

13 Advantages and Disadvantages of Genetic Engineering
The process of genetic engineering allows for the structure of genes to be altered. It is a deliberate modification which occurs through the direct manipulation of the genetic material of an organism. DNA is either added or subtracted to produce one or more new traits that were not found in that organism before.
With genetic engineering, it becomes possible to create plants that can resist herbicides while they grow. It also becomes possible to create new threats to our food supply or personal health because viruses and bacteria continue to adapt to the changes that are produced through this process.
Here are the advantages and disadvantages of genetic engineering to consider.
What Are the Advantages of Genetic Engineering?
1. It allows for a faster growth rate. Genetic engineering allows of plants or animals to be modified so their maturity can occur at a quicker pace. Engineering can allow this maturity to occur outside of the normal growth conditions that are favorable without genetic changes as well. Even if there is higher levels of heat or lower levels of light, it becomes possible to expand what can be grown in those conditions.
2. It can create an extended life. Genetic modification can help to create resistance to common forms of organism death. Pest resistance can be included into the genetic profiles of plants so they can mature as a crop without any further additives. Animals can have their genetic profiles modified to reduce the risks of common health concerns that may affect the breed or species. This creates the potential for an extended lifespan for each organism.
3. Specific traits can be developed. Plants and animals can have specific traits developed through genetic engineering that can make them more attractive to use or consumption. Different colors can be created to produce a wider range of produce. Animals can be modified to produce more milk, grow more muscle tissue, or produce different coats so that a wider range of fabrics can be created.
4. New products can be created. With genetic engineering, new products can be created by adding or combining different profiles together. One example of this is to take a specific product, such as a potato, and alter its profile so that it can produce more nutrients per kcal than without the genetic engineering. This makes it possible for more people to get what they need nutritionally, even if their food access is limited, and this could potentially reduce global food insecurity.
5. Greater yields can be produced. Genetic engineering can also change the traits of plants or animals so that they produce greater yields per plant. More fruits can be produced per tree, which creates a greater food supply and more profits for a farmer. It also creates the potential for using modified organisms in multiple ways because there is a greater yield available. Modified corn, for example, can be used for specific purposes, such as animal feed, ethanol, or larger cobs for human consumption.
6. Risks to the local water supply are reduced. Because farmers and growers do not need to apply as many pesticides or herbicides to their croplands due to genetic engineering, fewer applications to the soil need to occur. This protects the local watershed and reduces the risk of an adverse event occurring without risking the yield and profitability that is needed.
7. It is a scientific practice that has been in place for millennia. Humans in the past may not have been able to directly modify the DNA of a plant or animal in a laboratory, but they still practiced genetic engineering through selective breeding and cross-species or cross-breeding. People would identify specific traits, seek out other plants or animals that had similar traits, and then breed them together to create a specific result. Genetic engineering just speeds up this process and can predict an outcome with greater regularity.
What Are the Disadvantages of Genetic Engineering?
1. The nutritional value of foods can be less. When animals grow, and mature quickly, the nutritional value of that product can be reduced. This can be seen in poultry products today with the white striping that is found in meat products. That striping is a fat deposit that was created, often in the breast meat, because of the rapid growth of the bird. In chickens, Good Housekeeping reports that this can increase the fat content of the meat consumed by over 220%. At the same time, the amount of protein that is received is also reduced.
2. Pathogens adapt to the new genetic profiles. Genetic engineering can create a natural resistance against certain pathogens for plants and animals, but the natural evolutionary process is geared toward creating pathways. Bacteria and viruses evolve a resistance to the resistance that is created by the genetic engineering efforts. This causes the pathogens to become stronger and more resistant than they normally would be, potentially creating future health concerns that are unforeseen.
3. There can be negative side effects that are unexpected. Genetic engineering is guaranteed to make a change. Many of those changes are positive, creating more and healthier foods. Some of those changes, however, can be negative and unexpected. Making a plant become more tolerant to drought might also make that plant become less tolerant to direct sunlight. Animals may be modified to produce more milk, but have a shortened lifespan at the same time so farmers suffer a greater livestock.
4. The amount of diversity developed can be less favorable. At some point, genetically engineered plants and animals make it “into the wild” and interact with domestic species. This results in a crossing of “natural” and “artificial” organisms. The engineered organisms often dominate, resulting in only a modified species over several generations, reducing the diversity that is available.
5. Copyrighted genetic engineering can have costly consequences. Many companies copyright their genetic engineering processes or products to maintain their profitability. If a farmer plants genetically modified crops and the pollination process causes another farmer in the field over to have those modified crops grow, there have been precedents for legal actions against the “unauthorized” farmer. This can have several costly consequences, from fewer farmers wanting to work to a higher cost for the seeds that are planted.
6. This knowledge and technology can be easily abused. At the moment, genetic engineering in humans is being used to treat specific disorders that threaten the health or wellbeing of individuals. In time, the approach in humans could be like what is already being done with plants and animals. Genetic engineering can change specific traits, which could create human outcomes that are ethically questionable or easily abused.
The advantages and disadvantages of genetic engineering show that the results can be generally positive, but there must be controls in place to manage the negative when it occurs.
Genetic Engineering Advantages & Disadvantages

Genetic engineering has pros and cons.
Table of Contents
Through genetic engineering, scientists are able to move desirable genes from one plant or animal to another or from a plant to an animal or vice versa. (Ref. 1) By desirable , it means it can produce an outcome that is regarded as generally “beneficial” or “useful”. The organism that has undergone such genetic modification is referred to as “ genetically modified organism ” or GMO. In essence, genetic engineering is a technology wherein a specific gene can be selected and implanted into the recipient organism. The cell that received such an implant can, therefore, begin producing substances with the desired functions. Genetic engineering uses recombinant DNA, molecular cloning, and transformation.
Genetic engineering has become a mainstream part of our lives because of the many advantages involved. Here are some of them:
- Genetic engineering made it possible to create crop varieties regarded as “ more beneficial ”. Unlike selective breeding, modern genetic engineering is more gene-specific. One of the downsides of selective breeding is the possibility of generating traits that are less desirable. This is averted by modern genetic engineering that introduces specific genes. (Ref. 1) Since the process is rather straightforward, it is relatively faster than selective breeding (see previous tutorial ) in terms of coming up with crops with the desired traits. Examples of genetically-engineered plants with more desirable traits are drought-resistant plants, disease-resistant crops, plants that grow faster, and plants (e.g. legumes) fortified with more nutrients. (Ref.1, 2) The latter may be achieved by introducing genes that code for (1) trace-element-binding proteins, (2) overexpression of storage proteins already present, and/or (3) increased expression of proteins that are responsible for trace element uptake into plants. (Ref. 2)
- Organisms can be ‘ tailor-made ’ to show desirable characteristics. Genes can also be manipulated in trees, for example, to absorb more CO 2 and reduce the threat of global warming. Through genetic engineering, genetic disorders may also be fixed by replacing the faulty gene with a functional gene. Disease-carrying insects, such as mosquitoes, may be engineered into becoming sterile insects. This will help in curbing the spread of certain diseases, e.g. malaria and dengue fever.
- Genetic Engineering could increase genetic diversity and produce more variant alleles that could also be crossed over and implanted into other species. It is possible to alter the genetics of wheat plants to grow insulin as an example.Nevertheless, there are two sides to a coin. While genetic engineering is beneficial in ways mentioned above it is also implicated in certain eventualities deemed as “unpleasant” or disadvantageous.
- There are concerns over the inadvertent effects, such as the creation of food that can cause an allergic reaction, GMO that can cause harmful genetic effects, and genes moving from one species to another that is not genetically engineered. (Ref. 1) It has been shown that GMO crop plants can pass the beneficial gene along to a wild population. An example is the sunflowers genetically-engineered to fend off certain insects. They were observed to have transferred the gene to their weedy relatives. (Ref. 3) Nature is an extremely complex interrelated chain. Some scientists believe that introducing genetically-modified genes may have an irreversible effect with consequences yet unknown.
- Genetic engineering borderlines on many moral and ethical issues. One of the major questions raised is if humans have the right to manipulate the laws and course of nature.
Genetic engineering may be one of the greatest breakthroughs in recent history alongside the discovery of the atom and space flight. However, there are plausible risks involved. Thus, governments have produced legislation to control what sort of experiments are done involving genetic engineering.
Despite the strict regulation, genetic engineering progressed. It has led to many experimental breakthroughs over the years.
- At the Roslin Institute in Scotland, scientists successfully cloned an exact copy of a sheep, named ‘Dolly’, in July 1996. This was the first successful artificial cloning of a mammal. (Ref. 4)
- Scientists successfully manipulated the genetic sequence of a rat to grow a human ear on its back.
These procedures are essentially a form of “therapeutic cloning”. Embryonic cells can now be cloned. They are grown for health purposes, such as to obtain biological organs for transplantation. Cells are also cloned in the laboratory for research purposes. (Ref. 1) How about humans? Can a human individual be cloned? At this point in time, cloning a human individual is not possible. What can be cloned is the genotype but not the phenotype. (Ref. 4)
Genetic engineering has been made possible with the discovery of the complex and microscopic nature of DNA and its component nucleotides . For us to understand chromosomes and DNA more clearly, they can be mapped for future reference. More simplistic organisms such as fruit fly ( Drosophila ) have been chromosome-mapped due to their simplistic nature. They will require fewer genes to operate.
The process of genetic engineering involves splicing an area of a chromosome, a gene, that controls a certain characteristic of the body. The enzyme endonuclease is used to split a DNA sequence as well as split the gene from the rest of the chromosome. For example, this gene may be programmed to produce an antiviral protein. This gene is removed and can be placed into another organism. For example, it can be placed into a bacterial cell where it can be sealed into the DNA chain using ligase. When the chromosome is once again sealed the bacterial cell is now effectively re-programmed to replicate this new antiviral protein. The bacterium can continue to live a healthy life while genetic engineering by human intervention has manipulated it to produce the protein.
Select Advantage if it generally depicts the advantage of genetic engineering or Disadvantage if a disadvantage.
Send Your Results (Optional)

References:
- Genetically engineered foods: MedlinePlus Medical Encyclopedia. (2020). Medlineplus.Gov. https://medlineplus.gov/ency/article/002432.htm
- Lönnerdal, B. (May 2003). Genetically Modified Plants for Improved Trace Element Nutrition. J. Nutr. 133:1490S-1493S.https://www.biologyonline.com/articles/genetically-modified-plants-improved
- Ohio State University. (2002). Genetically Modified Crops May Pass Helpful Traits To Weeds, Study Finds – Biology Online Archive Article. Biology Articles, Tutorials & Dictionary Online. https://www.biologyonline.com/articles/genetically-modified-crops-may
- Ayala, F. J. (2015). Cloning humans? Biological, ethical, and social considerations. Proceedings of the National Academy of Sciences , 112 (29), 8879–8886. https://doi.org/10.1073/pnas.1501798112
Further Reading:
- Biomanufacturing . Biotech-Careers.Org. https://biotech-careers.org/job-areas/biomanufacturing
You will also like...
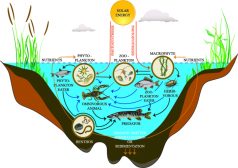
Freshwater Producers and Consumers
Freshwater ecosystem is comprised of four major constituents, namely elements and compounds, plants, consumers, and deco..
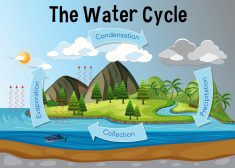
The Water Cycle
The water cycle (also referred to as the hydrological cycle) is a system of continuous transfer of water from the air, s..
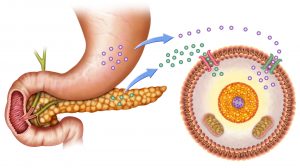
Sugar Homeostasis
The blood sugar level is regulated by two hormones. The mechanism behind this type of negative feedback control is descr..

Adaptation Tutorial
Adaptation, in biology and ecology, refers to the process or trait through which organisms or the populations in a habit..

Seed Plants
Seed plants are vascular plants. They differ from the other vascular plants in producing seeds that germinate into a new..

Freshwater Lentic Communities & Animals
This tutorial looks at some of the communities in freshwater lentic habitats. For instance, symbiosis occurs in a commun..
Home — Essay Samples — Science — Genetic Engineering — Exploring the Pros and Cons of Genetic Engineering
Exploring The Pros and Cons of Genetic Engineering
- Categories: Engineering Genetic Engineering
About this sample

Words: 571 |
Published: Feb 7, 2024
Words: 571 | Page: 1 | 3 min read
Table of contents
Introduction, pros of genetic engineering, cons of genetic engineering, regulation of genetic engineering.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr. Heisenberg
Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
1 pages / 408 words
2 pages / 1081 words
3 pages / 1416 words
7 pages / 3177 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Genetic Engineering
Genetic engineering is the manipulation of an organism's genetic material to produce desirable traits. It has been used in various fields such as agriculture, medicine, and environmental conservation. Genetic engineering has [...]
Introduction: In the realm of scientific innovation, genetic engineering stands at the forefront, offering unprecedented possibilities for enhancing human capabilities. The ethical implications of manipulating the very essence [...]
As genetic engineering continues to advance, the controversy surrounding its potential benefits and ethical considerations has become increasingly prominent. This essay aims to provide a comprehensive analysis of genetic [...]
In Gattaca's dystopia, the dangers of a society obsessed with genetic perfection are exposed. The film challenges viewers to reflect on the implications of genetic determinism, the erosion of personal identity, and the ethical [...]
Genetics is the most interesting part of science because it explains how certain traits are passed down by parents to their offspring. Gregor Mendel is considered the pioneer in explaining this theory of the genetics within [...]
Genetic Engineering is a powerful and potentially very dangerous too. To change the sequence of nucleotides of the DNA that code for the structure of a complex living organism, can have extremely ill effects although the [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
This page has been archived and is no longer updated
Genetically Modified Organisms (GMOs): Transgenic Crops and Recombinant DNA Technology
People have been altering the genomes of plants and animals for many years using traditional breeding techniques. Artificial selection for specific, desired traits has resulted in a variety of different organisms, ranging from sweet corn to hairless cats. But this artificial selection , in which organisms that exhibit specific traits are chosen to breed subsequent generations, has been limited to naturally occurring variations. In recent decades, however, advances in the field of genetic engineering have allowed for precise control over the genetic changes introduced into an organism . Today, we can incorporate new genes from one species into a completely unrelated species through genetic engineering, optimizing agricultural performance or facilitating the production of valuable pharmaceutical substances. Crop plants, farm animals, and soil bacteria are some of the more prominent examples of organisms that have been subject to genetic engineering.
Current Use of Genetically Modified Organisms
Table 1: Examples of GMOs Resulting from Agricultural Biotechnology
The pharmaceutical industry is another frontier for the use of GMOs. In 1986, human growth hormone was the first protein pharmaceutical made in plants (Barta et al ., 1986), and in 1989, the first antibody was produced (Hiatt et al ., 1989). Both research groups used tobacco, which has since dominated the industry as the most intensively studied and utilized plant species for the expression of foreign genes (Ma et al ., 2003). As of 2003, several types of antibodies produced in plants had made it to clinical trials. The use of genetically modified animals has also been indispensible in medical research. Transgenic animals are routinely bred to carry human genes, or mutations in specific genes, thus allowing the study of the progression and genetic determinants of various diseases.
Potential GMO Applications
Many industries stand to benefit from additional GMO research. For instance, a number of microorganisms are being considered as future clean fuel producers and biodegraders. In addition, genetically modified plants may someday be used to produce recombinant vaccines. In fact, the concept of an oral vaccine expressed in plants (fruits and vegetables) for direct consumption by individuals is being examined as a possible solution to the spread of disease in underdeveloped countries, one that would greatly reduce the costs associated with conducting large-scale vaccination campaigns. Work is currently underway to develop plant-derived vaccine candidates in potatoes and lettuce for hepatitis B virus (HBV), enterotoxigenic Escherichia coli (ETEC), and Norwalk virus. Scientists are also looking into the production of other commercially valuable proteins in plants, such as spider silk protein and polymers that are used in surgery or tissue replacement (Ma et al ., 2003). Genetically modified animals have even been used to grow transplant tissues and human transplant organs, a concept called xenotransplantation. The rich variety of uses for GMOs provides a number of valuable benefits to humans, but many people also worry about potential risks.
Risks and Controversies Surrounding the Use of GMOs
Despite the fact that the genes being transferred occur naturally in other species, there are unknown consequences to altering the natural state of an organism through foreign gene expression . After all, such alterations can change the organism's metabolism , growth rate, and/or response to external environmental factors. These consequences influence not only the GMO itself, but also the natural environment in which that organism is allowed to proliferate. Potential health risks to humans include the possibility of exposure to new allergens in genetically modified foods, as well as the transfer of antibiotic-resistant genes to gut flora.
Horizontal gene transfer of pesticide, herbicide, or antibiotic resistance to other organisms would not only put humans at risk , but it would also cause ecological imbalances, allowing previously innocuous plants to grow uncontrolled, thus promoting the spread of disease among both plants and animals. Although the possibility of horizontal gene transfer between GMOs and other organisms cannot be denied, in reality, this risk is considered to be quite low. Horizontal gene transfer occurs naturally at a very low rate and, in most cases, cannot be simulated in an optimized laboratory environment without active modification of the target genome to increase susceptibility (Ma et al ., 2003).
In contrast, the alarming consequences of vertical gene transfer between GMOs and their wild-type counterparts have been highlighted by studying transgenic fish released into wild populations of the same species (Muir & Howard, 1999). The enhanced mating advantages of the genetically modified fish led to a reduction in the viability of their offspring . Thus, when a new transgene is introduced into a wild fish population, it propagates and may eventually threaten the viability of both the wild-type and the genetically modified organisms.
Unintended Impacts on Other Species: The Bt Corn Controversy
One example of public debate over the use of a genetically modified plant involves the case of Bt corn. Bt corn expresses a protein from the bacterium Bacillus thuringiensis . Prior to construction of the recombinant corn, the protein had long been known to be toxic to a number of pestiferous insects, including the monarch caterpillar, and it had been successfully used as an environmentally friendly insecticide for several years. The benefit of the expression of this protein by corn plants is a reduction in the amount of insecticide that farmers must apply to their crops. Unfortunately, seeds containing genes for recombinant proteins can cause unintentional spread of recombinant genes or exposure of non-target organisms to new toxic compounds in the environment.
The now-famous Bt corn controversy started with a laboratory study by Losey et al . (1999) in which the mortality of monarch larvae was reportedly higher when fed with milkweed (their natural food supply) covered in pollen from transgenic corn than when fed milkweed covered with pollen from regular corn. The report by Losey et al . was followed by another publication (Jesse & Obrycki, 2000) suggesting that natural levels of Bt corn pollen in the field were harmful to monarchs.
Debate ensued when scientists from other laboratories disputed the study, citing the extremely high concentration of pollen used in the laboratory study as unrealistic, and concluding that migratory patterns of monarchs do not place them in the vicinity of corn during the time it sheds pollen. For the next two years, six teams of researchers from government, academia, and industry investigated the issue and concluded that the risk of Bt corn to monarchs was "very low" (Sears et al ., 2001), providing the basis for the U.S. Environmental Protection Agency to approve Bt corn for an additional seven years.
Unintended Economic Consequences
Another concern associated with GMOs is that private companies will claim ownership of the organisms they create and not share them at a reasonable cost with the public. If these claims are correct, it is argued that use of genetically modified crops will hurt the economy and environment, because monoculture practices by large-scale farm production centers (who can afford the costly seeds) will dominate over the diversity contributed by small farmers who can't afford the technology. However, a recent meta-analysis of 15 studies reveals that, on average, two-thirds of the benefits of first-generation genetically modified crops are shared downstream, whereas only one-third accrues upstream (Demont et al ., 2007). These benefit shares are exhibited in both industrial and developing countries. Therefore, the argument that private companies will not share ownership of GMOs is not supported by evidence from first-generation genetically modified crops.
GMOs and the General Public: Philosophical and Religious Concerns
In a 2007 survey of 1,000 American adults conducted by the International Food Information Council (IFIC), 33% of respondents believed that biotech food products would benefit them or their families, but 23% of respondents did not know biotech foods had already reached the market. In addition, only 5% of those polled said they would take action by altering their purchasing habits as a result of concerns associated with using biotech products.
According to the Food and Agriculture Organization of the United Nations, public acceptance trends in Europe and Asia are mixed depending on the country and current mood at the time of the survey (Hoban, 2004). Attitudes toward cloning, biotechnology, and genetically modified products differ depending upon people's level of education and interpretations of what each of these terms mean. Support varies for different types of biotechnology; however, it is consistently lower when animals are mentioned.
Furthermore, even if the technologies are shared fairly, there are people who would still resist consumable GMOs, even with thorough testing for safety, because of personal or religious beliefs. The ethical issues surrounding GMOs include debate over our right to "play God," as well as the introduction of foreign material into foods that are abstained from for religious reasons. Some people believe that tampering with nature is intrinsically wrong, and others maintain that inserting plant genes in animals, or vice versa, is immoral. When it comes to genetically modified foods, those who feel strongly that the development of GMOs is against nature or religion have called for clear labeling rules so they can make informed selections when choosing which items to purchase. Respect for consumer choice and assumed risk is as important as having safeguards to prevent mixing of genetically modified products with non-genetically modified foods. In order to determine the requirements for such safeguards, there must be a definitive assessment of what constitutes a GMO and universal agreement on how products should be labeled.
These issues are increasingly important to consider as the number of GMOs continues to increase due to improved laboratory techniques and tools for sequencing whole genomes, better processes for cloning and transferring genes, and improved understanding of gene expression systems. Thus, legislative practices that regulate this research have to keep pace. Prior to permitting commercial use of GMOs, governments perform risk assessments to determine the possible consequences of their use, but difficulties in estimating the impact of commercial GMO use makes regulation of these organisms a challenge.
History of International Regulations for GMO Research and Development
In 1971, the first debate over the risks to humans of exposure to GMOs began when a common intestinal microorganism, E. coli , was infected with DNA from a tumor-inducing virus (Devos et al ., 2007). Initially, safety issues were a concern to individuals working in laboratories with GMOs, as well as nearby residents. However, later debate arose over concerns that recombinant organisms might be used as weapons. The growing debate, initially restricted to scientists, eventually spread to the public, and in 1974, the National Institutes of Health (NIH) established the Recombinant DNA Advisory Committee to begin to address some of these issues.
In the 1980s, when deliberate releases of GMOs to the environment were beginning to occur, the U.S. had very few regulations in place. Adherence to the guidelines provided by the NIH was voluntary for industry. Also during the 1980s, the use of transgenic plants was becoming a valuable endeavor for production of new pharmaceuticals, and individual companies, institutions, and whole countries were beginning to view biotechnology as a lucrative means of making money (Devos et al ., 2007). Worldwide commercialization of biotech products sparked new debate over the patentability of living organisms, the adverse effects of exposure to recombinant proteins, confidentiality issues, the morality and credibility of scientists, the role of government in regulating science, and other issues. In the U.S., the Congressional Office of Technology Assessment initiatives were developed, and they were eventually adopted worldwide as a top-down approach to advising policymakers by forecasting the societal impacts of GMOs.
Then, in 1986, a publication by the Organization for Economic Cooperation and Development (OECD), called "Recombinant DNA Safety Considerations," became the first intergovernmental document to address issues surrounding the use of GMOs. This document recommended that risk assessments be performed on a case-by-case basis. Since then, the case-by-case approach to risk assessment for genetically modified products has been widely accepted; however, the U.S. has generally taken a product-based approach to assessment, whereas the European approach is more process based (Devos et al ., 2007). Although in the past, thorough regulation was lacking in many countries, governments worldwide are now meeting the demands of the public and implementing stricter testing and labeling requirements for genetically modified crops.
Increased Research and Improved Safety Go Hand in Hand
Proponents of the use of GMOs believe that, with adequate research, these organisms can be safely commercialized. There are many experimental variations for expression and control of engineered genes that can be applied to minimize potential risks. Some of these practices are already necessary as a result of new legislation, such as avoiding superfluous DNA transfer (vector sequences) and replacing selectable marker genes commonly used in the lab (antibiotic resistance) with innocuous plant-derived markers (Ma et al ., 2003). Issues such as the risk of vaccine-expressing plants being mixed in with normal foodstuffs might be overcome by having built-in identification factors, such as pigmentation, that facilitate monitoring and separation of genetically modified products from non-GMOs. Other built-in control techniques include having inducible promoters (e.g., induced by stress, chemicals, etc.), geographic isolation, using male-sterile plants, and separate growing seasons.
GMOs benefit mankind when used for purposes such as increasing the availability and quality of food and medical care, and contributing to a cleaner environment. If used wisely, they could result in an improved economy without doing more harm than good, and they could also make the most of their potential to alleviate hunger and disease worldwide. However, the full potential of GMOs cannot be realized without due diligence and thorough attention to the risks associated with each new GMO on a case-by-case basis.
References and Recommended Reading
Barta, A., et al . The expression of a nopaline synthase-human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Molecular Biology 6 , 347–357 (1986)
Beyer, P., et al . Golden rice: Introducing the β-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. Journal of Nutrition 132 , 506S–510S (2002)
Demont, M., et al . GM crops in Europe: How much value and for whom? EuroChoices 6 , 46–53 (2007)
Devlin, R., et al . Extraordinary salmon growth. Nature 371 , 209–210 (1994) ( link to article )
Devos, Y., et al . Ethics in the societal debate on genetically modified organisms: A (re)quest for sense and sensibility. Journal of Agricultural and Environmental Ethics 21 , 29–61 (2007) doi:10.1007/s10806-007-9057-6
Guerrero-Andrade, O., et al . Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Research 15 , 455–463(2006) doi:10.1007/s11248-006-0017-0
Hiatt, A., et al . Production of antibodies in transgenic plants. Nature 342 , 76–79 (1989) ( link to article )
Hoban, T. Public attitudes towards agricultural biotechnology. ESA working papers nos. 4-9. Agricultural and Development Economics Division, Food and Agricultural Organization of the United Nations (2004)
Jesse, H., & Obrycki, J. Field deposition of Bt transgenic corn pollen: Lethal effects on the monarch butterfly. Oecologia 125 , 241–248 (2000)
Losey, J., et al . Transgenic pollen harms monarch larvae. Nature 399 , 214 (1999) doi:10.1038/20338 ( link to article )
Ma, J., et al . The production of recombinant pharmaceutical proteins in plants. Nature Reviews Genetics 4 , 794–805 (2003) doi:10.1038/nrg1177 ( link to article )
Muir, W., & Howard, R. Possible ecological risks of transgenic organism release when transgenes affect mating success: Sexual selection and the Trojan gene hypothesis. Proceedings of the National Academy of Sciences 96 , 13853–13856 (1999)
Sears, M., et al . Impact of Bt corn on monarch butterfly populations: A risk assessment. Proceedings of the National Academy of Sciences 98 , 11937–11942 (2001)
Spurgeon, D. Call for tighter controls on transgenic foods. Nature 409 , 749 (2001) ( link to article )
Takeda, S., & Matsuoka, M. Genetic approaches to crop improvement: Responding to environmental and population changes. Nature Reviews Genetics 9 , 444–457 (2008) doi:10.1038/nrg2342 ( link to article )
United States Department of Energy, Office of Biological and Environmental Research, Human Genome Program. Human Genome Project information: Genetically modified foods and organisms, (2007)
- Add Content to Group
Article History
Flag inappropriate.


Email your Friend

- | Lead Editor: Bob Moss

Within this Subject (34)
- Applications in Biotechnology (4)
- Discovery of Genetic Material (4)
- DNA Replication (6)
- Gene Copies (5)
- Jumping Genes (4)
- RNA (7)
- Transcription & Translation (4)
Other Topic Rooms
- Gene Inheritance and Transmission
- Gene Expression and Regulation
- Nucleic Acid Structure and Function
- Chromosomes and Cytogenetics
- Evolutionary Genetics
- Population and Quantitative Genetics
- Genes and Disease
- Genetics and Society
- Cell Origins and Metabolism
- Proteins and Gene Expression
- Subcellular Compartments
- Cell Communication
- Cell Cycle and Cell Division
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
20 Genetic Engineering Pros and Cons for the Biotechnology Era
Genetic engineering is part of the brave new world of biotechnology, but is changing the genes of living things the right thing to do?
Green Coast is supported by its readers. We may earn an affiliate commission at no extra cost to you if you buy through a link on this page . Learn more .

As the third decade of the twenty-first century rolls on, advancements in genetic engineering, a leading area of biotechnology, continue to gain momentum. Humans are now capable of making applied and consistently transmitted changes to the genetic composition of living things, with impacts on organisms and our environment that are already being felt.
Genetic engineering certainly offers a lot of promise, but many people are concerned that it may harbor devastating consequences that humanity has not yet comprehended. In this article, we’ll take a closer look at the genetic engineering pros and cons, to help you evaluate if it is truly a good thing.
What is genetic engineering?
The National Human Genome Institute defines genetic engineering as a process that uses laboratory-based technologies to change or manipulate the packaged DNA (genes) of an organism. Genetic engineering uses invasive techniques to change the genetic makeup of cells.
By moving genes within and across species boundaries, scientists can obtain their desired observable traits in the host organism’s phenotype. This video from the Massachusetts Institute of Technology (MIT) explains the basics:
Scientists manipulate and modify sequences of DNA by removing them from organisms. They can also artificially synthesize RNA and complementary DNA strands using the polymerase chain reaction (PCR). Organisms can also be genetically modified (GM) through the removal of specific gene sequences, carried out using DNA-cutting enzymes known as restriction endonucleases.
Novel gene sequences can then be inserted or spliced into the DNA of a host organism. This is called recombinant technology. These genes encode instructions for the production of particular protein products. The host organism transcribes and translates the gene, leading to the production of the gene products with novel effects on the modified organism (gene expression).
What is a genetically modified organism?
A genetically modified organism or GMO is an organism that has undergone genetic engineering and is permanently different from the original wild-type or natural organism. This is because the genetic sequences added or removed change gene expression and protein production in the GMO organism leading to:
- An altered physical appearance
- The production of a specific substance, that may have positive or negative effects
- Increased resistance to a specific disease
- The eradication of specific genetic conditions in the organism or its offspring

A brief history of genetic engineering
The geneticists Stanley N. Cohen and Herbert W. Boyer were the first scientists to cut up DNA into fragments and insert gene sequences into an organism. In 1973 they successfully added new genes to the plasmid of E. Coli bacteria. A year later, Rudolf Jaenisch inserted foreign DNA into the genome of a mouse, creating the first GM animal.
By the end of the 1970s, the recombinant technology used by these scientists had already become commercialized with the production of human insulin by GM bacteria in 1982. Genetic engineering expanded to food crops and livestock, with GM food introduced to consumers in the 1990s. The Flavr Savr, a GM tomato introduced in 1994 was developed to have a longer shelf-life and by the early 21st century almost all the corn produced in the US was GM, with strained developed to be more resistant to drought and pests.
Genetic engineering technologies and techniques
Genetic engineering now uses several techniques to change the genes of living organisms. The methods vary in sophistication, cost, and application and certain jurisdictions may restrict their use. Here are some leading genetic engineering technologies:
- Recombination technology : This is the original form of genetic engineering that uses restriction enzymes that can cut sequences of DNA that are then inserted into the host organism’s genome.
- Zinc finger nucleases : This gene editing technology uses a special nuclease enzyme fused with a three base pair DNA-binding domains for more precise DNA binding site recognition when editing genes.
- CRISPR-Cas9 : Clustered Regularly Interspaced Short Palindromic Repeats or CRISPR- based genome editing is one of the most advanced forms of genetic engineering. It adapts a technique used by bacteria to evade genetic damage from viruses. CRISPR has become widespread because of its accessibility, low cost, and precision. It is thought to have been used in some extremely controversial experiments including gain-of-function virology research, and the gene editing of embryos.
- Base editing : This simpler gene editing technique makes single base substitutions without inducing risky double-stranded breaks in the genome. Double-strand breaks are a serious form of DNA damage that can lead to the deletion of genes, cell death, or the development of cancer. Base editing offers the promise of curing genetic diseases caused by errors in a single base in the DNA (single nucleotide polymorphisms).
- Prime editing: This contemporary form of gene editing uses an enzyme that can create single-strand breaks in DNA (Cas9 nickase) along with a complex (pegRNA) that acts as a template for the reverse transaction attachment of the desired bases to the site where the DNA has been broken. This avoids double-strand breaks and is considered safer for human therapeutic uses.
Genetic engineering applications
Because all living things have DNA the applications and implications of genetic engineering are understandably vast. The rapid standardization and commercialization of genetic engineering techniques have enabled companies to develop applications in diverse sectors spanning agriculture, healthcare, environmental conservation, and industry, without any public consultation or consent.
Key applications of genetic engineering include:
- The study of gene expression and function using experiments to add or delete gene sequences and portions.
- The development of pharmaceutical products like antibiotics, hormones, and antibodies using GMOs.
- The creation of disease, pest, and drought-hardy crops.
- The production of potent enzymes for industrial detergents, cheese production, and fermentation.
Pros of genetic engineering
Genetic engineering is a key driver of the biotechnology sector which advocates that altering the expression of genes can be targeted, controlled, and beneficial to life on Earth. Here are some benefits of genetic engineering:
1. A greater understanding of how DNA and genes work
Genetic engineering evolved from the study of DNA, and experiments were undertaken to understand how genes work. Once eminent scientists like Watson and Crick and Rosalind Franklin had established the molecular structure of DNA in the 1950s, work began in earnest to understand its function.
The Discovery of the four key classes of enzymes that acted on DNA, helicase, primase, DNA polymerase, and ligase, equipped scientists with tools to work with DNA, cutting and splicing it in specific places. This experimentation with DNA and its effects on gene expression underpin genetic engineering and has continued to expand the field of genetics.
2. The production of medicines
Biotechnology is already an integral part of healthcare and genetic engineering is used to produce medicines for many common conditions. Medicines that are produced by genetic engineering are either:
- Biological medicines that a GMO has produced.
- GMOs that are used as medicinal agents.
Essential medicines that are derived from GMOs or are GM products include:
- Immunoglobulins
- Monoclonal antibodies
- Hormones including insulin and growth hormone
- Pancreatic enzymes
Genetic engineering has made it possible to produce these humanized biological agents at scale so that patients can access them when needed. For example, recombinant insulin is now the main type of insulin used by Type I diabetics rather than the animal-derived insulins that were originally used to treat this condition.

3. A promise of cures for genetic diseases
The manipulation and editing of gene sequences using genetic engineering technologies offer an as-yet-unrealized promise of curing genetic diseases. Point mutation diseases such as sickle cell disease and hemophilia A and B, caused by errors in a single or a limited number of nucleotides in a gene sequence could be frankly cured by having the genetic error corrected.
This type of genetic engineering is gene therapy. Though gene therapy has captured the public imagination, offering hope for cures for devastating genetic diseases, this area of science is not adequately advanced to treat patients in a consistent, reliable, or safe manner.
4. Development of more productive and resilient crops
Genetic engineering is considered a key area of innovation in agriculture. After millennia of painstaking cross-pollination and plant breeding, genetic engineering can achieve dramatic changes in the characteristics and performance of crops almost instantly.
Genetic engineering has been used to develop crops with novel gene insertions that confer protection against pests and diseases or raise the crop’s tolerance to pesticide use. GM crops achieve their enhanced resilience by producing protein products that have these beneficial effects, like plants that express proteins known to inhibit pests.
New breeding techniques (NBT) routinely use genetic engineering alongside conventional breeding to produce crops that are more resilient and productive. Examples of genetically engineered crops include non-browning potatoes, mushrooms, and apples, soybeans with an improved oil profile, and flavor-enhanced fruit and vegetables.

5. Enhanced livestock breeds
Genetic engineering has also extended to livestock, with modern techniques like CRISPR used on pre-implantation embryos to alter specific traits and characteristics. Scientists implant the gene-edited embryos in the womb of a surrogate animal to gestate, producing a generation of transgenic animals with an altered genetic profile.
The gene editing of animals is understandably controversial and governments restrict the use of these animals as food in many parts of the world. Advocates for transgenic livestock point to the enhancements that can be achieved, which can improve livestock health, fertility, resilience, and the nutritional content of meat.
6. The creation of new commercial sectors
Genetic engineering has been integral to developing biotechnology as an industry worth over $1 trillion globally. Genetic engineering has gained significant commercial traction because various industries can apply it to increase their productivity and generate revenue.
Even industries where genetic engineering may not seem relevant may demand the complex biological substances that genetic engineering can produce. For example, genetic engineering is being used to develop enzymes and microbiological organisms that can digest oil spills .
The commercial sector is a key driver of innovation in genetic engineering technologies, but governments are also funding and investing in genetic engineering technologies that can provide solutions to health problems, food security, and environmental challenges.
7. Reduced resource consumption
Genetic engineering may develop crops and livestock breeds that demand less water, fertilizer, and feed. Healthier livestock requires less medicine and adding hardiness traits may mean that farmers can rear them in challenging environments that would normally be more resource and labor-intensive.

Enhancing crop yields and animal productivity also means that the resources used for cultivation go further, leading to cost savings for farmers. Consumers benefit from lower prices and foods that may have a longer shelf-life.
8. Eradicating diseases
Genetic engineering is currently being used to develop solutions for eradicating serious parasite-borne diseases like malaria . In sub-Saharan Africa, Asia, and Latin America, malaria is a significant health and economic burden, causing hundreds of thousands of deaths annually.
Genetic engineering that targets mosquitos and the plasmodium parasite that causes malaria could lead to the eradication of this devastating disease. Oxitec, a British company, has already released male GM mosquitoes that carry an inserted gene that encodes a protein which is fatal to their female offspring.
Cons of genetic engineering
Genetic engineering shows a lot of promise, but at what cost should humanity seek to realize its potential? Interference with genes has implications for every living thing on Earth, yet the knowledge and control of these technologies are in the hands of a tiny number of people.
Here are the important downsides of genetic engineering that everyone should know about:

1. The science of genetics is not settled
Genetics is an incredibly expansive area of biology, but it’s important to know that scientists do not fully understand how genes work. Geneticists are continually working in disparate niche areas of this field to contribute to the body of knowledge in this area and regularly publish discoveries that challenge the mainstream understanding of genetics.
Consider this; until recently scientists believed that 99 percent of DNA, which did not code for proteins, was junk. They focused most of the field of genetic engineering on just 1 percent of the total amount of DNA in a cell.
But errors in non-coding DNA have now been implicated in the development of cancers like leukemia and the emerging field of epigenetics has also challenged conventional thinking on the nature and purpose of DNA.

2. Some claims of genetic engineering may be overstated
The promise of genetics as a solution to food shortages and diseases has captured the public imagination. However, the realization of these promises may take many years to achieve, and in some cases, may not be fulfilled at all.
Genetic engineering is still extremely experimental, and for every success reported, there are thousands of failed experiments in the lab. Geneticists work with cells, tissues, enzymes, and proteins that may not always behave as predicted. This means that it can take decades to develop genetic engineering techniques that produce consistent and reliable results.
3. The long-term effects of genetic engineering are unknown
Because genetics is an evolving field, it is impossible to know the long-term effects of gene editing on species, individuals, populations, and the wider environment. This is particularly important for GM crops which may produce harmful protein products that harm wildlife and even accumulate in human tissues causing health problems.
The introduction of GM species into ecosystems may also add novel selection pressure to the environment. GM crop species have out-competed their wild-type equivalents , or transferred their genes to them via hybridization, leading to a loss of biodiversity.
4. Genetic engineering is expensive
Genetic engineering is extremely expensive because of its experimental nature. Gene editing uses some of the world’s most expensive laboratory equipment , reagents, and technical expertise. Genetic engineering routinely takes years of investment before scientists develop commercially viable and safe applications.
This is one of the key limitations of even the most promising gene therapies. Even when a technique is found to work on patients, the costs are so high that few people can access them. Running clinical trials is also very expensive meaning many gene therapies do not obtain the funding to be developed properly.
5. Genetic engineering can lead to unregulated gene expression
Genetic engineering has mastered the technique of inserting a transgene into a host species’ genome, but regulation of the expression of the gene is much more sophisticated. This means that the transgenic organism may over or under-produce the protein product of the inserted gene.
Under-expression of an inserted gene may mean that scientists don’t achieve the desired effect of genetic modification, while over-expression may be harmful to the GMO and animals or people that consume it. In transgenic swine , overexpression of an inserted growth hormone gene resulted in an enlargement of the heart, arthritis, abnormal skeletal growth, and renal disease.
6. Genetic engineering can introduce insertional mutations
By breaking and annealing the ends of DNA with new gene frequencies, geneticists can introduce errors and mutations in DNA that produce disease in the host organism. Insertional mutations may affect the genes that control essential biological processes producing metabolic diseases and cancers.
Worse still, these mutations may be transmissible to subsequent generations, creating new diseases that could be introduced to wild-type organisms through hybridization or mating.
7. Transgene mosaicism or sex linkage may mean that not all offspring carry an inserted gene
Some gene-editing processes are failures, because the GMO does not transmit the inserted gene to all of its offspring.
This is usually because of mosaicism, where only some cells of the organism carry the inserted gene, making its expression inconsistent. Alternatively, in animals or humans, the inserted gene may become sex-chromosome linked (Y-chromosome) so that only males carry the transgene.
8. Genetic engineering requires time and resource-consuming planning to be successful
Genetic engineering projects have to be carefully designed and constructed to achieve the successful insertion of a genetic sequence into a host organism. The high failure rate of these experiments is often because of errors in the inserted gene or its integration site.
Scientists therefore repeatedly model and test the transgene and its insertion site, which can use significant resources and may risk the health and welfare of host organisms.
9. Rogue agents could use genetic engineering for biological warfare or terrorism
Genetic engineering is already being used for controversial ‘gain-of-function’ research, where the genomes of bacteria and viruses are edited to increase their transmissibility or virulence. This research has been taking place in academic settings with the purported objective of investigating pathogens to prevent them from starting pandemics.
Genetic engineering is largely unregulated, with restrictions on access to this technology varying widely between countries. It is not inconceivable that rogue states or nefarious organizations could procure the equipment and expertise to develop bacteria, viruses, or parasites that could infect targeted populations.
10. Genetic engineering could be used for eugenics
Eugenics is an ideology that believes that human reproduction can be controlled to produce populations with pre-determined desirable traits and eliminate individuals with undesirable characteristics. It is a form of scientific racism and has been associated with atrocities and genocides throughout history.
Many advocates of genetic engineering of human beings, point to its potential to ‘improve’ the human race by eradicating genetic abnormalities and introducing desirable traits. Unfortunately, small but powerful groups with little accountability to the wider population may decide what desirable or undesirable hereditable traits are, irrespective of the implications of this course of action.
In the wrong hands, genetic engineering could pursue a eugenics agenda, focused on specific racial groups or populations of people.
11. Genetic engineering enables genes and organisms to be copyrighted
Genetic engineering is a new and fast-evolving area of science and the ethical and legal frameworks that surround it are relatively immature. A key example of this is the patenting of genes and organisms developed through genetic engineering, with companies enforcing ownership of seeds and animal breeds they have developed.
In many jurisdictions, alteration of the genome of an organism makes it a patented product and subject to copyright law. In India, this has been a hotly contested issue, with Monsanto recently winning a Supreme Court case regarding the patents of its GMO cotton seeds.

12. Significant ethical, cultural, and religious concerns surround the use of genetic engineering
Despite its advances and benefits, genetic engineering continues to face deep public antipathy. Many people believe that interfering with genes that are passed from generation to generation is taboo. Even with comprehension of the science and its benefits, they believe that altering living things at a genetic level will harm them.
Religious groups who believe that God created the Earth, perceive generic engineering as a defilement of the Creator’s intelligent design.
With few consistent legal or regulatory boundaries for genetic engineering, ethicists join them in the concern that most people have no say over introducing GMOs into the environment and the genetic engineering of human beings.
In conclusion
As you can see the pros and cons of genetic engineering are a weighty matter, certainly worthy of further investigation. This issue is not only about science but touches on human, animal, and environmental health and welfare ongoing. This means that the evaluation of the societal benefit of genetic engineering should be rigorous with high ethical standards and stringent regulation.
Articles you might also like

Depletion of Natural Resources: 11 Natural Resources That Show the Scale of the Problem

Waste Incineration Pros and Cons: is It a Good Idea?

Ocean Acidification: Solutions for the Most Serious Problem in Our Seas

The Most Effective Natural Bee Repellent

Carbon Cycle Steps: What is the Carbon Cycle and How Does It Work

What Are the Causes and Effects of Land Pollution and How Can We Prevent It?

The Top Environmental Issues in Japan and What They’re Doing to Address Them

5 of the Critical Water Scarcity Solutions Addressing the Water Crisis

35 Ways to Reduce Air Pollution and Boost Air Quality for All

What is Groundwater Pollution, and How Can it be Prevented?

Causes and Effects of Soil Degradation, Plus How to Protect Our Precious Earth
- OCR Gateway
Feeding the human race - OCR Gateway Potential benefits and risks of genetic engineering
Factors such as the increase in population, new pathogens and overhunting can result in food scarcity. Improved farming techniques, sustainable fisheries and biotechnology can help increase supply.
Part of Biology (Single Science) Global challenges
Potential benefits and risks of genetic engineering
There are many benefits to using genetic engineering close genetic engineering Process which involves the artificial transfer of genetic information from one donor cell or organism to another. . It is used in agriculture to do things such as, improve the yields close yield The mass of a crop produced. of important economic crops, and provide insect or pest resistance. It is also used in the medical field to create insulin, which can be used for treating diabetes close diabetes A serious disease in which the body is unable to regulate blood sugar. . But, as with most new technology, it also carries potential risks.
Benefits of genetic engineering:
- Genetic modification is a faster and more efficient way of getting the same results as selective breeding.
- Improve crop yields or crop quality, which is important in developing countries. This may help reduce hunger around the world.
- Introduce herbicide resistance, which results in less herbicides being used, as weeds are quickly and selectively killed.
- Insect and pest resistance can be developed and inserted into the plants. The plant produces toxins, which would discourage insects from eating the crop.
- Sterile insects could be created such as a mosquito. They would breed, which would lead to infertile offspring. This may help reduce the incidence and spread of diseases, such as malaria, dengue fever and the Zika virus.
Risks of genetic engineering:
- Transfer of the selected gene into other species. What benefits one plant may harm another.
- Some people believe it is not ethical to interfere with nature in this way. Also, GM crop seeds are often more expensive and so people in developing countries cannot afford them.
- GM crops could be harmful, for example toxins from the crops have been detected in some people's blood.
- GM crops could cause allergic reactions in people.
- Pollen produced by the plants could be toxic and harm insects that transfer it between plants.
Examples - Golden rice and herbicide resistance
Scientists have worked to develop genetically modified organisms that help meet the demand for food.
Examples of this include:
- golden rice that produce extra beta carotene that is used to make Vitamin A
- herbicide resistant crop plants
Golden rice
Scientists have added a gene to wild rice that makes it produce beta carotene. This changes the colour of the wild rice to a golden colour. Beta carotene is needed by humans in order to make Vitamin A. The advantage of golden rice is that it can be used in areas where Vitamin A deficiency is common and so can help prevent blindness. Some of the disadvantages of golden rice are:
- fears that it will crossbreed with and contaminate wild rice
- worries that GM organisms might harm people
- beta carotene levels aren't high enough to make a difference
- GM organisms can be expensive
Herbicide resistant crops
Scientists have added genes to crop plants that make them resistant to herbicides. Farmers can spray the entire field with herbicide and only the weeds will die. This reduces the quantity of herbicide that needs to be used. Potential disadvantages of this genetic modification include:
- the potential development of herbicide-resistant weeds
- loss of biodiversity as fewer weed species survive as a food and shelter source for animals
More guides on this topic
- Field investigations - OCR Gateway
- Monitoring and maintaining the environment - OCR Gateway
- QUIZ: Biodiversity and the effect of human interaction on ecosystems
- QUIZ: Food production
- Monitoring & maintaining health - Communicable diseases - OCR Gateway
- QUIZ: Communicable diseases activity 1
- QUIZ: Communicable diseases: prevention
- Treating, curing and preventing disease - OCR Gateway
- QUIZ: Vaccinations and antibiotics
- QUIZ: Bacterial growth and drug discovery
- Monoclonal antibodies - Higher - OCR Gateway
- Plant disease - OCR Gateway
- QUIZ: Plant disease
- Cancer and cardiovascular disease - Non-communicable - OCR Gateway
- Monitoring and maintaining health - Non-communicable - OCR Gateway
- QUIZ: Non-communicable diseases activity 1
- QUIZ: Non-communicable diseases: data analysis
- Sample exam questions - global challenges - OCR Gateway
Related links
- Biology: Exam-style questions
- Bitesize revision podcasts
- Personalise your Bitesize!
- Jobs that use Biology
- Save My Exams Subscription
- Tassomai Subscription
- Headsqueeze
- Revision Buddies Subscription
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Asian Bioeth Rev
- v.10(2); 2018 Jul
Risks and benefits of human germline genome editing: An ethical analysis
Giovanni rubeis.
Institute of the History, Philosophy and Ethics of Medicine, Ulm University, Parkstr. 11, 89073 Ulm, Germany
Florian Steger
With the arrival of new methods of genome editing, especially CRISPR/Cas 9, new perspectives on germline interventions have arisen. Supporters of germ line genome editing (GGE) claim that the procedure could be used as a means of disease prevention. As a possible life-saving therapy, it provides benefits that outweigh its risks. Opponents of GGE claim that the medical and societal risks, especially the use of GGE for genetic enhancement, are too high. In our paper, we analyze the risks and benefits of GGE. We show that the medical risk on an individual level might be reduced by further research in the near future so that they may be outweighed by the benefits. We also show that the societal risks of the procedure, i.e. genetic enhancement, are manageable by establishing a regulative framework before the GGE is implemented. Since the effects of modifying genes for the genepool of a given population are extremely difficult to model, the medical risks on the population level might be too high.
Introduction
For decades, potential interventions in the human germline for clinical purposes have been regarded as ethically impermissible. Recently, this strict view has come under review. With the arrival of new methods of genome editing, especially CRISPR/Cas 9, new perspectives on germline interventions have arisen. In their report Human Genome Editing: Science, Ethics, and Governance, the American National Academies of Science, Engineering, and Medicine have stated that clinical research using germline genome editing (GGE) in humans should be permitted (The National Academies 2017 ). In the long run, this may lead to the development of clinical applications. The Academies propose to limit GGE to severe cases of disease and disability where no alternative treatment is possible. It follows that although there are certain contexts where GGE could be used in a beneficial way, it should be used within narrow limits and with caution. GGE is mainly discussed in the context of disease prevention and infertility treatment (Ishii 2017a , b ; Long et al. 2014 ). Disease prevention is widely seen as a benefit that outweighs severe risks connected to GGE. Some even claim the preventative use of GGE to be a moral imperative; its application is an obligation for the sake of patients and future generations (Gyngell et al. 2017 ). In addition, GGE provides new perspectives for infertility patients who have no other option to create offspring that are genetically related to them. But there are also those who state that the medical as well as societal risks are too high, and argue, accordingly, for a full ban (Lanphier et al. 2015 ). Although there are no clinical applications of GGE available at the moment, it is important to have an intense ethical debate at this early stage in order to be prepared for coming developments, given the speed at which research efforts are now being conducted. A future clinical implementation of GGE needs an ethical framing which provides guidelines for clinicians.
The aim of our paper is to analyze the ethical implications of editing the human germline by using new procedures of genome editing. Editing somatic cells as an application of gene editing technology, and its ethical implications, is not the focus of our analysis. We discuss GGE as a possible clinical application, not as a research technique. Specifically, we attempt to provide an analysis of the risks and benefits that could arise from such an application. Weighing the risks and benefits of new technologies is important, but it is only one aspect of assessing technologies. We do not claim that our analysis is exhaustive or final; broader ethical analysis still needs to be done in order to thoroughly evaluate GGE. Although we focus on risks and benefits, many other aspects need to be taken into consideration, e.g. questions of reproductive autonomy or access to the procedure. Furthermore, questions of research ethics arise, as well as questions concerning regulations of the translational process. We addressed some of these questions elsewhere (Rubeis and Steger 2016 ). In this paper, we seek to produce a sound ethical evaluation of GGE in terms of its potential risks and benefits. The focus of our analysis is on this question: whether the benefits of GGE really outweigh the risks that are usually ascribed to a germline intervention? Apart from medical risks on the individual level like off-target mutations and genetic mosaicism, and medical risks on the population level, there are also societal risks like genetic enhancement. That means that the nature, aim, and risks of the possible applications have to be clarified. It has to be clear who benefits from the method and whether this benefit justifies the risks of its application.
Possible applications of GGE
In August 2017, the first therapeutic germline intervention using CRISPR/Cas9 was reported (Ma et al. 2017 ). A team from Oregon Health & Science University created zygotes by fertilizing healthy oocytes with sperm cells from a carrier of the MYBPC3 mutation. This mutation leads to hypertrophic cardiomyopathy, a heritable heart condition. By using CRISPR/Cas9, the team then corrected the genetic defect in the zygotes which led to the development of viable embryos. The majority of these embryos was mutation-free. Since the intervention in the germline did occur for research purposes, the embryos were not implanted in utero. This research shows that correcting a gene mutation in viable human embryos using genome editing methods is feasible. It confirms results from earlier research on human embryos by two Chinese research groups in 2015 and 2016 (Kang et al. 2016 ; Liang et al. 2015 ) and a team at the Francis-Crick-Institute in London (Callaway 2016 ).
There are various different applications of genome editing in germline therapy, especially by using CRISPR-based methods (Ishii 2017a , b ). All elements of the germline, oocytes, sperm cells, and embryos, can be edited. One possibility is the editing of oocytes. The method could be used to correct the mutation in the TUBB8-gene which is known to cause developmental arrest after fertilization. The method can be applied to the oocyte after retrieval. The edited and verified oocyte could then be used for an IVF. Furthermore, sperm cells can be edited through spermatogonial stem cell (SSC) editing. This procedure could be used for treating genetic infertility. By conducting a testicular biopsy, the SSCs can be retrieved and transfected with programmable nucleases. After genetic analysis and verification, the SSCs can be transferred back into the donor’s testes. The edited SSCs then trigger the production of mutation-free sperm cells. In order to ensure reproductive success, the resulting sperm cells could be used for in-vitro fertilization (IVF) or intra-cytoplasmic sperm injection (ICSI). Apart from sperm cells and oocytes, genome editing can be applied to zygotes, i.e. fertilized oocytes. This could be a therapeutic option for several monogenetic diseases such as Huntington’s disease or β-thalassemia. After fertilization through IVF or ICSI, the zygote can be microinjected with programmable nucleases. The resulting embryo could be tested by using preimplantation genetic diagnosis (PGD) to ensure that there are no off-target-effects. For the PGD, a blastomere biopsy three days post-fertilization or a trophectoderm biopsy four to five days post-fertilization could be used. After testing and verification, the embryo can be transferred in utero.
There are two possible applications discussed at the moment. The first possible application is infertility treatment. Oocyte editing and spermatogonial editing could be an option for patients suffering from genetic infertility. The second possible application is disease prevention. Also in this context, the editing of oocytes or sperm cells could be a possible application. Additionally, the editing of zygotes may be a way of correcting genetic errors that are likely to cause health problems later on.
The first studies using GGE on human embryos showed that there are severe medical risks (Liang et al. 2015 ; Kang et al. 2016 ). These risks are mainly due to off-target effects. Off-target effects occur when DNA double strand breaks are made at the wrong target site. The result is an inaccurate or incomplete editing, causing improper translocations, inversions or large deletions which can lead to point mutations (Ishii 2017b ). The latest research shows that off-target effects in human embryos, although still existing, can be minimized (Ma et al. 2017 ). Also, the occurrence of genetic mosaicism could be diminished to a minimum. Genetic mosaicism is the coexistence of edited cells and wild types, a condition that may lead to severe health conditions (Ishii 2017b ). Due to the multifactorial nature of the processes involved, the exact consequences of genetic mosaicism are hard to predict. However, clinical research is only now beginning. A lot of translational research is needed before GGE can be implemented in clinical practice. Although the latest results mean an enormous step forward, there is still a long way to go before these methods are safe enough for clinical application. Apart from the risks for the embryo and future child that is directly affected by GGE, there is another level of risks to consider. Genetic modifications of the human germ-line will be passed on to coming generations. This means that an off-target effect does not only affect one individual, but possibly many future individuals. How exactly off-target effects and genetic mosaicism would manifest in future generations, is almost impossible to predict which makes this risk incalculable. Therefore, a proper risk-assessment has to include coming generations as well.
Risks and benefits of GGE
At the moment, the empirical evidence shows that any clinical application would be too unsafe (Ishii 2017b ; Ma et al. 2017 ). However, with research efforts speeding up, clinical applications might be safe enough in the near future. Ethical evaluation of further implementation of these initiatives, as well as safety concerns in individual cases, is needed. One of these concerns is the effect of GGE on the population level (The National Academies 2017 ). Since germ cells are altered, the modified genes could be passed on to future generations (Ormond et al. 2017 ). Thus, the modified genes could spread within the human gene pool with yet unforeseeable consequences. For example, the genetic trait for sickle-cell anaemia also protects its carrier against malaria. Similarly, there may be as yet unknown positive effects to genes ordinarily considered pathogenic. On the other hand, modified genes which appear not to affect its carrier may turn out to be pathogenic over time. This aggravates safety concerns, since not only one individual, but many individuals or whole populations might be affected by possible pathogenic effects of the modified genes. Due to the complexity of gene frequency and microevolution, it is impossible to manage or even predict the impact of modified genes in future generations.
Another topic in the discussion is the specific way in which the human genome is altered through GGE. Some state that GGE implies a new level of interfering with nature that is to be considered as irresponsible. Therefore, the procedure crosses a line that should not be crossed (Lanphier et al. 2015 ). Others claim that the risk of misuse is too high (Baltimore et al. 2015 , Hildt 2016 ). Apart from disease prevention or infertility treatment, GGE could be used without any medical indication for enhancement purposes. Genetic enhancement is seen by many as societal risk because it could lead to the creation of two classes of humans, the enhanced and the non-enhanced. This would challenge the very ideas of justice and equality which are crucial to modern society. But there are also those that consider the implementation of GGE as ethically justified. Supporters of GGE see benefits of the method in two areas, disease prevention and infertility treatment (Gyngell et al. 2017 ; Savulescu et al. 2015 ).
As we have seen, GGE could be used in order to prevent hereditary monogenic disease like Huntington’s or β-thalassemia. As in the case of the carrier of the MYBPC3 mutation mentioned above, GGE would allow individuals with certain hereditary diseases to reproduce without passing on the disease. This would mean an immense benefit for individuals with a known genetic risk who want to have children. They could fulfil their wish to have children without the risk of severe health conditions or ailments. So far, couples with genetic risk depend on embryo selection, gamete donation, or adoption. Embryo selection, mostly through PGD, can be applied in certain cases to ensure that a certain genetic trait is not passed on. The method is elaborate, costly and often stressful for patients. It also implies the discarding of embryos with unwanted traits which is ethically questionable. Also, there are cases of dominant late-onset conditions like Huntington’s where a selection of embryos by using PGD is not possible. Thus, GGE could be used to prevent offspring from inheriting pathogenic genes.
Apart from disease prevention, GGE is also presented as a potential infertility treatment. One possible application is non-obstructive azoospermia (NOA) in male patients (Vij et al. 2018 ). For female patients with a ’missense mutation’ in the TUBB8 in oocytes, GGE could also be an option (Ishii 2017b ). So far, the only option for these patients to create offspring is through gamete donation. When it comes to gamete donation, legal restraints have to be considered apart from the effort and ethical implications. Since gamete donation is legally prohibited in many countries, the treatment is not always available. It furthermore implies that that the resulting child is not genetically related to both of the partners that apply the method. The fact that the genetic material of another individual is used conflicts with the wish for a child that is genetically related to both partners. Also, when it comes to adoption, this issue is to be considered. The wish for a child implies a child that is genetically related to both partners for most couples. Genetic relatedness, however, cannot be provided by adoption. GGE could be a viable alternative to PGD, gamete donation, and adoption. It circumvents the ethical and legal implications of these options by making a healthy genetically-related child a possibility for potential parents. As Gyngell et al. ( 2017 ) claim, for some reproduction partners GGE would be the only option to create offspring that is genetically related to both. It is, however, contestable that genetic relatedness is a strong argument for allowing genetic modifications (Baylis 2013 ).
Following the supporters of GGE, editing the germline would also provide benefits on a population level (Powell 2015 ). Correcting a pathogenic genetic mutation in the germ cells of an individual patient means that the genetic errors disappear from the germline. Thus, the mutation is prevented from spreading within the gene pool of a given population. Disease prevention would benefit future generations, in addition to the individual affected by the application of GGE. This public health benefit has led to the call for a genome-wise program of GGE (Powell 2015 ). And this argument suggests that we are morally obliged to improve health outcomes for future generations. In addition, the implementation of a large-scale program would lead to an eventual decrease in healthcare costs. Taken in its totality, supporters of GGE claim that in the light of its public health benefits, this pursuit should be regarded as a moral imperative (Gyngell et al. 2017 ).
In order to decide whether the expected benefits of GGE outweigh the risks, we have to analyze the arguments brought forward by opponents and supporters of the method. The argument that implementing GGE means interfering with nature and therefore crossing a line that should not be crossed is not well-founded, since modern medicine implies interfering with nature on many levels already. As long as security standards are followed, there is no reason why interfering with nature for medical purposes should be prohibited in this specific context when it is accepted and well-established in others. Apart from medical risks, which clearly have to be diminished through further research, opponents of GGE also identify societal risks. The main societal risk is seen in the possible misuse of GGE for non-medically indicated purposes such as genetic enhancement. There is an ongoing debate whether genetic enhancement is ethically acceptable. This an issue that surely needs a focused analysis that cannot be given here. Apart from the question, whether we should accept genetic enhancement as such, there is the argument that neither our societies nor our legal frameworks are prepared for its impact (Lanphier et al. 2015 ). However, we are already taking precautions against the enhancing use of reproductive technologies. When it comes to PGD for example, there are legal restrictions in place in most countries that prohibit any non-medically indicated use. An analogous legal framework for GGE could be created. This can be considered as a practical argument following which the possibility of genetic enhancement is not a strong argument against GGE because it can be regulated when needed. Rules and regulations could be implemented in order to guarantee that GGE is only used in medically indicated cases. The differences between the cases do not only result from the nature of the procedure applied, but also from the moral status of the agent involved. Whether a medical intervention is to be considered as treatment or enhancement depends on the specific circumstances of the case. Each case where GGE is applied would have to be evaluated by an ethics committee, since there is no overall criterion to separate treatment from enhancement. This would have to be a case-to-case decision. Thus, the argument following which the risk of misuse of GGE is too high has to be considered as non-convincing.
When considering the arguments in favor of GGE, we find that main benefit of GGE is seen in disease prevention for monogenic diseases (Gyngell et al. 2017 ; Powell 2015 ; Savulescu and Gyngell 2015 ). The argument implies that by using GGE, a future individual can be prevented from having a monogenic disease. This notion is valid with some restrictions. It is unproblematic as long as we deal with editing sperm cells or oocyte. In these cases, the pathogenic genes are eliminated or modified, so that they will not be transferred to the future individual. Thus, the future individual is prevented from inheriting the genetic defect. The matter is more complex, however, when we deal with zygotes. The edited zygote is only created because of the availability of GGE. The fact that GGE is available is the only reason a couple with pathogenic traits decides to create a zygote that is then immediately edited, thus producing an embryo without the pathogenic genetic trait. In other words, a zygote with a genetic defect is knowingly created in order to be edited at once. By stretching the boundaries of the term, this therapeutic procedure may also have the effect of disease prevention, particularly at the population level. The challenge here is the status of the zygote. If one considers the zygote as an individual, the term therapy may apply. If one considers the zygote as eukaryotic, diploid cell, it is unproblematic to call GGE disease prevention, since, analogous to sperm cell and oocyte-editing, a future individual is prevented from inheriting a monogenic disease. Given this definition, the term therapy would be misleading. The term therapy could only be used if an in vivo application of GGE was possible. Imagine the case where a life-threatening genetic defect is detected in an embryo in the womb by using prenatal genetic diagnosis. If GGE was applied here, given the technical feasibility which is unavailable at the moment, then we would deal with a life-saving therapy. Another possibility would be to classify GGE as an elaborate procedure of ART which is to fulfil one’s wish to have a child. Both reproduction partners know about their genetic conditions. They know that if they reproduce, their offspring could inherit a pathogenic trait. Instead of using alternatives like gamete donation or adoption, they decide to create a zygote with the pathogenic trait that is altered afterwards. That means that there was no immediate need for intervening in the genome in the first place. These terminological issues may seem purely academic, but they may shape the development of clinical practice. Given a publicly funded healthcare system, it is to be expected that the question, whether or not GGE should be covered by the public health scheme, will arise. Usually, methods of disease prevention and therapeutic measures are covered by the public health scheme, whereas not all procedures of ART are financed by the public. In this regard, it may become important to clarify the status of GGE.
As noted, GGE could also be used as a means of disease prevention on a population level. However, it is doubtful how a large-scale use of GGE should be implemented without coercive measures. In order to be thoroughly effective, GGE may have to be mandatory for all carriers of certain genetic traits who want to reproduce. That such a coercive, if not eugenic, measure would be implemented seems rather unlikely. Therefore, disease prevention on a population level may at most be considered as a side effect of GGE.
Do the benefits outweigh the risks?
It follows from our analysis that we can identify three types of risks: Firstly, there is a medical risk for the child that is created through GGE. Off-target effects and mosaicism are the crucial risk factors here. These risks are still prevalent despite recent refinement of GGE procedures. We are usually willing to take certain risks when a new method can be used to treat or prevent diseases that have so far been untreatable. As we have seen, the National Academies in the United States have suggested translational research on GGE which aims at a preventive use in cases of severe disease or disability where no alternative methods exist (The National Academies 2017 ). If we consider GGE as a possible means of disease prevention (notwithstanding the terminological difficulties outlined above), there are certain cases that fulfill these conditions, i.e. monogenic diseases like Huntington’s. Since these conditions are usually severe and since there are no treatment alternatives, translational research efforts are justified. The same holds for infertility treatment. GGE is a promising method that allows couples with a known genetic risk to create healthy offspring that is genetically related to both partners. It can be used as an infertility treatment in cases where a genetic defect leads to infertile oocytes or sperm cells. As an assisted reproduction technology, GGE is certainly an alternative to available methods. It renders gamete donation obsolete and allows infertile partners to have a child that is genetically related to both of them. Regarding both disease prevention and infertility treatment, the main goal of the translational research will have to be the reduction of off-target effects and genetic mosaicism. However, it will be difficult to decide when an acceptable level of risk is reached. Whether the benefits outweigh the risks in this regard, however, depends on the outcome of future translational research.
Secondly, there is a medical risk for future generations. Since interventions in the germline mean that the modified genetic trait is passed on, errors in editing may have negative effects on future individuals. It is extremely difficult to predict which consequences genetic mosaicism for example will generate in one individual. It is even harder, if not impossible to foresee the effects in two or three generations. It is doubtful, whether further research will be useful here since effects on whole populations and in future generations are very difficult, if not impossible to model. However, if we consider that only a few individuals will use GGE, the risks at the level of the population must be put into perspective. The number of GGE-treatments might be small enough as to have no effect on the genome of a whole population (The National Academies 2017 ).
Thirdly, there are societal risks. GGE could be used for non-medically indicated purposes, first and foremost genetic enhancement. The majority considers genetic enhancement as a societal hazard because it may compromise social justice and equality. However, this slippery-slope-argument suggests that the non-medically indicated use of GGE is a necessary consequence of its clinical implementation. This does not have to be the case since it is possible to create guidelines and regulations before GGE is implemented. This implies an intense ethical, legal, and public debate. Since the societal risks are manageable, they do not outweigh the benefits.
GGE offers promising possibilities for disease prevention and infertility treatment. The technique has been improved immensely in recent years and its medical risks have diminished. GGE might therefore be safe enough to be implemented in the clinic in the near future. Societal risk can easily be managed by implementing a regulative framework that limits GGE to medically-indicated uses. However, the effects of modified genes within the gene pool of a given population are unforeseeable and uncontrollable. It is almost impossible to predict which impact these modifications will have in future generations. When it comes to weighing risks and benefits, the question of scale is an important point. Given the treatment of one individual, we have sufficient data to assess the risks, e.g. for off-target mutations. This assessment becomes more difficult on a societal level, but is still possible, as long as a broad public debate takes place. But it is hard to see how such an assessment should be possible when the data simply cannot be retrieved sufficiently. Since the number of cases where GGE is applied will be small, there might not be an effect at all, but it is hard to say at the moment. Maybe future research will provide the means of calculating the effects of modified genes on a population level sufficiently. As long as this is not the case, the wish of few individuals to have a genetically related child does not outweigh the risks for a whole population.
Contributor Information
Giovanni Rubeis, Email: [email protected] .
Florian Steger, Email: [email protected] .
- Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, Corn JE, Daley GQ, Doudna JA, Fenner M, Greely HT, Jinek M, Martin GS, Penhoet E, Puck J, Stemberg SH, Weissman JS, Yamamoto KR. A prudent path forward for genomic engineering and germline gene modification. Science. 2015; 348 :36–38. doi: 10.1126/science.aab1028. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baylis F. The ethics of creating children with three genetic parents. Reproductive Biomedicine Online. 2013; 26 :531–534. doi: 10.1016/j.rbmo.2013.03.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Callaway, E. 2016. UK scientists gain licence to edit genes in human embryos. Nature 530. 10.1038/nature.2016.19270. [ PubMed ]
- Gyngell C, Douglas T, Savulescu J. The ethics of germline gene editing. Journal of Applied Philosophy. 2017; 34 :498–513. doi: 10.1111/japp.12249. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hildt, E. 2016. Human germline interventions–think first. Frontiers in Genetics 7. 10.3389/fgene.2016.00081. [ PMC free article ] [ PubMed ]
- Ishii T. Germ line genome editing in clinics: the approaches, objectives and global society. Briefings in Functional Genomics. 2017; 16 :46–56. doi: 10.1093/bfgp/elv053. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ishii T. Reproductive medicine involving genome editing: clinical uncertainties and embryological needs. Reproductive Biomedicine Online. 2017; 34 :27–31. doi: 10.1016/j.rbmo.2016.09.009. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kang X, He W, Huang Y, Yu Q, Chen Y, Gao X, Sun X, Fan Y. Introducing precise genetic modifications into human 3PN-embryos by CRISPR/Cas-mediated genome editing. Journal of Assisted Reproduction and Genetics. 2016; 33 :581–588. doi: 10.1007/s10815-016-0710-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don’t edit the human germ line. Nature. 2015; 519 :410–411. doi: 10.1038/519410a. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lu J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Zhou S, Ma W, Zhou C, Huang J. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & Cell. 2015; 6 :363–372. doi: 10.1007/s13238-015-0153-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014; 345 :1184–1188. doi: 10.1126/science.1254445. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ma H, Marti-Gutierrez N, S-W Park JW, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, Darby H, Van Dyken C, Li Y, Kang E, Park A-R, Kim D, Kim S-T, Gong J, Gu Y, Xu X, Battaglia D, Krieg SA, Lee DM, Wu DH, Wolf DP, Heitner SB, Izpisua Belmonte JC, Amato P, Kim J-S, Kaul S, Mitalipov S. Correction of a pathogenic gene mutation in human embryos. Nature. 2017; 548 :413–419. doi: 10.1038/nature23305. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ormond KE, Mortlock DP, Scholes DT, Bombard Y, Brody LC, Faucett WA, Garrison NA, Hercher L, Isassi R, Middleton A, Musunuru K, Shriner D, Virani A, Young CE. Human Germline Genome Editing. American Journal of Human Genetics. 2017; 101 :167–176. doi: 10.1016/j.ajhg.2017.06.012. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Powell, R. 2015. In genes we trust: Germline engineering, eugenics, and the future of the human genome. Journal of Medicine and Philosophy 40:669–695. 10.1093/jmp/jhv025. [ PubMed ]
- Rubeis G, Steger F. Genome Editing in der Pränatalmedizin. Eine medizinethische Analyse. In: Byrd SB, Hruschka J, Joerden JC, editors. Jahrbuch für Recht und Ethik - Annual Review of Law and Ethics . Berlin: Duncker & Humblot; 2016. pp. 143–159. [ Google Scholar ]
- Savulescu J, Gyngell C. The medical case for gene editing. Ethics in Biology, Engineering and Medicine. 2015; 6 :57–66. doi: 10.1615/EthicsBiologyEngMed.2015014314. [ CrossRef ] [ Google Scholar ]
- Savulescu J, Pugh J, Douglas T, Gyngell C. The moral imperative to continue gene editing research on human embryos. Protein & Cell. 2015; 6 :476–479. doi: 10.1007/s13238-015-0184-y. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- The National Academies of Sciences, Engineering, and Medicine. 2017. Human genome editing: science, ethics, and governance . 10.17226/24623. [ PubMed ]
- Vij SC, Sabanegh E, Jr, Ashok A. Biological therapy for non-obstructive azoospermia. Expert Opinion on Biological Therapy. 2018; 18 :19–23. doi: 10.1080/14712598.2018.1380622. [ PubMed ] [ CrossRef ] [ Google Scholar ]

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

14.4: Genetic Engineering - Risks, Benefits, and Perceptions
- Last updated
- Save as PDF
- Page ID 77491

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Summarize the mechanisms, risks, and potential benefits of gene therapy
- Identify ethical issues involving gene therapy and the regulatory agencies that provide oversight for clinical trials
- Compare somatic-cell and germ-line gene therapy
Many types of genetic engineering have yielded clear benefits with few apparent risks. Few would question, for example, the value of our now abundant supply of human insulin produced by genetically engineered bacteria. However, many emerging applications of genetic engineering are much more controversial, often because their potential benefits are pitted against significant risks, real or perceived. This is certainly the case for gene therapy, a clinical application of genetic engineering that may one day provide a cure for many diseases but is still largely an experimental approach to treatment.
Mechanisms and Risks of Gene Therapy
Human diseases that result from genetic mutations are often difficult to treat with drugs or other traditional forms of therapy because the signs and symptoms of disease result from abnormalities in a patient’s genome. For example, a patient may have a genetic mutation that prevents the expression of a specific protein required for the normal function of a particular cell type. This is the case in patients with Severe Combined Immunodeficiency (SCID), a genetic disease that impairs the function of certain white blood cells essential to the immune system.
Gene therapy attempts to correct genetic abnormalities by introducing a nonmutated, functional gene into the patient’s genome. The nonmutated gene encodes a functional protein that the patient would otherwise be unable to produce. Viral vectors such as adenovirus are sometimes used to introduce the functional gene; part of the viral genome is removed and replaced with the desired gene (Figure \(\PageIndex{1}\)). More advanced forms of gene therapy attempt to correct the mutation at the original site in the genome, such as is the case with treatment of SCID.

So far, gene therapies have proven relatively ineffective, with the possible exceptions of treatments for cystic fibrosisand adenosine deaminase deficiency, a type of SCID. Other trials have shown the clear hazards of attempting genetic manipulation in complex multicellular organisms like humans. In some patients, the use of an adenovirus vector can trigger an unanticipated inflammatory response from the immune system, which may lead to organ failure. Moreover, because viruses can often target multiple cell types, the virus vector may infect cells not targeted for the therapy, damaging these other cells and possibly leading to illnesses such as cancer. Another potential risk is that the modified virus could revert to being infectious and cause disease in the patient. Lastly, there is a risk that the inserted gene could unintentionally inactivate another important gene in the patient’s genome, disrupting normal cell cycling and possibly leading to tumor formation and cancer. Because gene therapy involves so many risks, candidates for gene therapy need to be fully informed of these risks before providing informed consent to undergo the therapy.
Gene Therapy Gone Wrong
The risks of gene therapy were realized in the 1999 case of Jesse Gelsinger, an 18-year-old patient who received gene therapy as part of a clinical trial at the University of Pennsylvania. Jesse received gene therapy for a condition called ornithine transcarbamylase (OTC) deficiency, which leads to ammonia accumulation in the blood due to deficient ammonia processing. Four days after the treatment, Jesse died after a massive immune response to the adenovirus vector. 1
Until that point, researchers had not really considered an immune response to the vector to be a legitimate risk, but on investigation, it appears that the researchers had some evidence suggesting that this was a possible outcome. Prior to Jesse’s treatment, several other human patients had suffered side effects of the treatment, and three monkeys used in a trial had died as a result of inflammation and clotting disorders. Despite this information, it appears that neither Jesse nor his family were made aware of these outcomes when they consented to the therapy. Jesse’s death was the first patient death due to a gene therapy treatment and resulted in the immediate halting of the clinical trial in which he was involved, the subsequent halting of all other gene therapy trials at the University of Pennsylvania, and the investigation of all other gene therapy trials in the United States. As a result, the regulation and oversight of gene therapy overall was reexamined, resulting in new regulatory protocols that are still in place today.
Exercise \(\PageIndex{1}\)
- Explain how gene therapy works in theory.
- Identify some risks of gene therapy.
Oversight of Gene Therapy
Presently, there is significant oversight of gene therapy clinical trials. At the federal level, three agencies regulate gene therapy in parallel: the Food and Drug Administration (FDA), the Office of Human Research Protection (OHRP), and the Recombinant DNA Advisory Committee (RAC) at the National Institutes of Health (NIH). Along with several local agencies, these federal agencies interact with the institutional review board to ensure that protocols are in place to protect patient safety during clinical trials. Compliance with these protocols is enforced mostly on the local level in cooperation with the federal agencies. Gene therapies are currently under the most extensive federal and local review compared to other types of therapies, which are more typically only under the review of the FDA. Some researchers believe that these extensive regulations actually inhibit progress in gene therapy research. In 2013, the Institute of Medicine (now the National Academy of Medicine) called upon the NIH to relax its review of gene therapy trials in most cases. 2 However, ensuring patient safety continues to be of utmost concern.
Ethical Concerns
Beyond the health risks of gene therapy, the ability to genetically modify humans poses a number of ethical issues related to the limits of such “therapy.” While current research is focused on gene therapy for genetic diseases, scientists might one day apply these methods to manipulate other genetic traits not perceived as desirable. This raises questions such as:
Exercise \(\PageIndex{2}\)
- Which genetic traits are worthy of being “corrected”?
- Should gene therapy be used for cosmetic reasons or to enhance human abilities?
- Should genetic manipulation be used to impart desirable traits to the unborn?
- Is everyone entitled to gene therapy, or could the cost of gene therapy create new forms of social inequality?
- Who should be responsible for regulating and policing inappropriate use of gene therapies?
The ability to alter reproductive cells using gene therapy could also generate new ethical dilemmas. To date, the various types of gene therapies have been targeted to somatic cells, the non-reproductive cells within the body. Because somatic cell traits are not inherited, any genetic changes accomplished by somatic-cell gene therapy would not be passed on to offspring. However, should scientists successfully introduce new genes to germ cells (eggs or sperm), the resulting traits could be passed on to offspring. This approach, called germ-line gene therapy, could potentially be used to combat heritable diseases, but it could also lead to unintended consequences for future generations. Moreover, there is the question of informed consent, because those impacted by germ-line gene therapy are unborn and therefore unable to choose whether they receive the therapy. For these reasons, the U.S. government does not currently fund research projects investigating germ-line gene therapies in humans.
Risky Gene Therapies
While there are currently no gene therapies on the market in the United States, many are in the pipeline and it is likely that some will eventually be approved. With recent advances in gene therapies targeting p53, a gene whose somatic cell mutations have been implicated in over 50% of human cancers, 3 cancer treatments through gene therapies could become much more widespread once they reach the commercial market.
Bringing any new therapy to market poses ethical questions that pit the expected benefits against the risks. How quickly should new therapies be brought to the market? How can we ensure that new therapies have been sufficiently tested for safety and effectiveness before they are marketed to the public? The process by which new therapies are developed and approved complicates such questions, as those involved in the approval process are often under significant pressure to get a new therapy approved even in the face of significant risks.
To receive FDA approval for a new therapy, researchers must collect significant laboratory data from animal trials and submit an Investigational New Drug (IND) application to the FDA’s Center for Drug Evaluation and Research (CDER). Following a 30-day waiting period during which the FDA reviews the IND, clinical trials involving human subjects may begin. If the FDA perceives a problem prior to or during the clinical trial, the FDA can order a “clinical hold” until any problems are addressed. During clinical trials, researchers collect and analyze data on the therapy’s effectiveness and safety, including any side effects observed. Once the therapy meets FDA standards for effectiveness and safety, the developers can submit a New Drug Application (NDA) that details how the therapy will be manufactured, packaged, monitored, and administered.
Because new gene therapies are frequently the result of many years (even decades) of laboratory and clinical research, they require a significant financial investment. By the time a therapy has reached the clinical trials stage, the financial stakes are high for pharmaceutical companies and their shareholders. This creates potential conflicts of interest that can sometimes affect the objective judgment of researchers, their funders, and even trial participants. The Jesse Gelsinger case (see Case in Point: Gene Therapy Gone Wrong ) is a classic example. Faced with a life-threatening disease and no reasonable treatments available, it is easy to see why a patient might be eager to participate in a clinical trial no matter the risks. It is also easy to see how a researcher might view the short-term risks for a small group of study participants as a small price to pay for the potential benefits of a game-changing new treatment.
Gelsinger’s death led to increased scrutiny of gene therapy, and subsequent negative outcomes of gene therapy have resulted in the temporary halting of clinical trials pending further investigation. For example, when children in France treated with gene therapy for SCID began to develop leukemia several years after treatment, the FDA temporarily stopped clinical trials of similar types of gene therapy occurring in the United States. 4 Cases like these highlight the need for researchers and health professionals not only to value human well-being and patients’ rights over profitability, but also to maintain scientific objectivity when evaluating the risks and benefits of new therapies.
Exercise \(\PageIndex{3}\)
- Why is gene therapy research so tightly regulated?
- What is the main ethical concern associated with germ-line gene therapy?
Key Concepts and Summary
- While gene therapy shows great promise for the treatment of genetic diseases, there are also significant risks involved.
- There is considerable federal and local regulation of the development of gene therapies by pharmaceutical companies for use in humans.
- Before gene therapy use can increase dramatically, there are many ethical issues that need to be addressed by the medical and research communities, politicians, and society at large.
- 1 Barbara Sibbald. “Death but One Unintended Consequence of Gene-Therapy Trial.” Canadian Medical Association Journal 164 no. 11 (2001): 1612–1612.
- 2 Kerry Grens. “Report: Ease Gene Therapy Reviews.” The Scientist , December 9, 2013. http://www.the-scientist.com/?articl...erapy-Reviews/ . Accessed May 27, 2016.
- 3 Zhen Wang and Yi Sun. “Targeting p53 for Novel Anticancer Therapy.” Translational Oncology 3 , no. 1 (2010): 1–12.
- 4 Erika Check. “Gene Therapy: A Tragic Setback.” Nature 420 no. 6912 (2002): 116–118.
Genetic engineering has its both advantages and disadvantages. Discuss.
Unauthorized use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Writing9 with appropriate and specific direction to the original content.
Include an introduction and conclusion
A conclusion is essential for IELTS writing task 2. It is more important than most people realise. You will be penalised for missing a conclusion in your IELTS essay.
The easiest paragraph to write in an essay is the conclusion paragraph. This is because the paragraph mostly contains information that has already been presented in the essay – it is just the repetition of some information written in the introduction paragraph and supporting paragraphs.
The conclusion paragraph only has 3 sentences:
- Restatement of thesis
- Prediction or recommendation
To summarize, a robotic teacher does not have the necessary disciple to properly give instructions to students and actually works to retard the ability of a student to comprehend new lessons. Therefore, it is clear that the idea of running a classroom completely by a machine cannot be supported. After thorough analysis on this subject, it is predicted that the adverse effects of the debate over technology-driven teaching will always be greater than the positive effects, and because of this, classroom teachers will never be substituted for technology.
Start your conclusion with a linking phrase. Here are some examples:
- In conclusion
- To conclude
- To summarize
- In a nutshell
Discover more tips in The Ultimate Guide to Get a Target Band Score of 7+ » — a book that's free for 🚀 Premium users.
- multilingual
- communicate
- cognitive skills
- cultural awareness
- opportunities
- globalized world
- linguistic abilities
- cultural exchange
- language proficiency
- language barrier
- foreign travel
- personal growth
- academic achievement
- self-confidence
- cross-cultural communication
- Check your IELTS essay »
- Find essays with the same topic
- View collections of IELTS Writing Samples
- Show IELTS Writing Task 2 Topics
Write a letter to a friend to ask him/her to develop a website for your business. In your letter: Give a brief description of the business Mention what you’d like to be there on the website Provide some ideas on the use of images on the website.
Some people say that the main environmental problem of our time is the loss of particular species of plants and animals. others say that there are more important environmental problems. discuss both these views and give your own opinion., some people believe that working hard is more important than creativity in terms of achieving job success. do you agree or disagree, in the past, people stored knowledge in books. nowadays people stored knowledge on the internet. do you think the advantages outweigh the disadvantages, in some countries, more and more people are becoming interested in finding out about the history of the house or building they live in. what are the reasons for this how can people research this.

IMAGES
VIDEO
COMMENTS
1. It allows for a faster growth rate. Genetic engineering allows of plants or animals to be modified so their maturity can occur at a quicker pace. Engineering can allow this maturity to occur outside of the normal growth conditions that are favorable without genetic changes as well.
Unlike selective breeding, modern genetic engineering is more gene-specific. One of the downsides of selective breeding is the possibility of generating traits that are less desirable. This is averted by modern genetic engineering that introduces specific genes. (Ref. 1) Since the process is rather straightforward, it is relatively faster than ...
Cons of Genetic Engineering / Disadvantages of Genetic Engineering. On the other hand, there are several types of potential health effects that could arise from the insertion of a novel gene into an organism. Critics disagree with the methods of genetic engineering because of: 1. The fear for unintended selection and any unwanted transfer of genes
1. Unpredictable Results. One of the primary disadvantages of genetic engineering is that it involves manipulating an organism's genes, which can produce unpredicted results. For example, genetically modified organisms (GMOs) may produce toxins and allergens or have random environmental impacts. 2.
Learning Objectives. Summarize the mechanisms, risks, and potential benefits of gene therapy. Identify ethical issues involving gene therapy and the regulatory agencies that provide oversight for clinical trials. Compare somatic-cell and germ-line gene therapy. Many types of genetic engineering have yielded clear benefits with few apparent risks.
3 A gene drive is a genetic engineering technology—adding, deleting, disrupting, or modifying genes—to rapidly spread a particular genetic trait to an entire offspring population. A gene drive ...
These regulations vary in scope, with some countries having stricter regulations than others. The advantages and disadvantages of these regulations are also a subject of debate. B. The Need for Stricter Regulation There is a need for stricter regulation of genetic engineering, particularly in human genetic engineering.
In 1971, the first debate over the risks to humans of exposure to GMOs began when a common intestinal microorganism, E. coli, was infected with DNA from a tumor-inducing virus (Devos et al ., 2007 ...
Essay includes three advantages and three disadvantages ... There are several disadvantages of genetic engineering. There are moral dilemmas with it and questions on whether a person's DNA should ...
5. Genetic engineering can lead to unregulated gene expression. Genetic engineering has mastered the technique of inserting a transgene into a host species' genome, but regulation of the expression of the gene is much more sophisticated. This means that the transgenic organism may over or under-produce the protein product of the inserted gene.
Potential disadvantages of this genetic modification include: the potential development of herbicide-resistant weeds loss of biodiversity as fewer weed species survive as a food and shelter source ...
Genetic engineering is the process of changing an organism's genetic material. Genetic engineering is also used in the processes of heredity reproduction (genetic engineering, 1). Genetic engineering alters the genes of an organism to give it desired characteristics, these techniques have been used in plants, animals, and even people.
The Advantages and Disadvantages of Genetic Engineering Genetic engineering has been a major topic of discussion ever since 'Dolly' the sleep was cloned. Its raises ethical, moral and religious questions due to the fact it is tampering with the makeup of organisms, and certain religions believe it is not our right to do this.
Answer: advantages : 1. Disease could be prevented by detecting people/plants/animals that are genetically prone to certain hereditary. Free Essay: Genetic Engineering / Advantages and Disadvantages During the latter stage stages of the 20th century, man harnessed the power of the atom, and...
Genetic engineering has become a hot topic recent years and people hold different views against the use of it. From my point of view, while genetic engineering may cause some problems, the benefits people can get from it still overweight disadvantages. It is undoubted that there are risks involved in developing genetic engineering.
Supporters of germ line genome editing (GGE) claim that the procedure could be used as a means of disease prevention. As a possible life-saving therapy, it provides benefits that outweigh its risks. Opponents of GGE claim that the medical and societal risks, especially the use of GGE for genetic enhancement, are too high.
This page titled 14.4: Genetic Engineering - Risks, Benefits, and Perceptions is shared under a CC BY license and was authored, remixed, and/or curated by OpenStax. Many types of genetic engineering have yielded clear benefits with few apparent risks. However, many emerging applications of genetic engineering are much more controversial, often ...
Genetically engineering foods is a relatively new practice, which means the long-term effects on safety are not yet clear. Many concerns about the disadvantages relate to human health.
Genetic Engineering: Advantages and Disadvantages essay. During the latter stage stages of the 20th century, man harnessed the power of the atom, and not long after, soon realised the power of genes. Genetic engineering is going to become a very mainstream part of our lives sooner or later, because there are so many possibilities advantages ...
Moreover, some medicines and vaccines are obtained throw genetic engineering process. An important breakthrough that genetic engineering can help society to fight with inherited diseases. Some genes can be modified before a baby is born improving its life. It could be said then that genetic engineering might cure some diseases.
The main steps in the process of genetic engineering: Enzymes are used to isolate (cut out) the required gene. This gene is inserted into a vector. The vector is usually a bacterial plasmid (a piece of circular DNA found inside bacterial cells) or a virus. The vector is used to insert the gene into the required cells of the target organism.
Additionally, Genetic Engineering has so many advantages. One of the advantages is that it can create new plant/crops by subtracting or combining different genome. Now, a great amount of plants/crops can be produced. For example, it helps a tree to produce more fruits than the usual yield.
Band 5. Genetic engineering has its both advantages and disadvantages. Discuss. One major advantage of genetic engineering is that you can choose the sex of your children . Imagine having the ability to choose what your child will look like before it is born. It is no longer. a.
Advantages and disadvantages of 3D food printing for PKU food production and formulations. Download CSV Display Table This technology could facilitate the creation of a wide array of appealing, low-phenylalanine foods, from meat analogues to snacks and desserts, thereby improving dietary adherence and quality of life for individuals with PKU.
Electric vehicle (EV) fast charging systems are rapidly evolving to meet the demands of a growing electric mobility landscape. This paper provides a comprehensive overview of various fast charging techniques, advanced infrastructure, control strategies, and emerging challenges and future trends in EV fast charging. It discusses various fast charging techniques, including inductive charging ...