
How to Format a Scientific Paper
#scribendiinc
Written by Joanna Kimmerly-Smith
You've done the research. You've carefully recorded your lab results and compiled a list of relevant sources. You've even written a draft of your scientific, technical, or medical paper, hoping to get published in a reputable journal. But how do you format your paper to ensure that every detail is correct? If you're a scientific researcher or co-author looking to get your research published, read on to find out how to format your paper.
While it's true that you'll eventually need to tailor your research for your target journal, which will provide specific author guidelines for formatting the paper (see, for example, author guidelines for publications by Elsevier , PLOS ONE , and mBio ), there are some formatting rules that are useful to know for your initial draft. This article will explore some of the formatting rules that apply to all scientific writing, helping you to follow the correct order of sections ( IMRaD ), understand the requirements of each section, find resources for standard terminology and units of measurement, and prepare your scientific paper for publication.
Format Overview
The four main elements of a scientific paper can be represented by the acronym IMRaD: introduction, methods, results, and discussion. Other sections, along with a suggested length,* are listed in the table below.
* Length guidelines are taken from https://www.elsevier.com/connect/11-steps-to-structuring-a-science-paper-editors-will-take-seriously#step6 .
Now, let's go through the main sections you might have to prepare to format your paper.
On the first page of the paper, you must present the title of the paper along with the authors' names, institutional affiliations, and contact information. The corresponding author(s) (i.e., the one[s] who will be in contact with the reviewers) must be specified, usually with a footnote or an asterisk (*), and their full contact details (e.g., email address and phone number) must be provided. For example:
Dr. Clara A. Bell 1, * and Dr. Scott C. Smith 2
1 University of Areopagitica, Department of Biology, Sometown, Somecountry
2 Leviathan University, Department of Biochemistry and Biomedical Sciences, Sometown, Somecountry
FORMATTING TIPS:
- If you are unsure of how to classify author roles (i.e., who did what), guidelines are available online. For example, American Geophysical Union (AGU) journals now recommend using Contributor Roles Taxonomy (CRediT), an online taxonomy for author contributions.
In this summary of your research, you must state your subject (i.e., what you did) and encapsulate the main findings and conclusions of your paper.
- Do not add citations in an abstract (the reader might not be able to access your reference list).
- Avoid using acronyms and abbreviations in the abstract, as the reader may not be familiar with them. Use full terms instead.
Below the abstract, include a list of key terms to help other researchers locate your study. Note that "keywords" is one word (with no space) and is followed by a colon:
Keywords : paper format, scientific writing.
- Check whether "Keywords" should be italicized and whether each term should be capitalized.
- Check the use of punctuation (e.g., commas versus semicolons, the use of the period at the end).
- Some journals (e.g., IEEE ) provide a taxonomy of keywords. This aids in the classification of your research.
Introduction
This is the reader's first impression of your paper, so it should be clear and concise. Include relevant background information on your topic, using in-text citations as necessary. Report new developments in the field, and state how your research fills gaps in the existing research. Focus on the specific problem you are addressing, along with its possible solutions, and outline the limitations of your study. You can also include a research question, hypothesis, and/or objectives at the end of this section.
- Organize your information from broad to narrow (general to particular). However, don't start too broad; keep the information relevant.
- You can use in-text citations in this section to situate your research within the body of literature.
This is the part of your paper that explains how the research was done. You should relate your research procedures in a clear, logical order (i.e., the order in which you conducted the research) so that other researchers can reproduce your results. Simply refer to the established methods you used, but describe any procedures that are original to your study in more detail.
- Identify the specific instruments you used in your research by including the manufacturer’s name and location in parentheses.
- Stay consistent with the order in which information is presented (e.g., quantity, temperature, stirring speed, refrigeration period).
Now that you've explained how you gathered your research, you've got to report what you actually found. In this section, outline the main findings of your research. You need not include too many details, particularly if you are using tables and figures. While writing this section, be consistent and use the smallest number of words necessary to convey your statistics.
- Use appendices or supplementary materials if you have too much data.
- Use headings to help the reader follow along, particularly if your data are repetitive (but check whether your style guide allows you to use them).
In this section, you interpret your findings for the reader in relation to previous research and the literature as a whole. Present your general conclusions, including an assessment of the strengths and weaknesses of the research and the implications of your findings. Resolve the hypothesis and/or research question you identified in the introduction.
- Use in-text citations to support your discussion.
- Do not repeat the information you presented in the results or the introduction unless it is necessary for a discussion of the overall implications of the research.
This section is sometimes included in the last paragraph of the discussion. Explain how your research fits within your field of study, and identify areas for future research.
- Keep this section short.
Acknowledgments
Write a brief paragraph giving credit to any institution responsible for funding the study (e.g., through a fellowship or grant) and any individual(s) who contributed to the manuscript (e.g., technical advisors or editors).
- Check whether your journal uses standard identifiers for funding agencies (e.g., Elsevier's Funder Registry ).
Conflicts of Interest/Originality Statement
Some journals require a statement attesting that your research is original and that you have no conflicts of interest (i.e., ulterior motives or ways in which you could benefit from the publication of your research). This section only needs to be a sentence or two long.
Here you list citation information for each source you used (i.e., author names, date of publication, title of paper/chapter, title of journal/book, and publisher name and location). The list of references can be in alphabetical order (author–date style of citation) or in the order in which the sources are presented in the paper (numbered citations). Follow your style guide; if no guidelines are provided, choose a citation format and be consistent .
- While doing your final proofread, ensure that the reference list entries are consistent with the in-text citations (i.e., no missing or conflicting information).
- Many citation styles use a hanging indent and may be alphabetized. Use the styles in Microsoft Word to aid you in citation format.
- Use EndNote , Mendeley , Zotero , RefWorks , or another similar reference manager to create, store, and utilize bibliographic information.
Appendix/Supplementary Information
In this optional section, you can present nonessential information that further clarifies a point without burdening the body of the paper. That is, if you have too much data to fit in a (relatively) short research paper, move anything that's not essential to this section.
- Note that this section is uncommon in published papers. Before submission, check whether your journal allows for supplementary data, and don't put any essential information in this section.
Beyond IMRaD: Formatting the Details
Aside from the overall format of your paper, there are still other details to watch out for. The sections below cover how to present your terminology, equations, tables and figures, measurements, and statistics consistently based on the conventions of scientific writing.
Terminology
Stay consistent with the terms you use. Generally, short forms can be used once the full term has been introduced:
- full terms versus acronyms (e.g., deoxyribonucleic acid versus DNA);
- English names versus Greek letters (e.g., alpha versus α); and
- species names versus short forms (e.g., Staphylococcus aureus versus S. aureus ).
One way to ensure consistency is to use standard scientific terminology. You can refer to the following resources, but if you're not sure which guidelines are preferred, check with your target journal.
- For gene classification, use GeneCards , The Mouse Genome Informatics Database , and/or genenames.org .
- For chemical nomenclature, refer to the International Union of Pure and Applied Chemistry (IUPAC) Compendium of Chemical Terminology (the Gold Book ) and the IUPAC–IUB Combined Commission on Biochemical Nomenclature .
- For marine species names, use the World Register of Marine Species (WoRMS) or the European Register of Marine Species (ERMS) .
Italics must be used correctly for scientific terminology. Here are a couple of formatting tips:
- Species names, which are usually in Greek or Latin, are italicized (e.g., Staphylococcus aureus ).
- Genes are italicized, but proteins aren't.
Whether in mathematical, scientific, or technical papers, equations follow a conventional format. Here are some tips for formatting your calculations:
- Number each equation you present in the text, inserting the number in parentheses.
X + Y = 1 (1)
- Check whether your target journal requires you to capitalize the word "Equation" or use parentheses for the equation number when you refer to equations within the text.
In Equation 1, X represents . . .
In equation (1), X represents . . .
(Note also that you should use italics for variables.)
- Try using MathType or Equation Editor in Microsoft Word to type your equations, but use Unicode characters when typing single variables or mathematical operators (e.g., x, ≥, or ±) in running text. This makes it easier to edit your text and format your equations before publication.
- In line with the above tip, remember to save your math equations as editable text and not as images in case changes need to be made before publication.
Tables and Figures
Do you have any tables, graphs, or images in your research? If so, you should become familiar with the rules for referring to tables and figures in your scientific paper. Some examples are presented below.
- Capitalize the titles of specific tables and figures when you refer to them in the text (e.g., "see Table 3"; "in Figure 4").
- In tables, stay consistent with the use of title case (i.e., Capitalizing Each Word) and sentence case (i.e., Capitalizing the first word).
- In figure captions, stay consistent with the use of punctuation, italics, and capitalization. For example:
Figure 1. Classification of author roles.
Figure 2: taxonomy of paper keywords
Measurements
Although every journal has slightly different formatting guidelines, most agree that the gold standard for units of measurement is the International System of Units (SI) . Wherever possible, use the SI. Here are some other tips for formatting units of measurement:
- Add spaces before units of measurement. For example, 2.5 mL not 2.5mL.
- Be consistent with your units of measure (especially date and time). For example, 3 hours or 3 h.
When presenting statistical information, you must provide enough specific information to accurately describe the relationships among your data. Nothing is more frustrating to a reviewer than vague sentences about a variable being significant without any supporting details. The author guidelines for the journal Nature recommend that the following be included for statistical testing: the name of each statistical analysis, along with its n value; an explanation of why the test was used and what is being compared; and the specific alpha levels and P values for each test.
Angel Borja, writing for Elsevier publications, described the statistical rules for article formatting as follows:
- Indicate the statistical tests used with all relevant parameters.
- Use mean and standard deviation to report normally distributed data.
- Use median and interpercentile range to report skewed data.
- For numbers, use two significant digits unless more precision is necessary.
- Never use percentages for very small samples.
Remember, you must be prepared to justify your findings and conclusions, and one of the best ways to do this is through factual accuracy and the acknowledgment of opposing interpretations, data, and/or points of view.
Even though you may not look forward to the process of formatting your research paper, it's important to present your findings clearly, consistently, and professionally. With the right paper format, your chances of publication increase, and your research will be more likely to make an impact in your field. Don't underestimate the details. They are the backbone of scientific writing and research.
One last tip: Before you submit your research, consider using our academic editing service for expert help with paper formatting, editing, and proofreading. We can tailor your paper to specific journal guidelines at your request.
Image source: 85Fifteen/ Unsplash.com
Let Us Format Your Paper to Your Target Journal’s Guidelines
Hire an expert academic editor , or get a free sample, about the author.
Joanna's passion for English literature (proven by her M.A. thesis on Jane Austen) is matched by her passion to help others with their writing (shown by her role as an in-house editor with Scribendi). She enjoys lively discussions about plot, character, and nerdy TV shows with her husband, and she loves singing almost as much as she loves reading. Isn't music another language after all?
Have You Read?
"The Complete Beginner's Guide to Academic Writing"
Related Posts

APA Style and APA Formatting

How to Research a Term Paper

How to Write a Thesis or Dissertation
Upload your file(s) so we can calculate your word count, or enter your word count manually.
We will also recommend a service based on the file(s) you upload.
English is not my first language. I need English editing and proofreading so that I sound like a native speaker.
I need to have my journal article, dissertation, or term paper edited and proofread, or I need help with an admissions essay or proposal.
I have a novel, manuscript, play, or ebook. I need editing, copy editing, proofreading, a critique of my work, or a query package.
I need editing and proofreading for my white papers, reports, manuals, press releases, marketing materials, and other business documents.
I need to have my essay, project, assignment, or term paper edited and proofread.
I want to sound professional and to get hired. I have a resume, letter, email, or personal document that I need to have edited and proofread.
Prices include your personal % discount.
Prices include % sales tax ( ).

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Sports Phys Ther
- v.7(5); 2012 Oct
HOW TO WRITE A SCIENTIFIC ARTICLE
Barbara j. hoogenboom.
1 Grand Valley State University, Grand Rapids, MI, USA
Robert C. Manske
2 University of Wichita, Wichita, KS, USA
Successful production of a written product for submission to a peer‐reviewed scientific journal requires substantial effort. Such an effort can be maximized by following a few simple suggestions when composing/creating the product for submission. By following some suggested guidelines and avoiding common errors, the process can be streamlined and success realized for even beginning/novice authors as they negotiate the publication process. The purpose of this invited commentary is to offer practical suggestions for achieving success when writing and submitting manuscripts to The International Journal of Sports Physical Therapy and other professional journals.
INTRODUCTION
“The whole of science is nothing more than a refinement of everyday thinking” Albert Einstein
Conducting scientific and clinical research is only the beginning of the scholarship of discovery. In order for the results of research to be accessible to other professionals and have a potential effect on the greater scientific community, it must be written and published. Most clinical and scientific discovery is published in peer‐reviewed journals, which are those that utilize a process by which an author's peers, or experts in the content area, evaluate the manuscript. Following this review the manuscript is recommended for publication, revision or rejection. It is the rigor of this review process that makes scientific journals the primary source of new information that impacts clinical decision‐making and practice. 1 , 2
The task of writing a scientific paper and submitting it to a journal for publication is a time‐consuming and often daunting task. 3 , 4 Barriers to effective writing include lack of experience, poor writing habits, writing anxiety, unfamiliarity with the requirements of scholarly writing, lack of confidence in writing ability, fear of failure, and resistance to feedback. 5 However, the very process of writing can be a helpful tool for promoting the process of scientific thinking, 6 , 7 and effective writing skills allow professionals to participate in broader scientific conversations. Furthermore, peer review manuscript publication systems requiring these technical writing skills can be developed and improved with practice. 8 Having an understanding of the process and structure used to produce a peer‐reviewed publication will surely improve the likelihood that a submitted manuscript will result in a successful publication.
Clear communication of the findings of research is essential to the growth and development of science 3 and professional practice. The culmination of the publication process provides not only satisfaction for the researcher and protection of intellectual property, but also the important function of dissemination of research results, new ideas, and alternate thought; which ultimately facilitates scholarly discourse. In short, publication of scientific papers is one way to advance evidence‐based practice in many disciplines, including sports physical therapy. Failure to publish important findings significantly diminishes the potential impact that those findings may have on clinical practice. 9
BASICS OF MANUSCRIPT PREPARATION & GENERAL WRITING TIPS
To begin it might be interesting to learn why reviewers accept manuscripts! Reviewers consider the following five criteria to be the most important in decisions about whether to accept manuscripts for publication: 1) the importance, timeliness, relevance, and prevalence of the problem addressed; 2) the quality of the writing style (i.e., that it is well‐written, clear, straightforward, easy to follow, and logical); 3) the study design applied (i.e., that the design was appropriate, rigorous, and comprehensive); 4) the degree to which the literature review was thoughtful, focused, and up‐to‐date; and 5) the use of a sufficiently large sample. 10 For these statements to be true there are also reasons that reviewers reject manuscripts. The following are the top five reasons for rejecting papers: 1) inappropriate, incomplete, or insufficiently described statistics; 2) over‐interpretation of results; 3) use of inappropriate, suboptimal, or insufficiently described populations or instruments; 4) small or biased samples; and 5) text that is poorly written or difficult to follow. 10 , 11 With these reasons for acceptance or rejection in mind, it is time to review basics and general writing tips to be used when performing manuscript preparation.
“Begin with the end in mind” . When you begin writing about your research, begin with a specific target journal in mind. 12 Every scientific journal should have specific lists of manuscript categories that are preferred for their readership. The IJSPT seeks to provide readership with current information to enhance the practice of sports physical therapy. Therefore the manuscript categories accepted by IJSPT include: Original research; Systematic reviews of literature; Clinical commentary and Current concept reviews; Case reports; Clinical suggestions and unique practice techniques; and Technical notes. Once a decision has been made to write a manuscript, compose an outline that complies with the requirements of the target submission journal and has each of the suggested sections. This means carefully checking the submission criteria and preparing your paper in the exact format of the journal to which you intend to submit. Be thoughtful about the distinction between content (what you are reporting) and structure (where it goes in the manuscript). Poor placement of content confuses the reader (reviewer) and may cause misinterpretation of content. 3 , 5
It may be helpful to follow the IMRaD format for writing scientific manuscripts. This acronym stands for the sections contained within the article: Introduction, Methods, Results, and Discussion. Each of these areas of the manuscript will be addressed in this commentary.
Many accomplished authors write their results first, followed by an introduction and discussion, in an attempt to “stay true” to their results and not stray into additional areas. Typically the last two portions to be written are the conclusion and the abstract.
The ability to accurately describe ideas, protocols/procedures, and outcomes are the pillars of scientific writing . Accurate and clear expression of your thoughts and research information should be the primary goal of scientific writing. 12 Remember that accuracy and clarity are even more important when trying to get complicated ideas across. Contain your literature review, ideas, and discussions to your topic, theme, model, review, commentary, or case. Avoid vague terminology and too much prose. Use short rather than long sentences. If jargon has to be utilized keep it to a minimum and explain the terms you do use clearly. 13
Write with a measure of formality, using scientific language and avoiding conjunctions, slang, and discipline or regionally specific nomenclature or terms (e.g. exercise nicknames). For example, replace the term “Monster walks” with “closed‐chain hip abduction with elastic resistance around the thighs”. You may later refer to the exercise as “also known as Monster walks” if you desire.
Avoid first person language and instead write using third person language. Some journals do not ascribe to this requirement, and allow first person references, however, IJSPT prefers use of third person. For example, replace “We determined that…” with “The authors determined that….”.
For novice writers, it is really helpful to seek a reading mentor that will help you pre‐read your submission. Problems such as improper use of grammar, tense, and spelling are often a cause of rejection by reviewers. Despite the content of the study these easily fixed errors suggest that the authors created the manuscript with less thought leading reviewers to think that the manuscript may also potentially have erroneous findings as well. A review from a second set of trained eyes will often catch these errors missed by the original authors. If English is not your first language, the editorial staff at IJSPT suggests that you consult with someone with the relevant expertise to give you guidance on English writing conventions, verb tense, and grammar. Excellent writing in English is hard, even for those of us for whom it is our first language!
Use figures and graphics to your advantage . ‐ Consider the use of graphic/figure representation of data and important procedures or exercises. Tables should be able to stand alone and be completely understandable at a quick glance. Understanding a table should not require careful review of the manuscript! Figures dramatically enhance the graphic appeal of a scientific paper. Many formats for graphic presentation are acceptable, including graphs, charts, tables, and pictures or videos. Photographs should be clear, free of clutter or extraneous background distractions and be taken with models wearing simple clothing. Color photographs are preferred. Digital figures (Scans or existing files as well as new photographs) must be at least 300dpi. All photographs should be provided as separate files (jpeg or tif preferred) and not be embedded in the paper. Quality and clarity of figures are essential for reproduction purposes and should be considered before taking images for the manuscript.
A video of an exercise or procedure speaks a thousand words. Please consider using short video clips as descriptive additions to your paper. They will be placed on the IJSPT website and accompany your paper. The video clips must be submitted in MPEG‐1, MPEG‐2, Quicktime (.mov), or Audio/Video Interface (.avi) formats. Maximum cumulative length of videos is 5 minutes. Each video segment may not exceed 50 MB, and each video clip must be saved as a separate file and clearly identified. Formulate descriptive figure/video and Table/chart/graph titles and place them on a figure legend document. Carefully consider placement of, naming of, and location of figures. It makes the job of the editors much easier!
Avoid Plagiarism and inadvertent lack of citations. Finally, use citations to your benefit. Cite frequently in order to avoid any plagiarism. The bottom line: If it is not your original idea, give credit where credit is due . When using direct quotations, provide not only the number of the citation, but the page where the quote was found. All citations should appear in text as a superscripted number followed by punctuation. It is the authors' responsibility to fully ensure all references are cited in completed form, in an accurate location. Please carefully follow the instructions for citations and check that all references in your reference list are cited in the paper and that all citations in the paper appear correctly in the reference list. Please go to IJSPT submission guidelines for full information on the format for citations.
Sometimes written as an afterthought, the abstract is of extreme importance as in many instances this section is what is initially previewed by readership to determine if the remainder of the article is worth reading. This is the authors opportunity to draw the reader into the study and entice them to read the rest of the article. The abstract is a summary of the article or study written in 3 rd person allowing the readers to get a quick glance of what the contents of the article include. Writing an abstract is rather challenging as being brief, accurate and concise are requisite. The headings and structure for an abstract are usually provided in the instructions for authors. In some instances, the abstract may change slightly pending content revisions required during the peer review process. Therefore it often works well to complete this portion of the manuscript last. Remember the abstract should be able to stand alone and should be as succinct as possible. 14
Introduction and Review of Literature
The introduction is one of the more difficult portions of the manuscript to write. Past studies are used to set the stage or provide the reader with information regarding the necessity of the represented project. For an introduction to work properly, the reader must feel that the research question is clear, concise, and worthy of study.
A competent introduction should include at least four key concepts: 1) significance of the topic, 2) the information gap in the available literature associated with the topic, 3) a literature review in support of the key questions, 4) subsequently developed purposes/objectives and hypotheses. 9
When constructing a review of the literature, be attentive to “sticking” or “staying true” to your topic at hand. Don't reach or include too broad of a literature review. For example, do not include extraneous information about performance or prevention if your research does not actually address those things. The literature review of a scientific paper is not an exhaustive review of all available knowledge in a given field of study. That type of thorough review should be left to review articles or textbook chapters. Throughout the introduction (and later in the discussion!) remind yourself that a paper, existing evidence, or results of a paper cannot draw conclusions, demonstrate, describe, or make judgments, only PEOPLE (authors) can. “The evidence demonstrates that” should be stated, “Smith and Jones, demonstrated that….”
Conclude your introduction with a solid statement of your purpose(s) and your hypothesis(es), as appropriate. The purpose and objectives should clearly relate to the information gap associated with the given manuscript topic discussed earlier in the introduction section. This may seem repetitive, but it actually is helpful to ensure the reader clearly sees the evolution, importance, and critical aspects of the study at hand See Table 1 for examples of well‐stated purposes.
Examples of well-stated purposes by submission type.
The methods section should clearly describe the specific design of the study and provide clear and concise description of the procedures that were performed. The purpose of sufficient detail in the methods section is so that an appropriately trained person would be able to replicate your experiments. 15 There should be complete transparency when describing the study. To assist in writing and manuscript preparation there are several checklists or guidelines that are available on the IJSPT website. The CONSORT guidelines can be used when developing and reporting a randomized controlled trial. 16 The STARD checklist was developed for designing a diagnostic accuracy study. 17 The PRISMA checklist was developed for use when performing a meta‐analyses or systematic review. 18 A clear methods section should contain the following information: 1) the population and equipment used in the study, 2) how the population and equipment were prepared and what was done during the study, 3) the protocol used, 4) the outcomes and how they were measured, 5) the methods used for data analysis. Initially a brief paragraph should explain the overall procedures and study design. Within this first paragraph there is generally a description of inclusion and exclusion criteria which help the reader understand the population used. Paragraphs that follow should describe in more detail the procedures followed for the study. A clear description of how data was gathered is also helpful. For example were data gathered prospectively or retrospectively? Who if anyone was blinded, and where and when was the actual data collected?
Although it is a good idea for the authors to have justification and a rationale for their procedures, these should be saved for inclusion into the discussion section, not to be discussed in the methods section. However, occasionally studies supporting components of the methods section such as reliability of tests, or validation of outcome measures may be included in the methods section.
The final portion of the methods section will include the statistical methods used to analyze the data. 19 This does not mean that the actual results should be discussed in the methods section, as they have an entire section of their own!
Most scientific journals support the need for all projects involving humans or animals to have up‐to‐date documentation of ethical approval. 20 The methods section should include a clear statement that the researchers have obtained approval from an appropriate institutional review board.
Results, Discussion, and Conclusions
In most journals the results section is separate from the discussion section. It is important that you clearly distinguish your results from your discussion. The results section should describe the results only. The discussion section should put those results into a broader context. Report your results neutrally, as you “found them”. Again, be thoughtful about content and structure. Think carefully about where content is placed in the overall structure of your paper. It is not appropriate to bring up additional results, not discussed in the results section, in the discussion. All results must first be described/presented and then discussed. Thus, the discussion should not simply be a repeat of the results section. Carefully discuss where your information is similar or different from other published evidence and why this might be so. What was different in methods or analysis, what was similar?
As previously stated, stick to your topic at hand, and do not overstretch your discussion! One of the major pitfalls in writing the discussion section is overstating the significance of your findings 4 or making very strong statements. For example, it is better to say: “Findings of the current study support….” or “these findings suggest…” than, “Findings of the current study prove that…” or “this means that….”. Maintain a sense of humbleness, as nothing is without question in the outcomes of any type of research, in any discipline! Use words like “possibly”, “likely” or “suggests” to soften findings. 12
Do not discuss extraneous ideas, concepts, or information not covered by your topic/paper/commentary. Be sure to carefully address all relevant results, not just the statistically significant ones or the ones that support your hypotheses. When you must resort to speculation or opinion, be certain to state that up front using phrases such as “we therefore speculate” or “in the authors' opinion”.
Remember, just as in the introduction and literature review, evidence or results cannot draw conclusions, just as previously stated, only people, scientists, researchers, and authors can!
Finish with a concise, 3‐5 sentence conclusion paragraph. This is not just a restatement of your results, rather is comprised of some final, summative statements that reflect the flow and outcomes of the entire paper. Do not include speculative statements or additional material; however, based upon your findings a statement about potential changes in clinical practice or future research opportunities can be provided here.
CONCLUSIONS
Writing for publication can be a challenging yet satisfying endeavor. The ability to examine, relate, and interlink evidence, as well as to provide a peer‐reviewed, disseminated product of your research labors can be rewarding. A few suggestions have been offered in this commentary that may assist the novice or the developing writer to attempt, polish, and perfect their approach to scholarly writing.
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
Welcome to the PLOS Writing Center
Your source for scientific writing & publishing essentials.
A collection of free, practical guides and hands-on resources for authors looking to improve their scientific publishing skillset.
ARTICLE-WRITING ESSENTIALS
Your title is the first thing anyone who reads your article is going to see, and for many it will be where they stop reading. Learn how to write a title that helps readers find your article, draws your audience in and sets the stage for your research!
The abstract is your chance to let your readers know what they can expect from your article. Learn how to write a clear, and concise abstract that will keep your audience reading.
A clear methods section impacts editorial evaluation and readers’ understanding, and is also the backbone of transparency and replicability. Learn what to include in your methods section, and how much detail is appropriate.
In many fields, a statistical analysis forms the heart of both the methods and results sections of a manuscript. Learn how to report statistical analyses, and what other context is important for publication success and future reproducibility.
The discussion section contains the results and outcomes of a study. An effective discussion informs readers what can be learned from your experiment and provides context for the results.
Ensuring your manuscript is well-written makes it easier for editors, reviewers and readers to understand your work. Avoiding language errors can help accelerate review and minimize delays in the publication of your research.
The PLOS Writing Toolbox
Delivered to your inbox every two weeks, the Writing Toolbox features practical advice and tools you can use to prepare a research manuscript for submission success and build your scientific writing skillset.
Discover how to navigate the peer review and publishing process, beyond writing your article.
The path to publication can be unsettling when you’re unsure what’s happening with your paper. Learn about staple journal workflows to see the detailed steps required for ensuring a rigorous and ethical publication.
Reputable journals screen for ethics at submission—and inability to pass ethics checks is one of the most common reasons for rejection. Unfortunately, once a study has begun, it’s often too late to secure the requisite ethical reviews and clearances. Learn how to prepare for publication success by ensuring your study meets all ethical requirements before work begins.
From preregistration, to preprints, to publication—learn how and when to share your study.
How you store your data matters. Even after you publish your article, your data needs to be accessible and useable for the long term so that other researchers can continue building on your work. Good data management practices make your data discoverable and easy to use, promote a strong foundation for reproducibility and increase your likelihood of citations.
You’ve just spent months completing your study, writing up the results and submitting to your top-choice journal. Now the feedback is in and it’s time to revise. Set out a clear plan for your response to keep yourself on-track and ensure edits don’t fall through the cracks.
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher.
Are you actively preparing a submission for a PLOS journal? Select the relevant journal below for more detailed guidelines.
How to Write an Article
Share the lessons of the Writing Center in a live, interactive training.
Access tried-and-tested training modules, complete with slides and talking points, workshop activities, and more.
How to Write and Publish a Research Paper for a Peer-Reviewed Journal
- Open access
- Published: 30 April 2020
- Volume 36 , pages 909–913, ( 2021 )
Cite this article
You have full access to this open access article

- Clara Busse ORCID: orcid.org/0000-0002-0178-1000 1 &
- Ella August ORCID: orcid.org/0000-0001-5151-1036 1 , 2
272k Accesses
15 Citations
719 Altmetric
Explore all metrics
Communicating research findings is an essential step in the research process. Often, peer-reviewed journals are the forum for such communication, yet many researchers are never taught how to write a publishable scientific paper. In this article, we explain the basic structure of a scientific paper and describe the information that should be included in each section. We also identify common pitfalls for each section and recommend strategies to avoid them. Further, we give advice about target journal selection and authorship. In the online resource 1 , we provide an example of a high-quality scientific paper, with annotations identifying the elements we describe in this article.
Similar content being viewed by others

Literature reviews as independent studies: guidelines for academic practice
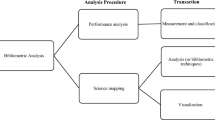
How to design bibliometric research: an overview and a framework proposal

Plagiarism in research
Avoid common mistakes on your manuscript.
Introduction
Writing a scientific paper is an important component of the research process, yet researchers often receive little formal training in scientific writing. This is especially true in low-resource settings. In this article, we explain why choosing a target journal is important, give advice about authorship, provide a basic structure for writing each section of a scientific paper, and describe common pitfalls and recommendations for each section. In the online resource 1 , we also include an annotated journal article that identifies the key elements and writing approaches that we detail here. Before you begin your research, make sure you have ethical clearance from all relevant ethical review boards.
Select a Target Journal Early in the Writing Process
We recommend that you select a “target journal” early in the writing process; a “target journal” is the journal to which you plan to submit your paper. Each journal has a set of core readers and you should tailor your writing to this readership. For example, if you plan to submit a manuscript about vaping during pregnancy to a pregnancy-focused journal, you will need to explain what vaping is because readers of this journal may not have a background in this topic. However, if you were to submit that same article to a tobacco journal, you would not need to provide as much background information about vaping.
Information about a journal’s core readership can be found on its website, usually in a section called “About this journal” or something similar. For example, the Journal of Cancer Education presents such information on the “Aims and Scope” page of its website, which can be found here: https://www.springer.com/journal/13187/aims-and-scope .
Peer reviewer guidelines from your target journal are an additional resource that can help you tailor your writing to the journal and provide additional advice about crafting an effective article [ 1 ]. These are not always available, but it is worth a quick web search to find out.
Identify Author Roles Early in the Process
Early in the writing process, identify authors, determine the order of authors, and discuss the responsibilities of each author. Standard author responsibilities have been identified by The International Committee of Medical Journal Editors (ICMJE) [ 2 ]. To set clear expectations about each team member’s responsibilities and prevent errors in communication, we also suggest outlining more detailed roles, such as who will draft each section of the manuscript, write the abstract, submit the paper electronically, serve as corresponding author, and write the cover letter. It is best to formalize this agreement in writing after discussing it, circulating the document to the author team for approval. We suggest creating a title page on which all authors are listed in the agreed-upon order. It may be necessary to adjust authorship roles and order during the development of the paper. If a new author order is agreed upon, be sure to update the title page in the manuscript draft.
In the case where multiple papers will result from a single study, authors should discuss who will author each paper. Additionally, authors should agree on a deadline for each paper and the lead author should take responsibility for producing an initial draft by this deadline.
Structure of the Introduction Section
The introduction section should be approximately three to five paragraphs in length. Look at examples from your target journal to decide the appropriate length. This section should include the elements shown in Fig. 1 . Begin with a general context, narrowing to the specific focus of the paper. Include five main elements: why your research is important, what is already known about the topic, the “gap” or what is not yet known about the topic, why it is important to learn the new information that your research adds, and the specific research aim(s) that your paper addresses. Your research aim should address the gap you identified. Be sure to add enough background information to enable readers to understand your study. Table 1 provides common introduction section pitfalls and recommendations for addressing them.

The main elements of the introduction section of an original research article. Often, the elements overlap
Methods Section
The purpose of the methods section is twofold: to explain how the study was done in enough detail to enable its replication and to provide enough contextual detail to enable readers to understand and interpret the results. In general, the essential elements of a methods section are the following: a description of the setting and participants, the study design and timing, the recruitment and sampling, the data collection process, the dataset, the dependent and independent variables, the covariates, the analytic approach for each research objective, and the ethical approval. The hallmark of an exemplary methods section is the justification of why each method was used. Table 2 provides common methods section pitfalls and recommendations for addressing them.
Results Section
The focus of the results section should be associations, or lack thereof, rather than statistical tests. Two considerations should guide your writing here. First, the results should present answers to each part of the research aim. Second, return to the methods section to ensure that the analysis and variables for each result have been explained.
Begin the results section by describing the number of participants in the final sample and details such as the number who were approached to participate, the proportion who were eligible and who enrolled, and the number of participants who dropped out. The next part of the results should describe the participant characteristics. After that, you may organize your results by the aim or by putting the most exciting results first. Do not forget to report your non-significant associations. These are still findings.
Tables and figures capture the reader’s attention and efficiently communicate your main findings [ 3 ]. Each table and figure should have a clear message and should complement, rather than repeat, the text. Tables and figures should communicate all salient details necessary for a reader to understand the findings without consulting the text. Include information on comparisons and tests, as well as information about the sample and timing of the study in the title, legend, or in a footnote. Note that figures are often more visually interesting than tables, so if it is feasible to make a figure, make a figure. To avoid confusing the reader, either avoid abbreviations in tables and figures, or define them in a footnote. Note that there should not be citations in the results section and you should not interpret results here. Table 3 provides common results section pitfalls and recommendations for addressing them.
Discussion Section
Opposite the introduction section, the discussion should take the form of a right-side-up triangle beginning with interpretation of your results and moving to general implications (Fig. 2 ). This section typically begins with a restatement of the main findings, which can usually be accomplished with a few carefully-crafted sentences.
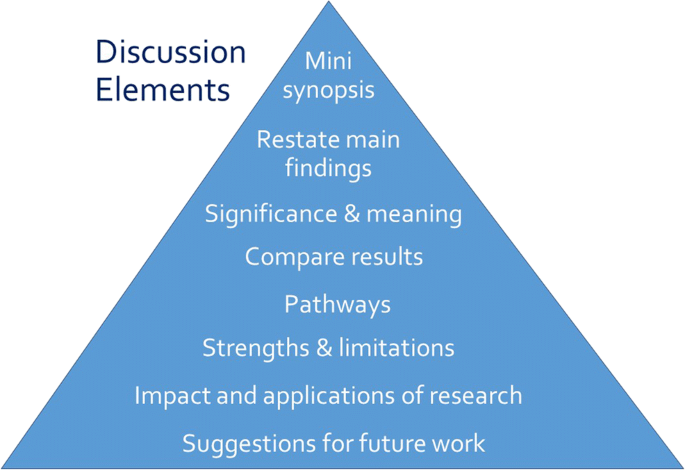
Major elements of the discussion section of an original research article. Often, the elements overlap
Next, interpret the meaning or explain the significance of your results, lifting the reader’s gaze from the study’s specific findings to more general applications. Then, compare these study findings with other research. Are these findings in agreement or disagreement with those from other studies? Does this study impart additional nuance to well-accepted theories? Situate your findings within the broader context of scientific literature, then explain the pathways or mechanisms that might give rise to, or explain, the results.
Journals vary in their approach to strengths and limitations sections: some are embedded paragraphs within the discussion section, while some mandate separate section headings. Keep in mind that every study has strengths and limitations. Candidly reporting yours helps readers to correctly interpret your research findings.
The next element of the discussion is a summary of the potential impacts and applications of the research. Should these results be used to optimally design an intervention? Does the work have implications for clinical protocols or public policy? These considerations will help the reader to further grasp the possible impacts of the presented work.
Finally, the discussion should conclude with specific suggestions for future work. Here, you have an opportunity to illuminate specific gaps in the literature that compel further study. Avoid the phrase “future research is necessary” because the recommendation is too general to be helpful to readers. Instead, provide substantive and specific recommendations for future studies. Table 4 provides common discussion section pitfalls and recommendations for addressing them.
Follow the Journal’s Author Guidelines
After you select a target journal, identify the journal’s author guidelines to guide the formatting of your manuscript and references. Author guidelines will often (but not always) include instructions for titles, cover letters, and other components of a manuscript submission. Read the guidelines carefully. If you do not follow the guidelines, your article will be sent back to you.
Finally, do not submit your paper to more than one journal at a time. Even if this is not explicitly stated in the author guidelines of your target journal, it is considered inappropriate and unprofessional.
Your title should invite readers to continue reading beyond the first page [ 4 , 5 ]. It should be informative and interesting. Consider describing the independent and dependent variables, the population and setting, the study design, the timing, and even the main result in your title. Because the focus of the paper can change as you write and revise, we recommend you wait until you have finished writing your paper before composing the title.
Be sure that the title is useful for potential readers searching for your topic. The keywords you select should complement those in your title to maximize the likelihood that a researcher will find your paper through a database search. Avoid using abbreviations in your title unless they are very well known, such as SNP, because it is more likely that someone will use a complete word rather than an abbreviation as a search term to help readers find your paper.
After you have written a complete draft, use the checklist (Fig. 3 ) below to guide your revisions and editing. Additional resources are available on writing the abstract and citing references [ 5 ]. When you feel that your work is ready, ask a trusted colleague or two to read the work and provide informal feedback. The box below provides a checklist that summarizes the key points offered in this article.

Checklist for manuscript quality
Data Availability
Michalek AM (2014) Down the rabbit hole…advice to reviewers. J Cancer Educ 29:4–5
Article Google Scholar
International Committee of Medical Journal Editors. Defining the role of authors and contributors: who is an author? http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authosrs-and-contributors.html . Accessed 15 January, 2020
Vetto JT (2014) Short and sweet: a short course on concise medical writing. J Cancer Educ 29(1):194–195
Brett M, Kording K (2017) Ten simple rules for structuring papers. PLoS ComputBiol. https://doi.org/10.1371/journal.pcbi.1005619
Lang TA (2017) Writing a better research article. J Public Health Emerg. https://doi.org/10.21037/jphe.2017.11.06
Download references
Acknowledgments
Ella August is grateful to the Sustainable Sciences Institute for mentoring her in training researchers on writing and publishing their research.
Code Availability
Not applicable.
Author information
Authors and affiliations.
Department of Maternal and Child Health, University of North Carolina Gillings School of Global Public Health, 135 Dauer Dr, 27599, Chapel Hill, NC, USA
Clara Busse & Ella August
Department of Epidemiology, University of Michigan School of Public Health, 1415 Washington Heights, Ann Arbor, MI, 48109-2029, USA
Ella August
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ella August .
Ethics declarations
Conflicts of interests.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Electronic supplementary material
(PDF 362 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Busse, C., August, E. How to Write and Publish a Research Paper for a Peer-Reviewed Journal. J Canc Educ 36 , 909–913 (2021). https://doi.org/10.1007/s13187-020-01751-z
Download citation
Published : 30 April 2020
Issue Date : October 2021
DOI : https://doi.org/10.1007/s13187-020-01751-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Manuscripts
- Scientific writing
- Find a journal
- Publish with us
- Track your research

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
13.1 Formatting a Research Paper
Learning objectives.
- Identify the major components of a research paper written using American Psychological Association (APA) style.
- Apply general APA style and formatting conventions in a research paper.
In this chapter, you will learn how to use APA style , the documentation and formatting style followed by the American Psychological Association, as well as MLA style , from the Modern Language Association. There are a few major formatting styles used in academic texts, including AMA, Chicago, and Turabian:
- AMA (American Medical Association) for medicine, health, and biological sciences
- APA (American Psychological Association) for education, psychology, and the social sciences
- Chicago—a common style used in everyday publications like magazines, newspapers, and books
- MLA (Modern Language Association) for English, literature, arts, and humanities
- Turabian—another common style designed for its universal application across all subjects and disciplines
While all the formatting and citation styles have their own use and applications, in this chapter we focus our attention on the two styles you are most likely to use in your academic studies: APA and MLA.
If you find that the rules of proper source documentation are difficult to keep straight, you are not alone. Writing a good research paper is, in and of itself, a major intellectual challenge. Having to follow detailed citation and formatting guidelines as well may seem like just one more task to add to an already-too-long list of requirements.
Following these guidelines, however, serves several important purposes. First, it signals to your readers that your paper should be taken seriously as a student’s contribution to a given academic or professional field; it is the literary equivalent of wearing a tailored suit to a job interview. Second, it shows that you respect other people’s work enough to give them proper credit for it. Finally, it helps your reader find additional materials if he or she wishes to learn more about your topic.
Furthermore, producing a letter-perfect APA-style paper need not be burdensome. Yes, it requires careful attention to detail. However, you can simplify the process if you keep these broad guidelines in mind:
- Work ahead whenever you can. Chapter 11 “Writing from Research: What Will I Learn?” includes tips for keeping track of your sources early in the research process, which will save time later on.
- Get it right the first time. Apply APA guidelines as you write, so you will not have much to correct during the editing stage. Again, putting in a little extra time early on can save time later.
- Use the resources available to you. In addition to the guidelines provided in this chapter, you may wish to consult the APA website at http://www.apa.org or the Purdue University Online Writing lab at http://owl.english.purdue.edu , which regularly updates its online style guidelines.
General Formatting Guidelines
This chapter provides detailed guidelines for using the citation and formatting conventions developed by the American Psychological Association, or APA. Writers in disciplines as diverse as astrophysics, biology, psychology, and education follow APA style. The major components of a paper written in APA style are listed in the following box.
These are the major components of an APA-style paper:
Body, which includes the following:
- Headings and, if necessary, subheadings to organize the content
- In-text citations of research sources
- References page
All these components must be saved in one document, not as separate documents.
The title page of your paper includes the following information:
- Title of the paper
- Author’s name
- Name of the institution with which the author is affiliated
- Header at the top of the page with the paper title (in capital letters) and the page number (If the title is lengthy, you may use a shortened form of it in the header.)
List the first three elements in the order given in the previous list, centered about one third of the way down from the top of the page. Use the headers and footers tool of your word-processing program to add the header, with the title text at the left and the page number in the upper-right corner. Your title page should look like the following example.

The next page of your paper provides an abstract , or brief summary of your findings. An abstract does not need to be provided in every paper, but an abstract should be used in papers that include a hypothesis. A good abstract is concise—about one hundred fifty to two hundred fifty words—and is written in an objective, impersonal style. Your writing voice will not be as apparent here as in the body of your paper. When writing the abstract, take a just-the-facts approach, and summarize your research question and your findings in a few sentences.
In Chapter 12 “Writing a Research Paper” , you read a paper written by a student named Jorge, who researched the effectiveness of low-carbohydrate diets. Read Jorge’s abstract. Note how it sums up the major ideas in his paper without going into excessive detail.

Write an abstract summarizing your paper. Briefly introduce the topic, state your findings, and sum up what conclusions you can draw from your research. Use the word count feature of your word-processing program to make sure your abstract does not exceed one hundred fifty words.
Depending on your field of study, you may sometimes write research papers that present extensive primary research, such as your own experiment or survey. In your abstract, summarize your research question and your findings, and briefly indicate how your study relates to prior research in the field.
Margins, Pagination, and Headings
APA style requirements also address specific formatting concerns, such as margins, pagination, and heading styles, within the body of the paper. Review the following APA guidelines.
Use these general guidelines to format the paper:
- Set the top, bottom, and side margins of your paper at 1 inch.
- Use double-spaced text throughout your paper.
- Use a standard font, such as Times New Roman or Arial, in a legible size (10- to 12-point).
- Use continuous pagination throughout the paper, including the title page and the references section. Page numbers appear flush right within your header.
- Section headings and subsection headings within the body of your paper use different types of formatting depending on the level of information you are presenting. Additional details from Jorge’s paper are provided.

Begin formatting the final draft of your paper according to APA guidelines. You may work with an existing document or set up a new document if you choose. Include the following:
- Your title page
- The abstract you created in Note 13.8 “Exercise 1”
- Correct headers and page numbers for your title page and abstract
APA style uses section headings to organize information, making it easy for the reader to follow the writer’s train of thought and to know immediately what major topics are covered. Depending on the length and complexity of the paper, its major sections may also be divided into subsections, sub-subsections, and so on. These smaller sections, in turn, use different heading styles to indicate different levels of information. In essence, you are using headings to create a hierarchy of information.
The following heading styles used in APA formatting are listed in order of greatest to least importance:
- Section headings use centered, boldface type. Headings use title case, with important words in the heading capitalized.
- Subsection headings use left-aligned, boldface type. Headings use title case.
- The third level uses left-aligned, indented, boldface type. Headings use a capital letter only for the first word, and they end in a period.
- The fourth level follows the same style used for the previous level, but the headings are boldfaced and italicized.
- The fifth level follows the same style used for the previous level, but the headings are italicized and not boldfaced.
Visually, the hierarchy of information is organized as indicated in Table 13.1 “Section Headings” .
Table 13.1 Section Headings
A college research paper may not use all the heading levels shown in Table 13.1 “Section Headings” , but you are likely to encounter them in academic journal articles that use APA style. For a brief paper, you may find that level 1 headings suffice. Longer or more complex papers may need level 2 headings or other lower-level headings to organize information clearly. Use your outline to craft your major section headings and determine whether any subtopics are substantial enough to require additional levels of headings.
Working with the document you developed in Note 13.11 “Exercise 2” , begin setting up the heading structure of the final draft of your research paper according to APA guidelines. Include your title and at least two to three major section headings, and follow the formatting guidelines provided above. If your major sections should be broken into subsections, add those headings as well. Use your outline to help you.
Because Jorge used only level 1 headings, his Exercise 3 would look like the following:
Citation Guidelines
In-text citations.
Throughout the body of your paper, include a citation whenever you quote or paraphrase material from your research sources. As you learned in Chapter 11 “Writing from Research: What Will I Learn?” , the purpose of citations is twofold: to give credit to others for their ideas and to allow your reader to follow up and learn more about the topic if desired. Your in-text citations provide basic information about your source; each source you cite will have a longer entry in the references section that provides more detailed information.
In-text citations must provide the name of the author or authors and the year the source was published. (When a given source does not list an individual author, you may provide the source title or the name of the organization that published the material instead.) When directly quoting a source, it is also required that you include the page number where the quote appears in your citation.
This information may be included within the sentence or in a parenthetical reference at the end of the sentence, as in these examples.
Epstein (2010) points out that “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive” (p. 137).
Here, the writer names the source author when introducing the quote and provides the publication date in parentheses after the author’s name. The page number appears in parentheses after the closing quotation marks and before the period that ends the sentence.
Addiction researchers caution that “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive” (Epstein, 2010, p. 137).
Here, the writer provides a parenthetical citation at the end of the sentence that includes the author’s name, the year of publication, and the page number separated by commas. Again, the parenthetical citation is placed after the closing quotation marks and before the period at the end of the sentence.
As noted in the book Junk Food, Junk Science (Epstein, 2010, p. 137), “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive.”
Here, the writer chose to mention the source title in the sentence (an optional piece of information to include) and followed the title with a parenthetical citation. Note that the parenthetical citation is placed before the comma that signals the end of the introductory phrase.
David Epstein’s book Junk Food, Junk Science (2010) pointed out that “junk food cannot be considered addictive in the same way that we think of psychoactive drugs as addictive” (p. 137).
Another variation is to introduce the author and the source title in your sentence and include the publication date and page number in parentheses within the sentence or at the end of the sentence. As long as you have included the essential information, you can choose the option that works best for that particular sentence and source.
Citing a book with a single author is usually a straightforward task. Of course, your research may require that you cite many other types of sources, such as books or articles with more than one author or sources with no individual author listed. You may also need to cite sources available in both print and online and nonprint sources, such as websites and personal interviews. Chapter 13 “APA and MLA Documentation and Formatting” , Section 13.2 “Citing and Referencing Techniques” and Section 13.3 “Creating a References Section” provide extensive guidelines for citing a variety of source types.
Writing at Work
APA is just one of several different styles with its own guidelines for documentation, formatting, and language usage. Depending on your field of interest, you may be exposed to additional styles, such as the following:
- MLA style. Determined by the Modern Languages Association and used for papers in literature, languages, and other disciplines in the humanities.
- Chicago style. Outlined in the Chicago Manual of Style and sometimes used for papers in the humanities and the sciences; many professional organizations use this style for publications as well.
- Associated Press (AP) style. Used by professional journalists.
References List
The brief citations included in the body of your paper correspond to the more detailed citations provided at the end of the paper in the references section. In-text citations provide basic information—the author’s name, the publication date, and the page number if necessary—while the references section provides more extensive bibliographical information. Again, this information allows your reader to follow up on the sources you cited and do additional reading about the topic if desired.
The specific format of entries in the list of references varies slightly for different source types, but the entries generally include the following information:
- The name(s) of the author(s) or institution that wrote the source
- The year of publication and, where applicable, the exact date of publication
- The full title of the source
- For books, the city of publication
- For articles or essays, the name of the periodical or book in which the article or essay appears
- For magazine and journal articles, the volume number, issue number, and pages where the article appears
- For sources on the web, the URL where the source is located
The references page is double spaced and lists entries in alphabetical order by the author’s last name. If an entry continues for more than one line, the second line and each subsequent line are indented five spaces. Review the following example. ( Chapter 13 “APA and MLA Documentation and Formatting” , Section 13.3 “Creating a References Section” provides extensive guidelines for formatting reference entries for different types of sources.)

In APA style, book and article titles are formatted in sentence case, not title case. Sentence case means that only the first word is capitalized, along with any proper nouns.
Key Takeaways
- Following proper citation and formatting guidelines helps writers ensure that their work will be taken seriously, give proper credit to other authors for their work, and provide valuable information to readers.
- Working ahead and taking care to cite sources correctly the first time are ways writers can save time during the editing stage of writing a research paper.
- APA papers usually include an abstract that concisely summarizes the paper.
- APA papers use a specific headings structure to provide a clear hierarchy of information.
- In APA papers, in-text citations usually include the name(s) of the author(s) and the year of publication.
- In-text citations correspond to entries in the references section, which provide detailed bibliographical information about a source.
Writing for Success Copyright © 2015 by University of Minnesota is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.

Cultural Relativity and Acceptance of Embryonic Stem Cell Research
Article sidebar.
Main Article Content
There is a debate about the ethical implications of using human embryos in stem cell research, which can be influenced by cultural, moral, and social values. This paper argues for an adaptable framework to accommodate diverse cultural and religious perspectives. By using an adaptive ethics model, research protections can reflect various populations and foster growth in stem cell research possibilities.
INTRODUCTION
Stem cell research combines biology, medicine, and technology, promising to alter health care and the understanding of human development. Yet, ethical contention exists because of individuals’ perceptions of using human embryos based on their various cultural, moral, and social values. While these disagreements concerning policy, use, and general acceptance have prompted the development of an international ethics policy, such a uniform approach can overlook the nuanced ethical landscapes between cultures. With diverse viewpoints in public health, a single global policy, especially one reflecting Western ethics or the ethics prevalent in high-income countries, is impractical. This paper argues for a culturally sensitive, adaptable framework for the use of embryonic stem cells. Stem cell policy should accommodate varying ethical viewpoints and promote an effective global dialogue. With an extension of an ethics model that can adapt to various cultures, we recommend localized guidelines that reflect the moral views of the people those guidelines serve.
Stem cells, characterized by their unique ability to differentiate into various cell types, enable the repair or replacement of damaged tissues. Two primary types of stem cells are somatic stem cells (adult stem cells) and embryonic stem cells. Adult stem cells exist in developed tissues and maintain the body’s repair processes. [1] Embryonic stem cells (ESC) are remarkably pluripotent or versatile, making them valuable in research. [2] However, the use of ESCs has sparked ethics debates. Considering the potential of embryonic stem cells, research guidelines are essential. The International Society for Stem Cell Research (ISSCR) provides international stem cell research guidelines. They call for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by ESC research.” [3] The ISSCR also publishes updates about culturing human embryos 14 days post fertilization, suggesting local policies and regulations should continue to evolve as ESC research develops. [4] Like the ISSCR, which calls for local law and policy to adapt to developing stem cell research given cultural acceptance, this paper highlights the importance of local social factors such as religion and culture.
I. Global Cultural Perspective of Embryonic Stem Cells
Views on ESCs vary throughout the world. Some countries readily embrace stem cell research and therapies, while others have stricter regulations due to ethical concerns surrounding embryonic stem cells and when an embryo becomes entitled to moral consideration. The philosophical issue of when the “someone” begins to be a human after fertilization, in the morally relevant sense, [5] impacts when an embryo becomes not just worthy of protection but morally entitled to it. The process of creating embryonic stem cell lines involves the destruction of the embryos for research. [6] Consequently, global engagement in ESC research depends on social-cultural acceptability.
a. US and Rights-Based Cultures
In the United States, attitudes toward stem cell therapies are diverse. The ethics and social approaches, which value individualism, [7] trigger debates regarding the destruction of human embryos, creating a complex regulatory environment. For example, the 1996 Dickey-Wicker Amendment prohibited federal funding for the creation of embryos for research and the destruction of embryos for “more than allowed for research on fetuses in utero.” [8] Following suit, in 2001, the Bush Administration heavily restricted stem cell lines for research. However, the Stem Cell Research Enhancement Act of 2005 was proposed to help develop ESC research but was ultimately vetoed. [9] Under the Obama administration, in 2009, an executive order lifted restrictions allowing for more development in this field. [10] The flux of research capacity and funding parallels the different cultural perceptions of human dignity of the embryo and how it is socially presented within the country’s research culture. [11]
b. Ubuntu and Collective Cultures
African bioethics differs from Western individualism because of the different traditions and values. African traditions, as described by individuals from South Africa and supported by some studies in other African countries, including Ghana and Kenya, follow the African moral philosophies of Ubuntu or Botho and Ukama , which “advocates for a form of wholeness that comes through one’s relationship and connectedness with other people in the society,” [12] making autonomy a socially collective concept. In this context, for the community to act autonomously, individuals would come together to decide what is best for the collective. Thus, stem cell research would require examining the value of the research to society as a whole and the use of the embryos as a collective societal resource. If society views the source as part of the collective whole, and opposes using stem cells, compromising the cultural values to pursue research may cause social detachment and stunt research growth. [13] Based on local culture and moral philosophy, the permissibility of stem cell research depends on how embryo, stem cell, and cell line therapies relate to the community as a whole. Ubuntu is the expression of humanness, with the person’s identity drawn from the “’I am because we are’” value. [14] The decision in a collectivistic culture becomes one born of cultural context, and individual decisions give deference to others in the society.
Consent differs in cultures where thought and moral philosophy are based on a collective paradigm. So, applying Western bioethical concepts is unrealistic. For one, Africa is a diverse continent with many countries with different belief systems, access to health care, and reliance on traditional or Western medicines. Where traditional medicine is the primary treatment, the “’restrictive focus on biomedically-related bioethics’” [is] problematic in African contexts because it neglects bioethical issues raised by traditional systems.” [15] No single approach applies in all areas or contexts. Rather than evaluating the permissibility of ESC research according to Western concepts such as the four principles approach, different ethics approaches should prevail.
Another consideration is the socio-economic standing of countries. In parts of South Africa, researchers have not focused heavily on contributing to the stem cell discourse, either because it is not considered health care or a health science priority or because resources are unavailable. [16] Each country’s priorities differ given different social, political, and economic factors. In South Africa, for instance, areas such as maternal mortality, non-communicable diseases, telemedicine, and the strength of health systems need improvement and require more focus. [17] Stem cell research could benefit the population, but it also could divert resources from basic medical care. Researchers in South Africa adhere to the National Health Act and Medicines Control Act in South Africa and international guidelines; however, the Act is not strictly enforced, and there is no clear legislation for research conduct or ethical guidelines. [18]
Some parts of Africa condemn stem cell research. For example, 98.2 percent of the Tunisian population is Muslim. [19] Tunisia does not permit stem cell research because of moral conflict with a Fatwa. Religion heavily saturates the regulation and direction of research. [20] Stem cell use became permissible for reproductive purposes only recently, with tight restrictions preventing cells from being used in any research other than procedures concerning ART/IVF. Their use is conditioned on consent, and available only to married couples. [21] The community's receptiveness to stem cell research depends on including communitarian African ethics.
c. Asia
Some Asian countries also have a collective model of ethics and decision making. [22] In China, the ethics model promotes a sincere respect for life or human dignity, [23] based on protective medicine. This model, influenced by Traditional Chinese Medicine (TCM), [24] recognizes Qi as the vital energy delivered via the meridians of the body; it connects illness to body systems, the body’s entire constitution, and the universe for a holistic bond of nature, health, and quality of life. [25] Following a protective ethics model, and traditional customs of wholeness, investment in stem cell research is heavily desired for its applications in regenerative therapies, disease modeling, and protective medicines. In a survey of medical students and healthcare practitioners, 30.8 percent considered stem cell research morally unacceptable while 63.5 percent accepted medical research using human embryonic stem cells. Of these individuals, 89.9 percent supported increased funding for stem cell research. [26] The scientific community might not reflect the overall population. From 1997 to 2019, China spent a total of $576 million (USD) on stem cell research at 8,050 stem cell programs, increased published presence from 0.6 percent to 14.01 percent of total global stem cell publications as of 2014, and made significant strides in cell-based therapies for various medical conditions. [27] However, while China has made substantial investments in stem cell research and achieved notable progress in clinical applications, concerns linger regarding ethical oversight and transparency. [28] For example, the China Biosecurity Law, promoted by the National Health Commission and China Hospital Association, attempted to mitigate risks by introducing an institutional review board (IRB) in the regulatory bodies. 5800 IRBs registered with the Chinese Clinical Trial Registry since 2021. [29] However, issues still need to be addressed in implementing effective IRB review and approval procedures.
The substantial government funding and focus on scientific advancement have sometimes overshadowed considerations of regional cultures, ethnic minorities, and individual perspectives, particularly evident during the one-child policy era. As government policy adapts to promote public stability, such as the change from the one-child to the two-child policy, [30] research ethics should also adapt to ensure respect for the values of its represented peoples.
Japan is also relatively supportive of stem cell research and therapies. Japan has a more transparent regulatory framework, allowing for faster approval of regenerative medicine products, which has led to several advanced clinical trials and therapies. [31] South Korea is also actively engaged in stem cell research and has a history of breakthroughs in cloning and embryonic stem cells. [32] However, the field is controversial, and there are issues of scientific integrity. For example, the Korean FDA fast-tracked products for approval, [33] and in another instance, the oocyte source was unclear and possibly violated ethical standards. [34] Trust is important in research, as it builds collaborative foundations between colleagues, trial participant comfort, open-mindedness for complicated and sensitive discussions, and supports regulatory procedures for stakeholders. There is a need to respect the culture’s interest, engagement, and for research and clinical trials to be transparent and have ethical oversight to promote global research discourse and trust.
d. Middle East
Countries in the Middle East have varying degrees of acceptance of or restrictions to policies related to using embryonic stem cells due to cultural and religious influences. Saudi Arabia has made significant contributions to stem cell research, and conducts research based on international guidelines for ethical conduct and under strict adherence to guidelines in accordance with Islamic principles. Specifically, the Saudi government and people require ESC research to adhere to Sharia law. In addition to umbilical and placental stem cells, [35] Saudi Arabia permits the use of embryonic stem cells as long as they come from miscarriages, therapeutic abortions permissible by Sharia law, or are left over from in vitro fertilization and donated to research. [36] Laws and ethical guidelines for stem cell research allow the development of research institutions such as the King Abdullah International Medical Research Center, which has a cord blood bank and a stem cell registry with nearly 10,000 donors. [37] Such volume and acceptance are due to the ethical ‘permissibility’ of the donor sources, which do not conflict with religious pillars. However, some researchers err on the side of caution, choosing not to use embryos or fetal tissue as they feel it is unethical to do so. [38]
Jordan has a positive research ethics culture. [39] However, there is a significant issue of lack of trust in researchers, with 45.23 percent (38.66 percent agreeing and 6.57 percent strongly agreeing) of Jordanians holding a low level of trust in researchers, compared to 81.34 percent of Jordanians agreeing that they feel safe to participate in a research trial. [40] Safety testifies to the feeling of confidence that adequate measures are in place to protect participants from harm, whereas trust in researchers could represent the confidence in researchers to act in the participants’ best interests, adhere to ethical guidelines, provide accurate information, and respect participants’ rights and dignity. One method to improve trust would be to address communication issues relevant to ESC. Legislation surrounding stem cell research has adopted specific language, especially concerning clarification “between ‘stem cells’ and ‘embryonic stem cells’” in translation. [41] Furthermore, legislation “mandates the creation of a national committee… laying out specific regulations for stem-cell banking in accordance with international standards.” [42] This broad regulation opens the door for future global engagement and maintains transparency. However, these regulations may also constrain the influence of research direction, pace, and accessibility of research outcomes.
e. Europe
In the European Union (EU), ethics is also principle-based, but the principles of autonomy, dignity, integrity, and vulnerability are interconnected. [43] As such, the opportunity for cohesion and concessions between individuals’ thoughts and ideals allows for a more adaptable ethics model due to the flexible principles that relate to the human experience The EU has put forth a framework in its Convention for the Protection of Human Rights and Dignity of the Human Being allowing member states to take different approaches. Each European state applies these principles to its specific conventions, leading to or reflecting different acceptance levels of stem cell research. [44]
For example, in Germany, Lebenzusammenhang , or the coherence of life, references integrity in the unity of human culture. Namely, the personal sphere “should not be subject to external intervention.” [45] Stem cell interventions could affect this concept of bodily completeness, leading to heavy restrictions. Under the Grundgesetz, human dignity and the right to life with physical integrity are paramount. [46] The Embryo Protection Act of 1991 made producing cell lines illegal. Cell lines can be imported if approved by the Central Ethics Commission for Stem Cell Research only if they were derived before May 2007. [47] Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization. [48] Spain’s approach differs still, with a comprehensive regulatory framework. [49] Thus, research regulation can be culture-specific due to variations in applied principles. Diverse cultures call for various approaches to ethical permissibility. [50] Only an adaptive-deliberative model can address the cultural constructions of self and achieve positive, culturally sensitive stem cell research practices. [51]
II. Religious Perspectives on ESC
Embryonic stem cell sources are the main consideration within religious contexts. While individuals may not regard their own religious texts as authoritative or factual, religion can shape their foundations or perspectives.
The Qur'an states:
“And indeed We created man from a quintessence of clay. Then We placed within him a small quantity of nutfa (sperm to fertilize) in a safe place. Then We have fashioned the nutfa into an ‘alaqa (clinging clot or cell cluster), then We developed the ‘alaqa into mudgha (a lump of flesh), and We made mudgha into bones, and clothed the bones with flesh, then We brought it into being as a new creation. So Blessed is Allah, the Best of Creators.” [52]
Many scholars of Islam estimate the time of soul installment, marked by the angel breathing in the soul to bring the individual into creation, as 120 days from conception. [53] Personhood begins at this point, and the value of life would prohibit research or experimentation that could harm the individual. If the fetus is more than 120 days old, the time ensoulment is interpreted to occur according to Islamic law, abortion is no longer permissible. [54] There are a few opposing opinions about early embryos in Islamic traditions. According to some Islamic theologians, there is no ensoulment of the early embryo, which is the source of stem cells for ESC research. [55]
In Buddhism, the stance on stem cell research is not settled. The main tenets, the prohibition against harming or destroying others (ahimsa) and the pursuit of knowledge (prajña) and compassion (karuna), leave Buddhist scholars and communities divided. [56] Some scholars argue stem cell research is in accordance with the Buddhist tenet of seeking knowledge and ending human suffering. Others feel it violates the principle of not harming others. Finding the balance between these two points relies on the karmic burden of Buddhist morality. In trying to prevent ahimsa towards the embryo, Buddhist scholars suggest that to comply with Buddhist tenets, research cannot be done as the embryo has personhood at the moment of conception and would reincarnate immediately, harming the individual's ability to build their karmic burden. [57] On the other hand, the Bodhisattvas, those considered to be on the path to enlightenment or Nirvana, have given organs and flesh to others to help alleviate grieving and to benefit all. [58] Acceptance varies on applied beliefs and interpretations.
Catholicism does not support embryonic stem cell research, as it entails creation or destruction of human embryos. This destruction conflicts with the belief in the sanctity of life. For example, in the Old Testament, Genesis describes humanity as being created in God’s image and multiplying on the Earth, referencing the sacred rights to human conception and the purpose of development and life. In the Ten Commandments, the tenet that one should not kill has numerous interpretations where killing could mean murder or shedding of the sanctity of life, demonstrating the high value of human personhood. In other books, the theological conception of when life begins is interpreted as in utero, [59] highlighting the inviolability of life and its formation in vivo to make a religious point for accepting such research as relatively limited, if at all. [60] The Vatican has released ethical directives to help apply a theological basis to modern-day conflicts. The Magisterium of the Church states that “unless there is a moral certainty of not causing harm,” experimentation on fetuses, fertilized cells, stem cells, or embryos constitutes a crime. [61] Such procedures would not respect the human person who exists at these stages, according to Catholicism. Damages to the embryo are considered gravely immoral and illicit. [62] Although the Catholic Church officially opposes abortion, surveys demonstrate that many Catholic people hold pro-choice views, whether due to the context of conception, stage of pregnancy, threat to the mother’s life, or for other reasons, demonstrating that practicing members can also accept some but not all tenets. [63]
Some major Jewish denominations, such as the Reform, Conservative, and Reconstructionist movements, are open to supporting ESC use or research as long as it is for saving a life. [64] Within Judaism, the Talmud, or study, gives personhood to the child at birth and emphasizes that life does not begin at conception: [65]
“If she is found pregnant, until the fortieth day it is mere fluid,” [66]
Whereas most religions prioritize the status of human embryos, the Halakah (Jewish religious law) states that to save one life, most other religious laws can be ignored because it is in pursuit of preservation. [67] Stem cell research is accepted due to application of these religious laws.
We recognize that all religions contain subsets and sects. The variety of environmental and cultural differences within religious groups requires further analysis to respect the flexibility of religious thoughts and practices. We make no presumptions that all cultures require notions of autonomy or morality as under the common morality theory , which asserts a set of universal moral norms that all individuals share provides moral reasoning and guides ethical decisions. [68] We only wish to show that the interaction with morality varies between cultures and countries.
III. A Flexible Ethical Approach
The plurality of different moral approaches described above demonstrates that there can be no universally acceptable uniform law for ESC on a global scale. Instead of developing one standard, flexible ethical applications must be continued. We recommend local guidelines that incorporate important cultural and ethical priorities.
While the Declaration of Helsinki is more relevant to people in clinical trials receiving ESC products, in keeping with the tradition of protections for research subjects, consent of the donor is an ethical requirement for ESC donation in many jurisdictions including the US, Canada, and Europe. [69] The Declaration of Helsinki provides a reference point for regulatory standards and could potentially be used as a universal baseline for obtaining consent prior to gamete or embryo donation.
For instance, in Columbia University’s egg donor program for stem cell research, donors followed standard screening protocols and “underwent counseling sessions that included information as to the purpose of oocyte donation for research, what the oocytes would be used for, the risks and benefits of donation, and process of oocyte stimulation” to ensure transparency for consent. [70] The program helped advance stem cell research and provided clear and safe research methods with paid participants. Though paid participation or covering costs of incidental expenses may not be socially acceptable in every culture or context, [71] and creating embryos for ESC research is illegal in many jurisdictions, Columbia’s program was effective because of the clear and honest communications with donors, IRBs, and related stakeholders. This example demonstrates that cultural acceptance of scientific research and of the idea that an egg or embryo does not have personhood is likely behind societal acceptance of donating eggs for ESC research. As noted, many countries do not permit the creation of embryos for research.
Proper communication and education regarding the process and purpose of stem cell research may bolster comprehension and garner more acceptance. “Given the sensitive subject material, a complete consent process can support voluntary participation through trust, understanding, and ethical norms from the cultures and morals participants value. This can be hard for researchers entering countries of different socioeconomic stability, with different languages and different societal values. [72]
An adequate moral foundation in medical ethics is derived from the cultural and religious basis that informs knowledge and actions. [73] Understanding local cultural and religious values and their impact on research could help researchers develop humility and promote inclusion.
IV. Concerns
Some may argue that if researchers all adhere to one ethics standard, protection will be satisfied across all borders, and the global public will trust researchers. However, defining what needs to be protected and how to define such research standards is very specific to the people to which standards are applied. We suggest that applying one uniform guide cannot accurately protect each individual because we all possess our own perceptions and interpretations of social values. [74] Therefore, the issue of not adjusting to the moral pluralism between peoples in applying one standard of ethics can be resolved by building out ethics models that can be adapted to different cultures and religions.
Other concerns include medical tourism, which may promote health inequities. [75] Some countries may develop and approve products derived from ESC research before others, compromising research ethics or drug approval processes. There are also concerns about the sale of unauthorized stem cell treatments, for example, those without FDA approval in the United States. Countries with robust research infrastructures may be tempted to attract medical tourists, and some customers will have false hopes based on aggressive publicity of unproven treatments. [76]
For example, in China, stem cell clinics can market to foreign clients who are not protected under the regulatory regimes. Companies employ a marketing strategy of “ethically friendly” therapies. Specifically, in the case of Beike, China’s leading stem cell tourism company and sprouting network, ethical oversight of administrators or health bureaus at one site has “the unintended consequence of shifting questionable activities to another node in Beike's diffuse network.” [77] In contrast, Jordan is aware of stem cell research’s potential abuse and its own status as a “health-care hub.” Jordan’s expanded regulations include preserving the interests of individuals in clinical trials and banning private companies from ESC research to preserve transparency and the integrity of research practices. [78]
The social priorities of the community are also a concern. The ISSCR explicitly states that guidelines “should be periodically revised to accommodate scientific advances, new challenges, and evolving social priorities.” [79] The adaptable ethics model extends this consideration further by addressing whether research is warranted given the varying degrees of socioeconomic conditions, political stability, and healthcare accessibilities and limitations. An ethical approach would require discussion about resource allocation and appropriate distribution of funds. [80]
While some religions emphasize the sanctity of life from conception, which may lead to public opposition to ESC research, others encourage ESC research due to its potential for healing and alleviating human pain. Many countries have special regulations that balance local views on embryonic personhood, the benefits of research as individual or societal goods, and the protection of human research subjects. To foster understanding and constructive dialogue, global policy frameworks should prioritize the protection of universal human rights, transparency, and informed consent. In addition to these foundational global policies, we recommend tailoring local guidelines to reflect the diverse cultural and religious perspectives of the populations they govern. Ethics models should be adapted to local populations to effectively establish research protections, growth, and possibilities of stem cell research.
For example, in countries with strong beliefs in the moral sanctity of embryos or heavy religious restrictions, an adaptive model can allow for discussion instead of immediate rejection. In countries with limited individual rights and voice in science policy, an adaptive model ensures cultural, moral, and religious views are taken into consideration, thereby building social inclusion. While this ethical consideration by the government may not give a complete voice to every individual, it will help balance policies and maintain the diverse perspectives of those it affects. Embracing an adaptive ethics model of ESC research promotes open-minded dialogue and respect for the importance of human belief and tradition. By actively engaging with cultural and religious values, researchers can better handle disagreements and promote ethical research practices that benefit each society.
This brief exploration of the religious and cultural differences that impact ESC research reveals the nuances of relative ethics and highlights a need for local policymakers to apply a more intense adaptive model.
[1] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[2] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[3] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk ; Kimmelman, J., Hyun, I., Benvenisty, N. et al. Policy: Global standards for stem-cell research. Nature 533 , 311–313 (2016). https://doi.org/10.1038/533311a
[4] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk
[5] Concerning the moral philosophies of stem cell research, our paper does not posit a personal moral stance nor delve into the “when” of human life begins. To read further about the philosophical debate, consider the following sources:
Sandel M. J. (2004). Embryo ethics--the moral logic of stem-cell research. The New England journal of medicine , 351 (3), 207–209. https://doi.org/10.1056/NEJMp048145 ; George, R. P., & Lee, P. (2020, September 26). Acorns and Embryos . The New Atlantis. https://www.thenewatlantis.com/publications/acorns-and-embryos ; Sagan, A., & Singer, P. (2007). The moral status of stem cells. Metaphilosophy , 38 (2/3), 264–284. http://www.jstor.org/stable/24439776 ; McHugh P. R. (2004). Zygote and "clonote"--the ethical use of embryonic stem cells. The New England journal of medicine , 351 (3), 209–211. https://doi.org/10.1056/NEJMp048147 ; Kurjak, A., & Tripalo, A. (2004). The facts and doubts about beginning of the human life and personality. Bosnian journal of basic medical sciences , 4 (1), 5–14. https://doi.org/10.17305/bjbms.2004.3453
[6] Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience , 28 (4), 589–603. https://doi.org/10.3233/RNN-2010-0543
[7] Socially, at its core, the Western approach to ethics is widely principle-based, autonomy being one of the key factors to ensure a fundamental respect for persons within research. For information regarding autonomy in research, see: Department of Health, Education, and Welfare, & National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978). The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research.; For a more in-depth review of autonomy within the US, see: Beauchamp, T. L., & Childress, J. F. (1994). Principles of Biomedical Ethics . Oxford University Press.
[8] Sherley v. Sebelius , 644 F.3d 388 (D.C. Cir. 2011), citing 45 C.F.R. 46.204(b) and [42 U.S.C. § 289g(b)]. https://www.cadc.uscourts.gov/internet/opinions.nsf/6c690438a9b43dd685257a64004ebf99/$file/11-5241-1391178.pdf
[9] Stem Cell Research Enhancement Act of 2005, H. R. 810, 109 th Cong. (2001). https://www.govtrack.us/congress/bills/109/hr810/text ; Bush, G. W. (2006, July 19). Message to the House of Representatives . National Archives and Records Administration. https://georgewbush-whitehouse.archives.gov/news/releases/2006/07/20060719-5.html
[10] National Archives and Records Administration. (2009, March 9). Executive order 13505 -- removing barriers to responsible scientific research involving human stem cells . National Archives and Records Administration. https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells
[11] Hurlbut, W. B. (2006). Science, Religion, and the Politics of Stem Cells. Social Research , 73 (3), 819–834. http://www.jstor.org/stable/40971854
[12] Akpa-Inyang, Francis & Chima, Sylvester. (2021). South African traditional values and beliefs regarding informed consent and limitations of the principle of respect for autonomy in African communities: a cross-cultural qualitative study. BMC Medical Ethics . 22. 10.1186/s12910-021-00678-4.
[13] Source for further reading: Tangwa G. B. (2007). Moral status of embryonic stem cells: perspective of an African villager. Bioethics , 21(8), 449–457. https://doi.org/10.1111/j.1467-8519.2007.00582.x , see also Mnisi, F. M. (2020). An African analysis based on ethics of Ubuntu - are human embryonic stem cell patents morally justifiable? African Insight , 49 (4).
[14] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics , 22 (2), 112–122. https://doi.org/10.1111/dewb.12324
[15] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics, 22(2), 112–122. https://doi.org/10.1111/dewb.12324
[16] Jackson, C.S., Pepper, M.S. Opportunities and barriers to establishing a cell therapy programme in South Africa. Stem Cell Res Ther 4 , 54 (2013). https://doi.org/10.1186/scrt204 ; Pew Research Center. (2014, May 1). Public health a major priority in African nations . Pew Research Center’s Global Attitudes Project. https://www.pewresearch.org/global/2014/05/01/public-health-a-major-priority-in-african-nations/
[17] Department of Health Republic of South Africa. (2021). Health Research Priorities (revised) for South Africa 2021-2024 . National Health Research Strategy. https://www.health.gov.za/wp-content/uploads/2022/05/National-Health-Research-Priorities-2021-2024.pdf
[18] Oosthuizen, H. (2013). Legal and Ethical Issues in Stem Cell Research in South Africa. In: Beran, R. (eds) Legal and Forensic Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32338-6_80 , see also: Gaobotse G (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[19] United States Bureau of Citizenship and Immigration Services. (1998). Tunisia: Information on the status of Christian conversions in Tunisia . UNHCR Web Archive. https://webarchive.archive.unhcr.org/20230522142618/https://www.refworld.org/docid/3df0be9a2.html
[20] Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[21] Kooli, C. Review of assisted reproduction techniques, laws, and regulations in Muslim countries. Middle East Fertil Soc J 24 , 8 (2020). https://doi.org/10.1186/s43043-019-0011-0 ; Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[22] Pang M. C. (1999). Protective truthfulness: the Chinese way of safeguarding patients in informed treatment decisions. Journal of medical ethics , 25(3), 247–253. https://doi.org/10.1136/jme.25.3.247
[23] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[24] Wang, Y., Xue, Y., & Guo, H. D. (2022). Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Frontiers in pharmacology , 13 , 1013740. https://doi.org/10.3389/fphar.2022.1013740
[25] Li, X.-T., & Zhao, J. (2012). Chapter 4: An Approach to the Nature of Qi in TCM- Qi and Bioenergy. In Recent Advances in Theories and Practice of Chinese Medicine (p. 79). InTech.
[26] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[27] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[28] Zhang, J. Y. (2017). Lost in translation? accountability and governance of Clinical Stem Cell Research in China. Regenerative Medicine , 12 (6), 647–656. https://doi.org/10.2217/rme-2017-0035
[29] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[30] Chen, H., Wei, T., Wang, H. et al. Association of China’s two-child policy with changes in number of births and birth defects rate, 2008–2017. BMC Public Health 22 , 434 (2022). https://doi.org/10.1186/s12889-022-12839-0
[31] Azuma, K. Regulatory Landscape of Regenerative Medicine in Japan. Curr Stem Cell Rep 1 , 118–128 (2015). https://doi.org/10.1007/s40778-015-0012-6
[32] Harris, R. (2005, May 19). Researchers Report Advance in Stem Cell Production . NPR. https://www.npr.org/2005/05/19/4658967/researchers-report-advance-in-stem-cell-production
[33] Park, S. (2012). South Korea steps up stem-cell work. Nature . https://doi.org/10.1038/nature.2012.10565
[34] Resnik, D. B., Shamoo, A. E., & Krimsky, S. (2006). Fraudulent human embryonic stem cell research in South Korea: lessons learned. Accountability in research , 13 (1), 101–109. https://doi.org/10.1080/08989620600634193 .
[35] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
[36] Association for the Advancement of Blood and Biotherapies. https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-cellular-therapies/international-competent-authorities/saudi-arabia
[37] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21 (1), 35. https://doi.org/10.1186/s12910-020-00482-6
[38] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
Culturally, autonomy practices follow a relational autonomy approach based on a paternalistic deontological health care model. The adherence to strict international research policies and religious pillars within the regulatory environment is a great foundation for research ethics. However, there is a need to develop locally targeted ethics approaches for research (as called for in Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6), this decision-making approach may help advise a research decision model. For more on the clinical cultural autonomy approaches, see: Alabdullah, Y. Y., Alzaid, E., Alsaad, S., Alamri, T., Alolayan, S. W., Bah, S., & Aljoudi, A. S. (2022). Autonomy and paternalism in Shared decision‐making in a Saudi Arabian tertiary hospital: A cross‐sectional study. Developing World Bioethics , 23 (3), 260–268. https://doi.org/10.1111/dewb.12355 ; Bukhari, A. A. (2017). Universal Principles of Bioethics and Patient Rights in Saudi Arabia (Doctoral dissertation, Duquesne University). https://dsc.duq.edu/etd/124; Ladha, S., Nakshawani, S. A., Alzaidy, A., & Tarab, B. (2023, October 26). Islam and Bioethics: What We All Need to Know . Columbia University School of Professional Studies. https://sps.columbia.edu/events/islam-and-bioethics-what-we-all-need-know
[39] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[40] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[41] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[42] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[43] The EU’s definition of autonomy relates to the capacity for creating ideas, moral insight, decisions, and actions without constraint, personal responsibility, and informed consent. However, the EU views autonomy as not completely able to protect individuals and depends on other principles, such as dignity, which “expresses the intrinsic worth and fundamental equality of all human beings.” Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[44] Council of Europe. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (ETS No. 164) https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164 (forbidding the creation of embryos for research purposes only, and suggests embryos in vitro have protections.); Also see Drabiak-Syed B. K. (2013). New President, New Human Embryonic Stem Cell Research Policy: Comparative International Perspectives and Embryonic Stem Cell Research Laws in France. Biotechnology Law Report , 32 (6), 349–356. https://doi.org/10.1089/blr.2013.9865
[45] Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[46] Tomuschat, C., Currie, D. P., Kommers, D. P., & Kerr, R. (Trans.). (1949, May 23). Basic law for the Federal Republic of Germany. https://www.btg-bestellservice.de/pdf/80201000.pdf
[47] Regulation of Stem Cell Research in Germany . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-germany
[48] Regulation of Stem Cell Research in Finland . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-finland
[49] Regulation of Stem Cell Research in Spain . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-spain
[50] Some sources to consider regarding ethics models or regulatory oversights of other cultures not covered:
Kara MA. Applicability of the principle of respect for autonomy: the perspective of Turkey. J Med Ethics. 2007 Nov;33(11):627-30. doi: 10.1136/jme.2006.017400. PMID: 17971462; PMCID: PMC2598110.
Ugarte, O. N., & Acioly, M. A. (2014). The principle of autonomy in Brazil: one needs to discuss it ... Revista do Colegio Brasileiro de Cirurgioes , 41 (5), 374–377. https://doi.org/10.1590/0100-69912014005013
Bharadwaj, A., & Glasner, P. E. (2012). Local cells, global science: The rise of embryonic stem cell research in India . Routledge.
For further research on specific European countries regarding ethical and regulatory framework, we recommend this database: Regulation of Stem Cell Research in Europe . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-europe
[51] Klitzman, R. (2006). Complications of culture in obtaining informed consent. The American Journal of Bioethics, 6(1), 20–21. https://doi.org/10.1080/15265160500394671 see also: Ekmekci, P. E., & Arda, B. (2017). Interculturalism and Informed Consent: Respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi, Romania) , 14 (2), 159–172.; For why trust is important in research, see also: Gray, B., Hilder, J., Macdonald, L., Tester, R., Dowell, A., & Stubbe, M. (2017). Are research ethics guidelines culturally competent? Research Ethics , 13 (1), 23-41. https://doi.org/10.1177/1747016116650235
[52] The Qur'an (M. Khattab, Trans.). (1965). Al-Mu’minun, 23: 12-14. https://quran.com/23
[53] Lenfest, Y. (2017, December 8). Islam and the beginning of human life . Bill of Health. https://blog.petrieflom.law.harvard.edu/2017/12/08/islam-and-the-beginning-of-human-life/
[54] Aksoy, S. (2005). Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. Journal of Medical Ethics , 31: 399-403.; see also: Mahmoud, Azza. "Islamic Bioethics: National Regulations and Guidelines of Human Stem Cell Research in the Muslim World." Master's thesis, Chapman University, 2022. https://doi.org/10.36837/ chapman.000386
[55] Rashid, R. (2022). When does Ensoulment occur in the Human Foetus. Journal of the British Islamic Medical Association , 12 (4). ISSN 2634 8071. https://www.jbima.com/wp-content/uploads/2023/01/2-Ethics-3_-Ensoulment_Rafaqat.pdf.
[56] Sivaraman, M. & Noor, S. (2017). Ethics of embryonic stem cell research according to Buddhist, Hindu, Catholic, and Islamic religions: perspective from Malaysia. Asian Biomedicine,8(1) 43-52. https://doi.org/10.5372/1905-7415.0801.260
[57] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[58] Lecso, P. A. (1991). The Bodhisattva Ideal and Organ Transplantation. Journal of Religion and Health , 30 (1), 35–41. http://www.jstor.org/stable/27510629 ; Bodhisattva, S. (n.d.). The Key of Becoming a Bodhisattva . A Guide to the Bodhisattva Way of Life. http://www.buddhism.org/Sutras/2/BodhisattvaWay.htm
[59] There is no explicit religious reference to when life begins or how to conduct research that interacts with the concept of life. However, these are relevant verses pertaining to how the fetus is viewed. (( King James Bible . (1999). Oxford University Press. (original work published 1769))
Jerimiah 1: 5 “Before I formed thee in the belly I knew thee; and before thou camest forth out of the womb I sanctified thee…”
In prophet Jerimiah’s insight, God set him apart as a person known before childbirth, a theme carried within the Psalm of David.
Psalm 139: 13-14 “…Thou hast covered me in my mother's womb. I will praise thee; for I am fearfully and wonderfully made…”
These verses demonstrate David’s respect for God as an entity that would know of all man’s thoughts and doings even before birth.
[60] It should be noted that abortion is not supported as well.
[61] The Vatican. (1987, February 22). Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation Replies to Certain Questions of the Day . Congregation For the Doctrine of the Faith. https://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html
[62] The Vatican. (2000, August 25). Declaration On the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells . Pontifical Academy for Life. https://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html ; Ohara, N. (2003). Ethical Consideration of Experimentation Using Living Human Embryos: The Catholic Church’s Position on Human Embryonic Stem Cell Research and Human Cloning. Department of Obstetrics and Gynecology . Retrieved from https://article.imrpress.com/journal/CEOG/30/2-3/pii/2003018/77-81.pdf.
[63] Smith, G. A. (2022, May 23). Like Americans overall, Catholics vary in their abortion views, with regular mass attenders most opposed . Pew Research Center. https://www.pewresearch.org/short-reads/2022/05/23/like-americans-overall-catholics-vary-in-their-abortion-views-with-regular-mass-attenders-most-opposed/
[64] Rosner, F., & Reichman, E. (2002). Embryonic stem cell research in Jewish law. Journal of halacha and contemporary society , (43), 49–68.; Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[65] Schenker J. G. (2008). The beginning of human life: status of embryo. Perspectives in Halakha (Jewish Religious Law). Journal of assisted reproduction and genetics , 25 (6), 271–276. https://doi.org/10.1007/s10815-008-9221-6
[66] Ruttenberg, D. (2020, May 5). The Torah of Abortion Justice (annotated source sheet) . Sefaria. https://www.sefaria.org/sheets/234926.7?lang=bi&with=all&lang2=en
[67] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[68] Gert, B. (2007). Common morality: Deciding what to do . Oxford Univ. Press.
[69] World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA , 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 Declaration of Helsinki – WMA – The World Medical Association .; see also: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research . U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
[70] Zakarin Safier, L., Gumer, A., Kline, M., Egli, D., & Sauer, M. V. (2018). Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. Journal of assisted reproduction and genetics , 35 (7), 1219–1225. https://doi.org/10.1007/s10815-018-1171-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063839/ see also: Riordan, N. H., & Paz Rodríguez, J. (2021). Addressing concerns regarding associated costs, transparency, and integrity of research in recent stem cell trial. Stem Cells Translational Medicine , 10 (12), 1715–1716. https://doi.org/10.1002/sctm.21-0234
[71] Klitzman, R., & Sauer, M. V. (2009). Payment of egg donors in stem cell research in the USA. Reproductive biomedicine online , 18 (5), 603–608. https://doi.org/10.1016/s1472-6483(10)60002-8
[72] Krosin, M. T., Klitzman, R., Levin, B., Cheng, J., & Ranney, M. L. (2006). Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical trials (London, England) , 3 (3), 306–313. https://doi.org/10.1191/1740774506cn150oa
[73] Veatch, Robert M. Hippocratic, Religious, and Secular Medical Ethics: The Points of Conflict . Georgetown University Press, 2012.
[74] Msoroka, M. S., & Amundsen, D. (2018). One size fits not quite all: Universal research ethics with diversity. Research Ethics , 14 (3), 1-17. https://doi.org/10.1177/1747016117739939
[75] Pirzada, N. (2022). The Expansion of Turkey’s Medical Tourism Industry. Voices in Bioethics , 8 . https://doi.org/10.52214/vib.v8i.9894
[76] Stem Cell Tourism: False Hope for Real Money . Harvard Stem Cell Institute (HSCI). (2023). https://hsci.harvard.edu/stem-cell-tourism , See also: Bissassar, M. (2017). Transnational Stem Cell Tourism: An ethical analysis. Voices in Bioethics , 3 . https://doi.org/10.7916/vib.v3i.6027
[77] Song, P. (2011) The proliferation of stem cell therapies in post-Mao China: problematizing ethical regulation, New Genetics and Society , 30:2, 141-153, DOI: 10.1080/14636778.2011.574375
[78] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[79] International Society for Stem Cell Research. (2024). Standards in stem cell research . International Society for Stem Cell Research. https://www.isscr.org/guidelines/5-standards-in-stem-cell-research
[80] Benjamin, R. (2013). People’s science bodies and rights on the Stem Cell Frontier . Stanford University Press.
Mifrah Hayath
SM Candidate Harvard Medical School, MS Biotechnology Johns Hopkins University
Olivia Bowers
MS Bioethics Columbia University (Disclosure: affiliated with Voices in Bioethics)
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .

International Journal of Research and Innovation in Social Science | IJRISS
ISSN – 2454-6186
DOI – 10.47772/IJRISS

Journal metrics
About the journal.
The International Journal of Research and Innovation in Social Science (ISSN 2454-6186), with the DOI 10.47772/IJRISS, operates as an accessible global journal with a focus on Social Studies and its Sub Topics . Authors are invited to submit the manuscript in various formats, including Research Papers, Review Papers, Informative Articles, Comparative Studies, Case Studies, Dissertation Chapters, Research Proposals, and Synopses. Our primary objective is to enlighten and establish connections within the worldwide community of social science scholars.
- DOI Number: 10.47772/IJRISS
- Open Access: All published papers are immediately freely available to read, download, and share .
- Rapid Publication: Rapid publication of papers and retaining a high-quality publication process.
- Nominal Fee: Nominal Publication Fee to Support Research Community.
- Connect: Connect the global social science community.
Cover Page
The primary aim of the International Journal of Research and Innovation in Social Science (IJRISS) revolves around the advancement of inventive research that actively contributes to the progression of the social sciences. This journal is committed to presenting original and empirical research that introduces fresh insights and problem-solving approaches to address societal issues. The journal’s editorial board is comprised of dedicated experts and researchers hailing from diverse corners of the globe, all working towards upholding the caliber and pertinence of the research disseminated through its pages.
The International Journal of Research and Innovation in Social Science (IJRISS) stands as an open-access publication, meaning that all articles are readily available online at no cost, facilitating easy accessibility for readers across the world. This inclusive approach ensures that the research showcased in the journal reaches a broader audience, consequently fostering the exchange of knowledge and ideas among the scholarly community and researchers.
Calls for papers
We extend an invitation to Scientists, Academicians, and Researchers to contribute their articles for publication in a peer-reviewed, open-access Social Science International Journal. The journal is published monthly and follows a rigorous refereeing process.
Research Area
Indexing/abstracted.

HOW WE WORK ?
Extensive & advanced integrations ui component for event & conference website, call for paper.
Unlock the opportunity to showcase your groundbreaking research by publishing with our esteemed journal
Subscribe to Our Newsletter
Sign up for our newsletter, to get updates regarding the Call for Paper, Papers & Research.
Email Address * Subscribe
Track Your Paper
Enter the following details to get the information about your paper
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Handwashing
- Hand Hygiene as a Family Activity
- Hand Hygiene FAQs
- Handwashing Facts
- Publications, Data, & Statistics
- Health Promotion Materials
- Global Handwashing Day
- Life is Better with Clean Hands Campaign
- Clinical Safety
- Healthcare Training
- Clean Hands Count Materials
About Handwashing
- Many diseases and conditions are spread by not washing hands with soap and clean, running water.
- Handwashing with soap is one of the best ways to stay healthy.
- If soap and water are not readily available, use a hand sanitizer with at least 60% alcohol to clean your hands.

Why it's important
Washing hands can keep you healthy and prevent the spread of respiratory and diarrheal infections. Germs can spread from person to person or from surfaces to people when you:
- Touch your eyes, nose, and mouth with unwashed hands
- Prepare or eat food and drinks with unwashed hands
- Touch surfaces or objects that have germs on them
- Blow your nose, cough, or sneeze into hands and then touch other people's hands or common objects
Key times to wash hands
You can help yourself and your loved ones stay healthy by washing your hands often, especially during these key times when you are likely to get and spread germs:
- Before, during, and after preparing food
- Before and after eating food
- Before and after caring for someone at home who is sick with vomiting or diarrhea
- Before and after treating a cut or wound
- After using the toilet
- After changing diapers or cleaning up a child who has used the toilet
- After blowing your nose, coughing, or sneezing
- After touching an animal, animal feed, or animal waste
- After handling pet food or pet treats
- After touching garbage
How it works
Washing your hands is easy, and it’s one of the most effective ways to prevent the spread of germs. Follow these five steps every time.
- Wet your hands with clean, running water (warm or cold), turn off the tap, and apply soap.
- Lather your hands by rubbing them together with the soap. Lather the backs of your hands, between your fingers, and under your nails.
- Scrub your hands for at least 20 seconds . Need a timer? Hum the “Happy Birthday” song from beginning to end twice.
- Rinse your hands well under clean, running water.
- Dry your hands using a clean towel or an air dryer.
Use hand sanitizer when you can't use soap and water
Washing hands with soap and water is the best way to get rid of germs in most situations. If soap and water are not readily available, you can use an alcohol-based hand sanitizer that contains at least 60% alcohol. You can tell if the sanitizer contains at least 60% alcohol by looking at the product label.
What you can do
CDC has health promotion materials to encourage kids and adults to make handwashing part of their everyday lives.
- Share social media graphics and messages.
- Print stickers and place clings on bathroom mirrors.
- Promote handwashing on or around Global Handwashing Day , celebrated each year on October 15.
- Distribute fact sheets to share information about hand hygiene for specific audiences.
- Frequent Questions About Hand Hygiene
- Hand Hygiene in Healthcare Settings
- The Life is Better with Clean Hands Campaign
Clean Hands
Having clean hands is one of the best ways to avoid getting sick and prevent the spread of germs to others.
For Everyone
Health care providers.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Submission guidelines
Format of articles, cover letter, revised manuscripts, tex/latex files, writing your manuscript, copy editing services, acknowledgements, author contributions, competing interests, data availability, ethics declarations, approval for animal experiments, approval for human experiments, consent to participate/consent to publish.
- Supplementary information
Figure legends
General figure guidelines, figures for peer review, figures for publication, statistical guidelines, chemical and biological nomenclature and abbreviations, gene nomenclature, characterisation of chemical and biomolecular materials, registered reports.
Scientific Reports publishes original research in two formats: Article and Registered Report. For Registered Reports, see section below . In most cases, we do not impose strict limits on word count or page number. However, we strongly recommend that you write concisely and stick to the following guidelines:
- Articles should ideally be no more than 11 typeset pages
- The main text should be no more than 4,500 words (not including Abstract, Methods, References and figure legends)
- The title should be no more than 20 words, should describe the main message of the article using a single scientifically accurate sentence, and should not contain puns or idioms
- The abstract should be no more than 200 words
For a definitive list of which limits are mandatory please visit the submission checklist page .
Please do not include any references in your Abstract. Make sure it serves both as a general introduction to the topic and as a brief, non-technical summary of the main results and their implications. Abstract should be unstructured, i.e. should not contain sections or subheadings.
We allow the use of up to 6 keywords/key phrases that can be used for indexing purposes. These should represent the main content of the submission.
Your manuscript text file should start with a title page that shows author affiliations and contact information, identifying the corresponding author with an asterisk. We recommend that each section includes an introduction of referenced text that expands on the background of the work. Some overlap with the Abstract is acceptable. Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript. In response to emerging information, advice, guidance and policy around artificial intelligence (AI), we have created a dedicated AI section in our Editorial Policy page . Please familiarize yourself with this content and comply with relevant policies.
For the main body of the text, there are no specific requirements. You can organise it in a way that best suits your research. However, the following structure will be suitable in many cases:
- Introduction
- Results (with subheadings)
- Discussion (without subheadings)
You should then follow the main body of text with:
- References (limited to 60 references, though not strictly enforced)
- Acknowledgements (optional)
- Data availability statement (mandatory)
- Additional Information (including a Competing Interests Statement)
- Figure legends (these are limited to 350 words per figure)
- Tables (maximum size of one page)
Please note, footnotes should not be used.
We do not automatically include page or line numbers in the materials sent to Editorial Board Members and reviewers. Please consider including those in your manuscript; this can help facilitate the evaluation of the paper and makes giving feedback on specific sections easier.
You may include a limited number of uncaptioned molecular structure graphics and numbered mathematical equations if necessary. Display items are limited to 8 ( figures and/or tables ). However, to enable typesetting of papers, we advise making the number of display items commensurate with your overall word length. So, for Articles of 2,000 words or less, we suggest including no more than 4 figures/tables. Please note that schemes should not be used and should be presented as figures instead.
Your submission must also include:
- A cover letter
- Individual figure files and optional supplementary information files
For first submissions (i.e. not revised manuscripts), you may incorporate the manuscript text and figures into a single file up to 3 MB in size. Whilst Microsoft Word is preferred we also accept LaTeX, or PDF format. Figures can be inserted in the text at the appropriate positions, or grouped at the end.
Supplementary information should be combined and supplied as a single separate file, preferably in PDF format.
A submission template is available in the Overleaf template gallery to help you prepare a LaTeX manuscript within the Scientific Reports formatting criteria.
In your cover letter, you should include:
- The affiliation and contact information of your corresponding author
- A brief explanation of why the work is appropriate for Scientific Reports
- The names and contact information of any reviewers you consider suitable
- The names of any referees you would like excluded from reviewing
Finally, you should state whether you have had any prior discussions with a Scientific Reports Editorial Board Member about the work described in your manuscript.
For revised manuscripts, you should provide all textual content in a single file, prepared using either Microsoft Word or LaTeX. Please note, we do not accept PDF files for the article text of revised manuscripts. Make sure you:
- Format the manuscript file as single-column text without justification.
- Number the pages using an Arabic numeral in the footer of each page.
- Use the default Computer Modern fonts for your text, and the 'symbols' font for any Greek characters.
- Supply any figures as individual files.
- Combine and supply any Supplementary Information as a separate file, preferably in PDF format.
- Include the title of the manuscript and author list in the first page of the Supplementary Information file.
If you do not wish to incorporate the manuscript text and figures into a single file, please provide all textual content in a separate single file, prepared using either Microsoft Word or LaTeX.
If you’re submitting LaTeX files, you can either use the standard ‘Article’ document class (or similar) or the wlscirep.cls file and template provided by Overleaf . For graphics, we recommend your use graphicx.sty. Use numerical references only for citations.
Our system cannot accept .bib files. If you prepare references using BibTeX (which is optional), please include the .bbl file with your submission (as a ‘LaTeX supplementary file’) in order for it to be processed correctly; this file is included automatically in the zip file generated by Overleaf for submissions. Please see this help article on Overleaf for more details.
Alternatively, you can make sure that the references (source code) are included within the manuscript file itself. As a final precaution, you should ensure that the complete .tex file compiles successfully on its own system with no errors or warnings, before submission.
Scientific Reports is read by a truly diverse range of scientists. Please therefore give careful thought to communicating your findings as clearly as possible.
Although you can assume a shared basic knowledge of science, please don’t expect that everyone will be familiar with the specialist language or concepts of your particular field. Therefore:
- Avoid technical jargon wherever possible, explaining it clearly when it is unavoidable.
- Keep abbreviations to a minimum, particularly when they are not standard.
- If you must use an abbreviation, make sure you spell it out fully in the text or legend the first time it appears.
- Clearly explain the background, rationale and main conclusions of your study.
- Write titles and abstracts in language that will be readily understood by any scientist.
We strongly recommend that you ask a colleague with different expertise to review your manuscript before you submit it. This will help you to identify concepts and terminology that non-specialist readers may find hard to grasp.
We don’t provide in-depth copy editing as part of the production process. So, if you feel your manuscript would benefit from someone looking at the copy, please consider using a copy editing or language editing service. You can either do this before submission or at the revision stage. You can also get a fast, free grammar check of your manuscript that takes into account all aspects of readability in English.
We have two affiliates who can provide you with these services: Nature Research Editing Service and American Journal Experts . As a Scientific Reports author, you are entitled to a 10% discount on your first submission to either of these.
Claim 10% off English editing from Nature Research Editing Service
Claim 10% off American Journal Experts
Please note that the use of an editing service is at your own expense, and doesn’t ensure that your article will be selected for peer-review or accepted for publication.
We don't impose word limits on the description of methods. Make sure it includes adequate experimental and characterisation data for others to be able to reproduce your work. You should:
- Include descriptions of standard protocols and experimental procedures.
- Only identify commercial suppliers of reagents or instrumentation when the source is critical to the outcome of the experiments.
- Identify sources for any kits you use in your procedures.
- Include any experimental protocols that describe the synthesis of new compounds.
- Use the systematic name of any new compound and put its bold Arabic numeral in the heading for the experimental protocol, indicating it thereafter by its assigned, bold numeral.
- Describe the experimental protocol in detail, referring to amounts of reagents in parentheses, when possible (eg 1.03 g, 0.100 mmol).
- Use standard abbreviations for reagents and solvents.
- Clearly identify safety hazards posed by reagents or protocols.
- Report isolated mass and percent yields at the end of each protocol.
If you’re reporting experiments on live vertebrates (or higher invertebrates), humans or human samples, you must include a statement of ethical approval in the Methods section (see our detailed requirements for further information on preparing these statements).
We don’t copy edit your references. Therefore, it’s essential you format them correctly, as they will be linked electronically to external databases where possible. At Scientific Reports , we use the standard Nature referencing style. So, when formatting your references, make sure they:
- Run sequentially (and are always numerical).
- Sit within square brackets.
- Only have one publication linked to each number.
- Only include papers or datasets that have been published or accepted by a named publication, recognised preprint server or data repository (if you include any preprints of accepted papers in your reference list, make sure you submit them with the manuscript).
- Include published conference abstracts and numbered patents, if you wish.
- Don’t include grant details and acknowledgements.
Sorry, we cannot accept BibTeX (.bib) bibliography files for references. If you are making your submission by LaTeX, it must either contain all references within the manuscript .tex file itself, or (if you’re using the Overleaf template) include the .bbl file generated during the compilation process as a ‘LaTeX supplementary file’ (see the "Manuscripts" section for more details).
In your reference list, you should:
- Include all authors unless there are six or more, in which case only the first author should be given, followed by 'et al.'.
- List authors by last name first, followed by a comma and initials (followed by full stops) of given names.
- Use Roman text for Article and dataset titles, with only the first word of the title having an initial capital and written exactly as it appears in the work cited, ending with a full stop.
- Use italics for book titles, giving all words in the title an initial capital.
- Use italics for journal and data repository names, abbreviating them according to common usage (with full stops).
- Use bold for volume numbers and the subsequent comma.
- Give the full page range (or article number), where appropriate.
Published papers:
Printed journals Schott, D. H., Collins, R. N. & Bretscher, A. Secretory vesicle transport velocity in living cells depends on the myosin V lever arm length. J. Cell Biol . 156 , 35-39 (2002).
Online only Bellin, D. L. et al . Electrochemical camera chip for simultaneous imaging of multiple metabolites in biofilms . Nat. Commun . 7 , 10535; 10.1038/ncomms10535 (2016).
For papers with more than five authors include only the first author’s name followed by ‘ et al. ’.
Books: Smith, J. Syntax of referencing in How to reference books (ed. Smith, S.) 180-181 (Macmillan, 2013).
Online material:
Babichev, S. A., Ries, J. & Lvovsky, A. I. Quantum scissors: teleportation of single-mode optical states by means of a nonlocal single photon. Preprint at https://arxiv.org/abs/quant-ph/0208066 (2002).
Manaster, J. Sloth squeak. Scientific American Blog Network http://blogs.scientificamerican.com/psi-vid/2014/04/09/sloth-squeak (2014).
Hao, Z., AghaKouchak, A., Nakhjiri, N. & Farahmand, A. Global integrated drought monitoring and prediction system (GIDMaPS) data sets. figshare https://doi.org/10.6084/m9.figshare.853801 (2014).
Please keep any acknowledgements brief, and don’t include thanks to anonymous referees and editors, or any effusive comments. You may acknowledge grant or contribution numbers. You should also acknowledge assistance from medical writers, proof-readers and editors.
You must supply an Author Contribution Statement as described in the Author responsibilities section of our Editorial and Publishing Policies .
Please be aware:
- The author name you give as the corresponding author will be the main contact during the review process and should not change.
- The information you provide in the submission system will be used as the source of truth when your paper is published.
You must supply a competing interests statement . If there is no conflict of interest, you should include a statement declaring this.
Your statement must be explicit and unambiguous, describing any potential competing interest (or lack thereof) for EACH contributing author. The information you provide in the submission system will be used as the source of truth when your paper is published.
Examples of declarations are:
Competing interests The author(s) declare no competing interests.
Competing interests Dr X's work has been funded by A. He has received compensation as a member of the scientific advisory board of B and owns stock in the company. He also has consulted for C and received compensation. Dr Y and Dr Z declare no potential conflict of interest.
You must include a Data Availability Statement in all submitted manuscripts (at the end of the main text, before the References section); see ' Availability of materials and data ' section for more information.
If your research includes human or animal subjects, you will need to include the appropriate ethics declarations in the Methods section of your manuscript.
For experiments involving live vertebrates and/or higher invertebrates, your Methods section must include a statement that:
- Identifies the institutional and/or licensing committee that approved the experiments, including any relevant details.
- Confirms that all experiments were performed in accordance with relevant named guidelines and regulations.
- Confirms that the authors complied with the ARRIVE guidelines.
For experiments involving human subjects (or tissue samples), your Methods section must include a statement that:
- Confirms that informed consent was obtained from all participants and/or their legal guardians.
Please note that:
- Study participant names (and other personally identifiable information) must be removed from all text/figures/tables/images.
- The use of coloured bars/shapes or blurring to obscure the eyes/facial region of study participants is not an acceptable means of anonymisation. For manuscripts that include information or images that could lead to identification of a study participant, your Methods section must include a statement that confirms informed consent was obtained to publish the information/image(s) in an online open access publication.
Supplementary Information
You should submit any Supplementary Information together with the manuscript so that we can send it to referees during peer-review. This will be published online with accepted manuscripts.
It’s vital that you carefully check your Supplementary Information before submission as any modification after your paper is published will require a formal correction.
Please avoid including any "data not shown" statements and instead make your data available via deposition in a public repository (see ' Availability of materials and data ' for more information).
If any data that is necessary to evaluate the claims of your paper is not available via a public depository, make sure you provide it as Supplementary Information.
We do not edit, typeset or proof Supplementary Information, so please present it clearly and succinctly at initial submission, making sure it conforms to the style and terminology of the rest of the paper.
To avoid any delays to publication, please follow the guidelines below for creation, citation and submission of your Supplementary Information:
You can combine multiple pieces of Supplementary Information and supply them as a single composite file. If you wish to keep larger information (e.g. supplementary videos, spreadsheets [.csv or .xlsx] or data files) as another separate file you may do so.
Designate each item as Supplementary Table, Figure, Video, Audio, Note, Data, Discussion, Equations or Methods, as appropriate. Number Supplementary Tables and Figures as, for example, "Supplementary Table S1". This numbering should be separate from that used in tables and figures appearing in the main article. Supplementary Note or Methods should not be numbered; titles for these are optional.
Refer to each piece of supplementary material at the appropriate point(s) in the main article. Be sure to include the word "Supplementary" each time one is mentioned. Please do not refer to individual panels of supplementary figures.
Use the following examples as a guide (note: abbreviate "Figure" as "Fig." when in the middle of a sentence): "Table 1 provides a selected subset of the most active compounds. The entire list of 96 compounds can be found as Supplementary Table S1 online." "The biosynthetic pathway of L-ascorbic acid in animals involves intermediates of the D-glucuronic acid pathway (see Supplementary Fig. S2 online). Figure 2 shows...".
Remember to include a brief title and legend (incorporated into the file to appear near the image) as part of every figure submitted, and a title as part of every table.
Keep file sizes as small as possible, with a maximum size of 50 MB, so that they can be downloaded quickly.
Supplementary video files should be provided in the standard video aspects: 4:3, 16:9, 21:9.
If you have any further questions about the submission and preparation of Supplementary Information, please email: [email protected] .
Please begin your figure legends with a brief title sentence for the whole figure and continue with a short description of what is shown in each panel. Use any symbols in sequence and minimise the methodological details as much as possible. Keep each legend total to no more than 350 words. Provide text for figure legends in numerical order after the references.
Please submit any tables in your main article document in an editable format (Word or TeX/LaTeX, as appropriate), and not as images. Tables that include statistical analysis of data should describe their standards of error analysis and ranges in a table legend.
Include any equations and mathematical expressions in the main text of the paper. Identify equations that are referred to in the text by parenthetical numbers, such as (1), and refer to them in the manuscript as "equation (1)" etc.
For submissions in a .doc or .docx format, please make sure that all equations are provided in an editable Word format. You can produce these with the equation editor included in Microsoft Word.
You are responsible for obtaining permission to publish any figures or illustrations that are protected by copyright, including figures published elsewhere and pictures taken by professional photographers. We cannot publish images downloaded from the internet without appropriate permission.
You should state the source of any images used. If you or one of your co-authors has drawn the images, please mention this in your acknowledgements. For software, you should state the name, version number and URL.
Number any figures separately with Arabic numerals in the order they occur in the text of the manuscript. Include error bars when appropriate. Include a description of the statistical treatment of error analysis in the figure legend.
Please do not use schemes. You should submit sequences of chemical reactions or experimental procedures as figures, with appropriate captions. You may include in the manuscript a limited number of uncaptioned graphics depicting chemical structures - each labelled with their name, by a defined abbreviation, or by the bold Arabic numeral.
Use a clear, sans-serif typeface (for example, Helvetica) for figure lettering. Use the same typeface in the same font size for all figures in your paper. For Greek letters, use a 'symbols' font. Put all display items on a white background, and avoid excessive boxing, unnecessary colour, spurious decorative effects (such as three-dimensional 'skyscraper' histograms) and highly pixelated computer drawings. Never truncate the vertical axis of histograms to exaggerate small differences. Ensure any labelling is of sufficient size and contrast to be legible, even after appropriate reduction. The thinnest lines in the final figure should be no smaller than one point wide. You will be sent a proof that will include figures.
- Figures divided into parts should be labelled with a lower-case, bold letter ( a, b, c and so on) in the same type size as used elsewhere in the figure.
- Lettering in figures should be in lower-case type, with only the first letter of each label capitalised.
- Units should have a single space between the number and the unit, and follow SI nomenclature (for example, ms rather than msec) or the nomenclature common to a particular field.
- Thousands should be separated by commas (1,000).
- Unusual units or abbreviations should be spelled out in full or defined in the legend.
- Scale bars should be used rather than magnification factors, with the length of the bar defined on the bar itself rather than in the legend.
In legends, please use visual cues rather than verbal explanations such as "open red triangles". Avoid unnecessary figures: data presented in small tables or histograms, for instance, can generally be stated briefly in the text instead. Figures should not contain more than one panel unless the parts are logically connected; each panel of a multipart figure should be sized so that the whole figure can be reduced by the same amount and reproduced at the smallest size at which essential details are visible.
At the initial submission stage, you may choose to upload separate figure files or to incorporate figures into the main article file, ensuring that any figures are of sufficient quality to be clearly legible.
When submitting a revised manuscript, you must upload all figures as separate figure files, ensuring that the image quality and formatting conforms to the specifications below.
You must supply each complete figure as a separate file upload. Multi-part/panel figures must be prepared and arranged as a single image file (including all sub-parts; a, b, c, etc.). Please do not upload each panel individually.
Please read the digital images integrity and standards section of our Editorial and Publishing Policies . When possible, we prefer to use original digital figures to ensure the highest-quality reproduction in the journal. When creating and submitting digital files, please follow the guidelines below. Failure to do so, or to adhere to the following guidelines, can significantly delay publication of your work.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
1. Line art, graphs, charts and schematics
For optimal results, you should supply all line art, graphs, charts and schematics in vector format, such as EPS or AI. Please save or export it directly from the application in which it was made, making sure that data points and axis labels are clearly legible.
2. Photographic and bitmap images
Please supply all photographic and bitmap images in a bitmap image format such as tiff, jpg, or psd. If saving tiff files, please ensure that the compression option is selected to avoid very large file sizes. Please do not supply Word or Powerpoint files with placed images. Images can be supplied as RGB or CMYK (note: we will not convert image colour modes).
Figures that do not meet these standards will not reproduce well and may delay publication until we receive high-resolution images.
3. Chemical structures
Please produce Chemical structures using ChemDraw or a similar program. All chemical compounds must be assigned a bold, Arabic numeral in the order in which the compounds are presented in the manuscript text. Structures should then be exported into a 300 dpi RGB tiff file before being submitted.
4. Stereo images
You should present stereo diagrams for divergent 'wall-eyed' viewing, with the two panels separated by 5.5 cm. In the final accepted version of the manuscript, you should submit the stereo images at their final page size.
If your paper contains statistical testing, it should state the name of the statistical test, the n value for each statistical analysis, the comparisons of interest, a justification for the use of that test (including, for example, a discussion of the normality of the data when the test is appropriate only for normal data), the alpha level for all tests, whether the tests were one-tailed or two-tailed, and the actual P value for each test (not merely "significant" or "P < 0.05"). Please make it clear what statistical test was used to generate every P value. Use of the word "significant" should always be accompanied by a P value; otherwise, use "substantial," "considerable," etc.
Data sets should be summarised with descriptive statistics, which should include the n value for each data set, a clearly labelled measure of centre (such as the mean or the median), and a clearly labelled measure of variability (such as standard deviation or range).
Ranges are more appropriate than standard deviations or standard errors for small data sets. Graphs should include clearly labelled error bars. You must state whether a number that follows the ± sign is a standard error (s.e.m.) or a standard deviation (s.d.).
You must justify the use of a particular test and explain whether the data conforms to the assumptions of the tests. Three errors are particularly common:
- Multiple comparisons: when making multiple statistical comparisons on a single data set, you should explain how you adjusted the alpha level to avoid an inflated Type I error rate, or you should select statistical tests appropriate for multiple groups (such as ANOVA rather than a series of t-tests).
- Normal distribution: many statistical tests require that the data be approximately normally distributed; when using these tests, you should explain how you tested your data for normality. If the data does not meet the assumptions of the test, you should use a non-parametric alternative instead.
- Small sample size: when the sample size is small (less than about 10), you should use tests appropriate to small samples or justify the use of large-sample tests.
You should identify molecular structures by bold, Arabic numerals assigned in order of presentation in the text. Once identified in the main text or a figure, you may refer to compounds by their name, by a defined abbreviation, or by the bold Arabic numeral (as long as the compound is referred to consistently as one of these three).
When possible, you should refer to chemical compounds and biomolecules using systematic nomenclature, preferably using IUPAC . You should use standard chemical and biological abbreviations. Make sure you define unconventional or specialist abbreviations at their first occurrence in the text.
You should use approved nomenclature for gene symbols, and employ symbols rather than italicised full names (for example Ttn, not titin). Please consult the appropriate nomenclature databases for correct gene names and symbols. A useful resource is Entrez Gene .
You can get approved human gene symbols from HUGO Gene Nomenclature Committee (HGNC), e-mail: [email protected] ; see also www.genenames.org .
You can get approved mouse symbols from The Jackson Laboratory, e-mail: [email protected] ; see also www.informatics.jax.org/mgihome/nomen .
For proposed gene names that are not already approved, please submit the gene symbols to the appropriate nomenclature committees as soon as possible, as these must be deposited and approved before publication of an article.
Avoid listing multiple names of genes (or proteins) separated by a slash, as in 'Oct4/Pou5f1', as this is ambiguous (it could mean a ratio, a complex, alternative names or different subunits). Use one name throughout and include the other at first mention: 'Oct4 (also known as Pou5f1)'.
Scientific Reports is committed to publishing technically sound research. Manuscripts submitted to the journal will be held to rigorous standards with respect to experimental methods and characterisation of new compounds.
You must provide adequate data to support your assignment of identity and purity for each new compound described in your manuscript. You should provide a statement confirming the source, identity and purity of known compounds that are central to the scientific study, even if they are purchased or resynthesised using published methods.
1. Chemical identity
Chemical identity for organic and organometallic compounds should be established through spectroscopic analysis. Standard peak listings (see formatting guidelines below) for 1H NMR and proton-decoupled 13C NMR should be provided for all new compounds. Other NMR data should be reported (31P NMR, 19F NMR, etc.) when appropriate. For new materials, you should also provide mass spectral data to support molecular weight identity. High-resolution mass spectral (HRMS) data is preferred. You may report UV or IR spectral data for the identification of characteristic functional groups, when appropriate. You should provide melting-point ranges for crystalline materials. You may report specific rotations for chiral compounds. You should provide references, rather than detailed procedures, for known compounds, unless their protocols represent a departure from or improvement on published methods.
2. Combinational compound libraries
When describing the preparation of combinatorial libraries, you should include standard characterisation data for a diverse panel of library components.
3. Biomolecular identity
For new biopolymeric materials (oligosaccharides, peptides, nucleic acids, etc.), direct structural analysis by NMR spectroscopic methods may not be possible. In these cases, you must provide evidence of identity based on sequence (when appropriate) and mass spectral characterisation.
4. Biological constructs
You should provide sequencing or functional data that validates the identity of their biological constructs (plasmids, fusion proteins, site-directed mutants, etc.) either in the manuscript text or the Methods section, as appropriate.
5. Sample purity
We request evidence of sample purity for each new compound. Methods for purity analysis depend on the compound class. For most organic and organometallic compounds, purity may be demonstrated by high-field 1H NMR or 13C NMR data, although elemental analysis (±0.4%) is encouraged for small molecules. You may use quantitative analytical methods including chromatographic (GC, HPLC, etc.) or electrophoretic analyses to demonstrate purity for small molecules and polymeric materials.
6. Spectral data
Please provide detailed spectral data for new compounds in list form (see below) in the Methods section. Figures containing spectra generally will not be published as a manuscript figure unless the data are directly relevant to the central conclusions of the paper. You are encouraged to include high-quality images of spectral data for key compounds in the Supplementary Information. You should list specific NMR assignments after integration values only if they were unambiguously determined by multidimensional NMR or decoupling experiments. You should provide information about how assignments were made in a general Methods section.
Example format for compound characterisation data. mp: 100-102 °C (lit. ref 99-101 °C); TLC (CHCl 3 :MeOH, 98:2 v/v): R f = 0.23; [α] D = -21.5 (0.1 M in n-hexane); 1 H NMR (400 MHz, CDCl 3 ): δ 9.30 (s, 1H), 7.55-7.41 (m, 6H), 5.61 (d, J = 5.5 Hz, 1H), 5.40 (d, J = 5.5 Hz, 1H), 4.93 (m, 1H), 4.20 (q, J = 8.5 Hz, 2H), 2.11 (s, 3H), 1.25 (t, J = 8.5 Hz, 3H); 13 C NMR (125 MHz, CDCl 3 ): δ 165.4, 165.0, 140.5, 138.7, 131.5, 129.2, 118.6, 84.2, 75.8, 66.7, 37.9, 20.1; IR (Nujol): 1765 cm- 1 ; UV/Vis: λ max 267 nm; HRMS (m/z): [M] + calcd. for C 20 H 15 C l2 NO 5 , 420.0406; found, 420.0412; analysis (calcd., found for C 20 H 15 C l2 NO 5 ): C (57.16, 57.22), H (3.60, 3.61), Cl (16.87, 16.88), N (3.33, 3.33), O (19.04, 19.09).
7. Crystallographic data for small molecules
If your manuscript is reporting new three-dimensional structures of small molecules from crystallographic analysis, you should include a .cif file and a structural figure with probability ellipsoids for publication as Supplementary Information. These must have been checked using the IUCR's CheckCIF routine, and you must include a PDF copy of the output with the submission, together with a justification for any alerts reported. You should submit crystallographic data for small molecules to the Cambridge Structural Database and the deposition number referenced appropriately in the manuscript. Full access must be provided on publication.
8. Macromolecular structural data
If your manuscript is reporting new structures, it should contain a table summarising structural and refinement statistics. Templates are available for such tables describing NMR and X-ray crystallography data. To facilitate assessment of the quality of the structural data, you should submit with the manuscript a stereo image of a portion of the electron density map (for crystallography papers) or of the superimposed lowest energy structures (≳10; for NMR papers). If the reported structure represents a novel overall fold, you should also provide a stereo image of the entire structure (as a backbone trace).
Registered Reports are original research articles which undergo peer-review prior to data collection and analyses. This format is designed to minimize publication bias and research bias in hypothesis-driven research, while also allowing the flexibility to conduct exploratory (unregistered) analyses and report serendipitous findings. If you intend to submit a Registered Report to Scientific Reports , please refer to detailed guidelines here .
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Articles are the main format for original research contributions to ... A useful set of articles providing general advice about writing and submitting scientific papers can be found on the SciDev ...
Publications share new research, results, and methods in a trusted format and advance scientific knowledge and practice (1,7). ... Writing a scientific paper — a brief guide for new investigators. Vaccine 2017; 35 (5) ... Abbasi K. Screening research papers by reading abstracts. BMJ 2004; 329 (7464) ...
Keywords: paper format, scientific writing. FORMATTING TIPS: Check whether "Keywords" should be italicized and whether each term should be capitalized. Check the use of punctuation (e.g., commas versus semicolons, the use of the period at the end). Some journals (e.g., IEEE) provide a taxonomy of keywords.
The present paper presents a brief historical background to medical publication and practical guidelines for writing scientific papers for acceptance in good journals. Key words: guidelines, scientific writing. INTRODUCTION. ... For clinical research papers, you have to provide information on how the participants were selected, identify the ...
A clear format will ensure that your research paper is understood by your readers. Follow: 1. Context — your introduction. 2. Content — your results. 3. Conclusion — your discussion. Plan ...
The main guidelines for formatting a paper in APA Style are as follows: Use a standard font like 12 pt Times New Roman or 11 pt Arial. Set 1 inch page margins. Apply double line spacing. If submitting for publication, insert a APA running head on every page. Indent every new paragraph ½ inch.
Conducting scientific and clinical research is only the beginning of the scholarship of discovery. ... The task of writing a scientific paper and submitting it to a journal for publication is a time‐consuming and often daunting task. 3,4 Barriers to effective writing ... It may be helpful to follow the IMRaD format for writing scientific ...
Delivered to your inbox every two weeks, the Writing Toolbox features practical advice and tools you can use to prepare a research manuscript for submission success and build your scientific writing skillset. Discover how to navigate the peer review and publishing process, beyond writing your article.
The vast majority of scientific journals follow the so-called "IMRAD" format, i.e. introduction, methods, results and discussion. Naturally, there are some exceptions to this rule, and you should always check the instructions for authors of the journal where you plan to submit your paper to ensure that this is indeed the recommended format.
Communicating research findings is an essential step in the research process. Often, peer-reviewed journals are the forum for such communication, yet many researchers are never taught how to write a publishable scientific paper. In this article, we explain the basic structure of a scientific paper and describe the information that should be included in each section. We also identify common ...
FORMAT FOR THE PAPER. Scientific research articles provide a method for scientists to communicate with other scientists about the results of their research. A standard format is used for these articles, in which the author presents the research in an orderly, logical manner. This doesn't necessarily reflect the order in which you did or thought ...
Do not use a period after your title or after any heading in the paper (e.g., Works Cited). Begin your text on a new, double-spaced line after the title, indenting the first line of the paragraph half an inch from the left margin. Fig. 1. The top of the first page of a research paper.
Set the top, bottom, and side margins of your paper at 1 inch. Use double-spaced text throughout your paper. Use a standard font, such as Times New Roman or Arial, in a legible size (10- to 12-point). Use continuous pagination throughout the paper, including the title page and the references section.
1. Introduction. A research paper communicates scientific work to a wide audience. Without publishing results, the important data collected, analyzed, and interpreted is inaccessible to the scientific community and hence of little or no value. In order to advance the science, researchers must share their results.
A majority of readers will find your paper via electronic database searches and those search engines key on words found in the title. Title FAQs. Format: The title should be centered at the top of page 1 (DO NOT use a title page - it is a waste of paper for our purposes); the title is NOT underlined or italicized.
This table describes how to format your research paper using either the MLA or APA guidelines. Be sure to follow any additional instructions that your teacher provides. 12-pt. Times Roman or Courier. For figures, however, use a sans serif font such as Arial. Leave one space after a period unless your teacher prefers two. Leave one space after a ...
T emplate and Guidelines for Writing a Scientific Paper. Thomas Kiebel, Michael Schulze, and Sebastian Zug. Institute of Embedded Systems and Operating Systems. Department of Distributed Systems ...
Create a research paper outline. Write a first draft of the research paper. Write the introduction. Write a compelling body of text. Write the conclusion. The second draft. The revision process. Research paper checklist. Free lecture slides.
In each paragraph, the first sentence defines the context, the body contains the new idea and the final sentence offers a conclusion. For the whole paper, the introduction sets the context, the ...
Information on manuscript types, including length constraints, can be found on our general information for authors page. The instructions below apply to an initial submission. For a manuscript submitted after peer review and revision, the same style guidelines apply, but we require slightly different file preparation - see instructions ...
Research paper format is an essential aspect of academic writing that plays a crucial role in the communication of research findings.The format of a research paper depends on various factors such as the discipline, style guide, and purpose of the research. It includes guidelines for the structure, citation style, referencing, and other elements of the paper that contribute to its overall ...
Research Papers: Presents an in-depth investigation and analysis of a research question. ... Adhere to specific guidelines for tables, figures, and graphs, including proper numbering, labeling, and citation. ... Publication-ready exporting ensures seamless integration into scientific papers and presentations. Mind the Graph empowers scientists ...
After considering the public's suggestions, panelists work to determine which treatments to recommend. They consider four factors: the overall strength of the evidence, the balance of benefits to potential harms, patients' values and preferences, and the applicability or generalizability of the evidence. They parse their recommendations ...
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
The International Journal of Research and Innovation in Social Science (ISSN 2454-6186), with the DOI 10.47772/IJRISS, operates as an accessible global journal with a focus on Social Studies and its Sub Topics.Authors are invited to submit the manuscript in various formats, including Research Papers, Review Papers, Informative Articles, Comparative Studies, Case Studies, Dissertation Chapters ...
Washing your hands is easy, and it's one of the most effective ways to prevent the spread of germs. Follow these five steps every time. Wet your hands with clean, running water (warm or cold), turn off the tap, and apply soap. Lather your hands by rubbing them together with the soap. Lather the backs of your hands, between your fingers, and ...
Format of articles. Scientific Reports publishes original research in two formats: ... Statistical guidelines. If your paper contains statistical testing, it should state the name of the ...
Predicting urban morphology based on local attributes is an important issue in urban science research. The deep generative models represented by generative adversarial network (GAN) models have achieved impressive results in this area. However, in such methods, the urban morphology is assumed to follow a specific probability distribution and be able to directly approximate the distribution via ...
Access the portal of NASS, the official source of agricultural data and statistics in the US, and explore various reports and products.