CBSE NCERT Solutions
NCERT and CBSE Solutions for free

Class 8 Science Assignments
We have provided below free printable Class 8 Science Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 8 Science . These Assignments for Grade 8 Science cover all important topics which can come in your standard 8 tests and examinations. Free printable Assignments for CBSE Class 8 Science , school and class assignments, and practice test papers have been designed by our highly experienced class 8 faculty. You can free download CBSE NCERT printable Assignments for Science Class 8 with solutions and answers. All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Science Class 8. Students can click on the links below and download all Pdf Assignments for Science class 8 for free. All latest Kendriya Vidyalaya Class 8 Science Assignments with Answers and test papers are given below.
Science Class 8 Assignments Pdf Download
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 8 Science . Students and teachers can download and save all free Science assignments in Pdf for grade 8th. Our expert faculty have covered Class 8 important questions and answers for Science as per the latest syllabus for the current academic year. All test papers and question banks for Class 8 Science and CBSE Assignments for Science Class 8 will be really helpful for standard 8th students to prepare for the class tests and school examinations. Class 8th students can easily free download in Pdf all printable practice worksheets given below.
Topicwise Assignments for Class 8 Science Download in Pdf

Advantages of Class 8 Science Assignments
- As we have the best and largest collection of Science assignments for Grade 8, you will be able to easily get full list of solved important questions which can come in your examinations.
- Students will be able to go through all important and critical topics given in your CBSE Science textbooks for Class 8 .
- All Science assignments for Class 8 have been designed with answers. Students should solve them yourself and then compare with the solutions provided by us.
- Class 8 Students studying in per CBSE, NCERT and KVS schools will be able to free download all Science chapter wise worksheets and assignments for free in Pdf
- Class 8 Science question bank will help to improve subject understanding which will help to get better rank in exams
Frequently Asked Questions by Class 8 Science students
At https://www.cbsencertsolutions.com, we have provided the biggest database of free assignments for Science Class 8 which you can download in Pdf
We provide here Standard 8 Science chapter-wise assignments which can be easily downloaded in Pdf format for free.
You can click on the links above and get assignments for Science in Grade 8, all topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here topic-wise Science Grade 8 question banks, revision notes and questions for all difficult topics, and other study material.
We have provided the best collection of question bank and practice tests for Class 8 for all subjects. You can download them all and use them offline without the internet.
Related Posts

Class 8 Hindi Assignments

Class 8 Kannada Assignments

Class 8 Assignments Download Pdf
NCERT Solutions for Class 6, 7, 8, 9, 10, 11 and 12
NCERT Solutions for Class 8 Science Chapter 4 Materials Metals and Non Metals
October 4, 2019 by Sastry CBSE
Topics and Sub Topics in Class 8 Science Chapter 4 Materials Metals and Non-Metals:
| 4 | Materials Metals and Non-Metals |
| 4.1 | Physical Properties of Metals and Non-metals |
| 4.2 | Chemical Properties of Metals and Non-metals |
| 4.3 | Uses of Metals and Non-metals |
Materials: Metals and Non-Metals Class 8 Science NCERT Textbook Questions
Question 1. Which of the following can be beaten into thin sheets? (a) Zinc (b) Phosphorus (c) Sulphur (d) Oxygen Answer: (a) Zinc
Question 2. Which of the following statements is correct? (a) All metals are ductile. (b) All non-metals are ductile. (c) Generally, metals are ductile. (d) Some non-metals are ductile. Answer: (c) Generally, metals are ductile
Question 3. Fill in the blanks. (a) Phosphorus is a very ____ non-metal. (b) Metals are _____ conductors of heat and _____ (c) Iron is ______ reactive than copper. (d) Metals react with acids to produce ______ gas. Answer: (a) reactive (b) good, electricity (c) more (d) hydrogen
Question 4. Mark ‘T’ if the statement is true and ‘F’ if it is false. (a) Generally, non-metals react with acids. (b) Sodium is a very reactive metal. (c) Copper displaces zinc from zinc sulphate solution. (d) Coal can be drawn into wires. Answer: (a) False (b) True (c) False (d) False
Question 5. Some properties are listed in the following Table. Distinguish between metals and non-metals on the basis of these properties.
| 1. Appearance | ||
| 2. Hardness | ||
| 3. Malleability | ||
| 4. Ductility | ||
| 5. Heat Conduction | ||
| 6. Conduction of Electricity |
| 1. Appearance | have metallic lustre | dull |
| 2. Hardness | hard | soft |
| 3. Malleability | malleable | non-malleable |
| 4. Ductility | ductile | non-ductile |
| 5. Heat Conduction | good conductors | bad conductors |
| 6. Conduction of Electricity | good conductors | bad conductors/insulators |
Question 6. Give reasons for the following. (a) Aluminium foils are used to wrap food items. (b) Immersion rods for heating liquids are made up of metallic substances. (c) Copper cannot displace zinc from its salt solution. (d) Sodium and potassium are stored in kerosene. Answer: (a) Aluminium is highly malleable and it can be easily beaten in sheets to make its foil for wrapping purposes. It is also soft and does not react with food items. That is why aluminium foils are used . to wrap food items. (b) Immersion rods made up of metallic substances because metals are good conductors of heat and electricity. They get hot very soon on the passage of electric current and warm the water. (c) Copper is less reactive than zinc. So it cannot displace zinc from its solution. (d) Sodium and potassium are highly reactive, so they are stored in kerosene.
Question 7. Can you store the lemon pickle in an aluminium utensil? Explain. Answer: No, we cannot store lemon pickle in an aluminium utensil because aluminium is a metal and metals readily react with acids to produce hydrogen. When aluminium comes in contact with lemon, which is acidic, would react to give hydrogen and the pickles will be spoiled.
Question 8. Match the substances given in column A with their uses given in column B.
| A | B |
| Gold | Thermometers |
| Iron | Electric wire |
| Aluminium | Wrapping food |
| Carbon | Jewellery |
| Copper | Machinery |
| Mercury | Fuel |
Answer: (i) (d) (ii) (e) (iii) (c) (iv) (f) (v) (b) (vi) (a)
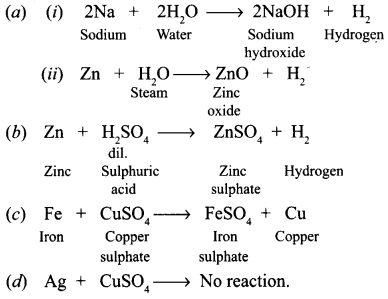
Question 9. What happens when (a) Dilute sulphuric acid is poured on a copper plate? (b) Iron nails are placed in a copper sulphate solution? Write word equations of the reactions involved. Answer: (a) No reaction will take place because copper is very less reactive. (b) Iron being more reactive than copper will replace copper from its solution and brown coating of copper is deposited on the iron nails. Also, the blue colour turns green. Iron + Copper sulphate (solution) → Iron sulphate (solution) + Copper
Question 10. Saloni took a piece of burning charcoal and collected the gas evolved in a test tube. (a) How will she find the nature of the gas? (b) Write down the word equations of all the reactions taking place in this process. (a) She can find the nature of the gas by using a wet litmus paper. After bringing the litmus paper in contact with the gas, if it turns the blue litmus paper into red, it is acidic. Similarly, if it turn the red litmus into blue, it is basic. (b) (i) Carbon + Oxygen → Carbon dioxide (ii) Carbon dioxide + Lime water → Milky
Question 11. One day Reeta went to a jeweller’s shop with her mother. Her mother gave an old gold jewellery to the goldsmith to polish. Next day when they brought the jewellery back, they found that there was a slight loss in its weight. Can you suggest a reason for the loss in weight? Answer: The gold jewellery is dipped into an acidic solution called aqua regia (a mixture of hydrochloric acid and nitric acid) for polishing. On dipping the gold jewellery in the acid solution, the outer layer of gold dissolves and the inner shiny layer appears. This causes a slight loss in its weight.
Materials: Metals and Non-Metals Class 8 Science NCERT Intext Activities Solved

| Object/Material | Change in Shape (Flattens/Breaks into Pieces) |
| Iron nail | Flattens |
| Coal piece | Breaks into pieces |
| Aluminium wire | Flattens |
| Pencil lead | Breaks into pieces |
Solution: This activity shows that iron and aluminium are malleable while coal piece and pencil lead are brittle. Thus, metals are malleable and non-metals are non-malleable.

| S. No. | Materials | Good Conductor/Poor Conductor |
| 1. | Iron rod/nail | Good conductor |
| 2. | Sulphur | Poor conductor |
| 3. | Coal piece | Poor conductor |
| 4. | Copper wire | Good conductor |
Solution: It shows that metals are good conductors of electricity and non-metals are poor conductors of electricity.
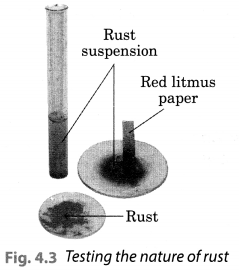
Activity 7 (NCERT Textbook, Page 50) Prepare a fresh solution of sodium hydroxide in a test tube by dissolving 3-4 pellets of it in 5 mL of water. Drop a piece of aluminium foil into it. Bring a burning matchstick near the mouth of the test tube. Observe carefully. Solution: We observed that a colourless gas is evolved which burns with a pop sound. This shows that aluminium react with bases on heating to produce hydrogen gas.
NCERT Solutions for Class 8 Science Chapter 4 – 1 Mark Questions and Answers
Question 1. Non-metals cannot be drawn into wires. Why ? [DAV2008] Answer: Non-metals are not ductile, therefore they cannot be drawn into wires.
Question 2. Complete the following equation : Zn + 2HCl ——-> __+ __ [MSE (Chandigarh) 2007] Answer: Zn + 2HCl ——-> ZnCl 2 + H 2
Question 3. Which of the following can be beaten into thin sheets ? [NCERT]
Answer: Zinc.
Question 4. The number of metals is much ………….. than non-metals. Answer: More.
Question 5. ……… are the good conductors of heat and electricity. Answer: Metals.
Question 6. Examples of metals are ………., ……. and ……… Answer: Iron, sodium and nickel.
Question 7. Examples of non-metals are ………. , …….. and ……. Answer: Sulphur, chlorine and oxygen.
Question 8. Explain the term ‘metallurgy’. Answer: Metallurgy is the science of extracting metals from their ores and purifying them for various uses.
Question 9. State general steps involved in metallurgy of a metal. Answer: The general steps of metallurgy are :
- Concentration of ore.
- Reduction of the metal compound.
- Refining of metal.
Question 10. Metals are (softer/harder) than non-metals. Answer: Harder.
Question 11. Most non-metals are (bad/good) conductors of heat. Answer: Bad.
Question 12. The property that allows the metals to be hammered into thin sheets is called (ductility/ malleability). Answer: Malleability.
Question 13. Melting point of most non-metals is (higher/lower) than metals. Answer: Lower.
Question 14. (Metals/non-metals) display lustre. Answer: Metals.
Question 15. Arrange the following metals in the order of their decreasing chemical activity : magnesium, potassium, iron, gold. Answer: Potassium, magnesium, iron, gold.
Question 16. Can copper displace iron from iron sulphate solution ? Give reasons. Answer: Copper cannot displace iron from iron sulphate because copper is less reactive than iron.
Question 17. (Platinum/iron) is the member of the family of noble metals. Answer: Platinum.
Question 18. Pure gold is (24/100) carats. Answer: 24.
Question 19. International standards of weights are made of (gold-silver/platinum-iridium) alloy. Answer: Platinum-iridium.
Question 20. Gold dissolves in (aqua regia/aqueous solution of silver nitrate). Answer: Aqua regia.
Question 21. Silver tarnishes due to (nitrogen oxides/hydrogen sulphide) in the air. Answer: Hydrogen sulphide.
Question 22. Why is aluminium used in making aeroplanes ? Answer: Aluminium is used in making aeroplanes, as it is light and has high resistance to corrosion when exposed to air which aircrafts demand the most.
Question 23. What type of oxides are formed by metals ? Answer: Metals form basic oxides.
Question 24. What type of oxides are formed by non-metals ? Answer: Non-metals form acidic or neutral oxides.
Question 25. How does phosphorus occur in nature ? Answer: Phosphorus occurs in nature in the combined state as it has strong affinity for oxygen.
Question 26. Give the different forms of silica in nature. Answer: Silica occurs in nature as ordinary sand, flint, quartz and opal.
Question 27. Which metal foil is used in packing of some medicine tablets ? Answer: Aluminium.
Question 28. Name the soft metal which can be cut with a knife. Answer: Sodium or potassium.
Question 29. Name the non-metal used in vulcanization. Answer: Sulphur.
Question 30. Name one metal which is not malleable. Answer: Zinc or arsenic.
Question 31. Name one non-metal which has lustre. Answer: Graphite or Iodine.
Question 32. What would happen to iron railings if they are not painted ? Answer: They will get rusted.
Question 33. Name the element commonly used for converting edible vegetable oils into vanaspati ghee. Answer: Hydrogen.
Question 34. Name the element used for making containers of dry cells. Answer: Zinc.
Question 35. Which metal is used for making radiators of cars ? Answer: Copper.
Question 36. Name the metal whose salt is used for making photographic films. Answer: Silver.
NCERT Solutions for Class 8 Science Chapter 4 – 2 Mark Questions and Answers
Question 1. White phosphorous has to be kept in water. Why ? [NCT2007] Answer: Phosphorus is to be kept in water to prevent its contact with air because it is highly reactive.
Question 2. Can you store lemon pickle in an aluminium utensils ? Explain. [NCERT] Answer: We cannot store acidic food stuffs in aluminium utensils because aluminium reacts with acids. The food gets spoilt.
Question 3. One day Reeta went to a jeweller’s shop with her mother. Her mother gave an old gold jewellery to the goldsmith to polish. Next day when they brought the jewellery back, they found that there was a slight loss in its weight. Can you suggest a reason for the loss in weight ? [NCERT] Answer: The goldsmith must have used acid to clean the gold jewellery and some gold must have dissolved in it. Therefore, there was loss in weight of the jewellery.
Question 4. Write short notes on
- Metallurgical processes
- Uses of common metals and non-metals
- Noble metals
- Concentration of the ore
- Reduction of metal compound to get free metal
- Uses of common metals and non-metals : Uses of metals – for making machinery, automobiles, industrial gadgets, building, bridges, cooking utensils, electrical gadgets, jewellery, sheets. Uses of non-metals – oxygen is used by plants and animals for their survival, nitrogen is used by plants for their growth, chlorine is used in water purification to kill germs, sulphur is used for making sulphuric acid, tincture iodine has antiseptic properties.
- Noble metals – Gold, silver and platinum are noble metals. They occur free in nature . and maintain their lustre for a long time. Platinum, gold and silver are used for making jewellery as they do not tarnish.
Question 5. Purity of gold is 15 carat. What is the percentage’of gold in the ornaments ? Answer: 24 carat purity of gold =100 ∴ 1 carat purity or gold = 100/24 15 carat purity of gold = (100*15)/24 = 62.5 %
Question 6. Give two uses of sulphur in chemical industry. Answer:
- It is used in the manufacture of sulphuric acid.
- It is used in the manufacture of carbon disulphide, which is used as an industrial solvent.
Question 7. How is sulphur useful in agriculture ? How is sulphur useful in medicine ? Answer: Sulphur powder is an excellent insecticide and fungicide. It is used in spraying fruit trees.
- Sulphur is the main constituent of skin ointments.
- Metallic sulphides of sulphur are used in the preparation of Ayurvedic medicines.
Question 8. Give two important uses of silver. Answer:
- It is used for making coins.
- Silver salts (silver bromide and silver iodide) are used for making photographic films.
Question 9. Give two uses of gold. Answer:
- Gold is used for making ornaments.
- Gold foils are used in the preparation of Ayurvedic medicines.
Question 10. Give two uses of platinum. Answer:
- It is used as a catalyst in the manufacture of sulphuric and nitric acid.
- Platinum catalytic converters use platinum as catalytic agent.
Question 11. Which of the following will form acidic oxide and why : P, K, Na, Ca? Answer: P (Phosphorus) will form acidic oxide because it is a non-metal.
Question 12. You are given two materials X and Y. On hammering X is flattened, but Y breaks. Which one is a metal ? Answer: X is a metal because it flattens, i.e., it is malleable.
Question 13. There are four materials A, B, C and D. A and D are hard and shiny, but B and C are dull and not very hard. Identify the metals and non-metals from A, B, C and D. Answer: A and D are metals. B and C are non-metals.
Question 14. Gaurav knows that wires can be made from copper and aluminium. He tries to make wire . from sulphur and carbon. Will he succeed ? Give reason also. Answer: No, he will not succeed because sulphur and carbon are non-metals. Non-metals are not ductile, that is, they cannot be drawn into wires.
NCERT Solutions for Class 8 Science Chapter 4 – 3 Mark Questions and Answers
Question 1.
- Identify the most reactive and least reactive metal amongst the followings : Al, K, Cu, Au.
- An iron knife kept dipped in blue copper sulphate solution changes to light green. Why ? Write the equation also. [KVS 2005]
- Most reactive metal is K and least reactive metal is Au.
- An iron knife kept dipped in blue copper sulphate solution changes to light green because iron replaces copper from copper sulphate and forms iron sulphate. This happens because iron is more reactive than copper. Fe + CuSO 4 ——> FeSO 4 + Cu
Question 2. Give reasons, why :
- Silver is used in jewellery.
- Copper is used in electrical wiring.
- Sodium is stored in kerosene oil.
- Silver does not corrode and it is malleable and ductile, therefore, it can be used in jewellery.
- Copper is used in electrical wiring because it is a good conductor of electricity.
- Sodium has low ignition temperature. It oxidises quickly and bums when exposed to air. It can only be stored in a liquid hydrocarbon like mineral oil or kerosene oil.
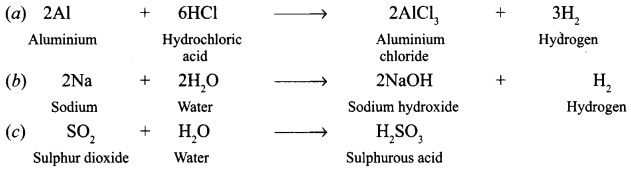
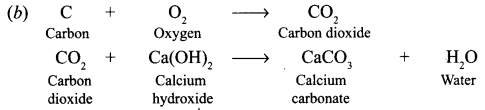
Question 3. Taking examples of magnesium and sulphur explain how metals and non-metals produce oxides with different characteristics. Answer: Magnesium bums in oxygen to form magnesium oxide, which dissolves in water to form magnesium hydroxide – an alkali. 2Mg + O 2 ——-> 2MgO MgO + H 2 O ——-> Mg(OH) 2 Magnesium hydroxide changes red litmus into blue. Sulphur bums in air to form sulphur dioxide, which dissolves in water to form sulphurous acid – an acid which turns blue litmus into red. S + O 2 ——> SO 2 SO 2 + H 2 O ——–> H 2 SO 3
Question 4. Compare the following chemical properties of metals and non-metals.
- Formation of ions
- Action with dilute acids
- Action with hydrogen.
- Formation of ions. Metals form cations whereas non-metals form anions.
- Action with dilute acids. Metals react with dilute mineral acid to liberate hydrogen. Non-metals do not react with dilute mineral acids.
- Action with hydrogen. Metals do not react with hydrogen but non-metals react with hydrogen to form hydrides.
NCERT Solutions for Class 8 Science Chapter 4 – 5 Mark Questions and Answers
Question 2.
- A copper spoon had fallen into a container containing dil.HCl. What would happen to it in three days time ? [DAV2008]
- Metals are used for making bells.
- We can’t use pure gold to make jewellery.
- Which metal is this ?
- Give the equation of the reaction taking place.
- The metallic oxide formed would be acidic or basic in nature ?
- Nothing will happen as copper does not react with hydrochloric acid.
- Metals have the property of sonorosity so they are used for making bells.
- Pure gold cannot be used for making jewellery because it is very soft.
- 2Mg + O 2 ——> 2MgO
- Basic in nature.
Question 3. Give reasons for the following : [KVS 2007]
- Silver is used in making mirrors.
- Aluminium is used to make electrical wire.
- Iron is used in construction of bridges and houses.
- Graphite is used as an electrode in the dry cell.
- Iron sheets are galvanised before use.
- Silver has the ability to reflect light, therefore, it is used for making mirrors.
- Aluminium is a good conductor of electricity, so, it is used for making electrical wires.
- Iron is a strong metal, therefore, it is mixed with concrete to make bridges and houses.
- Graphite is a good conductor of electricity, therefore, it is used as an electrode in dry cell.
- Iron sheets are galvanised before use so that they do not corrode.
Question 4. Which of the following statements is correct ? [NCERT]
- All metals are ductile.
- All non-metals are ductile.
- Generally, metals are ductile.
- Some non-metals are ductile.
Answer: Generally, metals are ductile.
Question 5. Fill in the blanks : [NCERT]
- Phosphoms is a very ………… non-metal.
- Metals are …….. conductors of heat and ………
- Iron is ……… reactive than copper.
- Metals react with acids to produce ………. gas.
- good, electricity
Question 6. Mark ‘T’ if the statement is true and ‘F’ if it is false. [NCERT]
- Generally, non-metals react with acids. ( )
- Sodium is a very reactive metal. ( )
- Copper displaces zinc from zinc sulphate solution. ( )
- Coal can be drawn into wires. ( )
Question 7. Some properties are listed in the following Table. Distinguish between metals and non-metals on the basis of these properties. [NCERT]
| Properties | Metals | Non-metals |
| 1. Appearance 2. Hardness 3. Malleability 4. Ductility 5. Heat conduction 6. Conduction of electricity |
| Properties | Metals | Non-metals |
| 1. Appearance | Solid at room temperature except mercury. | They are either solids or gases except bromine (liquid). |
| 2. Hardness | They are hard | They are brittle. |
| 3. Malleability | Malleable | Non-malleable |
| 4. Ductility | Ductile | Non-ductile |
| 5. Heat conduction | Good conductors | Bad conductors |
| 6. Conduction of electricity | Good conductors | Bad conductors |
Question 8. Give reasons for the following : [NCT 2010]
- Aluminium foils are used to wrap food items.
- Immersion rods for heating liquids are made up of metallic substances.
- Copper cannot displace zinc from its salt solution.
- Sodium and potassium are stored in kerosene.
- Aluminium is a highly malleable metal and can be made into foils. So, it can be used to wrap food items.
- Metals are good conductors of electricity, therefore, they are used for making immersion rods.
- Copper is less reactive than zinc. Therefore, it cannot displace zinc from its salt solution.
- Sodium and potassium are highly reactive metals. On exposure to air, they get oxidized. To avoid this they are stored in kerosene.
Question 9. Match the substances given in Column I with their uses given in Column II. [NCERT]
| Column I | Column II |
| (a) Gold | (i) Thermometers |
| (b) Iron | (ii) Electric wire |
| (c) Aluminium | (iii) Wrapping food |
| (d) Carbon | (iv) Jewellery |
| (e) Copper | (v) Machinery |
| (f) Mercury | (vi) Fuel |
| Column I | Column II |
| (a) Gold (b) Iron (c) Aluminium (d) Carbon (e) Copper (f) Mercury | (iv) Jewellery (v) Machinery (iii) Wrapping food (vi) Fuel (ii) Electric wire (i) Thermometers |
Question 10. What happens when [NCERT]
- Dilute sulphuric acid is poured on copper plate ?
- Iron nails are placed in copper sulphate solution ? Write word equations of the reactions involved.
- When sulphuric acid is poured on copper plate, copper sulphate and hydrogen gas are produced. Copper + Sulphuric acid ——-> Copper sulphate + Hydrogen (gas).
- When iron nails are placed in copper sulphate solution, iron sulphate and copper are formed. Iron + Copper sulphate ——-> Iron sulphate + Copper
Question 12. List different uses of metals that you come across in everyday life. Answer: Metals are used for making
- automobiles, aeroplanes, trains, etc.
- pins, cooking utensils, electrical gadgets.
- electrical wires.
- thin sheets used for wrapping of food items, medicines, etc.
Question 13. Choose appropriate words from the brackets and complete the statements.
- Noble gases are found in (free state/compound forms).
- Non-metals are generally (malleable/brittle).
- Potassium after combustion will form (acidic oxide/basic oxide).
- (Iodine/bromine) has antiseptic properties.
- German silver has (copper/silver) as major constituent.
- Basic oxide
Question 14. State whether the following statements are True or False :
- Sodium is more reactive than magnesium.
- Magnesium reacts with cold water.
- All metals exist in solid form at room temperature.
- Gallium has a low melting point.
- Gold is alloyed with copper to make it hard.
Question 17. A set of metals in order of their increasing chemical reactivity is given below : silver, copper, lead, iron, zinc, magnesium and sodium.
- Which of the above metals is stored in kerosene ?
- Which metals will react with cold water ?
- Which gas will be liberated when metals react with cold water ?
- Which of the metals will react with oxygen when heated ?
- Which of the metals become black in the presence of hydrogen sulphide ?
- Zinc, magnesium
NCERT Solutions for Class 8 Science Chapter 4 MCQs
Question 1. Which of the following properties is generally not shown by metals? (a) Ductility (b) Sonorous (c) Dullness (d) Electrical conduction Answer: (c)
Question 2. The most abundant element in the universe is (a) hydrogen (b) oxygen (c) helium (d) carbon Answer: (a)
Question 3. The ability of metals to be drawn into wires is known as (a) ductility (b) conductivity (c) malleability (d) sonorousity Answer: (a)
Question 4. The most abundant element in the earth crust is (a) iron (b) oxygen (c) silicon (d) aluminium Answer: (b)
Question 5. Galvanisation is a method qf protecting iron from rusting by coating with a thin layer of (a) silver (b) galium (c) zinc (d) aluminium Answer: (c)
Question 6. The most abundant ihetal in earth crust is (a) Cu (b) Al (c) Fe (d) Zn Answer: (b)
Question 7. An alloy is (a) a compound (b) a heterogeneous mixture (c) a homogeneous mixture (d) an element Answer: (c)
Question 8. In extraction of copper, the flux used is (a) FeO (b) Si02 (c) CaO (d) FeSi03 Answer: (b)
Question 9. Alloys are homogeneous mixtures of a metal with a metal or non-metal. Which among the following aljoys contain non-metal as one of its constituents? (a) Amalgam (b) Brass (c) Bronze (d) Steel Answer: (d)
Question 10. Which of the following is purest form of carbon? (a) Diamond (b) Graph’ite (c) Fullerenes (d) Charcoal Answer: (c)
Question 11. Which among the following alloys contain mercury as one of its constituents? (a) Alnico (b) Solder (c) Stainless steel (d) Zinc Amalgam Answer: (d)
Question 12. Which of the following methods is suitable for preventing an iron frying pan from rusting? (a) Applying paint (b) Applying grease (c) Applying a coating of zinc (d) all of these Answer: (c)
Question 13. Generally, non-metals are not conductors of electricity, which of the following is a good conductor of electricity? (a) Fullerenes (b) Graphite (c) Diamond (d) Sulphur Answer: (b)
Question 14. Food cans are coated with tin and not with zinc because (a) zinc is costlier than tin (b) zinc is less reactive than tin (c) zinc is more reactive than tin (d) zinc has a higher melting point than tin Answer: (a)
Question 15. Electrical wires have a coating of an insulating materials. The material, generally used is (a) sulphur (b) graphite (c) PVC (d) none of these Answer: (c)
Question 16. Which of the following non-metals is a liquid? (a) Sulphur (b) Phosphorus (c) Carbon (d) Bromine Answer: (d)
More CBSE Class 8 Study Material
- NCERT Solutions for Class 8 Maths
- NCERT Solutions for Class 8 Science
- NCERT Solutions for Class 8 Social Science
- NCERT Solutions for Class 8 English
- NCERT Solutions for Class 8 English Honeydew
- NCERT Solutions for Class 8 English It So Happened
- NCERT Solutions for Class 8 Hindi
- NCERT Solutions for Class 8 Sanskrit
NCERT Solutions
Free resources.
Quick Resources

Summer’s Ending, Learning’s Just Beginning! Soak 25% off off
on Annual Courses
Use code SUMMER25
Share this article

Table of Contents
Latest updates.

NCERT Solutions for Class 7 Science Chapter 17 Forests: Our Lifeline – Download PDF

NCERT Solutions for Class 7 Science Chapter 18: Wastewater Story – Download PDF

NCERT Solutions for Class 7 Science Chapter 10 2024: Respiration in Organisms

NCERT Solutions for Class 7 Science Chapter 15: Light

NCERT Solutions for Class 10 Science 2024 – Download PDF

NCERT Books for Class 12 Chemistry 2024: Download PDF

NCERT Solutions for Factorisation Exercise 14.1 Class 8 Maths

NCERT Books for Class 10 Maths 2025: Download Latest PDF

NCERT Solutions for Class 12 2024 – Physics, Chemistry, Maths, Biology

NCERT Solutions for Class 9 2024: Science, Maths, Social Science – Download PDF
Tag cloud :.
- entrance exams
- engineering
- ssc cgl 2024
- Written By Saif_Ansari
- Last Modified 05-03-2024
NCERT Solutions for Class 8 Science 2024 – Download PDF

Science is important for strengthening fundamental concepts in Class 8. Students need to have a thorough understanding of the chapters and their topics. But a lot of the time, students need help with a particular question or topic. In such times, having NCERT solutions is a lifesaver. NCERT Solutions for Class 8 Science helps students understand the fundamentals of Physics, Chemistry, and Biology. This will make Science exam preparation for Class 8 students easier and smoother.
In this article, we have provided the links to access the NCERT Solutions Class 8 Science. These NCERT solutions are designed by subject experts who have provided simple and understandable solutions to every problem. Students can find the answers to all the problems and questions in the NCERT textbook. To know more about NCERT Solutions for Class 8 Science, continue reading the article.

NCERT Solutions for Class 8 Science
NCERT Solutions for Class 8 Science is a comprehensive study material designed to help students understand the fundamental concepts of Science. It covers cell biology, force and pressure, and metals and non-metals. The solutions are presented step-by-step, making it easier for students to understand and learn the concepts.
With the help of NCERT Solutions for Class 8 Science, students can prepare for exams effectively and excel in their academic pursuits. By watching Embibe videos, students can reinforce their understanding of the concepts covered in the NCERT Solutions and enhance their learning experience.

Benefits of Referring to NCERT Solutions for Class 8 Science
The benefits of solving NCERT Solutions for Class 8 Science are:
- Offers In-depth Knowledge in Easy Language: All NCERT solutions have been solved by top academic experts at Embibe. The Class 8 Science NCERT Solutions have been formed so that any student can understand them.
- Strictly Follows the CBSE Curriculum: All Science Class 8 NCERT book questions have been solved step-by-step, taking care of the CBSE marking scheme.
- Cover all Fundamentals: The solutions have been explained with the help of principles, laws, and concepts from the chapter. We provide a valid explanation of why this is the correct answer.
- Helpful for Competitive Examinations: Solving these tricky questions requires in-depth knowledge and the strategy to arrive at the answer quickly. Solving these NCERT science solutions will help you learn to handle the questions in competitive examinations like JEE, NEET, and Olympiads.
- Help You Complete the Assignments on Time: It will help you complete your assignments. However, you should try to solve the questions independently. If you need help understanding, refer to these 8th Science Solutions. To improve practice, students can also refer to Class 8 science practice questions on Embibe.
NCERT Solutions for Class 8 Science: Points to Remember
Having a few important points to refer to while preparing for Class 8 Science is always helpful. Given below are some points to remember for Class 8 Science that will help you have a detailed preparation:
- Agriculture is a branch of Science dealing with the cultivation or growing of plants and raising animals that are useful to us in the field.
- Microbes are of five major kinds — bacteria, fungi, algae, protozoa and viruses.
- Synthetic plastic materials are obtained by gentle heating and moulding, such that the moulded materials cannot be reshaped by gentle heating.
- A substance which cannot be broken into two or more different substances by any physical or chemical means is called an element.
- Heating a complex organic substance without air so that it breaks up into simpler volatile fractions is called destructive distillation.
- The materials such as wood, coal, coke, hydrogen, petroleum gas, compressed natural gas, alcohol and ether are combustible materials.
Access NCERT Class 8 Science Chapter-wise Solutions
You can find NCERT Science Class 8 Solutions for all chapters at Embibe. We have provided solutions for all chapters from Chapters 1 to 18.
| Class 8 Science Chapter-wise Solutions | |
|---|---|

FAQs on NCERT Solutions for Class 8 Science
Given below are some frequently asked questions on NCERT Class 8 Science solutions:
Ans : We suggest that every student try to solve the questions first, and if they fail to do so then only they should refer to the solutions.
Ans: Referring to Science Class 8 NCERT Solutions will help you in the final exam. But apart from solving the NCERT Questions, one must also solve the previous year’s question papers, complete the assignments given by the teacher and take some online mock tests as it will help them to overcome stress during the final exam.
Ans : Students can find NCERT Class 8 practice questions on Embibe.
Ans : Students can access the NCERT Class 8 Science Solutions for on Embibe.
Ans : Yes, the NCERT Guide for Class 8 Science has been framed according to the CBSE Guidelines.
We hope this article on NCERT Class 8 Science solutions was helpful to you. For more information, stay tuned on Embibe .
Related Articles
NCERT Solutions for Class 7 Science Chapter 17 Forests Our Lifeline: Science is one of the main subjects included in the Class 7 curriculum. And...
NCERT Solutions for Class 7 Science Chapter 18: Wastewater Story: The 18th chapter of Class 7 Science is Wastewater Story. Top academic experts have prepared...
NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms: NCERT solutions are great study resources that help students solve all the questions associated...
NCERT Solutions for Class 7 Science Chapter 15: The NCERT Class 7 Science Chapter 15 is Light. It is one of the most basic concepts. Students...
NCERT Solutions for Class 10 Science: The National Council of Educational Research and Training (NCERT) publishes NCERT Solutions for Class 10 Science as a comprehensive...
NCERT Books for class 12 Chemistry: NCERT publishes chemistry class 12 books every year. The NCERT chemistry class 12 books are essential study material for...
NCERT Exercise 14.1 Class 8 Maths Solutions: NCERT solutions for Class 8 Maths chapter 14 exercise 14.1 - Factorisation provides answers to all of the chapter's...
NCERT Books for Class 10 Maths: The NCERT Class 10 Maths Book is a comprehensive study resource for students preparing for their Class 10 board exams....
NCERT Solutions for Class 12 2023-24: The NCERT Solutions for Class 12 are meant to help students understand what the subject holds. These solutions are...
NCERT Solutions for Class 9 2023-24: Students in Class 9 must start their exam preparation as per the syllabus as soon as possible. It will...
NCERT Solutions for Class 7 2024: Download PDF (Maths & Science)
NCERT Solutions for Class 7 2024: Class 7 is a foundational phase in students' lives and it is important to strengthen the fundamentals. NCERT solutions...
NCERT Solutions for Class 6 2024: Download PDF
NCERT Solutions for Class 6 2023-24: CBSE and many state education boards prescribe the NCERT curriculum for classes 1 to 12. The syllabus is curated to...
NCERT Books for Class 10 2024: Download PDF
NCERT Books for Class 10: The Central Board of Secondary Education (CBSE) prescribes the NCERT books for all classes. The Class 10 NCERT books are...
NCERT Books for Class 6 2024: Download PDF
NCERT Books for Class 6: Class 6 students must understand that their grades will significantly influence their future opportunities. Students in this period cultivate hobbies...
NCERT Class 11 English Books 2024
NCERT Class 11 English Books: The Central Board of Secondary Education (CBSE) follows the National Council of Educational Research and Training (NCERT) textbooks. English is...
NCERT Solutions for Class 10 Science Chapter 13 2024: Download PDF
NCERT Solutions for Class 10 Science Chapter 13 2023-24: CBSE students studying in Class 10 must go through all the chapters in detail for thorough...
NCERT Solutions for Class 12 Physics Chapter 9: Ray Optics and Optical Instruments
NCERT Solutions for Class 12 Physics Chapter 9 Ray Optics and Optical Instruments: The NCERT solutions for Class 12 Physics Chapter 9 provided by Embibe is...
NCERT Solutions for Class 12 Chemistry Chapter 8: D and F Block Elements
NCERT Solutions for Class 12 Chemistry Chapter 8 The D and F Block Elements: In this article, we will provide NCERT Solutions for Class 12...
NCERT Solutions for Class 9 Science 2024: Chapter 10; Gravitation
NCERT Solutions for Class 9 Science Chapter 10 Gravitation: Class 10 Science deals with Gravitation, which is an important topic. The chapter focuses on giving...
NCERT Solutions for Class 12 Chemistry Chapter 12: Aldehydes, Ketones and Carboxylic Acids
NCERT Solutions for Class 12 Chemistry Chapter 12: The chapter deals with Aldehydes, Ketones and Carboxylic Acids. Students need to prepare for the exams by referring...
NCERT Solutions for Class 12 Biology Chapter 3: Human Reproduction – Download PDF
NCERT Solutions for Class 12 Biology Chapter 3 2023-24: Biology is one of the most important subjects and can be a quite scoring one. Human...
NCERT Solutions for Class 12 Chemistry Chapter 2: Solutions – Download PDF
NCERT Solutions for Class 12 Chemistry Chapter 2: CBSE Class 12 Chemistry is divided into two parts - Part I contains 9 chapters, and Part...
NCERT Solutions for Class 12 Physics 2024 – Download PDF
NCERT Solutions for Class 12 Physics 2023-24: Class 12 is a crucial year, and students should use all the help that they can get. For...
NCERT Solutions for Class 11 Biology Chapter 14: Respiration in Plants – Download PDF
NCERT Solutions for Class 11 Biology Chapter 14: Chapter 14 of Class 11th Biology will help students understand the basic concepts related to Respiration In...
NCERT Solutions for Class 11 Chemistry Chapter 7: Equilibrium – Download PDF
NCERT Solutions for Class 11 Chemistry Chapter 7 Equilibrium: Chapter 7 of the Class 11 NCERT Chemistry is Equilibrium. It contains several interesting and challenging...
NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics: Download PDF
NCERT Solutions for Class 11 Physics Chapter 12 Thermodynamics: NCERT Solutions for Class 11 Physics are very important for students. NCERT Solutions for Chapter 12...
NCERT Solutions for Class 11 Biology Chapter 19 2024: Download PDF
NCERT Solutions for Class 11 Biology Chapter 19: Excretory Products and Their Elimination - is a valuable resource to help students score well in exams. Experts...

39 Insightful Publications

Embibe Is A Global Innovator

Innovator Of The Year Education Forever

Interpretable And Explainable AI

Revolutionizing Education Forever

Best AI Platform For Education

Enabling Teachers Everywhere

Decoding Performance

Leading AI Powered Learning Solution Provider

Auto Generation Of Tests

Disrupting Education In India

Problem Sequencing Using DKT

Help Students Ace India's Toughest Exams

Best Education AI Platform

Unlocking AI Through Saas

Fixing Student’s Behaviour With Data Analytics

Leveraging Intelligence To Deliver Results

Brave New World Of Applied AI

You Can Score Higher

Harnessing AI In Education

Personalized Ed-tech With AI

Exciting AI Platform, Personalizing Education

Disruptor Award For Maximum Business Impact

Top 20 AI Influencers In India

Proud Owner Of 9 Patents

Innovation in AR/VR/MR

Best Animated Frames Award 2024
Trending Searches
Previous Year Question Papers
Sample papers.
Adaptive Practice with Solutions To Help You Ace Important Topics for 8th NCERT Science

Practice Unlimited 8th NCERT Science Questions With Hints & Solutions
Enter mobile number.
By signing up, you agree to our Privacy Policy and Terms & Conditions
WorkSheets Buddy
Download Math, Science, English and Many More WorkSheets

CBSE Worksheets for Class 8 Science
CBSE Worksheets for Class 8 Science: One of the best teaching strategies employed in most classrooms today is Worksheets. CBSE Class 8 Science Worksheet for students has been used by teachers & students to develop logical, lingual, analytical, and problem-solving capabilities. So in order to help you with that, we at WorksheetsBuddy have come up with Kendriya Vidyalaya Class 8 Science Worksheets for the students of Class 8. All our CBSE NCERT Class 8 Science practice worksheets are designed for helping students to understand various topics, practice skills and improve their subject knowledge which in turn helps students to improve their academic performance. These chapter wise test papers for Class 8 Science will be useful to test your conceptual understanding.
Board: Central Board of Secondary Education(www.cbse.nic.in) Subject: Class 8 Science Number of Worksheets: 51
CBSE Class 8 Science Worksheets PDF
All the CBSE Worksheets for Class 8 Science provided in this page are provided for free which can be downloaded by students, teachers as well as by parents. We have covered all the Class 8 Science important questions and answers in the worksheets which are included in CBSE NCERT Syllabus. Just click on the following link and download the CBSE Class 8 Science Worksheet. CBSE Worksheets for Class 8 Science can also use like assignments for Class 8 Science students.
- Grade 8 Crop Production and Management Worksheets
- Grade 8 Microorganisms: Friend and Foe Worksheets
- Grade 8 Synthetic Fibres and Plastics Worksheets
- Grade 8 Materials: Metals and Non-Metals Worksheets
- Grade 8 Coal and Petroleum Worksheets
- Grade 8 Combustion and Flame Worksheets
- Grade 8 Conservation of Plants and Animals Worksheets
- Grade 8 Cell Structure and Functions Worksheets
- Grade 8 Reproduction in Animals Worksheets
- Grade 8 Reaching the Age of Adolescence Worksheets
- Grade 8 Force and Pressure Worksheets
- Grade 8 Friction Worksheets
- Grade 8 Sound Worksheets
- Grade 8 Chemical Effects of Electric Current Worksheets
- Grade 8 Some Natural Phenomena Worksheets
- Grade 8 Light Worksheets
- Grade 8 Stars and the Solar System Worksheets
- Grade 8 Pollution of Air and Water Worksheets
- CBSE Worksheets for Class 8 Science Air and water pollution Assignment
- CBSE Worksheets for Class 8 Science Cell Structure and Functions Assignment
- CBSE Worksheets for Class 8 Science Chemical Effect of Electric Current Part A Assignment
- CBSE Worksheets for Class 8 Science Chemical Effect of Electric Current Part B Assignment
- CBSE Worksheets for Class 8 Science Coal and Petroleum Part A Assignment
- CBSE Worksheets for Class 8 Science Coal and Petroleum Part B Assignment
- CBSE Worksheets for Class 8 Science Combustion and Flame Part A Assignment
- CBSE Worksheets for Class 8 Science Combustion and Flame Part B Assignment
- CBSE Worksheets for Class 8 Science Conservation of Plants and Animals Assignment
- CBSE Worksheets for Class 8 Science Crop Production and Management Assignment
- CBSE Worksheets for Class 8 Science Force And Pressure Part A Assignment
- CBSE Worksheets for Class 8 Science Force And Pressure Part B Assignment
- CBSE Worksheets for Class 8 Science Friction Assignment
- CBSE Worksheets for Class 8 Science Light Assignment
- CBSE Worksheets for Class 8 Science Materials-Metals and Non-Metals Part A Assignment
- CBSE Worksheets for Class 8 Science Materials-Metals and Non-Metals Part B Assignment
- CBSE Worksheets for Class 8 Science Micro Organism Assignment
- CBSE Worksheets for Class 8 Science Micro organisms Friend and Foe Assignment
- CBSE Worksheets for Class 8 Science Pollution of Air and Water Assignment
- CBSE Worksheets for Class 8 Science Reaching the Age of Adolescence Part A Assignment
- CBSE Worksheets for Class 8 Science Reaching the Age of Adolescence Part B Assignment
- CBSE Worksheets for Class 8 Science Reproduction in Animals Part A Assignment
- CBSE Worksheets for Class 8 Science Reproduction in Animals Part B Assignment
- CBSE Worksheets for Class 8 Science Some Natural Phenomena Part A Assignment
- CBSE Worksheets for Class 8 Science Some Natural Phenomena Part B Assignment
- CBSE Worksheets for Class 8 Science Sound Part A Assignment
- CBSE Worksheets for Class 8 Science Sound Part B Assignment
- CBSE Worksheets for Class 8 Science Stars And The Solar System Part A Assignment
- CBSE Worksheets for Class 8 Science Stars And The Solar System Part B Assignment
- CBSE Worksheets for Class 8 Science Stars And The Solar System Part C Assignment
- CBSE Worksheets for Class 8 Science Synthetic Fibers And Plant Assignment
- CBSE Worksheets for Class 8 Science Synthetic Fibers And Plastics Assignment
- CBSE Worksheets for Class 8 Science The Cell Assignment
- CBSE Worksheets for Class 8 Science Assignment 1
- CBSE Worksheets for Class 8 Science Assignment 2
- CBSE Worksheets for Class 8 Science Assignment 3
- CBSE Worksheets for Class 8 Science Assignment 4
- CBSE Worksheets for Class 8 Science Assignment 5
- CBSE Worksheets for Class 8 Science Assignment 6
- CBSE Worksheets for Class 8 Science Assignment 7
- CBSE Worksheets for Class 8 Science Assignment 8
- CBSE Worksheets for Class 8 Science Assignment 9
- CBSE Worksheets for Class 8 Science Assignment 10
- CBSE Worksheets for Class 8 Science Assignment 11
- CBSE Worksheets for Class 8 Science Assignment 12
- CBSE Worksheets for Class 8 Science Assignment 13
- CBSE Worksheets for Class 8 Science Assignment 14
- CBSE Worksheets for Class 8 Science Assignment 15
- CBSE Worksheets for Class 8 Science Assignment 16
- CBSE Worksheets for Class 8 Science Assignment 17
- CBSE Worksheets for Class 8 Science Assignment 18
Advantages of CBSE Class 8 Science Worksheets
- By practising NCERT CBSE Class 8 Science Worksheet , students can improve their problem solving skills.
- Helps to develop the subject knowledge in a simple, fun and interactive way.
- No need for tuition or attend extra classes if students practise on worksheets daily.
- Working on CBSE worksheets are time-saving.
- Helps students to promote hands-on learning.
- One of the helpful resources used in classroom revision.
- CBSE Class 8 Science Workbook Helps to improve subject-knowledge.
- CBSE Class 8 Science Worksheets encourages classroom activities.
Worksheets of CBSE Class 8 Science are devised by experts of WorksheetsBuddy experts who have great experience and expertise in teaching Maths. So practising these worksheets will promote students problem-solving skills and subject knowledge in an interactive method. Students can also download CBSE Class 8 Science Chapter wise question bank pdf and access it anytime, anywhere for free. Browse further to download free CBSE Class 8 Science Worksheets PDF .
Now that you are provided all the necessary information regarding CBSE Class 8 Science Worksheet and we hope this detailed article is helpful. So Students who are preparing for the exams must need to have great solving skills. And in order to have these skills, one must practice enough of Class 8 Science revision worksheets . And more importantly, students should need to follow through the worksheets after completing their syllabus. Working on CBSE Class 8 Science Worksheets will be a great help to secure good marks in the examination. So start working on Class 8 Science Worksheets to secure good score.
CBSE Worksheets for Class 8
Share this:.
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
Leave a Comment Cancel reply
Notify me of follow-up comments by email.
Notify me of new posts by email.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Important Question
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- Question Bank
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Previous Year Paper
- JEE Mock Test
- JEE Crash Course
- JEE Sample Papers
- Important Info
- SRM-JEEE Previous Year Paper
- SRM-JEEE Mock Test
- VITEEE Previous Year Paper
- VITEEE Mock Test
- BITSAT Previous Year Paper
- BITSAT Mock Test
- Manipal Previous Year Paper
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- AP EAMCET Mock Test
- COMEDK Previous Year Paper
- COMEDK Mock Test
- GUJCET Previous Year Paper
- GUJCET Mock Test
- KCET Previous Year Paper
- KCET Mock Test
- KEAM Previous Year Paper
- KEAM Mock Test
- MHT CET Previous Year Paper
- MHT CET Mock Test
- TS EAMCET Previous Year Paper
- TS EAMCET Mock Test
- WBJEE Previous Year Paper
- WBJEE Mock Test
- AMU Previous Year Paper
- AMU Mock Test
- CUSAT Previous Year Paper
- CUSAT Mock Test
- AEEE Previous Year Paper
- AEEE Mock Test
- UPSEE Previous Year Paper
- UPSEE Mock Test
- CGPET Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
Science Assignment 4 Worksheet for Class 8 PDF with Answers
In Class 8 Science there is a chapter “Science Assignment 4”, it is a crucial lesson for the students as they get to know all the basic topics of Science Assignment 4. Since it is an important lesson, students should take Science Assignment 4 Worksheet Class 8 to better develop an understanding of the concepts explained.
It is very important for Class 8 students to practise questions of the chapter Science Assignment 4 because it will help them create their own exam strategy to score well in the upcoming final examination.
In addition to that, Science Assignment 4 Worksheet Class 8 has quite interactive and creative tasks which boosts student’s creativity level.
Science Assignment 4 Worksheet With Solutions
The questions in the chapter Science Assignment 4 worksheet for Class 8 are provided with solutions. Through these solutions, students can easily solve all their doubts. By clearing the doubts of Science Assignment 4, students can build a strong foundation. Accordingly students can also score good marks in questions related to the chapter Science Assignment 4. The subject matter experts at Selfstudys has prepared Science Assignment 4 Worksheet With Solutions in a way that helps students answer all types of questions regardless of its difficulty.
Science Assignment 4 Worksheet Class 8 PDF
Class 8 students can easily download the Portable Document Format (PDF) of Science Assignment 4 worksheet with the help of Selfstudys website. This can help Class 8 students to understand all the topics and concepts of the chapter Science Assignment 4 which will help students increase their self-confidence level. A perfect level of self- confidence can easily decrease students' level of stress to prepare for the chapter Science Assignment 4.
How to Download Science Assignment 4 Worksheet Class 8?
To look through the questions included in Science Assignment 4 worksheet Class 8, students can follow the given steps. These steps are the easiest one that one can follow to download Science Assignment 4 Worksheet Class 8.
- Open the Selfstudys website.
- Bring the arrow towards the CBSE which can be seen in the navigation bar.
- A drop down menu will appear, select KVS NCERT CBSE Worksheet.
- A new page will appear, Class 8 from the list of classes.
- Click Science from the list of subjects.
- Again a new page will appear, now select Science Assignment 4 Worksheet from the list of chapters.
Features of Science Assignment 4 Worksheet Class 8
Before solving questions from Science Assignment 4 worksheet Class 8, students should understand what makes Science Assignment 4 Worksheet Class 8 PDF special. Features of the worksheet are discussed below:
- All Concepts are Covered: In Science Assignment 4 worksheet Class 8, all concepts and topics are covered in an elaborate manner in the questions format. Through this elaboration, students can understand all the topics of the chapter Earth in a better way.
- Explained in an Easy Language: Answers of Science Assignment 4 Worksheet Class 8 is explained in an easy language which helps students easily understand the process of answering questions.
- Varieties of Questions are Included: In the Class 8 Science Assignment 4 worksheet, varieties of questions are included. Through this students can solve all kinds of questions of the chapter Science Assignment 4.
- Eye Catching Format: Science Assignment 4 Worksheet of Class 8 is considered to be an eye catching one. This eye-catching format can attract many students to solve the questions of the chapter Science Assignment 4.
- Solutions are Provided: For all the questions in the worksheet of Class 8 Science Assignment 4, solutions are provided. Through the solutions, Class 8 students will be able to solve challenging questions which will help them develop a critical thinking capability.
- According to the Class 8 Syllabus: The questions in Class 8 Science Science Assignment 4 Worksheet are as per the Class 8 Syllabus and prescribed NCERT books. With the help of this, kids will be able to make their foundational understanding stronger.
How to Know If You're Ready for Science Assignment 4 Worksheet Class 8?
First of all Class 8 students need to complete the chapter Science Assignment 4 from the main Science book. After covering all the topics, definitions and concepts from the chapter, students are totally ready to solve the questions from Science Assignment 4 Worksheet Class 8. Solving the questions from the Class 8 worksheet can help students to increase their conceptual understanding of Science Assignment 4.
What Is Science Assignment 4 Worksheet Class 8 and How to Use It?
Science Assignment 4 worksheet Class 8 is mainly given to students to practise a variety of questions. After practising questions from Science Assignment 4 worksheet, students can also look through the answers. Answers for all questions can help students to improve their practising skills. These skills can help Class 8 students to increase their level of understanding.
Parents are advised to tell their Class 8rd kids to begin solving Science Assignment 4 Worksheet Class 8 the moment they finish their study of the chapter. Doing this, will help students brush up their all learning as well as be completed for upcoming annual exams or tests.
Advantages of Science Assignment 4 Worksheet Class 8
Solving questions from Science Assignment 4 worksheet Class 8, students can be benefited a lot. Those important advantages are:
- Boosts Confidence Level: To solve questions from the Class 8 Science Assignment 4 worksheet can help students to boost their confidence. A perfect level of confidence can help students to improve the study process.
- Assist the Preparation Process: Solving questions from the Class 8 Science Assignment 4 worksheet can help students in preparing for the chapter.
- Helps in Self Evaluation: Through Science Assignment 4 worksheet, students can easily evaluate themselves. According to the self evaluation process, students can easily improve their preparation.
- Builds a Strong Foundation: It is important for all students to solve questions from the chapter Science Assignment 4. Regular solving questions from the worksheet can help students to build a strong foundation for the chapter Science Assignment 4.
- Enhances the Learning Process: Constant solving of questions from Science Assignment 4 Worksheet can enhance a student's learning process so that students can understand all topics easily.
- Quick Revision: By solving questions from the Class 8 Science Assignment 4 worksheet, students can easily revise all the topics and concepts included in the chapter.
Why Science Assignment 4 Worksheet Class 8 Is Right for You?
It is a must for students to exercise questions from Science Assignment 4 worksheet Class 8 as perfectly right for them. With the help of Science Assignment 4 worksheet Class 8 questions, students can increase their capability of solving questions in a different and creative manner.
Tips to Understand All Questions of Science Assignment 4 Worksheet Class 8 in a Better Way:
Students should understand all questions of Science Assignment 4 worksheet Class 8 in a better way. Better understanding of questions can help students to score good marks in questions which are related to the chapter Science Assignment 4. Those important tips are:
- Finish Off The Chapter: First and foremost tip is to finish off the chapter Science Assignment 4. Students need to complete each and every topic included in the lesson.
- Practise Questions: After completing the chapter Science Assignment 4, students need to practise questions from the Class 8 worksheet. Routine practice of questions can help students in identifying their strengths and weaknesses.
- Note Down the Mistakes: While practising questions, it is very important that students note down their mistakes. Noting down the mistakes is very important as accordingly students can improve their preparation strategies.
- Correction of Mistakes: After noting down the mistakes, it is a must to correct all the mistakes made. Correction of mistakes can help students to solve worksheet questions in a better way.
- Maintain a Positive Attitude: While solving questions from the Class 8 Science Assignment 4 worksheet, students need to maintain a positive attitude. A positive attitude can help students to remove stress and anxiety while preparing for the chapter Science Assignment 4.
- Remain Focused: To understand questions of Science Assignment 4 worksheet, students need to remain focused while preparing for it.
Why Should Students Start Solving Science Assignment 4 Worksheet Class 8 From the PDF?
Science Assignment 4 Class 8 Worksheet is provided in the PDF so that students don’t need to search for them here and there. By solving the questions from Science Assignment 4 worksheet Class 8, students can understand the chapter in a fine way. Routine solving of questions from the Class 8 Science Worksheet can help students increase their comprehension skills. Comprehension skills will help in performing outstanding in the final examination.
What are Included in Science Assignment 4 Worksheet Class 8?
In Science Assignment 4 worksheet Class 8, questions from the chapter are included. After solving questions of the chapter Science Assignment 4, students can also go through the answers included in the worksheet. Answers to these questions of Science Assignment 4 are explained in a detailed manner. As it can also help teachers to make students understand in a better and elaborate way. Through this students can easily identify their skills and flaws for the Science chapter Science Assignment 4.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

One Last Step...

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.

AssignmentsBag.com
Assignments For Class 8 Science
Solve these Science Assignment for Class 8 PDF with answers. These Science Assignment for Class 8 PDF are prepared by our expert teachers according to the latest syllabus of the CBSE Board Exam. We have provided you CBSE Science Assignment for Class 8 PDF with answers to help students to make their preparation better.
Assignments for Class 8 Science have been developed for Standard 8 students based on the latest syllabus and textbooks applicable in CBSE, NCERT and KVS schools. Parents and students can download the full collection of class assignments for class 8 Science from our website as we have provided all topic wise assignments free in PDF format which can be downloaded easily. Students are recommended to do these assignments daily by taking printouts and going through the questions and answers for Grade 8 Science. You should try to do these test assignments on a daily basis so that you are able to understand the concepts and details of each chapter in your Science book and get good marks in class 8 exams.
Free PDF of CBSE Class 8 Science assignments PDF with Answers prepared by our expert from the latest edition of NCERT books. By practicing these Class 8 assignment will help in scoring more marks in your Examinations
Students can also download NCERT Solution free PDF for all subjects to prepare for their upcoming exams. You can also download NCERT Solutions for Class 8 Maths to help you to make your preparation better and score more marks in your examinations.
And more importantly, students have to follow through the assignments after completing their syllabus. So.start working on CBSE Class 8 Science assignment PDF will be a great help to get good marks in the examination.
Assignments for Class 8 Science as per CBSE NCERT pattern
All students studying in Grade 8 Science should download the assignments provided here and use them for their daily routine practice. This will help them to get better grades in Science exam for standard 8. We have made sure that all topics given in your textbook for Science which is suggested in Class 8 have been covered ad we have made assignments and test papers for all topics which your teacher has been teaching in your class. All chapter wise assignments have been made by our teachers after full research of each important topic in the textbooks so that you have enough questions and their solutions to help them practice so that they are able to get full practice and understanding of all important topics. Our teachers at https://www.assignmentsbag.com have made sure that all test papers have been designed as per CBSE, NCERT and KVS syllabus and examination pattern. These question banks have been recommended in various schools and have supported many students to practice and further enhance their scores in school and have also assisted them to appear in other school level tests and examinations. Its easy to take print of thee assignments as all are available in PDF format.
Some advantages of Free Assignments for Class 8 Science
- Solving Assignments for Science Class 8 helps to further enhance understanding of the topics given in your text book which will help you to get better marks
- By solving one assignments given in your class by Science teacher for class 8 will help you to keep in touch with the topic thus reducing dependence on last minute studies
- You will be able to understand the type of questions which are expected in your Science class test
- You will be able to revise all topics given in the ebook for Class 8 Science as all questions have been provided in the question banks
- NCERT Class 8 Science Workbooks will surely help you to make your concepts stronger and better than anyone else in your class.
- Parents will be able to take print out of the assignments and give to their child easily.
All free Printable practice assignments are in PDF single lick download format and have been prepared by Class 8 Science teachers after full study of all topics which have been given in each chapter so that the students are able to take complete benefit from the worksheets. The Chapter wise question bank and revision assignments can be accessed free and anywhere. Go ahead and click on the links above to download free CBSE Science Assignment for Class 8 pdf.
Free PDF download of Class 8 Science assignment pdf with answers created by master educators from the latest syllabus of CBSE Boards. By practicing these Class 8 Science assignments will help you to score more marks in your CBSE Board Examinations. We also give free NCERT Solutions and other study materials for students to make their preparation better.
Students who are searching for better solutions can download the Class 8 Science Assignment pdf with answers to assist you with revising the whole syllabus and score higher marks in your exam.
These Class 8 Science assignment pdf with answers pdf shows up with an answer key with step-by-step answers for students to comprehend the problem at each level and not retain it.
Since you are given all the essential data with respect to the 8th Standard Science Assignment and we trust this definite article is useful. So Students who are planning for the Exam should have to have better-solving skills. What’s more, to have these skills, one should rehearse enough of the 8th Standard Science Assignment. Also, most importantly, students should have to finish the assignments after completing their syllabus. Dealing with the 8th Standard Science Assignment will be more helpful to get good marks in the exam. So begin your preparation on Science Assignment for Class 8 pdf to score higher marks.

We hope this Science Assignment for Class 8 PDF with answers shared with you will help you to score good marks in your exam. We have also other study material like NCERT Solutions, NCERT Book, Exam Question, and simpler paper of Class 8. If You want to score higher in your exam, So practice all this study material and if you have any problem then, write it in the comment box and we will guide you as much as possible.
You can download free assignments for class 8 Science from https://www.assignmentsbag.com
You can get free PDF downloadable assignments for Grade 8 Science from our website which has been developed by teachers after doing extensive research in each topic.
On our website we have provided assignments for all subjects in Grade 8, all topic wise test sheets have been provided in a logical manner so that you can scroll through the topics and download the worksheet that you want.
You can easily get question banks, topic wise notes and questions and other useful study material from https://www.assignmentsbag.com without any charge
Yes all test papers for Science Class 8 are available for free, no charge has been put so that the students can benefit from it. And offcourse all is available for download in PDF format and with a single click you can download all assignments.
https://www.assignmentsbag.com is the best portal to download all assignments for all classes without any charges.
Related Posts

Assignments For Class 3 Kannada

Assignments For Class 3 Moral Science

Assignments For Class 9 Mathematics Area
Question and Answer forum for K12 Students

MCQ Questions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals with Answers
We have compiled the NCERT MCQ Questions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals with Answers Pdf free download covering the entire syllabus. Practice MCQ Questions for Class 8 Science with Answers on a daily basis and score well in exams. Refer to the Materials: Metals and Non-Metals Class 8 MCQs Questions with Answers here along with a detailed explanation.
Materials: Metals and Non-Metals Class 8 MCQs Questions with Answers
Choose the correct option.
Question 1. Metals are (a) shiny (b) hard (c) sonorous (d) all of these
Answer: (d) all of these

Question 2. Non-metals are (a) non-ductile (b) non-sonorous (c) non-malleable (d) all of these

Question 3. Which of the following is a non-metal? (a) Aluminium (b) Oxygen (c) Iron (d) Silver
Answer: (b) Oxygen
Question 4. Metalloids possess the properties of (a) metals (b) non-metals (c) both metals and non-metals (d) none of these
Answer: (c) both metals and non-metals
Question 5. The most reactive metal is (a) copper (b) zinc (c) potassium (d) gold
Answer: (c) potassium
Question 6. Non-metals are (a) generally gases (b) generally liquids (c) generally solids (d) generally solid and gases
Answer: (d) generally solid and gases
Question 7. Which of the following metal is stored in kerosene? (a) Sodium (b) Magnesium (c) Phosphorus (d) Zinc
Answer: (a) Sodium
Question 8. Metal oxides are (a) neutral (b) basic (c) acidic (d) all of these
Answer: (b) basic
Question 9. The non-metal which is liquid at room temperature is (a) bromine (b) chlorine (c) iodine (d) carbon
Answer: (a) bromine
Question 10. Which substance is used for making pencil lead? (a) Sulphur (b) Silicon (c) Graphite (d) Aluminium
Answer: (c) Graphite
Question 11. Which non-metal is used in making glass? (a) Graphite (b) Sulphur (c) Silica (d) None of these
Answer: (c) Silica
Question 12. Which metal is used in wrapping materials? (a) Aluminium (b) Zinc (c) Copper (d) None of these
Answer: (a) Aluminium
Question 13. The metal found in liquid state is (a) mercury (b) silver (c) calcium (d) sodium
Answer: (a) mercury
Question 14. The most ductile metal is (a) silver (b) gold (c) copper (d) aluminium
Answer: (b) gold
Question 15. Non-metals (a) react with water (b) do not react with water (c) both (a) and (b) (d) none of these
Answer: (b) do not react with water
Question 16. Which of the following metals are soft enough to be even cut with a knife? (a) Sodium (b) Potassium (c) Lithium (d) All of these
Answer: (d) All of these
Question 17. Which is the hardest substance? (a) Gold (b) Diamond (c) Aluminium (d) None of these
Answer: (b) Diamond
Question 18. Metals react with acids to produce respective salts with evolution of (a) hydrogen gas (b) oxygen gas (c) CO 2 gas (d) none of these
Answer: (a) hydrogen gas
Question 19. In displacement reactions (a) a more reactive metal displaces a less reactive metal. (b) a less reactive metal displaces a more reactive metal. (c) both (a) and (b) (d) none of these
Answer: (a) a more reactive metal displaces a less reactive metal.
Question 20. Which of the following non-metals are used in fertilisers? (a) Nitrogen (b) Phosphorus (c) Both (a) and (b) (d) None of these
Answer: (c) Both (a) and (b)
Question 21. When non-metal reacts with water (a) hydrogen gas is formed (b) carbon dioxide gas is formed (c) non-metals generally do not react with water. (d) none of these.
Answer: (c) non-metals generally do not react with water.
Question 22. The property of metals by which they can be beaten into thin sheets is called (a) malleability (b) ductility (c) conduction (d) expansion
Answer: (a) malleability
Question 23. Which one of the following is a metal? (a) C (b) N (c) Na (d) O
Answer: (a) C
Question 24. Which of the following does not show the property of malleability? (a) Iron (b) Graphite (c) Aluminium (d) Silver
Answer: (b) Graphite
Question 25. Which one of the following is a good conductor of electricity? (a) Iron (b) Plastic (c) Wood (d) Glass
Answer: (a) Iron
Question 26. The property of a metal by which it can be drawn into wires is called (a) conductivity (b) malleability (c) ductility (d) flexibility
Answer: (c) ductility
Question 27. The metals that produce ringing sounds are said to be (a) malleable (b) sonorous (c) lustrous (d) hard
Answer: (b) sonorous
Question 28. The solution of ash of magnesium ribbon in water is (a) acidic (b) basic (c) neutral (d) none of these
Question 29. When sulphur dioxide is dissolved in water (a) sulphur is formed (b) sulphur trioxide is formed (c) sulphuric acid is formed (d) sulphurous acid is formed
Answer: (d) sulphurous acid is formed
Question 30. What is the chemical formula of sulphurous acid? (a) H 2 SO 4 (b) SO 2 (c) SO 3 (d) H 2 SO 3
Answer: (d) H 2 SO 3
Question 31. Sodium metal is stored in (a) water (b) alcohol (c) kerosene (d) ether
Answer: (c) kerosene
Question 32. Which one of the following metals reacts vigorously with oxygen and water? (a) Sodium (b) Iron (c) Calcium (d) Magnesium
Question 33. What is the chemical formula of copper sulphate? (a) CuSO 4 (b) CuCO 3 (c) CuCl 2 (d) CuO
Answer: (a) CuSO 4
Question 34. Which gas is produced when metals react with acids? (a) Oxygen (b) Nitrogen (c) Hydrogen (d) Carbon dioxide
Answer: (c) Hydrogen
Question 35. Which one of the following does not react with acids? (a) Cu (b) Ni (c) Cr (d) O
Answer: (d) O
Question 36. Which one of the following gas burns with a ‘pop’ sound? (a) Oxygen (b) Hydrogen (c) Chlorine (d) Hydrogen sulphide
Answer: (b) Hydrogen
Question 37. Which one of the following is applied on wounds as an antiseptic? (a) Sodium (b) Iodine (c) Brass (d) All of these
Answer: (b) Iodine
Question 38. Which one of the following is a very reactive non-metal? (a) Sodium (b) Potassium (c) Carbon (d) Phosphorous
Answer: (d) Phosphorous
Fill in the blanks with suitable word/s.
Question 1. Metals are ______ of heat and ______
Answer: good conductors, electricity
Question 2. Iodine is a ______ having lustre.
Answer: non-metal
Question 3. ______ and ______ are kept in kerosene to avoid explosion.
Answer: Sodium, potassium
Question 4. Non-metal oxides are ______ in nature.
Answer: acidic
Question 5. _______ is more reactive than copper.
Answer: Zinc
Question 6. Sulphur forms ______ oxides.
Question 7. Magnesium forms ______ oxides.
Answer: basic
Question 8. _______ is less reactive than iron.
Answer: Copper
Question 9. All metals are hard except _______ and ______
Answer: sodium, potassium
Question 10. Metals have generally_______melting and boiling points.
Answer: high
Question 11. _______ are used in medicines as antiseptic.
Answer: Non-metals
Question 12. The only liquid metal is ______
Answer: mercury
Question 13. ______ is non-metal used in breathing by all living beings.
Answer: Oxygen
Question 14. The metal which produces hydrogen gas on reaction with dilute hydrochloric acid as well as sodium hydroxide solution is ______
Answer: aluminium
Question 15. _______ reacts with cold water vigorously.
Answer: Sodium
Question 16. ………………….. metal is used in car batteries.
Answer: Lead
Question 17. Zinc is used for galvanizing iron to protect it from …………………..
Answer: Rusting
Question 18. Liquid ………………….. is used as rocket fuel.
Answer: Hydrogen
Question 19. The oxides produced by the reaction of non-metals with oxygen are ………………….. in nature.
Answer: Acidic
Question 20. Metals give ………………….. gas when they react with acids.
Question 21. ………………….. is used in electroplating because of its high shiny appearance.
Answer: Chromium
True or False
Question 1. Metals are non-sonorous.
Answer: False
Question 2. Metals react with water.
Answer: True
Question 3. Non-metals cannot be converted into wires.
Question 4. The only liquid metal is bromine.
Question 5. Sodium and potassium do not react vigorously with water and oxygen.
Question 6. Basic solution turns red litmus into blue.
Question 7. Graphite is a good conductor of electricity.
Question 8. Generally, metallic oxides are basic and non-metallic oxides are acidic in nature.
Question 9. Chlorine is not a non-metal.
Question 10. Phosphorus is kept in water.
Question 11. All metals exist in solid form at room temperature.
Question 12. Rust formed on iron object is acidic in nature.
Question 13. Aluminium is more reactive than copper.
Question 14. Non-metals react with water to form a gas which burns with a ‘pop’ sound.
Question 15. All the gases are non-metals.
Question 16. Sodium is a very reactive metal.
Question 17. Rust formed on iron objects is basic in nature.
Question 18. All metals exist in solid form at room temperature.
Question 19. Non-metals react with oxygen to from basic or neutral oxides.
Question 20. Copper is less reactive than aluminium.
Question 21. Sodium is a hard metal that cannot be cut with a knife.
Question 22. Chlorine is a non-metal.
Question 23. Non-metals can be drawn into wires.
Question 24. Metals do not react with water.
Match the following
| Column I | Column II |
| 1. Malleable | (a) Can be transformed into wire |
| 2. Ductile | (b) For making crackers |
| 3. Oxygen | (c) Give sheets on hammering |
| 4. Copper | (d) For disinfecting water |
| 5. Sulphur | (e) All living beings inhale during breathing |
| 6. Diamond | (f) For making electric wires |
| 7. Sonority | (g) For making rails |
| 8. Iron | (h) Hardest non-metal |
| 9. Chlorine | (i) Ringing of bells |
| 10. Platinum | (j) Used in making ornaments |
| Column I | Column II |
| 1. Malleable | (c) Give sheets on hammering |
| 2. Ductile | (a) Can be transformed into wire |
| 3. Oxygen | (e) All living beings inhale during breathing |
| 4. Copper | (f) For making electric wires |
| 5. Sulphur | (b) For making crackers |
| 6. Diamond | (h) Hardest non-metal |
| 7. Sonority | (i) Ringing of bells |
| 8. Iron | (g) For making rails |
| 9. Chlorine | (d) For disinfecting water |
| 10. Platinum | (j) Used in making ornaments |
| Column A | Column B |
| 1. Metallic oxide | a. Turns red litmus into blue |
| 2. Sodium | b. Non-metal |
| 3. A liquid non-metal | c. Basic in nature |
| 4. Chlorine | d. Kept in kerosene |
| 5. Basic solution | e. Bromine |
| Column A | Column B |
| 1. Metallic oxide | c. Basic in nature |
| 2. Sodium | d. Kept in kerosene |
| 3. A liquid non-metal | e. Bromine |
| 4. Chlorine | b. Non-metal |
| 5. Basic solution | a. Turns red litmus into blue |
Hope the information shed above regarding NCERT MCQ Questions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals with Answers Pdf free download has been useful to an extent. If you have any other queries of CBSE Class 8 Science Materials: Metals and Non-Metals MCQs Multiple Choice Questions with Answers, feel free to reach us so that we can revert back to us at the earliest possible.
NCERT Books

NCERT Books for Class 8 Science PDF Download
NCERT Books Class 8 Science : The National Council of Educational Research and Training (NCERT) publishes Science textbooks for Class 8. The NCERT Class 8th Science textbooks are well known for it’s updated and thoroughly revised syllabus. The NCERT Science Books are based on the latest exam pattern and CBSE syllabus.
NCERT keeps on updating the Science books with the help of the latest question papers of each year. The Class 8 Science books of NCERT are very well known for its presentation. The use of NCERT Books Class 8 Science is not only suitable for studying the regular syllabus of various boards but it can also be useful for the candidates appearing for various competitive exams, Engineering Entrance Exams, and Olympiads.
NCERT Class 8 Science Books in English PDF Download
NCERT Class 8 Science Books are provided in PDF form so that students can access it at any time anywhere. Class 8 NCERT Science Books are created by the best professors who are experts in Science and have good knowledge in the subject.
NCERT Books for Class 8 Science – English Medium
The NCERT Science book is a single book and not divided into parts. The book is available in all English, Hindi & Urdu Medium. Students can click on the respective link to download the NCERT Class 8 book.
- Chapter 1: Crop Production and Management
NCERT Solutions for class 8 Science
- Chapter 2: Microorganisms: Friend and Foe
- Chapter 3: Synthetic Fibres and Plastics
- Chapter 4: Materials: Metals and Non-Metals
- Chapter 5: Coal and Petroleum
- Chapter 6: Combustion and Flame
- Chapter 7: Conservation of Plants and Animals
- Chapter 8: Cell – Structure and Functions
- Chapter 9: Reproduction in Animals
- Chapter 10: Reaching the Age of Adolescence
- Chapter 11: Force and Pressure
- Chapter 12: Friction
- Chapter 13: Sound
- Chapter 14: Chemical Effects of Electric Current
- Chapter 15: Some Natural Phenomena
- Chapter 16: Light
- Chapter 17: Stars and The Solar System
- Chapter 18: Pollution of Air and Water
NCERT Books for Class 8 Science – Hindi Medium
- अध्याय 1: फसल उत्पादन एवं प्रबंध
- अध्याय 2: सूक्ष्मजीव: मित्र एवं शत्रु
- अध्याय 3: संश्लेषित रेशे और प्लास्टिक
- अध्याय 4: पदार्थ: धातु और अधातु
- अध्याय 5: कोयला और पेट्रोलियम
- अध्याय 6: दहन और ज्वाला
- अध्याय 7: पौधे एवं जंतुओं का संरक्षण
- अध्याय 8: कोशिका – संरचना एवं प्रकार्य
- अध्याय 9: जंतुओं में जनन
- अध्याय 10: किशोरावस्था की ओर
- अध्याय 11: बल तथा दाब
- अध्याय 12: घर्षण
- अध्याय 13: ध्वनि
- अध्याय 14: विधुत धारा के रासानिक प्रभाव
- अध्याय 15: कुछ प्राकृतिक परिघटनाएँ
- अध्याय 16: प्रकाश
- अध्याय 17: तारे एवं सौर परिवार
- अध्याय 18: वायु तथा जल का प्रदूषण
The NCERT syllabus mainly focuses on this book to make it student-friendly to make it useful for both the students and the competitive exam aspirants. The book covers a detailed Science based on the syllabuses of various boards. NCERT Science Books for Class 8 is perfectly compatible with almost every Indian education state and central boards.
We hope that this detailed article on NCERT Books Class 8 Science helps you in your preparation and you crack the Class 8 exams or competitive exams with excellent scores.
Leave a Comment Cancel reply
You must be logged in to post a comment.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
UP Class 8th Science
Class 8th science (up), unit 1: latest advancements in science and technology, unit 2: man made objects, unit 3: structure of atom, unit 4: minerals and metals, unit 5: general introduction and classification of micro-organisms, unit 6: from cells to organs, unit 7: reproduction in animals, unit 8: adolescence, unit 9: disabilities, unit 10: crop production, unit 11: force and pressure, unit 12: light and optical instruments, unit 13: electric current, unit 14: magnetism, unit 15: carbon and its compounds, unit 16: alternate sources of energy, unit 17: computer.
- Grades 6-12
- School Leaders
Check Out Our 32 Fave Amazon Picks! 📦
Every product is independently selected by (obsessive) editors. Things you buy through our links may earn us a commission.
50 Exciting 4th Grade Science Projects and Experiments
Did you know you can make plastic from milk?

Nothing gets kids more excited for science than hands-on experiments! Watch your 4th grade science students’ eyes light up when they try some of these activities. You’ll find physics, biology, engineering, chemistry, and more. These projects are easy to set up and really help drive the learning home. Get ready for some science fun!
To help you find the right 4th grade science projects and activities, we’ve rated them all based on difficulty and materials:
Difficulty:
- Easy: Low or no-prep experiments you can do pretty much any time
- Medium: These take a little more setup or a longer time to complete
- Advanced: Experiments like these take a fairly big commitment of time or effort
- Basic: Simple items you probably already have around the house
- Medium: Items that you might not already have but are easy to get your hands on
- Advanced: These require specialized or more expensive supplies to complete
4th Grade Science Fair Projects
4th grade stem challenge science projects, 4th grade motion and energy science activities.
- More 4th Grade Science Projects and Experiments
These 4th grade experiments also work well as science fair projects. Try changing up the variables to turn it into a real experiment, then form a hypothesis and find out what happens.
Blow unpoppable bubbles

Difficulty: Easy / Materials: Medium
A soap bubble you can hold in your hand? It’s true! A little glycerin makes the soap bubble layers stronger, so you can even toss them gently from person to person.
Learn more: Unpoppable Bubbles Experiment at Learning Resources
Grow crystal names

No list of 4th grade science projects would be complete without crystals! Kids of all ages love growing crystals, making this an ideal way to learn about supersaturated solutions. The classic experiment gets a new twist when you have kids shape pipe cleaners into their own names first.
Learn more: Crystal Letters at Playdough to Plato
Grow bacteria in petri dishes
Difficulty: Medium / Materials: Medium
Your students will truly feel like scientists when they perform this classic experiment. They’ll prep the dishes with agar, swab different surfaces, and see what bacteria they grow. It’s gross science, but it’s also easy and impressive.
Learn more: Growing Bacteria at Steve Spangler Science
See coastal erosion in action

Here’s a cool experiment to include in your unit on oceans. Build a miniature coastline, then see how wave action erodes the shore.
Learn more: Erosion Experiment at Little Bins for Little Hands
Erupt a lemon volcano

Difficulty: Easy / Materials: Basic
Early chemistry experiments with acids and bases are always a lot of fun. This one uses the natural acids of lemon juice and adds a little food coloring to up the wow factor.
Learn more: Lemon Volcano at STEAM Powered Family
Sink and float to explore density

Adding items like salt or sugar to water changes its density, as does the temperature itself. Turn this into a 4th grade science fair project by experimenting with different solutions and forming hypotheses about the results.
Learn more: Salt Water Density at Science Kiddo
Discover a density rainbow
Difficulty: Medium / Materials: Basic
Colorful, simple, and impressive: It’s the trifecta of 4th grade science experiments! Wow your students by layering colored sugar water as you learn about density, adhesion, and cohesion.
Transform milk into plastic
Plastic seems incredibly modern, but people have been making casein plastic from milk for centuries. In this 4th grade science project, students experiment to create the formula for the best milk plastic. They’ll be amazed at the results!
Simulate an earthquake

The ground under our feet may feel solid, but an earthquake changes that pretty quickly. Use Jell-O to simulate the Earth’s crust, then see if you can build an earthquake-proof structure for a practical and fascinating 4th grade science fair project.
Learn more: Earthquake Simulation at Teaching Science
Test Sharpie solubility

Find out if Sharpie markers are really permanent with this 4th grade science project that uses the scientific method to explore solutes and solvents.
Learn more: Sharpie Solubility at Around the Kampfire

Find out if mood rings really work

Apply the rigors of the scientific method to mood rings ! Find out what makes mood rings change color, then see if they really reflect a person’s mood.
Learn more: Mood Rings Validity Test at Education.com
Create a new plant or animal

Kids will really get into this project, indulging their creativity as they invent a plant or animal that’s never been seen before. They’ll need to be able to explain the biology behind it all, though, making this an in-depth project you can tailor to any class.
Learn more: Create an Organism at I Love 2 Teach
Investigate decomposition

Difficulty: Easy / Materials: Easy
Yup, it’s gross … so kids will love it! Seal food items in a plastic bag and experiment to see what factors affect their decomposition, helped along by a heaping dose of mold.
Learn more: Decomposition at Mystery Science
Assemble a lung model
With just a few supplies including balloons and a plastic bottle, you can make an impressive working model of human lungs. This makes a very cool 4th grade science fair project.
Explore the causes of tooth decay
They hear it from their parents all the time, but this experiment will prove to your students once and for all what can happen to their teeth when exposed to different drinks such as soda and milk. This is one of those classic 4th grade science fair projects every kid should try.
For students who love to tinker, STEM challenges can spark incredible 4th grade science fair projects. Here are some of our favorites for this age group.
Engineer a drinking-straw roller coaster

STEM challenges are always a hit with kids. We love this one, which only requires basic supplies like drinking straws . ( Get more 4th grade STEM challenges here. )
Learn more: Drinking Straw Roller Coaster at Frugal Fun for Boys and Girls
Make a wigglebot

Who knew electricity could be so adorable? Explore the science behind batteries and motors by creating a simple “wigglebot.” Experiment with weights to throw the motor off balance and create fun designs.
Learn more: Wigglebot at Research Parent
Construct a working flashlight
You’ll only need a few supplies to guide your students in building their own LED flashlights. They’ll learn how electricity travels and the way circuits work. The slideshow available through the link makes this lesson a breeze for teachers too.
Learn more: DIY Flashlight at Mystery Science
Build a hovercraft

It’s not exactly the same model the military uses, but this simple hovercraft is a lot easier to build. An old CD and a balloon help demonstrate air pressure and friction in this fun 4th grade science experiment.
Learn more: DIY Hovercraft at Education.com
Create a smartphone projector

No projector in your classroom yet? No problem! Have your students help you construct one for your smartphone using a cardboard box and large magnifying glass . They’ll learn about convex lenses and how the brain processes images too.
Learn more: DIY Smartphone Projector at The STEM Laboratory
Set up a pulley system

The science of machines never fails to fascinate kids. In this experiment, they’ll design their own pulley system to make it easier to lift an object.
Learn more: DIY Pulley at 123 Homeschool 4 Me
Design a working elevator
Engineering activities make for amazing hands-on learning. Challenge your 4th grade students to build an elevator that can safely lift a certain amount of weight.
Make a model seismometer

Explore the science of seismology and learn how scientists study earthquakes and their effects. This model seismometer is easy to build and fun to experiment with.
Learn more: Model Seismometer at Science Sparks
Conduct an egg drop
Here’s one more classic to add to our list of 4th grade science experiments: the egg drop! The great thing about this project is that kids can do it at any age, with different materials and heights to mix it up. Hit the link below to get an egg drop project designed just for 4th graders.
Learn more: Egg Drop Challenge Ideas
Demonstrate Newton’s laws of motion with balloon rockets
Who doesn’t love balloon rockets?! Your students will have a blast(off) displaying Newton’s third law of motion while learning about physics.
Many 4th grade science standards include units on energy and motion. These energy science activities offer cool hands-on ways to spice up your classroom lessons.
Flick marbles to learn transfer of energy

This experiment is a bit of a thinker: What will happen when one moving marble hits several stationary marbles sitting in a row? Flick the first marble and find out!
Learn more: Marble Energy Transfer at Frugal Fun for Boys and Girls
See energy transfer in action with sports balls
Place a tennis ball on top of a basketball and bounce them together to see how energy transfers from one object to another. This one is very easy, and kids will love seeing how high they can get the balls to bounce!
Go an on energy scavenger hunt

Emphasize the fact that energy is all around us in one form or another with this easy, free printable energy science activity. For a more advanced version, help students identify each kind of energy (kinetic, stored, heat, etc.) they find.
Learn more: Energy Scavenger Hunt at The Science Penguin
See a heat-powered windmill demonstrate convection
Heat rises, and its interaction with cooler air creates convection currents. Find out how we can put convection to work for us with this 4th grade science craft project.
Capture waves in a bottle

Here’s a quick and easy way to show wave action in a no-mess way. You don’t need to add a little ship to the bottle, but it does make it more fun!
Learn more: Waves in a Bottle at What I Have Learned Teaching
Assemble a wave machine
Turn this one into a class cooperative activity, or try it as a science fair project idea. Either way, it’s an incredibly fascinating way to demonstrate the energy science of waves.
Use a Slinky to demonstrate types of waves
A Slinky is more than just a toy—it’s also a terrific science manipulative! Use it to see waves in motion, both longitudinal and transverse.
Watch gravity beads prove Newton’s laws

You’ll need a loooooooong string of beads for this experiment. Make your own by taping dollar-store strings together, or buy a long bead garland . Pile them in a cup and get the beads going; it’s fascinating to watch inertia and gravity at work.
Learn more: Gravity Beads at Teach Beside Me
Spin marble tops to learn about inertia

Glue together marbles in a variety of pyramidal patterns to form tops, then form hypotheses about which will spin best. Afterwards, kids will have fun new toys to play with!
Learn more: Marble Tops at KidsActivities.com
Visualize the second law of motion with soda cans
Newton’s second law, concerning acceleration, force, and mass, can be a little hard to understand. This easy 4th grade science demo makes it a little easier to visualize.
More 4th Grade Science Projects and Activities
Use these cool science experiments to encourage a love of science, at home or in the classroom!
Measure a magnet’s attraction force

Fourth grade science students already know that magnets attract metal objects. In this experiment, they’ll measure to see how close a magnet needs to be to an object for the attraction to work. Mix things up with different sizes of magnets and objects of various weights.
Learn more: Magnet Measurements at Ashleigh’s Education Journey
See light refraction in action

This seems more like a magic trick, but we promise it’s science! Make colors seem to appear and disappear, change numbers into letters, and more.
Learn more: Light Refraction at Ronyes Tech
“Draw” on water with dry-erase marker
This is another one of those mind-blowing science demos that kids will want to try over and over again. Draw on a shallow bowl or plate with dry-erase markers , then slowly add water. The marker (which is insoluble in water) will float to the top!
Paint with sunscreen

Prove that sunscreen really does provide protection from harmful UV rays. Turn this into a full-blown experiment by trying different SPFs or comparing it to other creams or lotions without SPF.
Learn more: Paint With Sunscreen at Team Cartwright
Become human sundials

Choose a sunny day and grab some sidewalk chalk—your students are about to become sundials! They’ll practice measuring skills and learn about the movement of the sun across the sky.
Learn more: Human Sundial at Rhythms of Play
Mine for chocolate chips

If you’re learning about mineral resources, this quick hands-on activity is an interesting way to explore the effects of mining. Kids have two minutes to find as many chocolate chips as they can in a cookie. Will they smash it up and destroy it entirely? Pick them out one by one? This experiment can lead to intriguing discussions.
Learn more: Mining for Chocolate Chips at Sarah’s STEM Stuff
Assemble an edible DNA model

Use licorice sticks, four different-colored candies or fruits, and toothpicks to build an edible strand of DNA. Learn about chemical bonds and the helix shape, then eat your creation!
Learn more: Edible DNA Model at wikiHow
Layer an edible soil model

Digging in the dirt is fun, but it’s even more fun when you can eat the dirt when you’re finished! Create edible soil-layer models, complete with gummy worms, for a simple earth science project. ( Find more edible science projects here. )
Learn more: Edible Soil Layers at Super Teacher Blog
Turn a penny green

Experiment with simple chemical reactions as you turn pennies green using vinegar. (Don’t forget to tell students that the Statue of Liberty is green for this very same reason!)
Learn more: Penny Reactions at Buggy and Buddy
Use marshmallows to explore Boyle’s law
Seeing Boyle’s law (which relates pressure and volume of gasses) in action makes it a little easier to understand and remember. This simple 4th grade science experiment uses marshmallows to make a great visual.
Learn more: Boyle’s Law at Hojo’s Teaching Adventures
Form ocean currents

Learning about oceanography? Demonstrate how ocean currents form using warm and cold water (and a few plastic sea creatures for extra fun!).
Learn more: Ocean Currents at Life Over C’s
Understand the impact of non-renewable resources

This is a neat Earth Day activity . Discuss the differences between renewable and non-renewable resources, then have your class form “companies” to “mine” non-renewable resources. As they compete, they’ll see how quickly the resources are used. It’s a great tie-in to energy conservation discussions.
Learn more: Non-Renewable Resources at The Owl Teacher
Explore blood components

Use simple kitchen supplies to create a jar full of “blood” that includes plasma, platelets, red blood cells, and white blood cells. (You can even snack on the blood cells along the way!)
Learn more: Blood Model at Almost Supermom
Create cool colors with candy
Learn about diffusion in the sweetest way! Grab a bag of Skittles for this quick and easy 4th grade science project.
Wow them with glowing water

Your students will ooh and aah at the result of this exploratory way to show phosphors in action with a black light, different types of water, and a highlighter. The results of this experiment might surprise both you and your students!
Learn more: Glowing Water Experiment at Cool Science Experiments Headquarters
Keep the STEM excitement going with these 25 Fantastic Free 4th Grade Math Games .
Plus, sign up for our newsletters to get all the latest teaching tips and ideas, straight to your inbox..

You Might Also Like
55 Terrific 3rd Grade Science Projects Anyone Can Do
Engage students in the classroom, or prep for the science fair! Continue Reading
Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
- NCERT Solutions
- NCERT Class 8
- NCERT 8 Science
- Chapter 4: Materials Metals And Non Metals
NCERT Solutions For Class 8 Science Chapter 4- Materials: Metals And Non Metals
Ncert solutions for class 8 science chapter 4 – free pdf download.
*According to the latest update on the CBSE Syllabus 2023-24, this chapter has been removed.
NCERT Solutions for Class 8 Science Chapter 4 Materials: Metals and Nonmetals provided here is one of the most important resources for Class 8 students. The metals and nonmetals solutions are given here so that students can clear their doubts easily and learn the chapter in a more efficient way. Students must learn the concepts of metals and nonmetals properly as it will be helpful for them in higher standards. All the NCERT Solutions for Class 8 Science are prepared according to the latest CBSE syllabus. Check the Chapter 4 Class 8 Science NCERT Solutions PDF given below.
BYJU’S brings you NCERT Solutions Class 8 Science , designed by our subject experts to facilitate a smooth and precise understanding of concepts. These CBSE Class 8 NCERT Solutions have detailed step-by-step explanations of questions given in the textbooks. It covers all the important concepts with detailed explanations to promote a better conceptual understanding in students.
NCERT Solutions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals
carouselExampleControls111

Previous Next
Access Answers to NCERT Class 8 Science Chapter 4 Materials – Metals and Non-Metals
Exercise Questions
1. Which of the following can be beaten into thin sheets?
(b) Phosphorus
(c) Sulphur
Answer is a) Zinc
Explanation:
Here, zinc is a metal with malleability and ductility whereas Phosphorus, Sulphur and Oxygen are nonmetals which lack malleability and ductility.
2. Which of the following statements is correct?
(a) All metals are ductile.
(b) All non-metals are ductile.
(c) Generally, metals are ductile.
(d) Some non-metals are ductile.
Answer is (c) Generally, metals are ductile.
Ductility is a property where a substance can be drawn into thin wires. Generally, metals are ductile with mercury as the exception.
3. Fill in the blanks.
(a) Phosphorus is a very _________non-metal.
(b) Metals are _________ conductors of heat and ____________ .
(c) Iron is ____________reactive than copper.
(d) Metals react with acids to produce ____________ gas.
(a) Phosphorus is a very reactive non-metal.
(b) Metals are good conductors of heat and electricity.
(c) Iron is more reactive than copper.
(d) Metals react with acids to produce hydrogen gas.
4. Mark ‘T’ if the statement is true and ‘F’ if it is false.
(a) Generally, non-metals react with acids. ( )
(b) Sodium is a very reactive metal. ( )
(c) Copper displaces zinc from zinc sulphate solution. ( )
(d) Coal can be drawn into wires. ( )
5. Some properties are listed in the following table. Distinguish between metals and non-metals on the basis of these properties.
| Lustrous | Dull | |
| Hard | Soft | |
| Have property of Malleability | Do not have a property of Malleability | |
| Have property of Ductility | Do not have the property of Ductility | |
| Good conductor of Heat | Bad Conductor of Heat | |
| Good conductor of Electricity | The bad conductor of Electricity |
6. Give reasons for the following.
(a) Aluminium foils are used to wrap food items.
(b) Immersion rods for heating liquids are made up of metallic substances.
(c) Copper cannot displace zinc from its salt solution.
(d) Sodium and potassium are stored in kerosene
a) Aluminium is malleable and can be drawn into thin sheets; hence aluminium foils are used to wrap food items
b) Immersion rods for heating liquids are made up of metallic substances because metals are good conductors of heat and electricity.
c) Copper cannot displace zinc from its salt solution because zinc is more reactive than copper.
d) Sodium and Potassium are highly reactive metals which readily reacts with atmospheric oxygen to catch fire; hence sodium and potassium are stored in kerosene.
7. Can you store lemon pickle in an aluminium utensil? Explain.
Pickle contains acids which react with aluminium metal to produce salt and hydrogen. Hence pickle is not stored in aluminium utensil.
8. Match the substances given in Column A with their uses given in Column B.
| A | B |
| (i) Gold | (d) Jewellery |
| (ii) Iron | (e) Machinery |
| (iii) Aluminium | (c) Wrapping food |
| (iv) Carbon | (f) Fuel |
| (v) Copper | (b) Electric wire |
| (vi) Mercury | (a) Thermometers |
9. What happens when
(a) Dilute sulphuric acid is poured on a copper plate?
(b) Iron nails are placed in a copper sulphate solution?
Write word equations of the reactions involved.
(i) No reaction occurs when dilute sulphuric acid is poured on a copper plate. However, when concentrated sulphuric acid is poured on a copper plate, hydrogen gas evolves along with the formation of blue coloured copper sulphate crystals. The chemical reaction for the reaction between concentrated sulfuric acid and copper is: Cu + H 2 SO 4 (conc.) -> CuSO 4 + H 2
ii) Iron, being more reactive, displaces copper from copper sulphate. In this reaction, the blue colour of copper sulphate fades and there is a deposition of copper on the iron nail.
Fe + CuSO 4 → FeSO 4 + Cu
10. Saloni took a piece of burning charcoal and collected the gas evolved in a test tube.
(a) How will she find the nature of the gas?
(b) Write down word equations of all the reactions taking place in this process.
a) In a test tube containing gas, add a few drops of water. Now cover the test tube and shake well. After shaking, test the solution with blue litmus. It will change from blue to red. Thus, gas is acidic in nature.
b) Charcoal reacts with oxygen to form carbon dioxide gas.

11. One day Reeta went to a jeweller’s shop with her mother. Her mother gave old gold jewellery to the goldsmith to polish. Next day when they brought the jewellery back, they found that there was a slight loss in its weight. Can you suggest a reason for the loss in weight?
In order to polish the gold ornament, it is to be dipped into a liquid called aqua regia (a mixture of hydrochloric acid and nitric acid). On getting immersed in aqua regia, the outer layer of gold dissolves and an inner shiny layer appears. The dissolving of the layer causes a reduction in the weight of the jewellery.
Topics covered in NCERT Solutions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals
| 4.1 | Physical Properties of Metals and Non-metals |
| 4.2 | Chemical Properties of Metals and Non-metals |
| 4.3 | Uses of Metals and Non-metals |
Students can utilise the NCERT Solutions for Class 8 available in Class-wise and chapter-wise formats for any quick references to comprehend important and complex topics. Further, these solutions also provide tips and tricks to score high marks in the CBSE Class 8 Science examination.
BYJU’S is India’s largest K-12 learning app with top-notch teachers from across the nation with excellent teaching skills. Find CBSE notes and question papers for various other subjects like Mathematics, Biology, Chemistry and many competitive exams as well. Enjoy learning with a great experience. Stay tuned to learn more about NCERT Solutions and preparation tips. To know more about NCERT Solutions , you can register with BYJU’S or download our app for simple and interesting content.
Frequently Asked Questions on NCERT Solutions for Class 8 Science Chapter 4
List the topics and subtopics covered in chapter 4 of ncert solutions for class 8 science., what are the uses of metals given in chapter 4 of ncert solutions for class 8 science, where can i find the ncert solutions for class 8 science chapter 4 online.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Best solution website
the best website ever.
best solution website and best learning app
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Talk to our experts
1800-120-456-456
Revision Notes for CBSE Class 8 Science
- Revision Notes

CBSE Class 8 Science Revision Notes
Science is a multi-disciplined academic field that helps students learn various concepts and topics. These professionally made Class 8 Science Notes by expert teachers at Vedantu can help students understand complex concepts related to all the chapters. Our Notes for Class 8 Science are designed to make even the toughest topic seem easy to learn for students. Based on the latest syllabus, students can use these Science Notes Class 8 and prepare well for their examination with ease.
NCERT Solutions Class 8 Science Notes PDF can be easily accessed online. You can also download NCERT Solutions for Class 8 Maths to help you to revise complete syllabus and score more marks in your examinations.
Detailed Overview of Class 8 Science Revision Notes
Class: | 8 |
Subject: | Science |
Number of Chapters: | 18 |
Content Type: | Text, Videos, Images and PDF Format |
Academic Year: | 2024-25 |
Medium: | English |
Available Materials: | Chapter Wise |
Other Materials |
Download CBSE Class 8 Science Revision Notes 2024-25 PDF
Also, check CBSE Class 8 Science revision notes for other chapters:
CBSE Class 8 Science Revision Notes | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

CBSE Quick Revision Notes for Class 8 Science - Free PDF Solutions
Having 8th Standard Science Notes in a PDF format is one of the most convenient ways to do last minute revision and leave no room for confusion regarding any topic. The best part is that these Notes for 8th Class Science are available for free download from the main website of Vedantu. All these revision notes are carefully prepared to explain to students each chapter in a detailed way and clear their queries in the best possible way. Get 8th Class Science Notes PDFs to study at your pace and convenience without worrying about a stable internet connection.
Revision Notes for Class 8 Science
Chapter 1 - Crop Production and Management - This chapter covers how the food is being produced through various agricultural practices. It also elaborates that crops are majorly categorized into two kinds in India as per the season, i.e., Kharif and rabi. All the primary practices of crop production are explained thoroughly.
Chapter 2 - Microorganisms – Friend and Foe - This chapter starts with the easy definition of microorganisms, and then its concepts are described with the help of various activities. After that, it elaborates how microorganism plays a vital role in our lives and causes multiple diseases to humans, animals and plants.
Chapter 3 - Synthetic Fibres and Plastics - It talks about synthetic fibres and their kinds (nylon, acrylic, polyester, and rayon). Apart from this, the characteristics of synthetic fibres are elaborated thoroughly. The chapter also introduces plastics, their properties and their effect on the environment.
Chapter 4 - Materials – Metals and Non-Metals - The chapter describes the physical and chemical properties and uses of metals and non-metals. To describe all these properties in an easy way, various activities are included in the notes.
Chapter 5 - Coal and Petroleum - This chapter is about natural resources and their categorization. It elaborates that coal, petroleum and natural gas are fossil fuels that are generated from the dead residues of living organisms millions of years ago. It also explains how coke, coal gas and coal tar are generated from coal and refining petroleum and other concepts of natural gas.
Chapter 6 - Combustion and Flame - It covers the topic of combustion and its different kinds. It is thoroughly elaborated with the help of activities. Topics like measures to control the fire, concept of flame and its structure, fuel, efficiency and harmful effects of burning the fuel are discussed in the chapter.
Chapter 7 - Conservation of Plants and Animals - This chapter focuses on deforestation and its cause, consequences of deforestation, ways to conserve wildlife and forests, etc. It also covers topics like flora and fauna, wildlife sanctuary, biosphere reserve, migration, national park, endemic species, reforestation and more.
Chapter 8 - Cell – Structure and Functions - The chapter explains that the cells are the smallest living part of organisms. It exhibits a wide range of shapes and sizes and how several cells differ from organism to organism. It also elaborates on cell structure and function.
Chapter 9 - Reproduction in Animals - In this chapter, two types of reproduction, i.e., sexual and asexual reproduction, are explained. The sexual reproduction topic is elaborated thoroughly, including male reproductive organs, female reproductive organs, fertilization, viviparous and oviparous animals and development of the embryo.
Chapter 10 - Reaching the Age of Adolescence - This chapter explains the role of hormones that trigger a change in the human body and transform a child into an adult. It also explains topics like Adolescence, puberty and changes occurring during puberty.
Chapter 11 - Force and Pressure - One of the most critical chapters explains how a force emerges due to the interaction of two items and how force can be a push or a pull. It can change the state of shape or motion of an item. Further, topics like contact forces, non-contact forces, pressure, the pressure applied by gases and liquids and atmospheric pressure are explained thoroughly.
Chapter 12 - Friction - It explains topics like the force of friction, features are affecting friction and how friction can be maximised and minimised and fluid friction.
Chapter 13 - Sound - As we know, sound plays a vital part in human lives by guiding us to communicate with each other. The chapter elaborates how sound is produced, how it travels, how we hear it and why some sounds are louder than others.
Chapter 14 - Chemical Effects of Electric Current - It describes that a few liquids are a perfect conductor of electricity and a few are a poor conductor of electricity. It also explains the topic of ‘chemical effects of electric current’ and ‘electroplating’.
Chapter 15 - Some Natural Phenomena - The chapter discusses the disastrous natural phenomena, i.e. earthquakes, tsunami and lighting. It also highlights some steps to reduce the destruction of such natural phenomena.
Chapter 16 - Light - Our world is full of colours and pretty things. In this chapter, the topic of light and how we can see things around us are covered. It elaborates on the law of reflection, regular and diffused reflection and numerous reflections. It further explains that sunlight is called white light and it consists of 7 colours. Even the human eye is described with a diagram. The chapter also covers steps to protect eyes and how visually impaired people can write and read.
Chapter 17 - Stars and The Solar System - It explains the celestial objects like the moon, stars and constellations thoroughly. Later, the solar system is also elaborated with the features of every planet. It also includes comets, asteroids, meteors and meteorites and artificial satellites.
Chapter 18 - Pollution of Air and Water - This chapter talks about the harmful changes in our surroundings because of air and water pollution. It explains the greenhouse effects and ways to reduce air and water pollutants and purify them.
Importance of Revision Notes CBSE Class 8 Science
Students of Class 8 have a detailed Science syllabus consisting of 18 chapters that deal with Physics, Chemistry, and Biology concepts. Students can learn about microorganisms, crop production, synthetic fibres, metals, non-metals, plant and animal conservation, reproduction, cell structure, and much more. They are also introduced to the concepts of Force, Pressure, Sound, Friction, Light, Chemical Effects of Electric Current, Pollution, Solar System, etc. These topics are covered in the important chapters of Class 8 Science. Students can download Class 8th Science Notes in order to get all the details of the chapters easily. They will be able to understand the concepts better and hence prepare for their examination.
All the concepts, topics, processes, functions, and principles are explained in detail by the Science experts at Vedantu. Students can read the notes and then delve deeper into the details of the chapters and gain a strong conceptual foundation. Science chapters of Class 8 will teach the students a lot about the different occurrences that take place around us. Students will be able to understand the chapters in detail with the help of these revision notes. Thus, downloading Science Notes for Class 8 PDF is one of the ideal choices for students.
Benefits of Class 8 Science Notes PDF
The Class 8 Science notes have been prepared by professionals at Vedantu in order to provide a conceptual foundation for the science chapters to Class 8 students. The topics and concepts have been explained in simple language. Thus, students are able to easily understand the details.
Vedantu’s experts have ensured that every single topic is explained with the help of examples. The notes provide detailed insights into the chapters. For instance, the Class 8 Science Chapter 1 Notes talk about the production of crops and different agricultural procedures. The students will be able to learn a lot of things from the notes.
The revision notes have been prepared as study materials for the students of Class 8. They can use the revision notes to prepare themselves for the examination and score good marks in the finals.
In case they miss out on classes at some point in time, students don’t have to worry about missing out on the chapters. They can quickly catch up with the rest of the class by referring to the revision notes.
Downloading quick revision notes for Science Class 8 will also enable the students to complete their revision way before their exams. They don’t have to go through the whole textbook in order to read the chapters. Just following the notes and reading the important parts will be enough.
Download Revision Notes for Class 8 Science to Prepare Well
Do you want to excel in your studies but need some help in Class 8 Science? For students looking for some assistance with the science chapters, Vedantu’s revision notes are the best source. Students can get all the details about the chapters from these notes and prepare the subject in the best way. Download the revision notes right now and gain a proper conceptual foundation in the chapters.
CBSE Class 8 Science Study Materials
|
|
|
|
|
|

FAQs on Revision Notes for CBSE Class 8 Science
Q1. Why is it essential to revise before your Class 8 Science exam?
Ans: There are multiple reasons why revision is essential for you before taking an exam. During the revision process, you grasp small details involved in the topic and improve your analytical skills. You even find out your weak and strong topics and accordingly work on them to score better. If you use our Class 8 Science Notes , you don’t need to go through the entire textbook to prepare for your exam.
Q2. Do Notes for Class 8 Science cover all the crucial points and topics?
Ans: Science is an incredibly diverse and interesting subject that allows students to learn about various topics in life. As these notes are well-prepared by the subject professionals, they covered every important topic and thoroughly explained the topic to leave no room for doubts. You can undoubtedly call these 8th Class Science Notes your best friend during the exam period.
Q3. Is Class 8 Science easy?
Ans: Several factors determine whether Class 8 is easy or not. This includes the students’ ability and how well they prepare for examinations and their comprehension ability. Hence, there isn’t a simple answer to this question. Students who can comprehend the reasoning, underlying processes and systems will find Class 8 Science easy to cope with and they will be able to score good grades in the subject. It's also important to practice to become familiar with all the different types of questions that can be asked from the topics.
Q4. How many chapters are there in the NCERT Class 8 Science book?
Ans: There are 18 chapters in the NCERT Class 8 textbook . As a result, students in Class 8 may learn a lot of intriguing material and develop their understanding of a variety of topics. Hence, students must pay attention to all the chapters and not skip any of them. They must also practice the problems judiciously if they wish to attain good grades in the Class 8 Science exam . Each concept is crucial and must be learnt properly.
Q5. Do I need to practice all the questions provided in the NCERT book?
Ans: Yes, you should practice all the questions in the Class 8 NCERT Science book . You can also use a variety of other reference books. However, for Class 8, the NCERT book is more than sufficient for good comprehension and the tests. Check out Vedantu 's Class 8 Science Notes for additional information. The NCERT is the optimum solution for addressing all the concepts and doubts of the students regarding the content of the syllabus of Class 8 Science . Solving all the problems will enable you to get acquainted with the format and the different problems related to your course.
Q6. Can I download the Class 8 Science notes?
Ans: Yes. Students can download the Class 8 Science Notes from Vedantu's website. These revision notes contain all the concepts and topics of all chapters of Class 8 Science . To download the notes for offline use, you should follow these steps.
Visit the page Revision Notes for CBSE Class 8 Science .
The webpage with Vedantu's chapter-wise revision notes for Class 8 Science will open.
To download the revision notes , click on the chapter for which you want to download the notes.
The page with the notes of that chapter will open up.
Click on the Download PDF button. The pdf will be saved on your device and you can view the notes offline.
Q7. Is Vedantu’s revision notes enough to get good marks in Class 8 Science?
Ans: The revision notes are created in such a way that pupils can readily comprehend the concepts. They can be used by students who are not able to get their questions answered during class time and want to do well in the Class 8 examinations . In terms of test preparation, these notes are among the finest self-study aids since they help students enhance their ability to solve problems. Students may access Vedantu's Class 8 Science Revision Notes from anywhere and at any time, without any time restrictions or restrictions on accessing them.
REVISION NOTES FOR CLASS 8
Cbse class 8 study materials, home tuitions in india.

45,000+ students realised their study abroad dream with us. Take the first step today
Here’s your new year gift, one app for all your, study abroad needs, start your journey, track your progress, grow with the community and so much more.

Verification Code
An OTP has been sent to your registered mobile no. Please verify

Thanks for your comment !
Our team will review it before it's shown to our readers.

Science Projects for Class 8

- Updated on
- Jan 15, 2024

Science and its applications are way beyond the textbook experiments and class activities we did as kids. But to boost the interest of students in science and help them learn the basics, these simple and interesting experiments and observation projects enable them to understand varied basic concepts of Science. Often class 8 students are asked to submit their science project by the end of the year, in which they have to choose a topic, carry out an experiment and state the conclusion of their chosen scientific principle through their science project. Read this blog to learn about the best and most interesting science projects for class 8 which can help you delve deeper into the magic of science while also getting a higher score in your science class!
This Blog Includes:
Best science projects for class 8, top 45 science models for class 8, science projects for class 8 for exhibitions, measuring glucose in food, boiling point of water, baking soda volcano, red cabbage indicator, potato battery , thermal conductivity of metals, pinhole camera, create your own biodiesel, astronomical telescope, cotton candy machine, create a working model of the human heart.
Download NCERT Solutions Class 7 Science Chapter 8 Important Questions and Answers PDF
For science projects for class 8, students are generally asked to perform an experiment, conduct an activity or design a working model which elucidates the principle that they have chosen. Thus, we will explain to you how you can perform simple experiments as well as create a working model.
Here is a list of top Science Models that you can try:
- Drop an egg to prove the first law of motion
- Assemble a Newton’s cradle
- Blow out a candle with a balloon
- Relight a candle without touching it
- Measure and compare lung capacity
- Build an infinity mirror
- Brew up some root beer
- Construct a cup holder
- Assemble a spring scale
- Extract bismuth from Pepto-Bismol
- Make a solar desalinator
- Perform a starch test with iodine
- Keep your hands warm
- Explore symbiosis with nitrogen-fixing bacteria
- Crash cars for science
- Discover the center of gravity
- Power up homemade batteries
- Examine the connection between personality and memory
- Concoct and test your own shampoo
- Fuel a film canister rocket
- Stand on a pile of paper cups
- Create a rainbow of flames
- Get your laundry really clean
- Test water quality
- Fingerprint analysis
- Create a roller coaster loop
- Extract your DNA
- Separate water into hydrogen and oxygen
- Build a circuit to detect ripe produce
- Discover the strength of interleaved paper
- Guide a growing plant through a maze
- Find out if peppermint improves reaction time
- Raise a hydraulic elevator
- Grow a carbon sugar snake
- Generate a Lichtenberg figure
- Teach a computer to play tic-tac-toe
- Cast animal tracks
- Construct a Rube Goldberg machine
- Block the sun’s UV rays
- Turn juice into spheres
- Measure your threshold of hearing
- Measure how body temperature and reaction time vary throughout the day
- Model the human cardiovascular system
- Use spin tests to discover how your eyes and ears affect your balance and dizziness
- Determine how much huddling reduces heat loss in warm-blooded animals
Here are some of the Science Projects for Class 8th Exhibitions that you can try:
The majority of the meals that we take in a day include carbohydrates or sugar. But the form in which we consume them is different and the one that is found inside the human body is different. In our body, glucose is broken down into simpler substances and you can opt for a glucose experiment for your Class 8 science project through which you can analyse the presence of carbohydrates in glucose and our food. As per your choice, collect some food items of daily life along with food dye, room temperature water, plastic glasses, knife and record your observations. You will also need glucose reading strips for this science project and create a positive strip by dipping a glucose strip into a sugar-based or glucose solution as well as a negative strip by dipping a glucose strip into the water. Then, test each of the foods you have taken with these two strips and record your observations.
Water in its pure form has a specific boiling point and if we keep on adding some solutions or chemicals to water, its boiling point tends to change. For this simple and interesting science project for Class 8, you need some basic household items, i.e. sugar, salt, lemon juice, oil, oranges, jaggery, tea, etc. Ask an adult for supervision and assistance in helping you test the boiling point of water while adding a different item every time. Record your observation for these items and create your Science project accordingly.
Having a homemade volcano is an amazing science science project. You need to build a cone shaped structure similar to a volcano with a container in the middle. Then in that container, add vinegar. Now, to demonstrate the working of the volcano, add baking soda along with some food colour to the vinegar. The volcano will erupt with effervescence which is generated due to the reaction of baking soda and vinegar thus releasing CO2 gas.

The red cabbage that we eat consists of a natural pH indicator that we can use to identify the nature of various substances. Since not many people are aware of this natural indicator, you can use red cabbage to create a unique Science project for Class 8. First, extract the juice of red cabbage and take some paper cups. Now, you need a specific amount of the following items in each paper cup:
- Baking Soda
- Lemon Juice
Now, pour the red cabbage juice into each cup until the colour of the liquid/powder [baking soda] changes. Note down your observations regarding the nature of acid and base in each item as indicated by the red cabbage and astonish your class with an amazing science experiment!
Do you know that the potato we use in our food can actually convert chemical energy into electrical energy? As a potato has many nutrients that act as chemicals in a particular setup, it can be used as an electrical battery and thus a unique working model for your Class 8 science project! You need a few potatoes to create your own potato battery and the process is quite simple. Take two potatoes, zinc and copper electrodes, 6 alligator clips and a small bulb/digital clock. First, insert the zinc and copper electrodes in the potatoes and then use alligator clips to attach the electrodes with the clock or bulb [adult supervision necessary].
You will see that the potatoes will charge electrodes and then the clock or the bulb will turn on automatically.
Amongst the most popular science projects for Class 8, you must have come across this one in day-to-day scenarios. Have you noticed that the pan in which we cook gets all heated up including the handles of it or the tong with which we cook our chapatis becomes hot? This is because of thermal conductivity in solids which means that the molecules of solids when heated up move in different directions and spread the heat. For your project, you can pick a bunch of solid items and check at which temperature the entire object gets heated up and up to which temperature.
You must have come across various high-end cameras, but a pinhole camera is something that you can construct from simple household products. The camera does not contain any type of lens rather it is a light-proof box which has a small hole on one of its sides. Light from one side will fall into it and then by using its effort, it will reflect the picture on the other side of it. the image formed by this lens is inverted, thus, it becomes a tree basis of your entire project.
Science Working Model for Class 8
Here is a list of Science Working Models for Class 8:
- Wind Turbine
- Biogas plant
Now, let’s pick up some of these and learn how to make these models:
Biodiesel is made from vegetable oil and animal-fat based diesel fuel including long-chain alkyl and esters. Biodiesels are made by chemically reacting lipids, e.g., vegetable oils, animals with alcohol to produce fatty acid esters. The project is for renewable energy, physics, biology.
A telescope is an optical instrument to make observations to make distant objects look closer with an arrangement of lenses or curved mirrors. The rays of light are collected and focus on resulting in magnified images.
A cotton candy machine follows the same framework everywhere in the world and you can make it right at home. When sugar is heated, it becomes liquid caramel. If liquid caramel is pushed through tiny holes to the outside by centrifugal force when the container continuously rotates, cotton candy is made by recrystallization.
One of the most famous working models is the human heart’s working model. The project only requires a physioball, PVC Pipe cap, balloons, and drip pipe. The project will barely take an hour to complete and you will be able to impress your teacher with it.
Drop an egg to prove the first law of motion Assemble a Newton’s cradle Blow out a candle with a balloon Relight a candle without touching it Measure and compare lung capacity
The majority of the meals that we take in a day include carbohydrates or sugar. But the form in which we consume them is different and the one that is found inside the human body is different. In our body, glucose is broken down into simpler substances and you can opt for a glucose experiment for your Class 8 science project through which you can analyse the presence of carbohydrates in glucose and our food.
Follow Leverage Edu for more interesting and informative articles on school education .
Team Leverage Edu
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Contact no. *
8th std 3rd term projects

Leaving already?
8 Universities with higher ROI than IITs and IIMs
Grab this one-time opportunity to download this ebook
Connect With Us
45,000+ students realised their study abroad dream with us. take the first step today..

Resend OTP in

Need help with?
Study abroad.
UK, Canada, US & More
IELTS, GRE, GMAT & More
Scholarship, Loans & Forex
Country Preference
New Zealand
Which English test are you planning to take?
Which academic test are you planning to take.
Not Sure yet
When are you planning to take the exam?
Already booked my exam slot
Within 2 Months
Want to learn about the test
Which Degree do you wish to pursue?
When do you want to start studying abroad.
September 2024
January 2025
What is your budget to study abroad?

How would you describe this article ?
Please rate this article
We would like to hear more.
Due: Wed Jul 3 11:59 pm Late submissions accepted until Fri Jul 5 11:59 pm
Assignment by Julie Zelenski, with modifications by Nick Troccoli, Chris Gregg, Katie Creel and Brynne Hurst
Along with this assignment, we've posted a page of extra bits practice problems with solutions. There is also an online practice site . These are very helpful to confirm your understanding of bit operators, binary, and more as you get started with this assignment!
Learning Goals
This assignment covers topics from the first few lectures and the first lab. You will be building your skills with:
- editing, compiling, testing, and debugging C programs under Unix
- writing code that manipulates bits and integers using the C bitwise and arithmetic operators
- working within/around the limits of integer representation
- understanding the ramifications of integer representations in real-world software
You shouldn't need material related to strings/pointers for this assignment - in other words, this assignment focuses just on the material related to bits and bytes.
This assignment asks you to complete three programs and one case study. Each program explores a particular aspect of bitwise or numeric manipulation:
- Automata has you model an evolving cell colony using a bitvector.
- UTF8 has you create unicode characters by constructing bit patterns.
- Saturated Arithmetic has you detect overflow in addition operations.
- The Ariane-5 Case Study has you examine the consequences of a real-world overflow bug.
The code you will write is short, but these small passages are mighty!
- The working on assignments page contains info about the assignment process.
- The collaboration policy page outlines permitted assignment collaboration, emphasizing that you are to do your own independent thinking, design, coding, and debugging. If you are having trouble completing the assignment on your own, please reach out to the course staff; we are here to help!
To get started on the assignment, clone the starter project using the command
The starter project contains the following:
- readme.txt : a text file where you will answer questions for the assignment
- sat.c . automata.c , utf8.c and Makefile : three partially-written programs that you will modify, and their Makefile for compiling
- custom_tests : the file where you will add custom tests for your programs
- SANITY.ini , sanity.py and prototypes.h : files to configure and run Sanity Check. You can ignore these.
- automata_soln , sat_soln and utf8_soln : executable solutions for the programs you will modify.
- tools : contains symbolic links to the sanitycheck and submit programs for testing and submitting your work. It also contains a new codecheck tool for checking for style and other common code issues.
Assignment Support: Through TA helper hours and the discussion forum, our focus will be on supporting you so that you can track down your own bugs. Please ask us how to best use tools (like the brand-new GDB!), what strategies to consider, and advice about how to improve your debugging process or track down your bug. We're happy to help you with these in order to help you drive your own debugging. For this reason, if you have debugging questions during helper hours, please make sure to gather information and explore the issue on your own first, and fill out the QueueStatus questions with this information. For instance, if your program is failing in certain cases, try to find a small replicable test case where the program fails; then, try using GDB to narrow down to what function/code block/etc. you think is causing the issue; then, further investigate just that area to try and find the bug. As another example, if your program is crashing, take a similar approach, but try using GDB and the backtrace command (check it out in lab!) to narrow in on the source of the crash. This information is important for the course staff to effectively help you with debugging. Starting with a future assignment, we will require this information when signing up for helper hours for debugging help , so please make sure to provide as much information as possible.
Tools and Testing
We have added a new tool, codecheck , that can check your code for common style and other issues that are detectable at compile-time. This check is NOT exhaustive; it is only meant to identify common style and other code issues. Check the course style guide and assignment spec for more code guidelines. You can run Codecheck like the sanitycheck and submit tools, from the tools folder, by running tools/codecheck , which runs it on all .c files in the current directory. You can also run it on individual files, like this:
Note that Codecheck does check for magic numbers , but for assign1 it may not align exactly with the best approach, as it considers anything above a certain numeric value automatically a "magic number". For this assignment, it's not necessarily stylistically bad if Codecheck shows any issue messages about magic numbers. But, you should still be vigilant and evaluate each suggestion from Codecheck to decide how you can make your code as clean as possible.
This assignment heavily emphasizes testing. For each program you write below, you should also add at least 3 to 5 additional tests of your own per program (total of at least 10) in the custom_tests file that show thoughtful effort to develop comprehensive test coverage. When you add a test, also document your work by including comments (on their own line, starting with # ) in the custom_tests file that remind you why you included each test and how the tests relate to one another. The tests supplied with the default SanityCheck are a start that you should build on, with the goal of finding and fixing any bugs before submitting, just like how a professional developer is responsible for vetting any code through comprehensive testing before adding it to a team repository.
The best way to approach testing on this assignment is, once you understand the expected program behavior but BEFORE you write any code, write some tests that cover various cases you can think of. This is because once you start writing code, you may start to think in terms of how your code works rather than how the code should work, meaning if you omit handling a case in your code, you may also omit covering that case in your testing. Thus, a good strategy is to write some tests before implementing anything, and then as you implement, you can add further tests. Use the tests as a way to gauge your progress and uncover bugs! We provide some testing recommendations in each problem section below.
How do you brainstorm what additional tests to consider? First review the cases supplied with default sanitycheck to see what is already covered. Then consider the full range of inputs/possibilities beyond those simple cases. If the variety of inputs is sufficiently small, you may be able to enumerate a test case for each possibility. In other situations, exhaustive coverage is not feasible, so you instead identify representative samples to validate the operation on specific cases. You should target ordinary cases as well as edge conditions and unusual inputs. For more advice, check out our guide to testing , from the assignments dropdown.
Review and Comment
When you inherit code to build on, you should first carefully review it to understand its behavior and plan how your code will fit into the existing program. You are given about 25 lines for each program and will add about a dozen lines of your own per program, so there is more starter code to review than new code to add.
Here, our provided code for each program processes the command-line arguments, handles user error in how the program is invoked, and has a few important details, particularly in converting string arguments to numbers. The triangle program from assign0 used the atoi function, which is simpler to use but has no capability to detect malformed inputs. For these programs, we are using the more robust, but complex, strtol . Read the function documentation at man strtol and review how the function is used in convert_arg .
Some questions you might consider as a self-test:
- How do the sample programs respond if invoked with missing arguments? excess arguments?
- When converting the user's string argument to a number, what base is passed to strtol ? In which bases does this allow the argument to be specified? How does the user's argument indicate which base to use?
- What does strtol do if it encounters an invalid character in the string to convert?
- How is the second argument to strtol used for error-detection? Why is it ok that the end variable is dereferenced when it is initialized to NULL ? If you did not want that error-detection, what do you pass as the second argument instead?
- What does strtol do if asked to convert a string whose value would be too large to be represented in a long ?
- Do you see anything unexpected or erroneous? We intend for our code to be bug-free; if you find otherwise, please let us know!
After reviewing the code, try experimenting with it! Build and run the programs, invoking them with different arguments to see how each is treated. Run the program under the debugger (check out our Debugger Guide under "Handouts") and step through code, printing intermediate values as you go. Observing the code in action is an excellent way to better understand it. Confirm your answers to the self-test questions above to verify your understanding.
We intentionally left off all the comments on the starter code, so you should add in documentation. Add an appropriate overview comment for the entire program, document each of the functions, and add inline comments where necessary to highlight any particularly tricky details.
1. Cellular Automata
here that gives an excellent overview of the cellular automata described in this problem. Feel free to watch it.--> A cellular automaton models the life cycle of a cell colony, which you can think of as a 1D array of cells, each of which can be either live or empty . Starting with an initial pattern, the automaton simulates the birth and death of future generations of cells using a set of simple rules. In this part of the assignment, you will implement a cellular automaton using a bitvector so that it uses memory as efficiently as possible.
We represent a 1D cell colony as a 64-bit unsigned long , with one bit for each cell. A 1 indicates the cell is live, and 0 indicates the cell is empty. Your program will advance the cells forward 1 generation, print out what the colony looks like, and then repeat, thereby printing out the cells' lifecycle.
For a given cell, the way we know whether it is live or empty in the next generation is by looking at its neighbors , the cells to its immediate left and right. The ruleset , which is a number specified by the user when they run the program, tells us, based on the state (live or empty) of a cell and its neighbors in the current generation, whether that cell is live or empty in the next generation. Using this bit vector representation, reading and updating a single cell is implemented with bitwise operations.
The automata program prints a number of iterations of the cell colony, but stops early if the cell colony does not change further. In the output, each line represents one generation, going from the first generation at the top to the last generation at the bottom. Below shows a sample run of the program for ruleset 77 - take a moment to run the sample solution (in the samples folder) and try out the automata program for yourself!
Pretty cool, huh? Since a ruleset is an 8-bit number, this means there are 256 different possible rulesets to dictate how cell generations evolve. There are some really cool outcomes you get depending on the ruleset. We'll let you explore these!
You are to implement these 2 functions for the automata program, both of which are called by the starter code.
Testing recommendations: look carefully at where you have special cases in advancing the generation, and set up test cases that explicitly poke at those issues.
Step 1: draw_generation
The draw_generation function takes a cell colony gen and outputs it as text. Specifically, it should output a row of 64 characters, one for each cell. A live cell is shown as an inverted box (a provided constant), and an empty cell is shown as a blank space. The generation is ordered such that the cell corresponding to the most significant bit is in the leftmost column and the least significant bit is in the rightmost column. You will need to iterate through the binary representation of gen and print out each cell individually. The key idea is that gen is an unsigned long , but we can use bit operators to access and manipulate its underlying binary representation. Remember to print a new line character once you have fully printed the generation! Once you complete this task, if you then run the program with any ruleset, it should print out one line for the first generation, another blank line for a generation of 0 (since that is what the advance function below initially returns), and then it will stop because the generation does not change:
Step 2: advance
The advance function takes a cell colony gen and returns a new cell colony bitvector which is gen advanced forward one generation. For each cell, it examines its neighborhood and uses the specified ruleset to determine the cell's state in the next generation. Here's how the process works and what you need to implement. Diagrams are taken from Daniel Shiffman's neat book The Nature of Code, Sections 7.1 and 7.2 .
A cell and its two neighbors form a "neighborhood":

A neighborhood is effectively a 3-bit number, a value from 0 to 7. For instance, the highlighted neighborhood here is 110 , which is the 3-bit number 6. The ruleset is an 8-bit number that dictates, for each of the 8 possible neighborhood values, whether that cell will be live or empty in the next generation. Specifically, bit 0 in the ruleset (the least significant bit) specifies whether cells with neighborhood 0 will be live or empty. Bit 1 in the ruleset (the second-least significant bit) specifies whether cells with neighborhood 1 will be live and empty. And so on. Here are the steps to determine whether a cell is alive or empty in the next generation:
- for a given cell, get its 3-bit neighborhood N
- look at bit N in the ruleset (note: bit 0 here is the least significant bit, and bit 7 the most significant)
- if that bit is 1, the cell is alive in the next generation. If that bit is 0, it is empty in the next generation.

A cool observation here is that a ruleset is ultimately an 8 bit number - in this diagram, it is the 01011010 which is 90. This diagram shows the possible neighborhood values along the top, and along the bottom it shows the bit in the ruleset at that index. The user specifies a ruleset number when they run the program, and this is passed in to advance , and you will use its underlying binary representation. Remember that even though ruleset is an unsigned char , it is really represented in binary, and you can access that binary representation using bitwise operators.
Another key idea is that we can alternate between treating a value as a binary representation and as a regular integer number. For example, we get a 3-bit neighborhood for a cell, but then treat it as a number to know which bit in the ruleset to look at.
Here are some final notes and tips:
- The leftmost and rightmost cells in a generation are special cases since they don't have 2 neighbors. You should pretend their non-existent neighbor is an empty cell. In other words, in the first diagram above, the rightmost cell would have neighborhood 010 .
- You will need to iterate through the passed-in generation and calculate each cell's updated state, adding those to a new generation you build up.
- Consider how you can use bit operations to do things like: isolating a given cell's 3-bit neighborhood as a number from 0 to 7, and looking at a specific bit in the ruleset.
- Keep in mind that cell generations are 64 bit unsigned longs , and number literals are by default 32 bit signed integers!
- Use sanity check and custom tests to compare your output to the sample solution
- If a generation doesn't match the solution, run GDB and put a breakpoint on advance (using continue to skip calls if necessary) and step through the call that returns the incorrect generation.
A C char is a one-byte data type, capable of storing 2 8 different bit patterns. The ASCII encoding standard ( man ascii ) establishes a mapping for those patterns to various letters, digits and punctuation, but is limited to 256 options, so only a subset are included. Unicode is ASCII's more cosmopolitan cousin, defining a universal character set that supports glyphs from a wide variety of world languages, both modern and ancient ( Egyptian hieroglyphics, the original emoji? ). This large number of characters requires a different system than just a single char , however. For this part of the assignment, you will implement the most popular Unicode encoding, UTF-8, which according to recent measurements , is used by over 93% of all websites (with known character encodings)!
Unicode maps each character to a code point, which is a hexadecimal number. There are over 1 million different code points in the Unicode standard! A character's code point is commonly written in the format U+NNNN , which signifies the hexadecimal value 0xNNNN .
However, the Unicode standard does not specify how to best encode these code points in binary. There are a variety of different possible Unicode encodings which specify, given a code point, how to actually store it. Why multiple encodings? Each may have a different priority, whether it is compatibility with other non-Unicode encodings, the locale it is intended for, space efficiency, etc. Some questions that may have different answers depending on the encoding are: should smaller code points take up the same amount of space as larger code points? If not, how can we tell how long the encoding is? And is it possible to preserve compatibility with other encodings, such as ASCII?
One of the most popular Unicode encodings is UTF-8. UTF-8 is popular partially because it preserves backwards compatibility with ASCII - in fact, it was designed such that ASCII is a subset of UTF-8, meaning that the characters represented in the ASCII encoding have the same encoding in ASCII and UTF-8. The UTF-8 encoding represents a code point using 1-4 bytes, depending on the size of the code point. If it falls in the range U+0000 to U+007F , its UTF-8 encoding is 1 byte long. If it falls in the range U+0080 to U+07FF , its UTF-8 encoding is two bytes long. And if it falls in the range U+0800 to U+FFFF , its UTF-8 encoding is 3 bytes long (there is a 4 byte range, but we'll be ignoring that for this assignment). The way UTF-8 works is it splits up the binary representation of the code point across these UTF-8 encoded byte(s) .
The utf8 program accepts code points as command line arguments and prints out their UTF-8 encoding and the actual character that encoding represents.
( Note: if your Unicode characters are not printing or appear garbled, but the encoded hex bytes are correct, you may need to set your terminal to expect UTF-8. Changing the configuration is OS/terminal-specific, but within 5 minutes of Googling we found utf8 advice for Putty , MobaXterm and Xterm . The default Mac OS X Terminal.app works by default. Note that sanity check should still pass even if your terminal is showing the wrong character.)
Your task is to implement the to_utf8 function that takes a single Unicode code point and constructs its sequence of UTF-8 encoded bytes. This function is called once for every argument specified by the user.
The to_utf8 function has two parameters; an unsigned short code_point and a byte array utf8_bytes . The function constructs the UTF-8 representation for the given code point and writes the sequence of encoded bytes into the array utf8_bytes . The first byte (e.g. leading byte) should go at index 0, and the remaining bytes (if any) should follow at index 1, etc. The utf8_bytes array is provided by the client and is guaranteed to be large enough to hold a full 3 bytes, although only 1 or 2 may be needed. While we will talk about C arrays in more depth later, it turns out that if you pass an array as a parameter, modifying the elements of that array parameter will modify the elements of the original array passed in, so the function above can pass back the byte(s) it creates. The function returns the number of bytes written to the array (either 1, 2, or 3). As an example, if code_point were 0xfc , utf8_bytes would be updated to store 0xc3 at index 0, and 0xbc at index 1, and to_utf8 would return 2.
Your to_utf8 function will need to be written to handle each of the 3 code point ranges and construct the corresponding UTF-8 encoding . You are allowed to use comparison operators to distinguish this. Your code will need to use bitmasks and bitwise operators to manually construct each byte for the UTF-8 encodings.
Testing recommendations: there are some 65,000 possible code points you could feed to utf8 , but given that they all flow through just a few distinct paths, having a test case targeted to hit each of those paths, with a little extra attention at the boundaries, should be sufficient to give you confidence about the rest.
1 Byte Encodings
If a code point is in the range U+0000 to U+007F , inclusive, its UTF-8 encoding is 1 byte. A code point in this range can be represented using at most 7 bits - called its significant bits . The significant bits are the bits that could actually store something meaningful for the code point. It's 7 here because, for a code point from 0 to 0x7f , it would need at most 7 bits to store a value in that range, which are the 7 least significant, so those are all that we care about. The UTF-8 encoding is one byte with its most significant bit 0 , and its remaining 7 bits as the seven significant bits from the code point.
Example: for code point 0x001f (which is 0001 1111 ), its UTF-8 encoding would be 0001 1111 ( 0x001f ) and to_utf8 would return 1.
2 Byte Encodings
If a code point is in the range U+0080 to U+07FF , inclusive, its UTF-8 encoding is 2 bytes. A code point in this range can be represented using at most 11 significant bits. The UTF-8 encoding is:
- The first byte has a leading 110 , followed by the 5 most significant bits in the code point
- The second byte has a leading 10 , followed by the remaining 6 significant bits in the code point
Example: for code point 0x00fc (which is 1111 1100 ), the first byte would be 1100 0011 ( 0xc3 ), the second byte would be 1011 1100 ( 0xbc ) and to_utf8 would return 2.
3 Byte Encodings
If a code point is in the range U+0800 to U+FFFF , inclusive, its UTF-8 encoding is 3 bytes. A code point in this range can be represented using at most 16 significant bits. The UTF-8 encoding is:
- The first byte has a leading 1110 , followed by the 4 most significant bits in the code point
- The second byte has a leading 10 , followed by the 6 next most significant bits in the code point
- The third byte has a leading 10 , followed by the final 6 significant bits in the code point
Example: for code point 0x4751 (which is 0100 0111 0101 0001 ), the first byte would be 1110 0100 ( 0xe4 ), the second byte would be 1001 1101 ( 0x9d ), the third byte would be 1001 0001 ( 0x91 ) and to_utf8 would return 3.
Here is a table containing the design described above. The xxx bits store the binary representation of the code point.
| Code Point Range | Significant Bits | UTF-8 Encoded Bytes (Binary) | |
|---|---|---|---|
| U+0000 to U+007F | 7 | ||
| U+0080 to U+07FF | 11 | 10xxxxxx | |
| U+0800 to U+FFFF | 16 | 10xxxxxx 10xxxxxx |
In a single-byte sequence, the high-order bit is always 0 , and the other 7 bits are the value of the code point itself. This is so the first 128 Unicode code points use the same single byte representation as the corresponding ASCII character!
For the multi-byte sequences, the first byte is called the leading byte , and the subsequent byte(s) are continuation bytes. The high-order bits of the leading byte indicate the number of total bytes in the sequence through the number of 1 s (for instance, the leading byte of an encoding for a code point from U+0080 to U+07FF starts with 110 , indicating the entire encoding is 2 bytes long, since there are two 1 s). The high-order bits of a continuation byte are always 10 . The bit representation of the code point is then divided across the low-order bits of the leading and continuation bytes.
Walkthrough Example: (paraphrased from Wikipedia UTF-8 page )
Consider encoding the Euro sign, €.
- The Unicode code point for € is U+20AC .
- The code point is within the range U+0800 to U+FFFF and will require a three-byte sequence. There are 14 significant bits in the binary representation of 0x20AC.
- Hex code point 20AC is binary 00100000 10101100 . Two leading zero bits of padding are used to fill to 16 bits. These 16 bits will be divided across the three-byte sequence.
- Let's calculate the leading byte. High-order bits are fixed: three 1s followed by a 0 indicate the three-byte sequence. The low-order bits store the 4 highest bits of the code point. This byte is 1110 0010 . The code point has 12 bits remaining to be encoded.
- Next, let's calculate the first continuation byte. High-order bits are fixed 10 , low-order bits store the next 6 bits of the code point. This byte is 10 000010 . The code point has 6 bits remaining to be encoded.
- Finally, let's calculate the last continuation byte. High-order bits are fixed 10 , low-order bits store the last 6 bits of the code point. This byte is 10 101100 .
- The three-byte sequence this results in is 1110 0010 10 000010 10 101100 , which can be more concisely written in hexadecimal, as e2 82 ac . The underscores indicate where the bits of the code point were distributed across the encoded bytes.
3. Saturating Arithmetic
The sat program performs a synthetic saturating addition operation. Saturating addition clamps the result into the representable range. Instead of overflowing with wraparound as ordinary two's-complement addition does, a saturating addition returns the type's maximum value when there would be positive overflow, and minimum when there would be negative overflow. Saturating arithmetic is a common feature in 3D graphics and digital signal processing applications.
The sat program accepts different numbers of arguments. With just one argument, it prints the representable range of numbers with that bitwidth:
Any additional specified arguments are added together using saturated arithmetic - for instance, if we add 126 and 5, the result overflows and sticks to the maximum value of 127.
You are to implement these 3 functions for the sat program. You are encouraged to call any of these functions from any of the others as needed to share logic!
For these 3 functions, you may not use the following in your implementations:
- No relational operators or math.h functions . You are prohibited from making any use of the relational operators. This means no use of < > <= >= . You may use != == and all other operators (arithmetic, logical, bitwise, ...). You also should not call any function from the floating point math.h library (e.g no pow , no exp2 ). These restrictions are intended to guide you to implement the operation via bitwise manipulation.
- No special cases based on bitwidth. Whether the value of bitwidth is 4, 64, or something in between, your functions must use one unified code path to handle any/all values of bitwidth without special-case handling. You should not use if switch ?: to divide the code into different cases based on the value of bitwidth. This doesn't mean that you can't use conditional logic (such as to separately handle overflow or non-overflow cases), but conditionals that dispatch based on the value of bitwidth or make a special case out of one or more bitwidths are disallowed.
- No loops or recursion. No loops or recursion at all. (In particular, no loop increment by one and stop at max.)
A solution that violates any of these restrictions will receive zero credit , so please verify your approach is in compliance!
Testing recommendations : your custom tests should cover a variety of different bitwidths, including extreme values, and on sums within range as well as those that overflow.
Step 1: signed_min
signed_min returns the smallest value representable in bitwidth bits. bitwidth is a number between 4 and 64, inclusive. Note, however, that the function returns a long ; your function needs to return the minimum value, but correctly represented in 64 bits, not just bitwidth bits. As an example, with a bitwidth of 4, the min is binary 1000 , which represents the value -8. We want to return the value -8 but represented using 64 bits, which would be 11111...1000 . As a hint, try writing out what the signed min values are for different bitwidths, and see if you notice a pattern in how to go from a given bitwidth to its signed min binary representation. Your goal is to determine an expression based on bitwidth that evaluates to the correct long value. Once you implement this function, you should be able to run sat with just the bitwidth argument and see the correct value outputted for the min.
Step 2: signed_max
signed_max returns the largest value representable in bitwidth bits. It works the same as signed_min , but for the largest value instead of the smallest. Like signed_min , the function returns a long ; your function needs to return the maximum value, but correctly represented in 64 bits, not just bitwidth bits. As an example, with a bitwidth of 4, the max is binary 0111 , which represents the value 7. We want to return the value 7 but represented using 64 bits, which would be 000....0111 . As a hint, try writing out what the signed max values are for different bitwidths, and see if you notice a pattern in how to go from a given bitwidth to its signed max binary representation. You may also notice similarities with your signed_min implementation. Once you implement this function, you should be able to run sat with just the bitwidth argument and see the correct value outputted for the max.
Step 3: sat_add
sat_add returns the saturated addition of the two operands which are guaranteed to fit in bitwidth bits. This means:
- If the sum of the operands does not overflow, we just return the sum
- If the sum of the operands overflows past the max, return the max
- If the sum of the operands overflows past the min, return the min
Remember that you may not use relational operators such as < or > .
The key idea is to try and develop a way to examine a value's binary representation to know whether it has overflowed its bitwidth. As a hint, try picking a bitwidth such as 4, and write out some addition operations that do and don't overflow. Then ask yourself - by looking at the operands and the sum, did this addition overflow? How did I know that? When is it possible for overflow to occur?
4. Case Study: Ariane-5
Once you have implemented your sat program, you will have a taste of functions that work around overflow (completing sat is not required to complete this part, but provides context). Overflow errors can cause many problems, which we saw some examples of in Lectures 3 and 4. These errors are not very common, but they can be disastrous when they occur. Read through this article explaining an issue experienced by the European Space Agency, and answer the following questions in the readme.txt file.
View Ariane-5 Article
1) What was the cause of the Ariane 5 rocket failure, and why was it not detected before the rocket was launched? Write your answer in 3-5 sentences.
2) Imagine that you are developing software for Ariane 6. You notice that Ariane 6 takes the average of two different sensors' measurements of horizontal velocity (which are stored as int s) by adding them together and dividing by 2, as in the following code:
What issues might arise from this calculation? How would you test for other potential overflow issues that could arise before the launch date? Write your answer in 3-5 sentences. Note: There are many possible answers to this question. Your answer should rely on critical reflection about appropriate testing strategies.
Submitting and Grading
Once you are finished working and have saved all your changes, check out the guide to working on assignments for how to submit your work. We recommend you do a trial submit in advance of the deadline to familiarize yourself with the process and allow time to work through any snags. You may submit as many times as you would like; we will grade the latest submission. Submitting a stable but unpolished/unfinished is like an insurance policy. If the unexpected happens and you miss the deadline to submit your final version, this previous submit will earn points. Without a submission, we cannot grade your work.
When you submit, you may optionally indicate that you do not plan to make a submission after the on-time deadline. This allows the staff to start grading some submissions as soon as the on-time deadline passes, rather than waiting until after the late period to start grading.
- When in doubt, it's fine to indicate that you may make a late submission, even if you end up submitting on time
- If you do indicate you won't submit late, this means once the on-time deadline passes, you cannot submit again. You can resubmit any time before the on-time deadline, however.
- If you want to change your decision, you can do so any time before the on-time deadline by resubmitting and changing your answer.
- If you know that you will not make a late submission, we would appreciate you indicating this so that we can grade assignments more quickly!
You should only need to modify the following files for this assignment: readme.txt , sat.c , automata.c , utf8.c , custom_tests
We would also appreciate if you filled out this homework survey to tell us what you think once you submit. We appreciate your feedback!
Below is the tentative grading rubric. We use a combination of automated tests and human review to evaluate your submission. More details are given in our page linked to from the Assignments dropdown explaining How assignments are graded .
Readme Answers (10 points)
Code Functionality (80 points)
- Sanity cases . (30 points) The default sanity check tests are used for sanity grading.
- Comprehensive cases . (45 points) We will thoroughly test on a variety of an additional inputs ranging from simple to complex, including edge conditions where appropriate.
- Custom sanitycheck tests (5 points) Your custom_tests file should include at least 10 tests of your own, 3-5 per program, that show thoughtful effort to develop comprehensive testing coverage. Please include comments that explain your choices. We will run your custom tests against your submission as well as review the cases to assess the strength of your testing efforts.
- Clean compile . (2 points) We expect your code to compile cleanly without warnings.
Code Quality (buckets weighted to contribute ~10 points)
The grader's code review is scored into buckets to emphasize the qualitative features of the review over the quantitative. The styleguide is a great overall resource for good program style. Here are some highlights for this assignment:
- Bitwise manipulation . We expect you to show proficiency in bitwise manipulation through clean construction and use of masks and proper application of the bitwise operations.
- Algorithms. Your chosen approach should demonstrate that you understand how to leverage bit patterns and numeric representation to directly and efficiently accomplish the task. Avoid unnecessary divergence and special-case code; unify into one general-purpose path wherever possible.
- Style and readability. We expect your code to be clean and readable. We will look for descriptive names, defined constants (not magic numbers!), and consistent layout. Be sure to use the most clear and direct C syntax and constructs available to you.
- Documentation. You should document both the code you wrote and what we provided. We expect program overview and per-function comments that explain the overall design along with sparing use of inline comments to draw attention to noteworthy details or shed light on a dense or obscure passage. The audience for the comments is your C-savvy peer.
Post-Assignment Check-in
How did the assignment go for you? We encourage you to take a moment to reflect on how far you've come and what new knowledge and skills you have to take forward. Once you finish this assignment, you will have completed your first C programs manipulating bits, and understood more about the limitations of integer representations! This assignment may definitely have a bit of a learning curve getting used to the write/compile/debug process in C and the command line. Celebrate this important milestone; the skills you develop now will pay off throughout the quarter!
To help you gauge your progress, for each assignment/lab, we identify some of its takeaways and offer a few thought questions you can use as a self-check on your post-task understanding. If you find the responses don't come easily, it may be a sign a little extra review is warranted. These questions are not to be handed in or graded. You're encouraged to freely discuss these with your peers and course staff to solidify any gaps in you understanding before moving on from a task. They could also be useful as review before exams.
- Consider all negative int s whose absolute value is an exact power of two (-8, -32 and so on). What do their bit patterns have in common?
- How can you determine if an integer bit pattern has a pair of consecutive 'on' bits? (Straightforward to do using a loop, but there is also a clever single expression...)
- How can integer limitations manifest themselves in real-world software?
- A treasure trove of bit wizardry curated by Sean Anderson, Stanford CS PhD.
To help approach this assignment, we've compiled some tips that may help with the various problems, as well as bit manipulation in general.
Don't use 0b -prefixed literals. There is no official support in C for numbers written in binary with 0b . We used this form in lecture to explicitly write out binary numbers, but it's not something you can use in standard C programs. Instead, you should... (see the next part)
Embrace hexadecimal. The base-16 system very conveniently translates to and from binary -- each byte is expressed in two hex digits, one hex digit for the 4 high-order bits, the other for the 4 low bits. Masks in hex or expressed via their construction are more evocative of the binary representation and help you avoid mistakes. For example, a mask to extract the sign bit from a 32-bit value can be cleanly expressed as 1 << 31 or 0x80000000 and both are quite readable. The mask also happens to be decimal 2147483648U , but who can tell? Would you notice if you had accidentally mistyped 2147486348U ? Say no to decimal, hex is your pal!
Learn your bitwise operators. It is wasteful to invoke the (expensive, floating-point) pow function to compute integer powers of two: 1 << 10 does a perfect job of computing pow(2, 10) and requires way fewer compute cycles. The bitwise operations are also the right tools to rearrange/isolate/change bits as opposed to the more indirect use of integer multiply/divide/mod with powers of two.
Aim for directness. There may be more direct ways to write certain bit manipulations. Here are a few:
No left-shift right-shift . In the code below, the first version right-shifts then immediately left-shifts back. This seems to just cycle the bits back to where they were originally, but in fact the right-shift will discard the lsb and the left-shift will then zero-fill. This is roundabout way to wipe the lsb, but the better approach is to just directly mask it off as in second version:
No redundant/unnecessary ops . Imagine your goal is to extract the 4 high-order bits of a byte and shift them down to the lower half-byte. You could do it with two steps (mask and shift), but in fact, the shift will already discard the low-order bits, so there's no need to mask them off first!
Know your identities/relationships . When putting together a bitwise construction, stop and consider whether there is a more direct way to accomplish the same thing, e.g.
At first you may find yourself using a longer expression than needed, but reviewing in a later pass, you can spot these issues and make them more concise. The goal is eventually to go straight to the most direct expressions from the beginning.
Frequently Asked Questions
I'm getting the error warning: left shift count >= width of type . what does this mean.
Code such as this will produce that warning:
An integer constant defaults to type int , so shifting a 32-bit int by 32 positions would effectively shift off all the bits, and thus the compiler warns about it. Assigning the result to a variable of type long (64 bits) doesn't help; you have to start with a 64-bit value if you intend to compute a 64-bit result. Read the next question!
What bitwidth and signedness does a numeric integer constant have?
An integer literal is a signed int by default. Suffixes can be added to the constant to dictate its type. A suffix of L indicates the type is long and U (either by itself or with L ) indicates it is unsigned. E.g. 1UL means a literal 1 that is an unsigned long.
What are the rules for integer promotion in C?
When assigning from narrower bitwidth (e.g. char, short) to a wider one, the source bytes are copied to the low-order bytes of the destination, and the upper bytes are assigned based on the promotion rules. If the source type was unsigned, the upper bytes of destination are zero-filled. If the source type was signed, its sign bit (the msb) is replicated across the upper bytes of destination (this is called sign extension). It may help to trace through this and confirm for yourself how/why this is value-preserving. If an operation is performed on two operands of mixed type, integer promotion is implicitly applied to the narrower first. It also the case (and a bit surprising, too) that integer promotion is also applied to types narrower than int when an operation is performed on them, even if no other wider type is involved. For example, the bitshift and invert operations promote the operand to an int, so bit-shifting a char will promote to an int before shifting, bit-inverting of a char similarly promotes, and the default result type of these expressions will be int. If the bitwidth of the operand was larger than int, the bitwidth will be unchanged by these promotion rules.
The Daily Show Fan Page

Explore the latest interviews, correspondent coverage, best-of moments and more from The Daily Show.
Extended Interviews

The Daily Show Tickets
Attend a Live Taping
Find out how you can see The Daily Show live and in-person as a member of the studio audience.
Best of Jon Stewart

The Weekly Show with Jon Stewart
New Episodes Thursdays
Jon Stewart and special guests tackle complex issues.
Powerful Politicos

The Daily Show Shop
Great Things Are in Store
Become the proud owner of exclusive gear, including clothing, drinkware and must-have accessories.
About The Daily Show

IMAGES
VIDEO
COMMENTS
Science Class 8 Assignments Pdf Download. We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 8 Science. Students and teachers can download and save all free Science assignments in Pdf for grade 8th. Our expert faculty have covered Class 8 important questions and answers for Science as per the latest ...
These solutions include chapter-wise & exercise-wise solutions to Class 8 Science textbook questions. The solutions are easy to understand and are provided in simple words. Diagrams are included for better understanding. CBSE Class 8 Science Solutions are given in a step-by-step manner to help students understand each concept easily.
The concepts of science syllabus form the backbone of higher classes. NCERT class 8 science solutions help to clear our basic concepts and make them very easy and interesting for the students. We prepared Class 8 science NCERT Solutions as per CBSE remodeled assessment structure. We have discussed extensively how are different food crops produced.
There are four materials A, B, C and D. A and D are hard and shiny, but B and C are dull and not very hard. Identify the metals and non-metals from A, B, C and D. Answer: A and D are metals. B and C are non-metals. Question 14. Gaurav knows that wires can be made from copper and aluminium.
NCERT Class 8 Science Solutions are meticulously crafted to help you comprehend the intricate details of combustion and flame, enhance your problem-solving abilities, and prepare effectively for your exams. Important Topics Covered in Chapter 4: Combustion and Flame. Types of Combustion. Structure of a Flame.
In this page you can find class 8 science ncert solutions , notes, assignments, worksheets etc. You can share the link to free Class 8 NCERT science solutions with your classmates as all study material is free of cost. How to learn class 8 science. First read and understand the notes. Try to go through the solved examples of of the chapter you ...
Register for our free webinar class with the best science tutor in India to get the most out of your studies. Vedantu is a platform that provides free NCERT Solution and other study materials for students. Subjects like Science, Maths, English, and more will become easier to study if you have access to NCERT Solutions for Class 8 Science, Maths, and other subjects.
NCERT Solutions for Class 8 Science is a comprehensive study material designed to help students understand the fundamental concepts of Science. It covers cell biology, force and pressure, and metals and non-metals. The solutions are presented step-by-step, making it easier for students to understand and learn the concepts.
Just click on the following link and download the CBSE Class 8 Science Worksheet. CBSE Worksheets for Class 8 Science can also use like assignments for Class 8 Science students. Grade 8 Crop Production and Management Worksheets. Grade 8 Microorganisms: Friend and Foe Worksheets. Grade 8 Synthetic Fibres and Plastics Worksheets.
Also, check CBSE Class 8 Science Important Questions for chapter-wise PDF for other chapters: CBSE Class 8 Science Important Questions with Answer PDF. Chapter 1 - Crop Production and Management. Chapter 2 - Microorganisms : Friend and Foe. Chapter 3 - Synthetic Fibres and Plastics. Chapter 4 - Materials : Metals and Non-Metals.
In Class 8 Science there is a chapter "Science Assignment 4", it is a crucial lesson for the students as they get to know all the basic topics of Science Assignment 4. Since it is an important lesson, students should take Science Assignment 4 Worksheet Class 8 to better develop an understanding of the concepts explained.
These Class 8 Science assignment pdf with answers pdf shows up with an answer key with step-by-step answers for students to comprehend the problem at each level and not retain it. Since you are given all the essential data with respect to the 8th Standard Science Assignment and we trust this definite article is useful. So Students who are ...
Practice MCQ Questions for Class 8 Science with Answers on a daily basis and score well in exams. Refer to the Materials: Metals and Non-Metals Class 8 MCQs Questions with Answers here along with a detailed explanation. Materials: Metals and Non-Metals Class 8 MCQs Questions with Answers. Choose the correct option.
Students can click on the respective link to download the NCERT Class 8 book. Chapter 1: Crop Production and Management. NCERT Solutions for class 8 Science. Chapter 2: Microorganisms: Friend and Foe. Chapter 3: Synthetic Fibres and Plastics. Chapter 4: Materials: Metals and Non-Metals.
Community questions. Embark on an exciting journey through Grade 8 Science! Enjoy captivating activity videos and stimulating practice exercises. From microscopic wonders to gravity-defying feats, this course will broaden your knowledge and ignite your curiosity!"
Science Assignment Class 8 (English Version) 4th Week 2021 | EV Science assignment 2021 class Eight week 48 Animals Phylum, Characteristics and living habita...
Dec 27, 2023. Nothing gets kids more excited for science than hands-on experiments! Watch your 4th grade science students' eyes light up when they try some of these activities. You'll find physics, biology, engineering, chemistry, and more. These projects are easy to set up and really help drive the learning home. Get ready for some science ...
Solution: (a) Phosphorus is a very reactive non-metal. (b) Metals are good conductors of heat and electricity. (c) Iron is more reactive than copper. (d) Metals react with acids to produce hydrogen gas. 4. Mark 'T' if the statement is true and 'F' if it is false. (a) Generally, non-metals react with acids.
Science is a multi-disciplined academic field that helps students learn various concepts and topics. These professionally made Class 8 Science Notes by expert teachers at Vedantu can help students understand complex concepts related to all the chapters. Our Notes for Class 8 Science are designed to make even the toughest topic seem easy to learn for students.
Science and its applications are way beyond the textbook experiments and class activities we did as kids. But to boost the interest of students in science and help them learn the basics, these simple and interesting experiments and observation projects enable them to understand varied basic concepts of Science. Often class 8 students are asked to submit their science project by the end of the ...
Assignment by Julie Zelenski, with modifications by Nick Troccoli, Chris Gregg, Katie Creel and Brynne Hurst. Along with this assignment, we've posted a page of extra bits practice problems with solutions. There is also an online practice site. These are very helpful to confirm your understanding of bit operators, binary, and more as you get ...
The source for The Daily Show fans, with episodes hosted by Jon Stewart, Ronny Chieng, Jordan Klepper, Dulcé Sloan and more, plus interviews, highlights and The Weekly Show podcast.