressing apraxia | ||
| Other: left/right disorientation, acalculia, limb apraxia, agraphia, and finger agnosia | Higher cortical features: limb or orobuccal apraxia, cortical sensory deficit, and alien limb phenomena | |
| Imaging features (MRI, FDG-PET, SPECT) | Predominant occipito-parietal or occipito-temporal atrophy, and hypometabolism or hypoperfusion | Asymmetric perirolandic, posterior frontal, parietal atrophy, and hypometabolism or hypoperfusion |
| Neuropathological associations | AD>CBD, LBD, TDP, JCD | CBD>PSP, AD, TDP |
a Consensus diagnostic criteria for posterior cortical atrophy per Crutch et al. ( 7 ) require at least three cognitive features and relative sparing of anterograde memory, speech-nonvisual language functions, executive functions, behavior, and personality. Diagnostic criteria for probable corticobasal syndrome per Armstrong et al. ( 8 ) require asymmetric presentation of at least two motor features and at least two higher cortical features. AD=Alzheimer’s disease; CBD=corticobasal degeneration; FDG-PET=[ 18 ]F-fluorodexoxyglucose positron emission tomography; JCD=Jakob-Creutzfeldt disease; LBD=Lewy body disease; PSP=progressive supranuclear palsy; SPECT=single-photon emission computed tomography; TDP=TDP–43 proteinopathy.
TABLE 1. Clinical features and neuropathological associations of posterior cortical atrophy and corticobasal syndrome a
In addition to a standard laboratory work-up for cognitive impairment, it is important to determine whether imaging of the brain provides evidence of neurodegeneration in a topographical distribution consistent with the clinical presentation. A first step in most cases would be to obtain an MRI of the brain that includes a high-resolution T 1 -weighted MRI sequence to assess potential atrophy, a T 2 /fluid-attenuated inversion recovery (FLAIR) sequence to assess the burden of vascular disease and rule out less likely etiological considerations (e.g., infection, autoimmune-inflammatory, neoplasm), a diffusion-weighted sequence to rule out subacute infarcts and prion disease (more pertinent to subacute or rapidly progressive cases), and a T 2 *-gradient echo or susceptibility weighted sequence to examine for microhemorrhages and superficial siderosis.
A lumbar puncture would serve two purposes. First, it would allow for the assessment of inflammation that might occur in HE, as approximately 80% of cases have some abnormality of CSF (i.e., elevated protein, lymphocytic pleiocytosis, or oligoclonal bands) ( 9 ). Second, in selected circumstances—particularly in cases with atypical nonamnesic clinical presentations or early-onset dementia in which AD is in the neuropathological differential diagnosis—we frequently pursue AD biomarkers of molecular neuropathology ( 10 , 11 ). This is most frequently accomplished with CSF analysis of amyloid-β-42, total tau, and phosphorylated tau levels. Amyloid positron emission tomography (PET) imaging, and most recently tau PET imaging, represent additional options that are approved by the U.S. Food and Drug Administration for clinical use. However, insurance often does not cover amyloid PET and currently does not reimburse tau PET imaging. [ 18 ]-F-fluorodeoxyglucose (FDG) PET and perfusion single-photon emission computed tomography imaging may provide indirect evidence for AD neuropathology via a pattern of hypometabolism or hypoperfusion involving the temporoparietal and posterior cingulate regions, though without molecular specificity. Pertinent to this case, a syndromic diagnosis of PCA is most commonly associated with underlying AD neuropathology—that is, plaques containing amyloid-β and neurofibrillary tangles containing tau ( 12 – 15 ).
The patient underwent MRI, demonstrating a minimal burden of T 2 /FLAIR hyperintensities and some degree of bilateral parietal volume loss with a left greater than right predominance ( Figure 2A ). There was relatively minimal medial temporal volume loss. Her basic laboratory work-up, including thyroid function, vitamin B 12 level, and treponemal antibody, was normal. She underwent a lumbar puncture; CSF studies revealed normal cell counts, protein, and glucose levels and low amyloid-β-42 levels at 165.9 pg/ml [>500 pg/ml] and elevated total and phosphorylated tau levels at 1,553 pg/ml [<350 pg/ml] and 200.4 pg/ml [<61 pg/ml], respectively.
FIGURE 2. MRI brain scan of the patient at presentation and 4 years later a
a Arrows denote regions of significant atrophy.
Considering This Additional Data, What Would Be an Appropriate Diagnostic Formulation?
For optimal clarity, we aim to provide a three-tiered approach to diagnosis comprising neurodegenerative clinical syndrome (e.g., primary amnesic, mixed amnesic and dysexecutive, primary progressive aphasia), level of severity (i.e., mild cognitive impairment; mild, moderate or severe dementia), and predicted underlying neuropathology (e.g., AD, Lewy body disease [LBD], frontotemporal lobar degeneration) ( 16 ). This approach avoids problematic conflations that cause confusion, for example when people equate AD with memory loss or dementia, whereas AD most strictly describes the neuropathology of plaques and tangles, regardless of the patient’s clinical symptoms and severity. This framework is important because there is never an exclusive, one-to-one correspondence between syndromic and neuropathological diagnosis. Syndromes arise from neurodegeneration that starts focally and progresses along the anatomical lines of large-scale brain networks that can be defined on the basis of both structural and functional connectivity, a concept detailed in the network degeneration hypothesis ( 17 ). It is important to note that neuropathologies defined on the basis of specific misfolded protein inclusions can target more than one large-scale network, and any given large-scale network can degenerate in association with more than one neuropathology.
The MRI results in this case support a syndromic diagnosis of PCA, with a posteriorly predominant pattern of atrophy. Given the patient’s loss of independent functioning in instrumental activities of daily living (ADLs), including driving and managing her finances, the patient would be characterized as having a dementia (also known as major neurocognitive disorder). The preservation of basic ADLs would suggest that the dementia was of mild severity. The CSF results provide supportive evidence for AD amyloid plaque and tau neurofibrillary tangle (NFT) neuropathology over other pathologies potentially associated with PCA syndrome (i.e., CBD, LBD, TDP-43 proteinopathy, and Jakob-Creutzfeldt disease) ( 13 , 14 ). The patient’s formulation would thus be best summarized as PCA at a level of mild dementia, likely associated with underlying AD neuropathology.
The patient’s symptoms progressed. One year after initial presentation, she had difficulty locating the buttons on her clothing or the food on her plate. Her word-finding difficulties worsened. Others observed stiffness of her right arm, a new symptom that was not present initially. She also had decreased ability using her right hand to hold everyday objects such as a comb, a brush, or a pen. On exam, she was noted to have rigidity of her right arm, impaired dexterity with her right hand for fine motor tasks, and a symmetrical tremor of the arms, apparent when holding objects or reaching. Her right hand would also intermittently assume a flexed, dystonic posture and would sometime move in complex ways without her having a sense of volitional control.
Four to 5 years after initial presentation, her functional status declined to the point where she was unable to feed, bathe, or dress herself. She was unable to follow simple instructions. She developed neuropsychiatric symptoms, including compulsive behaviors, anxiety, and apathy. Her right-sided motor symptoms progressed; she spent much of the time with her right arm flexed in abnormal postures or moving abnormally. She developed myoclonus of both arms. Her speech became slurred and monosyllabic. Her gait became less steady. She underwent a second MRI of the brain, demonstrating progressive bilateral atrophy involving the frontal and occipital lobes in addition to the parietal lobes and with more left > right asymmetry than was previously apparent ( Figure 2B ). Over time, she exhibited increasing weight loss. She was enrolled in hospice and ultimately passed away 8 years from the onset of symptoms.
Does Information About the Longitudinal Course of Her Illness Alter the Formulation About the Most Likely Underlying Neuropathological Process?
This patient developed clinical features characteristic of corticobasal syndrome over the longitudinal course of her disease. With time, it became apparent that she had lost volitional control over her right arm (characteristic of an alien limb phenomenon), and she developed signs more suggestive of basal ganglionic involvement (i.e., limb rigidity and possible dystonia). This presentation highlights the frequent overlap between neurodegenerative clinical syndromes; any given person may have elements of more than one syndrome, especially later in the course of a disease. In many instances, symptomatic features that are less prominent at presentation but evolve and progress can provide clues regarding the underlying neuropathological diagnosis. For example, a patient with primary progressive apraxia of speech or nonfluent-agrammatic primary progressive aphasia could develop the motor features of a progressive supranuclear palsy (PSP) clinical syndrome (e.g., supranuclear gaze impairment, axial rigidity, postural instability), which would suggest underlying PSP neuropathology (4-repeat tauopathy characterized by globose neurofibrillary tangles, tufted astrocytes, and oligodendroglial coiled bodies).
If CSF biomarker data were not suggestive of AD, the secondary elements of CBS would substantially increase the likelihood of underlying CBD neuropathology presenting with a PCA syndrome and evolving to a mixed PCA-CBS. But the CSF amyloid and tau levels are unambiguously suggestive of AD (i.e., very low amyloid-β-42 and very high p-tau levels), the neuropathology of which accounts for not only a vast majority of PCA presentations but also roughly a quarter of cases presenting with CBS ( 18 , 19 ). Thus, underlying AD appears most likely.
NEUROPATHOLOGY
On gross examination, the brain weighed 1,150 g, slightly less than the lower end of normal at 1,200 g. External examination demonstrated mild cortical atrophy with widening of the sulci, relatively symmetrical and uniform throughout the brain ( Figure 3A ). There was no evidence of atrophy of the brainstem or cerebellum. On cut sections, the hippocampus was mildly atrophic. The substantia nigra in the midbrain was intact, showing appropriate dark pigmentation as would be seen in a relatively normal brain. The remainder of the gross examination was unremarkable.
FIGURE 3. Mild cortical atrophy with posterior predominance and neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body a
a Panel A shows the gross view of the brain, demonstrating mild cortical atrophy with posterior predominance (arrow). Panel B shows the hematoxylin and eosin of the hippocampus at high power, demonstrating neurofibrillary tangles, granulovacuolar degeneration, and a Hirano body.
Histological examination confirmed that the neurons in the substantia nigra were appropriately pigmented, with occasional extraneuronal neuromelanin and moderate neuronal loss. In the nucleus basalis of Meynert, NFTs were apparent on hematoxylin and eosin staining as dense fibrillar eosinophilic structures in the neuronal cytoplasm, confirmed by tau immunohistochemistry (IHC; Figure 4 ). Low-power examination of the hippocampus revealed neuronal loss in the subiculum and in Ammon’s horn, most pronounced in the cornu ammonis 1 (CA1) subfield, with a relatively intact neuronal population in the dentate gyrus. Higher power examination with hematoxylin and eosin demonstrated numerous NFTs, neurons exhibiting granulovacuolar degeneration, and Hirano bodies ( Figure 3B ). Tau IHC confirmed numerous NFTs in the CA1 region and the subiculum. Amyloid-β IHC demonstrated occasional amyloid plaques in this region, less abundant than tau pathology. An α-synuclein stain revealed scattered Lewy bodies in the hippocampus and in the amygdala.
FIGURE 4. Tau immunohistochemistry demonstrating neurofibrillary tangles (staining brown) in the nucleus basalis of Meynert, in the hippocampus, and in the cerebral cortex of the frontal, temporal, parietal, and occipital lobes
In the neocortex, tau IHC highlighted the extent of the NFTs, which were very prominent in all of the lobes from which sections were taken: frontal, temporal, parietal and occipital. Numerous plaques on amyloid-β stain were likewise present in all cortical regions examined. The tau pathology was confined to the gray matter, sparing white matter. There were no ballooned neurons and no astrocytic plaques—two findings one would expect to see in CBD ( Table 2 ).
| Feature | Case of PCA/CBS due to AD | Exemplar case of CBD |
|---|---|---|
| Macroscopic findings | Cortical atrophy: symmetric, mild | Cortical atrophy: often asymmetric, predominantly affecting perirolandic cortex |
| Substantia nigra: appropriately pigmented | Substantia nigra: severely depigmented | |
| Microscopic findings | Tau neurofibrillary tangles and beta-amyloid plaques | Primary tauopathy |
| No tau pathology in white matter | Tau pathology involves white matter | |
| Hirano bodies, granulovacuolar degeneration | Ballooned neurons, astrocytic plaques, and oligodendroglial coiled bodies | |
| (Lewy bodies, limbic) |
a AD=Alzheimer’s disease; CBD=corticobasal degeneration; CBS=corticobasal syndrome; PCA=posterior cortical atrophy.
TABLE 2. Neuropathological features of this case compared with a case of corticobasal degeneration a
The case was designated by the neuropathology division as Alzheimer’s-type pathology, Braak stage V–VI (of VI), due to the widespread neocortical tau pathology, with LBD primarily in the limbic areas.
Our patient had AD neuropathology presenting atypically with a young age at onset (52 years old) and a predominantly visual-spatial and corticobasal syndrome as opposed to prominent amnesia. Syndromic diversity is a well-recognized phenomenon in AD. Nonamnesic presentations include not only PCA and CBS but also the logopenic variant of primary progressive aphasia and a behavioral-dysexecutive syndrome ( 20 ). Converging lines of evidence link the topographical distribution of NFTs with syndromic presentations and the pattern of hypometabolism and cortical atrophy. Neuropathological case reports and case series suggest that atypical AD syndromes arise in the setting of higher than normal densities of NFTs in networks subserving the functions compromised, including visual association areas in PCA-AD ( 21 ), the language network in PPA-AD ( 22 ), and frontal regions in behavioral-dysexecutive AD ( 23 ). In a large sample of close to 900 cases of pathologically diagnosed AD employing quantitative assessment of NFT density and distribution in selected neocortical and hippocampal regions, 25% of cases did not conform to a typical distribution of NFTs characterized in the Braak staging scheme ( 24 ). A subset of cases classified as hippocampal sparing with higher density of NFTs in the neocortex and lower density of NFTs in the hippocampus had a younger mean age at onset, higher frequency of atypical (nonamnesic) presentations, and more rapid rate of longitudinal decline than subsets defined as typical or limbic-predominant.
Tau PET, which detects the spatial distribution of fibrillary tau present in NFTs, has corroborated postmortem work in demonstrating distinct patterns of tracer uptake in different subtypes of AD defined by clinical symptoms and topographical distributions of atrophy ( 25 – 28 ). Amyloid PET, which detects the spatial distribution of fibrillar amyloid- β found in amyloid plaques, does not distinguish between typical and atypical AD ( 29 , 30 ). In a longitudinal study of 32 patients at early symptomatic stages of AD, the baseline topography of tau PET signal predicted subsequent atrophy on MRI at the single patient level, independent of baseline cortical thickness ( 31 ). This correlation was strongest in early-onset AD patients, who also tended to have higher tau signal and more rapid progression of atrophy than late-onset AD patients.
Differential vulnerability of selected large-scale brain networks in AD and in neurodegenerative disease more broadly remains poorly understood. There is evidence to support multiple mechanisms that are not mutually exclusive, including metabolic stress to key network nodes, trophic failure, transneuronal spread of pathological proteins (i.e., prion-like mechanisms), and shared vulnerability within network regions based on genetic or developmental factors ( 32 ). In the case of AD, cortical hub regions with high intrinsic functional connectivity to other regions across the brain appear to have high metabolic rates across the lifespan and to be foci of convergence of amyloid-β and tau accumulation ( 33 , 34 ). Tau NFT pathology appears to spread temporally along connected networks within the brain ( 35 ). Patients with primary progressive aphasia are more likely to have a personal or family history of developmental language-based learning disability ( 36 ), and patients with PCA are more likely to have a personal history of mathematical or visuospatial learning disability ( 37 ).
This case highlights the symptomatic heterogeneity in AD and the value of a three-tiered approach to diagnostic formulation in neurodegenerative presentations. It is important to remember that not all AD presents with amnesia and that early-onset AD tends to be more atypical and to progress more rapidly than late-onset AD. Multiple lines of evidence support a relationship between the burden and topographical distribution of tau NFT neuropathology and clinical symptomatology in AD, instantiating network-based neurodegeneration via mechanisms under ongoing investigation.
The authors report no financial relationships with commercial interests.
Supported by NIH grants K08 AG065502 (to Dr. Miller) and T32 HL007627 (to Dr. Miller).
The authors have confirmed that details of the case have been disguised to protect patient privacy.
1 Balasa M, Gelpi E, Antonell A, et al. : Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease . Neurology 2011 ; 76:1720–1725 Crossref , Medline , Google Scholar
2 Mercy L, Hodges JR, Dawson K, et al. : Incidence of early-onset dementias in Cambridgeshire, United Kingdom . Neurology 2008 ; 71:1496–1499 Crossref , Medline , Google Scholar
3 Kothbauer-Margreiter I, Sturzenegger M, Komor J, et al. : Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment . J Neurol 1996 ; 243:585–593 Crossref , Medline , Google Scholar
4 Nasreddine ZS, Phillips NA, Bédirian V, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment . J Am Geriatr Soc 2005 ; 53:695–699 Crossref , Medline , Google Scholar
5 Rizzo M, Vecera SP : Psychoanatomical substrates of Bálint’s syndrome . J Neurol Neurosurg Psychiatry 2002 ; 72:162–178 Crossref , Medline , Google Scholar
6 Rusconi E : Gerstmann syndrome: historic and current perspectives . Handb Clin Neurol 2018 ; 151:395–411 Crossref , Medline , Google Scholar
7 Crutch SJ, Schott JM, Rabinovici GD, et al. : Consensus classification of posterior cortical atrophy . Alzheimers Dement 2017 ; 13:870–884 Crossref , Medline , Google Scholar
8 Armstrong MJ, Litvan I, Lang AE, et al. : Criteria for the diagnosis of corticobasal degeneration . Neurology 2013 ; 80:496–503 Crossref , Medline , Google Scholar
9 Marshall GA, Doyle JJ : Long-term treatment of Hashimoto’s encephalopathy . J Neuropsychiatry Clin Neurosci 2006 ; 18:14–20 Link , Google Scholar
10 Johnson KA, Minoshima S, Bohnen NI, et al. : Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association . Alzheimers Dement 2013 ; 9:e-1–e-16 Crossref , Medline , Google Scholar
11 Shaw LM, Arias J, Blennow K, et al. : Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease . Alzheimers Dement 2018 ; 14:1505–1521 Crossref , Medline , Google Scholar
12 Alladi S, Xuereb J, Bak T, et al. : Focal cortical presentations of Alzheimer’s disease . Brain 2007 ; 130:2636–2645 Crossref , Medline , Google Scholar
13 Renner JA, Burns JM, Hou CE, et al. : Progressive posterior cortical dysfunction: a clinicopathologic series . Neurology 2004 ; 63:1175–1180 Crossref , Medline , Google Scholar
14 Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. : Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy . Neurology 2004 ; 63:1168–1174 Crossref , Medline , Google Scholar
15 Victoroff J, Ross GW, Benson DF, et al. : Posterior cortical atrophy: neuropathologic correlations . Arch Neurol 1994 ; 51:269–274 Crossref , Medline , Google Scholar
16 Dickerson BC, McGinnis SM, Xia C, et al. : Approach to atypical Alzheimer’s disease and case studies of the major subtypes . CNS Spectr 2017 ; 22:439–449 Crossref , Medline , Google Scholar
17 Seeley WW, Crawford RK, Zhou J, et al. : Neurodegenerative diseases target large-scale human brain networks . Neuron 2009 ; 62:42–52 Crossref , Medline , Google Scholar
18 Lee SE, Rabinovici GD, Mayo MC, et al. : Clinicopathological correlations in corticobasal degeneration . Ann Neurol 2011 ; 70:327–340 Crossref , Medline , Google Scholar
19 Whitwell JL, Jack CR Jr, Boeve BF, et al. : Imaging correlates of pathology in corticobasal syndrome . Neurology 2010 ; 75:1879–1887 Crossref , Medline , Google Scholar
20 Warren JD, Fletcher PD, Golden HL : The paradox of syndromic diversity in Alzheimer disease . Nat Rev Neurol 2012 ; 8:451–464 Crossref , Medline , Google Scholar
21 Hof PR, Archin N, Osmand AP, et al. : Posterior cortical atrophy in Alzheimer’s disease: analysis of a new case and re-evaluation of a historical report . Acta Neuropathol 1993 ; 86:215–223 Crossref , Medline , Google Scholar
22 Mesulam MM, Weintraub S, Rogalski EJ, et al. : Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia . Brain 2014 ; 137:1176–1192 Crossref , Medline , Google Scholar
23 Blennerhassett R, Lillo P, Halliday GM, et al. : Distribution of pathology in frontal variant Alzheimer’s disease . J Alzheimers Dis 2014 ; 39:63–70 Crossref , Medline , Google Scholar
24 Murray ME, Graff-Radford NR, Ross OA, et al. : Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study . Lancet Neurol 2011 ; 10:785–796 Crossref , Medline , Google Scholar
25 Ossenkoppele R, Lyoo CH, Sudre CH, et al. : Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer’s disease . Alzheimers Dement 2020 ; 16:335–344 Crossref , Medline , Google Scholar
26 Phillips JS, Das SR, McMillan CT, et al. : Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease . Hum Brain Mapp 2018 ; 39:691–708 Crossref , Medline , Google Scholar
27 Tetzloff KA, Graff-Radford J, Martin PR, et al. : Regional distribution, asymmetry, and clinical correlates of tau uptake on [18F]AV-1451 PET in atypical Alzheimer’s disease . J Alzheimers Dis 2018 ; 62:1713–1724 Crossref , Medline , Google Scholar
28 Xia C, Makaretz SJ, Caso C, et al. : Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease . JAMA Neurol 2017 ; 74:427–436 Crossref , Medline , Google Scholar
29 Formaglio M, Costes N, Seguin J, et al. : In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings . J Neurol 2011 ; 258:1841–1851 Crossref , Medline , Google Scholar
30 Lehmann M, Ghosh PM, Madison C, et al. : Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease . Brain 2013 ; 136:844–858 Crossref , Medline , Google Scholar
31 La Joie R, Visani AV, Baker SL, et al. : Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET . Sci Transl Med 2020 ; 12:12 Crossref , Google Scholar
32 Zhou J, Gennatas ED, Kramer JH, et al. : Predicting regional neurodegeneration from the healthy brain functional connectome . Neuron 2012 ; 73:1216–1227 Crossref , Medline , Google Scholar
33 Buckner RL, Sepulcre J, Talukdar T, et al. : Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease . J Neurosci 2009 ; 29:1860–1873 Crossref , Medline , Google Scholar
34 Hoenig MC, Bischof GN, Seemiller J, et al. : Networks of tau distribution in Alzheimer’s disease . Brain 2018 ; 141:568–581 Crossref , Medline , Google Scholar
35 Liu L, Drouet V, Wu JW, et al. : Trans-synaptic spread of tau pathology in vivo . PLoS One 2012 ; 7:e31302 Crossref , Medline , Google Scholar
36 Rogalski E, Johnson N, Weintraub S, et al. : Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives . Arch Neurol 2008 ; 65:244–248 Crossref , Medline , Google Scholar
37 Miller ZA, Rosenberg L, Santos-Santos MA, et al. : Prevalence of mathematical and visuospatial learning disabilities in patients with posterior cortical atrophy . JAMA Neurol 2018 ; 75:728–737 Crossref , Medline , Google Scholar
- Jeffrey Maneval , M.D. ,
- Kirk R. Daffner , M.D. ,
- Scott M. McGinnis , M.D.
- Seth A. Gale , M.A., M.D. ,
- C. Alan Anderson , M.D. ,
- David B. Arciniegas , M.D.

- Posterior Cortical Atrophy
- Corticobasal Syndrome
- Atypical Alzheimer Disease
- Network Degeneration
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Case Report of a 63-Year-Old Patient With Alzheimer Disease and a Novel Presenilin 2 Mutation
Wells, Jennie L. BSc, MSc, MD, FACP, FRCPC, CCRP *,† ; Pasternak, Stephen H. MD, PhD, FRCPC †,‡,§
* Department of Medicine, Division of Geriatric Medicine, Schulich School of Medicine and Dentistry, Western University
† St. Joseph’s Health Care London—Parkwood Institute
‡ Molecular Medicine Research Group, Robarts Research Institute
§ Department of Clinical Neurological Sciences, Schulich School of Medicine and Dentistry, University of Western Ontario, London, ON, Canada
The authors declare no conflicts of interest.
Reprints: Jennie L. Wells, BSc, MSc, MD, FACP, FRCPC, CCRP, Department of Medicine, Division of Geriatric Medicine, St. Joseph’s Health Care London—Parkwood Institute, Room A2-129, P.O. Box 5777 STN B, London, ON, Canada N6A 4V2 (e-mail: [email protected] ).
This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. http://creativecommons.org/licenses/by/4.0/
Early onset Alzheimer disease (EOAD) is a neurodegenerative dementing disorder that is relatively rare (<1% of all Alzheimer cases). Various genetic mutations of the presenilin 1 ( PSEN1 ) and presenilin 2 ( PSEN2 ) as well as the amyloid precursor protein (APP) gene have been implicated. Mutations of PSEN1 and PSEN2 alter γ-secretase enzyme that cleaves APP resulting in increase in the relative amount of the more amyloidogenic Aβ42 that is produced. 1
PSEN2 has been less studied than PSEN1 and fewer mutations are known. Here, we report a case of a 63-year-old woman (at the time of death) with the clinical history consistent with Alzheimer D, an autopsy with brain histopathology supporting Alzheimer disease (AD), congophylic angiopathy, and Lewy Body pathology, and whose medical genetic testing reveals a novel PSEN2 mutation of adenosine replacing cytosine at codon 222, nucleotide position 665 (lysine replacing threonine) that has never been previously reported. This suggests that genetic testing may be useful in older patients with mixed pathology.
CASE REPORT
The patient was referred to our specialty memory clinic at the age of 58 with a 2-year history of repetitiveness, memory loss, and executive function loss. Magnetic resonance imaging scan at age 58 revealed mild generalized cortical atrophy. She is white with 2 years of postsecondary education. Retirement at age 48 from employment as a manager in telecommunications company was because family finances allowed and not because of cognitive challenges with work. Progressive cognitive decline was evident by the report of deficits in instrumental activities of daily living performance over the past 9 months before her initial consultation in the memory clinic. Word finding and literacy skills were noted to have deteriorated in the preceding 6 months according to her spouse. Examples of functional losses were being slower in processing and carrying out instructions, not knowing how to turn off the stove, and becoming unable to assist in boat docking which was the couple’s pastime. She stopped driving a motor vehicle about 6 months before her memory clinic consultation. Her past medical history was relevant for hypercholesterolemia and vitamin D deficiency. She had no surgical history. She had no history of smoking, alcohol, or other drug misuse. Laboratory screening was normal. There was no first-degree family history of presenile dementia. Neurocognitive assessment at the first clinic visit revealed a Mini Mental State Examination (MMSE) score of 14/30; poor verbal fluency (patient was able to produce only 5 animal names and 1 F-word in 1 min) as well as poor visuospatial and executive skills ( Fig. 1 ). She had fluent speech without semantic deficits. Her neurological examination was pertinent for normal muscle tone and power, mild ideomotor apraxia on performing commands for motor tasks with no suggestion of cerebellar dysfunction, normal gait, no frontal release signs. Her speech was fluent with obvious word finding difficulties but with no phonemic or semantic paraphrasic errors. Her general physical examination was unremarkable without evidence of presenile cataracts. She had normal hearing. There was no evidence of depression or psychotic symptoms.

At the time of the initial assessment, her mother was deceased at age 79 after a hip fracture with a history long-term smoking and idiopathic pulmonary fibrosis. Her family believes that there is possible German and Danish descent on her father’s side. Her father was alive and well at age 80 at the time of her presentation with a history coronary artery disease. He is still alive and well with no functional or cognitive concerns at age 87 at the time of writing this report. Her paternal grandfather died at approximately age 33 of appendicitis with her paternal grandmother living with mild memory loss but without known dementia or motor symptoms until age 76, dying after complications of abdominal surgery. Her paternal uncle was diagnosed with Parkinson disease in his 40s and died at age 58. Her maternal grandmother was reported to be functionally intact, but mildly forgetful at the time of her death at age 89. The maternal grandfather had multiple myocardial infarctions and died of congestive heart failure at age 75. She was the eldest of 4 siblings (ages 44 to 56 at the time of presentation); none had cognitive problems. She had no children.
Because of her young age and clinical presentation with no personality changes, language or motor change, nor fluctuations, EOAD was the most likely clinical diagnosis. As visuospatial challenges were marked at her first visit and poor depth perception developing over time, posterior cortical variant of AD was also on the differential as was atypical presentation of frontotemporal dementias. Without fluctuations, Parkinsonism, falls, hallucinations, or altered attention, Lewy Body dementia was deemed unlikely. After treatment with a cholinesterase inhibitor, her MMSE improved to 18/30, tested 15 months later with stability in function. Verbal fluency improved marginally with 7 animals and 3 F-words. After an additional 18 months, function and cognition declined (MMSE=13/30) so memantine was added. The stabilizing response to the cholinesterase inhibitor added some degree of confidence to the EOAD diagnosis. In the subsequent 4 years, she continued to decline in cognition and function such that admission to a care facility was required with associated total dependence for basic activities of daily living. Noted by family before transfer to the long-term care facility were episodic possible hallucinations. It was challenging to know if what was described was misinterpretation of objects in view or a true hallucination. During this time, she developed muscle rigidity, motor apraxias, worsening perceptual, and language skills and became dependent for all activities of daily livings. At the fourth year of treatment, occasional myoclonus was noted. She was a 1 person assist for walking because of increased risk of falls. After 1 year in the care home, she was admitted to the acute care hospital in respiratory distress. CT brain imaging during that admission revealed marked generalized global cortical atrophy and marked hippocampal atrophy ( Fig. 2 ). She died at age 63 of pneumonia. An autopsy was performed confirming the cause of death and her diagnosis of AD, showing numerous plaques and tangles with congophilic amyloid angiopathy. In addition, there was prominent Lewy Body pathology noted in the amygdala.

Three years before her death informed consent was obtained from the patient and family to perform medical genetic testing for EOAD. The standard panel offered by the laboratory was selected and included PSEN1 , PSEN2 , APP, and apoE analysis. Tests related to genes related to frontotemporal dementia were not requested based on clinical presentation and clinical judgement. This was carried out with blood samples and not cerebrospinal fluid because of patient, family, and health provider preference. The results revealed a novel PSEN2 mutation with an adenosine replacing cytosine at nucleotide position 665, codon 222 [amino acid substation of lysine for threonine at position 221 (L221T)]. This PSEN2 variant was noted to be novel to the laboratory’s database, noting that models predicted that this variant is likely pathogenic. The other notable potentially significant genetic finding is the apoliprotein E genotype was Є 3/4 .
β-amyloid (Aβ) is a 38 to 43 amino acid peptide that aggregates in AD forming toxic soluble oligomers and insoluble amyloid fibrils which form plaques. Aβ is produced by the cleavage of the APP first by an α-secretase, which produces a 99 amino acid C-terminal fragment of APP, and then at a variable “gamma” position by the γ-secretase which releases the Aβ peptide itself. It is this second γ-cleavage which determines the length and therefore the pathogenicity of the Aβ peptides, with 42 amino acid form of Aβ having a high propensity to aggregate and being more toxic.
The γ-secretase is composed of at least 4 proteins, mAph1, PEN2, nicastrin, and presenilin . Of these proteins, presenilin has 2 distinct isoforms ( PSEN1 and PSEN2 ), which contain the catalytic site responsible for the γ-cleavage. PSEN mutants are the most common genetic cause of AD with 247 mutations described in PSEN1 and 48 mutations described in PSEN2 (Alzgene database; www.alzforum.org/mutations ). PSEN2 mutations are reported to be associated with AD of both early onset and variable age onset as well as with other neurodegenerative disorders such as Lewy Body dementia, frontotemporal dementia, Parkinson dementia, and posterior cortical atrophy. 2–4 In addition, PSEN2 has associations with breast cancer and dilated cardiomyopathy. 3
PSEN2 mutants are believed to alter the γ-secretase cleavage of APP increasing the relative amount of the more toxic Aβ42. The mean age of onset in PSEN2 mutations, is 55.3 years but the range of onset is surprisingly wide, spanning 39 to 83 years. Over 52% of cases are over 60 years. All cases have extensive amyloid plaque and neurofibrillary tangles, and many have extensive alpha-synuclein pathology as well. 5
In considering the novelty of this reported PSEN2 mutation, a literature search of Medline, the Alzgene genetic database of PSEN2 and the Alzheimer Disease and Frontotemporal Dementia Mutation Databases (AD&FTMD) were completed ( www.molgen.vib-ua.be/ADMutations ). The mutation presented here (L221T) has never been described before.
Although this mutation has not been described, we believe that it is highly likely to be pathogenic. This mutation is not conservative, as it replaces a lysine residue which is positively charged with threonine which is an uncharged polar, hydrophilic amino acid. The mutation itself occurs in a small cytoplasmic loop between transmembrane domain 4 and 5, which is conserved in the PSEN1 gene, and in PSEN2 is highly conserved across vertebrates, including birds and zebrafish all the way to Caenorhabditis elegans , but differs in Drosophila melanogaster (fruit fly) ( Fig. 3 ). We examined this mutation using several computer algorithms which examine the likelihood that a mutation will not be tolerated. Both SIFT ( http://sift.bii.a-star.edu.sg ) and PolyPhen-2.2.2 (HumVar) ( www.bork.embl-heidelberg.de/PolyPhen ) predicts that this variant is pathogenic. Interestingly, it is noted that PSEN1 mutations after amino acid 200 develop amyloid angiopathy. 5,7

This patient also had an additional risk factor for AD, being a heterozygote for the apoЄ4 allele. Among other mechanisms, its presence reduces clearance of Aβ42 from the brain and increases glial activation. 8 Although the apoЄ4 allele is known to lower the age of onset of dementia in late onset AD, it has not been clearly shown to influence age of onset of EOAD in a limited case series. 9 It should be noted that heterozygote state may have contributed to an acceleration of her course given the known metabolism of apoЄ4 and its association with accelerated cerebral amyloid and known reduction in age of onset. 10
Given that there is no definite family history of autosomal dominant early onset dementia, it is likely that her PSEN2 mutation was a new random event. With the unusually wide age of onset it is conceivable that one of her parents could still harbor this PSEN2 mutation. The patient’s father, however, is currently 87 and living independently at the time of writing this manuscript, making him highly unlikely to be an EOAD carrier. Nonpaternity is an alternate explanation for the lack of known first-degree relative with EOAD; however, this is deemed unlikely by the family member who provided the supplemental history. Her mother died at age 79, so she could conceivably carry our mutation but we do not have access to this genetic material. Without extensive testing of many family members it would be impossible to speculate about autosomal recessive form of gene expression. In addition, the genetic testing requested was limited to presenilins , APP, and apoE mutations. Danish heritage may add Familial Danish dementia as a remote consideration; however, Familial Danish dementia has a much different clinical presentation with long tract signs, cerebellar dysfunction, onset in the fourth decade as well as hearing loss and cataracts at a young age. 11 This disease has high autosomal dominant penetration which also makes it less likely in the patient’s context. This specific gene (chromosome 13) was not tested. The autopsy findings do not support this possibility. There are reports of Familial AD pedigrees in Germany, including a Volga pedigree with PSEN141I mutation in exon 5, but this is clearly separate from our mutation which is in exon 7. Our mutation was also not observed in a recent cohort of 23 German individuals with EOAD which underwent whole genome sequencing, but did find 2 carriers of the Volga pedigree. It is also possible that both the PSEN2 mutation and the ApoE genotype contributed to her disease and early onset presentation. This case illustrates the multiple pathology types which occur in individuals bearing PSEN2 mutations, and highlights the later ages in which patients can present with PSEN2 mutations. 12
ACKNOWLEDGMENT
The authors acknowledge Gwyneth Duhn, RN, BNSc, MSc, for her support of this paper.
- Cited Here |
- View Full Text | PubMed | CrossRef |
- Google Scholar
- PubMed | CrossRef |
Alzheimer disease; presenilin ; mutation
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Frontotemporal dementia knowledge scale: development and preliminary..., epileptic seizures in alzheimer disease: a review.
- Open access
- Published: 10 April 2023
Views of people living with dementia and their carers on their present and future: a qualitative study
- Danielle Nimmons 1 ,
- Jill Manthorpe 2 ,
- Emily West 3 ,
- Greta Rait 1 ,
- Elizabeth L Sampson 3 , 4 ,
- Steve Iliffe 1 &
- Nathan Davies 1 , 3
BMC Palliative Care volume 22 , Article number: 38 ( 2023 ) Cite this article
3223 Accesses
6 Citations
42 Altmetric
Metrics details
Dementia leads to multiple issues including difficulty in communication and increased need for care and support. Discussions about the future often happen late or never, partly due to reluctance or fear. In a sample of people living with dementia and carers, we explored their views and perceptions of living with the condition and their future.
Semi-structured interviews were conducted in 2018-19 with 11 people living with dementia and six family members in England. Interviews were audio-recorded, transcribed and analysed using reflexive thematic analysis.
Findings were explored critically within the theory of social death and three themes were developed: (1) loss of physical and cognitive functions, (2) loss of social identity, and (3) social connectedness. Most participants living with dementia and carers wanted to discuss the present, rather than the future, believing a healthy lifestyle would prevent the condition from worsening. Those with dementia wanted to maintain control of their lives and demonstrated this by illustrating their independence. Care homes were often associated with death and loss of social identity. Participants used a range of metaphors to describe their dementia and the impact on their relationships and social networks.
Focusing on maintaining social identity and connectedness as part of living well with dementia may assist professionals in undertaking advance care planning discussions.
Peer Review reports
Dementia is the most common neurodegenerative condition worldwide and is estimated to affect around 47 million people [ 1 ]. In the early stages people living with dementia often experience early memory loss but as the condition progresses communication becomes more difficult, behaviour can change, walking becomes difficult and there is an increasing need for assistance with activities of daily living [ 1 ]. In the media and general society, the condition is often associated with negative images [ 2 ] and it is not uncommon for people to experience and be affected by stigma [ 3 ]. A UK survey found 62% of adults were worried about dementia in some way, and felt a diagnosis would mean their ‘life was over’ [ 4 ].
Many professionals see it as important that people living with dementia plan for the future through a process of advance care planning (ACP), particularly as the condition is unpredictable, with people experiencing a staggered decline with bouts of good and bad health, sometimes resulting in hospital admission [ 5 ]. In the UK there is a general professional consensus that opportunities for discussions about the future should be offered regularly (including around the time of diagnosis) but must be tailored to the individual, depending on their view of the future and readiness to discuss it [ 6 ]. This respects that people may not want to discuss the future due to fear, worry or denial [ 7 ] or other reasons, even though it means conversations about choices and preferences may not be possible, leaving it to family and/or professionals to make decisions.
Discussions may also not take place due to organisational challenges, such as a lack of access to specialist or primary care [ 8 ], professional inexperience or lack of confidence [ 9 ]. Although there is professional advice that ACP discussions should occur regularly, this does not always happen due to inequity in care provision [ 10 ]. Previous reviews of the literature have recommended that professionals hold several ACP discussions with people living with dementia over a longer period and involve family and significant others as early as possible, including when it is more difficult for the person living with dementia to communicate [ 11 ]. Despite challenges, it is important to discuss the future as ACP in dementia can lead to an increased number of advance directives and greater concordance between the person with dementia’s preferences and relatives’ decisions on their behalf [ 12 , 13 ].
How people living with dementia view their futures is likely to vary. Some studies show negative views, for example, people may experience symptoms that leave them feeling unsure of themselves as the world becomes more unfamiliar [ 14 ]. While other studies have a more positive outlook, where people are not passive in how they experience the condition and use various strategies to cope with its challenges [ 15 ]. A recent Dutch study found those with early-stage dementia were willing to talk about their future, if given the opportunity [ 6 ]. They also felt it was important to live a meaningful life and maintain belongingness until end of life [ 6 ]. In recent years there has been an emphasis on framing the condition more positively and on people ‘living well’ with dementia [ 16 ], despite negatively held assumptions by the public [ 15 ]. Considering the varied views expressed in previous studies, the aim of our study was to explore, with an end-of-life focus, the views and perceptions of dementia and the future of people living with dementia in England.
Qualitative study using semi-structured interviews, analysed using reflexive thematic analysis [ 17 ].
Recruitment procedure
People living with dementia were purposively sampled from a range of sources including NHS memory services, general practice, carer or dementia organisations, and the National Institute for Health Research (NIHR) Join Dementia Research website. Potential participants from clinical services were identified and invited by a member of the clinical team. Interested and eligible participants either contacted the research team directly or with permission their details were passed to the research team. Recruitment invitations via non-clinical settings were sent by members of the research team or host organisation via email.
Inclusion and exclusion criteria
People living with dementia were eligible if they met the following criteria:
Clinical diagnosis of any type of dementia as categorised in ICD-10.
Capacity to provide written informed consent to take part in an interview.
Able to read and speak English.
Clinical teams checked eligibility, which included assessing if they felt potential participants had capacity. Experienced researchers trained in capacity assessment also assessed capacity when taking consent, at the start of the interviews and throughout. Those unable to provide informed written consent were not included. Family members were not recruited separately, only if the person with dementia wanted to be interviewed with them. Family members had to be adults, and able to consent to take part in an interview and to read and speak English.
Data collection
Interviews were conducted by either a female research assistant or a male senior researcher, both of whom had training in qualitative research methods. Interviews were guided by an interview schedule which was informed by relevant literature [ 18 , 19 , 20 ], and supplemented with vignettes to prompt discussion [ 21 ]. The interview schedule can be seen in the Supplementary material along with an example vignette. The interview schedule was piloted with the study’s Patient and Public Involvement (PPI) group consisting of former carers, and with the first two participants, and revised. Interviews were conducted with the person with dementia alone, or with a family member if preferred by the participant living with dementia. Where a family member was present and took part in the interview they were consented, and their responses are included in the analysis. During dyadic interviews, participants living with dementia were asked questions first to ensure their views and experiences were heard. Carers were able to contribute when they wanted to but this was not the focus of the interview. All interviews were audio-recorded and transcribed verbatim. A research assistant checked all transcripts against the original audio files for any discrepancies and data were anonymised.
Data analysis
Transcripts were imported into NVivo version 12 to facilitate analysis [ 22 ]. Reflexive thematic analysis was used to analyse data and develop themes [ 17 ]. One member of the research team (DN) coded all transcripts, while two others independently coded a further five. Through a series of discussions initial code lists were refined and initial themes developed by DN. Themes were then refined, while interpretation and meaning of each theme were discussed with the whole team (experienced in gerontology, primary care and palliative care), enabling them to contribute to findings and revise iteratively.
Following coding and the development of initial themes, we identified a similarity to some of the key themes within the concept of social death [ 23 ], a series of losses resulting in a disconnection from social life. Social death was first conceptualised in 1982 by Patterson in relation to slavery to describe how people can be considered unworthy and seen as dead when they are alive [ 24 ]. The theory has also been applied, at times controversially, to patients with chronic diseases, for example people living with dementia or, as it was then perceived, ‘suffering from dementia’ [ 23 ]. A recent concept analysis classified social death into three themes in relation to patients: the loss of social identity, loss of social relations, and deficiencies related to the inefficiency of the body and various diseases [ 25 ]. For example, as some chronic conditions advance, social roles change and people are unable to continue their previous social interactions as they are threatened by the body’s decline [ 23 ]. For some, losing social identity leads to exclusion or withdrawal from the wider community; associated with vulnerability, stigma and loss of physiological functioning [ 23 ]. In light of this, we shaped our findings and organised them within the three concepts underpinning the theory of social death to see if they offered an explanatory model. This enabled us to see if the theory of social death may offer insights into dementia experiences.
Ethics approval
Health Research Authority (HRA) ethical approval was received (London Queen Square Research Ethics Committee (18/LO/0408) on 10.04.2018. Written informed consent was provided by all participants.
Eleven people living with dementia were recruited, all of whom had capacity. Six were dyadic interviews with a family carer and four were individual interviews. During dyadic interviews, all carers provided answers to all questions. Participant characteristics are presented in Table 1 .
We arranged our themes under the three key concepts of social death theory [ 17 ], however we adapted the names of these concepts to reflect the participants’ words and the context of dementia: (1) loss of physical and cognitive functions, (2) loss of social identity, and (3) social connectedness. Table 2 provides an overview of the adapted concepts of the theory and our themes within them.
Loss of physical and cognitive functions
Across the interviews participants discussed their progressive decline, in particular the decline of their memory and cognitive functions. As discussed in the themes below, the loss of cognitive functions was often the focus of their discussion, rather than a change to physical functioning and abilities, although these often accompany advancing stages of dementia and indeed old age.
Staying present and the importance of maintaining self
Many participants wanted to discuss the present and focus on what they were still able to do, rather than discussing the future and anticipated limitations. This discussion of the present appeared to either be a representation of their role and importance within their family, still demonstrating a sense of purpose or use to others, or more simply as a representation of things they enjoyed doing and as a way of reflecting they still enjoy life:
“But I can still cook, can’t I? I can still wash up.“ Participant 03 “But other than that, I still play golf three times a day – a week” . Participant 02
Maintaining current levels of health and well-being was important to support their mood and some felt this was important to try to minimise the impact or delay the progression of their dementia:
“I mean this morning I’ve done yoga. And we do it – [husband] and I do it every week. And I feel great when I’ve done it. “ Participant 09 “ I still believe that at least I can delay and delay and delay it. ... And I’m still healthy, although I’ve got a heart condition.“ Participant 02
When asked about advanced dementia, many participants focussed on progressive memory loss which would lead to them relying on others such as their family. The narrative of dependence was often dominated by memory and less around physical function. Dementia was therefore seen as a condition of the mind (not the body) which would continue to deteriorate:
“I’m not sure what dementia is, I don’t know what it means. I know it’s a side of the brain and the mind…I have knowledge confirmed of what’s going on in my mind or my brain.” Participant 05
Participants believed that although their mind and memory will inevitably decline, by maintaining physical health they could still ‘get on’ with their lives and not be too restricted by the condition, for example, carrying out activities of daily living and ‘getting by’:
“What is important to me really is to ensure that my health is, continues to be good physically, that I manage and that I can carry on – getting by in terms of memory” . Participant 11
A few participants felt maintaining good physical health could help them ‘mask’ their dementia from other people, enabling them to still partake in enjoyable activities. As one participant said:
“I think I handled it quite well because, as I say, there’s no, there’s no visible sort of way that gives away the fact that you’re suffering with a disease” . Participant 10
This suggests this participant’s perception of dementia being a condition of the mind, instead of the body.
Masking their dementia was important to some participants who were worried about how others would treat or view them if they knew they had dementia. For example, one participant feared that others would point and talk about him if they could see that he had dementia:
“People will think I will get more sensitive if you see me with that [dementia], and particularly for someone, you know, talking to each other, point out, pointing at me…but I’m afraid .“ Participant 02
Discussing the future
Discussing the future is discussed within two subthemes: ‘interviewer as enabler of discussion’ and ‘discussing with others and planning’.
Interview as enabler of advance care planning and discussions around the future
Most participants did not want to discuss the future perhaps because they feared what it entailed, both in terms of losing their cognitive and physical functions, leading to increased need for support and care. They viewed dementia negatively and a condition that would only get worse “[It is] Irreversible. One-way traffic… “. However, for some this was as far as they wanted to initially go in discussing their dementia, with many diverting or closing down the conversation:
“I don’t think about anything really about the future. Okay, so have you… (interviewer) I’m happy as I am.” Participant 08
When asked directly about their future, most said they did not know what would lie ahead. However, as the interview progressed and rapport was built with the interviewer, many also recalled situations of people they knew approaching end of life. This included nursing a friend with terminal cancer, parents who had died in care homes, and friends with more advanced dementia:
So you talked about maybe a need for a conversation now about diagnosis and things. What, as a family, do you understand about maybe the later stages of dementia? (interviewer) ”Not very much ”. Carer of Participant 03
When directly asked, this carer said she did not know what happens in the advanced stages of dementia, however she later discussed a friend whose husband with more advanced dementia was moving to a nursing home:
“I’m getting a bit concerned this week because I have a friend in America with exactly the same problem with her husband. But I had a letter from her this week to say that he’s been taken into a home… through the memory clinic out there. And it’s all very, very distressing for her.“ Carer of Participant 03 (later)
These possible contradictions could suggest a degree of denial and that some carer participants perhaps knew more about later stages of dementia than they wanted to admit or felt uncomfortable talking about in the presence of their relative. Many carers seemed determined to focus on the present and not discuss the future, in part, due to fear and family experiences:
“I think because he [Participant 07] has seen his mum in the home and ... they were lying in the bed all day and nobody came to look. So this is what we have seen in the home and that is in the mind.“ Carer of Participant 07
The interviews appeared at times to enable discussions around the future and advance care planning for participants and their carers, possibly providing them with one of the first opportunities to engage in these conversations either as an individual or with their partner who was being interviewed with them. However, responses to advance care planning questions were always short or vague:
So if you were in that position, when you stopped eating, you’d stopped eating and drinking, and people – your family were trying to say, “No, no, you need to eat, you need to drink,” but you didn’t want to – what would you want them to think about in making that decision whether to stop or whether to think about those other options? (interviewer) ”Hmm, that’s difficult. I don’t know, if I didn’t know, I don’t know. If I didn’t know, I wouldn’t know, I’d have to wait and see what the problem was, you know, how I really, really felt ” Participant 08
This further indicates that participants did not want to talk about the future or were not ready to. This may have been for reasons such as fear, and/or the risk of causing sadness or distress for the person living with dementia. This often resulted in carer participants saying they would talk about these topics at some stage, without committing to a time:
“I mean he gets a bit upset when you start talking about these sorts of things. But I mean I know it has got to be sought after, you know, later on, but I mean, at the moment, he copes very well”. Carer of Participant 11
Planning for the future in discussions with family members
Some participants living with dementia felt it was not worth talking about the future, when they would not be able to understand what was going on around them towards end of life:
“ I mean it has come to my mind, but I just try not to sort of dwell on it really. I just think I’ll take it as it comes. What’s the point of worrying about, you know, all those things that you worry about when you think about what’s going to happen when I die?“ Participant 09
This was despite some family carer participants showing interest in discussing the topic, while the participant living with dementia did not want to. This also illustrates how the interview enabled discussions around planning with family:
“So, even I can picture this to what is lying ahead of us. But, yes, it would be nice to be prepared, or even he would like to – it will be good for him to know as well that what is there to look for” Carer of Participant 07
This could be because several participants living with dementia openly said they would leave end of life decisions to their family carer when the time came, and some carers wanted to be prepared for this:
“ You know, I just think I’m sure my children will sort it out if they have to. I don’t have any great thoughts about it. You know, I try not to because I, you know, I want to enjoy what life that I’ve got without being miserable all the time ”. Participant 09
The default position adopted by participants living with dementia for their carers to make end of life decisions in a future when they no longer have capacity, is another example of how participants living with dementia wanted to stay in the present. This could be viewed as avoidant or a legitimate strategy. It also shows participants relinquishing more active or decision-making roles within their families, onto their children or partners.
By focusing on the present, maintaining current health and well-being, and showing a reluctance to discuss the future, participants living with dementia seemed to be deciding to leave discussions about the future to others while trying in the present to minimise further decline, although they knew their condition would worsen. In some cases, this was felt by interviewers to manifest as anger, as reflected in this participant’s response to his carer who said they had not discussed care homes or later stages of dementia:
“No, this is the first time that it’s – we haven’t spoken about – because I didn’t think that I was about to pop my clogs (die).” Participant 03
Loss of social identity
Throughout the interviews there was an underlying discussion of social identity and how participants perceived and categorised themselves in relation to the progression of their dementia, other people and the world around them. Participants presented a narrative of independence and control to reduce the risk of social exclusion while the anticipated loss of independence was associated with advanced dementia and moving to care homes. We created the following themes within the category of loss of social identity: ‘Power, control and independence’ and ‘Perceptions on support and care homes’.
Power, control and independence
Apart from planning for the future, participants living with dementia wanted to be in control of their lives, mostly in respect of everyday activities. Several participants felt the future was now out of their control, which worried them:
“And people then getting worried about where they are and what they’re doing here and what’s going to happen next. And I’m not, I don’t have any control over it. I think it’s the feeling of lack of control that’s probably most worrying.” Participant 04
However, control was also beginning to be affected by their current limitations. This included no longer being able to drive, cook or go out unaccompanied; activities that many people take for granted:
“Well, you don’t have any choice. Once you’ve been diagnosed, they then say – “Hand in your [driving] licence .” Participant 05 “They told me, didn’t they, that it’s inadvisable to go out unaccompanied, I think, was the expression.” Participant 03
Declining independence seemed to be altering parts of their identity. This was often viewed negatively by those living with dementia, as they felt independence was eroding which prevented them from living their lives as fully as previously. However, participants understood these changes were necessary to ensure their safety:
“Well I can’t drive a car, which is another irritant. But I understand the necessity of stopping people like me driving, because we wouldn’t be safe on the road.” Participant 10
To balance this, carers often focused positively on what their relative could do:
“Well, you’re very independent, yes. You can do your shopping and you can read and you can eat, choose what you want to eat.” Carer of Participant 06
However, many participants living with dementia were now needing assistance in many aspects of their lives, usually from family carers:
“There’s very little I can do without her [wife] input, put it that way.” Participant 05
Several acknowledged they would need more help from family as their dementia advanced. Those who did not have family felt they would have to employ people to help them:
“No, I’ve really got no family that I can turn to. And, you know, they’ve moved on and I really, to some degree, now only got very minimal contact with my brother who lives in another part – he lives in [another part of UK]. So, so, so I would think that in terms of financial affairs, I’d have to employ someone specifically, you know, an accountant or someone to deal with that.” Participant 11
For some participants with substantial resources these were not new arrangements:
“We’ve got staff who look after the place. So you know, we’ve got a gardener, we’ve got a cleaner.” Participant 11
Several participants mentioned role-reversal, where their spouse or children were carrying out the roles/jobs that previously held by the person with dementia. This included roles traditionally seen as signifying independence, such as managing one’s finances:
“But you controlled your own money. I controlled mine and we both controlled ours. But recently I’ve had to do it all for you, haven’t I...” Carer of Participant 08
Again, many participants recognised that they could no longer carry out these duties and some recalled a time when they had held a more active role in the family:
“But, you know, I’ve always been a leader and I don’t mean democratically. I’ve always looked upon the girls and the wives- I bought this house off my mother-in-law…I’ve never found a problem looking after the mother-in-law, both financially, and you know, like elderly ladies have to have baths etc. I never found it a problem,” Participant 03
Not being able to carry out duties of responsibility was also seen as a loss of power by some:
“What she [carer] said that I still want to hold power – in my mind I can correct a little bit what she just mentioned. And I try to hold on – but no, I’m very, very easy-going in my mind, because what can we do if I’m not able to make any decision?” Participant 02
Another participant living with dementia discussed the situation of her mother who was also living with dementia. Evidently, she had arranged a personal alarm for her mother who did not wear it, as she considered it as a loss of independence:
“So she does have an alarm that we pay for, but she refuses to wear it. So there’s many incidences of independence which are almost there in spite of what I’m trying to put in place to help to show how, how much independence you have, which is silly, because you need to wear the alarm.” Participant 06
Perceptions of support and mixed perceptions of care homes
Most participants viewed care homes very negatively and associated them with death:
“That simply would be awful, a care home. Well I’d probably be dead. Most people in care home die, don’t they?” Participant 06
Some participants said they would rather die or be dead than move to a care home:
“I wouldn’t want that, yes. No, I’d sooner go and jump off a bridge.” Participant 11
Care homes were seen as institutions where people are removed from their usual social settings, losing their social roles and therefore their social identity. Participants living with relatives felt they would lose social interaction in a care home and be left alone for long periods:
“They [family] look after me. And I’ve company all the time, all the time. Then by the time, if I go to a home, then there won’t be company – in the morning, you are sleeping, if you’re there, you go to sleep and in the evening, you might get up – and by the time – well, if you get up or if you don’t, you go to sleep all the time, all the time.” Participant 07
The care home environment was seen as important and one participant felt a home would not be able to cater to their needs or that they would not be able to relate to other residents:
“Well, I don’t – I think the image of being in a place where I wouldn’t be able to – well, I wouldn’t be able to relate comfortably to the other people maybe. Or, if the place was going to be very basic and all the rest of it.” Participant 11
However, two participants (P9 and C11) recalled positive care home experiences involving their relatives:
“But I was very lucky to find two great homes for [mother] ” Participant 09
Although, they admitted their relative had to pay for good quality care, for example, by using the assets raised by selling their home, as is generally the case in the UK:
“And he went in with her – obviously he had to sell his house to pay, you know, help pay for it, you know, which is what happens with these now, these days, you know.” Carer of Participant 11
Other participants were also more open to the idea of living in a care home when their dementia progressed, although all indicated they would prefer to live at home for as long as possible:
“Yes, if the care home was like home, yes, I would be content to go there, but I can’t imagine them being as comfortable as – oh no, I mean the one that mum and dad was in, was very good.” Participant 10
Timing of a care home move was also discussed, and all participants felt it would happen when the person living with dementia could no longer do things for themselves, family could no longer cope with caring for them, or they had lost capacity, although for some it would be a last resort:
“ And to go into a care home would mess up the whole thing and be useless and I think the only thing to do is to die.” Participant 06
Social connectedness
Participants described their previous and current social networks. These relationships and networks were constantly changing and were affected by factors, such as their past employment, and interactions with others from outside their circles of family or friends. Participants used a range of metaphors to help describe their dementia and its impact on their relationships and social networks.
Social view of dementia: life legacy that can be maintained
Most participants reflected on their life, what it meant and how they did not want dementia to overshadow their achievements either academically, socially or just the person they had always been. Some referred to their successful working lives, for example in the military or public services. Some specifically mentioned how having a good memory had helped them at work, in contrast to the symptoms of their dementia:
“I had a very, very, very good memory. I had a very good military career which I was, as a result of my memory and expertise ” Participant 03
They discussed the idea of loss, in particular loss of what they perceived as their identity. For example, one participant described this loss in relation to their previous occupation:
“But, at that point, I was very, very aware that, you know, I was losing, really my vocabulary. That really bothered me, a big reader, works in libraries and so on. That was a big deal for me.” Participant 01
Participants seemed to not want to be defined by their dementia and therefore focused on past careers or current roles. This was important for their social identity. For instance, one participant described current volunteer work, what that involved and how it benefitted them. They emphasised how some people living with dementia continue to add value to society despite their dementia:
“I take a group of people round and talk about the history and about particular features of the cathedral. So that makes me, I do preparation the previous day to recall, because, after a month, for me, things slip away, so I just go through my prompt cards that I have about key facts and events. And the history of the cathedral. So that sort of refreshes the memory. I don’t read those when I go round, I do it all verbatim. So that’s that.” Participant 11
Social view of dementia: viewed by others and mixed metaphors of dementia and decline
Participants often used idioms, phrases or images through a series of different descriptions, and metaphors for describing their dementia and associated decline including:
“Well, you’re not firing on all cylinders, you know. It doesn’t mean you’re totally gaga” Participant 4 “something that sort of invades the brain really, I think.” Participant 09
However, some also used humour as a way of talking about their decline, possibly as a way of avoiding the reality of their condition:
“Well if I got to the stage where I needed it, physical and mental stage – that’s never going to happen to me! Immortal.” Participant 04
One participant described his family as a team, his daughters as ‘the managers’ and wife as ‘leader’:
“I’m alright because we’re a team, you see. And [my wife] is the team leader.” Participant 03
This participant was unable to carry out some activities, such as dealing with his finances or cooking. He relied on his family to do this and describing them as a team helped convey this. It seemed to be a way to help him cope with his gradual decline as he accepted the ‘team’ would have to do more for him and make more decisions for him as time passed. Being part of the ‘team’ also reflected his continued active involvement within his family and that he was not as dependent as he might be if he were outside the ‘team’, where he would have a less active role:
“ And I thought, “Oh gosh, I never want to get into a situation being old folk like that.” But it happens. But I’m sure I’m not going to do that to my team because they’re not going to allow me to do it.” Participant 03
Many discussed how they were perceived by others and did not want to be recognised as having dementia. This was accomplished by not letting others know about their diagnosis, only close relatives and friends, or by hoping that by simply looking at them no one would know they had dementia:
“I think I handled it quite well because, as I say, there’s no, there’s no visible sort of way that gives away the fact that you’re suffering with a disease.” Participant 10
For some this meant not disclosing their dementia, so people would not treat them differently:
“I thought I’m glad I haven’t told anybody, you know, because I mean it’s silly really, but, you know, I’ve kept – my friends say, when I’m taking the full, the full drug, you wouldn’t know, you’d never know. So I just wanted to keep it like that. I don’t want people doing things for me because they feel sorry for me.” Participant 09
One participant described having dementia as wearing a mask but acknowledged that one day the mask will have to be peeled off. When asked about the later stages of dementia, he replied:
“I think probably, nowadays, I think that I should be getting more happier, because by that time, probably I should give up – okay, I should peel off my mask.” Participant 02
We conceptualised our findings into three concepts or categories, which we adapted from the Social Death theory [ 17 ], namely: (1) loss of physical and cognitive functions, (2) loss of social identity, and (3) social connectedness. Our participants living with dementia were focused on the present instead of thinking about and discussing their future and maintaining current levels of health was seen as a way to prevent deterioration. The interview seemed in many instances to enable discussions about the future, which was the subject raised in the study information material, this was particularly noted by interviewers in fieldnotes when interviewing dyads. However, even after enabling conversations many responses were vague, indicating a reluctance to discuss specifics or personal matters in detail during the interview. Overall dementia was viewed negatively and seen primarily as a condition affecting the mind. There was some suggestion that being in control of their physical body was more achievable at the present and could mediate their cognitive decline. Control seemed to help maintain independence while a lack of independence was associated with perceptions of advanced dementia, reliance on family, and care home moves. Our participants did not want dementia to overshadow past achievements or define who they were, and varied metaphors were used to describe the condition.
Social Death theory has previously been applied to people living with dementia. However, this theory no longer fits with how dementia is often viewed or understood in countries such as the UK as reflected in our findings; where there is more emphasis on capabilities and independence, instead of loss and social exclusion, and living well with dementia. Although helpful to consider Social Death in relation to our findings, we felt it was important to critically consider it and modify it, considering key important concepts of it as part of our analysis.
We explored how using the concept of social death could help reveal how participants living with dementia and their carers perceived the future of their condition and professional interactions. Social death was a concept used several years ago in respect of dementia by Sweeting and Gilhooly who argued that people who are treated as socially dead hold certain characteristics, including those with lengthy fatal illnesses, very old people, and people with a loss of personhood [ 26 ]. Some have argued that social death deprives people the dignity of a meaningful death [ 27 ]. However, it is also argued that loss of social identity, loss of connectedness and loss of physical abilities must be extreme for social death to be valid [ 23 ] (which our participants did not have as their dementia was early stage). Although, several participants held ideas about advanced and disabling dementia through the experiences of others [ 28 ], for example, family members who had dementia.
People living with dementia used to be described in a negative, dehumanised way as passive actors and socially dead [ 29 ]. However, as Kitwood and others have proposed, personhood and citizenship can be facilitated when people with dementia are seen and treated as socially active.[ 16 ] Also, empowerment and facilitation may help people living with dementia maintain their social identity and enable them to be part of a community [ 30 ], as emphasised in the policy ambitions of living well with dementia [ 31 ]. This is important as deterioration and a loss of personhood are not just due to the dementia itself but also affected by how people with dementia are treated by others, e.g. by being infantilised, in what is termed ‘malignant social psychology’ [ 16 ]. Being socially active may include people living with dementia taking part in discussions or choices about everyday life and taking their preferences into consideration [ 30 ]. This may be why all participants in our interviews focused on positive elements of their social identity, including maintaining independence and control of their lives. Also, it may explain why discussions around advance care planning were not raised, due to fear of upsetting the person living with dementia. Empowering them by following their wishes may have to include respecting their wishes not to explore the topic.
Metaphors reflecting active shaping of narratives were often used by participants to explain their dementia or how they were coping with the condition. This included describing an active presentation of donning a ‘mask’ to present a non-disabled status and presenting the family as a ‘team’ rather than overbearing. Patients often use metaphors to tell their stories and assigning meaning to their illness, which provides a bridge between the image of their old life and new one [ 32 , 33 ]. For people living with dementia, choices of metaphors enable them to also preserve their social identity against negative cultural images of dependence, passivity and decline [ 33 ]. Metaphors with negative connotations are sometimes used by the media and scientific community to describe dementia, invoking fear in people [ 34 ], such as an ‘epidemic’ or ‘crisis’ [ 26 ]. People affected may be likened to victims and linked with a living death [ 34 ]. However, the use of metaphors in our participants’ narratives indicates more agency and autonomy in people living with dementia, enabling them to exercise voice and platform against the negative views of passivity and dependence associated with the condition [ 33 ].
Participants’ general reluctance to discuss the future and wish to live in the present links with previous research demonstrating these can be a barrier to advance care planning. A systematic review found patient factors such as ‘not being ready’, fear of death and denial were all reasons for lack of engagement, which sometimes could be explained in terms of coping [ 7 ]. This is also seen in other neurodegenerative conditions, such as Parkinson’s disease and multiple sclerosis, where it is unclear when is the optimal time to discuss the future and depends on similar patient factors [ 9 , 35 ].
Participants living with dementia did not want the condition to define them or be part of their legacy, which could instead focus on their contributions to society. Post and colleagues have proposed that living a meaningful life is challenged by having dementia, as cognition is associated with maintaining self-control, independence, and relationships [ 36 ]. Demonstrating a meaningful legacy is therefore important in maintaining social identity in dementia and could be promoted by professionals. Social identity is also important in preventing social disenfranchisement, where people get labelled as a ‘dementia patient’, and so need to manage their condition as well as their identities [ 37 ]. The implications for practice are that healthcare professionals should elicit and consider patients’ identities and illness narratives, which may aid discussions around the future.
We found some carers seemed more open to discussing the future than the person living with dementia. This may be because they are witnessing their relative’s decline and are anticipating a potential crisis (when the person with dementia will be less able to make decisions) and that decisions will be needed, as described in other studies [ 38 ]. Participants living with dementia trusted their family carers to make decisions on their behalf about their future care, when the time comes. This finding confirms other studies [ 6 ], where a high level of trust and confidence was placed in family members taking on end of life decisions with professionals and advocating for their relatives’ wishes [ 39 ]. However, end of life discussions are difficult for some carers of people living with dementia and other conditions, as well as in other decision-making areas, such as legal-financial matters, care home moves, and making plans if the carer become unable to provide care [ 40 ]. Most of these topics were brought up during interviews, however, future care scenarios had not been fully discussed within the family in our view and this aligns with other research, where family carers report feeling unprepared when end of life care is needed [ 41 ].
Most participants viewed care homes negatively, fearing the isolation, loss of autonomy and dependency they associated with them. Some views were based on family experiences from some years ago. Care homes were viewed as institutions where, de facto , people are removed from their usual social settings, but also lose their social roles and therefore their social identity [ 23 ]. This confirms other research using social identity perspective theory that argues that care homes restrict social identity, as represented by a loss or giving up of possessions, clothing and activities, that represent a loss of independence and autonomy [ 42 ]. However, Paddock’s study included care home residents without dementia who shared a commonly held downward social comparison with those with dementia. Those residents who were more ‘cognitively superior’ than those with dementia felt their positive sense of identity was hindered because of the association of care homes with severe cognitive impairment [ 42 ].
However, a few participants whose relatives had positive experiences were more willing to consider moving to a care home in the future. There is an increasing body of international research highlighting the needs of people living with dementia from their own perspective, including those living in residential care [ 43 ]. This includes measures that can be taken to improve quality of life in care homes. For example, boredom is commonly experienced by residents with dementia and therefore organised activities are encouraged to combat this [ 43 ]. A feeling of restriction is also common, which can be counterbalanced by providing choice, while a homely environment and meaningful relationships can combat loneliness [ 43 ]. Funding of such improvements could lead to better care homes, promote social identity and connectedness; and help reduce the negativity associated with them.
Strengths and limitations
This study explored the perspectives of people living with dementia, a group whose voices are often excluded from research and we explored a topic seldom researched. Our analysis was reflexive, our research team is diverse and from different disciplines including psychology, medicine, social work, and anthropology, which aided interpretation of results.
The study limitations should be noted. Only 11 people living with dementia were interviewed, which may not be representative of a wider population, however for this study we were much more guided by the concept of information power [ 44 ]. Information power encourages researchers to consider the richness of the dataset as opposed to the sample’s size and is recommended in recent discussions of thematic analysis [ 45 ]. No new themes were developed from the data during the eleventh interview, and there was strong, rich and clear dialogue between the researcher and participants. We therefore believe there was sufficient information power in this study and the sample size was adequate. People were excluded if they were not able to speak or read English, since we were not able to access interpreters, and further research should explore how findings might differ according to culture and ethnicity. Six of these interviews included a carer and this may have affected people’s frankness. A downside of dyadic interviews is that carers can ‘speak for’ or speak over the person living with dementia [ 41 ]. All those interviewed were able to provide written informed consent and we did not use communication aids or approaches to recruit participants with greater cognitive problems or other disabilities. Most of our sample was married and there were more men than women. Furthermore, socioeconomic data was not collected and differences according to income could not be analysed.
Conclusions
We found participants living with dementia wanted to focus on the present and maintain control of their lives, while also maintaining their social identity and connectedness. We discussed key themes in relation to how people living with dementia and their carers view the future of their condition, in the context of social death theory and concluded that this needs to be used with caution in contemporary practice, policy making and professional interactions. Our participants were more empowered than this theory might suggest and wanted to present narratives of agency and social identity rather than ‘social death’. Focusing on this may allow for more open discussion around advance care planning to address the implications of decline as the condition progresses, if people so wish.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. If someone wants to request data, please contact Dr Nathan Davies ([email protected]).
Abbreviations
- Advance care planning
National Institute for Health Research
Patient and Public Involvement
Health Research Authority
Emmady PD, Tadi P. Dementia. StatPearls. Treasure Island (FL)2022.
Low L-F, Purwaningrum F. Negative stereotypes, fear and social distance: a systematic review of depictions of dementia in popular culture in the context of stigma. BMC Geriatr. 2020;20(1):477.
Article PubMed PubMed Central Google Scholar
Milne A. The ‘D’ word: reflections on the relationship between stigma, discrimination and dementia. J Mental Health. 2010;19(3):227–33.
Article Google Scholar
YouGov. Are you worried about dementia? 2012 [13/04/2022]. Available from: https://yougov.co.uk/topics/politics/articles-reports/2012/05/22/are-you-worried-about-dementia .
Addington-Hall. Positive partnerships – palliative car for adults with severe mental health problems. Guy’s, King’s and St Thomas’s School of Medicine.; 2000.
Bolt SR, van der Steen JT, Khemai C, Schols JMGA, Zwakhalen SMG, Meijers JMM. The perspectives of people with dementia on their future, end of life and on being cared for by others: A qualitative study.Journal of Clinical Nursing.n/a(n/a).
van der Steen JT, van Soest-Poortvliet MC, Hallie-Heierman M, Onwuteaka-Philipsen BD, Deliens L, de Boer ME, et al. Factors associated with initiation of advance care planning in dementia: a systematic review. J Alzheimers Dis. 2014;40(3):743–57.
Article PubMed Google Scholar
Connolly A, Iliffe S, Gaehl E, Campbell S, Drake R, Morris J, et al. Quality of care provided to people with dementia: utilisation and quality of the annual dementia review in general practice. Br J Gen Pract. 2012;62(595):e91–8.
Koffman J, Penfold C, Cottrell L, Farsides B, Evans CJ, Burman R, et al. I wanna live and not think about the future” what place for advance care planning for people living with severe multiple sclerosis and their families? A qualitative study. PLoS ONE. 2022;17(5):e0265861.
Article CAS PubMed PubMed Central Google Scholar
England PH, Dementia P. 2019 [Available from: https://fingertips.phe.org.uk/profile-group/mental-health/profile/dementia/data#page/0/gid/1938133052/pat/6/par/E12000004/ati/102/are/E06000015/iid/91215/age/1/sex/4 .
Piers R, Albers G, Gilissen J, De Lepeleire J, Steyaert J, Van Mechelen W, et al. Advance care planning in dementia: recommendations for healthcare professionals. BMC Palliat Care. 2018;17(1):88.
Kelly AJ, Luckett T, Clayton JM, Gabb L, Kochovska S, Agar M. Advance care planning in different settings for people with dementia: a systematic review and narrative synthesis. Palliat Support Care. 2019;17(6):707–19.
Bosisio F, Sterie AC, Rubli Truchard E, Jox RJ. Implementing advance care planning in early dementia care: results and insights from a pilot interventional trial. BMC Geriatr. 2021;21(1):573.
Phinney A. Living with dementia from the patient’s perspective. J Gerontol Nurs. 1998;24(6):8–15.
Article CAS PubMed Google Scholar
de Boer ME, Hertogh CM, Dröes RM, Riphagen II, Jonker C, Eefsting JA. Suffering from dementia - the patient’s perspective: a review of the literature. Int Psychogeriatr. 2007;19(6):1021–39.
PubMed Google Scholar
Kitwood B. Dementia reconsidered, revisited: the person still comes first / original text by Tom Kitwood ; edited by Dawn Brooker. Second edition, new edition. ed: London : Open University Press / McGraw Hill Education; 2019.
Braun V, Clarke V. One size fits all? What counts as quality practice in (reflexive) thematic analysis? Qualitative Res Psychol. 2021;18(3):328–52.
Davies N, Maio L, Rait G, Iliffe S. Quality end-of-life care for dementia: what have family carers told us so far? A narrative synthesis. Palliat Med. 2014;28(7):919–30.
Mathew R, Davies N, Manthorpe J, Iliffe S. Making decisions at the end of life when caring for a person with dementia: a literature review to explore the potential use of heuristics in difficult decision-making. BMJ Open. 2016;6(e010416). https://doi.org/10.1136/bmjopen-2015-010416 .
Davies N, Schiowitz B, Rait G, Vickerstaff V, Sampson EL. Decision aids to support decision making in dementia care: a systematic review. Int Psychogeriaatrics. 2019;31(10):1403–19.
Hughes R, Huby M. The application of vignettes in social and nursing research. J Adv Nurs. 2002;37(4):382–6.
Zamawe FC. The implication of using NVivo Software in qualitative data analysis: evidence-based reflections. Malawi Med J. 2015;27(1):13–5.
Králová J. What is social death? Contemp Social Sci. 2015;10(3):235–48.
Fellman M. Int History Rev. 1984;6(2):328–30.
Google Scholar
Ghane G, Shahsavari H, Zare Z, Ahmadnia S, Siavashi B. Social death in patients: Concept analysis with an evolutionary approach. SSM - Population Health. 2021;14:100795.
Sweeting H, Gilhooly M. Dementia and the phenomenon of social death. Sociol Health Illn. 1997;19(1):93–117.
CARD C. Genocide and Social Death.Hypatia. 2003;18(1):63–79.
Kleinman A. The illness narratives: Suffering, healing, and the human condition1988.
Bartlett R, O’Connor D. From personhood to citizenship: broadening the lens for dementia practice and research. J Aging Stud. 2007;21(2):107–18.
Brannelly T. Sustaining citizenship: people with dementia and the phenomenon of social death. Nurs Ethics. 2011;18(5):662–71.
Care DoHaS. Living Well With Dementia: a national dementia strategy. 2009.
Becker G. Disrupted lives: how people create meaning in a Chaotic World. University of California Press Books; 1999.
Zimmermann M. Alzheimer’s Disease Metaphors as Mirror and Lens to the Stigma of Dementia. Lit Med. 2017;35(1):71–97.
Zeilig H. Dementia as a Cultural Metaphor. Gerontologist. 2013;54(2):258–67.
Nimmons D, Hatter L, Davies N, Sampson E, Walters K, Schrag A. Experiences of advance care planning in Parkinson’s disease and atypical parkinsonian disorders: a mixed methods systematic review. Eur J Neurol. 2020;27(10):1971–87.
Post. The concept of Alzheimer disease in a hypercognitive society. In Concepts of Alzheimer disease: Biological, clinical and cultural perspectives 2000.
Beard RL, Fox PJ. Resisting social disenfranchisement: negotiating collective identities and everyday life with memory loss. Soc Sci Med. 2008;66(7):1509–20.
St-Amant O, Ward-Griffin C, DeForge RT, Oudshoorn A, McWilliam C, Forbes D, et al. Making care decisions in home-based dementia care: why context matters. Can J Aging. 2012;31(4):423–34.
Poole M, Bamford C, McLellan E, Lee RP, Exley C, Hughes JC, et al. End-of-life care: a qualitative study comparing the views of people with dementia and family carers. Palliat Med. 2017;32(3):631–42.
Livingston G, Leavey G, Manela M, Livingston D, Rait G, Sampson E, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ. 2010;341:c4184.
Caron CD, Griffith J, Arcand M. End-of-life decision making in dementia: the perspective of family caregivers. Dementia. 2005;4(1):113–36.
Paddock K, Brown Wilson C, Walshe C, Todd C. Care Home Life and Identity: a qualitative case study. Gerontologist. 2018;59(4):655–64.
Article PubMed Central Google Scholar
Shiells K, Pivodic L, Holmerová I, Van den Block L. Self-reported needs and experiences of people with dementia living in nursing homes: a scoping review. Aging Ment Health. 2020;24(10):1553–68.
Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753–60.
Braun V, Clarke V. Thematic analysis: a practical guide. Sage; 2021.
Download references
Acknowledgements
We thank all participants for their time and willingness to share their experiences. We would also like to thank Tanisha DeSouza for conducting the interviews.
This work was supported by Alzhiemer’s Society [grant number: AS-JF-16b-012].
DN is funded by the National Institute of Health and Social care research (NIHR) as and In-Practice Fellow. The funding bodies played no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and affiliations.
Research Department of Primary Care and Population Health, Centre for Ageing and Population Studies, UCL, London, UK
Danielle Nimmons, Greta Rait, Steve Iliffe & Nathan Davies
Social Care Workforce Research Unit, King’s College London, London, UK
Jill Manthorpe
Division of Psychiatry, Marie Curie Palliative Care Research Department, Centre for Dementia Palliative Care Research, UCL, London, UK
Emily West, Elizabeth L Sampson & Nathan Davies
Barnet, Enfield and Haringey Mental Health Liaison Service, North Middlesex University Hospital NHS Trust, London, UK
Elizabeth L Sampson
You can also search for this author in PubMed Google Scholar
Contributions
DN made substantial contributions to the analysis of data, interpretation of the data; and drafted the work. JM made substantial contributions to conception, design and interpretation of data; and substantively revised the paper. EW made substantial contributions to the analysis and interpretation of data; and substantively revised it. GR made substantial contributions to conception, design and interpretation of data; and substantively revised the paper. ELS made substantial contributions to conception, design and interpretation of data; and substantively revised the paper. SI made substantial contributions to the interpretation of data; and substantively revised the paper. ND made substantial contributions to conception, design, acquisition, analysis and interpretation of data; and substantively revised the paper. All authors reviewed the manuscript.
Corresponding author
Correspondence to Danielle Nimmons .
Ethics declarations
The authors are solely responsible for the content and not Alzheimer’s Society or NIHR.
Ethical approval
and consent to participate: Research was performed in accordance with the Declaration of Helsinki and Health Research Authority (HRA), ethical approval was received (London Queen Square Research Ethics Committee (18/LO/0408) on 10.04.2018. Written informed consent was provided by all participants, who also provided consent for publication and all their details are anonymised.
Consent for publication
All participants gave informed consent for publication.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nimmons, D., Manthorpe, J., West, E. et al. Views of people living with dementia and their carers on their present and future: a qualitative study. BMC Palliat Care 22 , 38 (2023). https://doi.org/10.1186/s12904-023-01165-w
Download citation
Received : 28 October 2022
Accepted : 03 April 2023
Published : 10 April 2023
DOI : https://doi.org/10.1186/s12904-023-01165-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Social death theory
BMC Palliative Care
ISSN: 1472-684X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Last updated 27/06/24: Online ordering is currently unavailable due to technical issues. We apologise for any delays responding to customers while we resolve this. For further updates please visit our website: https://www.cambridge.org/news-and-insights/technical-incident
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert

- > Journals
- > Ageing & Society
- > Volume 38 Issue 10
- > Dementia in the workplace case study research: understanding...

Article contents
Introduction, dementia in the workplace case study research: understanding the experiences of individuals, colleagues and managers.
Published online by Cambridge University Press: 29 June 2017
This article reports case study research which addresses the gap in knowledge about dementia in the workplace. Receiving a diagnosis of dementia whilst still in employment may have negative consequences for a person's identity, further compounded by loss of employment. This study is the first to explore the employment-related experiences of people with dementia and their employers to determine the potential for continued employment post-diagnosis. Sixteen case studies centred on a person with dementia who was still in employment or had left in the previous 18 months. Each involved interviews with the person with dementia, a family member and a workplace representative. This triangulation of the data promoted rigour, allowing the experiences to be viewed through a variety of lenses to build a clear picture of each situation. Thematic analysis was carried out and three themes were developed: (a) dementia as experienced in the workplace; (b) work keeps me well; and (c) wider impact of dementia in the workplace. These findings have the potential to initiate changes to policy and practice related to supporting employees with dementia. The implications of this research are multifaceted and need to be considered in terms of the individuals’ wellbeing, organisational support, as well as the wider theoretical, economic and societal consequences of supporting an employee with dementia.
Ageing and work
As the population of developed countries ages, many organisations will find themselves faced with the challenges associated with an ageing workforce. In 2014, 75.3 per cent of all 50–64 year olds in the United Kingdom (UK) were in some form of employment; this proportion has been steadily increasing since the early 1990s, indicating that more people are working into later life. A further 12.1 per cent of people past state pension age are engaged in the labour market (Office for National Statistics 2014 ). In response to the population changes, governments across Europe and beyond are increasing the age of retirement (Organisation for Economic Co-operation and Development 2014 ). As an example of policy change, the UK government has removed the default retirement age of 65 and increased the statutory pension age to 66, rising eventually to 69 or 70 in order to extend working lives and reduce economic burden.
Working until later life can have positive financial impacts on the economy, businesses and individuals (Brown, Orszag and Snower Reference Brown, Orszag and Snower 2008 ). Similarly, there may be further benefits to the individual. A recent review found that continued work in later life has a role to play in promoting good physical and mental health (Staudinger et al . Reference Staudinger, Finkelstein, Calvo and Sivaramakrishnan 2016 ). Other research has shown this can result in improved wellbeing (Hershey and Henkins Reference Hershey and Henkens 2014 ) and, in some occupations, improved cognitive functioning (Fisher et al. Reference Fisher, Stachowski, Infurna, Faul, Grosch and Tetrick 2014 ). However, many researchers argue that organisations are not well equipped to support an ageing workforce and have uncovered negative attitudes towards older workers and poor practice for supporting older workers, including a lack of formal performance appraisal measures (Chartered Institute for Personnel and Development 2011 ; Danson and Gilmore Reference Danson and Gilmore 2012 ; VanDalen, Henkens and Wang Reference VanDalen, Henkens and Wang 2015 ). One concern is that employers have not considered the impact health problems, which have previously been associated with older age and retirement, may have on the workplace (McNamara Reference McNamara 2014 ). An example of this is dementia, which is most commonly considered a condition of later life, although it also affects younger people. This is often referred to as ‘early onset dementia’. The purpose of this paper is to report the findings of case study research which addresses the gap in knowledge about the work-related experiences of people diagnosed with dementia before their expected age of retirement.
Dementia in the workplace
Dementia is an umbrella term referring to a number of neurological disorders which cause a progressive decline in cognitive functioning. There are many different types of dementia, including Alzheimer's disease, vascular dementia and fronto-temporal dementia. Symptoms include memory impairment, reduced ability to learn new material, sensory impairments, speech and communication problems, and personality changes. Dementia most often occurs in people over the age of 65, however, it is estimated that around 40,000 people under the age of 65 in the UK currently have a diagnosis of dementia (Alzheimer's Society 2014 ). Younger people with dementia are more likely to be in employment when they receive their diagnosis as well as having other responsibilities such as mortgages, dependent children or ageing parents (Pipon-Young et al. Reference Pipon-Young, Lee, Jones and Guss 2012 ). This, coupled with the recent policy changes relating to state pension age and the push for early diagnosis of dementia by national dementia strategies (Department of Health 2013 ; Scottish Government 2013 ), means that the number of people who are still in employment when they are diagnosed with dementia will rise (Harris Reference Harris 2004 ).
A recent literature review reported a dearth of evidence concerning the experiences of people who develop dementia while in employment, and a widely held assumption that post-diagnosis employment was not possible (Ritchie et al. Reference Ritchie, Banks, Danson, Tolson and Borrowman 2015 ). It is widely recognised that younger people with dementia are more likely to be in employment at the time of their diagnosis ( e.g. Harris Reference Harris 2004 ). Bentham and La Fontaine ( Reference Bentham and La Fontaine 2005 ), focusing on services for younger people with dementia in the UK, noted that it is not unusual for people with early symptoms of dementia to be made redundant or to be dismissed for incompetence before they are diagnosed. They found that some younger people with dementia wished to remain in employment, and suggested that efforts should be made to persuade employers to support people to retain appropriate employment and/or to recognise dementia as a reason for early retirement so that pension rights and other benefits are not affected.
Previous research has highlighted that getting a diagnosis of dementia can be a lengthy process, especially for a younger person (Harris Reference Harris 2004 ; Roach et al. Reference Roach, Keady, Bee and Hope 2008 ), and it is common in this period for a person with dementia to either leave work or go on sick leave before they have a diagnosis due to the stress associated with trying to cope in the workplace without knowing what is wrong (Chaplin and Davidson Reference Chaplin and Davidson 2016 ; Ohman, Nygard and Borell Reference Ohman, Nygard and Borell 2001 ). The interaction of undiagnosed dementia with an ever-stricter social security regime threatens increasing numbers with sanctions, loss of welfare payments and poverty. Furthermore, after receiving a diagnosis, the majority of people with dementia do not see returning to work as an option (Ohman, Nygard and Borell Reference Ohman, Nygard and Borell 2001 ), stretching loss of income and financial disruption into the years ahead.
A number of authors highlight the need for occupational health professionals to be aware of the problems and to be proactive in helping people to gain a diagnosis and secure support to retain employment where possible (Chaston Reference Chaston 2010 ; Martin Reference Martin 2009 ; Mason Reference Mason 2008 ). Similarly, Alzheimer's charities have recognised this and have begun to produce guidelines and advice for employers to support employees who develop dementia ( e.g. Alzheimer's Society 2015 a ). However, there is a dearth of research focusing on the experiences of people with dementia in employment and the nature of help that may be required to support continued employment. It is thought that simple changes such as using memory aids, providing written information and quiet work areas could be useful (Ohman, Nygard and Borell Reference Ohman, Nygard and Borell 2001 ); nevertheless, this would be dependent on the type of job the individual does.
Continuing employment post-diagnosis of dementia could have many advantages for the individual and the wider organisation. Notwithstanding the financial benefits of continued employment, there may also be many social and psychological benefits for the individual. Much has been written about the problems associated with an unplanned early labour market exit in later life, including increased incidences of depression (Christ et al. Reference Christ, Lee, Fleming, LeBlanc, Arheart, Chung-Bridges, Caban and McCollister 2007 ), lower wellbeing (Bender Reference Bender 2012 ), a loss of identity (Gabriel, Gray and Goregaokar Reference Gabriel, Gray and Goregaokar 2010 ) and loss of social networks (Lancee and Radl Reference Lancee and Radl 2012 ). Continuing employment post-diagnosis could alleviate some of these potential issues at a difficult time in the person's life, allowing them time to adjust to their diagnosis and make plans for leaving employment at a later date. Similarly for employers, continuing employment prevents the immediate loss of skilled labour (Pejrova Reference Pejrova 2014 ), helps to promote a good working environment within the company as co-workers perceive their colleagues being treated fairly (Hashim and Wok Reference Hashim and Wok 2014 ) and may also promote the corporation as being socially responsible (Dibben et al. Reference Dibben, James, Cunningham and Smythe 2002 ). Theory and practice recognises that employers adopt strategies to retain core workers during recession, structural market changes and individual challenges (Doeringer and Piore Reference Doeringer and Piore 1985 ; Hakim Reference Hakim 1990 ), so it might be expected that efforts would be made to accommodate workers who develop symptoms of dementia where the enterprise has invested in the development of their human capital and where they still display value to the operations of the business.
Policy landscape
Given the changes in policy relating to retirement age, it is timely to explore the employment-related experiences of people who develop long-term chronic conditions such as dementia. The World Health Organisation ( 2012 ) has declared dementia an international public health priority and many governments across Europe have developed their own dementia strategies or plans ( e.g. Alzheimer Europe 2016 ; Department of Health 2013 ; Scottish Government 2013 ). The focus of these strategies is to implement policies which enable people with dementia to live well with the condition. Improving diagnosis rates, with a focus on earlier diagnosis, has been a starting point for many countries, as has improving the care of people with dementia post-diagnosis. Although employment for people with dementia is not explicitly addressed within these strategies, if a person with dementia is still in employment, accessing an early diagnosis and appropriate post-diagnostic support may allow them to continue employment. Much of contemporary policy in health and social care advocates a focus on the person with dementia. Accordingly, person-centred practice has become the service mantra and a feature of national dementia strategies and action plans (Department of Health 2013 ; Scottish Government 2013 ). Descriptions of person-centred approaches (McCormack Reference McCormack 2004 ) highlight the importance of knowing the person in terms of who they are, their relationships with others and the contexts through which their personhood is articulated.
Employment and, even more significantly, welfare policies emphasise the centrality of ‘work’ to the lives and contributions of members of society (House of Commons Work and Pensions Committee 2015 ) so that, while discrimination and employment laws have an important role to play in framing the environment for how workers developing symptoms of dementia are treated by employers and co-workers, there may well be overwhelming concerns over the financial, social security and status implications of diagnosis. The complexity of the social security system, especially for those without a diagnosis in a flexible labour market, undoubtedly emphasises the importance of retaining a job where possible.
Theoretical perspectives
A lack of public understanding and the associated stigma of dementia are a challenge faced by society in implementing the policies relating to living well with dementia. Experiencing stigma can have an impact on the psychological wellbeing of the person with dementia (Swaffer Reference Swaffer 2014 ) as well as the support and services they access (Milne Reference Milne 2010 ). A person's sense of self is closely tied to their occupation, and their perception of who they are is closely tied to what they do (Ross and Buehler Reference Ross, Buehler, Brewer and Hewstone 2004 ). Thus, when a person loses their job unexpectedly, they can suffer a loss of identity or sense of self. Furthermore, preserving identity in early stage dementia is thought to be linked closely to ‘being valued for what you do’ and ‘being valued for what you are’ (Steeman et al. Reference Steeman, Tournoy, Grypdonck, Godderis and De Casterlé 2013 ), which highlights the value that continued employment may have to a person with dementia. For an individual who is still in employment, how they manage stigma will be important for their social and emotional wellbeing post-diagnosis. Stigma may result in a person being reluctant to disclose their diagnosis to their employer and they may worry about facing discrimination within the workplace. However, continuing employment post-diagnosis may be an opportunity to challenge this stereotype and associated stigma, by highlighting the skills and abilities the person has retained, as well as providing continued social support. Goffman's ( Reference Goffman 1963 ) theory on stigma states that an individual can develop a ‘spoiled identity’ as a result of being stigmatised. Link and Phelan ( Reference Link and Phelan 2001 ) further defined stigma as the co-occurrence of four components: labelling, stereotyping, separation and discrimination. The presence of stigma or perceived stigma in the workplace may influence an individual's decision to continue working. However, if stigmatisation could be addressed and myths dispelled, post-diagnostic employment could be beneficial for individuals and feel achievable for employers.
Population ageing and the rising numbers of people diagnosed with dementia, coupled with the policy imperative for longer working lives, provides the backdrop to this study. Furthermore, the implicit message in the literature of a possible ambivalence towards dementia as a disability and suggestions of inequality are morally troubling. These reasons, coupled with the knowledge that social isolation and a lack of stimulation amplify problems for individuals beyond those associated with the dementia trajectory alone (Shankar et al. Reference Shankar, Hamer, McMunn and Steptoe 2013 ), confirm the need for this research.
This study aimed to explore the employment-related experiences of people with dementia, and attitudes of employers and/or co-workers towards supporting people with dementia, in order to identify the potential for continued employment post-diagnosis.
Case studies
Case study research involves an empirical investigation of a particular contemporary phenomenon within a real-life context using multiple sources of evidence (Robson Reference Robson 1993 ; Yin Reference Yin 2013 ). In this study, the phenomenon of interest was the experience of developing dementia during employment. The study was designed following consultation with people with dementia and their families who highlighted employment as an important and multifaceted issue for them following diagnosis. It was agreed that case study research was appropriate given the complexities associated with obtaining a diagnosis of dementia and working. It mattered to individuals that the study captured the experiences from their own perspective, close family and also of people in their workplace. Taking account of these stakeholder perspectives also aligned with recommendations in the literature (Baxter and Jack Reference Baxter and Jack 2008 ) and contributed to the triangulation of data, promoting confidence in interpretation of findings.
Each of the 16 case studies was based around an individual who has a diagnosis of dementia and was in paid employment or had left within the previous 18 months. The person with dementia (the participant) then nominated additional participants to inform the case study, including a family member or friend and a workplace representative.
Recruitment
A project reference group was established to support and inform the development of the project, including health-care professionals, representatives from employer organisations and research network volunteers from the Alzheimer's Society. The reference group supported recruitment of the study by acting as gatekeepers to a range of organisations. Recruitment occurred through a number of organisations including the Alzheimer's Society, Scottish Clinical Dementia Research Network, Alzheimer Scotland and the National Health Service. Potential participants were given written information about the study and either contacted the research team directly to volunteer or gave permission for their details to be passed to the research team to initiate contact. A member of the research team then contacted the participant to arrange an initial meeting to discuss the project before they made a decision to participate. Participants were requested to sign written consent forms and reassured that they may withdraw from the study at any time without explanation or redress. Interview times and venues were negotiated and interviewees were asked to verbally consent to the commencement of the audio recording at the start of the actual interview and could request cessation of recording at any point during the conversation. At the end of the interview, the participant with dementia was invited to provide contact information for a workplace representative to assist in the building of the case study. All participants were provided with written information sheets explaining the purpose of the study, detailing interview procedures and strategies to maintain confidentiality and their right to withdraw at any time. Opportunity to seek clarification was provided prior to obtaining written consent. Figure 1 shows the inclusion criteria for the case study participants.
Figure 1. Inclusion criteria for case study participants.
Data collection
Data collection involved individual interviews with the person with dementia, their family members, a workplace representative and other relevant individuals, including health-care professionals. Because the situation in each case study is different, interviews were based around topics rather than an interview schedule. These topics were: (a) employment; (b) first problems; (c) impact on work; (d) health; (e) support at work; and (f) family and home life. Interviews with participants with dementia utilised an original visual tool ( see Figure 2 ), printed on a laminated card, influenced by Timeline and Lifegrid approaches (Adriansen Reference Adriansen 2012 ; Blane Reference Blane 2003 ).
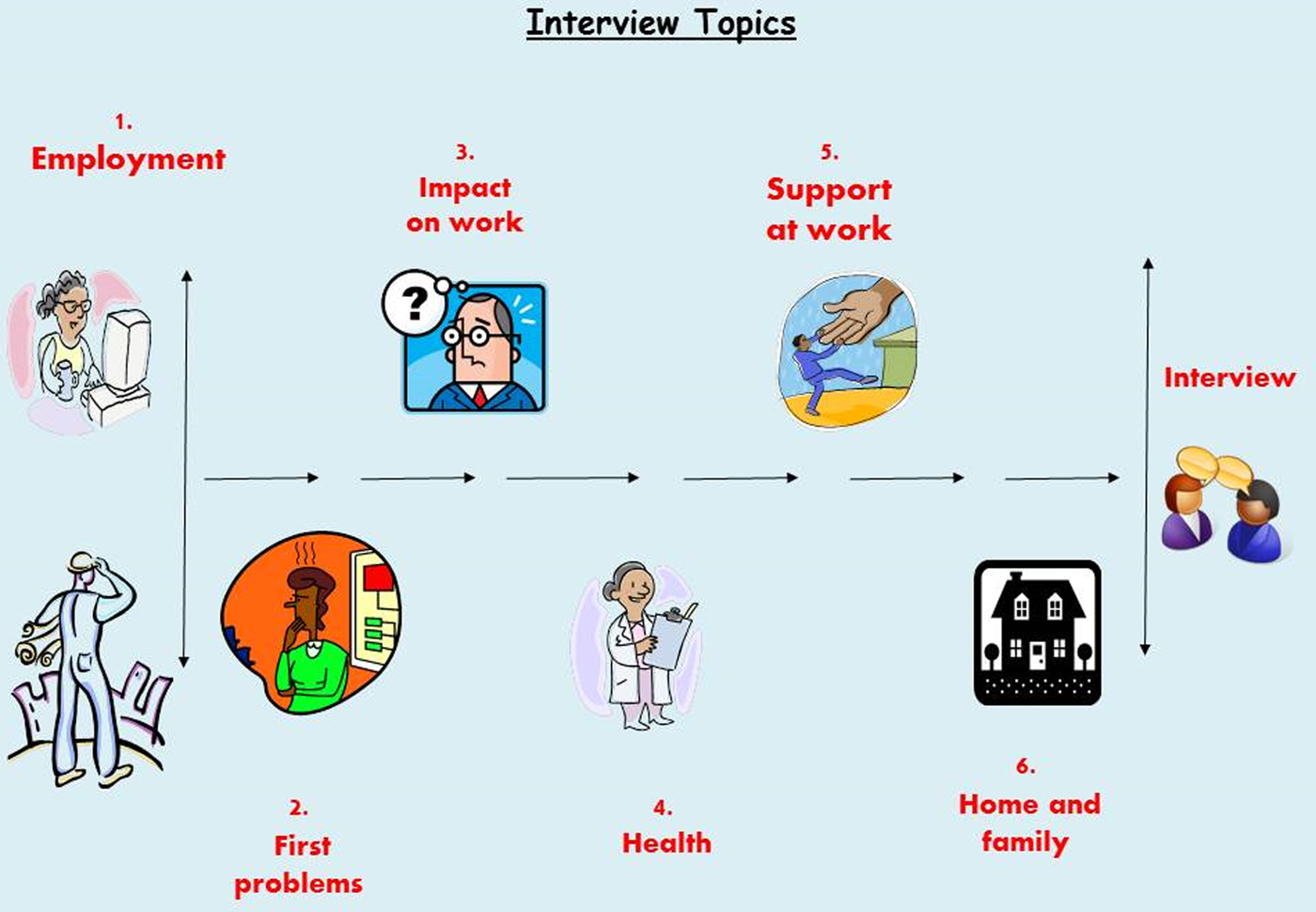
Figure 2. Interview topics.
This tool was piloted with volunteers with dementia who welcomed the visual prompts based on iconic symbols, as this helped them to assemble thoughts during the interview process and direct the flow of the conversation. The visualisation of the topic enabled participants to have an idea of what was coming next, also pausing to refer to the topic card was a dignified way for the person to influence the pace and focus of the interview. Flexibility was inherent in the flow of the interview topics and sometimes individuals returned to topics several times, and the interviewer could encourage moving on to another topic either by a verbal prompt or pointing to another image.
Data analysis
All interviews were audio recorded with participants’ consent, then transcribed and anonymised prior to analysis. Each case study was analysed individually and full descriptions of the individual case studies are published elsewhere (Tolson et al. Reference Tolson, Ritchie, Danson and Banks 2016 ). In order to explore the commonalities of experience of the participants, a thematic analysis was carried out across all the interviews included in the 16 case studies which is presented below. This followed Braun and Clarke's ( Reference Braun and Clarke 2006 ) six phases of thematic analysis. A coding schedule was developed after initial familiarisation of the data. This was then applied on a case-by-case basis, altering, revising and checking codes in a cyclical process. To promote rigour, two members of the research team were involved in this stage, in order to check agreement of the codes. Then, the research team met to discuss the codes and group them into emerging themes and consulted the project's Alzheimer's Society Research Network volunteers. These themes were then refined and developed until three overarching themes were clear in the data, each with a number of sub-themes. These themes are presented below.
Participants
Sixteen case studies were developed. Table 1 outlines the main characteristics for each participant and lists the relationship of the additional case study participants to the person with dementia. All participants were given a pseudonym to preserve anonymity.
Table 1. Overview of case study characteristics
All but one case study included a family member; however, there were issues with recruitment of workplace representatives in five case studies. Reasons for this varied, including the place of work having closed down so there were no contact details, and problems contacting and scheduling interviews with workplace representatives.
The analysis produced three themes in the data: Theme 1: Dementia as experienced in the workplace; Theme 2: Work keeps me well; and Theme 3: Wider impact of dementia in the workplace. Table 2 shows the development of these themes.
Table 2. Codes and theme development
Theme 1: Dementia as experienced in the workplace
Each case study presented a personal experience of dementia in the workplace. However, it was clear that employment was important to the participants and there were many shared experiences. This theme explores the experiences and implications of developing dementia whilst in employment, highlighting the supports which enabled continued employment but also the experiences of those who did not continue employment post-diagnosis.
Recognising dementia
The case studies highlighted the difficulties associated with the diagnosis of dementia, with six of the participants having long delays to their diagnosis. Emerging symptoms were often explained by situational factors or misdiagnosed as other disorders or mental health problems. During this period, many of the participants were continuing to work with awareness that ‘something was not right’ but with no understanding of what that might be.
So I've been stumbling around trying to make sense of it whilst, you know, professionals were going ‘oh it could be this, could be that’. (John, CS05, lecturer/journalist)
Throughout this period, employers were often aware of the problem their employee was having, although there was little consideration about the risks involved and little support offered to them. This potentially puts the person and other employees at unnecessary risk. Judgement of the participants’ abilities appeared to be based on their previous skills and abilities, with no understanding that there may be a serious underlying cause.
I phoned his immediate senior, who said yeah they had, they knew something was wrong but because he's got such knowledge of his craft … it was just like seen as an idiosyncrasy. (Phil's partner, CS16, off-shore safety inspector)
It was clear that in the pre-diagnosis period, little support was offered to the participants who were clearly struggling in their workplace. The longer the time period between the emergent symptoms and getting a diagnosis, the more likely it was that participants would have to go on sick leave before this was confirmed.
Symptoms experienced in the workplace
Participants reported a variety of symptoms which impacted on their employment, this included memory problems, problems with communication, visuo-spatial difficulties, and an impaired ability to learn and process information. The impact of the symptoms varied depending on the types of jobs and activities that the participants were employed to do. Some participants were able to cope with the symptoms they experienced in the workplace as they did not directly impact on their ability to carry out their job; for example, it may just take them longer to complete but their knowledge of the job was unimpaired.
That's why I'm comfortable at the moment carrying on working because I still know more than them [the team]. (Rose, CS08, team leader)
However, in many of the participants’ roles, their symptoms impacted directly on their ability to carry out their jobs; for example, the journalist found his communication skills were particularly affected which meant he was not able to perform well as a contributor on live radio programmes or the heavy goods vehicle (HGV) driver could not retain his licence post-diagnosis. This meant that, at an early stage, continued employment post-diagnosis was not possible for these participants in the jobs they were doing.
Leaving employment
The experience of leaving employment varied greatly between the participants; the majority who left did so after receiving their diagnosis, normally after being out of the workplace on sick leave for a period pre-diagnosis. However, two participants lost their jobs before their diagnosis because they were not performing well. In the cases where the participant was on sick leave when they received their diagnosis, returning to work was not given serious consideration by the employer, regardless of whether the participant wanted to return or not. Many of the organisations had a policy of offering redeployment or retirement in these situations. However, in the cases where this was available, participants felt that redeployment to a more suitable role was not seriously considered by their employers.
The first meeting we went to they said there were three options which were retirement, retirement due to ill health and redeployment but the redeployment, it was just mentioned and moved on. (Alison's husband, CS14, maternity care assistant)
Participants reported that they felt they had little control over the decisions for them to leave employment and many had long periods of uncertainty waiting for employers to make a decision about their future.
For all, leaving employment at this point was not something they had planned for and resulted in financial problems for many of the participants. However, for the two who lost their job before diagnosis, the immediate loss of income and difficulties in accessing benefits resulted in serious financial difficulties for their families and significant distress for the participant while going through the diagnostic process.
I've not had any money since September last year. (Mary, CS13, office manager)
Experiences varied greatly for all those participants who had left employment; however, it is clear that the support received could have been improved to assist the process of leaving employment and dealing with the associated paperwork for pensions and benefits. A number of the participants felt they had left employment on bad terms, either feeling pushed out or that they could have been better supported, which meant they experienced disappointment and resentment towards how their working life ended.
I'm desperately disappointed, humiliated and I've never been back to the place since, not to visit at all because I just felt I was squeezed out really. (Tom, CS10, projectionist)
Participants who had been on sick leave for a period before leaving employment felt that the communication from their employers was poor which contributed to their negative feelings about the process of leaving employment.
I was sat at home and no decisions made until much later on when they suddenly said ‘we're going to retire you’. (Edward, CS06, judge)
Leaving employment was not an easy process for any of the participants, and their experiences indicate that appropriate support for leaving employment was not available.
Supporting continued employment
The Equality Act 2010 (UK Government 2010 ) states that employers have a duty to make reasonable adjustments to the job description or work environment of an employee with a disability to allow them to retain or access employment. There was no direct mention of the Equality Act 2010 within the case studies; however, there were many examples of adjustments to participants’ roles with the aim of supporting their continued employment. Adjustments to the participants’ working patterns were common. Other common adjustments were the use of memory cues; for example, the use of calendars, diaries and mobile phones. Other case studies saw the participant having a change in their job description reflected in a reduction of the tasks they were required to do or the number of hours they worked. Successful adjustments which supported participants to continue working were often based on a good understanding of dementia, which for many came from attending a dementia-awareness session.
Something that we did notice that whenever he came to stairs he always seemed to hesitate and he was really concentrating and we weren't aware of that until the nurse came in and says no, he can't, he can't see straight lines … and the nurse gave us some hints and tips and how to do things (Paul's line manager, CS04, council officer)
This helped employers understand the specific difficulties their employee was facing and working together with the employee, and in some cases their families, they were able to develop well-placed and supportive adjustments. These adjustments focused on facilitating and retaining the skills the person with dementia had rather than focusing on what they could no longer do.
It was about looking at what she was capable of rather than looking at what she couldn't do. (Joan's line manager, CS12, head of business support)
A strong support network was important for all of the participants in the case studies, regardless of whether they continued or left work. Family members were an important source of support in all of the cases. They were important in the process of getting a diagnosis, often being the people who instigated the first trip to the general practitioner regarding changes they had noticed, and also communicating what adjustments might be required with the employers. Work colleagues were also an essential source of support. This included having close friends in the workplace, supportive colleagues and a good relationship with their line manager where they felt supported and understood.
I always made sure that if he needed help I would be there. I didn't want to be too over-compensating for him because he'd need to do it his self. (Paul's work colleague, CS04, council officer)
Support from family and work colleagues was integral to supporting the participants when they continued employment and to ensuring that the adjustments made worked well.
Theme 2: Work and wellbeing
This theme explores the impact of the employment situation on the physical, social and emotional wellbeing of the participants. The sub-themes discussed here are: managing symptoms better, staying connected and it was a relief to stop.
Managing symptoms better
Participants felt that employment provided challenges they would not have without working. This in turn fostered the belief that work was preserving their functioning or ability to manage their symptoms.
The more I do, the longer I'll keep some sort of good function there. (Joan, CS12, head of business support)
Other participants felt that the routine of working was beneficial for them, either in the routine of getting up and going to work each day or within the workplace following a set routine to carry out work tasks. Overall, the participants who continued working felt that, by doing so, it was helping them cope with their diagnosis and manage their symptoms. It is not clear from this information what is influencing the participants’ perception that work keeps them well, there may be many other factors involved which keeps them well enough to work, rather than work itself delivering the benefit. What this does show, nevertheless, is that the participants placed a value on being able to continue working.
Staying connected
For the seven participants who did continue working, although adapting to their diagnosis was difficult, it was viewed as a way of keeping connected with their social networks and allowing for some continuity in their lives at a time when many other things were changing.
What kept me going was, see the guys that I worked with, an absolutely fantastic group of guys, couldn't ask for better honestly and I think that's what really kept me going because even when I was off, the banter, I totally missed the banter. (Chris, CS09, engineer)
In contrast, participants who left work found it difficult to access age-appropriate services and, because so many of their social networks were linked to their workplace, leaving work had resulted in them losing contact with a lot of friends, especially for male participants. This had a negative effect on their social and psychological wellbeing, with many people feeling under-stimulated and isolated at home.
You feel quite remote you know because you've been taken out the work environment you know, meeting your mates every day and having a laugh and you know maybe have them for a beer at the weekends or something. I've lost track of my, they've moved away and I've lost track of my mates when I got diagnosed. (Michael, CS02, HGV driver)
This issue around feeling socially isolated particularly may link more widely to a loss of identity from leaving employment. For many participants, their identity was closely linked to their employment. The loss of work resulted in changes to the participants’ way of life and a period of adjustment.
I was babysitting for money when I was about 11; so I've been working since I was 11 but in paid work since I was 15. So it's a huge part of my life and you know that culture of work ethic and everything, so that took a bit of getting used not going to work. (Anna, CS07, nurse)
It was clear from the data that employment had a wider benefit to the participants in the study beyond financial remuneration. There were clear differences in outcome for the participants who continued employment and those who did not in terms of their social connections.
It was a relief to stop
It was clear that continued employment was not the best option for all of the participants. There was evidence that working was having a negative impact on participants’ wellbeing in three of the case studies, especially those who had a long delay to their diagnosis and little support in the workplace.
When she came in from her work she was stressed out every single day but when she stopped going to her work she was a lot better. (Myra's husband, CS15, office manager)
Participants reported increased stress as they attempted to continue performing in their job without support or understanding why they were struggling, and expressed relief at leaving work.
It was really the very understanding [line manager] and myself had a conversation about it and it was agreed that I would desist, that I would stop, though I have to confess it was a huge relief to me to be able to go ‘oh, enough is enough’. (John, CS01, lecturer)
In these situations, stopping work was the best option for the participants’ health and wellbeing. Performing poorly at work impacted on the participants’ confidence and they felt they were no longer able to continue. However, on reflection two of the three participants felt that with the correct support they could have continued working in some capacity.
Theme 3: Wider impact of dementia in the workplace
This theme examines the perspectives of the employer and the specific challenges that they faced when they were supporting a person with dementia in the workplace.
Doing the best we can
The employers who participated in the case studies expressed a desire to want to do the best they could to support their employees. This is in contrast to the proposition discussed previously that many participants felt they had left employment on bad terms, and that they could have been supported better. For most employers, they felt they had done the best they could by their employee and this involved showing compassion to their employees and treating them with respect, with pre-existing relationships at the centre of this.
Yeah, I'm your line manager, but then at the same time I'm more concerned about your own personal wellbeing and obviously in doing that I'll do my role as a manager, but also your friend. (John's line manager, CS01, lecturer)
It is important to note that an employer perspective was not included in all of the case studies. In these cases, employers either did not respond to invitations to participate or the participant themselves did not feel comfortable asking their employer to participate because of the circumstances surrounding them leaving employment. No employers had previous experience of supporting an employee with dementia so there was a feeling that they were learning as they went, revealing a lack of understanding of dementia, and importantly what the employee with dementia might be capable of. This inexperience was highlighted in a number of cases where, although the participant and/or family felt that the person could continue working in some capacity, there was a contrast with the employer's view that early retirement was the best option for the employee.
Do they want to offer her something else? Do they know the implications that are going to go with that? Is it easier for them to just to pension her off? (Myra's husband, CS15, office manager)
However, in the cases where the participants continued employment, the employers accessed support from existing disability management processes and dementia-awareness training which helped them discuss and implement appropriate adjustments. One example was the use of a health passport which is explained in the following quote:
[The health passport] is just an opportunity for him to be open and honest and tell me how work, how is his condition is affecting his work, so it can you know instigate a two-way conversation so if we need to make some adjustments then we can and at least I'm fully cognisant of his condition. (Jack's line manager, CS05, telephone engineer)
The majority of employers interviewed did not know where to access support external to their organisation, such as training or guidance on employment of a person with dementia.
I mean it would've been good if there had been some sort of expert there that you're able to pick up the phone and ask. (Jim's Human Resources representative, CS03, engineer)
These conflicting views between employers and workers of the optimal path for employees post-diagnosis may suggest a structural issue arising from power and other relations within the workplace but there was insufficient evidence or cases to reveal any systematic evidence to confirm or deny this speculation.
Protecting business needs
The employers also highlighted the challenges faced when trying to balance supporting their employee with dementia with running a successful business. At times maintaining the employee within the workforce had associated costs; for example, many participants acknowledged they were not performing at the same levels as they had before but, with the exception of one participant, their salary remained the same despite adjustments to job descriptions and working patterns. In many cases, the business absorbed these costs. It is of note that, in the majority of cases, employees who continued working were employed in a large organisation (over 250 employees). The exception to this was a small family business where the costs appeared to come secondary to the need to support their family member.
We didn't want to stop him doing it so we thought if we lose £20, £30, £100 what the hell does it matter you know, as long as he is keeping as, as, as much of his faculties going as he can but it, it's difficult you know ’cause your business head is saying you can't do this ’cause you're losing money here. (Ken's brother, CS11, shop owner)
When asked about preparing for the future or monitoring the person with dementia, very few of the line managers had thought about this. Again, this may reflect the lack of understanding of dementia and a support need for employers in the future.
I think I need to have that conversation with him just to say you know how, if, if there is a deterioration, whether it just be temporary or a gradual, how am I going to be best sort of informed. (Jack's line manager, CS05, telephone engineer)
This lack of forward planning may reflect a lack of understanding about the progressive nature of dementia, but may also be related to the fact that it is potentially a difficult situation for a line manager to confront so they were not comfortable talking about it.
Impact on colleagues
By extension, another issue that was highlighted within the case studies was the impact that having a colleague diagnosed with dementia had on co-workers. As well as experiencing increased workloads, there was an emotional impact on colleagues and line managers who worked with the participants. This included feelings of guilt over not spotting the signs of dementia in their colleague, worry about their own health as a result of learning about their colleague's diagnosis, and shock and sympathy for their colleague.
I would have liked to have thought that I was the one that recognised it you know. It's quite a complicated little thing when you, when you get into the, the different scenarios that could've transpired, did transpire etc . and how reflective you become and that after you look back you know. (John's line manager, CS01, lecturer)
There was also stress related to supporting a colleague with dementia, especially in the early stages pre-diagnosis when the problems were emerging and there was no understanding of what was causing the problem. At times this caused conflicts within the workplace, causing increased stress for the person with dementia, and those working with them.
It affected my mental health a bit because you do start to think ‘gosh, is it me’ and am I being unfair and am I doing, so it's horrendous for Myra and it's devastating for her family but it did have a big impact on everybody else. It has been really, really difficult. (Myra's line manager, CS15, office manager)
This shows the wide impact an employee being diagnosed with dementia can have in the workplace and highlights the need for support on some level for all the employees involved.
The findings revealed that continued employment post-diagnosis of dementia is possible; however, this was not a common experience across all 16 case studies. There are a number of factors which need to be considered in supporting people with dementia with employment and this can be complex to manage. It was clear from the data that there were differences in the outcomes of those who continued working and those who left after diagnosis in terms of their overall wellbeing and support they received. This highlights the need for appropriate support for those diagnosed with dementia both to continue employment and to facilitate a supported exit from employment if necessary.
Employment-related support for people with dementia
This study has shown that, when well-supported, many people with dementia can continue working, and by continuing in a job they have done for many years they are retaining their skills and continuing to contribute economically and socially to society.
While there was evidence of the specific supports that were helpful in assisting people with dementia to continue working, there was no clear consensus. What was deemed appropriate support was linked to the type of employment, the symptoms the person experienced and the resources the company had to provide support. This highlights the need to have a person-centred approach to supporting continued employment, in line with the recommendations for post-diagnostic support ( e.g. Scottish Government 2013 ).
Although the specific supports used varied, it appeared that employers who successfully facilitated their employee to continue working adopted a person-centred approach to assessing their needs and abilities, and drew on existing disability management policies in the absence of policies which specifically addressed dementia. Employers who take a proactive attitude towards health and disability in the workplace are commonly more successful in retaining their employees who experience health problems. In these organisations, timely workplace adjustments, guided by vocational rehabilitation advice, are most effective at supporting employees with health problems (Waddell, Burton and Kendall Reference Waddell, Burton and Kendall 2008 ). Such actions might be expected where there was a high degree of occupational and enterprise-specific skills pre-diagnosis, as the embodiment of these within the employee could well represent significant human capital investment and continuing value to the employer (Doeringer and Piore Reference Doeringer and Piore 1985 ; Pollert Reference Pollert 1988 ). This approach appeared to be successful in a number of the case studies where organisations supported their employee to continue working, with the focus very much on what they can still do, rather than what they cannot.
Workplace education and dementia-awareness sessions were found to be useful to those organisations which did promote continued employment by supporting colleagues to understand the abilities, challenges and how best to support their co-worker. While no previous research has evaluated the usefulness of workplace dementia-awareness sessions, there is growing evidence for the positive outcome associated with mental health education in the workplace (Wagner et al. Reference Wagner, Koehn, White, Harder, Schultz, Williams-Whitt, Warje, Dionne, Koehoorn and Pasca 2016 ).
Nevertheless, in some occupations continued employment in the same work was not possible for health and safety reasons; for example, the HGV driver could no longer drive and the nurse recognised it was not safe for her to continue practising. It was also clear from the findings that delays in the diagnosis process proved a barrier for continuing employment for many. The challenges of getting a timely diagnosis of dementia have been widely reported ( e.g. Hayo Reference Hayo 2015 ; Johannessen and Möller Reference Johannessen and Möller 2011 ; Mendez Reference Mendez 2006 ). However, for an individual who is still in work, this can be crucial to whether continued employment is possible. The UK Equality Act (UK Government 2010 ) prohibits discrimination against people with disabilities. Yet, without a diagnosis of dementia, individuals may not be able to benefit from the provision of the Act. The findings revealed just that: the longer it took to get a diagnosis, the more likely it was that the individual had left work or gone on sick leave due to the increased stress and pressure of trying to cope with the symptoms experienced without the appropriate support. Similar to the findings of the Ohman, Nygard and Borell ( Reference Ohman, Nygard and Borell 2001 ) study, after receiving a diagnosis, it was more likely that this triggered the process of early retirement rather than returning to work with support.
The majority of the participants who left, including the two people on sick leave, felt they would have been able to continue working in some capacity but did not have the opportunity. Previous research has shown that younger people with dementia often face challenges in how other people view their abilities and they can be thought of as unable to continue with their normal activities (Clemerson, Walsh and Isaac Reference Clemerson, Walsh and Isaac 2014 ). In the present study, when employers were not fully aware of the capabilities of the person with dementia and the supports available to them, then facilitating continued employment was not considered.
Although the employers’ perspective was to balance a desire to do the best for their employees with the needs of the business, it was clear that a lack of knowledge and experience with dementia for the employers meant that the opportunity for continued employment was not always considered fully. Large organisations with the internal resources and developed disability management policies appeared to adapt to supporting an employee with dementia by adopting a general disability management approach, focused on the abilities the person retained. There is little research evidence on how small and medium-sized enterprises support employees with health problems and disabilities, highlighting the need for more support and external services which can be accessed by these organisations (Waddell, Burton and Kendall Reference Waddell, Burton and Kendall 2008 ).
Leaving employment had a wider impact on the psychological, social and emotional wellbeing of the participants. Those participants who felt they had no control over leaving work, either because their illness meant that it was not possible to continue and no support was offered or because they felt pushed out by their employers, were more likely to have financial problems and have fewer activities and social arrangements organised for their retirement. Previous research has indicated that having control over the decision to retire is important for wellbeing in retirement. If retirement is perceived to be forced, either due to reaching statutory retirement age or through ill health, there is a negative impact on wellbeing (Hershey and Henkins Reference Hershey and Henkens 2014 ). Although the aim of this research was to investigate the potential for continued employment, it has highlighted that continued employment is not possible or appropriate for every individual with dementia and appropriate support for leaving work and adjusting to retirement is just as important as supporting continued employment post-diagnosis.
Theoretical implications
There were clear benefits to the participants and their families in this study of continuing employment. Much of this related to the idea that employment provided a challenge, a way of staying connected and of feelings of doing something useful and worthwhile. For many of the participants, continuing employment helped them to address the stereotype of dementia being associated with older age, therefore reducing the stigma they experienced. However, other participants did experience stigma which left them feeling isolated and this had a negative impact on their wellbeing. It is clear from the data presented here that how the participants responded to stigma was important for their continued wellbeing. Goffman ( Reference Goffman 1963 ), in his book Stigma: Notes on the Management of Spoiled Identity , states that withdrawing from society is a common way of dealing with stigma, but can lead to isolation, anxiety and depression. This was evident in some cases where losing employment compounded their spoiled identity, becoming further stigmatized by leaving the labour market. The participants’ response in these situations was to withdraw from their previous social lives and activities, which had a further negative impact on overall wellbeing.
Other responses to stigma listed by Goffman include using it as a learning experience, highlighting other skills and abilities to compensate, or joining self-help groups and clubs to foster a sense of belonging (Goffman Reference Goffman 1963 ). The case study examples supported this also, where participants stayed engaged within their community either through continued employment or volunteering. In these instances, there were clear benefits to the participants, both in terms of their feelings about their diagnosis, and for their wellbeing. For many, although their employers felt they were doing the best they could for the individual after their diagnosis, stigma of dementia may explain why the option of redeployment was not considered as an option for continuing employment. This may stem from a lack of understanding of dementia within the general population, which needs to be addressed to create an enabling environment for continued employment.
Where this research may be important is by challenging the public perceptions of dementia. People with dementia are commonly labelled and stereotyped negatively which does not accurately portray the experience of dementia (Swaffer Reference Swaffer 2014 ). However, by continuing employment, the participants in this study have demonstrated that they are still able to make a contribution to work and to society, and by doing so they are addressing the stereotypes of dementia and showing that people with dementia can continue to live well post-diagnosis.
Policy implications
This analysis links to current policy promoting the ‘living well with dementia’ agenda ( e.g. Scottish Government 2013 ). The findings highlight some of the issues surrounding employment for people with dementia which have not previously been considered in policy documentation. Future policy revisions should be cognisant of this to ensure that practitioners are aware of the implications of maintaining employment for a person with dementia and that the appropriate person-centred support plan is developed should the individual with dementia wish to continue working. This will become all the more important in coming years as policies relating to increased retirement age are implemented with the potential of more people developing dementia while still in the workforce. Moreover, the World Alzheimer Report 2016 makes a strong call to improve health care for people with dementia (Alzheimer's Disease International 2016 ). Reflecting on the results presented here, it is argued that for people with early onset dementia especially, this should include improved occupational health provision.
Similarly, it is not just dementia-focused policy which needs to address the issues raised in this article; government initiatives, such as the recent UK Government publication Fuller Working Lives (Department of Work and Pensions 2014 ), should address the wider implications of supporting an employee with age-related disorders in the workplace and ensure employers are equipped to support and identify employees who develop dementia whilst still in work. Strategies such as ‘dementia friends’ and ‘dementia-friendly communities’ (Alzheimer's Society 2015 b , 2016 ) go some way to increasing awareness of dementia within the community and businesses; however, similar education and awareness-raising initiatives targeting employers may be useful.
Strengths and limitations
Previous studies which report on the employment of people with dementia have not focused on the support required to continue working, instead prioritising first symptoms and problems experienced and the process of leaving work (Chaplin and Davidson Reference Chaplin and Davidson 2016 ; Ohman, Nygard and Borell Reference Ohman, Nygard and Borell 2001 ). A key strength of this research is the case study design which gathered data from differing perspectives on the same situation. This approach allowed for fuller understanding of each situation, acknowledging that a person being diagnosed with dementia will also have an impact on the people close to them. However, it is limited by a workplace representative not being included in every case study. There were a variety of reasons for this, including the person with dementia having no contact with their previous workplace, and there were challenges in some cases trying to engage with workplace participants with busy work schedules. The researchers are aware that the experiences uncovered within this study are unique to the participants in the case studies and make no claims of generalisation. However, the strength of this research has been to establish that issues surrounding employment for people with dementia are an important and timely area of research. The depth and quality of information gathered in each case study is testament to the willingness of participants to share their experiences. Further investigation of the issues needs to be carried out, including developing a deeper understanding of the factors which support continued employment, as well as how employment and exit from employment may be managed as the disease progresses.
Ethical issues
Given the sensitivity of the topic of discussion, a number of ethical considerations needed to be addressed from the outset of the study. It was unclear how willing people would be to discuss their experiences, and especially to facilitate access to a workplace representative. If the person with dementia was still in employment, allowing a researcher access to the workplace would mean that, firstly, they would have to disclose their diagnosis but, secondly, it could potentially highlight problems in their performance or the researcher could become privy to information about their employment that they were not aware of, such as their manager disclosing that their employment would be terminated. In order to safeguard against the potentiality of disclosing sensitive information between the case study participants, the procedure stated that workplace interviews were only conducted once all contact with the participant and their family was concluded. Additionally, safeguards were needed to manage the distress which could potentially be caused by discussing the sensitive topics of getting diagnosed, employment (or loss of), and the impact on family and finances, both for the researchers and the participants in the form of debriefing and signposting to services if required. Despite the complex ethical issues which had to be addressed, this study demonstrated that, in the correct and supported circumstances, it is possible to engage people with dementia and their families in topics which are of a sensitive nature.
This study is the first to reveal the profound impact that employment-related issues have on the lives of people with dementia. It is clear that continued employment post-diagnosis can be beneficial for people with dementia; however, this may be complex to support. Incorporating the views of people with dementia, their family members and workplace representatives within the case studies provided a rich description of each unique situation. The implications of this research are wide ranging, and these findings have the potential to influence future policy and practice relating to the employment of people with dementia post-diagnosis. Practitioners involved in diagnosis and post-diagnostic support, employers and employment support organisations need to be aware of the potential for continued employment in order to ensure that appropriate support is available to facilitate both continued employment and the process of leaving employment. Given the current policy environment, this research is timely in realising the potential impact an ageing workforce may have on society. From a theoretical perspective, supporting continued employment may be protective to an individual's identity and challenge the stigma associated with dementia. The increasing casualisation and flexibility of the labour market means that, for many, loss of a long-term position can mean enforced exclusion and isolation from employment and income. Retention with their existing employer can help to avoid some of the negative impacts of the onset of dementia, as this research has revealed; for others in the peripheral labour market or in self-employment, society needs to develop different means of support for those where working is still possible and promotes wellbeing. Further research is required to understand fully the problems relating to the employment of people with dementia. In particular, research is required to strengthen the evidence for workplace supports identified in this study and to understand how an employee can be supported over time in the workplace as their dementia progresses.
Acknowledgements
This project was funded by an Alzheimer's Society Research Grant (Number 180, PG-2012-199). The authors wish to thank the Alzheimer's Society for their funding and support for the project. The authors are grateful to the interviewees for their time and participation in the project and for their permission to publish extracts from the interviews. The authors report no conflicts of interest.
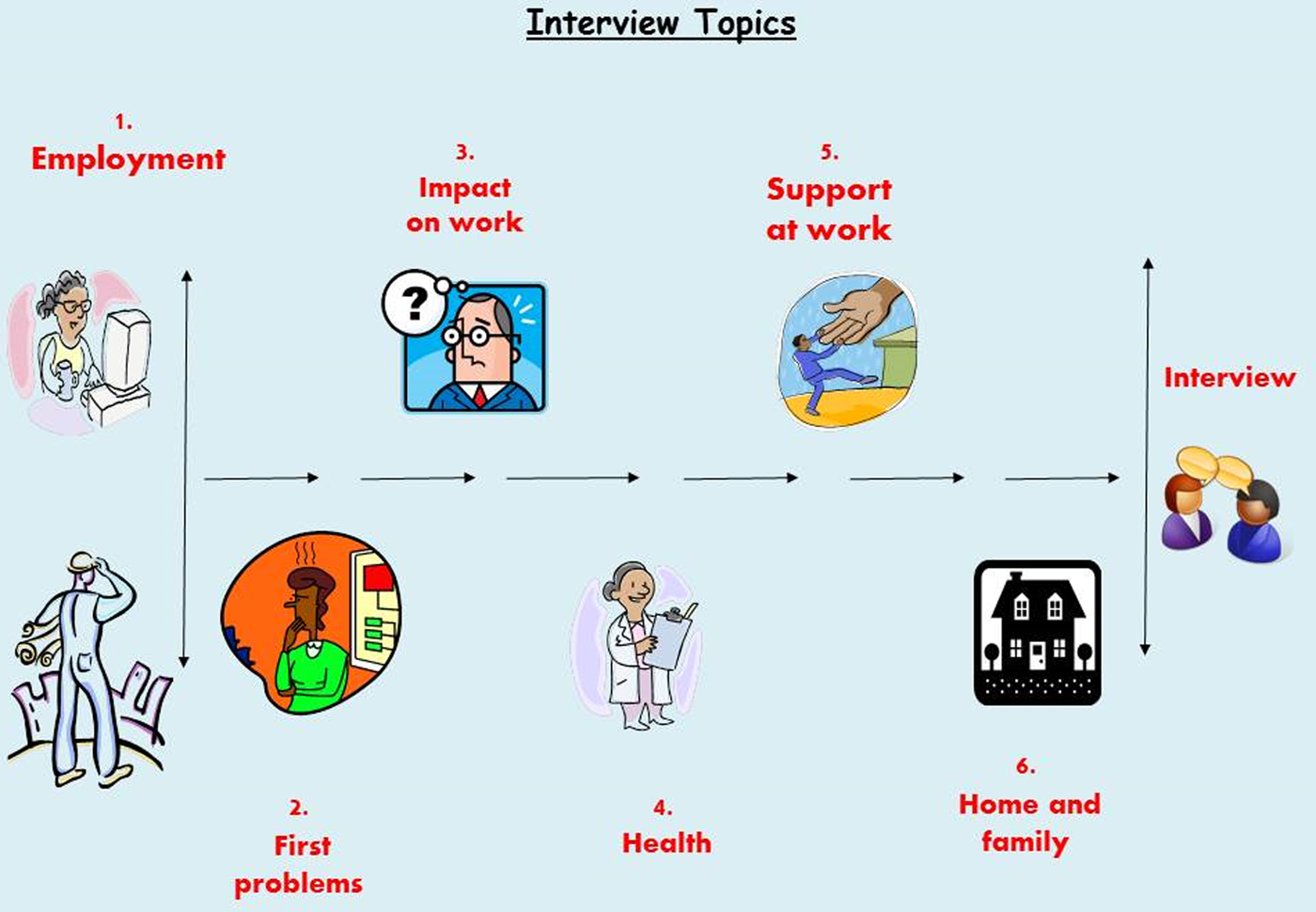
This article has been cited by the following publications. This list is generated based on data provided by Crossref .
- Google Scholar
View all Google Scholar citations for this article.
Save article to Kindle
To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
- Volume 38, Issue 10
- LOUISE RITCHIE (a1) , DEBBIE TOLSON (a1) and MIKE DANSON (a2)
- DOI: https://doi.org/10.1017/S0144686X17000563
Save article to Dropbox
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox .
Save article to Google Drive
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive .
Reply to: Submit a response
- No HTML tags allowed - Web page URLs will display as text only - Lines and paragraphs break automatically - Attachments, images or tables are not permitted
Your details
Your email address will be used in order to notify you when your comment has been reviewed by the moderator and in case the author(s) of the article or the moderator need to contact you directly.
You have entered the maximum number of contributors
Conflicting interests.
Please list any fees and grants from, employment by, consultancy for, shared ownership in or any close relationship with, at any time over the preceding 36 months, any organisation whose interests may be affected by the publication of the response. Please also list any non-financial associations or interests (personal, professional, political, institutional, religious or other) that a reasonable reader would want to know about in relation to the submitted work. This pertains to all the authors of the piece, their spouses or partners.

Case study 3: Joan
Download the Full Case Study for Joan PDF file (58KB)
Download the Vignette for Joan PDF file (49KB)
Name: Joan O’Leary
Gender: Female
Ethnicity: White Irish
Religion: Catholic
Disability: dementia with Lewy Bodies
First language: English
Family: estranged and in Ireland
Location: village in South West
Joan lives in a house in a small village. She has lived there for 28 years. She has two dogs and 11 chickens. Joan has always been quite private but is well known in her village. She goes to the nearby town on the bus to church, visits the local shop and community café, and goes away frequently. Joan goes for a long walk with her dogs each day. Joan hadn’t been to her GP for 6 years.
The café owner, Margaret, was worried about Joan. Joan has appeared very forgetful and disorientated. She has been seen in the village wearing her slippers and her neighbours have seen her out late at night with her dogs. Margaret went to Joan’s house and wasn’t allowed in but thought there was quite a strong smell.
Margaret phoned the GP in the village who went round to Joan’s house and persuaded her to have some tests. She has been diagnosed with moderate dementia with Lewy Bodies.
The GP phoned social services and you go out to do an assessment.
Download the Ecogram for Joan PDF file (48KB)
You told me that you didn’t really want to have an assessment. I explained that your GP had asked me to talk to you about what is happening for you at the moment because you have been diagnosed with dementia. You agreed to the assessment but you don’t want to have lots of information about you written down. These are the main things that you were happy for me to know.
At the moment you have capacity to make decisions about whether to have support or not. In the future you may find it more difficult to make decisions or not be able to make a particular decision. We talked about you arranging a lasting power of attorney and also doing an advanced care plan.
What’s important to you?
You said that you are quite a private person. You have two dogs and 11 chickens. You like to walk the dogs, go to the café and go to church in Lyme Regis. You take eggs to the café for Margaret (the owner) to sell.
You are a talented artist and have a small pottery shed and kiln in the garden.
You like to plan trips to different local areas and travel using the bus.
Your family is in Ireland and you haven’t seen them for many years. Your Catholic faith is important to you. You are a Eucharistic minister and you take holy communion to parishioners who can’t get to church.
What’s happening for you at the moment?
Your GP asked me to come and see you because you have been diagnosed with moderate dementia with Lewy Bodies.
You told me that you understand this is similar to Parkinson’s disease. You have noticed that your muscles have been aching and that you are not sleeping well.
The GP explained to me that you might find that you are unsteady at times or have tremors, and you might have some hallucinations, as these are common with this kind of dementia.
Margaret told me that you have been into the village wearing your slippers and that your neighbours have noticed you being out late at night which you agreed is unusual for you. However, you also told me that what you do is your own business. We talked about the risks that are attached to you going out late or not wearing appropriate clothing. You told me that you don’t think it is a problem, as you have always walked a lot and always find your way home. Also you said that you have the dogs with you when you are out and they know their way home. You already used reminders around the house so you said you would put a reminder on the front door to check your coat and shoes.
We talked about how you were managing day to day. You told me that you think you are managing well. However you haven’t been able to put the rubbish out or to clean out the chickens. You also have not been able to clean the bathroom. This is because you sometimes feel dizzy and your arms ache.
What is the impact on you?
You are concerned that people, like me, might start interfering with your life because of your illness.
What would you like to happen in the future?
You said that you want to continue to live in your home, looking after your animals, and doing the things that you currently do. We talked about what you think will happen and you said that you knew you will become less well. If you did need help in the future, you would like to arrange this yourself. We talked about having a personal assistant in the future.
How might we achieve this?
We talked about the importance of you having the right support in the future. You agreed that I could share this assessment with Margaret and your GP so that if you are not well enough to ask for advice or assistance in the future, they can ring the council. You will discuss a lasting power of attorney for finances, and health and care for Margaret. And you will do an advanced care plan with your GP. You will also talk to your GP about any medical support that might be available now, for example memory clinic and help with sleeping.
We also called your Parish Priest, Father Philip, and let him know that you have been diagnosed with dementia. He will ask a parishioner to give you a lift to church if you aren’t able to go on the bus.
You agreed to some help with keeping the animals and putting the rubbish out. Margaret and you will make an advert to put in the café window.
What strengths and support networks do you have to help you?
You said you are a very independent person. You have managed your home and finances for many years. You are managing well despite your illness and have strategies in place for things you might forget. You are active and have wide interests and talents. You also earn money through selling eggs. You contribute to the community through the parish. You are familiar with your local area and are used to travelling around.
You have a good relationship with Margaret, with your parish priest and with your GP. They are all willing and able to offer support.
Social care assessor conclusion
You have recently been diagnosed with dementia. This is starting to have an impact on your life and because of the nature of the illness the impact will increase as time goes on.
You have a lot of strengths to draw on and a good support network. You want to remain independent and, although there are some concerns about how you are managing, these are currently relatively minor and you have identified how to minimise them.
It is important that you plan ahead so that you remain in control of what happens and so that you can continue to achieve what matters to you for as long as possible.
Eligibility decision
You are not currently eligible for care and support under the Care Act 2014 because you can currently manage all day to day activities, apart from needing some help with maintaining your home. However, we have done a care and support plan that says what you will do to get help with the house and to plan for the future.
What’s happening next
See care and support plan .
Download the care and support plan document PDF file (178KB)

- Equal opportunities
- Complaints procedure
- Terms and conditions
- Privacy policy
- Cookie policy
- Accessibility

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 27 June 2024
Incident traumatic spinal cord injury and risk of Alzheimer’s disease and related dementia: longitudinal case and control cohort study
- Paul Lin 1 , 2 ,
- Neil Kamdar 2 ,
- Gianna M. Rodriguez ORCID: orcid.org/0000-0002-1655-9402 3 ,
- Christine Cigolle 2 ,
- Denise Tate ORCID: orcid.org/0000-0001-5210-3704 3 &
- Elham Mahmoudi ORCID: orcid.org/0000-0002-9746-8165 1 , 2
Spinal Cord ( 2024 ) Cite this article
7 Altmetric
Metrics details
- Alzheimer's disease
- Epidemiology
- Health policy
- Outcomes research
Study design
Retrospective case/control longitudinal cohort study
Prevalent traumatic spinal cord injury (TSCI) is associated with Alzheimer’s disease and related dementia (ADRD). We examined the hazard ratio for ADRD after incident TSCI and hypothesized that ADRD hazard is greater among adults with incident TSCI compared with their matched control of adults without TSCI.
Using 2010–2020 U.S. national private administrative claims data, we identified adults aged 45 years and older with probable (likely and highly likely) incident TSCI ( n = 657). Our controls included one-to-ten matched cohort of people without TSCI ( n = 6553).
We applied Cox survival models and adjusted them for age, sex, years of living with certain chronic conditions, exposure to six classes of prescribed medications, and neighborhood characteristics of place of residence. Hazard ratios were used to compare the results within a 4-year follow-up.
Our fully adjusted model without any interaction showed that incident TSCI increased the risk for ADRD (HR = 1.30; 95% CI, 1.01–1.67). People aged 45–64 with incident TSCI were at high risk for ADRD (HR = 5.14; 95% CI, 2.27–11.67) and no significant risk after age 65 (HR = 1.20; 95% CI, .92–1.55). Our sensitivity analyses confirmed a higher hazard ratio for ADRD after incident TSCI at 45–64 years of age compared with the matched controls.
Conclusions
TSCI is associated with a higher hazard of ADRD. This study informs the need to update clinical guidelines for cognitive screening after TSCI to address the heightened risk of cognitive decline and to shed light on the causality between TSCI and ADRD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Plasma extracellular vesicle tau and TDP-43 as diagnostic biomarkers in FTD and ALS

Acceptable performance of blood biomarker tests of amyloid pathology — recommendations from the Global CEO Initiative on Alzheimer’s Disease

APOE4 homozygosity represents a distinct genetic form of Alzheimer’s disease
Data availability.
The data that support the findings of this study are available from OptumInsight but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of OptumInsight.
Fann JR, Bombardier CH, Richards JS, Tate DG, Wilson CS, Temkin N, et al. Depression after spinal cord injury: comorbidities, mental health service use, and adequacy of treatment. Arch Phys Med Rehabilit. 2011;92:352–60.
Article Google Scholar
Krause JS, Saunders LL. Health, secondary conditions, and life expectancy after spinal cord injury. Arch Phys Med Rehabilit. 2011;92:1770–5.
Tate DG, Forchheimer M, Rodriguez G, Chiodo A, Cameron AP, Meade M, et al. Risk factors associated with neurogenic bowel complications and dysfunction in spinal cord injury. Arch Phys Med Rehabilit. 2016;97:1679–86.
Tate DG, Wheeler T, Lane GI, Forchheimer M, Anderson KD, Biering-Sorensen F, et al. Recommendations for evaluation of neurogenic bladder and bowel dysfunction after spinal cord injury and/or disease. J spinal Cord Med. 2020;43:141–64.
Article PubMed PubMed Central Google Scholar
Peterson MD, Berri M, Lin P, Kamdar N, Rodriguez G, Mahmoudi E, et al. Cardiovascular and metabolic morbidity following spinal cord injury. Spine J. 2021;21:1520–7.
Peterson MD, Meade MA, Lin P, Kamdar N, Rodriguez G, Krause JS, et al. Psychological morbidity following spinal cord injury and among those without spinal cord injury: the impact of chronic centralized and neuropathic pain. Spinal Cord. 2022;60:163–9.
Rodriguez G, Berri M, Lin P, Kamdar N, Mahmoudi E, Peterson MD. Musculoskeletal morbidity following spinal cord injury: a longitudinal cohort study of privately-insured beneficiaries. Bone. 2021;142:115700.
Article PubMed Google Scholar
Sargent L, Smitherman J, Sorenson M, Brown R, Starkweather A. Cognitive and physical impairment in spinal cord injury: a scoping review and call for new understanding. J Spinal Cord Med. 2023;46:343–6.
Alcántar-Garibay OV, Incontri-Abraham D, Ibarra A. Spinal cord injury-induced cognitive impairment: a narrative review. Neural Regen Res. 2022;17:2649.
Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J Spinal Cord Med. 2020;43:88–97.
Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27:669–82.
Allison D, Ditor D. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53:14–8.
Article CAS PubMed Google Scholar
Song HL, Shim S, Kim DH, Won SH, Joo S, Kim S, et al. β‐Amyloid is transmitted via neuronal connections along axonal membranes. Ann Neurol. 2014;75:88–97.
Stites SD, Harkins K, Rubright JD, Karlawish J. Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild Alzheimer’s disease dementia, and normal cognition. Alzheimer Dis Associated Disord. 2018;32:276.
Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol Aging. 2014;35:S74–S8.
Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: a perspective on potential mechanisms and mediators. J Neurochem. 2010;115:551–62.
Article CAS PubMed PubMed Central Google Scholar
Speck CE, Kukull WA, Brenner DE, Bowen JD, McCormick WC, Teri L, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6:366–9.
Jensen MP, Molton IR, Groah SL, Campbell ML, Charlifue S, Chiodo A, et al. Secondary health conditions in individuals aging with SCI: terminology, concepts and analytic approaches. Spinal Cord. 2012;50:373–8.
Hall OT, McGrath RP, Peterson MD, Chadd EH, DeVivo MJ, Heinemann AW, et al. The Burden of Traumatic Spinal Cord Injury in the United States: Disability-Adjusted Life Years. Arch Phys Med Rehabil. 2019;100:95–100.
Torrandell-Haro G, Branigan GL, Brinton RD, Rodgers KE. Association between specific type 2 diabetes therapies and risk of alzheimer’s disease and related dementias in propensity-score matched type 2 diabetic patients. Front Aging Neurosci. 2022;14:878304.
Cao S, Fisher DW, Yu T, Dong H. The link between chronic pain and Alzheimer’s disease. J Neuroinflamm. 2019;16:1–11.
Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL. Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther. 2017;9:1–9.
Cancelli I, Beltrame M, Gigli GL, Valente M. Drugs with anticholinergic properties: cognitive and neuropsychiatric side-effects in elderly patients. Neurological Sci. 2009;30:87–92.
Dublin S, Walker RL, Gray SL, Hubbard RA, Anderson ML, Yu O, et al. Prescription opioids and risk of dementia or cognitive decline: a prospective cohort study. J Am Geriatrics Soc. 2015;63:1519–26.
Wang Y-C, Tai P-A, Poly TN, Islam MM, Yang H-C, Wu C-C, et al. Increased risk of dementia in patients with antidepressants: a meta-analysis of observational studies. Behav Neurol. 2018;2018:5315098.
He Q, Chen X, Wu T, Li L, Fei X. Risk of dementia in long-term benzodiazepine users: evidence from a meta-analysis of observational studies. J Clin Neurol. 2019;15:9–19.
Mahmoudi E, Lin P, Peterson MD, Meade MA, Tate DG, Kamdar N. Traumatic spinal cord injury and risk of early and late onset Alzheimer’s disease and related dementia: large longitudinal study. Arch Phys Med Rehabilit. 2021;102:1147–54.
Yeh T-S, Ho Y-C, Hsu C-L, Pan S-L. Spinal cord injury and Alzheimer’s disease risk: a population-based, retrospective cohort study. Spinal Cord. 2018;56:151–7.
Huang S-W, Wang W-T, Chou L-C, Liou T-H, Lin H-W. Risk of dementia in patients with spinal cord injury: a nationwide population-based cohort study. J Neurotrauma. 2017;34:615–22.
Powell WR, Buckingham WR, Larson JL, Vilen L, Yu M, Salamat MS, et al. Association of neighborhood-level disadvantage with Alzheimer disease neuropathology. JAMA Netw open. 2020;3:e207559-e.
Clinformatics Data Mart - Optum. 2021. https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf .
User Guide Optum Clinformatics Data Mart Database. 2017. https://web.uri.edu/pharmacy/files/USER-GUIDE-V-02-27-2017.pdf .
Melendez R, Clarke P, Khan A, Gomez-Lopez I, Li M, Chenoweth M. National Neighborhood Data Archive (NaNDA): Socioeconomic status and demographic characteristics of zip code tabulation areas, United States, 2008-2017. 2021. Available from: https://www.openicpsr.org/openicpsr/project/120462/version/V1/view .
Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33:1131–8.
Turner RS, Stubbs T, Davies DA, Albensi BC. Potential new approaches for diagnosis of alzheimer’s disease and related dementias. Front Neurol. 2020;11:496.
Fillit H, Geldmacher DS, Welter RT, Maslow K, Fraser M. Optimizing coding and reimbursement to improve management of Alzheimer’s disease and related dementias. J Am Geriatrics Soc. 2002;50:1871–8.
Sachdeva R, Gao F, Chan CC, Krassioukov AV. Cognitive function after spinal cord injury: a systematic review. Neurology. 2018;91:611–21.
Hagen E, Rekand T, Gilhus N, Gronning M. Diagnostic coding accuracy for traumatic spinal cord injuries. Spinal Cord. 2009;47:367–71.
Chamberlain JD, Ronca E, Brinkhof MW. Estimating the incidence of traumatic spinal cord injuries in Switzerland: use of administrative data to identify potential coverage error in a cohort study. Swiss Med Wkly. 2017;147:w14430–w.
PubMed Google Scholar
Lin P-J, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimers Dement. 2010;6:334–41.
Download references
Acknowledgements
Authors acknowledge Liz Katz for her assistance in editing the manuscript.
This research was developed under a grant from the Craig Neilsen Foundation (Grant Application # 721097) and the Department of Defence (Grant Application# W81XWH2110751).
Author information
Authors and affiliations.
Department of Family Medicine, Michigan Medicine, University of Michigan, Ann Arbor, MI, USA
Paul Lin & Elham Mahmoudi
Institute for Healthcare Policy and Innovation, Michigan Medicine, University of Michigan, Ann Arbor, MI, USA
Paul Lin, Neil Kamdar, Christine Cigolle & Elham Mahmoudi
Department of Physical Medicine and Rehabilitation, Michigan Medicine, University of Michigan, Ann Arbor, MI, USA
Gianna M. Rodriguez & Denise Tate
You can also search for this author in PubMed Google Scholar

Contributions
PL conducted the data analysis and drafted and revised the data and methods sections. NK revised the manuscript. JR validated the cohort selection and revised the manuscript. CC assisted in cohort selection and revised the manuscript. DT revised the manuscript. EM designed the study, wrote the research design, and drafted and revised the manuscript.
Corresponding author
Correspondence to Elham Mahmoudi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
This research project used human data in agreement with the Declaration of Helsinki. Since we used secondary de-identified data, receiving informed consent was not applicable. The study was deemed exempt from our Institutional Review Board approval (IRB#HUM00192830) because administrative claims data are de-identified for research purposes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental file, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Lin, P., Kamdar, N., Rodriguez, G.M. et al. Incident traumatic spinal cord injury and risk of Alzheimer’s disease and related dementia: longitudinal case and control cohort study. Spinal Cord (2024). https://doi.org/10.1038/s41393-024-01009-1
Download citation
Received : 24 July 2023
Revised : 04 June 2024
Accepted : 11 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1038/s41393-024-01009-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Advanced Life Support
- Endocrinology
- Gastroenterology
- Infectious disease
- Intensive care
- Palliative Care
- Respiratory
- Rheumatology
- Haematology
- Endocrine surgery
- General surgery
- Neurosurgery
- Ophthalmology
- Plastic surgery
- Vascular surgery
- Abdo examination
- Cardio examination
- Neurolo examination
- Resp examination
- Rheum examination
- Vasc exacmination
- Other examinations
- Clinical Cases
- Communication skills
- Prescribing

Dementia case study with questions and answers

Dementia case study with questions and answers
Common dementia exam questions for medical finals, OSCEs and MRCP PACES
The case below illustrates the key features in the assessment of a patient with dementia or undiagnosed memory decline. It works through history, examination and investigations – click on the plus symbols to see the answers to each question
Part 1: Mavis
- Mavis is an 84-year old lady, referred to you in the memory clinic for assessment of memory impairment. She attends in the company of her son and daughter-in-law.
- On the pre-clinic questionnaire her son has reported a severe deterioration in all aspects of her cognition over the past 12 months.
- The patient herself acknowledges that there have been memory problems, but feels it is just her short term memory that is an issue.
Question 1.
- To begin the history, start broadly. Build rapport and establish both the patient’s view on memory impairment (if any) and the family’s (or other collateral history).
- Patient’s (and collateral) view of memory decline
- Biographical history
- Objective view of memory decline (e.g. knowledge of current affairs)
- Impact of memory decline on day-to-day living and hobbies
- Social history, including safety and driving
- General medical history (especially medications)
- See below for details on these…
Question 2.
- Is it for everything or are specific details missed out/glossed over?
- Try to pin down specific details (e.g. names of people/places).
- At what time in chronological order do things start to get hazy?
Question 3.
- If under 12 years this will lead to additional point being awarded on some cognitive tests
- Ask about long term memories, e.g. wedding day or different jobs
- Then move on to more recent memories, e.g. last holiday
Question 4.
- If your patient watches the news/read newspapers on a regular basis, ask them to recount the headlines from the past few days.
- Be sure to look for specifics to prevent your patient masking memory deficiencies with broad statements. For example: “The government are incompetent, aren’t they?!” should be clarified by pinning down exactly why they are incompetent, for example: “Jeremy Hunt”.
- If they like to read, can they recall plotlines from current books or items from magazines?
- If they watch TV, can they recount recent plot lines from soaps, or formats of quiz shows?
Question 5.
- Ask about hobbies and other daily activities, and whether or not these have declined recently.
- If your patient no longer participates in a particular hobby, find out why: is it as a result of a physical impairment (e.g. arthritis making cooking difficult), or as the result of a loss of interest/ability to complete tasks (e.g. no longer able to complete crosswords/puzzles).
- Once you have a good idea of the memory decline itself, begin to ask about other features. Including a social and general medical history.
Question 6.
- Review their social history and current set-up, and also subjective assessments from both patient and family over whether or not the current arrangements are safe and sustainable as they are.
- Previous and ongoing alcohol intake
- Smoking history
- Still driving (and if so, how safe that is considered to be from collateral history)
- Who else is at home
- Any package of care
- Upstairs/downstairs living
- Meal arrangements (and whether weight is being sustained).
- Of all these issues, that of driving is perhaps one of the most important, as any ultimate diagnosis of dementia must be informed (by law) to both the DVLA and also the patient’s insurers. If you feel they are still safe to drive despite the diagnosis, you may be asked to provide a report to the DVLA to support this viewpoint.
Now perform a more generalised history, to include past medical history and – more importantly – a drug history.
Question 7.
- Oxybutynin, commonly used in primary care for overactive bladder (anticholinergic side effects)
- Also see how the medications are given (e.g. Dossett box)
- Are lots of full packets found around the house?
Part 2: The History
On taking a history you have found:
- Mavis was able to give a moderately detailed biographical history, but struggled with details extending as far back as the location of her wedding, and also her main jobs throughout her life.
- After prompting from her family, she was able to supply more information, but it was not always entirely accurate.
- Her main hobby was knitting, and it was noted that she had been able to successfully knit a bobble hat for her great-grand child as recently as last month, although it had taken her considerably longer to complete than it might have done a few years previously, and it was a comparatively basic design compared to what she has been able to create previously.
- She has a few children living in the area, who would frequently pop in with shopping, but there had been times when they arrived to find that she was packed and in her coat, stating that she was “just getting ready to go home again”.
- She had been helping occasionally with the school run, but then a couple of weekends ago she had called up one of her sons – just before she was due to drive over for Sunday lunch – and said that she could not remember how to drive to his house.
- Ever since then, they had confiscated her keys to make sure she couldn’t drive. Although she liked to read the paper every day, she could not recall any recent major news events. Before proceeding to examine her, you note that the GP referral letter has stated that her dementia screen investigations have been completed.
Question 8.
- Raised WCC suggests infection as a cause of acute confusion
- Uraemia and other electrolyte disturbances can cause a persistent confusion.
- Again, to help rule out acute infection/inflammatory conditions
- Liver failure can cause hyperammonaemia, which can cause a persistent confusion.
- Hyper- or hypothyroidism can cause confusion.
- B12 deficiency is an easily missed and reversible cause of dementia.
- This looks for space occupying lesions/hydrocephalus which may cause confusion.
- This can also help to determine the degree of any vascular component of an ultimately diagnosed dementia.
Part 3: Examination
- With the exception of age-related involutional changes on the CT head (noted to have minimal white matter changes/small vessel disease), all the dementia screen bloods are reassuring.
- You next decide to perform a physical examination of Mavis.
Question 9.
- Important physical findings that are of particular relevance to dementia, are looking for other diseases that may have an effect on cognition.
- To look for evidence of stroke – unlikely in this case given the CT head
- Gait (shuffling) and limb movements (tremor, rigidity, bradykinesia)
- Affect is also important here and may also point to underlying depression
- Pay attention to vertical gaze palsy, as in the context of Parkinsonism this may represent a Parkinson plus condition (e.g. progressive supranuclear palsy).
- It is also useful to look at observations including blood pressure (may be overmedicated and at risk of falls from syncope) and postural blood pressure (again, may indicate overmedication but is also associated with Parkinson plus syndromes e.g. MSA)
Part 4: Cognitive Testing
- On examination she is alert and well, mobilising independently around the clinic waiting room area. A neurological examination was normal throughout, and there were no other major pathologies found on a general examination.
- You now proceed to cognitive testing:
Question 10.
- Click here for details on the MOCA
- Click here for details on the MMSE
- Click here for details on the CLOX test
Part 5: Diagnosis
- Mavis scores 14/30 on a MOCA, losing marks throughout multiple domains of cognition.
Question 11.
- Given the progressive nature of symptoms described by the family, the impairment over multiple domains on cognitive testing, and the impact on daily living that this is starting to have (e.g. packing and getting ready to leave her own home, mistakenly believing she is somewhere else), coupled with the results from her dementia screen, this is most likely an Alzheimer’s type dementia .
Question 12.
- You should proceed by establishing whether or not Mavis would like to be given a formal diagnosis, and if so, explain the above.
- You should review her lying and standing BP and ECG, and – if these give no contraindications – suggest a trial of treatment with an acetylcholinesterase inhibitor, such as donepezil.
- It is important to note the potential side effects – the most distressing of which are related to issues of incontinence.
- If available, put her in touch with support groups
- Given the history of forgetting routes before even getting into the care, advise the patient that she should stop driving and that they need to inform the DVLA of this (for now, we will skip over the depravation of liberty issues that the premature confiscation of keys performed by the family has caused…)
- The GP should be informed of the new diagnosis, and if there are concerns over safety, review by social services for potential support should be arranged.
- Follow-up is advisable over the next few months to see whether the trial of treatment has been beneficial, and whether side effects have been well-tolerated.
Now click here to learn more about dementia
Perfect revision for medical students, finals, osces and mrcp paces, …or click here to learn about the diagnosis and management of delirium.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Quantitative gait analysis in mild cognitive impairment, dementia, and cognitively intact individuals: a cross-sectional case-control study
Affiliations.
- 1 Faculty of Physical Therapy, Mahidol University, Nakhon Pathom, Thailand.
- 2 Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, TX, USA.
- 3 Allied Health Research Unit, University of Central Lancashire, Preston, UK.
- 4 Division of Neurology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. [email protected].
- PMID: 36151524
- PMCID: PMC9502583
- DOI: 10.1186/s12877-022-03405-9
Background: Cognitive age-related decline is linked to dementia development and gait has been proposed to measure the change in brain function. This study aimed to investigate if spatiotemporal gait variables could be used to differentiate between the three cognitive status groups.
Methods: Ninety-three older adults were screened and classified into three groups; mild cognitive impairment (MCI) (n = 32), dementia (n = 31), and a cognitively intact (n = 30). Spatiotemporal gait variables were assessed under single- and dual-tasks using an objective platform system. Effects of cognitive status and walking task were analyzed using a two-way ANCOVA. Sub-comparisons for between- and within-group were performed by one-way ANCOVA and Paired t-tests. Area Under the Curve (AUC) of Receiver Operating Characteristics (ROC) was used to discriminate between three groups on gait variables.
Results: There were significant effects (P < 0.05) of cognitive status during both single and dual-task walking in several variables between the MCI and dementia and between dementia and cognitively intact groups, while no difference was seen between the MCI and cognitively intact groups. A large differentiation effect between the groups was found for step length, stride length, and gait speed during both conditions of walking.
Conclusions: Spatiotemporal gait variables showed discriminative ability between dementia and cognitively intact groups in both single and dual-tasks. This suggests that gait could potentially be used as a clinical differentiation marker for individuals with cognitive problems.
Keywords: Classification; Dementia; Gait; Mild cognitive impairment.
© 2022. The Author(s).
PubMed Disclaimer
Conflict of interest statement
The authors declare they have no competing interests.
Similar articles
- Spatiotemporal gait parameter fluctuations in older adults affected by mild cognitive impairment: comparisons among three cognitive dual-task tests. Du S, Ma X, Wang J, Mi Y, Zhang J, Du C, Li X, Tan H, Liang C, Yang T, Shi W, Zhang G, Tian Y. Du S, et al. BMC Geriatr. 2023 Sep 27;23(1):603. doi: 10.1186/s12877-023-04281-7. BMC Geriatr. 2023. PMID: 37759185 Free PMC article.
- Measuring gait speed to better identify prodromal dementia. Grande G, Triolo F, Nuara A, Welmer AK, Fratiglioni L, Vetrano DL. Grande G, et al. Exp Gerontol. 2019 Sep;124:110625. doi: 10.1016/j.exger.2019.05.014. Epub 2019 Jun 4. Exp Gerontol. 2019. PMID: 31173841 Review.
- The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Bahureksa L, Najafi B, Saleh A, Sabbagh M, Coon D, Mohler MJ, Schwenk M. Bahureksa L, et al. Gerontology. 2017;63(1):67-83. doi: 10.1159/000445831. Epub 2016 May 13. Gerontology. 2017. PMID: 27172932 Free PMC article. Review.
- The motor signature of mild cognitive impairment: results from the gait and brain study. Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, Muir-Hunter SW. Montero-Odasso M, et al. J Gerontol A Biol Sci Med Sci. 2014 Nov;69(11):1415-21. doi: 10.1093/gerona/glu155. Epub 2014 Sep 2. J Gerontol A Biol Sci Med Sci. 2014. PMID: 25182601 Free PMC article.
- Spatial variability during gait initiation while dual tasking is increased in individuals with mild cognitive impairment. Boripuntakul S, Lord SR, Brodie MA, Smith ST, Methapatara P, Wongpakaran N, Sungkarat S. Boripuntakul S, et al. J Nutr Health Aging. 2014 Mar;18(3):307-12. doi: 10.1007/s12603-013-0390-3. J Nutr Health Aging. 2014. PMID: 24626760
- Machine Learning Model for Mild Cognitive Impairment Stage Based on Gait and MRI Images. Park I, Lee SK, Choi HC, Ahn ME, Ryu OH, Jang D, Lee U, Kim YJ. Park I, et al. Brain Sci. 2024 May 9;14(5):480. doi: 10.3390/brainsci14050480. Brain Sci. 2024. PMID: 38790458 Free PMC article.
- Impact of distinct cognitive domains on gait variability in individuals with mild cognitive impairment and dementia. Ofori E, Delgado F, James DL, Wilken J, Hancock LM, Doniger GM, Gudesblatt M. Ofori E, et al. Exp Brain Res. 2024 Jul;242(7):1573-1581. doi: 10.1007/s00221-024-06832-9. Epub 2024 May 16. Exp Brain Res. 2024. PMID: 38753043
- Curve Walking Reveals More Gait Impairments in Older Adults with Mild Cognitive Impairment than Straight Walking: A Kinect Camera-Based Study. Seifallahi M, Galvin JE, Ghoraani B. Seifallahi M, et al. J Alzheimers Dis Rep. 2024 Mar 15;8(1):423-435. doi: 10.3233/ADR-230149. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 38549633 Free PMC article.
- Biological and Physical Performance Markers for Early Detection of Cognitive Impairment in Older Adults. Kerminen H, Marzetti E, D'Angelo E. Kerminen H, et al. J Clin Med. 2024 Jan 30;13(3):806. doi: 10.3390/jcm13030806. J Clin Med. 2024. PMID: 38337499 Free PMC article. Review.
- Clinical improvements in temporospatial gait variables after a spinal tap test in individuals with idiopathic normal pressure hydrocephalus. Bovonsunthonchai S, Witthiwej T, Vachalathiti R, Hengsomboon P, Thong-On S, Sathornsumetee S, Ngamsombat C, Chawalparit O, Muangpaisan W, Richards J. Bovonsunthonchai S, et al. Sci Rep. 2024 Jan 24;14(1):2053. doi: 10.1038/s41598-024-52516-3. Sci Rep. 2024. PMID: 38267518 Free PMC article.
- Taylor ME, Close JCT. Dementia. Handb Clin Neurol. 2018;159:303–321. doi: 10.1016/B978-0-444-63916-5.00019-7. - DOI - PubMed
- Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. - DOI - PMC - PubMed
- Ru M, Brauer M, Lamarque JF, Shindell D: Exploration of the global burden of dementia attributable to PM2.5: What do we know based on current evidence? Geohealth 2021, 5(5):e2020GH000356. - PMC - PubMed
- Mayer F, Remoli G, Bacigalupo I, Palazzesi I, Piscopo P, Bellomo G, Canevelli M, Corbo M, Vanacore N, Lacorte E. A systematic review of studies reporting a decreasing trend in the incidence and prevalence of dementia. Minerva Med. 2021;112(4):430–440. doi: 10.23736/S0026-4806.21.07454-1. - DOI - PubMed
- Dharmasaroja PA, Assanasen J, Pongpakdee S, Jaisin K, Lolekha P, Phanasathit M, Cheewakriengkrai L, Chotipanich C, Witoonpanich P, Pitiyarn S, et al. Etiology of Dementia in Thai Patients. Dement Geriatr Cogn Dis Extra. 2021;11(1):64–70. doi: 10.1159/000515676. - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Systematic Review
- Open access
- Published: 19 June 2024
Predictive models of Alzheimer’s disease dementia risk in older adults with mild cognitive impairment: a systematic review and critical appraisal
- Xiaotong Wang 1 ,
- Shi Zhou 1 ,
- Niansi Ye 1 ,
- Yucan Li 1 ,
- Pengjun Zhou 1 ,
- Gao Chen 1 &
- Hui Hu 1 , 2 , 3
BMC Geriatrics volume 24 , Article number: 531 ( 2024 ) Cite this article
285 Accesses
7 Altmetric
Metrics details
Mild cognitive impairment has received widespread attention as a high-risk population for Alzheimer’s disease, and many studies have developed or validated predictive models to assess it. However, the performance of the model development remains unknown.
The objective of this review was to provide an overview of prediction models for the risk of Alzheimer’s disease dementia in older adults with mild cognitive impairment.
PubMed, EMBASE, Web of Science, and MEDLINE were systematically searched up to October 19, 2023. We included cohort studies in which risk prediction models for Alzheimer’s disease dementia in older adults with mild cognitive impairment were developed or validated. The Predictive Model Risk of Bias Assessment Tool (PROBAST) was employed to assess model bias and applicability. Random-effects models combined model AUCs and calculated (approximate) 95% prediction intervals for estimations. Heterogeneity across studies was evaluated using the I 2 statistic, and subgroup analyses were conducted to investigate sources of heterogeneity. Additionally, funnel plot analysis was utilized to identify publication bias.
The analysis included 16 studies involving 9290 participants. Frequency analysis of predictors showed that 14 appeared at least twice and more, with age, functional activities questionnaire, and Mini-mental State Examination scores of cognitive functioning being the most common predictors. From the studies, only two models were externally validated. Eleven studies ultimately used machine learning, and four used traditional modelling methods. However, we found that in many of the studies, there were problems with insufficient sample sizes, missing important methodological information, lack of model presentation, and all of the models were rated as having a high or unclear risk of bias. The average AUC of the 15 best-developed predictive models was 0.87 (95% CI: 0.83, 0.90).
Most published predictive modelling studies are deficient in rigour, resulting in a high risk of bias. Upcoming research should concentrate on enhancing methodological rigour and conducting external validation of models predicting Alzheimer’s disease dementia. We also emphasize the importance of following the scientific method and transparent reporting to improve the accuracy, generalizability and reproducibility of study results.
Registration
This systematic review was registered in PROSPERO (Registration ID: CRD42023468780).
Peer Review reports
Introduction
According to the WHO, more than 55 million people worldwide (8.1% of women and 5.4% of men over 65) are currently estimated to be living with dementia. This number is estimated to increase to 78 million by 2030 and 139 million by 2050 [ 1 ].So the World Health Organization approved “the Global Action Plan on the Public Health Response to Dementia 2017–2025” at the World Health Assembly in May 2017, which proposes strategies for the prevention and treatment of dementia and provides guidance on improving the quality of life of people living with dementia, their families and caregivers [ 2 ]. Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 60–80% of dementia. It has been reported that every patient with AD dementia experiences mild cognitive impairment (MCI), a stage considered to be the transition between normal ageing and dementia. Statistics show that the prevalence of MCI is about 8% in people aged 65 to 69 years, rising to 15% in people aged 70 to 79 years, 25% in people aged 80 to 84 years, and 37% in people aged 85 years and older [ 3 ], however, because the current diagnostic and treatment model of cognitive impairment in the elderly has not yet been perfected, coupled with the lack of awareness of the importance of treatment among patients and their families as well as uncertainty about the effectiveness of MCI treatment [ 4 ], Existing statistics may significantly underestimate the actual prevalence of MCI in older adults, and these factors also highlight the challenges faced in early screening and intervention services for AD dementia. It has been suggested that without intervention at the MCI stage, about 15% of people with MCI will develop AD dementia after two years [ 5 ]. However, effective intervention at this stage can delay cognitive decline [ 6 ]. In the face of the challenges of global ageing, screening and treatment of older adults with MCI should receive more attention, and dementia risk prediction is crucial for identifying at-risk populations.
Several studies have indicated that risk prediction models can assist healthcare professionals in identifying patients who are at high risk of cognitive decline. For instance, Wang et al. [ 7 ]. developed a predictive model to assess the risk of MCI in normal community-dwelling older adults. Another meta-study by Gopisankar et al. [ 8 ]. analyzed risk factors for MCI in Chinese older adults and developed a new hybrid model by updating and evaluating three existing models and applying a deep neural network analysis. This new model demonstrated higher predictive performance in assessing the incidence of dementia. An et al. [ 9 ]. conducted a meta-analysis and identified critical indicators of objectively measured cognitive impairment in individuals who have reported experiencing subjective cognitive deterioration. They also developed risk prediction models under two scenarios to identify individuals more likely to experience clinical progression.
However, the prediction models themselves have generated some controversial discussions, such as a meta-analysis by Huang et al. [ 10 ] to assess the predictive performance of multivariable prediction models for cognitive decline in older adults, which showed that the usefulness of the models was not very high. Li et al. [ 11 ] found that the prediction of individual disease risk varied significantly between different types of machine learning and statistical models with almost the same level of discrimination. However, the different views generated by the above studies are mainly caused by the significant differences in the existing models regarding data sources, sample sizes, and data processing and analysis methods. At present, there is yet to be a consensus on the most effective model. Considering that filling this research gap will be the key to predicting the progression of the disease in older adults with MCI and the implementation of timely and effective diagnosis and treatment, the present study will comprehensively analyze the published dementia-related risk prediction models.
This systematic review and critical evaluation was carried out to: 1) Provide a comprehensive summary of the best-performing multivariable predictive models in all current studies,2) Summarise models relevant data and methodological issues in model development and validation for 16 studies, and 3) Explore whether machine learning and traditional modelling approaches may affect model performance. The outcomes of this study aim to enhance the dependability and precision of AD dementia risk prediction models, thereby informing future efforts in predictive modeling and validation.
This study strictly followed the CHARMS checklist for systematically evaluating predictive modelling studies [ 12 ]. PROBAST [ 13 , 14 ] was used to assess the bias and applicability of the predictive modelling studies. We used various methods to obtain estimates and confidence intervals for each study’s optimal model performance measures. For data extraction, we prioritized the performance statistics that were derived using the most convincing validation methods (in increasing order of confidence: external validation, i.e., evaluation in independent populations, internal validation such as Bootstrap validation, cross-validation, random training-test splits and time splits, and the same data as in the development process). Performance statistics (in increasing order of confidence: external validation, i.e., evaluation in an independent population; internal validation, such as Bootstrap validation, cross-validation, randomized training-test splits and time splits, and evaluation with the same data as in the development process) [ 15 ], and finally we merged and summarized the data.
Literature search
Our comprehensive search across PubMed, EMBASE, Web of Science (WOS), and MEDLINE, spanning from each database’s start to October 19, 2023, focused on identifying models designed to estimate the likelihood of AD dementia in individuals 60 years and above who have MCI. Furthermore, we retrieve by using search filters that recognize predictive modelling studies [ 16 ]. The filters have been validated to have high sensitivity in retrieving clinical prediction model studies. A comprehensive inventory of the search terms utilized is available in Appendix File 1 .
Eligibility criteria
This analysis encompassed all primary research that either created or confirmed multivariable prediction models (with a minimum of two predictors). A thorough delineation of the study’s population, the primary model being evaluated, the model used for comparison, the outcomes, the time frame, and the context (PICOTS) is depicted in Table 1 .
The literature inclusion criteria were as follows:
Study population: older adults with MCI with a mean age of 60 years or older.
Study content: Studies on risk prediction models for the progression of MCI to AD dementia.
Study type: cross-sectional surveys, case-control studies, and cohort studies.
Outcome metrics: compliance with the diagnostic criteria for AD(includes the NINCDS-ADRDA [ 17 ]diagnostic criteria, any version“Diagnostic and Statistical Manual of Mental Disorders” [ 18 ], NIA [ 19 ], IWG [ 20 ], standardized neuropsychological tests and clinician’s diagnosis or a combination of these criteria.
Literature exclusion criteria were as follows:
Studies on specific populations, such as some specific diseases (e.g., organic diseases such as stroke, epilepsy, and Parkinson’s).
The number of predictors was less than 2.
Duplicate publications.
Unofficial publications such as conference abstracts, academic papers, and so on.
The language of the article needed to be English.
Screening process
Screening was performed using Endnote X9 software. Initially, two independent reviewers (XW, PZ) screened titles and abstracts for predictive modelling studies based on inclusion and exclusion criteria, with a third reviewer (NY) participating when necessary. After consensus was reached, the full-text literature was independently searched and screened by two reviewers (XW, PZ); in addition, we conducted a manual examination of the reference lists in the selected studies to identify additional studies that might be pertinent [ 21 ].
Data extraction
In this scientific investigation, the data extraction was carried out independently by two researchers, XW and PZ. Use CHARMS [ 12 ] to design standardized data extraction forms. The critical information extracted followed the principles of PICOTS, i.e., number of subjects included, data source, predictors (e.g., patient characteristics, imaging or biological markers), model status (e.g., performance, modelling status, and model presentation), and outcome metrics (e.g., measurement tools for AD dementia and duration of follow-up). In addition, information including author names, year of publication, type of study, and statistical information (e.g., treatment of missing data and selection of predictors, treatment of continuous variables) was also collected. Finally, we calculated the minimum sample size by pmsampsize package in R. We reviewed the supplementary material of the articles to ensure that all information about the articles could be extracted in its entirety. (see Appendix Table 6 ).
In order to analyze the predictive ability of each model, this study intends to analyze the following metrics: Confusion Matrix, Accuracy, Sensitivity, Specificity, Precision, F1 Score, AUC, DCA to evaluate the clinical applicability of the model were extracted.1) Discrimination, which refers to the model’s ability to discriminate between people with AD dementia and those without AD correctly, is often measured by the Consistency Statistics (C-index) and the AUC. The AUC is a measure of the accuracy of the model, the closer the AUC is to 1, the better the diagnostic effectiveness of the model [ 11 ]; 2) Calibration, the degree of accuracy of probability prediction is called “calibration degree”, is a way to measure the size of the difference between the probability predicted by the algorithm and the actual result, also called consistency, goodness-of-fit, mainly through the Hosmer-Lemeshow test and the goodness-of-fit curve evaluation [ 21 ]; 3)Clinical validity evaluation metrics, DCA, this approach serves to assess clinical predictive models, diagnostic examinations, and molecular markers. It aligns with the practical demands of clinical decision-making processes, often being more prevalent in external validations [ 22 ]. In addition to the above several conventional metrics, include the Confusion Matrix, Accuracy, Sensitivity, Specificity, F1-Score, and Brier Score [ 23 ]. (see Appendix Table 7 )
If inconsistencies are found during the data extraction process, NY will adjudicate these inconsistencies. Our study was guided by a systematic review and meta-analysis of preferred reporting items (TRIPOD-SRMA). For a complete list of search terms used.
Risk of bias assessment
This review applies the Risk of Bias Assessment Tool (PROBAST) [ 14 ] to assess the risk of bias (ROB) and the applicability of prediction models. PROBAST consists of four domains: participants, predictors, outcomes and analysis. Each question can be answered as “yes”, “probably yes”, “probably no”, “no”, or “no information”, As long as one of the domains is answered “no” or “probably not”, the domain is considered high risk; as long as the question in each domain is answered “yes” or “probably yes”, it will be defined as low risk. The overall ROB was deemed low when each domain consistently exhibited a low ROB. Conversely, the ROB was categorized as unclear, in cases where one or several domains exhibit an uncertain ROB, while the remaining domains are assessed as low in ROB. The applicability evaluation was similar to that of ROB, but only the first three domains were used to evaluate the applicability of the predictive model. The first two researchers (XW and PZ) assessed independently, and finally, the third reviewer, NY, made the judgment.
Statistical analysis
We used the R version 4.3.1 for the meta-analysis. Different from the conventional Meta-analysis, due to the significant heterogeneity of the predictive models, we directly used the random effects model [ 13 , 22 ], the AUC of the models and the calculated (approximate) 95% prediction intervals were combined and estimated, heterogeneity was quantified using the I 2 statistic, where p < 0.05 and I 2 > 50% signify statistically significant heterogeneity [ 23 ]. This statistic indicates the extent of variation across studies attributable to heterogeneity. To further analyze this variation, we divided the studies into two subgroups: those using machine learning modelling and those employing traditional modeling. Additionally, we utilized a funnel plot to illustrate the risk of bias.
Selection process
The PRISMA flowchart illustrates our process for searching and selecting literature. In our search, we gathered a total of 3,337 potentially relevant records, sourced from PubMed (2,920 records), EMBASE (1,461 records), Medline (1,910 records), Web of Science (2,979 records), and manual searches (12 records). We removed 3,563 records identified as duplicates, leaving 5,626 unique records for initial review based on titles and abstracts. Of these, 5,579 were subsequently excluded from title and abstract evaluation. Ultimately, we thoroughly reviewed 43 full-text articles, and 16 of these met our criteria for inclusion in this study. The literature screening process is shown in Fig. 1 .

Systematic reviews and Meta-Analyses (PRISMA) flowchart of literature searching and selection
Summary of findings
Study designs and population.
All prediction models ( n = 16) were development models. The majority ( n = 13) were retrospective cohort studies, and three [ 24 , 25 , 26 ] were prospective cohort studies. One study [ 24 ] was from a medical examination centre. One study [ 25 ] was from a memory clinic. One study [ 26 ] was recruited from a community-based primary health care centre, two [ 27 , 28 ] were multicenter retrospective cohort studies, and 11 studies [ 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ] were from the ADNI database in North America. In addition, there was one study [ 29 ] from the NACC (The National Alzheimer’s Coordinating Center) in the United States, two studies [ 24 , 26 ] from China, and two other studies from Korea [ 25 ] and Spain [ 30 ] respectively. In our prediction modelling, the populations studied varied in size, ranging from 102 to 2,611 individuals, and the prevalence of MCI progressing to AD dementia ranged from 15.03 to 52.22%. Detailed characteristics are shown in Appendix Table 6 .
We identified more than 400 candidate predictors and 94 final variables in our predictive model, divided into four main types: demographic characteristics, health-related risk factors, cognitive scores, and various biomarkers. The following 16 predictors were used at least twice as predictor variables in the model: MMSE, age, FAQ, ADAS, ApoE4, education level, hippocampal volume, CDR, AVLT, gender, p-tau, Aβ amyloid, cortical thickness, and ADL, age ( n = 10, 62%), MMSE ( n = 10, 62%), and FAQ ( n = 62, 14%) were the most common predictors. (See Fig. 2 .)

: An overview of the most commonly used predictors in AD dementia risk prediction models. *MMSE: Mini-Mental State Examination; FAQ: Functional Activities Questionnaire; ADAS, Alzheimer’s disease assessment scale-cognitive subscale; APOE4, Apolipoprotein E 4 allele; p-tau: Highly phosphorylated tau protein; CDR, Clinical dementia rating; ADL: Activity of daily living
Missing data and continuous variables
Three studies used an entire case study approach [ 24 , 27 , 31 ], one study used median imputation [ 32 ], one study used machine learning for imputation [ 39 ], one study [ 29 ] directly deleted missing data, and the remaining ten studies [ 25 , 26 , 30 , 31 , 34 , 35 , 36 , 37 , 38 ]did not indicate how the missing values were handled. Four studies [ 24 , 26 , 30 , 32 ] converted continuous variables to categorical variables, six studies [ 25 , 27 , 28 , 36 , 37 , 38 ] retained continuous variables, and the remaining five studies [ 29 , 31 , 33 , 34 , 35 , 39 ] did not indicate how the variable transformation was performed. Regarding the choice of variable screening methods, two models [ 35 , 39 ] chose LASSO to screen variables, two studies [ 30 , 38 ] chose RF for screening, and the other studies ( n = 10) chose COX regression, complete subset regression, and MRMR, in addition to one study [ 28 ] that did not specify the screening method.
Modelling method and follow-up duration
Most of the forecasting models ( n = 11) [ 24 , 25 , 26 , 28 , 30 , 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39 ] were developed using machine learning, two studies [ 27 , 31 ] modeled Cox proportional risk regression models, two studies [ 29 , 32 ]combined a mixture of modeling approaches for hybrid modeling, and one study [ 28 ] did not indicate the model type. The forecast period was 2–7 years, most studies [ 25 , 26 , 27 , 28 , 31 , 32 , 35 , 36 , 37 , 38 , 39 ] ( n = 9) predicted the AD dementia time for medium-term forecast (3–5 years), two models [ 24 , 34 ] focused on the short-term forecast (1–2 years), and 3 models [ 29 , 30 , 32 ] were for the long term forecast (5–10 years).
Model performance and validation
Two models have been externally validated [ 32 , 35 ], and the rest have only been internally validated. Among the internally validated models, one model [ 38 ]used random partitioning, one model [ 31 ] used bootstrapping, and eight models [ 24 , 25 , 26 , 27 , 30 , 34 , 35 , 36 , 37 ] used cross-validation, of which three models [ 28 , 32 , 37 ]used hierarchically nested cross-validation, and two models [ 29 , 33 ] used combinatorial methods. Regarding model performance, all models reported discrimination except for one model [ 39 ], which did not provide discrimination. Fifteen models had an AUC greater than 0.70, and one model [ 29 ] had an AUC ranging from 0.50 ? ∼ 0.69. In addition, eight models [ 25 , 27 , 28 , 30 , 31 , 34 , 36 , 37 , 38 ] reported calibration or predictive model fit goodness-of-fit curves, and all reported models were calibrated. The sensitivity of the models was 0.62 ? ∼ 1, and the specificity was 0.69 ? ∼ 0.98. Accuracy ranged from 0.50 to 0.99%. (See appendix Table 7 )
Model presentation
Five models [ 25 , 28 , 30 , 31 , 35 , 39 ] were presented as scoring systems, one model [ 34 ] was presented as an equation formula, one model [ 38 ] was presented as a graphical scoring method, one model [ 27 ] was presented as a web-based calculator and an app, one model [ 32 ] was presented as a column-line graph, and the other six models [ 24 , 26 , 29 , 33 , 36 , 37 ] were not reported.
Risk of bias and applicability
Fourteen models were rated high, and two [ 25 , 34 ] had an unclear ROB (see Appendix Tables 8 and 9 ). We found a model with a low risk of bias, but without external validation [ 29 ]. Therefore, we classified them as high ROB. Two models [ 30 , 32 ] were judged to have high ROB in the participant, mainly because the study population did not represent the model’s target population. Most models were judged to have unclear ROB in the outcome, mainly due to some interference between the outcome measures and perhaps the predictors. Four models [ 24 , 27 , 28 , 37 ] had high ROBs in the analysis domain related to insufficient sample size, and nine models [ 25 , 26 , 29 , 31 , 33 , 35 , 37 , 38 , 39 ] rated as unclear were mainly related to the unclear on the handling of variables.
Regarding applicability, five models [ 31 , 33 , 36 , 37 , 39 ] were rated as having an unclear concern of applicability in the domain of participants. Two models [ 29 , 30 ] were highly biased in the outcome domain as long as they were related to a mismatch between the outcome indicator and the systematically evaluated question. This implies that the settings or participant demographics in these predictive modeling studies might not align perfectly with the context of our research question. Overall, most of the model ( n = 14, 85%) had a high ROB, and about half of the models ( n = 7, 43%) had a concern of applicability that was unclear or high.
Meta-analysis of validation models
We performed a meta-analysis including 15 development models reporting AUC and their 95% confidence intervals (95% CI). The Li et al. [ 39 ] study was excluded due to missing AUC. Pooled analysis of the 15 studies showed that we combined all AUCs using a random-effects model, with an AUC of 0.86 (95% CI: 0.82, 0.90) and an I 2 of 95% ( p < 0.01) (Fig. 3 .), suggesting high heterogeneity.
In addition, we conducted subgroup analyses by grouping the traditional regression model, and ML showed that the effect size of the traditional regression model subgroup (0.89; 95% CI: 0.86,0.93) was more significant than that of the traditional regression model subgroup (0.77; 95% CI: 0.71,0.74) (Fig. 4 .), and the results suggest that different modelling may be a potential heterogeneity of study results Reason. Publication bias was evaluated using funnel plots (see Appendix Fig. 3 ). The distribution of the scatter plot appeared largely symmetric, indicating an absence of notable publication bias in the prediction models analyzed.

Forest plot of meta-analysis of pooled AUC estimates for 15 validation models. *95% CI, 95% confidence interval; ML: machine learning
Forest plot subgroup analysis of pooled AUC estimates for 15 validation models. *95% CI, 95% confidence interval; ML: machine learning
Principal findings
This research offers a comprehensive analysis of predictive models aimed at determining the risk of AD dementia in elderly with MCI, examining 16 different models. These models, formulated in both community and clinical environments, primarily target the elderly population, including those attending memory clinics. Notably, Five studies opted for Random Forest (RF) as the primary modeling tool during model construction. This choice was primarily due to RF’s ability to robustly handle high-dimensional data and demonstrate strong generalization capabilities [ 40 ]. Additionally, two studies employed the Cox Proportional Hazards Model (COX), exhibiting exceptional survival analysis performance [ 28 ]. Researchers also favored fusion models as they combine the strengths of multiple models, significantly enhancing prediction accuracy [ 29 , 32 ]. Support Vector Machine (SVM) was often used in some studies to classify data [ 33 ]. Cyclic Neural Networks (RNN) demonstrated unique advantages in capturing time dependencies, while Artificial Neural Networks (ANN) stood out for their powerful learning capabilities [ 41 ]. When dealing with large-scale datasets, eXtreme Gradient Boosting (XGBoost) exhibited efficiency, and the Variational Bayes (VB) method provided valuable uncertainty estimations [ 25 ]. Each modeling approach possesses unique advantages and different application scenarios.
Then, we conducted a subgroup analysis of ML versus traditional modelling approaches and found that ML algorithms were more effective than traditional regression models in outcome prediction. However, it has been argued [ 42 ] that the reliance on large amounts of data for machine-learning approaches may limit their effectiveness in small sample datasets. Reinke et al. [ 43 ] compared classical and ML approaches to develop dementia risk prediction models and found that ML did not outperform logistic regression, confirming the importance of sample size. This also shows that the data and features determine the upper bound of the predictive model, and the model and algorithm only help the research to keep approaching the upper limit.
Furthermore, by summarizing the most used predictors in prediction models for the stage from MCI to AD dementia, our findings are inconsistent with An et al. [ 9 ], mainly due to inconsistencies in the study population. Although the close association of cognitive biomarkers with the pathology of the disease makes them overall superior to epidemiology and neuropsychology, we observed that four of the top five predictors were non-cognitive biomarkers. Only ApoE4 was a cognitive biomarker, which may result from some scholars’ preference to use relatively valid demographic characteristics and neuropsychological scales due to cost considerations as predictor variables. Given this, we suggest that future studies develop more diverse predictive models based on different clinical settings, community environments, and individual circumstances.
Most of the studies were developing new models, and our assessment of them found that the predictive power of the models ranged from moderate to excellent. However, these models consistently have a high or unclear ROB. With three models [ 26 , 28 , 31 ] showing low ROB in all but the analysis, suggesting that the models performed well in terms of study design and data collection but had problems in terms of statistical analysis, commonly such as insufficient sample size, insufficient consideration of model overfitting issues, use of missing data and unclear treatment of continuous variables [ 44 ], it is likely to result in good performance on the training set but poor performance on the test set or in real-world applications. This means that while the model learns the features of the training data well, it cannot generalize to new data effectively, dramatically reducing the model’s usefulness and reliability [ 45 ]. Although predictive modelling holds some promise for improving AD dementia prevention and intervention, given the insufficient evidence, it is yet to be possible to recommend any predictive model for widespread use in practice.
Challenges and opportunities
This comprehensive analysis identified certain methodological shortcomings in the integrated development or validation of predictive models.
First, although many models have been internally validated and calibrated, only a few have been externally validated. It is worth noting that predictive models usually outperform external validation in model development data, but external validation is more convincing than internal validation [ 46 ]. Therefore, to ensure the generalizability of the models, we emphasize the importance of using different datasets as much as possible to validate the existing model’s performance. In validation studies, we need to verify that the model’s performance (discrimination and calibration, especially discrimination) on new data is close to the performance on the data on which it was developed [ 47 ], and the assessment of model usefulness requires a clinical judgment; furthermore, in machine learning the performance of a model may undergo the concept drift, over time, and thus continuous validation and updating of the predictive model is necessary to ensure applicability to new populations.
In addition, we found that about half of the models suffered from direct deletion of missing values and incomplete reporting. Failure to treat missing data appropriately usually leads to biased effect estimates because missing data can distort the performance of a predictive model if it is correlated with other variables [ 48 ]. Missing value imputation methods are categorized into deletion, simple imputation, multiple imputation, and algorithmic imputation, while multiple imputation [ 49 ] and Miss Forest [ 50 ] are currently the more recommended methods. About half of the studies transformed continuous variables into binary or multi-class classification. However, many researchers have hotly debated the treatment of continuous-type variables. From a statistical point of view, downgrading continuous variables to categorical variables, especially binary classification is highly likely to result in the loss of data information and reduced prediction performance. However, from the clinical point of view, it is easier to quickly determine the outcome of the patients [ 51 , 52 , 53 ]. Therefore, which method should be taken should be considered according to the purpose of the study, the method used, and the data.
In addition, we note that different methods were used to screen the model’s predictor variables. If the number of variables is too high, the model may overfit the training data, decreasing predictive performance on new data, and too few may lead to poor model performance [ 54 ]. Thus, it is essential to ensure that the model captures the key features of the data while keeping the model concise. There are three general categories of variable selection methods: filter, wrapper, and embedding [ 55 ]. The most appropriate method for selecting predictors still needs to be discovered. However, for data with many features (or data that exhibit multicollinearity), it is expected to use regularized regression (often also known as penalized models or shrinkage methods) to impose restrictions and thus reduce the occurrence of overfitting. In addition, the sample size is also closely related to the variables. In addition to the traditional 10-EPV estimation method, sample size calculation tools have been developed to estimate the sample size of clinical prediction models [ 56 ].
Furthermore, the practical application of risk models should take into account their cost-effectiveness. Typically, models that incorporate high-cost predictors tend to exhibit greater predictive accuracy compared to solely depending on the judgment of clinicians [ 57 , 58 ]. However, model feasibility and cost constraints can limit model use, particularly in primary care [ 59 ]. Model simplicity and measurement reliability are essential for developing clinically useful prognostic models. The current study shows that clinical judgment frequently demonstrates comparable or superior performance compared to predictive models, and some predictors may instead limit the use of these models due to their invasive nature, the high cost of the tests, and the non-routine nature of the tests [ 60 ]. Therefore, we suggest that future studies need to consult with clinical experts on the one hand for their opinions and insights, including the interpretability and applicability of these variables in actual clinical settings, in addition, continuously updating current models by mining predictors with more vital incremental value (exploring non-cognitive biomarkers with modifiable properties and more common biomarkers) to identify patients at high risk of AD dementia more effectively.
Finally, we found that the included studies needed more model presentation, incomplete regression equations and lack of clarity about the intended use of the model used. An inadequate presentation of research studies not only represents a significant squandering of research resources but also obstructs future activities such as validation, updating, recalibration, and providing direction for clinical practice. In terms of model presentation, in addition to providing complete model equations, there are many forms, such as scoring systems, column-line graphs, web calculators, and apps. In addition, Bonnett et al. [ 61 ] point out that even if models do not perform very well, they may still be of clinical utility. Therefore, indicating that the specific intended use of a predictive model (i.e., when or where they will be used in an investigation and for whom they will be used) may be equally relevant may be helpful.
Advantages and limitations
This study represents the first comprehensive and integrated assessment of AD dementia risk prediction models for elderly with MCI. Critical features were assembled through an extensive literature search, meticulous screening, and standardized data extraction, thus providing valuable research information for primary healthcare systems and clinical healthcare professionals. This approach lays the foundation for more effective construction and external validation of future predictive models. Furthermore, this study conducted a risk of bias (ROB) assessment and applicability assessment of the prediction model using the PROBAST tool, alongside subgroup and bias analyses, constituting another significant strength in our study.
However, this review is subject to certain limitations. First, despite identifying multiple models that predict conditions in similar populations, a comprehensive meta-analysis of the discrimination and calibration of these models was not feasible due to the lack of detailed calibration reports. Second, the “best model” selection might have overlooked some essential information due to incomplete data extraction. Additionally, despite using random-effects models, there was still a high heterogeneity among the included studies, mainly because there were also unadjustable differences between the patient environments used for study design, measurement methods, and patient characteristics. Heterogeneity is also usually accepted in Meta-analyses of predictive models, but future subgroup analyses of its variability in relevant settings and populations by meta are necessary.
Future research
To help make clinical decisions founded on the most robust available evidence and to identify the most effective models advocated or utilized for predicting the risk of AD dementia in older adults with MCI. However, we are unable to recommend any specific model due to several reasons. Firstly, nearly all predictive models reviewed exhibited unclear or high risk of bias (ROBs), and the included developmental models required further external validation. Additionally, the significant heterogeneity among the included models, the use of non-standardized statistical analysis methods, incomplete data in model reports, and a lack of analysis regarding the clinical application value all contribute to the challenge of selecting the optimal model. Finally, while the average AUC of our best-developed model has achieved 0.87, some even exceeding 0.98, these findings may not fully translate into actual medical practice [ 62 ]. This is because although AUC is a widely utilized metric for evaluating the performance of predictive models, it only partially represents their actual efficacy. Pursuing excessively high values under the receiver operating characteristic AUC maybe over-optimization and potential distortion of the models [ 63 ].
Based on these methodological shortcomings, we make the following recommendations. First, models should be externally validated several times in different populations, and sample sizes must be adequately considered. Second, when data are missing, interpolation should be performed using multiple interpolation or machine learning. Third, predictive variables with incremental solid value should be mined based on clinical feasibility and applicability, and preventing overfitting should be emphasized in the predictive model. Fourth, it is clear that medical predictive modelling aims to construct a predictive tool with practical application value. Therefore, when constructing a medical predictive model, it is necessary to start from the practical point of view and give full consideration to the application prospect of the model. At the same time, we also need to recognize the importance of diversified evaluation, as far as possible, sensitivity, specificity, calibration index, net benefit, and DCA for comprehensive evaluation.
Conclusions
We identified 16 predictive models, most of the researchers reported excellent discrimination in their study. However, for various reasons, the risk of bias in nearly all models was high or unclear. Consequently, this finding implies that the predictive performance of these models might be overestimated, their accuracy in practical application to the target population remains questionable, and currently, we cannot endorse any of these predictive models for clinical practice. Additionally, our exploration of potential predictors, translating evidence into new insights for clinical practice. Future studies on predictive modelling of AD dementia risk in older adults should adhere to methodological guidelines and prioritize practicality and cost-effectiveness in model evaluation, thereby facilitating disease progression identification in older adults with MCI.
Data availability
All data generated or analyzed during this study are included in the Appendix. The corresponding author can provide the code upon request.
Abbreviations
- Alzheimer’s disease
Area under curve
Alzheimer’s disease assessment scale-cognitive subscale
Apolipoprotein E 4 allele
Highly phosphorylated tau protein
Activity of daily living
Clinical dementia rating
Decision curve analysis
- Mild cognitive impairment
Mini-Mental State Examination
Functional Activities Questionnaire
Random forests
Machine learning
Least absolute shrinkage and selection operator
Max-Relevance and Min-Redundancy
Risk of bias
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46.
Article PubMed PubMed Central Google Scholar
Soria Lopez JA, González HM, Léger GC. Alzheimer’s disease. Handb Clin Neurol. 2019;167:231–55.
Article PubMed Google Scholar
2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598–1695.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–35.
Aigbogun MS, Stellhorn R, Hartry A, Baker RA, Fillit H. Treatment patterns and burden of behavioral disturbances in patients with dementia in the United States: a claims database analysis. BMC Neurol. 2019;19:33.
Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2022;59:1721–9.
Article Google Scholar
Wang B, Shen T, Mao L, Xie L, Fang QL, Wang XP. Establishment of a risk prediction model for mild cognitive impairment among Elderly Chinese. J Nutr Health Aging. 2020;24(3):255–61.
Article CAS PubMed Google Scholar
Geethadevi GM, Peel R, Bell JS, Cross AJ, Hancock S, Ilomaki J, et al. Validity of three risk prediction models for dementia or cognitive impairment in Australia. Age Ageing. 2022;51(12):afac307.
An R, Gao Y, Huang X, Yang Y, Yang C, Wan Q. Predictors of progression from subjective cognitive decline to objective cognitive impairment: a systematic review and meta-analysis of longitudinal studies. Int J Nurs Stud. 2023;149:104629.
Huang J, Zeng X, Hu M, Ning H, Wu S, Peng R, et al. Prediction model for cognitive frailty in older adults: a systematic review and critical appraisal. Front Aging Neurosci. 2023;15:1119194.
Li Y, Sperrin M, Ashcroft DM, van Staa TP. Consistency of variety of machine learning and statistical models in predicting clinical risks of individual patients: longitudinal cohort study using cardiovascular disease as exemplar. BMJ. 2020;371:m3919.
Snell KIE, Levis B, Damen JAA, Dhiman P, Debray TPA, Hooft L, et al. Transparent reporting of multivariable prediction models for individual prognosis or diagnosis: checklist for systematic reviews and meta-analyses (TRIPOD-SRMA). BMJ. 2023;381:e073538.
Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A Tool to assess risk of Bias and Applicability of Prediction Model studies: explanation and elaboration. Ann Intern Med. 2019;170(1):W1–33.
Debray TP, Damen JA, Snell KI, Ensor J, Hooft L, Reitsma JB, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ. 2017;356:i6460.
Debray TP, Damen JA, Riley RD, Snell K, Reitsma JB, Hooft L, et al. A framework for meta-analysis of prediction model studies with binary and time-to-event outcomes. Stat Methods Med Res. 2019;28(9):2768–86.
Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS. 2012;7:e32844.
Article CAS Google Scholar
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology.1984;34(7): 939–44.
Hilliard RB, Spitzer RL. Change in criterion for paraphilias in DSM-IV-TR. Am J Psychiatry. 2002;159(7):1249.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
Platzbecker U, Fenaux P, Adès L, Giagounidis A, Santini V, van de Loosdrecht AA, et al. Proposals for revised IWG 2018 hematological response criteria in patients with MDS included in clinical trials. Blood. 2019;133(10):1020–30.
Article CAS PubMed PubMed Central Google Scholar
Damen JAA, Moons KGM, van Smeden M, Hooft L. How to conduct a systematic review and meta-analysis of prognostic model studies. Clin Microbiol Infect. 2023;29(4):434–40.
Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users’ guides to the Medical Literature. JAMA. 2017;318(14):1377–84.
Xie Y, Yu Z. Models and prediction, how and what? Ann Transl Med. 2020;8(4):75.
Zhao X, Sui H, Yan C, Zhang M, Song H, Liu X, Yang J. Machine-based learning shifting to Prediction Model of Deteriorative MCI due to Alzheimer’s Disease - A two-year Follow-Up investigation. Curr Alzheimer Res. 2022;19(10):708–15.
Chun MY, Park CJ, Kim J, Jeong JH, Jang H, Kim K, et al. Prediction of conversion to dementia using interpretable machine learning in patients with amnestic mild cognitive impairment. Front Aging Neurosci. 2022;14:898940.
Kuang J, Zhang P, Cai T, Zou Z, Li L, Wang N et al. Prediction of transition from mild cognitive impairment to Alzheimer’s disease based on a logistic regression-artificial neural network-decision tree model. Geriatr Gerontol Int. 2021;43–7.
van Maurik IS, Vos SJ, Bos I, Bouwman FH, Teunissen CE, Scheltens P, et al. Alzheimer’s Disease Neuroimaging Initiative. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol. 2019;18(11):1034–44.
Chen J, Chen G, Shu H, Chen G, Ward BD, Wang Z, et al. Alzheimer’s Disease Neuroimaging Initiative. Predicting progression from mild cognitive impairment to Alzheimer’s disease on an individual subject basis by applying the CARE index across different independent cohorts. Aging. 2019;11(8):2185–201.
Bucholc M, Titarenko S, Ding X, Canavan C, Chen T. A hybrid machine learning approach for prediction of conversion from mild cognitive impairment to dementia. Expert Syst Appl. 2023;217:119541.
Mallo SC, Valladares-Rodriguez S, Facal D, Lojo-Seoane C, Fernández-Iglesias MJ, Pereiro AX. Neuropsychiatric symptoms as predictors of conversion from MCI to dementia: a machine learning approach. Int Psychogeriatr. 2020;32(3):381–92.
Lee SJ, Ritchie CS, Yaffe K, Stijacic Cenzer I, Barnes DE. A clinical index to predict progression from mild cognitive impairment to dementia due to Alzheimer’s disease. PLoS ONE. 2014;9(12):e113535.
Grassi M, Rouleaux N, Caldirola D, Loewenstein D, Schruers K, Perna G, et al. Alzheimer’s Disease Neuroimaging Initiative. A Novel ensemble-based machine learning algorithm to predict the Conversion from mild cognitive impairment to Alzheimer’s Disease using Socio-demographic characteristics, clinical information, and neuropsychological measures. Front Neurol. 2019;10:756.
Mubeen AM, Asaei A, Bachman AH, Sidtis JJ, Ardekani BA. Alzheimer’s Disease Neuroimaging Initiative. A six-month longitudinal evaluation significantly improves accuracy of predicting incipient Alzheimer’s disease in mild cognitive impairment. J Neuroradiol. 2017;44(6):381–7.
Lee G, Nho K, Kang B, Sohn KA, Kim D. For Alzheimer’s Disease Neuroimaging Initiative. Predicting Alzheimer’s disease progression using multi-modal deep learning approach. Sci Rep. 2019;9(1):1952.
Li HT, Yuan SX, Wu JS, Gu Y, Sun X. Predicting Conversion from MCI to AD combining Multi-modality Data and based on Molecular Subtype. Brain Sci. 2021;11(6):674.
Hojjati SH, Ebrahimzadeh A, Khazaee A, Babajani-Feremi A. Alzheimer’s Disease Neuroimaging Initiative. Predicting conversion from MCI to AD using resting-state fMRI, graph theoretical approach and SVM. J Neurosci Methods. 2017;282:69–80.
Korolev IO, Symonds LL, Bozoki AC. Alzheimer’s Disease Neuroimaging Initiative. Predicting Progression from mild cognitive impairment to Alzheimer’s dementia using clinical, MRI, and plasma biomarkers via Probabilistic Pattern classification. PLoS ONE. 2016;11(2):e0138866.
Velazquez M, Lee Y. Alzheimer’s Disease Neuroimaging Initiative. Random forest model for feature-based Alzheimer’s disease conversion prediction from early mild cognitive impairment subjects. PLoS ONE. 2021;16(4):e0244773.
Li H, Liu Y, Gong P, Zhang C, Ye J. Alzheimers Disease Neuroimaging Initiative. Hierarchical interactions model for predicting mild cognitive impairment (MCI) to Alzheimer’s Disease (AD) conversion. PLoS ONE. 2014;9(1):e82450.
Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284(6):603–19.
Deo Rc. Machine learning in Medicine. Circulation. 2015;132(20):1920–30.
Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. 2019;110:12–22.
Reinke C, Doblhammer G, Schmid M, Welchowski T. Dementia risk predictions from German claims data using methods of machine learning. Alzheimers Dement. 2023;19(2):477–86.
Grant SW, Collins GS, Nashef SAM. Statistical primer: developing and validating a risk prediction model. Eur J Cardiothorac Surg. 2018;54(2):203–8.
Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. Bmj.2020;369:m1328.
Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605.
Bellou V, Belbasis L, Konstantinidis AK, Tzoulaki I, Evangelou E. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ. 2019;367:l5358.
Van Calster B, Steyerberg EW, Wynants L, van Smeden M. There is no such thing as a validated prediction model. BMC Med. 20 23 ;21(1):70.
Li Q, Yao X. Échevin. How good is machine learning in Predicting all-cause 30-Day hospital readmission? Evidence from Administrative Data. Value Health. 2020;23(10):1307–15.
Zhou Z, Lin C, Ma J, Towne SD, Han Y, Fang Y. The association of social isolation with the risk of Stroke among Middle-aged and older adults in China. Am J Epidemiol. 2019;188(8):1456–65.
Stekhoven DJ, Bühlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8.
Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-depth mining of clinical data: the construction of clinical prediction model with R. Ann Transl Med. 2019;7(23):796.
Liang J, Bi G, Zhan C. Multinomial and ordinal logistic regression analyses with multi-categorical variables using R. Ann Transl Med. 2020;8(16):982.
Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575.
Gu HQ, Liu C. Clinical prediction models: evaluation matters. Ann Transl Med. 2020;8(4):72.
Riley RD, Ensor J, Snell KIE, Harrell FE Jr, Martin GP, Reitsma JB, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441.
Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a life-table model. JAMA Oncol. 2018;4(11):1504–10.
Colunga-Lozano LE, Foroutan F, Rayner D, De Luca C, Hernández-Wolters B, Couban R et al. Clinical judgment shows similar and sometimes superior discrimination compared to prognostic clinical prediction models. A systematic review. J Clin Epidemiol. 2023.
Blum MR, Øien H, Carmichael HL, Heidenreich P, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of Transitional Care services after hospitalization with heart failure. Ann Intern Med. 2020;172(4):248–57.
Bonnett LJ, Snell KIE, Collins GS, Riley RD. Guide to presenting clinical prediction models for use in clinical settings. BMJ. 2019;365:l737.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Cancer. 2015;112(2):251–9.
Wilson J, Chowdhury F, Hassan S, Harriss EK, Alves F, Dahal P, et al. Prognostic prediction models for clinical outcomes in patients diagnosed with visceral leishmaniasis: protocol for a systematic review. BMJ Open. 2023;13(10):e075597.
Crawford SM. Goodhart’s law: when waiting times became a target, they stopped being a good measure. BMJ. 2017;359:j5425.
Download references
This paper is supported by The National Natural Science Fund (grant number: 81973921).
Author information
Authors and affiliations.
College of Nursing, Hubei University of Chinese Medicine, Wuhan, China
Xiaotong Wang, Shi Zhou, Niansi Ye, Yucan Li, Pengjun Zhou, Gao Chen & Hui Hu
Engineering Research Center of TCM Protection Technology and New Product Development for the Elderly Brain Health, Ministry of Education, Wuhan, China
Hubei Shizhen Laboratory, Wuhan, China
You can also search for this author in PubMed Google Scholar
Contributions
XW, PZ, and GC were instrumental in conceptualizing and designing the study. The protocol was formulated by XW and PZ, who also undertook the article screening and results reporting. YL, SZ, and NY offered analytical consultations and data verification. The initial draft of the manuscript was prepared by XW, with NY and HH providing critical reviews and revisions. All contributors sanctioned the final manuscript for publication and committed to being responsible for every aspect of the work.
Corresponding author
Correspondence to Hui Hu .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval has been reviewed by the Medical Ethics Committee of Hubei University of Traditional Chinese Medicine (2019IEC003). Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, X., Zhou, S., Ye, N. et al. Predictive models of Alzheimer’s disease dementia risk in older adults with mild cognitive impairment: a systematic review and critical appraisal. BMC Geriatr 24 , 531 (2024). https://doi.org/10.1186/s12877-024-05044-8
Download citation
Received : 24 November 2023
Accepted : 06 May 2024
Published : 19 June 2024
DOI : https://doi.org/10.1186/s12877-024-05044-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Predictive model
- Systematic review
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Introduction
- Conclusions
- Article Information
The timeline for participant 1 represents the index, the early terminator for whom matches were sought. Other timelines represent the candidate pool of individuals matched exactly to the index based on age at metformin initiation, gender, and whether metformin was the first diabetes drug prescribed in the Kaiser Permanente Northern California (KPNC) health care system. Orange boxes indicate the person-time that would be contributed for each participant if matched, with follow-up time starting at the age of metformin termination for the index. Blue boxes indicate metformin prescriptions, and black squares indicate the end of contributed person-time due to censoring. eGFR indicates estimated glomerular filtration rate.
The flowchart displays inclusions and exclusions for the analytic sample in the Kaiser Permanente Northern California (KPNC) cohort and exposure definition for early terminators and matched routine users. The final sample eligible for matching is shown in the 2 boxes at the bottom. Note that this does not match the sample sizes in Table 1 given that not every early terminator could be matched and some early terminators were matched with other early terminators prior to early termination. eGFR indicates estimated glomerular filtration rate.
Sample sizes differed across mediation analyses due to data availability for mediators.
eFigure 1. Conceptual Model
eFigure 2. Dementia-Free Probability Curve
eTable 1. Match Quality Table for Main Analysis
eTable 2. Match Quality Table for Sensitivity Analysis With Tighter Matches
eTable 3. Characteristics of the Creatinine-Based Analytic Sample, Overall and for Early and Nonearly Terminators
eTable 4. Characteristics of the Analytic Sample Limited to High-Adherence Participants, Overall and for Early and Nonearly Terminators
eTable 5. Characteristics of the Analytic Sample Using Tighter Matching Criteria, Overall and for Early and Nonearly Terminators
eTable 6. Characteristics of the Analytic Sample Limited to Early Terminators With <2 y Follow-Up, Overall and for Early and Nonearly Terminators
eTable 7. Exponentiated Coefficients for All Models
eTable 8. Electronic Health Record Data
eTable 9. Prescriptions
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zimmerman SC , Ferguson EL , Choudhary V, et al. Metformin Cessation and Dementia Incidence. JAMA Netw Open. 2023;6(10):e2339723. doi:10.1001/jamanetworkopen.2023.39723
Manage citations:
© 2024
- Permissions
Metformin Cessation and Dementia Incidence
- 1 Department of Epidemiology and Statistics, University of California, San Francisco
- 2 Kaiser Permanente Division of Research, Oakland, California
- 3 Now with Kaiser Permanente Research Bank, Oakland, CA
- 4 Department of Clinical Pharmacy, University of California, San Francisco
- 5 Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles
- 6 Department of Public Health Sciences, University of California, Davis
- 7 Department of Epidemiology, George Washington University Milken Institute School of Public Health, Washington, District of Columbia
- 8 Department of Epidemiology, Boston University, Boston, Massachusetts
Question Is cessation of metformin therapy associated with dementia incidence, and is the association mediated by hemoglobin A 1c (HbA 1c ) level or insulin use?
Findings This cohort study of 12 220 early terminators and 29 126 routine users of metformin found that cessation of metformin therapy without abnormal kidney function markers was associated with 1.21 times the hazard of dementia diagnosis compared with continuation of therapy or cessation with abnormal kidney function markers. This association was minimally mediated by increases in HbA 1c level and not mediated by insulin use 1 or 5 years after metformin cessation.
Meaning The findings of this study suggest that metformin cessation is associated with increased dementia incidence and that mechanisms other than glucose control or insulin use may mediate this association.
Importance Prior studies suggested that metformin may be associated with reduced dementia incidence, but associations may be confounded by disease severity and prescribing trends. Cessation of metformin therapy in people with diabetes typically occurs due to signs of kidney dysfunction but sometimes is due to less serious adverse effects associated with metformin.
Objective To investigate the association of terminating metformin treatment for reasons unrelated to kidney dysfunction with dementia incidence.
Design, Setting, and Participants This cohort study was conducted at Kaiser Permanente Northern California, a large integrated health care delivery system, among a cohort of metformin users born prior to 1955 without history of diagnosed kidney disease at metformin initiation. Dementia follow-up began with the implementation of electronic health records in 1996 and continued to 2020. Data were analyzed from November 2021 through September 2023.
Exposures A total of 12 220 early terminators, individuals who stopped metformin with normal estimated glomerular filtration rate (eGFR), were compared with routine metformin users, who had not yet terminated metformin treatment or had terminated (with or without restarting) after their first abnormal eGFR measurement. Early terminators were matched with routine users of the same age and gender who had diabetes for the same duration.
Main outcomes and measures The outcome of interest was all-cause incident dementia. Follow-up for early terminators and their matched routine users was started at age of termination for the early terminator. Survival models adjusted for sociodemographic characteristics and comorbidities at the time of metformin termination (or matched age). Mediation models with HbA 1c level and insulin usage 1 and 5 years after termination tested whether changes in blood glucose or insulin usage explained associations between early termination of metformin and dementia incidence.
Results The final analytic sample consisted of 12 220 early terminators (5640 women [46.2%]; mean [SD] age at start of first metformin prescription, 59.4 [9.0] years) and 29 126 routine users (13 582 women [46.6%]; mean [SD] age at start of first metformin prescription, 61.1 [8.9] years). Early terminators had 1.21 times the hazard of dementia diagnosis compared with routine users (hazard ratio, 1.21; 95% CI, 1.12 to 1.30). In mediation analysis, contributions to this association by changes in HbA 1c level or insulin use ranged from no contribution (0.00 years; 95% CI, −0.02 to 0.02 years) for insulin use at 5 years after termination to 0.07 years (95% CI, 0.02 to 0.13 years) for HbA 1c level at 1 year after termination, suggesting that the association was largely independent of changes in HbA 1c level and insulin usage.
Conclusions and Relevance In this study, terminating metformin treatment was associated with increased dementia incidence. This finding may have important implications for clinical treatment of adults with diabetes and provides additional evidence that metformin is associated with reduced dementia risk.
Type 2 diabetes occurs among an increasing fraction of people aged older than 65 years in the US, and diabetes is associated with increased dementia risk. 1 , 2 Approved in 1995 in the US, 3 metformin (dimethylbiguanide) has been the preferred first-line agent for type 2 diabetes since 2006. 4 - 6 Metformin treatment reduces incidence of diabetes complications and diabetes-related and all-cause mortality. 7 Metformin may also reduce dementia risk by improved glucose control or by mechanisms unrelated to diabetes, including activation of adenosine monophosphate–activated protein kinase, which may mimic starvation, 7 or by inhibition of aromatase, which may be associated with lower blood pressure. 8 , 9
Previous randomized clinical trials found that metformin treatment improved cognition and lowered dementia risk in people with type 2 diabetes, but this may reflect cognitive benefits of glucose lowering independent of the agent used. 10 In contrast, the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) randomized clinical trial 11 , 12 found no evidence of cognitive benefit for an intensive glucose-control strategy (glycated hemoglobin A 1c [HbA 1c ] target level: <6.0%), which increased exposure to antidiabetes drugs, including metformin, compared with an HbA 1c target level of 7.0% to 7.9%. Prior observational studies found that initiating metformin treatment was associated with a benefit in dementia risk, including a benefit compared with other antidiabetes drugs. 10 , 13 However, confounding by diabetes severity and duration may bias associations. 10 Additionally, increasing metformin use 14 , 15 and decreasing age-specific dementia incidence in the US 16 complicate observational comparisons between metformin and other agents. 10 Furthermore, some prior studies compared prevalent users with nonusers, which can lead to immortal person-time and confounding biases. 17
Individuals may terminate metformin for several reasons. 18 , 19 Gastrointestinal adverse effects are more common with metformin than with other antidiabetes agents, 20 leading to lower adherence 18 and replacement with other antidiabetes agents in one-fifth of early users. 21 , 22 Metformin may also be terminated because it is associated with increased mortality in people with kidney dysfunction, a common type 2 diabetes complication. 21 , 23 , 24 Current recommendations are to discontinue metformin when the estimated glomerular filtration rate (eGFR) decreases to less than 30 or 45 mL/min/1.73 m 2 of height, depending on benefits and risks of continued use. 3 Examining metformin cessation may mitigate confounding by disease severity and cohort effects associated with studies of metformin initiation.
We evaluated the association between termination of metformin treatment for reasons unrelated to kidney dysfunction and dementia incidence using an emulated trial design. 25 , 26 We compared individuals in the Kaiser Permanente Northern California (KPNC) health care system who terminated metformin without abnormal kidney function markers with individuals who continued metformin therapy or stopped after abnormal kidney function. We investigated whether metformin was associated with reduced risk of dementia and whether this association was mediated predominantly by mechanisms other than improved glucose control or insulin use.
This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. 33 Human participant approval was granted by University of California, San Francisco, and KPNC, Mid-Atlantic States institutional review boards. All participants provided informed consent.
KPNC is an integrated health care delivery system with 4.6 million members. 27 Older adult KPNC members are similar to the general older adult population of Northern California with respect to chronic conditions (diabetes, hypertension, heart disease, and asthma), other risk factors (smoking, obesity, and sedentary behavior), 28 and demographics; extremes of the income distribution are underrepresented. 28 - 30
This study used deidentified survey results and electronic health records from KPNC members who had used metformin; were dementia free; were born prior to January 1, 1955; and had completed 1 of 2 harmonized health surveys: (1) the California Men’s Health Survey, 31 offered 2002 to 2003 to men aged 45 to 69 years in January 2000 who had been KPNC members for at least 1 year, and (2) the Kaiser Permanente Research Program on Genes, Environment, and Health survey, offered 2007 to 2009 to adults who had been KPNC members for at least 2 years. 32 Dementia follow-up began with the implementation of electronic health records in 1996 and continued to 2020.
Participants were followed up from the first availability of electronic health records (starting in 1996) until administrative censoring, age 90 years, death, the start of a 90-day membership gap, or dementia diagnosis. Each participant was assigned an administrative censoring date randomly chosen between January 30, 2020, and March 31, 2020, so that date of birth could not be inferred. Death dates were obtained from the KPNC mortality database, which combines KPNC clinical and administrative, National Death Index, California State death, and Social Security Administration records. Details on diagnostic, laboratory, prescription record, diabetes registry, and sociodemographic variables are given in the eMethods in Supplement 1 and eTables 8 and 9 Supplement 2 . Race and ethnicity were self-reported. See original race and ethnicity categories, along with other details on race and ethnicity reporting, in the eMethods in Supplement 1 . These were collapsed into the following categories for consistency between survey and other data sources and because participants could endorse multiple categories: Asian, Black, Hispanic or Latino, White, and other or uncertain. An algorithm (validated by comparing against several sources, including the KPNC virtual data warehouse) was used to assign 1 adjudicated race or ethnicity category to individuals who endorsed more than 1 category, with the following prioritization order: Black, Hispanic, Asian, and Native American. Race and ethnicity was derived from KPNC health plan databases for individuals who endorsed no categories. Race and ethnicity were assessed by KPNC to improve patient care and for research purposes.
Exposures were categorized as follows: First, the age of first documented abnormal kidney function after initiating metformin was determined for each individual in a cohort of metformin users. Early terminators were defined as individuals who stopped using metformin without prior history of abnormal kidney function. A routine user was any individual who remained on metformin at the age when the matched early terminator ceased using metformin. No information from times after the cessation time was used to define exposure status. Follow-up for routine users ended at censoring or when the individual became an early terminator. We excluded individuals who stopped using metformin but had no antidiabetes medication prescriptions within 1 month, given that prescription nonadherence may be a sign of incipient dementia or particularly good control of blood glucose with nonpharmacologic interventions. Such individuals would not be comparable to individuals who remained on metformin.
Analyses were conducted using R statistical software version 4.0.4 (R Project for Statistical Computing). All P values calculated were 2-sided, with a significance threshold of α = .05. Data were analyzed from November 2021 through September 2023. Figure 1 and eFigure 1 in Supplement 1 provide a conceptual model of the emulated trial design used. An emulated trial design is intended to replicate key features of a randomized trial. Estimated associations are analogous to intent-to-treat estimates given that we compared early terminators with the routine user group, which includes individuals who stopped taking metformin after kidney dysfunction markers were found. Each early terminator was matched with up to 4 individuals using incidence density sampling. For the purposes of the match, these individuals were routine users, but they could go on to be early terminators themselves at an older age. Matching was performed such that no matches were repeated, with exact matching on gender and whether metformin was the first diabetes drug prescribed in KPNC. A distance measure was calculated with age of metformin initiation, diabetes duration (years since first diagnosis of diabetes in the registry), and HbA 1c level using the sum of absolute differences, with HbA 1c level multiplied by 10 to be on approximately the same scale as age. Among individuals eligible based on exact matching criteria, matches were assigned by selecting a routine user with the minimum distance to the early terminator. Matches were rejected if matched pairs ages of metformin initiation differed by more than 5 years, the difference in HbA 1c level was greater than 0.5%, or the difference in diabetes duration was greater than 5 years. If no match meeting these criteria could be obtained for an early terminator, that individual was excluded from the analysis. Matches were iteratively chosen such that early terminators received matches before being considered for subsequent matches. To prevent immortal person-time bias, an early terminator and the matched routine user began follow-up time at the age that the early terminator stopped metformin treatment. Individuals were not eligible for matching after dementia diagnosis.
We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs of dementia diagnosis using time since metformin termination (or equivalent for routine users) as the time scale. To provide a simple interpretation of associations, we also present HRs and 95% CIs from accelerated failure time (AFT) models with a Weibull-distributed waiting time. We present HRs adjusted only for matching variables (minimally adjusted models) and HRs additionally adjusted for demographic characteristics and comorbidities at the time of metformin initiation (fully adjusted models).
For matched pairs, covariates were ascertained at the age of the early terminator’s metformin cessation. Models adjusted for demographic characteristics (gender, age at metformin initiation, race and ethnicity, educational attainment, nativity, parental nativity, and survey language) and low-density lipoprotein and HbA 1c levels at the time of metformin termination. We additionally adjusted for history of abnormal kidney function prior to metformin initiation as a binary variable, baseline use of antilipemic agents, insulin use, nonmetformin oral hypoglycemic use, diabetes status, and whether metformin was the first diabetes drug prescribed. We adjusted for cardiovascular disease history and number of categories of events (hypertension or secondary hypertension, ischemic heart disease, heart attack, and stroke) and cancer history .
Causal mediation analysis 34 - 36 assessed whether the association between early metformin termination and dementia was mediated by changes in HbA 1c level or insulin prescription status measured approximately 1 year after early metformin termination (specifically, mean HbA 1c level during the period 8-16 months after termination) or at the same age among matched routine users. Individuals who got dementia, died, were censored prior to mediator measurement, or did not have mediators measured were excluded from mediation analyses. In additional analyses, we used HbA 1c level (specifically, mean levels at 4 years, 8 months to 5 years, 4 months) and change in insulin prescription status at 5 years after early metformin termination as mediators. Mediation analyses used AFT models with linear mediator models with the same covariates as the fully adjusted model. We assessed for exposure-mediator interaction in outcome models with a prespecified threshold of α = .05 for significance. We calculated total, natural direct, and natural indirect effects of metformin termination on time to dementia and 95% CIs.
The following sensitivity analyses were performed: First, we repeated analyses using creatinine instead of eGFR. Gender-specific cutoffs of 1.4 mg/dL (124 μmol/L) or greater for women and 1.5 mg/dL (133 μmol/L) or greater for men were used. Second, we restricted analysis to participants with medication adherence exceeding 80% (eMethods in Supplement 1 ). Third, we further restricted differences between early and routine users, rejecting matches with differences greater than 0.1% in HbA 1c level or 1 year of diabetes duration. Finally, we restricted analysis to early terminators who had initiated metformin within the prior 2 years, a time frame too short for diabetes progression to be likely.
The final analytic sample consisted of 12 220 early terminators (5640 women [46.2%]; mean [SD] at start of first metformin prescription, 59.4 [9.0] years; 1642 Asian [13.4%], 1004 Black [8.2%], 1819 Hispanic [14.9%], and 7663 White [62.7%]) and 29 126 routine users (13 582 women [46.6%]; mean [SD] age at start of first metformin prescription, 61.1 [8.9] years; 4674 Asian [16.0%], 2131 Black [7.3%], 3832 Hispanic [13.2%], and 18 286 White [62.8%]) ( Table 1 ). Figure 2 shows the number of individuals meeting inclusion and exclusion criteria. Table 1 gives additional demographic information. At the age of matching among 29 126 matched pairs, 18 686 pairs (64.2%) had an HbA 1c level within 0.1 percentage points of each other, 17 563 pairs (60.3%) had ages within 1 year of each other, and 18 064 pairs (62.0%) had a duration with diabetes within 1 year of each other (eTables 1 and 2 in Supplement 1 ). There were 724 routine users and 281 early terminators who reached age 90 years, 5172 routine users and 3434 early terminators who died, and 6286 routine users and 2181 early terminators who left the Kaiser system. See eTables 3 through 6 in Supplement 1 for the final analytic samples for sensitivity analyses. Cox models were stratified by age at metformin initiation and gender. Proportional hazard assumptions were met at a significance threshold of .05.
In minimally adjusted models, early terminators had 1.21 times the hazard of dementia diagnosis compared with routine users (HR, 1.21; 95% CI, 1.15-1.28). In a fully adjusted model, early terminators had 1.21 times the hazard of dementia diagnosis compared with routine users (HR, 1.21; 95% CI, 1.12-1.30) ( Table 2 ; eFigure 2 and eTable 7 in Supplement 1 ). For the purposes of comparisons with studies of metformin initiation, the reciprocal of the fully adjusted HR was 0.83 (95% CI, 0.76-0.90). When estimated with AFT models, this association translated to an accelerated time to dementia of 0.89 (95% CI, 0.83-0.96) in the minimally adjusted model and 0.89 (95% CI, 0.82-0.97) in the fully adjusted model with early termination compared with routine use. Sensitivity analyses yielded similar results to the main analysis. Table 2 provides a summary of main results and sensitivity analyses.
Figure 3 presents mediation analyses. Exposure-mediator interactions were not significant for any models and were not included. Total estimated acceleration of dementia diagnosis ranged from 0.91 years (95% CI, −0.57 to 2.42 years) for HbA 1c level at 1 year to 1.75 years (95% CI, 0.15 to 3.44 years) for HbA 1c level at 5 years. The mediated acceleration contributed by changes in HbA 1c level or insulin use ranged from no contribution (0.00 years (95% CI, −0.02 to 0.02 years) for insulin use at 5 years to 0.07 years (95% CI, 0.02 to 0.13 years) for HbA 1 c level at 1 year, suggesting that a small fraction of the acceleration of dementia diagnosis could be attributed to measured changes in HbA 1c level or insulin use. Additional estimates and 95% CIs are presented in Figure 3 .
This cohort study found that terminating metformin treatment was associated with increased dementia incidence in a diverse cohort of older adults. Most of this association was not accounted for by increases in HbA 1c levels or insulin use 1 or 5 years after cessation of treatment with metformin.
This work has several important implications. First, findings corroborate the largely consistent evidence from other observational studies showing an association between metformin use and lower dementia incidence. A major advantage of our design was that it may have reduced potential for confounding by indication or cohort effects present in initiation designs given that all individuals in our analyses were metformin users. In addition, the very large sample size and long follow-up provided fairly precise estimates. Previously meta-analyzed results 10 indicated that people with diabetes who receive metformin had 0.76 times the hazard of Alzheimer disease compared with people with diabetes not receiving metformin (HR, 0.76; 95% CI, 0.60-0.97). This meta-analyzed result included control groups of patients with diabetes receiving sulphonylurea therapy or no drug therapy or any patients with diabetes not receiving metformin. A prior study 10 , 37 found an HR of 0.82 (95% CI, 0.52-1.28) comparing metformin with sulphonylurea therapy. Thus, our result of 0.83 times the hazard of dementia with routine use compared with early termination (reciprocal of the fully adjusted HR, 0.83; 95% CI, 0.76-0.90) is consistent with the literature on metformin treatment and dementia incidence.
Second, these results may have potential implications for diabetes treatment in late life. For individuals with diabetes at particularly high risk of dementia, such as carriers of APOE ε4 or individuals with a family history of dementia, it may be particularly beneficial to find ways to manage or mitigate gastrointestinal adverse effects (eg, switching to slower-release formulations of metformin or taking the medication with food in the evening) 20 instead of replacing metformin with other agents given that participants in this study remained on antidiabetes drugs after early termination with metformin. Formally evaluating the heterogeneity of the metformin estimate across known risk factors for dementia is an important extension of our work. Given considerable interest in drug repurposing for dementia, further confirmatory work would be required to extrapolate to prediabetic or nondiabetic populations. 38 , 39
Our analysis emulates an intent-to-treat analysis: we did not compare early terminators only with individuals who stayed on metformin indefinitely or until censoring, nor did we only compare early terminators with individuals who terminated due to kidney dysfunction. Such separate comparisons would have the same biases that as-treated analyses have 40 because, assuming that discontinuing metformin is not associated with dementia, early terminators would be expected to have increased risk of dementia compared with those who did not terminate simply because some early terminators will develop kidney dysfunction. Similarly, early terminators as a group would be expected to have reduced risk of dementia compared with individuals who terminated due to signs of kidney dysfunction because not all early terminators will develop kidney dysfunction. Thus, both such comparisons would be biased metformin was not associated with dementia.
This study has several strengths. First, while we did not use a standard quasiexperimental design, 41 we leveraged the fact that gastrointestinal adverse effects are a common reason for discontinuation of metformin but are likely unrelated to diabetes progression or dementia risk. Second, all individuals were metformin initiators, mitigating the potential for cohort effects and confounding by indication. Additionally, beginning follow-up at the age of early termination of metformin use prevents immortal person-time bias. 17
This study also has several limitations. First, dementia diagnosis was obtained based on medical records, and recording of such diagnoses likely follows onset of pathology. Second, this was a complete case analysis; thus, the main analysis and mediation samples differed in size, although estimates for total effect sizes were consistent in both analyses. Third, we did not evaluate numerous and relevant potential axes of heterogeneity, such as race, ethnicity, or duration of metformin use. Our reported associations are means over the distributions of the history of metformin use and thus would not be appropriate for clinicians for specific patient recommendations. Fourth, as stated previously, data on the precise reason for termination of metformin were not available. Metformin initiation is contraindicated in severe liver disease and heart failure. However, because metformin is beneficial for people with diabetes with congestive heart failure, chronic liver disease, or reduced kidney function (eGFR, 45-60 mL/min/1.73 m 2 ), 42 we did not consider these reasons for termination in routine users. It is possible that our characterization of early metformin terminators may have misclassified some individuals. However, in a sensitivity analysis that examined only individuals who terminated use within the first 2 years of therapy, we had comparable results. In this analysis, we would be assuming that very early termination was less likely to be due to kidney dysfunction. Fifth, due to data limitations, we could not address every potential source of bias, such as deprescribing to improve quality of life in individuals with frailty, and we did not consider time-updated mediator outcome confounding. However, these are different confounding biases than would be present in studies of treatment initiation that indicate a comparable benefit of metformin. If censoring due to reaching age 90 years, death, or KPNC membership gap and another risk factor associated with dementia both affect treatment and outcome status, effect estimates could be biased. Early terminators were more likely to die during follow-up, so expected bias would likely be to reduce the estimated effect size in the association of early termination with dementia risk. Relatedly, to ensure privacy, all data related to time were provided with age as the time scale as opposed to calendar time. However, it is possible that calendar time was associated with non-kidney-disease–related metformin cessation. Sixth, the applicability of these results relies on the assumption that individuals experiencing significant gastrointestinal or other adverse effects were not also more likely to experience diabetes progression for other reasons. Factors such as high dietary carbohydrate consumption could be associated with adverse effect prevalence and diabetes progression. However, confounders of metformin discontinuation due to adverse effects were likely different than those for metformin initiation, and our estimates for terminating metformin were similar to estimates of initiating metformin.
In this cohort study of metformin users, terminating metformin treatment was associated with increased dementia incidence, corroborating prior observational research that initiating metformin was associated with reduced risk of dementia. This finding has important implications for the clinical management of diabetes.
Accepted for Publication: September 14, 2023.
Published: October 25, 2023. doi:10.1001/jamanetworkopen.2023.39723
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Zimmerman SC et al. JAMA Network Open .
Corresponding Author: Sarah F. Ackley, PhD, Department of Epidemiology, Boston University, Boston, MA, Talbot Building, 715 Albany St, Boston, MA 02118 ( [email protected] ).
Author Contributions: Mr Zimmerman and Dr Ackley had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zimmerman, Oni-Orisan, Whitmer, Power, Ackley.
Acquisition, analysis, or interpretation of data: Zimmerman, Ferguson, Choudhary, Ranatunga, Oni-Orisan, Hayes-Larson, Duarte Folle, Mayeda, Gilsanz, Power, Schaefer, Glymour, Ackley.
Drafting of the manuscript: Zimmerman, Ferguson, Choudhary, Ackley.
Critical review of the manuscript for important intellectual content: Zimmerman, Ranatunga, Oni-Orisan, Hayes-Larson, Duarte Folle, Mayeda, Whitmer, Gilsanz, Power, Schaefer, Glymour, Ackley.
Statistical analysis: Zimmerman, Ferguson, Hayes-Larson, Whitmer, Ackley.
Obtained funding: Power, Glymour.
Administrative, technical, or material support: Zimmerman, Choudhary, Duarte Folle, Power.
Supervision: Whitmer, Glymour, Ackley.
Conflict of Interest Disclosures: Mr Zimmerman reported owning stock from AbbVie Inc, Gilead Sciences LLC, CRISPR Therapeutics, and Abbott Laboratories outside the submitted work. Dr Mayeda reported receiving grants from the NIA and California Department of Public Health during the conduct of the study. Dr Whitmer reported receiving grants from the National Institutes of Health (NIH) and personal fees from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study. Dr Gilsanz reported receiving grants from the NIA during the conduct of the study. Dr Power reported receiving grants from the NIH during the conduct of the study and providing prior service as a paid member of the Biogen Healthy Climate, Healthy Lives Scientific Advisory Council. No other disclosures were reported.
Funding/Support: The study was funded by grants R01AG057869 to Mr Zimmerman, Dr Power, and Dr Glymour; K99AG075317 to Dr Hayes-Larson; and K99AG073454 to Dr Ackley from the National Institutes of Health National Institute on Aging.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 3 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Neurol
- v.15(1); 2019 Jan

Risk of Dementia in Long-Term Benzodiazepine Users: Evidence from a Meta-Analysis of Observational Studies
a Department of Out-Patient, West China Hospital, Sichuan Uniwersity, Chengdu, China.
Xiaohua Chen
b Department of Nursing, West China Hospital, Sichuan Uniwersity, Chengdu, China.
Xiaofan Fei
c Department of Pharmacy, West China Hospital, Sichuan Uniwersity, Chengdu, China.
Associated Data
Background and purpose.
There is conflicting evidence in the literature on the association between benzodiazepines (BDZs) and the risk of dementia. This meta-analysis aimed to determine the relationship between the long-term usage of BDZs and the risk of dementia.
The PubMed and Embase databases were systematically searched for relevant publications up to September 2017. The literature search focused on observational studies that analyzed the relationship between the long-term use of BDZs and the risk of dementia. Pooled rate ratios (RRs) with 95% confidence interval (CI) were assessed using a random-effects model. The robustness of the results was checked by performing subgroup and sensitivity analyses.
Ten studies were included: six case–control and four cohort studies. The pooled RR for developing dementia was 1.51 (95% CI=1.17–1.95, p =0.002) in patients taking BDZ. The risk of dementia was higher in patients taking BDZs with a longer half-life (RR=1.16, 95% CI=0.95–1.41, p =0.150) and for a longer time (RR=1.21, 95% CI=1.04–1.40, p =0.016).
Conclusions
This meta-analysis that pooled ten studies has shown that BDZ significantly increases the risk of dementia in the elderly population. The risk is higher in patients taking BDZ with a longer half-life (>20 hours) and for a longer duration (>3 years).
INTRODUCTION
Benzodiazepines (BDZs) enhance the efficacy of the neurotransmitter gamma-aminobutyric acid (GABA) at the GABA-A receptor so as to induce sedative, hypnotic, anxiolytic, anticonvulsant, and muscle relaxant properties. 1 Various studies have been conducted to understand the association between BDZ and dementia. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 A few well-conducted prospective cohort studies have found an increased risk of dementia in users of long-acting BDZ, whereas subsequent studies found no such association.
The type of BDZ activity (long acting or short acting) and the time period of taking the drug (long term or short term) were also considered in previous analyses. A few studies have inferred that the long-term use of BDZ could increase the risk of dementia, whereas its short-term use might not. 2 , 8 , 10 Moreover, long-acting BDZ but not short-acting BDZ might be related to an increased risk of dementia. 7 , 9 , 11
To resolve these discrepancies, the current pooled meta-analysis was designed to establish the association between the long-term use of BDZ and the risk of dementia, in terms of both the duration of action and type of BDZ taken.
Search strategy
A systematic search strategy of the following electronic databases was used to identify all published studies on the association between BDZ use and the risk of dementia: Medline (via Ovid), Embase, and the Cochrane Library. The search terms and keywords were altered in accordance with the specifications of the individual databases. The search strategies are given in the Supplementary Material 1 (in the online-only Data Supplement). No restrictions were placed on publication language. The database search was performed on January 22, 2018. The reference lists of identified articles were checked for additional publications, while the corresponding authors were contacted to obtain additional information about both published and unpublished studies. The present study was reported according to PRISMA guidelines for reporting meta-analyses.
Study selection
The results obtained from searching the three databases were exported into EndNote X8.0.1 software to identify and remove potential duplicate studies that appeared in more than one database. All of the resulting unique studies were exported into an Excel spreadsheet for initial screening, which involved the titles and/or Abstracts of the unique studies in Excel being checked by two authors (T.W. and L.L.) to exclude any clearly irrelevant studies. For secondary screening, the full text was read to decide on inclusion or exclusion by two authors (T.W. and X.C.) independently based on the selection criteria of the meta-analysis. Disagreement was resolved by a third author (Q.H.) who examined the studies independently.
Inclusion and exclusion criteria
Studies were eligible for inclusion if they met the following inclusion criteria: 1) a case–control or cohort design, 2) exposure of interest was BDZ intake, 3) outcome was incidence of dementia, and 4) relative risks or odds ratios (ORs) with corresponding 95% confidence interval (CI) reported or could be estimated (from the raw data in the published article).
Data extraction
The primary studies were reviewed by two authors independently to evaluate their relevance for inclusion in the current pooled analysis. The following data points were then extracted for each study: 1) author name, publication year, and study site, 2) study design, 3) number of study subjects, and numbers of study subjects on BDZ and having dementia, 4) effect estimates with CIs, 5) assessment of exposure (BDZ usage) and outcome (dementia), and 6) confounding factors adjusted, if applicable.
Quality assessment
Any inconsistencies in opinion between the two independent authors were resolved by an analysis of the published article by a third author. The Newcastle-Ottawa Scale (NOS) was used to measure the quality of each study. This scale considers the three factors of selection (4 points), comparability (2 points), and outcome/exposure (3 points), with the study quality being highest for the maximum score of 9 points, moderate for a score of 7 or 8 points, and low for a score ≤6 points.
Data synthesis and analysis
The rate ratio (RR) was used as the effect estimate in pooling. Both cohort and case–control studies were allowed since the risk of dementia is low and the RR in prospective cohort studies will mathematically approximate the OR. A random-effects model (the DerSimonian-Laird method) was chosen for pooling the effect estimates of individual studies, since it was assumed that the effect sizes underlying different studies are drawn from a distribution rather than representing a common effect size shared by all studies. The overall pooled RR estimate was calculated by comparing ever BDZ users with never BDZ users. Heterogeneity was assessed in various ways, including visual examination of a forest plot, the Cochrane Q test, and the I 2 statistic. A Q statistic with a p value of <0.01 and I 2 value >50% was considered to indicate heterogeneity. Subgroup analyses were performed to assess the sources of heterogeneity according to study design (cohort vs. case–control studies) and study quality.
A secondary analysis was performed to determine the effects on dementia of the duration of BDZ usage (effect estimate for long-term usage was obtained by comparing longterm BDZ usage with short-term BDZ usage) and the half-life of BDZ (effect estimate for the use of long-acting BDZ was obtained by comparing the use of long-acting BDZ with the use of other types of BDZ: ultra-short-, short-, and medium-term-acting BDZ). A subgroup analysis could not be conducted based on the duration of BDZ use and BDZ pharmacokinetics since these are not primary estimates and these effect estimates are not reported for all studies. Therefore, a secondary pooled analysis was performed to draw further inferences on the use of BDZ.
The test for interaction developed by Altman and Bland 12 is useful for assessing if there is a significant difference between two effect estimates (E1 and E2) obtained from a subgroup analysis. The result of the test for interaction is interpreted based on the p interaction value, and the z value is calculated as:
The p interaction value is obtained by referring the ratio (z) to a table for a normal distribution, and p interaction >0.05 indicates that there is no good evidence for a different treatment effect across two effect estimates or vice versa.
A sensitivity analysis was performed to assess the influence of each individual study on the pooled effect estimate. Studies were depicted in a forest plot in ascending order of difference between the individual effect size and the pooled estimate in order to depict the results of the sensitivity analysis. Publication bias was assessed via visual inspection of a funnel plot and the Begg and Mazumdar 13 adjusted rank correlation test. The statistical analyses were performed using the Comprehensive Meta-Analysis software (version 2, Biostat, Englewood, NJ, USA).
Search results
The 3,030 references identified included 3,026 articles (258 from Medline, 2,551 from Embase, and 217 from the Cochrane Library) found by searching the electronic databases up to January 2018, plus 4 articles found by screening the reference lists of the included articles ( Fig. 1 ). We excluded 358 duplicates using EndNote reference manager and 2,623 studies found to be ineligible after reading titles and Abstracts. Accordingly, we retrieved 49 references for further assessment. We excluded 39 references since 20 did not report separate data on BDZs, 16 studies did not assess the casual relationship between BDZ use and dementia, and 3 did not report original data. The ten studies remaining for inclusion in the meta-analysis comprised four cohort and six case–control studies.

Study characteristics
The 4 cohort studies ( Table 1 ) and 6 case–control studies ( Table 2 ) involved 171,939 subjects and 42,025 dementia cases. The follow-up period was 4–22 years in these studies, and the publication period was 2002–2017. The mean age of patients in all of the included studies was older than 70 years, with the exception of Gallacher et al. 2 reporting a mean age of 61 years. This meant that all of the included results were for elderly patients; the mean ages in the individual studies are presented in Table 1 and and2. 2 . The studies included more females than males, with the exception of Gallacher et al. 2 studying male patients only.
| Study name | Mean age/ M to F ratio | F/u period (years) | Definition of BDZ use | BDZ Class and Components | BDZ use assessment | Dementia assessment | Study population (BDZ users/ non BDZ users) | Total dementia cases (BDZ users/ non BDZ users) | NOS quality rating |
|---|---|---|---|---|---|---|---|---|---|
| Shash et al. 2016 (France) | 73 (0.25) | 8 | • Prevalent use was defined as any report of BDZ use at baseline | All classes of benzodiazepines and their derivative drugs, including anxiolytic, hypnotic and sedative antiepileptic, and myorelaxants | Patient reported | Medical records | 7,130 (1,246/5,884) | 647 (151/496) | 7 |
| • Incident use was defined as BDZ use during the study | |||||||||
| • Long half-life BDZ-t1/2 >20 hrs | |||||||||
| Gray et al. 2016 (USA) | 74 (NR) | 7.3 | • Ever exposure to BDZ (any) | 1) BDZs-temazepam, diazepam, clonazepam, triazolam, lorazepam, alprazolam, flurazepam, oxazepam, chlordiazepoxide, clorazepate | Computerized pharmacy data | Clinician | 3,434 (NA/NA) | 797 (NA/NA | 8 |
| • At each time point during F/u, the cumulative exposure to BDZ is calculated by summing all BDZ use in the previous 10 years | 2) Non-BDZ hypnotics-zolpidem, eszopiclone, zaleplon | ||||||||
| • Categorized further based on cumulative use as no use, 1–30, 31–120, or ≥121 (long term intake) TSDDs | |||||||||
| Gallacher et al. 2012 (UK) | 61 (All subjects are males) | 22 | • Ever exposure to BDZ (any) | BDZ | Medical record | Clinician | 1,188 (103/1,085) | 93 (22/71) | 6 |
| • Long term intake defined as >4 years of BDZ use | |||||||||
| Billioti de Gage et al. 2012 (France) | 72 (0.89) | 15 | • New BDZ users defined as subjects without declared BDZ use at baseline to 3 years and declared BDZ use between 3 and 5 year | Alprazolam,bromazepam, chlordiazepoxide, clobazam, clonazepam, clorazepate, clotiazepam, diazepam, estazolam, flunitrazepam, loflazepate, loprazolam, lormetazepam, nitrazepam, nordazepam, prazepam, oxazepam, temazepam, tetrazepam, tofizopam, triazolam, zolpidem, and zopiclone | Patient reported | Clinician | 1,063 (95/968) | 253 (30/223) | 7 |
| • Non users are defined as subjects without any declared use of BDZ at all 5 years from start of study |
BDZ: benzodiazepine, F: female, F/u: follow-up, M: male, NA: nor applicable, NOS: New Castle Ottawa Scale, NR: not rated, TSSDs: total standardized daily doses.
| Study name | Mean age/ M to F ratio | Study period (years) | Definition of BDZ use | BDZ Class and Components | BDZ use assessment | Dementia assessment | Study population (cases/controls) | BDZ users in cases/controls | NOS quality rating |
|---|---|---|---|---|---|---|---|---|---|
| Chan et al. 2017 (Hong Kong) | 87 (0.28) | 9.8 | • Ever exposure to BDZ (any) | Alprazolam, bromazepam, lormetazepam, chlordiazepoxide, clorazepate, diazepam, flunitrazepam, flurazepam, lorazepam, midazolam, nitrazepam, triazolam, clonazepam, clobazam | Computerized medical records | Clinician | 273 (91/182) | 40/81 | 7 |
| • Exposure density assessed as PDD-total doses in milligrams divided by the total duration of use in days by all subjects in the cohort | |||||||||
| • Long term use of BDZ is defined as PDD ≥1,096 | |||||||||
| • Long half-life BDZ–t1/2 >20 hrs | |||||||||
| Gomm et al. 2016 (Germany) | 75 (0.55) | 4.7 | • Regular BDZ (any) users are defined as if subjects had at least one prescription of BDZs in each of four sequential quarters during the observation time before the index date | Study did not specify the specific BDZ used, we assume that all approved BDZs were used for the analysis | Computerized medical records | Computerized medical records | 105,725 (21,145/84,580) | 1,719/4,940 | 7 |
| • No use is defined as no prescription in any quarter during the observation time before the index date | |||||||||
| • Patients with occasional BDZR use (i.e., non regular BDZ use) were excluded | |||||||||
| Imfeld et al. 2015 (UK) | 79 (0.55) | 10 | • Ever exposure to BDZ (any) | All classes of BDZs and BDZ derivatives | Medical records | Medical records | 19,272 (9,636/9,636) | 2,872/2,576 | 7 |
| • Long half-life BDZ-t1/2 >24 hrs | |||||||||
| • Long term use defined as >150 BDZ prescriptions | |||||||||
| Wu et al. 2009 (Taiwan) | 75 (0.86) | 4 | • BDZs exposure status was assessed in subjects before the case index date | All classes of BDZs and BDZ derivatives | Medical records | Medical records | 5,405 (779/4,626) | 267/852 | 7 |
| • Long-term BDZs users, defined as receiving BDZs for more than 180 days within an 1-year period | |||||||||
| Wu et al. 2011 (Taiwan) | 77 (0.94) | 9.1 | • BDZs exposure status was assessed in subjects before the case index date | Study did not specify the specific BDZs used, we assume that all approved BDZs were used for the analysis | Medical records | Medical records | 25,140 (8,434/16,706) | 3,113/2,628 | 7 |
| • Long-term BDZs users, defined as receiving BDZs for more than 180 days within an 1-year period | |||||||||
| Lagnaoui et al. 2002 (France) | 74 (0.54) | 11 | • Ever users if exposed to BDZs at least once before the index date and nonusers if not | Alpidem, alprazolam, bromazepam, chlordiazepoxide, clobazam, clonazepam, clorazepate, clotiazepam, diazepam, estazolam, flunitrazepam, loflazepate, loprazolam, lormetazepam, nitrazepam, nordazepam, prazepam, oxazepam, temazepam | Patient reported | Medical records | 3,309 (150/3,159) | 97/1,714 | 6 |
| • Former use of BDZ is defined as BZD use had ended within the past 2 or 3 years (former use) |
BDZ: benzodiazepine, BDZR: benzodiazepine related z-substance, F: female, M: male, NOS: New Castle Ottawa Scale, PDD: prescribed daily doses.
The 6 case–control studies involved 159,124 participants who were followed up for 4–11 years, comprising 8,108 BDZ users in 40,235 dementia cases and 12,791 BDZ users in 109,889 controls. Only one of the six case–control studies found a negative relationship between BDZ use and the risk of dementia. 11 Two studies each were conducted in Taiwan and Europe, and one each in UK and Hong Kong ( Table 2 ).
The 4 cohort studies were published between 2011 and 2016 and involved 12,815 participants. These studies had follow-up periods ranging from 7 to 22 years, and involved 1,790 dementia cases and more than 1,500 BDZ users. A negative relationship between BDZ use and the risk of dementia was found in one study. 9 The four cohort studies comprised two conducted in France and one each in the UK and USA ( Table 1 ).
Exposure to BDZ assessment in included studies
None of the included studies focused on a single class or category of BDZ. Patients were classified as being exposed to BDZ if they took any BDZ for a prespecified duration. However, the included studies performed ad-hoc analyses according to various categories of BDZ. The method of exposure assessment differed across the included studies. The use of BDZ was analyzed using medical records in five of the six case-control studies, and self-reported in the sixth study, while medical records were used in two of the four cohort studies, with self-reporting in the other two. Further information about the BDZ use and assessment is given in Table 1 and and2 2 .
Quality assessment results
The NOS scores resulted in eight of the ten studies being categorized as of moderate quality, and the remaining two studies categorized as of low quality. 2 , 5 These results were as expected since all of the included studies were observational in nature and prone to selection bias. Detailed results of the quality assessment are given in the Supplementary Materials 2 (in the online-only Data Supplement). Despite the mediocre overall quality of the included studies, the present pooled meta-analysis results are reliable.
Pooled RR estimates: ever BDZ use vs. never BDZ use
The heterogeneity parameter ( p heterogeneity <0.05, I 2 =97%) was observed to be significant, and so a random-effects model was chosen over a fixed-effects model. The combined analysis of the ten studies inferred that patients with ever BDZ use were associated with a considerable increase in the risk of dementia (RR=1.51, 95% CI=1.17–1.95, p =0.002) compared to patients with never BDZ use. The multivariable-adjusted RR estimate and 95% CI of each study and the pooled RR are shown in Fig. 2 .

Subgroup analysis
No significant difference in the two pooled RR values was found when the studies were grouped into those of moderate quality (RR=1.44, Cochrane Q=302.5, p heterogeneity <0.05, I 2 =97.7%) and low quality (RR=1.87, Cochrane Q=1.2, p heterogeneity =0.280, I 2 =14.5%) ( p interaction =0.64) ( Fig. 3 ), nor when the studies were grouped according to the study design (case–control and cohort studies) ( p interaction =0.43). However, the positive relationship between BDZ use and the risk of dementia was stronger in the case–control studies (RR=1.57, Cochrane Q=294, p heterogeneity <0.05, I 2 =98.3%) than in the cohort studies (RR=1.26, Cochrane Q=6.4, p heterogeneity =0.091, I 2 =53.5%) ( Fig. 4 ).

Long-term BDZ use
The long-term use of BDZ and the risk of dementia were assessed in four studies. 2 , 4 , 8 , 10 Long-term BDZ use was significantly associated with an increased risk of dementia (RR=1.21, Cochrane Q=3.4, p heterogeneity =0.330, I 2 =12.7%) when compared to the short-term use of BDZ ( Fig. 5 ).

Use of long-acting BDZ
The use of long-acting BDZ and the risk of dementia were assessed in four studies. 7 , 9 , 10 , 11 The use of long-acting BDZ was not strongly related to the risk of dementia (RR=1.16, Cochrane Q=13.3, p heterogeneity <0.05, I 2 =77.6%) when compared to patients taking short- or medium-term-acting BDZ ( Fig. 6 ).

Publication bias
The p values for Begg's ( p =0.21) and Egger's ( p =0.49) tests indicated that publication bias was not present, and the visual inspection of the funnel plot also did not reveal any asymmetry ( Fig. 7 ).

Sensitivity analysis
The robustness of the analysis results was tested by performing a sensitivity analysis in which the overall effect size was measured by removing one study at a time. This analysis produced no significant variation in the pooled RR when excluding either the outlier study of Chan et al. 11 (due to a very small sample) (RR=1.57, 95% CI=1.20–2.06) or any of the other studies (RR=1.37–1.58), thereby confirming the robustness of the present results ( Fig. 8 ).

This meta-analysis pooled ten studies and found that BDZ significantly increases the risk of dementia in the elderly population. This effect was greater in patients using BDZ with a longer half-life (>20 hours half-life) and taking BDZ for a longer duration (>3 years).
The positive relationship between BDZ use and the risk of dementia was stronger in the case–control studies (RR=1.57) than in the cohort studies (RR=1.26). Such a trend is usually observed when effect estimates for adverse effects are compared between case–control and cohort studies. A methodological review performed by Golder et al. 14 found that the estimates for adverse effects were slightly larger (although not significantly) for case–control than cohort studies. It is generally considered that case–control studies have a higher risk of bias than cohort studies and are more susceptible to selection and recall bias.
The RR values for long-term BDZ use (=1.21) and the use long-acting BDZ (=1.16) were lower than the primary pooled RR (ever BDZ user vs. never BDZ user; RR=1.51) estimate because the comparator to estimate RR differed for the three estimates, which meant they could not be compared.
The long-term use of BDZ is associated with the accumulation of generalized cognitive deficits that lead to an increased risk of dementia in long-term BDZ users compared to short-term BDZ users. Withdrawal symptoms could be observed in short-term BDZ users, but no accumulation of cognitive deficits. The trend observed in the present analysis is consistent with the explanation given above.
This studies included the following three categories of BDZ half-life values: 1) short-acting BDZs (half-life <12 h) such as midazolam and triazolam, 2) intermediate-acting BDZs (half-life=12–24 h) such as alprazolam, clonazepam, lorazepam, oxazepam, temazepam, lormetazepam, and flunitrazepam, and 3) long-acting BDZs (half-life >24 h) such as chlordiazepoxide, flurazepam, diazepam, nitrazepam, and quazepam. The pooled analysis showed that the use of long-acting BDZ is associated with a nonsignificant increase in the risk of dementia compared to patients taking short- or medium-term-acting BDZ.
The results of the meta-analysis should be interpreted carefully since statistically significant heterogeneity was observed in the included studies. The source of heterogeneity in the pooled studies was not revealed in the predefined subgroup analysis. However, the study characteristics in Table 1 and and2 2 provide interesting insights that might explain the observed heterogeneity. The sample sizes of the included studies ranged widely, from 273 to 105,725, and studies with smaller samples had effect estimates with wider 95% CIs or RRs of less than 1. Pooling studies with differing sample sizes might lead to heterogeneity. However, other sources of heterogeneity should not be ignored, including the definition of BDZ exposure and the diagnostic method used to confirm the presence of dementia in the included studies.
A few recently published studies have found no association between BDZ and dementia. Despite this, the pooled evidence shows that risk of dementia is high in patients taking BDZ. 10 , 11 Three associated processes might have impacted the development of dementia in patients using BDZ. One characteristic is that BDZ reduces the level of beta-site amyloid precursor protein-cleaving enzyme 1 and the c-secretase activity that subsequently slows down the buildup of amyloid-beta oligomers in the brain. 15 , 16 Such a possible positive effect along with an antiglutamatergic action of BDZ has never been confirmed. 17 Another possible influencing process is the presence of astrocytes at the amyloid plaques in patients with predementia lesions having GABA-secreting activity, which will enhance the deleterious cognitive effects of BDZ. 18 Another major process via which BDZs might result in dementia is decreasing the brain activation level. 19
The association between BDZs and dementia could be a reverse-causation bias, since the main indications for BDZ (insomnia and anxiety) can also be prodromal of dementia disorders. 6 The main hurdle with observational studies is collecting data to support the possibility of reverse causation over a long period. 20
However, we suggest that bias due to reverse causation was highly unlikely in the present analysis, since BDZs were taken for more than 10 years before the diagnosis of dementia. 2 The relationship between BDZ use and dementia can be considered a marker of the increased risk of the occurrence of dementia, and not as the main cause. However, some studies have recommended that before diagnosing dementia, data from previous years should be analyzed to see if there is a strong correlation between the symptoms of dementia and BDZ prescriptions. 21 , 22 , 23 Such an association can be inferred as a confounding factor based on indication and reverse causation. If reverse causation is a factor, the association of BDZ with dementia should be stronger in short-term users than in long-term past users. Imfeld et al. 10 suggested that in patients using BDZs for <1 year before a diagnosis have an increased risk of Alzheimer's and vascular dementia compared with patients using BDZs for 2–4 years before a dementia diagnosis.
The Caerphilly Prospective Study of Gallacher et al. 2 found a significant positive association between BDZ use and dementia. Those authors also reported that the relationship was strongest for cumulative use over >4 years (OR=4.38, 95% CI=1.15–16.75). The very long follow-up was an undisputable strength of the present study, even if its low statistical power precluded several subanalyses for assessing the plausibility of reverse causation.
In addition to an increased risk of dementia, there is also evidence that BDZs increase the risk of injurious falls, fractures, acute respiratory failure, and delirium, all of which reduce a patient's quality of life and increases the financial burden. 24 , 25 , 26 The ten studies published on the topic were not consistent, with one inferring a protective effect of BDZ, 27 nine finding an increase in dementia disorders in BDZ users, 2 , 3 , 4 , 5 , 6 , 28 , 29 and the tenth finding no relationship. 10 These findings suggest that BDZs should be prescribed only when there is an utmost need for treatment.
A BDZ still might be the drug of choice for treating insomnia in elderly patients, with careful selection of the particular BDZ according to clinical guidelines. Short-acting BDZs should be prescribed as a short-term therapy and progressively withdrawn to avoid cumulative effects such as deceased cognition.
This study was subject to some limitations: 1) all of included studies were observational, which may lead to recall bias, 2) few data on the duration of action of BDZs were included in the studies, and 3) the specific roles of individual BDZs and doses could not be determined since the required data were not reported.
In conclusion, this meta-analysis has yielded evidence that BDZ use is associated with dementia. This association is stronger in people using long-acting BDZs for longer durations. We suggest that ultra-short-acting BDZs should be prescribed and then tapered off while using other therapies in order to avoid dependence and other long-term adverse events. Further prospective long-term studies are required to eliminate reverse-causation bias and to confirm the presence of an association between BDZ and dementia.
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2019.15.1.9 .
NOS quality rating of included studies

COMMENTS
Presentation of Case. Dr. David L. Perez: A 62-year-old, left-handed man was seen in the memory disorders clinic of this hospital because of memory loss, personality changes, and odd behavior ...
CASE PRESENTATION. A 55-year-old right-handed woman presented with a 3-year history of cognitive changes. Early symptoms included mild forgetfulness—for example, forgetting where she left her purse or failing to remember to retrieve a take-out order her family placed—and word-finding difficulties.
Three years before her death informed consent was obtained from the patient and family to perform medical genetic testing for EOAD. The standard panel offered by the laboratory was selected and included PSEN1, PSEN2, APP, and apoE analysis.Tests related to genes related to frontotemporal dementia were not requested based on clinical presentation and clinical judgement.
A nested case control study of early onset clinically diagnosed Alzheimer's disease within an established cohort also found TBI was a risk factor, ... Weight loss in midlife and dementia risk . A meta-analysis of seven RCTs (468 participants) and 13 longitudinal studies (551 participants) of overweight and obese adults without dementia, mean ...
The mean age of onset in PSEN2 mutations, is 55.3 years but the range of onset is surprisingly wide, spanning 39 to 83 years. Over 52% of cases are over 60 years. All cases have extensive amyloid plaque and neurofibrillary tangles, and many have extensive alpha-synuclein pathology as well. 5.
We conducted this study using qualitative methods (interviews, focus groups, and observations) and qualitative case study analysis to understand approaches used by Care Team Navigators to address caregiver burden among caregivers of people with dementia (Figure 1). This study took place within the Care Ecosystem program at the University of ...
We used a case study design, with thematic narrative analysis applied to interview data, to describe the experiences of a wife and son caring for a husband/father with semantic dementia. Using this approach, we identified four themes: (a) living with routines, (b) policing and protecting, (c) making connections, and (d) being adaptive and flexible.
Covering the spectrum of cognitive decline in aging using illustrative cases, from mild impairment to dementia, this set of case studies offers a wide-ranging guide for trainees and clinicians. This second volume includes updated research diagnostic criteria and details of new imaging technology, including novel biomarkers such as PET amyloid ...
Summary. An 85-year-old woman with hypertension and hyperlipidemia presented with gradual and progressive cognitive impairment for more than 2 years, involving cognitive domains of memory, executive function, visuospatial and mood. She has short-term memory loss such as forgetting whether she has eaten or showered.
This collection of case studies from around the world illustrates both common and unusual causes of dementia, emphasizing clinical reasoning, integrative thinking and problem-solving skills. ... The aim is to reinforce diagnostic skills through careful analysis of individual presenting patterns, and to guide treatment decisions, using state-of ...
We used a case study design, with thematic narrative analysis applied to interview data, to describe the experiences of a wife and son caring for a husband/father with semantic dementia. Using this approach, we identified four themes: (a) living with routines, (b) policing and protecting, (c) making connections, and (d) being adaptive and flexible.
Design. Qualitative study using semi-structured interviews, analysed using reflexive thematic analysis [].Recruitment procedure. People living with dementia were purposively sampled from a range of sources including NHS memory services, general practice, carer or dementia organisations, and the National Institute for Health Research (NIHR) Join Dementia Research website.
Methods: Qualitative methods (interviews, focus groups, and case study analysis) were used to identify care navigator approaches used to address caregiver burden in dementia as part of a dementia care navigation program. Results: Care navigators targeted caregiver burden by focusing on strategies to reduce caregiver guilt and frustration ...
This study explored the approaches to dementia case-finding being implemented in acute hospitals in the East of England and explored the views of primary and secondary care staff regarding the benefits and challenges of case-finding for dementia. Our analysis showed that hospitals had developed and implemented different processes in terms of ...
In conclusion, this dementia case study analysis provides valuable insights and lessons for healthcare professionals, caregivers, and families dealing with dementia. It highlights the importance of early detection and diagnosis, as well as the need for comprehensive care plans tailored to the individual's needs.
Each of the 16 case studies was based around an individual who has a diagnosis of dementia and was in paid employment or had left within the previous 18 months. The person with dementia (the participant) then nominated additional participants to inform the case study, including a family member or friend and a workplace representative.
These three case studies help you to consider different situations that people with dementia face. They are: Raj, a 52 year old with a job and family, who has early onset dementia. Bob and Edith, an older married couple who both have dementia and are struggling to cope, along with their family. Joan, an older woman, who lives alone and has just ...
Disability: dementia with Lewy Bodies. First language: English. Family: estranged and in Ireland. Location: village in South West. Joan lives in a house in a small village. She has lived there for 28 years. She has two dogs and 11 chickens. Joan has always been quite private but is well known in her village. She goes to the nearby town on the ...
Study design. Retrospective case/control longitudinal cohort study. Objectives. Prevalent traumatic spinal cord injury (TSCI) is associated with Alzheimer's disease and related dementia (ADRD).
The study team undertook analysis of the full set of data for each case study site individually followed by cross-case analysis. Interview, focus group and training observation data were analysed using the thematic analysis method, template analysis [ 31 , 32 ] using NVivo 11 [ 33 ].
Part 1: Mavis. Mavis is an 84-year old lady, referred to you in the memory clinic for assessment of memory impairment. She attends in the company of her son and daughter-in-law. On the pre-clinic questionnaire her son has reported a severe deterioration in all aspects of her cognition over the past 12 months.
This study aimed to investigate if spatiotemporal gait variables could be used to differentiate between the three cognitive status groups. Methods: Ninety-three older adults were screened and classified into three groups; mild cognitive impairment (MCI) (n = 32), dementia (n = 31), and a cognitively intact (n = 30).
Mild cognitive impairment has received widespread attention as a high-risk population for Alzheimer's disease, and many studies have developed or validated predictive models to assess it. However, the performance of the model development remains unknown. The objective of this review was to provide an overview of prediction models for the risk of Alzheimer's disease dementia in older adults ...
One study adopted 18F-florbetaben PET imaging in Down syndrome patients, suggesting potential role of amyloid imaging in identifying population at risk of dementia. 17 Similar study was conducted on patients with Down syndrome, but with 18F-florbetapir tracer. 18 An attempt to differentiate Down Syndrome pathology from AD has also been made ...
Key Points. Question Is cessation of metformin therapy associated with dementia incidence, and is the association mediated by hemoglobin A 1c (HbA 1c) level or insulin use?. Findings This cohort study of 12 220 early terminators and 29 126 routine users of metformin found that cessation of metformin therapy without abnormal kidney function markers was associated with 1.21 times the hazard of ...
Forest plot representing RR estimates with 95% CIs of each study and combined RR based on 10 studies (6 case-control and 4 cohort studies). Squares indicate RR in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the length of horizontal lines represents the 95% CI.