- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Medical Studies

How to Write a Medical Case Study Report
Last Updated: April 18, 2024 Fact Checked
This article was medically reviewed by Mark Ziats, MD, PhD and by wikiHow staff writer, Jennifer Mueller, JD . Dr. Mark Ziats is an Internal Medicine Physician, Scientist, Entrepreneur, and the Medical Director of xBiotech. With over five years of experience, he specializes in biotechnology, genomics, and medical devices. He earned a Doctor of Medicine degree from Baylor College of Medicine, a Ph.D. in Genetics from the University of Cambridge, and a BS in Biochemistry and Chemistry from Clemson University. He also completed the INNoVATE Program in Biotechnology Entrepreneurship at The Johns Hopkins University - Carey Business School. Dr. Ziats is board certified by the American Board of Internal Medicine. There are 15 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 187,903 times.
You've encountered an interesting and unusual case on your rounds, and a colleague or supervising physician says, "Why don't you write up a case study report?" If you've never written one before, that might sound intimidating, but it's a great way to get started in medical writing. Case studies always follow a standard structure and format, so the writing is very formulaic once you get the hang of it. Read on for a step-by-step guide to writing your first case study report.
What is a case study report?

- Medical students or residents typically do the bulk of the writing of the report. If you're just starting your medical career, a case study report is a great way to get a publication under your belt. [2] X Research source

- If the patient is a minor or is incapable of giving informed consent, get consent from their parents or closest relative. [4] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source
- Your hospital likely has specific consent forms to use. Ask your supervising physician if you're not sure where to get one.
- Some journals also have their own consent form. Check your target journal's author or submission information to make sure. [5] X Research source
How is a case study report structured?

- Even though the introduction is the first part of a case study report, doctors typically write it last. You'll have a better idea of how to introduce your case study to readers after you've written it.
- Your abstract comes at the top, before the introduction, and provides a brief summary of the entire report. Unless your case study is published in an open-access journal, the abstract is the only part of the article many readers will see.

- Many journals offer templates and checklists you can use to make sure your case study includes everything necessary and is formatted properly—take advantage of these! Some journals, such as BMJ Case Reports , require all case studies submitted to use their templates.
Drafting Your Medical Case Study Report

- Patient description
- Chronological case history
- Physical exam results
- Results of any pathological tests, imaging, or other investigations
- Treatment plan
- Expected outcome of treatment
- Actual outcome of treatment

- Why the patient sought medical help (you can even use their own words)
- Important information that helped you settle on your diagnosis
- The results of your clinical examination, including diagnostic tests and their results, along with any helpful images
- A description of the treatment plan
- The outcome, including how and why treatment ended and how long the patient was under your care [11] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- You will need references to back up symptoms of the condition, common treatment, and the expected outcome of that common treatment.
- Use your research to paint a picture of the usual case of a patient with a similar condition—it'll help you show how unusual and different your patient's case is.
- Generally, aim for around 20 references—no fewer than 15, but no more than 25. [13] X Trustworthy Source PubMed Central Journal archive from the U.S. National Institutes of Health Go to source

- Close your discussion section with a summary of the lessons learned from the case and why it's significant to consider when treating similar cases in the future.
- Outline any open questions that remain. You might also provide suggestions for future research.

- In your conclusion, you might also give suggestions or recommendations to readers based on what you learned as a result of the case.
- Some journals don't want a separate conclusion section. If that's the case for one of your target journals, just move this paragraph to the end of your discussion section.
Polishing Your Report for Submission to Publishers

- Most titles are fewer than 10 words long and include the name of the disease or condition treated.
- You might also include the treatment used and whether the outcome was successful. When deciding what to include, think about the reason you wrote the case study in the first place and why you think it's important for other clinicians to read.

- Made a significant intellectual contribution to the case study report
- Was involved in the medical care of the patient reported
- Can explain and defend the data presented in the report
- Has approved the final manuscript before submission for publication

- Keep in mind that the abstract is not just going to be the first thing people read—it will often be the only thing people read. Make sure that if someone is going to walk away having only read the abstract, they'll still get the same message they would have if they read the whole thing.
- There are 2 basic types of abstract: narrative and structured. A narrative abstract is a single paragraph written in narrative prose. A structured abstract includes headings that correspond with the sections of the paper, then a brief summary of each section. Use the format preferred by your target journal.

- Look for keywords that are relevant to your field or sub-field and directly related to the content of your article, such as the name of the condition or specific treatments you used.
- Most journals allow 4-8 keywords but check the submission guidelines of your target journal to make sure.

- Blur out the patient's face as well as any tattoos, birthmarks, or unrelated scars that are visible in diagnostic images.

- It's common to thank the patient, but that's up to you. Even if you don't, include a statement indicating that you have the patient's written, informed consent to publish the information.
- Read the journal's submission guidelines for a definition of what that journal considers a conflict of interest. They're generally the same, but some might be stricter than others. [22] X Research source

- If you're not familiar with the citation style used by your target journal, check online for a guide. There might also be one available at your hospital or medical school library.
- Medical librarians can also help with citation style and references if you run into something tricky—don't just wing it! Correct citation style insures that readers can access the materials you cite.

- It's also a good idea to get a beta reader who isn't a medical professional. Their comments can help you figure out where you need to clarify your points.
- Read a lot of case studies published in your target journals—it will help you internalize the tone and style that journal is looking for.
Submitting Your Report to Publishers

- Look into the background and reputation of journals before you decide to submit to them. Only seek publication from reputable journals in which articles go through a peer-review process.
- Find out what publishing fees the journals charge. Keep in mind that open-access journals tend to charge higher publishing fees. [26] X Research source
- Read each journal's submission and editorial guidelines carefully. They'll tell you exactly how to format your case study, how long each section should be, and what citation style to use. [27] X Research source
- For electronic journals that only publish case reports, try BMJ Case Reports , Journal of Medical Case Reports , or Radiology Case Reports .

- If your manuscript isn't suitable for the journal you submitted to, the journal might offer to forward it to an associated journal where it would be a better fit.
- When your manuscript is provisionally accepted, the journal will send it to other doctors for evaluation under the peer-review process.
- Most medical journals don't accept simultaneous submissions, meaning you'll have to submit to your first choice, wait for their decision, then move to the next journal on the list if they don't bite.

- Along with your revised manuscript, include a letter with your response to each of the reviewer's comments. Where you made revisions, add page numbers to indicate where the revisions are that address that reviewer's comments.
- Sometimes, doctors involved in the peer review process will indicate that the journal should reject the manuscript. If that's the case, you'll get a letter explaining why your case study report won't be published and you're free to submit it elsewhere.

- Some journals require you to have your article professionally copy-edited at your own cost while others do this in-house. The editors will let you know what you're responsible for.

- With your acceptance letter, you'll get instructions on how to make payment and how much you owe. Take note of the deadline and make sure you pay it as soon as possible to avoid publication delays.
- Some journals will publish for free, with an "open-access option" that allows you to pay a fee only if you want open access to your article. [32] X Research source

- Through the publishing agreement, you assign your copyright in the article to the journal. This allows the journal to legally publish your work. That assignment can be exclusive or non-exclusive and may only last for a specific term. Read these details carefully!
- If you published an open-access article, you don't assign the copyright to the publisher. The publishing agreement merely gives the journal the right to publish the "Version of Record." [33] X Research source
How do I find a suitable case for a report?

- A rare disease, or unusual presentation of any disease
- An unusual combination of diseases or conditions
- A difficult or inconclusive diagnosis
- Unexpected developments or responses to treatment
- Personal impact
- Observations that shed new light on the patient's disease or condition

- There might be other members of your medical team that want to help with writing. If so, use one of these brainstorming sessions to divvy up writing responsibilities in a way that makes the most sense given your relative skills and experience.
- Senior doctors might also be able to name some journals that would potentially publish your case study. [36] X Research source
Expert Q&A
You Might Also Like

- ↑ https://www.elsevier.com/connect/authors-update/the-dos-and-donts-of-writing-and-publishing-case-reports
- ↑ https://www.bmj.com/content/350/bmj.h2693
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686928/
- ↑ https://health.usf.edu/medicine/internalmedicine/im-impact/~/media/B3A3421F4C144FA090AE965C21791A3C.ashx
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6476221/
- ↑ https://www.springer.com/gp/authors-editors/authorandreviewertutorials/writing-a-journal-manuscript/title-abstract-and-keywords/10285522
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597880/
- ↑ https://thelancet.com/pb/assets/raw/Lancet/authors/tl-info-for-authors.pdf
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-017-1351-y
- ↑ https://guides.himmelfarb.gwu.edu/casereports
- ↑ https://casereports.bmj.com/pages/authors/
- ↑ https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-7-239
- ↑ https://research.chm.msu.edu/students-residents/writing-a-case-report
- ↑ https://authorservices.taylorandfrancis.com/publishing-your-research/moving-through-production/copyright-for-journal-authors/#
About This Article

Medical Disclaimer
The content of this article is not intended to be a substitute for professional medical advice, examination, diagnosis, or treatment. You should always contact your doctor or other qualified healthcare professional before starting, changing, or stopping any kind of health treatment.
Read More...
To start a medical case study report, first choose a title that clearly reflects the contents of the report. You’ll also need to list any participating authors and develop a list of keywords, as well as an abstract summarizing the report. Your report will need to include an introduction summarizing the context of the report, as well as a detailed presentation of the case. Don’t forget to include a thorough citation list and acknowledgements of anyone else who participated in the study. For more tips from our Medical co-author, including how to get your case study report published, keep reading! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Sep 5, 2020
Did this article help you?
Asfia Banu Pasha
Apr 10, 2017
Jun 20, 2021
Mar 1, 2017

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Level up your tech skills and stay ahead of the curve
- Open access
- Published: 27 November 2013
A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes
- Richard A Rison 1
Journal of Medical Case Reports volume 7 , Article number: 239 ( 2013 ) Cite this article
130k Accesses
61 Citations
13 Altmetric
Metrics details
Case reports are a time-honored, important, integral, and accepted part of the medical literature. Both the Journal of Medical Case Reports and the Case Report section of BioMed Central Research Notes are committed to case report publication, and each have different criteria. Journal of Medical Case Reports was the world’s first international, PubMed-listed medical journal devoted to publishing case reports from all clinical disciplines and was launched in 2007. The Case Report section of BioMed Central Research Notes was created and began publishing case reports in 2012. Between the two of them, thousands of peer-reviewed case reports have now been published with a worldwide audience. Authors now also have Cases Database, a continually updated, freely accessible database of thousands of medical case reports from multiple publishers. This informal editorial outlines the process and mechanics of how and when to write a case report, and provides a brief look into the editorial process behind each of these complementary journals along with the author’s anecdotes in the hope of inspiring all authors (both novice and experienced) to write and continue writing case reports of all specialties. Useful hyperlinks are embedded throughout for easy and quick reference to style guidelines for both journals.
Peer Review reports
Introduction: the importance of case reports
Case reports are a time-honored tradition in the medical profession. From Hippocrates (460 B.C. to 370 B.C.), and even arguably further back since the papyrus records of ancient Egyptian medicine (c. 1600 B.C.) to modern day, physicians of all specialties have described interesting cases involving all specialties [ 1 , 2 ]. Published case reports provide essential information for optimal patient care because they can describe important scientific observations that are missed or undetected in clinical trials, and provide individual clinical insights thus expanding our knowledge base [ 3 ].
The publication of case reports has indeed become a standard lexicon of the medical literature. Examples abound. Few practicing physicians would not know for instance the significance and subsequent discovery of a disease whose first description in 1981 began with the title in the medical case report literature as: “A preliminary communication on extensively disseminated Kaposi’s sarcoma in a young homosexual man” [ 4 ]. There is no neurologist that I know who is unfamiliar with the disease whose description began in 1817 by James Parkinson (1755 to 1824) with the title “An essay on the shaking palsy.” [ 5 ].
Yes, both of the above-mentioned famous diseases (the acquired immunodeficiency syndrome and Parkinson’s disease) were first described in the case study format. The act of recording, discussion with colleagues, and publishing our clinical observations with patients remains essential to the art of medicine and patient care. As Osler once said “Always note and record the unusual…Publish it. Place it on permanent record as a short, concise note. Such communications are always of value.” [ 6 ].
But how and when should we do this? Early case reports were little more than personal communications between colleagues about unique and interesting patients seen in their respective medical practices. This anecdotal reporting has evolved into an accepted form of scholarly publication with the ability to rapidly disseminate knowledge to a broad medical audience [ 7 ] using the generally accepted format of a title, abstract, introduction (background), case presentation, discussion, conclusions, and references. Many biomedical journals publish case reports and provide authors with guidelines that provide instruction for acceptance criteria, content, and format and give advice on relevant patient case reports that merit publication [ 3 ].
There are already many well-written published articles on how and when to write a good case report (please see Recommended further reading section at the end). I will not re-invent the wheel, but within this editorial I hope to provide an informal guide on how and when to write a case report for BioMed Central (BMC), in particular the Journal of Medical Case Reports ( JMCR ) and BioMed Central Research Notes ( BMCRN ). The utility of the newly created Cases Database will also be discussed. Relevant and useful website links will be used throughout to allow the reader easy access to further information on BMC requirements. I also hope to impart to the reader a brief overview of case report editorial flow in both JMCR and BMCRN along with the complementary relationship between both journals. I will also give anecdotes of how I personally approach things.
Definitions
What exactly is a case report? From peer-reviewed journals to Wikipedia (and yes, I read Wikipedia like we all do) definitions are readily available and generally agreed upon. A simple online search shows the following definition from “thefreedictionary.com” [ 8 ]: “Case Report A report of a single case of a disease, usually with an unexpected presentation, which typically describes the findings, clinical course, and prognosis of the case, often accompanied by a review of other cases previously reported in the biomedical literature to put the reported case in context.” Wikipedia [ 9 ] has this to say: “In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient, but usually describe an unusual or novel occurrence. Some case reports also contain a literature review of other reported cases.” Whether one uses the above definitional references or older more classic ones [ 10 ], all are in agreement.
How to start: the patient
Things start at the bedside or in the office with the most important person involved: the patient. Patients and their stories (including from their friends, coworkers, and family) are our portal to writing the case report. Patients (both in-patients and out-patients) are assessed, we confer with colleagues, appropriate investigations then follow, and treatment if possible begins. If I encounter an in-patient on call then I follow him or her throughout his or her hospitalization and, I hope, timely discharge. The patient is then followed and reexamined in the office over the course of time to see how the clinical course evolves. I usually wait 6 months over the course of multiple visits before I actually begin to write a case report so as to allow enough time for the clinical course to play out. Of course if the patient is hospitalized with an acute and rapid illness then this time may be much shorter, but I still follow him or her with daily neurologic examinations.
Collegial discussion and the Internet: our modern day water cooler
When an interesting condition is encountered in either the hospital or the office setting, I discuss the case in person with both my local neurology colleagues and colleagues of other specialties to see if they have encountered before the clinical scenario that I am dealing with at the time. This is usually a quick face-to-face nursing station conversation. If the case is particularly challenging then I will contact my local university colleagues for their opinion (especially if an urgent transfer needs to be arranged). I then “hit the books”, or at least I used to. Nowadays I usually “hit the keyboards” which are plentiful at every hospital nursing station and in my office. Indeed, the Internet seems to have become our modern day replacement for office water cooler conversations. Since it is readily available (and free to me because I am a member of the staff) in the hospital in which I see patients and in my office, I usually start with UpToDate® [ 11 ] and then click the links to individual references. Further reading is then supplemented by both PubMed [ 12 ] (free) and Cases Database (also free) [ 13 ] (see later). If I feel that a particular patient warrants a case report, then I continue to read more and more. There are also medical list servers and medical online communities to which one can post a case with de-identified images online and petition the advice of colleagues worldwide. I use both Neurolist [ 14 ] (a membership-only service, but membership is free) and The American Academy of Neurology (AAN) for my specialty and/or subspecialties [ 15 ] (also a membership-only service, the fee of which comes out of my yearly AAN dues). Another useful list server is sermo® [ 16 ], which has free membership. Teaching grand rounds at one’s local university or hospital, poster presentations, and simple discussion with professors giving lectures at local seminars are also good (and previously “traditional”) places to start. I have always preferred an in-person encounter to discuss a case with a colleague or professor, but given the current day and age (daily workload, travel costs, time away from the office and family, and so on), I have found Internet-based discussion (keeping all patient information anonymous of course) very helpful.
The BMC series, JMCR , and BMCRN : a brief history
The BMC series is a group of open access, peer-reviewed journals that spans most areas of biological and clinical research. There are currently 65 journals in the series, including (alphabetically) BMC Anesthesiology to BMC Women’s Health. Some of these publish case reports within their respective disciplines, and some do not [ 17 ].
JMCR is an online, open access journal under BMC auspices dedicated mainly to the publication of high quality case reports, and aims to contribute to the expansion of current medical knowledge (please see specific publication criteria below). It was created and founded by Michael Kidd and colleagues in 2007 and at the time was believed to be the world’s first international medical journal devoted to publishing case reports from all clinical disciplines. In the 5 years since its launch, JMCR has published over 2000 case reports. In 2011, case reports were downloaded from the journal’s website over 1,500,000 times [ 18 ].
BMCRN is also an online, open access journal under BMC auspices publishing scientifically sound research across all fields of biology and medicine. The journal provides a home for short publications, case series, and incremental updates to previous work with the intention of reducing the loss suffered by the research community when such results remain unpublished. BMCRN began publishing case reports in 2012 and now has a dedicated section for case reports [ 19 ].
Please read on to see the complementary relationship of case reporting between the two journals, how they relate to other journals in the BMC series, and further information on editorial work flow including specific publication criteria.
Cases Database: an invaluable resource
Since the launch of JMCR in 2007 and the more recent introduction of case reports to the BMCRN , which aims to have a broader scope, BMC has acknowledged and continues to acknowledge the value of case reports to the scientific literature. To further strengthen this commitment, BMC in conjunction with Michael Kidd have developed the invaluable new resource of Cases Database, a continually updated, freely accessible database of thousands of medical case reports from multiple other publishers, including Springer, British Medical Journal, and PubMed Central. By aggregating case reports and facilitating comparison, Cases Database provides a simple resource to clinicians, researchers, regulators and patients to explore content and identify emerging trends [ 20 ].
http://www.casesdatabase.com/
I find Cases Database indispensable when I research a particular patient’s condition. It is very helpful in seeing if a particular condition has been reported before and what treatment the authors have performed. It is an invaluable resource which can be used to check and see if previous cases have been reported before and how other authors have managed their patients with similar clinical conditions. When I last checked, Cases Database had in its repository 27,915 peer-reviewed medical case reports from 250 journals (!) [ 13 ]. Cases Database is quickly becoming my first go to when reading about a patient’s condition and symptoms.
When to write a case report
How does one determine when to write an actual case report? What constitutes and what are the criteria for publication? Different journals have different criteria, but here are the criteria for JMCR and BMCRN .
JMCR [ 21 ] publishes original and interesting case reports that contribute significantly to medical knowledge. Manuscripts must meet one of the following criteria: unreported or unusual side effects or adverse interactions involving medications; unexpected or unusual presentations of a disease; new associations or variations in disease processes; presentations, diagnoses and/or management of new and emerging diseases; an unexpected association between diseases or symptoms; an unexpected event in the course of observing or treating a patient; findings that shed new light on the possible pathogenesis of a disease or an adverse effect.
http://www.jmedicalcasereports.com/authors/instructions/casereport
BMCRN [ 22 ] has somewhat different publication criteria: BMCRN considers medical case reports that describe any clinical case. Case reports submitted to BMCRN do not need to be novel, but must be authentic cases and have some educational value along with representing at least an incremental advance in the field. BMCRN will not consider case reports describing preventive or therapeutic interventions because these generally require stronger evidence.
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport
Neither BMCRN nor JMCR will consider case reports where there are ethical concerns.
JMCR and BMCRN have the following definitions that authors should know: a single case report, two case reports, or a case series (greater than two reported cases). Both journals follow this format and accept submissions with these title structures.
I tend to classify case reports in my mind generally as follows: diagnosis-related, management-related, or both [ 10 ]. Either type should have clear and concise take-home messages and teaching points. I personally keep a stack of charts labeled “Curious Cases” on a bookshelf within my small office next to my desk which is always within my field of view at work, adhering to the “out of sight, out of mind” principle. Over the years that space has grown and, admittedly, I have cases dating back over the entire span of my years in practice (now over 13 years) which I simply have not gotten around to yet (!).
BMC editorial workflow for case reports: a brief glimpse
If a BMC Series journal editorial team considers a submitted case report unsuitable for their respective specialty journal (and now a growing list of Springer journals that BMC is now affiliated with), the authors are given the option to transfer their manuscript to BMCRN . If this option is exercised, then the BMC editorial team (usually the Case Report Section Editor for BMCRN in conjunction with the appropriate Associate Editor) determines if the manuscript is suitable for BMCRN or if it is more suitable for JMCR (based on the criteria listed above). The manuscripts will then be forwarded on to the respective Deputy and/or Associate Editors for peer review depending on which of the journals the author(s) agree(s) to. Peer reviewers are solicited (usually at least one at BMCRN and at least two at JMCR ). The peer review comments (which are open and identifiable at JMCR and blinded at BMCRN ) are then usually sent to the authors for appropriate revisions and rebuttals (unless it is felt that the manuscript should be rejected outright, at which time the editorial office sends the authors an explanatory letter). After these revisions and rebuttals have been performed, the revised manuscript and rebuttals are sent back to the respective editors for a final decision and recommendations. These decisions and recommendations are then forwarded on to the Editor-in-Chief for final approval for publication. At JMCR , manuscripts are professionally copyedited before being sent off to the production team for publication, whereas at BMCRN the authors are requested to obtain their own professional copyediting (if needed) before publication (the respective costs being reflected within the different article processing charges for both journals). When the manuscripts are published in both journals, they are in the preliminary form before being converted to the final form after production.
Author satisfaction consistently ranks high for the overall process in both journals.
The actual case report
Now let us discuss the brass tacks of writing the actual case report by going through the individual sections that will comprise the manuscript. I will present them in a sequence that matches the journals’ website requirements and provide easily accessible hyperlinks to both respective journals.
The first page of the manuscript should be a dedicated title page, including the title of the article. The title should be a clear and short description of the case with a list of the full names, institutional addresses and email addresses for all authors. There should always be at least one corresponding author who is clearly identified. Abbreviations within the title should always be avoided.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-title
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#title
I usually end the title with “…: a case report” or “…: two case reports” or “…: a case series”. I also try to avoid any puns or overly cute wording within the title and try to keep things strictly descriptive and clear. The title needs to accurately describe the case – after all, this may be all that someone reads. If a cute or clever title is used that obscures what the case is really about, then it may be even less likely that the manuscript is read.
The Abstract should be “short and sweet”. It should not exceed 350 words. Abbreviations or references within the Abstract should not be used. The Abstract should be structured into three sections: Background, an introduction about why this case is important and needs to be reported. Please include information on whether this is the first report of this kind in the literature; Case presentation, brief details of what the patient(s) presented with, including the patient’s age, sex and ethnic background; Conclusions, a brief conclusion of what the reader should learn from the case report and what the clinical impact will be. Is it an original case report of interest to a particular clinical specialty of medicine or will it have a broader clinical impact across medicine? Are any teaching points identified?
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-abstract
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#abstract
I find this is the most important part because this is often all that people will read and its availability will allow easy retrieval from electronic databases and help researchers decide their level of interest in the case report. The Abstract should be a concise and condensed version of the case report and should include the same main sections of the main text and be as succinct as possible [ 3 ]. This is the last thing that I usually write as it tends to flow easily after I have invested my time in thought and writing of the manuscript.
This section is comprised of three to ten keywords representing the main content of the article. It is important for indexing the manuscript and easy online retrieval.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-keywords
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-keywords
Introduction (Background)
The Introduction ( JMCR ) or Background ( BMCRN ) section should explain the background of the case, including the disorder, usual presentation and progression, and an explanation of the presentation if it is a new disease. If it is a case discussing an adverse drug interaction the Introduction should give details of the drug’s common use and any previously reported side effects. It should also include a brief literature review. This should give an introduction to the case report from the standpoint of those without specialist knowledge in the area, clearly explaining the background of the topic. It should end with a very brief statement of what is being reported in the article.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-intro
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#background
The Introduction or Background serves as the sales pitch for the rest of the manuscript. It should be concise and salient [ 3 ] and immediately attract the reader’s attention to entice him or her to read on.
Case presentation
This should present all relevant details concerning the case. The Case presentation section should contain a description of the patient’s relevant demographic information (without adding any details that could lead to the identification of the patient); any relevant medical history of the patient; the patient's symptoms and signs; any tests that were carried out and a description of any treatment or intervention. If it is a case series, then details must be included for all patients. This section may be broken into subsections with appropriate subheadings.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-case
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#presentation
This is one of the most integral sections. The case should be described in a concise and chronological order. One should usually begin with the primary complaint, salient history (including significant family, occupational, and other social history along with any significant medications taken or allergies), followed by the physical examination, starting with the vital signs presented at the examination, along with pertinent investigations and results. There should be enough detail (but not too much) for the reader to establish his or her own conclusions about the validity. It should contain only pertinent information and nothing superfluous or confusing [ 3 ].
This is an optional section in JMCR for additional comments that provide additional relevant information not included in the case presentation, and that put the case in context or that explain specific treatment decisions.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-discussion
This section should evaluate the patient case for accuracy, validity, and uniqueness and compare and contrast the case report with the published literature. The authors should briefly summarize the published literature with contemporary references [ 3 ].
Although this section is optional in JMCR (and not even listed separately on the BMCRN guidelines website), I find that most authors write this section, or an expanded conclusions section incorporating the elements listed above.
I personally write a separate discussion section and conclusions section for each case report that I author.
Conclusions
This should state clearly the main conclusions of the case report and give a clear explanation of their importance and relevance. Is it an original case report of interest to a particular clinical specialty of medicine or will it have a broader clinical impact across medicine? Information should be included on how it will significantly advance our knowledge of a particular disease etiology or drug mechanism (if appropriate).
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-conclusion
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#conclusions
This should be short and concise with clear take-home messages and teaching points [ 3 ].
Patient’s perspective
This section is an opportunity for patients to add a description of a case from their own perspective. The patients should be encouraged to state what originally made them seek medical advice, give a description of their symptoms, whether the symptoms were better or worse at different times, how tests and treatments affected them, and how the problem is now. This section can be written as deemed appropriate by the patients, but should not include identifying information that is irrelevant to the case reported. As medicine becomes more person-centered, the voice of the individual patient becomes even more important, both to assist in clinical decision making, and for medical education.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-patients
This optional section is unique to JMCR , and I believe adds an important new dimension to the traditional case report. Most authors still do not yet take advantage of this, but I hope as time goes on and more and more open access case report manuscripts are published that this section will be routinely used, not just in JMCR but also in BMCRN and all other BMC clinical journals. I recall one manuscript in particular where the patient himself was requesting publication as soon as possible because of his terminal disease. He wanted his message out there and be available to all to read before he died.
List of abbreviations
When abbreviations are used in the text they should be defined in the text at first use, and a list of abbreviations can be provided, which should precede the Competing interests and Authors’ contributions sections.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-abbreviations
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-abbreviations
Both JMCR and BMCRN publish case reports over a wide range of medical and surgical specialties, and it is important for the reader who may not be within that particular specialty to readily access a quick list of commontechnical abbreviations. Also, given the open access nature of both journals, please keep in mind that nonmedical professionals may read the manuscript as well.
This section is compulsory for BMC. It should provide a statement to confirm that the patient has given their informed consent for the case report to be published. The written consent should not routinely be sent in along with the manuscript submission (because of patient privacy issues), but the BMC editorial office may request copies of the consent documentation at any time. The following wording is recommended: “Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.” If the individual described in the case report is a minor, or unable to provide consent, then consent must be sought from his or her parents or legal guardians. In these cases, the statement in the ‘Consent’ section of the manuscript should be amended accordingly. Please keep in mind that manuscripts will not be peer reviewed if a statement of patient consent is not present.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-consent
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#consent
In practice, I always start with written consent from the patient. If the patient is incapacitated or deceased, then I obtain consent from the patient’s next-of-kin. Once this is obtained then I place it in the patient’s chart for safe keeping. I find that most patients and family members are quite agreeable to publication as long as their details are anonymous. BMC has very clear and explicit consent criteria and consent forms in multiple languages. I always keep a consent form within my office (and carry a few in my doctor’s handbag for hospital consults) for ready access. After I have obtained consent, I place it in the patient’s chart and keep it my office.
If the patient has died, then I try to obtain consent from the patient’s next-of-kin. This is usually done via telephone or postal mail. If the deceased patient’s family is amenable (and usually they are), then I send them (I never use email when it comes to patient-identifying information) the pre-filled out consent form in their language with a return envelope and paid for postage via the postal service. If I am unable to obtain consent this way in a case involving a patient who has died, then I write in the Consent section the following: “Written informed consent could not be obtained from the deceased patient’s next-of-kin for publication of this case report and accompanying images despite all reasonable attempts. Every effort has been made to protect the patient’s identity and there is no reason to believe that our patient would have objected to publication.”
If the patient was last known to be living but untraceable (or mentally incapacitated without next-of-kin consent), then I just simply do not publish the case.
For further information, please see JMCR and BMCRN website consent section hyperlinks as listed above.
Authors’ information
This section includes any relevant information about the author(s) that may aid the reader’s interpretation of the article and understanding of the standpoint of the author(s). This may include details about the authors’ qualifications, current positions they hold at institutions or societies, or any other relevant background information. Please refer to authors using their initials. Note this section should not be used to describe any competing interests.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-information
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-information
In practice, I have frankly also personally used this section to advertise my services and “tout” my certifications and subspecialties (along with any co-authors and affiliated institutions) to my surrounding local community. This has in turn given me a modest increase in business (which has been completely non-monetary to date), usually in the form of email-based queries, many of which come from patients outside of my locality.
Acknowledgements
Authors should acknowledge anyone who contributed towards the article by making substantial contributions to conception, design, acquisition of data, or analysis and interpretation of data, or who was involved in drafting the manuscript or revising it critically for important intellectual content, but who does not meet the criteria for authorship. Also included should be the source(s) of funding for each author, and for the manuscript preparation. Authors must describe the role of the funding body, if any, in the: design, collection, analysis, and interpretation of data; writing of the manuscript; and decision to submit the manuscript for publication. Please also acknowledge anyone who contributed materials essential for the study. If a language editor has made significant revision of the manuscript, I recommend that you acknowledge the editor by name, where possible. Authors may also like to acknowledge (anonymously) the patient on whom the case report is based. If a scientific (medical) writer is used, this person should be included in the Acknowledgements section, including their source(s) of funding. Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-acknowledgements
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-acknowledgements
I have had colleagues who do not want to participate in the actual writing of the manuscript or do any actual “work” who have instead preferred to be mentioned in this section only.
Authors must search for and cite published case reports that are relevant to the case they are presenting. There should be no more than 15 references usually, although BMC does publish manuscripts with more references particularly if there is an extended literature review. Unless it is of historic interest, please keep the references as contemporary as feasible (for example, within the last 5 years or so). Please avoid excessive referencing.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-references
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-references
Cover letter
This is a separate document that should be written and uploaded with the main manuscript submission. I usually write this after I have written the Abstract. The cover letter should be addressed to the Editor-in-Chief in a formal manner and include all of the authors’ contact information. It should clearly and concisely state the title of the manuscript, and why the authors feel that their case report should be published based on any already available literature on the topic at hand. From an editor’s viewpoint, the cover letter is exceptionally important as that is the first thing that he or she reads and serves as the gateway to the Abstract and then the rest of the manuscript.
BMC author academy: help for all
Both JMCR and BMCRN have a large number of non-native English-speaking authors. Since JMCR and BMCRN are both BMC publications whose editorial offices are based in England, the language of publication is of course English. The BMC author academy is a joint program by BMC and Edanz [ 23 ] aimed at equipping writers for successful publication. Their materials have been developed from training workshops that Edanz gives to researchers worldwide and are not just limited to case reports. BMC recommends Edanz for authors who want to have their manuscript edited by a native speaker of English who is a scientific expert. Edanz provides scientific editing and related services that raise the quality of manuscripts to the standard needed to be understood at peer review.
http://www.biomedcentral.com/authors/authoracademy
I find that most non-native English-speaking authors have their manuscripts reviewed informally by a native English-speaking colleague and/or friend who is usually mentioned within the Acknowledgements section. This is understandable to keep costs down. However, please be aware that poor grammar and frequent spelling mistakes can be an impediment to editorial work flow and peer review. The editorial staff for both JMCR and BMCRN are acutely aware and sensitive to this given the large number of international submissions. At both JMCR and BMCRN , submitted manuscripts with questionable grammar and spelling are returned back to the authors by the editorial staff if it is felt that the grammar and spelling mistakes would impede peer review. If these issues are minor and it is felt that they would not impede peer review, then the manuscripts are sent off to peer reviewers (when appropriate).
Final checklist and the rule of C s
After I have completed a case report, I like to run through my long-winded (but useful) “rule of Cs” which is as follows.
Is it C lear, C oncise, and C oherent? Does it C onvey your message? Have you used C ases Database to look for any previously similar reported cases, and included them, if appropriate, in your references? Have you C onferred with your C olleagues on the C ontent? Will it C ause the reader to be C urious? Did you obtain C onsent? Does it C ontain all of the necessary information? Does it C omply with BM C guidelines? Do you think that it may need C opyediting? Do your C o-authors C oncur with the C ompleted paper? C an you C ut anything unnecessary out? Are your findings likely to be a C oincidence or by C hance alone? If so, then mention this in the Discussion section. Is the writing style C onsistent? Many times I find co-authored manuscripts have different writing styles within the same paper depending on who wrote what section. There should be a C entral, C orresponding author who is in C harge and oversees all of this. Is the C ase report written in a C hronological fashion with respect to the patient’s history and C hain of events? Is there anything that can be C ut out and have it still C ontain the C ompulsory information? Is it C oncise? Have you C onveyed C uriosity for your C ase report within your C over letter to the editorial team? Remember: your C over letter is the sales pitch to the editorial team! Make it C ount! Have you used within the manuscript C opyrighted information from another source? If so, do you need and/or have permission for use? After C ompletion, wait a C ouple of days before final submission to C lear your mind and read the manuscript again to C atch any mistakes that you may have made while you were C aught up in the C ompletion of it. Are the references C ontemporary? C an it be C omprehended by the average (“ C ”) reader? Remember, both JMCR and BMCRN are open access and freely available to anyone with an Internet C onnection and C omputer. C ast as wide a net as possible and C apture your C olleagues’ and other readers’ C uriosity. And first and foremost as a C linician: was the C are of your patient C ompetent and C ompassionate? (that is, are there any ethical concerns that may preclude peer review and publication?).
Summary and parting advice
Case reporting can be fun and a lifelong hobby, both for novice and experienced authors alike. It is now integral and widely accepted within published medical literature and today’s electronic information and data-sharing age. By following the above recommended steps and general overview, I hope to encourage BMC authors to continue to write and submit manuscripts to both JMCR and BMCRN . After your manuscript is complete, please follow the rule of “Cs”, especially “ C lear, C oncise, C oherent, C onsent, C ompassion, and C ompetence”, which will be appreciated by both reviewers and editors. Do not be afraid to obtain help from native English speakers for your manuscript. Also, please adhere to deadlines and follow instructions given by the editorial office, especially regarding any revisions. Editors read many different manuscripts and the longer it takes to get back a manuscript after revisions have been requested the less fresh that manuscript is in mind. Lastly, consider volunteering as an Associate Editor and/or reviewer within your specialty for both journals. I do for both, and the experience has improved both my writing and editing skills and daily interactions with patients.
Recommended further reading
I recommend the following further instructive reading on how and when to write a case report: References [ 3 , 7 , 10 , 24 ] (the last referenced article is in German, but one should readily be able to obtain an English translation if needed through a local librarian. It is well worth reading.)
I also recommend the following instructive BMC-related editorials and commentaries concerning the modern-day importance of case reports: References 2, 18, and 19.
Breasted J: The Edwin Smith Surgical Papyrus. 1930, Chicago: Chicago University Press
Google Scholar
Rison RA: Neurology case reporting: a call for all. J Med Case Reports. 2011, 5: 113-10.1186/1752-1947-5-113.
Article PubMed Central Google Scholar
Cohen H: How to write a case report. Am J Health Syst Pharm. 2006, 63: 1888-1892. 10.2146/ajhp060182.
Article PubMed Google Scholar
Gottleib GJ, Rogoz A, Vogel JV, Friedman-Kien A, Rywlin AM, Weiner EA, Ackerman AB: A preliminary communication on extensively disseminated Kaposi’s sarcoma in a young homosexual man. Am J Dermatopathol. 1981, 3: 111-114. 10.1097/00000372-198100320-00002.
Article Google Scholar
Goetz CG: The history of Parkinson’s disease: early clinical descriptions and neurological therapies. Cold Spring Harb Perspect Med. 2011, 1 (1): a008862-
Article PubMed PubMed Central Google Scholar
Thayer WS: Osler, The Teacher Sir William Osler, Bart. 1920, Baltimore: Johns Hopkins Press, 51-52.
Carleton HA, Webb ML: The case report in context. Yale J Biol Med. 2012, 85: 93-96.
PubMed PubMed Central Google Scholar
Weblink: “ http://medical-dictionary.thefreedictionary.com/case+report ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://en.wikipedia.org/wiki/Case_report ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Peh WCG, Ng KH: Writing a case report. Singapore Med J. 2010, 51 (1): 10-
CAS PubMed Google Scholar
Weblink: “ http://www.uptodate.com/home ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.ncbi.nlm.nih.gov/pubmed ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.casesdatabase.com/ ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.neurolist.com/ ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.aan.com/ ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.sermo.com/ ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.biomedcentral.com/authors/bmcseries ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Kidd MR, Saltman DC: Case reports at the vanguard of 21 st century medicine. J Med Case Reports. 2012, 6: 156-10.1186/1752-1947-6-156.
Cabán-Martinez AJ, Beltrán WF: Advancing medicine one research note at a time: the educational value in clinical case reports. BMC Res Notes. 2012, 5: 293-10.1186/1756-0500-5-293.
Weblink: “ http://www.casesdatabase.com/about ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.jmedicalcasereports.com/ ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.biomedcentral.com/bmcresnotes ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Weblink: “ http://www.edanzediting.com/ ” Accessed on August 11 th , 2013 “” Accessed on August 11th, 2013
Schneemann M, Ruggieri F: [Publish your case report]. [ Article in German ] Praxis ( Bern 1994 ) 2013. 102 (5): 253-259. doi:10.1024/1661-8157/a001229. quiz 60–61
Download references
I thank Professor Michael R. Kidd for his valuable advice and comments on this manuscript.
Author information
Authors and affiliations.
Presbyterian Intercommunity Hospital Health Stroke Program, Los Angeles County Medical Center, University of Southern California Keck School of Medicine, 12401 Washington Blvd, Whittier, CA, 90602, USA
Richard A Rison
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Richard A Rison .
Additional information
Competing interests.
A competing interest exists when one’s interpretation of data or presentation of information may be influenced by a personal or financial relationship with other people or organizations. Authors must disclose any financial competing interests and should also reveal any non-financial competing interests that may cause embarrassment were they to become public after the publication of the manuscript. Authors are required to complete a declaration of competing interests. All competing interests that are declared will be listed at the end of published article. Where an author gives no competing interests, the listing should read “The author(s) declare that they have no competing interests”.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-competing
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-competing
I do not usually find any problems with competing interests in the case reports that I publish, but the section should always be completed in our era and in the spirit of complete disclosure.
Authors’ contributions
In order to give appropriate credit to each author of a paper, the individual contributions of authors to the manuscript should be specified in this section.
An ‘author’ is generally considered to be someone who has made substantive intellectual contributions to a published study. To qualify as an author one should: 1) have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) have been involved in drafting the manuscript or revising it critically for important intellectual content; and 3) have given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship. All contributors who do not meet the criteria for authorship should be listed in an Acknowledgements section. Examples of those who might be acknowledged include a person who provided purely technical help, writing assistance, or a department chair who provided only general support.
http://www.jmedicalcasereports.com/authors/instructions/casereport#formatting-contributions
http://www.biomedcentral.com/bmcresnotes/authors/instructions/casereport#formatting-contributions
I have found over the years a trend towards multi-authored case report manuscripts by many different individuals involved in the care of a patient(s). In my setting, it is usually me, a medical student or resident, a second-opinion tertiary colleague, and/or a pathologist or radiologist (if applicable). But I also recognize that there are situations that warrant more co-authors. The above criteria though for co-authorship should always be followed, and I have seen editorial situations where peer reviewers (including Associate Editors) have questioned what they felt was excessive authorship.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Rison, R.A. A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes . J Med Case Reports 7 , 239 (2013). https://doi.org/10.1186/1752-1947-7-239
Download citation
Received : 30 August 2013
Accepted : 07 October 2013
Published : 27 November 2013
DOI : https://doi.org/10.1186/1752-1947-7-239
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 January 2023
A student guide to writing a case report
- Maeve McAllister 1
BDJ Student volume 30 , pages 12–13 ( 2023 ) Cite this article
28 Accesses
Metrics details
As a student, it can be hard to know where to start when reading or writing a clinical case report either for university or out of special interest in a Journal. I have collated five top tips for writing an insightful and relevant case report.
A case report is a structured report of the clinical process of a patient's diagnostic pathway, including symptoms, signs, diagnosis, treatment planning (short and long term), clinical outcomes and follow-up. 1 Some of these case reports can sometimes have simple titles, to the more unusual, for example, 'Oral Tuberculosis', 'The escapee wisdom tooth', 'A difficult diagnosis'. They normally begin with the word 'Sir' and follow an introduction from this.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Guidelines To Writing a Clinical Case Report. Heart Views 2017; 18 , 104-105.
British Dental Journal. Case reports. Available online at: www.nature.com/bdj/articles?searchType=journalSearch&sort=PubDate&type=case-report&page=2 (accessed August 17, 2022).
Chate R, Chate C. Achenbach's syndrome. Br Dent J 2021; 231: 147.
Abdulgani A, Muhamad, A-H and Watted N. Dental case report for publication; step by step. J Dent Med Sci 2014; 3 : 94-100.
Download references
Author information
Authors and affiliations.
Queen´s University Belfast, Belfast, United Kingdom
Maeve McAllister
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maeve McAllister .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McAllister, M. A student guide to writing a case report. BDJ Student 30 , 12–13 (2023). https://doi.org/10.1038/s41406-023-0925-y
Download citation
Published : 30 January 2023
Issue Date : 30 January 2023
DOI : https://doi.org/10.1038/s41406-023-0925-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Writing a Case Report
This page is intended for medical students, residents or others who do not have much experience with case reports, but are planning on writing one.
What is a case report? A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Case reports are commonly of the following categories :
- Rare diseases
- Unusual presentation of disease
- Unexpected events
- Unusual combination of diseases or conditions
- Difficult or inconclusive diagnosis
- Treatment or management challenges
- Personal impact
- Observations that shed new light on a disease or condition
- Anatomical variations
It is important that you recognize what is unique or interesting about your case, and this must be described clearly in the case report.
Case reports generally take the format of :
1. Background
2. Case presentation
3. Observations and investigation
4. Diagnosis
5. Treatment
7. Discussion
Does a case report require IRB approval?
Case reports typically discuss a single patient. If this is true for your case report, then it most likely does not require IRB approval because it not considered research. If you have more than one patient, your study could qualify as a Case Series, which would require IRB review. If you have questions, you chould check your local IRB's guidelines on reviewing case reports.
Are there other rules for writing a case report?
First, you will be collecting protected health information, thus HIPAA applies to case reports. Spectrum Health has created a very helpful guidance document for case reports, which you can see here: Case Report Guidance - Spectrum Health
While this guidance document was created by Spectrum Health, the rules and regulations outlined could apply to any case report. This includes answering questions like: Do I need written HIPAA authorization to publish a case report? When do I need IRB review of a case report? What qualifies as a patient identifier?
How do I get started?
1. We STRONGLY encourage you to consult the CARE Guidelines, which provide guidance on writing case reports - https://www.care-statement.org/
Specifically, the checklist - https://www.care-statement.org/checklist - which explains exactly the information you should collect and include in your case report.
2. Identify a case. If you are a medical student, you may not yet have the clinical expertise to determine if a specific case is worth writing up. If so, you must seek the help of a clinician. It is common for students to ask attendings or residents if they have any interesting cases that can be used for a case report.
3. Select a journal or two to which you think you will submit the case report. Journals often have specific requirements for publishing case reports, which could include a requirement for informed consent, a letter or statement from the IRB and other things. Journals may also charge publication fees (see Is it free to publish? below)
4. Obtain informed consent from the patient (see " Do I have to obtain informed consent from the patient? " below). Journals may have their own informed consent form that they would like you to use, so please look for this when selecting a journal.
Once you've identified the case, selected an appropriate journal(s), and considered informed consent, you can collect the required information to write the case report.
How do I write a case report?
Once you identify a case and have learned what information to include in the case report, try to find a previously published case report. Finding published case reports in a similar field will provide examples to guide you through the process of writing a case report.
One journal you can consult is BMJ Case Reports . MSU has an institutional fellowship with BMJ Case Reports which allows MSU faculty, staff and students to publish in this journal for free. See this page for a link to the journal and more information on publishing- https://lib.msu.edu/medicalwriting_publishing/
There are numerous other journals where you can find published case reports to help guide you in your writing.
Do I have to obtain informed consent from the patient?
The CARE guidelines recommend obtaining informed consent from patients for all case reports. Our recommendation is to obtain informed consent from the patient. Although not technically required, especially if the case report does not include any identifying information, some journals require informed consent for all case reports before publishing. The CARE guidelines recommend obtaining informed consent AND the patient's perspective on the treatment/outcome (if possible). Please consider this as well.
If required, it is recommended you obtain informed consent before the case report is written.
An example of a case report consent form can be found on the BMJ Case Reports website, which you can access via the MSU library page - https://casereports.bmj.com/ . Go to "Instructions for Authors" and then "Patient Consent" to find the consent form they use. You can create a similar form to obtain consent from your patient. If you have identified a journal already, please consult their requirements and determine if they have a specific consent form they would like you to use.
Seek feedback
Once you have written a draft of the case report, you should seek feedback on your writing, from experts in the field if possible, or from those who have written case reports before.
Selecting a journal
Aside from BMJ Case Reports mentioned above, there are many, many journals out there who publish medical case reports. Ask your mentor if they have a journal they would like to use. If you need to select on your own, here are some strategies:
1. Do a PubMed search. https://pubmed.ncbi.nlm.nih.gov/
a. Do a search for a topic, disease or other feature of your case report
b. When the results appear, on the left side of the page is a limiter for "article type". Case reports are an article type to which you can limit your search results. If you don't see that option on the left, click "additional filters".
c. Review the case reports that come up and see what journals they are published in.
2. Use JANE - https://jane.biosemantics.org/
3. Check with specialty societies. Many specialty societies are affiliated with one or more journal, which can be reviewed for ones that match your needs
4. Search through individual publisher journal lists. Elsevier publishes many different medical research journals, and they have a journal finder, much like JANE ( https://journalfinder.elsevier.com/ ). This is exclusive to Elsevier journals. There are many other publishers of medical journals for review, including Springer, Dove Press, BMJ, BMC, Wiley, Sage, Nature and many others.
Is it free to publish ?
Be aware that it may not be free to publish your case report. Many journals charge publication fees. Of note, many open access journals charge author fees of thousands of dollars. Other journals have smaller page charges (i.e. $60 per page), and still others will publish for free, with an "open access option". It is best practice to check the journal's Info for Authors section or Author Center to determine what the cost is to publish. MSU-CHM does NOT have funds to support publication costs, so this is an important step if you do not want to pay out of pocket for publishing
*A more thorough discussion on finding a journal, publication costs, predatory journals and other publication-related issues can be found here: https://research.chm.msu.edu/students-residents/finding-a-journal
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. 2013. The CARE guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med . 2:38-43. doi: 10.7453/gahmj.2013.008
Riley DS, Barber MS, Kienle GS, AronsonJK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. 2017. CARE guidelines for case reports: explanation and elaboration document . J Clin Epidemiol . 89:218-234. doi: 10.1016/j.jclinepi.2017.04.026
Guidelines to writing a clinical case report. 2017. Heart Views . 18:104-105. doi: 10.4103/1995-705X.217857
Ortega-Loubon C, Culquichicon C, Correa R. The importance of writing and publishing case reports during medical education. 2017. Cureus. 9:e1964. doi: 10.7759/cureus.1964
Writing and publishing a useful and interesting case report. 2019. BMJ Case Reports. https://casereports.bmj.com/pages/wp-content/uploads/sites/69/2019/04/How-to-write-a-Case-Report-DIGITAL.pdf
Camm CF. Writing an excellent case report: EHJ Case Reports , Case of the Year 2019. 2020. European Heart Jounrnal. 41:1230-1231. https://doi.org/10.1093/eurheartj/ehaa176
*content developed by Mark Trottier, PhD
Unfortunately we don't fully support your browser. If you have the option to, please upgrade to a newer version or use Mozilla Firefox , Microsoft Edge , Google Chrome , or Safari 14 or newer. If you are unable to, and need support, please send us your feedback .
We'd appreciate your feedback. Tell us what you think! opens in new tab/window
The do’s and don’ts of writing and publishing case reports
March 6, 2023 | 5 min read

Lessons from a recent Researcher Academy webinar
As a method of documenting a single clinical observation, case reports offer timely and valuable information, especially with regards to rare diseases. They show medical professionals how fellow practitioners have acted in similar situations and thus aid in the decision-making process by sharing best practices. Not only do they significantly contribute to the knowledge pool, they also help add to a researcher’s own publication portfolio. Producing a good case report requires much more than just an interesting case, however.
To assist researchers with this task, Professors Oliver Kurzai and Adilia Warris, editors of the journal Medical Mycology Case Reports opens in new tab/window shared tips on writing high impact case reports in the latest Researcher Academy webinar opens in new tab/window . We are pleased to share here some quick do’s and don’ts from the webinar.
Tell a story
The best way to compose a case report is to tell a story. This can be accomplished by arranging the events in chronological order, being specific about your differential diagnostic considerations, elucidating the arguments for your clinical decision-making process, and following up to round off the story neatly. This will create an imaginary journey where your readers can follow every development of the case and understand why you have performed specific tests or made certain decisions during a particular treatment.
Get the details right
Make sure to describe the relevant signs and symptoms which have resulted in the differential diagnosis, both positive and negative in order to provide readers with the context in which you have made your decisions. You can also include in your case reports descriptions of actual values for blood test results, detailed dosages for medications prescribed or other variables that should be taken into account with respect to the outcome of the situation.
Employ pictures/figures where relevant
A picture, as they say, is worth a thousand words, especially for case reports where findings can be clearly and efficiently illustrated via images. Don’t make use of pictures without justification, however – do so only if they have a function. For example, macroscopic and microscopic images of a newly-identified causative microorganism are an essential whereas a picture of the model you have clearly explained elsewhere in the text may be overkill.
Formulate short and sharp titles
The title is the first selling point of your case report. Therefore, you would want it to be interesting and something that grasps the reader’s attention. Make sure you phrase it concisely, but still in an eye-catching way. Take a look at the examples shared by the speakers below.
An OK title: " Treatment of cerebral mucormycosis with drug therapy alone: a case report"
Versus a compelling title: "Successful outcome of cerebral mucormycosis with drug therapy alone"
Secure written consent from patient
Due to its nature of being a detailed description of an individual patient’s clinical presentation and therapy, a case report almost always contains information that could be traced back to the individual in question. Thus, a written, informed consent from the patient is a key requirement for the publication. Keep in mind that your patient is your partner in completing a case report, therefore make sure to discuss the report proactively with them including being explicit about any potential images that you are going to use, especially if they show or could identify the patient.
Don’t write your case report before doing your homework
If your case is not unique or interesting enough, there is a high chance that it will not be published. Even when your case is unique but is not well-documented or misses some crucial diagnostic elements, the same outcome might still ensue. This is not only a waste of your precious time but also a discouragement which might prevent you from producing more case reports in the future. To avoid this outcome, make sure to carry out careful research before writing your case reports. Make sure it meets all necessary characteristics and requirements before spending a lot of time and effort on the writing part.
Don’t publish a case report without the patient’s consent
As explained above, informed patient consent is mandatory for the publication of your case reports. Ignoring this requirement can result in a rejection for your work and worse, ruin your relationship and reputation with patients. However, there is an exception for publishing a case report without patient consent when the benefit of publication toward to society outweighs potential harm for individual. This happens when the case report contains an extremely important public health message but impossible to obtain informed consent despite all efforts as the patient has died, for example.
Don’t forget, moreover that clinical practitioners are not required to, and should not reveal personal patient information to a journal that is not relevant to the case.
Don’t include everything
“Less is more” goes the popular adage… It is not recommended to provide an extensive overview or discuss every single aspect of the patient’s disease in the introduction and conclusion. This will only serve to disengage readers and will distract them from the main ideas you want to communicate. If you want to give a focused introduction and discussion, make sure your case report mentions only the key messages and information related and relevant to these points.
To learn more about other insightful tips on how to write influential case reports and more importantly to get them published, you can watch the full webinar recording at the Researcher Academy opens in new tab/window . If, after doing so you still have unresolved questions, why not post in the Researcher Academy Mendeley group opens in new tab/window , where the team will endeavour to find expert answers for you.
Contributor

Marketing and Communications Intern
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Write for Us
- BMJ Journals
You are here
- Volume 21, Issue 1
- What is a case study?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Roberta Heale 1 ,
- Alison Twycross 2
- 1 School of Nursing , Laurentian University , Sudbury , Ontario , Canada
- 2 School of Health and Social Care , London South Bank University , London , UK
- Correspondence to Dr Roberta Heale, School of Nursing, Laurentian University, Sudbury, ON P3E2C6, Canada; rheale{at}laurentian.ca
https://doi.org/10.1136/eb-2017-102845
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
What is it?
Case study is a research methodology, typically seen in social and life sciences. There is no one definition of case study research. 1 However, very simply… ‘a case study can be defined as an intensive study about a person, a group of people or a unit, which is aimed to generalize over several units’. 1 A case study has also been described as an intensive, systematic investigation of a single individual, group, community or some other unit in which the researcher examines in-depth data relating to several variables. 2
Often there are several similar cases to consider such as educational or social service programmes that are delivered from a number of locations. Although similar, they are complex and have unique features. In these circumstances, the evaluation of several, similar cases will provide a better answer to a research question than if only one case is examined, hence the multiple-case study. Stake asserts that the cases are grouped and viewed as one entity, called the quintain . 6 ‘We study what is similar and different about the cases to understand the quintain better’. 6
The steps when using case study methodology are the same as for other types of research. 6 The first step is defining the single case or identifying a group of similar cases that can then be incorporated into a multiple-case study. A search to determine what is known about the case(s) is typically conducted. This may include a review of the literature, grey literature, media, reports and more, which serves to establish a basic understanding of the cases and informs the development of research questions. Data in case studies are often, but not exclusively, qualitative in nature. In multiple-case studies, analysis within cases and across cases is conducted. Themes arise from the analyses and assertions about the cases as a whole, or the quintain, emerge. 6
Benefits and limitations of case studies
If a researcher wants to study a specific phenomenon arising from a particular entity, then a single-case study is warranted and will allow for a in-depth understanding of the single phenomenon and, as discussed above, would involve collecting several different types of data. This is illustrated in example 1 below.
Using a multiple-case research study allows for a more in-depth understanding of the cases as a unit, through comparison of similarities and differences of the individual cases embedded within the quintain. Evidence arising from multiple-case studies is often stronger and more reliable than from single-case research. Multiple-case studies allow for more comprehensive exploration of research questions and theory development. 6
Despite the advantages of case studies, there are limitations. The sheer volume of data is difficult to organise and data analysis and integration strategies need to be carefully thought through. There is also sometimes a temptation to veer away from the research focus. 2 Reporting of findings from multiple-case research studies is also challenging at times, 1 particularly in relation to the word limits for some journal papers.
Examples of case studies
Example 1: nurses’ paediatric pain management practices.
One of the authors of this paper (AT) has used a case study approach to explore nurses’ paediatric pain management practices. This involved collecting several datasets:
Observational data to gain a picture about actual pain management practices.
Questionnaire data about nurses’ knowledge about paediatric pain management practices and how well they felt they managed pain in children.
Questionnaire data about how critical nurses perceived pain management tasks to be.
These datasets were analysed separately and then compared 7–9 and demonstrated that nurses’ level of theoretical did not impact on the quality of their pain management practices. 7 Nor did individual nurse’s perceptions of how critical a task was effect the likelihood of them carrying out this task in practice. 8 There was also a difference in self-reported and observed practices 9 ; actual (observed) practices did not confirm to best practice guidelines, whereas self-reported practices tended to.
Example 2: quality of care for complex patients at Nurse Practitioner-Led Clinics (NPLCs)
The other author of this paper (RH) has conducted a multiple-case study to determine the quality of care for patients with complex clinical presentations in NPLCs in Ontario, Canada. 10 Five NPLCs served as individual cases that, together, represented the quatrain. Three types of data were collected including:
Review of documentation related to the NPLC model (media, annual reports, research articles, grey literature and regulatory legislation).
Interviews with nurse practitioners (NPs) practising at the five NPLCs to determine their perceptions of the impact of the NPLC model on the quality of care provided to patients with multimorbidity.
Chart audits conducted at the five NPLCs to determine the extent to which evidence-based guidelines were followed for patients with diabetes and at least one other chronic condition.
The three sources of data collected from the five NPLCs were analysed and themes arose related to the quality of care for complex patients at NPLCs. The multiple-case study confirmed that nurse practitioners are the primary care providers at the NPLCs, and this positively impacts the quality of care for patients with multimorbidity. Healthcare policy, such as lack of an increase in salary for NPs for 10 years, has resulted in issues in recruitment and retention of NPs at NPLCs. This, along with insufficient resources in the communities where NPLCs are located and high patient vulnerability at NPLCs, have a negative impact on the quality of care. 10
These examples illustrate how collecting data about a single case or multiple cases helps us to better understand the phenomenon in question. Case study methodology serves to provide a framework for evaluation and analysis of complex issues. It shines a light on the holistic nature of nursing practice and offers a perspective that informs improved patient care.
- Gustafsson J
- Calanzaro M
- Sandelowski M
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.
Read the full text or download the PDF:
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to present patient...
How to present patient cases
- Related content
- Peer review
- Mary Ni Lochlainn , foundation year 2 doctor 1 ,
- Ibrahim Balogun , healthcare of older people/stroke medicine consultant 1
- 1 East Kent Foundation Trust, UK
A guide on how to structure a case presentation
This article contains...
-History of presenting problem
-Medical and surgical history
-Drugs, including allergies to drugs
-Family history
-Social history
-Review of systems
-Findings on examination, including vital signs and observations
-Differential diagnosis/impression
-Investigations
-Management
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence. 1
The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the patient’s condition and further management can be planned accordingly. 2 To give a high quality presentation you need to take a thorough history. Consultants make decisions about patient care based on information presented to them by junior members of the team, so the importance of accurately presenting your patient cannot be overemphasised.
As a medical student, you are likely to be asked to present in numerous settings. A formal case presentation may take place at a teaching session or even at a conference or scientific meeting. These presentations are usually thorough and have an accompanying PowerPoint presentation or poster. More often, case presentations take place on the wards or over the phone and tend to be brief, using only memory or short, handwritten notes as an aid.
Everyone has their own presenting style, and the context of the presentation will determine how much detail you need to put in. You should anticipate what information your senior colleagues will need to know about the patient’s history and the care he or she has received since admission, to enable them to make further management decisions. In this article, I use a fictitious case to …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £33 / $40 / €36 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
- Open access
- Published: 27 June 2011
The case study approach
- Sarah Crowe 1 ,
- Kathrin Cresswell 2 ,
- Ann Robertson 2 ,
- Guro Huby 3 ,
- Anthony Avery 1 &
- Aziz Sheikh 2
BMC Medical Research Methodology volume 11 , Article number: 100 ( 2011 ) Cite this article
787k Accesses
1063 Citations
37 Altmetric
Metrics details
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we reflect on the different types of case study design, the specific research questions this approach can help answer, the data sources that tend to be used, and the particular advantages and disadvantages of employing this methodological approach. The paper concludes with key pointers to aid those designing and appraising proposals for conducting case study research, and a checklist to help readers assess the quality of case study reports.
Peer Review reports
Introduction
The case study approach is particularly useful to employ when there is a need to obtain an in-depth appreciation of an issue, event or phenomenon of interest, in its natural real-life context. Our aim in writing this piece is to provide insights into when to consider employing this approach and an overview of key methodological considerations in relation to the design, planning, analysis, interpretation and reporting of case studies.
The illustrative 'grand round', 'case report' and 'case series' have a long tradition in clinical practice and research. Presenting detailed critiques, typically of one or more patients, aims to provide insights into aspects of the clinical case and, in doing so, illustrate broader lessons that may be learnt. In research, the conceptually-related case study approach can be used, for example, to describe in detail a patient's episode of care, explore professional attitudes to and experiences of a new policy initiative or service development or more generally to 'investigate contemporary phenomena within its real-life context' [ 1 ]. Based on our experiences of conducting a range of case studies, we reflect on when to consider using this approach, discuss the key steps involved and illustrate, with examples, some of the practical challenges of attaining an in-depth understanding of a 'case' as an integrated whole. In keeping with previously published work, we acknowledge the importance of theory to underpin the design, selection, conduct and interpretation of case studies[ 2 ]. In so doing, we make passing reference to the different epistemological approaches used in case study research by key theoreticians and methodologists in this field of enquiry.
This paper is structured around the following main questions: What is a case study? What are case studies used for? How are case studies conducted? What are the potential pitfalls and how can these be avoided? We draw in particular on four of our own recently published examples of case studies (see Tables 1 , 2 , 3 and 4 ) and those of others to illustrate our discussion[ 3 – 7 ].
What is a case study?
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the central tenet being the need to explore an event or phenomenon in depth and in its natural context. It is for this reason sometimes referred to as a "naturalistic" design; this is in contrast to an "experimental" design (such as a randomised controlled trial) in which the investigator seeks to exert control over and manipulate the variable(s) of interest.
Stake's work has been particularly influential in defining the case study approach to scientific enquiry. He has helpfully characterised three main types of case study: intrinsic , instrumental and collective [ 8 ]. An intrinsic case study is typically undertaken to learn about a unique phenomenon. The researcher should define the uniqueness of the phenomenon, which distinguishes it from all others. In contrast, the instrumental case study uses a particular case (some of which may be better than others) to gain a broader appreciation of an issue or phenomenon. The collective case study involves studying multiple cases simultaneously or sequentially in an attempt to generate a still broader appreciation of a particular issue.
These are however not necessarily mutually exclusive categories. In the first of our examples (Table 1 ), we undertook an intrinsic case study to investigate the issue of recruitment of minority ethnic people into the specific context of asthma research studies, but it developed into a instrumental case study through seeking to understand the issue of recruitment of these marginalised populations more generally, generating a number of the findings that are potentially transferable to other disease contexts[ 3 ]. In contrast, the other three examples (see Tables 2 , 3 and 4 ) employed collective case study designs to study the introduction of workforce reconfiguration in primary care, the implementation of electronic health records into hospitals, and to understand the ways in which healthcare students learn about patient safety considerations[ 4 – 6 ]. Although our study focusing on the introduction of General Practitioners with Specialist Interests (Table 2 ) was explicitly collective in design (four contrasting primary care organisations were studied), is was also instrumental in that this particular professional group was studied as an exemplar of the more general phenomenon of workforce redesign[ 4 ].
What are case studies used for?
According to Yin, case studies can be used to explain, describe or explore events or phenomena in the everyday contexts in which they occur[ 1 ]. These can, for example, help to understand and explain causal links and pathways resulting from a new policy initiative or service development (see Tables 2 and 3 , for example)[ 1 ]. In contrast to experimental designs, which seek to test a specific hypothesis through deliberately manipulating the environment (like, for example, in a randomised controlled trial giving a new drug to randomly selected individuals and then comparing outcomes with controls),[ 9 ] the case study approach lends itself well to capturing information on more explanatory ' how ', 'what' and ' why ' questions, such as ' how is the intervention being implemented and received on the ground?'. The case study approach can offer additional insights into what gaps exist in its delivery or why one implementation strategy might be chosen over another. This in turn can help develop or refine theory, as shown in our study of the teaching of patient safety in undergraduate curricula (Table 4 )[ 6 , 10 ]. Key questions to consider when selecting the most appropriate study design are whether it is desirable or indeed possible to undertake a formal experimental investigation in which individuals and/or organisations are allocated to an intervention or control arm? Or whether the wish is to obtain a more naturalistic understanding of an issue? The former is ideally studied using a controlled experimental design, whereas the latter is more appropriately studied using a case study design.
Case studies may be approached in different ways depending on the epistemological standpoint of the researcher, that is, whether they take a critical (questioning one's own and others' assumptions), interpretivist (trying to understand individual and shared social meanings) or positivist approach (orientating towards the criteria of natural sciences, such as focusing on generalisability considerations) (Table 6 ). Whilst such a schema can be conceptually helpful, it may be appropriate to draw on more than one approach in any case study, particularly in the context of conducting health services research. Doolin has, for example, noted that in the context of undertaking interpretative case studies, researchers can usefully draw on a critical, reflective perspective which seeks to take into account the wider social and political environment that has shaped the case[ 11 ].
How are case studies conducted?
Here, we focus on the main stages of research activity when planning and undertaking a case study; the crucial stages are: defining the case; selecting the case(s); collecting and analysing the data; interpreting data; and reporting the findings.
Defining the case
Carefully formulated research question(s), informed by the existing literature and a prior appreciation of the theoretical issues and setting(s), are all important in appropriately and succinctly defining the case[ 8 , 12 ]. Crucially, each case should have a pre-defined boundary which clarifies the nature and time period covered by the case study (i.e. its scope, beginning and end), the relevant social group, organisation or geographical area of interest to the investigator, the types of evidence to be collected, and the priorities for data collection and analysis (see Table 7 )[ 1 ]. A theory driven approach to defining the case may help generate knowledge that is potentially transferable to a range of clinical contexts and behaviours; using theory is also likely to result in a more informed appreciation of, for example, how and why interventions have succeeded or failed[ 13 ].
For example, in our evaluation of the introduction of electronic health records in English hospitals (Table 3 ), we defined our cases as the NHS Trusts that were receiving the new technology[ 5 ]. Our focus was on how the technology was being implemented. However, if the primary research interest had been on the social and organisational dimensions of implementation, we might have defined our case differently as a grouping of healthcare professionals (e.g. doctors and/or nurses). The precise beginning and end of the case may however prove difficult to define. Pursuing this same example, when does the process of implementation and adoption of an electronic health record system really begin or end? Such judgements will inevitably be influenced by a range of factors, including the research question, theory of interest, the scope and richness of the gathered data and the resources available to the research team.
Selecting the case(s)
The decision on how to select the case(s) to study is a very important one that merits some reflection. In an intrinsic case study, the case is selected on its own merits[ 8 ]. The case is selected not because it is representative of other cases, but because of its uniqueness, which is of genuine interest to the researchers. This was, for example, the case in our study of the recruitment of minority ethnic participants into asthma research (Table 1 ) as our earlier work had demonstrated the marginalisation of minority ethnic people with asthma, despite evidence of disproportionate asthma morbidity[ 14 , 15 ]. In another example of an intrinsic case study, Hellstrom et al.[ 16 ] studied an elderly married couple living with dementia to explore how dementia had impacted on their understanding of home, their everyday life and their relationships.
For an instrumental case study, selecting a "typical" case can work well[ 8 ]. In contrast to the intrinsic case study, the particular case which is chosen is of less importance than selecting a case that allows the researcher to investigate an issue or phenomenon. For example, in order to gain an understanding of doctors' responses to health policy initiatives, Som undertook an instrumental case study interviewing clinicians who had a range of responsibilities for clinical governance in one NHS acute hospital trust[ 17 ]. Sampling a "deviant" or "atypical" case may however prove even more informative, potentially enabling the researcher to identify causal processes, generate hypotheses and develop theory.
In collective or multiple case studies, a number of cases are carefully selected. This offers the advantage of allowing comparisons to be made across several cases and/or replication. Choosing a "typical" case may enable the findings to be generalised to theory (i.e. analytical generalisation) or to test theory by replicating the findings in a second or even a third case (i.e. replication logic)[ 1 ]. Yin suggests two or three literal replications (i.e. predicting similar results) if the theory is straightforward and five or more if the theory is more subtle. However, critics might argue that selecting 'cases' in this way is insufficiently reflexive and ill-suited to the complexities of contemporary healthcare organisations.
The selected case study site(s) should allow the research team access to the group of individuals, the organisation, the processes or whatever else constitutes the chosen unit of analysis for the study. Access is therefore a central consideration; the researcher needs to come to know the case study site(s) well and to work cooperatively with them. Selected cases need to be not only interesting but also hospitable to the inquiry [ 8 ] if they are to be informative and answer the research question(s). Case study sites may also be pre-selected for the researcher, with decisions being influenced by key stakeholders. For example, our selection of case study sites in the evaluation of the implementation and adoption of electronic health record systems (see Table 3 ) was heavily influenced by NHS Connecting for Health, the government agency that was responsible for overseeing the National Programme for Information Technology (NPfIT)[ 5 ]. This prominent stakeholder had already selected the NHS sites (through a competitive bidding process) to be early adopters of the electronic health record systems and had negotiated contracts that detailed the deployment timelines.
It is also important to consider in advance the likely burden and risks associated with participation for those who (or the site(s) which) comprise the case study. Of particular importance is the obligation for the researcher to think through the ethical implications of the study (e.g. the risk of inadvertently breaching anonymity or confidentiality) and to ensure that potential participants/participating sites are provided with sufficient information to make an informed choice about joining the study. The outcome of providing this information might be that the emotive burden associated with participation, or the organisational disruption associated with supporting the fieldwork, is considered so high that the individuals or sites decide against participation.
In our example of evaluating implementations of electronic health record systems, given the restricted number of early adopter sites available to us, we sought purposively to select a diverse range of implementation cases among those that were available[ 5 ]. We chose a mixture of teaching, non-teaching and Foundation Trust hospitals, and examples of each of the three electronic health record systems procured centrally by the NPfIT. At one recruited site, it quickly became apparent that access was problematic because of competing demands on that organisation. Recognising the importance of full access and co-operative working for generating rich data, the research team decided not to pursue work at that site and instead to focus on other recruited sites.
Collecting the data
In order to develop a thorough understanding of the case, the case study approach usually involves the collection of multiple sources of evidence, using a range of quantitative (e.g. questionnaires, audits and analysis of routinely collected healthcare data) and more commonly qualitative techniques (e.g. interviews, focus groups and observations). The use of multiple sources of data (data triangulation) has been advocated as a way of increasing the internal validity of a study (i.e. the extent to which the method is appropriate to answer the research question)[ 8 , 18 – 21 ]. An underlying assumption is that data collected in different ways should lead to similar conclusions, and approaching the same issue from different angles can help develop a holistic picture of the phenomenon (Table 2 )[ 4 ].
Brazier and colleagues used a mixed-methods case study approach to investigate the impact of a cancer care programme[ 22 ]. Here, quantitative measures were collected with questionnaires before, and five months after, the start of the intervention which did not yield any statistically significant results. Qualitative interviews with patients however helped provide an insight into potentially beneficial process-related aspects of the programme, such as greater, perceived patient involvement in care. The authors reported how this case study approach provided a number of contextual factors likely to influence the effectiveness of the intervention and which were not likely to have been obtained from quantitative methods alone.
In collective or multiple case studies, data collection needs to be flexible enough to allow a detailed description of each individual case to be developed (e.g. the nature of different cancer care programmes), before considering the emerging similarities and differences in cross-case comparisons (e.g. to explore why one programme is more effective than another). It is important that data sources from different cases are, where possible, broadly comparable for this purpose even though they may vary in nature and depth.
Analysing, interpreting and reporting case studies
Making sense and offering a coherent interpretation of the typically disparate sources of data (whether qualitative alone or together with quantitative) is far from straightforward. Repeated reviewing and sorting of the voluminous and detail-rich data are integral to the process of analysis. In collective case studies, it is helpful to analyse data relating to the individual component cases first, before making comparisons across cases. Attention needs to be paid to variations within each case and, where relevant, the relationship between different causes, effects and outcomes[ 23 ]. Data will need to be organised and coded to allow the key issues, both derived from the literature and emerging from the dataset, to be easily retrieved at a later stage. An initial coding frame can help capture these issues and can be applied systematically to the whole dataset with the aid of a qualitative data analysis software package.
The Framework approach is a practical approach, comprising of five stages (familiarisation; identifying a thematic framework; indexing; charting; mapping and interpretation) , to managing and analysing large datasets particularly if time is limited, as was the case in our study of recruitment of South Asians into asthma research (Table 1 )[ 3 , 24 ]. Theoretical frameworks may also play an important role in integrating different sources of data and examining emerging themes. For example, we drew on a socio-technical framework to help explain the connections between different elements - technology; people; and the organisational settings within which they worked - in our study of the introduction of electronic health record systems (Table 3 )[ 5 ]. Our study of patient safety in undergraduate curricula drew on an evaluation-based approach to design and analysis, which emphasised the importance of the academic, organisational and practice contexts through which students learn (Table 4 )[ 6 ].
Case study findings can have implications both for theory development and theory testing. They may establish, strengthen or weaken historical explanations of a case and, in certain circumstances, allow theoretical (as opposed to statistical) generalisation beyond the particular cases studied[ 12 ]. These theoretical lenses should not, however, constitute a strait-jacket and the cases should not be "forced to fit" the particular theoretical framework that is being employed.
When reporting findings, it is important to provide the reader with enough contextual information to understand the processes that were followed and how the conclusions were reached. In a collective case study, researchers may choose to present the findings from individual cases separately before amalgamating across cases. Care must be taken to ensure the anonymity of both case sites and individual participants (if agreed in advance) by allocating appropriate codes or withholding descriptors. In the example given in Table 3 , we decided against providing detailed information on the NHS sites and individual participants in order to avoid the risk of inadvertent disclosure of identities[ 5 , 25 ].
What are the potential pitfalls and how can these be avoided?
The case study approach is, as with all research, not without its limitations. When investigating the formal and informal ways undergraduate students learn about patient safety (Table 4 ), for example, we rapidly accumulated a large quantity of data. The volume of data, together with the time restrictions in place, impacted on the depth of analysis that was possible within the available resources. This highlights a more general point of the importance of avoiding the temptation to collect as much data as possible; adequate time also needs to be set aside for data analysis and interpretation of what are often highly complex datasets.
Case study research has sometimes been criticised for lacking scientific rigour and providing little basis for generalisation (i.e. producing findings that may be transferable to other settings)[ 1 ]. There are several ways to address these concerns, including: the use of theoretical sampling (i.e. drawing on a particular conceptual framework); respondent validation (i.e. participants checking emerging findings and the researcher's interpretation, and providing an opinion as to whether they feel these are accurate); and transparency throughout the research process (see Table 8 )[ 8 , 18 – 21 , 23 , 26 ]. Transparency can be achieved by describing in detail the steps involved in case selection, data collection, the reasons for the particular methods chosen, and the researcher's background and level of involvement (i.e. being explicit about how the researcher has influenced data collection and interpretation). Seeking potential, alternative explanations, and being explicit about how interpretations and conclusions were reached, help readers to judge the trustworthiness of the case study report. Stake provides a critique checklist for a case study report (Table 9 )[ 8 ].
Conclusions
The case study approach allows, amongst other things, critical events, interventions, policy developments and programme-based service reforms to be studied in detail in a real-life context. It should therefore be considered when an experimental design is either inappropriate to answer the research questions posed or impossible to undertake. Considering the frequency with which implementations of innovations are now taking place in healthcare settings and how well the case study approach lends itself to in-depth, complex health service research, we believe this approach should be more widely considered by researchers. Though inherently challenging, the research case study can, if carefully conceptualised and thoughtfully undertaken and reported, yield powerful insights into many important aspects of health and healthcare delivery.
Yin RK: Case study research, design and method. 2009, London: Sage Publications Ltd., 4
Google Scholar
Keen J, Packwood T: Qualitative research; case study evaluation. BMJ. 1995, 311: 444-446.
Article CAS PubMed PubMed Central Google Scholar
Sheikh A, Halani L, Bhopal R, Netuveli G, Partridge M, Car J, et al: Facilitating the Recruitment of Minority Ethnic People into Research: Qualitative Case Study of South Asians and Asthma. PLoS Med. 2009, 6 (10): 1-11.
Article Google Scholar
Pinnock H, Huby G, Powell A, Kielmann T, Price D, Williams S, et al: The process of planning, development and implementation of a General Practitioner with a Special Interest service in Primary Care Organisations in England and Wales: a comparative prospective case study. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). 2008, [ http://www.sdo.nihr.ac.uk/files/project/99-final-report.pdf ]
Robertson A, Cresswell K, Takian A, Petrakaki D, Crowe S, Cornford T, et al: Prospective evaluation of the implementation and adoption of NHS Connecting for Health's national electronic health record in secondary care in England: interim findings. BMJ. 2010, 41: c4564-
Pearson P, Steven A, Howe A, Sheikh A, Ashcroft D, Smith P, the Patient Safety Education Study Group: Learning about patient safety: organisational context and culture in the education of healthcare professionals. J Health Serv Res Policy. 2010, 15: 4-10. 10.1258/jhsrp.2009.009052.
Article PubMed Google Scholar
van Harten WH, Casparie TF, Fisscher OA: The evaluation of the introduction of a quality management system: a process-oriented case study in a large rehabilitation hospital. Health Policy. 2002, 60 (1): 17-37. 10.1016/S0168-8510(01)00187-7.
Stake RE: The art of case study research. 1995, London: Sage Publications Ltd.
Sheikh A, Smeeth L, Ashcroft R: Randomised controlled trials in primary care: scope and application. Br J Gen Pract. 2002, 52 (482): 746-51.
PubMed PubMed Central Google Scholar
King G, Keohane R, Verba S: Designing Social Inquiry. 1996, Princeton: Princeton University Press
Doolin B: Information technology as disciplinary technology: being critical in interpretative research on information systems. Journal of Information Technology. 1998, 13: 301-311. 10.1057/jit.1998.8.
George AL, Bennett A: Case studies and theory development in the social sciences. 2005, Cambridge, MA: MIT Press
Eccles M, the Improved Clinical Effectiveness through Behavioural Research Group (ICEBeRG): Designing theoretically-informed implementation interventions. Implementation Science. 2006, 1: 1-8. 10.1186/1748-5908-1-1.
Article PubMed Central Google Scholar
Netuveli G, Hurwitz B, Levy M, Fletcher M, Barnes G, Durham SR, Sheikh A: Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005, 365 (9456): 312-7.
Sheikh A, Panesar SS, Lasserson T, Netuveli G: Recruitment of ethnic minorities to asthma studies. Thorax. 2004, 59 (7): 634-
CAS PubMed PubMed Central Google Scholar
Hellström I, Nolan M, Lundh U: 'We do things together': A case study of 'couplehood' in dementia. Dementia. 2005, 4: 7-22. 10.1177/1471301205049188.
Som CV: Nothing seems to have changed, nothing seems to be changing and perhaps nothing will change in the NHS: doctors' response to clinical governance. International Journal of Public Sector Management. 2005, 18: 463-477. 10.1108/09513550510608903.
Lincoln Y, Guba E: Naturalistic inquiry. 1985, Newbury Park: Sage Publications
Barbour RS: Checklists for improving rigour in qualitative research: a case of the tail wagging the dog?. BMJ. 2001, 322: 1115-1117. 10.1136/bmj.322.7294.1115.
Mays N, Pope C: Qualitative research in health care: Assessing quality in qualitative research. BMJ. 2000, 320: 50-52. 10.1136/bmj.320.7226.50.
Mason J: Qualitative researching. 2002, London: Sage
Brazier A, Cooke K, Moravan V: Using Mixed Methods for Evaluating an Integrative Approach to Cancer Care: A Case Study. Integr Cancer Ther. 2008, 7: 5-17. 10.1177/1534735407313395.
Miles MB, Huberman M: Qualitative data analysis: an expanded sourcebook. 1994, CA: Sage Publications Inc., 2
Pope C, Ziebland S, Mays N: Analysing qualitative data. Qualitative research in health care. BMJ. 2000, 320: 114-116. 10.1136/bmj.320.7227.114.
Cresswell KM, Worth A, Sheikh A: Actor-Network Theory and its role in understanding the implementation of information technology developments in healthcare. BMC Med Inform Decis Mak. 2010, 10 (1): 67-10.1186/1472-6947-10-67.
Article PubMed PubMed Central Google Scholar
Malterud K: Qualitative research: standards, challenges, and guidelines. Lancet. 2001, 358: 483-488. 10.1016/S0140-6736(01)05627-6.
Article CAS PubMed Google Scholar
Yin R: Case study research: design and methods. 1994, Thousand Oaks, CA: Sage Publishing, 2
Yin R: Enhancing the quality of case studies in health services research. Health Serv Res. 1999, 34: 1209-1224.
Green J, Thorogood N: Qualitative methods for health research. 2009, Los Angeles: Sage, 2
Howcroft D, Trauth E: Handbook of Critical Information Systems Research, Theory and Application. 2005, Cheltenham, UK: Northampton, MA, USA: Edward Elgar
Book Google Scholar
Blakie N: Approaches to Social Enquiry. 1993, Cambridge: Polity Press
Doolin B: Power and resistance in the implementation of a medical management information system. Info Systems J. 2004, 14: 343-362. 10.1111/j.1365-2575.2004.00176.x.
Bloomfield BP, Best A: Management consultants: systems development, power and the translation of problems. Sociological Review. 1992, 40: 533-560.
Shanks G, Parr A: Positivist, single case study research in information systems: A critical analysis. Proceedings of the European Conference on Information Systems. 2003, Naples
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2288/11/100/prepub
Download references
Acknowledgements
We are grateful to the participants and colleagues who contributed to the individual case studies that we have drawn on. This work received no direct funding, but it has been informed by projects funded by Asthma UK, the NHS Service Delivery Organisation, NHS Connecting for Health Evaluation Programme, and Patient Safety Research Portfolio. We would also like to thank the expert reviewers for their insightful and constructive feedback. Our thanks are also due to Dr. Allison Worth who commented on an earlier draft of this manuscript.
Author information
Authors and affiliations.
Division of Primary Care, The University of Nottingham, Nottingham, UK
Sarah Crowe & Anthony Avery
Centre for Population Health Sciences, The University of Edinburgh, Edinburgh, UK
Kathrin Cresswell, Ann Robertson & Aziz Sheikh
School of Health in Social Science, The University of Edinburgh, Edinburgh, UK
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sarah Crowe .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
AS conceived this article. SC, KC and AR wrote this paper with GH, AA and AS all commenting on various drafts. SC and AS are guarantors.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Crowe, S., Cresswell, K., Robertson, A. et al. The case study approach. BMC Med Res Methodol 11 , 100 (2011). https://doi.org/10.1186/1471-2288-11-100
Download citation
Received : 29 November 2010
Accepted : 27 June 2011
Published : 27 June 2011
DOI : https://doi.org/10.1186/1471-2288-11-100
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Case Study Approach
- Electronic Health Record System
- Case Study Design
- Case Study Site
- Case Study Report
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
How to make an oral case presentation to healthcare colleagues
The content and delivery of a patient case for education and evidence-based care discussions in clinical practice.

BSIP SA / Alamy Stock Photo
A case presentation is a detailed narrative describing a specific problem experienced by one or more patients. Pharmacists usually focus on the medicines aspect , for example, where there is potential harm to a patient or proven benefit to the patient from medication, or where a medication error has occurred. Case presentations can be used as a pedagogical tool, as a method of appraising the presenter’s knowledge and as an opportunity for presenters to reflect on their clinical practice [1] .
The aim of an oral presentation is to disseminate information about a patient for the purpose of education, to update other members of the healthcare team on a patient’s progress, and to ensure the best, evidence-based care is being considered for their management.
Within a hospital, pharmacists are likely to present patients on a teaching or daily ward round or to a senior pharmacist or colleague for the purpose of asking advice on, for example, treatment options or complex drug-drug interactions, or for referral.
Content of a case presentation
As a general structure, an oral case presentation may be divided into three phases [2] :
- Reporting important patient information and clinical data;
- Analysing and synthesising identified issues (this is likely to include producing a list of these issues, generally termed a problem list);
- Managing the case by developing a therapeutic plan.

Specifically, the following information should be included [3] :
Patient and complaint details
Patient details: name, sex, age, ethnicity.
Presenting complaint: the reason the patient presented to the hospital (symptom/event).
History of presenting complaint: highlighting relevant events in chronological order, often presented as how many days ago they occurred. This should include prior admission to hospital for the same complaint.
Review of organ systems: listing positive or negative findings found from the doctor’s assessment that are relevant to the presenting complaint.
Past medical and surgical history
Social history: including occupation, exposures, smoking and alcohol history, and any recreational drug use.
Medication history, including any drug allergies: this should include any prescribed medicines, medicines purchased over-the-counter, any topical preparations used (including eye drops, nose drops, inhalers and nasal sprays) and any herbal or traditional remedies taken.
Sexual history: if this is relevant to the presenting complaint.
Details from a physical examination: this includes any relevant findings to the presenting complaint and should include relevant observations.
Laboratory investigation and imaging results: abnormal findings are presented.
Assessment: including differential diagnosis.
Plan: including any pharmaceutical care issues raised and how these should be resolved, ongoing management and discharge planning.
Any discrepancies between the current management of the patient’s conditions and evidence-based recommendations should be highlighted and reasons given for not adhering to evidence-based medicine ( see ‘Locating the evidence’ ).
Locating the evidence
The evidence base for the therapeutic options available should always be considered. There may be local guidance available within the hospital trust directing the management of the patient’s presenting condition. Pharmacists often contribute to the development of such guidelines, especially if medication is involved. If no local guidelines are available, the next step is to refer to national guidance. This is developed by a steering group of experts, for example, the British HIV Association or the National Institute for Health and Care Excellence . If the presenting condition is unusual or rare, for example, acute porphyria, and there are no local or national guidelines available, a literature search may help locate articles or case studies similar to the case.

Giving a case presentation
Currently, there are no available acknowledged guidelines or systematic descriptions of the structure, language and function of the oral case presentation [4] and therefore there is no standard on how the skills required to prepare or present a case are taught. Most individuals are introduced to this concept at undergraduate level and then build on their skills through practice-based learning.
A case presentation is a narrative of a patient’s care, so it is vital the presenter has familiarity with the patient, the case and its progression. The preparation for the presentation will depend on what information is to be included.
Generally, oral case presentations are brief and should be limited to 5–10 minutes. This may be extended if the case is being presented as part of an assessment compared with routine everyday working ( see ‘Case-based discussion’ ). The audience should be interested in what is being said so the presenter should maintain this engagement through eye contact, clear speech and enthusiasm for the case.
It is important to stick to the facts by presenting the case as a factual timeline and not describing how things should have happened instead. Importantly, the case should always be concluded and should include an outcome of the patient’s care [5] .
An example of an oral case presentation, given by a pharmacist to a doctor, is available here .
A successful oral case presentation allows the audience to garner the right amount of patient information in the most efficient way, enabling a clinically appropriate plan to be developed. The challenge lies with the fact that the content and delivery of this will vary depending on the service, and clinical and audience setting [3] . A practitioner with less experience may find understanding the balance between sufficient information and efficiency of communication difficult, but regular use of the oral case presentation tool will improve this skill.
Tailoring case presentations to your audience
Most case presentations are not tailored to a specific audience because the same type of information will usually need to be conveyed in each case.
However, case presentations can be adapted to meet the identified learning needs of the target audience, if required for training purposes. This method involves varying the content of the presentation or choosing specific cases to present that will help achieve a set of objectives [6] . For example, if a requirement to learn about the management of acute myocardial infarction has been identified by the target audience, then the presenter may identify a case from the cardiology ward to present to the group, as opposed to presenting a patient reviewed by that person during their normal working practice.
Alternatively, a presenter could focus on a particular condition within a case, which will dictate what information is included. For example, if a case on asthma is being presented, the focus may be on recent use of bronchodilator therapy, respiratory function tests (including peak expiratory flow rate), symptoms related to exacerbation of airways disease, anxiety levels, ability to talk in full sentences, triggers to worsening of symptoms, and recent exposure to allergens. These may not be considered relevant if presenting the case on an unrelated condition that the same patient has, for example, if this patient was admitted with a hip fracture and their asthma was well controlled.
Case-based discussion
The oral case presentation may also act as the basis of workplace-based assessment in the form of a case-based discussion. In the UK, this forms part of many healthcare professional bodies’ assessment of clinical practice, for example, medical professional colleges.
For pharmacists, a case-based discussion forms part of the Royal Pharmaceutical Society (RPS) Foundation and Advanced Practice assessments . Mastery of the oral case presentation skill could provide useful preparation for this assessment process.
A case-based discussion would include a pharmaceutical needs assessment, which involves identifying and prioritising pharmaceutical problems for a particular patient. Evidence-based guidelines relevant to the specific medical condition should be used to make treatment recommendations, and a plan to monitor the patient once therapy has started should be developed. Professionalism is an important aspect of case-based discussion — issues must be prioritised appropriately and ethical and legal frameworks must be referred to [7] . A case-based discussion would include broadly similar content to the oral case presentation, but would involve further questioning of the presenter by the assessor to determine the extent of the presenter’s knowledge of the specific case, condition and therapeutic strategies. The criteria used for assessment would depend on the level of practice of the presenter but, for pharmacists, this may include assessment against the RPS Foundation or Pharmacy Frameworks .
Acknowledgement
With thanks to Aamer Safdar for providing the script for the audio case presentation.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal . You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
[1] Onishi H. The role of case presentation for teaching and learning activities. Kaohsiung J Med Sci 2008;24:356–360. doi: 10.1016/s1607-551x(08)70132–3
[2] Edwards JC, Brannan JR, Burgess L et al . Case presentation format and clinical reasoning: a strategy for teaching medical students. Medical Teacher 1987;9:285–292. doi: 10.3109/01421598709034790
[3] Goldberg C. A practical guide to clinical medicine: overview and general information about oral presentation. 2009. University of California, San Diego. Available from: https://meded.ecsd.edu/clinicalmed.oral.htm (accessed 5 December 2015)
[4] Chan MY. The oral case presentation: toward a performance-based rhetorical model for teaching and learning. Medical Education Online 2015;20. doi: 10.3402/meo.v20.28565
[5] McGee S. Medicine student programs: oral presentation guidelines. Learning & Scholarly Technologies, University of Washington. Available from: https://catalyst.uw.edu/workspace/medsp/30311/202905 (accessed 7 December 2015)
[6] Hays R. Teaching and Learning in Clinical Settings. 2006;425. Oxford: Radcliffe Publishing Ltd.
[7] Royal Pharmaceutical Society. Tips for assessors for completing case-based discussions. 2015. Available from: http://www.rpharms.com/help/case_based_discussion.htm (accessed 30 December 2015)
You might also be interested in…

How to demonstrate empathy and compassion in a pharmacy setting

Be more proactive to convince medics, pharmacists urged

How pharmacists can encourage patient adherence to medicines
A step-by-step guide to causal study design using real-world data
- Open access
- Published: 19 June 2024
Cite this article
You have full access to this open access article

- Sarah Ruth Hoffman 1 ,
- Nilesh Gangan 1 ,
- Xiaoxue Chen 2 ,
- Joseph L. Smith 1 ,
- Arlene Tave 1 ,
- Yiling Yang 1 ,
- Christopher L. Crowe 1 ,
- Susan dosReis 3 &
- Michael Grabner 1
319 Accesses
Explore all metrics
Due to the need for generalizable and rapidly delivered evidence to inform healthcare decision-making, real-world data have grown increasingly important to answer causal questions. However, causal inference using observational data poses numerous challenges, and relevant methodological literature is vast. We endeavored to identify underlying unifying themes of causal inference using real-world healthcare data and connect them into a single schema to aid in observational study design, and to demonstrate this schema using a previously published research example. A multidisciplinary team (epidemiology, biostatistics, health economics) reviewed the literature related to causal inference and observational data to identify key concepts. A visual guide to causal study design was developed to concisely and clearly illustrate how the concepts are conceptually related to one another. A case study was selected to demonstrate an application of the guide. An eight-step guide to causal study design was created, integrating essential concepts from the literature, anchored into conceptual groupings according to natural steps in the study design process. The steps include defining the causal research question and the estimand; creating a directed acyclic graph; identifying biases and design and analytic techniques to mitigate their effect, and techniques to examine the robustness of findings. The cardiovascular case study demonstrates the applicability of the steps to developing a research plan. This paper used an existing study to demonstrate the relevance of the guide. We encourage researchers to incorporate this guide at the study design stage in order to elevate the quality of future real-world evidence.
Similar content being viewed by others

Examples of Applying Causal-Inference Roadmap to Real-World Studies

Selection Mechanisms and Their Consequences: Understanding and Addressing Selection Bias
Assessing causality in epidemiology: revisiting Bradford Hill to incorporate developments in causal thinking
Avoid common mistakes on your manuscript.
1 Introduction
Approximately 50 new drugs are approved each year in the United States (Mullard 2022 ). For all new drugs, randomized controlled trials (RCTs) are the gold-standard by which potential effectiveness (“efficacy”) and safety are established. However, RCTs cannot guarantee how a drug will perform in a less controlled context. For this reason, regulators frequently require observational, post-approval studies using “real-world” data, sometimes even as a condition of drug approval. The “real-world” data requested by regulators is often derived from insurance claims databases and/or healthcare records. Importantly, these data are recorded during routine clinical care without concern for potential use in research. Yet, in recent years, there has been increasing use of such data for causal inference and regulatory decision making, presenting a variety of methodologic challenges for researchers and stakeholders to consider (Arlett et al. 2022 ; Berger et al. 2017 ; Concato and ElZarrad 2022 ; Cox et al. 2009 ; European Medicines Agency 2023 ; Franklin and Schneeweiss 2017 ; Girman et al. 2014 ; Hernán and Robins 2016 ; International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2022 ; International Society for Pharmacoepidemiology (ISPE) 2020 ; Stuart et al. 2013 ; U.S. Food and Drug Administration 2018 ; Velentgas et al. 2013 ).
Current guidance for causal inference using observational healthcare data articulates the need for careful study design (Berger et al. 2017 ; Cox et al. 2009 ; European Medicines Agency 2023 ; Girman et al. 2014 ; Hernán and Robins 2016 ; Stuart et al. 2013 ; Velentgas et al. 2013 ). In 2009, Cox et al. described common sources of bias in observational data and recommended specific strategies to mitigate these biases (Cox et al. 2009 ). In 2013, Stuart et al. emphasized counterfactual theory and trial emulation, offered several approaches to address unmeasured confounding, and provided guidance on the use of propensity scores to balance confounding covariates (Stuart et al. 2013 ). In 2013, the Agency for Healthcare Research and Quality (AHRQ) released an extensive, 200-page guide to developing a protocol for comparative effectiveness research using observational data (Velentgas et al. 2013 ). The guide emphasized development of the research question, with additional chapters on study design, comparator selection, sensitivity analyses, and directed acyclic graphs (Velentgas et al. 2013 ). In 2014, Girman et al. provided a clear set of steps for assessing study feasibility including examination of the appropriateness of the data for the research question (i.e., ‘fit-for-purpose’), empirical equipoise, and interpretability, stating that comparative effectiveness research using observational data “should be designed with the goal of drawing a causal inference” (Girman et al. 2014 ). In 2017 , Berger et al. described aspects of “study hygiene,” focusing on procedural practices to enhance confidence in, and credibility of, real-world data studies (Berger et al. 2017 ). Currently, the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) maintains a guide on methodological standards in pharmacoepidemiology which discusses causal inference using observational data and includes an overview of study designs, a chapter on methods to address bias and confounding, and guidance on writing statistical analysis plans (European Medicines Agency 2023 ). In addition to these resources, the “target trial framework” provides a structured approach to planning studies for causal inferences from observational databases (Hernán and Robins 2016 ; Wang et al. 2023b ). This framework, published in 2016, encourages researchers to first imagine a clinical trial for the study question of interest and then to subsequently design the observational study to reflect the hypothetical trial (Hernán and Robins 2016 ).
While the literature addresses critical issues collectively, there remains a need for a framework that puts key components, including the target trial approach, into a simple, overarching schema (Loveless 2022 ) so they can be more easily remembered, and communicated to all stakeholders including (new) researchers, peer-reviewers, and other users of the research findings (e.g., practicing providers, professional clinical societies, regulators). For this reason, we created a step-by-step guide for causal inference using administrative health data, which aims to integrate these various best practices at a high level and complements existing, more specific guidance, including those from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and the International Society for Pharmacoepidemiology (ISPE) (Berger et al. 2017 ; Cox et al. 2009 ; Girman et al. 2014 ). We demonstrate the application of this schema using a previously published paper in cardiovascular research.
This work involved a formative phase and an implementation phase to evaluate the utility of the causal guide. In the formative phase, a multidisciplinary team with research expertise in epidemiology, biostatistics, and health economics reviewed selected literature (peer-reviewed publications, including those mentioned in the introduction, as well as graduate-level textbooks) related to causal inference and observational healthcare data from the pharmacoepidemiologic and pharmacoeconomic perspectives. The potential outcomes framework served as the foundation for our conception of causal inference (Rubin 2005 ). Information was grouped into the following four concepts: (1) Defining the Research Question; (2) Defining the Estimand; (3) Identifying and Mitigating Biases; (4) Sensitivity Analysis. A step-by-step guide to causal study design was developed to distill the essential elements of each concept, organizing them into a single schema so that the concepts are clearly related to one another. References for each step of the schema are included in the Supplemental Table.
In the implementation phase we tested the application of the causal guide to previously published work (Dondo et al. 2017 ). The previously published work utilized data from the Myocardial Ischaemia National Audit Project (MINAP), the United Kingdom’s national heart attack register. The goal of the study was to assess the effect of β-blockers on all-cause mortality among patients hospitalized for acute myocardial infarction without heart failure or left ventricular systolic dysfunction. We selected this paper for the case study because of its clear descriptions of the research goal and methods, and the explicit and methodical consideration of potential biases and use of sensitivity analyses to examine the robustness of the main findings.
3.1 Overview of the eight steps
The step-by-step guide to causal inference comprises eight distinct steps (Fig. 1 ) across the four concepts. As scientific inquiry and study design are iterative processes, the various steps may be completed in a different order than shown, and steps may be revisited.
A step-by-step guide for causal study design
Abbreviations: GEE: generalized estimating equations; IPC/TW: inverse probability of censoring/treatment weighting; ITR: individual treatment response; MSM: marginal structural model; TE: treatment effect
Please refer to the Supplemental Table for references providing more in-depth information.
1 Ensure that the exposure and outcome are well-defined based on literature and expert opinion.
2 More specifically, measures of association are not affected by issues such as confounding and selection bias because they do not intend to isolate and quantify a single causal pathway. However, information bias (e.g., variable misclassification) can negatively affect association estimates, and association estimates remain subject to random variability (and are hence reported with confidence intervals).
3 This list is not exhaustive; it focuses on frequently encountered biases.
4 To assess bias in a nonrandomized study following the target trial framework, use of the ROBINS-I tool is recommended ( https://www.bmj.com/content/355/bmj.i4919 ).
5 Only a selection of the most popular approaches is presented here. Other methods exist; e.g., g-computation and g-estimation for both time-invariant and time-varying analysis; instrumental variables; and doubly-robust estimation methods. There are also program evaluation methods (e.g., difference-in-differences, regression discontinuities) that can be applied to pharmacoepidemiologic questions. Conventional outcome regression analysis is not recommended for causal estimation due to issues determining covariate balance, correct model specification, and interpretability of effect estimates.
6 Online tools include, among others, an E-value calculator for unmeasured confounding ( https://www.evalue-calculator.com /) and the P95 outcome misclassification estimator ( http://apps.p-95.com/ISPE /).
3.2 Defining the Research question (step 1)
The process of designing a study begins with defining the research question. Research questions typically center on whether a causal relationship exists between an exposure and an outcome. This contrasts with associative questions, which, by their nature, do not require causal study design elements because they do not attempt to isolate a causal pathway from a single exposure to an outcome under study. It is important to note that the phrasing of the question itself should clarify whether an association or a causal relationship is of interest. The study question “Does statin use reduce the risk of future cardiovascular events?” is explicitly causal and requires that the study design addresses biases such as confounding. In contrast, the study question “Is statin use associated with a reduced risk of future cardiovascular events?” can be answered without control of confounding since the word “association” implies correlation. Too often, however, researchers use the word “association” to describe their findings when their methods were created to address explicitly causal questions (Hernán 2018 ). For example, a study that uses propensity score-based methods to balance risk factors between treatment groups is explicitly attempting to isolate a causal pathway by removing confounding factors. This is different from a study that intends only to measure an association. In fact, some journals may require that the word “association” be used when causal language would be more appropriate; however, this is beginning to change (Flanagin et al. 2024 ).
3.3 Defining the estimand (steps 2, 3, 4)
The estimand is the causal effect of research interest and is described in terms of required design elements: the target population for the counterfactual contrast, the kind of effect, and the effect/outcome measure.
In Step 2, the study team determines the target population of interest, which depends on the research question of interest. For example, we may want to estimate the effect of the treatment in the entire study population, i.e., the hypothetical contrast between all study patients taking the drug of interest versus all study patients taking the comparator (the average treatment effect; ATE). Other effects can be examined, including the average treatment effect in the treated or untreated (ATT or ATU).When covariate distributions are the same across the treated and untreated populations and there is no effect modification by covariates, these effects are generally the same (Wang et al. 2017 ). In RCTs, this occurs naturally due to randomization, but in non-randomized data, careful study design and statistical methods must be used to mitigate confounding bias.
In Step 3, the study team decides whether to measure the intention-to-treat (ITT), per-protocol, or as-treated effect. The ITT approach is also known as “first-treatment-carried-forward” in the observational literature (Lund et al. 2015 ). In trials, the ITT measures the effect of treatment assignment rather than the treatment itself, and in observational data the ITT can be conceptualized as measuring the effect of treatment as started . To compute the ITT effect from observational data, patients are placed into the exposure group corresponding to the treatment that they initiate, and treatment switching or discontinuation are purposely ignored in the analysis. Alternatively, a per-protocol effect can be measured from observational data by classifying patients according to the treatment that they initiated but censoring them when they stop, switch, or otherwise change treatment (Danaei et al. 2013 ; Yang et al. 2014 ). Finally, “as-treated” effects are estimated from observational data by classifying patients according to their actual treatment exposure during follow-up, for example by using multiple time windows to measure exposure changes (Danaei et al. 2013 ; Yang et al. 2014 ).
Step 4 is the final step in specifying the estimand in which the research team determines the effect measure of interest. Answering this question has two parts. First, the team must consider how the outcome of interest will be measured. Risks, rates, hazards, odds, and costs are common ways of measuring outcomes, but each measure may be best suited to a particular scenario. For example, risks assume patients across comparison groups have equal follow-up time, while rates allow for variable follow-up time (Rothman et al. 2008 ). Costs may be of interest in studies focused on economic outcomes, including as inputs to cost-effectiveness analyses. After deciding how the outcome will be measured, it is necessary to consider whether the resulting quantity will be compared across groups using a ratio or a difference. Ratios convey the effect of exposure in a way that is easy to understand, but they do not provide an estimate of how many patients will be affected. On the other hand, differences provide a clearer estimate of the potential public health impact of exposure; for example, by allowing the calculation of the number of patients that must be treated to cause or prevent one instance of the outcome of interest (Tripepi et al. 2007 ).
3.4 Identifying and mitigating biases (steps 5, 6, 7)
Observational, real-world studies can be subject to multiple potential sources of bias, which can be grouped into confounding, selection, measurement, and time-related biases (Prada-Ramallal et al. 2019 ).
In Step 5, as a practical first approach in developing strategies to address threats to causal inference, researchers should create a visual mapping of factors that may be related to the exposure, outcome, or both (also called a directed acyclic graph or DAG) (Pearl 1995 ). While creating a high-quality DAG can be challenging, guidance is increasingly available to facilitate the process (Ferguson et al. 2020 ; Gatto et al. 2022 ; Hernán and Robins 2020 ; Rodrigues et al. 2022 ; Sauer 2013 ). The types of inter-variable relationships depicted by DAGs include confounders, colliders, and mediators. Confounders are variables that affect both exposure and outcome, and it is necessary to control for them in order to isolate the causal pathway of interest. Colliders represent variables affected by two other variables, such as exposure and outcome (Griffith et al. 2020 ). Colliders should not be conditioned on since by doing so, the association between exposure and outcome will become distorted. Mediators are variables that are affected by the exposure and go on to affect the outcome. As such, mediators are on the causal pathway between exposure and outcome and should also not be conditioned on, otherwise a path between exposure and outcome will be closed and the total effect of the exposure on the outcome cannot be estimated. Mediation analysis is a separate type of analysis aiming to distinguish between direct and indirect (mediated) effects between exposure and outcome and may be applied in certain cases (Richiardi et al. 2013 ). Overall, the process of creating a DAG can create valuable insights about the nature of the hypothesized underlying data generating process and the biases that are likely to be encountered (Digitale et al. 2022 ). Finally, an extension to DAGs which incorporates counterfactual theory is available in the form of Single World Intervention Graphs (SWIGs) as described in a 2013 primer (Richardson and Robins 2013 ).
In Step 6, researchers comprehensively assess the possibility of different types of bias in their study, above and beyond what the creation of the DAG reveals. Many potential biases have been identified and summarized in the literature (Berger et al. 2017 ; Cox et al. 2009 ; European Medicines Agency 2023 ; Girman et al. 2014 ; Stuart et al. 2013 ; Velentgas et al. 2013 ). Every study can be subject to one or more biases, each of which can be addressed using one or more methods. The study team should thoroughly and explicitly identify all possible biases with consideration for the specifics of the available data and the nuances of the population and health care system(s) from which the data arise. Once the potential biases are identified and listed, the team can consider potential solutions using a variety of study design and analytic techniques.
In Step 7, the study team considers solutions to the biases identified in Step 6. “Target trial” thinking serves as the basis for many of these solutions by requiring researchers to consider how observational studies can be designed to ensure comparison groups are similar and produce valid inferences by emulating RCTs (Labrecque and Swanson 2017 ; Wang et al. 2023b ). Designing studies to include only new users of a drug and an active comparator group is one way of increasing the similarity of patients across both groups, particularly in terms of treatment history. Careful consideration must be paid to the specification of the time periods and their relationship to inclusion/exclusion criteria (Suissa and Dell’Aniello 2020 ). For instance, if a drug is used intermittently, a longer wash-out period is needed to ensure adequate capture of prior use in order to avoid bias (Riis et al. 2015 ). The study team should consider how to approach confounding adjustment, and whether both time-invariant and time-varying confounding may be present. Many potential biases exist, and many methods have been developed to address them in order to improve causal estimation from observational data. Many of these methods, such as propensity score estimation, can be enhanced by machine learning (Athey and Imbens 2019 ; Belthangady et al. 2021 ; Mai et al. 2022 ; Onasanya et al. 2024 ; Schuler and Rose 2017 ; Westreich et al. 2010 ). Machine learning has many potential applications in the causal inference discipline, and like other tools, must be used with careful planning and intentionality. To aid in the assessment of potential biases, especially time-related ones, and the development of a plan to address them, the study design should be visualized (Gatto et al. 2022 ; Schneeweiss et al. 2019 ). Additionally, we note the opportunity for collaboration across research disciplines (e.g., the application of difference-in-difference methods (Zhou et al. 2016 ) to the estimation of comparative drug effectiveness and safety).
3.5 Quality Control & sensitivity analyses (step 8)
Causal study design concludes with Step 8, which includes planning quality control and sensitivity analyses to improve the internal validity of the study. Quality control begins with reviewing study output for prima facie validity. Patient characteristics (e.g., distributions of age, sex, region) should align with expected values from the researchers’ intuition and the literature, and researchers should assess reasons for any discrepancies. Sensitivity analyses should be conducted to determine the robustness of study findings. Researchers can test the stability of study estimates using a different estimand or type of model than was used in the primary analysis. Sensitivity analysis estimates that are similar to those of the primary analysis might confirm that the primary analysis estimates are appropriate. The research team may be interested in how changes to study inclusion/exclusion criteria may affect study findings or wish to address uncertainties related to measuring the exposure or outcome in the administrative data by modifying the algorithms used to identify exposure or outcome (e.g., requiring hospitalization with a diagnosis code in a principal position rather than counting any claim with the diagnosis code in any position). As feasible, existing validation studies for the exposure and outcome should be referenced, or new validation efforts undertaken. The results of such validation studies can inform study estimates via quantitative bias analyses (Lanes and Beachler 2023 ). The study team may also consider biases arising from unmeasured confounding and plan quantitative bias analyses to explore how unmeasured confounding may impact estimates. Quantitative bias analysis can assess the directionality, magnitude, and uncertainty of errors arising from a variety of limitations (Brenner and Gefeller 1993 ; Lash et al. 2009 , 2014 ; Leahy et al. 2022 ).
3.6 Illustration using a previously published research study
In order to demonstrate how the guide can be used to plan a research study utilizing causal methods, we turn to a previously published study (Dondo et al. 2017 ) that assessed the causal relationship between the use of 𝛽-blockers and mortality after acute myocardial infarction in patients without heart failure or left ventricular systolic dysfunction. The investigators sought to answer a causal research question (Step 1), and so we proceed to Step 2. Use (or no use) of 𝛽-blockers was determined after discharge without taking into consideration discontinuation or future treatment changes (i.e., intention-to-treat). Considering treatment for whom (Step 3), both ATE and ATT were evaluated. Since survival was the primary outcome, an absolute difference in survival time was chosen as the effect measure (Step 4). While there was no explicit directed acyclic graph provided, the investigators specified a list of confounders.
Robust methodologies were established by consideration of possible sources of biases and addressing them using viable solutions (Steps 6 and 7). Table 1 offers a list of the identified potential biases and their corresponding solutions as implemented. For example, to minimize potential biases including prevalent-user bias and selection bias, the sample was restricted to patients with no previous use of 𝛽-blockers, no contraindication for 𝛽-blockers, and no prescription of loop diuretics. To improve balance across the comparator groups in terms of baseline confounders, i.e., those that could influence both exposure (𝛽-blocker use) and outcome (mortality), propensity score-based inverse probability of treatment weighting (IPTW) was employed. However, we noted that the baseline look-back period to assess measured covariates was not explicitly listed in the paper.
Quality control and sensitivity analysis (Step 8) is described extensively. The overlap of propensity score distributions between comparator groups was tested and confounder balance was assessed. Since observations in the tail-end of the propensity score distribution may violate the positivity assumption (Crump et al. 2009 ), a sensitivity analysis was conducted including only cases within 0.1 to 0.9 of the propensity score distribution. While not mentioned by the authors, the PS tails can be influenced by unmeasured confounders (Sturmer et al. 2021 ), and the findings were robust with and without trimming. An assessment of extreme IPTW weights, while not included, would further help increase confidence in the robustness of the analysis. An instrumental variable approach was employed to assess potential selection bias due to unmeasured confounding, using hospital rates of guideline-indicated prescribing as the instrument. Additionally, potential bias caused by missing data was attenuated through the use of multiple imputation, and separate models were built for complete cases only and imputed/complete cases.
4 Discussion
We have described a conceptual schema for designing observational real-world studies to estimate causal effects. The application of this schema to a previously published study illuminates the methodologic structure of the study, revealing how each structural element is related to a potential bias which it is meant to address. Real-world evidence is increasingly accepted by healthcare stakeholders, including the FDA (Concato and Corrigan-Curay 2022 ; Concato and ElZarrad 2022 ), and its use for comparative effectiveness and safety assessments requires appropriate causal study design; our guide is meant to facilitate this design process and complement existing, more specific, guidance.
Existing guidance for causal inference using observational data includes components that can be clearly mapped onto the schema that we have developed. For example, in 2009 Cox et al. described common sources of bias in observational data and recommended specific strategies to mitigate these biases, corresponding to steps 6–8 of our step-by-step guide (Cox et al. 2009 ). In 2013, the AHRQ emphasized development of the research question, corresponding to steps 1–4 of our guide, with additional chapters on study design, comparator selection, sensitivity analyses, and directed acyclic graphs which correspond to steps 7 and 5, respectively (Velentgas et al. 2013 ). Much of Girman et al.’s manuscript (Girman et al. 2014 ) corresponds with steps 1–4 of our guide, and the matter of equipoise and interpretability specifically correspond to steps 3 and 7–8. The current ENCePP guide on methodological standards in pharmacoepidemiology contains a section on formulating a meaningful research question, corresponding to step 1, and describes strategies to mitigate specific sources of bias, corresponding to steps 6–8 (European Medicines Agency 2023 ). Recent works by the FDA Sentinel Innovation Center (Desai et al. 2024 ) and the Joint Initiative for Causal Inference (Dang et al. 2023 ) provide more advanced exposition of many of the steps in our guide. The target trial framework contains guidance on developing seven components of the study protocol, including eligibility criteria, treatment strategies, assignment procedures, follow-up period, outcome, causal contrast of interest, and analysis plan (Hernán and Robins 2016 ). Our work places the target trial framework into a larger context illustrating its relationship with other important study planning considerations, including the creation of a directed acyclic graph and incorporation of prespecified sensitivity and quantitative bias analyses.
Ultimately, the feasibility of estimating causal effects relies on the capabilities of the available data. Real-world data sources are complex, and the investigator must carefully consider whether the data on hand are sufficient to answer the research question. For example, a study that relies solely on claims data for outcome ascertainment may suffer from outcome misclassification bias (Lanes and Beachler 2023 ). This bias can be addressed through medical record validation for a random subset of patients, followed by quantitative bias analysis (Lanes and Beachler 2023 ). If instead, the investigator wishes to apply a previously published, claims-based algorithm validated in a different database, they must carefully consider the transportability of that algorithm to their own study population. In this way, causal inference from real-world data requires the ability to think creatively and resourcefully about how various data sources and elements can be leveraged, with consideration for the strengths and limitations of each source. The heart of causal inference is in the pairing of humility and creativity: the humility to acknowledge what the data cannot do, and the creativity to address those limitations as best as one can at the time.
4.1 Limitations
As with any attempt to synthesize a broad array of information into a single, simplified schema, there are several limitations to our work. Space and useability constraints necessitated simplification of the complex source material and selections among many available methodologies, and information about the relative importance of each step is not currently included. Additionally, it is important to consider the context of our work. This step-by-step guide emphasizes analytic techniques (e.g., propensity scores) that are used most frequently within our own research environment and may not include less familiar study designs and analytic techniques. However, one strength of the guide is that additional designs and techniques or concepts can easily be incorporated into the existing schema. The benefit of a schema is that new information can be added and is more readily accessed due to its association with previously sorted information (Loveless 2022 ). It is also important to note that causal inference was approached as a broad overarching concept defined by the totality of the research, from start to finish, rather than focusing on a particular analytic technique, however we view this as a strength rather than a limitation.
Finally, the focus of this guide was on the methodologic aspects of study planning. As a result, we did not include steps for drafting or registering the study protocol in a public database or for communicating results. We strongly encourage researchers to register their study protocols and communicate their findings with transparency. A protocol template endorsed by ISPOR and ISPE for studies using real-world data to evaluate treatment effects is available (Wang et al. 2023a ). Additionally, the steps described above are intended to illustrate an order of thinking in the study planning process, and these steps are often iterative. The guide is not intended to reflect the order of study execution; specifically, quality control procedures and sensitivity analyses should also be formulated up-front at the protocol stage.
5 Conclusion
We outlined steps and described key conceptual issues of importance in designing real-world studies to answer causal questions, and created a visually appealing, user-friendly resource to help researchers clearly define and navigate these issues. We hope this guide serves to enhance the quality, and thus the impact, of real-world evidence.
Data availability
No datasets were generated or analysed during the current study.
Arlett, P., Kjaer, J., Broich, K., Cooke, E.: Real-world evidence in EU Medicines Regulation: Enabling Use and establishing value. Clin. Pharmacol. Ther. 111 (1), 21–23 (2022)
Article PubMed Google Scholar
Athey, S., Imbens, G.W.: Machine Learning Methods That Economists Should Know About. Annual Review of Economics 11(Volume 11, 2019): 685–725. (2019)
Belthangady, C., Stedden, W., Norgeot, B.: Minimizing bias in massive multi-arm observational studies with BCAUS: Balancing covariates automatically using supervision. BMC Med. Res. Methodol. 21 (1), 190 (2021)
Article PubMed PubMed Central Google Scholar
Berger, M.L., Sox, H., Willke, R.J., Brixner, D.L., Eichler, H.G., Goettsch, W., Madigan, D., Makady, A., Schneeweiss, S., Tarricone, R., Wang, S.V., Watkins, J.: and C. Daniel Mullins. 2017. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 26 (9): 1033–1039
Brenner, H., Gefeller, O.: Use of the positive predictive value to correct for disease misclassification in epidemiologic studies. Am. J. Epidemiol. 138 (11), 1007–1015 (1993)
Article CAS PubMed Google Scholar
Concato, J., Corrigan-Curay, J.: Real-world evidence - where are we now? N Engl. J. Med. 386 (18), 1680–1682 (2022)
Concato, J., ElZarrad, M.: FDA Issues Draft Guidances on Real-World Evidence, Prepares to Publish More in Future [accessed on 2022]. (2022). https://www.fda.gov/drugs/news-events-human-drugs/fda-issues-draft-guidances-real-world-evidence-prepares-publish-more-future
Cox, E., Martin, B.C., Van Staa, T., Garbe, E., Siebert, U., Johnson, M.L.: Good research practices for comparative effectiveness research: Approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: The International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report–Part II. Value Health. 12 (8), 1053–1061 (2009)
Crump, R.K., Hotz, V.J., Imbens, G.W., Mitnik, O.A.: Dealing with limited overlap in estimation of average treatment effects. Biometrika. 96 (1), 187–199 (2009)
Article Google Scholar
Danaei, G., Rodriguez, L.A., Cantero, O.F., Logan, R., Hernan, M.A.: Observational data for comparative effectiveness research: An emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat. Methods Med. Res. 22 (1), 70–96 (2013)
Dang, L.E., Gruber, S., Lee, H., Dahabreh, I.J., Stuart, E.A., Williamson, B.D., Wyss, R., Diaz, I., Ghosh, D., Kiciman, E., Alemayehu, D., Hoffman, K.L., Vossen, C.Y., Huml, R.A., Ravn, H., Kvist, K., Pratley, R., Shih, M.C., Pennello, G., Martin, D., Waddy, S.P., Barr, C.E., Akacha, M., Buse, J.B., van der Laan, M., Petersen, M.: A causal roadmap for generating high-quality real-world evidence. J. Clin. Transl Sci. 7 (1), e212 (2023)
Desai, R.J., Wang, S.V., Sreedhara, S.K., Zabotka, L., Khosrow-Khavar, F., Nelson, J.C., Shi, X., Toh, S., Wyss, R., Patorno, E., Dutcher, S., Li, J., Lee, H., Ball, R., Dal Pan, G., Segal, J.B., Suissa, S., Rothman, K.J., Greenland, S., Hernan, M.A., Heagerty, P.J., Schneeweiss, S.: Process guide for inferential studies using healthcare data from routine clinical practice to evaluate causal effects of drugs (PRINCIPLED): Considerations from the FDA Sentinel Innovation Center. BMJ. 384 , e076460 (2024)
Digitale, J.C., Martin, J.N., Glymour, M.M.: Tutorial on directed acyclic graphs. J. Clin. Epidemiol. 142 , 264–267 (2022)
Dondo, T.B., Hall, M., West, R.M., Jernberg, T., Lindahl, B., Bueno, H., Danchin, N., Deanfield, J.E., Hemingway, H., Fox, K.A.A., Timmis, A.D., Gale, C.P.: beta-blockers and Mortality after Acute myocardial infarction in patients without heart failure or ventricular dysfunction. J. Am. Coll. Cardiol. 69 (22), 2710–2720 (2017)
Article CAS PubMed PubMed Central Google Scholar
European Medicines Agency: ENCePP Guide on Methodological Standards in Pharmacoepidemiology [accessed on 2023]. (2023). https://www.encepp.eu/standards_and_guidances/methodologicalGuide.shtml
Ferguson, K.D., McCann, M., Katikireddi, S.V., Thomson, H., Green, M.J., Smith, D.J., Lewsey, J.D.: Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): A novel and systematic method for building directed acyclic graphs. Int. J. Epidemiol. 49 (1), 322–329 (2020)
Flanagin, A., Lewis, R.J., Muth, C.C., Curfman, G.: What does the proposed causal inference Framework for Observational studies Mean for JAMA and the JAMA Network Journals? JAMA (2024)
U.S. Food and Drug Administration: Framework for FDA’s Real-World Evidence Program [accessed on 2018]. (2018). https://www.fda.gov/media/120060/download
Franklin, J.M., Schneeweiss, S.: When and how can Real World Data analyses substitute for randomized controlled trials? Clin. Pharmacol. Ther. 102 (6), 924–933 (2017)
Gatto, N.M., Wang, S.V., Murk, W., Mattox, P., Brookhart, M.A., Bate, A., Schneeweiss, S., Rassen, J.A.: Visualizations throughout pharmacoepidemiology study planning, implementation, and reporting. Pharmacoepidemiol Drug Saf. 31 (11), 1140–1152 (2022)
Girman, C.J., Faries, D., Ryan, P., Rotelli, M., Belger, M., Binkowitz, B., O’Neill, R.: and C. E. R. S. W. G. Drug Information Association. 2014. Pre-study feasibility and identifying sensitivity analyses for protocol pre-specification in comparative effectiveness research. J. Comp. Eff. Res. 3 (3): 259–270
Griffith, G.J., Morris, T.T., Tudball, M.J., Herbert, A., Mancano, G., Pike, L., Sharp, G.C., Sterne, J., Palmer, T.M., Davey Smith, G., Tilling, K., Zuccolo, L., Davies, N.M., Hemani, G.: Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 11 (1), 5749 (2020)
Hernán, M.A.: The C-Word: Scientific euphemisms do not improve causal inference from Observational Data. Am. J. Public Health. 108 (5), 616–619 (2018)
Hernán, M.A., Robins, J.M.: Using Big Data to emulate a target Trial when a Randomized Trial is not available. Am. J. Epidemiol. 183 (8), 758–764 (2016)
Hernán, M., Robins, J.: Causal Inference: What if. Chapman & Hall/CRC, Boca Raton (2020)
Google Scholar
International Society for Pharmacoeconomics and Outcomes Research (ISPOR): Strategic Initiatives: Real-World Evidence [accessed on 2022]. (2022). https://www.ispor.org/strategic-initiatives/real-world-evidence
International Society for Pharmacoepidemiology (ISPE): Position on Real-World Evidence [accessed on 2020]. (2020). https://pharmacoepi.org/pub/?id=136DECF1-C559-BA4F-92C4-CF6E3ED16BB6
Labrecque, J.A., Swanson, S.A.: Target trial emulation: Teaching epidemiology and beyond. Eur. J. Epidemiol. 32 (6), 473–475 (2017)
Lanes, S., Beachler, D.C.: Validation to correct for outcome misclassification bias. Pharmacoepidemiol Drug Saf. (2023)
Lash, T.L., Fox, M.P., Fink, A.K.: Applying Quantitative bias Analysis to Epidemiologic data. Springer (2009)
Lash, T.L., Fox, M.P., MacLehose, R.F., Maldonado, G., McCandless, L.C., Greenland, S.: Good practices for quantitative bias analysis. Int. J. Epidemiol. 43 (6), 1969–1985 (2014)
Leahy, T.P., Kent, S., Sammon, C., Groenwold, R.H., Grieve, R., Ramagopalan, S., Gomes, M.: Unmeasured confounding in nonrandomized studies: Quantitative bias analysis in health technology assessment. J. Comp. Eff. Res. 11 (12), 851–859 (2022)
Loveless, B.: A Complete Guide to Schema Theory and its Role in Education [accessed on 2022]. (2022). https://www.educationcorner.com/schema-theory/
Lund, J.L., Richardson, D.B., Sturmer, T.: The active comparator, new user study design in pharmacoepidemiology: Historical foundations and contemporary application. Curr. Epidemiol. Rep. 2 (4), 221–228 (2015)
Mai, X., Teng, C., Gao, Y., Governor, S., He, X., Kalloo, G., Hoffman, S., Mbiydzenyuy, D., Beachler, D.: A pragmatic comparison of logistic regression versus machine learning methods for propensity score estimation. Supplement: Abstracts of the 38th International Conference on Pharmacoepidemiology: Advancing Pharmacoepidemiology and Real-World Evidence for the Global Community, August 26–28, 2022, Copenhagen, Denmark. Pharmacoepidemiology and Drug Safety 31(S2). (2022)
Mullard, A.: 2021 FDA approvals. Nat. Rev. Drug Discov. 21 (2), 83–88 (2022)
Onasanya, O., Hoffman, S., Harris, K., Dixon, R., Grabner, M.: Current applications of machine learning for causal inference in healthcare research using observational data. International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Atlanta, GA. (2024)
Pearl, J.: Causal diagrams for empirical research. Biometrika. 82 (4), 669–688 (1995)
Prada-Ramallal, G., Takkouche, B., Figueiras, A.: Bias in pharmacoepidemiologic studies using secondary health care databases: A scoping review. BMC Med. Res. Methodol. 19 (1), 53 (2019)
Richardson, T.S., Robins, J.M.: Single World Intervention Graphs: A Primer [accessed on 2013]. (2013). https://www.stats.ox.ac.uk/~evans/uai13/Richardson.pdf
Richiardi, L., Bellocco, R., Zugna, D.: Mediation analysis in epidemiology: Methods, interpretation and bias. Int. J. Epidemiol. 42 (5), 1511–1519 (2013)
Riis, A.H., Johansen, M.B., Jacobsen, J.B., Brookhart, M.A., Sturmer, T., Stovring, H.: Short look-back periods in pharmacoepidemiologic studies of new users of antibiotics and asthma medications introduce severe misclassification. Pharmacoepidemiol Drug Saf. 24 (5), 478–485 (2015)
Rodrigues, D., Kreif, N., Lawrence-Jones, A., Barahona, M., Mayer, E.: Reflection on modern methods: Constructing directed acyclic graphs (DAGs) with domain experts for health services research. Int. J. Epidemiol. 51 (4), 1339–1348 (2022)
Rothman, K.J., Greenland, S., Lash, T.L.: Modern Epidemiology. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia (2008)
Rubin, D.B.: Causal inference using potential outcomes. J. Am. Stat. Assoc. 100 (469), 322–331 (2005)
Article CAS Google Scholar
Sauer, B.V.: TJ. Use of Directed Acyclic Graphs. In Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide , edited by P. Velentgas, N. Dreyer, and P. Nourjah: Agency for Healthcare Research and Quality (US) (2013)
Schneeweiss, S., Rassen, J.A., Brown, J.S., Rothman, K.J., Happe, L., Arlett, P., Dal Pan, G., Goettsch, W., Murk, W., Wang, S.V.: Graphical depiction of longitudinal study designs in Health Care databases. Ann. Intern. Med. 170 (6), 398–406 (2019)
Schuler, M.S., Rose, S.: Targeted maximum likelihood estimation for causal inference in Observational studies. Am. J. Epidemiol. 185 (1), 65–73 (2017)
Stuart, E.A., DuGoff, E., Abrams, M., Salkever, D., Steinwachs, D.: Estimating causal effects in observational studies using Electronic Health data: Challenges and (some) solutions. EGEMS (Wash DC) 1 (3). (2013)
Sturmer, T., Webster-Clark, M., Lund, J.L., Wyss, R., Ellis, A.R., Lunt, M., Rothman, K.J., Glynn, R.J.: Propensity score weighting and trimming strategies for reducing Variance and Bias of Treatment Effect estimates: A Simulation Study. Am. J. Epidemiol. 190 (8), 1659–1670 (2021)
Suissa, S., Dell’Aniello, S.: Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 29 (9), 1101–1110 (2020)
Tripepi, G., Jager, K.J., Dekker, F.W., Wanner, C., Zoccali, C.: Measures of effect: Relative risks, odds ratios, risk difference, and ‘number needed to treat’. Kidney Int. 72 (7), 789–791 (2007)
Velentgas, P., Dreyer, N., Nourjah, P., Smith, S., Torchia, M.: Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Agency for Healthcare Research and Quality (AHRQ) Publication 12(13). (2013)
Wang, A., Nianogo, R.A., Arah, O.A.: G-computation of average treatment effects on the treated and the untreated. BMC Med. Res. Methodol. 17 (1), 3 (2017)
Wang, S.V., Pottegard, A., Crown, W., Arlett, P., Ashcroft, D.M., Benchimol, E.I., Berger, M.L., Crane, G., Goettsch, W., Hua, W., Kabadi, S., Kern, D.M., Kurz, X., Langan, S., Nonaka, T., Orsini, L., Perez-Gutthann, S., Pinheiro, S., Pratt, N., Schneeweiss, S., Toussi, M., Williams, R.J.: HARmonized Protocol Template to enhance reproducibility of hypothesis evaluating real-world evidence studies on treatment effects: A good practices report of a joint ISPE/ISPOR task force. Pharmacoepidemiol Drug Saf. 32 (1), 44–55 (2023a)
Wang, S.V., Schneeweiss, S., Initiative, R.-D., Franklin, J.M., Desai, R.J., Feldman, W., Garry, E.M., Glynn, R.J., Lin, K.J., Paik, J., Patorno, E., Suissa, S., D’Andrea, E., Jawaid, D., Lee, H., Pawar, A., Sreedhara, S.K., Tesfaye, H., Bessette, L.G., Zabotka, L., Lee, S.B., Gautam, N., York, C., Zakoul, H., Concato, J., Martin, D., Paraoan, D.: and K. Quinto. Emulation of Randomized Clinical Trials With Nonrandomized Database Analyses: Results of 32 Clinical Trials. JAMA 329(16): 1376-85. (2023b)
Westreich, D., Lessler, J., Funk, M.J.: Propensity score estimation: Neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J. Clin. Epidemiol. 63 (8), 826–833 (2010)
Yang, S., Eaton, C.B., Lu, J., Lapane, K.L.: Application of marginal structural models in pharmacoepidemiologic studies: A systematic review. Pharmacoepidemiol Drug Saf. 23 (6), 560–571 (2014)
Zhou, H., Taber, C., Arcona, S., Li, Y.: Difference-in-differences method in comparative Effectiveness Research: Utility with unbalanced groups. Appl. Health Econ. Health Policy. 14 (4), 419–429 (2016)
Download references
The authors received no financial support for this research.
Author information
Authors and affiliations.
Carelon Research, Wilmington, DE, USA
Sarah Ruth Hoffman, Nilesh Gangan, Joseph L. Smith, Arlene Tave, Yiling Yang, Christopher L. Crowe & Michael Grabner
Elevance Health, Indianapolis, IN, USA
Xiaoxue Chen
University of Maryland School of Pharmacy, Baltimore, MD, USA
Susan dosReis
You can also search for this author in PubMed Google Scholar
Contributions
SH, NG, JS, AT, CC, MG are employees of Carelon Research, a wholly owned subsidiary of Elevance Health, which conducts health outcomes research with both internal and external funding, including a variety of private and public entities. XC was an employee of Elevance Health at the time of study conduct. YY was an employee of Carelon Research at the time of study conduct. SH, MG, and JLS are shareholders of Elevance Health. SdR receives funding from GlaxoSmithKline for a project unrelated to the content of this manuscript and conducts research that is funded by state and federal agencies.
Corresponding author
Correspondence to Sarah Ruth Hoffman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Hoffman, S.R., Gangan, N., Chen, X. et al. A step-by-step guide to causal study design using real-world data. Health Serv Outcomes Res Method (2024). https://doi.org/10.1007/s10742-024-00333-6
Download citation
Received : 07 December 2023
Revised : 31 May 2024
Accepted : 10 June 2024
Published : 19 June 2024
DOI : https://doi.org/10.1007/s10742-024-00333-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Causal inference
- Real-world data
- Confounding
- Non-randomized data
- Bias in pharmacoepidemiology
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 27 June 2024
Operationalizing and digitizing person-centered daily functioning: a case for functionomics
- Esther R.C. Janssen 1 , 2 , 3 ,
- Ilona M. Punt 4 ,
- Johan van Soest 5 , 6 ,
- Yvonne F. Heerkens 7 , 8 ,
- Hillegonda A. Stallinga 9 ,
- Huib ten Napel 10 ,
- Lodewijk W. van Rhijn 11 ,
- Barend Mons 12 , 13 ,
- Andre Dekker 6 ,
- Paul C. Willems 4 &
- Nico L.U. van Meeteren 14 , 15
BMC Medical Informatics and Decision Making volume 24 , Article number: 184 ( 2024 ) Cite this article
Metrics details
An ever-increasing amount of data on a person’s daily functioning is being collected, which holds information to revolutionize person-centered healthcare. However, the full potential of data on daily functioning cannot yet be exploited as it is mostly stored in an unstructured and inaccessible manner. The integration of these data, and thereby expedited knowledge discovery, is possible by the introduction of functionomics as a complementary ‘omics’ initiative, embracing the advances in data science. Functionomics is the study of high-throughput data on a person’s daily functioning, that can be operationalized with the International Classification of Functioning, Disability and Health (ICF).
A prerequisite for making functionomics operational are the FAIR (Findable, Accessible, Interoperable, and Reusable) principles. This paper illustrates a step by step application of the FAIR principles for making functionomics data machine readable and accessible, under strictly certified conditions, in a practical example. Establishing more FAIR functionomics data repositories, analyzed using a federated data infrastructure, enables new knowledge generation to improve health and person-centered healthcare. Together, as one allied health and healthcare research community, we need to consider to take up the here proposed methods.
Peer Review reports
Introduction
Omics research and the definition of functionomics.
An ever-increasing amount of health, healthcare and related research data on a person’s daily functioning is collected by a variety of stakeholders: people themselves, healthcare professionals and researchers, among others. By joint analysis of these data a tremendous amount of information can be derived from these data and has the potential to revolutionize person-centered prevention and healthcare, potentially improving health and life expectancy. Within the fields of oncology, radiology and genetics, computerized analysis of high-throughput data has already shown benefits for the personalization and optimization of healthcare [ 1 , 2 , 3 ]. These initiatives are often referred to as ‘omics’ research, where analyses of big data on genes (genomics), RNA (transcriptomics), proteins (proteomics), metabolites (metabolomics), and imaging (radiomics) are performed to advance personalized and preventive health and healthcare [ 4 , 5 ]. To make this possible, ‘omics’ initiatives rely more and more on machine actionable data.
In this study we propose the integration of a new ‘omics’, namely functionomics. The current ‘omics’ research field focusses on biomedical or internal exposures, whilst functionomics can specifically contribute to eliciting interactions with personal and general external exposures (Table 1 ).
The concept of functioning and its underlying phenomena are globally described by the World Health Organization (WHO) in the International Classification of Functioning, Disability and Health (ICF) [ 9 ] (Fig. 1 ).
The ICF-framework
The ICF is used internationally in different types of interdisciplinary healthcare and social research settings, as well as to inform health policy development [ 12 , 13 ]. Therefore the ICF is an ideal framework for providing a common format for making functionomics data machine actionable in an international setting.
Until now the potential of functionomics data cannot yet be fully exploited, as they are usually stored in an unstructured manner in all sorts of mostly inaccessible data-silos. The lack of machine actionable data makes it difficult for people themselves as well as for outsiders (those not involved in the data collection and storage) to access, understand, analyze, interpret, and reuse these data. Imagine your own hard drive which holds all sorts of research datasets which cannot be accessed or understood by others. This prohibits joint analysis of data, causing dilution of information and loss of valuable knowledge which may result in suboptimal clinical decisions and ultimately less effective care [ 14 ]. Therefore, a transition towards the integration of functionomics as an additional ‘omics’ initiative, and at the same time embracing the advances in data science and information technology (IT), is necessary. Integrating functionomics in health, healthcare, education and research practice has an additional benefit on top of the other ‘omics’, as it provides a means to capture a more holistic view of health, rather than the limited biomedical view. Functionomics research can specifically contribute to eliciting interactions with personal and general external exposures (Fig. 2 ) and to broaden the scope of person-centered healthcare.
The human exposome reflects the totality of internal and external exposures within a human life cycle. Current ‘omics’ research field focus solely on biomedical or internal exposures, whilst functionomics can specifically contribute to eliciting interactions with personal and general external exposures. (adapted from Vrijheid et al. [ 15 ])
Functionomics in the context of allied health professionals
Allied healthcare disciplines are well-positioned to pioneer a functionomics initiative, as these disciplines generate large amounts of data that can be captured within the ICF. Allied healthcare comprises a large group of health professionals, that are not physicians, with the core focus of enabling people to enjoy optimal functioning in their daily lives (e.g., physiotherapists, dieticians, speech therapists). For example: there are currently annually 3.84 million people treated by approximately 35 000 physiotherapists in the Netherlands and there are 560 000 physiotherapists in the European Union (EU) [ 16 , 17 ] and 216 920 in the United States (US) [ 18 ]. If we assume that the average physiotherapist generates approximately 0.1 GB of data per patient, [ 19 ] we can estimate a data volume of roughly 375 terabytes per year in the Netherlands, 5.9 petabytes in the EU and 2.4 petabytes in the US. Translating these data into information that is actionable at the point of care and subsequently using that information to guide prognosis, diagnosis, prevention, and treatment pave the way towards more adequate and personalized physiotherapy [ 20 , 21 ]. However, this huge amount of functionomics data can only be processed by machines. Subsequently, acceleration of knowledge generation can only be achieved by making data machine actionable.
Barriers to implementation
To enable functionomics research, there are four major challenges in data collection, processing and storage that need to be addressed: 1) variability in data collection and storage strategies, 2) lack of implementation of community data standards, 3) ethical and social dilemmas like patient privacy issues, and 4) interoperability between IT systems [ 22 ]. In this paper we will focus on suggesting solutions for these challenges, where we will focus on the problem that functionomics data are currently not machine actionable as they are collected in a mostly unstructured manner and stored in inaccessible data-silos. The potential to compromise patient privacy when linking records across data silos is an additional complicating factor (challenge 3). These issues could be resolved by creating a federated functionomics data infrastructure before functionomics research can live up to its full potential and will be discussed in this paper.
Transition from data storage to data use
A robust data infrastructure between the many data silos is a prerequisite for any ‘omics’ initiative, as it allows joint analysis of multiple data sources. Such a data infrastructure relies on usage of a ontology and data processing. Particularly, data should be transformed following the FAIR (Findable, Accessible, Interoperable, and Reusable) principles [ 23 ]. FAIR principles are internationally promoted as best practice in data management, with examples of successful application in other types of ‘omics’ initiatives [ 24 ]. FAIR principles are recommended by organizations like WHO, G20, European Commission, and European Open Science Cloud [ 25 ]. Computational ontologies and Semantic Web technologies, are strongly recommended methods to help achieve FAIR data [ 26 ]. Applying these methods will provide citizens, health professionals and researchers with machine readable data that can be analyzed via a federated data infrastructure. These concepts are currently under-utilized, as many are unfamiliar with them and what they can bring to daily life challenges up to clinical practice quests.Moreover, many of the prerequisites for making functionomics data FAIR are currently not available in this field. Combined efforts are needed to resolve these issues.
Therefore, the aim of this article is to provide a step-by-step guide on how to implement and utilize FAIR functionomics data, by proposing a method for creating an ontology based on the ICF and introducing internationally advocated concepts (FAIR principles operationalized through Semantic Web technology) for making data machine actionable. In the discussion we will address remaining issues for making data FAIR within the domain of functionomics.
Materials and methods
In this study we used a single database example from a retrospective cohort study, to walk through the steps of creating FAIR data ready for federated analysis. The data were collected with the goal of developing a decision-support system to aid in the personalization of the perioperative care pathway by identifying which patients are at risk for worse short- and long-term outcomes [ 27 ]. This study was assessed by the local medical ethical committee AzM/UM (METC AzM/UM) and was considered not applicable to the Medical Research Involving Human Subject Act (number 2019 − 1426). In Table 2 , we provide the reader with a glossary of some fundamental terms and abbreviations used in this paper.
A practical example
We will describe the first steps in the methodological process to develop a FAIR functionomics database, using the above mentioned dataset, by: A) creating a computational ontology using the ICF, B) making data machine readable, C) publishing data on the Semantic Web to transform clinical data into FAIR and linked data, and D) analyzing data (queried) using a federated learning infrastructure (Fig. 3 ). The letters A till D in Fig. 3 are used throughout the Methods and Results sections to delimit the different steps in the process.
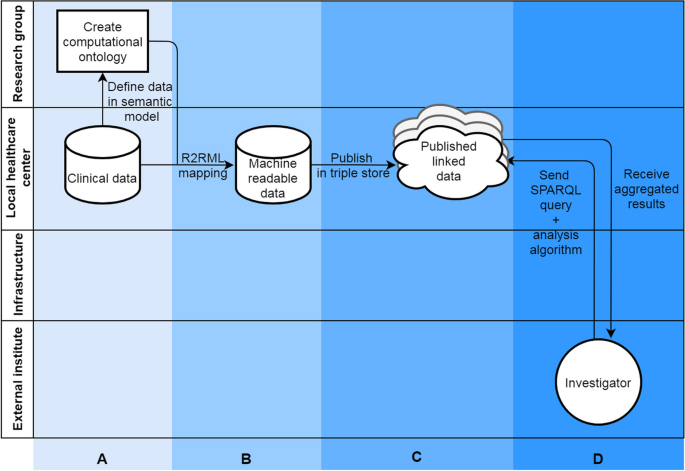
FAIRification process in a practical example. Section A: data prepping, section B: make data linkable, section C: publish FAIR data, section D: query FAIR data. If process A, B and C are repeated by different clinics multiple published linked datasets will arise that can be queried
Case description
Routine clinical data from 160 adult patients were collected during the perioperative care period for patients with degenerative disorders of the lumbar spine opting for fusion surgery. The database contained a set of diverse variables: patient demographic characteristics, patient-reported pain and functioning (including activities), and other clinical outcome measures (Table 3 ).
An ontology was formulated (column A, Fig. 3 ) to make the data from case study interoperable. The created ontology only provides classes for concepts in our used case. It should be viewed as an example of how the allied health research community can approach building a functionomics ontology. In an ontology, a research field agrees on formal definitions of the terms in the domain and relations among them and are expressed in machine readable language [ 28 ]. A machine readable language means that computers can easily find, ‘read’ and understand data, without manual intervention. In our study, we used the open access Protégé (Stanford University, Stanford, CA, USA) software, which incorporates current standards for developing machine readable ontologies: Resource Description Framework Schema (RDFS) and the Web Ontology Language (OWL). Herein we combined terms from existing terminologies in the biomedical field to give universally agreed-upon definitions and structure to our dataset: SNOMED-CT, and Units of Measurement Ontology (UO). The ICF was used as an upper level class structure for our ontology. We added classes from SNOMED-CT and UO to define specific concepts that were available in these ontologies for variables in our dataset (e.g., age, sex). For biopsychosocial variables that could not be defined using the existing ontologies, we formulated a new class. The basic idea of this mapping process was to link each data structure (row, columns and values) within the database to its corresponding component (concept, property, relationship). The way variables are interlinked was defined within the ontology and was based on clinical expertise and understanding of these relationships by the authors. These components were developed using feedback loops with experts in the field of lumbar spinal fusion (LSF), perioperative care and the ICF. The reader should keep in mind that this is only an example, ontologies are flexible and can easily incorporate new variables and relationships or adjust existing variables/relationships. Ideally an ontology should be based on international community standards and consensus.
Semantic web technologies
Semantic Web technologies are an extension of the World Wide Web (WWW) and provide people with a means of publishing and storing data on the Web. Within the Semantic Web, data are described in triples, based on the Resource Description Framework (RDF; column B, Fig. 3 ). A triple consists of three components, namely: a subject, a predicate and an object. Each of these components has a semantic definition, defined within the ontology. These three components from the defined ontology are combined to make a triple, for example see Table 4 :
In a relational database, all variables within a two-dimensional table (e.g., csv file, excel file, SPSS file) have a relation to each other, which needs to be defined in the process of making data machine readable. In this study we used R2RML descriptions to transform our data into RDF triples using the Ontop software package. Once in the dataset all data were transformed into RDF triples, the triples were stored on a web platform called GraphDB (Ontotext, Sofia, Bulgaria) running on the hospitals’ intranet (column C, Fig. 3 ). We checked the triple mapping using the visual graph interface of GraphDB. The intranet is a private part of the WWW, accessible only to employees of the hospital. The GraphDB instance held the RDF triples and hosts a REST API to receive requests to query the data hosted in the GraphDB instance. The universal language that can be used to query data transformed into RDF triples is SPARQL.
The Personal Health Train (PHT) [ 10 ] federated infrastructure allows a researcher or other external parties to perform analyses on data from multiple GraphDB instances or data silos without physically having access to the data (column D, Fig. 3 ). Through the REST API in the PHT infrastructure a researcher can send their analysis to one or more data stations communicating with a central PHT server. Subsequently the analysis is performed locally in data stations (e.g. hospitals, physiotherapy practices) and only aggregated results are sent back to the researcher via the same infrastructure. This infrastructure can be utilized to send all different types of data analyses – queries and algorithms – to the data stations, like quality assessment, prediction modelling or effectiveness calculations. A SPARQL query and algorithm for performing a simple count of gender was written and performed via the federated infrastructure.
To assess the FAIRness of the data (e.g., the degree to which the digital resource adheres to the FAIR data principles) the data was analyzed using the FAIRMetrics [ 11 ]. We used the standardized FAIR maturity indicators manual assessment, which assesses Findability, Accessibility, Interoperability and Reusability of the resource using thirtyfour indicators.
As this paper aimed to provide guidance on how to implement functionomics in clinical practice, a step-by-step tutorial of the described results was created in our GitHub repository: https://github.com/ERCJanssen/Functionomics . Readers can use this tutorial including dummy data similar to the real dataset to recreate the same steps themselves.
We developed an ontology describing basic concepts, relationships and properties within the preoperative context of a patient deciding forLSF (column A, Fig. 3 ).The ICF was used as the upper level hierarchy of classes for this ontology, containing 1,596 classes. We added ‘new’ lower level concepts to the ICF structure when these concepts, defining the variables in our dataset, were not available in the ICF. We mapped all variables from our dataset as concepts in the ontology reusing concepts from well-known published ontologies, wherever possible (e.g., SNOMED CT and UO).If no appropriate concepts or relationships were available in existing ontologies, which was often the case for data about a patient’s daily functioning, new concepts were added. In total we added 42 classes and 10 predicates to the ontology. From this process we can see that many of these ‘new’ concepts about a patient’s daily functioning can appropriately be mapped to the ICF hierarchy. This ontology was published on Github (Fig. 4 ). The ontology can be (re)used and fine-tuned by others to fit their data on a person’s daily functioning.
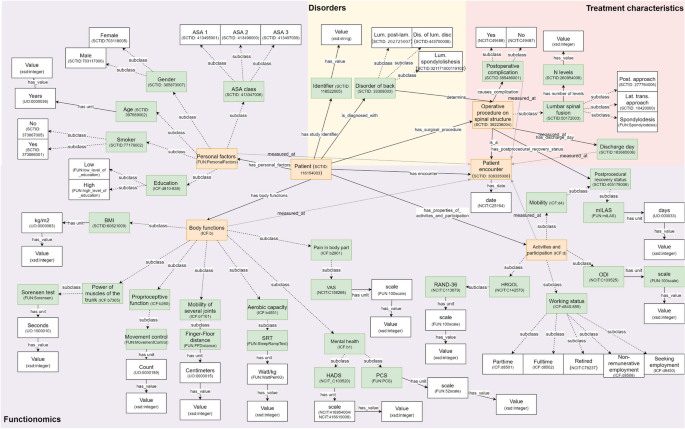
Basic concepts and relationships within the example dataset, defined within different existing ontologies. Abbreviations: ASA American society of anesthesiology, BMI Body mass index, DIS Disease, HADS Hospital anxiety and depression scale, HRQOL Health related quality of life, KG Kilograms, LAM Laminectomy, LAT Lateral, LUM Lumbar, mILAS Modified iowa level of assistance scale, ODI Oswestry disability index, PCS Pain catastrophizing scale, POST Posterior, SRT Steep ramp test, TRANS Transversal, VAS Visual analogue scale
Using semantic web technology
To transform the .csv dataset into machine readable data (RDF triples) we made an R2RML script (column B, Fig. 3 ). This script reads the .csv file, using the previously created ontology, and is translated it into 74 triples. An example of the mapping file is shown in Fig. 5 . The full mapping can be found on GitHub.
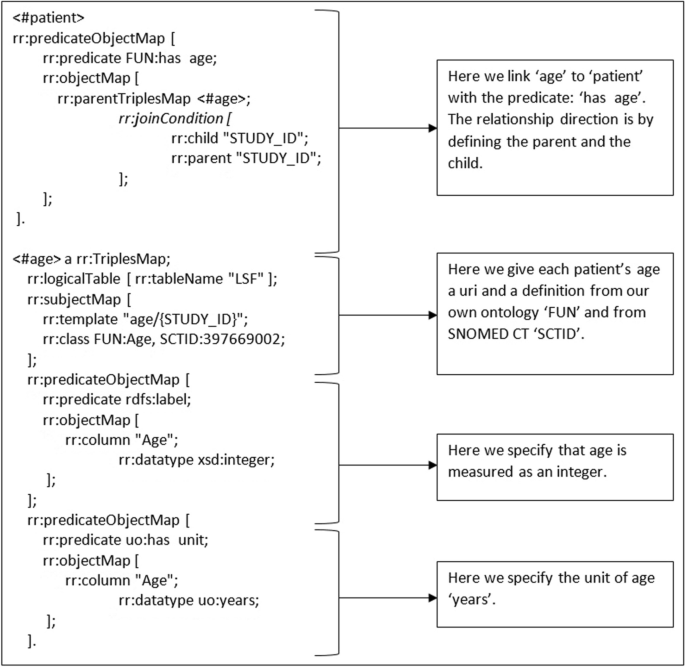
Example of an R2RML mapping to FAIRify functionomics data
The RDF mapping and data were published in a GraphDB instance on a local server, which linked the data repository to the web (column C, Fig. 3 ). From this point on, data could be analyzed by external parties by linking to the GraphDB instance via the PHT infrastructure (column D, Fig. 3 ).The PHT infrastructure allows the researcher to perform analysis without having to physically collect the data in a central server [ 29 ]. To perform such an action we linked two computers via internet in a password secured infrastructure to prevent unauthorized access to the data. A researcher then sent the example SPARQL query and algorithm to our GraphDB instance via the PHT infrastructure from their own computer. The results of this query were calculated locally in our local GraphDB instance. Subsequently, the aggregated results – frequencies of gender - of this simple query were sent back the researcher via the PHT infrastructure: N females = 101, N males = 59.
FAIRness of the data is described in GitHub repository. The main focus of this example was on the interoperability part of the FAIR principles, as such the scoring for FAIRness metrics on policies is low.From the applicable indicators we scored 7/8 for findability, 2/3 for accessibility, 7/7 for interoperability and 1/4 for reusability.
Recent history shows the usefulness of big data analysis in personalizing healthcare through ‘omics’ research in many medical fields [ 5 , 30 , 31 ]. In our practical example we redefined functionomics to include data on daily functioning of a person and showed how it can be operationalized and used, here in a clinical setting. A functionomics ontology for the specific setting and population of the example was created, based on the ICF. Both biomedical and psychosocial data were transformed into a machine readable language (RDF) and published on the web (Graph DB instance). Next, these data were queried (using SPARQL) and gender counts were generated via an analytic algorithm. This paper and the tutorial in the accompanying GitHub repository enables others to familiarize themselves with the proposed approach, establish their own functionomics data station and send all different types of analyses to these stations. Using this approach we will be able to create a network of linked FAIR functionomics datasets.
From its conception many scientific breakthroughs have been established through ‘omics’ research. For example, in the recent years, radiomics has made a serious impact on personalization of radiotherapy, due to firm investment in available IT and statistical solutions [ 2 ]. This has resulted in multiple scientific and clinical advancements; for example, an internationally validated prediction model for cancer survival has been developed and new knowledge on tumor phenotypes has been generated [ 31 , 32 ]. However, when considering the whole human exposome, major concepts are often not included in these ‘omics’ research types: the specific and general external exposome, and a considerable amount of the biopsychosocial aspect of the internal exposome. To further improve health and healthcare research, all elements of the human exposome should be included, informed by a biopsychosocial perspective.
In our practical example, we suggested how to operationalize this transition towards functionomics by using the ICF for the development of an appropriate ontology. The ICF is an international framework and terminology often used by allied healthcare professions to describe and organize data on a patient’s daily functioning. However, the transition from the ICF to a functionomics ontology requires to solve some major gaps in knowledge established in our study. Firstly, a classification of personal factors is lacking in the current ICF class hierarchy and – although different articles are published with preliminary lists - the WHO has decided to refrain from a classification of personal factors in the near future [ 9 ]. Secondly, no predicates were available in the ICF to establish relationships between classes. Thirdly, some concepts are hard to map within the current ICF class hierarchy, as they involve multiple ICF classes. In the community there is disagreement on methods of measuring functioning and how to map different concepts to the ICF [ 33 ]. Mapping the perception of one’s quality of life, for example, has led to some discussion about its position in the class hierarchy in our practical example as well as in other research, [ 34 ] even when applying the linking rules of the ICF [ 35 ]. Without consensus on this issue, it will remain difficult to make functionomics data FAIR. Therefore, we propose to address these gaps in knowledge in an international and interdisciplinary collaboration, to enable structured capture of real-world functionomics data. By addressing these issues, we can make functionomics operational, firstly in datasets and field examples, and step by step around the globe.
Making data FAIR has scientific value with a tremendous impact on population health, healthcare and the economy. The cost of not making data FAIR comes at a high price; annually around €100 billion is lost due to missed innovation opportunities [ 36 ]. We invest large amounts of time and effort in data capturing, but these data are only operable for single-use purposes, as they are mostly captured in an – when considering a global scale – unstructured and inaccessible manner. The FAIR principles, operationalized in Semantic Web Technology, guide the development of a global infrastructure and tooling to make all health and research data optimally reusable for machines and people alike resulting in the internet of FAIR data and services, where data, far more divergent than just health and research, can be found, accessed, and (re)used by anyone [ 25 ]. Accomplishing this will revolutionize the scientific and societal value of this data.
A major advantage of applying Semantic Web technologies and building a functionomics ontology is the ability to link different silos of data and concurrently to query them. In our example an external researcher was able to query our data without it leaving the data silo based in the hospital. Moreover, the researcher only received the aggregated results and not the individual patient data, meaning it is privacy preserving. Applying these techniques can help to solve the issues of physical data integration.
Ultimately, this approach could lead to ‘digital twins’, where one would be in the possession of very detailed biopsychosocial information of a person over time and relate them to similar persons who already underwent diagnostic, prophylactic and/or therapeutic interventions for their health challenges and very accurately predict their health outcomes [ 37 ].
Possible barriers for implementation of functionomics
An important issue that we have not addressed in this paper is unstructured, free text, data describing a person’s functioning. Often data on functioning are not collected in a structured manner, as from our example. Concepts of functioning, including the influencing contextual factors (personal and environmental factors), are hard to capture in a cohesive whole using measurement tools. There are two ways we can deal with this issue. The first one is investing in making functionomics data more structured, for example by creating new validated measurement tools and implementing these tools in standard clinical and research practice. However, as mentioned above, data on functioning is very context sensitive, using measurement tools we may lose this context and may not accurately present the patient’s perspective [ 38 ]. The second approach is to apply free text mining, like natural language processing (NLP), to extract meaningful concepts from the free text and convert them to structured formats.
Our current science landscape does not promote data and knowledge sharing [ 39 ]. This issue is inherent to putting great value on impact factors, publication numbers and grant acquisition. A major worry for many is that when data are shared too early, others will foreshadow their work [ 40 ]. Another issue is the analysis of privacy sensitive healthcare data, stored at many different locations. Functionomics data are often collected on the same person by different healthcare providers, social organizations or even by people themselves. Combining these privacy sensitive data repositories for functionomics research requires a privacy-preserving approach. By using federated learning techniques we could largely solve this issue, as it enables local analysis of data with only aggregated results leaving the place of storage, through privacy-by-design. Still, it is obligatory to gain informed consent of any individual to use their healthcare data for research purposes. This would not be feasible in the proposed system, as different types of queries could be sent to the data silo on a daily basis. A tiered informed consent may be a viable solution. Here people grant permission for the (research) purposes of their choice, but not for all [ 41 ].
Another thing to keep in mind is that FAIR is not equal to Open: The ‘A’ in FAIR stands for ‘Accessible under well-defined conditions’ [ 42 ]. Even when publishing data on the Semantic Web it is still stored locally on a ‘private’ network. The ‘owner’ of the data can still control who gets access to them, in our case through the PHT network, for example by requiring password authentication and authorization. In contrast, opening up data (Open Access) yields most benefits, as it provides researchers access to large amounts of data to analyze.
The next steps in functionomics
Big data analysis is not only a way to improve the robustness of science today, but can drive new scientific discovery of tomorrow. The analysis of big data on functionomics will give valuable insight in how to move forward in personalizing healthcare. For this, an internationally accepted functionomics ontology should be built, capturing all relevant data from the ICF, open access mapping scripts, a trustworthy data infrastructure and international agreements on data usage policies. Therefore, we call to action to all stakeholders in functionomics to contribute to a new ontology and participate in making their (own) data more FAIR.
In this study functionomics as the study of high-throughput data on daily functioning, as defined and objectified in the ICF, is introduced. Functionomics research can have great benefits for health and person-centered healthcare, thus improving health of people and with people. Investments, by an international community in the domain of functionomics, in the proposed IT solutions for big data analysis - FAIR principles through Semantic Web technologies - are necessary to achieve this. Together, as one united health and care (research) community, we need to make serious efforts to take up the proposed methods.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity. Data are, however, available from the authors upon reasonable request and with permission from the local medical ethical committee (METC UM/AzM). Data are located in controlled access data storage at Maastricht UMC+.
Cusumano D, Dinapoli N, Boldrini L, Chiloiro G, Gatta R, Masciocchi C, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med. 2018;123(4):286–95.
Article PubMed Google Scholar
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Reviews Clin Oncol. 2017;14(12):749–62.
Article Google Scholar
Rhrissorrakrai K, Koyama T, Parida L. Watson for genomics: moving personalized medicine forward. Trends Cancer. 2016;2(8):392–5.
Ibrahim R, Pasic M, Yousef GM. Omics for personalized medicine: defining the current we swim in. Expert Rev Mol Diagn. 2016;16(7):719–22.
Article CAS PubMed Google Scholar
Micheel CM, Nass SJ, Omenn GS, editors. Evolution of translational omics: lessons learned and the path forward. Washington (DC): National Academies Press (US); 2012.
Vailati-Riboni M, Palombo V, Loor JJ. What are omics sciences? In: Ametaj BN, editor. Periparturient diseases of dairy cows: a systems biology approach. Cham: Springer International Publishing; 2017. p. 1–7.
Google Scholar
Research N, Transcriptomics. Nature Research; https://www.nature.com/subjects/transcriptomics .
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–77.
Heerkens YF, de Weerd M, Huber M, de Brouwer CPM, van der Veen S, Perenboom RJM, et al. Reconsideration of the scheme of the international classification of functioning, disability and health: incentives from the Netherlands for a global debate. Disabil Rehabil. 2017;40(5):603–11.
Beyan O, Choudhury A, Soest Jv, Kohlbacher O, Zimmermann L, Stenzhorn H, et al. Distributed analytics on sensitive medical data: the personal health train. Data Intell. 2020;2(1–2):96–107.
Wilkinson MD, Sansone S-A, Schultes E, Doorn P, Bonino da Silva Santos LO, Dumontier M. A design framework and exemplar metrics for FAIRness. Sci Data. 2018;5(1):180118.
Article PubMed PubMed Central Google Scholar
Stucki G. International Classification of Functioning, disability, and health (ICF): a promising framework and classification for rehabilitation medicine. Am J Phys Med Rehabil. 2005;84(10):733–40.
Kostanjsek N. Use of The International Classification of Functioning, Disability and Health (ICF) as a conceptual framework and common language for disability statistics and health information systems. BMC Public Health. 2011;11 Suppl 4(Suppl 4):S3. https://doi.org/10.1186/1471-2458-11-S4-S3 .
Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood). 2014;33(7):1163–70.
Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014;69(9):876–8.
(KNGF) KNGvF. FysioFacts. https://www.kngf.nl/KNGF/Missie+%26+Visie/feiten--cijfers.html ; 2019.
Eurostat. Healthcare personnel statistics - dentists, pharmacists and physiotherapists https://ec.europa.eu/eurostat/statistics-explained/index.php/Healthcare_personnel_statistics_-_dentists,_pharmacists_and_physiotherapists 2020.
Elflein J, Number of physical therapists in the U.S. 2001–2016 https://www.statista.com/statistics/185731/number-of-physical-therapists-in-the-us-since-2001/#:~:text=Number%20of%20physical%20therapists%20in%20the%20U.S.%202001%2D2016&text=In%202001%2C%20there%20were%20126%2C450,were%20216%2C920%20physical%20therapists%20employed.2016
Lustberg T, van Soest J, Jochems A, Deist T, van Wijk Y, Walsh S, et al. Big data in radiation therapy: challenges and opportunities. Br J Radiol. 2017;90(1069):20160689.
Lambin P, Zindler J, Vanneste BGL, De Voorde LV, Eekers D, Compter I, et al. Decision support systems for personalized and participative radiation oncology. Adv Drug Deliv Rev. 2017;109:131–53.
Bates DW, Saria S, Ohno-Machado L, Shah A, Escobar G. Big data in health care: using analytics to identify and manage high-risk and high-cost patients. Health Aff. 2014;33(7):1123–31.
Skripcak T, Belka C, Bosch W, Brink C, Brunner T, Budach V, et al. Creating a data exchange strategy for radiotherapy research: towards federated databases and anonymised public datasets. Radiother Oncol. 2014;113(3):303–9.
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding principles for scientific data management and stewardship. Sci Data. 2016;3(1):160018.
Berrios DC, Beheshti A, Costes SV. FAIRness and usability for open-access omics data systems. AMIA Annu Symp Proc. 2018;2018:232–41.
PubMed PubMed Central Google Scholar
Mons B, Neylon C, Velterop J, Dumontier M, da Silva Santos LOB, Wilkinson MD. Cloudy, increasingly FAIR; revisiting the FAIR data guiding principles for the European open Science cloud. Inform Serv Use. 2017;37:49–56.
Traverso A, van Soest J, Wee L, Dekker A. The radiation oncology ontology (ROO): publishing linked data in radiation oncology using semantic web and ontology techniques. Med Phys. 2018;45(10):e854–62.
Janssen ER, Osong B, van Soest J, Dekker A, van Meeteren NL, Willems PC, Punt IM. Exploring Associations of Preoperative Physical Performance With Postoperative Outcomes After Lumbar Spinal Fusion: A Machine Learning Approach. Arch Phys Med Rehabil. 2021;102(7):1324–1330.e3. https://doi.org/10.1016/j.apmr.2021.02.013 .
Gruber TR. A translation approach to portable ontology specifications. Knowl Acquisition. 1993;5(2):199–220.
Choudhury A, Janssen E, Bongers BC, van Meeteren NLU, Dekker A, van Soest J. Colorectal cancer health and care quality indicators in a federated setting using the personal health train. BMC Med Inf Decis Mak. 2024;24(1):121.
Jochems A, Deist TM, van Soest J, Eble M, Bulens P, Coucke P, et al. Distributed learning: developing a predictive model based on data from multiple hospitals without data leaving the hospital – a real life proof of concept. Radiother Oncol. 2016;121(3):459–67.
Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5(1):4006.
Jochems A, Deist TM, El Naqa I, Kessler M, Mayo C, Reeves J, et al. Developing and validating a survival prediction model for NSCLC patients through distributed learning across 3 countries. Int J Radiat Oncol*Biol*Phys. 2017;99(2):344–52.
Article PubMed Central Google Scholar
Alford VM, Ewen S, Webb GR, McGinley J, Brookes A, Remedios LJ. The use of the international classification of functioning, disability and health to understand the health and functioning experiences of people with chronic conditions from the person perspective: a systematic review. Disabil Rehabil. 2015;37(8):655–66.
Cieza A, Stucki G. Content comparison of health-related quality of life (HRQOL) instruments based on the international classification of functioning, disability and health (ICF). Qual Life Res. 2005;14(5):1225–37.
Stucki G. ICF linking rules: an update based on lessons learned. J Rehabil Med. 2005;37(4):212–8.
Mons B. Invest 5% of research funds in ensuring data are reusable. Nature. 2020;578(7796):491.
Bruynseels K, Santoni de Sio F, van den Hoven J. Digital Twins in Health Care: Ethical Implications of an Emerging Engineering Paradigm. Front Genet. 2018;9:31.
Newman-Griffis DR, Hurwitz MB, McKernan GP, Houtrow AJ, Dicianno BE. A roadmap to reduce information inequities in disability with digital health and natural language processing. PLOS Digit Health. 2022;1(11):e0000135.
Tenopir C, Dalton ED, Allard S, Frame M, Pjesivac I, Birch B, et al. Changes in data sharing and data reuse practices and perceptions among scientists worldwide. PLoS ONE. 2015;10(8):e0134826.
Popkin G. Data sharing and how it can benefit your scientific career. Nature. 2019;569(7756):445–7.
Eisenhauer ER, Tait AR, Rieh SY, Arslanian-Engoren CM. Participants’ understanding of informed consent for biobanking: a systematic review. Clin Nurs Res. 2019;28(1):30–51.
Landi A, Thompson M, Giannuzzi V, Bonifazi F, Labastida I, da Silva Santos LOB, Roos M. The A of FAIR–as open as possible, as closed as necessary. Data Intell. 2020;2(1–2):47–55.
Download references
Acknowledgements
We would like to thank dr. A.J. Kittelson for proof reading the paper and providing us with valuable feedback.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Radboud Institute for Health Sciences, IQ health, Radboud university medical centre, Nijmegen, The Netherlands
Esther R.C. Janssen
School of Allied Health, HAN University of Applied Sciences, Nijmegen, The Netherlands
Department of Orthopedic Surgery, VieCuri Medical Centre, Tegelseweg 210, Venlo, 5912 BL, The Netherlands
Department of Orthopedics and Research School Caphri, Maastricht University Medical Centre+, Maastricht, The Netherlands
Ilona M. Punt & Paul C. Willems
Brightlands Institute for Smart Society (BISS), Faculty of Science and Engineering (FSE), Maastricht University, Heerlen, The Netherlands
Johan van Soest
Department of Radiation Oncology (Maastro), GROW-School for Oncology, Maastricht University Medical Centre+, Maastricht, The Netherlands
Johan van Soest & Andre Dekker
Dutch Institute of Allied Health Care (NPi), Amersfoort, The Netherlands
Yvonne F. Heerkens
Research Group Occupation & Health, HAN University of Applied Sciences, Nijmegen, The Netherlands
University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
Hillegonda A. Stallinga
RIVM/ Dutch WHO-FIC Collaborating Centre, Bilthoven, The Netherlands
Huib ten Napel
Department of Orthopedic surgery, Utrecht University, Utrecht, The Netherlands
Lodewijk W. van Rhijn
Leiden University Medical Centre, Leiden, The Netherlands
Barend Mons
GO FAIR International Support & Coordination Office (GFISCO), Leiden, The Netherlands
Top Sector Life Sciences and Health (Health~Holland), The Hague, The Netherlands
Nico L.U. van Meeteren
Department of Anesthesiology, Erasmus Medical Centre, Rotterdam, The Netherlands
You can also search for this author in PubMed Google Scholar
Contributions
EJ, IP, JvS and NvM contributed to the conception of functionomics and design of the work. EJ and JvS performed data acquisition and analysis. IP, JvS, YH, HS, HtN, LvR, PW and NvM helped interpret the data according to the ICF ontology. JvS, AD, BM and NvM helped interpret the data in the context of FAIR data and semantic web technology. EJ drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Esther R.C. Janssen .
Ethics declarations
Ethics approval and consent to participate.
This study was performed in accordance with the Declaration of Helsinki. This study was assessed by the local medical ethical committee AzM/UM (METC AzM/UM) and was considered not applicable to the Medical Research Involving Human Subject Act (number 2019 − 1426). Participant informed consent was obtained under the declaration of no objection.
Consent for publication
Competing interests.
Johan van Soest reports a relationship with Medical Data Works B.V. that includes: equity or stocks. Andre Dekker reports a relationship with Medical Data Works B.V. that includes: equity or stocks. The other authors have no competing interests to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Janssen, E.R., Punt, I.M., van Soest, J. et al. Operationalizing and digitizing person-centered daily functioning: a case for functionomics. BMC Med Inform Decis Mak 24 , 184 (2024). https://doi.org/10.1186/s12911-024-02584-2
Download citation
Received : 04 April 2023
Accepted : 21 June 2024
Published : 27 June 2024
DOI : https://doi.org/10.1186/s12911-024-02584-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Real-world data
- Interoperability
- Information technology
- Personalized healthcare
BMC Medical Informatics and Decision Making
ISSN: 1472-6947
- General enquiries: [email protected]
This paper is in the following e-collection/theme issue:
Published on 26.6.2024 in Vol 8 (2024)
Evaluating ChatGPT-4’s Accuracy in Identifying Final Diagnoses Within Differential Diagnoses Compared With Those of Physicians: Experimental Study for Diagnostic Cases
Authors of this article:

Original Paper
- Takanobu Hirosawa 1 , MD, PhD ;
- Yukinori Harada 1 , MD, PhD ;
- Kazuya Mizuta 1 , MD ;
- Tetsu Sakamoto 1 , MD ;
- Kazuki Tokumasu 2 , MD, PhD ;
- Taro Shimizu 1 , MD, MPH, MBA, PhD
1 Department of Diagnostic and Generalist Medicine, Dokkyo Medical University, Tochigi, Japan
2 Department of General Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
Corresponding Author:
Takanobu Hirosawa, MD, PhD
Department of Diagnostic and Generalist Medicine
Dokkyo Medical University
880 Kitakobayashi
Mibu-cho, Shimotsuga
Tochigi, 321-0293
Phone: 81 282861111
Email: [email protected]
Background: The potential of artificial intelligence (AI) chatbots, particularly ChatGPT with GPT-4 (OpenAI), in assisting with medical diagnosis is an emerging research area. However, it is not yet clear how well AI chatbots can evaluate whether the final diagnosis is included in differential diagnosis lists.
Objective: This study aims to assess the capability of GPT-4 in identifying the final diagnosis from differential-diagnosis lists and to compare its performance with that of physicians for case report series.
Methods: We used a database of differential-diagnosis lists from case reports in the American Journal of Case Reports , corresponding to final diagnoses. These lists were generated by 3 AI systems: GPT-4, Google Bard (currently Google Gemini), and Large Language Models by Meta AI 2 (LLaMA2). The primary outcome was focused on whether GPT-4’s evaluations identified the final diagnosis within these lists. None of these AIs received additional medical training or reinforcement. For comparison, 2 independent physicians also evaluated the lists, with any inconsistencies resolved by another physician.
Results: The 3 AIs generated a total of 1176 differential diagnosis lists from 392 case descriptions. GPT-4’s evaluations concurred with those of the physicians in 966 out of 1176 lists (82.1%). The Cohen κ coefficient was 0.63 (95% CI 0.56-0.69), indicating a fair to good agreement between GPT-4 and the physicians’ evaluations.
Conclusions: GPT-4 demonstrated a fair to good agreement in identifying the final diagnosis from differential-diagnosis lists, comparable to physicians for case report series. Its ability to compare differential diagnosis lists with final diagnoses suggests its potential to aid clinical decision-making support through diagnostic feedback. While GPT-4 showed a fair to good agreement for evaluation, its application in real-world scenarios and further validation in diverse clinical environments are essential to fully understand its utility in the diagnostic process.
Introduction
Diagnostic error and feedback.
A well-developed diagnostic process is fundamental to medicine. Diagnostic errors [ 1 ], which include missed, incorrect, or delayed diagnoses [ 2 ], result in severe misdiagnosis-related harm, affecting up to 795,000 patients annually in the United States [ 3 ]. These errors often stem from a failure to correctly identify an underlying condition [ 4 , 5 ]. Enhancing the diagnostic process is crucial, with diagnostic feedback playing a key role [ 6 ]. The feedback enables physicians to assess their diagnostic accuracy and adjust their subsequent clinical decisions accordingly [ 7 ]. Common diagnostic feedback methods include self-reflection [ 8 , 9 ], peer review [ 1 ], and clinical decision support systems (CDSSs), which aim to enhance decision-making at the point of care [ 10 ]. Unlike the retrospective nature of self and peer review processes, feedback from CDSSs is provided in real-time [ 11 ], offering immediate support and guidance during the diagnostic process. This timely feedback is particularly advantageous in fast-paced clinical settings where timely decision-making is critical.
CDSSs and Artificial Intelligence
CDSSs are categorized into 2 main types: knowledge-based and nonknowledge-based systems [ 10 ]. Knowledge-based CDSSs rely on established medical knowledge including clinical guidelines, expert protocols, and information on drug interactions. In contrast, nonknowledge-based systems, particularly those using artificial intelligence (AI), leverage advanced algorithms, machine learning, and statistical pattern recognition. Unlike their rule-based counterparts, these systems adapt over time, continuously refining their insights and recommendations. The rapid integration of AI into CDSSs highlights the growing importance of advanced technologies in health care [ 12 ]. In recent years, generative AI through large language models (LLMs) has been reshaping health care, offering improvements in diagnostic accuracy, treatment planning, and patient care [ 13 , 14 ]. AI systems, emulating human cognition, continuously learn from new data [ 15 ]. They assist health care professionals by analyzing complex patient data, thereby enhancing clinical decision-making and patient outcomes [ 10 ].
Growing Importance of Generative AI
In this context of rapidly integrating AI into CDSSs, generative AIs have marked a new era in digital health. LLMs are advanced AI algorithms trained on extensive textual data, enabling them to process and generate human-like text, thereby providing valuable insights to medical diagnostics. Several generative AI tools are now available to the public, including Bard (currently Gemini) by Google [ 16 , 17 ], LLM Meta AI 2 (LLaMA2) by Meta AI [ 18 ], and ChatGPT, developed by OpenAI [ 19 ]. These AI tools, which use LLMs, have successfully passed national medical licensing exams without specific training or reinforcement [ 20 ], demonstrating their potential in medical diagnostics. Among these, ChatGPT stands out as one of the most extensively researched generative AI applications in health care [ 21 ]. Specifically, in diagnostics, a recent study has shown that these generative AI systems, particularly ChatGPT with GPT-4, demonstrate excellent diagnostic capability when answering clinical vignette questions [ 22 ]. Additionally, other studies, including our own, have assessed AI systems’ performance in one aspect of the diagnostic process, generating differential diagnosis lists [ 23 - 25 ]. While broader studies compare a variety of state-of-the-art models, our analysis focuses on the distinct capabilities and impacts of these specific tools within medical diagnostics.
Generative AI Systems in the Diagnostic Process
The diagnostic process involves collecting clinical information, forming a differential diagnosis, and refining it through continuous feedback [ 26 ]. This feedback consists of patient outcomes, test results, and final diagnoses [ 27 , 28 ]. Similar to traditional CDSSs, generative AI systems can enhance this feedback loop [ 29 ]. However, a gap previously existed in the systematic comparison of differential diagnoses with final diagnoses through a feedback loop [ 27 ]. Given this background, it remains less explored how effectively these AI systems integrate their feedback into clinical workflow. To address this gap, exploring how generative AI systems provide feedback by comparing final diagnoses with differential-diagnosis lists represents a straightforward and viable first step. This study used differential diagnosis lists to assess diagnostic accuracy. This approach was chosen to mimic a key aspect of the clinical decision-making process, where physicians often narrow down a broad list of potential diagnoses to determine the most likely one. This method reflects a critical use case for AI in health care, potentially speeding up and refining diagnostic accuracy. In our previous short communication, we reported that the fourth generation ChatGPT (GPT-4) showed very good agreement with physicians in evaluating the lists for a limited number of case reports published from our General Internal Medicine (GIM) department [ 30 ]. Building on this research, this study focused on assessing the capability of GPT-4 in identifying the final diagnosis from differential-diagnosis lists for comprehensive case report series, compared with those of physicians. Furthermore, this research aimed to demonstrate the role of generative AI, particularly GPT-4, in enhancing the diagnostic learning cycle through effective feedback mechanisms.
We conducted an experimental study using GPT-4 and the differential-diagnosis lists generated by 3 AI systems inputting into case descriptions. The research was conducted at the Department of Generalist and Diagnostic Medicine (GIM), Dokkyo Medical University, Tochigi, Japan. Our research methodology encompassed preparing a data set for differential-diagnosis lists and the corresponding final diagnoses, assessing these lists using GPT-4, and having physicians evaluate the lists. Figure 1 illustrates this study flow.

Ethical Considerations
Since we used a database extracted from published case reports, obtaining ethical approval was not applicable.
Database of Differential-Diagnosis Lists and Final Diagnoses
We used our data set from a previous study (TH, YH, KM, T Sakamoto, KT, T Shimizu. Diagnostic performance of generative artificial intelligences for a series of complex case reports. unpublished data, November 2023). From the PubMed search, we identified a total of 557 case reports. We excluded the nondiagnosed cases (130 cases) and the pediatric cases, aged younger than 10 years (35 cases). The exclusion criteria were based on the previous research for CDSS [ 31 ]. After the exclusion, we included 392 case reports. The case reports were brushed up as case descriptions to focus on the diagnosis. The authors typically defined the final diagnoses. Through inputting into the case descriptions and systematic prompt, 3 generative AI systems—GPT-4, Google Bard (currently Google Gemini), and LLaMA2 chatbot—generated the top 10 differential-diagnosis lists. The AI systems used were not trained for any additional medical use or reinforced. The main investigator (TH) conducted the entire process, with validation provided by another investigator (YH). Through this process, this data set included differential diagnosis lists corresponding to case descriptions and final diagnoses from case reports in the American Journal of Case Reports . Detailed lists of differential diagnoses and their final diagnoses are shown in Multimedia Appendix 1 .
GPT-4 Assessment of the Differential-Diagnosis Lists
In selecting the generative AI systems for evaluation, we focused on GPT-4 due to its distinct architectural frameworks and widespread use in the field of health care research. GPT-4, developed by OpenAI, is notable for its advanced natural language processing capabilities and extensive training data set, making it particularly relevant for health care [ 32 ]. We used the August 3 version and September 25 version of GPT-4 to evaluate differential diagnosis lists. The access date was from September 11, 2023, to October 6, 2023. A structured prompt was crafted to ascertain whether GPT-4 could identify the final diagnosis within a list and its position if present. The prompt required direct copying and pasting of the final diagnoses and differential diagnosis lists from our data set. We assessed the inclusion of the final diagnosis in the list (Yes=1, No=0) and its position. The prompt selection was a preliminary investigation. To ensure unbiased output, each session was isolated by deactivating chat history and training controls and restarting GPT-4 before every new evaluation. We obtained a single output from GPT-4 for each differential diagnosis list. The details of this structured prompt in this study are expounded in Multimedia Appendix 2 .
Physician Assessment of the Differential-Diagnosis Lists
For comparison, 2 independent physicians (KM and T Sakamoto) also evaluated the differential diagnosis lists. The presence of the final diagnosis within the differential diagnosis lists was marked with a 1 or 0. A “1” was marked when the lists precisely and acceptably identified the final diagnosis [ 33 ], further ranking it from 1 to 10 based on its placement. A “0” indicated its absence. Discrepancies between the evaluations of the 2 physicians were resolved by another physician (KT). Notably, the physicians were blinded to which AI generated the lists they assessed. We selected 3 independent physicians, specializing in GIM. Selection was based on expertise in diagnostic processes and familiarity with AI technologies in health care. All physicians underwent a brief guidance session to familiarize themselves with the evaluation criteria and objectives of the study to ensure consistent assessment standards.
The primary outcome was defined as the κ coefficient for interrater agreement between GPT-4 and the physicians’ evaluations for the differential-diagnosis lists generated by 3 AI systems including GPT-4, Google Bard (currently Google Gemini), and LLaMA2 chatbot. The secondary outcomes were defined as the κ coefficients for interrater agreement between GPT-4 and the physicians’ evaluations for the differential diagnosis lists generated by each AI system. Additionally, another secondary outcome was defined as the ranking patterns between GPT-4’s evaluation and that of physicians.
Statistical Analysis
Analytical procedures were conducted using R (version 4.2.2; The R Foundation for Statistical Computing). The agreement between different evaluations was quantified using the Cohen κ coefficient through the irr package in R. Agreement strength was categorized as per Cohen κ benchmarks: values under 0.40 indicated poor agreement; values between 0.41 and 0.75 showed fair to good agreement; and values ranging from 0.75 to 1.00 denoted very good agreement [ 34 ]. The 95% CIs were used to quantify uncertainty. Additionally, we compared ranking patterns between GPT-4’s evaluation and that of physicians [ 35 ].
Overall Evaluation
This study involved 3 generative AI systems—GPT-4, Google Bard (currently Google Gemini), and LLaMA2 chatbot—outputting differential-diagnosis lists for 392 case descriptions, resulting in a total of 1176 lists. In 825 lists where physicians included a final diagnosis, GPT-4 matched 636 lists and did not match 189 lists. Conversely, in 351 lists where physicians did not include a final diagnosis, GPT-4 matched 330 lists and did not match 21 lists. In total, GPT-4’s evaluations matched the physicians’ evaluations in 966 out of 1176 lists (82.1%). Cohen κ coefficient was 0.63 (95% CI 0.56-0.69), indicating a fair to good agreement between GPT-4 and the physicians’ evaluations. GPT-4 omitted the final diagnosis in 16.1% (n=189) of cases, contrasting with physicians’ evaluations that included these diagnoses. Table 1 shows GPT-4’s evaluations concurred with the physicians’ evaluations. Table 2 details the κ coefficient for interrater agreement between GPT-4 and the physicians’ evaluations. The representative input used in GPT-4’s evaluations is illustrated in Figure 2 , and the corresponding output is shown in Figure 3 . A formed data set is shown in Multimedia Appendix 3 .
| Variables | GPT-4 | Total (N=1176) | |
| Matched | Did not match | ||
| Inclusion of final diagnosis | 636 | 189 | 825 |
| Noninclusion of final diagnosis | 330 | 21 | 351 |
| Differential-diagnosis lists generator | Cohen κ coefficient (95% CI) | Strength of agreement [ ] | Number of differential-diagnosis lists |
| All | 0.63 (0.56-0.69) | Fair to good | 1176 |
| GPT-4 | 0.47 (0.39-0.56) | Fair to good | 392 |
| Google Bard | 0.67 (0.52-0.73) | Fair to good | 392 |
| LLaMA2 chatbot | 0.63 (0.52-0.73) | Fair to good | 392 |
a Currently Google Gemini.
b LLaMA2: LLM Meta AI 2.

Evaluation of Each Generative AI
The κ coefficients for differential-diagnosis lists generated by GPT-4, Google Bard (currently Google Gemini), and LLaMA2 chatbot were 0.47 (95% CI 0.39-0.56), 0.67 (95% CI 0.52-0.73), and 0.63 (95% CI 0.52-0.73), respectively. All κ coefficients indicated a fair to good agreement between GPT-4 and the physicians’ evaluations.
Comparison of Ranking Patterns Between GPT-4 and Physicians
Both GPT-4’s evaluation and that of physicians showed a general trend of decreasing frequency as the rank increases. Figure 4 shows the comparisons of ranking patterns between GPT-4 and physicians.

Evaluation Between Physicians
Physicians’ evaluations (KM and T Sakamoto) for the differential diagnosis lists showed very good agreement, with concordance in 88.8% (n=1044) of cases. The κ coefficient was 0.75 (95% CI 0.46-0.99).
Principal Results
This experimental study highlights several key findings. First, GPT-4’s evaluations matched those of physicians in more than 82% (n/N=966/1176) of the cases, demonstrating fair to good agreement according to κ coefficient values. These results imply that GPT-4’s accuracy in identifying the final diagnosis within differential-diagnosis lists is comparable to that of physicians. Unlike traditional CDSSs, generative AI systems, including GPT-4, are capable of performing multiple roles in the diagnostic process including formulating and assessing differential diagnoses. These capabilities highlight GPT-4’s potential to streamline diagnostics in clinical settings by expediting diagnostic feedback [ 36 ]. Our study design focuses on GPT-4’s ability to refine and validate pre-existing diagnostic considerations as supplementary tools for medical diagnostics. This scenario is akin to real-world clinical settings where generative AI systems could verify and support physicians’ final diagnostic decisions. By assessing the AI’s accuracy in this context, we can better understand its potential role and limitations in practical medical applications. Furthermore, in medical education, generative AI tools, like GPT-4, can offer students valuable self-learning opportunities. They provide timely feedback in the form of final diagnoses [ 37 ], enabling them to cross-reference with reliable sources for verification [ 38 ].
Second, GPT-4 failed to identify the final diagnosis in 16% (n/N=189/1176) of differential-diagnosis lists, even though these diagnoses were recognized by the evaluating physicians. Notably, despite achieving very good agreement among physicians, GPT-4 did not reach similar levels of concordance. This discrepancy highlights potential areas for improving the system’s ability to interpret and analyze complex medical data. This discrepancy arises primarily from GPT-4’s reliance on textual patterns and word associations within the provided differential diagnosis lists. Unlike physicians, who use a comprehensive medical knowledge base and clinical experience, an inherent limitation in generative AI systems like GPT-4 is their reliance on existing data patterns and textual association. To mitigate these discrepancies, continuous development in generative AI systems for health care is needed. Additionally, future research should focus on enhancing the medical training of these systems. This will enhance the generative AI systems’ diagnostic feedback, making it more adaptable to real clinical settings.
Third, regarding evaluation at what rank in the differential-diagnosis list was the final diagnosis found, both GPT-4 and physicians exhibited a trend of decreasing frequency. This suggests GPT-4’s diagnosis ranking shows a similar trend to physicians’ diagnosis ranking. Moreover, all 3 generative AI systems, including GPT-4, Google Bard (currently Google Gemini), and LLaMA2 chatbot, prioritized the most likely diagnoses at the top of the list, leading to a natural decrease in frequency as less-probable diagnoses are ranked lower. Therefore, generative AI systems showed the potential not only to generate differential diagnosis lists for clinical cases but also to evaluate these lists as feedback.
Fourth, an examination of the differential diagnosis lists generated by 3 different AI systems showed the overlap in the 95% CI for the κ coefficients across the 3 AI platforms. One might hypothesize that GPT-4 would exhibit improved performance when evaluating differential-diagnosis lists it generated itself. However, observed results may stem from the inherent variability in generative AI outputs including GPT-4. This inherent variability underscores the challenge of maintaining a consistent standard of accuracy and reliability in the outputs from generative AI systems. Even when evaluating differential-diagnosis lists generated by itself, GPT-4’s performance did not markedly surpass that of lists generated by other AI systems. Additionally, the observed performance differences may be partially due to version inconsistencies. The generation of differential diagnosis lists used an earlier version of GPT-4 (March 24). Subsequent evaluations used later versions (August 3 and September 25). Different versions of generative AI systems can exhibit varied capabilities and outputs, potentially impacting the accuracy and consistency of diagnostic evaluations. This highlights the need for ongoing updates and version alignment in clinical AI applications to maintain reliability.
Limitations
This study has several limitations. First, GPT-4’s role was limited to identifying the final diagnosis within the differential diagnosis list. The current binary evaluation method has not been a well-established approach to evaluating diagnostic performance by other CDSSs. Another study used a 5-grade level of accuracy for a variety number of differentials [ 39 ]. Investigating more complex outcomes, such as quantitative evaluations and additional clinical suggestions, might yield different results. Second, our inputs to GPT-4 consisted only of the final diagnoses and the differential diagnosis list, without the case descriptions that generated these lists. Further research should examine what types of input enhance AI systems’ performance the most. Third, there was a nonnegligible risk associated with generative AI systems, including GPT-4, regarding their capacity to inadvertently learn from and replicate the information contained in publicly available case reports. Fourth, the data set was sourced from a single case reports journal and generated by 3 AI systems. Future research would benefit from using real-world scenarios [ 40 ]. Expanding the data set to include a more diverse range of AI systems is also advisable.
Regarding limitations for generative AI systems, like GPT-4, there is currently no approval for their use as CDSSs. Furthermore, GPT-4 operates as a fee-based application, which could potentially limit its accessibility to the wider public. Additionally, the reliability of generative AI systems can vary based on the input data it was trained on. If it is not exposed to diverse clinical scenarios during its training, it may not be as effective in real-world diagnostic situations [ 41 ]. Moreover, while AI tools can assist, they do not replace the nuanced judgments and decision-making processes of human physicians [ 42 , 43 ]. Additionally, the rapid evolution of AI means that our findings may become outdated as Google Bard and LLaMA2 were updated to the new LLM model, Google Gemini and LLaMA3, respectively [ 17 , 44 ]. Finally, overreliance on AI without critical review could lead to diagnostic errors [ 45 ].
Comparison With Prior Work
In our previous study involving GPT-4 [ 30 ], we observed a very good agreement with physicians in identifying final diagnoses within the differential-diagnosis lists, achieving a 95.9% agreement rate (236 out of 246 lists; κ=0.86). In contrast, this study demonstrated a fair to good agreement rate of 82.1% (966/1176 lists; κ=0.63). Despite using the same evaluation methods in both studies, the observed decrease in the agreement can be attributed to several factors: the source of case reports (GIM-published vs a broader range of case reports), the generators of differential diagnoses (physicians, GPT-3/GPT-4 vs GPT-4/Google Bard [currently Gemini]/LLaMA2 chatbot), and the volume of lists assessed (246 lists vs 1176 lists).
Future Directions
Future studies explore the potential of integrating GPT-4 and similar AI systems into real-world clinical settings. This could involve developing interfaces that allow these AI systems to interact directly with electronic health records, providing real-time diagnostic feedback to physicians. Additionally, research could focus on tailoring these AI systems for specialized medical fields, where their ability to process vast amounts of data could significantly aid in complex case analysis. Another vital area for future research is the ethical implications of AI in medicine [ 43 ], particularly in patient data privacy, AI decision transparency, and the impact of AI-assisted diagnostics on physician-patient relationships.
Furthermore, further research should also investigate the optimal use of AI technologies, including the exploration of both chatbot interfaces and application programming interface functionalities. A more detailed examination of application programming interface settings, such as adjustable parameters including temperature and Top P, could be invaluable. This investigation would provide clearer guidelines on when and how to use different AI tools effectively, considering both scientific evidence and effectiveness.
Moreover, our future research will focus on refining the evaluation of AI-generated differential diagnoses by incorporating more sophisticated and validated psychometric methods as the next diagnostic step. We propose to adopt methodologies for assessing the quality of differential diagnoses. This approach will allow us not only to compare AI-generated outputs with those from physicians but also to treat it as a form of Turing test—evaluating whether AI can match or surpass human performance in diagnostic tasks without being distinguishable from them [ 46 ].
Conclusions
GPT-4 demonstrated a fair to good agreement in identifying the final diagnosis from differential-diagnosis lists, comparable to physicians for case report series. By reliably identifying diagnoses, GPT-4 can provide on-time feedback by comparing final diagnoses with differential-diagnosis lists. Therefore, this study suggests that generative AI systems have the potential to assist physicians in the diagnostic process by providing reliable and efficient feedback, thereby contributing to improved clinical decision-making and medical education. However, it is imperative to recognize that these findings are based on experimental studies. Real-world scenarios could present unique challenges, and further validations in diverse clinical environments are essential before broad implementation can be recommended.
Acknowledgments
This research was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant 22K10421). This study was conducted using resources from the Department of Diagnostics and Generalist Medicine at Dokkyo Medical University.
Authors' Contributions
TH, YH, KM, T Sakamoto, KT, and T Shimizu contributed to the study concept and design. TH performed the statistical analyses. TH contributed to the drafting of the manuscript. YH, KM, T Sakamoto, KT, and T Shimizu contributed to the critical revision of the manuscript for relevant intellectual content. All the authors have read and approved the final version of the manuscript.
Conflicts of Interest
None declared.
The differential-diagnosis generated by 3 artificial intelligences used in this study and the final diagnosis.
Structured prompt used in this study.
Formed data set used in this study.
- Balogh EP, Miller BT, Ball JR. Improving Diagnosis in Health Care. Washington, DC. National Academies Press; 2015.
- Graber M. Diagnostic errors in medicine: a case of neglect. Jt Comm J Qual Patient Saf. 2005;31(2):106-113. [ CrossRef ] [ Medline ]
- Newman-Toker DE, Nassery N, Schaffer AC, Yu-Moe CW, Clemens GD, Wang Z, et al. Burden of serious harms from diagnostic error in the USA. BMJ Qual Saf. 2024;33(2):109-120. [ CrossRef ] [ Medline ]
- Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. [ CrossRef ] [ Medline ]
- Schiff GD, Hasan O, Kim S, Abrams R, Cosby K, Lambert BL, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. [ CrossRef ] [ Medline ]
- Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. [ CrossRef ] [ Medline ]
- Meyer AND, Singh H. The path to diagnostic excellence includes feedback to calibrate how clinicians think. JAMA. 2019;321(8):737-738. [ CrossRef ] [ Medline ]
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475. [ CrossRef ] [ Medline ]
- Mamede S, Schmidt HG. Reflection in medical diagnosis: a literature review. Health Prof Educ. 2017;3(1):15-25. [ CrossRef ]
- Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Rubins D, McCoy AB, Dutta S, McEvoy DS, Patterson L, Miller A, et al. Real-time user feedback to support clinical decision support system improvement. Appl Clin Inform. 2022;13(5):1024-1032. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Haug CJ, Drazen JM. Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med. 2023;388(13):1201-1208. [ CrossRef ] [ Medline ]
- Liu J, Wang C, Liu S. Utility of ChatGPT in clinical practice. J Med Internet Res. 2023;25:e48568. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. 2023;23(1):689. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Collins C, Dennehy D, Conboy K, Mikalef P. Artificial intelligence in information systems research: a systematic literature review and research agenda. Int J Inf Manage. 2021;60:102383. [ CrossRef ]
- Patrizio A. Google Gemini (formerly Bard). TechTarget. Mar 2024. URL: https://www.techtarget.com/searchenterpriseai/definition/Google-Bard [accessed 2024-06-01]
- Sundar PD. Introducing Gemini: our largest and most capable AI model. Google. Dec 06, 2023. URL: https://blog.google/technology/ai/google-gemini-ai/#sundar-note [accessed 2024-06-01]
- Touvron H, Martin L, Stone K, Albert P, Almahairi A, Babaei Y. Llama 2: open foundation and fine-tuned chat models. arXiv. Preprint posted online July 18, 2023. [ CrossRef ]
- Achiam J, Adler S, Agarwal S. GPT-4 Technical Report. arXiv. Preprint posted online March 15, 2023. [ CrossRef ]
- Sai S, Gaur A, Sai R, Chamola V, Guizani M, Rodrigues JJPC. Generative AI for transformative healthcare: a comprehensive study of emerging models, applications, case studies, and limitations. IEEE Access. 2024;12:31078-31106. [ CrossRef ]
- Cascella M, Montomoli J, Bellini V, Bignami E. Evaluating the feasibility of ChatGPT in healthcare: an analysis of multiple clinical and research scenarios. J Med Syst. 2023;47(1):33. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Han T, Adams LC, Bressem KK, Busch F, Nebelung S, Truhn D. Comparative analysis of multimodal large language model performance on clinical vignette questions. JAMA. 2024;331(15):1320-1321. [ CrossRef ] [ Medline ]
- Hirosawa T, Kawamura R, Harada Y, Mizuta K, Tokumasu K, Kaji Y, et al. ChatGPT-generated differential diagnosis lists for complex case-derived clinical vignettes: diagnostic accuracy evaluation. JMIR Med Inform. 2023;11:e48808. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hirosawa T, Mizuta K, Harada Y, Shimizu T. Comparative evaluation of diagnostic accuracy between Google Bard and physicians. Am J Med. Nov 2023;136(11):1119-1123.e18. [ CrossRef ] [ Medline ]
- Kanjee Z, Crowe B, Rodman A. Accuracy of a generative artificial intelligence model in a complex diagnostic challenge. JAMA. 2023;330(1):78-80. [ CrossRef ] [ Medline ]
- Price RB, Vlahcevic ZR. Logical principles in differential diagnosis. Ann Intern Med. 1971;75(1):89-95. [ CrossRef ] [ Medline ]
- Fernandez Branson C, Williams M, Chan TM, Graber ML, Lane KP, Grieser S, et al. Improving diagnostic performance through feedback: the diagnosis learning cycle. BMJ Qual Saf. 2021;30(12):1002-1009. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Rosner B, Zwaan L, Olson A. Imagining the future of diagnostic performance feedback. Diagnosis (Berl). 2023;10(1):31-37. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Filiberto A, Leeds I, Loftus T. Editorial: machine learning in clinical decision-making. Front Digit Health. 2021;3:784495. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Mizuta K, Hirosawa T, Harada Y, Shimizu T. Can ChatGPT-4 evaluate whether a differential diagnosis list contains the correct diagnosis as accurately as a physician? Diagnosis (Berl). 2024. [ CrossRef ] [ Medline ]
- Graber ML, Mathew A. Performance of a web-based clinical diagnosis support system for internists. J Gen Intern Med. 2007;23(S1):37-40. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nori H, King N, McKinney S, Carignan D, Horvitz E. Capabilities of GPT-4 on medical challenge problems. arXiv. Preprint posted online March 20, 2024. [ CrossRef ]
- Krupat E, Wormwood J, Schwartzstein RM, Richards JB. Avoiding premature closure and reaching diagnostic accuracy: some key predictive factors. Med Educ. Nov 2017;51(11):1127-1137. [ CrossRef ] [ Medline ]
- Fleiss J, Levin B, Paik M. Statistical Methods for Rates and Proportions. Hoboken, NJ. John Wiley & Sons; 2003.
- Webber W, Moffat A, Zobel J. A similarity measure for indefinite rankings. ACM Trans Inf Syst. Nov 23, 2010;28(4):1-38. [ CrossRef ]
- Hattie J, Timperley H. The power of feedback. Rev Educ Res. 2016;77(1):81-112. [ FREE Full text ] [ CrossRef ]
- Chamberland M, Setrakian J, St-Onge C, Bergeron L, Mamede S, Schmidt HG. Does providing the correct diagnosis as feedback after self-explanation improve medical students diagnostic performance? BMC Med Educ. 2019;19(1):194. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Abd-Alrazaq A, AlSaad R, Alhuwail D, Ahmed A, Healy PM, Latifi S, et al. Large language models in medical education: opportunities, challenges, and future directions. JMIR Med Educ. 2023;9:e48291. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bond WF, Schwartz LM, Weaver KR, Levick D, Giuliano M, Graber ML. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27(2):213-219. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Painter A, Hayhoe B, Riboli-Sasco E, El-Osta A. Online symptom checkers: recommendations for a vignette-based cinical evaluation standard. J Med Internet Res. 2022;24(10):e37408. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Wiens J, Saria S, Sendak M, Ghassemi M, Liu VX, Doshi-Velez F, et al. Do no harm: a roadmap for responsible machine learning for health care. Nat Med. 2019;25(9):1337-1340. [ CrossRef ] [ Medline ]
- Karches KE. Against the iDoctor: why artificial intelligence should not replace physician judgment. Theor Med Bioeth. 2018;39(2):91-110. [ CrossRef ] [ Medline ]
- Ethics and governance of artificial intelligence for health: WHO guidance. World Health Organization. 2021. URL: https://www.who.int/publications/i/item/9789240029200 [accessed 2024-06-12]
- Build the future of AI with Meta Llama 3 2024. Meta AI. 2024. URL: https://llama.meta.com/llama3/ [accessed 2024-05-24]
- Passi S, Vorvoreanu M. Overreliance on AI literature review. Microsoft. 2022. URL: https://www.microsoft.com/en-us/research/publication/overreliance-on-ai-literature-review/ [accessed 2024-06-12]
- Pinar SA, Cicekli I, Akman V. Turing test: 50 years later. Minds Mach. 2000;10(4):50. [ FREE Full text ] [ CrossRef ]
Abbreviations
| artificial intelligence |
| clinical decision support system |
| general internal medicine |
| LLM Meta AI 2 |
| large language model |
Edited by A Mavragani; submitted 08.04.24; peer-reviewed by A Rodman, C Zhang; comments to author 24.04.24; revised version received 28.04.24; accepted 04.05.24; published 26.06.24.
©Takanobu Hirosawa, Yukinori Harada, Kazuya Mizuta, Tetsu Sakamoto, Kazuki Tokumasu, Taro Shimizu. Originally published in JMIR Formative Research (https://formative.jmir.org), 26.06.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR Formative Research, is properly cited. The complete bibliographic information, a link to the original publication on https://formative.jmir.org, as well as this copyright and license information must be included.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Community Med
- v.12(2); May-Aug 2005
TEACHING TIPS: TWELVE TIPS FOR MAKING CASE PRESENTATIONS MORE INTERESTING *
1. set the stage.
Prepare the audience for what is to come. If the audience is composed of people of mixed expertise, spend a few minutes forming them into small mixed groups of novices and experts. Explain that this is an opportunity for the more junior to learn from the more senior people. Tell them that the case to be presented is extremely interesting, why it is so and what they may learn from it. The primary objective is to analyze the clinical reasoning that was used rather than the knowledge required, although the acquisition of such knowledge is an added benefit of the session. A “well organized case presentation or clinicopathological conference incorporates the logic of the workup implicitly and thus makes the diagnostic process seem almost preordained”.
A psychiatry resident began by introducing the case as an exciting one, explaining the process and dividing the audience into teams mixing people with varied expertise. He urged everyone to think in ‘real time’ rather than jump ahead and to refrain from considering information that is not normally available at the time: for example, a laboratory report that takes 24 hours to obtain be assessed in the initial workup.
2. PROVIDE ONLY INITIAL CUES AT FIRST
Give them the first two to live cues that were picked up in the first minute or two of the patient encounter either verbally, or written on a transparency. For example, age, sex race and reason for seeking medical help. Ask each group to discuss their first diagnostic hypotheses. Experts and novices will learn a great deal from each other at this stage and the discussions will be animated. The initial cues may number only one or two and hypothesis generation occurs very quickly even in the novices. Indeed, the only difference between the hypotheses of novices and those of experts is in the degree of refinement, not in number.
It is Saturday afternoon and you are the psychiatric emergency physician. A 25-year-old male arrives by ambulance and states that he is feeling suicidal. Groups talked for 4 minutes before the resident called for order to commence step three.
3. ASK FOR HYPOTHESES AND WRITE THEM UP ON THE BLACKBOARD
Call for order and ask people to offer their suggested diagnoses and write these up on a board or transparency.
The following hypotheses were suggested by the groups and the resident wrote them on a flip chart: depression, substance abuse, recent social stressors-crisis, adjustment disorder, organic problem, dysthymia, schizophrenia, bipolar affective disorder. The initial three or four bits of information generated eight hypotheses.
4. ALLOW THE AUDIENCE TO ASK FOR INFORMATION
After all hypotheses have been listed instruct the audience to ask for the information they need to confirm or refute these hypotheses. Do not allow them to ‘jump the gun’ by asking for a test result, for example, that would not have been received within the time frame that is being re-lived. There will be a temptation to move too fast and the exercise is wasted if information is given too soon. Recall that the purpose is to help them go through a thinking process which requires time.
Teachers participating in this exercise will receive much diagnostic information about students’ thinking at this stage. Indeed, an interesting teaching session can be conducted by simply asking students to generate hypotheses without proceeding further. There is evidence to suggest that when a diagnosis is not considered initially it is unlikely to be reached over time, Hence it is worth spending time with students to discuss the hypotheses they generate before they proceed with an enquiry.
Directions to the group were to determine what questions they would like to ask, based on gender, age and probabilities, to support or exclude the listed diagnostic possibilities. A sample of question follow:
- Does he work? No, he's unemployed.
- Does he drink? one to three beers a week.
- Why now? He's been feeling worse and worse for the last 3 weeks.
- Social support? He gives alone. Has no girlfriend.
- Appearance? Looks his age. Not shaved today. No shower in 3 days.
- Cultural background? Refugee from Iraq. Muslim.
- How did he get here? He spent 4 years in a refugee camp after spending 4 months walking to Pakistan from Iraq. He left Iraq to avoid military service.
- Suicide thoughts? Increasing the last 3 weeks. He was admitted in December and has been taking chloral hydrate.
This step took 13 minutes.
5. HAVE THE AUDIENCE RE-FORMULATE THEIR LIST OF HYPOTHESES
After enough information has been gained to proceed, ask them to resume their discussion about the problem and reformulate their diagnostic hypotheses in light of the new information. Instruct them to discuss which pieces of information changed the working diagnosis and why. Call for order again and ask people what they now think.
After allowing the group to talk for a few minutes, the resident asked them if there was enough information to strike off any hypotheses or if new hypotheses should be added to the list. One more possibility was added, post-traumatic stress disorder (PTSD). One group's list of priorities was major affective disorder with psychosis, schizophrenia, personality disorder. Another group also placed affective disorder first followed by organic mood disorder.
This step took 25 minutes.
6. FACILITATE A DISCUSSION ABOUT REASONING
Alter the original lists of hypotheses on the board in light of the discussion, or allow one member from each group to alter their own lists. By the use of open-ended questions encourage a general discussion about the reasons a group has for preferring one diagnosis over another.
A general discussion ensued about reasons for these priorities. Then the list was altered so that it read: schizophrenia, personality disorder, PTSD, major affective disorder with psychosis, organic mood disorder.
7. ALLOW ANOTHER ROUND OF INFORMATION SEEKING
Continue with another round of information and small-group discussion or else allow the whole group to interact. By giving information only when asked for and only in correct sequence, each person is challenged to think through the problem.
More information was sought, such as: form of speech? eye contact? affect? substance use? After 5 minutes the resident asked if there were only lab tests they would like. The group asked for thyroid stimulating hormone, T4, electrolytes and were given the results. They also asked for the results of the physical examination and were told that the pulse was 110 and the thyroid was enlarged. At this point some hypotheses were removed from the list.
8. ASK GROUPS TO REACH A FINAL DIAGNOSIS
When there is a lull in the search for information, ask the groups to reach consensus on their final diagnosis, given the information they have. Allow discussion within the groups.
9. CALL FOR EACH GROUP'S FINAL DIAGNOSIS
On each group's list of hypothesis, star or underline the final diagnosis.
The group decided that the most likely diagnosis was affective disorder with psychosis, the actual working diagnosis of the patient.
10. ASK FOR MANAGEMENT OPTIONS
If there is enough time, ask them to form small groups again to discuss treatment options, or conduct the discussion as a large group. Again ask for the reasons why one approach is preferred over another. Particularly ask the experts in the room for their reasoning so that the novices can learn from them.
11. SUMMARIZE
By the time the end is in sight the audience will be so involved that they will not wish to leave. However, 5 minutes before time, call for order and summarize the session. Highlight the key points that have been raised and refer to the objective of the session.
We are now at the end of our time. You have all had the opportunity to use your clinical reasoning skills to generate several hypotheses which are shown on the board. Initially you thought it possible that this man could have any one of a number of diagnoses including depression, substance abuse, adjustment disorder with depressed mood, organic mood disorder or post-traumatic stress disorder. With further information the possible diagnosis shifted to include schizophrenia and personality disorder as well as depression with psychotic features. Finally the diagnosis of depression or mood disorder with psychosis was most strongly supported because of the history of consistently depressed mood over several months, along with disturbed sleep, poor appetite, weight loss, decreased energy and diminished interest in most activities. The initially abnormal thyroid test proved to be a red herring so organic mood disorder related to hyper- or hypo-thyroidism was excluded. Additionally absence of vivid dreams involving a traumatic event made a diagnosis of post-traumatic stress disorder unlikely. Although a diagnosis of schizophrenia could not be totally excluded, this seemed less likely given the findings.
12. CLOSE THE SESSION WITH POSITIVE FEEDBACK
In some respects, but only some, teaching is like acting and one should strive to leave them not laughing as you go, but feeling that they have learned something.
The more novice members of the group have learned from the more experienced and all your suggestions have been valid. It has been interesting for me to follow your reasoning and compare it with mine when I actually saw this man. You have given me a different perspective as you thought of things I had not, and I thank you for your participation.
Although case presentation should be a major learning experience for both novice and experienced physicians they are often conducted in a stultifying way that defies thought. We have presented a series of steps which, if followed, guarantee active participation from the audience and ensure that if experts are in the room their expertise is used. Physicians have been moulded to believe that teaching means telling and, as a consequence, adopt a remote listening stance during case presentations. Indeed the back row often use the time to catch up on much needed sleep! Changing the format requires courage. We urge you to try out these steps so that both you and your audience will learn from and enjoy the process.

COMMENTS
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
First steps. Begin by sitting down with your medical team to discuss the interesting aspects of the case and the learning points to highlight. Ideally, a registrar or middle grade will mentor you and give you guidance. Another junior doctor or medical student may also be keen to be involved. Allocate jobs to split the workload, set a deadline ...
5. Complete your introduction and conclusion after you've written the body. Since these sections summarize key points of your case study, it's best to write them last. The introduction gives a brief overview of the basic condition of your patient and the problem you'll address in your case study.
A clinical case report or case study is a means of disseminating new knowledge gained from clinical practice. Medical practitioners often come across patient cases that are different or unusual such as a previously unknown condition, a complication of a known disease, an unusual side effect or adverse response to a mode of treatment, or a new ...
All those previous examples show how important is case report in the advancement of medical practice. The case report might be in the tail of the hierarchy of evidence-based medicine but if properly selected and appropriately reported it might stand a better chance of publication in high impact journals than even a clinical trial.
It is best to simply tell the story and let the outcome speak for itself. With these points in mind, let's begin the process of writing the case study: Title page: Title: The title page will contain the full title of the article. Remember that many people may find our article by searching on the internet.
Writing a case report is an excellent way of documenting these findings for the wider medical community—sharing new knowledge that will lead to better and safer patient care. For many medical students and junior doctors, a case report may be their first attempt at medical writing. A published case report will look impressive on your ...
Case reports are a time-honored tradition in the medical profession. From Hippocrates (460 B.C. to 370 B.C.), and even arguably further back since the papyrus records of ancient Egyptian medicine (c. 1600 B.C.) to modern day, physicians of all specialties have described interesting cases involving all specialties [1, 2].Published case reports provide essential information for optimal patient ...
Sometimes case reports include a short literature review, if you think it is worthwhile, include it. 2. Describe your patient and follow the diagnostic pathway. For example - Patient X is a 10 ...
A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. The most important aspect of a case report, i.e. the reason you would go to the trouble of writing one, is that the case is sufficiently unique, rare or interesting such that other medical professionals will learn something from it.
Don't publish a case report without the patient's consent. As explained above, informed patient consent is mandatory for the publication of your case reports. Ignoring this requirement can result in a rejection for your work and worse, ruin your relationship and reputation with patients. However, there is an exception for publishing a case ...
A 53 year old man presents to clinic with swelling of his hands and a uric acid of 12. 15. A 58-year-old woman presents to clinic with difficulty walking. 16. A 49-year-old woman is seen with an abnormal Nerve Conduction Study. 17. A 55-year-old woman is seen because of her right knee is "giving out". 18.
Step 2 - Select a Journal. What target audience (doctors & patients) would best benefit from the info? If indexed in PubMed, it is peer reviewed and locatable. Some journals do NOT publish case reports. FIRST double-check the journal's "Author's Guidelines," or "Author's Instructions" to see "Types of Articles".
Case study is a research methodology, typically seen in social and life sciences. There is no one definition of case study research.1 However, very simply… 'a case study can be defined as an intensive study about a person, a group of people or a unit, which is aimed to generalize over several units'.1 A case study has also been described as an intensive, systematic investigation of a ...
Structure Your Report. Once you have selected the journal of submission, carefully reread the author instructions to structure your submission. The JACC: Case Reports authors instructions suggest a specific structure for a clinical case: history of presentation, physical examination, past medical history, differential diagnosis, investigations, management (medical/interventions), discussion ...
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence. 1 The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the ...
Case studies can help others (e.g., students, other organizations, employees) learn about • new concepts, • best practices, and • situations they might face. Writing a case study also allows you to critically examine your organizational practices. Examples The following pages provide examples of different types of case study formats. ...
A case study is a detailed analysis of a specific topic in a real-world context. It can pertain to a person, place, event, group, or phenomenon, among others. The purpose is to derive generalizations about the topic, as well as other insights. Case studies find application in academic, business, political, or scientific research.
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the ...
A case presentation is a narrative of a patient's care, so it is vital the presenter has familiarity with the patient, the case and its progression. The preparation for the presentation will depend on what information is to be included. Generally, oral case presentations are brief and should be limited to 5-10 minutes.
A case study was selected to demonstrate an application of the guide. An eight-step guide to causal study design was created, integrating essential concepts from the literature, anchored into conceptual groupings according to natural steps in the study design process. ... This bias can be addressed through medical record validation for a random ...
Introduction. For many doctors and other healthcare professionals, writing a case report represents the first effort at getting articles published in medical journals and it is considered a useful exercise in learning how to write scientifically due to similarity of the basic methodology.1 Case reports aim to convey a clinical message.2,3 Despite different types of case reports, they all aim ...
An ontology was formulated (column A, Fig. 3) to make the data from case study interoperable. The created ontology only provides classes for concepts in our used case. It should be viewed as an example of how the allied health research community can approach building a functionomics ontology. ... This study was assessed by the local medical ...
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table.
Background: The potential of artificial intelligence (AI) chatbots, particularly ChatGPT with GPT-4 (OpenAI), in assisting with medical diagnosis is an emerging research area. However, it is not yet clear how well AI chatbots can evaluate whether the final diagnosis is included in differential diagnosis lists. Objective: This study aims to assess the capability of GPT-4 in identifying the ...
1. SET THE STAGE. Prepare the audience for what is to come. If the audience is composed of people of mixed expertise, spend a few minutes forming them into small mixed groups of novices and experts. Explain that this is an opportunity for the more junior to learn from the more senior people. Tell them that the case to be presented is extremely ...