

Polycystic kidney disease
Citation, doi, disclosures and case data.
At the time the case was submitted for publication Shervin Sharifkashani had no recorded disclosures.
Presentation
Bilateral flank pain, nausea and vomiting, and hematuria.
Patient Data
There are numerous cysts in both kidneys with hyperdense foci within some of the cysts compatible with hemorrhage. There are also a few scattered small calculi within the kidneys and a few small hypodense foci in hepatic lobes more consistent with small liver cysts.
4 case questions available
Q: what is the main clinical symptom of autosomal dominant kidney disease? show answer
A: Hematuria.
Q: what is the most sensitive imaging modality for the diagnosis of autosomal dominant polycystic kidney disease? show answer
Q: what is the most hazardous complication of autosomal dominant kidney disease? show answer
A: Intracranial arterial aneurysm.
Q: what is the preferred imaging modality for follow-up of the autosomal dominant polycystic kidneys disease? show answer
Case Discussion
A case is a 50-year-old man who came to the emergency room with the clinical symptoms of flank pain and hematuria . The u reteral s tones suspected and non-contrast abdomen and pelvic MDCT were requested. On performed MDCT, numerous cysts of different sizes and a few scattered small stones with small hyperdense foci compatible with hemorrhage within some of the cysts in both kidneys were detected. A few small hypodense foci in hepatic lobes compatible with small cysts are also seen. Decreased kidneys' parenchyma interposed the cysts were also seen. Findings were compatible with polycystic kidney disease .
A high-resolution ultrasound exam, MDCT, and MRI all can be useful for the diagnosis of polycystic kidney disease patients but the MRI is considered highly sensitive and specific in some of the recent studies for diagnosis and also a follow-up of kidneys status autosomal dominant polycystic kidney disease . These patients are also at greater risk of intracranial arterial aneurysm and MRA also is the preferred imaging modality for follow-up of the patients from this point of view.
- Pei Y, Hwang Y, Conklin J et al. Imaging-Based Diagnosis of Autosomal Dominant Polycystic Kidney Disease. JASN. 2014;26(3):746-53. doi:10.1681/asn.2014030297 - Pubmed
- Jiang T, Wang P, Qian Y et al. A Follow-Up Study of Autosomal Dominant Polycystic Kidney Disease with Intracranial Aneurysms Using 3.0T Three-Dimensional Time-Of-Flight Magnetic Resonance Angiography. Eur J Radiol. 2013;82(11):1840-5. doi:10.1016/j.ejrad.2013.01.024 - Pubmed
0 public playlists include this case
Related radiopaedia articles.
- Autosomal dominant polycystic kidney disease
- Autosomal recessive polycystic kidney disease
- Intracranial aneurysm (overview)
- Loin pain haematuria syndrome
- MR angiography
- Renal tract calculi (summary)
- Salt and pepper sign (autosomal recessive polycystic kidney disease)
- Simple hepatic cyst
- Ureteric calculi
- Urolithiasis
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Genetic identification of inherited cystic kidney diseases for implementing precision medicine: a study protocol for a 3-year prospective multicenter cohort study
Affiliations.
- 1 Department of Internal Medicine, Hallym University College of Medicine, Seoul, South Korea.
- 2 Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea.
- 3 Department of Internal Medicine, National Medical Center, Seoul, South Korea.
- 4 Department of Internal Medicine, Kangbuk Samsung Hospital, Seoul, South Korea.
- 5 Department of Internal Medicine, Busan Paik Hospital, Busan, South Korea.
- 6 Department of Internal Medicine, Keimyung University School of Medicine, Daegu, South Korea.
- 7 Department of Internal Medicine, Chonnam National University Medical School, Gwangju, South Korea.
- 8 Department of Pediatrics, Seoul National University Children's Hospital, Seoul, South Korea.
- 9 Department of Pediatrics, Hallym University College of Medicine, Seoul, South Korea.
- 10 Department of Biomedical Sciences, Korea University College of Medicine, Seoul, South Korea.
- 11 Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea. [email protected].
- 12 Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, South Korea. [email protected].
- PMID: 33407230
- PMCID: PMC7786983
- DOI: 10.1186/s12882-020-02207-8
Background: Inherited cystic kidney disease is a spectrum of disorders in which clusters of renal cysts develop as the result of genetic mutation. The exact methods and pipelines for defining genetic mutations of inherited cystic kidney disease are not clear at this point. This 3-year, prospective, multicenter, cohort study was designed to set up a cohort of Korean patients with inherited cystic kidney disease, establish a customized genetic analysis pipeline for each disease subtype, and identify modifying genes associated with the severity of the disease phenotype.
Methods/design: From May 2020 to May 2022, we aim to recruit 800 patients and their family members to identify pathogenic mutations. Patients with more than 3 renal cysts in both kidneys are eligible to be enrolled. Cases of simple renal cysts and acquired cystic kidney disease that involve cyst formation as the result of renal failure will be excluded from this study. Demographic, laboratory, and imaging data as well as family pedigree will be collected at baseline. Renal function and changes in total kidney volume will be monitored during the follow-up period. Genetic identification of each case of inherited cystic kidney disease will be performed using a targeted gene panel of cystogenesis-related genes, whole exome sequencing (WES) and/or family segregation studies. Genotype-phenotype correlation analysis will be performed to elucidate the genetic effect on the severity of the disease phenotype.
Discussion: This is the first nationwide cohort study on patients with inherited cystic kidney disease in Korea. We will build a multicenter cohort to describe the clinical characteristics of Korean patients with inherited cystic kidney disease, elucidate the genotype of each disease, and demonstrate the genetic effects on the severity of the disease phenotype.
Trial registration: This cohort study was retrospectively registered at the Clinical Research Information Service ( KCT0005580 ) operated by the Korean Center for Disease Control and Prevention on November 5th, 2020.
Keywords: Cohort study; Cystic kidney disease; Genetic association studies; Genotype; Glomerular filtration rate; High-throughput nucleotide sequencing; Phenotype.
PubMed Disclaimer
Conflict of interest statement
The authors declare that they have no competing interests.
Genetic analysis pipeline. A total…
Genetic analysis pipeline. A total of 800 probands with iCKD will be enrolled…
Similar articles
- Baseline characteristics of the Korean genetic cohort of inherited cystic kidney disease. Cho JM, Park HC, Lee JW, Ryu H, Kim YC, Ahn C, Lee KB, Kim YH, Han S, Kim Y, Bae EH, Kang HG, Park E, Jeong K, Kang S, Choi J, Oh KH, Oh YK. Cho JM, et al. Kidney Res Clin Pract. 2023 Sep;42(5):617-627. doi: 10.23876/j.krcp.23.097. Epub 2023 Sep 27. Kidney Res Clin Pract. 2023. PMID: 37813524 Free PMC article.
- Genetic spectrum of renal disease for 1001 Chinese children based on a multicenter registration system. Rao J, Liu X, Mao J, Tang X, Shen Q, Li G, Sun L, Bi Y, Wang X, Qian Y, Wu B, Wang H, Zhou W, Ma D, Zheng B, Shen Y, Chen Z, Luan J, Wang X, Wang M, Dang X, Wang Y, Wu Y, Hou L, Sun S, Li Q, Liu X, Bai H, Yang Y, Shao X, Li Y, Zheng S, Han M, Liu C, Cao G, Zhao L, Qiu S, Dong Y, Zhu Y, Wang F, Zhang D, Li Y, Zhao L, Yang C, Luo X, Chen L, Jiang X, Zhang A, Xu H; for “Internet Plus” Nephrology Alliance of National Center for Children's Care. Rao J, et al. Clin Genet. 2019 Nov;96(5):402-410. doi: 10.1111/cge.13606. Epub 2019 Jul 25. Clin Genet. 2019. PMID: 31328266
- A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Bullich G, Domingo-Gallego A, Vargas I, Ruiz P, Lorente-Grandoso L, Furlano M, Fraga G, Madrid Á, Ariceta G, Borregán M, Piñero-Fernández JA, Rodríguez-Peña L, Ballesta-Martínez MJ, Llano-Rivas I, Meñica MA, Ballarín J, Torrents D, Torra R, Ars E. Bullich G, et al. Kidney Int. 2018 Aug;94(2):363-371. doi: 10.1016/j.kint.2018.02.027. Epub 2018 May 22. Kidney Int. 2018. PMID: 29801666
- Inherited renal cystic diseases. Kim B, King BF Jr, Vrtiska TJ, Irazabal MV, Torres VE, Harris PC. Kim B, et al. Abdom Radiol (NY). 2016 Jun;41(6):1035-51. doi: 10.1007/s00261-016-0754-3. Abdom Radiol (NY). 2016. PMID: 27167233 Review.
- Current insights into renal ciliopathies: what can genetics teach us? Arts HH, Knoers NV. Arts HH, et al. Pediatr Nephrol. 2013 Jun;28(6):863-74. doi: 10.1007/s00467-012-2259-9. Epub 2012 Jul 25. Pediatr Nephrol. 2013. PMID: 22829176 Free PMC article. Review.
- Protocol for the nationwide registry of patients with polycystic kidney disease: japanese national registry of PKD (JRP). Nakatani S, Kawano H, Sato M, Hoshino J, Nishio S, Miura K, Sekine A, Suwabe T, Hidaka S, Kataoka H, Ishikawa E, Shimazu K, Uchiyama K, Fujimaru T, Moriyama T, Kurashige M, Shimabukuro W, Hattanda F, Kimura T, Ushio Y, Manabe S, Watanabe H, Mitobe M, Seta K, Shimada Y, Kai H, Katayama K, Ichikawa D, Hayashi H, Hanaoka K, Mochizuki T, Nakanishi K, Tsuchiya K, Horie S, Isaka Y, Muto S; JRP collaborators. Nakatani S, et al. Clin Exp Nephrol. 2024 May 11. doi: 10.1007/s10157-024-02509-3. Online ahead of print. Clin Exp Nephrol. 2024. PMID: 38734869
- Factors Associated With the Development and Severity of Polycystic Liver in Patients With Autosomal Dominant Polycystic Kidney Disease. Kim Y, Park HC, Ryu H, Kim YC, Ahn C, Lee KB, Kim YH, Han S, Bae EH, Jeong K, Choi J, Oh KH, Oh YK. Kim Y, et al. J Korean Med Sci. 2023 Sep 25;38(38):e296. doi: 10.3346/jkms.2023.38.e296. J Korean Med Sci. 2023. PMID: 37750370 Free PMC article.
- Cystic Diseases of the Kidneys: From Bench to Bedside. Raina R, Lomanta F, Singh S, Anand A, Kalra R, Enukonda V, Barat O, Pandher D, Sethi SK. Raina R, et al. Indian J Nephrol. 2023 Mar-Apr;33(2):83-92. doi: 10.4103/ijn.ijn_318_21. Epub 2023 Feb 20. Indian J Nephrol. 2023. PMID: 37234435 Free PMC article. Review.
- Mayo imaging classification is a good predictor of rapid progress among Korean patients with autosomal dominant polycystic kidney disease: results from the KNOW-CKD study. Park HC, Hong Y, Yeon JH, Ryu H, Kim YC, Lee J, Kim YH, Chae DW, Chung W, Ahn C, Oh KH, Oh YK. Park HC, et al. Kidney Res Clin Pract. 2022 Jul;41(4):432-441. doi: 10.23876/j.krcp.21.261. Epub 2022 Mar 3. Kidney Res Clin Pract. 2022. PMID: 35286789 Free PMC article.
- Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol. 2016;12(3):133–146. - PMC - PubMed
- Dillman JR, Trout AT, Smith EA, Towbin AJ. Hereditary renal cystic disorders: imaging of the kidneys and beyond. Radiographics. 2017;37(3):924–946. - PubMed
- Mitchison HM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol. 2017;241(2):294–309. - PubMed
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364(16):1533–1543. - PMC - PubMed
- Braun DA, Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol. 2017;9(3):a028191. - PMC - PubMed
Publication types
- Search in MeSH
Associated data
- CRiS/KCT0005580
Related information
Grants and funding.
- 2019-ER-7304-00/Korea Centers for Disease Control and Prevention
- 2019-ER-7304-01/Korea Centers for Disease Control and Prevention
LinkOut - more resources
Full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Acquired Cystic Kidney Disease
- First Online: 10 August 2017
Cite this chapter

- Eugene Y. H. Chan MRCPCH, FHKAM(Paed) 4 &
- Bradley A. Warady MD 5
773 Accesses
2 Citations
Acquired cystic kidney disease (ACKD) is a well-described condition in the adult population, which occurs primarily in patients with end-stage renal disease (ESRD). In contrast to inherited cystic kidney disease, ACKD is characterized by the presence of multiple small cysts in small kidneys. Whereas pediatric data pertaining to the development of ACKD is limited, a high incidence (21.6–45.8%) of the disorder has been described in children on chronic dialysis. An increased frequency of ACKD is also associated with a longer duration of dialysis. Although most patients with ACKD are asymptomatic, the condition can be complicated by the development of renal cell carcinoma (RCC). Since early RCC detection can lead to improved long-term outcome, routine surveillance should be considered in pediatric dialysis and transplant recipients.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
Acquired cystic kidney disease: an under-recognized condition in children with end-stage renal disease.

Cystic Diseases of the Kidney

Renal Cystic Diseases
Dunnill M, Millard P, Oliver D. Acquired cystic disease of the kidneys: a hazard of long-term intermittent maintenance haemodialysis. J Clin Pathol. 1977;30(9):868–77.
Article CAS PubMed PubMed Central Google Scholar
Grantham JJ. Acquired cystic kidney disease. Kidney Int. 1991;40(1):143–52.
Article CAS PubMed Google Scholar
Ishikawa I, editor. Acquired cystic disease: mechanisms and manifestations. Semin Nephrol. 1991;11(6):671–84. Elsevier.
Google Scholar
Konda R, Sato H, Hatafuku F, Nozawa T, Ioritani N, Fujioka T. Expression of hepatocyte growth factor and its receptor C-met in acquired renal cystic disease associated with renal cell carcinoma. J Urol. 2004;171(6):2166–70.
Oya M, Mikami S, Mizuno R, Marumo K, Mukai M, Murai M. C-jun activation in acquired cystic kidney disease and renal cell carcinoma. J Urol. 2005;174(2):726–30.
Article PubMed Google Scholar
Narasimhan N, Golper TA, Wolfson M, Rahatzad M, Bennett WM. Clinical characteristics and diagnostic considerations in acquired renal cystic disease. Kidney Int. 1986;30(5):748–52.
Sieniawska M, Roszkowska-Blaim M, Welc-Dobies J. Acquired cystic kidney disease in renal insufficiency. Child Nephrol Urol. 1990;11(1):20–4.
Matson MA, Cohen EP. Acquired cystic kidney disease: occurrence, prevalence, and renal cancers. Medicine. 1990;69(4):217–26.
Hogg RJ. Acquired renal cystic disease in children prior to the start of dialysis. Pediatr Nephrol. 1992;6(2):176–8.
Leichter HE, Dietrich R, Salusky IB, Foley J, Cohen AH, Kangarloo H, et al. Acquired cystic kidney disease in children undergoing long-term dialysis. Pediatr Nephrol. 1988;2(1):8–11.
Mattoo TK, Greifer I, Geva P, Spitzer A. Acquired renal cystic disease in children and young adults on maintenance dialysis. Pediatr Nephrol. 1997;11(4):447–50.
Querfeld U, Schneble F, Wradzidlo W, Waldherr R, Tröger J, Schärer K. Acquired cystic kidney disease before and after renal transplantation. J Pediatr. 1992;121(1):61–4.
Lien Y-HH, Hunt KR, Siskind MS, Zukoski C. Association of cyclosporin A with acquired cystic kidney disease of the native kidneys in renal transplant recipient. Kidney Int. 1993;44(3):613–6.
Denton MD, Magee CC, Ovuworie C, Mauiyyedi S, Pascual M, Colvin RB, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int. 2002;61(6):2201–9.
Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37(10):1469–89.
Delahunt B, Srigley JR, Montironi R, Egevad L. Advances in renal neoplasia: recommendations from the 2012 International Society of Urological Pathology Consensus Conference. Urology. 2014;83(5):969–74.
Cheung CY, Lam MF, Lee KC, Chan GSW, Chan KW, Chau KF, et al. Renal cell carcinoma of native kidney in Chinese renal transplant recipients: a report of 12 cases and a review of the literature. Int Urol Nephrol. 2011;43(3):675–80.
Article PubMed PubMed Central Google Scholar
Mehra R, Smith SC, Divatia M, Amin MB. Emerging entities in renal neoplasia. Surg Pathol Clin. 2015;8(4):623–56.
Chen K, Huang HH, Aydin H, Tan YH, Lau WK, Cheng CW, et al. Renal cell carcinoma in patients with end-stage renal disease is associated with more favourable histological features and prognosis. Scand J Urol. 2015;49(3):200–4.
Karam G, Kälble T, Alcaraz A, Aki F, Budde K, Humke U. Guidelines on renal transplantation of the European Association of Urology (EAU). 2014. https://www.dropbox.com/s/xxuyvtt561cimt0/EAU%20Renal%20Transplantatnation%20guideline.pdf?dl=0 .
Frascà GM, Sandrini S, Cosmai L, Porta C, Asch W, Santoni M, et al. Renal cancer in kidney transplanted patients. J Nephrol. 2015;28(6):659–68.
Weibl P, Klatte T, Kollarik B, Waldert M, Schüller G, Geryk B, et al. Interpersonal variability and present diagnostic dilemmas in Bosniak classification system. Scand J Urol Nephrol. 2011;45(4):239–44.
Heilbrun ME, Remer EM, Casalino DD, Beland MD, Bishoff JT, Blaufox MD, et al. ACR Appropriateness Criteria indeterminate renal mass. J Am Coll Radiol. 2015;12(4):333–41.
Download references
Author information
Authors and affiliations.
Princess Margaret Hospital, Hong Kong, China
Eugene Y. H. Chan MRCPCH, FHKAM(Paed)
Division of Pediatric Nephrology, University of Missouri, Kansas City School of Medicine, Children’s Mercy Hospital, Kansas City, MO, USA
Bradley A. Warady MD
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eugene Y. H. Chan MRCPCH, FHKAM(Paed) .
Editor information
Editors and affiliations.
Division of Pediatric Nephrology, University of Missouri, Kansas City School of Medicine, Children’s Mercy Hospital, Kansas City, Missouri, USA
Bradley A Warady
Division of Pediatric Nephrology, Center for Pediatrics and Adolescent Medicine, University of Heidelberg, Heidelberg, Germany
Franz Schaefer
Division of Pediatric Nephrology, Department of Pediatrics, Stanford University School of Medicine, Lucile Packard Children’s Hospital Stanford, Stanford, California, USA
Steven R. Alexander
Rights and permissions
Reprints and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Chan, E.Y.H., Warady, B.A. (2017). Acquired Cystic Kidney Disease. In: Warady, B., Schaefer, F., Alexander, S. (eds) Pediatric Dialysis Case Studies. Springer, Cham. https://doi.org/10.1007/978-3-319-55147-0_44
Download citation
DOI : https://doi.org/10.1007/978-3-319-55147-0_44
Published : 10 August 2017
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-55145-6
Online ISBN : 978-3-319-55147-0
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: cystic kidney diseases in children and adults: from diagnosis to etiology and back.

- 1 University of Zagreb School of Medicine, Zagreb, Croatia
- 2 Division of Nephrology, Dialysis and Transplantation, Department of Pediatrics, University Hospital Center Zagreb, Zagreb, Croatia
- 3 Department of Nephrology, Arterial Hypertension, Dialysis and Transplantation, University Hospital Center Zagreb, Zagreb, Croatia
- 4 Institute of Human Genetics, Center for Molecular Medicine Cologne, and Center for Rare and Hereditary Kidney Disease, Cologne, University Hospital of Cologne, Cologne, Germany
Editorial on the Research Topic Cystic kidney diseases in children and adults: from diagnosis to etiology and back
Renal cysts are often regarded as the most common abnormality associated with kidney disease ( 1 , 2 ). They are encountered in both adults and children, as isolated findings or as part of a more complex clinical condition ( 3 – 5 ). Isolated kidney cysts in adults sometimes require evaluation for kidney cancer or simple cysts may occur as a sign of age-related kidney tissue degeneration in the absence of any underlying specific kidney disease. Recent advances in understanding the underlying mechanisms have led to the concept of renal ciliopathies with more than 100 genes associated with ciliary dysfunction, resulting in conditions such as polycystic kidney disease (PKD), tuberous sclerosis complex (TSC) and nephronophthisis complex (NPHC), which may be associated with various extrarenal phenotypes ( Figure 1 ) ( 6 – 8 ). In addition to progressive CKD, these disorders are characterized by a variety of additional symptoms such as hepatic impairment, vision problems, developmental delays, intellectual disabilities, and skeletal abnormalities, which inconsistently present throughout the course of the disease ( 4 , 5 , 7 ). Furthermore, the significant phenotypic overlap makes it difficult to differentiate specific disorders, often necessitating genetic testing to reach a definite diagnosis ( 9 ). Despite a multitude of clinical and translational studies, in the majority of cases it is still challenging or even impossible to predict the individual clinical course, necessitating regular follow-up of the patients and a timely response in terms of treatment, which remains mostly symptomatic ( 10 ).
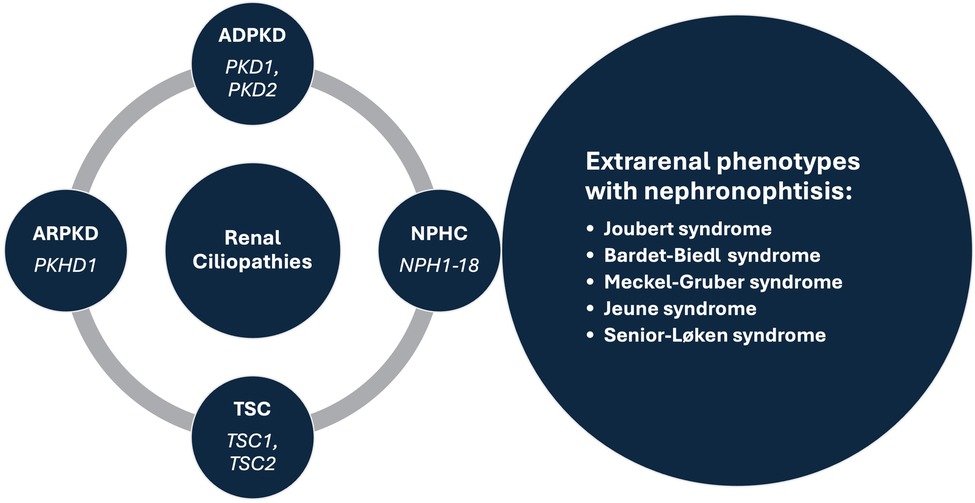
Figure 1 . Prominent syndromes and associated genes within the renal ciliopathies concept. ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; NPHC, nephronophthisis complex; TSC, tuberous sclerosis complex.
The present special issue contains seven noteworthy articles describing engaging cases of children and adults with various disorders having a common denominator in the form of kidney cysts, systematically reviewing the current literature on the clinical characteristics of an HNF1B gene variant and biomarkers of kidney disease progression in autosomal dominant PKD (ADPKD), investigating the outcome of fetal renal cystic disease and exploring the utility of magnetic resonance imaging-based kidney volume assessment for risk stratification in children with ADPKD.
In more detail, Simičić Majce et al . describe a nonconsanguineous family with three members affected by BBS caused by compound heterozygous mutations in the BBS12 gene. Despite identical genotypes, the affected family members demonstrated significant diversity in clinical characteristics (different expressivity) of the BBS phenotype emphasizing the importance of genetic testing for the early diagnosis of this rare ciliopathy. Similarly, Fištrek Prlić et al. present two clinically distinct cases of autosomal dominant tubulointerstitial kidney disease (ADTKD) diagnosed only after genetic testing, along with an extensive review of the literature and a comprehensive overview of the condition. Both patients had uninformative renal ultrasound and urinalysis findings with only elevated serum creatinine levels indicating a kidney disease. An adult patient with a positive family history of CKD had no other symptoms, while an adolescent boy with an unremarkable family history had psychomotor impairment with epilepsy. After the testing they were diagnosed with MUC1 -related ADTKD and 17q12 microdeletion syndrome causing the loss of one copy of the transcription factor HNF1B and 14 additional genes, respectively, highlighting the importance of clinical awareness in diagnosing this syndrome. Finally, the third case report by Kasahara et al. advocates an interesting option to treat the chronic pain experienced by more than half of patients with ADPKD. They describe an adolescent girl with persistent pain associated with multiple renal cysts that prevented her from participating in daily activities. After being diagnosed with attention deficit disorder (ADHD) and appropriate treatment for this condition being initiated, she experienced significant pain relief and better control of her hypertension. Therefore, in patients with ADPKD it may be important to recognize concomitant ADHD and consider a trial of ADHD medications when chronic pain associated with ADPKD is present.
In line with the exploration of important associations between cystic kidney disease and other disorders a systematic review by Nittel et al. examined the prevalence of neurodevelopmental disorders (NDD) in patients with 17q12 microdeletions vs. HNF1B point mutations. The results of a diligent literature search revealed that NDDs are frequently observed in HNF1B -associated diseases, especially in the common 17q12 microdeletion, and should hence become a routine part of clinical care for patients with HNF1B -related diseases. On the other hand, a systematic review by Sorić Hosman et al. provided a critical overview of previously examined serum and urine biomarkers with a potential for predicting disease progression or response to therapy in patients with ADPKD. A comprehensive literature review identified several prognostic molecules that are involved in various processes central to the development of the disease, such as tubular injury, inflammation, metabolism, renin-angiotensin, or vasopressin system adjustments. Interestingly, the most accurate predictive models have been achieved when incorporating such serum and urine biomarkers with the Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) score which combines underlying genetic mutations and clinical risk factors, or with the Mayo Imaging Classification (MIC) which is based on age- and height-adjusted total kidney volume (TKV) measured by magnetic resonance imaging (MRI).
MRI-based kidney volume assessment was further investigated by Yilmaz et al. in a multicenter, cross-sectional, and case-controlled study involving 89 children and adolescents with a genetically confirmed and detailly characterized diagnosis of ADPKD. The study patients were stratified according to the innovative Leuven Imaging Classification (LIC) into different risk categories, with those in the highest risk category having an increased incidence of hypertension and a higher prevalence of PKD1 mutations. Therefore, the study advocates the use of MRI for the measurement of TKV in the pediatric population, in addition to the use of ambulatory blood pressure monitoring to recognize those with hypertension.
Finally, Botero-Calderon et al. presented a retrospective study evaluating clinical and imaging data, genetic testing results and postnatal follow-up outcomes of infants identified in utero with bilateral renal cystic disease at a single referral center over a period of 11 years. Among 17 patients with suspected renal ciliopathy, the most common diagnosis was autosomal recessive PKD (ARPKD, n = 4), followed by Bardet-Biedl syndrome (BBS, n = 3), autosomal dominant polycystic disease (ADPKD, n = 2), HNF1B-related disease ( n = 2), and Meckel-Gruber syndrome (MKS, n = 2), while four cases were not genetically resolved. In terms of postnatal management, the study revealed that the vast majority of neonatal survivors with renal ciliopathies are directed to the care of a pediatric nephrologist, while this proportion is much lower in those with genetically unresolved enlarged, echogenic kidneys, stressing the need for structured management programs for prenatally identified kidney disease.
In conclusion, our research topic provides a contemporary overview of current practices, unmet clinical needs and research gaps regarding the broad spectrum of renal ciliopathies that may be useful to a wide range of physicians and researchers dealing with these complex disorders.
Author contributions
LL: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. IV: Writing – review & editing, Conceptualization. MF: Writing – review & editing, Conceptualization. BB: Writing – review & editing, Conceptualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kwatra S, Krishnappa V, Mhanna C, Murray T, Novak R, Sethi SK, et al. Cystic diseases of childhood: a review. Urology . (2017) 110:184–91. doi: 10.1016/j.urology.2017.07.040
PubMed Abstract | Crossref Full Text | Google Scholar
2. De Groof J, Dachy A, Breysem L, Mekahli D. Cystic kidney diseases in children. Archives de Pédiatrie . (2023) 30(4):240–6. doi: 10.1016/j.arcped.2023.02.005
Crossref Full Text | Google Scholar
3. Mensel B, Kühn JP, Kracht F, Völzke H, Lieb W, Dabers T, et al. Prevalence of renal cysts and association with risk factors in a general population: an MRI-based study. Abdominal Radiology . (2018) 43(11):3068–74. doi: 10.1007/s00261-018-1565-5
4. McConnachie DJ, Stow JL, Mallett AJ. Ciliopathies and the kidney: a review. Am J Kidney Dis . (2021) 77(3):410–9. doi: 10.1053/j.ajkd.2020.08.012
5. Gambella A, Kalantari S, Cadamuro M, Quaglia M, Delvecchio M, Fabris L, et al. The landscape of HNF1B deficiency: a syndrome not yet fully explored. Cells . (2023) 12(2):307. doi: 10.3390/cells12020307
6. Modarage K, Malik SA, Goggolidou P. Molecular diagnostics of ciliopathies and insights into novel developments in diagnosing rare diseases. Br J Biomed Sci . (2022) 79. doi: 10.3389/bjbs.2021.10221
7. Devlin LA, Sayer JA. Renal ciliopathies. Curr Opin Genet Dev . (2019) 56:49–60. doi: 10.1016/j.gde.2019.07.005
8. Kurschat CE, Müller RU, Franke M, Maintz D, Schermer B, Benzing T. An approach to cystic kidney diseases: the clinician’s view. Nat Rev Nephrol . (2014) 10(12):687–99. doi: 10.1038/nrneph.2014.173
9. Lam BL, Leroy BP, Black G, Ong T, Yoon D, Trzupek K. Genetic testing and diagnosis of inherited retinal diseases. Orphanet J Rare Dis . (2021) 16(1):514. doi: 10.1186/s13023-021-02145-0
10. Yu ASL, Landsittel DP. Biomarkers in polycystic kidney disease: are we there? Adv Kidney Dis Health . (2023) 30(3):285–93. doi: 10.1053/j.akdh.2022.12.009
Keywords: cystic kidney disease, autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD), nephronophtisis complex (NPHC), Bardet Biedl syndrome (BBS)
Citation: Lamot L, Vuković Brinar I, Fištrek Prlić M and Beck B (2024) Editorial: Cystic kidney diseases in children and adults: from diagnosis to etiology and back. Front. Pediatr. 12:1401593. doi: 10.3389/fped.2024.1401593
Received: 15 March 2024; Accepted: 29 March 2024; Published: 10 April 2024.
Edited and Reviewed by: Michael L. Moritz , University of Pittsburgh, United States
© 2024 Lamot, Vuković Brinar, Fištrek Prlić and Beck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lovro Lamot [email protected]
This article is part of the Research Topic
Cystic Kidney Diseases in Children and Adults: From Diagnosis to Etiology and Back
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Single-center experience of pediatric cystic kidney disease and literature review.
1. Introduction
1.1. cystic kidney disease as part of the ciliopathies spectrum, 1.2. a condensed overview of cystic kidney disease(s), 2. materials and methods, 2.1. study design, 2.2. data collection, 2.3. diagnostic criteria and definitions, 2.3.1. autosomal dominant polycystic disease, 2.3.2. autosomal recessive polycystic disease, 2.3.3. multicystic dysplastic kidney, 2.3.4. tuberous sclerosis complex, 2.3.5. joubert syndrome, 2.3.6. bardet–biedl syndrome, 2.3.7. nephronophthisis complex, 2.3.8. isolated renal cyst, 2.3.9. recurrent uti (ruti), vesicoureteral reflux (vur) and antibiotic prophylaxis, 2.3.10. hypertension, 2.3.11. proteinuria, 2.4. data analysis, 2.5. ethics statement, 3.1. characteristics of the patients with cystic kidney disease, 3.2. presenting symptoms and signs of cystic kidney disease, 3.3. the renal ultrasound findings, 3.4. vur, (r)uti and antibiotic prophylaxis, 3.5. genetic testing, 3.6. progression to chronic kidney disease (ckd), end-stage renal disease (esrd), and renal replacement therapy (rrt), 3.7. follow-up of patients, 3.8. administered medications and surgical interventions during the follow-up, 3.9. extrarenal cysts and manifestations, 4. discussion, 4.1. ultrasound, 4.2. hypertension, 4.4. genetic testing, 4.5. progression to ckd, esrd and follow-up of patients, 4.6. limitations of the study, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Loftus, H.; Ong, A.C.M. Cystic kidney diseases: Many ways to form a cyst. Pediatr. Nephrol. 2012 , 28 , 33–49. [ Google Scholar ] [ CrossRef ]
- König, J.C.; Titieni, A.; Konrad, M. The NEOCYST Consortium Network for Early Onset Cystic Kidney Diseases—A Comprehensive Multidisciplinary Approach to Hereditary Cystic Kidney Diseases in Childhood. Front. Pediatr. 2018 , 6 , 24. [ Google Scholar ] [ CrossRef ]
- Kurschat, C.E.; Müller, R.-U.; Franke, M.; Maintz, D.; Schermer, B.; Benzing, T. An approach to cystic kidney diseases: The clinician’s view. Nat. Rev. Nephrol. 2014 , 10 , 687–699. [ Google Scholar ] [ CrossRef ]
- McConnachie, D.J.; Stow, J.L.; Mallett, A.J. Ciliopathies and the Kidney: A Review. Am. J. Kidney Dis. 2021 , 77 , 410–419. [ Google Scholar ] [ CrossRef ]
- A Devlin, L.; A Sayer, J. Renal ciliopathies. Curr. Opin. Genet. Dev. 2019 , 56 , 49–60. [ Google Scholar ] [ CrossRef ]
- Modarage, K.; Malik, S.A.; Goggolidou, P. Molecular Diagnostics of Ciliopathies and Insights into Novel Developments in Diagnosing Rare Diseases. Br. J. Biomed. Sci. 2022 , 79 , 10221. [ Google Scholar ] [ CrossRef ]
- Phu, M.D.; Bross, S.; Burkhalter, M.D.; Philipp, M. Limitations and opportunities in the pharmacotherapy of ciliopathies. Pharmacol. Ther. 2021 , 225 , 107841. [ Google Scholar ] [ CrossRef ]
- Butler, T.; Kennedy, L.; Buttino, L.; Juberg, R.C. Prenatal Sonographic Renal Findings Associated with Trisomy 13. J. Diagn. Med. Sonogr. 1992 , 8 , 262–265. [ Google Scholar ] [ CrossRef ]
- Barakat, A.Y.; Butler, M.G. Renal and urinary tract abnormalities associated with chromosome aberrations. Int. J. Pediatr. Nephrol. 1987 , 8 , 215–226. [ Google Scholar ]
- Northrup, H.; Krueger, D.A.; Roberds, S.; Smith, K.; Sampson, J.; Korf, B.; Kwiatkowski, D.J.; Mowat, D.; Nellist, M.; Povey, S.; et al. Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013 , 49 , 243–254. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Henske, E.P.; Jozwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2016 , 2 , 16035. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sekine, A.; Hidaka, S.; Moriyama, T.; Shikida, Y.; Shimazu, K.; Ishikawa, E.; Uchiyama, K.; Kataoka, H.; Kawano, H.; Kurashige, M.; et al. Cystic Kidney Diseases That Require a Differential Diagnosis from Autosomal Dominant Polycystic Kidney Disease (ADPKD). J. Clin. Med. 2022 , 11 , 6528. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chetty, S. Multicystic dysplastic kidney. Am. J. Obstet. Gynecol. 2021 , 225 , B21–B22. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Harris, M.; Schuh, M.P.; McKinney, D.; Kaufman, K.; Erkan, E. Whole Exome Sequencing in a Population with Severe Congenital Anomalies of Kidney and Urinary Tract. Front. Pediatr. 2022 , 10 , 898773. [ Google Scholar ] [ CrossRef ]
- Harris, P.C.; Torres, V.E. Polycystic Kidney Disease. Annu. Rev. Med. 2009 , 60 , 321–337. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cordido, A.; Besada-Cerecedo, L.; García-González, M.A. The Genetic and Cellular Basis of Autosomal Dominant Polycystic Kidney Disease—A Primer for Clinicians. Front. Pediatr. 2017 , 5 , 279. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Prim. 2018 , 4 , 50. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Willey, C.J.; Blais, J.D.; Hall, A.K.; Krasa, H.B.; Makin, A.J.; Czerwiec, F.S. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 2016 , 32 , 1356–1363. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Harris, P.C.; Torres, V.E. Polycystic Kidney Disease, Autosomal Dominant. In GeneReviews ® ; University of Washington: Seattle, WA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1246/ (accessed on 1 December 2023).
- Marlais, M.; Cuthell, O.; Langan, D.; Dudley, J.; Sinha, M.D.; Winyard, P.J.D. Hypertension in autosomal dominant polycystic kidney disease: A meta-analysis. Arch. Dis. Child. 2016 , 101 , 1142–1147. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Massella, L.; Mekahli, D.; Paripović, D.; Prikhodina, L.; Godefroid, N.; Niemirska, A.; Ağbaş, A.; Kalicka, K.; Jankauskiene, A.; Mizerska-Wasiak, M.; et al. Prevalence of Hypertension in Children with Early-Stage ADPKD. Clin. J. Am. Soc. Nephrol. 2018 , 13 , 874–883. [ Google Scholar ] [ CrossRef ]
- Kuo, I.Y.; Chapman, A. Intracranial Aneurysms in Adpkd: How Far Have We Come? Clin. J. Am. Soc. Nephrol. 2019 , 14 , 1119–1121. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bergmann, C. ARPKD and early manifestations of ADPKD: The original polycystic kidney disease and phenocopies. Pediatr. Nephrol. 2014 , 30 , 15–30. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dillman, J.R.; Trout, A.T.; Smith, E.A.; Towbin, A.J. Hereditary Renal Cystic Disorders: Imaging of the Kidneys and Beyond. RadioGraphics 2017 , 37 , 924–946. [ Google Scholar ] [ CrossRef ]
- Lu, H.; Galeano, M.C.R.; Ott, E.; Kaeslin, G.; Kausalya, P.J.; Kramer, C.; Ortiz-Brüchle, N.; Hilger, N.; Metzis, V.; Hiersche, M.; et al. Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat. Genet. 2017 , 49 , 1025–1034. [ Google Scholar ] [ CrossRef ]
- Liebau, M.C. Early clinical management of autosomal recessive polycystic kidney disease. Pediatr. Nephrol. 2021 , 36 , 3561–3570. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shneider, B.L.; Magid, M.S. Liver disease in autosomal recessive polycystic kidney disease. Pediatr. Transplant. 2005 , 9 , 634–639. [ Google Scholar ] [ CrossRef ]
- Gimpel, C.; Avni, E.F.; Breysem, L.; Burgmaier, K.; Caroli, A.; Cetiner, M.; Haffner, D.; Hartung, E.; Franke, D.; König, J.; et al. Imaging of Kidney Cysts and Cystic Kidney Diseases in Children: An International Working Group Consensus Statement. Radiology 2019 , 290 , 769–782. [ Google Scholar ] [ CrossRef ]
- Simms, R.J.; Hynes, A.M.; Eley, L.; Sayer, J.A. Nephronophthisis: A Genetically Diverse Ciliopathy. Int. J. Nephrol. 2011 , 2011 , 527137. [ Google Scholar ] [ CrossRef ]
- Wolf, M.T. Nephronophthisis and related syndromes. Curr. Opin. Pediatr. 2015 , 27 , 201–211. [ Google Scholar ] [ CrossRef ]
- Parisi, M.; Glass, I. Joubert Syndrome. In GeneReviews ® ; University of Washington: Seattle, WA, USA, 1993. [ Google Scholar ]
- Braun, D.A.; Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2016 , 9 , a028191. [ Google Scholar ] [ CrossRef ]
- Randle, S.C. Tuberous Sclerosis Complex: A Review. Pediatr. Ann. 2017 , 46 , e166–e171. [ Google Scholar ] [ CrossRef ]
- Gallo-Bernal, S.; Kilcoyne, A.; Gee, M.S.; Paul, E. Cystic kidney disease in tuberous sclerosis complex: Current knowledge and unresolved questions. Pediatr. Nephrol. 2022 , 38 , 3253–3264. [ Google Scholar ] [ CrossRef ]
- Dixon, B.P.; Hulbert, J.C.; Bissler, J.J. Tuberous Sclerosis Complex Renal Disease. Nephron Exp. Nephrol. 2010 , 118 , e15–e20. [ Google Scholar ] [ CrossRef ]
- Hemachandar, R. Bardet–Biedl syndrome: A rare cause of end stage renal disease. Int. J. Appl. Basic Med. Res. 2015 , 5 , 70–72. [ Google Scholar ] [ CrossRef ]
- Forsyth, R.; Gunay-Aygun, M. Bardet-Biedl Syndrome Overview. In GeneReviews ® ; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1363/ (accessed on 31 July 2023).
- Forsythe, E.; Sparks, K.; Best, S.; Borrows, S.; Hoskins, B.; Sabir, A.; Barrett, T.; Williams, D.; Mohammed, S.; Goldsmith, D.; et al. Risk Factors for Severe Renal Disease in Bardet–Biedl Syndrome. J. Am. Soc. Nephrol. 2016 , 28 , 963–970. [ Google Scholar ] [ CrossRef ]
- Goel, N.; Morris, J.K.; Tucker, D.; de Walle, H.E.K.; Bakker, M.K.; Kancherla, V.; Marengo, L.; Canfield, M.A.; Kallen, K.; Lelong, N.; et al. Trisomy 13 and 18—Prevalence and mortality—A multi-registry population based analysis. Am. J. Med. Genet. Part A 2019 , 179 , 2382–2392. [ Google Scholar ] [ CrossRef ]
- Silverman, S.G.; Pedrosa, I.; Ellis, J.H.; Hindman, N.M.; Schieda, N.; Smith, A.D.; Remer, E.M.; Shinagare, A.B.; Curci, N.E.; Raman, S.S.; et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 2019 , 292 , 475–488. [ Google Scholar ] [ CrossRef ]
- Dell, K.M. The Spectrum of Polycystic Kidney Disease in Children. Adv. Chronic Kidney Dis. 2011 , 18 , 339–347. [ Google Scholar ] [ CrossRef ]
- Ferro, F.; Vezzali, N.; Comploj, E.; Pedron, E.; Di Serafino, M.; Esposito, F.; Pelliccia, P.; Rossi, E.; Zeccolini, M.; Vallone, G. Pediatric cystic diseases of the kidney. J. Ultrasound 2019 , 22 , 381–393. [ Google Scholar ] [ CrossRef ]
- Chaubal, R.; Pokhriyal, S.C.; Deshmukh, A.; Gupta, U.; Chaubal, N. Multicystic Dysplastic Kidney Disease: An In-Utero Diagnosis. Cureus 2023 , 15 , e37786. [ Google Scholar ] [ CrossRef ]
- Valente, E.M.; Marsh, S.E.; Castori, M.; Dixon-Salazar, T.; Bertini, E.; Al-Gazali, L.; Messer, J.; Barbot, C.; Woods, C.G.; Boltshauser, E.; et al. Distinguishing the four genetic causes of jouberts syndrome–related disorders. Ann. Neurol. 2005 , 57 , 513–519. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- A Parisi, M.; Doherty, D.; Chance, P.F.; A Glass, I. Joubert syndrome (and related disorders) (OMIM 213300). Eur. J. Hum. Genet. 2007 , 15 , 511–521. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Beales, P.L.; Elcioglu, N.; Woolf, A.S.; Parker, D.; A Flinter, F. New criteria for improved diagnosis of Bardet-Biedl syndrome: Results of a population survey. J. Med. Genet. 1999 , 36 , 437–446. [ Google Scholar ] [ CrossRef ]
- Melluso, A.; Secondulfo, F.; Capolongo, G.; Capasso, G.; Zacchia, M. Bardet-Biedl Syndrome: Current Perspectives and Clinical Outlook. Ther. Clin. Risk Manag. 2023 , 19 , 115–132. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Soliman, N.; Otto, E.; Allen, S.J.; Sheba, M.; Gawdat, G.; Hildebrandt, F.; Nabhan, M.M.; Badr, A.M.; Fadda, S.; El-Kiky, H. Clinical characterization and NPHP1 mutations in nephronophthisis and associated ciliopathies: A single center experience. Saudi J. Kidney Dis. Transplant. 2012 , 23 , 1090–1098. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hildebrandt, F.; Otto, E.; Rensing, C.; Nothwang, H.G.; Vollmer, M.; Adolphs, J.; Hanusch, H.; Brandis, M. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat. Genet. 1997 , 17 , 149–153. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saunier, S.; Calado, J.; Heilig, R.; Silbermann, F.; Benessy, F.; Morin, G.; Konrad, M.; Broyer, M.; Gubler, M.-C.; Weissenbach, J.; et al. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum. Mol. Genet. 1997 , 6 , 2317–2323. [ Google Scholar ] [ CrossRef ]
- Olbrich, H.; Fliegauf, M.; Hoefele, J.; Kispert, A.; Otto, E.; Volz, A.; Wolf, M.T.; Sasmaz, G.; Trauer, U.; Reinhardt, R.; et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat. Genet. 2003 , 34 , 455–459. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Otto, E.A.; Schermer, B.; Obara, T.; O’Toole, J.F.; Hiller, K.S.; Mueller, A.M.; Ruf, R.G.; Hoefele, J.; Beekmann, F.; Landau, D.; et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 2003 , 34 , 413–420. [ Google Scholar ] [ CrossRef ]
- Mollet, G.; Salomon, R.; Gribouval, O.; Silbermann, F.; Bacq, D.; Landthaler, G.; Milford, D.; Nayir, A.; Rizzoni, G.; Antignac, C.; et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat. Genet. 2002 , 32 , 300–305. [ Google Scholar ] [ CrossRef ]
- Otto, E.; Hoefele, J.; Ruf, R.; Mueller, A.M.; Hiller, K.S.; Wolf, M.T.; Schuermann, M.J.; Becker, A.; Birkenhäger, R.; Sudbrak, R.; et al. A Gene Mutated in Nephronophthisis and Retinitis Pigmentosa Encodes a Novel Protein, Nephroretinin, Conserved in Evolution. Am. J. Hum. Genet. 2022 , 71 , 1161–1167. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- A Sayer, J.; Otto, E.A.; O’Toole, J.F.; Nurnberg, G.; A Kennedy, M.; Becker, C.; Hennies, H.C.; Helou, J.; Attanasio, M.; Fausett, B.V.; et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 2006 , 38 , 674–681. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Valente, E.M.; Silhavy, J.L.; Brancati, F.; Barrano, G.; Krishnaswami, S.R.; Castori, M.; A Lancaster, M.; Boltshauser, E.; Boccone, L.; Al-Gazali, L.; et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 2006 , 38 , 623–625. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lurbe, E.; Cifkova, R.; Cruickshank, J.K.; Dillon, M.J.; Ferreira, I.; Invitti, C.; Kuznetsova, T.; Laurent, S.; Mancia, G.; Morales-Olivas, F.; et al. Management of high blood pressure in children and adolescents: Recommendations of the European Society of Hypertension. J. Hypertens. 2009 , 27 , 1719–1742. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cystic Renal Disease in Children: A Broad Spectrum from Simple Cyst to End Stage Renal Failure. Available online: http://turkjnephrol.org/en/cystic-renal-disease-in-children-a-broad-spectrum-from-simple-cyst-to-end-stage-renal-failure-136848 (accessed on 3 January 2024).
- Evaluation of Proteinuria in Children. Available online: https://www.medilib.ir/uptodate/show/6091 (accessed on 3 January 2024).
- Dupont, V.; Kanagaratnam, L.; Sigogne, M.; Bechade, C.; Lobbedez, T.; Portoles, J.; Rieu, P.; Drame, M.; Touré, F. Outcome of polycystic kidney disease patients on peritoneal dialysis: Systematic review of literature and meta-analysis. PLoS ONE 2018 , 13 , e0196769. [ Google Scholar ] [ CrossRef ]
- Okyere, P.; Ephraim, R.K.; Okyere, I.; Attakorah, J.; Serwaa, D.; Essuman, G.; Abaka-Yawson, A.; Adoba, P. Demographic, diagnostic and therapeutic characteristics of autosomal dominant polycystic kidney disease in Ghana. BMC Nephrol. 2021 , 22 , 156. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fencl, F.; Janda, J.; Bláhová, K.; Hříbal, Z.; Štekrová, J.; Puchmajerová, A.; Seeman, T. Genotype–phenotype correlation in children with autosomal dominant polycystic kidney disease. Pediatr. Nephrol. 2009 , 24 , 983–989. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barroso-Gil, M.; Olinger, E.; Sayer, J.A. Molecular genetics of renal ciliopathies. Biochem. Soc. Trans. 2021 , 49 , 1205–1220. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gall, E.C.-L.; Torres, V.E.; Harris, P.C. Genetic Complexity of Autosomal Dominant Polycystic Kidney and Liver Diseases. J. Am. Soc. Nephrol. 2017 , 29 , 13–23. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Al-Hamed, M.H.; Kurdi, W.; Alsahan, N.; Alabdullah, Z.; Abudraz, R.; Tulbah, M.; Alnemer, M.; Khan, R.; Al-Jurayb, H.; Alahmed, A.; et al. Genetic spectrum of Saudi Arabian patients with antenatal cystic kidney disease and ciliopathy phenotypes using a targeted renal gene panel. J. Med. Genet. 2016 , 53 , 338–347. [ Google Scholar ] [ CrossRef ]
- Alamir, A.; Al Rasheed, S.A.; Al Qahtani, A.T.; Almosa, M.S.; Aljehani, N.D.; Alanazi, E.D.; Almutairi, K.A.; Al Rasheed, S.A.; Almutairi, K.A. The Outcome of Multicystic Dysplastic Kidney Disease Patients at King Abdulaziz Medical City in Riyadh. Cureus 2023 , 15 , e37994. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kopač, M.; Kordič, R. Associated Anomalies and Complications of Multicystic Dysplastic Kidney. Pediatr. Rep. 2022 , 14 , 375–379. [ Google Scholar ] [ CrossRef ]
- Iorio, P.; Heidet, L.; Rutten, C.; Garcelon, N.; Audrézet, M.-P.; Morinière, V.; Boddaert, N.; Salomon, R.; Berteloot, L. The “salt and pepper” pattern on renal ultrasound in a group of children with molecular-proven diagnosis of ciliopathy-related renal diseases. Pediatr. Nephrol. 2020 , 35 , 1033–1040. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Awazu, M. Isolated Nocturnal Hypertension in Children. Front. Pediatr. 2022 , 10 , 823414. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chapman, A.B.; Stepniakowski, K.; Rahbari-Oskoui, F. Hypertension in Autosomal Dominant Polycystic Kidney Disease. Adv. Chronic Kidney Dis. 2010 , 17 , 153–163. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Rechter, S.; Bammens, B.; Schaefer, F.; Liebau, M.C.; Mekahli, D. Unmet needs and challenges for follow-up and treatment of autosomal dominant polycystic kidney disease: The paediatric perspective. Clin. Kidney J. 2018 , 11 , i14–i26. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wicher, D.; Obrycki, Ł.; Jankowska, I. Autosomal Recessive Polycystic Kidney Disease—The Clinical Aspects and Diagnostic Challenges. J. Pediatr. Genet. 2020 , 10 , 1–8. [ Google Scholar ] [ CrossRef ]
- Ariceta, G.; Buj, M.J.; Furlano, M.; Martínez, V.; Matamala, A.; Morales, M.; Robles, N.R.; Sans, L.; Villacampa, F.; Torra, R. Recommendations for the management of renal involvement in the tuberous sclerosis complex. Nefrologia 2019 , 40 , 142–151. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Malaga-Dieguez, L.; Spencer, R.; Pehrson, L.J.; Vento, S.; Menzer, K.; Devinsky, O.; Trachtman, H. Early manifestations of renal disease in patients with tuberous sclerosis complex. Int. J. Nephrol. Renov. Dis. 2017 , 10 , 91–95. [ Google Scholar ] [ CrossRef ]
- Orosz, P.; Kollák, Z.; Pethő, Á.; Fogarasi, A.; Reusz, G.; Hadzsiev, K.; Szabó, T. The Importance of Genetic Testing in the Differential Diagnosis of Atypical TSC2-PKD1 Contiguous Gene Syndrome—Case Series. Children 2023 , 10 , 420. [ Google Scholar ] [ CrossRef ]
- Reyna-Fabián, M.E.; Alcántara-Ortigoza, M.A.; Hernández-Martínez, N.L.; Berumen, J.; Jiménez-García, R.; Gómez-Garza, G.; Angel, A.G.-D. TSC2/PKD1 contiguous gene syndrome, with emphasis on a case with an atypical mild polycystic kidney phenotype and a novel genetic variant. Nefrologia 2019 , 40 , 91–98. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cadnapaphornchai, M.A.; McFann, K.; Strain, J.D.; Masoumi, A.; Schrier, R.W. Prospective Change in Renal Volume and Function in Children with ADPKD. Clin. J. Am. Soc. Nephrol. 2009 , 4 , 820–829. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kilis-Pstrusinska, K. Urinary Tract Infections in Children with Chronic Kidney Disease. In Urogenital Infections and Inflammations ; Naber, K.G., Ed.; German Medical Science GMS Publishing House: Berlin, Germany, 2018. [ Google Scholar ] [ CrossRef ]
- Idrizi, A.; Barbullushi, M.; Koroshi, A.; Dibra, M.; Bolleku, E.; Bajrami, V.; Xhaferri, X.; Thereska, N. Urinary Tract Infections in polycystic Kidney Disease. Med. Arch. 2011 , 65 , 213–215. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sklar, A.H.; Caruana, R.J.; Lammers, J.E.; Strauser, G.D. Renal Infections in Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 1987 , 10 , 81–88. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Harris, T. Is It Ethical to Test Apparently “Healthy” Children for Autosomal Dominant Polycystic Kidney Disease and Risk Medicalizing Thousands. Front. Pediatr. 2018 , 5 , 291. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zahid, R.; Akram, M.; Rafique, E. Prevalence, risk factors and disease knowledge of polycystic kidney disease in Pakistan. Int. J. Immunopathol. Pharmacol. 2020 , 34 , 2058738420966083. [ Google Scholar ] [ CrossRef ]
- Chapman, A.B.; Devuyst, O.; Eckardt, K.-U.; Gansevoort, R.T.; Harris, T.; Horie, S.; Kasiske, B.L.; Odland, D.; Pei, Y.; Perrone, R.D.; et al. Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015 , 88 , 17–27. [ Google Scholar ] [ CrossRef ]
- Treger, T.D.; Chowdhury, T.; Pritchard-Jones, K.; Behjati, S. The genetic changes of Wilms tumour. Nat. Rev. Nephrol. 2019 , 15 , 240–251. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lustosa-Mendes, E.; dos Santos, A.P.; Vieira, T.P.; Ribeiro, E.M.; Rezende, A.A.; Fett-Conte, A.C.; Cavalcanti, D.P.; Félix, T.M.; Monlleó, I.L.; Gil-Da-Silva-Lopes, V.L. Identification of genomic imbalances in oral clefts. J. Pediatr. 2020 , 97 , 321–328. [ Google Scholar ] [ CrossRef ]
- Coppieters, F.; Lefever, S.; Leroy, B.P.; De Baere, E. CEP290, a gene with many faces: Mutation overview and presentation of CEP290base. Hum. Mutat. 2010 , 31 , 1097–1108. [ Google Scholar ] [ CrossRef ]
- Meyer, K.J.; Whitmore, S.S.; Burnight, E.R.; Riker, M.; Mercer, H.E.; Ajose, A. Genetic Modifiers of Cep290-Mediated Retinal Degeneration. bioRxiv 2022 . [ Google Scholar ] [ CrossRef ]
- Brancati, F.; Barrano, G.; Silhavy, J.L.; Marsh, S.E.; Travaglini, L.; Bielas, S.L.; Amorini, M.; Zablocka, D.; Kayserili, H.; Al-Gazali, L.; et al. CEP290 Mutations Are Frequently Identified in the Oculo-Renal Form of Joubert Syndrome–Related Disorders. Am. J. Hum. Genet. 2007 , 81 , 104–113. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stein, Q.; Westemeyer, M.; Darwish, T.; Pitman, T.; Hager, M.; Tabriziani, H.; Curry, K.; Collett, K.; Raible, D.; Hendricks, E. Genetic Counseling in Kidney Disease: A Perspective. Radiology 2023 , 5 , 100668. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vaisitti, T.; Bracciamà, V.; Faini, A.C.; Del Prever, G.M.B.; Callegari, M.; Kalantari, S.; Mioli, F.; Romeo, C.M.; Luca, M.; Camilla, R.; et al. The role of genetic testing in the diagnostic workflow of pediatric patients with kidney diseases: The experience of a single institution. Hum. Genom. 2023 , 17 , 10. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aron, A.W.; Dahl, N.K.; Besse, W. A Practical Guide to Genetic Testing for Kidney Disorders of Unknown Etiology. Kidney360 2022 , 3 , 1640–1651. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Snoek, R.; Stokman, M.F.; Lichtenbelt, K.D.; van Tilborg, T.C.; Simcox, C.E.; Paulussen, A.D.; Dreesen, J.C.; van Reekum, F.; Lely, A.T.; Knoers, N.V.; et al. Preimplantation Genetic Testing for Monogenic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020 , 15 , 1279–1286. [ Google Scholar ] [ CrossRef ]
- Murphy, E.L.; Droher, M.L.; DiMaio, M.S.; Dahl, N.K. Preimplantation Genetic Diagnosis Counseling in Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 2018 , 72 , 866–872. [ Google Scholar ] [ CrossRef ]
- Groopman, E.E.; Rasouly, H.M.; Gharavi, A.G. Genomic medicine for kidney disease. Nat. Rev. Nephrol. 2018 , 14 , 83–104. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chaperon, J.L.; Wemmer, N.M.; McKanna, T.A.; Clark, D.M.; Westemeyer, M.A.; Gauthier, P.; Bai, Y.; Coleman, J.M. Preimplantation Genetic Testing for Kidney Disease-Related Genes: A Laboratory’s Experience. Am. J. Nephrol. 2021 , 52 , 684–690. [ Google Scholar ] [ CrossRef ]
- Thompson, W.S.; Babayev, S.N.; McGowan, M.L.; Kattah, A.G.; Wick, M.J.; Bendel-Stenzel, E.M.; Chebib, F.T.; Harris, P.C.; Dahl, N.K.; Torres, V.E.; et al. State of the Science and Ethical Considerations for Preimplantation Genetic Testing for Monogenic Cystic Kidney Diseases and Ciliopathies. J. Am. Soc. Nephrol. 2023 , 35 , 235–248. [ Google Scholar ] [ CrossRef ]
- McEwan, P.; Wilton, H.B.; Ong, A.C.M.; Ørskov, B.; Sandford, R.; Scolari, F.; Cabrera, M.-C.V.; Walz, G.; O’reilly, K.; Robinson, P. A model to predict disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD): The ADPKD Outcomes Model. BMC Nephrol. 2018 , 19 , 37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gimpel, C.; Bergmann, C.; Mekahli, D. Correction to: The wind of change in the management of autosomal dominant polycystic kidney disease in childhood. Pediatr. Nephrol. 2021 , 37 , 687–688. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chebib, F.T.; Nowak, K.L.; Chonchol, M.B.; Bing, K.; Ghanem, A.; Rahbari-Oskoui, F.F.; Dahl, N.K.; Mrug, M. Polycystic Kidney Disease Diet. Clin. J. Am. Soc. Nephrol. 2023 . [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Messchendorp, A.L.; Meijer, E.; Boertien, W.E.; Engels, G.E.; Casteleijn, N.F.; Spithoven, E.M.; Losekoot, M.; Burgerhof, J.G.; Peters, D.J.; Gansevoort, R.T. Urinary Biomarkers to Identify Autosomal Dominant Polycystic Kidney Disease Patients with a High Likelihood of Disease Progression. Kidney Int. Rep. 2017 , 3 , 291–301. [ Google Scholar ] [ CrossRef ]
- Hosman, I.S.; Roić, A.C.; Prlić, M.F.; Brinar, I.V.; Lamot, L. Predicting autosomal dominant polycystic kidney disease progression: Review of promising Serum and urine biomarkers. Front. Pediatr. 2023 , 11 , 1274435. [ Google Scholar ] [ CrossRef ]
- Grantham, J.; Cook, L.; Torres, V.; Bost, J.; Chapman, A.; Harris, P.; Guay-Woodford, L.; Bae, K. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008 , 73 , 108–116. [ Google Scholar ] [ CrossRef ]
- Perrone, R.D.; Mouksassi, M.-S.; Romero, K.; Czerwiec, F.S.; Chapman, A.B.; Gitomer, B.Y.; Torres, V.E.; Miskulin, D.C.; Broadbent, S.; Marier, J.F. Total Kidney Volume Is a Prognostic Biomarker of Renal Function Decline and Progression to End-Stage Renal Disease in Patients with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. Rep. 2017 , 2 , 442–450. [ Google Scholar ] [ CrossRef ]
- Breysem, L.; De Keyzer, F.; Schellekens, P.; Dachy, A.; De Rechter, S.; Janssens, P.; Vennekens, R.; Bammens, B.; Irazabal, M.V.; Van Ongeval, C.; et al. Risk Severity Model for Pediatric Autosomal Dominant Polycystic Kidney Disease Using 3D Ultrasound Volumetry. Clin. J. Am. Soc. Nephrol. 2023 , 18 , 581–591. [ Google Scholar ] [ CrossRef ]
- Vidal, E.; Ray, P.E. Acute kidney injury during the first week of life: Time for an update? Pediatr. Nephrol. 2024 . [ Google Scholar ] [ CrossRef ]
- Schinzel, A.; Schmid, W.; Fraccaro, M.; Tiepolo, L.; Zuffardi, O.; Opitz, J.M.; Lindsten, J.; Zetterqvist, P.; Enell, H.; Baccichetti, C.; et al. The ‘Cat Eye Syndrome’: Dicentric Small Marker Chromosome Probably Derived from a No.22 (Tetrasomy 22pter to Q11) Associated with a Characteristic Phenotype. Report of 11 Patients and Delineation of the Clini-cal Picture. Hum. Genet. 1981 , 57 , 148–158. [ Google Scholar ] [ CrossRef ]
- Chen, T.-J.; Song, R.; Janssen, A.; Yosypiv, I.V. Cytogenomic aberrations in isolated multicystic dysplastic kidney in children. Pediatr. Res. 2021 , 91 , 659–664. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Izzi, C.; Dordoni, C.; Econimo, L.; Delbarba, E.; Grati, F.R.; Martin, E.; Mazza, C.; Savoldi, G.; Rampoldi, L.; Alberici, F.; et al. Variable Expressivity of HNF1B Nephropathy, from Renal Cysts and Diabetes to Medullary Sponge Kidney through Tubulo-interstitial Kidney Disease. Kidney Int. Rep. 2020 , 5 , 2341–2350. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Drube, J.; Wan, M.; Bonthuis, M.; Wühl, E.; Bacchetta, J.; Santos, F.; Grenda, R.; Edefonti, A.; Harambat, J.; Shroff, R.; et al. Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat. Rev. Nephrol. 2019 , 15 , 577–589. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Caba, L.; Florea, L.; Braha, E.E.; Lupu, V.V.; Gorduza, E.V. Monitoring and Management of Bardet-Biedl Syndrome: What the Multi-Disciplinary Team Can Do. J. Multidiscip. Health 2022 , 15 , 2153–2167. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stergiou, G.S.; Alamara, C.V.; Salgami, E.V.; Vaindirlis, I.N.; Dacou-Voutetakis, C.; Mountokalakis, T.D. Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press. Monit. 2005 , 10 , 143–147. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016 , 34 , 1887–1920. [ Google Scholar ] [ CrossRef ]
- Chapter 3: Management of progression and complications of CKD. Kidney Int. Suppl. 2013 , 3 , 73–90. [ CrossRef ]
- Guay-Woodford, L.M.; Bissler, J.J.; Braun, M.C.; Bockenhauer, D.; Cadnapaphornchai, M.A.; Dell, K.M.; Kerecuk, L.; Liebau, M.C.; Alonso-Peclet, M.H.; Shneider, B.; et al. Consensus Expert Recommendations for the Diagnosis and Management of Autosomal Recessive Polycystic Kidney Disease: Report of an International Conference. J. Pediatr. 2014 , 165 , 611–617. [ Google Scholar ] [ CrossRef ]
- Anarat, A.; Testa, S.; Jankauskiene, A.; Caldas-Afonso, A.; Niaudet, P.; Mir, S.; Bakkaloglu, A.; Enke, B.; Montini, G.; Wingen, A.M. The ESCAPE Trial Group Strict Blood-Pressure Control and Progression of Renal Failure in Children. N. Engl. J. Med. 2009 , 361 , 1639–1650. [ Google Scholar ] [ CrossRef ]
- Mattoo, T.K.; Shaikh, N.; Nelson, C.P. Contemporary Management of Urinary Tract Infection in Children. Pediatrics 2021 , 147 , e2020012138. [ Google Scholar ] [ CrossRef ]
- Psooy, K. Multicystic dysplastic kidney (MCDK) in the neonate: The role of the urologist. Can. Urol. Assoc. J. 2016 , 10 , 18–24. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jawa, N.A.; Rosenblum, N.D.; Radhakrishnan, S.; Pearl, R.J.; Levin, L.; Matsuda-Abedini, M. Reducing Unnecessary Imaging in Children with Multicystic Dysplastic Kidney or Solitary Kidney. Pediatrics 2021 , 148 , e2020035550. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Simpson, J.M.; Williams, G.J.; Lowe, A.; Reynolds, G.J.; McTaggart, S.J.; Hodson, E.M.; Carapetis, J.R.; Cranswick, N.E.; Smith, G.; Irwig, L.M.; et al. Antibiotic Prophylaxis and Recurrent Urinary Tract Infection in Children. N. Engl. J. Med. 2009 , 361 , 1748–1759. [ Google Scholar ] [ CrossRef ]
- Shaikh, N.; Hoberman, A.; Keren, R.; Gotman, N.; Docimo, S.G.; Mathews, R.; Bhatnagar, S.; Ivanova, A.; Mattoo, T.K.; Moxey-Mims, M.; et al. Recurrent Urinary Tract Infections in Children with Bladder and Bowel Dysfunction. Pediatrics 2016 , 137 , e20152982. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Diamond, D.A.; Mattoo, T.K. Endoscopic Treatment of Primary Vesicoureteral Reflux. N. Engl. J. Med. 2012 , 366 , 1218–1226. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bachmann-Gagescu, R.; Dempsey, J.C.; Bulgheroni, S.; Chen, M.L.; D’Arrigo, S.; Glass, I.A.; Heller, T.; Héon, E.; Hildebrandt, F.; Joshi, N.; et al. Healthcare recommendations for Joubert syndrome. Am. J. Med. Genet. Part A 2019 , 182 , 229–249. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pradilla, M.J.B.; Ballesté, T.M.; Torra, R.; Aubá, F.V. Recommendations for imaging-based diagnosis and management of renal angiomyolipoma associated with tuberous sclerosis complex. Clin. Kidney J. 2017 , 10 , 728–737. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dudley, J.; Winyard, P.; Marlais, M.; Cuthell, O.; Harris, T.; Chong, J.; Sayer, J.; Gale, D.P.; Moore, L.; Turner, K.; et al. Clinical practice guideline monitoring children and young people with, or at risk of developing autosomal dominant polycystic kidney disease (ADPKD). BMC Nephrol. 2019 , 20 , 148. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gimpel, C.; Bergmann, C.; Bockenhauer, D.; Breysem, L.; Cadnapaphornchai, M.A.; Cetiner, M.; Dudley, J.; Emma, F.; Konrad, M.; Harris, T.; et al. International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat. Rev. Nephrol. 2019 , 15 , 713–726. [ Google Scholar ] [ CrossRef ]
- Sweeney, W.E.; Avner, E.D. Polycystic Kidney Disease, Autosomal Recessive. In GeneReviews ® ; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1326/ (accessed on 21 February 2024).
- Forsythe, E.; Beales, P.L. Bardet–Biedl syndrome. Eur. J. Hum. Genet. 2012 , 21 , 8–13. [ Google Scholar ] [ CrossRef ]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020 , 76 , S1–S107. [ Google Scholar ] [ CrossRef ] [ PubMed ]
| Diagnosis | ADPKD | ARPKD | BBS | IRC | JS | MCDK | NPHC | T13 | TSC | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (N, %) | 21 18.8% | 10 8.9% | 2 1.8% | 15 13.4% | 2 1.8% | 56 50.0% | 2 1.8% | 1 0.9% | 3 2.7% | 112 100.0% |
| Lost to follow-up (N, %) | 11 52.4% | 2 20.0% | 0 | 8 53.3% | 0 | 23 41.1% | 1 50.0% | 0 | 0 | 45 40.2% |
| Transition to adult care (N, %) | 3 14.3% | 3 30.0% | 0 | 0 | 0 | 1 1.8% | 0 | 0 | 1 33.3% | 8 7.1% |
| Follow-up in years (median (IQR)) | 3.0 (1.5–5.0) | 4.0 (2.0–8.0) | - | 3.0 (1.0–6.5) | - | 3.0 (1.0–6.3) | - | - | - | 3.0 (1.0–7.0) |
| Years without medications or medical interventions (median (IQR)) | 3.0 (1.0–5.0) | 0.0 (0.0–1.8) | - | 2.0 (1.0–6.5) | - | 2.5 (1.0–6.3) | - | - | - | 2.0 (0.0–5.3) |
| Number of ambulant visits (median (IQR)) | 4.0 (2.0–8.5) | 7.5 (1.0–25.0) | - | 5.0 (2.0–8.5) | - | 6.0 (1.0–12.8) | - | - | - | 5.0 (2.0–14.0) |
| Number of hospitalizations (median (IQR)) | 0.0 (0.0–1.0) | 2.0 (1.0–5.0) | - | 0.0 (0.0–1.0) | - | 1.0 (1.0–2.0) | - | - | - | 1.0 (0.0–2.0) |
| , ]. | |
| Serial monitoring blood pressure (BP) at least once a year in patients with ADPKD (might be applicable for other CyKD, except IRC) [ ]. Prefer ambulatory BP monitoring [ ]. If hypertension has developed adhere to specific guidelines [ ]. | |
| Consider starting antihypertensive medications at a lower antihypertensive treatment threshold (ninetieth percentile for age, sex, and height, which equals 130/85 mmHg on clinic measurements for those ≥16 years of age) if CKD has developed [ ]. Prefer ACEi or ARB [ ]. Consider aggressive blood pressure control (24 h mean arterial blood pressure below the 50th percentile for age, height, and sex) [ ]. | |
| Perform urinalysis and cultures when UTI is suspected [ ]. Consider VCUG and DMSA kidney scans after the first febrile UTI, especially in patients with MCDK [ , ]. Consider antimicrobial prophylaxis, especially with BBD [ , ]. Consider surgical intervention with VUR [ ]. | |
| ADPKD: consider only if there a new clinical events (UTIs, hematuria, abdominal pain) [ ]. ARPKD: consider prenatally every 2–3 weeks for serial assessment of the renal size and amniotic fluid volume, confirm diagnosis postnatally, and perform abdominal ultrasound at the age of 5 years [ ]. BBS: consider yearly screening for renal tract malformations (e.g., dysplasia, agenesis, cysts, scarring) [ ]. IRC: consider at least one follow-up US to rule out the development of another CyKD [ ]. MCDK: consider serial measurements with defined intervals to ensure adequate compensatory hypertrophy of the contralateral kidney [ ]. JS: consider periodic evaluation during the abdominal ultrasounds performed to follow spleen size [ ]. TSC: consider US follow-up of renal lesions every 1–2 years [ ]. | |
| Consider urine ACR every 1–2 years in patients with ADPKD (might be applicable for other CyKD, except IRC) [ ]. Consider creatinine, BUN and cystatin C when available at least once a year in patients with BBS (might be applicable for other CyKD, except IRC) [ ]. If CKD has developed, adhere to specific recommendations [ ]. | |
| Consider dietary sodium (salt) restriction, increased fluid intake, no protein restriction, and maintaining normal weight in patients with ADPKD (might be applicable for other CyKD, except IRC) [ ]. To optimize weight gain and growth in patients with ARPKD provide aggressive nutritional intervention, including supplementary feedings [ ]. Consider weight management with exercise and diet in patients with BBS [ , ]. If CKD has developed, adhere to specific recommendations [ ]. |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Grlić, S.; Gregurović, V.; Martinić, M.; Davidović, M.; Kos, I.; Galić, S.; Fištrek Prlić, M.; Vuković Brinar, I.; Vrljičak, K.; Lamot, L. Single-Center Experience of Pediatric Cystic Kidney Disease and Literature Review. Children 2024 , 11 , 392. https://doi.org/10.3390/children11040392
Grlić S, Gregurović V, Martinić M, Davidović M, Kos I, Galić S, Fištrek Prlić M, Vuković Brinar I, Vrljičak K, Lamot L. Single-Center Experience of Pediatric Cystic Kidney Disease and Literature Review. Children . 2024; 11(4):392. https://doi.org/10.3390/children11040392
Grlić, Sara, Viktorija Gregurović, Mislav Martinić, Maša Davidović, Ivanka Kos, Slobodan Galić, Margareta Fištrek Prlić, Ivana Vuković Brinar, Kristina Vrljičak, and Lovro Lamot. 2024. "Single-Center Experience of Pediatric Cystic Kidney Disease and Literature Review" Children 11, no. 4: 392. https://doi.org/10.3390/children11040392
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
You are here
Polycystic kidney disease, table of contents, about polycystic kidney disease, signs and symptoms, complications, preparing for your appointment.
Polycystic kidney disease (also called PKD) causes numerous cysts to grow in the kidneys. These cysts are filled with fluid. If too many cysts grow or if they get too big, the kidneys can become damaged. PKD cysts can slowly replace much of the kidneys, reducing kidney function and leading to kidney failure.
In the United States about 600,000 people have PKD, which is the fourth leading cause of kidney failure. Men and women are equally at risk for the disease. It causes about 5% of all kidney failure.
Most people do not develop symptoms until they are 30 to 40 years old. About 25% of PKD patients have a so-called floppy valve in the heart, and may experience a fluttering or pounding in the chest as well as chest pain. These symptoms almost always disappear on their own but may be the first hint that someone has PKD.
High blood pressure is the most common sign of PKD. Occasionally, patients may develop headaches related to high blood pressure or their doctors may detect high blood pressure during a routine physical exam. Because high blood pressure can cause kidney damage, it is very important to treat it. In fact, treatment of high blood pressure can help slow or even prevent kidney failure.
- Back or side pain
- An increase in the size of the abdomen
- Blood in the urine
- Frequent bladder or kidney infections
- High blood pressure
- Fluttering or pounding in the chest
PKD runs in families. It is an inherited disorder that is passed from parents to children through genes. Genes are the basic elements of heredity. At conception, children receive a set of genes from each parent. They determine many characteristics such as hair color and eye color. Genes can also determine the likelihood of developing a disease.
A genetic disease can happen if one or both parents pass abnormal genes to a child. This happens through something called dominant inheritance or recessive inheritance.
Dominant inheritance If one parent has the disease and passes an abnormal gene to the child, it is called dominant inheritance. Each child has a 50% chance of getting the disease. The risk is the same for every child, regardless of how many children develop the disease.
Recessive inheritance If both parents carry the abnormal gene, and both parents pass an abnormal gene to the child, it is called recessive inheritance. In this situation, every child has a 25% chance of getting the disease.
Autosomal dominant PKD
Also called PKD or ADPKD
This form of the disease is passed from parent to child by dominant inheritance. In other words, only one copy of the abnormal gene is needed to cause the disease. Symptoms usually begin between the ages of 30 and 40, but they can begin earlier, even in childhood. ADPKD is the most common form of PKD. In fact, about 90% of all PKD cases are ADPKD.
Infantile or autosomal recessive PKD
Also called ARPKD
This form of the disease is passed from parent to child by recessive inheritance. Symptoms can begin in the earliest months of life, even in the womb. It tends to be very serious, progresses rapidly, and is often fatal in the first few months of life. This form of ARPKD is extremely rare. It occurs in 1 out of 25,000 people.
Acquired cystic kidney disease
Also called ACKD
ACKD can happen in kidneys with long-term damage and severe scarring, so it is often associated with kidney failure and dialysis. About 90 percent of people on dialysis for 5 years develop ACKD. People with ACKD usually seek help because they notice blood in their urine. This is because the cysts bleed into the urinary system, which discolors urine.
Individuals with PKD who are concerned about passing the disease to their children may want to consult a genetics counselor to help them with family planning. Many university medical centers have this service.
Most women with PKD (80%) have successful and uneventful pregnancies. However, some women with PKD have an increased risk for serious complications for themselves and their babies, especially for those who also have:
- Decreased kidney function
About 40% of pregnant women with PKD, who also have high blood pressure, develop a condition called pre-eclampsia (or toxemia). This is a life-threatening disorder for both the mother and baby, and can develop suddenly and without warning. Due to this increased risk, women with PKD, particularly those who also have high blood pressure, should be followed closely during their pregnancy by their doctor.
PKD can affect other organs besides the kidney. People with PKD may have cysts in their liver, pancreas, spleen, ovaries, and large bowel. Cysts in these organs usually do not cause serious problems, but can in some people. PKD can also affect the brain or heart. If PKD affects the brain, it can cause an aneurysm. An aneurysm is a bulging blood vessel that can burst, resulting in a stroke or even death. If PKD affects the heart, the valves can become floppy, resulting in a heart murmur in some patients.
About 50% of people with PKD will have kidney failure by age 60, and about 60% will have kidney failure by age 70. People with kidney failure will need dialysis or a kidney transplant. Certain people have an increased risk of kidney failure including:
- Patients with high blood pressure
- Patients with protein or blood in their urine
- Women with high blood pressure who have had 3 or more pregnancies
Looking for more info about PKD?
Join our kidney community for valuable tips and resources to support your PKD journey.
Ultrasound is the most reliable, inexpensive and non-invasive way to diagnose PKD. If someone at risk for PKD is older than 40 years and has a normal ultrasound of the kidneys, they probably do not have PKD. Occasionally, a CT scan (computed tomography scan) and MRI (magnetic resonance imaging) may detect smaller cysts that cannot be found by an ultrasound. MRI is used to measure and monitor the volume and growth of kidneys and cysts.
In some situations, genetic testing might also be done. This involves a blood test that checks for abnormal genes that cause the disease. Genetic testing is not recommended for everyone. The test is costly, and it also fails to detect PKD in about 15% of people who have it. However, genetic testing can be useful when a person:
- has an uncertain diagnosis based on imaging tests
- has a family history of PKD and wants to donate a kidney
- is younger than 30-years old with a family history of PKD and a negative ultrasound, and is planning to start a family
At present, there is no cure for PKD. However, a lot of research is being done. Recent studies suggest that drinking plain water throughout the day and avoiding caffeine in beverages can slow the growth of cysts. Research is also helping us understand the genetic basis of PKD.
Studies also suggest that some treatments may slow the rate of kidney disease in PKD, but further research is needed before these treatments can be used in patients. In the meantime, many supportive treatments can be done to control symptoms, help slow the growth of cysts, and prevent or slow down the loss of kidney function in people with PKD. These include:
- Careful control of blood pressure
- Prompt treatment with antibiotics for bladder or kidney infection
- Drinking lots of fluid when blood in the urine is first noted
- Medication to control pain (talk to your doctor about which over-the-counter medicines are safe to take if you have kidney disease)
- Healthy lifestyle with regard to quitting smoking, exercise, weight control and reduced salt intake
- Drinking lots of plain water throughout the day
- Avoiding caffeine in all beverages
In April 2018, the FDA approved a new drug called tolvaptan for the treatment of autosomal dominant polycystic kidney disease (ADPKD). The drug can be used to help slow kidney function decline in adults at risk for this type of PKD. You can speak with a healthcare professional for more information about this treatment and to see if it’s right for you.
At present, no specific diet is known to prevent cysts from developing in patients with PKD. Reducing salt intake helps control blood pressure in patients with PKD who have high blood pressure. A diet low in fat and moderate in calories is recommended to maintain a healthy weight. Speak to your doctor or a dietitian about other changes to your diet, such as avoiding caffeine.
Physical exercise is recommended for people with PKD, however exercises that are potentially harmful to the kidney, such as contact sports, should be avoided. It is important not to become too dehydrated during any physical activity.
In addition to your primary care doctor, you should also find a nephrologist who has experience treating patients with PKD. You and your healthcare professional can work together to choose treatment options that are best for you.

Questions to ask
You and your healthcare professional will need to work together to make treatment choices that are best for you. Here are some questions to help you begin a discussion. It is helpful to write your questions down before your appointments, and bring the list with you. Doing so will help you make the best use of your time together.
- Do I have PKD?
- What type of PKD do I have?
- Does my PKD affect any other organs?
- Can my PKD be cured?
- What are my treatment options?
- How long will treatment last?
- Are there any risks or side effects associated with my treatment?
- Should I also see a nephrologist (kidney doctor)? Will you be partnering with a nephrologist about my care?
- How much experience do you have treating PKD?
- Are there any clinical trials I should think about?
National Kidney and Urologic Disease Information Clearinghouse (NKUDIC) , a service of the National Institute of Diabetes, Digestive and Kidney Diseases. 800.891.5390
Polycystic Kidney Disease Foundation 8330 Ward Parkway, Suite 510 Kansas City, MO 64114 (800) PKD-CURE
This content is provided for informational use only and is not intended as medical advice or as a substitute for the medical advice of a healthcare professional.
Save this content:
Share this content:
Is this content helpful?
Back to top:
- Study protocol
- Open access
- Published: 06 January 2021
Genetic identification of inherited cystic kidney diseases for implementing precision medicine: a study protocol for a 3-year prospective multicenter cohort study
- Hayne Cho Park 1 ,
- Hyunjin Ryu 2 ,
- Yong-Chul Kim 2 ,
- Curie Ahn 3 ,
- Kyu-Beck Lee 4 ,
- Yeong Hoon Kim 5 ,
- Yunmi Kim 5 ,
- Seungyeup Han 6 ,
- Yaerim Kim 6 ,
- Eun hui Bae 7 ,
- Seong Kwon Ma 7 ,
- Hee Gyung Kang 8 ,
- Yo Han Ahn 8 ,
- Eujin Park 9 ,
- Kyungjo Jeong 10 ,
- Jaewon Lee 10 ,
- Jungmin Choi 10 ,
- Kook-Hwan Oh 2 &
- Yun Kyu Oh ORCID: orcid.org/0000-0001-8632-5743 2 , 11
BMC Nephrology volume 22 , Article number: 2 ( 2021 ) Cite this article
4143 Accesses
7 Citations
1 Altmetric
Metrics details
Inherited cystic kidney disease is a spectrum of disorders in which clusters of renal cysts develop as the result of genetic mutation. The exact methods and pipelines for defining genetic mutations of inherited cystic kidney disease are not clear at this point. This 3-year, prospective, multicenter, cohort study was designed to set up a cohort of Korean patients with inherited cystic kidney disease, establish a customized genetic analysis pipeline for each disease subtype, and identify modifying genes associated with the severity of the disease phenotype.
Methods/design
From May 2020 to May 2022, we aim to recruit 800 patients and their family members to identify pathogenic mutations. Patients with more than 3 renal cysts in both kidneys are eligible to be enrolled. Cases of simple renal cysts and acquired cystic kidney disease that involve cyst formation as the result of renal failure will be excluded from this study. Demographic, laboratory, and imaging data as well as family pedigree will be collected at baseline. Renal function and changes in total kidney volume will be monitored during the follow-up period. Genetic identification of each case of inherited cystic kidney disease will be performed using a targeted gene panel of cystogenesis-related genes, whole exome sequencing (WES) and/or family segregation studies. Genotype-phenotype correlation analysis will be performed to elucidate the genetic effect on the severity of the disease phenotype.
This is the first nationwide cohort study on patients with inherited cystic kidney disease in Korea. We will build a multicenter cohort to describe the clinical characteristics of Korean patients with inherited cystic kidney disease, elucidate the genotype of each disease, and demonstrate the genetic effects on the severity of the disease phenotype.
Trial registration
This cohort study was retrospectively registered at the Clinical Research Information Service ( KCT0005580 ) operated by the Korean Center for Disease Control and Prevention on November 5th, 2020.
Inherited cystic kidney disease (iCKD) is a hereditary disorder in which clusters of cysts develop within the kidneys [ 1 , 2 ]. Approximately 100 genes involved in renal cystogenesis are known to result in dysfunction of a hair-like organelle called the cilium [ 3 ]. Therefore, iCKD is otherwise called ciliopathy [ 4 , 5 ]. iCKD encompasses autosomal-dominant polycystic kidney disease (ADPKD), tuberous sclerosis complex [ 6 ], von Hippel-Lindau disease, autosomal-dominant tubulointerstitial kidney disease (ADTKD) [ 7 ], and pediatric diseases such as autosomal recessive polycystic kidney disease (ARPKD) [ 8 ] and nephronophthisis (NPHP) [ 9 ]. There are still many other disorders in which causative mutations have not been found by molecular diagnosis.
Although iCKDs are caused by genetic derangement, most are diagnosed by clinical impressions other than molecular diagnosis. However, clinical diagnosis is not always easy because iCKDs often share common clinical manifestations. Therefore, genetic testing is important to establish a correct diagnosis and treatment. A recent report by Bullich et al. demonstrated that they could confirm diagnosis in 32% of cases with unspecified clinical diagnosis by establishing a kidney gene panel [ 10 ]. They also showed that genetic testing changed the clinical diagnosis in 2% of cases. Therefore, genetic testing should be the most important venue to confirm diagnosis and establish precision medicine.
Moreover, the exact methods and pipelines to find genetic mutations in iCKDs are not clear at this point. It may be reasonable to perform targeted exome sequencing or Sanger sequencing of PKD1 and PKD2 to define pathogenic mutations in well-known clinical phenotypes such as ADPKD. However, our previous study demonstrated that approximately 20% of patients with typical ADPKD did not reveal causative germline mutations by targeted exome sequencing of PKD1 and PKD2 [ 11 ]. In addition, extrarenal manifestations often do not follow renal manifestations. For example, the severity of polycystic liver accompanying ADPKD does not always correlate with the severity of renal disease [ 12 ]. Therefore, modifying genetic effects or gene dosage effects may play a role in determining the severity of renal and extrarenal phenotypes in ADPKD [ 13 ]. In addition, apart from typical ADPKD, there are patients with atypical polycystic kidney disease who either do not show concordant features within the family, do not have typical imaging features of ADPKD, or have discordant disease severity between renal volume and renal function [ 14 ]. Mutations in GANAB and DNAJB11 are known to cause a mild phenotype of polycystic kidney and liver disease [ 15 , 16 ]. However, the exact prevalence and prognosis of atypical polycystic kidney disease is unknown at this point. Last, iCKDs in the pediatric population are typically rare diseases, and their molecular diagnoses are even more difficult. Therefore, building a cohort of iCKDs is necessary to reveal their genetic characteristics.
Therefore, we designed a 3-year prospective, multicenter, cohort study to establish a cohort of Korean iCKD patients, establish a customized genetic analysis pipeline that can genotype each iCKD and identify the modifying genes associated with the severity of the disease phenotype.
Study design and settings
This is a 3-year prospective, multicenter, cohort study to elucidate genotype-phenotype associations among iCKD patients. A total of 11 medical centers from 9 tertiary hospitals in Korea will participate in this study. Seven centers will enroll and collect data from adult patients, and 4 centers will enroll pediatric patients. We established a research team, statistical analysis team, database team, sequencing and biobanking team, and genetic analysis team to perform this large nationwide project. The research team is composed of 26 clinicians and 15 clinical research coordinators from 11 medical centers. The role of the research team is to recruit eligible patients and collect clinical data. The statistical analysis team supports the calculation of sample size, distribution of enrollment according to iCKD subclasses, and statistical analysis. The database team collects clinical and genetic data from each patient and builds an electronic case report form to store and manage the dataset. The database team also performs imaging analysis quantitatively and qualitatively from nonenhanced computed tomography (CT) or sonography. The team for sequencing and biobanking is outsourced to Macrogen, Inc. to collect whole blood from each medical center and perform initial genetic analysis to produce sequencing data. The residual DNA samples will be prepared for biobanking after quantity and quality checks. Finally, the genetic analysis team is composed of bioinformaticians to interpret the results of sequencing data and to determine the pathogenicity of each variant.
Study participants
A total of 800 participants are planned to be enrolled from May 19, 2019 to May 18, 2022. Patients with ≥3 renal cysts in both kidneys are eligible to be enrolled. Those who are not able to give informed consent or are pregnant will be excluded from enrollment. Cases of simple renal cysts and acquired cystic kidney disease that involve cyst formation as the result of renal failure will also be excluded from this study. However, patients with end-stage kidney disease who are receiving renal replacement therapy due to iCKD can be enrolled.
The patients will be classified into typical ADPKD, atypical polycystic kidney disease, and other iCKDs after enrollment. Typical ADPKD is defined according to Pei-Ravine criteria as previously described [ 17 ]. Atypical polycystic kidney disease is defined either when the case is typical ADPKD but the patient does not have a family history of polycystic kidney disease or when the imaging phenotype is atypical as follows: unilateral, asymmetric, segmental, lopsided, bilateral or unilateral atrophic kidneys [ 18 ]. Other iCKDs are rare disease entities in children and adolescents. The other iCKDs include but are not limited to the tuberous sclerosis complex, von Hippel-Lindau disease, ADTKD, ARPKD, HNF-1β -related disease and NPHP. Among the 800 participants, approximately 650 patients with typical ADPKD, 90 patients with atypical polycystic kidney disease, and 60 patients with other iCKDs will be enrolled.
The parents, siblings, or children of the enrolled patients are recommended to participate in the study by giving whole blood samples for a segregation study. We will collect family samples when genetic diagnosis is undetermined, genotype-phenotype severity is not matched, and when the extrarenal manifestation is severe. We will also collect family samples from the families with more than 3 affected individuals to define gene penetrance and modify gene effects.
Data collection at enrollment
Demographic data, including age, sex, height and weight, will be collected. The age of diagnosis of iCKD and associated symptoms at initial diagnosis will be collected. Medical history of diabetes, hypertension, cardiovascular disease, and stroke will be investigated. Family history of iCKD, diabetes, hypertension, chronic kidney disease, dialysis, and death will be evaluated. In particular, a genetic tree will be drawn upon enrollment including 3 generations (affected and unaffected individuals). The presence of renal and extrarenal complications and their types will be recorded. Medication data, including on antihypertensive drugs and glucose-lowering therapy, will be collected. Blood pressure will be checked upon enrollment in the office. All patients will be asked to fill out the following questionnaires upon enrollment: 5 Level version of European Quality of Life 5 Dimensions questionnaire (EQ-5D-5L, adult subjects) and Pediatric Quality of Life Inventory TM (PedsQL 4.0 Generic Core Scales, pediatric subjects) to assess the quality of life of the affected patients, Patient Health Questionnaire-9 (PHQ-9) to evaluate depressive symptoms, and the modified Subjective Global Assessment (mSGA) to assess the nutritional status of the subjects.
Laboratory assessment included complete blood cell counts (white blood cells, hemoglobin, platelets), blood urea nitrogen and serum creatinine, total calcium and phosphorus, serum sodium, potassium, chloride, total carbon dioxide, total cholesterol, serum albumin, uric acid, highly sensitive C-reactive protein, urinalysis with microscopy, spot urine protein to creatinine ratio, random urine uric acid, calcium, phosphorus, sodium, potassium, chloride, and osmolality. Genetic samples will be collected once during the study period for genetic analysis. Approximately 18 mL of whole blood will be collected in 3 EDTA bottles for each adult participant, 6 mL for each family member and 4–5 mL for each child participant. The collected blood samples will be refrigerated at 4 °C until delivery to the sequencing company. The sequencing company will extract DNA from the whole blood and aliquots in several tubes to store at − 70 °C before sequencing or biobanking.
Kidney imaging will be performed at enrollment. If the patients have already undergone imaging studies within 1 year, the patients can undergo other imaging studies within a 2-year interval. Adult patients will undergo a nonenhanced kidney CT, and children and adolescents will undergo kidney sonography.
Data collection, monitoring, and follow up
The total study scheme and annual assessment plan are depicted in Table 1 . Annual laboratory assessment will be performed after enrollment for 2 years. The laboratory assessment includes the complete blood cell counts, blood urea nitrogen and serum creatinine, serum calcium and phosphorus, serum uric acid, urinalysis with microscopy, and spot urine protein to creatinine ratio. Kidney imaging will be performed every 2 years to calculate the rate of total kidney volume growth.
Electronic case report forms will be developed, including demographic sheets, laboratory assessments, volumetry, and genetic analysis information. The electronic case report form will be opened to the participating researchers and clinical research coordinators to fill out and modify patient information. A family tree will be drawn and stored in electronic case report form by scanning the sheet.
Evaluation of renal function
Renal function will be evaluated upon enrollment and every year thereafter. For the adult patients, renal function will be measured using the estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [ 19 ]. If the patients are in end-stage renal disease or receive renal replacement therapy upon enrollment, renal function will be evaluated retrospectively to calculate the renal function decline rate. For children, renal function will be measured using the estimated glomerular filtration rate calculated by the Schwartz equation [ 20 ].
Imaging analysis
The patients will be classified into typical and atypical cystic kidney disease. Nonenhanced kidney CT will be performed in adult patients. The patients will be encouraged to take water before the CT exams to accurately distinguish the liver and stomach anatomically. All image files from the nonenhanced CT will be retrieved to a workstation and inspected to confirm complete coverage of both kidneys and liver. Images will be reconstructed into 5 mm sections in axial images and 3 mm sections in both coronal and sagittal sections before volume measurements. The total kidney volume will be measured by a professionally educated radiologist. Total kidney volume will be measured by 2 methods: the stereologic method by using semiautomatic volumetry software (ImageJ version 1.5a, https://imagej.nih.gov/ij/ ) [ 21 ] and the manual method by using the Mayo ellipsoid method [ 18 , 22 ]. Expanded imaging classification will be applied to typical and atypical polycystic kidney cases [ 23 ]. The sonographic images and their interpretations will be collected from pediatric patients. The number and distribution of cysts and their characteristics will be reported in our case report form. The radiologist will also measure the muscle area to assess the nutritional status as previously reported [ 24 ].
Genetic pipeline
We will use a stepwise approach to confirm genetic diagnosis. First, we will use a targeted gene panel for the screening method. We designed a targeted gene panel (Twist Bioscience, San Francisco, CA, USA) encompassing 0.5 megabases, including 89 genes related to cystogenesis or ciliopathy as well as genes that are associated with extrarenal phenotypes such as liver cysts (Table 2 ). Twist technology has provided high-quality target enrichment probes to cover target genes uniformly and efficiently [ 25 ]. Targeted exon capture will be performed on genomic DNA samples using a Twist custom panel kit followed by 101 base paired-end sequencing on an Illumina NovaSeq6000 platform (Illumina, San Diego, CA, USA). Sequence reads will be aligned to the human reference genome (GRCh37/hg19) using BWA-MEM and further processed to call single nucleotide variants and indels following the GATK Best Practices workflow [ 26 ]. All variants covered by independent sequence reads with a depth of 8x or greater will be annotated with ANNOVAR. All variants will be visualized in silico to eliminate false positives. Additional genetic testing will be performed if the pathogenic mutations were not found using a targeted gene panel or if the patients have severe renal or extrarenal phenotypes compared to other family members. If the patients are clinically classified as having typical ADPKD but pathogenic mutations are not found using a targeted gene panel, the patients will undergo targeted resequencing with long-range polymerase chain reaction (PCR) combined with multiplex ligation-dependent probe amplification (MLPA) to detect large deletions. WES will take place in the following cases: 1) if the patients are clinically classified as typical ADPKD but no pathogenic mutations are found using a previous method, 2) if the patients are clinically classified as atypical polycystic kidney disease or other iCKD but pathogenic mutations are not found using a targeted gene panel, 3) if the patients present with a severe phenotype and variants cannot explain the severity, or 4) if the patients show extremely different phenotypes compared to other family members. Familial segregation analysis will also take place to define the pathogenicity of variants. A schematic representation of the genetic workflow is shown in Fig. 1 .
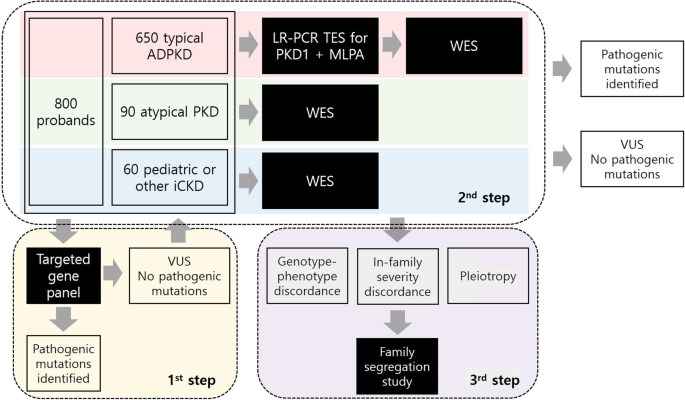
Genetic analysis pipeline. A total of 800 probands with iCKD will be enrolled in the study. They will be classified into typical ADPKD, atypical polycystic kidney disease, or other/pediatric iCKDs. For the first screening genetic test, a targeted gene panel of 89 cytogenesis-related genes will be applied to the total population. All the variants will be analyzed by bioinformaticians to identify pathogenic mutations. For those with variants of undetermined significance (VUS) or no variants found by the gene panel, different genetic approaches will be taken for each class of iCKD. For those with typical ADPKD, targeted exome sequencing of PKD1 after long-range PCR combined with MLPA will be performed to identify pathogenic mutations. If the mutations are not found by this method, WES will take place. For those with atypical polycystic kidney disease or pediatric iCKD, WES will be performed to identify pathogenic mutations. For the last step of genetic diagnosis, a family segregation study will be performed to elucidate the cause of genotype-phenotype discordance, in-family severity discordance, or discordance between renal and extrarenal manifestations. Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; iCKD, inherited cystic kidney disease; PCR, polymerase chain reaction; MLPA, multiplex ligation-dependent probe amplification; PKD, polycystic kidney disease; VUS, variant of undetermined significance; WES, whole exome sequencing
Genotype-phenotype correlation analysis
Statistical analyses will be performed using a recent version of SPSS software (IBM Corp., Armonk, NY, USA). A linear regression model will be performed to identify the correlation between genotype and clinical parameters, including total kidney volume and estimated glomerular filtration rate. The cases will be classified into 5 classes using the Mayo imaging classification before analysis. The cases will also be classified into 6 groups according to the chronic kidney disease stages. Analysis of covariance, the Mann-Whitney test, and the chi-square test will be performed to compare variables between groups. The modifier effect of multiple genes on renal and extrarenal manifestations will also be assessed. A P value < 0.05 will be considered statistically significant.
This study is the first prospective, multicenter cohort study that will evaluate the genetic profiles and their clinical correlation among patients with iCKDs in Korea. There are some international cohorts to define the genetic characteristics of various cystic kidney diseases and their association with phenotypes. The Network for Early Onset Cystic Kidney Disease (NEOCYST) is a government-funded multicenter network that collects clinical and genetic data to understand the underlying pathogenesis of hereditary cystic kidney disease [ 27 ]. The Consortium for Radiologic Imaging Study of Polycystic kidney disease (CRISP) group prospectively collected clinical, radiological, and genetic data to perform genotype-phenotype studies [ 28 , 29 ]. The Toronto Genetic Epidemiology Study of Polycystic kidney disease (TGESP) also examined the prevalence of different mutation classes and their association with phenotypes [ 30 ]. In Korea, there have only been single-center driven cohort studies for specific diseases. However, there has not been a nationwide multicenter iCKD network to collect epidemiologic, clinical, radiological, and genetic data prospectively. This multicenter iCKD cohort will establish a concrete database and biobank of the Korean iCKD population from which genotype-phenotype association studies can be performed.
Since the clinical diagnosis of iCKD is not always easy, genetic characterization will help to confirm the diagnosis of each iCKD case and to elucidate the heterogeneity of disease manifestations within the family. Since there are over 100 genes that can result in ciliopathies, Sanger sequencing or targeted exome sequencing of a few genes can be time-consuming and costly. The targeted gene panel approach through parallel sequencing of targeted subsets of disease-specific genes may be an effective screening method for iCKD cases. Recent papers have also reported the effectiveness of gene panels and subsequent WES approaches in confirming genetic diagnosis [ 10 , 31 , 32 ]. Therefore, we designed a targeted gene panel for the initial screening method to find causal variants. The gene panel can be designed and customized for research purposes. We included 13 genes associated with Joubert syndrome, 27 genes associated with polycystic liver, 8 genes associated with ADPKD, 1 gene associated with ARPKD, 11 genes associated with NPHP, 3 genes associated with Alport syndrome, 2 genes associated with ADTKD, 2 genes associated with tuberous sclerosis complex and 19 other ciliopathy-related genes in our targeted gene panel. The composition of the gene panel will help us not only identify causal variants for renal cystic disease but also explain the heterogeneity of extrarenal manifestations in the same disease.
Patient recruitment from secondary and tertiary hospitals across the country will represent the Korean cohort of iCKDs. The sample size of 800 should provide sufficient statistical power to address the heterogeneity of typical ADPKD, atypical polycystic kidney disease, and pediatric iCKDs. In particular, the establishment of a pediatric iCKD subcohort and atypical polycystic kidney disease cohort can be helpful in defining pathogenic mutations in each group because they are so rare, and genetic diagnosis of each case can be difficult without building a multicenter cohort. The five well-organized study teams (research team, statistical analysis team, database team, sequencing and biobanking team, and genetic analysis team) of this study will facilitate the study process. Various other factors, such as central electronic case report forms, researcher meetings, study nurse meetings, comprehensive study analyses and regular monitoring, will keep the quality of this study as high as possible.
Potential limitations include the observational nature of the study and short duration of follow-up. Although we will recruit approximately 15% of the total iCKD population in Korea, we cannot exclude potential selection bias since most of the patients will be recruited through secondary and tertiary hospitals.
In summary, we will establish a prospective genetic cohort of iCKDs in Korea with 800 pedigrees in which we collect demographic and clinical data as well as family tree and laboratory follow-up data. We will establish a genetic pipeline in typical ADPKD, atypical polycystic kidney disease, and pediatric iCKD cohorts and analyze genotype-phenotype correlations in renal and extrarenal manifestations. This study will help us implement precision medicine for Korean iCKD patients.
Availability of data and materials
Not applicable.
Abbreviations
Autosomal dominant polycystic kidney disease
Autosomal dominant tubulointerstitial kidney disease
Autosomal recessive polycystic kidney disease
Chronic Kidney Disease Epidemiology Collaboration
Consortium for Radiologic Imaging Study of Polycystic Kidney Disease
Computed tomography
5 Level version of European Quality of life 5 Dimensions questionnaire
Endoplasmic reticulum
Inherited cystic kidney disease
Meckel syndrome
Multiplex ligation-dependent probe amplification
Modified Subjective Global Assessment
Network for Early Onset Cystic Kidney Disease
Nephronophthisis
Orofaciodigital syndrome
Polymerase chain reaction
Pediatric Quality of Life inventory
Patient Health Questionnaire-9
Toronto Genetic Epidemiology Study of Polycystic kidney disease
Variant of undetermined significance
Whole exome sequencing
Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol. 2016;12(3):133–46.
Article CAS PubMed PubMed Central Google Scholar
Dillman JR, Trout AT, Smith EA, Towbin AJ. Hereditary renal cystic disorders: imaging of the kidneys and beyond. Radiographics. 2017;37(3):924–46.
Article PubMed Google Scholar
Mitchison HM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol. 2017;241(2):294–309.
Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364(16):1533–43.
Braun DA, Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol. 2017;9(3):a028191.
Article CAS PubMed Google Scholar
Bissler JJ, Christopher Kingswood J. Renal manifestation of tuberous sclerosis complex. Am J Med Genet C: Semin Med Genet. 2018;178(3):338–47.
Article Google Scholar
Zaucke F, Boehnlein JM, Steffens S, Polishchuk RS, Rampoldi L, Fischer A, Pasch A, Boehm CW, Baasner A, Attanasio M, et al. Uromodulin is expressed in renal primary cilia and UMOD mutations result in decreased ciliary uromodulin expression. Hum Mol Genet. 2010;19(10):1985–97.
Bergmann C. Early and severe polycystic kidney disease and related Ciliopathies: an emerging field of interest. Nephron. 2019;141(1):50–60.
Srivastava S, Molinari E, Raman S, Sayer JA. Many genes-one disease? Genetics of Nephronophthisis (NPHP) and NPHP-associated disorders. Front Pediatr. 2017;5:287.
Bullich G, Domingo-Gallego A, Vargas I, Ruiz P, Lorente-Grandoso L, Furlano M, Fraga G, Madrid A, Ariceta G, Borregan M, et al. A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int. 2018;94(2):363–71.
Kim H, Park HC, Ryu H, Kim H, Lee HS, Heo J, Lee C, Kim NKD, Park WY, Hwang YH, et al. Genetic characteristics of Korean patients with autosomal dominant polycystic kidney disease by targeted exome sequencing. Sci Rep. 2019;9(1):16952.
Article PubMed PubMed Central CAS Google Scholar
Chebib FT, Jung Y, Heyer CM, Irazabal MV, Hogan MC, Harris PC, Torres VE, El-Zoghby ZM. Effect of genotype on the severity and volume progression of polycystic liver disease in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;31(6):952–60.
Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van't Hoff WG, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75(8):848–55.
Lanktree MB, Haghighi A, di Bari I, Song X, Pei Y. Insights into autosomal dominant polycystic kidney disease from genetic studies. Clin J Am Soc Nephrol. 2020; 20:CJN.02320220. https://doi.org/10.2215/CJN.02320220 . Online ahead of print.
Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, et al. Mutations in GANAB, encoding the Glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98(6):1193–207.
Cornec-Le Gall E, Olson RJ, Besse W, Heyer CM, Gainullin VG, Smith JM, Audrezet MP, Hopp K, Porath B, Shi B, et al. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet. 2018;102(5):832–44.
Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–12.
Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–72.
Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–83.
Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4(11):1832–43.
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(1):529.
Article PubMed PubMed Central Google Scholar
Bevilacqua MU, Hague CJ, Romann A, Sheitt H, Vasilescu DM, Yi TW, Levin A. CT of kidney volume in autosomal dominant polycystic kidney disease: accuracy, reproducibility, and radiation dose. Radiology. 2019;291(3):660–7.
Bae KT, Shi T, Tao C, Yu ASL, Torres VE, Perrone RD, Chapman AB, Brosnahan G, Steinman TI, Braun WE, et al. Expanded imaging classification of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2020;31(7):1640–51.
Gomez-Perez SL, Haus JM, Sheean P, Patel B, Mar W, Chaudhry V, McKeever L, Braunschweig C. Measuring abdominal circumference and skeletal muscle from a single cross-sectional computed tomography image: a step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr. 2016;40(3):308–18.
Leproust E. New double-stranded twist on NGS adds more depth to each run. In: Genetic Engineering & Biotechnology News, vol. 39; 2019. p. 60–2.
Google Scholar
Genome Analysis Toolkit. The Broad Institute, Cambridge. 2020. https://software.broadinstitute.org/gatk/ Accessed 8 Nov 2020.
Konig JC, Titieni A, Konrad M, Consortium N. Network for early onset cystic kidney diseases-a comprehensive multidisciplinary approach to hereditary cystic kidney diseases in childhood. Front Pediatr. 2018;6:24.
Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18(7):2143–60.
Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, Eckloff BW, Ward CJ, Winearls CG, Torres VE, et al. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol. 2012;23(5):915–33.
Hwang YH, Conklin J, Chan W, Roslin NM, Liu J, He N, Wang K, Sundsbak JL, Heyer CM, Haider M, et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(6):1861–8.
Al-Hamed MH, Kurdi W, Alsahan N, Alabdullah Z, Abudraz R, Tulbah M, Alnemer M, Khan R, Al-Jurayb H, Alahmed A, et al. Genetic spectrum of Saudi Arabian patients with antenatal cystic kidney disease and ciliopathy phenotypes using a targeted renal gene panel. J Med Genet. 2016;53(5):338–47.
Al Alawi I, Al Salmi I, Al Rahbi F, Al Riyami M, Al Kalbani N, Al Ghaithi B, Al Mawali A, Sayer JA. Molecular genetic diagnosis of Omani patients with inherited cystic kidney disease. Kidney Int Rep. 2019;4(12):1751–9.
Download references
Acknowledgments
This work has been supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2019-ER-7304-00, 2019-ER-7304-01). The funding body has no role in the design of the study, collection, analysis, interpretation of data, or writing of the manuscript.
Author information
Authors and affiliations.
Department of Internal Medicine, Hallym University College of Medicine, Seoul, South Korea
Hayne Cho Park
Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea
Hyunjin Ryu, Yong-Chul Kim, Kook-Hwan Oh & Yun Kyu Oh
Department of Internal Medicine, National Medical Center, Seoul, South Korea
Department of Internal Medicine, Kangbuk Samsung Hospital, Seoul, South Korea
Kyu-Beck Lee
Department of Internal Medicine, Busan Paik Hospital, Busan, South Korea
Yeong Hoon Kim & Yunmi Kim
Department of Internal Medicine, Keimyung University School of Medicine, Daegu, South Korea
Seungyeup Han & Yaerim Kim
Department of Internal Medicine, Chonnam National University Medical School, Gwangju, South Korea
Eun hui Bae & Seong Kwon Ma
Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, South Korea
Hee Gyung Kang & Yo Han Ahn
Department of Pediatrics, Hallym University College of Medicine, Seoul, South Korea
Department of Biomedical Sciences, Korea University College of Medicine, Seoul, South Korea
Kyungjo Jeong, Jaewon Lee & Jungmin Choi
Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, South Korea
You can also search for this author in PubMed Google Scholar
Contributions
HCP participated in the design of the study, data acquisition, data analysis and drafting of the manuscript. HR and CA participated in the design of the study, interpretation of data and revision of the manuscript. Y-CK, K-BL, YHK, YK, SH, YK, EhB, and SKM participated in data acquisition from adult patients and monitoring of data to improve accuracy. HGK, YHA, EP participated in data acquisition from pediatric patients and monitoring of data to improve accuracy. KJ, JL, and JC participated in the design of the study, interpretation of the data and drafting of the manuscript. K-HO participated in revising the manuscript and in the final approval of the manuscript for publication. YKO participated in the design of the study, gathered the study collaborators and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Yun Kyu Oh .
Ethics declarations
Ethics approval and consent to participate.
This study has been registered at Clinical Research Information Service (KCT0005580). In addition, the study has been approved by the institutional review board of each participating center: the institutional review boards of Seoul National University College of Medicine/Seoul National University Hospital (H-1907-067-1047), Chonnam National University Hospital (CNUH-2019-276), Kangbuk Samsung Hospital (KBSMC 2019–07-029), Inje University Busan Paik Hospital (19–0151), Seoul Metropolitan Government Seoul National University Boramae Medical Center (30–2019-104), Hallym University Kangnam Sacred Heart Hospital (2019–07-015), Keimyung University Dongsan Hospital (DSMC 2019–07–055-008), and Seoul National University Bundang Hospital (B-1910/572–403). We will gather written informed consent from all the patients before enrollment. According to the good clinical practice guidelines and regional rules of the Institutional Review Board, we will collect informed consent from legally authorized representatives of pediatric patients if the patients are under the age of 6, and we will collect the form from both the patients and their legally authorized representatives when the patients are between 6 and 18. In the case when the family members are participating in the study by giving their blood samples, written informed consent will also be collected from the family members. We will also gather informed consent forms from the patients and their family members whose blood samples or DNA left over after genetic analysis will be sent to the National Biobank of Korea for public use.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Park, H.C., Ryu, H., Kim, YC. et al. Genetic identification of inherited cystic kidney diseases for implementing precision medicine: a study protocol for a 3-year prospective multicenter cohort study. BMC Nephrol 22 , 2 (2021). https://doi.org/10.1186/s12882-020-02207-8
Download citation
Received : 27 November 2020
Accepted : 10 December 2020
Published : 06 January 2021
DOI : https://doi.org/10.1186/s12882-020-02207-8

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cohort study
- Cystic kidney disease
- High-throughput nucleotide sequencing
- Genetic association studies
- Glomerular filtration rate
BMC Nephrology
ISSN: 1471-2369
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 13 August 2022
Clinical efficacy of intraoperative real time ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage in the treatment of parapelvic cysts
- Ting Huang 1 ,
- Qing Yang 1 ,
- Haixiao Wu 1 ,
- Desheng Zhu 1 ,
- Yang Hu 1 &
BMC Surgery volume 22 , Article number: 315 ( 2022 ) Cite this article
1261 Accesses
1 Citations
Metrics details
This study aims to investigate the efficacy and safety of intraoperative real time ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage in the treatment of parapelvic cysts, and to review recently published relevant literature.
This is a retrospective study in which the clinical data of 47 patients who underwent flexible ureteroscopic holmium laser incision and internal drainage of parapelvic cysts in our center from March 2017 to March 2021 were retrospectively analyzed. A literature search was conducted to review and summarize relevant reports on endoscopic treatment of parapelvic cysts published in the past 10 years.
Among 47 patients with parapelvic cysts who underwent flexible ureteroscopic holmium laser incision and internal drainage, 12 (25.53%) cases had a typical cyst wall bulging into the collecting system under flexible ureteroscope. As the cyst wall was thin and translucent in these cases, ultrasound was not used during the operation. The cysts of the remaining 35 patients were located with the aid of intraoperative real time ultrasound, and all underwent successful operation. No serious surgical complications occurred after surgery. The patients were followed up for 12–24 months after operation. The cyst in one case was observed larger than its original size before operation, so recurrence was considered. In another two cases, the diameters of the cysts were more than half of their original diameters before operation. Thus, the efficacy was poor in the three cases. For the remaining 44 cases, there was no obvious cyst observed or the diameter of the cysts was less than half their preoperative level.
The approach of ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage in the treatment of parapelvic cysts is safe and effective, which helps to solve the problem of localization of atypical parapelvic cysts on endoscopic findings.
Peer Review reports
Introduction
Renal cyst, a common lesion of kidney, has a population prevalence of approximately 10% [ 1 ]. Most renal cysts are simple, asymptomatic, and can be incidentally detected by routine physical examination. Only a small proportion of them require treatment [ 2 ]. At present, the treatment of simple renal cysts is quite mature, and there are many choices in terms of operation management. However, parapelvic cyst, as a rare disease, accounts for only a small proportion of renal cysts, and there is no uniform standard for operation management due to the special location and complex adjacent structures. Laparoscopic deroofing is the most effective and minimally invasive method of treating renal cysts. However, the specific anatomical structure of parapelvic cysts increases difficulty in operation and the associated surgical complications. Flexible ureteroscope for treatment of parapelvic cysts is recommended in guideline and has become a growing trend [ 3 ]. In recent years, although the use of flexible ureteroscopy in the treatment of renal cystic diseases has become more and more popular, there exists rare relevant literature due to its low prevalence and limited surgery indication. We retrospectively analyzed the clinical data of 47 patients with parapelvic cysts treated with ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage from March 2017 to March 2021, and analyzed the safety and efficacy of this procedure and summarized relevant literature published since its application.
The clinical data of 47 patients with parapelvic cyst treated with ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage in our center from March 2017 to March 2021 were retrospectively analyzed, including preoperative data (general information of patients, location and size of cysts, whether accompanied by stones, etc.), intraoperative data (operation time, whether the cyst performance was typical, etc.), postoperative data and follow-up (postoperative hospital stay, complications, cyst recurrence, etc.) and statistical analysis.
Inclusion criteria were as follows: (1) maximal diameter of renal cyst > 4 cm; (2) symptoms such as lumbago, hematuria, kidney stones, obvious symptoms of the collecting system caused by a parapelvic cyst; (3) endogenous cysts mainly convex to renal hilus as determined by imaging. Exclusion criteria were as follows: (1) patients with a Bosniak classification on CT imaging of grade III and IV; (2) patients with severe cardiac, hepatic, pulmonary and brain dysfunction, without tolerance to general anesthesia; (3) patients thought to have a severe urinary tract infection or with ureteral stricture.
Preoperative examinations included laboratory examinations such as blood and urine routines, electrolytes, renal function, coagulation, and routine biochemistry tests and imaging examinations such as urinary ultrasound, urinary CT + enhancement, and excretory phase imaging.
Surgical methods
Two weeks before surgery, an F6 double-J tube (COOK, USA) was preset to facilitate passage of the ureteroscope, and all patients were treated with the following procedure: general anesthesia, lithotomy position, removal of the preset ureteral stent zebra guide, transurethral insertion of the ureteroscope into the renal pelvis under direct vision, indwelling guide wire and then removal of ureteroscope, placement of an dilator sheath (COOK, USA) to the ureteropelvic junction along the guide wire, placement of a flexible ureteroscope through the dilator sheath, exploration of the renal pelvis and calyces, and identification of the extent of the capsule wall. Typical endoscopic findings were darker color of the weak part of the capsule wall and bulging of part of the mucosa into the renal pelvis (Fig. 1 ). Patients combined with stones were first treated with holmium laser to break the stones and then remove them with a stone basket. If no typical endoscopic findings were obtained, intraoperative real time ultrasound was applied for precise positioning. With hyperechogenicity shown at the flexible ureteroscope forepart, it was confirmed that the end of the flexible ureteroscope was close to the weakness of the cyst wall beside the renal pelvis. Cyst was drained from the central radial fenestration of the weak site of the cyst wall to the boundary of the weak cyst wall by Holmium laser (power: 60–80 W), then the cyst could be communicated with the collecting system. Usually, under the tension of the cyst wall, the incision of the incised cyst wall showed a state of natural opening. Meanwhile, the blending of pale-yellow cyst fluid and transparent saline used to wash the flexible ureteroscopes visual field was visible. Moreover, the cyst wall was incised until it was about 1 cm away from the renal parenchyma, or the incising would stop when the cyst wall gradually thickened and obvious blood vessel distribution was found. Holmium laser was used for hemostasis when there was bleeding at the incision site. The phenomenon of “smoking” was observed when the capsule wall was punctured (Fig. 2 ). An F6 double-J tube was indwelled, with its proximal end located in the fenestrated cyst and its distal end located in the bladder. The indwelling catheter was removed 1–3 days after surgery, the double-J tube was removed 1 month after surgery, and the surgical result was reexamined by CT 1 and 12 months after surgery.

Endoscopic typical findings were darker color of the weak part of the capsule wall and bulging of part of the mucosa into the renal pelvis
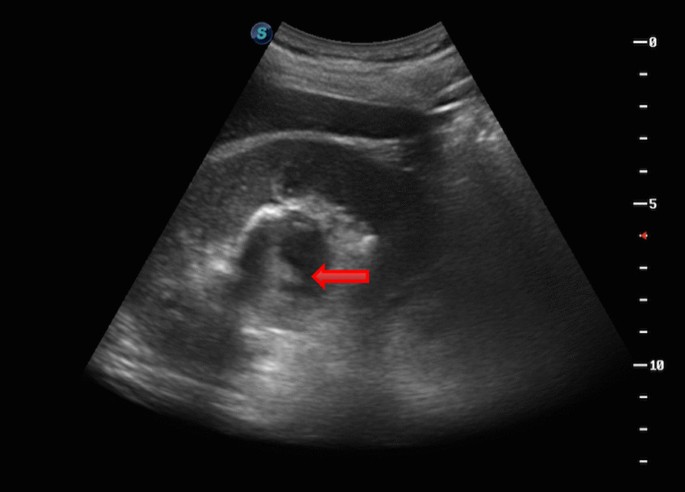
“Smoking” phenomenon was observed when the capsule wall was punctured
Statistical analysis
IBM SPSS statistics v26.0. software was used to analyze the extracted data. Quantitative variables were compared by using the t test. Continuous data are expressed as the mean and range. Te K-S test was used to check whether the preoperative and postoperative data conformed to a normal distribution, and a t test was used to verify whether the data conformed to a normal distribution. Otherwise, the rank sum test was used. p < 0.05 was defined as a statistically significant difference.
Since BASIRI et al. [ 4 ] first used internal incision and drainage of ureteral cyst to treat a case of parapelvic cyst in 2010 and reported the treatment, many scholars have also conducted relevant exploration. By searching PubMed and the Web of Science databases with the term “Parapelvic Cysts”, we found 13 articles on endoscopic surgery related to parapelvic cyst and summarized them.
The general clinical data of the group of patients, aged 58.45 ± 10.86 years, were collected. The lesions were located on the left side in 26 cases, on the right side in 19 cases, and on both sides in 2 cases (one of the cysts was 1.5 cm in diameter, and the affected side was decided to undergo contralateral surgery). The diameter of preoperative lesions was 4.78 ± 1.02 (3.5–7.8) cm. 17 patients presented with lumbar and abdominal discomfort, 4 patients had hematuria as symptoms, and 26 patients were found to be asymptomatic during physical examination. There were 2 cases of hydronephrosis caused by the lesion, one case of nephrydrosis and one case of hydronephrosis of the upper calyx. Additionally, 5 patients with ipsilateral renal calculi received concurrent flexible ureteroscopic holmium laser lithotripsy, and postoperative reexamination showed that all achieved stone-clearing effect. A special case of suprarenal paracalyceal cyst with stenosis of the suprarenal calyceal neck was treated with holmium laser incision of the narrow calyceal neck, followed by incision of the cyst wall after searching for the cyst. By summarizing the clinical data of 47 patients (Table 1 ), it was found that 6 patients had postoperative lumbar and abdominal pain and 4 had significant gross hematuria, all of which were Clavien-Dindo grade I postoperative complications [ 5 ] and were relieved after symptomatic treatment with drugs. All patients were successfully discharged 1–3 days after operation. During a follow-up of 12–24 months after operation, 1 cyst was larger than its original size before operation, and recurrence was considered. Meanwhile, the diameters of the cysts in two cases were more than half of their original diameters before operation. Poor efficacy was considered for the three cases [ 6 , 7 ]. The cysts of the remaining 44 patients showed no obvious cyst or the cysts were reduced to less than half of their original size before operation (Fig. 3 a and b). The maximum diameter of the cysts in 47 patients before and after surgery was compared, which showed a statistical difference (t = 19.631, p < 0.01); diameter variation of the cysts post operation between the maximum diameter of cysts larger (n = 38) than 4 cm and that less (n = 9) than 4 cm was compared, and there was a statistical difference (t = 19.631, p < 0.01).
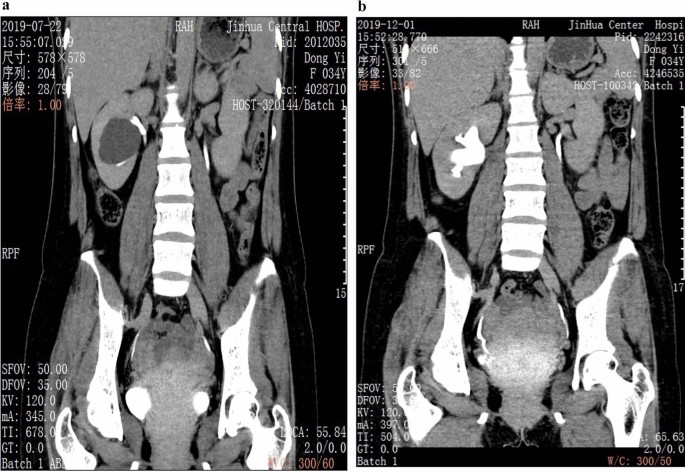
a The parapelvic cyst was shown in excretory phase imaging of preopretive urinary CT. b CT reexamined 4 months after surgery, the parapelvic cyst disappeared
A total of 13 articles (except for those about laparoscopic techniques) on surgical procedures related to endoscopic parapelvic cysts were identified by searching the databases (Table 2 ). 11 were retrospective studies, one was prospective study and one did not mention its study category. One study performed percutaneous ureteroscopic laser decortication, one study performed ablation, and the remaining 11 studies performed flexible ureteroscopic laser endopyelotomy, of which five studies used some auxiliary measures (real time ultrasound included) to help locate renal cyst. A total of five cases of serious postoperative complications occurred, including nephrocolic fistula, urogenic urethral stricture and persistent urinary leakage, with a recurrence rate of 0–26.7% [ 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 ].
Parapelvic cysts show particularity in terms of location, because they are adjacent to renal pedicle. Compared with common simple renal cysts, parapelvic cysts are more likely to result in compression symptoms of the collecting system or renal pedicle vessels when they are rather small. Therefore, the indications for parapelvic cyst surgery should be appropriately relaxed, and it is not necessary to deliberately follow the treatment indication of simple renal cysts > 5 cm [ 21 ] or ≥ 4 cm [ 22 ] in diameter, especially for those with symptoms such as hydronephrosis and hematuria should have surgery within a time limit.
At present, there are many means to treat simple renal cysts, including open surgery, puncture and aspiration with or without sclerosing agent, laparoscopic decortication, puncture, retrograde endoscopic drainage and even robot-assisted laparoscopic stripping [ 1 ]. However, these treatment methods are not completely applicable to parapelvic cysts. The use of laparoscopic renal cyst decortication in the treatment of parapelvic cysts requires adequate exposure of the renal pedicle. The operation is complex and risky, and most cysts are not significantly convex, so it is difficult to locate them. The use of puncture aspiration in the treatment of parapelvic cysts also faces the risk of causing damage to renal pedicle. It has been reported that the recurrence rate of percutaneous ethanol injection is as high as 72% [ 23 ], which can easily lead to ureteropelvic junction obstruction, while the recurrence rate of simple puncture aspiration is even higher. Inverse endoscopic cystotomy seems to be more suitable for the anatomical characteristics of parapelvic cysts, and has the advantages of minimal invasion and repeatability.
Similar to the management of simple renal cysts, relevant examinations and Bosniak classification of cysts should be performed, and the nature of cysts should be systematically assessed. Imaging examination of the urinary excretory phase is helpful to differentiate parapelvic cysts from hydronephrosis [ 24 ]. The aforementioned preoperative examinations are necessary to diagnose the disease. For example, Sabrina H Rossi et al. [ 25 ] reported that a case of parapelvic cyst with hydronephrosis was misdiagnosed as simple hydronephrosis, in which the patient underwent ureteral stenting but the symptoms recurred several months after stent removal. Ruslan Korets et al. [ 20 ] reported a case of persistent urinary leakage after calyceal ablation. All patients included in this study completed preoperative examinations including urinary CT + enhanced and excretory phase imaging. The key to the treatment of parapelvic cysts with flexible ureteroscopic holmium laser incision and internal drainage is to identify the location of cyst and the optimal entry point. However, only a small proportion of the cysts will have a cyst wall bulging into the collecting system under endoscope, showing a typical thin and translucent performance. Most parapelvic cysts are difficult to locate by the naked eye under endoscope. Therefore, intraoperative real-time ultrasound is used to assist in locating the optimal entry point of the cyst. At present, 11 reports have elaborated the localization of parapelvic cysts under intraoperative real-time ultrasound-assisted flexible ureteroscopy. In this study, 12 cases (25.53%) had typical performance under endoscope, and the optimal entry points of the cysts of the remaining 35 cases were located under ultrasound-assisted and the operations were successfully completed.
According to relevant literature, the significance of the technology of intraoperative real-time ultrasound assisted flexible ureteroscope for localization of parapelvic renal cysts has been affirmed by most of the scholars. However, for the current retrospective studies of small sample size, the recurrence rate of cyst post operation varies widely (0–26.7%), which may be related to factors such as different case inclusion criteria and surgical operation criteria. In addition, an interesting finding in the research shows that the patients with a cyst diameter larger than 4 cm may have better benefits. The reason may be that large-diameter cysts allow a wider range of fenestration space during incising process. Therefore, a better communication will be formed. As for the key points of the intraoperative real-time ultrasound assisted flexible ureteroscopes parapelvic cyst open operation, we can make the following conclusions: the range of intraoperative fenestration should be as large as possible, and should be stopped until it is about 1 cm away from the renal parenchyma [ 26 ], to avoid uncontrollable bleeding due to the injury. During operation, it is necessary to avoid blood vessel deformation. If a small amount of uncontrolled hemorrhage is found under microscope, for example, holmium laser can be used for hemostasis; after fenestration during operation, the blending phenomenon between the cystic fluid and the transparent saline that is used to wash the flexible ureteroscopes visual field can be observed until the cystic fluid and the washed-down fluid are completely fused due to the fact that the cystic fluid is not completely transparent, most of which is usually pale-yellow. After fenestration, one end of the double-J tube is put into the cyst cavity [ 27 ]. One the one hand, the recurrence of the cyst after operation can be avoided; on the other hand, it is helpful to drain the cyst fluid.
The current study has some limitations due to the retrospective nature of the study and the absence of a control group. Considering the low incidence of the disease and limitation of single-center studies on the subjects, it is necessary to conduct multicenter, large sample and longer follow-up studies to further verify our conclusions in the future.
As mentioned above, it can be found that intraoperative real-time ultrasound assisted flexible ureteroscopic holmium laser incision and internal drainage in the treatment of renal cystic diseases have some advantages, such as safe and reliable efficacy, less surgical injury, faster postoperative recovery after surgery and less complications in the perioperative period, especially the better outcomes for the patients with cyst diameter greater than 4 cm, which can be found in the combined short-term studies of small sample size according to the literature reported in recent years and the practice research of the cases. The technology of intraoperative real-time ultrasound assisted flexible ureteroscope for localization of parapelvic renal cysts contributes to treating atypical parapelvic renal cysts accurately with flexible ureteroscopes and avoids iatrogenic injury caused by blind incision. Therefore, the clinical surgical therapeutic effect for patients is good with low cyst recurrence rate in the short term. However, it still needs further study, observation and follow-up based on large sample data to investigate the long-term efficacy and complication prevalence of the operation.
Availability of data and materials
The datasets used during this study available from the corresponding author on reasonable request.
Eissa A, El Sherbiny A, Martorana E, et al. Non-conservative management of simple renal cysts in adults: a comprehensive review of literature. Minerva Urol Nefrol. 2018;70(2):179–92. https://doi.org/10.23736/S0393-2249.17.02985-X .
Article PubMed Google Scholar
Choi JD. Clinical characteristics and long-term observation of simple renal cysts in a healthy Korean population. Int Urol Nephrol. 2016;48:319–24.
Article Google Scholar
Gao X. Pararenal cyst and sinus cyst. In: Guo YL, editor. Chinese urology and andrology disease diagnosis and treatment guidelines. 2019th ed. Beijing: Science Press; 2020. p. 672.
Google Scholar
Basiri A, Hosseim SR, Tousi VN, et al. Uretemscopic management of symptomatic, simple parapelvic renal cyst. J Endourol. 2010;24(4):537–40.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. https://doi.org/10.1097/SLA.0b013e3181b13ca2 .
Glassberg K. Renal dysplasis and cystic disease of the kidney. Campbell’s Urol. 2002;8:1443–95.
Yoder BM, Wolf JS Jr. Long-term outcome of laparoscopic decortication of peripheral and peripelvic renal and adrenal cysts. J Urol. 2004;171:583–7.
Yan KW, Tian XF, Meng N, et al. Flexible ureteroscopy with ultrasound guidance for the treatment of parapelvic renal cysts: a complementary approach for locating the cystic wall. BMC Urol. 2022;22(1):7.
Chen H, Chen G, Pan Y, Jin X. Minimally invasive surgeries in the management of renal parapelvic cysts: a retrospective comparative study. Urol J. 2021;18(4):389–94.
PubMed Google Scholar
Chen Y, Wang R, Shen X, et al. Ultrasonography-assisted flexible ureteroscope for the treatment of parapelvic renal cysts: a comparison between the 1470-nm diode laser and the holmium laser. Exp Therapeutic Med. 2021;21(2):172.
Article CAS Google Scholar
Huang B, Lu G, Tu W, et al. Factors influencing surgical outcome in retrograde management of parapelvic renal cysts. J Endourol. 2021;35(4):466–72.
Wen J, Xu G, He G, et al. The clinical efficacy and safety of flexible ureteroscopic treatment for parapelvic renal cyst and secondary renal stone. Urol J. 2020;17(3):243–7. https://doi.org/10.22037/uj.v0i0.5441 .
Kang N, Guan X, Song L, et al. Simultaneous treatment of parapelvic renal cysts and stones by flexible ureterorenoscopy with a novel four-step cyst localization strategy. Int Braz J Urol. 2018;44(5):958–64. https://doi.org/10.1590/S1677-5538.IBJU.2018.0074 .
Article PubMed PubMed Central Google Scholar
Wang Z, Zeng X, Chen C, et al. Methylene blue injection via percutaneous renal cyst puncture used in flexible ureteroscope for treatment of parapelvic cysts: a modified method for easily locating cystic wall. Urology. 2019;125:243–7. https://doi.org/10.1016/j.urology.2018.11.014 .
Jia Hu, Isse DN, Jun Y, et al. Percutaneous ureteroscopy laser unroofing-a minimally invasive approach for renal cyst treatment. Sci Rep. 2017;7:14445.
Mao XiaWa Xu, HuiFeng GW, et al. Ureteroscopic management of asymptomatic and symptomatic simple parapelvic renal cysts. BMC Urol. 2015;15:48.
Zhao Q, Huang S, Li Q, et al. Treatment of parapelvic cyst by internal drainage technology using ureteroscope and holmium laser. West Indian Med J. 2015;64(3):230–5. https://doi.org/10.7727/wimjopen.2014.220.Epub .
Yu W, Zhang D, He X, et al. Flexible ureteroscopic management of symptomatic renal cystic diseases. J Surg Res. 2015;196(1):118.
Luo Q, Zhang X, Chen H, et al. Treatment of renal parapelvic cysts with a flexible ureteroscope. Int Urol Nephrol. 2014;46(10):1903–8. https://doi.org/10.1007/s11255-014-0741-y .
Korets R, Mues AC, Gupta M. Minimally invasive percutaneous ablation of parapelvic renal cysts and caliceal diverticula using bipolar energy. J Endourol. 2011;25(5):769–73. https://doi.org/10.1089/end.2010.0525 .
Arisan S, Dalkilinc A, Caskurlu T, et al. Laparoscopic unroofing and aspiration-sclerotherapy in the management of symptomatic simple renal cysts. Sci World J. 2006;6:2296–301.
Shao Q, Xu J, Adams T, et al. Comparison of aspiration-sclerotherapy versus laparoscopic decortication in management of symptomatic simple renal cysts. J Xray Sci Technol. 2013;21:419–28.
Zerem E, Imamovic G, Omerovic S. Symptomatic simple renal cyst: comparison of continuous negative-pressure catheter drainage and single-session alcohol sclerotherapy. AJR Am J Roentgenol. 2008;190:1193–7.
Schoots IG, Zaccai K, Hunink MG, et al. Bosniak classification for complex renal cysts reevaluated: a systematic review. J Urol. 2017;198(1):12–21. https://doi.org/10.1016/j.juro.2016.09.160 .
Rossi SH, Koo B, Riddick A, et al. Different successful management strategies for obstructing renal parapelvic cysts. Urol Int. 2018;101:366–8.
Shen J, Chen Y, Wang R. Efficacy and complication of flexible ureteroscopic holmium laser incision for simple renal cysts: a retrospective study. J Endourol. 2019;33(11):881–6.
Zewu Z, Hequn C, Yu C, Yang L, Zhongqing Y, Zhiyong C, Feng Z. Long-term outcome after flexible ureteroscopy with holmium laser for simultaneous treatment of a single renal cyst and ipsilateral renal stones. J Int Med Res. 2019;47(8):3601–12.
Download references
Acknowledgements
Not applicable.
This work is supported by scientific research project of Zhejiang medical and health science and technology plan project (Grant No.2022RC285). The foundation provided financial support for publication charges. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. All the funds were granted to Dr. Ting Huang for the charge of article-processing charge, manuscript modifications fee.
Author information
Authors and affiliations.
Department of Urology, Affiliated Jinhua Hospital, Zhejiang University School of Medicine, Jinhua, 321000, China
Ting Huang, Qing Yang, Haixiao Wu, Desheng Zhu, Yang Hu & Min Xu
You can also search for this author in PubMed Google Scholar
Contributions
TH carried out the initial analyses, QY wrote the main manuscript text. H-XW reviewed and revised the manuscript. D-SZ coordinated and supervised data collection. YH prepared figures and tables.MX critically reviewed and revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Min Xu .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the institutional ethics committee (202252, Jinhua Hospital Affiliated to Zhejiang University institutional ethics committee, China). Informed consent was obtained from all participants. All patients signed the written informed consent. Patients’ identification information had been removed. The study is in line with the Declaration of Helsinki.
Consent for publication
The authors obtained the written informed consent for publication from these patients.
Competing interests
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huang, T., Yang, Q., Wu, H. et al. Clinical efficacy of intraoperative real time ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage in the treatment of parapelvic cysts. BMC Surg 22 , 315 (2022). https://doi.org/10.1186/s12893-022-01763-0
Download citation
Received : 07 May 2022
Accepted : 05 August 2022
Published : 13 August 2022
DOI : https://doi.org/10.1186/s12893-022-01763-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Flexible ureteroscope
- Holmium laser
- Parapelvic cyst
BMC Surgery
ISSN: 1471-2482
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Patient Care & Health Information
- Diseases & Conditions
- Chronic kidney disease
- What is kidney disease? An expert explains
Learn more from kidney doctor Andrew Bentall, M.D.
I'm Dr. Andrew Bentall, a kidney doctor at Mayo Clinic. I look after patients with kidney disease, either in the early stages, or with more advanced kidney disease considering dialysis and transplantation as treatment options. In this video, we'll cover the basics of chronic kidney disease. What is it? Who gets it? The symptoms, diagnosis and treatment. Whether you are looking for answers for yourself or for someone you love, we're here to give you the best information available.
Chronic kidney disease is a disease characterized by progressive damage and loss of function in the kidneys. It's estimated that chronic kidney disease affects about one in seven American adults. And most of those don't know they have it. Before we get into the disease itself, let's talk a little bit about the kidneys and what they do. Our kidneys play many important roles keeping our bodies in balance. They remove waste and toxins, excess water from the bloodstream, which is carried out of the body in urine. They helped to make hormones to produce red blood cells, and they turn vitamin D into its active form, so it's usable in the body.
There are quite a few things that can cause or put you at higher risk for chronic kidney disease. Some of them are not things that can be avoided. Your risk is simply higher if you have a family history of certain genetic conditions like polycystic kidney disease or some autoimmune diseases like lupus or IgA nephropathy. Defects in the kidney structure can also cause your kidneys to fail, and you have an increased risk as you get older. Sometimes, other common medical conditions can increase your risk. Diabetes is the most common cause of kidney disease. Both type 1 and type 2 diabetes. But also heart disease and obesity can contribute to the damage that causes kidneys to fail. Urinary tract issues and inflammation in different parts of the kidney can also lead to long-term functional decline. There are things that are more under our control: Heavy or long-term use of certain medications, even those that are common over-the-counter. Smoking can also be a contributing factor to chronic kidney disease.
Often there are no outward signs in the earlier stages of chronic kidney disease, which is grouped into stages 1 through 5. Generally, earlier stages are known as 1 to 3. And as kidney disease progresses, you may notice the following symptoms. Nausea and vomiting, muscle cramps, loss of appetite, swelling via feet and ankles, dry, itchy skin, shortness of breath, trouble sleeping, urinating either too much or too little. However, these are usually in the later stages, but they can also happen in other disorders. So don't automatically interpret this as having kidney disease. But if you're experiencing anything that concerns you, you should make an appointment with your doctor.
Even before any symptoms appear, routine blood work can indicate that you might be in the early stages of chronic kidney disease. And the earlier it's detected, the easier it is to treat. This is why regular checkups with your doctor are important. If your doctor suspects the onset of chronic kidney disease, they may schedule a variety of other tests. They may also refer you to a kidney specialist, a nephrologist like myself. Urine tests can reveal abnormalities and give clues to the underlying cause of the chronic kidney disease. And this can also help to determine the underlying issues. Various imaging tests like ultrasounds or CT scans can be done to help your doctor assess the size, the structure, as well as evaluate the visible damage, inflammation or stones of your kidneys. And in some cases, a kidney biopsy may be necessary. And a small amount of tissue is taken with a needle and sent to the pathologist for further analysis.
Treatment is determined by what is causing your kidneys to not function normally. Treating the cause is key, leading to reduced complications and slowing progression of kidney disease. For example, getting better blood pressure control, improved sugar control and diabetes, and reducing weight are often key interventions. However, existing damage is not usually reversible. In some conditions, treatment can reverse the cause of the disease. So seeking medical review is really important. Individual complications vary, but treatment might include high blood pressure medication, diuretics to reduce fluid and swelling, supplements to relieve anemia, statins to lower cholesterol, or medications to protect your bones and prevent blood vessel calcification. A lower-protein diet may also be recommended. It reduces the amount of waste your kidneys need to filter from your blood. These can not only slow the damage of kidney disease, but make you feel better as well. When the damage has progressed to the point that 85 to 90 percent of your kidney function is gone, and they no longer work well enough to keep you alive, it's called end-stage kidney failure. But there are still options. There's dialysis, which uses a machine to filter the toxins and remove water from your body as your kidneys are no longer able to do this. Where possible, the preferred therapy is a kidney transplant. While an organ transplant can sound daunting, it's actually often the better alternative, and the closest thing to a cure, if you qualify for a kidney transplant.
If you have kidney disease, there are lifestyle choices. Namely quit smoking. Consuming alcohol in moderation. If you're overweight or obese, then try to lose weight. Staying active and getting exercise can help not only with your weight, but fatigue and stress. If your condition allows, keep up with your routine, whether that's working, hobbies, social activities, or other things you enjoy. It can be helpful to talk to someone you trust, a friend or relative who's good at listening. Or your doctor could also refer you to a therapist or social worker. It can also be helpful to find a support group and connect with people going through the same thing. Learning you have chronic kidney disease and learning how to live with it can be a challenge. But there are lots of ways to help you to be more comfortable for longer before more drastic measures are needed. And even then, there is plenty of hope. If you'd like to learn even more about chronic kidney disease, watch our other related videos or visit mayoclinic.org. We wish you well.
Chronic kidney disease, also called chronic kidney failure, involves a gradual loss of kidney function. Your kidneys filter wastes and excess fluids from your blood, which are then removed in your urine. Advanced chronic kidney disease can cause dangerous levels of fluid, electrolytes and wastes to build up in your body.
In the early stages of chronic kidney disease, you might have few signs or symptoms. You might not realize that you have kidney disease until the condition is advanced.
Treatment for chronic kidney disease focuses on slowing the progression of kidney damage, usually by controlling the cause. But, even controlling the cause might not keep kidney damage from progressing. Chronic kidney disease can progress to end-stage kidney failure, which is fatal without artificial filtering (dialysis) or a kidney transplant.
- How kidneys work
One of the important jobs of the kidneys is to clean the blood. As blood moves through the body, it picks up extra fluid, chemicals and waste. The kidneys separate this material from the blood. It's carried out of the body in urine. If the kidneys are unable to do this and the condition is untreated, serious health problems result, with eventual loss of life.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- A Book: The Body's Keepers
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of chronic kidney disease develop over time if kidney damage progresses slowly. Loss of kidney function can cause a buildup of fluid or body waste or electrolyte problems. Depending on how severe it is, loss of kidney function can cause:
- Loss of appetite
- Fatigue and weakness
- Sleep problems
- Urinating more or less
- Decreased mental sharpness
- Muscle cramps
- Swelling of feet and ankles
- Dry, itchy skin
- High blood pressure (hypertension) that's difficult to control
- Shortness of breath, if fluid builds up in the lungs
- Chest pain, if fluid builds up around the lining of the heart
Signs and symptoms of kidney disease are often nonspecific. This means they can also be caused by other illnesses. Because your kidneys are able to make up for lost function, you might not develop signs and symptoms until irreversible damage has occurred.
When to see a doctor
Make an appointment with your doctor if you have signs or symptoms of kidney disease. Early detection might help prevent kidney disease from progressing to kidney failure.
If you have a medical condition that increases your risk of kidney disease, your doctor may monitor your blood pressure and kidney function with urine and blood tests during office visits. Ask your doctor whether these tests are necessary for you.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Healthy kidney vs. diseased kidney
A typical kidney has about 1 million filtering units. Each unit, called a glomerulus, joins a tubule. The tubule collects urine. Conditions such as high blood pressure and diabetes harm kidney function by damaging these filtering units and tubules. The damage causes scarring.
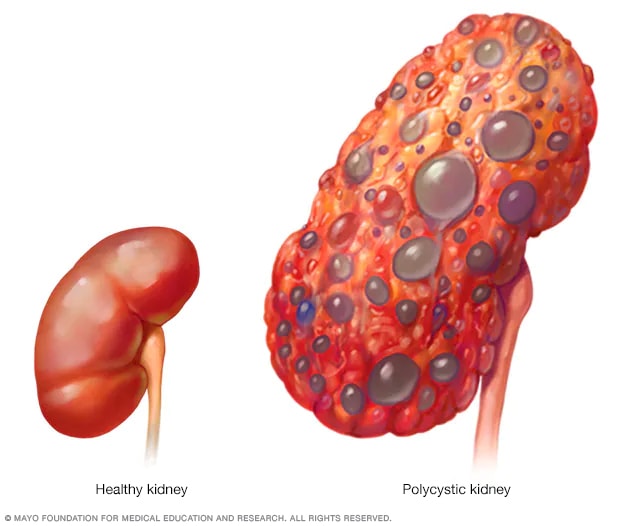
- Polycystic kidney
A healthy kidney (left) eliminates waste from the blood and maintains the body's chemical balance. With polycystic kidney disease (right), fluid-filled sacs called cysts develop in the kidneys. The kidneys grow larger and gradually lose the ability to function as they should.
Chronic kidney disease occurs when a disease or condition impairs kidney function, causing kidney damage to worsen over several months or years.
Diseases and conditions that cause chronic kidney disease include:
- Type 1 or type 2 diabetes
- High blood pressure
- Glomerulonephritis (gloe-mer-u-low-nuh-FRY-tis), an inflammation of the kidney's filtering units (glomeruli)
- Interstitial nephritis (in-tur-STISH-ul nuh-FRY-tis), an inflammation of the kidney's tubules and surrounding structures
- Polycystic kidney disease or other inherited kidney diseases
- Prolonged obstruction of the urinary tract, from conditions such as enlarged prostate, kidney stones and some cancers
- Vesicoureteral (ves-ih-koe-yoo-REE-tur-ul) reflux, a condition that causes urine to back up into your kidneys
- Recurrent kidney infection, also called pyelonephritis (pie-uh-low-nuh-FRY-tis)
Risk factors
Factors that can increase your risk of chronic kidney disease include:
- Heart (cardiovascular) disease
- Being Black, Native American or Asian American
- Family history of kidney disease
- Abnormal kidney structure
- Frequent use of medications that can damage the kidneys
Complications
Chronic kidney disease can affect almost every part of your body. Potential complications include:
- Fluid retention, which could lead to swelling in your arms and legs, high blood pressure, or fluid in your lungs (pulmonary edema)
- A sudden rise in potassium levels in your blood (hyperkalemia), which could impair your heart's function and can be life-threatening
- Heart disease
- Weak bones and an increased risk of bone fractures
- Decreased sex drive, erectile dysfunction or reduced fertility
- Damage to your central nervous system, which can cause difficulty concentrating, personality changes or seizures
- Decreased immune response, which makes you more vulnerable to infection
- Pericarditis, an inflammation of the saclike membrane that envelops your heart (pericardium)
- Pregnancy complications that carry risks for the mother and the developing fetus
- Irreversible damage to your kidneys (end-stage kidney disease), eventually requiring either dialysis or a kidney transplant for survival
To reduce your risk of developing kidney disease:
- Follow instructions on over-the-counter medications. When using nonprescription pain relievers, such as aspirin, ibuprofen (Advil, Motrin IB, others) and acetaminophen (Tylenol, others), follow the instructions on the package. Taking too many pain relievers for a long time could lead to kidney damage.
- Maintain a healthy weight. If you're at a healthy weight, maintain it by being physically active most days of the week. If you need to lose weight, talk with your doctor about strategies for healthy weight loss.
- Don't smoke. Cigarette smoking can damage your kidneys and make existing kidney damage worse. If you're a smoker, talk to your doctor about strategies for quitting. Support groups, counseling and medications can all help you to stop.
- Manage your medical conditions with your doctor's help. If you have diseases or conditions that increase your risk of kidney disease, work with your doctor to control them. Ask your doctor about tests to look for signs of kidney damage.
Chronic kidney disease care at Mayo Clinic
Living with chronic kidney disease?
Connect with others like you for support and answers to your questions in the Transplants support group on Mayo Clinic Connect, a patient community.
Transplants Discussions

1594 Replies Fri, Jun 07, 2024

1672 Replies Fri, Jun 07, 2024

21 Replies Tue, Jun 04, 2024
- Goldman L, et al., eds. Chronic kidney disease. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. http://www.clinicalkey.com. Accessed April 27, 2021.
- Chronic kidney disease (CKD). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd#:~:text=Chronic kidney disease (CKD) means,family history of kidney failure. Accessed April 26, 2021.
- Rosenberg M. Overview of the management of chronic kidney disease in adults. https://www.uptodate.com/contents/search. Accessed April 26, 2021.
- Chronic kidney disease (CKD) symptoms and causes. National Kidney Foundation. https://www.kidney.org/atoz/content/about-chronic-kidney-disease. Accessed April 26, 2021.
- Chronic kidney disease. Merck Manual Professional Version. https://www.merckmanuals.com/professional/genitourinary-disorders/chronic-kidney-disease/chronic-kidney-disease?query=Chronic kidney disease. Accessed April 26, 2021.
- Ammirati AL. Chronic kidney disease. Revista da Associação Médica Brasileira. 2020; doi:10.1590/1806-9282.66.S1.3.
- Chronic kidney disease basics. Centers for Disease Control and Prevention. https://www.cdc.gov/kidneydisease/basics.html. Accessed April 26, 2021.
- Warner KJ. Allscripts EPSi. Mayo Clinic; April 21, 2021.
- Office of Patient Education. Chronic kidney disease treatment options. Mayo Clinic; 2020.
- Chronic kidney disease: Is a clinical trial right for me?
- Eating right for chronic kidney disease
- Effectively managing chronic kidney disease
- Kidney biopsy
- Kidney disease FAQs
- Low-phosphorus diet: Helpful for kidney disease?
- MRI: Is gadolinium safe for people with kidney problems?
- Renal diet for vegetarians
Associated Procedures
- Deceased-donor kidney transplant
- Hemodialysis
- Kidney transplant
- Living-donor kidney transplant
- Nondirected living donor
- Peritoneal dialysis
- Preemptive kidney transplant
News from Mayo Clinic
- Mayo Clinic Minute: Why Black Americans are at higher risk of chronic kidney disease March 05, 2024, 05:00 p.m. CDT
- Mayo Clinic Minute: Can extra salt hurt your kidneys? Feb. 16, 2024, 04:00 p.m. CDT
- Mayo Clinic Minute: Using AI to predict kidney failure in patients with polycystic kidney disease April 06, 2023, 04:00 p.m. CDT
- Mayo Clinic Q and A: Understanding chronic kidney disease March 23, 2023, 12:35 p.m. CDT
- Mayo Clinic Minute: Game-changing treatment for chronic kidney disease could slow down progression of the disease March 06, 2023, 04:01 p.m. CDT
- Science Saturday: Seeking a cellular therapy for chronic kidney disease Nov. 12, 2022, 12:00 p.m. CDT
- Science Saturday: Mayo Clinic researchers integrate genomics into kidney disease diagnosis, care Sept. 17, 2022, 11:00 a.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
- Open access
- Published: 26 April 2024
Clinical manifestation, epidemiology, genetic basis, potential molecular targets, and current treatment of polycystic liver disease
- Amir Ali Mahboobipour 1 ,
- Moein Ala ORCID: orcid.org/0000-0001-5951-4864 2 ,
- Javad Safdari Lord 3 &
- Arash Yaghoobi 3 , 4
Orphanet Journal of Rare Diseases volume 19 , Article number: 175 ( 2024 ) Cite this article
557 Accesses
1 Altmetric
Metrics details
Polycystic liver disease (PLD) is a rare condition observed in three genetic diseases, including autosomal dominant polycystic liver disease (ADPLD), autosomal dominant polycystic kidney disease (ADPKD), and autosomal recessive polycystic kidney disease (ARPKD). PLD usually does not impair liver function, and advanced PLD becomes symptomatic when the enlarged liver compresses adjacent organs or increases intra-abdominal pressure. Currently, the diagnosis of PLD is mainly based on imaging, and genetic testing is not required except for complex cases. Besides, genetic testing may help predict patients’ prognosis, classify patients for genetic intervention, and conduct early treatment. Although the underlying genetic causes and mechanisms are not fully understood, previous studies refer to primary ciliopathy or impaired ciliogenesis as the main culprit. Primarily, PLD occurs due to defective ciliogenesis and ineffective endoplasmic reticulum quality control. Specifically, loss of function mutations of genes that are directly involved in ciliogenesis, such as Pkd1 , Pkd2, Pkhd1, and Dzip1l, can lead to both hepatic and renal cystogenesis in ADPKD and ARPKD. In addition, loss of function mutations of genes that are involved in endoplasmic reticulum quality control and protein folding, trafficking, and maturation, such as PRKCSH , Sec63, ALG8 , ALG9 , GANAB , and SEC61B, can impair the production and function of polycystin1 (PC1) and polycystin 2 (PC2) or facilitate their degradation and indirectly promote isolated hepatic cystogenesis or concurrent hepatic and renal cystogenesis. Recently, it was shown that mutations of LRP5, which impairs canonical Wnt signaling, can lead to hepatic cystogenesis. PLD is currently treated by somatostatin analogs, percutaneous intervention, surgical fenestration, resection, and liver transplantation. In addition, based on the underlying molecular mechanisms and signaling pathways, several investigational treatments have been used in preclinical studies, some of which have shown promising results. This review discusses the clinical manifestation, complications, prevalence, genetic basis, and treatment of PLD and explains the investigational methods of treatment and future research direction, which can be beneficial for researchers and clinicians interested in PLD.
Introduction
PLD is a shared presentation of several genetic diseases such as ADPKD, ARPKD, and ADPLD [ 1 , 2 , 3 , 4 ]. PLD is generally a rare medical condition mainly observed together with polycystic kidney disease (PKD) rather than alone [ 5 , 6 , 7 ]. Unlike PKD, which can finally progress to end-stage renal disease (ESRD), PLD does not impair liver function, but instead, liver enlargement physically compresses the adjacent organs and increases intra-abdominal mechanical pressure, which can cause most of the symptoms and necessitates treatment in symptomatic cases [ 8 , 9 ]. Although PLD remains asymptomatic in a considerable proportion of patients [ 9 ].
Currently, PLD is diagnosed based on imaging modalities such as ultrasonography, computed tomography (CT) scan, and magnetic resonance imaging (MRI). Identification of more than 20 hepatic cysts commonly confirms the diagnosis of PLD [ 7 , 9 , 10 ]. Due to the widespread use of abdominal imaging for various purposes, asymptomatic PLD or early-stage PLD is usually diagnosed as an incidental finding in many cases [ 7 ]. Pathological assessment can show many fluid-filled cysts whose lining is covered by cholangiocytes [ 9 , 10 ]. In addition to the vast genetic heterogeneity among cases, already-known genetic variants do not explain all cases and the responsible genes in a substantial group of patients still need to be discovered [ 11 ].
In this review, we dissect the clinical manifestation, complications, prevalence, genetic basis, and treatment of PLD. In addition, we discuss the investigational methods of treatment and future research direction based on the underlying molecular mechanisms. As this article comprehensively discusses all dimensions of the topic, it can be helpful for researchers, scientists, and clinicians who wish to know the latest findings regarding PLD.
Clinical presentation and epidemiological characteristics
Clinical presentation and epidemiological characteristics of adpkd.
PKD is a genetic disorder that causes the growth of fluid-filled cysts in the kidneys and damages the surrounding tissues [ 12 , 13 ]. ADPKD is the most common form of PKD and the most frequent hereditary kidney disease, which finally progresses to ESRD [ 14 ]. A meta-analysis of 8 epidemiological studies revealed that the prevalence of ADPKD is approximately 2.7 per 10,000 individuals [ 15 ]. ADPKD can present with hypertension, pain, hematuria, urinary tract infection, proteinuria, liver cysts, intracranial aneurysms, heart valve insufficiency, and mitral valve prolapse [ 14 ]. Although this disease is inherited monogenetically, it is phenotypically and genetically heterogeneous [ 12 , 13 ]. Progressive renal fibrosis in ADPKD is often associated with extrarenal abnormalities such as cystogenesis in the liver, seminal vesicle, pancreas, and arachnoid membrane, abdominal herniation, intracranial aneurysms, and cardiac abnormalities [ 2 , 12 , 13 ]. Hepatic cysts are the most common extrarenal manifestations of ADPKD, and the incidence of hepatic cysts among patients with ADPKD was shown to gradually increase with aging [ 1 , 2 ]. Among 129 patients with ADPKD in one study, 62.8% of participants developed PLD [ 16 ]. Despite renal cysts, hepatic cysts do not develop in utero and mainly manifest after puberty [ 1 ]. In addition, age was an independent predictor of hepatic cysts in patients with ADPKD [ 1 , 17 ]. Moreover, female gender, number of pregnancies, severity of renal cystic disease, and renal functional impairment were positively associated with the progression of PLD in patients with ADPKD [ 1 , 17 ]. Another study comprising 241 patients with ADPKD and 119 patients with ADPLD indicated that female patients with ADPKD had larger height-adjusted total liver volume (TLV) compared with female patients with ADPLD [ 18 ]. Surprisingly, the study reported that among patients with ADPKD, younger females (≤ 51 years) had greater liver volumes than older females (> 51 years), reminding the importance of female sex hormones in the development of liver cysts [ 18 ].
Consistent with the effect of female gender and pregnancy in hepatic cyst growth [ 1 , 17 ], it was found that estrogen receptor and insulin-like growth factor 1 (IGF1) receptor were markedly upregulated in hepatic cyst epithelium, and 17β-estradiol and IGF1 significantly promoted liver cyst-derived epithelial cell proliferation [ 19 ].
Comparing the clinical characteristics of 19 patients with isolated ADPLD and 34 patients with ADPKD revealed that [ 20 ]: 1) development of liver cysts was significantly correlated with female gender in both ADPLD and ADPKD; 2) Patients with ADPLD had greater numbers and larger sizes of liver cysts but experienced fewer morbidities; 3) Liver cyst decompressions were significantly more frequent among patients with ADPLD, and serious hepatic complications necessitating liver transplantation were more common in ADPKD [ 20 ].
Clinical presentation and epidemiological characteristics of ARPKD
Autosomal recessive polycystic kidney disease (ARPKD) is a less common form of PKD. Its prevalence is estimated to be 1 in 20,000 live births [ 21 ]. ARPKD usually manifests during pregnancy or childhood, leading to premature death [ 22 ]. Of 50 patients with ARPKD, 24% were diagnosed before birth and 66% were diagnosed before 1 year of age, with hypertension as the most common symptom [ 22 ]. ARPKD is characterized by the development of multiple cysts in the kidneys and liver, as well as other complications such as pulmonary hypoplasia and hypertension [ 22 , 23 ]. Hepatic complications are also frequently detected in patients with ARPKD, including hepatic fibrosis, hepatosplenomegaly, portal hypertension, cholangitis, variceal bleeding, ascites, hepatic and bile duct cysts, and hepatic fibrosis [ 3 , 22 , 23 ]. Particularly, cholangitis, portal hypertension, and subsequent variceal bleeding, splenomegaly, and thrombocytopenia are the main and most severe hepatic complications of ARPKD [ 24 ]. Liver cysts have been observed in the ultrasonography of 23.1% of patients with ARPKD [ 3 ]. Among 32 patients with ARPKD and pathogenic variants of the Pkhd1 gene, one-third exhibited prenatal anomalies, and five died within the first year of life due to respiratory failure [ 25 ]. Another cross-sectional study, which analyzed 49 patients with ARPKD and a mean age of 21.4 ± 3.3 years, reported that fourteen (31%) patients underwent kidney transplantation and six patients (13%) underwent liver transplantation or both liver and kidney transplantation [ 26 ].
Clinical presentation and epidemiological characteristics of ADPLD
The incidence of ADPLD seems to be 1.01 per 100,000 person-years and most cases are detected between 30 and 50 years of age [ 7 , 27 ]. ADPLD is characterized by abnormal liver enlargement, which physically compresses the adjacent organs [ 27 ]. Patients with isolated ADPLD mainly present with abdominal pain, abdominal distension, dyspepsia, and dyspnea, and less than 20% of patients may remain asymptomatic [ 9 ]. Compared with individuals with a negative or indeterminate diagnosis of ADPLD, those with ADPLD were shown to have slightly higher serum levels of alkaline phosphatase, gamma-glutamyl transferase, and total bilirubin and lower serum levels of total cholesterol and triglyceride [ 27 ]. It has also been observed that female patients with ADPLD develop more advanced liver cysts compared with male patients [ 27 ]. The hepatic cysts in patients with ADPLD originate from the proliferating biliary microhamartomas and peribiliary glands [ 27 ]. In addition to cyst hemorrhage, rupture, and infection, the growing hepatic cysts may compress the neighboring organs and cause serious complications, such as portal vein obstruction, common bile duct obstruction, and inferior vena cava occlusion, that often necessitate urgent medical intervention [ 8 , 9 ]. PLD is also accompanied by increased mechanical pressure on the abdominal wall, which considerably elevates the risk of abdominal herniation [ 28 ]. A study comprising 484 patients with PLD reported that 40.1% of patients developed abdominal hernia, particularly umbilical hernia [ 28 ]. Therefore, the management of ADPLD mainly aims to reduce liver volume or prevent liver enlargement. However, these compressive symptoms due to liver enlargement are the main symptoms in ADPLD, they can all be expected in ADPKD and ARPKD since PLD is a common manifestation of all of these diseases.
In addition, several classification systems, such as Schnelldorfer classification (Supplementary Table 1 ), Gigot classification (Supplementary Table 2 ), and Qian classification, have been developed to categorize disease severity and symptomatic phase in PLD [ 29 , 30 ]. Schnelldorfer and Gigot classifications consider the size and number of cysts and normal liver parenchyma [ 29 , 30 ]. Schnelldorfer classification also considers portal vein or hepatic vein occlusion for categorization and relates symptom burden to the number of affected liver segments [ 30 ]. However, Qian classification simply categorizes patients with PLD into 5 grades based on the number of liver cysts and the presence of symptoms [ 27 ].
Genetic basis
Genetic basis of adpkd.
ADPKD is caused by mutations in either Pkd1 or Pkd2 gene, which encode polycystin-1 (PC1) and polycystin-2 (PC2), respectively. PC1 and PC2 are involved in the development and maintenance of kidney cells, and their mutations can lead to the growth of fluid-filled cysts [ 31 ].
Mutations of Pkd1 gene on chromosome 16p13.3 and Pkd2 gene on chromosome 4q22.1 account for almost 80% and 15% of ADPKD cases. The remaining 5–10% of ADPKD cases are not genetically determined or occur due to rare mutations at other loci [ 31 ]. Some cases of PKD can be explained by mutations in at least one of the endoplasmic reticulum protein-encoding genes. The loss of any of these genes, such as GANAB, DNAJB11, and ALG9, results in the production of non-functional PC1 [ 31 , 32 , 33 ].
GANAB , also known as Pkd3 , encodes the alpha subunit of glucosidase II. The main function of glucosidase II is to promote protein folding by catalyzing the hydrolysis of glucose residues of immature glycoproteins. GANAB mutation can disrupt protein maturation and cell surface localization of PC1 and PC2 [ 34 ]. Studies have shown that GANAB variants cause mild polycystic kidney and liver cysts in most patients [ 35 ]. DNAJB11 is a co-factor of binding immunoglobulin protein (BiP), which is a major chaperone in the endoplasmic reticulum and regulates the folding, trafficking, and degradation of secreted and membrane proteins [ 36 ]. DNAJB11 deletion was shown to impair PC1 maturation and trafficking [ 36 ]. Likewise, heterozygous loss of function mutation of the ALG9 gene, which encodes an enzyme needed for adding specific mannose molecules to produce N-glycan precursors in the endoplasmic reticulum, can impair PC1 maturation and lead to the development of kidney and liver cysts [ 33 ].
Pkd1 or Pkd2 deletion promotes renal tubular cell proliferation, which was shown to be associated with higher intracellular concentrations of Ca 2+37 . PC2 mainly localizes on the endoplasmic reticulum, primary cilia, and plasma membrane, acts as a cation channel, and forms the PC1-PC2 complex in a 1:3 ratio [ 38 , 39 ]. PC2 acts as an ion channel on the plasma membrane and allows a small but detectable Ca 2+ influx in renal primary cilia; therefore, mutated PC2 is deemed to decrease intracellular Ca 2+ concentration [ 40 ]. PC2 acts as a potassium channel in the endoplasmic reticulum to facilitate potassium–calcium counterion exchange for inositol trisphosphate–mediated endoplasmic reticulum Ca 2+ release [ 41 ]. PC2 also directly functions as a calcium-activated, high-conductance ER channel mediating Ca 2+ release from the endoplasmic reticulum [ 42 ], and Pkd2 knockout impairs Ca 2+ release from the endoplasmic reticulum in kidney cells [ 41 ]. In addition, PC1 was shown to decrease Ca 2+ leak from the endoplasmic reticulum and increase endoplasmic reticulum Ca 2+ uptake [ 43 , 44 ]. It has been hypothesized that PC1 may physically block cation transfer by PC2 [ 39 , 45 ]. Membrane depolarization and increased intraciliary Ca 2+ concentration both can activate monovalent cation transfer by PC2 39 . In addition, PC2 is needed for PC1 localization in the cilia, and PC2 deletion not only promotes cystogenesis but also inhibits ciliary localization of PC1 [ 46 ]. Furthermore, Yao et al . reported that Pkd1 knockout can enhance PC2 expression by upregulating GRP94, an endoplasmic reticulum chaperone [ 47 ]. Enhancing Pkd2 expression in Pkd1 -mutant cells may improve PC1 trafficking or promote the formation of heteromeric PC1-PC2 protein complexes (Table 1 and Fig. 1 ) [ 48 ].
The role of PLD-causing genes in cholangiocytes. As shown in the figure, PLD-causing genes are primarily involved in ciliogenesis and quality control of protein folding, transport, and maturation in the endoplasmic reticulum
The morphological assessment of hepatic cyst epithelium in patients with ADPKD illuminated that small (< 1 cm) hepatic cysts had normal epithelium, medium-sized (1–3 cm) hepatic cysts had rare or shortened cilia, and large (> 3 cm) hepatic cysts lacked both primary cilia and microvilli [ 19 ]. Normally, primary cilia are assumed to promote cellular quiescence and delay cell cycle progression to the S or M phase [ 49 ]. In addition, ciliary disassembly was shown to induce cell-cycle reentry [ 49 ]. Consistently, it was shown that decreased ciliogenesis in cancer cells enhances their proliferative capacity and promotes their invasive behavior [ 50 ].
The classical hypothesis for cyst formation claims that in addition to a germline inactivating mutation in one allele of the Pkd gene, there is somatic inactivation (referred to as the second hit) in another allele, causing the complete loss of polycystin expression. However, recent studies claimed that the function of the Pkd gene has a threshold for cystogenesis [ 51 , 52 ]. Based on this hypothesis, complete loss of Pkd1 function is not required, and partial malfunctioning of Pkd1 is enough to induce cystogenesis [ 53 ]. Consistently, many individuals with ADPKD still have residual PC1 expression because they carry missense rather than inactivating mutations [ 54 ]. Thus, promoting the expression of the normal Pkd1 allele may improve ADPKD even in the presence of an abnormal allele. The type of mutation not only determines the development and penetrance of ADPKD but also explains the severity of cystogenesis [ 16 ]. A study with 129 participants with ADPKD revealed that mutation position and mutation type (truncating mutation: nonsense, frameshift, and splicing mutation; or non-truncating mutation: substitution) can affect the severity of hepatic cystogenesis, and patients with PKD1 nonsense mutations exhibit more severe hepatic cystogenesis [ 16 ]. Furthermore, in this study, ADPKD patients with Pkd1 nonsense mutation located closer to the 5ʹ end of Pkd1 gene were more likely to have a maximum diameter index value of hepatic cyst ≥ 6 cm [ 16 ].
Genetic basis of ARPKD
ARPKD is caused by mutations in the polycystic kidney and hepatic disease 1 ( Pkhd1 ) gene, which encodes fibrocystin/polyductin. Different variants of the Pkhd1 gene (missense and truncating mutations) cause most cases of ARPKD. The mRNA of Pkhd1 is alternatively spliced to generate multiple transcripts [ 55 , 56 ]. Pkhd1 knockout was shown to promote cholangiocyte proliferation in vitro [ 57 ] . Furthermore, it was found that Pkhd1 knockout induces connective tissue growth factor (CTGF) production by cholangiocytes, which can induce hepatic fibrosis [ 57 ]. Similar to PC1, fibrocystin forms a complex with PC2 on the plasma membrane and participates in Ca 2+ transfer [ 58 ]. Previously, it was found that the COOH terminal of fibrocystin interacts with the NH2 terminal of PC2. The lack of fibrocystin decreased PC2 expression, but Pkd2 deletion did not alter fibrocystin expression 59 . These findings suggest that fibrocystin binds to PC2 and maintains its normal levels, thereby preventing cystogenesis (Table 1 and Fig. 1 ) [ 59 ].
In another study, it was shown that children with clinically moderate ARPKD had a mutation in the Dzip1l gene [ 60 ]. Similar to the Pkhd1 gene, the Dzip1l gene is involved in ciliogenesis [ 61 ]. Dzip1l deletion downregulated ciliogenesis or led to the formation of dysmorphic cilia in mice [ 61 ] . Dzip1l gene encodes a ciliary transition zone protein that is responsible for ciliary membrane translocation of PC1 and PC2 (Table 1 and Fig. 1 ) [ 60 ].
Genetic basis of ADPLD
Mutations in PRKCSH or Sec63 genes have been implicated in the development of ADPLD [ 62 ]. PRKCSH or Sec63 mutations are found in approximately 40% of patients with isolated ADPLD [ 9 ]. PRKCSH and Sec63 genes encode glucosidase IIβ and SEC63p, respectively, and are involved in endoplasmic reticulum quality control [ 62 ]. They are responsible for carbohydrate processing and folding and translocation of newly synthesized glycoproteins [ 62 ]. As a chaperone-like molecule, glucosidase II binds to the C-terminal domain of PC2 and inhibits Herp-mediated ubiquitination and subsequent degradation of PC2 [ 62 ]. Likewise, PRKCSH or Sec63 deletion was shown to impair normal PC1 folding and accelerate its ubiquitination and proteasomal degradation [ 63 ]. Sec63 conducts the post-translational transport of proteins in the endoplasmic reticulum (Table 1 and Fig. 1 ) [ 64 ]. Consistently, proteasome inhibition by MG132 and carfilzomib, two proteasome inhibitors, markedly upregulated PC1 and promoted cyst-lining cell apoptosis [ 63 ].
Using whole-exome sequencing data from 102 unrelated patients, Choi et al . demonstrated that heterozygous loss of function mutations in 3 additional genes, ALG8 , GANAB , and SEC61B , are also linked to ADPLD [ 32 ]. Using in vitro experiments, they also indicated that similar to PRKCSH and SEC63 , ALG8 , GANAB , and SEC61B are related to protein biogenesis pathway in the endoplasmic reticulum and loss of function mutation of each one of these genes results in defective maturation and trafficking of PC1 (Table 1 and Fig. 1 ) [ 32 ].
A recent study has shown that heterozygous mutations of the low-density lipoprotein receptor-related protein 5 ( LRP5 ) gene, particularly p.R1188W variant, can lead to ADPLD; however, another study reported that some variants of LRP5 , such as rs724159825, can also lead to ADPKD [ 65 , 66 ]. Mechanistically, LRP5 mutations were shown to impair canonical signaling of Wnt3α and promote the expression of several proliferative genes such as adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), and leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5), transcription factor v-myc avian myelocytomatosis viral oncogene homolog (c-Myc), and cyclin D1 (Table 1 and Fig. 1 ) [ 66 ].
Is genetic testing helpful in the diagnosis and treatment of PLD?
Currently, genetic screening is not widely used to confirm ADPKD, ARPKD, and ADPLD as their imaging characteristics and clinical presentations are distinct and there are few differential diagnoses [ 67 ]. On the other hand, already known disease-causing genetic mutations include a wide spectrum and still do not explain a considerable proportion of cases, particularly in ADPLD [ 68 ]. In addition, it has been shown that the affected gene or the type of mutation cannot significantly alter the phenotype of PLD [ 67 ]. Therefore, current guidelines do not recommend routine genetic testing for PLD [ 67 ].
However, genetic testing is not necessary to confirm ADPKD, ARPKD, and ADPLD or enough to rule out these diseases; it may help categorize patients and potentially identify those eligible for future modalities of genetic intervention. Furthermore, a recent study reported that genetic confirmation can predict the risk of hospitalization in both isolated and non-isolated PLD [ 69 ]. Specifically, the study indicated that mutation carriers were significantly younger when waitlisting for liver transplantation and first hospitalization compared to patients without genetic diagnosis; however, current imaging classifications could not differentiate between severe and moderate courses [ 69 ].
Genetic testing can also be helpful when patients come with atypical presentations, which mimic other diseases and make diagnosis complex for clinicians [ 68 ]. In addition, genetic testing is the last resort when patients present with clinical symptoms or complications, but their cyst number in imaging still does not satisfy the diagnostic criteria for ADPLD or ADPKD [ 68 ]. On the other hand, with recent findings and future advances toward the pharmacological and genetic interventions for ADPLD, ADPKD, and ARPKD, genetic testing can allow early diagnosis and management of these diseases. Early diagnosis and management can considerably improve patients’ outcome and prevent serious complications [ 68 ]. Therefore, future studies may define new applications for genetic testing of PLD.
Potential molecular targets for treating PLD
Pkd1 and Pkd2 mutations have been linked to deregulated activation of proliferative signaling pathways. Indeed, decreased intracellular Ca 2+ concentration following impaired function of PC2 is believed to be responsible for activating proliferative pathways 70 . Intracellular Ca 2+ depletion can activate adenylyl cyclase 5, which in turn upregulates intracellular cyclic adenosine monophosphate (cAMP) levels [ 70 ]. Increased cAMP can subsequently overactivate protein kinase A (PKA)/Ras/extracellular signal-regulated kinases (ERK)/hypoxia-inducible factor α (HIF-α) pathway, promote vascular endothelial growth factor A (VEGF-A) expression, and enhance angiogenesis for cholangiocyte proliferation [ 71 , 72 ]. Consistently, adenylyl cyclase 5 inhibition and knockout both significantly reduced hepatic cystogenesis in Pkd knockout mice [ 70 ]. Likewise, VEGF receptor inhibition was shown to inhibit liver cyst growth in pkd2 (WS25/ −) mice [ 73 ], and serum levels of VEGF were positively correlated with total cyst volume but negatively correlated with creatinine clearance in patients with ADPKD [ 74 ]. Moreover, PKA inhibition in liver cyst epithelial cells decreased VEGF expression and ERK1/2 activation [ 71 ]. ERK inhibition also reduced the proliferation of liver cyst epithelial cells [ 71 ].
Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is also aberrantly activated in ADPKD and contributes to epithelial cell proliferation [ 75 , 76 ]. It was shown that JAK2 expression strongly increases in ADPKD and JAK2 blockade reduces cyst growth. JAK2 is a key kinase that most likely contributes to cyst growth by activating STAT as a transcription factor [ 77 ].
Similar to the JAK/STAT signaling pathway, dysregulated mechanistic target of rapamycin (mTOR), Wnt, and Hippo signaling pathways have also been implicated in the pathogenesis of ADPKD. It was shown that the mTOR pathway is abnormally activated in cyst-forming epithelial cells in patients with ADPKD and in the mice model of ADPKD [ 78 ]. Rapamycin, an mTOR inhibitor, was shown to effectively suppress cystogenesis in two mouse models of PKD. Moreover, treatment with rapamycin markedly decreased native polycystic kidney size in patients with ADPKD who received kidney transplants [ 78 ].
Similarly, it has been indicated the lack of PC2 can overactivate the Wnt/β-catenin pathway in murine embryonic fibroblasts, renal epithelia, and isolated collecting duct cells [ 79 ]. In addition, inhibition of the Wnt/β-catenin pathway prevented renal cyst formation and prolonged survival in a mice model of ADPKD [ 79 ]. Similarly, non-canonical Wnt/planar cell polarity (PCP) pathway has been implicated in the proliferative response after Pkhd1 mutation in ARPKD [ 80 ]. Wnt can also bind to the extracellular domain of PC1, thereby inducing PC2-dependent Ca 2+ influx in epithelial cells [ 81 ]. Pathogenic mutations in Pkd1 and Pkd2 were shown to abrogate PC1-PC2 complex formation, reduce cell surface localization of PC1, and hinder PC2 activation by Wnt molecule 81 . Besides, mutations in several PLD-causing genes, such as LRP5 , Sec63 , and Pkhd1, were shown to impair Wnt signaling pathway, which makes it interesting for further investigation [ 66 , 80 , 82 ].
Previously, it has been reported that overactivation of Hippo/Yes-associated protein (YAP) and their transcriptional target four-jointed ( Fjx1 ) is a major driver of cystogenesis in ADPKD [ 83 ]. Consistently, it was shown that simultaneous knockout of Fjx1 decelerates renal fibrosis, alleviates renal inflammation, and preserves renal function in mice with Pkd1 deletion; however , Fjx1 knockout did not markedly inhibit cyst formation [ 84 ].
As PC1-PC2 complex deficiency leads to decreased intracellular Ca 2+ concentration, activation of transient receptor potential vanilloid (Trpv4), a calcium-entry channel in cholangiocytes, has been proposed as a therapeutic option 86 . In-vitro experiments showed that Trpv4 activation increases intracellular Ca 2+ concentration and decreases cholangiocyte proliferation and cyst growth in 3-dimensional culture [ 85 ]. In vivo, Trpv4 activation significantly reduced renal cystic area and non-significantly reduced liver cysts [ 85 ]. Similarly, it was found that Trpv4 activation downregulates cAMP levels and decelerates the progression of ARPKD in rats [ 86 ].
Using tissues from patients with ADPLD and in vivo and in vitro experiments, it was shown that increased HDAC6-mediated ubiquitination and deregulated autophagy of ciliogenic proteins such as ADP-ribosylation factor-like protein 3 (ARL3) and ADP-ribosylation factor-like protein 13B (ARL13B) in cholangiocytes promote hepatic cystogenesis [ 87 , 88 ]. In addition, inhibition of autophagy was shown to promote ciliary localization of ARL3 and ARL13B, recover cholangiocyte ciliogenesis, and inhibit uncontrolled proliferation of cholangiocytes [ 87 , 88 ]. Interestingly, it was indicated that increased autophagic removal of miR-345 potentiates hepatic cystogenesis in PLD [ 89 ]. miR-345 is a non-coding RNA that targets and downregulates cell cycle and proliferation-related genes such as cell division cycle 25A ( CDC25A ), cyclin-dependent kinase 6, E2F transcription factor 2, and proliferating cell nuclear antigen [ 89 ]. These findings point out the importance of autophagy as a therapeutic target in PLD.
Inhibition of protein SUMOylation with S-adenosylmethionine or protein NEDDylation with pevonedistat, as post-translational events, hindered hepatic cystogenesis in the experimental model of PLD [ 90 , 91 ]. Inhibition of autophagy by hydroxychloroquine also suppressed the proliferation of PLD cholangiocytes in vitro and decreased hepatic cystogenesis in a rat model of ADPKD [ 88 ]. Pioglitazone and telmisartan can act as peroxisome proliferator-activated receptor γ (PPAR-γ) agonists. Activating PPAR-γ signaling pathway by pioglitazone or telmisartan reduced liver size and decreased PLD progression in the rat model of ARPKD [ 92 , 93 ]. Previously, it was found that CDC25A is overexpressed in the cholangiocytes of patients with PLD or PKD and in rats with PKD [ 94 ]. Furthermore, Cdc25A ± Pkhd1 del2/del2 mice, with nearly 50% decreased Cdc25A expression, had 33% reduction in liver weight compared with Pkhd1 del2/del2 mice 95 . Consistently, a CDC25A inhibitor like vitamin K3 or PM-20 diminished liver and kidney cystogenesis in Pkd2 WS25/− mice model 95 .
Discovery of new disease-causing mutations and identification of the signaling pathways that mediate cystogenesis can provide new therapeutic targets for PLD.
PLD usually does not impair liver function. Therefore, the latest European Association for the Study of the Liver (EASL) guideline limited the treatment indication to symptomatic patients whose symptoms are attributable to cysts and liver enlargement [ 67 ].
Treatment options available for PLD can be classified into three categories: pharmacological treatment (especially somatostatin analogs), radiological or percutaneous intervention, and surgery [ 67 ]. Since liver size is a prognostic marker in PLD, the efficacy of therapeutic strategies is usually measured by changes in TLV. For this purpose, CT or MRI is the gold standard for liver volume measurement in patients with PLD [ 95 ].
EASL decision-making flowchart suggests somatostatin analogs for PLD patients with numerous scattered small-to-medium-sized cysts. Surgical resection is the treatment of choice if these cysts are clustered in a few liver segments. Aspiration sclerotherapy and cyst fenestration are recommended or a single giant cyst and multiple superficial large cysts, respectively. Finally, liver transplantation may be the last solution for massive PLD that severely affects the quality of life [ 67 ]. Here, we discuss the treatment strategies and the latest evidence.
Pharmacological treatment
Cyclic adenosine monophosphate (cAMP) is a principal regulator of cholangiocyte proliferation and fluid secretion. Octreotide, as a somatostatin analog, binds to the somatostatin receptor, reduces cAMP levels in cholangiocytes and serum, and prevents cyst growth [ 4 ]. Several randomized controlled trials (RCTs) investigated the efficacy of pharmacotherapy, especially long-acting analogs of somatostatin, in patients with PLD (Table 2 ) [ 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 ]. They demonstrated that somatostatin analogs can reduce TLV compared to placebo [ 96 , 97 , 98 , 99 , 100 , 101 ].
In a phase three RCT conducted by van Aerts et al ., 175 PLD patients (as an external manifestation of ADPKD) with at least 2000 mL liver volume were included. The intervention group received 120 mg of lanreotide every 28 days via subcutaneous injection. After 120 weeks, compared with the control group, height-adjusted TLV decreased by 5.91% (95% CI: -9.18 to -2.63; p -value < 0.001); however, the symptom severity score did not significantly differ between the two groups. The main serious adverse event, probably related to lanreotide, was liver cyst infection in 6.5% of patients in the intervention group. They concluded that long-term treatment with lanreotide can reduce liver growth in this setting [ 100 ]. Moreover, this benefit could be seen in short-term therapy with lanreotide [ 96 ]. In another study, changing the lanreotide dose from 90 to 120 mg in non-responders, which was administered subcutaneously every four weeks for one year, stopped the increase in TLV. Thus, the efficacy of lanreotide may be dose-dependent [ 105 ].
A recently published systematic review and meta-analysis on RCTs (mainly administering octreotide 40 mg or lanreotide 120 mg every 28 days with at least a six-month follow-up) confirmed the effectiveness of somatostatin analogs for PLD treatment [ 96 , 97 , 98 , 99 , 100 , 101 , 106 ]. They are associated with a lower liver growth rate (mean difference = -6.37%, 95% CI: -7.90 to -4.84; p -value < 0.001) compared to the control group. This effect is also seen for total kidney volume (mean difference = -3.66%, 95% CI: -5.35 to -1.97; p -value < 0.001). However, they do not significantly affect eGFR decline (mean difference = -0.96 mL/min./1.73 m2, 95% CI: -2.38 to 0.46; p -value = 0.19). Regarding adverse events, biliary complications, gastrointestinal symptoms, and cyst infection occurred more frequently in the somatostatin group than in the control group [ 106 ].
Some studies showed that cessation of treatment (drug holiday) with somatostatin analogs can lead to the recurrence of cyst growth [ 107 , 108 ]. Meanwhile, retreatment with somatostatin analogs after a drug holiday was as effective as the first cycle of treatment regarding TLV reduction. Therefore, intermittent doses of somatostatin analogs can be considered in a subset of patients [ 108 ].
Other drugs also showed a promising potential to reduce liver volume in animal studies, but their efficacy was disappointing in clinical trials [ 103 , 104 ]. In polycystic rats, ursodeoxycholic acid (UDCA) has been shown to stop hepatic cystogenesis by increasing intracellular calcium levels [ 109 ]. However, 24 weeks of treatment with oral UDCA (15–20 mg/kg/day) did not decrease TLV in patients with PLD ( p -value = 0.49). Despite this fact, post hoc analysis showed that in patients with ADPKD, UDCA decreased liver cyst volume growth [ 103 ]. Thus, further studies are needed to evaluate the efficacy of UDCA in PLD. mTOR inhibitors such as everolimus and sirolimus, best known for their roles in cancer therapy and kidney transplant, demonstrated their effectiveness in the preclinical setting [ 78 , 110 , 111 , 112 ]. Nevertheless, clinical trials did not support their efficacy for PLD. An add-on trial showed that the combination of everolimus and octreotide is not superior to octreotide alone in reducing TLV (-3.8% vs. -3.5% respectively, p -value = 0.73) [ 104 ].
As mentioned previously, the number of pregnancies and female gender are associated with the number and size of hepatic cysts in ADPKD [ 17 ]. Estrogen stimulates cholangiocyte proliferation by activating the extracellular signal-regulated kinase (ERK) signaling pathway [ 113 ]. A case report mentioned that in a 59-year-old woman with breast cancer and ADPLD, treatment with tamoxifen, 20 mg once daily for five years, markedly decreased the volume of liver cysts from 311 to 22 mL [ 114 ]. In addition, each year of exposure to estrogen-containing oral contraceptives was associated with 1.45% higher height-adjusted TLV among premenopausal women with PLD [ 115 ]. Moreover, postmenopausal estrogen therapy in women with ADPKD was significantly associated with a selective increase in total liver volume but not with kidney volume [ 116 ]. Furthermore, an ongoing RCT in the Netherlands evaluates the efficacy of a gonadotropin-releasing hormone (GnRH) agonist in pre-menopausal women with PLD (NCT05478083).
Ultimately, gene therapy may be the future landscape for PLD treatment. PC1 is a large membrane glycoprotein, which is too huge to be modified by gene therapy. However, a recently published animal study concluded that only a tiny piece of this protein could be enough to prevent the disease. A transgenic expression of 200 amino acid-long fragment of PC1 dramatically suppressed kidney cystogenesis in a Pkd1 -knockout murine model. This finding opens a new insight into the gene therapy of ADPKD [ 117 ].
Percutaneous or radiological intervention
Cyst aspiration and sclerosis are recommended for PLD patients with a symptomatic large cyst (> 5 cm) [ 95 ]. In this method, the interventionist aspirates cystic fluid and then injects sclerosing agents such as ethanol, tetracycline, or minocycline to destroy the cyst wall epithelium [ 118 , 119 , 120 ]. A systematic review including 526 patients showed that this procedure reduced cyst size by 76%-100% and eliminated the symptoms of PLD in 56%-100% of patients. However, not all patients had PLD, and the recurrence rate was not reported [ 121 ]. Besides, PLD patients usually have multiple cysts, and this method does not apply to most PLD patients.
Transcatheter arterial embolization (TAE) is another percutaneous procedure that utilizes an embolic agent to occlude the supplying arteries [ 122 ]. In a retrospective cohort study with 244 PLD patients, TAE significantly reduced liver volume by 9.2% after one year of the procedure [ 123 ]. Moreover, Yan et al . observed an approximately 15% decrease in TLV in 13 patients with PLD 6–12 months following TAE [ 124 ]. Meanwhile, Yang et al . reported that among 18 PLD patients who underwent TAE, the failure rate was around 70% [ 125 ]. It is why the EASL guideline has not recommended TAE for PLD patients [ 67 ].
Surgical management
For superficial large hepatic cysts, cyst fenestration can be considered in symptomatic PLD patients [ 95 ]. This technique consists of cyst fluid aspiration and surgical deroofing, mostly through laparoscopic surgery. Compared to aspiration sclerotherapy, the main advantage of this method is that multiple cysts can be treated in one session [ 126 ]. In a meta-analysis of 62 studies on patients with or without PLD, symptoms alleviated in 90% of patients after laparoscopic fenestration; however, subgroup analysis showed that symptom recurrence rate and the complication rate are as high as 34% and 29% among patients with PLD, respectively [ 127 ]. Additionally, an ongoing RCT aims to compare the efficacy of aspiration sclerotherapy with laparoscopic fenestration in patients with large symptomatic hepatic cysts (NCT05500157).
When the cysts are limited to a few hepatic segments, hepatic resection can be a therapeutic approach for PLD. However, hepatectomy should only be performed in severely symptomatic patients who are not suitable candidates for liver transplantation [ 67 ]. Although it can remarkably reduce liver volume and relieve symptoms, the morbidity rate is up to 50% [ 126 ]. Among 186 patients with PLD, the mortality rate of surgical treatment was 2.7%, and 21% of patients experienced major complications after dual therapy with hepatectomy and fenestration [ 128 ]. Furthermore, hepatectomy can complicate future liver transplantation since it causes abdominal adhesion [ 129 ].
Finally, the only cure for patients with PLD is liver transplantation. Liver transplantation has a better prognosis in PLD than in chronic liver failure or hepatocellular carcinoma (5-year patient survival rate 85%) [ 130 ]. However, liver transplantation is not commonly used for patients with PLD since the number of liver donors is limited, and PLD is not a medical emergency and has a low mortality rate [ 95 ]. One of the available allocation systems is the model of end-stage liver disease (MELD) score. However, this model has been validated for cirrhosis. In the PLD setting, liver transplantation is considered for patients with extensive PLD whose quality of life is severely affected by the liver disease, or who experience serious complications, such as recurrent cyst infections, portal hypertension, variceal bleeding, and severe malnutrition, and when other interventions fail or are not suitable. Moreover, in patients with creatinine clearance less than 30 ml/min surgeons can consider combined liver and kidney transplantation [ 67 ]. One of the reasons for hepatorenal transplantation in patients with PLD/PKD is malnutrition and cachexia due to the compressive effect of the liver on the stomach. Malnutrition is a dangerous complication of PLD that can be seen in severe cases, especially in cases where there is concurrent renal failure [ 67 , 131 ]. In the study by Coquillard and colleagues, the 5-year survival rate of patients with PLD/PKD who underwent hepatorenal transplantation was 90%. In contrast, the 5-year survival rate of PLD patients who underwent liver transplantation was 77%, and that of patients who underwent hepatorenal transplantation for other reasons was 67%. The authors speculate that the difference in survival between the two groups PLD/PKD and PLD was caused by the difference in transplant indication, as the transplant indication for patients with PLD/PKD was mostly poor renal function [ 131 ].
PLD is caused by different genes and can be observed alone or in combination with PKD. Primarily, PKD occurs due to defective ciliogenesis and ineffective endoplasmic reticulum quality control of ciliogenic proteins. Currently, PLD is mainly diagnosed by imaging and treated by surgical fenestration, resection, and liver transplantation in advanced stages. Future genetic interventions based on recent findings about the genetic basis of PLD may open a new chapter for research and bring hope to patients. An increasing number of studies are now uncovering the genetic basis and subsequent signaling pathways and mechanisms that are responsible for hepatic cystogenesis. Identification of the underlying genetic mutations and subsequent alterations in cellular signaling pathways can help develop new therapeutic options and decrease the need for liver transplantation. In addition, clinical trials have shown that pharmacological intervention might be helpful to some extent, and previous in vivo studies have indicated the involvement of several signaling pathways in the development of PLD. By targeting these signaling pathways, more satisfactory results may be obtained in clinical trials.
Availability of data and materials
Not applicable.
Everson GT. Hepatic cysts in autosomal dominant polycystic kidney disease. Mayo Clin Proc. 1990;65(7):1020–5. https://doi.org/10.1016/s0025-6196(12)65165-9 .
Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):173–80.
Article PubMed Google Scholar
Burgmaier K, Kilian S, Bammens B, et al. Clinical courses and complications of young adults with autosomal recessive polycystic kidney disease (ARPKD). Sci Rep. 2019;9(1):7919.
Article PubMed PubMed Central Google Scholar
Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′, 5′-cyclic monophosphate. Gastroenterology. 2007;132(3):1104–16.
Article CAS PubMed Google Scholar
Dalgaard OZ. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med Scand Suppl. 1957;328:1–255.
CAS PubMed Google Scholar
Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis. 1983;2(6):630–9. https://doi.org/10.1016/s0272-6386(83)80044-4 .
Suwabe T, Chamberlain AM, Killian JM, et al. Epidemiology of autosomal-dominant polycystic liver disease in Olmsted county. JHEP Reports. 2020;2(6): 100166.
Macutkiewicz C, Plastow R, Chrispijn M, et al. Complications arising in simple and polycystic liver cysts. World J Hepatol. 2012;4(12):406.
Van Keimpema L, De Koning DB, Van Hoek B, et al. Patients with isolated polycystic liver disease referred to liver centres: clinical characterization of 137 cases. Liver Int. 2011;31(1):92–8.
Patel A, Chapman AB, Mikolajczyk AE. A practical approach to polycystic liver disease. Clinical liver disease. 2019;14(5):176.
Schönauer R, Baatz S, Nemitz-Kliemchen M, et al. Matching clinical and genetic diagnoses in autosomal dominant polycystic kidney disease reveals novel phenocopies and potential candidate genes. Genet Med. 2020;22(8):1374–83.
Bergmann C. ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr Nephrol. 2015;30(1):15–30. https://doi.org/10.1007/s00467-013-2706-2 .
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–301. https://doi.org/10.1016/s0140-6736(07)60601-1 .
Oh YK, Park HC, Ryu H, Kim Y-C, Oh K-H. Clinical and genetic characteristics of Korean autosomal dominant polycystic kidney disease patients. Korean J Intern Med. 2021;36(4):767.
Article CAS PubMed PubMed Central Google Scholar
Solazzo A, Testa F, Giovanella S, et al. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): A meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS ONE. 2018;13(1): e0190430.
Kataoka H, Watanabe S, Sato M, et al. Predicting liver cyst severity by mutations in patients with autosomal-dominant polycystic kidney disease. Hep Intl. 2021;15:791–803.
Article Google Scholar
Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990;11(6):1033–7.
van Aerts RM, Kievit W, de Jong ME, et al. Severity in polycystic liver disease is associated with aetiology and female gender: results of the International PLD Registry. Liver Int. 2019;39(3):575–82.
Alvaro D, Onori P, Alpini G, et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol. 2008;172(2):321–32.
Hoevenaren IA, Wester R, Schrier RW, et al. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int. 2008;28(2):264–70.
Bergmann C. Genetics of autosomal recessive polycystic kidney disease and its differential diagnoses. Front Pediatr. 2018;5:221.
Dorval G, Boyer O, Couderc A, et al. Long-term kidney and liver outcome in 50 children with autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2021;36:1165–73.
Büscher R, Büscher AK, Weber S, et al. Clinical manifestations of autosomal recessive polycystic kidney disease (ARPKD): kidney-related and non-kidney-related phenotypes. Pediatr Nephrol. 2014;29:1915–25.
Hartung EA, Wen J, Poznick L, Furth SL, Darge K. Ultrasound elastography to quantify liver disease severity in autosomal recessive polycystic kidney disease. J Pediatr. 2019;209:107–15.
Ishiko S, Morisada N, Kondo A, Nagai S, Aoto Y, Okada E, Rossanti R, Sakakibara N, Nagano C, Horinouchi T, Yamamura T, Ninchoji T, Kaito H, Hamada R, Shima Y, Nakanishi K, Matsuo M, Iijima K, Nozu K. Clinical features of autosomal recessive polycystic kidney disease in the Japanese population and analysis of splicing in PKHD1 gene for determination of phenotypes. Clin Exp Nephrol. 2022;26(2):140–53. https://doi.org/10.1007/s10157-021-02135-3 .
Burgmaier K, Kilian S, Bammens B, et al. Clinical courses and complications of young adults with Autosomal Recessive Polycystic Kidney Disease (ARPKD). Scientific reports. May 28 2019;9(1):7919. doi: https://doi.org/10.1038/s41598-019-43488-w
Qian Q, Li A, King BF, et al. Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37(1):164–71.
Barten TR, Bökkerink RAM, Venderink W, Gevers TJ, Ten Broek RP, Drenth JP. Abdominal wall hernia is a frequent complication of polycystic liver disease and associated with hepatomegaly. Liver Int. 2022;42(4):871–8.
Gigot JF, Jadoul P, Que F, et al. Adult polycystic liver disease: is fenestration the most adequate operation for long-term management? Ann Surg. 1997;225(3):286–94. https://doi.org/10.1097/00000658-199703000-00008 .
Schnelldorfer T, Torres VE, Zakaria S, Rosen CB, Nagorney DM. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann Surg. 2009;250(1):112–8. https://doi.org/10.1097/SLA.0b013e3181ad83dc .
Cornec-Le Gall E, Torres VE, Harris PC. Genetic Complexity of Autosomal Dominant Polycystic Kidney and Liver Diseases. J Am Soc Nephrol. 2018;29(1):13–23. https://doi.org/10.1681/asn.2017050483 .
Besse W, Dong K, Choi J, et al. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest. 2017;127(5):1772–85. https://doi.org/10.1172/jci90129 .
Besse W, Chang AR, Luo JZ, et al. ALG9 mutation carriers develop kidney and liver cysts. J Am Soc Nephrol. 2019;30(11):2091–102.
Janssen MJ, Waanders E, Woudenberg J, Lefeber DJ, Drenth JP. Congenital disorders of glycosylation in hepatology: the example of polycystic liver disease. J Hepatol. 2010;52(3):432–40. https://doi.org/10.1016/j.jhep.2009.12.011 .
Delbarba E, Econimo L, Dordoni C, et al. Expanding the variability of the ADPKD-GANAB clinical phenotype in a family of Italian ancestry. J Nephrol. 2022;35(2):645–52. https://doi.org/10.1007/s40620-021-01131-w .
Cornec-Le Gall E, Olson RJ, Besse W, et al. Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am J Hum Genet. 2018;102(5):832–44. https://doi.org/10.1016/j.ajhg.2018.03.013 .
Talbi K, Cabrita I, Schreiber R, Kunzelmann K. Gender-Dependent Phenotype in Polycystic Kidney Disease Is Determined by Differential Intracellular Ca2+ Signals. Int J Mol Sci. 2021;22(11):6019.
Brill AL, Ehrlich BE. Polycystin 2: A calcium channel, channel partner, and regulator of calcium homeostasis in ADPKD. Cell Signal. 2020;66: 109490.
Douguet D, Patel A, Honoré E. Structure and function of polycystins: insights into polycystic kidney disease. Nat Rev Nephrol. 2019;15(7):412–22.
Kleene SJ, Kleene NK. Inward Ca2+ current through the polycystin-2-dependent channels of renal primary cilia. American Journal of Physiology-Renal Physiology. 2021;320(6):F1165–73.
Padhy B, Xie J, Wang R, Lin F, Huang C-L. Channel function of polycystin-2 in the endoplasmic reticulum protects against autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2022;33(8):1501–16.
Koulen P, Cai Y, Geng L, et al. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4(3):191–7.
Weber KH, Lee EK, Basavanna U, et al. Heterologous expression of polycystin-1 inhibits endoplasmic reticulum calcium leak in stably transfected MDCK cells. American Journal of Physiology-Renal Physiology. 2008;294(6):F1279–86.
Hooper K, Boletta A, Germino G, Hu Q, Ziegelstein R, Sutters M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. American Journal of Physiology-Renal Physiology. 2005;289(3):F521–30.
Su Q, Hu F, Ge X, et al. Structure of the human PKD1-PKD2 complex. Science. 2018;361(6406):eaat9819.
Walker RV, Keynton JL, Grimes DT, et al. Ciliary exclusion of Polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nat Commun. 2019;10(1):4072.
Yao Q, Outeda P, Xu H, et al. Polycystin-1 dependent regulation of polycystin-2 via GRP94, a member of HSP90 family that resides in the endoplasmic reticulum. FASEB J. 2021;35(10): e21865.
Lakhia R, Ramalingam H, Chang CM, et al. PKD1 and PKD2 mRNA cis-inhibition drives polycystic kidney disease progression. Nat Commun. Aug 15 2022;13(1):4765. doi: https://doi.org/10.1038/s41467-022-32543-2
Goto H, Inaba H, Inagaki M. Mechanisms of ciliogenesis suppression in dividing cells. Cell Mol Life Sci. 2017;74:881–90.
Kojima R, Hassan E, Ozawa F, et al. Abnormal accumulation of OFD1 in endometrial cancer with poor prognosis inhibits ciliogenesis. Oncol Lett. 2022;24(1):1–9.
Lanktree MB, Haghighi A, di Bari I, Song X, Pei Y. Insights into Autosomal Dominant Polycystic Kidney Disease from Genetic Studies. Clin J Am Soc Nephrol. 2021;16(5):790–9. https://doi.org/10.2215/cjn.02320220 .
Ong AC, Harris PC. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int. 2015;88(4):699–710. https://doi.org/10.1038/ki.2015.207 .
Hajarnis S, Lakhia R, Yheskel M, et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat Commun. Feb 16 2017;8:14395. doi: https://doi.org/10.1038/ncomms14395
Lanktree MB, Guiard E, Akbari P, et al. Patients with Protein-Truncating PKD1 Mutations and Mild ADPKD. Clin J Am Soc Nephrol. 2021;16(3):374–83. https://doi.org/10.2215/cjn.11100720 .
Onuchic LF, Furu L, Nagasawa Y, et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70(5):1305–17. https://doi.org/10.1086/340448 .
Boddu R, Yang C, O’Connor AK, et al. Intragenic motifs regulate the transcriptional complexity of Pkhd1/PKHD1. J Mol Med (Berl). 2014;92(10):1045–56. https://doi.org/10.1007/s00109-014-1185-7 .
Tsunoda T, Kakinuma S, Miyoshi M, et al. Loss of fibrocystin promotes interleukin-8-dependent proliferation and CTGF production of biliary epithelium. J Hepatol. 2019;71(1):143–52.
Wang S, Zhang J, Nauli SM, et al. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27(8):3241–52.
Kim I, Fu Y, Hui K, et al. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19(3):455–68.
Lu H, Galeano MCR, Ott E, et al. Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat Genet. 2017;49(7):1025–34. https://doi.org/10.1038/ng.3871 .
Wang C, Li J, Takemaru K-I, Jiang X, Xu G, Wang B. Centrosomal protein Dzip1l binds Cby, promotes ciliary bud formation, and acts redundantly with Bromi to regulate ciliogenesis in the mouse. Development. 2018;145(6):dev164236.
Gao H, Wang Y, Wegierski T, et al. PRKCSH/80K-H, the protein mutated in polycystic liver disease, protects polycystin-2/TRPP2 against HERP-mediated degradation. Hum Mol Genet. 2010;19(1):16–24.
Fedeles SV, Tian X, Gallagher A-R, et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet. 2011;43(7):639–47.
Jung S-j, Kim H. Emerging view on the molecular functions of Sec62 and Sec63 in protein translocation. Int J Mol Sci. 2021;22(23):12757.
Cnossen WR, Te Morsche RH, Hoischen A, et al. LRP5 variants may contribute to ADPKD. Eur J Hum Genet. 2016;24(2):237–42.
Cnossen WR, te Morsche RH, Hoischen A, et al. Whole-exome sequencing reveals LRP5 mutations and canonical Wnt signaling associated with hepatic cystogenesis. Proc Natl Acad Sci U S A. 2014;111(14):5343–8. https://doi.org/10.1073/pnas.1309438111 .
Liver EAftSot. EASL Clinical Practice Guidelines on the management of cystic liver diseases. J Hepatol. 2022;77(4):1083–108.
Boerrigter MM, Bongers E, Lugtenberg D, Nevens F, Drenth JPH. Polycystic liver disease genes: Practical considerations for genetic testing. Eur J Med Genet. 2021;64(3): 104160. https://doi.org/10.1016/j.ejmg.2021.104160 .
Sierks D, Schönauer R, Friedrich A, et al. Modelling polycystic liver disease progression using age-adjusted liver volumes and targeted mutational analysis. JHEP reports : innovation in hepatology. 2022;4(11): 100579. https://doi.org/10.1016/j.jhepr.2022.100579 .
Spirli C, Mariotti V, Villani A, Fabris L, Fiorotto R, Strazzabosco M. Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease. J Hepatol. 2017;66(3):571–80.
Spirli C, Okolicsanyi S, Fiorotto R, et al. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138(1):360–71 e7.
Spirli C, Morell CM, Locatelli L, et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56(6):2363–74.
Amura CR, Brodsky KS, Groff R, Gattone VH, Voelkel NF, Doctor RB. VEGF receptor inhibition blocks liver cyst growth in pkd2 (WS25/−) mice. Am J Physiol Cell Physiol. 2007;293(1):C419–28.
Reed BY, Masoumi A, Elhassan E, et al. Angiogenic growth factors correlate with disease severity in young patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;79(1):128–34.
Fragiadaki M, Lannoy M, Themanns M, et al. STAT5 drives abnormal proliferation in autosomal dominant polycystic kidney disease. Kidney Int. 2017;91(3):575–86. https://doi.org/10.1016/j.kint.2016.10.039 .
Weimbs T, Olsan EE, Talbot JJ. Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. Jakstat. Apr 1 2013;2(2):e23650. doi: https://doi.org/10.4161/jkst.23650
Patera F, Cudzich-Madry A, Huang Z, Fragiadaki M. Renal expression of JAK2 is high in polycystic kidney disease and its inhibition reduces cystogenesis. Sci Rep. Mar 14 2019;9(1):4491. doi: https://doi.org/10.1038/s41598-019-41106-3
Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103(14):5466–71. https://doi.org/10.1073/pnas.0509694103 .
Li A, Xu Y, Fan S, Meng J, Shen X, Xiao Q, Li Y, Zhang L, Zhang X, Wu G, Liang C, Wu D. Canonical Wnt inhibitors ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI Insight. 2018;3(5):e95874. https://doi.org/10.1172/jci.insight.95874 .
Richards T, Modarage K, Dean C, et al. Atmin modulates Pkhd1 expression and may mediate autosomal recessive polycystic kidney disease (ARPKD) through altered non-canonical Wnt/Planar cell polarity (PCP) signalling. Biochim Biophys Acta Mol Basis Dis. 2019;1865(2):378–90.
Kim S, Nie H, Nesin V, et al. The polycystin complex mediates Wnt/Ca(2+) signalling. Nat Cell Biol. 2016;18(7):752–64. https://doi.org/10.1038/ncb3363 .
Müller L, Funato Y, Miki H, Zimmermann R. An interaction between human Sec63 and nucleoredoxin may provide the missing link between the SEC63 gene and polycystic liver disease. FEBS Lett. 2011;585(4):596–600.
Happé H, van der Wal AM, Leonhard WN, et al. Altered Hippo signalling in polycystic kidney disease. J Pathol. 2011;224(1):133–42.
Formica C, Happé H, Veraar KA, et al. Four-jointed knock-out delays renal failure in an ADPKD model with kidney injury. J Pathol. 2019;249(1):114–25.
Gradilone SA, Masyuk TV, Huang BQ, et al. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology. 2010;139(1):304–14 e2.
Pyrshev K, Stavniichuk A, Tomilin V, et al. TRPV4 functional status in cystic cells regulates cystogenesis in autosomal recessive polycystic kidney disease (ARPKD) during variations in dietary potassium. Physiology. 2023;38(S1):5729507.
Masyuk AI, Masyuk TV, Trussoni CE, Pirius NE, LaRusso NF. Autophagy promotes hepatic cystogenesis in polycystic liver disease by depletion of cholangiocyte ciliogenic proteins. Hepatology. 2022;75(5):1110–22.
Masyuk AI, Masyuk TV, Lorenzo Pisarello MJ, et al. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology. 2018;67(3):1088–108.
Masyuk T, Masyuk A, Trussoni C, et al. Autophagy-mediated reduction of miR-345 contributes to hepatic cystogenesis in polycystic liver disease. JHEP Reports. 2021;3(5): 100345.
Lee-Law PY, Olaizola P, Caballero-Camino FJ, et al. Targeting UBC9-mediated protein hyper-SUMOylation in cystic cholangiocytes halts polycystic liver disease in experimental models. J Hepatol. 2021;74(2):394–406.
Lee-Law PY, Olaizola P, Caballero-Camino FJ, et al. Inhibition of NAE-dependent protein hyper-NEDDylation in cystic cholangiocytes halts cystogenesis in experimental models of polycystic liver disease. UEG Journal. 2021;9(7):848–59.
Yoshihara D, Kugita M, Sasaki M, et al. Telmisartan ameliorates fibrocystic liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. PLoS ONE. 2013;8(12): e81480.
Yoshihara D, Kurahashi H, Morita M, Kugita M, Hiki Y, Aukema HM, Yamaguchi T, Calvet JP, Wallace DP, Nagao S. PPAR-gamma agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300(2):F465–74. https://doi.org/10.1152/ajprenal.00460.2010 .
Masyuk TV, Radtke BN, Stroope AJ, et al. Inhibition of Cdc25A suppresses hepato-renal cystogenesis in rodent models of polycystic kidney and liver disease. Gastroenterology. 2012;142(3):622–33 e4.
van Aerts RM, van de Laarschot LF, Banales JM, Drenth JP. Clinical management of polycystic liver disease. J Hepatol. 2018;68(4):827–37.
van Keimpema L, Nevens F, Vanslembrouck R, et al. LANREOTIDE REDUCES THE VOLUME OF POLYCYSTIC LIVER: A RANDOMIZED, DOUBLE-BLIND, PLACEBOCONTROLLED TRIAL: 56. Hepatology. 2009;50:328A-329A.
Google Scholar
Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–61.
Caroli A, Antiga L, Cafaro M, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5(5):783–9.
Pisani A, Sabbatini M, Imbriaco M, et al. Long-term effects of octreotide on liver volume in patients with polycystic kidney and liver disease. Clin Gastroenterol Hepatol. 2016;14(7):1022–30 e4.
van Aerts RM, Kievit W, D’Agnolo HM, et al. Lanreotide reduces liver growth in patients with autosomal dominant polycystic liver and kidney disease. Gastroenterology. 2019;157(2):481–91 e7.
Hogan MC, Chamberlin JA, Vaughan LE, et al. Pansomatostatin agonist pasireotide long-acting release for patients with autosomal dominant polycystic kidney or liver disease with severe liver involvement: a randomized clinical trial. Clin J Am Soc Nephrol. 2020;15(9):1267–78.
Wijnands TF, Gevers TJ, Lantinga MA, Te Morsche RH, Schultze Kool LJ, Drenth JP. Pasireotide does not improve efficacy of aspiration sclerotherapy in patients with large hepatic cysts, a randomized controlled trial. Eur Radiol. 2018;28:2682–9.
D’Agnolo HM, Kievit W, Takkenberg RB, et al. Ursodeoxycholic acid in advanced polycystic liver disease: a phase 2 multicenter randomized controlled trial. J Hepatol. 2016;65(3):601–7.
Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. J Hepatol. 2013;59(1):153–9.
Temmerman F, Ho TA, Vanslembrouck R, et al. Lanreotide reduces liver volume, but might not improve muscle wasting or weight loss, in patients with symptomatic polycystic liver disease. Clin Gastroenterol Hepatol. 2015;13(13):2353–9 e1.
Suwabe T, Barrera FJ, Rodriguez-Gutierrez R, Ubara Y, Hogan MC. Somatostatin analog therapy effectiveness on the progression of polycystic kidney and liver disease: a systematic review and meta-analysis of randomized clinical trials. PLoS ONE. 2021;16(9): e0257606.
Chrispijn M, Nevens F, Gevers T, et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35(2):266–74.
van Aerts RM, Kolkman M, Kievit W, Gevers TJ, Nevens F, Drenth JP. Drug holiday in patients with polycystic liver disease treated with somatostatin analogues. Ther Adv Gastroenterol. 2018;11:1756284818804784.
Munoz-Garrido P, Marin JJ, Perugorria MJ, et al. Ursodeoxycholic acid inhibits hepatic cystogenesis in experimental models of polycystic liver disease. J Hepatol. 2015;63(4):952–61.
Temmerman F, Chen F, Libbrecht L, et al. Everolimus halts hepatic cystogenesis in a rodent model of polycystic-liver-disease. World J Gastroenterol. 2017;23(30):5499.
Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han: SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant. 2006;21(3):598–604.
Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease J Am Soc Nephrol. 2005;16:46–51.
CAS Google Scholar
Alvaro D, Onori P, Metalli VD, et al. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36(2):297–304.
Aapkes SE, Bernts LHP, van den Berg M, Gansevoort RT, Drenth JPH. Tamoxifen for the treatment of polycystic liver disease: A case report. Medicine (Baltimore). 2021;100(32):e26797. https://doi.org/10.1097/MD.0000000000026797 .
van Aerts RM, Bernts LH, Gevers TJ, et al. Estrogen-containing oral contraceptives are associated with polycystic liver disease severity in premenopausal patients. Clin Pharmacol Ther. 2019;106(6):1338–45.
Sherstha R, McKinley C, Russ P, et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology. 1997;26(5):1282–6.
Onuchic L, Padovano V, Schena G, et al. The C-terminal tail of polycystin-1 suppresses cystic disease in a mitochondrial enzyme-dependent fashion. Nat Commun. 2023;14(1):1790.
Yamada N, Shinzawa H, Ukai K, et al. Treatment of symptomatic hepatic cysts by percutaneous instillation of minocycline hydrochloride. Dig Dis Sci. 1994;39:2503–9.
van Keimpema L, de Koning DB, Strijk SP, Drenth JP. Aspiration–sclerotherapy results in effective control of liver volume in patients with liver cysts. Dig Dis Sci. 2008;53:2251–7.
Moorthy K, Mihssin N, Houghton P. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl. 2001;83(6):409.
CAS PubMed PubMed Central Google Scholar
Wijnands TF, Görtjes AP, Gevers TJ, et al. Efficacy and safety of aspiration sclerotherapy of simple hepatic cysts: a systematic review. Am J Roentgenol. 2017;208(1):201–7.
Ubara Y, Takei R, Hoshino J, et al. Intravascular embolization therapy in a patient with an enlarged polycystic liver. Am J Kidney Dis. 2004;43(4):733–8.
Hoshino J, Ubara Y, Suwabe T, et al. Intravascular embolization therapy in patients with enlarged polycystic liver. Am J Kidney Dis. 2014;63(6):937–44.
Yan J, Zhang J, Yuan K, et al. Transarterial embolisation with bleomycin and N-butyl-2-cyanoacrylate–Lipiodol mixture for symptomatic polycystic liver disease: preliminary experience. Clinical Radiology. 2019;74(12):975. e11–975. e16.
Yang J, Ryu H, Han M, et al. Comparison of volume-reductive therapies for massive polycystic liver disease in autosomal dominant polycystic kidney disease. Hepatol Res. 2016;46(2):183–91.
Drenth J, Chrispijn M, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52(6):2223–30.
Bernts LH, Echternach SG, Kievit W, Rosman C, Drenth JP. Clinical response after laparoscopic fenestration of symptomatic hepatic cysts: a systematic review and meta-analysis. Surg Endosc. 2019;33:691–704.
Chebib FT, Harmon A, Mira MVI, et al. Outcomes and durability of hepatic reduction after combined partial hepatectomy and cyst fenestration for massive polycystic liver disease. J Am Coll Surg. 2016;223(1):118–26 e1.
Baber JT, Hiatt JR, Busuttil RW, Agopian VG. A 20-year experience with liver transplantation for polycystic liver disease: does previous palliative surgical intervention affect outcomes? J Am Coll Surg. 2014;219(4):695–703.
Doshi SD, Bittermann T, Schiano TD, Goldberg DS. Waitlisted candidates with polycystic liver disease are more likely to be transplanted than those with chronic liver failure. Transplantation. 2017;101(8):1838.
Coquillard C, Berger J, Daily M, et al. Combined liver–kidney transplantation for polycystic liver and kidney disease: analysis from the United Network for Organ Sharing dataset. Liver Int. 2016;36(7):1018–25.
Download references
Acknowledgements
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Tracheal Diseases Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
Amir Ali Mahboobipour
Experimental Medicine Research Center, School of Medicine, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Department of Medical Genetics, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Javad Safdari Lord & Arash Yaghoobi
School of Biological Science, Institute for Research in Fundamental Sciences (IPM), Tehran, Iran
Arash Yaghoobi
You can also search for this author in PubMed Google Scholar
Contributions
AAM searched the literature and wrote the draft. MA conceptualized the study, searched the literature, wrote the draft, and critically edited the article. JSL searched the literature and wrote the draft. AY searched the literature and wrote the draft. All authors studied the last version of the article and approved it.
Corresponding author
Correspondence to Moein Ala .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors of this manuscript declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mahboobipour, A.A., Ala, M., Safdari Lord, J. et al. Clinical manifestation, epidemiology, genetic basis, potential molecular targets, and current treatment of polycystic liver disease. Orphanet J Rare Dis 19 , 175 (2024). https://doi.org/10.1186/s13023-024-03187-w
Download citation
Received : 04 July 2023
Accepted : 17 April 2024
Published : 26 April 2024
DOI : https://doi.org/10.1186/s13023-024-03187-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cystogenesis
- Polycystic liver disease
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

The diagnostic value of ultrasound in cystic kidney diseases
Children’s Hospital, University of Duisburg-Essen, Hufelandstr. 55, 45122 Essen, Germany
Birgitta Kranz
Peter f. hoyer.
Renal cysts in childhood can be found in a variety of diseases, which can be congenital or acquired, or renal cysts may be part of a multiorgan disease or restricted to the kidneys only. Ultrasonography is the first-line diagnostic tool and is informative in many cases. However, there is a broad spectrum in the sonographic appearance of renal cysts, and family or genetic studies, a search for extrarenal organ involvement, or additional imaging modalities may be required to make a definitive diagnosis. The aim of this article is to summarize the diagnostic potential and limitations of ultrasonography and depict typical examples of the most important cystic entities.
Introduction
Cysts are defined as spherical, fluid-filled, thin-walled structures that may be single or multiple. With the widespread availability of ultrasound, renal cysts in children can be diagnosed during the mother’s pregnancy or early childhood. There is no universally accepted classification for renal cysts, and according to a recent textbook: “it appears likely that cystic diseases of the kidney will be repeatedly reclassified with future insights into their pathogenesis” [ 1 ]. We have followed a proposal to classify developmental disorders of the kidneys to different stages of nephrogenesis [ 2 ], as follows:.
Renal cystic diseases classified to the time they develop in relation to the stage of nephrogenesis
- Multicystic dysplasia
- As part of syndromes
- With obstruction
- Autosomal recessive
- Autosomal dominant
- Nephronophthisis
- Medullary cystic disease
- Isolated cysts
- Acquired renal cysts
- Cysts within tumors
- Metabolic diseases
It is the aim of this paper to summarize the technical requirements and sonographic images of renal cystic diseases and give examples of entities that should be familiar to the pediatric nephrologist.
Technical requirements
Ultrasound fulfills the requirements of an ideal diagnostic tool in childhood: it does not expose the patient to radiation or contrast media, it can be repeated easily, it does not need patient preconditioning, and it offers good sensitivity and specificity. In addition, ultrasound allows family screening when indicated, with the same advantages. However, standardized and continuous operator training is essential, as it may reduce the “operator dependency” of ultrasound.
The kidneys can be visualized from both sides in a supine position. In older patients, the examination is mostly completed from the back with the patient in the prone position. Because of the well-defined interface to the surrounding tissue, ultrasound identification of cysts allows their visualization down to a size of 1 mm.
Modern ultrasound equipment should include probes suitable to depict the spectrum of renal diseases through the whole pediatric age range. This requires a sector probe and a linear probe of at least 8 MHz for infants and a 4-MHz sector probe for adolescents. Cysts in the kidneys are usually identified with the B-mode scan only, but modern technical facilities such as Doppler or harmonic mode may allow easier orientation or increase sensitivity.
It is important to emphasize that ultrasound in children with renal cysts should not be restricted to the kidneys, because multiorgan involvement in systemic cystic disease or syndromes should always be anticipated and included in the diagnostic workup.
Other imaging modalities may add additional information, such as magnetic resonance imaging (MRI) in extrarenal organ involvement or within interventional studies [ 3 , 4 ]; radiological studies may help depict urological pathologies, such as vesicoureteral reflux; and scintigraphy helps measure renal function [ 5 ]. The growing knowledge of the genetic basis of cystic kidney diseases allows identification of hereditary origin [ 6 ].
Renal cystic diseases
Multicystic renal dysplasia . Multicystic renal dysplasia or multicystic dysplastic kidney is the most common cystic malformation of the kidney among infants. It is found in approximately 1 in 4,000 live births [ 7 ]. On ultrasound, the multicystic kidney typically consists of several variable-sized cysts without identifiable renal parenchyma between these cysts (Fig. 1 a,b). The ureter is atretic in most cases, and the multicystic kidney does not show residual function. Bilateral disease is fatal in the newborn period, but usually only one kidney is affected. Most cases of multicystic renal dysplasia are suspected during prenatal ultrasound examination and should be followed after birth. Ultrasound often allows differentiation from severe hydronephrosis. However, in doubtful cases, scintigraphy to detect residual function or drainage may be indicated [ 5 ]. As involution of the multicystic dysplastic kidney is frequently observed [ 8 ], which can be followed sonographically, routine nephrectomy is not recommended and is reserved for some cases with hypertension or malignant transformation (Fig. 1 c), or cases with an exceptionally large cystic kidney (Fig. 1 d). As even after sonographic involution a remnant of the multicystic kidney can be expected, long-term follow-up seems advisable [ 9 ].

Multicystic dysplasia: a macroscopic appearance; b typical example of sonographic appearance; c multicystic kidney with nephroblastoma ( marked with calipers ); d giant-size multicystic kidney (approximately 900-ml volume) in a 2-year-old girl, crossing the midline (transverse section, * vertebral column)
The contralateral kidney should receive special attention, as this kidney should hypertrophy to guarantee normal renal function and has been shown to have an increased incidence (20–40%) of minor malformations, such as vesicoureteral reflux [ 10 – 12 ]. Usually, multicystic dysplastic kidney is an incidental finding, but familial occurrence has been described [ 13 ].
Renal dysplasia with cysts. Renal dysplasia is a histological entity with undifferentiated parenchyma with or without cysts. Renal histology is characterized by poorly differentiated glomeruli, atypical tubuli, or nonrenal-tissue-like cartilage [ 14 ]. Renal dysplasia can affect one or both kidneys or may be segmental in some cases. Renal function of the affected kidney is more or less reduced; in bilateral cases, progressive loss of function may lead to renal failure. In large series, renal dysplasia is one of the leading causes of end-stage renal failure in childhood [ 15 ]. Therefore, monitoring renal function is more important than repeated ultrasound examinations.
Renal dysplasia with cysts may occur sporadically or as part of a variety of syndromes. A recent textbook of pediatric nephrology lists 78 syndromes, many of which can be suspected after recognition of associated malformations [ 16 ]. Therefore, siblings of some affected children may need to be screened to exclude familial forms of renal dysplasia.
A typical sonographic feature of renal dysplasia is the lack of normal renal architecture, especially the differentiation between cortex and medulla. Echogenicity of a dysplastic kidney is usually enhanced, and cysts may be rare or numerous (Fig. 2 a,b). Sonographic appearance does not necessarily correlate with renal function (Fig. 2 c,d).

Renal dysplasia with cysts: a dysplastic kidney with a single and small cyst (13-year-old boy, creatinine 2.0 mg/dl); b dysplastic kidney with numerous cysts (2-week-old boy, end-stage renal failure within first year of life); c , d dysplastic kidneys with cysts and different function ( c 6-year-old patient, creatinine 0.64 mg/dl; d 5-week-old patient, creatinine 2.0 mg/dl, end-stage renal failure at 6 months)
It should be stressed that a distinct phenotype cannot be expected regularly within the same syndrome, as shown in Fig. 3 a–d. In these three siblings with a mutation of the hepatocyte nuclear factor (HNF)-1β encoded by the TCF2 -gene are shown. The phenotypic differences are obvious between right and left kidney in one patient (Fig. 3 a,b) and between the siblings (Fig. 3 c,d). HNF-1β mutations are found in up to 30% of unselected children with renal dysplasia and are frequently associated with maturity-onset diabetes of the young (MODY) type 5 and urogenital abnormalities [ 17 ].

Phenotypic variability of renal dysplasia and cysts in a family with three siblings and hepatocyte nuclear factor (HNF)1β-mutation (mother has renal cysts, creatinine of 1.5 mg/dl and uterus bicornis): a , b index case (boy, end-stage renal failure within 10 months) ( a multicystic dysplasia on the right side; b dysplasia without cysts on the left side); c sister, creatinine 1.0. mg/dl at the age of 1 year, dysplasia with cysts; d intrauterine enlarged kidneys in a third child, resembling autosomal recessive polycystic kidney disease (ARPKD)

Renal dysplasia with cysts and urinary tract dilation ( CAKUT congenital anomalies of kidney and urinary tract): a , b boy with urethral valves and impaired renal function (creatinine 1.5 mg/dl) at the age of 6 weeks ( a dilated ureters and thickened bladder wall, b dysplastic right kidney with small cysts); c girl with duplex collecting system: the small upper kidney pole shows cystic dysplasia and the lower pole normal parenchyma; d upper kidney pole in a duplex collecting system, with lack of corticomedullary differentiation in the upper pole, indicating dysplasia without cysts
Renal dysplasia with or without cysts in combination with urinary tract abnormalities is called CAKUT (congenital anomalies of kidney and urinary tract) [ 18 – 20 ]. In Fig. 4 a,b, a dysplastic kidney in combination with urethral valves is shown. Figure 4 c, d depicts two children with duplication of the ureter and ureterocele with dysplastic parenchyma of the upper renal pole, in Fig. 4 c with and in Fig. 4 d without cysts.
Autosomal recessive polycystic kidney disease (ARPKD) . Autosomal recessive polycystic kidney disease (ARPKD) is caused by mutations in the PKHD1 -gene on chromosome 6 [ 21 – 23 ]. The clinical spectrum of ARPKD ranges from intrauterine death to early renal failure and hypertension or preserved renal function into adulthood [ 24 ]. In pre- and postnatal ultrasound, the kidneys appear grossly enlarged, with increased echogenicity and reduced corticomedullary differentiation [ 25 , 26 ] (Fig. 5 a–d). Renal cysts are confined to the collecting ducts and are usually so small that their size in infancy is close to the resolution capacity of ultrasound.

Autosomal recessive polycystic kidney disease (ARPKD) in neonates: a 2-week-old boy with typical echogenic spots representing small cysts; b 2-week-old girl with subcapsular brush-like appearance of dilated collecting ducts; c girl with giant-sized kidneys and ARPKD; d same patient as in c : transverse abdominal section with enlarged kidneys touching in the midline (* vertebral column)
In advanced cases, renal cysts may become bigger, and progressive hepatic fibrosis will lead to portal hypertension with splenomegaly (Fig. 6 a–d). Follow-up ultrasound is requested mainly to monitor hepatic fibrosis and portal hypertension.

Autosomal recessive polycystic kidney disease (ARPKD) in advanced cases: a 8-year-old boy with peritoneal dialysis and portal hypertension – left longitudinal view with an enlarged spleen, free abdominal dialysis fluid, and polycystic left kidney; b cholangiodysplasia in the same patient (later received successful combined kidney–liver transplantation); c 9-year-old girl with thickened periportal echogenicity as a sign of periportal fibrosis (transverse liver scan); d numerous macroscopic cysts in the kidney of the same girl (cysts are much larger than in neonates with ARPKD)
The variability of organ involvement in ARPKD is only partially understood [ 27 ] but is in part caused by the combination of mutations in the fibrocystin gene [ 28 ].
Autosomal dominant polycystic kidney disease (ADPKD) . Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of cystic disease, with a frequency of 1 in 800 live births and is caused by mutations in the PKD1 gene on chromosome 16 or the PKD2 gene on chromosome 4 [ 21 ]. With prenatal ultrasound, increased echogenicity of the renal cortex with increased corticomedullary differentiation is often found, but these findings are not specific [ 25 ]. In these cases, a family study is helpful, albeit most cases in childhood will be examined due to a positive family history. Clinical symptoms of ADPKD, as with hypertension and progressive enlargement of the kidneys and renal failure, are mainly seen in adult patients and are more severe in PKD1 -related cases [ 3 ]. In childhood, cyst formation can be detected in increasing number and size, which develop in an apparently normal kidney (Fig. 7 a,b). However, cyst formation is a developing process, and a significant number of children with ADPKD will not show cysts before the second decade of life [ 29 ]. Renal failure is exclusively seen in individuals with severe kidney enlargement [ 30 ].

Typical sonographic image of autosomal dominant polycystic kidney disease (ADPKD): a 10-year-old girl (father ADPKD); b 11-year-old boy (father ADPKD)
In some cases of ADPKD, tuberous sclerosis cannot be distinguished by kidney morphology [ 31 ]. This has been identified as a contiguous gene on chromosome 16 [ 32 ]. These cases have to be diagnosed with additional investigations on extrarenal manifestation, such as brain MRI, to detect cerebral hamartomas [ 5 ].
A special subgroup of patients with ADPKD may exhibit symptoms early in life [ 33 ], and it can be sonographically confused with ARPKD (Fig. 8 a–d). Outcome in this subgroup of children with ADPKD seems to be better than in children with ARPKD [ 34 ]. Classification in dominant or recessive cystic kidney disease therefore always requires family studies.

Autosomal dominant polycystic kidney disease (ADPKD) resembling autosomal recessive polycystic kidney disease (ARPKD): a 3-year-old boy with enlarged kidneys and small cysts; b father (38 years) of patient in a with multiple liver cysts and ADPKD; c 3-week-old girl, oligohydramnios, hypertension, small cysts in large kidneys, with a calculated total kidney volume of 90–100 ml (normal value < 40 ml according to [ 49 ] – mother ADPKD); d isolated spleen cyst in the same girl
Extrarenal manifestation of ADPKD may involve mitral valve ballooning, cerebral aneurysm, or cyst formation of other parenchymal organs, such as the liver, spleen (Fig. 8 b,d), or pancreas [ 35 ]. As in ARPKD, the variability of ADPKD is only partially understood, with genetic, environmental, and hormonal modifiers identified so far [ 27 ]. In families with recessive or dominant forms of polycystic kidney disease, siblings should be screened, albeit prenatal ultrasound is not always conclusive [ 36 ].
Nephronophthisis . The nephronophthisis complex consists of several heterogeneous autosomal recessive diseases in which gene products are linked with cilia or centrosomes [ 37 ]. The sonographic appearance of nephronophthisis at an early stage is nonspecific, with normal-shaped kidneys [ 38 ]. A reduction in corticomedullary differentiation can be detected early. Albeit nephronophthisis is a cystic kidney disease, cysts are seldom found at the initial stage of disease and mostly do not appear before end-stage renal failure is reached (Figs. 9 a–d).

Juvenile nephronophthisis – sonographic appearance: normal or reduced kidney size, enhanced echogenicity of the renal cortex, and reduced corticomedullary differentiation (progressive with renal failure): a 13-year-old boy, creatinine 1.1 mg/dl; b 16-year-old boy (brother of patient in a ), creatinine 2.4 mg/dl; c 16-year-old girl, end-stage renal failure; d 9-year-old boy, end-stage renal failure (cysts are a late sign)
Diagnosis of nephronophthisis with sonography alone is ambiguous, and the final diagnosis is made in combination with the typical clinical signs and extrarenal manifestations and is confirmed by genetic testing [ 39 ].
Medullary cystic kidney disease (MCKD) . MCKD is an autosomal dominant disorder with cystic dilation of the medullary part of the collecting ducts, which presents with gout and hyperuricemia in some cases and usually does not present before adulthood. So far, two loci on chromosomes 1 and 16 have been located. For the latter, mutations in the UMOD gene have been described [ 40 ]. Ultrasound examination is nonspecific, similar to nephronophthisis.
Isolated cysts . Whereas benign cysts are found in up to 50% of the population older than 50 years, it is a rare finding in children [ 41 ]. Isolated cysts may be small or large (Fig. 10 a–d). Multiple or bilateral cysts should always prompt suspicion to ADPKD and initiate family studies or follow-up examinations.

Isolated renal cysts with normal renal function and absence of familiar cystic kidney disease: a 3-month-old boy, isolated cyst of the right kidney – family members normal; b 15-year-old girl, incidental finding of an isolated cyst of the left kidney; c 3-year-old boy, isolated large cyst (size 10 × 9 × 6 cm , approximately 270 ml) ; d same boy as in c after surgery (* shows former cyst)
Acquired cystic kidney disease . Patients on dialysis often develop multiple cysts in their native kidney, even if the underlying disease is not cystic (Fig. 11 ). The pathogenesis of this cyst formation is not clearly understood, but the increased incidence of renal carcinomas in acquired cystic disease requires frequent and life-long monitoring of these kidneys [ 42 , 43 ]. Regular follow-up and computed tomography (CT) or MRI in doubtful cases is advised, and removal of the nonfunctioning kidney should be considered in select cases [ 44 ].

Acquired renal cysts in end-stage renal failure: 16-year-old girl with Alport syndrome and peritoneal dialysis from the age of 2 years
Cysts within tumors . It is of obvious importance to emphasize that tumors might harbor some degree of cystic parenchyma with variable expression [ 45 ]. This includes multilocular cystic nephroma and cystic variants of clear-cell sarcoma, renal cell carcinoma, nephroblastoma, or mesoblastic nephroma and should always be differentiated from benign lesions (Fig. 12 a–f).

Cysts in renal tumors: a 9-year-old girl, small Wilms tumor with cysts; b 4-year-old boy, huge Wilms tumour with cysts of left kidney; c 2-week-old boy, mixed cystic and solid variant of a mesoblastic nephroma; d huge Wilms tumour with multiple cysts (transverse section); e , f 3-year-old boy, large kidney with multiple cysts – not a tumour but cystic dysplasia on histology; e transverse ultrasound, f magnetic resonance imaging
Miscellaneous . Cysts have been described in kidneys of patients with metabolic diseases such as glutaric acidemia type II or carnitine palmitoyltransferase type II deficiency or congenital disorders of glycosylation [ 46 , 47 ].
Ultrasound is convenient to use through the entire pediatric age range, as it does not need sedation or preconditioning and does not expose the child to radiation. Modern equipment allows the diagnosis of nearly all variants of cystic kidney disease. Therefore, ultrasound is the very first diagnostic tool and will guide further diagnostic workup with additional imaging studies or genetic testing. However, knowledge of the phenotypic variety of cystic renal diseases [ 48 ] is essential to correlate the sonographic appearance within the clinical and genetic context. Training in ultrasound, or at least knowledge of sonographic interpretation, is part of the training in pediatric nephrology, and we advise every trainee to concentrate on this rewarding technique for the benefit of their patients.
Acknowledgment
The authors thank R. Kolditz for excellent technical support and C. Bergmann for critically reviewing the manuscript. This work was supported by “Forschungsunterstützungskreis Kindernephrologie e.V.”, Essen, Germany.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 03 June 2024
Cystatin C and the misdiagnosis of CKD in older adults
- Andrew D. Rule ORCID: orcid.org/0000-0003-0338-7784 1 &
- Richard J. Glassock 2
Nature Reviews Nephrology ( 2024 ) Cite this article
85 Accesses
8 Altmetric
Metrics details
- Chronic kidney disease
The use of cystatin C-inclusive equations will continue to propagate the unnecessary overdiagnosis of chronic kidney disease (CKD) in older people. Cystatin C is less biologically specific for CKD than is serum creatinine, inflates the risks of adverse outcomes compared to measured glomerular filtration rate, and does not establish chronicity at a single time point.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Eriksen, B. O. et al. GFR in healthy aging: an individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J. Am. Soc. Nephrol. 31 , 1602–1615 (2020).
Article CAS PubMed PubMed Central Google Scholar
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105 , S117–S314 (2024).
Article Google Scholar
Liu, P. et al. Accounting for age in the definition of chronic kidney disease. JAMA Intern. Med. 181 , 1359–1366 (2021).
Article PubMed PubMed Central Google Scholar
Jonsson, A. J., Lund, S. H., Eriksen, B. O., Palsson, R. & Indridason, O. S. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int. 98 , 1286–1295 (2020).
Article CAS PubMed Google Scholar
Kleeman, S. O. et al. Cystatin C is glucocorticoid responsive, directs recruitment of Trem2+ macrophages, and predicts failure of cancer immunotherapy. Cell Genomics 3 , 100347 (2023).
Keddis, M. T. et al. GFR estimated with creatinine rather than cystatin C is more reflective of the true risk of adverse outcomes with low GFR in kidney transplant recipients. Nephrol. Dial. Transplant. 38 , 1898–1906 (2023).
Rule, A. D., Bailey, K. R., Lieske, J. C., Peyser, P. A. & Turner, S. T. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. 83 , 1169–1176 (2013).
Fu, E. L. et al. Association of low glomerular filtration rate with adverse outcomes at older age in a large population with routinely measured cystatin C. Ann. Intern. Med. 177 , 269–279 (2024).
Article PubMed Google Scholar
Asghar, M. S. et al. Age-based versus young-adult thresholds for nephrosclerosis on kidney biopsy and prognostic implications for CKD. J. Am. Soc. Nephrol. 34 , 1421–1432 (2023).
Denic, A. et al. An improved method for estimating nephron number and the association of resulting nephron number estimates with chronic kidney disease outcomes. J. Am. Soc. Nephrol. 34 , 1264–1278 (2023).
Download references
Acknowledgements
The authors’ work is supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Author information
Authors and affiliations.
Division of Nephrology and Hypertension, Mayo Clinic, Rochester, Minnesota, USA
Andrew D. Rule
Department of Medicine, Geffen School of Medicine at UCLA, Los Angeles, California, USA
Richard J. Glassock
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andrew D. Rule .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rule, A.D., Glassock, R.J. Cystatin C and the misdiagnosis of CKD in older adults. Nat Rev Nephrol (2024). https://doi.org/10.1038/s41581-024-00852-y
Download citation
Published : 03 June 2024
DOI : https://doi.org/10.1038/s41581-024-00852-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
The role of advanced practice nurses in improving healthcare outcomes for patients with chronic kidney disease: A scoping review protocol
- Nozu, Hanako
- Tamura, Haruka
- Kudo, Takemi
- Araki, Tomoko
- Sato, Hidetaka
- Watanabe, Takao
- Sasagawa, Isoji
Introduction The number of patients with chronic kidney disease is increasing worldwide; previous studies have suggested that advanced practice nurses, including nurse practitioners and clinical nurse specialists, with expert practice skills can provide high-quality care and solve complex healthcare problems. In general, nurse practitioners are generalist nurses who work as autonomous clinicians with whole personal care. Clinical nurse specialists, in contrast, are nurses with advanced nursing knowledge and skills for individuals or specific populations. Their roles are independent and different; however, similarities exist in their role in potentially improving healthcare outcomes. Although two previous studies described the role of nephrology nurse practitioners, they were systematic reviews, and their outcomes were limited. To clarify the overall aspect of advanced practice nurses' role, it is necessary to extract the studies illustrating advanced practice nurses' practices for patients with chronic kidney disease. Objective This study aims to map the literature describing the role of advanced practice nurses in improving healthcare outcomes for patients with chronic kidney disease. Materials and methods This scoping review will be conducted using the Joanna Briggs Institute methodology for scoping review. Online databases will be searched across MEDLINE (PubMed), CINAHL (EBSCOhost), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, and Web of Science. Only studies published in English will be included, and no date limit will be set. Chronic kidney disease, renal replacement therapy, and advanced practice nurses as keywords and related search terms will be used. Two independent reviewers will screen the title and abstract/full-text; in case of discrepancy, a third reviewer will make the final decision. The results will be extracted and presented following the review question concerning the study characteristics, patients' characteristics, condition of chronic kidney disease, and role of advanced practice nurses.

IMAGES
VIDEO
COMMENTS
Presentation of Case. Dr. Weizhen Tan: A 19-year-old man was evaluated in the nephrology clinic of this hospital because of kidney cysts. The patient had been well until 3 years before the current ...
Atypical or unilateral polycystic kidney disease is a rare entity that is found incidentally and is characterized on imaging as asymmetric or unilateral distribution of cysts confined to the kidneys. We present a case of an incidental finding of atypical polycystic kidney disease in a 72-year-old male. Computed tomography imaging showed ...
A high-resolution ultrasound exam, MDCT, and MRI all can be useful for the diagnosis of polycystic kidney disease patients but the MRI is considered highly sensitive and specific in some of the recent studies for diagnosis and also a follow-up of kidneys status autosomal dominant polycystic kidney disease. These patients are also at greater ...
In ADPKD cohorts enriched for rapidly progressive disease, such as HALT Progression of Polycystic Kidney Disease (HALT-PKD) study and Consortium for Radiologic Imaging Study of PKD, PKD1 and PKD2 variants account for approximately 78% and 15% of cases. In the remaining approximately 7%, no pathogenic variant was identified 5.By contrast, when ADPKD was studied in a large, unselected cohort ...
Autosomal dominant polycystic kidney disease (ADPKD) is the most common cystic kidney disease in adults and tends to present as innumerable cysts with symmetric involvement of both kidneys. ADPKD, classified as a ciliopathy, progresses to renal insufficiency and end-stage renal failure in 50% of cases [1]. In rare instances, ADPKD can be ...
Renal cystic disease (RCD) refers to a group of pathologic conditions associated with the development of renal cysts. These conditions may present in children and adults with extrarenal symptoms and have genetic or non-inherited etiologies. The renal cysts caused by these conditions are some of the most common renal abnormalities and can vary from being clinically insignificant to resulting in ...
Keywords: Cystic, Kidney disease, Neoplasm AbSTRACT Renal cysts are the lesion which can bother to patient, treating nephrologists and urologist. Spectrum of presentation ranges from benign to malignant, familial to sporadic. In this case, we present a young 34-year-old female who initially came with a large
Genetic studies have revealed a large number of gene mutations that can lead to the development of cystic kidney disease, but interpretation of genetic findings is complex and requires knowledge ...
Cystic kidney disease causes cysts (sacs of fluid) to form in or around the kidneys. There are many types of cystic kidney disease. Some are the result of abnormal genes; others start during fetal development or as a result of kidney failure. Both adults and infants can have cystic kidney disease.
The two main types of polycystic kidney disease, caused by different genetic flaws, are: Autosomal dominant polycystic kidney disease (ADPKD). Signs and symptoms of ADPKD often develop between the ages of 30 and 40. In the past, this type was called adult polycystic kidney disease, but children can develop the disorder.
In adults, renal angiomyolipomas and RCC may have cystic components.2. Polycystic kidney disease- presents with3. High blood pressure Back pain or flank pain. An increase in size of the abdomen Blood in urine Frequent bladder or kidney infection Or may be asymptomatic. A Case Report Of Polycystic Kidney Disease.
Renal function and changes in total kidney volume will be monitored during the follow-up period. Genetic identification of each case of inherited cystic kidney disease will be performed using a targeted gene panel of cystogenesis-related genes, whole exome sequencing (WES) and/or family segregation studies.
During the procedure, it was noted that the left kidney of the female fetus contained approximately six large, simple cysts. The left kidney measured 3.47 cm in length, nearly double the size of the right kidney, which measured 1.91 cm in length. The right kidney appeared sonographically to be normal . During the examination, the sonographer ...
PKD is associated with enlarged kidneys and cyst formation in other parts of the body. In ACKD, the kidneys are normal sized or smaller and cysts do not form in other parts of the body. Between 10 and 20 percent of people with ACKD develop kidney tumors, which in some cases are cancerous. ACKD often has no symptoms.
Introduction. Acquired cystic disease-associated renal cell carcinoma (ACD-RCC) is a new subtype in the light of the 2016 World Health Organization (WHO) classification, which occurred in end-stage renal disease (ESRD) patients ().Dialysis is one of the measures to aid or replace the renal function in ESRD patients, which may result in the development of acquired cystic kidney disease (ACKD ...
Acquired cystic kidney disease (ACKD) is a well-described condition in the adult population, which occurs primarily in patients with end-stage renal disease (ESRD). In contrast to inherited cystic kidney disease, ACKD is characterized by the presence of multiple small cysts in small kidneys. Whereas pediatric data pertaining to the development ...
Editorial on the Research Topic Cystic kidney diseases in children and adults: from diagnosis to etiology and back. Renal cysts are often regarded as the most common abnormality associated with kidney disease (1, 2).They are encountered in both adults and children, as isolated findings or as part of a more complex clinical condition (3-5).Isolated kidney cysts in adults sometimes require ...
Introduction: Pediatric cystic kidney disease (CyKD) includes conditions characterized by renal cysts. Despite extensive research in this field, there are no reliable genetics or other biomarkers to estimate the phenotypic consequences. Therefore, CyKD in children heavily relies on clinical and diagnostic testing to predict the long-term outcomes. Aim: A retrospective study aimed to provide a ...
Studies also suggest that some treatments may slow the rate of kidney disease in PKD, but further research is needed before these treatments can be used in patients. In the meantime, many supportive treatments can be done to control symptoms, help slow the growth of cysts, and prevent or slow down the loss of kidney function in people with PKD.
Inherited cystic kidney disease is a spectrum of disorders in which clusters of renal cysts develop as the result of genetic mutation. The exact methods and pipelines for defining genetic mutations of inherited cystic kidney disease are not clear at this point. This 3-year, prospective, multicenter, cohort study was designed to set up a cohort of Korean patients with inherited cystic kidney ...
1.Introduction. Congenital anomalies of the kidney and urinary tract are the leading cause of end stage renal disease in children, and hydronephrosis is the most common fetal urinary tract anomaly detected by prenatal ultrasound, and it is seen in 0.2-2 % of pregnancies.
The overall objective of this study is to define genes causing cystic kidney disease and to determine the degree to which variants at these cystic kidney disease genes underlie the clinical diversity of these disorders. This will provide an insight into the disease mechanisms, and explore novel mutation screening methods.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of inherited kidney cystic disease and appears as a heterogeneous disease with different progression rates, even within the same family. The assessment of ADPKD progression is critical to allow early initiation of kidney-protective measures and specific treatment. Therefore, surrogate prognostic biomarkers are ...
This study aims to investigate the efficacy and safety of intraoperative real time ultrasound-assisted flexible ureteroscopic holmium laser incision and internal drainage in the treatment of parapelvic cysts, and to review recently published relevant literature. This is a retrospective study in which the clinical data of 47 patients who underwent flexible ureteroscopic holmium laser incision ...
Chronic kidney disease is a disease characterized by progressive damage and loss of function in the kidneys. It's estimated that chronic kidney disease affects about one in seven American adults. ... With polycystic kidney disease (right), fluid-filled sacs called cysts develop in the kidneys. The kidneys grow larger and gradually lose the ...
Clinical presentation and epidemiological characteristics of ADPKD. PKD is a genetic disorder that causes the growth of fluid-filled cysts in the kidneys and damages the surrounding tissues [12, 13].ADPKD is the most common form of PKD and the most frequent hereditary kidney disease, which finally progresses to ESRD [].A meta-analysis of 8 epidemiological studies revealed that the prevalence ...
Medullary cystic kidney disease (MCKD). MCKD is an autosomal dominant disorder with cystic dilation of the medullary part of the collecting ducts, which presents with gout and hyperuricemia in some cases and usually does not present before adulthood. So far, two loci on chromosomes 1 and 16 have been located.
The use of cystatin C-inclusive equations will continue to propagate the unnecessary overdiagnosis of chronic kidney disease (CKD) in older people. Cystatin C is less biologically specific for CKD ...
Introduction The number of patients with chronic kidney disease is increasing worldwide; previous studies have suggested that advanced practice nurses, including nurse practitioners and clinical nurse specialists, with expert practice skills can provide high-quality care and solve complex healthcare problems. In general, nurse practitioners are generalist nurses who work as autonomous ...