Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 11 October 2023

Research progress of electronic nose technology in exhaled breath disease analysis
- Ying Li 1 , 2 ,
- Xiangyang Wei ORCID: orcid.org/0000-0001-6809-9455 1 , 2 ,
- Yumeng Zhou 1 ,
- Jing Wang 3 &
- Rui You 1 , 2
Microsystems & Nanoengineering volume 9 , Article number: 129 ( 2023 ) Cite this article
6650 Accesses
7 Citations
1 Altmetric
Metrics details
- Electrical and electronic engineering
Exhaled breath analysis has attracted considerable attention as a noninvasive and portable health diagnosis method due to numerous advantages, such as convenience, safety, simplicity, and avoidance of discomfort. Based on many studies, exhaled breath analysis is a promising medical detection technology capable of diagnosing different diseases by analyzing the concentration, type and other characteristics of specific gases. In the existing gas analysis technology, the electronic nose (eNose) analysis method has great advantages of high sensitivity, rapid response, real-time monitoring, ease of use and portability. Herein, this review is intended to provide an overview of the application of human exhaled breath components in disease diagnosis, existing breath testing technologies and the development and research status of electronic nose technology. In the electronic nose technology section, the three aspects of sensors, algorithms and existing systems are summarized in detail. Moreover, the related challenges and limitations involved in the abovementioned technologies are also discussed. Finally, the conclusion and perspective of eNose technology are presented.

Similar content being viewed by others

A Clinical Breathomics Dataset
New perspectives on ‘Breathomics’: metabolomic profiling of non-volatile organic compounds in exhaled breath using DI-FT-ICR-MS
Recent developments in wearable breath sensors for healthcare monitoring
Introduction.
Human exhaled gas is composed of 150 mL of ‘dead space gas’ and approximately 350 mL of ‘alveolar gas’ 1 . ‘Alveolar gas’ refers to the headspace gas of human blood, which can dynamically reflect the trend of blood metabolism 2 . Exhaled gases of healthy humans contain nitrogen, oxygen, carbon dioxide, water vapor, rare gases, and various compounds produced during metabolism 3 , 4 , 5 , 6 . These compounds contain trace amounts of volatile organic compounds (VOCs) and some nonvolatile components, usually between one trillionth (ppt) and one millionth (ppm) 7 . Various gases have different types, concentrations, volatilities, fat solubilities, diffusion rates in the blood circulation, passing rates through alveolar cell membranes, and other characteristics 8 . When one or more gas concentration exceed a certain range or some specific gases are produced, they often cause changes in the body’s disease or metabolic function 9 , 10 , 11 . Significant changes in breath markers can be detected in many diseases, among which Helicobacter pylori breath detection has become a clinical basis 12 , 13 , and exhaled NO detection can also be used as an auxiliary means of asthma clinical 14 .
As noninvasive medical diagnostic and therapeutic technologies continue to advance, exhaled breath analysis is the most likely alternative to noninvasive and portable health diagnosis. It has the advantages of being noninvasive, painless, safe and convenient, and simple operation. Moreover, it can also avoid the discomfort and embarrassment caused by blood and urine tests. In summary, breath analysis is a highly a promising medical detection technology 15 , 16 , 17 . Thousands of different gases contained in human exhaled breath are products of human metabolism and exposure to exogenous compounds. These exhaled breath biomarkers can characterize the effects of external factors on human health. By testing the relative levels of certain biomarkers, the health status of the human body can potentially be determined. The detection of human exhaled breath is usually based on mass spectrometry and gas chromatography. However, this related equipment is expensive, complicated to operate, and not portable enough, which limits its practical application in the field of breath diagnosis 18 , 19 . Unlike the traditional methods of testing human exhalation described above, the electronic nose (eNose) usually does not require expensive components or skilled operators. In addition, the operation time is relatively short, with results available in a few minutes.
eNose is an intelligent system that combines a cross-sensitive chemical sensor array with an effective set of pattern recognition algorithms to detect, identify or quantify various gases/odors. First, a series of gas-sensitive sensors with good resolution and selectivity to the target analytes are selected to form a sensor array. Then, the response curve of this sensor array is obtained through a data acquisition card to extract feature parameters after denoising of these response signals. Finally, the extracted feature parameters are fed into the pattern recognition system to identify the type and concentration information of the gas/odor. The utilization of eNose technology in noninvasively diagnosing human exhalation provides significant advantages, such as low technical costs and excellent discrimination capabilities.
With the continuous development of gas sensing technology and artificial intelligence, the human exhaled breath detection method based on eNose technology has the potential for large-scale early diagnostic screening and long-term monitoring and diagnosis. eNose technology has the advantages of miniaturization, easy integration, economic benefits, and simple operation. The development of eNose technology in the field of health care has greatly expanded 20 . The application of eNose in clinical medicine mainly includes early screening of various cancers 21 , lung diseases, such as pneumonia and upper respiratory tract infection 22 , diabetes 23 , identification of bacterial pathogens 24 , and microbial metabolites released from superficial wounds 25 .
After nearly three decades of development, eNose technology has made great progress. However, several challenges persist. One such challenge is the presence of the drift phenomenon, where the sensor response and pattern recognition algorithm (PRA) gradually deviate over time. This drift hinders the alignment between the sensor response and the algorithm’s performance, leading to decreased matching accuracy. Furthermore, the collected data from sensor arrays for the same detection target consist of multivariate time series signals with complex structures. In addition, a priori response functions and accurate mathematical models for gas-sensitive sensors are difficult to obtain due to the complexity of the response mechanism. Consequently, researchers still rely on empirical approaches when choosing signal processing and pattern recognition algorithms. These unresolved issues have impeded the widespread utilization and advancement of eNose technology. Therefore, exploring and researching solutions for real-time, fast, efficient, and accurate gas identification within the eNose domain remains an imperative research direction.
Here, an overview and analysis of the research conducted on eNose technology for noninvasive breath diagnosis is presented. Its working schematic diagram is shown in Fig. 1 . In this review, the significance of utilizing human exhaled breath as a diagnostic tool for various diseases is initially highlighted. The correlation between certain diseases and specific biomarkers present in human exhaled breath are elucidated. Then, several existing methods for detecting expiratory breath and their underlying principles are summarized and demonstrated. Through a comparative analysis of their practical advantages and limitations, the expiratory breath detection method based on eNose emerges as an ideal noninvasive diagnostic approach. In the subsequent section, the gas sensors and PRA used within the eNose system are two technological aspects that serve as crucial components, and each are thoroughly discussed. Then, the research progress of eNose technology for disease breath analysis is introduced, and the applications of eNose technology in this field are provided. Finally, the main challenges existing at present and the prospect of future development are presented.
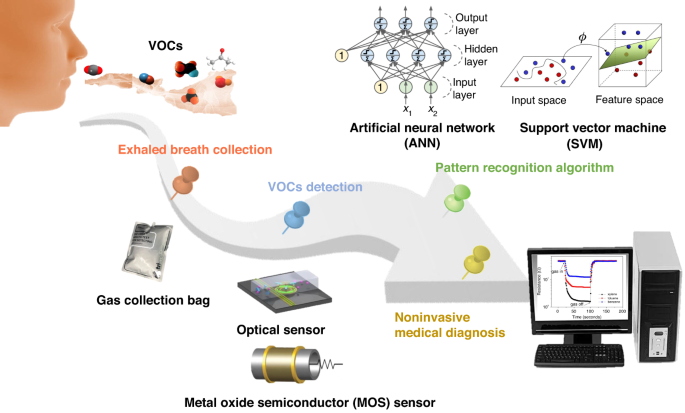
Schematic diagram of the noninvasive breath detection via the eNose system
Application of human exhaled breath components in disease diagnosis
Exhalation is a process of gas exchange between the human body and the outside environment. It is one of the most important metabolic activities of organisms. Exhaled gas contains much information related to body health. In 1971, Linus et al. published a significant article in which more than 200 ppm levels of VOCs were detected in exhaled gas through gas chromatography 26 . This discovery paved the way for various methods of exhalation analysis. With the development of exhaled breath analysis and detection, the study of VOC biomarkers in human exhaled breath for metabolic diseases has attracted wide attention. Currently, more than 3000 different VOCs have been identified in breath samples 19 , 27 , 28 , 29 , with over 500 VOCs detected in single breath samples 27 , 30 , 31 .
In addition, inorganic and organic compounds have also been found in human exhaled breath. Inorganic compounds in human exhaled breath include nitric oxide (NO), carbon monoxide (CO), ammonia (NH 3 ), and hydrogen sulfide (H 2 S). Organic compounds mainly include hydrocarbons (such as ethane, pentane, and isoprene), oxygen-containing compounds (such as acetone, alcohols, and aldehydes), nitrogen-containing compounds (such as dimethylamine and trimethylamine) and sulfur-containing compounds (such as methyl mercaptan, ethyl mercaptan, and dimethyl sulfide) 4 , 32 , 33 , 34 . The prevalent compounds detected in human exhaled breath are summarized in Table 1 , as well as their corresponding disease types and exhaled breath concentrations observed in healthy people. These are expected to become potential biomarkers for disease diagnosis.
Inorganic compounds, such as NO, have been used as biomarkers of lung inflammation and have shown potential in the study of various lung diseases. Their clinical value for the diagnosis of patients with lung cancer (LC) is considerable 35 . As shown in Fig. 2a , breath samples were collected from healthy people (H) and LC patients. The H subjects exhibited a considerably higher count of individuals with exhaled breath NO levels below 20 ppb compared to the LC group. Furthermore, the H subjects demonstrated a maximum level of exhaled breath NO below 60 ppb, while the LC group showed a maximum level of exhaled breath NO surpassing 100 ppb. Exhaled NO detection has been approved by the U.S. Food and Drug Administration as a diagnostic criterion for asthma, thus positioning it as a valuable adjunctive tool for asthma assessment and treatment 36 . Exhaled CO may be associated with obstructive sleep apnea (OSA), a common sleep-disordered breathing disorder characterized by recurrent complete or partial collapse of the upper airway during sleep 37 . The resulting intermittent hypoxia can lead to airway inflammation and oxidative stress. Endogenous CO is mainly a byproduct of heme oxygenase-catalyzed heme degradation 38 . It is a marker of oxidative stress. Studies have shown elevated levels of exhaled circulating CO in patients with OSA 39 . The exhaled CO content in patients with different types of OSA is demonstrated in Fig. 2b .

a NO content in exhaled breath of H subjects (green) and LC patients (red) 35 . Copyright 2021 MDPI. b Different types of OSA in patients with exhaled CO content diagram 39 . b1–b4 Four OSA patients with different degrees of physical health. Copyright 2017 the American Physiological Society. c Comparison of the content of aldehydes in the exhaled breath of H subjects and patients 55 . c1 Statistical results of the detection of aldehydes in the exhaled breath of H subjects (blue) and LC patients (red). c2 Exhaled breath samples of uremic hemodialysis (HD) patients (symbol ○ ), chronic renal insufficiency or chronic renal failure (CRI/CRF) patients (symbol ×) and H subjects (symbol □). Copyright 2022 MDPI. d Ammonia metabolism 55 . d1 The urea cycle. d2 Hemodialysis. Copyright 2011 Informa UK Limited
Hydrocarbons are compounds derived from lipid peroxidation 40 and can serve as biomarkers of oxidative stress 2 . Oxidative stress is the most frequent pathological state in major diseases such as asthma, chronic obstructive pulmonary disease (COPD) and LC. They can be characterized by chronic inflammation and oxidative stress, which can be diagnosed by endogenous volatiles 41 , 42 . Specifically, most of the VOCs in COPD are aldehydes or hydrocarbons 43 . The saturated aldehydes in the exhaled breath of patients with LC showed distinctive disparities compared to those of the H subjects (Fig. 2c 1, red bars). Oxidative stress metabolites are considered to be the main components of abnormal exhaled breath in LC 44 . Additionally, hydrocarbons, such as methane, ethane and pentane, can serve as biomarkers for asthma, breast cancer, liver disease, and intestinal and colon-related diseases 34 , 45 . The exhaled breath of breast cancer patients contains volatile alkanes (such as pentane, hexane and long-chain alkanes) and alkane derivatives, which are derived from oxidative stress associated with breast cancer lesions 30 or induced activation of polymorphic cytochrome mixed oxidase 46 . Isoprene is the main hydrocarbon found in human exhaled gas 34 and is associated with cholesterol metabolism 45 .
Many studies have shown that acetone is one of the most abundant VOCs in human respiration 4 , 47 , 48 , 49 . The research results show that acetone in human exhaled breath can be used as the main characteristic marker of diabetes due to its high sensitivity and specificity 50 . Ketones in the human body are produced when the liver decomposes fat and are special intermediate products of fat metabolism. Among them, 3-β-hydroxybutyric acid and acetoacetic acid are not volatile; thus, the ketone present in exhaled air is mainly acetone. The concentration of acetone in the exhaled breath of diabetic patients can reach 2–6 times higher than that of the H subjects, as shown in Table 1 51 , 52 , 53 . Ethanol and methanol in the human body are derived from microbial fermentation of carbohydrates in the gastrointestinal tract 34 , 54 . Increased levels of reactive oxygen species in cancer cells promote lipid peroxidation, leading to the production of various aldehydes 55 . Therefore, the content of ethanol, ketones and aldehydes in the exhaled breath of cancer patients is significantly higher than that of the H subjects 56 . In addition, formaldehyde has also been proposed as a marker for LC 7 .
Ammonia is the main nitrogen-containing volatile compound. Abnormal levels of ammonia in breath are associated with liver or kidney dysfunction 57 , which could also be used to diagnose peptic ulcers of the stomach or duodenum caused by Helicobacter pylori 58 . Additionally, elevated concentrations of dimethylamine and trimethylamine are detected in the exhaled breath of uremic patients (Fig. 2c 2) 59 , 60 , 61 . There are two different modes of ammonia metabolism in the human body: the urea cycle and hemodialysis. The detailed process is presented in Fig. 2d . Endogenous ammonia is a product of protein metabolism and is converted to urea in the liver and subsequently eliminated by the glomerulus (urea cycle); this results in its depletion in the exhaled breath of the H subjects. However, in patients with impaired renal function, the proportion of ammonia in the exhaled breath is elevated, indicating an altered exhaled breath profile. Remarkably, hemodialysis treatment has been found to effectively reduce the level of ammonia 7 .
Sulfur compounds found within the human body are derived from the incomplete metabolism of methionine through the transamination pathway. They serve as the main markers for liver failure 62 . Remarkably, patients who have undergone liver transplantation or are affected by liver disease exhibit comparatively high concentrations of sulfur compounds in their exhaled breath. Specifically, the exhaled breath of individuals with liver disease shows significant increases in the levels of dimethyl sulfide, acetone, 2-butanone and 2-pentanone 63 . Importantly, liver disease is an important extraoral cause of halitosis 62 . In fact, approximately 85% of halitosis cases stem from lesions located within the oropharynx, such as tongue coating, gingivitis, periodontitis, and tonsillitis. These conditions are associated with sulfur-containing compounds, such as hydrogen sulfide, methyl mercaptan, and dimethyl sulfide 64 , 65 .
Exhaled breath analysis technology
Exhaled breath analysis in academic research entails the utilization of several prevalent techniques. Notably, gas chromatography (GC) and mass spectrometry (MS) are extensively used, relying on substantial analytical instruments. Another prevalent approach is cavity ring-down spectroscopy (CRDS) based on spectral analysis. Additionally, gas sensor analysis grounded in electrochemical principles constitutes a significant methodological avenue 18 , 66 . Herein, a concise summary of the detection methods and underlying principles specific to each technique is provided below.
GC separates various components based on their differential distribution coefficients in the relative motion of two phases. In terms of reliability, GC is recognized as the best standard solution for gas detection 67 . The acetone content in the breath of diabetic patients can be effectively analyzed by GC (Fig. 3a ). Currently, gas detection methods utilizing GC primarily include thermal desorption-gas chromatography (TD-GC) 68 , gas chromatography-hydrogen flame ionization detector (GC-FID) 50 , gas chromatography-ion mobility spectrometry (GC-IMS) 69 and gas chromatography-mass spectrometry (GC-MS) 62 . The distribution of the acetone-butanol-ethanol (ABE) fermentation substrate was tested based on the GC-FID method, as well as the product concentration (Fig. 3b 1-2). However, due to the limited qualitative capacity of GC, it was imperative to combine GC with other detectors for more precise analysis. Furthermore, GC exhibits drawbacks, such as lengthy detection times, complex operational mechanisms, and the requirement for skilled personnel, causing it to be less suitable for point-of-care testing in medical diagnostics 70 .
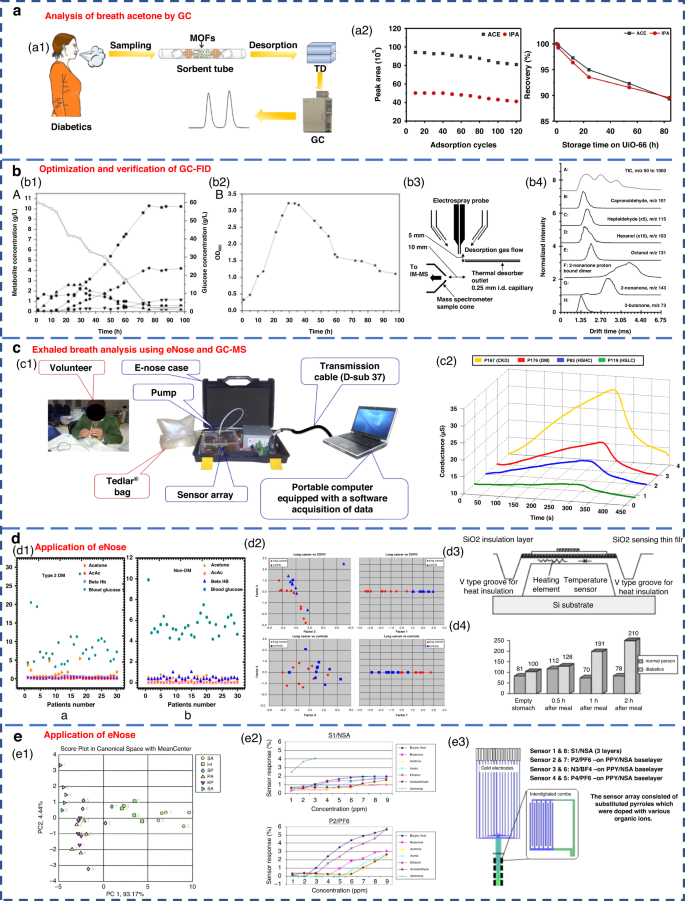
a Acetone content in the breath of diabetic patients analyzed by GC 68 . Copyright 2019 Springer Nature. b Optimization and verification of the GC-FID determination method. b1 , b2 Concentration distribution of ABE fermentation substrate and product 50 . Copyright 2014 Oxford University Press. b3 , b4 Detection of VOCs in breath using thermal desorption electrospray ionization-IMS-MS 73 . Copyright 2021 American Chemical Society. c Exhaled breath analysis using eNose and GC-MS 63 . Copyright 2018 Elsevier. c1 Experimental setup of the eNose system. c2 Electrical conductance changes in the presence of 4 VOC samples using the MQ-137 sensor. d Application of eNose in the exhaled breath of diabetic, NSCLC and COPD patients. d1 Scatter plot for plasma breath acetone in type 2 diabetic (left) and nondiabetic mellitus patients (right) 23 . Copyright 2019 MDPI. d2 eNose results for the discrimination of patients with NSCLC and COPD 75 . Copyright 2009 Elsevier. d3 , d4 Novel method for diabetes diagnosis based on eNose 77 . Copyright 1997 Elsevier. e Application of eNose in upper respiratory tract infection and wound bacteria detection. e1 Identification of upper respiratory bacterial pathogens with eNose 24 . Copyright 2009 John Wiley & Sons. e2 , e3 Development of CP sensor arrays for wound monitoring 25 . Copyright 2008 Elsevier
MS entails the ionization of gases into charged particles via an ion source, followed by their separation based on the mass-to-charge ratio utilizing electric and magnetic fields. It has the advantages of fast response and no pretreatment. At present, proton transfer reaction-mass spectrometry (PTR-MS) 71 , selective ion flow tube-mass spectrometry (SIFT-MS) 72 and IMS-MS 73 are widely employed for exhaled gas analysis. Thermal desorption electrospray ionization-IMS-MS can also be used to detect VOCs in breath (Fig. 3b 3-4). Tarik’s research team performed noninvasive diagnosis of chronic kidney disease, diabetes, and H subjects using eNose and GC-MS coupled analysis 63 . Breath samples were measured with an eNose system specifically developed for breath analysis purposes (Fig. 3c 1). Typical responses produced by the MQ-137 sensor in the presence of different breath samples (chronic kidney disease, diabetics and H subjects with high/low creatinine) are shown in Fig. 3c 2. However, many kinds of trace gases are present in human exhaled breath, which leads to the inevitable formation of numerous ionic clusters. Consequently, when there were components with identical mass-to-charge ratios in exhaled breath samples, clearly distinguishing these components by MS alone was difficult. In addition, MS has a great requirement of a high vacuum level within the test chamber. Therefore, the equipment structure is complex, limiting the development of portability and miniaturization.
CRDS stands out for its remarkable sensitivity. It is widely used in the trace detection of gases as well as absorption spectroscopy of molecules, atoms and clusters. Wang et al. from Mississippi State University first used CRDS technology to systematically study acetone in human exhaled gas and its correlation with blood glucose concentration in 2010 74 . CRDS leverages gas-specific optical absorption peaks to detect trace gases. Moreover, it is not affected by the laser intensity fluctuation. However, its utilization is constrained by the availability of laser light sources and high reflectivity mirrors. Acquiring CRDS instruments for multiple wavelength ranges can be challenging. Additionally, the equipment needs to be highly calibrated and is expensive.
The abovementioned three methods have high requirements for experimental instruments and environmental conditions. Typically, the detection and analysis processes take a long time and cannot be monitored in real time. Additionally, the equipment structures are complex, impeding progress in terms of portability and miniaturization. Furthermore, the large cost associated with these methods hinders their widespread adoption and development across various fields.
Compared with the above methods, the gas sensor analysis method can quickly obtain qualitative and quantitative gas detection results. They provide high sensitivity, small size, ease of packaging, and low price. By using a sensor array comprising multiple sensors, collaborative analysis of gas samples can also be achieved. Based on the principles of biological olfaction, eNose technology utilizes gas sensor arrays and PRA for gas detection and has shown excellent performance and significant application potential. Notably, it has been applied in clinical medicine, including early screening of diverse cancers 75 , lung diseases 76 , diabetes 77 , bacterial pathogen identification 78 , 79 , and in the analysis of microbial metabolites from superficial wounds 25 , 80 .
Respiratory acetone levels were investigated in diabetic and nondiabetic patients by using an eNose system (Fig. 3d 1, 3-4). As expected, diabetic patients exhibited high levels of respiratory acetone (greater than 0.8 ppm) compared to their nondiabetic counterparts (less than 0.8 ppm). The applications of eNose in distinguishing non-small cell lung cancer (NSCLC) and COPD patients are shown in Fig. 3d 2. The eNose system was able to distinguish the LC patients from the COPD patients and H subjects from the breath test experimental results. This result confirmed that eNose has the potential to become a noninvasive diagnostic tool for LC patients in the future. Recent studies have demonstrated the ability of eNose technology to test for bacterial infections (Fig. 3e 1). The eNose analysis exhibited the ability not only to detect common upper respiratory pathogens but also to discriminate between bacterial species when compared to the control group. Moreover, eNose sensor arrays based on conductive polymers can also be used for wound monitoring (Fig. 3e 2-3).
Breath analysis technology based on eNose possesses the advantages of high sensitivity, rapid response, real-time monitoring, and user-friendly portability. As a noninvasive diagnostic model, it presents an ideal approach for the rapid screening of diseases through breath detection. The eNose system consists of two key technologies: the sensor array responsible for the detection of chemical substances and the algorithm for providing the analytical software model within the system.
Gas sensors for eNose systems
eNose technology relies on gas sensors to obtain the composition information of gas samples. To enable precise detection of breath-related diseases with complex components, integration of multiple specific sensors into a sensing array is needed to achieve high-precision detection 81 . In the field of exhalation analysis, sensor arrays have been recognized for their considerable application potential 82 . In the field of clinical practice, several types of gas sensors find widespread utilization in eNose systems. These include the following: chemical resistance sensors, such as metal oxide semiconductor (MOS) sensors and conductive polymer (CP) sensors; the widely used piezoelectric sensors, such as quartz crystal microbalance (QCM) sensors and surface acoustic wave (SAW) sensors; electrochemical (EC) sensors; and optical sensors 19 , 70 , 82 , 83 , 84 , 85 . Typical schematics are shown in Fig. 4 .
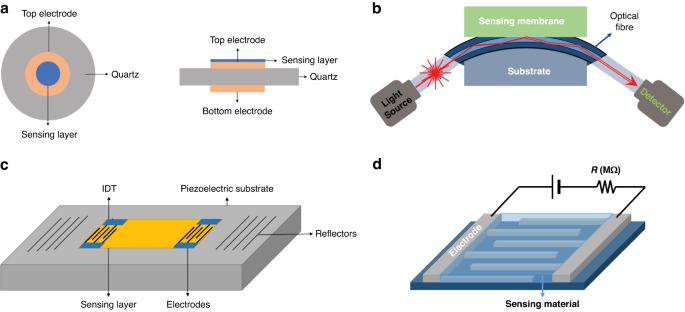
Schematic view of a typical QCM sensor ( a ), fiber-optic sensor ( b ), SAW gas sensor ( c ), and chemical resistance gas sensor ( d ) 129 . Copyright 2019 MDPI
Chemical resistance gas sensor
The MOS sensor, a member of the chemical resistance gas sensor category, is the most commonly used sensor type used in eNose systems 7 , 81 . It has the advantages of high sensitivity, rapid response, miniaturization, low cost, user-friendliness, and good compatibility with microelectronic processes 18 . MOS sensors operate by utilizing the adsorption of the targeted gas to modify the conductivity of the semiconductor material. According to the difference in charge carriers, they can be divided into N-type and P-type semiconductor materials. Notably, these two semiconductor materials have different sensing responses to reducing gas and oxidizing gas, as shown in Table 2 .
At present, a range of MOS sensing materials, such as SnO 2 , ZnO, CuO, TiO 2 , WO 3 , NiO, In 2 O 3 , WO 3 , TiO 2 , Fe 2 O 3 , and MoO 3 , are commonly used to detect various gases, such as acetone, ethanol, formaldehyde, H 2 S, NH 3 , NO 2 , and CO 19 , 28 , 82 , 83 . The performance of the MOS sensor is influenced by the morphology of the sensing material as well as surface additives. Semiconductor materials are generally polycrystalline materials containing lattice gaps between the crystalline structures. During the charge transport process, the grain boundary barrier affects the material resistance to a certain extent. Therefore, the selectivity and sensitivity to the target gas can be increased by increasing the porosity or reducing the grain size to the nanoscale level; these methods expand the specific surface area and generates oxygen-rich vacancies 2 , 18 , 83 . The sensitivity can be defined as R a /R g (for reducing gases) or R g /R a (for oxidizing gases), where R a represents the resistance of the gas sensor in the reference gas (generally air) and R g represents the resistance of the gas sensor in the reference gas containing the target gas 86 .
Nanostructured materials, such as nanowires, nanosheets, nanospheres, and nanopetals, have been used for VOC detection 87 , 88 , 89 , 90 , 91 . Additionally, modifying the surface of the material by adding a certain number of additives is another way to enhance the performance of MOS sensors and improve their selectivity, sensitivity and response speed 2 , 83 . Examples of such additives include Pt-In 2 O 3 , Pt-Fe 2 O 3 , Co-SnO 2 , Au-ZnO, Si-WO 3 92 , 93 , 94 , 95 , 96 , and composite metal oxides, such as La 2 O 3 -SnO 2 , In-WO 3 -SnO 2 , and ZnO-SnO 2 97 , 98 , 99 . Chen et al. designed and developed gravure-printed WO 3 /Pt-modified rGO (reduced Graphene Oxide) nanosheets for the detection of acetone 88 . As shown in Fig. 5a , the transient response to 10 ppm acetone was shown for three different samples and provided response/recovery times of approximately 15.2/9.6 s and 14.1/6.8 s for WO 3 /GNs and WO 3 /Pt-GNs, respectively. Notably, the gas response/recovery times were much lower than those of WO 3 /GMs. The fast response recovery characteristics were attributed to the large number of p-n junction active sites present at the WO 3 /rGO interface, which facilitated the rapid charge carrier transport into the conduction band. Liu’s group designed an acetone gas sensor based on a porous platinum (Pt)-doped In 2 O 3 nanofiber structure (Fig. 5b ) 92 . Similar work was performed by Zhang’s group to design and fabricate an acetone sensor based on nanosized Pt-loaded Fe 2 O 3 nanocubes (Fig. 5c ) 93 . Additionally, Homayoonnia et al. developed metal-organic framework (MOF)-based nanoparticles for VOC detection (Fig. 5d ) 89 .
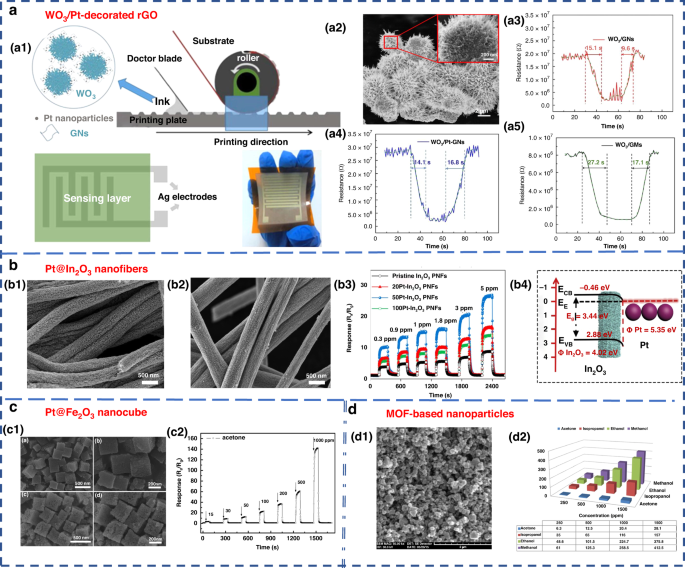
a WO 3 /Pt-decorated rGO nanosheets for the detection of acetone 88 . a1 Schematic representation of the gravure printing process. a2 SEM image. a3 – a5 Response transient of the gas sensor based on WO 3 /GNs, WO 3 /Pt-GNs and WO 3 /GMs samples to 10 ppm acetone at 200 °C. Copyright 2017 Elsevier. b In 2 O 3 nanofiber-functionalized Pt catalysts 92 . b1 , b2 SEM images. b3 Linear relationship between response and acetone concentrations. b4 Schematic illustration of the energy band of Pt-In 2 O 3 PNFs. Copyright 2019 Elsevier. c Acetone gas sensor based on nanosized Pt-loaded Fe 2 O 3 nanocubes 93 . c1 SEM images of pure Fe 2 O 3 (top) and Pt-Fe 2 O 3 (bottom). c2 Response curve of Pt-Fe 2 O 3 exposed to a high concentration of acetone at 139 °C. Copyright 2019 Elsevier. d MOF-based nanoparticles for VOC detection 89 . d1 SEM image. d2 Sensor sensitivity for methanol, ethanol, isopropanol and acetone at different concentrations of 250, 500, 1000 and 1500 ppm. Copyright 2016 Elsevier
CP sensors are also chemical resistance sensors 100 that provide high sensitivity, high selectivity and the ability to function at room temperature 19 . The material properties of CP are similar to those of some metal and inorganic semiconductor materials, while retaining the flexibility of the polymer and having the advantage of easy processing and synthesis 101 . Common examples of CPs include polypyrrole (PPy), polyaniline (PANI), and polythiophene (PT) 102 , 103 , 104 . Researchers have explored the potential of CP sensors within eNose for detecting VOCs. Chatterjee et al. developed an eNose system by integrating 5 carbon nanotube (CNT)-based CP nanocomposite (CPC) sensors with a CNT sensor 105 . The system was able to successfully detect 18 different LC VOC biomarkers at the ppm level; thus, its application performance was confirmed. João et al. used the commercial Cyranose 320 (Sensigent, Baldwin Park, CA, USA) eNose device to effectively distinguish asthma patients through the analysis of their breath VOCs. The device utilized a NoseChip nanocomposite array consisting of 32 CP sensors. The sensor consisted of a carbon black film dispersed in a polymer matrix, which was deposited onto two metal electrodes to form an electrical connection. The relative resistance change of sensors was measured upon exposure to VOCs 106 . Finnegan et al. proposed a miniature, low-cost, and battery-free wearable eNose based on a CP sensor array 107 . This device could be used to detect 6 VOCs: pyridine, tetrahydrofuran, ethanol, methanol, acetic acid and ammonium hydroxide 107 .
Piezoelectric gas sensor
SAW and QCM sensors are two widely used piezoelectric sensors in eNose applications 19 . SAW sensors use the mutual conversion of electrical energy and mechanical energy to generate sound waves through piezoelectric materials. When sound waves propagate through the piezoelectric substrate or on the surface of the piezoelectric substrate, any change in the propagation path characteristics leads to changes in the SAW characteristics, which can be associated with the measured physical (or chemical) quantities 82 . SAW technology has evident advantages of high sensitivity and low energy consumption. However, the process of manufacturing patterned metal electrodes on piezoelectric substrates is expensive and complex, requiring specialized equipment. Additionally, it is very sensitive to environmental factors, such as temperature and humidity, limiting its application 85 , 108 .
FundaKus et al. studied the molecular recognition properties of Calix arene-modified gold nanorods (AuNR) and silver nanoclusters (AgNC) on the surface of SAW transducers (Fig. 6a ) 4 . The sensitivity of the modified sensor was 6–8 times higher when used to detect acetone, ethanol, chloroform, n-hexane, toluene and isoprene. The use of zeolitic imidazolate framework (ZIF) nanocrystals as a sensitive layer in SAW-based sensor arrays was developed by Fabio et al. As shown in Fig. 6b , it could detect and identify three diabetes-related breath markers of acetone, ethanol and ammonia with a detection limit of 5 ppm 109 .
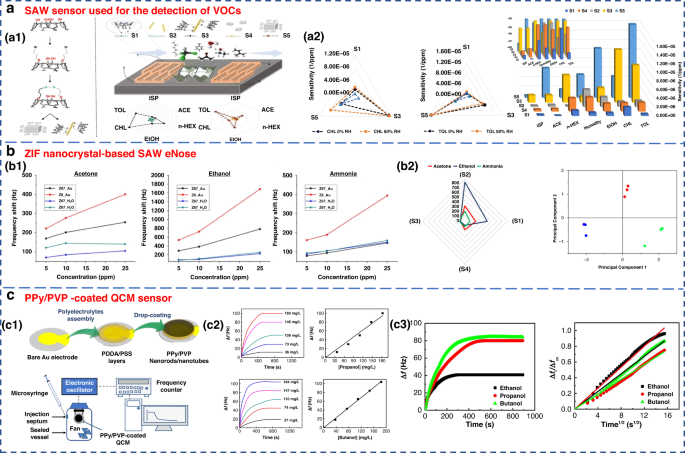
a SAW sensor used for the detection of VOCs 182 . a1 Surface reaction mechanism diagram of the SAW sensor. a2 Sensitivity of sensors S1–S5 to 6 gases under 50% relative humidity (RH). Copyright 2021 Elsevier. b ZIF nanocrystal-based SAW eNose to detect diabetes in human breath 109 . b1 Calibration curves of sensors S1, S2, S3 and S4 for acetone (left), ethanol (middle) and ammonia (right). b2 Radial representation of the sensor array responses to 10 ppm of acetone, ethanol and ammonia. Copyright 2018 MDPI. c Gas sensing properties of a PPy/PVP nanorod/nanotube-coated QCM sensor 171 . c1 Illustration of PPy/PVP nanorod/nanotube film formation. c2 Time-dependent frequency change of the QCM sensor when exposed to different concentrations of 1-propanol and 1-butanol and their calibration curves. c3 Frequency change of the QCM sensor against exposure time for a constant concentration of ethanol, 1-propanol and 1-butanol vapor (184 mg L −1 ) and plot of Δft/Δf∞ against the square root of time. Copyright 2021 Elsevier
QCM is a type of bulk acoustic wave (BAW) device made of quartz, which is mainly cut by AT 110 . It has received considerable attention due to its high precision and sensitivity 111 , 112 . As a piezoelectric mass sensor, QCM measures changes in the resonance frequency when specific gas molecules are adsorbed on the sensing material’s surface. By measuring the change in resonance frequency, the mass or concentration of a specific gas adsorbed can be quantified 70 , 81 . The sensing performance of QCM depends on the physical or chemical properties of coating materials, such as zeolites, CNTs and polymers, which have been used to detect gases on the surface of QCM 82 . A QCM sensor coated with a colloidal PPy/poly(N-vinylpyrrolidone) (PPy/PVP) nanorod/nanotube film was used for the detection of alcohol vapors (Fig. 6c ). This sensor showed good detection sensitivity for alcohol vapor.
Electrochemical sensor
The EC sensor operates by analyzing the concentration of the gas being measured. It detects changes in the current generated by the oxidation or reduction reaction of gas molecules on the surface of the catalytic electrode. This type of sensor is particularly effective in detecting electrochemically active gases 113 , 114 . However, it has a lower sensitivity to a variety of compounds, especially aromatic hydrocarbons 115 . Obermeier et al. developed an eNose system composed of three different EC sensors. As shown in Fig. 7a , it could be used to detect ppb levels of exhaled aldehydes and airway inflammation markers, such as CO and NO 116 . The Nazir group developed a hexanol-terminated AuNP-based eNose system for detecting limonene (Fig. 7b ), a biomarker of exhaled breath found in patients with cirrhosis. The detection results of this system provided an R 2 value of 0.99. The qualitative and quantitative detection results were close to those of GC-MS 117 .
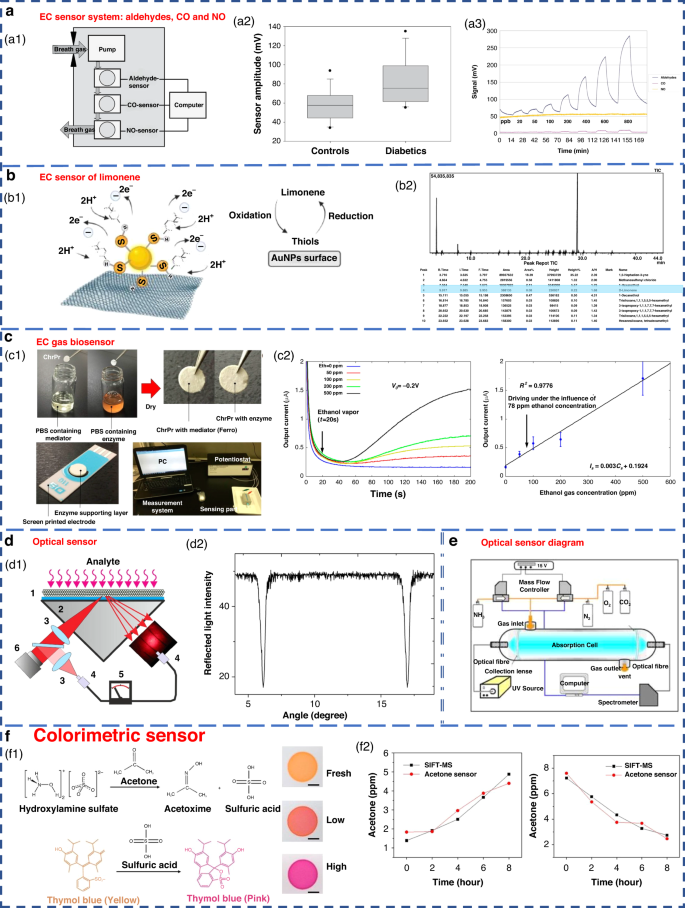
a EC sensor system for breath analysis of aldehydes, CO and NO 171 . a1 Schematic of the sensor system. a2 Comparison of the aldehyde signals from the breath of controls and diabetic patients. a3 Response of the sensor system to dry aldehyde standards (20–800 ppbV) in clean ambient air. Copyright 2015 IOP Publishing. b EC sensor of limonene using thiol-capped gold nanoparticles 117 . b1 Schematic diagram of limonene oxidation at the electrode surface. b2 Screening of limonene via GC-MS. Copyright 2022 Elsevier. c An EC gas biosensor based on enzymes immobilized on chromatography paper 120 . c1 Synthesis of the sensitive materials and flow chart of the sensor fabrication. c2 Typical current responses of modified chromatography paper enzyme electrodes for several ethanol gaseous concentrations. Copyright 2017 MDPI. d Optical sensors with high sensitivity and fast response 122 . d1 Schematic of the experimental setup. d2 Measured angular dependence of the reflected light intensity. Copyright 2015 Elsevier. e Experimental device diagram of ammonia sensing using an optical sensor 183 . Copyright 2009 Elsevier. f Colorimetric sensor for detecting exhaled acetone 127 . Copyright 2021 American Chemical Society
Some EC sensors for breath gas detection are enzyme sensors 118 , 119 , 120 , 121 . Due to the specific reactivity of enzymes, they have high sensitivity and high selectivity. However, an enzyme is sensitive to temperature and needs to be stored at low temperature. Furthermore, the enzyme sensor is disposable and cannot be repeatedly tested 83 . An EC gas biosensor based on an enzyme immobilized on chromatographic paper is shown in Fig. 7c . Ethanol vapor could be measure in the concentration range of 50–500 ppm.
Optical gas sensor
Optical sensors have the advantages of high sensitivity, good selectivity, and rapid response. They also have the ability to monitor chemical and physical parameters on a large scale 122 , 123 , 124 . These sensors can operate in colorimetric, fluorescence, chemiluminescence or scattering modes, converting the optical changes generated by the interaction between the analyte and the biometric substance into measurable signals 49 , 82 .
In recent years, there have been highly sensitive fast response gas sensors based on light reflection at the glass-photonic crystal interface (Fig. 7d ), which have a sensitivity of 1 ppm for NH 3 , a rise time response of 100 ms, and a recovery time of approximately 10 s. A schematic diagram of the optical sensor ammonia sensing experimental setup is shown in Fig. 7e . However, the optical sensor equipment system is complex and costly to operate 125 . Additionally, the optical system results can be easily affected by external factors, such as physical damage and sunlight; this greatly limits its miniaturization and portability 49 , 100 .
Colorimetric sensors are optical sensors that produce visible visual color changes when affected by external stimuli. Gold, silver, copper and other nanoparticles are widely used in colorimetric sensing because of their favorable optical properties 83 . Colorimetric acetone sensors have shown promising application potential in detecting human exhaled VOCs due to their advantages of simple production and rapid detection capabilities (Fig. 7f ) 126 , 127 .
Summary of this chapter
From the perspective of medical diagnosis, the ideal sensor array in eNose should have the advantages of high sensitivity, stable performance, rapid response, simple portability, reusability and low cost 19 , 83 . The results of the relevant studies are summarized in terms of chemical resistance gas sensors, piezoelectric gas sensors and electrochemical sensors in Table 3 . Relevant target analytes, practical detection ranges and detection limits are also detailed.
Pattern recognition algorithm used within the eNose system
Pattern recognition refers to identifying trends or specific patterns in data 81 . The core processing technology in the eNose system involves the qualitative or quantitative analysis of gas information obtained by a sensor array through a machine learning algorithm 85 , 128 . However, in real-world disease breath diagnosis, the eNose system must deal with a diverse array of complex and trace gases. To address this challenge, researchers have incorporated appropriate multivariate analysis technology into the algorithm components of the eNose system, resulting in improved selectivity in multivariate scenarios. This approach effectively mitigates the problem of low cross-sensitivity and poor selectivity observed in existing gas sensors 19 . In addition, for various diseases, the detection limits of the corresponding markers are different (Table 1 ). A single sensor has difficulty meeting the detection limits of different markers alone, and the use of a sensor array of the eNose system effectively solves this problem. Then, the gas information obtained by the sensor array is qualitatively or quantitatively analyzed by a machine learning algorithm to meet the practical application of the eNose system in the field of human breath. The practical application of PRA in assisting eNose for disease breath diagnosis in recent years is generalized in Table 4 . Abbreviations in Table 4 are summarized in Table 5 .
Gas sensor arrays in the eNose system are typically analyzed using classical machine learning algorithms, such as principal component analysis (PCA) 6 , 82 , 115 , 129 , 130 , linear discriminant analysis (LDA) 6 , 19 , 82 , 130 , 131 , support vector machine (SVM) 2 , 6 , 19 , 70 , 130 , 132 , 133 , decision tree (DT) 2 , 130 , K-nearest neighbor (KNN) 2 , 6 , 19 , 130 , 134 , cluster analysis (CA) 115 , canonical discriminant analysis (CDA) 115 , partial least squares regression (PLS) 63 , and others.
Ensemble learning is a machine learning strategy independent of the algorithm 135 . It can combine a group of weak learners to form a strong one. The generation method of the learner can be roughly divided into two categories: Boosting, in which there is a strong dependence between individual learners and serial generation can only be used; and bagging, in which there is no strong dependence between individual learners, and parallel generation can be used. Paleczek et al. proposed a diabetic breath detection method based on the XGBoost algorithm (Fig. 8a ). The system had high selectivity for low concentrations of acetone. Its accuracy and recall rates were 99% and 100%, respectively, which were superior to those of other commonly used algorithms (such as SVM, KNN and DT) 136 .
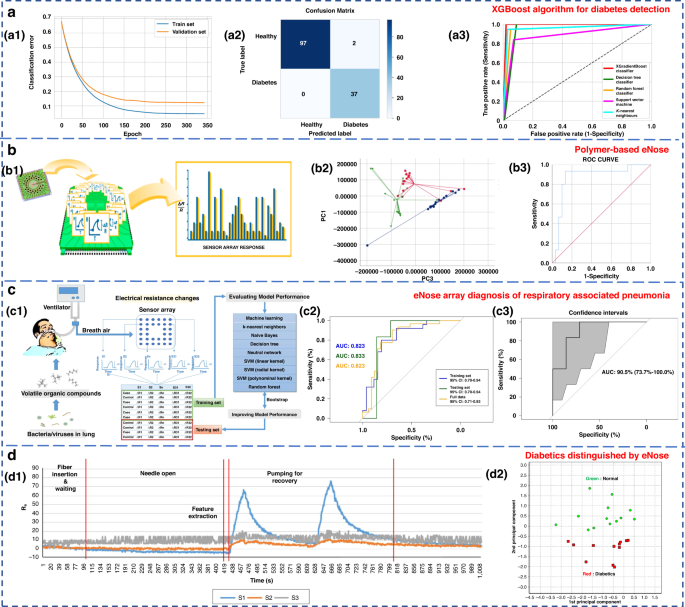
a Artificial breath classification using the XGBoost algorithm for diabetes detection 136 . a1 XGBoost learning curves. a2 XGBoost classifier confusion matrix. a3 ROC comparison of different algorithms. Copyright 2021 MDPI. b Role of polymer-based eNose in the detection of head and neck cancer from exhaled breath 137 . b1 Working principal scheme of Cyranose 320. b2 Two-dimensional PCA with 2 composite factors. b3 ROC curve with line of identity of the breath print discriminant function (representing PC1 and PC3). Copyright 2022 MDPI. c eNose sensor array signal diagnosis of respiratory-associated pneumonia 141 . c1 Flow diagram of this study. c2 Area under the receiver operating curve (AUC) for VAP in the training set, testing set, and full dataset. c3 AUC for VAP in the testing set, with the 95% confidence interval. Copyright 2020 Springer Nature. d Diabetics distinguished by using eNose 144 . d1 Sensor response of breath samples of the control group. d2 PCA result of measured breath samples. Copyright 2018 John Wiley & Sons
To investigate the potential of eNose in detecting head and neck cancer through exhaled breath analysis, Roberta’s research team used Cyranose 320 for sampling, as depicted in Fig. 8b 137 . In the PCA diagram, patients with head and neck cancer formed distinct clusters in relation to both the control group and patients with allergic rhinitis. The three groups were successfully discriminated with a typical discriminant analysis, and a cross-validation accuracy of 75.1% ( p < 0.01) was achieved. The area under the receiver operating characteristic (ROC) curve for identifying patients with head and neck tumors from other groups reached 0.87. In conclusion, eNose technology exhibits promising application potential in diagnostic contexts. Lei et al. proposed a high-precision PCA-SVE ensemble learning framework that combined 11 four-type gas sensors to form an eNose system for rapid noninvasive exhalation diagnosis of LC 135 . A set of single machine learning models with excellent performance, including SVM, DT, random forest (RF), logistic regression and KNN, were selected to construct the PCA-SVE framework. Experiments were performed on 214 exhaled breath samples (98 LC patients and 116 H subjects). The accuracy, sensitivity and specificity of the proposed framework were 95.75%, 94.78% and 96.96%, respectively.
Due to their strong self-learning and adaptive ability, as well as nonlinear expression ability, neural networks often have better analysis results than traditional machine learning methods when dealing with complex and trace human exhaled breath data. The commonly used neural networks in the eNose systems are artificial neural networks (ANNs) 115 , multilayer perceptron neural networks (MLPs) 138 , convolutional neural networks (CNNs) 138 , 139 , 140 , and radial basis functions (RBFs) 115 . Chen et al. diagnosed ventilator-associated pneumonia (VAP) by sensor arrays and machine learning technology (Fig. 8c ) 141 . Eight algorithms, including KNN, naive Bayes, DT, neural network, SVM (including linear kernel, polynomial kernel and radial basis kernel), and RF, were used. The results were verified by using real exhaled samples from VAP patients ( n = 33) and a control group ( n = 26), with an average accuracy of 0.81 ± 0.04, a sensitivity of 0.79 ± 0.08, and a specificity of 0.83 ± 0.00 136 . Hendrick et al. identified tuberculosis by using a sensor array combined with a pattern recognition method. The classification effects of SVM, XGBoost, ANN and RF were researched. The accuracy rates were 92%, 88.24%, 94.87% and 84.24%, respectively 142 , 143 .
Jin et al. selected four kinds of semiconductor chemical sensors with different sensitive materials (Au/N-SnO 2 , Au/N-WO 3 , N-WO 3 and N-SnO 2 ) and constructed a 20-sensor array operating at five different temperatures (245, 285, 310, 325, and 340 °C) 144 . The work is shown in Fig. 8d . PCA and Euclidean distance were used to identify the best-performing sensor array combination and enabled the accurate detection of five types of VOC gases, including acetone. Twenty-five real exhalation samples (12 diabetic patients and 13 H subjects) were successfully distinguished. Although classical machine learning methods are simple to design and have a relatively fixed framework with few parameters, their generalization ability is weak. Consequently, it is difficult to accurately identify the gas atmosphere in high-noise environments, such as exhaled breath detection.
By imitating the cognitive process of the human brain, the neural network achieves high-precision recognition and analysis of the target by designing parameters, such as the number of network layers, the number of neurons, and the activation functions. Typically, the performance of neural networks improves with an increase in the number of data samples acquired 130 .
Development of the eNose system
eNose has a documented history dating back to 1964 145 , when Wilkens and Hartman used electrodes to chemically react with gases to simulate the olfactory process of organisms. Since then, a large number of experts and scholars have been attracted to this field and carried out research.
A significant breakthrough in eNose research occurred during the annual meeting of the European Chemical Sensing Research Organization held at the University of Warwick, England in 1987 146 . At this meeting, researchers from the University of Warwick presented a paper on gas sensors that introduced the concept of ‘pattern recognition’ and discussed the feasibility of using sensors for detecting both composite and simple gases. Following several years of exploration in eNose-related technologies, the same research group published another article in 1994, in which the concept of ‘eNose’ was proposed and defined in detail 146 . According to these studies, eNose is a biomimetic detection instrument composed of a sensor array that can react with multiple gases, and a specific identification methodology enable the identification and classification of individual or compound gases. The introduction of this concept signaled the transition of eNose technology from a phase of growth period to one of maturity, leading to a stage of steady development. In the same year, the world witnessed the emergence of the first commercial ‘eNose’ instrument.
In recent years, due to the continuous development of eNose technology, remarkable progress has been achieved in the food, medicine, agriculture and other light industries. The Nahid group used an eNose system to classify the maturity of berries into five levels in 2020 147 . ANN, PCA and LDA were applied to the recognition mode of the sensor array. Among them, the performance of ANN was the best, achieving a 100% discrimination rate for blackberry and 88.3% for bayberry. PCA achieved discrimination rates of 97% for blackberry and 93% for bayberry, while LDA exhibited the lowest efficacy (Fig. 9a ). Cevoli et al. used an eNose equipped with six MOS sensors and ANN methods to successfully classify Italian cheese (Fig. 9b ). The final accuracy was 100% 148 .
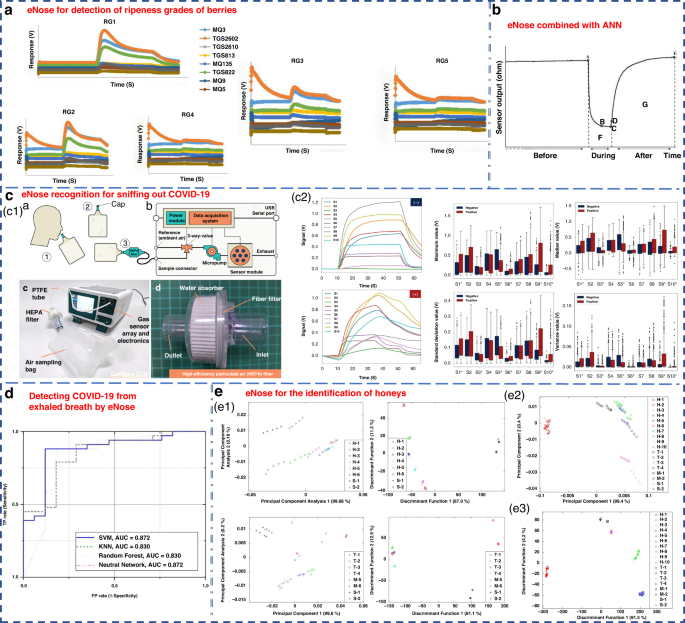
a eNose for the detection of ripeness grades of berries 147 . Copyright 2022 John Wiley & Sons. b eNose combined with ANN for the classification of pecorino cheese 148 . Copyright 2011 Elsevier. c A rapid noninvasive eNose based on breath-fingerprint recognition for sniffing out COVID-19 152 . Copyright 2022 Springer Nature. d Clinical studies of detecting COVID-19 from exhaled breath by eNose 153 . Copyright 2022 Springer Nature. e eNose sensor for the identification of different honeys 155 . Separate plot of 32 eNose sensor results ( e1 ) for honey assessment by using LDA ( e2 ) and PCA ( e3 ). Copyright 2011 MDPI
Machado et al. utilized the Cyranose 320 eNose to analyze the exhaled gas composition of 14 patients with bronchial cancer and 45 H subjects 21 . By combining with SVM, it achieved an accuracy of 72% and specificity of 92% for LC detection. The Cyranose 320 eNose was also used to distinguish NSCLC, COPD, and H control subjects. The results showed that the olfactory characteristics of LC patients could be distinguished from those of COPD patients and H subjects 75 . Horvath et al. utilized an eNose system to distinguish different VOCs produced by ovarian cancer and normal tissues. It obtained a remarkable recognition accuracy of 100% when using 15 samples for each tissue type 149 .
Recently, Wang’s group from Zhejiang University applied an eNose to detect pests during crop storage and early bollworm infestation in cotton 150 . It could effectively distinguish healthy crops from pest-infested crops 151 . Dian et al. developed a rapid noninvasive eNose based on expiratory breath fingerprinting recognition for sniffing out COVID-19 152 . Notably, the eNose system exhibited high levels of systematic detection accuracy (88–95%), sensitivity (86–94%), and specificity (88–95%), as shown in Fig. 9c . These findings indicated the potential of the use of GeNose C19 as a highly effective breath testing device for rapid COVID-19 screening. In a related study, the outcomes of COVID-19 detection within a local hospital were detailed utilizing a developed electronic setup incorporating commercial VOC gas sensors 153 . ROC curves were generated for a cohort of 50 samples, consisting of 33 COVID-19-infected patients and 17 H. Four detection algorithms of SVM, KNN, RF, and neural network, were examined, as illustrated in Fig. 9d .
Chen et al. proposed a novel eNose model based on a virtual array SAW sensor 154 . The image recognition method and improved neural network were utilized to analyze the output response of the sensor. This eNose system successfully detected 11 LC-related marker VOCs and achieved promising diagnostic results in hospitalized patients. Zakaria et al. utilized an eNose system comprising 32 sensors combined with probabilistic neural networks (PNNs) to differentiate honey from various floral sources, pseudo-honey and syrup (Fig. 9e ). It was able to compositionally classify different samples with an accuracy of 92.59% 155 .
Through the above research, the emergence of various commercial eNoses and self-developed eNoses have been widely used in various fields. According to the analysis of the literature in recent years, the application of the eNose system in the field of clinical medicine is increasing. In addition to the early cancer screening, bacterial pathogen identification and analysis of superficial wound microorganisms mentioned in the manuscript, several research teams have also developed respiratory tests for COVID-19 in the last three years 156 , 157 . The Helicobacter pylori breath test is also widely used in clinical practice 158 . The sensors and algorithms complement each other. Based on these test results, the high integration of gas sensor arrays and intelligent algorithms in the future will provide great prospects for the application of eNose systems in the field of respiratory diagnosis.
Conclusion and perspective
In the pursuit of early diagnosis and timely treatment of diseases, breath testing has gained considerable attention due to its inherent safety, noninvasiveness, and convenience. eNose is capable of providing rapid qualitative or semiquantitative results and considered an ideal device for swift breath screening in disease detection. In this review, a comprehensive examination of gas sensor arrays and pattern recognition algorithms employed in eNose systems that have been widely utilized for expiratory diagnosis in recent years is presented.
The widespread clinical application of eNose systems requires the synchronized advancement of physiological mechanisms and sensing technologies. The primary challenge is achieving selective detection within the complex human exhaled environment while avoiding the impact of other VOCs and humidity. Therefore, it is essential to further improve the selectivity of the eNose system. Furthermore, to ensure their suitability for the human expiratory environment in clinical applications, the influence of high humidity needs to be addressed. This can be accomplished by further exploring potential biochemical and metabolic mechanisms underlying expiratory markers while considering the pathological conditions of patients.
Additionally, the selection of appropriate sensing materials and processing techniques for gas sensors within eNose systems should be guided by the device’s intended purpose and operational requirements. The implementation of targeted pattern recognition algorithms will enable the identification of correlations between the sensor response signals and physiological indicators and can improve the robustness of the exhaled biomarkers for clinical diagnosis. Moving forward, the high integration of gas sensor arrays and intelligent algorithms holds great promise for enhancing the applications of eNose systems in the field of breath diagnosis.
Mukhopadhyay, R. Don’t waste your breath. Anal. Chem. 76 , 273 A–276 A (2004).
Google Scholar
Kim, C. et al. Recent trends in exhaled breath diagnosis using an artificial olfactory system. Biosensor 11 , 337 (2021).
Schubert, J. K. et al. Breath analysis in critically ill patients: potential and limitations. Expert Rev. Mol. Diagn. 4 , 619–629 (2004).
Miekisch, W. et al. Diagnostic potential of breath analysis—focus on volatile organic compounds. Clin. Chim. Acta 347 , 25–39 (2004).
D’Amico, A. et al. Olfactory systems for medical applications. Sens. Actuators B Chem. 130 , 458–465 (2008).
Paleczek, A. et al. Review of the algorithms used in exhaled breath analysis for the detection of diabetes. J. Breath. Res. 16 , 026003 (2022).
Guntner, A. T. et al. Breath sensors for health monitoring. ACS Sens 4 , 268–280 (2019).
Sehnert, S. S. et al. Breath biomarkers for detection of human liver diseases: preliminary study. Biomarkers 7 , 174–187 (2002).
Cao, W. et al. Breath analysis: potential for clinical diagnosis and exposure assessment. Clin. Chem. 52 , 800–811 (2006).
Arasaradnam, R. P. et al. Next generation diagnostic modalities in gastroenterology–gas phase volatile compound biomarker detection. Aliment. Pharmacol. Ther. 39 , 780–789 (2014).
Wilson, A. D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 5 , 140–163 (2015).
Atherton, J. C. et al. The urea breath test for Helicobacter pylori. Gut 35 , 723 (1994).
Gisbert, J. P. et al. 13C‐urea breath test in the diagnosis of Helicobacter pylori infection–a critical review. Aliment. Pharmacol. Ther. 20 , 1001–1017 (2004).
Menzies, G. A. et al. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur. Respir. J. 55 , 3 (2020).
Francesco, F. D. et al. Breath analysis: trends in techniques and clinical application. Microchem. J. 79 , 405–410 (2005).
Dummer, J. et al. Analysis of biogenic volatile organic compounds in human health and disease. Trends Anal. Chem. 30 , 960–967 (2011).
Chen, T. et al. Exhaled breath analysis in disease detection. Clin. Chim. Acta 515 , 61–72 (2021).
Saasa, V. et al. Sensing technologies for detection of acetone in human breath for diabetes diagnosis and monitoring. Diagnostics 8 , 12 (2018).
Behera, B. et al. Electronic nose: a non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath. Res. 13 , 024001 (2019).
Wilson, A. D. et al. Advances in electronic-nose technologies developed for biomedical applications. Sensors 11 , 1105–1176 (2011).
Machado, R. F. et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Resp. Crit. Care. 171 , 1286–1291 (2005).
Pcrsaud, K. C. Medical applications of odor-sensing devices. Int. J. Low. Extr. Wound 4 , 50–56 (2005).
Saasa, V. et al. Blood ketone bodies and breath acetone analysis and their correlations in type 2 diabetes mellitus. Diagnostics 9 , 224 (2019).
Lai, S. Y. et al. Identification of upper respiratory bacterial pathogens with the electronic nose. Laryngoscope 112 , 975979 (2010).
Bailey, A. L. P. S. et al. Development of conducting polymer sensor arrays for wound monitoring. Sens. Actuators B Chem. 131 , 5–9 (2008).
Pauling, L. et al. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl Acad. Sci. USA 68 , 2374–2376 (1971).
Smolinska, A. et al. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PloS One 9 , e95668 (2014).
Rydosz, A. Sensors for enhanced detection of acetone as a potential tool for noninvasive diabetes monitoring. Sensors 18 , 2298 (2018).
Licht, J. C. et al. Potential of the electronic nose for the detection of respiratory diseases with and without infection. Int. J. Mol. Sci. 21 , 9416 (2020).
Barash, O. et al. Differentiation between genetic mutations of breast cancer by breath volatolomics. Oncotarget 6 , 44864 (2015).
Xu, J. et al. Wearable biosensors for non-invasive sweat diagnostics. Biosensors 11 , 245 (2021).
Mazzone, P. J. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J. Thorac. Oncol. 3 , 774–780 (2008).
Dent, A. G. et al. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 5 , S540 (2013).
Das, S. et al. Significance of exhaled breath test in clinical diagnosis: a special focus on the detection of diabetes mellitus. J. Med. Biol. Eng. 36 , 605–624 (2016).
Li, J. et al. Measurement of exhaled nitric oxide in 456 lung cancer patients using a ringdown FENO analyzer. Metabolites 11 , 352 (2020).
Ai, Y. et al. Cavity ringdown spectroscopy of nitric oxide in the ultraviolet region for human breath test. J. Breath. Res. 14 , 037101 (2020).
Chan, A. S. L. et al. Obstructive sleep apnoea–an update. Intern. Med. J. 40 , 102–106 (2010).
Ryter, S. W. et al. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am. J. Respir. Cell Mol. Biol. 41 , 251–260 (2009).
Kis, A. et al. Exhaled carbon monoxide levels in obstructive sleep apnoea. J. Breath. Res. 13 , 036012 (2019).
Schwoebel, H. et al. Phase-resolved real-time breath analysis during exercise by means of smart processing of PTR-MS data. Anal. Bioanal. Chem. 401 , 2079–2091 (2011).
Cazzola, M. et al. Analysis of exhaled breath fingerprints and volatile organic compounds in COPD. COPD Res. Pract. 1 , 1–8 (2015).
Ratiu, I. A. et al. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma and COPD. J. Clin. Med. 10 , 32 (2020).
Christiansen, A. et al. A systematic review of breath analysis and detection of volatile organic compounds in COPD. J. Breath. Res. 10 , 034002 (2016).
Natale, D. C. et al. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Act. 824 , 1–17 (2014).
Vasilescu, A. et al. Exhaled breath biomarker sensing. Biosens. Bioelectron. 182 , 113193 (2021).
Phillips, M. et al. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 170 , 343–350 (2018).
Agapiou, A. et al. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. Trends Anal. Chem. 66 , 158–175 (2015).
King, J. et al. A mathematical model for breath gas analysis of volatile organic compounds with special emphasis on acetone. J. Math. Biol. 63 , 959–999 (2011).
MathSciNet MATH Google Scholar
Beduk, T. et al. Breath as the mirror of our body is the answer really blowing in the wind? Recent technologies in exhaled breath analysis systems as non-invasive sensing platforms. Trends Anal. Chem. 143 , 116329 (2021).
Lin, X. Q. et al. Optimization and validation of a GC–FID method for the determination of acetone-butanol-ethanol fermentation products. J. Chromatogr. Sci. 52 , 264–270 (2014).
Righettoni, M. et al. Breath acetone monitoring by portable Si: WO 3 gas sensors. Anal. Chim. Acta 738 , 69–75 (2012).
Liu, W. et al. Understanding the noble metal modifying effect on In 2 O 3 nanowires: highly sensitive and selective gas sensors for potential early screening of multiple diseases. Nanoscale Horiz. 4 , 1361–1371 (2019).
Tai, H. et al. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 318 , 128104 (2020).
Karunagaran, M. et al. Volatile organic compounds in human breath. Indian. J. Dent. Res. 33 , 100 (2022).
Sutaria, S. R. et al. Lipid peroxidation produces a diverse mixture of saturated and unsaturated aldehydes in exhaled breath that can serve as biomarkers of lung cancer—a review. Metabolites 12 , 561 (2022).
Rudnicka, J. et al. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer 135 , 123–129 (2019).
Navas, M. J. et al. Human biomarkers in breath by photoacoustic spectroscopy. Clin. Chim. Acta 413 , 1171–1178 (2012).
Hibbard, T. et al. Breath ammonia analysis: clinical application and measurement. Crit. Rev. Anal. Chem. 41 , 21–35 (2011).
Lin, Y. J. et al. Application of the electronic nose for uremia diagnosis. Sens. Actuators B Chem. 76 , 177–180 (2001).
Shirasu, M. et al. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 150 , 257–266 (2011).
Hsu, C. N. et al. Association of trimethylamine, trimethylamine N-oxide, and dimethylamine with cardiovascular risk in children with chronic kidney disease. J. Clin. Med. 9 , 336 (2020).
Van, D. V. S. et al. GC–MS analysis of breath odor compounds in liver patients. J. Chromatogr. B. 875 , 344–348 (2008).
Tarik, S. et al. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 257 , 178–188 (2018).
Van, D. V. S. et al. Halitosis associated volatiles in breath of healthy subjects. J. Chromatogr. B. 853 , 54–61 (2007).
Campisi, G. et al. Halitosis: could it be more than mere bad breath? J. Emerg. Med. 6 , 315–319 (2011).
Ti, Q. Z. et al. Combined utilization of analysis instruments: trace impurity detection for purity xenon. IEEE 3 , 1327–1331 (2015).
Selvaraj, R. et al. Advances in mid-infrared spectroscopy-based sensing techniques for exhaled breath diagnostics. Molecules 25 , 2227 (2020).
Yu, L. Q. et al. Metal-organic frameworks for the sorption of acetone and isopropanol in exhaled breath of diabetics prior to quantitation by gas chromatography. Microchim Acta 186 , 1–6 (2019).
Allers, M. et al. Measurement of exhaled volatile organic compounds from patients with chronic obstructive pulmonary disease (COPD) using closed gas loop GC-IMS and GC-APCI-MS. J. Breath. Res. 10 , 026004 (2016).
Lekha, S. et al. Recent advancements and future prospects on e-nose sensors technology and machine learning approaches for non-invasive diabetes diagnosis: a review. IEEE Rev. Biomed. Eng. 14 , 127–138 (2020).
Schwarz, K. et al. Breath acetone—aspects of normal physiology related to age and gender as determined in a PTR-MS study. J. Breath. Res. 3 , 027003 (2009).
Dummer, J. F. et al. Accurate, reproducible measurement of acetone concentration in breath using selected ion flow tube-mass spectrometry. J. Breath. Res. 4 , 046001 (2010).
Reynolds, J. C. et al. Detection of volatile organic compounds in breath using thermal desorption electrospray ionization-ion mobility-mass spectrometry. Anal. Chem. 82 , 2139–2144 (2010).
Wang, C. et al. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens J. 10 , 54–63 (2010).
Dragonieri, S. et al. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung cancer 64 , 166–170 (2009).
Farraia, M. V. et al. The electronic nose technology in clinical diagnosis: a systematic review. Porto Biomed. J. 4 , 4 (2019).
Ping, W. et al. A novel method for diabetes diagnosis based on electronic nose. Biosens. Bioelectron. 12 , 1031–1036 (1997).
Lai, S. Y. et al. Identification of upper respiratory bacterial pathogens with the electronic nose. Laryngoscope 112 , 975 (2002).
Geffen, W. H. et al. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. J. Breath. Res. 10 , 036001 (2016).
Yan, J. et al. Electronic nose feature extraction methods: a review. Sensors 15 , 27804–27831 (2015).
Karakaya, D. et al. Electronic nose and its applications: a survey. Int. J. Autom. Comput. 17 , 179–209 (2020).
Alizadeh, N. et al. Breath acetone sensors as non-invasive health monitoring systems: a review. IEEE Sens J. 20 , 5–31 (2019).
Obeidat, Y. The most common methods for breath acetone concentration detection: a review. IEEE Sens J. 21 , 14540–14558 (2021).
Nazemi, H. et al. Advanced micro-and nano-gas sensor technology: a review. Sensors 19 , 1285 (2019).
Hotel, O. et al. A review of algorithms for SAW sensors e-nose based volatile compound identification. Sens. Actuators B Chem. 255 , 2472–2482 (2018).
Wang, C. et al. Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10 , 2088–2106 (2010).
Li, Y. et al. Graphene/polyaniline electrodeposited needle trap device for the determination of volatile organic compounds in human exhaled breath vapor and A549 cell. RSC Adv. 7 , 11959–11968 (2017).
Chen, L. et al. Fully gravure-printed WO 3 /Pt-decorated rGO nanosheets composite film for detection of acetone. Sens. Actuators B Chem. 255 , 1482–1490 (2018).
Homayoonnia, S. et al. Design and fabrication of capacitive nanosensor based on MOF nanoparticles as sensing layer for VOCs detection. Sens. Actuators B Chem. 237 , 776–786 (2016).
Nugent, P. et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO 2 separation. Nat 495 , 80–84 (2013).
Righettoni, M. et al. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 18 , 163–171 (2015).
Liu, W. et al. Rationally designed mesoporous In 2 O 3 nanofibers functionalized Pt catalysts for high-performance acetone gas sensors. Sens. Actuators B Chem. 298 , 126871 (2019).
Zhang, S. et al. An acetone gas sensor based on nanosized Pt-loaded Fe 2 O 3 nanocubes. Sens. Actuators B Chem. 290 , 58–67 (2019).
Xu, Y. et al. Highly sensitive and selective electronic sensor based on Co catalyzed SnO 2 nanospheres for acetone detection. Sens. Actuators B Chem. 304 , 127237 (2020).
Wang, P. et al. Ultraselective acetone-gas sensor based ZnO flowers functionalized by Au nanoparticle loading on certain facet. Sens. Actuators B Chem. 288 , 1–11 (2019).
Güntner, A. T. et al. Guiding ketogenic diet with breath acetone sensors. Sensors 18 , 3655 (2018).
Tammanoon, N. et al. Highly sensitive acetone sensors based on flame-spray-made La 2 O 3 -doped SnO 2 nanoparticulate thick films. Sens. Actuators B Chem. 262 , 245–262 (2018).
Tomer, V. K. et al. Rapid acetone detection using indium loaded WO 3 /SnO 2 nanohybrid sensor. Sens. Actuators B Chem. 253 , 703–713 (2017).
Asal, M. et al. Acetone gas sensing features of zinc oxide/tin dioxide nanocomposite for diagnosis of diabetes. Mater. Res. Express 6 , 095093 (2019).
Park, S. Y. et al. Chemoresistive materials for electronic nose: progress, perspectives, and challenges. Info Mat. 1 , 289–316 (2010).
Nambiar, S. et al. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 26 , 1825–1832 (2011).
Bai, H. et al. Gas sensors based on conducting polymers. Sensors 7 , 267–307 (2007).
Adhikari, B. et al. Polymers in sensor applications. Prog. Polym. Sci. 29 , 699–766 (2004).
Park, S. J. et al. Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 9 , 155 (2017).
Chatterjee, S. et al. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 1 , 4563–4575 (2013).
Cavaleiro, R. J. et al. Exhaled breath condensate volatilome allows sensitive diagnosis of persistent asthma. Allergy 74 , 527–534 (2019).
Finnegan, J. et al. Wireless, Battery Free Wearable electronic nose [C]//Frontiers in Biomedical Devices. ASME 84815 , V001T04A005 (2022).
Nam, J. et al. A conductive liquid-based surface acoustic wave device. Lab Chip 16 , 3750–3755 (2016).
Bahos, F. A. et al. ZIF nanocrystal-based Surface Acoustic Wave (SAW) electronic nose to detect diabetes in human breath. Biosensors 9 , 4 (2018).
Länge, K. Bulk and surface acoustic wave sensor arrays for multi-analyte detection: a review. Sensors 19 , 5382 (2019).
Zhang, D. et al. Polypyrrole-modified tin disulfide nanoflower-based quartz crystal microbalance sensor for humidity sensing. IEEE Sens J. 19 , 9166–9171 (2019).
Julian, T. et al. Intelligent mobile electronic nose system comprising a hybrid polymer-functionalized quartz crystal microbalance sensor array. ACS Omega 5 , 29492–29503 (2020).
Diouf, A. et al. A novel electrochemical sensor based on ion imprinted polymer and gold nanomaterials for nitrite ion analysis in exhaled breath condensate. Talanta 209 , 120577 (2020).
Gholizadeh, A. et al. Toward point-of-care management of chronic respiratory conditions: Electrochemical sensing of nitrite content in exhaled breath condensate using reduced graphene oxide. Microsyst. Nanoeng. 3 , 1–8 (2017).
Wilson, A. D. et al. Applications and advances in electronic-nose technologies. Sensors 9 , 5099–5148 (2009).
Obermeier, J. et al. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J. Breath. Res. 9 , 016008 (2015).
Nazir, N. U. et al. Electrochemical sensing of limonene using thiol capped gold nanoparticles and its detection in the real breath sample of a cirrhotic patient. J. Electroanal. Chem. 905 , 115977 (2022).
Bagchi, S. et al. Development and characterization of carbonic anhydrase-based CO 2 biosensor for primary diagnosis of respiratory health. IEEE Sens J. 17 , 1384–1390 (2017).
Chien, P. J. et al. Biochemical gas sensors (biosniffers) using forward and reverse reactions of secondary alcohol dehydrogenase for breath isopropanol and acetone as potential volatile biomarkers of diabetes mellitus. Anal. Chem. 89 , 12261–12268 (2017).
Kuretake, T. et al. An electrochemical gas biosensor based on enzymes immobilized on chromatography paper for ethanol vapor detection. Sensors 17 , 281 (2017).
Motooka, M. et al. Improvement in limit of detection of enzymatic biogas sensor utilizing chromatography paper for breath analysis. Sensors 18 , 440 (2018).
Kuchyanov, A. S. et al. Highly sensitive and fast response gas sensor based on a light reflection at the glass-photonic crystal interface. Opt. Commun. 351 , 109–114 (2015).
Manap, H. et al. Ammonia sensing and a cross sensitivity evaluation with atmosphere gases using optical fiber sensor. Proc. Chem. 1 , 959–962 (2019).
Natale, D. C. et al. Porphyrins-based opto- electronic nose for volatile compounds detection. Sens. Actuators B Chem. 65 , 220–226 (2000).
Hodgkinson, J. et al. Optical gas sensing: a review. Meas. Sci. Technol. 24 , 012004 (2012).
Keshvari, F. et al. Sensitive and selective colorimetric sensing of acetone based on gold nanoparticles capped with l-cysteine. J. Iran. Chem. Soc. 13 , 1411–1416 (2016).
Wang, D. et al. Colorimetric sensor for online accurate detection of breath acetone. ACS Sens 6 , 450–453 (2020).
Cao, J. et al. Drift compensation on massive online electronic-nose responses. Chemosensors 9 , 78 (2021).
Nazemi, H. et al. Advanced micro- and nano-gas sensor technology: a review. Sensors 19 , 1285 (2019).
Chen, H. et al. Gas Recognition in E-nose System: A Review. IEEE T BIOMED CIRC S . 2022.
Siegel, A. P. et al. Analyzing breath samples of hypoglycemic events in type 1 diabetes patients: towards developing an alternative to diabetes alert dogs. J. Breath. Res. 11 , 026007 (2017).
Maho, P. et al. Olfactive robot for gas discrimination over several months using a new opto electronic nose. In 2019 IEEE ISOEN . 1–3 (IEEE, 2109).
Boubin, M. et al. Microcontroller implementation of support vector machine for detecting blood glucose levels using breath volatile organic compounds. Sensors 19 , 2283 (2019).
Patikar, S. et al. An approach towards prediction of diabetes using modified Fuzzy K nearest neighbor[C]//2020. IEEE GUCON 2020 , 73–76 (2020).
Liu, L. et al. Detection of lung cancer with electronic nose using a novel ensemble learning framework. J. Breath. Res. 15 , 026014 (2021).
Paleczek, A. et al. Artificial breath classification using XGBoost algorithm for diabetes detection. Sensors 21 , 4187 (2021).
Anzivino, R. et al. The role of a polymer-based E-nose in the detection of head and neck cancer from exhaled breath. Sensors 22 , 6485 (2022).
Wei, G. et al. Development of a LeNet-5 gas identification CNN structure for electronic nose. Sensors 19 , 217 (2019).
Peng, P. et al. Gas classification using deep convolutional neural networks. Sensors 18 , 157 (2018).
MathSciNet Google Scholar
Zhang, H. et al. A novel convolutional recurrent neural network based algorithm for fast gas recognition in electronic nose system. In 2018 IEEE EDSSC . 1–2 (IEEE, 2018).
Chen, C. Y. et al. Diagnosis of ventilator-associated pneumonia using electronic nose sensor array signals: solutions to improve the application of machine learning in respiratory research. Respir. Res. 21 , 1–12 (2020).
Hendrick, H. et al. Non-invasive method for tuberculosis exhaled breath classification using electronic nose. IEEE Sens J. 21 , 11184–11191 (2021).
Marzorati, D. et al. MOS sensors array for the discrimination of lung cancer and at-risk subjects with exhaled breath analysis. Chemosensors 9 , 209 (2021).
Jeon, J. Y. et al. Sensor array optimization techniques for exhaled breath analysis to discriminate diabetics using an electronic nose. Etri J. 40 , 802–812 (2018).
Wilkens, H. An electronic analog for the olfactory process. Ann. N. Y. Acad. Sci. 29 , 372–378 (2010).
Julian, W. et al. A brief history of electronic nose. Sens. Actuators B Chem. 18 , 211–220 (1994).
Nahid, A. et al. Detection of ripeness grades of berries using an electronic nose. Food Sci. Nutr. 8 , 120–123 (2020).
Cevoli, C. et al. Classification of Pecorino cheeses using electronic nose combined with artificial neural network and comparison with GC–MS analysis of volatile compounds. Food Chem. 129 , 1315–1319 (2011).
GyRgy, H. et al. Different volatile signals emitted by human ovarian carcinoma and healthy tissue. Fut. Oncol. 6 , 1043–1049 (2010).
Zhou, B. et al. Electronic nose detection of cotton pests at flowering stage. Acta Agric. Engin 36 , 194–200 (2020).
Wang, J. et al. Research progress of electronic nose in detecting crop pests and diseases. Jiangsu Agric. Sci. 47 , 143–148 (2019).
Nurputra, D. K. et al. Fast and noninvasive electronic nose for sniffing out COVID-19 based on exhaled breath-print recognition. npj Digit. Med. 5 , 115 (2022).
Kwiatkowski, A. et al. Clinical studies of detecting COVID-19 from exhaled breath with electronic nose. Sci. Rep. 12 , 15990 (2022).
Chen, X. et al. A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas. Sci. Tech. 16 , 1535 (2005).
Maz, J. M. A biomimetic sensor for the classification of honeys of different floral origin and the detection of adulteration. Sensors 11 , 112–119 (2011).
Aikaterini, L. et al. A method for the identification of COVID-19 biomarkers in human breath using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. EClinicalMedicine 42 , 101207 (2021).
Jing, L. et al. Electronic nose development and preliminary human breath testing for rapid, non-invasive COVID-19 detection. ACS Sens 8 , 2309–2318 (2023).
Guang, J. S. et al. An ultrasensitive fluorescent breath ammonia sensor for noninvasive diagnosis of chronic kidney disease and helicobacter pylori infection. Chem. Eng. J. 440 , 135979 (2022).
Brannelly, N. T. et al. The measurement of ammonia in human breath and its potential in clinical diagnostics. Crit. Rev. Anal. Chem. 46 , 490–501 (2016).
Sánchez, C. et al. Use of electronic nose for diagnosis of digestive and respiratory diseases through the breath. Biosensors 9 , 35 (2019).
Das, S. et al. Non-invasive monitoring of human health by exhaled breath analysis: a comprehensive review. J. Electrochem. Soc. 167 , 037562 (2020).
Sánchez, V. C. et al. Graphene-doped tin oxide nanofibers and nanoribbons as gas sensors to detect biomarkers of different diseases through the breath. Sensors 20 , 7223 (2020).
Som, S. et al. Mechanisms linking metabolism of Helicobacter pylori to 18O and 13C-isotopes of human breath CO 2 . Sci. Rep. 5 , 1–9 (2015).
Polag, D. et al. Long-term monitoring of breath methane. Sci. Total. Environ. 624 , 69–77 (2018).
Ozato, N. et al. Association between breath methane concentration and visceral fat area: a population-based cross-sectional study. J. Breath. Res. 14 , 026008 (2020).
Perez, G. D. et al. Towards the determination of isoprene in human breath using substrate-integrated hollow waveguide mid-infrared sensors. J. Breath. Res. 8 , 026003 (2004).
Guntner, A. T. et al. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens 1 , 528–535 (2016).
Shin, H. et al. Surface activity-tuned metal oxide chemiresistor: toward direct and quantitative halitosis diagnosis. ACS Nano 15 , 14207–14217 (2021).
Yoon, J. W. et al. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 17 , 3537–3557 (2017).
Tonezzer, M. Selective gas sensor based on one single SnO 2 nanowire. Sens. Actuators B Chem. 288 , 53–59 (2019).
Torad, N. L. et al. Gas sensing properties of polypyrrole/poly (N-vinylpyrrolidone) nanorods/nanotubes-coated quartz-crystal microbalance sensor. Synth. Met 282 , 116935 (2021).
Tirzïte, M. et al. Detection of lung cancer with electronic nose and logistic regression analysis. J. Breath. Res. 13 , 016006 (2018).
Van, D. G. R. et al. Training and validating a portable electronic nose for lung cancer screening. J. Thorac. Oncol. 13 , 676–681 (2018).
Marzorati, D. et al. A Metal Oxide Gas Sensors Array for Lung Cancer Diagnosis Through Exhaled Breath Analysis[C]//2019 IEEE EMBC . 1584-1587 (2019).
Rodríguez, A. M. et al. Ultrafast gas chromatography coupled to electronic nose to identify volatile biomarkers in exhaled breath from chronic obstructive pulmonary disease patients: a pilot study. Biomed. Chromatogr. 33 , e4684 (2019).
Tenero, L. et al. Electronic nose in discrimination of children with uncontrolled asthma. J. Breath. Res. 14 , 046003 (2020).
Peters, Y. et al. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut 69 , 1169–1172 (2020).
Keulen, V. K. E. et al. Volatile organic compounds in breath can serve as a non‐invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 51 , 334–346 (2020).
Moor, C. C. et al. Exhaled breath analysis by use of electronic nose technology: a novel diagnostic tool for interstitial lung disease. Eur. Respir. J. 1 , 57 (2021).
Shan, B. et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 14 , 12125–12132 (2020).
Binson, V. A. et al. Design and development of an e-nose system for the diagnosis of pulmonary diseases. Acta Bioeng. Biomech. 23 , 1 (2021).
Kus, F. et al. Surface acoustic wave (SAW) sensor for volatile organic compounds (VOCs) detection with calix [4] arene functionalized Gold nanorods (AuNRs) and silver nanocubes (AgNCs). Sens. Actuators B Chem. 330 , 129402 (2021).
Manap, H. et al. Ammonia sensing and a cross sensitivity evaluation with atmosphere gases using optical fiber sensor. Proc. Chem. 1 , 959–962 (2009).
Download references
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC) (No. U21A6003), Beijing Nova Program (No. Z211100002121075), Key R&D Program of Shandong Province, China (2022CXPT045), and Qin Xin Talents Cultivation Program of Beijing Information Science & Technology University (No. QXTCP A202101). Additionally, this study was also supported by the Beijing Laboratory of Biomedical Testing Technology and Instruments.
Author information
Authors and affiliations.
School of Instrument Science and Opto-Electronics Engineering, Beijing Information Science and Technology University, Beijing, 100192, China
Ying Li, Xiangyang Wei, Yumeng Zhou & Rui You
Laboratory of Intelligent Microsystems, Beijing Information Science and Technology University, Beijing, 100192, China
Ying Li, Xiangyang Wei & Rui You
School of Electronics and Information Engineering, Changchun University of Science and Technology, Changchun, 130022, China
You can also search for this author in PubMed Google Scholar
Contributions
Y.L. and X.Y.W. investigated the literature and conceived and prepared the manuscript and figures. Y.M.Z. and J.W. helped to conduct literature surveys and write the manuscript. X.Y.W. and R.Y. supervised the writing of the manuscript and revised the paper.
Corresponding authors
Correspondence to Xiangyang Wei or Rui You .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, Y., Wei, X., Zhou, Y. et al. Research progress of electronic nose technology in exhaled breath disease analysis. Microsyst Nanoeng 9 , 129 (2023). https://doi.org/10.1038/s41378-023-00594-0
Download citation
Received : 11 July 2023
Revised : 16 August 2023
Accepted : 17 August 2023
Published : 11 October 2023
DOI : https://doi.org/10.1038/s41378-023-00594-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Dohyung Kim
- Seung Hwan Ko
Communications Materials (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
ThinkIR: The University of Louisville's Institutional Repository
Home > ETD > 2707
Electronic Theses and Dissertations
Electronic nose for analysis of volatile organic compounds in air and exhaled breath..
Zhenzhen Xie , University of Louisville Follow
Date on Master's Thesis/Doctoral Dissertation
Document type.
Doctoral Dissertation
Degree Name
Chemical Engineering
Degree Program
Chemical Engineering, PhD
Committee Chair
Fu, Xiao-an
Committee Co-Chair (if applicable)
Nantz, Michael
Committee Member
Watters, James
Willing, Gerold
Sumanasekera, Gamini
Exhaled breath is a complex mixture containing numerous volatile organic compounds (VOCs) at trace levels (ppb to ppt) including hydrocarbons, alcohols, ketones, aldehydes, esters and other non-volatile compounds. Different patterns of VOCs have been correlated with various diseases. The concentration levels of VOCs in exhaled breath depend on an individual subject’s health status. Therefore, breath analysis has great potential for clinical diagnostics, monitoring therapeutic progress and drug metabolic products. Even though up to 3000 compounds may be detected in breath, the matrix of exhaled breath is less complex than that of blood or other body fluids. Breath analysis can be performed on people irrespective of age, gender, lifestyle, or other confounding factors. Breath gas concentration can be related to VOC concentrations in blood via mathematical modeling; for example, as in blood alcohol testing. Since exhaled breath samples are easy to collect and online instruments are commercially available, VOC analysis in exhaled breath appears to be a promising tool for noninvasive detection and monitoring of diseases. Breath analysis has been very successful in identifying cancer, diabetes and other diseases by using a chemiresistor sensor array to detect biomarkers. The objective of this research project is to develop sensor arrays ― or so-called electronic nose ― for analysis of VOCs in air and exhaled breath. In this dissertation, we have investigated both commercial and synthesized thiol functionalized gold nanoparticles (AuNPs) as sensing materials for analysis of VOCs in air and exhaled breath. The advantages of these sensors include very high sensitivity, selectivity for detection of target analytes and operation at ambient temperature. The synthesis and material characterization of new thiols and AuNPs for increasing sensitivity and selectivity have been studied. Selected commercial thiols and in-house synthesized new functional thiols have been used to modify AuNP-based sensors for detection of VOCs in air and exhaled breath. The interdigitated electrodes (IDE) used for the sensors were fabricated by microelectromechanical systems (MEMS) microfabrication technologies. The sensor arrays were characterized by measuring the resistance difference from vacuum and different spiked analyte concentrations in air and breath samples. Air samples and breath samples were collected using Tedlar bags, and analyzed using the thiol functionalized AuNP sensors. The analysis of air samples provides a reference for analysis of exhaled breath samples. The sensors have demonstrated a low detection limit of 0.1 ppbv of acetone and ethanol in dry air and exhaled breath. The concentrations of acetone in air and exhaled breath were determined by a silicon microreactor approach. The measurements of acetone by the microreactor approach were correlated with the sensor signals. The intellectual thrust of this research is the rational design of an electronic nose for analysis of VOCs in exhaled breath, which offers a new frontier in medical diagnostics because of its non-invasive and inexpensive characteristics.
Recommended Citation
Xie, Zhenzhen, "Electronic nose for analysis of volatile organic compounds in air and exhaled breath." (2017). Electronic Theses and Dissertations. Paper 2707. https://doi.org/10.18297/etd/2707
Since July 24, 2017
Included in
Engineering Commons
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Author Corner
- Collection Policy
- License Agreements
- ThinkIR Electronic Resource Guide
- Submit Research
Related Links
- Guidelines for the Preparation and Processing of Theses and Dissertations (School of Interdisciplinary & Graduate Studies) ( PDF )
- University of Louisville Libraries Research Assistance and Instruction
- Nonexclusive License to Electronically Disseminate UofL ETD ( PDF )
- Data Management Guides for Theses and Dissertations
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Mol Sci

Potential of the Electronic Nose for the Detection of Respiratory Diseases with and without Infection
Johann-christoph licht.
1 Division of Respiratory Medicine, Department of Pediatrics, Hospital for Sick Children, Toronto, ON M5G 1X8, Canada; [email protected]
2 Translational Medicine Research Program, Hospital for Sick Children Research Institute, Toronto, ON M5G 1X8, Canada
3 Department of Immunology, University of Toronto, Toronto, ON M5S 1A8, Canada
Hartmut Grasemann
Respiratory tract infections are common, and when affecting the lower airways and lungs, can result in significant morbidity and mortality. There is an unfilled need for simple, non-invasive tools that can be used to screen for such infections at the clinical point of care. The electronic nose (eNose) is a novel technology that detects volatile organic compounds (VOCs). Early studies have shown that certain diseases and infections can result in characteristic changes in VOC profiles in the exhaled breath. This review summarizes current knowledge on breath analysis by the electronic nose and its potential for the detection of respiratory diseases with and without infection.
1. Introduction
Human-exhaled breath contains over 3000 volatile organic compounds (VOCs) in gas phase, which are detectable by different laboratory methods such as gas chromatography and mass spectrometry. Exhaled VOCs include molecules such as alkanes, benzene derivatives, acetone, dimethyl sulfide, phenol, and aromatic compounds [ 1 ]. The composition of these has been found to be altered in an increasing number of medical conditions including cancers [ 2 , 3 ] and inflammatory bowel disease (IBD), for example [ 4 , 5 , 6 ]. With respect to the analysis of VOCs, technological advancements during recent years have resulted in the development of chemical sensing and identification devices that can capture the signatures or patterns of VOC mixtures. These ‘electronic noses’ (eNoses), mimic mammalian olfactory senses by being able to detect a ‘breathprint’ of VOC mixtures, as opposed to identifying their individual molecular constituents [ 1 ]. An eNose, as previously defined by others, is an instrument which comprises an array of electronic chemical sensors and an appropriate pattern-recognition system, capable of recognizing simple or complex odors [ 7 ]. eNoses can identify different complex odors by comparing the incoming odor with previously learnt patterns [ 8 ] by creating so called breathprints. Readings occur when VOCs react at the surfaces of the eNose sensors, causing a change in conductivity of the sensors [ 9 ]. These are then detected by transducers and converted into electrical signals that create specific VOC signatures [ 7 ]. Several distinct eNose technologies have been developed. These include the Aeonose, which uses micro hotplate metal-oxide sensors [ 9 ], the BIONOTE eNose based on QCM sensors utilizing anthocyanin-coated gold electrodes [ 10 , 11 ], the Cyranose 320, using a carbon black-polymer sensor array [ 12 ], the Tor Vergata eNose, using quartz crystal microbalances (QCM) covered with metalloporphyrins [ 13 ], the Common Invent eNose using metal oxide semiconductor sensors [ 14 ], the Owlstone Lonestar eNose based on field asymmetric ion mobility spectrometry [ 15 ], and the SpiroNose, using cross-reactive metal-oxide semiconductor sensors [ 16 ]. eNoses detect mixtures of VOCs to create breathprints—they do not generally identify individual molecular compounds. The use of other analytic methods, primarily gas chromatography-mass spectrometry (GC-MS), being used in parallel or in addition to eNose measurement, are being explored to help identify the specific biomarkers responsible for the changes in breathprints and to test the eNoses for accuracy in detecting certain conditions. The use of eNose plus GC-MS will be contextualized in more detail in subsequent chapters.
Detection of VOCs in exhaled air is showing immense promise for improving diagnostic and screening standards of certain lung and airway diseases. Lung cancer is among the most commonly diagnosed malignancies and also among the leading causes of death worldwide [ 17 ]. In lung cancer, common methods of detection—imaging by chest radiography (X-ray), computerized tomography (CT) or magnetic resonance imaging (MRI), and bronchoscopy—carry many limitations from a mass screening perspective [ 3 ]. Interestingly, recent studies have shown that certain VOCs, including isopropanol, acetone, pentane, and benzene can serve as biomarkers for lung cancer [ 18 , 19 ]. Various eNose models have been used to date, to discriminate breathprints of lung cancer patients from those of healthy subjects [ 18 , 20 , 21 , 22 , 23 ]. Positive findings were supported with robust, reproducible data consistent across several groups, and have also shown differentiation of lung cancer from other respiratory diseases [ 18 , 21 , 24 , 25 , 26 ], suggesting that eNoses could be used as clinical screening tools. In addition, there is early evidence to show that VOC patterns detected by eNose can also predict response to novel cancer treatments, as demonstrated in patients with advanced non-small cell lung cancer and anti-programmed cell death 1 (anti-PD-1) immunotherapy [ 16 ]. The potential of eNose technology in the detection of lung cancer will be addressed in more detail below.
Electronic noses were developed for olfactory analysis in commercial settings such as food quality control, environmental monitoring, military purposes and, more relevant for this review, also in areas of research with focus on diagnosis of disease [ 27 ]. With regards to potential relevance for infectious diseases, biosensors have proven to be an effective method to sense foodborne pathogens, such as Salmonella contaminating packaged meat [ 28 ]. As early as in 1997, Gibson et al., reported detection of certain microorganisms from plate cultures [ 29 ] and in 2004, Pavlou et al., found that an eNose could detect Mycobacterium tuberculosis (TB) in human sputum [ 30 ]. A 14-sensor conducting polymer array eNose discriminated between M. tuberculosis, M. avium, M. scrofulaceum , P. aeruginosa cultures, and non-infected control samples in vitro. Using principal component analysis (PCA), 100% of TB cultures were identified and discriminated from other bacterial cultures [ 30 ]. In a study comparing healthy subjects and tuberculosis patients, an eNose discriminated the two groups with high sensitivity and specificity of 95.9% and 98.5% respectively [ 31 ]. Furthermore in 2006, Thaler and Hanson showed that patients with bacterial rhinosinusitis, caused by either Staphylococcus aureus (SA) or Pseudomonas aeruginosa (PA), could be distinguished by eNose from patients without infection, allowing for correct diagnosis in 72% of cases [ 32 ].
With advances in technology, there is now accumulating evidence that eNoses have the potential to address significant unmet clinical needs in both discrimination of one disease from another, as well as in the timely detection of airway infections in patients with underlying respiratory diseases at the point of care (POC) [ 33 , 34 , 35 ]. In recent years, eNoses have advanced in their discriminative accuracy from being able to detect differences between specific disease groups, to achieving similar results as diagnostic tests such as exhaled nitric oxide (F E NO) and pulmonary function testing for asthmatics [ 36 ]. As this technology is being developed and investigated in comparison to established tests, it is important to critically examine factors that may influence its diagnostic performance across disease groups. In this review, we will summarize current knowledge relevant to the potential roles of eNose technologies in respiratory diseases including lung cancer, asthma, COPD, cystic fibrosis (CF), primary ciliary dyskinesia (PCD), and non-CF bronchiectasis.
2. eNose Technology in Respiratory Disease and Infection
2.1. lung cancer.
Late diagnosis of lung cancer contributes to its high lethality; only about 15% of patients are diagnosed with early stage disease, five-year survival rate is low, and over half of all lung cancer patients die within one year of diagnosis [ 37 , 38 ]. There is an unmet need for simple, affordable, and accessible innovative tools for the (early) detection of lung cancer, and eNose technology has emerged as such a tool. Tirzīte et al., utilized a Cyranose 320 eNose to compare breath profiles of 252 lung cancer patients to those of 223 patients without cancer [ 38 ]. Cancers included squamous cell cancer, adenocarcinoma, undifferentiated non-small cell lung cancer, small cell lung cancer, and large cell lung cancer [ 38 ]. Non-smokers and smokers with or without lung cancer were compared. 128/133 cancer patients who were non-smokers and 114/119 of those who were smokers were diagnosed correctly by eNose (sensitivities of 96.2% and 95.8%, respectively) [ 38 ]. In a similar study, van de Goor et al., using an Aeonose device in 60 lung cancer patients and 107 healthy controls, obtained a diagnostic accuracy of 83% with a sensitivity of 83% and specificity of 84% [ 9 ]. The study included small cell and non-small cell lung cancer patients. Here, the authors suggested to utilize the eNose in combination with low-dose CT scans, with the aim of reducing false-positive results by CT imaging alone [ 9 ]. McWilliams et al., in an earlier study, utilized a Cyranose 320 to discriminate lung cancer patients from high-risk control subjects [ 26 ]. Exhaled breath from 191 subjects including 25 with lung cancers and 166 high-risk smokers were analyzed by a Cyranose 320. Patients with squamous cell carcinoma, adenocarcinoma, small cell lung cancer, and non-small cell lung carcinoma were included [ 26 ]. VOC breathprints could discriminate lung cancer patients from high risk controls with >80% accuracy [ 26 ]. Interestingly, a cheaper, alternative eNose technology called BIONOTE, which differs from the Cyranose and Aeonose in its working principle, sensing material, sensor array composition, and molecular selectivity, produced similar results [ 11 ]. In this study, 100 high-risk individuals participating in a screening program for lung cancer were included. Cancers identified included squamous cell carcinoma, adenocarcinoma, and undefined lung cancer [ 11 ]. Partial least square discriminant analysis (PLS-DA) [ 39 ] was used for analysis of the eNose data. BIONOTE sensitivity and specificity were reported at 86% and 95%, respectively, with an area under the receiver operator characteristic curve (AUROC) of 0.87 [ 11 ].
Frequent screening tests are of great importance for individuals exposed to asbestos because of the lifetime increased risk for malignant pleural mesothelioma (MPM). In a study on detection of MPM, Lamote et al. utilized a combination of four different eNoses—Cyranose 320, Tor Vergata eNose, Owlstone Lonestar eNose, and Common Invent eNose—and GC-MS [ 40 ]. The aim of this cross-sectional, case-control study was to investigate the accuracy of eNose and GC-MS in discriminating healthy controls ( n = 16), asymptomatic asbestos-exposed subjects (AEx, n = 19), patients with benign asbestos-related disease (ARD, n = 15), and MPM patients ( n = 14). Data were analyzed using AUROC graphs, and the final eNose breathprints were established by merging the sensor data of all four eNoses [ 40 ]. GC-MS and eNose differentiated MPM from healthy controls with 71.4% and 65.2% accuracy, MPM vs. AEx with 97.0% and 73.1% accuracy, and MPM vs. AEx + ARD with 93.8% and 73.7% accuracy, respectively [ 40 ]. Thus, in this study GC-MS outperformed eNose by >20% accuracy in discriminating between MPM vs. HC and MPM vs. AEx + ARD. Nevertheless, these findings are still promising as the main advantages of eNoses are ease of use and accessibility, as well as lower costs. Further developments and improvements of eNose devices and a combination of eNose and GC-MS technologies should be explored to further improve detection accuracy of various malignancies. As illustrated by Lamote et al., implementing these or similar modalities could make screening of asymptomatic, high-risk individuals faster and more cost-effective, which may allow for earlier interventions leading to improved management and clinical outcomes.
2.2. Asthma
Diagnosing asthma, as well as differentiating between eosinophilic, neutrophilic or other asthma endotypes, can be challenging. Dragonieri et al. investigated whether people with an established diagnosis of asthma could be discriminated from controls by eNose, and whether different degrees of asthma severity could also be identified. Subjects inspired VOC-filtered air by tidal breathing for 5 min, and a single expiratory vital capacity was collected into a Tedlar bag, which was subsequently sampled by a Cyranose 320 [ 41 ]. Based on individual’s breathprints, the Cyranose was able to separate mild asthma from controls. Patients with mild asthma could also be distinguished from those with severe asthma, though less distinctly (cross-validation value (CVV) of 65%) [ 41 ]. Plaza et al., performed a cross-sectional proof-of-concept study comparing VOC breathprints in different asthma subtypes [ 42 ]. Exhaled air from 52 patients with persistent asthma was analyzed by a Cyranose 320. Eosinophilic, neutrophilic, and paucigranulocytic inflammatory asthma phenotypes were characterized by inflammatory cell counts in induced sputum. Breathprints were significantly different in eosinophilic compared to both neutrophilic (accuracy 73%, p -value = 0.008, AUROC 0.92), and paucigranulocytic asthma (accuracy 74%, p -value = 0.004, AUROC, 0.79), and neutrophilic was different from the paucigranulocytic phenotype (accuracy 90%, p -value = 0.001, AUROC 0.88), supporting the concept of using an eNose as an alternative to sputum cytology. Plaza et al.’s observations were consistent with similar studies. Ibrahim et al., reported an 83% accuracy discriminating eosinophilic from non-eosinophilic asthma, and 72% for distinguishing neutrophilic from non-neutrophilic phenotypes, using GC-MS to detect exhaled VOCs [ 43 ]. Wagener et al., also used an eNose to differentiate eosinophilic from non-eosinophilic asthma breathprints in 27 patients with an accuracy of 85% and AUROC of 99% [ 44 ]. Interestingly, a similarly high accuracy was found by van der Schee et al., in predicting the response to corticosteroid therapy in 25 asthma patients. eNose was more accurate than sputum eosinophil counts (AUROC 0.883, p -value = 0.008 vs. AUROC 0.610, p -value = 0.441 respectively) or F E NO (0.545, p -value = 0.751) [ 45 ]. In further support of the above findings, exhaled breath samples from adults with severe asthma of the “U-BIOPRED” (Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes) cohort were used in a longitudinal multicenter study by Brinkman and colleagues [ 46 ]. Here, severe asthma phenotypes were assessed over time using both clinical characteristics and exhaled metabolomic breathprints, revealing three eNose-derived disease clusters ( n = 26/33/19). A four-eNose panel was used, including the Tor Vergata, Cyranose 320, Owlstone Lonestar, and Common Invent eNose. At baseline and at 12–18 month follow-up visits, F E NO, spirometry, and induced sputum marker values were obtained. Asthma patients falling into each of these clusters showed differing clinical characteristics, such as systemic inflammatory markers, circulating eosinophil and neutrophil counts, and oral corticosteroid use. These data supported the notion that exhaled VOCs in asthma may be associated with systemic and local eosinophilic inflammation and may help to close the gap between clinical and laboratory tests in phenotyping severe asthma [ 46 ]. There is also evidence that the eNose can distinguish patients based on their current level of asthma control. In a recent cross-sectional study by Tenero et al., 28 children with asthma were categorized into controlled ( n = 9), partially controlled ( n = 7), or uncontrolled ( n = 12) groups [ 47 ]. A Cyranose 320 discriminated between healthy controls ( n = 10) plus controlled asthma (non-symptomatic) and partially-controlled plus uncontrolled asthma (symptomatic) with an AUROC of 0.85, and a sensitivity and specificity of 0.79 and 0.84, respectively [ 47 ].
eNose technology for asthma diagnosis and phenotyping also showed promising results when compared to conventional testing methods. Montuschi et al., compared the diagnostic accuracy of a Tor Vergata eNose, F E NO, and pulmonary function testing. Twenty-seven patients with intermittent or mild persistent asthma and 24 healthy subjects were studied. Exhaled breath was collected in Tedlar bags following a 2 h period of fasting. GC-MS was performed to confirm differences in VOC patterns between groups, and to confirm that exhaled breath samples remained stable within 48 h from collection [ 36 ]. eNose alone was able to discriminate between asthma and healthy controls in 87.5% of cases, outperforming F E NO (79.2%), spirometry (70.8%), and the combination of F E NO and spirometry (83.3%). The combination of eNose analysis of exhaled alveolar air with F E NO had the highest diagnostic accuracy for asthma (95.8%) [ 36 ]. No correlation was found between the eNose results, F E NO, and lung function in asthma or healthy controls [ 36 ].
Bannier et al. investigated the potential of an eNose for accurate diagnosis of lung disease by comparing patients with asthma, CF, and healthy controls [ 48 ]. This cross-sectional study in children 6 years of age or older included 20 with moderate to severe asthma, 13 with an established diagnosis of CF, and 22 healthy controls [ 48 ]. Asthma was defined as presenting with typical respiratory symptoms in combination with reversible airways obstruction on pulmonary function testing [ 49 ]. Almost all children enrolled (54/55) were able to perform the measurements. An Aeonose eNose showed high accuracy in differentiating asthma from CF (AUROC 0.90, sensitivity 89%, specificity 91%) and CF from controls (AUROC 0.87, sensitivity 85%, specificity 77%), while the accuracy was lower when discriminating asthma from healthy controls (AUROC 0.79, sensitivity 74%, specificity 91%) [ 48 ]. Discrimination between different diseases, i.e., asthma and CF, showed similar results to a report by Fens et al. for adults with asthma or COPD (88%) [ 50 ]. This study did not account for different subtypes of asthma and was limited by a relatively small sample size.
Finally, Brinkman et al., utilized eNose in combination with GC-MS to differentiate between stable and unstable episodes of asthma [ 51 ]. A panel of four eNoses was again used, for which the data were merged to produce a final, combined breathprint. 23 patients with mild to moderate asthma were included and exhaled breath profiles measured at baseline, loss of control, and recovery. PCA of eNose data showed 95% distinction between asthma at baseline and at loss of control, and 86% between loss of control and recovery. In comparison, GC-MS data showed much lower classification accuracies of only 68% for baseline vs. loss of control, and 77% for loss of control vs. recovery [ 51 ]. GC-MS detected exhaled metabolites that were significantly associated with sputum eosinophils. This study is one of the first to compare these two VOC-detecting technologies to longitudinally monitor exhaled breath profiles during worsening and subsequent recovery of asthma control. Three specific compounds of interest, methanol, acetonitrile, and bicyclo [2.2.2]octan-1-ol, 4-methyl were identified by GC-MS [ 51 ]. Interestingly, the composite eNose technologies were superior in their discrimination between controlled and uncontrolled asthma, when compared to GC-MS, but eNose findings did not correlate with sputum eosinophil and neutrophil percentages, which was different from the GC-MS results [ 51 ]. This may indicate a potential advantage of using both detection strategies together. The advantage of the eNose lies in detecting smaller changes in exhaled VOC profiles that may not be detected by GC-MS (i.e., broader sensitivity), whereas GC-MS has the ability to pick up more specific biomarker signals associated with changes in local inflammation during asthma flare-ups [ 51 ].
2.3. Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) and asthma are both common and despite the fact that they are different disease entities, there can be significant clinical overlap between the two. The potential for accurate diagnosis of COPD and discrimination from asthma by exhaled breath profiles was first studied by Fens et al. [ 50 ]. This cross-sectional study included 21 asthmatics with fixed and 39 with reversible airways obstruction, as well as 40 patients with a diagnosis of COPD. While asthma with reversible or fixed airway obstruction could not be distinguished based on breathprints, both asthma with fixed obstruction and asthma with reversible airway obstruction were significantly different from COPD (accuracy of 88% and 83%, respectively) [ 50 ]. These findings suggested that eNose may represent a diagnostic option for patients having overlapping symptoms between fixed-obstruction asthma and COPD. In addition to discriminating from asthma, recent data suggested that eNose technology may be able to detect flare-ups or exacerbations of COPD (ECOPD). An ECOPD is characterized by a burst of pulmonary and systemic inflammation, and is usually the result of bacterial or viral infection [ 52 , 53 ]. These events can significantly influence disease progression as well as morbidity [ 54 ] and mortality [ 55 ]. Potential pathogenic micro-organisms (PPMs) in sputum or bronchoalveolar lavage (BAL) are only identifiable in up to 50% of patients experiencing an ECOPD [ 56 , 57 ]. Shafiek et al. utilized a Cyranose 320 to discriminate between infectious vs. non-infectious ECOPD or pneumonia, and showed differences in VOC breathprints [ 33 ]. Among ECOPD patients, the eNose could discriminate infected vs. non-infected COPD patients with a 75% success ratio, 88% sensitivity, and 60% specificity [ 33 ]. These findings may allow for a novel strategy in diagnosing ECOPD associated with bacterial infections in routine clinical practice, rather than depending solely on clinical diagnosis.
There are several indexes of COPD severity and disease progression, including the six-minute walk test distance (6MWD), body mass index (BMI), airflow obstruction, dyspnea, and exercise (BODE), that can be used to assess the functional status of COPD patients. Since many of these tests are limited by patient compliance, space and time (e.g., availability of a 30 m hallway to perform 6MWD), Finamore et al. investigated whether VOC analysis by eNose could predict the functional status and its variation over time in COPD patients [ 58 ]. In this monocentric prospective study with one-year follow-up, patients performed pulmonary function testing, arterial blood gas analysis, bioimpedance, 6MWD, and VOC analysis by eNose in 63 patients. A BIONOTE eNose was used, and partial least square discriminant analysis (PLS-DA) to calculate outcomes-predictive accuracy, sensitivity, and specificity [ 58 ]. The eNose predicted BODE scores with 86% accuracy, and quartiles of normalized 6MWD (n6MWD) with 79% accuracy. Reference quartiles of n6MWD to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification were as follows: 22–111 m/m 2 corresponds to GOLD class A, 112–145 m/m 2 to B, 146–165 m/m 2 to C, and 166–215 m/m 2 to D (quartiles 1–4) [ 58 ]. A change in n6MWD after one year by more than the median value of decline was predicted with an accuracy of 86% by eNose vs. 52% by GOLD classification alone, and 78% by both measures combined. These data supported that eNose technology could be further developed as a simple, and inexpensive tool to assess COPD functional status. To tangibly illustrate costs, in this study for example, an eNose analysis vs. a 6MWD represented a difference of €10 vs. €50, respectively [ 58 ].
Finally, van Velzen et al. performed a randomized controlled trial measuring exhaled breath profiles in COPD patients with and without exacerbations over a period of three years, comparing eNose and GC-MS [ 59 ]. This study included 31 patients with COPD exacerbations and 37 with stable COPD, and found significant differences between breath profiles of these patients. eNose discriminated patients with stable disease from ECOPD with an accuracy of 75%, which was very similar to GC-MS (71%) [ 59 ]. GC-MS analysis yielded ten compounds of significance in discriminating between the two groups. Similar to Brinkman et al. [ 46 ], this study utilized a panel of four eNoses, for which the data were merged. The Common Invent eNose drove the discriminative signal in detecting exacerbations most definitively [ 59 ]. The similar accuracies in detecting stable and exacerbated COPD states by these technologies is encouraging. In their work, Van Velzen et al. highlighted their approach of GC-MS and eNose technologies complementing one another: GC-MS can identify specific compounds needed to inform the fine-tuning of metabolite-specific sensor arrays on an eNose, for more precise recognition of disease-specific VOC profiles [ 59 ]. This or similar types of combined approaches will hopefully accelerate the improvement of eNose technologies to the point where they can be used as diagnostic point-of-care tools in clinical practice.
2.4. Cystic Fibrosis, Bronchiectasis and Primary Ciliary Dyskinesia
Lung disease in cystic fibrosis (CF) and primary ciliary dyskinesia (PCD) share similarities as both are genetic diseases associated with neutrophil-dominated airway inflammation, recurrent and chronic bacterial infections, retention of suppurative airway secretion, development of bronchiectasis, and chronic loss of lung function [ 60 , 61 , 62 ]. In CF, airway secretions are dehydrated due to water/electrolyte imbalance; secretions become difficult to clear and provide optimal conditions for bacterial infections. In PCD, defective ciliary motion leads to disturbed mucociliary clearance, which also results in recurrent and persistent sinorespiratory infections [ 62 ]. Although similar in their clinical presentation, CF and PCD are different entities, and several studies have shown differences in VOC breath profiles between CF and PCD [ 35 , 63 ]. eNose technology has also been able to detect differences based on bacterial colonization and disease exacerbation in these diseases. In a study of 50 children, 25 with CF and 25 with PCD, Paff et al. could show that breathprints from healthy controls differed from both CF and PCD (AUROC of 0.76 and 0.80 respectively), and PCD differed from CF as well (AUROC of 0.77) [ 64 ]. The authors speculated that distinct inflammatory and metabolic processes in CF or PCD airways would generate different volatile metabolites, and thus explain the differences seen by eNose. In PCD, these metabolites may be comprised of inflammatory cytokines such as interleukin (IL)-8 in combination with lower DNA content in airway secretions compared to CF, as well as lower proteolytic enzyme levels [ 65 , 66 ]. The investigators also observed that pulmonary exacerbations altered exhaled breath profiles [ 64 ]. Both PCD and CF are diseases in which early diagnosis, frequent monitoring, and aggressive treatment of airway infections help preserve lung function over time [ 64 , 67 ]. Therefore, non-invasive techniques to detect or monitor respiratory infections are becoming increasingly important not only for patients unable to expectorate sputum due to younger age, but also for those on effective therapies such as cystic fibrosis transmembrane conductance regulator (CFTR) targeting drugs [ 68 ].
A few studies have explored the potential role of eNose in detecting airway colonization with pathogens. Fungal infections with Aspergillus fumigatus were identified by way of an eNose in studies by de Heer et al. [ 69 ]. In the setting of invasive pulmonary aspergillosis (IA) in patients with prolonged chemotherapy-induced neutropenia (PCIN), they initially showed that patients with PCIN and IA presented with characteristic exhaled breath profiles [ 69 ]. In a more recent study by the same group, using a Cyranose 320 they showed that A. fumigatus airway colonization in patients with CF also led to a distinct breathprint [ 70 ]. 27 CF patients, of whom nine were colonized with A. fumigatus , were correctly classified by eNose with a cross-validated accuracy of 89%. eNose data were analyzed using PCA, the factors of which were then used for linear canonical discriminant analysis (LCDA). Overall, eNose-generated breathprints of CF patients with and without A. fumigatus colonization were significantly different [ 70 ]. They highlighted the previously-identified in vitro biomarker specific for A. fumigatus -induced invasive disease and colonization, 2-pentylfuran by GC-MS analysis [ 70 , 71 ].
One of the most common opportunistic pathogens leading to chronic bacterial lung infections in CF is Pseudomonas aeruginosa (PA). Persistent PA infection is known to be associated with increased morbidity and mortality in patients with CF [ 72 ]. Distinct eNose breath profiles of chronic PA infection were reported in CF patients by Joensen et al. (sensitivity and specificity of 71.4% and 63.3%, respectively, and AUROC of 0.69) [ 73 ]. In this cross-sectional case-control study 64 patients with CF, 21 with PCD, and 21 healthy controls were included [ 73 ]. Breathprints of CF patients with and without chronic infections by other pathogens, including Achromobacter xylosoxidans or Stenotrophomonas maltophilia , were not different (AUROC of 0.59). Significant differences were also not found between breath profiles of PCD patients with or without chronic PA infection [ 73 ]. Findings by Robroeks et al. support these observations; here, CF patients with PA colonization were discriminated via GC-MS from non-colonized patients on the basis of 14 exhaled VOCs [ 35 ]. Based on these VOCs, 100% discrimination was achieved between the two groups [ 35 ]. This work validates the concept of PA-specific VOCs that can be screened for by VOC-sensing instruments such as eNoses or GC-MS. Of relevance to studies suggesting that eNoses might be able to detect PA or other infections in exhaled breath by pattern analysis, recent studies have shown that specific VOCs can also be identified in fluid samples obtained from airways of CF patients. Nasir et al. analyzed volatile molecules from CF bronchoalveolar lavage (BAL) fluid using two-dimensional GC-time-of-flight-MS [ 74 ]. Utilizing nine specific volatile molecules, PA-positive ( n = 7) were distinguished from PA-negative ( n = 53) BAL samples with an AUROC of 0.86. Similar results were seen for Staph. aureus (SA)-positive and -negative samples [ 74 ]. Finally, eNoses have also shown potential in detecting infection in people with non-CF bronchiectasis. This chronic respiratory disease that is increasingly recognized in Europe and the United States [ 75 , 76 ], is characterized by irreversible dilation of the bronchi and by chronic airway inflammation [ 77 ], and similar to CF, PA airway infection contributes to morbidity as well [ 78 ]. In a study of 73 clinically stable patients with bronchiectasis by Suarez-Cuartin et al., using a Cyranose 320, airway infection produced different breath profiles compared to uninfected, with an accuracy of 72.1% and AUROC of 0.75 [ 34 ]. Further, breath profiles from subjects infected with PA were different from other pathogens (accuracy of 89.2%, AUROC of 0.96), or no infection patients (72.7%, AUROC of 0.82). Thus, these findings suggest the potential of an eNose to identify specific bacterial airway infections such as PA, regardless of underlying disease.
To summarize, the currently published data suggest that eNoses may be able to distinguish between exhaled breath profiles of patients with CF, PCD, and bronchiectasis, and to detect certain infections with pathogens such as A. fumigatus and P. aeruginosa (PA) [ 34 , 64 , 69 , 70 , 73 ]. This has potential implications for transforming patient care in the near future by implementing eNoses at the clinical point-of-care for early and accurate detection of infections. Further studies using eNose in combination with technologies able to identify specific molecular markers, such as GC-MS, are needed to help improve current eNose technologies. This could be done by adding sensors for specific VOC compounds identified by GC-MS to the sensor arrays on an eNose, for more precise recognition of disease-specific VOC profiles, as discussed above [ 59 ]. Utilizing more sensor data-points (e.g., 158 sensors in the 4-eNose-platform vs. 32 in the Cyranose 320) is an alternative strategy [ 46 ].
3. Future Directions and Need for Future VOC-based Studies
Current results of detecting both respiratory and non-respiratory diseases by eNose, as well as specific infections in some conditions, are promising. Rapid improvements in eNose technologies may overcome their current limitations, as newer generations of eNoses are being upgraded with more advanced sensor technologies and data analysis systems [ 28 ]. With this, new areas of research may evolve. As an example, recent work has demonstrated the ability of the eNose to diagnose different types of interstitial lung diseases (including cryptogenic organizing pneumonia, idiopathic pulmonary fibrosis, and connective tissue disease-associated ILD) [ 79 , 80 ]. eNoses are also becoming increasingly utilized to detect biomarkers of various types of malignancies outside of the respiratory system, including colorectal cancer, and Barrett’s esophagus, the precursor to esophageal adenocarcinoma [ 81 , 82 ].
Further, studies investigating VOC metabolomics have also yielded promising results in respiratory and non-respiratory conditions. By combining the eNose with GC-MS, detecting individual VOCs may not only improve sensitivity and specificity, but also allow for the detection of novel, previously unrecognized biomarkers and biological pathways ( Figure 1 ). Several studies have already taken this approach. For example, Rodriguez-Aguilar et al. used an eNose coupled with GC-MS to identify and match specific VOCs to breathprints obtained by eNose from patients with COPD [ 83 ], identifying biomarkers of COPD in real time. VOC biomarkers of pulmonary oxygen toxicity have also become identifiable when combining eNose and GC-MS in a study of scuba divers by Wingelaar et al. [ 84 ]. Research in CF suggests that VOC breath profiles identify SA infection; by using GC-MS, breath VOC profiles were classified, and distinguished SA-infected and non-infected CF patients with 100% sensitivity and 80% specificity [ 85 ]. Potential biomarkers specific for SA detection are isovaleric acid and methylbutanal [ 86 , 87 ]. In the aforementioned study of A. fumigatus colonization of patients with CF by de Heer et al., GC-MS analysis was suggested to complement eNose testing [ 70 ]. Definitive exhaled biomarkers of A. fumigatus infection, including 2-pentylfuran as well as monoterpenes and sesquiterpenes have been identified [ 88 ]. It is possible that factors other than A. fumigatus metabolites such as host inflammatory responses to A. fumigatus , exposure to antimicrobial therapy or corticosteroids, or more-severe CF lung disease, also contribute to the VOC patterns detectable by eNose in these patients [ 70 ]. However, VOC breathprints detectable by eNose seem to be disease-specific as inflammatory airway diseases such as asthma, COPD, CF, and PCD can all be discriminated by this technology [ 41 , 50 , 64 , 73 ].

Complimentary analysis of volatile organic compounds by gas chromatography-mass spectrometry (GC-MS) (top) and eNose detection (bottom) [ 89 ]. GC-MS can be utilized to identify specific VOC biomarkers that make up eNose-detected breath profiles. Figures reproduced with permission [ 89 ].
4. Conclusions
Taking advantage of detecting VOCs exhaled in human breath, eNose technology has enormous potential to improve or offer alternative solutions to current diagnostic tests for respiratory diseases. eNoses provide increasingly accurate and sensitive discriminative power to help differentiate between health and disease, sub-types of diseases and also disease activity and control ( Table 1 ). In addition, eNose technology may represent a non-invasive tool to detect infections as they occur in patients with respiratory diseases including lung malignancies, asthma, COPD, CF, and PCD. While the eNose has the potential to be used as a screening tool at the clinical point-of-care, its integration with specific analytic methods such as GC-MS will help identify new biomarkers of disease and disease control.
Summary of key studies presented in this review.
| Publication [Ref.] | Disease | Number of Patients/Total Study Participants | Type of VOC Detection Device | Main Findings |
|---|---|---|---|---|
| Tirzīte et al. [ ] | Lung cancer | 252/475 | Cyranose 320 | High-risk controls vs. cancer Sensitivity: 96%, specificity: 92% Non-smokers vs. cancer Sensitivity: 96%, specificity: 91% |
| Van de Goor et al. [ ] | Lung cancer | 52/144 | Aeonose | High-risk controls vs. cancer Sensitivity: 83%, specificity: 84% |
| McWilliams et al. [ ] | Lung cancer | 25/191 | Cyranose 320 | High-risk controls vs. cancer Sensitivity: 81.3%, specificity: 88% |
| Rocco et al. [ ] | Lung cancer | 23/100 | BIONOTE eNose | High-risk controls vs. cancer Sensitivity: 86%, specificity: 95% |
| Lamote et al. [ ] | Lung cancer (malignant pleural mesothelioma, [MPM]) | 35/64 (19 asymptomatic asbestos-exposed subjects [AEx] + 16 control, 14 MPM, 15 benign disease [ARD]) | Common Invent, Owlstone Lonestar, Cyranose 320, Tor Vergata eNoses + GC-MS | AEx subjects vs. MPM eNose: 97% accuracy GC-MS: 97% accuracy MPM vs. AEx + ARD eNose: 74% accuracy GC-MS: 94% |
| De Vries et al. [ ] | Lung cancer | 143/143 | SpiroNose | Responders vs. Non-responders to anti-PD-1 therapy Sensitivity: 81%, specificity: 50% |
| Dragonieri et al. [ ] | Asthma | 20/40 | Cyranose 320 | Mild asthma vs. young controls Cross-validation: 100% Severe asthma vs. old controls Cross-validation: 90% |
| Plaza et al. [ ] | Asthma | 52/52 | Cyranose 320 | Eosinophilic vs. neutrophilic Accuracy: 73%, AUROC: 0.92 Eosinophilic vs. paucigranulocytic Accuracy: 74%, AUROC: 0.79 Neutrophilic vs. paucigranulocytic Accuracy: 89%, AUROC: 0.88 |
| Brinkman et al. [ ] | Asthma | 78/78 | Common Invent, Owlstone Lonestar, Cyranose 320, Tor Vergata eNoses | Inflammatory phenotypes in severe asthma Three distinct clusters ( = 26, = 33, = 19) |
| Van der Schee et al. [ ] | Asthma | 25/45 | Cyranose 320 | Asthma vs. controls Sensitivity: 80%, specificity: 65% |
| Tenero et al. [ ] | Asthma | 28/38 | Cyranose 320 | Non-symptomatic asthma (control + controlled asthma) vs. symptomatic asthma (partially controlled + uncontrolled asthma) Sensitivity: 0.79, specificity: 0.84 |
| Montuschi et al. [ ] | Asthma | 27/51 | Tor Vergata eNose + GC-MS | Asthma vs. controls eNose: 87.5% accuracy GC-MS: “significantly different” |
| Brinkman et al. [ ] | Asthma | 23/23 | Common Invent, Owlstone Lonestar, Cyranose 320, Tor Vergata eNoses + GC-MS | eNose: baseline vs. loss of control: 95% accuracy loss of control vs. recovery: 86% GC-MS: baseline vs. loss of control: 68% accuracy loss of control vs. recovery: 77% |
| Bannier et al. [ ] | Asthma & CF | 33/55 (20 asthma, 13 CF) | Aeonose | Asthma vs. CF Sensitivity: 0.89, specificity: 0.77 CF vs. controls Sensitivity: 0.85, specificity: 0.77 Asthma vs. controls Sensitivity: 0.84, specificity: 0.91 |
| Fens et al. [ ] | Asthma & COPD | 60 asthma, 40 COPD | Cyranose 320 | COPD vs. fixed asthma Sensitivity: 85%, specificity: 90% COPD vs. classical asthma Sensitivity: 91%, specificity: 90% |
| Shafiek et al. [ ] | COPD | 143/173 (90 Exacerbated COPD [ECOPD], 50 stable COPD [SCOPD]) | Cyranose 320 | SCOPD vs. controls Sensitivity: 72%, specificity: 70% ECOPD vs. controls Sensitivity: 66%, specificity: 80% ECOPD vs. SCOPD Sensitivity: 89%, specificity: 48% |
| Finnamore et al. [ ] | COPD | 63/63 | BIONOTE eNose | BODE functional status predicted via eNose Sensitivity: 0.71, specificity: 0.93 |
| Van Velzen et al. [ ] | COPD | 31/68 (31 ECOPD, 37 COPD) | Common Invent, Owlstone Lonestar, Cyranose 320, Tor Vergata eNoses + GC-MS | ECOPD vs. COPD eNose: Accuracy: 75% GC-MS: Accuracy: 71% |
| Paff et al. [ ] | CF & PCD | 50/73 (25 CF, 25 PCD) | Cyranose 320 | CF vs. controls Sensitivity: 84%, specificity: 65% PCD vs. controls Sensitivity: 88%, specificity: 52% CF vs. PCD Sensitivity: 84%, specificity: 60% |
| Joensen et al. [ ] | CF & PCD | 85/106 (64 CF, 21 PCD) | Cyranose 320 | CF with (PA) vs. CF without PA Sensitivity: 71.4%, specificity: 63.3% No sig. difference between: CF with non-PA infection vs. CF without infection & PCD with PA/other infection vs. PCD without infection |
| De Heer et al. [ ] | CF | 27/27 | Cyranose 320 | CF with ( = 9) and without ( = 18) Sensitivity: 78%, specificity: 94% |
| Suarez-Cuartin et al. [ ] | Bronchiecta-sis | 73/73 | Cyranose 320 | Bronchiectasis with PA vs. Bronchiectasis without PA Sensitivity: 92%, specificity: 85% |
A. fumigatus: Aspergillus fumigatus, AEx: asymptomatic former asbestos-exposed, ARD: benign asbestos-related diseases, AUROC: area under the receiver operating characteristic curve, BODE: body mass index, airflow obstruction, dyspnea, and exercise, CF: cystic fibrosis, COPD: chronic obstructive pulmonary disease, ECOPD: exacerbated COPD, eNose: electronic nose, GC-MS: gas chromatography-mass spectrometry, MPM: malignant pleural mesothelioma, PA: Pseudomonas aeruginosa, PCD: primary ciliary dyskinesia, PCIN: prolonged chemotherapy-induced neutropenia, SCOPD: stable COPD, U-BIOPRED: Unbiased biomarkers for the prediction of respiratory disease outcomes, VOCs: volatile organic compounds.
Abbreviations
| AEx | Asymptomatic former asbestos-exposed subjects |
| ARD | Benign asbestos-related diseases |
| AUROC | Area under the receiver operator characteristic |
| BAL | Broncho-alveolar lavage |
| BODE | Body mass index, obstruction, dyspnea, and exercise |
| CD | Crohn’s disease |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| COPD | Chronic obstructive pulmonary disorder |
| CVV | Cross-validation value |
| ECOPD | Exacerbations of COPD |
| eNose | Electronic nose |
| GC-MS | Gas chromatography-mass spectrometry |
| IA | Invasive pulmonary aspergillosis |
| IBD | Inflammatory bowel disease |
| LCDA | Linear canonical discriminant analysis |
| LRA | Logistic regression analysis |
| MPM | Malignant pleural mesothelioma |
| PA | |
| PCA | Principal component analysis |
| PCD | Primary ciliary dyskinesia |
| PCIN | Prolonged chemotherapy-induced neutropenia |
| PLS-DA | Partial least square discriminant analysis |
| POC | Point of care |
| PPMs | Potential pathogenic micro-organisms |
| SA | |
| TB | |
| UC | Ulcerative colitis |
| VOCs | Volatile organic compounds |
| 6MWD | Six-minute walk test distance |

Author Contributions
Conceptualization: J.-C.L. and H.G.; writing—original draft preparation: J.-C.L.; writing—review and editing: H.G. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

- OPUS at UTS
- UTS Digital Thesis Collection
- UTS PhD & Masters Theses
An Efficient Electronic Nose System for Odour Analysis and Assessment
- Recently Added
- In Progress
- Open Access
| Field | Value | Language |
|---|---|---|
| dc.contributor.author | Zhang, Wentian | |
| dc.date.accessioned | 2020-04-23T05:39:52Z | |
| dc.date.available | 2020-04-23T05:39:52Z | |
| dc.date.issued | 2020 | |
| dc.identifier.uri | ||
| dc.description | University of Technology Sydney. Faculty of Engineering and Information Technology. | en_AU |
| dc.description.abstract | An electronic nose (e-nose) is capable of identifying chemical compounds through sensing and analysing odour molecules. As a type of machine olfaction, e-nose plays a significant role in the odour analysis area and has received considerable attention from researchers all over the world. The e-nose system comprises a set of active gas sensors that detect the odour and transduce the chemical vapours into electrical signals. The odour "fingerprint" captured by the gas sensors can then be analysed and identified with pattern analysis methods, e.g., Principal Component Analysis (PCA), Cluster Analysis (CA), Support Vector Machine (SVM), and Artificial Neural Networks (ANNs). E-nose has been extensively applied in the areas of agriculture, medical diagnosis, environmental monitoring and protection, food safety, the military, cosmetics and pharmaceuticals. In order to meet the growing demand from the global odour analysis market, a novel e-nose system, which has a high-efficiency and low-cost odour analysis, was designed and built in this dissertation through collaboration with different research areas. Firstly, inspired by the knowledge of the human olfactory system, an automated fault monitoring and alarming electronic nose (e-nose) system, named “NOS.E”, for odour detection and identification has been designed. This design is based on reliable hardware and software designs as well as an airflow intake system design which is the most significant part of NOS.E. Just as the air inhalations are important and necessary activities for the olfactory perception by controlling the airflow in the human olfactory system, the airflow control design is a crucial and essential element to guarantee the precise odour analysis procedure in the e-nose system. Different parts of the NOS.E are built together under a particular control logic, which was designed to improve the e-nose test efficiency by saving operation time. In addition, the fault detection and alarming design generates a high-reliability performance for the e-nose by constantly monitoring the working status of the air intake system, to make sure all the actuators are working under the guidance of the proposed control logic. A novel e-nose data pre-processing method, based on a recently developed nonparametric kernel-based modelling (KBM) approach is presented. The experimental results show that when extracting the derivative-related features from signals collected by the NOS.E, the proposed non-parametric KBM odour data pre-processing method achieves more reliable and stable pre-processing results compared with other pre-processing methods such as wavelet package correlation filter (WPCF), mean filter (MF), polynomial curve fitting (PCF) and locally weighted regression (LWR). Moreover, this dissertation also proposes a novel e-nose pattern analysis algorithm, which is a hybrid of genetic algorithm (GA) and supervised fuzzy support vector machine (FSVM). GA was used to select the informative features and the optimal model parameters of FSVM. FSVM was used as a fitness evaluation criterion and the sequent odour classifier, which would reduce the outlier effects to provide a robust classifier which has a steady classification accuracy. In addition, several studies were conducted with the NOS.E system. The first was to evaluate the performance of NOS.E based on data collected from different types of alcohols. A comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GC◊GC-TOFMS) was used to provide the standard comparison for the evaluation in this study. The second study focused on the effectiveness of KBM data pre-processing method and FSVM odour pattern analysis method. The third study explores the potential to implement NOS.E in the biomedical engineering area, while the fourth study applied NOS.E in the wildlife protection area by rapidly identifying legal from illegal wildlife parts. As a proof-of-concept test, water buffalo horn and rhinoceros horn samples were selected as the test targets in this study. The study results indicated the reliability and effectiveness of the developed NOS.E system. The NOS.E system is able to be applied to various applications based on the user-friendly and rapid odour analysis tests. Moreover, the NOS.E has the potential to be a universal odour analysis platform implemented in different applications. | en_AU |
| dc.format | Thesis (PhD) | |
| dc.language.iso | en_AU | en_AU |
| dc.relation | https://opus.lib.uts.edu.au/bitstream/10453/140210/2/02whole.pdf | |
| dc.rights | The author owns the copyright in this thesis including all reproduction and reuse rights for the work. The work may not be altered without the permission of the copyright owner. Attribution is essential when quoting or paraphrasing from this thesis. | |
| dc.rights | au.edu.uts.lib/ppc | |
| dc.rights | info:eu-repo/semantics/openAccess | |
| dc.title | An Efficient Electronic Nose System for Odour Analysis and Assessment | en_AU |
| dc.type | Thesis | en_AU |
| utslib.copyright.status | open_access |
Download statistics for the last 12 months
Not enough data to produce graph
ORIGINAL RESEARCH article
Machine learning analysis of electronic nose in a transdiagnostic community sample with a streamlined data collection approach: no links between volatile organic compounds and psychiatric symptoms.

- 1 Laureate Institute for Brain Research, Tulsa, OK, United States
- 2 Department of Computer Science, Tandy School of Computer Science, University of Tulsa, Tulsa, OK, United States
- 3 Department of Community Medicine, Oxley College of Health Sciences, University of Tulsa, Tulsa, OK, United States
- 4 Department of Mathematics, College of Engineering & Natural Sciences, University of Tulsa, Tulsa, OK, United States
- 5 Department of Psychiatry, School of Medicine, University of California San Diego, San Diego, CA, United States
Non-intrusive, easy-to-use and pragmatic collection of biological processes is warranted to evaluate potential biomarkers of psychiatric symptoms. Prior work with relatively modest sample sizes suggests that under highly-controlled sampling conditions, volatile organic compounds extracted from the human breath (exhalome), often measured by an electronic nose (“e-nose”), may be related to physical and mental health. The present study utilized a streamlined data collection approach and attempted to replicate and extend prior e-nose links to mental health in a standard research setting within large transdiagnostic community dataset (N = 1207; 746 females; 18–61 years) who completed a screening visit at the Laureate Institute for Brain Research between 07/2016 and 05/2018. Factor analysis was used to obtain latent exhalome variables, and machine learning approaches were employed using these latent variables to predict three types of symptoms independent of each other (depression, anxiety, and substance use disorder) within separate training and a test sets. After adjusting for age, gender, body mass index, and smoking status, the best fitting algorithm produced by the training set accounted for nearly 0% of the test set’s variance. In each case the standard error included the zero line, indicating that models were not predictive of clinical symptoms. Although some sample variance was predicted, findings did not generalize to out-of-sample data. Based on these findings, we conclude that the exhalome, as measured by the e-nose within a less-controlled environment than previously reported, is not able to provide clinically useful assessments of current depression, anxiety or substance use severity.
Introduction
Volatomics, the study of volatile metabolites ( 1 ) (such as ethanol and amino acids) and emanations from the human body, especially human breath known as exhalome, is an area of active investigation ( 2 ). The human exhalome contains more than 1,800 volatile organic compounds (VOCs) ( 3 ), which researchers hope can elucidate the inner workings and status of bodily functions. This hope for exhalome as a measure of physical and mental health status is well-grounded as a growing literature links various types of psychopathology with altered central and peripheral markers of bodily processing within the bidirectional brain–body context ( 4 – 7 ). Few exhalome studies have examined the extent to which VOCs are altered as a function of psychopathology and investigations such as the present study could help fill this gap in the field.
Some have argued that the brain itself is an endocrine gland that triggers stress responses ( 8 ). VOC patterns in the breath may shed light on brain–body dysfunction ( 9 ). For example, upper respiratory tract infections such as influenza are linked to stress ( 10 ) that could be associated with dysfunctional changes in exhaled breath composition. Moreover, impaired quality of life ( 11 ) and presence of obsessive compulsive and bipolar disorders ( 12 ) are also linked to upper respiratory tract infections. More research is warranted to investigate the utility of exhalome for indexing clinical symptoms and potentially, differentiating between particular psychiatric disorders.
In psychiatry, assessment of illness severity relies almost entirely on report by the affected individual or by a mental health provider, which can be subject to a number of different biases. The identification of quantitative measures of illness severity, which is based on the underlying biological processes that are affected by the disorder, would be a major advance in the field. The measures could have broad applicability for the selection of individuals for treatment and the monitoring of the efficacy of different behavioral or pharmacological interventions. Moreover, these types of measures would build a much stronger case for hypothesis testing and reaching objective, evidence-based conclusions ( 13 ). As many clinical symptoms are transdiagnostic, or present across multiple mental disorders ( e.g. , insomnia, appetite change, negative mood, concentration difficulties), identifying non-invasive biological markers of dimensional as well as categorical symptom clusters could improve mental health screening and intervention efforts ( 14 ).
Modern breath analysis can be traced back to the seminal work of Pauling et al. ( 15 ) wherein they showed the presence of a colorful cast of compounds in human breath, using gas–liquid partition chromatography ( 16 ). Two classes of instruments have traditionally been used for breath analysis: (1) gas chromatographic technologies coupled with a mass spectrometric detector (GC–MS); and (2) electronic “nose” also known as “e-nose” ( 17 ). Early studies ( 18 ) used GC–MS technologies that are relatively expensive, difficult to use, and require specially-trained and field-experienced technicians to operate ( 19 ). Over the past two decades, exhalome researchers have increasingly employed easy-to-use, non-invasive, relatively fast, and low-cost tools ( 16 ) to investigate links between exhalome and symptoms of physical and mental health disorders ( 12 , 13 , 19 ). In contrast to GC–MS technology, e-nose devices enable researchers to easily study “smell-prints” (the molecular pattern of chemical compounds recorded by the sensors inside the device) derived from various VOCs using pattern recognition and modern machine learning methods ( 16 ).
E-nose-driven metrics have shown potential in differentiating case/control groups, especially in respiratory diseases ( 16 ). For example, Dragonieri et al. ( 20 ) distinguished between controls and people with asthma (with 10 subjects per group, four different groups), without the need to observe intricate molecular components of the breath. One of the main differences between GC–MS and an e-nose is that e-nose researchers do not need to confront a long list of compounds and their concentrations within a particular sample, but instead need to know to what degree a detected smell-print matches a known compound pattern ( 21 ). From an evolutionary biology perspective, this process is roughly similar to the way the human olfactory system has evolved since it does not directly recognize the presence of a particular chemical compound; rather, it senses a pattern similar to what it has already been experienced by the brain without knowing what particular chemical compound has implemented that pattern on the sensory organ ( 17 , 22 ). The study of human breath, as well as potential biomarkers it may actualize to monitor brain health and functions, has not been fully investigated within large samples using robust statistical methods ( 13 ). Additional research in this area could pave the way for the establishment of breath analysis in the diagnosis of various psychiatric symptoms.
According to a recent review ( 22 ), biological sources of the VOCs in respiration measured by e-nose devices are known to some extent. In the work of Bajtarevic et al. ( 23 ), isoprene, acetone, methanol, and benzene were employed as biomarkers of lung cancer. The concentrations of these VOCs decreased in patients compared to healthy subjects due to uncontrolled creation of new and unnecessary lung cells as well as retention of old damaged cells ( 24 ). However, Sánchez et al. ( 22 ) also noted that the VOCs present in exhaled breath are not necessarily produced by endogenous biochemical processes ( e.g. , acetonitrile is commonly found in the breath of smokers, occurring exogenously).
Breath analysis has shown some success in indexing a variety of physiological symptoms ( 13 , 17 ) within modestly sized samples, demonstrating that candidate VOCs can plausibly index the presence of certain disorders within individuals. For instance, e-nose technology has distinguished between: (1) smokers and non-smokers ( 21 ); (2) mild/severe asthmatics from non-asthmatics ( 20 ); (3) smokers with and without lung cancer ( 25 ); and (4) individuals with Alzheimer’s disease, Parkinson’s disease, and healthy controls ( 26 , 27 ). Moreover, altered levels of nitric oxide in the breath have been associated with: (1) cardiovascular, neurological, and respiratory disorders ( 13 ); and (2) increased negative affect (anxiety, depression) and stress via weakening of the immune system ( 28 – 33 ). Further research is warranted to determine whether a broader spectrum of VOCs beyond nitric oxide is implicated in psychiatric symptoms ( 13 ).
There has been attempts to standardize e-nose instruments and sampling procedures and highlight the potential technical issues for exhalome research ( 34 ). However, there are some gaps to be filled in the exhalome literature, including, small and non-representative sample sizes and failure to account for non-linearity of data arising from the measurement of exhaled breath ( 13 , 35 ). In addition, variability in e-nose detectors (whether commercially available or custom-built in labs), which are typically constructed with a small number of sensor arrays ( 14 ) could limit the resolution to detect complex VOC patterns in breath samples. Although the recommended e-nose analysis pipeline for breath analysis consists of “data acquisition, data pre-processing including data reduction/feature selection, generation of a pattern recognition algorithm in a training set, and testing of the algorithm in a validation set” ( 36 ), some studies do not perform external validation or confirmation of their findings, thereby limiting reliability and validity of their reports ( 37 ).
In investigations where a cohort design is analyzed and individuals with physical and/or mental disorder comorbidities will be involved, it is postulated that conventional unsupervised methods like principal component analysis (PCA), which has been widely used thus far in exhalome studies ( 16 ), will have difficulty differentiating between cases and controls ( 37 ). Although researchers recommend that supervised dimension reduction techniques such as partial least squares discriminant analysis (PLS-DA) be used in such designs, the combination of PCA and linear discriminant analysis (LDA) tends to yield more consistent results ( 36 ). Furthermore, with respect to clinically relevant prediction/classification, no published exhalome studies have employed a substantial heterogeneous sample of individuals to identify whether exhaled breath patterns can differentiate transdiagnostic clinical symptoms ( e.g. , negative affect, anxiety, substance use, and depression). In order for a particular VOC pattern to be a useful biomarker of impairment, it must be sensitive and specific, distinguishing abnormal from normal functioning. In addition to data analysis concerns, issues regarding the collection of e-nose data are crucial to address. For e-nose to be more widely tested in research settings, hardware must be easy and straightforward to use and validated in less-controlled environments ( e.g. , outpatient clinic or hospital setting).
The goal of this study was to identify whether patterns of human exhalome collected with a straightforward sampling approach and extracted by modern instruments (e-nose) and analyzed by machine learning approaches can replicate prior work linking VOC patterns to depression and anxiety symptoms ( 38 ). As some research has attempted to show that breath patterns vary as a function of gender and age ( 39 ), we also incorporated gender and age as factors in our analysis. As more women than men suffer from mood and anxiety disorders ( 40 ), breath patterns may show differential classification for men and women. To measure exact breath composition patterns, we utilized an e-nose (Cyranose 320; Smiths Detection, Pasadena, CA, USA) with 32 sensors to improve VOC detection accuracy and reliability ( 21 ). Furthermore, we investigated whether these goals are achievable under less-controlled, simpler sampling conditions without the need for sophisticated equipment such as VOC filters, separated air tubes, valves, medical air capsules and controlled sampling room conditions.
Participants
A total of 1,550 participants (947 female; ages 18–66 years) were recruited via fliers, radio, and internet advertisements from the greater Tulsa, OK area and completed a screening visit at Laureate Institute for Brain Research (LIBR) between 07/01/2016 and 05/21/2018 to determine further eligibility for various ongoing studies at LIBR. Participants with psychosis or cognitive impairments or medical conditions causing neuropsychiatric disorders were excluded. Written and informed consent was obtained from all participants, and the study was approved by Western IRB, WIRB Protocol No. 20101611. Participants received compensation for their participation.
During their screening visit, participants completed a demographics questionnaire (to obtain age, gender, and nicotine smoking status) as well as the Patient Health Questionnaire 9 (PHQ-9) ( 41 ), the Drug Abuse Screening Test (DAST-10) ( 42 , 43 ) and the Overall Anxiety Severity and Impairment Scale (OASIS) ( 44 ) to index symptoms of depression, substance use disorder, and anxiety respectively. Body mass index (BMI) was calculated by using an InBody370 Impedance Body Composition Analyzer (InBody Co., Ltd., South Korea). After excluding participants with incomplete/unknown smoking status data, 1,207 participants were included in the analysis. Although formal sample size estimation was not performed prior to study start, a sample of 1,207 subjects was sufficient to detect an effect with Cohen’s d of 0.081 with 80% power and significance level of 0.05. When queried about their nicotine use status, 36% ( n = 435) were found to be current smokers and 64% ( n = 772) were found to be non-smokers. The consort diagram for participant inclusion in this work is presented in Figure 1 . Demographic and clinical characteristics of the final participants involved in this study are presented in Table 1 . All participants were instructed to abstain from any food, drink, and chewing gum consumption, except for water, within 2 hrs of breath sample collection, and refrain from smoking and brushing their teeth.
Figure 1 Consort-like diagram for participant inclusion, according to research inclusion criteria.
Table 1 Participant characteristics.
Technology and Hardware
A commercially available e-nose (Cyranose 320; Smiths Detection, Pasadena, CA, USA), was utilized to acquire exhaled VOC patterns (sampling procedure below). This e-nose utilizes 32 sensors and on-board pattern recognition algorithms to detect chemical vapors of interest to produce a “smell print”. As these sensors are semi-selective for various compounds, all of them will respond to the mixture (breath sample) in varying degrees. Sample differentiation is organized by basic properties (polarity, hydrogen bonding, acidity/basicity, etc. ) rather than by specific compounds ( e.g. , oxygen, nitric oxide, carbon dioxide) meaning that it seeks an established pattern of compounds on the sensor array and not each specific chemical compound alone. First the e-nose is initiated by registering levels of ambient air (baseline measurement) over time. Next, when VOCs from exhaled breath pass over organic insulated polymer within the e-nose, polymer swelling produces a change in electrical resistance output as a function of time for each sensor. Raw signals are output as a function of resistance (ohms) by time (seconds) for baseline and registered breath. These signals are then processed to compute percent signal change from baseline for each of the 32 sensors.
Sampling Procedure
After participants provided informed consent and completed questionnaires, they provided one exhaled breath sample, collected in sampling bags comprised of Nalophane film (16 in) and a 3-in poly-tetra-fluoro-ethylene tube attached as a mouth-piece for the participant (and also as an interface to be attached to the e-nose). Nalophane bags were employed, because they are cost-effective, reliable and well-suited for exhalome investigations ( 45 ). The breath sample collection procedure was administered by a trained research assistant. Participants were instructed to take a deep breath and blow one vital capacity exhalation into a sampling bag. A baseline metric of ambient air from the e-nose equipment (6 min) was obtained in accordance to manufacturer’s guidelines. The bag was secured and connected to the e-nose to sample the exhaled breath for duration of 1 min. This procedure was followed by a subsequent ambient air collection (1 min) and then the second breath sample measurement was performed for another 1 min from the same sampling bag in the same manner described above.
Data Preprocessing
First, raw e-nose breath signals were corrected for baseline drift ( 25 ) by fitting a five-degree polynomial to the signal acquired from each of the 32 e-nose sensors. Second, percent signal change (PSC) from baseline was computed as the corrected breath signal divided by the corrected baseline signal, with the highest value during the first breath sample as the metric of interest for each of the 32 sensors. Third, a regression model was fitted using an 11th-order polynomial with respect to each participant’s e-nose collection date to correct long-term temporal drift ( 36 ). Fourth, to correct for heterogeneity in overall VOC concentration magnitude across participants, a double standardization procedure was performed on the data: (1) all participants’ breath samples were standardized (z-scored) on one sensor; and (2) all 32 sensor responses were standardized on one participant’s breath sample. Fifth, as several sensor responses were significantly correlated, principal components analysis (PCA) was applied as an exploratory machine learning method to reduce data dimensions and generate linearly independent breath factors. All 32 principal components were used for data analysis. Details regarding data preprocessing steps are presented in the Supplementary Material .
Statistical Analysis
We computed intraclass correlations (ICCs) ( 46 ) based on responses from both breath sample draws for each sensor to evaluate the short-term stability of sensor readings. Large ICCs would indicate relatively stable measurements, implying little difference between selecting the first or second sample draw. Conversely, small ICCs would be indicative of rapidly decaying or unstable sensor measurements.
Figure 2 illustrates the machine learning analysis pipeline used in the present study, wherein transformed PSC e-nose data were related to demographic and clinical variables. In machine learning, it is common to perform cross-validation so that a model is repeatedly trained and tested on the dataset to obtain robust performance and accuracy results. A training set is used in order to let the machine “learn” from the data (fit the model to data) and a test set is used for evaluating the fitted model on training data in terms of accuracy (how close the model’s output is to the real data).
Figure 2 Statistical data analysis and machine learning pipeline.
Supervised machine learning algorithms were applied to the transformed PSC data using the R and Python statistics platforms. Specifically, Random Forest (RF) ( 47 – 49 ), Support Vector Machine (SVM) ( 50 ), and linear/logistic regression learning algorithms with varying hyperparameters and nested cross-validation ( 51 , 52 ) (five-fold for both the inner and outer loops) were applied to the questionnaire, BMI, and e-nose data. SVM solves an optimization problem to find support vectors which are a subset of points from the training dataset, and the decision boundary is calculated based on these support vectors. On the other hand, RF is an ensemble learning method that is constructed by multiple bagged decision trees. To evaluate model accuracy, Area Under the receiver operating characteristic Curve (AUC) and R 2 values were used. The primary variables of interest related to e-nose VOCs were three mental health variables: PHQ-9, OASIS and DAST-10 scores. Other measures included were age, sex, BMI, and nicotine smoking status. We also attempted to replicate the results from Cheng et al. ( 21 ), who differentiated subject smoking status based on the first two PCs.
Furthermore, nested validation was applied for model hyperparameter tuning and feature selection ( e.g. , selecting number of trees in RF or regularization term in SVM) in each inner loop iteration ( 53 ). The dataset was first divided into five disjointed and equally-sized subsets or “folds”. There were two nested loops (inner and outer loops) within this pipeline (see Figure 2 ) and on each repetition, one of the folds was used as the test/validation set, whereas the remaining folds were treated as the training set. Although the divisions were established by randomization for regression problems (age, BMI and mental health scores), one-way analysis of variance (ANOVA) test was implemented to ensure these subsets had the same population mean of the dependent variable; stratified division was applied for classification problems (gender and smoking status). Both loops were iterated five times to evaluate and cross validate results. In each run of this nested CV structure, prediction performance was measured, and the model with the best accuracy was specified as the final result of this pipeline.
The machine learning pipeline ( Figure 2 ) was applied to depression, anxiety, and addiction variables of interest including PHQ-9, OASIS, and DAST-10 ( Figure 3 ). In general, although some of the algorithms learned to predict different psychiatric symptoms, explaining as much as 20% of the variance (blue bars, Figure 3 ), these models did not generalize to the test set (orange bars, Figure 3 ). For the independent test dataset, very little variance was accounted for, and in one case, the prediction was worse than just predicting the mean of the test sample (negative variance accounted for). Supplementary Material provides additional illustrations of machine learning analyses. This pattern of results is most consistent with model overfitting to the training dataset. The R 2 value of the test dataset being smaller than or near 0 (orange bars, Figure 3 ) indicates that the fitted model is worse or not much better than the null hypothesis (a model that always predicts the mean value for any input). Models for age and BMI have similar prediction performance as those for mental health variables ( Figure 4 ).
Figure 3 Model performance (R 2 values) in predicting PHQ-9, OASIS, and DAST-10 using Linear Model, Random Forest (RF), and Support Vector Machine (SVM) algorithms. Error bars represent standard deviations of R 2 values.
Figure 4 Model performance (R 2 values) in predicting age and BMI using Linear Model, Random Forest (RF), and Support Vector Machine (SVM) algorithms. Error bars represent standard deviations of R 2 values.
Similar to predicting continuous outcome variables, evidence for overfitting was observed when predicting dichotomous outcomes: smoking status and gender. Area under the curve (AUC) was used to measure model performance for predicting smoking status and gender. Cross-validated AUC in the training data (blue bars, Figure 5 ) was above 0.5 (red-dashed line) for all methods. However, the AUC on independent test data (orange bars, Figure 5 ) was consistent with 0.5 AUC, which is the null value of no discrimination capacity to distinguish between positive and negative classes. Our main focus was to assess the generalizability of machine learning models that use the sensor measurements directly ( Figures 3 – 5 ). As a secondary analysis, we also attempted to reproduce the results from ( 21 ), which differentiated smoking status based on the first two PCs with highest variance. In our data, no cluster(s) were present to separate smokers from non-smokers in the two-dimensional PC space ( Figure 6 ).
Figure 5 Model performance (AUC values) in predicting smoking status and gender using Linear Model, Random Forest (RF), and Support Vector Machine (SVM) algorithms. Error bars represent standard deviations of AUC values.
Figure 6 Principal component analysis plot of smoking status.
The ICC values indicated a strong reliability of e-nose measurements within a short period of time. The sensor value ICCs are between 0.91 and 0.99, with the exception of Sensor 8, which produced an ICC of 0.80. Together, these values show the stability ( 54 ) of sensor reading between selecting the first and second sample draws.
The current investigation employed multiple machine learning approaches on a sizable community sample of 1,207 individuals to further previous research linking e-nose exhalome to assessment of psychiatric symptom severity in less-controlled research settings. Although our results showed that machine learning algorithms using cross-validation were able to achieve accuracy and account for variance above the null hypothesis in the training sample, the models did not generalize to an independent test sample, which is evidence for overfitting. Thus, we found no generalizable relationship between e-nose factors or PCA factors and the mental health symptoms of depression, anxiety, or substance use.
The main strength of the current study over previous studies is the larger sample size. This is the largest e-nose study ever conducted on a psychiatric population; our current sample of over 1,200 participants is substantially larger than published exhalome research (typically less than 100 subjects) ( 36 , 55 ). Ligor et al. ( 56 ) used solid phase microextraction-gas chromatography combined with mass spectrometry (SPME-GC/MS) analyses with 484 subjects for selecting potential lung cancer biomarkers, which was the next largest sample we identified. A second strength of our study is the application of multiple analysis pathways suggested previously by researchers in the field. We utilized a rigorous machine learning paradigm with an iterative nested cross-validation approach, which involved splitting the dataset into training and testing sets on each iteration of model building and evaluation processes. To assess overfitting, we included an independent dataset to test final models. Overfitting could be a main culprit in overlooking the possible presence of false positives in prior work ( 57 ). A third strength is our use of multiple psychiatric symptoms to attempt to identify e-nose metrics as novel biomarkers in assessing and predicting mental illness. Exclusive reliance on self-report and traditional methods for diagnosis, treatment and monitoring of psychiatric symptoms is a current challenge in the field. There is a need for simple measures to predict the severity of these symptoms and develop more accessible and non-invasive biomarkers for this purpose in medicine and psychiatry.
In addition to possible overfitting in previous smaller studies, the lack of replication in the current study could also be due to the “winner’s curse” phenomena that has been observed in association studies: early studies tend to report a result with a substantial effect size, which is less likely to be seen in subsequent replication studies i.e. , GWAS and epidemiological investigations. In the long run, regression toward the mean would be a more achievable result expected to be observed ( 58 ). Furthermore, the sampling method to collect breath samples practiced in this work was different compared to previous works: in contrast to applying sophisticated sampling tubes, filters and special valves to breath data collection, we employed an accessible sampling setup within a conventional research/medical facility setting. Another factor limiting replication could be the dimensional design of the present study as opposed to categorical group comparisons (cases versus controls) reported in prior works; it is possible that effects become larger when comparing extremes of a phenotype ( e.g. , healthy control versus symptomatic patient).
A limitation of the current study is our use of e-nose hardware, which is cheaper, more easily measured, and more easily used biomarker, but is less accurate than gas chromatography and mass spectrometry (GC–MS) technologies ( 59 , 60 ). Unlike GC–MS, which detects specific chemicals and molecules within the breath, e-nose detects patterns of chemical compositions detected over sensor arrays, performing “smell-print” recognition ( 20 ). A second limitation is the possible presence of psychiatric comorbidities among participants, which might have impacted detection of particular symptom(s). Third, this study was cross-sectional, with data collected at only one time point during a screening session; employing similar analysis strategies on longitudinal e-nose and symptom data may result in more effective prediction of future illness severity.
Another possible limitation was that the breath sample was collected from the mouth using a relatively simple procedure; it might be the case that having stricter control or collecting samples from alternate airways, e.g. , nasal passages, may yield different sets of smell prints and resulting outcomes might change. Although some previous studies used more sophisticated devices or VOC-filtered room air for breath sample collection control ( 20 , 21 , 25 ), this study focused on investigating an easy, quick, and relatively inexpensive approach to evaluate mental health status. Another potential limitation of the breath sampling procedures in the present study might be when participants hold their breath. The breath-hold process involves anatomic dead space ( 61 ), and it has been shown that exhalation rate and breath-hold affect the levels of exhaled VOCs detected by the Cyranose 320 ( 62 ). Furthermore, although the effect of diurnal variations on the breath sample VOCs detected by e-nose has been investigated ( 63 , 64 ), these variations were not observed in our pipeline ( Figure. S14 in Supplement ).
The present study did not replicate prior studies linking e-nose breath metrics to mental health variables within the context of a less-controlled sampling environment than the GC–MS ( 21 , 25 ). Given the limitations of this study, more work is needed to investigate whether e-nose technologies utilizing higher resolution sensor arrays and more sensitive materials can aid in the development of novel biomarkers to track psychiatric symptom severity.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Western Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MP developed the research question and conducted the research and supervised the project, as well as revised and edited the manuscript. BX and MM as joint first co-authors, performed the research, wrote the manuscript, and revised it. RK is the machine learning expert and supervised the data analysis pipeline and revised the manuscript. JS is field expert and revised the manuscript. BM is machine learning expert and revised the manuscript. SS revised the manuscript and gave feedback on data analysis. All authors contributed to the article and approved the submitted version.
This research was supported by the Laureate Institute for Brain Research and the National Institute of General Medical Sciences (P20GM121312, MP, RK).
Conflict of Interest
MP is an advisor to Spring Care, Inc., a behavioral health startup. He has received royalties for an article about methamphetamine in UpToDate. The author is supported by a grant from the National Institute of Mental Health (R01 MH101453), from the National Institute on Drug Abuse (U01 DA041089).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to express their appreciation to the participants who contributed to provide the invaluable data for this research. Also, special thanks to research assistants at Laureate Institute for Brain Research for their assistance with data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.503248/full#supplementary-material
1. Taware R, Taunk K, Pereira JA, Dhakne R, Kannan N, Soneji D, et al. Investigation of urinary volatomic alterations in head and neck cancer: a non-invasive approach towards diagnosis and prognosis. Metabolomics (2017) 13:111. doi: 10.1007/s11306-017-1251-6
CrossRef Full Text | Google Scholar
2. Sinues PM-L, Zenobi R, Kohler M. Analysis of the exhalome: a diagnostic tool of the future. Chest (2013) 144:746–9. doi: 10.1378/chest.13-1106
PubMed Abstract | CrossRef Full Text | Google Scholar
3. de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, Khalid T, et al. A review of the volatiles from the healthy human body. J Breath Res (2014) 8:014001. doi: 10.1088/1752-7155/8/1/014001
4. Goldsmith D, Rapaport M, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry (2016) 21:1696. doi: 10.1038/mp.2016.3
5. Kaczkurkin AN, Moore TM, Calkins ME, Ciric R, Detre JA, Elliott MA, et al. Common and dissociable regional cerebral blood flow differences associate with dimensions of psychopathology across categorical diagnoses. Mol Psychiatry (2018) 23:1981. doi: 10.1038/mp.2017.174
6. Roffman JL, Petruzzi LJ, Tanner AS, Brown HE, Eryilmaz H, Ho NF, et al. Biochemical, physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial. Mol Psychiatry (2018) 23:316. doi: 10.1038/mp.2017.41
7. Sjögren M, Nielsen ASM, Hasselbalch KC, Wøllo M, Hansen JS. A systematic review of blood-based serotonergic biomarker in Bulimia Nervosa. Psychiatry Res (2019) 279:155–71. doi: 10.1016/j.psychres.2018.12.167
8. Sapolsky RM. Stress and the brain: individual variability and the inverted-u. Nat Neurosci (2015) 18:1344. doi: 10.1038/nn.4109
9. Kataoka H, Saito K, Kato H, Masuda K. Noninvasive analysis of volatile biomarkers in human emanations for health and early disease diagnosis. Bioanalysis (2013) 5:1443–59. doi: 10.4155/bio.13.85
10. Cohen S. Psychological stress, immunity, and upper respiratory infections. Curr Dir psychol Sci (1996) 5:86–9. doi: 10.1111/1467-8721.ep10772808
11. Linder JA, Singer DE. Health-related quality of life of adults with upper respiratory tract infections. J Gen Internal Med (2003) 18:802–7. doi: 10.1046/j.1525-1497.2003.21246.x
12. Gutiérrez-Segura J, Cruz-Calderon S, Bedoya-Arias J, López-Pineda J, Chacón-Zuluaga A, Montilla- Trejos C, et al. Upper respiratory tract infections and bipolar affective and obsessive-compulsive disorders in colombia, 2009-2016: An ecological study. Int J Infect Dis (2018) 73:149. doi: 10.1016/j.ijid.2018.04.3752
13. Tonacci A, Sansone F, Pala AP, Conte R. Exhaled breath analysis in evaluation of psychological stress: A short literature review. Int J Psychol (2019) 54(5):589–97. doi: 10.1002/ijop.12494
14. Di Natale C, Paolesse R, Comandini P, Pennazza G, Martinelli E, Rullo S, et al. Identification of schizophrenic patients by examination of body odor using gas chromatography-mass spectrometry and a cross-selective gas sensor array. Med Sci Monit (2005) 11:CR366–75.
PubMed Abstract | Google Scholar
15. Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci (1971) 68:2374–6. doi: 10.1073/pnas.68.10.2374
16. Dragonieri S, Pennazza G, Carratu P, Resta O. Electronic nose technology in respiratory diseases. Lung (2017) 195(2):157–65. doi: 10.1007/s00408-017-9987-3
17. Di Francesco F, Fuoco R, Trivella MG, Ceccarini A. Breath analysis: trends in techniques and clinical applications. Microchem J (2005) 79:405–10. doi: 10.1016/j.microc.2004.10.008
18. Wilson HK. Breath analysis: physiological basis and sampling techniques. Scandinavian J Work Environ Health (1986) 12(3):174–92. doi: 10.5271/sjweh.2159
19. Wilson AD, Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors (2011) 11:1105–76. doi: 10.3390/s110101105
20. Dragonieri S, Schot R, Mertens BJ, Le Cessie S, Gauw SA, Spanevello A, et al. An electronic nose in the discrimination of patients with asthma and controls. J Allergy Clin Immunol (2007) 120:856–62. doi: 10.1016/j.jaci.2007.05.043
21. Cheng Z, Warwick G, Yates D, Thomas P. An electronic nose in the discrimination of breath from smokers and non-smokers: a model for toxin exposure. J Breath Res (2009) 3:036003. doi: 10.1088/1752-7155/3/3/036003
22. Sánchez C, Santos JP, Lozano J. Use of electronic noses for diagnosis of digestive and respiratory diseases through the breath. Biosensors (2019) 9:35. doi: 10.3390/bios9010035
23. Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer (2009) 9:348. doi: 10.1186/1471-2407-9-348
24. Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, et al. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev (2012) 112:5949–66. doi: 10.1021/cr300174a
25. McWilliams A, Beigi P, Srinidhi A, Lam S, MacAulay CE. Sex and smoking status effects on the early detection of early lung cancer in high-risk smokers using an electronic nose. IEEE Trans Biomed Eng (2015) 62:2044–54. doi: 10.1109/TBME.2015.2409092
26. Bach J-P, Gold M, Mengel D, Hattesohl A, Lubbe D, Schmid S, et al. Measuring compounds in exhaled air to detect alzheimer’s disease and parkinson’s disease. PloS One (2015) 10:e0132227. doi: 10.1371/journal.pone.0132227
27. Tisch U, Schlesinger I, Ionescu R, Nassar M, Axelrod N, Robertman D, et al. Detection of Alzheimer’s and Parkinson’s disease from exhaled breath using nanomaterial-based sensors. Nanomedicine (2013) 8:43–56. doi: 10.2217/nnm.12.105
28. Ritz T, Trueba AF, Liu J, Auchus RJ, Rosenfield D. Exhaled nitric oxide decreases during academic examination stress in asthma. Ann Am Thoracic Soc (2015) 12:1638–45. doi: 10.1513/AnnalsATS.201504-213OC
29. Ritz T, Trueba AF, Simon E, Auchus RJ. Increases in exhaled nitric oxide after acute stress: association with measures of negative affect and depressive mood. Psychosom Med (2014) 76:716–25. doi: 10.1097/PSY.0000000000000118
30. Srivastava N, Barthwal M, Dalal P, Agarwal A, Nag D, Seth P, et al. A study on nitric oxide, β-adrenergic receptors and antioxidant status in the polymorphonuclear leukocytes from the patients of depression. J Affect Disord (2002) 72:45–52. doi: 10.1016/S0165-0327(01)00421-9
31. Trueba AF, Rosenfield D, Oberdörster E, Vogel PD, Ritz T. The effect of academic exam stress on mucosal and cellular airway immune markers among healthy and allergic individuals. Psychophysiology (2013a) 50:5–14. doi: 10.1111/j.1469-8986.2012.01487.x
32. Trueba AF, Rosenfield D, Smith NB, Gorena TL, Ritz T. Social support as a predictor exhaled nitric oxide in healthy individuals across time. Int J Psychophysiol (2014) 93:356–62. doi: 10.1016/j.ijpsycho.2014.05.011
33. Trueba AF, Smith NB, Auchus RJ, Ritz T. Academic exam stress and depressive mood are associated with reductions in exhaled nitric oxide in healthy individuals. Biol Psychol (2013b) 93:206–12. doi: 10.1016/j.biopsycho.2013.01.017
34. Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respirat J (2017) 49(4):1600965. doi: 10.1183/13993003.00965-2016
35. Yan J, Guo X, Duan S, Jia P, Wang L, Peng C, et al. Electronic nose feature extraction methods: A review. Sensors (2015) 15:27804–27831\. doi: 10.3390/s151127804
36. Bikov A, Lázár Z, Horvath I. Established methodological issues in electronic nose research: how far are we from using these instruments in clinical settings of breath analysis? J Breath Res (2015) 9:034001. doi: 10.1088/1752-7155/9/3/034001
37. Leopold JH, Bos LD, Sterk PJ, Schultz MJ, Fens N, Horvath I, et al. Comparison of classification methods in breath analysis by electronic nose. J Breath Res (2015) 9:046002. doi: 10.1088/1752-7155/9/4/046002
38. Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M. Elevated plasma nitrate levels in depressive states. J Affect Disord (2001) 63:221–4. doi: 10.1016/S0165-0327(00)00164-6
39. Lourenço C, Turner C. Breath analysis in disease diagnosis: methodological considerations and applications. Metabolites (2014) 4:465–98. doi: 10.3390/metabo4020465
40. Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnormal Psychol (1998) 107:128. doi: 10.1037/0021-843X.107.1.128
41. Kroenke K, Spitzer RL, Williams JB. The phq-9: validity of a brief depression severity measure. J Gen Internal Med (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
42. Skinner HA. The drug abuse screening test. Addictive Behav (1982) 7:363–71. doi: 10.1016/0306-4603(82)90005-3
43. Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the drug abuse screening test. J Subst Abuse Treat (2007) 32:189–98. doi: 10.1016/j.jsat.2006.08.002
44. Norman SB, Hami Cissell S, Means-Christensen AJ, Stein MB. Development and validation of an overall anxiety severity and impairment scale (oasis). Depression Anxiety (2006) 23:245–9. doi: 10.1002/da.20182
45. Ghimenti S, Lomonaco T, Bellagambi F, Tabucchi S, Onor M, Trivella MG, et al. Comparison of sampling bags for the analysis of volatile organic compounds in breath. J Breath Res (2015) 9:047110. doi: 10.1088/1752-7155/9/4/047110
46. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. psychol Bull (1979) 86:420. doi: 10.1037/0033-2909.86.2.420
47. Breiman L. Random forests. Mach Learn (2001) 45:5–32. doi: 10.1023/A:1010933404324
48. Liaw A, Wiener M. Classification and regression by random forest. R News (2002) 2:18–22.
Google Scholar
49. Ho TK. Random decision forests. In: Proceedings of 3rd international conference on document analysis and recognition (IEEE) , vol. 1. (1995). p. 278–82.
50. Cortes C, Vapnik V. Support-vector networks. Mach Learn (1995) 20:273–97. doi: 10.1007/BF00994018
51. Ekhtiari H, Kuplicki R, Yeh H-w, Paulus MP. Physical characteristics not psychological state or trait characteristics predict motion during resting state fmri. Sci Rep (2019) 9:419. doi: 10.1038/s41598-018-36699-0
52. Krstajic D, Buturovic LJ, Leahy DE, Thomas S. Cross-validation pitfalls when selecting and assessing regression and classification models. J Cheminform (2014) 6:10. doi: 10.1186/1758-2946-6-10
53. James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning . New York: Springer (2013) Vol. 112 p. 18.
54. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropract Med (2016) 15(2):155–63. doi: 10.1016/j.jcm.2016.02.012
55. Oakley-Girvan I, Davis SW. Breath based volatile organic compounds in the detection of breast, lung, and colorectal cancers: A systematic review. Cancer Biomarkers (2018) 21:29–39. doi: 10.3233/CBM-170177
56. Ligor T, Pater Ł., Buszewski B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J Breath Res (2015) 9:027106. doi: 10.1088/1752-7155/9/2/027106
57. Gillan CM, Whelan R. What big data can do for treatment in psychiatry. Curr Opin Behav Sci (2017) 18:34–42. doi: 10.1016/j.cobeha.2017.07.003
58. Ioannidis JP. Why most discovered true associations are inflated. Epidemiology (2008) 19:640–8. doi: 10.2307/25662607
59. Lamote K, Brinkman P, Vandermeersch L, Vynck M, Sterk PJ, Van Langenhove H, et al. Breath analysis by gas chromatography-mass spectrometry and electronic nose to screen for pleural mesothelioma: a cross-sectional case-control study. Oncotarget (2017) 8:91593. doi: 10.18632/oncotarget.21335
60. Takeo E, Sasano R, Shimma S, Bamba T, Fukusaki E. Solid-phase analytical derivatization for gas-chromatography–mass-spectrometry-based metabolomics. J Biosci Bioengineer (2017) 124:700–6. doi: 10.1016/j.jbiosc.2017.07.006
61. Quinn M, Rizzo A. Anatomy, Anatomic Dead Space . StatPearls Publishing: Treasure Island (FL (2019). Available at: http://europepmc.org/abstract/MED/28723045 .
62. Bikov A, Hernadi M, Korosi BZ, Kunos L, Zsamboki G, Sutto Z, et al. Expiratory flow rate, breath hold and anatomic dead space influence electronic nose ability to detect lung cancer. BMC Pulm Med (2014) 14:202. doi: 10.1186/1471-2466-14-202
63. Kunos L, Bikov A, Lazar Z, Korosi BZ, Benedek P, Losonczy G, et al. Evening and morning exhaled volatile compound patterns are different in obstructive sleep apnoea assessed with electronic nose. Sleep Breath (2015) 19(1):247–53. doi: 10.1007/s11325-014-1003-z 2015/03//.
64. Liotino V, Dragonieri S, Quaranta V, Carratu P, Ranieri T, Resta O. Influence of circadian rhythm on exhaled breath profiling by electronic nose. J Biol Regulat Homeostatic Agents (2018) 32(5):1261–5.
Keywords: machine learning, data mining, electronic nose, mental health, exhalomes, computational psychiatry
Citation: Xu B, Moradi M, Kuplicki R, Stewart JL, McKinney B, Sen S and Paulus MP (2020) Machine Learning Analysis of Electronic Nose in a Transdiagnostic Community Sample With a Streamlined Data Collection Approach: No Links Between Volatile Organic Compounds and Psychiatric Symptoms. Front. Psychiatry 11:503248. doi: 10.3389/fpsyt.2020.503248
Received: 07 October 2019; Accepted: 24 August 2020; Published: 16 September 2020.
Reviewed by:
Copyright © 2020 Xu, Moradi, Kuplicki, Stewart, McKinney, Sen and Paulus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin P. Paulus, [email protected]
† These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- Simple Search
- Advanced Search
- Deposit an Item
- Deposit Instructions
- Instructions for Students
| Lee, Kyra SoHyun (2021) Dissertation (Ph.D.), California Institute of Technology. doi:10.7907/j5e1-k535. Chapter 1 When coated with a polymer surface layer and suspended on 3-D textured glass electrodes, the hybrid combination of polymer and graphene yields sensitive chemiresistive vapor sensors. The expansion and contraction of the polymer layer when it absorbs/reacts with the VOCs, is proposed to produce tremendous train on the suspended graphene. Hence, when VOCs permeates into the polymer layer, sizable electrical resistive changes as folds and creases is induced in the graphene due to its high gauge factor. The hybrid suspended polymer/Gr sensor exhibits substantial responses to polar organic vapors, especially pyridine, while also exhibiting reversibility and the potential future tunability in the types of polymers used as the reactive surface layer. Chapter 2 Various polar and non-polar functional groups were covalently bonded onto MoS2 yielding incredibly sensitive chemiresistive vapor sensors. The VOCs' interaction to the functional end groups produced tremendous signal, while also exhibiting reproducibility and reversibility. Future work will further standardize the sensors while also exploring tunability in the types of groups used. Chapter 3 This chapter reflects the very start of my PhD research, and one of the important lessons to learn about the electronic nose. It is an example that I wish my predecessors taught me (all had graduated by the time I began my research) that I hope to pass onto future nose users. It is just one example of many projects that had similar end result. Many key lessons can be learned for future nose users. Readers can choose to skip reading this.
|











COMMENTS
In the electronic nose technology section, the three aspects of sensors, algorithms and existing systems are summarized in detail. Moreover, the related challenges and limitations involved in the ...
In the last two decades, improvements in materials, sensors and machine learning technologies have led to a rapid extension of electronic nose (EN) related research topics with diverse applications. The food and beverage industry, agriculture and forestry, medicine and health-care, indoor and outdoor monitoring, military and civilian security systems are the leading fields which take great ...
Machine learning methods enable the electronic nose (E-Nose) for precise odor identification with both qualitative and quantitative analysis. Advanced machine learning methods are crucial for the E-Nose to gain high performance and strengthen its capability in many applications, including robotics, food engineering, environment monitoring, and medical diagnosis. Recently, many machine learning ...
The objective of this research project is to develop sensor arrays ― or so-called electronic nose ― for analysis of VOCs in air and exhaled breath. In this dissertation, we have investigated both commercial and synthesized thiol functionalized gold nanoparticles (AuNPs) as sensing materials for analysis of VOCs in air and exhaled breath.
the Electronic Nose for the Sensitive Detection of VOC . Thesis by . Kyra SoHyun Lee. In Partial Fulfillment of the Requirements for the degree of Chemistry . CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena, California . 2020 Defended June 4, 2020. ii 2020 .
Here, we review the recent advances in E-Nose machine learning techniques, with a focus on three important aspects: (1) feature extraction, (2) modeling, and (3) gas sensor drift compensation. By surveying machine learning methods for different E-Nose applications, this work tries to evaluate the performance of an E-Nose in existing ...
In this paper, the researchers intended to review the electronic nose. E-nose is a technology that has the capability to recognize odor and it mimics the human nose. Using Optical Sensor System, Mass Spectrometry, Ion Mobility Spectrometry, Gas Chromatography, and Infrared Spectroscopy are the different technologies in electronic nose. Metal-oxide, Photoionization detector, Catalytic bead ...
MS-Thesis, The University of Tennessee, Knoxville, TN, 1989. ... The electronic nose is a natural match for physiologically motivated odor analysis. Both the olfactory system and the electronic ...
Recent advances in the field of electronic noses (e-noses) have led to new developments in both sensors and feature extraction as well as data processing techniques, providing an increased amount of information. Therefore, feature selection has become essential in the development of e-nose applications. Sophisticated computation techniques can be applied for solving the old problem of sensor ...
Electronic nose is an important area of biological olfaction simulation and odour perceptron. This book identifies sensor drift, concept drift, sensor discreteness, and disturbance as the key challenges and presents efficient algorithms in signal processing, machine learning, and pattern recognition.
The electronic nose (eNose) is a novel technology that detects volatile organic compounds (VOCs). Early studies have shown that certain diseases and infections can result in characteristic changes in VOC profiles in the exhaled breath. This review summarizes current knowledge on breath analysis by the electronic nose and its potential for the ...
Download thesis Adobe PDF (13.55 MB) View statistics. Full metadata record Abstract: An electronic nose (e-nose) is capable of identifying chemical compounds through sensing and analysing odour molecules. ... Firstly, inspired by the knowledge of the human olfactory system, an automated fault monitoring and alarming electronic nose (e-nose ...
Citation: Xu B, Moradi M, Kuplicki R, Stewart JL, McKinney B, Sen S and Paulus MP (2020) Machine Learning Analysis of Electronic Nose in a Transdiagnostic Community Sample With a Streamlined Data Collection Approach: No Links Between Volatile Organic Compounds and Psychiatric Symptoms. Front. Psychiatry 11:503248. doi: 10.3389/fpsyt.2020.503248
This chapter reflects the very start of my PhD research, and one of the important lessons to learn about the electronic nose. It is an example that I wish my predecessors taught me (all had graduated by the time I began my research) that I hope to pass onto future nose users. It is just one example of many projects that had similar end result.
An electronic nose (E-nose) is a technology fundamentally inspired by the human nose, designed to detect, recognize, and differentiate specific odors or volatile components in complex and chaotic environments. ... If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is ...
An electronic nose (E-Nose) is a system designed for sensing and identifying gases and odors through chemical sensors [2], [3]. E-Nose stimulates the individual's sense of smell [4]. E-nose is composed of two main sub-systems which are feature extraction and artificial intelligence (AI).
The electronic nose (e-nose) is a new technology with many potential applications in rhinology. ... Thaler ER: Candidate's thesis: the diagnostic utility of an electronic nose: rhinologic applications. Laryngoscope 2002; 112: 1533-42. Article PubMed Google Scholar ...
In this Thesis, an electronic nose instrument was used to analyze and classify different herbs. The sensor responses were evaluated by Principal Component Analysis (PCA), Artificial Neural ...
2,4,6-trichloroanisole (TCA) is mainly responsible for cork taint in wine, which causes significant economic losses; therefore, the wine and cork industries demand an immediate, economic, noninvasive and on-the-spot solution. In this work, we present a novel prototype of an electronic nose (e-nose) using an array of digital and analog metal-oxide gas sensors with a total of 31 signals, capable ...
The system we investigated is the electronic nose and tongue system, which is designed to emulate human chemosensory system. The system is capable of detecting pollutants, such as volatile organic compounds (VOCs), ammonia and carbon dioxide in the air, and arsenic (As(III)) in the water. ... Open Access Theses. 1259. https://docs.lib.purdue ...
For odor measurements, an electronic nose (e-nose) is a new type of detection equipment, its working mode is similar to the mammal olfactory system (Persaud and Dodd, 1982). The device is mainly composed of a sensor array simulating olfactory cells and machine learning algorithms simulating the working mode of the brain (Gu et al., 2020).
electronic nose and a human nose (adapted from Craven et al., 1996). 10 Figure 1.5 Operational principle of a static headspace analysis in a sampling vial and the area (Ai) of a sensor response (adapted from www2.nose-network.org). 15 Figure 1.6 Some of the avalilable methods of analysis for data from sensor arrays (adapted from Jurs, 2000). 16
and quality evaluation of food items. Currently, sensory instruments like e-nose (electronic nose) and e-tongue (electronic tongue) are also being implemented to aid in both descriptive and naïve panel evaluations. The present study employed all three techniques for evaluating the sensory attributes of selected food and fruit items.
The library catalog is the most comprehensive list of UT Austin theses and dissertations. Since 2010, the Office of Graduate Studies at UT Austin has required all theses and dissertations to be made publicly available in Texas ScholarWorks; however, authors are able to request an embargo of up to seven years. Embargoed ETDs will not show up in ...