- See us on facebook
- See us on instagram
- See us on twitter

Research Studies
Currently Recruiting or Active Research Studies
Please download the document below for our current recruiting studies organized by age range.
Study Title
Study description, spark (simons powering autism research) study.
Available in English and Spanish.
If you or your child has a professional diagnosis of autism, Stanford University invites you to learn more about SPARK, a new online research study sponsored by the Simons Foundation Autism Research Initiative. The mission of SPARK is clear: speed up research and advance understanding of autism by creating the nation’s largest autism study. Joining SPARK is simple – register online and provide a DNA sample via a saliva collection kit in the comfort of your own home. Together, we can help spark a better future for all individuals and families affected by autism.
Register by contacting us at [email protected] or online at www.sparkforautism.org/stanford .
SPARK está trabajando para fomentar la investigación y mejorar nuestra comprensión del autismo. Stanford y más de 30 de las principales escuelas de medicina y centros de investigación del autismo del país forman parte de este esfuerzo.
- Participar en SPARK es gratis y se puede hacer completamente desde casa.
- Muchas de las encuestas de SPARK aportan informes personalizados.
- Los participantes serán notificados en caso de haber otras oportunidades de investigación.
- Los individuos con autismo podrán recibir códigos de regalo de Amazon por un valor de hasta 50 dólares (uno por familia) después de la recepción de sus muestras de saliva.
Para inscribirse en SPARK: https://sparkforautism.org/Stanford/ES
La inscripción suele llevar unos 20 minutos y puede empezar y parar si lo necesita. Una vez que se registre y complete unos cuestionarios en línea, le enviaremos un kit para recolectar saliva a su domicilio. Para obtener más información, envíe un correo electrónico a [email protected]
Language Treatment Trial for Children with Autism
Researchers at Stanford University are currently recruiting children with autism spectrum disorder to identify MRI-based markers of response to treatment with Pivotal Response Treatment (PRT) targeting language abilities. Children with autism spectrum disorder between the ages of 2 and 4 years 11 months are invited to participate. This study involves up to a 5 month time commitment. The participant must be willing to complete cognitive and behavioral assessments (such as IQ and language testing) and be able to either sleep (young children) or lie still in the scanner during an MRI. After a successful MRI, the participant will be randomized into the PRT trial or DTG (Delayed Treatment Group). PRT will consist of 16 weekly, 60-90 minute sessions of parent training in PRT over a 16 week time period. DTG will consist of your child’s treatments as usual in the community and measurements and questionnaires will need to be filled out on three study visits over the course of the 16 weeks. After completion of the DTG, the participant will be offered PRT parent training sessions similar to the PRT group. There is no cost to participate in the study. If you would like to participate or if you have any questions please call (650) 736-1235 or email: [email protected] to discuss the study in more detail.
2 and 4 years,11 months
Targeting the Neurobiology of Restricted and Repetitive Behaviors in Children with Autism Using N-acetylcysteine Randomized Control Trial
We are recruiting children autism to participate in a study examining the treatment effects of an over-the-counter dietary supplement on the brain.
Eligibility: Children with autism spectrum disorder who -
· are aged between 3 and 12 years old
· exhibit restricted and repetitive behaviors
· will drink N-acetyl cysteine dissolved in water
· will undergo brain scanning (asleep or awake) with magnetic resonance imaging (MRI)
· will undergo brain scanning with electroencephalography (EEG)
The study will take place over 3 to 6 visits (some remotely over Zoom) and the approximate time required is about 10 to 12 hours. Individuals that are able to complete both of the MRI/EEG sessions will be compensated $50.
You can find more information about our NAC studies at https://redcap.link/NACforAutism .
If you have any questions please call 650-736-1235 or email: [email protected] .
3 to 12 years
Autism Center of Excellence Sleep Study
Dear Parents,
We are excited to tell you about a new research study for children. We are looking to partner with parents who have children that are between the ages of 4 and 17 years old, with and without an Autism Spectrum Disorder (ASD) diagnosis.
What is involved?
- In-person cognitive and behavioral assessments
- Day-time Electroencephalogram (EEG)
- In-home, 2 night sleep monitoring session
- Collection of saliva to measure cortisol and melatonin levels
- Wearing a watch device that tracks sleep and daily activity
What will I receive if I participate?
- Research sleep report and behavioral testing summary upon request
- $50 for each in-person visit to Stanford and $100 for the 2 night in-home sleep assessment
Treatment extension study:
- If your child has ASD, sleep difficulties, and ages 8-17, they may also qualify for sleep medication trials
Interested in participating or want to learn more? Click Here!
If you would like to reach out to our team directly with any questions, please contact our team below!
Email: [email protected]
650-498-7215
4 to 17 years
Pregnenolone Randomized Controlled Trial
Neurosteroid Pregnenolone Treatment for Irritability in Adolescents with Autism
Medication treatments for core symptoms of autism spectrum disorder (ASD) continue to be unmet medical needs. The only medications approved by the U.S. Food and Drug Administration (FDA) for the treatment of individuals with ASD are effective in treating irritability and associated aggressive behaviors, but these medications can also cause severe long-term side effects such as diabetes and involuntary motor movements. Therefore, effective medications with more tolerable side effect profiles are highly desirable. This profile is consistent with pregnenolone (PREG). PREG belongs to a new class of hormones known as neurosteroids, which have been shown to be effective in treating various psychiatric conditions including bipolar depression and schizophrenia. As compared to currently FDA-approved medications, our preliminary data suggested that PREG may represent a potentially effective and well-tolerated agent for treating irritability in individuals with ASD. In addition, our experience suggests that PREG might be helpful in improving selected core symptoms such as social deficits and sensory abnormalities of ASD. This study provides the opportunity to further explore the usefulness of PREG in the treatment of irritability and some core symptoms of ASD. We are performing a 12-week randomized double-blind controlled pilot trial to examine the effectiveness of orally administered PREG in reducing irritability and associated behaviors in adolescents with ASD. In this study, we also aim to examine the usefulness of biomarkers (blood levels of neurosteroids, eyetracking and brain wave recording) in predicting treatment response and assessing biologic changes with PREG treatment.
Link to study in Stanford's Clinical Trials Directory
14 to 25 years
Trial of Center-Based vs. In-Home Pivotal Response Treatment (PRT) in Autism (PRT-HvC)
Do you have a child (2-5 years old) with autism and want an intensive center-based or in-home intervention?
Stanford University researchers are recruiting children with autism and their parents to participate in a study examining the effectiveness of a center-based vs. in-home Pivotal Response Treatment (PRT) program in targeting social communication abilities in young children with autism.
Participants must:
- Be diagnosed with Autism Spectrum Disorder
- Be between the ages of 2 years and 5 years 11 months
- Be able to attend 3-hour research treatment sessions 4 days per week and participate in parent training
Based on behavioral screening assessments, children who are eligible will be randomly assigned to either center-based intervention, in-home intervention, or treatment as usual. Those assigned to the treatment-as-usual group will receive treatment after the 16–week period is completed.
Call 650-736-1235 or email [email protected] to learn more.
https://clinicaltrials.gov/ct2/show/NCT04899544
2 to 5 years
Improving Access to Pivotal Response Treatment (PRT) via Telehealth Parent Training
There is an urgent need for improved access to effective autism treatments. With advances in technology, distance learning models have particular promise for families who cannot access evidence-based parent training locally or may be on long wait-lists for behavioral treatments. Pivotal Response Treatment (PRT) is an established treatment for autism spectrum disorder (ASD); however, a telehealth PRT model has not yet been evaluated in a controlled trial. This study will examine the effects of training parents in PRT via secure video conferencing and investigate 1) whether parents can learn via telehealth to deliver PRT in the home setting (PRT-T) and 2) whether their children will show greater improvement in functional communication skills compared to children in a waitlist control group. Participants will include 40 children age 2 to 5 years with ASD and significant language delay. Eligible children will be randomly assigned to either PRT-T or waiting list. Weekly 60-minute parent training sessions will be delivered for 12 weeks via secure video conferencing software by a PRT-trained study therapist. Link: https://clinicaltrials.gov/ct2/show/NCT04042337
Note: Participants must live at least 200 miles away from Stanford University (i.e., this study is geared towards out-of-state families or families living at a distance)
A Center Based Randomized Controlled Trial of Pivotal Response Treatment for Preschoolers With Autism
Researchers at Stanford University are currently recruiting children with autism and their parents to participate in a study examining the effectiveness of a center-based Pivotal Response Treatment (PRT) program in targeting social communication abilities in young children with autism. We are currently recruiting children diagnosed with ASD and social communication deficits, aged 2:0 to 3:11 years. Children who are eligible based on behavioral screening assessments will be randomly assigned to either an immediate treatment (PRT) group or a delayed treatment group (DTG). If randomized into the PRT group, the 12-week treatment will consist of a combination of one weekly 60-minute individual parent training session and 12 weekly hours (approximately 3 hours per day for 4 days per week) with your child in a center-based group preschool environment at Stanford University. If randomized into the delayed treatment group, the children will wait 12 weeks to receive the PRT treatment and continue any treatment they are receiving as usual in the community. The cost of clinic-based services varies based on individual family health insurance plans.
For more information, please call (650) 736-1235 or email [email protected] to discuss the study in more detail.
2 and 3 years,11 months
Natural History Study of Individuals with Autism and Germline Heterozygous PTEN Mutations
The goal of this study is to gain a better understanding of PTEN mutation syndromes to identify early markers and ultimately effective interventions for autism spectrum disorder. Individuals 18 months or older are eligible to participate if they have been diagnosed with PTEN hamartoma tumor syndrome. The study involves five visits over a two year period. Three of the visits occur on-site at a study location. The other two visits occur as phone calls. The on-site visits include a blood draw, physical/neurological exams and behavioral testing.
Study Webpage
18 months and older
Active Studies, not Recruiting
An open-label pilot study of esomeprazole in children with autism.
Researchers at Stanford University are currently examining the effectiveness of esomeprazole in improving social communication deficits in children with Autism Spectrum Disorder (ASD). Esomeprazole is currently FDA-approved for children ages 1 and up for gastroesophageal reflux disease (GERD) and has been identified as a potential treatment for improving social communication in children with ASD. Children with ASD ages 2 through 6 years are invited to participate. The child must be willing to take esomeprazole orally for at least 8 weeks, complete diagnostic and behavioral assessments, and be free of serious medical problems. There is also an optional research blood draw. The study will require visits to Stanford University and the parent/caregiver will be required to complete questionnaires for each visit.
For more information, please go to https://is.gd/ASDstudy , call (650) 736-1235, or email [email protected] .
2 to 6 years
Vasopressin Treatment Trial for Children with Autism
The purpose of this clinical trial is to investigate the effectiveness of vasopressin nasal spray for treating symptoms associated with autism. Vasopressin is a hormone that is produced naturally within the body and has been implicated in regulating social behaviors. It has been proposed that administration of the hormone may also help improve social functioning in individuals with autism.
Link to study at clinicaltrials.gov
6 to 17 years

Autism Spectrum Disorder
The National Institute of Mental Health (NIMH) , a component of the National Institutes of Health ( NIH ), is a leading federal funder of research on ASD .
What is autism spectrum disorder?
Autism spectrum disorder (ASD) refers to a group of complex neurodevelopment disorders caused by differences in the brain that affect communication and behavior. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—a guide created by the American Psychiatric Association used to diagnose health conditions involving changes in emotion, thinking, or behavior (or a combination of these)—people with ASD can experience:
- Challenges or differences in communication and interaction with other people
- Restricted interests and repetitive behaviors
- Symptoms that may impact the person's ability to function in school, work, and other areas of life
ASD can be diagnosed at any age but symptoms generally appear in early childhood (often within the first two years of life). Doctors diagnose ASD by looking at a person's behavior and development. The American Academy of Pediatrics recommends that all children get screened for developmental delays and behaviors often associated with ASD at their 18- and 24-month exams.
The term “spectrum” refers to the wide range of symptoms, skills, and levels of ability in functioning that can occur in people with ASD. ASD affects every person differently; some may have only a few symptoms and signs while others have many. Some children and adults with ASD are fully able to perform all activities of daily living and may have gifted learning and cognitive abilities while others require substantial support to perform basic activities. A diagnosis of ASD includes Asperger syndrome, autistic disorder, childhood disintegrative disorder, and pervasive developmental disorder not otherwise specified that were once diagnosed as separate disorders.
In addition to differences or challenges with behavior and difficulty communicating and interacting with others, early signs of ASD may include, but are not limited to:
- Avoiding direct eye contact
- Delayed speech and language skills
- Challenges with nonverbal cues such as gestures or body language
- Showing limited interest in other children or caretakers
- Experiencing stress when routines change
Scientists believe that both genetics and environment likely play a role in ASD. ASD occurs in every racial and ethnic group, and across all socioeconomic levels. Males are significantly more likely to develop ASD than females.
People with ASD also have an increased risk of having epilepsy. Children whose language skills regress early in life—before age 3—appear to have a risk of developing epilepsy or seizure-like brain activity. About 20 to 30 percent of children with ASD develop epilepsy by the time they reach adulthood.
Currently, there is no cure for ASD. Symptoms of ASD can last through a person's lifetime, and some may improve with age, treatment, and services. Therapies and educational/behavioral interventions are designed to remedy specific symptoms and can substantially improve those symptoms. While currently approved medications cannot cure ASD or even treat its main symptoms, there are some that can help with related symptoms such as anxiety, depression, and obsessive-compulsive disorder. Medications are available to treat seizures, severe behavioral problems, and impulsivity and hyperactivity.
How can I or my loved one help improve care for people with autism spectrum disorder?
Consider participating in a clinical trial so clinicians and scientists can learn more about ASD and related conditions. Clinical trials are studies that use human volunteers to look for new or better ways to diagnose, treat, or cure diseases and conditions.
All types of volunteers are needed—people with ASD, at-risk individuals, and healthy volunteers—of all different ages, sexes, races, and ethnicities to ensure that study results apply to as many people as possible, and that treatments will be safe and effective for everyone who will use them.
For information about participating in clinical research visit NIH Clinical Research Trials and You . Learn about clinical trials currently looking for people with ASD at Clinicaltrials.gov .
Where can I find more information about autism spectrum disorder? The following resources offer information about ASD and current research: American Academy of Pediatrics Centers for Disease Control and Prevention (CDC) Eunice Kennedy Shriver National Institute of Child Health and Human Development Interagency Autism Coordinating Committee (IACC) National Center for Advancing Translational Sciences National Institute on Deafness and Other Communication Disorders National Institute of Environmental Health Sciences The National Task Group on Intellectual Disabilities and Dementia Practices (NTG) Additional organizations offer information, research news, and other resources about ASD for individuals and caregivers, such as support groups. These organizations include: Association for Science in Autism Treatment Autism National Committee (AUTCOM) Autism Network International (ANI) Autism Research Institute (ARI) Autism Science Foundation Autism Society Autism Speaks, Inc.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
Autism Spectrum Disorder
What is asd.
Autism spectrum disorder (ASD) is a neurological and developmental disorder that affects how people interact with others, communicate, learn, and behave. Although autism can be diagnosed at any age, it is described as a “developmental disorder” because symptoms generally appear in the first 2 years of life.
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) , a guide created by the American Psychiatric Association that health care providers use to diagnose mental disorders, people with ASD often have:
- Difficulty with communication and interaction with other people
- Restricted interests and repetitive behaviors
- Symptoms that affect their ability to function in school, work, and other areas of life
Autism is known as a “spectrum” disorder because there is wide variation in the type and severity of symptoms people experience.
People of all genders, races, ethnicities, and economic backgrounds can be diagnosed with ASD. Although ASD can be a lifelong disorder, treatments and services can improve a person’s symptoms and daily functioning. The American Academy of Pediatrics recommends that all children receive screening for autism. Caregivers should talk to their child’s health care provider about ASD screening or evaluation.
What are the signs and symptoms of ASD?
The list below gives some examples of common types of behaviors in people diagnosed with ASD. Not all people with ASD will have all behaviors, but most will have several of the behaviors listed below.
Social communication / interaction behaviors may include:
- Making little or inconsistent eye contact
- Appearing not to look at or listen to people who are talking
- Infrequently sharing interest, emotion, or enjoyment of objects or activities (including infrequent pointing at or showing things to others)
- Not responding or being slow to respond to one’s name or to other verbal bids for attention
- Having difficulties with the back and forth of conversation
- Often talking at length about a favorite subject without noticing that others are not interested or without giving others a chance to respond
- Displaying facial expressions, movements, and gestures that do not match what is being said
- Having an unusual tone of voice that may sound sing-song or flat and robot-like
- Having trouble understanding another person’s point of view or being unable to predict or understand other people’s actions
- Difficulties adjusting behaviors to social situations
- Difficulties sharing in imaginative play or in making friends
Restrictive / repetitive behaviors may include:
- Repeating certain behaviors or having unusual behaviors, such as repeating words or phrases (a behavior called echolalia)
- Having a lasting intense interest in specific topics, such as numbers, details, or facts
- Showing overly focused interests, such as with moving objects or parts of objects
- Becoming upset by slight changes in a routine and having difficulty with transitions
- Being more sensitive or less sensitive than other people to sensory input, such as light, sound, clothing, or temperature
People with ASD may also experience sleep problems and irritability.
People on the autism spectrum also may have many strengths, including:
- Being able to learn things in detail and remember information for long periods of time
- Being strong visual and auditory learners
- Excelling in math, science, music, or art
What are the causes and risk factors for ASD?
Researchers don’t know the primary causes of ASD, but studies suggest that a person’s genes can act together with aspects of their environment to affect development in ways that lead to ASD. Some factors that are associated with an increased likelihood of developing ASD include:
- Having a sibling with ASD
- Having older parents
- Having certain genetic conditions (such as Down syndrome or Fragile X syndrome)
- Having a very low birth weight
How is ASD diagnosed?
Health care providers diagnose ASD by evaluating a person’s behavior and development. ASD can usually be reliably diagnosed by age 2. It is important to seek an evaluation as soon as possible. The earlier ASD is diagnosed, the sooner treatments and services can begin.
Diagnosis in young children
Diagnosis in young children is often a two-stage process.
Stage 1: General developmental screening during well-child checkups
Every child should receive well-child check-ups with a pediatrician or an early childhood health care provider. The American Academy of Pediatrics recommends that all children receive screening for developmental delays at their 9-, 18-, and 24- or 30-month well-child visits, with specific autism screenings at their 18- and 24-month well-child visits. A child may receive additional screening if they have a higher likelihood of ASD or developmental problems. Children with a higher likelihood of ASD include those who have a family member with ASD, show some behaviors that are typical of ASD, have older parents, have certain genetic conditions, or who had a very low birth weight.
Considering caregivers’ experiences and concerns is an important part of the screening process for young children. The health care provider may ask questions about the child’s behaviors and evaluate those answers in combination with information from ASD screening tools and clinical observations of the child. Read more about screening instruments on the Centers for Disease Control and Prevention (CDC) website.
If a child shows developmental differences in behavior or functioning during this screening process, the health care provider may refer the child for additional evaluation.
Stage 2: Additional diagnostic evaluation
It is important to accurately detect and diagnose children with ASD as early as possible, as this will shed light on their unique strengths and challenges. Early detection also can help caregivers determine which services, educational programs, and behavioral therapies are most likely to be helpful for their child.
A team of health care providers who have experience diagnosing ASD will conduct the diagnostic evaluation. This team may include child neurologists, developmental pediatricians, speech-language pathologists, child psychologists and psychiatrists, educational specialists, and occupational therapists.
The diagnostic evaluation is likely to include:
- Medical and neurological examinations
- Assessment of the child’s cognitive abilities
- Assessment of the child’s language abilities
- Observation of the child’s behavior
- An in-depth conversation with the child’s caregivers about the child’s behavior and development
- Assessment of age-appropriate skills needed to complete daily activities independently, such as eating, dressing, and toileting
Because ASD is a complex disorder that sometimes occurs with other illnesses or learning disorders, the comprehensive evaluation may include:
- Blood tests
- Hearing test
The evaluation may lead to a formal diagnosis and recommendations for treatment.
Diagnosis in older children and adolescents
Caregivers and teachers are often the first to recognize ASD symptoms in older children and adolescents who attend school. The school’s special education team may perform an initial evaluation and then recommend that a child undergo additional evaluation with their primary health care provider or a health care provider who specialize in ASD.
A child’s caregivers may talk with these health care providers about their child’s social difficulties, including problems with subtle communication. For example, some children may have problems understanding tone of voice, facial expressions, or body language. Older children and adolescents may have trouble understanding figures of speech, humor, or sarcasm. They also may have trouble forming friendships with peers.
Diagnosis in adults
Diagnosing ASD in adults is often more difficult than diagnosing ASD in children. In adults, some ASD symptoms can overlap with symptoms of other mental health disorders, such as anxiety disorder or attention-deficit/hyperactivity disorder (ADHD).
Adults who notice signs of ASD should talk with a health care provider and ask for a referral for an ASD evaluation. Although evaluation for ASD in adults is still being refined, adults may be referred to a neuropsychologist, psychologist, or psychiatrist who has experience with ASD. The expert will ask about:
- Social interaction and communication challenges
- Sensory issues
- Repetitive behaviors
- Restricted interests
The evaluation also may include a conversation with caregivers or other family members to learn about the person’s early developmental history, which can help ensure an accurate diagnosis.
Receiving a correct diagnosis of ASD as an adult can help a person understand past challenges, identify personal strengths, and find the right kind of help. Studies are underway to determine the types of services and supports that are most helpful for improving the functioning and community integration of autistic transition-age youth and adults.
What treatment options are available for ASD?
Treatment for ASD should begin as soon as possible after diagnosis. Early treatment for ASD is important as proper care and services can reduce individuals’ difficulties while helping them build on their strengths and learn new skills.
People with ASD may face a wide range of issues, which means that there is no single best treatment for ASD. Working closely with a health care provider is an important part of finding the right combination of treatment and services.
A health care provider may prescribe medication to treat specific symptoms. With medication, a person with ASD may have fewer problems with:
- Irritability
- Repetitive behavior
- Hyperactivity
- Attention problems
- Anxiety and depression
Read more about the latest medication warnings, patient medication guides, and information on newly approved medications at the Food and Drug Administration (FDA) website .
Behavioral, psychological, and educational interventions
People with ASD may be referred to a health care provider who specializes in providing behavioral, psychological, educational, or skill-building interventions. These programs are often highly structured and intensive, and they may involve caregivers, siblings, and other family members. These programs may help people with ASD:
- Learn social, communication, and language skills
- Reduce behaviors that interfere with daily functioning
- Increase or build upon strengths
- Learn life skills for living independently
Other resources
Many services, programs, and other resources are available to help people with ASD. Here are some tips for finding these additional services:
- Contact your health care provider, local health department, school, or autism advocacy group to learn about special programs or local resources.
- Find an autism support group. Sharing information and experiences can help people with ASD and their caregivers learn about treatment options and ASD-related programs.
- Record conversations and meetings with health care providers and teachers. This information may help when it’s time to decide which programs and services are appropriate.
- Keep copies of health care reports and evaluations. This information may help people with ASD qualify for special programs.
How can I find a clinical trial for ASD?
Clinical trials are research studies that look at new ways to prevent, detect, or treat diseases and conditions. The goal of clinical trials is to determine if a new test or treatment works and is safe. Although individuals may benefit from being part of a clinical trial, participants should be aware that the primary purpose of a clinical trial is to gain new scientific knowledge so that others may be better helped in the future.
Researchers at NIMH and around the country conduct many studies with patients and healthy volunteers. We have new and better treatment options today because of what clinical trials uncovered years ago. Be part of tomorrow’s medical breakthroughs. Talk to your health care provider about clinical trials, their benefits and risks, and whether one is right for you.
To learn more or find a study, visit:
- NIMH’s Clinical Trials webpage : Information about participating in clinical trials
- Clinicaltrials.gov: Current Studies on ASD : List of clinical trials funded by the National Institutes of Health (NIH) being conducted across the country
Where can I learn more about ASD?
Free brochures and shareable resources.
- Autism Spectrum Disorder : This brochure provides information about the symptoms, diagnosis, and treatment of ASD. Also available en español .
- Digital Shareables on Autism Spectrum Disorder : Help support ASD awareness and education in your community. Use these digital resources, including graphics and messages, to spread the word about ASD.
Federal resources
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Neurological Disorders and Stroke
- National Institute on Deafness and Other Communication Disorders
- Centers for Disease Control and Prevention (CDC)
- Interagency Autism Coordinating Committee
- MedlinePlus (also available en español )
Research and statistics
- Science News About Autism Spectrum Disorder : This NIMH webpage provides press releases and announcements about ASD.
- Research Program on Autism Spectrum Disorders : This NIMH program supports research focused on the characterization, pathophysiology, treatment, and outcomes of ASD and related disorders.
- Statistics: Autism Spectrum Disorder : This NIMH webpage provides information on the prevalence of ASD in the U.S.
- Data & Statistics on Autism Spectrum Disorder : This CDC webpage provides data, statistics, and tools about prevalence and demographic characteristics of ASD.
- Autism and Developmental Disabilities Monitoring (ADDM) Network : This CDC-funded program collects data to better understand the population of children with ASD.
- Biomarkers Consortium - The Autism Biomarkers Consortium for Clinical Trials (ABC-CT) : This Foundation for the National Institutes of Health project seeks to establish biomarkers to improve treatments for children with ASD.
Last Reviewed: February 2024
Unless otherwise specified, the information on our website and in our publications is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.
Unraveling the Endocannabinoid System: Exploring Its Therapeutic Potential in Autism Spectrum Disorder
- Open access
- Published: 14 May 2024
- Volume 26 , article number 20 , ( 2024 )
Cite this article
You have full access to this open access article

- Ankit Jana 1 ,
- Arnab Nath 2 ,
- Palash Sen 3 ,
- Swikriti Kundu 4 ,
- Badrah S. Alghamdi 5 , 6 ,
- Turki S. Abujamel 7 , 8 ,
- Muhammad Saboor 9 ,
- Chan Woon-Khiong 1 ,
- Athanasios Alexiou ORCID: orcid.org/0000-0002-2206-7236 10 , 11 , 12 , 13 ,
- Marios Papadakis ORCID: orcid.org/0000-0002-9020-874X 14 ,
- Mohammad Zubair Alam 5 , 15 &
- Ghulam Md Ashraf ORCID: orcid.org/0000-0002-9820-2078 9
187 Accesses
Explore all metrics
The salient features of autism spectrum disorder (ASD) encompass persistent difficulties in social communication, as well as the presence of restricted and repetitive facets of behavior, hobbies, or pursuits, which are often accompanied with cognitive limitations. Over the past few decades, a sizable number of studies have been conducted to enhance our understanding of the pathophysiology of ASD. Preclinical rat models have proven to be extremely valuable in simulating and analyzing the roles of a wide range of established environmental and genetic factors. Recent research has also demonstrated the significant involvement of the endocannabinoid system (ECS) in the pathogenesis of several neuropsychiatric diseases, including ASD. In fact, the ECS has the potential to regulate a multitude of metabolic and cellular pathways associated with autism, including the immune system. Moreover, the ECS has emerged as a promising target for intervention with high predictive validity. Particularly noteworthy are resent preclinical studies in rodents, which describe the onset of ASD-like symptoms after various genetic or pharmacological interventions targeting the ECS, providing encouraging evidence for further exploration in this area.
Similar content being viewed by others

Prenatal Exposure to COVID-19 mRNA Vaccine BNT162b2 Induces Autism-Like Behaviors in Male Neonatal Rats: Insights into WNT and BDNF Signaling Perturbations

An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder

Autism Spectrum Disorders and ADHD: Overlapping Phenomenology, Diagnostic Issues, and Treatment Considerations
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) refers to a group of neurodevelopmental disorders that involve significant difficulties in communication and social interaction, as well as the presence of restricted, repetitive, and stereotyped patterns of behavior. Along with these defining features, ASD is commonly associated with a rage of comorbidities, including aggression, hyperactivity, seizures, depression, sleep disturbances, gastrointestinal problems, and immunological malfunction. The global incidence rate of ASD is estimated to be approximately 1%, with a male-to-female ratio of approximately 3:1 (Werling & Geschwind, 2013 ). Despite its significant public health impact and high prevalence rates, the pathogenesis of ASD remains poorly understood, in large part due to ASD’s complicated genetic and environmental interactions. Recent literature suggests that ASD is characterized by impairments in synaptic function, which are believed to contribute to the core symptoms of the disorder. Consequent to the observed synaptic impairments in ASD, the endocannabinoid system (ECS) has gained significant attention as a potential contributor to the initiation and/or progression of the disorder. This is because the ECS has the ability to modulate a variety of synaptic mechanisms, including neurotransmission, synaptic currents, inhibition (E/I balance), and neuroplasticity. Moreover, the ECS has been implicated in several processes that are frequently affected in individuals with ASD, such as social communication, motor control, repetitive behaviors, emotional control, as well as learning, and memory (Zou et al., 2021 ).
ECS comprises cannabinoid receptors CB1, found as a neuronal target of the psychoactive ingredient of Cannabis sativa , 9-tetrahydrocannabinol (THC), and CB2, and their endogenous lipid ligands, i.e., the endocannabinoids (eCBs). These eCBS include anandamide (AEA) and 2-arachidonoylglycerol (2-AG), which are synthesized on demand and function as retrograde neurotransmitters (Pascucci et al., 2020 ; Su et al., 2021 ). It is noteworthy that the ECS provides a critical link between the immune system and the central nervous system (CNS). CB2 receptors are primarily found on immune cells and modulate the immune system, whereas CB1 receptors are found abundantly in the CNS (particularly in the hippocampus, cerebral cortex, basal ganglia, and cerebellum), and peripheral nervous system (PNS).
The involvement of the ECS in ASD extends beyond its influence on synaptic function and neuroplasticity. Indeed, emotional and behavioral responses to social and environmental stimuli as well as modulation of learning, memory, seizure susceptibility, and circadian rhythm, are also thought to be regulated by the ECS in ASD (Marco & Laviola, 2012 ; Marsicano & Lutz, 2006 ; Rubino et al., 2008 ; Trezza & Vanderschuren, 2008 ; Trezza et al., 2012 ). This highlights the broad impact that the ECS has on various physiological and behavioral processes that are disrupted in individuals with ASD. In addition to preclinical studies, human neuroimaging research has also uncovered the relationships between polymorphisms in the CB1 receptor gene, CNR1 , and social reward sensitivity, suggesting that variations in CB1 receptors could contribute to ASD-related irregularities in social reward processing. Furthermore, postmortem analysis of the brains diagnosed with ASD has revealed lower CB1 receptor expression, adding further support to the notion that the ECS plays a key role in the pathogenesis of ASD (Aishworiya et al., 2022 ; Baron-Cohen, 2004 ; Chakrabarti et al., 2006 ; Domschke et al., 2008 ). Regardless of the fact that these data imply the involvement of the ECS in ASD, there is still a dearth of research exploring the role of ECS in ASD, and our knowledge of the EC signaling in ASD remains limited.
This review focuses on studies investigating ECS alterations and the effects of pharmacological modulation of the ECS in animal models of ASD. In addition, the potential of the ECS as a therapeutic target for ASD is discussed.
Genetic Model of ASD and ECS
Variations in the genes encoding CB1 receptors have a significant impact on social behavior in individuals. The observed reduction in CB1 receptors levels among ASD patients suggests a direct association between them. However, studies have shown that CB1 receptor ligand AEA is present in relatively lower amounts in ASD children, whereas the 2-AG level remains unchanged (Pietropaolo et al., 2020 ).
The CB2 receptor was first identified in 1993 through cDNA-based polymerase chain reaction (PCR) clone of the human promyelocytic leukemic line HL60, using degenerative primers (Munro et al., 1993 ). CB2 receptors belong to the G-protein-coupled receptor family and are composed of an internal C-terminal, three extracellular and three intracellular loops, seven transmembrane domains, and an external N-terminal. CB2 receptors show approximately 44% amino acid sequence similarity to CB1 receptors with hydrophobic domains 1, 2, 5, 6, 7, and the extracellular domain of both receptors sharing at least 50% similarity in amino acid sequence (Matsuda, 1997 ). The CNR2 gene is highly conserved across different taxa. The human CNR2 gene consists of a single translated exon flanked by 5' and 3' untranslated regions and a single untranslated exon (Sipe et al., 2005 ). However, transcriptional products of mammalian CB2 receptor genes (CNR2) vary among species. The mouse (23 kb) and rat (20 kb) CB2 receptor genes are almost four times shorter than the human CB2 receptor gene (90 kb). The mouse CB2 receptor gene is transcribed into two mRNAs from three exons, whereas the rat CB2 receptor gene can be spliced into four mRNAs from three exons (Cording & Bateup, 2023 ; Onaivi et al., 2013 ). Compared to human CB2 receptors, the amino acid sequence homology is lower between human and mouse CB2 receptors (82%) than between human and rat CB2 receptors (93%). Although polymorphism of the CNR2 gene is not well studied, but it may be associated with depression in humans. However, Karsak et al. reported that CNR2 gene polymorphism correlates with osteoporosis and other autoimmune diseases (Karsak et al., 2005 ).
Further studies in the Japanese population showed that Q63R polymorphism of the CNR2 gene is linked with alcoholism (Ishiguro et al., 2007 ) and depression (Onaivi et al., 2013 ). CNR2 gene expression in peripheral immune cells prevents inflammation and neuronal damage and exerts specific changes in the central nervous system. Activated glial cells, NK cells, and monocytes show the highest levels of CB2 receptors indicating that CB2 receptors may be a key player in cytokines release and immune cell migration during different pathophysiological conditions. CB2 receptors have been found to be upregulated during inflammation in other brain-associated cells and have been shown to play a vital role in reducing depression in rodents (Morcuende et. al 2022 , Garcia-Gutierrezet. al 2018 , Onaivi et. al 2008 ). Furthermore, the expression level of the CNR2 gene has been shown to increase in the hippocampus of offspring exposed to VPA (Onaivi, 2006 ). The evidence presented suggests that overexpression of CNR2 gene could be a potential therapeutic approach for treating inflammation and depression in autistic children. CNR2 and FAAH genes are closely linked in mice and humans. It has been shown that anandamide-deactivating enzyme FAAH inhibition can ameliorate social disabilities in ASD-related models BTBR and fmr1 −/− mice (Wei et al., 2016 ). It is likely that bidirectional modulation of CNR2 and FAAH genes would likely increase social interaction and reduce anxiety and neuroinflammatory responses in autistic children.
In addition, Fragile X Syndrome (FXS) is one of the significant monogenetic reasons for ASD. FXS occurs due to mutation in the Fmr1 gene on the X chromosome, which leads to reduction or absence of the FMRP protein (Zou et al., 2021 ). FMRP is instrumental for the normal development of synapses in the brain, and its absence or reduction can cause various symptoms such as developmental delay, anxiety, intellectual and physical disabilities, and repetitive behaviors among others (Garber et al., 2008 ). FXS also leads to ASD in at least 30% of cases, and the Fmr1 knockout mouse is considered a model system for FXS (Hagerman et al., 2010 ; Kazdoba et al., 2014 ). Patients suffering from FXS have an impaired endocannabinoid signaling system (Zhang & Alger, 2010 ). Moreover, studies have shown that modulation of either CB1 or CB2 receptors in the Fmr1 knockout mouse can improve some behavioral symptoms associated with ASD (Arnau Busquets-Garcia et al., 2013 ). JZL184 increases CB1 receptors through the 2-AG signaling pathway, and its application in Fmr1 knockout mouse led to decrease in behavioral abnormalities (Fig. 1 ) (Arnau Busquets-Garcia et al., 2013 ; Jung et al., 2012 ). In addition, the deletion of the CB1 receptor gene (CN1R) or pharmacological blockage of CB1 receptors resulted in reduced cognitive and seizure-related neurological problems in Fmr1 knockout mouse. However, rimonabant, a promising CB1 targeting drug, has been associated with severe adverse effects (Maria Gomis-González et al., 2016 ). Modulation of AEA levels may be a potential therapeutic approach. FAAH inhibitors increase the level of AEA, and their application improves cognitive and social behavioral problems in Fmr1 knockout mice (Fig. 1 ) (Qin et al., 2015 ). Furthermore, treatment with the CB2 receptor agonist AM630 has shown to ameliorate anxiety and audiogenic seizure behaviors in the Fmr1 knockout mouse model (Fig. 1 ). Therefore, drugs that can alter the efficiency of CB2 receptors may be a potential therapeutic target to cure ASD-related behavioral traits (Busquets-Garcia et al., 2013 ).

Simplified representation of the endocannabinoid (EC) system upon nerve stimulus. The endocannabinoids (AEA and 2-AG) trigger the CB1 receptors of presynaptic neurons. 2-AG is generated through hydrolysis of DAG by the DAGLα and DAGLβ enzymes, whereas AEA is synthesized through the action of NAPE-PLD enzyme. Membrane depolarization or nerve stimulation elevates the intracellular Ca 2+ level that induces the 2-AG and AEA production in postsynaptic neurons. Retrograde attachment of AEA and 2-AG to CB1 receptors initiates downstream pathways that lowers Ca 2+ , reduces neurotransmitter release and leads to endocannabinoid degradation via MAGL and FAAH. Rimonabant and NESS3027 are two potential inhibitors of CB1 receptors. CB2 receptors are preferentially found in immune cells and reduce IL-1 expression. AM630 blocks CB2 receptors
Neuroligins (NLGNs) are a group of postsynaptic cell adhesion molecules. They control the maturity and function of excitatory and inhibitory synapses in the mammalian brain (Jamain et al., 2008 ; Südhof, 2008 ). Mutations of NLGN3 and NLGN4 in humans are associated with seizures, X-linked intellectual disability, and autistic behavior. In particular, the Arg 451 Cys (R451C) missense mutation of NLGN3 has been linked with ASD in humans. Similarly, NLGN3 mutant mice with the R 451 C mutation show impaired social communication, increased synaptic inhibition in the somatosensory cortex, and excitatory transmission in the hippocampus (Etherton et al., 2011 ). NLGN3 mutant mice model not only expresses partial characteristics of ASD condition, but it also provides sufficient information about synaptic gene regulation and ASD (Radyushkin et al., 2009 ). Studies on NLGN3R451C knock-in and NLGN3 knockout mouse models have shown that disruption of tonic EC signaling mediated by CB1 receptors in the hippocampus and the somatosensory cortex causes autistic behaviors (Zamberletti et al., 2017 ). Although CB2 receptors do not have a direct role in controlling NLGNs associated ASD-like phenotypes, a combination of drugs modulating both CB1 and CB2 receptors may be a possible pharmacological approach to mitigate ASD-related symptoms. Alteration in CB2 receptors activity and AEA metabolism have been observed in blood monocyte-derived macrophages and peripheral blood mononuclear cells of ASD patients (Siniscalco et al., 2013 ). This evidence suggests that CB2 and other endocannabinoid signaling compounds may play a critical role in influencing ASD-related symptoms. Nevertheless, zebrafish and humans share similarities in the endocannabinoid pathway (Bailone et al., 2022 ), including CB1, CB2 receptors, as well as key enzymes of the endocannabinoid system, such as prostaglandin-endoperoxide synthase 2, fatty acid amide hydrolase, and transient receptor potential Cation Channel 1A (Elphick, 2012 ; Klee et al., 2012 ; Lam et al., 2006 ). Studies using the zebrafish model have established that the CB2 inverse agonist JTE‐907 acts as an anxiogenic agent, while the non‐selective CB agonist WIN 55,212 has anxiolytic effects (Hasumi et al., 2020 ; Prasad et al., 2020 ).
Role of ECS in Pathophysiology of ASD
Uncovering the etiopathogenesis of ASD is extremely challenging because this ailment arises from a complex interplay of multiple genetic and environmental factors that act through a multitude of complicated disease mechanisms, such as imbalances between neuronal excitation and inhibition and hypo- and/or hyper-connectivity (Pardo & Eberhart, 2007 ). As mentioned earlier, the genetics of ASD are extremely diverse, involving hundreds of ASD susceptibility genes (Roux et al., 2019 ). The intricacy appears to preclude any simple characterization of pathophysiological mechanisms that can explain the interactions and permutative effects of polygenic mutations, as well as the role of environmental impact (McOmish et al., 2014 ). However, successful understanding of the etiopathology of ASD can immediately lead to the discovery of new therapeutic options that can treat the root cause of ASD. Currently, existing therapeutic interventions for ASD patients only target peripheral symptoms such as anxiety, irritability, aggression, and seizures, and they are treated with anxiolytics, antipsychotics, and anticonvulsants, in that order. These symptom-focused treatments do not address the root cause of ASD, and they are linked with severe side effects that are particularly undesirable in children. To solve the pressing need for better treatments, animal research is focused on identifying new molecular targets for potent interventions by dissecting prevalent ASD etiopathological pathways.
The ECS has demonstrated pathogenetic significance and potential as a novel therapeutic target in our search for shared contributing factors for ASD.
Firstly, CB1Rs are extensively expressed in the brain (Mackie, 2005 ). Second, the ECS has emerged as an essential modulator of neuronal function. Endocannabinoid signaling affects synaptogenesis and neural interconnectedness throughout development. Impairment of these pathways underlies the pathophysiology of autism spectrum disorder. As shown in Fig. 2 , CB1 receptors are present presynaptically in both GABAergic and glutamatergic neurons. They are triggered by endogenous ligands such as AEA and 2-AG (Berghuis et al., 2007 ; FREUND et al., 2003 ). After membrane depolarization or excitation of metabotropic receptors, endogenous cannabinoids including AEA and 2-AG are generated at the postsynaptic location. This generation is caused by calcium elevation, which can induce plasma membrane lipid reconfiguration. The biosynthetic enzyme N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) produces AEA, while diacylglycerol lipase alpha (DAGL-a) produces 2-AG (FREUND et al., 2003 ). These endogenous cannabinoids retrogradely attach to presynaptic CB1 receptors, activating multiple intracellular pathways that lower intracellular calcium levels and restrict neurotransmitter release. Endogenous cannabinoids are eliminated through a reuptake mechanism. AEA is decomposed by fatty acid amide hydrolase (FAAH), and 2-AG by monoacyl-glycerol lipase (MAGL).

Role of Endocannabinoid System in Pathophysiology of ASD. CB1 and CB2 receptors can work as a potential therapeutic target of ASD. Rimonabant & NESS0327 targets CB1; whereas AM630 targets CB2
In recent times, behavioral conditions including depression, autism, and schizophrenia have been linked to dopamine abnormalities. Evidence from neurochemistry demonstrates that activation of CB1R expression on GABA neurons in the ventral tegmental area (VTA) reduces GABAergic transmission, which in turn increases dopaminergic neurotransmission in the nucleus accumbens (NAc) (Sperlágh et al., 2009 ). Clarifying the connection between ECS and DA in ASD may aid in improving our knowledge of the etiopathogenesis of the disorder and may lead to the development of new treatment approaches, as dopamine signaling anomalies have been linked to ASD in both animal models (Pascucci et al., 2020 ) and autistic individuals (Su et al., 2021 ).
Neuroimmunology of ASD and ECS
Microglia and macrophage are considered to be the key immune cells in repairing CNS injuries and infections, as they mediate phagocytosis of pathogens and initiate neuroinflammatory responses by releasing cytokines such as IL-1, IL-6, TNFα, etc. (Janda et al., 2018 ; Yang et al., 2010 ). In recent times, neuroimmunologists have been largely focusing on microglia, the resident immune population of brain parenchyma, which is classified as mononuclear phagocytes, including monocyte-derived cells, dendritic cells, peripheral and CNS associated macrophages (Gomez Perdiguero et al., 2013 ; Prinz et al., 2011 ). Microglia are derived from a common pool immune progenitors found in the fetal yolk sac, which also give rises to astrocytes and oligodendrocytes (Ginhoux et al., 2010 , 2013 ). After entering the CNS at embryonic day 9.5, microglia arrive in the CNS before astrocytes and even before the commencement of true cortical neurogenesis, which begins at approximately embryonic day 12 (Hughes et al., 2023 ; Miller & Gauthier, 2007 ).
Microglial Involvement and Biology of ASD
Positron emission tomography (PET) and post-mortem analyses have both showed high levels of neuroinflammation and increased microglia activation in the brains of individuals with ASD, indicating the microglial involvement in ASD (Morgan et al., 2010 ; Vargas et al., 2005 ). Recently, a distinct microglial signature has been observed from large-scale transcriptomic data analysis from post-mortem cerebral cortex (Suzuki et al., 2013 ). Changes in synaptic density have also been observed in post-mortem ASD brain tissues (Hutsler & Zhang, 2010 ), and in ASD mouse models (Comery et al., 1997 ; Hughes et al., 2023 ; Tang et al., 2014 ; Wang et al., 2017 ). These changes may be due to defects in developmental synaptic pruning (Hansel, 2019 ). Indeed, current evidence suggests that microglia may contribute to ASD progression through dysregulation of synaptic pruning (Di Marco et al., 2016 ; Lenz & Nelson, 2018 ). This hypothesis is supported by the finding that inhibition of microglia autophagy increases synaptic density and reduces sociability in mice (Kim et al., 2017 ). Studies on the mouse model of Rett syndrome (RTT), a syndromic form of ASD caused by mutations in the methyl-CpG binding protein 2 (MECP-2) encoding gene, provide additional support for the involvement of microglia in ASD pathogenesis (Lombardi et al., 2015 ). One model of RTT showed that neuronal loss of MECP-2 caused increased synaptic engulfment by microglia in subsequent stages of disease, although microglia themselves did not exhibit any loss (Schafer et al., 2016 ). This suggests that neuronal loss of MECP-2 alone is sufficient to induce aberrant microglial activity.
In ASD, aggregated alpha-synuclein released from dying dopaminergic neurons activates microglia, leading to high production of proinflammatory cytokines and reactive oxygen species (ROS) which is one of the hallmarks of ASD. Transforming growth factor beta (TGF-β) is one of the factors involved in ASD. Lower levels of TGF-β have been observed in ASD children with high behavioral scores. In addition, macrophage inhibitory factor (MIF), which plays a role in the neural and endocrine systems, is also associated with ASD (Fingerle-Rowson & Bucala, 2001 ). Two polymorphisms in the promoter region of MIF linked with autism have been observed in genotypic studies of 1000 families (Grigorenko et al., 2008 ). Differences in NK cell activity have also been observed in ASD patients with some studies showing reduced cytotoxic activity of NK cells in ASD children compared to control. Toll-like receptors (TLRs) expressed by monocytes also act as markers of ASD. TLR-2 and TLR-4 stimulation of monocytes produce proinflammatory cytokines in ASD individuals compared to controls. However, TLR-9 stimulation showed decreased production of proinflammatory cytokines in ASD compared with non-ASD patients, suggesting that monocytes from ASD children have different response in stimulating innate immunity compared with controls. Recent studies on post-mortem brain and spinal cord samples from 11 individual with ASD have revealed high activation of microglia and astroglia, as well as increased levels of cytokines monocyte chemotactic protein-1 (MCP-1) and TGF-beta compared to control (Vargas et al., 2005 ). After measuring the cytokine levels in brain samples of individuals with ASD in comparison to age and sex-matched non-ASD individuals, there was a significant increase in proinflammatory and Th1 cytokines. These studies provide a clear insight into the immune status of ASD, and due to its anti-inflammatory activity, the ECS can be considered a promising tool for modulating microglial involvement in ASD.
Microglial-Endocannabinoid Signaling and ASD
Preclinical evidence supporting a role of ECS signaling in ASD comes from studies in rodent models of MIA and neuroinflammation (Hughes et al., 2023 ; Salloum-Asfar et al., 2023 ). For example, the production of MIA-based IL-17 in response to the innate immune stimulator polyinosine:polycytidylic acid [poly(I:C)] induces abnormal cortex development and ASD-like sociability deficits in mouse offspring (Gunn et al., 2016 ). Recent in vivo and in vitro studies suggest that ECS plays an outstanding role in communication between the nervous and immune systems during neuronal damage and inflammation in the CNS. Some studies have reported the proinflammatory role of cannabis in protecting against the activation of microglia with a complex mechanism (Bailone et al., 2022 ; Killestein et al., 2003 ; Maestroni, 2004 ). Moreover, proinflammatory cytokines such as IL-6, IL-12, and TNF-α can cause neuroinflammation and neurodegeneration (Wang et al., 2015 ). The activation of ECS has been identified as one of the mechanisms that protect against the detrimental effects of these proinflammatory cytokines. During inflammation, 2-AG, which is a ligand of endocannabinoid receptor, is released from various immune cells and induces neuroprotection through several mechanisms by binding to endocannabinoid receptor (Zou & Kumar, 2018 ). Microglial cells and astrocytes produce 2-AG in response to intracellular Ca +2 and glutamate receptor stimulation, which stimulates purinergic P2X7 receptor (Carrier et al., 2004 ; Hu et al., 2022 ). The ECS, specially CB2 receptor, mediates T and B lymphocytes proliferation, apoptosis, macrophage-mediated killing of sensitized cells, production of inflammatory cytokines by the inhibiting cyclic AMP/ Protein kinase A (PKA) pathways, migration of B cells, and cytokines induction. Dendritic cells also have the capability to undergo cannabinoid-induced apoptosis due to their immunosuppressive properties (Do et al., 2004 ; Hu et al., 2022 ). So, ECS can be a crucial target for ASD therapies due to its anti-inflammatory and immunosuppressive effects. Targeting the ES receptor can lead to side effects like inhibition of learning and memory, hence the ES CB2 receptor, which is present in microglia, is being targeted nowadays. High levels of mRNA and protein of the CB2 receptor have been observed in the blood of autistic children, suggesting its essential role in ASD (Hu et al., 2022 ; Siniscalco et al., 2013 ). Various pharmacological molecules can be used to reduce microglia-mediated neurodegeneration and inflammation and beta-amyloid induced neurotoxicity by modulating various ECS receptors like CB1, CB2 and unknown receptors. In the future, we should focus on altered eCB signaling in microglia and the mechanism by which it yields protective and detrimental effects in CNS to provide improved therapies for ASD patients(Kibret et al., 2023 ).
CBS as a Potential Therapeutic Target of ASD
Several animal models of ASD have shown variations in ECS functionality through various techniques. The Fmr1-KO mouse model of FXS, a monogenic developmental condition linked to ASD, has been investigated the most regarding the ECS in relation to ASD models. Since FXS also lacks therapies, researchers have also explored the ECS for potential medications. In Fmr1-KO mice, aberrations and imbalances in EC signaling have been observed, indicating that their correction via 2-AG, AEA, and cannabinoid CB1 and CB2 receptors may have medical benefits. First, the link between hippocampus mGluR activation and CB mobilization was strengthened in Fmr1-KO mice, while CB1R expression was unaffected (Zamberletti et al., 2017 ). In addition, an increase in striatal diacylglycerol lipase activity was observed (Maccarrone et al., 2010 ). Furthermore, the use of the drug JZL184 (which inhibits the breakdown of 2-AG by MAGL) to enhance 2-AG signaling corrected hyperactivity and anxiety in Fmr1-KO mice (Jung et al., 2012 ). In addition, it has been demonstrated that modulating AEA signaling can improve certain behavioral characteristics of Fmr1-deficient animals. In a study, a single injection of the FAAH blocker URB597 improved unpleasant memory and anxiety-like behavior in Fmr1-deficient mice without impairing their social behavior (Qin et al., 2015 ). In contrast, Wei et al . discovered that acute injection of URB597 to inhibit FAAH completely corrected the social deficit in Fmr1 deletion mice, indicating that boosting AEA activity at CB1 receptors may exhibit a prosocial behavior effect in mouse models of ASD (Wei et al., 2016 ). Furthermore, activation of either CB1 or CB2 receptors was found to alleviate certain behavioral symptoms of Fmr1-deficient animals. In the murine model, genetic and pharmacological inhibition of CB1 receptors with the CB1 receptor antagonist/inverse agonist rimonabant reversed cognitive impairments, epilepsy susceptibility, and nociceptive desensitization. Biochemically, CB1 receptor inhibition in the hippocampus of Fmr1 mutant mice corrected the overactivation of mTOR signaling and dendritic spine formation. Intriguingly, treatment with the CB2 inverse agonist AM630 had no effect on anxiety-like behavior or audiogenic seizure susceptibility, indicating that CB1 and CB2 receptors play distinct roles in the behavioral symptoms of FXS (Busquets-Garcia et al., 2013 ). A recent study by Gomis-Gonzales et al. validated the favorable effect of blocking CB1 receptors on the cognitive function of Fmr1 knockout mice. The authors demonstrated that low doses of rimonabant and the neutral antagonist NESS0327 prevented cognitive abnormalities in Fmr1 knockout mice, as determined by the novel object recognition test. Interestingly, the cognitive benefits of rimonabant were associated with the restoration of mGluR-LTD in the hippocampus of Fmr1-deficient animals (Gomis-González et al., 2016 ).
In addition, the VPA rat model has been widely used to assess the potential implications of the ECS in ASD. Rats treated with a single injection of VPA on gestational day 12.5 (GD 12.5) exhibited decreased mRNA expression of the enzyme primarily responsible for producing 2-AG DAGL, in the cerebellum, and enhanced activity of the 2-AG-catabolizing enzyme, MAGL in the hippocampus (Kerr et al., 2013 ). Gene expression of CB1 and CB2 receptors were unaffected; however, rats prenatally exposed to VPA showed altered expression of phosphorylated CB1 receptor in the amygdala, hippocampus, and dorsal striatum, with no alterations in the prefrontal cortex, cerebellum, and nucleus accumbens (Mangiatordi et al., 2023 ; Servadio et al., 2016 ). Modifications were also observed in the expression of other receptor targets for ECs, namely PPAR and GPR55 in the frontal cortex and PPAR and GPR55 in the hippocampus (Kerr et al., 2013 ). AEA and its congeners, oleoylethanolamide (OEA), and palmitoylethanolamide (PEA) were increased in the hippocampus of VPA-exposed rats immediately after social exposure, indicating that prenatal VPA exposure may have altered AEA signaling in response to interpersonal stimuli (Kerr et al., 2013 ). Rats exposed to VPA exhibited alterations in AEA metabolism from early life to adulthood. In fact, decreased expression of NAPE-PLD and increased expression of FAAH were observed in whole brains of rats treated with VPA (Servadio et al., 2016 ).
It is worth noting that increasing AEA signaling by inhibiting its degradation has been shown to alleviate the behavioral phenotype resulting from prenatal VPA exposure. Specifically, a systemic injection of the FAAH antagonist PF3845 at a dose of 10 mg/kg was found to ameliorate the social impairment observed in male mice exposed to VPA (Kerr et al., 2016 ). In comparison, PF3845 had no effect on the social behavior of female mice exposed to VPA, suggesting that FAAH inhibition may induce sexually dimorphic behaviors in VPA-exposed female mice (Kerr et al., 2016 ). Likewise, URB597 treatment improved the interaction problems of VPA-exposed pups in the homing test and reversed their social deficiencies in the three-chamber and social play behavior tests (Mechoulam, 2023 ; Servadio et al., 2016 ).
Moreover, recently Schiavi et al. examined the role of endocannabinoid neurotransmission in autism-like feautures in Fmr1-Δexon 8 rats. Their study revealed reduced anandamide in the hippocampus and elevated 2-arachidonoylglycerol (2-AG) in the amygdala of these rats. Increasing anandamide levels lessened cognitive abnormalities, while blocking amygdalar 2-AG transmission improved sociability in the rats, as demonstrated by behavioral tests (Schiavi et al., 2023 ). Considering the pivotal role of endocannabinoids in the etiopathology of ASD described in this article, it is not astonishing that researchers have investigated the therapeutic potential of certain phytocannabinoids, such as cannabidiol (Hill et al., 2023 ; Parrella et al., 2023 ; Shani Poleg et al., 2019 ). Although cannabidiol has only a weak affinity for CB1 receptors, it has been found to inhibit FAAH, which is responsible for the breakdown of AEA (Cristino et al., 2020 ; Parrella et al., 2023 ). This is thought to be particularly beneficial for individuals with ASD, who have been shown to have lower levels of AEA (Aishworiya et al., 2022 ; Aran et al., 2019 ). According to preliminary findings, cannabidiol has reduced symptoms of hyperactivity, self-injurious behaviors, anxiety, and sleep problems in ASD children. Recent clinical trials have not only demonstrated the effectiveness of cannabidiol in treating ASD symptoms, but also cognitive symptoms in individuals with FXS, without any adverse effects (Heussler et al., 2019 ; Tartaglia et al., 2019 ).
Although post-natal LPS injection is not widely accepted as a model of ASD, additional studies suggest that suppressing FAAH may be a treatment option for diseases characterized by reduced social behavior. In mice exposed postnatally to LPS, disruptions to the ECS have been described (Doenni et al., 2016 ; Mondal et al., 2023 ). Early-life inflammation caused by a single LPS administration on post-natal day (PND) 14 impaired both male and female adolescent social play and non-play behavior. Interpersonal impairments caused by LPS were associated with decreased CB1 receptor binding, higher AEA levels, and, interestingly, elevated FAAH activity in the amygdala. Prior to the social interaction test, oral administration of 1 mg/kg of the FAAH inhibitor PF-04457845 restored LPS-induced abnormalities in social behavior. A similar improvement was noticed following direct administration of PF-04457845 into the basolateral amygdala, suggesting that altered AEA signaling in this brain area play an important role in transmitting LPS-induced social deficits in at least female mice (Doenni et al., 2016 ; Shamabadi et al., 2024 ).
Medical Cannabinoid and Risks
Agonists and antagonists of CB1 and CB2 have the potential to act as drug targets for ASD. However, there are several challenges in designing CB2 receptors-modulating drugs to alleviate ASD symptoms. CB2 receptors, like other lipid-binding receptors, bind to multiple non-specific ligands, making it difficult to design specific agonist and antagonist ligands for CB2 receptors due to off-target effects. The high abundance of CB1 receptors and other lipid-based endocannabinoid receptors also leads to more non-specific binding of CB2 ligands with CB1 receptors, triggering different downstream signaling pathways. Therefore, specific agonists or antagonists targeting CB2 receptors need to be synthesized and undergo multiple in vitro, in vivo, and clinical trials to minimize potential side effects (Atwood & Mackie, 2010 ). Different agonists of CB2 receptors can target other downstream regulatory molecules to modify their function. CB2 agonists display distinct of functional selectivity (Atwood et al., 2012a , 2012b ; Mechoulam, 2023 ; Pinapati et al., 2024 ). For instance, aminoalkylindoles (e.g., WIN55,212-2, AM1241, etc.), which are common CB2 receptor agonists, cannot block calcium channels and do not have any role in CB2 receptor internalization. However, these molecules can activate the MAP kinase pathway and stimulate beta-arrestin2 (Shoemaker et al., 2005 ). They can recruit beta-arrestin2 to the plasma membrane and initiate different downstream signaling pathways. While blocking calcium channels and receptor internalization are crucial components of regulating any signaling pathway, aminoalkylindoles fail to do so (Nguyen et al., 2012 ). Therefore, standard CB2 receptor agonists can only control a portion of the signaling pathway. Similarly, inverse agonists and antagonists of CB2 receptors can also exhibit similar functional selectivity. Inverse agonists are drugs that selectively couple receptors to one type of downstream signaling molecule while reducing their association with other signaling molecules. Different inverse agonists can target different signaling molecules, so it is important to specify which molecules are being regulated and how they affect ASD before using these drugs as potential therapeutics. For example, commonly used CB2 receptor inverse agonists like SR144258 selectively target CB2 receptor internalization, whereas other like AM630 have a neutral effect (Atwood et al., 2012a , 2012b ; Oka et al., 2005 ). It is important to specify the regulatory molecules and their impact on ASD for every agonist and antagonist before using them as potential therapeutics. Further research, animal trials and clinical trials are needed to focus on these areas. Furthermore, functional efficacy is also an essential parameter for the application of any drugs, as the density of receptors and downstream signaling molecules determine the practical impact of ligands. Moreover, functional efficacy varies in different cellular and physiological conditions in GPCR targeting drugs (Efron & Taylor, 2023 ; Strange, 2002 ). In the case of in vitro studies, transfected cells of CB2 receptors are used to evaluate the functional efficacy of ligands. However, the density of CB2 receptors is usually low in physiological conditions. Therefore, it is important to note that any agonists that work effectively in vitro may only act as partial agonists in physiological conditions. On the other hand, antagonists may behave as a reverse agonists in different physiological conditions, which could be additional challenge to the effective function of drugs particularly in cases where there is a higher density of receptors or downstream signaling molecules (Fong & Heymsfield, 2009 ). However, the likelihood of this problem occurring in CB2 receptor antagonists is minimal due to their low physiological density. While drugs targeting CB1 receptors are commonly used to treat different brain pathophysiological diseases, drugs targeting CB2 receptors may have a less significant off-target effect due to their low density in normal physiological conditions. Beside this, using cannabinoid-based drugs to treat children and adolescent patients affected by ASD has raised many social and legal controversies (Bou Khalil, 2012 ). During childhood and adolescence, several critical brain development processes occur, and it is believed that cannabinoid-based drugs may have adverse effects on the brain (Poleg et al., 2019 ) or induce cannabis addiction. Some clinical data also support these arguments (Bailone et al., 2022 ; Parrella et al., 2023 ; Shani Poleg et al., 2019 ; Salloum-Asfar et al., 2023 ). For instance, a preclinical rodent study found that chronic application of CB2 receptors agonist WIN 55,212-2 during puberty resulted in severe behavioral disturbance in adulthood (Schneider & Koch, 2003 ; Schneider et al., 2008 ).
From the cell biological and neuroimmunological perspective, targeting CB2 receptors with agonists and antagonists could offer new therapeutic options for ameliorating ASD-related symptoms. Promising results have been observed in preclinical and in vitro studies. However, the available clinical trials data is disappointing, with many drugs being withdrawn or failing to reach their primary pharmacological targets. This disparity between preclinical and clinical data highlights the need for further pharmacological studies to determine the clinical and functional efficacy of CB2 receptor-based drugs. To develop possible drugs, humanoid rodent models or 3D organoid models could be used for in vitro studies, while considering all aspects of CB2 receptor pharmacological properties. With careful research and development, CB2-based therapeutics may soon become available in clinics, providing a potential cure for ASD patients.
Conclusion & Perspectives
Clinical and preclinical evidence clearly supports the role of the ECS in the etiopathogenesis of ASD and its potential for medication development. According to Geschwind’s “many genes, similar pathways” concept (Geschwind, 2008 ), evidence from ASD-related mouse lines and pharmacological interventions targeting the ECS in wild-type animals suggests that an imbalance in ECS signaling is a possible common etiopathological route of this complex condition. This is consistent with the ECS's substantial modulatory effect on neural functioning and cognitive maturation. However, this field remains unexplored, and many researchers have emphasized the need to understand how ECS failure contributes to aberrant brain maturation. The use of neurons derived from induced pluripotent stem cells is anticipated to provide new insights, as it has already contributed to our understanding of the etiology of various neuropsychiatric disorders, in order to distinguish the significance of ECS in abnormal brain growth from fully developed synaptic functionality (Wen et al., 2014 ; Yeh & Levine, 2017 ).
Although preclinical data suggest that modifying the ECS through the pharmaceutical therapies may be useful for alleviating ASD symptoms, no definitive conclusions can be drawn due to the early stage of research. Evidence suggests that increasing AEA signaling by inhibiting its disintegration promotes prosocial behavior in several mouse models of ASD. Furthermore, acute or chronic inhibition of CB1 receptors has been shown to have positive effects on cognitive impairments in animal models of FXS. Interestingly, medication was systematically administered in most investigations. However, changes in the ECS documented in mouse models of ASD seem to vary depending on the specific brain area studied, suggesting a potential diverse contribution to ASD-like manifestations. If so, it is unlikely that any prospective treatment method would rely on a single targeted molecule.
In this review, we aim to demonstrate that the ECS’s role in ASD is a near foregone conclusion, based on the vast amount of data presented here. However, we do not intend to imply that the ECS alone can explain the etiopathology of ASD. On the contrary, we, along with other experts in the field, are convinced that any comprehensive understanding of ASD must incorporate parallel pathogenic elements. This complex neurodevelopmental disorder is essentially caused by intricate interactions between parallel systems that regulate brain development. The ECS may provide a clue to the identity other major players and the complexity of the situation. Accordingly, drug design should seek new molecular pathways for multi-target pharmacology.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
2-Arachidonoylglycerol
Three dimensional
- Autism spectrum disorder
Adenosine triphosphate
Brain-derived neurotrophic factor
Complementary DNA
CB2 receptor genes
Central nervous system
Diacylglycerol lipase alpha
Endocannabinoids
- Endocannabinoid system
Fatty acid amide hydrolase
Fragile X syndrome
G-protein-coupled receptor 55
Interleukin 1
Interleukin 12
Interleukin 17
Interleukin 1β
Interleukin 6
Lipopolysaccharide
Monoacyl-glycerol lipase
Methyl-CpG binding protein 2
Group I metabotropic glutamate receptor-dependent long-term depression
Macrophage inhibitory factor
Messenger RNA
Mammalian target of rapamycin
N-acyl phosphatidylethanolamine-specific phospholipase D
Natural killer cells
Neuroligins
Oleoylethanolamide
Polymerase chain reaction
Palmitoylethanolamide
Position emission tomography
Post-natal day
Peripheral nervous system
Peroxisome proliferator-activated receptors
Rett syndrome
Transforming growth factor beta
9-Tetrahydrocannabinol
Tandem-pore domain halothane-inhibited K + channel 1
Toll-like receptor
Tumor necrosis factor-alpha
Valproic acid
Aishworiya, R., Valica, T., Hagerman, R., & Restrepo, B. (2022). An update on psychopharmacological treatment of autism spectrum disorder. Neurotherapeutics, 19 (1), 248–262. https://doi.org/10.1007/s13311-022-01183-1
Article CAS PubMed PubMed Central Google Scholar
Aran, A., Eylon, M., Harel, M., Polianski, L., Nemirovski, A., Tepper, S., Schnapp, A., Cassuto, H., Wattad, N., & Tam, J. (2019). Lower circulating endocannabinoid levels in children with autism spectrum disorder. Molecular Autism, 10 (1), 1–11.
Article Google Scholar
Atwood, B. K., & Mackie, K. (2010). CB2: A cannabinoid receptor with an identity crisis. British Journal of Pharmacology, 160 (3), 467–479.
Atwood, B. K., Straiker, A., & Mackie, K. (2012a). CB2: Therapeutic target-in-waiting. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 38 (1), 16–20. https://doi.org/10.1016/j.pnpbp.2011.12.001
Article CAS PubMed Google Scholar
Atwood, B. K., Wager-Miller, J., Haskins, C., Straiker, A., & Mackie, K. (2012b). Functional selectivity in CB2 cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of CB2 ligands. Molecular Pharmacology, 81 (2), 250–263.
Bailone, R. L., Fukushima, H. C. S., De Aguiar, L. K., & Borra, R. C. (2022). The endocannabinoid system in Zebrafish and its potential to study the effects of Cannabis in humans. Laboratory Animal Research, 38 (1), 1–12.
Baron-Cohen, S. (2004). Autism: Research into causes and intervention. Pediatric Rehabilitation, 7 (2), 73–78.
Article PubMed Google Scholar
Berghuis, P., Rajnicek, A. M., Morozov, Y. M., Ross, R. A., Mulder, J., Urbán, G. M., Monory, K., Marsicano, G., Matteoli, M., Canty, A., Irving, A. J., Katona, I., Yanagawa, Y., Rakic, P., Lutz, B., Mackie, K., & Harkany, T. (2007). Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science, 316 (5828), 1212–1216. https://doi.org/10.1126/science.1137406
Bou Khalil, R. (2012). Would some cannabinoids ameliorate symptoms of autism? European Child & Adolescent Psychiatry, 21 (4), 237–238.
Busquets-Garcia, A., Gomis-González, M., Guegan, T., Agustín-Pavón, C., Pastor, A., Mato, S., Pérez-Samartín, A., Matute, C., De La Torre, R., & Dierssen, M. (2013). Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nature Medicine, 19 (5), 603–607.
Carrier, E. J., Kearn, C. S., Barkmeier, A. J., Breese, N. M., Yang, W., Nithipatikom, K., Pfister, S. L., Campbell, W. B., & Hillard, C. J. (2004). Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Molecular Pharmacology, 65 (4), 999–1007. https://doi.org/10.1124/mol.65.4.999
Chakrabarti, B., Kent, L., Suckling, J., Bullmore, E., & Baron-Cohen, S. (2006). Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. European Journal of Neuroscience, 23 (7), 1944–1948.
Comery, T. A., Harris, J. B., Willems, P. J., Oostra, B. A., Irwin, S. A., Weiler, I. J., & Greenough, W. T. (1997). Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proceedings of the National Academy of Sciences USA, 94 (10), 5401–5404.
Article CAS Google Scholar
Cording, K. R., & Bateup, H. S. (2023). Altered motor learning and coordination in mouse models of autism spectrum disorder. Frontiers in Cellular Neuroscience, 17 , 1270489. https://doi.org/10.3389/fncel.2023.1270489
Cristino, L., Bisogno, T., & Di Marzo, V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews Neurology, 16 (1), 9–29. https://doi.org/10.1038/s41582-019-0284-z
Di Marco, B., Bonaccorso, C. M., Aloisi, E., Antoni, S., & Catania, M. V. (2016). Neuro-inflammatory mechanisms in developmental disorders associated with intellectual disability and autism spectrum disorder: A neuro-immune perspective. CNS & Neurological Disorders-Drug Targets, 15 (4), 448–463.
Do, Y., McKallip, R. J., Nagarkatti, M., & Nagarkatti, P. S. (2004). Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. The Journal of Immunology, 173 (4), 2373–2382. https://doi.org/10.4049/jimmunol.173.4.2373
Doenni, V. M., Gray, J. M., Song, C. M., Patel, S., Hill, M. N., & Pittman, Q. J. (2016). Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain, Behavior, and Immunity, 58 , 237–247. https://doi.org/10.1016/j.bbi.2016.07.152
Domschke, K., Dannlowski, U., Ohrmann, P., Lawford, B., Bauer, J., Kugel, H., Heindel, W., Young, R., Morris, P., & Arolt, V. (2008). Cannabinoid receptor 1 (CNR1) gene: Impact on antidepressant treatment response and emotion processing in major depression. European Neuropsychopharmacology, 18 (10), 751–759.
Efron, D., & Taylor, K. (2023). Medicinal Cannabis for paediatric developmental, behavioural and mental health disorders. International Journal of Environmental Research and Public Health, 20 (8), 5430.
Elphick, M. R. (2012). The evolution and comparative neurobiology of endocannabinoid signalling. Philosophical Transactions of the Royal Society b: Biological Sciences, 367 (1607), 3201–3215.
Etherton, M., Földy, C., Sharma, M., Tabuchi, K., Liu, X., Shamloo, M., Malenka, R. C., & Südhof, T. C. (2011). Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proceedings of the National Academy of Sciences USA, 108 (33), 13764–13769.
Fingerle-Rowson, G. R., & Bucala, R. (2001). Neuroendocrine properties of macrophage migration inhibitory factor (MIF). Immunology and Cell Biology, 79 (4), 368–375. https://doi.org/10.1046/j.1440-1711.2001.01024.x
Fong, T. M., & Heymsfield, S. B. (2009). Cannabinoid-1 receptor inverse agonists: Current understanding of mechanism of action and unanswered questions. International Journal of Obesity, 33 (9), 947–955. https://doi.org/10.1038/ijo.2009.132
Freund, T. F., Katona, I., & Piomelli, D. (2003). Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews, 83 (3), 1017–1066. https://doi.org/10.1152/physrev.00004.2003
Garber, K. B., Visootsak, J., & Warren, S. T. (2008). Fragile X syndrome. European Journal of Human Genetics, 16 (6), 666–672.
Garcia-Gutierrez, M. S., Navarrete, F., Navarro, G., Reyes-Resina, I., Franco, R., Lanciego, J. L., Giner, S., Manzanares, J. (2018). Alterations in gene and protein expression of cannabinoid cb2 and gpr55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics . (2018) 15 , 796–806. https://doi.org/10.1007/s13311-018-0610-y
Geschwind, D. H. (2008). Autism: Many genes, common pathways? Cell, 135 (3), 391–395. https://doi.org/10.1016/j.cell.2008.10.016
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., Mehler, M. F., Conway, S. J., Ng, L. G., & Stanley, E. R. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 330 (6005), 841–845.
Ginhoux, F., Lim, S., Hoeffel, G., Low, D., & Huber, T. (2013). Origin and differentiation of microglia. Frontiers in Cellular Neuroscience, 2013 (7), 45.
Google Scholar
Gomez Perdiguero, E., Schulz, C., & Geissmann, F. (2013). Development and homeostasis of “resident” myeloid cells: The case of the microglia. Glia, 61 (1), 112–120. https://doi.org/10.1002/glia.22393
Gomis-González, M., Busquets-Garcia, A., Matute, C., Maldonado, R., Mato, S., & Ozaita, A. (2016). Possible therapeutic doses of cannabinoid type 1 receptor antagonist reverses key alterations in fragile X syndrome mouse model. Genes (basel) . https://doi.org/10.3390/genes7090056
Grigorenko, E. L., Han, S. S., Yrigollen, C. M., Leng, L., Mizue, Y., Anderson, G. M., Mulder, E. J., de Bildt, A., Minderaa, R. B., Volkmar, F. R., Chang, J. T., & Bucala, R. (2008). Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics, 122 (2), e438-445. https://doi.org/10.1542/peds.2007-3604
Gunn, J. K., Rosales, C. B., Center, K. E., Nuñez, A., Gibson, S. J., Christ, C., & Ehiri, J. E. (2016). Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. British Medical Journal Open, 6 (4), e009986. https://doi.org/10.1136/bmjopen-2015-009986
Hagerman, R., Hoem, G., & Hagerman, P. (2010). Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Molecular Autism, 1 (1), 1–14.
Hansel, C. (2019). Deregulation of synaptic plasticity in autism. Neuroscience Letters, 688 , 58–61. https://doi.org/10.1016/j.neulet.2018.02.003
Hasumi, A., Maeda, H., & Yoshida, K.-I. (2020). Analyzing cannabinoid-induced abnormal behavior in a zebrafish model. PLoS ONE, 15 (10), e0236606.
Heussler, H., Cohen, J., Silove, N., Tich, N., Bonn-Miller, M. O., Du, W., O’Neill, C., & Sebree, T. (2019). A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. Journal of Neurodevelopmental Disorders, 11 (1), 1–9.
Hill, M. N., Haney, M., Hillard, C. J., Karhson, D. S., & Vecchiarelli, H. A. (2023). The endocannabinoid system as a putative target for the development of novel drugs for the treatment of psychiatric illnesses. Psychological Medicine, 53 (15), 7006–7024.
Article PubMed PubMed Central Google Scholar
Hu, C., Li, H., Li, J., Luo, X., & Hao, Y. (2022). Microglia: Synaptic modulator in autism spectrum disorder. Front Psychiatry, 13 , 958661. https://doi.org/10.3389/fpsyt.2022.958661
Hughes, H., Moreno, R., & Ashwood, P. (2023). Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain, Behavior, and Immunity, 108 , 245–254.
Hutsler, J. J., & Zhang, H. (2010). Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Research, 1309 , 83–94.
Ishiguro, H., Iwasaki, S., Teasenfitz, L., Higuchi, S., Horiuchi, Y., Saito, T., Arinami, T., & Onaivi, E. (2007). Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. The Pharmacogenomics Journal, 7 (6), 380–385.
Jamain, S., Radyushkin, K., Hammerschmidt, K., Granon, S., Boretius, S., Varoqueaux, F., Ramanantsoa, N., Gallego, J., Ronnenberg, A., & Winter, D. (2008). Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences USA, 105 (5), 1710–1715.
Janda, E., Boi, L., & Carta, A. R. (2018). Microglial phagocytosis and its regulation: A therapeutic target in Parkinson’s disease? Frontiers in Molecular Neuroscience, 11 , 144.
Jung, K.-M., Sepers, M., Henstridge, C. M., Lassalle, O., Neuhofer, D., Martin, H., Ginger, M., Frick, A., DiPatrizio, N. V., & Mackie, K. (2012). Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature Communications, 3 (1), 1–11.
Karsak, M., Cohen-Solal, M., Freudenberg, J., Ostertag, A., Morieux, C., Kornak, U., Essig, J., Erxlebe, E., Bab, I., & Kubisch, C. (2005). Cannabinoid receptor type 2 gene is associated with human osteoporosis. Human Molecular Genetics, 14 (22), 3389–3396.
Kazdoba, T. M., Leach, P. T., Silverman, J. L., & Crawley, J. N. (2014). Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable & Rare Diseases Research, 3 (4), 118–133.
Kerr, D. M., Downey, L., Conboy, M., Finn, D. P., & Roche, M. (2013). Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behavioural Brain Research, 249 , 124–132. https://doi.org/10.1016/j.bbr.2013.04.043
Kerr, D. M., Gilmartin, A., & Roche, M. (2016). Pharmacological inhibition of fatty acid amide hydrolase attenuates social behavioural deficits in male rats prenatally exposed to valproic acid. Pharmacological Research, 113 (Pt A), 228–235. https://doi.org/10.1016/j.phrs.2016.08.033
Kibret, B. G., Canseco-Alba, A., Onaivi, E. S., & Engidawork, E. (2023). Crosstalk between the endocannabinoid and mid-brain dopaminergic systems: Implication in dopamine dysregulation. Frontiers in Behavioral Neuroscience, 17 , 1137957.
Killestein, J., Hoogervorst, E. L., Reif, M., Blauw, B., Smits, M., Uitdehaag, B. M., Nagelkerken, L., & Polman, C. H. (2003). Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. Journal of Neuroimmunology, 137 (1–2), 140–143. https://doi.org/10.1016/s0165-5728(03)00045-6
Kim, H. J., Cho, M. H., Shim, W. H., Kim, J. K., Jeon, E. Y., Kim, D. H., & Yoon, S. Y. (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry, 22 (11), 1576–1584. https://doi.org/10.1038/mp.2016.103
Klee, E. W., Schneider, H., Clark, K. J., Cousin, M. A., Ebbert, J. O., Hooten, W. M., Karpyak, V. M., Warner, D. O., & Ekker, S. C. (2012). Zebrafish: A model for the study of addiction genetics. Human Genetics, 131 (6), 977–1008.
Lam, C., Rastegar, S., & Strähle, U. (2006). Distribution of cannabinoid receptor 1 in the CNS of zebrafish. Neuroscience, 138 (1), 83–95.
Lenz, K. M., & Nelson, L. H. (2018). Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Frontiers in Immunology, 9 , 698. https://doi.org/10.3389/fimmu.2018.00698
Lombardi, L. M., Baker, S. A., & Zoghbi, H. Y. (2015). MECP2 disorders: From the clinic to mice and back. The Journal of Clinical Investigation, 125 (8), 2914–2923. https://doi.org/10.1172/jci78167
Maccarrone, M., Rossi, S., Bari, M., De Chiara, V., Rapino, C., Musella, A., Bernardi, G., Bagni, C., & Centonze, D. (2010). Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology, 35 (7), 1500–1509. https://doi.org/10.1038/npp.2010.19
Mackie, K. (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. In R. G. Pertwee (Ed.), Cannabinoids (pp. 299–325). Springer.
Chapter Google Scholar
Maestroni, G. J. (2004). The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. The FASEB Journal, 18 (15), 1914–1916. https://doi.org/10.1096/fj.04-2190fje
Mangiatordi, G. F., Cavalluzzi, M. M., Delre, P., Lamanna, G., Lumuscio, M. C., Saviano, M., Majoral, J.-P., Mignani, S., Duranti, A., & Lentini, G. (2023). Endocannabinoid degradation enzyme inhibitors as potential antipsychotics: A medicinal chemistry perspective. Biomedicines, 11 (2), 469.
Marco, E. M., & Laviola, G. (2012). The endocannabinoid system in the regulation of emotions throughout lifespan: A discussion on therapeutic perspectives. Journal of Psychopharmacology, 26 (1), 150–163.
Marsicano, G., & Lutz, B. (2006). Neuromodulatory functions of the endocannabinoid system. Journal of Endocrinological Investigation, 29 (3), 27.
CAS PubMed Google Scholar
Matsuda, L. A. (1997). Molecular aspects of cannabinoid receptors. Critical Reviews™ in Neurobiology, 11 (2–3), 143.
McOmish, C. E., Burrows, E. L., & Hannan, A. J. (2014). Identifying novel interventional strategies for psychiatric disorders: Integrating genomics, ‘enviromics’ and gene–environment interactions in valid preclinical models. British Journal of Pharmacology, 171 (20), 4719–4728. https://doi.org/10.1111/bph.12783
Mechoulam, R. (2023). A delightful trip along the pathway of cannabinoid and endocannabinoid chemistry and pharmacology. Annual Review of Pharmacology and Toxicology, 63 , 1–13.
Miller, F. D., & Gauthier, A. S. (2007). Timing is everything: Making neurons versus glia in the developing cortex. Neuron, 54 (3), 357–369. https://doi.org/10.1016/j.neuron.2007.04.019
Mondal, A., Sharma, R., Abiha, U., Ahmad, F., Karan, A., Jayaraj, R. L., & Sundar, V. (2023). A Spectrum of solutions: Unveiling non-pharmacological approaches to manage autism spectrum disorder. Medicina, 59 (9), 1584.
Morcuende, A., García-Gutiérrez, M. S., Tambaro, S., Nieto, E., Manzanares, J., & Femenia, T. (2022). Immunomodulatory role of CB2 receptors in emotional and cognitive disorders. Frontiers in Psychiatry , 13 , 866052.
Morgan, J. T., Chana, G., Pardo, C. A., Achim, C., Semendeferi, K., Buckwalter, J., Courchesne, E., & Everall, I. P. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological Psychiatry, 68 (4), 368–376.
Munro, S., Thomas, K. L., & Abu-Shaar, M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365 (6441), 61–65.
Nguyen, P. T., Schmid, C. L., Raehal, K. M., Selley, D. E., Bohn, L. M., & Sim-Selley, L. J. (2012). β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biological Psychiatry, 71 (8), 714–724. https://doi.org/10.1016/j.biopsych.2011.11.027
Oka, S., Yanagimoto, S., Ikeda, S., Gokoh, M., Kishimoto, S., Waku, K., Ishima, Y., & Sugiura, T. (2005). Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. Journal of Biological Chemistry, 280 (18), 18488–18497. https://doi.org/10.1074/jbc.M413260200
Onaivi, E. S. (2006). Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology, 54 (4), 231–246.
Onaivi, E. S., Ishiguro, H., Gong, J. P., Patel, S., Meozzi, P. A., Myers, L., Perchuk, A., Mora, Z., Tagliaferro, P. A., Gardner, E., Brusco, A., Akinshola, B. E., Hope, B., Lujilde, J., Inada, T., Iwasaki, S., Macharia, D., Teasenfitz, L., Arinami, T., Uhl, G. R. (2008). Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One . 3 , e1640. https://doi.org/10.1371/journal.pone.0001640
Onaivi, E., Ishiguro, H., Sgro, S., & Leonard, C. (2013). Cannabinoid receptor gene variations in drug addiction and neuropsychiatric disorders. Journal of Drug and Alcohol Research, 2 (1), 1–11.
Pardo, C. A., & Eberhart, C. G. (2007). The neurobiology of autism. Brain Pathology, 17 (4), 434–447. https://doi.org/10.1111/j.1750-3639.2007.00102.x
Parrella, N.-F., Hill, A. T., Enticott, P. G., Barhoun, P., Bower, I. S., & Ford, T. C. (2023). A systematic review of cannabidiol trials in neurodevelopmental disorders. Pharmacology Biochemistry and Behavior, 230 , 173607. https://doi.org/10.1016/j.pbb.2023.173607
Pascucci, T., Colamartino, M., Fiori, E., Sacco, R., Coviello, A., Ventura, R., Puglisi-Allegra, S., Turriziani, L., & Persico, A. M. (2020). P-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the BTBR mouse. Brain Sciences, 10 (4), 233.
Pietropaolo, S., Bellocchio, L., Bouzón-Arnáiz, I., & Yee, B. K. (2020). The role of the endocannabinoid system in autism spectrum disorders: Evidence from mouse studies. Progress in Molecular Biology and Translational Science, 173 , 183–208.
Pinapati, K. K., Vidya, S., Khan, M. F., Mandal, D., & Banerjee, S. (2024). Gut bacteria, endocannabinoid system, and marijuana addiction: Novel therapeutic implications. Health Sciences Review, 10 , 100144.
Poleg, S., Golubchik, P., Offen, D., & Weizman, A. (2019). Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89 , 90–96. https://doi.org/10.1016/j.pnpbp.2018.08.030
Prasad, A., Crowe, M., Burton, G., & McGrew, L. (2020). Anxiolytic effects of cannabinoid receptor agonists in the Zebrafish species, Danio Rerio. The FASEB Journal, 34 (S1), 1–1.
Prinz, M., Priller, J., Sisodia, S. S., & Ransohoff, R. M. (2011). Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nature Neuroscience, 14 (10), 1227–1235. https://doi.org/10.1038/nn.2923
Qin, M., Zeidler, Z., Moulton, K., Krych, L., Xia, Z., & Smith, C. B. (2015). Endocannabinoid-mediated improvement on a test of aversive memory in a mouse model of fragile X syndrome. Behavioural Brain Research, 291 , 164–171.
Radyushkin, K., Hammerschmidt, K., Boretius, S., Varoqueaux, F., El-Kordi, A., Ronnenberg, A., Winter, D., Frahm, J., Fischer, J., & Brose, N. (2009). Neuroligin-3-deficient mice: Model of a monogenic heritable form of autism with an olfactory deficit. Genes, Brain and Behavior, 8 (4), 416–425.
Roux, S., Bailly, Y., & Bossu, J. L. (2019). Regional and sex-dependent alterations in Purkinje cell density in the valproate mouse model of autism. NeuroReport, 30 (2), 82–88. https://doi.org/10.1097/wnr.0000000000001164
Rubino, T., Realini, N., Castiglioni, C., Guidali, C., Vigano, D., Marras, E., Petrosino, S., Perletti, G., Maccarrone, M., & Di Marzo, V. (2008). Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cerebral Cortex, 18 (6), 1292–1301.
Salloum-Asfar, S., Elsayed, A. K., & Abdulla, S. A. (2023). Chapter 6—Potential approaches and recent advances in biomarker discovery in autism spectrum disorders. In A. S. El-Baz & J. S. Suri (Eds.), Neural engineering techniques for autism spectrum disorder (pp. 121–145). Academic Press.
Schafer, D. P., Heller, C. T., Gunner, G., Heller, M., Gordon, C., Hammond, T., Wolf, Y., Jung, S., & Stevens, B. (2016). Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. eLife . https://doi.org/10.7554/eLife.15224
Schiavi, S., Manduca, A., Carbone, E., Buzzelli, V., Rava, A., Feo, A., Ascone, F., Morena, M., Campolongo, P., Hill, M. N., & Trezza, V. (2023). Anandamide and 2-arachidonoylglycerol differentially modulate autistic-like traits in a genetic model of autism based on FMR1 deletion in rats. Neuropsychopharmacology, 48 (6), 897–907. https://doi.org/10.1038/s41386-022-01454-7
Schneider, M., & Koch, M. (2003). Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology, 28 (10), 1760–1769.
Schneider, M., Schömig, E., & Leweke, F. M. (2008). PRECLINICAL STUDY: Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addiction Biology, 13 (3–4), 345–357.
Servadio, M., Melancia, F., Manduca, A., di Masi, A., Schiavi, S., Cartocci, V., Pallottini, V., Campolongo, P., Ascenzi, P., & Trezza, V. (2016). Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid. Translational Psychiatry, 6 (9), e902. https://doi.org/10.1038/tp.2016.182
Shamabadi, A., Karimi, H., Arabzadeh Bahri, R., Motavaselian, M., & Akhondzadeh, S. (2024). Emerging drugs for the treatment of irritability associated with autism spectrum disorder. Expert Opinion on Emerging Drugs . https://doi.org/10.1080/14728214.2024.2313650
Shoemaker, J. L., Ruckle, M. B., Mayeux, P. R., & Prather, P. L. (2005). Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. Journal of Pharmacology and Experimental Therapeutics, 315 (2), 828–838.
Siniscalco, D., Sapone, A., Giordano, C., Cirillo, A., de Magistris, L., Rossi, F., Fasano, A., Bradstreet, J. J., Maione, S., & Antonucci, N. (2013). Cannabinoid receptor type 2, but not type 1, is up-regulated in peripheral blood mononuclear cells of children affected by autistic disorders. Journal of Autism and Developmental Disorders, 43 (11), 2686–2695. https://doi.org/10.1007/s10803-013-1824-9
Sipe, J. C., Arbour, N., Gerber, A., & Beutler, E. (2005). Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: Possible risk for autoimmune disorders. Journal of Leukocyte Biology, 78 (1), 231–238.
Sperlágh, B., Windisch, K., Andó, R. D., & Vizi, E. S. (2009). Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochemistry International, 54 (7), 452–457.
Strange, P. G. (2002). Mechanisms of inverse agonism at G-protein-coupled receptors. Trends in Pharmacological Sciences, 23 (2), 89–95.
Su, T., Yan, Y., Li, Q., Ye, J., & Pei, L. (2021). Endocannabinoid system unlocks the puzzle of autism treatment via microglia. Frontiers in Psychiatry, 12 , 734837.
Südhof, T. C. (2008). Neuroligins and neurexins link synaptic function to cognitive disease. Nature, 455 (7215), 903–911.
Suzuki, K., Sugihara, G., Ouchi, Y., Nakamura, K., Futatsubashi, M., Takebayashi, K., Yoshihara, Y., Omata, K., Matsumoto, K., & Tsuchiya, K. J. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry, 70 (1), 49–58.
Tang, G., Gudsnuk, K., Kuo, S.-H., Cotrina, M. L., Rosoklija, G., Sosunov, A., Sonders, M. S., Kanter, E., Castagna, C., & Yamamoto, A. (2014). Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron, 83 (5), 1131–1143.
Tartaglia, N., Bonn-Miller, M., & Hagerman, R. (2019). Treatment of fragile X syndrome with cannabidiol: A case series study and brief review of the literature. Cannabis and Cannabinoid Research, 4 (1), 3–9.
Trezza, V., Damsteegt, R., Manduca, A., Petrosino, S., Van Kerkhof, L. W., Pasterkamp, R. J., Zhou, Y., Campolongo, P., Cuomo, V., & Di Marzo, V. (2012). Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. Journal of Neuroscience, 32 (43), 14899–14908.
Trezza, V., & Vanderschuren, L. J. (2008). Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (berl), 197 (2), 217–227.
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., & Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology, 57 (1), 67–81. https://doi.org/10.1002/ana.20315
Wang, M., Li, H., Takumi, T., Qiu, Z., Xu, X., Yu, X., & Bian, W.-J. (2017). Distinct defects in spine formation or pruning in two gene duplication mouse models of autism. Neuroscience Bulletin, 33 (2), 143–152.
Wang, W. Y., Tan, M. S., Yu, J. T., & Tan, L. (2015). Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med, 3 (10), 136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
Wei, D., Dinh, D., Lee, D., Li, D., Anguren, A., Moreno-Sanz, G., & Piomelli, D. (2016). Enhancement of anandamide-mediated endocannabinoid signaling corrects autism-related social impairment. Cannabis and Cannabinoid Research, 1 (1), 81–89. https://doi.org/10.1089/can.2015.0008
Wen, Z., Nguyen, H. N., Guo, Z., Lalli, M. A., Wang, X., Su, Y., Kim, N.-S., Yoon, K.-J., Shin, J., & Zhang, C. (2014). Synaptic dysregulation in a human iPS cell model of mental disorders. Nature, 515 (7527), 414–418.
Werling, D. M., & Geschwind, D. H. (2013). Sex differences in autism spectrum disorders. Current Opinion in Neurology, 26 (2), 146.
Yang, I., Han, S. J., Kaur, G., Crane, C., & Parsa, A. T. (2010). The role of microglia in central nervous system immunity and glioma immunology. Journal of Clinical Neuroscience, 17 (1), 6–10.
Yeh, M. L., & Levine, E. S. (2017). Perspectives on the role of endocannabinoids in autism spectrum disorders. OBM Neurobiology, 1 (2), 5.
Zamberletti, E., Gabaglio, M., & Parolaro, D. (2017). The endocannabinoid system and autism spectrum disorders: Insights from animal models. International Journal of Molecular Sciences, 18 (9), 1916.
Zhang, L., & Alger, B. E. (2010). Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. Journal of Neuroscience, 30 (16), 5724–5729.
Zou, M., Liu, Y., Xie, S., Wang, L., Li, D., Li, L., Wang, F., Zhang, Y., Xia, W., & Sun, C. (2021). Alterations of the endocannabinoid system and its therapeutic potential in autism spectrum disorder. Open Biology, 11 (2), 200306.
Zou, S., & Kumar, U. (2018). Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. International Journal of Molecular Sciences . https://doi.org/10.3390/ijms19030833
Download references
Acknowledgements
This research work was funded by the Institutional Fund Projects under grant no. (IFFPP-24-22). Therefore, authors gratefully acknowledge technical and financial support from Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia.
Open Access funding enabled and organized by Projekt DEAL. This research work was funded by the Institutional Fund Projects under Grant No. (IFFPP-24-22). Therefore, authors gratefully acknowledge technical and financial support from Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia. Open Access funding enabled and organized by Projekt DEAL. This work was supported by the University of Witten-Herdecke Germany.
Author information
Authors and affiliations.
Department of Biological Sciences, National University of Singapore, Singapore, 117558, Singapore
Ankit Jana & Chan Woon-Khiong
Department of Developmental Biology and Genetics, Indian Institute of Science, Bangalore, Karnataka, 560012, India
School of Biosciences, Indian Institute of Technology, Kharagpur, West Bengal, 721302, India
Siksha Bhavana, Visva-Bharati University, Bolpur, West Bengal, 731235, India
Swikriti Kundu
Pre-Clinical Research Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
Badrah S. Alghamdi & Mohammad Zubair Alam
Department of Physiology, Neuroscience Unit, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Badrah S. Alghamdi
Vaccines and Immunotherapy Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
Turki S. Abujamel
Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Department of Medical Laboratory Sciences, College of Health Sciences, and Research Institute for Medical and Health Sciences, University of Sharjah, P.O. Box 27272, Sharjah, United Arab Emirates
Muhammad Saboor & Ghulam Md Ashraf
University Centre for Research & Development, Chandigarh University, Chandigarh-Ludhiana Highway, Mohali, Punjab, India
Athanasios Alexiou
Department of Research & Development, Funogen, Athens, Greece
Department of Research & Development, AFNP Med, 1030, Vienna, Austria
Department of Science and Engineering, Novel Global Community Educational Foundation, Hebersham, NSW, 2770, Australia
Department of Surgery II, University Hospital Witten-Herdecke, University of Witten-Herdecke, Heusnerstrasse 40, 42283, Wuppertal, Germany
Marios Papadakis
Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Mohammad Zubair Alam
You can also search for this author in PubMed Google Scholar
Contributions
Ankit Jana: Investigation, Writing—original draft, Writing—review & editing, Visualization, Conceptualization. Arnab Nath: Investigation, Writing—original draft, Writing—review & editing. Palash Sen: Investigation, Writing—original draft. Swikriti Kundu: Writing—review & editing, Artwork. Badrah S. Alghamdi: Artwork, Turki S. Abujamel: Writing—review & editing. Muhammad Saboor: Writing—review & editing. Chan Woon Khiong: Writing—review & editing. Athanasios Alexiou: Writing—review & editing. Marios Papadakis: Conceptualization, Supervision. Mohammad Zubair Alam: Writing-review & editing. Ghulam Md Ashraf: Conceptualization, Supervision.
Corresponding authors
Correspondence to Marios Papadakis or Ghulam Md Ashraf .
Ethics declarations
Competing interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
Consent for Publication
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Jana, A., Nath, A., Sen, P. et al. Unraveling the Endocannabinoid System: Exploring Its Therapeutic Potential in Autism Spectrum Disorder. Neuromol Med 26 , 20 (2024). https://doi.org/10.1007/s12017-024-08781-6
Download citation
Received : 08 December 2023
Accepted : 04 March 2024
Published : 14 May 2024
DOI : https://doi.org/10.1007/s12017-024-08781-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anandamide (AEA)
- 2-Arachidonoylglycerol (2-AG)
- Find a journal
- Publish with us
- Track your research

- About Autism
- Is It Autism?
- Starting Intervention
- Prenatal Factors
- Autism Assessment Tools
- Diagnostic Checklist
- Autism Treatment Evaluation Checklist (ATEC)
- Understanding and Treating Self-Injurious Behavior Tool
- Autism Support
- Expert Webinars
- Newly Diagnosed
- Studies Seeking Participants
- ARI-Funded Research Studies 2023
- ARI Think Tanks
- Participate in Studies
- ARI-Funded Research By Year
- Mission Statement
- Board of Directors
- Scientific Advisory Board
- National Autism History Museum Hours
- ARI's Latest Accomplishments
- Annual Reports
- Financials - Audit Reports/990s
- Donate Cryptocurrency
- Donate Stock/Mutual Funds

What is Autism?
Autism is a developmental disorder with symptoms that appear within the first three years of life. Its formal diagnostic name is autism spectrum disorder. The word “spectrum” indicates that autism appears in different forms with varying levels of severity. That means that each individual with autism experiences their own unique strengths, symptoms , and challenges.
Understanding more about ASD can help you better understand the individuals who are living with it.

How autism spectrum disorders are described
Psychiatrists and other clinicians rely on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) to define autism and its symptoms. The DSM-5 definition recognizes two main symptom areas:
- Deficits in social communication and interaction
- Restricted, repetitive behaviors, interests, or activities
These symptoms appear early in a child’s development—although diagnosis may occur later. Autism is diagnosed when symptoms cause developmental challenges that are not better explained by other conditions.
The definition of autism has been refined over the years. Between 1995 and 2011, the DSM-IV grouped Asperger’s Syndrome and Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS) with autism. Asperger’s syndrome was an autism spectrum disorder marked by strong verbal language skills and, often, high intellectual ability. PDD-NOS was a more general diagnosis for people who did not fit clearly into the other two categories.
However, the DSM-5 no longer recognizes Asperger’s syndrome or PDD-NOS as separate diagnoses. Individuals who would previously have received either of these diagnoses may now receive a diagnosis of autism spectrum disorder instead.
Autism symptoms and behaviors
Individuals with autism may present a range of symptoms, such as:
- Reduced eye contact
- Differences in body language
- Lack of facial expressions
- Not engaging in imaginative play
- Repeating gestures or sounds
- Closely focused interests
- Indifference to temperature extremes
These are just a few examples of the symptoms an individual with autism may experience. Any individual could have some, all, or none of these symptoms. Keep in mind that having these symptoms does not necessarily mean a person has autism. Only a qualified medical professional can diagnose autism spectrum disorder.
Most importantly, an individual with autism is first and foremost an individual. Learning about the symptoms can help you start to understand the behaviors and challenges related to autism, but that’s not the same as getting to know the individual. Each person with autism has their own strengths, likes, dislikes, interests, challenges, and skills, just like you do.
How autism is diagnosed
There is no known biological marker for autism. That means that no blood or genetic test can diagnose the disorder. Instead, clinicians rely on observation, medical histories, and questionnaires to determine whether an individual has autism.
Physicians and specialists may use one or several of the following screening tools :
- Modified Checklist for Autism in Toddlers , Revised (M-CHAT), a 20-question test designed for toddlers between 16 and 30 months old.
- The Ages and Stages Questionnaire (ASQ) , a general developmental screening tool with sections targeting specific ages used to identify any developmental challenges a child may have.
- Screening Tool for Autism in Toddlers and Young Children (STAT) , an interactive screening tool, comprising 12 activities that assess play, communication, and imitation.
- Parents’ Evaluation of Developmental Status (PEDS) is a general developmental parent-interview form that identifies areas of concern by asking parents questions.
The American Academy of Pediatrics encourages autism screening for all children at their 18 and 24-month well-child checkups. Parents and caregivers can also ask their pediatrician for an autism screening if they have concerns. In rare cases, individuals with autism reach adulthood before receiving a diagnosis. However, most individuals receive an autism diagnosis before the age of 8.
Prevalence of autism
For many years, a diagnosis of autism was rare, occurring in just one child out of 2,000. One reason for this was the diagnostic criteria. Autism was not clearly defined until 1980 when the disorder was included in the DSM-III. Before that time, some cases of autism spectrum disorder may have been mistaken for other conditions.
Since the ’80s, the rate of autism has increased dramatically around the world. In March 2020, the US Federal Centers for Disease Control announced that 1 in every 54 children in the United States is affected by autism.
Although autism is more likely to affect boys than girls, children of all genders have been diagnosed with ASD. Several recent studies investigate the impact of race, ethnicity, and socioeconomic disparities on the diagnosis of autism spectrum disorder. 1,2,3,4
A short history of autism
Researchers have been working on autism and autism-like disorders since the 1940s. At that time, autism studies tended to be small in scale and used varying definitions of the disorder. Autism was also sometimes lumped in with other conditions.
Focused research into ASD became more common in the 1980s when the DSM-III established autism as a distinct diagnosis. Since then, researchers have explored the causes, symptoms, comorbidities, efficacy of treatments, and many other issues related to autism.
Researchers have yet to discover a cause for autism. Many of the ideas put forth thus far have been disproven. Likely a combination of genetic , neurological , and environmental factors are at work, which is the case with many psychiatric disorders and conditions.
Autism Prognosis
Autism is a lifelong condition, and a wide variety of treatments can help support people with ASD. The symptoms and comorbidities—conditions occurring in the same individual—are treatable. Early intervention delivers the best results. Parents and caregivers should seek out the advice of a qualified medical professional before starting any autism treatment.
Advances in understanding autism, its symptoms, and comorbidities have improved outcomes for individuals with autism. In recent years, more children with autism have attended school in typical classrooms and gone on to live semi-independently. However, the majority remain affected to some degree throughout their lifetime.
Co-occurring conditions
When a person has more than two or more disorders, these conditions are known as comorbidities. Several comorbidities are common in people with autism.
These include:
- Gastrointestinal and immune function disorders
- Metabolic disorders
- Sleep disorders
Identifying co-occurring conditions can sometimes be a challenge because their symptoms may be mimicked or masked by autism symptoms. However, diagnosing and identifying these conditions can help avoid complications and improve the quality of life for individuals with autism.
Autism in pop culture
Movies and books featuring characters with autism have helped bring autism spectrum disorder into the public consciousness. Some have ignited controversy; others have increased the public’s general understanding of autism. A few have done both. At ARI, we hope that people will rely on evidence-based research to understand autism spectrum disorder better.
Learn more about autism spectrum disorder by watching one of our expert-led webinars . They help you learn about ASD from clinicians, researchers, and therapists who research autism and support individuals with ASD.
- Donohue MR, Childs AW, Richards M, Robins DL. Race influences parent report of concerns about symptoms of autism spectrum disorder. Autism . 2019;23(1):100-111. doi:10.1177/1362361317722030
- Durkin MS, Maenner MJ, Baio J, et al. Autism Spectrum Disorder Among US Children (2002-2010): Socioeconomic, Racial, and Ethnic Disparities. Am J Public Health . 2017;107(11):1818-1826. doi:10.2105/AJPH.2017.304032
- Newschaffer CJ. Trends in Autism Spectrum Disorders: The Interaction of Time, Group-Level Socioeconomic Status, and Individual-Level Race/Ethnicity. Am J Public Health . 2017;107(11):1698-1699. doi:10.2105/AJPH.2017.304085
- Yingling ME, Hock RM, Bell BA. Time-Lag Between Diagnosis of Autism Spectrum Disorder and Onset of Publicly-Funded Early Intensive Behavioral Intervention: Do Race-Ethnicity and Neighborhood Matter?. J Autism Dev Disord . 2018;48(2):561-571. doi:10.1007/s10803-017-3354-3

Motor Skills and Executive Function in Autism
autismAdmin 2024-05-08T16:09:01-05:00 May 7th, 2024 | Back to School , Early Intervention , Educational Therapies , Executive Function , Health , Parenting , Sensory , Social Skills , Webinar |
Learn about emerging research on the relationship between the development of motor skills and executive function in autistic children. Handouts are online HERE The

Editorial – Addressing delays: proactive parent-led interventions during waiting periods
Melanie Glock 2024-04-28T15:40:41-05:00 December 6th, 2023 | News |
The wait for an autism diagnosis and subsequent intervention can be highly stressful for many families, especially when access to needed health and educational services also hinges on the approval of insurance

Prenatal exposure to cannabis may increase likelihood of autism
Melanie Glock 2024-04-28T15:45:52-05:00 August 29th, 2023 | News |
Cannabis use during pregnancy may alter placental and fetal DNA methylation (the process of turning genes “on” and “off”) in ways that increase the likelihood of autism spectrum disorder (ASD) or other
New multi-national study adds to evidence linking alterations of the gut microbiome to autism
Melanie Glock 2024-04-28T15:46:00-05:00 August 29th, 2023 | News |
Strong new evidence linking alterations of the gut microbiome to autism spectrum disorders (ASD) comes from a new multi-national study by James Morton and colleagues. In the study, researchers in North America,

Sleep problems in infancy associated with ASD, autism traits, and social attention alterations
Melanie Glock 2024-04-28T15:47:35-05:00 July 20th, 2023 | News |
A new study from the United Kingdom indicates that sleep problems in infancy may help to predict later social skills deficits, autism traits, and autism diagnoses in children. Jannath Begum-Ali and colleagues

Preemptive therapy prior to autism diagnosis may be highly cost-effective
Melanie Glock 2024-04-28T15:47:42-05:00 July 17th, 2023 | News |
Preemptive therapy for infants who display early symptoms of autism may be highly cost-effective, according to a new study from Australia. Leonie Segal and colleagues based their economic analysis on a 2021
Privacy Overview
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New study links autism spectrum disorder to disrupted developmental dopamine
by Eileen Leahy, Chhavi Chauhan, PhD, Elsevier
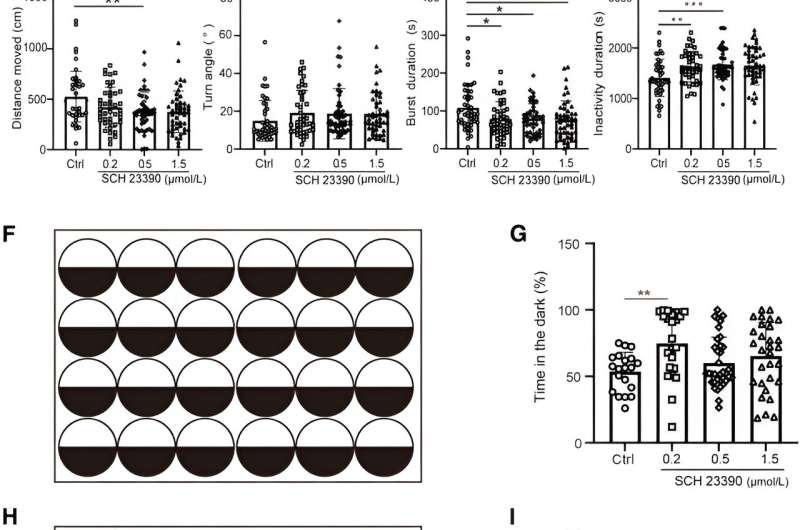
Recent evidence suggests that dopamine plays a crucial role in neural development. In a novel study, investigators demonstrated the link between disrupted developmental dopamine signaling and autism spectrum disorder (ASD).
Their findings underscore the importance of studying developmental signaling pathways to understand the etiology of ASD, paving the way for potential targeted interventions. Their findings appear in The American Journal of Pathology .
Lead investigators Lingyan Xing, Ph.D., and Gang Chen, Ph.D., Key Laboratory of Neuroregeneration of Jiangsu and the Ministry of Education, Co-innovation Center of Neuroregeneration, NMPA Key Laboratory for Research and Evaluation of Tissue Engineering Technology Products, Nantong University, explain, "While dopamine is commonly recognized as a neurotransmitter, its significance in the developmental aspects of autism is largely unexplored. Recent studies have highlighted the crucial roles of dopamine and serotonin in development and their importance in the construction of neural circuits.
"In addition, studies have indicated that the use of dopamine-related drugs during pregnancy is associated with an increased risk of autism in children. Armed with these tantalizing clues, we embarked on a mission to bridge the gap between dopamine's known functions and its potential impact on neurodevelopmental disorders, particularly autism. Our quest was to uncover a novel therapeutic target that could revolutionize the way we approach autism treatment."
Investigators studied the role of disrupted dopaminergic signaling in the etiology of ASD by integrating human brain RNA sequencing transcriptome analysis and a zebrafish model, recognized for its high degree of conservation with humans.
To analyze the developmental deficits in ASD systematically, two large publicly available data sets were retrieved from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database and RNA sequencing data from Arkinglab. Transcriptome analysis of human brains revealed significant correlations between changes in dopaminergic signaling pathways and neural developmental signaling in patients with autism. This suggests a potential link between disrupted developmental dopamine signaling and autism pathology.
To explore this link further researchers used the zebrafish model to study the effects of disrupted dopaminergic signaling on neural circuit development. They found that perturbations in developmental dopaminergic signaling led to neural circuit abnormalities and behavioral phenotypes reminiscent of autism in zebrafish larvae. The study also uncovered a potential mechanism by which dopamine impacts neuronal specification through the modulation of integrins.
Dr. Chen comments, "We were surprised by the extent of the impact that dopaminergic signaling has on neuronal specification in zebrafish, potentially laying the groundwork for circuit disruption in autism-related phenotype. Furthermore, the unexpected involvement of integrins as downstream targets of dopaminergic signaling provides new insights into the mechanisms underlying neurodevelopmental disorders."
Dr. Xing concludes, "This research sheds light on the role of dopamine in neural circuit formation during early development, specifically in the context of autism. Understanding these mechanisms could lead to novel therapeutic interventions targeting dopaminergic signaling pathways to improve outcomes in individuals with autism and other neurodevelopmental disorders."
ASD is a developmental disorder that usually manifests itself in early childhood. Although clinical outcomes vary greatly from case to case, autism is characterized by both a restricted interest in social interaction and repetitive behavior. This coincides with disruptions in brain connectivity shown by diffusion tension imaging.
Studies have shown that several neurodevelopmental processes may be affected in ASD, including neurogenesis, neural migration, axon pathfinding, and synaptic formation, all of which can lead to neural circuit disruption.
Explore further
Feedback to editors
New analysis estimates the effects of race-neutral lung function testing on patients, hospitals, and beyond
4 hours ago

World-first trial shows benefits of finding and treating undiagnosed asthma and COPD
8 hours ago

Acetaminophen shows promise in warding off acute respiratory distress syndrome, organ injury in patients with sepsis
11 hours ago

Modular communicative leadless ICD found to be safe and exceeds performance expectations
May 18, 2024

Creativity and humor shown to promote well-being in older adults via similar mechanisms

Sweet taste receptor affects how glucose is handled metabolically by humans

Better medical record-keeping needed to fight antibiotic overuse, studies suggest

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses

A long-term ketogenic diet accumulates aged cells in normal tissues, new study shows
May 17, 2024

Gut bacteria enhance cancer immunotherapy in mouse study
Related stories.

Metabolism of autism reveals developmental origins
May 10, 2024
The hunt for disrupted brain signals behind autism
Nov 14, 2022
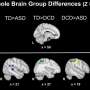

AI could help in the early diagnosis of autism, study finds
Dec 20, 2023
Understanding the role of a new enzyme in the development of autism spectrum disorder
Jan 4, 2024
Vitamin D alters developing neurons in the brain's dopamine circuit, finds study
May 25, 2023
Unlocking the secrets of the brain's dopaminergic system
Dec 5, 2023
Recommended for you
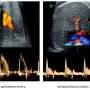
Study opens the door to designing therapies to improve lung development in growth-restricted fetuses

Primary health coverage found to have prevented more than 300,000 child deaths in four Latin American countries
May 16, 2024

Simple learning test may be used to diagnose autism at just six months of age
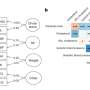
Genetics, environment and health disparities linked to increased stress and mental health challenges during adolescence

Likelihood of kids and young people smoking and vaping linked to social media use

Study finds brain wiring predicted adolescents' emotional health during COVID-19 pandemic
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cambridge Open

Genetic contributions to autism spectrum disorder
1 Nic Waals Institute, Lovisenberg Diaconal Hospital, Oslo, Norway
2 Department of Mental Disorders, Norwegian Institute of Public Health, Oslo, Norway
3 Department of Psychology, PROMENTA Research Center, University of Oslo, Oslo, Norway
M. Niarchou
4 Vanderbilt Genetics Institute, Vanderbilt University Medical Center, TN, USA
A. Starnawska
5 The Lundbeck Foundation Initiative for Integrative Psychiatric Research, iPSYCH, Denmark
6 Department of Biomedicine, Aarhus University, Denmark
7 Center for Genomics for Personalized Medicine, CGPM, and Center for Integrative Sequencing, iSEQ, Aarhus, Denmark
8 College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, UAE
C. van der Merwe
9 Stanley Center for Psychiatric Research, The Broad Institute of MIT and Harvard, MA, USA
10 Department of Psychiatry, Autism Research Centre, University of Cambridge, UK
Autism spectrum disorder (autism) is a heterogeneous group of neurodevelopmental conditions characterized by early childhood-onset impairments in communication and social interaction alongside restricted and repetitive behaviors and interests. This review summarizes recent developments in human genetics research in autism, complemented by epigenetic and transcriptomic findings. The clinical heterogeneity of autism is mirrored by a complex genetic architecture involving several types of common and rare variants, ranging from point mutations to large copy number variants, and either inherited or spontaneous ( de novo ). More than 100 risk genes have been implicated by rare, often de novo , potentially damaging mutations in highly constrained genes. These account for substantial individual risk but a small proportion of the population risk. In contrast, most of the genetic risk is attributable to common inherited variants acting en masse , each individually with small effects. Studies have identified a handful of robustly associated common variants. Different risk genes converge on the same mechanisms, such as gene regulation and synaptic connectivity. These mechanisms are also implicated by genes that are epigenetically and transcriptionally dysregulated in autism. Major challenges to understanding the biological mechanisms include substantial phenotypic heterogeneity, large locus heterogeneity, variable penetrance, and widespread pleiotropy. Considerable increases in sample sizes are needed to better understand the hundreds or thousands of common and rare genetic variants involved. Future research should integrate common and rare variant research, multi-omics data including genomics, epigenomics, and transcriptomics, and refined phenotype assessment with multidimensional and longitudinal measures.
Definition of autism
Kanner defined autism in 1943 with detailed case descriptions of children showing social aloofness, communication impairments, and stereotyped behaviors and interests, often accompanied by intellectual disability (ID) (Kanner, 1943 ). A year later, Asperger independently published an article on children presenting marked difficulties in social communication and unusually circumscribed and intense interests, despite advanced intellectual and language skills (Asperger, 1944 ). Three decades later, Wing and Gould united Asperger and Kanner's descriptions and conceptualized a spectrum of autistic conditions (Wing and Gould, 1978 , 1979 ).
The onset of autism is during the first years of life, although symptoms may not be fully apparent or recognized until later (American Psychiatric Association, 2013 ). Autism is a heterogeneous and complex group of conditions with considerable variation in core symptoms, language level, intellectual functioning, and co-occurring psychiatric and medical difficulties. Subtype diagnoses such as childhood autism and Asperger's syndrome were previously used to specify more homogeneous presentations, but were unstable over time within individuals and used unreliably by clinicians (Lord et al., 2020 ). Current editions of the major diagnostic manuals have replaced the subtypes with an overarching autism spectrum disorder diagnosis and instead require specification of key sources of heterogeneity; language level, intellectual functioning, and co-occurring conditions (APA, 2013 ; World Health Organization, 2018 ).
Epidemiology
Prevalence estimates of autism have steadily increased from less than 0.4% in the 1970s to current estimates of 1–2% (Fombonne, 2018 ; Lyall et al., 2017 ). The increase is largely explained by broadening diagnostic criteria to individuals without ID and with milder impairments, and increased awareness and recognition of autistic traits (Lord et al., 2020 ; Taylor et al., 2020 ). There are marked sex and gender differences in autism (Halladay et al., 2015 ; Warrier et al., 2020 ). The male-to-female ratio is approximately 4:1 in clinical and health registry cohorts but closer to 3:1 in general population studies with active case-finding (Loomes, Hull, & Mandy, 2017 ) and 1–2:1 in individuals with moderate-to-severe ID (Fombonne, 1999 ; Yeargin-Allsopp et al., 2003 ). The mechanisms underlying the sex difference are mostly unknown, and hypotheses include a female protective effect (aspects of the female sex conferring resilience to risk factors for autism), prenatal steroid hormone exposure, and social factors such as underdiagnosis and misdiagnosis in women (Ferri, Abel, & Brodkin, 2018 ; Halladay et al., 2015 ).
Co-occurring conditions are the rule rather than the exception, estimated to affect at least 70% of people with autism from childhood (Lai et al., 2019 ; Simonoff et al., 2008 ). Common co-occurring conditions include attention-deficit hyperactivity disorder (ADHD), anxiety, depression, epilepsy, sleep problems, gastrointestinal and immune conditions (Davignon, Qian, Massolo, & Croen, 2018 ; Warrier et al., 2020 ). There is an elevated risk of premature mortality from various causes, including medical comorbidities, accidental injury, and suicide (Hirvikoski et al., 2016 ).
Autism is also associated with positive traits such as attention to detail and pattern recognition (Baron-Cohen & Lombardo, 2017 ; Bury, Hedley, Uljarević, & Gal, 2020 ). Further, there is wide variability in course and adulthood outcomes with regard to independence, social relationships, employment, quality of life, and happiness (Howlin & Magiati, 2017 ; Mason et al., 2020 ; Pickles, McCauley, Pepa, Huerta, & Lord, 2020 ). Rigorous longitudinal studies and causally informative designs are needed to determine the factors affecting developmental trajectories and outcomes.
Environmental factors
Twin studies suggest that 9–36% of the variance in autism predisposition might be explained by environmental factors (Tick, Bolton, Happé, Rutter, & Rijsdijk, 2016 ). There is observational evidence for association with pre- and perinatal factors such as parental age, asphyxia-related birth complications, preterm birth, maternal obesity, gestational diabetes, short inter-pregnancy interval, and valproate use (Lyall et al., 2017 ; Modabbernia, Velthorst, & Reichenberg, 2017 ). Mixed results are reported for pregnancy-related nutritional factors and exposure to heavy metals, air pollution, and pesticides, while there is strong evidence that autism risk is unrelated to vaccination, maternal smoking, or thimerosal exposure (Modabbernia et al., 2017 ). It is challenging to infer causality from observed associations, given that confounding by lifestyle, socioeconomic, or genetic factors contributes to non-causal associations between exposures and autism. Many putative exposures are associated with parental genotype (e.g. obesity, age at birth) (Gratten et al., 2016 ; Taylor et al., 2019a , Yengo et al., 2018 ), and some are associated both with maternal and fetal genotypes (e.g. preterm birth) (Zhang et al., 2017 ). Studies triangulating genetically informative designs are needed to disentangle these relationships (Davies et al., 2019 ; Leppert et al., 2019 ; Thapar & Rutter, 2019 ).
Twin and pedigree studies
In 1944, Kanner noted that parents shared common traits with their autistic children, introducing the ‘broader autism phenotype’ (i.e. sub-threshold autistic traits) and recognizing the importance of genetics (Harris, 2018 ; Kanner, 1944 ). Thirty years later, twin studies revolutionized the field of autism research (Ronald & Hoekstra, 2011 ).
Twin studies were the first to demonstrate the heritability of autism. In 1977, the first twin-heritability estimate was published, based on a study of 10 dizygotic (DZ) and 11 monozygotic (MZ) pairs (Folstein & Rutter, 1977 ). Four out of the 11 MZ pairs (36%) but none of the DZ pairs were concordant for autism. Subsequently, over 30 twin studies have been published, further supporting the high heritability of autism (Ronald & Hoekstra, 2011 ). A meta-analysis of seven primary twin studies reported that the heritability estimates ranged from 64% to 93% (Tick et al., 2016 ). The correlations for MZ twins were at 0.98 [95% confidence interval (CI) 0.96–0.99], while the correlations for DZ twins were at 0.53 (95% CI 0.44–0.60) when the autism prevalence rate was assumed to be 5% (based on the broader autism phenotype) and increased to 0.67 (95% CI 0.61–0.72) when the prevalence was 1% (based on the stricter definition) (Tick et al., 2016 ). Additionally, family studies have found that the relative risk of a child having autism relates to the amount of shared genome with affected relatives ( Fig. 1 ) (Bai et al., 2019 ; Constantino et al., 2013 ; Georgiades et al., 2013 ; Grønborg, Schendel, & Parner, 2013 ; Risch et al., 2014 ; Sandin et al., 2014 ).

Relative risk of autism by degree of relatedness with a person with autism. Relative risk for full and half siblings, and full cousins was provided in Hansen et al. ( 2019 ). Relative risk for half first cousins was estimated based on Xie et al. ( 2019 ). GS, genome shared.
Early twin and pedigree studies demonstrated that the biological relatives of individuals with autism who did not meet the criteria for an autism diagnosis themselves commonly showed elevated autistic traits such as communication and social interaction difficulties (Le Couteur et al., 1996 ), indicating that the heritability is not restricted to the traditional diagnostic boundaries of autism. Twin studies also indicate that although social communication and repetitive behavior trait dimensions each show strong heritability, there is a limited genetic correlation between them (e.g. for a review, see Ronald & Hoekstra, 2011 ). Further, twin studies have found substantial genetic overlap between autistic traits and symptoms of other psychiatric conditions, including language delay (e.g. Dworzynski et al., 2008 ), ID (e.g. Nishiyama et al., 2009 ), ADHD (e.g. Ronald, Edelson, Asherson, & Saudino, 2010 ), and anxiety (e.g. Lundström et al., 2011 ) (for a review, see Ronald & Hoekstra, 2014 ). Moreover, twin and family studies indicate that the sibling recurrence rate of autism is lower in female than male siblings (Palmer et al., 2017 ; Werling & Geschwind, 2015 ), suggesting the female protective effect hypothesis as a potential explanation for the male preponderance in the diagnosis of autism. The hypothesis was supported by results showing that the siblings of autistic females had a higher likelihood of high autistic trait scores and autism than the siblings of autistic males (Ferri et al., 2018 ; Palmer et al., 2017 ; Robinson, Lichtenstein, Anckarsäter, Happé, & Ronald, 2013 ), consistent with females having a higher liability threshold.
Genetic variants differ in the frequency at which they occur in the population (e.g. rare v. common), the type (i.e. SNPs/CNVs/translocations and inversions/indels), and whether they are inherited or de novo . Here, we summarize the findings on genetic risk for autism from linkage and candidate gene studies, common and rare genetic variation studies, epigenomics, and transcriptomics. A glossary of important terms is in Box 1 .
Candidate gene association study: A study that examines the association between a phenotype and a genetic variant chosen a priori based on knowledge of the gene's biology or functional impact.
Complex trait: A trait that does not follow Mendelian inheritance patterns, but is likely the result of multiple factors including a complex mixture of variation within multiple genes.
Copy number variant (CNV): Deletion or duplication of large genomic regions.
de novo mutation: A mutation that is present in the offspring but is either absent in parents or is present only in parental germ cells.
DNA methylation (DNAm): Epigenetic modification of DNA characterized by the addition of a methyl group (-CH 3 ) to the 5 th position of the pyrimidine ring of cytosine base resulting in 5-methylcytosine (5mC).
Epigenetics: The science of heritable changes in gene regulation and expression that do not involve changes to the underlying DNA sequence.
Epigenome-Wide Association Study (EWAS): A study that investigates associations between DNA methylation levels quantified at tens/hundreds of thousands of sites across the human genome, and the trait of interest.
Genome-Wide Association Study (GWAS): A study scanning genome-wide genetic variants for associations with a given trait.
Genetic correlation: An estimate of the proportion of variance shared between two traits due to shared genetics.
Heritability: An estimate of the proportion of variation in a given trait that is due to differences in genetic variation between individuals in a given population.
Heritability on the liability scale : A heritability estimate adjusted for the population prevalence of a given binary trait, typically disorders.
Genetic linkage studies: A statistical method of mapping genes of heritable traits to their chromosomal locations by using chromosomal co-segregation with the phenotype.
Mendelian inheritance: When the inheritance of traits is passed down from parents to children and is controlled by a single gene for which one allele is dominant and the other recessive.
Methylation Quantitative Trait Locus (mQTL): A SNP at which genotype is correlated with the variation of DNA methylation levels at a nearby ( cis- mQTL) or distal ( trans- mQTL) site.
Phenotype: The observable characteristics of an individual.
Polygenic risk score (PRS): An estimate of an individual's genetic liability for a condition calculated based on the cumulative effect of many common genetic variants.
Single nucleotide polymorphism (SNP): A single base pair change that is common (>1%) in the population.
Single nucleotide variant (SNV): A variation in a single nucleotide without any limitation of frequency.
SNP heritability: The proportion of variance in a given phenotype in a population that is attributable to the additive effects of all SNPs tested. Typically, SNPs included have a minor allele frequency >1%.
Linkage and candidate gene studies
Initial linkage studies were conducted to identify chromosomal regions commonly inherited in affected individuals. Susceptibility loci implicated a range of regions, but only two have been replicated (Ramaswami & Geschwind, 2018 ): at chromosome 20p13 (Weiss, Arking, Daly, & Chakravarti, 2009 ) and chromosome 7q35 (Alarcón, Cantor, Liu, Gilliam, & Geschwind, 2002 ). Lack of replication and inconsistent findings were largely due to low statistical power (Kim & Leventhal, 2015 ). Candidate gene association studies identified over 100 positional and/or functional candidate genes for associations with autism (Bacchelli & Maestrini, 2006 ). However, there was no consistent replication for any of these findings (Warrier, Chee, Smith, Chakrabarti, & Baron-Cohen, 2015 ), likely due to limitations in study design (e.g. low statistical power, population diversity, incomplete coverage of variation within the candidate genes, and false positives arising from publication bias) (Ioannidis, 2005 ; Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001 ). The advancement of genome-wide association studies (GWAS) and next-generation sequencing techniques has significantly enhanced gene and variant discovery.
Common genetic variation
The SNP-heritability (proportion of variance attributed to the additive effects of common genetic variants) of autism ranges from 65% in multiplex families (Klei et al., 2012 ) to 12% in the latest Psychiatric Genomics Consortium GWAS ( Fig. 2 a ) (Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium, 2017 ; Grove et al., 2019 ). Variation is largely attributable to sample heterogeneity and differences in methods used to estimate SNP-heritability.

Variance explained by different classes of genetic variants in autism. ( a ) Donut chart of the variance explained by different classes of variants. The narrow-sense heritability (82.7%, Nordic average, shades of green) has been estimated using familial recurrence data from Bai et al. ( 2019 ). The total common inherited heritability (12%) has been estimated using LDSC-based SNP-heritability (additive) from Grove et al. ( 2019 ) and the total rare inherited heritability (3%) has been obtained from Gaugler et al. ( 2014 ). The currently unexplained additive heritability is thus 67.7% (total narrow-sense heritability minus common and rare inherited heritabilities combined). This leaves a total of 17.3% of the variance to shared and unique environmental estimates (Bai et al., 2019 ). The term environmental refers to non-additive and non-inherited factors that contribute to variation in autism liability. Of this, de novo missense and protein-truncating variants (Satterstrom et al., 2020 ) and variation in non-genic regions (An et al., 2018 ) together explain 2.5% of the variance. Whilst de novo variation can be inherited in some cases (germline mutation in the parent) and thus shared between siblings, it is unlikely that this will be shared by other related individuals, and thus unlikely to be included in the narrow-sense heritability in Bai et al. ( 2019 ). This is likely to be a lower-bound of the estimate as we have not included the variance explained by de novo structural variants and tandem repeats. Additionally, non-additive variation accounts for ~4% of the total variance (Autism Sequencing Consortium et al., 2019 ). Thus, ~11% of the total variance is currently unaccounted for, though this is likely to be an upper bound. ( b ) The variance explained is likely to change in phenotypic subgroups. For instance, the risk ratio for de novo protein-truncating variants in highly constrained genes (pLI > 0.9) is higher in autistic individuals with ID compared to those without ID (point estimates and 95% confidence intervals provided; Kosmicki et al., 2017 ). ( c ) Similarly, the proportion of the additive variance explained by common genetic variants is higher in autistic individuals without ID compared to autistic individuals with ID (Grove et al., 2019 ). Point estimates and 95% confidence intervals provided.
Early GWASs of autism were underpowered, partly due to overestimating potential effect sizes. Grove et al. ( 2019 ) conducted a large GWAS of autism combining data from over 18 000 autistic individuals and 27 000 non-autistic controls and an additional replication sample. They identified five independent GWAS loci ( Fig. 3 ). Another recent study (Matoba et al., 2020 ) identified a further novel locus by meta-analyzing the results from Grove et al. ( 2019 ) with over 6000 case-pseudocontrol pairs from the SPARK cohort by employing a massively parallel reporter assay to identify a potential causal variant (rs7001340) at this locus which regulates DDH2 in the fetal brain. The sample sizes are still relatively small compared to other psychiatric conditions (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2020 ; Howard et al., 2019 ), though ongoing work aims to double the sample size and identify additional loci.

Karyogram showing the 102 genes implicated by rare variant findings at a false discovery rate of 0.1 or less (Satterstrom et al., 2020 ) and the five index SNPs identified in GWAS (Grove et al., 2019 ) of autism.
Using genetic correlations and polygenic score analyses, studies have identified modest shared genetics between autism and different definitions of autistic traits in the general population (Askeland et al., 2020 ; Bralten et al., 2018 ; Robinson et al., 2016 ; Taylor et al., 2019 b ). There is some evidence for developmental effects, with greater shared genetics in childhood compared to adolescence (St Pourcain et al., 2018 ). These methods have also identified modest polygenic associations between autism and other neurodevelopmental and mental conditions such as schizophrenia, ADHD, and major depressive disorder, related traits such as age of walking, language delays, neuroticism, tiredness, and self-harm, as well as risk of exposure to childhood maltreatment and other stressful life events (Brainstorm Consortium et al., 2018 ; Bulik-Sullivan et al., 2015 ; Grove et al., 2019 ; Hannigan et al., 2020 ; Lee et al., 2019 , b ; Leppert et al., 2019 ; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013 ; Warrier & Baron-Cohen, 2019 ). Notably, autism is positively genetically correlated with measures of intelligence and educational attainment (EA) (Bulik-Sullivan et al., 2015 ; Grove et al., 2019 ), an observation supported by polygenic score association (Clarke et al., 2016 ). Polygenic Transmission Disequilibrium Tests have identified an over-transmission of polygenic scores for EA, schizophrenia, and self-harm from parents to autistic children, but an absence of such over-transmission to non-autistic siblings (Warrier & Baron-Cohen, 2019 ; Weiner et al., 2017 ), suggesting that these genetic correlations are not explained by ascertainment biases or population stratification. However, a genetic correlation does not necessarily imply a causal relationship between the two phenotypes and may simply index biological pleiotropy. Causal inference methods such as Mendelian randomization can be used to disentangle such relationships (Davies et al., 2019 ; Pingault et al., 2018 ).
The relatively low SNP-heritability in autism compared to other psychiatric conditions may partly be due to phenotypic heterogeneity. In an attempt to reduce phenotypic heterogeneity, Chaste et al. ( 2015 ) identified 10 phenotypic combinations to subgroup autistic individuals. Family-based association analyses did not identify significant loci, and SNP-heritability for the subgroups was negligent. It is unclear if reducing phenotypic heterogeneity increases genetic homogeneity, and investigating this in larger samples is warranted. Another study identified no robust evidence of genetic correlation between social and non-social (restricted and repetitive behavior patterns) autistic traits (Warrier et al., 2019 ). A few studies have investigated the common variant genetic architecture of social and non-social autistic traits in individuals with autism (Alarcón et al., 2002 ; Cannon et al., 2010 ; Cantor et al., 2018 ; Lowe, Werling, Constantino, Cantor, & Geschwind, 2015 ; Tao et al., 2016 ; Yousaf et al., 2020 ) and in the general population (St Pourcain et al., 2014 ; Warrier et al., 2018 , 2019 ), but replication of the identified loci is needed.
Diagnostic classification is another source of heterogeneity: SNP-heritability of Asperger's syndrome (ICD-10 diagnosis) was twice (0.097 ± 0.001) that of childhood autism and unspecified pervasive developmental disorders (Grove et al., 2019 ) [due to overlap in subtype diagnoses, a hierarchy was used: childhood autism>atypical autism>Asperger's syndrome>unspecified subtypes (Grove et al., 2019 )]. Supporting this, polygenic scores for intelligence and EA had larger loadings in the Asperger's syndrome and childhood autism subgroups compared to other subgroups (Grove et al., 2019 ). Additionally, the SNP-heritability of autism (all subtypes) without co-occurring ID diagnosis (0.09 ± 0.005) was three times that of autism with ID (Grove et al., 2019 ) ( Fig. 2 c ).
Rare genetic variation
Rare genetic variants confer significant risk in the complex etiology of autism. They are typically non-Mendelian, with substantial effect sizes and low population attributable risk. It is estimated that ~10% of autistic individuals have been diagnosed with an identifiable rare genetic syndrome characterized by dysmorphia, metabolic, and/or neurologic features (Carter & Scherer, 2013 ; Tammimies et al., 2015 ). Associated syndromes include the 15q11-q13 duplication of the Prader-Willi/Angelman syndrome, fragile X syndrome, 16p11.2 deletion syndrome, and 22q11 deletion syndrome (Sztainberg & Zoghbi, 2016 ). Prevalence estimates for autism vary widely between genetic syndromes; for example, 11% in 22q11.2 deletion syndrome and 54% in Cohen's syndrome (Richards, Jones, Groves, Moss, & Oliver, 2015 ). Of note, estimating the prevalence of autism in the context of genetic syndromes is complex (Havdahl et al., 2016 ; Richards et al., 2015 ).
The rate of gene discovery in autism is a linear function of increasing sample size (De Rubeis et al., 2014 ). Early studies implicated nine genes in the first 1000 autism cases (Neale et al., 2012 ; Sanders et al., 2012 ), increasing to 27 and 33 associated genes from separate analyses of Simons Simplex Collection and Autism Sequencing Consortium (ASC) samples (De Rubeis et al., 2014 ; Iossifov et al., 2014 ). Integrating these samples using the TADA framework implicated a total of 65 autism genes (Sanders et al., 2015 ).
The MSSNG initiative analyzed whole genomes from 5205 individuals ( N cases = 2636), and identified 61 autism-risk genes, of which 18 were new candidates (Yuen et al., 2017 ). More recently, the largest whole-exome sequencing analysis to date conducted by the ASC ( N = 35 584, N cases = 11 986) identified 102 autism-associated genes ( Fig. 3 ), many of which are expressed during brain development with roles in the regulation of gene expression and neuronal communication (Satterstrom et al., 2020 ). Rare CNVs and SNVs associated with autism have pleiotropic effects, thus increasing the risk for other complex disorders such as schizophrenia, ADHD, ID, and epilepsy (Gudmundsson et al., 2019 ; Satterstrom et al., 2019 , 2020 ).
CNVs can impact one or multiple genes and can occur at common or rare frequencies in a population. All CNVs associated with autism have been rare. Recurrent CNVs are among the most convincing rare inherited risk variations for autism, and have a prevalence of about 3% in affected patients (Bourgeron, 2016 ). In comparison, approximately 4–10% of autistic individuals have de novo deletions or duplications (Bourgeron, 2016 ; Pinto et al., 2010 ; Sebat et al., 2007 ) frequently mapped to established risk loci 1q21.1, 3q29, 7q11.23, 15q11.2-13, and 22q11.2 (Sanders et al., 2015 ). A higher global frequency of de novo CNVs is observed in idiopathic autism cases from simplex families (10%) compared to multiplex families (2%) and controls (1%) (Halladay et al., 2015 ; Itsara et al., 2010 ; Sebat et al., 2007 ). Inherited CNVs can be present in unaffected siblings and parents, suggesting a model of incomplete penetrance dependent on the dosage sensitivity and function of the gene(s) they affect (Vicari et al., 2019 ).
Damaging SNVs include nonsense, frameshift, and splice site mutations (collectively referred to as protein-truncating variants, or PTVs), and missense variants. Rare inherited variants have a smaller average effect size and reduced penetrance compared to de novo pathogenic mutations. Early studies on whole exomes from trios established a key role for de novo germline mutations in autism. Whilst analysis in smaller sample sizes indicated only modest increase in de novo mutation rates in autism cases (Neale et al., 2012 ), the rate rose significantly in excess of expectation as the sample size increased (De Rubeis et al., 2014 ; Iossifov et al., 2014 ). Most recently, the ASC observed a 3.5-fold case enrichment of damaging de novo PTVs and a 2.1-fold enrichment for damaging de novo missense variants (Satterstrom et al., 2020 ), concluding that all exome de novo SNVs explain 1.92% of the variance in autism liability (Satterstrom et al., 2020 ) ( Fig. 2 a ).
Comparatively, the ASC discovered a 1.2-fold enrichment of rare inherited damaging PTVs in cases compared to unaffected siblings (Satterstrom et al., 2020 ). Similarly, recent whole-genome analysis found no excess of rare inherited SNVs, and no difference in the overall rate of these variants in affected subjects compared to unaffected siblings (Ruzzo et al., 2019 ).
New advancements
It is estimated that de novo mutations in protein-coding genes contribute to risk in ~30% of simplex autism cases (Yuen et al., 2017 ; Zhou et al., 2019 ). However, recent work has also shown that de novo mutations in non-coding regions of the genome (particularly gene promoters) contribute to autism (An et al., 2018 ; Zhou et al., 2019 ). Adapting machine learning techniques may be key to providing novel neurobiological insights to the genetic influences on autism in the future (An et al., 2018 ; Ruzzo et al., 2019 ; Zhou et al., 2019 ). Additionally, rare tandem repeat expansions in genic regions are more prevalent among autism cases than their unaffected siblings, with a combined contribution of ~2.6% to the risk of autism (Trost et al., 2020 ).
Common and rare variant interplay
The largest component of genetic risk is derived from common variants of additive effect with a smaller contribution from de novo and rare inherited variation ( Fig. 2 a ) (de la Torre-Ubieta, Won, Stein, & Geschwind, 2016 ; Gaugler et al., 2014 ). Notably, KMT2E was implicated in both the latest GWAS (Grove et al., 2019 ) and exome sequencing (Satterstrom et al., 2020 ) analyses. It is hypothesized that common genetic variation in or near the genes associated with autism influences autism risk, although current sample sizes lack the power to detect the convergence of the two (Satterstrom et al., 2020 ).
Whilst higher SNP-heritability is observed in autistic individuals without ID ( Fig. 2 b ), de novo PTVs in constrained genes are enriched in autistic individuals with ID ( Fig. 2 a ). However, the genetic architecture of autism is complex and diverse. For example, common genetic variants also contribute to risk in autistic individuals with ID and in autistic individuals carrying known large-effect de novo variants in constrained genes (Weiner et al., 2017 ). Furthermore, an excess of disruptive de novo variants is also observed in autistic individuals without co-occurring ID compared to non-autistic individuals (Satterstrom et al., 2020 ).
Epigenetics
DNA methylation (DNAm), an epigenetic modification, allows for both genetic and environmental factors to modulate a phenotype (Martin & Fry, 2018 ; Smith et al., 2014 ). DNAm affects gene expression, regulatory elements, chromatin structure, and alters neuronal development, functioning, as well as survival (Kundaje et al., 2015 ; Lou et al., 2014 ; Peters et al., 2015 ; Sharma, Klein, Barboza, Lohdi, & Toth, 2016 ; Yu et al., 2012 ; Zlatanova, Stancheva, & Caiafa, 2004 ). Additionally, putative prenatal environmental risk factors impact the offspring's methylomic landscape (Anderson, Gillespie, Thiele, Ralph, & Ohm, 2018 ; Cardenas et al., 2018 ; Joubert et al., 2016 ), thus providing a plausible molecular mechanism to modulate the neurodevelopmental origins of autism.
Autism Epigenome-Wide Association Study (EWAS) meta-analysis performed in blood from children and adolescents from SEED and SSC cohorts ( N cases = 796, N controls = 858) identified seven differentially methylated positions (DMPs) associated ( p < 10 × 10 −05 ) with autism, five of them also reported to have brain-based autism associations. The associated DMPs annotated to CENPM , FENDRR , SNRNP200 , PGLYRP4 , EZH1 , DIO3 , and CCDC181 genes, with the last site having the largest effect size and the same direction of association with autism across the prefrontal cortex, temporal cortex, and cerebellum (Andrews et al., 2018 ). The study reported moderate enrichment of methylation Quantitative Trait Loci (mQTLs) among the associated findings, suggesting top autism DMPs to be under genetic control (Andrews et al., 2018 ). These findings were further extended by the MINERvA cohort that added 1263 neonatal blood samples to the meta-analysis. The SEED-SSC-MINERvA meta-EWAS identified 45 DMPs, with the top finding showing the consistent direction of association across all three studies annotated to ITLN1 (Hannon et al., 2018 ). The MINERvA sample was also used for EWAS of autism polygenic score, hypothesizing that the polygenic score-associated DNAm variation is less affected by environmental risk factors, which can confound case–control EWAS. Elevated autism polygenic score was associated with two DMPs ( p < 10 × 10 −06 ), annotated to FAM167A / C8orf12 and RP1L1 . Further Bayesian co-localization of mQTL results with autism GWAS findings provided evidence that several SNPs on chromosome 20 are associated both with autism risk and DNAm changes in sites annotated to KIZ , XRN2 , and NKX2-4 (Hannon et al., 2018 ). The mQTL effect of autism risk SNPs was corroborated by an independent study not only in blood, but also in fetal and adult brain tissues, providing additional evidence that autism risk variants can act through DNAm to mediate the risk of the condition (Hammerschlag, Byrne, Bartels, Wray, & Middeldorp, 2020 ).
Since autism risk variants impact an individual's methylomic landscape, studies that investigate DNAm in the carriers of autism risk variants are of interest to provide insight into their epigenetic profiles. A small blood EWAS performed in 52 cases of autism of heterogeneous etiology, nine carriers of 16p11.2del, seven carriers of pathogenic variants in CHD8 , and matched controls found that DNAm patterns did not clearly distinguish autism of the heterogeneous etiology from controls. However, the homogeneous genetically-defined 16p11.2del and CHD8 +/− subgroups were characterized by unique DNAm signatures enriched in biological pathways related to the regulation of central nervous system development, inhibition of postsynaptic membrane potential, and immune system (Siu et al., 2019 ). This finding highlights the need to combine genomic and epigenomic information for a better understanding of the molecular pathophysiology of autism.
It must be noted that a very careful interpretation of findings from peripheral tissues is warranted. DNAm is tissue-specific and therefore EWAS findings obtained from peripheral tissues may not reflect biological processes in the brain. Using the mQTL analytical approach may reduce this challenge, as mQTLs are consistently detected across tissues, developmental stages, and populations (Smith et al., 2014 ). However, not all mQTLs will be detected across tissues and will not necessarily have the same direction of effect (Smith et al., 2014 ). Therefore, it is recommended that all epigenetic findings from peripheral tissues are subjected to replication analyses in human brain samples, additional experimental approaches, and/or Mendelian randomization to strengthen causal inference and explore molecular mediation by DNAm (Walton, Relton, & Caramaschi, 2019 ).
EWASs performed in post-mortem brains have typically been conducted using very small sample sizes, due to limited access to brain tissue (Ladd-Acosta et al., 2014 ; Nardone et al., 2014 ). One of the largest autism EWAS performed in post-mortem brains (43 cases and 38 controls) identified multiple DMPs ( p < 5 × 10 −05 ) associated with autism (31 DMPs in the prefrontal cortex, 52 in the temporal cortex, and two in the cerebellum) (Wong et al., 2019 ), and autism-related co-methylation modules to be significantly enriched for synaptic, neuronal, and immune dysfunction genes (Wong et al., 2019 ). Another post-mortem brain EWAS reported DNAm levels at autism-associated sites to resemble the DNAm states of early fetal brain development (Corley et al., 2019 ). This finding suggests an epigenetic delay in the neurodevelopmental trajectory may be a part of the molecular pathophysiology of autism.
Overall, methylomic studies of autism provide increasing evidence that common genetic risk variants of autism may alter DNAm across tissues, and that the epigenetic dysregulation of neuronal processes can contribute to the development of autism. Stratification of study participants based on their genetic risk variants may provide deeper insight into the role of aberrant epigenetic regulation in subgroups within autism.
Transcriptomics
Transcriptomics of peripheral tissues.
Gene expression plays a key role in determining the functional consequences of genes and identifying genetic networks underlying a disorder. One of the earliest studies on genome-wide transcriptome (Nishimura et al., 2007 ) investigated blood-derived lymphoblastoid cells gene expression from a small set of males with autism ( N = 15) and controls. Hierarchical clustering on microarray expression data followed by differentially expressed gene (DEG) analysis revealed a set of dysregulated genes in autism compared to controls. This approach was adopted (Luo et al., 2012 ) to investigate DEGs in a cohort of 244 families with autism probands (index autism case in a family) known to carry de novo pathogenic or variants of unknown significance and discordant sibling carriers of non-pathogenic CNVs. From genome-wide microarray transcriptome data, this study identified significant enrichment of outlier genes that are differentially expressed and reside within the proband rare/ de novo CNVs. Pathway enrichment of these outlier genes identified neural-related pathways, including neuropeptide signaling, synaptogenesis, and cell adhesion. Distinct expression changes of these outlier genes were identified in recurrent pathogenic CNVs, i.e. 16p11.2 microdeletions, 16p11.2 microduplications, and 7q11.23 duplications. Recently, multiple independent genome-wide blood-derived transcriptome analysis (Filosi et al., 2020 ; Lombardo et al., 2018 ; Tylee et al., 2017 ) showed the efficiency of detecting dysregulated genes in autism, including aberrant expression patterns of long non-coding RNAs (Sayad, Omrani, Fallah, Taheri, & Ghafouri-Fard, 2019 ).
Transcriptomics of post-mortem brain tissue
Although blood-derived transcriptome can be feasible to study due to easy access to the biological specimen, blood transcriptome results are not necessarily representative of the transcriptional machinery in the brain (GTEx Consortium, 2017 ). Hence, it is extremely hard to establish a causal relationship between blood transcriptional dysregulations and phenotypes in autism. A landmark initiative by Allen Brain Institute to profile human developing brain expression patterns (RNA-seq) from post-mortem tissue enabled neurodevelopmental research to investigate gene expression in the brain (Sunkin et al., 2013 ). Analyzing post-mortem brain tissue, multiple studies identified dysregulation of genes at the level of gene exons impacted by rare/ de novo mutations in autism (Uddin et al., 2014 ; Xiong et al., 2015 ), including high-resolution detection of exon splicing or novel transcript using brain tissue RNA sequencing (RNA-seq). High-resolution RNA-seq enabled autism brain transcriptome analysis on non-coding elements, and independent studies identified an association with long non-coding RNA and enhancer RNA dysregulation (Wang et al., 2015 ; Yao et al., 2015 ; Ziats & Rennert, 2013 ).
Although it is difficult to access post-mortem brain tissue from autistic individuals, studies of whole-genome transcriptome from autism and control brains have revealed significantly disrupted pathways ( Fig. 4 ) related to synaptic connectivity, neurotransmitter, neuron projection and vesicles, and chromatin remodeling pathways (Ayhan & Konopka, 2019 ; Gordon et al., 2019 ; Voineagu et al., 2011 ). Recently, an integrated genomic study also identified from autism brain tissue a component of upregulated immune processes associated with hypomethylation (Ramaswami et al., 2020 ). These reported pathways are in strong accordance with numerous independent autism studies that integrated genetic data with brain transcriptomes (Courchesne, Gazestani, & Lewis, 2020 ; Uddin et al., 2014 ; Yuen et al., 2017 ). A large-scale analysis of brain transcriptome from individuals with autism identified allele-specific expressions of genes that are often found to be impacted by pathogenic de novo mutations (Lee et al., 2019 a ). The majority of the studies are in consensus that genes that are highly active during prenatal brain development are enriched for clinically relevant mutations in autism (Turner et al., 2017 ; Uddin et al., 2014 ; Yuen et al., 2017 ). Recently, a large number (4635) of expression quantitative trait loci were identified that were enriched in prenatal brain-specific regulatory regions comprised of genes with distinct transcriptome modules that are associated with autism (Walker et al., 2019 ).

Most commonly reported three pathways (Ayhan & Konopka, 2019 ; Gordon et al., 2019 ; Voineagu et al., 2011 ) associated with autism. ( a ) The synaptic connectivity and neurotransmitter pathway involves genes (yellow rectangular box) within presynaptic and postsynaptic neurons. Neurotransmitter transport through numerous receptors is an essential function of this pathway; ( b ) the chromatin remodeling pathway involves binding of remodeling complexes that initiate the repositioning (move, eject, or restructure) of nucleosomes that potentially can disrupt gene regulation; and ( c ) the neural projection pathway [adapted from Greig, Woodworth, Galazo, Padmanabhan, & Macklis ( 2013 )] involves the projection of neural dendrite into distant regions and the migration of neuronal cells through ventricular (VZ) and subventricular zones (SVZ) into the different cortical layers (I-VI).
Single-cell transcriptomics
Recent advancement of single-cell transcriptomics enables the detection of cell types that are relevant to disorder etiology. A recent case–control study conducted single-cell transcriptomics analysis on 15 autism and 16 control cortical post-mortem brain tissues generating over 100 000 single-cell transcriptomics data (Velmeshev et al., 2019 ). Cell-type analysis revealed dysregulations of a specific group of genes in cortico-cortical projection neurons that correlate with autism severity (Velmeshev et al., 2019 ). Deciphering cell-type identification has future implications, in particular for the implementation of precision medicine. However, single-cell technology is at very early stages of development and computationally it is still very complex to classify cell-type identity.
The emergence of CRISPR/Cas9 genome editing technology can potentially become an effective tool in future therapeutics of genetic conditions associated with autism. Although introducing and reversing DNA mutation is becoming a mature technology within in vitro systems, much work needs to be done for in vivo use of genome editing. Single-cell OMICs is another emerging field that has the potential to decipher developmental (spatio-temporally) brain cell types that are associated with autism. Identifying cell clusters and defining cell identity is a major computational challenge. Artificial intelligence can significantly improve these computational challenges to identify the molecular associations of autism at the single-cell level.
Clinical and therapeutic implications
In some, but not all, best practice clinical guidelines, genetic tests such as fragile X testing, chromosomal microarray, and karyotype testing are part of the standard medical assessment in a diagnostic evaluation of autism to identify potentially etiologically relevant rare genetic variants (Barton et al., 2018 ). The guidelines vary with respect to whether genetic testing is recommended for all people with autism, or based on particular risk factors, such as ID, seizures, or dysmorphic features. The DSM-5 diagnosis of autism includes a specifier for associated genetic conditions (APA, 2013 ). Although genetic test results may not usually have consequences for treatment changes, the results could inform recurrence risk and provide families with access to information about symptoms and prognosis. In the future, gene therapy, CRISPR/Cas9, and genome editing technologies may lead to the gene-specific design of precision medicine for rare syndromic forms of autism (Benger, Kinali, & Mazarakis, 2018 ; Gori et al., 2015 ).
Given that a substantial proportion of the genetic liability to autism is estimated to be explained by the cumulative effect of a large number of common SNPs, polygenic scores have gained traction as potential biomarkers. However, the predictive ability of polygenic scores from the largest autism GWAS to date is too low to be clinically useful. The odds ratio when comparing the top and bottom polygenic score decile groups is only 2.80 (95% CI 2.53–3.10) (Grove et al., 2019 ). Additionally, polygenic scores based on the samples of European ancestry do not translate well in populations with diverse ancestry (Palk, Dalvie, de Vries, Martin, & Stein, 2019 ).
Genetic testing can in the future become useful for informing screening or triaging for diagnostic assessments or identifying who may be more likely to respond to which type of intervention (Wray et al., 2021 ). Genetics may also help identify individuals with autism who are at a high risk of developing co-occurring physical and mental health conditions or likely to benefit from treatments of such conditions. A top research priority for autistic people and their families is addressing co-occurring mental health problems (Autistica, 2016 ), which may sometimes be the primary treatment need as opposed to autism per se . Genomics may also be helpful to repurpose existing treatments and better identify promising treatments. There are active clinical trials to repurpose drugs in autism (Hong & Erickson, 2019 ). Moreover, genetics can be used to identify social and environmental mediating and moderating factors (Pingault et al., 2018 ), which could inform interventions to improve the lives of autistic people.
Notably, there are important ethical challenges related to clinical translation of advances in genetics, including concerns about discriminatory use, eugenics concerning prenatal genetic testing, and challenges in interpretation and feedback (Palk et al., 2019 ). People with autism and their families are key stakeholders in genetic studies of autism and essential to include in discussions of how genetic testing should be used.
Conclusions and future directions
Recent large-scale and internationally collaborative investigations have led to a better understanding of the genetic contributions to autism. This includes identifying the first robustly associated common genetic variants with small individual effects (Grove et al., 2019 ) and over 100 genes implicated by rare, mostly de novo , variants of large effects (Sanders et al., 2015 ; Satterstrom et al., 2020 ). These and other findings show that the genetic architecture of autism is complex, diverse, and context-dependent, highlighting a need to study the interplay between different types of genetic variants, identify genetic and non-genetic factors influencing their penetrance, and better map the genetic variants to phenotypic heterogeneity within autism.
Immense collaborative efforts are needed to identify converging and distinct biological mechanisms for autism and subgroups within autism, which can in turn inform treatment (Thapar & Rutter, 2020 ). It is crucial to invest in multidimensional and longitudinal measurements of both core defining traits and associated traits such as language, intellectual, emotional, and behavioral functioning, and to collaboratively establish large omics databases including genomics, epigenomics, transcriptomics, proteomics, and brain connectomics (Searles Quick, Wang, & State, 2020 ). Indeed, large-scale multi-omic investigations are becoming possible in the context of large population-based family cohorts with rich prospective and longitudinal information on environmental exposures and developmental trajectories of different neurodevelopmental traits. Finally, novel methods (Neumeyer, Hemani, & Zeggini, 2020 ) can help investigate causal molecular pathways between genetic variants and autism and autistic traits.
Acknowledgements
We thank the Psychiatric Genomics Consortium, Anders Børglum, and Elise Robinson for their support and advice.
Financial support
Alexandra Havdahl was supported by the South-Eastern Norway Regional Health Authority (#2018059, career grant #2020022) and the Norwegian Research Council (#274611 PI Ted Reichborn-Kjennerud and #288083 PI Espen Røysamb). Maria Niarchou was supported by Autism Speaks (#11680). Anna Starnawska was supported by The Lundbeck Foundation Initiative for Integrative Psychiatric Research, iPSYCH, Denmark (R155-2014-1724). Varun Warrier is supported by the Bowring Research Fellowship (St. Catharine's College, Cambridge), the Templeton World Charity Foundation, Inc., the Autism Research Trust, and the Wellcome Trust. Celia van der Merwe is supported by the Simons Foundation NeuroDev study (#599648) and the NIH R01MH111813 grant.
Conflict of interest

FPG Sponsored Proposal Pipeline
Research on autism spectrum disorders (r03 clinical trial optional).
The purpose of this Funding Opportunity Announcement (FOA) is to encourage research grant applications to support research designed to elucidate the etiology, epidemiology, diagnosis, and optimal means of service delivery in relation to Autism Spectrum Disorders (ASD). An R03 grant supports small, discrete, well-defined projects that can be completed in two years and that require limited resources. R03 applications may include development of new research methodologies or technology, secondary analysis of existing data, and pilot or feasibility studies. Preliminary data are not required, particularly in applications proposing pilot or feasibility studies. Applicants pursuing exploratory/developmental research to support early and conceptual stages of project development should consider the companion R21 FOA, PA-21-200 . Applicants pursuing larger studies in established scientific areas where preliminary data are expected should consider the companion R01 FOA, PA-21-201 .
Application Deadline:
- Request new password

Are Autistic People More Likely to Speak Up at Work?
Research suggests that autistic people are more likely to raise concerns on the job..
Posted May 13, 2024 | Reviewed by Devon Frye
- What Is Autism?
- Find a therapist to help with autism
- In general, autistic people are more likely than others to be unemployed or underemployed.
- Yet autistic people may be more likely to voice concerns about organizational dysfunction, research suggests.
- This heightened sensitivity to injustice and malpractice could make autistic people valuable employees.
Do you find yourself being the person who raises concerns at work? If so, and you’re autistic , you’re not alone.
A consistent theme that has cropped up in my work with autistic clients is that they tend to have a strong sense of social justice; this means they try to help colleagues when needed and ensure their workplaces are implementing best practices. Despite their general discomfort with communication and their dislike of drawing attention to themselves, my autistic clients often tell me that their need to do what they consider to be the right thing overrides their fear of doing so.
“I’ll stay quiet during a meeting, even when I’ve got something to contribute," my client Shona told me. "But if I feel that someone is being picked on in any way, I’ll very vociferously offer my opinion."

Another client, Anita, described a strong need to make sure people were “doing what was right” from a very young age. “I work as a nurse now," she explained. "There are procedures in place to protect people’s safety but colleagues still cut corners. This is a black-and-white issue to me, and I’ll report anyone who’s not following the rules as it endangers patients.”
“I’ve become the go-to person at work for when something needs to be sorted out,” Stella shared. “As a manager, colleagues know that I’m never going to sit by and let bad practice go unnoticed. Much as it causes me stress to speak my mind, it would be far more stressful to not intervene if I witness waste, bad behaviour, or inefficiency.”
Why People Do and Don't Speak Up
We might think that intervening if we’re aware of wrongdoing is a natural response—but in practice, people often succumb to the “ bystander effect ,” 1 which can lead them to avoid becoming involved. Reasons for staying silent include the belief that someone else will intervene, being influenced by how concerned other people are about the situation, a lack of confidence in their abilities to intervene, or concern over how they will be judged for taking action. 2
Recent research 3 supports what my clients have told me and suggests that autistic people may be more likely to voice concerns when made aware of inefficient processes and dysfunctional practices in the workplace than non-autistic people. In an online survey, 33 autistic employees and 34 nonautistic employees were presented with examples of potential workplace situations, which contained an example of workplace dysfunction, including an ethical issue or example of operational inefficiency. Both groups were asked to evaluate the hypothetical examples at work and state what, if any, action they would take if they were aware of ethical issues or inefficiencies.
Why were autistic participants more likely to express a desire to take action? It might be because autistic people tend to be less worried about what others think when making moral judgments. 4 It could also be the case that autistic people internalise a different set of cultural beliefs and psychological rules in their earlier developmental stages, which makes them less likely to be influenced by others. 5
Previous research has found that autistic people are more likely to make moral decisions based on the consequences of their behaviour as opposed to their emotional response to the situation. 6 In hypothetical research scenarios, this typically involves making difficult moral decisions that may negatively affect a small number of people in service of a greater good. In real life, as the example of my client, Anita, shows, this might involve reporting a colleague’s or organisation’s poor practices to protect others from harm.
What This Means for Autistic People in the Workforce
Taken together, these findings suggest that when autistic people see injustices, inappropriateness, or inefficiency, there’s less “noise”—meaning ingrained social values or concern over how they will come across to others—to distract them from taking action. As the authors of the most recent study suggest, employing people who are ready to raise concerns about both minor and major workplace issues or malpractice can have a positive impact on the workplace. This is especially important to note because autistic people are more likely than non-autistic people to be unemployed or under-employed. 7
Some large companies, including Ford, Google, and Microsoft, have long realised the positive impact that autistic employees can have. This new research highlights the value that autistic employees bring at an organisational level for companies that care about protecting their employees and customers and acting in an efficient, professional, and ethical manner.
1. Latane, Bibb, and John M. Darley. "Group inhibition of bystander intervention in emergencies." Journal of personality and social psychology 10.3 (1968): 215.
2. Ross, Lee, and Richard E. Nisbett. The person and the situation: Perspectives of social psychology . Pinter & Martin Publishers, 2011.
3. Hartman, LM, Farahani, M, Moore, A. et. Al (2023) Organizational benefits of neurodiversity: preliminary findings on autism and the bystander effect. Autism Research, 16(10): 1989-2001
4. Frith, Uta, and Chris Frith. "Reputation management: In autism, generosity is its own reward." Current biology 21.24 (2011): R994-R995.
5. Hartman, LM, Farahani, M, Moore, A. et. Al (2023) Organizational benefits of neurodiversity: preliminary findings on autism and the bystander effect. Autism Research, 16(10): 1989-2001
6. Brewer, R., Marsh, A. A., Catmur, C., Cardinale, E. M., Stoycos, S., Cook, R., & Bird, G. (2015). The impact of autism spectrum disorder and alexithymia on judgments of moral acceptability. Journal of abnormal psychology , 124 (3), 589.
7. Howlin, P. (2013). Social disadvantage and exclusion: adults with autism lag far behind in employment prospects. Journal of the American Academy of Child & Adolescent Psychiatry , 52 (9), 897-899.

Claire Jack, Ph.D. , is a hypnotherapist, life coach, researcher, and training provider who specialises in working with women with autism spectrum disorder (ASD). She was herself diagnosed with ASD in her forties.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 05 September 2022
A capabilities approach to understanding and supporting autistic adulthood
- Elizabeth Pellicano ORCID: orcid.org/0000-0002-7246-8003 1 , 2 ,
- Unsa Fatima 1 ,
- Gabrielle Hall 1 ,
- Melanie Heyworth 1 , 3 ,
- Wenn Lawson 1 ,
- Rozanna Lilley ORCID: orcid.org/0000-0001-6143-8805 1 ,
- Joanne Mahony 1 &
- Marc Stears 4
Nature Reviews Psychology volume 1 , pages 624–639 ( 2022 ) Cite this article
24k Accesses
47 Citations
319 Altmetric
Metrics details
- Autism spectrum disorders
There is little comprehensive research into autistic adulthood, and even less into the services and supports that are most likely to foster flourishing adult autistic lives. This limited research is partly because autism is largely conceived as a condition of childhood, but this focus of research has also resulted from the orthodox scientific approach to autism, which conceptualizes autistic experience almost entirely as a series of biologically derived functional deficits. Approaching autism in this way severely limits what is known about this neurodevelopmental difference, how research is conducted and the services and supports available. In this Review, we adopt an alternative research strategy: we apply Martha Nussbaum’s capabilities approach, which focuses on ten core elements of a thriving human life, to research on autistic adulthood. In doing so, we identify areas where autistic adults thrive and where they often struggle, and highlight issues to which researchers, clinicians and policymakers should respond. The resulting picture is far more complex than conventional accounts of autism imply. It also reveals the importance of engaging autistic adults directly in the research process to make progress towards genuinely knowing autism and supporting flourishing autistic lives.
Similar content being viewed by others
Modelling resilience in adolescence and adversity: a novel framework to inform research and practice

Mental health challenges faced by autistic people
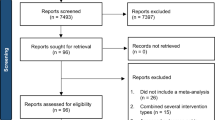
Efficacy of psychosocial interventions for Autism spectrum disorder: an umbrella review
Introduction.
Autism is a lifelong neurodevelopmental difference that influences the way a person interacts and communicates with others and experiences the world around them 1 . For decades, autism research focused predominantly on autistic children 2 , in line with the very earliest descriptions of autism 3 , 4 and the tendency for society to depict autism as a disability of childhood 5 . The result is a substantial lack of understanding about the opportunities and challenges that autistic adults face in building their futures, achieving their goals and living satisfying and fulfilling lives. These issues clearly matter, however, and in the past decade there has been an increase in publications on autistic adulthood, a new journal specifically dedicated to autism in adulthood, a notable increase in funding dedicated to adult-related issues 6 and numerous policy interventions designed to assist autistic adults to live good lives 7 .
Serious obstacles nevertheless continue to prevent researchers, clinicians, educators, policymakers and the broader public from fully grasping the nature of contemporary autistic adulthood. Overcoming these obstacles is vital not only because they constrain understanding but because they also hinder efforts to inform and transform the services and supports that might enhance autistic adults’ lives.
Paramount among these obstacles is the orthodox approach taken in conventional autism research, in which there is an overfocus on ‘deficits’ or ‘impairments’ of autistic adulthood and an overemphasis on specific attributes of individuals as opposed to the broader contexts in which autistic adults live 8 , 9 . This conventional research paradigm derives both from long-standing conventions in medicine, which prioritize a putatively objective standard of ‘bodily health’ over a subjective understanding of ‘well-being’ 10 , and from the developmental psychopathology literature, which stresses the importance of ‘patterns of maladaptation’ in shaping the life course of autistic people 11 . Consequently, individual autistic adults’ behavioural, cognitive and neural functionings are frequently compared with some typical or ‘normal’ level of ability that is held as the ideal ‘state of health’ 9 ; interventions and treatments typically aim to remediate these apparent shortcomings to align functioning with the accepted norm. This narrow focus on deficits results in a radically constrained understanding of the experiences that shape autistic lives, limiting the range of supports and services to those that seek to ‘change the individual’ rather than consider how to ‘change the world’. Conventional research efforts are also routinely conducted without meaningful input from autistic people themselves 12 , meaning that often the wrong questions are posed and findings are misinterpreted. Research of this kind can be said to be ‘lost in translation’ 13 . As such, most research on autism prioritizes researcher-defined normative life goals without discovering how much they matter to a diverse range of autistic people 14 , 15 .
In this Review, we — a team of autistic and non-autistic researchers — propose an alternative way of approaching adult autism research. First, we provide some context by briefly discussing the diagnosis and developmental trajectories of autistic adults. Next, we describe Nussbaum’s capabilities approach 16 , 17 , which outlines ten central capabilities that enable people, whether autistic or non-autistic, to lead lives that are of value to them on their own terms rather than to meet a predetermined normative standard set by others. We then examine each of the ten capabilities in the context of available autism research. This approach enables us to evaluate the opportunities and challenges facing autistic adults, the forces shaping them and the ways in which services and other interventions might enhance the quality of their lives.
Diagnosis and developmental trajectory
Adult diagnosis of autism first became available in the 1980s (ref. 18 ) and was further encouraged by changes in the Diagnostic and Statistical Manual of Mental Disorders , fifth edition (DSM-5) (refs. 1 , 19 ) several decades later. Many autistic adults initially seek their diagnosis following concerns about social relationships and mental health, sometimes precipitated by a personal crisis or by the diagnosis of their own children. For many, this search for diagnostic clarity is preceded by decades of feeling ‘different’ and of relationship or employment difficulties 20 , 21 . Challenges to adult autism diagnosis are discussed in Box 1 .
A growing number of adults self-identify as autistic without a formal diagnosis 22 . This self-identification is controversial in research and clinical communities but is often accepted in the autistic community, in part because, even in high-income countries, autistic adults often remain undiagnosed 2 , 23 , 24 and, even when formally diagnosed, are only minimally supported 2 , 7 , 23 , 24 , 25 . Those diagnosed later in life may have higher self-reported autistic traits and poorer quality of life, especially mental health, than those diagnosed in childhood 26 .
Following the normative tendencies of the conventional approach to autism research, the vast majority of studies that have examined the developmental trajectories of autistic adults diagnosed in childhood focus on areas thought to be critical for achieving ‘good’ adult outcomes. In longitudinal studies, these outcomes are often defined in terms of a set of standard ‘life achievements’, on which autistic adults typically fare badly 14 , 15 . For example, autistic adults with and without intellectual disability followed from childhood are less likely than non-autistic people to hold down a job, live independently or have friends and intimate relationships 2 , 14 , 15 . Other longitudinal studies have examined whether people remain ‘autistic’ (that is, meet instrument and/or clinical thresholds for autism) as they move from childhood into adulthood. These studies show that the diagnostic status of individuals diagnosed in childhood generally endures into adulthood 15 , 27 , with the exception of a minority of individuals who no longer display sufficient core autistic features to warrant a clinical diagnosis, which is sometimes described as an ‘optimal outcome’ 28 . Yet despite initial variability, many people show little change in researcher-defined ‘autistic symptoms’ as they move into adulthood 29 , potentially placing them at greater risk for poor psychosocial outcomes in adulthood 30 .
More detailed research on the quality of life of autistic adults also largely focuses on the achievement of standard life outcomes, irrespective of whether those outcomes are considered meaningful by autistic adults themselves 31 , 32 . Studies that have complemented standard, researcher-defined measures with more subjective, autistic person-led measures (such as quality of life) consistently demonstrate that outcomes are more positive when subjective factors are accounted for 14 , 15 . For example, an autistic person who is highly dependent on others for their care — a so-called ‘poor outcome’ according to the standard framework — might nevertheless be happy and subjectively enjoy a very good quality of life. Another autistic person who no longer meets the diagnostic criteria for autism — a so-called ‘good’ outcome — might struggle to find their way in the world and feel different and distant from others. Approaches that focus on researcher-defined measures in this way limit understanding and risk failing to grant autistic people the dignity, agency and respect they deserve.
In considering how to respond to these limitations, it is helpful to establish two clear aims. First, research into autistic adulthood must recognize that people’s life chances (opportunities each individual has to improve their quality of life) are shaped by a range of factors beyond the person, consistent with an ecological perspective 33 . That is, quality of life is influenced both by biological factors at the heart of the conventional medical model and a broader set of contextual factors as stressed by the social model of disability 34 . Second, no one, autistic or not, has high quality of life if their life goals are primarily set by others. Thus, quality of life should not be measured by a standard set of outcomes judged to be important by researchers, clinicians or policymakers. Instead, the goals of each individual’s varied human life should be at least partly set by the person themselves 35 .
Box 1 Challenges for autism diagnosis in adulthood
In most countries, adults seeking an autism assessment and diagnosis face severe challenges, and the individual is expected to initiate and navigate the process 24 . Although there are published guidelines 7 , 294 , major differences exist between guidelines and actual experience 295 . Adults seeking diagnosis report lengthy waiting times and prohibitive costs 2 , 24 , and encounter clinicians who lack a nuanced understanding of autism 75 , 174 . Further, the guidelines are far from standardized in their recommendations for the use of adult diagnostic tools and there is much variation in practice 2 , 7 , 294 .
The process of adult autism diagnosis is also challenging owing to difficulties in recovering early developmental history and the self-reported tendency of many autistic adults to use strategies (masking or camouflaging) to minimize autistic features 274 , 275 . Although autistic adults of all genders have been reported to mask 275 , it is more often reported among women 296 , which could be one reason why twice as many men present to adult diagnostic services 297 . These findings dovetail with a growing recognition of gender bias in autism diagnosis 2 , 7 .
More research concerning adult autism diagnosis is needed. For example, little is known about the diagnostic experiences of autistic adults with intellectual disability 24 , about how autism is identified in different cultural contexts or about adult autistic experiences in the Global South 298 . It is likely that autistic adults in many low and middle-income countries do not have access to formal diagnosis, post-diagnostic supports or the positive transformations in self-understanding and connections to a peer community that often accompany diagnosis 181 , 217 , 261 .
A capabilities approach to autistic lives
Martha Nussbaum’s 16 , 17 capabilities approach to quality of life, which has been widely used to analyse social disadvantage in multiple settings, satisfies both of the aims outlined above. First, according to the capabilities approach, a human ‘capability’ is not an intrinsic ability that a person has or does not have solely by virtue of who they are. Instead, ‘capability’ refers to the actual opportunity to be or do something that is facilitated or constrained by features of the person and by the broader contexts in which a person is embedded. The relevant contexts can include close family and household influences; everyday community interactions; educational institutions; economic factors, including the cost of living; services and supports, including accessibility and performance of healthcare institutions; and the broader social and political context, including social attitudes towards autism. Second, flourishing human lives are characterized by a set of these capabilities which enable a person to achieve any number of a range of outcomes, rather than by the attainment of a small number of pre-specified outcomes. These capabilities are considered foundations for a range of doings and beings; they shape what a person can do and, critically, who and how they can be in the world. Capabilities are not a narrow or specific set of achievements, nor are they possessions. Similarly, capabilities cannot be ranked or interpreted by a group of people, such as professionals, or reduced to a single score on a standardized scale. Instead, they refer to the preconditions for a broad range of ways of living.
According to Nussbaum, there are ten central capabilities that most people need if they are to be able to choose and create lives that are meaningful and fulfilling on their own terms 16 , 17 (Table 1 ). In what follows, we outline how analysing the life chances of autistic adults through this lens can enable a far richer understanding of autistic adults’ lives of all abilities (see Box 2 ) than the conventional research approach. We do so by highlighting the strengths and challenges of autistic adults in each of the ten central capabilities, and their causes, and consider the potential supports, services and changes in societal attitudes that might help to transform those challenges into strengths. Analysing these capabilities provides a way to examine the lives of autistic adults without narrow normative judgement, while also directing attention to issues that require intervention and support. Readers are advised that some of this material may be distressing and evoke difficult past associations.
Box 2 Inclusivity and the capabilities approach
The capabilities approach focuses on the real opportunities that are open to each person to live in ways that are meaningful to them. Applying such an approach to research on autistic adulthood enables identification of the ways in which autistic people can thrive on their own terms and the nature of the obstacles to this thriving. Diverging from more conventional medical frameworks, the key to this approach is the value of personal autonomy: the belief that all people, including autistic people, should enjoy the right to be at least ‘part author’ of their own lives 35 and that their quality of life should always be measured, at least in part, according to their own aspirations.
Although widely used in other settings 299 , the capabilities approach is novel in the context of autism, partly because it has previously been suggested that this sort of autonomy-inflected approach is ill-suited to a substantial proportion of the autistic community 300 . Non-speaking autistic people, those with intellectual disabilities and/or those with very high support needs have sometimes been considered unable to communicate or conceptualize their precise wishes in the ways the capabilities approach seems to require. From this perspective, the capabilities approach is applicable only to those who can make and articulate judgements about their own life purposes and not to the entire autistic population.
Some have called for a fine-grained approach to the heterogeneity within autism, suggesting that the autism spectrum should be split into those for whom an autonomy-inflected approach could be appropriately applied and those for whom the traditional medical model may be better suited 300 . Similarly, others have called for the creation of a separate ‘profound’ or ‘severe autism’ diagnostic category for those with the most severe impairments 7 , 301 .
We do not believe that we need to be this pessimistic. There is no clear scientific basis for segmenting the autism spectrum in the way that proponents of a separate ‘severe’ or ‘profound’ autism label suggest. Moreover, doing so poses grave risks, potentially excluding people deemed ‘severe’ or ‘profound’ from the concern, dignity and respect offered to others 302 , 303 . Nonetheless, it is crucial for future research into autistic quality of life to consider people of all abilities. Such research should investigate whether augmentative and alternative communication can enable those with higher support needs to make their needs and desires known 304 . Future research should also examine the effectiveness of available long-term services and supports to enable those with the greatest needs to fulfil key aspects of quality of life. This work would acknowledge the inevitable complexities of deploying the capabilities approach in these instances while recognizing that it remains possible to develop a broad and subtle framework for the evaluation of quality of life across the whole autistic community.
The first central capability is “being able to live to the end of a human life of normal length; not dying prematurely, or before one’s life is so reduced as to be not worth living” 17 . Autistic adults are currently at a substantial disadvantage in this capability. There are persistent patterns of premature mortality in the autistic population 36 , 37 . Autistic people are twice as likely to die prematurely as non-autistic people 36 , 37 , 38 , and this risk is greater for autistic women 36 , 38 (but see ref. 37 ) and those with intellectual disability 36 , 37 , 38 . The lives of autistic people are, on average, 16 years shorter than those of non-autistic people 36 . The risk of death is elevated in autistic people who experience poor physical health or chronic illness (including epilepsy) 36 , 37 , 38 , 39 . Little is known about the influence of social and economic factors, including access to healthcare, on these mortality rates, but it is widely hypothesized that an important contributor is the extent to which physicians listen to, and learn from, their autistic patients 40 .
Among the specific causes of premature mortality, there is a higher risk of suicide 41 , 42 . Suicide attempts are more frequent and more likely to result in death in autistic people than in non-autistic people 36 , 37 , 43 , 44 , 45 , possibly owing to co-occurring psychiatric conditions 36 . Research focused on understanding why autistic people are at increased risk of self-harm and suicide has identified individual risk markers common to those in the general population, including (younger) age 46 , low mood and rumination 47 . More work is needed to understand potentially unique risk markers for increased suicidality in autistic people, including broader interpersonal causes (such as thwarted belonging and perceived burdensomeness) which might mediate associations between autistic traits and suicidality 48 , and systemic issues (such as clinicians’ lack of knowledge 49 ).
More generally, autistic quality of life in older adulthood (adults aged 50 years and older 50 ) — albeit as assessed using normative measures — is seen as considerably poorer than that in non-autistic older adults 51 . Social isolation and loneliness are major issues for all older adults, leading to greater risk of dementia and other serious medical conditions 52 . Both social isolation and loneliness might disproportionately influence older autistic adults, who might be more prone to reclusiveness 53 , despite many autistic adults describing a longing for interpersonal connection 54 . For example, in a study in which autistic adults’ experiences of growing older were elicited, one autistic participant said “I think I’m a born loner, quite frankly … Maybe I’m not the kind of person to have a life. Oh, I’d love it, with a person that would understand me” 54 . There are few longitudinal and participatory studies focusing on autistic older people, including under-represented populations who might have poorer life satisfaction. Thus, little is known about how autistic adults can be supported to live a full and satisfying life into old age in diverse sociocultural contexts 55 , 56 .
Bodily health
The second central capability is “being able to have good health, including reproductive health; to be adequately nourished; to have adequate shelter” 17 . Once again, the evidence suggests that autistic adults are disadvantaged in this regard. Co-occurring physical conditions are common across the autistic lifespan 57 , 58 , 59 and are more prevalent than in the general population for almost all conditions assessed 43 , 58 , 59 , even when lifestyle factors are considered 58 . Autistic adults with intellectual disability have distinctive needs 59 and might be especially vulnerable to poor physical health 60 .
Risks for most physical health conditions are further exacerbated for autistic women 58 , 61 . Understanding the mechanisms for these differences in health outcomes is critical for reducing these inequalities. Moreover, further clarifying the temporal development of these health problems should inform how interventions are designed to prevent and treat them 62 . There are at present very few studies on autistic people’s reproductive health. Autistic women report challenging experiences with menstruation, including a cyclical amplification of sensory differences and difficulties with emotional regulation 63 , 64 , and autistic women are at greater risk for pregnancy complications 65 . Autistic women also report significant deterioration in everyday quality of life during menopause 66 . None of these concerns have yet been investigated in depth. Likewise, there are no studies specifically addressing the reproductive health experiences of autistic men, those with intellectual disability and/or those who are non-speaking; no studies have adopted a less gender-binary approach to reproductive health in autistic adults. This absence of research potentially leaves crucial areas of experience unsupported by clinicians and other policy interventions.
Autistic adults also face barriers to healthcare 67 , 68 , 69 . Despite greater healthcare utilization, medication use and higher healthcare costs than the general population 70 , autistic adults report more unmet health needs 71 , lower utilization of preventative care 71 and more frequent use of emergency departments 71 , 72 than non-autistic adults. Healthcare settings are often inaccessible to autistic adults, with significant risk of sensory and social overwhelm , miscommunication and lack of autistic-informed care 67 , 73 . Autistic people also experience reduced coordination of care compared with non-autistic people, particularly during the transition from paediatric to adult services 74 . Thus, autistic adults are often left to fend for themselves in navigating the healthcare system 75 , resulting in negative healthcare experiences and feelings of distrust 66 , 67 .
Autistic adults also report poor patient–provider communication (in both directions): autistic adults often face difficulties identifying and articulating their physical health symptoms 76 and professionals often do not appreciate the need to adapt their communication style for autistic patients and do not take their autistic patients’ concerns seriously 67 , 68 , 71 . Clinicians’ limited knowledge of 68 , 69 and lack of confidence in 75 understanding autistic adults’ specific needs further exacerbate these difficulties. Some tools have been developed to assess barriers to healthcare access experienced by autistic adults from their own perspective 71 or from their caregiver’s or healthcare provider’s perspective 77 . The person-related, provider-related and system-related barriers identified using these tools should facilitate future research that seeks to improve the care and health of autistic people 71 , 78 . However, research designed in collaboration with autistic people is needed to assess the most effective ways of improving their healthcare experiences 56 , 67 , 78 .
Many other external factors influence autistic adults’ physical health, such as access to affordable, appropriate housing. Initial studies suggest that autistic adults might be over-represented in homeless communities at rates substantially higher (12–18% 79 , 80 ) than adult population prevalence estimates (1% 81 ). The range of challenges facing autistic adults might predispose them to homelessness, and reduced social support networks might compound other risk factors, including unemployment, making it difficult for autistic adults to exit homelessness.
Other housing challenges also influence this crucial capability. Compared with other people with disabilities, autistic adults are less likely to live independently, leaving them vulnerable to the inadequacies of institutionalized housing. Formal institutional living and similar settings that purport to be community-based, but are often only nominally so 82 , have been criticized for displacing people from their families and communities and for providing poor and unresponsive services to residents 83 , 84 . Nonetheless, autistic adults continue to be over-represented in more restrictive and segregated settings 85 .
In sum, the bodily health of autistic adults is severely compromised at present in many regards, owing to failings in clinical provision and in the broader social and economic context within which they must lead their lives.
Bodily integrity
The third capability is that people should be “able to move freely from place to place; to be secure against violent assault; having opportunities for sexual satisfaction and for choice in matters of reproduction” 17 . This capability is underpinned by a person’s right to make decisions about their body.
There are good reasons to be concerned about autistic disadvantage in accessing this capability. Autistic children are at substantial risk of experiencing multiple forms and repeated occurrences of victimization and abuse 86 , and this vulnerability persists into adulthood 87 , 88 , 89 , 90 . In particular, there are elevated rates of sexual victimization in autistic compared with non-autistic adults 89 , 90 , especially in autistic women 91 , 92 , 93 and those who identify as a gender minority 92 or as a member of the LGBTQI+ community 94 . This increased vulnerability might be exacerbated by the fact that autistic people often have reduced access to good quality, effective sexual education 95 , which can impart vital protective knowledge, as well as by broader structural inequalities (for example, lack of access to healthcare 67 , 68 , 69 ).
Autistic adults also experience increased rates of physical assault 87 , 92 and domestic violence, largely perpetrated by people known to them 90 . Autistic women, particularly those who report multiple traumatic experiences, emphasize deeply distressing betrayals of trust 91 and how they often “just couldn’t see it coming” 93 . Worryingly, these already high victimization rates are likely to be an underestimate: autistic adults are less likely to report experiences of violence to the police 87 or even to confide in others 87 . Autistic adults who experience victimization therefore receive neither the requisite mental health support nor the critical social support that could reduce the likelihood of developing post-traumatic symptoms.
Concerns about physical safety also influence the ability to move freely. Many autistic adults want to be able to access work and go about their daily activities within their communities 96 , and parents often want this independence for their children too 96 . Yet both groups worry about safety. Use of public transportation can be challenging for autistic adults owing to lack of accessibility 97 and difficulties with wayfinding and traffic judgement 98 . Furthermore, despite research showing that autistic drivers are more rule-abiding than non-autistic drivers 99 and are no more likely to be at fault for a police-reported car crash 100 , few autistic people take up driving 101 , partly because of perceived difficulties in spatial awareness, motor coordination, processing speed and executive function 96 . Consequently, autistic adults can remain reliant on their parents. As one autistic adult expressed in a focus group on understanding autistic adults’ transportation needs and barriers: “If I want to go shopping in the middle of the day I can’t. I have to wait for my mom to come home from work” 96 .
Finding a balance between autonomy and safety is critical. Autistic children and adults can be more susceptible to wandering 102 , 103 , and parents sometimes advocate the use of measures such as tracking devices 104 . Yet wandering can occur for many reasons 102 and is often purposeful 104 . Researchers and activists warn of the negative impact surveillance technologies can have on people’s independence and urge investment in alternatives such as community supports and safety skills training 104 , 105 .
Bodily integrity is inextricably linked to other capabilities. Violations of bodily integrity have adverse effects on other capabilities 106 , including mental health 107 , bodily health, interpersonal relationships and sense of agency. Threats to bodily integrity are also likely to influence autistic people’s sense of sexual well-being and their freedom to achieve it. Long-held views of autistic people being uninterested in sexual experiences 108 have been firmly quashed by research showing that autistic adults desire sexual relationships to a similar extent as non-autistic adults 109 , 110 . Autistic adults in satisfying relationships are more likely to report greater sexual satisfaction, just like non-autistic adults 111 . They also identify with a wider range of sexual orientations 94 , 109 , 112 and gender identities 113 , 114 , 115 , 116 , their sexual ‘debuts’ occur at a later age 117 and they have fewer lifetime sexual experiences 112 than non-autistic adults. The lack of qualitative studies on the realities of autistic adults’ sexual lives limits understanding, despite the fact that this topic is prioritized by the autistic community 118 .
Senses, imagination and thought
The fourth capability focuses on being “able to use the senses, to imagine, think, and reason — and to do these things in … a way informed and cultivated by an adequate education … being able to use imagination and thought in connection with experiencing and producing [creative] works … Being able to have pleasurable experiences and to avoid nonbeneficial pain” 17 . The dominance of the conventional medical model has meant that autism is often associated with deficits in this regard 119 . There is often a presumption that autistic adults will struggle with higher-order cognition or have low intelligence owing to poor performance on standard intelligence tests 120 . This stereotype persists even though there is little evidence for it in the everyday experience of the autistic population 121 . There is an even greater presumption of low intelligence in autistic people who are non-speaking or do not use traditional forms of communication 122 , who are routinely under-recruited in research 123 . Similarly, researchers, clinicians and educators have long presumed that creative and imaginative skills and aspirations are limited in autistic people 124 .
However, the predominant use of standard intelligence tests can lead to an underestimation of autistic people’s intellectual ability 120 , particularly in non-speaking people 125 . Autistic people have also been shown to excel at producing novel responses on creative tasks 126 and are increasingly recognized for their creative talents 127 , with major companies investing in autistic people’s ‘out-of-the-box’ thinking 128 . These strengths have been linked to autistic people’s different way of perceiving the world, including detail-focused processing style 129 and enhanced perceptual abilities 130 , which might be underpinned by heightened sensory perception 131 .
Nevertheless, autistic people are, in general, poorly served by the educational environments that might further enhance this capability 132 . They regularly encounter sensory overwhelm within the physical school environment 133 , struggle with complex social expectations and interactions 134 , experience bullying and social isolation 135 , and are stigmatized by a presumption of low competence 136 . Moreover, limited attention is given to their specific needs, strengths and preferences 132 , 137 , including by school staff who lack confidence in supporting autistic students 138 . Being excluded from 139 or not completing 140 school can have persisting negative effects on mental health and well-being.
Increasing numbers of autistic adults are enrolling in higher education 141 , but barriers exist there too. Autistic adults rarely receive relevant supports and accommodations, partly because they are hesitant to disclose their diagnosis or find it difficult to reach out for help 141 and partly owing to the absence of formal transition planning 142 . Consequently, autistic adults are at high risk of dropping out of university 143 . There is also limited research on the destinations of autistic students who complete higher education 144 , so it is unclear how to best respond to these challenges.
The senses, imagination and thought capability also emphasizes the importance of being able to take pleasure from sensory experiences. Although research tends to focus on the challenges that autistic sensory differences — such as experiences of sensory overload — bring to people’s everyday lives 145 , sensory stimuli can also be a source of pleasure 146 , 147 . For example, one autistic adult reported enjoying “touching metal a lot … cold smooth metal is, like, just amazing” 147 . There is also evidence that autistic adults with limited spoken communication in a supported living environment find joy in the everyday, for example in the sound of the washing machine on the last spin or the feel of bubbles while dishwashing 146 , 148 .
However, these distinctive sources of pleasure are often pathologized. This is captured by the debate over certain ‘repetitive motor stereotypies’ such as hand-flapping 1 , which have been reclaimed by autistic adults as ‘stimming’ 149 . These behaviours tend to be perceived as an individual problem with no clear purpose or function that prevent the person from learning skills and interacting with others 150 . Stimming behaviours are often the target behaviour for interventions that promote ‘calm’ or ‘quiet’ hands 151 (cf. ref. 152 ). However, there is very little evidence that stimming behaviours are harmful to autistic people or their peers (the same cannot be said for self-injurious behaviours, which might also be purposeful but are nevertheless harmful to the person). In fact, it now seems likely that stimming behaviours can serve as a source of pleasure or reassurance or a form of self-regulation 149 .
The next capability is defined as “[b]eing able to have attachments to things and people outside ourselves; to love those who love and care for us, to grieve at their absence; in general, to love, to grieve, to experience longing … not having one’s emotional development blighted by fear and anxiety” 17 . The empirical literature shows that autistic adults have more difficulties recognizing others’ emotions 153 , 154 and identifying and describing their own emotions (alexithymia) than non-autistic people 155 , 156 . However, emerging work suggests a far more nuanced picture: autistic adults describe feeling emotions and empathy intensely 157 and often experience deeply satisfying emotional lives 158 .
At their most extreme, the conventionally reported difficulties with emotions were thought to preclude autistic people from the capacity to love or desire meaningful romantic and intimate relationships 159 . However, research is inconsistent with this claim 160 . Romantically involved autistic adults report high relationship satisfaction 93 , 161 . The strong bonds that autistic adults report with their partners, particularly with those who are also autistic 160 , extend to their autistic children, with whom they describe an intense connection and love 162 .
These reports speak strongly against an understanding of autism as a ‘disorder’ of affect. Rather than lack of interest, autistic adults often cite significant challenges with initiating and maintaining romantic relationships 154 , including difficulties reading and interpreting others’ emotions 161 , which can impact their capacity to remain romantically involved. The stereotyped assumptions of non-autistic people that autistic people are uninterested in interpersonal relationships might also be an obstacle 163 . These challenges can intensify feelings of loneliness and are linked to significant negative emotional experiences and poor mental health 164 . Autistic adults who desire intimate connection but whose needs are unfulfilled might be at particular risk of depression and low self-worth 164 , 165 .
This loneliness, depression and poor self-perception can take a substantial toll on mental health and well-being 164 , 166 , 167 . A substantial proportion of autistic adults experience a co-occurring psychiatric condition during their lifetime, with anxiety and mood disorders being the most common 168 , 169 . Rates of co-occurring psychiatric conditions are somewhat lower for autistic adults with intellectual disability 170 , but these rates might be underestimated owing to a lack of detailed understanding in how best to characterize and measure mental health in this context 168 . The risk of developing mood disorders increases with age 168 and autistic adults are at elevated risk of developing post-traumatic stress disorder 107 . Some mental health problems in autistic adults have been attributed to everyday discrimination and internalized stigma 171 .
The reliance on mental health assessments and diagnostic criteria that were established in non-autistic people 168 , 172 , 173 and a lack of necessary expertise among health professionals 174 might result in an overestimation or underestimation of mental ill health in the autistic population 173 . Some autistic characteristics might overshadow indicators of mental health conditions (for example, social withdrawal and sleep disturbance are common to both autism and depression), suggesting that co-occurring mental health conditions might go unrecognized 173 , 175 . Similarly, mental health diagnoses might overshadow an autism diagnosis, resulting in misdiagnosis 175 .
Mental health difficulties in autistic adults are likely to be compounded by the inadequacies of formal and informal supports. Autistic adults report a significantly higher number of unmet support needs than the general population 25 , struggle to obtain appropriate post-diagnostic support 176 and face challenges in accessing individually tailored treatment for mental health problems 25 , 176 . As one autistic adult put it: “I haven’t requested any, because people like me don’t get support” 25 . There is a clear need for mental health interventions that are adapted to autistic people’s needs and preferences 176 .
Practical reason
The next capability, practical reason, is defined as “being able to form a conception of the good and to engage in critical reflection about the planning of one’s own life” 17 . The three key elements of this capability — choosing what one wants to do, critically reflecting on that choice and making a plan to realize it — are fundamental to making full use of all the other capabilities.
It is sometimes assumed that people with cognitive disability, including some autistic people, are incapable of practical reason, failing even at the initial task of deciding what it is that they value or desire 177 . Autistic people were traditionally thought to have impaired self-awareness 178 . A substantial minority of autistic adults have co-occurring intellectual disability (29% 179 ) and some do not use speech to communicate 180 , which can make it difficult for others to gain insight into their thinking. However, research demonstrates that autistic people have a deep capacity to reflect on many aspects of the self, regardless of their intellect or communication preferences 181 , 182 .
The practical reason capability also requires people to be able to reflect critically on their choices, and to change their mind. Here, it seems that autistic people might approach decision-making differently to non-autistic people 183 , 184 . Autistic adults make more logically consistent, rational decisions 185 , are more circumspect in their decision-making, sample more information prior to making a decision 186 , are less susceptible to social influence 187 and are more deliberative in their reasoning 188 , 189 .
However, first-hand accounts suggest that such an approach to decision-making can have its disadvantages. For example, autistic people report challenges changing their decisions, especially if the change is unanticipated or requires a shift in routine 190 . Indeed, autistic people’s tendency to focus intensely on topics or objects of interests ( monotropism ) 191 can make it difficult to ‘move on’ or ‘change gears’ 192 . Interrupting activities after such states of flow and difficulties starting new activities (autistic inertia) can lead to pervasive and often debilitating effects on autistic adults 192 , including on their ability to design and execute a plan.
Many of the above skills come under the broader umbrella of executive function (higher-order processes that underpin goal-directed activity and enable individuals to respond flexibly to change and plan their actions accordingly) 193 . Problems with planning, organization and future-oriented thinking are common in autistic adults 189 , are linked to adaptive difficulties 194 , 195 , might be compounded by particular contexts (such as in parenting 196 or the workplace 197 ) and are perceived to be real obstacles to achieving desired outcomes 198 . Interventions and supports that focus on planning and decision-making are scarce, but those that do exist are associated with gains in executive function-related behaviours in real-world settings 199 .
Affiliation
The next capability is “being able to live with and toward others, to recognise and show concern for other human beings, to engage in various forms of social interaction … and having the social bases of self-respect and nonhumiliation; being able to be treated as a dignified being whose worth is equal to that of others” 17 . Simply put, that the person is respected as a social being 17 . Prima facie this might be the capability in which autistic adults might be expected to be at the greatest disadvantage. After all, the term ‘autism’ comes from the Greek autos , meaning both ‘self’ and ‘by itself’, and autistic people are often described as preferring a life of self-isolation 163 . Dominant characterizations suggest that autistic people lack the motivation 200 and/or cognitive building blocks 201 for social interaction, which prevents them from establishing and maintaining the types of reciprocal relationships that are fundamental for this capability.
Research has repeatedly shown that autistic children and adolescents have fewer reciprocal friendships 202 , 203 , are often on the periphery of social networks 202 , 203 and spend less time with their friends outside school than their non-autistic counterparts 204 . Autistic adolescents also report a growing awareness of feeling different from others despite wanting to ‘fit in’ 205 , 206 , and frequently experience social exclusion and bullying 135 , which might exacerbate their challenges in making and keeping friends. These patterns persist into adulthood 207 . It is therefore unsurprising that many interventions in adolescence and early adulthood focus on formal social skills training 208 , 209 , with the aim of equipping autistic people to manage everyday social relationships on their own terms and, thereby, secure this capability.
However, such interventions fail to appreciate that autistic sociality is shaped by the sociocultural context in which people are embedded 208 , 210 , 211 . Autistic people can and do have fulfilling connections with others, even if negotiating those relationships can be challenging 93 . They are drawn to those who accept them for who they are 154 , 159 , 161 and with whom they do not have to mask their autistic ways 212 , 213 . These friendships include (but are not restricted to) autistic-to-autistic interactions 214 , 215 . As one participant reported in a study on autistic adults’ experiences of loneliness and social relationships: “though many of us have only met each other three to four times, it feels as if we have known each other forever. Because all of a sudden you are in a community with someone where you are on the same wavelength … it is a really strong experience” 216 . Such autistic-to-autistic interactions promote self-understanding 181 , 214 , 217 , positive self-identity 217 , 218 and well-being 219 .
Isolation owing to the COVID-19 pandemic has also revealed that autistic people long for social connection in the same way as everyone else, both in terms of close, trusting relationships and fleeting, incidental interactions. As one autistic interviewee said when describing their lockdown experience: “I didn’t realise how important that incidental human contact was to me. It was so incidental that it never really registered on my radar until it was gone” 167 . Autistic people’s need for human connection and the extent to which social isolation plays a role in autistic people’s mental health distress have been underestimated by conventional accounts.
The double empathy problem 220 suggests that there is a misalignment between the minds of autistic and non-autistic people. This misalignment leads to a lack of reciprocity in cross-neurotype interactions and is the source of social communication difficulties between autistic and non-autistic people 221 , 222 . Empirical evidence suggests that non-autistic people have difficulties understanding the minds and behaviours of autistic people 221 , 222 , and that they are unwilling to interact with autistic people on the basis of initial judgements or interactions 221 , 222 , 223 . Thus, non-autistic people also interact less successfully with autistic people, compared with other non-autistic people 224 .
These cross-neurotype interaction difficulties can lead to stereotyping of and discrimination against autistic people. Although non-autistic people tend to deny feeling negatively inclined towards autistic people 225 , autistic people often report experiencing bullying, exclusion and discrimination. Attitudinal research has shown that considerable implicit biases are present, even among non-autistic people who report no explicit biases 226 , suggesting they may be unaware that they have negative attitudes towards autistic people. These implicit, negative biases are likely to be difficult to shift using short-term educational training programmes 227 . Such discrimination and stigma constitute a substantial barrier for autistic people seeking to develop social connections. Discrimination and stigma could be countered by widespread public acceptance campaigns (including those developed with autistic people 228 ), and programmes that increase the number of everyday interactions between autistic and non-autistic people 229 , 230 .
Other species
The eighth capability requires that humans are “able to live with concern for and in relation to animals, plants and the world of nature” 17 . Prominent autistic naturalists (such as Temple Grandin) and environmentalists (such as Greta Thunberg) have captured the public’s attention 231 . Yet there is remarkably little written about autistic people’s connections to nature and non-human animals.
Research with parents of autistic children has revealed that natural elements (such as sand, mud, leaves, twigs and water) can keep children engrossed for extended periods of time 232 . Some autistic children also prefer interacting with animals over inanimate objects and humans 233 , and report strong attachments to pets 234 . Studies have therefore focused on the potential therapeutic benefits of interacting with nature for children, with some purporting to show ‘reduced autistic severity’ or improvements in family functioning following interaction with trained animals 235 .
Research with autistic adults also reveals benefits of interacting with animals and nature 236 . Nature and gardening are two of the interests most reported by autistic adults, particularly women, and the pursuit of these interests is positively associated with subjective well-being 237 . In a study using photovoice methodology , images of natural scenes were frequently included among the photographs shared by autistic adults, demonstrating the importance of nature in contributing to a good autistic life 238 . Autistic adults’ autobiographies reveal the emotional depth of these connections to nature 239 , which some autistic people say offer respite from the intensity of an often inhospitable social world.
The capability of play emphasizes the right to be “able to laugh, to play, to enjoy recreational activities” 17 . This capability is one in which autistic adults might excel. Researchers and clinicians often refer to autistic people’s passions and interests as ‘highly restricted’, ‘perseverative’ or ‘circumscribed’, or as ‘obsessions’ or ‘fixations’, and as differing qualitatively (in content) and quantitatively (in intensity) from the interests of non-autistic people 240 . Yet autistic testimony attests that these passions are often a great source of joy and enjoyment 241 , which situates them within the play capability. Intense interests are common in autistic people 237 , 242 and become more diverse over time 243 . They are not limited to the sciences or computers, as popular stereotypes suggest 244 , but extend broadly to a range of areas 237 , 242 and might be more idiosyncratic in autistic adults with limited spoken language and/or intellectual disabilities 245 .
Autistic adults often view their capacity to pursue their passions as an advantage 181 , 237 , 241 , 246 that can be affirming and have positive implications for identity and self-concept 243 . Indeed, one autistic participant, who once “owned about 15,000 CDs,” celebrated the capacity “to be intense in stuff” 181 . Passions and interests have been likened to experiences of flow 237 , 247 and to monotropism 191 , which are driven by intrinsic (interest and knowledge) rather than extrinsic (prestige or achievement) motivation 237 . Finding others who share similar interests can form the basis of long-lasting friendships 93 . Nevertheless, exceptionally high intensity of engagement may, in some circumstances, negatively impact well-being 237 .
The generally positive effects of engaging in one’s interests also extend to taking part in recreational activities. Autistic adults report relatively high levels of weekly participation in exercise and hobbies 248 . However, they participate in conventional social and recreational activities to a lesser extent than the general population 249 , despite saying these are important to them 250 . Future research should consider the possible reasons for this disparity and the constraints that autistic adults face when engaging in meaningful and satisfying leisure activities. Inaccessible and inhospitable environments might be barriers for autistic adults 251 , and the effectiveness of programmes designed to support such participation appear to be limited 251 , 252 . Enhancing the play capability is important because engaging in recreational activities might buffer the relationship between perceived stress and quality of life 253 .
Control over one’s environment
The final capability emphasizes the importance of “being able to participate effectively in political choices that govern one’s life … being able to hold property and having property rights on an equal basis with others; having the right to seek employment on an equal basis with others; having the freedom from unwarranted search and seizure” 17 .
There is virtually no research on autistic adults’ engagement in mainstream political processes. Individuals with intellectual disability are less likely to vote than the general population 254 , especially if they live in supported accommodation rather than with family 255 . They often lack support and accessible information for political engagement 255 , 256 and are even explicitly told they cannot vote due to their intellectual disability 256 . More research is needed on autistic citizenship to identify precisely how these obstacles can be overcome 256 .
Extant data suggest that autistic people might be more politically disengaged than non-autistic people. This suggestion stands in contrast to high-profile autistic activists and political commentators, such as Australia’s Grace Tame and Eric Garcia from the United States, and increasing autistic involvement in self-advocacy since the 1990s. The autistic self-advocacy movement grew out of the self-advocacy efforts of people with intellectual and developmental disabilities in the United States and the United Kingdom 257 , and is perhaps epitomized most by Jim Sinclair’s 258 foundational essay (‘Don’t Mourn For Us’) which implored parents not to see their autistic child as a tragedy but, instead, to embrace their differences. Autistic and neurodiversity activists now promote individual self-advocacy, harnessing self-understanding and knowledge to ensure that individuals have greater control over their own lives. Such individual self-advocacy is complemented by collective advocacy, sometimes led by organizations run by and for autistic people (for example, Autistic Self-Advocacy Network ), where autistic people collectively campaign on a range of issues 259 , 260 and come together in dedicated autistic spaces and events 261 . Consequently, self-advocates have begun to shift conceptions of autism from a disorder that needs to be eradicated, prevented or ‘fixed’ to a distinct way of being, which demands acceptance and emphasizes human rights and a positive autistic identity and culture 261 , 262 , 263 , 264 , 265 , 266 , 267 .
There is much for autistic self-advocates to campaign about. Autistic people’s opportunities are constrained by others’ unjustified assumptions about their capacity 268 . Autistic adults are at far greater risk of prejudice, stigmatization and discrimination in many facets of their lives, such as education 141 , 269 , health 40 , 72 , care 270 , intimate relationships 271 , community 171 , justice 272 and work 273 . Moreover, to navigate a world that is not typically set up for them, autistic adults often (consciously or unconsciously) hide or mask aspects of their autistic self 274 , 275 to keep themselves safe or adjust their abilities through ‘compensation’ 276 . Such adaptation can come at serious personal cost, including poor mental and physical health 277 , 278 , negative self-perceptions 275 , 278 and autistic burnout 279 , 280 .
Work provides a particularly constrained environment. Autistic people face substantial challenges in gaining and sustaining meaningful employment, even relative to other disabled people 281 , 282 , 283 , despite possessing a range of skills that might be prized by employers 127 , 246 , 282 , 283 . Autistic adults who do obtain employment are often in positions that fail to match up with their abilities (malemployment) or for which they are overqualified (underemployment) 284 . They can also face challenges maintaining employment 285 , owing to inhospitable work environments 286 , negative experiences with (and sometimes bullying by) colleagues 281 , failure to have their needs and preferences met 287 , and experiences of discrimination, including following the disclosure of an autism diagnosis 288 . There is growing interest in paid short-term autism-specific employment programmes or internships, which are designed to reduce barriers to employment for autistic jobseekers, introduce them to workplace life and provide training in job-relevant skills 289 , 290 . These initiatives show promising effects on autistic trainees’ occupational self-efficacy 289 , 290 but deserve sustained attention to determine whether they help autistic adults to secure and maintain suitable employment in the longer term. Research is also needed on what constitutes a successful employment outcome according to autistic people themselves, and how it should be measured 291 .
Summary and future directions
Autistic people deserve to live long, healthy and creative lives of their own design. Just like all people, they need to be equipped with a set of fundamental capabilities to do so. In this Review, we have examined the lives and life chances of autistic adults through Nussbaum’s capabilities 16 , 17 lens. Doing so allows us to escape the narrowly normative focus on specific life outcomes and to consider the broader foundations for a range of possible good autistic lives. When approached in this way, the literature suggests that there are some capabilities in which autistic people have the potential to excel despite conventional stereotypes to the contrary, such as emotions, affiliation, play, connections to other species, practical reason and control over one’s own environment. At the same time, the literature suggests that in these capability areas and others (especially life, bodily health and integrity), autistic adults are often constrained by a range of social, economic and other environmental disadvantages and barriers, which prohibit them from enjoying a good life that they have the right to expect.
This Review suggests two clear directions for future research. First, it will be important for researchers to more clearly identify these externally shaped disadvantages and find ways to alleviate them. That is, once researchers are collectively equipped with a fuller understanding of what currently prevents autistic adults from enjoying a particular capability, they should be able to begin the task of removing those constraints so that further opportunities are provided. Second, it will be equally important to encourage autistic people themselves to reflect further on the capabilities to which they aspire and the obstacles which they believe obstruct them. The capabilities reviewed here are only a starting point and further amendment might be needed to capture the breadth and specificity of autistic experience (see ref. 292 ). Determining what autistic capabilities to add to this list can be resolved only through research that is genuinely participatory (see Box 3 ); that is, research that places the interests of autistic adults first and takes their own experience and expertise as seriously as any other input.
Box 3 New agendas and approaches to autism research
Despite the large literature on autism since it was first identified in the 1940s, this research generally does not have a positive, meaningful impact on the day-to-day lives of autistic people and their allies. There has been an extensive focus on underlying biological questions and relatively little research on the design of services and supports, the social contexts within which autistic people live or the policy settings that influence their quality of life. Through advocacy and other means, autistic people are increasingly making it clear that they are dissatisfied with this mix and, in line with the emphases of the capabilities approach, want the massive public investment in autism research to provide a greater direct return 305 . They want to address the imbalance in current autism research: research that has a direct impact on the daily lives of autistic people should be valued as much as research on the underlying biology and causes of autism 306 .
Crucially, autistic people also want to have greater input into research decisions 307 , 308 , 309 . Autism research has traditionally been designed and conducted by non-autistic people. Autistic people, their family members and even practitioners have rarely been involved in the decision-making processes that shape research and its application 12 , 13 , beyond being passive research participants. This limited involvement in research has begun to change in the past decade. There is a slow but growing movement towards collaborating with autistic people and their allies as part of the research process, such that autistic researchers and community members are actively involved in making decisions about research 308 , 309 . These decisions can include what kind of research is done, how it is done, how research results are interpreted and how the findings are used.
Such participatory research has a long history outside autism research 310 . In these contexts, participatory processes that draw on the ‘practical wisdom’ of non-scientists have been shown to have a dramatic effect on both the research agenda and the effectiveness of the research 311 . Participation itself can take many forms, ranging from being a consultant on a research project to sitting on a formal advisory board, being a full collaborative partner or even leading projects. The critical issue in participatory research is who makes the research decisions. In research involving community members only to a minimal extent (for example, through consultation), the researchers are typically in control. When that involvement deepens, researchers relinquish control to share decision-making power with community members.
There are some excellent examples of autism research that uses participatory approaches 40 , 181 , 312 , 313 , but it is still very much in its infancy. Although there is much enthusiasm for involving autistic people in the decisions that influence them 314 , 315 , researchers can be worried about how time-consuming participatory research can be, can find it hard to relinquish control in research decision-making and worry that community members might introduce bias into otherwise rigorous research processes. These concerns could lead to tokenism when community involvement is attempted 312 . Instead, researchers and community members need to appreciate that they each have different ‘experiential expertise’ 316 ; they must take that expertise seriously to enable valuable insights for those involved in the research and for the research itself 317 .
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th ed. (American Psychiatric Publishing, 2013).
Howlin, P. Adults with autism: changes in understanding since DSM-III. J. Autism Dev. Disord. 51 , 4291–4308 (2021).
Article PubMed PubMed Central Google Scholar
Kanner, L. Autistic disturbances of affective contact. Nerv. Child 2 , 217–250 (1943).
Google Scholar
Asperger, H. in Autism and Asperger Syndrome (ed. Frith, U.) 37–92 (Cambridge Univ. Press, 1944).
Stevenson, J., Harp, B. & Gernsbacher, M. Infantilizing autism. Disabil. Stud. Q. https://doi.org/10.18061/dsq.v31i3.1675 (2011).
Cervantes, P. E. et al. Trends over a decade in NIH funding for autism spectrum disorder services research. J. Autism Dev. Disord. 51 , 2751–2763 (2021).
Article PubMed Google Scholar
Lord, C. et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 399 , 10321 (2021).
Leadbitter, K., Buckle, K. L., Ellis, C. & Dekker, M. Autistic self-advocacy and the neurodiversity movement: implications for autism early intervention research and practice. Front. Psychiatry https://doi.org/10.3389/fpsyg.2021.635690 (2021).
Article Google Scholar
Pellicano, E. & den Houting, J. Annual research review: shifting from “normal science” to neurodiversity in autism science. J. Child. Psychol. Psychiatry 63 , 381–396 (2022).
Brock, D. In The Quality of Life (eds Nussbaum, M. & Sen, A.) 95–132 (Oxford Univ. Press, 1993).
Sroufe, L. A. & Rutter, M. The domain of developmental psychopathology. Child. Dev. 55 , 17–29 (1984).
Jivraj, J., Sacrey, L.-A., Newton, A., Nicholas, D. & Zwaigenbaum, L. Assessing the influence of researcher–partner involvement on the process and outcomes of participatory research in autism spectrum disorder and neurodevelopmental disorders: a scoping review. Autism 18 , 782–793 (2014).
Pellicano, E., Dinsmore, A. & Charman, T. Views on researcher–community engagement in autism research in the United Kingdom: a mixed-methods study. PLoS ONE 9 , e109946 (2014).
Henninger, N. A. & Taylor, J. L. Outcomes in adults with autism spectrum disorders: a historical perspective. Autism 17 , 103–116 (2013).
Howlin, P. & Magiati, I. Autism spectrum disorder: outcomes in adulthood. Curr. Opin. Psychiatry 30 , 69–76 (2017).
Nussbaum, M. Women and Human Development: The Capabilities Approach (Cambridge Univ. Press, 2000).
Nussbaum, M. Creating Capabilities (Harvard Univ. Press, 2011). This foundational text is a primer on Nussbaum’s capabilities approach, which is an innovative model for assessing human development .
Lai, M. C. & Baron-Cohen, S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2 , 1013–1027 (2015).
Lord, C. et al. Autism spectrum disorder. Nat. Rev. Dis. Primer 6 , 5 (2020).
Crane, L. et al. Autism diagnosis in the United Kingdom: perspectives of autistic adults, parents and professionals. J. Autism Dev. Disord. 48 , 3761–3772 (2018).
Geurts, H. M. & Jansen, M. D. A retrospective chart study: the pathway to a diagnosis for adults referred for ASD assessment. Autism 16 , 299–305 (2011).
Lewis, L. F. Exploring the experience of self-diagnosis of autism spectrum disorder in adults. Arch. Psychiatr. Nurs. 30 , 574–580 (2016).
Lewis, L. A mixed methods study of barriers to formal diagnosis of autism spectrum disorder in adults. J. Autism Dev. Disord. 47 , 2410–2424 (2017).
Huang, Y., Arnold, S. R. C., Foley, K.-R. & Trollor, J. N. Diagnosis of autism in adulthood: a scoping review. Autism 24 , 1311–1327 (2020).
Camm-Crosbie, L., Bradley, L., Shaw, R., Baron-Cohen, S. & Cassidy, S. People like me don’t get support: autistic adults’ experiences of support and treatment for mental health difficulties, self-injury and suicidality. Autism 23 , 1431–1441 (2019).
Atherton, G., Edisbury, E., Piovesan, A. & Cross, L. Autism through the ages: a mixed methods approach to understanding how age and age of diagnosis affect quality of life. J. Autism Dev. Disord. https://doi.org/10.1007/s10803-021-05235-x (2021).
Woolfenden, S., Sarkozy, V., Ridley, G. & Williams, K. A systematic review of the diagnostic stability of autism spectrum disorder. Res. Autism Spectr. Disord. 6 , 345–354 (2012).
Fein, D. A. et al. Optimal outcome in individuals with a history of autism. J. Child. Psychol. Psychiatry 54 , 195–205 (2013).
Simonoff, E. et al. Trajectories in symptoms of autism and cognitive ability in autism from childhood to adult life: findings from a longitudinal epidemiological cohort. J. Am. Acad. Child. Adolesc. Psychiatry 59 , 1342–1352 (2020).
Zimmerman, D., Ownsworth, T., O’Donovan, A., Roberts, J. & Gullo, M. J. High-functioning autism spectrum disorder in adulthood: a systematic review of factors related to psychosocial outcomes. J. Intellect. Dev. Disabil. 43 , 2–19 (2018).
McConachie, H. et al. What is important in measuring quality of life? Reflections by autistic adults in four countries. Autism Adulthood 2 , 4–12 (2020).
Ne’eman, A. When disability is defined by behavior, outcome measures should not promote ‘passing’. AMA J. Ethics 23 , E569–E575 (2021). This opinion piece presents a powerful call to researchers to rethink the measures they use to evaluate autistic people’s outcomes using a neurodiversity lens .
Bronfenbrenner, U. in International Encyclopedia of Education Vol. 3 2nd ed. (eds. Husen, T. & Postlethwaite, N.) 1643–1647 (Elsevier, 1994).
Oliver, M. The Politics of Disablement (MacMillan, 1990).
Raz, J. The Morality of Freedom (Oxford Univ. Press, 1986).
Hirvikoski, T. et al. Premature mortality in autism spectrum disorder. Br. J. Psychiatry 208 , 232–238 (2016).
Hwang, Y. I., Srasuebkul, P., Foley-K-R, Arnold, S. & Trollor, J. N. Mortality and cause of death of Australians on the autism spectrum. Autism Res. 12 , 806–815 (2019).
Woolfenden, S., Sarkozy, V., Ridley, G., Coory, M. & Williams, K. A systematic review of two outcomes in autism spectrum disorder: epilepsy and mortality. Dev. Med. Child. Neurol. 54 , 306–312 (2012).
Bishop-Fitzpatrick, L. et al. Using machine learning to identify patterns of lifetime health problems in decedents with autism spectrum disorder. Autism Res. 11 , 1120–1128 (2018).
Nicolaidis, C. et al. “Respect the way I need to communicate with you”: healthcare experiences of adults on the autism spectrum. Autism 19 , 824–831 (2015). This qualitative study, which adopts a community-based participatory approach, presents a thorough analysis of the individual-level, provider-level and system-level factors that impact autistic people’s interactions with the healthcare system .
Cassidy, S. et al. Suicidal ideation and suicide plans or attempts in adults with Asperger’s syndrome attending a specialist diagnostic clinic: a clinical cohort study. Lancet Psychiatry 1 , 142–147 (2014).
Hedley, D. & Uljarević, M. Systematic review of suicide in autism spectrum disorder: current trends and implications. Curr. Dev. Disord. Rep. 5 , 65–76 (2018).
Hand, B. N., Angell, A. M., Harris, L. & Carpenter, L. A. Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism 24 , 755–764 (2020).
Kirby, A. V. et al. A 20-year study of suicide death in a statewide autism population. Autism Res. 12 , 658–666 (2019).
Kõlves, K., Fitzgerald, C., Nordentoft, M., Wood, S. J. & Erlangsen, A. Assessment of suicidal behaviors among individuals with autism spectrum disorder in Denmark. JAMA Netw. Open. 4 , e2033565 (2021).
Hand, B. N., Benevides, T. W. & Carretta, H. J. Suicidal ideation and self-inflicted injury in medicare enrolled autistic adults with and without co-occurring intellectual disability. J. Autism Dev. Disord. 50 , 3489–3495 (2019).
South, M. et al. Unrelenting depression and suicidality in women with autistic traits. J. Autism Dev. Disord. 50 , 3606–3619 (2020).
Pelton, M. K. et al. Understanding suicide risk in autistic adults: comparing the Interpersonal Theory of Suicide in autistic and non-autistic samples. J. Autism Dev. Disord. 50 , 3620–3637 (2020).
Jager-Hyman, S., Maddox, B. B., Crabbe, S. R. & Mandell, D. S. Mental health clinicians’ screening and intervention practices to reduce suicide risk in autistic adolescents and adults. J. Autism Dev. Disord. 50 , 3450–3461 (2020).
Roestorf, A. et al. “Older adults with ASD: the consequences of aging”: insights from a series of special interest group meetings held at the International Society for Autism Research 2016–2017. Res. Autism Spectr. Disord. 63 , 3–12 (2019).
van Heijst, B. C. & Geurts, H. M. Quality of life in autism across the lifespan: a meta-analysis. Autism 19 , 158–167 (2015).
National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System (National Academies Press, 2020).
Hwang, Y., Foley, K. & Trollor, J. Aging well on the autism spectrum: the perspectives of autistic adults and carers. Int. Psychogeriatr. 29 , 2033–2046 (2017).
Hickey, A., Crabtree, J. & Stott, J. “Suddenly the first fifty years of my life made sense”: experiences of older people with autism. Autism 22 , 357–367 (2018).
Michael, C. Why we need research about autism and ageing. Autism 20 , 515–516 (2016).
Warner, G., Parr, J. R. & Cusack, J. Workshop report: establishing priority research areas to improve the physical health and well-being of autistic adults and older people. Autism Adulthood 1 , 20–26 (2019). This study, in collaboration with members of the autistic and autism communities, identifies 11 priority areas for research on the physical health and well-being of autistic adults and older people .
Rydzewska, E., Dunn, K. & Cooper, S.-A. Umbrella systematic review of systematic reviews and meta-analyses on comorbid physical conditions in people with autism spectrum disorder. Br. J. Psychiatry 218 , 10–19 (2021).
Weir, E., Allison, C., Warrier, V. & Baron-Cohen, S. Increased prevalence of non-communicable physical health conditions among autistic adults. Autism 25 , 681–694 (2020).
Gilmore, D., Harris, L., Longo, A. & Hand, B. N. Health status of medicare-enrolled autistic older adults with and without co-occurring intellectual disability: an analysis of inpatient and institutional outpatient medical claims. Autism 25 , 266–274 (2021).
Dunn, K. D., Rydzewska, E., Macintyre, C., Rintoul, J. & Cooper, S.-A. The prevalence and general health status of people with intellectual disabilities and autism co-occurring together — a total population study. J. Intellect. Dev. Disabil. 63 , 277–285 (2019).
Kassee, C. et al. Physical health of autistic girls and women: a scoping review. Mol. Autism 11 , 84 (2020).
Rubenstein, E. & Bishop-Fitzpatrick, L. A matter of time: the necessity of temporal language in research on health conditions that present with autism spectrum disorder. Autism Res. 12 , 20–25 (2019).
Simantov, T. et al. Medical symptoms and conditions in autistic women. Autism 26 , 373–388 (2021).
Steward, R., Crane, L., Roy, E., Remington, A. & Pellicano, E. “Life is much more difficult to manage during periods”: autistic experiences of menstruation. J. Autism Dev. Disord. 48 , 4287–4292 (2018).
Sundelin, H. E., Stephansson, O., Hultman, C. M. & Ludvigsson, J. F. Pregnancy outcomes in women with autism: a nationwide population-based cohort study. Clin. Epidemiol. 10 , 1817–1826 (2018).
Moseley, R. L., Druce, T. & Turner-Cobb, J. M. ‘When my autism broke’: a qualitative study spotlighting autistic voices on menopause. Autism 24 , 1423–1437 (2020).
Bradshaw, P., Pellicano, E., van Driel, M. & Urbanowicz, A. How can we support the healthcare needs of autistic adults without intellectual disability? Curr. Dev. Disord. Rep. 6 , 45–56 (2019).
Mason, D. et al. A systematic review of what barriers and facilitators prevent and enable physical healthcare services access for autistic adults. J. Autism Dev. Disord. 49 , 3387–3400 (2019).
Walsh, C., Lydon, S., O’Dowd, E. & O’Connor, P. Barriers to healthcare for persons with autism: a systematic review of the literature and development of a taxonomy. Dev. Neurorehabilit. 23 , 413–430 (2020).
Zerbo, O. et al. Health care service utilization and cost among adults with autism spectrum disorders in a US integrated health care system. Autism Adulthood 1 , 27–36 (2018).
Nicolaidis, C. et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic–community partnership. J. Gen. Intern. Med. 28 , 761–769 (2013).
Tregnago, M. K. & Cheak-Zamora, N. C. Systematic review of disparities in health care for individuals with autism spectrum disorders in the United States. Res. Autism Spectr. Disord. 6 , 1023–1031 (2012).
Micai, M. et al. Autistic adult health and professional perceptions of it: evidence from the ASDEU project. Front. Psychiatry 12 , 614102 (2021).
Anderson, K. A., Sosnowy, C., Kuo, A. A. & Shattuck, P. T. Transition of individuals with autism to adulthood: a review of qualitative studies. Pediatrics 141 , S318–S327 (2018).
Unigwe, S. et al. GPs’ perceived self-efficacy in the recognition and management of their autistic patients: an observational study. Br. J. Gen. Pract. 67 , e445–e452 (2017).
Fiene, L. & Brownlow, C. Investigating interoception and body awareness in adults with and without autism spectrum disorder. Autism Res. 8 , 709–716 (2015).
Walsh, C. et al. Development and preliminary evaluation of a novel physician-report tool for assessing barriers to providing care to autistic patients. BMC Health Serv. Res. 21 , 873 (2021).
Walsh, C., O’Connor, P., Walsh, E. & Lydon, S. A systematic review of interventions to improve healthcare experiences and access in autism. Rev. J. Autism Dev. Disord. https://doi.org/10.1007/s40489-021-00279-2 (2021).
Churchard, A., Ryder, M., Greenhill, A. & Mandy, W. The prevalence of autistic traits in a homeless population. Autism 23 , 665–676 (2019).
Evans, R. The Life We Choose: Shaping Autism Services in Wales (National Autistic Society, 2011).
Brugha, T. et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch. Gen. Psychiatry 68 , 459–465 (2011).
Autistic Self Advocacy Network. ASAN Toolkit on Improving Home and Community-Based Services (HCBS) https://autisticadvocacy.org/policy/toolkits/hcbs/ (2011).
Mandell, D. A house is not a home: the great residential divide in autism care. Autism 21 , 810–811 (2017).
Paode, P. Housing for Adults with Autism and/or Intellectual and Developmental Disabilities: Shortcomings of Federal Programs (Daniel Jordan Fiddle Foundation, 2020).
Allely, C. S. A systematic PRISMA review of individuals with autism spectrum disorder in secure psychiatric care: prevalence, treatment, risk assessment and other clinical considerations. J. Crim. Psychol. 8 , 58–79 (2018).
Mandell, D. S., Walrath, C. M., Manteuffel, B., Sgro, G. & Pinto-Martin, J. A. The prevalence and correlates of abuse among children with autism served in comprehensive community-based mental health settings. Child Abuse Negl. 29 , 1359–1372 (2005).
Gibbs, V. et al. Experiences of physical and sexual violence as reported by autistic adults without intellectual disability: rate, gender patterns and clinical correlates. Res. Autism Spectr. Disord. 89 , 101866 (2021).
Weiss, J. A. & Fardella, M. A. Victimisation and perpetration experiences of adults with autism. Front. Psychiatry 9 , 203 (2018).
Brown-Lavoie, S. M., Viecili, M. A. & Weiss, J. A. Sexual knowledge and victimization in adults with autism spectrum disorders. J. Autism Dev. Disord. 44 , 2185–2196 (2014).
Griffiths, S. et al. The vulnerability experiences quotient (VEQ): a study of vulnerability, mental health and life satisfaction in autistic adults. Autism Res. 12 , 1516–1528 (2019).
Bargiela, S., Steward, R. & Mandy, W. The experiences of late-diagnosed women with autism spectrum conditions: an investigation of the female autism phenotype. J. Autism Dev. Disord. 46 , 328–3294 (2016).
Reuben, K. E., Stanzione, C. M. & Singleton, J. L. Interpersonal trauma and posttraumatic stress in autistic adults. Autism Adulthood 3 , 247–256 (2021).
Sedgewick, F., Crane, L., Hill, V. & Pellicano, E. Friends and lovers: the relationships of autistic women in comparison to neurotypical women. Autism Adulthood 1 , 112–123 (2019).
Pecora, L. A., Hancock, G. I., Mesibov, G. B. & Stokes, M. A. Characterising the sexuality and sexual experiences of autistic females. J. Autism Dev. Disord. 49 , 4834–4846 (2019).
Solomon, D., Panatalone, D. W. & Faja, S. Autism and adult sex education: a literature review using the information–motivation–behavioral skills framework. Sex. Disabil. 37 , 339–351 (2019).
Lubin, A. & Feeley, C. Transportation issues of adults on the autism spectrum: findings from focus group discussions. Transp. Res. Rec. 2542 , 1–8 (2016).
Kersten, M., Coxon, K., Lee, H. & Wilson, N. J. Independent community mobility and driving experiences of adults on the autism spectrum: a scoping review. Am. J. Occup. Ther. 74 , 7405205140 (2020).
Deka, D., Feeley, C. & Lubin, A. Travel patterns, needs, and barriers of adults with autism spectrum disorder: report from a survey. Transp. Res. Rec. 2542 , 9–16 (2016).
Chee, D. Y.-T. et al. Viewpoints on driving of individuals with and without autism spectrum disorder. Dev. Neurorehabilit. 18 , 26–36 (2015).
Curry, A. E. et al. Comparison of motor vehicle crashes, traffic violations, and license suspensions between autistic and non-autistic adolescent and young adult drivers. J. Am. Acad. Child. Adolesc. Psychiatry 60 , 913–923 (2021).
Curry, A. E., Yerys, B. E., Huang, P. & Metzger, K. B. Longitudinal study of driver licensing rates among adolescents and young adults with autism spectrum disorder. Autism 22 , 479–488 (2018).
Anderson, C. J. et al. Occurrence and family impact of elopement in children with autism spectrum disorders. Pediatrics 130 , 870–877 (2012).
Solomon, O. & Lawlor, M. C. “And I look down and he is gone”: narrating autism, elopement and wandering in Los Angeles. Soc. Sci. Med. 94 , 106–114 (2013). This anthropological research analyses narratives from African American mothers of autistic children about wandering, highlighting the cultural context of these phenomena in a group under-represented in research .
Ne’eman, A. Safety versus autonomy: advocates for autistic children split over tracking devices. Vox https://www.vox.com/the-big-idea/2016/12/17/13993398/safety-autonomy-avonte-tracking-autism-wandering-schumer (2016).
Baggs, A. Wandering. Ballastexistenz https://ballastexistenz.wordpress.com/2005/08/01/wandering/ (2005).
Wolff, J. & de-Shalit, A. Disadvantage (Oxford Univ. Press, 2007).
Rumball, F., Happé, F. & Grey, N. Experience of trauma and PTSD symptoms in autistic adults: risk of PTSD development following DSM-5 and non-DSM-5 traumatic life events. Autism Res. 13 , 2122–2132 (2020).
Bertilsdotter Rosqvist, H. Becoming an ‘autistic couple’: narratives of sexuality and couplehood within the Swedish Autistic self-advocacy movement. Sex. Disabil. 32 , 351–363 (2014).
Dewinter, J., De Graaf, H. & Begeer, S. Sexual orientation, gender identity and romantic relationships in adolescents and adults with autism spectrum disorder. J. Autism Dev. Disord. 47 , 2927–2934 (2017).
Gilmour, L., Smith, V. & Schalomon, M. in Comprehensive Guide to Autism (eds Patel, V. B., Preedy, V. R. & Martin, C. R.) 569–584 (Springer, 2014).
Byers, E. & Nichols, S. Sexual satisfaction of high-functioning adults with autism spectrum disorder. Sex. Disabil. 32 , 365–382 (2014).
Bush, H. H. Dimensions of sexuality among young women, with and without autism, with predominantly sexual minority identities. Sex. Disabil. 37 , 275–292 (2019).
George, R. & Stokes, M. A. Gender identity and sexual orientation in autism spectrum disorder. Autism 22 , 970–982 (2018).
Strang, J. F. et al. Increased gender variance in autism spectrum disorders and attention deficit hyperactivity disorder. Arch. Sex. Behav. 43 , 1525–1533 (2014).
Van Der Miesen, A. I. R., Hurley, H. & De Vries, A. L. C. Gender dysphoria and autism spectrum disorder: a narrative review. Int. Rev. Psychiatry 28 , 70–80 (2016).
Warrier, V. et al. Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals. Nat. Comm. 11 , 3959 (2020).
Barnett, J. P. & Maticka-Tyndale, E. Qualitative exploration of sexual experiences among adults on the autism spectrum: implications for sex education. Perspect. Sex. Reprod. Health 47 , 171–179 (2015).
Dewinter, J., van der Miesen, A. I. R. & Graham Holmes, L. INSAR Special Interest Group Report: stakeholder perspectives on priorities for future research on autism, sexuality and intimate relationships. Autism Res. 13 , 1248–1257 (2020).
Dinishak, J. The deficit view and its critics. Disabil. Stud. Q https://doi.org/10.18061/dsq.v36i4.5236 (2016).
Dawson, M., Soulieres, I., Gernsbacher, M. A. & Mottron, L. The level and nature of autistic intelligence. Psychol. Sci. 18 , 657–662 (2007). This pioneering study refutes the long-standing view that the majority of autistic people are intellectually impaired .
Goldberg Edelson, M. Are the majority of children with autism mentally retarded? A systematic evaluation of the data. Focus. Autism Other Dev. Disabil. 21 , 66–83 (2006).
Alvares, G. A. et al. The misnomer of ‘high functioning autism’: intelligence is an imprecise predictor of functional abilities at diagnosis. Autism 24 , 221–232 (2020).
Russell, G. et al. Selection bias on intellectual ability in autism research: a cross-sectional review and metaanalysis. Mol. Autism 10 , 9 (2019).
Craig, J. & Baron-Cohen, S. Creativity and imagination in autism and Asperger syndrome. J. Autism Dev. Disord. 29 , 319–326 (1999).
Courchesne, V., Meilleur, A.-A. S., Poulin-Lord, M.-P., Dawson, M. & Soulières, I. Autistic children at risk of being underestimated: school-based pilot study of a strength-informed assessment. Mol. Autism 6 , 12 (2015).
Kasirer, A. & Mashal, N. Verbal creativity in autism: comprehension and generation of metaphoric language in high-functioning autism spectrum disorder and typical development. Front. Hum. Neurosci. 8 , 615 (2014).
de Schipper, E. et al. Functioning and disability in autism spectrum disorder: a worldwide survey of experts. Autism Res. 9 , 959–969 (2016).
Austin, R. D. & Pisano, G. P. Neurodiversity as a competitive advantage. Harv. Bus. Rev. 95 , 96–103 (2017).
Happé, F. & Vital, P. What aspects of autism predispose to talent? Philos. Trans. R. Soc. Lond. B Biol. Sci. 364 , 1369–1375 (2009).
Mottron, L., Dawson, M., Soulières, I., Hubert, B. & Burack, J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 36 , 27–43 (2006).
Proff, I., Williams, G. L., Quadt, L. & Garfinkel, S. N. Sensory processing in autism across exteroceptive and interoceptive domains. Psychol. Neurosci. 15 , 105–130 (2021).
Goodall, C. ‘I felt closed in and like I couldn’t breathe’: a qualitative study exploring the mainstream educational experiences of autistic young people. Autism Dev. Lang. Impair. 3 , 1–16 (2018).
Jones, E. K., Hanley, M. & Riby, D. M. Distraction, distress and diversity: exploring the impact of sensory processing differences on learning and school life for pupils with autism spectrum disorders. Res. Autism Spectr. Disord. 72 , 101515 (2020).
Williams, E., Gleeson, K. & Jones, B. E. How pupils on the autism spectrum make sense of themselves in the context of their experiences in a mainstream school setting: a qualitative metasynthesis. Autism 23 , 8–28 (2019).
Maïano, C., Normand, C. L., Salvas, M. C., Moullec, G. & Aime, A. Prevalence of school bullying among youth with autism spectrum disorders: a systematic review and meta-analysis. Autism Res. 9 , 601–615 (2016).
Biklen, D. Presuming competence, belonging, and the promise of inclusion: the US experience. Prospects 49 , 233–247 (2020).
Makin, C., Hill, V. & Pellicano, E. The primary-to-secondary school transition for children on the autism spectrum: a multi-informant mixed-methods study. Autism Dev. Lang. Impair. 2 , 1–18 (2017).
Robertson, K., Chamberlain, B. & Kasari, C. General education teachers’ relationships with included students with autism. J. Autism Dev. Disord. 33 , 123–130 (2003).
Brede, J., Remington, A., Kenny, L., Warren, K. & Pellicano, E. Excluded from school: examining the educational experiences of students on the autism spectrum. Autism Dev. Lang. Impair. 2 , 1–20 (2017).
Ringbom, I. et al. Psychiatric disorders diagnosed in adolescence and subsequent long-term exclusion from education, employment or training: longitudinal national birth cohort study. Br. J. Psychiatry 220 , 148–153 (2021).
Nuske, A., Rillotta, F., Bellon, M. & Richdale, A. Transition to higher education for students with autism: a systematic literature review. J. Divers. High. Educ. 12 , 280–295 (2019).
Cai, R. Y. & Richdale, A. L. Educational experiences and needs of higher education students with autism spectrum disorder. J. Autism Dev. Disord. 46 , 31–41 (2016).
Cage, E. & Howes, J. Dropping out and moving on: a qualitative study of autistic people’s experiences of university. Autism 24 , 1664–1675 (2020).
Kuder, S. J. & Accardo, A. What works for college students with autism spectrum disorder. J. Autism Dev. Disord. 48 , 722–731 (2018).
Tavassoli, T., Miller, L. J., Schoen, S. A., Nielsen, D. M. & Baron-Cohen, S. Sensory over-responsivity in adults with autism spectrum conditions. Autism 18 , 428–432 (2014).
Baggs, A. Cultural commentary: up in the clouds and down in the valley: my richness and yours. Disabil. Stud. Q. 30 , 1052 (2010). In this commentary, autistic thinker and activist Mel Baggs implores researchers to view autistic people, especially those who think and communicate in non-traditional ways, as fully human .
Robertson, A. E. & Simmons, D. R. The sensory experiences of adults with autism spectrum disorder: a qualitative analysis. Perception 44 , 569–586 (2015).
Gaudion, K., Hall, A., Myerson, J. & Pellicano, E. A designer’s approach: how can autistic adults with learning disabilities be involved in the design process? CoDesign 11 , 49–69 (2015).
Kapp, S. et al. Autistic adults’ views and experiences of stimming. Autism 23 , 1782–1792 (2019).
DiGennaro-Reed, F. D., Hirst, J. M. & Hyman, S. R. Assessment and treatment of stereotypic behavior in children with autism and other developmental disabilities: a thirty year review. Res. Autism Spectr. Disord. 6 , 422–430 (2012).
Tereshko, L., Ross, R. K. & Frazee, L. The effects of a procedure to decrease motor stereotypy on social interactions in a child with autism spectrum disorder. Res. Autism Spectr. Disord. 14 , 367–377 (2021).
Bascom, J. in Loud Hands: Autistic People, Speaking (ed. Autistic Self Advocacy Network) 177–182 (Autistic Press, 2012).
Uljarević, M. & Hamilton, A. Recognition of emotions in autism: a formal meta-analysis. J. Autism Dev. Disord. 43 , 1517–1526 (2013).
Yew, R. Y., Samuel, P., Hooley, M., Mesibov, G. B. & Stokes, M. A. A systematic review of romantic relationship initiation and maintenance factors in autism. Pers. Relatsh. 28 , 777–802 (2021).
Bird, G. & Cook, R. Mixed emotions: the contribution of alexithymia to the emotional symptoms of autism. Transl. Psychiatry 3 , e285 (2013).
Kinnaird, E., Stewart, C. & Tchanturia, K. Investigating alexithymia in autism: a systematic review and meta-analysis. Euro. Psychiatry 55 , 80–89 (2019).
Smith, A. The empathy imbalance hypothesis of autism: a theoretical approach to cognitive and emotional empathy in autistic development. Psychol. Res. 59 , 489–510 (2009).
Hume, R. & Burgess, H. “I’m human after all”: autism, trauma and affective empathy. Autism Adulthood 3 , 221–229 (2021).
Sala, G., Hooley, M. & Stokes, M. A. Romantic intimacy in autism: a qualitative analysis. J. Autism Dev. Disord. 50 , 4133–4147 (2020).
Strunz, S. et al. Romantic relationships and relationship satisfaction among adults with Asperger syndrome and high-functioning autism. J. Clin. Psychol. 73 , 113–125 (2017).
Smith, R., Netto, J., Gribble, N. C. & Falkmer, M. ‘At the end of the day, it’s love’: an exploration of relationships in neurodiverse couples. J. Autism Dev. Disord. 51 , 3311–3321 (2021).
Dugdale, A.-S., Thompson, A. R., Leedham, A., Beail, N. & Freeth, M. Intense connection and love: the experiences of autistic mothers. Autism 25 , 1973–1984 (2021).
Jaswal, V. K. & Akhtar, N. Being vs. appearing socially uninterested: challenging assumptions about social motivation in autism. Behav. Brain Sci. 42 , 1–73 (2018). This perspective piece challenges the notion that autism is fundamentally characterized by diminished social motivation and urges researchers to take autistic testimony seriously in research .
Mazurek, M. O. Loneliness, friendship, and well-being in adults with autism spectrum disorders. Autism 18 , 223–232 (2014).
Smith, I. C. & White, S. W. Socio-emotional determinants of depressive symptoms in adolescents and adults with autism spectrum disorder: a systematic review. Autism 24 , 995–1010 (2020).
Hedley, D., Uljarević, M., Wilmot, M., Richdale, A. & Dissanayake, C. Understanding depression and thoughts of self-harm in autism: a potential mechanism involving loneliness. Res. Autism Spectr. Disord. 46 , 1–7 (2018).
Pellicano, E. et al. COVID-19, social isolation and the mental health of autistic people and their families: a qualitative study. Autism 26 , 914–927 (2022).
Hollocks, M. J., Lerh, J. W., Magiati, I., Meiser-Stedman, R. & Brugha, T. S. Anxiety and depression in adults with autism spectrum disorder: a systematic review and meta-analysis. Psychol. Med. 49 , 559–572 (2019).
Hudson, C. C., Hall, L. & Harkness, K. L. Prevalence of depressive disorders in individuals with autism spectrum disorder: a meta-analysis. J. Abnorm. Child Psychol. 47 , 165–175 (2019).
Bishop-Fitzpatrick, L. & Rubenstein, E. The physical and mental health of middle aged and older adults on the autism spectrum and the impact of intellectual disability. Res. Autism Spectr. Disord. 63 , 34–41 (2019).
Botha, M. & Frost, D. M. Extending the minority stress model to understand mental health problems experienced by the autistic population. Soc. Ment. Health 10 , 20–34 (2018).
Cassidy, S. et al. Measurement properties of tools used to assess depression in adults with and without autism spectrum conditions: a systematic review. Autism Res. 11 , 738–754 (2018).
Lai, M.-C. et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6 , 819–829 (2019).
Crane, L., Davidson, I., Prosser, R. & Pellicano, E. Understanding psychiatrists’ knowledge, attitudes and experiences in identifying and supporting their patients on the autism spectrum: an online survey. BJPsych Open 5 , e33 (2019).
Au-Yeung, S. K. et al. Experience of mental health diagnosis and perceived misdiagnosis in autistic, possibly autistic and non-autistic adults. Autism 23 , 1508–1518 (2019).
Adams, D. & Young, K. A systematic review of the perceived barriers and facilitators to accessing psychological treatment for mental health problems in individuals on the autism spectrum. Rev. J. Autism Dev. Disord. 49 , 33873400 (2020).
Stancliffe, R. J. in Choice, Preference, and Disability (eds. Stancliffe, R. J. et al.) 3–26 (Springer, 2020).
Frith, U. & Happé, F. Theory of mind and self-consciousness: what is it like to be autistic? Mind Lang. 14 , 1–22 (1999).
Rydzewska, E. et al. Prevalence of long-term health conditions in adults with autism: observational study of a whole country population. BMJ Open 8 , e023945 (2018).
Holyfield, C., Drager, K. D. R., Kremkow, J. M. D. & Light, J. Systematic review of AAC intervention research for adolescents and adults with autism spectrum disorder. Augment. Altern. Comm. 33 , 201–212 (2017).
Lilley, R. et al. “A way to be me”: autobiographical reflections of late-diagnosed autistic adults. Autism https://doi.org/10.1177/13623613211050694 (2021).
McGeer, V. Autistic self-awareness. Philos. Psychiatr. Psychol. 11 , 235–251 (2004).
Damiano, C. R. et al. Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions. J. Neurodev. Disord. 4 , 1866–1955 (2012).
Vella, L. et al. Understanding self-reported difficulties in decision-making by people with autism spectrum disorders. Autism 22 , 549–559 (2018).
Gosling, C. J. & Moutier, S. Brief report: Risk-aversion and rationality in autism spectrum disorders. J. Autism Dev. Disord. 48 , 3623–3628 (2018).
Brosnan, M., Chapman, E. & Ashwin, C. Adolescents with autism spectrum disorder show a circumspect reasoning bias rather than ‘jumping to conclusions’. J. Autism Dev. Disord. 44 , 513–520 (2014).
Cage, E., Pellicano, E., Shah, P. & Bird, G. Reputation management: evidence for ability but reduced propensity in autism. Autism Res. 6 , 433–442 (2013).
Brosnan, M., Lewton, M. & Ashwin, C. Reasoning on the autism spectrum: a dual process theory account. J. Autism Dev. Disord. 46 , 2115–2125 (2016).
Shah, P., Catmur, C. & Bird, G. Emotional decision-making in autism spectrum disorder: the roles of interoception and alexithymia. Mol. Autism 7 , 43 (2016).
Lawson, W. Understanding and Working with the Spectrum of Autism: An Insider’s View (Jessica Kingsley, 2001).
Murray, D., Lesser, M. & Lawson, W. Attention, monotropism and the diagnostic criteria for autism. Autism 9 , 139–156 (2005). This autistic-led article presents ‘monotropism’, an alternative account of autistic cognition that is widely discussed in the autistic community but has received little attention from established autism researchers .
Buckle, K. L., Leadbitter, K., Poliakoff, E. & Gowen, E. “No way out except from external intervention”: first-hand accounts of autistic inertia. Front. Psychol. 12 , 631596 (2021).
Demetriou, E. A. et al. Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatry 23 , 1198–1204 (2018).
Kenny, L., Cribb, S. & Pellicano, E. Childhood executive function predicts later autistic features and adaptive behaviours in young autistic people: a 12-year prospective study. J. Abnorm. Child. Psychol. 47 , 1089–1099 (2019).
Pugliese, C. E. et al. Longitudinal examination of adaptive behavior in autism spectrum disorders: influence of executive function. J. Autism Dev. Disord. 46 , 467–477 (2016).
Pohl, A. L., Crockford, S. K., Blakemore, M., Allison, C. & Baron-Cohen, S. A comparative study of autistic and non-autistic women’s experience of motherhood. Mol. Autism 11 , 3 (2020).
Woolard, A. et al. Perception of work ability is related to social anxiety and executive function in autistic adults. Autism 25 , 2124–2134 (2021).
Cribb, S., Kenny, L. & Pellicano, E. “I definitely feel more in control of my life”: the perspectives of young autistic people and their parents on emerging adulthood. Autism 23 , 1765–1781 (2019).
Oswald, T. M. et al. A pilot randomized controlled trial of the ACCESS Program: a group intervention to improve social, adaptive functioning, stress coping, and self-determination outcomes in young adults with autism spectrum disorder. J. Autism Dev. Disord. 48 , 1742–1760 (2018).
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S. & Schultz, R. T. The social motivation theory of autism. Trends Cogn. Sci. 16 , 231–239 (2012).
Baron-Cohen, S. Mindblindness: An Essay on Autism and Theory of Mind (MIT Press/Bradford Books, 1995).
Cresswell, L., Hinch, R. & Cage, E. The experiences of peer relationships amongst autistic adolescents: a systematic review of the qualitative evidence. Res. Autism Spectr. Disord. 61 , 45–60 (2019).
Petrina, N., Carter, M. & Stephenson, J. The nature of friendship in children with autism spectrum disorders: a systematic review. Res. Autism Spectr. Disord. 8 , 111–126 (2014).
Shattuck, P. T., Orsmond, G. I., Wagner, M. & Cooper, B. P. Participation in social activities among adolescents with an autism spectrum disorder. PLoS ONE 6 , e27176 (2011).
Bottema-Beutel, K., Cuda, J., Kim, S. Y., Crowley, S. & Scanlon, D. High school experiences and support recommendations of autistic youth. J. Autism Dev. Disord. 50 , 3397–3412 (2020).
Humphrey, N. & Lewis, S. “Make me normal”: the views and experiences of pupils on the autistic spectrum in mainstream secondary schools. Autism 12 , 23–46 (2008).
Sedgewick, F., Leppanen, J. & Tchanturia, K. The Friendship Questionnaire, autism and gender differences: a study revisited. Mol. Autism 10 , 40 (2019).
Bottema-Beutel, K., Park, H. & Kim, S. Y. Commentary on social skills training curricula for individuals with ASD: social interaction, authenticity and stigma. J. Autism Dev. Disord. 48 , 953–964 (2018).
Spain, D. & Blainey, S. H. Group social skills interventions for adults with high-functioning autism spectrum disorders: a systematic review. Autism 19 , 874–886 (2015).
Bertilsdotter Rosqvist, H., Brownlow, C. & O’Dell, L. “What’s the point of having friends?” Reformulating notions of the meaning of friends and friendship among autistic people. Disabil. Stud. Q. https://doi.org/10.18061/dsq.v35i4.3254 (2015).
Ochs, E. & Solomon, O. Autistic sociality. Ethos 38 , 69–92 (2010).
Forster, S. & Pearson, A. “Bullies tend to be obvious”: autistic adults’ perceptions of friendship and the concept of ‘mate crime’. Disabil. Soc. 35 , 1103–1123 (2020).
Crompton, C. J., Hallett, S., Ropar, D., Flynn, E. & Fletcher-Watson, S. ‘I never realised everybody felt as happy as I do when I am around autistic people’: a thematic analysis of autistic adults’ relationships with autistic and neurotypical friends and family. Autism 24 , 1438–1448 (2020).
Bertilsdotter Rosqvist, H. Doing things together: exploring meanings of different forms of sociality among autistic people in an autistic work space. Alter 13 , 168–178 (2019).
Sinclair, J. Cultural commentary: being autistic together. Disabil. Stud. Q . https://dsq-sds.org/article/view/1075/1248 (2010).
Elmose, M. Understanding loneliness and social relationships in autism: the reflections of autistic adults. Nord. Psychol. 72 , 3–22 (2020).
Crane, L., Hearst, C., Ashworth, M., Davies, J. & Hill, E. L. Supporting newly identified or diagnosed autistic adults: an initial evaluation of an autistic-led programme. J. Autism Dev. Disord. 51 , 892–905 (2021).
Cooper, K., Smith, L. G. E. & Russell, A. Social identity, self-esteem, and mental health in autism. Eur. J. Soc. Psychol. 47 , 844–854 (2017).
Milton, D. & Sims, T. How is a sense of well-being and belonging constructed in the accounts of autistic adults? Disabil. Soc. 31 , 520–534 (2016).
Milton, D. On the ontological status of autism: the ‘double empathy problem’. Disabil. Soc. 27 , 883–887 (2012). This influential paper proposes a misalignment between the minds of autistic and non-autistic people and highlights the limitations in many non-autistic people’s efforts to communicate with autistic people .
Davis, R. & Crompton, C. J. What do new findings about social interaction in autistic adults mean for neurodevelopmental research? Perspect. Psychol. Sci. 16 , 649–653 (2021).
Mitchell, P., Sheppard, E. & Cassidy, S. Autism and the double empathy problem: implications for development and mental health. Br. J. Dev. Psychol. 39 , 1–18 (2021).
Sasson, N. J. et al. Neurotypical peers are less willing to interact with those with autism based on thin slice judgments. Sci. Rep. 7 , 40700 (2017).
Crompton, C. J. et al. Neurotype-matching, but not being autistic, influences self and observer ratings of interpersonal rapport. Front. Psychol. 11 , 586171 (2020).
Kuzminski, R. et al. Linking knowledge and attitudes: determining neurotypical knowledge about and attitudes towards autism. PLoS ONE 14 , e0220197 (2019).
Dickter, C. L., Burk, J. A., Zeman, J. L. & Taylor, S. C. Implicit and explicit attitudes toward autistic adults. Autism Adulthood 2 , 144–151 (2020).
Jones, D. R., DeBrabander, K. M. & Sasson, N. J. Effects of autism acceptance training on explicit and implicit biases toward autism. Autism 25 , 1246–1261 (2021).
Gillespie-Lynch, K. et al. If you want to develop an effective autism training, ask autistic students to help you. Autism https://doi.org/10.1177/13623613211041006 (2021).
den Houting, J., Botha, M., Cage, E., Jones, D. R. & Kim, S. Y. Shifting stigma about autistic young people. Lancet Child. Adolesc. Health 5 , P839–P841 (2021).
Shand, A. J., Close, S. A. D. & Shah, P. Greater autism knowledge and contact with autistic people are independently associated with favourable attitudes towards autistic people. Exp. Results 1 , e46 (2020).
Taylor, E. C., Livingston, L. A., Callan, M. J., Hanel, P. H. P. & Shah, P. Do autistic traits predict pro-environmental attitudes and behaviors, and climate change belief? J. Environ. Psychol. 76 , 101648 (2021).
Li, D. et al. Exposure to nature for children with autism spectrum disorder: benefits, caveats, and barriers. Health Place. 55 , 71–79 (2019).
Prothmann, A., Ettrich, C. & Prothmann, S. Preference for, and responsiveness to, people, dogs and objects in children with autism. Anthrozoös 22 , 161–171 (2009).
Carlisle, G. K. The social skills and attachment to dogs of children with autism spectrum disorder. J. Autism Dev. Disord. 45 , 1137–1145 (2015).
O’Haire, M. E. Animal-assisted intervention for autism spectrum disorder: a systematic literature review. J. Autism Dev. Disord. 43 , 1606–1622 (2013).
Atherton, G. & Cross, L. Seeing more than human: autism and anthropomorphic theory of mind. Front. Psychol. 9 , 528 (2018).
Grove, R., Hoekstra, R. A., Wierda, M. & Begeer, S. Special interests and subjective wellbeing in autistic adults. Autism Res. 11 , 766–775 (2018).
Lam, G. Y. H., Holden, E., Fitzpatrick, M., Mendez, L. R. & Berkman, K. “Different but connected”: participatory action research using photovoice to explore well-being in autistic young adults. Autism 24 , 1246–1259 (2020).
Davidson, J. & Smith, M. in Emotions Matter: A Relations Approach to Emotions (eds Spencer D., Walby, K. & Hunt, A.) 260–280 (Univ. of Toronto, 2012).
Turner-Brown, L. M., Lam, K. S. L., Holtzclaw, T. N., Dichter, G. S. & Bodfish, J. W. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism 15 , 437–456 (2011).
Winter-Messiers, M. From tarantulas to toilet brushes: understanding the special interest areas of children and youth with Asperger’s syndrome. Remedial Spec. Educ. 28 , 140–152 (2007).
Kirchner, J., Ruch, W. & Dziobek, I. Brief report: character strengths in adults with autism spectrum disorder without intellectual impairment. J. Autism Dev. Disord. 46 , 3330–3337 (2016).
Mercier, C., Mottron, L. & Belleville, S. A psychosocial study on restricted interests in high-functioning persons with pervasive developmental disorders. Autism 4 , 406–425 (2000).
Draaisma, D. Stereotypes of autism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364 , 1475–1480 (2009).
Gaudion, K. & Pellicano, E. The triad of strengths: a strengths-based approach for designing with autistic adults with additional learning disabilities. Lect. Notes Comput. Sci. 9746 , 266–280 (2016).
Russell, G. et al. Mapping the autistic advantage from the accounts of adults diagnosed with autism: a qualitative study. Autism Adulthood 1 , 124–133 (2019).
Csikszentmihalyi, M. Flow: The Psychology of Optimal Experience (Harper and Row, 1990).
Orsmond, G. I., Krauss, M. W. & Seltzer, M. M. Peer relationships and social and recreational activities among adolescents and adults with autism. J. Autism Dev. Disord. 34 , 245–256 (2004).
Stacey, T.-L., Froude, E. H., Trollor, J. & Foley, K.-R. Leisure participation and satisfaction in autistic adults and neurotypical adults. Autism 116 , 993–1004 (2019).
Shea, L. L., Verstreate, K., Nonnemacher, S., Song, W. & Salzer, M. S. Self-reported community participation experiences and preferences of autistic adults. Autism 25 , 1295–1306 (2021).
Cameron, L., Borland, R. L., Tonge, B. J. & Gray, K. M. Community participation in adults with autism: a systematic review. J. Appl. Res. Intellect. Disabil. 35 , 421–447 (2022).
Tanner, K., Hand, B. N., O’Toole, G. & Lane, A. E. Effectiveness of interventions to improve social participation, play, leisure and restricted and repetitive behaviors in people with autism spectrum disorder: a systematic review. Am. J. Occup. Ther. 69 , 6905180010 (2015).
Bishop-Fitzpatrick, L., Smith DaWalt, L., Greenberg, J. S. & Mailick, M. R. Participation in recreational activities buffers the impact of perceived stress on quality of life in adults with autism spectrum disorder. Autism Res. 10 , 973–982 (2017).
Emerson, E. B., Davies, I., Spencer, K. & Malam, S. Adults with Learning Difficulties in England 2003/4 (National Statistics, 2005).
Keeley, H., Redley, M., Holland, A. J. & Clare, I. C. H. Participation in the 2005 general election by adults with intellectual disabilities. J. Intellect. Disabil. Res. 52 , 175–181 (2008).
Armstrong, R. Political engagement in the 2019 UK general election of patients with autism and/or a learning disability detained in a psychiatric hospital. Tizard Learn. Disabil. Rev. 26 , 77–86 (2021).
Fleischer, D. Z. & Zames, F. The Disability Rights Movement: from Charity to Confrontation (Temple Univ. Press, 2001).
Sinclair, J. Don’t mourn for us. Autism Network International http://www.autreat.com/dont_mourn.html (1993). This foundational text for the neurodiversity movement from groundbreaking autistic activist Jim Sinclair challenges the notion that parental grief is the inevitable consequence of having an autistic child .
Kras, J. F. The ‘ransom notes’ affair: when the neurodiversity movement came of age. Disabil. Stud. Q. 30 , 1065 (2010).
Neumeier, S. M. & Brown, L. X. Z. in Autistic Community and the Neurodiversity Movement (ed. Kapp, S.) 195–210 (Palgrave Macmillan, 2020).
Dekker, M. On our own terms: emerging autistic culture. AUTSCAPE http://www.autscape.org/2015/programme/handouts/Autistic-Culture-07-Oct-1999.pdf (1999).
Baggs, M. in Autistic Community and the Neurodiversity Movement (ed. Kapp, S.) 77–86 (Palgrave Macmillan, 2020).
Garcia, E. We’re Not Broken: Changing the Autism Conversation (Houghton Mifflin Harcourt, 2021).
Kapp, S. Autistic Community and the Neurodiversity Movement: Stories from the Frontline (Palgrave Macmillan, 2020). This exceptional compendium presents a collection of papers on the history and ideas of the neurodiversity movement from neurodivergent contributors and others .
Ne’eman, A. & Bascom, J. Autistic self advocacy in the developmental disability movement. Am. J. Bioeth. 20 , 25–27 (2020).
Silberman, S. Neurotribes: The Legacy of Autism and How to Think Smarter About People Who Think Differently (Allen & Unwin, 2015).
Walker, N. & Raymaker, D. M. Toward a neuroqueer future: an interview with Nick Walker. Autism Adulthood 3 , 5–10 (2020).
Späth, E. M. A. & Jongsma, K. R. Autism, autonomy and authenticity. Med. Health Care Philos. 23 , 73–80 (2020).
MacLeod, A., Allan, J., Lewis, A. & Robertson, C. “Here I come again”: the cost of success for higher education students diagnosed with autism. Int. J. Incl. Educ. 22 , 683–697 (2018).
Anderson, C. & Butt, C. Young adults on the autism spectrum: the struggle for appropriate services. J. Autism Dev. Disord. 48 , 3912–3925 (2018).
Brosnan, M. & Gavin, J. The impact of stigma, autism label and wording on the perceived desirability of the online dating profiles of men on the autism spectrum. J. Autism Dev. Disord. 51 , 4077–4085 (2021).
Rava, J., Shattuck, P., Rast, J. & Roux, A. The prevalence and correlates of involvement in the criminal justice system among youth on the autism spectrum. J. Autism Dev. Disord. 47 , 340–346 (2017).
Lindsay, S., Osten, V., Rezai, M. & Bui, S. Disclosure and workplace accommodations for people with autism: a systematic review. Disabil. Rehabil. 43 , 597–610 (2021).
Cook, J., Hull, L., Crane, L. & Mandy, W. Camouflaging in autism: a systematic review. Clin. Psychol. Rev. 89 , 102080 (2021).
Pearson, A. & Rose, K. A conceptual analysis of autistic masking: understanding the narrative of stigma and the illusion of choice. Autism Adulthood 3 , 52–60 (2021). This work is a compelling account of autistic masking that highlights its developmental, adaptive and contextual nature .
Livingston, L. A., Shah, P. & Happé, F. Compensatory strategies below the behavioural surface in autism: a qualitative study. Lancet Psychiatry 6 , 766–777 (2019).
Bradley, L., Shaw, R., Baron-Cohen, S. & Cassidy, S. Autistic adults’ experiences of camouflaging and its perceived impact on mental health. Autism Adulthood 3 , 320–329 (2021).
Cage, E. & Troxell-Whitman, Z. Understanding the reasons, contexts and costs of camouflaging for autistic adults. J. Autism Dev. Disord. 49 , 1899–1911 (2019).
Higgins, J., Arnold, S., Weise, J., Pellicano, E. & Trollor, J. Defining autistic burnout through experts by lived experience: grounded Delphi method investigating #AutisticBurnout. Autism 25 , 2356–2369 (2021).
Raymaker, D. M. et al. “Having all of your internal resources exhausted beyond measure and being left with no clean-up crew”: defining autistic burnout. Autism Adulthood 2 , 132–143 (2020).
Scott, M. et al. Factors impacting employment for people with autism spectrum disorder: a scoping review. Autism 23 , 869–901 (2019).
Bury, S. M., Hedley, D., Uljarević, M. & Gal, E. The autism advantage at work: a critical and systematic review of current evidence. Res. Dev. Disabil. 105 , 103750 (2020).
Scott, M. et al. Employers’ perception of the costs and the benefits of hiring individuals with autism spectrum disorder in open employment in Australia. PLoS ONE 12 , e0177607 (2017).
Baldwin, S., Costley, D. & Warren, A. Employment activities and experiences of adults with high-functioning autism and Asperger’s disorder. J. Autism Dev. Disord. 44 , 2440–2449 (2014).
Chan, W. et al. Factors associated with sustained community employment among adults with autism and co-occurring intellectual disability. Autism 22 , 794–803 (2018).
Black, M. H. et al. Perspectives of key stakeholders on employment of autistic adults across the United States, Australia, and Sweden. Autism Res. 12 , 1648–1662 (2019).
Hayward, S. M., McVilly, K. R. & Stokes, M. A. “I would love to just be myself”: what autistic women want at work. Autism Adulthood 1 , 297–305 (2019).
Thompson-Hodgetts, S., Labonte, C., Mazumder, R. & Phelan, S. Helpful or harmful? A scoping review of perceptions and outcomes of autism diagnostic disclosure to others. Res. Autism Spectr. Disord. 77 , 101598 (2020).
Flower, R. L., Hedley, D., Spoor, J. R. & Dissanayake, C. An alternative pathway to employment for autistic job-seekers: a case study of a training and assessment program targeted to autistic job candidates. J. Vocat. Educ. Train. 71 , 407–428 (2019).
Remington, A., Heasman, B., Romualdez, A. & Pellicano, E. Experiences of autistic and non-autistic individuals participating in a corporate internship scheme. Autism 26 , 201–216 (2021).
Taylor, J. L. When is a good outcome actually good? Autism 21 , 918–919 (2017).
Robeyns, I. Conceptualising well-being for autistic persons. J. Med. Ethics 42 , 383–390 (2016). This article from a political philosopher asks whether the capabilities approach is a useful theory of well-being for autistic people .
Fardella, M. A., Riosa, P. B. & Weiss, J. A. A qualitative investigation of risk and protective factors for interpersonal violence in adults on the autism spectrum. Disabil. Soc. 33 , 1460–1481 (2018).
Hayes, J., Ford, T., Rafeeque, H. & Russell, G. Clinical practice guidelines for diagnosis of autism spectrum disorder in adults and children in the UK: a narrative review. BMC Psychiatry 18 , 222 (2018).
Scattoni, M. L. et al. Real-world experiences in autistic adult diagnostic services and post-diagnostic support and alignment with services guidelines: results from the ASDEU study. J. Autism Dev. Disord. 51 , 4129–4146 (2021).
Hull, L., Petrides, K. V. & Mandy, W. The female autism phenotype and camouflaging: a narrative review. Rev. J. Autism Dev. Disord. 7 , 306–317 (2020).
Leedham, A., Thompson, A., Smith, R. & Freeth, M. “I was exhausted trying to figure it out”: the experiences of females receiving an autism diagnosis in middle to late adulthood. Autism 24 , 135–146 (2020).
de Leeuw, A., Happé, F. & Hoekstra, R. A. A conceptual framework for understanding the cultural and contextual factors on autism across the globe. Autism Res. 13 , 1029–1050 (2020).
Wehmeyer, M. L. The importance of self-determination to the quality of life of people with intellectual disability: a perspective. Int. J. Environ. Res. Public. Health 17 , 7121 (2020). This paper presents a powerful analysis on the importance of self-determination to the lives of people with intellectual and developmental disabilities .
Article PubMed Central Google Scholar
Baron-Cohen, S. The concept of neurodiversity is dividing the autism community. Scientific American https://blogs.scientificamerican.com/observations/the-concept-of-neurodiversity-is-dividing-the-autism-community/ (2019).
National Council on Severe Autism. NCSA position statement on diagnostic labels. NCSA https://www.ncsautism.org/dsm (2021).
Ne’eman, A. & Pellicano, E. Neurodiversity as politics. Hum. Dev. 66 , 149–157 (2022).
Pukki, H. et al. Autistic perspectives on the future of clinical autism research. Autism Adulthood https://doi.org/10.1089/aut.2022.0017 (2022).
Lam, G. Y. H., Sabnis, S., Valcarlos, M. M. & Wolgemuth, J. R. A critical review of academic literature constructing wellbeing in autistic adults. Autism Adulthood 3 , 61–71 (2021). This excellent review elucidates how the idea of well-being is conceptualized in the academic literature, demonstrating how such understandings constrain the possibilities of living a good life for autistic people .
Robertson, S. M. Neurodiversity, quality of life, and autistic adults: shifting research and professional focuses onto real-life challenges. Disabil. Stud. Q. 30 , 1069 (2010).
Pellicano, E., Dinsmore, A. & Charman, T. What should autism research focus upon? Community views and priorities from the UK. Autism 18 , 756–770 (2014). This large-scale consultation with members of the autistic and autism communities demonstrates the disconnect between what gets researched and what community members want to be researched .
Botha, M. Academic, activist or advocate? Angry, entangled and emerging: a critical reflection on autism knowledge production. Front. Psychol. 12 , 727542 (2021). This piece is a powerful reflection into how the differing roles of autistic activist and autism researcher can intersect to inform autism research .
Fletcher-Watson, S. et al. Making the future together: shaping autism research through meaningful participation. Autism 23 , 943–953 (2019). This co-produced paper identifies five topics relevant to building a community of practice in participatory research, offering a useful guide for early-career and more senior researchers .
Nicolaidis, C. et al. Collaboration strategies in nontraditional community-based participatory research partnerships: lessons from an academic–community partnership with autistic self-advocates. Prog. Community Health Partnersh. 5 , 143–150 (2011).
Cornwall, A. & Jewkes, R. What is participatory research? Soc. Sci. Med. 41 , 1667–1676 (1995).
The best research is produced when researchers and communities work together. Nature 562 , 7 (2018).
Jose, C. et al. “We are the stakeholders with the most at stake”: scientific and autism community co-researchers reflect on their collaborative experience in the CONNECT project. Res. Involv. Engagem. 6 , 58 (2020).
Benevides, T. W. et al. Listening to the autistic voice: mental health priorities to guide research and practice in autism from a stakeholder-driven project. Autism 24 , 822–833 (2020).
den Houting, J., Higgins, J., Isaacs, K., Mahony, J. & Pellicano, E. “I’m not just a guinea pig”: academic and community perceptions of participatory autism research. Autism 25 , 148–163 (2021).
Pickard, H., Pellicano, E., den Houting, J. & Crane, L. Participatory autism research: early career and established researchers’ views and experiences. Autism 26 , 75–87 (2022).
Milton, D. Autistic expertise: a critical reflection on the production of knowledge in autism studies. Autism 18 , 794–802 (2014).
Pellicano, E. et al. “I knew she’d get it, and get me”: participants’ perspectives of a participatory autism research project. Autism Adulthood 4 , 120–129 (2022).
Download references
Acknowledgements
The authors thank L. Crane, J. Lowe and D. Tan for their extremely helpful comments on a previous version of this manuscript. This work has been funded through an Australian Research Council Future Fellowship awarded to E.P. (FT190100077). The views expressed are the views of the authors alone and do not necessarily represent the views of their organizations or funding sources.
Author information
Authors and affiliations.
Macquarie School of Education, Macquarie University, Sydney, New South Wales, Australia
Elizabeth Pellicano, Unsa Fatima, Gabrielle Hall, Melanie Heyworth, Wenn Lawson, Rozanna Lilley & Joanne Mahony
Department of Clinical, Educational and Health Psychology, University College London, London, UK
- Elizabeth Pellicano
Reframing Autism, Sydney, New South Wales, Australia
- Melanie Heyworth
UCL Policy Lab, University College London, London, UK
Marc Stears
You can also search for this author in PubMed Google Scholar
Contributions
This Review was a collaboration between non-autistic researchers (E.P., U.F., R.L. and M.S.) and autistic researchers (G.H., M.H., W.L. and J.M.), who all actively participated in making decisions about the Review. E.P. and M.S. identified the theoretical framework in discussion with U.F., G.H., M.H., W.L., R.L. and J.M.; E.P., U.F., G.H., M.H., W.L., R.L. and J.M. identified the search terms; U.F. and E.P. conducted the literature searches. All authors identified areas of interest from across and within the capabilities and read and reflected on the existing literature in those areas, focusing in particular on the aspects of relevant papers that were least and most compelling and the next steps for research. E.P. and M.S. wrote the original draft of the manuscript. All authors contributed to reviewing and editing the manuscript. The analytic approach was informed by the authors’ training in education (E.P., U.F. and R.L.), psychology (E.P. and W.L.), anthropology (R.L.), nursing (G.H.), history (J.M.) and political philosophy (M.S.), as well as positionalities as autistic researchers and advocates (G.H., M.H., W.L. and J.M.). These participatory processes ensured that the Review was approached through a strengths-based, rather than deficits-based, lens.
Corresponding author
Correspondence to Elizabeth Pellicano .
Ethics declarations
Competing interests.
E.P. reports grants from the Australian Government Department of Education, Skills and Employment, the Australian Research Council, Australia’s National Disability and Insurance Agency’s Information, Linkages and Capacity Building Program, Australia’s National Health and Medical Research Council, Australia’s Cooperative Research Centre for Living with Autism (Autism CRC) and Simons Foundation Autism Research Initiative, and has received honoraria for invited talks from the International Society for Autism Research and Aspect Australia. G.H. reports grants from the Australian Government Department of Education, Skills and Employment, and sits on the Board of Directors for Amaze, the peak organization for autistic people and their families in Victoria, Australia, and the Disability Advisory Council for Australia’s Victorian State Government, for which she receives meeting attendance payments. M.H. is CEO of the not-for-profit organization Reframing Autism Ltd and co-chair of the Australasian Autism Research Council (unremunerated), and reports grants from the Australian Government Department of Education, Skills and Employment, and Australia’s National Disability and Insurance Agency’s Information, Linkages and Capacity Building Program. W.L. reports grants from the Australian Government Department of Education, Skills and Employment, and Autism CRC. He is a member of the Australasian Autism Research Council (unremunerated), a participant and advisor for Autism CRC and an ambassador for the I CAN Network, and receives royalties from books and occasional fees for workshop and invited addresses. R.L. reports grants from the Australian Government Department of Education, Skills and Employment, and Autism CRC. M.S. reports grants from the Paul Ramsay Foundation and from the University of Sydney, is an Associate Fellow at the Said Business School, Oxford and assists fundraising efforts with various philanthropic groups in his role as Director of the UCL Policy Lab. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Psychology thanks Vanessa Bal, who co-reviewed with Ellen Wilkinson; Brenna Maddox; and Ari Ne’eman for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A term used by autistic people to describe a state caused by excessive sensory or social stimulation.
A state that occurs when a person’s sensory system becomes overwhelmed, possibly owing to difficulties processing and integrating perceptual information, causing significant distress.
When a person accepts negative stereotypes about autism and applies them to themselves.
A cognitive theory of autism, which suggests that the primary feature of autism is a tendency for a singular attentional focus.
An optimal state in which a person becomes fully immersed in an activity, resulting in intense concentration, creative engagement and the loss of awareness of time and self.
A qualitative research methodology in which participants take photographs to illustrate, and possibly prompt discussion of, their experiences.
A community-driven term describing a highly debilitating condition involving exhaustion, withdrawal, executive function problems and generally reduced functioning, with increased manifestation of autistic traits.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Pellicano, E., Fatima, U., Hall, G. et al. A capabilities approach to understanding and supporting autistic adulthood. Nat Rev Psychol 1 , 624–639 (2022). https://doi.org/10.1038/s44159-022-00099-z
Download citation
Accepted : 26 July 2022
Published : 05 September 2022
Issue Date : November 2022
DOI : https://doi.org/10.1038/s44159-022-00099-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A comparison of attitudes and knowledge towards autism based on adult sibling experiences.
- Victoria Morris
- Gillian Hendry
- Carrie Ballantyne
Journal of Adult Development (2024)
Positive and Negative Sexual Cognitions of Autistic Individuals
- Marta García-Barba
- Shana Nichols
- E. Sandra Byers
Sexuality and Disability (2024)
Autistic Women’s Experiences of the Perinatal Period: A Systematic Mixed Methods Review
- Verity Westgate
- Olivia Sewell
- Heather O’Mahen
Review Journal of Autism and Developmental Disorders (2024)
Mental Health Professionals’ Experiences of Adapting Mental Health Interventions for Autistic Adults: A Systematic Review and Thematic Synthesis
- Laura Moore
- Fionnuala Larkin
- Sarah Foley
Journal of Autism and Developmental Disorders (2023)
The Foundations of Autistic Flourishing
Current Psychiatry Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Signs and Symptoms
- Living with Autism Spectrum Disorder
- Frequently Asked Questions (FAQs)
- Data and Statistics on Autism Spectrum Disorder
- Autism Materials and Resources
- Diagnosis ASD
- Information on ASD for Healthcare Providers
- Acceptance Month Partner Toolkit
- 2023 Community Report on Autism
- Autism Data Visualization Tool
Treatment and Intervention for Autism Spectrum Disorder
- Current treatments for autism spectrum disorder (ASD) seek to reduce symptoms that interfere with daily functioning and quality of life.
- Treatments can be given in education, health, community, or home settings, or a combination of settings.
- As individuals with ASD leave high school and grow into adulthood, additional services can help improve health and daily functioning, and facilitate social and community engagement.

Types of Treatments
There are many types of treatments available. These treatments generally can be broken down into the following categories, although some treatments involve more than one approach:
- Developmental
- Educational
- Social-relational
- Pharmacological
- Psychological
- Complementary and alternative
Behavioral approaches
Behavioral approaches focus on changing behaviors by understanding what happens before and after the behavior. Behavioral approaches have the most evidence for treating symptoms of ASD. They have become widely accepted among educators and healthcare professionals and are used in many schools and treatment clinics. A notable behavioral treatment for people with ASD is called applied behavior analysis (ABA) . ABA encourages desired behaviors and discourages undesired behaviors to improve a variety of skills. Progress is tracked and measured.
Two ABA teaching styles are discrete trial training (DTT) and pivotal response training (PRT) .
- DTT uses step-by-step instructions to teach a desired behavior or response. Lessons are broken down into their simplest parts, and desired answers and behaviors are rewarded. Undesired answers and behaviors are ignored.
- PRT takes place in a natural setting rather than clinic setting. The goal of PRT is to improve a few "pivotal skills" that will help the person learn many other skills. One example of a pivotal skill is being able to initiate communication with others.
Developmental approaches
Developmental approaches focus on improving specific developmental skills, such as language skills or physical skills, or a broader range of interconnected developmental abilities. Developmental approaches are often combined with behavioral approaches.
The most common developmental therapy for people with ASD is speech and language therapy . Speech and language therapy helps to improve the person's understanding and use of speech and language. Some people with ASD communicate verbally. Others may communicate through the use of signs, gestures, pictures, or an electronic communication device.
Occupational therapy teaches skills that help the person live as independently as possible. Skills may include dressing, eating, bathing, and relating to people. Occupational therapy can also include
- Sensory integration therapy to help improve responses to sensory input that may be restrictive or overwhelming.
- Physical therapy can help improve physical skills, such as fine movements of the fingers or larger movements of the trunk and body.
The Early Start Denver Model (ESDM) is a broad developmental approach based on the principles of ABA. It is used with children 12–48 months of age. Parents and therapists use play, social exchanges, and shared attention in natural settings to improve language, social, and learning skills.
Educational approaches

Educational treatments are given in a classroom setting. One type of educational approach is the Treatment and Education of Autistic and Related Communication-Handicapped Children (TEACCH) approach. TEACCH is based on the idea that people with autism thrive on consistency and visual learning. It provides teachers with ways to adjust the classroom structure and improve academic and other outcomes. For example, daily routines can be written or drawn and placed in clear sight. Boundaries can be set around learning stations. Verbal instructions can be complemented with visual instructions or physical demonstrations.
Social-relational approaches
Social-relational treatments focus on improving social skills and building emotional bonds. Some social-relational approaches involve parents or peer mentors.
- The Developmental, Individual Differences, Relationship-Based model (also called DIR or "Floor Time") encourages parents and therapists to follow the interests of the individual to expand opportunities for communication.
- The Relationship Development Intervention (RDI) model involves activities that increase motivation, interest, and abilities to participate in shared social interactions.
- Social Stories provide simple descriptions of what to expect in a social situation.
- Social skills groups provide opportunities for people with ASD to practice social skills in a structured environment.
Pharmacological approaches
Important to know.
Some medications treat co-occurring symptoms (those that happen along with ASD) and can help people with ASD function better. For example, medication might help manage high energy levels, inability to focus, or self-harming behavior, such as head banging or hand biting. Medication can also help manage co-occurring psychological conditions, such as anxiety or depression, in addition to medical conditions such as seizures, sleep problems, or stomach or other gastrointestinal problems.
It is important to work with a doctor who has experience in treating people with ASD when considering the use of medication. This applies to both prescription medication and over-the-counter medication. Individuals, families, and doctors must work together to monitor progress and reactions to be sure any negative side effects of the medication do not outweigh the benefits.
Psychological approaches
Psychological approaches can help people with ASD cope with anxiety, depression, and other mental health issues. Cognitive-behavior therapy (CBT) is one psychological approach that focuses on learning the connections between thoughts, feelings, and behaviors. During CBT, a therapist and the individual work together to identify goals and then change how the person thinks about a situation to change how they react to the situation.
Complementary and alternative treatments
Some people with ASD and their families use treatments that do not fit into any of the other categories. These treatments are known as complementary and alternative treatments. Complementary and alternative treatments are often used to supplement more traditional approaches. They might include special diets, herbal supplements, chiropractic care, animal therapy, arts therapy, mindfulness, or relaxation therapies. Individuals and families should always talk to their doctor before starting a complementary and alternative treatment.
Additional ASD Treatment Options
For more information on ASD treatment options, please check out these additional resources:
- American Academy of Pediatrics Council on Children with Disabilities
- Autism Society
- Interagency Autism Coordinating Committee (IACC)
- National Institute on Child Health and Human Development
- Hyman SL, Levy SE, Myers SM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics . 2020;145(1):e20193447.
Autism Spectrum Disorder (ASD)
Autism spectrum disorder (ASD) is a developmental disability that can cause significant social, communication and behavioral challenges. CDC is committed to continuing to provide essential data on ASD and develop resources that help identify children with ASD as early as possible.
For Everyone
Health care providers, public health.

IMAGES
COMMENTS
About the journal. Research in Autism Spectrum Disorders (RASD) publishes high quality empirical articles and reviews that contribute to a better understanding of Autism Spectrum Disorders (ASD) at all levels of description; genetic, neurobiological, cognitive, and behavioral. The primary focus of the journal is to …. View full aims & scope.
ARI donors support research that has practical application in the evolution of autism understanding and the lives of autistic people. Last fall, ARI awarded more than $400,000 in grants to fund research on evidence-based therapeutic interventions and underlying biological mechanisms. Our Latest Research Grant Recipients.
Some research has suggested that in the context of autism, depressive illnesses such as bipolar disorder can present atypically . Combined with other study ( 25 ) suggesting that interventions targeting depressive symptoms might also impact on core autistic features, the possibility that autism and depression or depressive symptoms might be ...
Polygenic risk for autism, attention-deficit hyperactivity disorder, schizophrenia, major depressive disorder, and neuroticism is associated with the experience of childhood abuse. Mol Psychiatry ...
Autism spectrum disorders are a group of neurodevelopmental disorders that are characterized by impaired social interaction and communication skills, and are often accompanied by other behavioural ...
Autism is a neurodevelopmental disorder that typically begins to manifest in early childhood and affects social communication and behaviours throughout the life span. Autism and other neurodevelopmental disorders have seen a tremendous influx of interest from the scientific community in the past 60 years.
Autism is a major, peer-reviewed, international journal, published 8 times a year, publishing research of direct and practical relevance to help improve the quality of life for individuals with autism or autism-related disorders. It is interdisciplinary in nature, … | View full journal description. This journal is a member of the Committee on ...
Overview. Journal of Autism and Developmental Disorders is a leading scholarly publication that focuses on all aspects of autism spectrum disorders and related developmental disabilities. Seeks to advance the understanding of autism, including potential causes and prevalence, diagnosis advancements, and effective clinical care, education, and ...
Advances in autism research, 2021: continuing to decipher the secrets of autism. 1427. trimester average and maximal daily exposure to ne air. fi. particulate matter of diameter ≤2.5 μm (PM2.5 ...
Autism and attention deficit hyperactivity disorder (ADHD) frequently coexist, but prevalence reports exhibit significant variability based on population characteristics and assessment methods. In the present study, parents and teachers reported a similar 3% prevalence of autism and ADHD traits, with an estimated comorbid diagnosis prevalence ...
Welcome to the website of the Autism and Developmental Disorders Research Program (ADDRP), Lucile Packard Children's Hospital at Stanford University. This Stanford autism research program is based in the Department of Psychiatry and Behavioral Sciences at the Stanford University School of Medicine. ADDRP focuses on improving the quality of life of individuals with autism spectrum disorder and ...
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted interests and repetitive behaviors . ... Ongoing research continues to deepen our understanding of potential etiologic mechanisms in ASD, but currently no single unifying cause has been elucidated. ...
Abstract. In this paper, we provide an update on autism spectrum disorder (ASD), including epidemiology, etiopathogenesis, clinical presentation, instrumental investigations, early signs, onset patterns, neuropsychological hypotheses, treatments, and long-term outcome. The prevalence of this condition has increased enormously over the last few ...
Children with autism spectrum disorder between the ages of 2 and 4 years 11 months are invited to participate. This study involves up to a 5 month time commitment. The participant must be willing to complete cognitive and behavioral assessments (such as IQ and language testing) and be able to either sleep (young children) or lie still in the ...
The National Institute of Mental Health (NIMH), a component of the National Institutes of Health (), is a leading federal funder of research on ASD. What is autism spectrum disorder? Autism spectrum disorder (ASD) refers to a group of complex neurodevelopment disorders caused by differences in the brain that affect communication and behavior.
Autism spectrum disorders (ASD) are a diverse group of conditions. They are characterised by some degree of difficulty with social interaction and communication. Other characteristics are atypical patterns of activities and behaviours, such as difficulty with transition from one activity to another, a focus on details and unusual reactions to sensations.
Research Program on Autism Spectrum Disorders: This NIMH program supports research focused on the characterization, pathophysiology, treatment, and outcomes of ASD and related disorders. Statistics: Autism Spectrum Disorder: This NIMH webpage provides information on the prevalence of ASD in the U.S.
The salient features of autism spectrum disorder (ASD) encompass persistent difficulties in social communication, as well as the presence of restricted and repetitive facets of behavior, hobbies, or pursuits, which are often accompanied with cognitive limitations. Over the past few decades, a sizable number of studies have been conducted to enhance our understanding of the pathophysiology of ...
Autism spectrum disorder — or autism — is a neurodevelopmental disorder that typically manifests in young children. ... in both research and practice. Autism spectrum disorder — or autism ...
What is Autism? Autism is a developmental disorder with symptoms that appear within the first three years of life. Its formal diagnostic name is autism spectrum disorder. The word "spectrum" indicates that autism appears in different forms with varying levels of severity. That means that each individual with autism experiences their own ...
In a novel study, investigators demonstrated the link between disrupted developmental dopamine signaling and autism spectrum disorder (ASD). Their findings underscore the importance of studying ...
This data table provides a collection of information from peer-reviewed autism prevalence studies. Information reported from each study includes the autism prevalence estimate and additional study characteristics (e.g., case ascertainment and criteria). A PubMed search was conducted to identify studies published at any time through September ...
Screening recommendations. Research has found that autism spectrum disorder (ASD) can sometimes be detected at 18 months or younger. By age 2 years, a diagnosis by an experienced professional can be considered very reliable. 1 However, many children do not receive a final diagnosis until they are much older. This delay means that children with ...
Abstract. Autism spectrum disorder (autism) is a heterogeneous group of neurodevelopmental conditions characterized by early childhood-onset impairments in communication and social interaction alongside restricted and repetitive behaviors and interests. This review summarizes recent developments in human genetics research in autism ...
Key points. Diagnosing autism spectrum disorder (ASD) usually relies on two main sources of information: parents' or caregivers' descriptions of their child's development and a professional's observation of the child's behavior. The American Psychiatric Association's Diagnostic and Statistical Manual, Fifth Edition (DSM-5) provides standardized ...
The purpose of this Funding Opportunity Announcement (FOA) is to encourage research grant applications to support research designed to elucidate the etiology, epidemiology, diagnosis, and optimal means of service delivery in relation to Autism Spectrum Disorders (ASD).
Autism Research, 16(10): 1989-2001. 4. Frith, Uta, and Chris Frith. "Reputation management: In autism, generosity is its own reward." ... The impact of autism spectrum disorder and alexithymia on ...
A matter of time: the necessity of temporal language in research on health conditions that present with autism spectrum disorder. Autism Res. 12 , 20-25 (2019). Article PubMed Google Scholar
The most common developmental therapy for people with ASD is speech and language therapy. Speech and language therapy helps to improve the person's understanding and use of speech and language. Some people with ASD communicate verbally. Others may communicate through the use of signs, gestures, pictures, or an electronic communication device.