- Open access
- Published: 07 May 2020

Zebrafish as an alternative animal model in human and animal vaccination research
- Ricardo Lacava Bailone 1 , 2 ,
- Hirla Costa Silva Fukushima 3 ,
- Bianca Helena Ventura Fernandes 4 ,
- Luís Kluwe De Aguiar 5 ,
- Tatiana Corrêa 6 ,
- Helena Janke 6 ,
- Princia Grejo Setti 6 ,
- Roberto De Oliveira Roça 2 &
- Ricardo Carneiro Borra 6
Laboratory Animal Research volume 36 , Article number: 13 ( 2020 ) Cite this article
20k Accesses
62 Citations
1 Altmetric
Metrics details
Much of medical research relies on animal models to deepen knowledge of the causes of animal and human diseases, as well as to enable the development of innovative therapies. Despite rodents being the most widely used research model worldwide, in recent decades, the use of the zebrafish ( Danio rerio ) model has exponentially been adopted among the scientific community. This is because such a small tropical freshwater teleost fish has crucial genetic, anatomical and physiological homology with mammals. Therefore, zebrafish constitutes an excellent experimental model for behavioral, genetic and toxicological studies which unravels the mechanism of various human diseases. Furthermore, it serves well to test new therapeutic agents, such as the safety of new vaccines. The aim of this review was to provide a systematic literature review on the most recent studies carried out on the topic. It presents numerous advantages of this type of animal model in tests of efficacy and safety of both animal and human vaccines, thus highlighting gains in time and cost reduction of research and analyzes.
Introduction
The role of the immune system is to protect a body against bacterial, viral, or any foreign antigen invasions. In order to improve protection, vaccination is used to boost immunity against diseases caused by microorganisms. It typically contains a less virulent agent that triggers a reaction, thus, stimulating a body’s immune system to recognize it as foreign. In the process, a body’s defense mechanism learns to recognize and destroy a microorganism, its toxins or surface proteins [ 94 ] every time an invasion is identified. The use of vaccination is important because it promotes the stimulation of the body’s defense mechanisms and the development of both individual and collective immunity. Vaccination can act on specific (adaptive) and nonspecific (innate) immune responses unlike immunostimulants which only act on innate response. In addition, it should be noted the role vaccines play in controlling diseases as preventative as well as non-therapeutic measures. Therefore, the body is able to produce antibodies that recognize, signal and neutralize pathogens or particular cellular responses which detect the specific antigens with high efficiency and affinity. As a result, vaccines protect the body against future infections [ 27 ] thus reducing the need for the use of antibiotics and other types of drugs.
Despite the study of immunology in fish being more recent compared to those of humans and in animals, the concepts and techniques used are similar [ 60 ]. The study of the use of vaccines in fish is an area of fast-growing. As aquaculture expands and the need to control pathogens becomes more pressing, the commercial vaccination of different varieties of fish is already a reality in many countries. It aids in the prevention of diseases that could pose health risks to the shoal as well as in avoiding the economic losses due to mortality caused by infection. It reduces the contamination of water bodies by the excessive use of antibiotics, and the reduction of final fish product quality [ 5 , 24 , 42 , 79 , 100 ].
The Zebrafish model has been widely used in both animal and human health research and, more recently, in aquaculture too. In spite of rodents being the most widely used research model in the world, in recent decades the use of the zebrafish ( Danio rerio ) model has exponentially increased among the scientific community. It follows the principle of 3Rs (replacement, reduction, and refinement) as required by a multiplicity of national and international regulatory bodies. Furthermore, the use of zebrafish model results in a reduction of time and use of resources when compared to those more established animals’ models. It also provides a greater informational and predictive capacity when compared to in vitro results [ 53 ]. Thus, using the zebrafish model, it is possible to replace and reduce the use of mammals in research as well as mitigate problems related to the welfare of those animals. Furthermore, zebrafish is used as confirmatory models of the positive previously obtained results, thus, having the ability to refine the findings [ 2 ]. A review of the literature was carried out aiming at presenting the most recent information on vaccination of fish, which brings to light the advantages of this animal model in tests of efficacy and safety of both animal and human vaccines.
Material and methods
The present study was based on a systematic literature review carried out using databases such as Science Direct, Google Scholar and SciELO (Scientific Electronic Library Online). Emphasis was given on identifying publications using search words and terms containing ‘human vaccination’ and ‘animal vaccination’. Particularly, the main key-words searched included ‘Zebrafish model’, ‘vaccine safety’, ‘diseases’, ‘infection’ and ‘toxicology’. Initially, 99 publications were identified which included books, rulings and articles published by international scientific journals of high impact factor. The publications were selected according to relevance and timeliness. 19% of the articles used were published in the last year, 65% in the last 5 years, and 89% published in the last 10 years.
Zebrafish model and vaccines testing
Vaccination safety.
When devising immunization experiments, challenge trials for vaccine development evaluate the efficacy and safety of the vaccine against different pathogens. These are normally assessed using animal models, mainly mammals, which are often imprecise in reflecting human diseases [ 93 ], not to mention time consuming, and require a large number of animals. Moreover, the mortality and clinical signs as well as laboratory tests are usually analyzed to evaluate the innate (non-specific) or adaptive (specific) immune system response. As in mammals, Zebrafish has a well-maintained adaptive immune system composed of T and B lymphocytes that develop from the thymus and kidneys respectively. However, in relation to the development of memory lymphocytes, fish seem to have memory cells of the type B and T [ 78 ]. Yet, there has not been enough data to confirm that in Zebrafish. Zebrafish also presents the enzyme system involved in the process of genetic rearrangement that originates the B (BCR) and T (TCR) lymphocyte receptors. As in humans, Zebrafish has recombination activator genes that control the rearrangement of gene segments V, D and J to produce the diversity of antibodies and lymphocyte receptors. In addition, the zebrafish’s immune system has only approximately 300,000 antibody-producing B cells, making it three orders of magnitude smaller than mice and five orders simpler than humans [ 48 ].
The efficiency of the humoral response increases due to the increased affinity of the antibodies. Affinity maturation of antibody responses is less efficient in cold-blooded vertebrates compared to mammals. Despite this, in zebrafish, data revealed that specific nucleotides in regions of the BCR receptor were target of directed mutations. Therefore it was suggested that activation-induced deaminase and affinity maturation contributed to the diversification of antibodies also in fish [ 56 ]. Immunization of teleost fish with the TNP-KLH antigen (linked to trinitrophenyl to keyhole limpet hemocyanin), for example, induced the production of specific low affinity antibodies, which were replaced in 5 weeks by antibodies of intermediate affinity, and after 15 weeks, by antibodies with greater affinity for the antigen [ 28 , 97 ].
Among the immunological tests, the most frequent ones are: complete hematological analysis by counting erythrocytes; thrombocytes and leukocytes; differential white cell count; hematocrit; glucose; organ histology, and immunological essays such as serology, specific antibody titration, and agglutination [ 4 , 29 , 57 ]. Furthermore, toxicity tests can be also conducted using zebrafish such as embryotoxicity, hepatotoxicity, neurotoxicity, endocrine toxicity, genotoxicity, among others as proposed by Bailone et al. [ 3 ].
Up to now, these tests have been conducted using rodents, but in recent decades, the Zebrafish model has proved to be an important tool in the studies of infections and immunological responses. This model has the advantage of having OECD-specific guidelines for safety evaluation of chemical compounds (acute toxicity), which is performed within 96 h [ 65 ]. In addition, observations can be made in real-time allowing for the monitoring of embryogenesis (Fig. 1 ) as well as regarding the effects of vaccines in relation to cardiovascular, hepatic, nervous, and endocrine, not to mention, behavioral aspects too [ 18 , 40 ].

Embryos of zebrafish 0, 6, 24 and 48 h’ post-fertilization. Larvae of zebrafish 72 and 96 h post-fertilization
Prior to vaccines being tested on humans, livestock or pets, these should be assessed using animal models to avoid causing them harm, including death, especially in the case of immunosuppressed organisms, children and the elderly [ 26 ]. As for vaccination in humans, for example, about 0.4 to 1.9 people per million who had been vaccinated with BCG against tuberculosis may have developed the disease through vaccine contagion. For hepatitis B, 1 in 600,000 people vaccinated may have presented a severe allergic reaction (anaphylaxis). In the case of vaccine against poliomyelitis, vaccine contagion happened to 1 in every 3.6 million vaccinated. Moreover, to combat yellow fever, the vaccine contagion and seizures happened to 1 in 22 million and internal hemorrhages happened to 1 in 450,000. Thence, the occurrence of side effects is very rare. Side effect reactions in humans may also be observed to be caused by other vaccines such as yellow fever, measles, mumps, rubella, chicken pox, diphtheria and tetanus. The most common symptoms are seizures, severe allergic reactions, meningitis, encephalitis [ 26 ]. Although these risks are irrelevant when compared to damages that could be caused by the non-use of a vaccine, the toxicology, the side effects and immunization at different concentrations ought to be adequately tested.
Thus, the Zebrafish model has the advantage of a researcher to follow in real-time the fish’s development from its embryogenesis to full organ development which is reached about 36 h after fertilization. This allows for a vaccine’s effect on all the major organs precursors to be closely studied [ 53 ] such as using immunohistology (Fig. 2 ).
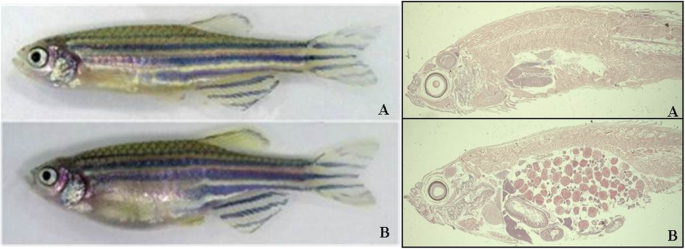
Histology of adult zebrafish (hematoxylin eosin). a Male. b Female
Zebrafish and mammalian toxicity (Lethal concentration – LC 50 ) profiles are surprisingly similar for a range of substances specified in Table 1 below. Therefore, toxicity studies support the effectiveness of using the zebrafish model for the purpose of testing these substances. Furthermore, they can be extrapolated to the active ingredients present in the vaccine, and enabling quick parallel studies of vaccine reactions in humans and zebrafish.
Advantages of zebrafish model in vaccination tests
Compared to other vertebrates, zebrafish have extra biological advantages including high fecundity, external fertilization, optical transparency and rapid development. Moreover, Zebrafish possess a highly developed immune system that is remarkably similar to the human one. Therefore, it is expected that the majority of the signaling pathways and molecules involved in the immune response of mammals would also exist and behave similarly in fish [ 89 ]. Consequently, the presence in fish of elements of innate and adaptive immunity enables research in infectious processes, being susceptible to infections by gram-negative and gram-positive bacteria, protozoa, viruses, fungi and mycobacteria.
The development of special cloning, mutagenesis and transgenesis techniques allowed the identification of a significant number of mutants. Commercial mutant zebrafish lines and the recently developed CRISPR/Cas9 genome modification system provide the means to create knockout zebrafish for studying individual genes at a whole organism level [ 66 ]. Non-pigmenting mutants such as Casper zebrafish have also helped improve visibility of internal organs [ 92 ]. In addition it is easy to generate transgenic zebrafish with ‘reporter genes’ to facilitate analysis in live fish [ 87 ]. Because the zebrafish genome is conserved in humans, information obtained from zebrafish studies may lead to translational results in humans [ 38 ].
Examples of mutant animals displaying human-like diseases are numerous such as: sapje, which has the gene homologous to that of Duchenne muscular dystrophy; dracula , related to erythropoietic protoporphyria; van Gogh, model of the DiGeorge syndrome; and gridlock , which induces coarctation of the aorta [ 47 ]. Research in tumor suppressor genes p53 and apc ( adenomatous polyposis coli) is another area of great interest . The importance of the p53 gene in human carcinogenesis is well recognized and recent studies have shown zebrafish as an excellent model for assessing the presence (or not) of gene stability. Lymphoid leukemia, melanoma and hepato-carcinoma have already been described in zebrafish thus confirming that the molecular mechanisms involved are similar to those of humans [ 49 ].
Regarding the administration of vaccines, in view of the different routes of applications presented in animals and humans, the zebrafish model still allows the immunization of embryos, facilitated by its transparency, using glass needles (Figs. 3 and 4 ). Interestingly, the fact that the fish’s adaptive immune system does not reach maturity up to 4 weeks after fertilization allows them to be used without the need for immunosuppression in the embryonic stages [ 32 ] in the case, for example, of tumor xenograft experiments.

a Vitelline Yolk Injection (24 HPF), Magnifying Glass Nikon SMZ745, 50X; B) Vitelline Yolk Injection (24 h.p.f.), Magnifying Glass Nikon SMZ745, 50X
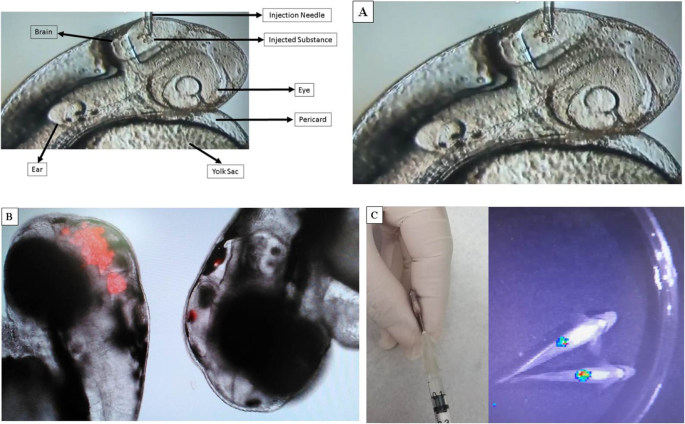
a 24 HPF Zebrafish Embryo Brain Injection, Nikon Microscope; b Brain injection of turbo-red substance into a 24 HPF zebrafish embryo; c Luciferin-labeled 4 T1 tumor cell bioluminescence in 3-month-old animals
In zebrafish larvae, a rapid systemic infection can be initiated by direct microinjection of a bacterial suspension into the bloodstream. Alternatively, a more localized infection may be induced by the injection of microbes into the muscle tail or the hindbrain ventricle [ 6 ]. For high transfer rate, the microbes can be readily injected into the yolk for the first few hours after fertilization. However, it is important to keep in mind that the yolk lacks immune cells, and therefore the bacteria are able to grow freely before invading the larval tissues [ 51 ].
Several transgenic zebrafish lines containing fluorescent markers in different cells of the immune system have been developed to visualize host-microbe interactions in the transparent larvae. For example, recruitment of fluorescent neutrophils to the site of bacterial infection (which can also be labeled with fluorescence) could be easily followed and quantified in real time. Yet, so far, researchers have focused primarily on larval infection patterns [ 51 ].
Fish vaccines
In the prevention of disease outbreaks causing mortalities in aquaculture, similarly to any other animal production system, vaccination is essential. Thus, the use of vaccines for that purpose could be improved based on the results from the studies performed in zebrafish [ 89 ]. The development of vaccines for aquaculture has been an important milestone for guaranteeing a continuous safe and high standard animal health production system. In recent years, zebrafish models have been chosen as the preferred model in the production of fish vaccination experiments against several pathogens that cause losses in aquaculture around the world such as bacteriosis and viruses. One of the most important pathogen studies applied to fishing production is attributed to Guo et al. [ 35 ]. They analyzed the protective efficacy of four iron-related recombinant proteins and their single-walled carbon nanotube encapsulated counterparts against the Aeromonas hydrophila infection in zebrafish. They observed that the immune response was increased after vaccination. Guo et al. [ 34 ] also studied Edwardsiella tarda which is an important intracellular pathogenic bacterium that causes the infectious disease Edwardsiellosis in fish. They proved that live E. tarda vaccine enhanced innate immunity by metabolic modulation in zebrafish.
Vibrio anguillarum , a bacterium that causes vibriosis, was also studied by Ye et al. [ 98 ] who observed the maternal transfer and protection role in zebrafish offspring following vaccination of the brood stock with a live attenuated V. anguillarum vaccine. They proved that the development of immune cells was enhanced and the maternally-derived antibody could protect early embryos and larvae from the attack of specific pathogens via vaccination with a live attenuated vaccine. Furthermore, Liu et al. [ 50 ] analyzed the profiling immune response in zebrafish intestine, skin, spleen and kidney when immersion vaccinated was used with a live attenuated V. anguillarum vaccine. Immersion, or bath vaccination, is a common practice in aquaculture, because of it being convenient as mass vaccination giving sufficient protection. The fish is submerged in water with a sub lethal concentration of the bacteria for a specific time. Liu et al. [ 50 ] observed that antibodies were either produced at antigen-contact tissues or in immune organs. Zhang et al. [ 101 ] studied Th17-like immune response in fish mucosal tissues after administration of live attenuated V. anguillarum via different vaccination routes. When compared to injection vaccination, immersion vaccination elicited intense Th17-like immune responses in the gut tissue of zebrafish. Vibrio vulnificus , that is an aquatic pathogen that can cause primary sepsis and soft tissue infection, was also tested during an experimentation of zebrafish’s reaction to vaccine. It was concluded that CpG oligodeoxynucleotides, a type of essential immunomodulators, protected zebrafish against Vibrio vulnificus induced infection [ 15 ].
Francisella noatunensis is a bacterium that causes granulomatous disease in freshwater and marine fish, and remains an unsolved problem for the aquaculture sector as no efficient vaccines are yet available. Lagos et al. [ 46 ] studied the immunomodulatory properties of Concholepas concholepas hemocyanin against francisellosis in a zebrafish model, proving that his adjuvant was a potential one for aquaculture vaccines. Moreover, Brudal et al. [ 11 ] observed that vaccination with outer membrane vesicles from F. noatunensis reduced the development of francisellosis in a zebrafish model.
Streptococcus sp. has also been studied with the Zebrafish model. Streptococcus parauberis is the major infectious agent of streptococcosis in olive flounder ( Paralichthys olivaceus ). Kim et al. [ 45 ], studying the identification of novel immunogenic proteins against S. parauberis by reverse vaccinology using zebrafish model, identified 41 vaccine candidates against S. parauberis. Furthermore, Streptococcus iniae was studied by Membrebe et al. [ 58 ] testing the protective efficacy of Streptococcus iniae derived enolase against Streptococcal infection in zebrafish model. In that study, enolase protein was evaluated to induce cross-protective immunity against S. iniae and S. parauberis which are major pathogens causing streptococcosis in fish.
Further to the aforementioned examples, many other diseases have been investigated with the Zebrafish model. For example, Rhabdovirus, which is one of the most important diseases in salmonids, is a virus that causes hemorrhagic viral septicemia [ 44 , 64 ]. Listeria monocytogenes [ 19 , 20 ]; Piscirickettsia salmonis which causes salmonid rickettsia sepsis (Tandberg et al. [ 83 ]); and in adjuvant test to improve the efficacy of vaccines [ 44 ], among others [ 82 ].
Animals and human vaccines
The zebrafish model has been used not only in aquaculture, but also in veterinary and human medicine. So far, it has become one of the major model systems used in modern biomedical research [ 51 ]. According to Torraca et al. [ 86 ], zebrafish can be also used as a model for pathogenesis and host defense, modeling many human diseases, such as tuberculosis, Staphylococcus aureus and Shigella infection, among others, as well as model to investigate immune cells, infection and inflammation of different kind of human diseases.
Torraca et al. [ 86 ] posited that zebrafish could also be used as a model for Tuberculosis which is a devastating infectious disease worldwide and with no current prospect of efficient prevention. Tuberculosis is an infectious disease caused by bacilli from the Mycobacterium tuberculosis complex. It is estimated that up to one third of the world’s population is infected with M. tuberculosis and have active tuberculosis, which often develops decades after the primary infection. Annually about two million people perish of tuberculosis and, so far, due to the lack of well-established animal models, such a disease has been difficult to study [ 51 ].
An infection by Mycobacterium marinum in adult zebrafish resembles that of human tuberculosis, as demonstrated by Myllymäki et al. [ 62 ]. Those authors proved that the M. marinum infection model in adult zebrafish was suitable for preclinical screening of tuberculosis immune’s responses and vaccines. It was also a promising new model for tuberculosis vaccine research, including the pre-clinical identification of vaccine antigens [ 16 , 17 , 36 , 41 , 61 , 67 ];). Other species of Mycobacterium have also been studied, such as M. bovis [ 52 , 73 ] and M. abscessos [ 7 ]. M. bovis is most common in cattle, but also affects humans. M. bovis Bacillus Calmette-Guérin vaccine is currently available as a prophylactic tool for preventing the disease. It has been shown to be efficient in preventing disseminated forms of tuberculosis in children; however, its efficiency is limited in areas where individuals have had prior exposure to environmental mycobacteria, and its efficacy decreased with a host’s age [ 55 ].
Moreover, teleost models offer an expanding platform for the understanding of mycobacterial infections and those mechanisms that offer the greatest potential to enhance host protection [ 37 ]. The models make it possible to screen the host and bacterial factors that modify the disease and facilitate the search for new therapeutic agents. It has recently been shown that zebrafish can also be used for the potential screening of DNA-based vaccines and, in particular, for identifying novel antigens protecting against mycobacteria [ 67 ]. Therefore, using the Zebrafish model is expected to accelerate the understanding of the pathogenesis of tuberculosis which would lead to the development of better vaccines. Yet, the usefulness of this model is not limited to tuberculosis, which as seen before it could benefit research for many other important infectious diseases [ 51 ].
Similarly, this model also helps to elucidate bacterial infections in animals and humans by Aeromonas hydrophila [ 91 ], Pseudomonas aeruginosa [ 74 ], Escherichia coli nonpathogenic [ 63 ], E. coli CFT073 [ 95 ], Listeria monocytogenes [ 80 , 81 ], Myroides odoratimimus [ 72 ], Cronobacter turicensis [ 25 ], Streptococcus agalactiae [ 70 , 96 ], Streptococcus iniae and Streptococcus pyogenes [ 59 , 76 , 77 ], among others [ 12 , 85 ].
Shigella is a major cause of dysentery worldwide, accounting for up to 165 million cases of shigellosis each year [ 23 ]. Yet, despite there not existing vaccine available as yet, the human and animal challenge–rechallenge trials with virulent Shigella as well as observational studies in Shigella-endemic areas are promising. The incidence of the disease decreased following Shigella’s infection which pointsto a biological feasibility of a vaccine [ 54 ]. Phalipon et al. [ 71 ] as well as Mani et al. [ 54 ] proposed that adult zebrafish could be used to study the immune response to Shigella, which is crucial to understanding the crosstalk between Shigella and T-lymphocytes [ 75 ] thus this being relevant in the development of vaccine strategies. Studies have also been conducted with Zebrafish model to promote a vaccine against Salmonella, which produces gastroenteritis that causes massive morbidity and mortality in adults and children in developing countries. Howlader et al. [ 39 ] proved that zebrafish was an excellent model for the study of vaccines using successive immersion triple vaccines with the single serotype Salmonella. Typhimurium and Salmonella entereditis induced protective efficacy against a high dose (10 8 CFU/ml) of infection by these pathogens.
Other microorganisms of importance such as fungi which can cause pathologies in humans, such as Candida albicans [ 10 ], Cryptococcus neoformans [ 8 , 84 ] and Mucor circinelloides [ 90 ] have also been the subject of study with teleosts. In addition, viruses such as Herpes simplex [ 13 , 31 ]; human norovirus [ 88 ]; Vesicular stomatitis [ 33 ]; hepatite C [ 21 , 22 ]; Chikungunya [ 1 , 9 , 14 , 68 ]; Sindib [ 69 ] and Influenza A [ 30 ] are some of the human viruses already studied by the zebrafish model in both embryos and larvae.
Conclusions
The use of the Zebrafish model for the production of vaccines with application for both animals and humans, despite already being a reality, is still underused. This model is an important tool for the development of new safe vaccines against diseases which do not yet have preventive treatment, or for which the existing vaccines are not so effective. Thus, previous screening tests with zebrafish have been proven to be effective in preliminary phases prior to testing with mammalians. Despite the evidence from the literature indicating that science in this field is in its infancy, when compared to other animal models used in research, teleost models have proved to be effective in the elucidation of the infection and immunological responses to the diverse animal and human pathogens. In addition, the reduced financial cost and time frame needed for testing are another attractive regarding the use of zebrafish. Thus, it is expected its use would expand in the coming years.
Availability of data and materials
See Materials and Methods section;
Aleksejeva E, Houel A, Briolat V, Levraud JP, Langevin C, Boudinot P. Zebrafish Plzf transcription factors enhance early type I IFN response induced by two non-enveloped RNA viruses. Dev Comp Immunol. 2016;57:48–56. https://doi.org/10.1016/j.dci.2015.12.016 .
Article CAS PubMed Google Scholar
Bailone RL, Aguiar LK, Roça RO, Borra RC, Corrêa T, Janke H, et al. Zebrafish as an animal model for food safety research: trends in the animal research. Food Biotechnol. 2019a;33(4):283–302. https://doi.org/10.1080/08905436.2019.1673173 .
Article CAS Google Scholar
Bailone RL, Fukushima H, Roça R, Corrêa T, Janke H, Setti P, et al. Potenciais usos do Modelo animal zebrafish Danio rerio em pesquisas na medicina veterinária. Rev Educ Cont Med Vet e Zoo CRMV-SP. 2019b; Just Accepted.
Bailone RL, Martins ML, Mouriño JLP, Vieira FN, Pedrotti FS, Nunes GC, et al. Hematology and agglutination titer after polyvalent immunization and subsequent challenge with Aeromonas hydrophila in Nile tilapia ( Oreochromis niloticus ). Arch Med Vet. 2010;42(3):221–7 https://www.redalyc.org/pdf/1730/173016376015.pdf Accessed 27 Feb 2020.
Article Google Scholar
Bao P, Sun X, Liu Q, Zhang Y, Liu X. Synergistic effect of a combined live Vibrio anguillarum and Edwardsiella piscicida vaccine in turbot. Fish Shellfish Immunol. 2019;88:84–90. https://doi.org/10.1016/j.fsi.2019.02.014 .
Benard EL, Van der Sar AM, Ellett F, Lieschke GJ, Spaink HP, Meijer AH. Infection of zebrafish embryos with intracellular bacterial pathogens. JoVE (J Vis Exp). 2012;61:e3781. https://doi.org/10.3791/3781 .
Bernut A, Dupont C, Sahuquet A, Herrmann JL, Lutfalla G, Kremer L. Deciphering and imaging pathogenesis and cording of Mycobacterium abscessus in zebrafish embryos. JoVE (J Vis Exp). 2015;103:e53130. https://doi.org/10.3791/53130 .
Bojarczuk A, Miller KA, Hotham R, Lewis A, Ogryzko NV, Kamuyango AA, et al. Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci Rep. 2016;5(1):1–6. https://doi.org/10.1038/srep2148 .
Briolat V, Jouneau L, Carvalho R, Palha N, Langevin C, Herbomel P, et al. Contrasted innate responses to two viruses in zebrafish: insights into the ancestral repertoire of vertebrate IFN-stimulated genes. J Immunol. 2014;192(9):4328–41. https://doi.org/10.4049/jimmunol.1302611 .
Brothers KM, Newman ZR, Wheeler RT. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell. 2011;10(7):932–44. https://doi.org/10.1128/EC.05005-11 .
Article CAS PubMed PubMed Central Google Scholar
Brudal E, Lampe EO, Reubsaet L, Roos N, Hegna IK, Thrane IM, et al. Vaccination with outer membrane vesicles from Francisella noatunensis reduces development of francisellosis in a zebrafish model. Fish Shellfish Immunol. 2015;42(1):50–7. https://doi.org/10.1016/j.fsi.2014.10.025 .
Brudal E, Ulanova LS, Lampe EO, Rishovd AL, Griffiths G, Winther-Larsen HC. Establishment of three Francisella infections in zebrafish embryos at different temperatures. Infect Immun. 2014;82(6):2180–94. https://doi.org/10.1128/IAI.00077-14 .
Burgos JS, Ripoll-Gomez J, Alfaro JM, Sastre I, Valdivieso F. Zebrafish as a new model for herpes simplex virus type 1 infection. Zebrafish. 2008;5(4):323–33. https://doi.org/10.1089/zeb.2008.0552 .
Burnham LA, Jaishankar D, Thompson JM, Jones KS, Shukla D, Tiwari V. Liposome-mediated herpes simplex virus uptake is glycoprotein-D receptor-independent but requires heparan sulfate. Front Microbiol. 2016;7:973. https://doi.org/10.3389/fmicb.2016.00973 .
Article PubMed PubMed Central Google Scholar
Chen H, Zhang L, Li S, Ling K, Chen X, Lin C. CpG-ODN 2007 protects zebrafish against Vibrio vulnificus -induced infection. bioRxiv. 2019:780742. https://doi.org/10.1101/780742 .
Cen J, Jia ZL, Zhu CY, Wang XF, Zhang F, Chen WY, Liu KC, Li, SY, Zhang, Y. Particulate matter (PM10) induces cardiovascular developmental toxicity in zebrafish embryos and larvae via the ERS, Nrf2 and Wnt pathways. Chemosphere. 2020. p. 126288.
Cheng T, Kam JY, Johansen MD, Oehlers SH. High content analysis of granuloma histology and neutrophilic inflammation in adult zebrafish infected with Mycobacterium marinum . Micron. 2020;129:102782. https://doi.org/10.1016/j.micron.2019.102782 .
Article PubMed Google Scholar
Cornet C, Calzolari S, Miñana-Prieto R, Dyballa S, Van Doornmalen E, Rutjes H, et al. ZeGlobalTox: an innovative approach to address organ drug toxicity using zebrafish. Int J Mol Sci. 2017;18(4):864. https://doi.org/10.3390/ijms18040864 .
Article CAS PubMed Central Google Scholar
Ding C, Fan E, Wang S, Guo L, Li J, Liu Q. A potential aquaculture vaccine vector: evaluation of a double-gene attenuated Listeria monocytogenes in zebrafish ( Danio rerio ). Aquaculture. 2017;479:311–20. https://doi.org/10.1016/j.aquaculture.2017.04.018 .
Ding C, Liu Q, Li J, Ma J, Wang S, Dong Q, et al. Attenuated Listeria monocytogenes protecting zebrafish ( Danio rerio ) against vibrio species challenge. Microb Pathog. 2019;132:38–44. https://doi.org/10.1016/j.micpath.2019.03.040 .
Ding CB, Zhang JP, Zhao Y, Peng ZG, Song DQ, Jiang JD. Zebrafish as a potential model organism for drug test against hepatitis C virus. PLoS One. 2011;6(8):e22921. https://doi.org/10.1371/journal.pone.0022921 .
Ding CB, Zhao Y, Zhang JP, Peng ZG, Song DQ, Jiang JD. A zebrafish model for subgenomic hepatitis C virus replication. Int J Mol Med. 2015;35(3):791–7. https://doi.org/10.3892/ijmm.2015.2063 .
Duggan GM, Mostowy S. Use of zebrafish to study Shigella infection. Dis Model Mech. 2018;11(2):dmm032151. https://doi.org/10.1242/dmm.032151 .
Faber MN, Holland JW, Secombes CJ. Vaccination strategies and IgM responses against PKD in rainbow trout. Fish Shellfish Immunol. 2019;91:423. https://doi.org/10.1016/j.fsi.2019.04.159 .
Fehr A, Eshwar AK, Neuhauss SC, Ruetten M, Lehner A, Vaughan L. Evaluation of zebrafish as a model to study the pathogenesis of the opportunistic pathogen Cronobacter turicensis . Emerg Microbes Infect. 2015;4(1):1–9. https://doi.org/10.1038/emi.2015.29 .
Ferrairo F. Os riscos reais da vacina. Revista Superinteressante. 2015; https://super.abril.com.br/saude/os-riscos-reais-da-vacina/ Accessed 27 Feb 2020.
Figueiredo HCP, Castro GAC, Leal CAG, Netto LN. Uso de vacinas na piscicultura: verdades, mitos e perspectivas. Panorama da Aquicultura. 2009;19(115):22–31 https://panoramadaaquicultura.com.br/uso-de-vacinas-na-piscicultura-verdades-mitos-e-perspectivas/ Accessed 27 Feb 2020.
Google Scholar
Fillatreau S, Six A, Magadan S, Castro R, Sunyer JO, Boudinot P. The astonishing diversity of Ig classes and B cell repertoires in teleost fish. Front Immunol. 2013;4:28. https://doi.org/10.3389/fimmu.2013.00028 .
Fukushima HCS, Leal CAG, Cavalcante RB, Figueiredo HCP, Arijo S, Moriñigo MA, et al. Lactococcus garvieae outbreaks in Brazilian farms Lactococcosis in Pseudoplatystoma sp.–development of an autogenous vaccine as a control strategy. J Fish Dis. 2017;40(2):263–72.
Gabor KA, Goody MF, Mowel WK, Breitbach ME, Gratacap RL, Witten PE, et al. Influenza a virus infection in zebrafish recapitulates mammalian infection and sensitivity to anti-influenza drug treatment. Dis Model Mech. 2014;7(11):1227–37. https://doi.org/10.1242/dmm.014746 .
Ge R, Zhou Y, Peng R, Wang R, Li M, Zhang Y, et al. Conservation of the STING-mediated cytosolic DNA sensing pathway in zebrafish. J Virol. 2015;89(15):7696–706. https://doi.org/10.1128/JVI.01049-15 .
Granato M, Nüsslein-Volhard C. Fishing for genes controlling development. Curr Opin Genet Dev. 1996;6(4):461–8. https://doi.org/10.1016/S0959-437X(96)80068-2 .
Guerra-Varela J, Baz-Martinez M, Da Silva-Alvarez S, Losada AP, Quiroga MI, Collado M, et al. Susceptibility of zebrafish to vesicular stomatitis virus infection. Zebrafish. 2018;15(2):124e132. https://doi.org/10.1089/zeb.2017.1499 .
Guo C, Peng B, Song M, Wu CW, Yang MJ, Zhang JY, et al. Live Edwardsiella tarda vaccine enhances innate immunity by metabolic modulation in zebrafish. Fish Shellfish Immunol. 2015;47(2):664–73. https://doi.org/10.1016/j.fsi.2015.09.034 .
Guo Z, Lin Y, Wang X, Fu Y, Lin W, Lin X. The protective efficacy of four iron-related recombinant proteins and their single-walled carbon nanotube encapsulated counterparts against Aeromonas hydrophila infection in zebrafish. Fish Shellfish Immunol. 2018;82:50–9. https://doi.org/10.1016/j.fsi.2018.08.009 .
Harjula SKE, Saralahti AK, Ojanen MJ, Rantapero T, Uusi-Mäkelä MI, Nykter M, et al. Characterization of immune response against Mycobacterium marinum infection in the main hematopoietic organ of adult zebrafish ( Danio rerio ). Dev Comp Immunol. 2020;103:103523. https://doi.org/10.1016/j.dci.2019.103523 .
Hodgkinson JW, Belosevic M, Elks PM, Barreda DR. Teleost contributions to the understanding of mycobacterial diseases. Dev Comp Immunol. 2019. https://doi.org/10.1016/j.dci.2019.02.011 .
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498. https://doi.org/10.1038/nature12111 .
Howlader DR, Sinha R, Nag D, Majumder N, Mukherjee P, Bhaumik U, et al. Zebrafish as a novel model for non-typhoidal salmonella pathogenesis, transmission and vaccine efficacy. Vaccine. 2016;34(42):5099–106. https://doi.org/10.1016/j.vaccine.2016.08.077 .
Jarque S, Ibarra J, Rubio-Brotons M, García-Fernández J, Terriente J. Multiplex analysis platform for endocrine disruption prediction using zebrafish. Int J Mol Sci. 2019;20(7):1739. https://doi.org/10.3390/ijms20071739 .
Ji J, Torrealba D, Thwaite R, Gomez AC, Parra D, Roher N. Nanostructured TNFα protein targets the zebrafish ( Danio rerio ) immune system through mucosal surfaces and improves the survival after Mycobacterium marinum lethal infection. Aquaculture. 2019;510:138–49. https://doi.org/10.1016/j.aquaculture.2019.05.050 .
Kamalii A, Prabu E, Ruby P, Ahilan B. Advanced developments in fish vaccination. J Aquacult Trop. 2018;33(1/2):101–9 https://search.proquest.com/openview/78c8d674d1ff36d2c136f6dc355bf1b8/1?pq-origsite=gscholar&cbl=506338 Accessed 27 Feb 2020.
Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharm Ther. 2007;82(1):70–80. https://doi.org/10.1038/sj.clpt.6100223 .
Kavaliauskis A, Arnemo M, Speth M, Lagos L, Rishovd AL, Estepa A, et al. Protective effect of a recombinant VHSV-G vaccine using poly (I: C) loaded nanoparticles as an adjuvant in zebrafish ( Danio rerio ) infection model. Dev Comp Immunol. 2016;61:248–57. https://doi.org/10.1016/j.dci.2016.04.010 .
Kim YS, Yoon NK, Karisa N, Seo SH, Lee JS, Yoo SS, et al. Identification of novel immunogenic proteins against Streptococcus parauberis in a zebrafish model by reverse vaccinology. Microb Pathog. 2019;127:56–9. https://doi.org/10.1016/j.micpath.2018.11.053 .
Lagos L, Tandberg JI, Becker MI, Winther-Larsen HC. Immunomodulatory properties of Concholepas concholepas hemocyanin against francisellosis in a zebrafish model. Fish Shellfish Immunol. 2017;67:571–4. https://doi.org/10.1016/j.fsi.2017.06.046 .
Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8(5):353. https://doi.org/10.1038/nrg2091 .
Litman GW, Cannon JP, Dishaw JL. Reconstructing immune phylogeny: new perspectives. Nat Rev Immunol. 2005;5(11):866–79. https://doi.org/10.1038/nri1712 .
Liu S, Steven DL. Zebrafish models for cancer. Ann Rev Pathol: Mech. 2011. https://doi.org/10.1146/annurev-pathol-011110-130330 .
Liu X, Wu H, Liu Q, Wang Q, Xiao J, Chang X, et al. Profiling immune response in zebrafish intestine, skin, spleen and kidney bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol. 2015;45(2):342. https://doi.org/10.1016/j.fsi.2015.04.028 .
Lohi O, Parikka M, Rämet M. The zebrafish as a model for paediatric diseases. Acta Paediatr. 2013;102(2):104–10. https://doi.org/10.1111/j.1651-2227.2012.02835.x .
López V, Risalde MA, Contreras M, Mateos-Hernández L, Vicente J, Gortázar C, et al. Heat-inactivated Mycobacterium bovis protects zebrafish against mycobacteriosis. J Fish Dis. 2018;41(10):1515–28. https://doi.org/10.1111/jfd.12847 .
MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov. 2015;14(10):721. https://doi.org/10.1038/nrd4627 .
Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34:2887–94. https://doi.org/10.1016/j.vaccine.2016.02.075 .
Mantilla Galindo A, Ocampo M, Patarroyo MA. Experimental models used in evaluating anti-tuberculosis vaccines: the latest advances in the field. Expert Rev Vaccines. 2019;18(4):365–77.
Marianes AE, Zimmerman AM. Targets of somatic hypermutation within immunoglobulin light chain genes in zebrafish. Immunology. 2011;132(2):240–55. https://doi.org/10.1111/j.1365-2567.2010.03358.x .
McFetridge R, Sobanjo-ter Meulen A, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2015;33(24):2793–9. https://doi.org/10.1016/j.vaccine.2015.04.025 .
Membrebe JD, Yoon NK, Hong M, Lee J, Lee H, Park K, et al. Protective efficacy of Streptococcus iniae derived enolase against streptococcal infection in a zebrafish model. Vet Immunol Immunopathol. 2016;170:25–9. https://doi.org/10.1016/j.vetimm.2016.01.004 .
Miller JD, Neely MN. Zebrafish as a model host for streptococcal pathogenesis. Acta Trop. 2004;91(1):53–68. https://doi.org/10.1016/j.actatropica.2003.10.020 .
Muktar Y, Tesfaye S, Tesfaye B. Present status and future prospects of fish vaccination: a review. J Vet Sci Technol. 2016;2:299. https://doi.org/10.4172/2157-7579.1000299 .
Myllymäki H, Niskanen M, Luukinen H, Parikka M, Rämet M. Identification of protective postexposure mycobacterial vaccine antigens using an immunosuppression-based reactivation model in the zebrafish. Dis Model Mech. 2018;11(3):dmm033175. https://doi.org/10.1242/dmm.033175 .
Myllymäki H, Niskanen M, Oksanen KE, Sherwood E, Ahava M, Parikka M, et al. Identification of novel antigen candidates for a tuberculosis vaccine in the adult zebrafish ( Danio rerio ). PLoS One. 2017;12(7):e0181942. https://doi.org/10.1371/journal.pone.0181942 .
Nguyen-Chi M, Phan QT, Gonzalez C, Dubremetz JF, Levraud JP, Lutfalla G. Transient infection of the zebrafish notochord with E. coli induces chronic inflammation. Dis Model Mech. 2014;7(7):871–82. https://doi.org/10.1242/dmm.014498 .
Novoa B, Romero A, Mulero V, Rodriguez I, Fernandez I, Figueras A. Zebrafish ( Danio rerio ) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV). Vaccine. 2006;24:5806–16. https://doi.org/10.1016/j.vaccine.2006.05.015 .
OECD. Test no. 236: fish embryo acute toxicity OECD guidelines for the testing of chemicals; 2013. OECD/OCDE N236 Last Modified July 26, 2013. https://www.oecd-ilibrary.org/docserver/9789264203709-en.pdf?expires=1554216347&id=id&accname=guest&checksum=98B3CA87CA423D51D70FAF3B708EA660 . Accessed 27 Feb 2020.
Ojanen M. Reverse genetics to study immunity against mycobacteria in zebrafish ( Danio rerio ); 2019. https://trepo.tuni.fi/bitstream/handle/ 10024/105069/978-952-03-0995-4.pdf?sequence= 1&isAllowed=y Accessed 27 Feb 2020.
Oksanen KE, Halfpenny NJ, Sherwood E, Harjula SKE, Hammarén MM, Ahava MJ, et al. An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine. 2013;31(45):5202–9. https://doi.org/10.1016/j.vaccine.2013.08.093 .
Palha N, Guivel-Benhassine F, Briolat V, Lutfalla G, Sourisseau M, Ellett F, et al. Real-time whole-body visualization of chikungunya virus infection and host interferon response in zebrafish. PLoS Pathog. 2013;9(9):e1003619. https://doi.org/10.1371/journal.ppat.1003619 .
Passoni G, Langevin C, Palha N, Mounce BC, Briolat V, Affaticati P, et al. Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes. Dis Model Mech. 2017;10(7):847e857. https://doi.org/10.1242/dmm.029231 .
Patterson H, Saralahti A, Parikka M, Dramsi S, Trieu-Cuot P, Poyart C, et al. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev Comp Immunol. 2012;38(3):447–55. https://doi.org/10.1016/j.dci.2012.07.007 .
Phalipon A, Mulard LA, Sansonetti PJ. Vaccination against shigellosis: is it the path that is difficult or is it the difficult that is the path? Microbes Infect. 2008;10(9):1057–62. https://doi.org/10.1016/j.micinf.2008.07.016 .
Ravindran C, Varatharajan GR, Raju R, Vasudevan L, Anantha SR. Infection and pathogenecity of Myroides odoratimimus (NIOCR-12) isolated from the gut of grey mullet ( Mugil cephalus (Linnaeus, 1758)). Microb Pathog. 2015;88:22–8. https://doi.org/10.1016/j.micpath.2015.08.001 .
Risalde MA, López V, Contreras M, Mateos-Hernández L, Gortázar C, de la Fuente J. Control of mycobacteriosis in zebrafish ( Danio rerio ) mucosally vaccinated with heat-inactivated Mycobacterium bovis . Vaccine. 2018;36(30):4447–53. https://doi.org/10.1016/j.vaccine.2018.06.042 .
Rocker AJ, Weiss AR, Lam JS, Van Raay TJ, Khursigara CM. Visualizing and quantifying Pseudomonas aeruginosa infection in the hindbrain ventricle of zebrafish using confocal laser scanning microscopy. J Microbiol Methods. 2015;117:85–94. https://doi.org/10.1016/j.mimet.2015.07.013 .
Salgado-Pabón W, Konradt C, Sansonetti PJ, Phalipon A. New insights into the crosstalk between Shigella and T lymphocytes. Trends Microbiol. 2014;22(4):192–8. https://doi.org/10.1016/j.tim.2014.02.002 .
Saralahti A. A zebrafish model for host-pathogen interactions in streptococcal infections; 2019. https://trepo.tuni.fi/bitstream/handle/10024/105436/978-952-03-0981-7.pdf?sequence=1 Accessed 27 Feb 2020.
Saralahti A, Rämet M. Zebrafish and streptococcal infections. Scand J Immunol. 2015;82(3):174–83. https://doi.org/10.1111/sji.12320 .
Scapigliati G, Fausto AM, Picchietti S. Fish lymphocytes: an evolutionary equivalent of mammalian innate-like lymphocytes? Front Immunol. 2018;9:971. https://doi.org/10.3389/fimmu.2018.00971 .
Shahin K, Shinn AP, Metselaar M, Ramirez-Paredes JG, Monaghan SJ, Thompson KD, et al. Efficacy of an inactivated whole-cell injection vaccine for nile tilapia, Oreochromis niloticus (L), against multiple isolates of Francisella noatunensis subsp. orientalis from diverse geographical regions. Fish Shellfish Immunol. 2019;89:217–27. https://doi.org/10.1016/j.fsi.2019.03.071 .
Shan Y, Fang C, Cheng C, Wang Y, Peng J, Fang W. Immersion infection of germ-free zebrafish with Listeria monocytogenes induces transient expression of innate immune response genes. Front Microbiol. 2015;6:373. https://doi.org/10.3389/fmicb.2015.00373 .
Shan Y, Zhang Y, Zhuo X, Li X, Peng J, Fang W. Matrix metalloproteinase-9 plays a role in protecting zebrafish from lethal infection with Listeria monocytogenes by enhancing macrophage migration. Fish Shellfish Immunol. 2016;54:179–87. https://doi.org/10.1016/j.fsi.2016.04.003 .
Sullivan C, Matty MA, Jurczyszak D, Gabor KA, Millard PJ, Tobin DM, Kim CH. Infectious disease models in zebrafish. In: Methods in cell biology, vol. 138: Academic; 2017. p. 101–36. https://doi.org/10.1016/bs.mcb.2016.10.005 .
Tandberg J, Oliver C, Lagos L, Gaarder M, Yáñez AJ, Ropstad E, Winther-Larsen HC. (2017). Membrane vesicles from Piscirickettsia salmonis induce protective immunity and reduce development of salmonid rickettsial septicemia in an adult zebrafish model. Fish Shellfish Immu. 2017;67:189–98. https://doi.org/10.1016/j.fsi.2017.06.015 .
Tenor JL, Oehlers SH, Yang JL, Tobin DM, Perfect JR. Live imaging of host-parasite interactions in a zebrafish infection model reveals cryptococcal determinants of virulence and central nervous system invasion. MBio. 2015;6(5):e01425–15. https://doi.org/10.1128/mBio.01425-15 .
Toh MC, Goodyear M, Daigneault M, Allen-Vercoe E, Van Raay TJ. Colonizing the embryonic zebrafish gut with anaerobic bacteria derived from the human gastrointestinal tract. Zebrafish. 2013;10(2):194–8. https://doi.org/10.1089/zeb.2012.0814 .
Torraca V, Gomes MC, Sarris M, Mostowy S. Meeting report: zebrafish infection and immunity 2019. Lab Anim. 2019;48(10):284–7. https://doi.org/10.1038/s41684-019-0397-4 .
Tsang M. Zebrafish: a tool for chemical screens. Birth Defects Res C Embryo Today. 2010;90(3):185–92. https://doi.org/10.1002/bdrc.20183 .
Van Dycke J, Ny A, Conceição-Neto N, Maes J, Hosmillo M, Cuvry A, et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019;15(9):e1008009. https://doi.org/10.1371/journal.ppat.1008009 .
Varela M, Figueras A, Novoa B. Modelling viral infections using zebrafish: innate immune response and antiviral research. Antivir Res. 2017;139:59–68. https://doi.org/10.1016/j.antiviral.2016.12.013 .
Voelz K, Gratacap RL, Wheeler RT. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides . Dis Model Mech. 2015;8(11):1375–88. https://doi.org/10.1242/dmm.019992 .
Wang Y, Ren Z, Fu L, Su X. Two highly adhesive lactic acid bacteria strains are protective in zebrafish infected with Aeromonas hydrophila by evocation of gut mucosal immunity. J Appl Microbiol. 2016;120(2):441–51. https://doi.org/10.1111/jam.13002 .
White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2(2):183–9. https://doi.org/10.1016/j.stem.2007.11.002 .
WHO. 2016. Human challenge trials for vaccine development: regulatory considerations. Expert Committee on Biological Standardisation. October. Geneva. https://www.who.int/biologicals/expert_committee/Human_challenge_Trials_IK_final.pdf . Accessed 27 Feb 2020.
WHO. Health topics: vaccines; 2019. https://www.who.int/topics/vaccines/en/ > Accessed 27 Feb 2020.
Wiles TJ, Norton JP, Smith SN, Lewis AJ, Mobley HL, Casjens SR, et al. A phyletically rare gene promotes the niche-specific fitness of an E. coli pathogen during bacteremia. PLoS Pathog. 2013;9(2):e1003175. https://doi.org/10.1371/journal.ppat.1003175 .
Wu XM, Cao L, Hu YW, Chang MX. Transcriptomic characterization of adult zebrafish infected with Streptococcus agalactiae . Fish Shellfish Immunol. 2019;94:355–72. https://doi.org/10.1016/j.fsi.2019.09.040 .
Ye J, Kaattari IM, Kaattari SL. The differential dynamics of antibody subpopulation expression during affinity maturation in a teleost. Fish Shellfish Immunol. 2011;30(1):372–7. https://doi.org/10.3389/fimmu.2018.00971 .
Ye N, Wu H, Zhang Y. Maternal transfer and protection role in zebrafish ( Danio rerio ) offspring following vaccination of the brood stock with a live attenuated Vibrio anguillarum vaccine. Aquac Res. 2016;47(11):3667–78. https://doi.org/10.1111/are.12821 .
Zhang C, Willett C, Fremgen T. Zebrafish: an animal model for toxicological studies. Curr Protoc Toxicol. 2003;17(1):1–7. https://doi.org/10.1002/0471140856.tx0107s17 .
Zhang D, Thongda W, Li C, Zhao H, Beck BH, Mohammed H, et al. More than just antibodies: protective mechanisms of a mucosal vaccine against fish pathogen Flavobacterium columnare . Fish Shellfish Immunol. 2017;71:160–70. https://doi.org/10.1016/j.fsi.2017.10.001 .
Zhang H, Shen B, Wu H, Gao L, Liu Q, Wang Q, et al. Th17-like immune response in fish mucosal tissues after administration of live attenuated Vibrio anguillarum via different vaccination routes. Fish Shellfish Immunol. 2014;37(2):229–38. https://doi.org/10.1016/j.fsi.2014.02.007 .
Download references
Acknowledgements
Not applicable.
There was no funding;
Author information
Authors and affiliations.
Ministry of Agriculture, Livestock and Supply, Federal Inspection Service, São Carlos, SP, Brazil
Ricardo Lacava Bailone
São Paulo State University, Botucatu, SP, Brazil
Ricardo Lacava Bailone & Roberto De Oliveira Roça
Health and Biological Sciences Center, Federal University, Federal University of São Carlos, São Carlos, SP, Brazil
Hirla Costa Silva Fukushima
Technical Directorate for Teaching and Research Support, São Paulo University, São Paulo, SP, Brazil
Bianca Helena Ventura Fernandes
Department of Food Technology and Innovation, Harper Adams University, Newport, UK
Luís Kluwe De Aguiar
Department of Genetic and Evolution, Federal University of São Carlos, São Carlos, SP, Brazil
Tatiana Corrêa, Helena Janke, Princia Grejo Setti & Ricardo Carneiro Borra
You can also search for this author in PubMed Google Scholar
Contributions
Bailone (Mean author); Fukushima (Zebrafish review); Fernandes (Photographies); Aguiar (Translator); Corrêa, Junke and Setti (Bibliographic review help, vaccine review, team group of Laboratory of Applied Immunology); Roça (Bailone’s Supervisor, review final corrections); Borra (Bailone’s Co-supervisor, review final corrections). The authors read and approved the final manuscript.
Corresponding author
Correspondence to Ricardo Lacava Bailone .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Bailone, R.L., Fukushima, H.C.S., Ventura Fernandes, B. et al. Zebrafish as an alternative animal model in human and animal vaccination research. Lab Anim Res 36 , 13 (2020). https://doi.org/10.1186/s42826-020-00042-4
Download citation
Received : 06 January 2020
Accepted : 19 March 2020
Published : 07 May 2020
DOI : https://doi.org/10.1186/s42826-020-00042-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- 3Rs, Animal health
- Human health
- Vaccine safety
Laboratory Animal Research
ISSN: 2233-7660
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Themed Issues
- High-Impact Collection
- Infographics
- Author Guidelines
- Open Access Options
- Self-Archiving Policy
- Why Publish with Us?
- About Animal Frontiers
- About the American Society of Animal Science
- Editorial Board
- Advertising & Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, common fish species used as model species, general features of zebrafish, why do zebrafish make such good animal models, zebrafish as a model for metabolic diseases, zebrafish as a model animal for diet-induced obesity, zebrafish as model for glucose metabolism and type 2 diabetes mellitus, zebrafish as model for dyslipidemia and atherosclerosis diseases, zebrafish as a model for nonalcoholic fatty liver disease and other liver disorders, zebrafish as a model for the study of intestinal diseases and host–microbe interactions, acknowledgments, literature cited.
- < Previous
The use of zebrafish ( Danio rerio ) as biomedical models
These authors contributed equally to this work.
- Article contents
- Figures & tables
- Supplementary Data
Tsegay Teame, Zhen Zhang, Chao Ran, Hongling Zhang, Yalin Yang, Qianwen Ding, Minxu Xie, Chenchen Gao, Yongan Ye, Ming Duan, Zhigang Zhou, The use of zebrafish ( Danio rerio ) as biomedical models, Animal Frontiers , Volume 9, Issue 3, July 2019, Pages 68–77, https://doi.org/10.1093/af/vfz020
- Permissions Icon Permissions
Because of its fully sequenced genome, easy genetic manipulation, high fecundity, external fertilization and rapid development, and nearly transparent embryo, zebrafish are a unique model animal for biomedical research, including studies of biological processes and human diseases.
Zebrafish have all the main organs involved in the process of metabolism and can be used to study several human metabolic disorders such as nonalcoholic fatty liver disease, type 2 diabetes mellitus, dyslipidemia, and other hepatic diseases.
With innovation and improvement of molecular techniques, zebrafish will continue to be an important biomedical model in the future.
Various animal species have important roles as experimental models to advance biomedical research. Animal models provide consistency and validity of research results from in vitro studies or studies with rodents. Zebrafish has become a popular animal model for biomedical research. As shown in Figure 1 , the number of publications per year on zebrafish as a model for biomedical research has been significantly increasing in recent years. One reason that zebrafish are an important biomedical model is because zebrafish embryos are transparent and they develop outside of the uterus. This unique developmental process allows scientists to study the details of development starting from fertilization and continuing throughout development. Innovation and development of molecular techniques in the later 20th century allowed zebrafish to be used as a model organism in almost all aspects of biology throughout the world. This review focuses on the use of zebrafish as a biomedical model in areas mainly related to diet-induced diseases, metabolic disorders, liver diseases, and intestinal diseases in humans.

The number of publications in PubMed per year when searching with the keywords “zebrafish” and “Biomedical.”
For more than 200 years, scientists used fish as model species with goldfish ( Carassius auratus ) the oldest model species. Goldfish were primarily used for applied studies of aquatic toxicology. Additional fish species have also been used, including zebrafish ( Danio rerio ), goldfish (Carassius auratus) , medaka ( Oryzias latipes ), roach ( Rutilus rutilus ), three-spined stickleback ( Gasterosteus aculeatus ), pufferfish ( Takifugu rubripes ), and the swordtail ( Xiphophorus hellerii ) ( Ribas and Piferrer, 2014 ). Every fish species has its unique advantages and disadvantages. For instance, goldfish have been used to study growth, stress, immunology, and reproduction. Medaka fish were the most popular species of fish used to study genetics, reproduction, and development. In recent years, the popularity of zebrafish as a model has increased due to its suitable features for many research areas.
Danio rerio the Latin name for zebrafish formerly called Brachydanio rerio is a small tropical freshwater fish originating in the Ganges River and its tributaries in northern India ( Tavares and Santos Lopes, 2013 ). In the natural habitat, zebrafish are usually found near the bottom of the water to minimize attack by predators. The morphology of male and female zebrafish is shown in Figure 2 .

Adult male and female AB strain of zebrafish, adapted from https://www.asianscientist.com/2014/12/in-the-lab/zebrafish-switch-sex/ with minor modification.
Currently, zebrafish are considered as a suitable model to investigate development, genetics, immunity, behavior, physiology, and nutrition. According to its feeding habits, zebrafish are classified as omnivores and they eat a variety of foods (euryphagous). During experimental trials, scientists use different types and levels of dietary feeds. The same amounts of ingredients are used for adult and larvae zebrafish. Moreover, the feeds and feeding regimes implemented by some laboratories for rearing zebrafish are varied and, in some cases, are implemented without formal evaluation ( Castranova et al., 2011 ; Gonzales and Law, 2013 ).
In the laboratory, to get reasonable research results, zebrafish should receive the appropriate type and level of dietary nutrients. Most of the time researchers use different commercial diets for zebrafish, but several commercial diets have undefined nutritional composition and may have an effect on experimental results ( Gonzales and Law, 2013 ). In addition, the dietary requirement for larvae and adults are different in the amount and composition of ingredients. In research studies, it is important to use a standard diet with adequate nutritional composition and known ingredients, which promote optimum growth and physiological status of the fish and to minimize the contribution of unintended nutritional effects on experimental results. The following diet formulas ( Tables 1 and 2 ) were developed in our laboratory and give consistent experimental results with zebrafish. We recommend that researchers use these dietary formulas in their studies with zebrafish.
Dietary formula for zebrafish larvae (5 to 29 d post fertilization)
| . | Basic feed . | High sugar . | High fat . | Low nitrogen . |
|---|---|---|---|---|
| Raw material (g/100 g diet) | ||||
| Casein | 46.00 | 46.00 | 46.00 | 32.00 |
| Gelatin | 11.00 | 11.00 | 11.00 | 8.00 |
| Dextrin | 22.00 | 31.00 | 10.00 | 32.00 |
| Lard oil | – | – | 8.00 | – |
| Soybean oil | 3.50 | 8.00 | 6.00 | |
| Cod liver oil | 3.50 | 2.00 | 4.00 | 4.00 |
| Soy lecithin | 2.00 | 2.00 | 2.00 | 2.00 |
| Lysine | 0.37 | 0.37 | 0.37 | – |
| VC phosphate | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Calcium dihydrogen phosphate | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium alginate | 4.00 | 4.00 | 4.00 | 4.00 |
| Zeolite powder | 4.93 | 0.93 | 3.93 | 9.30 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition analysis | ||||
| Crude protein (estimated) | 48.09 | 48.09 | 48.09 | 33.75 |
| Crude fat (estimated) | 9.01 | 4.01 | 22.01 | 12.01 |
| Nitrogen-free extract (estimated) | 22.00 | 31.00 | 10.00 | 32.00 |
| Total energy (KJ/g) | 15.13 | 14.75 | 18.02 | 15.53 |
| . | Basic feed . | High sugar . | High fat . | Low nitrogen . |
|---|---|---|---|---|
| Raw material (g/100 g diet) | ||||
| Casein | 46.00 | 46.00 | 46.00 | 32.00 |
| Gelatin | 11.00 | 11.00 | 11.00 | 8.00 |
| Dextrin | 22.00 | 31.00 | 10.00 | 32.00 |
| Lard oil | – | – | 8.00 | – |
| Soybean oil | 3.50 | 8.00 | 6.00 | |
| Cod liver oil | 3.50 | 2.00 | 4.00 | 4.00 |
| Soy lecithin | 2.00 | 2.00 | 2.00 | 2.00 |
| Lysine | 0.37 | 0.37 | 0.37 | – |
| VC phosphate | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Calcium dihydrogen phosphate | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium alginate | 4.00 | 4.00 | 4.00 | 4.00 |
| Zeolite powder | 4.93 | 0.93 | 3.93 | 9.30 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition analysis | ||||
| Crude protein (estimated) | 48.09 | 48.09 | 48.09 | 33.75 |
| Crude fat (estimated) | 9.01 | 4.01 | 22.01 | 12.01 |
| Nitrogen-free extract (estimated) | 22.00 | 31.00 | 10.00 | 32.00 |
| Total energy (KJ/g) | 15.13 | 14.75 | 18.02 | 15.53 |
1 Vitamin premix (g/kg): thiamine, 0.438; riboflavin, 0.632; pyridoxine·HCl, 0.908; d -pantothenic acid, 1.724; nicotinic acid, 4.583; biotin, 0.211; folic acid, 0.549; vitamin B 12 , 0.001; inositol, 21.053; menadione sodium bisulfite, 0.889; retinyl acetate, 0.677; cholecalciferol, 0.116; dl -α-tocopherol-acetate, 12.632.
2 Mineral premix (g/kg): CoCl 2 ·6H 2 O, 0.074; CuSO 4 ·5H 2 O, 2.5; FeSO 4 ·7H 2 O, 73.2; NaCl, 40.0; MgSO 4 ·7H 2 O, 284.0; MnSO 4 ·H 2 O, 6.50; KI, 0.68; Na 2 SeO 3 , 0.10; ZnSO 4 ·7H 2 O, 131.93; cellulose, 501.09. (Unpublished data; formulated in our zebrafish laboratory.)
Dietary formula for zebrafish (1 to 3 mo of age)
| . | Basic feed . | High Sugar . | High fat . | Low nitrogen . |
|---|---|---|---|---|
| Raw material (g/100g diet) | ||||
| Casein | 40.00 | 40.00 | 40.00 | 28.00 |
| Gelatin | 10.00 | 10.00 | 10.00 | 7.00 |
| Dextrin | 28.00 | 38.00 | 16.00 | 38.50 |
| Lard oil | – | – | 8.00 | – |
| Soybean oil | 6.00 | 2.00 | 8.00 | 6.00 |
| Lysine | 0.33 | 0.33 | 0.33 | – |
| VC phosphate | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Calcium dihydrogen phosphate | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium alginate | 2.00 | 2.00 | 2.00 | 2.00 |
| Microcrystalline cellulose | 4.00 | 4.00 | 4.00 | 4.00 |
| Zeolite powder | 6.97 | 0.97 | 8.97 | 11.80 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition analysis | ||||
| Crude protein (estimated) | 42.19 | 42.19 | 42.19 | 29.53 |
| Crude fat (estimated) | 6.01 | 2.01 | 16.01 | 6.01 |
| Nitrogen-free extract (estimated) | 28.00 | 38.00 | 16.00 | 38.50 |
| Total energy (KJ/g) | 14.02 | 14.18 | 15.77 | 13.65 |
| . | Basic feed . | High Sugar . | High fat . | Low nitrogen . |
|---|---|---|---|---|
| Raw material (g/100g diet) | ||||
| Casein | 40.00 | 40.00 | 40.00 | 28.00 |
| Gelatin | 10.00 | 10.00 | 10.00 | 7.00 |
| Dextrin | 28.00 | 38.00 | 16.00 | 38.50 |
| Lard oil | – | – | 8.00 | – |
| Soybean oil | 6.00 | 2.00 | 8.00 | 6.00 |
| Lysine | 0.33 | 0.33 | 0.33 | – |
| VC phosphate | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix | 0.20 | 0.20 | 0.20 | 0.20 |
| Calcium dihydrogen phosphate | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium alginate | 2.00 | 2.00 | 2.00 | 2.00 |
| Microcrystalline cellulose | 4.00 | 4.00 | 4.00 | 4.00 |
| Zeolite powder | 6.97 | 0.97 | 8.97 | 11.80 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition analysis | ||||
| Crude protein (estimated) | 42.19 | 42.19 | 42.19 | 29.53 |
| Crude fat (estimated) | 6.01 | 2.01 | 16.01 | 6.01 |
| Nitrogen-free extract (estimated) | 28.00 | 38.00 | 16.00 | 38.50 |
| Total energy (KJ/g) | 14.02 | 14.18 | 15.77 | 13.65 |
The amount of feed varies across the different growth stages of the fish and is dependent on the stage of growth. From 5 d post fertilization, zebrafish larvae are mostly fed zooplanktons such as paramecium and rotifers and young larvae can be fed with artificial food up to 100 μm in size or live feed. For adult fish, the size of the dry food can range from 300 to 400 μm ( Avdesh et al., 2012 ). The size of the dry food can increase with increasing size of the fish. The commonly practiced feeding ratio of zebrafish is about 4% of its bodyweight. Overfeeding may increase the concentration of nitrate in the water and affect the physiology of the fish. In addition, overeating may cause death of the fish.
The criteria to select animal models for biomedical research are directly related to the final goal of the research. The use of zebrafish as a biomedical model was suggested by George Streisinger and colleagues at the University of Oregon, who launched the modern era for zebrafish in the field of biomedical research ( Clark and Ekker, 2015 ). Zebrafish are popular animal models because they have numerous advantages over other species. The most advantageous features of zebrafish are a fully sequenced genome, easy manipulation of its genome, high fecundity, short generation time (about 3 mo), rapid embryonic development (24 hr), and external fertilization. The translucent zebrafish embryo allows study of the different developmental stages starting from the early stage of embryogenesis. In addition, zebrafish embryos form complete organ systems, including heart, intestine and blood vessels within 48 hr after fertilization. More than 10,000 mutants in protein-coding genes have been generated ( Howe et al., 2013 ) and several transgenic lines of zebrafish have been made to study human diseases. The availability of multiple strains of zebrafish is another important advantage of this species. In addition, it is also very affordable to maintain a large number of zebrafish in a relatively small amount of laboratory space. Although zebrafish require relatively easy management, special attention must be paid to ensuring a healthy diet and adequate water quality to optimize fish health and growth. While there are several strains of zebrafish in the world, the most widely used strains in biomedical research are AB, Casper, Ekkwill, Nadia, Wild Indian Karyotype, wild-caught, and Tubingen. According to the ZFIN website, more than 800 biological laboratories around the world conduct basic and applied research with zebrafish ( https://zfin.org/search?q=Zebrafish+laboratories&category ). Many of these laboratories use zebrafish to study human diseases, including neural disorders, cancer, infectious diseases, cardiovascular diseases, kidney diseases, diabetes, blindness, deafness, digestive diseases, hematopoiesis, and muscle disorders.
Mutant zebrafish have been established by knocking out or knocking in specific genes. These alterations create novel biomedical models. For example, if the patient has a disease related to metabolism, different mutations in zebrafish genes related to metabolism can be made and then changes in gene expression can be monitored using different molecular techniques. The short generation time of zebrafish makes it difficult to produce stable transgenic adults or homozygous mutant embryos, which usually requires about 4 months. Recently, scientists have developed many technologies to expedite the transgenic process ( Burger et al., 2016 ). The presence or absence of genomic duplication events in zebrafish makes it complicated to study some human diseases such as diabetes mellitus. Zebrafish are also important for developing new therapies or screening novel drugs to treat or prevent human diseases.
Even though zebrafish are an important biomedical model, they have some limitations, including the dissimilarity of some organs like the respiratory system and the reproductive system. Thus, it is difficult to use zebrafish as a model for respiration or reproduction in humans. In addition, because zebrafish live in an aquatic habitat, screening of some water soluble drugs in zebrafish is another limitation.
There are several examples of human diseases that have been successfully modeled in zebrafish such as Duchenne muscular dystrophy, human melanoma, acute lymphoblastic leukemia, polycystic kidney disease, nephronophthisis, acute kidney injury, Parkinson’s disease, Huntington’s disease, Alzheimer disease, myocardial infarction, and some metabolic diseases. As shown in Figure 3 , in addition to genomic similarity, the presence of conserved organs and organ systems between human and zebrafish contributes to development of a number of successful models of human diseases.

Some of the conserved organ systems between zebrafish and humans (adapted from http://www.intl.upm.edu.my/article/zebrafish_replace_lab_rat-30977 with minor modification).
We will focus on the common human metabolic diseases successfully modeled in zebrafish, including obesity, type 2 diabetes mellitus, nonalcoholic steatohepatitis, and atherosclerosis. Disturbance of the normal process of converting food to energy in the cell results in different metabolic disorders. Even though zebrafish and humans have differences in basic nutrient requirements, different metabolic mechanisms may not be needed. To keep the balance between the production and utilization of energy several organs are involved, including the brain, intestines, liver, skeletal muscle, and adipose tissue. Whole animal models are needed to study the entire process of metabolism. Zebrafish are an appropriate model to study metabolic dysfunction because they have all the organs involved in energy homeostasis and metabolism including appetite and insulin regulation and a lipid storage system which is conserved with that found in humans ( Nishio et al., 2012 ).
A report from World Health Organization indicated that, of the metabolism-related human diseases, cardiovascular disease is currently the most predominant fatal disease ( Lozano et al., 2012 ). Obesity ( Ng et al., 2014 ), type 2 diabetes mellitus, and nonalcoholic fatty liver disease ( LaBrecque et al., 2014 ) increase the risk of cardiovascular disease. Because zebrafish and humans have similar metabolic organs (including the digestive organs, adipose tissue, and muscle), zebrafish are a popular model to study metabolic disorders. In addition, the availability of several new tools and approaches such as talens, CRISPR/Cas9 ( Wu et al., 2018 ), compound treatment ( Poureetezadi et al., 2016 ), mass spectrometry-based polar metabolomics and lipidomics ( Zhang et al., 2018 ), and in vivo imaging of fluorescent dyes ( Minchin et al., 2018 ) make it possible to investigate the molecular mechanisms of metabolic processes in zebrafish.
Researchers have also used zebrafish as a model organism to study different types of metabolic diseases such as congenital errors of metabolism, hyper- and hypothyroidism, disorders of the hypothalamus–pituitary–adrenal axis, dysregulation of the circadian clock, and cancer metabolism ( Gut et al., 2017 ). In this review, our emphasis will be on diet-induced metabolic disorders.
Utilization of zebrafish in diet-induced obesity studies was first developed by Oka et al. ( 2010 ) by feeding adult zebrafish Artemia nauplii . In these studies, the fish showed increased body mass index, developed hepatic steatosis, hypertriglyceridemia, and dysregulation of some lipid metabolism genes. Chen et al. (2018) fed zebrafish a diet of high cholesterol, which resulted in increased body weight, increased triglyceride levels, and lipid deposition in the liver. Over nutrition of zebrafish with high fat from different sources or cholesterol also lead to hyperglycemia and ectopic lipid accumulation, increased body weight, increased adipose tissue, cardiovascular overload, and steatosis ( Forn-Cuní et al., 2015 ). Landgraf et al. (2017) used zebrafish to compare the result of overfeeding with normal and high-fat diets on obesity development. They concluded that both diets showed an increase in adipose tissue and the fish fed the normal fat diet developed obesity, but these fish were metabolically healthy. The other fish fed a high-fat diet were unhealthy. Similar with the above findings, in our laboratory, we also found that larvae and adult zebrafish fed a high-fat diet developed hepatic steatosis as shown in Figure 4 . In zebrafish, diet-induced obesity is also used to estimate the type of food and effect of nutrient compounds on development, testing, and discovering different drugs to prevent or treat obesity and by altering fat metabolism. The diet-induced obesity zebrafish model overfed with Artemia shares common pathophysiological pathways with mammalian obesity and can be used to identify putative pharmacological targets of human obesity ( Oka et al., 2010 ). Therefore, the diet-induced obesity approach allows us to understand the disease in the context of systematic obesity, hence mimicking the most common process occurring in humans affected by this condition.

High-fat diets (HFD) induced hepatic steatosis in adult and larval zebrafish. (a) Adult zebrafish (1 mo old) and larval zebrafish (5 d post fertilization). (b) Representative liver histology image by Haemotoxylin and Eosin (H&E) staining and oil red O (ORO) staining of adult zebrafish fed with a control diet or HFD for 4 wk. The scale bar is 50 μm. (c) Representative intestinal histology image by H&E staining and ORO staining of adult zebrafish fed with a control diet or HFD for 4 wk. The scale bar is 100 μm. (d) Representative image of whole-mount ORO staining in zebrafish larvae fed control diet and HFD for 7 d. The scale bar is 200 μm. (Unpublished data from our zebrafish laboratory.)
The main cause for development of diabetes mellitus is the failure of pancreatic β-cells to produce insulin, which leads to insulin deficiency. These functions and processes are conserved between zebrafish and humans. Zebrafish exposure to hypercaloric and high-fat diets quickly induces obesity and obesity‐related disease, and activates metabolic pathways very similar to their human counterparts. If glucose is available in the diet, insulin is produced by the pancreas, and gluconeogenesis is inhibited through the down-regulation of genes involved in the pathway. In the absence of glucose in the bloodstream, gluconeogenesis is induced by the action of glucagon. Capiotti et al. (2014) revealed that zebrafish immersed in a high-glucose solution (111 mM) for 14 d were able to increase by 41% froctosamine (glycated protein) levels from the eyes, decreased amounts of mRNA for insulin receptors in muscle, and developed hyperglycemia. Zang et al. (2017) developed a zebrafish model of type 2 diabetes mellitus by overfeeding a high-calorie diet (408 calories per fish per day). Using gene expression profiling in the liver and pancreas, a common pathway for development of type 2 diabetes mellitus was seen between zebrafish and humans. The relationship between age and type 2 diabetes mellitus was developed by Connaughton et al. (2016) and revealed that young zebrafish (4 to 11 mo olds) developed hyperglycemia slower than old zebrafish with increasing concentrations of glucose. The glucose concentration of homeostasis organs can be increased by immersing zebrafish embryos in a glucose solution. Gleeson et al. (2007) showed that immersion of adult zebrafish in a 1% glucose solution for 24 hr increase blood glucose up to 400 mg/dL. The two transgenic models of insulin resistance established by Zang et al. (2017) were skeletal muscle insulin resistance achieved by transgenic expression of a dominant-negative IGF-I receptor in skeletal muscle. In the second model, insulin resistance was attained via liver--specific knockdown of the insulin receptor gene using CRISPR/Cas9 ( Yin et al., 2015 ). These results revealed that zebrafish are a suitable model to study glucose-induced human disease. Marín-Juez et al. (2014) also developed a zebrafish model for hyperinsulinemia by injecting human recombinant insulin in zebrafish larvae. These studies demonstrated upregulation of the negative immune modulator protein tyrosine phosphatase non receptor type 6 in insulin-resistant larvae. Recent research results of Yang et al. (2018) showed that mutant zebrafish with a knockout in insulin receptor a and b genes when fed a high-carbohydrate (41%) diet showed hyperglycemia, reduced growth hormone signaling, increased visceral adiposity, and fatty liver development, which are similar signs to the human lipodystrophy disease. The glucose level in zebrafish can be measured using two hand-held glucose meters designed for use in humans with diabetics ( Eames et al., 2010 ). Additionally, fasting for performing postprandial glucose and intraperitoneal glucose tolerance tests can be used. There are several methods of measuring insulin levels in zebrafish, including measuring the insulin mRNA expression level by q-PCR ( Michel et al., 2016 ), insulin antibody for immunostaining ( Kimmel et al., 2015 ), or semi-quantitative dot-blot ( Olsen et al., 2012 ). Insulin sensitivity can also be assessed by intraperitoneal injection of insulin in hyperglycemic zebrafish ( Capiotti et al., 2014 ).
Increasing the level of cholesterol, triglycerides, or high-density lipoprotein cholesterol resulted in dyslipidemia, and in turn, led to development of atherosclerosis. Since the nutritional requirements of zebrafish are known, several researchers established different models by changing the standard diet (such as feeding zebrafish a high-fat diet to develop obesity, hyperglycemia, and dyslipidemia) to induce metabolic stress on the fish. The histopathological changes showed by zebrafish fed a high level of cholesterol are very similar with the symptoms shown in human atherosclerosis ( Fang and Miller, 2012 ). Formulation of a high-cholesterol diet is also important for the study of dyslipidemia ( Oka et al., 2010 ). Miyares et al. (2014) described lipid and lipoprotein metabolism using the zebrafish embryo yolk metabolism stages and concluded that incorporation of exogenous fatty acids into the circulatory system was dependent on lipoprotein production in the system.
Nonalcoholic fatty liver disease is not related to overconsumption of alcohol. It is the accumulation of excess fat in the liver, and this can lead to steatosis, steatohepatitis, fibrosis, corrihosis, and hepatocellular carcinoma. This disease can develop and be associated with insulin resistance, high-fat diets, drug-induced liver injuries, and metabolic syndromes. Several research results show that zebrafish also develop hepatic steatosis when exposed to hepatotoxic chemicals, fasting and excessive dietary fat, cholesterol, or carbohydrate. These mechanisms are similar in zebrafish and humans. Interestingly, publication of the first paper on zebrafish development ( Roosen, 1937 ) investigated the effect of different toxins, alcohol, and different levels of carbohydrate or fat diets on zebrafish embryos, larvae, and adult developmental stages. The application of toxins to the fish tank is a simple technique and this technique makes zebrafish a popular model to study chemical screening mechanisms.
The zebrafish liver resembles the human liver in cellular structure, function, and genetics. This observation led investigators to use zebrafish to study the detailed embryological and genetics associated with development of the human liver, as well as liver disorders and potential therapies for liver diseases. Development of liver tumors in zebrafish using carcinogenic substances and comparison with gene expression in tumors of human livers first pointed to the importance of zebrafish as an appropriate biomedical model. Tonin et al. (2018) showed that zebrafish immersed in 6% fructose lead to the formation of hepatic steatosis in a manner similar to the symptoms shown in humans fed a high-carbohydrate diet. Using a differential feeding strategy, Yang et al. (2019) showed that over feeding resulted in development of fatty liver and hastened the carcinogenic process. In addition, the hormone leptin, which is responsible for obesity, was unregulated in the oncogenic and overfed zebrafish. They also found that, by downregulating leptin signaling, it is possible to reduce the muscle wasting phenotype. Development of a mutated gene foie gras in zebrafish initiated scientists to study development of hepatic steatosis and the associated molecular mechanisms. In addition, development of gonzo mutant zebrafish showed that development of alcohol-induced hepatic steatosis was mediated by sterol response element binding protein transcription factors ( Passeri et al., 2009 ). Shimada et al. (2015) applied transcriptomic and proteomic methods using a model of diet-induced obesity in the liver of zebrafish to isolate genes responsible for the formation of hepatic steatosis. In these studies, fatty acid binding protein 3 and transcription factors (E2F) were upregulated in hepatic steatosis zebrafish. Howarth et al. (2013) developed two models using zebrafish to investigate either tunicamycin- or ethanol-provoked steatosis which leads to liver failure. They prevented ethanol-induced steatosis by blocking activation of sterol response element binding proteins using mutant zebrafish. In these studies, even without lipid accumulation, hepatocyte dysfunction occurred. Recent research from Imran et al. (2018) using zebrafish larvae to test the involvement of membrane remodeling in hepatotoxicity showed that co-exposure of obese zebrafish larvae to benzo[a]pyrene and ethanol induced in vivo hepatotoxicity through membrane remodeling. This result led scientists to develop a therapy for nonalcoholic fatty liver disease and associated risk factors.
The intestine of zebrafish is a long tube like structure, which has been divided into the intestine bulb, mid-intestine, and posterior intestine that folds twice in the abdominal cavity. The absorptive enterocytes, goblet cells, and endocrine cells are the three cell types that have differentiated from the intestine epithelium. Since innovation of forward genetic screening techniques, many scientists have used zebrafish as a model to study the physiology, function, and diseases of the human intestine. The entire intestinal track opens at 6 d post fertilization and at this time larvae start to feed on small aquatic animals ( Brugman, 2016 ). At this stage of development, the intestine of the fish is easily visible and its morphology can be observed with a microscope. Because of its transparent body, many researchers have developed a zebrafish model of intestinal inflammation. Ji et al. (2018) developed a zebrafish model to evaluate how bioactive compounds are taken up by the intestine. They concluded that bioactive compounds are able to cross the intestinal mucosal barriers and pass through the lamina propria to reach the muscle. Arias-Jayo et al. (2018) showed that zebrafish fed a high-fat diet of 10% (w/w) cocoa butter added to the normal diet resulted in intestinal inflammation via activation of NF-κβ. The intestinal barrier was also damaged and there was an increase in mucin production by goblet cells. Oehlers et al. (2011a) developed a model with zebrafish embryos infected with salmonella, and showed that depletion of the bacterial detector proteins NOD1 and NOD2 reduced expression of the dual oxidase in the intestinal epithelial. This also weakened the ability of the fish to reduce the intracellular burden of bacteria. Overall, this finding was a good model for Crohn’s disease in humans.
Zebrafish have also been used to study host–microbe interactions in the digestive system. Recent studies of the intestinal microbiome in zebrafish with a mutation in gene myd88 demonstrated that changes due to the microbiome in the body (especially the intestinal leukocytes) are dependent on the immune adaptor gene myd88 ( Koch et al., 2018 ). Raising germ-free zebrafish to investigate the effect of microbiota on the innate immune system has also been studied ( Kostic et al., 2013 ) and the contribution of gut microbes on fatty acid absorption was studied by Semova et al. (2012) . In these studies with zebrafish, the fish with microbes in their gut had increased fatty acid absorption, higher accumulation of fats in the liver and the body when compared with germ-free zebrafish. The gut microbiota of human and zebrafish are different. Valenzuela et al. (2018) showed that germ-free zebrafish larvae can be colonized by human gut microorganisms, such as Clostridioides difficile and Bacillus . This result opened an interesting area to study interactions between these microorganisms and the host. The role of the gut microbiota on host biology is similar between zebrafish and mammals and, in both species, intestinal microbiota participate in the education of the immune system, maturation of the gut, and promotion of nutrient metabolism in the host ( Bates et al., 2007 ).Therefore, zebrafish are an important model to further explore intestinal diseases and related aspects of gut biology.
Zebrafish are an important biomedical model in every aspect of biology. Zebrafish have several suitable features for developmental, physiological, and genetic studies including external fertilization and the transparent nature of embryo. The large degree of functional conservation of morphology, genetics, and physiology between zebrafish and humans makes zebrafish an attractive model for several human disorders and development of potential therapies for humans. Advancement of nanotechnologies and molecular techniques also contributes to the use of zebrafish to study different diseases in humans. In this review, we emphasized some biomedical areas where zebrafish are a popular model to investigate the mechanisms and processes associated with metabolic diseases, including diet-induced obesity, type 2 diabetes mellitus, dyslipidemia and atherosclerosis, liver-related diseases, and intestinal diseases. Scientists have also used zebrafish to develop new therapies to treat and prevent these important human diseases.
This work was supported by the National Natural Science Foundation of China (31872584, 3180131599, 31702354, 31602169, 31672294, 31572633), the Beijing earmarked fund for Modern Agro-industry Technology Research System (SCGWZJ 20191104-4), and Innovation Capability Support Program of Shaanxi (2018TD-021).
About the Authors

Tsegay Teame is a PhD student in the laboratory of Dr. Zhigang Zhou and the Innovation Team of Aquatic Animal Feed at the Feed Research Institute, Chinese Academy of Agricultural Science, Beijing, China. His research focuses on the effects of high-fat diet on the gut microbiota of zebrafish. He received his M.Sc. in Aquaculture and Fisheries from Ambo University, Ethiopia.

Zhen Zhang received his PhD from the Chinese Academy of Agricultural Sciences, Beijing, China. He is currently a postdoctoral trainee in the laboratory of Dr. Zhigang Zhou and the Innovation Team of Aquatic Animal Feed at the Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China.

Chao Ran received his PhD from Auburn University, Alabama, USA. He is currently associate professor in the Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China.

Yalin Yang received her PhD from the Chinese Academy of Agricultural Sciences, Beijing, China and is currently deputy researcher of Biochemistry and Molecular Biology in the Innovation Team of Aquatic Animal Feed, at the Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China.

Qianwen Ding received her MSc from the Chinese Academy of Agricultural Sciences, Beijing, China. She is currently a research assistant in the Key Laboratory for Feed Biotechnology of the Ministry of Agriculture at the Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China.

Mingxu Xie received her MSc from Dalian Ocean University, Dalian, China. She is currently a research assistant in the Key Laboratory for Feed Biotechnology of the Ministry of Agriculture at the Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China.

Hongling Zhang received her PhD from the Chinese Academy of Agricultural Sciences, Beijing, China. She is currently a postdoctoral trainee in the laboratory of Dr. Zhigang Zhou, China-Norway Joint Lab on Fish Gastrointestinal Microbiota, Feed Research Institute at the Chinese Academy of Agricultural Sciences, Beijing, China.

Chenchen Gao received her BSc from the Yunnan Normal University, Yunnan, China. She is currently a research assistant in the Key Laboratory for Feed Biotechnology of the Ministry of Agriculture, Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, China.

Ye Yong’an is currently the chairman of the Committee of Hepatology of the World Federation of Chinese Medicine Societies. As one of the most prominent hepatologists in the field of viral hepatitis B in China, he has been dedicated to the treatment of chronic liver diseases with integrative approaches of traditional Chinese herbal medicines plus modern therapies for decades. Funded by the National Health Commission of the P.R. China, he has presided over five large-scaled clinical studies nationwide, from which he had established a series of innovative therapeutic protocols for chronic hepatitis B and made a significant contribution to the control of viral hepatitis B in P.R. China. Moreover, he is also well known in treating refractory diseases such as liver cirrhosis, and gastrointestinal cancers with Chinese herbal medicines.

Min Duan received his PhD from the Institute of Hydrobiology, Chinese Academy of Science. He was a postdoctoral fellow in the East China Sea fisheries Research Institute, Chinese Academy of Fishery Science/Shanghai Ocean University. His research interest is in fish ecology. Currently, he is working as an associate researcher at the Institute of Hydrology, Chinese Academy of Science, Hubei, China

Zhigang Zhou is the Director of the Aqua Feed Innovation Research Team, Feed Research Institute of Chinese Academy of Agricultural Sciences, Head of the China-Norway Joint Lab on Fish Gut Microbiota, and Associate Chairman of the International Research Consortium on Gastrointestinal Microbiota in Aquatic Animals. His current research interests focus on understanding the interaction between gut microbiota and the piscine host. He is also interested in microbial products to support sustainable aquaculture.
Arias-Jayo ]]], N. , L. Abecia , L. Alonso-Sáez , A. Ramirez-Garcia , A. Rodriguez , and M.A. Pardo . 2018 . High-fat diet consumption induces microbiota dysbiosis and intestinal inflammation in zebrafish . Microb. Ecol . 76 : 1089 – 1101 . doi: 10.1007/s00248-018-1198-9
Google Scholar
Avdesh ]]], A. , M. Chen , M.T. Martin-Iverson , A. Mondal , D. Ong , S. Rainey-Smith , K. Taddei, M. Lardelli, D.M. Groth, G. Verdile, and R.N. Martins. 2012 . Regular care and maintenance of a Zebrafish ( Danio rerio ) laboratory: an introduction . J. Vis. Exp . 69 : e4196 , doi: 10.3791/4196
Bates ]]], J.M. , J. Akerlund , E. Mittge , and K. Guillemin . 2007 . Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota . Cell Host Microbe 2 : 371 – 382 . doi: 10.1016/j.chom.2007.10.010
Brugman ]]], S . 2016 . The zebrafish as a model to study intestinal inflammation . Dev. Comp. Immunol . 64 : 82 – 92 . doi: 10.1016/j.dci.2016.02.020
Burger ]]], A. , H. Lindsay , A. Felker , C. Hess , C. Anders , E. Chiavacci , J. Zaugg , L.M. Weber , R. Catena , M. Jinek , et al . 2016 . Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes . Development , 143 ( 11 ): 2025 – 2037 doi: 10.1242/dev.134809
Capiotti ]]], K.M. , R. Antonioli , Jr , L.W. Kist , M.R. Bogo , C.D. Bonan , and R.S. Da Silva . 2014 . Persistent impaired glucose metabolism in a Zebrafish hyperglycemia model . Comp. Biochem. Physiol. B. Biochem. Mol. Biol . 171 : 58 – 65 . doi: 10.1016/j.cbpb.2014.03.005
Castranova ]]], D. , A. Lawton , C. Lawrence , D.P. Baumann , J. Best , J. Coscolla , A. Doherty , J. Ramos , J. Hakkesteeg , C. Wang , et al. 2011 . The effect of stocking densities on reproductive performance in laboratory zebrafish ( Danio rerio ) . Zebrafish 8 : 141 – 146 . doi: 10.1089/zeb.2011.0688
Chen ]]], B. , Y.M. Zheng , and J.P. Zhang . 2018 . Comparative study of different diets-induced NAFLD models of zebrafish . Front. Endocrinol. (Lausanne) . 9 : 366 . doi: 10.3389/fendo.2018.00366
Clark ]]], K.J., and S.C. Ekker . 2015 . How zebrafish genetics informs human biology . Nat. Educ . 8 ( 4 ): 3 .
Connaughton ]]], V.P. , C. Baker , L. Fonde , E. Gerardi , and C. Slack . 2016 . Alternate immersion in an external glucose solution differentially affects blood sugar values in older versus younger zebrafish adults . Zebrafish 13 : 87 – 94 . doi: 10.1089/zeb.2015.1155
Eames ]]], S.C. , L.H. Philipson , V.E. Prince , and M.D. Kinkel . 2010 . Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis . Zebrafish 7 : 205 – 213 . doi: 10.1089/zeb.2009.0640
Fang ]]], L., and Y.I. Miller . 2012 . Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids . Free Radic. Biol. Med . 53 : 1411 – 1420 . doi: 10.1016/j.freeradbiomed.2012.08.004
Forn-Cuní ]]], G. , M. Varela , C.M. Fernández-Rodríguez , A. Figueras , B. Novoa . 2015 . Liver immune responses to inflammatory stimuli in a diet‐ induced obesity model of zebrafish . J Endocrinol 224 : 159 – 170 . doi: 10.1530/JOE-14-0398
Gleeson ]]], M. , V. Connaughton , and L.S. Arneson . 2007 . Induction of hyperglycaemia in zebrafish ( Danio rerio ) leads to morphological changes in the retina . Acta Diabetol . 44 : 157 – 163 . doi: 10.1007/s00592-007-0257-3
Gonzales ]]], J.M., Jr , and S.H. Law . 2013 . Feed and feeding regime affect growth rate and gonadosomatic index of adult zebrafish ( Danio rerio ) . Zebrafish 10 : 532 – 540 . doi: 10.1089/zeb.2013.0891
Gut ]]], P. , S. Reischauer , D.Y.R. Stainier , and R. Arnaout . 2017 . Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease . Physiol. Rev . 97 : 889 – 938 . doi: 10.1152/physrev.00038.2016
Howarth ]]], D.L. , C. Yin , K. Yeh , and K.C. Sadler . 2013 . Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae . Zebrafish 10 : 199 – 210 . doi: 10.1089/zeb.2012.0821
Howe ]]], K. , M.D. Clark , C.F. Torroja , J. Torrance , C. Berthelot , M. Muffato , J.E. Collins , S. Humphray , K. McLaren , L. Matthews , et al. 2013 . The zebrafish reference genome sequence and its relationship to the human genome . Nature 496 : 498 – 503 . doi: 10.1038/nature12111
Imran ]]], M. , O. Sergent , A. Tete , I. Gallais , M. Chevanne , D. Lagadic-Gossmann , and N. Podechard . 2018 . Membrane remodeling as a key player of the hepatotoxicity induced by co-exposure to benzo[a]pyrene and ethanol of obese zebrafish larvae . Biomolecules 8 : 26 . doi: 10.3390/biom8020026
Ji ]]], J. , R. Thwaite , and N. Roher . 2018 . Oral intubation of adult zebrafish: a model for evaluating intestinal uptake of bioactive compounds . J. Vis. Exp . 139 : e58366 .
Kimmel ]]], R.A. , S. Dobler , N. Schmitner , T. Walsen , J. Freudenblum , and D. Meyer . 2015 . Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment . Sci. Rep . 5 : 14241 . doi: 10.1038/srep14241
Koch ]]], B.E.V. , S. Yang , G. Lamers , J. Stougaard , and H.P. Spaink . 2018 . Intestinal microbiome adjusts the innate immune setpoint during colonization through negative regulation of myd88 . Nat. Commun . 9 : 4099 . doi: 10.1038/s41467-018-06658-4
Kostic ]]], A.D. , M.R. Howitt , and W.S. Garrett . 2013 . Exploring host-microbiota interactions in animal models and humans . Genes Dev . 27 : 701 – 718 . doi: 10.1101/gad.212522.112
LaBrecque ]]], D.R. , Z. Abbas , F. Anania , P. Ferenci , A.G. Khan , K.L. Goh , S.S. Hamid , V. Isakov , M. Lizarzabal , M.M. Peñaranda , et al. 2014 . World gastroenterology organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis . J. Clin. Gastroenterol . 48 ( 6 ): 467 – 473 .
Landgraf ]]], K. , S. Schuster , A. Meusel , A. Garten , T. Riemer , D. Schleinitz , W. Kiess , and A. Körner . 2017 . Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity . BMC Physiol . 17 : 4 . doi: 10.1186/s12899-017-0031-x
Lozano ]]], R. , M. Naghavi , K. Foreman , S. Lim , K. Shibuya , V. Aboyans , J. Abraham , T. Adair , R. Aggarwal , S.Y. Ahn , et al. 2012 . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010 . Lancet 380 : 2095 – 2128 . doi: 10.1016/S0140-6736(12)61728-0
Marín-Juez ]]], R. , S. Jong-Raadsen , S. Yang , and H.P. Spaink . 2014 . Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish . J. Endocrinol . 222 : 229 – 241 . doi: 10.1530/JOE-14-0178
Michel ]]], M. , P.S. Page-McCaw , W. Chen , and R.D. Cone . 2016 . Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish . Proc. Natl. Acad. Sci. USA . 113 : 3084 – 3089 . doi: 10.1073/pnas.1513212113
Minchin ]]], J.E.N. , C.M. Scahill , N. Staudt , E.M. Busch-Nentwich , and J.F. Rawls . 2018 . Deep phenotyping in zebrafish reveals genetic and diet-induced adiposity changes that may inform disease risk . J. Lipid Res . 59 : 1536 – 1545 . doi: 10.1194/jlr.D084525
Miyares ]]], R.L. , V.B. de Rezende , and S.A. Farber . 2014 . Zebrafish yolk lipid processing: a tractable tool for the study of vertebrate lipid transport and metabolism . Dis. Model. Mech . 7 : 915 – 927 . doi: 10.1242/dmm.015800
Ng ]]], M. , T. Flerning , M. Robinson , B. Thornson , N. Graetz , C. Margono , E.C. Mvllany , S. Biryvkov , S. Abbafati , S.F. Abera , et al . 2014 . Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013 . The Lancet , 384 ( 9945 ): 766 – 781 . doi: 10.1016/S0140-6736(14)60460-8
Nishio ]]], S. , Y. Gibert , L. Berekelya , L. Bernard , F. Brunet , E. Guillot , J.C. Le Bail , J.A. Sánchez , A.M. Galzin , G. Triqueneaux , et al. 2012 . Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner . Mol. Endocrinol . 26 : 1316 – 1326 . doi: 10.1210/me.2011-1180
Oehlers ]]], S.H. , M.V. Flores , K.S. Okuda , C.J. Hall , K.E. Crosier , and P.S. Crosier . 2011a . A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents . Dev. Dyn . 240 : 288 – 298 . doi: 10.1002/dvdy.22519
Oka ]]], T. , Y. Nishimura , L. Zang , M. Hirano , Y. Shimada , Z. Wang , N. Umemoto , J. Kuroyanagi , N. Nishimura , and T. Tanaka . 2010 . Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity . BMC Physiol . 10 : 21 . doi: 10.1186/1472-6793-10-21
Olsen ]]], A.S. , M.P. Sarras Jr. , A. Leontovich , and R.V. Intine . 2012 . Heritable transmission of diabetic metabolic memory in zebrafish correlates with DNA hypomethylation and aberrant gene expression . Diabetes 61 : 485 – 491 . doi: 10.2337/db11-0588
Passeri ]]], M.J. , A. Cinaroglu , C. Gao , and K.C. Sadler . 2009 . Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation . Hepatology 49 : 443 – 452 . doi: 10.1002/hep.22667
Poureetezadi ]]], S.J. , C.N. Cheng , J.M. Chambers , B.E. Drummond , and R.A. Wingert . 2016 . Prostaglandin signaling regulates nephron segment patterning of renal progenitors during zebrafish kidney development . eLife 5 : e17551 .
Ribas ]]], L. , and P. Francesc . 2014 . The zebrafish ( Danio rerio ) as a model organism, with emphasis on applications for finfish aquaculture research . J. Rev. Aquac . 6 ( 4 ): 209 – 240 .
Roosen ]]], R.E . 1937 . Observations of the early development of the zebrafish, Brachydanio rerio . Anat. Rec . 70 : 103 .
Semova ]]], I. , J.D. Carten , J. Stombaugh , L.C. Mackey , R. Knight , S.A. Farber , and J.F. Rawls . 2012 . Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish . Cell Host Microbe 12 : 277 – 288 . doi: 10.1016/j.chom.2012.08.003
Shimada ]]], Y. , S. Kuninaga , M. Ariyoshi , B. Zhang , Y. Shiina , Y. Takahashi , N. Umemoto , Y. Nishimura , H. Enari , and T. Tanaka . 2015 . E2F8 promotes hepatic steatosis through FABP3 expression in diet-induced obesity in zebrafish . Nutr. Metab. (Lond) . 12 : 17 . doi: 10.1186/s12986-015-0012-7
Tavares ]]], B. and S. Santos Lopes . 2013 . The importance of zebrafish in biomedical research . Acta Med. Port . 26 ( 5 ): 583 – 592 .
Tonin ]]], F.J. , A. Raquel , O.H. , Thais , T.R. da Silveira , and C. Uribe-Cruz . 2018 . Experimental model of hepatic steatosis by fructose in adult zebrafish: a pilot study . Clin. Biomed. Res . 38 ( 2 ): 151 – 154 .
Valenzuela ]]], M.J. , M. Caruffo , Y. Herrera , D.A. Medina , M. Coronado , C.G. Feijóo , S. Muñoz , D. Garrido , M. Troncoso , G. Figueroa , et al. 2018 . Evaluating the capacity of human gut microorganisms to colonize the zebrafish larvae ( Danio rerio ) . Front. Microbiol . 9 : 1032 . doi: 10.3389/fmicb.2018.01032
Wu ]]], R.S. , I.I. Lam , H. Clay , D.N. Duong , R.C. Deo , and S.R. Coughlin . 2018 . A rapid method for directed gene knockout for screening in G0 zebrafish . Dev. Cell 46 : 112 – 125.e4 . doi: 10.1016/j.devcel.2018.06.003 . https://www.asianscientist.com/2014/12/in-the-lab/zebrafish-switch-sex/
Yang ]]], Q. , C. Yan , X. Wang , and Z. Gong . 2019 . Leptin induces muscle wasting in kras -driven hepatocellular carcinoma (HCC) model in zebrafish . Dis. Mod. Mech . 12(2): dmm038240. doi:10.1242/dmm.038240
Yang ]]], B.Y. , G. Zhai , Y.L. Gong , J.Z. Su , X.Y. Peng , G.H. Shang , D. Han , J.Y. Jin , H.K. Liu , Z.Y. Du , et al. 2018 . Different physiological roles of insulin receptors in mediating nutrient metabolism in zebrafish . Am. J. Physiol. Endocrinol. Metab . 315 : E38 – E51 . doi: 10.1152/ajpendo.00227.2017
Yin , L. , Maddison , L.A. , Li , M. , Kara , N. , LaFave , M.C. , Varshney , G.K. , S.M. Burgess and J.G. Patton . 2015 . Multiplex conditional mutagenesis using transgenic expression of Cas9 and sgRNAs . Genetics 200 : 431 – 441 . doi: 10.1534/genetics.115.176917
Zang ]]], L. , Y. Shimada , and N. Nishimura . 2017 . Development of a novel zebrafish model for type 2 diabetes mellitus . Sci. Rep . 7 : 1461 . doi: 10.1038/s41598-017-01432-w
Zhang ]]], M. , J.S. Di Martino , R.L. Bowman , N.R. Campbell , S.C. Baksh , T. Simon-Vermot , I.S. Kim , P. Haldeman , C. Mondal , V. Yong-Gonzales , et al. 2018 . Adipocyte-derived lipids mediate melanoma progression via FATP proteins . Cancer Discov . 8 : 1006 – 1025 . doi: 10.1158/2159-8290.CD-17-1371
Author notes
| Month: | Total Views: |
|---|---|
| June 2019 | 279 |
| July 2019 | 288 |
| August 2019 | 255 |
| September 2019 | 461 |
| October 2019 | 619 |
| November 2019 | 678 |
| December 2019 | 491 |
| January 2020 | 537 |
| February 2020 | 613 |
| March 2020 | 466 |
| April 2020 | 333 |
| May 2020 | 432 |
| June 2020 | 576 |
| July 2020 | 596 |
| August 2020 | 600 |
| September 2020 | 618 |
| October 2020 | 866 |
| November 2020 | 763 |
| December 2020 | 602 |
| January 2021 | 653 |
| February 2021 | 717 |
| March 2021 | 984 |
| April 2021 | 744 |
| May 2021 | 833 |
| June 2021 | 896 |
| July 2021 | 864 |
| August 2021 | 714 |
| September 2021 | 1,141 |
| October 2021 | 1,204 |
| November 2021 | 1,029 |
| December 2021 | 875 |
| January 2022 | 823 |
| February 2022 | 834 |
| March 2022 | 1,139 |
| April 2022 | 1,167 |
| May 2022 | 1,148 |
| June 2022 | 1,082 |
| July 2022 | 882 |
| August 2022 | 779 |
| September 2022 | 1,332 |
| October 2022 | 1,765 |
| November 2022 | 1,505 |
| December 2022 | 1,146 |
| January 2023 | 1,685 |
| February 2023 | 1,708 |
| March 2023 | 2,061 |
| April 2023 | 2,045 |
| May 2023 | 1,577 |
| June 2023 | 924 |
| July 2023 | 804 |
| August 2023 | 873 |
| September 2023 | 1,392 |
| October 2023 | 1,462 |
| November 2023 | 1,144 |
| December 2023 | 804 |
| January 2024 | 926 |
| February 2024 | 1,148 |
| March 2024 | 1,393 |
| April 2024 | 940 |
| May 2024 | 933 |
| June 2024 | 212 |
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations

- Online ISSN 2160-6064
- Print ISSN 2160-6056
- Copyright © 2024 American Society of Animal Science
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement
Zebrafish, a biological model for pharmaceutical research for the management of anxiety
- Published: 09 February 2023
- Volume 50 , pages 3863–3872, ( 2023 )
Cite this article

- Amir Modarresi Chahardehi ORCID: orcid.org/0000-0002-9767-6255 1 ,
- Yasaman Hosseini ORCID: orcid.org/0000-0002-7524-3301 1 ,
- Seyed Mohammad Mahdavi 2 &
- Iman Naseh 1
758 Accesses
2 Citations
Explore all metrics
The zebrafish ( Danio rerio ) is a valuable animal model rapidly becoming more commonly used in pharmaceutical studies. Due to its low-cost maintenance and high breeding potential, the zebrafish is a suitable substitute for most adult rodents (mice and rats) in neuroscience research. It is widely used in various anxiety models. This species has been used to develop a conceptual framework for anxiety behavior studies with broad applications in the laboratory, including the study of herbal and chemical drugs. This review discusses the latest studies of anxiety-related behavior in the zebrafish model.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Zebrafish Models of Anxiety-Like Behaviors

Zebrafish Model for Neurotoxic Drug Screening: Methodologies and Protocols
Screening selected medicinal plants for potential anxiolytic activity using an in vivo zebrafish model
Rennekamp AJ, Peterson RT (2015) 15 years of zebrafish chemical screening. Curr Opin Chem Biol 24:58–70. https://doi.org/10.1016/j.cbpa.2014.10.025
Article CAS PubMed Google Scholar
Lorenzetti S, Altieri I, Arabi S, Balduzzi D, Bechi N, Cordelli E et al (2011) Innovative non-animal testing strategies for reproductive toxicology: the contribution of italian partners within the EU project ReProTect. Ann Ist Super Sanita 47(4):429–444. https://doi.org/10.4415/ANN_11_04_16
Ahmad F, Noldus LPJJ, Tegelenbosch RAJ, Richardson MK (2012) Zebrafish embryos and larvae in behavioural assays. Behaviour 149(10–12):1241–1281. https://doi.org/10.1163/1568539X-00003020
Article Google Scholar
Modarresi Chahardehi A, Ibrahim D, Abolhassani F, Sulaiman SF (2012) Antidepressant-like effect of extracts from Urtica dioica in mice model of depression. in Proceedings of The Annual International Conference, Syiah Kuala University-Life Sciences & Engineering Chapter
Modarresi Chahardehi A, Ibrahim D, Abolhassani F, Sulaiman SF (2013) Antidepressant-like effects of selected crude extracts of Pilea microphylla in mice model of depression. Am J Agric Biol Sci 8(1):75
Biney RP, Benneh CK, Kyekyeku JO, Ameyaw EO, Boakye-Gyasi E, Woode E (2018) Attenuation of anxiety behaviours by xylopic acid in mice and zebrafish models of anxiety disorder. J pharm biosci 07–16
Tarsaei M, Peyrovan ZS, Mahdavi SM, Modarresi Chahardehi A, Vafaie R, Haidari MH (2022) Effects of 2.45 GHz non-ionizing radiation on anxiety-like behavior, gene expression, and corticosterone level in male rats. J Lasers Med Sci 13:e56–e56
Kalueff AV, Echevarria DJ, Stewart AM (2014) Gaining translational momentum: more zebrafish models for neuroscience research. Prog Neuropsychopharmacol Biol Psychiatry 55:1–6. https://doi.org/10.1016/j.pnpbp.2014.01.022
Article PubMed Google Scholar
Craven R (2011) The risky business of drug development in neurology. Lancet Neurol 10(2):116–117. https://doi.org/10.1016/S1474-4422(11)70004-7
Pankevich DE, Altevogt BM, Dunlop J, Gage FH, Hyman SE (2014) Improving and accelerating drug development for nervous system disorders. Neuron 84(3):546–553. https://doi.org/10.1016/j.neuron.2014.10.007
Article CAS PubMed PubMed Central Google Scholar
Jesuthasan S (2012) Fear, anxiety, and control in the zebrafish. Dev Neurobiol 72(3):395–403. https://doi.org/10.1002/dneu.20873
Fraser LM, Brown RE, Hussin A, Fontana M, Whittaker A, O’Leary TP et al (2010) Measuring anxiety- and locomotion-related behaviours in mice: a new way of using old tests. Psychopharmacology 211(1):99–112. https://doi.org/10.1007/s00213-010-1873-0
Lezak KR, Missig G, Carlezon WA Jr (2017) Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci 19(2):181–191. https://doi.org/10.31887/DCNS.2017.19.2/wcarlezon
Article PubMed PubMed Central Google Scholar
Basnet RM, Zizioli D, Taweedet S, Finazzi D, Memo M (2019) Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 7(1). https://doi.org/10.3390/biomedicines7010023
Dugatkin LA, Wilson DS (1993) Fish behaviour, partner choice experiments and cognitive ethology. Rev Fish Biol Fish 3(4):368–372. https://doi.org/10.1007/BF00043386
Blaser RE, Chadwick L, GC McGinnis (2010) Behavioral measures of anxiety in zebrafish (Danio rerio) . Behav Brain Res 208(1):56–62. https://doi.org/10.1016/j.bbr.2009.11.009
Stewart A, Cachat J, Wong K, Gaikwad S, Gilder T, DiLeo J et al (2010) Homebase behavior of zebrafish in novelty-based paradigms. Behav Processes 2010. 85(2):198–203. https://doi.org/10.1016/j.beproc.2010.07.009
Kysil EV, Meshalkina DA, Frick EE, Echevarria DJ, Rosemberg DB, Maximino C et al (2017) Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14(3):197–208. https://doi.org/10.1089/zeb.2016.1415
d’Amora M, Giordani S (2018) The utility of zebrafish as a model for screening developmental neurotoxicity. Front Neurosci 12:976. https://doi.org/10.3389/fnins.2018.00976
Geng Y, Peterson RT (2019) The zebrafish subcortical social brain as a model for studying social behavior disorders. Dis Model Mech 12(8):dmm039446. https://doi.org/10.1242/dmm.039446
Alsop D, Vijayan MM (2008) Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol 294(3):R711–R719. https://doi.org/10.1152/ajpregu.00671.2007
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF et al (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205(1):38–44. https://doi.org/10.1016/j.bbr.2009.06.022
Speedie N, Gerlai R (2008) Alarm substance induced behavioral responses in zebrafish (Danio rerio) . Behav Brain Res 188(1):168–177. https://doi.org/10.1016/j.bbr.2007.10.031
Langova V, Vales K, Horka P, Horacek J (2020) The role of zebrafish and laboratory rodents in schizophrenia research. Front Psychiatry 11. https://doi.org/10.3389/fpsyt.2020.00703
Hajebi A, Motevalian SA, Rahimi-Movaghar A, Sharifi V, Amin-Esmaeili M, Radgoodarzi R, Hefazi M (2018) Major anxiety disorders in Iran: prevalence, sociodemographic correlates and service utilization. BMC Psychiatry 18(1):261. https://doi.org/10.1186/s12888-018-1828-2
Modarresi Chahardehi A, Mousavi L, Hasan S (2017) The effectiveness of psychiatric therapy against malaysian cancer patients with depressive disorders: a systematic review.Jurnal Psikologi Malaysia, 31(1)
Ostovar S, Modarresi Chahardehi A, Mohd Hashim IH, Othman A, Kruk J, Griffiths MD (2022) Prevalence of psychological distress among cancer patients in southeast asian countries: a systematic review. Eur J Cancer Care 31(6):e13669. https://doi.org/10.1111/ecc.13669
Aina Y, Susman JL (2006) Understanding comorbidity with depression and anxiety disorders. J Am Osteopath Assoc 106(5 Suppl 2):S9–14
PubMed Google Scholar
Cachat JM, Stewart A, Utterback E, Kyzar E, Hart PC, Carlos D et al (2011) Deconstructing Adult Zebrafish Behavior with Swim Trace Visualizations, in Zebrafish Neurobehavioral Protocols, AV Kalueff, JM Cachat, Editors, Humana Press: Totowa, NJ. 191–201
Bystritsky A, Khalsa SS, Cameron ME, Schiffman J (2013) Current diagnosis and treatment of anxiety disorders. 38:30–571
Munir S, Takov V (2020) Generalized anxiety disorder (GAD), in StatPearls. Treasure Island (FL)
Wittchen HU, F Jacobi (2005) Size and burden of mental disorders in Europe - A critical review and appraisal of 27 studies. Eur Neuropsychopharmacol 15(4):357–376. https://doi.org/10.1016/j.euroneuro.2005.04.012
Bushnell GA, Gaynes BN, Compton SN, Dusetzina SB, Olfson M, Sturmer T (2019) Incident substance use disorder following anxiety disorder in privately insured youth. J Adolesc Health 65(4):536–542. https://doi.org/10.1016/j.jadohealth.2019.05.007
Lai HM, Cleary M, Sitharthan T, Hun GE (2015) Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: a systematic review and meta-analysis. Drug Alcohol Depend 154:1–13. https://doi.org/10.1016/j.drugalcdep.2015.05.031
Dehghani Z, Mahdavi SM, Modarresi Chahardehi A, Mansouri V, Jahani Sherafat S (2022) The Effect of 2.45 GHz Electromagnetic Fields on fear memory extinction in male rats. J Lasers Med Sci 13:e52–e52
Kavan MG, Elsasser GN, Barone EJ (2012) The physician’s role in managing acute stress disorder. Am Fam Physician 86(7):643–649
Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35(1):105–135. https://doi.org/10.1038/npp.2009.109
Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE (2009) What is an anxiety disorder? Depress Anxiety 26(12):1066–1085. https://doi.org/10.1002/da.20633
Fairbrother N, Corbyn B, Thordarson DS, Ma A, Surm D (2019) Screening for perinatal anxiety disorders: room to grow. J Affect Disord 250:363–370. https://doi.org/10.1016/j.jad.2019.03.052
Colwill RM, Creton R (2011) Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci 22(1):63–73. https://doi.org/10.1515/rns.2011.008
Association AP (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub
Stewart AM, Ullmann JF, Norton WH, Parker MO, Brennan CH, Gerlai R, Kalueff AV (2015) Molecular psychiatry of zebrafish. Mol Psychiatry 20(1):2–17. https://doi.org/10.1038/mp.2014.128
Lucki I (1998) The spectrum of behaviors influenced by serotonin. Biol Psychiatry 44(3):151–162. https://doi.org/10.1016/s0006-3223(98)00139-5
Schneider H, Fritzky L, Williams J, Heumann C, Yochum M, Pattar K et al (2012) Cloning and expression of a zebrafish 5-HT(2 C) receptor gene. Gene 502(2):108–117. https://doi.org/10.1016/j.gene.2012.03.070
Spence R, Gerlach G, Lawrence C, Smith C (2008) The behaviour and ecology of the zebrafish, Danio rerio . Biol Rev Camb Philos Soc 83(1):13–34. https://doi.org/10.1111/j.1469-185X.2007.00030.x
Jayaram KC (1999) Freshwater Fishes of the Indian Region. Narendra Publishing House, Delhi
Google Scholar
Modarresi Chahardehi A, Arsad H, Lim V (2020) Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants 9(10):1345. https://doi.org/10.3390/plants9101345
Article CAS PubMed Central Google Scholar
Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS et al (2013) Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10(1):70–86. https://doi.org/10.1089/zeb.2012.0861
Varga ZK, Zsigmond A, Pejtsik D, Varga M, Demeter K, Mikics E et al (2018) The swimming plus-maze test: a novel high-throughput model for assessment of anxiety-related behaviour in larval and juvenile zebrafish (Danio rerio) Sci Rep, 2018. 8(1):16590. https://doi.org/10.1038/s41598-018-34989-1
Cheng RK, Krishnan S, Jesuthasan S (2016) Activation and inhibition of tph2 serotonergic neurons operate in tandem to influence larval zebrafish preference for light over darkness. Sci Rep 6(1):20788. https://doi.org/10.1038/srep20788
Benchoula K, Khatib A, Jaffar A, Ahmed QU, Sulaiman W, Wahab RA, El-Seedi HR (2019) The promise of zebrafish as a model of metabolic syndrome. Exp Anim 68(4):407–416. https://doi.org/10.1538/expanim.18-0168
Akhter A, Kumagai R, Roy SR, Ii S, Tokumoto M, Hossain B et al (2016) Generation of transparent zebrafish with fluorescent ovaries: a living visible model for reproductive biology. Zebrafish 13(3):155–160. https://doi.org/10.1089/zeb.2015.1116
Gao XY, Li K, Jiang LL, He MF, Pu CH, Kang D, Xie J (2017) Developmental toxicity of auranofin in zebrafish embryos. J Appl Toxicol 37(5):602–610. https://doi.org/10.1002/jat.3410
Ali S, Champagne DL, Spaink HP, Richardson MK (2011) Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Res C Embryo Today 93(2):115–133. https://doi.org/10.1002/bdrc.20206
Kalueff AV, Stewart AM, Gerlai R (2014) Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35(2):63–75. https://doi.org/10.1016/j.tips.2013.12.002
Kato T, Kubota M, Kasahara T (2007) Animal models of bipolar disorder. Neurosci Biobehav Rev 31(6):832–842. https://doi.org/10.1016/j.neubiorev.2007.03.003
Gut P, Reischauer S, Stainier DYR, Arnaout R (2017) Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol Rev 97(3):889–938. https://doi.org/10.1152/physrev.00038.2016
Hsu CH, Wen ZH, Lin CS, Chakraborty C (2007) The zebrafish model: use in studying cellular mechanisms for a spectrum of clinical disease entities. Curr Neurovasc Res 4(2):111–120. https://doi.org/10.2174/156720207780637234
Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E et al (2000) The syntenic relationship of the zebrafish and human genomes. Genome Res 10(9):1351–1358. https://doi.org/10.1101/gr.144700
Norton WHJ (2019) Screening for drugs to reduce aggression in zebrafish. Neuropharmacology 156:107394
Beekhuijzen M, de Koning C, Flores-Guillen ME, de Vries-Buitenweg S, Tobor-Kaplon M, van de Waart B, Emmen H (2015) From cutting edge to guideline: a first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod Toxicol 56:64–76. https://doi.org/10.1016/j.reprotox.2015.06.050
Singleman C, NG Holtzman (2011) Heart dissection in larval, juvenile and adult zebrafish, Danio rerio . J Vis Exp 55. https://doi.org/10.3791/3165
Lardelli M (2008) Using zebrafish in human disease research: some advantages, disadvantages and ethical considerations. in Proceedings of 2008 ANZCCART Conference, Auckland, New Zealand
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90(1):54–58. https://doi.org/10.1016/j.physbeh.2006.08.026
Lundegaard PR, Anastasaki C, Grant NJ, Sillito RR, Zich J, Zeng Z et al (2015) MEK inhibitors reverse cAMP-Mediated anxiety in zebrafish. Chem Biol 22(10):1335–1346. https://doi.org/10.1016/j.chembiol.2015.08.010
Parker MO, Annan LV, Kanellopoulos AH, Brock AJ, Combe FJ, Baiamonte M et al (2014) The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Prog Neuropsychopharmacol Biol Psychiatry 55:94–100
Dias GP, Cavegn N, Nix A, do Nascimento Bevilaqua MC, Stangl D, Zainuddin MSA et al (2012) The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid Med Cell Longev 2012:541971
Campbell S, Marriott M, Nahmias C, MacQueen GM (2004) Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 161(4):598–607. https://doi.org/10.1176/appi.ajp.161.4.598
Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R (2007) Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci 262(1–2):77–84. https://doi.org/10.1016/j.jns.2007.06.028
Moberg GP, Mench JA (2000) The biology of animal stress: basic principles and implications for animal welfare. Wallingford, UK, New York, NY, USA: CABI Pub. xiii, p 377
Book Google Scholar
Stewart AM, Gaikwad S, Kyzar E, Kalueff AV (2012) Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Res 1451:44–52. https://doi.org/10.1016/j.brainres.2012.02.064
Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV (2012) Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology 62(1):135–143. https://doi.org/10.1016/j.neuropharm.2011.07.037
Zabegalov KN, Khatsko SL, Lakstygal AM, Demin KA, Cleal M, Fontana BD et al (2019) Abnormal repetitive behaviors in zebrafish and their relevance to human brain disorders. Behav Brain Res 367:101–110. https://doi.org/10.1016/j.bbr.2019.03.044
Miller N, R Gerlai (2007) Quantification of shoaling behaviour in zebrafish (Danio rerio) . Behav Brain Res 184(2):157–166. https://doi.org/10.1016/j.bbr.2007.07.007
Golla A, Østby H, Kermen F (2020) Chronic unpredictable stress induces anxiety-like behaviors in young zebrafish. Sci Rep 10(1):10339. https://doi.org/10.1038/s41598-020-67182-4
Blaser RE, Rosemberg DB (2012) Measures of anxiety in zebrafish ( Danio rerio ): dissociation of black/white preference and novel tank test. PLoS ONE 7(5):e36931. https://doi.org/10.1371/journal.pone.0036931
Sackerman J, Donegan JJ, Cunningham CS, Nguyen NN, Lawless K, Long A et al (2010) Zebrafish behavior in novel environments: effects of acute exposure to anxiolytic compounds and choice of Danio rerio line. Int J Comp Psychol 23(1):43–61
Quadros VA, Silveira A, Giuliani GS, Didonet F, Silveira AS, Nunes ME et al (2016) Strain- and context-dependent behavioural responses of acute alarm substance exposure in zebrafish. Behav Processes 122:1–11. https://doi.org/10.1016/j.beproc.2015.10.014
Maximino C, Araujo J, Leao LK, Grisolia AB, Oliveira KR, Lima MG et al (2011) Possible role of serotoninergic system in the neurobehavioral impairment induced by acute methylmercury exposure in zebrafish (Danio rerio) . Neurotoxicol Teratol 33(6):727–734. https://doi.org/10.1016/j.ntt.2011.08.006
Steenbergen PJ, Richardson MK, Champagne DL (2011) Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav Brain Res 222(1):15–25. https://doi.org/10.1016/j.bbr.2011.03.025
Collymore C, Tolwani RJ, Rasmussen S (2015) The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio) . J Am Assoc Lab Anim Sci 54(3):280–285
PubMed PubMed Central Google Scholar
Song C, Yang L, Wang J, Chen P, Li S, Liu Y et al (2016) Building neurophenomics in zebrafish: Effects of prior testing stress and test batteries. Behav Brain Res 311:24–30. https://doi.org/10.1016/j.bbr.2016.05.005
Maximino C, de Brito TM, Colmanetti R, Pontes AA, de Castro HM, de Lacerda RI et al (2010) Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res 210(1):1–7. https://doi.org/10.1016/j.bbr.2010.01.031
Stewart A, Wu N, Cachat J, Hart P, Gaikwad S, Wong K et al (2011) Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog Neuropsychopharmacol Biol Psychiatry 35(6):1421–1431. https://doi.org/10.1016/j.pnpbp.2010.11.035
Engeszer RE, Ryan MJ, Parichy DM (2004) Learned social preference in zebrafish. Curr Biol 14(10):881–884. https://doi.org/10.1016/j.cub.2004.04.042
Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB (2006) Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio . Aquaculture 258(1):565–574. https://doi.org/10.1016/j.aquaculture.2006.04.020
Article CAS Google Scholar
Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB (2009) Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 297(1–4):157–162
Ahmed O, Seguin D, Gerlai R (2011) An automated predator avoidance task in zebrafish. Behav Brain Res 216(1):166–171. https://doi.org/10.1016/j.bbr.2010.07.028
Bass SL, Gerlai R (2008) Zebrafish ( Danio rerio ) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res 186(1):107–117. https://doi.org/10.1016/j.bbr.2007.07.037
Echevarria DJ, Hammack CM, Pratt DW, Hosemann JD (2008) A novel behavioral test battery to assess global drug effects using the zebrafish. Int J Comp Psychol 21:19–34. https://doi.org/10.46867/ijcp.2008.21.01.02
Comim CM, Pereira JG, Steckert A, Petronilho F, Barichello T, Quevedo J, Dal-Pizzol F (2009) Rivastigmine reverses habituation memory impairment observed in sepsis survivor rats. Shock 32(3):270–271. https://doi.org/10.1097/SHK.0b013e31819963c4
Reyhanian N, Volkova K, Hallgren S, Bollner T, Olsson PE, Olsen H, Hallstrom IP (2011) 17alpha-Ethinyl estradiol affects anxiety and shoaling behavior in adult male zebra fish (Danio rerio) . Aquat Toxicol 105(1–2):41–48. https://doi.org/10.1016/j.aquatox.2011.05.009
Biney RP, Benneh CK, Adongo DW, Ameyaw EO, Woode E (2021) Evidence of an antidepressant-like effect of xylopic acid mediated by serotonergic mechanisms. Psychopharmacology 238(8):2105–2120. https://doi.org/10.1007/s00213-021-05835-6
DePasquale C, Leri J (2018) The influence of exercise on anxiety-like behavior in zebrafish (Danio rerio) Behav Processes 157:638–644. https://doi.org/10.1016/j.beproc.2018.04.006
Hamilton TJ, Morrill A, Lucas K, Gallup J, Harris M, Healey M et al (2017) Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci Rep 7(1):15081. https://doi.org/10.1038/s41598-017-15374-w
Maximino C, Benzecry R, Oliveira KRM, de Jesus Oliveira Batista E, Herculano AM et al (2012) A comparison of the light/dark and novel tank tests in zebrafish. Behaviour 149(10–12):1099–1123. https://doi.org/10.1163/1568539X-00003029
Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R (2009) Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav 8(6):586–599. https://doi.org/10.1111/j.1601-183X.2009.00488.x
Way GP, Kiesel AL, Ruhl N, Snekser JL, McRobert SP (2015) Sex differences in a shoaling-boldness behavioral syndrome, but no link with aggression. Behav Processes 113:7–12. https://doi.org/10.1016/j.beproc.2014.12.014
Ruhl N, McRobert SP (2005) The effect of sex and shoal size on shoaling behaviour in Danio rerio . J Fish Biol 67(5):1318–1326. https://doi.org/10.1111/j.0022-1112.2005.00826.x
Gumm JM, Snekser JL, Iovine MK (2009) Fin-mutant female zebrafish ( Danio rerio ) exhibit differences in association preferences for male fin length. Behav Processes 80(1):35–38. https://doi.org/10.1016/j.beproc.2008.09.004
Download references
Author information
Authors and affiliations.
Cognitive Neuroscience Research Center, AJA University of Medical Sciences, Tehran, Iran
Amir Modarresi Chahardehi, Yasaman Hosseini & Iman Naseh
Department of Bioscience and Biotechnology, Malek Ashtar University of Technology (MUT), Tehran, Iran
Seyed Mohammad Mahdavi
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Amir Modarresi Chahardehi, and Yasaman Hosseini; Investigation: Iman Naseh; Writing-original draft preparation: Amir Modarresi Chahardehi; Writing-review and editing: Amir Modarresi Chahardehi, and Seyed Mohammad Mahdavi; Supervision: Seyed Mohammad Mahdavi.
Corresponding authors
Correspondence to Amir Modarresi Chahardehi or Seyed Mohammad Mahdavi .
Ethics declarations
Competing interest.
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Chahardehi, A.M., Hosseini, Y., Mahdavi, S.M. et al. Zebrafish, a biological model for pharmaceutical research for the management of anxiety. Mol Biol Rep 50 , 3863–3872 (2023). https://doi.org/10.1007/s11033-023-08263-1
Download citation
Received : 11 August 2022
Accepted : 10 January 2023
Published : 09 February 2023
Issue Date : April 2023
DOI : https://doi.org/10.1007/s11033-023-08263-1

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anxiety disorders
- Behavioral tests
- Neuroscience
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Zebrafish articles from across Nature Portfolio
Zebrafish (Danio rerio) is a small freshwater fish that is an extensively studied vertebrate model organism. It can be bred rapidly in large numbers, is amenable to genetics, and has a clear embryo, making it attractive to study vertebrate development, physiology and disease.
Latest Research and Reviews
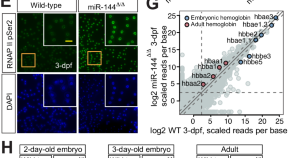
The miR-144/Hmgn2 regulatory axis orchestrates chromatin organization during erythropoiesis
Differentiation of stem and progenitor cells is a highly regulated process. Here, the authors uncover miR-144 and its target Hmgn2 as the backbone of the genetic regulatory circuit that controls the terminal differentiation of erythrocytes in vertebrates.
- Dmitry A. Kretov
- Leighton Folkes
- Daniel Cifuentes
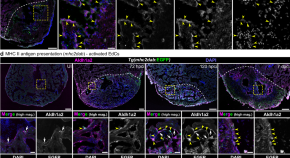
Antigen presentation plays positive roles in the regenerative response to cardiac injury in zebrafish
An adequate immune response is necessary to promote heart regeneration. Here, the authors identified a link between antigen presentation, immune cells, and endocardial cells during the regenerative response to cardiac injury in the adult zebrafish.
- João Cardeira-da-Silva
- Qianchen Wang
- Didier Y. R. Stainier
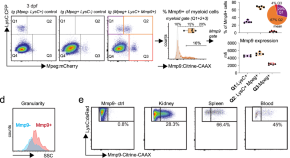
Comparative transcriptomics coupled to developmental grading via transgenic zebrafish reporter strains identifies conserved features in neutrophil maturation
Maturation of innate immune cells is a graded stereotypic process which is often conserved across species. Here authors label distinct neutrophil leukocyte developmental stages via generating combinations of transgenic zebrafish reporter strains, followed by transcriptome analysis of different neutrophil maturation stages and comparison to the gene expression profile of developing neutrophils from humans and mice.
- Stefanie Kirchberger
- Mohamed R. Shoeb
- Martin Distel
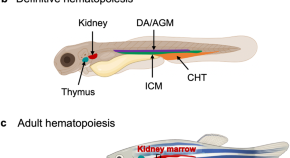
Exploring hematopoiesis in zebrafish using forward genetic screening
- Hyemin Song
- Unbeom Shin
- Yoonsung Lee
A systematic review of the impact of environmental enrichment in zebrafish
Environmental enrichment is a home-based intervention that mimics natural habitats for laboratory-housed animals. This systematic review of 27 environmental enrichment protocols finds consistent benefits for zebrafish welfare while raising the importance of a standardizing protocol to improve reproducibility of results.
- Matheus Gallas-Lopes
- Radharani Benvenutti
- Matheus Marcon
Uncovering developmental time and tempo using deep learning
A deep learning-based method that uses microscopy images to stage embryos and analyze developmental time.
- Nikan Toulany
- Hernán Morales-Navarrete
- Patrick Müller
News and Comment
In vivo traction force microscopy
Embryogram allows stress measurements during migration of the lateral line primordium in zebrafish embryos.
Optogenetics in the hot seat
Optogenetic and thermogenetic tools have been limited to applications for single-state control of cellular processes. A single-component optogenetic tool was found to act as both a temperature sensor and a photoreceptor, enabling multi-state control of developmental signaling.
- Maxwell Z. Wilson
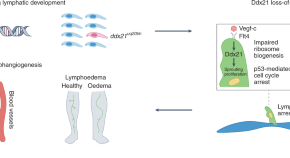
An RNA helicase swirls in lymphangiogenesis
How lymphatic vessels arise from veins is still poorly understood. A study reports the discovery of a ribosome biogenesis regulator Ddx21 as a previously unappreciated specific factor that is important for the first steps of lymphatic but not blood vessel development. The finding may lead to better strategies to selectively target lymphangiogenesis.
- Severin Mühleder
- Rui Benedito

A tonic for moonshine
- Grant Miura
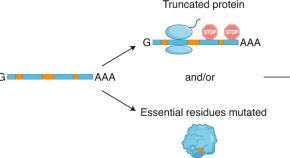
Crispants take the spotlight
A new CRISPR/Cas9 method that can generate F0 mutant zebrafish has the potential to cut costs, spare time, and reduce animal use for researchers interested in screening loss-of-function alleles in vivo.
- Wouter Masselink
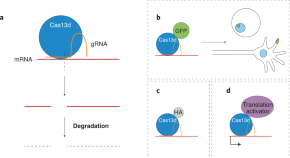
A CRISPR cut for messenger RNAs
To achieve knockdown rather than knockout of particular genes, a new paper demonstrates a CRISPR/Cas13 method that can efficiently edit mRNA in zebrafish, medaka, killifish, and mouse embryos.
- Rebecca Leech
- Karuna Sampath
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Zebrafish as a Model for Fish Diseases in Aquaculture
The use of zebrafish as a model for human conditions is widely recognized. Within the last couple of decades, the zebrafish has furthermore increasingly been utilized as a model for diseases in aquacultured fish species. The unique tools available in zebrafish present advantages compared to other animal models and unprecedented in vivo imaging and the use of transgenic zebrafish lines have contributed with novel knowledge to this field. In this review, investigations conducted in zebrafish on economically important diseases in aquacultured fish species are included. Studies are summarized on bacterial, viral and parasitic diseases and described in relation to prophylactic approaches, immunology and infection biology. Considerable attention has been assigned to innate and adaptive immunological responses. Finally, advantages and drawbacks of using the zebrafish as a model for aquacultured fish species are discussed.
1. Introduction
1.1. background.
The zebrafish is extensively used as a vertebrate model for human diseases [ 1 , 2 , 3 , 4 , 5 , 6 ] and other conditions such as aging, development and genetics [ 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ]. The small fish presents many advantages as a model; therefore, it cannot be overlooked and has not been since Dr. George Streisinger convinced the world in the 1980s of the wonders of the zebrafish [ 17 , 18 , 19 ]. The main advantages to date include optical transparency at early stages of life, a short generation time, continuous egg production, a large number of offspring, fast development, availability of a wide spectrum of mutant/transgenic lines, willingness of zebrafish scientists to share, ease of genetic manipulations and relatively cheap facilities. In particular, the ability to visualize processes inside the fish has consolidated this model as unique for intravital imaging—unmatched by any other animal model [ 13 , 20 ].
Within the last two decades, the small fish has not only been used as a model for human conditions but also for fish conditions. In particular, fish diseases and immune responses have comprised the focus of the investigations when zebrafish were used as a model for fish. Relevant disease-causing pathogens are naturally those that are important for aquaculture productions and to utilize zebrafish as a model for fish appears straightforward. The genetic distance, however, is relatively large between some of the fish species and while zebrafish and carp belong to the same family, zebrafish and salmonids are quite distant ( Figure 1 ). Nonetheless, zebrafish have contributed to important knowledge regarding fish diseases that are relevant for various production species and some of the investigations mentioned in this review have utilized the unique optical feasibility of real-time observation of processes in vivo.

Evolutionary relationship between zebrafish and a selection of fish species used for production. Modified from Berthelot et al. (2014) [ 21 ].
Fish disease outbreaks represent a key problem for the aquaculture industry as they can cause severe economic losses due to morbidity and mortality. A primary cause of such catastrophic epidemics is typically the high rearing densities used in modern intensive fish aquaculture, which facilitate the transfer and spread of pathogenic organisms. Chronic diseases are an additional concern in modern fish farming. Maintaining fundamental hygienic conditions as well as implementing vaccination strategies to improve fish health are, therefore, prerequisites for controlling diseases and should be included in management procedures. Regular surveillance of the health status of the farmed fish is also crucial for effective disease management, enabling a fast response to disease outbreaks and, as a consequence, reducing morbidity and mortality in the infected stocks [ 22 , 23 ]. New knowledge on immunological mechanisms and pathways as well as testing new treatments can, with relative ease and within a short period of time, be obtained in zebrafish and can benefit novel vaccine development and aid in treatment regimes.
The zebrafish belongs to the bony fishes (teleosts or Teleostei) together with most extant ray-finned fish. It belongs to the same family as carp (Cyprinidae) and is more distantly related to other fish used in production such as salmonids, channel catfish and cod ( Figure 1 ). These fish and the zebrafish were separated evolutionary more than 150 M years ago [ 15 ], which is a relatively short time compared to the separation of mammals and teleosts, which took place approximately 400 M years ago. Zebrafish share the teleost genome duplication that occurred during the evolution of the ray-finned fish. They are, however, much smaller than fish used for production with a weight of 0.5–0.9 g and a fork length from 22–38 mm [ 23 , 24 , 25 , 26 ]. The females are able to spawn up to more than 200 eggs every 1–14 days [ 27 , 28 ], which is very useful for repetition of experiments and statistically powerful investigations. Zebrafish can furthermore survive at a temperature range of 6–38 °C but prefer ~28 °C [ 29 ].
The use of zebrafish as a model for aquacultured fish species has increased enormously since the review by Dham et al. 2006 [ 15 ] and Ribas et al. 2014 [ 23 ] and recent studies are included and described in this review. The majority of studies are conducted on bacterial and viral diseases ( Table 1 ). Parasitic diseases are underrepresented ( Table 1 ) but parasites are also more complicated to work with. Ectoparasites, for example, cannot be injected, which is common procedure for bacteria and viruses, while other parasites are species-specific and will not infect zebrafish. The lack of techniques to cultivate a wide spectrum of parasites in the laboratory represents another obstacle making experimentation problematic. Even infections with the most studied parasite in zebrafish, Ichthyophthirius multifiliis , which is of huge economic importance for the aquaculture industry, are more complicated to work with in the zebrafish model compared to classical infections in, e.g., rainbow trout ( Oncorhynchus mykiss ) [ 30 , 31 , 32 ], carp ( Cyprinus carpio ) [ 33 , 34 ] or channel catfish ( Ictalurus punctatus ) [ 35 , 36 ]. Zebrafish are more resilient and are only susceptible towards the parasite, when a stress factor is applied during the infection process. In the described studies, overcrowding was used and approximately 10 zebrafish per litre of water provided enough stress to facilitate ichthyophthiriosis. Zebrafish are naturally more resistant towards the parasite and this illustrates how even a fish to fish model can present species-specific differences related to infection biology and immune responses.
Published papers mentioned in this review divided into three different focus areas.
| Focus Area | Bacteria | Virus | Parasites | |||
|---|---|---|---|---|---|---|
| N° | % | N° | % | N° | % | |
| Prophylactic approaches | 12 | 30 | 5 | 14.7 | 0 | 0 |
| Immunology | 17 | 42.5 | 21 | 61.8 | 4 | 57.1 |
| Infection biology | 11 | 27.5 | 8 | 23.5 | 3 | 42.9 |
| Total | 40 | 100 | 34 | 100 | 7 | 100 |
The research mentioned in this review on zebrafish as a model organism for aquacultured fish is focused on economically important diseases caused by bacteria, viruses and parasites and is divided into three focus areas; prophylactic approaches, immunology and infection biology. The studies were primarily focused on immunology represented with 42.5%, 61.8% and 57.1% of the papers on bacteria, viruses and parasites, respectively, whereas investigations on prophylactic approaches only represented 30%, 14.7% and 0%, respectively ( Table 1 ). This distribution indicates that basic knowledge on immunological mechanisms in fish disease biology still is an ongoing and very important field for aquaculture research, while few prophylactic means are tested and perhaps species-specific characteristics prevent researchers from conducting vaccine experiments in the zebrafish model. Knowledge within fish immunology has lagged behind mammalian immunology because of missing tools. The opportunity for using front-line tools in zebrafish has probably contributed to the increase in papers on subjects such as infection kinetics and immunological mechanisms. There is an obvious desire for more knowledge and a deeper understanding of the induced immune responses by the pathogens with an aspect on how to use this information to combat the diseases in aquaculture.
1.2. Prophylactic Approaches
Treatments with chemicals, antibiotics or prophylactics have been the traditional way to fight fish infections. In recent times, however, vaccines for fish have emerged as the most efficient and promising solution [ 37 , 38 ]. Zebrafish must be susceptible towards the disease under investigation to represent a good model for prophylactic studies and several studies on protection have been conducted [ 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 ]. The zebrafish has also been used in a few studies for treatment purposes and has contributed with new knowledge for control of diseases [ 54 , 55 ].
1.3. Immunology
The immune system of fish is more primitive compared to humans [ 56 ]. Many basic functions and cell types are similar and fish also possess innate and adaptive immune mechanisms. Fish, however, do not have lymph nodes and the helper T-cell responses including Th1, Th2 and Th17 are not as clearly defined as they are in mammals [ 52 , 57 ]. Although it has been demonstrated that fish immunoglobulins are functional [ 32 , 58 , 59 ], the response of fish to immune challenges is strongly based on the innate immune response [ 56 , 60 ]. There are significant variations in the immune system between different fish species, and one of the more spectacular ones is that Atlantic cod ( Gadus morhua ) lacks the antigen-presenting major histocompatibility complex (MHC) II [ 61 ]. This molecule is, in other vertebrates, a part of the development of the classical adaptive immune response against bacterial and parasitic infections through the activation of CD4 + T-cells. MHC II malfunction is generally considered to lead to major immunodeficiency and death [ 62 ]. In 2005, a third group of antibodies, different to the classical fish antibodies IgD and IgM, called IgT/IgZ was identified in rainbow trout and zebrafish, respectively, and has since then been recognized in other fish species [ 63 , 64 , 65 ]. Rainbow trout also has multiple forms of C3 molecules as part of the complement system [ 66 , 67 ]. These immunological differences illustrate inter-species variation and emphasize that care has to be taken using one fish as a model for another fish species. For further information on the fish immune system please see [ 67 , 68 , 69 ].
During the first four days after fertilization, the zebrafish exhibits no adaptive immunity markers [ 70 ]. Many studies on innate immunology are conducted at this stage, both because no bias from adaptive immune mechanisms occurs and due to the fact that the larvae until 120 h post fertilization are not considered an experimental animal and no animal experimentation license is thus required. At four days after fertilization, expression of the genes activating recombination, rag1 and rag2 , ensues [ 71 ], and T and B cells are developed for future use in the adaptive system [ 72 , 73 ]. Complete functionality of the adaptive immune system takes 4 to 6 weeks to develop [ 74 ]. The zebrafish is highly useful as a model system in this regard because of the simplicity of using specific life stages to examine certain aspects of immunological maturation and function.
Bacterial and especially viral infections are associated with interferon (IFN) responses [ 48 , 75 , 76 , 77 , 78 , 79 ]. For example, an infection with infectious hematopoietic necrosis virus (IHNV) is often lethal and is linked to a delayed and inefficient IFN response [ 48 ]. Therefore, many studies focus on this molecule or other molecules influencing the production of IFN. The immunological investigations mentioned in this review have been dedicated to innate and adaptive immunity using classical transcription analyses [ 40 , 41 , 42 , 43 , 44 , 48 , 55 , 76 , 77 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 ], gnotobiotic (germ-free) [ 81 ] and transgenic fish lines for functional studies [ 89 , 91 , 97 , 98 , 99 ], but also visualization of the behaviour of professional phagocytes [ 100 ].
1.4. Infection Biology
Few investigations have been conducted on infection biology of important fish diseases in zebrafish. The model is, however, very suitable for this purpose, since it is feasible to visualize real-time in vivo how an infection spreads and how the immune system reacts. Transparent and gnotobiotic zebrafish have been used to study these aspects [ 82 , 101 ]. Different infection routes have furthermore been applied to study the natural and laboratory-induced kinetics of diseases [ 102 , 103 , 104 ]. Finally, pathogen tropism [ 105 ] and infections at different temperatures [ 106 ] have been investigated to learn the biology of the infectious agents.
2. Fish Diseases in Aquaculture Studied in the Zebrafish Model
2.1. bacteria.
Bacterial diseases in fish have caused problems as long as aquaculture has existed. Fish producers are currently facing the same problems as other animal producers with increasing resistance against antibiotics and other treatments [ 107 ]. Therefore, it is of vital importance to continue investigating the dynamics of bacterial diseases in fish, vaccines and the immunological responses of the fish to discover and develop new tools to control the infections. A summary of the studies mentioned in this section is found in Table 2 .
Studies on selected bacterial pathogens relevant for aquaculture utilizing zebrafish as an in vivo model.
| Pathogen | Focus Area | Zebrafish Stage | Reference |
|---|---|---|---|
| Prophylactic approaches | Adult Larvae | Cui et al. 2010 [ ] Hortle et al. 2019 [ ] | |
| Immunology | Larvae Adult Adult Larvae Larvae Larvae | Davis et al. 2002 [ ] Meijer et al. 2005 [ ] Swaim et al. 2006 [ ] Hortle et al. 2019 [ ] Niu et al. 2019 [ ] Ogryzko et al. 2019 [ ] | |
| Infection biology | Adult Larvae Adult Larvae Larvae Adult, larvae | Prouty et al. 2003 [ ] Cosma et al. 2006 [ ] Harriff et al. 2007 [ ] Takaki et al. 2013 [ ] Johansen et al. 2018 [ ] Johansen et al. 2018 [ ] | |
| Prophylactic approaches | Adult Adult Adult Adult Adult Larvae Larvae Larvae | Zhang et al. 2012 [ ] Zhang et al. 2013 [ ] Liu et al. 2014 [ ] Zhang et al. 2014 [ ] Gao et al. 2014 [ ] Silva et al. 2014 [ ] Caruffo et al. 2015 [ ] Zhang et al. 2017 [ ] | |
| Immunology | Adult Adult Adult Adult Adult Larvae | Zhang et al. 2012 [ ] Zhang et al. 2013 [ ] Liu et al. 2014 [ ] Zhang et al. 2014 [ ] Gao et al. 2014 [ ] Oyarbide et al. 2015 [ ] | |
| Infection biology | Larvae Larvae Adult Adult | O’Toole et al. 2004 [ ] Oyarbide et al. 2015 [ ] Liu et al. 2015 [ ] Schmidt et al. 2017 [ ] | |
| Immunology | Adult Adult Adult, larvae | Lin et al. 2007 [ ] Lin et al. 2009 [ ] Da’as et al. 2011 [ ] | |
| Prophylactic approaches | Adult, larvae | Korbut et al. 2016 [ ] | |
| Immunology | Larvae | Sieger et al. 2009 [ ] | |
| Infection biology | Larvae | Jank et al. 2015 [ ] | |
| Prophylactic approaches | Larvae | Solís et al. 2015 [ ] | |
| Immunology | Larvae | Solís et al. 2015 [ ] |
2.1.1. Mycobacterium marinum
M. marinum is a fish pathogen causing a chronic granulomatous disease similar to mammalian mycobacteriosis. The prevalence of M. marinum has increased worldwide with the intensification of fish farming and ornamental fish production [ 39 ] but the majority of studies using the zebrafish and M. marinum model have been conducted to study human medicine. A single prophylactic study documented that zebrafish immunized with a live attenuated L1D M. marinum mutant were protected following a challenge with a virulent M. marinum strain [ 39 ]. A recent study demonstrated that treatment with aspirin, which targets platelet activation, reduced the mycobacterial infection in zebrafish [ 54 ].
More research has been conducted on the immunological responses of zebrafish infected with the bacterium. Despite the fact that the embryos do not yet have lymphocytes, an M. marinum infection led to formation of macrophage aggregates with pathological signs of granulomas and activation of granuloma-specific Mycobacterium genes [ 108 ]. Therefore, infections in larvae initiated granuloma formation solely in the context of innate immunity [ 108 ]. A transcriptomic analysis showed that many genes related to immune responses, especially inflammatory genes, were up-regulated and the authors inferred that only some genes related to adaptive immunity were activated and that the reaction towards the bacterium therefore induced a specific response [ 80 ]. It has been shown that adaptive responses are critical to fight the pathogen. Rag1 mutant zebrafish, which lack the ability to activate recombination and thereby are deficient in functional B and T lymphocytes, were hypersusceptible towards the infection [ 97 ]. Reduction in the mycobacterial burden is dependent on macrophages and granuloma formation, which provides evidence that platelet activation induced by M. marinum compromises protective host immunity to the infection [ 54 ]. The usefulness of the transgenic line with fluorescently labelled macrophages and neutrophils [ 20 ] was recently demonstrated in a study visualizing in vivo dynamic processes in zebrafish larvae infected with M. marinum . Processes included immune cell migration, host/pathogen relationship and cell death [ 109 ]. Another transgenic line with interleukin-1β (Il1β) fluorescence was used to document early innate immune proinflammatory responses. In the same study, antimicrobial nitric oxide (NO) was shown to be involved in host protective mechanisms [ 98 ].
The zebrafish has also been used as a model for M. marinum pathogenesis and host/bacterium interactions [ 103 , 110 , 111 ]. Clinical pathology included the formation of granuloma-like lesions and the bacterium established either an acute or a chronic infection based upon inoculum. Infections were studied through the natural route using bath exposures and it was observed that the gastrointestinal track was the primary route of infection [ 102 ]. The M. marinum infection-induced lipid metabolism was furthermore studied using the zebrafish model [ 112 , 113 ].
2.1.2. Vibrio anguillarum
V. anguillarum affects salt- and occasionally freshwater fish all over the world [ 114 ] and a relatively large amount of research has been conducted on this pathogen in zebrafish. Vaccination studies in zebrafish showed that the fish are able to acquire protection against V. anguillarum and a Th17-like response is induced following bath exposure with a live attenuated V. anguillarum strain [ 40 , 41 , 43 ]. It was documented that the live attenuated strain induced notable mucosal immune responses in the intestine with participation of neutrophils and macrophages [ 42 ]. The same research group tested a live attenuated combination vaccine against Edwardsiella tarda and V. anguillarum in zebrafish and turbot and achieved a high level of protection, especially against V. anguillarum [ 44 ]. Phage treatment also proved to be effective against vibriosis, when tested in zebrafish [ 115 ]. Some species of yeast isolated from the gut were used as probiotics against V. anguillarum infections and mortality was consequently reduced. It was suggested that gut colonization could be involved in the protective effect [ 116 ]. A functional compound, phenazine-1-carboxylic acid, derived from Pseudomonas aeruginosa strain PA31x was demonstrated to inhibit the growth of V. anguillarum and proved efficient as treatment against vibriosis in zebrafish [ 117 ].
Several studies using immunization with the live attenuated V. anguillarum have been conducted to investigate the immunological responses responsible for protection [ 40 , 41 , 42 , 43 , 44 ]. Following a challenge with wildtype pathogenic V. anguillarum , protection induced by the attenuated bacterium was confirmed and genes encoding pro-inflammatory factors such as Il1 and Il8 were found to be up-regulated 1–7 days post-vaccination, while the expression of mhcII increased 7 days post-vaccination [ 40 ]. The triggering of a MyD88-dependent signalling pathway in the intestine implied that the flagellum was the most important antigen in the attenuated vaccine. Professional phagocytes were found to participate in antigen recognition and sampling following vaccination and inflammation was observed in the intestine [ 42 ]. Furthermore, genes encoding factors participating in the Th17-like pathway were found to be up-regulated in the spleen and in mucosal tissues [ 41 , 43 ] and a Toll-like receptor and Mhc I and II signalling pathways were activated in the spleen and liver [ 44 ]. Another study using gnotobiotic zebrafish larvae revealed a downregulation of genes encoding Nfκb, Il1β, Mpo, Tlr4, Tlr22 and the authors suggested that V. anguillarum eluded the larvae’s innate immune defences as a “stealth mechanism” during the first stages of infection [ 81 ].
The infection kinetics of V. anguillarum in zebrafish have been investigated using transparent zebrafish to visualize the spread of a green fluorescent protein (GFP)-tagged V. anguillarum [ 101 ]. The bacterium was found to initially infect skin and intestinal surfaces. Another study used gnotobiotic zebrafish larvae and found colonization of the intestine [ 81 ]. Spread of infection with V. anguillarum was also compared through bath exposure and peritoneal injection [ 104 ]. The pathogen was found in the blood at all sampling points after injection, whereas only mucosal surfaces were affected initially following infection by bath. The authors emphasized, based on the results, how important mucosal immune defence mechanisms are. A similar study described that skin-injured zebrafish were more susceptible to a V. anguillarum infection and that the infection in such cases travelled much faster to internal organs and the blood stream [ 118 ].
2.1.3. Aeromonas salmonicida
Infections with A. salmonicida in Norway comprised a major problem for the salmon industry until a vaccine was developed in 1993 [ 119 ]. The bacterium still causes problems all over the world and vaccines are not equally effective in all countries. A few studies solely on immunology have been conducted with this pathogen in zebrafish. One study described that infections with A. salmonicida induced expression of genes encoding the acute phase proteins serum amyloid a (Saa), Hepcidin and Haptoglobin [ 82 ], which is similar to the response of, e.g., salmon infected with the same pathogen [ 120 ]. This correlation indicates that zebrafish may be a suitable model for A. salmonicida infections. Intelectins also take part in the response against the bacterium in zebrafish [ 83 ] and following injection with a live A. salmonicida , significant mast cell degranulation was observed [ 121 ].
2.1.4. Yersinia ruckeri
Y. ruckeri is a freshwater bacterium that infects salmonids [ 122 ]. Fish are routinely vaccinated against it but vaccines or management procedures are not optimal and there is room for improvement. A few studies have been conducted with Y. ruckeri in zebrafish. An inactivated vaccine was produced from a GFP-tagged Y. ruckeri and antigen uptake following bath immunization was visualized in transparent zebrafish at different life stages [ 45 ]. Common for all stages was that the intestine was a major location of antigen processing.
One study focused on the protective immunological response in zebrafish infected with Y. ruckeri , which was found to require Ifn-γ [ 84 ].
The infection biology of Y. ruckeri has also been investigated and the mode of action of the toxic effector antifeeding prophage 18 of the prophage tail-like protein translocation machinery was found to impair blastomere cell behaviour in zebrafish embryos [ 123 ].
2.1.5. Flavobacterium psychrophilum
This bacterium is responsible for the bacterial cold-water disease and the rainbow trout fry syndrome in freshwater salmonids. Only one study was found on this pathogen in the zebrafish model and this may be due to the bacterium preferring temperatures below 16 °C [ 124 ]. Two bacterins based on different F. psychrophilum isolates were tested and differences in innate immune responses visualized by neutrophil migration in zebrafish larvae were found [ 46 ].
No naturally occurring virus has been discovered in zebrafish [ 125 , 126 ]; however, a range of fish viruses are nonetheless able to infect both larval and adult zebrafish. Similar to F. psychrophilum , the optimal temperature is often less than the 28 °C preferred by zebrafish. Despite that, much has been learned about the zebrafish viral immune responses, the infection biology and the pathologies of the viruses. In this review, four important viral fish diseases have been included ( Table 3 ); spring viraemia carp virus (SVCV), IHNV, infectious pancreatic necrosis virus (IPNV) and viral haemorrhagic septicemia virus (VHSV). They are all RNA viruses and SVCV, IHNV and VHSV belong to the rhabdoviridae family, whereas IPNV belongs to the family birnaviridae.
Studies on viruses relevant for aquaculture utilizing zebrafish as an in vivo model.
| Pathogen | Focus Area | Zebrafish Stage | Reference |
|---|---|---|---|
| SVCV | Prophylactic approaches | Adult Adult | Encinas et al. 2013 [ ] García-Valtanen et al. 2014 [ ] |
| Immunology | Adult Adult, larvae Adult, larvae Larvae Adult Larvae Adult Larvae Adult Adult, larvae Adult, larvae Larve Adult Adult Adult | Levraud et al. 2007 [ ] Aggad et al. 2009 [ ] Aggad et al. 2010 [ ] López-Muños et al. 2010 [ ] Encinas et al. 2013 [ ] Varela et al. 2014a [ ] Varela et al. 2014b [ ] Candel et al. 2015 [ ] Li et al. 2015 [ ] Pereiro et al. 2015 [ ] Varela et al. 2016 [ ] Espín-Palazón et al. 2016 [ ] Feng et al. 2016 [ ] Liu et al. 2019 [ ] Medina-Gali et al. 2019 [ ] | |
| Infection biology | Adult Adult | Sanders et al. 2003 [ ] Wang et al. 2017 [ ] | |
| IHNV | Immunology | Adult, larvae Adult, larvae Larvae | Aggad et al. 2009 [ ] Aggad et al. 2010 [ ] Briolat et al. 2014 [ ] |
| Infection biology | Adult Larvae Larvae | LaPatra et al. 2000 [ ] Liu et al. 2002 [ ] Ludwig et al. 2011 [ ] | |
| VHSV | Prophylactic approaches | Adult Adult Adult | Novoa et al. 2006 [ ] Chinchilla et al. 2013 [ ] Kavaliauskis et al. 2015 [ ] |
| Immunology | Adult Adult, Larvae Adult | Encinas et al. 2010 [ ] Dios et al. 2010 [ ] Estepa and Coll 2015 [ ] | |
| Infection biology | Adult | Kim et al. 2015 [ ] | |
| IPNV | Infection biology | Adult Adult | Seeley et al. 1977 [ ] LaPatra et al. 2000 [ ] |
2.2.1. SVCV
SVCV is prevalent worldwide and is associated with haemorrhaging in cyprinids, especially in common carp ( Cyprinus carpio ) [ 127 , 128 , 129 ]. SVCV outbreaks usually occur during the spring, when the water temperature rises [ 130 ], and cause high mortality in young fish, with mortality rates up to 90% [ 127 ], leading to significant economic losses for the aquaculture industry. The vast amount of studies on SVCV utilizing zebrafish as a model appears reasonable, since carp and zebrafish belong to the same family and are thereby closely related ( Figure 1 ). Basic biological features are thus very conserved between the two species. Only a few studies on preventive measures have been conducted and Encinas et al. (2013) identified, using a pathway-targeted microarray, genes and transcription factors implicated in viral shutoff and/or host survival responses after SVCV infection, which may contribute to the development of novel drug-based prevention methodologies [ 55 ]. A second investigation discovered that zebrafish beta-defensin 2 (zfBD2) has antiviral activity, immunomodulatory properties and is a potent viral DNA vaccine molecular adjuvant [ 47 ].
Several studies on evolutionary aspects of the immune system have been conducted in zebrafish challenged with virus and these include studies of type III Ifn as the ancestral antiviral system of vertebrates [ 75 ]. Functional studies have also been conducted for Ifns in zebrafish and two types of Ifns were shown to be induced after challenge with SVCV and IHNV and it was demonstrated that the different Ifns bound to two different receptors [ 76 , 77 ]. It has furthermore been shown that even though larvae possess protective antiviral Ifns, three-day old larvae were unable to mount a protective response following infection by the natural water-borne route [ 85 ]. Ifn-induced proteins with tetratricopeptide repeats (Ifits) also have conserved antiviral functions as in humans and were induced in zebrafish after challenge with SVCV [ 86 ]. In mammals, interferon regulatory factors (IRFs) regulate interferons. Zebrafish were utilized to demonstrate that zebrafish Irf4 was regulated by signal transducer and activator of transcription 6 (Stat6) and c-Rel [ 88 ] and that fish and mammals have evolved a similar Irf-dependent regulatory mechanism, fine-tuning Ifn gene activation [ 92 ]. An inhibitory effect was observed for some zebrafish Irfs at lower concentrations and a synergistic effect at higher concentrations [ 92 ]. An Ifn-inducing substance, 7-(6-(2-methyl-imidazole))-coumarin (D5) was found to elicit an innate immune response in non-viral infected zebrafish by up-regulating the expression of interferon genes ( Ifnγ , Ifnφ1 , Ifnφ2 and Rig-1 ) and to inhibit SVCV replication after administration in infected fish [ 93 ]. Genes and transcription factors involved in different pathways have been identified, which were suggested to be implicated in suppression of the virus and/or host survival responses [ 55 ]. Zebrafish larvae were employed to visualize the damage caused by an infection with SVCV. Cellular processes, such as transendothelial migration of leukocytes, were demonstrated and virus-induced pyroptosis of macrophages and Il1β release could be observed in individual cells. In the zebrafish model, it was possible to identify exactly which cells were infected with the virus. Detailed host/pathogen interactions were discovered and the results of the study provided a more thorough understanding of the immune mechanisms implicated in the disease [ 99 ]. The negative regulators of LPS signalling, Md1 and Rp105 form complexes that directly interact with the Md2-Tlr4 pattern recognition receptor complex. A functional study using genetic inhibition of zebrafish Md1 and Rp105 revealed that Md1 or Rp105 deficiency impaired the expression of genes encoding pro-inflammatory and antiviral molecules. This led to increased susceptibility to viral infection and it was thereby demonstrated that these molecules had an important function for the regulation of innate immunity [ 87 ]. Nk-lysins are antimicrobial proteins produced by cytotoxic T lymphocytes and natural killer cells with a broad antimicrobial spectrum. Out of four identified nk-lysin genes in zebrafish, two were found to be up-regulated following an SVCV challenge [ 89 ]. Perforins are known in mammals to be involved in granule-dependent cell death. Genes encoding 6 perforins were identified in the zebrafish genome and one, Prf19b, which is mainly produced by myeloid cells, was involved in an antiviral defence, inducing protection after an in vivo infection with SVCV [ 90 ]. In some viral diseases, the viruses use Tnfα to their benefit. After a challenge with SVCV in zebrafish larvae, it was found that Tnfα blocked the host autophagic response, which is required for viral clearance [ 91 ]. A proteomic approach disclosed that proteins of the vitellogenin family (Vtg) and the grass carp reovirus-induced gene (Gig) proteins were up-regulated during SVCV infection, highlighting that these proteins are important in the antiviral response.
Only a few studies have been conducted in zebrafish on the infection biology of SVCV. It has been demonstrated that zebrafish were susceptible to infection by SVCV at 15–24 °C through the natural route—the water body—when 10(3) to 10(5) plaque-forming units per millilitre (PFU/mL) of water was used. Mortality was highest at the lower temperatures [ 106 ]. A transcriptomic analysis described changes and tissue-specific impacts caused by SVCV in vivo, which brought the understanding on host/pathogen interactions forward [ 131 ].
2.2.2. IHNV
The first study in zebrafish on infections with IHNV was conducted in 2000 [ 132 ]. Adult zebrafish were infected with IHNV and IPNV and progression of the diseases was compared. It was found that the kinetics of hematopoietic defects between IHNV and IPNV infections diverged but common for both diseases were that the fish recovered 6 days after infection. IHNV is associated with a delayed and insufficient Ifn response and is normally lethal. A typical signature of Ifn-stimulated genes (Isgs) was observed in another study after challenge with IHNV and Chikungunya virus (CHIKV) in zebrafish larvae, but was stronger after challenge with CHIKV. Some inflammatory genes were induced through Ifn-independent pathways by IHNV and not by CHIKV and the fish recovered from CHIKV but not from IHNV. It was therefore demonstrated how host/virus interactions in zebrafish led to protective and non-protective antiviral innate immune responses [ 48 ].
Infection biology of this virus in zebrafish has been investigated to a limited extent. A novel form of zebrafish fibronectin (Fn2) on the cell surface was found to mediate IHNV attachment and cell entry [ 133 ]. Ludwig et al. (2011) fully explored the benefits of using zebrafish as a model to study IHNV infections. The authors took advantage of transgenic lines and visibility in the larval stage and described primary targets of the virus, reversibility of viral damage and spread of the disease in a whole vertebrate body [ 105 ].
2.2.3. VHSV
VHSV is one of the most economically important viral diseases of rainbow trout and other farmed fish species [ 134 ]. In 2006, the first study of a VHSV infection in zebrafish was conducted and adult zebrafish proved to be susceptible via the injection and the bath route at 15 °C [ 49 ]. An attenuated virus was used for immunization and induced protection against VHSV at 15 °C, illustrating that zebrafish are able to mount a protective response even at a low temperature. A study on adjuvant efficacy has also been conducted using zebrafish and VHSV infections [ 50 ].
Five studies have focused on the immune response of zebrafish against VHSV. The first study in 2010 used gene expression analyses and described genes (such as those encoding complement components) that contributed to the early molecular events occurring in the fish surfaces during initial infections [ 94 ]. At the same time, it was discovered that temperature significantly affected the immune response of the fish. At 15 °C, zebrafish did not show altered gene expression after challenge, which they did at 28 °C. Furthermore, it was shown that Mx was important in the innate anti-viral response in the larvae [ 95 ] and that zebrafish IgM antibodies were key players in the acquired immunity [ 53 ]. A later study in 2015, also using expression data, uncovered that long-term VHSV survivors maintained molecular/cellular memories of viral encounters by modifying the expression levels of innate multigene families and, at the same time, had specific adaptive antibodies [ 96 ]. In our laboratory, we are currently investigating, using the transgenic line with fluorescently marked neutrophils, how these cells take part in the battle against VHSV and/or control the damage induced by the virus.
Investigations of VHSV infection biology have been conducted in several fish species [ 135 ] and this may be the reason for finding only a few studies in zebrafish. Regarding virulence, it was found that specific residues in the 3’-UTR of VHSV have a promoter function and are important for virulence in cells and pathogenicity in fish [ 136 ]. We are currently also investigating the kinetics and the tropism of the virus in vivo by inoculating zebrafish larvae with a transgenic VHSV virus, which induces red fluorescence in infected cells.
In 1977, the first study using zebrafish as a model for viral infections was conducted. Results showed that injected IPNV was transmitted to the eggs (vertical transfer), and that this transmission occurred via females alone [ 137 ]. It was later confirmed that zebrafish also could acquire the infection by the natural route through the water body [ 132 ].
2.3. Parasites
Only a few investigations have been conducted in zebrafish with parasites, which are economically important for the fish production industry ( Table 4 ).
Studies on parasite pathogens relevant for aquaculture utilizing zebrafish as an in vivo model.
| Pathogen | Focus Area | Zebrafish Stage | Reference |
|---|---|---|---|
| Adult Adult Adult Adult | Jørgensen 2016 [ ] Christoffersen et al. 2016 [ ] Jørgensen 2016 [ ] Jørgensen et al. 2018 [ ] | ||
| Adult Adult | Cherry 2003 [ ] Jørgensen 2016 [ ] | ||
| Larvae | Dóró et al. 2019 [ ] |
2.3.1. I. multifiliis
I. multifiliis is a single-celled freshwater parasite and a major problem all over the world in fish aquaculture [ 139 ]. The parasite is a generalist capable of infecting many species of fish and can, within two weeks, cause up to 100% mortality in production facilities. From 2016, several studies have described the immune response of zebrafish against I. multifiliis and it was documented that zebrafish were susceptible towards the parasite and were able to acquire immunological immunity [ 51 ]. Subsequently, regulation of immune-relevant genes was investigated and the main findings included an upregulation of the Th2-associated genes il4/13 and ighm in immune fish and il4/13 and Saa in naïve fish [ 52 , 140 ], which similarly have been observed in fish species used in production [ 31 , 32 , 141 , 142 ]. The transgenic line of zebrafish expressing GFP in connection with neutrophils has also been exploited to investigate the cellular responses against the parasite as well as host/parasite dynamics [ 100 ]. Neutrophils were highly attracted to infected sites 24 h after infection (quantified by counting an increase in the number of neutrophils) but the number of cells decreased during the next two days even though the parasites grew bigger and caused more damage. A video recording showed that the parasites ingested and neutralized functionally active neutrophils and thereby reduced the number of these cells. The most recent study investigated gene expression and the neutrophil response in naïve and I. multifiliis -immune zebrafish [ 52 ]. The number of neutrophils attracted to infection loci were highest in protected fish 24 h after infection even though it was documented that virtually no parasites were left on those fish, which was in contrast to naïve fish that hosted many parasites. It was hypothesized that parasites in the naïve fish ingested a large number of neutrophils, which resulted in the observed reduction in the number of cells. The gene cxcl8 , which encodes a known chemoattractant of neutrophils, was not upregulated in the tissues with accumulations of neutrophils and a Cxcl8-independent sub-population of neutrophils was proposed to have comprised the responders.
The zebrafish was used as a host to study the infection biology of I. multifiliis for the first time in 2003. Here, zebrafish were found to be less susceptible to the parasite than channel catfish and it was concluded that zebrafish were suitable as a more resistant model for this disease [ 143 ].
2.3.2. Trypanosoma carassii
Trypanosomes cause sleeping sickness in humans and a close relative T. carassii is a natural parasite of carp, zebrafish and some non-cyprinid freshwater fish. In 2019, visualization of the swimming behaviour of T. carassii parasites was documented in zebrafish for the first time [ 144 ].
3. Discussion
From the number of studies discussed in this review, the use of zebrafish as a model has contributed tremendously to knowledge on bacterial and viral diseases that are important for fish aquaculture. This is valuable for a more comprehensive understanding of host/pathogen interactions as well as for development of novel control measures. For both types of diseases, transcription analysis is a common tool to decipher immunological responses but with regard to bacterial diseases, the use of fluorescence-tagged bacteria is especially suitable to study the infection biology and real-time local immune responses in vivo in the zebrafish model. Only recently has such an approach been applied for viruses and such investigations may be more frequently encountered in the future. Studying host/parasite relationships in the zebrafish model has only recently begun. The applicability of the model may, however, never reach that of bacterial and viral diseases because of the difficulties working with parasites. Nonetheless, the model has already contributed with unique data, which are impossible to obtain in other fish species.
3.1. Advantages of Using the Zebrafish as a Model
The use of the robust zebrafish as a model represents obvious advantages compared to experimentation in the much larger fish species used for production. The small size benefits especially analyses of whole-body immune responses or infection patterns using technologies such as in vivo imaging (which can be conducted to cellular resolution), whole-mount in situ hybridization or whole-mount immunohistochemistry. The transparency of the larvae represents another advantage for visual documentation of, e.g., pathogen tropisms or tissue damage as a consequence of pathogen assault. To fully unravel the complicated host/pathogen interactions, real-time in vivo experimental infections are a prerequisite. Some studies use zebrafish as a model because the fish is small and easy to manage and breed and do not utilize any of the unique tools available. These tools (in vivo imaging, transgenic lines including fluorescence-labelling of cells, receptors, signalling molecules or tissues) are not widely available in other fish species. A variety of transgenic lines have been utilized in the mentioned studies but other relevant lines are available such as the one resistant to viral or bacterial infections [ 23 , 145 , 146 ], providing a tool to further study immune mechanisms in relation to disease. The more frequently used transgenic strains are those with a fluorescence marker on neutrophils and/or macrophages [ 20 ], where innate cell behaviours in vivo can be analysed, which is otherwise impossible in production fish. Another point of interest is that gnotobiotic zebrafish have been produced and exploited to follow microbial infections of sterile larval fish in real-time.
Zebrafish are able to survive at temperatures from 6–38 °C, making them perfect to study infections at various temperatures. Consequently, fish pathogens thriving at temperatures on both sides of the preferred 28 °C can be studied in the zebrafish and a few studies mentioned in this review took advantage of this.
Apart from transferring specific results of zebrafish genomics, biology and immunology to aquacultured species, a prospect of transferring research approaches such as morpholino-induced knockdown, the CRISPR knockout technology and transgenesis is also valuable.
The genetic similarity between zebrafish and production fish is greater than between zebrafish and mammals and the small fish is widely used as a model for mammalian biology and therefore, appears even more appropriate as a model for other fish species when taking species-specific differences into account. The zebrafish seems to be an ideal model for carp since the genetic distance between these species is so small.
3.2. Drawbacks of Using the Zebrafish as a Model
Even though the fish species to species genetic variation is less than from zebrafish to mammals, differences do exist and zebrafish are, for example, less disposed for stress and anatomically different from rainbow trout [ 147 ]. The zebrafish does not possess a true stomach [ 148 ], potentially reducing the applicability of results to aquacultured species that have one. However, enzymes specific to the vertebrate stomach are represented in zebrafish regardless of its anatomy [ 149 ]. A drawback in some cases is its small size, which makes it difficult to obtain blood in sufficient amounts and limits the amount of tissue and cell populations available for analyses. Some immune cell types such as natural killer cells are poorly characterized [ 78 ].
The feasibility of acclimatizing zebrafish to a desired temperature also comes at a price. Conducting investigations at a lower or higher temperature than 28 °C may have a consequence on the ability of the zebrafish immune system to react properly and a biased response may be expected, as well as a biased period of time until morbidity or mortality due to a pathogen is reached.
Breeding protocols are not standardized between facilities and inbreeding and outbreeding crosses may compromise the fitness of the zebrafish. As a consequence, experiments may be difficult to repeat from one laboratory to another [ 150 ].
The zebrafish may not be useful as a model for marine fish species such as Atlantic salmon ( Salmo salar ) under certain conditions. It does not tolerate salinities of more than approximately 10 ppt (personal observation) and the economically important salmon louse ( Lepeophtheirus salmonis ), which thrives at salinities >27 ppt [ 151 ], appears problematic to study in zebrafish.
4. Conclusions
There are more advantages than drawbacks using the zebrafish as a model for diseases in aquacultured fish species and much has been learned within the last couple of decades. Especially when the unique tools in zebrafish are exploited in vivo in real-time, novel host/pathogen interactions are documented and immune mechanisms are disclosed. Studying processes “live” in a whole vertebrate organism reveal novel insights contributing to basic science but hopefully, also contributing with valuable knowledge for the development of new treatments and prophylactic means.
This research received no external funding.
KLAS® Performance Report, February 2022
KLAS® Performance Report, February 2022 research is focused on assessing whether shared smart devices meet clinical healthcare needs.
KLAS® Performance Report, February 2022 research is focused on assessing whether shared smart devices meet clinical healthcare needs. Zebra enterprise healthcare mobile computers were evaluated on how well these devices perform, increase operational efficiency, enhance patient care, and meet the needs of clinical workflows.
* KLAS Research is a healthcare IT data and insights company founded in 1994. They provide accurate, honest, and impartial research on the software and services used by healthcare providers and payers all over the world. Performance Insights – Zebra Technologies – Utilizing Mobile Computers to Elevate Efficiency and Patient Care February 2022
Content Type
White Paper
- Mobile Computers
Consult an Expert
Our team of partners and sales associates know the ins and outs of every industry to deliver tailored solutions to customers. Let us connect you with a Zebra expert in your area to select and implement the right hardware offerings that best fit your business needs.
Talk to a Partner
Need more information on what solution is right for your needs a partner can help., contact sales, ready to integrate zebra solutions into your systems contact us to get started..

Connect with our team
- About Zebra
- Story Hub/Newsroom
- Corporate Social Responsibility
- Global Locations
- Success Stories
Support Resources
- Support and Downloads
- Contact Support
- Request a Repair
- Product Warranty Information
- Developer Portal
- Report a Potential Security Vulnerability or Concern
Stay up to date with Zebra.
Sign up for our newsletter.
Legal Terms of Use Privacy Policy Supply Chain Transparency
ZEBRA and the stylized Zebra head are trademarks of Zebra Technologies Corp., registered in many jurisdictions worldwide. All other trademarks are the property of their respective owners. ©2024 Zebra Technologies Corp. and/or its affiliates.

IMAGES
VIDEO
COMMENTS
Zebrafish have been a tractable animal model for identifying developing neurons and the in vivo architecture of the brain, from neurogenesis at the early neural plate stage to the adult brain (Fig ...
Introduction. For use in genetic studies as an animal model, zebrafish was initially introduced by Streisinger and colleagues 1 in the early 1980s. Large-scale N-ethyl-N-nitrosourea (ENU) mutagenesis was conducted in combination with extensive phenotypic screening 2,3.Further phenotypic characterization of these ENU mutations in most of the major organ systems was performed 4.
Much of medical research relies on animal models to deepen knowledge of the causes of animal and human diseases, as well as to enable the development of innovative therapies. Despite rodents being the most widely used research model worldwide, in recent decades, the use of the zebrafish (Danio rerio) model has exponentially been adopted among the scientific community. This is because such a ...
The zebrafish. A small aquatic vertebrate, the zebrafish (Danio rerio) is rapidly becoming a new popular model organism in biomedical research (Figures 1 and and2 2) 1-5.Major universities and research centers worldwide have established zebrafish facilities, and the US National Institutes of Health have recently constructed the world's biggest zebrafish research center, able to house ...
The use of zebrafish (Danio rerio) in biomedical research has expanded at a tremendous rate over the last two decades.Along with increases in laboratories using this model, we are discovering new and important diseases. We review here the important pathogens and diseases based on some 20 years of research and findings from our diagnostic service at the NIH-funded Zebrafish International ...
Zebrafish (Danio rerio) is emerging as an increasingly successful model for translational research on human neurological disorders. In this review, we appraise the high degree of neurological and ...
fi. This review addresses the use of zebra fish as an animal model for biomedical research, mainly in developmental disorders, mental disorders, and communication between the brain and organs. In ...
This review focuses on the use of zebrafish as a biomedical model in areas mainly related to diet-induced diseases, metabolic disorders, liver diseases, and intestinal diseases in humans. Figure 1. The number of publications in PubMed per year when searching with the keywords "zebrafish" and "Biomedical.".
The zebrafish (Danio rerio) is a valuable animal model rapidly becoming more commonly used in pharmaceutical studies. Due to its low-cost maintenance and high breeding potential, the zebrafish is a suitable substitute for most adult rodents (mice and rats) in neuroscience research. It is widely used in various anxiety models. This species has been used to develop a conceptual framework for ...
Ecology, Social Behavior, and Conservation in Zebras. Daniel I. Rubenstein. department of ecology and evolutionary biology, princeton university, princeton, new jersey, usa. I. I NTRODUCTION. One ...
The century-old idea that stripes make zebras cryptic to large carnivores has never been examined systematically. We evaluated this hypothesis by passing digital images of zebras through species-specific spatial and colour filters to simulate their appearance for the visual systems of zebras' primary predators and zebras themselves. We also measured stripe widths and luminance contrast to ...
The zebrafish (Danio rerio) is a versatile model organism that has been used in biomedical research for several decades to study a wide range of biological phenomena. There are many technical ...
The zebrafish is increasingly recognized as a model organism for translational research into human neuropathology. The zebrafish brain exhibits fundamental resemblance with human neuroanatomical and neurochemical pathways, and hallmarks of human brain pathology such as protein aggregation, neuronal degeneration and activation of glial cells, for example, can be modeled and recapitulated in the ...
Abstract and Figures. Abstract Zebra fish (Diano riro) is becoming a popular animal for studying human developments and diseases. This is animal has become widely accepted due to its genetic and ...
Research Open Access 27 Feb 2024 Nature Communications. Volume: 15, P: 1792. ... a new paper demonstrates a CRISPR/Cas13 method that can efficiently edit mRNA in zebrafish, medaka, killifish, and ...
We looked for research papers available in ScienceDirect, Google Scholar, Science Citation Index (SCI-Expanded) of Web of Science using the term (microplastic OR microplastics OR nanoplastics OR nanoplastic) AND (zebrafish OR zebra fish OR Danio rerio) and selected 38 research papers in which zebrafish was used as an experimental organism to study the effects of MPs/NPs (Table 1).
Introduction. The zebra mussel (Dreissena polymorpha), a native of the Black and Caspian Seas region in southeastern Europe, is one of the most aggressive and successful aquatic invaders of freshwaters in the northern hemisphere (Karatayev et al., 2006).Originally introduced to the Laurentian Great Lakes region in the mid-1980s (Hebert et al., 1991), zebra mussels rapidly spread throughout the ...
ABSTRACT. The zebrafish, Danio rerio (Hamilton, 1822), has recently emerged as a model. organism for genetic research and for the study of v ertebrate development. It is also. extensively used to ...
Search for more papers by this author. David L Strayer, David L Strayer. Cary Institute of Ecosystem Studies, Millbrook, NY ... In the 20 years since zebra mussels (Dreissena polymorpha) first appeared in North America, they have become one of our most widespread and abundant freshwater animals, and have fundamentally transformed freshwater ...
with design ideas while capturing interaction. In this paper, we describe a technology probe called Zebra, which aimed at exploring the design of an observation tool for fieldwork with busy professionals. We deployed Zebra in the coffee ro om of our lab and observed researchers' reac tions to the proposed concepts
1.1. Background. The zebrafish is extensively used as a vertebrate model for human diseases [1,2,3,4,5,6] and other conditions such as aging, development and genetics [7,8,9,10,11,12,13,14,15,16].The small fish presents many advantages as a model; therefore, it cannot be overlooked and has not been since Dr. George Streisinger convinced the world in the 1980s of the wonders of the zebrafish ...
Overview. KLAS® Performance Report, February 2022 research is focused on assessing whether shared smart devices meet clinical healthcare needs. Zebra enterprise healthcare mobile computers were evaluated on how well these devices perform, increase operational efficiency, enhance patient care, and meet the needs of clinical workflows.
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on ZEBRA MUSSEL. Find methods information, sources, references or conduct a literature review on ZEBRA ...