An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Literature Review on the Role of Uterine Fibroids in Endometrial Function
Deborah e. ikhena.
1 Department of Obstetrics and Gynecology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Serdar E. Bulun
Uterine fibroids are benign uterine smooth muscle tumors that are present in up to 8 out of 10 women by the age of 50. Many of these women experience symptoms such as heavy and irregular menstrual bleeding, early pregnancy loss, and infertility. Traditionally believed to be inert masses, fibroids are now known to influence endometrial function at the molecular level. We present a comprehensive review of published studies on the effect of uterine fibroids on endometrial function. Our goal was to explore the current knowledge about how uterine fibroids interact with the endometrium and how these interactions influence clinical symptoms. Our review shows that submucosal fibroids produce a blunted decidualization response with decreased release of cytokines critical for implantation such as leukocyte inhibitory factor and cell adhesion molecules. Furthermore, fibroids alter the expression of genes relevant for implantation, such as bone morphogenetic protein receptor type II, glycodelin, among others. With regard to heavy menstrual bleeding, fibroids significantly alter the production of vasoconstrictors in the endometrium, leading to increased menstrual blood loss. Fibroids also increase the production of angiogenic factors such as basic fibroblast growth factor and reduce the production of coagulation factors resulting in heavy menses. Understanding the crosstalk between uterine fibroids and the endometrium will provide key insights into implantation and menstrual biology and drive the development of new and innovative therapeutic options for the management of symptoms in women with uterine fibroids.
Introduction
Uterine fibroids are the most common gynecologic tumor, present in up to 80% of all women by the age of 50. 1 While most uterine fibroids do not cause symptoms, some women can experience severe symptoms that significantly impact their quality of life. Fibroid symptoms include heavy and irregular menstrual bleeding with accompanying anemia, pelvic pain, dysmenorrhea, dyspareunia, increased urinary frequency, infertility, early pregnancy loss, among others. 2 , 3 Fibroids are the leading indication for hysterectomy in the United States and account for up to US$34.4 billion dollars annually in health-care costs. 4
The effects of fibroids on fertility were formerly believed to be exclusively as a result of their size; however, this perspective has changed as our understanding of fibroid pathogenesis at the molecular level has broadened. Fibroids influence endometrial gene expression through paracrine interactions. Additionally, the effect of fibroids on the endometrium is global and not localized to the endometrium overlying the fibroid itself. 5 We conducted a review of the literature to evaluate and discuss what is currently known about how uterine fibroids interact with the endometrium and how these interactions lead to clinical symptoms, specifically infertility, miscarriage, and heavy menstrual bleeding.
We performed a comprehensive review of the literature on uterine fibroids, the influences they exert on endometrial function, and the potential mechanisms through which these lead to the impaired implantation. PubMed and Google Scholar websites were used to identify relevant articles. Search terms such as “uterine fibroids,” “leiomyoma,” and “endometrium” were used in combination with “implantation,” “heavy menstrual bleeding,” “irregular menses,” “recurrent pregnancy loss,” “miscarriage,” “early pregnancy loss,” “infertility,” “subfertility,” and “fertility outcomes.” References from these articles were used to identify additional sources.
Only reports written in English were included in the literature review. We placed no restrictions on year of publication; we included all publications from the earliest database dates until March 2017. We described and expanded on what is currently known about the relationship between uterine fibroids and the endometrium as it pertains to fertility and menstrual bleeding.
Cellular Origins of Uterine Fibroids
Uterine fibroids are monoclonal tumors believed to arise from a single fibroid stem cell within the myometrium. 6 Three cell populations have been identified in uterine fibroids: fully differentiated fibroid smooth muscle cells, a cell population with intermediate characteristics, and fibroid stem cells. 7 Both myometrium and fibroid tissue have side population cells that possess cell surface markers characteristic of stem cells. 7 , 8 Fibroid stem cells are critical for fibroid growth and expansion. In fact, in a murine model, tumors composed only of fully differentiated or intermediate populations of fibroid cells demonstrate significantly slower growth rates than those tumors composed of fibroid stem cells. 7
It appears that fibroid stem cells occur as a result of a genetic hit to a myometrial stem cell, such as point mutations in the mediator complex subunit 12 ( MED12 ) gene or chromosomal rearrangements that affect the expression of the high-mobility group AT-hook 2 ( HMGA2 ) gene. 6 Chromosomal rearrangements involving HMGA2 on the long arm of chromosome 12 are believed to play a role in the induction of fibroid stem cells and fibroid tumorigenesis, especially in larger tumors. 6 , 9 Additionally, some fibroid stem cells possess MED12 mutations that have not been identified in the myometrial stem cell population. 10 Introduction of a MED12 mutation in murine uterine tissue has been shown to give rise to fibroid-like tumor formation. 11 These findings suggest that a genetic hit may be important for the initiation of fibroid tumors and their growth. Ethnicity and environmental factors are believed to play a role in tumorigenesis. Endocrine-disrupting chemicals (EDCs) have been shown to interfere with growth and differentiation in different stem cell types. Recent studies suggest that exposure to EDCs may lead to genetic alterations in stem cells, which may be important in fibroid tumorigenesis. 12 - 14 With regard to ethnicity, studies reveal that the number of tumor-initiating myometrial stem cells is directly correlated with the likelihood of developing uterine fibroids, with the highest number being present in African American women with uterine fibroids and the lowest in Caucasian women without uterine fibroids. 15
Fibroids are hormonally responsive tumors. Mature fibroid cells possess estrogen receptors, and estradiol is associated with increased proliferation of uterine fibroid smooth muscle cells. 16 , 17 Uterine fibroids not only respond to systemic steroids but also to local steroids biosynthesized by aromatase within the fibroid itself. 18 Despite the hormonally dependent nature of fibroids, fibroid stem cells express low levels of estrogen and progesterone receptors, suggesting that steroid hormones utilize a paracrine mechanism to exert their tropic effects on fibroid stem cells ( Figure 1 ).

Illustration of stem cell populations in the myometrium and fibroid tissue. Stem cells are self-renewing and are involved in the proliferation of both normal myometrium and fibroid tissue. It is thought that a genetic hit, such as a mutation in the MED12 gene, can lead to the transformation of a myometrial stem cell into a fibroid stem cell. Fibroids are hormone-responsive tissues. However, fibroid stem cells, which are mainly responsible for proliferation and fibroid growth, are devoid of estrogen and progesterone receptors. Thus, stem cell replication and growth is likely regulated via paracrine signals, which lead to fibroid growth. ERα indicates estrogen receptor alpha; PR, progesterone receptor.
An important signaling pathway implicated in promoting fibroid growth is the wingless-type Mouse Mammary Tumor Virus (MMTV) integration site Wingless Type (WNT)/β-catenin pathway. 19 Because β-catenin targets the MED12 subunit, mutations in the MED12 gene can lead to alterations in the interactions between MED12 and β-catenin leading to inhibition of β-catenin transactivation in response to WNT signaling. 20 In the WNT/β-catenin pathway, secreted WNT proteins bind to frizzled family cell surface receptors, leading to decreased β-catenin degradation in the cytoplasm and a subsequent increase in nuclear β-catenin. 2 , 21 In the murine model, increased β-catenin level seen with increasing parity is correlated with the number of fibroid-like tumors present in the uteri of such mice, which exhibit both histologic and molecular characteristics of fibroids. 22 However, in this study, it was unclear whether the increased β-catenin level or the increased parity of these mice is the primary driver of the increase in fibroid-like tumors. Recent data show that MED12 knockdown in human fibroid cells leads to decreased cell proliferation via downregulation of the WNT/β-catenin signaling pathway. 23
Additionally, activation of the WNT/β-catenin pathway leads to increased levels of transforming growth factor β3 (TGF-β3). Fibroid cells secrete markedly elevated levels of TGF-β3 in a steroid-responsive manner when compared to myometrial cells. 24 Transforming growth factor β3 has also been shown to play a key role in cell proliferation and deposition of extracellular matrix. 22 Taylor and colleagues have demonstrated that TGF-β3 secreted by fibroid cells exerts paracrine effects on endometrial stromal cells (ESCs) and epithelial cells. 5 , 25 , 26
Clinical Fertility Outcomes in the Presence of Uterine Fibroids
One in every 10 women seeking fertility treatment has uterine fibroids. 27 - 29 The effect of uterine fibroids on infertility is largely dependent on the location of the fibroid, with submucosal and intramural fibroids having the most significant impact.
Submucosal fibroids
Submucosal fibroids, which impinge into the uterine cavity, have been associated with impaired reproductive outcomes. In 2008, Klatsky et al performed a systematic review showing that women with submucosal fibroids had lower implantation rates (3.0%-11.5% vs 14%-30%) and a higher incidence of early pregnancy loss (47% vs 22%) compared to women without fibroids. 30 - 33 A meta-analysis by Pritts et al found that women with submucosal fibroids had significantly lower implantation rates, pregnancy rates, ongoing pregnancies, and live birth rates. In this meta-analysis, submucosal fibroids were associated with an increased risk of spontaneous abortion. 34 Although most of these data are from retrospective or prospective cohort studies, the consensus is to surgically remove submucosal fibroids in a woman who is actively pursuing pregnancy, regardless of other symptoms.
Intramural fibroids
Fibroids located within the wall of the myometrium are known as intramural fibroids. The data on the relationship between intramural fibroids and infertility are inconclusive at best. A meta-analysis by Pritts et al found higher rates of spontaneous abortions and significantly lower rates of implantation, ongoing pregnancies, and live births in women with intramural fibroids. 34 In 2017, Christopoulos et al showed decreased pregnancy rates after in vitro fertilization (IVF) in women with noncavity-distorting fibroids. Sagi-Dain and colleagues observed a similar trend in recipients of donor oocytes with uterine fibroids. 35 , 36 However, in this study, oocyte recipients with intramural fibroids received a significant lower percent of good quality embryos and this was not controlled for in the results. However, other studies show data to the contrary. Klatsky et al also studied pregnancy rates in recipients of donor oocytes and noted no difference in implantation or clinical pregnancy rates between women with and without uterine fibroids. 37 Additionally, the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation clinical trial by the Reproductive Medicine Network showed no difference in conception and live birth rates in women with noncavity-distorting intramural fibroids. 38 Given the conflicting data, there is still some debate about the clinical effect of noncavity-distorting intramural fibroids. Current data suggest that if a clinical effect is present, it may be unmasked by and as a result more clinically relevant for IVF cycles than with ovarian stimulation and intrauterine insemination.
In addition to the controversy on fibroid location, there is still some debate as to whether the degree of the detrimental effect of uterine fibroids’ endometrial function correlates with the size of the fibroid. A 2008 meta-analysis by Pritts et al both show no difference in effect due to fibroid size or number on outcomes. 34 However, fibroid size was only reported by 5 of the studies included in this meta-analysis.
The effect of myomectomy
These observations, specifically in the case of submucosal fibroids, raise the question of whether myomectomy leads to an improvement in fertility and early pregnancy outcomes. A 2013 Cochrane review concluded that hysteroscopic myomectomy improves clinical pregnancy rates with timed intercourse from a baseline of 21% to 39%. 39 Although these findings suggest that hysteroscopic myomectomy provides a clinical benefit in the presence of a submucosal fibroid, more data from randomized controlled trials with larger populations are needed to better understand the effect of hysteroscopic myomectomy on endometrial function and implantation.
Effect of Uterine Fibroids on the Endometrium and Implantation
The narrow time period during which the endometrium is receptive to implantation of the embryo is known as the window of implantation (WOI). The WOI occurs between 7 and 10 days following the luteinizing hormone surge and it is when the endometrium prepares for the attachment of the blastocyst. 40 The steps necessary for successful implantation are apposition, adhesion, and invasion. A complex series of interactions between various processes are necessary for these steps to occur and any aberrations can result in recurrent implantation failure, early pregnancy loss, or infertility. The effects of uterine fibroids on implantation are summarized in Figure 2 and described in detail below.

Diagram summarizing the effects of submucosal and intramural fibroids on implantation. BMP2 indicates bone morphogenetic protein 2; HOXA10, Homeobox A10; IL-1β, interleukin 1 beta; IL-11, interleukin 11; LIF, leukemia inhibitory factor; TGF-β3, transforming growth factor beta 3; VEGF, vascular endothelial growth factor.
Cell adhesions molecules, homeobox genes, and other gene expression
Transcription factors known as homeobox genes, specifically homeobox A10 ( HOXA10 ) and homeobox A11 ( HOXA11 ), are expressed in the female reproductive system and are important for implantation. 41 In mice, HOXA10 expression increased during the WOI. Knockout mice for HOXA10 are infertile due to implantation failure, specifically embryos from HOXA10 knockout mice are able to grow normally in wild-type mice, demonstrating that the defect lies with endometrial receptivity and not with the embryos themselves. 42
The HOXA10 expression is decreased in the endometrium of women with submucosal fibroids. This decrease in HOXA10 expression is most prominent in the endometrium overlying the submucosal fibroid but is also observed throughout the endometrium. 5
Decreased expression of HOXA10 and the cell adhesion molecule E-cadherin have been described in the endometrium of women with noncavity-distorting intramural uterine fibroids during the WOI. 43 In fact, 68.8% of women with fibroids have low mid-secretory phase HOXA10 protein expression. 44 Furthermore, it appears that this decrease in HOXA10 expression reverses following myomectomy. Interestingly, the same study failed to show any improvement in HOXA10 expression following myomectomy for submucosal fibroids. 45 Bone morphogenetic protein type II (BMP2) mediates HOXA10 expression; thus, increased endometrial resistance to BMP2 may contribute to the low HOXA10 expression in the endometrium of these patients 25 ( Figure 2 ).
Ben-Nagi and colleagues evaluated levels of glycodelin, osteopontin, interleukin (IL) 6, IL-10, and tumor necrosis factor (TNF) α in uterine flushings of women with and without submucosal fibroids during the WOI. They found lower levels of glycodelin and IL-10 in uterine flushings from the mid-luteal endometrium of women with uterine fibroids and no differences in osteopontin, IL-6, and TNFα compared to women without fibroids. 46 However, this was a study of uterine flushings and the accuracy of the correlation between uterine flushings and secretions from ESCs is unclear.
Horcajadas et al performed gene expression analysis on endometrial tissue from women with or without intramural uterine fibroids during the WOI. They identified 3 genes that are dysregulated in women with intramural fibroids >5 cm compared to controls: glycodelin and aldehyde dehydrogenase 3 family member B2. 47 Glycodelin was dysregulated in women with intramural fibroids <5 cm. This suggests that larger fibroids may have a more profound effect on endometrial gene expression; however, additional studies are needed to better elucidate this point.
The rise in progesterone following ovulation is responsible for decidualization of the endometrium, which is marked by increasing amounts of prostaglandins and vascular endothelial growth factor (VEGF). 48 These prostaglandins and VEGF increase vessel permeability in endometrial blood vessels allowing for extravasation of polymorphonuclear cells, which also produce cytokines important for implantation, including leukocyte inhibitory factor (LIF).
Another effect of progesterone and estrogen on ESCs is the secretion of decidual markers such as prolactin and insulin-like growth factor-binding protein 1, which are associated with IL-11. 49 , 50 IL-11 is essential for implantation. 51 Both LIF and IL-11 are pleiotropic cytokines belonging to the IL-6 family and have been noted to be essential for embryo implantation in the murine model. Both LIF and IL-11 bind to ligand-specific receptors, LIFR and IL-11R, and share the same signal transduction target, gp130. The gp130 signaling pathway is important for embryo implantation, 41 , 52 with inactivation of gp130 in a murine model resulting in implantation failure. 53
The LIF-deficient mice show a complete failure of implantation due to defective decidualization. Interestingly, embryos from LIF-deficient mice are unable to implant in the endometrium of LIF-deficient mice, but they are able to implant in the endometrium of wild-type mice. 54 In humans, LIF expression increases in the luteal phase and peaks during the implantation window; however, in the presence of submucosal fibroids, the luteal phase increase in LIF protein expression is blunted. 55 Clinically, deregulation of LIF production in the secretory endometrium has been associated with unexplained infertility and recurrent abortions. 56
Interleukin 11 is essential for sustained decidualization. The IL-11-deficient mice are able to begin decidualization but cannot sustain or complete the decidual response, thus leading to pregnancy loss by day 8. 51 , 57 In humans, IL-11 plays a role in the regulation of trophoblast invasion, and low levels of IL-11 are associated with decreased numbers of uterine natural killer (NK) cells in the secretory endometrium. 58 , 59 The production of IL-11 is decreased during the WOI in the presence of submucosal fibroids. 55 Because of its known role in trophoblast invasion and decidualization, reduction in IL-11 may lead to defective implantation; however, further study is needed to determine the clinical correlation.
Growth factors
Progesterone induces the secretion of BMP2 and its downstream target wingless-type MMTV integration site family, member 4 (WNT4) by ESCs. 60 This occurs via decidual signals from TGF-β3 family proteins such as heparin-binding epidermal growth factor. 61 The endometrium in BMP2-deficient mice is unable to undergo decidual differentiation due to the absence of BMP2 production. 62 , 63 Furthermore, although embryo attachment is possible in BMP2-deficient mice, impaired decidual differentiation leads to defective implantation and pregnancy loss. 60 , 63 When exposed to progesterone, WNT4 knockout mice have defective implantation as a result of impaired ESC survival and decidualization. 64 The activation of BMP2 in response to progesterone appears to be necessary for WNT4 activation and subsequently implantation.
In humans, BMP2 resistance is one of the proposed mechanisms by which submucosal fibroids impair implantation. Submucosal fibroids secrete high levels of TGF-β3, which downregulates BMP receptor type II expression in ESC and subsequently leads to ESC resistance to BMP2. 25 This resistance to BMP2 negatively affects cell proliferation and differentiation, causing impaired decidualization and implantation site formation. 63 Given the essential role of BMP2 and its downstream targets in decidualization and successful implantation, endometrial resistance to BMP2 in the presence of uterine fibroids has the potential to result in suboptimal decidualization and defective implantation. Clinically, this may manifest as a higher incidence of spontaneous abortions and a lower rate of implantation.
Immune cells
The progesterone-dependent increase in VEGF and prostaglandin secretion seen with decidualization promotes extravasation of immune cells into the endometrium. These cells consist mainly of macrophages and NK cells. 65 Macrophages produce cytokines, such as LIF, which as described above are essential for implantation. 58 , 66 Furthermore, macrophages play an integral role in trophoblast invasion and placental development. 67
The NK cells are the principal immune cells present during the WOI and are important for immune tolerance, angiogenesis, trophoblast migration, and invasion. 68 The NK cells produce pro-angiogenic factors such as VEGF and placental growth factor, which regulate maternal–uterine vasculature remodeling and trophoblast invasion. 69 , 70 Mice deficient in NK cells are still fertile, but their pregnancies are marked by fetal loss, severe intrauterine growth restriction, and preeclampsia. 71
Mid-secretory endometrium of women with uterine fibroids compared to women without fibroids show an increase in macrophage density and a decrease in the density of NK cells 72 ( Figure 2 ). These abnormalities in macrophage and NK cell density result in altered endometrial function and may impede endometrial receptivity to implantation.
Mechanical stretch, uterine wall contractility, and implantation
Uterine fibroids can place tremendous stress and stretch on the nearby myometrium and overlying endometrium, proportionate to the size and location of the fibroid. This increase in uterine stretch results in abnormal gene expression. 73 - 75
These abnormalities in gene expression, together with the physical presence of fibroids, contribute to impaired uterine contractility. Recent studies have implicated uterine contractility in implantation. Abnormal uterine contractions and peristalsis during the mid-luteal phase have been observed on cine magnetic resonance imaging of women with uterine fibroids. 76 Yoshino et al further described lower pregnancy rates in women with intramural fibroids and a higher frequency of uterine peristalsis during the WOI. In that study, 10 of the 29 women with intramural fibroids in the low-frequency peristalsis group achieved pregnancy compared to none of the 22 women in the high-frequency peristalsis group. 77 Although it was a small study, the data suggest that abnormal uterine peristalsis may play a role in implantation and pregnancy outcomes in women with intramural fibroids. However, additional larger studies are needed before these clinical relevance of these data can be determined.
Heavy Menstrual Bleeding and Dysmenorrhea Associated With Uterine Fibroids
Abnormal uterine bleeding is one of the most common symptoms in women with uterine fibroids. Normal menses occurs every 24 to 35 days. The American Congress of Obstetrics and Gynecology (ACOG) defines heavy menstrual bleeding as diagnosed when bleeding exceeds 80 mL, however, for clinical purposes, any level of menstrual bleeding which causes distress to the patient is managed as heavy menstrual bleeding. 78 The quantity of bleeding experienced with each menses depends on a complex interplay of vasoconstriction, angiogenesis, and coagulation. The most common type of abnormal uterine bleeding observed with fibroids is excessive menstrual bleeding that is frequently accompanied by dysmenorrhea. 2
Endothelin-1 (ET-1) and prostaglandin F 2alpha (PGF 2α ) are the 2 most important vasoconstrictors involved in menstruation. 79 Endothelin-1 is a potent vasoconstrictor that stimulates mitogenesis and myometrial contraction. 80 In the endometrium, ET-1 plays a role in spiral arteriole vasoconstriction and thus blood flow. Significantly higher levels of ET-1 are expressed in the endometrium compared to the myometrium and fibroid tissue. Endothelin-1 exerts its effects via its receptors ET A -R and ET B -R. Higher levels of ET A -R are found in fibroid tissue relative to the myometrium, but the opposite is observed for ET B -R. The alterations in receptor levels suggest that ET function is aberrant in the presence of uterine fibroids. 81 The altered myometrial expression of ET A -R and ET B -R may result in abnormal uterine contractions leading to defective vasoconstriction and increased menstrual blood flow, especially in the setting of intramural fibroids. Consistent with these data, the endometrial stroma in women with fibroids and heavy menstrual bleeding have been shown to have dilated endometrial stromal venous spaces compared to normal controls. This supports the idea that defective vasoconstriction is one of the mechanisms by which heavy menstrual bleeding occurs. 82 Prostaglandin F 2alpha receptors are present in normal myometrium and regulate uterine contractions. Uterine fibroids have increased PGF 2α production, which is accompanied by disordered uterine contraction and may play a role in the greater menstrual blood loss observed in women with uterine fibroids. 65
When compared to normal endometrium, fibroids overexpress basic fibroblast growth factor (bFGF), an important regulator of angiogenesis. 83 Concurrently, the endometrial stroma of women with uterine fibroids expresses increased levels of basic fibroblast growth factor receptor 1 (FGFR1). 83 The combined increase in the expression of bFGF and FGFR1 may play a role in abnormal angiogenesis and excess bleeding during menses observed in women with uterine fibroids ( Figure 3 ).

Diagram summarizing the effects of submucosal and intramural fibroids on bleeding. ATIII indicates antithrombin III; bFGF, basic fibroblast growth factor; BMPR-2, bone morphogenetic receptor type II; ET-1, endothelin-1; FGFR1, basic fibroblast growth factor receptor 1; PAI1, plasminogen activator inhibitor 1; PGF2α, prostaglandin F 2-alpha; TGF-β3, transforming growth factor beta 3; TM, thrombomodulin.
As described previously, fibroids secrete TGF-β3. Increased TGF-β3 secretion impedes production of coagulation and thrombosis factors, such as thrombomodulin, antithrombin III, and plasminogen activator inhibitor 1. Therefore, disproportionately higher levels of TGF-β3 secreted by fibroids inhibit expression of genes related to fibrinolytic and anticoagulant activity, which results in heavy menstrual bleeding ( Figure 3 ).
Our understanding of the intricate communication between uterine fibroids and the endometrium continues to grow. Although a clear link exists between uterine fibroids and heavy menstrual bleeding, a causative relationship between uterine fibroids and fertility is less clear given that both conditions are relatively common. There is consensus that submucosal fibroids, which distort the uterine cavity, are associated with infertility and early pregnancy loss and should be removed in patients with infertility. In contrast, the clinical significance of intramural fibroids remains controversial.
Submucosal and intramural fibroids both exert significant effects on endometrial gene expression and function. The downstream effects of excessive TGF-β3 secretion from uterine fibroids influence the entire endometrium. This leads to decreased production of transcription factors necessary for implantation during the WOI and aberrant production of coagulation factors during menses. Fibroids also exert their effect on the endometrium through altered gene expression and changes to the immune environment and vasoconstrictive factors.
Despite the significant strides that have been made in this field in recent years, further study is warranted to better understand the crosstalk between uterine fibroids and the endometrium. Such knowledge has the potential to lead to new therapeutic options for the management of symptomatic uterine fibroids.
Authors’ Note: DEI designed the review, performed the literature search, and wrote the manuscript. SEB designed the manuscript, supervised and performed revisions, and critically discussed and reviewed the complete manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH grants, POI-HD57877 and R37-HD38691 (to S.E.B) and the Friends of Prentice (to D.E.I).
- Neoplasms by Histologic Type
- Muscle Tissue Neoplasms
- Connective and Soft Tissue Neoplasms
A Comprehensive Review of Uterine Fibroids: Pathogenesis, Diagnosis, Treatment, And Future Perspectives
- November 2023
- Journal of Population Therapeutics and Clinical Pharmacology 30(18):1961–1974
- 30(18):1961–1974

- University of Karachi

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Int J Environ Res Publ Health

- INT J GYNECOL OBSTET
- Emma Giuliani

- Erica E. Marsh

- Geum Seon Sohn
- Yong Man Kim

- J Basic Clin Physiol Pharmacol
- Manoj Kumar
- Nihar Ranjan Bhoi

- BIOMED PHARMACOTHER
- Linjiao Chen

- Christine P West
- George A Vilos
- Angelos G Vilos

- Surg Tech Int
- Marcel Grube

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

MARIA SYL D. DE LA CRUZ, MD, AND EDWARD M. BUCHANAN, MD
Am Fam Physician. 2017;95(2):100-107
Patient information : A handout on this topic is available at https://familydoctor.org/familydoctor/en/diseases-conditions/uterine-fibroids.html .
Author disclosure: No relevant financial affiliations.
Uterine fibroids are common benign neoplasms, with a higher prevalence in older women and in those of African descent. Many are discovered incidentally on clinical examination or imaging in asymptomatic women. Fibroids can cause abnormal uterine bleeding, pelvic pressure, bowel dysfunction, urinary frequency and urgency, urinary retention, low back pain, constipation, and dyspareunia. Ultrasonography is the preferred initial imaging modality. Expectant management is recommended for asymptomatic patients because most fibroids decrease in size during menopause. Management should be tailored to the size and location of fibroids; the patient's age, symptoms, desire to maintain fertility, and access to treatment; and the experience of the physician. Medical therapy to reduce heavy menstrual bleeding includes hormonal contraceptives, tranexamic acid, and nonsteroidal anti-inflammatory drugs. Gonadotropin-releasing hormone agonists or selective progesterone receptor modulators are an option for patients who need symptom relief preoperatively or who are approaching menopause. Surgical treatment includes hysterectomy, myomectomy, uterine artery embolization, and magnetic resonance–guided focused ultrasound surgery.
Uterine fibroids, or leiomyomas, are the most common benign tumors in women of reproductive age. 1 Their prevalence is age dependent; they can be detected in up to 80% of women by 50 years of age. 2 Fibroids are the leading indication for hysterectomy, accounting for 39% of all hysterectomies performed annually in the United States. 3 Although many are detected incidentally on imaging in asymptomatic women, 20% to 50% of women are symptomatic and may wish to pursue treatment. 4
WHAT IS NEW ON THIS TOPIC: UTERINE FIBROIDS
Compared with total laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy, vaginal hysterectomy is associated with shorter operative time, less blood loss, shorter paralytic ileus time, and shorter hospitalization.
In 2014, the U.S. Food and Drug Administration recommended limiting the use of laparoscopic power morcellation to reproductive-aged women who are not candidates for en bloc uterine resection. Morcellation should not be used in women with suspected or known uterine cancer.
An estimated 15% to 33% of fibroids recur after myomectomy, and approximately 10% of women undergoing myomectomy will undergo a hysterectomy within five to 10 years.
| Ultrasonography is the recommended initial imaging modality for diagnosis of uterine fibroids. | C | , |
| Management of uterine fibroids should be tailored to the size and location of fibroids; the patient's age, symptoms, desire to preserve fertility, and access to therapy; and the physician's experience. | C | , |
| Expectant management is appropriate for women with asymptomatic uterine fibroids. | C | |
| In women undergoing hysterectomy for treatment of uterine fibroids, the least invasive approach possible should be chosen. | B | , |
Epidemiology and Etiology
Fibroids are benign tumors that originate from the uterine smooth muscle tissue (myometrium) whose growth is dependent on estrogen and progesterone. 5 , 6 Fibroids are rare before puberty, increase in prevalence during the reproductive years, and decrease in size after menopause. 6 Aromatase in fibroid tissue allows for endogenous production of estradiol, and fibroid stem cells express estrogen and progesterone receptors that facilitate tumor growth in the presence of these hormones. 5 Protective factors and risk factors for fibroid development are listed in Table 1 . 7 – 9 The major risk factors for fibroid development are increasing age (until menopause) and African descent. 7 , 8 Compared with white women, black women have a higher lifetime prevalence of fibroids and more severe symptoms, which can affect their quality of life. 10
| Increased parity | African descent |
| Late menarche (older than 16 years) | Age greater than 40 years |
| Smoking | Early menarche (younger than 10 years) |
| Use of oral contraceptives | Family history of uterine fibroids |
| Nulliparity | |
| Obesity |
Clinical Features
Uterine fibroids are classified based on location: subserosal (projecting outside the uterus), intramural (within the myometrium), and submucosal (projecting into the uterine cavity). The symptoms and treatment options are affected by the size, number, and location of the tumors. 11 The most common symptom is abnormal uterine bleeding, usually excessive menstrual bleeding. 12 Other symptoms include pelvic pressure, bowel dysfunction, urinary frequency and urgency, urinary retention, low back pain, constipation, and dyspareunia. 13
Uterine fibroids may be associated with infertility, and some experts recommend that women with infertility be evaluated for fibroids, with potential removal if the tumors have a submucosal component. 14 However, there is no evidence from randomized controlled trials to support myomectomy to improve fertility. 15 One meta-analysis included two studies that showed improvement in spontaneous conception rates in women who underwent myomectomy for submucosal fibroids (relative risk [RR] = 2.034; 95% confidence interval [CI], 1.081 to 3.826; P = .028). 16 However, no statistically significant difference was noted in the ongoing pregnancy/live birth rate. Women with intramural fibroids had no differences in pregnancy rates after undergoing myomectomy. Although studies have had conflicting results on the change in fibroid size during pregnancy, 17 , 18 a large retrospective study of women with uterine fibroids found a significantly increased risk of cesarean delivery compared with a control group (33.1% vs. 24.2%), as well as increases in the risk of breech presentation (5.3% vs. 3.1%), pre-term premature rupture of membranes (3.3% vs. 2.4%), delivery before 37 weeks' gestation (15.1% vs. 10.5%), and intrauterine fetal death with growth restriction (3.9% vs. 1.5%). 19 Therefore, fibroids in pregnant women warrant additional maternal and fetal surveillance.
In the postpartum period, women with fibroids have an increased risk of postpartum hemorrhage secondary to an increased risk of uterine atony. 20 The risk of malignancy for uterine fibroids is very low; the prevalence of leiomyosarcoma is estimated at about one in 400 (0.25%) women undergoing surgery for fibroids. 21 Because the natural course of fibroids involves growth and regression, enlarging fibroids are not an indication for removal. 22 , 23
The evaluation of fibroids is based mainly on the patient's presenting symptoms: abnormal menstrual bleeding, bulk symptoms, pelvic pain, or findings suggestive of anemia. Fibroids are sometimes found in asymptomatic women during routine pelvic examination or incidentally during imaging. 24 In the United States, ultrasonography is the preferred initial imaging modality for fibroids. 4 Transvaginal ultrasonography is about 90% to 99% sensitive for detecting uterine fibroids, but it may miss subserosal or small fibroids. 25 , 26 Adding sonohysterography or hysteroscopy improves sensitivity for detecting submucosal myomas. 25 There are no reliable means to differentiate benign from malignant tumors without pathologic evaluation. Some predictors of malignancy on magnetic resonance imaging include age older than 45 years (odds ratio [OR] = 20), intratumoral hemorrhage (OR = 21), endometrial thickening (OR = 11), T2-weighted signal heterogeneity (OR = 10), menopausal status (OR = 9.7), and nonmyometrial origin (OR = 4.9). 27 , 28 Risk factors for leiomyosarcoma include radiation of the pelvis, increasing age, and use of tamoxifen, 29 , 30 which has implications for surgical management of fibroids. Table 2 includes the differential diagnosis of uterine masses. 31
| Adenomyosis |
| Ectopic pregnancy |
| Endometrial carcinoma |
| Endometrial polyp |
| Endometriosis |
| Metastatic disease |
| Pregnancy |
| Uterine carcinosarcoma (considered an epithelial neoplasm) |
| Uterine fibroids |
| Uterine sarcoma (leiomyosarcoma, endometrial stromal sarcoma, mixed mesodermal tumor) |
Treatment of uterine fibroids should be tailored to the size and location of the tumors; the patient's age, symptoms, desire to maintain fertility, and access to treatment; and the physician's experience 4 , 11 ( Table 3 32 – 42 and Table 4 4 , 16 , 34 , 38 , 40 – 44 ) . The ideal treatment satisfies four goals: relief of signs and symptoms, sustained reduction of the size of fibroids, maintenance of fertility (if desired), and avoidance of harm. Figure 1 presents an algorithm for the management of uterine fibroids. 4
| Gonadotropin-releasing hormone agonists | Preoperative treatment to decrease size of tumors before surgery or in women approaching menopause | Decrease blood loss, operative time, and recovery time | Long-term treatment associated with higher cost, menopausal symptoms, and bone loss; increased recurrence risk with myomectomy | Depends on subsequent procedure |
| Levonorgestrel-releasing intrauterine system (Mirena) | Treats abnormal uterine bleeding, likely by stabilization of endometrium | Most effective medical treatment for reducing blood loss; decreases fibroid volume | Irregular uterine bleeding, increased risk of device expulsion | Yes, if discontinued after resolution of symptoms |
| Nonsteroidal anti-inflammatory drugs | Anti-inflammatories and prostaglandin inhibitors | Reduce pain and blood loss from fibroids | Do not decrease fibroid volume; gastrointestinal adverse effects | Yes |
| Oral contraceptives | Treat abnormal uterine bleeding, likely by stabilization of endometrium | Reduce blood loss from fibroids; ease of conversion to alternate therapy if not successful | Do not decrease fibroid volume | Yes, if discontinued after resolution of symptoms |
| Selective progesterone receptor modulators , | Preoperative treatment to decrease size of tumors before surgery or in women approaching menopause | Decrease blood loss, operative time, and recovery time; not associated with hypoestrogenic adverse effects | Headache and breast tenderness, progesterone receptor modulator–associated endometrial changes; increased recurrence risk with myomectomy | Depends on subsequent procedure |
| Tranexamic acid (Cyklokapron) , | Antifibrinolytic therapy | Reduces blood loss from fibroids; ease of conversion to alternate therapy | Does not decrease fibroid volume; medical contraindications | Yes |
| Hysterectomy | Surgical removal of the uterus (transabdominally, transvaginally, or laparoscopically) | Definitive treatment for women who do not wish to preserve fertility; transvaginal and laparoscopic approach associated with decreased pain, blood loss, and recovery time compared with transabdominal surgery | Surgical risks higher with transabdominal surgery (e.g., infection, pain, fever, increased blood loss and recovery time); morcellation with laparoscopic approach increases risk of iatrogenic dissemination of tissue | No |
| Magnetic resonance–guided focused ultrasound surgery | In situ destruction by high-intensity ultrasound waves | Noninvasive approach; shorter recovery time with modest symptom improvement | Heavy menses, pain from sciatic nerve irritation, higher reintervention rate | Unknown |
| Myomectomy | Surgical or endoscopic excision of tumors | Resolution of symptoms with preservation of fertility | Recurrence rate of 15% to 30% at five years, depending on size and extent of tumors | Yes |
| Uterine artery embolization | Interventional radiologic procedure to occlude uterine arteries | Minimally invasive; avoids surgery; short hospitalization | Recurrence rate > 17% at 30 months; postembolization syndrome | Unknown |
| Asymptomatic women | Clinical surveillance |
| Infertile women with distorted uterine cavity (i.e., submucosal fibroids) who desire future fertility | Myomectomy |
| Symptomatic women who desire future fertility | Medical treatment or myomectomy , , |
| Symptomatic women who do not desire future fertility but wish to preserve the uterus | Medical treatment, myomectomy, uterine artery embolization, magnetic resonance–guided focused ultrasound surgery , , – |
| Symptomatic women who want definitive treatment and do not desire future fertility | Hysterectomy by least invasive approach possible , |
EXPECTANT THERAPY
About 3% to 7% of untreated fibroids in premenopausal women regress over six months to three years, and most decrease in size at menopause. Because there is minimal concern for malignancy in women with asymptomatic fibroids, watchful waiting is preferred - for management. 4 There are no studies that support - surveillance with imaging or repeat imaging in asymptomatic women with fibroids. 4 , 11
MEDICAL THERAPY
Hormonal Contraceptives . Women who use combined oral contraceptives have significantly less self-reported menstrual blood loss after 12 months compared with placebo. 33 However, the levonorgestrel-releasing intra-uterine system (Mirena) results in a significantly greater reduction in menstrual blood loss at 12 months vs. oral contraceptives (mean reduction = 91% vs. 13% per cycle; P < .001). 33 In six prospective observational studies, reported expulsion rates of intrauterine devices were between zero and 20% in women with uterine fibroids. 45 There is a lack of high-quality evidence regarding oral and injectable progestin for uterine fibroids. 46 – 48
Tranexamic Acid . Tranexamic acid (Cyklokapron) is an oral nonhormonal antifibrinolytic agent that significantly reduces menstrual blood loss compared with placebo (mean reduction = 94 mL per cycle; 95% CI, 36 to 151 mL). 37 , 38 One small nonrandomized study reported a higher rate of fibroid necrosis in patients who received tranexamic acid compared with untreated patients (15% vs. 4.7%; OR = 3.60; 95% CI, 1.83 to 6.07; P = .0003), with intralesional thrombi in one-half of the 22 cases involving fibroid necrosis (manifesting as apop-totic cellular debris with inflammatory cells, and usually hemorrhage). 49 However, in a systematic review of four studies with 200 patients who received tranexamic acid, none of the studies detailed the adverse effects of fibroid necrosis or thrombus formation. 50
Nonsteroidal Anti-inflammatory Drugs . Another medical option for the treatment of uterine fibroids is a non-steroidal anti-inflammatory drug. These agents significantly reduce blood loss (mean reduction = 124 mL per cycle; 95% CI, 62 to 186 mL) and improve pain relief compared with placebo, 34 but are less effective in decreasing blood loss compared with the levonorgestrel-releasing intrauterine system or tranexamic acid at three months. 51
Hormone Therapy . Gonadotropin-releasing hormone (GnRH) agonists and selective progesterone receptor modulators (SPRMs) are options for patients who need temporary relief from symptoms preoperatively or who are approaching menopause. Preoperative administration of GnRH agonists (e.g., leuprolide [Lupron], goserelin [Zoladex], triptorelin [Trelstar Depot]) increases hemoglobin levels preoperatively by 1.0 g per dL (10 g per L) and postoperatively by 0.8 g per dL (8 g per L), as well as significantly decreases pelvic symptom scores. 32 Adverse effects resulting from the hypoestrogenized state, including hot flashes (OR = 6.5), vaginitis (OR = 4.0), sweating (OR = 8.3), and change in breast size (OR = 7.7), affect the long-term use of these agents. 32
Compared with placebo, the SPRM mife-pristone (Mifeprex) significantly decreases heavy menstrual bleeding (OR = 18; 95% CI, 6.7 to 47) and improves fibroid-specific quality of life, but does not affect fibroid volume. 35 Ulipristal (Ella) is an SPRM approved as a contraceptive in the United States but used in other countries for the treatment of fibroids in adult women who are eligible for surgery. Compared with placebo, a 5-mg dose of ulipristal significantly reduces mean blood loss (94% vs. 48% per cycle; 95% CI, 55% to 83%; P < .001), decreases fibroid volume by more than 25% (85% vs. 45%; 95% CI, 4% to 39%; P = .01), and induces amenorrhea in significantly more patients (94% vs. 48%; 95% CI, 50% to 77%; P < .001). 52 Treatment is limited to three months of continuous use. The most common adverse effects include headache and breast tenderness. The advantage of SPRMs over GnRH agonists for preoperative adjuvant therapy is their lack of hypoestrogenic adverse effects and bone loss. However, SPRMs can result in progesterone receptor modulator–associated endometrial changes, although these seem to be benign. 36
Other Agents . Other, less-studied options for the treatment of uterine fibroids include aromatase inhibitors and estrogen receptor antagonists. Aromatase inhibitors (e.g., letrozole [Femara], anastrozole [Arimidex], fadrozole [not available in the United States]) block the synthesis of estrogen. Limited data have shown that they help reduce fibroid size as well as decrease menstrual bleeding, with adverse effects including hot flashes, vaginal dryness, and musculoskeletal pain. 53 , 54 Overall, there is insufficient evidence to support the use of aromatase inhibitors for the treatment of uterine fibroids. 55 Selective estrogen receptor modulators act as partial estrogen receptor agonists in bone, cardiovascular tissue, and the endometrium. In a small prospective trial of 18 patients, tamoxifen did not reduce fibroid size or uterine volume, but did reduce menstrual blood loss by 40% to 50% and decrease pelvic pain compared with the control group. 56 Based on its adverse effects (e.g., hot flashes, dizziness, endometrial thickening), the authors concluded that its risks outweigh its marginal benefits for fibroid treatment. Another selective estrogen receptor modulator, raloxifene (Evista), has also shown inconsistent results, with two of three studies included in a Cochrane review showing significant benefit. 57
Hysterectomy . Hysterectomy provides a definitive cure for women with symptomatic fibroids who do not wish to preserve fertility, resulting in complete resolution of symptoms and improved quality of life. Hysterectomy by the least invasive approach possible is the most effective treatment for symptomatic uterine fibroids. 39 Vaginal hysterectomy is the preferred technique because it provides several statistically significant advantages, including shorter surgery time than total laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (70 minutes vs. 151 minutes vs. 130 minutes, respectively), decreased blood loss (183 mL vs. 204 mL vs. 358 mL), shorter hospitalization (51 hours vs. 77 hours vs. 77 hours), and shorter paralytic ileus time (19 hours vs. 28 hours vs. 26 hours); however, vaginal hysterectomy is limited by the size of the myomatous uterus. 43 Abdominal hysterectomy is an alternative approach, but the balance of risks and benefits must be individualized to each patient. 44
The laparoscopic extraction of the uterus may be performed with morcellation, whereby a rotating blade cuts the tissue into small pieces. This technique has come under scrutiny because of concerns about iatrogenic dissemination of benign and malignant tissue. The U.S. Food and Drug Administration recommends limiting the use of laparoscopic morcellation to reproductive-aged women who are not candidates for en bloc uterine resection. 58 The American College of Obstetricians and Gynecologists recommends morcellation as an option, but emphasizes the importance of informed consent and notes that the technique should not be performed in women with suspected or known uterine cancer. 59 , 60 Approximately one in 10 women have new symptoms after hysterectomy with bilateral salpingo-oophorectomy. 61
Myomectomy . Hysteroscopic myomectomy is the preferred surgical procedure for women with submucosal fibroids who wish to preserve their uterus or fertility. It is optimal for submucosal fibroids less than 3 cm when more than 50% of the tumor is intracavitary. 62 Laparoscopy is associated with less postoperative pain at 48 hours, less risk of postoperative fever (OR = 0.44; 95% CI, 0.26 to 0.77), and shorter hospitalization (mean of 67 fewer hours; 95% CI, 55 to 79 hours) compared with open myomectomy. 41 An estimated 15% to 33% of fibroids recur after myomectomy, and approximately 10% of women who undergo this procedure will have a hysterectomy within five to 10 years. 24
Uterine Artery Embolization . Uterine artery embolization is an option for women who wish to preserve their uterus or avoid surgery because of medical comorbidities or personal preference. 4 It is an interventional radiologic procedure in which occluding agents are injected into one or both of the uterine arteries, limiting blood supply to the uterus and fibroids. Compared with hysterectomy and myomectomy, uterine artery embolization has a significantly decreased length of hospitalization (mean of three fewer days), decreased time to normal activities (mean of 14 days), and a decreased likelihood of blood transfusion (OR = 0.07; 95% CI, 0.01 to 0.52). 42 Long-term studies show a reoperation rate of 20% to 33% within 18 months to five years. 24 Contraindications include pregnancy, active uterine or adnexal infections, allergy to intravenous contrast media, and renal insufficiency. The most common complication is postembolization syndrome, which is characterized by mild fever and pain, and vaginal expulsion of fibroids. 63
There is insufficient evidence on the effect of uterine artery embolization on future fertility. An observational study of 26 women treated with uterine artery embolization and 40 treated with hysterectomy found no difference in live birth rates. 42 In a retrospective study with five years of follow-up in women who received uterine artery embolization for fibroids, 27 (4.2%) had one (n = 20) or more (n = 7) pregnancies after uterine artery embolization. 64 Of these pregnancies, there were 15 miscarriages and 19 live births, 79% of which were cesarean deliveries because of complications. Further studies are needed on fertility outcomes after uterine artery embolization so that patients can be counseled appropriately.
Myolysis . Myolysis is a minimally invasive procedure targeting the destruction of fibroids via a focused energy delivery system such as heat, laser, or more recently, magnetic resonance–guided focused ultrasound surgery (MRgFUS). A study of 359 women treated with MRgFUS showed improved scores on the Uterine Fibroid Symptoms Quality of Life questionnaire at three months that persisted for up to 24 months ( P < .001). 40 In another study comparing women who underwent MRgFUS with those who underwent total abdominal hysterectomy, the groups had similar improvement in quality-of-life scores at six months, but the MRgFUS group had significantly fewer complications (14 vs. 33 events; P < .0001). 65 In a five-year follow-up study of 162 women, the reoperative rate was 59%. 66 Overall, this less-invasive procedure is well tolerated, although risks include localized pain and heavy bleeding. 40 Spontaneous conception has occurred in patients after MRgFUS, but further studies are needed to examine its effect on future fertility. 67
This article updates a previous article on this topic by Evans and Brunsell. 68
Data Sources: A PubMed search was completed in Clinical Queries using the key terms leiomyoma, uterine fibroids, diagnosis, management, power morcellation, and guidelines. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, the Database of Abstracts of Reviews of Effects, Essential Evidence Plus, and the National Guideline Clearinghouse database. Search date: October 25, 2015.
Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104(2):393-406.
Zimmermann A, Bernuit D, Gerlinger C, et al. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12(1):6.
Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198(1):34.e1-34.e7.
Vilos GA, Allaire C, Laberge PY, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2015;37(2):157-181.
Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344-1355.
Ishikawa H, Ishi K, Serna VA, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-2442.
Ross RK, Pike MC, Vessey MP, et al. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives [published correction appears in Br Med J (Clin Res Ed) . 1986;293(6553):1027]. Br Med J (Clin Res Ed). 1986;293(6543):359-362.
Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312-324.
Chiaffarino F, Parazzini F, La Vecchia C, et al. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol. 1999;106(8):857-860.
Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt). 2013;22(10):807-816.
Munro MG, Storz K, Abbott JA, et al. Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137-148.
Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet. 2015;131(2):117-122.
Bukulmez O, Doody KJ. Clinical features of myomas. Obstet Gynecol Clin North Am. 2006;33(1):69-84.
Carranza-Mamane B, Havelock J, Hemmings R, et al. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can. 2015;37(3):277-288.
Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev. 2012;11:CD003857.
Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215-1223.
De Vivo A, Mancuso A, Giacobbe A, et al. Uterine myomas during pregnancy: a longitudinal sonographic study. Ultrasound Obstet Gynecol. 2011;37(3):361-365.
Hammoud AO, Asaad R, Berman J, et al. Volume change of uterine myomas during pregnancy: do myomas really grow?. J Minim Invasive Gynecol. 2006;13(5):386-390.
Stout MJ, Odibo AO, Graseck AS, et al. Leiomyomas at routine second-trimester ultrasound examination and adverse obstetric outcomes. Obstet Gynecol. 2010;116(5):1056-1063.
Klatsky PC, Tran ND, Caughey AB, et al. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198(4):357-366.
Knight J, Falcone T. Tissue extraction by morcellation: a clinical dilemma. J Minim Invasive Gynecol. 2014;21(3):319-320.
ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
Practice Committee of American Society for Reproductive Medicine; Society of Reproductive Surgeons. Myomas and reproductive function. Fertil Steril. 2008;90(5 suppl):S125-S130.
Singh SS, Belland L. Contemporary management of uterine fibroids: focus on emerging medical treatments [published correction appears in Curr Med Res Opin . 2016;32(4):797]. Curr Med Res Opin. 2015;31(1):1-12.
Dueholm M, Lundorf E, Hansen ES, et al. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186(3):409-415.
Cicinelli E, Romano F, Anastasio PS, et al. Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol. 1995;85(1):42-47.
Thomassin-Naggara I, Dechoux S, Bonneau C, et al. How to differentiate benign from malignant myometrial tumours using MR imaging. Eur Radiol. 2013;23(8):2306-2314.
Bonneau C, Thomassin-Naggara I, Dechoux S, et al. Value of ultra-sonography and magnetic resonance imaging for the characterization of uterine mesenchymal tumors. Acta Obstet Gynecol Scand. 2014;93(3):261-268.
Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, et al. The effect of anastrazole on symptomatic uterine leiomyomata. Obstet Gynecol. 2007;110(3):643-649.
Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253-1255.
Fleischer AC, James AE, Millis JB, et al. Differential diagnosis of pelvic masses by gray scale sonography. AJR Am J Roentgenol. 1978;131(3):469-476.
Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy. BJOG. 2002;109(10):1097-1108.
Sayed GH, Zakherah MS, El-Nashar SA, et al. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynaecol Obstet. 2011;112(2):126-130.
Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;1:CD000400.
Tristan M, Orozco LJ, Steed A, et al. Mifepristone for uterine fibroids. Cochrane Database Syst Rev. 2012;8:CD007687.
Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409-420.
Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000(4):CD000249.
Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding. Obstet Gynecol. 2010;116(4):865-875.
Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015(8):CD003677.
Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110(2 pt 1):279-287.
Bhave Chittawar P, Franik S, et al. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014(10):CD004638.
Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014(12):CD005073.
Sesti F, Cosi V, Calonzi F, et al. Randomized comparison of total laparoscopic, laparoscopically assisted vaginal and vaginal hysterectomies for myomatous uteri. Arch Gynecol Obstet. 2014;290(3):485-491.
Hwang JL, Seow KM, Tsai YL, et al. Comparative study of vaginal, laparoscopically assisted vaginal and abdominal hysterectomies for uterine myoma larger than 6 cm in diameter or uterus weighing at least 450 g. Acta Obstet Gynecol Scand. 2002;81(12):1132-1138.
Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids. Contraception. 2010;82(1):41-55.
Venkatachalam S, Bagratee JS, Moodley J. Medical management of uterine fibroids with medroxyprogesterone acetate (Depo Provera). J Obstet Gynaecol. 2004;24(7):798-800.
Verspyck E, Marpeau L, Lucas C. Leuprorelin depot 3.75 mg versus lynestrenol in the preoperative treatment of symptomatic uterine myomas. Eur J Obstet Gynecol Reprod Biol. 2000;89(1):7-13.
Ichigo S, Takagi H, Matsunami K, et al. Beneficial effects of dienogest on uterine myoma volume. Arch Gynecol Obstet. 2011;284(3):667-670.
Ip PP, Lam KW, Cheung CL, et al. Tranexamic acid-associated necrosis and intralesional thrombosis of uterine leiomyomas. Am J Surg Pathol. 2007;31(8):1215-1224.
Peitsidis P, Koukoulomati A. Tranexamic acid for the management of uterine fibroid tumors. World J Clin Cases. 2014;2(12):893-898.
Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164(3):879-883.
Carbonell Esteve JL, Acosta R, Heredia B, et al. Mifepristone for the treatment of uterine leiomyomas. Obstet Gynecol. 2008;112(5):1029-1036.
Hilário SG, Bozzini N, Borsari R, et al. Action of aromatase inhibitor for treatment of uterine leiomyoma in perimenopausal patients. Fertil Steril. 2009;91(1):240-243.
Gurates B, Parmaksiz C, Kilic G, et al. Treatment of symptomatic uterine leiomyoma with letrozole. Reprod Biomed Online. 2008;17(4):569-574.
Song H, Lu D, Navaratnam K, et al. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev. 2013(10):CD009505.
Sadan O, Ginath S, Sofer D, et al. The role of tamoxifen in the treatment of symptomatic uterine leiomyomata—a pilot study. Eur J Obstet Gynecol Reprod Biol. 2001;96(2):183-186.
Deng L, Wu T, Chen XY, et al. Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst Rev. 2012(10):CD005287.
U.S. Food and Drug Administration. Updated: laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDAsafety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm . Accessed January 20, 2016.
American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery . Accessed January 20, 2016.
Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma. Gynecol Oncol. 2015;137(1):167-172.
Carlson KJ, Miller BA, Fowler FJ. The Maine women's health study: I. outcomes of hysterectomy. Obstet Gynecol. 1994;83(4):556-565.
Camanni M, Bonino L, Delpiano EM, et al. Hysteroscopic management of large symptomatic submucous uterine myomas. J Minim Invasive Gynecol. 2010;17(1):59-65.
Goodwin SC, Spies JB. Uterine fibroid embolization. N Engl J Med. 2009;361(7):690-697.
Hirst A, Dutton S, Wu O, et al. A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. Health Technol Assess. 2008;12(5):1-248.
Taran FA, Tempany CM, Regan L, et al.; MRgFUS Group. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol. 2009;34(5):572-578.
Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247-251.
Rabinovici J, David M, Fukunishi H, et al.; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93(1):199-209.
Evans P, Brunsell S. Uterine fibroid tumors: diagnosis and treatment. Am Fam Physician. 2007;75(10):1503-1508.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2017 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- U.S. Department of Health & Human Services

Call the OWH HELPLINE: 1-800-994-9662 9 a.m. — 6 p.m. ET, Monday — Friday OWH and the OWH helpline do not see patients and are unable to: diagnose your medical condition; provide treatment; prescribe medication; or refer you to specialists. The OWH helpline is a resource line. The OWH helpline does not provide medical advice.
Please call 911 or go to the nearest emergency room if you are experiencing a medical emergency.
Uterine fibroids

Fibroids are muscular tumors that grow in the wall of the uterus (womb). Fibroids are almost always benign (not cancerous). Not all women with fibroids have symptoms. Women who do have symptoms often find fibroids hard to live with. Some have pain and heavy menstrual bleeding. Treatment for uterine fibroids depends on your symptoms.
What are fibroids?
Fibroids are muscular tumors that grow in the wall of the uterus (womb). Another medical term for fibroids is leiomyoma (leye-oh-meye-OH-muh) or just "myoma". Fibroids are almost always benign (not cancerous). Fibroids can grow as a single tumor, or there can be many of them in the uterus. They can be as small as an apple seed or as big as a grapefruit. In unusual cases they can become very large.
Why should women know about fibroids?
About 20 percent to 80 percent of women develop fibroids by the time they reach age 50. Fibroids are most common in women in their 40s and early 50s. Not all women with fibroids have symptoms. Women who do have symptoms often find fibroids hard to live with. Some have pain and heavy menstrual bleeding. Fibroids also can put pressure on the bladder, causing frequent urination, or the rectum, causing rectal pressure. Should the fibroids get very large, they can cause the abdomen (stomach area) to enlarge, making a woman look pregnant.
Who gets fibroids?
There are factors that can increase a woman's risk of developing fibroids.
- Age. Fibroids become more common as women age, especially during the 30s and 40s through menopause. After menopause, fibroids usually shrink.
- Family history. Having a family member with fibroids increases your risk. If a woman's mother had fibroids, her risk of having them is about three times higher than average.
- Ethnic origin. African-American women are more likely to develop fibroids than white women.
- Obesity. Women who are overweight are at higher risk for fibroids. For very heavy women, the risk is two to three times greater than average.
- Eating habits. Eating a lot of red meat (e.g., beef) and ham is linked with a higher risk of fibroids. Eating plenty of green vegetables seems to protect women from developing fibroids.
Where can fibroids grow?
Most fibroids grow in the wall of the uterus. Doctors put them into three groups based on where they grow:
- Submucosal (sub-myoo-KOH-zuhl) fibroids grow into the uterine cavity.
- Intramural (ihn-truh-MYOOR-uhl) fibroids grow within the wall of the uterus.
- Subserosal (sub-suh-ROH-zuhl) fibroids grow on the outside of the uterus.
Some fibroids grow on stalks that grow out from the surface of the uterus or into the cavity of the uterus. They might look like mushrooms. These are called pedunculated (pih-DUHN-kyoo-lay-ted) fibroids.
What are symptoms of fibroids?
Most fibroids do not cause any symptoms, but some women with fibroids can have:
- Heavy bleeding (which can be heavy enough to cause anemia ) or painful periods
- Feeling of fullness in the pelvic area (lower stomach area)
- Enlargement of the lower abdomen
- Frequent urination
- Pain during sex
- Lower back pain
- Complications during pregnancy and labor, including a six-time greater risk of cesarean section
- Reproductive problems, such as infertility , which is very rare
What causes fibroids?
No one knows for sure what causes fibroids. Researchers think that more than one factor could play a role. These factors could be:
- Hormonal (affected by estrogen and progesterone levels)
- Genetic (runs in families)
Because no one knows for sure what causes fibroids, we also don't know what causes them to grow or shrink. We do know that they are under hormonal control — both estrogen and progesterone. They grow rapidly during pregnancy, when hormone levels are high. They shrink when anti-hormone medication is used. They also stop growing or shrink once a woman reaches menopause.
Can fibroids turn into cancer?
Fibroids are almost always benign (not cancerous). Rarely (less than one in 1,000) a cancerous fibroid will occur. This is called leiomyosarcoma. (leye-oh-meye-oh-sar-KOH-muh) Doctors think that these cancers do not arise from an already-existing fibroid. Having fibroids does not increase the risk of developing a cancerous fibroid. Having fibroids also does not increase a woman's chances of getting other forms of cancer in the uterus.
What if I become pregnant and have fibroids?
Women who have fibroids are more likely to have problems during pregnancy and delivery. This doesn't mean there will be problems. Most women with fibroids have normal pregnancies. The most common problems seen in women with fibroids are:
- Cesarean section. The risk of needing a c-section is six times greater for women with fibroids.
- Baby is breech. The baby is not positioned well for vaginal delivery.
- Labor fails to progress.
- Placental abruption. The placenta breaks away from the wall of the uterus before delivery. When this happens, the fetus does not get enough oxygen.
- Preterm delivery.
Talk to your obstetrician if you have fibroids and become pregnant. All obstetricians have experience dealing with fibroids and pregnancy. Most women who have fibroids and become pregnant do not need to see an OB who deals with high-risk pregnancies.
How do I know for sure that I have fibroids?
Your doctor may find that you have fibroids when you see her or him for a regular pelvic exam to check your uterus, ovaries, and vagina. The doctor can feel the fibroid with her or his fingers during an ordinary pelvic exam, as a (usually painless) lump or mass on the uterus. Often, a doctor will describe how small or how large the fibroids are by comparing their size to the size your uterus would be if you were pregnant. For example, you may be told that your fibroids have made your uterus the size it would be if you were 16 weeks pregnant. Or the fibroid might be compared to fruits, nuts, or a ball, such as a grape or an orange, an acorn or a walnut, or a golf ball or a volleyball.
Your doctor can do imaging tests to confirm that you have fibroids. These are tests that create a "picture" of the inside of your body without surgery. These tests might include:
- Ultrasound – Uses sound waves to produce the picture. The ultrasound probe can be placed on the abdomen or it can be placed inside the vagina to make the picture.
- Magnetic resonance imaging (MRI) – Uses magnets and radio waves to produce the picture
- X-rays – Uses a form of radiation to see into the body and produce the picture
- Cat scan (CT) – Takes many X-ray pictures of the body from different angles for a more complete image
- Hysterosalpingogram (hiss-tur-oh-sal-PIN-juh-gram) (HSG) or sonohysterogram (soh-noh-HISS-tur-oh-gram) – An HSG involves injecting x-ray dye into the uterus and taking x-ray pictures. A sonohysterogram involves injecting water into the uterus and making ultrasound pictures.
You might also need surgery to know for sure if you have fibroids. There are two types of surgery to do this:
- Laparoscopy (lap-ar-OSS-koh-pee) – The doctor inserts a long, thin scope into a tiny incision made in or near the navel. The scope has a bright light and a camera. This allows the doctor to view the uterus and other organs on a monitor during the procedure. Pictures also can be made.
- Hysteroscopy (hiss-tur-OSS-koh-pee) – The doctor passes a long, thin scope with a light through the vagina and cervix into the uterus. No incision is needed. The doctor can look inside the uterus for fibroids and other problems, such as polyps. A camera also can be used with the scope.
What questions should I ask my doctor if I have fibroids?
- How many fibroids do I have?
- What size is my fibroid(s)?
- Where is my fibroid(s) located (outer surface, inner surface, or in the wall of the uterus)?
- Can I expect the fibroid(s) to grow larger?
- How rapidly have they grown (if they were known about already)?
- How will I know if the fibroid(s) is growing larger?
- What problems can the fibroid(s) cause?
- What tests or imaging studies are best for keeping track of the growth of my fibroids?
- What are my treatment options if my fibroid(s) becomes a problem?
- What are your views on treating fibroids with a hysterectomy versus other types of treatments?
A second opinion is always a good idea if your doctor has not answered your questions completely or does not seem to be meeting your needs.
How are fibroids treated?
Most women with fibroids do not have any symptoms. For women who do have symptoms, there are treatments that can help. Talk with your doctor about the best way to treat your fibroids. She or he will consider many things before helping you choose a treatment. Some of these things include:
- Whether or not you are having symptoms from the fibroids
- If you might want to become pregnant in the future
- The size of the fibroids
- The location of the fibroids
- Your age and how close to menopause you might be
If you have fibroids but do not have any symptoms, you may not need treatment. Your doctor will check during your regular exams to see if they have grown.
Medications
If you have fibroids and have mild symptoms, your doctor may suggest taking medication. Over-the-counter drugs such as ibuprofen or acetaminophen can be used for mild pain. If you have heavy bleeding during your period, taking an iron supplement can keep you from getting anemia or correct it if you already are anemic.
Several drugs commonly used for birth control can be prescribed to help control symptoms of fibroids. Low-dose birth control pills do not make fibroids grow and can help control heavy bleeding. The same is true of progesterone-like injections (e.g., Depo-Provera®). An IUD (intrauterine device) called Mirena® contains a small amount of progesterone-like medication, which can be used to control heavy bleeding as well as for birth control.
Other drugs used to treat fibroids are "gonadotropin releasing hormone agonists" (GnRHa). The one most commonly used is Lupron®. These drugs, given by injection, nasal spray, or implanted, can shrink your fibroids. Sometimes they are used before surgery to make fibroids easier to remove. Side effects of GnRHas can include hot flashes, depression, not being able to sleep, decreased sex drive, and joint pain. Most women tolerate GnRHas quite well. Most women do not get a period when taking GnRHas. This can be a big relief to women who have heavy bleeding. It also allows women with anemia to recover to a normal blood count. GnRHas can cause bone thinning, so their use is generally limited to six months or less. These drugs also are very expensive, and some insurance companies will cover only some or none of the cost. GnRHas offer temporary relief from the symptoms of fibroids; once you stop taking the drugs, the fibroids often grow back quickly.
If you have fibroids with moderate or severe symptoms, surgery may be the best way to treat them. Here are the options:
- Myomectomy (meye-oh-MEK-tuh-mee) – Surgery to remove fibroids without taking out the healthy tissue of the uterus. It is best for women who wish to have children after treatment for their fibroids or who wish to keep their uterus for other reasons. You can become pregnant after myomectomy. But if your fibroids were imbedded deeply in the uterus, you might need a cesarean section to deliver. Myomectomy can be performed in many ways. It can be major surgery (involving cutting into the abdomen) or performed with laparoscopy or hysteroscopy. The type of surgery that can be done depends on the type, size, and location of the fibroids. After myomectomy new fibroids can grow and cause trouble later. All of the possible risks of surgery are true for myomectomy. The risks depend on how extensive the surgery is.
- Hysterectomy (hiss-tur-EK-tuh-mee) – Surgery to remove the uterus. This surgery is the only sure way to cure uterine fibroids. Fibroids are the most common reason that hysterectomy is performed. This surgery is used when a woman's fibroids are large, if she has heavy bleeding, is either near or past menopause, or does not want children. If the fibroids are large, a woman may need a hysterectomy that involves cutting into the abdomen to remove the uterus. If the fibroids are smaller, the doctor may be able to reach the uterus through the vagina, instead of making a cut in the abdomen. In some cases hysterectomy can be performed through the laparoscope. Removal of the ovaries and the cervix at the time of hysterectomy is usually optional. Women whose ovaries are not removed do not go into menopause at the time of hysterectomy. Hysterectomy is a major surgery. Although hysterectomy is usually quite safe, it does carry a significant risk of complications. Recovery from hysterectomy usually takes several weeks.
- Endometrial ablation (en-doh-MEE-tree-uhl uh-BLAY-shuhn) – The lining of the uterus is removed or destroyed to control very heavy bleeding. This can be done with laser, wire loops, boiling water, electric current, microwaves, freezing, and other methods. This procedure usually is considered minor surgery. It can be done on an outpatient basis or even in a doctor's office. Complications can occur, but are uncommon with most of the methods. Most people recover quickly. About half of women who have this procedure have no more menstrual bleeding. About three in 10 women have much lighter bleeding. But, a woman cannot have children after this surgery.
- Myolysis (meye-OL-uh-siss) – A needle is inserted into the fibroids, usually guided by laparoscopy, and electric current or freezing is used to destroy the fibroids.
- Have fibroids that are causing heavy bleeding
- Have fibroids that are causing pain or pressing on the bladder or rectum
- Don't want to have a hysterectomy
- Don't want to have children in the future
What new treatments are available for uterine fibroids?
The following methods are not yet standard treatments, so your doctor may not offer them or health insurance may not cover them.
- Radiofrequency ablation uses heat to destroy fibroid tissue without harming surrounding normal uterine tissue. The fibroids remain inside the uterus but shrink in size. Most women go home the same day and can return to normal activities within a few days.
- Anti-hormonal drugs may provide symptom relief without bone-thinning side effects.
More information on uterine fibroids
For more information about uterine fibroids, call womenshealth.gov at 1-800-994-9662 (TDD: 888-220-5446) or contact the following organizations:
- American College of Obstetricians and Gynecologists Phone: 202-638-5577
- Center for Uterine Fibroids Phone: 800-722-5520
- National Institute of Child Health and Human Development, NIH, HHS Phone: 800-370-2943 (TDD: 888-320-6942)
- Steve Eisinger, M.D., F.A.C.O.G., Professor of Family Medicine, Professor of Obstetrics and Gynecology, University of Rochester School of Medicine and Dentistry
- HHS Non-Discrimination Notice
- Language Assistance Available
- Accessibility
- Privacy Policy
- Disclaimers
- Freedom of Information Act (FOIA)
- Use Our Content
- Vulnerability Disclosure Policy
- Kreyòl Ayisyen
A federal government website managed by the Office on Women's Health in the Office of the Assistant Secretary for Health at the U.S. Department of Health and Human Services.
1101 Wootton Pkwy, Rockville, MD 20852 1-800-994-9662 • Monday through Friday, 9 a.m. to 6 p.m. ET (closed on federal holidays).

Uterine Fibroids
Medically reviewed by Drugs.com. Last updated on Jun 5, 2024.
- Español
What are uterine fibroids?
Uterine fibroids are growths found inside your uterus. Uterine fibroids are benign (not cancer) and may also be called myomas or leiomyomas. Uterine fibroids often appear in groups, or you may have only one. They can be small or large, and they can grow. Fibroids likely will not spread to other parts of your body. They may grow when you are pregnant and shrink after you no longer have a monthly period.
What increases my risk for uterine fibroids?
The cause of uterine fibroids is not clear. Ask your healthcare provider about these and other risk factors for uterine fibroids:
- A family history of uterine fibroids
- Increased hormone levels
- Menstrual periods starting before age 13
- Too much body weight
- Not having children
- Drinking alcohol
What are the signs and symptoms of uterine fibroids?
Symptoms depend on the size, type, and number of fibroids you have. Symptoms also depend on where the fibroids are inside your uterus:
- Heavy or painful menstrual bleeding that lasts more than 1 week
- Pelvic pressure and pain
- Urge to urinate often
- Constipation or pain when you have a bowel movement
- Increased pelvic pain during sex
How are uterine fibroids diagnosed?
Your healthcare provider will examine you and ask about your symptoms. Tell the provider if any women if your family have had uterine fibroids. You may also need any of the following:
- A pelvic exam is also called an internal or vaginal exam. During a pelvic exam, a speculum is gently placed into your vagina. A speculum is a tool that opens your vagina. This lets your provider see your cervix (bottom part of your uterus). With gloved hands, your provider will check the size and shape of your uterus and ovaries.
- An ultrasound uses sound waves to show pictures on a monitor. An ultrasound may be done to show your uterus and fibroids. The ultrasound device may be moved over your abdomen. Instead, the device may be placed in your vagina.
- A biopsy is a tissue sample of a fibroid that your healthcare provider takes from your uterus for testing.
How are uterine fibroids treated?
Watchful waiting may be recommended if your signs and symptoms are mild. The following treatments may shrink your fibroids and decrease your symptoms:
- Medicines that decrease hormones may help shrink your fibroids and decrease menstrual bleeding.
- Oral contraceptives can help control menstrual bleeding.
- NSAIDs help decrease swelling and pain or fever. This medicine is available with or without a doctor's order. NSAIDs can cause stomach bleeding or kidney problems in certain people. If you take blood thinner medicine, always ask your healthcare provider if NSAIDs are safe for you. Always read the medicine label and follow directions.
- A procedure may be done to stop blood flow to the fibroids to help shrink them. This will help decrease your symptoms.
- Surgery may be used to remove your fibroids and leave your uterus in place. Surgery may instead be used to remove your fibroids and uterus.
Treatment options
The following list of medications are related to or used in the treatment of this condition.
- Lupron Depot
View more treatment options
How can I help prevent uterine fibroids?
- Maintain a healthy weight. Extra weight can increase your risk for fibroids. Talk to your healthcare provider about a healthy weight for you. Your provider can help you create a healthy weight loss plan, if needed.
- Limit or do not drink alcohol, as directed. Alcohol can increase your risk for fibroids. A drink of alcohol is 12 ounces of beer, 1½ ounces of liquor, or 5 ounces of wine. Ask your healthcare provider for information if you need help to quit drinking alcohol.
When should I seek immediate care?
- Your heart begins to race, and you feel faint.
- You begin to pass large blood clots from your vagina.

When should I call my doctor or gynecologist?
- Your symptoms do not go away, or they get worse.
- You feel weak and are more tired than usual.
- You do not feel like your bladder is empty after you urinate. You also may urinate small amounts more often.
- You have questions or concerns about your condition or care.
Care Agreement
© Copyright Merative 2024 Information is for End User's use only and may not be sold, redistributed or otherwise used for commercial purposes.
Learn more about Uterine Fibroids
- Medications for Neoplasia, Estrogen Dependent
- Medications for Uterine Fibroids
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Medical Disclaimer
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
The uphill battle to provide noninvasive treatment for fibroids
By Suzanne LeBlang May 29, 2024

I f an effective treatment is available for one common disease in men, and that same treatment is available for a different common disease in women, a logical expectation would be that the treatments are equally accessible to men and women. That is not the current reality.
The treatment I am talking about is focused ultrasound. It has been approved by the Food and Drug Administration for a range of conditions, including prostate cancer, fibroids, liver tumors, essential tremor and Parkinson’s related tremor, osteoid osteoma, and pain from bone metastases. It works by using sound waves to precisely target and destroy tissue deep within the body without incisions or radiation. It’s akin to using a magnifying glass to converge the sun’s rays to a small spot on a leaf, causing enough heat to induce an actual flame. A focused ultrasound beam can be precisely focused on target tissue deep within the body to create heat to kill tissue, and the tissue in between is spared.
advertisement
For years, there were no good options for treating fibroids other than surgery, which many women were not interested in due to the risks, cost, and recovery time — not to mention the possible loss of their ability to have a baby if a hysterectomy was required. Minimally invasive procedures, utilizing catheters and probes that pierce through the skin or the cervix, exist such as uterine artery embolization and radiofrequency ablation, but they still carry risks and may require weeks of recovery. Medications can be helpful in temporarily shrinking fibroids and reducing some of the symptoms, but there are undesirable side effects associated with the decrease in hormone levels.
The FDA first approved focused ultrasound as a treatment of uterine fibroids in 2004. I had the privilege of performing the first commercial focused ultrasound treatment of uterine fibroids in the country. It was performed inside an MRI scanner; the patient was fully awake. She walked out of the medical center an hour after the procedure without any incisions and returned to her normal activities the next day.
Sign up for First Opinion
The smartest thinkers in life sciences on what's happening — and what's to come
Now, here is where the irony begins. Almost 300,000 men in the United States are diagnosed with prostate cancer each year; and millions live with enlarged prostate glands (benign prostatic hyperplasia), which can interfere with urination. They have access to more than 170 sites that offer focused ultrasound therapy, which is an FDA-approved treatment for both conditions. Medicare and most commercial insurers cover about 80% of the cost of this procedure.
More than 25 million women in the U.S. live with uterine fibroids, growths in the uterus that can cause a range of debilitating symptoms, including excessive menstrual bleeding, severe pain in the pelvis and abdomen, and infertility. They have access to just seven sites offering FDA-approved focused ultrasound treatment, and only one private insurance carrier covers the procedure. The average out-of-pocket cost for treatment is around $40,000, making it inaccessible to most women.
A similar gap exists worldwide . Although more than 200,000 focused ultrasound treatments have been performed for fibroids and about 100,000 treatments for prostate disease, there are only 319 treatment sites for fibroids compared to 432 treatment sites for prostate cancer. One of the main reasons for this gap comes from the penalty of fibroids being the first indication approved, resulting in more scrutiny in the regulatory approval process, reimbursement approval process, and adoption by clinicians. There is more hesitation to adopt a disruptive technology that few clinicians were trained in. But it did pave the way for other clinical trials using focused ultrasound to move forward, leading to more indications approved by the FDA, reimbursed by insurance, and adopted by clinicians.
Additionally, there is a lack of representation among decision-makers who choose whether insurance pays for focused ultrasound for fibroids and how accessible it is. Few women are in leadership positions within focused ultrasound companies and insurance companies, and even fewer are African American women, who are more likely to have fibroids. This disparity is seen across health care, so much so that the White House recently announced a new initiative prioritizing women’s health research .
Related: Heat waves associated with increased risk of preterm birth in the U.S.
Twenty years after focused ultrasound was approved as a treatment for uterine fibroids, it is still the only noninvasive treatment for women with this condition. This technology has nine FDA-approved indications , yet this groundbreaking treatment remains hard to access for most women with fibroids in the U.S.
One of the most formidable challenges to making this therapy more accessible has been getting insurers to cover it, and thus few manufacturers are willing to enter the space. Despite extensive data demonstrating long-term efficacy and safety, insurance companies continue to dither by asking for additional evidence. Their reluctance is driven by cost concerns. Sixteen years ago I presented data showing the benefits of focused ultrasound for fibroids to insurers, but they believed treating a smaller subset of women with hysterectomy or even minimally invasive techniques is economically advantageous compared to offering focused ultrasound to the broader population of women with uterine fibroids.
The slow adoption of focused ultrasound for treating uterine fibroids isn’t for lack of women interested in the treatment. A survey published in the Journal of Women’s Health reported that when 1,000 U.S. women with fibroids were presented with descriptions of various treatments, 60% rated focused ultrasound as their top choice.
The U.S. health system is past due for change. Insurers must provide coverage for focused ultrasound as a treatment for uterine fibroids, and more manufacturers must enter the U.S. market, providing affordable systems for hospitals and clinics. It is imperative to seize this opportunity with the national spotlight on women’s health with the White House Initiative on Women’s Health Research to ensure that focused ultrasound becomes not just a promise but a reality for all women battling uterine fibroids.
Suzanne LeBlang, M.D., is a neuroradiologist who has performed hundreds of focused ultrasound procedures, the director of clinical relationships at the Focused Ultrasound Foundation, serves on the faculty at the University of Miami Miller School of Medicine, and is a clinical affiliate associate professor of medicine at the Florida Atlantic University Schmidt College of Medicine.
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the author reprints, suzanne leblang.
Health insurance
reproductive health
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

Curing rare childhood diseases will falter unless Congress steps up

How faith leaders like me can help address America’s obesity epidemic

STAT Plus: The inside story of how Lykos’ MDMA research went awry

STAT Plus: FDA advisory panel votes overwhelmingly against MDMA therapy for PTSD

STAT Plus: Elevance PBM’s president out as customers complain of prescription chaos
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The conservative and interventional treatment of fibroids
Affiliation.
- 1 Städtisches Klinikum Karlsruhe, Department of Gynecology and Obstetrics, Frauenklinik des Evangelischen Krankenhauses Köln Weyertal, Städtisches Klinikum Karlsruhe, Institute of Diagnostic and Interventional Radiology, FUS Center, Dachau Medical Center.
- PMID: 25597366
- PMCID: PMC4298239
- DOI: 10.3238/arztebl.2014.0877
Background: Fibroids are the most common benign tumors in women. One-third of all women of reproductive age undergo treatment for symptomatic fibroids. In recent years, the spectrum of available treatments has been widened by the introduction of new drugs and interventional procedures.
Methods: Selective literature review on the treatment of uterine fibroids, including consideration of several Cochrane Reviews.
Results: Fibroids can be treated with drugs, interventional procedures (uterine artery embolization [UAE] and focused ultrasound treatment [FUS]), and surgery. The evidence regarding the various available treatments is mixed. All methods improve symptoms, but only a few comparative studies have been performed. A meta-analysis revealed that recovery within 15 days is more common after laparoscopic enucleation than after open surgery (odds ratio [OR], 3.2). A minimally invasive hysterectomy, or one performed by the vaginal route, is associated with a shorter hospital stay and a more rapid recovery than open transabdominal hysterectomy. UAE is an alternative to hysterectomy for selected patients. The re-intervention rates after fibroid enucleation, hysterectomy, and UAE are 8.9-9%, 1.8-10.7%, and 7-34.6%, respectively. The main drugs used to treat fibroids are gonadotropin-releasing hormone analogs and selective progesterone receptor modulators.
Conclusion: Multiple treatment options are available and enable individualized therapy for symptomatic fibroids. The most important considerations in the choice of treatment are the question of family planning and, in some cases, the technical limitations of the treatments themselves.
PubMed Disclaimer
Fibroid locations (schematic): a) subserosal…
Fibroid locations (schematic): a) subserosal fibroid, b) submucosal fibroid, c) intramural fibroid, d)…
Ultrasonography: 1 Submucosal posterior wall…
Ultrasonography: 1 Submucosal posterior wall fibroid distorting the endometrial cavity 2 Myometrium 3…
Intracavitary fibroid before hysteroscopic resection…
Intracavitary fibroid before hysteroscopic resection (left), hysteroscopic enucleation of a fibroid (right). (1,…
Incision of the myometrium for…
Incision of the myometrium for laparoscopic enucleation of a fibroid 1 Fibroid 2…
- Missing treatment option. Strunkg H, Marinova M. Strunkg H, et al. Dtsch Arztebl Int. 2015 May 1;112(18):328. doi: 10.3238/arztebl.2015.0328a. Dtsch Arztebl Int. 2015. PMID: 26037469 Free PMC article. No abstract available.
- In reply. Boosz A, Müller A. Boosz A, et al. Dtsch Arztebl Int. 2015 May 1;112(18):328. doi: 10.3238/arztebl.2015.0328b. Dtsch Arztebl Int. 2015. PMID: 26037470 Free PMC article. No abstract available.
Similar articles
- Therapeutic management of uterine fibroid tumors: updated French guidelines. Marret H, Fritel X, Ouldamer L, Bendifallah S, Brun JL, De Jesus I, Derrien J, Giraudet G, Kahn V, Koskas M, Legendre G, Lucot JP, Niro J, Panel P, Pelage JP, Fernandez H; CNGOF (French College of Gynecology and Obstetrics). Marret H, et al. Eur J Obstet Gynecol Reprod Biol. 2012 Dec;165(2):156-64. doi: 10.1016/j.ejogrb.2012.07.030. Epub 2012 Aug 29. Eur J Obstet Gynecol Reprod Biol. 2012. PMID: 22939241 Review.
- Management of symptomatic fibroids: conservative surgical treatment modalities other than abdominal or laparoscopic myomectomy. Istre O. Istre O. Best Pract Res Clin Obstet Gynaecol. 2008 Aug;22(4):735-47. doi: 10.1016/j.bpobgyn.2008.01.010. Epub 2008 Mar 7. Best Pract Res Clin Obstet Gynaecol. 2008. PMID: 18328788 Review.
- EMAS position statement: management of uterine fibroids. Pérez-López FR, Ornat L, Ceausu I, Depypere H, Erel CT, Lambrinoudaki I, Schenck-Gustafsson K, Simoncini T, Tremollieres F, Rees M; EMAS. Pérez-López FR, et al. Maturitas. 2014 Sep;79(1):106-16. doi: 10.1016/j.maturitas.2014.06.002. Epub 2014 Jun 9. Maturitas. 2014. PMID: 24975954
- Uterine artery embolization as a treatment option for uterine myomas. Marshburn PB, Matthews ML, Hurst BS. Marshburn PB, et al. Obstet Gynecol Clin North Am. 2006 Mar;33(1):125-44. doi: 10.1016/j.ogc.2005.12.009. Obstet Gynecol Clin North Am. 2006. PMID: 16504811 Review.
- The effect of a gynecologist-interventional radiologist relationship on selection of treatment modality for the patient with uterine myoma. Zurawin RK, Fischer JH 2nd, Amir L. Zurawin RK, et al. J Minim Invasive Gynecol. 2010 Mar-Apr;17(2):214-21. doi: 10.1016/j.jmig.2009.12.015. J Minim Invasive Gynecol. 2010. PMID: 20226411
- Should acupuncture become a complementary therapy in the treatment of uterine fibroid: a systematic review and meta-analysis of randomized controlled trials. Ren Y, Zhang J, Wu W, Yuan Y, Wang J, Tang Y, Liao Y, Liu X. Ren Y, et al. Front Med (Lausanne). 2023 Dec 13;10:1268220. doi: 10.3389/fmed.2023.1268220. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38152298 Free PMC article.
- Serum Calcium and Magnesium Levels in Women with Uterine Fibroids in Southwest Nigeria: a Cross-sectional Study. Adeboje-Jimoh F, Okunade KS, Olorunfemi G, Oluwole AA, Olamijulo JA. Adeboje-Jimoh F, et al. Biol Trace Elem Res. 2024 Jun;202(6):2501-2508. doi: 10.1007/s12011-023-03873-z. Epub 2023 Sep 27. Biol Trace Elem Res. 2024. PMID: 37758981
- Serum Calcium and Magnesium Levels in Women with Uterine Fibroids at a University Teaching Hospital in Southwest Nigeria: A Comparative Cross-Sectional Study. Adeboje-Jimoh F, Okunade KS, Olorunfemi G, Olamijulo JA. Adeboje-Jimoh F, et al. Res Sq [Preprint]. 2023 May 4:rs.3.rs-2877359. doi: 10.21203/rs.3.rs-2877359/v1. Res Sq. 2023. PMID: 37205458 Free PMC article. Updated. Preprint.
- Deep learning enables automated MRI-based estimation of uterine volume also in patients with uterine fibroids undergoing high-intensity focused ultrasound therapy. Theis M, Tonguc T, Savchenko O, Nowak S, Block W, Recker F, Essler M, Mustea A, Attenberger U, Marinova M, Sprinkart AM. Theis M, et al. Insights Imaging. 2023 Jan 5;14(1):1. doi: 10.1186/s13244-022-01342-0. Insights Imaging. 2023. PMID: 36600120 Free PMC article.
- Improvement of fibroid-associated symptoms and quality of life after US-guided high-intensity focused ultrasound (HIFU) of uterine fibroids. Tonguc T, Recker F, Ganslmeier J, Strunk HM, Pieper CC, Ramig O, Welz S, Egger EK, Mutschler N, Warwas L, Essler M, Mustea A, Conrad R, Marinova M. Tonguc T, et al. Sci Rep. 2022 Dec 7;12(1):21155. doi: 10.1038/s41598-022-24994-w. Sci Rep. 2022. PMID: 36476975 Free PMC article.
- Ciavattini A, Di Giuseppe J, Stortoni P, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int. 2013:173–184. - PMC - PubMed
- Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12 - PMC - PubMed
- Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002;17:1424–1430. - PubMed
- Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev. 2012 Nov 14; CD003857. - PubMed
- Ciavattini A, Clemente N, Delli Carpini G, Di Giuseppe J, Giannubilo SR, Tranquilli AL. Number and size of uterine fibroids and obstetric outcomes. J Matern Fetal Neonatal Med. 2014;5:1–5. - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Deutsches Aerzteblatt International
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Adrenal Schwannoma Presenting as an Incidentaloma in a Patient with Uterine Fibroids and Cholelithiasis: a Rare Case Report
- Case Report
- Published: 29 May 2024
Cite this article

- Utkarsh Singh 1 ,
- Shubhajeet Roy 1 ,
- Kushagra Gaurav ORCID: orcid.org/0000-0002-5212-4203 2 ,
- Akshay Anand 2 ,
- Sumaira Qayoom 3 &
- Abhinav A. Sonkar 2
Explore all metrics
Schwannomas, which are benign mesenchymal tumors derived from Schwann cells, are common in the central nervous system. While they are commonly seen in the extremities and head-neck area, their presence in visceral organs, particularly the adrenals, is uncommon. Adrenal schwannomas are frequently discovered incidentally, offering a diagnostic difficulty because of their uncommon presentation. A 46-year-old woman initially sought treatment for symptoms related to uterine fibroids and biliary stones. Diagnostic imaging uncovered an adrenal incidentaloma, necessitating a laparoscopic right adrenalectomy. The mass was determined to be an adrenal schwannoma based on its spindle-shaped cells and S-100 immunohistochemistry positivity. The patient’s symptoms improved, and she was discharged with stable vital signs. Preoperative diagnosis of adrenal schwannomas is difficult and requires histological confirmation. When diagnosing non-secreting adrenal tumors with unusual radiology, surgeons should investigate for schwannoma. Post-resection adrenal schwannoma follow-up studies are scarce; however, they imply a low risk of recurrence or metastasis.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
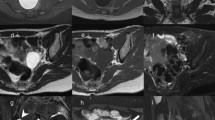
Magnetic resonance imaging findings of cystic ovarian tumors: major differential diagnoses in five types frequently encountered in daily clinical practice
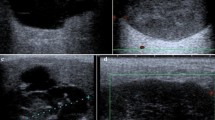
The differential diagnosis of parotid gland tumors with high-resolution ultrasound in otolaryngological practice
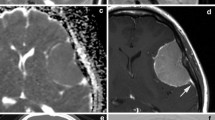
Variants of meningiomas: a review of imaging findings and clinical features
Data availability.
Data supporting this study are included within the article and/or supporting materials.
Shivalingaiah PH, Kumar P, Bajoria S (2018) Adrenal schwannoma treated with open adrenalectomy: a case report. Indian J Surg Oncol 9(1):83–85. https://doi.org/10.1007/s13193-017-0715-5
Article PubMed Google Scholar
Yorita K, Naroda T, Tamura M, Ito S, Nakatani K (2022) Adrenal schwannoma in an elderly man: a case report and literature review. Intern Med 61(1):65–69. https://doi.org/10.2169/internalmedicine.7026-21
Kapoor A, Morris T, Rebello R (2011) Guidelines for the management of the incidentally discovered adrenal mass. Can Urol Assoc J 5(4):241–247. https://doi.org/10.5489/cuaj.11135
Article PubMed PubMed Central Google Scholar
Zhou J, Zhang D, Li W, Zhou L, Xu H, Zheng S, Wang C (2019) Primary adrenal schwannoma: a series of 31 cases emphasizing their clinicopathologic features and favorable prognosis. Endocrine 65(3):662–674. https://doi.org/10.1007/s12020-019-01992-z
Article CAS PubMed Google Scholar
Mohiuddin Y, Gilliland MG (2013) Adrenal schwannoma: a rare type of adrenal incidentaloma. Arch Pathol Lab Med 137(7):1009–1014. https://doi.org/10.5858/arpa.2012-0291-RS
Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR (2004) The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 25:309–340. https://doi.org/10.1210/er.2002-0031
Komori T (2016) The 2016 WHO Classification of Tumours of the Central Nervous System: the major points of revision. Neurol Med Chir (Tokyo) 57(7):301–311. https://doi.org/10.2176/nmc.ra.2017-0010
Article Google Scholar
Lloyd RV, Osamura RY, Kloppel G, Rosai J (2017) WHO Classification of tumours: pathology and genetics of tumours of endocrine organs. IARC, Lyon, pp 176. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Endocrine-Organs-2017 . Accessed 22 Aug 2023
Bancos I, Prete A (2021) Approach to the patient with adrenal incidentaloma. J Clin Endocrinol Metab 106(11):3331–3353. https://doi.org/10.1210/clinem/dgab512
Lau SK, Spagnolo DV, Weiss LM (2006) Schwannoma of the adrenal gland: report of two cases. Am J Surg Pathol 30(5):630–634. https://doi.org/10.1097/01.pas.0000194739.80174.26
Bhattacharya S, Kumar A, Chatterjee U, Pal DK (2021) Adrenal schwannoma: an uncommon incidentaloma. Indian J Pathol Microbiol 64(2):379–381. https://doi.org/10.4103/IJPM.IJPM_526_20
(2002) NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements 19(2):1–25. Available from: https://pubmed.ncbi.nlm.nih.gov/14768652/ . Accessed 22 Aug 2023
Târcoveanu E, Dimofte G, Bradea C, Moldovanu R, Vasilescu A, Anton R, Ferariu (2009) Adrenal schwannoma. JSLS 13(1):116–9. Available from: https://pubmed.ncbi.nlm.nih.gov/19366556/ . Accessed 22 Aug 2023
Yang CY, Chou CW, Lin MB, Li CF (2009) Schwannomas of the left adrenal gland and posterior mediastinum. J Chin Med Assoc 72(2):83–87. https://doi.org/10.1016/S1726-4901(09)70028-0
Download references
Author information
Authors and affiliations.
Faculty of Medical Sciences, King George’s Medical University, Lucknow, Uttar Pradesh, India
Utkarsh Singh & Shubhajeet Roy
Department of General Surgery, King George’s Medical University, Shah Mina Shah Road, Chowk, Lucknow, 226003, Uttar Pradesh, India
Kushagra Gaurav, Akshay Anand & Abhinav A. Sonkar
Department of Pathology, King George’s Medical University, Lucknow, Uttar Pradesh, India
Sumaira Qayoom
You can also search for this author in PubMed Google Scholar
Contributions
Patient treatment: KG, AA, and AAS; first draft: US and SR. Pathological analysis: SQ. All the authors read and approved the final draft.
Corresponding author
Correspondence to Kushagra Gaurav .
Ethics declarations
Iec approval.
Not applicable.
Informed Consent
Written informed consent was taken to publish the patient data and photographs in an anonymous format.
Financial/Non-financial Interests
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Singh, U., Roy, S., Gaurav, K. et al. Adrenal Schwannoma Presenting as an Incidentaloma in a Patient with Uterine Fibroids and Cholelithiasis: a Rare Case Report. Indian J Surg Oncol (2024). https://doi.org/10.1007/s13193-024-01969-z
Download citation
Received : 12 October 2023
Accepted : 21 May 2024
Published : 29 May 2024
DOI : https://doi.org/10.1007/s13193-024-01969-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adrenal schwannoma
- Retroperitoneal mass
- Uterine fibroids
- Cholelithiasis
- Laparoscopic adrenalectomy
- Find a journal
- Publish with us
- Track your research
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Combined treatment of uterine leiomyosarcoma with gamma secretase inhibitor mk-0752 and chemotherapeutic agents decreases cellular invasion and increases apoptosis.

Simple Summary
1. introduction, 2. materials and methods, 2.1. cell lines and cell culture, 2.2. inhibitory concentrations of mk-0752, docetaxel, doxorubicin, and gemcitabine, 2.3. transwell invasion assays, 2.4. cell cycle analysis, 2.5. cellular proliferation, 2.6. reverse transcription—quantitiative pcr, 2.7. rna sequencing, 2.8. statistical analysis, 3.1. combination of mk-0752 and chemotherapeutics decreases ulms cell viability, 3.2. exposure to combinations of mk-0752 and chemotherapeutics impacts ulms cellular invasion, 3.3. treatment of ulms cells with mk-0752 or mk-0752 in combination with chemotherapeutic agents affects the cell cycle, 3.4. treatment of ulms cells with mk-0752 decreases proliferation only in the sk-lms-1 cell line, 3.5. treatment with mk-0752 alone and in combination with chemotherapeutics yields significant changes in differentially expressed genes, 3.6. cytotoxic treatment has limited effects on the differences in gene expression and altered pathways in ulms cells between sk-lms-1 and sk-ut-1b, 3.7. mk-0752 exposure decreases cell cycle and dna replication pathway activity, 3.8. expression of notch pathway effectors is decreased and expression of components of the gamma secretase complex are increased after exposure to the combination of mk-0752 and chemotherapeutic agents, 4. discussion, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Rizzo, A.; Pantaleo, M.A.; Saponara, M.; Nannini, M. Current status of the adjuvant therapy in uterine sarcoma: A literature review. World J. Clin. Cases 2019 , 7 , 1753–1763. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rizzo, A.; Nannini, M.; Astolfi, A.; Indio, V.; De Iaco, P.; Perrone, A.M.; De Leo, A.; Incorvaia, L.; Di Scioscio, V.; Pantaleo, M.A. Impact of Chemotherapy in the Adjuvant Setting of Early Stage Uterine Leiomyosarcoma: A Systematic Review and Updated Meta-Analysis. Cancers 2020 , 12 , 1899. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Roberts, M.E.; Aynardi, J.T.; Chu, C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol. Oncol. 2018 , 151 , 562–572. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Seagle, B.L.; Sobecki-Rausch, J.; Strohl, A.E.; Shilpi, A.; Grace, A.; Shahabi, S. Prognosis and treatment of uterine leiomyosarcoma: A National Cancer Database study. Gynecol. Oncol. 2017 , 145 , 61–70. [ Google Scholar ] [ CrossRef ]
- George, S.; Serrano, C.; Hensley, M.L.; Ray-Coquard, I. Soft Tissue and Uterine Leiomyosarcoma. J. Clin. Oncol. 2018 , 36 , 144–150. [ Google Scholar ] [ CrossRef ]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023 , 21 , 181–209. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gupta, A.A.; Yao, X.; Verma, S.; Mackay, H.; Hopkins, L.; Sarcoma Disease Site Group and the Gynecology Cancer Disease Site Group. Systematic chemotherapy for inoperable, locally advanced, recurrent, or metastatic uterine leiomyosarcoma: A systematic review. Clin. Oncol. 2013 , 25 , 346–355. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gupta, A.A.; Yao, X.; Verma, S.; Mackay, H.; Hopkins, L. Chemotherapy (gemcitabine, docetaxel plus gemcitabine, doxorubicin, or trabectedin) in inoperable, locally advanced, recurrent, or metastatic uterine leiomyosarcoma: A clinical practice guideline. Curr. Oncol. 2013 , 20 , e448–e454. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2022 , 23 , 1044–1054. [ Google Scholar ] [ CrossRef ]
- Omura, G.A.; Major, F.J.; Blessing, J.A.; Sedlacek, T.V.; Thigpen, J.T.; Creasman, W.T.; Zaino, R.J. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983 , 52 , 626–632. [ Google Scholar ] [ CrossRef ]
- Hensley, M.L.; Blessing, J.A.; Mannel, R.; Rose, P.G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol. Oncol. 2008 , 109 , 329–334. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hensley, M.L.; Maki, R.; Venkatraman, E.; Geller, G.; Lovegren, M.; Aghajanian, C.; Sabbatini, P.; Tong, W.; Barakat, R.; Spriggs, D.R. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: Results of a phase II trial. J. Clin. Oncol. 2002 , 20 , 2824–2831. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abedin, Y.; Gabrilovich, S.; Alpert, E.; Rego, E.; Begum, S.; Zhao, Q.; Heller, D.; Einstein, M.H.; Douglas, N.C. Gamma Secretase Inhibitors as Potential Therapeutic Targets for Notch Signaling in Uterine Leiomyosarcoma. Int. J. Mol. Sci. 2022 , 23 , 5980. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, X.; Gong, L.; Ou, R.; Zheng, Z.; Chen, J.; Xie, F.; Huang, X.; Qiu, J.; Zhang, W.; Jiang, Q.; et al. Sequential combination therapy of ovarian cancer with cisplatin and γ-secretase inhibitor MK-0752. Gynecol. Oncol. 2016 , 140 , 537–544. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cook, N.; Basu, B.; Smith, D.-M.; Gopinathan, A.; Evans, J.; Steward, W.P.; Palmer, D.; Propper, D.; Venugopal, B.; Hategan, M.; et al. A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br. J. Cancer 2018 , 118 , 793–801. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schott, A.F.; Landis, M.D.; Dontu, G.; Griffith, K.A.; Layman, R.M.; Krop, I.; Paskett, L.A.; Wong, H.; Dobrolecki, L.E.; Lewis, M.T.; et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin. Cancer Res. 2013 , 19 , 1512–1524. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cokol-Cakmak, M.; Bakan, F.; Cetiner, S.; Cokol, M. Diagonal Method to Measure Synergy Among Any Number of Drugs. J. Vis. Exp. 2018 , 136 , 57713. [ Google Scholar ]
- Babichev, Y.; Kabaroff, L.; Datti, A.; Uehling, D.; Isaac, M.; Al-Awar, R.; Prakesch, M.; Sun, R.X.; Boutros, P.C.; Venier, R.; et al. PI3K/AKT/mTOR inhibition in combination with doxorubicin is an effective therapy for leiomyosarcoma. J. Transl. Med. 2016 , 14 , 67. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012 , 9 , 676–682. [ Google Scholar ] [ CrossRef ]
- Kim, K.H.; Sederstrom, J.M. Assaying Cell Cycle Status Using Flow Cytometry. Curr. Protoc. Mol. Biol. 2015 , 111 , 28.6.1–28.6.11. [ Google Scholar ] [ CrossRef ]
- Hoffman, L.M.; Fouladi, M.; Olson, J.; Daryani, V.M.; Stewart, C.F.; Wetmore, C.; Kocak, M.; Onar-Thomas, A.; Wagner, L.; Gururangan, S.; et al. Phase I trial of weekly MK-0752 in children with refractory central nervous system malignancies: A pediatric brain tumor consortium study. Childs Nerv. Syst. 2015 , 31 , 1283–1289. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Krop, I.; Demuth, T.; Guthrie, T.; Wen, P.Y.; Mason, W.P.; Chinnaiyan, P.; Butowski, N.; Groves, M.D.; Kesari, S.; Freedman, S.J.; et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 2012 , 30 , 2307–2313. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, T.R. SK-UT-1B, a human tumorigenic diploid cell line. Cancer Genet. Cytogenet. 1988 , 33 , 77–81. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chapel, D.B.; Nucci, M.R.; Quade, B.J.; Parra-Herran, C. Epithelioid Leiomyosarcoma of the Uterus: Modern Outcome-based Appraisal of Diagnostic Criteria in a Large Institutional Series. Am. J. Surg. Pathol. 2022 , 46 , 464–475. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yousefi, H.; Bahramy, A.; Zafari, N.; Delavar, M.R.; Nguyen, K.; Haghi, A.; Kandelouei, T.; Vittori, C.; Jazireian, P.; Maleki, S.; et al. Notch signaling pathway: A comprehensive prognostic and gene expression profile analysis in breast cancer. BMC Cancer 2022 , 22 , 1282. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Edwards, A.; Brennan, K. Notch Signalling in Breast Development and Cancer. Front. Cell Dev. Biol. 2021 , 9 , 692173. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- LeBon, L.; Lee, T.V.; Sprinzak, D.; Jafar-Nejad, H.; Elowitz, M.B. Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. eLife 2014 , 3 , e02950. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Luo, Z.; Mu, L.; Zheng, Y.; Shen, W.; Li, J.; Xu, L.; Zhong, B.; Liu, Y.; Zhou, Y. NUMB enhances Notch signaling by repressing ubiquitination of NOTCH1 intracellular domain. J. Mol. Cell Biol. 2020 , 12 , 345–358. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pongjantarasatian, S.; Nowwarote, N.; Rotchanakitamnuai, V.; Srirodjanakul, W.; Saehun, R.; Janebodin, K.; Manokawinchoke, J.; Fournier, B.P.J.; Osathanon, T. A γ-Secretase Inhibitor Attenuates Cell Cycle Progression and Invasion in Human Oral Squamous Cell Carcinoma: An In Vitro Study. Int. J. Mol. Sci. 2022 , 23 , 8869. [ Google Scholar ] [ CrossRef ]
- Patrad, E.; Niapour, A.; Farassati, F.; Amani, M. Combination treatment of all-trans retinoic acid (ATRA) and γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis induction in the human gastric cancer cell line. Cytotechnology 2018 , 70 , 865–877. [ Google Scholar ] [ CrossRef ]
- Grottkau, B.E.; Chen, X.; Friedrich, C.C.; Yang, X.; Jing, W.; Wu, Y.; Cai, X.; Liu, Y.; Huang, Y.; Lin, Y. DAPT enhances the apoptosis of human tongue carcinoma cells. Int. J. Oral. Sci. 2009 , 1 , 81–89. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rasul, S.; Balasubramanian, R.; Filipović, A.; Slade, M.J.; Yagüe, E.; Coombes, R.C. Inhibition of gamma-secretase induces G2/M arrest and triggers apoptosis in breast cancer cells. Br. J. Cancer 2009 , 100 , 1879–1888. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lambert, L.A.; Qiao, N.; Hunt, K.K.; Lambert, D.H.; Mills, G.B.; Meijer, L.; Keyomarsi, K. Autophagy: A novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res. 2008 , 68 , 7966–7974. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghelli Luserna di Rora, A.; Ferrari, A.; Imbrogno, E.; Robustelli, V.; Papayannidis, C.; Abbenante, M.C.; Marconi, G.; Cavo, M.; Martinelli, G. Enhance the Efficacy of Doxorubicin Destabilizing the G2/M Checkpoint with a CHK1/CHK2 Inhibitor Pre-Treatment on Acute Lymphoblastic Leukemia. Blood 2017 , 130 , 5049. [ Google Scholar ]
- Shin, H.-J.; Kwon, H.-K.; Lee, J.-H.; Gui, X.; Achek, A.; Kim, J.-H.; Choi, S. Doxorubicin-induced necrosis is mediated by poly-(ADP-ribose) polymerase 1 (PARP1) but is independent of p53. Sci. Rep. 2015 , 5 , 15798. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teoh, S.L.; Das, S. Notch Signalling Pathways and Their Importance in the Treatment of Cancers. Curr. Drug Targets 2018 , 19 , 128–143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Albain, K.; Czerlanis, C.; Rajan, P.; Zlobin, A.; Godellas, C.; Bova, D.; Lo, S.; Robinson, P.; Sarker, S.; Gaynor, E.; et al. Abstract PD05-12, Combination of Notch Inhibitor MK-0752 and Endocrine Therapy for Early Stage ERα + Breast Cancer in a Presurgical Window Pilot Study. Cancer Res. 2010 , 70 , PD05-12. [ Google Scholar ] [ CrossRef ]
- Xu, R.; Shimizu, F.; Hovinga, K.; Beal, K.; Karimi, S.; Droms, L.; Peck, K.K.; Gutin, P.; Iorgulescu, J.B.; Kaley, T.; et al. Molecular and Clinical Effects of Notch Inhibition in Glioma Patients: A Phase 0/I Trial. Clin. Cancer Res. 2016 , 22 , 4786–4796. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mizuma, M.; Rasheed, Z.A.; Yabuuchi, S.; Omura, N.; Campbell, N.R.; de Wilde, R.F.; De Oliveira, E.; Zhang, Q.; Puig, O.; Matsui, W.; et al. The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical models. Mol. Cancer Ther. 2012 , 11 , 1999–2009. [ Google Scholar ] [ CrossRef ]
- Ivanova, O.M.; Anufrieva, K.S.; Kazakova, A.N.; Malyants, I.K.; Shnaider, P.V.; Lukina, M.M.; Shender, V.O. Non-canonical functions of spliceosome components in cancer progression. Cell Death Dis. 2023 , 14 , 77. [ Google Scholar ] [ CrossRef ]
- Eymin, B. Targeting the spliceosome machinery: A new therapeutic axis in cancer? Biochem. Pharmacol. 2021 , 189 , 114039. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lopez, G.; Braggio, D.; Zewdu, A.; Casadei, L.; Batte, K.; Bid, H.K.; Koller, D.; Yu, P.; Iwenofu, O.H.; Strohecker, A.; et al. Mocetinostat combined with gemcitabine for the treatment of leiomyosarcoma: Preclinical correlates. PLoS ONE 2017 , 12 , e0188859. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Choy, E.; Ballman, K.; Chen, J.; Dickson, M.A.; Chugh, R.; George, S.; Okuno, S.; Pollock, R.; Patel, R.M.; Hoering, A.; et al. SARC018_SPORE02, Phase II Study of Mocetinostat Administered with Gemcitabine for Patients with Metastatic Leiomyosarcoma with Progression or Relapse following Prior Treatment with Gemcitabine-Containing Therapy. Sarcoma 2018 , 2018 , 2068517. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016 , 7 , 27–31. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, H.S.; Lee, Y.S.; Kim, D.K. Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology 2009 , 84 , 300–309. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Geles, K.G.; Gao, Y.; Giannakou, A.; Sridharan, L.; Yamin, T.-T.; Zhang, J.; Karim, R.; Bard, J.; Piche-Nicholas, N.; Charati, M.; et al. NOTCH3-targeted antibody drug conjugates regress tumors by inducing apoptosis in receptor cells and through transendocytosis into ligand cells. Cell Rep. Med. 2021 , 2 , 100279. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aburjania, Z.; Jang, S.; Whitt, J.; Jaskula-Stzul, R.; Chen, H.; Rose, J.B. The Role of Notch3 in Cancer. Oncologist 2018 , 23 , 900–911. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Xiu, M.; Zeng, X.; Shan, R.; Wen, W.; Li, J.; Wan, R. Targeting Notch4 in Cancer: Molecular Mechanisms and Therapeutic Perspectives. Cancer Manag. Res. 2021 , 13 , 7033–7045. [ Google Scholar ] [ CrossRef ]
- Bose, S.; Schwartz, G.K.; Ingham, M. Novel Therapeutics in the Treatment of Uterine Sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2022 , 42 , 900–909. [ Google Scholar ] [ CrossRef ]
| SK-UT-1B | SK-LMS-1 | |||
|---|---|---|---|---|
| Agents | IC (µM) | IC (µM) | IC (µM) | IC (µM) |
| MK-0752 | 4.02 × 10 | 3.66 × 10 | 1.35 × 10 | 8.12 × 10 |
| Docetaxel | 5.50 × 10 | 2.40 × 10 | 1.00 × 10 | 8.70 × 10 |
| Doxorubicin | 7.10 × 10 | 3.70 × 10 | 3.01 × 10 | 2.80 × 10 |
| Gemcitabine | 2.00 × 10 | 1.40 × 10 | 6.00 × 10 | 2.00 × 10 |
| SK-UT-1B | SK-LMS-1 | |||
|---|---|---|---|---|
| Agents | IC (X) | IC (µM) | IC (X) | IC (µM) |
| MK-0752 + Docetaxel | - | - | 0.80 | M: 1.35 × 10 , Doce: 1.20 × 10 |
| MK-0752 + Doxorubicin | 0.07 | M: 7.30 × 10 , Doxo: 1.00 × 10 | 0.80 | M: 3.09 × 10 , Doxo: 6.90 × 10 |
| MK-0752 + Gemcitabine | 1.05 | - | 0.30 | M: 8.12, Gem: 3.50 × 10 |
| MK-0752 + Gemcitabine + Docetaxel | 0.70 | M: 5.04, Gem: 2.50 × 10 , Doce: 6.92 × 10 | 0.50 | M: 8.03, Gem: 3.30 × 10 , Doce: 6.70 × 10 |
| SK-UT-1B | SK-LMS-1 | ||||||
|---|---|---|---|---|---|---|---|
| Treatment (Ref) | Treatment | Fold Change | p-Value | Treatment (Ref) | Treatment | Fold Change | p-Value |
| Doce | MK + Gem + Doce | 1.66 | 0.48 | Doce | MK + Doce | 1.17 | 0.25 |
| Doxo | MK + Doxo | 1.16 | 0.39 | Doce | MK + Gem + Doce | 0.81 | 0.13 |
| Gem | MK + Gem + Doce | 0.75 | 0.24 | Doxo | MK + Doxo | 0.82 | 0.7 |
| Gem | MK + Gem | 1.88 | 0.24 | ||||
| Gem | MK + Gem + Doce | 0.88 | 0.58 | ||||
| MK + Doce | MK + Gem + Doce | 0.68 | 0.13 | ||||
| MK + Gem | MK + Gem + Doce | 0.47 | |||||
| SK-UT-1B | SK-LMS-1 | |||
|---|---|---|---|---|
| Treatment | Fold Change | p-Value | Fold Change | p-Value |
| MK-0752 | 1.09 | 0.58 | 0.63 | |
| MK-0752 + Docetaxel | --- | --- | 1.07 | 0.91 |
| MK-0752 + Doxorubicin | 0.99 | 0.70 | 1.01 | 0.94 |
| MK-0752 + Gemcitabine + Docetaxel | 1.30 | 0.83 | 1.20 | 0.09 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Abedin, Y.; Fife, A.; Samuels, C.-A.; Wright, R.; Murphy, T.; Zhang, X.; Alpert, E.; Cheung, E.; Zhao, Q.; Einstein, M.H.; et al. Combined Treatment of Uterine Leiomyosarcoma with Gamma Secretase Inhibitor MK-0752 and Chemotherapeutic Agents Decreases Cellular Invasion and Increases Apoptosis. Cancers 2024 , 16 , 2184. https://doi.org/10.3390/cancers16122184
Abedin Y, Fife A, Samuels C-A, Wright R, Murphy T, Zhang X, Alpert E, Cheung E, Zhao Q, Einstein MH, et al. Combined Treatment of Uterine Leiomyosarcoma with Gamma Secretase Inhibitor MK-0752 and Chemotherapeutic Agents Decreases Cellular Invasion and Increases Apoptosis. Cancers . 2024; 16(12):2184. https://doi.org/10.3390/cancers16122184
Abedin, Yasmin, Alexander Fife, Cherie-Ann Samuels, Rasheena Wright, Trystn Murphy, Xusheng Zhang, Emily Alpert, Emma Cheung, Qingshi Zhao, Mark H. Einstein, and et al. 2024. "Combined Treatment of Uterine Leiomyosarcoma with Gamma Secretase Inhibitor MK-0752 and Chemotherapeutic Agents Decreases Cellular Invasion and Increases Apoptosis" Cancers 16, no. 12: 2184. https://doi.org/10.3390/cancers16122184
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
Abstract. Uterine fibroids are benign uterine smooth muscle tumors that are present in up to 8 out of 10 women by the age of 50. Many of these women experience symptoms such as heavy and irregular menstrual bleeding, early pregnancy loss, and infertility. Traditionally believed to be inert masses, fibroids are now known to influence endometrial ...
A Comprehensive Review Of Uterine Fibroids: Pathog enesis, Diagnosis, Treatment, And Future Perspectives Vol.30 No.18(2023): JPTCP ( 196 1-1 97 4) Page | 1970
Uterine fibroids (leiomyomas and myomas) are the most common benign gynecological condition in patients presenting with abnormal uterine bleeding, pelvic masses causing pressure or pain, infertility and obstetric complications. Almost a third of women with fibroids need treatment due to symptoms. Objectives: In this review we present all currently available treatment modalities for uterine ...
a temporary reduction in both uterine size and broid symp-toms. This review aims to comprehensively cover the main pharmacological treatment options that were considered or currently investigated for their role in UF management in the literature. 2 Uterine Fibroid (UF) Pathogenesis The prevailing model for UF pathogenesis invokes the
Improvement in symptom severity and quality of life (QoL) are critical concerns for women with fibroids as they evaluate treatment options. This systematic review analyzed available evidence regarding minimally invasive approaches to fibroid treatment and compared validated QoL and fibroid-associated symptom scores before and after treatment. A comprehensive search was conducted using PubMed ...
Some studies have associated obesity with uterine fibroids. A literature review performed by Qin et al., 2021, including relevant literature from 1992 to 2020, ... surgery is the standard of uterine fibroid treatment worldwide, as it has proven to be effective (Florence and Fatehi, 2022).
By the time they reach 50 years of age, nearly 70% of white women and more than 80% of black women will have had at least one fibroid; severe symptoms develop in 15 to 30% of these women. 1,2 ...
A systematic review was conducted using MEDLINE, Embase, PubMed and Google Scholar to identify studies published since database inception and May 1, 2020. Prospective and retrospective studies with at least ten patients were considered eligible if they included treatment of symptomatic UF patients and reported QoL measures using the uterine ...
Background and Objectives: Uterine myomas represent one of the most prevalent pathologies affecting the female population. These benign neoplasms originate from the smooth muscular cells of the uterus, and they can be either single or multiple. Often associated with debilitating symptoms such as pelvic heaviness, pain, constipation, and urinary dysfunctions, the surgical management of ...
Uterine fibroids are benign uterine smooth muscle tumors that are present in up to 8 out of 10 women by the age of 50. ... Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. ... Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the ...
Newer minimally invasive techniques provide treatment options for symptomatic uterine fibroids while allowing uterus preservation. The objective of this review was to analyze the efficacy of uterine-preserving, minimally invasive treatment modalities in reducing fibroid-related bleeding. A comprehensive search was conducted of PubMed, Embase, PsycINFO, ClinicalTrials.gov, Scopus, and Cochrane ...
Surgical treatment includes hysterectomy, myomectomy, uterine artery embolization, and magnetic resonance-guided focused ultrasound surgery. Uterine fibroids, or leiomyomas, are the most common ...
Uterine fibroids are tumors. "Uterine fibroids are tumors of the uterus. The majority of them are benign," said Dr. Gillispie-Bell, noting "they are basically an abnormal growth. It's one cell that has grown quite a bit to develop the tumor.". And while uterine fibroids "are usually benign, there is a cancerous type called ...
Uterine fibroids. Fibroids are muscular tumors that grow in the wall of the uterus (womb). Fibroids are almost always benign (not cancerous). Not all women with fibroids have symptoms. Women who do have symptoms often find fibroids hard to live with. Some have pain and heavy menstrual bleeding. Treatment for uterine fibroids depends on your ...
Uterine fibroids are benign (not cancer) and may also be called myomas or leiomyomas. Uterine fibroids often appear in groups, or you may have only one. They can be small or large, and they can grow. Fibroids likely will not spread to other parts of your body. They may grow when you are pregnant and shrink after you no longer have a monthly period.
The average out-of-pocket cost for treatment is around $40,000, making it inaccessible to most women. A similar gap exists worldwide. Although more than 200,000 focused ultrasound treatments have ...
Medical therapies are divided into hormonal and non-hormonal treatments; however, long-term, safe, and effective treatments in the treatment of uterine fibroids are limited. In addition to established therapies, there is an increasing number of studies investigating the effect of substances such as vitamin D or green tea extract on uterine ...
Methods: Selective literature review on the treatment of uterine fibroids, including consideration of several Cochrane Reviews. Results: Fibroids can be treated with drugs, interventional procedures (uterine artery embolization [UAE] and focused ultrasound treatment [FUS]), and surgery. The evidence regarding the various available treatments is ...
A 46-year-old woman initially sought treatment for symptoms related to uterine fibroids and biliary stones. Diagnostic imaging uncovered an adrenal incidentaloma, necessitating a laparoscopic right adrenalectomy. ... Nakatani K (2022) Adrenal schwannoma in an elderly man: a case report and literature review. Intern Med 61(1):65-69. https ...
Due to limited effective therapeutics for uterine leiomyosarcoma (uLMS), the impact of the gamma secretase inhibitor (GSI) MK-0752 with common chemotherapeutics was explored in uLMS. MTT assays were performed on two human uLMS cell lines, SK-UT-1B and SK-LMS-1, using MK-0752, docetaxel, doxorubicin, and gemcitabine, individually and in combination, to determine cell viability after treatment.