Greater Good Science Center • Magazine • In Action • In Education

11 Questions to Ask About COVID-19 Research
Debates have raged on social media, around dinner tables, on TV, and in Congress about the science of COVID-19. Is it really worse than the flu? How necessary are lockdowns? Do masks work to prevent infection? What kinds of masks work best? Is the new vaccine safe?
You might see friends, relatives, and coworkers offer competing answers, often brandishing studies or citing individual doctors and scientists to support their positions. With so much disagreement—and with such high stakes—how can we use science to make the best decisions?
Here at Greater Good , we cover research into social and emotional well-being, and we try to help people apply findings to their personal and professional lives. We are well aware that our business is a tricky one.
Summarizing scientific studies and distilling the key insights that people can apply to their lives isn’t just difficult for the obvious reasons, like understanding and then explaining formal science terms or rigorous empirical and analytic methods to non-specialists. It’s also the case that context gets lost when we translate findings into stories, tips, and tools, especially when we push it all through the nuance-squashing machine of the Internet. Many people rarely read past the headlines, which intrinsically aim to be relatable and provoke interest in as many people as possible. Because our articles can never be as comprehensive as the original studies, they almost always omit some crucial caveats, such as limitations acknowledged by the researchers. To get those, you need access to the studies themselves.
And it’s very common for findings and scientists to seem to contradict each other. For example, there were many contradictory findings and recommendations about the use of masks, especially at the beginning of the pandemic—though as we’ll discuss, it’s important to understand that a scientific consensus did emerge.
Given the complexities and ambiguities of the scientific endeavor, is it possible for a non-scientist to strike a balance between wholesale dismissal and uncritical belief? Are there red flags to look for when you read about a study on a site like Greater Good or hear about one on a Fox News program? If you do read an original source study, how should you, as a non-scientist, gauge its credibility?
Here are 11 questions you might ask when you read about the latest scientific findings about the pandemic, based on our own work here at Greater Good.
1. Did the study appear in a peer-reviewed journal?
In peer review, submitted articles are sent to other experts for detailed critical input that often must be addressed in a revision prior to being accepted and published. This remains one of the best ways we have for ascertaining the rigor of the study and rationale for its conclusions. Many scientists describe peer review as a truly humbling crucible. If a study didn’t go through this process, for whatever reason, it should be taken with a much bigger grain of salt.
“When thinking about the coronavirus studies, it is important to note that things were happening so fast that in the beginning people were releasing non-peer reviewed, observational studies,” says Dr. Leif Hass, a family medicine doctor and hospitalist at Sutter Health’s Alta Bates Summit Medical Center in Oakland, California. “This is what we typically do as hypothesis-generating but given the crisis, we started acting on them.”
In a confusing, time-pressed, fluid situation like the one COVID-19 presented, people without medical training have often been forced to simply defer to expertise in making individual and collective decisions, turning to culturally vetted institutions like the Centers for Disease Control (CDC). Is that wise? Read on.
2. Who conducted the study, and where did it appear?
“I try to listen to the opinion of people who are deep in the field being addressed and assess their response to the study at hand,” says Hass. “With the MRNA coronavirus vaccines, I heard Paul Offit from UPenn at a UCSF Grand Rounds talk about it. He literally wrote the book on vaccines. He reviewed what we know and gave the vaccine a big thumbs up. I was sold.”
From a scientific perspective, individual expertise and accomplishment matters—but so does institutional affiliation.
Why? Because institutions provide a framework for individual accountability as well as safety guidelines. At UC Berkeley, for example , research involving human subjects during COVID-19 must submit a Human Subjects Proposal Supplement Form , and follow a standard protocol and rigorous guidelines . Is this process perfect? No. It’s run by humans and humans are imperfect. However, the conclusions are far more reliable than opinions offered by someone’s favorite YouTuber .
Recommendations coming from institutions like the CDC should not be accepted uncritically. At the same time, however, all of us—including individuals sporting a “Ph.D.” or “M.D.” after their names—must be humble in the face of them. The CDC represents a formidable concentration of scientific talent and knowledge that dwarfs the perspective of any one individual. In a crisis like COVID-19, we need to defer to that expertise, at least conditionally.
“If we look at social media, things could look frightening,” says Hass. When hundreds of millions of people are vaccinated, millions of them will be afflicted anyway, in the course of life, by conditions like strokes, anaphylaxis, and Bell’s palsy. “We have to have faith that people collecting the data will let us know if we are seeing those things above the baseline rate.”
3. Who was studied, and where?
Animal experiments tell scientists a lot, but their applicability to our daily human lives will be limited. Similarly, if researchers only studied men, the conclusions might not be relevant to women, and vice versa.
Many psychology studies rely on WEIRD (Western, educated, industrialized, rich and democratic) participants, mainly college students, which creates an in-built bias in the discipline’s conclusions. Historically, biomedical studies also bias toward gathering measures from white male study participants, which again, limits generalizability of findings. Does that mean you should dismiss Western science? Of course not. It’s just the equivalent of a “Caution,” “Yield,” or “Roadwork Ahead” sign on the road to understanding.
This applies to the coronavirus vaccines now being distributed and administered around the world. The vaccines will have side effects; all medicines do. Those side effects will be worse for some people than others, depending on their genetic inheritance, medical status, age, upbringing, current living conditions, and other factors.
For Hass, it amounts to this question: Will those side effects be worse, on balance, than COVID-19, for most people?
“When I hear that four in 100,000 [of people in the vaccine trials] had Bell’s palsy, I know that it would have been a heck of a lot worse if 100,000 people had COVID. Three hundred people would have died and many others been stuck with chronic health problems.”
4. How big was the sample?
In general, the more participants in a study, the more valid its results. That said, a large sample is sometimes impossible or even undesirable for certain kinds of studies. During COVID-19, limited time has constrained the sample sizes.
However, that acknowledged, it’s still the case that some studies have been much larger than others—and the sample sizes of the vaccine trials can still provide us with enough information to make informed decisions. Doctors and nurses on the front lines of COVID-19—who are now the very first people being injected with the vaccine—think in terms of “biological plausibility,” as Hass says.
Did the admittedly rushed FDA approval of the Pfizer-BioNTech vaccine make sense, given what we already know? Tens of thousands of doctors who have been grappling with COVID-19 are voting with their arms, in effect volunteering to be a sample for their patients. If they didn’t think the vaccine was safe, you can bet they’d resist it. When the vaccine becomes available to ordinary people, we’ll know a lot more about its effects than we do today, thanks to health care providers paving the way.
5. Did the researchers control for key differences, and do those differences apply to you?
Diversity or gender balance aren’t necessarily virtues in experimental research, though ideally a study sample is as representative of the overall population as possible. However, many studies use intentionally homogenous groups, because this allows the researchers to limit the number of different factors that might affect the result.
While good researchers try to compare apples to apples, and control for as many differences as possible in their analyses, running a study always involves trade-offs between what can be accomplished as a function of study design, and how generalizable the findings can be.

The Science of Happiness
What does it take to live a happier life? Learn research-tested strategies that you can put into practice today. Hosted by award-winning psychologist Dacher Keltner. Co-produced by PRX and UC Berkeley’s Greater Good Science Center.
- Apple Podcasts
- Google Podcasts
You also need to ask if the specific population studied even applies to you. For example, when one study found that cloth masks didn’t work in “high-risk situations,” it was sometimes used as evidence against mask mandates.
However, a look beyond the headlines revealed that the study was of health care workers treating COVID-19 patients, which is a vastly more dangerous situation than, say, going to the grocery store. Doctors who must intubate patients can end up being splattered with saliva. In that circumstance, one cloth mask won’t cut it. They also need an N95, a face shield, two layers of gloves, and two layers of gown. For the rest of us in ordinary life, masks do greatly reduce community spread, if as many people as possible are wearing them.
6. Was there a control group?
One of the first things to look for in methodology is whether the population tested was randomly selected, whether there was a control group, and whether people were randomly assigned to either group without knowing which one they were in. This is especially important if a study aims to suggest that a certain experience or treatment might actually cause a specific outcome, rather than just reporting a correlation between two variables (see next point).
For example, were some people randomly assigned a specific meditation practice while others engaged in a comparable activity or exercise? If the sample is large enough, randomized trials can produce solid conclusions. But, sometimes, a study will not have a control group because it’s ethically impossible. We can’t, for example, let sick people go untreated just to see what would happen. Biomedical research often makes use of standard “treatment as usual” or placebos in control groups. They also follow careful ethical guidelines to protect patients from both maltreatment and being deprived necessary treatment. When you’re reading about studies of masks, social distancing, and treatments during the COVID-19, you can partially gauge the reliability and validity of the study by first checking if it had a control group. If it didn’t, the findings should be taken as preliminary.
7. Did the researchers establish causality, correlation, dependence, or some other kind of relationship?
We often hear “Correlation is not causation” shouted as a kind of battle cry, to try to discredit a study. But correlation—the degree to which two or more measurements seem connected—is important, and can be a step toward eventually finding causation—that is, establishing a change in one variable directly triggers a change in another. Until then, however, there is no way to ascertain the direction of a correlational relationship (does A change B, or does B change A), or to eliminate the possibility that a third, unmeasured factor is behind the pattern of both variables without further analysis.
In the end, the important thing is to accurately identify the relationship. This has been crucial in understanding steps to counter the spread of COVID-19 like shelter-in-place orders. Just showing that greater compliance with shelter-in-place mandates was associated with lower hospitalization rates is not as conclusive as showing that one community that enacted shelter-in-place mandates had lower hospitalization rates than a different community of similar size and population density that elected not to do so.
We are not the first people to face an infection without understanding the relationships between factors that would lead to more of it. During the bubonic plague, cities would order rodents killed to control infection. They were onto something: Fleas that lived on rodents were indeed responsible. But then human cases would skyrocket.
Why? Because the fleas would migrate off the rodent corpses onto humans, which would worsen infection. Rodent control only reduces bubonic plague if it’s done proactively; once the outbreak starts, killing rats can actually make it worse. Similarly, we can’t jump to conclusions during the COVID-19 pandemic when we see correlations.
8. Are journalists and politicians, or even scientists, overstating the result?
Language that suggests a fact is “proven” by one study or which promotes one solution for all people is most likely overstating the case. Sweeping generalizations of any kind often indicate a lack of humility that should be a red flag to readers. A study may very well “suggest” a certain conclusion but it rarely, if ever, “proves” it.
This is why we use a lot of cautious, hedging language in Greater Good , like “might” or “implies.” This applies to COVID-19 as well. In fact, this understanding could save your life.
When President Trump touted the advantages of hydroxychloroquine as a way to prevent and treat COVID-19, he was dramatically overstating the results of one observational study. Later studies with control groups showed that it did not work—and, in fact, it didn’t work as a preventative for President Trump and others in the White House who contracted COVID-19. Most survived that outbreak, but hydroxychloroquine was not one of the treatments that saved their lives. This example demonstrates how misleading and even harmful overstated results can be, in a global pandemic.
9. Is there any conflict of interest suggested by the funding or the researchers’ affiliations?
A 2015 study found that you could drink lots of sugary beverages without fear of getting fat, as long as you exercised. The funder? Coca Cola, which eagerly promoted the results. This doesn’t mean the results are wrong. But it does suggest you should seek a second opinion : Has anyone else studied the effects of sugary drinks on obesity? What did they find?
It’s possible to take this insight too far. Conspiracy theorists have suggested that “Big Pharma” invented COVID-19 for the purpose of selling vaccines. Thus, we should not trust their own trials showing that the vaccine is safe and effective.
But, in addition to the fact that there is no compelling investigative evidence that pharmaceutical companies created the virus, we need to bear in mind that their trials didn’t unfold in a vacuum. Clinical trials were rigorously monitored and independently reviewed by third-party entities like the World Health Organization and government organizations around the world, like the FDA in the United States.
Does that completely eliminate any risk? Absolutely not. It does mean, however, that conflicts of interest are being very closely monitored by many, many expert eyes. This greatly reduces the probability and potential corruptive influence of conflicts of interest.
10. Do the authors reference preceding findings and original sources?
The scientific method is based on iterative progress, and grounded in coordinating discoveries over time. Researchers study what others have done and use prior findings to guide their own study approaches; every study builds on generations of precedent, and every scientist expects their own discoveries to be usurped by more sophisticated future work. In the study you are reading, do the researchers adequately describe and acknowledge earlier findings, or other key contributions from other fields or disciplines that inform aspects of the research, or the way that they interpret their results?

Greater Good’s Guide to Well-Being During Coronavirus
Practices, resources, and articles for individuals, parents, and educators facing COVID-19
This was crucial for the debates that have raged around mask mandates and social distancing. We already knew quite a bit about the efficacy of both in preventing infections, informed by centuries of practical experience and research.
When COVID-19 hit American shores, researchers and doctors did not question the necessity of masks in clinical settings. Here’s what we didn’t know: What kinds of masks would work best for the general public, who should wear them, when should we wear them, were there enough masks to go around, and could we get enough people to adopt best mask practices to make a difference in the specific context of COVID-19 ?
Over time, after a period of confusion and contradictory evidence, those questions have been answered . The very few studies that have suggested masks don’t work in stopping COVID-19 have almost all failed to account for other work on preventing the disease, and had results that simply didn’t hold up. Some were even retracted .
So, when someone shares a coronavirus study with you, it’s important to check the date. The implications of studies published early in the pandemic might be more limited and less conclusive than those published later, because the later studies could lean on and learn from previously published work. Which leads us to the next question you should ask in hearing about coronavirus research…
11. Do researchers, journalists, and politicians acknowledge limitations and entertain alternative explanations?
Is the study focused on only one side of the story or one interpretation of the data? Has it failed to consider or refute alternative explanations? Do they demonstrate awareness of which questions are answered and which aren’t by their methods? Do the journalists and politicians communicating the study know and understand these limitations?
When the Annals of Internal Medicine published a Danish study last month on the efficacy of cloth masks, some suggested that it showed masks “make no difference” against COVID-19.
The study was a good one by the standards spelled out in this article. The researchers and the journal were both credible, the study was randomized and controlled, and the sample size (4,862 people) was fairly large. Even better, the scientists went out of their way to acknowledge the limits of their work: “Inconclusive results, missing data, variable adherence, patient-reported findings on home tests, no blinding, and no assessment of whether masks could decrease disease transmission from mask wearers to others.”
Unfortunately, their scientific integrity was not reflected in the ways the study was used by some journalists, politicians, and people on social media. The study did not show that masks were useless. What it did show—and what it was designed to find out—was how much protection masks offered to the wearer under the conditions at the time in Denmark. In fact, the amount of protection for the wearer was not large, but that’s not the whole picture: We don’t wear masks mainly to protect ourselves, but to protect others from infection. Public-health recommendations have stressed that everyone needs to wear a mask to slow the spread of infection.
“We get vaccinated for the greater good, not just to protect ourselves ”
As the authors write in the paper, we need to look to other research to understand the context for their narrow results. In an editorial accompanying the paper in Annals of Internal Medicine , the editors argue that the results, together with existing data in support of masks, “should motivate widespread mask wearing to protect our communities and thereby ourselves.”
Something similar can be said of the new vaccine. “We get vaccinated for the greater good, not just to protect ourselves,” says Hass. “Being vaccinated prevents other people from getting sick. We get vaccinated for the more vulnerable in our community in addition for ourselves.”
Ultimately, the approach we should take to all new studies is a curious but skeptical one. We should take it all seriously and we should take it all with a grain of salt. You can judge a study against your experience, but you need to remember that your experience creates bias. You should try to cultivate humility, doubt, and patience. You might not always succeed; when you fail, try to admit fault and forgive yourself.
Above all, we need to try to remember that science is a process, and that conclusions always raise more questions for us to answer. That doesn’t mean we never have answers; we do. As the pandemic rages and the scientific process unfolds, we as individuals need to make the best decisions we can, with the information we have.
This article was revised and updated from a piece published by Greater Good in 2015, “ 10 Questions to Ask About Scientific Studies .”
About the Authors
Jeremy Adam Smith
Uc berkeley.
Jeremy Adam Smith edits the GGSC’s online magazine, Greater Good . He is also the author or coeditor of five books, including The Daddy Shift , Are We Born Racist? , and (most recently) The Gratitude Project: How the Science of Thankfulness Can Rewire Our Brains for Resilience, Optimism, and the Greater Good . Before joining the GGSC, Jeremy was a John S. Knight Journalism Fellow at Stanford University.
Emiliana R. Simon-Thomas
Emiliana R. Simon-Thomas, Ph.D. , is the science director of the Greater Good Science Center, where she directs the GGSC’s research fellowship program and serves as a co-instructor of its Science of Happiness and Science of Happiness at Work online courses.
You May Also Enjoy

Why Is COVID-19 Killing So Many Black Americans?

In a Pandemic, Elbow Touches Might Keep Us Going

How to Form a Pandemic Pod

How Does COVID-19 Affect Trust in Government?

Why Your Sacrifices Matter During the Pandemic

How to Keep the Greater Good in Mind During the Coronavirus Outbreak

Conducting research during the COVID-19 pandemic
Advice from psychological researchers on protecting participants, animals and research plans.

COVID-19 is not just altering everyday life; it’s also upending psychological research. As universities and colleges across the country go virtual, researchers are scrambling to protect their human participants and animal subjects, their scholarship and their careers.
“The research that will be affected first are studies that involve bringing groups of people together in close proximity,” says Jeff Zacks, PhD, of Washington University in St. Louis, who chairs APA’s Board of Scientific Affairs (BSA). “But this is going to slow everybody down for 2020.”
To mitigate the impact, Zacks, his fellow BSA members and other experts offer the following advice.
Prepare to work remotely
Check in with your program officer, modify your research and analysis, protect your human participants and animal subjects, cross-train staff, maintain communication with your team, do the things that you never have time for, support junior colleagues, keep things in perspective.
For information about how the pandemic will affect existing and future research awards and other information about National Institutes of Health (NIH) research, see the NIH FAQ and NIH’s information for applicants and recipients of NIH funding .
For similar information about National Science Foundation (NSF) research, see the NSF FAQ .
The Council on Government Relations is compiling a list of institutional and agency responses to the pandemic.
Have an idea for research about preventing or treating COVID-19? See NSF’s Dear Colleague Letter about how to submit a research proposal.
Additional Information
- Psychologist leads innovative approach to tackle psychological toll of COVID-19
- COVID-19 isn’t just a danger to older people’s physical health
Contact APA Office of Public Affairs
You may also like.
Office of the Dean for Research

COVID-19 Research Proposals
Princeton University has authorized funding to support faculty research projects that consider biomedical, health-related and fundamental science related to the COVID pandemic, as well as those that impact corresponding policy, social, and economic topics.
Read the full details in this email from Dean for Research Pablo Debenedetti . Proposals were due by 11 p.m. on Sunday, April 5, 2020 and awards were announced on Friday, April 10, 2020.

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 16 June 2020
COVID-19 impact on research, lessons learned from COVID-19 research, implications for pediatric research
- Debra L. Weiner 1 , 2 ,
- Vivek Balasubramaniam 3 ,
- Shetal I. Shah 4 &
- Joyce R. Javier 5 , 6
on behalf of the Pediatric Policy Council
Pediatric Research volume 88 , pages 148–150 ( 2020 ) Cite this article
147k Accesses
83 Citations
19 Altmetric
Metrics details
The COVID-19 pandemic has resulted in unprecedented research worldwide. The impact on research in progress at the time of the pandemic, the importance and challenges of real-time pandemic research, and the importance of a pediatrician-scientist workforce are all highlighted by this epic pandemic. As we navigate through and beyond this pandemic, which will have a long-lasting impact on our world, including research and the biomedical research enterprise, it is important to recognize and address opportunities and strategies for, and challenges of research and strengthening the pediatrician-scientist workforce.
The first cases of what is now recognized as SARS-CoV-2 infection, termed COVID-19, were reported in Wuhan, China in December 2019 as cases of fatal pneumonia. By February 26, 2020, COVID-19 had been reported on all continents except Antarctica. As of May 4, 2020, 3.53 million cases and 248,169 deaths have been reported from 210 countries. 1
Impact of COVID-19 on ongoing research
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical research, or redirected research to COVID-19. Most clinical trials, except those testing life-saving therapies, have been paused, and most continuing trials are now closed to new enrollment. Ongoing clinical trials have been modified to enable home administration of treatment and virtual monitoring to minimize participant risk of COVID-19 infection, and to avoid diverting healthcare resources from pandemic response. In addition to short- and long-term patient impact, these research disruptions threaten the careers of physician-scientists, many of whom have had to shift efforts from research to patient care. To protect research in progress, as well as physician-scientist careers and the research workforce, ongoing support is critical. NIH ( https://grants.nih.gov/policy/natural-disasters/corona-virus.htm ), PCORI ( https://www.pcori.org/funding-opportunities/applicant-and-awardee-faqs-related-covid-19 ), and other funders acted swiftly to provide guidance on proposal submission and award management, and implement allowances that enable grant personnel to be paid and time lines to be relaxed. Research institutions have also implemented strategies to mitigate the long-term impact of research disruptions. Support throughout and beyond the pandemic to retain currently well-trained research personnel and research support teams, and to accommodate loss of research assets, including laboratory supplies and study participants, will be required to complete disrupted research and ultimately enable new research.
In the long term, it is likely that the pandemic will force reallocation of research dollars at the expense of research areas funded prior to the pandemic. It will be more important than ever for the pediatric research community to engage in discussion and decisions regarding prioritization of funding goals for dedicated pediatric research and meaningful inclusion of children in studies. The recently released 2020 National Institute of Child Health and Development (NICHD) strategic plan that engaged stakeholders, including scientists and patients, to shape the goals of the Institute, will require modification to best chart a path toward restoring normalcy within pediatric science.
COVID-19 research
This global pandemic once again highlights the importance of research, stable research infrastructure, and funding for public health emergency (PHE)/disaster preparedness, response, and resiliency. The stakes in this worldwide pandemic have never been higher as lives are lost, economies falter, and life has radically changed. Ultimate COVID-19 mitigation and crisis resolution is dependent on high-quality research aligned with top priority societal goals that yields trustworthy data and actionable information. While the highest priority goals are treatment and prevention, biomedical research also provides data critical to manage and restore economic and social welfare.
Scientific and technological knowledge and resources have never been greater and have been leveraged globally to perform COVID-19 research at warp speed. The number of studies related to COVID-19 increases daily, the scope and magnitude of engagement is stunning, and the extent of global collaboration unprecedented. On January 5, 2020, just weeks after the first cases of illness were reported, the genetic sequence, which identified the pathogen as a novel coronavirus, SARS-CoV-2, was released, providing information essential for identifying and developing treatments, vaccines, and diagnostics. As of May 3, 2020 1133 COVID-19 studies, including 148 related to hydroxychloroquine, 13 to remdesivir, 50 to vaccines, and 100 to diagnostic testing, were registered on ClinicalTrials.gov, and 980 different studies on the World Health Organization’s International Clinical Trials Registry Platform (WHO ICTRP), made possible, at least in part, by use of data libraries to inform development of antivirals, immunomodulators, antibody-based biologics, and vaccines. On April 7, 2020, the FDA launched the Coronavirus Treatment Acceleration Program (CTAP) ( https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap ). On April 17, 2020, NIH announced a partnership with industry to expedite vaccine development ( https://www.nih.gov/news-events/news-releases/nih-launch-public-private-partnership-speed-covid-19-vaccine-treatment-options ). As of May 1, 2020, remdesivir (Gilead), granted FDA emergency use authorization, is the only approved therapeutic for COVID-19. 2
The pandemic has intensified research challenges. In a rush for data already thousands of manuscripts, news reports, and blogs have been published, but to date, there is limited scientifically robust data. Some studies do not meet published clinical trial standards, which now include FDA’s COVID-19-specific standards, 3 , 4 , 5 and/or are published without peer review. Misinformation from studies diverts resources from development and testing of more promising therapeutic candidates and has endangered lives. Ibuprofen, initially reported as unsafe for patients with COVID-19, resulted in a shortage of acetaminophen, endangering individuals for whom ibuprofen is contraindicated. Hydroxychloroquine initially reported as potentially effective for treatment of COVID-19 resulted in shortages for patients with autoimmune diseases. Remdesivir, in rigorous trials, showed decrease in duration of COVID-19, with greater effect given early. 6 Given the limited availability and safety data, the use outside clinical trials is currently approved only for severe disease. Vaccines typically take 10–15 years to develop. As of May 3, 2020, of nearly 100 vaccines in development, 8 are in trial. Several vaccines are projected to have emergency approval within 12–18 months, possibly as early as the end of the year, 7 still an eternity for this pandemic, yet too soon for long-term effectiveness and safety data. Antibody testing, necessary for diagnosis, therapeutics, and vaccine testing, has presented some of the greatest research challenges, including validation, timing, availability and prioritization of testing, interpretation of test results, and appropriate patient and societal actions based on results. 8 Relaxing physical distancing without data regarding test validity, duration, and strength of immunity to different strains of COVID-19 could have catastrophic results. Understanding population differences and disparities, which have been further exposed during this pandemic, is critical for response and long-term pandemic recovery. The “Equitable Data Collection and Disclosure on COVID-19 Act” calls for the CDC (Centers for Disease Control and Prevention) and other HHS (United States Department of Health & Human Services) agencies to publicly release racial and demographic information ( https://bass.house.gov/sites/bass.house.gov/files/Equitable%20Data%20Collection%20and%20Dislosure%20on%20COVID19%20Act_FINAL.pdf )
Trusted sources of up-to-date, easily accessible information must be identified (e.g., WHO https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov , CDC https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html , and for children AAP (American Academy of Pediatrics) https://www.aappublications.org/cc/covid-19 ) and should comment on quality of data and provide strategies and crisis standards to guide clinical practice.
Long-term, lessons learned from research during this pandemic could benefit the research enterprise worldwide beyond the pandemic and during other PHE/disasters with strategies for balancing multiple novel approaches and high-quality, time-efficient, cost-effective research. This challenge, at least in part, can be met by appropriate study design, collaboration, patient registries, automated data collection, artificial intelligence, data sharing, and ongoing consideration of appropriate regulatory approval processes. In addition, research to develop and evaluate innovative strategies and technologies to improve access to care, management of health and disease, and quality, safety, and cost effectiveness of care could revolutionize healthcare and healthcare systems. During PHE/disasters, crisis standards for research should be considered along with ongoing and just-in-time PHE/disaster training for researchers willing to share information that could be leveraged at time of crisis. A dedicated funded core workforce of PHE/disaster researchers and funded infrastructure should be considered, potentially as a consortium of networks, that includes physician-scientists, basic scientists, social scientists, mental health providers, global health experts, epidemiologists, public health experts, engineers, information technology experts, economists and educators to strategize, consult, review, monitor, interpret studies, guide appropriate clinical use of data, and inform decisions regarding effective use of resources for PHE/disaster research.
Differences between adult and pediatric COVID-19, the need for pediatric research
As reported by the CDC, from February 12 to April 2, 2020, of 149,760 cases of confirmed COVID-19 in the United States, 2572 (1.7%) were children aged <18 years, similar to published rates in China. 9 Severe illness has been rare. Of 749 children for whom hospitalization data is available, 147 (20%) required hospitalization (5.7% of total children), and 15 of 147 required ICU care (2.0%, 0.58% of total). Of the 95 children aged <1 year, 59 (62%) were hospitalized, and 5 (5.3%) required ICU admission. Among children there were three deaths. Despite children being relatively spared by COVID-19, spread of disease by children, and consequences for their health and pediatric healthcare are potentially profound with immediate and long-term impact on all of society.
We have long been aware of the importance and value of pediatric research on children, and society. COVID-19 is no exception and highlights the imperative need for a pediatrician-scientist workforce. Understanding differences in epidemiology, susceptibility, manifestations, and treatment of COVID-19 in children can provide insights into this pathogen, pathogen–host interactions, pathophysiology, and host response for the entire population. Pediatric clinical registries of COVID-infected, COVID-exposed children can provide data and specimens for immediate and long-term research. Of the 1133 COVID-19 studies on ClinicalTrials.gov, 202 include children aged ≤17 years. Sixty-one of the 681 interventional trials include children. With less diagnostic testing and less pediatric research, we not only endanger children, but also adults by not identifying infected children and limiting spread by children.
Pediatric considerations and challenges related to treatment and vaccine research for COVID-19 include appropriate dosing, pediatric formulation, and pediatric specific short- and long-term effectiveness and safety. Typically, initial clinical trials exclude children until safety has been established in adults. But with time of the essence, deferring pediatric research risks the health of children, particularly those with special needs. Considerations specific to pregnant women, fetuses, and neonates must also be addressed. Childhood mental health in this demographic, already struggling with a mental health pandemic prior to COVID-19, is now further challenged by social disruption, food and housing insecurity, loss of loved ones, isolation from friends and family, and exposure to an infodemic of pandemic-related information. Interestingly, at present mental health visits along with all visits to pediatric emergency departments across the United States are dramatically decreased. Understanding factors that mitigate and worsen psychiatric symptoms should be a focus of research, and ideally will result in strategies for prevention and management in the long term, including beyond this pandemic. Social well-being of children must also be studied. Experts note that the pandemic is a perfect storm for child maltreatment given that vulnerable families are now socially isolated, facing unemployment, and stressed, and that children are not under the watch of mandated reporters in schools, daycare, and primary care. 10 Many states have observed a decrease in child abuse reports and an increase in severity of emergency department abuse cases. In the short term and long term, it will be important to study the impact of access to care, missed care, and disrupted education during COVID-19 on physical and cognitive development.
Training and supporting pediatrician-scientists, such as through NIH physician-scientist research training and career development programs ( https://researchtraining.nih.gov/infographics/physician-scientist ) at all stages of career, as well as fostering research for fellows, residents, and medical students willing to dedicate their research career to, or at least understand implications of their research for, PHE/disasters is important for having an ongoing, as well as a just-in-time surge pediatric-focused PHE/disaster workforce. In addition to including pediatric experts in collaborations and consortiums with broader population focus, consideration should be given to pediatric-focused multi-institutional, academic, industry, and/or government consortiums with infrastructure and ongoing funding for virtual training programs, research teams, and multidisciplinary oversight.
The impact of the COVID-19 pandemic on research and research in response to the pandemic once again highlights the importance of research, challenges of research particularly during PHE/disasters, and opportunities and resources for making research more efficient and cost effective. New paradigms and models for research will hopefully emerge from this pandemic. The importance of building sustained PHE/disaster research infrastructure and a research workforce that includes training and funding for pediatrician-scientists and integrates the pediatrician research workforce into high-quality research across demographics, supports the pediatrician-scientist workforce and pipeline, and benefits society.
Johns Hopkins Coronavirus Resource Center. Covid-19 Case Tracker. Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html (2020).
US Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. FDA News Release . https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (2020).
Evans, S. R. Fundamentals of clinical trial design. J. Exp. Stroke Transl. Med. 3 , 19–27 (2010).
Article Google Scholar
Antman, E. M. & Bierer, B. E. Standards for clinical research: keeping pace with the technology of the future. Circulation 133 , 823–825 (2016).
Food and Drug Administration. FDA guidance on conduct of clinical trials of medical products during COVID-19 public health emergency. Guidance for Industry, Investigators and Institutional Review Boards . https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency (2020).
National Institutes of Health. NIH clinical trials shows remdesivir accelerates recovery from advanced COVID-19. NIH New Releases . https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19#.XrIX75ZmQeQ.email (2020).
Radcliffe, S. Here’s exactly where we are with vaccines and treatments for COVID-19. Health News . https://www.healthline.com/health-news/heres-exactly-where-were-at-with-vaccines-and-treatments-for-covid-19 (2020).
Abbasi, J. The promise and peril of antibody testing for COVID-19. JAMA . https://doi.org/10.1001/jama.2020.6170 (2020).
CDC COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. Morb. Mortal Wkly Rep . 69 , 422–426 (2020).
Agarwal, N. Opinion: the coronavirus could cause a child abuse epidemic. The New York Times . https://www.nytimes.com/2020/04/07/opinion/coronavirus-child-abuse.html (2020).
Download references
Author information
Authors and affiliations.
Department of Pediatrics, Division of Emergency Medicine, Boston Children’s Hospital, Boston, MA, USA
Debra L. Weiner
Harvard Medical School, Boston, MA, USA
Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
Vivek Balasubramaniam
Department of Pediatrics and Division of Neonatology, Maria Fareri Children’s Hospital at Westchester Medical Center, New York Medical College, Valhalla, NY, USA
Shetal I. Shah
Division of General Pediatrics, Children’s Hospital Los Angeles, Los Angeles, CA, USA
Joyce R. Javier
Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
You can also search for this author in PubMed Google Scholar
Contributions
All authors made substantial contributions to conception and design, data acquisition and interpretation, drafting the manuscript, and providing critical revisions. All authors approve this final version of the manuscript.
Pediatric Policy Council
Scott C. Denne, MD, Chair, Pediatric Policy Council; Mona Patel, MD, Representative to the PPC from the Academic Pediatric Association; Jean L. Raphael, MD, MPH, Representative to the PPC from the Academic Pediatric Association; Jonathan Davis, MD, Representative to the PPC from the American Pediatric Society; DeWayne Pursley, MD, MPH, Representative to the PPC from the American Pediatric Society; Tina Cheng, MD, MPH, Representative to the PPC from the Association of Medical School Pediatric Department Chairs; Michael Artman, MD, Representative to the PPC from the Association of Medical School Pediatric Department Chairs; Shetal Shah, MD, Representative to the PPC from the Society for Pediatric Research; Joyce Javier, MD, MPH, MS, Representative to the PPC from the Society for Pediatric Research.
Corresponding author
Correspondence to Debra L. Weiner .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Pediatric Policy Council are listed below Author contributions.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Weiner, D.L., Balasubramaniam, V., Shah, S.I. et al. COVID-19 impact on research, lessons learned from COVID-19 research, implications for pediatric research. Pediatr Res 88 , 148–150 (2020). https://doi.org/10.1038/s41390-020-1006-3
Download citation
Received : 07 May 2020
Accepted : 21 May 2020
Published : 16 June 2020
Issue Date : August 2020
DOI : https://doi.org/10.1038/s41390-020-1006-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Catalysing global surgery: a meta-research study on factors affecting surgical research collaborations with africa.
- Thomas O. Kirengo
- Hussein Dossajee
- Nchafatso G. Obonyo
Systematic Reviews (2024)
Lessons learnt while designing and conducting a longitudinal study from the first Italian COVID-19 pandemic wave up to 3 years
- Alvisa Palese
- Stefania Chiappinotto
- Carlo Tascini
Health Research Policy and Systems (2023)
Pediatric Research and COVID-19: the changed landscape
- E. J. Molloy
- C. B. Bearer
Pediatric Research (2022)
Cancer gene therapy 2020: highlights from a challenging year
- Georgios Giamas
- Teresa Gagliano
Cancer Gene Therapy (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
A proposal for long-term COVID-19 control
Download the full working paper
Subscribe to Global Connection
Universal vaccination, prophylactic drugs, rigorous mitigation, and international cooperation, william a. haseltine william a. haseltine chair and ceo - access health international, trustee - the brookings institution @wmhaseltine.
August 20, 2021
Introduction
Four successive waves of COVID-19 have buffeted the United States for the past year and a half. With each wave, we have bet on different measures to push us through: First, public health measures, then drugs and treatments, and now, with our fifth wave, we hold out hope for vaccine-led recovery. But from the outset, we have underestimated this virus and its ability to maneuver the public health battleground; it is escaping the best defenses we are able to muster and finding new avenues of attack.
In this paper, I propose a multimodal strategy for long-term COVID control, one that sets up multiple barriers of protection so that we are able to not only contain SARS-CoV-2 and eliminate COVID-19 as a major life-threatening disease, but also return to a new social and economic life. The strategy uses the best of what we have on hand today—a rapidly growing arsenal of vaccines and antiviral drugs and public health measures— with an eye towards future improvements and developments.
The most immediate priority should be supporting additional research on the molecular biology of SARS-CoV-2, of which we still know surprisingly little. This is particularly important since there is great likelihood that COVID-19 will become endemic . Unlike the viruses that cause smallpox or polio, SARS-CoV-2 has demonstrated an impressive ability to adapt and thrive in both humans and animals, including our much-loved pet, cats and dogs. Even if we can eliminate the disease from our own communities, it is unlikely we can do the same across the globe and for all our animal populations at the same time.
The best we can hope for is containment of COVID-19 at levels we can tolerate both personally and economically. We have to use all the tools we have at our disposal— being aware of the inequities and disparities from country to country and within countries—that have made this and other diseases so hard to address.
Related Content
John Nkengasong
January 21, 2021
Matthew M. Kavanagh, David Dollar
June 7, 2021
Philip Schellekens
April 2, 2021
Global Economy and Development
AEI, Washington DC
12:45 pm - 1:45 pm EDT
Janice C. Eberly, Carol Graham, Stephen Kissler, Jón Steinsson
June 20, 2024
Online only
9:00 am - 10:15 am EDT
Research proposals to address COVID-19 challenges sought
Washington University’s McDonnell International Scholars Academy and Social Policy Institute (SPI) seek proposals from WashU researchers and their international partners to identify and address the challenges of COVID-19 through artificial intelligence, technology and big data.
This is the second year the Social Policy Institute and McDonnell Academy have partnered to provide seed grants for international research to capitalize on the strengths of both institutions and to further establish Washington University as a leader in global research.
Leaders anticipate providing funding for up to three proposals at $25,000 each in this round, with support from the Mastercard Impact Fund , in collaboration with the Mastercard Center for Inclusive Growth. Proposals are due Feb. 26. For more information, visit the McDonnell International Scholars Academy site .
Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.
You Might Also Like
Latest from the Record
Announcements.
Parking shares latest update
Staff leadership program applications due May 31
Peace Park planting May 18
Eleven alumni earn Fulbright awards
University members selected for Focus St. Louis leadership class
High school teachers join WashU faculty in the lab
Leah Rae Czerniewski, biomedical engineering doctoral student, 34
Ruth Levinsohn Siteman, philanthropist, 92
Stan H. Braude, professor of practice in Arts & Sciences, 62
Research Wire
Surprising phosphate finding in asteroid sample
Chen awarded two Scialog grants to study the molecular basis of cognition
OpenAI awards grant to improve machine learning models
The View From Here
Washington people.
Amy Zhou
Sadie Williams Clayton
Caitlyn Collins
Who Knew WashU?
Who Knew WashU? 1.27.21
Who Knew WashU? 1.13.21
Who Knew WashU? 12.9.20
Global Health (GHWG)
Content from global health (ghwg) working group, do you want to write a covid dissertation.

Professor Sophie Harman, a member of our Global Health Working Group, gives some advice about coming up with dissertation topics related to COVID.
Part of the joy and point of writing a dissertation is for students to come up with their own subject and research question. Both students and supervisors know this is often the most painful part of the process (second only to the week before deadline – start early, marathon not a sprint etc!). I know good supervisors can support students writing dissertations in all manner of subjects and this is what makes it so rewarding. However, in a year where we’re all dealing with increased pressure, demands on our time, and managing screen headaches, I thought I’d put my 15 years global health politics experience to good use and make some suggestions/pointers to help you when a student comes to you as says the inevitable: [1]
‘I was thinking of writing my dissertation on COVID-19’
Below are 10 suggested questions with suggested literature and methods, covering institutions, security, race, policy, vaccines, gender, aesthetics, expertise, knowledge. These by no means cover everything and by no means prescribe how I think a dissertation on that topic should be written. If helpful, see them as jump-off points to think about these topics. The only caution I have is make sure all projects are only focused on the start/first 6 months of COVID-19 – we are only at the end of the beginning. This is also a pre-emptive move to stop you getting your students to email me for ideas.
Institutions and global governance
1. Is the WHO capable of preventing and responding to major pandemics?
Literature: WHO, IHR, GOARN, global health security + previous outbreaks (Ebola, pandemic flu, HIV/AIDS)
Methods: Case Studies – look at the tools/instruments e.g. IHR, GOARN, Regional offices etc
2. Why did states pursue different responses to the COVID-19 outbreak?
Literature: Global health security, state compliance in IR, international law and international organisations
Methods: Pick two contrasting case studies e.g. England/Scotland, Canada/US, Germany/UK, Sweden/Denmark and then look at different levels of policy and decision making per chapter – Global, National, Regional/local and rationales behind decisions from – expert evidence, speeches, policy decisions, policy timelines
3. How can we understand the gender dimensions of COVID-19?
Literature: Gender and global health, Feminist IPE, Black Feminism, WPS (if looking at violence)
Methods: Explore 1 – 3 key themes from the literature – Care and domestic burden, Health Care Workers, Domestic violence in depth. Depending on networks and contacts, could run focus groups (ethics! And definitely NOT if doing violence), or analyse survey data – lots of surveys done on this and the raw data is always made available if have the skills to play with it.
Political economy
4. Are states the main barrier to vaccine equity?
Literature: Vaccine access and nationalism, access to treatment, IPE of health and trade, pharmaceutical companies, Bill and Melinda Gates Foundation
Methods: Look at the different stages of vaccine development for 2/3 trials and consider the role of States (where putting money, public statements, any actions e.g. email hacks), Researchers (where get money from, how collaborating, knowledge sharing), Institutions (CEPI, GAVI, WHO), and the Private Sector (pharma and foundations – who’s investing, what is their return – and private security companies – who protects the commodity?). Think: interests, investment, barriers/opportunities.
Security and foreign policy
5. Were state security strategies prepared for major pandemics prior to COVID-19? If not, why not?
Literature: Global health security, securitisation and desecuritisation of health
Methods: 2 – 3 state case studies or 1 in detail, think about Strategy, Training/Preparedness, Actors. Content analysis of security strategies and defence planning and budget allocations, speeches, training, simulations etc.
6. What is the role of images in responding to outbreaks?
Literature: Aesthetics and IR, behaviour change communication and images in public health
Methods: 3 case studies on different types of images in COVID-19, e.g. 1. Global public health messaging; 2. National public health messaging; 3. Community Expression – OR pick one of these options and explore in depth.
Race and racism
7. Could the racial inequalities of COVID-19 been foreseen and prevented?
Literature: Racism and global health, racism and domestic health systems, Black Feminism, Critical Trans Politics
Method: Option 1 – look maternal health as a proxy in three case study countries e.g. Brazil, US, UK; Option 2 – pick one country and look at three health issues prior to COVID-19 e.g. Maternal Health, Diabetes, Heart Disease.
Knowledge, discourse, and experts
8. Is COVID-19 the biggest global pandemic of a generation?
Literature: Postcolonial/decolonial theory, poststructuralism, Politics of HIV/AIDS, pandemic flu
Method: Discourse analysis around ‘once in a lifetime rhetoric’ – who says it, when, and why; contrast with discourse around COVID-19 from countries with previous outbreaks e.g. Sierra Leone, DRC, China, Indonesia, South Africa, Brazil (you’ll need to be selective as can’t measure discourse from all states! Think through why you make your choices here and how they relate to each other) OR contrast COVID-19 with a previous pandemic, e.g. HIV/AIDS
9. What knowledge counts in COVID-19?
Literature: Postcolonial/decolonial theory, post-structuralism, IR and Global Health, politics of experts
Methods: Review lessons learned from previous outbreaks (there are lots of source material on this after Ebola and SARS for example) and how they led to changes/what learned for COVID-19; Stakeholder mapping and/or network analysis – Who are the experts? Look at backgrounds, types of knowledge and expertise, did they work on the Ebola response/HIV/AIDS in the early 2000s for example?; Case Study – UK/US – where have high concentration of public health experts and institutions, export knowledge to low and middle income countries, evidence of importing knowledge from these countries, especially given the experience?
UK/State responses
10. How can we understand/explain the first 6 months of the US/UK/Sweden/Australia/South Africa/China/Brazil/you choose! response to COVID-19?
WARNING! This is the question that could descend into a polemic so approach with absolute caution. I would strongly advise against, but have included to give a clearer steer.
The key with this question is to remember you are not submitting a public health or epidemiology dissertation, so bear in mind you probably don’t have the skills and knowledge to assess what was a good/bad public health decision (other than obvious ones such as PPE stocks for example). What you do have the skills to do is to look at the politics as to why a decision was taken and how it was taken – investigate what the different recommendations/guidance suggested, who followed/ignored/subverted it and what outcomes this produced.
Literature: health policy, public policy, state compliance IR
Methods: 1. Global – map what global advice there was and how did the state follow (or not) in preparedness and response and what was the rationale for doing so – political circumstances at the time, stated rationale for decision, who was making decision; 2. National – key public health decisions, commodities, social-economic consequences – how were these planned for/overlooked and why. To look at these two levels may require mixed methods of global and national policy timelines, stakeholder analysis, content analysis of speeches and recommendations, mapping changes to data presentation and access.
[1] For the first two years of my career I supervised countless projects loosely based around ‘Is the War in Iraq illegal?’ I’m hoping some of the variety here will stop two years of ‘Is the UK government’s respond to COVID-19 a national scandal?’ or ‘Is the WHO fit for purpose?’ – two great topics, but tiresome after a bit.
Reproduced with kind permission from Global Politics Unbound at QMU.
Photo by iMattSmart on Unsplash
BISA is entirely self-funded
Your donations help us to support the International Studies community. Choose to donate towards free memberships for Global South scholars, conference bursaries or student experience events. Then receive updates on how your donation has helped.
- Executive Committee
- Membership Terms and Conditions
- Working group end of year report 2022/23
- Anti-bribery and corruption policy
- Anti-money laundering and counter-terrorism policy
- Anti-slavery and human trafficking policy
- Code of Conduct
- Complaints procedure
- Conflict of interest policy
- Constitution - Charitable Incorporated Organisation
- Cookie policy
- Data retention policy
- Duties of trustees
- Donations, sponsorship and fundraising policy
- Equality and diversity policy
- Political campaigning and lobbying
- Privacy standard
- Statement on academic freedom
- Working group guidance
- Global South countries
- Main in-person conference
- Virtual conference
- Face-to-Face Activity Fund - Working groups
- Early Career Small Research Grants
- Founders Fund application form
- Learning and Teaching Small Grant
- Working group end of year report
- Working group grant application
- Astropolitics Working Group
- Equality, diversity and inclusion policy
- Best Article in the Review of International Studies Prize
- Distinguished Contribution Prize nomination form
- Equality, Diversity and Inclusion Prize nomination form
- L.H.M. Ling Outstanding First Book Prize nomination form
- Michael Nicholson Thesis Prize nomination form
- New Voices In Cultural Relations Prize nomination form
- Susan Strange Best Book Prize nomination form
- Working Group of the Year Prize nomination form
- Nomination form for teaching prizes
- Early Career Excellence In Teaching International Studies Prize
- Excellence In Teaching International Studies By A Postgraduate Student Prize
- Review of International Studies
- European Journal of International Security
- Our book series
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

The impact of COVID-19 on research
a Department of Pediatric Urology and Pediatric Surgery, Hopital Pellegrin-Enfants, CHU Bordeaux, France
b Service de chirurgie et urologie pédiatrique, hôpital Lapeyronie, CHU de Montpellier et Université de Montpellier, France
G.M.A. Beckers
c Department of Urology, Section of Pediatric Urology, AmsterdamUMC, Location VUmc, Amsterdam, the Netherlands
d Indiana University, 702 Barnhill Drive, Suite 4230, Indianapolis, IN, USA
A.J. Nieuwhof-Leppink
e Department of Medical Psychology and Social Work, Urology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, PO box 85090, 3508 AB, Utrecht, the Netherlands
Magdalena Fossum
f Department of Pediatric Surgery, Copenhagen University Hospital Rigshospitalet, DK-2100, Denmark
g Department of Women's and Children's Health, Bioclinicum, Floor 10, Karolinska Institutet, SE-171 76, Stockholm, Sweden
K.W. Herbst
h Division of Urology, Department of Research, Connecticut Children's Medical Center, Hartford, CT, USA
i Hospital for Sick Chidlren, Univeristy of Toronto, Canada
Coronavirus disease 2019 (COVID-19) has swept across the globe causing hundreds of thousands of deaths, shutting down economies, closing borders and wreaking havoc on an unprecedented scale. It has strained healthcare services and personnel to the brink in many regions and will certainly deeply mark medical research both in the short and long-term.
Prior to the COVID pandemic, virology research (including influenza) represented less than 2% of all biomedical research. However, the number of laboratories and investigators that have pivoted to address COVID related research questions is astonishing, likely comprising 10–20% of current biomedical investigation, showing the incredible adaptability of the research community [ 1 ]. The multinational support rapidly infused for COVID-19 research is in the billions of euros [ 2 ]. The sharing of research findings and research data has never been as rapid and efficient [ 3 ]. The crisis has also brought disease, health, and healthcare back to the forefront of societal issues, and will have a lasting impact on public spending. However, with all this optimism and focus, there is a downside.
To begin, the COVID-19 crisis has led to a massive influx of publications. Not only are specialty journals being flooded with submissions by authors being unwittingly granted much needed writing time, but publications on COVID have literally inundated us. More than 20,000 papers have been published since December 2019, many in prestigious journals. There are also an increasing number of studies being uploaded to preprint servers, such as BioRxiv, for rapid dissemination prior to any peer review. However, we cannot assume that the time and quality available for peer review is able to keep pace with the explosion of publication. There is need for increased caution in the wake of this massive influx of submissions, especially since we are increasingly seeing these results being picked up by the media and diffused to a less attuned audience. In recent weeks, several prestigious journals, including the Lancet and the New England Journal of Medicine, have published retractions of earlier and potentially major COVID-related findings [ 4 , 5 ]. On June 15, 2020, The New York Times highlighted potential lapses in the peer review process affecting major scientific journals [ 6 ].
We must strive to improve scientific quality always. The current debate over the use of hydroxychloroquine further illustrates the undermining of the scientific process when faced with global desperation for ready-made truths and solutions [ 4 , 7 , 8 ]. Science needs time, and good science needs a lot of it for data to grow and knowledge to evolve, but this process is ill-prepared to handle the rush for solutions to the COVID crises.
Moreover, just as COVID-19 has shown social, racial, and economic health disparities, the pandemic seems also to have accentuated existing gender inequalities within the field of research [ 9 ]. Indeed, early analyses suggest that female academics are publishing less and starting fewer research projects than their male peers. This might be an effect of the lockdown and the fact that more women than are men are juggling caring for families and children despite both “working” from home [ 10 , 11 ].
Travel, social, and funding restrictions will also take a serious toll on scientific research worldwide. Research staff and resources have been purposely and purposefully prioritized to COVID-19 activities above all else. Distancing and transmission issues have caused most non-COVID clinical research to be suspended, causing a reduction in recruitment of research subjects and a delay in data entry into clinical trial databases [ 12 ]. Research-related hiring has been suspended because of travel restrictions and young researchers might soon find themselves out of a job if their subject is not the pandemic. Indeed, though government-funded medical research bodies worldwide say they are committed to maintaining the continuity and breadth of biomedical research, how the economic downfall will influence government spending remains to be seen. Furthermore, research funding that relies on public fundraising is expected to drop substantially and many researchers will see a significant decrease in funding opportunities [ 13 ]. The global impact the crisis will have on the economy makes it hard to imagine that future research funding will not be substantially affected.
During this crisis, many resources were understandably redirected toward preparing for and caring for COVID-19 patients, but the collateral damage to so many patients with non-COVID-19 medical conditions that did not receive, or failed to seek, treatment will surely emerge [ 14 ]. Finally, children have also paid a high price for the redirecting of medical resources, with delays in their medical and surgical management, as well as vaccinations [ 15 , 16 ]. This may be especially problematic when many aspects of pediatric care is based on their developmental clock, which even the pandemic cannot stop. Whether this was the best option will certainly be analyzed in retrospect. Congenital anomalies alone account for over 400,000 deaths worldwide every year, and inflict a considerable burden both on children, families, and healthcare systems [ 17 ]. Thus, it is essential that funding for medical research does not follow the same pattern with a disproportionate decrease in funding for non-COVID research including pediatric and developmental urology.
COVID-19 has already changed the world, not only because of the disease itself, but because of the long-term effects of the world's reaction to the pandemic. While the pandemic may have brought with it some silver linings, it is crucial that the scientific community conduct current and future research broadly and openly, lest future pandemic preparedness in research repeat the hard-fought lessons of today.

Research Proposal and Award Operations During COVID-19 Pandemic
The Sponsored Programs Office remains operational and continues to process proposals and manage awards. Proposals, awards and outgoing subawards related to COVID-19 are being prioritized.
To ensure COVID-19 related actions get prioritized, please ensure the following:
- Proposals: The PI must submit projects using Cayuse, include “ COVID-19 ” in the project title, provide budget/budget justification and certify the proposal in Cayuse. PI’s should also send an email to [email protected] identifying the proposal as addressing COVID-19.
- Awards/Award Agreements: SPO will prioritize negotiation of COVID-19 awards if we are on notice that the award is for COVID-19. Health System Contracts will negotiate/process industry sponsored human clinical trials. SPO will review/negotiate all other clinical trials, including award coming from industry sponsors using federal funds. PI’s should also send an email to [email protected] identifying the proposal as addressing COVID-19.
- Outgoing subawards: “COVID-19” should be in the title of the project.
Agency specific updated on submission deadlines:
- NIH released a notice that for all proposals with March and April deadlines may be submitted any time through May 1
- NSF published a list of funding opportunities for which they have extended the deadline
- USDA describes their deadline extensions here
- DOE guidance on submission deadlines can be found here
- The Department of Defense recommends speaking directly to program officers to request an extension, or check FOA amendments for agency-issued extensions
Related to COVID-19
You might also like.

Quick Links

Office Units

Frontiers welcomes research proposals about COVID-19
An outbreak of a respiratory disease began in Wuhan, China in December 2019 and the causative agent was discovered in January 2020 to be a novel betacoronovirus of the same subgenus as SARS-CoV and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronavirus disease 2019 (COVID-19) has rapidly disseminated worldwide, with clinical manifestations ranging from mild respiratory symptoms to severe pneumonia and a fatality rate estimated around 2%. Person to person transmission is occurring both in the community and healthcare settings. The World Health Organization (WHO) has recently declared the COVID-19 epidemic a public health emergency of international concern. The ongoing outbreak presents many clinical and public health management challenges due to limited understanding of viral pathogenesis, risk factors for infection, natural history of disease including clinical presentation and outcomes, prognostic factors for severe illness, period of infectivity, modes and extent of virus inter-human transmission, as well as effective preventive measures and public health response and containment interventions. There are no antiviral treatment nor vaccine available but fast track research and development efforts including clinical therapeutic trials are ongoing across the world. Managing this serious epidemic requires the appropriate deployment of limited human resources across all cadres of health care and public health staff, including clinical, laboratory, managerial and epidemiological data analysis and risks assessment experts. Therefore, integrated operational research and intervention, learning from experiences across different fields and settings should contribute towards better understanding and managing COVID-19. This Research Topic aims to highlight interdisciplinary research approaches deployed during the COVID-19 epidemic, addressing knowledge gaps and generating evidence for its improved management and control. It will incorporate critical, theoretically informed and empirically grounded original research contributions using diverse approaches, experimental, observational and intervention studies, conceptual framing, expert opinions and reviews from across the world. The Research Topic proposes a multi-dimensional approach to improving the management of COVID-19 with scientific contributions from all areas of virology, immunology, clinical microbiology, epidemiology, therapeutics, as well as infection prevention and public health risk assessment and management studies. Submissions are welcome for the following article types: original research, review, mini-reviews, systematic reviews, research protocol, opinion and hypothesis. We particularly welcome contributions that include, but are not limited to, the following topics: • SARS-CoV-2 genome structure, encoded proteins, replication properties, viral pathogenesis, comparative phylogenetic and viral receptor binding analysis within the betacoronavirus genus, e.g. SARS-CoV, MERS-CoV; • SARS-CoV-2 antiviral susceptibility and antigen diversity; • Natural history of COVID-19 clinical disease spectrum in different populations and analysis of intrinsic and extrinsic prognostic factors, including sero-epidemiological studies in the general population; • Host factors, including host genetics and immunological variabilities, and their association with disease severity; • Modes and dynamics of SARS-CoV-2 transmission in household, workplace, closed community and healthcare settings, including role of super-spreading events; • Systematic reviews and meta-analysis of COVID-19 epidemiological studies and surveillance data as well as modelling studies and analytical methods for risk assessment studies; • Effectiveness of COVID-19 infection prevention and control procedures including personal protective equipment and airborne/aerosol versus droplet isolation precautions for specific healthcare settings and epidemiological stages of the outbreak; • Effectiveness of novel public health interventions for containment or impact mitigation of the COVID-19 outbreak, including social distancing and quarantine measures; • Clinical accuracy and effectiveness of rapid SARS-CoV-2 diagnostics and serological assays; • Pre-clinical development and clinical trials of therapeutic agents for COVID-19; • Pre-clinical development and clinical trials of COVID-19 candidate vaccines; • Evaluation of methods for COVID-19 epidemiological surveillance, contact tracing studies and public health risk assessment, including digital health solutions and modelling/forecasting studies; • Clinical immunology of COVID-19, including specific antibody and cellular immune responses to SARS-CoV-2 and protective immunity. As this is a multidisciplinary Research Topic, we ask the authors to choose the appropriate Topic Editor for sections upon submission. For any questions please contact the editorial office ( [email protected] ). ***Due to the exceptional nature of the COVID-19 situation, Frontiers is waiving all article publishing charges for COVID-19 related research.***
Keywords : Coronavirus, microbiology, immunology, public health, COVID-19, SARS-CoV-2, infection prevention and control, epidemiology, transmission studies, antiviral therapy
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
The research topic was first published here .
Never miss an update from Science|Business: Newsletter sign-up
Related News
Get the science|business newsletters.
Sign up for the Funding Newswire
Sign up for the Policy Bulletin
Sign up for The Widening
Funding Newswire subscription

Follow where the public and private R&I money is going and which collaborative opportunities you can pursue.
Subscribe today
Manage my subscription
Find out more about the Science|Business Network

Why join?
Become a member
- Funding Newswire
- The Widening
- R&D Policy
- Why subscribe?
- Testimonials
- Become a member
- Network news
- Strategic advice
- Sponsorships
- EU Projects
- Enquire today

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Challenges During Review of COVID-19 Research Proposals: Experience of Faculty of Medicine, Ain Shams University Research Ethics Committee, Egypt
Affiliations.
- 1 Faculty of Medicine, Ain Shams University, Cairo, Egypt.
- 2 Misr International University, US Naval Medical Research Unit No.3 (NAMRU-3), Cairo, Egypt.
- 3 Faculty of Medicine, October 6 University, Giza, Egypt.
- PMID: 34805197
- PMCID: PMC8596560
- DOI: 10.3389/fmed.2021.715796
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC). From April 10, 2020, until October 13, 2020, the FMASU REC reviewed 98 COVID-19 research protocols. This article addressed the question of how to face an overwhelming amount of research submitted to the REC while applying the required ethical principles. Ethical challenges included a new accelerated mode of review, online meetings, balance of risks vs. benefits, measures to mitigate risks, co-enrolment in different studies, protection of a vulnerable COVID-19 population, accelerated decisions, online research, how to handle informed consent during the pandemic, and justification of placebo arm.
Keywords: COVID-19 research; Faculty of Medicine Ain Shams University; accelerated review; ethical challenges; research ethics committees.
Copyright © 2021 Marzouk, Sharawy, Nakhla, El Hodhod, Gadallah, El-Shalakany, Elwakil, Moussa, Ismail and Tash.
PubMed Disclaimer
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FMASU REC Workload from January…
FMASU REC Workload from January 1st, 2008 to October 13th, 2020.
Similar articles
- Surveying the Indian research ethics committee response to the COVID-19 pandemic. Shetty YC, Ramalingam S, Koli P, Shanmugam K, Seetharaman R. Shetty YC, et al. Dev World Bioeth. 2023 Aug 4. doi: 10.1111/dewb.12417. Online ahead of print. Dev World Bioeth. 2023. PMID: 37540074
- Key ethical issues encountered during COVID-19 research: a thematic analysis of perspectives from South African research ethics committees. Burgess T, Rennie S, Moodley K. Burgess T, et al. BMC Med Ethics. 2023 Feb 15;24(1):11. doi: 10.1186/s12910-023-00888-y. BMC Med Ethics. 2023. PMID: 36793067 Free PMC article.
- Ethical Characteristics of Research Proposals Related to COVID-19 Pandemic in Nepal: A Retrospective Review. Ghimire N, Hamal PK, Panthee A, Vaidya A, Khadka M, Mahato NK, Karn MK, Verma S, Dhimal M, Ghimire P, Gyanwali P. Ghimire N, et al. J Nepal Health Res Counc. 2021 Apr 23;19(1):148-153. doi: 10.33314/jnhrc.v19i1.3373. J Nepal Health Res Counc. 2021. PMID: 33934150 Review.
- Research Ethics Committees in Laboratory Medicine. Beshir L. Beshir L. EJIFCC. 2020 Nov 20;31(4):282-291. eCollection 2020 Nov. EJIFCC. 2020. PMID: 33376468 Free PMC article. Review.
- Ethical considerations in malaria research proposal review: empirical evidence from 114 proposals submitted to an Ethics Committee in Thailand. Adams P, Prakobtham S, Limphattharacharoen C, Vutikes P, Khusmith S, Pengsaa K, Wilairatana P, Kaewkungwal J. Adams P, et al. Malar J. 2015 Sep 14;14:342. doi: 10.1186/s12936-015-0854-5. Malar J. 2015. PMID: 26370243 Free PMC article.
- A comparative ethical analysis of the Egyptian clinical research law. Martin S, Ancillotti M, Slokenberga S, Matar A. Martin S, et al. BMC Med Ethics. 2024 Apr 30;25(1):48. doi: 10.1186/s12910-024-01040-0. BMC Med Ethics. 2024. PMID: 38689214 Free PMC article.
- Research ethics review during the COVID-19 pandemic: An international study. Salamanca-Buentello F, Katz R, Silva DS, Upshur REG, Smith MJ. Salamanca-Buentello F, et al. PLoS One. 2024 Apr 16;19(4):e0292512. doi: 10.1371/journal.pone.0292512. eCollection 2024. PLoS One. 2024. PMID: 38626030 Free PMC article.
- Marzouk D, Abd El Aal W, Saleh A, Sleem H, Khyatti M, Mazini L, et al. . Overview on health research ethics in Egypt and North Africa. Eur J Public Health. (2014) 24(Suppl. 1):87–91. 10.1093/eurpub/cku110 - DOI - PubMed
- Sisi Passes Law Regulating Clinical Medical Research in Egypt . Egypt: Independent Gazette (2020). Available online at: https://egyptindependent.com/sisi-passes-law-regulating-clinical-medical... (accessed February 28, 2021)
- Available, online at: https://clinicaltrials.gov/ct2/results?cond=COVID-19 (accessed March 3, 2021)
- Guidance for Managing Ethical Issues in Infectious Disease Outbreaks. Geneva: World Health Organization; (2016). Available online at: https://apps.who.int/iris/bitstream/handle/10665/250580/9789241549837-en... (accessed November 10, 2020)
- Ethical Standards for Research During Public Health Emergencies: Distilling Existing Guidance to Support COVID-19 R&D . Geneva: World Health Organization; (2020). Available online at: https://apps.who.int/iris/bitstream/handle/10665/331507/WHO-RFH-20.1-eng... (accessed November 10, 2020)
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- Frontiers Media SA
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Methodologies for COVID-19 research and data analysis
This collection has closed and is no longer accepting new submissions.

This BMC Medical Research Methodology collection of articles has not been sponsored and articles undergo the journal’s standard peer-review process overseen by our Guest Editors, Prof. Dr. Livia Puljak (Catholic University of Croatia in Zagreb, Croatia) and Prof. Dr. Martin Wolkewitz (University of Freiburg, Germany).
Delays in reporting and publishing trial results during pandemics: cross sectional analysis of 2009 H1N1, 2014 Ebola, and 2016 Zika clinical trials
Pandemic events often trigger a surge of clinical trial activity aimed at rapidly evaluating therapeutic or preventative interventions. Ensuring rapid public access to the complete and unbiased trial record is...
- View Full Text
Open science saves lives: lessons from the COVID-19 pandemic
In the last decade Open Science principles have been successfully advocated for and are being slowly adopted in different research communities. In response to the COVID-19 pandemic many publishers and research...
Clinical research activities during COVID-19: the point of view of a promoter of academic clinical trials
During the COVID-19 emergency, IRST IRCCS, an Italian cancer research institute and promoter of no profit clinical studies, adapted its activities and procedures as per European and national guidelines to main...
Instruments to measure fear of COVID-19: a diagnostic systematic review
The COVID-19 pandemic has become a source of fear across the world. Measuring the level or significance of fear in different populations may help identify populations and areas in need of public health and edu...
A risk assessment tool for resumption of research activities during the COVID-19 pandemic for field trials in low resource settings
The spread of severe acute respiratory syndrome coronavirus-2 has suspended many non-COVID-19 related research activities. Where restarting research activities is permitted, investigators need to evaluate the ...
Outbreaks of publications about emerging infectious diseases: the case of SARS-CoV-2 and Zika virus
Outbreaks of infectious diseases generate outbreaks of scientific evidence. In 2016 epidemics of Zika virus emerged, and in 2020, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a p...
Analysis of clinical and methodological characteristics of early COVID-19 treatment clinical trials: so much work, so many lost opportunities
The COVID-19 pandemic continues to rage on, and clinical research has been promoted worldwide. We aimed to assess the clinical and methodological characteristics of treatment clinical trials that have been set...
The unintended consequences of COVID-19 mitigation measures matter: practical guidance for investigating them
COVID-19 has led to the adoption of unprecedented mitigation measures which could trigger many unintended consequences. These unintended consequences can be far-reaching and just as important as the intended o...
Short-term real-time prediction of total number of reported COVID-19 cases and deaths in South Africa: a data driven approach
The rising burden of the ongoing COVID-19 epidemic in South Africa has motivated the application of modeling strategies to predict the COVID-19 cases and deaths. Reliable and accurate short and long-term forec...
Incorporating and addressing testing bias within estimates of epidemic dynamics for SARS-CoV-2
The disease burden of SARS-CoV-2 as measured by tests from various localities, and at different time points present varying estimates of infection and fatality rates. Models based on these acquired data may su...
COVID-19-related medical research: a meta-research and critical appraisal
Since the start of the COVID-19 outbreak, a large number of COVID-19-related papers have been published. However, concerns about the risk of expedited science have been raised. We aimed at reviewing and catego...
Use of out-of-hospital cardiac arrest registries to assess COVID-19 home mortality
In most countries, the official statistics for the coronavirus disease 2019 (COVID-19) take account of in-hospital deaths but not those that occur at home. The study’s objective was to introduce a methodology ...
Strengthening policy coding methodologies to improve COVID-19 disease modeling and policy responses: a proposed coding framework and recommendations
In recent months, multiple efforts have sought to characterize COVID-19 social distancing policy responses. These efforts have used various coding frameworks, but many have relied on coding methodologies that ...
Predictive accuracy of a hierarchical logistic model of cumulative SARS-CoV-2 case growth until May 2020
Infectious disease predictions models, including virtually all epidemiological models describing the spread of the SARS-CoV-2 pandemic, are rarely evaluated empirically. The aim of the present study was to inv...
Alternative graphical displays for the monitoring of epidemic outbreaks, with application to COVID-19 mortality
Classic epidemic curves – counts of daily events or cumulative events over time –emphasise temporal changes in the growth or size of epidemic outbreaks. Like any graph, these curves have limitations: they are ...
The Correction to this article has been published in BMC Medical Research Methodology 2020 20 :265
COVID19-world: a shiny application to perform comprehensive country-specific data visualization for SARS-CoV-2 epidemic
Data analysis and visualization is an essential tool for exploring and communicating findings in medical research, especially in epidemiological surveillance.
Social network analysis methods for exploring SARS-CoV-2 contact tracing data
Contact tracing data of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is used to estimate basic epidemiological parameters. Contact tracing data could also be potentially used for assessin...
Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children
COVID-19, the disease caused by the highly infectious and transmissible coronavirus SARS-CoV-2, has quickly become a morbid global pandemic. Although the impact of SARS-CoV-2 infection in children is less clin...
Statistical design of Phase II/III clinical trials for testing therapeutic interventions in COVID-19 patients
Because of unknown features of the COVID-19 and the complexity of the population affected, standard clinical trial designs on treatments may not be optimal in such patients. We propose two independent clinical...
Rapid establishment of a COVID-19 perinatal biorepository: early lessons from the first 100 women enrolled
Collection of biospecimens is a critical first step to understanding the impact of COVID-19 on pregnant women and newborns - vulnerable populations that are challenging to enroll and at risk of exclusion from ...
Disease progression of cancer patients during COVID-19 pandemic: a comprehensive analytical strategy by time-dependent modelling
As the whole world is experiencing the cascading effect of a new pandemic, almost every aspect of modern life has been disrupted. Because of health emergencies during this period, widespread fear has resulted ...
A four-step strategy for handling missing outcome data in randomised trials affected by a pandemic
The coronavirus pandemic (Covid-19) presents a variety of challenges for ongoing clinical trials, including an inevitably higher rate of missing outcome data, with new and non-standard reasons for missingness....
Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID-19 patients: a multistate approach
The clinical progress of patients hospitalized due to COVID-19 is often associated with severe pneumonia which may require intensive care, invasive ventilation, or extracorporeal membrane oxygenation (ECMO). T...
COVID-19 prevalence estimation by random sampling in population - optimal sample pooling under varying assumptions about true prevalence
The number of confirmed COVID-19 cases divided by population size is used as a coarse measurement for the burden of disease in a population. However, this fraction depends heavily on the sampling intensity and...
Coronavirus disease 2019 (COVID-19): an evidence map of medical literature
Since the beginning of the COVID-19 outbreak in December 2019, a substantial body of COVID-19 medical literature has been generated. As of June 2020, gaps and longitudinal trends in the COVID-19 medical litera...
Group testing performance evaluation for SARS-CoV-2 massive scale screening and testing
The capacity of the current molecular testing convention does not allow high-throughput and community level scans of COVID-19 infections. The diameter in the current paradigm of shallow tracing is unlikely to ...
Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19
The research community reacted rapidly to the emergence of COVID-19. We aimed to assess characteristics of journal articles, preprint articles, and registered trial protocols about COVID-19 and its causal agen...

Using online technologies to improve diversity and inclusion in cognitive interviews with young people
We aimed to assess the feasibility of using multiple technologies to recruit and conduct cognitive interviews among young people across the United States to test items measuring sexual and reproductive empower...
Towards reduction in bias in epidemic curves due to outcome misclassification through Bayesian analysis of time-series of laboratory test results: case study of COVID-19 in Alberta, Canada and Philadelphia, USA
Despite widespread use, the accuracy of the diagnostic test for SARS-CoV-2 infection is poorly understood. The aim of our work was to better quantify misclassification errors in identification of true cases of...
The semi-automation of title and abstract screening: a retrospective exploration of ways to leverage Abstrackr’s relevance predictions in systematic and rapid reviews
We investigated the feasibility of using a machine learning tool’s relevance predictions to expedite title and abstract screening.
Social media as a recruitment platform for a nationwide online survey of COVID-19 knowledge, beliefs, and practices in the United States: methodology and feasibility analysis
The COVID-19 pandemic has evolved into one of the most impactful health crises in modern history, compelling researchers to explore innovative ways to efficiently collect public health data in a timely manner....
Current methods for development of rapid reviews about diagnostic tests: an international survey
Rapid reviews (RRs) have emerged as an efficient alternative to time-consuming systematic reviews—they can help meet the demand for accelerated evidence synthesis to inform decision-making in healthcare. The s...
Methodological challenges of analysing COVID-19 data during the pandemic
Marie Skłodowska-Curie Actions
Developing talents, advancing research
Projects researching COVID–19, SARS-CoV-2 and related topics
The current COVID-19 outbreak has not caught EU-funded research off guard. The Marie Skłodowska-Curie Actions (MSCA) of the European Commission are supporting outstanding researchers in finding solutions to challenges posed by the novel coronavirus disease COVID-19 and other infectious diseases.
This page will be regularly updated with MSCA projects, results and testimonials relevant to COVID-19, SARS-CoV-2 and related topics.
DIAGNOSTICS AND TREATMENTS (including vaccines)
Diabetic nephropathy modelling in hesc-derived 3d kidney organoids.
EPIORGABOLISM is studying how SARS-Co-V2, the coronavirus responsible for the 2019 novel coronavirus disease (COVID-19), interacts with and infects kidney cells. Together with the lung, the kidney is one of the main organs affected by the COVID-19 disease. Dr Carmen Hurtado, researcher of EPIORGABOLISM, is currently generating human kidney organoids from human pluripotent stem cells.
The use of human organoids allows to test treatments against coronavirus in an agile way, dramatically reducing the time human drug trials take. Hurtado is part of international research team has identified a drug capable of blocking the effects of the SARS-CoV-2 virus. The findings have been partially supported by EPIORGABOLISM and published in the journal ‘Cell’.
Find out more
- Trial drug shows promise in fighting coronavirus
- Watch the testimonial of Carmen Hurtado , researcher of the EPIORGABOLISM project.
Host switching pathogens, infectious outbreaks and zoonosis; a Marie Sklodowska-Curie Training Network
HONOURs is teaching 15 talented young researchers, including coronavirologists, to become “preparedness-experts”. The project involves 11 laboratories, all at the forefront of novel virus investigations and characterizations. HONOUR reacted in January 2020, immediately after the emergence of COVID-19, by starting work on SARS-CoV-2. A synthetic biology virus culture system was developed to swiftly evaluate therapy options, next to rapid tests to determine virus shedding on location. The quality of protective immunity was evaluated, and a search started on the most suitable animal model to battle the virus and provide therapy options. HONOURs is devoting its expert knowledge to fight this coronavirus and provide therapy options.
- HONOURs: Virus Outbreak Preparedness and COVID-19
- Visit the HONOURs website
INnate-ImmunomeTabolIsm as Antiviral TargEt
The global COVID-19 pandemic highlights an urgent need for innovation in the development of novel antiviral strategies and therapies. INITIATE has recruited 15 young PhD candidates to become experts in the field of antiviral immunometabolism, with a focus on RNA viruses – including coronaviruses. While it is clear that viral replication, metabolic pathways, and host immune responses are tightly interconnected, the host molecular pathways that impact viral pathogenesis are not well-defined. With the emergence of COVID-19, eight of the INITIATE projects have included SARS-CoV-2 in their research programs to understand coronavirus molecular virology, the role of the host immune response in driving COVID-19 immunopathogenesis and the potential of targeting host metabolism as therapeutic strategies.
Organoids for Virus Research - An innovative training-ETN programme
ORGANOVIR is contributing to COVID-19 research in a variety of ways, and several of its researchers are currently working on the development of new antivirals to combat the disease. Researchers at KU Leuven (Belgium) are studying the way in which coronaviruses evolve, and are searching out possible targets for further remedies. The project also investigating active substances – or a combination of them – in existing medicines that could be effective against SARS-CoV-2. ORGANOVIR is also conducting pre-clinical tests for a vaccine against COVID-19 using a technology based on the yellow fever vaccine.
In parallel, a group of researchers at the Jagiellonian University (Poland) is studying the infection on the single-cell and tissue level in different organs and cell types, working on virus inhibitors and collaborating with companies to create a point of care diagnostics based on different platforms. The group is also studying the course of the pandemic in Poland and monitoring the virus variability in the country.
ORGANOVIR’s coordinators have been intensively working on clinical and diagnostic tasks and set up new COVID-19 research at the Amsterdam UMC (The Netherlands). This has resulted in the launch of COVID-KIDS, a study on immunity in children, and the use of 3D culture models for COVID-19 studies.
- Read about the COVID-19 activities of ORGANOVIR partners
- Read the testimonial of Mariana Guedes , researcher for the OrganoVir project
- Read the testimonial of Thuc Do , researcher for the OrganoVir project
- Air-liquid interface cultures of nasal epithelial cells to investigate factors critical for viral entry into host cells
MECHANISMS OF INFECTION, IMMUNE REACTIONS AND HOST-PATHAGEN INTERACTION
Unravelling species barriers of coronaviruses.
COV RESTRIC targeted the precise mechanisms that allow coronaviruses to jump across species. Dr Stephanie Pfänder, researcher of COV RESTRIC, worked on various virological aspects of emerging viruses – with a focus on emerging coronaviruses. Her work has the potential to lead to novel strategies to protect cells against coronavirus infection. This is crucial to fight the COVID-19 pandemic – and to help insulate society against future coronavirus outbreaks.
- Read the testimonial of Stephanie Pfänder , researcher of the COV RESTRIC project.
- Host proteins involved in species barriers of viral infections
DIGITAL TOOLS, DATA AND MODELLING
Research and innovation staff exchange network of european data scientists.
The NeEDS consortium is currently focusing on the emerging data challenges that come with the COVID-19 pandemic. In Spain, the first cases of the COVID-19 pandemic were confirmed late February 2020 and data started to be collected daily by the different regions. Data and Data Science tools turned out to be crucial to assist decision makers in this highly uncertain context. NeEDS and the scientific collaborations they enjoy were fundamental to create a working group of data scientists from different European universities, which has developed an Artificial Intelligence tool to provide short-term predictions of the pandemic’s evolution. With this novel methodology, NeEDS as contributed to the cooperative efforts coordinated by the Spanish Commission of Mathematics to support data-driven decision making related to the COVID19 pandemic. In a recent interview , Project Coordinator Dolores Romero Morales has reflected on the potential of the NeEDS expertise and the efforts of tackling these data challenges within the team. The consortium is tackling other important Data Science questions, e.g., using spatial data to support COVID19 information apps or addressing the pressing data privacy needs.
- Read about the COVID-19 activities of NeEDS and its partners
- On Sparse Ensemble Methods: An Application to Short-Term Predictions of the Evolution of COVID-19
- Read the testimonials of Remedios Sillero, Cristina Molero and Sandra Benitez , seconded researchers for the NeEDS project.
Pan-genome Graph Algorithms and Data Integration
Researchers involved in PANGAIA are investigating how massive amounts of genome sequence data can be ordered and analysed for their use in biomedicine. Their work has important implications in areas such as bacteria and virus research, investigation of drug resistance mechanisms and vaccine development: big data technology can help to identify the characteristics of new strains of viruses such as SARS-CoV-2 and bacteria by comparing their genomes.
- Identifying large data sets to help coronavirus research
- Identifying pathogenic genes in virus strains at a glance
Modelling Infectious Diseases in Dynamic, relocated, refugee populations
In order to assist policy-makers in mitigating outbreaks, MIDIDP has created realistic models to simulate the spread of infectious diseases in under-vaccinated refugee populations in Europe and neighbouring countries. Dr Hasan Güçlü, researcher of MIDIDP, has created a model that simulates the spread of COVID-19 in populations with variable demographics.
- Read the testimonial of Hasan Güçlü , researcher of the MIDIDP project.
PUBLIC HEALTH, PREPAREDNESS AND RESPONSE
Disability and disease during the 1918 influenza pandemic: implications for preparedness policies.
As the current COVID-19 pandemic shows, people with disabilities are at increased risk for complications and death as they are often neglected in epidemic responses. Dr Jessica Dimka, researcher of DIS2 , is exploring disability as a risk factor in pandemics. Using the 1918 Spanish influenza pandemic as a model, the project seeks to promote more equitable public health plans and interventions. Dimka points out that people with disabilities must be considered in all pandemic strategies: their lives, livelihoods and rights are not expendable.
- Read the testimonial of Jessica Dimka , researcher of the DIS2 project.
MULTIDISCIPLINARY PROJECTS
Protecting human rights and public health in global pandemics.
THEMIS is an interdisciplinary research project that reacts to the increasing occurrence of global pandemics, like the caused by the present COVID-19 disease, and restrictive public health measures taken to respond to these threats. Using a rights-based approach, Dr Patrycja Dąbrowska-Kłosińska, researcher of THEMIS, intends to create a better understanding of how to prepare for, and respond to, global pandemics.
The project seeks to offer a vital reference for policy-making at national, regional and global levels – one that prioritises fair pandemic preparedness to cross-border health threats. The project has offered critical guidance during the current COVID-19 pandemic, which has required a previously unimagined scale of coordinated, public health-control measures as well as consideration of human-rights implications worldwide.
- Read the testimonial of Patrycja Dąbrowska-Kłosińska , researcher of the THEMIS project.
Martí I Franquès COFUND
Since the emergence of COVID-19, several fellows involved in the Martí Franquès Programme (MFP) have been working on solutions to the current crisis. Researchers are developing an epidemiological mathematical model that infers the status of the epidemic, thereby monitoring and estimating the impact of interventions on the spread of COVID-19.
In parallel, another group of researchers is implementing an original virtual screening protocol to reposition approved drugs. This would allow predicting which of them could inhibit the main protease of the virus (M-pro), a key target for antiviral drugs given its essential role in the virus replication.
- Read the testimonial of Benjamin Steinegger , whose research is developing a mathematical framework to monitor and estimate the impact of interventions on the COVID-19 pandemic.
Project outcomes
- Modelling the impact of interventions on the spread of COVID-19
- Prediction of novel inhibitors of the main protease of SARS-CoV-2
- See all the results relevant to COVID-19 produced by MFP fellows
The launch of a new industrial PhD programme at EPFL
Several fellows involved in the EPFLinnovators project are working on solutions to COVID-19 since the start of the crisis. Teams of researchers are developing subunit vaccines against the SARS-CoV-2 virus, investigating the potential use of cyclodextrin derivatives to prevent and treat the infections caused by SARS-CoV-2, and analysing the mechanical aspects of SARS-CoV-2 entry into cells.
- Read the testimonial of Xiaomeng Hu , researcher of the EPFLInnovators project.
- Subunit vaccines against SARS-CoV-2
- Non-toxic cyclodextrin derivative against viruses at micromolar concentration
- Variations in clathrin mediated endocytosis on a mammalian cell membrane
SOCIAL BEHAVIOUR AND IMPACT
Leading fellows.
Over the last decade, the reliance on online products and services has steadily increased, but since the beginning of the COVID-19 pandemic it has escalated to an unprecedented level. Dr Matthew Dennis, researcher of the LEaDing Fellows COFUND project at TU Delft (the Netherlands), examines the ethical implications and value trade-offs as societies attempt to transition across the digital divide. His project highlights that an ethical reflection on this digital transition is urgently needed, as digital solutions to problems generated by COVID-19 may create winners and losers – likely disproportionately affecting vulnerable users. By addressing these issues, the pandemic may foster the kind of social and political interconnectedness that was envisioned at the start of the crisis.
- Read the testimonial of Matthew James Dennis , researcher of the LEaDing Fellows project.
MSCA on social media
The MSCA social media are continuously updated with testimonials of MSCA fellows, supervisors, coordinators and projects working to find solutions to challenges posed by COVID-19 and other infectious diseases.
- MSCA on Twitter
- MSCA Facebook page
Related content
Related information, want to give your feedback about this page, thanks for your feedback.
We are happy to see that your experience was positive. Don't forget to share the pages you like with your friends and colleagues.
If you need to ask a question, please contact Europe direct .
York Graduate Research School
Covid impact statement
An optional impact statement to explain to your examiners how your project/thesis has changed as a consequence of Covid-19 restrictions.
Many PGRs will have had to adapt their research project, sometimes significantly, in response to Covid-19 restrictions and this may be a cause of concern. Be reassured that adapting research projects in the light of unforeseen circumstances is a normal part of research and you will not be disadvantaged for doing so, as long as you are still able to meet the criteria for the relevant award ( section 2 of the Policy on Research Degrees ).
If you believe the pandemic is having or has had a significant negative impact on your personal circumstances (for example, led to ill-health or a challenging domestic situation) you should request a leave of absence or extension on those grounds. As always, you can seek independent advice from the Graduate Student's Association advice team.
Challenges and context
If you started on or before 31 March 2021 and will submit from December 2020 onwards, you will have the option of submitting a short impact statement to give contextual information about the effect of the Covid-19 restrictions on your research project/thesis. Submitted statements will be shared by PGR Administration with your examiners, who may explore the statement in an oral examination.
The statement enables you to explain challenges, for example:
- difficulty or delay in collecting or analysing data due to the closure of/restrictions on laboratories/other specialist facilities/expertise, curtailed/cancelled fieldwork due to travel restrictions or social distancing measures
- reduced data in one or more theis chapters, and/or thesis chapters that are shorter and/or not as closely linked as might be expected
You can also explain how the planned (i.e. pre-Covid-19 restrictions) research would have fitted into the thesis’ narrative and the steps you took to address the challenges arising from the Covid-19 restrictions, in terms of adjusting the scope, design or phasing of their research project/thesis, for example:
- one or more changes of research topic
- a change in emphasis from empirical to theoretical research
- a change of research location (fieldwork, archive, etc)
- a change a method (e.g. running experiments remotely rather than in person, using simulation, moving from in-person data collection to online data collection, analysing existing data sets)
- altering the timing of, or substituting, one or more experiments.
Submit an impact statement
You should complete the impact statement just before you submit your thesis for examination. Please upload the completed impact statement (as a PDF file) with your thesis.
[email protected] +44 (0)1904 325962 Student Hub, Information Centre Basement, Market Square
REVIEW article
Challenges during review of covid-19 research proposals: experience of faculty of medicine, ain shams university research ethics committee, egypt.

- 1 Faculty of Medicine, Ain Shams University, Cairo, Egypt
- 2 Misr International University, US Naval Medical Research Unit No.3 (NAMRU-3), Cairo, Egypt
- 3 Faculty of Medicine, October 6 University, Giza, Egypt
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC). From April 10, 2020, until October 13, 2020, the FMASU REC reviewed 98 COVID-19 research protocols. This article addressed the question of how to face an overwhelming amount of research submitted to the REC while applying the required ethical principles. Ethical challenges included a new accelerated mode of review, online meetings, balance of risks vs. benefits, measures to mitigate risks, co-enrolment in different studies, protection of a vulnerable COVID-19 population, accelerated decisions, online research, how to handle informed consent during the pandemic, and justification of placebo arm.
Introduction
The majority of the RECs in North African countries are registered with the Office for Human Research Protections and have Federal Wide Assurance (FWA) active numbers ( 1 ). Egypt has a National Ethics Committee, active institutional committees and the Egyptian Medical Research Regulation Law for the regulation of clinical trials and human research ethics issued December 2020; the Egyptian National Ethical Committee collaborates with the United Nations Educational, Scientific and Cultural Organization ( 2 ).
The FMASU REC was established in October 2007, to review research conducted at the Faculty of Medicine, Ain Shams University, in Cairo, Egypt. It holds a Federal Wide Assurance Number (FWA 00017585). The committee trained 220 staff members as reviewers working in 32 faculty departments over 23 training events. Since its establishment, the FMASU REC reviewed 414 international and multicentre projects, 5,033 theses, and free research. Since April 10, 2020, the FMASU REC reviewed 98 COVID-19 research studies, Figure 1 .
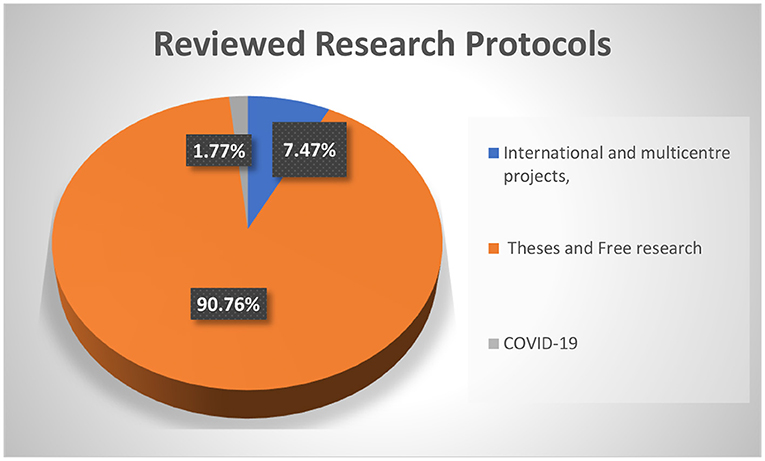
Figure 1 . FMASU REC Workload from January 1st, 2008 to October 13th, 2020.
During the COVID-19 pandemic in the year 2020, investigators began research to understand the novel virus, its epidemiology and pathogenicity, as well as to find ways for prevention and control, including discovering a treatment and/or vaccine. Worldwide, more than 4,900 studies and trials have been registered on “ Clinicaltrials.gov ” since the start of the pandemic ( 3 ). This rise in the number of emerging COVID-19 research projects has recently resulted in an overwhelming number of research project submissions to RECs.
To ensure ethical research during the COVID-19 pandemic, the World Health Organization (WHO) summarized the key universal ethical standards that should be adhered to by researchers, review bodies, funders, publishers, and manufacturers during a pandemic ( 4 ), identifying the main ethical standards as scientific validity, reasonable risk-benefit ratio, fair and voluntary participation, and independent review ( 5 ).
The European Network of Research Ethics Committees (EUREC) issued a statement that was adopted by the EUREC Board on April 27, 2020, stressing the fact that the administrative processes for reviewing research protocols during the COVID-19 pandemic must be accelerated and simplified if these protocols are related to the treatment, prevention or diagnosis of infections caused by SARS-CoV-2 ( 6 ). However, all research must be guided by the principle that RECs will not compromise the quality of the review process under these extraordinarily stressful circumstances. Furthermore, an accelerated procedure cannot be at the expense of the safety of research participants. The recognized ethical principles must always be respected, and the free and informed consent procedure must remain in accordance with international and national regulations.
In Egypt, there are 115 university hospitals ( 7 ) conducting research on human beings which needs approval from institutional RECs which are either internationally recognized and have an international FWA number and/or are registered with the Ministry of Health and Population (MOHP), or are newly developed and have been submitted for registry with the MOHP. International projects need other final approval from the MOHP REC.
The Clinical Medical Research Regulation Law (the Egyptian law for the conduct of research) was issued on December 23, 2020; its bylaws are currently being written. The Egyptian law is aligned with international guidelines for health research ethics review. The research studies protocols described in this manuscript were submitted during the period April 10 to October 13, 2020, to Ain Shams REC and were reviewed according to the international guidelines.
This article tried to answer the question of how the REC can effectively apply ethical principles when faced with an overwhelming number of research projects submitted.
The aim of this article is to report the challenges encountered and the experience of the FMASU REC during review of COVID-19 research, starting from April 10 to October 13, 2020, and to give an overview about the challenges that the committee faced and how it overcame them.
Modified Standard Operating Procedures
The article tried to illustrate how a governmental university REC in a low-middle income country modified its standard operating procedures (SOPs) to cope with a fast-track review of the overwhelming COVID-19 research and continue reviewing non-COVID-19 research. During the first wave of the pandemic there were no international guidelines for reviewing COVID-19 research. This challenge was increased by a scarcity of information about the disease, governmental lockdown periods, and lack of extra budget allocation.
The FMASU REC was confronted by multiple challenges in reviewing COVID-19 research during the pandemic, necessitating out-of-the box solutions to maintain an effective, accelerated review while at the same time practicing the ethical principles required.
The researchers, as well as the REC members, were encouraged to transform these challenges into opportunities. The SOPs followed by the REC were updated, regarding protocol submission changes to adopt digital submission route, rather than hard-copy paper submissions. Training of the employees on the use of digital technology for submissions and archiving followed. An electronic signature for the head of the committee was introduced. An accelerated, fast-track mode for reviewing protocols was adopted to cope with the pace of the pandemic. While the reviewers had previously been allowed up to 1 month for response for randomized controlled trials (RCTs), they were now expected to respond within one-seven days during the pandemic. The expedited process was meant to help in generating desperately needed knowledge out of the submitted research.
The REC resorted to on-line conferencing to overcome the inability to hold face-to-face meetings due to the Faculty lockdown. The first wave of research was received on April 10, 2020, with seven research projects reviewed in two online meetings over 3 days, including five clinical trials and two observational studies. That new mode of reviewing was dynamic, accelerated, and fast-tracked, in accordance with international guidelines and the REC's updated SOPs.
As the number of submitted research protocols increased rapidly, the REC had to increase the frequency of meetings to every other day, then every week or 2 weeks instead of the previous monthly schedule. Reviewing was done in a shorter time as the institution and investigators were expecting a rapid response. The usual review process that was adhered to by the REC was as follows: all the above minimal-risk protocols were initially reviewed by two reviewers and then discussed in full board meetings. To acquire a faster review track of the COVID-19-related proposals, clinical trials, or repurposed drugs, the number of initial reviewers was increased to three members, then discussed in full board online meetings. The number of members attending the virtual meetings ranged from 9 to 13 members, out of the 15 members comprising the committee.
For this article we reviewed the list of all protocols submitted to FMASU REC for review during the COVID-19 time from April 10, 2020, until October 13, 2020. We listed the titles, principal investigators (PI) names, date of submission and date of response to the investigators. We analyzed the frequencies from these data. We also reviewed the meeting minutes to pin out the most interesting and challenging issues that were discussed during the meetings. Additionally, we reviewed the current procedures and changes that were instituted to the SOPs. Finally, we asked the members to provide their input about their concerns in the review process during the COVID-19 time. Last, we incorporated all the data into the article.
Review Processes
Reviewing research during COVID-19 pandemic lockdown included shortened average duration of review, rapid request for clarifications and reply of investigators and quick provision of decisions. The duration of review was shortened to a minimum of 1 day and a maximum of 7 days. Before the pandemic, for more than minimal risk (low risk studies) and commercially-sponsored studies, the REC adopted a two-reviewer system, followed by discussion by the full Board. During the pandemic, to accomplish a shorter review time, this system was replaced by a three-member review, followed by the online meeting. As for minimal risk studies, the pre-pandemic system was also a two-reviewer, expedited review system, while during the pandemic for COVID-19 protocols, two reviewers were still assigned to review each protocol, but in a shorter reviewing period of 1-7 days.
To overcome the challenges of the short review time, the reviewing process of COVID-19 research stressed the rationale or justification as tackled by many researchers all over the world, the research question, hypothesis, social value, and benefits to the community. The novelty of the research idea was also an important point of discussion during the review process.
“Good science is itself an ethical requirement, as it is meaningless to apply ethical principles to a scientifically flawed product or plan. Bad science can only be bad ethics” ( 8 ). A rigorous revision of the research methodology was conducted, including study design, sample size and type, study procedures, randomization, and blinding. Many studies needed redesigning to specify inclusion and exclusion criteria of subjects, or to rigorously define the diagnosis of mild, moderate, and severe COVID-19 cases according to the National Guidelines reported and updated by the Egyptian MOHP.
Ensuring the well-being of researchers and research participants in the context of a pandemic was a very important objective of the REC. For research participants' well-being, hospital beds and equipment disinfection were under control of the Infection Control Unit, thus conforming to all international guidelines. As for mental health studies (some included healthcare professionals as well as patients), the committee recommended providing medical assistance for those who had high scores (e.g., recommending adding a paragraph in the questionnaire telling participants that if they had high scores for depression, to seek medical consultation). As for researchers, the REC insisted on following international, local governmental, and institutional recommendations. They were supplied with personal protective equipment including surgical, N95 masks, face shields, and goggles.
The REC was rigorous about sample size calculation in the protocol, urging the investigators to have a precise, predefined sample-size calculation, in order to receive REC approval of the study. As a standard procedure, the investigators were required, as a prerequisite before submission to the REC, to refer to the accredited statistics unit at the Department of community medicine in FMASU, to obtain the sample size calculation for any research. While some researchers chose to conduct a pilot study and a convenience sampling because of lack of sufficient data for new or repurposed drugs, the investigators had to provide strong justifications in such instances. The REC required an interim analysis and power calculation, to see if the sample size was large enough, or needed revision to find out if the efficacy of the tested drugs had been reached.
Special Concerns During Review
The balance of risks and benefits is a pivotal element for the protection of human subjects in research. The REC exerted a great effort to mitigate research risks to provide maximum possible protection of research participants. The REC members spent long hours reviewing recent COVID 19-related publications, with special emphasis on adverse events reported involving drugs under trial and drug interactions. Clarifications were required on how to minimize the risks of these side effects, and suggestions were offered to the researchers. For instance, REC recommended additional investigations such as a baseline electrocardiogram, complete blood counts, requested exclusion of high-risk participants, or recommended increased frequency of monitoring visits.
The COVID-19 submitted protocols posted a novel risk-benefit evaluation to the reviewers. For example, in many instances, the REC could not ignore the risk to the researchers who had to interact face-to-face with COVID-19 patients in intensive care units (ICU). The REC had to ensure performance of enrolment and study procedures in unconventional circumstances, such as instances when researchers might not have been allowed in the research setting. The ICU physicians and nurses had to be trained to perform the study procedures. The PI had to keep close contact with the ICU staff and get monitoring reports about the enrolled participants. Whenever possible, the PI could see the patients following the standard safety infection control precautions.
One of the challenges faced by the FMASU REC was the lack of or insufficient animal studies and combining of Phases II and III for testing new drugs. The REC did not encounter Phase I protocols at that time. While the side effects and risks of the proposed therapies were not yet fully studied, Phase II usually includes more patients, and combining Phases II and III usually results in larger sample sizes than Phase II alone.
The REC responded by evaluating the risks and benefits, while maintaining strict requirements for risk minimization. The direct, potential patient benefit was the hope for effectiveness of new drugs, and the indirect benefit was withdrawing drugs from the list of potential drugs if proven non-effective.
The REC faced a big challenge with the large number of protocols, exceeding by far the routine work of the committee. Repurposed drugs, innovative drugs, and vaccines necessitate enormous steps to be approved. During the first 6 months of the pandemic, there was a relatively greater flow of RCTs, Phase III (2/5.85%) and Phase II (15/0.52%), compared to the period before the pandemic. The majority of studies were low risk observational studies (79.86%). Low risk studies are usually reviewed in an expedited manner by two reviewers, but in a shorter reviewing period of 1-7 days. The duration of the initial review was shortened to a minimum of 1 day and a maximum of 7 days compared to a minimum 1-month clinical trial review time, according to the REC SOPs. The total review time in the first rush of protocols during the pandemic was 1 week, but later the total review time was within 1 month, depending on how rapidly the investigators responded to the REC's comments.
The REC members devoted all their time to pandemic work, as most of the submitted protocols, even the non-commercial research, were to find an effective treatment for the emerging COVID-19 disease, either through using a repurposed drug, steroids, or antiviral drugs used earlier in management of Ebola virus, HCV, HIV, or antimalarial drugs. The flow of routine research as multicentre studies between Egyptian research centers, international centers and single center, non-commercial international project submissions, amendments, renewals and theses was slower than before the pandemic, but was reviewed in the same, accelerated manner.
The thesis topics submitted during the early wave of the COVID-19 pandemic were not yet related to the pandemic, but later in 2020 the topics were related to COVID-19.
The refusal rate was minimal; one study was deferred until more information could be obtained, as per the REC reviewers' request. Further details cannot be mentioned in this manuscript for protection of the confidentiality of the research topics.
The most frequent types of research submitted to the REC during the COVID-19 pandemic were observational studies (76.86%) to know the nature of this new emerging disease, followed by Phase III RCTs (5.85%), trying to find the most effective treatment through novel or repurposed drugs, such as drugs previously used in diseases other than COVID-19 such as EBOLA, Hepatitis C Virus, Human Immune Deficiency Virus and malaria Table 1 .
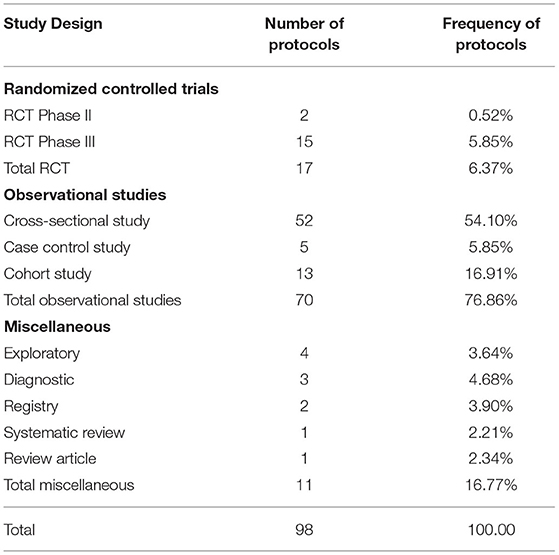
Table 1 . Frequency of clinical trial phases reviewed by FMASU REC from April to October 2020.
The protocols might not have been written as state of the art, as investigators submitted them in an expedited manner. This necessitated more frequent and more rapid than usual communication between the FMASU REC and the investigators. In some instances, the REC required the investigators to provide more information to be able to make informed decisions. The direct communication between the REC members and the investigators was effective in enhancing the quality of the protocols and their scientific validity, in view of the scarce and controversial information concerning COVID-19.
Defining the target population of COVID-19 patients and the vulnerability of this population was another challenge. The investigators had to define their study population with regard to the severity of the disease, the state of consciousness, and addressing what the REC defined as a new vulnerability group; the COVID-19 patients were desperate for treatment and might have agreed to participate in any research project without proper consideration. The protection of moderate and severe cases of COVID-19, as vulnerable groups, was extremely important to the REC. Therefore, the REC insisted that mild and moderate cases give consent for themselves and did not allow a legally-authorized representative to give consent on their behalf.
Allowing enrolment of severe disease cases engendered a wide range of discussion. Although the severe cases were in great need of the benefit of any drug at a time when no proven cure was available, the opposing committee members were hesitant to allow severely ill cases to be enrolled in the studies, if the investigators could not provide enough preliminary evidence of a potential direct benefit. Some investigators requested that in severe cases, the ICU manager might sign the consent on behalf of the subjects, but the REC refused this idea and insisted that the patient be conscious enough to give his or her own consent. Otherwise, the investigators would have to obtain the legal guardian's consent outside the isolation hospital due to rules on who was allowed in an isolation hospital.
Reviewing Telemedicine Studies
The review process had previously been accomplished through hard copies and online communication, as per the preference of the involved REC reviewers. During the pandemic, the shift to electronic communication became mandatory among researchers, the REC administrative office, the REC board, and the reviewers. The institution administration, as well, supported this shift and provided online meeting platforms in support of the digital transformation.
The COVID-19 pandemic resulted in greater use of online surveys and telemedicine. Telemedicine for clinical care started in 2016 at the Faculty of Medicine, Ain Shams University in the Neurology Department to help communication with patients being seen in clinical practice and was later extended to include other departments. To counteract the effects of the lockdown due to COVID-19, FMASU offers different telemedicine services, including consultation and outpatient clinics. Seven departments offer these services: Family Medicine, Clinical Oncology, Internal Medicine, Psychiatry, Paediatrics, Geriatrics and Obstetrics and Gynaecology. Services are offered through a secure link, where data, images and laboratory results can be uploaded and stored in the patient's medical record. The REC received this new type of telemedicine research as another challenge, Table 2 . The use of online surveys to study the behavioral and psychiatric well-being of the community by different age groups, different study populations and in different places, was a new type of research for the REC to review, constituting 13% of the submitted COVID-19 research at that time and the second most frequent type of studies to be reviewed after therapeutic research (18%). The lack of experience in reviewing this type of research was challenging. Breeching of confidentiality and assurance were the major concerns. Additionally, telemedicine services are not common in developing countries like Egypt due to high illiteracy rate, 24.6% in July 2019 as announced by the Central Agency for Public Mobilization and Statistics ( 9 ). While the REC tried to ensure optimization of the research protocol and data collection and follow up, the REC experienced difficulties in interpretation of the PIs or physicians' instructions and data collection by phone. However, the REC also requested that the patient have an educated relative beside him to ensure proper comprehension of the instructions of the PI and for easier communication.
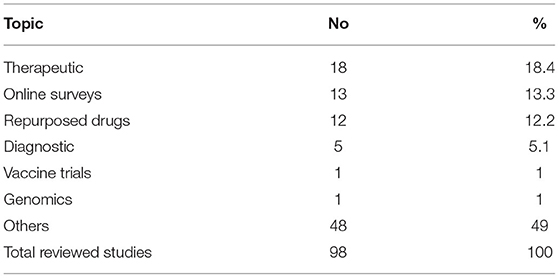
Table 2 . Most common COVID-19 research topics reviewed by FMASU REC from April to October 2020.
Ain Shams University includes eight hospitals and several health centers. The total number of beds affiliated with the university is 2,300. Research is allowed in all hospitals and health centers except the specialized hospitals (the Specialized Hospital on within the FMASU campus and the Obour Specialized Hospital in Obour city).
During the first wave of the pandemic from February to October 2020, two main hospitals were transformed into isolation hospitals for moderate and severe adult cases, Obour Specialized Hospital, and the Geriatric Hospital; one new hospital was established, Al Maidany Hospital. Ain Shams student dorms were transformed into hospitals to receive moderate COVID-19 cases. The Internal Medicine and Surgery Departments were allocated for adult COVID-19 cases and the Paediatric Department for pediatric COVID-19 cases. The rest of the hospitals offered the same services as usual, except that every patient being admitted was instructed to do a Polymerase Chain Reaction (PCR) test before admission. If the PCR was positive, the patient would be transferred to Obour Specialized Isolation Hospital.
Ain Shams University designated some of its hospitals and ICUs as “Isolation Hospitals” for the management of COVID-19 patients, which became the target for many, if not all, of the COVID-19 research studies. In view of the overwhelming number of research protocols, more than one research protocol targeted the same population in the same isolation hospital or ICU. In a few instances, the same PI was involved in more than one study. While there are no regulations against this, the REC was concerned about the involvement of the same patient in more than one study. The FMASU REC did not allow enrolment of subjects in more than one ongoing clinical trial. This decision conflicts with Cinnella and Gertner who reported that co-enrolment does not affect the safety of patients, the study outcome, or side effects, provided that the inclusion and exclusion criteria are appropriately set ( 10 , 11 ).
Before COVID-19, the REC stressed on selecting the appropriate control groups for the study, especially regarding how healthy controls were selected. For the COVID-19 protocols the controls were usually sick patients, hence risk mitigation was the major issue. During review of COVID-19 protocols, the REC noticed that several RCTs control groups did not receive interventional medication and had similar characteristics and inclusion criteria, e.g., the severity of the disease. To decrease the number of subjects included in the studies, the REC suggested the use of the same set of controls, whenever applicable and possible, in more than one study. This meant that the subjects were enrolled only once, and their data was shared with other investigators performing their studies at the same time in the same place. The intervention arm, however, included different subjects, receiving different medications according to the trial they were enrolled in. The committee also required the intervention group to control group ratio to be 1:1, and not more, in order to avoid enrolment of more subjects than required in the RCTs.
In many clinical trials placebo control arm is recommended, especially where the effect of the drug is still not well-documented, or as obvious as in cancer research where a drug causes shrinkage of the tumor ( 12 ). For the placebo-controlled trials, the REC decided that all participants must receive the updated standard of care treatment as per the Egyptian MOHP protocol for COVID-19 patients, while the participants receive the new drug under trial as add-on therapy. This way the clinical trial design was an add-on design, where the controls received the standard care MOHP protocol, rather than receiving a sham medication. The REC thought that this could minimize the risk, although the standard protocols at the start of the pandemic had no clear evidence of benefit.
The inclusion of the Egyptian MOHP COVID-19 management protocol was a challenge to the investigators, as the treatment protocol was constantly updated according to new information arising in that arena. The FMASU REC recommended that the standard of care set by the Egyptian MOHP always be updated in the submitted protocols and provided to all COVID-19 study participants.
Regarding the clinical trial endpoints, the FMASU REC had lengthy discussions on the use of measurable achievement vs. patient-related outcomes in the pandemic research. Several protocols used “the time to clinical improvement” as the measure for outcome. Clinical improvement in some studies was defined as the time from randomization to either an improvement of two points on a six or eight-category ordinal scale. Some members of the committee considered the scale as very subjective and required the use of more objective or measurable outcomes. The FMASU REC resorted to the time to clinical improvement scale, in addition to the patient-related outcome scales, the investigators should use more objective outcome measures in their studies, such as persistent positive PCR tests after treatment, or time until the emergence of antibodies against the novel virus. The use of objective tests as outcomes would be a dynamic process as new information is published.
Ensuring the provision of a clear informed consent form was a big challenge for the committee, and certainly for the investigators as well. The motivational force behind the willingness of the patients to enroll in the clinical trials is complex, and therapeutic misconception had to be clearly avoided in the consent language. Due to the scarcity of available information about the virus and the use of novel drugs, the committee ensured that the investigators simplify the information provided to the participants in a manner that the participants could comprehend, as there were so many unknown facts about the virus. The committee understood how challenging it was to describe and explain unknown risks to the potential participants. Still, the FMASU REC required the explanation of risks to be clear and in a language the participants could understand. Furthermore, the prospect of direct benefits was in no way to be promised or overestimated. The FMASU REC members conducted a meticulous review of the wording of the informed consent to ensure the message was clearly communicated to the potential participants and their guardians, regarding the lack of scientific evidence of the efficacy of the used drugs, as well as the unknown side effects, while still providing convincing rationale for the performance of the study.
Additional minor concerns in some of the studies included the completion of a diary for drug doses to be completed by the patients. The FMASU REC was concerned about how a severely ill participant would record his/her daily doses of drugs under trial, especially for the non-educated participants. The FMASU REC requested that this be confined to moderate or highly educated cases only.
The FMASU REC requested the timely reporting of all adverse events as soon as they occurred, not only the serious ones as per the standard procedures which assign a medical monitor in all COVID-19 clinical trials. The medical monitor should be a medical doctor, not involved in the study, but who observes the progress of the study and provides reports to the FMASU REC in case of adverse events. In studies assessing psychological risks to healthcare workers, the FMASU REC requested that participants who tested positive on screening, receive free management and referral to receive psychiatric help if proven to be suffering from anxiety or depression.
Common modifications and clarifications requested by the FMASU REC were the inclusion criteria and the age range of the recruited subjects. Enrolment of subjects not receiving other medicines under trial was a challenge during review. Detailed data of the study procedures were requested in many submitted research studies. More frequent progress reports were requested.
To mitigate risks, more frequent electrocardiograms, complete blood counts, x-rays, and chest Computed Tomographies were requested to safeguard against serious adverse events of the drugs under investigation, such as Hydroxychloroquine and other repurposed drugs. Regarding the control groups in the RCTs, the REC recommended that controls obtain the standard MOHP protocol for COVID-19 management. The REC requested monthly progress reports and swift notification of serious adverse events.
The innovative approaches adopted by the REC in the earlier wave of the COVID-19 pandemic were acceleration of submission of pre-requisite paperwork needed for application, increased frequency of virtual meetings, expansion of meeting agendas, direct contact with the PIs by phone and fast-track review within 1-7 days (compared to the usual 1-month review of clinical trials according to the SOPs.
FMASU REC experience, being active for 13 years since 2007 would be beneficial for other Egyptian RECs, numbering approximately 85 committees in 2021 with variable experience. Seventy Egyptian RECS are linked through a non-governmental body named the Egyptian Network of Research Ethics Committees (also known as ENREC), established in 2008, that enhances REC networking, standardizing the SOPs among RECs all over Egypt, the exchange of knowledge of research review challenges and obstacles, as well as finding solutions through regular annual meetings ( 13 ). Thirty-five RECs are registered with the MOHP.
Data confidentiality is fundamental in both COVID-19 and non-COVID-19 research to protect the life, health, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information of research subjects. It has been practiced since the establishment of the committee according to the Declaration of Helsinki ( 14 ).
During the review of COVID-19 research, the REC was more diligent in reviewing the parts of the protocol where the investigators detailed the precautions for data confidentiality, including databases and computer files, as well as paper copies of questionnaires and informed consents. REC members followed the REC review checklist to review the protocols with confidentiality adequately listed in the checklist. Digital tracking technologies were not allowed, so there were no concerns regarding generated data confidentiality.
Some of the strengths of this analysis at the organizational level were REC resilience and at the research level, the researchers continued conduct of research in spite of the discussed challenges in order to generate knowledge for this new disease and to accomplish investigator career progress. Regarding the research participants: COVID-19 investigation results, including PCR, lab and radiology and all medications given to research participants were provided free of charge. Participants were offered the autonomy to participate in research under strict REC oversight during that period, which was characterized by little and misinformation. Regarding inclusiveness and diversity, participants included healthcare professionals, literate and illiterate patients, and the elderly without discrimination in research enrolment while maintaining equity of healthcare.
Our research prioritization during the COVID-19 pandemic is in line with that of Kheng-Wei Yeoh and Ketan Shah, who provided recommendations for RECs on research prioritization and fast-tracking research, without compromising the participants' safety and well-being. Priority should be given to research that helps find a cure for the patients, while other research should be re-evaluated for public health concerns and precautions incorporated into the studies. As for the design, the authors recommend incorporating the study design into clinical care and look for new information due to the increased demand ( 15 ).
The Pan American Health Organization (PAHO), website on April 15, 2020 published guidance for the development of SOPs for RECs for the review of research during the COVID-19 pandemic. In addition to the revised SOPs, the PAHO recommended that RECs should accelerate reviews and initiate a system for follow up on COVID-19 research ( 16 ).
Due to confidentiality issues, the authors provided minimal details about the studies. We would have liked to expand on the types of research, but many of the studies included new drugs about which we could not provide details. Another limitation is that this article's scope is limited to the performance of the REC and the challenges it faced, rather than a predesigned research study or survey.
During the COVID-19 pandemic the FMASU REC was overwhelmed with a huge number of COVID-19 -related research protocols. The increased amount of research protocols to be reviewed in a short time presented several logistic and ethical challenges. The committee had to adopt different methods of review to ensure adherence to the ethical principles. The ethics training background of the members proved beneficial to balance the risks and benefits to the patients among novel ethical dilemmas.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This study was self-funded by the authors, who have not received any monetary or financial support for the writing and publishing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the efforts of all the FMASU REC members and office staff during the first wave of the COVID-19 pandemic and FMASU administration's support.
Abbreviations
RECs, Research ethics committees; EUREC, European Network of Research Ethics Committees; FMASU REC, Faculty of Medicine, Ain Shams University Research Ethics Committee; ICU, Intensive care units; MOHP, Ministry of Health and Population; PAHO, Pan American Health Organization; PCR, Polymerase Chain Reaction; PI, Principal investigator; RCTs, Randomized controlled trials; SOPs, Standard operating procedures; WHO, World Health Organization.
1. Marzouk D, Abd El Aal W, Saleh A, Sleem H, Khyatti M, Mazini L, et al. Overview on health research ethics in Egypt and North Africa. Eur J Public Health . (2014) 24(Suppl. 1):87–91. doi: 10.1093/eurpub/cku110
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sisi Passes Law Regulating Clinical Medical Research in Egypt. Egypt: Independent Gazette (2020). Available online at: https://egyptindependent.com/sisi-passes-law-regulating-clinical-medical-research-in-egypt/ (accessed February 28, 2021)
Google Scholar
3. Available, online at: https://clinicaltrials.gov/ct2/results?cond=COVID-19 (accessed March 3, 2021)
4. Guidance for Managing Ethical Issues in Infectious Disease Outbreaks . Geneva: World Health Organization (2016). Available online at: https://apps.who.int/iris/bitstream/handle/10665/250580/9789241549837-eng.pdf?sequence=1&isAllowed=y (accessed November 10, 2020)
5. Ethical Standards for Research During Public Health Emergencies: Distilling Existing Guidance to Support COVID-19 R&D. Geneva: World Health Organization (2020). Available online at: https://apps.who.int/iris/bitstream/handle/10665/331507/WHO-RFH-20.1-eng.pdf?sequence=1&isAllowed=y (accessed November 10, 2020)
6. Position of the European Network of Research Ethics Committees (EUREC) on the Responsibility of Research Ethics Committees during the COVID-19 Pandemic (2020). Available online at: https://ancei.es/wp-content/uploads/2020/05/EUREC-Positionpaper_COVID_19.pdf (accessed December, 2020)
7. Ministry of Higher Education and Scientific Research. Available online at: http://portal.mohesr.gov.eg/ar-eg/Pages/Home.aspx (accessed June, 2021)
8. Molyneux M. New ethical considerations in vaccine trials. Hum Vaccines Immunother . (2017) 13:2160–3. doi: 10.1080/21645515.2016.1272744
9. Central Agency for Public Mobilization and Statistics (CAPMAS) (2019). Available online at: https://dailynewsegypt.com/2020/09/08/egypts-illiteracy-rate-decreased-to-24-6-in-july-2019-capmas/ (accessed August 2, 2021)
10. Cinnella G. Enrolling patients into multiple trials: it is time for glasnost. Crit Care Med . (2015) 43:485–6. doi: 10.1097/CCM.0000000000000756
11. Gertner E. COVID-19 trial co-enrolment and subsequent enrolment. Lancet . (2020) 396:461–2. doi: 10.1016/S0140-6736(20)31537-3
12. Schilsky RL. Placebos in Cancer Clinical Trials (2018). Available online at: https://www.cancer.net/research-and-advocacy/clinical-trials/placebos-cancer-clinical-trials (accessed February 28, 2021)
13. Egyptian Network of Research Ethics Committees (2008). Available online at: http://www.enrec.org (accessed July, 2021)
14. WMA Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects (2021). Available online at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed July, 2021)
15. Kheng-Wei Y, Ketan S. Research ethics during a pandemic (COVID-19). Int Health . (2021) 13:374–5. doi: 10.1093/inthealth/ihaa054
16. Available, online at: https://iris.paho.org/handle/10665.2/52086 (accessed August 1, 2021)
Keywords: research ethics committees, Faculty of Medicine Ain Shams University, COVID-19 research, ethical challenges, accelerated review
Citation: Marzouk D, Sharawy I, Nakhla I, El Hodhod M, Gadallah H, El-Shalakany A, Elwakil R, Moussa MM, Ismail A and Tash FM (2021) Challenges During Review of COVID-19 Research Proposals: Experience of Faculty of Medicine, Ain Shams University Research Ethics Committee, Egypt. Front. Med. 8:715796. doi: 10.3389/fmed.2021.715796
Received: 27 May 2021; Accepted: 29 September 2021; Published: 02 November 2021.
Reviewed by:
Copyright © 2021 Marzouk, Sharawy, Nakhla, El Hodhod, Gadallah, El-Shalakany, Elwakil, Moussa, Ismail and Tash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diaa Marzouk, diaamarzouk@med.asu.edu.eg
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Introduction
- Article Information
Data Sharing Statement
- Incomplete Sentence in Discussion Section JAMA Network Open Correction February 8, 2023
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Llor C , Moragas A , Maier M. Evaluation of Publication of COVID-19–Related Articles Initially Presented as Preprints. JAMA Netw Open. 2022;5(12):e2245745. doi:10.1001/jamanetworkopen.2022.45745
Manage citations:
© 2024
- Permissions
Evaluation of Publication of COVID-19–Related Articles Initially Presented as Preprints
- 1 Research Unit for General Practice, Department of Public Health, University of Southern Denmark, Odense, Denmark
- 2 Primary Care Research Institute Jordi Gol, Barcelona, Spain
- 3 Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Barcelona, Spain
- 4 Jaume I Health Centre, Institut Català de la Salut, University Rovira i Virgili, Catalonia, Spain
- 5 Department of General Practice and Family Medicine, Centre for Public Health, Medical University of Vienna, Vienna, Austria
- Correction Incomplete Sentence in Discussion Section JAMA Network Open
Since the launch of the medRxiv preprint server in 2019, the dissemination of research as preprints has grown rapidly, largely facilitated by the COVID-19 pandemic. 1 Notwithstanding, this unprecedented increase in preprints has been subject to criticism, mainly because of reliability concerns owing to their lack of peer review. In 2020, Abdill et al 2 reported that 62.6% of bioRxiv preprints were later published in scientific journals, considering a time frame of at least 1 year. However, other studies 3 , 4 have highlighted the low percentage of medRxiv preprints subsequently published in journals, with publication rates of 14.0% after 0 to 12 months 3 and 10.6% after 6 to 19 months. 4 In an analysis of COVID-19–related preprints posted on 3 servers, Añazco et al 5 observed that 5.7% were published in a journal 3 to 8 months after their preprint posting. To our knowledge, no recent studies have analyzed whether journal publication rates of medRxiv preprints have changed. Therefore, we conducted this study to evaluate the subsequent journal publication of COVID-19–related preprint articles posted on medRxiv in 2020.
This cross-sectional study did not require institutional review board approval or informed consent because it used publicly available data, in accordance with 45 CFR §46. The study followed the STROBE reporting guideline.
In March 2022, we searched preprints on medRxiv and included all papers on COVID-19 in the infectious diseases subject area that were posted between January 1 and December 31, 2020. We repeated this search in October 2022. Two of us (C.L. and A.M.) completed and verified both searches.
We checked whether a preprint was already published in a peer-reviewed journal by measuring the proportion of medRxiv preprints flagged as published. We recorded the journal names and obtained the journal rankings by measuring their quartile according to the updated 2021 Journal Citation Reports, 6 in which quartiles 1 and 4 indicate the top 25% and bottom 25% of journals in a particular category, respectively.
Results are presented as counts and percentages. Descriptive statistical analyses were conducted using Excel software, version 16.0 (Microsoft Corp).
In this study, we identified 3343 COVID-19–related preprints posted on medRxiv in 2020. Our March 2022 search indicated that 1712 of those preprints (51.2%) were subsequently published in the peer-reviewed literature; this number increased to 1742 (52.1%) when we repeated the search in October 2022. Not considering January 2020, in which only 1 article on COVID-19 was posted, the rate of subsequent publication in a scientific journal ranged from 43.5% (94 of 216 preprints; observed in March 2020) to 60.6% (177 of 292 preprints posted in August 2020). The Table shows the top 25 of 579 peer-reviewed journals in which these preprints were published; 827 preprints (47.5%) were subsequently published in quartile 1 journals ( Figure ).
Researchers are able to communicate their findings immediately by posting papers on preprint servers, which has become even more important during the COVID-19 pandemic. In this cross-sectional study, we observed that slightly more than half of the preprints related to COVID-19 posted on medRxiv in 2020 were later published in peer-reviewed journals as of October 2022. This publication rate is only slightly greater than that observed 7 months earlier in March 2022, which suggests that a substantial change in the proportion of papers subsequently published in peer-reviewed journals is not expected in the future. Another notable finding of this study is the high quality of the journals in which these articles were subsequently published, as nearly half of the preprints were published in quartile 1 journals.
This study has the limitations of having analyzed only preprints related to COVID-19 posted on a single preprint server and relying only on the medRxiv indication of a related published article, which may have resulted in undercounting. The publication rate of preprints on other topics may be different. Future studies aimed at evaluating publication rates in other areas of medical science are needed.
Accepted for Publication: October 19, 2022.
Published: December 8, 2022. doi:10.1001/jamanetworkopen.2022.45745
Correction: This article was corrected on February 8, 2023, to correct a sentence in the Discussion concerning the study’s limitations.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 Llor C et al. JAMA Network Open .
Corresponding Author: Carl Llor, MD, PhD, Research Unit for General Practice, Department of Public Health, University of Southern Denmark, J.B. Winsløws Vej 9, DK-5000 Odense C, Denmark ( [email protected] ).
Author Contributions: Dr Llor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Llor, Maier.
Acquisition, analysis, or interpretation of data: Llor, Moragas.
Drafting of the manuscript: Llor.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Llor.
Administrative, technical, or material support: Llor, Moragas.
Supervision: Moragas, Maier.
Conflict of Interest Disclosures: Dr Llor reported receiving grants from Abbott Diagnostics during the conduct of the study. No other disclosures were reported.
Data Sharing Statement: See the Supplement .
Additional Contributions: We thank Josh Gray, MD (Duke Univesity Health System), for helping with the reanalysis of our data. He was not financially compensated for his contribution.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Learning sustainability: post-graduate students’ perceptions on the use of social media platforms to enhance academic writing.

1. Introduction
2. literature review, 2.1. online communication through social media for educational purposes, 2.2. academic writing, 2.3. social media, academic writing, and the fourth goal of sustainable development, 3. research questions.
- How do post-graduate students perceive the role of social media platforms in enhancing their academic writing?
- What are the obstacles to the use of social media platforms that hinder enhancing academic writing?
4. Methodology
4.1. participants, 4.2. research approach, 4.3. research procedure, 4.4. data collection and analysis.
- What social media tools were used by you in writing the research plan?
- Which of these tools do you prefer to use? Why?
- Describe your experience using your favorite tool in some detail.
- What do you think of the use of these tools in terms of their usefulness in improving the quality of your writing of the research plan? Why?
- From your point of view, what are the disadvantages of using these tools that may negatively affect the quality of your writing of the research plan? Why?
- From your point of view, what are the obstacles to using these tools that may reduce their usefulness in improving the quality of your writing of the research plan? Why?
- Do you prefer using social media tools or traditional methods of communicating with the supervisor regarding the development of the research plan? Why?
- Familiarizing yourself with your data: The authors of the study were the interviewers. Therefore, they were very familiar with the data.
- Generating initial codes: The initial interesting codes from the data were identified. The codes were then assessed in a meaningful way regarding the research questions.
- Searching for themes: Here the analysis re-focused on the codes on a broader level, which is generating themes. In this phase, different codes are sorted into potential themes.
- Reviewing themes: The initial list of themes was refined, and some candidate themes that did not have enough data to support them were ignored.
- Defining and naming themes: In this phase, the data were defined by identifying the essence of what each theme was about and were refined by determining what aspect of the data each theme captured. Table 2 illustrates the themes and frequencies of the interview data revealed from the thematic analysis.
- Producing the report: This phase involved the final analysis under the themes and write-up of the final report. The final report of the data analysis and the concluded findings will be reviewed in the following section.
5. Findings and Discussion
6. limitation, 7. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Graham, L.; Berman, J.; Bellert, A. Sustainable Learning: Inclusive Practices for 21st Century Classrooms ; Cambridge University Press: Sydney, Australia, 2015. [ Google Scholar ]
- UNESCO. Incheon Declaration: Education 2030: Towards Inclusive and Equitable Quality Education and Lifelong Learning for All ; UNESCO: Paris, France, 2015. [ Google Scholar ]
- Al Mulhim, E.N. Technology Fatigue During the COVID-19 Pandemic: The Case of Distance Project-Based Learning Environments. Turk. Online J. Distance Educ. 2023 , 24 , 234–245. [ Google Scholar ] [ CrossRef ]
- Alshammary, F.M.; Alhalafawy, W.S. Sustaining Enhancement of Learning Outcomes across Digital Platforms during the COVID-19 Pandemic: A Systematic Review. J. Pos. Sch. Psyc. 2022 , 6 , 2279–2301. [ Google Scholar ]
- Ismaeel, D.A.; Al Mulhim, E.N. E-teaching Internships and TPACK during the Covid-19 Crisis: The Case of Saudi Pre-service Teachers. Int. J. Instr. 2022 , 15 , 147–166. [ Google Scholar ] [ CrossRef ]
- Vision 2030. National Transformation Program. Available online: https://www.vision2030.gov.sa/en/vision-2030/vrp/national-transformation-program/ (accessed on 15 April 2023).
- Glazer, H.R.; Breslin, M.; Wanstreet, C.E. Online professional and academic learning communities: Faculty perspectives. Q. Rev. Distance Educ. 2013 , 14 , 123–130. [ Google Scholar ]
- Thormann, J.; Fidalgo, P. Guidelines for online course moderation and community building from a student’s perspective. J. Online Learn. Teach. 2014 , 10 , 374–388. [ Google Scholar ]
- Froment, F.; García González, A.J.; Bohórquez, M.R. The Use of Social Networks as a Communication Tool between Teachers and Students: A Literature Review. Turk. Online J. Educ. Technol. TOJET 2017 , 16 , 126–144. [ Google Scholar ]
- Vlachopoulos, D.; Makri, A. Online communication and interaction in distance higher education: A framework study of good practice. Int. Rev. Educ. 2019 , 65 , 605–632. [ Google Scholar ] [ CrossRef ]
- Habibi, A.; Mukminin, A.; Riyanto, Y.; Prasojo, L.D.; Sulistiyo, U.; Sofwan, M.; Saudagar, F. Building an online community: Student teachers’ perceptions on the advantages of using social networking services in a teacher education program. Turk. Online J. Distance Educ. 2018 , 19 , 46–61. [ Google Scholar ] [ CrossRef ]
- Chugh, R.; Ruhi, U. Social media in higher education: A literature review of Facebook. Edu. Info. Tech. 2018 , 23 , 605–616. [ Google Scholar ] [ CrossRef ]
- Faramarzi, S.; Tabrizi, H.H.; Chalak, A. Telegram: An instant messaging application to assist distance language learning. Teach. Engl. Technol. 2019 , 19 , 132–147. [ Google Scholar ]
- Aghajani, M.; Adloo, M. The Effect of Online Cooperative Learning on Students’ Writing Skills and Attitudes through Telegram Application. Int. J. Instr. 2018 , 11 , 433–448. [ Google Scholar ] [ CrossRef ]
- VanDoorn, G.; Eklund, A.A. Face to Facebook: Social media and the learning and teaching potential of symmetrical, synchronous communication. J. Univ. Teach. Learn. Pract. 2013 , 10 , 1–14. [ Google Scholar ] [ CrossRef ]
- Northey, G.; Bucic, T.; Chylinski, M.; Govind, R. Increasing student engagement using asynchronous learning. J. Mark. Educ. 2015 , 37 , 171–180. [ Google Scholar ] [ CrossRef ]
- Dewey, J. How We Think: A Restatement of the Relation of Reflective Thinking to the Educative Process ; D.C. Heath & Co Publishers: Boston, MA, USA, 1993. [ Google Scholar ]
- Piaget, J. The Psychology of the Child ; Basic Books: New York, NY, USA, 1972. [ Google Scholar ]
- Vygotsky, L. Mind in Society ; Harvard University Press: Cambridge, MA, USA, 1978. [ Google Scholar ]
- Kelm, O.R. Social media: It’s what students do. Bus. Commun. Q. 2011 , 74 , 505–520. [ Google Scholar ] [ CrossRef ]
- Churcher, K.; Downs, E.; Tewksbury, D. “Friending” Vygotsky: A Social Constructivist Pedagogy of Knowledge Building through Classroom Social Media Use. J. Eff. Teach. 2014 , 14 , 33–50. [ Google Scholar ]
- Schrader, D.E. Constructivism and Learning in the Age of Social Media: Changing Minds and Learning Communities. New Dir. Teach. Learn. 2015 , 144 , 23–35. [ Google Scholar ] [ CrossRef ]
- Akpan, V.I.; Igwe, U.A.; Mpamah, I.B.I.; Okoro, C.O. Social constructivism: Implications on teaching and learning. Br. J. Edu. 2020 , 8 , 49–56. [ Google Scholar ]
- Buriro, G.A.; Charan, A.A. Social media tools at developing academic writing skills. UICELL Conf. Proc. 2018 , 2 , 29–37. [ Google Scholar ]
- Scott, C.E.; Ritter, N.L.; Fowler, R.M.; Franks, A.D. Developing a community of academic writers: Using social media to support academic accountability, motivation, and productivity. J. Lit. Technol. 2019 , 20 , 61–96. [ Google Scholar ]
- Seneviratne, L.C. An Intervention Using Digital Social Media to Support Academic Writing of University Students: A case study. Available online: https://research.usq.edu.au/download/18d93bd589ccde0d3b5b95e600ee9d06efbd1d9a943116acbc0a780f5856e943/3486702/Seneviratne_2018_whole.pdf (accessed on 20 March 2023).
- Zheng, B. Social Media and Classroom Writing: Participation, Interaction, and Collaboration. Available online: https://www.proquest.com/openview/f69e557a16831d2576ba1fc607fde189/1?pq-origsite=gscholar&cbl=18750 (accessed on 27 July 2023).
- Sun, Y.C. Extensive writing in foreign-language classrooms: A blogging approach. Innov. Educ. Teach. Int. 2010 , 47 , 327–339. [ Google Scholar ] [ CrossRef ]
- Alsamadani, H.A. The Effectiveness of Using Online Blogging for Students’ Individual and Group Writing. Int. Educ. Stud. 2018 , 11 , 44–51. [ Google Scholar ] [ CrossRef ]
- Yeboah, J.; Ewur, G.D. The impact of WhatsApp messenger usage on students performance in Tertiary Institutions in Ghana. J. Educ. Pract. 2014 , 5 , 157–164. [ Google Scholar ]
- Abdul Fattah, S.F.E.S.A. The Effectiveness of Using WhatsApp Messenger as One of Mobile Learning Techniques to Develop Students’ Writing Skills. J. Educ. Pract. 2015 , 6 , 115–127. [ Google Scholar ]
- Lin, W.C.; Yang, S.C. Exploring students’ perceptions of integrating Wiki technology and peer feedback into English writing courses. Engl. Teach. Pract. Crit. 2011 , 10 , 88–103. [ Google Scholar ]
- Li, X.; Chu, S.K.; Ki, W.W.; Woo, M.M. Using a wiki-based collaborative process writing pedagogy to facilitate collaborative writing among Chinese primary school students. Australas. J. Educ. Technol. 2012 , 28 , 159–181. [ Google Scholar ] [ CrossRef ]
- Woo, M.M.; Chu SK, W.; Li, X. Peer-feedback and revision process in a wiki mediated collaborative writing. Educ. Technol. Res. Dev. 2013 , 61 , 279–309. [ Google Scholar ] [ CrossRef ]
- Baishya, D.; Maheshwari, S. WhatsApp Groups in Academic Context: Exploring the Academic Uses of WhatsApp Groups among the Students. Contemp. Educ. Technol. 2020 , 11 , 31–46. [ Google Scholar ] [ CrossRef ]
- Iqbal, M.Z.; Alradhi, H.I.; Alhumaidi, A.A.; Alshaikh, K.H.; AlObaid, A.M.; Alhashim, M.T.; AlSheikh, M.H. Telegram as a tool to supplement online medical education during COVID-19 crisis. Acta Inform. Med. 2020 , 28 , 94. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Suárez-Lantarón, B.; Deocano-Ruíz, Y.; García-Perales, N.; Castillo-Reche, I.S. The Educational Use of WhatsApp. Sustainability 2022 , 14 , 10510. [ Google Scholar ] [ CrossRef ]
- Yılmazsoy, B.; Kahraman, M.; Köse, U. Negative Aspects of Using Social Networks in Education: A Brief Review on WhatsApp Example. J. Educ. Technol. Online Learn. 2020 , 3 , 69–90. [ Google Scholar ] [ CrossRef ]
- Revere, L.; Kovach, J.V. Online Technologies for Engaged Learning A Meaningful Synthesis for Educators. Q. Rev. Distance Educ. 2011 , 12 , 113–124. [ Google Scholar ]
- González-Padilla, D.A.; Tortolero-Blanco, L. Social media influence in the COVID-19 pandemic. Int. Braz. J. Urol. 2020 , 46 , 120–124. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Global Education Monitoring Report Team (UNESCO). Global Education Monitoring Report 2023: Technology in Education: A Tool on whose Terms? United Nations Educational, Scientific and Cultural Organization: Paris, France, 2023. [ Google Scholar ]
- Al Mulhim, E.N.; Zaky, Y.A.M. Sustainability in E-Learning: E-Books and Academic Procrastination among Secondary Students. Sustainability 2023 , 15 , 14668. [ Google Scholar ] [ CrossRef ]
- Fülöp, M.T.; Breaz, T.O.; Topor, I.D.; Ionescu, C.A.; Dragolea, L.L. Challenges and perceptions of e-learning for educational sustainability in the “new normality era”. Front. Psychol. 2023 , 14 , 1104633. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Prasetyanto, D.; Rizki, M.; Sunitiyoso, Y. Online Learning Participation Intention after COVID-19 Pandemic in Indonesia: Do Students Still Make Trips for Online Class? Sustainability 2022 , 14 , 1982. [ Google Scholar ] [ CrossRef ]
- Geith, C.; Vignare, K. Access to Education with Online Learning and Open Educational Resources: Can they Close the Gap? On. Lear. 2019 , 12 , 105–126. [ Google Scholar ] [ CrossRef ]
- Sobaih, A.E.E.; Moustafa, M.A.; Ghandforoush, P.; Khan, M. To use or not to use? Social media in higher education in developing countries. Com. Hum. Behav. 2016 , 58 , 296–305. [ Google Scholar ] [ CrossRef ]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006 , 3 , 77–101. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Demographic Data | n | Percentages | |
|---|---|---|---|
| Gender | Males | 4 | 40% |
| Females | 6 | 60% | |
| Age range | 22–27 years old | 8 | 80% |
| 28–31 years old | 2 | 20% | |
| Specialization in Masters | Educational Technology | 10 | 100% |
| Specialization in Bachelor’s degree | Science | 1 | 10% |
| Math | 2 | 20% | |
| Computer Sciences | 4 | 40% | |
| Arabic | 1 | 10% | |
| Early Childhood | 1 | 10% | |
| Social Studies | 1 | 10% | |
| Main Themes | Sub Themes | Frequency | Percentages |
|---|---|---|---|
| Social media tools | 7 | 70% | |
| Microsoft Teams | 4 | 40% | |
| Zoom | 3 | 30% | |
| 2 | 20% | ||
| Telegram | 1 | 10% | |
| Blackboard | 1 | 10% | |
| Benefits | Easy communication | 7 | 70% |
| Transcending the limits of space | 6 | 60% | |
| Instant feedback | 5 | 50% | |
| Repeat review files | 5 | 50% | |
| Interactive guidance | 5 | 50% | |
| Save time and effort | 5 | 50% | |
| File and resource sharing | 5 | 50% | |
| Transcending the limits of time | 4 | 40% | |
| Diverse file format | 3 | 30% | |
| Communicate experienced researchers | 3 | 30% | |
| Favorite method of holding meetings | Distance | 6 | 60% |
| Blended | 4 | 40% | |
| Obstacles | Technical problems | 5 | 50% |
| Poor face-to-face communication skills | 3 | 30% | |
| Distracting | 3 | 30% | |
| Lack of technical skills | 1 | 10% | |
| Burden | 1 | 10% |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Al Mulhim, E.N.; Ismaeel, D.A. Learning Sustainability: Post-Graduate Students’ Perceptions on the Use of Social Media Platforms to Enhance Academic Writing. Sustainability 2024 , 16 , 5587. https://doi.org/10.3390/su16135587
Al Mulhim EN, Ismaeel DA. Learning Sustainability: Post-Graduate Students’ Perceptions on the Use of Social Media Platforms to Enhance Academic Writing. Sustainability . 2024; 16(13):5587. https://doi.org/10.3390/su16135587
Al Mulhim, Ensaf Nasser, and Dina Ahmed Ismaeel. 2024. "Learning Sustainability: Post-Graduate Students’ Perceptions on the Use of Social Media Platforms to Enhance Academic Writing" Sustainability 16, no. 13: 5587. https://doi.org/10.3390/su16135587
Article Metrics
Further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
By continuing to browse the site you are agreeing to our use of cookies and similar tracking technologies described in our privacy policy .
Voice of the Discipline
News and publications.
Access AHA news and publications supporting the work of historians.
Stay up-to-date with the AHA

June 25, 2024
AHA Members Co-author Article on SCOTUS and Gun Control
AHA members Holly Brewer (Univ. of Maryland) and Laura F. Edwards (Princeton Univ.) have co-authored an article for Washington Monthly…

June 24, 2024
Action Alert Opposing Ohio SB 83

June 21, 2024
AHA Signs On to CIE Letter Urging HEA-Title VI Funding for FY 2025

June 18, 2024
Welcome to the AHA’s New Website
The American Historical Review is the flagship journal of the AHA and the journal of record for the historical discipline in the United States, bringing together scholarship from every major field of historical study.
Perspectives on History is the newsmagazine of the AHA and is the principal source for news and information about the discipline of history. Since 1962, Perspectives has promoted our work by publishing articles and commentary on all aspects of the historical discipline.
History in Focus Podcast

Environmental Crisis and Recovery
Collaborative history + revisiting marion thompson wright, aha booklets.
The AHA publishes booklets that address a diversity of topics to serve the needs of history students and historians in all professions. Our publications include career advice for history graduates, overviews and syntheses of current historical topics and fields, and guides to teaching and learning in history.
For the Press
The AHA is pleased to provide resources for journalists and press. If you are a member of the media and would like to submit a request for a referral or interview, please email [email protected] . Please provide any pertinent deadlines and we will do our best to accommodate your request. The AHA can find you a historian for any topic, and assists with dozens of inquiries each year.
The AHA encourages the reading of history with periodic reading challenges.
Permission to Use AHA Copyrighted Material
All material published by the American Historical Association in any medium is protected by copyright.
Join the AHA
The AHA brings together historians from all specializations and all work contexts, embracing the breadth and variety of activity in history today.
- How it works
- Pay for essays
- Do my homework
- Term Paper Writing Service
- Do my assignment
- Coursework help
- Our Writers

How to write a research proposal: a detailed guide for students

A professional writer with ten years of experience and a Ph.D. in Modern History, Catharine Tawil writes engaging and insightful papers for academic exchange. With deep insight into the impact of historical events on the present, she provides a unique perspective in giving students a feel for the past. Her writing educates and stimulates critical thinking, making her a treasure to those wading through the complexities of history.
How to write a research proposal? Although writing academic papers and completing projects is part of the routine of any young learner, this assignment can often be troublesome. Still, if you are looking for professional research proposal guidelines, you’ve come to the right place. In this post, we’ll go down the rabbit hole and discover all the best ways to complete this assignment easily and quickly.
Research proposal: meaning and description
Research proposals are not easy to write. However, if you follow our tips and tricks, you will achieve all your academic goals. As a rule, you need to develop a strong research proposal before you start working on your research paper. In other words, it’s like preparing a list of ingredients for cooking your main course.
Your paper will generally contain a topic (well, that’s the most straightforward component), your research questions, methodology, and the significance of the chosen field. However, the requirements might differ depending on your academic level and the overall complexity of your paper.
Why a good research proposal matters
What do I need to compose a proposal writing? Many bright minds ask this question. The answer is that it has many goals. First and foremost, it allows learners to clarify their ideas and get approval from their teachers or professors. The second thing about this writing is that it helps students create a well-structured and properly formatted paper before diving too deep into the research paper. As a rule, you must submit your proposal before you start working on your research project, thesis, or dissertation.
Top components of a research proposal
Your research proposal must cover many experts. For your convenience, we’ve prepared a list of top features you are expected to have in this type of writing.
- Topic: Similar to a research paper, your proposal must have a precise topic. It should be understandable, fresh, and sharp. And, of course, it should be focused on your research for 100%.
- Intro: This is background info for your topic. In most cases, it highlights the importance of your study and describes the research objectives.
- Literature review: What literature is related to the chosen niche? What unresolved questions does your field have? Why do you need to conduct the research? Your literature review helps you prove that you have already conducted the primary research and understand the selected topic well. This point is a must-to-write for all research proposals.
- Research questions: What questions will your study address? Make sure they are unique, measurable, and achievable.
- Methodology: In this field, you must specify the methods and techniques you will use to collect and process your findings.
- Timeline: Every project has its deadlines and milestones. You will need to create an approximate schedule for completing your assignment. This schedule must include time for collecting information, advanced data analysis, and writing.
- Reference: As with the other academic assignments, you must develop a reference list for your paper proposal.
- Appendices: This is the best place to provide your supplementary artifacts and other materials.
How can you understand your research problem
Now that you have a better understanding of a research proposal, what’s next? Below is a simple step-by-step solution for writing a research proposal.
Spotlighting the research gap
Identifying a research gap for a research proposal involves several stages. Firstly, you will need to review existing literature in your field. This will allow you to pinpoint areas where knowledge is lacking or contradictory. For example, you can search for some unanswered questions that require more investigation. After that, you may consider recent developments or emerging trends that created new gaps in existing research. Finally, you should critically evaluate your expertise and interests.
Developing your main research question
Developing your main research question for a research proposal usually involves the following critical steps:
- Brainstorming ideas and narrowing down your topic to a specific field.
- Clarify the gaps you’ve discovered in the previous stage.
- Formulate your main research question.
- Double-check your question for relevance and clarity.
- Refine your research question and make sure it fully aligns with your goals.
Choosing your top-notch research objectives
Now, it’s time to dive into the ocean of your research proposal objectives. Although there might be too many goals, you must select only the most important ones. Moreover, it requires careful consideration and strategic planning. The best approach to this task is to start by identifying the main purpose of your research. After that, you may try to connect your goals with the main questions and the research gap. Not to mention, make sure to focus only on realistic goals during your objective’s research design.
Working on your literature review
If you look at any good-written research proposal example, you will notice that it always has a literature review section. To complete it easily, feel free to follow these easy steps:
- Gather articles and texts that address these themes.
- Critically analyze and synthesize the findings from the chosen sources.
- Organize your literature review thematically or chronologically.
- Conclude by summarizing the current state of knowledge and explaining your proposed research's contribution.
Selecting the research methodology
When choosing the most fitting research methodology, consider the following parameters:
- Define the research questions to guide your methodology selection.
- Evaluate different research methodologies (for example, you can choose quantitative, qualitative, or mixed methods.)
- Consider practical factors, including resources, time constraints, and access to participants.
- Choose a methodology that aligns with your research goals and theoretical framework.
- Explain how the chosen methodology enables you to collect relevant data and generate insights for your research proposal.
Developing the introduction
There is nothing new about the introduction being one of the most impactful parts of any academic paper. To succeed in writing your assignment, you must follow many rules and requirements. So, here is a quick start on how to complete this part like a real pro.
Collecting the background information
Writing a research proposal is never an easy task. However, a good introduction with properly arranged background information is one of the keys to success. You can begin by introducing the broad study area and its significance in the academic or practical realm. After that, you can provide historical context or foundational theories relevant to the research topic. It is always a brilliant idea to summarize past research and scholarly discussions related to the subject and highlight your key findings and knowledge gaps. After all, connect your background info and the proposed research. You can also add a smooth transition to your problem statements and research proposal purpose.
Arranging the study context
This part of writing is one of the most complicated. However, the tips below might help you cope with it more easily.
- Describe your research's social, cultural, economic, or environmental context.
- Identify any trends, developments, or events that underscore the relevance of the research significance.
- Highlight any specific challenges or opportunities presented by the research context.
- Emphasize the need to address the identified research problem.
Describing the research problem and objectives
This section articulates the specific research problem the proposal aims to address. The research problem is the focal point of the study, representing the gap or issue in knowledge that the research seeks to explore. Always make sure your objectives provide clear and measurable targets for the study.
Demonstrating the significance of the study
All excellent research proposal examples can boast of having an outstanding demonstration of the significance of the study. And you can do that, too! For these purposes, follow this easy schema:
- Discuss the broader significance of the research topic within its field or discipline, emphasizing its relevance and timeliness.
- Identify the potential contributions that the proposed research is expected to make.
Composing the research methodology
You might feel tired of all the rules, but it’s not the time to give up. Your research proposal template desperately needs a sound research methodology. So, let’s get started!
Determining the right research design
When determining the right research design for your proposal, consider the following tips:
- Clarify your research questions and objectives.
- Explore various research designs and methodologies that can fit the research proposal structure well. This approach will help you identify the best options for your research aims.
- Consider practical constraints. You need to consider time, budget, access to participants or data, and ethical considerations.
- Pilot-test your research design. It is always good to conduct a pilot study or small-scale test to assess the feasibility and effectiveness of your chosen research design in the primary stage of the research proposal template development.
Choosing the research participants
Always consider the practical aspects of participant selection, such as accessibility and feasibility. Finally, be transparent about the participant selection process in your research proposal.
Collecting data: your most fitting methods
Any student is required to select the most suitable methods of data collection for their assignment. Here are a few tips for your convenience:
- Choose methods that best capture the data needed to address your research questions and objectives.
- Assess the practicality of each method in terms of resources, time, and access to participants or data sources.
- Select methods that yield data with high reliability and validity.
The truth is that every research proposal format requires diverse methods of collecting data. So, always adapt them to your specific writing.
Creating the plan for data analysis
In this step, you will outline the specific techniques and procedures you will use to analyze your research data. This includes selecting appropriate statistical or qualitative analysis methods and organizing and coding your data. The data analysis plan should align with your research objectives. This is the essential feature of all research proposals.
Ensuring the validity and reliability of your research
Ensuring validity and reliability means making sure your research methods accurately measure what they're supposed to and produce consistent results. Remember that ensuring research study validity is a must for this type of academic paper.
Crafting the literature review
According to research proposal format rules, your paper must contain a literature review. Some students believe that completing this task during the data collection step is easier, while others admit they usually craft it at the final steps of writing a proposal. Anyway, the process usually has several steps.
Choosing the relevant literature
Identifying the foundational studies and key sources that will guide your research is essential. Although you can look for some research proposal examples, it is still good to follow this plan during writing:
- Search academic databases and scholarly sources related to your research topic.
- Look for key studies, articles, and books that provide insights into your research area.
- Focus on literature that directly addresses your research questions and objectives.
Summarizing the literature lists
Once you've gathered relevant literature for your paper proposal, synthesizing it will be your next step. Here's how to approach it:
- Summarize the main findings and arguments of the chosen literature list.
- Identify common themes, trends, and patterns in different sources.
- Analyze how the literature contributes to the understanding of your research topic.
Evaluating the literature sources
Now, it's high time to evaluate the resources’ quality and relevance critically:
- Assess each source's credibility, relevance, and methodological rigor for your proposal writing.
- Consider the strengths and weaknesses of different research methods used in the literature.
Exploring the research gaps
At the final step of working with your literature sources, you need to find out the areas for further investigation:
- Evaluate the existing literature to identify unanswered questions.
- Look for contradictions, inconsistencies, or limitations in the current research.
- Consider emerging trends, recent developments, or advancements in your field.
Now, the work with literature lists for your research proposal is done.
Note: Failure to effectively analyze your sources is one of the most common mistakes in writing a research proposal, so don’t underestimate it.
Creating the conclusion
Every research proposal has a conclusion. Although many students don’t like this part of writing, it is still necessary to pay attention to it. Here is how to do that.
Summarizing your findings
- Condense the primary outcomes of your study into clear statements.
- Make sure that your summary captures the essential research findings derived from your research.
Adding recommendations for future research
- Suggest areas for further investigation based on the limitations identified in your research proposal.
- Offer suggestions or directions for future research.
Showing off the implications of your study
- Discuss the broader significance and relevance of your study's findings for theory, practice, or policy.
- Articulate the potential impact or practical applications of your research results.
Note: The conclusion is a must-have writing piece in every research proposal format. Always add it to your paper.
Composing the abstract
If you look at any professionally written research proposal example, you will discover it also has an abstract.
Offering a concise study overview
Start your abstract with a brief overview of the study. In other words, you can provide a snapshot of your paper’s purpose and significance. You will also need to summarize the main topic and objectives. If you are feeling a bit lost in what to write in this part, read the introduction of your research proposal once more - you will find some basic info to write about.
Introducing the research problem and objectives
Regardless of your writing style, it is crucial to add your research paper objectives to your abstract:
- Clearly present your research problem and objectives.
- Define the research problem, emphasizing its importance.
- Clearly state the specific research questions that your study aims to address.
Detailing the research methodology
- Ensure the credibility of your study by providing a detailed overview of your research methodology.
- Describe your research design and methods used while composing a research proposal briefly.
- Justify your methodology choice.
- Briefly outline your data collection, analysis, and interpretation procedures.
Recapping the research findings
- Summarize your key findings.
- Outline the main outcomes of your study in clear and straightforward language.
- Highlight how your findings address the research objectives and contribute to filling the research gap.
Note: According to research proposal format requirements, all these abstract parts are obligatory.
Style and formatting
If you look for a fresh and up-to-date research proposal example online, you will likely discover they all have different formats. However, there are still some rules you are expected to follow.
Following the research proposal guidelines
Employing clear and concise writing, proofreading and editing the proposal, conclusion .
Of course, writing papers is usually a tricky process. Fortunately, you can always get professional help and pay for essay online. Still, if you want to complete it by yourself, remember about these critical aspects.
Most universities and colleges have a solid view of the components of the proposal:
- Introduction;
- Literature Review;
- Methodology;
- Research Timeline;
- References;
- Appendices.
A perfectly written research proposal is a roadmap for the entire research project. In other words, it guides the researcher in defining objectives, methods, and expected outcomes. A research proposal is also necessary to secure funding, gain approval from ethics committees, and attract collaborators or participants.
Final thoughts and recommendations
The best recommendation for creating this type of writing is to begin far in advance and follow all the professor’s requirements. Still, if you have an urgent deadline or writing difficulties, you can always rely on the Write my paper for me professional service. So, how to write a research proposal? It’s up to you!
What is the main goal of a research proposal?
The purpose of a research proposal is to outline the planned research project, including its objectives, methodology, and significance.
How long should a typical research proposal be?
Research proposal length typically ranges from 1500 to 2500 words.
What are the common mistakes to avoid when composing a research proposal?
The list of mistakes includes unclear objectives, inadequate literature review, and lack of coherence in the methodology.
Can I revise a research proposal after submission?
Yes, a research proposal can be revised after submission based on reviewer feedback or research plan changes.
How is it better to ensure the validity and reliability of my research study?
Use appropriate research designs and methods, maintain consistency in data collection and analysis, and address potential sources of bias.
Related posts

8 Answers on How to Write an Annotated Bibliography

How to write a research proposal: top tips for busy students

Exploring the Creative Universe of Jenny Parks Illustration 🎨
What are you waiting for?
You are a couple of clicks away from tranquility at an affordable price!
Public ranks long-term challenges and health determinants as top priorities for new EU
To enhance public health, the post-election European Union (EU) should prioritise long-term challenges such as climate change and the ageing population, as well as factors that influence our health, according to a new report . The findings, derived from a seven-month public debate led by the European Observatory on Health Systems and Policies, highlight a collective call for the EU to play a more significant role in health.
The report, which is based on the public debate commissioned by the European Commission’s Directorate General for Health and Food Safety ( DG SANTE ), outlines the key priorities and actions desired by citizens and stakeholders from a wide range of sectors and mostly from Europe. The analysis included more than 800 responses in conference polls and a survey, plus comprehensive inputs across three webinars .
The large and participatory initiative allowed to collect public opinion on nine critical health topics: health security; determinants of health; health system transformation; the health workforce; universal health coverage; digital solutions and AI; performance and resilience; long-term challenges like climate change and ageing; and the EU’s global role in health.
The public’s calls for action – including across sectors
Participants called for the European Commission to coordinate across its different policy branches. Collaborating across sectors is considered key to deliver health priorities, making the concepts of ‘ Health in All Policies’ and ‘Health for All Policies ’ important tools for addressing the determinants of health. Interestingly, the topics which garnered the highest consensus in the discussion framework were those least controlled by the health sector alone.
Significant measures should be taken to mitigate the health impacts of environmental risks, including promoting environmental health and supporting health equity through integrated policies. Participants also considered addressing the needs of an ageing population essential, by improving health services and ensuring that health systems are prepared to meet the demands of older adults.
The public opinion suggested several actions to achieve universal health coverage (UHC) across the EU, such as ensuring equal access to comprehensive health care services for all EU citizens and financial protection for all. Other recommendations ranged from establishing a common minimum coverage package and a European health insurance scheme to focusing on underserved groups, improving health literacy, and including mental health in UHC policies.
What role for the EU?
Participants highlighted the importance of EU legal frameworks and instruments in promoting and safeguarding health, such as funding and technical support. They advocated both for new tools and for better implementation and coordination of existing mechanisms.
Aligning educational standards was raised as a key topic in the context of addressing shortages of health workers , regional disparities and managing the demands for new skills. Better addressing health workforce needs and improving their working conditions to mitigate existing gaps was also discussed. There was consensus on the need for EU approaches to health workforce issues, including better coordination of initiatives and pursuit of EU wide policies.
Digital solutions , health security and strengthening the EU’s global voice and leadership were widely discussed but ranked slightly lower. Possible explanations outlined in the report include the “transversal nature of digital solutions, which voters may have perceived as a means to achieving other priorities”. The COVID-19 pandemic and sustained EU action on health security may have elicited some voters to opt for other topics that have received less policy attention in recent years.
Survey, webinars, and conference polls
Media Contacts
Débora Miranda
Communications and Dissemination Officer
Subscribe to our newsletter
Skip to Content
Other ways to search:
- Events Calendar
Elevate your fall fellowship proposal through RIO's Writing & Peer Editing Program, starting July 16
Elevate your fall fellowship proposal through rio's writing & peer editing program, starting july 16.
The Research and Innovation Office (RIO) announces the launch of the fourth annual Fall Fellowships Writing & Peer Editing Program: ACLS, NHC, Fulbright and More , facilitated by RIO Proposal Writer/Editor Donna Axel. Faculty are encouraged to attend a brief 30-minute kickoff on Tuesday, July 16 at either 10 a.m. or 4 p.m. to determine if the program is right for them.
The virtual program is designed to assist faculty in submitting a proposal to one of the many fellowships with a fall deadline—including the American Council of Learned Societies (ACLS) , National Humanities Center (NHC) , Stanford (SHC) , Guggenheim , Fulbright and more. In the process, participants will also learn how to reuse, repurpose and recycle proposals at a later date or for other opportunities.
From July through September, program participants will draft proposals and participate in a peer editing workshop to hone the narrative portion of fellowship applications typically due in September and November.
Learn More about the Fall Fellowships Writing & Peer Editing Program
Bonus session
September 10 Faculty Panel: Fellowship Writing Success Stories
Three CU Boulder faculty members will share their success stories—and their processes—for applying for fellowships:
- Ashleigh Lawrence-Sanders (History) , awarded the American Council of Learned Societies Fellowship (2023)
- Elspeth Dusinberre (Classics) , awarded the Guggenheim Fellowship (2023)
- Emmanuel David (Women & Gender Studies) , awarded the National Humanities Center Fellowship (2022)
Program overview
- Attend a Kickoff on Tuesday, July 16 to determine if the program is appropriate for your needs. Register for 10 a.m. Kickoff Register for 4 p.m. Kickoff
- Attend one of the “Categories & Requirements” sessions taking place on Thursday, July 25 to review specific fellowship opportunities. Register for 10 a.m. Session Register for 4 p.m. Session
- Meet 1-on-1 with Donna and/or send her your draft one to two weeks prior to submitting your draft for the peer review workshop.
- Prepare a draft narrative for review by committed colleagues one week in advance of your peer review workshop.
- Peer review 1–4 of your colleagues’ proposals in advance of the Peer Editing Workshop.
- Participate in at least one peer review workshop (Aug.–Sept., TBD by faculty).
- Optional : As part of participating in this program, and on a first-come-first-served basis, Donna Axel offers to review your draft after you have incorporated any suggestions from the peer-review process.
- Optional : Attend the panel event where faculty share their success stories on Sept. 10. Register Here
I couldn’t have managed the fellowship application process last year without the help of the fellowship writing program. I benefited enormously from Donna’s expert and valuable suggestions at every step of the way; she helped me improve my various proposals, re-craft them for different fellowships, and also think about additional programs to which I might apply for funding. The peer editing program was also incredibly helpful and resulted not only in much better proposals but also in real friendships with colleagues across campus. Donna’s guidance in writing proposals geared toward readers outside my field has also helped me think more critically and productively about how best to craft public presentations that are compelling to a broader audience. The work I was fortunate enough to do with Donna and the fellowship writing program has improved my entire professional being and career trajectory."
— Elspeth Dusinberre , Professor, Classics; Selected as a Guggenheim Fellow 2023-2024.
My participation in RIO’s peer editing fellowship program made a world of difference for me as I prepared my external fellowship applications. I received useful and concrete feedback from the other faculty members in the program, and Donna Axel really helped me shape my proposal with her comments and suggestions. I’ve used my application for several fellowship programs and have worked with Donna to tailor the prose for different funding opportunities. I feel like my application writing skills and my understanding of the entire grant application process have improved immensely thanks to Donna’s expert advice and close attention to detail.”
— Emmanuel David , Associate Professor, Women & Gender Studies; Selected as a National Humanities Center Fellow 2022-2023.
View More Testimonials
Search Faculty Experts
Research and expertise across CU Boulder.
- Research Institutes
Our 12 research institutes conduct more than half of the sponsored research at CU Boulder.
- Research Computing
A carefully integrated cyberinfrastructure supports CU Boulder research.
- Research Centers
More than 75 research centers span the campus, covering a broad range of topics.
Research Development, Institutes & Centers
- Research Development
- Shared Instrumentation Network
- Office of Postdoctoral Affairs
- Research & Innovation Office Bulletin
Research Administration
- Office of Contracts and Grants
- Research Integrity (Compliance)
- Human Research & IRB
- Office of Animal Resources
- Research Tools
- Research Professor Series
Partnerships & Innovation
- Innovation & Entrepreneurship
- Venture Partners (formerly Technology Transfer Office)
- Industry & Foundation Relations
- AeroSpace Ventures
- Grand Challenge
- Center for National Security Initiatives

IMAGES
VIDEO
COMMENTS
Untill the pr eparation of this proposal (3 Apr il 2020), more than 1,010, 000 cases of. COVID-19 have been repo rted in more than two hundred countries and territories, resulting. in over 53,000 ...
When hundreds of millions of people are vaccinated, millions of them will be afflicted anyway, in the course of life, by conditions like strokes, anaphylaxis, and Bell's palsy. "We have to have faith that people collecting the data will let us know if we are seeing those things above the baseline rate.". 3.
For similar information about National Science Foundation (NSF) research, see the NSF FAQ. The Council on Government Relations is compiling a list of institutional and agency responses to the pandemic. Have an idea for research about preventing or treating COVID-19? See NSF's Dear Colleague Letter about how to submit a research proposal.
In this paper, I propose a multimodal strategy for long-term COVID control, one that sets up multiple barriers of protection so that we are able to not only contain SARS-CoV-2 and eliminate COVID ...
COVID-19 pandemic has severely impacted the crude, stock market, gold and metals and almost all areas of the global market [ 1 ]. Large research laboratories and corporate houses are working with a high speed to develop medicines and vaccines for the prevention and treatment of this dreaded disease. To deal with these current health management ...
COVID-19 Research Proposals. Princeton University has authorized funding to support faculty research projects that consider biomedical, health-related and fundamental science related to the COVID pandemic, as well as those that impact corresponding policy, social, and economic topics. Read the full details in this email from Dean for Research ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
Thinking about COVID-19 and the literature review It is less likely in the short-term that the crisis will impact on your literature - which is theoretical or empirical and tends to have a long lead into publication. However, there is great deal of research at the moment around the impact of COVID-19 on family life, healthcare and education.
NIH aims to actualize the response to the COVID-19 pandemic by supporting research to understand SARS-CoV-2 and mitigate the threat of COVID-19 for the health of all people. NIH will build on existing research initiatives—and accelerate the development of new ones—that are focused on the five research priorities detailed in this strategic plan.
Introduction. Four successive waves of COVID-19 have buffeted the United States for the past year and a half. With each wave, we have bet on different measures to push us through: First, public ...
Washington University's McDonnell International Scholars Academy and Social Policy Institute (SPI) seek proposals from WashU researchers and their international partners to identify and address the challenges of COVID-19 through artificial intelligence, technology and big data.. This is the second year the Social Policy Institute and McDonnell Academy have partnered to provide seed grants ...
Literature: Aesthetics and IR, behaviour change communication and images in public health. Methods: 3 case studies on different types of images in COVID-19, e.g. 1. Global public health messaging; 2. National public health messaging; 3. Community Expression - OR pick one of these options and explore in depth.
The sixth question concerns how COVID-19 should be treated and what treatment options should be made available. COVID-19 is a self-limiting disease in more than 80% of patients. Severe pneumonia occurred in about 15% of cases as revealed in studies with large cohorts of patients. The gross case fatality is 3.4% worldwide as of February 25, 2020.
The impact of COVID-19 on research. Coronavirus disease 2019 (COVID-19) has swept across the globe causing hundreds of thousands of deaths, shutting down economies, closing borders and wreaking havoc on an unprecedented scale. It has strained healthcare services and personnel to the brink in many regions and will certainly deeply mark medical ...
Building on the work of other agencies, the proposal aims to: vaccinate at least 40 percent of the population in all countries by the end of 2021 and at least 60 percent by the first half of 2022, track and insure against downside risks, and. ensure widespread testing and tracing, maintain adequate stocks of therapeutics, and enforce public ...
Proposals: The PI must submit projects using Cayuse, include "COVID-19" in the project title, provide budget/budget justification and certify the proposal in Cayuse.PI's should also send an email to [email protected] identifying the proposal as addressing COVID-19.; Awards/Award Agreements: SPO will prioritize negotiation of COVID-19 awards if we are on notice that the award is for COVID-19.
By Communication from Frontiers. An outbreak of a respiratory disease began in Wuhan, China in December 2019 and the causative agent was discovered in January 2020 to be a novel betacoronovirus of the same subgenus as SARS-CoV and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronavirus disease 2019 (COVID-19) has rapidly ...
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, A …
Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. COVID-19, the disease caused by the highly infectious and transmissible coronavirus SARS-CoV-2, has quickly become a morbid global pandemic. Although the impact of SARS-CoV-2 infection in ...
THEMIS is an interdisciplinary research project that reacts to the increasing occurrence of global pandemics, like the caused by the present COVID-19 disease, and restrictive public health measures taken to respond to these threats. Using a rights-based approach, Dr Patrycja Dąbrowska-Kłosińska, researcher of THEMIS, intends to create a ...
Covid impact statement. An optional impact statement to explain to your examiners how your project/thesis has changed as a consequence of Covid-19 restrictions. Many PGRs will have had to adapt their research project, sometimes significantly, in response to Covid-19 restrictions and this may be a cause of concern.
The COVID-19 pandemic has caused widespread infection, school closures, and high rates of job loss. Much of the current research has focused on the clinical features of COVID-19 infection, but the family well-being consequences of COVID-19 are less well documented. The goal of the current study is to describe parent and child well-being
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC). From April 10, 2020, until October 13 ...
While this meta-analysis supports an urgent need for intervention and recovery efforts aimed at improving child and adolescent well-being, it also highlights that individual differences need to be considered when determining targets for intervention (eg, age, sex, exposure to COVID-19 stressors). Research on the long-term effect of the COVID-19 ...
Llor and colleagues [1] evaluated how many of the 3343 COVID-19-related preprints posted on medRxiv in 2020 were subsequently published in peer-reviewed journals, finding 1712 (51.2%) had corresponding journal articles as of March 2022, 1742 (52.1%) as of October 2022.
Academic writing is a vital element in any post-graduate study. Therefore, it is crucial to harness all the tools and capabilities available to serve this purpose. These capabilities include the digital tools that characterize the current era. This paper aims to explore post-graduate students' perceptions of the use of social media platforms to enhance academic writing during the COVID-19 ...
Stay up-to-date with the AHA View All News The American Historical Review is the flagship journal of the AHA and the journal of record for the historical discipline in the United States, bringing together scholarship from every major field of historical study. Learn More Perspectives on History is the newsmagazine…
Note: The conclusion is a must-have writing piece in every research proposal format. Always add it to your paper. Composing the abstract. If you look at any professionally written research proposal example, you will discover it also has an abstract. Offering a concise study overview. Start your abstract with a brief overview of the study.
To enhance public health, the post-election European Union (EU) should prioritise long-term challenges such as climate change and the ageing population, as well as factors that influence our health, according to a new report. The findings, derived from a seven-month public debate led by the European Observatory on Health Systems and Policies, highlight a collective call for the EU to play a ...
The Research and Innovation Office (RIO) announces the launch of the fourth annual Fall Fellowships Writing & Peer Editing Program: ACLS, NHC, SHC and More, facilitated by RIO Proposal Writer/Editor Donna Axel. Faculty are encouraged to attend a brief 30-minute kickoff on Tuesday, July 16 at either 10 a.m. or 4 p.m. to determine if the program is right for them.