- New QB365-SLMS
- NEET Materials
- JEE Materials
- Banking first yr Materials
- TNPSC Materials
- DIPLOMA COURSE Materials
- 5th Standard Materials
- 1st Standard - CVBHSS Materials
- 2nd Standard - CVBHSS Materials
- 3rd Standard - CVBHSS Materials
- 4th Standard - CVBHSS Materials
- 5th Standard - CVBHSS Materials
- 12th Standard Materials
- 11th Standard Materials
- 10th Standard Materials
- 9th Standard Materials
- 8th Standard Materials
- 7th Standard Materials
- 6th Standard Materials
- 12th Standard CBSE Materials
- 11th Standard CBSE Materials
- 10th Standard CBSE Materials
- 9th Standard CBSE Materials
- 8th Standard CBSE Materials
- 7th Standard CBSE Materials
- 6th Standard CBSE Materials
- Tamilnadu Stateboard
- Scholarship Exams
- Scholarships


CBSE 12th Standard Chemistry Subject Solution Case Study Questions With Solution 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
12th Standard CBSE
Final Semester - June 2015
Read the passage given below and answer the following questions: The concentration of a solute is very important in studying chemical reactions because it determines how often molecules collide in solution and thus indirectly determine the rate of reactions and the conditions at equilibrium. There are several ways to express the amount of solute present in a solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Concentration can be expressed in terms of molarity, molality, parts per million, mass percentage, volume percentage, etc. The following questions are multiple choice questions. Choose the most appropriate answer: (i) The molarity (in mol L -1 ) of the given solution will be
(ii) Which of the following is correct relationship between mole fraction and molality?
(iii) Which of the following is temperature dependent?
(iv) Which of the following is true for an aqueous solution of the solute in terms of concentration?
Read the passage given below and answer the following questions: At 298 K, the vapour pressure of pure benzene, C 6 H 6 is 0.256 bar and the vapour pressure of pure toluene C 6 H 5 CH 3 is 0.0925 bar. Two mixtures were prepared as follows: (i) 7.8 g of C 6 H 6 + 9.2 g of toluene (ii) 3.9 g of C 6 H 6 + 13.8 g of toluene The following questions are multiple choice questions. Choose the most appropriate answer: (i) The total vapour pressure (bar) of solution 1 is
(ii) Which of the given solutions have higher vapour pressure?
(iii) Mole fraction of benzene in vapour phase in solution 1 is
(iv) Solution I is an example of a/an
Read the passage given below and answer the following questions: An ideal solution may be defined as the solution which obeys Raoult's law exactly over the entire range of concentration. The solutions for which vapour pressure is either higher or lower than that predicted by Raoult's law are called non-ideal solutions. Non-ideal solutions can show either positive or negative deviations from Raoult's law depending on whether the A-B interactions in solution are stronger or weaker than A - A and B - B interactions. The following questions are multiple choice questions. Choose the most appropriate answer: (i) Which of the following solutions is/are ideal solution(s)? (i) Bromoethane and iodoethane (ii) Acetone and chloroform (iii) Benzene and acetone (iv)n-heptane and n-hexane
(ii) Which of the following is not true for positive deviations?
(iii) For water and nitric acid mixture which of the given graph is correct?
(iv) Water- HCl mixture I. shows positive deviations II. forms minimum boiling azeotrope III. shows negative deviations IV. forms maximum boiling azeotrope
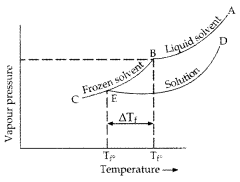
Read the passage given below and answer the following questions: The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties. For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 20°C is 17.5 mm of Hg.) The following questions are multiple choice questions. Choose the most appropriate answer: (i) Relative lowering of vapour pressure for the given solution is
(ii) The vapour pressure (mm of Hg) of solution will be
(iii) Mole fraction of sugar in the solution is
(iv) The vapour pressure (mm of Hg) of water at 293 K when 25 g of glucose is dissolved in 450 g of water is
Read the passage given below and answer the following questions: Few colligative properties are: (a) relative lowering of vapour pressure: depends only on molar concentration of solute (mole fraction) and independent of its nature. (b) depression in freezing point: it is proportional to the molal concentration of solution. (c) elevation of boiling point: it is proportional to the molal concentration of solute. (d) osmotic pressure: it is proportional to the molar concentration of solute. A solution of glucose is prepared with 0.052 g at glucose in 80.2 g of water. (K f = 1.86 K kg mol -1 and K b = 5.2 K kg mol -1 ) The following questions are multiple choice questions. Choose the most appropriate answer: (i) Molality of the given solution is
(ii) Boiling point for the solution will be
(iii) The depression in freezing point of solution will be
(iv) Mole fraction of glucose in the given solution is
*****************************************
Cbse 12th standard chemistry subject solution case study questions with solution 2021 answer keys.
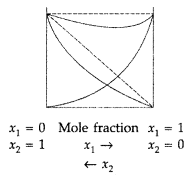
(i) (d) : Density of solution = 1.202 g/mL Volume of solution = \(\frac{100 \mathrm{~g}}{1.202 \mathrm{~g} / \mathrm{mL}}=83.2 \mathrm{~mL}\) Molarity = \(\frac{n_{\mathrm{KI}}}{\text { Volume of solution in } \mathrm{L}}\) \(=\frac{0.120 \mathrm{~mol}}{0.0832 \mathrm{~L}}=1.4423 \mathrm{~mol} \mathrm{~L}^{-1}\) (ii) (a): \(x_{2}=\frac{n_{2}}{n_{1}+n_{2}} ; x_{1}=\frac{n_{1}}{n_{1}+n_{2}} ; \frac{x_{2}}{x_{1}}=\frac{n_{2}}{n_{1}}\) \(\frac{x_{2}}{x_{1}}=\frac{m_{2} / M_{2}}{m_{1} / M_{1}}=\frac{m_{2}}{m_{1}} \times \frac{M_{1}}{M_{2}}\) ...(i) Molality = \(\frac{n_{2}}{m_{1}}=\frac{m_{2}}{M_{2} \times m_{1}}\) ...(ii) From(i) and (ii), m = \(\frac{x_{2}}{x_{1}} \times \frac{1}{M_{1}} ; x_{1}=1-x_{2}\) Hence. x 2 = \(\frac{m M_{1}}{1+m M_{1}}\) (iii) (a) : Mass does not depend on temperature while volume does. Hence, molarity depends on temperature. (iv) (b): 1M solution contains 1 mole of solute in less than 1000 g of the solvent whereas 1 m solution has 1 mole of the solute in 1000 g of the solvent.
(i) (b) : Moles of C 6 H 6 = \(\frac{7.8}{78}=0.1\) Mole C 6 H 5 CH 3 = \(\frac{9.2}{92}=0.1\) Mole fraction of C 6 H 6 = \(\frac{0.1}{0.1+0.1}=0.5\) => Mole fraction of C 6 H 5 CH 3 = 0.5 Vapour pressure of toluene = Vapour pressure of pure toluene x mole fraction of toluene = 0.0925 x 0.5 = 0.04625 Vapour pressure of benzene = 0.256 x 0.5 = 0.128 Total vapour pressure of solution = 0.17425 (ii) (a) : Moles of benzene in solution-II = \(\frac{3.9}{78}=0.05\) Moles of toluene in solution-II = \(\frac{13.8}{92}=0.15\) Vapour pressure of solution = 0.256 x 0.05 + 0.0925 x 0.15 = 0.0128 + 0.013875 = 0.026675 (iii) (c) : Mole fraction of benzene in vapour phase \(y_{\text {benzene }}=\frac{p_{\text {benzene }}}{P_{\text {total }}}=\frac{0.128}{0.17425}=0.734\) (iv) (a) : Benzene and toluene form an ideal solution.
(i) (d) : II represents negative deviations and III represents positive deviations. (ii) (b): For positive deviations \(p_{A}>p_{A}^{\circ} x_{A} \text { and } p_{B}>p_{B}^{\circ} x_{B}\) (iii) (b) : Water and nitric acid mixture shows negative deviations from Raoult's law, hence \(p_{A}<p_{A}^{\circ} x_{A} \text { and } p_{B}<p_{B}^{\circ} x_{B}\) (iv) (d): Water-HCl mixture shows negative deviations from Raoult's law and solutions showing negative deviations from ideal behaviour form maximum boiling azeotrope.
(i) (a) : Vapour pressure of water \(\left(p_{A}^{\circ}\right)\) = 17.5 mm of Hg Lowering of vapour pressure \(\left(p_{A}^{\circ}-p_{A}\right)\) = 0.061 Relative lowering of vapour pressure \(=\frac{p_{A}^{\circ}-p_{A}}{p_{A}^{\circ}}=\frac{0.061}{17.5}=0.00348\) (ii) (c): P = Vapour pressure of solvent - lowering in vapour pressure = 17.5 - 0.061 = 17.439 mm of Hg (iii) (a): \(\frac{p_{A}^{\circ}-p_{A}}{p_{A}^{\circ}}=x_{B}=0.00348\) Hence, mole fraction of sugar = 0.00348 (iv) (b): \(\frac{p_{A}^{\circ}-p_{A}}{p_{A}^{\circ}}=x_{B}=\frac{w_{B} \times M_{A}}{M_{B} \times w_{A}}\) \(\frac{17.5-p_{A}}{17.5}=\frac{25 \times 18}{450 \times 180}=5.56 \times 10^{-3}\) \(17.5-p_{A}=17.5 \times 5.56 \times 10^{-3}\) \(17.5-p_{A}=0.0973\) P = 17.40 mm Hg
(i) (b) : m \(=\frac{0.052}{180} \times \frac{1000}{80.2}=0.0036\) (ii) (c): \(\Delta T_{b}=K_{b} \times m=5.2 \times 0.0036=0.0187 \mathrm{~K}\) \(T_{b}=373+0.0187=373.0187 \mathrm{~K} \approx 373.02 \mathrm{~K}\) (iii) (d): \(\Delta T_{f}=K_{f} \times m=1.86 \times 0.0036=0.067 \mathrm{~K}\) (iv) (a): Moles of glucose \(=\frac{0.052}{180}=0.00028\) Moles 0f water = \(\frac{80.2}{18}=4.455\) Mole fraction of glucose = \(\frac{0.00028}{4.45+0.00028}=6.28 \times 10^{-5}\)
Related 12th Standard CBSE Chemistry Materials
12th standard cbse syllabus & materials, cbse 12th physics wave optics chapter case study question with answers, cbse 12th physics ray optics and optical instruments chapter case study question with answers, cbse 12th physics nuclei chapter case study question with answers, cbse 12th physics moving charges and magnetism chapter case study question with answers, cbse 12th physics electromagnetic induction chapter case study question with answers, cbse 12th physics atoms chapter case study question with answers, 12th physics alternating current chapter case study question with answers cbse, 12th maths vector algebra chapter case study question with answers cbse, 12th maths three dimensional geometry chapter case study question with answers cbse, 12th maths probability chapter case study question with answers cbse, 12th maths linear programming chapter case study question with answers cbse, 12th maths differential equations chapter case study question with answers cbse, 12th maths continuity and differentiability chapter case study question with answers cbse, 12th maths application of integrals chapter case study question with answers cbse, class 12th economics - non-competitive markets case study questions and answers 2022 - 2023.

Class VI to XII
Tn state board / cbse, 3000+ q&a's per subject, score high marks.

12th Standard CBSE Study Materials

12th Standard CBSE Subjects


Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Case Study Questions for Class 12 Chemistry Chapter 2 Solutions
- Last modified on: 2 months ago
- Reading Time: 5 Minutes

| Board | CBSE |
| Useful for | Class 12 Students |
| Subject | Chemistry |
| Chapter | Chapter 2 Solutions |
| Type of Questions | Case Study |
| Answers Provided | Yes |
| Format | Question-Answer Format |
| Important Link |
Table of Contents
There is Case Study Questions in class 12 Chemistry in session 2020-21. For the first time, the board has introduced the case study questions in the board exam. The first two questions in the board exam question paper will be based on Case Study and Assertion & Reason. The first question will have 5 MCQs out of which students will have to attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four. Here are the questions based on case study.
Case Study Question 1:
Read the passage given below and answer the following questions:
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example ofcolligative properties.
For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg. (Vapour pressure of water at 20 0 C is 17.5 mm of Hg)
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Relative lowering of vapour pressure for the given solution is (a) 0.00348 (b) 0.061 (c) 0.122 (d) 1.75
(ii) The vapour pressure (mm of Hg) of solution will be (a) 17.5 (b) 0.61 (c) 17.439 (d) 0.00348
(iii) Mole fraction of sugar in the solution is (a) 0.00348 (b) 0.9965 (c) 0.061 (d) 1.75
If weight of sugar taken is 5 g in 108 g of water then molar mass of sugar will be (a) 358 (b) 120 (c) 240 (d) 400
(iv) The vapour pressure (mm of Hg) of water at 293K when 25g of glucose is dissolved in 450 g of water is (a) 17.2 (b) 17.4 (c) 17.120 (d) 17.02
Related Posts
Download cbse books.
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
7 thoughts on “ Case Study Questions for Class 12 Chemistry Chapter 2 Solutions ”
Why answers are not given ???
Answers uploaded
can you share the pictures of solutions too??
Sure! Please wait for some time.
where are sols.,
Click on answer, it will expand.
can you share solutions of these also
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
CBSE Expert
CBSE Class 12 Chemistry Case Study Questions PDF
Case studies play a pivotal role in CBSE Class 12 Chemistry, as they enable students to apply theoretical knowledge to real-life scenarios. CBSE Class 12 Chemistry Case Study Questions PDF section introduces the significance of case studies in enhancing analytical skills and understanding complex chemical reactions.
Case studies challenge students to think critically, analyze experimental data, and devise problem-solving strategies. They provide a deeper understanding of chemical principles and their practical applications, fostering a holistic learning experience. Familiarize yourself with the structure of case study questions to streamline your preparation. Each case study presents a unique chemical problem, encouraging students to identify relevant concepts and devise accurate solutions.
Table of Contents
Class 12 Chemistry Case Study Questions
CBSE Class 12 Chemistry question paper will have case study questions too. These case-based questions will be objective type in nature. So, Class 12 Chemistry students must prepare themselves for such questions. First of all, you should study NCERT Textbooks line by line, and then you should practice as many questions as possible.

Chapter-wise Solved Case Study Questions for Class 12 Chemistry
| Click Below | |
|---|---|
Class 12 students should go through important Case Study problems for Chemistry before the exams. This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year’s exams. The solutions have been designed in a manner to help the grade 12 students understand the concepts and also easy-to-learn solutions.
Tips to Excel in CBSE Class 12 Chemistry Examinations
Excel in your Chemistry exams with these practical tips.
A. Regular Practice with Case Studies
Consistent practice with case study questions enhances your ability to tackle complex problems. Dedicate time to solving various case studies to build confidence.
B. Understanding Analytical Skills
Develop strong analytical skills to approach case studies logically. Break down complex problems into simpler components and analyze them step-by-step.
C. Time Management Strategies
Allocate sufficient time for each case study during the exam. Practice time management in mock tests to complete the paper within the stipulated time.
Best Books for Class 12 Chemistry
Strictly as per the new term-wise syllabus for Board Examinations to be held in the academic session 2024 for class 12 Multiple Choice Questions based on new typologies introduced by the board- Stand-Alone MCQs, MCQs based on Assertion-Reason Case-based MCQs. Include Questions from CBSE official Question Bank released in April 2024 Answer key with Explanations What are the updates in the book: Strictly as per the Term wise syllabus for Board Examinations to be held in the academic session 2024. Chapter-wise -Topic-wise Multiple choice questions based on the special scheme of assessment for Board Examination for Class 12th Chemistry.

Mastering CBSE Class 12 Chemistry case study questions is crucial for excelling in the exams. Embrace case studies as a valuable learning tool, and with practice, you’ll ace your Chemistry exams with confidence.
Benefits of Utilizing the CBSE Class 12 Chemistry Case Study PDF
- Enhanced Learning Experience : The case study PDF offers practical examples and scenarios, making the learning process engaging and relatable for students.
- Application of Theoretical Concepts : It enables students to apply theoretical knowledge to practical situations, honing their problem-solving and analytical skills.
- Real-World Relevance : By connecting classroom learning to real-life applications, students can grasp the practical significance of chemistry in various industries.
- Critical Thinking Development : Analyzing case studies encourages students to think critically and make informed decisions based on chemical principles.
- Exam Preparation : Exposure to case studies aids in better preparation for chemistry examinations by providing a comprehensive understanding of the subject.
The CBSE Class 12 Chemistry case study PDF brings a refreshing perspective to the world of education. By intertwining theoretical knowledge with practical applications, it equips students to face real-world challenges with confidence. The diverse case studies provide invaluable insights, encouraging students to explore chemistry beyond the classroom and make a positive impact on society.
What is the CBSE Class 12 Chemistry case study PDF?
The CBSE Class 12 Chemistry case study PDF is a curated document by CBSE, presenting real-life applications of chemistry concepts for students to understand the subject’s practical relevance.
How does the case study PDF benefit students?
The case study PDF enhances the learning experience, fosters critical thinking, promotes application-based learning, and prepares students for examinations.
Are the case studies diverse in content?
Yes, the case studies cover various branches of chemistry, including organic, inorganic, physical, environmental, and analytical chemistry.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
CBSE NCERT Solutions
NCERT and CBSE Solutions for free
Case Study Class 12 Chemistry With Questions Answers
In Coming Exams, CBSE will ask two Case Study Questions in the CBSE class 12 Chemistry questions paper. Each theme will have five questions and students will have a choice to attempt any four of them. Here are some example questions Based On Case Study Problems:
Question-1 Read the passage given below and answer any four out of the following questions: Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e.g., urea. On a large scale, ammonia is manufactured by Haber’s process. In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated with hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH– ions. The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base.

The following questions are multiple-choice questions. Choose the most appropriate choice 1. On a small scale, ammonia is obtained from ammonium salts which decompose when treated with 1.caustic soda 2.calcium chloride 3.sodium hydroxide 4.sodium chloride
2.The optimum conditions for the production of ammonia are a pressure of 1. 200*105 Pa 2. 400*105 Pa 3. 100*105 Pa 4. 300*105 Pa
3. The catalyst which is used in the preparation of NH3 by Haber’s process 1. Mg2O3 + K2O 2. Al2O3 + K2O 3. NaO3 + K2O 4. None of these 4. The ammonium molecule has: 1. five bond pair and two lone pair 2. four lone pair and one bond pair 3. three bond pair and one lone pair 4. three bond pair and two lone pair 5. A compound reacts with ammonia to form deep colour solution, identify the compound 1. Au2+ 2. Cu2+ 3. Al3+
Questions-2 Read the passage and answer any four out of the following questions: Colloidal particles always carry an electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative. The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electrodispersion of metals. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place. When silver nitrate solution is added to the potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium, and negatively charged colloidal solution results. acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer. The presence of equal and similar charges on colloidal particles is largely responsible for providing stability to the colloidal solution. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
1. Assertion and reason both are correct statements and reason is correct explanation for assertion 2. Assertion and reason both are correct statements but reason is not correct explanation for assertion 3. Assertion is correct statement and reason is wrong statement 4. Assertion is wrong statement but reason is correct statement
1. Assertion: The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution. Reason: The repulsive forces between charged particles having the same charge prevent them from aggregating and provide stability.
2. Assertion: The first layer is mobile in Helmholtz electrical double layer. Reason: The potential difference between the fixed layer and the diffused layer of opposite charges is called zeta potential.
3. Assertion: The sol particle in the colloid has a charge. Reason: The charge in sol is due to electron capture by sol particles during the electrodispersion of metals.
4. Assertion: Methylene blue sol is a negatively charged sol. Reason: When KI solution is added to AgNO3 solution, positively charged sol formed.
5. Assertion: If FeCl3 is added to an excess of hot water, a positively charged sol of hydrated ferric oxide is formed. Reason: When ferric chloride is added to NaOH a negatively charged sol is obtained with adsorption of OH- ions.
Question-3 .Read the passage and answer the following questions: The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to be ionically arising purely from electrostatic interactions between the metal ion and the ligand. Ligands are treated as point charges in case of anions or point dipoles in case of neutral molecules. The five d orbitals in an isolated gaseous metal atom/ion have the same energy, i.e., they are degenerate. In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal d orbitals and the electrons (or negative charges) of the ligands. This splitting of the degenerate levels due to the presence of ligands in a definite geometry is termed crystal field splitting and the energy separation is denoted by Δ0. The colour in the coordination compounds can be readily explained in terms of the crystal field theory. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. 1.Assertion and reason both are correct statements and reason is correct explanation for assertion. 2. Assertion and reason both are correct statements but reason is not correct explanation for assertion. 3. Assertion is correct statement but reason is wrong statement. 4. Assertion is wrong statement but reason is correct statement.
1. Assertion: The dx2-y2 and dz2 orbitals which point towards the axes along the direction of the ligand will experience more repulsion. Reason: The dxy, dyz and dxz orbitals which are directed between the axes will be lowered in energy.
2. Assertion: The complex [Ti(H2O)6]3+, which is red in colour. Reason: The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron.
3. Assertion: Ligands for which Δ0Δ0 < P are known as weak field ligands and form high spin complexes. Reason: If Δ0 > P, then the fourth electron enters one of the eg orbitals giving the configuration t2g3 eg1.
4. Assertion: In tetrahedral coordination entity formation, the d orbital splitting is inverted and is smaller as compared to the octahedral field splitting. Reason: Spectrochemical series is based on the absorption of light by complexes with different ligands.
5. Assertion: The crystal field model is successful in explaining the formation, structures, colour and magnetic properties of coordination compounds. Reason: The anionic ligands are found at the low end of the spectrochemical series.
Answer Key:
1. (b) Assertion and reason both are correct statements and reason is not correct explanation for assertion. 2. (c) Assertion is correct statement but reason is wrong statement. 3. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. 4. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. 5. (d) Assertion is wrong statement but reason is correct statement.
Question-4 Read the passage and answer any four out of the following question Alfred Werner (1866-1919), a Swiss chemist was the first to formulate his ideas about the structures of coordination compounds. Werner proposed the concept of a primary valence and a secondary valence for a metal. The coordination entity constitutes a central metal atom or ion bonded to a fixed number of ions or molecules. In a coordination entity, the atom/ion to which a fixed number of ions/groups are bound in a definite geometrical arrangement around it is called the central atom or ion. The ions or molecules bound to the central atom/ion in the coordination entity are called ligands. Ligands may be simple ions such as Cl-, small molecules such as H2O or NH3, larger molecules such as H2NCH2CH2NH2 or N(CH2CH2NH2)3 or even macromolecules, such as protein. Ligands are unidentate, bidentate and polydentate. The coordination number (CN) of a metal ion in a complex is the number of ligand donor atoms to which the metal is directly bonded.
In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
1. Assertion and reason both are correct statements and reason is correct explanation for assertion 2. Assertion and reason both are correct statements but reason is not correct explanation for assertion 3. Assertion is correct statement but reason is wrong statement 4. Assertion is wrong statement but reason is correct statement
1. Assertion: Binary compounds such as CrCl3, have a primary valence of 3. Reason: Coordinate compound metals show only one type of linkage that is primary linkage.
2. Assertion: CoCl3(NH3)3 is a coordination entity in which the cobalt ion is surrounded by three ammonia molecules and three chloride ion. Reason: The central atom/ion in the coordination entities: [NiCl2(H2O)4] is Ni2+.
3. Assertion: H2NCH2CH2NH2 (ethane-1,2-diamine) ligand is said to be didentate. Reason: Didentate ligands are bind through two donor atoms.
4. Assertion: The complex ions, [PtCl6]2- the coordination number of Pt is 4. Reason: Ligand which can ligate through two different atoms is called ambidentate ligand.
5. Assertion: EDTA can bind through two nitrogen and four oxygen atoms to a central metal ion. Reason: The number of ligating groups attach to an atom is called the denticity of the ligand.
1. (c) Assertion is correct statement but reason is wrong statement 2. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion 3. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion 4. (d) Assertion is wrong statement but reason is correct statement 5. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion
Question-5 Read the passage given below and answer any four out of the following questions: Nitrogen differs from the rest of the members of group 15 due to its smaller size, high electronegativity, high ionisation enthalpy, and non-availability of d orbitals. Nitrogen has a unique ability to form pπ-pπ multiple bonds with itself. Nitrogen exists as a diatomic molecule with a triple bond one s and two p between the two atoms. Phosphorus, arsenic and antimony from single bonds as P–P, As–As and Sb–Sb while bismuth forms metallic bonds in an elemental state. Dinitrogen is produced commercially by the liquefaction and fractional distillation of air. Liquid dinitrogen (b.p. 77.2 K) distils out first leaving behind liquid oxygen (b.p. 90 K). In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite. Dinitrogen is a colourless, odourless, tasteless and non-toxic gas. It has two stable isotopes 14N and 15N. It has very low solubility in water. The main use of dinitrogen is in the manufacture of ammonia and other industrial chemicals containing nitrogen.
The following questions are multiple-choice questions. choose the most appropriate answer
1. N–N bond is weaker than the single P–P bond because 1. high interelectronic repulsion of the bonding electrons 2. high interelectronic repulsion of the non-bonding electrons 3. no repulsion between bonding electrons 4. no repulsion between non-bonding electrons 2. Very pure nitrogen can be obtained by the 1. thermal decomposition of sodium 2. thermal decomposition of barium azide 3. thermal decomposition of ammonium dichromate 4. both (a) and (b) 3. Dinitrogen is rather inert at room temperature because of 1. low bond enthalpy of N≡≡N bond 2. high bond enthalpy of N≡≡N bond 3. low freezing point 4. low boiling point 4. Dinitrogen combines with dioxygen only at very high temperature (at about 2000 K) to form 1. nitric oxide 2. nitrate 3. nitrites 4. nitric acid 5. Liquid dinitrogen is used as a refrigerant to 1. preserve biological materials 2. preserve food items 3. in cryosurgery 4. all of these
1. (b) high interelectronic repulsion of the non-bonding electrons 2. (d) both (a) and (b) 3. (b) high bond enthalpy of N≡≡N bond 4. (a) nitric oxide 5. (d) all of these
Question-6 Read the following passage and answer any four out of the following questions: Transition metal oxides are generally formed by the reaction of metals with oxygen at high temperatures. The highest oxidation number in the oxides coincides with the group number. In vanadium, there is a gradual change from the basic V2O3 to less basic V2O4 and to amphoteric V2O5. V2O4 dissolves in acids to give VO2+ salts. Potassium dichromate is a very important chemical used in the leather industry and as an oxidant for the preparation of many azo compounds. Dichromates are generally prepared from chromate. Sodium dichromate is more soluble than potassium dichromate. The latter is, therefore, prepared by treating the solution of sodium dichromate with potassium chloride. Sodium and potassium dichromates are strong oxidising agents; sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry. Potassium dichromate is used as a primary standard in volumetric analysis.
The following questions are multiple-choice questions. Choose the most appropriate answer.
1. All transition metal reacts with oxygen to form MO oxide except 1. scandium 2. vanadium 3. cupper 4. zinc 2. As the oxidation number of a metal increases, ionic character 1. increases 2. decreases 3. remain the same 4. none of these 3. The shape of chromate ion is 1. tetrahedral 2. pyramidal 3. square planer 4. triangular 4. Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of 1. FeCr2O 2. FeCr2O 4 3. Na2CrO 4 4. Na2Cr2O 7 5. The oxo cations stabilise VIV 1. VO 2. VO 4+ 3. VO 2+ 4. all of these
Related Posts

HTML Fundamentals Class 10 Computer Science Notes and Questions
Cbse class 12 writing skills long compositions factual description writing, cbse class 12 english unseen passages with answers.

Serving Quality Education
Case Study Question 1 on Solutions – Chapter 2 CBSE Class 12 Chemistry
- May 14, 2022
- Chemistry , CBSE , Class 12
Case-based Questions
Read the given passages and answer the questions that follow :
The spontaneous flow of the solvent through a semipermeable membrane from a pure solvent to a solution or from a dilute solution to a concentrated solution is called osmosis. The phenomenon of osmosis can be demonstrated by taking two eggs of the same size. In an egg, the membrane below the shell and around the egg material is semi- permeable. The outer hard shell can be removed by putting the egg in dilute hydrochloric acid. After removing the hard shell, one egg is placed in distilled water and the other in a saturated salt solution. After some time, the egg placed in distilled water swells-up while the egg placed in salt solution shrinks.
The external pressure applied to stop the osmosis is termed as osmotic pressure (a Colligative property). Reverse osmosis takes place when the applied external pressure becomes larger than the osmotic pressure.
1. What do you expect to happen when red blood corpuscles (RBC’s) are placed in 0.5% NaCl solution?
Ans. RBC’s are isotonic with 0.9% NaCl solution, so they will swell and may even burst when placed in 0.5% NaCl solution.
2. Which one of the following will have higher osmotic pressure in 1 M KCl or 1 M urea solution?
Ans. 1 M KCl will have higher osmotic pressure because its dissociates to give K + and Cl – ions while urea does not dissociate into ions in the solution.
3. Name one SPM which can be used in the process of reverse osmosis.
Ans. Cellulose acetate placed on a suitable support.
4. What are isotonic solutions?
Ans. Solutions having equal osmotic pressure are called isotonic solutions.
5. Write van’t Hoff equation for dilute solution.
Ans. p V = nRT ,
Where, p = Osmotic pressure, n = Number of moles, V = Volume of solution in litre, R = Gas constant ,
T = Temperature
Related Posts
Ncert solutions class 11 maths chapter 9 exercise 9.2 straight lines.
- January 13, 2024
Handwritten Notes of Chapter 2, Physics, Electrical Potential and Capacitance
- August 20, 2023
EMF, Chapter 6, Worksheet- 1 (2023-24)
- July 9, 2023
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment
NCERT Solutions for Class 6, 7, 8, 9, 10, 11 and 12
Important Questions for Class 12 Chemistry Chapter 2 Solutions Class 12 Important Questions
December 6, 2019 by Bhagya
Solutions Class 12 Important Questions Very Short Answer Type
Question 1. Differentiate between molarity and molality of a solution. (All India 2010) Answer: The distinction between molarity and molality. Molarity : It is the number of moles of solute dissolved in 1 litre of solution. It is temperature dependent. M = \(\frac{\omega \times 1000}{\text { mol.mass } \times V}\) Molality : It is the number of moles of solute dissolved in 1 kg of the solvent. m = \(\frac{\omega \times 1000}{M_{2} \times W}\) The relationship between molarity and molality is m = \(\frac{\mathrm{M}}{d-\frac{\mathrm{MM}_{2}}{1000}}\) When molality = molarity, we get, 1 = \(\frac{1}{d-\frac{\mathrm{MM}_{2}}{1000}}\) or d – \(\frac{\dot{\mathrm{MM}}_{2}}{1000}\) = 1 ∴ d = \(1+\frac{\mathrm{MM}_{2}}{1000}\) Molarity is temperature dependent while molarity is not. For very dilute solution, the factor MM 2 /1000 can be neglected in comparison to 1. Hence molality will be same to molarity when density d = 1. Molality is independent of temperature, whereas molarity is a function of temperature because volume depends on temperature and mass does not.
Question 2. What type of semiconductor is obtained when silicon is doped with arsenic? (Delhi 2010) Answer: n-type semiconductor.
Question 3. What is meant by ‘reverse osmosis’? (All India 2011) Answer: If a pressure higher than the osmotic pressure is applied on the solution, the solvent will flow from the solution into the pure solvent through semipermeable membrane. This process is called reverse osmosis (R.O.).
Question 4. What are isotonic solutions? (Delhi 2014) Answer: An isotonic solution is a kind of solution with the same salt concentration as blood and cells. Those solutions which are exerting same osmotic pressure under similar conditions (For example 0.9% NaCl solution by mass volume is Isotonic with human blood).
Question 5. Some liquids on mixing form ‘azeotropes’. What are ‘azeotropes’? (Delhi 2014) Answer: The liquid mixture having a definite composition and boiling like a pure liquid without change in composition is called as azeotrope.
Question 6. What type of intermolecular attractive interaction exists in the pair of methanol and acetone? (Delhi 2014) Answer: Solute-solvent dipolar interactions exist in the pair of methanol and acetone.
Question 7. Out of BaCl 2 and KCl, which one is more effective in causing coagulation of a negatively charged colloidal Sol? Give reason. (Delhi 2015) Answer: BaCl 2 is more effective in causing coagulation because it has double +ve charge than K+.
Solutions Class 12 Important Questions Short Answer Type – I [SA-I]
Question 8. Differentiate between molality and molarity of a solution. What is the effect of change in temperature of a solution on its molality and molarity? (Delhi 2009) Answer: Distinction between molarity and molality. Molarity : It is the number of moles of solute dissolved in 1 litre of solution. It is temperature dependent. M = \(\frac{\omega \times 1000}{\text { mol.mass } \times V}\) Molality : It is the number of moles of solute dissolved in 1 kg of the solvent. m = \(\frac{\omega \times 1000}{M_{2} \times W}\) The relationship between molarity and molality is m = \(\frac{\mathrm{M}}{d-\frac{\mathrm{MM}_{2}}{1000}}\) When molality = molarity, we get, 1 = \(\frac{1}{d-\frac{\mathrm{MM}_{2}}{1000}}\) or d – \(\frac{\dot{\mathrm{MM}}_{2}}{1000}\) = 1 ∴ d = \(1+\frac{\mathrm{MM}_{2}}{1000}\) Molarity is temperature dependent while molarity is not. For very dilute solution, the factor MM 2 /1000 can be neglected in comparison to 1. Hence molality will be same to molarity when density d = 1. Molality is independent of temperature, whereas molarity is a function of temperature because volume depends on temperature and mass does not.
Question 10. Define the terms, ‘osmosis’ and ‘osmotic pressure’. What is the advantage of using osmotic pressure as compared to other colligative properties for the determination of molar masses of solutes in solutions? (All India 2010) Answer: Osmosis : The net spontaneous flow of the solvent molecules from the solvent to the solution or from a less concentrated solution to a more concentrated solution through a semipermeable membrane is called osmosis. Osmotic pressure : The minimum excess pressure that has to be applied on the solution to prevent the entry of the solvent into the solution through the semipermeable membrane is called the osmotic pressure. The osmotic pressure method has the advantage that it uses molarities instead of molalities and it can be measured at room temperature.
Question 11. A 1.00 molal aqueous solution of trichloroacetic acid (CCl 3 COOH) is heated to its boiling point. The solution has the boiling point of 100.18°C. Determine the van’t Hoff factor for trichloroacetic acid. (K b for water = 0.512 K kg mol -1 ) (Delhi 2012) Answer: As ΔT b = iK b m (100.18 – 100) °C = i × 0.512 K kg mol -1 × 1 m 0.18 K = i × 0.512 K kg mol -1 × 1 m ∴ i = 0.3
Question 12. Define the following terms : (i) Mole fraction (ii) Isotonic solutions (iii) van’t Hoff factor (iv) Ideal solution (Delhi 2012) Answer: (i) Mole fraction : Mole fraction is the ratio of number of moles of one component to the total number of moles in a mixture. (ii) Isotonic solution : Two solutions having same osmotic pressure at a given temperature are called Isotonic solutions. (iii) van’t Hoff factor : van’t Hoff factor is expressed as : i = \(\frac{\text { normal molar mass }}{\text { abnormal molar mass }}\) (iv) Ideal solution : The solution which obeys Raoult’s law under all conditions is known as an ideal solution.
Question 13. Explain why aquatic species are more comfortable in cold water rather than in warm water. (Comptt. Delhi 2012) Answer: Aquatic species need dissolved oxygen for breathing. As solubility of gases decreases with increase of temperature, less oxygen is available in summer in the lake. Hence the aquatic species feel more comfortable in winter (low temperature) when the solubility of oxygen is higher.
Question 14. State Raoult’s law. How is it formulated for solutions of non-volatile solutes? (Comptt. Delhi 2012) Answer: Raoult’s Law : Raoult’s Law states that “for a solution of volatile liquids, the partial vapour of each component in the solution is directly proportional to its mole fraction”. Thus for component 1 : p 1 = p 1 0 X 1 where [p 1 0 is vapour pressure of pure component 1] For component 2 : p 2 = P 2 0 X 2 According to Dalton’s law of partial pressure P Total = P 2 + P 2 ⇒ P T = p 1 0 X 1 + p 2 0 X 2 ⇒ P T = p 1 0 (1 – X 2 ) + p 2 0 X 2 ⇒ P T = p 1 0 + (p 2 0 – p 1 0 )X 2
Question 15. State Henry’s law and mention two of its important applications. (Comptt. All India 2012) Answer: Henry’s law : Henry’s law states that “The partial pressure of the gas in vapour phase is proportional to the mole fraction of the gas in the solution “, Applications of Henry’s law :
- To increase the solubility of CO 2 in soft drinks and soda water, the bottle is sealed under high pressure.
- To avoid a dangerous medical condition called bends, scuba divers use oxygen diluted with less soluble helium gas.
Question 16. Why do gases nearly always tend to be less soluble in liquids as the temperature is raised? (Comptt. All India 2012) Answer: This is because the dissolution of gas in liquid is an exothermic process. The solubility should decrease with increase in temperature.
Question 17. 18 g of glucose, C 6 H 12 O 6 (Molar mass – 180 g mol -1 ) is dissolved in 1 kg of water in a sauce pan. At what temperature will this solution boil? (K b for water = 0.52 K kg mol -1 , boiling point of pure water = 373.15 K) (Delhi 2013) Answer: We know that : Elevation of boiling point ∆T b \(\frac{\mathrm{W}_{\mathrm{B}}}{\mathrm{M}_{\mathrm{B}}} \times \frac{100 \times \mathrm{K}_{\mathrm{b}}}{\mathrm{wt} . \text { of solvent }}\) Given: W B = 18 g M B = Formula of glucose is C 6 H 12 O 6 = 6 × 12 + 12 + 6 × 16 = 180 Wt. of solvent = 1 kg or 1000 g, K b = 0.52 K kg mol -1 Hence, ∆T b = \(\frac{18 g}{180} \times \frac{1000 \times 0.52}{1000 g}\) = 0.52 K ∴B.P of the solution = 373.15 + 0.052 = 373.202 K
Question 19. Derive expression for Raoult’s law when the solute is non-volatile. (Comptt. Delhi 2013) Answer: Raoult’s law : Raoult’s law states that “for a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction”. Thus for component 1 : p 1 = p 1 0 X 1 where [p 1 0 is vapour pressure of pure component 1] For component 2 : p 2 = p 2 0 X 2 ccording to Dalton’s law of partial pressure P Total = P 1 + P 2 ⇒ P T = p 1 0 X 1 + p 2 0 X 2 ⇒ P T = p 1 0 ( 1 – X 2 ) + p 2 0 X 2 ∴ P T = p 1 0 + (p 2 0 – p 1 0 )X 2
Question 20. Calculate the mass of compound (molar mass = 256 g mol -1 ) to be dissolved in 75 g of benzene to lower its freezing point by 0.48 K (K f = 5.12 K kg mol -1 ). (Delhi 2014) Answer: Given : ∆T f = 0.48 K, W 1 = 75g, M 2 = 256 g mol -1 W 2 =? Using formula, W 2 = \(\frac{\mathrm{M}_{2} \times \mathrm{W}_{1} \times \Delta \mathrm{T}_{f}}{1000 \times \mathrm{K}_{f}}\) = \(\frac{256 \times 75 \times 0.48}{1000 \times 5.12}\) = 1.8 g
Question 21. Define an ideal solution and write one of its characteristics. (Delhi 2014) Answer: Those solutions which are obeying Raoult’s law are called ideal solutions. An ideal solution is a solution in which no volume change and no enthalpy change takes place on mixing the solute and the solvent in any proportion. Characteristic of an ideal solution : There will be no change in enthalpy ∆H mix = 0, ∆V mix = 0, ∆P mix = 0
Question 22. State Henry’s law. What is the effect of temperature on the solubility of a gas in a liquid? (Delhi 2014) Answer: Henry’s law : Henry’s law states that, “The solubility of a gas in a liquid at a particular temperature is directly proportional to the pressure of the gas in equilibrium with the liquid at that temperature.” Solubility of gas decreases with increase of temperature at the same pressure.
Question 23. State Raoult’s law for the solution containing volatile components. What is the similarity between Raoult’s law and Henry’s law? (Delhi 2014) Answer: Raoult’s law : “In a solution, the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the vapour pressure of that component in pure state.” Similarity between Raoult’s law and Henry’s law is that the partial pressure or vapour pressure of the volatile component (gas) is directly proportional to the mole fraction of that component in the solution.
Question 24. How is the vapour pressure of a solvent affected when a non-volatile solute is dissolved in it? (Comptt. Delhi 2014) Answer: The vapour pressure of a solvent decreases when a non-volatile solute is dissolved in it because some solvent molecules are replaced by the molecules of solute.
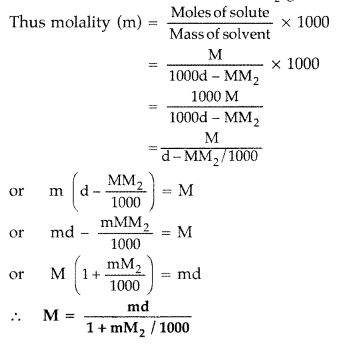
Question 26. What is meant by positive deviations from Raoult’s law? Give an example. What is the sign of ∆ mix H for positive deviation? (Delhi 2015) Answer: In positive deviations, the partial vapour pressure of each component A and B of a solution and the total pressure of the solution is higher than the vapour pressure calculated from Raoult’s law. For example, Water and Ethanol. In case of positive deviations, ∆ mix H > 0 (Positive)
Question 27. Define azeotropes. What type of azeotrope is formed by positive deviation from Raoult’s law? Given an example. (Delhi) 2015 Answer: Azeotropes : Liquid mixture which distills without change in compositions are called azeotropic mixtures or Azeotropes. In positive deviations from Raoult’s law, minimum boiling point azeotropic mixture is formed. For example, 95% ethanol + 5% water.
Question 28. (i) On mixing liquid X and liquid Y, volume of the resulting solution decreases. What type of deviation from Raoult’s law is shown by the resulting solution? What change in temperature would you observe after mixing liquids X and Y? (ii) What happens when we place the blood cell in water (hypotonic solution)? Give reason. (All India 2015) Answer: (i) Volume decreases by mixing X and Y. It shows negative deviations from Raoult’s law. There will be rise in temperature. (∆H mix < 0) (ii) Blood cell will swell due to osmosis as water enters the cell.
Question 29. Define osmotic pressure of a solution. How is the osmotic pressure related to the concentration of a solute in a solution? (Comptt. Delhi 2015) Answer: Osmotic pressure : It is the external pressure which is applied on the side solution which is sufficient to prevent the entry of the solvent through semi-permeable membrane. According to the Boyle-van’t Hoff Law, the osmotic pressure (π) of a dilute solution is directly proportional to its molar concentration provided temperature is constant. π ∝ C (At constant temperature) π ∝ CT (At constant concentration) π = CRT (R = Solution constant) or, π = \(\frac{n}{v} \mathrm{RT}\)
Question 30. Define the following terms : (i) Mole fraction (x) (ii) Molality of a solution (m) (Comptt. All India 2015) Answer: (i) Mole fraction : Mole fraction of a constituent is the fraction obtained by dividing number of moles of that constituent by the total number of moles of all the constituents present in the solution. It is denoted by ‘x’. Example : x 1 = \(\frac{\text { No. of moles of } x_{1}}{\text { Total no. of moles }}=\frac{n x_{1}}{n x_{1}+n x_{2}}\)
(ii) Molality of a solution : Molality of a solution is defined as the number of moles of the solute dissolved in 1000 grams (1 kg) of the solvent. It is denoted by’m’. m = \(\frac{w \times 1000}{\mathrm{M} \times \mathrm{W}}\) Where w = Weight of solute in grams M = Molecular mass of solute W = Weight of solvent in grams
Question 31. (i) Gas (A) is more soluble in water than Gas (B) at the same temperature. Which one of the two gases will have the higher value of K H (Henry’s constant) and why? (ii) In non-ideal solution, what type of deviation shows the formation of maximum boiling azeotropes? (All India 2016) Answer: (i) Gas (B) will have higher value of K H (Henry’s constant) than Gas (A) at the same temperature because lesser the solubility of a gas in a given solvent, higher will be the value of K H for a gas. K H = \(\frac{\text { Partial pressure of gas }}{\text { Mole fraction of gas in the solution }}\) (ii) Negative deviations from Raoult’s law show the formation of maximum boiling azeotropes.
Question 32. What is osmotic pressure? Why it is a colligative property? (Comptt Delhi 2016) Answer: The excess pressure applied on solution side to stop the process of osmosis. Because it depends upon the number of solute particles but not on their nature.
Question 33. Define osmotic pressure. How is osmotic pressure related to the concentration of a solute in a solution? (Comptt. All India 2016) Answer: Osmotic pressure is the measure of excess pressure applied on solution side to stop the process of osmosis. Osmotic pressure is directly proportional to the conentration of solute in solution π ∝ c
Question 34. Define the following terms: (Delhi 2017) (i) Colligative properties (ii) Molality (m) Answer: (i) Colligative properties. All those properties which depend on the number of solute particles irrespective of the nature of solute are called as colligative properties. (ii) Molality (m). Number of moles of solute dissolved per kg of the solvent.
Question 35. Define the following terms: (i) Abnormal molar mass (ii) van’t Hoff factor (i) (Delhi 2017) Answer: (i) Abnormal molar mass. If the molar mass calculated by using any of colligative properties tends to be different than theoretically expected molar mass, it is called abnormal molar mass. (ii) van’t Hoff factor (i). Extent of dissociation or association or ratio of the observed colligative property to calculated colligative property. i = \(\frac{\text { Observed colligative property }}{\text { Theoretical colligative property }}\)
Question 36. Define the following terms: (i) Ideal solution (ii) Molarity (M) (Delhi 2017) Answer: (i) Ideal solution: The solution that obeys Raoults ’ Law over the entire range of concentration. (ii) Molarity is the number of moles of solute dissolved per litre of solution or M = \(\frac{w_{b} \times 1000}{\mathrm{M}_{b} \times \mathrm{Volume}(\mathrm{mL})}\)

Solutions Class 12 Important Questions Short Answer Type – II [SA – II]
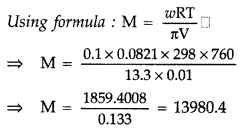
Question 39. Calculate the freezing point depression expected for 0.0711 m aqueous solution of Na2S04. If this solution actually freezes at – 0.320°C, what would be the value of Van’t Hoff factor? (Ky for water is 1.86°C mol -1 ) (Delhi 2009) Answer: Given : Molality, m = 0.0711 m ΔT f = – 0.320°C K f = 1.86°C f = ? Substituting these values in the formula, we get ΔT f = i K f m or i = \(\frac{\Delta \mathrm{T}_{f}}{\mathrm{K}_{f} \mathrm{m}}\) or i = \(\frac{-0.320}{1.86 \times 0.0711}=\frac{-0.320}{0.132246}\) = -2.4
Question 40. A solution prepared by dissolving 1.25 g of oil of winter green (methyl salicylate) in 99.0 g of benzene has a boiling point of 80.31°C. Determine the molar mass of this compound. (B.P. of pure benzene = 80.10°C and K b for benzene = 2.53°C kg mol -1 ) (Delhi 2010) Answer: Given : w 2 = 1.25 g, w 1 = 99 g ΔT b = 80.31 – 80.10°C = 0.21°C K b = 2.53°C kg mol -1 According to the formula : M 2 = \(\frac{1000 \mathrm{K}_{b} w_{2}}{w_{1} \Delta \mathrm{T}_{b}}\) Substituting these values in the formula, we get M 2 = \(\frac{1000 \times 2.53 \times 1.25}{99 \times 0.21}=\frac{3162.5}{20.79}\) = 152 g mol -1
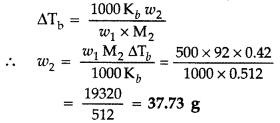
Question 44. 15 g of an unknown molecular substance was dissolved in 450 g of water. The resulting solution freezes at -0.34° C. What is the molar mass of the substance? (K f for water = 1.86 K kg mol -1 ). (All India 2010) Answer: Given : w 2 = 15 g, w 1 = 450 g ΔT f = -0.34°C CK f = 1.86 K kg mol -1 M 2 =? Substituting these values in the formula M 2 = \(\frac{1000 \times \mathrm{K}_{f} w_{2}}{w_{1} \Delta \mathrm{T}_{f}}\) ∴ M 2 = \(\frac{1000 \times 1.86 \times 15}{450 \times 0.34}\) or M 2 = \(\frac{27900}{153}\) = 182.53 2 153 ∴ Molar mass of the substance, M 2 = 182.53 g
Question 46. Calculate the amount of KCl which must beadded to 1 kg of water so that the freezing point is depressed by 2K. (K f for water = 1.86 K kg mol -1 ) (Delhi 2012) Answer: Since one mol of KCl gives 2 mole particles, the value of i = 2, ΔT f , = 2K, K f = 1.86 kg mol -1 Applying equation, ΔT f = iK f m = \(\frac{\Delta \mathrm{T}_{f}}{i \mathrm{K}_{f}}=\frac{2}{2 \times 1.86}=\frac{1}{1.86}\) = 0.54 mol kg -1 ∴ 0.54 mole of KC1 should be added to 1 kg of water Molar mass of KCl = 39 + 35.5 = 74.5 g ∴ Amount of KCl = 0.54 × 74.5 g = 40.05 g
Question 47. A solution of glycerol (C 3 H 8 O 3 ) in water was prepared by dissolving some glycerol in 500 g of water. This solution has a boiling point of 100.42 °C while pure water boils at 100 °C. What mass of glycerol was dissolved to make the solution? (Delhi 2012) Answer: ΔT b = (100.42 – 100)°C = 0.42°C or 0.42 K ΔT b = K b m 0.42 = 0.512 × \(\frac{\mathrm{W}_{2}}{92} \times \frac{1000}{500}\) W 2 = \(\frac{0.42 \times 92 \times 500}{0.512 \times 1000}=\frac{4.83}{0.128}\) = 37.7 g where W 2 is the weight of the solute.
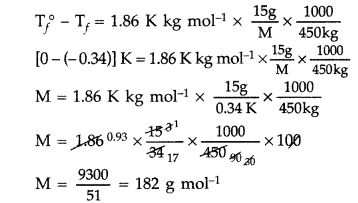
Question 51. The partial pressure of ethane over a saturated solution containing 6.56 × 10 -2 g of ethane is 1 bar. If the solution contains 5.0 × 10 -2 g of ethane, then what will be the partial pressure of the gas? (Comptt. All India 2012) Answer: Applying the Henry’s law, m = K H × p In first case, 6.56 × 10 -2 = K H × 1 K H = 6.56 × 10 -2 g bar -1 Putting the value of KH in the second case, we get 5 × 10 -2 g = 6.56 × 10 -2 g bar -1 × p ∴ p = \(\frac{5 \times 10^{-2}}{6.56 \times 10^{-2} \mathrm{g} \mathrm{bar}}\) = 0.762 bar
Question 52. Determine the osmotic pressure of a solution prepared by dissolving 2.5 × 10 -2 g of K 2 SO 4 in 2L of water at 25° C, assuming that it is completely dissociated. (R = 0.0821 L atm K -1 mol -1 , Molar mass of K 2 SO 4 = 174 g mol -1 ). (Delhi 2013) Answer: We know, π = iCRT ⇒ π = \(\frac{i \mathrm{n} \mathrm{RT}}{\mathrm{V}}\) ⇒ π = i × \(\frac{w}{M} \times \frac{1}{V} R T\) Given : w = 2.5 × 10 -2 g = 0.025 g V = 2L, T = 25°C = 298 K M = K 2 SO 4 = 2 × 39 + 32 + 4 × 16 = 174 g mol -1 K 2 SO 4 = dissociates completely as K 2 SO 4 → 2K + + SO 4 2- ∴ Ions produced = 3 i.e., i = 3 Hence, π = 3 × \(\frac{0.025 \mathrm{g}}{174 \mathrm{g} \mathrm{mol}^{-1}} \times \frac{1}{2 \mathrm{L}}\) × 0.0821 L atm K -1 mol -1 × 298 K ∴ π = 527 × 10 -3 atm
Question 53. The partial pressure of ethane over a saturated solution containing 6.56 × 10 -2 g of ethane is 1 bar. If the solution were to contain 5.0 × 10 -2 g of ethane, then what will be the partial pressure of the gas? (Comptt. Delhi 2013) Answer: Applying the Henry’s law, m = K H × p In first case, 6.56 × 10 -2 = K H × 1 K H = 6.56 × 10 -2 g bar -1 Putting the value of KH in the second case, we get 5 × 10 -2 g = 6.56 × 10 -2 g bar -1 × p ∴ p = \(\frac{5 \times 10^{-2}}{6.56 \times 10^{-2} \mathrm{g} \mathrm{bar}}\) = 0.762 bar
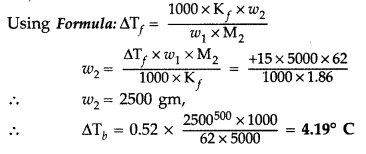
Question 57. 45 g of ethylene glycol (C 2 H 6 O 2 ) is mixed with 600 g of water. Calculate (i) the freezing point depression and (ii) the freezing point of the solution (Given : K f of water = 1.86 K kg mol -1 ) (Comptt. Delhi 2015) Answer: (i) Given : w = 45 g, W = 600 g, K f = 1.86 K kg mol -1 , Δ T f = ? Using the formula for freezing point depression, ΔT f = \(\mathrm{K}_{f} \frac{w \times 1000}{m \times \mathrm{W}}\) m of ethylene glycol (C 2 H 6 O 2 ) = 2 × 12 + 6 × 1 + 2 × 16 = 24 + 6 + 32 = 62 g mol -1 Substituting above values in formula, ΔT f = \(\frac{1.86 \mathrm{K} \mathrm{kg} \mathrm{mol}^{-1} \times 45 \mathrm{g} \times 1000 \mathrm{g} \mathrm{kg}^{-1}}{62 \mathrm{g} \mathrm{mol}^{-1} \times 600 \mathrm{g}}\) = \(\frac{837}{372}\) ∴ ΔT f = 2.25 K (ii) ΔT f = T f 0 – T f Where, T f 0 = Freezing point of pure water ⇒ T f = 273.15 – 2.25 K ∴ T f = 270.9 K (Freezing point of the solution)
Question 58. A 5 percent solution (by mass) of cane-sugar (M.W. 342) is isotonic with 0.877% solution of substance X. Find the molecular weight of X. (Comptt. All India 2015) Answer: Given : W (mass) of cane-sugar = 5% means 5 g Molar mass of cane-sugar (M) = 342 g mol -1 Mass of isotonic substance X = 0.877% means 0.877 g Molar mass of X = ? Using formula, \(\frac{\mathrm{W}_{\text { canesugar }}}{\mathrm{M}_{\text { cane sugar }}}=\frac{\mathrm{W}_{\mathrm{X}}}{\mathrm{M}_{\mathrm{X}}} \quad \Rightarrow \frac{5 \mathrm{g}}{342 \mathrm{g} \mathrm{mol}^{-1}}=\frac{0.877 \mathrm{g}}{\mathrm{M}_{\mathrm{X}}}\) or M x = \(\frac{0.877 \mathrm{g} \times 342 \mathrm{g} \mathrm{mol}^{-1}}{5 \mathrm{g}} \Rightarrow \frac{299.934 \mathrm{g} \mathrm{mol}^{-1}}{5 \mathrm{g}}\) ∴ M x = 59.9 ≈ 60 g mol -1
Solutions Class 12 Important Questions Long Answer Type (LA)
Question 64. (a) Define the following terms : (i) Mole fraction (ii) Van’t Hoff factor (b) 100 mg of a protein is dissolved in enough water to make 10.0 mL of a solution. If this solution has an osmotic pressure of 13.3 mm Hg at 25°C, what is the molar mass of protein? (R = 0.0821 L atm mol -1 K -1 and 760 mm Hg = 1 atm) (All India 2009) Answer: (a) (i) Mole fraction : Mole fraction of a constituent is the fraction obtained by dividing number of moles of that constituent by the total number of moles of all the constituents present in the solution. It is denoted by ‘x’. Example : x i = \(\frac{\text { No. of moles of } x_{1}}{\text { Total no. of moles }}=\frac{n x_{1}}{n x_{1}+n x_{2}}\) (ii) Van’t Hoff factor : It is the ratio of the observed colligative property to the theoretical value. It is denoted by ‘i’. i = \(\frac{\text { Observed colligative property }}{\text { Theoretical colligative property }}\)
Question 65. (a) What is meant by : (t) Colligative properties (ii) Molality of a solution (b) What concentration of nitrogen should be present in a glass of water at room temperature? Assume a temperature of 25° C, a total pressure of 1 atmosphere and mole fraction of nitrogen in air of 0.78. [K H for nitrogen = 8.42 × 10 -7 M/mm Hg] (All India 2009) Answer: (a) (i) Colligative properties : Those properties of ideal solutions which depend only on the number of particles of the solute dissolved in a definite amount of the solvent and do not depend on the nature of solute are called colligative properties. (ii) Molality of a solution : Molality of a solution is defined as the number of moles of the solute dissolved in 1000 grams (1 kg) of the solvent. It is denoted by’m’. m = \(\frac{w \times 1000}{\mathrm{M} \times \mathrm{W}}\) Where ‘w = Weight of solute in grams M = Molecular mass of solute W = Weight of solvent in grams
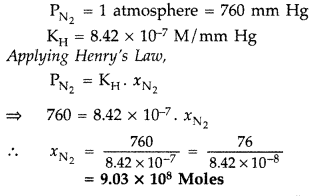
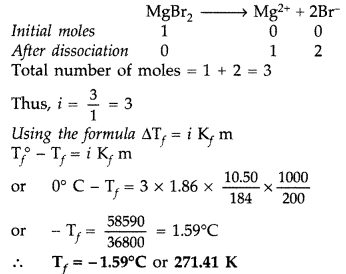
Question 66. (a) Differentiate between molarity and molality for a solution. How does a change in temperature influence their values? (b) Calculate the freezing point of an acqueous solution containing 10.50 g of MgBr 2 in 200 g of water. (Molar mass of MgBr 2 = 184 g) (K f for water = 1.86 K kg mol -1 ) (Delhi 2011) Answer: (a) Distinction between molarity and molality. Molarity : It is the number of moles of solute dissolved in 1 litre of solution. It is temperature dependent. M = \(\frac{\omega \times 1000}{\text { mol.mass } \times V}\) Molality : It is the number of moles of solute dissolved in 1 kg of the solvent. m = \(\frac{\omega \times 1000}{M_{2} \times W}\) The relationship between molarity and molality is m = \(\frac{\mathrm{M}}{d-\frac{\mathrm{MM}_{2}}{1000}}\) When molality = molarity, we get, 1 = \(\frac{1}{d-\frac{\mathrm{MM}_{2}}{1000}}\) or d – \(\frac{\dot{\mathrm{MM}}_{2}}{1000}\) = 1 ∴ d = \(1+\frac{\mathrm{MM}_{2}}{1000}\) Molarity is temperature dependent while molarity is not. For very dilute solution, the factor MM 2 /1000 can be neglected in comparison to 1. Hence molality will be same to molarity when density d = 1. Molality is independent of temperature, whereas molarity is a function of temperature because volume depends on temperature and mass does not.
Question 67. (a) Define the terms osmosis and osmotic pressure. Is the osmotic pressure of a solution a colligative property? Explain. (b) Calculate the boiling point of a solution prepared by adding 15.00 g of NaCl to 250.0 g of water. (K b for water = 0.512 K kg mol -1 , Molar mass of NaCl = 58.44 g) (Delhi 2011) Answer: Osmosis : The net spontaneous flow of the solvent molecules from the solvent to the solution or from a less concentrated solution to a more concentrated solution through a semipermeable membrane is called osmosis. Osmotic pressure : The minimum excess pressure that has to be applied on the solution to prevent the entry of the solvent into the solution through the semipermeable membrane is called the osmotic pressure. The osmotic pressure method has the advantage that it uses molarities instead of molalities and it can be measured at room temperature.
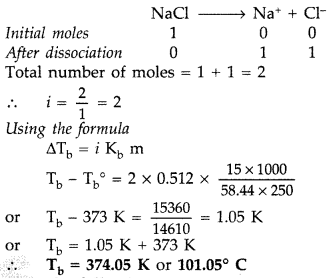
Question 68. (a) State the following : (i) Henry’s law about partial pressure of a gas in a mixture. (ii) Raoult’s law in its general form in reference to solutions. (b) A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 mL of water has an osmotic pressure of 0.335 torr at 25°C. Assuming the gene fragment is a non-electrolyte, determine its molar mass. (All India 2011) Answer: (a) (i) Henry’s law : “The solubility of a gas in a liquid at a particular temperature is directly proportional to the pressure of the gas in equilibrium with the liquid at that temperature.” Applications of Henry’s law : • In the production of carbonated beverages which are prepared under high pressure. • Deep sea divers depend upon compressed air for their oxygen supply.
(ii) Raoult’s law : For a solution of volatile liquids the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. P = P°x Non-ideal solution shows positive and negative deviations from Raoult’s law. Positive deviation from Raoult’s law : The total vapour pressure for any solution is greater than the corresponding ideal solution of same composition. Such behaviour is called positive deviation. Example : Mixtures of ethanol + cyclohexane Mixture of acetone + carbon disulphide Negative deviation from Raoult’s law: When the total vapour pressure will be less than corresponding vapour pressure, then it is termed as negative deviation. Example : Chloroform + Benzene Chloroform + Diethylether
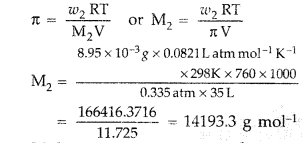
Question 69. (a) Differentiate between molarity and molality in a solution. What is the effect of temperature change on molarity and molality in a solution? (b) What would be the molar mass of a compound if 6.21 g of it dissolved in 24.0 g of chloroform form a solution that has a boiling point of 68.04°C. The boiling point of pure chloroform is 61.7°C and the boiling point elevation constant, K b for chloroform is 3.63°C/m. (Delhi 2011) Answer: (a) Distinction between molarity and molality. Molarity : It is the number of moles of solute dissolved in 1 litre of solution. It is temperature dependent. M = \(\frac{\omega \times 1000}{\text { mol.mass } \times V}\) Molality : It is the number of moles of solute dissolved in 1 kg of the solvent. m = \(\frac{\omega \times 1000}{M_{2} \times W}\) The relationship between molarity and molality is m = \(\frac{\mathrm{M}}{d-\frac{\mathrm{MM}_{2}}{1000}}\) When molality = molarity, we get, 1 = \(\frac{1}{d-\frac{\mathrm{MM}_{2}}{1000}}\) or d – \(\frac{\dot{\mathrm{MM}}_{2}}{1000}\) = 1 ∴ d = \(1+\frac{\mathrm{MM}_{2}}{1000}\) Molarity is temperature dependent while molarity is not. For very dilute solution, the factor MM 2 /1000 can be neglected in comparison to 1. Hence molality will be same to molarity when density d = 1. Molality is independent of temperature, whereas molarity is a function of temperature because volume depends on temperature and mass does not.
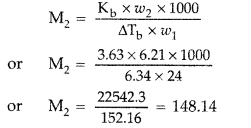
Question 70. (a) Define the following terms : (i) Mole fraction (ii) Ideal solution (b) 15.0 g of an unknown molecular material is dissolved in 450 g of water. The resulting solution freezes at – 0.34°C. What is the molar mass of the material? (K f for water = 1.86 K kg mol -1 ) (All India 2011) Answer: (a) (i) Mole fraction : Mole fraction is the ratio of number of moles of one component to the total number of moles in a mixture. X A = \(\frac{n_{\mathrm{A}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}\), X B = \(\frac{n_{\mathrm{B}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}\) (ii) Ideal solution : The solution which obeys Raoult’s law under all conditions is known as an ideal solution.
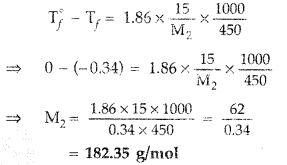
Question 71. (a) Explain the following : (i) Henry’s law about dissolution of a gas in a liquid (ii) Boiling point elevation constant for a solvent (b) A solution of glycerol (C 3 H 8 O 3 ) in water was prepared by dissolving some glycerol in 500 g of water. This solution has a boiling point of 100.42°C. What mass of glycerol was dissolved to make this solution? (K b for water = 0.512 K kg mol -1 ) (All India 2011) Answer: (a) (i) Henry’s law : The law states “that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.” (ii) Boiling point elevation constant for a solvent or molal elevation constant may be defined as the elevation in the boiling point when the molality of the solution is unity.
(b) ΔT b = (100.42 – 100)°C = 0.42°C or 0.42 K ΔT b = K b m 0.42 = 0.512 × \(\frac{\mathrm{W}_{2}}{92} \times \frac{1000}{500}\) W 2 = \(\frac{0.42 \times 92 \times 500}{0.512 \times 1000}=\frac{4.83}{0.128}\) = 37.7 g where W 2 is the weight of the solute.
Question 72. (a) State Raoult’s law for a solution containing volatile components. How does Raoult’s law become a special case of Henry’s law? (b) 1.00 g of a non-electrolyte solute dissolved in 50 g of benzene lowered the freezing point of benzene by 0.40 K. Find the molar mass of the solute. (K f for benzene = 5.12 K kg mol -1 ) (All India 2013) Answer: Raoult’s law: For a solution of volatile liquids the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. P = P°x Non-ideal solution shows positive and negative deviations from Raoult’s law. (i) Positive deviation from Raoult’s law : The total vapour pressure for any solution is greater than the corresponding ideal solution of same composition. Such behaviour is called positive deviation. Example : Mixtures of ethanol + cyclohexane Mixture of acetone + carbon disulphide (ii) Negative deviation from Raoult’s law : When the total vapour pressure will be less than corresponding vapour pressure, then it is termed as negative deviation. Example : Chloroform + Benzene Chloroform + Diethvlether According to Raoult’s law P A = P A ° × x A According to Henry’s law P A = K H × x A Thus both laws are identical and differ by their proportionality constants.
(b) We know that M 2 = \(\frac{1000 \mathrm{K}_{f} w_{2}}{w_{1} \mathrm{DT}_{f}}\) Given : w 2 = 1.0 g, w 1 = 50 g, ΔT f = 0.40 K f = 5.12 K kg mol -1 ∴ M 2 = \(\frac{1000 g \mathrm{kg}^{-1} \times 5.12 \mathrm{kg} \mathrm{mol}^{-1} \times 1.0 \mathrm{g}}{50 \mathrm{g} \times 0.40 \mathrm{K}}\) = 256 g mol -1
Question 73. (a) Define the following terms : (i) Ideal solution (ii) Azeotrope (iii) Osmotic pressure (b) A solution of glucose (C 6 H 12 O 6 ) in water is labelled as 10% by weight. What would be the molality of the solution? (Molar mass of glucose = 180 g mol -1 ) (All India 2013) Answer: (a) (i) Ideal solution : An ideal solution is that which obeys Raoult’s law and in which the intermolecular interactions between the different components are of same magnitude as that is found in pure components. (ii) Azeotrope : I t is a type of liquid mixture having a definite composition and boiling like a pure liquid, (distills without change in compositions) (iii) Osmotic pressure : The minimum excess pressure that has to be applied on the solution to prevent the entry of the solvent into the solution through semi- permeable membrane is called osmotic pressure.
(b) 10% of glucose means 10 g of solute in 100 g of solvent ∴ Mass of solute = 10 g Mass of solvent = 100 – 10 = 90 g = \(\frac{90}{1000}\) kg Molar mass of glucose = 180 g mol -1 No. of moles of \(\frac{10}{100}=\frac{1}{18}\) mole ∴ Molarity = \(\frac{\text { No. of moles of solute }}{\text { mass of solvent in } \mathrm{kg}}\) = \(\frac{1}{18} \times \frac{1000}{90}=\frac{100}{162}\) = 0.67 mol kg -1 = 0.6 m
Question 74. (a) The vapour pressures of benzene and toluene at 293 K are 75 mm Hg and 22 mm Hg respectively. 23.4 g of benzene and 64.4 g of toluene are mixed. If the two form an ideal solution, calculate the mole fraction of benzene in the vapour phase assuming that the vapour pressures are in equilibrium with the liquid mixture at this temperature. (b) What is meant by +ve and -ve deviations from Raoult’s law and how is the sign of AH solution related to +ve and -ve deviations from Raoult’s law? (Comptt. All India 2013) Answer: (a) Given : Mass of benzene = 23.4 g Molar mass of benzene = C 6 H 6 = 12 × 6 + 6 = 78g mol -1 Mass of toluene = 64.4 g Molar mass of toluene = C 6 H 5 CH 3 = 12 × 7 + 8 = 92g mol -1 Moles of Benzene = \(\frac{23.4}{78}\) = 0.3 mole Moles of toluene = \(\frac{64.4}{92}\) = 0.7 mole Vapour pressure of benzene, P B = x B × P = 0.3 × 75 = 22.5 mm Vapour pressure of toluene, P T = x T × P = 0.7 × 22 = 15.4 mm Total vapour pressure = 22.5 + 15.4 = 37.9 mm ∴ Mole fraction of benzene = \(\frac{22.5}{37.9}\) = 0.59
(b) +ve and -ve deviations : Raoult’s law: For a solution of volatile liquids the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. P = P°x Non-ideal solution shows positive and negative deviations from Raoult’s law. (i) Positive deviation from Raoult’s law : The total vapour pressure for any solution is greater than the corresponding ideal solution of same composition. Such behaviour is called positive deviation. Example : Mixtures of ethanol + cyclohexane Mixture of acetone + carbon disulphide (ii) Negative deviation from Raoult’s law : When the total vapour pressure will be less than corresponding vapour pressure, then it is termed as negative deviation. Example : Chloroform + Benzene Chloroform + Diethvlether According to Raoult’s law P A = P A ° × x A According to Henry’s law P A = K H × x A Thus both laws are identical and differ by their proportionality constants. If it is higher, the solution exhibits positive deviation and if it is low, it exhibits negative deviation from Raoult’s law. For positive deviation Δ mix H = +ve For negative devation Δ mix H = -ve
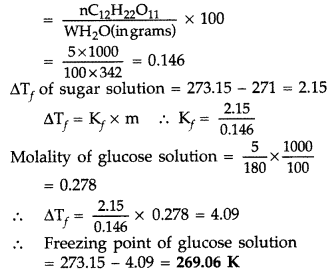
(b) Henry’s law : Henry’s law states that, “The solubility of a gas in a liquid at a particular temperature is directly proportional to the pressure of the gas in equilibrium with the liquid at that temperature.” Solubility of gas decreases with increase of temperature at the same pressure.
Question 76. (a) Define the following terms : (i) Molarity (ii) Molal elevation constant (K b ) (b) A solution containing 15 g urea (molar mass = 60 g mol -1 ) per litre of solution in water has the same osmotic pressure (isotonic) as a solution of glucose (molar mass = 180 g mol -1 ) in water. Calculate the mass of glucose present in one litre of its solution. (All India 2014) Answer: (a) (i) Molarity is the number of moles of solute dissolved in one litre of solution. (ii) Molal elevation constant may be defined as the elevation in boiling point when the molality of solution is unity i.e. 1 mole of solute is dissolved in 1 kg of the solvent.
(b) For urea, concentration = \(\frac{15}{60}\) moles/lt. For glucose, concentration = \(\frac{w}{180}\) moles/lt. ∵ Solutions are isotonic ∴ \(\frac{w}{180}=\frac{15}{60}\) ∴ w = \(\frac{15 \times 180}{60}\) = 45 g
Question 77. (a) What type of deviation is shown by a mixture of ethanol and acetone? Give reason. (b) A solution of glucose (molar mass = 180 g mol -1 ) in water is labelled as 10% (by mass). What would be the molality and molarity of the solution? (Density of solution = 1.2 g mL -1 ) (All India 2014) Answer: (a) Since acetone is nearly non-polar in nature and ethanol is polar in nature therefore, no interaction occurs between acetone and ethanol, the number of molecules increases, which shows positive deviation.
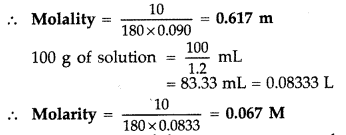
Question 78. (a) What is van’t Hoff factor? What types of values can it have if in forming the solution, the solute molecules undergo (i) Dissociation? (ii) Association? (b) How many mL of a 0.1 M HCl solution are required to react completely with 1 g of a mixture of Na 2 CO 3 and NaHCO 3 containing equimolar amounts of both? (Molar mass : Na 2 CO 3 = 106 g, NaHCO 3 = 84 g) (Comptt. All India 2014) Answer: (a) (i) Van’t Hoff factor : It is defined as the ratio of the experimental value of the colligative property to the calculated value of the colligative property . i = \(\frac{\text { Experimental value }}{\text { Calculated value }}\) If there is dissociation of the solute in the solution, the Van’t Hoff factor T’ will be greater than one i.e. i > 1. It means observed colligative property will be greater than calculated value. (ii) Association : If there is association of solute in the solution, the Van’t Hoff factor ‘f’ will be less than one i.e. i < 1. Thus, observed colligative property will be less than the calculated value.
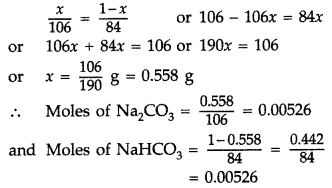
Question 79. (a) Define (i) Mole fraction (Hi) Raoult’s law (b) Assuming complete dissociation, calculate the expected freezing point of a solution prepared by dissolving 6.00 g of Glauber’s salt, Na 2 SO 4 .10H 2 O in 0.100 kg of water. (K f for water = 1.86 K kg mol -1 , Atomic masses : Na = 23, S = 32, O = 16, H = 1) (Comptt. All India 2014) Answer: (a) (i) Mole fraction : Mole fraction is the ratio of number of moles of one component to the total number of moles in a mixture. (ii) Molality of a solution : Molality of a solution is defined as the number of moles of the solute dissolved in 1000 grams (1 kg) of the solvent. It is denoted by’m’. m = \(\frac{w \times 1000}{\mathrm{M} \times \mathrm{W}}\) Where ‘w = Weight of solute in grams M = Molecular mass of solute W = Weight of solvent in grams
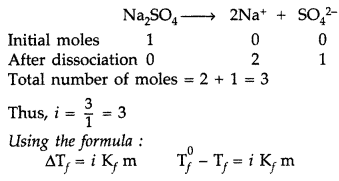
(b) (i) 2M glucose will have a higher boiling point than 1M glucose because elevation in boiling point is a colligative property which depends upon the number of particles in the solution which is more in the case of 2M glucose solution. (ii) When the external pressure applied becomes more than the osmotic pressure of the solution, then the solvent will flow from the solution into the pure solvent through the semi-permeable membrane. The process is called reverse osmosis (RO).
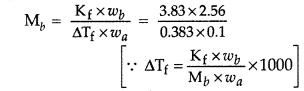
(b) (i) If RBCs are placed in contact with 1.2% NaCl solution, then the osmotic pressure of 1.2% NaCl becomes higher than that of RBCs due to which water present inside the cells moves into the NaCl solution which results in shrinkage of RBCs. (ii) Reverse process will take place if RBCs are kept in contact with 0.4% NaCl solution which has less osmotic pressure ’ due to which water moves into RBCs and they will swell.
(b) (i) Molality (m): Number of moles of solute dissolved per kg of the solvent. (ii) Abnormal molar mass: If the molar mass calculated by using any of the colligative properties comes to be different than theoretically expected molar mass.
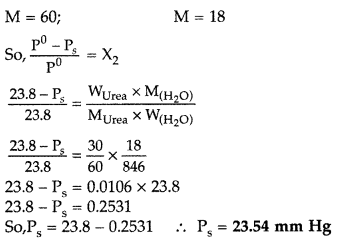
| (i) They obey Raoult’s law over the enitre range of concentration. | (i) They do no obey Raoult’s law over the entire range of concentration |
| (ii) Neither the heat is evolved or absorbed during dissolution | (ii) Heat is evolved or absorbed during dissolution |
| (iii) Δ H = 0 Δ V = 0 | (iii) Δ H is not equal to 0. Δ V is not equal to 0. |
Question 84. (a) Explain why on addition of 1 mol glucose to 1 litre water the boiling point of water increases. (b) Henry’s law constant for CO 2 in water is 1.67 × 10 8 Pa at 298 K. Calculate the number of moles of CO 2 in 500 ml of soda water when packed under 2.53 × 10 5 Pa at the same temperature. (Comptt. All India 2017) Answer: (a) Glucose is a non-volatile solute, therefore, addition of glucose to water lowers the vapour pressure of water as a result of which boiling point of water increases.
Question 85. (a) Define the following terms : (i) Ideal solution (ii) Osmotic pressure (b) Calculate the boiling point elevation for a solution prepared by adding 10 g CaCl 2 to 200 g of water, assuming that CaCl 2 is completely dissociated. (K b ) for water = 0.512 K kg mol -1 ; Molar mass of CaCl 2 = 111 g mol -1 ) (Comptt. All India 2017) Answer: (a) (i) Ideal solution : The solutions which obey Raoult’s law over the entire range of concentration are known as ideal solutions. (ii) The minimum excess pressure that has to be applied on the solution to prevent the entry of the solvent into the solution through the semipermeable membrane is called the osmotic pressure.

Important Questions for Class 12 Chemistry
Free resources.
NCERT Solutions
Quick Resources

Class 12 Chemistry Case Study Questions Chapter 1 The Solid State
- Post author: studyrate
- Post published:
- Post category: Class 12
- Post comments: 0 Comments
In Class 12 Boards there will be Case studies and Passage Based Questions will be asked, So practice these types of questions. Study Rate is always there to help you. Free PDF Downloads of CBSE Class 12 Chemistry Chapter 1 The Solid State Case Study and Passage-Based Questions with Answers were Prepared Based on the Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Case Study Questions The Solid State to know their preparation level.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 12 Chemistry Paper, There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
The Solid State Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 12 Chemistry Chapter 1 The Solid State
Case Study/Passage-Based Questions
Case Study 1: In a hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them. A space-filling model of this structure, called hexagonal close-packed (hep), is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Each one of these three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in a relative position.
(I) The number of atoms in this hep unit cell is
| (a) 4 | (b) 6 | (c) 12 | (d) 17 |
Answer: (b) 6
(ii) The empty space in this hep unit cell is
| (a) 74% | (b) 47.6% | (c) 32% | (d) 26% |
Answer:(d) 26%
(iii) In hexagonal close packing of spheres in three-dimensions
| (a) in one unit cell, there are six octahedral voids and all are completely inside the unit cell |
| (b) in one unit cell, there are six octahedral voids out of which three are completely inside the unit cell and the other three are from contributions of octahedral voids which are partially inside the unit cell |
| (c) in one unit cell, there are 12 tetrahedral voids, all are completely inside the unit cell. |
| in one unit cell there are 12 octahedral voids and all are completely inside the unit cell |
Answer:(a) in one unit cell, there are six octahedral voids and all are completely inside the unit cell
Case Study 2: In an assembly of atoms or molecules, a solid phase is formed whenever the interatomic attractive forces significantly exceed the disruptive thermal forces and thus restrict the mobility of atoms, forcing them into more or less fixed positions. From energy considerations, it is evident that in such solids the atoms or molecules will always attempt to assume highly ordered structures which are characterised by symmetry. Depending on the nature of the active interatomic forces, all solids may be subdivided into the following categories: Ionic solids: These solids consist of positively and negatively charged ions arranged in a regular fashion throughout the solid. These solids are veryhard and brittle, have very high melting points and have high enthalpies of vaporisation, e.g., NaCl, MgO, KCl, LiCl etc. Covalent solids: In these solids, the constituent particles are atoms which are linked together by a continuous system of covalent bonds. These bonds are strong and directional in nature. The covalent crystals are hard, have high melting points, are poor conductors of electricity. Diamond is a typical example of covalent solids. Metallic solids : In these solids, the constituent particles are positive ions immersed in a sea of mobile electrons. Metallic solids may be hard as well as soft. They are good conductors of heat and electricity e.g., common metals such as nickel, copper and alloys. Molecular solids : In these the constituent particles are molecules. The molecules are held together by dispersion forces or London forces, dipole-dipole forces or hydrogen bonds. In these questions (Q. No. i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Molecular solids are characterized by low melting point. Reason: Molecular solids are made up of covalent molecules.
Answer:(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(ii) Assertion: Ionic solids are characterized by high melting and boiling point. Reason: Ionic solids have coulombic forces of attraction between their ions.
Answer:(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(iii) Assertion: Covalent solids are insulators of electricity. Reason: Covalent solids are constituted by ions.
Answer:(c) Assertion is correct statement but reason is wrong statement.
(iv) Assertion: Diamond and graphite do not have the same covalent structure. Reason: Silicon carbide is typical example of network solid.
Hope the information shed above regarding Case Study and Passage Based Questions for Class 12 Chemistry Chapter 1 The Solid State with Answers Pdf free download has been useful to an extent. If you have any other queries about the CBSE Class 12 Chemistry The Solid State Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible.

You Might Also Like
Class 12 physics assertion reason questions chapter 4 moving charges and magnetism, class 12 maths: case study of chapter 12 linear programming pdf download, class 12 chemistry case study questions chapter 14 biomolecules, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

The Topper Combo Flashcards
- Contains the Latest NCERT in just 350 flashcards.
- Colourful and Interactive
- Summarised Important reactions according to the latest PYQs of NEET(UG) and JEE
No thanks, I’m not interested!
No products in the cart.
This website mtg.in will down for maintenance on Jun 07, 2024 from 10:00 am to 12:00 pm, please do not place any order after 09:30 am till the services are restored.

- Computer Science Olympiad (ICSO) Books
- English Olympiad (IEO) Books
- GK Olympiad (IGKO) Books
- Maths Olympiad (IMO) Books
- Science Olympiad (NSO) Books
- Olympiads Combo Packs
- SST Olympiad (ISSO) Books
- Hindi Olympiad (IHO) Books
- International Computer Science Olympiad (ICSO) Books
- Commerce Olympiad (ICO) Books
- NEET Champion
- NEET Previous Years Paper
- NEET Sample Paper
- NEET Crash Course
- Online Tests
- NCERT Fingertips
- NEET Biology Books
- NEET Physics Books
- NEET Chemistry Books
- Assertion & Reason
- AIIMS Previous Years Paper
- GK & Aptitude
- JIPMER Previous Years Paper
- JEE Main Guide
- JEE PREVIOUS YEARS PAPER
- JEE Sample Papers
- JEE Crash Course
- JEE Champion
- JEE Mathematics Books
- JEE Physics Books
- JEE Chemistry Books
- KCET Books – Karnataka CET
- KEAM Books – Kerala Engineering Exam
- WBJEE Books – West Bengal JEE
- BITSAT Exam Books
- MHT CET Books – Maharashtra CET
- Live Classes
- Recorded Classes
- Monthly Magazine – Single Issue
- Monthly Magazines – Subscription
- Monthly Magazine: Older Volumes
- Foundation Course Class 6
- Foundation Course Class 7
- Foundation Course Class 8
- Foundation Course Class 9
- Foundation Course Class 10
- NCERT Solutions Class 6
- NCERT Solutions Class 7
- NCERT Solutions Class 8
- NCERT Solutions Class 9
- NCERT Solutions Class 10
- NCERT Solutions Class 11
- NCERT Solutions Class 12
- CBSE Class 9 Books
- CBSE Class 10 Reference Books
- CBSE Class 10 Sample Paper Books
- CBSE Class 10 Previous Year Solved Paper Books
- CBSE Class 11 Books
- CBSE Reference Books for Class 12
- CBSE Previous Year Papers class 12 Books
- CBSE Sample Papers for Class 12
- Competitive Books
- Olympiad Books
- Govt. Exams
- 21 Science Crossword Puzzles
- 51 English Grammar Worksheets
- IGKO Online Test Programme
- Literature Books
- Vedic Mathematics
- Science Skill Development
- Mathematics Skill Development
- English Skill Development
- Summer Programme Combo
- Psychometric Online Test
- General ebooks

Click on the image to zoom in
Read reviews (0)
Write a review
Score More Case Study Chapter wise Practice Questions Chemistry Class-12
₹ 125.00 Original price was: ₹125.00. ₹ 93.75 Current price is: ₹93.75. (25% Off)
Discount offer on this book in a bundle, click to view
ScoreMore Case Study Chapter-wise Practice Questions Chemistry Class-12 is curated with 800+ Chapter-wise MCQs related to Case study/ Passage based and Assertion & Reason with hints and explanations based on latest pattern of CBSE (2020-2021).
88 in stock
Your security guaranteed
- Description
- Table of content
- Additional information
- Reviews (0)
MTG ScoreMore Case Study Based Sample Questions Chemistry Class 12 is specially designed to help students get familiar with solving these new patterns of questions. The book covers 800 + sample questions for practice with detailed explanations to each question. Practising these questions will definitely help students to get an edge in their CBSE preparations.
For the academic year 2020-21 CBSE has incorporated more Objective type/MCQ based questions which will focus on measuring critical thinking ability of students.
The new pattern of questions includes Case study based questions, Passage based questions, Assertion and Reason type questions. In Case study based/ Passage based questions, students will be expected to answer questions after reading a given paragraph or a passage. Assertion and Reason type question is just another way of checking the clarity of one’s concept.
- 1. The Solid State
- 2. Solutions
- 3. Electrochemistry
- 4. Chemical Kinetics
- 5. Surface Chemistry
- 6. General Principles and Processes of Isolation of Elements*
- 7. The p-Block Elements
- 8. The d- and f-Block Elements
- 9. Coordination Compounds
- 10. Haloalkanes and Haloarenes
- 11. Alcohols, Phenols and Ethers
- 12. Aldehydes, Ketones and Carboxylic Acids
- 14. Biomolecules
- 15. Polymers*
- 16. Chemistry in Everyday Life*
- *This chapter is not a part of the Board Examination 2020-21 syllabus
| ISBN13 | 9789390801381 |
|---|---|
| Author | MTG Editorial Board |
| Edition | 2020-21 |
| Pages | 152 |
| Classes | Class 12 |
| Exams | CBSE Boards, CUET |
| Subjects | Chemistry |
| Weight | 250gm |
There are no reviews yet.
Your email address will not be published. Required fields are marked *
Your review *
Name *
Email *
Save my name, email, and website in this browser for the next time I comment.
Customers prefer to buy this combination offer...

Score More Case Study Chapter wise Practice Questions Combo-Phy,Chem, Bio,Maths Class-12
Total Price = ₹ 500.00
Combo Price = ₹ 375.00

Score More Case Study Chapter wise Practice Questions Biology Class-12

Score More Case Study Chapter wise Practice Questions Maths Class-12

Score More Case Study Chapter wise Practice Questions Physics Class-12
- Notifications
- Institutional Queries
Username or Email Address
Class Select Class Class 1 Class 2 Class 3 Class 4 Class 5 Class 6 Class 7 Class 8 Class 9 Class 10 Class 11 Class 12 Pre-Nursery
Phone number
Class Select Class Class 1 Class 2 Class 3 Class 4 Class 5 Class 6 Class 7 Class 8 Class 9 Class 10 Class 11 Class 12 Others Pre-Nursery
Country * Afghanistan Åland Islands Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belau Belgium Belize Benin Bermuda Bhutan Bolivia Bonaire, Saint Eustatius and Saba Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo (Brazzaville) Congo (Kinshasa) Cook Islands Costa Rica Croatia Cuba Curaçao Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Eswatini Ethiopia Falkland Islands Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Honduras Hong Kong Hungary Iceland India Indonesia Iran Iraq Ireland Isle of Man Israel Italy Ivory Coast Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Kuwait Kyrgyzstan Laos Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia Moldova Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island North Korea North Macedonia Northern Mariana Islands Norway Oman Pakistan Palestinian Territory Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Reunion Romania Russia Rwanda São Tomé and Príncipe Saint Barthélemy Saint Helena Saint Kitts and Nevis Saint Lucia Saint Martin (Dutch part) Saint Martin (French part) Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia/Sandwich Islands South Korea South Sudan Spain Sri Lanka Sudan Suriname Svalbard and Jan Mayen Sweden Switzerland Syria Taiwan Tajikistan Tanzania Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom (UK) United States (US) United States (US) Minor Outlying Islands Uruguay Uzbekistan Vanuatu Vatican Venezuela Vietnam Virgin Islands (British) Virgin Islands (US) Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe
Mobile number *
Email address *
Password *
Lost your password? Please enter your username or email address. You will receive a link to create a new password via email.
Username or email
Reset password
Just remembered? Log In Instead
Don’t have an account? Register with us
myCBSEguide
- Mathematics
- Class 12 Maths Case...
Class 12 Maths Case Study Questions
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
Class 12 Maths question paper will have 1-2 Case Study Questions. These questions will carry 5 MCQs and students will attempt any four of them. As all of these are only MCQs, it is easy to score good marks with a little practice. Class 12 Maths Case Study Questions are available on the myCBSEguide App and Student Dashboard .
Why Case Studies in CBSE Syllabus?
CBSE has introduced case study questions in the CBSE curriculum recently. The purpose was to make students ready to face real-life challenges with the knowledge acquired in their classrooms. It means, there was a need to connect theories with practicals. Whatsoever the students are learning, they must know how to apply it in their day-to-day life. That’s why CBSE is emphasizing case studies and competency-based education .
Case Study Questions in Maths
Let’s have a look over the class 12 Mathematics sample question paper issued by CBSE, New Delhi. Question numbers 17 and 18 are case study questions.
Focus on concepts
If you go through each MCQ there, you will find that the theme/case study is common but the questions are based on different concepts related to the theme. It means, that if you have done ample practice on the various concepts, you can solve all these MCQs in minutes.
Easy Questions with a Practical Approach
The difficulty level of the questions is average or say easy in some cases. On the other hand, you get four options to choose from. So, you get two levels of support to get full marks with very little effort.
Practice Questions Regularly
Most of the time we feel that it’s easy and neglect it. But in the end, we have to pay for this negligence. This may happen here too. Although it’s easy to score good marks on the case study questions if you don’t practice such questions, you may lose your marks. So, we suggest students should practice at least 30-40 such questions before writing the board exam.
12 Maths Case-Based Questions
We are giving you some examples of case study questions here. We have arranged hundreds of such questions chapter-wise on the myCBSEguide App. It is the complete guide for CBSE students. You can download the myCBSEguide App and get more case study questions there.
Case Study Question – 1
- A is a diagonal matrix
- A is a scalar matrix
- A is a zero matrix
- A is a square matrix
- If A and B are two matrices such that AB = B and BA = A, then B 2 is equal to
Case Study Question – 2
- 4(x 3 – 24x 2 + 144x)
- 4(x 3 – 34x 2 + 244x)
- x 3 – 24x 2 + 144x
- 4x 3 – 24x 2 + 144x
- Local maxima at x = c 1
- Local minima at x = c 1
- Neither maxima nor minima at x = c 1
- None of these
Case Study Questions Matrices -1
Answer Key:
Case Study Questions Matrices – 2
Read the case study carefully and answer any four out of the following questions: Once a mathematics teacher drew a triangle ABC on the blackboard. Now he asked Jose,” If I increase AB by 11 cm and decrease the side BC by 11 cm, then what type of triangle it would be?” Jose said, “It will become an equilateral triangle.”
Again teacher asked Suraj,” If I multiply the side AB by 4 then what will be the relation of this with side AC?” Suraj said it will be 10 cm more than the three times AC.
Find the sides of the triangle using the matrix method and answer the following questions:
- (a) 3 × 3
Case Study Questions Determinants – 01
DETERMINANTS: A determinant is a square array of numbers (written within a pair of vertical lines) that represents a certain sum of products. We can solve a system of equations using determinants, but it becomes very tedious for large systems. We will only do 2 × 2 and 3 × 3 systems using determinants. Using the properties of determinants solve the problem given below and answer the questions that follow:
Three shopkeepers Ram Lal, Shyam Lal, and Ghansham are using polythene bags, handmade bags (prepared by prisoners), and newspaper envelopes as carrying bags. It is found that the shopkeepers Ram Lal, Shyam Lal, and Ghansham are using (20,30,40), (30,40,20), and (40,20,30) polythene bags, handmade bags, and newspapers envelopes respectively. The shopkeeper’s Ram Lal, Shyam Lal, and Ghansham spent ₹250, ₹270, and ₹200 on these carry bags respectively.
- (b) Shyam Lal
- (a) Ram Lal
Case Study Questions Determinants – 02
Case study questions application of derivatives.
- R(x) = -x 2 + 200x + 150000
- R(x) = x 2 – 200x – 140000
- R(x) = 200x 2 + x + 150000
- R(x) = -x 2 + 100 x + 100000
- R'(x) > 0
- R'(x) < 0
- R”(x) = 0
- (a) -x 2 + 200x + 150000
- (a) R'(x) = 0
- (c) 257, -63
Case Study Questions Vector Algebra
- tan−1(5/12)
- tan−1(12/3)
- (b) 130 m/s
- (a) tan−1(5/12)
- (b) 170 m/s
More Case Study Questions
These are only some samples. If you wish to get more case study questions for CBSE class 12 maths, install the myCBSEguide App. It has class 12 Maths chapter-wise case studies with solutions.
12 Maths Exam pattern
Question Paper Design of CBSE class 12 maths is as below. It clearly shows that 20% weightage will be given to HOTS questions. Whereas 55% of questions will be easy to solve.
| 1. | Exhibit memory of previously learned material by recalling facts, terms, basic concepts, and answers. Demonstrate understanding of facts and ideas by organizing, comparing, translating, interpreting, giving descriptions, and stating main ideas | 44 | 55 |
| 2. | Solve problems to new situations by applying acquired knowledge, facts, techniques and rules in a different way. | 20 | 25 |
| 3. | Examine and break information into parts by identifying motives or causes. Make inferences and find evidence to support generalizations | 16 | 20 |
| Present and defend opinions by making judgments about information, the validity of ideas, or quality of work based on a set of criteria. | |||
| Compile information together in a different way by combining elements in a new pattern or proposing alternative solutions | |||
| 80 | 100 |
- No. chapter-wise weightage. Care to be taken to cover all the chapters
- Suitable internal variations may be made for generating various templates keeping the overall weightage to different forms of questions and typology of questions the same.
Choice(s): There will be no overall choice in the question paper. However, 33% of internal choices will be given in all the sections
| Periodic Tests ( Best 2 out of 3 tests conducted) | 10 Marks |
| Mathematics Activities | 10 Marks |
12 Maths Prescribed Books
- Mathematics Part I – Textbook for Class XII, NCERT Publication
- Mathematics Part II – Textbook for Class XII, NCERT Publication
- Mathematics Exemplar Problem for Class XII, Published by NCERT
- Mathematics Lab Manual class XII, published by NCERT
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- CBSE Reduced Syllabus Class 10 (2020-21)
- CBSE Class 10 Maths Sample Paper 2020-21
- Case Study Class 10 Maths Questions
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
1 thought on “Class 12 Maths Case Study Questions”
plz provide solutions too
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Sandeep Garg Textbook Solution
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- HOTS Question
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- JEE Crash Course
- JEE Previous Year Paper
- Important Info
- JEE Mock Test
- JEE Sample Papers
- SRM-JEEE Mock Test
- VITEEE Mock Test
- BITSAT Mock Test
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- COMEDK Previous Year Paper
- GUJCET Previous Year Paper
- KCET Previous Year Paper
- KEAM Previous Year Paper
- Manipal Previous Year Paper
- MHT CET Previous Year Paper
- WBJEE Previous Year Paper
- AMU Previous Year Paper
- TS EAMCET Previous Year Paper
- SRM-JEEE Previous Year Paper
- VITEEE Previous Year Paper
- BITSAT Previous Year Paper
- UPSEE Previous Year Paper
- CGPET Previous Year Paper
- CUSAT Previous Year Paper
- AEEE Previous Year Paper
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
- STATE WISE BOOKS
- ENGINEERING EXAM
- SCHOLARSHIP OLYMPIAD
- STATE BOOKS

CBSE Class 12 Maths Case Study Questions With Solutions
CBSE Class 12 Mathematics Case Study Questions are introduced this year in the updated CBSE Board Exam Pattern. According to that this year the candidates need to prepare for the case study problems along with the questions that are legacy of CBSE Board.
In Class XIIth Mathematics Case Study Questions there are problems based on Objective types of questions, Assertions and Reason, and cae based problems. These problems have the intention to examine the students' overall understanding of the subject. If you are preparing for the Maths board exam then this is the right place for you.
Here, we have provided the complete set of CBSE class 12 maths case study questions With Solutions. These are developed by the subject matter expert. If you want to score good marks in the board papers then you should practice these Case based problems. It is absolutely free of cost.
Download PDF CBSE Class 12 Maths Case Study From the Given links. It is in chapter wise format.
| Class 12 Maths Chapter 2 – Inverse Trigonometric Functions Case Study | |
| Class 12 Maths Chapter 7 – Integrals Case Study | |
Class 12th Mathematics Important Formulas, MCQ, Case-Based, Assertion and Reason
| Chapter 1 – Relations and Functions (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 2 – Inverse Trigonometric Functions (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 3 – Matrices (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 4 – Determinants (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 5 – Continuity and Differentiability (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 6 – Application of Derivatives (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 7 – Integrals (MCQ) | |
| Chapter 8 – Application of Integrals (MCQ) | |
| Chapter 9 – Differential Equations (MCQ) | |
| Chapter 10 – Vector Algebra (MCQ) | |
| Chapter 11 – Three Dimensional Geometry (MCQ) | |
| Chapter 12 – Linear Programming (MCQ, Case-Based, Assertion & Reasoning) | |
| Chapter 13 – Probability (MCQ) |
Class 12th Maths Case Study
In class 12th Maths Case Study the questions are based on the real world scenarios. A passage filled with information or data is provided to the students. On the basis of that paragraph upto 5 questions are developed which should be answered by the students. To answer them they need to read the passage carefully and then pay attention to the given data to solve such questions.
These types of problems are generally known as case based questions which can be only solved by referring to the given paragraph.
Class 12 Maths Important Formulas
Knowing about the Maths Important Formulas is a crucial part of solving the Maths questions. Formulas help in solving the problems more efficiently and faster. Therefore the PDF file that we have provided here consists of all the basics and Important formulas of maths. It will help in revisions and solving the questions more accurately and easily.
Every chapter has its own topics and formulas so the PDF has been divided into chapter wise format. And if you access them from this place then you will be able to get all the formulas and other questions separately.
Class 12 Maths Assertion and Reason MCQs
Class 12th Maths Assertion and Reason MCQs PDF with Solutions are also given here. It is the most important part of Case Study Questions because scoring good marks in this section is not that much hard. If your basic concepts are clear. Because most of the time such types of questions are directly prepared by referring to the concepts.
Furthermore, the assertion and reason MCQs are solved by applying the distinct approaches. For instance, to answer these questions first candidates need to verify the Assertion (Statement) and then the reason if both are correct, then learners need to verify whether both statement and reason support each other or not.
There are a total of 5 sections in CBSE Class 12 Maths Questions Term 1. Section A contains 1 mark, Section B contains 2 marks, Section C contains 3 marks, Section D contains 4 marks and the last Section E contains 5 marks. A total of 5 questions are given in these sections.
No, In Term 1 exam there will be a total of 5 questions in each case study of class 12 maths, out of which 4 are compulsory to solve.

CBSE 12th 2024-25 : Physics Official Competency Focused Practice Questions released by CBSE

CBSE 12th 2024-25 : Chemistry Official Competency Focused Practice Questions released by CBSE

CBSE Class 12 Marks Verification Window Last Date Today: Apply Soon, Direct Link Here

CBSE Class 12 Result 2024: Application for Verification of Marks Starts; Last Date 21st May – Check Complete Process

CBSE Class 12 Result 2024 Latest Update : Verification of Marks, Revaluation & Photocopy of Answer Sheet; Check Complete Process
CBSE Class 12th Result 2024 Out : Supplementary, Re-verification & Re-evaluation Date Released; Check Details Here

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.

- NCERT Solutions
- NCERT Class 12
- NCERT 12 Chemistry
NCERT Solutions for Class 12 Chemistry
Download chapter-wise ncert solutions for class 12 chemistry.
NCERT Solutions for Class 12 Chemistry are drafted by the faculty at BYJU’S to help students learn all the complex concepts efficiently. Each and every question from the NCERT Textbook is answered in a systematic format to help students learn in a shorter duration. NCERT Solutions are prepared following vast research to make it easier for the students to complete the entire syllabus before the exams. Though various study materials are available online, it is very important for the students to identify their individual needs, and find the appropriate material accordingly. For this purpose, we at BYJU’S have provided both online and offline formats of the solutions, completely based on the latest syllabus of the CBSE board.
Students can access the chapter-wise solutions on this page from the links provided below. NCERT Solutions for Class 12 Chemistry Chapter 2 PDF Download option can be used by the students while learning the chapters from the textbook. NCERT Solutions Class 12 Chemistry (specific chapter) can be downloaded by clicking the links which are provided below.
NCERT Solutions for Class 12 Chemistry (Chapter-wise)
- Chapter 1: Solutions
- Chapter 2: Electrochemistry
- Chapter 3: Chemical Kinetics
- Chapter 4: The d & f Block Elements
- Chapter 5: Coordination Compounds
- Chapter 6: Haloalkanes and Haloarenes
- Chapter 7: Alcohols, Phenols, and Ethers
- Chapter 8: Aldehydes, Ketones, and Carboxylic Acids
- Chapter 9: Amines
- Chapter 10: Biomolecules
The following chapters have been removed from the NCERT Class 12 Chemistry textbook 2023-24.
- The Solid State
- Surface Chemistry
- General Principles and Processes of Isolation of Elements
- The p-Block Elements
- Chemistry in Everyday Life
Understanding the concepts during class hours might be difficult for the students. To overcome this issue, students must make use of the reference materials which are available online to get their doubts cleared. Regular practice of concepts in which they are weak will make them more confident from the exam point of view. Students are advised to read the chapter from the NCERT textbook and answer the exercise questions present in them for strong conceptual knowledge. The problem-solving and analytical thinking skills among students will be developed if the students answer the questions by referring to the NCERT Solutions available at BYJU’S.
Download NCERT Chemistry Class 12 Solutions PDF to boost your final exam preparation.
NCERT Solutions Class 12 Chemistry
NCERT Solutions Class 12 Chemistry is an important subject as it contains many chemical reactions and problems which are of more marks when it comes to exams. Scoring good marks in this subject is possible only if students learn the chapters on a regular basis. Even numericals are present in the chapters, so noting down the important formulas and understanding the method of solving them is an important step here. The NCERT Solutions contain an explanation for each reaction so that it will be easier for the students while preparing for the exams. Students will also be able to revise the chapters and recall them easily while answering the complex questions in the exam.
NCERT Solutions Class 12 Chemistry Chapter Details and Exercises
Chapter 1: the solid state.
This chapter explains the general characteristics of solid state, crystal lattice, classification of solids , imperfections in solids and unit cell. Matter is of three states – solid, liquid and gas. Solids are again divided into – Crystalline and Amorphous. Crystalline has a definite shape, whereas amorphous has no form. NCERT Solutions is a perfect guide to acquire a firm grip over these concepts. To strengthen the concepts further, students can also access the NCERT Exemplar Solutions which are available at BYJU’S.
Also access the following resources for Class 12 Chapter 1 The Solid State at BYJU’S:
- The Solid State Class 12 Notes Chapter 1
- Chemistry Revision Notes for Class 12 Chapter 1 the Solid State
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 1 – Solid State
- Chapter I – Solid State
Chapter 2: Solutions
A solution is a mixture of two or more components. This chapter helps students to understand the concentration of solutions, types of solutions, the vapour pressure of liquid solutions, solubility of gases and solids in a liquid, ideal and non ideal solutions and Raoult’s Law. Various problems based on finding the molarity, mole fraction, mass percentage and Henry’s Law constant are also present here. These problems are not only important for the Class 12 exams but also are of higher importance in competitive exams like JEE Mains, JEE Advanced etc.
Topics Covered in Class 12 CBSE Chemistry Chapter 2 Solutions :
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, colligative properties – relative lowering of vapour pressure, elevation of B.P., depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, and abnormal molecular mass.
Also access the following resources for Class 12 Chapter 2 Solutions at BYJU’S:
- Solutions Class 12 Notes Chapter 2
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 2 – Solutions
Chapter 3: Electrochemistry
Electrochemistry is defined as the branch of Chemistry which deals with the relationship between electrical and chemical energy produced in a redox reaction and their conversion. The concepts which are covered in this chapter are – electrochemical cells, Galvanic cells , Nernst equation, conductance of electrolytic solutions, electrolytic cells and electrolysis, batteries, fuel cells and corrosion. Students who are not able to solve the numerical problems can also refer to the NCERT Solutions and answer them effortlessly.
Topics Covered in Class 12 CBSE Chemistry Chapter 3 Electrochemistry :
Redox reactions; conductance in electrolytic solutions, specific and molar conductivity variations of conductivity with concentration, Kohlrausch’s Law, electrolysis and laws of electrolysis (elementary idea), dry cell – electrolytic cells and Galvanic cells; lead accumulator, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, fuel cells; corrosion.
Also access the following resources for Class 12 Chapter 3 Electrochemistry at BYJU’S:
- Electrochemistry Class 12 Notes Chapter 3
- Electrochemistry Formulas for NEET
- MCQs on Electrochemistry
- Chemistry Revision Notes for Class 12 Chapter 3 Electrochemistry
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 3 – ElectroChemistry Solutions
Chapter 4: Chemical Kinetics
This chapter will provide you with a good understanding of the rate of chemical reaction, Arrhenius equation , dependence on the rate of reaction and collision theory of chemical reaction. Chemical Kinetics is a branch of Chemistry which deals with the rate of chemical reaction, the factors affecting it and the mechanism of the reaction. We have 3 types in accordance to the rate of reaction – Instantaneous reactions, Slow reactions and Moderately Slow reactions.
Topics Covered in Class 12 CBSE Chemistry Chapter 4 Chemical Kinetics :
Rate of a reaction (average and instantaneous), factors affecting rates of reaction: concentration, temperature, catalyst; order and molecularity of a reaction; rate law and specific rate constant, integrated rate equations and half life (only for zero and first order reactions); concept of collision theory (elementary idea, no mathematical treatment).
Also access the following resources for Class 12 Chapter 4 Chemical Kinetics at BYJU’S:
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 4 – Chemical Kinetics
- Chemical Kinetics
- Chemical Kinetics Class 12 Notes Chapter 4
- Chemistry Revision Notes for Class 12 Chapter 4 Chemical Kinetics
Chapter 5: Surface Chemistry
Surface chemistry deals with important features like catalysis, adsorption and colloids which comprises gel and emulsion. After going through this chapter, students will understand the interfacial phenomenon and its significance, adsorption and its classification, mechanism of adsorption and the factors controlling adsorption. Further solving the textbook questions along with previous year question papers will boost the exam preparation of CBSE students.
Also access the following resources for Class 12 Chapter 5 Surface Chemistry at BYJU’S:
- Surface Chemistry Class 12 Notes Chapter 5
- Chapter 5 – Surface Chemistry
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 5 – Surface Chemistry Solutions
- MCQs on Surface Chemistry
- Chemistry Revision Notes for Class 12 Chapter 5 Surface Chemistry
Chapter 6: General Principles and Processes of Isolation of Elements
Metallurgy is a scientific and technological process which is followed to isolate the metal from its ores. Apart from explaining the processes and reactions of metal extraction, students will also learn about the fundamental principles and developments which would occur in this field. Aluminium is the most abundant metal which is found on the earth’s crust which is 8.3% by weight. So cleaning the ore, i.e., removal of particles like clay, sand etc., is known as concentration or dressing of the ore.
Also access the following resources for Class 12 Chapter 6 General Principles and Processes of Isolation of Elements at BYJU’S:
- General Principles and Processes of Isolation of Elements Class 12 Notes Chapter 6
- Chapter 6 – General Principles and Processes of Isolation of Elements
- Chemistry Revision Notes for Class 12 Chapter 6 General Principles and Processes of Isolation of Elements
- General principles and Processes of Isolation of Elements MCQ
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 6 – General Principles And Processes Of Isolation Of Elements
Chapter 7: The p-Block Elements
The history of the p-block elements has a history which takes us back to the 19th century. Group 13, 14, 15, 16, 17 and 18 elements are known as p-block elements. They exist in three physical states – metal, non metal and metalloids. For a better clarification of these concepts, students can refer to the NCERT Solutions available at BYJU’S. The clear explanation of each and every concept will help students attain good marks in the final exam.
Also access the following resources for Class 12 Chapter 7 The p-Block Elements at BYJU’S:
- Chapter 7 – P – Block Elements
- MCQs on p-Block Elements for NEET
- The p-Block Elements Class 12 Notes Chapter 7
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 7 – The P Block Elements
Chapter 8: The d- and f-Block Elements
The elements which are lying between the s and p-block elements are called as d-block or transition elements . The inner transition series are called as f-block elements. This chapter introduces concepts such as the general properties of transition elements, variation in ionic and atomic size of transition metals, physical properties, ionization enthalpies, magnetic properties and oxidation states. Students will get a clear idea about the electronic configuration, general characteristics and properties of important compounds in this chapter.
Topics Covered in Class 12 CBSE Chemistry Chapter 8 The d- and f-Block Elements :
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation. Preparation and properties of K 2 Cr 2 O 7 and KMnO 4 . Lanthanoids: electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction. Actinoids: Electronic configuration, oxidation states.
Also access the following resources for Class 12 Chapter 8 The d- and f-Block Elements at BYJU’S:
- The d-and f-Block Elements Class 12 Notes Chapter 8
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 8 – The D Block And F Block Elements
- MCQ on D and F Block Elements
- Chapter 8 – D and F Block Elements
Chapter 9: Coordination Compounds
Coordination compounds is a challenging area in the modern inorganic chemistry. In this chapter, students will be able to learn about Werner’s Theory of Coordination Compounds, definitions of important terms, nomenclature, isomerism , bonding, stability, importance and applications of coordination compounds. They will also study about the bonding in meta carbonyls which is important for the exams. These concepts are important for CBSE exams and competitive exams so more importance should be given when it comes to scoring marks.
Topics Covered in Class 12 CBSE Chemistry Chapter 9 The Coordination Compounds:
Coordination compounds: Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds, bonding; isomerism, importance of coordination compounds (in qualitative analysis, extraction of metals and biological systems).
Also access the following resources for Class 12 Chapter 9 Coordination Compounds at BYJU’S:
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 9 – Coordination Compounds
- Coordination Compounds MCQ
- Chemistry Revision Notes for Class 12 Chapter 9 the Coordination Compounds
- Coordination Compounds Class 12 Notes Chapter 9
Chapter 10: Haloalkanes and Haloarenes
The halogen derivatives of hydrocarbons are called haloalkanes. They are classified based on the number of hydrogen atoms present in them. The aromatic compounds in which the halogens are attached directly to the carbon atom of the aromatic ring are called haloarenes. In this chapter, students will also get an idea about the methods of preparation, chemical and physical properties and the organohalogen compounds uses. The reactions involved in the preparation of haloarenes and haloalkanes are explained clearly in this chapter to help students perform well in the exams.
Topics Covered in Class 12 CBSE Chemistry Chapter 10 Haloalkanes and Haloarenes :
Haloalkanes: Nomenclature, nature of C-X bond, physical and chemical properties, mechanism of substitution reactions. Haloarenes: Nature of C-X bond, substitution reactions (directive influence of halogen for monosubstituted compounds only). Uses and environmental effects of – dichloromethane, trichloromethane, tetrochloromethane, iodoform, freons, DDT.
Also access the following resources for Class 12 Chapter 10 Haloalkanes and Haloarenes at BYJU’S:
- Haloalkanes and Haloarenes Class 12 Notes Chapter 10
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 10 – Haloalkanes And Haloarenes
- Chapter 10 – Haloalkanes and Haloarenes
- Chemistry Revision Notes for Class 12 Chapter 10 Haloalkanes and Haloarenes
- Haloalkanes and Haloarenes MCQs
Chapter 11: Alcohols, Phenols and Ethers
The c lassification of alcohols and phenols are based on the number of -OH groups present. Compounds which have one -OH group are called monohydride alcohols and phenols. The compounds which have two, three or more -OH groups are called dihydric, trihydric or polyhydric alcohols and phenols. Students will study about the reactions involved in the process of making alcohols from phenols, alcohols and ethers. It will also help students to learn about the physical properties of alcohols, phenols and ethers.
Topics Covered in Class 12 CBSE Chemistry Chapter 11 Alcohols, Phenols and Ethers :
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only); identification of primary, secondary and tertiary alcohols; mechanism of dehydration, uses, some important compounds – methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophilic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Also access the following resources for Class 12 Chapter 11 Alcohols, Phenols and Ethers at BYJU’S:
- Alcohols, Phenols and Ethers Class 12 Notes Chapter 11
- Important Notes For NEET Chemistry – Alcohols, Phenols and Ethers
- Chemistry Revision Notes for Class 12 Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 11 – Alcohols Phenols And Ethers
- MCQ on Alcohols, Phenols and Ethers
Chapter 12: Aldehydes, Ketones and Carboxylic Acids
In organic chemistry, aldehydes, ketones and carboxylic acids are of utmost importance. Aldehydes and Ketones can be obtained by the hydration of alkynes, ozonolysis of alkenes and by the oxidation of alcohols . Carboxylic acids can be obtained by the oxidation of aldehydes or primary alcohols. This chapter is very important and carries more marks in the board exam. For this purpose, students have to learn all the concepts and revise them on a regular basis for a good score.
Topics Covered in Class 12 CBSE Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids :
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, and mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes; uses. Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Also access the following resources for Class 12 Chapter 12 Aldehydes, Ketones and Carboxylic Acids at BYJU’S:
- Aldehydes, Ketones and Carboxylic Acids Class 12 Notes Chapter 12
- Aldehydes, Ketones and Carboxylic Acids MCQs
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 12 – Aldehydes Ketones And Carboxylic Acids
- Chemistry Revision Notes for Class 12 Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Chapter 13: Amines
The derivatives of ammonia are amines which are obtained by the replacement of hydrogen. From this chapter, students will be able to understand the nomenclature, structure and properties of amines . Amines are an important organic compound which contains nitrogen. Numerous examples of determining the basicity of amines, the reaction and synthesis of amines are explained briefly in this chapter. Students will obtain a good hold on these concepts by answering the questions present at the end of the chapter.
Topics Covered in Class 12 CBSE Chemistry Chapter 13 Amines :
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, and identification of primary, secondary and tertiary amines. Cyanides and Isocyanides will be mentioned at relevant places in context. Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry
Also access the following resources for Class 12 Chapter 13 Amines at BYJU’S:
- Amines Class 12 Notes Chapter 13
- MCQs on Amines
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 13 – Amines
Chapter 14: Biomolecules
The organic compounds which are present as essential constituents in different cells of the living organism are called biomolecules . These include proteins, carbohydrates, vitamins, enzymes and nucleic acids. The interaction of biomolecules constitute the molecular logic of life processes. Simple molecules such as mineral salts and vitamins play an important role in the function of organisms. The structure and functions of the biomolecules are covered in this chapter as per the latest CBSE guidelines.
Topics Covered in Class 12 CBSE Chemistry Chapter 14 Biomolecules :
Carbohydrates: Classification (aldoses and ketoses), monosaccharides (glucose and fructose), oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); importance. Proteins: Elementary idea of α – amino acids, peptide bond, polypeptides, proteins, primary structure, secondary structure, tertiary structure and quaternary structure (qualitative idea only), denaturation of proteins; enzymes. Vitamins: Classification and functions. Nucleic Acids: DNA and RNA .
Also access the following resources for Class 12 Chapter 14 Biomolecules at BYJU’S:
- Biomolecules Class 12 Notes Chapter 14
- MCQ On Biomolecules
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 14 – Biomolecules
Chapter 15: Polymers
This chapter includes the concepts like – monomer, polymer and polymerisation. Its classification is based on the source, structure and polymerisation. The types of polymerisation are – addition polymerisation and condensation polymerisation. The important concepts of this chapter are explained clearly in the NCERT textbook. Students who aspire to score good marks in the exams are recommended to learn this chapter thoroughly and have a clear idea of these concepts.
Also access the following resources for Class 12 Chapter 15 Polymers at BYJU’S:
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 15 – Polymers
- Polymers Class 12 Notes Chapter 15
- Polymers MCQs
Chapter 16: Chemistry in Everyday Life
The sphere of human life is influenced by Chemistry. The principles of Chemistry have benefitted humans in a lot of ways. The concepts which are discussed in this chapter are – drugs and their classification , drug target interaction, the therapeutic action of different classes of drugs, chemicals in food and cleansing agents. Chemistry is a tough subject, and it requires lots of practice to remember the chemical reactions and formulas. So making use of the NCERT Solutions at BYJU’S will provide you with a strong grip on the important topics.
Also access the following resources for Class 12 Chapter 16 Chemistry in Everyday Life at BYJU’S:
- Chemistry in Everyday Life Class 12 Notes Chapter 16
- MCQs on Chemistry in Everyday Life
- Chapter 16 – Chemistry in Everyday Life
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 16 – Chemistry Solutions In Everyday Life
Each chapter from the NCERT Class 12 textbook contains a lot of concepts which might cause more stress in the students’ mind. So it is necessary to first know the tips and tricks to remember all the concepts efficiently. Students can download NCERT Class 12 Books here.
Merits of BYJU’S NCERT Solutions for Class 12 Chemistry
The Class 12 NCERT Solutions for Chemistry provided by BYJU’S feature:
- In-depth explanations for all logical reasoning questions.
- Step-by-step processes for solving numerical value questions.
- Concise and to-the-point answers to all theoretical questions.
- Verified answers from top-class subject experts.
- Free download option is available for Chemistry 12 NCERT Solutions PDF .
Note: CBSE Class 12 Chemistry students can bookmark this page in their browser to easily access NCERT Solutions for Class 12 Chemistry in the future.
CBSE Marking Scheme 2023-24
Class 12 is a crucial stage in the life of a student. To help them grasp all the concepts effectively, the CBSE board has divided the entire course into sections having marks according to the exam pattern. All the chapters are divided based on the marks weightage and importance from the CBSE exams perspective. The purpose of doing this is to provide a quality education for Class 12 students and help them achieve their future goals. It also provides a strong foundation of basic concepts which are important for the exams.
| 1 | Solutions | 7 |
| 2 | Electrochemistry | 9 |
| 3 | Chemical Kinetics | 7 |
| 4 | d-and f-Block Elements | 7 |
| 5 | Coordination Compounds | 7 |
| 6 | Haloalkanes and Haloarenes | 6 |
| 7 | Alcohols, Phenols and Ethers | 6 |
| 8 | Aldehydes, Ketones and Carboxylic Acids | 8 |
| 9 | Amines | 6 |
| 10 | Biomolecules | 7 |
Think NCERT Solutions, Think BYJU’S
BYJU’S NCERT Solutions are considered by many as the best solutions for CBSE students. The accuracy and reliability of the content provided here are unparalleled. Students will be able to complete the NCERT Class 12 Chemistry Syllabus and revise them accordingly to score good marks in the exams. Some key attributes of the NCERT Solutions provided by us are listed below.
- The solutions are drafted by top-notch faculty
At BYJU’S, top-notch faculty design solutions in order to help students perform well in the CBSE exams. They make it easier for the students to understand the concepts and grasp them. The solutions are 100% accurate as they completely follow the CBSE syllabus and guidelines.
- Regular practice helps students answer difficult questions
The solutions at BYJU’S are student-friendly because the faculty make use of simple language to help students understand the concepts. All the difficult questions are explained with many examples so that students do not face any further doubts about the concept. The solutions not only help students to gain marks but will also help them in their higher education.
- Best study material for reference
Students who are not able to answer the questions from the textbook can refer to the NCERT Solutions available at BYJU’S. The solutions are prepared in order to assist students with the various types of questions that would appear in the exam. It helps them to understand the method of approaching questions in the final exam.
After going through our Class 12 Chemistry NCERT Solutions, Also Explore:
- NCERT Exemplar Problems for Class 12 Chemistry
- CBSE Revision Notes for Class 12 Chemistry
Have any questions/issues regarding our NCERT Solutions for Class 12 Chemistry? Our support team is always available to resolve your queries. Register with BYJU’S and get in touch with them NOW!
Frequently Asked Questions on NCERT Solutions for Class 12 Chemistry
How many chapters are present in the ncert solutions for class 12 chemistry , is the ncert solutions for class 12 chemistry important for the students, what are the advantages of using byju’s ncert solutions for class 12 chemistry , can i use the ncert solutions for class 12 chemistry while revising the concepts for the exam.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
CBSE Case Study Questions for Class 12 Maths Relations and Functions Free PDF

Mere Bacchon, you must practice the CBSE Case Study Questions Class 12 Maths Relations and Functions in order to fully complete your preparation . They are very very important from exam point of view. These tricky Case Study Based Questions can act as a villain in your heroic exams!
I have made sure the questions (along with the solutions) prepare you fully for the upcoming exams. To download the latest CBSE Case Study Questions , just click ‘ Download PDF ’.
CBSE Case Study Questions for Class 12 Maths Relations and Functions PDF
Mcq set 1 -, mcq set 2 -, checkout our case study questions for other chapters.
- Chapter 2 Inverse Trigonometric Functions Case Study Questions
- Chapter 3 Matrices Case Study Questions
- Chapter 4 Determinants Case Study Questions
- Chapter 5 Continuity and Differentiability Case Study Questions
How should I study for my upcoming exams?
First, learn to sit for at least 2 hours at a stretch
Solve every question of NCERT by hand, without looking at the solution.
Solve NCERT Exemplar (if available)
Sit through chapter wise FULLY INVIGILATED TESTS
Practice MCQ Questions (Very Important)
Practice Assertion Reason & Case Study Based Questions
Sit through FULLY INVIGILATED TESTS involving MCQs. Assertion reason & Case Study Based Questions
After Completing everything mentioned above, Sit for atleast 6 full syllabus TESTS.
Comments are closed.
Contact Form
Privacy Policy
Talk to our experts
1800-120-456-456
CBSE Class 12 Important Questions for 2024-25
- Important Questions

CBSE Class 12 Important Questions for Maths, Physics, Chemistry and Biology - Free PDF Download
Class 12 is an important phase for the students. After this year of the academic curriculum, they will enter professional courses and pursue a career. It means the students who have chosen the science stream will need to strengthen their conceptual foundation well. Download the Class 12 Important Questions for Maths, Biology, Chemistry, and Physics to practice and strengthen your concepts. This is how you can ace the board and competitive exams.
The CBSE (Central Board of Secondary Education) board examinations are a gateway to India's esteemed universities. The CBSE Important Questions Class 12 solutions in free PDF download at Vedantu includes chapterwise solutions for Science (Physics, Chemistry, and Biology) and Maths subjects. This section includes CBSE Class 12 Important Questions Chapterwise Solutions with step-by-step explanations. Chapter-wise important questions Class 12 with answers are available for free PDF download on Vedantu.
The CBSE Class 12 important chapterwise solutions include commonly asked questions in previous years' papers of Maths Physics, Chemistry, and Biology. As per the analysis of previous year question paper patterns, our subject experts at Vedantu have prepared these important questions. These important question of Physics class 12 also include concepts from the latest updated curriculum of CBSE, thus ensuring students a thorough revision. Students can refer to materials offered by Vedantu to score high marks, including.
Download CBSE Class 12 Important Questions 2024-25 for All Subjects PDF
Access free essential Class 12 questions for all subjects to enhance your preparation. Utilize the solutions to understand how to tackle these questions and improve your proficiency in the respective subjects. Sharpen your skills by practicing with CBSE Important Questions Class 12, increasing your chances of scoring higher in exams.
CBSE Class 12 Important Questions |
|
|
|
|
|
|
|
Important Questions for CBSE Class 12
Important Questions for Class 12 CBSE Board
These Class 12 questions and solutions will enable students to build a strong foundation in the Maths and Sciences and ensure a rigorous preparation for their upcoming Class 12 CBSE board exams.
Not only that but by working through these question bank class 12 solutions in a chapter-wise format, students will excel in various competitive exams held outside the comfort of their classrooms like the JEE, NEET, and different engineering entrance exams. Since concepts to each topic as prescribed in the syllabus are broken down step-by-step, students will be able to understand the material prescribed in their Class 12 syllabus with ease and confidence.
Benefits of CBSE Important Questions Class 12
It provides you with various strategies to prepare for your board examinations and other competitive exams.
You will get all the Class 12 questions compiled in one place and will also help you to assess your preparation.
You will get to know the important topics with a hi̱gher probability of being asked in your examinations.
These important questions are compiled by subject matter experts who have analyzed the pattern of the Class 12 Board exams and understand the mindset of the examiner.
Since all the questions are prepared by referring to the NCERT textbooks, these are according to the latest syllabus of the CBSE.
You will come across several practice questions that are not present in your textbooks.
These Class 12 questions are extremely useful for revision before your exams.
By solving these questions, you will come across topics that you might have skipped while studying or you might need to go through again.
Vedantu Gives You the Competitive Edge
The class 12 English important questions break down concepts but they don't teach them from scratch. For those who are looking for additional help with their CBSE preparations, Vedantu provides personalized online tuition from the comfort of one's home in the core science subjects. This means no more hassles or travels after school hours and studying whenever you want, wherever you want. As long as you have an iPad, tablet, desktop PC, Smartphone, or any electronic device which supports WiFi and media playback, you can access our wide range of live streaming tuitions with the click (or tap) of a button. Internal tests and regular assessments are conducted by our teachers to keep a track of a students' progress, and live lessons are recorded for later playback and viewing by Vedantu students.
Are you ready to experience the Vedantu edge today? Simply log on to Vedantu.com and have fun learning.
Importance of CBSE Important Questions Class 12 for All Subjects
The subjects taught in the Class 12 syllabus need the highest support from the complete study material. Without the proper study material, one will not be able to comprehend the content and context of the chapters. Once the chapters are prepared well, students will also need a platform to judge their preparation level and find out the gaps.
This is where the Class 12 questions are framed by the experts following the latest CBSE syllabus for all subjects will be the best bet. These questions can be solved at home to practise and to strengthen the preparation level. Students will also be able to use these questions as the perfect guide to finding out which part of a particular chapter or subject needs more attention.
The Important Questions Class 12 with Answers will also help students to learn how to frame the right answers for the fundamental questions. The simpler format and stepwise method of explaining the formulas, concepts, and contexts will help students focus on the topics well and increase their answering skills perfectly.
Advantages of Important Questions Class 12 for All Subjects
Focus on the chapters you want to study and complete preparing them. Proceed to solve the CBSE important questions Class 12 available here and refer to the solutions. Test your preparation level by comparing your answers with the solutions.
Resolve doubts related to these questions by referring to the solutions instantly. Take a step ahead in your preparation by clarifying your queries faster.
Follow the easier answering format developed by the experts. These Class 12 question formats have been compiled following the latest CBSE guidelines for all subjects of Class 12. Hence, you can score more by sticking to these answering formats and staying ahead of the competition.
Get more probable questions from these lists and broaden your preparation.
Download CBSE Class 12 Important Questions for All Subjects PDF
Get the free Economics Class 12 Important Questions and for other subjects and focus on your preparation. Learn how to solve such questions from the solutions and develop your answering skills in these subjects. Practise answering the English Important Questions Class 12 and learn to score more in the exams.
CBSE Class 12 Study Materials
Study materials for CBSE Class 12 students are available here. These resources are designed to help you understand and excel in your academic subjects. Whether you're preparing for exams or looking to enhance your knowledge, our materials cover a range of topics from various subjects. Access these materials to make your learning experience more effective and enjoyable.
|
|
|
|
|
|
|
Vedantu's CBSE Class 12 Important Questions for 2024-25 serve as a valuable resource for students. These curated questions cover key topics, providing a focused approach to exam preparation. With a user-friendly interface, students can easily access and practice these essential questions, ensuring a thorough understanding of the curriculum. The emphasis on CBSE guidelines ensures relevance and alignment with exam patterns. This resource proves beneficial for class 12 students, offering a strategic tool to enhance their exam readiness. Vedantu's commitment to quality education is evident through these thoughtfully compiled and accessible class 12 important questions.

FAQs on CBSE Class 12 Important Questions for 2024-25
1. What are the benefits of Practising Important Questions for CBSE Class 12 Board Exam?
Vedantu curates a set of important questions for Class 12 subjects. Students must practice these questions as they provide ample revision and help students in scoring well in the subject. These questions are solved by highly experienced subject matter experts. By referring to the important questions PDF for Class 12 Maths, Physics, Chemistry, and Biology, students will be able to practice for board exams as well as competitive exams like JEE Main and Advanced and NEET, etc. These important question PDFs give an idea of what kinds of questions to expect in the exams.
2. Where can I avail Important Questions for Class 12 CBSE Subjects?
Vedantu is a premier online learning platform which provides a well-prepared set of Important Questions for Class 12- all major subjects. Students can rely on Vedantu as it offers exceptional exam materials and relevant questions that can be asked in the exams. Vedantu provides Important Questions for Class 12 with solutions so that students do not face any difficulty attempting these questions. These solutions are provided by subject experts. These questions aid in exam preparation and provide thorough revision during board exams. You can download the free PDFs of Important Questions for CBSE Class 12 subjects including Maths, Physics, Chemistry and Biology.
3. What are the main Subjects for which Class 12 CBSE students can avail important Questions?
At Vedantu, students can find question banks for all the core subjects of CBSE Class 12. These extra questions PDF ensure that students do not miss any important questions from exam point of view while studying. The main subjects for which students can find Important Questions Class 12 PDF are Maths, Physics, Chemistry, Biology, Business Studies and Economics. Students will score excellent marks by downloading the free PDFs of solved important questions and practising them during exams.
4. Is solving Important Questions enough for Scoring Well in Board Exams?
While solving important questions PDFs available on platforms like Vedantu is a great way to boost scores in board exams, students must not completely rely on them. Students must focus primarily on improving their concepts. For this, reading NCERT textbooks concepts is a must. Moreover, students should practise each and every problem given in the textbooks. In case of any difficulties in solving the problems, students can refer to the solutions PDFs provided by the best online educational platforms like Vedantu.
5. How to make the best use of the Important questions?
Once you have studied any chapter from your textbook, solve all the questions present in your textbook. You can refer to Vedantu’s Solutions to get the best possible solutions to questions in your NCERT books. Once you have solved all the questions in your textbook, start solving important question of Class 12 . Mark the questions you are unable to solve and go through the related topics in your textbooks and your notes. Make sure to go through every question in the Important Questions.
6. How can I download study material for Class 12?
You can download the study material for Class 12 on Vedantu. Here, you will discover relevant content in simple and easy language. Follow the provided steps to download the study material.
First, visit the official website of Vedantu. On this website, you will discover the learning materials including notes, significant questions, summaries of different concepts, and textbook questions with answers.
At the same time, you’ll see a list containing all the subjects of Class 12.
Choose your subject and click on that subject’s icon.
After selecting the subject, choose the chapters for which you require the content.
Click on the download option to avail the study stuff.
7. How can you top in Class 12?
Many Class 12 students aspire to get top ranks in their respective streams. They search for different tips so they can accomplish their dream. Here are some tips to help you with this.
It is recommended to start your preparation at the beginning of the academic year. This will allow you to study and complete every chapter on time.
Try to attend every lecture at your school.
Revise concepts when you reach your home.
Never hesitate to ask your queries from the respective subject teacher.
Practise the NCERT textbook questions and answers to have a grip on the subject.
Practise sample papers to understand the exam question paper format.
8. How to make a study plan for Class 12?
Here’s how you can make a study plan for Class 12.
First, you must know the time when you feel like studying. Some students may prefer studying at night. While some may like studying early in the morning.
Make changes to your current study timetable. Make sure that your timetable includes breaks between study hours.
Try to avoid studying for longer durations. As this will exhaust your mind.
Take out at least one hour a day to study each subject. You can change the study duration depending on the difficulty level of the subject.
Start studying those subjects or chapters first which you find easy.
9. What are the advantages of referring to the NCERT books for Class 12?
There are numerous advantages of referring to the NCERT books for Class 12. These are as follows.
The NCERT Books of Class 12 helps students to clarify their concepts of different chapters in different subjects.
The content written in the NCERT books for Class 12 is easy to comprehend so that the students may not find any difficulties while reading the chapters.
These books are created by professionals who have curated the content after doing deep research on various topics.
The NCERT books provide accurate and clear information about the chapters. Easy NCERT solutions are available on Vedantu for Class 12 all chapters and subjects.
10. Is Class 12 difficult?
The difficulty level of Class 12 varies from student to student. Some students who are excellent in academics will find this class easy. However, some students may find Class 12 tough due to a lack of knowledge about the chapters taught to them. The students who are weak in academics are recommended to commence studying at an early phase. This way will help them to read and understand each and every concept of the chapter thoroughly. Additionally, they must clarify their study-related issues with their mentors. Moreover, they should do a proper revision before any class test or exam and analyze their preparation level. By following these suggestions, Class 12 will become easy for all the students.
HOME TUITIONS IN INDIA
- Skip to main content
- Skip to search
- Skip to footer
Products and Services
Now available: ccna v1.1 exam topics.

CCNA certification
Validate your knowledge and skills in network fundamentals and access, IP connectivity, IP services, security fundamentals, and more. Take your IT career in any direction by earning a Cisco Certified Network Associate (CCNA) certification.
Your career in networking begins with CCNA
Take your IT career in any direction by earning a CCNA. CCNA validates a broad range of fundamentals for all IT careers - from networking technologies, to security, to software development - proving you have the skills businesses need to meet market demands.
Networking fundamentals
Showcase your knowledge of networking equipment and configuration. Be able to troubleshoot connectivity issues and effectively manage networks.
IP Services
Demonstrate your ability to configure routing for different IP versions and describe the purpose of redundancy protocols. Be able to interpret the components of a routing table.
Security fundamentals
Understand threats and ways to prevent them. Identify key elements of a security program, like user awareness and training. Demonstrate practical skills like setting up secure access to devices and networks.
Understand how automation affects network management, and compare traditional networks with controller-based networking. Leverage APIs, and understand configuration management tools.

CCNA Certification
How it works, no formal prerequisites.
CCNA is an asset to IT professionals of all experience levels, but learners often benefit from one or more years of experience implementing and administering Cisco solutions.
Example learner profiles
- Individuals looking to move into the IT field
- IT professionals looking to stand out in the job market
- IT professionals looking to enrich their current roles with additional networking skills
Getting started
To earn this certification, you’ll need to pass a single required exam.
A variety of resources are available to help you study - from guided learning to self-study and a community forum.
Unlock your career potential
Because CCNA covers so many IT fundamentals, it’s a great way to stand out no matter where your career takes you.
Potential roles
Network engineer.
Apply a range of technologies to connect, secure, and automate complex networks.
Network administrator
Install, maintain, monitor, and troubleshoot networks and keep them secure.
Help desk administrator
Diagnose and troubleshoot technical issues for clients and employees.
Alumni testimonials
Ccna moved elvin up the career ladder.

"Passing that CCNA exam triggered a chain of events I could never have predicted. First, I was a student, then a teacher, then a Cisco instructor, and I eventually became a Cisco VIP."
Elvin Arias Soto, CloudOps engineer
CCNA, CCDP, CCDA, CCNP, CCIE
Certifications give Kevin instant credibility at work

"People always want to know who they're talking to. They want to know if you’re qualified. Certifications give you instant credibility."
Kevin Brown, CyberOps analyst
CCNA, CyberOps Associate
Ben made a career change with a Cisco certification

"I chose to pursue Cisco certifications because I knew it would put me in the best position to start a career in networking."
Ben Harting, Configuration engineer
Maintain your certification
Your certification is valid for three years. You can renew with Continuing Education credits or retake exams before they expire.
CCNA essentials webinar series
Learn what to expect from the CCNA exam, and chart your path to certification success.
CCNA certification guide
Get familiar with Cisco’s learning environment, find study resources, and discover helpful hints for earning your CCNA.
CCNA Prep Program
Packed with 50+ hours of resources, webinars, and practice quizzes, CCNA Prep On Demand is your ultimate study buddy.
Enhance your learning journey
Stay up to date.
Get the latest news about Cisco certifications, plus tools and insights to help you get where you want to go.
CCNA community
Not sure where to begin? Head to the Cisco CCNA community to get advice and connect with experts.

IMAGES
VIDEO
COMMENTS
A solution of glucose is prepared with 0.052 g at glucose in 80.2 g of water. (K f = 1.86 K kg mol -1 and K b = 5.2 K kg mol -1) The following questions are multiple choice questions. Choose the most appropriate answer: (i) Molality of the given solution is. (a) 0.0052 m. (b) 0.0036 m.
Case Study Question 1: Read the passage given below and answer the following questions: The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example ofcolligative properties.
Class 12 Chemistry Case Study Questions for Term 1 exam includes The Solid State, The P block elements, Haloalkanes and Haloarenes, Biomolecules, etc. Questions for all these chapters are given in the PDF file that are available here for free to download. Term 1 exam is about to be held in November-December this year.
This will help them to understand the type of Case Study questions that can be asked in Grade 12 Chemistry examinations. Our expert faculty for standard 12 Chemistry have designed these questions based on the trend of questions that have been asked in last year's exams. The solutions have been designed in a manner to help the grade 12 ...
Here, we have provided case-based/passage-based questions for Class 12 Chemistry Chapter 2 Solutions. Case Study/Passage-Based Questions. Case Study 1: At 298 K, the vapour pressure of pure benzene, C 6 H 6 is 0.256 bar and the vapour pressure of pure toluene C 6 H 5 CH 3 is 0.0925 bar. Two mixtures were prepared as follows:
In Coming Exams, CBSE will ask two Case Study Questions in the CBSE class 12 Chemistry questions paper. Each theme will have five questions and students will have a choice to attempt any four of them. Here are some example questions Based On Case Study Problems: Question-1 Read the passage given below and answer any four out of the following ...
The solutions are in the hint manner as well as contain full examples too, refer to the link to access the Case Study on Solutions Class 12 Chemistry with Solutions in PDF - it's free. Features of Class 12 Solutions Case Study Questions . Certain features of Class 12 Solutions Case Study Questions are - Available for free 24×7
Ans. The solution will show negative deviation from Raoult's law. 5. Which type of deviation will be shown by the solution, if yAB < y AA. Ans. Solution will show positive deviation. Solutions - Chapter 2 CBSE Class 12 Chemistry Case-based Questions Read the given passages and answer the questions that follow: A solution which obeys Raoult ...
Ans. pV = nRT, Where, p = Osmotic pressure, n = Number of moles, V = Volume of solution in litre, R = Gas constant, T = Temperature. Case Study Question 1 on Solutions - Chapter 2 CBSE Class 12 Chemistry Case-based Questions Read the given passages and answer the questions that follow: The spontaneous flow of the solvent through a ...
CBSE Case Study Class 12: Here, you will get class 12 case study questions and answers for maths, physics, chemistry and biology pdf at free of cost. Along with you can also download case study questions for class 12 chapter wise for getting higher marks in board exams.
Class 12 Chemistry case study-based questions play a vital role in your overall understanding of the subject. They enable you to: Apply Theoretical Knowledge: Case studies allow you to apply the concepts you have learned in real-life situations, bridging the gap between theory and practical application. Develop Analytical Skills: By critically ...
Class 12 Chemistry : Case Study Questions of Solutions | Boards 2023Chemistry Exposed - Important Topicshttps://youtu.be/hW8HMmFCpaUPhysical Chemistry by Bha...
CBSE will ask Case Study Questions class 12 Chemistry in session 2020-21. These will be the first two questions in the board exam question paper. The first question will have 5 MCQs out of which students will attempt any 4 questions. The second question will carry 5 Assertion & Reason type questions with the choice to attempt any four.
Question 7. Out of BaCl 2 and KCl, which one is more effective in causing coagulation of a negatively charged colloidal Sol? Give reason. (Delhi 2015) Answer: BaCl 2 is more effective in causing coagulation because it has double +ve charge than K+.. Solutions Class 12 Important Questions Short Answer Type - I [SA-I] Question 8. Differentiate between molality and molarity of a solution.
Here, we have provided case-based/passage-based questions for Class 12 Chemistry Chapter 1 The Solid State. Case Study/Passage-Based Questions. Case Study 1: In a hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three ...
Discount offer on this book in a bundle, click to view. ScoreMore Case Study Chapter-wise Practice Questions Chemistry Class-12 is curated with 800+ Chapter-wise MCQs related to Case study/ Passage based and Assertion & Reason with hints and explanations based on latest pattern of CBSE (2020-2021). See Complete Description of Product. Qty: -. +.
More Case Study Questions. These are only some samples. If you wish to get more case study questions for CBSE class 12 maths, install the myCBSEguide App. It has class 12 Maths chapter-wise case studies with solutions. 12 Maths Case Study Questions 12 Maths Exam pattern. Question Paper Design of CBSE class 12 maths is as below.
Class 12th Maths Case Study. In class 12th Maths Case Study the questions are based on the real world scenarios. A passage filled with information or data is provided to the students. On the basis of that paragraph upto 5 questions are developed which should be answered by the students.
BST Class 12 Case studies: You already know that as per new pattern , questions based on case study can be asked in exam .These type of questions are introduced to check students ability to understand and apply his/her knowledge to given situation . Do not fear the questions based on case study. If you are well prepared and have through understanding of chapter, those questions will not be ...
Merits of BYJU'S NCERT Solutions for Class 12 Chemistry. The Class 12 NCERT Solutions for Chemistry provided by BYJU'S feature: In-depth explanations for all logical reasoning questions. Step-by-step processes for solving numerical value questions. Concise and to-the-point answers to all theoretical questions.
Mere Bacchon, you must practice the CBSE Case Study Questions Class 12 Maths Relations and Functions in order to fully complete your preparation.They are very very important from exam point of view. These tricky Case Study Based Questions can act as a villain in your heroic exams!. I have made sure the questions (along with the solutions) prepare you fully for the upcoming exams.
The main subjects for which students can find Important Questions Class 12 PDF are Maths, Physics, Chemistry, Biology, Business Studies and Economics. Students will score excellent marks by downloading the free PDFs of solved important questions and practising them during exams. 4.
CCNA certification. Validate your knowledge and skills in network fundamentals and access, IP connectivity, IP services, security fundamentals, and more. Take your IT career in any direction by earning a Cisco Certified Network Associate (CCNA) certification. Schedule exam Why certify?