October 25, 2023

The World Solved Acid Rain. We Can Also Solve Climate Change
Lessons from how we tackled acid rain can be applied to our world today
By Hannah Ritchie

Thomas Fuchs
The world feels like it’s being set alight; wildfires in Canada and Europe, floods in China, and a never-ending stream of recording-breaking heat waves have garnered numerous headlines.
The feeling that time is quickly running out is very real . And it’s easy to believe that the world cannot tackle big environmental problems. This sense of helplessness is something that I have personally battled for more than a decade. But that feeling is a barrier to action: Nothing has changed when we’ve called for action before, so why should we expect any different this time?
But our past efforts tell us there is hope. The world has solved large environmental problems that seemed unsurmountable at the time. In my role at Our World in Data, I’ve spent years looking at how these problems have evolved, and I think that it’s worth studying these issues, not only for hope, but to understand what went right and what can help us face today’s crises. An eye-opening example is acid rain; studying how the world tackled this geopolitically divisive problem can give us some insights into how we can tackle climate change today.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
It has mostly slipped from the public conversation, but acid rain was the leading environmental problem of the 1990s. At one point, it was one of the biggest bilateral diplomatic issues between the United States and Canada.
Acid rain—precipitation with high levels of sulfuric or nitric acids—is mostly caused by sulfur dioxide, a gas that is produced when we burn coal. It had severe effects on ecosystems. It dissolved old sculptures , stripped forests of their leaves, leached soils of their nutrients, and polluted rivers and lakes . Emissions from the U.K. would blow over to Sweden and Norway; emissions from the U.S. would blow over to Canada. Just like climate change, it crossed borders, and no country could solve it on its own.
This is a classic game theory problem; outcomes don’t only depend on the actions of one country but on the actions of the others too. Countries will only act if they know that others are willing to do the same. This time, they did act collectively. Government officials signed international agreements , placed emissions limits on power plants and started to reduce coal burning. Interventions were incredibly effective. In Europe, sulfur dioxide emissions fell by 84 percent and in the U.S. by 90 percent . Some countries have reduced them by more than 98 percent.
We did something similar with the ozone layer. The ozone hole was a big coordination problem. No single country was responsible for the world’s emissions of ozone-depleting substances. So there was little upside and some downside to countries taking the lead on their own. They would spend money and implement unpopular environmental policies without making much of a dent in the global problem. The only way to cut emissions substantially was for many countries to join in. It relied on international collaboration. Yet the world solved it. After countries signed the Montreal Protocol, emissions of ozone-depleting substances fell by more than 99 percent .
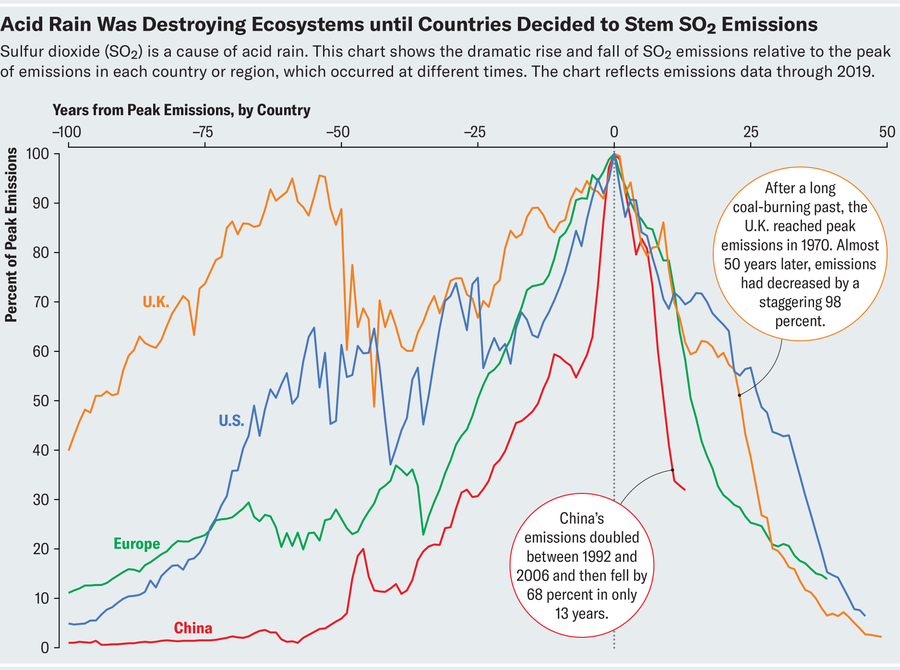
Credit: John Knight; Source: Data Explorer: Air Pollution, Our World in Data
What we learned from tackling acid rain and the ozone hole can be applied to tackling climate change overall.
First, the cost of technology really matters. The cost-benefit ratio of desulfurization technologies was key to solving acid rain. The cost of installing scrubbers was significant but not budget-breaking. If they had come at a huge cost, countries wouldn’t have made the switch.
Similarly, cheap low-carbon technologies are essential for climate change. Low-carbon technologies used to be expensive, but in the last decade the price of solar energy has fallen by more than 90 percent . The price of wind energy by more than 70 percent. Battery costs have tumbled by 98 percent since 1990, bringing the cost of electric cars down with them. Globally, one in every seven new cars sold is electric . In Europe, one in every five, and in China one in every three.
At the same time, countries are waking up to the potential costs of not moving to clean energy, whether in the form of climate damages—at home or overseas—or being tied to volatile fossil fuel markets.
Second, climate agreements and targets take time to evolve. Negotiations are long. The ozone hole and acid rain were not fixed with the first international agreements on the table. The initial targets were too modest to make a large enough difference . But over time, countries increased their ambitions, amended their agreements and reached for those higher goals.
This is a basic principle of the Paris climate agreement. Countries agreed to step up their commitments to keep global temperature rise below 1.5 degrees Celsius or 2 degrees C . While this has been happening, it definitely hasn’t happened fast enough. The world is on track for an increase of around 2.6 degrees C by 2100. That’s extremely bad. But it’s still a degree lower than where we were heading in 2016. Governments have increased action and increased their target numbers too. And just like with acid rain or the ozone hole, they need to keep aiming higher. If every country fulfilled its pledges, the world would keep temperature rise to 2 degrees C. If they met their net-zero commitments on time, we could sneak below it.
Finally, the stance of elected officials matters more than their party affiliation. Environmental issues do not have to be so politically divisive. Acid rain was a bipartisan divide in the U.S. under Ronald Reagan’s presidency. But it wasn’t a Democrat who finally took action; it was his Republican successor, George H.W. Bush. Before taking office, Bush pledged to be the “environmental president,” a bold stance for many right-wing leaders today, but one that we need to see repeated if we are going to make and reach these loftier goals. In the U.K., there is strong public support for net-zero emissions even among the political right. Margaret Thatcher—arguably one of the U.K.’s most right-wing leaders ever—was one of the earliest to take climate change seriously .
Former German chancellor Angela Merkel is a modern example of a pro-climate conservative leader. A scientist by training , Merkel always acknowledged the threats of climate change, gaining the title of “climate chancellor.” In the late 1990s she led the first U.N. climate conferences and the Kyoto Protocol. In 2007, she convinced G8 leaders to set binding emission reduction targets. It's wrong to frame environmental problems as right-left wing issues. If we’re going to tackle climate change, we need to overcome this divide.
Climate change is not the perfect parallel for the environmental problems we’ve solved before. It will be harder; we should be honest about that. It means rebuilding the energy, transport and food systems that underpin the modern world. It will involve every country, and almost every sector. But change is happening, even if it doesn’t hit the headlines. To accelerate action, we need to have the expectation that things can move faster. That’s where past lessons come in; we should use them to understand that these expectations are not unrealistic. Change can happen quickly, but not on its own; we need to be the ones to drive it.
This is an opinion and analysis article, and the views expressed by the author or authors are not necessarily those of Scientific American.
A version of this article with the title “What We Learned from Acid Rain" was adapted for inclusion in the January 2024 issue of Scientific American.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Acid rain and its environmental effects: Recent scientific advances
- More information: Publisher Index Page (via DOI)
- Open Access Version: Publisher Index Page
- Download citation as: RIS | Dublin Core
The term ‘acid rain’ refers to atmospheric deposition of acidic constituents that impact the earth as rain, snow, particulates, gases, and vapor. Acid rain was first recognized by Ducros (1845) and subsequently described by the English chemist Robert Angus Smith (Smith, 1852) whose pioneering studies linked the sources to industrial emissions and included early observations of deleterious environmental effects (Smith, 1872). Smith's work was largely forgotten until the mid-20th century when observations began to link air pollution to the deposition of atmospheric sulfate (SO 4 2− ) and other chemical constituents, first near the metal smelter at Sudbury, Ontario, Canada, and later at locations in Europe, North America, and Australia (Gorham, 1961). Our modern understanding of acid rain as an environmental problem caused largely by regional emissions of sulfur dioxide (SO 2 ) and nitrogen oxides (NO x ) stems from observations in the 1960s and early 1970s in Sweden by Svante Odén (Odén, 1976), and in North America by Gene Likens and colleagues (Likens and Bormann, 1974). These scientists and many who followed showed the link to emissions from coal-fired power plants and other industrial sources, and documented the environmental effects of acid rain such as the acidification of surface waters and toxic effects on vegetation, fish, and other biota.
| Publication type | Article |
|---|---|
| Publication Subtype | Journal Article |
| Title | Acid rain and its environmental effects: Recent scientific advances |
| Series title | Atmospheric Environment |
| DOI | 10.1016/j.atmosenv.2016.10.019 |
| Volume | 146 |
| Year Published | 2016 |
| Language | English |
| Publisher | Pergamon Press |
| Publisher location | Oxford |
| Contributing office(s) | New York Water Science Center |
| Description | 4 p. |
| First page | 1 |
| Last page | 4 |
| Google Analytic Metrics |
Acid Rain and Our Ecosystem
More than 150 years after acid rain was first identified, scientists now see success in recovery from its damaging effects
Cassandra Willyard
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/acid-rain-black-crust-gravestones-Madison-Street-Cemetery-Hamilton-New-York-631.jpg)
Geologist Rich April climbs the small hill behind Colgate University and makes his way into the cemetery. He stops before a white marble pillar erected in 1852. The inscription is nearly illegible. Over time, any stone exposed to the elements will weather, April explains, but this marble has weathered unnaturally fast. The culprit? Acid rain.
April pulls a vial of acid from his pocket to demonstrate. He unscrews the cap and lets a few drops leak onto the stone, where they fizz and bubble. The rain that fell throughout the Northeast in the latter half of the 20th century wasn’t as acidic as the liquid in April’s vial, but the principle is the same. Acid eats marble. Given enough time, it can erase even words meant to last an eternity.
The effects of acid rain extend far beyond graveyards. Acid rain destroyed fish populations in lakes and streams, harmed fragile soils and damaged millions of acres of forest worldwide.
These far-reaching effects illustrate the profound impact air pollution can have on the land. But the story of acid rain is also a tale of how understanding air pollution can lead to solutions. Due to overwhelming scientific evidence linking power plant emissions to acid rain and acid rain to the death of lakes, new regulations have dramatically cut emissions and cleaned up the rain that falls on the United States.
The term ‘acid rain’ was coined in the mid-1800s, when Robert Angus Smith, a Scottish chemist working in London, noticed that rain tended to be more acidic in areas with more air pollution and that buildings crumble faster in areas where coal is burned. But it took another century for scientists to realize that acid rain was a widespread environmental problem. Scandinavian scientists began to document acidic damage to lakes and streams in the 1950s. In 1963, Gene Likens, then at Dartmouth, and colleagues began collecting and testing the pH of rainwater in New Hampshire’s White Mountains as part of an ecosystem study. They were surprised to find that it was quite acidic, but they didn’t have much basis for comparison; at that time, scientists weren’t regularly measuring the pH of rainwater.
Likens took a job at Cornell a few years later and set up instruments to collect rainwater in the Finger Lakes region and soon observed that the rain in New York was roughly as acidic as rain in New Hampshire. “That was the first clue that we had that this might be some kind of a regional phenomenon,” he says. But neither Likens nor his colleagues had a clear idea what the cause might be.
Likens won a fellowship that took him to Sweden in 1969, a serendipitous event, he says, because he met Svante Odén, a scientist at Uppsala University who had observed the same trends in Sweden that Likens had been observing in the Northeastern United States. Odén had his finger on a potential cause. “He was trying to build a case that [acid rain] might be due to emissions coming from the more industrialized areas of Europe,” Likens recalls.
Likens and his colleagues traced the emissions from coal-fired power plants and examined satellite and aircraft data, and they found a similar long-distance link. “Sure enough, the emissions were coming primarily from Midwestern states like Indiana, Ohio, Illinois and Kentucky,” Likens recalls. “They were making their way literally thousands of kilometers to New England and southeastern Canada and coming back down as acids.”
He reported his findings in Science in 1974, and the story was immediately picked up by newspapers. The phone didn’t stop ringing for months, Likens recalls. “It was that media exposure that really put acid rain on the map in North America.”
Acid rain occurs, Likens and Odén and other scientists realized, when sulfur dioxide and nitrogen oxide enter the atmosphere and react with water to form sulfuric and nitric acids. Natural sources of these gases exist—volcanoes, for instance, belch out sulfur dioxide—but the vast majority comes from the burning of fossil fuels, especially by coal-fired power plants. The tall smokestacks allow pollution to travel long distances. According to studies conducted by Likens and his colleagues, normal rainwater has a pH of 5.2. During the 1970s and 1980s, when acid rain was at its worst, scientists recorded pH levels as low as 2.1, roughly 1,000 times more acidic.

Acid rain affected many parts of the United States, but the Northeast suffered the most ecological damage. The Adirondack Mountains proved especially susceptible. Many soils contain calcium carbonate or other minerals that can neutralize acid rain before it seeps into lakes and streams. “Unfortunately the Adirondacks have almost none,” April says. As a result, lakes and streams quickly became acidic, killing fish and other aquatic animals.
In the late 1970s, researchers surveyed 217 lakes above 2,000 feet in the Adirondacks and found that 51 percent were highly acidic. The news was so grim that scientists began attempting to breed more acid-tolerant strains of trout. One New York State employee compared the area to Death Valley. A decade later, a larger study that included 849 lakes higher than 1,000 feet found that 55 percent were either completely devoid of life or on the brink of collapse.
As the scientific evidence linking acid rain to power plant emissions and ecological damage mounted, battles erupted among industry, scientists and environmentalists. “The 1980s is a period I call the ‘acid rain wars,’” Likens says. “There was huge rancorous nasty controversy.” Environmentalists from Greenpeace climbed power plant smokestacks and hung banners in protest; scientists testified before Congress about the link between emissions and acid rain, the severity of the effects, and whether proposed legislation would have an impact; and the power industry questioned the science and argued that regulations would drive electricity rates sky high.
Congress passed several amendments to the Clean Air Act in 1990 that cut emissions of sulfur dioxide through a cap-and-trade scheme. The goal was a 50 percent reduction in sulfur dioxide emissions from 1980 levels. That goal was achieved in 2008, two years before the deadline, which was set for 2010. Sulfur dioxide emissions fell from 17.3 million tons in 1980 to 7.6 million tons in 2008, less than the 8.95 million tons required by 2010.
The effect has been remarkable. Doug Burns, a scientist at the U.S. Geological Survey in Troy, New York, who directs the National Acid Precipitation Assessment Program, says the rain falling in the Northeast today is about half as acidic as it was in the early 1980s. Consequently, surface waters have become less acidic and fragile ecosystems are beginning to recover.
In many places, however, recovery has been painfully slow. Scientists now know that acid rain not only acidified lakes and streams, it also leached calcium from forest soils. That calcium depletion has had devastating effects on trees, especially sugar maples and red spruce. Acid rain leaches calcium from the needles of red spruce, making them more susceptible to cold. It also leaches calcium and magnesium from the soil, which can stress sugar maples. In addition, acid rain allows aluminum to accumulate in the soil. When trees take up aluminum, their roots can become brittle.
Some researchers have tried adding calcium back into the forests to speed recovery. April is currently involved in one such experiment in the Adirondacks. Over the past four and a half years, the calcium has penetrated only the top 15 centimeters of forest soil. “It takes a really long time for [the calcium] to get back down into the soil,” April says, so it won’t be a quick fix.
April would like to see sulfur dioxide and other emissions curtailed even further. “We still have acid rain coming in,” he says. “Some lakes look like they might be ready to come back, and if we cut the emissions more they would.”
Princeton University’s Michael Oppenheimer, who was a key player in the acid wars as chief scientist for the conservation group Environmental Defense Fund, agrees. “I think sulfur dioxide and nitrogen oxide need to be effectively eliminated,” he says. “We ought to head towards zero and see how close we can get.”
Although some effects of acid rain are lingering, most scientists consider it an environmental success story. “Science identified the problem. Science provided the guidelines for how to try to resolve the problem,” Likens says. “The success is that we have taken action as a society to try to deal with the problem.”
Get the latest Science stories in your inbox.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 12 February 2024
Impact of simulated acid rain on chemical properties of Nyalau series soil and its leachate
- Mohamad Hilmi Ibrahim 1 ,
- Susilawati Kasim 2 ,
- Osumanu Haruna Ahmed 3 ,
- Mohd. Rashid Mohd. Rakib 4 ,
- Nur Aainaa Hasbullah 4 &
- Md. Tariqul Islam Shajib 5 nAff6
Scientific Reports volume 14 , Article number: 3534 ( 2024 ) Cite this article
1195 Accesses
Metrics details
- Environmental sciences
Greenhouse gases can cause acid rain, which in turn degrades soil chemical properties. This research was conducted to determine the effects of simulated acid rain (SAR) on the chemical properties of Nyalau series ( Typic paleudults ). A 45-day laboratory leaching and incubation study (control conditions) was conducted following standard procedures include preparing simulated acid rain with specific pH levels, followed by experimental design/plan and systematically analyzing both soil and leachate for chemical changes over the 45-day period. Six treatments five of which were SAR (pH 3.5, 4.0, 4.5, 5.0, and 5.5) and one control referred to as natural rainwater (pH 6.0) were evaluated. From the study, the SAR had significant effects on the chemical properties of the soil and its leachate. The pH of 3.5 of SAR treatments decreased soil pH, K + , and fertility index. In contrast, the contents of Mg 2+ , Na + , SO 4 2− , NO 3 − , and acidity were higher at the lower SAR pH. Furthermore, K + and Mg 2+ in the leachate significantly increased with increasing acidity of the SAR. The changes in Ca 2+ and NH 4 + between the soil and its leachate were positively correlated (r = 0.84 and 0.86), whereas the changes in NO 3 − negatively correlated (r = − 0.82). The novelty of these results lies in the discovery of significant alterations in soil chemistry due to simulated acid rain (SAR), particularly impacting soil fertility and nutrient availability, with notable positive and negative correlations among specific ions where prolonged exposure to acid rain could negatively affect the moderately tolerant to acidic and nutrient-poor soils. Acid rain can negatively affect soil fertility and the general soils ecosystem functions. Long-term field studies are required to consolidate the findings of this present study in order to reveal the sustained impact of SAR on tropical forest ecosystems, particularly concerning soil health, plant tolerance, and potential shifts in biodiversity and ecological balance.
Similar content being viewed by others
The effects of soil freeze–thaw processes on water and salt migrations in the western Songnen Plain, China
Effects of rainfall amount and frequencies on soil net nitrogen mineralization in Gahai wet meadow in the Qinghai-Tibetan Plateau
Management of soil pH promotes nitrous oxide reduction and thus mitigates soil emissions of this greenhouse gas
Introduction.
Acid deposition poses several threats to ecosystems by affecting plant health, diversity and structure, including processes and functions in the ecosystem 1 , 2 . Acid deposition is defined as accumulation of undesired chemical compounds in the atmosphere at toxic concentrations 3 . Acid deposits are materials (solids, liquids and gases) occurring in excess quantities from the average amount and present at the lowest layer of the atmosphere 4 . Acid deposition in the atmosphere can be attributed to diverse chemical compounds originating from fossil fuel combustion, agriculture, mining, and manufacturing activities. Acid deposition is a global threat that has been shown to result in various environmental and human health hazards such as depleting essential nutrients and increasing toxic metals, which can lead to reduced plant growth and biodiversity 5 , 6 , 7 .
Acid deposits refer to rain, snow, fog, particulates, and gases, whereas acid rain only refers to rainwater at pH below 5.6 8 , 9 . Acid rain mainly consists of sulfur dioxide (SO 2 ) and nitrogen oxides (NOx) forming acidic compounds, whereas other greenhouse gases like Cl − and CO 2 , linked to climate change and global warming. These gases undergo complex chemical reactions in the atmosphere after which they fall to the earth’s surface as wet or dry deposition 10 . According to Zhang et al. 11 , acid rain with a pH of 5.6 is deemed normal as atmospheric CO 2 at a pressure of 101 kPa and temperature of 20 °C lowers rainwater pH from 7 to 5.6. This normalcy shifts when gases like N 2 O and SO 2 contribute to a further decrease in pH below 5.6 due to increasing hydrogen ion concentrations.
Soil fertility and soil physico-chemical properties such as soil nutrients for plant growth and production, are commonly affected by prolonged exposure to acid deposition 12 , 13 . Several scientific reports have demonstrated that acid deposition may disrupt nutrient cycling in soil habitats, particularly by deteriorating soil physico-chemical properties, especially its fertility 14 , 15 , 16 , 17 . For example, soil nutrient leaching in White Mountain National Forest in the Central New Hampshire, US, resulted from acid deposition 18 . In addition, other studies on the impact of acid deposition on ecosystems have revealed that this phenomenon affects species richness and diversity 19 , 20 and hydrological cycle, including water quality 21 .
More than that, this acidic precipitation lowers the soil pH, a process termed soil acidification. Research by Yang et al. 22 shows that acidification leads to nutrient leaching, particularly of calcium and magnesium, while increasing the solubility and toxicity of metals like aluminum and lead. This results in reduced soil fertility and damage to plant root systems, adversely affecting plant growth and crop yields, as noted by Dai et al. 23 . Furthermore, soil acidification disrupts microbial communities, impacting critical processes like decomposition and nutrient cycling 24 .
Soil leaching is defined as the movement of nutrients from the upper soil profile to its lower depths 25 . Leaching typically causes soil pH to decrease with decreasing base cations concentrations. When acid deposition occurs, there is an increase in the solubility of heavy metals and Al mobilization in soils 26 . To this effect, accumulation of H + ions reduces soil pH while increasing the solubility of heavy metals and Al mobilization. The leaching of macronutrients occurs due to the replacement of H + ions by acid rain, which increases soil acidity to levels that compromise fertility 27 . This phenomenon of soil acidification is not just theoretical; it has been observed on a large scale, for instance, in Southern China, where soil acidification was documented after 20 years of continuous exposure to acid rain 28 , 29 .
The mineral acid soils in Sarawak, Malaysia belong to four major series, namely Bekenu, Nyalau, Merit, and Stom series 30 . Nyalau series are the soils contaminated with eroded material from upslope areas with high content of sesquoxides 31 . According to Tan et al. 32 , Nyalau series belongs to Typic paleudults , therefore it is classified as acidic soils, with pH between 4.3 and 4.8 and CEC values below 24 cmol kg −1 . The textural class of these soils is sandy clay loam with brownish yellow to yellow colouration. In Malaysia, the cumulative acid loading from the atmosphere to terrestrial ecosystems has been on the increase since 2010–2019 33 . As a result, SO 2 and N 2 O composition in some states in Malaysia are 0.66 and 0.17 ppm, respectively 34 , while the pH of rainwater in selected industrial areas in Malaysia have reached 4.32 35 . EANET 36 reported the annual rainwater pH at Petaling Jaya, Tanah Rata, Danum Valley, and Kuching, Malaysia as 4.15, 5.01, 5.21, and 5.43, respectively.
According to Department of Environment of Malaysia (unpublished data), the total SO 2 emission in Malaysia was 0.25 ppm in 2020. Although this value is less than those of other countries, precautions should be taken to manage this occurrence to prevent it from increasing in severity. Although there are studies on simulated acid rain on soils in other areas 26 , 27 , 37 , 38 , there is dearth of information on the effect of SAR in Nyalau soils and its leachate. This study is important because the Nyalau series is not widely known. The Nyalau series, a tropical soil, is unique for its high sand content, strong acidity, and poor nutrient retention, making it challenging for agriculture but crucial for soil studies. Its characteristics and study are valuable for soil science and geology and contribute to our understanding of soil composition and geological history in certain regions facing the problem of acid rain.
This study embodies three objectives that significantly centre on the effects of simulated acid rain on chemistry and properties of Nyalau series ( Typic paleudults ) soil and its leachate. Firstly, the objective of the study is to identify the possibility of significant differences in soil fertility index and soil evaluation factor of Nyalau series soils when exposed to SAR. Secondly, the objective of the study seeks to ascertain the possibility of significant differences in the chemical properties of Nyalau series soils and its leachate when exposed to SAR. Finally, the study strives to examine the correlation and cluster between soil and leachate chemical properties across SAR pH. Soil fertility index and soil evaluation factor were used as key indicators to determine the effects of SAR on the fertility of Nyalau series.
Materials and methods
Soil collection, preparation and analysis.
The topsoil (0–20 cm depth) of Nyalau series from the undisturbed/minimal human intervention or alteration agricultural field, Universiti Putra Malaysia, Bintulu Campus, Sarawak, (03° 12.721′ N, 113° 4.477′ E) was collected from 10 points apart then bulked together using a spade until approximately 50 kg of soil (Fig. 1 ). The soil was collected in transparent plastic bags and transported to the laboratory, where it was air-dried in room temperature for a few days to a week and sieved to pass a 2 mm mesh. The initial chemical properties of the soil samples were determined using standard procedures as adopted from Tan 39 , for pH, Allen et al. 40 for CEC, K + , Ca 2+ , Na + , Mg 2+ and P, Keeney and Nelson method 41 for NO 3 − and NH 4 + , Rowell 42 , for acidity, Al 3+ , and H + and Cheftetz et al. 43 for soil organic matter and total organic carbon (Table 1 ).
Location of the soil sampling sites in Universiti Putra Malaysia, Bintulu, Sarawak. Sampling were conducted ramdomly from several points in study sites.
Leaching experiment design and setup
The experiment was conducted using 18 polyethylene soil columns having 16 cm diameter and 28 cm depth and fitted with 26 holes (3 mm in diameter) at the bottom. The holes evenly distributed in a uniform circular pattern for optimal drainage. Analytical grade tissue paper was placed at the bottom of the column (to prevent soil loss) after which the column was filled with 270 g soil. Soil bulk density 44 at the the undisturbed agricultural field site was first quantified, and the value was used to estimate the quantity of soil (i.e. soil without water content) to be used/ correspond with soil compaction in each column. This resulted in each empty soil column being filled with 270 g of air-dried soil, to simulate the natural condition of the Nyalau soil at the study sites. A tray was placed underneath each soil column to collect leachate.
Treatment preparation and application
The soil in the columns were exposed to SAR by applying water with pH of 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0.The pH 6.0 served as natural rainwater (control treatment). The selected SAR pH values of 3.5, 4.0, 5.0 and 5.5 were chosen to represent a range of acid deposition scenarios, from extreme to more moderate conditions enabling the study of soil responses under different environmental stress levels. A pH of 3.5 represents the worst-case scenario for acid rain worldwide and indicates the most severe environmental impacts. The other values, 4.0, 5.0 and 5.5, serve as projections ranging from extreme acidity to normal rainwater conditions. This range provides a comprehensive understanding of how different acidity levels can affect ecosystems, making the study relevant to real-world scenarios.
Water with varying pH levels was prepared by adding 0.1 molar H 2 SO 4 and HNO 3 in a 3:2 volume-to-volume ratio to distilled water, after which the pH was adjusted to the desired level 45 . The chemical properties of the SAR are presented in Table 2 . Each treatment had three replications; thus, the total experimental units were 18. The experimental units were arranged in a completely randomized design (CRD) with aset up of 6 m × 4 m room having a 76% relative humidity and a temperature of 21 °C. Approximately 318 mL of SAR were applied to each soil column and this volume was based on the field capacity of the soil using a drip system operating at a flow rate of 2.71 mL s −1 . The soil in the leaching columns were exposed to the SAR once every three days for 45 days (15 applications in total) at 8 pm. SAR application interval was based on average monthly/yearly rainfall events in Bintulu (MMD, Unpublished data), Sarawak, Malaysia. At the end of the experiment, the soil and its leachate were collected for chemical analysis.
Analysis of selected chemical properties of Nyalau series
After the incubation experiment, the soil samples in the columns were collected, air-dried, and sieved to pass through a 2 mm sieve for chemical analysis. The soil pH was measured in distilled water at a soil/water ratio of 1:2.5 39 . The CEC in mg/kg of the soil was determined using 1 M ammonium acetate buffered at pH 7. Exchangeable base cations were extracted using 100 mL of 1 M ammonium acetate buffered at pH 7, after which the filtrates were analyzed to determine the concentrations of exchangeable K, Ca, Na, and Mg using Flame Atomic Absorption Spectrometery (AAS) (iCE 300, Thermo Fisher Scientific®, NSW, Australia). The concentration of available P in the soil filtrate was determined using a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) operated at 820 nm wavelength after extracting the soils using Bray’s solution (0.03 N of ammonium fluoride, NH 4 Fl in 0.025 N of HCI) 40 .
Soil available NO 3 − and NH 4 + were determined using Keeney and Nelson method 41 followed by steam distillation 40 . Soil acidity, Al 3+ , and H + were determined using the titration method 42 . The soil available sulfate was extracted using 0.5 M of NaHCO 3 , after which the extract was analyzed using ion chromatograph IC-MS (AI300, PerkinElmer Inc., USA). The loss-on-ignition (LOI) method was used to determine soil organic matter and total organic carbon 43 . A 5 g of oven-dried sample (dried at 6 °C for 24 h) was weighed into a porcelain dish, placed in a muffle furnace, and heated at 300 °C for 1 h to determine soil organic matter content.
The Soil Fertility Index (SFI; Eq. 1 ) and Soil Evaluation Factor (SEF; Eq. 2 ) of Nyalau series were calculated using the formulas of Moran et al. 46 and Lu et al. 47 , respectively.
Analysis of selected chemical properties of leachate
The leachate pH was measured using a pH meter (S220, Thermo Fisher Scientific®, USA) whereas electric conductivity (EC), salinity, and total dissolved solids were determined using EC meter (S70, Mettler Toledo Co., USA). Exchangeable cations were determined using AAS (AA5000, PerkinElmer Inc., USA) whereas nitrite (NO 2− ), phosphate (PO 4 3− ), nitrate (NO 3 − ), and ammonium (NH 4 + ) were measured using UV-spectrophotometer (DR 2010, Hach©, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was used to detect between-treatment before after which treatment means were compare dusing Duncan’s New Multiple Range Test (post-hoc analysis) at p ≤ 0.05. Pearson’s correlation analysis was conducted to determine the relationship between the chemical properties of the soil and its leachate. In addition, Pearson’s correlation analysis was performed to analyze the response of soil and leachate variables across the pH of SARtreatment. The statistical analysis was performed using SAS version 9.4 48 .
Hierarchical cluster analysis (CA) was performed to find out similar groups of soil properties depending of origin (one soil type) and concentration. The CA was performed on the various chemical properties simulated acid rain and leachate, using a distance cluster between 15 and 20 49 , 50 . A distance criterion between two variables express how closely correlate within the group. Two cluster analyses by means of hierarchical dendrograms were performed by using SPSS 28.0 (IBM SPSS Statistics, USA) applied to the SAR and soil leachate. All these analysis collectively allowed for interpreting how SAR treatments affected soil and leachate composition, guiding conclusions on acid rain's impact.
Effects of simulated acid rain treatments on soil properties
Soil pH, K + , SFI and SEF significantly decreased with increasing acidity of SAR. As example, significant decrease in soil pH and SFI (2.21% reduction) were recorded when the soil was exposed to SAR with pH 4.0 and pH 3.5. Potassium ions in the soil decreased from 0.037 to 0.019 mg kg −1 (48.64% reduction). Contrastingly, Mg 2+ , Na + , SO 4 2− , NO 3 − , and soil acidity significantly increased with increasing acidity of the SAR. Relative to control (natural rainwater) the soil which was exposed to SAR with a pH of 3.5 increased Mg 2+ , Na + , SO 4 2− , NO 3 − , and acidity by 193.33%, 101.30%, 46.2%, 18.65% and 22.02%, respectively. Furthermore, significant reduction was observed in the level of Al 3+ , H + , and Zn 2+ in soils exposed to SAR with pH 5.0. However, the K + , Ca 2+ and Zn 2+ cations decreased with increasing acidity of SAR (pH 4.0 and below). Similarly, available P in the soil significantly reduced from 1.62 mg kg −1 at SAR of pH 6.0 to 1.43 mg kg −1 at pH of 4.5, whereas SAR with pH 3.5 recorded an available P value of 1.57 mg kg −1 . Furthermore, the Soil CEC, Ca 2+ , Fe 2+ , and NH 4 + fluctuated across the SAR treatments whereas SEF generally remained unchanged (Table 3 ).
Effects of simulated acid rain (SAR) treatments on leachate properties
There was significant increase in K + and Mg 2+ concentrations in leachate as SAR levels were decreased from 4.0 to 3.5 (Table 4 ). K + ions increased from 5.62 mg L −1 (SAR at pH 6.0) to 6.65 mg L −1 (SAR at pH 3.5) whereas Mg 2+ ions increased from 0.72 mg L −1 (SAR at pH 6.0) to 0.83 mg L −1 (SAR at pH 3.5). The Na + in the leachate significantly increased from 1.92 to 4.63 mg L −1 with increasing SAR acidity. The continued acidification reduced Na + in the leachate to 2.68 mg L −1 (pH 3.5). The leachate of PO 4 2− concentration did not significantly differences regardless of SAR pH. Other variables fluctuated across the SAR pH (Table 4 ).
Relationship between soil and leachate properties
The relationship between the soil and its leachate properties was analyzed to determine acid deposition's effect on nutrients leaching or retention by the Nyalau series. The Pearson’s correlation analysis revealed that the changes in Ca 2+ and NH 4 + between the soil and its leachate positively correlated and the Pearson’s correlation coefficient (r) values were 0.84 and 0.86, respectively. However, the NO 3 − in the soil and its leachate was correlated negatively (r = − 0.82). The correlation for the other variables were not significant (Fig. 2 ).
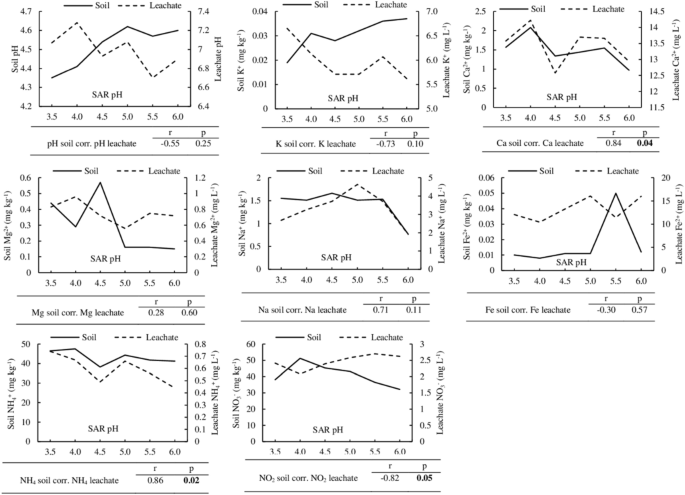
Trends of selected soil and leachate properties of Nyalau series ( Typic paleudults ) soil after exposure to simulated acid rain. Correlation analysis was conducted, and the relationship was indicated by the Pearson’s correlation coefficient (r) and probability level significant at p ≤ 0.05.
Cluster analysis for soil and leachate properties
The findings of CA are presented in two hierarchical dendrograms representing soil (Fig. 3 A) and leachate (Fig. 3 B). The dendrogram for soil comprise 3 clusters (Fig. 3 A). NH 4 + and NO 3 − comprise first cluster and SO 4 2− , SFI, CEC and SEF comprise the second cluster and are associated with a low distance criterion around 1. The rest of the chemical properties acidity, pHwater, pH KCl , H + , Na + , Ca 2+ , Mg 2+ , Al 3+ , Cu 2+ , K + , Zn 2+ and Fe 2+ form the third cluster and they are associated in a very low distance at around 1. In Fig. 3 B, the first cluster contains Cu 2+ ,NO 3 − ,PO 4 2− , NO 2 − , NH 4 + , Mg 2+ , Cl − , Salinity and S 2− and they are positioned at a very low distance around 1. In the second cluster, pH, K + , Ca 2+ and Fe 2+ form a group with a distance of CA below 3 whereas electrical conductivity (EC) is placed separately than cluster 1 and 2 with a high distance criteria at 25.
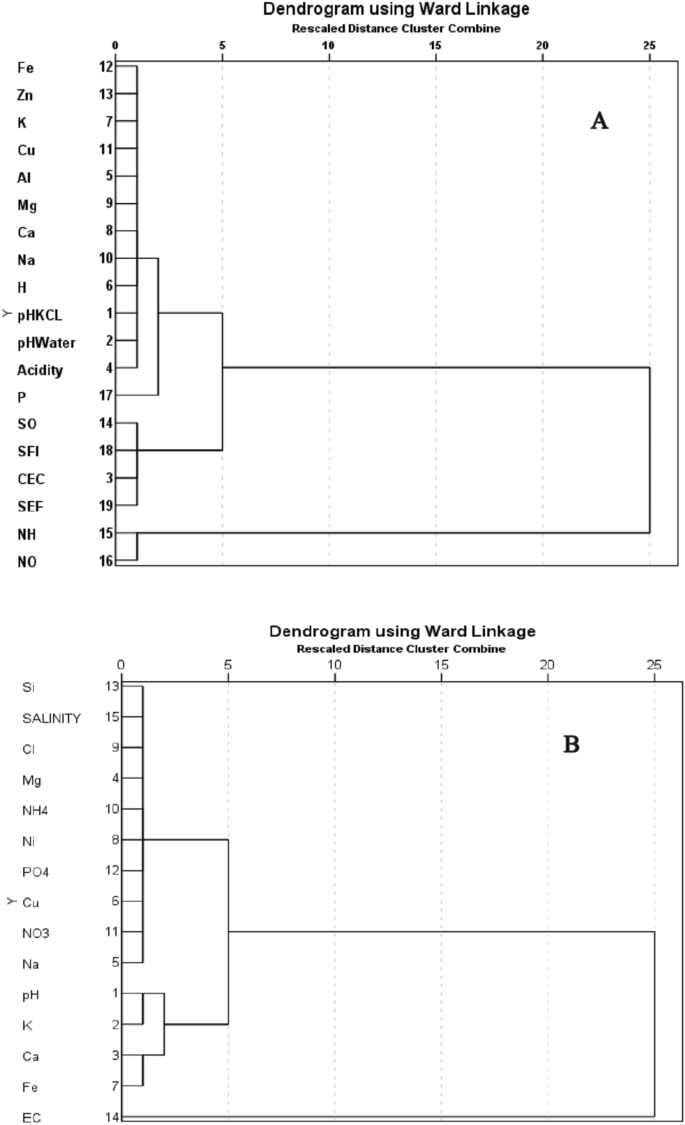
Hierarchical dendrogram for chemicals properties found in soil ( A ) and leachate ( B ) using Ward’s method.
Simulated acid rain and natural rainwater on soil properties of Nyalau series
Generally, the SAR treatments, including control, initially decreased soil pH (4.84). The pH ofsoilwith SAR pH below 4.5 (Table 3 ) was significantly low and this may cause reduction in the soil fertility index. Additionally, the soil exchangeable Al 3+ and H + were significantly increased because aluminium hydrolysis increases with increasing soil acidity. For example, a complete hydrolysis of one mole Al 3+ ions produces three moles H + ions to further decrease soil pH and this chemical reaction reduces soil CEC. This finding corroborates that of Zhang et al. 11 who explained that acid rain increases soil acidity and H + ions, leading to loss of mineral structure. Loss in mineral structure has been implicated in soil fertility decline. Wei et al. 51 also reported that acid rain reduces soil fertility because it reduces soil pH and cation retention capacity.
Although soils have strong pH buffering capacity, the SO 4 2− , H + , NO 3 − , and NH 4 + in acid rain favour the dominance of H + ions on the soil exchange sites such that soil CEC is disproportionately dominated by hydrogen ions instead of base cations, especially K. Significant leaching of K + in soil with SAR at pH 3.5 was expected due to the high acidity ofthis treatment. The dominance of stronger complementary adsorbed cations at the soil exchange sites could partly explain the loss of K into the leachate 26 , 51 . Acidic rainwater gradually diminishes exchangeable cations in topsoil because it facilitates changes in the nutrient pool and leaching of nutrients from the soil profile 44 . This observation is supported by Zhang et al. 11 , who reported significantly higher effluent K + concentration of SAR at pH 3 and below.
The low SAR pH were responsible for low variations in Ca 2+ , Mg 2+ , Na + , acidity, NH 4 + , and SO 4 2− in the soils compared with soil treated with natural rainwater. This finding is similar to that of Rampazzo and Blum 52 who reported that exposing parent rock material to acid rain, inspite of having 30–80% calcite, reduced CEC and base saturation, particularly Ca contents. This suggests the fertility and the overall productivity of soils will decline if they are exposed to acid deposition for a long time. A notable reduction in soil pH enhances the solubility of aluminium, consequently elevating the concentration of Al 3+ ions in the leachate. This finding aligns with Mulder et al. 53 observation, where they reported the phytotoxic effects due to increased dissolution of Al 3+ in soil leaching experiments conducted in both the Netherlands and New Hampshire, USA.
Soil Zn 2+ solubility has increase with decreasing pH (3.5–6.0) because the solubility of Zn decreases with increasing soil pH. High levels of soil contamination, with soluble Zn 2+ reaching 19,570 mg/kg and Cu 2+ up to 322.4 mg/kg 38 , enhance the phytoavailability of heavy metals 14 , leading to increased uptake by plants. The very acidic SAR treatments increased soil exchangeable sulfate 46.20% because of sulfate adsorption to form sulfuric acid which upon decomposing, releases H + and SO 4 2− ion. This reaction occurs at low soil pH 54 . Soil available ammonium increase with increasing acidity of SAR. The increase in NH 4 + concentration is consistent with the report of Johnson et al. 55 , who demonstrated that acid rain increases nitrogen mineralization and nitrification in forest soils.
Simulated acid rain and natural rainwater on leachate properties of Nyalau series
Leachate pH was highest with lower SAR pH compared with natural rainwater. According to De Walle et al. 56 , the increase in leachate pH was due to the accumulation of base cations, especially Ca 2+ and Mg 2+ (Table 4 ). This result also explains the movement of Ca 2+ and Mg 2+ down the soil profile, corroborating the results of Zhang et al. 11 on Latosol of Southern China. Low electrical conductivity and salinity values were recorded with lower SAR pH because the accumulation of base cations in the leachate increased the EC of the soil. The base cations in the leachate of the lower SAR pHs were higher than with the natural rainwater (Table 4 ). This present study suggests that acid rain causes leaching of the bases and this could cause ground water pollution through enrichment through lost nutrients from the soil profile.
Overall implication of varying simulated acid rain on soil and leachate properties
The incubation of Nyalau soil series with SAR generally had negative effects on pH, K, Fe and NO 3 of the soil and its leachate. This includes a decrease in soil pH, indicating increased acidity, and reductions in the concentrations of potassium (K), iron (Fe), and nitrate (NO 3 ) in the soil. The results indicate that when the pH of SAR decreases from 6.0 to 3.5, the pH and potassium (K) content in the soil and leachate also decrease. This is confirmed by the data in Tables 3 and 4 . The increased soil acidity with the low pH SAR is related to high H + concentration. The accumulation of H + from acid deposition increased the soil acidity 27 , 51 . Increase in the soil acidity through acid deposition might have affected the solubility of heavy metals such as Fe, as observed in the soil with low SAR pH. Furthermore, acidic pollutants can cause P fixation by Al and Fe in soils 57 and this explain low available P content in this present study (Table 3 ). The positive relationship between soil and leachate for Ca 2+ and NH 4 + was due to insufficient time (45 days) for leaching of cations from the soil. This slower leaching rate is due to the complex interplay of physical, chemical, and environmental factors within the soil. Essentially, these ions are not as readily mobilized or washed out of the soil compared to other elements, indicating a delayed response to the leaching process influenced by soil composition and conditions 58 .
More than that the similarities of SO 4 2− , SFI, CEC and SEF in hierarchical dendrograms of soil have shown that the fertility of Nyalau series soil have also influenced by SO 4 2− . We believe it was happening because of the presence of sulphuric acid (H 2 SO 4 ) from SAR treatments. Our argument is consistent with finding in Table 3 recorded higher SO 4 2− content under low SAR pH treatments. Similar study reported by Hüttl and Frielinghaus 59 in Eastern, Germany who shows that air pollutant or acid rain content with H 2 SO 4 could reduce the soil fertility accelerating soil acidification. In the leachate hierarchical dendrograms, there are similarities of soil water pH, K and Ca. This results reliable comes from accumulation of base cation while exposure to SAR as discussing in previous section.
Management implication of simulated acid rain on soil and leachate properties
Even with a short incubation study (45 days), we found a 2.21% reduction in the fertility of Nyalau series and 5.43% reduction in soil acidity as compared when exposed to natural rainwater (control treatment). The lower SFI of the soil in the present study (11.94) compared with research on a secondary forest in Lundu, Sarawak, by Perumal et al. 60 where SFI of 19.63 was recorded, indicates the prolonged negative impact of acid rain on soil fertility. These results showed that acid rain impacted soil and leachate properties, and it is possible that prolonged acid rain exposure will further modify soils of the Nyalau series detrimentally.
Therefore, for a comprehensive understanding of acid rain's effects, a long term study, possibly over a year, is recommended. This allows for observing long-term ecological and soil changes. Complementing this with advanced modeling would provide a holistic view, predicting future impacts and aiding in effective environmental management strategies, crucial for sustaining ecosystems and agricultural productivity in the face of environmental changes. Therefore, understanding the prolonged impacts of acid rain on soil properties is not only an ecological necessity but also crucial for human sustainability.
The study focused on the impact of simulated acid rain (SAR) on the Nyalau series soil, examining a range of acidity levels from less acidic (pH 5.5 and 5.0) to more acidic (pH 4.0 and 3.5). It was found that with increasing acidity, especially at pH 3.5, the soil experienced significant changes: a decrease in pH, potassium, and fertility, and an increase in magnesium, sodium, sulfate, nitrate, and overall acidity. The leachate from the soil also showed increased levels of potassium and magnesium, indicating a leaching effect that could lead to nutrient deficiencies for plants. The study also noted a positive correlation between changes in calcium and ammonium levels in both soil and leachate, and a negative correlation in nitrate levels, highlighting complex interactions between soil acidity and nutrient dynamics.
The results of our study have important practical implications for both land management and environmental policy. Land managers are suggested to regularly conduct comprehensive soil health assessments, especially in areas vulnerable to acid rain or soil acidification. These assessments should go beyond simply measuring pH and consider chemical properties such as K + , Mg 2+ and NO 3 − to inform soil treatment plans. In terms of policy, the observed deleterious effects of acidic treatments on soil properties call for stricter pollution regulations to curb acid rain, and the data could further guide the establishment of safe areas for agriculture and forestry based on the resilience of soils to acidification.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Irvine, I. C., Greaver, T., Phelan, J., Sabo, R. D. & Van Houtven, G. Terrestrial acidification and ecosystem services: Effects of acid rain on bunnies, baseball, and Christmas trees. Ecosphere 8 , e01857. https://doi.org/10.1002/ecs2.1857 (2017).
Article Google Scholar
Debnath, B. & Ahammed, G. J. Effect of acid rain on plant growth and development: Physiological and molecular interventions. In Contaminants in Agriculture (eds Naeem, M. et al. ) 103–114 (Springer, 2020). https://doi.org/10.1007/978-3-030-41552-5_5 .
Chapter Google Scholar
NADP (National Atmospheric Deposition Program). National trends network. Illinois State Water Survey, Champaign, IL, U.S.A. Retrieved from http://nadp.sws.uiuc.edu/NTN (Accessed 4 December 2019) (2005).
US-EPA (United State Environmental Protection Agency). Report of Acid Rain Program Progress 2004 (Washington, United State, 2005).
Bhatti, N., Streets, D. G. & Foell, W. K. Acid rain in Asia. Environ. Manag. 16 , 541–562. https://doi.org/10.1007/BF02394130 (1992).
Article ADS Google Scholar
Burns, D. A., Aherne, J., Gay, D. A. & Lehmann, C. Acid rain and its environmental effects: Recent scientific advances. Atmos. Environ. 146 , 1–4. https://doi.org/10.1016/j.atmosenv.2016.10.019 (2016).
Article CAS ADS Google Scholar
Nicoletti, G., Arcuri, N., Nicoletti, G. & Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 89 , 205–213. https://doi.org/10.1016/j.enconman.2014.09.057 (2015).
Charola, A. E. & Ware, R. Acid deposition and the deterioration of stone: A brief review of a broad topic. Geol. Soc. Lond. Spec. Publ. 205 , 393–406. https://doi.org/10.1144/GSL.SP.2002.205.01.28 (2002).
Dubey, S. Acid rain-the major cause of pollution: its causes, effects and solution. Int. J. Sci. Eng. Technol. 2 , 772–775 (2013).
Google Scholar
Saxena, P. & Sonwani, S. Criteria air pollutants: Chemistry, sources, and sinks. In Criteria Air Pollutants and Their Impact on Environmental Health (eds Saxena, P. & Sonwani, S.) 7–48 (Springer, 2019). https://doi.org/10.1007/978-981-13-9992-3_2 .
Zhang, X., Jiang, H., Jin, J., Xu, X. & Zhang, Q. Analysis of acid rain patterns in northeastern China using a decision tree method. Atmos. Environ. 46 , 590–596. https://doi.org/10.1016/j.atmosenv.2011.03.004 (2012).
Ouyang, X. J. et al. Effect of simulated acid rain on potential carbon and nitrogen mineralization in forest soils. Pedosphere 18 , 503–514. https://doi.org/10.1016/S1002-0160(08)60041-7 (2008).
Article CAS Google Scholar
Lal, N. Effects of acid rain on plant growth and development. J. Sci. Technol. 11 , 85–108 (2016).
Kim, A. Y., Kim, J. Y., Ko, M. S. & Kim, K. W. Acid rain impact on phytoavailability of heavy metals in soils. Geosyst. Eng. 13 , 133–138. https://doi.org/10.1080/12269328.2010.10541320 (2010).
Kirk, G. J., Bellamy, P. H. & Lark, R. M. Changes in soil pH across England and Wales in response to decreased acid deposition. Glob. Change Biol. 16 , 3111–3119. https://doi.org/10.1111/j.1365-2486.2009.02135.x (2010).
Stevens, C. J., Thompson, K., Grime, J. P., Long, C. J. & Gowing, D. J. Contribution of acidification and eutrophication to declines in species richness of calcifuge grasslands along a gradient of atmospheric nitrogen deposition. Funct. Ecol. 24 , 478–484. https://doi.org/10.1111/j.1365-2435.2009.01663.x (2010).
Jonard, M. et al. Deterioration of Norway spruce vitality despite a sharp decline in acid deposition: A long-term integrated perspective. Glob. Change Biol. 18 , 711–725. https://doi.org/10.1111/j.1365-2486.2011.02550.x (2012).
Likens, G. E. & Bailey, S. W. The discovery of acid rain at the Hubbard Brook Experimental Forest: a story of collaboration and long-term research. In USDA Forest Service Experimental Forests and Ranges 463–482 (Springer, New York, NY, 2014). https://doi.org/10.1007/978-1-4614-1818-4_20
Maskell, L. C., Smart, S. M., Bullock, J. M., Thompson, K. E. N. & Stevens, C. J. Nitrogen deposition causes widespread loss of species richness in British habitats. Glob. Change Biol. 16 , 671–679. https://doi.org/10.1111/j.1365-2486.2009.02022.x (2010).
Simkin, S. M. et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. 113 , 4086–4091. https://doi.org/10.1073/pnas.1515241113 (2016).
Article CAS PubMed PubMed Central ADS Google Scholar
Park, J. H., Duan, L., Kim, B., Mitchell, M. J. & Shibata, H. Potential effects of climate change and variability on watershed biogeochemical processes and water quality in Northeast Asia. Environ. Int. 36 , 212–225. https://doi.org/10.1016/j.envint.2009.10.008 (2010).
Article CAS PubMed Google Scholar
Yang, S. et al. Effectiveness of amendments on re-acidification and heavy metal immobilization in an extremely acidic mine soil. J. Environ. Monit. 13 (7), 1876. https://doi.org/10.1039/c1em10028a (2011).
Dai, P. et al. Alleviating soil acidification and increasing the organic carbon pool by long-term organic fertilizer on tobacco planting soil. Agronomy 11 (11), 2135. https://doi.org/10.3390/agronomy11112135 (2021).
Araújo, R. A. Advances in soil engineering: sustainable strategies for rhizosphere and bulk soil microbiome enrichment. Frontiers in Bioscience-Landmark, 27(6), 195 (2022). https://doi.org/10.31083/j.fbl2706195 .
Bashir, M. A. et al. Soil survey techniques determine nutrient status in soil profile and metal retention by calcium carbonate. Catena 173 , 141–149. https://doi.org/10.1016/j.catena.2018.10.015 (2019).
Zheng, S. A., Zheng, X. & Chen, C. Leaching behavior of heavy metals and transformation of their speciation in polluted soil receiving simulated acid rain. PLoS ONE 7 , e49664. https://doi.org/10.1371/journal.pone.0049664 (2012).
Article PubMed PubMed Central ADS Google Scholar
Zhang, J. E., Yu, J., Ouyang, Y. & Xu, H. Impact of simulated acid rain on trace metals and aluminum leaching in Latosol from Guangdong Province, China. Soil Sedim. Contam. Int. J. 23 , 725–735. https://doi.org/10.1080/15320383.2014.866934 (2014).
Wen, X. J., Duan, C. Q. & Zhang, D. C. Effect of simulated acid rain on soil acidification and rare earth elements leaching loss in soils of rare earth mining area in Southern Jiangxi Province of China. Environ. Earth Sci. 69 , 843–853. https://doi.org/10.1007/s12665-012-1969-4 (2013).
Tian, D. & Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 10 , 024019. https://doi.org/10.1088/1748-9326/10/2/024019 (2015).
Theng, C. S. Keys to Soil Classification in Sarawak. Technical paper 10, 47 pp. Soil Division, Department of Agriculture, Sarawak (1993).
Sabang, J., Kendawang, J. J. & Lee, H. S. Soil characteristics of an abandoned shifting cultivation land in Sarawak, Malaysia. Tropics 10 , 251–263. https://doi.org/10.3759/tropics.10.251 (2000).
Tan, S. et al. Review of soils on the 52 ha long term ecological research plot in mixed dipterocarp forest at Lambir, Sarawak, Malaysian Borneo. Tropics 18 , 61–86. https://doi.org/10.3759/tropics.18.61 (2009).
EANET (Acid Deposition Monitoring Network in East Asia). Report on the State of Acid Deposition in East Asia. (Niigata, Japan, 2020).
Mohamed, R. M. S. R., Rahim, A. F. H. & Kassim, A. H. M. A monitoring of air pollutants (CO, SO 2 and NO) in ambient air near an industrial area. In: MATEC Web of Conferences Vol. 47, p. 05022 (EDP Sciences, 2016).
Norela, S., Nurfatihah, M. Z., Mainon, A. & Ismail, B. S. Wet deposition in the residential area of the Nilai industrial park in Negeri Sembilan, Malaysia. App. Sci. J. 7 , 170–179 (2009).
CAS Google Scholar
EANET (Acid Deposition Monitoring Network in East Asia). Report on Data 2011. (Niigata, Japan, 2013)
Du, Y. J., Wei, M. L., Reddy, K. R., Liu, Z. P. & Jin, F. Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 271 , 131–140. https://doi.org/10.1016/j.jhazmat.2014.02.002 (2014).
Li, J., Jia, C., Lu, Y., Tang, S. & Shim, H. Multivariate analysis of heavy metal leaching from urban soils following simulated acid rain. Microchem. J. 122 , 89–95. https://doi.org/10.1016/j.microc.2015.04.015 (2015).
Tan, K. H. Soil Sampling, Preparation, and Analysis 2nd edn. (CRC Press, 2005).
Book Google Scholar
Allen, S. E., Grimshaw, H. M., Parkinson, J. A. & Quarmby, C. Chemical Analysis of Ecological Materials (Blackwell Scientific Publications, 1989).
Keeney, D. R. & Nelson, D. W. Nitrogen-inorganic forms. In Methods of Soil Analysis Part 2 (eds Page, A. L., Keeney, D. R., Baker, D. E. et al. ) (ASA and SSAA, 1982).
Rowell, D. L. Soil Science: Method and Applications (Pearson Education, Inc., 1994).
Cheftetz, B., Hatcher, P. H., Hadar, Y. & Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 25 , 776–785. https://doi.org/10.2134/jeq1996.00472425002500040018x (1996).
Brady, N. C. & Weil, R. R. The Nature and Properties of Soils 13th edn. (Pearson Education, Inc., 2002).
Ayers, G. P., Leong, C. P., Gillett, R. W. & Lim, S. F. Rainwater composition and acidity at five sites in Malaysia in 1996. Water Air Soil Pollut. 133 , 15–30. https://doi.org/10.1023/A:1012967614759 (2002).
Moran, E. F. et al. Effects of soil fertility and land-use on forest succession in Amazonia. For. Ecol. Manage 139 , 93–108. https://doi.org/10.1016/S0378-1127(99)00337-0 (2000).
Lu, D., Moran, E. & Mausel, P. Linking Amazonian secondary succession forest growth to soil properties. Land Degrad. Dev. 13 , 331–343. https://doi.org/10.1002/ldr.516 (2002).
Statistical Analysis System, SAS. SAS/STAT software (Version 9.2) [Statistical software]. https://www.sas.com/en_us/software/stat.html (2009).
Shajib, M. T. I., Hansen, H. C. B., Tao, L. & Holm, P. E. Metals in surface-specific urban runoff in Beijing, China. Environ. Pollut. 248 , 584–598. https://doi.org/10.1016/j.envpol.2019.02.039 (2019).
Shajib, M. T. I., Hansen, H. C. B., Tao, L. & Holm, P. E. Rare earth element in surface specific urban runoff in Northern Beijing. Sci. Total Environ. 717 , 136969. https://doi.org/10.1016/j.scitotenv.2020.136969 (2020).
Article CAS PubMed ADS Google Scholar
Wei, H. et al. Soil pH responses to simulated acid rain leaching in three agricultural soils. Sustainability 12 , 280. https://doi.org/10.3390/su12010280 (2020).
Rampazzo, N. & Blum, W. E. Changes in chemistry and mineralogy of forest soils by acid rain. Water Air Soil Pollut. 61 , 209–220. https://doi.org/10.1007/BF00482605 (1992).
Mulder, J., Van Breemen, N. & Eijck, H. C. Depletion of soil aluminium by acid deposition and implications for acid neutralization. Nature 337 , 247–249. https://doi.org/10.1038/337247a0 (1989).
Sokolova, T. A. & Alekseeva, S. A. Adsorption of sulfate ions by soils (a review). Eurasian Soil Sci. 41 , 140–148. https://doi.org/10.1134/S106422930802004X (2008).
Johnson, D. W., Turner, J. & Kelly, J. M. The effects of acid rain on forest nutrient status. Water Resour. Res. 18 , 449–461. https://doi.org/10.1029/WR018i003p00449 (1982).
De Walle, D. R., Ribblett, G. C., Helvey, J. D. & Kochenderfer, J. Laboratory investigation of soil solution chemistry from six Appalachian Forest floor types subjected to simulated acid rain. J. Environ. Qual. 14 , 234–240. https://doi.org/10.1007/BF00284237 (1985).
David, M. B. & Driscoll, C. T. Aluminum speciation and equilibria in soil solution of a haplorthod in the Adirondack Mountain (New York, U.S.A.). Geoderma 33 , 297–318. https://doi.org/10.1016/0016-7061(84)90031-4 (1984).
Zarabi, M. & Jalali, M. Leaching of nitrogen and base cations from calcareous soil amended with organic residues. Environ. Technol. 33 (14), 1577–1588. https://doi.org/10.1080/09593330.2011.638675 (2012).
Hüttl, R. F. & Frielinghaus, M. Soil fertility problems—An agriculture and forestry perspective. Sci. Total Environ. 143 , 63–74. https://doi.org/10.1016/0048-9697(94)90533-9 (1994).
Perumal, M., Wasli, M. E., Ying, H. S., Lat, J. & Sani, H. Association between soil fertility and growth performance of planted Shorea macrophylla (de Vriese) after enrichment planting at rehabilitation sites of Sampadi Forest Reserve, Sarawak, Malaysia. Int. J. For. Res. https://doi.org/10.1155/2017/6721354 (2017).
Download references
Acknowledgements
The authors would like to acknowledge Department of Environment of Malaysia and Malaysia Meteorological Department for the providing atmospheric concentration data on 2020 and Bintulu temperature and rainfall data. We also aknowlwdge the technical support provided by Muhamad Fuad Ibrahim, Palanivell Perumal, UPMKB staff Arni Japar, Awang Marzuki Awang Mustapha, Elizabeth Andrew Anyah, and Awangku Ahmad Nizam Awang during the conduct of the research.
This research was funded by the Universiti Malaysia Sarawak under PILOT Research Grant Scheme (UNI/F07/PILOT/85193/2022), Ministry of Higher Education Education (Malaysia) under the Fundamental Research Grant Scheme (FRGS: 5523701), Universiti Putra Malaysia under the Research University Grant Scheme (RUGS: 9199765), Ministry of the Environment (Japan) under the Environmental Research and Technology Development Fund (B-0801), and Mitsubishi Corporation Trust Fund (6380500).
Author information
Md. Tariqul Islam Shajib
Present address: Department of Natural Resources and Environmental Design, North Carolina Agricultural and Technical State University, Greensboro, NC, USA
Authors and Affiliations
Agrotechnology Programme, Faculty of Resources Science and Technology, Universiti Malaysia Sarawak, 94300, Kota Samarahan, Sarawak, Malaysia
Mohamad Hilmi Ibrahim
Department of Land Management, Faculty of Agriculture, Universiti Putra Malaysia, 43400, Serdang, Selangor Darul Ehsan, Malaysia
Susilawati Kasim
Universiti Islam Sultan Sharif Ali, Kampus Sinaut, Km 33 Jln Tutong Kampong Sinaut, Tutong, TB1741, Negara Brunei Darussalam
Osumanu Haruna Ahmed
Faculty of Sustainable Agriculture, Universiti Malaysia Sabah, 90000, Sandakan, Sabah, Malaysia
Mohd. Rashid Mohd. Rakib & Nur Aainaa Hasbullah
Division of Soil, Water and Environment, Care to People Denmark, 2400, Copenhagen, NV, Denmark
You can also search for this author in PubMed Google Scholar
Contributions
[I.M.H] conceptualized the study, developed the main research framework, and was primarily responsible for the laboratory leaching and incubation study. He also contributed to data curation, methodology, and wrote the original draft of the manuscript. [K.S] played a pivotal role in data analysis and interpretation. She employed the necessary statistical tools to determine the correlations and were responsible for generating all the data charts and figures. [Author B] also assisted in writing and revising the manuscript, ensuring the technical details were articulated effectively. [A.O.H] managed the logistics and resources for the entire study. He supervised the application of SAR treatments to the soil samples and were instrumental in ensuring that standard procedures were adhered to. [Author C] also played a role in manuscript revision, particularly overseeing the accuracy and authenticity of the methodology. [M.R.M.R., H.N.A and I.M.T] contributed to the literature review and provided essential insights into the implications of the research findings, particularly concerning tropical forest ecosystems. They also assisted in drafting the discussion and conclusion sections of the manuscript and provided critical feedback for overall improvement.
Corresponding author
Correspondence to Mohamad Hilmi Ibrahim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ibrahim, M.H., Kasim, S., Ahmed, O.H. et al. Impact of simulated acid rain on chemical properties of Nyalau series soil and its leachate. Sci Rep 14 , 3534 (2024). https://doi.org/10.1038/s41598-024-52758-1
Download citation
Received : 26 October 2023
Accepted : 23 January 2024
Published : 12 February 2024
DOI : https://doi.org/10.1038/s41598-024-52758-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Advertisement
Acid rain and air pollution: 50 years of progress in environmental science and policy
- Open access
- Published: 21 September 2019
- Volume 49 , pages 849–864, ( 2020 )
Cite this article
You have full access to this open access article

- Peringe Grennfelt ORCID: orcid.org/0000-0002-0896-7924 1 ,
- Anna Engleryd 2 ,
- Martin Forsius 3 ,
- Øystein Hov 4 ,
- Henning Rodhe 5 &
- Ellis Cowling 6
98k Accesses
193 Citations
627 Altmetric
116 Mentions
Explore all metrics
Because of its serious large-scale effects on ecosystems and its transboundary nature, acid rain received for a few decades at the end of the last century wide scientific and public interest, leading to coordinated policy actions in Europe and North America. Through these actions, in particular those under the UNECE Convention on Long-range Transboundary Air Pollution, air emissions were substantially reduced, and ecosystem impacts decreased. Widespread scientific research, long-term monitoring, and integrated assessment modelling formed the basis for the policy agreements. In this paper, which is based on an international symposium organised to commemorate 50 years of successful integration of air pollution research and policy, we briefly describe the scientific findings that provided the foundation for the policy development. We also discuss important characteristics of the science–policy interactions, such as the critical loads concept and the large-scale ecosystem field studies. Finally, acid rain and air pollution are set in the context of future societal developments and needs, e.g. the UN’s Sustainable Development Goals. We also highlight the need to maintain and develop supporting scientific infrastructures.
Similar content being viewed by others
The legacy from the 50 years of acid rain research, forming present and future research and monitoring of ecosystem impact

A History of Air Quality Management

Implications of Current Knowledge on Nitrogen Deposition and Impacts for Policy, Management and Capacity Building Needs: CLRTAP
Avoid common mistakes on your manuscript.
Introduction
Acid rain was one of the most important environmental issues during the last decades of the twentieth century. It became a game changer both scientifically and policy-wise. For some time, particularly during the 1980s, acid rain was by many considered to be one of the largest environmental threats of the time. Observations of fish extinction in Scandinavian surface waters and forest dieback on the European Continent were top stories in the news media. Even in North America acid rain received large public and policy attention.
During the cold war, with almost no contacts between East and West, acid rain broke the ice and formed an opening for scientific and political collaboration, resulting in a treaty under the United Nations’ Economic Commission for Europe (UNECE), the Convention on Long-range Transboundary Air Pollution (often mentioned as CLRTAP but in this paper we call it the Air Convention) signed in 1979. Eight protocols have been signed under the Air Convention committing parties to take far-reaching actions, not only with respect to acid rain but also with respect to several other air pollution problems (Table 1 ). Emissions of all key air pollutants have been reduced significantly and for the most important acidifying compound, sulphur dioxide, emissions in Europe have decreased by 80% or more since the peaks around 1980–1990 (Fig. 1 ).
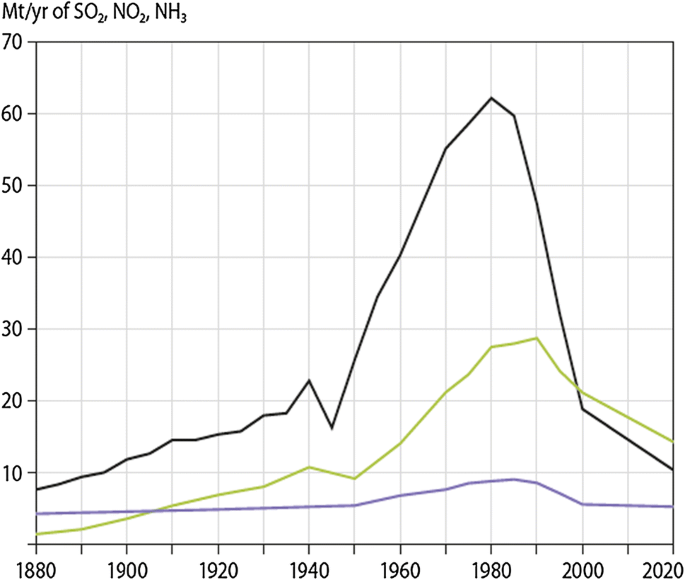
European emissions of sulphur dioxide (SO 2 —black), nitrogen oxides (NO x , calculated as NO 2 —green) and ammonia (NH 3 —blue) 1880–2020 (updated from Fig. 2 in Schöpp et al. 2003 )
In this paper, we present and discuss how the acid rain problem became a key environmental issue among industrial countries from the late 1960s and the following decades (Fig. 2 ). We view the problem from a science-to-policy interaction perspective, based on a Symposium in Stockholm in the autumn 2017 organised to manifest 50 years of international air pollution science and policy development. The Symposium involved both a testimony from a number of those involved in science and policy during the first decades of the history but also a discussion of what we have learned and how the experience can be used in the future. Further information about the symposium and its outcome can be found at http://acidrain50years.ivl.se .
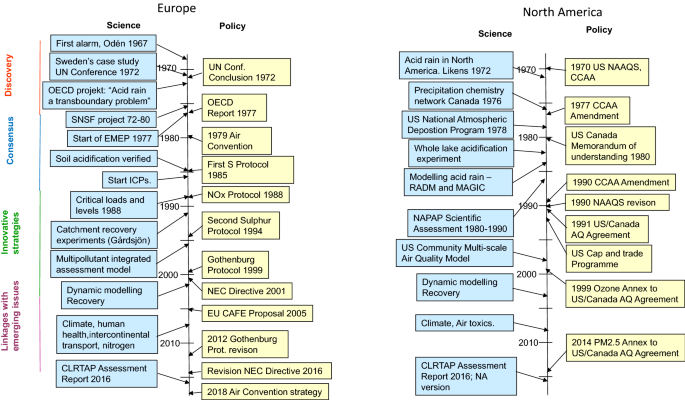
The timeline of science and policy interactions in Europe and North America 1967–2018. (updated from Driscoll et al. 2012). Abbreviations not occurring in text. NAAQS: National Ambient Air Quality Standards under the US Clean Air Act; CCAA: Canadian Clean Air Act; RADM: Regional Atmospheric Deposition Model; MAGIC Model of Acidification of Groundwater in Catchments. It should be mentioned that Canada and US are both parties to the Air Convention and they have also signed and ratified most of its protocols
Our historical review will be limited to some of the issues brought up at the Symposium. For more information on the early history see Cowling ( 1982 ). A comprehensive description of the acid rain history has recently been published by Rothschild ( 2018 ). The history of the first 30 years of the science–policy interactions under the Air Convention is also described in Sliggers and Kakebeeke ( 2004 ).
Short historical review
The discovery and the early acid rain history.
In a deliberatively provocative article in the Swedish newspaper Dagens Nyheter in October 1967, entitled “An Insidious Chemical Warfare Among the Nations of Europe”, the Swedish scientist Svante Odén (Fig. 3 ) described a new and threatening environmental problem—Acid Rain. He pointed to the significant decrease in pH of rainwater and surface waters that had occurred over the previous decade and linked it to the large and increasing emissions of sulphur dioxide in Europe.

Svante Odén around 1970 (photo Ellis B. Cowling)
The discovery received immediate attention by the Swedish government and, a few weeks after Odén’s article, the minister of industry presented the issue at the Organisation for Economic Cooperation and Development (OECD), but it did not receive any political attention at that time. The issue was also brought up in OECD’s Air Pollution Management Committee by the Swedish delegate Göran Persson. Also, here the message was met by scepticism and the common opinion among the members in the committee was that sulphur dioxide was a local problem, which easily could be solved by tall stacks. It was not until Persson felt he was going to “loose the case” he “played his last card” and pointed to the observations of intercontinental transport of radioactivity from the Chinese nuclear bomb experiments. The opinion then changed and the meeting agreed that acid rain might be an issue to look into. From now on, OECD and the western world realised that air pollution might be a problem of international political dimensions.
Odén’s discoveries were to a large extent based on the regional precipitation networks that were running in Sweden and Europe. In 1947, the Swedish scientist Hans Egnér set up a Swedish network to investigate the importance of atmospheric deposition for the fertilisation of crops. In 1954, the network was expanded forming the European Air Chemistry Network (EACN) through initiatives by Egnér, Carl Gustav Rossby, and Erik Eriksson (Egnér and Eriksson 1955 ; see also Engardt et al. 2017 ). Data from these networks together with a Scandinavian surface water network set up by Odén in 1961 formed the basis for Odén’s observations on the ongoing acidification (Odén 1968 ).
Acid rain and many of its ecological effects were, however, recognised long before 1967–1968. In fact, many features of the acid rain phenomenon were first discovered by an English chemist, Robert Angus Smith, in the middle of the nineteenth century! In 1852, Smith published a detailed report on the chemistry of rain in and around the city of Manchester, England. Twenty years later, in a very detailed book titled “Air and Rain: The Beginnings of a Chemical Climatology”, Smith first used the term “acid rain” and enunciated many of the principal ideas that are part of our present understanding of this phenomenon (Smith 1872 ). Unfortunately, however, Smith’s pioneering book was substantially ignored by nearly every subsequent investigator.
In Norway salmon catches decreased substantially in the early 1900s and in 1927, Professor Knut Dahl hypothesised that acidification of surface waters could be a factor of importance for the extinction of fish. Later Alf Dannevig assumed that “The acidity of a lake is dependent on the acidity of the rainwater and the contributions from the soil” (Dannevig 1959 ).
Based on detailed field observations and experimental studies both in England and in Canada, beginning in 1955 and continuing through 1963, Eville Gorham and his colleagues built a significant foundation for contemporary understanding of the causes of acid precipitation and its impacts on aquatic ecosystems, agricultural crops, soils, and even human health (Gorham 1981 ; Cowling 1982 ). Thus, Gorham and his colleagues as well as Dahl and Dannevig had discovered major aspects of the causes of contemporary changes in the chemistry of atmospheric emissions and deposition and their effects on aquatic ecosystems.
But these pioneering contributions, like those of Smith a century earlier, were not generally recognised—neither by scientists nor by society in general. Gorham’s researches, like those of Smith a century before, were met by what Gorham himself acknowledged as a “thundering silence”, not only by the scientific community, but also by the public at large.
It was not until 1967 and 1968 when Svante Oden published both his deliberatively provocative article in Dagens Nyheter and his carefully documented Ecological Committee Report (Odén 1968 ) that the acid rain problem was brought to both public and scientific considerations. The report included a huge body of scientific and policy-relevant evidence that long-distance transport and deposition of acidifying pollutants were causing significant environmental and ecological impacts, even in countries far away from pollutant-emitting source areas in other countries.
The Swedish case study and the OECD project
Two years after Odén’s article, the Swedish government decided to prepare a “case study” as a contribution to the UN Conference on the Human–Environment in Stockholm 1972 (Royal Ministry of Foreign Affairs and Royal Ministry of Agriculture 1972 ). Bert Bolin at the Stockholm University was appointed chair of the study, which included Svante Odén, Henning Rodhe, and Lennart Granat as authors. The report included a broad environmental assessment of the sulphur emission problem including sources, atmospheric and surface water chemistry, and effects on ecosystems and materials. Finally, it also included scenarios and estimated costs for environmental damage and control; in fact it was probably the first full systems analysis of an environmental problem.
In the report, a first estimate was made of the relative contributions of domestic and foreign emissions to the sulphur deposition in Sweden (Rodhe 1972 ). Estimates were also made of the effects of sulphur emissions on excess mortality and showed that 50% of the Swedish lakes and rivers would reach a critical pH level within 50 years (assuming continuation of present emission trends). Even if some aspects of the report received criticism, the overall case study was well received by the UN conference and in its final report (see http://www.un-documents.net/aconf48-14r1.pdf ) regional air pollution was explicitly mentioned (§85) with a citation of the Swedish study.
The Swedish initiative in the OECD resulted in a collaborative project to investigate the nature and magnitude of the transboundary transport of emitted sulphur dioxide over Western Europe, in which 11 countries participated. To initiate the project, a Nordic organisation on scientific research, Nordforsk, was asked to plan and develop methodologies for the investigation. Scientists and institutions from Norway, Sweden, Denmark, and Finland established an expert group in April 1970, which became central for the development and implementation of the OECD project. The Norwegian Institute for Air Research (NILU) offered through its director Brynulf Ottar to coordinate the project. The project included emission inventories, measurements of atmospheric concentrations, and deposition, together with model development and application for the assessment of the transport. A key part of the model calculations was to prepare the so-called “blame matrices”, through which the transport of pollutants between countries could be quantified.
The main conclusion from the OECD project, published in 1977, was that “Sulphur compounds do travel long distances in the atmosphere and the air quality in any European country is measurably affected by emissions from other European countries” (OECD 1977 ). Even if there still were hesitations about the magnitude of the transport, the common opinion was that transboundary transport of air pollution is an issue that needs collaboration across national borders. These conclusions paved the road for a pan-European scientific collaboration on air pollution, the European Monitoring and Evaluation Programme (EMEP) starting in 1977. The findings from the project also formed the basis for the Air Convention (Table 1 ). EMEP was already from the beginning included in the Convention as a key element, strongly contributing to the scientific credibility of the policy work.
Threats to forests boosted the interest
In 1980, the German scientist Bernhard Ulrich warned that European forests were seriously threatened from atmospheric deposition of sulphur. From his long-term experiments in the Solling area, he concluded that the high deposition of atmospheric pollutants had seriously changed the soil chemistry (Ulrich et al. 1980 ). Ulrich pointed to the links between sulphur deposition and the release of inorganic aluminium. His findings became a policy issue not only in Germany but in Europe as a whole, and even in North America. The alarms—often exaggerated—went like a wildfire through media and changed many attitudes throughout Europe. Newspapers were filled with photos of dying forests, in particular from “The Black Triangle”, the border areas between Poland, East Germany, and Czechoslovakia, characterised by large combustion of brown coal with high sulphur content. Forest inventories showed crown thinning and other effects on forests, but it became difficult to finally determine that acid deposition was the (only) cause for the observed effects.
The increasing interest in regional air pollution also paved the way for the first international agreement on emission control under the Air Convention. As a start, countries with a large interest in taking actions formed a “club” under the Convention, aiming for a 30% reduction in emissions. This ambition then became the basis for the first emission reduction protocol, the Sulphur Protocol signed in 1985. While Germany and some other West European countries acted almost immediately on the alarms, the progress in emission control in Eastern Europe was very slow during the 1980s, even though several of these countries signed the protocol. In fact, substantial decrease in emissions did not take place in the East until after the break-down of the communist regimes and the industrial collapse around 1990.
Critical loads and advanced policies
One of the most well-known characteristics for the control of the acid rain problem is the concept of Critical Loads (Nilsson 1986 ; Nilsson and Grennfelt 1988 ). The Executive Body, the highest decision-making body of the Air Convention, decided in 1988 that new negotiations on the control of sulphur and nitrogen emissions should be based on critical loads, and all parties to the Convention were requested to prepare their own critical load maps. The Netherlands offered to take a lead and prepared mapping manuals and initiated an international network, which became crucial for the scientific and policy acceptance of the concept (Hettelingh et al. 1991 ; De Vries et al. 2015 ; Fig. 4 ). (The critical loads concept is further discussed later in the paper)
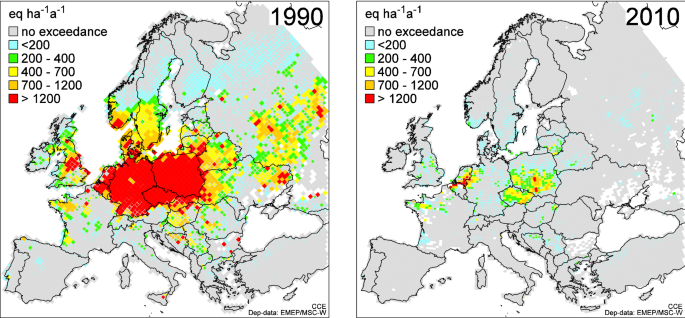
The outcome of emission control of SO2, NOx, and NH3 between 1990 and 2010 presented as maps on exceedance of critical loads of acidity. Such maps have played an important role for illustrating outcomes of future policies as well as of actions taken (from Maas and Grennfelt 2016 )
When critical loads became a basis for further protocols, Integrated Assessment Models (IAMs) offered a method to calculate how to achieve a prescribed ecosystem effect reduction in the most cost-effective way. A couple of different approaches were developed, but the model at the International Institute for Applied Systems Analysis (IIASA) became the official model on which the Second Sulphur Protocol signed in 1994 was agreed (Hordijk 1995 ).
When revising or developing a new protocol for nitrogen oxides the concept could, however, not be used in the same way as for sulphur and acid deposition, since the NO x emissions contributed to several effects and, in addition, a strategy would need to take additional compounds into account. Instead, a more advanced approach was suggested by which both several effects and several compounds could be considered simultaneously (Grennfelt et al. 1994 , Fig. 5 ). IIASA and other bodies under the Air Convention were asked to develop an integrated assessment model that fitted into a broader approach and a more comprehensive model was developed, which made it possible to simultaneously take into account the effects of acidic deposition, nitrogen deposition, and ozone—the so-called multi-pollutant, multi-effect approach. The calculations became the basis for the Gothenburg Protocol (GP) that was signed in 1999 (Amann et al. 1999 ). The GP and the parallel EU National Emissions Ceilings (NEC) Directive from 2001 outlined control measures for 2010 and beyond.
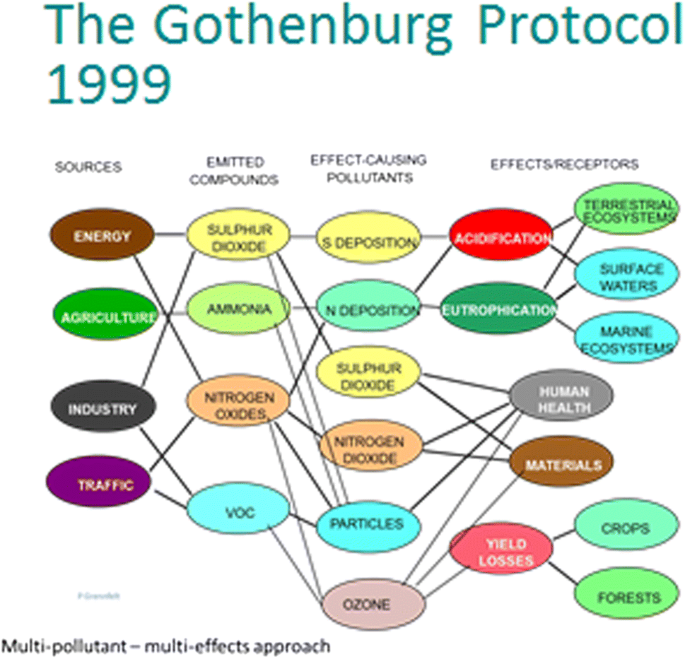
Links between sources and effects used as an illustration in the preparation of the Gothenburg Protocol. From Grennfelt et al. 1994
After 2000—Health effects and integration with other policies became main drivers
The basis for the GP was almost entirely ecosystem effects. Around 2000, however, public health effects from air pollution became increasingly important. Large epidemiological studies indicated that air pollution was a significant source of premature deaths and that particles were a main cause of the health effects (WHO 2018 ). When the European Commission started its work to revise the NEC directive, health effects became central and the Air Convention followed. Further studies have supported the role of air pollution for health effects and when the GP was finally revised in 2012, health effects dominated as a policy driver for the establishment of national emission ceilings, and for the first time particulate matter was included in an international protocol (Reis et al. 2012 ).
When considering further actions after signing the GP in 1999, it was realised that for some pollutants under the Air Convention, emission control needed to be considered over larger geographic scales than Europe and North America alone. Ozone was of particular importance, since long-term objectives in the form of critical levels and public health standards could not be reached without taking into account sources outside the areas considered so far. Future policies therefore needed to include the ozone precursors methane and to some extent carbon monoxide. A task force on Hemispheric Transport of Air Pollution (HTAP) was set up under the Convention in 2004, with a primary objective to quantify the intercontinental transport of pollutants. The outcome of its work clearly showed the importance of considering air pollution in a wider geographic perspective than had been done so far (Dentener et al. 2010 ).
Climate change has for more than a decade become an issue of increasing interest for air pollution science and policy. In many cases, the emission sources are the same and there are obvious co-benefits (and some trade-offs) in handling them together. One aspect that has received large interest is the option to decrease short-term temperature increase through control measures directed towards atmospheric pollutants that also contribute to the warming of the atmosphere, in particular black carbon and methane (for methane both by itself but also as a tropospheric ozone precursor) (Ramanathan et al. 2001 ). Compounds contributing to both air pollution effects and to the radiation balance in the atmosphere have been named Short Lived Climate Pollutants (SLCPs). SLCPs thus also include compounds that are cooling the atmosphere, i.e. small secondary aerosols, e.g. sulphate particles. Recent research has focused on a better understanding of these compounds’ contribution to both air pollution and climate as well as on opportunities for selective control of these compounds (e.g. Sand et al. 2016 ).
Reactive nitrogen species are another group of compounds that has received increased attention after the turn of the century. Around 2006 several initiatives were taken in Europe, including a special task force on Reactive Nitrogen under the Air Convention, a large-scale EU project on nitrogen, and the preparation of a European Nitrogen Assessment (Sutton et al. 2011 ). Here nitrogen was considered both as a traditional atmospheric pollutant and within a societal and industrial context. A cascade perspective, where one fixed nitrogen molecule could contribute to a series of effects before it returns to molecular nitrogen again, was introduced (Galloway et al. 2003 ). The studies have pointed to the importance of the agricultural sector for the intensification of reactive nitrogen cycling, determined by food production mechanisms and dietary choices.
North America
In North America, the acid rain problem developed to a large extent in parallel with the situation in Europe. Lake acidification became already from the beginning a main driver, and monitoring programmes were set up both in the United States and Canada (Driscoll et al. 2010 ). The US National Atmospheric Deposition programme (NADP) started in 1976 and is still running. Both countries have taken part in the Air Convention activities and have signed most of the protocols and achieved decreases in SO 2 emissions of the order of 80% between 1980 and 2015. The US has however taken a different approach with respect to policy in comparison to Europe. Instead of developing a strategy based on integrated assessment modelling, it was decided to establish an emissions trading programme for the large electric generation sources under the Clean Air Act (See also UNECE 2016 ).
Characteristics of the science–policy interactions
In this section we will, from a science–policy perspective, briefly discuss some characteristics of the history of acid rain and transboundary air pollution that have become central for the international collaboration, not only on air pollution but also for international environmental collaboration in general. We will bring up monitoring, modelling, and data collection (including field experiments and long-term studies carried out in order to understand and quantify effects to ecosystems), development of bridging concepts that have served the implementation of strategies, and finally the dynamics in the science–policy interactions.
Monitoring, modelling, and data collection
Monitoring of atmospheric concentrations, deposition, and ecosystem effects has been a key for understanding the causes, impact, and trends in acid rain, both in Europe and North America and later in other geographic areas (Table 2 ). The original EMEP network has since the start over 40 years ago formed a broad atmospheric monitoring system. The originally established simple monitoring stations have over time been complemented with more advanced monitoring, and some stations are today advanced atmospheric chemistry platforms with continuous collection of a multitude of atmospheric parameters (Fig. 6 ). The EMEP database is nowadays widely used for a variety of scientific purposes including computation of long-term trends, exposure estimates, and as a basis for modelling. EMEP has also become a model for monitoring networks related to other geographical regions, conventions, and purposes. One example is the acid deposition monitoring network in East Asia (EANET). It is obvious that having a qualified centre for data collection and storage, standardisation, and intercalibration of methods has served the international policy system extremely well. Its open nature is part of the success. The financial support to EMEP, regulated through a separate protocol, has been fundamental for the development and progress of the monitoring activities.

Atmospheric monitoring stations have been of importance for understanding the long-range transport and chemical conversions of atmospheric pollutants. Pallas air pollution background station in Northern Finland (Photo Martin Forsius)
Monitoring of air pollution effects in a systematic way under the Air Convention started a few years later than EMEP and was organised through so-called International Cooperative Programmes (ICPs). Separate programmes were set up for forests, waters, vegetation (primarily ozone), materials, and integrated monitoring. A separate ICP was set up for developing critical load methodologies and coordinating European-scale mapping activities (ICP Modelling and Mapping). The ICPs are of great importance for general understanding of the magnitude and geographical distribution of the effects and for showing how decreases in emissions have led to beneficial conditions in ecosystems and decreased material corrosion (Maas and Grennfelt 2016 ). Ecosystem monitoring is also important for the development and verification of ecosystem models. Since their start, the responsibility for the ICPs has been taken by different parties of the Air Convention (Table 2 ). The distributed responsibility has been of large importance for the establishment of networks of monitoring sites among the Convention parties, but the system has not had a stable financial support in the same way as for EMEP. This has resulted in the lack of a common source for easily accessible data or adequate resources for standardisation and intercalibration.
Monitoring and other data collection (i.e. emissions and critical loads) under the Air Convention are responsibilities of every country, and data are then used for the assessments on the Convention level as well as for the development of EU air pollution policies. The bottom-up process in data collection is important for the development of national expertise and, not the least, for the establishment of national policies. In this way, direct communication links between the science and the policy levels within countries have evolved.
Numerical modelling of atmospheric pollution is also a long-term commitment under EMEP. The atmospheric chemistry models are necessary for the understanding of the nature of transboundary transport but also to make budget estimates of the exchange of pollutants over Europe and North America, and later on a hemispheric scale. The Meteorological Synthesizing Centre West at the Norwegian Meteorological Institute together with the Eastern Centre in Moscow took the lead in this work. In addition to calculating transboundary fluxes, the centres are important for coordinating modelling efforts done by other groups, forming a basis for scrutinising models and support further modelling.
Field experiments and long-term studies—a way to understand processes and trends, and to visualise the problems
Some of the most important and reliable findings regarding acid rain and its effects on ecosystems emanate from long-term field experiments. These experiments, which are known from the sites where they are run, include Hubbard Brook (US), Solling (Germany), Risdalsheia (Norway) and Lake Gårdsjön (Sweden) (Fig. 7 ). The studies there have shown how acid deposition and the impact of other air pollutants have changed the ecosystems, but also how ecosystems respond to decreased emissions (e.g. Wright et al. 1988 ; Likens et al. 1996 ). A central feature in all these field experiments was the establishment of ion budgets, from which the chemical effects on acid deposition can be analysed and understood (Reuss et al. 1987 ).

Field experiments have played an important role for the overall understanding of the interactions between atmospheric deposition and ecosystem effects. The photo illustrates the covered catchment experiment to study the recovery of ecosystems at reduced emissions in Risdalsheia Norway (Photo NIVA)
In the intense research period during the 1970s and 1980s, a number of large-scale research programmes and experiments of temporary nature were set up, some of them in connection with the above-mentioned sites. The first research programme of some magnitude was the Norwegian programme “Acid precipitation—effects on forest and fish” (SNSF), which run between 1972 and 1980 (Overrein et al. 1981 ). At that time the scientific understanding was limited, and the programme received a lot of attention. The results were important for the general acceptance that long-distance transport of sulphur caused acidification of surface waters, with a serious die-off of fresh water fish populations (salmon and trout) as a main consequence. On the other hand, the studies on Norwegian forests did not give any significant evidence for acid rain effects. The SNSF project was a joint effort across disciplinary and organisational boundaries, with scientists mainly from the research institute sectors outside of traditional academia. This project served as a model for later research programmes and provided educational opportunities for a new generation of scientists working together on all aspects of the acid rain issue—emissions and their control, atmospheric transport and deposition, impact on ecosystems, health and materials, and finally development of pollutant-control policies.
The long-term field experiments served another important task. The sites became exhibition platforms, at which policymakers, experts, scientific journalists, and leaders of non-governmental organisations (NGOs) and others can be informed about the problem directly on site. During the most intense period in the 1980s and early 1990s, politicians and industry leaders, often directly involved in decisions on the highest levels, visited many of these experimental sites. For example, US congress members travelled across Europe to see and understand the issue in preparation for the 1990 amendment of the Clean Air Act.
Bridging concepts and approaches
Concepts developed, such as critical loads and similar approaches, formed links between science and policy, and were essential for the understanding and scientific legitimacy of the policy measures. These concepts also formed a basis for priority setting in agreements under the Convention and the EU, but also to some extent for national policies. Even “acid rain” can be considered as a bridging concept. While the acidity from sulphur and nitrogen compounds is threatening ecosystems through a chemical change, the expression also gives the impression of a threat to the life-giving rain, a fundamental necessity for life on Earth.
The quantification of transboundary fluxes was very important politically. The establishment of national budgets and so-called blame matrices formed the first bridging concept. The development of mathematical models to calculate source–receptor relations was a scientific challenge but when the annual tables were prepared showing the interdependence between countries with respect to atmospheric emissions and deposition, they served as an important basis for the need for common action. Anton Eliassen, the leader of the modelling centre at the EMEP Meteorological Synthesising Centre West (MSC-W) during many years (the Eastern center is in Moscow—MSC-E), was key to this development as well as for the communication of the results to policymakers.
As earlier mentioned, critical loads played an outstanding role for the development of the more advanced strategies leading to the Second Sulphur Protocol and the GP. Critical loads formed a successful link between science and policy that became crucial for the negotiations and agreements. The concept, first discussed in 1982, was taken from the original idea to application quite quickly during the 1980s. The Swedish expert Jan Nilsson was a key leader for the success of the concept, and the Nordic Council of Ministers played a unique role for forming the links between science and policy. Through a series of workshops involving both key scientists and key policymakers, the concept gained the legitimacy on which policies were developed. According to Jan Nilsson, it all started with requests from both industry and negotiators to have a sounder base for emission control, something that could express the long-term objectives for emission control policies. The concept was first met by scepticism, not least from scientists, but after a couple of workshops, the interest turned around and the concept became widely accepted (Nilsson 1986 ; Nilsson and Grennfelt 1988 ). When critical loads were included in the plans for the next rounds of the sulphur and nitrogen protocols in 1988, it changed the way the Air Convention operated.
The application of the critical loads concept has encouraged intense research over several decades where the main objective has been to find simple chemical parameters that can mimic the (often biological) real effects or effect risks. For lake acidification, where the effects of dissolved aluminium on fish often were chosen as the main biological effect, the acidity of the water, mostly expressed as acid neutralising capacity (ANC), is used (e.g. Henriksen et al. 1989 ; Forsius et al. 2003 ; Posch et al. 2012 ). For forests, where the toxicity of aluminium to tree roots is considered as critical, the Al 3+ to Ca 2+ ratio in soil water has become the main effect parameter (Sverdrup et al. 1990 ; de Vries et al. 1994 ).
Integrated assessment modelling (IAM) also has been a bridging concept. The idea of applying systems analysis goes back to the work at IIASA in the beginning of 1980s. A conceptual model was formulated by Joseph Alcamo, Pekka Kauppi, and Maximilian Posch for the interactions between emissions, their control (including costs), and the effects on ecosystems (Alcamo et al. 1984 ). Their work of bringing together the scientific knowledge to a comprehensive systems analysis tool formed a new way of framing environmental policies. Under the leadership of Leen Hordijk, the new idea was introduced to and accepted by the policy side, which had asked for more targeted methods for policies than simple percentage decreases in amounts of emissions. IAMs as a policy-supporting concept was then taken further by Markus Amann, who led the development of the more advanced RAINS (later GAINS) models that were used as a basis for the GP and later agreements (Amann et al. 2011 ). From the strategies strictly directed at ecosystem effects, the approach is now widened to include health effects, local air pollution impact, climate policies, and reactive nitrogen.
All the bridging concepts are to varying degrees dependent on underlying models, assumptions, and simplifications. For these to be accepted among policymakers, it is important to keep transparency and confidence in the underlying data and to scientifically evaluate and scrutinise them. This is particularly important for the IAMs, which are the final step in a chain of inputs (Fig. 8 ). The models have often been criticised, not least from industry and other stakeholders that are questioning the priorities that result from the IAM calculations. IIASA, as a provider of the model calculations, has, however, been transparent, and countries and stakeholders have always had the option to re-check data and take this into account when developing their own negotiation positions.
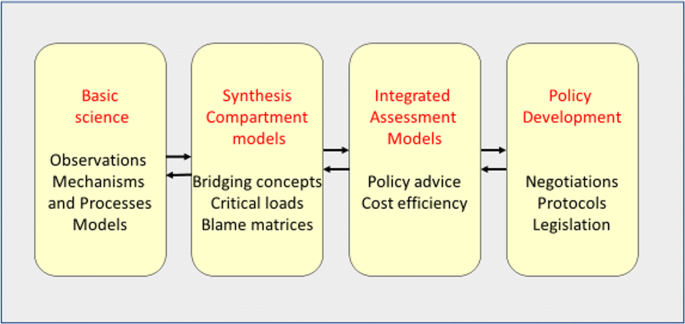
The scientific support to regional air pollution policies consists today of a series of steps. The policy side may often only see the integrated assessment step and not realise that the legitimacy of the use of scientific support builds on an advanced system of underlying research and development
Forming science–policy credibility
In all interactions between science and policy, it becomes crucially important to maintain scientific credibility. The close involvement of scientists has been a signature of the Air Convention. Scientists have always had a role at the policy meetings, communicating results from basic scientific research over outcomes of monitoring and inventories to presenting options for control strategies. Scientists have in this way taken the responsibility to move scientific knowledge into the policy system and presenting results in a way that has been understandable and useful for the policy work. The role of the scientists has been as honest brokers , not that of issue advocates to follow the terminology of Pielke ( 2007 ). The leadership from the policy side and its sensitivity to changes in the underlying science and observations of new problems have also been important, and have resulted in repeated changes in the framings of the Air Convention to adapt to new situations: going from an initial framing around sulphur and acidification, through extension to eutrophication, human health, materials, crops, biological diversity, and finally to links to climate, urban air quality, and societal changes. A balanced interplay between the two communities has in this way been developed and maintained over time.
Another factor is the building of networks. The strong networks of scientists and policymakers pushed the politicians. The whole field of international diplomacy during these four decades of the Convention is built on incremental developments forming protocols of increasing capability to solve specific environmental issues by cutting emissions in a cost-effective way.
Future challenges
New approaches necessary.
International air pollution control is by many considered as a success story. However, the success is in many ways limited to Europe and North America and a few additional industrialised countries (including Japan and Australia), where emissions of sulphur dioxide, nitrogen oxides, VOCs, and some other compounds have been decreased significantly (Maas and Grennfelt 2016 ). But even in the areas, where air pollution has been a top priority for several decades, air pollution remains a problem. Ecosystem effects, which were the main reason for the establishment of the Convention, are to some extent reduced, but the acidification effects of historical emissions will remain for decades (Wright et al. 2005 ; Johnson et al. 2018 ) and the emissions of ammonia have so far only been reduced by 20–30% in Europe and even less in North America. Looking at health effects, it is difficult to talk about success, when hundreds of thousands of inhabitants on both continents are predicted to meet an earlier death due to air pollution.
But the problem is even larger and more urgent when looking outside the traditional industrialised world. The focus is today on the large urban regions in the countries that are facing rapid population growth and industrialisation. Although large efforts now are being made to decrease sulphur emissions in China—the world’s leading sulphur emitter—major challenges remain. In India and several other countries, sulphur emissions are still increasing. Estimates indicate that more than four million people die prematurely due to outdoor air pollution globally ( https://www.who.int/airpollution/ambient/health-impacts/en/ ). It is assumed that fine particles (PM2.5) are a main cause for the health effects. The new and great challenge is therefore to control air pollution in relation to health risks, in particular by decreasing exposure to the small particles.
There is, however, a risk that control measures will only to a limited extent focus on the right sources and the right measures. In Paris, several air pollution episodes with high concentrations of particles have occurred during recent years. At first, these episodes were considered to be caused essentially by local emissions. More thorough analysis has, however, shown that they were to a large extent caused by regional emissions and buildup of high concentrations over several days when urban emissions of oxides of nitrogen from traffic mix with ammonium emissions from surrounding agricultural areas to form particulate nitrate. Similar situations are also often encountered in urban regions in developing countries, e.g. by agricultural waste burning, and need to be considered. Air pollution problems are, as previously mentioned, also linked to intercontinental and hemispheric scales.
It is also obvious that the research communities within air pollution and climate change need to work more closely together. Health aspects are of importance both from air pollution and climate change perspectives, and heat waves carry poor air quality as winds are often very low and the atmospheric boundary layer stagnant. During heat waves, the soil and vegetation dry up and increase the likelihood of fires, which also can cause severe air pollution, as seen in wildfires around the world (e.g. California in 2018).
Despite the large progress in atmospheric and air pollution science, basic questions still need further investigations to develop the best policies. Such areas include a better understanding of health effects from air pollution, nitrogen effects to ecosystems, and air pollution interactions with climate through carbon storage in ecosystems and impacts on radiation balances. Modelling is a scientific area where much progress has been made and where increased computer power, as in climate change research, has allowed integration of atmospheric chemistry into the climate models formulated as Earth system models, coupling the atmosphere, ocean, the land surface, cryosphere, biogeochemical cycles, and human activities together. This has allowed studying air pollution and climate change simultaneously. The modelling approach can be further developed when observations are designed to map Earth system component boundaries to understand and quantify the flows and interactions between different compartments, including terrestrial and aquatic ecosystems. Air pollution should be an integrated part of such models. In this context, global-scale concepts such as “planetary boundaries” and “trajectories of the Earth system vs. planetary thresholds” have been developed (Rockström et al. 2009 ; Steffen et al. 2018 ).
Solutions are available; driving forces and investments are lacking
In 2016, the Air Convention launched a scientific report “Towards Cleaner Air”, in which the actual air pollution situation within the UNECE region was updated (Maas and Grennfelt 2016 ). The report also presented future challenges and ways forward to solve the air pollution problems. It also showed that solutions are available for most of the identified problems at affordable costs below the health and ecosystem benefits of the control actions.
Even if solutions are available, many parts of the world are facing large problems in implementing them. There are several reasons, but often there is a lack of knowledge and resources. This is particularly true in many developing countries. Another reason is the lack of political interest. Air pollution is still not of top priority among politicians, even if there is overwhelming evidence that air pollution is one of the most common causes of shortened life expectancies. Another reason may be that other interests (e.g., industry and agriculture) are forming strong lobbying forces delaying actions.
Air pollution is a problem that cannot be seen in isolation. Future policies need to take into account climate change and climate change policies. Whereas some air pollutants—in particular black carbon particles—contribute to warming, others, including sulphate particles, tend to cool the climate. A reduction in sulphur dioxide emissions, although highly desirable from health and ecosystems perspectives, will therefore contribute to warming. On the other hand, a reduction of black carbon will be a win–win solution. It is also important to see air pollution control in the perspective of sector policies, such as energy, agriculture, transportation, and urban planning in order to meet the challenges to decrease air pollution problems.
Internationally coordinated actions and infrastructures are keys for success
The perspective of international cooperation on air pollution is changing. Policy development is no longer limited to long-range transport in line with that developed under the Air Convention. The ranking of air pollution as a top ten cause of premature deaths in the world has given high priority to the issue within fora such as the WHO and UN Environment. Both organisations have adopted resolutions calling for actions (WHO 2015 ; UN Environment 2017 ). Additional initiatives are taken by other organisations, such as the World Meteorological Organisation (WMO), the Climate and Clean Air Coalition (CCAC), and the Arctic Monitoring and Assessment Programme (AMAP). WMO is particularly important as a global technical agency for weather and climate observations, research and services, and it is rapidly developing its regional and global capacities in Earth system observations, modelling, and predictions to the benefit of mitigating a range of environmental threats and for global use. The research is done in large programmes like Global Atmosphere Watch (GAW) and the World Weather Research Programme (WWRP). Even if the starting point and modes of action can be different, all initiatives are aiming for the same goal, cleaner air. It is also worth mentioning the initiative taken by the International Law Commission, under which a proposal for a Law for the Protection of the Atmosphere has been prepared ( http://legal.un.org/ilc/summaries/8_8.shtml ) but in the current international atmosphere there is a lack of political support to implement it. Our hope is that the situation will change soon—the initiative is too important to fail.
The UN has put forward a very strong agenda in order to reach the Sustainable Development Goals (SDGs), and air pollution is an integral part of several of the SGDs, like goal No 2: No Hunger, No 3: Good health and well-being, No 6: Clean Water, No 7: Affordable and clean energy, No 9: Industry, innovation and infrastructure, No 11: Sustainable cities and communities, No 13: Climate action, No 14: Life below water, No 15: Life on Land, No 16: Peace and Justice, and No 17: Partnerships for the Goals. The approach taken to develop multiple pollutant—multiple impacts protocols under the Air Convention can serve as important learning ground to meet the ambitions of many of the SDGs. Air pollution plays an integral role in the evolution of the food production and ecosystem services, the health of the population, the shape of the energy and transportation systems, and the availability of clean water. Climate change is a very significant common and cross-cutting factor.
The Air Convention has taken some steps in promoting air pollution on a wider scale. Due to its long history and well-developed structure, it has taken a role of making sure that international organisations having air pollution on its agenda are aware of each other and to invite to further collaboration and development. Initiatives are taken both within the formal Convention structure and through dedicated workshops (UNECE 2018 ; Engleryd and Grennfelt 2018 ). The approach developed under the Air Convention, which has proven successful in linking scientific evidence, monitoring, and integrated assessment modelling directed towards cost-effective solutions, may also serve as a working model for environmental problems in other fields.
These new international initiatives have a strong emphasis on policy development. The experience from the 50 years of international air pollution development is the value of well-defined scientific objectives and activities supporting policy. The increased interest from WHO and UN Environment is welcome and there are expectations of an active role from these organisations in combatting the situation in many parts of the world. However, for these organisations, air pollution is just one of several priority areas, and priorities may change. Further, none of these organisations are likely able to set up advanced infrastructures with respect to emission inventories, monitoring, and research. Here WMO needs to live up to its mission and capitalise on global research and development efforts and improve the global operational capability to observe, analyse, and forecast the development of the Earth system and its components, air pollution being an important part. This is in line with the WMO strategic plan and with fast growing capabilities in some countries and in global centres like The European Centre for Medium Range Weather Forecast (ECMWF). WMO, through GAW, is also developing a research-driven operational system (IG3IS) for top-down determination of greenhouse gas emissions, to complement the usual bottom-up-based inventories where emission factors and fuel consumption or production statistics form the basis for the emission estimates ( https://library.wmo.int/doc_num.php?explnum_id=4981 ). The Air Convention and the science support for the policy work there has been a model for the WMO ambitions on a global basis. However, current investments in these new capabilities are not enough to get the societal return they would offer.
Therefore, we see a need for developing long-lasting infrastructures that can continuously develop science-based control policy options, potentially as part of a wider network of global observatories for comprehensive monitoring of interactions between the planet’s surface and atmosphere (Kulmala 2018 ). Such a network should be able to support policies from local to the global levels. The challenge is how to organise and raise resources for scientific support on a wider scale. Financial institutions such as the World Bank and/or regional banks may step in and make sure that control measures and investments are made on a sound basis with respect to global air pollution.
There is also a need to mobilise new generations of scientists, scientists that are willing to cross boundaries and focus on thematic problems and to build legitimacy among policymakers (e.g. Bouma 2016 ). Today we have more developed and stronger political institutions to handle environmental problems, which may make it harder for scientists and individuals to influence and make a difference. It is also important to mobilise new generations of dedicated policymakers. Unfortunately, we also see that politicians often are questioning science and seeing science as just a special interest. Public awareness may be a key for forming stronger interests and put pressure on decision-makers. During the acid rain history, NGOs played an important role in driving the awareness at a wider scale than local or national actions and could be important for a more global movement towards cleaner air. We also see the need for a deeper responsibility not only from politicians but also from industry. The so-called “diesel gate” exposed the cynic view from parts of the industry to peoples’ health, which hopefully will not occur in the future. Instead we hope that it was an eye-opener and that industry instead can play a role as a forerunner and a positive power for a cleaner atmosphere.
Final remarks
The Acid Rain history taught us that when science, policy, industry, and the public worked together, the basis was formed for the successful control of, what was considered, one of the largest environmental problems towards the end of the last century. We learnt from experience that science-based policy advice worked well when the best available knowledge was provided, and used to understand the specific problems, generate, and evaluate the policy options and monitor the outcomes of policy implementation.
However, the world does not look the same today, and we cannot just apply the ways the international science community worked together then on today’s problems. But there are lessons to be learnt. Most important is the building of mutual trust between science advisers and policymakers, and that both communities are honest about their values and goals. In this way, a fruitful discussion around critical topics within society can be formed. The advice works best when it is guided by the ideal of co - creation of knowledge and policy options between scientists and policymakers (SAPEA 2019 ).
Amann M., I. Bertok, J. Cofala, F. Gyarfas, C. Heyes, Z. Klimont, and W. Schöpp. 1999. Integrated assessment modelling for the protocol to abate acidification, eutrophication and ground-level ozone in Europe, Lucht & Energie 132, November 1999.
Amann, M., I. Bertok, J. Borken-Kleefeld, J. Cofala, C. Heyes, L. Höglund-Isaksson, Z. Klimont, B. Nguyen, et al. 2011. Cost-effective control of air quality and greenhouse gases in Europe: Modeling and policy applications. Environmental Modelling & Software 26 (12): 1489–1501.
Article Google Scholar
Alcamo, J., P. Kauppi, M. Posch and E. Runca. 1984. Acid rain in Europe: A framework to assist decision making. Working paper WP-84-32, International Institute for Applied Systems Analysis, Laxenburg, Austria.
Bouma, J. 2016. Implications of the nexus approach when assessing water and soil quality as a function of solid and liquid waste management. In Environmental resource management and the nexus approach , ed. H. Hettiarachchi and R. Ardakanian, 179–209. New York: Springer.
Chapter Google Scholar
Cowling, E.B. 1982. Acid precipitation in a historical perspective. Environmental Science & Technology 16: 110A–123A.
Article CAS Google Scholar
Dannevig, A. 1959. Influence of precipitation on river acidity and fish populations. Jeger og Fisker 3: 116–118.
Google Scholar
Dentener, F., T. Keating, and H. Akimoto. 2010. Hemispheric transport of air pollution, part A. air pollution studies no. 17, Convention on long-range transboundary air pollution, UNECE.
De Vries, W., G.J. Reinds, and M. Posch. 1994. Assessment of critical loads and their exceedance on European forests using a one-layer steady-state model. Water, Air, and Soil Pollution 72: 357–394.
De Vries, W., J.-P. Hettelingh, and M. Posch, eds. 2015. Critical loads and dynamic risk assessments: Nitrogen, acidity and metals in terrestrial and aquatic ecosystems. Environmental pollution series, Vol. 25. Dordrecht: Springer. ISBN 978-94-017-9507-4; https://doi.org/10.1007/978-94-017-9508-1 .
Driscoll, C.T., E.B. Cowling, P. Grennfelt, J.M. Galloway, and R.L. Dennis. 2010. Integrated assessment of ecosystem effects of atmospheric deposition: Lessons available to be learned. EM Magazine , November 2010: 6–13.
Engleryd, A. and P. Grennfelt. 2018. Saltsjöbaden VI workshop. Clean air for a sustainable future—goals and challenges. Nordic Council of Ministers, TemaNord 2018:540, Copenhagen, ISSN 0908-6692. http://saltsjobaden6.ivl.se/finalreport.4.14bae12b164a305ba11c38f.html .
Egnér, H., and E. Eriksson. 1955. Current data on the chemical composition of air and precipitation. Tellus 7: 134–139.
Engardt, M., D. Simpson, M. Schwikowski, and L. Granat. 2017. Deposition of sulphur and nitrogen in Europe 1900-2050. Model calculations and comparison to historical observations. Tellus B 69: 1328945. https://doi.org/10.1080/16000889.2017.1328945 .
Forsius, M., J. Vuorenmaa, J. Mannio, and S. Syri. 2003. Recovery from acidification of Finnish lakes: Regional patterns and relations to emission reduction policy. Science of the Total Environment 310: 121–132.
Galloway, J.N., J.D. Aber, J.W. Erisman, S.P. Seitzinger, R.W. Howarth, E.B. Cowling, and B.J. Cosby. 2003. The nitrogen cascade. BioScience 53 (4): 341–356.
Grennfelt, P., Ø. Hov, and R.G. Derwent. 1994. Second generation abatement strategies for NO X , NH 3 , SO 2 and VOC. Ambio 23: 425–433.
Gorham, E. 1981. Scientific understanding of atmosphere-biosphere Interactions: A historical review. Chapter 2. In Toward a better understanding of the ecological consequences of fossil fuel combustion . National Research Council, 9–21. 1981. Washington, DC: The National Academies Press. https://doi.org/10.17226/135 .
Henriksen, A., L. Lien, B.O. Rosseland, T.S. Traaen, and I.S. Sevaldrud. 1989. Lake acidification in Norway—present and predicted fish status. Ambio 18: 314–321.
Hettelingh, J.-P., R.J. Downing, and P.A.M. de Smet. 1991. Mapping critical loads for Europe, CCE Status Report 1 1991, Coordination Center for Effects, National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Hordijk, L. 1995. Integrated assessment models as a basis for air pollution negotiations. Water, Air, and Soil pollution 85: 249–260.
Johnson, J., E. Graf Pannatier, S. Carnicelli, C. Cecchini, N. Clarke, N. Cools, K. Hansen, H. Meesenburg, et al. 2018. The response of soil solution chemistry in European forests to decreasing acid deposition. Global Change Biology 24: 3603–3619.
Kulmala, M. 2018. Build a global Earth observatory. Nature 553: 21–23.
Likens, G.E., C.T. Driscoll, and D.C. Buso. 1996. Long-term effects of acid rain: Response and recovery of a forested ecosystem. Science 272: 244–246.
Maas R. and P. Grennfelt, eds. 2016. Towards cleaner air. Scientific assessment report 2016. EMEP steering body and Working Group on effects of the convention on long-range transboundary air pollution, Oslo.
Nilsson J., ed. 1986. Critical loads for nitrogen and sulphur. Report from a Nordic working group, Nordic Council of Ministers, Miljø rapport 1986:11, Stockholm.
Nilsson, J. and P. Grennfelt, eds. 1988. Critical loads for sulphur and nitrogen. Report of a workshop held in Skokloster, Nordic Council of Ministers, Miljø rapport 1988:15, Stockholm.
Odén, S. 1968. The acidification of air precipitation and its consequences in the natural environment. Bulletin Ecological Research Commission. NFR. Translation Consultants Ltd., Arlington VA.
OECD. 1977. The OECD programme on long range transport of air pollutants . Paris: Measurements and Findings.
Overrein, L.N., H.M. Seip, and A. Tollan, eds. 1981. Acid precipitation–effects on forest and fish: Final report of the SNSF-project 1972-1980. Oslo Norway.
Pielke, R.A. Jr. 2007. The honest broker. Making sense of science in policy and politics. Cambridge: Cambridge University Press.
Posch, M., J. Aherne, M. Forsius, and M. Rask. 2012. Past, present and future exceedance of critical loads of acidity for surface waters in Finland. Environmental Science and Technology 46: 4507–4514.
Ramanathan, V., P.J. Crutzen, J.T. Kiehl, and D. Rosenfeld. 2001. Review paper: Aerosols, climate, and the hydrological cycle. Science 294: 2119–2124.
Reis, S., P. Grennfelt, Z. Klimont, M. Amann, H. Apsimon, J.-P. Hettelingh, M. Holland, A.-C. LeGall, et al. 2012. From acid rain to climate change. Science 338 (6111): 1153–1154.
Reuss, J.O., B.J. Cosby, and R.F. Wright. 1987. Chemical processes governing soil and water acidification. Nature 329: 27–32.
Rockström, J., W. Steffen, K. Noone, et al. 2009. A safe operating space for humanity. Nature 461: 472–475.
Rodhe, H. 1972. A study of the sulfur budget for the atmosphere over Northern Europe. Tellus 24: 128–138.
CAS Google Scholar
Rothschild, R.E. 2018. Poisonous skies. Acid rain and the globalization of pollution . Chicago: University of Chicago Press. ISBN 9780226634852.
Royal Ministry of Foreign Affairs and Royal Ministry of Agriculture. 1972. Air pollution across national boundaries. The impact on the environment of sulfur in air and precipitation. In Sweden’s case study for the United Nations’ Conference on the Human Environment, Stockholm.
Sand, M., T.K. Berntsen, K. von Salzen, M.G. Flanner, J. Langner, and D.G. Victor. 2016. Response of Arctic temperature to changes in emissions of short-lived climate forcers. Nature Climate Change 6: 286–289. https://doi.org/10.1038/nclimate2880 .
SAPEA. 2019. Making sense of science for policy under conditions of complexity and uncertainty. Science Advice for Policy by European Academies, 1–186, Berlin. https://doi.org/10.26356/MASOS .
Schöpp, W., M. Posch, S. Mylona, and M. Johansson. 2003. Long-term development of acid deposition (1880–2030) in sensitive freshwater regions in Europe. Hydrology and Earth System Sciences 7: 436–446. https://doi.org/10.5194/hess-7-436-2003 .
Sliggers, J. and W. Kakebeeke, eds. 2004. Clearing the air. 25 years of the convention on long-range transboundary air pollution. United Nations Economic Commission for Europe, Geneva 1–167.
Smith, R.A. 1872. Air and Rain, the Beginnings of a Chemical Climatology . London: Longmans Green.
Steffen, W., J. Rockström, K. Richardson, T.M. Lenton, C. Folke, D. Liverman, C.P. Summerhayes, A.D. Barnosky, et al. 2018. Trajectories of the earth system in the anthropocene. PNAS 115 (33): 8252–8259. https://doi.org/10.1073/pnas.1810141115 .
Sverdrup, H., W. de Vries, and A. Henriksen. 1990. Mapping critical loads, Nord 1990:98 . Copenhagen: Nordic Council of Ministers.
Sutton, M.A., C.M. Howard, J.W. Erisman, G. Billen, A. Bleeker, P. Grennfelt, H. van Grinsven, and B. Grizzetti (eds.). 2011. The European nitrogen assessment . Cambridge: Cambridge University Press.
Ulrich, B., R. Mayer, and P.K. Khanna. 1980. Chemical changes due to acid precipitation in a loess‐derived soil in Central Europe. Soil Science 130: 193–199.
UNECE. 2016. Towards cleaner air. Scientific assessment report 2016: North America. United Nations, Geneva. http://www.unece.org/index.php?id=42947&L=0 .
UNECE. 2018. Report from 38th session of the executive body for the convention on long-range transboundary air pollution Geneva 10–14 December 2018, Annex, 18–21, http://www.unece.org/fileadmin/DAM/env/documents/2018/Air/EB/ECE_EB.AIR_142-1902903E.pdf .
UN Environment. 2017. Preventing and reducing air pollution to improve air quality globally on air quality. Third UN Environment Assembly, 4–6 December 2017, Resolution 8. https://papersmart.unon.org/resolution/uploads/k1800222.english.pdf .
WHO. 2015. Resolution WHA68.8 health and the environment: addressing the health impact of air pollution. 68th World Health Assembly World Health Organisation, http://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_R8-en.pdf .
WHO. 2018. https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health .
Wright, R.F., E. Lotse, and A. Semb. 1988. Reversibility of acidification shown by whole-catchment experiments. Nature 334: 670–675.
Wright, R.F., L. Camarero, B.J. Cosby, R.C. Ferrier, M. Forsius, R. Helliwell, A. Jenkins, J. Kopáček, et al. 2005. Recovery of acidified European surface waters. Environmental Science and Technology 39: 64A–72A.
Download references
Acknowledgement
Open access funding provided by The Swedish Environmental Protection Agency. The Symposium could not have been arranged and this paper written without financial support from the Nordic Council of Ministers, the Swedish Environmental Protection Agency, the Mistra Foundation, and other organisations and institutes in the Nordic countries. We are also grateful to all participants of the Symposium and their contributions with background material for this paper. We also thank the two anonymous reviewers of the manuscript. Their comments greatly improved the quality of this paper.
Author information
Authors and affiliations.
IVL Swedish Environmental Research Institute, PO Box 53021, 40014, Gothenburg, Sweden
Peringe Grennfelt
Swedish Environmental Protection Agency, Virkesvägen 2F, 10648, Stockholm, Sweden
Anna Engleryd
Finnish Environment Institute, Latokartanonkaari 11, 00790, Helsinki, Finland
Martin Forsius
The Norwegian Meteorological Institute, P.O. Box 43, Blindern, 0313, Oslo, Norway
Øystein Hov
Department of Meteorology, Stockholm University, 10691, Stockholm, Sweden
Henning Rodhe
Department of Forestry and Environmental Resources, NC State University, 5211 Glenhope Court, Cary, NC, 27511, USA
Ellis Cowling
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Peringe Grennfelt .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Grennfelt, P., Engleryd, A., Forsius, M. et al. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 49 , 849–864 (2020). https://doi.org/10.1007/s13280-019-01244-4
Download citation
Received : 16 May 2019
Revised : 16 August 2019
Accepted : 19 August 2019
Published : 21 September 2019
Issue Date : April 2020
DOI : https://doi.org/10.1007/s13280-019-01244-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Air pollution
- Critical loads
- Integrated assessment modelling
- Policy development
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Special Collections
- Author Guidelines
- Submission Site
- Open Access
- Reasons to submit
- About BioScience
- Journals Career Network
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Potentially Offensive Content
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
- < Previous
Acid Rain: Researchers Addressing Its Lingering Effects
- Article contents
- Figures & tables
- Supplementary Data
Lesley Evans Ogden, Acid Rain: Researchers Addressing Its Lingering Effects, BioScience , Volume 68, Issue 11, November 2018, Page 928, https://doi.org/10.1093/biosci/biy113
- Permissions Icon Permissions
Acid rain, linked to fossil fuel–burning emissions, featured prominently in 1970 s to 1990 s headlines. Concern over dying lakes triggered international solution seeking, but as emissions-reducing agreements were enacted, attention to the problem faded. But the legacy of acid rain remains. Despite water chemistry's return to normal pH levels, many biological communities remain altered. The problem, explains David Schindler, professor emeritus at University of Alberta, is that although sulfur oxides were controlled, “We did nothing to control oxides of nitrogen.” Nitric acid remains high enough to deplete soil calcium in many parts of eastern Canada and elsewhere.
Now, Canadian scientists are exploring whether biological recovery for one key fish food—a freshwater shrimp that disappeared completely in many acidified lakes—might be given a helping hand. Heading the study is Mike Rennie, Canada Research Chair in Freshwater Ecology and Fisheries at Lakehead University in Northwestern Ontario and Research Fellow at Canada's International Institute for Sustainable Development Experimental Lakes Area (IISD-ELA), where the experiment is taking place. Since 1968, this research hub, renowned for experimental manipulations of whole lakes, has drawn scientists internationally.
ELA’s isolated soft-water lakes had escaped acidification. So unlike many contaminated lakes elsewhere, where biological community composition preacidification was poorly documented, “Here we were able to add the acid right in the lake experimentally and show how the whole ecosystem responded,” Rennie says. In acidification experiments, a scientist protectively suited up “rather like Darth Vader,” piped a sulfuric acid solution, and mixed it by boat propeller into lake 223, says Schindler, ELA founding director. Over 7 years, researchers reduced pH from 6.8 to 5.0. Neighboring lake 224, untreated, was their experimental control. Although lab studies suggested a 5.0 pH would not harm trout, in this ecosystem context, it did. By pH 5.6, most trout food—including tiny organisms requiring calcium to form their exoskeleton—had died, their protective coatings dissolved.
Photographs of starving fish helped persuade policymakers to legislate more rigorous air-quality standards. Reduced emissions addressed the root cause, but decades of acid rain had already transformed many limnological food webs. Crustaceans such as Mysis relicta, a freshwater shrimp and keystone species as vital trout food, were extirpated from some lakes. Some fish starved or switched to different prey. But even today, brook trout and lake trout populations in thousands of North American and European lakes have yet to fully recover.
After nearly a half century, lake 223 is no longer acidic. The chemistry and other ecosystem components look normal, says Rennie, with one key caveat. “When you look at lake trout in this lake, they grow much slower than they used to.” That, his team hypothesizes, is because Mysis are still missing, even though they are abundant in nearby lake 224. In lake 223, with Mysis missing, fish had few other prey options. So the team wants to know: Can Mysis reintroductions jumpstart the ecosystem?
IISD-ELA food web biologist Cyndy Desjardins spent last summer characterizing the “before” conditions. Then, in spring 2018, 10,000 Mysis, painstakingly counted by hand, were introduced into lake 223. Students, using white trays divided into quadrats, ferried critters from one side to the other as they counted them one by one, explains Desjardins. It took three people four nights to count them. Another 10,000 Mysis were to be added in October. The crew will then study what happens next.
John Smol, heading the Paleoecological Environmental Assessment and Research Lab at Queen's University in Kingston, Ontario, has studied acid rain impacts since the 1980 s across Eastern Canada, New York's Adirondacks, and elsewhere. Lake sediment cores are his proxy time machine, incorporating fossils, radioisotopic decay, and modeled pH–biodiversity relationships to create a timeline of how chemistry affects biology. Bottlenecks caused by extirpations such as that of Mysis may be a biological missing piece in recovery of some formerly acidified lake systems, explains Smol, but chemical missing pieces may be critical too. A deficiency in dissolved calcium—stripped out by acidification—may continue to be a roadblock to recovery for zooplankton and other organisms that require this structurally supportive element to survive and thrive. Moreover, says Smol, “a lot of other things have happened besides acidification, not least of which is climate change, which is also affecting lakes quite dramatically.” Full biological recovery may be overly optimistic given that environmental change is a moving target. Nevertheless, the return of Mysis, Rennie's team hopes, may restore at least one piece of the ever-changing puzzle.
Lesley Evans Ogden is a scientist-turned multimedia storyteller based near Vancouver, Canada. Say hello at www.lesleyevansogden.com or on Twitter @ljevanso.
| Month: | Total Views: |
|---|---|
| October 2018 | 17 |
| November 2018 | 285 |
| December 2018 | 88 |
| January 2019 | 61 |
| February 2019 | 74 |
| March 2019 | 75 |
| April 2019 | 200 |
| May 2019 | 197 |
| June 2019 | 69 |
| July 2019 | 70 |
| August 2019 | 52 |
| September 2019 | 96 |
| October 2019 | 151 |
| November 2019 | 159 |
| December 2019 | 122 |
| January 2020 | 107 |
| February 2020 | 145 |
| March 2020 | 144 |
| April 2020 | 173 |
| May 2020 | 62 |
| June 2020 | 80 |
| July 2020 | 44 |
| August 2020 | 47 |
| September 2020 | 99 |
| October 2020 | 91 |
| November 2020 | 111 |
| December 2020 | 96 |
| January 2021 | 49 |
| February 2021 | 44 |
| March 2021 | 141 |
| April 2021 | 87 |
| May 2021 | 96 |
| June 2021 | 21 |
| July 2021 | 14 |
| August 2021 | 23 |
| September 2021 | 28 |
| October 2021 | 56 |
| November 2021 | 55 |
| December 2021 | 36 |
| January 2022 | 50 |
| February 2022 | 108 |
| March 2022 | 118 |
| April 2022 | 166 |
| May 2022 | 103 |
| June 2022 | 76 |
| July 2022 | 49 |
| August 2022 | 35 |
| September 2022 | 77 |
| October 2022 | 116 |
| November 2022 | 89 |
| December 2022 | 60 |
| January 2023 | 31 |
| February 2023 | 43 |
| March 2023 | 96 |
| April 2023 | 84 |
| May 2023 | 53 |
| June 2023 | 36 |
| July 2023 | 38 |
| August 2023 | 33 |
| September 2023 | 44 |
| October 2023 | 83 |
| November 2023 | 36 |
| December 2023 | 55 |
| January 2024 | 48 |
| February 2024 | 63 |
| March 2024 | 58 |
| April 2024 | 87 |
| May 2024 | 65 |
| June 2024 | 10 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1525-3244
- Copyright © 2024 American Institute of Biological Sciences
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Acid rain, explained
The fossil fuels that humans burn for energy can come back to haunt us as acid rain.
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth. Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4 .
Causes of acid rain
Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants , factories, and automobiles.
When humans burn fossil fuels , sulfur dioxide (SO 2 ) and nitrogen oxides (NO x ) are released into the atmosphere. Those air pollutants react with water, oxygen, and other substances to form airborne sulfuric and nitric acid. Winds may spread these acidic compounds through the atmosphere and over hundreds of miles . When acid rain reaches Earth, it flows across the surface in runoff water, enters water systems, and sinks into the soil.

A virtual tree graveyard of Norway spruce in Poland bears the scars of acid rain. Caused when rain droplets absorb air pollution like sulfur and nitrogen oxides, acid rain weakens trees by dissolving nutrients in the soil before plants can use them.
Effects of acid rain
Sulfur dioxide and nitrogen oxides are not primary greenhouse gases that contribute to global warming , one of the main effects of climate change ; in fact, sulfur dioxide has a cooling effect on the atmosphere. But nitrogen oxides contribute to the formation of ground-level ozone , a major pollutant that can be harmful to people. Both gases cause environmental and health concerns because they can spread easily via air pollution and acid rain.
Acid rain has many ecological effects, especially on lakes, streams, wetlands, and other aquatic environments. Acid rain makes such waters more acidic, which results in more aluminum absorption from soil, which is carried into lakes and streams. That combination makes waters toxic to aquatic animals. ( Learn more about the effects of water pollution .)
Some species can tolerate acidic waters better than others. However, in an interconnected ecosystem, what affects some species eventually affects many more throughout the food chain, including non-aquatic species such as birds .
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of essential nutrients such as calcium and cause aluminum to be released in the soil, which makes it hard for trees to take up water . Acids also harm tree leaves and needles.
The effects of acid rain, combined with other environmental stressors, leave trees and plants less healthy and more vulnerable to cold temperatures, insects, and disease. The pollutants may also inhibit trees' ability to reproduce. Some soils are better able to neutralize acids than others. But in areas where the soil's "buffering capacity" is low, such as parts of the U.S. Northeast, the harmful effects of acid rain are much greater.
Acid deposits damage physical structures such as limestone buildings and cars. And when it takes the form of inhalable fog, acid precipitation can cause health problems in people, including eye irritation and asthma.
What can be done?
The only way to fight acid rain is by curbing the release of the pollutants that cause it. This means burning fewer fossil fuels and setting air-quality standards.
In the U.S., the Clean Air Act of 1990 targeted acid rain, putting in place pollution limits that helped cut sulfur dioxide emissions 88 percent between 1990 and 2017. Air-quality standards have also driven U.S. emissions of nitrogen dioxide down 50 percent in the same time period. These trends have helped red spruce forests in New England and some fish populations , for example, recover from acid rain damage. But recovery takes time, and soils in the northeastern U.S. and eastern Canada have only recently shown signs of stabilizing nutrients .
Acid rain problems will persist as long as fossil fuel use does, and countries such as China that have relied heavily on coal for electricity and steel production are grappling with those effects. One study found that acid rain in China may have even contributed to a deadly 2009 landslide . China is implementing controls for sulfur dioxide emissions, which have fallen 75 percent since 2007 —but India's have increased by half.
Related Topics
You may also like.

Inside the Hidden World of Jaguars

This deadly fungus is hitchhiking its way across the world

Is a shoe-free home really better? Scientists may have an answer.

Are dust storms getting worse? Here’s why they’re so destructive.

Meet the marvelous creatures that bring soil to life
- Environment
- Paid Content
- Photography
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

Acid Rain: Scourge of the Past or Trend of the Present?
Find related stories on NSF's Long-Term Ecological Research Program at this link .
Acid rain. It was a problem that largely affected U.S. eastern states. It began in the 1950s when Midwest coal plants spewed sulfur dioxide and nitrogen oxides into the air, turning clouds--and rainfall--acidic.
As acid rain fell, it affected everything it touched, leaching calcium from soils and robbing plants of important nutrients. New England's sugar maples were among the trees left high and dry.
Acid rain also poisoned lakes in places like New York's Adirondack Mountains, turning them into a witches' brew of low pH waters that killed fish and brought numbers of fish-eating birds like loons to the brink.
Then in 1970, the U.S. Congress imposed acid emission regulations through the Clean Air Act, strengthened two decades later in 1990. By the 2000s, sulfate and nitrate in precipitation had decreased by some 40 percent.
Has acid rain now blown over? Or is there a new dark cloud on the horizon?
In findings recently published in the journal Water Resources Research , Charles Driscoll of Syracuse University and the National Science Foundation's (NSF) Hubbard Brook Long Term Ecological Research (LTER) site in New Hampshire reports that the reign of acid rain is far from over.
It's simply "shape-shifted" into a different form.
Hubbard Brook is one of 26 NSF LTER sites across the nation and around the world in ecosystems from deserts to coral reefs to coastal estuaries.
Co-authors of the paper are Afshin Pourmokhtarian of Syracuse University, John Campbell of the U.S. Forest Service in Durham, N.H., and Katharine Hayhoe of Texas Tech University. Pourmokhtarian is the lead author.
Acid rain was first identified in North America at Hubbard Brook in the mid-1960s, and later shown to result from long-range transport of sulfur dioxide and nitrogen oxides from power plants.
Hubbard Brook research influenced national and international acid rain policies, including the 1990 Clean Air Act amendments.
Researchers at Hubbard Brook have continued to study the effects of acid rain on forest growth and on soil and stream chemistry.
Long-term biogeochemical measurements, for example, have documented a decline in calcium levels in soils and plants over the past 40 years. Calcium is leaching from soils that nourish trees such as maples. The loss is primarily related to the effects of acid rain (and acid snow).
Now, Hubbard Brook LTER scientists have discovered that a combination of today's higher atmospheric carbon dioxide (CO 2 ) level and its atmospheric fallout is altering the hydrology and water quality of forested watersheds--in much the same way as acid rain.
"It's taken years for New England forests, lakes and streams to recover from the acidification caused by atmospheric pollution," says Saran Twombly, NSF program director for long-term ecological research.
"It appears that these forests and streams are under threat again. Climate change will likely return them to an acidified state. The implications for these environments, and for humans depending on them, are severe."
Climate projections indicate that over the 21st century, average air temperature will increase at the Hubbard Brook site by 1.7 to 6.5 degrees Celsius, with increases in annual precipitation ranging from 4 to 32 centimeters above the average from 1970-2000.
Hubbard Brook scientists turned to a biogeochemical model known as PnET-BGC to look at the effects of changes in temperature, precipitation, solar radiation and atmospheric CO 2 on major elements such as nitrogen in forests.
The model is used to evaluate the effects of climate change, atmospheric deposition and land disturbance on soil and surface waters in northern forest ecosystems.
It was created by linking the forest-soil-water model PnET-CN with a biogeochemical sub-model, enabling the incorporation of major elements like calcium, nitrogen, potassium and others.
The results show that under a scenario of future climate change, snowfall at Hubbard Brook will begin later in winter, snowmelt will happen earlier in spring, and soil and stream waters will become acidified, altering the quality of water draining from forested watersheds.
"The combination of all these factors makes it difficult to assess the effects of climate change on forest ecosystems," says Driscoll.
"The issue is especially challenging in small mountain watersheds because they're strongly influenced by local weather patterns."
The Hubbard Brook LTER site has short, cool summers and long, cold winters. Its forests are made up of northern hardwood trees like sugar maples, American beeches and yellow birches. Conifers--mostly balsam firs and red spruces--are more abundant at higher elevations.
The model was run for Watershed 6 at Hubbard Brook. "This area has one of the longest continuous records of meteorology, hydrology and biogeochemistry research in the U.S.," says Pourmokhtarian.
The watershed was logged extensively from 1910 to 1917; it survived a hurricane in 1938 and an ice storm in 1998.
It may have more to weather in the decades ahead.
The model showed that in forest watersheds, the legacy of an accumulation of nitrogen, a result of acid rain, could have long-term effects on soil and on surface waters like streams.
Changes in climate may also alter the composition of forests, says Driscoll. "That might be very pronounced in places like Hubbard Brook. They're in a transition forest zone between northern hardwoods and coniferous red spruces and balsam firs."
The model is sensitive to climate that is changing now--and climate changes expected to occur in the future.
In scenarios that result in water stress, such as decreases in summer soil moisture due to shifts in hydrology, the end result is further acidification of soil and water.
Related links
- NSF LTER Network
- NSF Hubbard Brook LTER Site
- Science, Engineering and Education for Sustainability NSF-Wide Investment (SEES)
Research areas
- All Publications
- Priorities Magazine Spring 2018
- The Next Plague and How Science Will Stop It
- Priorities Magazine Winter 2018
- Priorities Magazine Fall 2017
- Little Black Book of Junk Science
- Priorities Magazine Winter 2017
- Should You Worry About Artificial Flavors Or Colors?
- Should You Worry About Artificial Sweeteners?
- Summer Health and Safety Tips
- How Toxic Terrorists Scare You With Science Terms
- Adult Immunization: The Need for Enhanced Utilization
- Should You Worry About Salt?
- Priorities Magazine Spring 2016
- IARC Diesel Exhaust & Lung Cancer: An Analysis
- Teflon and Human Health: Do the Charges Stick?
- Helping Smokers Quit: The Science Behind Tobacco Harm Reduction
- Irradiated Foods
- Foods Are Not Cigarettes: Why Tobacco Lawsuits Are Not a Model for Obesity Lawsuits
- The Prevention and Treatment of Osteoporosis: A Review
- Are "Low Dose" Health Effects of Chemicals Real?
- The Effects of Nicotine on Human Health
- Traditional Holiday Dinner Replete with Natural Carcinogens - Even Organic Thanksgiving Dinners
- A Primer On Dental Care: Quality and Quackery
- Nuclear Energy and Health And the Benefits of Low-Dose Radiation Hormesis
- Priorities in Caring for Your Children: A Primer for Parents
- Endocrine Disrupters: A Scientific Perspective
- Good Stories, Bad Science: A Guide for Journalists to the Health Claims of "Consumer Activist" Groups
- A Comparison of the Health Effects of Alcohol Consumption and Tobacco Use in America
- Moderate Alcohol Consumption and Health
- Irradiated Foods Fifth Edition
- Media/Contact
- Write For Us
Whatever Happened to Acid Rain?
Related articles.
Those of us who are non-millennials may remember back to the 1970s, 1980s, and 1990s when the hottest environmental issue was acid rain. In fact, acid rain generated as much controversy and international conflicts as the big environmental issue of today, climate change, with scientists, policymakers, and politicians engaging in heated battles over this issue.

But the issue seems to have disappeared since that time. Is the reason for this that we actually solved a pressing environmental problem, or did acid rain simply get pushed down the priority list as new, more urgent environmental issues came to the forefront?
What is Acid Rain?
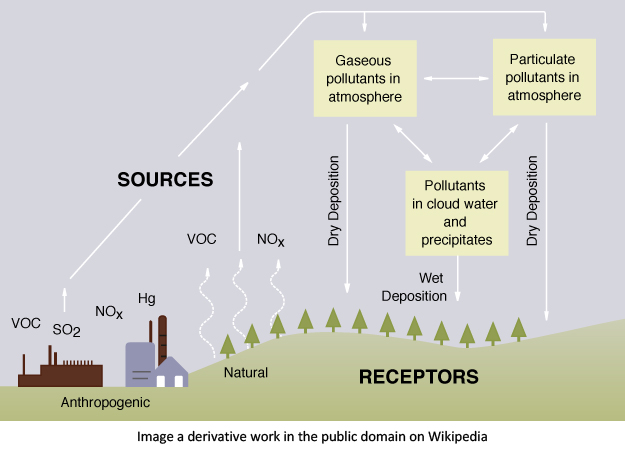
Acid rain is rain or any other type of precipitation, including snow or fog, that is unusually acidic. Acid rain is caused by sulfur dioxide, or nitrogen dioxide emissions released into the air and react with water molecules before falling to the ground as rain or snow. Most sulfur or nitrogen dioxide comes from electrical power plants, with a smaller amount coming from cars and other vehicles and natural sources such as volcanoes and wildfires. Both emissions, move by circulating air and wind, can travel long distances so that acid rain may be found in areas far from its source.
Acid rain can create highly acidic soils, adversely affecting the growth of forests and crops. Acidic waters can result in the death of fish and other aquatic species. Acid rain also enhances the deposition of mercury, which has adverse effects on human health. Direct impacts on human health have also been documented. A severe episode of acid fog, the Great London Smog of 1952, resulted in an increase in the daily average death rate from 252 to approximately 1,000, and acid fog was r esponsible for several severe air pollution episodes in southern California in the 1980s.
- 1852 - Scientists identified a relationship between acid rain and air pollution in Manchester, England.
- 1972 - Scientists discovered rain deposited in the White Mountains in New Hampshire was acidic.
- 1980 - Congress passed the Acid Deposition Act establishing an 18-year assessment and research program on acid rain.
- 1983 - National Academy of Sciences (NAS) issues a draft report saying that acid rain is a real problem that needs to be addressed.
- 1990 - Congress passed a series of amendments to the Clean Air Act establishing a cap-and-trade system designed to control sulfur dioxide emissions. A more traditional regulatory program was established to control nitrogen dioxide emissions.
What is cap-and-trade?
“We devised a cap-and-trade approach, written into the 1990 Clean Air Act. It required cutting overall sulfur emissions in half, but let each company decide how to make the cuts. Power plants that lowered their pollution more than required could sell those extra allowances to other plants. A new commodities market was born.”
Environmental Defense Fund
Cap-and-trade is a market-based solution to environmental concerns developed with all stakeholders, the regulators, environmental advocates, and businesses.
- EPA sets a cap, or a limit, on the total amount of sulfur dioxide allowed to be emitted by all electric-generating power plants in the U.S.
- Allowances are “authorizations to emit” allocated to every power plant.
- An allowance market was set up that allows power plants and others to buy or sell allowances throughout the year.
- Power plants are given the flexibility to choose their own options to reduce emissions, such as adding emission controls, using more advanced technologies, switching to new fuels, using banked allowances, or buying allowances from the market.
- At the end of every compliance period, each power plant must have enough allowances to cover their sulfur dioxide emissions, or the EPA fines them.
This system gives businesses strong financial incentives to cut emissions. Electrical power plants emitted 778 thousand tons of sulfur dioxide in 2020 , well below the permanent cap of 8.95 million tons.
What Happened?
A 93% reduction in annual sulfur dioxide emissions between 1990 and 2019!
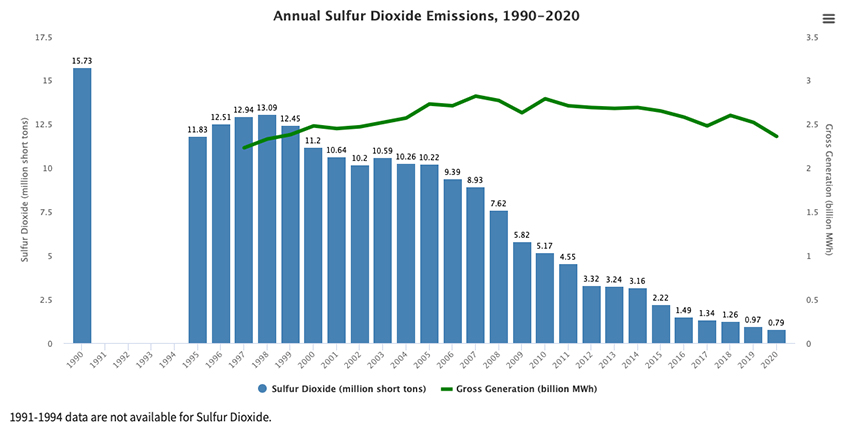
There was also an 87% reduction in annual nitrogen dioxide emissions between 1990 and 2019.
Significant decreases occurred in acid rain nationwide – wet sulfate deposition, an indicator of acid rain, decreased by 68% between 1989 and 2019.
Why Such Little Attention?
The acid rain issue has been called “the greatest green success story of the past decade” by The Economist in their article “The Invisible Green Hand.” Two factors may explain why we heard so much about acid rains' harmful effects and so little about our successful mitigation. First, economic solutions often become baked in, integrated into our economy, creating an invisible fix not apparent to most people. Second, journalists just don’t like to cover good news stores – it doesn’t gather our attention and emotional responses the way bad news does.
Conclusions
The acid rain story should be studied by every person interested in environmental policy. The cap-and-trade approach is currently being used to help solve climate change in the U.S. and globally. For example , California instituted a cap-and-trade policy for carbon emissions, leading to a steady decline in carbon dioxide emissions. The European Union capped carbon dioxide emissions from some industrial sources, which led to a 29% reduction from 2005 to 2018. China, the world’s largest emitter of greenhouse gases, began a carbon cap-and-trade program in 2017.
On a broader level, acid rain should give us hope for the future that the government, private sector, and non-profits, working with technological solutions, can solve today’s pressing problems. Acid rain was an issue that seemed unsolvable 40 years ago, but today is held up as an example as to what is achievable.
[1] The EPA’s website , from which the graphic was taken, has several interactive charts showing the significant improvement in our air over the last 30 years.
Sources: Environmental Defense Fund. How Cap and Trade Works , How Economics solved acid rain
U.S. Environmental Protection Agency: Acid Rain Program , Acid Rain Program Results
View the discussion thread.

By Susan Goldhaber MPH
Susan Goldhaber, M.P.H., is an environmental toxicologist with over 40 years’ experience working at Federal and State agencies and in the private sector, emphasizing issues concerning chemicals in drinking water, air, and hazardous waste. Her current focus is on translating scientific data into usable information for the public.
Latest from Susan Goldhaber MPH :

- Distillations Podcast

Distillations podcast
Whatever Happened to Acid Rain?
It’s complicated.

Remember acid rain? If you were a kid in the 1980s like our hosts were, the threat of poison falling from the sky probably made some kind of impression on your consciousness. But thanks to the work of scientists, government, the media, and the pope—that’s right, the pope—the problem was fixed! Well, mostly fixed is probably more accurate.
This complicated story spans 27 years, six U.S. presidents, and ecologist Gene Likens’s entire career. Discover the insidious details in the second chapter of our three-part series on environmental success stories.
Hosts : Alexis Pedrick and Elisabeth Berry Drago Senior Producer : Mariel Carr Producer : Rigoberto Hernandez Audio Engineer: James Morrison Additional audio was recorded by David G. Rainey. Image of Gene Likens by Phil Bradshaw of FreshFly . Our theme music was composed by Zach Young. Additional music courtesy of the Audio Network .
Research Notes
We interviewed Rachel Rothschild, a former Science History Institute research fellow and Rumford Scholar, about her book, “Poisonous Skies: Acid Rain and the Globalization of Pollution.” To research this episode we read her 2015 dissertation, A Poisonous Sky: Scientific Research and International Diplomacy on Acid Rain . We also read Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Global Warming by Naomi Oreskes and Erik Conway (Bloomsbury, 2010).
We interviewed Gene Likens at Hubbard Brook Experimental Forest in New Hampshire in 2015 with Glenn Holsten and FreshFly . We interviewed him again in May 2018.
These are the archival news clips we used as they appear in the episode:
The following are the archival news clips we used as they appear in the episode:
Bettina Gregory, Tom Jarriel, and Bill Zimmerman. ABC Evening News , December 14, 1978.
Walter Cronkite and Jim Kilpatrick. “Environment: The Earth Revisited/Acid Rain.” CBS Evening News , September 11, 1979.
Robert Bazell and John Chancellor. “Special Segment: Acid Rain.” NBC Evening News , May 9, 1980.
“The MacNeil/Lehrer Report: Acid Rain,” NewsHour Productions, American Archive of Public Broadcasting (Boston: WGBH; Washington, DC: Library of Congress), aired May 26, 1980, on PBS, http://americanarchive.org/catalog/cpb-aacip_507-pk06w9754b.
“The MacNeil/Lehrer NewsHour,” NewsHour Productions, American Archive of Public Broadcasting (Boston: WGBH; Washington, DC: Library of Congress), aired on June 30, 1988, on PBS, http://americanarchive.org/catalog/cpb-aacip_507-b56d21s53c.
Tom Brokaw and Robert Hager. “Air Pollution: George Bush.” NBC Evening News , November 15, 1990 .
ABC Evening News, December 14, 1978: In the Adirondack Mountains of New York the lakes are so clear they mirror the forest around them. One might think pollution could never taint this mountain paradise, but it has. The fish have died in this lake. The rain has turned the water acid. Scientists say particles of sulfur are carried by these clouds and when it rains it pours a mild sulfuric acid into lakes like this one. The experts say power plants discharge most of the sulfur into the air. And what goes up these smoke stacks, must come down.
Alexis : Hi, I’m Alexis Pedrick.
Lisa : And I’m Lisa Berry Drago, and this is Distillations , coming to you from the Science History Institute.
Alexis : Each episode of Distillations takes a deep dive into a moment of science-related history in order to shed some light on the present. Today we’re talking about acid rain, in the second installment of a three-part series about environmental success stories.
Lisa : Our last episode, “Whatever Happened to the Ozone Hole?” is available on our website: Distillations DOT ORG, through Apple Podcasts, or wherever else you get your podcasts!
Alexis: In the early 1960s American scientists discovered a new environmental threat called acid rain, but most people didn’t become aware of it until almost 1980. Lisa, do you remember learning about acid rain?
Lisa : Yeah, but I’m not sure I knew what it was when I was a kid. I think I thought it had something to do with Guns N’ Roses, sort of acid-washed jeans, November rain…
Alexis : Same. Same. I think I had to do a school project on it and I remember reading this book about acid rain and all these terrible things that happened with it. But then it was raining outside and I was fine. And I didn’t melt. So I had no concept of, ‘is this a threat or not?’.
Lisa : Right. We definitely got rained on in the 80s.
Alexis : Right. And we survived. So…
Lisa : So why didn’t we find out about this problem sooner? What happened in this nearly two- decade-long gap? And what led to that ABC evening news clip we just heard from December of 1978?
Alexis: If you listened to our show about the ozone hole, you’ll remember that we told you how to solve any environmental problem in five easy steps.
Lisa : And of course…we actually learned that it’s far more complicated than that, but we’re going to follow the steps again anyway.
Alexis : So here we go: step number one: figure out the problem.
Lisa : Step two: get your evidence.
Alexis : Step three: inform the public.
Lisa: Step four: you have get industry onboard
Alexis: Step five: implement policy.
Lisa : Acid rain took a long time to resolve in the United States, and there were a lot more roadblocks and slowdowns than with the ozone hole, but you’re gonna hear all of it, so let’s get started.
Chapter 1: Figure out the Problem
Lisa: Chapter One. Figure out the problem.
Alexis : Compared to the ozone hole, acid rain took a bit longer to get under control in the U.S.
–like, a couple decades longer. It was first discovered in North America in 1963, but it took until 1980 before the media really jumped in, and until 1990 until there was any kind of resolution.
Ecologist Gene Likens was there the whole time. And we met up with him where it all started. In a pristine forest in the mountains of New England.
Likens: I’ve always said that I can’t believe that I’ve been paid for all these years to work here. I mean come on! It’s too nice, it’s too beautiful and yet they pay me to work here.
Alexis: Gene Likens is standing by a stream in Hubbard Brook Experimental Forest, in the White Mountains of New Hampshire. These woods have been his laboratory since 1953. When they set up Hubbard Brook, Likens and his colleagues thought of themselves kind of like doctors, and the forest ecosystem as a patient.
Likens: We had the idea that we could use the chemistry of the water flowing out of this watershed ecosystem much like a physician uses the chemistry of our blood and urine. If the physician measures the chemistry of my blood or urine and sees that something is wrong then he has some idea that my system isn’t functioning properly.
Alexis : In 1963 Likens and his colleagues were looking at the rain. And what they found was startling.
Likens : This is where we discovered acid rain. Our very first samples was roughly 100 times more acidic than we thought the rain ought to be. We didn’t have any idea why it was so acid or where it might have come from or how long it had been there? We didn’t know any of those fundamental answers.
Alexis : Likens found some of those answers by connecting with another scientist on another continent. Just a handful of years after his discovery, Likens crossed paths with a scientist in Sweden who had recently discovered acid rain in Scandinavia. His name? Svante Oden.
Likens: And Svante said, “I’m going tonight on the overnight train from Stockholm to Oslo, Norway, and would you like to go along?” And I said “oh sure, why not?” So we took the overnight train together, and sat up and talked most of the night.
Alexis: Oden told Likens that he thought that the pollution in Scandinavia was coming from more industrialized parts of Europe, and this information helped Likens connect some dots.
Lisa : It’s like he had to talk to someone else from across the globe to understand what was happening in his own little corner of the world. And this is a bigger theme in science I think we hear again and again. You have to step outside of your framework to see the big picture.
Alexis : Exactly. No one is ever just working on one thing in isolation by themselves. Lots of people are working on the same thing all over the world and they benefit from talking to each other.
Likens: It was just one of those serendipitous events where something happens and helps you understand what’s going on much more clearly than you might’ve otherwise.
Alexis: Likens went back to the U.S. and continued monitoring acid rain. Then in 1974, eleven years after he first discovered it, he decided he had enough evidence to write an article with his colleague Herbert Bormann for the academic journal Science . It was called “ Acid Rain: A Serious Environmental Problem .” By this point Likens had moved to upstate New York and had also found acid rain in the Adirondacks.
Likens: The paper was saying, “This is not something unique to Hubbard Brook but is a much more regional problem.”
Alexis : The paper said that acid had been falling in the Northeast for 20 years. But the biggest revelation was that tall smokestacks hundreds of miles away in the Midwest were to blame.
Emissions from burning coal was a major source of the problem.
Likens: The Midwest is emitting large quantities of sulfur and nitrogen Oxides. It gets carried to the atmosphere and then deposited here whenever it rains and snows. So it’s like somebody throwing their garbage out and then the garbage falling on your property and you don’t like it much.
Alexis : The idea that pollution could travel such distances was a new revelation. And the irony of it all was that the culprit—those tall smoke stacks—were originally created as a solution to another pollution problem.
Donora, Pennsylvania News Clip: Residents have difficulty breathing the murky air. 20 died. 400 others are stricken with respiratory illness. A local zinc plant is suspected of emitting poison smoke is closed down. An epidemic of pneumonia is feared in the wake of Donora’s deadly rain of smog.
Alexis : Donora was a small mill town in western Pennsylvania. Back in the 1940, their zinc plant, like most plants at the time, had a short smoke stack, and it was pumping out a poisonous combination of carbon monoxide, sulfur dioxide, and metal dust. In 1948 the town suffered a smog attack that killed twenty people and made seven thousand more sick. The disaster alerted people to the hazards of air pollution, and it eventually helped trigger the 1970 Clean Air Act. But it also raised the height of smokestacks.
Lisa : Tall smokestacks helped towns like Donora, they whisked clouds of pollution out of their backyards. But unfortunately they just sent them to other people backyards, further away.
Likens : And what that really did was convert a local soot problem to a more regional soot problem. It just took the push from here and emitted it at a higher level and then it was swept away by the winds and the atmosphere.
Chapter 2: Get Evidence Alexis: Chapter 2: Get evidence.
Lisa : By the time Gene Likens and Herbert Bormann published that paper in 1974, they’d been monitoring the issue for more than a decade.
Alexis : So maybe you’re wondering what they were doing all that time. I mean we certainly were.
Lisa : The answer is gathering that evidence. First they went to some of the most remote places in the world to try to get a baseline estimate of what the acidity, or pH, of rain should be. They had to go places without human activity or any smokestacks—tall or short. Just a few of the places they went were Southern Chile and remote parts of China and Australia. They traveled for a month by boat to get to an island in the middle of the Indian Ocean called Amsterdam Island.
Through it all they learned that the default pH of rain is 5.1. The samples they were measuring back home were at least a hundred times more acidic than that. Here’s how an ABC news clip explained what these numbers meant.
ABC Evening News, December 14, 1978: A pH of 7 would be neutral, the lower the reading the more acidic it is. This sample of rainwater from the summit reads 3.3, which is just about as acidic as grapefruit juice.
Lisa : Likens’s world travels really proved that the rain truly was too acidic. His research also proved that the pollution that caused acid rain really was coming from industry in the rust belt.
Likens : We tried to follow isotopic tracers in the emissions from smokestacks in the Midwest. We followed plumes in small airplanes and vehicles on the ground. We went to enormous lengths to try to answer those questions.
Lisa : Ten years in it seemed like the science was pretty clear. Likens and his team felt confident in their research and they published their article. Some of what they hoped for started to come true. The New York Times quickly picked up their story and the scientific community in the U.S. started paying attention to acid rain.
Likens: That paper changed my life forever because it was published on the front page of the New York Times . I had colleagues all over the world calling me saying, “Likens, what is this? What’s going on?”
Lisa: Environmental scientists definitely took notice. But so did plenty of other people, many with their own agendas.
Likens : There was lots of pushback saying, “Well, it’s not us.” You know, “We didn’t do it. It’s not us. There is no such thing as acid rain.” I can remember many times when there would be a meeting or I might be giving a talk and someone, a denier type would stand up and say, “There’s no such thing as acid rain.” And I would say, “Have you ever collected a sample of rain and analyzed it?” The answer was always no. I said, “Try it sometime. You might be surprised what you find out.”
Rachel Rothschild: There was this pretty dramatic response from the coal industries, who were thought to be the most serious contributors to the problem.
Lisa: Rachel Rothschild is a historian of environmental science and technology and a former research fellow at the Science History Institute. She’s finishing up a book called Poisonous Skies: Acid Rain and the Globalization of Pollution. She’s studied the pushback against acid rain science, and one of the things she’s uncovered is how quickly the coal industry realized that
Likens’s research could be a threat to them.
Rothschild: They, in fact, launched some of the most serious and extensive research efforts on acid rain in the hope of vindicating themselves, and it set up a very interesting confrontation between industry scientists and environmental scientists in the late 1970s and into the 1980s.
Lisa : So we’ve been here in step two, gathering evidence with Gene Likens, thinking we were alone with him.
Alexis : But it turns out these steps—which we made up by the way—aren’t secret! Other people can jump in and gather evidence too!
Lisa: So with acid rain step two is multi-pronged: first you have to gather your evidence, then wait for someone else to dispute or distort it, and meanwhile they’re gathering their evidence, and then you have to dispute the counter-evidence. When the attacks came they were often aimed right at Gene Likens.
Likens: It was bad. It was really nasty. I had a contract put out on me. It was…Did I tell you this story before? If so I apologize. Oh my goodness, I hadn’t thought about any of this in a long time, really painful.
Lisa : A coal-backed policy group tried to carry out what we can only describe as a “scientific hit” on Gene Likens. Okay, maybe that’s a little extreme, but they put out a call to discredit his research on acid rain—they called him by name—and offered to pay four hundred thousand dollars to anyone who could do the job.
Likens : That was the call. Show that he is wrong. So yeah, it was pretty unsettling and pretty shocking. It wasn’t a contract on my life, but it was a contract on my career, which in some ways almost was as important as my life. I mean, not really but you know what I mean? It’s what I do. It’s what I am. It’s what I’m all about. I grew up on a small farm in northern Indiana. I was a farm boy. I just thought the world worked a little differently and I kept finding out it didn’t. I thought all this
science rode around like knights on big white horses and I found out it didn’t work that way. Answers could be purchased and they were. All that was greatly disturbing to me.
Alexis : So I think this is a good place to stop because this is a pattern we’ve seen before, right?
The naiveté of scientists playing by the rules, but they don’t really understand all of them. Or
they see rules that aren’t there. They are just in their lab doing their thing and not really thinking about how to play this larger game.
Lisa : What happens when the research hits the real world? Yeah, the game can change a lot.
Alexis : Exactly.
Lisa : It’s partly that naïve sense of playing by the rules maybe? That might help certain scientists when they get to a crisis point, because in the end they have the science to go back to.
Likens: Why did we keep persevering? [laughs] Because I’m a scientist and because I am searching for the truth and because in science we search for the truth. We rarely find it, but we search for the truth.
Lisa: The contract Likens is talking about was put out by one of the biggest sources of counter- research—a coal trade group called the Edison Electric Institute. Their research arm was called EPRI, or the Electric Power Research Institute. Their job was to refute any science that made them look bad, and they were desperate to find some other industry to blame acid rain on.
Rothschild: They were hoping that they might find that, say, logging or other forestry practices, for example, might result in increased acidity in the soil.
Lisa : So EPRI scientists conducted a study in the Adirondacks to get alternative evidence, alternative facts if you will, but they couldn’t find any. So they distorted the evidence.
Rothschild: I would say they misrepresented the evidence and tried to convey that there was more uncertainty than there actually was and tried to use evidence that simply supported a different kind of proposition, to say that actually acid rain wasn’t the problem at all.
Lisa : That scientific hit never paid off. Remember how Gene Likens spend those eleven years of gathering evidence?
Likens: It all started with measurements and was bolstered by continuing high quality measurements so that when the attacks came we were able to lay our data out there and say, “Go at it and show that it’s wrong,” and nobody was ever able to do that.
Chapter 3: Let the Public Know
Lisa: Chapter three. Let the public know.
Alexis : Gene Likens learned that his data was crucial, but it was not going to speak for itself. So he had to learn how to talk to the public and the naysayers. When he wrote that pivotal paper in 1974 he consciously chose the term “acid rain” because he thought it would get people’s attention, and he was right.
Likens : We thought and argued long and hard about whether we should use that as a title. I’m really glad that we did because it brought public attention to the issue in ways, and I’m a scientist, so I’m not supposed to care about that, but in terms of the management of this serious environmental issue it helped. Because you can walk in the rain, you can sing in the rain, you can dance in the rain, but if the rain is acid you might think about it very differently than you would have otherwise.
Alexis: In the late 70s and early 80s television played a crucial role in getting the American public to know and care about acid rain. Robert Bazell worked at NBC news for 38 years. He was the chief science correspondent during the 1980s.
Robert Bazell : Well the media landscape was that there were three networks and most of America watched one of the three every night. There was no cable television.
Newspapers were not going out of business for all the things we think about now. And of course, there was no Internet. It was a very different world, and there was an enormous amount of impact from those stories that were on television.
CBS Evening News, September 11, 1979: Well, as far as I’m concerned the lake is dead. Period. There’s no swallows around, the swallows have left almost two weeks early this year.
Alexis: This is one of the earliest stories on Acid Rain, from 1980.
NBC Evening News, May 9, 1980: Now there are no fish, no lily pads. In fact, there is no life visible in Woods Lake. It was killed by a new type of pollution which is affecting many parts of the world. It’s called acid rain.
Alexis : 38 years later Bazell still remembers reporting it.
Bazell: We were always looking for stories, and this one was an important one, obviously, for the reasons that you just heard in that clip. Fish were dying, trees were dying. It was a visual story, which makes it very impactful for television. Made it a very easy story to tell. You could see what was happening, it wasn’t an obscure concept.
Alexis : Everyone was talking about acid rain, from TV reporters—like Robert Bazell—to cartoons, to the Pope. That’s right, the Pope. In 1985 Gene Likens visited Pope John Paul II, who went on to address acid rain in his encyclical. So the media helped. But it also might have hurt.
The MacNeil/Lehrer Report, May 26, 1980 [Jim Lehr] : There is a new environmental fear alive in the land, the fear of something called “acid rain.” Reports of its presence and its danger come from everywhere.
Alexis : This is Jim Lehrer, in a 1980 clip from the Macneil/Lehrer Report , the precursor to PBS Newshour . On the show Lehrer holds what is basically a debate. On one side is Douglas Costle, Jimmy Carter’s EPA administrator, and on the other is a man named William Poundstone. He’s the executive vice president of Consolidated Coal—one of the country’s biggest coal companies. Throughout the show Costle lays out well-established facts about acid rain and Poundstone disputes them. Or more accurately, he evades and distorts them.
The MacNeil/Lehrer Report, May 26, 1980 [Charlayne Hunter-Gault] : Mr. Costle, what has brought you to your present state of alarm?
Douglas Costle: I think the single most important thing that happened this year was that scientists from all around the world came to me and they said, in effect, “there`s a lot we still do not know about acid rain, but we know enough now to know that we should not be making the problem worse.”
Lehrer : Mr. Poundstone, what do you think of Mr. Costle`s position on acid rain?
Poundstone : There is no issue that the rainfall is acid. But we go beyond that point, and we start to diverge.
Lehrer : In other words, you will concede that there is such a thing as acid rain?
Poundstone : Yes, sir. The rain—
Lehrer : And it’s a damaging—it has serious repercussions when it hits the ground?
Poundstone : I have not said that. I have said the rain is acid.
Lisa and Alexis: “Ohhhhhhh” do you see what’s going on here? I think we can all see what’s going on here.
Poundstone : And there’s a great deal of argument and evidence that must be heard on this issue. The English Electricity Board, the EPRI people as well—
Lehrer : Who are the EPRI people?
Poundstone : That is the research arm of the Electric Power Research Institute.
Lehrer : I see. All right.
Poundstone : They have some $22 million a year in research activity, and I think in these areas are doing more than anyone.
Alexis: Poundstone’s goal was to discredit acid rain science, and this interview made it seem like there was no scientific consensus at all. If you’ve been paying attention to this podcast you already know this is what EPRI was all about. But Jim Lehrer takes everything both men say at face value, seemingly encouraging his viewers to do the same. Imagine you’re sitting at home watching this on the news, they’re the same to you. But they’re not the same.
Lisa : We see this kind of false equivalence all the time. Especially with environmental issues.
Alexis : Right, so that’s why Douglas Costle spent a lot of time playing defense during the Lehrer interview, but he still managed to squeeze in the fact that there was an attainable solution: older power plants could be retrofitted with a technological fix to reduce their emissions.
Lisa : I’m just speculating here, but it seems like that interview must have caught him off-guard, like it felt like a big setback. Just three weeks after this interview Douglas Costle said this on the ABC evening news:
ABC Evening News, June 18, 1980 [Douglas Costle]: I don’t want to sound too cynical, but I have never seen an industry that is a part of the problem, be the first to acknowledge a problem. Or the extent of their own involvement in it.
Alexis : Despite all of this it seemed like things were moving ahead. President Carter signed the acid precipitation act of 1980, which promised to address the problem within ten years. Things were looking up. And then this happened.
Ronald Regan Election Speech Jan. 20. 1981: In this present crisis, government is not the solution to our problem; government is the problem.
Chapter 4: Implement Policy
Alexis : Chapter four. Implement policy.
Lisa : Or, in the case of acid rain: intentionally waste a decade not implementing any policy!
Alexis: It turns out elections have consequences.
Rothschild: So Reagan had really campaigned, much like President Trump did recently, on this idea of deregulating the environment and making sure that environmental regulations weren’t getting in the way of economic development and growth. When he came into office, he very quickly transformed the Environmental Protection Agency.
Lisa : Douglas Costle didn’t last long in Reagan’s EPA. Instead the president brought on one of EPRI’s top scientists—remember them? Another new EPA pick banned the use of the term acid rain. In short, Reagan was not good for the environment. He did, however, invite a team of scientists to brief him on the issue at the White House in 1983. The group was led by Gene Likens.
Likens : At the end, President Reagan sat back in his chair and he looked around the room and he said, I’ll never forget this quote, “Well, gentleman it’s clear to me that my undergraduate education did not prepare me for such complicated issues.” I thought, “Wow.” But any rate we made our case and that was in September of 1983 and on January the Director of Management and Budget made the pronouncement that, “no, we’re not going to deal with acid rain. It’s too expensive to do so. We’ll study it instead.”
It was an amazing experience to go to the White House and to brief the president and the full cabinet, but not to see something happen.
Lisa : In 1986 Reagan suffered a backlash in the midterm elections, and results sent a message that he needed a different approach to environmental issues. So he signed the Montreal Protocol for the ozone hole, and the Sophia Protocol, an international accord aimed at reducing nitrogen oxides to combat acid rain. The environment became a huge campaign issue in the 1988 election.
Michael Dukakis attack ad: For seven and half years George Bush personally weakened regulations on corporate polluters. And now suddenly George Bush tells you he is going to be the environmentalist president. Do you believe that?
Lisa : On the left was Michael Dukakis, who obviously did not win. But Rachel Rothschild says he made a lasting impact.
Rothschild: So, Dukakis really placed environment at the forefront of his political platform during the election, and in many ways forced President Bush to move to the left on that issue and make a decisive break with President Reagan.
Lisa: Part of the public’s anxiety was a growing awareness of something called global warming.
Rothschild: In the summer of 1988 there were congressional hearings about the possibility that carbon dioxide was increasing the planet’s temperature with the potential for catastrophic results to the environment.
The MacNeil/Lehrer Report, June 30, 1988 Congressional hearing [Daniel Albritton, NOOA] If greenhouse gases continue to grow unabated…
[Rep. Claudine Schneider [R] Rhode Island]: There is a very high, high risk of irreversible, and catastrophic impact looming on the horizon.
Rothschild : And that I think, for the first time for many Americans, raised the specter of large scale planetary threats from fossil fuels. And so acid rain, in comparison, almost seemed much more solvable.
Likens: I often wondered if I was just banging my head against the wall for no value. But that didn’t turn out to be the case, did it? Because in 1990 under amazing conditions a Republican president signed the 1990 Clean Air Act into legislation.
NBC Evening News, Nov 15, 1990: What the President is calling for would be the first improvement of the clean air law in 12 years.
President George H. W. Bush : We’ve seen a stalemate. It’s time to clear the air. Acid rain must be stopped and that’s what we all care about.
President George H. W. Bush, address to Congress February 9, 1989: Because the time for study alone has passed and the time for action is now.
Likens : The Congress, both the House and the Senate had voted overwhelmingly, it wasn’t unanimous, but it was overwhelmingly in favor of that action, the 1990 Clean Air Act Amendments. So being able to be there in 1963 and make the discovery for North American about the occurrence of acid rain, and then all those tough years in between to 1990 when our country took legislative action, was very satisfying, and maybe is unique. I don’t know.
Lisa : Bush implemented what is now known as “cap-and-trade.” It essentially lets companies buy and sell the rights to pollute. It was a perfect free-market solution for a Republican, environmentalist president.
Alexis : You might have noticed that we left out the “get industry on board” step, that’s because, well, they never really got on board, per se, eventually they just had to yield to the change in policy.
Chapter 5: What Does Success Look Like?
Alexis: The cap and trade program was a cost-effective solution, and it stemmed the worst environmental impacts. The rain at Hubbard Brook is 80% less acid now than it was in 1963. But there are still areas of the country that are still at risk or haven’t fully recovered, so it’s a success story, but it’s complicated.
Lisa : The lesson of Gene Likens is the same lesson of Hubbard Brook forest. The mountains of New Hampshire do not exists in a vacuum and neither does Gene Likens and his science.
Alexis: Right. Exactly. And we’ve seen this—
Lisa: Both of them are touched by industry, and social concerns, and money and power and all of that stuff.
Alexis : And by the way, Gene Likens still has not given up the fight.
Likens: No way. And I still don’t. I’m in my mid-eighties and I’m not giving up yet. Here I am talking to you.
Alexis : Distillations is more than a podcast. We’re also a multimedia magazine.
Lisa : You can find our videos, our blog, and our print stories at Distillations DOT org.
Alexis : And you can also follow the Science History Institute on Facebook, Twitter, and Instagram.
Lisa : This episode was produced by Mariel Carr and Rigo Hernandez. Additional sound was recorded by Dave Rainey.
Alexis : This show was mixed by James Morrison and our theme music was composed by Zach Young.
Lisa : For Distillations I’m Lisa Berry Drago.
Alexis : And I’m Alexis Pedrick.
Alexis and Lisa : Thanks for listening!
Listen to more episodes

The Word for Blue
From Homer’s Odyssey to the internet’s great dress debate, our perception of the color blue has both fascinated and frustrated us.

Exploring ‘Health Equity Tourism’
With a new public interest in health equity research, who is actually receiving recognition and funding in the field?

The Mothers of Gynecology
Why are Black women in America three times more likely to die during childbirth than White ones?
Never Miss an Episode
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.
Acid rain: Causes, effects and solutions
How acid rain affects nearly everything it touches, and what we can do about it.

Solutions and prevention
Additional resources, bibliography.
Acid rain, or acid deposition, is a broad term that includes any form of precipitation that contains acidic components, such as sulfuric acid or nitric acid. The precipitation is not necessarily wet or liquid; the definition includes dust, gases, rain, snow, fog and hail. The type of acid rain that contains water is called wet deposition. Acid rain formed with dust or gases is called dry deposition.
The precipitation is not necessarily wet or liquid; the definition includes dust, gasses, rain, snow, fog and hail. The type of acid rain that contains water is called wet deposition. Acid rain formed with dust or gasses is called dry deposition.
Causes of acid rain
The term acid rain was coined in 1852 by Scottish chemist Robert Angus Smith, according to the Royal Society of Chemistry, which calls him the "father of acid rain." Smith decided on the term while examining rainwater chemistry near industrial cities in England and Scotland. He wrote about his findings in 1872 in the book " Air and Rain: The Beginnings of a Chemical Climatology ."
In the 1950s, scientists in the United States started studying the phenomenon, and in the 1960s and early 1970s, acid rain became recognized as a regional environmental issue that affected Western Europe and eastern North America.
Though manmade pollutants are currently affecting most acidic precipitation, natural disasters can be a factor as well. For example, volcanoes can cause acid rain by blasting pollutants into the air. These pollutants can be carried around the world in jet streams and turned into acid rain far from the volcano. After an asteroid supposedly wiped out the dinosaurs 65.5 million years ago, sulfur trioxide was blasted into the air. When it hit the air, it turned into sulfuric acid, generating a downpour of acid rain.
Even before that, over 4 billion years ago, it is suspected that the air may have had 10,000 times as much carbon dioxide as today. Geologists from the University of Wisconsin-Madison backed up this theory by studying rocks and publishing the results in a 2008 issue of the journal Earth and Planetary Science Letters. "At [those levels of carbon dioxide], you would have had vicious acid rain and intense greenhouse [effects]. That is a condition that will dissolve rocks," said study team member John Valley.
Sulfur dioxide (SO2) and nitrogen oxides (NOx) released into the air by fossil-fuel power plants, vehicles and oil refineries are the biggest cause of acid rain today, according to the Environmental Protection Agency (EPA). Two thirds of sulfur dioxide and one fourth of nitrogen oxide found in the atmosphere come from electric power generators.
A chemical reaction happens when sulfur dioxide and nitrogen oxides mix with water, oxygen and other chemicals in the air. They then become sulfuric and nitric acids that mix with precipitation and fall to the ground. Precipitation is considered acidic when its pH level is about 5.2 or below. The normal pH of rain is around 5.6.
Environmental affects of acid rain
Acid rain affects nearly everything. Plants, soil, trees, buildings and even statues can be transformed by the precipitation.
Acid rain has been found to be very hard on trees. It weakens them by washing away the protective film on leaves, and it stunts growth. A United States Environmental Protection Agency ( EPA ) study showed that acid rain is particularly hard on trees.
"By providing the only preserved soil in the world collected before the acid rain era, the Russians helped our international team track tree growth for the first time with changes in soil from acid rain," said Greg Lawrence, a U.S. Geological Survey scientist. "We've known that acid rain acidifies surface waters, but this is the first time we've been able to compare and track tree growth in forests that include soil changes due to acid rain."
Acid rain can also change the composition of soil and bodies of water, making them uninhabitable for local animals and plants. For example, healthy lakes have a pH of 6.5 or higher. As acid rain raises the level of acidity, fish tend to die off. Most fish species can't survive a water pH of below 5. When the pH becomes a 4, the lake is considered dead, according to National Atmospheric Deposition Program .
It can additionally deteriorate limestone and marble buildings and monuments, like gravestones.
There are several solutions to stopping human-caused acid rain. Regulating the emissions coming from vehicles and buildings is an important step, according to the EPA. This can be done by restricting the use of fossil fuels and focusing on more renewable energy sources such as solar and wind power.
Related: How do solar panels work?
Also, each person can do their part by reducing their vehicle use. Using public transportation, walking, riding a bike or carpooling is a good start, according to the EPA. People can also reduce their use of electricity, which is widely created with fossil fuels , or switch to a solar plan. Many electricity companies offer solar packages to their customers that require no installation and low costs.

It is also possible to prevent acid rain forming, by adding lime deposits to major water sources. This method has been used to neutralize the Ph levels in the water, which reduced the acidity, for thousands of years, the LA Times reported . These so-called "liming" operations have also been used to restore wildlife. In Wales, a liming operation was conducted in 2003 to restore salmon to the Wye river. The water had become too acidic for the fish to survive, causing them to disappear from the river 18 years earlier, Young People's Trust for the Environment , a U.K. non-profit organization, reported.
Discover key facts about acid rain on Young Peoples Trust for the Environment , watch this National Geographic video about the role of fossil fuels and pollution in creating acid rain, and learn more about what the WWF is doing to reduce emissions.
- Peringe Grennfelt, Anna Engleryd, Martin Forsius, Øystein Hov, Henning Rodhe & Ellis Cowling: Acid rain and air pollution: 50 years of progress in environmental science and policy
- Douglas A.Burns, Julian Aherne, David A.Gay, Christopher M.B.Lehmann: Acid rain and its environmental effects: Recent scientific advances
- Lesley Evans Ogden: Acid Rain: Researchers Addressing Its Lingering Effects
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
This year's hurricane season could see 25 named storms, NOAA says in record-breaking forecast
Earth from space: Rare phenomenon transforms African thunderstorm into giant ethereal 'jellyfish'
Epidurals may lower risk of complications after birth, study hints
Most Popular
- 2 100-foot 'walking tree' in New Zealand looks like an Ent from Lord of the Rings — and is the lone survivor of a lost forest
- 3 Save $400 on Unistellar's new smart binoculars during their early bird Kickstarter
- 4 10 'breathtaking' photos of our galaxy from the 2024 Milky Way Photographer of the Year contest
- 5 James Webb telescope finds carbon at the dawn of the universe, challenging our understanding of when life could have emerged
- 2 Shigir Idol: World's oldest wood sculpture has mysterious carved faces and once stood 17 feet tall
- 3 What is the 3-body problem, and is it really unsolvable?
- 4 Razor-thin silk 'dampens noise by 75%' — could be game-changer for sound-proofing homes and offices
- 5 Neanderthals and humans interbred 47,000 years ago for nearly 7,000 years, research suggests

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
- Effects of Acid Rain
On this Page:
- Effects of Acid Rain on Ecosystems
Effects of Acid Rain on Materials
Human health, the effects of acid rain on ecosystems.

An ecosystem is a community of plants, animals and other organisms along with their environment including the air, water and soil. Everything in an ecosystem is connected. If something harms one part of an ecosystem – one species of plant or animal, the soil or the water – it can have an impact on everything else.
Effects of Acid Rain on Fish and Wildlife
The ecological effects of acid rain are most clearly seen in aquatic environments, such as streams, lakes, and marshes where it can be harmful to fish and other wildlife. As it flows through the soil, acidic rain water can leach aluminum from soil clay particles and then flow into streams and lakes. The more acid that is introduced to the ecosystem, the more aluminum is released.
Some types of plants and animals are able to tolerate acidic waters and moderate amounts of aluminum. Others, however, are acid-sensitive and will be lost as the pH declines. Generally, the young of most species are more sensitive to environmental conditions than adults. At pH 5, most fish eggs cannot hatch. At lower pH levels, some adult fish die. Some acidic lakes have no fish. Even if a species of fish or animal can tolerate moderately acidic water, the animals or plants it eats might not. For example, frogs have a critical pH around 4, but the mayflies they eat are more sensitive and may not survive pH below 5.5.
Effects of Acid Rain on Plants and Trees
Dead or dying trees are a common sight in areas effected by acid rain. Acid rain leaches aluminum from the soil. That aluminum may be harmful to plants as well as animals. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
At high elevations, acidic fog and clouds might strip nutrients from trees’ foliage, leaving them with brown or dead leaves and needles. The trees are then less able to absorb sunlight, which makes them weak and less able to withstand freezing temperatures.
Buffering Capacity
Many forests, streams, and lakes that experience acid rain don’t suffer effects because the soil in those areas can buffer the acid rain by neutralizing the acidity in the rainwater flowing through it. This capacity depends on the thickness and composition of the soil and the type of bedrock underneath it. In areas such as mountainous parts of the Northeast United States, the soil is thin and lacks the ability to adequately neutralize the acid in the rain water. As a result, these areas are particularly vulnerable and the acid and aluminum can accumulate in the soil, streams, or lakes.
Episodic Acidification
Melting snow and heavy rain downpours can result in what is known as episodic acidification. Lakes that do not normally have a high level of acidity may temporarily experience effects of acid rain when the melting snow or downpour brings greater amounts of acidic deposition and the soil can’t buffer it. This short duration of higher acidity (i.e., lower pH) can result in a short-term stress on the ecosystem where a variety of organisms or species may be injured or killed.
Nitrogen Pollution
It’s not just the acidity of acid rain that can cause problems. Acid rain also contains nitrogen, and this can have an impact on some ecosystems. For example, nitrogen pollution in our coastal waters is partially responsible for declining fish and shellfish populations in some areas. In addition to agriculture and wastewater, much of the nitrogen produced by human activity that reaches coastal waters comes from the atmosphere.
- Learn more about Nitrogen Pollution
- EPA's Chesapeake Bay Program Office
Not all acidic deposition is wet . Sometimes dust particles can become acidic as well, and this is called dry deposition . When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and other manmade structures, and damage their surfaces. The acidic particles corrode metal and cause paint and stone to deteriorate more quickly. They also dirty the surfaces of buildings and other structures such as monuments.
The consequences of this damage can be costly:
- damaged materials that need to be repaired or replaced,
- increased maintenance costs, and
- loss of detail on stone and metal statues, monuments and tombstones.
Other Effects of SO 2 and NO X
In the atmosphere, SO 2 and NO X gases can be transformed into sulfate and nitrate particles, while some NO X can also react with other pollutants to form ozone. These particles and ozone make the air hazy and difficult to see through. This affects our enjoyment of national parks that we visit for the scenic view such as Shenandoah and the Great Smoky Mountains.
- Learn more about Visibility and Regional Haze
Walking in acid rain, or even swimming in a lake affected by acid rain, is no more dangerous to humans than walking in normal rain or swimming in non-acidic lakes. However, when the pollutants that cause acid rain —SO 2 and NO X, as well as sulfate and nitrate particles— are in the air, they can be harmful to humans.
SO 2 and NO X react in the atmosphere to form fine sulfate and nitrate particles that people can inhale into their lungs. Many scientific studies have shown a relationship between these particles and effects on heart function, such as heart attacks resulting in death for people with increased heart disease risk, and effects on lung function, such as breathing difficulties for people with asthma.
Learn more about:
- Sulfur Dioxide
- Nitrogen Oxides
- Particulate Matter (PM)
In addition, NO X emissions also contribute to ground level ozone, which is also harmful to human health .
- Learn more about Ground Level Ozone
- Clean Air Markets
- Clean Air Status and Trends Network (CASTNET)
- National Atmospheric Deposition Program (NADP)
- Long-Term Monitoring (LTM) Network
- Acid Rain Home
- What is Acid Rain?
- Acid Rain Program
- Acid Rain Program Results

Why dimming the Sun would be an effective tool in the fight against climate change
Lecturer in Earth Sciences, UCL
Disclosure statement
Peter Irvine receives funding from Horizon Europe. He acts as a scientific advisor to the Degrees Initiative, an NGO which funds developing world research into solar radiation modification geoengineering.
University College London provides funding as a founding partner of The Conversation UK.
View all partners
It’s becoming increasingly clear that we will fail to meet our climate goals. We were already at 1.26°C of warming in 2022 and are on track to blow through 1.5°C in the mid-2030s . Research even suggests that current climate policy will lead to more than 2.5°C of warming by the end of this century.
Warming of this magnitude would devastate vulnerable communities and ecosystems around the world. It’s time we consider something radically new that could stop climate change in its tracks.
After powerful volcanic eruptions, like Tambora (Indonesia) in 1815 and Pinatubo (Philippines) in 1991, global temperatures dip for a few years. Major eruptions create a hazy layer of microscopic particles in the upper atmosphere that last for several years, dimming the Sun temporarily. We could copy this effect to fight climate change.
The Earth is warmed by the Sun, but it is kept warm by greenhouse gases that trap the heat our planet gives off. The warming effect of our CO₂ emissions could be countered by creating a persistent, artificial haze like those seen following major volcanic eruptions. Research has found that we would only need to dim the Sun by around 1% to cool the planet by 1°C.
This may sound unlikely. But every engineering assessment to date has concluded that it would be feasible and relatively cheap to do using a fleet of high-flying jets to release reflective particles into the upper atmosphere.
So we could dim the Sun – but should we?

Cooling the planet would work
Dimming the Sun wouldn’t perfectly reverse climate change. The Sun’s warming effect is strongest during the day, in the summer and at the Tropics, whereas greenhouse gases warm everywhere and at all times.
However, we could create an even cooling effect across the world by adjusting where we release the particles. Research suggests that such an approach would greatly reduce climate risks .
Rising temperatures really matter. Species around the world are on the move , tracking familiar temperatures polewards as the planet warms up. But many won’t be able to keep pace with the changing climate and others have nowhere to go, so extinctions are projected to increase .
We are also seeing extreme heat that is edging closer to the absolute limits of the human body , putting lives at risk and limiting outdoor work.
As the planet heats up, warmer air is drawing more moisture from the soil in dry times, and dumping more out at once when it rains. This is making dry regions drier, wet regions wetter, and is intensifying both droughts and floods across the world.
Dimming the Sun would offset this effect. But it would still alter global wind and rainfall patterns.
Research indicates that this would mean smaller rainfall changes overall. However, a small minority of places could see more pronounced changes in rainfall compared to what they would face under climate change. Climate models disagree on the details of regional rainfall changes, so it’s unclear at this stage which regions would see the greatest change.
Blocking some sunlight would also be an effective way of keeping icy parts of the world frozen. Rising temperatures are causing the Antarctic and Greenland ice sheets to melt at an accelerating rate, driving up the global sea level . Climate change is also thawing permafrost (frozen soil that stores vast amounts of carbon) leading to the emission of more of methane and CO₂.

Side effects
Although dimming the Sun could keep the Earth cool, it would not deal with the root of the climate problem: the buildup of CO₂ and other greenhouse gases in the atmosphere. CO₂ not only warms the planet, it also acidifies the ocean , making it harder for corals and other creatures to form their shells. Dimming the Sun wouldn’t change this.
It would bring about some side effects as well. This hazy layer of particles would make the sky a little whiter . And if we copy volcanic eruptions by releasing sulphate particles to the upper atmosphere, then we’d also be adding to the acid rain problem .
These particles could impact the ozone layer too, which protects us from harmful UV rays. Research suggests that adding more sulphate particles to the upper atmosphere would delay the slow recovery of the ozone hole.
These side effects are a concern. But they pale in comparison to the impacts of climate change. A recent study found that the benefit of reduced extreme heat for human health could outweigh the health impacts of these side-effects by more than 50 to 1.
Paul Crutzen, who won a Nobel prize in 1995 for solving the chemistry of the ozone hole, was well aware of these side effects but nevertheless argued that we should start taking the idea of dimming the Sun seriously. In an article from 2006, he stressed that it would be best to cut CO₂ emissions rapidly so that we wouldn’t need to consider dimming the Sun at all. However, he lamented that “currently, this looks like a pious wish”.
Symptoms matter
It’s becoming increasingly clear that this “pious wish” isn’t coming true. Since Crutzen’s 2006 article, CO₂ emissions have surged by more than 15%. We just aren’t cutting emissions fast enough to prevent climate change from wreaking terrible damage.
Dimming the Sun would not address the root cause of the climate disease, and we must keep pushing to cut emissions, but a growing body of evidence suggests that it would work surprisingly well at treating the symptoms.
However, this is not so surprising. Ice melts when it is warm, hotter air carries more moisture and heat has a direct impact on life. We are far from knowing enough to recommend dimming the Sun today, but if countries don’t start taking this idea seriously we may miss a valuable opportunity to reduce the risks of climate change.

Don’t have time to read about climate change as much as you’d like? Get a weekly roundup in your inbox instead. Every Wednesday, The Conversation’s environment editor writes Imagine, a short email that goes a little deeper into just one climate issue. Join the 20,000+ readers who’ve subscribed so far.
- Climate change
- Carbon dioxide (CO2)
- Global warming
- Solar geoengineering
- Greenhouse gas emissions (GHG)
- Give me perspective

Head of School, School of Arts & Social Sciences, Monash University Malaysia

Chief Operating Officer (COO)

Clinical Teaching Fellow

Data Manager

Director, Social Policy
share this!
June 5, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Toxic forever chemicals are on the rise in Lake Michigan and have been detected in all of the Great Lakes
by Michael Hawthorne, Chicago Tribune

Toxic forever chemicals are on the rise in Lake Michigan, an alarming finding that reflects how the Great Lakes act like sponges soaking up pollution from near and far.
Rain and contaminated air are major sources of the contamination detected by a team of researchers from Indiana University and Canada's top environmental agency. So are discharges from sewage treatment plants and industries.
The new study found airborne concentrations of PFAS—per- and polyfluoroalkyl substances—are much higher near Chicago and other urban areas than at rural monitoring stations in northern Michigan and upstate New York. Previous research recorded similar patterns for flame retardants and other toxic chemicals .
But unlike many other contaminants, PFAS in rain were consistent throughout the Great Lakes region, likely because the chemicals are so widespread in the environment.
Levels detected in rain were the same near Chicago and at Sleeping Bear Dunes National Lakeshore, 223 miles northeast across Lake Michigan near Traverse City.
As the most comprehensive tracking of PFAS in the lakes to date, the study provides another example of how it is impossible to avoid exposure to the chemicals—some of which build up in human blood , cause cancer and other diseases and take years to leave the body.
"We need to take a broader approach to control sources releasing PFAS into the atmosphere and into bodies of water," Marta Venier, an environmental chemist at Indiana University and co-author of the study, said in an interview. "Eventually that pollution ends up in the lakes."
PFAS are called forever chemicals because their bonds of carbon and fluorine are nearly impossible to break—a quality that makes them attractive to manufacturers of products resistant to grease, heat, stains and water. But for decades 3M, DuPont and other PFAS makers hid from government regulators and the public what the corporations knew about the health risks.
In April, President Joe Biden's administration required every U.S. water utility to begin routinely testing for several PFAS in drinking water. Any utility that exceeds newly adopted federal limits will get five years to overhaul treatment plants to filter the compounds out of tap water.
Based on limited testing conducted by the U.S. Environmental Protection Agency and some states, thousands of utilities face expensive upgrades to their treatment plants. For now, though, it appears Chicago and other Illinois communities that depend on Lake Michigan for drinking water will not be required to do anything other than test for the chemicals.
Testing by the Chicago Department of Water Management and the Illinois EPA detected forever chemicals in treated Lake Michigan water but at levels below the new federal standards.
All told the Great Lakes provide drinking water to more than 40 million people in the United States and Canada, including 6.6 million in Illinois.
The new study found all of the lakes are contaminated with two PFAS that initially drew attention from scientists and regulators: perfluorooctane sulfonic acid (PFOS), used by 3M for decades to make Scotchgard stain repellent, and perfluorooctanoic acid (PFOA), sold to DuPont by 3M to manufacture Teflon coatings for cookware, clothing and wiring.
PFOS and PFOA no longer are made in the United States. Chemical manufacturers claimed other versions containing fewer carbon-fluorine bonds would be safer, but their own studies found the alternatives are just as dangerous, if not more so.
Levels of two alternative PFAS, known as PFBA and PFBS, are increasing in Lake Michigan and Lake Superior, the Indiana University and Canadian researchers found. PFOS, the original Scotchgard chemical, also is on the rise in the two lakes.
Lake Ontario had the highest PFAS concentrations, likely because it is downstream from the other Great Lakes. The chemicals also are flushing out of Lake Ontario more rapidly because it empties into the St. Lawrence Seaway and the Atlantic Ocean.
Venier said she welcomes the Biden administration's drinking water regulations for PFOA, PFOS and a handful of other forever chemicals. At the same time, she noted, industry has put some 15,000 PFAS into the marketplace during the past half-century and federal regulators have continued to approve new versions.
"We know enough about these chemicals," Venier said. "It's a matter of how much is enough to decide to stop putting more of them into our environment."
2024 Chicago Tribune. Distributed by Tribune Content Agency, LLC.
Explore further
Feedback to editors

Success follows failure less often than expected, study finds
8 minutes ago

Astronomers observe giant outburst of a distant X-ray binary
24 minutes ago

Astrophysicists calculate the likelihood that Earth was exposed to cold harsh interstellar clouds 2 million years ago
2 hours ago

Improved prime editing system makes gene-sized edits in human cells at therapeutic levels
4 hours ago

New method could allow multi-robot teams to autonomously and reliably explore other planets
22 hours ago

Virgin Galactic completes final spaceflight before two-year pause
Jun 9, 2024

Study finds fresh water and key conditions for life appeared on Earth a half-billion years earlier than thought
Jun 8, 2024

Saturday Citations: Praising dogs; the evolution of brown fat; how SSRIs relieve depression. Plus: Boeing's Starliner
Nonreciprocal quantum batteries exhibit remarkable capacities and efficiency

New method optimizes lithium extraction from seawater and groundwater
Relevant physicsforums posts, the secrets of prof. verschure's rosetta stones.
Jun 6, 2024
Is it possible to transform an electric thunderstorm into an EMP storm?
Jun 4, 2024
Jacchia Atmospheric Model
Jun 3, 2024
Iceland warming up again - quakes swarming
Mount ibu, indonesia erupts.
May 29, 2024
Adirondack Mountains and earthquakes
May 23, 2024
More from Earth Sciences
Related Stories

'Forever chemicals' found to rain down on all five Great Lakes
May 16, 2024

US says two 'forever chemicals' are hazardous, tells polluters to pay
Apr 19, 2024

EPA sets strict limit on PFAS 'forever chemicals' in US drinking water
Apr 10, 2024

US announces tough tap water standards for 'forever chemicals'

Q&A: What the EPA limits on 'forever chemicals' in water mean
Apr 16, 2024
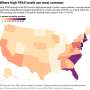
Ways you can filter out harmful PFAS from drinking water at home
Apr 22, 2024
Recommended for you

California wildfire pollution killed 52,000 in a decade: study
Jun 7, 2024

Wildfire smoke reached 99% of US lakes in 2019–2021: Study introduces 'lake-smoke day' metric

Scientists develop new method to estimate electrical parameters of regular pulse bursts in lightning

Faster alerts for California megaquakes: Early-warning system gets major upgrade
Coming in from the cold: Study reveals widespread negative experiences for women in polar research

New study finds Earth warming at record rate, but no evidence of climate change accelerating
Jun 5, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Gout and Diet: A Comprehensive Review of Mechanisms and Management
Yingling zhang.
1 School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
2 Engineering Research Center of Shanghai College for TCM New Drug Discovery, Shanghai 201203, China
3 Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
Associated Data
Data available in a publicly accessible repository.
Gout is well known as an inflammatory rheumatic disease presenting with arthritis and abnormal metabolism of uric acid. The recognition of diet-induced systemic metabolic pathways have provided new mechanistic insights and potential interventions on gout progression. However, the dietary recommendations for gouty patients generally focus on food categories, with few simultaneous considerations of nutritional factors and systemic metabolism. It is worthwhile to comprehensively review the mechanistic findings and potential interventions of diet-related nutrients against the development of gout, including purine metabolism, urate deposition, and gouty inflammation. Although piecemeal modifications of various nutrients often provide incomplete dietary recommendations, understanding the role of nutritional factors in gouty development can help patients choose their healthy diet based on personal preference and disease course. The combination of dietary management and medication may potentially achieve enhanced treatment effects, especially for severe patients. Therefore, the role of dietary and nutritional factors in the development of gout is systematically reviewed to propose dietary modification strategies for gout management by: (1) reducing nutritional risk factors against metabolic syndrome; (2) supplementing with beneficial nutrients to affect uric acid metabolism and gouty inflammation; and (3) considering nutritional modification combined with medication supplementation to decrease the frequency of gout flares.
1. Introduction
Throughout history, gouty disease has always been strongly associated with abundant foods and immoderate alcohol intake. Gout is even known as the “king’s disease” and symbolized social status in ancient times, as only the upper class could afford to consume wine and meats [ 1 ]. Nevertheless, gout currently has been well established as a global health problem and has gained attention due to its increasing incidence rate, multiple metabolic comorbidities, and high premature mortality [ 2 ]. Gout is well known as a phlogistic arthritis that is associated with hyperuricemia and elevation of urate in tissues. The increased urate causes the generation of monosodium urate (MSU) crystals, and MSU crystal deposition in and around the first metatarsophalangeal joint, knee, and fingers represents a clinical sign of gout [ 3 ]. The clinical symptoms of gout develop in several stages, including asymptomatic hyperuricemia, MSU crystal formation, intermittent gout and chronic gout [ 4 ]. Effective gout management mainly relies on the use of therapeutic strategies to control uric acid levels or achieve crystal dissolution. While current clinical principles based on medicinal management for gout have been well implemented [ 5 ], dietary modification and lifestyle changes have also been recommended for gout patients, since a suboptimal diet and obesity/diabetes-diseases of affluence contribute significantly to the risk of developing gout [ 5 , 6 , 7 ], increasing the burden of medical expenses. At present, dietary recommendations have been updated worldwide, and nutritional science for the management of gout has advanced dramatically [ 8 , 9 ]. Historical dietary recommendations for gouty patients tend to emphasize the concepts of “high-” and “low-” levels of the same nutrient, but the role of nutritional elements in gout is difficult to classify by a single beneficent or harmful criterion because problems of systemic metabolism arise when the balance between nutrient intake and consumption is disturbed. This means that although dietary management is considered to be an essential aspect of gout therapeutic strategies [ 2 ], a potential dietary mechanism in gout development is out of date or incomprehensive, and a systemic overview of dietary and nutritional factors of gout is needed for well-designed dietary management based on research practice. Moreover, studies have shown that dietary factors mainly focus on food classification, while diet-induced systemic metabolism is rarely mentioned in the progression of gout. It is worthwhile to comprehensively review the mechanistic findings of diets aimed at purine metabolism, urate deposition and gouty inflammation. Our acknowledgment of gout-related nutritional factors can provide a theoretical basis for on-target and comprehensive dietary guidelines for gout patients with different complications or at different stages.
2. Role of Dietary Consumption in the Progression of Gouty Diseases
2.1. uric acid disturbance: emphasis on purines, 2.1.1. formation of uric acid.
Purines are the basic components of nucleotides needed for building DNA and RNA within mammalian cells, and purine nucleotides, such as guano-sine-5′-triphosphate and adenosine triphosphate (ATP), are essential for regulating energy metabolism and intracellular functions, respectively. Disorders of purine metabolism are associated with considerable variations in the concentration of serum uric acid (SUA) because uric acid is the ultimate product of purine catabolism in humans. Purine source analyses show that nearly two-thirds of purines in the body are endogenous, and the remaining purines that enter the body via foods are known as exogenous purines [ 10 ]. As a direct source of exogenous nucleotides and uric acid, dietary purines are vital for maintaining the balance of purine metabolism in mammalian cells by a coordinated process of de novo biosynthesis, the salvage pathway, and purine inter-conversion or degradation. As shown in Figure 1 , these exogenous purines can be dephosphorylated into nucleosides in the body as part of the digestive course, accompanied by the oxidative release of free bases [ 11 ]. Subsequently, the degraded bases can be recycled into nucleotides in the tissues via the purine salvage pathway or wholly degraded into uric acid mainly within the liver or the small intestine [ 11 , 12 ]. Adenosine monophosphate (AMP) and guanine monophosphate (GMP) are usually the predominant forms of purine nucleosides derived from foods. The enzymatic degradation process uses deaminase and GMP reductase to convert AMP and GMP, respectively, to inosine monophosphate (IMP) [ 13 ]. AMP/GMP can also be dephosphorylated to generate adenosine/guanosine in a process catalyzed by nucleosidase. Both IMP and adenosine are then processed into inosine. The transformation of inosine to hypoxanthine is catalyzed by purine nucleoside phosphorylase, and eventually hypoxanthine utilizes the dual oxidation of xanthine oxidase (XO) to produce uric acid. Additionally, the conversion of guanosine into uric acid can be catalyzed by guanine deaminase following guanine formation by nucleotidase [ 13 ]. When purine overload in the body empowers the body’s ability to manage it, excessive uric acid can accumulate in the bloodstream. This condition presenting with an elevated SUA concentration is known as hyperuricemia, and gout induced by hyperuricemia is deemed to be the metabolic disease linked to purines. All meats and edible plants contain purines, and some foods contain higher concentrations. Thus, overindulgent intake of a high-purine diet, including seafoods and animal offal, can trigger the excessive accumulation of purine metabolites, giving rise to the excessive accumulation of uric acid in the body [ 5 ]. In addition, some purine-free drinks can accelerate the promotion of purine degradation; for example, alcohol intake consumes large amounts of ATP to produce AMP in the liver, leading to the rapid occurrence of increased SUA levels [ 14 ]. The consumption of yeast-rich foods, such as bread and yeast drinks, can lead to a high colonization of Saccharomyces cerevisiae in the gut [ 15 ], which can gradually elevate the secretion of uric acid in the host.

Potential mechanisms of diet-induced gout progression in humans. Diets provide abundant raw materials of purine, which is mainly metabolized in the liver, promoting uric acid production. Meanwhile, it can interfere with the intestinal environment, homeostasis, and urate transport to induce high levels of uric acid, leading to hyperuricemia and ultimately to gout. Additionally, gouty inflammation is caused by IL-1β production after the activation of NLRP3 by macrophages that ingest MSU crystals, and a second signal is required in humans by stimulating the activation of TLR signaling pathways that can be induced by diets. Moreover, neutrophil infiltration and diet-induced low-grade inflammatory states will exacerbate gouty inflammation. AMP, adenosine monophosphate. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain. GMP, guanine monophosphate. IL, interleukin. IMP, inosine monophosphate. LPS, lipopolysaccharide. MSU, monosodium urate. MyD88, myeloid differentiation factor88. NF-κB, nuclear factor kappa B. NLRP3, pyrin domain-containing protein 3. TLR, toll-like receptor. TNF, tumor necrosis factor. XO, xanthine oxidase.
2.1.2. The Excretion of Uric Acid
Under normal conditions, nearly 90% of uric acid is reabsorbed into the human body, and the remaining uric acid is excreted in the feces and urine [ 16 ]. When purines exceed the limit value for normal production and catabolism, the synthesis and excretion of uric acid are out of balance, and the circulating uric acid level is elevated. The kidneys are responsible for eliminating approximately two-thirds of circulating uric acid, with the remaining one-third excreted by the intestine and gut microbiota [ 12 ]. Diets could affect uric acid excretion by regulating the excretory function of the kidney and intestine. Because foods can come into contact with the intestinal tract and regulate intestinal homeostasis [ 17 ], dietary factors are involved in intestinal urate handling mechanisms. Endogenous uric acid from the bloodstream or as a constituent of saliva, bile, or peptic juices transfer from the enterocyte cytoplasm into the intestine tissues. In this process, enterocyte urate transporters are critical for maintaining urate homeostasis in the intestine, and an enterocyte-specific deficiency of these transporters, including ATP-binding cassette transporter G2 (ABCG2/BCRP) and NPT5 (SLC17A4), can impair the enterocyte urate transport process, which is also affected by exogenous metabolism, such as dietary fat and sugar [ 18 ]. Similarly, a study of 8709 participants suggested that high simple sugar exposure was found to interfere with the ability of SLC2A9 (encoding GLUT9) to mediate renal uric acid excretion without additive genotype-specific interaction [ 19 ]. After entering the intestinal tissue, uric acid can be degraded into nitrogen or CO 2 by the uricase activity found in the gut microbiota. The associations between diet-induced gut microbiota reconstruction and the progression of hyperuricemia/gout have been highlighted in recent research, as evidenced by the fact that long-term adherence to the typical Western diet caused an obvious reduction in the diversity of the gut microbiota, particularly those that degrade uric acid and produce metabolites known to benefit uric acid excretion [ 20 ]. For example, as a microbiota-derived metabolite, the short-chain fatty acid butyric acid was thought to promote intestinal uric acid excretion [ 21 ]; however, a fat-rich diet reduces the abundance of beneficial bacteria that produce short-chain fatty acids [ 20 ]. In addition, the diet is mainly designed to provide calories for energy expenditure, and the related energy metabolism and metabolites produced as a result of dietary modification can affect uric acid excretion. By way of illustration, a ketogenic diet converts energy metabolism substrates from sugars to fats with the production of large ketone bodies such as acetyl acetate and β-hydroxybutyrate (BHB) [ 22 ], which induce fluid acidification and cause uric acid precipitation. The production of BHB inhibits uric acid excretion by competing for binding sites of uric acid transporters.
2.2. Uric Acid Disturbance: Emphasis on Purines
Monosodium urate crystals formation occurs when the urate concentrations continue to rise beyond the point required for spontaneous generation (nucleation). The increased volume of MSU deposition can aggravate the progression of symptomatic gout [ 23 ]. Diets that contribute to excessive SUA levels can induce the formation and deposition of MSU crystals, and other factor changes, such as diet-induced fluid acidification and salt deposition, also promote the growth of urate crystallization [ 24 ]. Alcohol consumption and fasting can induce elevated lactic acid levels to decrease the local pH to create an acidic condition, which might be a risk factor for MSU deposition, as the increased concentrations of calcium ions in an acidic environment aggravate the decrease in MSU crystal solubility [ 25 ].
2.3. Gouty Inflammation
Some observations show that in some circumstances, uric acid can show antioxidant properties in the form of urate. However, soluble urate acts as a proinflammatory stimulus to fuel the maturation and production of interleukin (IL)-1β [ 26 ], thus strongly driving acute gouty inflammation and giving rise to chronic long-term inflammatory consequences of the disease. It is known that the pathogenesis of gouty inflammation involves the cleavage of C5 and generation of C5a and C5b-9 on the surface of urate crystals [ 27 ]. Urate crystal deposition is also extensively recognized as a danger signal for the influx of innate immune cells [ 28 ]. Mechanistically, MSU crystal-induced inflammatory gouty flares are caused by the activation of pyrin domain-containing protein 3 inflammasome (NLRP3) with consequent IL-1β secretion from macrophages and neutrophils, resulting in acute inflammatory responses, intense pain and joint swelling [ 28 ]. The initiation of NLRP3-dependent IL-1β activation includes a priming signal associated with nuclear factor κB (NF-κB) and a secondary signal of caspase-1 assembly activation; thus, MSU crystals alone are insufficient to induce IL-1β secretion. Additionally, other endogenous costimulatory factors, such as myeloid-related protein-8/-14 in phagocytes, can increase MSU crystals-mediated IL-1β secretion in a TLR-4-dependent pathway [ 27 ].
Diets also have a significant impact on the systemic phenotype of the innate immune system. For example, a Western diet or meat-based patterns intersect with a low-grade inflammatory response, which permanently biases the immune system toward a proinflammatory phenotype [ 29 ], fueling gouty inflammation. In contrast, the Mediterranean diet or vegetable- and fruit-based patterns have been reported to prevent systemic inflammation and gouty flares [ 30 ]. A fiber-rich diet has been proven to rapidly resolve the urate crystal-mediated inflammatory response in a gout-like mouse model [ 31 ]. Diet intervention affecting the neutrophil inflammasome has been explored. For example, a ketogenic diet alleviates urate crystal-induced gouty flares by increasing BHB, which can block NLRP3/caspase-1-dependent IL-1β expression in neutrophils and urate crystal-activated macrophages, reducing inflammatory neutrophil recruitment [ 32 ].
3. Nutrient Element-Richness and Structure Determine the Role of Dietary Factors in Gout
A large amount of clinical evidence, shown in Table 1 , indicated the close connection between adherence to the described dietary patterns and the risk of gout-related metabolic disorders, hyperuricemia, and metabolic syndrome. Commonly described dietary patterns, such as a high-carbohydrate diet, a high-protein diet, and a high-unsaturated fat diet, actually represent the proportional and structural collocation of dietary nutrient elements [ 33 ]. It has been shown that the beneficial dietary patterns against hyperuricemia usually contain a higher intake of vitamins, fiber, and unsaturated fatty acids and are often supplemented with appropriate amounts of minerals and high-quality protein, promoting a health state in which systemic metabolism is prone to disease improvement [ 34 , 35 ]. These observations can be explained by the fact that important nutrients in foods can be considered determinants of dietary factors in gouty development. For example, the Western diet is characterized by a high level of sugar, while fiber richness in the Dietary Approaches to Stop Hypertension (DASH) diet can increase satiety to reduce sugar intake from other high-energy foods and play a vital role in decreasing gout incidence [ 34 ]. Therefore, the influence of dietary factors on gouty disease is the effect of nutrient element-richness and structures on systemic metabolism. Understanding the potential mechanisms of nutrients, as shown in Figure 2 , in gout development can facilitate an understanding of the overall nutritional balance needed for the prevention or treatment of gout.

Nutrition-induced systemic metabolism involved in gouty disease. Metabolites of fat, carbohydrate and protein and the resulting metabolic diseases promote the development of gout, including changing intestinal flora, accelerating purine metabolism, promoting MSU deposition, activating macrophages, and inhibiting uric acid excretion. ADP, adenosine diphosphate. AMP, adenosine monophosphate. ATP, adenosine triphosphate. FFAs, free fatty acids. F6P, fructose 6 phosphate. KHK, ketohexokinase. LPS, lipopolysaccharide. MSU, monosodium urate. NAFLD, nonalcoholic fatty liver disease. NAFPD, nonalcoholic fatty pancreas disease. TG, triglyceride. TLR, toll-like receptor. UA, uric acid. XO, xanthine oxidase.
Dietary intervention associated with the improvement of serum uric acid and indicators of metabolic syndrome.
| Intervention Group | Control Group | Participants | Period | Major Findings |
|---|---|---|---|---|
| Low-carbohydrate (≤20 g/day) and high-fat diet [ ] | Habitual diet (carbohydrate ≤ 20 g/day) | 30 heathy persons (ages ≥ 18 years) | 3 weeks | UAM: urate significantly ↑ in the LCHF group MS in the LCHF group: •apolipoprotein B, TC, HDL-C significantly ↑ •FFA and urea significantly ↑ •mean plasma LDL-C ↑ |
| DASH diet with low, medium, and high sodium levels [ ] | The average American diet | 103 subjects (average age of 51.5 years) with pre- or stage 1 hypertension | 30 days | UAM: •mean SUA ↓ in the DASH diet group vs. the control group •SUA ↓ in medium and high sodium intake when aggregated across both diets |
| Fruit-rich and soybean products diet (Group 1) [ ] | Standard diet for hyperuricemia (Group 2) | 187 Chinese adults (ages 20 to 59 years) with asymptomatic hyperuricemia | 3 months | UAM: SUA ↓ in the Group 1 and Group 2 vs. baseline MS: •HDL-C significantly ↑ in the Group 1 vs. the baseline •BMI, TC and TG significantly ↓ in the Group 2 vs. the baseline |
| Low-salt diet followed by a high-salt diet [ ] | / | 90 subjects with similar dietary habits (ages 18 to 65 years) | 17 days | UAM: •PUA significantly ↑ in the low-salt diet group and PUA significantly ↓ in the high-salt diet group vs. baseline •24 h UUA significantly ↓ in the low-salt diet group and the high-salt diet group vs. baseline |
| 2 apples/day for 8 weeks, and then after a 4-weeks Washout period, consumed 500 mL of control beverage daily for a further 8 weeks (Group 1), or received the intervention foods in the reverse order (Group 2) [ ] | / | 40 healthy and mildly hypercholesterolemic Volunteers (ages 29 to 65 years) | 20 weeks | UAM: SUA ↑ in the Group 1 vs. the Group 2 MS: TC, LDL-C, TG and ICAM-1 significantly ↓ in the Group 1 vs. the Group 2 |
| Regular cola (SSSD); Diet cola; Isocaloric semiskimmed milk; Water [ ] | / | 47 overweight and obese adults (ages 20 to 50 years) | 6 months | UAM: PUA significantly ↑ in the SSSD group vs. other groups MS: •VAT significantly ↑ in the SSSD group vs. other beverages, and in liver fat of more than two-fold •Plasma TG ↑ in the SSSD group vs. the milk, the diet cola and the water group |
| High-carbohydrate diet (CARB); High-protein diet (PROT); High-unsaturated fat diet (UNSAT) [ ] | / | 163 subjects (ages ≥ 30 years) | 6 weeks | UAM: •SUA ↓ in PROT group vs. baseline •SUA significantly ↓ in PROT group vs. the CARB and UNSAT group |
| Pakistani almonds (PA); American almonds (AA) [ ] | No intervention | 150 patients with coronary artery disease (ages 55 to 63 years) | 12 weeks | UAM: •SUA ↓ in the PA group and the AA group at week 6 and week 12 vs. the NI group |
| High-carbohydrate and high/low-glycemic index diet (CG/Cg); low-carbohydrate and high/low-glycemic index diet (cG/cg) [ ] | / | 163 overweight or obese adults without cardiovascular disease (ages ≥ 30 years) | 5 weeks | UAM: •PUA ↓ in the Cg group and PUA ↑ in the cG group vs. baseline •PUA ↓ in the Cg group vs. the CG group •PUA ↓ in the cg group vs. the cG group •PUA ↑ in the cG group vs. the CG group |
| Yogurt with 300 g/day of probiotic [ ] | Regular yogurt | 44 metabolic syndrome patients (ages 20 to 65 years) | 8 weeks | UAM: •SUA ↓in the probiotic yogurt group •significantly changes in UA level MS in the probiotic yogurt group: •MDA and oxidized LDL ↓ •TAC ↑ |
| Fruit and vegetable (FV)-rich diet; DASH diet [ ] | Typical American diet | 459 subjects with blood pressure (<160 mmHg, 80–95 mmHg) (ages ≥ 30 years) | 8 weeks | UAM: •SUA ↓ in the FV group and SUA ↓ in the DASH group •effects increased in DASH group with increasing baseline SU levels |
| 100% orange juice; caffeine-free cola [ ] | / | 26 healthy adults have a habitual three-meals-per-day structure (ages 20 to 45 years) | 2 weeks | UAM: SUA significantly ↓ and UUA significant ↑ in the orange juice group vs. baseline MS: daylong glycemia and glucose variability significantly ↑, 24 h insulin secretion and serum potassium levels significantly ↓ in the cola group vs. orange juice group |
| High-resistant starch with low-protein flour staple (Group 1) [ ] | Protein-restriction diet | 75 patients with early type 2 diabetic nephropathy (ages 18 to 80 years) | 12 weeks | UAM: SUA ↓ in the Group 1 MS: fasting BG, HbA1c, TC and TG significantly ↓ in the Group 1; serum superoxide dismutase level b2-microglobulin ↑ in the Group 1 |
| Sugar-sweetened soda or reduced-fat milk [ ] | / | 30 overweight or obese subjects (males, ages 13 to 18 years) | Not specified | UAM after the milk intake phase: UA significantly ↓ MS after the milk intake phase: systolic blood pressure significantly ↓ after the milk intake phase |
| DASH diet followed by self-directed grocery purchases (DDG) or the reverse order (SDG) [ ] | / | 43 gouty participants without taking urate lowering therapy (ages ≥ 18 years) | 8 weeks | UAM: •SUA ↓ in the DDG group during Period 1 •SUA ↓ in the SDG group and SUA ↓ in the DDG group after crossover (Period 2) MS: total spot urine sodium excretion ↓ in the DDG group |
| Standard metabolic diet (beef, fish, or chicken) [ ] | / | 15 healthy subjects (ages 18 to 70 years) | Not specified | UAM: •SUA significantly ↑ for each diet phase, and beef was associated with lower SUA than chicken or fish •fish was associated with significant UUA ↑ than beef or chicken •calcium oxalate significantly ↑ in the beef diet phase vs. the chicken diet phase |
| 3 servings of 100% naturally sweetened orange juice (OJ)/day [ ] | 3 servings of sucrose-sweetened beverages (sucrose-SB)/day | 20 healthy and overweight women (ages 25 to 40 years) | 2 weeks | UAM: PUA significantly ↑ in the sucrose-SB group, and PUA ↓ in the OJ group vs. AUC of baseline MS: •BW significantly ↑ in the sucrose-SB group vs. baseline •BW ↑ in the sucrose-SB group vs. OJ group •Matsuda insulin sensitivity index ↓ in both group |
| High-fructose corn syrup (HFCS): 0% (aspartame sweetened), 10%, 17.5%, 25% Ereq-HFCS [ ] | / | 187 participants (ages 18 to 40 years) | 2 weeks | UAM: 24-h mean PUA significantly ↓ in 10%, 17.5% and 25% HFCS group vs. the 0% group MS: postprandial TG and fasting LDL-C significantly ↑ in 10%, 17.5% and 25% HFCS group vs. the 0% group |
| Tomatoes [ ] | / | 35 Caucasian women (ages 18 to 25 years) | 4 weeks | UAM: PUA ↓ vs. baseline MS: mean BW, fasting BG, TG, C ↓ vs. baseline |
| High-calcium fat-free milk session and followed by consumption of low-Ca control session (HC group) or the reverse order (LC group) [ ] | / | 14 type 2 diabetes subjects with habitual low calcium intake (ages 20 to 59 years) | 32 weeks | UA: SUA ↓ in the HC group and SUA significantly ↑ in the LC group MS: •25-hydroxyvitamin D significantly ↑, fructosamine and parathormone significantly ↓ in the HC group • 25-hydroxy-vitamin D significantly ↑ in the HC group vs. the LC group • Hb1Ac significantly ↑ and HOMA2-%B significantly ↓ in the LC group |
| 500 mL orange beverage (OB)/day [ ] | Not consume OB | 30 healthy volunteers (average age of 33.9 years) | 2 weeks | UAM: PUA significantly ↓ in the OB intervention phase vs. both of baseline and washout phase MS: • ORAC ↑ while CAT, TBARS and -reactive protein ↓ in the OB intervene phase vs. baseline •CAT, TBARS and oxidized LDL ↓ after the wash out phase vs. baseline |
| High-fructose or high-glucose diet [ ] | / | 32 healthy but centrally overweight men (ages 18 to 50 years) | 10 weeks | UAM: SUA ↑ in the fructose group SUA ↓ in the glucose group MS: •the risk of insulin resistance ↑ in the fructose diet group vs. the glucose diet •BG, TAG and biochemical assays of liver function ↑ in both group |
| Diet rich in whole grain (WG) products for 3 weeks followed by red meat (RM), or the reverse order [ ] | / | 20 healthy adults (ages 20 to 60 years) | 10 weeks | UAM: SUA significantly ↑ during RM intervention MS: •BMI, body fat mass and BW significantly ↓ in the WG group compared to baseline and after washout •creatinine significantly ↑ during RM intervention GB: • appearing after WG intervention • sp. ↑ after RM intervention |
| Low-fat and restricted-calorie diet; Low-carbohydrate and non–restricted-calorie diet Mediterranean and restricted-calorie; [ ] | / | 235 participants with moderate obesity (ages 40 to 65 years) | 24 months | UAM: •SUA ↓ at 6 months and 24 months among all participants •the effect of SUA ↓ in all group was positively correlated with baseline MS: BW, HDL-C, TC: HDL-C, TG, insulin resistance significant improved in all three groups |
| 1.5 L of a mineral water with 2.673 mg HCO /L [ ] | The same amount of water with 98 mg HCO /L | 34 patients with multiepisodic calcium oxalate urolithiasis (average age of 52.7 years) | Not specified | UAM in the intervention group: •UUA supersaturation, significant ↓ •pH -value in the intervention group, significant ↑ ( < 0.001) |
| Total energy value: 40% from carbohydrates, 30% from proteins and 30% from lipids, <300 mg/day of fatty acids and cholesterol (RESMENA group) [ ] | Total energy value: 55% from carbohydrates, 15% from proteins and other treatments were the same as the intervention group | 41 women and 52 men with metabolic syndrome (ages 40 to 65 years) | 6 months | UAM: SUA significantly ↑ in the control group vs. baseline MS: •waist circumference, BMI, BW, waist: hip ratio, android fat mass and alanine aminotransferase and aspartate aminotransferase significantly ↓ in RESMENA group vs. baseline • glucose and aminotransferase significantly ↑ in the control group •LDL-C and HDL-C significantly ↑ in treatment groups vs. baseline |
| Isocaloric diets: 30% of energy from animal (AP) or plant (PP) protein [ ] | / | 44 type 2 diabetes patients (ages 18 to 80 years) | 6 weeks | UAM: SUA ↓ in both groups MS: •M-value of insulin sensitivity significantly ↑ in the AP group vs. baseline •TC, LDL-C, HDL-C ↓ in both groups •fasting nonesterified fatty acids significant ↓ in the PP group vs. baseline •CRP significantly ↓ in the AP group |
| DASH diet [ ] | The typical American diet. | 103 prehypertensive or hypertensive adults (ages ≥ 22 years) | 90 days | UAM: •SUA ↓ at 30 and 90 days in the DASH group •SUA ↓ at 30 and 90 days in the DASH group when participants with baseline SUA ≥6 mg/dL |
| Soy protein trial: soy protein group (soy protein and isoflavones); isoflavone group (milk protein and isoflavone); Soy flour trial: whole soy group (soy flour); daidzein group (low-fat milk powder and daidzein) [ ] | Soy protein trial: milk protein Soy flour trial: low-fat milk powder | 450 postmenopausal women with either prediabetes or prehypertension (ages 48 to 65 years) | 6 months | UAM: •SUA significantly ↓ in the soy flour and soy protein groups (SCF group) compared with the isoflavone and daidzein groups and the milk placebo groups (MP group) •UA net decrease and UA% decrease between the SCF group and the MP group |
| Drinking filtered soup (250 g of fresh + 1000 mL water) at least an hour before breakfast every other day [ ] | / | 5168 subjects (ages ≥ 40 years) | 6 weeks | UAM: SUA significantly ↓ in the intervention group |
| Rice bran oil plus a standard diet (RBO) [ ] | Sunflower oil plus a standard diet (SO) | 40 patients with severe CAD undergoing angioplasty (ages 30 to 70 years) | 8 weeks | UAM: SUA ↓ in the RBO group MS: TG, BG, TC, LDL and TNF-α ↓ in the RBO group |
| The powders of lotus root and cucumber (first, they were squeezed into juices, and then freeze-dried under vacuum) in warm water [ ] | / | 25 men and 9 women (ages > 60 years) | 30 days | UAM: •PUA ↓ in both of lotus root group and cucumber group MS: •plasma glutathione peroxidase ↑ in both of lotus root group and cucumber group •blood mononuclear cell DNA damage ↓ in the lotus root group |
Note: data are from clinical trials that have been included in PubMed since 2012. A direct search was used to search for the following terms: “diet and uric acid” or “diet and gout” or “food and uric acid” or “food and gout”. A total of 462 articles were obtained. After following these exclusion criteria—repetitive articles, acute trials, dietary supplements, combination of drugs and food, questionnaire survey, exercise interference, and mismatched intervention subjects—a total of 32 articles showed the effect of diet on uric acid and other indicators of metabolic syndrome. ↑—increase; ↓—decrease; AA—amino acid; AUC—lower area under the curves; BG—blood glucose; BW—body weight; CRP— C -reactive protein; DASH—Dietary Approaches to Stop Hypertension; Ereq—energy requirement; HDL—high density lipoprotein; ICAM-1—intercellular cell adhesion molecule-1; LDL—low density lipoprotein; MDA—malondialdehyde; MS—indicators of metabolic syndrome; OR—odds ratio; PUA—plasma uric acid; SUA—serum uric acid; TBARS—thiobarbituric acid reactive substance. TC—total cholesterol; TG—triglyceride; UAM—indicators of uric acid metabolism; UUA—urine uric acid.
3.1. Energy-Type Nutrition Overload Can Induce Hyperuricemia and Inflammation
3.1.1. high fat.
Dietary fat is primarily metabolized into triglycerides within intestines and packaged as chylomicrons for delivery to peripheral tissues, where adipocytes further transfer triglycerides into free fatty acids (FFAs) for energy uptake and storage [ 67 ]. A small amount of FFAs can be absorbed by the liver tissue, together with lipids remaining in the chylomicron [ 67 ]. High-fat foods can evoke the pleasure of eating and promote the individual’s desire to consume more energy-dense diets [ 68 ], which culminates in the overproduction of FFAs. Free fatty acids-mediated metabolic events initiate acute onset of gouty disease in the presence of MSU crystals deposited in the joint. The interaction of FFAs with TLR2 synergized with MSU crystals leads to the release of IL-1β induced by ASC/caspase 1 [ 69 ].
High fat consumption can cause excessive accumulation of triglycerides, inducing increased fat mass and obesity. It has been reported that overweight/obesity was connected with 60% of hyperuricemia cases in a clinical trial of 14,624 adults [ 70 ], possibly due to lipid metabolic disorder promoting purine metabolism by elevating XO activity [ 71 ]. Hypertrophy and hyperplasia of adipocytes are associated with higher oxygen consumption, which evokes hypoxic damage in other tissues, resulting in the chronic inflammation of obesity [ 72 ]. Nonalcoholic fatty liver disease and nonalcoholic fatty pancreatic disease subsequently occur when lipid overload occurs in the liver and pancreatic tissue, causing metabolic dysfunction in both and affecting acid-base balance. Metabolic acidosis further promotes hypercalciuria, low urine pH and hypocitraturia, predisposing patients to MSU crystal deposition and calcium renal stone formation [ 73 ]. In response to excessive FFAs circulation, insulin secreted from pancreatic β-cells upregulates the expression of renal urate transporters, including GLUT9 and URAT1, and decreases ABCG2 levels, promoting high SUA levels [ 18 ]. Uric acid conversely induces lipid accumulation and insulin resistance (IR), thereby forming a vicious cycle of uric acid and insulin [ 74 ].
Prior to the onset of IR and obesity, high fat intake has been found to upregulate the expression of reactive oxygen species (ROS) in adipose tissue and liver, along with the metabolic disturbance of adipocytes and the dysregulation of adipokine release [ 75 ], promoting or aggravating MSU-mediated NF-κB-dependent inflammation. For example, leptin levels secreted from adipose tissues were elevated in patients with gouty inflammation, and leptin can facilitate MSU-induced acute gout-related proinflammatory cytokine production in macrophages and synoviocytes [ 76 ]. The key adipocyte-derived chemokines McP-1 and LTB4 recruit proinflammatory macrophages to induce inflammation amplification, thus aggravating gouty inflammation [ 77 ]. Furthermore, a high-fat diet changes gut microbiota composition, leading to reduced microbiota diversity and an increased ratio of Firmicutes to Bacteroidetes and to the reduction of microbiota-derived beneficial metabolites such as butyric acid, which further aggravates gouty arthritis [ 20 ].
3.1.2. High Sugar
Sugars are the most abundant macromolecules in nature and can be classified according to their structure into monosaccharides, complex carbohydrates, and glycoconjugates [ 78 ]. They are the primary carbon source for ATP production and cellular biosynthesis. Sugars from diets can be absorbed as glucose, galactose or fructose in the liver portal circulation. The liver and gut normally process galactose and fructose into lactate, glucose and organic acids through gluconeogenesis, glycogenolysis, aerobic oxidation and other pathways [ 78 ]. High sugar consumption might initiate metabolic disease processes accompanied with hyperglycemia, IR, and fat accumulation. Moreover, high sugar intake in obese patients increases serum urate and decreases the percent of uric acid to creatinine clearance, indicating a close association between hyperuricemia and a high sugar diet [ 79 ]. Sugar-sweetened beverages containing high-fructose corn syrup and sucrose or almost equal amounts of fructose and glucose, which account for approximately one-third of added sugar consumption in the diets of American adults [ 80 ], have been thought to be closely connected with a high prevalence of hyperuricemia in Western countries [ 81 ]. Long-term high sugar consumption has been found to accelerate the accumulation of uric acid and promote MSU deposition in fly renal tubules, suggesting that a similar problem may occur in human excretory systems under dietary challenges [ 82 ]. In a follow-up study of 650 participants, the results confirmed that a high-sugar diet participates in kidney dysfunction and uric acid metabolism disorders [ 82 ].
The metabolic effects of sugar are distinct from those of starch principally because of the fructose component. Studies of dietary sugar intervention in animals and humans have demonstrated that overconsumption of fructose, but not glucose, can manifest multiple traits of metabolic syndrome [ 83 , 84 , 85 ], indicating that fructose might be responsible for high sugar-driven hyperuricemia and gout [ 86 ]. Fructose metabolism starts within the small intestinal tissue, where fructose can be absorbed by the facilitative hexose transporter GLUT5 (SLC2A5) and converted by ketohexokinase. Notably, exposure to high fructose increases the intestinal villus length to expand the surface area of intestinal cells that can absorb more nutrients from food [ 87 ], possibly aggravating high fructose-mediated metabolic disorders via overconsumption of nutrients. Excessive fructose consumption-induced gouty syndrome is related to the altered gut microbiota and its metabolites and induces inflammation and fatty acid disorders. Since high fructose intake induces the proliferation of mucus-degrading bacteria in the gut microbiota, decreased mucus glycoproteins can promote intestinal barrier damage and pathogen invasion [ 88 ].
Fructose-derived metabolites can be transferred from the intestinal tissues to the liver and systemic circulation, and the redundant fructose can also directly reach the hepatic tissue or enter the systemic circulation when the intestinal clearance capacity reaches an upper limit [ 89 ]. Fructose culminates the main rate-limiting step of glycolysis and is rapidly converted into ketohexokinase to generate fructose-1-phosphate, which is further metabolized into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. The acute fructose overload in the liver leads to ATP degradation and a decrease in ATP synthesis, both of which lead to an increase in AMP levels and stimulation of AMP deaminase activity, subsequently accelerating the formation of uric acid with consequent hyperuricemia. More notably, the costly fructose metabolism can also result in renal inflammation and fibrosis and form kidney stones due to calcium salt precipitation in high-fructose diet-fed mice [ 90 ]. Alarmingly, a normal physiological concentration of fructose in the kidney still causes a risk of defective elimination of uric acid and activation of renal inflammation by increasing the expression of intercellular adhesion molecule-1 in the serum and endothelial cells [ 91 ]. Therefore, carbohydrate restrictions, especially fructose intake, have been regarded as an efficient diet intervention for gouty patients to modulate the disease state. It has been shown that the intake of fructose-rich fruits could bring about a temporary upregulation of uric acid. However, the moderate consumption of these foods over a long time can facilitate the excretion of uric acid, which could be correlated with the alkalization of body fluids [ 92 ]. Therefore, it is always suggested that limiting fructose-rich soft drinks or reducing the consumption of high-fructose drinks rather than fruits is better for gout improvement.
3.1.3. High Protein
Proteins are biomacromolecules formed by folding long chains of amino acids, and dietary protein digestion in the gastrointestinal tract can provide amino acid dipeptides and tripeptides for cellular metabolism and protein synthesis in muscle or other tissues. High protein consumption is well known for its promotion of energy expenditure and urea synthesis. In addition, high dietary protein intake can also affect uric acid homeostasis, since protein digestion can generate several amino acids, such as glutamine, glycine and threonine, to induce purine synthesis, promoting the development of hyperuricemia [ 93 ]. A cohort study with 193,676 participants further revealed that higher nondairy animal protein consumption results in a disturbance of uric acid metabolism, including a reduced level of citrate and a higher level of uric acid and acidic urine, which subsequently promotes uric acid stone formation [ 94 ]. In addition, long-term high dietary protein intake has been shown to have adverse effects on uric acid elimination due to increased intraglomerular pressure and flow in kidney tissues [ 95 ]. The severity of glomerular damage has been significantly attributed to elevated SUA levels; hence, dietary recommendations for gouty patients often suggest that excessive protein intake should be restricted to avoid placing kidney tissue under undue stress.
Studies have also shown inconsistent effects of dietary proteins from different sources [ 96 ]. Overconsumption of animal proteins is linked to an elevated prevalence of gout, whereas overconsumption of plant protein (soybeans and soy products) or dairy product intake are associated with a reduced risk [ 8 , 96 ]. When compared with foods rich in plant proteins, an animal protein-rich diet promotes acidic urine production and uric acid stone formation [ 94 ]. Conversely, substitution of plant-based protein for a carbohydrate-rich diet can attenuate IR and compensatory hyperinsulinemia [ 97 ], thus improving the renal clearance of urate. Therefore, choosing appropriate dietary protein sources and controlling the amount of protein intake might represent an effective intervention for the improvement of gouty diseases.
3.2. Adequate Consumption of Essential Nutritional Elements Leads to Beneficial Effects against Gout
3.2.1. vitamins.
Vitamins are essential trace elements that act as regulators of physiological and pathological functions, such as participating in immune responses, antioxidant activities and redox reactions. It has been demonstrated that an adequate intake of vitamin supplements or consumption of vitamin-rich fruits and vegetables seems to be a valid approach for hyperuricemia and gout treatment. Vitamins such as vitamin A, vitamin E, and vitamin C show beneficial effects against oxidative stress and inflammation, as well as effectively decreasing SUA levels [ 98 , 99 , 100 ], and the same uric acid-lowering effect also appears in a vitamin D-rich diet [ 101 ]. Additionally, vitamin E is also considered a membrane stabilizer that inhibits MSU crystal-induced hemolysis [ 102 ]. Many studies performed in humans and animals have shown that vitamin C (l-ascorbic acid) consumption can affect uric acid reabsorption and excretion to reduce SUA levels [ 100 ]. Both uric acid and vitamin C can be reabsorbed in the proximal tubule via anion-exchange transport, and vitamin C overload can competitively suppress the reabsorption of uric acid [ 103 ] in the filtrate. Meanwhile, its downregulation of URAT1 activity and/or Na + -dependent anion cotransporter could promote uric acid excretion [ 100 ]. The uricosuric function of vitamin C also appears to directly act on the glomerulus by reducing glomerular microvascular ischemia and increasing afferent arteriole dilation, thus increasing the glomerular uric acid filtration rate [ 104 ]. Furthermore, vitamin C reduces the incidence of gout by alleviating the NF-κB/NLRP3-related inflammatory response to MSU deposition [ 105 ].
3.2.2. Minerals
Minerals, including potassium, zinc, calcium, copper, iron, and selenium are micronutrients that are essential for body metabolism [ 73 ], and deficiencies or excesses of these micronutrients are potentially hazardous occurrences that might be involved in the development of gout. It is well known that dietary potassium consumption has obvious diuretic and natriuretic effects, and even a minor potassium insufficiency triggers an impairment in the kidney’s capacity to secrete sodium chloride and retain sodium [ 106 ], resulting in renal dysfunction, while long-term routine potassium replenishment aggravates thiazide diuretic-mediated elevation of uric acid [ 107 ]. A similar facilitation effect was observed when iron accumulation triggers increased saturated transferrin-mediated XO activity [ 108 , 109 ]. Minerals also have a crucial role in maintaining acid-base balance. This has been attributed to keeping urine electrically neutral by regulating the secretion of anions such as chloride, sulfate, and phosphate in kidney tissues [ 73 ]. For instance, urinary calcium loss is a crucial risk factor that can trigger calcium stone formation and cause a uric acid excretion disorder [ 73 ]. Normal calcium intake can decrease the potential risk of kidney stone formation and is conducive to uric acid elimination in renal tissue [ 110 , 111 ].
3.2.3. Fibers
Dietary fibers refer to plant-derived carbohydrates that are resistant to hydrolyzation or assimilation in the upper gastrointestinal tract, and fibers trapped in the gut can increase intestinal viscosity and satiety, as well as reduce gastric emptying rate and regulate intestinal conduction [ 112 ]. Correspondingly, dietary fibers reduce the intake and absorption of high-energy food [ 113 ]. The consumption of dietary fibers can manage glucose and lipid metabolism to regulate energy balance [ 113 , 114 ]. More importantly, a lack of these fibers leads to a slower recovery of gut dysbiosis, and dietary fiber supplementation is able to improve the composition of the gut microbiota [ 115 ], suggesting a close connection between fiber and intestinal flora disorder in gout patients. The fiber fermentation process by gut microbiota accompanies the release of microbiota-driven metabolites (short-chain fatty acids, SCFAs) that show beneficial effects on the host’s health [ 116 ]. After dietary fiber intake, acetate is the most abundant microbiota-derived SCFAs in the blood and can quickly resolve MSU-induced inflammation by promoting caspase-dependent apoptosis of neutrophils and the excretion of the anti-inflammatory IL-10 against LPS-induced inflammation [ 31 ]. Butyrate is another SCFAs mainly produced by microbiotal fermentation of indigestible fibers, and has been shown to improve lipid accumulation in the liver and pancreas, thereby reducing uric acid metabolism abnormalities by XO activation [ 71 ]. Butyrate can also decrease the activation of NF-κB induced by LPS and the translation or transcription of IL-1β by inhibiting histone deacetylases in human peripheral blood mononuclear cells [ 117 ]. Therefore, the consumption of more fiber-rich whole grains, vegetables and fruits is beneficial for regulating gastrointestinal homeostasis, reducing the intake of unhealthy foods and reshaping the gut microbiota. Meanwhile, the salutary metabolites produced by the microbiota-induced digestion of dietary fiber can regulate the inflammatory state of gouty patients and reduce uric acid production, all of which are conducive to the management of gout.
4. Recommended Nutritional Management and Its Combination with Drug Therapy
The close association of specific foods with SUA levels, as shown in Table 1 , illustrates that the essential impact of food on health is ascribed to the synthesized effects of the nutrients from food, specifically the dominant or beneficial nutritional factors determining the final performance. Taking dairy products as an example, the increased intake of dairy products, especially those with low fat, can reduce the incidence of gout [ 8 ]. Late season skim milk, which contains higher levels of orotic acid than early season skim milk, has a preferential impact on the excretion of uric acid [ 118 ]. Moreover, glycomacropeptide and G600 milk fat addition in skim milk can greatly and effectively relieve joint pain and the frequency of gout flares [ 119 ].
The dietary management for gouty patients is commonly self-prescribed and centers around the control of purine sources, such as reduced consumption of purine-rich foods, which theoretically attenuates uric acid production; but patients often fail to adhere to recommendations in the long term due to the limited palatability of purine-free diets [ 34 , 37 ]. Although piecemeal modifications of the various yet limited numbers of nutrients often provide incomplete dietary recommendations [ 34 ], attention should be given to nutrient richness and structure to avoid the burden of inappropriate dosage. For example, the plasma concentrations of vitamin C saturation ranges daily from 200 to 400 mg, implying that exceeding the recommended supplemental dose has little effect on the consequences [ 120 ]. More importantly, taking high-dose and long-term supplements of vitamin C may be associated with adverse effects, and the resulting excessive uric acid excretion could elevate the risk of kidney stones in gouty patients [ 121 , 122 ]. As shown in Figure 3 , recommended nutritional interventions should emphasize the necessity for appropriate supplementation of plant-derived fibers and dairy-derived protein and persuade patients to avoid high-compensation consumption of refined saturated fats and carbohydrates [ 34 ]. The typical dietary patterns include a DASH and Mediterranean diet, both of which are comprised of fruits, vegetables, and low-fat dairy products with reductions in total and saturated fats. Increasing evidence supports that consuming a DASH diet can continuously attenuate SUA in hyperuricemia patients and reduce the incidence of gout in participants [ 34 , 45 ]. Similar SUA-lowering effects have been observed in a research investigations of the Mediterranean diet [ 58 ]. Moreover, intervention with the DASH diet combined with adequate sodium and plant-derived protein shows more beneficial functions in reducing SUA levels [ 33 , 37 ].

Recommended food-derived nutritional interventions with anti-gouty mechanisms. Dietary management recommendations for gout patients include appropriate intake of fiber, minerals, and vitamins, as well as the selection of high-quality sugars, fats, and proteins, which are usually of plant origin. In addition, the consumption of products containing probiotics helps regulate intestinal homeostasis in patients with gout. ↑—increase; ↓—decrease; →—maintain; UA—uric acid; HQ—high-quality.
Most cases of dietary management related to uric acid reduction are reported in the nongouty population (as shown in Table 1 ). Dietary modification against gouty disease is commonly selected as an adjunct therapeutic strategy with medicinal drug therapy [ 10 ]; however, clinical trial data on the effects of dietary modifications combined with pharmacological interventions on gout are mostly lacking, as shown in Table 2 . By way of illustration, colchicine and nonsteroidal anti-inflammatory agents (NSAIDs) have been used to treat acute gouty inflammation. Their combined use with dietary micronutrient supplementation can remedy micronutrient deficiency induced by NSAIDs and colchicine [ 123 , 124 ]. Allopurinol, a first-line drug used as a xanthine oxidase inhibitor against gout, requires high protein replenishment to facilitate renal clearance [ 125 ]. Therefore, our recommendation is for individuals to follow a healthy diet for prevention purposes, and for patients with mild gout, we recommend the DASH and Mediterranean diet, which focus on plant-based components. Additionally, we recommend a reduction in the consumption of high-fat foods (fast food and cream products), especially foods with trans fatty acids (such as margarine and butter), and for individuals to pay attention to the amount of nutrient supplements consumed. For patients with severe gout, dietary modification and medication should be combined, and health care providers should remind patients of food–drug interactions to achieve synergistic effects.
Combination of nutrition modification and drug supplementation.
| Medicine | Dietary Intervention | Participants | Time | Major Findings |
|---|---|---|---|---|
| Lesinurad [ ] | High-fat and high-calorie meal | 16 healthy men (ages 18 to 55 years) | 6 days | •C ↓ vs. the fasted phase •serum urate-lowering effect and renal clearance ↑ vs. the fasted phase •absorption was slightly delayed vs. fasted phase |
| Lesinurad [ ] | Moderate-fat diet | 16 nonobese men (ages 18 to 55 years) | 10 days | •T 4 h delay •C ↓ in the fed state vs. the fasted phase |
| Colchicine [ ] | Seville orange juice or grapefruit juice | 44 nonobese adults (ages 18 to 45 years) | 4 days | •C and AUC ↓ in the seville orange juice group vs. the nonjuice group •T occurred 1 h delay compared with in the seville orange juice group vs. the nonjuice group |
| Febuxostat [ ] | High-fat breakfast | 68 healthy adults (ages 18 to 55 years) | Not specified | •C and AUC ↓ under feeding conditions vs. fasting conditions •SUA concentrations ↓ after treatment with febuxostat (80 mg) |
| Etoricoxib [ ] | High-fat meal | 12 healthy adults (ages 50 to 64 years) | 10 days | •the rate of absorption ↓ in the fed phase vs. the fasted phase •T occurred with an approximately 2 h delay in the fed phase vs. the fasted phase |
| Allopurinol/oxipurinol [ ] | High-protein or low-protein diet | 6 healthy adults (ages 20 to 30 years) | 28 days | •plasma AUC significantly ↑ in the high-protein diet group •renal clearance significantly ↓ in the high-protein diet group |
| Allopurinol [ ] | Low-purine diet | 60 hypertensive patients with high SUA levels (average age of 54.4 years) | 36 weeks | •SUA significantly ↓ in the intervention groups •6 months after the intervention, SUA shows an elevation tendency in the low-purine diet + medication group and medication-only group •6 months after the intervention, SUA shows a continuous drop in the low-purine diet group |
Note: Data are from clinical trials that have been included in PubMed. A direct search was used to search for the following terms: “diet” or “food” with “drug” or “medicine” or “treatment” with “gout” or “hyperuricemia”; “diet” or “food” with commonly used clinical anti-gout drugs including “zurampic (lesinurad)”, “colchicine”, “febuxostat”, “allopurinol”, “probenecid”, “aspirin”, “pegloticase”, “benzbromarone” and “etoricoxib”. A total of 751 articles were obtained. After following these exclusion criteria—repetitive articles, dietary supplements, drug interactions, formulation improvement, exercise interference, nonmarket food and mismatched intervention subjects—a total of 7 articles showed the effect of the combination of diet and medication in the treatment of gout. ↑—increase; ↓—decrease; AUC—area under curve; C max —maximal plasma concentration; SUA—serum uric acid; T max —time to reach C max .
5. Conclusions and Future Perspective
Our study has shown the important role of diet in gout development and management and how dietary adjustments based on nutrient composition should be an important component of routine care for gout. Gout patients are commonly prone to choose health management by dietary modification because diet changes can instantly affect gout flares via multiple signaling pathways. Diet-induced systemic metabolic pathways, including purine, lipid, and glucose metabolism, as well as energy balance and gut microbiota changes, have provided new mechanistic insights and potential interventions for gout progression. Since foods are eventually metabolized into multiple nutrients for metabolic homeostasis in the body, dietary modification might represent an appropriate nutritional regulation for gout patients or for potential patients to effectively reduce the incidence of gout. The critical role of nutritional factors on gout development also supported the following recommended nutritional modification strategies: (1) reducing nutritional risk factors against metabolic syndrome; (2) supplementing with beneficial nutrients to affect uric acid metabolism and gouty inflammation; and (3) considering nutritional modification combined with medication supplementation to decrease the frequency of gout flares. Our consistent principle is that, in terms of diet, nutritional balance should be analyzed from the point of view of enrichments and structures of nutritional elements. Evidence supports that a low-fat, low-carb, plant-based dietary intervention is suitable for gouty patients; however, we need to pay specific attention to the golden rule of healthy dietary intake, that is, moderation. In addition, we advocate the combination of medications and dietary modification for gouty patients in therapy, and caution that they should note the impact of nutritional factors on drug pharmacokinetics and pharmacodynamics. However, many human studies focusing on the relationship of food and SUA levels often do not involve gouty patients. A limited number of clinical research studies explore food/nutrition and gout or anti-gout drugs, resulting in limited being drawn conclusions. In conclusion, the dietary mechanisms and nutritional basis provide scientific evidence for the prevention and improvement of gouty diseases, and dietary modifications based on effective regulatory mechanisms may be a promising strategy to reduce the high prevalence of gout.
Acknowledgments
We would like to thank Peili Rao, Yannan Zheng and Yijie Song, for their revision suggestions of figures.
Funding Statement
This research was funded by the Key-Area Research and Development Program of Guangdong Province (2020B1111110003).
Author Contributions
Y.X. and H.X. initiated the idea and performed the study. Y.Z. and S.C. wrote the manuscript. Y.Z., S.C., M.Y., Y.X. and H.X. discussed and revised the manuscript. H.X. has final responsibility for all parts of this manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
In this paper, we present and discuss how the acid rain problem became a key environmental issue among industrial countries from the late 1960s and the following decades (Fig. 2).We view the problem from a science-to-policy interaction perspective, based on a Symposium in Stockholm in the autumn 2017 organised to manifest 50 years of international air pollution science and policy development.
Acid rain and acidification research are indeed a multidisciplinary field. This field evolved from the first attempts to mitigate acid freshwater in the 1920s, then linking acid rain to the acidification in late 1950s, to the broad project-concepts on cause and effect from the late 1960s. Three papers from 1974, 1976 and 1988 demonstrate a ...
The world is on track for an increase of around 2.6 degrees C by 2100. That's extremely bad. But it's still a degree lower than where we were heading in 2016. Governments have increased action ...
According to 2012 progress report of US. EPA (2013), The Impacts of major global. environmental problems such as acid. rain, acid dep osition, depletion of ozone. layer and health and ...
Acid rain (AR) can be defined as a combination of dry and wet deposition from the atmosphere having higher than normal concentrations of nitric (HNO 3), sulfuric acids (H 2 SO 4), and acidifying compounds which lead to a decrease in the pH of rainwater to less than 5.61.In 1845, AR was first been mentioned by Ducros, although a detailed study of AR was conducted by Robert Angus Smith and ...
While normal rainwater is slightly acidic at a pH of 5.6, by 1980 the average rainfall in the United States was at a pH level of 4.6, about ten times more acidic and trending more acidic. The effects of increasing acidity were widespread. Acid rain negatively affects aquatic and terrestrial life, damages structures by corroding metal, paint and ...
First, with the development of plants, they may also develop resistance to acid rain, such as surface wax and antioxidant (defense) systems (Simon and Hutchinson, 1986; Li and Liang, 2019), which make them better to adapt to acid rain. Second, long-term research was scarce in our database and several few long-term studies showed positive or ...
Acid rain penetrates through soil particles, washing away the basic cations of the soil, ... The research progress of the effect of acid rain on plant. World Science Technology Research and Development, 26 (2004), pp. 36-41, 10.1016/S1872-2032(06)60052-8. View in Scopus Google Scholar.
The wet and dry deposition of acidic substances from the atmosphere to landscapes ( acid rain) is a serious environmental problem, negatively impacting aquatic and terrestrial ecosystems. Fog water can be more acidic than rain at a specific site. Because legislation has reduced emissions of sulfur and nitrogen oxides, the precursors to the ...
Acid rain can dissolve certain more soluble elements from the soil, like aluminum. The dissolved aluminum begins to accumulate and can reach toxic levels as it enters local streams and wetlands. Acid rain also removes important nutrients from the soil, such as calcium, potassium, and magnesium. The lack of nutrients can negatively affect the ...
The term 'acid rain' refers to atmospheric deposition of acidic constituents that impact the earth as rain, snow, particulates, gases, and vapor. Acid rain was first recognized by Ducros (1845) and subsequently described by the English chemist Robert Angus Smith (Smith, 1852) whose pioneering studies linked the sources to industrial emissions and included early observations of deleterious ...
According to studies conducted by Likens and his colleagues, normal rainwater has a pH of 5.2. During the 1970s and 1980s, when acid rain was at its worst, scientists recorded pH levels as low as ...
Greenhouse gases can cause acid rain, which in turn degrades soil chemical properties. This research was conducted to determine the effects of simulated acid rain (SAR) on the chemical properties ...
Learn the cause and effect of acid rain. Read environmental news articles on how acid rain takes nutrients from the soil, leads to stunted forests and more. ... according to new research ...
The discovery and the early acid rain history. In a deliberatively provocative article in the Swedish newspaper Dagens Nyheter in October 1967, entitled "An Insidious Chemical Warfare Among the Nations of Europe", the Swedish scientist Svante Odén (Fig. 3) described a new and threatening environmental problem—Acid Rain.He pointed to the significant decrease in pH of rainwater and ...
Acid rain, linked to fossil fuel-burning emissions, featured prominently in 1970 s to 1990 s headlines. Concern over dying lakes triggered international solution seeking, but as emissions-reducing agreements were enacted, attention to the problem faded. ... Since 1968, this research hub, renowned for experimental manipulations of whole lakes ...
February 28, 2019. • 5 min read. Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits ...
July 25, 2012. Long-Term Ecological Research Program at this link. Acid rain. It was a problem that largely affected U.S. eastern states. It began in the 1950s when Midwest coal plants spewed sulfur dioxide and nitrogen oxides into the air, turning clouds--and rainfall--acidic. As acid rain fell, it affected everything it touched, leaching ...
Acid rain also enhances the deposition of mercury, which has adverse effects on human health. Direct impacts on human health have also been documented. A severe episode of acid fog, the Great London Smog of 1952, resulted in an increase in the daily average death rate from 252 to approximately 1,000, and acid fog was r esponsible for several ...
To research this episode we read her 2015 dissertation, A Poisonous Sky: Scientific Research and International Diplomacy on Acid Rain. We also read Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Global Warming by Naomi Oreskes and Erik Conway (Bloomsbury, 2010).
Acid rain is any form of precipitation that contains acidic components, such as sulfuric or nitric acid. ... Neanderthals and humans interbred 47,000 years ago for nearly 7,000 years, research ...
Effects of Acid Rain on Materials. Not all acidic deposition is wet.Sometimes dust particles can become acidic as well, and this is called dry deposition.When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and other manmade structures, and damage their surfaces.
Research even suggests that current climate policy will lead to more than 2.5°C of warming by the end of ... then we'd also be adding to the acid rain problem. ... In an article from 2006, ...
Rain and contaminated air are major sources of the contamination detected by a team of researchers from Indiana University and Canada's top environmental agency. So are discharges from sewage ...
The amount and strength of base for ZrO2 and Mg(OH)2 catalysts in hot compressed water at 380 °C and 30 MPa were investigated. The effect of acid poisoning on the rate of 2,5-hexanedione cyclization, which was used as an indicator of acid adsorption on base sites, was analyzed by using the Langmuir equation, and differences in basicity between both catalysts were discussed. Both catalysts ...
Introduction. Heartburn is an uncomfortable, burning feeling in the chest, behind the breastbone, or in the upper part of the abdomen that sometimes spreads to the throat. 1 It is specifically related to the reflux of gastric acid through the lower esophageal sphincter, which is a typical symptom of gastroesophageal reflux disease (GERD). Some patients with GERD might also present with ...
Aging Well Allergies Cancer Care & Prevention Chronic Pain Cold, Flu & Respiratory Illnesses Diabetes & Endocrinology Digestive Ear, Nose & Throat Eye Care Infectious Disease Lung Oral Health ...
Gout is well known as an inflammatory rheumatic disease presenting with arthritis and abnormal metabolism of uric acid. The recognition of diet-induced systemic metabolic pathways have provided new mechanistic insights and potential interventions on gout progression. However, the dietary recommendations for gouty patients generally focus on ...