Advances in Melanoma and Other Skin Cancers Research
Metastatic melanoma cells.
NCI-funded researchers are working to advance our understanding of how to treat melanoma and other skin cancers. Much progress has been made in treating people with melanoma that has spread in their bodies ( metastatic melanoma). Yet many people still don't benefit from the newest drugs, and others may relapse after initially successful treatment.
This page highlights some of the latest research in treatment for melanoma and other skin cancers, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and current research findings from recent studies.

Melanoma Treatment
Surgery remains the standard treatment for early-stage melanoma and may also be used as part of therapy for more advanced disease. Over the last two decades, researchers have also developed treatments that target certain mutations in melanoma cells or that harness the body’s immune system to attack melanoma.
Both of these approaches— targeted therapies and immunotherapies —have led to dramatic improvements in survival for patients with melanoma over the last decade. Researchers are continuing to explore ways to make these treatments more effective for more patients.
Targeted therapies
Targeted therapies use drugs or other substances to attack specific types of cancer cells with less harm to normal cells. About half of people with melanoma that has metastasized or can’t be removed with surgery ( unresectable melanoma) have mutations in the BRAF gene in their tumors. These mutations result in abnormal B-Raf proteins that can cause uncontrolled growth of melanoma cells.
Drugs have been developed that block the effects of these altered B-Raf proteins. Other new drugs block proteins that work together with altered B-Raf proteins to promote cancer cell growth. These include proteins produced by the MEK genes. The combination of blocking both B-Raf and MEK has been found to be particularly successful in treating melanoma that has a mutation in the BRAF gene. Three such combinations are approved for people with metastatic or unresectable melanoma that has mutations in the BRAF gene:
- dabrafenib (Tafinlar) and t rametinib (Mekinist)
- encorafenib (Braftovi) and b inimetinib (Mektovi)
- vemurafenib (Zelboraf) and c obimetinib (Cotellic)
However, although these drug combinations may be effective at first, most people develop resistance to them within a year. Researchers are studying how melanoma cells manage to grow in the presence of these targeted therapies, with the goal of finding ways to overcome treatment resistance. Ideas being tested include new drug combinations and drugs that target the B-Raf pathway in different ways than existing drugs.
Immune checkpoint inhibitors
Immunotherapies are treatments that help the body’s immune system fight cancer more effectively. Melanoma, unlike most other cancer types, tends to have a high number of genetic mutations that can be recognized by the immune system. This makes it more likely that melanoma will respond to immunotherapy.
One type of immunotherapy, called immune checkpoint inhibition , has shown impressive results in some people with advanced melanoma. Four immune checkpoint inhibitors are approved for the treatment of metastatic or unresectable melanoma:
- i pilimumab (Yervoy)
- pembrolizumab (Keytruda)
- nivolumab (Opdivo)
- atezolizumab (Tecentriq) , when used in combination with two targeted drugs
The combination of ipilimumab and nivolumab is also approved for some patients with metastatic or unresectable melanoma. In the study that led to its approval, more than half of the people who received the combination were alive 5 years after treatment . Another clinical trial showed that this combination can also shrink melanoma that has spread to the brain in some patients.
The combination of nivolumab with a type of immune checkpoint inhibitor called relatlimab also improved the amount of time people with advanced melanoma lived without their cancer getting worse . This combination received FDA approval in 2022, under the name Opdualag , for people aged 12 or older with untreated metastatic or unresectable melanoma.
Scientists are looking for ways for more people to have success with these drugs. Unfortunately, even when used in combination, immune checkpoint inhibitors don't work for all patients with metastatic or unresectable melanoma. However, patients whose tumors do shrink or disappear often have responses that last for years. Researchers are now testing ways to increase the number of people with melanoma who benefit from this type of treatment, such as:
- combining immune checkpoint inhibitors with immunostimulant s. Immunostimulants are medicines that increase the ability of the immune system to fight infection and disease. In a small clinical trial that combined pembrolizumab with an immunostimulant, tumors shrank in almost 80% of people who received the two treatments together . Larger trials of this and other combinations of immunotherapy drugs are underway.
- testing new and existing immune checkpoint inhibitors in combination with targeted therapies and other types of drugs.
- modifying people’s gut microbes before treatment with an immune checkpoint inhibitor. For example, a study led in part by NCI researchers found that changing some people’s gut microbes could make their melanoma more likely to shrink during treatment with an immune checkpoint inhibitor.
Learning what treatments to give first
Melanoma researchers are also looking to understand how best to use existing therapies. One pressing question had been whether it is better for people who have advanced melanoma with mutations in the BRAF gene to receive targeted drugs or immune checkpoint inhibitors first.
An NCI-sponsored trial, DREAMseq, has helped answer this question. Patients with advanced melanoma were randomly assigned to receive either a combination of B-Raf-targeted drugs or a combination of immune checkpoint inhibitors. When their cancer recurred, they received the other combination. The study found that more people who received the immune checkpoint inhibitor combination first were still alive 2 years later than people who received the targeted drugs first.
Scientists are also searching for biomarkers in melanoma that can predict which tumors might respond to other immunotherapies or drug combinations.
Harnessing the body’s immune cells
Adoptive cell therapy. Another type of immunotherapy, called adoptive cell therapy (ACT), is also being tested in patients with metastatic melanoma. In ACT, T cells (a type of immune cell) are given to a patient to help the body fight diseases, such as cancer.
One type of ACT, called tumor-infiltrating lymphocyte (TIL) therapy, received FDA approval in 2024 for the treatment of advanced melanoma that has recurred after treatment with either targeted therapy or an immunotherapy drug. TIL therapies are personalized treatments in which immune cells are collected from a patient’s tumors, treated to make them better at killing cancer cells, and infused back to the patient.
The approved TIL therapy, called lifileucel (Amtagvi), is the first approved cellular therapy for any type of solid tumor.
Work at NCI and elsewhere is now focused on identifying the TILs taken from a tumor that are likely to be best at killing cancer cells, and on engineering TILs to last longer in the challenging environment within a tumor.
Researchers are also looking for ways to make ACT work for more patients with melanoma.
- One idea being tested is the use of immune cells that have been collected from patients, genetically altered to make them better at killing cancer cells, and then infused back into patients. Such therapies include CAR T cells, a type of treatment where a patient's T cells are changed in the lab so they will attack cancer cells . Researchers are also testing other ways to boost the ability of T cells to kill tumor cells.
- Another idea is to find common proteins that are present in many people's tumors. This could allow for the creation of “off-the-shelf” T-cell therapies that don’t have to be made on a custom basis for each patient.
Immunotherapy following surgery
Adjuvant therapy is additional cancer treatment given after primary surgical treatment. Nivolumab, ipilimumab, and pembrolizumab have all been approved as adjuvant therapies for melanoma that has spread to nearby lymph nodes but can be removed with surgery. In clinical trials, all three immune checkpoint inhibitors reduced the risk of recurrence for some patients when given after surgery, although many patients experienced serious side effects.
Another study tested pembrolizumab in patients with early-stage melanoma that has not spread to the lymph nodes but had a high risk of doing so. It found that giving pembrolizumab after surgery reduced the chance of the cancer coming back or spreading elsewhere in the body . However, the treatment can cause significant side effects.
More studies are needed to understand how to identify the people with this type of high-risk, early-stage melanoma who would benefit the most from such treatment. Strategies such as adding personalized vaccines to immunotherapy for people with melanoma at high risk of recurring after surgery are also being tested.
Researchers are also exploring whether immune checkpoint inhibitors might be more effective if given before surgery. One NCI-sponsored trial found that people who received pembrolizumab both before and after surgery had a substantially lower risk of their cancer coming back than those who only received adjuvant treatment.
Rare Melanoma Types
Some rare types of melanoma have lagged behind melanoma of the skin in terms of advances in treatment. These include intraocular (uveal) melanoma , which starts in the eye; desmoplastic melanoma , a rare form of melanoma of the skin; mucosal melanoma, which begins in the mucosal membranes , such as the linings of the nose and mouth; and acral melanoma, which starts in the body's extremities like the palms of the hands, soles of the feet, and nailbeds.
Recent small clinical trials suggest that some of these types of melanoma may respond to immunotherapies. One NCI-sponsored trial tested pembrolizumab in people with desmoplastic melanoma . Initial results from this trial showed that the drug shrinks both tumors that can be removed surgically and those that cannot. The trial participants are still being tracked to see if pembrolizumab improves how long they live overall.
Immune checkpoint inhibitors have been less effective in intraocular melanoma than in other types of melanoma. However, a different type of immunotherapy called a bispecific fusion protein has shown promise for treating this rare cancer. These drugs bind to melanoma cells and the body’s own immune cells at the same time, to bring them together. This allows the immune cells to kill the melanoma cells.
One such drug, called tebentafusp (Kimmtrak) , was approved by the FDA in 2022 to treat metastatic intraocular melanoma with certain gene mutations .
Merkel Cell Carcinoma
Another rare type of skin cancer, called Merkel cell carcinoma (MCC), has been shown to be the most sensitive of any tumor type to treatment with a single immune checkpoint inhibitor. In 2017, an immunotherapy called avelumab (Bavencio) received the first-ever FDA approval for a drug to treat MCC. In addition, more than half of patients with MCC in a small clinical trial had their tumors shrink or disappear during treatment with pembrolizumab, which received FDA approval for the treatment of MCC in 2018.
In 2023, a third immunotherapy drug called retifanlimab (Zynyz) received FDA approval for the treatment of MCC that has recurred or spread elsewhere in the body. Other immunotherapy drugs and combinations of these drugs are currently being tested in this rare cancer type.
Treatment for Advanced Basal Cell Carcinoma and Squamous Cell Carcinoma
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of the skin are the most common cancers in the United States. They rarely spread to other organs and are seldom fatal. However, every year many people are diagnosed with advanced BCC or SCC.
For people with BCC or SCC that has not spread, surgery remains the mainstay of treatment. But less-intensive versions of radiation therapy have been developed for people who can’t tolerate surgery for larger tumors, such as those who are elderly or frail.
Recent breakthroughs in targeted therapies and immunotherapies have changed the way people with advanced BCC and SCC are treated. FDA-approved treatments now include:
- cemiplimab (Libtayo) for some people with metastatic or locally advanced SCC that can't be removed with surgery. Cemiplimab is also being tested as a treatment given before surgery for some people whose cancer can be removed.
- pembrolizumab for some people with recurrent or metastatic SCC
- cemiplimab for some people with advanced BCC whose tumors have become resistant to targeted therapy
Ongoing research seeks to build on these breakthroughs such as:
- Identifying better ways to give the targeted drugs sonidegib (Odomzo) and vismodegib (Erivedge) which can control tumors for a long time in some people with BCC, but to which resistance often develops. In addition, side effects can cause some patients who need to take the drugs for a long time to stop taking them. Changing when and how much of these drugs are given, may both delay the development of resistance and make them easier to tolerate.
New clinical trials are now testing other immunotherapy drugs and combinations in SCC and BCC.
NCI-Supported Research Programs
Many NCI-funded researchers at the NIH campus, and across the United States and world, are seeking ways to address melanoma and other skin cancers more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts for melanoma and other skin cancers.
Scientists in the Division of Cancer Epidemiology and Genetics (DCEG) study families in which multiple members have developed certain cancers. In collaboration with the Melanoma Genetics Consortium (GenoMEL), DCEG researchers are searching for new genes in both melanoma-prone families and through a genome-wide association study to find genes that may increase the risk of melanoma.
The Skin Specialized Programs of Research Excellence (Skin SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Skin SPORE program’s main focus is on melanoma research activities, but it also includes projects in other skin cancer types, such as cutaneous T-cell lymphoma.
NCI's National Clinical Trials Network (NCTN) is a collection of organizations and clinicians that coordinates and supports cancer clinical trials at more than 3,000 sites across the United States and Canada. NCTN currently has a variety of trials testing treatments for skin cancer .
The Division of Cancer Control and Population Sciences (DCCPS) oversees the Cancer Trends Progress Report, an online report that tracks the nation's progress against cancer from prevention through end of life. Topics in the report that inform melanoma and skin cancer research are sun-protective behavior , indoor and outdoor tanning , and sunburn . The division’s Health Behaviors Research Branch (HBRB) supports research in the area of sun protection and reducing indoor tanning practices, through both measurement and intervention studies.
Clinical Trials
NCI funds and oversees both early- and late-stage clinical trials to develop new treatments and improve patient care. Trials are available for melanoma prevention and treatment and non-melanoma skin cancer prevention and treatment .
Melanoma and Other Skin Cancers Research Results
The following are some of our latest news articles about research on melanoma and other skin cancers:
- First Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma
- Rare Melanoma Very Likely to Respond to Treatment with Pembrolizumab
- Immunotherapy before Surgery Appears Effective for Some with Melanoma
- Androgen Receptor May Explain Sex Differences in Melanoma Treatment Response
- Study Adds to Debate about Screening for Melanoma
- Opdualag Becomes First FDA-Approved Immunotherapy to Target LAG-3
View the full list of Melanoma and Other Skin Cancers Research Results and Study Updates .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Melanoma Skin Cancer Detection Using Recent Deep Learning Models
- PMID: 34891892
- DOI: 10.1109/EMBC46164.2021.9631047
Melanoma is considered as one of the world's deadly cancers. This type of skin cancer will spread to other areas of the body if not detected at an early stage. Convolutional Neural Network (CNN) based classifiers are currently considered one of the most effective melanoma detection techniques. This study presents the use of recent deep CNN approaches to detect melanoma skin cancer and investigate suspicious lesions. Tests were conducted using a set of more than 36,000 images extracted from multiple datasets. The obtained results show that the best performing deep learning approach achieves high scores with an accuracy and Area Under Curve (AUC) above 99%.
PubMed Disclaimer
Similar articles
- Skin cancer classification via convolutional neural networks: systematic review of studies involving human experts. Haggenmüller S, Maron RC, Hekler A, Utikal JS, Barata C, Barnhill RL, Beltraminelli H, Berking C, Betz-Stablein B, Blum A, Braun SA, Carr R, Combalia M, Fernandez-Figueras MT, Ferrara G, Fraitag S, French LE, Gellrich FF, Ghoreschi K, Goebeler M, Guitera P, Haenssle HA, Haferkamp S, Heinzerling L, Heppt MV, Hilke FJ, Hobelsberger S, Krahl D, Kutzner H, Lallas A, Liopyris K, Llamas-Velasco M, Malvehy J, Meier F, Müller CSL, Navarini AA, Navarrete-Dechent C, Perasole A, Poch G, Podlipnik S, Requena L, Rotemberg VM, Saggini A, Sangueza OP, Santonja C, Schadendorf D, Schilling B, Schlaak M, Schlager JG, Sergon M, Sondermann W, Soyer HP, Starz H, Stolz W, Vale E, Weyers W, Zink A, Krieghoff-Henning E, Kather JN, von Kalle C, Lipka DB, Fröhling S, Hauschild A, Kittler H, Brinker TJ. Haggenmüller S, et al. Eur J Cancer. 2021 Oct;156:202-216. doi: 10.1016/j.ejca.2021.06.049. Epub 2021 Sep 8. Eur J Cancer. 2021. PMID: 34509059
- Integrating Patient Data Into Skin Cancer Classification Using Convolutional Neural Networks: Systematic Review. Höhn J, Hekler A, Krieghoff-Henning E, Kather JN, Utikal JS, Meier F, Gellrich FF, Hauschild A, French L, Schlager JG, Ghoreschi K, Wilhelm T, Kutzner H, Heppt M, Haferkamp S, Sondermann W, Schadendorf D, Schilling B, Maron RC, Schmitt M, Jutzi T, Fröhling S, Lipka DB, Brinker TJ. Höhn J, et al. J Med Internet Res. 2021 Jul 2;23(7):e20708. doi: 10.2196/20708. J Med Internet Res. 2021. PMID: 34255646 Free PMC article. Review.
- Melanoma diagnosis using deep learning techniques on dermatoscopic images. Jojoa Acosta MF, Caballero Tovar LY, Garcia-Zapirain MB, Percybrooks WS. Jojoa Acosta MF, et al. BMC Med Imaging. 2021 Jan 6;21(1):6. doi: 10.1186/s12880-020-00534-8. BMC Med Imaging. 2021. PMID: 33407213 Free PMC article.
- Past and present of computer-assisted dermoscopic diagnosis: performance of a conventional image analyser versus a convolutional neural network in a prospective data set of 1,981 skin lesions. Sies K, Winkler JK, Fink C, Bardehle F, Toberer F, Buhl T, Enk A, Blum A, Rosenberger A, Haenssle HA. Sies K, et al. Eur J Cancer. 2020 Aug;135:39-46. doi: 10.1016/j.ejca.2020.04.043. Epub 2020 Jun 10. Eur J Cancer. 2020. PMID: 32534243
- Computational neural network in melanocytic lesions diagnosis: artificial intelligence to improve diagnosis in dermatology? Aractingi S, Pellacani G. Aractingi S, et al. Eur J Dermatol. 2019 Apr 1;29(S1):4-7. doi: 10.1684/ejd.2019.3538. Eur J Dermatol. 2019. PMID: 31017580 Review.
- Comprehensive analysis of clinical images contributions for melanoma classification using convolutional neural networks. Rios-Duarte JA, Diaz-Valencia AC, Combariza G, Feles M, Peña-Silva RA. Rios-Duarte JA, et al. Skin Res Technol. 2024 May;30(5):e13607. doi: 10.1111/srt.13607. Skin Res Technol. 2024. PMID: 38742379 Free PMC article.
- Maltol has anti-cancer effects via modulating PD-L1 signaling pathway in B16F10 cells. Han NR, Park HJ, Ko SG, Moon PD. Han NR, et al. Front Pharmacol. 2023 Sep 5;14:1255586. doi: 10.3389/fphar.2023.1255586. eCollection 2023. Front Pharmacol. 2023. PMID: 37731735 Free PMC article.
- Deep Learning in Dermatology: A Systematic Review of Current Approaches, Outcomes, and Limitations. Jeong HK, Park C, Henao R, Kheterpal M. Jeong HK, et al. JID Innov. 2022 Aug 23;3(1):100150. doi: 10.1016/j.xjidi.2022.100150. eCollection 2023 Jan. JID Innov. 2022. PMID: 36655135 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
Linkout - more resources.
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Developing a prognostic model for skin melanoma based on the persistent tumor mutation burden and determining IL17REL as a therapeutic target
- Open access
- Published: 20 June 2024
- Volume 150 , article number 313 , ( 2024 )
Cite this article
You have full access to this open access article

- Mingze Xu 1 na1 ,
- Xinyi Ma 1 na1 ,
- Yuchong Wang 1 na1 ,
- Ziqin Yu 2 ,
- Xiaoli Zheng 3 ,
- Haiying Dai 1 &
- Chunyu Xue 1
One popular and well-established marker for the immune checkpoint blockade (ICB) response is tumor mutation burden (TMB). Persistent TMB (pTMB), a subset of TMB, provides a better indicator to predict patient ICB therapy outcomes, as shown by some studies. Immune checkpoint drugs have significantly changed how melanoma is treated in recent years.
In this study, we integrated the TCGA-SKCM database and data of pTMB of TCGA from the paper that first mentioned pTMB and analyzed mutational and Immune characteristics associated with pTMB level in SKCM. Next, the predictive DEGs were identified the subgroups of pTMB by Cox regression and LASSO analyses to construct a pTMB-related signature. Finally, the expression and Biological functions of signature genes was detected, and further validated in vitro assay.
In the current research, we explored the mutational and immunological features related to the level of TMB in cutaneous melanoma (CM). The high-pTMB subgroup exhibited an increasing incidence of gene changes and higher levels of immune cell infiltration. Subsequently, we established a pTMB-related signature based on the predictive DEGs and found the biological features and immune-associated variables between two distinct risk groups. Lastly, the results of the clinical sample validation demonstrated that the expression of IL17REL was down-regulated in the collected samples of individuals with CM. The in vitro assay results indicated that IL17REL effectively suppressed the proliferation, clonality, and migration of CM cells.
In conclusion, we have developed a prediction model associated with TMB and subsequently validated the potential influence of IL17REL on Overall Survival (OS) in patients diagnosed with melanoma.
Avoid common mistakes on your manuscript.
Skin cancer developing from melanocyte stem cells and fully differentiated melanocytes is known as cutaneous melanoma (CM), a highly aggressive dermal carcinoma (Centeno et al. 2023 ). Melanomas, including around 1 in 5 skin cancers, are estimated to have affected approximately 325,000 individuals worldwide in 2020. Skin cancers, the most frequently diagnosed category globally, are anticipated to be responsible for over 1.5 million new cases in 2020 (Arnold et al. 2022 ). In recent years, immune checkpoint inhibitors have brought about a significant paradigm shift in treating melanoma, particularly in cases of advanced melanoma (Serratì et al. 2022 ). The accepted standard adjuvant therapy for managing and treating CM (stage III or IV) is using an inhibitor of the programmed cell death protein − 1 (PD-1) (Patrinely et al. 2021 ; Carlino et al. 2021 ). Although better results have been linked to immune checkpoint blockade (ICB), roughly half of patients do not see long-term benefits (Jie et al. 2022 ). Various biomarkers, such as tumor neoantigen burden (TNB) (Luo et al. 2022 ) and tumor mutation burden (TMB) (Mcgrail et al. 2021 ), have been reported for utilization in predicting ICB response; however, the findings of these indicators do not reliably predict the clinical outcome of patients. Building innovative and reliable prediction technologies and tools is necessary for precise individual assessment and pre-selection of suitable therapies for patients.
The primary biomarker for identifying cancer patients who can benefit therapeutically from ICB is the high TMB (Jung et al. 2023 ). Within the tumor mutation burden framework, all mutations are considered to be of similar significance, with variations observed solely in terms of mutation quantity. In the context of immunogenicity, specific mutations exert greater influence than others (Leung and Mcgranahan 2023 ). Hence, it is not always feasible for TMB to consistently demonstrate clinical efficacy in predicting the response to cancer immunotherapy. The concept of persistent TMB (pTMB), which denotes mutations that always elicit immune tumor control throughout the progression of tumors, was initially introduced by Niknafs et al. ( 2023 ). Significantly, the study's authors highlighted that pTMB has superior predictive capabilities for tumor ICB response compared to TMB. This finding offers novel perspectives for the precise prognosis of patients with CM.
This study examines the pTMB features of melanoma patients by analyzing the data from the Gene Expression Database (GEO), the Cancer Genome Atlas Program (TCGA), and all relevant scientific data from research by Niknafs et al. We developed a signature for predicting melanoma prognosis and response to chemotherapy and immunotherapy using Cox-Lasso regression based on the discovered differential genes with prognostic importance, allowing for individualized patient treatment regimens.
Materials and methods
Samples of melanoma patients.
The collection of clinical samples from the patients conformed to the requirements stated in the Declaration of Helsinki. Before donating tumor tissue, all patients provided their informed consent by signing the necessary documentation. The surgeries were performed based on clinical indications; only residual tumor material was contributed to the research. The sample of patients consisted of three individuals. Pathological biopsies were conducted to diagnose two cases.
Furthermore, extensive resection of the primary tumor was performed. The other patients were diagnosed during the surgery via frozen section pathology. A pathologist sectioned the surgically excised tumor tissues in the operation room. The western blotting assay was performed, for which the tumor and the normal tissue portions were collected and cryopreserved from the patients.
Data collection and processing
The gene expression profile of TCGA- SKCM (log 2 (FPKM + 1) conversion) was downloaded from the R package "TCGA biolinks". The TCGA official post-correction survival information (OS) and clinical data (including gender, age, grade, stage, etc.) were collected from a published work by Liu et al. ( 2018 ). The data of pTMB of TCGA was obtained from (Niknafs et al. 2023 ), which included 107 patients of SKCM (skin cutaneous melanoma). Moreover, the GSE65904 expression data set for model validation was retrieved from the GEO database ( https://www.ncbi ). Exclusion criteria were used for patients lacking survival information or had insufficient clinical data.
Mutational characteristics associated with pTMB level in SKCM
The pTMB was divided into two subgroups, high-pTMB(H-pTMB) and low-pTMB(L-pTMB), and was defined by an optimal cutoff value. Subsequently, the Kaplan–Meier (KM) survival analysis was used for the prognosis of the two subgroups. Using the R "maftools" package, the waterfall plot of somatic mutations in the pTMB subtypes was generated. Additionally, the R "ggplot2" package was employed to create a correlation dot plot, illustrating the associations between pTMB and several factors, including TMB, TNB, homologous recombination deficiency (HRD), and chromosomal instability (CIN). The TMB, HRD, and TNB data for cutaneous melanoma were obtained from a study by Thorsson et al. ( 2018 ), and the CIN statistics were retrieved from the research done by Drews et al. ( 2022 ). Further examination was conducted to assess the correlation between various subgroups of pTMB and the clinicopathological characteristics of cutaneous melanoma.
Immune characteristics associated with pTMB level in SKCM
A comprehensive investigation was conducted to assess the relationship between various pTMB groups and immunological status in SKCM patients. The calculations on the ESTIMATEScore, ImmuneScore, TumorPurity, and StromalScore of both the low- and high-pTMB subgroups were done using the R package "ESTIMATE" (estimation of stromal and immune cells in malignant tumor tissues using expression data). For the quantification of the relative frequencies of the types of cells that had infiltrated the tumor-immune microenvironment (TIME), a ssGSEA (single-sample gene set enrichment analysis) method was used. The gene set of the infiltrating immune cell types identified in each TIME was acquired from a study by Charoentong et al. ( 2017 ). The R package "GSVA" (gene set variation analysis) was used to compare immune pathways at various pTMB levels. To generate heat maps, the R package "ComplexHeatmap" was used.
Establishing and validating the pTMB-related signature
The DEGs (differentially expressed genes) were identified between the subgroups of pTMB. The limma algorithm was used ( p < 0.05). Subsequently, a univariate Cox regression analysis was conducted to determine the predictive DEGs. Using the "glmnet" package in R software, regression analysis was done by LASSO (Least Absolute Shrinkage and Selection Operator). This identified the signature genes and eliminated the overfitting issue. Patients' risk scores were determined by evaluating the level of expression for each prognostic gene with its related coefficient of regression.
where exp i represents the gene expression level, β i indicates the estimated regression coefficient value, and n represents the number of signature genes.
Patients diagnosed with SKCM were classified into two categories, namely high-risk and low-risk groups. This was based on the respective median risk scores. Subsequently, the survminer (survival analysis and visualization) R package was used to evaluate the OS of high- and low-risk categories of SKCM-classified patients. The curve of time-dependent ROC (receiver operating characteristic) was determined using the R "survminer" and "timeROC" utilities. In addition, univariate and multivariate Cox analyses were conducted to determine the predictive risk scores and independent prognostic values. Moreover, the formula that calculated the risk score for cohort validation was also used here. Afterward, the multivariate and univariate Cox analysis assessed the risk score independence as a prognostic determinant for patients afflicted with cutaneous malignant melanoma.
Differences in biological characteristics between the prognostic signature low- and high-risk groups
Based on a p- value cutoff of < 0.05, the prognostic markers of high-risk and low-risk groups' distinct pathways were evaluated, which was analyzed using the R "GSVA" package. Moreover, the heat maps were generated using the R "ComplexHeatmap" package.
Establishment of a nomogram model and clinical correlation analysis
Based on age, sex, clinical stage, Breslow depth, and pTMB-related features, a nomogram was constructed. To increase the clinical validation value even further, the actual and expected probabilities of 1, 3, and 5-year OS have been determined using calibration curves. The discriminatory capacity of each component to SKCM was analyzed using the ROC.
To analyze the differential expression among the tumor and normal samples in SKCM, the association between RiskScore and clinical characteristics was examined. Additionally, signature genes' predictive value and clinical importance were assessed to validate and identify potential candidate genes.
Immune-associated characteristic differences and assessment of the drug sensitivity
The study investigated the microenvironmental variations of tumors concerning the prognostic hallmark low- and high-risk subgroups using a ssGSEA method. Gene sets specifying TIME invading immune system cells were obtained explicitly from the research conducted by Charoentong et al. ( 2017 ). The 29 gene sets representing immunological properties were taken from a study by He et al. ( 2018 ). The enrichment level of immunological characteristics between low- and high-risk subgroups of predictive signature was then qualified using the ssGSEA algorithm. Following this, a systematic search was conducted for expression profiles of ICB genes that are accessible to the public and provide comprehensive clinical data. Thus, the study included two immunotherapy cohorts: one with metastatic melanoma patients receiving anti-PD- 1 antibody treatment (referred to as Cohort PRJEB23709 (Gide et al. 2019 )) and another involving melanoma patients receiving anti-CTLA- 4 antibody intervention (referred to as Cohort phs000452.v2.p1).
SubMap compared expression profiles to determine treatment impact. Therefore, the SubMap algorithm predicted the probability of anti-PD- 1 and anti-CTLA- 4 therapy responses. The data and its associated annotations were from 47 cutaneous malignant melanoma patients from published research by Lux et al. ( 2017 ).
Western blot
Tumor cells or tissues were taken from patients and subjected to protein extract. Normal cells and tissues (non-cancerous) were also collected for comparison. Proteins were extracted by radio immunoprecipitation assay (RIPA; Shanghai Life Mode Engineering, Shanghai, China) and phenylmethylsulfonyl fluoride (PMSF; Shanghai Life Mode Engineering, Shanghai, China). The BCA protein assay kit (bicinchoninic acid; Shanghai Dongsheng Biotechnology, Shanghai, China) assessed the extracted protein concentration.
The PVDF (polyvinylidene difluoride) membrane, of 0.22 μm size (Millipore ISEQ00010, USA), was incubated overnight at 4 °C with an anti-IL17REL (1:1000, Thermo Scientific). Subsequently, the membrane was incubated with a 1:2000 dilution of the secondary antibody conjugated with horseradish peroxidase (HRP; Abcam, Cambridge, UK). The detection and visualization of the protein were carried out using the Prime Western Blotting Detection Reagent (Cytiva, UK). The ChemiDoc MP imaging system (Tanon 4800, Shanghai, China) detected chemiluminescence, and the ImageJ software was used to analyze the bands' gray values.
Real-time quantitative PCR (RT-qPCR)
Utilizing the TRIzol reagent (Invitrogen, Waltham, MA, USA), total RNA was isolated from the cells of each group. Afterward, the RNA sample was subjected to reverse transcription using the reverse transcription kit (Tiangen Biotechnology, Beijing, China). The 2 × SYBR Green qPCR Master Mix (Shanghai Dongsheng Biotechnology, Shanghai, China) was used. The internal control used in this study was β-actin . The relative expression of the gene was calculated using the 2-ΔΔCt technique. The primers utilized are given in Table 1 .
Cell culture and transfection
A375 (catalog number CL-0014) and A875 (catalog number CL-0255) were brought from Procell Life Science & Tech-nology.Co.,Ltd, and they were cultured in Dulbecco's Modified Eagle Medium (DMEM; Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (FBS). After adding the FBS, 100 U / mL of penicillin and 100 g / mL of streptomycin were introduced to ensure sterility. Both cell lines were incubated at standard growth conditions.
The plasmids overexpressing the IL17REL gene and their corresponding negative controls were obtained from Generay Biotech (China). Lipofectamine 2000 (Invitrogen, USA) was used for transfecting the human A375 and A875 cells.
Measurement of the proliferation of cells
The BeyoClick™ EDU-55 cell proliferation detection kit (Beyotime, China) was prepared per the manufacturer's instructions. The kit provided a 5-Ethynyl-2′-deoxyuridine (Edu) solution from which a working solution was prepared and added to cells for 2 h. The cells were then fixed using a 4% paraformaldehyde solution and eventually treated with a 0.3% Triton X-100 permeability solution in a dark environment for 30 min. Hoechst nuclear fluorescence microscopy was used to detect EdU-stained cells.
Measurement of intracellular ROS in cells
The production of intracellular reactive oxygen species (ROS) was quantified using a commercially available ROS detection kit (Beyotime, China). Concisely, 3 × 10 5 cells were cultured in a 6-well plate and incubated overnight in standard growth conditions. The cells were subjected to staining using a concentration of 10 µM of DCFH-DA at 37 °C for 30 min. The images were taken and quantified.
Colony formation assay
The A375 and A875 cell lines were seeded in a 6-well plate. Following a 14-day incubation period, the cells were treated with 100% methanol for fixation and subjected to staining with a 0.5% solution of crystal violet. Eventually, the colonies were systematically counted, and images were taken.
Transwell assay
To assess the capacity of cells for transwell invasion, a volume of 100 µL containing 5 × 10 4 cells in incomplete DMEM medium (serum-free) was introduced into transwell inserts (Corning, USA). As a nutritional attractant, 10% FBS was added to serum-free DMEM and put in the lower section of the transwell experiment. The cells on the bottom surface were preserved with 4% poly-formaldehyde (Beyotime, China) for 30 min after conducting a 16-h invasion experiment. Subsequently, for 30 min, these cells were stained with a 0.4% crystal violet solution (Beyotime, China). After removing cells from the top surface, the cells on the bottom were quantified by microscopic observation.
Wound healing
The cells were gently scraped using a pipette, following the fusing of cellular components into a 6-well plate. Photographs were captured at the time points of 0 h and 24 h after the act of scratching.
Statistical analysis
The R software (version 4.1.2) was used for statistical analysis. For the significant data analysis (like expression, infiltration ratio, and various eigenvalues, etc.), the two groups of samples were compared for differences via the Wilcoxon signed rank and compared differences between multiple groups of samples through the Kruskal–Wallis.
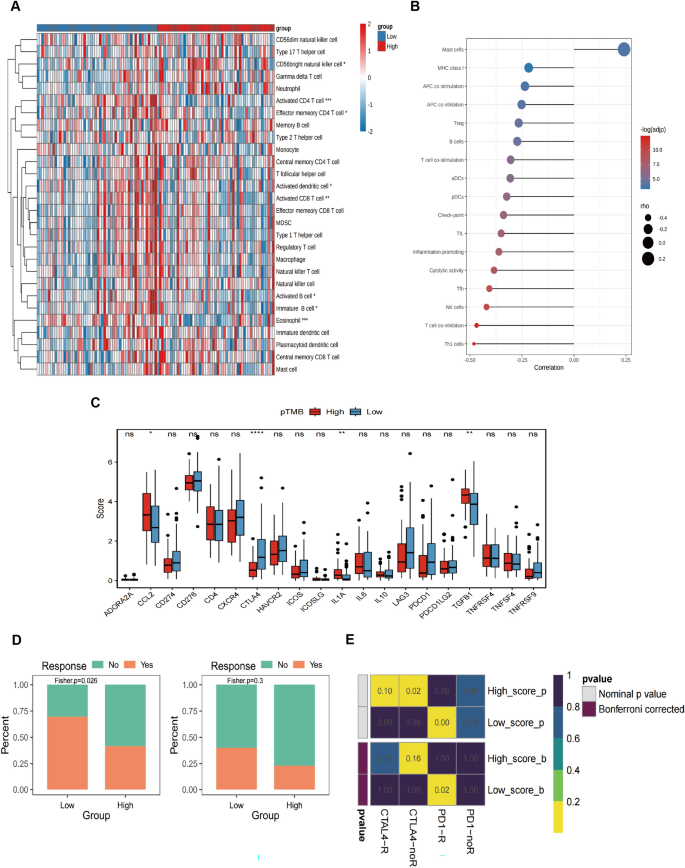
Using an optimal cutoff value based on pTMB enables the differential classification of groups into L-pTMB and H-pTMB categories (Supplementary Table 1). The findings demonstrated that the H-pTMB subgroup showed a significantly greater survival rate than the L-pTMB subgroup, as shown in Fig. 1 A. The proportion of gene mutations in the L-pTMB was much lower than in the H-pTMB, according to somatic mutation data of various pTMB levels (Fig. 1 B). The correlation analysis of pTMB with TMB, HRD, TNB, and CIN was also performed, and its results indicated that TMB, HRD, and TNB were positively associated with pTMB. In contrast, pTMB was negatively associated with CIN (Fig. 1 C). The distribution of clinicopathological features of pTMB and cutaneous melanoma showed significant differences between gender and pTMB (Supplementary Fig. 1A). This is unlike TMB, which was found to increase significantly with age regardless of gender in large sample data analyses (Li et al. 2022 ; Chalmers et al. 2017 ). This may indicate the advantage of pTMB in elderly CM.
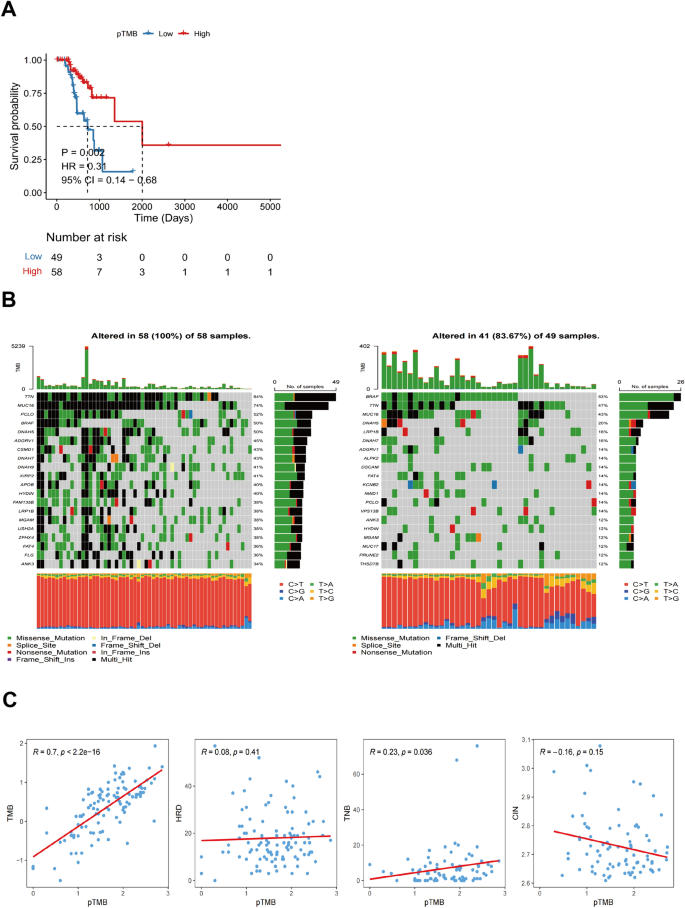
Shows the mutational characteristics linked to the pTMB level in SKCM. A Prognostic values of pTMB in SKCM. B Somatic mutations in different pTMB levels. ( C ) The relationship between different mutational markers and pTMB
The results of StromalScore, ImmuneScore, ESTIMATEScore, and TumorPurity for different pTMB levels were insignificant (Supplementary Fig. 1B). Immune cell subsets were quantified using the ssGSEA method. The findings revealed that the H-pTMB group had considerably more significant levels of infiltration of immature B cells, activated CD8 + T cells, activated CD4 + T cells, and other subsets (Fig. 2 A). Upon evaluating the expression of CD274 , CTLA4 , and other immune checkpoint genes at different pTMB levels, it was seen that the H-pTMB subgroup showed a significantly higher expression of CD274 , CTLA4 , and ICOS (Fig. 2 B). The H-pTMB group may thus be more responsive to immunotherapy, according to our inference. According to the GSVA differences, the H-pTMB group had a considerably high enrichment of the regulatory pathway of autophagy (Fig. 2 C). Autophagy is directly linked to the control of the immune response in tumors. It is also essential for the proper function and survival of the immune system's effector and developmental T cells. The extracellular matrix (ECM)-receptor interaction pathway, linked to tumor resistance, was significantly higher in the L- pTMB group.
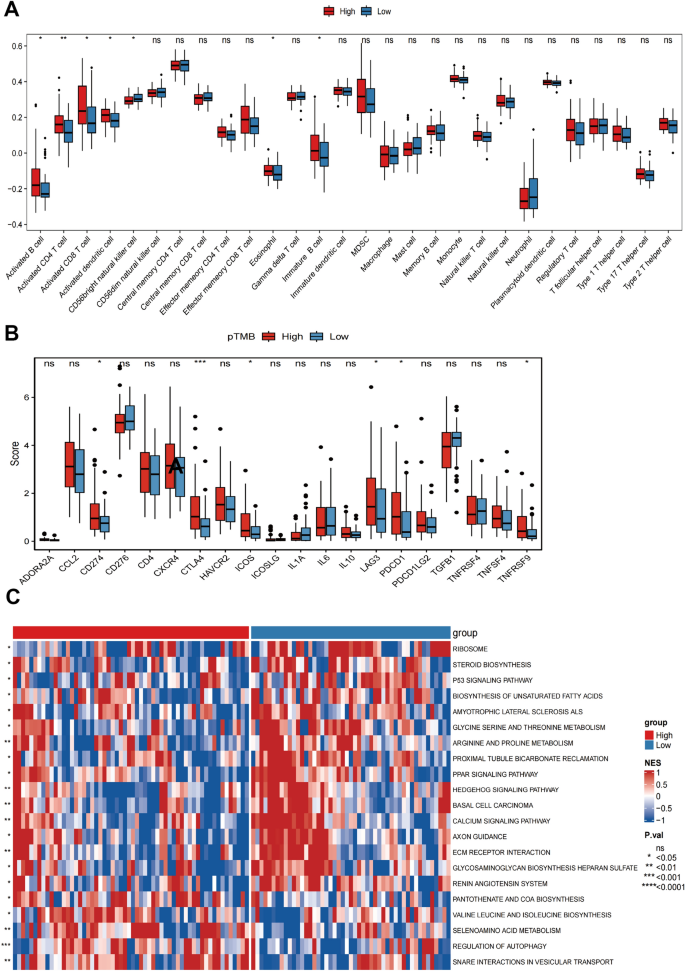
Immunological characteristics associated with pTMB concentration in SKCM. A ssGSEA immune infiltration with different pTMB levels. B Immune checkpoints with different pTMB levels. C GSVA of the immune pathway with varying levels of pTMB
Identification of pTMB-related signature
R software's "limma" package detected DEGs among the low-pTMB and high-pTMB subgroups. A comprehensive analysis revealed that a total of 2292 genes had differential expression patterns, with 1490 genes being up-regulated and 802 genes being down-regulated (Supplementary Table 2).
The univariate Cox regression analysis based on pTMB characteristics found 972 prognostic genes ( p < 0.05). Following the intersection with differential genes, 217 genes having prognostic significance were found. The forest plot was constructed using the top 20 genes with the lowest p -values (Fig. 3 A). Due to the extensive number of genes, which poses challenges for clinical identification, the LASSO regression model to refine the focus and determine the trajectory of the independent variables under study (Fig. 3 B). As lambda increased, independent variable coefficients gradually decreased to zero. The RiskScore, which is the gene-based survival risk score model, was constructed using 7 LASSO-coefficient-carrying genes, including IL17REL , SDC3 , RHOBTB2 , GSTA4 , MDFI , PTK7 , and FGF18 , based on the lambda value using LASSO. The confidence interval associated with each lambda value proves that the model achieves optimality when the number of genes is 7. This further supports the reliability of the candidate gene selection process. The RiskScore models were constructed using a ten-fold cross-validation approach, wherein the coefficients and expression levels of 7 specific genes were utilized to evaluate their influence on the OS outcome.

Establishing and validating the signature associated with pTMB. A Top 20 DEGs with prognostic values. B LASSO COX regression(with optimal lambda) identifying 7 host genes. C Patient statuses and expression patterns for seven host genes in the training cohort's high- and low-risk categories. The K-M survival curve and the AUC curve have distinct outcomes. D Status and expression patterns of seven host genes in the testing cohort's high- and low-risk patient groups. K-M survival curve and ROC curve demonstrating dissimilar outcomes
Based on median RiskScore, TCGA- SKCM cohort samples were divided into low- and high-risk categories (Supplementary Table 3). The KM survival analysis indicates that high-risk patients had a significantly lower OS than low-risk individuals. Additionally, the TCGA-SKCM cohort's OS could be predicted by the RiskScore, AUCs (area under the curve) for 1, 2, and 3 years were 0.819, 0.857, and 0.816, correspondingly (Fig. 3 C).
Using the same technique as the validation set GSE65904, the RiskScore model created using the TCGA-SKCM cohort was assessed for stability. The results demonstrated that low-risk SKCM had more substantial survival benefits, consistent with the TCGA-SKCM cohort (Fig. 3 C).
The univariate Cox regression analysis shows a strong association between TCGA-SKCM cohort risk score and OS (TCGA-SKCM cohort: HR = 5.99, 95% CI = 2.42–14.80, p < 0.001; GSE65904 cohort: HR = 1.67, 95% CI = 1.13–2.48, p = 0.01). Multivariate Cox regression analysis showed that risk score constituted an independent OS predictor (TCGA-SKCM cohort: HR = 9.72, 95% CI = 3.08–30.71, p < 0.001; GSE65904 cohort: HR = 1.63, 95% CI = 1.10–2.42, p = 0.02) (Fig. 4 A). A detailed investigation of the clinical applicability of the risk score signature. The present study observed that no clinical indicators exhibited positive findings in the independent prognostic analysis. Consequently, all the clinical indicators and risk scores were utilized to construct nomogram models and generate a calibration curve. The results are depicted in Fig. 4 B.
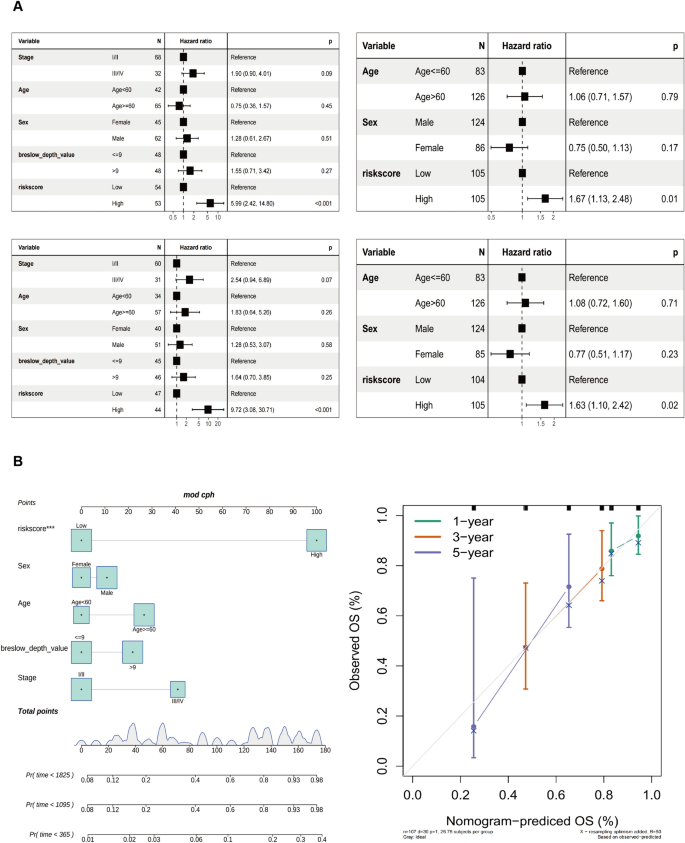
Development of the nomogram model. A Univariate and B multivariate Cox regression analyses for the risk score and other clinical variables of the training cohort. C Univariate and D multivariate Cox regression analysis for the risk score and other clinical characteristics of the testing cohort. E The construction of the nomogram aimed to develop a predictive model for estimating the probabilities of survival at 1-, 3-, and 5-year intervals. F Correction curve showing the consistency between predicted survival possibilities and observed survival rate
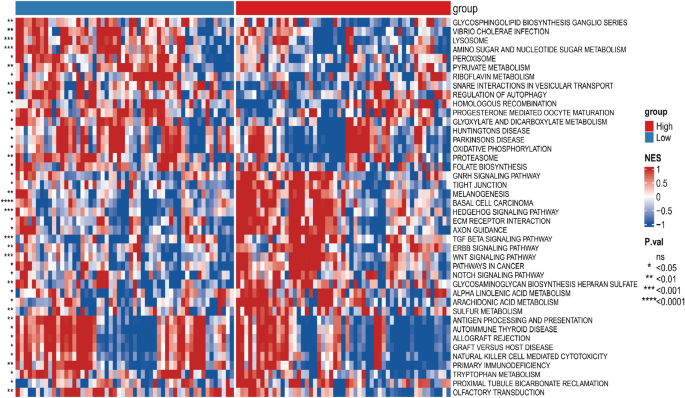
The biological traits of low- and high-risk subgroups were assessed using GSVA.
The enrichment proportion of basal cell carcinoma patients in the high-risk group was significantly higher than in the low-risk group.
The enrichment fraction was significantly higher in the high-risk subgroup than in the low-risk subgroup in basal cell carcinoma, melanogenesis, and other pathways (Fig. 5 ).
Differences in the biological features of the pTMB-related signature
Immune-associated characteristic differences and prediction of potential drug therapy between the prognostic signature low- and high-risk groups
Several immune cell subsets were quantified using ssGSEA, showing that high-risk subgroups had more CD56 bright natural killer cell infiltration. In contrast, the activated CD4 + T cells and eosinophils invaded the low-risk subgroup (Fig. 6 A). The difference in the immune function characteristics revealed that the levels of mast cells were considerably elevated in the high-risk subgroup. On the other hand, the low-risk subgroup had more NK cells, T cell co-inhibition, and Th1 cells (Fig. 6 B). Checkpoint expression analysis showed a statistically significant increase of CTLA4 in the low-risk subgroup (Fig. 6 C).
Variations in immune-related characteristics and possible drug treatment prediction in high-risk and low-risk populations. A Immune cell infiltration in high-risk and low-risk groups. B The graph displays the variations in immune function features between the low-risk and high-risk cohorts. C Boxplot showing immune checkpoint expression differences between low- and high-risk SKCM patients. D Drug sensitivity in low- and high-risk SKCM, including CTLA4 and PD-1. E Heat map for the response possibility of anti-PD-1 and anti-CTLA-4 treatment in the two risk groups
The melanoma treatment-associated sensitivity analysis conducted on the low- and high-risk subgroups indicates that the low-risk subgroup exhibited more positive responses to CTLA4 and PD-1 therapy than the high-risk subgroup (Fig. 6 D). Figure 6 E shows that the Submap approach compared the immunotherapy efficacy. The findings indicated a substantial similarity between the low-risk subgroup and the data from PD-1 treatment, implying that this group exhibited sensitivity to immunotherapy.
Expression of signature genes in SKCM
The differentially expressed genes were evaluated in normal and tumor samples to validate the risk-scoring model. For this, seven potential genes were investigated. The findings showed that in SKCM, the expression of FGF18 , MDFI , GSTA4 , and IL17REL were lowered. In contrast, SKCM increased the expression levels of the remaining two genes, except for PTK7 , which did not show a statistically significant difference (Fig. 7 A). The prognostic value of 6 genes in SKCM was analyzed and revealed that just the IL17REL gene exhibited expression levels in SKCM that correlated with OS. Specifically, it was shown that IL17REL expression was relatively low in SKCM cases, indicating a poor prognosis (Fig. 7 B). Therefore, validation of a prognostic signature by examining the effect of the IL17REL gene in tumor tissues and cell lines was done.
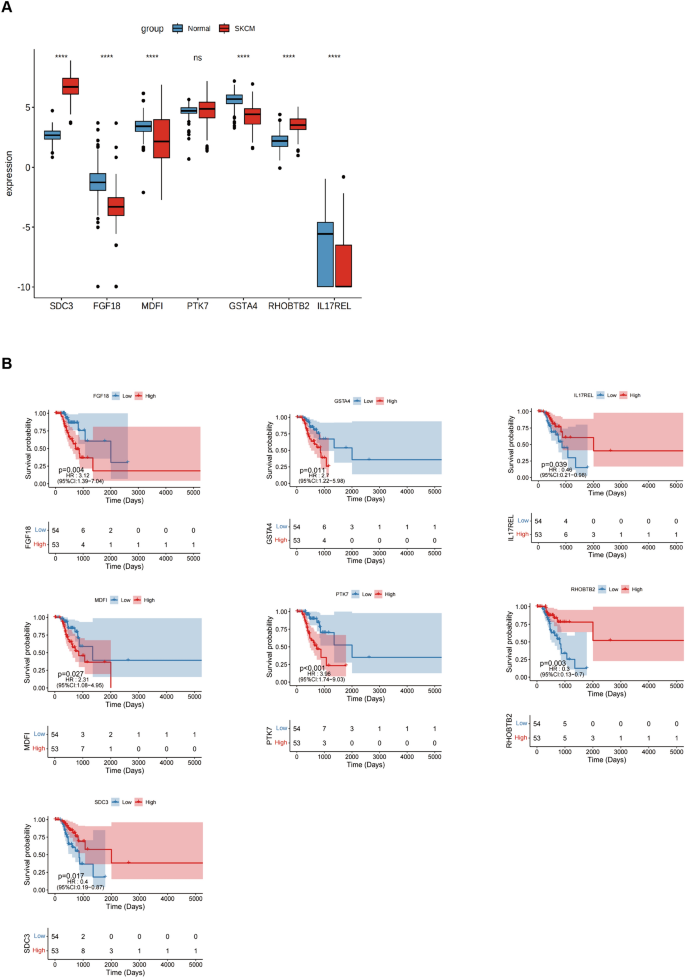
Expression of signature genes in SKCM. A Differentially expressed genes were evaluated on samples obtained from both normal and malignant tissues. B The K-M survival curve was produced to analyze the 7 host genes
Biological functions of the selected gene
IL17REL expression was detected in 3 in situ collected CM tissues, and the results from western blot (WB) showed a decrease in IL17REL gene expression, consistent with the bioinformatics analysis (Fig. 8 A). To further verify the effect of the IL17REL gene on the prognosis of CM, the overexpression efficiency of the IL17REL gene in human A375 and A875 cells was conducted using qRT-PCR and WB (Figure), and OE—IL17REL-1 and OE—IL17REL-2 were selected for further investigations (Fig. 8 B). The findings from the EdU experiment demonstrated that the upregulation of IL17REL had a detrimental effect on the proliferative capacity of malignant melanoma (MM) cells (Fig. 8 C). The levels of ROS generation in MM cells exhibited a reduction after the overexpression of IL17REL (Fig. 8 D). In addition, it was observed that the overexpression of IL17REL resulted in the suppression of MM cell migration, as evidenced by the findings from wound healing and transwell experiment (Fig. 8 E). The A375 and A875 cells overexpressed IL17REL had significantly higher cell proliferation and colony formation than the normal control cells (Fig. 8 F).
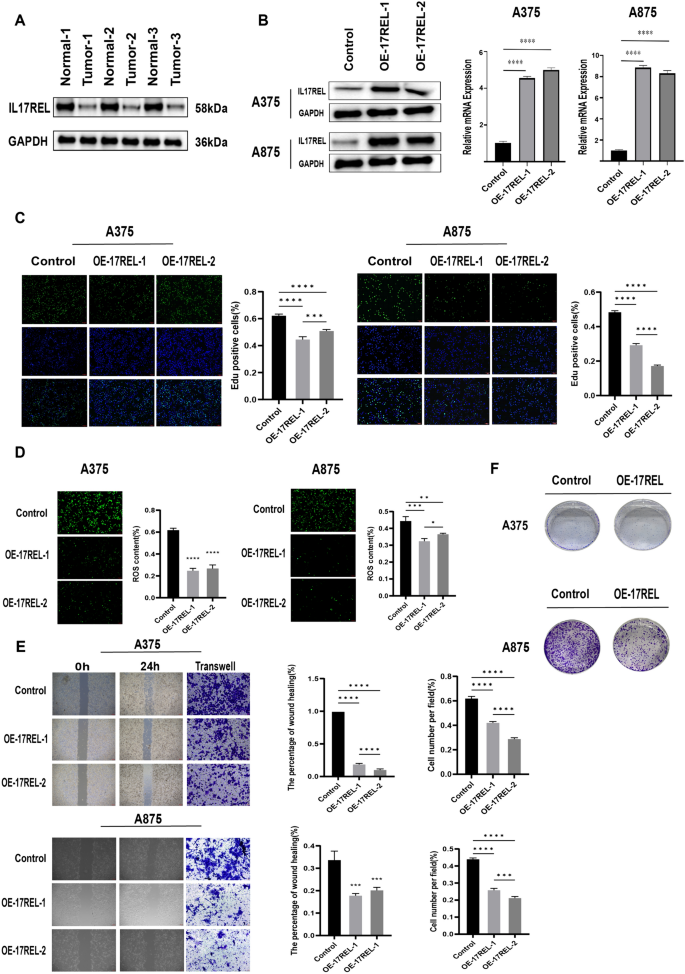
IL17REL inhibited the proliferation, clone, and migration in vivo and in vitro. A The expression of IL17REL was down-regulated in CM samples. B IL17REL was overexpressed in A375 and A875 cells. C OE-IL17REL inhibited proliferation in A375 and A875 cells.( D OE-IL17REL decreases ROS in A375 and A875 cells. E , F OE-IL17REL inhibited migration, invasion, and clone in A375 and A875 cells
Melanoma exhibits a notable degree of immunogenicity owing to its elevated load of genetic mutations and neoantigens, which can potentially trigger the commencement of the tumor cell elimination phase (Kalaora et al. 2022 ; Puig-Saus et al. 2023 ). The correlation between tumor mutation burden and the response to ICB has been widely acknowledged, making it a valuable predictive technique for assessing the outcomes of patients undergoing ICB treatment (Liu et al. 2019 ). The immunoediting theory suggests that, when subjected to immunotherapy, cancer cells acquire resistance against effector immune cells by favoring the growth of clones with reduced immunogenicity. According to a study, neoantigen loss was seen as a result of either the removal of tumor subclones or the loss of copy numbers (Łuksza et al. 2022 ). The observed loss was associated with the emergence of acquired resistance to immune checkpoint therapy (Anagnostou et al. 2017 ). In summary, these findings demonstrate the importance of accurately forecasting the clonality and heterogeneity of neoantigens.
Niknafs et al., 2023 , reported a collection of mutations characterized as "persistent" because of their reduced susceptibility to lose or develop immunoediting during tumor progression. These mutations primarily manifest as genomic and chromosomal deletions, constituting a minor proportion (10%) of somatic mutations. They defined it as pTMB, which refers to the cumulative count of single-copy and multi-copy mutations. The Whole Exome Sequencing (WES) research conducted on a collection of tumor samples both before and after ICB treatment revealed that persistent mutations exhibited a reduced tendency to induce subclonal loss during tumor progression within ICB (Davoli et al. 2017 ). Furthermore, there was no association observed between tumor clonal heterogeneity and the presence of persistent mutations. The researchers assessed the varying reclassification of cancer in 33 distinct tumor types. The results revealed that the average reclassification rate for the low/ high TMB subgroup, compared to the persistent low/high TMB subgroup, was 33%. This suggests that TMB and pTMB varied across all forms of cancer. In clinical applications, pTMB is better than TMB in predicting ICB response, and the authors further suggest that the predictive capacity of TMB to clinical outcomes primarily depends upon persistent mutations. The measurement of pTMB is an innovative method and has potential application in predicting ICB treatment outcomes in CM.
The present investigation examined the clinical and immunological associations between pTMB and CM, identified prognostically significant differentially expressed genes based on pTMB characteristics, developed a risk scoring system using pTMB-related gene modules, and investigated their prognostic utility, biological distinctions among various groups, and immune attributes for predicting potential therapeutic strategies for CM. Combined with candidate genes' differential expression and predictive value, further validation of the model in tissue specimens and in vitro experiments was done.
Among the pTMB-associated immune features, immature B cells, activated B cells, activated CD8 + T cells, and activated CD4 + T cells in the high- pTMB subgroup were up-regulated. Studies have shown that activated CD8 + and CD4 + T cells boost anti-tumor immunity. These cells are crucial in promoting the density and targeting of CD4 + /CD8 + effector T cells, enhancing immunotherapy's efficacy (Hirschhorn et al. 2023 ; Virassamy et al. 2023 ). There is a correlation between elevated levels of B-cell infiltration within the tumor microenvironment and favorable clinical outcomes in melanoma patients who undergo immunotherapy (Cabrita et al. 2020 ; Helmink et al. 2020 ). A comparison of immune checkpoint gene expression across multiple pTMB modes was performed. CD274 , CTLA4 , and ICOS exhibited a statistically significant upregulation in the high-pTMB subgroup.
Moreover, the high-pTMB subgroup showed a substantial rise in the regulatory pathway of autophagy, as seen by the GSVA results of various pTMB modes. Autophagy is essential for immune system development and affects T cell survival and function, influencing the control of immunological responses against tumors (Xia et al. 2021 ; Debnath et al. 2023 ). The ECM-receptor interaction pathway exhibited a significant enrichment in the group with low—p TMB. This enrichment was found to be connected with the development of drug resistance in tumors (Holle et al. 2016 ). Based on the study mentioned above, it is postulated that individuals with CM who exhibit high levels of pTMB may potentially be more sensitive to immunotherapy.
The current research used a risk score based on pTMB gene expression in multiple modes to construct a prognosis model and identified the differential genes with prognostic significance using analysis of different features. The results demonstrated this model's efficacy in accurately predicting patients' OS. Also, a decreased expression of the IL17REL gene was linked to worse outcomes in individuals with CM. This finding supports the validation of our proposed prognostic signature from a clinical standpoint. IL17REL encodes an IL17RE-like protein, and IL17RE is the least understood member of the IL17R family. In genome-wide association (GWAS) studies (Franke et al. 2010 ) and whole exon sequencing studies (Hu et al. 2021 ), IL17REL was found to be strongly correlated with the development of inflammatory bowel disease (IBD). Recent evidence indicates a strong association between IL17REL and the prognostic evaluation of HPV- associated head and neck squamous cell cancer (Yanan et al. 2023 ; Sun, et al. 2023 ). There needs to be more understanding regarding the expression and role of IL17REL in many cell types, particularly in tumor cells. Our study contributes novel insights to the realm of melanoma research.
In summary, this study has effectively developed and validated a predictive model linked to pTMB and has confirmed the possible impact of IL17REL on OS in individuals with melanoma. However, more experimental investigations are required to elucidate the precise molecular mechanism behind this association. Due to insufficient patient data, the study was incomplete regarding pre- and post-immunotherapy data for both the low and high-risk subgroups. Thus, the predictive models of pTMB can enhance the accuracy of patient survival predictions and serve as a valuable foundation for personalized decision-making in clinical settings.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Anagnostou V, Smith KN, Forde PM et al (2017) Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 7(3):264–276
Article CAS PubMed Google Scholar
Arnold M, Singh D, Laversanne M et al (2022) Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol 158(5):495–503
Article PubMed PubMed Central Google Scholar
Cabrita R, Lauss M, Sanna A et al (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577(7791):561–565
Carlino MS, Larkin J, Long GV (2021) Immune checkpoint inhibitors in melanoma. Lancet 398(10304):1002–1014
Centeno PP, Pavet V, Marais R (2023) The journey from melanocytes to melanoma. Nat Rev Cancer 23(6):372–390
Chalmers ZR, Connelly CF, Fabrizio D et al (2017) Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9(1):34
Charoentong P, Finotello F, Angelova M et al (2017) Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 18(1):248–262
Davoli T, Uno H, Wooten EC et al (2017) Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355(6322):eaaf8399
Debnath J, Gammoh N, Ryan KM (2023) Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol 24(8):560–575
Drews RM, Hernando B, Tarabichi M et al (2022) A pan-cancer compendium of chromosomal instability. Nature 606(7916):976–983
Article CAS PubMed PubMed Central Google Scholar
Franke A, Balschun T, Sina C et al (2010) Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nat Genet 42(4):292–294
Gide TN, Quek C, Menzies AM et al (2019) Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/Anti-CTLA-4 combined therapy. Cancer Cell 35(2):238
He Y, Jiang Z, Chen C et al (2018) Classification of triple-negative breast cancers based on Immunogenomic profiling. J Exp Clin Cancer Res 37(1):327
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577(7791):549–555
Hirschhorn D, Budhu S, Kraehenbuehl L et al (2023) T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 186(7):1432
Holle AW, Young JL, Spatz JP (2016) In vitro cancer cell-ECM interactions inform in vivo cancer treatment. Adv Drug Deliv Rev 97:270–279
Hu S, Vich Vila A, Gacesa R et al (2021) Whole exome sequencing analyses reveal gene-microbiota interactions in the context of IBD. Gut 70(2):285–296
CAS PubMed Google Scholar
Jie X, Chen Y, Zhao Y et al (2022) Targeting KDM4C enhances CD8+ T cell mediated antitumor immunity by activating chemokine CXCL10 transcription in lung cancer. J Immunother Cancer 10(2):e003716
Jung J, Heo YJ, Park S (2023) High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: a real-world pan-tumor analysis. J Immunother Cancer 11(4):e006454
Kalaora S, Nagler A, Wargo JA et al (2022) Mechanisms of immune activation and regulation: lessons from melanoma. Nat Rev Cancer 22(4):195–207
Leung M, Mcgranahan N (2023) Persistence is key: refining immunotherapy response prediction. Immunity 56(3):472–474
Li CH, Haider S, Boutros PC (2022) Age influences on the molecular presentation of tumours. Nat Commun 13(1):208
Liu J, Lichtenberg T, Hoadley KA et al (2018) An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 173(2):400–416
Liu D, Schilling B, Liu D et al (2019) Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med 25(12):1916–1927
Łuksza M, Sethna ZM, Rojas LA et al (2022) Neoantigen quality predicts immunoediting in survivors of pancreatic cancer. Nature 606(7913):389–395
Luo K, Liu S, Shen X et al (2022) Integration of cancer stemness and neoantigen load to predict responsiveness to anti-PD1/PDL1 therapy [J]. Front Cell Dev Biol 10:1003656
McGrail DJ, Pilié PG, Rashid NU, Voorwerk L et al (2021) High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 32(5):661–672
Niknafs N, Balan A, Cherry C et al (2023) Persistent mutation burden drives sustained anti-tumor immune responses. Nat Med 29(2):440–449
Patrinely JR, Johnson R, Lawless AR et al (2021) Chronic immune-related adverse events following adjuvant Anti-PD-1 therapy for high-risk resected melanoma. JAMA Oncol 7(5):744–748
Article PubMed Google Scholar
Puig-Saus C, Sennino B, Peng S et al (2023) Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy. Nature 615(7953):697–704
Roh W, Chen P-L, Reuben A et al (2017) Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aah3560
Serratì S, Guida M, Di Fonte R et al (2022) Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer 21(1):20
Thorsson V, Gibbs DL, Brown SD et al (2018) The immune landscape of cancer. Immunity 48(4):812–830
Virassamy B, Caramia F, Savas P et al (2023) Intratumoral CD8+ T cells with a tissue-resident memory phenotype mediate local immunity and immune checkpoint responses in breast cancer. Cancer Cell 41(3):585
Xia H, Green DR, Zou W (2021) Autophagy in tumour immunity and therapy. Nat Rev Cancer 21(5):281–297
Yanan L, Hui L, Zhuo C et al (2023) Comprehensive analysis of mitophagy in HPV-related head and neck squamous cell carcinoma. Sci Rep 13(1):7480
Yuhan Sun M, Khan AAK, Mangiola S et al (2023) IL17RB and IL17REL expression are associated with improved prognosis in HPV-infected head and neck squamous cell carcinomas. Pathogens 12(4):572
Download references
This study was funded by National Natural Science Foundation of China (Grant No.81871578) and supported by Sichuan Science and Technology Program (No.2020YFSY0030).
Author information
Mingze Xu, Xinyi Ma and Yuchong Wang have contributed equally to this work as co-first authors.
Authors and Affiliations
Department of Plastic Surgery, Changhai Hospital, Naval Military Medical University, 168 Changhai Road, Shanghai, 200433, People’s Republic of China
Mingze Xu, Xinyi Ma, Yuchong Wang, Haiying Dai & Chunyu Xue
Department of Radiology, Changhai Hospital, Naval Military Medical University, Shanghai, China
Basic Medical School, Southwest Medical University, Luzhou, Sichuan, China
Xiaoli Zheng
You can also search for this author in PubMed Google Scholar
Contributions
CX and HD designed the project and assays. MX and XM conducted the experimental assays. YW and XZ conducted the bioinformatic and statistical analysis. MX and DH collected the melanoma tissues. MX and XM wrote the paper and CX revised it. All authors have approved the final manuscript.
Corresponding authors
Correspondence to Haiying Dai or Chunyu Xue .
Ethics declarations
Conflict of interest.
None of the authors has a financial interest in any of the products or devices mentioned in this article.
Ethical approval and consent to participate
The study was approved by the Ethics Committee of the Changhai Hospital and was carried out according to the procedure of Changhai Hospital clinical research center biobank experiment.All patients who provided clinical specimens signed the written informed consent form.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2024_5843_MOESM1_ESM.png
Supplementary file1 Supplementary Figure 1 A The distribution of clinicopathological features of pTMB levels and cutaneous melanoma, B The results of StromalScore,ImmuneScore, ESTIMATEScore, and TumorPurity for different pTMB levels (PNG 172 KB)
432_2024_5843_MOESM2_ESM.xlsx
Supplementary file2 Supplementary Table 1: The differential classification of groups into L-pTMB and H-pTMB categories. (XLSX 14 KB)
Supplementary file3 Supplementary Table 2: The DEGs among the low-pTMB and high-pTMB subgroups. (TXT 2447 KB)
432_2024_5843_moesm4_esm.xlsx.
Supplementary file4 Supplementary Table 3: The differential classification of groups into low- and high-risk categories. (XLSX 1857 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Xu, M., Ma, X., Wang, Y. et al. Developing a prognostic model for skin melanoma based on the persistent tumor mutation burden and determining IL17REL as a therapeutic target. J Cancer Res Clin Oncol 150 , 313 (2024). https://doi.org/10.1007/s00432-024-05843-x
Download citation
Received : 23 April 2024
Accepted : 07 June 2024
Published : 20 June 2024
DOI : https://doi.org/10.1007/s00432-024-05843-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cutaneous melanoma
- Prognostic model
- Persistent tumor mutation burden
- Therapeutic target
- Gene signature
- Find a journal
- Publish with us
- Track your research
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.

Preprints with The Lancet is part of SSRN´s First Look, a place where journals identify content of interest prior to publication. Authors have opted in at submission to The Lancet family of journals to post their preprints on Preprints with The Lancet. The usual SSRN checks and a Lancet-specific check for appropriateness and transparency have been applied. Preprints available here are not Lancet publications or necessarily under review with a Lancet journal. These preprints are early stage research papers that have not been peer-reviewed. The findings should not be used for clinical or public health decision making and should not be presented to a lay audience without highlighting that they are preliminary and have not been peer-reviewed. For more information on this collaboration, see the comments published in The Lancet about the trial period, and our decision to make this a permanent offering, or visit The Lancet´s FAQ page, and for any feedback please contact [email protected] .
Analysis of Global Skin Cancer Epidemiology in 2022 and Correlation with Dermatologist Density
27 Pages Posted: 20 Jun 2024
Samir Salah
La Roche-Posay Laboratoire Dermatologique
Delphine Kerob
Khaled ezzedine.
Henri Mondor University Hospital
Puneet Khurana
FutureBridge
Deepthi Balan
Thierry passeron.
CHU de Nice
Background: Melanoma and non-melanoma skin cancers represent a significant public health burden, with skin cancers being the most frequently diagnosed type of cancer worldwide. Methods: This study used GLOBOCAN 2022 data from the International Agency for Research on Cancer (IARC) to analyze melanoma and non-melanoma skin cancer (NMSC) rates in 185 countries. IARC dataset excluded basal cell carcinomas (BCC) from NMSC incidence but included them in mortality estimates. Data from the U.S. Centers for Disease Control and Prevention (CDC) for 2018 were utilized to assess melanoma risk across different ethnic groups. The correlation between dermatologist density and skin cancer incidence-to-mortality rates was evaluated to identify national health policy profiles. Findings: In 2022, there were 331,722 melanoma cases and 58,667 related deaths globally. Approximately 1.2 million cases of NMSC were also reported. Despite a lower mortality rate, NMSC caused more deaths (69,416 vs. 58,667) due to higher incidence. Gender and age analysis showed an equal incidence of melanoma around age 54 between males and females (relative risk of 0.98), with incidence rates diverging thereafter. By age 85 and older, the relative risk for males reached 2.14. Non-Hispanic whites had the highest melanoma incidence, with a relative risk of 35 compared to non-Hispanic blacks. Mapping melanoma incidence and mortality-to-incidence ratios with dermatologist densities revealed varied country profiles, indicating different impacts and strategies against melanoma. Australia, the United Kingdom, and Canada showed successful cancer management despite having a relatively low density of dermatologists. Interpretation: Enhanced surveillance and deeper investigations into effective management strategies remain crucial for addressing melanoma and NMSC. Engaging other healthcare professionals and implementing consumer-oriented campaigns to improve awareness, early detection, and patient education, especially among vulnerable populations, are key for better disease management. Funding: This study was funded by La Roche-Posay laboratoire dermatologique. Declaration of Interest: Dr Kerob and Mr Salah are employees of L’Oreal. Pr Ezzedine has served as a consultant for La Roche Posay, L'Oréal, Incyte, MSD, BMS, Abbvie, Pfizer and Pierre Fabre. Pr Passeron has served as a consultant for La Roche Posay, L'Oréal, SVR, Symrise, Isis Pharma, Bioderma, Beiersdorf, ISDIN, Pierre Fabre and Hyphen. Dr Khurana and Mr Balan are employees of FutureBridge.
Keywords: skin cancer, non melanoma skin cancer, melanoma, basal cell carcinoma, squamous cell carcinoma
Suggested Citation: Suggested Citation
Samir Salah (Contact Author)
La roche-posay laboratoire dermatologique ( email ), henri mondor university hospital ( email ), futurebridge ( email ), chu de nice ( email ), click here to go to thelancet.com, paper statistics, related ejournals, preprints with the lancet.
Subscribe to this free journal for more curated articles on this topic
New Approach to Treating Melanoma
- Download PDF Copy
An international research team, led by Professor Wenbo Bu of Fudan University and Distinguished Professor Dayong Jin of the University of Technology Sydney , discovered an innovative method to effectively treat cancer by reactivating suppressed metabolic pathways in cancer cells, as published in the prestigious journal Nature Nanotechnology .

Image Credit: crystal light/Shutterstock.com
The researchers employed tyrosine, a common amino acid, as a nanomedicine to alter the metabolism of melanoma , a fatal skin cancer, and prevent the disease from spreading.
Australia has the highest skin cancer rate in the world. This novel technique could be coupled with existing therapies to improve the treatment of melanoma. The approach could potentially be used to treat other forms of cancer.
Tyrosine’s bioavailability is restricted in living beings. However, the researchers employed a novel nanotechnology approach to packaging it into small particles known as nanomicelles. These particles are attracted to cancer cell membranes and rapidly break down, increasing absorption.
The researchers then tested the novel therapy in mice and human-derived melanoma cells in the lab, discovering that the tyrosine nanomicelles awoke dormant metabolic pathways, induced melanin formation, and suppressed tumor development.
Uncontrolled rapid growth is a key feature that distinguishes cancer cells from normal cells. In cancer cells some metabolic pathways are over-activated, and others are suppressed, to create the environment necessary for rapid spread. Dayong Jin, Distinguished Professor, University of Technology Sydney
He added, “ While a few metabolism-based drugs for cancer have been developed previously, such as aromatase inhibitors impeding estrogen synthesis in breast cancer and HK2 inhibitors targeting glycolysis in various cancers, these work by suppressing over-activate metabolic pathways. ”
Professor Bu noted, “ Our research shows for the first time that cancer can be stopped by reactivating metabolic pathways that are dormant. And this can be done using simple nutrients, such as amino acids, sugars, and vitamins, which are safe, readily available and well tolerated. ”
Related Stories
- Study spotlights differences based on race and gender in melanoma diagnosis, outcomes
- UC San Diego Health launches personalized immunotherapy for metastatic melanoma
- In-vivo imaging to quantify collagen morphology in preclinical melanoma models during immunotherapy
Different nutrients will have varying effects on cancer. Melanoma cells arise from melanocytes, which are skin cells that create melanin. Tyrosine is required to create melanin and can enhance melanin production , which explains its effectiveness with melanoma.
The reactivation of melanin synthesis causes the melanoma cell to lower glycolysis, the process of turning sugar into energy, which is believed to be the reason for its anti-cancer effects.
Melanoma cells are also susceptible to heat stress. The researchers discovered that by combining tyrosine nanomicelle therapy with near-infrared laser treatment, they could remove melanoma in mice within six days, and it did not return during the study time.
The findings point to a possible fresh frontier in using nanomedicine for cancer treatment.
Chen, Y. et al. (2024) Nutrient-delivery and metabolism reactivation therapy for melanoma. Nature Nanotechnology . doi:10.1038/s41565-024-01690-6
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Amino Acid , Breast Cancer , Cancer , Cancer Treatment , Cell , Drugs , Estrogen , Glycolysis , heat , Melanin , Melanoma , Melanoma Cells , Metabolism , Nanomedicine , Nanotechnology , Nutrients , Research , Skin , Skin Cancer , Skin Cells , Stress , Technology , Tumor , Tyrosine , Vitamins
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

Revolutionizing Life Science: An Interview with SCIEX on ASMS, the SCIEX 7500+ System, and AI-Driven Quantitation
Jose Castro-Perez and Chris Lock, SCIEX
In our latest interview, News Medical speaks with SCIEX, a global leader in life science analytical technologies, about their exciting announcements at ASMS, the SCIEX 7500+ System, and how they utilize AI quantitation software to streamline solutions.

From Discovery Biology to ELRIG Chair
Melanie Leveridge
In this interview, we speak with Melanie Leveridge, Vice President of Discovery Biology at AstraZeneca and Chair of the Board for ELRIG UK, to discuss her extensive career in the pharmaceutical industry, her role in fostering scientific innovation, and her vision for ELRIG's future.

Breathing New Life into Diagnostics: Plasmion's SICRIT Technology
Revolutionizing Non-Invasive Diagnostics with Plasmion’s SICRIT Breath Analysis.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

- Introduction
- Conclusions
- Article Information
Estimated incidence per 100 000 person-years at age 60 years is shown by birth year (1908-1983). All curves are on the log 10 scale. These and additional curves are shown in eFigure 11 in Supplement 1 . Tick marks on the x-axis indicate start years for consecutive social generations: 1928-1945, Silent Generation; 1946-1964, Baby Boomers; 1965-1980, Generation X. Shaded areas indicate 95% CIs.
Estimated incidence per 100 000 person-years at age 60 years is shown by birth year (1908-1983). All curves are on the log 10 scale. These and additional curves are shown in eFigure 13 in Supplement 1 . Tick marks on the x-axis indicate start years for consecutive social generations: 1928-1945, Silent Generation; 1946-1964, Baby Boomers; 1965-1980, Generation X. Shaded areas indicate 95% CIs.
Gen X indicates Generation X. Shaded areas indicate 95% CIs.
Fitted cohort pattern curves by cancer site are adjusted for race and ethnicity as described in the Methods, plotted on a natural log scale, and sorted from largest (top) to smallest (bottom). IRR indicates incidence rate ratio; NHL, non-Hodgkins lymphoma.
Arrow plots indicate magnitude and direction of change from older to younger generations. Corresponding percentage changes and 95% CIs are also graphed in eFigure 19 in Supplement 1 .
eMethods. Statistical Methods
eTable 1. Organ-Specific Site Code Used in the Classification of Cancer Sites Using SEER*Stat 8.4.0
eTable 2. Age-Standardized Incidence Rates and Cancer Cases by Sex, Race and Ethnicity, United States, 35-84 Years Old, 1992-2018
eFigure 1. Observed Rates in Females
eFigure 2. APC Fitted Values in Females
eFigure 3. Observed Rates in Males
eFigure 4. APC Fitted Values in Males
eFigure 5. Lack of Fit (LOF) in Females
eFigure 6. Lack of Fit (LOF) in Males
eFigure 7. Higher-Order Deviations vs Lack of Fit (LOF) in Females
eFigure 8. Higher-Order Deviations vs Lack of Fit (LOF) in Males
eFigure 9. Local Drifts in Females
eFigure 10. Local Drifts in Males
eFigure 11. Fitted Cohort Patterns (FCPs) by Cancer Site, Race, and Ethnicity: Females
eFigure 12. Estimated Annual Percentage Change (EAPC) of the Fitted Cohort Pattern (FCP): Females
eFigure 13. Fitted Cohort Patterns (FCPs) by Cancer Site, Race, and Ethnicity: Males
eFigure 14. Estimated Annual Percentage Change (EAPC) of the Fitted Cohort Pattern (FCP): Males
eFigure 15. Average Incidence at Age 60: Generation X vs Baby Boomers
eFigure 16. Average Incidence at Age 60: Baby Boomers vs the Silent Generation
eFigure 17. Average Incidence at Age 60: Silent vs Greatest Generations
eFigure 18. Site-Adjusted Cancer Incidence Rate Ratios (IRRs) for Non-Hispanic Black, Hispanic, and Asian or Pacific Islander vs Non-Hispanic White by Sex and Social Generation
eFigure 19. Percent Changes in Incidence of Leading Cancers at Age 60 Years per 100 000 Person-Years in Successive Generations
Data Sharing Statement

See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Rosenberg PS , Miranda-Filho A. Cancer Incidence Trends in Successive Social Generations in the US. JAMA Netw Open. 2024;7(6):e2415731. doi:10.1001/jamanetworkopen.2024.15731
Manage citations:
© 2024
- Permissions
Cancer Incidence Trends in Successive Social Generations in the US
- 1 Division of Cancer Epidemiology and Genetics, Biostatistics Branch, National Cancer Institute, Rockville, Maryland
Question Is cancer incidence in successive social generations in the US slowing or growing?
Findings In this cohort study of 3.8 million patients with cancer ascertained by the Surveillance, Epidemiology, and End Results Program, members of Generation X born between 1965 and 1980 have been experiencing larger per-capita increases in the incidence of leading cancers combined than any prior generation born between 1908 and 1964.
Meaning These findings suggest that based on current trajectories, cancer incidence in the US might remain high for decades.
Importance The incidence of some cancers in the US is increasing in younger age groups, but underlying trends in cancer patterns by birth year remain unclear.
Objective To estimate cancer incidence trends in successive social generations.
Design, Setting, and Participants In this cohort study, incident invasive cancers were ascertained from the Surveillance, Epidemiology, and End Results (SEER) program’s 13-registry database (November 2020 submission, accessed August 14, 2023). Invasive cancers diagnosed at ages 35 to 84 years during 1992 to 2018 within 152 strata were defined by cancer site, sex, and race and ethnicity.
Exposure Invasive cancer.
Main Outcome and Measures Stratum-specific semiparametric age-period-cohort (SAGE) models were fitted and incidence per 100 000 person-years at the reference age of 60 years was calculated for single-year birth cohorts from 1908 through 1983 (fitted cohort patterns [FCPs]). The FCPs and FCP incidence rate ratios (IRRs) were compared by site for Generation X (born between 1965 and 1980) and Baby Boomers (born between 1946 and 1964).
Results A total of 3.8 million individuals with invasive cancer (51.0% male; 8.6% Asian or Pacific Islander, 9.5% Hispanic, 10.4% non-Hispanic Black, and 71.5% non-Hispanic White) were included in the analysis. In Generation X vs Baby Boomers, FCP IRRs among women increased significantly for thyroid (2.76; 95% CI, 2.41-3.15), kidney (1.99; 95% CI, 1.70-2.32), rectal (1.84; 95% CI, 1.52-2.22), corpus uterine (1.75; 95% CI, 1.40-2.18), colon (1.56; 95% CI, 1.27-1.92), and pancreatic (1.39; 95% CI, 1.07-1.80) cancers; non-Hodgkins lymphoma (1.40; 95% CI, 1.08-1.82); and leukemia (1.27; 95% CI, 1.03-1.58). Among men, IRRs increased for thyroid (2.16; 95% CI, 1.87-2.50), kidney (2.14; 95% CI, 1.86-2.46), rectal (1.80; 95% CI, 1.52-2.12), colon (1.60; 95% CI, 1.32-1.94), and prostate (1.25; 95% CI, 1.03-1.52) cancers and leukemia (1.34; 95% CI, 1.08-1.66). Lung (IRR, 0.60; 95% CI, 0.50-0.72) and cervical (IRR, 0.71; 95% CI, 0.57-0.89) cancer incidence decreased among women, and lung (IRR, 0.51; 95% CI, 0.43-0.60), liver (IRR, 0.76; 95% CI, 0.63-0.91), and gallbladder (IRR, 0.85; 95% CI, 0.72-1.00) cancer and non-Hodgkins lymphoma (IRR, 0.75; 95% CI, 0.61-0.93) incidence decreased among men. For all cancers combined, FCPs were higher in Generation X than for Baby Boomers because gaining cancers numerically overtook falling cancers in all groups except Asian or Pacific Islander men.
Conclusions and Relevance In this model-based cohort analysis of incident invasive cancer in the general population, decreases in lung and cervical cancers in Generation X may be offset by gains at other sites. Generation X may be experiencing larger per-capita increases in the incidence of leading cancers than any prior generation born in 1908 through 1964. On current trajectories, cancer incidence could remain high for decades.
Is cancer incidence in the US—ie, the number of newly diagnosed cases per capita per year—slowing or growing? Notably, while the incidence of certain cancers is declining, 1 others are increasing in younger age groups (aged <50 years). 2 These temporal patterns encompass a diversity of neoplasms and vary over time by demographic factors, including age, sex, and race and ethnicity. A more fundamental question is: are we collectively experiencing lower cancer incidence as we age than the generation of our parents?
Analyses that focus on birth years and social generations can provide insights into cancer prevention and health care accessibility and highlight persistent demographic and socioeconomic inequalities that span generations. Nevertheless, tracking the history of cancer in the US is challenging because cancers evolve over the lifetimes of individuals, 3 yet the observational period of contemporary cancer registries spans at most a few decades. 4 Hence, we must reconstruct the longitudinal history of cancer in the population from a time-limited series of cross-sectional observations. To understand the overarching trends, it is imperative to assemble these snapshots into a cohesive longitudinal narrative for a variety of cancer types in diverse demographic groups.
With the use of new statistical methods, 5 , 6 we can now obtain single-year reconstructions of cancer incidence by age, period, and birth cohort with unprecedented precision. Our goal is to model the shifting landscape of cancer from one birth year to the next for leading cancers in men and women by race and ethnicity. From this ensemble, we hope to glean insights into the overall trajectory of cancer in the US from the Greatest Generation (born from 1908 through 1927) through Generation X (born from 1965 through 1980).
Our cohort study was based on publicly available, deidentified data and, therefore, the National Cancer Institute institutional review board determined it exempt from review and informed consent. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
We collected case and person-years at risk data from the Surveillance, Epidemiology, and End Results (SEER) program’s 13-registery database using SEER*Stat, version 8.4.0. Data ascertained for the November 2020 submission were retrieved on August 14, 2023 (eTable 1 in Supplement 1 ). Using these data, we assembled 152 rate matrix Lexis diagrams 7 covering 50 single years of age (35-84 years) and 27 calendar years (1992-2018), spanning 76 single-year birth cohorts (1908-1983) for 21 leading invasive cancers in women and men within 4 racial and ethnic categories: Asian or Pacific Islander, Hispanic, non-Hispanic Black, and non-Hispanic White. The definitions of race and ethnicity follow the criteria established by the SEER program. 8 In brief, the SEER program uses an algorithm to recode detailed race and origin variables in the SEER incidence data. The 21 leading invasive cancers were esophageal, stomach, gallbladder, liver, pancreatic, colon, rectal, kidney, bladder, leukemia, non-Hodgkin lymphoma (NHL), myeloma, brain and central nervous system (hereafter, brain), thyroid, lung, melanoma of skin (hereafter, melanoma), breast, ovarian, corpus uterine (hereafter, corpus), cervix uterine (hereafter, cervical), and prostate (eTable 2 in Supplement 1 ).
We analyzed each Lexis diagram using semiparametric age-period-cohort (SAGE) analysis. 6 The basic idea is to denoise the observed Lexis diagrams upfront by using a contemporary nonparametric procedure, 5 then fit the new age-period-cohort (APC) model to the smoothed Lexis diagrams. 9 The new APC model contains parameters that describe how expected rates vary as a function of birth cohort, accounting for age and calendar period effects. We used the model parameters to estimate the fitted cohort pattern (FCP). The FCP estimates the absolute incidence of a cancer at an arbitrary reference age (here, 60 years) for each birth year in the Lexis diagram. 9 Additional details are provided in the eMethods in Supplement 1 .
To further characterize FCP features, we fitted Joinpoint models 10 to the FCPs, allowing up to 5 segments, each with 10 or more birth years. We also computed mean FCPs and corresponding variances for the following social generations: Greatest Generation (1908-1927), Silent Generation (1928-1945), Baby Boomers (1946-1964), and Generation X (1965-1980). We had too few data points to produce estimates for Millennials (1981-1996).
To compare and contrast mean FCP values across generations, we fitted log-linear models to the generational differences in site-specific FCPs among men and women separately for the Silent vs Greatest Generations, Baby Boomers vs the Silent Generation, and Generation X vs Baby Boomers. We adjusted for race and ethnicity with non-Hispanic White as the reference group and weighted the data inversely with the estimated variance of each difference.
The SEER registries do not record the parents’ birth year for each cancer case. However, on average, the parents of Generation X are Baby Boomers and members of the Silent Generation, and the parents of Baby Boomers are members of the Silent and Greatest Generations. To see this assumption, subtract 20 through 29 years (mean maternal age at birth during our study period 11 ) from the end points of each generation, yielding 1917-1944 for Baby Boomers, which straddles the Greatest and Silent Generations (eg, 1917-1927 and 1928-1944, respectively), and 1936-1960 for Generation X, which straddles the Silent Generation and Baby Boomers (eg, 1936-1945 and 1946-1960, respectively). Similarly, the proxy parents of the Millennial generation (1981-1996) straddle the Baby Boomers and Generation X (eg, 1952-1964 and 1965-1976, respectively). In our study, we make no adjustments for multiple testing.
In total, we analyzed 3.8 million cases of incident cancer occurring over 521 million person-years (eTable 2 in Supplement 1 ). Overall, 51.0% of individuals were male (compared with 49.0% female) and 71.5% were non-Hispanic White (compared with 8.6% Asian or Pacific Islander, 9.5% Hispanic, and 10.4% non-Hispanic Black).
The SAGE analysis produced optimally smoothed single-year estimates of incidence for 80 female strata (observed Lexis diagrams, eFigure 1 in Supplement 1 ; smoothed APC fitted rates, eFigure 2 in Supplement 1 ) and 72 male strata (observed, eFigure 3 in Supplement 1 ; smoothed, eFigure 4 in Supplement 1 ). For the majority, lack of fit (LOF) (females, eFigure 5 in Supplement 1 ; males, eFigure 6 in Supplement 1 ) was negligible or small relative to the corresponding annual fluctuations (females, eFigure 7 in Supplement 1 ; males, eFigure 8 in Supplement 1 ). The most prominent LOF was observed for liver, rectal, and brain cancers and melanoma in females and liver, brain, thyroid, and prostate cancers in males. Time trends by age (local drifts) were significant in all strata (females, eFigure 9 in Supplement 1 ; males, eFigure 10 in Supplement 1 ). As shown previously, 6 LOF had little effect on the local drifts for the Lexis diagrams included in our study. Hence, the model fits appear adequate. 6
eFigure 11 in Supplement 1 presents FCPs by cancer site for women by race and ethnicity. Six of these FCPs, including pancreatic, colon, kidney, thyroid, lung, and cervical, are also shown in Figure 1 A through F, respectively. The statistical efficiency of the SAGE analysis yielded partially or fully nonoverlapping curves within most FCPs; the conclusions below were obtain by visual inspection.
Differences by race and ethnicity strongly depended on birth cohort. There were marked declines in cervical FCPs across all racial and ethnic groups. Incidence rates were highest among the Greatest Generation in non-Hispanic Black women for esophageal, pancreatic, and colon cancers and myeloma; in Hispanic women for gallbladder cancer; in Asian or Pacific Islander women for stomach and liver cancers; and in non-Hispanic White women for bladder, breast, ovarian, and corpus cancers and leukemia, NHL, and melanoma. Patterns by race and ethnicity were similar for the Silent Generation. Patterns changed with the Baby Boomers. Notable changes included convergence of esophageal cancers in non-Hispanic Black and non-Hispanic White women; steep declines for stomach cancer in Asian or Pacific Islander women; and steep increases in corpus cancer for Asian or Pacific Islander, Hispanic, and non-Hispanic Black women. In Generation X women, the FCPs consistently increased for liver, colon, rectal, kidney, thyroid, and corpus cancers ( Figure 1 ; eFigure 11 in Supplement 1 ). Estimates of the corresponding estimated annual percentage changes (EAPCs) of the FCPs obtained by Joinpoint analysis are shown in eFigure 12 in Supplement 1 .
eFigure 13 in Supplement 1 presents FCPs by cancer site for men. Six of these FCPs, including pancreatic, colon, kidney, thyroid, lung, and prostate, are also shown in Figure 2 A through F, respectively. For thyroid cancer, increases by cohort were slower in Asian or Pacific Islander, Hispanic, and non-Hispanic Black men compared with women, and peak incidence for lung cancer occurred earlier in men compared with women. The FCPs for prostate cancer were parallel across racial and ethnic groups, being highest in non-Hispanic Black and lowest in Asian or Pacific Islander men. In Generation X men, the FCPs were consistently increasing for colon, rectal, kidney, and thyroid cancers ( Figure 2 ; eFigure 13 in Supplement 1 ). eFigure 14 in Supplement 1 provides the corresponding estimated EAPCs.
Figure 3 presents the sum of the cancer site–specific FCPs (20 sites in women and 18 in men) by sex and race and ethnicity. Among men, incidence at age 60 years declined through the Greatest and Silent Generations in all 4 racial and ethnic groups. Subsequently, incidence increased for the Baby Boomers. Except for Asian or Pacific Islander men, incidence continued to increase in Generation X.
In contrast, among women, incidence was comparatively stable among members of the Greatest and Silent Generations and then increased beginning with the Baby Boomers for non-Hispanic White individuals ( Figure 3 A), then the Silent Generation for Hispanic and Asian or Pacific Islander ( Figure 3 C and D) and Generation X for non-Hispanic Black individuals ( Figure 3 B). Within each racial and ethnic group, the large male excess in the Greatest Generation diminished in subsequent generations. By Generation X, Hispanic and non-Hispanic White women had a similar incidence to their male counterparts ( Figure 3 A and C), and Asian or Pacific Islander women had a higher incidence compared with their male counterparts ( Figure 3 D). In the non-Hispanic Black group, the male excess persisted.
eFigure 15 in Supplement 1 presents arrow plots of mean FCP values by cancer site for Generation X vs Baby Boomers stratified by sex and race and ethnicity. Within each stratum, the FCP for Generation X was higher than the corresponding FCP for Baby Boomers at some sites and lower at others. Similar heterogeneity was observed in arrow plots of Baby Boomers vs the Silent Generation (eFigure 16 in Supplement 1 ) and the Silent vs Greatest Generations (eFigure 17 in Supplement 1 ).
To synthesize these data, we estimated FCP incidence rate ratios (IRRs) by fitting log-linear models to the estimated FCPs in eFigures 15 to 17 in Supplement 1 . For example, for Generation X vs Baby Boomer women, we synthesized the 80 arrows shown in panels eFigure 15A to D in Supplement 1 . Each arrow, which is indexed by site and race and ethnicity, contributed 1 difference or IRR to the modeled data. The estimated variances of the differences were used as weights.
Figure 4 presents forest plots of the site-specific IRRs between successive social generations adjusted for race and ethnicity in women and men. Point estimates are shown for non-Hispanic White individuals. Estimates for other racial and ethnic groups are similar (eFigure 18 in Supplement 1 ). Site-specific IRRs varied markedly by generation except for the brain.
The incidence of many cancers increased significantly in members of Generation X vs Baby Boomers ( Figure 4 ). Among women, increases were observed for the following cancers: thyroid (IRR, 2.76, 95% CI, 2.41-3.15), kidney (IRR, 1.99; 95% CI, 1.70-2.32), rectal (IRR, 1.84; 95% CI, 1.52-2.22), corpus (IRR, 1.75; 95% CI, 1.40-2.18), colon (IRR, 1.56; 95% CI, 1.27-1.92), NHL (IRR, 1.40, 95% CI 1.08-1.82), pancreatic (IRR, 1.39; 95% CI, 1.07-1.80), and leukemia (IRR, 1.27; 95% CI, 1.03-1.58). Among men, increases were observed for the following cancers: thyroid (IRR, 2.16; 95% CI, 1.87-2.50), kidney (IRR, 2.14; 95% CI, 1.86-2.46), rectal (IRR, 1.80; 95% CI, 1.52-2.12), colon (IRR, 1.60; 95% CI, 1.32-1.94), leukemia (IRR, 1.34; 95% CI, 1.08-1.66), and prostate (IRR, 1.25; 95% CI, 1.03-1.52). Other cancers decreased, including lung (IRR, 0.60; 95% CI, 0.50-0.72) and cervical (IRR, 0.71; 95% CI, 0.57-0.89) among women and lung (IRR, 0.51; 95% CI, 0.43-0.60), NHL (IRR, 0.75; 95% CI, 0.61-0.93), liver (IRR, 0.76; 95% CI, 0.63-0.91), and gallbladder (IRR, 0.85; 95% CI, 0.72-1.00) among men.
Figure 5 summarizes incidence at age 60 years for leading cancers combined, averaged over birth years within successive generations. eFigure 19 in Supplement 1 plots the corresponding percentage changes. Comparing the Silent vs Greatest Generations ( Figure 5 A), combined incidence decreased significantly in both sexes and all 4 racial and ethnic groups. The decreases were greater for men compared with women. For example, incidence decreased by 41.0% (95% CI, −43.9% to −38.1%) in non-Hispanic White men vs 5.6% (95% CI, −6.6% to −4.6%) in non-Hispanic White women. In contrast, comparing Baby Boomers with the Silent Generation ( Figure 5 B), combined incidence had a mixed pattern, decreasing in some groups, eg, by 5.4% (95% CI, −6.1% to −4.8%) and 10.6% (95% CI, −11.6% to −9.5%), respectively, in non-Hispanic White women and men, but increasing in others, eg, by 13.0% (95% CI, 11.1%-14.9%) among Hispanic women.
Comparing Generation X with Baby Boomers ( Figure 5 C), combined incidence increased in all groups except for Asian or Pacific Islander men. The increases ranged from 5.5% (95% CI, 1.4%-9.7%) in non-Hispanic Black women to 28.0% (95% CI, 23.5%-32.5%) in Hispanic women. The increases ranged from 10.4% (95% CI, 0.7%-20.1%) in Hispanic men to 13.7% (95% CI, 2.4%-24.9%) in non-Hispanic White men. The increase among non-Hispanic Black men was 13.7% (95% CI, −1.9% to 29.2%). Incidence decreased by 8.2% (95% CI, −15.6% to −0.9%) among Asian or Pacific Islander men. Except for Asian or Pacific Islander men, these increases were larger than any observed in prior social generations (eg, point estimates in Figure 5 C are larger than corresponding point estimates in Figure 5 A and B). In absolute terms, non-Hispanic Black men in Generation X had the highest combined incidence at 1561 cases per 100 000 person-years (95% CI, 1493-1629 cases per 100 000 person-years), while Asian or Pacific Islander men had the lowest at 519 cases per 100 000 person-years (95% CI, 474-563 cases per 100 000 person-years).
Male Baby Boomers had a lower combined incidence than their proxy parents (Greatest and Silent Generation birth cohorts from 1917 to 1944) ( Figure 5 D), with the greatest reduction of 28.8% (95% CI, −31.0 to −26.7%) in non-Hispanic Black men. The decreases among men were statistically significant in the other 3 racial and ethnic groups (Asian or Pacific Islander, −16.8% [95% CI, −19.5% to −14.2%]; Hispanic, −11.3% [95% CI, −14.0% to −8.6%]; non-Hispanic White, −20.8% [95% CI, −21.9% to −19.7%]). Female Baby Boomers had a mixed pattern: non-Hispanic White women, 7.0% lower (95% CI, −7.7% to −6.3%); essentially unchanged in non-Hispanic Black women, 0.7% higher (95% CI, −1.3% to 2.7%); Hispanic women, 12.1% higher (95% CI, 9.8%-14.4%); Asian or Pacific Islander women, 7.7% higher (95% CI, 5.4%-10.0%).
In contrast, members of Generation X had a higher combined incidence than their proxy parents (Silent Generation and Baby Boomers birth cohorts from 1936 to 1960) ( Figure 5 E) in all demographic groups except for Asian or Pacific Islander men. These increases were statistically significant in all 4 racial and ethnic groups among women (Asian or Pacific Islander, 20.1% [95% CI, 15.7%-24.4%]; Hispanic, 34.9% [95% CI, 29.8%-40.0%]; non-Hispanic Black, 6.1% [95% CI, 1.7%-10.5%]; non-Hispanic White, 15.1% [95% CI, 13.1%-17.2%]) and in Hispanic (14.1%; 95% CI, 3.6%-24.5%) and non-Hispanic White (11.9%; 95% CI, 0.7%-23.0%) men. Similarly, the proxy parents of the Millennials (Baby Boomer and Generation X birth cohorts from 1952 to 1976) also had higher cancer incidence than the proxy parents of Generation X in all demographic groups except Asian or Pacific Islander males ( Figure 5 F). The increases were statistically significant in Asian or Pacific Islander (11.1%; 95% CI, 8.9%-13.2%), Hispanic (17.8%; 95% CI, 15.4%-20.2%), and non-Hispanic White (4.5%; 95% CI, 3.6%-5.5%) women and in Hispanic (9.0%; 95% CI, 4.1%-14.0%) and non-Hispanic White (4.7%; 95% CI, 0.4%-9.0%) men.
In this cohort study, we asked whether we, collectively, are experiencing lower cancer incidence as we age than our parents. Using SAGE analysis, we were able to reconstruct the cancer experience of social generations from the Greatest Generation through Generation X and use selected birth years from the 2 preceding social generations as a proxy for the corresponding parental generation. To arrive at an overall conclusion, we used a simple summary measure: the incidence of leading cancers combined (20 sites in women and 18 sites in men).
For Baby Boomers (1946-1964) vs their proxy parents (1917-1944), the answer was yes for men (ie, progress) and mixed for women ( Figure 5 D). For Generation X (1965-1980) vs their proxy parents (1936-1960), the answer was no in all groups except for Asian or Pacific Islander males ( Figure 5 E).
The increases in cancer incidence in members of Generation X vs their proxy parents were substantial, especially among Hispanic women (a 34.9% increase) and men (a 14.1% increase). In contrast, the corresponding increases among non-Hispanic White women and men were 15.1% and 11.9%, respectively. We obtained similar results when we compared the Generation X to Baby Boomer social generations ( Figure 5 C). Furthermore, between the Greatest Generation and Generation X, the historical male cancer excess narrowed (non-Hispanic Black and non-Hispanic White men), declined to parity (Hispanic men), or reversed (Asian or Pacific Islander men) ( Figure 3 ).
Our conclusions are more concerning than previously reported increases in cancer incidence in younger age groups. 2 , 12 Those results, based on local drifts, describe the slope of the FCP curve in consecutive blocks of P birth years, where P is the total number of calendar years in the analysis (eg, P = 19 in Sung et al 2 ; P = 27 in our analyses). 9 For this reason, local drifts provide lagging and conservative indicators of changes from one birth year to the next. Furthermore, using SAGE analysis, we were able to estimate absolute FCP values for single-year birth cohorts and consecutive changes in incidence per birth year vs the 10-year overlapping cohort relative risks reported by Sung et al. 2
The substantial increases we identified in Generation X vs both the Baby Boomers and their proxy parents surprised us. Numerous preventable causes of cancer have been identified. 13 Cancer control initiatives have led to substantial declines in tobacco consumption. 14 Screening is well accepted for precancerous lesions of the colon, rectum, cervix, uterus, and breast. 15 However, other suspected carcinogenic exposures are increasing. 16 - 18
Unfortunately, as shown in our detailed comparative analysis of Generation X vs Baby Boomers, gaining cancers have numerically overtaken falling cancers. Among Generation X women, statistically significant declines in lung and cervical cancers have been overtaken by significant increases in thyroid, kidney, rectal, corpus, colon, pancreatic, and ovarian cancer and NHL and leukemia ( Figure 4 C). Among Generation X men, declines in lung, liver, and gallbladder cancers and NHL have been overtaken by gains in thyroid, kidney, rectal, colon, and prostate cancers and leukemia ( Figure 4 F).
Some portion of these increases can be attributed to rising obesity rates 19 and increasingly sedentary lifestyles. 20 - 23 Another portion might be explained by changes in cancer registry policies and International Statistical Classification of Diseases and Related Health Problems, 10th Revision (World Health Organization) classifications, 24 leading to inclusion of relatively indolent lesions in more recent periods that might not have been diagnosed as cancer in earlier periods. Furthermore, radiologic diagnoses have become more common following widespread deployment of sophisticated medical imaging technologies, 25 especially for thyroid 26 , 27 and kidney 28 , 29 cancers. We chose not to exclude any leading cancer site from our summaries because our granular estimates are freely available (eFigures 15-17 in Supplement 1 ).
Our results beg the question of what the cancer experience may be like among the 72 million Millennials (1981-1996) when they enter their 40s, 50s, and 60s. On one hand, our analysis shows that the proxy parents of the Millennials are experiencing as much or more cancer than the proxy parents of Generation X ( Figure 5 F). This increase is concerning because of shared cancer-predisposing lifestyle factors and exposures. On the other hand, thanks to the global investment in cancer research, there are tremendous opportunities to prospectively reduce the Millennials’ future cancer burden.
The American Cancer Society, 30 Centers for Disease Control and Prevention, 31 and World Health Organization 13 advocate a series of preventive actions to diminish cancer risks. These include reducing tobacco and alcohol use, increasing physical activity, improving dietary habits, and promoting breastfeeding. These recommendations can also reduce heart disease 32 and cognitive decline. 33
Unfortunately, universal implementation of these recommendations in the US is a work in progress. The Black-to-White cancer mortality gap narrowed following passage of the Patient Protection and Affordable Care Act. 34 , 35 However, income inequality, 36 underinsurance, 37 - 39 food swamps and deserts, 40 , 41 deficits in the built environment, 42 and other factors make it difficult for everyone to eat healthy and stay active. 30 Taken together, these findings indicate that for many people in the US, a healthy lifestyle remains, to various degrees, an unattainable privilege rather than a fundamental right. The extent to which lifestyle disparities explain rising generational cancer rates in our data and falling life expectancies in other studies 43 is unclear and, in our view, merits further study.
Our study has 2 major limitations. First, the numbers of less common cancers in the SEER 13-registry database among Asian or Pacific Islander, Hispanic, and non-Hispanic Black men and women are limited, especially for esophageal and gallbladder cancers and melanoma. Second, our conclusions derive from modeling. Even so, we believe that our detailed analysis of 3.8 million individuals with invasive cancers in 152 distinct strata mitigated many potential biases. Furthermore, at most cancer sites, the birth cohort effects were substantial, and the LOF was relatively small. For this reason, we believe that it is appropriate to draw conclusions from our FCP estimates. However, it is important to appreciate that the FCP incorporates backward projection for older cohorts and forward projection for younger cohorts; in other words, it is very much a model-based quantity.
The models in this cohort study suggest that Generation X is experiencing larger per-capita increases in the incidence of leading cancers combined than any prior generation born from 1908 through 1964. In addition, the rate of leading cancers appears to be as high or higher in the proxy parents of the Millennials than the proxy parents of Generation X. Therefore, if the Millennials’ cancer experience follows the estimated trajectory of their proxy parents, cancer incidence in the US could remain unacceptably high for decades to come.
Accepted for Publication: April 8, 2024.
Published: June 10, 2024. doi:10.1001/jamanetworkopen.2024.15731
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Rosenberg PS et al. JAMA Network Open .
Corresponding Author: Philip S. Rosenberg, PhD, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NCI Shady Grove, Room 7E-130, 9609 Medical Center Dr, Bethesda, MD 20892 ( [email protected] ).
Author Contributions: Drs Rosenberg and Miranda-Filho had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: All authors.
Administrative, technical, or material support: Miranda-Filho.
Supervision: Rosenberg.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (Drs Rosenberg and Miranda-Filho) and through an appointment to the National Cancer Institute Oak Ridge Institute for Science and Education Research Participation Program under contract DE-SC0014554 from the Department of Energy (Dr Miranda-Filho).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript. The Division of Cancer Epidemiology and Genetics, National Cancer Institute, reviewed and approved the manuscript and approved the decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 21 February 2024
A precise model for skin cancer diagnosis using hybrid U-Net and improved MobileNet-V3 with hyperparameters optimization
- Umesh Kumar Lilhore 1 ,
- Sarita Simaiya 1 ,
- Yogesh Kumar Sharma 2 ,
- Kuldeep Singh Kaswan 3 ,
- K. B. V. Brahma Rao 2 ,
- V. V. R. Maheswara Rao 4 ,
- Anupam Baliyan 1 ,
- Anchit Bijalwan 5 &
- Roobaea Alroobaea 6
Scientific Reports volume 14 , Article number: 4299 ( 2024 ) Cite this article
1918 Accesses
2 Citations
1 Altmetric
Metrics details
- Cancer imaging
- Skin cancer
Skin cancer is a frequently occurring and possibly deadly disease that necessitates prompt and precise diagnosis in order to ensure efficacious treatment. This paper introduces an innovative approach for accurately identifying skin cancer by utilizing Convolution Neural Network architecture and optimizing hyperparameters. The proposed approach aims to increase the precision and efficacy of skin cancer recognition and consequently enhance patients' experiences. This investigation aims to tackle various significant challenges in skin cancer recognition, encompassing feature extraction, model architecture design, and optimizing hyperparameters. The proposed model utilizes advanced deep-learning methodologies to extract complex features and patterns from skin cancer images. We enhance the learning procedure of deep learning by integrating Standard U-Net and Improved MobileNet-V3 with optimization techniques, allowing the model to differentiate malignant and benign skin cancers. Also substituted the crossed-entropy loss function of the Mobilenet-v3 mathematical framework with a bias loss function to enhance the accuracy. The model's squeeze and excitation component was replaced with the practical channel attention component to achieve parameter reduction. Integrating cross-layer connections among Mobile modules has been proposed to leverage synthetic features effectively. The dilated convolutions were incorporated into the model to enhance the receptive field. The optimization of hyperparameters is of utmost importance in improving the efficiency of deep learning models. To fine-tune the model's hyperparameter, we employ sophisticated optimization methods such as the Bayesian optimization method using pre-trained CNN architecture MobileNet-V3. The proposed model is compared with existing models, i.e., MobileNet, VGG-16, MobileNet-V2, Resnet-152v2 and VGG-19 on the “HAM-10000 Melanoma Skin Cancer dataset". The empirical findings illustrate that the proposed optimized hybrid MobileNet-V3 model outperforms existing skin cancer detection and segmentation techniques based on high precision of 97.84%, sensitivity of 96.35%, accuracy of 98.86% and specificity of 97.32%. The enhanced performance of this research resulted in timelier and more precise diagnoses, potentially contributing to life-saving outcomes and mitigating healthcare expenditures.
Similar content being viewed by others
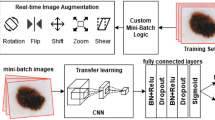
AI outperformed every dermatologist in dermoscopic melanoma diagnosis, using an optimized deep-CNN architecture with custom mini-batch logic and loss function
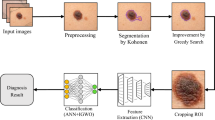
Skin cancer diagnosis (SCD) using Artificial Neural Network (ANN) and Improved Gray Wolf Optimization (IGWO)

DUNEScan: a web server for uncertainty estimation in skin cancer detection with deep neural networks
Introduction.
Skin cancer is considered one of the most widespread and significant forms of cancer globally, with its occurrence consistently rising in recent years. The prompt and precise identification of a medical condition is crucial for successfully implementing treatment strategies and enhancing patient results. Historically, dermatologists have traditionally depended on their professional knowledge and visual examination of skin lesions in order to detect possible malignancies. Nevertheless, this procedure is inherently subjective and prone to diagnostic inaccuracies 1 . In response to all these barriers, Computer-Aided Diagnosis (CAD) frameworks significantly improved medical dermatology. Deep learning models that fall into the category of "convolutional neural networks," also known as "CNNs," have shown remarkable promise for improving the accuracy of skin cancer recognition 2 . Figure 1 presents an image of a skin cancer sample.
Skin cancer sample 23 .
Melanoma and non-melanoma are the two primary subtypes of skin cancer, distinguished based on the cell that is transformed into a cancerous form. Various machine learning and deep learning frameworks and methodologies have been suggested to detect, classify, and segment skin cancer. Examples of these include Support Vector Machines (SVM), Decision Trees (DT), Fuzzy C-means, Recurrent Neural Networks (RNN), Convolutional Neural Networks (CNN) and Deep Neural Networks (DNN) 3 . CNN is a widely employed algorithm for automated feature learning and extraction, known for its exceptional performance in detection tasks 4 . Moreover, the training stage for deep learning networks often necessitates a substantial volume of data. Therefore, using transfer learning to refine a pre-existing network on comparable tasks can reduce training complexity, expedite convergence, and decrease training duration 5 .
Additionally, it is possible to utilize pre-trained deep learning models as feature extractors without additional training, provided they have already been trained on tasks or domains that are similar or related 6 . The deep learning model may acquire features that contain noise, thereby impacting the accuracy of the final classification. This can be attributed to the inclusion of non-relevant features and the naturally high dimensionality of the characteristics learned. Therefore, utilizing optimization techniques, specifically metaheuristic methods, can provide a viable approach for selecting elements. This approach involves identifying and choosing the most pertinent features, thereby enhancing the precision of recognition 7 .
This research paper introduces a comprehensive methodology to handle the complex issues related to skin cancer detection. This research presents a novel skin cancer recognition model based on optimized CNN architecture. This research utilizes deep learning and advanced optimization techniques to improve skin cancer detection's precision and effectiveness. The motivation behind this research originates from the critical requirement to deal with the primary healthcare problem related to dermatological cancer. Millions of people worldwide have skin cancer, and the disease's incidence is still rising. The prompt and precise identification of a condition or disease is crucial to administer the correct therapy and, finally, to preserve human life. Conventional diagnostic techniques, dependent on individual observation, are subjective and may change in precision based on the training and experience of the medical professional 8 .
The presence of subjectivity and the possibility of error underscore the utmost significance of advancing the development of more accurate and automated mechanisms for identifying skin cancer. The primary motivation behind our research stems from the pressing demand for enhanced techniques in detecting skin cancer. We harness their capabilities by harnessing deep learning and optimization methodologies to address this objective. Furthermore, the emergence of deep learning methods, i.e., CNNs, has shown the capacity for transformation in analyzing healthcare images. The capability of CNNs to identify complicated characteristics from visuals and to make decisions based on information presents an exciting chance for improvement in the discipline of skin diseases 9 . The convergence of urgent medical necessity and state-of-the-art technology constitutes the primary impetus for our investigation, compelling us to construct a sophisticated model for identifying skin cancer that harnesses the capabilities of deep learning and optimization methodologies.
The primary contributions of our research encompass innovative model architecture, meticulous hyperparameter optimization, persuasive experimental findings, and significant clinical implications. These collective achievements propel the current state of advancement in dermatological examinations and contribute to the enhancement of the treatment of patients. The proposed research presents several significant contributions to the domain of skin cancer detection and healthcare:
We enhance the learning procedure of deep learning by integrating Standard U-Net and Improved MobileNet-V3 with optimization techniques, which allows the model to accomplish better differentiation among malignant and benign skin cancers.
Our research focuses on the meticulous examination of hyperparameter optimization, a crucial factor that substantially influences the efficacy of deep learning models. Advanced optimization techniques, i.e., Bayesian optimization and grid search, are utilized to optimize the hyperparameter of the model effectively.
The optimization of hyperparameters is of utmost importance in enhancing the efficiency of deep learning models. To fine-tune the model's hyperparameter, we employ sophisticated optimization methods such as the Bayesian optimization method using pre-trained CNN architectures, i.e., MobileNet-V3, on the “HAM-10000 Melanoma Skin Cancer” dataset.
The proposed hybrid MobileNet-V3 model outperforms existing techniques based on high precision of 97.84%, sensitivity of 96.35%, accuracy of 98.86%, and specificity of 97.32%.
The article is organized in the following way: after an introductory part that provides an overview of the motivation and aims, a comprehensive examination of existing literature on skin cancer detection is presented in section two. The methodology section three offers a thorough account of the procedures employed for data collection, preliminary processing, and the development of a hybrid architecture specifically designed to detect skin cancer. Additionally, it includes a detailed explanation of the process of hyperparameter optimization. Section four provides a detailed description of the experimental setup, presents the results and performance indicators obtained, and compares them to existing methods. An extensive examination of the consequences, advantages, and drawbacks of the proposed method follows. The conclusion section five serves the purpose of combining the main findings of the research and highlighting their potential clinical significance.
Literature review
Previous research was investigated concerning the categorization of Skin Cancer using deep learning techniques. In the context of the categorization of Skin Cancer utilizing deep learning techniques, scholars generated skin lesion images sourced from publicly available websites.
Data augmentation methods were employed alongside five-fold cross-validation methods to enhance the dataset. The investigators conducted experiments to evaluate the performance of pre-trained VGG-16, ResNet50, and InceptionNet-V3 models in classifying Skin Cancer diseases. The ResNet50 model was selected due to its superior accuracy rate compared to the other models. The ResNet50 model achieves an F1 Score of 0.82, as reported in reference 10 . The researchers used the Xception training model with the Grad-Cam and the LIME algorithm techniques to predict skin cancer 11 . The Xception and the DenseNet models have implemented an integrated strategy utilizing unity. The research involved the analysis of the efficiency scores retrieved from experiments conducted on a publicly accessible dataset. These experiments were carried out using this suggested ensemble strategy. The results indicated an average recall, precision, F1 score, and accuracy rate of 86.74%, 85.8%, 86.24%, and 88.93%, respectively.
The article 12 presents research on the utilization of YOLOv4-DarkNet along with active contour techniques to localize and segment melanoma. The method was evaluated on the International Skin Imaging Collaboration (ISIC) datasets for 2016 and 2018. The values of one and the reported Jaccard coefficient were 1 and 0.989, respectively. The paper 13 discusses the topology of FC-DPN segmentation. The construction of the network involved the implementation of a dual-path and fully convolutional architecture. In the revised ISIC 2017 test dataset, the suggested approach achieved a Jaccard index of 81.32% and an average dice coefficient of 87.2%.
Similarly, for the PH2 dataset, a Jaccard index of 84.3% and an average dice coefficient of 91.76% were obtained. The paper discusses a CNN model for the classification of skin cancer 14 . The dataset was initially gathered and subsequently categorized into four distinct groups of skin cancer images. Later, augmentation techniques were employed to expand the dataset's size. During the testing phase, the algorithm developed by the researchers achieved an accuracy of 96.28%, surpassing the accuracy of the GoogleNet and MobileNet models by 1.76% and 1.12%, respectively.
The application of deep learning techniques for skin cancer detection has been the subject of considerable investigation in recent academic literature. Several deep learning techniques, including CNNs, have been utilized to categorize skin lesions as either cancerous or non-cancerous precisely. The authors of 15 present a unique deep learning network called the Dual Optimisation Utilising Deep Learning Network. This network was designed for skin cancer identification using dermoscopic images and achieved an impressive precision of 98.76%. This paper presents a thorough examination of conventional and deep-learning methodologies employed in the prompt identification of skin cancer. It critically evaluates the performance of these techniques and examines the datasets utilized for training and testing purposes 16 . The authors have developed a deep learning system using a CNN to detect melanoma lesions. This system has demonstrated superior performance regarding diagnostic accuracy compared to existing methods, as reported in reference 17 . The research proposes using convolutional-based neural networks with deep reinforcement learning to identify cancer-affected skin. It aims to overcome the challenges related to generalizability by implying the adoption of ensemble models to achieve optimal output, as indicated by reference 18 .
Recent studies have utilized various types of classification and preliminary processing methods to conduct morphological change examines on grey-level skin cancer images. These images were obtained from the PH2 repository and were subjected to classification and clustering procedures using a pre-trained Levenberg-Mean neural network 6 . Transfer learning has been utilized to forecast skin cancer visuals from the HAM10000 database precisely using the MobileNet CNN 19 . The recent research on the application of CNN in predicting skin cancer has identified significant challenges in incorporating the entire spectrum of the patient population and various melanoma subtypes 20 . In addition, ongoing efforts are being made to develop optimization algorithms that enhance accuracy by adjusting hyperparameters 21 . Moreover, recent studies have put forth methods to identify instances of shortcut development during the training of models based on convolutional neural networks on both the ISIC Storage collection. Recent research has also emphasized using comprehensible deep-learning techniques for the multi-class separation and categorization of skin lesion images 22 .
Predicting skin diseases using AI holds enormous promise for better early detection and treatment results 23 . This study offered a new way to improve skin disease prediction models by addressing class imbalance utilizing data balancing using class weighting and transfer learning approaches. They have also investigated the usefulness of data balancing using class weighting to boost TL performance for skin disease prediction. Experiments and performance evaluations have yielded important insights and outcomes, proving that the suggested method works. Optimized kernel-enhanced Resnet-dropped extended short-term memory technique for varicose vein disease detection was addressed in 24 . After processing 11,350 photos of leg veins, the experiment was evaluated using metrics such as accuracy, precision, kappa, mean square error (MSE), and time complexity. With a time complexity of 25.36 ms, a kappa of 95.77%, a precision of 98.69%, and an MSE of 1.73, the suggested model attained an accuracy of 98.5%.
Although skin cancer is dangerous, it is successfully treatable if caught early. A procedure is involved in the suggested approach 25 . Improving the quality and extracting useful information from the raw photos is the first step in the pre-processing phase. The next step is to feed the pre-processed pictures into a GRU Network, a deep-learning model that excels at capturing sequential information. The GRU Network was fine-tuned by the author using an improved version of the Orca Predation Algorithm (OPA). In comparison to previous approaches, the GRU/IOPA system achieved better results in terms of accuracy (0.96), sensitivity (0.95), specificity (0.97), PPV (0.95), and NPV (0.96). When compared to more conventional methods, these findings show that the suggested technique is superior in detecting skin cancer.
In order to better identify skin lesions or cancer, researchers have built an upgraded machine-learning framework 26 . This study segments and categorizes skin diseases and cancers using a machine-learning framework and an optimization method derived from fruit flies. The proposed method provides enhanced classification efficiency with a 98% accuracy rate, 99% specificity, 96% sensitivity, 95% JSI, and 99% DSC.
In 27 , we looked at a framework for multi-class skin disease classification that uses an LSTM, a sophisticated CNN, and MobileNet-V2 to improve the reliability and accuracy of cancer diagnoses. The proposed method leverages CNNs' self-learning discriminative features from unprocessed skin photos and long short-term memory (LSTM) capability to manage multi-class classification problems. The hybrid model showed promise in the experiments for improving the accuracy and efficiency of skin disease classification across a variety of categories.
The authors of 28 laid up a two-pronged approach to skin lesion categorization. First, during the image pre-processing phase, two distinct techniques were suggested for picture segmentation and feature extraction. Finally, S-MobileNet, a model for a deep convolutional neural network, is being developed with the intention of classifying seven distinct skin lesion types. When it comes to skin lesion picture classification in the HAM10000 dataset, the suggested deep learning-based S-MobileNet model is the way to go, according to experimental results.
In 29 , the authors provide a deep-learning architecture that can identify Melanoma and multi-class skin cancers. Four main phases make up the suggested architecture: pre-processing images, extracting and fusing features, selecting features, and finally, classification. Using the picture luminance data, a new method for enhancing contrast was suggested. The next step is to train two pre-trained deep models, DensNet-201 and DarkNet-53, using transfer learning to modify them with respect to a residual block at the end. The last step in the classification process was to use machine learning classifiers on the features that were chosen. For this experiment, we used two datasets: ISIC2018 and ISIC2019 29 , 30 . This dataset achieved the highest precision of 85.4%, whereas the other achieved 98.80%. Table 1 presents a comparative analysis of existing skin cancer research using a deep learning model.
Materials and methods
Skin cancer dataset.
This research utilizes the online ‘HAM-10000’ skin cancer dataset 33 . The dataset HAM-10000, referred to as 'Human against the machine', with 10,000 images used for training, is a collection of images of skin lesions utilized in skincare research. The dataset comprises visual representations of diverse dermatological disorders, encompassing Melanoma, nevus, and several related afflictions. The provided dataset is used to classify and diagnose skin diseases. As mentioned earlier, deep learning models trained on the dataset can discern different types of skin cancer, including malignant tumours that may exhibit malignant characteristics.
This dataset contains 10,015 dermatoscopic images with size (450*600) pixels. The dataset comprises seven diagnostic Skin cancer categories. The dataset classes include classes from zero to six, names as ‘melanoma’, ‘melanocytic nevus’, ‘basal cell carcinoma’, ‘actinic keratosis’, ‘benign keratosis’, ‘dermatofibroma’ and ‘vascular lesion’. Figure 2 presents the details of skin cancer classes and counts, and Fig. 3 illustrates the gender-wise distribution of skin cancer infection, including a 54% male, 45.5% Female and 0.5% unknown patient distribution in the dataset.
Skin cancer classes and counts.
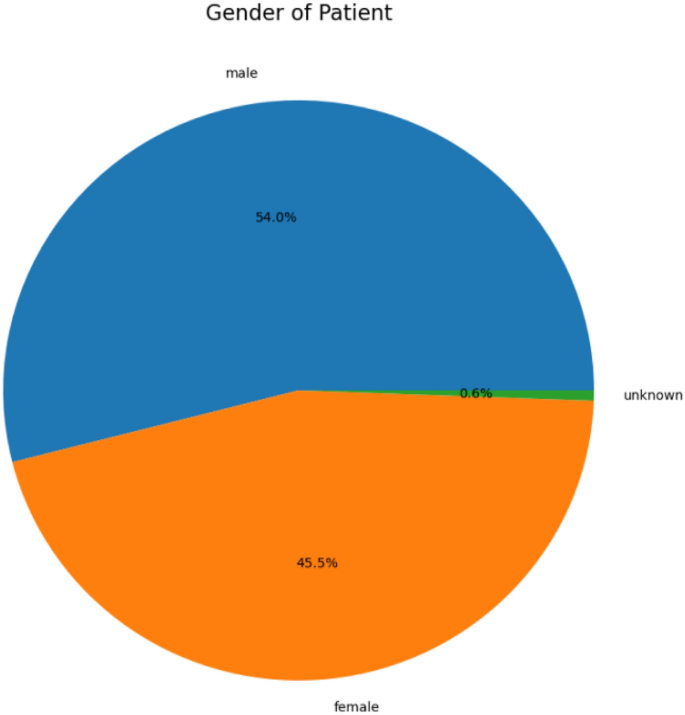
Gender-based skin cancer distribution.
Data pre-preprocessing
A critical first step in getting a skin cancer dataset ready for training a skin cancer identification framework is data preliminary processing. In addition to promising that the data is in a format appropriate for training, proper data pre-processing can enhance model performance 31 . The skin cancer dataset is pre-processed using the following crucial steps.
Data augmentation
Since there are unequal numbers of images in each category, data balancing is used to balance all types before the training procedure. Data augmentation techniques are used for data balancing. In this process, the following ranges are used: (a) rotation 25 degrees; (b) width and height shifting 15%; (c) shearing 15%; (d) employing flipping in both the vertical and horizontal directions; and (5) adjusting brightness within the [0.9: 1.5] range. Data augmentation is also applied to the images during the learning and optimization stages to prevent over-fitting and boost diversity. Table 2 presents the parameters used for the skin cancer dataset. After pre-processing, the images of (450*600) pixels were converted into 192*256 pixels. Figure 4 presents data augmentation results. Table 3 shows the data count after the data pre-processing steps for class 0 to class 6.
Data augmentation ( a ) image resizing, ( b ) zooming.
We use Eq. ( 1 ) for horizontal flipping operation, i.e., x-axis, 2 for rotation and 3 for shifting, 4 for shearing, and 5 for zooming. In these equations, h represents the rotating angle, t x represents the amount of shifting in tandem with the x-axis, t y represents the shifting in tandem with the y-axis, sh x represents the shear factor in tandem with the x-axis, sh y represents the shear factor together the y-axis, and C x represents the zoom factor together the x-axis, and C y represents the zoom factor in tandem the y-axis 32 . Here, FM is the flipping Matrix, RM is the rotation Matrix, SM is the shifting Matrix, SheM is the shearing Matrix, and ZM is the zooming Matrix.
Proposed model architecture
The proposed model is based on Standard U-Net and Improved MobileNet-V3 with optimization techniques, i.e., the Bayesian optimization method. Standard U-Net performs semantic segmentation activities in the proposed model, and MobileNet-V3 is used as a Feature Extractor. We also utilize a Bayesian optimization method for hyperparameter optimization. This method can identify the most promising hyperparameters by prioritizing those that have shown favourable outcomes in previous results. Figure 5 shows the architecture of the proposed hybrid model.
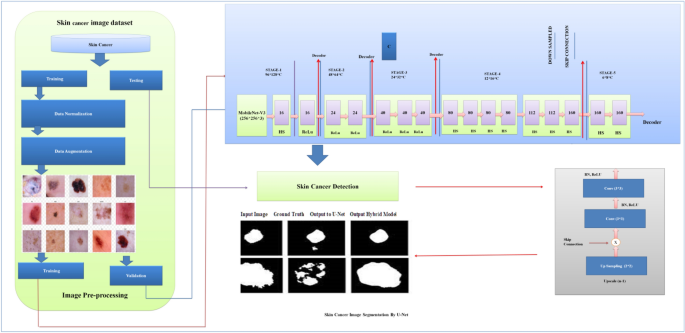
Architecture of proposed hybrid model.
Improved MobileNet-V3
In the proposed hybrid model, the existing MobileNet-V3 model is improved. The Improved MobileNet-V3 worked as an encoder in the proposed skin cancer prediction and segmentation model. The steps below enhanced the MobileNet-V3 structure for skin cancer recognition and segmentation. Figure 6 presents the architecture of the proposed MobileNet-V3 31 .
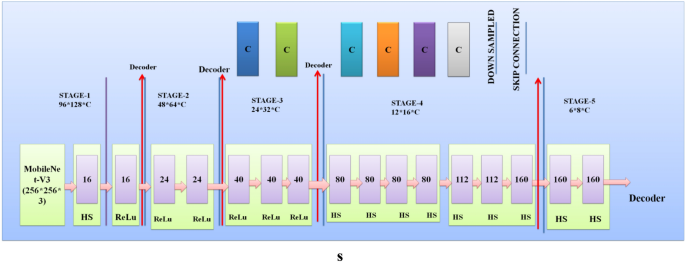
Architecture of proposed MobileNet-V3.
Architectural level Improvements: The following fundamental changes were performed at the existing MobileNet-V3 architecture 32 .
Deeper Model A deeper MobileNet-V3 model's depth (layer counts) is determined by the model's structural preferences. MobileNet-V3 is available in two predetermined sizes: "large" or "small," with "large" featuring more layers and greater capacity than "small." The "large" variant of MobileNet-V3 generally includes 157 layers. Depthwise separable convolution layers and flipped residuals using growing and squeeze-and-excitation (SE) segments are formed.
Wider Model MobileNet-V3 predominantly employs the flipped residual block and SE block. A depthwise convolution component is subsequently followed by a point-wise convolution layer to form the inverted residual block. The point-wise convolution changes the width (number of channels). Adjust the point-wise convolution layer output channels to widen a layer. This is typically accomplished by multiplying the total amount of channels through a scaling factor. Modulating channel-wise dependencies is done with the SE block. Although it cannot naturally regulate layer width, it can be utilized on layers to enhance model performance and adjust to particular tasks. Fully connected layers (FCL), Global average pooling (GAP), and element-wise scaling (EMS) comprise the SE block. The number of channels present in the map of input features is used as a criterion to establish the size of the SE block. MobileNet-V3 allows you to modify the range of channels within the starting convolutional layer with the last classification layer to adjust the total width of the resulting model for skin cancer analysis.
Attention Method We substituted the crossed-entropy loss function of the Mobilenet-v3 mathematical framework with a bias loss function that enhanced accuracy. The model's SE component was replaced with the adequate channel attention (ECA) component to achieve parameter reduction. Integrating cross-layer connections among Mobile modules has been proposed to leverage synthetic features effectively. The dilated convolutions were incorporated into the model to enhance the receptive field.
Regularization method
The following Regulization techniques were applied to improve the MobileNet-V3 model 32 .
Dropout Implement dropout layers inside the MobileNet-V3 structure to prevent overfitting throughout training. Dropout arbitrarily eliminates a portion of the neurons throughout each forward pass to improve the model's generalization ability.
L2 regularization's weight decay Applying a weight decay component to the model's loss function for the L2 regularization's weight decay step. Weight decay promotes the reduction of model weights, preventing unnecessary complexity and overfitting.
Normalization method
The following Normalization techniques were applied for improvements in the MobileNet-V3 model.
Batch Normalization To stabilize training, employ batch normalization layers to normalize the activations throughout each mini-batch. Batch normalization can boost the model convergence process and boost training.
Layer Normalization We have applied a layer normalization method that helps
Standard U-Net architecture
The Standard U-Net architecture is a convolutional neural network specifically developed for semantic segmentation tasks. The algorithm demonstrates exceptional proficiency in partitioning regions of interest within medical images, rendering it highly suitable for the analysis of skin cancer. The Standard U-Net architecture is distinguished by incorporating skip connections and embedded skip procedures, which efficiently capture multi-scale context-relevant data 33 .
Four Standard U-Net patterns, identical to U-Net, are used to complete the segmentation assignment. Batch normalization and GeLU for the hidden activation mechanism are utilized in the additional configuration, while the architecture described in 10 is used in the initial setup. Batch normalization (BN) mainly creates batches of a specific size. Along with batch normalization and the GeLU hidden activation function, the third and fourth patterns use VGG-19 and DenseNet-201 as their backbone. In the case of configuration four, deep supervision is not enabled. Figure 7 presents the standard U-Net architecture.
Standard U-Net architecture.
Bayesian optimization method
Bayesian optimization is an approach that employs probabilistic algorithms to search accurately for the most practical combination of hyperparameters to support a model built using deep learning. It functions by analyzing an ambiguous objective function within this instance, which evaluates the model's effectiveness as a Gaussian procedure, enabling it to make well-informed choices about where to start sampling the target function subsequently. The following describes the operation of Bayesian optimization in the hyperparameter optimization for skin cancer 6 .
Step 1: Initial Random Sampling To create an initial substitute framework for the objective function, Bayesian optimization usually begins with a few random specimens taken through the hyperparameter space. The function's behaviour can be partially understood because of these arbitrary specimens.
Step 2: Surrogate Model The algorithm generates a probabilistic substitute model using the initial specimens, generally a Gaussian procedure (GP). The GP prototypes the objective function's allocation and predicts the function's behaviour throughout the hyperparameter area. This substitute model encompasses average forecasting and an uncertainty determination (variance) at every point within the hyperparameter space.
Step 3: Acquisition Function Bayesian optimization utilizes an acquisition function for identifying the subsequent objective function specimen location. Typical acquisition functions consist of Probability of Improvement (PI), Expected Improvement (EI), and Upper Confidence Bound (UCB). These features balance examinations (sample collection in spaces of uncertainty) and exploitation (sampling in spots that are likely to produce more effective results).
Step 4: Next Sample Selection The acquisition function directs the selection of the subsequent collection of hyperparameters to be assessed. The method chooses hyperparameter values that maximize the acquisition function, indicating promising regions within the hyperparameter space for enhancing the objective function.
Step 5: Evaluate Objective Function The chosen hyperparameters are utilized for training and evaluating a deep learning framework via the skin cancer dataset. The objective function (including model precision and loss) gets calculated according to the model's efficiency.
Step 6: Update Surrogate Model: After assessing the objective function based on the chosen hyperparameter, the procedure upgrades the substitute model (GP) along with the newly acquired data point, including the evaluation results, to minimize uncertainty within the substitute model.
Step 7: Iteration Steps 3 through 6 are carried out for an established amount of iterations or until convergence is achieved. The method improves the substitute model iteratively and chooses a hyperparameter to enhance the model's accuracy.
Step 8: Final Result The Bayesian optimization method produces the hyperparameters, which generate the best objective function importance according to the model's behaviour.
- Transfer learning
ImageNet is used as a pre-training dataset for the MobileNet-V3 model, and then the Ham-10000 skin cancer dataset is used to fine-tune the model's parameters. Transfer learning enables the predictive algorithm to utilize features acquired within a broader context before becoming proficient in the skin cancer recognition assignment 34 .
Pseudo code for the proposed model
Algorithm 1 presents the steps for skin cancer analysis with the hybrid U-Net and MobileNet-V3 models 35 .

Skin Cancer analysis with Hybrid U-Net and MobileNet-V3.
Performance measuring parameters
We use parameters like accuracy, precision, F-measure, the area under the curve (AUC), and confusion metrics 6 , 31 , 32 , 33 to determine how to determine the present model and the one we have suggested works. The components of the confusion matrix, including TP (true positive), TN (true negative), FP (false positive), and FN (false negative), have been utilized in its construction. The subsequent passage comprehensively explains the previously referenced statistics 34 .
Precision (Prc) This illustrates the proportion of individuals who test positive correctly (true positives) and incorrectly (false positives) concerning the total number of positive individuals as described in Eq. ( 6 ).
Recall (RC) or Sensitivity The calculation of recall, also known as true positive rate 35 , involves dividing the number of true positives by the total number of instances that ought to be considered anticipated as positive, as described in Eq. ( 7 ).
F-Measure (FMs) The F-score, often referred to as the 'F1 score' or 'F-measure' 36 , serves as an empirical metric for assessing the effectiveness of a deep learning model. The integration of recall and precision results in an integrated score, as described in Eq. ( 8 ).
Accuracy (ACR) The evaluation of classification models often involves the consideration of various metrics, with accuracy being one of them 37 . In a more formal context, accuracy can be defined as the proportion of correct predictions our model makes, as described in Eq. ( 9 ).
Area Under the Curve (AUC) AUC refers to measuring the extent or size of two areas under a curve, which refers to the region bounded by the curve and the coordinate axes 33 , 38 . To determine this area, one can divide it into infinitesimally small rectangles and sum their areas. By incorporating the limit of this summation, as the rectangles become infinitely small, the total area under the curve can be calculated.
Ethical approval and consent to participate
No ethical approval is required, and the authors consent to participate in the paper.
Experimental results and evaluation
This section covers the simulation results of the existing and proposed model.
Experimental setup
The proposed and existing techniques, i.e., MobileNet, Resnet-152v2, VGG-16, MobileNet-V2, and VGG-19, were implemented using software and hardware components. Table 4 presents the parameters used in experimental analysis for proposed and existing models. Table 5 shows the Configuration parameters of Standard U-Net architecture used in a proposed hybrid model for image segmentation 39 , 40 , 41 , 42 , 43 .
Simulation results and discussion
The HAM-10000 Skin cancer dataset serves as the training ground for the proposed model and several existing deep learning models, i.e., MobileNet, Resnet-152v2, VGG-16, MobileNet-V2, and VGG-19. The dataset was divided into training, testing and validation with a ratio of 70:15:15. The experimental results and discussion are as follows.
Table 6 presents the experimental results of the testing dataset for 100 epochs for existing and proposed models. The combined utilization of the U-Net and MobileNet-V3 models yielded exceptional results in classifying skin cancer diseases.
Table 7 presents the experimental results of the validation dataset for 100 epochs for existing and proposed models. The proposed model demonstrated validation results with an accuracy of 98.03%, wherein the precision, recall, and F1-score all surpassed 94%.
Visualization of simulation results
Figures 8 , 9 , 10 present a visualization of simulation results for the proposed model on the “HAM-10000” skin cancer dataset. Figure 8 presents the Confusion Matrix of the Proposed Model, Fig. 9 illustrates the Model Accuracy and Loss of the Proposed Model, and Fig. 10 shows an ROC Curve of the Proposed Model. Figures 11 , 12 13 present a visualization of simulation results for the MobileNet Model on the “HAM-10000” skin cancer dataset. Figure 11 presents the Confusion Matrix of the MobileNet Model, Fig. 12 illustrates the Model Accuracy and Loss of the MobileNet Model, and Fig. 13 shows an ROC Curve of the MobileNet Model.
Confusion matrix of proposed model.
Model accuracy and loss of proposed model.
ROC curve of proposed model.
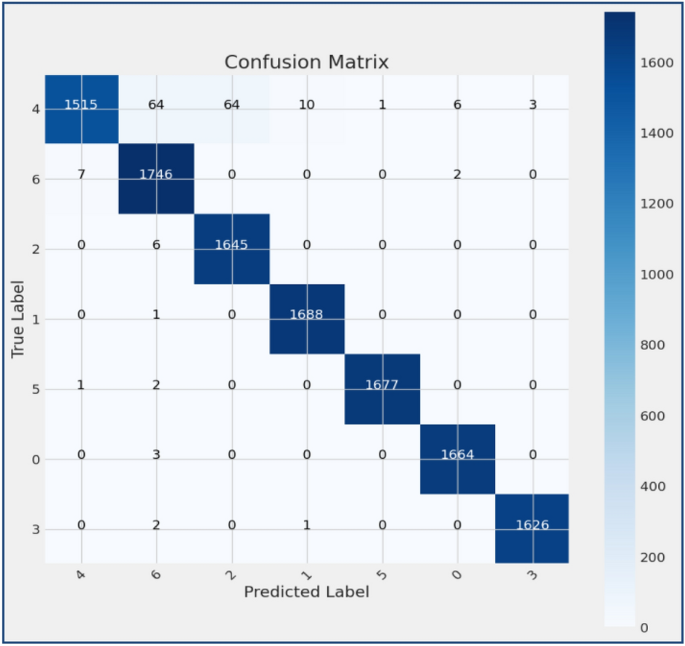
Confusion matrix of existing MobileNet model.
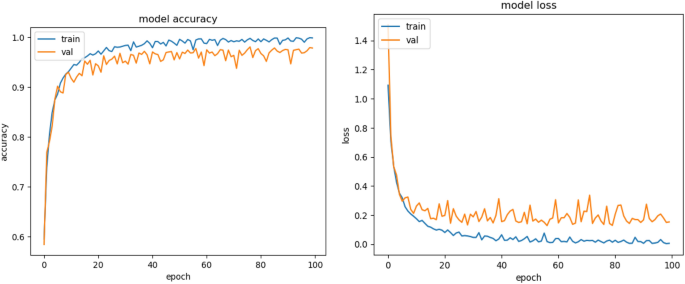
Model accuracy and loss of Existing MobileNet model.
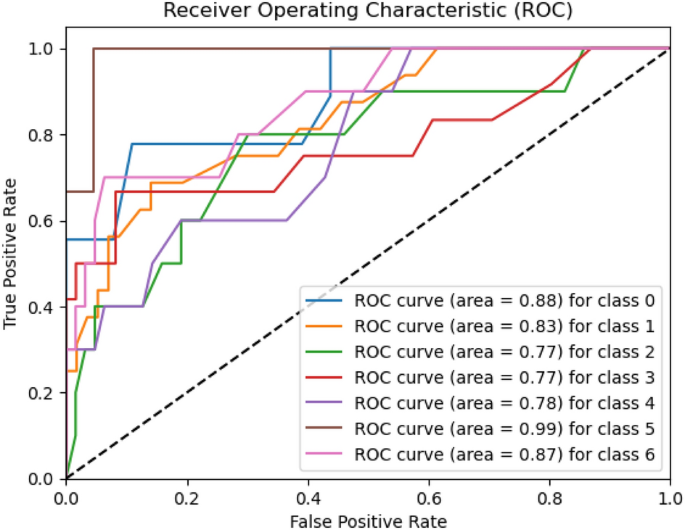
ROC curve of existing MobileNet model.
Figures 14 , 15 , 16 present a visualization of simulation results for the MobileNet-V2 Model on the “HAM-10000” skin cancer dataset. Figure 14 presents the Confusion Matrix of the MobileNet-V2 Model, Fig. 15 shows the Model Accuracy and Loss of the MobileNet-V2 Model, and Fig. 16 illustrates an ROC Curve of the MobileNet-V2 Model.
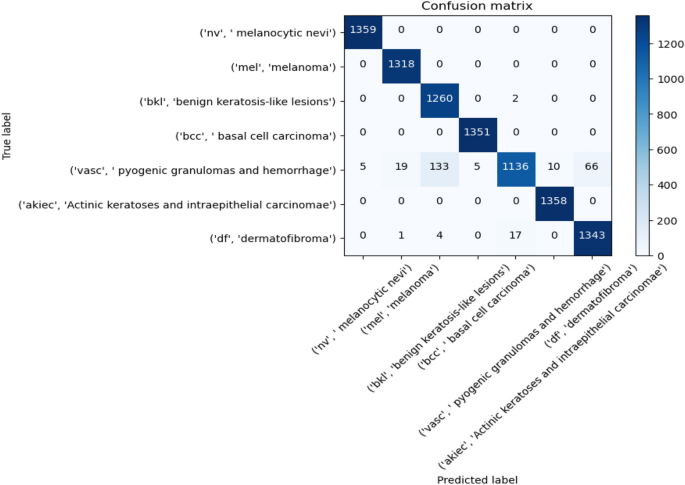
Confusion matrix existing MobileNet-V2 model.
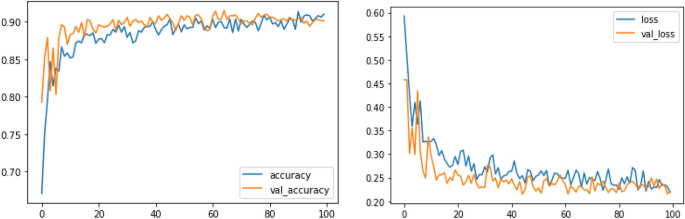
Model training, validation accuracy and loss existing MobileNet-V2 model.
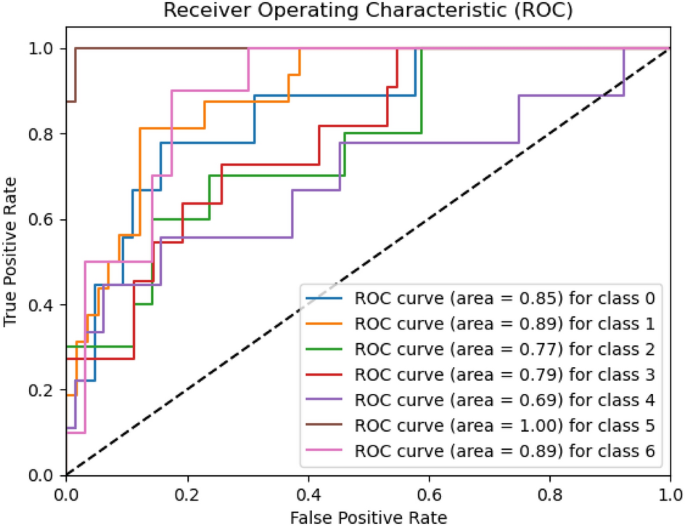
ROC curve of existing MobileNet-V2 model.
Figures 17 , 18 , 19 present a visualization of simulation results for the VGG-16 Model on the “HAM-10000” skin cancer dataset. Figure 17 presents the Confusion Matrix of the VGG-16 Model; Fig. 18 illustrates the Model Accuracy and Loss of the VGG-16; Fig. 19 shows an ROC Curve of the VGG-16 Model.
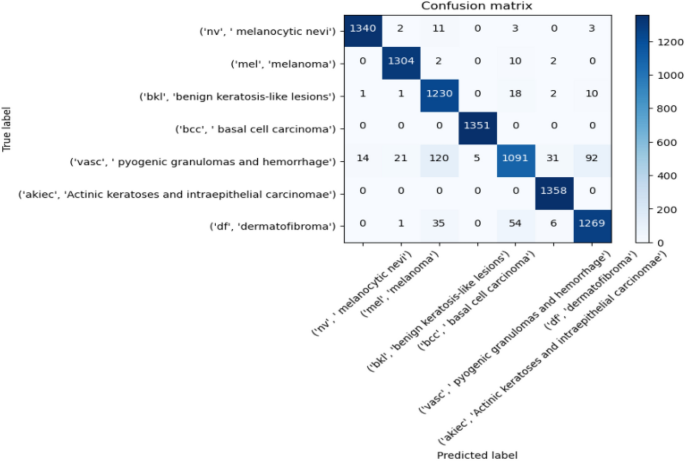
Confusion matrix of existing VGG-16 model.
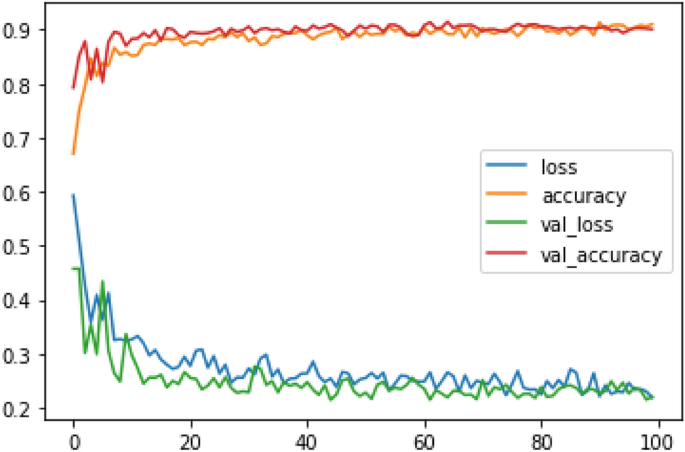
Model training, validation accuracy and loss existing VGG-16 model.
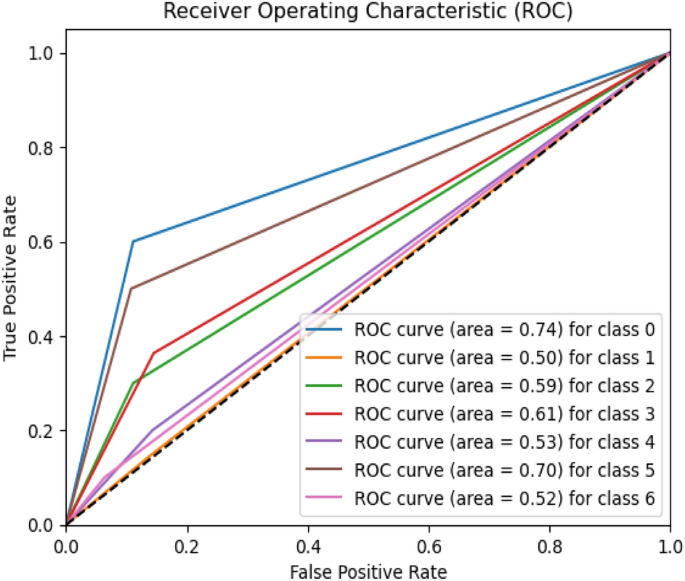
ROC curve of existing VGG-16 model.
Figures 20 , 21 , 22 present a visualization of simulation results for the VGG-19 Model on the “HAM-10000” skin cancer dataset. Figure 20 presents the Confusion Matrix of the VGG-19 Model; Fig. 21 illustrates the Model Accuracy and Loss of the VGG-19; Fig. 22 shows an ROC Curve of the VGG-19 Model. Figures 23 , 24 , 25 present a visualization of simulation results for the Resnet-152v2Model on the “HAM-10000” skin cancer dataset. Figure 23 presents the Confusion Matrix of the Resnet-152v2 Model; Fig. 24 illustrates the Model accuracy and loss of the Resnet-152v2; Fig. 25 shows an ROC Curve of the Resnet-152v2 Model. Based on the analysis of the results, it’s clearly proven that the proposed model has better simulation outcomes than existing models.
Confusion matrix of existing VGG-19 model.
Model training accuracy and validation of existing VGG-19 model.
ROC curve of existing VGG-19 model.
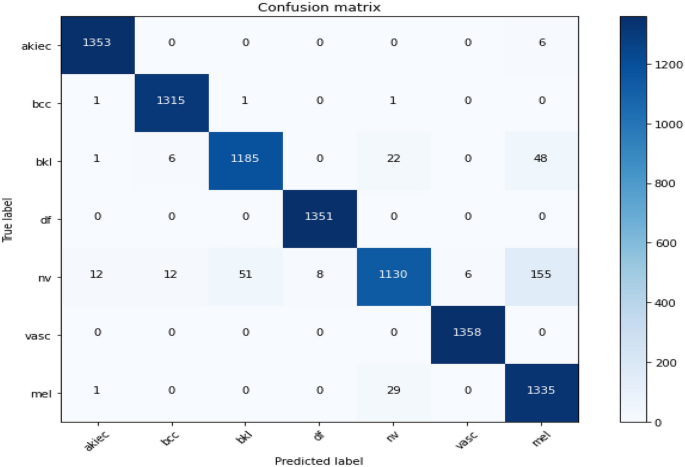
Confusion matrix of resnet-152v2.
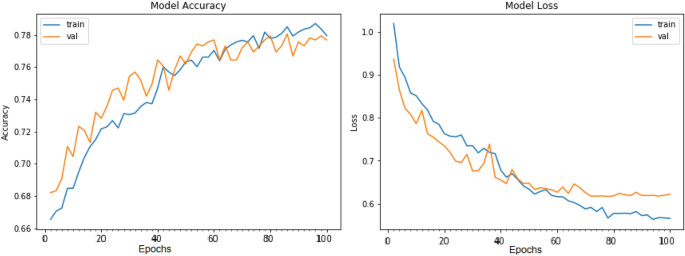
Training and validation accuracy, loss results of resnet-152v2.
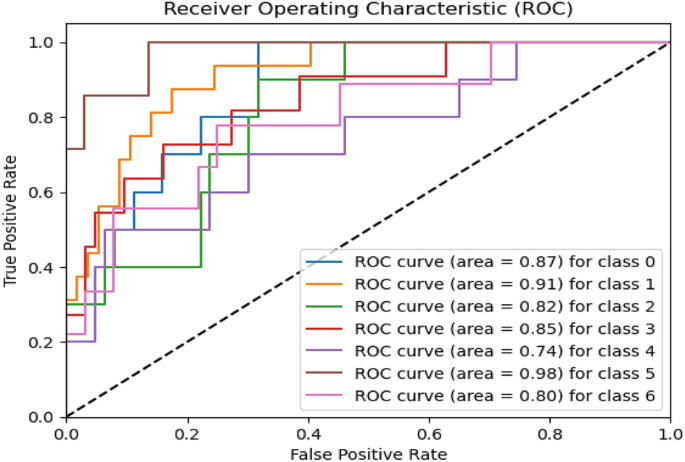
ROC-curve of resnet-152v2.
Results and discussion
The HAM-10000 Skin cancer dataset is utilized for the proposed model and several existing deep learning models, i.e., MobileNet, Resnet-152v2, VGG-16, MobileNet-V2, and VGG-19. Table 6 presents the experimental results of the testing dataset for 100 epochs for existing and proposed models. The combined utilization of the U-Net and MobileNet-V3 models yielded exceptional results in classifying skin cancer diseases. The model demonstrated a set of validation results with an accuracy of 98.86%, wherein the precision, recall, and F1-score all surpassed 95%. The model's capability to distinguish between cancerous and non-cancerous tumours is demonstrated by the ROC-AUC score of 98.45%, indicating excellent performance. The model consistently demonstrated a strong performance on the test set, achieving an accuracy rate of 98.45%.
The evaluation metrics, precision, accuracy, recall, ROC-AUC and F1-score, exhibited remarkable performance on the testing dataset, surpassing 95% for each metric. The ROC-AUC result of 98.45% reaffirms the model's robustness compared to the proposed model, existing MobileNet achieved, reaching 89% for each performance metric. Resnet-152V2 was achieved, surpassing 88% for each performance metric; VGG-16 was achieved, exceeding 89% for each performance metric. MobileNet-V2 achieved, exceeding 90% for each performance metric, and VGG-19 achieved, surpassing 90.50% for each performance metric. These results indicate that the proposed model achieved outstanding results over existing deep learning models.
Table 7 presents the experimental results of the validation dataset for 100 epochs for existing and proposed models. The proposed model demonstrated validation results with an accuracy of 98.03%, wherein the precision, recall, and F1-score all surpassed 94%. The model's capability to distinguish between cancerous and non-cancerous tumours is demonstrated by the ROC-AUC score of 97.09%, indicating excellent performance. The evaluation results show precision, accuracy, recall, ROC-AUC, and F1-score, exhibiting remarkable performance on the validation dataset, surpassing 94% for each metric. The ROC-AUC result of 97.09% reaffirms the model's robustness. As compared to the proposed model, the existing model, i.e., MobileNet achieved, surpassing 87% for each performance metric, Resnet-152V2 achieved, surpassing 87.02% for each performance metric VGG-16 achieved, reaching 88% for each performance metric, MobileNet-V2 achieved, exceeding 89% for each performance metric, and VGG-19 achieved, surpassing 88.50% for each performance metric. These results indicate that the proposed model achieved outstanding results over existing deep learning models.
Figures 8 , 9 , 10 present a visualization of simulation results for the proposed model, Figs. 11 , 12 , 13 present a visualization of simulation results for the MobileNet Model, Figs. 14 , 15 , 16 present a visualization of simulation results for the MobileNet-V2 Model, Figs. 17 , 18 , 19 shows a visualization of simulation results for the VGG-16 Model, Figs. 20 , 21 , 22 present a visualization of simulation results for the VGG-19 Model on the “HAM-10000” skin cancer dataset. Similar Figs. 23 , 24 , 25 present a visualization of simulation results for the Resnet-152v2Model on the “HAM-10000” skin cancer dataset. Based on the analysis of the results, it’s clearly proven that the proposed model has better simulation outcomes than existing models. Existing models such as MobileNet, VGG-16, MobileNet-V2, ResNet-152v2, and VGG-19 did not perform as well as the Hybrid U-Net, and Improved MobileNet-V3 model with hyperparameter optimization did on the "HAM-10000 Melanoma Skin Cancer dataset." There are several reasons for this.
Architecture of U-Net The U-Net architecture has gained recognition for its efficacy in the field of image segmentation, particularly in tasks related to skin cancer detection, where the objective is to delineate skin tumours from the tissue that surrounds them accurately. The U-Net's capacity to effectively capture intricate details and delineations contributes to enhanced feature extraction and superior classification outcomes.
Improved MobileNet-V3 MobileNet-V3 is a CNN architecture that has been specifically developed to prioritize both efficiency and accuracy. The enhanced iteration of the dataset takes into account alterations and refinements that render it better suited for the unique attributes of the skin malignancies dataset.
Feature extraction by Hybrid Model The hybrid models were created by integrating the advantageous features of both U-Net and MobileNet-V3. The provided system offers precise segmentation masks, whereas MobileNet-V3 efficiently extracts features from the segmented regions. The integration of these characteristics enhances the model's comprehension of images related to skin cancer.
Optimization of Hyperparameter The utilization of hyperparameter optimization techniques enables the refinement of the model's parameters and structural decisions, specifically tailored to the skin cancer dataset. In comparison with models with standard hyperparameters, this led to better performance and broad applicability.
Ablation analysis
We performed ablation research to determine the effect of critical parameters in the proposed skin cancer analysis model. To check the proposed model performance, we have conducted an ablation analysis with various conditions, which include (a) with and without Hybrid U-Net, (b) with and without MobileNet-V3, (c) with and without Augmentation, (d) with and without Batch Normalization, (e) with and without Hyperparameters. After methodically removing and testing with every single subsequent component eliminated, the following observations were found.
Various factors on the skin dataset were compared in the ablation analysis, as shown in Table 8 and Fig. 26 . For example, when it came to identifying complicated characteristics inside skin lesions, performance metrics dropped when the Hybrid U-Net was not used. There has been a significant gain in accuracy, suggesting that the Hybrid U-Net is vital in lowering the model's false positive rate and improving its capacity to detect cancerous tumours accurately. Improving MobileNet-V3 is crucial for efficient feature extraction since its absence significantly reduced total accuracy. There is strong evidence from the improved accuracy that Improved MobileNet-V3 helps cut down on false positives.
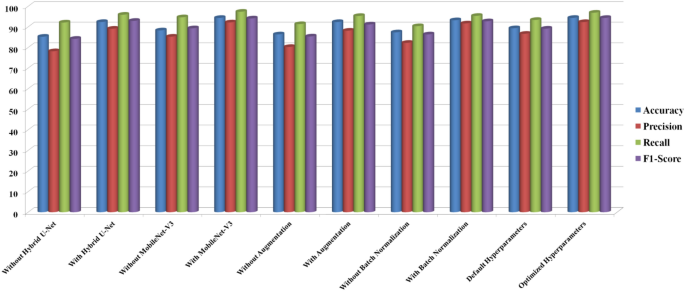
Comparative analysis of ablation analysis for different parameters on skin dataset.
Results showed that model resilience was reduced in the absence of data augmentation, highlighting the need for dataset augmentation for improved generalizability. Increases in accuracy and F1-score demonstrate how data augmentation enhances the model's capacity to categorize cancerous tumours accurately. A decrease in overall model performance and a slowdown in training convergence were the outcomes of removing batch normalization layers. Improving the model's capacity to distinguish between benign and malignant lesions and stabilizing the training process are both achieved using batch normalization, as seen by the significant gains in accuracy and F1-score. We found that the model's sensitivity to distinguishing between benign and malignant lesions is greatly affected by the ideal values of the hyperparameters. These enhanced precision and accuracy show how critical hyperparameter adjustment is for getting the most out of a model. All of the steps and parts of our skin cancer diagnostic methodology are vital, as these results show. In addition to shedding light on the model, the ablation research provides valuable information for making future upgrades and enhancements. Here are some practical ways to improve the model's diagnostic accuracy and reliability: observe the changes in performance measures.
Conclusion and future works
The proposed research integrates Hybrid U-Net and Improved MobileNet-V3 features to formulate a new hybrid model for skin cancer detection. Our model, showcasing superior diagnostic accuracy, emphasizes the pivotal roles of Hybrid U-Net and Improved MobileNet-V3 through a comprehensive assessment involving an ablation study and a comparison with state-of-the-art models. Beyond delivering a high-performing model, the study offers insightful directions for future research, contributing to the advancements in automated skin cancer detection. The observed enhancements, coupled with our competitive standing against current models, suggest a potentially significant therapeutic impact. According to the authors, this study introduces new possibilities for research and development in medical image analysis, promising faster and more accurate skin cancer diagnostics.
This research introduces an innovative skin cancer detection method incorporating U-Net and MobileNetV3 architecture, employing hyperparameter optimization strategies. By combining advanced deep learning techniques and meticulous parameter optimization, the result is a highly accurate and efficient system for diagnosing skin cancer. This work significantly improves skincare and medical services by providing a valuable tool for skin specialists and doctors to enhance the timely detection of skin malignancies and improve patient outcomes. The proposed model is compared with existing models, namely MobileNet, VGG-16, MobileNet-V2, Resnet-152v2, and VGG-19 on the "HAM-10000 Melanoma Skin Cancer dataset".
To fine-tune the model's hyperparameter, we employ sophisticated optimization methods such as the Bayesian optimization method using the pre-trained CNN architecture MobileNet-V3. Empirical findings demonstrate that the proposed optimized hybrid MobileNet-V3 model outperforms existing skin cancer detection and segmentation techniques, achieving high precision (97.84%), sensitivity (96.35%), accuracy (98.86%), and specificity (97.32%). The enhanced performance of this research can lead to timelier and more precise diagnoses, potentially contributing to life-saving outcomes and mitigating healthcare expenditures. The developed model holds significant potential in terms of clinical relevance, as suggested by its precision and recall scores, making it a valuable tool for dermatologists in the early detection of skin cancer. The elevated accuracy level presents a potential avenue for mitigating misdiagnoses and improving patient outcomes.
In the proposed model, computational demands during training and inference may arise as a consequence of integrating two complicated designs. This limitation should be taken into account, particularly in situations when resources are limited. Also, interpretability is a common issue for deep learning models like the proposed model. In clinical situations, the model's interpretability is limited because, despite its good performance, comprehending the precise elements underlying the classification judgments might be difficult.
In order to reduce computing complexity, future studies should concentrate on improving the model. To improve the practicality of deployment, it may be worthwhile to investigate model compression approaches or lightweight designs. Solving problems with interpretability might be crucial. Healthcare providers may gain confidence and knowledge of the model's decision-making process with the use of explainable AI approaches. Future research in the area of automated skin cancer detection will be guided by this balanced approach, which adds to the current conversation in the field. Future research should explore incorporating supplementary clinical data, developing interpretability methods tailored for medical professionals, and addressing the ethical implications associated with implementing artificial intelligence models for skin cancer diagnosis. Our commitment to innovation remains central as we aim to make a significant impact within dermatology. While the proposed model exhibits exemplary performance on the HAM-10000 dataset, it is crucial to recognize that real-world medical records may show unforeseen variations. Additional validation is necessary to extend the model's capabilities to datasets and situations in healthcare that have not been previously encountered.
Data availability
The data supporting the research results of the present research can be obtained by contacting the corresponding author.
Abbreviations
- Deep learning
International skin imaging collaboration
Adaptive moment optimization algorithm
Fully connected
Convolution neural network
Batch normalization
Support vector machine
Rectified linear unit
Ismail, W. N. & Alsalamah, H. A. Efficient Harris Hawk optimization (HHO)-based framework for accurate skin cancer prediction. Mathematics 11 (16), 3601 (2023).
Article Google Scholar
Mohakud, R. & Dash, R. A hybrid model for classification of skin cancer images after segmentation. Int. J. Image Graph. 31 , 2550022 (2023).
Ogundokun, R. O. et al. Enhancing skin cancer detection and classification in dermoscopic images through concatenated MobileNetV2 and xception models. Bioengineering 10 (8), 979 (2023).
Article PubMed PubMed Central Google Scholar
Nancy, V. A., Osvin, P. P., Arya, M. S. & Shamreen Ahamed, B. Comparative research and analysis on skin cancer detection using machine learning and deep learning algorithms. Multim. Tools Appl. 82 , 1–45 (2023).
Sharma, G., & Raman, C. An optimized predictive model based on deep neural network for detection of skin cancer and oral cancer. In 2023 2nd International Conference for Innovation in Technology (INOCON) , 1–6 (IEEE, 2023).
Behara, K., Bhero, E. & Agee, J. T. Skin lesion synthesis and classification using an improved DCGAN classifier. Diagnostics 13 , 2635 (2023).
Mampitiya, L. I., Rathnayake, N. & De Silva, S. Efficient and low-cost skin cancer detection system implementation with a comparative research between traditional and CNN-based models. J. Comput. Cogn. Eng. 2 (3), 226–235 (2023).
Google Scholar
Akilandasowmya, G., Nirmaladevi, G., Suganthi, S. U. & Aishwariya, A. Skin cancer diagnosis: Leveraging deep hidden features and ensemble classifiers for early detection and classification. Biomed. Signal Process. Control 88 , 105306 (2023).
Salih, O. & Duffy, K. J. Optimization convolutional neural network for automatic skin lesion diagnosis using a genetic algorithm. Appl. Sci. 13 (5), 3248 (2023).
Article CAS Google Scholar
Tabrizchi, H., Parvizpour, S. & Razmara, J. An improved VGG model for skin cancer detection. Neural Process. Lett. 55 (4), 3715–3732 (2023).
Balaha, H. M. & Hassan, A.E.-S. Skin cancer diagnosis based on deep transfer learning and sparrow search algorithm. Neural Comput. Appl. 35 (1), 815–853 (2023).
Anupama, C. S. S. et al. Sand cat swarm optimization with deep transfer learning for skin cancer classification. Comput. Syst. Sci. Eng. 47 , 2 (2023).
Shamsi, A. et al. A novel uncertainty-aware deep learning technique with an application on skin cancer diagnosis. Neural Comput. Appl. 35 (30), 22179–22188 (2023).
Adla, D. et al. A full-resolution convolutional network with a dynamic graph cut algorithm for skin cancer classification and detection. Healthcare Analytics 3 , 100154 (2023).
Rajeshwari, J. & Sughasiny, M. Skin cancer severity prediction model based on modified deep neural network with horse herd optimization. Optic. Memory Neural Netw. 31 (2), 206–222 (2022).
Shinde, R. K. et al. Squeeze-mnet: Precise skin cancer detection model for low computing IOT devices using transfer learning. Cancers 15 (1), 12 (2022).
Prasad, V., Emil Selvan, G. S. R. & Ramkumar, M. P. ADTBO: Aquila driving training-based optimization with deep learning for skin cancer detection. Imag. Sci. J. 4 , 1–19 (2023).
Mohakud, R. & Dash, R. Skin cancer image segmentation utilizing a novel EN-GWO based hyper-parameter optimized FCEDN. J. King Saud Univ. Comput. Inf. Sci. 34 (10), 9889–9904 (2022).
Shetty, B. et al. Skin lesion classification of dermoscopic images using machine learning and convolutional neural network. Sci. Rep. 12 (1), 18134 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Barburiceanu, S., & R. Terebeș. Automatic detection of melanoma by deep learning models-based feature extraction and fine-tuning strategy. In IOP Conference Series: Materials Science and Engineering , Vol. 1254, 012035 (IOP Publishing, 2022).
Houssein, E. H., Emam, M. M. & Ali, A. A. An optimized deep learning architecture for breast cancer diagnosis based on improved marine predators algorithm. Neural Comput. Appl. 34 (20), 18015–18033 (2022).
Gouda, W., Sama, N. U., Al-Waakid, G., Humayun, M. & Jhanjhi, N. Z. Detection of skin cancer based on skin lesion images using deep learning. Healthcare 10 (7), 1183 (2022).
El Gannour, O. et al. Improving skin diseases prediction through data balancing via classes weighting and transfer learning. Bull. Electr. Eng. Inf. 13 (1), 628–637 (2024).
Arunkumar, M., Mohanarathinam, A. & Subramaniam, K. Detection of varicose vein disease using optimized kernel Boosted ResNet-Dropped long Short term Memory. Biomed. Signal Process. Control 87 , 105432 (2024).
Zhang, L. et al. A deep learning outline aimed at prompt skin cancer detection utilizing gated recurrent unit networks and improved orca predation algorithm. Biomed. Signal Process. Control 90 , 105858 (2024).
Sonia, R. et al. Segmenting and classifying skin lesions using a fruit fly optimization algorithm with a machine learning framework. Automatika 65 (1), 217–231 (2024).
Deshmukh, A. A. et al. Multi-class skin diseases classification using hybrid deep convolutional neural network. Int. J. Intell. Syst. Appl. Eng. 11 (10s), 11–22 (2023).
Sulthana, R., Chamola, V., Hussain, A., Hussain, Z. & Albalwy, F. A novel end-to-end deep convolutional neural network based skin lesion classification framework. Expert Syst. Appl. 246 , 123056 (2023).
Bibi, S. et al. MSRNet: Multi-class skin lesion recognition using additional residual block based fine-tuned deep models information fusion and best feature selection. Diagnostics 13 (19), 3063 (2023).
Awotunde, J. B. et al. An enhanced hyper-parameter optimization of a convolutional neural network model for leukemia cancer diagnosis in a smart healthcare system. Sensors 22 (24), 9689 (2022).
Article ADS PubMed PubMed Central Google Scholar
Abdar, M. et al. Uncertainty quantification in skin cancer classification using three-way decision-based Bayesian deep learning. Comput. Biol. Med. 135 , 104418 (2021).
Article PubMed Google Scholar
Kilicarslan, S., Celik, M. & Sahin, Ş. Hybrid models based on genetic algorithm and deep learning algorithms for nutritional Anemia disease classification. Biomed. Signal Process. Control 63 , 102231 (2021).
Sayed, G. I., Soliman, M. M. & Hassanien, A. E. A novel melanoma prediction model for imbalanced data using optimized SqueezeNet by bald eagle search optimization. Comput. Boil. Med. 136 , 104712 (2021).
Tan, T. Y., Zhang, L. & Lim, C. P. Intelligent skin cancer diagnosis using improved particle swarm optimization and deep learning models. Appl. Soft Comput. 84 , 105725 (2019).
ElGhany, S. A., Ibraheem, M. R., Alruwaili, M. & Elmogy, M. Diagnosis of various skin cancer lesions based on fine-tuned ResNet50 deep network. Comput. Mater. Cont. 68 , 1 (2021).
Pham, T. C., et al. Improving binary skin cancer classification based on best model selection method combined with optimizing full connected layers of Deep CNN. In 2020 International conference on multimedia analysis and pattern recognition (MAPR) , 1–6. (IEEE, 2020).
Gouda, W., Sama, N. U., Al-Waakid, G., Humayun, M. & Jhanjhi, N. Z. Detection of skin cancer based on skin lesion images using deep learning.". Healthcare 10 (7), 1183 (2022).
Nour, A., & Boubakeur, B. Convolutional neural network strategy for skin cancer lesions classifications and detections. In Proceedings of the 11th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics , 1–9 (2020).
Tan, T. Y., Zhang, L., Neoh, S. C. & Lim, C. P. Intelligent skin cancer detection using enhanced particle swarm optimization. Knowl. Syst. 158 , 118–135 (2018).
Khan, M. A., Akram, T., Zhang, Y. D., Alhaisoni, M., Al Hejaili, A., Shaban, K. A., & Zayyan, M. H. SkinNet‐ENDO: Multi-class skin lesion recognition using deep neural network and Entropy‐Normal distribution optimization algorithm with ELM. Int. J. Imaging Syst. Technol. (2023).
Ajmal, M. et al. BF2SkNet: Best deep learning features fusion-assisted framework for multi-class skin lesion classification. Neural Comput. Appl. 35 (30), 22115–22131 (2023).
Dillshad, V., Khan, M. A., Nazir, M., Saidani, O., Alturki, N., & Kadry, S. D2LFS2Net: Multi‐class skin lesion diagnosis using deep learning and variance‐controlled Marine Predator optimization: An application for precision medicine. CAAI Trans. Intell. Technol. (2023).
Hussain, M. et al. SkinNet-INIO: Multi-class skin lesion localization and classification using fusion-assisted deep neural networks and improved nature-inspired optimization algorithm. Diagnostics 13 (18), 2869 (2023).
Download references
This research received no external funding.
Author information
Authors and affiliations.
Department of Computer Science and Engineering, Chandigarh University, Mohali, Punjab, 140413, India
Umesh Kumar Lilhore, Sarita Simaiya & Anupam Baliyan
Department of Computer Science and Engineering, Koneru Lakshmaiah Education Foundation, Greenfield, Vaddeswaram, Guntur, AP, India
Yogesh Kumar Sharma & K. B. V. Brahma Rao
School of Computing Science and Engineering, Galgotias University, Greater Noida, Uttar Pradesh, India
Kuldeep Singh Kaswan
Departmentt of Computer Science and Engineering, Shri Vishnu Engineering College for Women (A), Bhimavaram, India
V. V. R. Maheswara Rao
Arba Minch University, Arba Minch, Ethiopia
Anchit Bijalwan
Department of Computer Science, College of Computers and Information Technology, Taif University, P. O. Box 11099, 21944, Taif, Saudi Arabia
Roobaea Alroobaea
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Ideas and overarching research goals and aims by S.S. and A.B.; Methodology: Development and design of methodology by U.K.L. and V.V.R.M.R.; Software: Programming, software development by Y.K.S. and A.B.; Investigation and analysis: Conducting a research and investigation process and result analysis by; K.S.K.R. and K.B.V.B.R.; Supervision: Guidance and supervision for the research by U.K.L. Authors provide support for publication.
Corresponding author
Correspondence to Anchit Bijalwan .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kumar Lilhore, U., Simaiya, S., Sharma, Y.K. et al. A precise model for skin cancer diagnosis using hybrid U-Net and improved MobileNet-V3 with hyperparameters optimization. Sci Rep 14 , 4299 (2024). https://doi.org/10.1038/s41598-024-54212-8
Download citation
Received : 29 September 2023
Accepted : 09 February 2024
Published : 21 February 2024
DOI : https://doi.org/10.1038/s41598-024-54212-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health care
- Convolution Neural Network
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Author Biographies
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Synergistic effect of human papillomavirus and environmental factors on skin squamous cell carcinoma, basal cell carcinoma, and melanoma: insights from a taiwanese cohort.

Simple Summary
Share and cite.
Chen, C.-C.; Luo, C.-W.; Tsai, S.C.-S.; Huang, J.-Y.; Yang, S.-F.; Lin, F.C.-F. Synergistic Effect of Human Papillomavirus and Environmental Factors on Skin Squamous Cell Carcinoma, Basal Cell Carcinoma, and Melanoma: Insights from a Taiwanese Cohort. Cancers 2024 , 16 , 2284. https://doi.org/10.3390/cancers16122284
Chen C-C, Luo C-W, Tsai SC-S, Huang J-Y, Yang S-F, Lin FC-F. Synergistic Effect of Human Papillomavirus and Environmental Factors on Skin Squamous Cell Carcinoma, Basal Cell Carcinoma, and Melanoma: Insights from a Taiwanese Cohort. Cancers . 2024; 16(12):2284. https://doi.org/10.3390/cancers16122284
Chen, Chun-Chia, Ci-Wen Luo, Stella Chin-Shaw Tsai, Jing-Yang Huang, Shun-Fa Yang, and Frank Cheu-Feng Lin. 2024. "Synergistic Effect of Human Papillomavirus and Environmental Factors on Skin Squamous Cell Carcinoma, Basal Cell Carcinoma, and Melanoma: Insights from a Taiwanese Cohort" Cancers 16, no. 12: 2284. https://doi.org/10.3390/cancers16122284
Article Metrics
Further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Sensors (Basel)

New Trends in Melanoma Detection Using Neural Networks: A Systematic Review
Due to its increasing incidence, skin cancer, and especially melanoma, is a serious health disease today. The high mortality rate associated with melanoma makes it necessary to detect the early stages to be treated urgently and properly. This is the reason why many researchers in this domain wanted to obtain accurate computer-aided diagnosis systems to assist in the early detection and diagnosis of such diseases. The paper presents a systematic review of recent advances in an area of increased interest for cancer prediction, with a focus on a comparative perspective of melanoma detection using artificial intelligence, especially neural network-based systems. Such structures can be considered intelligent support systems for dermatologists. Theoretical and applied contributions were investigated in the new development trends of multiple neural network architecture, based on decision fusion. The most representative articles covering the area of melanoma detection based on neural networks, published in journals and impact conferences, were investigated between 2015 and 2021, focusing on the interval 2018–2021 as new trends. Additionally presented are the main databases and trends in their use in teaching neural networks to detect melanomas. Finally, a research agenda was highlighted to advance the field towards the new trends.
1. Introduction
Melanoma (Me) is known as the deadliest type of skin cancer [ 1 ], the incidence of its occurrence increasing for both men and women worldwide every year [ 2 , 3 ]. According to Sun X. et al. [ 4 ] the main cause of Me occurrence is exposure to ultraviolet radiation. Due to this excessive exposure, some mutations that occur at the level of melanocytes can lead to Me genesis. Even though it is one of the deadliest types of skin cancers, many studies showed that early detection of Me leads to its treatment in 90% of cases [ 5 ]. Currently, the standard method of Me diagnosis is visual analysis by a specialist. However, this method can be time-consuming. Moreover, it can lead to misdiagnosis due to the complexity of providing the diagnosis. The following aspects need to be considered: the number of parameters that need to be analyzed (color, shape, texture, edge, asymmetry, etc.), the fatigue, and the lack of experience of the specialist [ 6 , 7 , 8 ]. In most cases, the dermoscopic images are acquired and analyzed by the dermatologist, thus achieving a maximum of 84% examination accuracy (ACC) [ 9 , 10 ], which is insufficient. Therefore, the help of a computer-aided diagnosis (CAD) system for Me diagnosis from images is more than necessary [ 11 ].
Over time, a lot of researchers have put their ideas together to try to develop an automatic Me detection system based on machine learning (ML) that provides a quick result with high ACC, even if the complexity of skin lesion (SL) images analysis presented many problems [ 12 , 13 ]. In reality, it is a rather complex task to find a suitable diagnosis algorithm due to the presence of artifacts, such as the presence of hair around or even in the lesion, different lesion dimensions, color and shapes, the presence of blood vessels, and other artifacts [ 14 ], as seen in Figure 1 .

Artifacts in Me images collected from the ISIC 2016 dataset [ 14 ]: ( a – c )—presence of hair, ( d )—presence of blood vessels, ( e , f )—presence of oil drops.
The inconveniences caused by these factors led the authors to expand their research a lot but, in principle, most approaches use the same classical method in which the first step is the preprocessing step, followed by segmentation, feature extraction, and then the classification step. The main workflow of the classical method is as shown in Figure 2 .

Methods workflow for Me detection: ( a ) classical method, ( b ) NN approach.
The preprocessing step consists of applying primary operations such as the following: noise removal, data augmentation, resizing, brightness grayscale transformation or brightness corrections, binarization, and, mainly, intensity and contrast enhancement [ 15 ]. As the Me images have a high variability of content, the segmentation step is a much-debated topic and a difficult task. This step represents the part of the algorithm that makes possible the image splitting into several sets of pixels [ 16 ], with the extraction of regions of interest (RoI) by an automatic or semiautomatic process as the end result [ 17 ]. Among the most commonly used techniques for Me detection and segmentation are artificial neural network-based methods (NNs). Considering the variability of Me images, the first-mentioned method ( Figure 2 a) cannot provide the best results. After the segmentation, the feature extraction step is usually applied. This task consists of reducing the dimensions of the data representation such that this becomes more administrable. Thus, data processing becomes faster and easier, without losing important information. Even so, it is known as a large consumer of resources due to the high number of variables. Generally, if the feature extraction is well done, the detection ACC will increase significantly [ 16 ]. In the past, most authors [ 18 , 19 , 20 ] used the ABCD (Asymmetry, Border, Color, Differential structure) rule as a feature extraction-based method for Me detection, while presently others use deep learning (DL) techniques to make the feature extraction better. The last, and the most discussed step in our review, is the classification step. The goal of this step is to assign a class to an RoI from an image. Manual classification is hard and time-consuming and therefore the interest for developing an accurate automatic classification algorithm increased in last years.
Nowadays, whether it is about segmentation, feature extraction, or classification, the tendency is to use the benefits of Artificial Intelligence (AI) using NN and DL techniques to obtain more accurate results. The main goal of AI is the reproduction of human intelligence, with applications in domains such as autonomous vehicles, search engines, art creation, or medical diagnosis. In the case of Me detection by applying AI, promising results were obtained, reaching a level where only visual inspection of SL is no longer a reliable solution. Known as a subset of the AI, the classical ML algorithms were proposed first as a solution for automatic Me detection. Mainly, ML uses the previous experience to improve the given results [ 21 ]. The system first extracts the needed features to create the training data. After the training data are obtained, supervised or unsupervised learning is used in the learning process. Generally, most papers used the supervised learning models, being more accurate. As has been observed also in other areas in which it is applied, the classical ML-based methods showed promising results, but also some limitations. For example, a large amount of data are needed to train the system, the learning phase takes a long time, and ML presents a high error-susceptibility. Thus, the authors turned their attention to NN and DL techniques.
NNs consist of a collection of neurons that simulates the function of neurons in a human being. In such a network, the neurons are connected to each other, each connection being assigned a weight, helping the neurons to give the necessary output. The authors prefer the NNs because they present benefits, such as distributed memory, the possibility of giving good results with a small amount of information, or the possibility of parallel processing. For training, the system error is calculated by taking the difference between the predicted value and the output target. Using this calculated error, the system adjusts its weights until the error is minimized.
Most Me detection papers used the feedforward and the recurrent NNs to obtain a high ACC result. Better results were obtained by the authors by using DL models such as CNN or Recurrent NN. The CNNs are NNs with at least one convolution layer. At present, different applications including Me detection systems obtain the best results.
The main aim of this work is the analysis of new trends of approaches used in the automatic SL detection field (especially Me). The paper focuses on presenting the growth trend of using NN techniques when developing such a system. The rest of the paper is organized as follows. Section 2 , named Materials and Methods, presents the search strategy for motivation and selection of the recent relevant papers to establish the new trends in the Me detection by NN. Section 3 addresses the main DSs used in the selected articles, focusing on public DSs. The most important NNs used today for Me detection, classification, and segmentation are described and analyzed in Section 4 . Section 5 presents the new directions of NN implementation in Me detection, taking into consideration individual NNs, multiple NN configurations based on decision fusion, and hybrid configurations consisting of NNs and other intelligent classifiers. Finally, a Discussion section ( Section 6 ) compares the results of this paper with other similar review/survey papers highlighting the novelties.
2. Materials and Methods
Although the papers that addressed Me detection and NN use separately are older and their research is well-established, the study of Me detection by NN algorithms is relatively recent ( Figure 3 a). As we considered the new trends in Me detection using NNs, we searched the following DSs: Web of Science, Scopus, and PubMed between 2015 and 2021 considering the following topics: melanoma, skin lesions, artificial intelligence, machine learning, deep learning, and convolutional neural networks. The search was split between combinations of keywords using the “AND” connector: CNN AND Me ( Figure 3 a), DL AND Me ( Figure 3 b), ML AND Me ( Figure 3 c), and AI AND Me ( Figure 3 d). It can be observed that the increase in research is exponential in the cases of CNN AND Me, DL AND Me, and AI AND Me and quasilinear in the case of ML AND Me. The number of publications identified according to the search in the database is labeled on the y-axis in Figure 3 .

Searches for important terms in the Web of Science, Scopus, and PubMed DBs between 2015 and 2021 with the AND connector: ( a ) CNN AND Me, ( b ) DL AND Me, ( c ) ML AND Me, and ( d ) AI AND Me.
As many as 300 full-text papers were analyzed from Web of Science, Scopus, and PubMed, of which we selected 134 research papers for this review. The main criteria for paper selection were: the recent period, new trends in Me detection by the aid of NN, visibility, and impact of contributions (publishing in high-rank conferences and journals, number of citations). The most representative articles covering melanoma detection based on neural networks, published in journals and impact conferences, were investigated between 2015 and 2021 (92% of references), focusing on the interval 2018–2021 (80% of references) as a recent period. In terms of new trends of using NNs for detection, segmentation, and classification of Me, we noticed the following directions: systems using one single CNN most often modified and adapted for Me, systems using multiple CNNs, and systems using CNN combined with other classifiers. Details will be given in Section 5 . Although the number of citations is relative, in general for older papers it is higher than for new ones (2021). However, obviously, there are exceptions. Due to this, we did not set a threshold for the number of citations. We had in mind that most papers follow what we have stated as new trends and obviously have a reasonable number of citations. The high-rank of the journal refers to Category Quartile Q1, Q2, and the Journal Impact Factor greater than 2.2 in Web of Science 2020. About 50% of the total references meet this criterion. For the systematic review and meta-analysis, we used a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram ( Figure 4 ).

PRISMA flow diagram of our research.
Most relevant papers concerning the aspects of new trends in the last period (related to Me, DSs, NNs, decision fusion, and combined networks) are detailed in Section 3 , Section 4 and Section 5 . To compare every analyzed paper, the important statistical performances are presented. The performance evaluation metrics most used in SL detection, segmentation, and classification are the following: Accuracy, Precision, Sensitivity, Specificity, F1-score, and Jaccard index. The formulas are listed in Table 1 , where TP is true positive, TN—true negative, FP—false positive, and FN—false negative cases. The emphasis was on accuracy (ACC), F1 score (F1—Dice Coefficient), and Jaccard index (IoU—Intersection over Union).
Performance indicators used in the review.
Indicator | Formula | Indicator | Formula |
|---|---|---|---|
| Accuracy | Sensitivity | ||
| Precision | Specificity | ||
| Dice Coefficient | Jaccard index |
3. Datasets Used in Melanoma Detection
The systems presented in this study are based on AI, which means that they are meant to learn from one or more DSs (both small and large ones). The DSs were built in collaboration with doctors/medical specialists. These DSs are composed of high-quality, well-selected images, previously analyzed, labeled, and potentially segmented by medical specialists from the respective domain. Our study aims to present the growth trend of such automated systems able to diagnose, segment, or detect certain SLs (especially Me) based on existing papers in the literature. The outcome of these papers was possible because of some existing public DSs. In this section, we will present some of the popular DSs which were used in a lot of papers from the SL domain. Among these DSs, we can find PH2, ISIC 2016, 2017, 2018, 2019 challenge DSs, HAM10000, DermNet Atlas, Dermatology Atlas, DermIs, and MED-NODE ( Table 2 ).
Skin lesions DSs frequently used in Me detection.
| DS Name | Reference | Availability | SL | Me |
|---|---|---|---|---|
| PH2 | [ ] | Publicly available | 200 | 40 |
| ISIC 2016 | [ ] | Publicly available | 900 | 273 |
| ISIC 2017 | [ ] | Publicly available | 2000 | 374 |
| ISIC 2018, HAM10000 | [ , ] | Publicly available | 10,015 | 1113 |
| ISIC 2019 | [ , , ] | Publicly available | 25,333 | 4522 |
| ISIC 2020 | [ ] | Publicly available | 33,126 | 584 |
| DERMQUEST | [ ] | Publicly available | 126 | 66 |
| MED-NODE | [ ] | Publicly available | 170 | 100 |
| DERMNET | [ ] | Publicly available | 22,500 | 635 |
| DERMIS | [ , ] | Publicly available | 397 | 146 |
| DERMOFIT | [ ] | Purchase only | 1300 | 76 |
One of the most used dermoscopic databases (DB) in certain papers is PH2. As specified in [ 22 ], this DB was built in Portugal at Hospital Pedro Hispano as a collaboration between multiple medical entities. The images from this DB contain a total number of 200 dermoscopic images (80 common nevi, 80 atypical, and 40 Me). The images are 8-bit RGB color images with a resolution of 768 × 560 pixels, carefully selected by taking into consideration the quality, resolution, and dermoscopic features. For each image in the DB, the manual segmentation and the clinical diagnosis of the SL as well as the identification of other important dermoscopic criteria are available.
Other important DSs used in this area are provided by ISIC (International Skin Imaging Collaborative) which provides expertly annotated DSs containing digital SL images of different versions (2016, 2017, 2018, 2019, and 2020) to facilitate CAD of multiple SL diseases [ 23 , 24 ]. These DSs were used at the International Symposia in Biomedical Imaging (ISBI).
ISIC 2016 DS [ 14 ] contains 900 dermoscopic lesion images in JPEG format, with EXIF data stripped as training data and 379 images with the same format as testing data. The images from this DS have a resolution between 576 × 768 and 2848 × 4288, which means that, in some cases, resizing operations might be needed.
ISIC 2017 [ 25 ] contains a total number of 2750 SLs where 2150 can be used as training data and 600 can be used for testing data. The resolutions of these images are between 540 × 722 and 4499 × 6748. Like in the previous DS, in some cases, resizing operations might be needed.
The ISIC 2018 challenge DS [ 25 ] was used for Skin Lesion Analysis towards the Melanoma Detection challenge [ 26 ]. The DS is quite large (about 10.4 GB), and it contains 2594 images and 12,970 corresponding ground truth response masks (5 for each image) as training data and 1000 images (about 2.2 GB) as testing data. The SL are RGB images in JPG format and the masks are grayscale images in the PNG format [ 27 ]. The ISIC 2018 challenge was composed of three challenge tasks. Within the first two tasks, the participants were using 2594 images already presented, while, within the last task, representing a classification task, the participants used HAM10000 DS, which of course is another very popular DS, publicly available through ISIC archives. HAM10000 is composed of 10,015 images out of which 1113 are Me. All images in the DS are in JPEG format (8-bit color depth) and were all manually cropped with the lesion centered to 800 × 600 px at 72DPI and manual histogram corrections applied to enhance visual contrast and color reproduction [ 28 ]. ISIC 2019 and ISIC 2020 are new variants of ISIC DSs with more and more images in comparison with previous ones [ 23 ].
Another popular DS used in skin cancer detection systems is MED-NODE DS, which contains 70 Me and 100 nevus images from the digital image archive of the department of dermatology, University Medical Center Groningen [ 29 ].
Dermofit image library is a DS, property of the University of Edinburgh, which can be used only in medical imaging research. The DS is composed of 1300 high-quality SL images and contains ten different classes including Me (76), Melanocytic Nevus/Mole (331), Seborrhoeic Keratosis (257), Basal Cell Carcinomas (239), etc. [ 30 ]. Each image in this DS is a normal RGB captured with a quality SLR camera under controller (ring flash) indoor lighting. The images were labeled based on expert opinion (dermatologists and dermatopathologists) and binary segmentation masks, marking the lesions themselves, are also included. To access this DS, there is a need for a one-time purchase-only license.
DermNet Skin Disease Atlas is another DS used in research related to skin lesions detection, segmentation, and classification problems. This DS is composed of over 22,000 images (only 21,844 found as relevant) divided into 23 types of skin diseases (superclasses) [ 31 ]. The images are of the RGB type in JPEG format and the resolutions vary from image to image [ 32 ].
DermIS is also a publicly available dermoscopic image DS, widely used in the literature for SL detection, segmentation, and classification purposes and it is composed of a total number of 300 Me images [ 33 ]. The DS is available on [ 34 ] and provides the ability to search for dermoscopic images by category (face, hands, legs, etc.).
Another popular DS used for SL detection, segmentation, and classification was Dermquest which is an online medical atlas for dermatologists and dermatologist-based healthcare professionals [ 35 ]. The DS was publicly available (it is not currently) and contained over 22,000 clinical images.
Table 2 gives a summary of the properties of the DB/DS used in the studied references and illustrates the availability of the discussed and primarily used DSs identified in our study/survey.
The ISIC archives, PH2, HAM10000, MED-NODE, DermIS, and Dermquest DSs are free and publicly available for SL diagnosis research. Watermarks usually mean noise in the images when it comes to DL systems which are oriented towards learning different patterns. Therefore, researchers willing to access the high-quality images without watermarks from DermNet will need to purchase a license. This is one of the reasons we observed that DermNet is not widely used (it can be seen in Figure 5 and Figure 6 ), even if it is a large data set where the non-watermarked, high-quality images might make the difference in the DL process.

Frequently DSs used in Me detection between 2018 and 2020.

The four most used DSs for Me detection in 2021 (percentage).
According to our study, as can be seen in Figure 5 (focusing on the period 2018–2020, as new trends) and Figure 5 (for 2021), the most widely used DSs in SL diagnosis research are the ones included in ISIC archives (containing also {"type":"entrez-nucleotide","attrs":{"text":"H10000","term_id":"874822","term_text":"H10000"}} H10000 ). The first reason is that these DSs are quite consistent and very well labeled by domain experts, and the second reason might be the annual challenges posed by consistent prices. The second place is occupied by PH2, which is a small DB but, according to our study, the trend is to use small DSs for system/solution validation and to use large DSs for learning such as with DL and TL (transfer learning) systems. As can be seen in Section 4 , data augmentation is frequently used. For the year 2021, a separate evaluation (based on percentage) is presented in Figure 6 . It can be observed that the trend is maintained (54% ISIC and 30% PH2).
4. Neural Networks Used in Melanoma Detection, Segmentation, and Classification
According to the current study related to SL detection, segmentation, and classification papers in the literature, it turned out that the majority of these kinds of tasks used NNs, CNNs, DCNNs (Deep Convolutional Neural Networks), and TL for NNs. It can be observed that the trend throughout the years, in general, and not strictly related to SL diagnosis systems, is that researchers used to design deep networks with a lot of hidden layers (either convolutional or fully connected layers) to obtain better results. It is normal that, when this happened at first, the time complexity for training, classification, detection, or segmentation was somehow neglected, all works being more focused on better statistical performance (required by diagnostic specialists). As a consequence, the majority of works related to Me detection, segmentation and/or classification systems are based on NNs. Table 3 illustrates the most used NNs in such applications. As we are mostly interested in the usage trend of NNs used in Me diagnosis, this section presents the architecture of the basic NNs widely used in these kinds of applications.
Family of NNs used for Me diagnosis used in references.
| NN family | Representatives | References |
|---|---|---|
| ResNet | ResNet 34, ResNet 50, SEResNet 50, ResNet 101, ResNet 152, FCRN | [ , , , , , , , , , , , , , , , ] |
| Inception/GoogLeNet | GoogLeNet (Inception v2), InceptionResNet-v2, Inception v3, Inception v4 | [ , , , , , , , , , , , ] |
| U-Net | U-Net | [ , , , , , , , , , , , , ] |
| GAN | GAN, SPGGAN, DCGAN, DDGAN, LAPGAN, PGAN | [ , , , , , , , , , , ] |
| DenseNet | DenseNet 121, DenseNet 161, DenseNet 169, DenseNet 201 | [ , , , , , , , , , ] |
| AlexNet | AlexNet | [ , , , , , , , ] |
| Xception | Xception | [ , , , , , , ] |
| EfficientNet | EfficientNet, EfficientNetB5, EfficientNetB6 | [ , , , , , , , ] |
| VGG | VGG 16, VGG 19 | [ , , , , , , , ] |
| NASNet | NASNet, NASNet-Large | [ , , , ] |
| MobileNet | MobileNet, MobileNet2 | [ , , , ] |
| YOLO | YOLO v3, YOLO v4, YOLO v5 | [ , , ] |
| FrNet | FrNet | [ ] |
| Mask R_CNN | Mask R_CNN | [ ] |
Following the investigation of the Web of Science DB between the years 2018 and 2020 ( Figure 7 ), it can be found that the most used NN in the detection of Me were those in the family ResNet, followed by the families: VGG, GoogLeNet, and AlexNet. For the year 2021, the tendency is for ResNet and VGG networks ( Figure 8 ). Figure 7 marks the number of appearances in the years 2018, 2019, and 2020, and Figure 8 the percentage of appearances in 2021 (unfinished year).

Frequently NNs used in Me detection between 2018 and 2020.

The most used NNs for Me detection in 2021 (percentage).
4.1. AlexNet
AlexNet [ 93 ] is one of the first CNNs widely used in SL classification tasks via TL. The basic architecture ( Figure 9 ) is composed of eight layers, out of which five are convolutional layers (Conv) and three are fully connected layers (FC). The first and second layers are followed by Max Pooling layers (MPX) and Local Response Normalization (LRN), while the third, fourth, and fifth are followed by ReLU (Rectified Linear Units) [ 94 ]. The last layer (Softmax layer) has 1000 neurons and is used for the classification task (1000 classes). The number of layers specified in the above architecture is not what makes AlexNet special. AlexNet replaced the Tanh function with ReLU for speed enhancement in terms of training time. In Figure 9 , at each layer, the number of neurons is specified.

AlexNet basic architecture.
For example, in 2018, the authors in [ 45 ] trained AlexNet using TL, together with three other architectures: GoogLeNet, ResNet, and VGGNet to achieve a better ACC in such classification tasks. By training AlexNet to classify SL, the authors obtained an average ACC of about 85%. Other research papers such as: [ 12 , 73 , 74 ] used the trained AlexNet for SL diagnosis.
4.2. GoogLeNet/Inception
GoogLeNet, also named Inception v1, is a CNN proposed by researchers at Google in 2014 [ 95 ]. Its architecture was the winner of the ILSVRC 2014 image classification challenge (ImageNet Large Scale Visual Recognition Challenge 2014) and performed better in terms of error rate compared with previous winners: AlexNet in 2012 and ZG-Net in 2013. New features of GoogLeNet are the following: 1 × 1 convolution, global average pooling, an Inception module, and an auxiliary classifier for training. The 1 × 1 convolution blocks were introduced to decrease the number of parameters in general (weights and biases), which of course led to a depth increase of the architecture. The network’s basic block is the Inception module, where 1 × 1, 3 × 3, 5 × 5 convolutions, and 3 × 3 Max Pooling blocks perform in parallel. The outputs of these blocks are concatenated and fed to the next layer. The Inception module was introduced since different convolutions blocks of different sizes handle objects better at multiple scales. Figure 10 illustrates the components of the Inception module used in GoogLeNet.

Inception module used in GoogLeNet.
A simplified architecture of GoogLeNet is 22 layers deep ( Figure 11 ). The network takes a color image (RGB) of size 224 × 224 pixels as input and provides the classification result (out of 1000 classes) as output, using a Softmax layer of 1000 neurons. Another important aspect to mention is that all convolutions inside the architecture use ReLU as an activation function.

GoogleNet architecture’s simplified block diagram.
For example, the authors in [ 45 ] used the first version of GoogLeNet (Inception v1) as the basic CNN from which they started TL for SL diagnosis. Additionally, the authors in [ 5 ] (published in 2020) trained Goog-LeNet for the Me classification task, which shows that this architecture added a lot of value with its newly introduced features. Recently, a series of published SL diagnosis systems used newer versions of GoogLeNet. For instance [ 43 ] related to Me and the nevus SL classification task uses the Inception v3 NN [ 96 ] with 42 layers deep. Figure 12 illustrates the overall architecture of the Inception v3 network.

Inception v3 basic architecture.
An important observation is that Batch Norm (Batch Normalization) and ReLU blocks are used after each convolution. The basic idea of Inception v3 NN and what makes it more special than the first version (GoogLeNet—Inception v1) is to reduce the number of connections/parameters without decreasing the network efficiency. This is one of the reasons why researchers also investigate the performance of this CNN in their applications. Inception v3 uses “Factorizing convolutions” by replacing the 5 × 5 convolution filter represented in Figure 10 with two convolution filters 3 × 3. This procedure reduces the number of parameters from 25 to 18. The same technique was also used in VGG Net [ 97 ]. Another important novelty introduced by Inception v3 is related to factorization into asymmetric convolutions which means that a 3 × 3 convolution filter will be replaced by one 3 × 1 convolution filter followed by one 1 × 3 convolution filter.
4.3. VGG Networks
VGG is a NN family with the first representative VGG 16, which is widely used in SL diagnosis. VGG16 [ 98 ] is slightly similar to, but larger, than AlexNet, being 16 layers deep and containing only small 3 × 3 convolution filters ( Figure 13 ). For instance, the authors in [ 45 , 47 , 53 ] used a TL technique to train VGG 16 to achieve SL diagnosis.

VGG 16 network architecture [ 98 ].
VGG 16 model achieves a 92.7% top-5 test ACC in ImageNet BD (14 million images belonging to 1000 classes) and was the winner of ILSVRC-2014. With this model, an improvement can be seen over AlexNet, since it replaces large filters such as 11 × 11 and 5 × 5 with multiple smaller 3 × 3 filters, making the network deeper (ascending trend for obtaining a better ACC). The same behavior of “Factorizing Convolutions” was also used in GoogLeNet Inception v3.
VGG 19, shown in Figure 14 [ 98 ], is another VGG network used in SL (especially Me) diagnostic research papers in the literature. This time, the model becomes deeper (19 layers, out of which 3 are fully connected layers). According to our survey, examples of paper works related to SL diagnosis are [ 43 , 47 ]. Both papers mentioned as examples were published in 2020 and represent comparative studies between multiple networks to find the most accurate and precise ones for SL diagnosis tasks. What can also be noticed is that, in terms of compared networks, apart from VGG 16 and VGG 19, other deeper networks such as ResNet-50 (50 layers deep) and DenseNet-201 (201 layers deep) are involved. This means that the trend in using NNs for SLs diagnosis is to use deeper networks to achieve better ACC and precision. Of course, this can lead to more and more network parameters and large computation time in terms of the learning task, which will continue to be a subject of research.

VGG 19 network architecture [ 98 ].
4.4. ResNet
As we mentioned in the previous sections, the general trend for segmentation, detection, and classification tasks is to use deeper NNs. However, it was demonstrated that, as we go deeper with more and more layers with “plain” networks, the training error will start to increase over time. Therefore, very deep NNs are in general hard to train because of vanishing and exploding gradients kind of problems. To avoid this issue, researchers introduced “skip connections” in the networks which allow them to take the activation from one layer and feed it to another layer, even much deeper in the NN. This allows building “Residual” networks, instead of “Plain” networks, thus building very deep NNs (over hundreds of layers deep). The newly introduced “Residual” network [ 99 ] solves the problem of the vanishing gradient in deep NNs by allowing the shortcut presented in Figure 15 . In this way, the gradient can flow through. With this new feature, ResNet won first place in the ILSVRC 2015 competition with an error rate of 3.57%. It also won the COCO 2015 competition for detection and segmentation problems.

Residual block.
According to our search related to SLs diagnosis ( Table 3 ), in terms of the ResNet family, the most used NNs for detection, segmentation, and classification task, are ResNet-34, ResNet-50, ResNet-101, and ResNet-152. As can be seen in Figure 16 , ResNet-152 is a 152-layer-deep CNN composed of residual blocks which solve the vanishing gradient issue when training deep NNs. An example of an SL diagnosis paper that uses ResNet-34 is [ 47 ]. Another residual network used in SL diagnosis tasks is ResNet-50 (50 layers deep), which was used for instance in [ 41 , 43 , 47 , 50 ], all published in 2020. A residual network 101 layers deep, used in SL diagnosis, is ResNet-101 ([ 5 , 42 , 43 , 50 ], all published in 2020). Of course, there are also other studies, such as [ 38 , 39 , 48 , 96 ], that use a deeper “residual” network (representing the trend of using more deeper networks for better ACC) called ResNet-152 (152 layers deep).

ResNet-152 basic architecture.
4.5. YOLO Networks
YOLO (You Only Look Once) is a CNN widely used in real-time object detection tasks and commonly used network in Me detection papers (usually YOLO v3 and YOLO v4). According to [ 100 ], YOLO is a “new approach to object detection” by using a single NN to “predict bounding boxes and class probabilities directly from full images in one evaluation”. YOLO is composed of 24 convolutional layers followed by two fully connected layers which were pre-trained on ImageNet DB, similarly to other commonly used networks. As can be seen in Figure 17 [ 101 ], the network contains some alternating 1 × 1 convolution filters which are mainly used to reduce the features space from the preceding layers. This looks similar to what GoogLeNe—Inception v3 introduced. There are multiple versions of YOLO, out of which, according to our research, the most used CNNs for Me detection tasks are YOLO v3 and YOLO v4 [ 88 ].

YOLO v3 architecture [ 101 ].
YOLO v3 is an incremental improvement of the previous YOLO v2 which was based on DarkNet-19 network. According to the authors in [ 102 ], the network is bigger than YOLO v2, with increased ACC, and is fast enough. The authors proposed a hybrid approach between DarkNet-19 and a residual network (inspired from ResNet). The new architecture is based on 53 convolutional layers called DarkNet-53. As we already mentioned, YOLO v3 is used in Me detection tasks. For instance, the authors in [ 89 , 90 ] used YOLO v3 for benign/malignant Me or seborrheic keratosis detection. There is also the YOLO v4 version with an increasing speed, used in Me detection and segmentation [ 88 ].
4.6. Xception Network
Xception is another CNN used in SL diagnosis tasks. For instance, new related papers are [ 42 , 43 ], both being published in 2020. According to [ 103 ], this network was inspired by GoogLeNet Inception NN also developed by Google researchers and was meant to obtain better performance by replacing the standard Inception modules with depthwise separable convolutions. The Xception architecture ( Figure 18 ), which outperforms Inception v3, contains 36 convolutional layers structured in 14 modules, all with residual connections around them [ 104 ].

Xception network architecture.
4.7. MobileNet
MobileNet is a type of NN designed for mobile and embedded vision applications [ 105 ]. Since this CNN is deployed on mobile devices, memory usage should be taken seriously into consideration. Therefore, to decrease the complexity and to reduce the model size, the architecture is based on depthwise separable convolution blocks, as in the case of Xception NN described in the previous section.
There are multiple versions of MobileNet, out of which, according to this research, MobileNet-v1 and MobileNet-v2 are the most used in SL diagnosis papers. For instance [ 43 , 47 ], both published in 2020, use MobileNet-v1; meanwhile, newer papers such as [ 87 ] use MobileNet-v2 (deeper and improved version of MobileNet-v1) in such applications.
As we already mentioned, MobileNet NN reduces the complexity and number of network parameters using depthwise separable convolutions (1 × 1 convolution applied on each of the RGB channels). However, it also uses pointwise convolution with a 1 × 1 kernel (depth equal to the number of channels of the image) which iterates through every single point. To this end, MobileNet-v1 uses 13 blocks composed of depthwise separable convolution and pointwise convolution. However, researchers were focused on obtaining better results. Therefore, MobileNet-v2 came about as an improved version of MobileNet-v1. The first important change was marked by the fact that the network is now composed of 17 bottleneck blocks, each of them containing an expansion module, a depthwise separable convolution, and a pointwise convolution. The expansion block was introduced to increase the size of the representation within the bottleneck block to allow the NN to learn a richer function. The pointwise convolution will then “down” project the data so that they reach the initial size. Another important issue introduced in MobileNet-v2 is the residual connections around the bottleneck blocks, to solve the “vanishing gradient” problem, as in the case of ResNet. Of course, both versions end with a Max Pooling layer, followed by Fully Connected layers, and finally followed by a Softmax layer.
4.8. EfficientNet
As we have already mentioned in previous sections, researchers tend to obtain better results in terms of ACC and other performance metrics. For this to happen, the trend is to design deeper CNNs. For example, ResNet can be scaled up to ResNet 200 by increasing the number of layers. Authors in [ 106 ] propose a novel model scaling approach that uses compound coefficients to scale up CNNs in a more structured manner. This method uniformly scales each dimension with a fixed set of scaling coefficients. The authors also demonstrated the effectiveness of the proposed method on scaling up MobileNets and ResNets. In the same paper, they also build different versions of EfficientNet (EfficientNet B0–B7), all of them with better ACC than the networks with which they were compared. Another example of a recent paper [ 47 ] used EfficientNet to improve ACC for pigmented SL classification. The architecture ( Figure 19 ) is based on MBConv blocks (inverted residual blocks), originally applied on MobileNet-v2 [ 107 ].

EfficientNet architecture [ 107 ].
4.9. DenseNet
DenseNet is a CNN family often used in SL diagnosis. Examples of papers using DenseNet (especially DenseNet-201) are ([ 1 , 41 , 47 ], all of them published in 2020). Therefore, DenseNet represents a trend for recently published papers because of its efficiency and better ACC. The reason is that, in the initial paper [ 108 ], the authors introduced densely connected layers, thus modifying the standard CNN architecture as in Figure 20 . In DenseNet, each layer is fed with additional inputs from all preceding layers and provides its own input/feature map to all subsequent layers. In this way, each layer obtains knowledge from previous layers. Therefore, it is obvious that this becomes more powerful than ResNet, obtaining a stronger gradient flow, more diversified features, and a smaller network size. DenseNet-121, DenseNet-169, DenseNet-201, and DenseNet-264 are DenseNet networks presented in different works.

Five-layer DenseNet architecture [ 108 ].
4.10. U-Net
Recent papers such as [ 58 ] used U-Net CNN for SL segmentation. As can be seen in Figure 20 , U-Net has a “U” form, being composed of 23 convolutional layers. After each max pooling operation, the number of feature channels is increased by the previous number of feature channels, multiplied by two. The number of channels is increased until it reaches 1024 and then starts to decrease (dividing by 2, after each 2 × 2 up-conv block). This architecture contains four sections: the encoder, the bottleneck, the decoder, and the skip connections ( Figure 21 ). The bottleneck layer is a section between the down-sampling path (encoder) and up-sampling path (decoder), containing the smallest size of the feature map and the biggest number of filters. The skip connections are between the corresponding blocks of the encoder and decoder.

U-Net architecture [ 110 ].
According to the original paper [ 109 ], U-Net achieved very good performance on very different biomedical segmentation applications. This is one of the important reasons why researchers tend to use it in Me detection and segmentation-related papers.
4.11. Generative Adversarial Network
The Generative Adversarial Network (GAN) is another type of artificial NN that was used in the design of Me and SL diagnosis and segmentation systems. The GAN is composed of two different networks (main blocks), as can be seen in Figure 22 . The first one is the generator network which learns how to generate real-like data, while the second one is a discriminator network which learns how to detect fake data and not to classify them as real data. Both networks are competing and playing an adversarial zero-sum game [ 111 ]. The main blocks try to optimize objective functions. GAN was proposed for image synthesis tasks. Starting from this idea, the GAN is used in melanoma segmentation as a generative model based on supervised learning.

GAN standard network architecture.
According to our research, examples of research papers in this domain are [ 64 , 65 , 66 , 112 ], all of them proposing modified variants of GANs, such as SPGGAN (Self-attention Progressive Growing of Generative Adversarial Network), DCGAN (Deep Convolutional Generative Adversarial Network), DDGAN (Deeply Discriminated Generative Adversarial Network), LAPGAN (Laplacian Generative Adversarial Network), etc. There were other research papers involving the combination of GANs with other CNNs, such as Xception, Inception v3, etc. One example is [ 52 ], which presents an ensemble strategy of group decision for an accurate diagnosis.
5. Current Trends in Designing Skin Lesions Diagnosis Systems
As we mentioned earlier SL and, especially, Me are frequent and dangerous diseases. Simple and early detection might represent an important aspect for treating Me. That is why researchers are still looking for new, more effective methods for the early detection of melanoma. Therefore, this study tries to concentrate on the trends in designing systems dedicated to SL/Me detection, segmentation, and classification. Most of the recent papers are based on NNs but there are also other studies based on classic classifiers such as KNN and SVM. In terms of new trends of using NNs for detection, segmentation, and classification of Me, we noticed the following: systems using one single CNN most often modified and adapted for Me, systems using multiple CNNs, and systems using CNN combined with other classifiers. Figure 23 illustrates the percentage of research papers per year, between 2017 and 2021 which had the highest impact in terms of designing future such systems. It can be seen that most of the important papers related to SL/Me detection, segmentation, and classification were released in 2020. The year 2021 is also promising, since, even if it is not over, with an indexing delay, it captured our attention in terms of importance.

Percent of research papers per year with the highest impact for the new trends in Me detection by NN.
It can be observed the fact that almost all important CNNs were learned with TL techniques using different DSs already mentioned in previous chapters. There were also some studies in which the authors designed their own CNNs or modified existing ones. Our review shows the fact that researchers in this domain were interested in almost all important CNNs including the state-of-the-art: AlexNet, GoogLeNet, VGG, ResNet, Xception, U-Net, DenseNet, MobileNet, YOLO, different types of GANs, and others.
The trend is that researchers, similar to the case of other domains, experimented with different small CNNs and then transited to more complex and deep ones; for example, a transition to ResNet and to other networks using residual connections to improve performance-related indexes.
As we already mentioned, such systems are designed using:
- One CNN, most often modified and using TL technique;
- Multiple CNNs (combined CNN by data fusion into a global classifier);
- One or multiple CNNs combined with other classifiers;
- Other techniques/classifiers.
5.1. Melanoma Detection Using One Convolutional Neural Network
Many papers present Me and other SL detection systems designed using only one CNN. Most of them studied different CNNs to compare the obtained results and then to select the one matching the best performances. For instance, in [ 45 ] a DL-based approach for SL classification via different individual CNN architectures such as AlexNet, GoogLeNet, VGG, and ResNet, on ISIC 2017 DS is illustrated.
An example of a support system based on NN to help physicians improve their results in categorizing the seven most common pigmented SLs is described in [ 47 ]. The paper compares eight deep NNs (VGG16, VGG 19, ResNet 34, ResNet 50, SEResNet 50 (Squeeze-and-Excitation ResNet 50), ResNet 101, EfficientNet B5, and MobileNet) in different training conditions, using images randomly taken from ISIC and HAM1000 DSs. The authors in [ 113 ] proposed a method for automated Me detection and segmentation using a modified deep regional convolutional NN to reduce the investigation area and the fuzzy C means algorithm for precise segmentation. The dermoscopic images were from ISIC 2016 DS.
Existing networks modified to achieve a more accurate one are presented in [ 114 ]. The authors proposed a modified U-Net version. This new structure took advantage by combining DenseNet and ResNet to improve the performance of U-Net in SL segmentation. The convolutional layers of the encoder are intercalated with context modules containing dense connections. These modules are residual blocks. Similarly, the up-sampling layers of the decoder are intercalated with Localize modules. The new skip connection between the decoder and encoder is named the Dense Skip Connection.
A new trend in designing such systems with more accurate results in SL detection is represented by 3D CNN. For example, the authors in [ 115 ] proposed a 3D fully CNN named Hyper-net to achieve a more accurate segmentation of Me from hyperspectral pathology images. The hyperspectral images, as input for Hyper-net, are represented by cubes of size 256 × 256 × 16. The authors combine the dilated convolution for multi-scale features with the standard convolution. Between encoder and decoder blocks there is a fusion path. The output of the decoder is a 3D cube with the same size as the input 3D cube. To enhance the training efficiency, residual learning was inspired by V-net [ 116 ].
Another new direction in the use of NN for Me detection is the preprocessing tasks. An example of such a network can be seen in [ 117 ], a recently published paper that proposed an encoder–decoder CNN for hair removal ( Figure 24 ).

The schematic architecture of the proposed system for hair removal from skin lesion images from [ 117 ].
5.2. Melanoma Detection Using Multiple Convolutional Neural Networks (Combined)
Combining multiple networks into a complex system can lead to improved SL detection and classification performances. From this point of view, we distinguish two tendencies: (i) the use of several networks, separately, for different functions (detection, segmentation, and classification), either in cascade or in parallel, and (ii) the use of several networks for the same function and the combination of individual decisions, by fusion, for the final decision. For instance, the authors in [ 50 ] provided a solution for precise SL analysis by proposing a multi-task DL framework based on a Feature Pyramid Network (FPN), Region Proposal Network (RPN), and three subnets (for classification, detection, and segmentation). The subnets are fed with the outcomes from FPN and RPN (determining the RoI) and they run in parallel to obtain a combined and more precise result for skin lesions analysis and prediction. The framework is based on the design of a loss function based on focal loss (RPN loss function) and the Jaccard distance to solve the SL classes imbalance issue for the image DS. The ISIC 2016 and ISIC 2017 challenge DS were used.
The new diagnosis system presented in [ 38 ] is a solution for Me detection based on DL techniques. The system contains two main modules: (RoI detection using Mask R_CNN and RoI classification, using TL for ResNet152 which was previously trained with ImageNet DB).
Two CNNs are also combined in [ 1 ] to perform an accurate classification (95% ACC) of SL. As can be seen in Figure 25 , the image containing an SL flows through the first CNN (encoder–decoder type) designed for segmentation purposes, and afterwards, the segmented SL is considered as the input in the next CNN composed of merged dense blocks, for classification. The experiments were conducted using the HAM10000 DS.

The architecture of the proposed system for skin lesion classification [ 1 ].
A similar system design was proposed in [ 41 ], a recent paper proposing a combination of two modules: a segmentation module used as a pre-requisite and a classification module. The difference here is that, first, the authors experimented with multiple DSs such as ISIC 2016, ISIC 2017, and ISIC 2018, and second, multiple CNNs were involved and analyses were conducted for each of them. The SL segmentation is performed by FrCN and the classification is performed by the following NN: Inception v3, ResNet 50, Inception-ResNet v2, and DenseNet 201. Case studies of two, three, and seven classes are considered.
The authors in [ 42 ] proposed a generalized architecture for multi-class classification of skin cancer. This paper covers five convolutional NN architectures for different experiments, such as: Xception, NASNet-Large, Inception-RestNet v2, Inception v3, ResNeXt101 and the ensembles: Inception-ResNet v2 + Xception, Inception v3 + Xception, Inception-ResNet v2 + ResNetXt 101 + Xception and Inception-ResNet v2 + ResNetXt 101. Experiments were performed using the HAM10000 DS and the best ACC was obtained for ResNetXt 101 + Inception-ResNet v2 (92.83%).
Creating a complex multi-network system based on the fusion of decisions of individual NNs with the help of a final NN can increase the detection performance of Me. For example, the authors in [ 5 ] proposed a Me detection system characterized by the following new aspects: (a) use of multiple CNN as individual classifiers, (b) use of a hybrid structure which makes a decision fusion between four CNN-based classifiers and a classifier based on texture features, (c) use of another CNN considered as a global classifier having as input the probabilities of individual classifiers (considered as weights). The CNNs used were: a custom NN, GoogLeNet, ResNet-101, NasNet-Large, and a Perceptron ( Figure 26 ).

Multi-network system architecture based on decision fusion for Me detection [ 5 ].
Similar to the authors of [ 5 ], the authors in [ 45 ] proposed an ensemble of CNNs (GoogLeNet, AlexNet, ResNet, and VGGNet) for SL classification based on the CNN output interpretation, demonstrating that it is a meaningful approach.
A complex system for Me and SL diagnosis based on multi-CNN and a voting scheme is proposed in [ 52 ]. As seen in Figure 27 , the classification system is based on multiple sub-modules, each of them voting a single dermoscopic image and providing a value. The maximum value is then compared with a threshold. In case it is smaller than the threshold, the classification will be performed by a group decision conducted by a large module (Vote) composed of other CNNs. Thus, a final, more accurate decision related to the classification is obtained.

Ensemble strategy of the group decision [ 52 ].
Through the appropriate use of several NNs, it is possible to move from subjective classification decisions of individual networks to a decision considered more objective of the global classifier also represented by an NN [ 6 ]. In this specific research paper, as can be seen in Figure 28 , the authors proposed a system based on a decision taken from multiple NNs. This system is based on six classifiers (NN-based) connected to two operational levels. The first level contains five subjective (individual) classifiers, while in the second level, there is a Perceptron-type classifier that decides whether the final decision is a Me or not. The final decision is based on the learning-adjusted weights from the first level. In the learning phase, a weight is assigned to each subjective classifier, according to the classification accuracy. In the testing phase, the outputs of these classifiers are the probabilities offered. The convolution law of the final classifier is made up of the weights and probabilities of the subjective classifiers. It is considered that the final classifier is an objective one. The subjective classifiers are the following: (a) two NN namely ResNet 101 and AlexNet, (b) two perceptrons having as inputs LBP histogram and HOG, respectively, and (c) an ABCD-based classifier with a GAN for primary segmentation.

The architecture of the Me classification system proposed in [ 6 ], based on several NNs connected on two levels of classification.
5.3. Systems Designed Using Convolutional Neural Networks Combined with Other Classifiers/Techniques
As we mentioned earlier, there are SL diagnosis systems designed using CNNs combined with other techniques/classifiers to obtain better results in terms of statistic performance-related indexes. An example of this is [ 118 ], which aims to provide two solutions (benign/malignant) for a precise and optimum classification of SL by proposing two corresponding systems. The architecture of both systems specifies a lesion segmentation block based on HLPSO (Hybrid Learning Particle Swarm Optimization) and modified K-means as a common initial block. The first system is then composed of other two blocks: feature selection block (based on HLPSO, KIRSCH, and SLBP) and SL classification block (based on KNN and SVM). The second system is an adaptive one, based on evolving DCNN (CNN driven by HLPSO for parameters/hyper-parameters optimizations).
Another example of such a diagnosis system is [ 90 ], which aims to provide a solution for the SL detection and segmentation system based on YOLO v3 for SL detection and GrabCut algorithm for accurate segmentation. YOLO v3 was chosen for the detection part since it already proved to be much faster and has better precision and accuracy in detection than other methods such as RCNN (region-based convolutional neural network)/Fast-RCNN/Faster-RCNN. The system being proposed also contains a preprocessing module able to process the image (e.g., hair removal) before the SL detection phase and the segmentation phase.
The authors in [ 119 ] aim to provide a solution for designing a Me classification system based on CNN and a custom new regularizer for controlling the complexity of the classifier and thus making it more accurate. The results are indeed more accurate and precise when compared with other works from the existing literature. Similarly, another research paper [ 59 ] proposed a Me diagnosis system based on a combination between CNN and intelligent classifiers based on texture features. As seen in Figure 29 , this paper presents an architecture based on a segmentation block using U-Net, a feature extraction block using a color feature, HOG, LBP, and a classification block using RF (random forest), SVM, KNN, and NB (Naive Bayes).

The schematic architecture of the skin lesion classification system based on CNN for the segmentation, feature extraction, and intelligent classification [ 59 ].
The authors in [ 43 ] proposed a new solution for SL classification system based on handcrafted features (color, texture, etc.) fused with features learned by TL on pre-trained CNNs such as VGG 16, VGG 19, MobileNet, ResNet 50, Inception v3, Xception, DenseNet 201, MobileNet v1, and MobileNet v2. The fusion block identifies the most important features and passes them to the classification block which is based on Linear Regression, SVM, and a Relevant Vector Machine. Experiments on the system proposed in Figure 30 were conducted on ISIC 2018 DS and performance results were analyzed for each mentioned CNN. The best results were obtained by the one using MobileNet v2 (about 90% ACC in the testing phase).

The architecture of the SL classification system proposed in [ 43 ].
In terms of Me detection and segmentation systems, a recent important paper is considered [ 88 ], where YOLO v4 was used for Me detection and an Active Contour Segmentation approach for Me segmentation. As DSs, ISIC 2016 and 2018 were used. It can be seen that recent papers aim to use real-time object detection methods such as YOLO v3 and YOLO v4 to achieve Me detection.
A synthesis of the characteristics of the most important papers regarding the trends of using NN in Me and SL detection is presented in Table 4 .
Synthesis of the most important papers regarding the trends of using NN in Me and SL detection.
| Ref/ Year | Goal/Novelty | Description | NN Type/Function | Data Set | Me or SL + Me | Data Aug. | Performance Indicators (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| ACC | F1 | IoU | |||||||
| [ ]/ 2018 | DL-based approach for SL classification via the fusion of different individual CNN architectures. | Ensemble of CNNs with different fusion-based methods and selection of the best performing one. | GoogLeNet, Alexnet, ResNet, VGGNet/ classification | ISIC 2017 | SL + Me | Yes | 90.30 | NA | NA |
| [ ]/ 2019 | Pipeline architecture for SL segmentation, combining YOLO v3 and the GrabCut algorithm. | Combining YOLO v3 and the GrabCut Algorithm for SL segmentation. | YOLOv3/ detection and segmentation | PH2, ISIC 2017 | SL + Me | NA | 92.99 to 97.00 | 84.26 to 88.13 | 74.81 to 79.54 |
| [ ]/ 2019 | A DL method is proposed for automated Me detection and segmentation using dermoscopic images. | Skin refinement, localization of Me region, and, finally, segmentation of Me (fuzzy C means). | Deep region-CNN/detection and segmentation | ISIC 2016 | Me | NA | 94.80 | 95.89 | 93.00 |
| [ ]/ 2019 | New FCNN architecture for SL segmentation—DermoNet. | FCNN contains densely connected convolutional blocks and skip connections. | FCNN—DermoNet/ segmentation | PH2, ISIC 2016, ISIC 2017 | SL + Me | Yes | NA | 89.40 to91.50 | 82.50 to 85.30 |
| [ ]/ 2019 | Model enhanced by employing a multi-stage segmentation approach. | FCNN based on U-Net with batch normalization. | FCNN/ segmentation | ISIC 2018 | SL + Me | Yes | NA | 90.00 | 83.00 |
| [ ]/ 2019 | Encoder–decoder structure with an intermediate module (attention module). | The architecture contains three modules: the encoder that extracts features from a raw image; the decoder that generates the SL classes; the attention module for guiding the decoder to attend at different locations. | Encoder–Decoder | ISIC 2017 | SL + Me | NA | 72.3 | NA | NA |
| [ ]/ 2020 | New deep CNN-based model for face skin disease classification using a triplet loss function. | Fine-tuning layers of ResNet152 and InceptionResNet-v2. | ResNet152, Inception ResNet-v2/classification | From a hospital in Wuhan China | SL + Me | NA | 87.42 | NA | NA |
| [ ]/ 2020 | A new method called a “Lesion classifier” is derived from pixel-wise classification. | Encoder–Decoder Network Connected through skip pathways. Softmax modules for output. | Encoder–Decoder/ detection and segmentation | ISIC 2017, PH2 | Me | Yes | 95.00 | 92.00 | NA |
| [ ]/ 2020 | New skin image classification method using multi-tree genetic programming. | Various local and global features are extracted from skin cancer images. The classification method uses genetic programming. | NA/ classification | PH2, Dermofit | SL + Me | NA | 96.42 to 80.64 | NA | NA |
| [ ]/ 2020 | New scheme for Me localization and segmentation using YOLOv4 and active contour segmentation. Detecting multiple Me presented in a single image. | The skin refinement step removes the unnecessary artifacts automatically. A framework consisting of three phases: skin enhancement, Me localization, and Me segmentation. | YOLO v4/ detection and segmentation | ISIC 2016, ISIC 2018 | SL + Me | Yes | 94.00 | 92.00 | 96 |
| [ ]/ 2020 | DL-based CAD system with precise SL boundary segmentation and accurate classification for clinical diagnosis of SL | Cascaded full resolution CNN for segmentation and Inception-v3, ResNet-50, Inception-ResNet-v2, and DenseNet-201 for classification. | DCNN/ segmentation and classification | ISIC 2016, ISIC 2017, ISIC 2018 | SL + Me | Yes | 87.74 to89.28 | 77.84 to 81.28 | NA |
| [ ]/ 2020 | Me detection using an optimized set of Gabor-based features and a fast MNN classifier. | Gabor features combined with a fast (Multi-Level Neural Network) MNN. | MNN/ classification | PH2 | Me | NA | 97.50 | NA | NA |
| [ ]/ 2020 | YOLO v3 algorithm combining with two-phase segmentation based on the graph theory using minimal spanning tree concept and L-type fuzzy-based approximations. | YOLO v3 for Me detection and segmentation based on graph theory. | YOLOv3/ detection and segmentation | PH2, ISIC 2017, ISIC 2019 | Me | NA | 93.38–97.50 | 87.89–93.97 | 79.84–88.64 |
| [ ]/ 2020 | Fusing method that employs relevant mutual information obtained from handcraft and DL features obtained from DCNN. | ABCD rule combining with DCNN features employing mutual information measurements. | VGG-16, VGG-19, MobileNet v1, ResNet-50, Inception v3, Xception, DenseNet-201/ classification | HAM10000 | SL + Me | Yes | 92.40 | 90.00 | NA |
| [ ]/ 2020 | Integration of different NNs into a global fusion-based decision system. For the fusion weights, there are used the results, obtained by each NN. | A global classifier is implemented considering individual classifiers as the proposed NNs. The global classifier used partial decision fusion. | CNN, GoogLeNet, ResNet101, NasNet-Large, Perceptron/ classification | PH2, ISIC 2019 | SL + Me | Yes | 88.33 to 93.33 | 86.79 to 92.31 | NA |
| [ ]/ 2020 | Optimal CNN to predict skin cancer. | A new technique of using an improved whale optimization algorithm for optimizing the structure of CNN for skin cancer detection. | Optimized CNN/ detection | Dermquest, DermIS | SL + Me | NA | 95 | NA | NA |
| [ ]/ 2020 | An objective classifier containing five subjective classifiers (two texture-based classifiers with perceptrons and three NNs end-to-end type) for Me detection. | A multi-NN-based system containing six NNs and feature extraction algorithms. The final classifier is also an NN. | Perceptrons coupled with feature extraction, GAN, ResNet, AlexNet/ segmentation, and classification | PH2, ISIC 2019 | Me | Yes | 97.50 | 97.40 | NA |
| [ ]/ 2020 | Establishing how DL frameworks trained in large DSs can help non-dermatologists improve their performance in categorizing pigmented SL. | The performances of eight DCNNs are compared in different training conditions. | VGG16, VGG19, ResNet34, 50, 101 SEResNet50, EfficientNetB5, MobileNet/ classification | HAM10000 | SL + Me | NA | 75.73 to 84.73 | NA | NA |
| [ ]/ 2020 | New CNN architecture for SL segmentation, with an attention mechanism and high-resolution feature maps. | Proposed CNN with K consecutive HRFB (high-resolution feature block) for SL segmentation with more accurate SL boundaries. | CNN with HRFB/ segmentation | PH2, ISIC 2016, ISIC 2017 | SL + Me | Yes | 93.80 to 94.90 | 86.20 to91.90 | 78.30 to 85.80 |
| [ ]/ 2020 | Improved U-Net for SL segmentation. | The architecture is proposed with a modified U-Net, in which a bilinear interpolation method is used for up-sampling with a block of convolution layers followed by parametric ReLU. | U-net/ segmentation | NA | SL + Me | Yes | 94.00 | 88.00 | NA |
| [ ]/ 2020 | A variant of the particle swarm optimization algorithm, HLPSO, for SL segmentation and classification. | Combining HLPSO with DCNN and a K-Means clustering algorithm. | DCNN/ classification and segmentation | ISIC 2017 | SL + Me | NA | 91.37 | NA | 73.15 |
| [ ]/ 2020 | Global-Part CNN, considering the local information and global information with equal importance. | Ensemble of two CNNs for local and global information, based on data fusion. | Ensemble of two CNN/ classification | ISIC 2016, ISIC 2017 | SL + Me | Yes | 85.70 to 92.50 | NA | NA |
| [ ]/ 2021 | New model, ASCU-Net (Attention Gate, Spatial and Channel Attention U-Net) using convolutional block attention modules for SL segmentation. | Due to the attention module, ASCU-Net accelerates the learning phase. | ASCU-Net based on U-Net and triple attention mechanism/ segmentation | PH2, ISIC 2016, ISIC 2017 | SL + Me | Yes | 95.40 | 90.80 | 84.50 |
| [ ]/ 2021 | Design of a new DCNN model with multiple filter sizes—Classification of Skin Lesions Network (CSLNet). | Fewer filters, parameters, and layers to improve SL classification performances. | DCNN (CSLNet)/ classification | ISIC 2017, ISCI 2018, ISIC 2019 | SL + Me | Yes | 89.58 to93.25 | 89.75 to 93.47 | 81.50 to 88.20 |
| [ ]/ 2021 | New NN based on Efficient-B5. | A deeper, wider and higher resolution NN for Me classification based on fine-grained feature representations. | Efficient-B5/ classification | ISIC 2020 | Me | NA | NA | NA | NA |
| [ ]/ 2021 | Testing different NN for recognition of pigmented SL | Testing different NN for recognition of pigmented SL | ResNet50, DenseNet121, VGG16/ classification | ISIC, HAM10000,PH2, BCN20000, SKINL2 | SL + Me | Yes | NA | NA | NA |
| [ ]/ 2021 | An extensive analysis of twelve CNN architectures and eleven public images DBs. | An extensive analysis of twelve CNN architectures and eleven public image DBs for automatic Me automatic diagnosis. | DenseNet121, 169, 201, Inceptionv3, v4, ResNet50, InceptionResNet v2, Xception, VGG16, 19, Mo-bileNet, and NASNetMobile/detection | PH2, ISIC 2016, ISIC 2017, HAM10000, MED-NODE, MSK1, 2, 3, 4, UDA 1, 2. | Me | Yes | NA | NA | NA |
| [ ]/ 2021 | Combining the MobileNetV2 with the Spiking Neural Network (SNN) into a DCNN for the classification. | Three NNs connected into an intelligent decision support system for skin cancer detection. | Autoencoder, MobileNetv2, SNN/ classification | ISIC | Me | Yes | 95.27 | NA | NA |
| [ ]/ 2021 | New and efficient adaptive dual attention module (ADAM) for automated skin lesion segmentation. | The proposed ADAM modules are integrated into a dual encoder architecture. | Dual encoder + ADAM/ segmentation | ISIC 2017, ISIC 2018 | SL + Me | Yes | 96.36 | 91.63 | 84.70 |
| [ ]/ 2021 | New Siamese NN and architecture named Tensorial Regression Process to detect SL evolution. | A pair of SL images are compared to detect the possible evolution of SL to Me. To this end, a segmentation loss is incorporated into NN as a regularization term. | Siamese NN/ detection and segmentation | Sydney Melanoma Diagnostic Centre | SL + Me | NA | 74.10 | NA | NA |
| [ ]/ 2021 | SL augmentation DS by StyleGAN and DenseNet201 for classification. | Two NNs are used to improve SL classification: a special GAN for data augmentation and DenseNet 201 for classification with a special strategy of TL | GAN (StyleGAN). DenseNet201/ classification | ISIC 2018, ISIC 2019 | SL + Me | Yes | 93.64 | NA | NA |
5.4. Systems Designed Using Other Techniques
Our study of course identified Me and other skin lesion diagnosis systems using other techniques apart from convolutional NNs. An example of such a paper would be [ 33 ], which aims to provide a solution for designing an SL segmentation system based on the Artificial Bee Colony algorithm for obtaining an optimum threshold value for Me detection for the segmentation phase. From an architecture point of view, the system is composed of three modules: the preprocessing (applying median filter), the application of the ABC algorithm for finding the optimum threshold value to be used for Me detection, and the segmentation module.
Another example of such a paper is [ 120 ], which tries to provide a solution to the problem of having multiple small skin lesion DSs (less training data towards the classification of Me) by introducing a TL framework called TrCSVM (Transfer Constituent Support Vector Machine) which can transfer knowledge retrieved from a source training set to multiple target training sets, thus obtaining a more efficient classification model capable of classifying various SLs. This framework is based on FBDA (Feature-Based Domain Adaptation) and uses SVM and TrAdaBoost (Transfer AdaBoost).
6. Discussion
The paper presented the most used techniques based on NN for detection, classification, and segmentation of SL and, especially, Me. The focus was on new trends in such applications. To this end, we analyzed 134 references, most of them from the period 2017–2021. The most performant new systems for Me detection contain multiple DCNNs selected on a performance criterion and grouped either with each other, based on the fusion of decision, or with other classifiers based on texture, shape, and color features. In this way, we move from subjective classifications, specific to individual classifiers (NN), to a more objective classification, that of the global classifier. This classifier considers, according to pre-established criteria, the decisions of the subjective classifiers, but makes its own decision. The individual classifiers should be chosen so that the objective classifier can compensate for possible individual classification errors. Another interesting combination of NN would be the pipeline type, based on jobs; for example, the first network performs primary processing, the second segmentation, and the third classification. There were also implementations of new networks based on the introduction in the structure of a known network, as intermediate modules, smaller networks. The performances obtained depend both on the proposed network solution and on the DS used (including the selected images).
The vast majority of analyzed papers were selected from Web of Science as the most trusted publisher global citation DB. Searches were focused on the following criteria: (a) topics as Me and NNs, (b) new trends (papers between 2017 and 2021), (c) the number of citations, (d) impact factors for journals, and (e) rate of ISI indexing for proceedings papers. We identified eight review or survey papers between 2018 and 2021 [ 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 ]. Table 5 highlights the characteristics of these articles and the differences of our article, marked as positive aspects or contributions.
Recent review/survey papers on similar topics.
| Paper/Year | Description | Period | No. of References | Our Differences |
|---|---|---|---|---|
| [ ]/2018 | A critical and analytical survey of different algorithms for performing segmentation of SL. | 2007–2018 | 29 | New period (2017–2021). Focused on Me and NNs. More references. Focused on new trends (including 2021). |
| [ ]/2018 | Medical (general) image segmentation and classification using CNN. | 2010–2018 | 96 | New period (2017–2021). Focused on Me and NNs. More references. |
| [ ]/2018 | SL classification using CNNs. | 2012–2018 | 33 | New period (2017–2021). Focused on Me and NNs. More references. Focused on new trends (including 2021). |
| [ ]/2019 | Different methods for cancer detection including skin cancers: classical methods (ABCD, different features) and NNs. | 1993–2019 | 167 | A modern approach based on ML and NNs. New period (2017–2021). Focused on Me and NNs. Focused on new trends (including 2021). |
| [ ]/2020 | Investigating: DBs, Me types, DL techniques, reference sources, and index. | 2004–2020 | 95 | Focused on Me and NNs. More references. Focused on new trends (including 2021). |
| [ ]/2020 | Survey of the recent architectures of deep CNNs (general). Analysis of CNN’s internal structures. | 1982–2020 | 253 | Focused on Me and NNs. Systems of multiple NNs and decision fusion as new trends. |
| [ ]/2021 | Methods for detecting skin cancer from SL images. | 2011–2020 | 135 | Focused on Me and NNs. More references. Focused on new trends (including 2021). |
| [ ]/2021 | A systematic review of DL techniques for the early detection of skin cancer. | 1993–2021 | 82 | Focused on Me and NNs. More references. Focused on new trends (including 2021). |
As already mentioned above, over the years, numerous studies have been conducted on this topic. In 2009, Fernandez Alcon et al. [ 18 ] analyzed the SL pigment and performed Me diagnosis with an automatic imaging system proposed by the authors. The detection ACC was improved by combining the classification results with information such as gender, skin type, or the age of the patient. First, the segmentation, background correction, and threshold-based segmentation are analyzed. Then, the ABCD-based method is used to complete the feature extraction step. In the end, pattern recognition is used to perform the classification in Me and non-Me lesions. From the Dermnet dataset (DS), 152 images were used to evaluate the system, from which 107 were Me images and 45 benign SL images. The ACC given by the system was 86% [ 18 ].
In 2011, Capdehourat, G. et al. [ 19 ] proposed an ML-based approach that classifies SL as malignant or benign. In the preprocessing step, the authors used the already well-known Dullrazor algorithm, developed by Lee, T. et al. [ 142 ] to remove the hair present in the lesion. In the segmentation step, the Otsu method, which performs automatic image thresholding, was used with the specification from the authors that this method failed in certain pathological cases. Texture, color, and geometrical features were extracted in the feature extraction step. For the classification step, AdaBoost with C4.5 decision trees was used. According to the authors, this system performance was analyzed by calculating the specificity (77%) and sensitivity (90%). Two years later, Razmjooy, N. [ 143 ] et al. proposed another ML-based system that helps with Me detection. In the preprocessing step, a new algorithm for hair removal is used, other than Dullrazor. As specified by the authors, the hair removal algorithm consists first in applying canny edge detection. Then, a thicken operation, dilatation operation, and addition to the original image are used. The segmentation step is based on morphological operations while, for the feature extraction step, new features, based on asymmetry and irregular border quantification, are applied. An ACC of 95% was given by the Support Vector Machine (SVM) used as a classifier.
The authors in [ 20 ] developed a system based on the same classical method. They preprocessed the images by applying noise removal techniques and used the threshold-based method for image segmentation. ABCD rule and Principal Component Analysis (PCA) are then used to extract the features. The classification made with the help of SVM showed an ACC of 82.2%, a specificity of 86.93%, and a sensitivity of 77%. The evaluation of the system was made on 282 images (133 Me images and 149 benign images) selected from several DSs such as Dermquest, Dermnet, and Dermis.
Starting with 2015, the attention of most researchers turned to DL methods. Codella N. et al. [ 144 ] combined the SVM algorithm with sparse coding and DL techniques to develop a system that reaches a 93.1% ACC in the case of an Me class, an atypical class, and a benign class. As for feature extraction, a pre-trained Caffe CNN (Convolutional Neural Network) was used. The performance evaluation of the system was conducted on the International Skin Imaging Collaboration (ISIC) DS.
The authors in [ 145 ], proposed a system based on two main steps: preprocessing and classification. For the classification, a pre-trained CNN that contains two convolutional layers was used. The system showed an 81% ACC on Me detection.
Pomponiu V. et al. [ 146 ], just like in [ 144 ], developed an SL detection system that used a CNN as a feature extractor showing an ACC of 93.64%, a specificity of 95.18%, and a sensitivity of 92.1%. After the data augmentation was conducted, an AlexNet pre-trained CNN was used to extract the features and a K-nearest neighbor (KNN) algorithm was used for classification.
An NN ensemble method for Me detection was proposed by Xie F. et al. [ 147 ] in 2016. This paper presents a system that primarily has three steps. The first step is the segmentation step that is conducted with the help of a self-generating NN. The second step is the feature extraction step using PCA, followed by the classification step that is performed by using the NN ensemble method. The proposed classifier refers to a combination of Fuzzy NN and backpropagation NN. The performance evaluation on two different DSs provided by a local hospital showed an ACC of 94.17% and a sensitivity of 95%.
Some authors, such as Attia M. et al. [ 148 ] have only addressed the subject of Me segmentation. The authors used CNN and Recurrent NNs (RNN) to develop a high ACC of Me segmentation system. The proposed architecture contains seven convolutional layers that represent the autoencoder part and four recurrent layers. A total of 900 images obtained from ISBI 2016 challenge [ 14 ] were used for evaluating the algorithm. A Jaccard Index of 93% and segmentation ACC of 98% were obtained.
Li Y. et al. [ 149 ] also used DL techniques to detect Me. Two fully CNNs, named the Lesion Feature Network and Lesion Index Network, were used to complete the feature extraction, segmentation, and classification steps. Calculation of the distance heat map is then conducted to improve the detection. For the feature extraction step, a straightforward CNN (Lesion Index Network) was used. The evaluation was performed on ISIC 2017 DS [ 25 ]. In the case of the Lesion Index Network, the obtained ACC for image classification and segmentation was 91.2% while the obtained Jaccard index was 75.3%. Regarding the Lesion Feature Network, the performance was evaluated in terms of sensitivity and precision (69.3% and 42.2%) [ 149 ].
A study for CNN optimization study was performed by Zhang L. et al. [ 35 ] in 2019. The purpose of this study was to improve the training of network weights and biases by applying a meta-heuristic procedure. To minimize the learning error, the authors proposed the whale optimization algorithm. To evaluate the system ACC (91%), Dermrequest and Dermis DSs were used.
DL techniques were also used by Milton [ 25 ], where the following NNs: SENet154, InceptionResNetV2, PNASNet-5-Large, and InceptionV4 were applied, tested, and compared. The third mentioned one showed the best results (76% validation score) when applied on ISIC 2018 DS [ 25 , 28 ].
A more complex system configuration containing a global decision system that integrates the most commonly used DL methods was proposed in [ 5 ]. So, an NN-based method, three CNN-based methods, including NasNet-Large, GoogLeNet, and ResNet-101, and a classical ML-based method were combined to set the fusion weights. The system was evaluated on PH2 [ 22 ] and ISIC 2019 [ 25 , 28 , 150 ] DSs. The best ACC was obtained on PH2 DS (95%), while the ACC obtained on ISIC 2019 was 93%.
7. Conclusions
Neural networks as part of AI algorithms are increasingly being researched in imaging applications as a support system for diagnosing SL and detecting Me. New DBs and even challenges regarding the classification of SL are constantly appearing. That is why there is interest in improving these classifiers for detecting and tracking the evolution of SL even from a distance, with great accuracy. The best results were obtained using multiple NNs for different functions and decision fusion. Observing the tendency for increasing use of neural networks in detecting Me, we can say that this area of interest and the manner of solving problems are objectives of great interest in the integration of artificial intelligence in medicine. The use of NN in the detection of melanoma may be involved in a support system for the dermatologist who ultimately has to decide to either indicate a biopsy if at least one of the dermatologist’s diagnoses and the support system (a helpful method) indicates Me or to investigate if there is another type of cancerous lesion. In the latter case, the system can be taught to detect other types of malignant SLs. However, the system cannot make final decisions on its own. Given the evolutionary trends of neural networks, it is expected that such systems will increase their performance by using improved, adapted, and combined networks. A future direction to follow is the use of these systems to detect Me that develops under the nails, which is currently a more complicated case of diagnosis. We do not know of such an algorithm and we have not found it in the literature. If the nail is still transparent, an image enhancement algorithm can be used to separate the Me from the nail. If the Me has attacked the nail, the network must be learned with the nail.
Abbreviations
| ABCD | Asymmetry Borders-Colors-Dermatoscopic Structures |
| ACC | Accuracy |
| CAD | Computer Aided Diagnosis |
| CNN | Convolutional Neural Network |
| DB | Database |
| DCNN | Deep Convolutional Neural Network |
| DL | Deep Learning |
| DS | Dataset |
| F1 | Dice Coefficient (F1 Score) |
| FCN | Fully Convolutional Network |
| FPN | Feature Pyramid Network |
| HLPSO | Hybrid Learning Particle Swarm Organization |
| ILSVRC | ImageNet Large Scale Visual Recognition Challenge |
| IoU | Intersection-Over-Union, Jaccard Index |
| ISIC | International Skin Imaging Collaborative |
| KNN | K-Nearest Neighbor |
| Me | Melanoma |
| ML | Machine Learning |
| MNN | Multi-level Neural Network |
| NN | Neural Network |
| PCA | Principal Component Analysis |
| PSO | Particle Swarm Optimization |
| RCNN | Deep region based convolutional neural network |
| ReLU | Rectified Linear Units |
| RGB | Red-Green-Blue |
| RPN | Region Proposal Network |
| SL | Skin lesion |
| SVM | Support Vector Machine |
| TL | Transfer learning |
| YOLO | You Only Look Once |
Author Contributions
D.P. conceived the paper and revealed the new trends in melanoma detection. M.E.-K. contributed to artificial neural network investigation. H.E.-K. studied the databases of skin lesions. L.I. selected the references and edited the paper. All authors have read and agreed to the published version of the manuscript.
This research received no external funding. The founding was supported by the University POLITEHNICA of Bucharest.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Melanoma, a malignant tumor arising from melanocytes, is a rare disease, affecting only 22.1 out of 100,000 people in the US (Cancer statistics from the Center for Disease Control and Prevention). However, it is also a very deadly disease accounting for 75% of skin cancer deaths though it only accounts for 4% of skin cancer cases.
Cutaneous melanoma (CM) is a malignant tumor formed from pigment-producing cells called melanocytes. It is one of the most aggressive and fatal forms of skin malignancy. In the last decades, CM's incidence has gradually risen, with 351,880 new cases in 2015. Since the 1960s, its incidence has increased steadily, in 2019, with approximately ...
This paper presents a detailed systematic review of deep learning techniques for the early detection of skin cancer. Research papers published in well-reputed journals, relevant to the topic of skin cancer diagnosis, were analyzed. Research findings are presented in tools, graphs, tables, techniques, and frameworks for better understanding.
There are associations between skin cancers and those viral infectious diseases like acquired immune deficiency syndrome (AIDS). It has been observed that the risk of progression of non-melanoma skin cancer increases 3 to 5 times in AIDS patients [21]. Moreover, it has been documented that the incidence of BCC is 11.4 times more common in HIV ...
Human skin cancer is the most common and potentially life-threatening form of cancer. Melanoma skin cancer, in particular, exhibits a high mortality rate. Early detection is crucial for effective treatment. Traditionally, melanoma is detected through painful and time-consuming biopsies. This research introduces a computer-aided detection technique for early melanoma diagnosis-sis. In this ...
Introduction. In 2020, a grim milestone was reached when the global melanoma incidence passed 300,000 newly diagnosed cases in a single year 1. The most common form of this cancer, cutaneous ...
The melanoma contributes 1.6% of the new cancer cases and 0.6% of deaths due to cancer worldwide 1. As per the American Cancer Society, there is an estimation of 96,480 melanoma related new cases ...
Cutaneous melanoma causes 55 500 deaths annually. The incidence and mortality rates of the disease differ widely across the globe depending on access to early detection and primary care. Once melanoma has spread, this type of cancer rapidly becomes life-threatening. For more than 40 years, few treatment options were available, and clinical trials during that time were all unsuccessful.
Lesion parameters such as symmetry, color, size, shape, etc. are used to detect skin cancer and to distinguish benign skin cancer from melanoma. This paper presents a detailed systematic review of deep learning techniques for the early detection of skin cancer. Research papers published in well-reputed journals, relevant to the topic of skin ...
Melanoma is the most serious type of skin cancer. It develops when skin cells multiply rapidly as a consequence of mutations in their DNA caused by UV exposure. Melanomas originate in the pigment ...
Study Adds to Debate about Screening for Melanoma. Posted: May 4, 2022. Regular skin cancer screening leads to many diagnoses of very early-stage melanomas, results from a new study suggest. The results add to a debate about whether screening is fueling an overdiagnosis of melanoma in the United States. Opdualag Becomes First FDA-Approved ...
The Skin SPORE program's main focus is on melanoma research activities, but it also includes projects in other skin cancer types, such as cutaneous T-cell lymphoma. NCI's National Clinical Trials Network (NCTN) is a collection of organizations and clinicians that coordinates and supports cancer clinical trials at more than 3,000 sites across ...
This study presents the use of recent deep CNN approaches to detect melanoma skin cancer and investigate suspicious lesions. Tests were conducted using a set of more than 36,000 images extracted from multiple datasets. The obtained results show that the best performing deep learning approach achieves high scores with an accuracy and Area Under ...
Skin cancer developing from melanocyte stem cells and fully differentiated melanocytes is known as cutaneous melanoma (CM), a highly aggressive dermal carcinoma (Centeno et al. 2023).Melanomas, including around 1 in 5 skin cancers, are estimated to have affected approximately 325,000 individuals worldwide in 2020.
Identifying melanoma at the early stages of diagnosis is imperative as early detection can exponentially increase one's chances of cure. The paper first proposes a literature survey of multiple methods used for performing skin cancer classification. Our methodology consists of using Convolutional Neural Network (CNN) to identify and diagnose the skin cancer using the IS IC dataset containing ...
This article presents research on automated skin cancer detection using image processing and machine learning techniques. This paper focuses on melanoma, the deadliest form of skin cancer, and highlights the limitations of clinical observation. This study uses Convolutional Neural Networks (CNNs) and feature extraction to accurately classify skin cancers as malignant or benign. The proposed ...
EPIDEMIOLOGY OF MELANOMA. According to the Surveillance Epidemiology and End Results data, melanoma is the sixth most common fatal malignancy in the United States, responsible for 4% of all cancer deaths and 6 of every 7 skin cancer-related deaths.1 A thorough understanding about incidence trends, mortality rates, risk factors, and genetics of this deadly cancer is of critical importance in ...
Background: Melanoma and non-melanoma skin cancers represent a significant public health burden, with skin cancers being the most frequently diagnosed type of cancer worldwide. Methods: This study used GLOBOCAN 2022 data from the International Agency for Research on Cancer (IARC) to analyze melanoma and non-melanoma skin cancer (NMSC) rates in ...
Skin cancer, the most common human malignancy1,2,3, is primarily diagnosed visually, beginning with an initial clinical screening and followed potentially by dermoscopic analysis, a biopsy and ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Melanoma is the deadliest form ...
Introduction. Skin cancer is one of the most common types of cancer worldwide, and its incidence has been increasing over the past few decades (Abarca and Chávez Citation 2023).According to the World Health Organization (WHO), there are around 3 million cases of non-melanoma skin cancer and more than 132 cases of melanoma skin cancer diagnosed each year globally (Organization Citation 2017).
In the past few years many researches investigated algorithms to diagnose skin cancer lesions where melanoma is the deadliest type of skin cancer. In this paper, we propose three different methods for binary classification (melanoma and nevus) using smartphone images since there is a lack in research on it.
Melanoma vaccines: Vaccines to treat melanoma are being studied in clinical trials. These vaccines are, in some ways, like the vaccines used to prevent diseases such as polio, measles, and mumps that are caused by viruses. Such vaccines usually contain weakened viruses or parts of a virus that can't cause the disease.
Image Credit: crystal light/Shutterstock.com . The researchers employed tyrosine, a common amino acid, as a nanomedicine to alter the metabolism of melanoma, a fatal skin cancer, and prevent the ...
Abstract. Melanoma is a relentless type of skin cancer which involves myriad signaling pathways which regulate many cellular processes. This makes melanoma difficult to treat, especially when identified late. At present, therapeutics include chemotherapy, surgical resection, biochemotherapy, immunotherapy, photodynamic and targeted approaches.
Key Points. Question Is cancer incidence in successive social generations in the US slowing or growing?. Findings In this cohort study of 3.8 million patients with cancer ascertained by the Surveillance, Epidemiology, and End Results Program, members of Generation X born between 1965 and 1980 have been experiencing larger per-capita increases in the incidence of leading cancers combined than ...
The recent research on the application of CNN in predicting skin cancer has identified significant challenges in incorporating the entire spectrum of the patient population and various melanoma ...
E arly detection is key to treating melanoma, and social media can improve people's ability to identify early warning signs of the deadly skin cancer, according to a new study by University of ...
Human papillomavirus (HPV) has been implicated in various cancers, including those affecting the skin. The study assessed the long-term risk of skin cancer associated with HPV infection in Taiwan region, using data from the National Health Insurance Research Database between 2007 and 2015. Our analysis revealed a significant increase in skin cancer risk among those with HPV, particularly for ...
Due to its increasing incidence, skin cancer, and especially melanoma, is a serious health disease today. The high mortality rate associated with melanoma makes it necessary to detect the early stages to be treated urgently and properly. ... Similarly, another research paper proposed a Me diagnosis system based on a combination between CNN and ...