Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality
Affiliations.
- 1 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 2 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 3 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 4 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 5 St Luke's Radiation Oncology Network, St. James's Hospital, Dublin, Ireland. Electronic address: [email protected].
- 6 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 7 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- 8 Nuffield Department of Population Health, University of Oxford, Oxford, UK. Electronic address: [email protected].
- PMID: 35367784
- PMCID: PMC9096622
- DOI: 10.1016/j.ctrv.2022.102375
Background: Adjuvant and neoadjuvant breast cancer treatments can reduce breast cancer mortality but may increase mortality from other causes. Information regarding treatment benefits and risks is scattered widely through the literature. To inform clinical practice we collated and reviewed the highest quality evidence.
Methods: Guidelines were searched to identify adjuvant or neoadjuvant treatment options recommended in early invasive breast cancer. For each option, systematic literature searches identified the highest-ranking evidence. For radiotherapy risks, searches for dose-response relationships and modern organ doses were also undertaken.
Results: Treatment options recommended in the USA and elsewhere included chemotherapy (anthracycline, taxane, platinum, capecitabine), anti-human epidermal growth factor 2 therapy (trastuzumab, pertuzumab, trastuzumab emtansine, neratinib), endocrine therapy (tamoxifen, aromatase inhibitor, ovarian ablation/suppression) and bisphosphonates. Radiotherapy options were after breast conserving surgery (whole breast, partial breast, tumour bed boost, regional nodes) and after mastectomy (chest wall, regional nodes). Treatment options were supported by randomised evidence, including > 10,000 women for eight treatment comparisons, 1,000-10,000 for fifteen and < 1,000 for one. Most treatment comparisons reduced breast cancer mortality or recurrence by 10-25%, with no increase in non-breast-cancer death. Anthracycline chemotherapy and radiotherapy increased overall non-breast-cancer mortality. Anthracycline risk was from heart disease and leukaemia. Radiation-risks were mainly from heart disease, lung cancer and oesophageal cancer, and increased with increasing heart, lung and oesophagus radiation doses respectively. Taxanes increased leukaemia risk.
Conclusions: These benefits and risks inform treatment decisions for individuals and recommendations for groups of women.
Keywords: Adjuvant treatments; Breast cancer; Neoadjuvant treatments; Treatment benefits; Treatment harms.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.

Publication types
- Systematic Review
- Breast Neoplasms* / drug therapy
- Breast Neoplasms* / pathology
- Chemotherapy, Adjuvant
- Neoadjuvant Therapy
- Tamoxifen / therapeutic use
Grants and funding
- A21133/CRUK_/Cancer Research UK/United Kingdom
Advertisement
Advancements in Oncologic Surgery of the Breast: A Review of the Literature
- Published: 27 February 2024
Cite this article

- Tiffany J. Nevill 1 ,
- Kelly C. Hewitt 2 &
- Rachel L. McCaffrey 2
42 Accesses
7 Altmetric
Explore all metrics
Purpose of Review
This review article is an update on the state of surgical options in the treatment of breast cancer. We seek to provide readers with the best practices for oncologically safe and cosmetically superior breast surgery.
Recent Findings
This article reviews the latest technologies for partial mastectomy, advances in and oncologic safety of mastectomy including skin and nipple sparing techniques, and a review of oncoplastic breast surgery techniques.
Our goal is to inform all surgeons who treat patients with breast cancer that many options remain available for the surgical treatment of breast cancer.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

The Optimal Approach to Post-Mastectomy and Post-Lumpectomy Breast Reconstruction

Overview of Oncoplastic Breast Surgery Techniques for the Treatment of Breast Cancer with Review of Normal and Abnormal Postsurgical Imaging Findings

Mastectomy: Simple (Total), Modified, and Classical Radical
Data availability.
No datasets were generated or analyzed during the current study.
Halsted WSI. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46(1):1–19. https://doi.org/10.1097/00000658-190707000-00001 .
Article MathSciNet CAS PubMed PubMed Central Google Scholar
Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–75. https://doi.org/10.1056/NEJMoa020128 .
Article PubMed Google Scholar
Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333(22):1444–55. doi: https://doi.org/10.1056/nejm199511303332202 .
Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–73. https://doi.org/10.1056/nejm198503143121101 .
Article CAS PubMed Google Scholar
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. https://doi.org/10.1056/NEJMoa022152 .
de Boniface J, Szulkin R, Johansson ALV. Survival after breast conservation vs mastectomy adjusted for comorbidity and socioeconomic status: a Swedish national 6-year follow-up of 48 986 women. JAMA Surg. 2021;156(7):628–37. https://doi.org/10.1001/jamasurg.2021.1438 .
Article PubMed PubMed Central Google Scholar
Elmore LC, Dietz JR, Myckatyn TM, Margenthaler JA. The landmark series: mastectomy trials (skin-sparing and nipple-sparing and reconstruction landmark trials). Ann Surg Oncol. 2021;28(1):273–80. https://doi.org/10.1245/s10434-020-09052-x .
Margenthaler JA, Dietz JR, Chatterjee A. The landmark series: breast conservation trials (including oncoplastic breast surgery). Ann Surg Oncol. 2021;28(4):2120–7. https://doi.org/10.1245/s10434-020-09534-y .
Kapoor MM, Patel MM, Scoggins ME. The wire and beyond: recent advances in breast imaging preoperative needle localization. Radiographics. 2019;39(7):1886–906. https://doi.org/10.1148/rg.2019190041 .
Black DM, Mittendorf EA. Landmark trials affecting the surgical management of invasive breast cancer. Surg Clin North Am. 2013;93(2):501–18. https://doi.org/10.1016/j.suc.2012.12.007 .
Mascaro A, Farina M, Gigli R, Vitelli CE, Fortunato L. Recent advances in the surgical care of breast cancer patients. World J Surg Oncol. 2010;8:5. https://doi.org/10.1186/1477-7819-8-5 .
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. https://doi.org/10.1056/nejmoa020989 .
De La Cruz L, Blankenship SA, Chatterjee A, Geha R, Nocera N, Czerniecki BJ, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann Surg Oncol. 2016;23(10):3247–58. https://doi.org/10.1245/s10434-016-5313-1 .
Gwark S, Kim HJ, Kim J, Chung IY, Kim HJ, Ko BS, et al. Survival after breast-conserving surgery compared with that after mastectomy in breast cancer patients receiving neoadjuvant chemotherapy. Ann Surg Oncol. 2023;30(5):2845–53. https://doi.org/10.1245/s10434-022-12993-0 .
Hartmann-Johnsen OJ, Kåresen R, Schlichting E, Nygård JF. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: a registry-based follow-up study of Norwegian women primary operated between 1998 and 2008. Ann Surg Oncol. 2015;22(12):3836–45. https://doi.org/10.1245/s10434-015-4441-3 .
Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119(7):1402–11. https://doi.org/10.1002/cncr.27795 .
Wrubel E, Natwick R, Wright GP. Breast-conserving therapy is associated with improved survival compared with mastectomy for early-stage breast cancer: a propensity score matched comparison using the National Cancer Database. Ann Surg Oncol. 2021;28(2):914–9. https://doi.org/10.1245/s10434-020-08829-4 .
van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158–70. https://doi.org/10.1016/s1470-2045(16)30067-5 .
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi: https://doi.org/10.6004/jnccn.2022.0030 .
Bleicher RJ, Ruth K, Sigurdson ER, Daly JM, Boraas M, Anderson PR, Egleston BL. Breast conservation versus mastectomy for patients with T3 primary tumors (>5 cm): a review of 5685 medicare patients. Cancer. 2016;122(1):42–9. https://doi.org/10.1002/cncr.29726 .
Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553–64. https://doi.org/10.1016/j.ijrobp.2013.11.012 .
Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract Radiat Oncol. 2016;6(5):287–95. https://doi.org/10.1016/j.prro.2016.06.011 .
Gradishar WJ, Moran MS, et al.: NCCN clinical practice guidelines in oncology: breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2024). Accessed Feb 3 2024.
ACS Clinical Research Program; Alliance for Clinical Trials in Oncology; Nelson HDH, Kelly K. Operative standards for cancer surgery: volume I: breast, lung, pancreas, colon. LWW; 2015.
Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA: A Cancer Journal for Clinicians. 2002;52(5):277–300. doi: https://doi.org/10.3322/canjclin.52.5.277 .
Shirazi S, Hajiesmaeili H, Khosla M, Taj S, Sircar T, Vidya R. Comparison of wire and non-wire localisation techniques in breast cancer surgery: a review of the literature with pooled analysis. Medicina (Kaunas). 2023;59(7). doi: https://doi.org/10.3390/medicina59071297 .
Farha MJ, Simons J, Kfouri J, Townsend-Day M. SAVI Scout® system for excision of non-palpable breast lesions. Am Surg. 2023;89(6):2434–8. https://doi.org/10.1177/00031348221096576 .
Liang DH, Black D, Yi M, Luo CK, Singh P, Sahin A, et al. Clinical outcomes using magnetic seeds as a non-wire, non-radioactive alternative for localization of non-palpable breast lesions. Ann Surg Oncol. 2022;29(6):3822–8. https://doi.org/10.1245/s10434-022-11443-1 .
Srour MK, Kim S, Amersi F, Giuliano AE, Chung A. Comparison of wire localization, radioactive seed, and Savi Scout(®) radar for management of surgical breast disease. Breast J. 2020;26(3):406–13. https://doi.org/10.1111/tbj.13499 .
Choe AI, Ismail R, Mack J, Walter V, Yang AL, Dodge DG. Review of variables associated with positive surgical margins using scout reflector localizations for breast conservation therapy. Clin Breast Cancer. 2022;22(2):e232–8. https://doi.org/10.1016/j.clbc.2021.07.003 .
Kelly BN, Webster AJ, Lamb L, Spivey T, Korotkin JE, Henriquez A, et al. Magnetic seeds: an alternative to wire localization for nonpalpable breast lesions. Clin Breast Cancer. 2022;22(5):e700–7. https://doi.org/10.1016/j.clbc.2022.01.003 .
Jones C, Lancaster R. Evolution of operative technique for mastectomy. Surg Clin North Am. 2018;98(4):835–44. https://doi.org/10.1016/j.suc.2018.04.003 .
Surgeons TASoB: Performance and practice guidelines for mastectomy. 2018. https://breastsurgeons.org/docs/statements/Performance-and-Practice-Guidelines-for-Mastectomy.pdf . Accessed.
Morrow M, Jagsi R, Alderman AK, Griggs JJ, Hawley ST, Hamilton AS, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–6. https://doi.org/10.1001/jama.2009.1450 .
Article CAS PubMed PubMed Central Google Scholar
Hill EL, Ochoa D, Denham F, Merrill A, Lin-Duffy MF, Wilson AB, et al. The Angel Wings Incision: a novel solution for mastectomy patients with increased lateral adiposity. Breast J. 2019;25(4):687–90. https://doi.org/10.1111/tbj.13301 .
van Zeelst LJ, Ten Wolde B, van Eekeren R, Volders JH, de Wilt JHW, Strobbe LJA. Quilting following mastectomy reduces seroma, associated complications and health care consumption without impairing patient comfort. J Surg Oncol. 2022;125(3):369–76. https://doi.org/10.1002/jso.26739 .
Drivas E, Gachabayov M, Kajmolli A, Stadlan Z, Felsenreich DM, Castaldi M. Quilting suture technique after mastectomy: a meta-analysis. Am Surg. 2023;89(12):6045–52. https://doi.org/10.1177/00031348231173995 .
Baker JL, Dizon DS, Wenziger CM, Streja E, Thompson CK, Lee MK, et al. “Going Flat” after mastectomy: patient-reported outcomes by online survey. Ann Surg Oncol. 2021;28(5):2493–505. https://doi.org/10.1245/s10434-020-09448-9 .
Anderson C, Islam JY, Elizabeth Hodgson M, Sabatino SA, Rodriguez JL, Lee CN, et al. Long-term satisfaction and body image after contralateral prophylactic mastectomy. Ann Surg Oncol. 2017;24(6):1499–506. https://doi.org/10.1245/s10434-016-5753-7 .
Kim H, Yoon TI, Kim S, Lee SB, Kim J, Chung IY, et al. Age-related incidence and peak occurrence of contralateral breast cancer. JAMA Netw Open. 2023;6(12):e2347511. https://doi.org/10.1001/jamanetworkopen.2023.47511 .
Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. J Am Coll Surg. 2009;209(1):123–33. https://doi.org/10.1016/j.jamcollsurg.2009.02.061 .
Romanoff A, Zabor EC, Stempel M, Sacchini V, Pusic A, Morrow M. A Comparison of patient-reported outcomes after nipple-sparing mastectomy and conventional mastectomy with reconstruction. Ann Surg Oncol. 2018;25(10):2909–16. https://doi.org/10.1245/s10434-018-6585-4 .
Hieken TJ, Boolbol SK, Dietz JR. Nipple-sparing mastectomy: indications, contraindications, risks, benefits, and techniques. Ann Surg Oncol. 2016;23(10):3138–44. https://doi.org/10.1245/s10434-016-5370-5 .
Garcia-Etienne CA, Cody Iii HS, 3rd, Disa JJ, Cordeiro P, Sacchini V. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J. 2009;15(4):440- https://doi.org/10.1111/j.1524-4741.2009.00758.x
Agarwal S, Agarwal S, Neumayer L, Agarwal JP. Therapeutic nipple-sparing mastectomy: trends based on a national cancer database. The American Journal of Surgery. 2014;208(1):93–8. https://doi.org/10.1016/j.amjsurg.2013.09.030 .
Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V, Veronesi P. Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications. Breast. 2017;34 Suppl 1(Suppl 1):S82-s4. doi: https://doi.org/10.1016/j.breast.2017.06.034 .
De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–9. https://doi.org/10.1245/s10434-015-4739-1 .
Donovan CA, Harit AP, Chung A, Bao J, Giuliano AE, Amersi F. Oncological and surgical outcomes after nipple-sparing mastectomy: do incisions matter? Ann Surg Oncol. 2016;23(10):3226–31. https://doi.org/10.1245/s10434-016-5323-z .
Stolier AJ, Wang J. Terminal duct lobular units are scarce in the nipple: implications for prophylactic nipple-sparing mastectomy. Ann Surg Oncol. 2008;15(2):438–42. https://doi.org/10.1245/s10434-007-9568-4 .
Piato JR, de Andrade RD, Chala LF, de Barros N, Mano MS, Melitto AS, et al. MRI to predict nipple involvement in breast cancer patients. AJR Am J Roentgenol. 2016;206(5):1124–30. https://doi.org/10.2214/ajr.15.15187 .
Ponzone R, Maggiorotto F, Carabalona S, Rivolin A, Pisacane A, Kubatzki F, et al. MRI and intraoperative pathology to predict nipple-areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur J Cancer. 2015;51(14):1882–9. https://doi.org/10.1016/j.ejca.2015.07.001 .
Agha RA, Al Omran Y, Wellstead G, Sagoo H, Barai I, Rajmohan S, et al. Systematic review of therapeutic nipple-sparing<i>versus</i>skin-sparing mastectomy. BJS Open. 2019;3(2):135–45. https://doi.org/10.1002/bjs5.50119 .
Eisenberg RE, Chan JS, Swistel AJ, Hoda SA. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J. 2014;20(1):15–21. https://doi.org/10.1111/tbj.12199 .
Amara D, Peled AW, Wang F, Ewing CA, Alvarado M, Esserman LJ. Tumor involvement of the nipple in total skin-sparing mastectomy: strategies for management. Ann Surg Oncol. 2015;22(12):3803–8. https://doi.org/10.1245/s10434-015-4646-5 .
Galimberti V, Morigi C, Bagnardi V, Corso G, Vicini E, Fontana SKR, et al. Oncological outcomes of nipple-sparing mastectomy: a single-center experience of 1989 patients. Ann Surg Oncol. 2018;25(13):3849–57. https://doi.org/10.1245/s10434-018-6759-0 .
Mitchell SD, Willey SC, Beitsch P, Feldman S. Evidence based outcomes of the American Society of Breast Surgeons Nipple Sparing Mastectomy Registry. Gland Surg. 2018;7(3):247–57. https://doi.org/10.21037/gs.2017.09.10 .
Orzalesi L, Casella D, Santi C, Cecconi L, Murgo R, Rinaldi S, et al. Nipple sparing mastectomy: surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016;25:75–81. https://doi.org/10.1016/j.breast.2015.10.010 .
Smith BL, Tang R, Rai U, Plichta JK, Colwell AS, Gadd MA, et al. Oncologic safety of nipple-sparing mastectomy in women with breast cancer. J Am Coll Surg. 2017;225(3):361–5. https://doi.org/10.1016/j.jamcollsurg.2017.06.013 .
Daar DA, Abdou SA, Rosario L, Rifkin WJ, Santos PJ, Wirth GA, Lane KT. Is there a preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143(5):906e-e919. https://doi.org/10.1097/prs.0000000000005502 .
Peled AW, Peled ZM. Nerve preservation and allografting for sensory innervation following immediate implant breast reconstruction. Plast Reconstr Surg Glob Open. 2019;7(7):e2332. https://doi.org/10.1097/gox.0000000000002332 .
Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048–53.
Citgez B, Yigit B, Bas S. Oncoplastic and reconstructive breast surgery: a comprehensive review. Cureus. 2022. https://doi.org/10.7759/cureus.21763
Ayoub Z, Strom EA, Ovalle V, Perkins GH, Woodward WA, Tereffe W, et al. A 10-year experience with mastectomy and tissue expander placement to facilitate subsequent radiation and reconstruction. Ann Surg Oncol. 2017;24(10):2965–71. https://doi.org/10.1245/s10434-017-5956-6 .
Kaufman CS. Increasing role of oncoplastic surgery for breast cancer. Curr Oncol Rep. 2019;21(12):111. https://doi.org/10.1007/s11912-019-0860-9 .
Silverstein MJ, Mai T, Savalia N, Vaince F, Guerra L. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol. 2014;110(1):82–9. https://doi.org/10.1002/jso.23641 .
Macmillan RD, McCulley SJ. Oncoplastic breast surgery: what, when and for whom? Curr Breast Cancer Rep. 2016;8:112–7. https://doi.org/10.1007/s12609-016-0212-9 .
Crown A, Wechter DG, Grumley JW. Oncoplastic breast-conserving surgery reduces mastectomy and postoperative re-excision rates. Ann Surg Oncol. 2015;22(10):3363–8. https://doi.org/10.1245/s10434-015-4738-2 .
Kosasih S, Tayeh S, Mokbel K, Kasem A. Is oncoplastic breast conserving surgery oncologically safe? A meta-analysis of 18,103 patients. Am J Surg. 2020;220(2):385–92. https://doi.org/10.1016/j.amjsurg.2019.12.019 .
André C, Holsti C, Svenner A, Sackey H, Oikonomou I, Appelgren M, et al. Recurrence and survival after standard versus oncoplastic breast-conserving surgery for breast cancer. BJS Open. 2021;5(1). doi: https://doi.org/10.1093/bjsopen/zraa013 .
Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer (Dove Med Press). 2017;9:521–30. https://doi.org/10.2147/bctt.S113742 .
Kopkash K, Clark P. Basic oncoplastic surgery for breast conservation: tips and techniques. Ann Surg Oncol. 2018;25(10):2823–8. https://doi.org/10.1245/s10434-018-6604-5 .
Yang JD, Lee JW, Cho YK, Kim WW, Hwang SO, Jung JH, Park HY. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (Part 1): Volume Displacement. J Breast Cancer. 2012;15(1):1. https://doi.org/10.4048/jbc.2012.15.1.1 .
Cantürk NZ, Şimşek T, Özkan GS. Oncoplastic breast-conserving surgery according to tumor location. Eur J Breast Health. 2021;17(3):220–33. https://doi.org/10.4274/ejbh.galenos.2021.2021-1-2 .
Download references
Author information
Authors and affiliations.
Department of Surgery, Franciscan Health, Olympia Fields, Chicago, IL, USA
Tiffany J. Nevill
Department of Surgery, Vanderbilt University Medical Center, 2220 Pierce Avenue, Preston Research Building 597, Nashville, TN, 37232-2730, USA
Kelly C. Hewitt & Rachel L. McCaffrey
You can also search for this author in PubMed Google Scholar
Contributions
TN and RM wrote the main manuscript. KH reviewed the manuscript for clinical content and contributed to table formation. All reviewed the final manuscript prior to submission.
Corresponding author
Correspondence to Rachel L. McCaffrey .
Ethics declarations
Competing interests.
The authors of this review article have no conflicts of interest to disclose.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Nevill, T.J., Hewitt, K.C. & McCaffrey, R.L. Advancements in Oncologic Surgery of the Breast: A Review of the Literature. Curr Breast Cancer Rep (2024). https://doi.org/10.1007/s12609-024-00537-2
Download citation
Accepted : 18 February 2024
Published : 27 February 2024
DOI : https://doi.org/10.1007/s12609-024-00537-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast surgery
- Breast cancer
- Oncoplastic surgery
- Oncologic safety
- Find a journal
- Publish with us
- Track your research
- Research article
- Open access
- Published: 16 December 2019
Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review
- Claire H. Li 1 na1 ,
- Vassiliki Karantza 1 ,
- Gursel Aktan 1 &
- Mallika Lala 1 na1
Breast Cancer Research volume 21 , Article number: 143 ( 2019 ) Cite this article
22k Accesses
79 Citations
5 Altmetric
Metrics details
Metastatic triple-negative breast cancer (mTNBC), an aggressive histological subtype, has poor prognosis. Chemotherapy remains standard of care for mTNBC, although no agent has been specifically approved for this breast cancer subtype. Instead, chemotherapies approved for metastatic breast cancer (MBC) are used for mTNBC (National Comprehensive Cancer Network Guidelines [NCCN] v1.2019). Atezolizumab in combination with nab-paclitaxel was recently approved for programmed death-ligand 1 (PD-L1)–positive locally advanced or metastatic TNBC. Published historical data were reviewed to characterize the efficacy of NCCN-recommended (v1.2016) agents as first-line (1L) and second-line or later (2L+) treatment for patients with locally recurrent inoperable or metastatic TNBC (collectively termed mTNBC herein).
A systematic literature review was performed, examining clinical efficacy of therapies for mTNBC based on NCCN v1.2016 guideline recommendations. Data from 13 studies, either published retrospective mTNBC subgroup analyses based on phase III trials in MBC or phase II trials in mTNBC, were included.
A meta-analysis of mTNBC subgroups from three phase III trials in 1L MBC reported pooled objective response rate (ORR) of 23%, median overall survival (OS) of 17.5 months, and median progression-free survival (PFS) of 5.4 months with single-agent chemotherapy. In two subgroup analyses from a phase III study and a phase II trial ( n = 40 each), median duration of response (DOR) to 1L chemotherapy for mTNBC was 4.4–6.6 months; therefore, responses were not durable. A meta-analysis of seven cohorts showed the pooled ORR for 2L+ chemotherapy was 11% (95% CI, 9–14%). Median DOR to 2L+ chemotherapy in mTNBC was also limited (4.2–5.9 months) per two subgroup analyses from a phase III study. No combination chemotherapy regimens recommended by NCCN v1.2016 for treatment of MBC showed superior OS to single agents.
Conclusions
Chemotherapies have limited effectiveness and are associated with unfavorable toxicity profiles, highlighting a considerable unmet medical need for improved therapeutic options in mTNBC. In addition to the recently approved combination of atezolizumab and nab-paclitaxel for PD-L1–positive mTNBC, new treatments resulting in durable clinical responses, prolonged survival, and manageable safety profile would greatly benefit patients with mTNBC.
Introduction
Breast cancer (BC) is the most common malignant neoplasm in females; an estimated 266,120 new diagnoses and 40,920 related deaths occurred in the USA in 2018 [ 1 ]. Approximately 10–20% of BCs do not express estrogen and progesterone receptors and lack amplification/overexpression of the human epidermal growth factor 2 receptor (HER2) [ 2 , 3 , 4 ]; therefore, they are known as triple-negative breast cancers (TNBCs) and constitute an aggressive histologic subtype. In patients with locally recurrent inoperable or metastatic disease (collectively referred to as mTNBC in this article), treatment options have primarily been chemotherapies based on recommended therapeutic approaches (National Comprehensive Cancer Network [NCCN] v1.2019 guidelines and the European School of Oncology-European Society for Medical Oncology [ESO-ESMO] 2018 guidelines) for metastatic breast cancer (MBC) [ 5 , 6 ]. In particular, anthracyclines, taxanes, capecitabine, and more recently, eribulin are commonly used as monotherapy or in combination with other agents and as standard/control arms in registration trials of new/investigational agents for TNBC. Anthracyclines and taxanes are both recommended, unless contraindicated, as first-line (1L) treatments for patients who have not previously received these agents as neoadjuvant or adjuvant treatment [ 5 , 6 ]. The efficacy of anthracyclines in mTNBC has been inferred from earlier studies that involved patients with MBC in which the TNBC subpopulation was not distinctly defined (mostly because of the absence of HER2 status reporting) [ 7 ]. Compared with taxanes, anthracyclines have not demonstrated overall survival (OS) benefit in mTNBC [ 8 ]. Because data on the effectiveness of anthracyclines are not available in the mTNBC population and anthracyclines and taxanes are generally considered similarly effective, anthracyclines are not discussed further in this review.
Overall prognosis for patients with mTNBC is worse than for the other BC subtypes, and more effective therapeutic options are needed. In a pooled analysis of two phase III trials in MBC, inferior outcomes were reported with 1L or later line physician choice of chemotherapy for patients with mTNBC than for the overall MBC population [ 9 ]. Chemotherapies are generally associated with unfavorable adverse events (AEs), more so in combination, that can lead to treatment discontinuation. Because combination regimens have not prolonged OS compared with monotherapies, the approach recommended by the NCCN v1.2019/ESO-ESMO 2018 guidelines [ 5 , 6 ] for the treatment of MBC (including mTNBC) remains sequential use of single-agent chemotherapy. Based on recent evidence that atezolizumab plus nab-paclitaxel improves progression-free survival (PFS), this combination was recently granted accelerated approval by the US Food and Drug Administration (FDA) in patients with programmed death-ligand 1 (PD-L1)–positive (immune cell score, IC 1+) TNBC [ 5 , 10 , 11 ]. In general, clinical trials conducted only in patients with mTNBC are limited. No phase III trials have been conducted to specifically evaluate single agents as treatment for mTNBC in any line of therapy, and only a limited number of phase III trials have been conducted to evaluate combination therapies in the mTNBC population. The purpose of the current evidence synthesis was to systematically characterize the efficacy of commonly used chemotherapies, defined herein to be agents recommended in the NCCN v1.2016 guidelines (which were current at the time of this analysis) [ 12 ], as 1L and second-line or later (2L+) treatment for patients with mTNBC, thereby providing a summary of available historical data.
Systematic review
A systematic review of the literature was conducted to synthesize objective response rate (ORR), duration of response (DOR), PFS, and OS of commonly used chemotherapies as 1L or 2L+ treatment for patients with mTNBC. Commonly used chemotherapies were defined as agents recommended in the NCCN v1.2016 guidelines for the treatment of MBC (including mTNBC) as single agents or combinations thereof, including the combination of paclitaxel and bevacizumab [ 12 ]. Clinical trial results published in English between January 1, 1996, and August 21, 2016, were identified by searching the PubMed (MEDLINE), Cochrane, and Embase databases (Additional file 1 : Table S1, Additional file 2 : Table S2, Additional file 3 : Table S3). Identified publications were then manually screened for inclusion. Reports of phase III trials in either mTNBC or MBC (with mTNBC subgroup outcomes) populations, recent (2010 and later) phase II trials in mTNBC-only populations, and retrospective or meta-analyses of mTNBC subgroups based on phase III MBC trials were included. Details of the search inclusion and exclusion criteria are presented in Fig. 1 . Studies published after 21 August 2016 were evaluated separately for relevance based on recent guideline updates and were included for completeness [ 10 , 14 , 15 , 16 , 17 , 18 , 19 ].
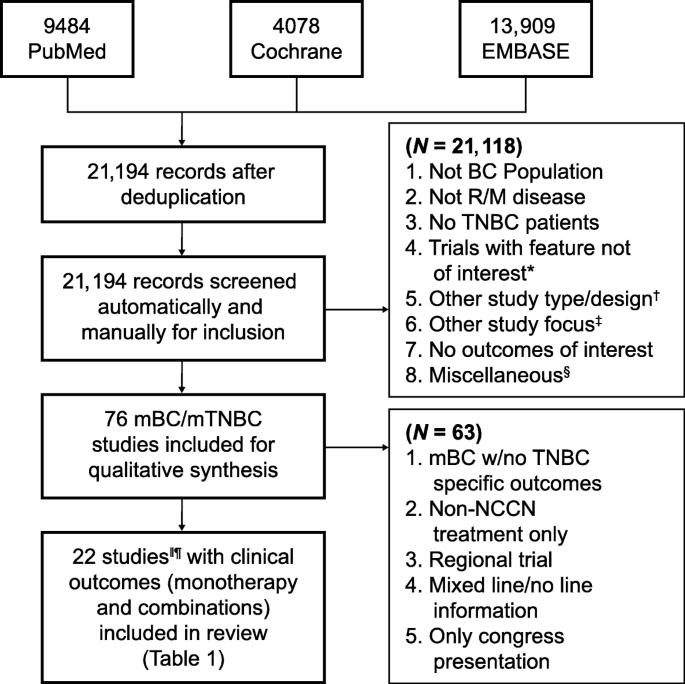
Study selection process for the systematic literature review and meta-analysis of breast cancer ( BC ). *Exclusions include not phase II, not phase III or phase II with triple-negative breast cancer ( TNBC ) focus, phase II not TNBC focus, not phase II or phase III, and not TNBC focus phase II/I. † Exclusions include review articles, other study types, not recurrent/metastatic ( R/M ) of phase III/II data, and not TNBC-specific R/M. ‡ Exclusions include non-cancer outcomes focus, only quality-of-life data, study protocol, surgical intervention, model development, and only AE data. § Exclusions include other language, older report of the same study, and reference unavailable. ‖ Results from one study (phase III trial, study 301) based on internal communication with sponsor (Eisai); not published results. ¶ Results from Twelves et al.’s [ 9 ] and Pivot et al.’s [ 13 ] studies are both included based on the reported different treatment line outcomes
Study selection
There was substantial heterogeneity in the inclusion of 1L and 2L+ populations, between and within identified studies, with many studies including mixed patient populations in terms of prior therapy and current line of treatment. Studies were first classified by line of treatment (1L, 2L+, mixed line). Only those that reported clinical efficacy outcomes in mTNBC populations in which the majority of patients (≥ 80%) were given 1L or 2L+ treatment with chemotherapy, as single-agent and in combination regimens, were included in the review. Reports of clinical trials that were conducted regionally (limited to one geographic location) in a non-White population and reports that were limited to presentation at a congress but not published were excluded from the review.
Data analysis and meta-analysis
Clinical outcomes, including ORR and OS, were qualitatively represented by monotherapy as 1L and 2L+ therapy, as shown in Figs. 3 and 4 . Meta-analyses were performed to synthesize the pooled ORRs for single-agent chemotherapy among studies of 2L+ treatment. Inverse-variance fixed-effects and random-effects meta-analyses were explored. A DerSimonian and Laird random-effects model was used to account for between-trial heterogeneity; this model assumes that the true treatment effects of the included studies follow a distribution around an overall mean [ 20 ]. The sample size, ORR, 95% confidence interval (95% CI) for each treatment and study, and pooled ORR (95% CI) are presented as forest plots, per PRISMA guidelines [ 21 ]. The ORRs were re-estimated using the all-patients-as-treated (APaT) population to ensure common definition across studies. The ORR proportions were transformed to a logit scale to calculate 95% CIs and then transformed back to proportions.
Data sources and software
The PubMed, Cochrane, and Embase databases were searched for eligible studies/publications; Microsoft Office Excel (Redmond, WA, USA) was used to synthesize study records. As necessary, trial eligibility criteria were compared against the criteria listed on ClinicalTrials.gov . Meta-analyses of ORR were conducted in R (version 3.1.3) using the metafor package [ 22 ]. Qualitative graphical analyses of ORR, DOR, OS, and PFS across identified trials were performed using R (version 3.2.5).
A total of 21,194 references were collected from combined literature searches of PubMed, Cochrane, and Embase databases after filtering duplicate records (Fig. 1 ). From those references, 76 studies complied with the key inclusion criteria from qualitative synthesis. Of these 76 trials, 63 were excluded, as described in the “ Results ” section (Fig. 1 ). Finally, 21 studies that reported clinical outcomes of interest with chemotherapies for patients with mTNBC were reviewed in detail and are reported herein.
A summary of study outcomes of all included studies is given in Table 1 . ORRs based on the APaT populations were calculated to facilitate comparisons across studies. ORRs were re-estimated based on the APaT population (i.e., number of responders divided by number of patients composing the APaT population for studies in which ORR was reported based on the evaluable or intention-to-treat population [ITT]). The clinical outcomes for patients with mTNBC treated with NCCN-recommended (v1.2016) agents [ 6 , 12 ] as either 1L or 2L+ therapy were further separated based on whether the investigation therapy was monotherapy or combination therapy (Table 1 ).
Description of the study outcomes with NCCN-recommended (v1.2016) agents
Monotherapy.
No published data from randomized controlled phase III trials with single-agent chemotherapy as 1L or later lines of treatment for mTNBC were found. Thirteen published reports (disregarding congress presentations) of retrospective subgroup analyses in patients with mTNBC based on phase III trials in MBC or phase II trials in mTNBC with limited sample size were identified, considering all lines of treatment. Of these, six studies reported clinical efficacy outcomes in the 1L mTNBC patient population, as summarized in Table 1 [ 24 , 25 , 27 , 28 , 32 ]. Treatments included capecitabine, taxanes (docetaxel, paclitaxel), eribulin, ixabepilone, or platinum (carboplatin, cisplatin). Furthermore, nine studies, also summarized in Table 1 , reported clinical efficacy outcomes in the 2L+ mTNBC patient population; treatments included capecitabine, carboplatin, cisplatin, or eribulin [ 9 , 13 , 23 , 25 , 26 , 27 , 30 , 31 , 33 ].
Among the six studies on 1L treatment, five had published outcomes [ 24 , 25 , 27 , 28 , 32 ]. For one study (phase III trial, study 301), clinical outcomes for the mTNBC subgroup were available via internal communication. Notably, a meta-analysis of the mTNBC subgroups from three phase III trials in 1L MBC [ 28 ] reported a pooled ORR of 23% and median OS of 17.5 months. In trial 301, which compared eribulin with capecitabine for the treatment of MBC, in the mTNBC subgroup, ORR for 1L eribulin and capecitabine was 10% and 12%, respectively.
In addition, four phase II trials conducted to investigate single-agent chemotherapies for mTNBC with sample sizes of 28–69 were identified; the reported ORR ranged from 12 to 30%, and a median OS of 13.1 months was reported in only one [ 24 ] of these phase II trials. In studies that reported response duration (two subgroup analyses from a phase III study (study 301) and one phase II trial, all limited in sample size [ n = 40 each]), the median DOR to 1L chemotherapy in mTNBC ranged from 4.4 to 6.6 months (Table 1 ) [ 32 ]. Qualitative analyses of the sample sizes, ORR, and OS are shown graphically in Figs. 3 b and 4 b.
Second-line or later
Among the nine studies on 2L+ treatment, seven phase III studies in MBC reported clinical efficacy outcomes for mTNBC subgroups [ 9 , 13 , 23 , 26 , 30 , 31 , 33 ]. In these studies, ORR ranged from 9 to 18%; median OS, from 8.1 to 15.2 months. The median DOR to 2L+ chemotherapy in mTNBC was only available from two subgroup analyses of a phase III study (study 301) and ranged from 4.2 to 5.9 months. Two additional phase II studies reported ORR of 6% and 11.8% with platinum (cisplatin/carboplatin) [ 25 , 27 ]. A meta-analysis of ORR reported for seven cohorts from six of these studies (mTNBC subgroup analyses from five phase III trials in MBC and two phase II trials in mTNBC) resulted in a pooled ORR of 11% (95% CI, 9–14%) for chemotherapy in Fig. 2 . Qualitative analyses of the sample sizes, ORR, and OS are shown graphically in Figs. 3 a and 4 a.
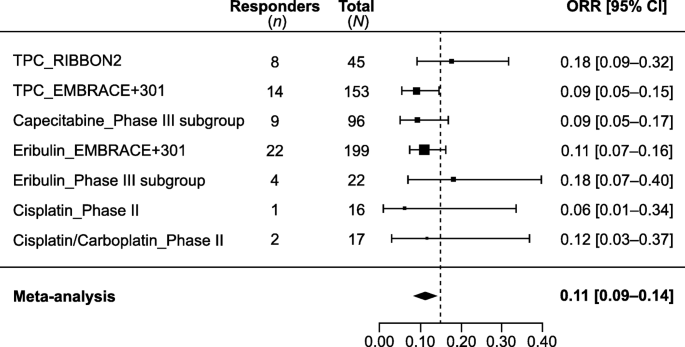
Historical objective response rate (ORR) with chemotherapy in 2L+ mTNBC. Meta-analysis of the seven cohorts from six studies reporting ORR with single-agent chemotherapy in second or later line treatment settings
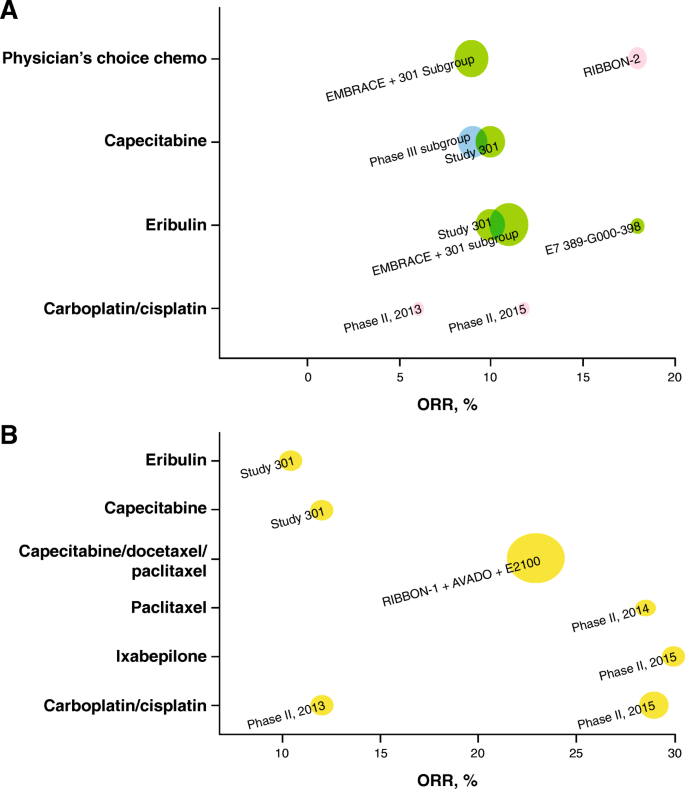
Graphical representations of objective response rates ( ORR s) for a trials of NCCN-recommended (v1.2016) second-line ( 2L ) plus monotherapy (including studies mixed with first-line [ 1L ]), and b trials of NCCN-recommended (v1.2016) first-line monotherapy; the size of the bubble is proportional to the study size (all-patients-as-treated population), and the color of the bubble indicates the line of therapy. Yellow = 1L, green = 2L–3L+, pink = 2L, blue = 1L–3L+ (including studies with ≤ 15% 1L patients). Study 301 result is based on internal communication with trial sponsor (Eisai); not published results
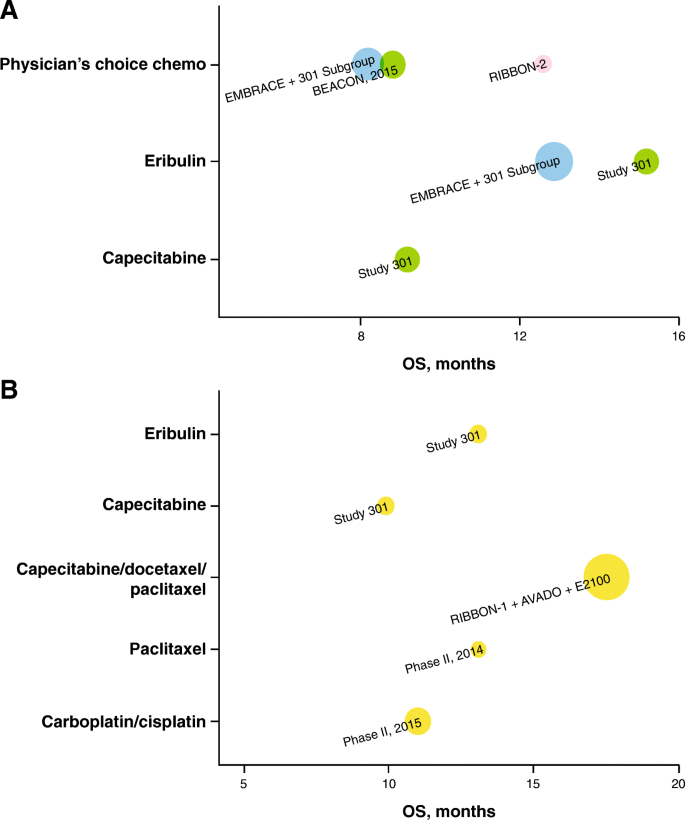
Graphical representation of overall survival (OS) for a trials of NCCN-recommended (v1.2016) second-line ( 2L ) plus monotherapy (including studies mixed with first-line [ 1L ]), and b trials of NCCN-recommended (v1.2016) 1L monotherapy; the size of the bubble is proportional to the study size (all-patients-as-treated population), and the color of the bubble indicates the line of therapy. Yellow = 1L, green = 2L–3L+, pink = 2L, blue = 1L–3L+ (including studies with ≤ 15% 1L patients). Study 301 result is based on internal communication with trial sponsor (Eisai); not published results. OS from phase II 2015 study is based on a total of 86 patients, including 80% 1L and 20% 2L+ patients
Combination therapy
Eleven published clinical studies reported efficacy outcomes in patients with mTNBC treated with NCCN-recommended (v1.2016) combination regimens [ 6 , 12 ], either as chemotherapy-only regimens or in combination with bevacizumab (Table 1 ) [ 28 , 30 , 31 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 ]. Only one phase III trial conducted specifically in the mTNBC population was identified, which evaluated the combination of gemcitabine, carboplatin, and iniparib/placebo as 1L–third-line (3L) treatment [ 40 ]. The overall reported ORR was 32%, and median OS was 11.1 months. In the 1L setting ( n = 149), median PFS and OS were 4.6 and 13.9 months, respectively, whereas in the 2L+ setting ( n = 109), median PFS and OS were 2.9 and 8.1 months, respectively.
In addition to monotherapy, as described previously herein, the meta-analysis of the mTNBC subgroups from three phase III trials in 1L MBC [ 28 ] also reported pooled outcomes for chemotherapy and bevacizumab combinations, including the NCCN-recommended (v1.2016) paclitaxel + bevacizumab regimen. In these studies, ORR was 42%; median OS, 18.9 months. Furthermore, two phase III trials in MBC included 20–25% patients with mTNBC and reported outcomes in their mTNBC subgroups [ 28 , 41 ] In addition, four phase II trials were also identified that investigated combination regimens in mTNBC [ 35 , 36 , 38 , 39 ]. Wide ranges of ORR (14.8–64.3%), median PFS (4.8–8.2 months), and median OS (16.5–21.5 months) were reported across these studies, in which trial designs varied and sample sizes were small (18–46 patients).
Two phase III trials in patients with MBC reported outcomes for mTNBC subgroups treated with ixabepilone + capecitabine [ 30 , 31 ]: median OS, 4.1–4.2 months, and ORR, 27% (reported in one study). An additional phase II study conducted specifically in the 2L+ mTNBC population was identified [ 37 ], which reported an ORR of 60% ( n = 50) with the paclitaxel + carboplatin combination.
The current standard of care for management of mTNBC is chemotherapy, although no chemotherapy agent is specifically approved for TNBC. Instead, chemotherapies approved for MBC (all subtypes) are also used for the treatment of mTNBC (NCCN v1.2019 guidelines and ESO-ESMO guidelines 2018) [ 5 , 6 ]. With the advent of immunotherapies, atezolizumab in combination with nab-paclitaxel was recently approved for PD-L1–positive locally advanced or metastatic TNBC [ 11 ]. In general, the number of clinical trials conducted only in patients with mTNBC is limited. Considering NCCN-recommended (v1.2016) treatments, there were no published phase III trials to specifically evaluate single-agent chemotherapy in mTNBC in any line of treatment and only one phase III trial that evaluated combination chemotherapy in mTNBC [ 40 ]. The most commonly used treatments were taxanes, capecitabine, and, more recently, eribulin. These agents were also used as standard/control arms in registration trials of new/investigational agents for mTNBC. The current systematic literature review was performed to determine effectiveness of treatments recommended for MBC in the NCCN v1.2016 guidelines, when used either as 1L or 2L+ therapy for mTNBC [ 6 , 12 ].
The wide range of ORRs (6–29% with single agents; 14.8–64.3% with combination regimens) to NCCN-recommended (v1.2016) therapies used as 1L and 2L+ treatments for mTNBC highlights a need for more precise determination of the efficacy of these therapies to inform clinical practice. The data reviewed here suggest that the variability in ORRs was not fully attributed to differences in the effectiveness of available therapies. Small study size and heterogeneity in the characteristics of the enrolled patients (reflective of real-world clinical settings) were also significant factors. Moreover, the observed responses were generally not durable and did not necessarily translate to survival benefit. A key focus of this review was to summarize clinical outcomes taking into consideration the heterogeneity among studies caused by mixed-line patient populations and different therapeutic approaches. Historical studies identified via a systematic literature search were categorized based on the patient population being closer to 1L or later line of treatment and the regimen being monotherapy or combination.
No published results of randomized controlled phase III trials in mTNBC in 1L or later lines of treatment were found for single-agent chemotherapy. Published reports of either retrospective mTNBC subgroup analyses based on phase III trials in MBC or phase II trials in mTNBC with limited sample size were identified. These formed the evidence base in this review of historical data. A notable meta-analysis of the mTNBC subgroups from three phase III trials in 1L MBC [ 28 ] reported a pooled ORR of 23% and a median OS of 17.5 months with chemotherapy. Among available historical data, this study is regarded as the most relevant to efficacy outcomes from available 1L treatments. The recent TNT trial also reported similar clinical outcomes (31–34% ORRs and median OS of 12 months) in 2L+ mTNBC subgroups treated with carboplatin or docetaxel [ 19 ].
Although achieving clinical response is important, long-term clinical benefit of a treatment is linked with durability of the response. In two subgroup analyses from a phase III study (study 301) and one phase II trial [ 32 ], all limited in sample size ( n = 40 each), that reported response duration, median DOR to 1L chemotherapy for mTNBC ranged from 4.4 to 6.6 months (Table 1 ), indicating that the responses were not durable. Considering later lines (2L+) of treatment, the efficacy of chemotherapies was lower than in the 1L setting. Based on a meta-analysis of seven cohorts, the pooled ORR for chemotherapy was 11% (95% CI, 9–14%) [ 13 , 23 , 25 , 26 , 27 , 30 ]. Median DOR to 2L+ chemotherapy in mTNBC was also limited, ranging from 4.2 to 5.9 months, based on two subgroup analyses from a phase III study.
NCCN-recommended (v1.2016) combination regimens (including paclitaxel + bevacizumab) have not been proven superior to single-agent chemotherapy in terms of OS [ 5 ]. Only one global phase III trial of a combination regimen was found. This trial evaluated the gemcitabine, carboplatin, and iniparib/placebo combination as 1L–3L treatment for mTNBC [ 40 ]. Although the ORR to gemcitabine + carboplatin (32%) exceeded clinical response rates seen with monotherapy, the combination did not prolong OS (median OS, 11.1 months in 1L–3L) but was instead accompanied by higher toxicity (86% of patients had AEs of grade 3 or higher toxicities, and 10% discontinued treatment because of AEs).
In addition, the meta-analysis of the mTNBC subgroups from three phase III trials in 1L MBC [ 28 ] also reported pooled outcomes for chemotherapy and bevacizumab combinations with an ORR of 42%, which is higher than that for monotherapy, but a median OS (18.9 months) similar to that with monotherapy. The meta-analysis included patients treated with bevacizumab in combination with several chemotherapies, among which only the bevacizumab + paclitaxel combination is recommended by the NCCN v1.2016 guidelines. For 2L+ treatment of patients with mTNBC, although the ORR for combination therapies was superior to that of monotherapy, survival (median OS, 4.1–4.2 months) was poor. The current NCCN v1.2019 guidelines continue to state that the recommended approach to treatment of mTNBC remains sequential use of single-agent chemotherapy, except in patients with PD-L1–positive mTNBC, for whom atezolizumab plus nab-paclitaxel may be considered [ 5 ].
Not only do commonly used chemotherapies for MBC result in short-lived responses in patients with mTNBC, but they are also associated with toxicity, such as myelosuppression and neuropathy, which can compromise quality of life and lead to early treatment discontinuation. A pooled analysis of two phase III trials in patients with MBC (including mTNBC) receiving either single-agent physician’s choice chemotherapy (~ 70% had capecitabine) or eribulin as 2L+ treatment reported inferior outcomes with 1L or later-line chemotherapy for mTNBC than with overall MBC (ORR, 10.3% vs 16.4%; OS, 8.2 vs 12.8 months; PFS, 2.6 vs 3.4 months for chemotherapy of physician’s choice and ORR, 12.0% vs 14.9%; OS, 12.9 vs 15.2 months; PFS, 2.8 vs 4.0 months for eribulin) [ 9 ]. The study also reported that 47% and 66% of patients, respectively, for physician choice chemotherapy and eribulin, had treatment-emergent AEs of grades 3 to 4 toxicity, with neutropenia and leukopenia being the most prominent, whereas discontinuations because of treatment-emergent AEs were 13.6% and 11.3%, respectively [ 9 ]. The RIBBON-1 phase III trial [ 42 ] in patients with MBC (including mTNBC) treated in the 1L setting reported that 22% of participants in the capecitabine cohort and 38% in the taxane cohort had AEs of grade 3–5 toxicity, with sensory neuropathy, neutropenia, and venous thromboembolism being the most common. The rates of discontinuations because of AEs were 11.9% and 7.8%, respectively.
Specifically in the mTNBC population, AEs and treatment discontinuations because of toxicity have been reported in phase II studies as follows: ixabepilone (1L treatment), 45% of patients had AEs of grade ≥ 3 toxicity (neutropenia and leukopenia most common) with 20% discontinuations because of AEs [ 32 ]; paclitaxel (1L treatment), 10.7% discontinuations because of AEs [ 24 ]; and platinum (carboplatin/cisplatin, 1L or 2L treatment), 11.6% discontinuations because of AEs [ 27 ]. However, caution is required when drawing conclusions regarding the therapeutic index of different agents based on grade 3 or 4 toxicities, given that in some cases these toxicities may have minimal clinical consequence (e.g., grade 3 neutropenia in the absence of infection) whereas other chronic grade 2 toxicities may be intolerable or have a substantial impact on a patient’s quality of life.
New agents and agents in development
New treatment options for mTNBC are emerging with the advent of immune checkpoint programmed death 1 (PD-1)/PD-L1 inhibitors, antibody drug conjugates (ADCs), and other immune therapies under investigation that could become essential for the treatment of mTNBC, either as monotherapy or in combination with other agents (Table 2 ). Targeted therapies and other chemotherapies under investigation, mostly in phase II studies, as 1L and later lines of treatment for mTNBC are primarily single arm and often include mixed-line patient populations; hence, efficacy outcomes are challenging to interpret [ 24 , 43 , 44 , 45 , 46 , 47 ].
Immune checkpoint inhibitors
Compared with nab-paclitaxel alone, atezolizumab in combination with nab-paclitaxel prolonged PFS in patients with mTNBC (ITT population: median PFS of 7.2 months vs 5.5 months; Table 2 ) in the IMpassion 130 trial. Median PFS among the subpopulation of that trial with PD-L1–positive tumors was 7.5 months in the atezolizumab group and 5.0 months in the placebo group [ 10 ]. PD-L1 positivity in that trial was determined using the Ventana PD-L1 [SP142] immunohistochemical assay (Roche Diagnostics USA) and was defined based on the percentage of PD-L1–expressing immune cells as a percentage of tumor area: IC3 (≥ 10%), IC2 (≥ 5% to < 10%), IC1 (≥ 1% and < 5%), and IC0 (< 1%). Combination atezolizumab plus nab-paclitaxel is now approved by the FDA for the treatment of PD-L1–positive (IC1+) mTNBC (with PD-L1 positivity established using an FDA-approved test) and is included in the most recent NCCN v1.2019 guidelines [ 5 , 11 ]. Results of KEYNOTE-355, a phase III study of pembrolizumab in combination with one of (nab)-paclitaxel, gemcitabine, or carboplatin as 1L therapy for mTNBC, are pending.
Immune checkpoint inhibitors are also being investigated for monotherapy, and atezolizumab and pembrolizumab both have shown durable responses but in limited patient subsets. Results from the single-arm atezolizumab monotherapy trial in mTNBC were promising, with an ORR of 26% and 7% in the 1L and 2L+ settings, respectively; median DOR was 21 months (range 8+ to 26+ months) in the 1L setting, and DOR ranged from 3 to 13+ months in the 2L+ setting [ 48 ]. In KEYNOTE-086, a phase II study of pembrolizumab monotherapy for heavily pretreated mTNBC reported an overall ORR of 5% in a 2L+ subset of patients. The median DOR was 6.3 months (range, 1.2+ to 10.3+ months), with a median PFS and OS of 2 months and 8.9 months, respectively [ 49 ].
PARP inhibitors
When the NCCN guidelines were updated in 2018 and 2019, after this systematic review was conducted, two poly adenosine diphosphate (ADP) ribose polymerase (PARP) inhibitors, olaparib and talazoparib, were added for the treatment of germline BRCA -mutated HER2-negative MBC [ 50 ]. In the recent phase 3 OlympiAD trial of single-agent olaparib versus physician choice chemotherapy as 1L+ treatment for patients with germline BRCA -mutant and HER2-negative MBC (50% of patients with mTNBC), use of olaparib showed improvement in ORR (60% vs 29%) and median PFS (7.0 months vs 4.2 months) compared with chemotherapy [ 17 ]. Similarly, the EMBRACA trial of talazoparib versus chemotherapy as a 2L+ treatment in a similar patient population (45% mTNBC) reported that, compared with chemotherapy, talazoparib conferred a significantly higher ORR (62.6% vs 27.2%; P < 0.001) and significantly longer median PFS (8.6 months vs 5.6 months; P < 0.001) (Table 2 ) [ 16 ].
AKT inhibitors
Addition of AKT inhibitors to chemotherapy is also being investigated as 1L treatment for patients with mTNBC. A recent combination trial of the AKT inhibitor ipatasertib plus paclitaxel as 1L treatment for mTNBC (LOTUS trial) reported a median PFS of 6.2 months with the ipatasertib combination (vs 4.9 months with the placebo combination; P = 0.037; Table 2 ). After a follow-up of 23 months, median OS was 23.1 months with ipatasertib (vs 18.4 months with placebo plus paclitaxel) and the 1-year OS rate increased from 70 to 83% with the addition of ipatasertib; OS seemed to be independent of PTEN expression status [ 14 , 15 ]. Furthermore, the AKT inhibitor AZD5363 (capivasertib) is being investigated in combination with paclitaxel in patients with previously untreated mTNBC (PAKT) [ 51 ]. After a median follow-up of 18.2 months, PFS and OS were both longer with capivasertib plus paclitaxel than with placebo plus paclitaxel (PFS, 5.9 months vs 4.2 months; OS, 19.1 months vs 12.6 months).
Antibody drug conjugates
Among ADCs, on February 5, 2016, the FDA granted breakthrough therapy designation to sacituzumab govitecan (IMMU-132) as 3L treatment for mTNBC based on the results of a phase I/II clinical trial, which demonstrated an ORR of 34%, a median PFS of 5.5 months, and a median OS of 12.7 months [ 52 ]. In the EMERGE phase II trial with the 3L+ mTNBC subpopulation treated with another ADC, glembatumumab vedotin (GV), reporting an ORR of 18% (vs 0% for the chemotherapy-treated counterparts), these figures were 40% and 0%, respectively, for patients with mTNBC overexpressing glycoprotein NMB (gpNMB) [ 53 ]. There was a suggestion of possible improvement in survival (PFS and OS) with GV compared with chemotherapy in this population of the EMERGE study (PFS: 3.5 months vs 1.5 months; OS, 10.0 months vs 5.5 months) [ 53 ]. However, a recent trial of GV versus capecitabine in a similar population of patients with gpNMB-overexpressing mTNBC (METRIC) did not meet its primary PFS objective, with no improvement in PFS with GV compared with capecitabine, and no OS benefit [ 54 ].
Limitations
No mTNBC-specific randomized controlled trials directly comparing NCCN-recommended (v1.2016) chemotherapies for the treatment of MBC were identified in this search, allowing only indirect comparison between studies. Furthermore, no phase III trial studying single-agent chemotherapy for the treatment of mTNBC in any line of therapy was found. Given that results from only one global phase III trial to evaluate combination chemotherapy in mTNBC are available [ 40 ], retrospective (and in one case prospective [ 41 ]) subgroup analyses of the mTNBC subpopulation from larger phase III MBC trials and smaller phase II trials, including single-arm trials, were included in this evidence synthesis. Furthermore, for the meta-analysis of 2L+ chemotherapies, quantitative adjustment for differences in patient characteristics across trials was not feasible because of the paucity of such historical trials. It should also be noted that these clinical trial results are representative of a very select group of patients with mTNBC. Therefore, worse outcomes are likely in the general population of patients, many of whom would not meet the stringent eligibility criteria specified in these clinical trials (e.g., exclusion of patients with brain metastases at screening, exclusion of patients with early recurrences in first-line studies).
Adequately controlled historical data on the treatment of mTNBC are limited, which may be attributed to the lack of therapies specific to mTNBC. Among the available historical data, commonly used chemotherapies have demonstrated limited durability of response, limited survival benefit, and challenging toxicity profiles, suggesting a considerable unmet medical need in mTNBC. The recent approval of the combination of nab-paclitaxel and atezolizumab for the treatment of PD-L1–positive (IC1+) mTNBC is a positive development for a subset of patients with mTNBC (41% by the Ventana PD-L1 [SP142] assay). However, therapeutic regimens that result in improved, sustainable clinical responses and longer survival, along with more manageable safety profiles, are still needed for patients with mTNBC, including those with PD-L1–negative tumors. Ongoing and future studies with immune therapies, targeted agents, and ADCs, either as monotherapy or combination treatment, can provide new opportunities for improved outcomes in patients with this difficult-to-treat BC subtype.
Availability of data and materials
Not applicable.
Abbreviations
Second-line or higher
Adverse event
All patients as treated
Breast cancer
Confidence interval
Duration of response
European School of Oncology-European Society for Medical Oncology
US Food and Drug Administration
Glycoprotein NMB
Glembatumumab vedotin
Metastatic breast cancer
- Metastatic triple-negative breast cancer
National Comprehensive Cancer Network
Objective response rate
Overall survival
Programmed death 1
Programmed death-ligand 1
Progression-free survival
Triple-negative breast cancer
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Article PubMed Google Scholar
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–8.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502.
Article CAS PubMed Google Scholar
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): breast cancer (Version 1.2019). Accessed 8 July 2019.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29:1634–57.
Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer. 2016;10:25–36.
CAS PubMed PubMed Central Google Scholar
Piccart-Gebhart MJ, Burzykowski T, Buyse M, Sledge G, Carmichael J, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–6.
Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, Awada A. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148:553–61.
Article CAS PubMed PubMed Central Google Scholar
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–21.
TECENTRIQ® (atezolizumab) injection, for intravenous use [prescribing information]. South San Francisco, CA, USA: Genentech, Inc.; 2019.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): breast cancer (Version 1.2016). Accessed 8 July 2019.
Pivot X, Marme F, Koenigsberg R, Guo M, Berrak E, Wolfer A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol. 2016;27:1525–31.
Dent R, Seock-Ah IM, Espie M, Blau S, Tan AR, et al. Overall survival (OS) update of the double-blind placebo (PBO)-controlled randomized phase 2 LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for locally advanced/metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2018;36(15_suppl):1008.
Article Google Scholar
Kim SB, Dent R, Im SA, Espie M, Blau S, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360–72.
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–63.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
Robson ME, Tung N, Conte P, Im SA, Senkus E, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–66.
Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Article PubMed PubMed Central Google Scholar
Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112:2710–9.
Aftimos P, Polastro L, Ameye L, Jungels C, Vakili J, et al. Results of the Belgian expanded access program of eribulin in the treatment of metastatic breast cancer closely mirror those of the pivotal phase III trial. Eur J Cancer. 2016;60:117–24.
Awada A, Bondarenko IN, Bonneterre J, Nowara E, Ferrero JM, et al. A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann Oncol. 2014;25:824–31.
Baselga J, Gomez P, Greil R, Braga S, Climent MA, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–92.
Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–75.
Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–9.
Miles DW, Dieras V, Cortes J, Duenne AA, Yi J, O'Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24:2773–80.
Perez EA, Awada A, O'Shaughnessy J, Rugo HS, Twelves C, et al. Etirinotecan pegol (NKTR-102) versus treatment of physician's choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1556–68.
Pivot XB, Li RK, Thomas ES, Chung HC, Fein LE, et al. Activity of ixabepilone in oestrogen receptor-negative and oestrogen receptor-progesterone receptor-human epidermal growth factor receptor 2-negative metastatic breast cancer. Eur J Cancer. 2009;45:2940–6.
Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–63.
Tredan O, Campone M, Jassem J, Vyzula R, Coudert B, et al. Ixabepilone alone or with cetuximab as first-line treatment for advanced/metastatic triple-negative breast cancer. Clin Breast Cancer. 2015;15:8–15.
von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1269–78.
Article CAS Google Scholar
Brodowicz T, Lang I, Kahan Z, Greil R, Beslija S, et al. Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses. Br J Cancer. 2014;111:2051–7.
Dieras V, Campone M, Yardley DA, Romieu G, Valero V, et al. Randomized, phase II, placebo-controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple-negative breast cancer. Ann Oncol. 2015;26:1904–10.
Fan Y, Xu BH, Yuan P, Ma F, Wang JY, et al. Docetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann Oncol. 2013;24:1219–25.
Halim IIA, El-Sadda W, El-Ebrashy M, El Ashry M. Phase II study of weekly paclitaxel and carboplatin followed by oral navelbine in patients with triple negative metastatic/recurrent breast cancer previously treated with adjuvant anthracycline and taxotere. J Clin Oncol. 2016;34(15_suppl):e12557.
Li Q, Li Q, Zhang P, Yuan P, Wang J, et al. A phase II study of capecitabine plus cisplatin in metastatic triple-negative breast cancer patients pretreated with anthracyclines and taxanes. Cancer Biol Ther. 2015;16:1746–53.
Liao Y, Fan Y, Wan Y, Li J, Peng L. Acceptable but limited efficacy of capecitabine-based doublets in the first-line treatment of metastatic triple-negative breast cancer: a pilot study. Chemotherapy. 2013;59:207–13.
O'Shaughnessy J, Schwartzberg L, Danso MA, Miller KD, Rugo HS, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–7.
Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015;33:2361–9.
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–60.
Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–23.
Duda DG, Ziehr DR, Guo H, Ng M, Barry WT, et al. Effect of cabozantinib treatment on circulating immune cell populations in patients with metastatic triple-negative breast cancer (TNBC). J Clin Oncol. 2016;34(15_suppl):1093.
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961–9.
Tolaney SM, Tan S, Guo H, Barry W, Van AE, et al. Phase II study of tivantinib (ARQ 197) in patients with metastatic triple-negative breast cancer. Investig New Drugs. 2015;33:1108–14.
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44.
Emens LA, Braiteh FS, Cassier P, Delord J-P, Eder JP, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res. 2015;75(15_suppl):2859.
Google Scholar
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J Clin Oncol. 2017;35(15_suppl):1008.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): breast cancer (Version 1.2018). Accessed 8 July 2019.
Schmid P, Abraham J, Chan S, Wheatley D, Brunt M, et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J Clin Oncol. 2018;36(15_suppl):1007.
Bardia A, Vahdat L, Diamond J, Kalinsky K, O'Shaughnessy J, et al. Abstract GS1–07: Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate, as ≥3rd-line therapeutic option for patients with relapsed/refractory metastatic triple-negative breast cancer (mTNBC): efficacy results [abstract]. Cancer Res. 2018;78(suppl 4):Abstract GS1-07.
Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer. J Clin Oncol. 2015;33:1609–19.
Vahdat LT, Forero-Torres A, Schmid P, Blackwell K, Telli ML, et al. A randomized international phase 2b study of the antibody-drug conjugate (ADC) glembatumumab vedotin (GV) in gpNMB-overexpressing, metastatic, triple-negative breast cancer (mTNBC). J Clin Oncol. 2019;79(4_suppl):P6–20 01.
Download references
Acknowledgements
The authors thank the study 301 research team. Medical writing and/or editorial assistance was provided by Amy McQuay, PhD, of the ApotheCom pembrolizumab team (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author information
Claire H. Li and Mallika Lala contributed equally to this work.
Authors and Affiliations
Merck & Co., Inc., Kenilworth, NJ, USA
Claire H. Li, Vassiliki Karantza, Gursel Aktan & Mallika Lala
You can also search for this author in PubMed Google Scholar
Contributions
CHL, VK, and ML conceived and designed the analysis; CHL, GA, and ML collected the data; CHL, GA, and ML analyzed the data; and CHL, VK, GA, and ML interpreted the data. All authors were involved in the drafting, critical review, and approval of the final manuscript and the decision to submit for publication.
Corresponding author
Correspondence to Mallika Lala .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
CHL, VK, GA, and ML have consented to the publication of this manuscript.
Competing interests
CHL, VK, GA, and ML are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:.
Table S1. PubMed search queries.
Additional file 2:
Table S2. Cochrane search queries.
Additional file 3:
Table S3. Embase search queries.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Li, C.H., Karantza, V., Aktan, G. et al. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res 21 , 143 (2019). https://doi.org/10.1186/s13058-019-1210-4
Download citation
Received : 24 January 2019
Accepted : 15 October 2019
Published : 16 December 2019
DOI : https://doi.org/10.1186/s13058-019-1210-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Chemotherapy
- Immune checkpoint inhibitor
- PARP inhibitor
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 18 May 2024
Disparities in quality of life among patients with breast cancer based on surgical methods: a cross-sectional prospective study
- Yi Wang 1 ,
- Yibo He 1 ,
- Shiyan Wu 1 &
- Shangnao Xie 1
Scientific Reports volume 14 , Article number: 11364 ( 2024 ) Cite this article
229 Accesses
Metrics details
- Breast cancer
- Quality of life
To determine the impact of breast conservation on quality of life and identify treatment-related and other demographic factors associated with post-breast cancer treatment quality of life. A prospective study was conducted on 392 women who underwent breast cancer surgery at Hangzhou Cancer Hospital from January 1, 2013, to December 31, 2022. Operable breast cancer patients who had completed all treatments except endocrine therapy were included. Patients with tumor recurrence/metastasis, bilateral or male breast cancer, and other primary malignancies were excluded. After enrollment, patients were asked to complete the BREAST-Q scale, and their pathological and medical records were reviewed. Analysis of variance was used to compare the quality of life scores among the groups. Univariate and multivariate linear regression analyses were performed to identify independent factors associated with quality of life scores in different domains. Participants completed the BREAST-Q scale at a median of 4.6 years after surgery. Quality of life scores varied based on the therapeutic strategy. Breast conservation has significant advantages over mastectomy in terms of breast satisfaction, psychosocial, and sexual well-being. Compared to oncoplastic breast-conserving surgery, mastectomy was independently associated with decreased breast satisfaction, psychosocial, and sexual well-being, while conventional breast-conserving surgery showed comparable outcomes to oncoplastic breast-conserving surgery in terms of these factors. Breast conservation leads to an improvement in quality of life compared to mastectomy. Oncoplastic breast-conserving surgery does not lead to a decrease in quality of life compared to conventional breast-conserving surgery and offers better outcomes compared to mastectomy.
Similar content being viewed by others

Acute health-related quality of life outcomes and systemic inflammatory markers following contemporary breast cancer surgery

Health-related quality of life and its influencing factors in patients with breast cancer based on the scale QLICP-BR

Primary breast cancer and health related quality of life in Spanish women: The EpiGEICAM case-control study
Introduction.
Breast cancer is a prevalent global malignancy 1 , and breast-conserving surgery (BCS) with adjuvant radiotherapy (RT) is a well-established treatment for early-stage breast cancer 2 , 3 . However, up to 30% of BCS recipients express dissatisfaction with their postoperative appearance, necessitating corrective interventions 4 . In the 1980s, European surgeons introduced "oncoplastic breast-conserving surgery" (OBCS), which incorporates plastic surgery techniques for post-BCS breast defect reconstruction 5 .
While OBCS offers satisfactory long-term oncological results and broadens treatment possibilities for patients who would typically undergo mastectomies 6 , it involves more extensive incisions, additional tissue manipulation, and potential flap reconstruction in comparison to conventional breast-conserving surgery (cBCS) 7 , 8 . The procedures involved in OBCS are more complex, time-consuming, and costly. Given these complexities, is it still worthwhile to pursue breast conservation by OBCS? Some researchers have proposed whether the use of OBCS should be reduced 9 .
Understanding the impact on the quality of life of breast cancer survivors is crucial given its significant influence on medical decision-making 10 , 11 . Despite the widespread utilization of OBCS to conserve the breast and enhance its aesthetics, research on its impact on quality of life is limited and complicated due to the variability of surgical approaches. Consequently, this study aimed to assess the effect of breast conservation by OBCS on the quality of life of patients with operable breast cancer treated at Hangzhou Cancer Hospital from January 1, 2013, to December 31, 2022, and to elucidate the treatment and demographic factors associated with postoperative quality of life.
Materials and methods
This prospective, cross-sectional, case–control study was conducted at a single center. The inclusion criteria were operable breast cancer patients treated at Hangzhou Cancer Hospital between January 1, 2013, and December 31, 2022, who had completed all treatments except endocrine therapy and provided participation consent. The exclusion criteria were patients with tumor recurrence/metastasis, bilateral or male breast cancer, or other primary malignancies. Participants were categorized into two groups: BCS group (cBCS with RT subgroup and OBCS with RT subgroup), and unilateral MAST group (MAST with RT subgroup and MAST without RT subgroup). This study utilized the BREAST-Q scale 12 , which includes separate modules for BCS and MAST without reconstruction. The BCS module was used for the OBCS with RT subgroup because OBCS in this study predominantly referred to oncoplastic lumpectomy/glandular remodeling. BREAST-Q assesses six distinct domains: satisfaction with breasts, psychosocial well-being, physical well-being, sexual well-being, satisfaction with overall outcome, and satisfaction with care. Due to the elapsed time between surgery and questionnaire completion in this study, the domains of satisfaction with the overall outcome and satisfaction with care were excluded. Each domain was scored on a scale from 0 to 100, with higher scores indicating an enhanced quality of life. Differences in BREAST-Q scores were categorized as small (2–3 points), moderate (4–7 points), and large (8–10 points) 13 . Patient characteristics, collected using the questionnaire, included employment status, educational level, marital status, and economic status. Patients’ medical and pathological records were reviewed to determine the disease tumor, node, and metastasis (TNM) staging 14 , erythroblastic oncogene B (ERBB2; formerly HER2/neu or HER2) status, hormone receptor status, and body mass index (BMI). Information on surgery, chemotherapy (yes/no), RT, and endocrine therapy (yes/no) was obtained using a questionnaire in conjunction with medical records. The lymphedema status (yes/no) was assessed using the questionnaire's question regarding arm swelling. This study was approved by the Ethics Committee of Hangzhou Cancer Hospital, and all participants provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and followed the guidelines of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) reporting guidelines.
Statistical analysis
The experimental data were statistically analyzed using SPSS (version 29.0) software, and categorical covariates were expressed as numbers (percentages). Analysis of variance (ANOVA) was used to compare quality of life scores among the different groups. Univariate and multivariate linear regression analyses were used to determine the independent factors associated with the quality of life scores in each domain. Variables with two-tailed P ≤ 0.15 in the univariate analysis were included in the multivariate analysis model using a stepwise method to establish the final multivariate model. Differences with P < 0.05 were considered statistically significant.
Ethics approval and consent to participate
This study was reviewed and approved by the ethics committee of Hangzhou Cancer Hospital (approval number: [hzch-2023] HS no.007). Written informed consent was obtained from every patient.
Patient enrollment
After screening, 623 eligible patients were invited, 456 provided written informed consent and completed the survey, but three were found to not meet the inclusion criteria after enrollment. After excluding 61 participants who only completed a brief questionnaire, a total of 392 patients’ data were included in the statistical analysis.
Patient, disease, and treatment characteristics
The interval between surgery and scale completion averaged 4.6 years (range: 0.33 to 9.83 years). Patient characteristics are detailed in Table 1 . Majority were married, employed, had moderate economic status (income ¥30,000–200,000 per year), and high school or higher education. At surgery, 324 (82.7%) patients had a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) within the normal range (18.5 to 23.9 kg/m 2 ), and 56 (14.3%) patients had a BMI of 24 kg/m 2 or above. Among the patients, 39 (9.9%) had stage 0 breast cancer, 154 (39.3%) had stage I breast cancer, 158 (40.3%) had stage II breast cancer, and 41 (10.5%) had stage III breast cancer. The lesions on imaging before surgery of 253 (64.5%) patients measured two centimeters or less, 134 (34.2%) two to five centimeters, and 5 (1.3%) more than five centimeters. Chemotherapy was administered to 293 (74.7%) patients, with 121(30.9%) receiving neoadjuvant chemotherapy, and 273 (69.6%) patients received hormone therapy.
Treatment details including surgery, RT, and lymphedema are presented in Table 1 . Among the patients, 88 (22.4%) underwent OBCS, 51 (13.0%) underwent cBCS, and 253 (64.5%) underwent unilateral MAST, among which 100 (25.5%) patients who underwent unilateral MAST received postoperative RT. All patients underwent axillary surgery, with 255 (65.1%) patients undergoing sentinel lymph node biopsy only and 137 (34.9%) patients undergoing axillary lymph node dissection. 61 (15.6%) patients reported having lymphedema.
BREAST-Q results by breast surgery strategy
Figure 1 illustrates unadjusted mean BREAST-Q scores by breast surgery strategy. Satisfaction with breasts, psychosocial well-being and sexual well-being were significantly different among the groups ( P < 0.001). BCS group showed higher scores in satisfaction with breasts (61.70), psychosocial well-being (76.01), physical well-being (83.52) and sexual well-being (55.06), while the scores for MAST group is lower (satisfaction with breasts: 57.30, psychosocial well-being: 70.83, physical well-being: 82.40 and sexual well-being: 49.21).
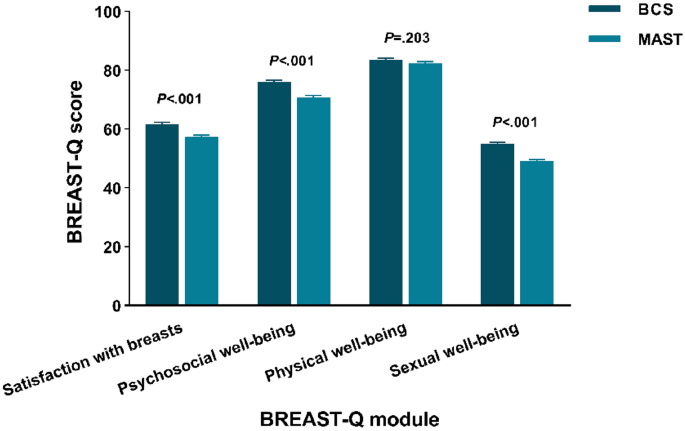
Unadjusted BREAST-Q mean scores by breast surgery strategy. BCS: breast-conserving surgery; MAST: mastectomy.
Satisfaction with breasts
Higher scores in satisfaction with breasts correlated independently with age ≥ 60 (β = 4.662; 95% CI = 2.345 to 6.979; P < 0.001) and patient-reported income ≥ 200,000 (β = 5.068; 95% CI = 2.781 to 7.356; P < 0.001). Lower scores were associated with BMI ≥ 24 (β = − 2.528; 95% CI = − 4.977 to − 0.079; P = 0.043), axillary dissection (β = − 4.875; 95% CI = − 6.704 to − 3.046; P < 0.001) and MAST (β = − 3.927; 95% CI = − 5.741 to − 2.113; P < 0.001) (Fig. 2 A). Patient-reported income < 30,000 and lymphedema showed significance only in univariate analysis. Other factors exhibited no significant association.
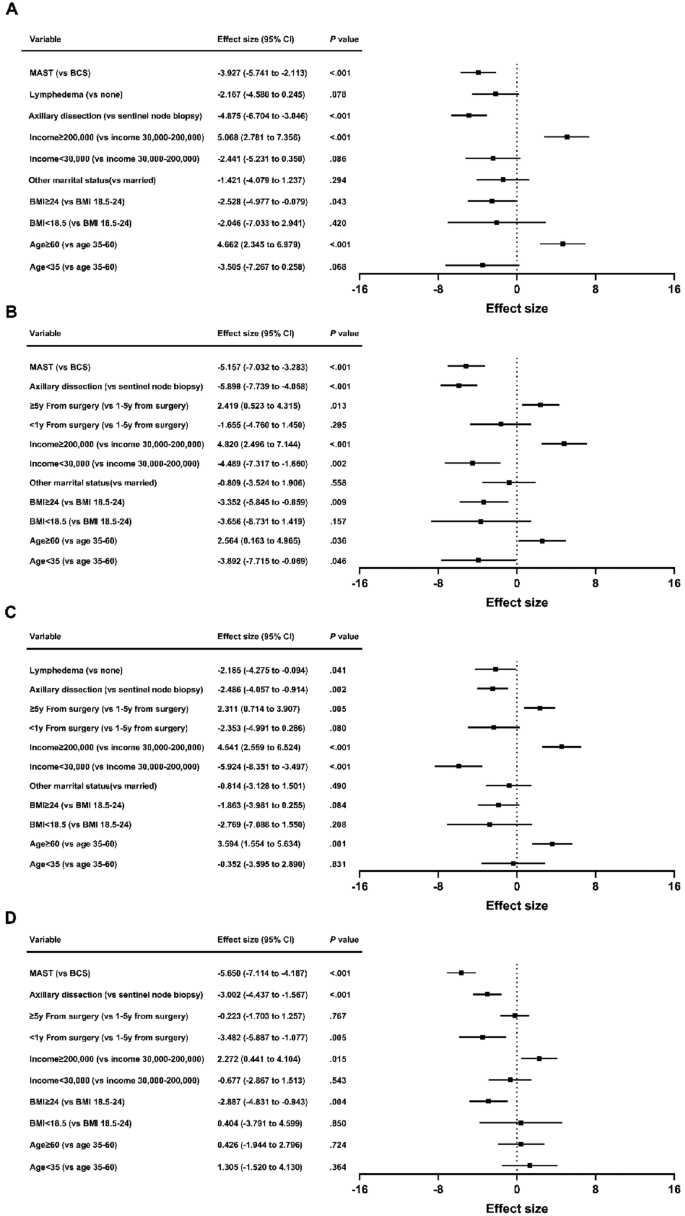
Patient and treatment factors associated with breast satisfaction ( A ), psychosocial well-being ( B ), physical well-being ( C ) and sexual well-being ( D ) scores by breast surgery strategy. MAST: mastectomy; BCS: breast-conserving surgery; BMI: body mass index; CI: confidence interval.
Psychosocial well-being
Better psychosocial well-being correlated with age ≥ 60 (β = 2.564; 95% CI = 0.163 to 4.965; P = 0.036), patient-reported income ≥ 200,000 (β = 4.820; 95% CI = 2.496 to 7.144; P < 0.001), and ≥ 5y from surgery (β = 2.419; 95% CI = 0.523 to 4.315; P = 0.013). Poor psychosocial well-being was linked to age < 35 (β = − 3.892; 95% CI = − 7.715 to − 0.069; P = 0.046), BMI ≥ 24 (β = − 3.352; 95% CI = − 5.845 to − 0.859; P = 0.009), patient-reported income < 30,000 (β = − 4.489; 95% CI = − 7.317 to − 1.660; P = 0.002), axillary dissection (β = − 5.898; 95% CI = − 7.739 to − 4.058; P < 0.001) and MAST (β = − 5.157; 95% CI = − 7.032 to − 3.283; P < 0.001) (Fig. 2 B). Chemotherapy was only significant in univariate analysis. Other variables showed no significant association.
Physical well-being
Factors associated with better physical well-being were age ≥ 60 (β = 3.594; 95% CI = 1.554 to 5.634; P = 0.001), patient-reported income ≥ 200,000 (β = 4.541; 95% CI = 2.559 to 6.524; P < 0.001), and ≥ 5y from surgery (β = 2.311; 95% CI = 0.714 to 3.907; P = 0.005). Conversely, patient-reported income < 30,000 (β = − 5.924; 95% CI = − 8.351 to − 3.497; P < 0.001), axillary dissection (β = − 2.486; 95% CI = − 4.057 to − 0.914; P = 0.002) and lymphedema (β = − 2.185; 95% CI = − 4.275 to − 0.094; P = 0.041) were associated with poorer physical well-being (Fig. 2 C). < 1y from surgery was only significant in univariate analysis. Other factors lacked significant association.
Sexual well-being
Multivariate analysis indicated lower sexual well-being scores with BMI ≥ 24 (β = − 2.887; 95% CI = − 4.831 to − 0.943; P = 0.004), < 1y from surgery (β = − 3.482; 95% CI = − 5.887 to − 1.077; P = 0.005), axillary dissection (β = − 3.002; 95% CI = − 4.437 to − 1.567; P < 0.001), and MAST (β = − 5.650; 95% CI = − 7.114 to − 4.187; P < 0.001). Patient-reported income ≥ 200,000 (β = 2.272; 95% CI = 0.441 to 4.104; P = 0.015) correlated with elevated sexual well-being (Fig. 2 D). Lymphedema was significant in univariate analysis. Other variables exhibited no significant correlation.
BREAST-Q results by local therapy strategy
To assess if there were enhancements in quality of life among women who underwent OBCS, we performed similar analyses among the subgroups. Figure 3 illustrates unadjusted mean BREAST-Q scores by local therapy strategy. All four domains were significantly different ( P < 0.05). OBCS with RT group showed highest scores in satisfaction with breasts (61.99), psychosocial well-being (76.27) and sexual well-being (55.53). cBCS with RT group yielded the highest physical well-being score (84.10). The lowest domain scores were in MAST with RT group (satisfaction with breasts: 53.11, psychosocial well-being: 65.49, physical well-being: 79.89 and sexual well-being: 46.24).
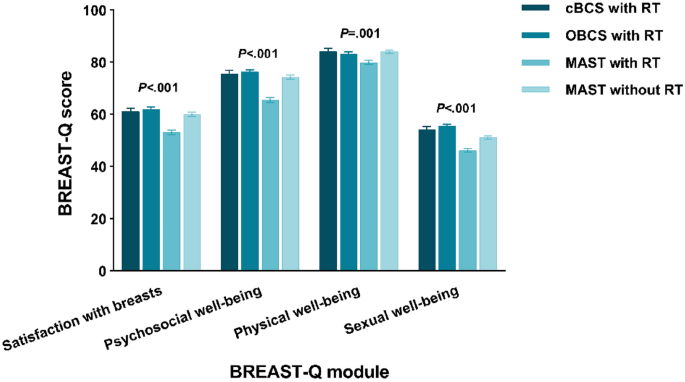
Unadjusted BREAST-Q mean scores by local therapy strategy. RT: radiotherapy; cBCS: conventional breast-conserving surgery; OBCS: oncoplastic breast-conserving surgery; MAST: mastectomy.
Multivariate analysis indicated that MAST with RT was associated with poor breast satisfaction (β = − 8.381; 95% CI = − 10.858 to − 5.905; P < 0.001), psychosocial well-being (β = − 11.491; 95% CI = − 14.039 to − 8.943; P < 0.001), physical well-being (β = − 3.607; 95% CI = − 5.782 to − 1.432; P = 0.001) and sexual well-being (β = − 9.493; 95% CI = − 11.454 to − 7.533; P < 0.001). MAST without RT was associated with decreased breast satisfaction (β = − 2.536; 95% CI = − 4.817 to − 0.255; P = 0.029), psychosocial well-being (β = − 3.171; 95% CI = − 5.487 to − 0.855; P = 0.007) and sexual well-being (β = − 4.739; 95% CI = − 6.530 to − 2.947; P < 0.001). cBCS with RT was not associated with BREAST-Q scores on univariate or multivariate analysis. The statistically significant factors correlated with BREAST-Q scores were mostly consistent with the outcomes of the breast surgery models (Fig. 4 ).
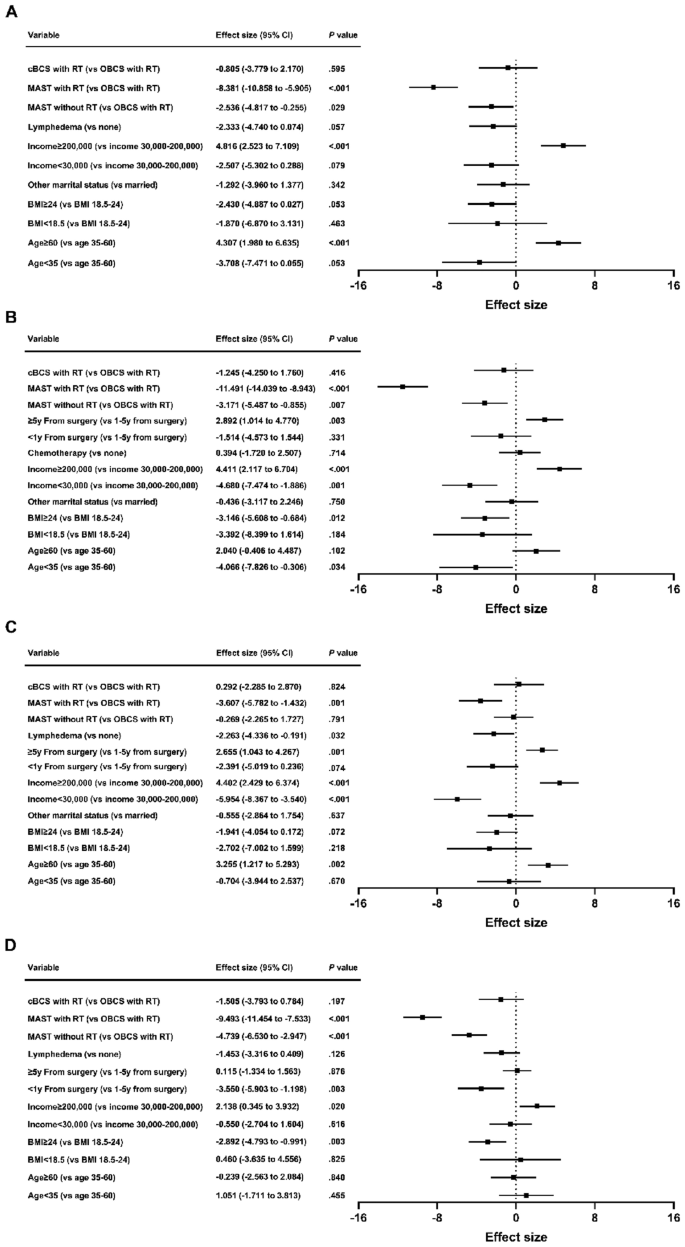
Patient and treatment factors associated with breast satisfaction ( A ), psychosocial well-being ( B ), physical well-being ( C ) and sexual well-being ( D ) scores by local therapy strategy. cBCS: conventional breast-conserving surgery; OBCS: oncoplastic breast-conserving surgery; MAST: mastectomy; RT: radiotherapy; BMI: body mass index; CI: confidence interval.
The rates of BCS and breast reconstruction after mastectomy are significantly lower in China than in Western countries 15 . One contributing factor is that Chinese women typically have smaller breast sizes than women in Western countries, while presenting with larger breast tumor volumes at the time of initial diagnosis, making BCS challenging. Additionally, some Chinese patients adhere to outdated beliefs and have concerns about potential impacts on treatment outcomes or cancer recurrence associated with BCS. OBCS provides acceptable long-term oncological outcomes and has extended treatment options for patients who would traditionally be candidates for mastectomies 6 . In recent years, there has been a clear change in the emphasis of surgical oncology in China, with a growing emphasis on utilizing modern oncoplastic surgical techniques to perform more breast conserving surgeries. Given the increasing prevalence of OBCS, it is essential to examine its impact on quality of life.
In this single-center prospective study, discernible disparities in quality of life surfaced among patients with breast cancer undergoing various local treatment strategies within ten years of surgery. Patients opting for more extensive surgery, particularly when combined with RT, experienced diminished quality of life; satisfaction with breasts; and psychosocial, physical, and sexual well-being. This aligns with findings from prior studies. Engel et al.’s study 16 has shown that patients undergoing BCS reports a higher quality of life compared to those opting for mastectomy. This improvement is often linked to the conservation of the breast and the associated psychological advantages. BCS enables breast conservation, leading to enhanced body image and self-esteem. Patients undergoing BCS may experience less psychological distress and enjoy better psychosocial well-being due to breast conservation. Additionally, BCS has a lesser impact on sexual well-being in comparison to mastectomy, as it retains natural breast tissue.
This study’s findings concur with those of Otsuka et al.’s study 17 in that oncoplastic surgery improved satisfaction with breasts. However, in Otsuka et al.’s study, the quality of life score was not elevated by OBCS (major breast surgery: 154.5 ± 24.6; minor breast surgery: 159.0 ± 20.8; OBCS: 158.7 ± 14.0). Although differences exist between major breast surgery and OBCS, the difference is not pronounced. In the present study, psychosocial and sexual well-being scores were elevated compared to MAST. Additionally, patients who underwent OBCS had better physical well-being scores than those who underwent MAST with RT and equal physical well-being scores than those who underwent MAST without RT. This may be attributable to the omission of RT, reduced chemotherapy and lymphedema in the MAST without RT group. Previous studies 18 , 19 have highlighted RT, chemotherapy, and lymphedema as adverse determinants of quality of life.
Rose et al. 20 suggested that patients who underwent OBCS showed significant improvement in the “psychosocial well-being” module compared to cBCS, while no significant differences were observed between the two groups in the “physical health,” “breast satisfaction,” and “sexual health” modules. Furthermore, a meta-analysis 21 indicated improved quality of life with OBCS compared with cBCS in patients with early-stage breast cancer, with better physical and psychological well-being, higher self-esteem, and a more stable body image, leading to improved social and emotional functioning. However, the clinical studies included in the meta-analysis were predominantly small- sample studies from single centers, and the surgical approaches varied. This study identified no significant differences in any of the quality of life modules between the patients who underwent OBCS and those who underwent cBCS, which is consistent with the findings of de Oliveira-Junior et al 22 . This may be because the present study’s follow-up time was longer, and several aspects of OBCS will decline over time 23 . In our study, the tumor lesion on imaging before surgery averaged 2.11 ± 0.67 cm in OBCS subgroup, and 1.62 ± 0.52 cm in cBCS subgroup. Smaller lesions are more likely to undergo cBCS, resulting in comparable cosmetic outcomes between the two surgical groups. Moreover, the limited number of BCS patients in our study is a significant factor that limits the ability to detect differences in quality of life between OBCS and cBCS subgroups.
In addition to the type of surgery, other clinical factors such as BMI (≥ 24), income (< 30,000), < 1y from surgery, axillary dissection, and lymphedema were negatively correlated with quality of life. Identifying these risk factors can facilitate early postoperative intervention and ultimately improve the postoperative quality of life of patients with breast cancer. Age (≥ 60) and ≥ 5y from surgery were associated with enhanced quality of life. Breast cancer patients can experience significant effects from the disease itself and the ongoing adjuvant therapies, both after diagnosis and during the treatment process 24 . These are all factors that lead to decreased quality of life within 5 years, especially within 1 year, rather than ≥ 5y after surgery. Moreover, good economic status was associated with better satisfaction with breasts, and psychosocial, physical, and sexual well-being. Patients with improved financial circumstances can access higher-quality healthcare services, opt for more expensive treatment options that may improve aesthetic outcomes. The financial advantage also affords patients more opportunities for supportive care, counseling, and resources to manage the challenges of breast cancer treatment and recovery, resulting in a decrease in stress, anxiety, and depression. These enhancements can have a positive impact on patients’ self-perception, confidence, and overall satisfaction with their breast appearance, all of which are closely connected to sexual health and intimacy. Notably, other studies 25 , 26 found an association between economic status and quality of life.
This study has some limitations. It was a cross-sectional, single-time, survey-based prospective study; therefore, the baseline quality of life of patients before surgery was not recorded, which may have influenced their choice of surgical approach and postoperative quality of life. Additionally, this study did not identify patients who chose MAST due to refusal of BCS; patients who selected MAST based on personal preferences may have different quality-of-life scores. Furthermore, this study did not include patients with postmastectomy breast reconstructions, which may improve quality of life of postmastectomy patients. Finally, given that this was a single-center small-sample study, studies with larger sample sizes are required to further confirm the findings of this study. Nevertheless, patient-reported questionnaires can provide basic information on quality of life and assist in identifying potential areas requiring intervention during the patient’s survival period.
OBCS is an acceptable option for patients with larger tumors who are not suitable for cBCS because it allows them to conserve their breasts 6 . This study demonstrated that patients who had their breast conserved reported a higher quality of life compared to mastectomy patients. Despite extensive incisions, additional tissue manipulation, and potential flap reconstruction, patients who underwent OBCS did not report a lower quality of life than those who underwent cBCS. Furthermore, they experienced significantly enhanced quality of life compared with patients who underwent MAST, particularly in the domains of satisfaction with breasts, psychosocial well-being, and sexual well-being. Quality of life data should be incorporated into decision support tools to assist patients with breast cancer in selecting the surgical approach, and discussions with patients should include information regarding quality of life to ensure that they understand the long-term impacts of different surgical approaches. This is particularly crucial because most patients with breast cancer have an extended postoperative survival period. Our data can support further improvements in Chinese breast surgical care for better survival and quality of life.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to Chinese law but are available from the corresponding author on reasonable request.
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249 (2021).
Article PubMed Google Scholar
Fisher, B. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 347 (16), 1233–1241 (2002).
Veronesi, U. et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 347 (16), 1227–1232 (2002).
Clough, K. B., Cuminet, J., Fitoussi, A., Nos, C. & Mosseri, V. Cosmetic sequelae after conservative treatment for breast cancer: Classification and results of surgical correction. Ann. Plast. Surg. 41 (5), 471–481 (1998).
Article CAS PubMed Google Scholar
Audretsch, W. et al. Oncoplatic surgery in breast conserving therapy and flap supported operability. In Proceedings of the Annual Symposium on Breast Surgery and Body Contouring. Santa Fe, New Mexico (1993).
Calabrese, C. et al. Oncoplastic conservative surgery for breast cancer: Long-term outcomes of our first ten years experience. Eur. Rev. Med. Pharmacol. Sci. 22 (21), 7333–7342 (2018).
CAS PubMed Google Scholar
Mohamedahmed, A. Y. Y. et al. Comparison of surgical and oncological outcomes between oncoplastic breast-conserving surgery versus conventional breast-conserving surgery for treatment of breast cancer: A systematic review and meta-analysis of 31 studies. Surg. Oncol. 42 , 101779 (2022).
Knowles, S. et al. An alternative to standard lumpectomy: A 5-year case series review of oncoplastic breast surgery outcomes in a Canadian setting. Can. J. Surg. 63 (1), E46–E51 (2020).
Article PubMed PubMed Central Google Scholar
Bonci, E. A., Anacleto, J. C. & Cardoso, M. J. Sometimes it is better to just make it simple. De-escalation of oncoplastic and reconstructive procedures. Breast. 69 , 265–273 (2023).
Ohsumi, S. et al. Factors associated with health-related quality-of-life in breast cancer survivors: Influence of the type of surgery. Jpn. J. Clin. Oncol. 39 (8), 491–496 (2009).
Luini, A. et al. The evolution of the conservative approach to breast cancer. Breast. 16 (2), 120–129 (2007).
Pusic, A. L. et al. Development of a new patient-reported outcome measure for breast surgery: The BREAST-Q. Plast. Reconstr. Surg. 124 (2), 345–353 (2009).
Voineskos, S. H., Klassen, A. F., Cano, S. J., Pusic, A. L. & Gibbons, C. J. Giving meaning to differences in BREAST-Q scores: Minimal important difference for breast reconstruction patients. Plast. Reconstr. Surg. 145 (1), 11e–20e (2020).
American Joint Committee on Cancer. AJCC Cancer Staging Manual 8th edn, 589–628 (Springer, 2017).
Google Scholar
Chen, Y. et al. Current trends of breast reconstruction after mastectomy for breast cancer patients in China: A survey report. Zhonghua Zhong Liu Za Zhi. 36 (11), 851–857 (2014).
PubMed Google Scholar
Engel, J., Kerr, J., Schlesinger-Raab, A., Sauer, H. & Hölzel, D. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J. 10 (3), 223–231 (2004).
Otsuka, S., Watanabe, N., Sasaki, Y. & Shimojima, R. Postoperative courses of breast reconstruction using inferior adipofascial tissue repair. Breast Cancer. 22 (6), 570–577 (2015).
Chu, C. N., Hu, K. C., Wu, R. S. & Bau, D. T. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy. BMC Cancer. 21 (1), 330 (2021).
Article CAS PubMed PubMed Central Google Scholar
Mokhtari-Hessari, P. & Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes. 18 (1), 338 (2020).
Rose, M. et al. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res. Treat. 180 (1), 247–256 (2020).
Aristokleous, I. & Saddiq, M. Quality of life after oncoplastic breast-conserving surgery: A systematic review. ANZ J. Surg. 89 (6), 639–646 (2019).
de Oliveira-Junior, I. et al. Oncoplastic surgery in breast-conserving treatment: Patient profile and impact on quality of life. Breast Care 16 (3), 243–253 (2021).
Maguire, P. D., Adams, A. & Nichols, M. A. Oncoplastic surgery and radiation therapy for breast conservation: Early outcomes. Am. J. Clin. Oncol. 38 (4), 353–357 (2015).
Ohsumi, S., Shimozuma, K., Kuroi, K., Ono, M. & Imai, H. Quality of life of breast cancer patients and types of surgery for breast cancer—current status and unresolved issues. Breast Cancer. 14 (1), 66–73 (2007).
Bowen, D. J. et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res. Treat. 106 (1), 85–95 (2007).
Lathan, C. S. et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J. Clin. Oncol. 34 (15), 1732–1740 (2016).
Download references
Disclaimers
The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Hangzhou Cancer Hospital is intended nor should be inferred.
This research was financed by the Medical and Health Research Project of Zhejiang Province, China (No. 2023KY964).
Author information
Authors and affiliations.
Division of Breast Surgery, Department of Surgical Oncology, Hangzhou Cancer Hospital, Zhejiang, China
Yi Wang, Yibo He, Shiyan Wu & Shangnao Xie
You can also search for this author in PubMed Google Scholar
Contributions
YW and SX contributed to the conception, design, wrote the manuscript and analyzed the data; YH was responsible for the execution and for data collection; and SW supervised the study. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Shangnao Xie .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wang, Y., He, Y., Wu, S. et al. Disparities in quality of life among patients with breast cancer based on surgical methods: a cross-sectional prospective study. Sci Rep 14 , 11364 (2024). https://doi.org/10.1038/s41598-024-62105-z
Download citation
Received : 05 October 2023
Accepted : 14 May 2024
Published : 18 May 2024
DOI : https://doi.org/10.1038/s41598-024-62105-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Operable breast cancer
- Oncoplastic surgery
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Conclusions
- Article Information
Evidence reviews for the USPSTF use an analytic framework to visually display the key questions that the review will address in order to allow the USPSTF to evaluate the effectiveness and safety of a preventative service. The questions are depicted by linkages that relate interventions and outcomes. A dashed line indicates a health outcome that immediately follows an intermediate outcome. For additional details see the US Preventive Services Task Force Procedure Manual. 13
Reasons for exclusion: Design: Study did not use an included design. Outcomes: Study did not have relevant outcomes or had incomplete outcomes. Comparator: Study used an excluded comparator. Intervention: Study used an excluded intervention/screening approach. Population: Study was not conducted in an average-risk population. Timing: Study only reported first (prevalence) round screening follow-up. Publication type: Study was published in non–English-language or only available in an abstract. Quality: Study did not meet criteria for fair or good quality. Setting: Study was not conducted in a setting relevant to US practice. KQ indicates key question.
DBT indicates digital breast tomosynthesis; DM, digital mammography; and RR, relative risk.
a From random-effects restricted maximum likelihood model.
eMethods. Literature Search Strategies for Primary Literature
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Quality Assessment Criteria
eTable 3. Included Studies and Their Ancillary Publications
eTable 4. Screen-Detected DCIS Diagnosed in Studies Comparing Digital Breast Tomosynthesis and Digital Mammography
eFigure 1. Pooled Analysis of Screen-Detected Invasive Cancers Diagnosed in Trials Comparing Digital Breast Tomosynthesis and Digital Mammography
eFigure 2. Pooled Analysis of Interval Cancers Diagnosed in Trials Comparing Digital Breast Tomosynthesis and Digital Mammography
eFigure 3. Cumulative Probability of False-Positive Biopsy in One NSRI Using BCSC Data Comparing Annual vs Biennial Screening with DBT or DM
eFigure 4. Cumulative Probability of False-Positive Recall in One NSRI Using BCSC Data Comparing Annual vs Biennial Screening with DBT or DM
eFigure 5. Cumulative Probability of False-Positive Recall or Biopsy in One NSRI Using BCSC Data Comparing Annual vs Biennial Screening with DBT or DM, among Women with Extremely Dense Breasts
- USPSTF Recommendation: Screening for Breast Cancer JAMA US Preventive Services Task Force April 30, 2024 This 2024 Recommendation Statement from the US Preventive Services Task Force recommends biennial screening mammography for women aged 40 to 74 years (B recommendation) and concludes that evidence is insufficient to assess the balance of benefits and harms of screening mammography in women 75 years or older (I statement) and of screening using ultrasonography or MRI in women with dense breasts on a negative mammogram (I statement). US Preventive Services Task Force; Wanda K. Nicholson, MD, MPH, MBA; Michael Silverstein, MD, MPH; John B. Wong, MD; Michael J. Barry, MD; David Chelmow, MD; Tumaini Rucker Coker, MD, MBA; Esa M. Davis, MD, MPH; Carlos Roberto Jaén, MD, PhD, MS; Marie Krousel-Wood, MD, MSPH; Sei Lee, MD, MAS; Li Li, MD, PhD, MPH; Carol M. Mangione, MD, MSPH; Goutham Rao, MD; John M. Ruiz, PhD; James J. Stevermer, MD, MSPH; Joel Tsevat, MD, MPH; Sandra Millon Underwood, PhD, RN; Sarah Wiehe, MD, MPH
- USPSTF Report: Collaborative Modeling to Compare Breast Cancer Screening Strategies JAMA US Preventive Services Task Force April 30, 2024 This modeling study uses Cancer Intervention and Surveillance Modeling Network models and national data on breast cancer incidence, mammography performance, treatment effects, and other-cause mortality in US women without previous cancer diagnoses to estimate outcomes of various mammography screening strategies. Amy Trentham-Dietz, PhD, MS; Christina Hunter Chapman, MD, MS; Jinani Jayasekera, PhD, MS; Kathryn P. Lowry, MD; Brandy M. Heckman-Stoddard, PhD, MPH; John M. Hampton, MS; Jennifer L. Caswell-Jin, MD; Ronald E. Gangnon, PhD; Ying Lu, PhD, MS; Hui Huang, MS; Sarah Stein, PhD; Liyang Sun, MS; Eugenio J. Gil Quessep, MS; Yuanliang Yang, MS; Yifan Lu, BASc; Juhee Song, PhD; Diego F. Muñoz, PhD; Yisheng Li, PhD, MS; Allison W. Kurian, MD, MSc; Karla Kerlikowske, MD; Ellen S. O’Meara, PhD; Brian L. Sprague, PhD; Anna N. A. Tosteson, ScD; Eric J. Feuer, PhD; Donald Berry, PhD; Sylvia K. Plevritis, PhD; Xuelin Huang, PhD; Harry J. de Koning, MD, PhD; Nicolien T. van Ravesteyn, PhD; Sandra J. Lee, ScD; Oguzhan Alagoz, PhD, MS; Clyde B. Schechter, MD, MA; Natasha K. Stout, PhD; Diana L. Miglioretti, PhD, ScM; Jeanne S. Mandelblatt, MD, MPH
- Toward More Equitable Breast Cancer Outcomes JAMA Editorial April 30, 2024 Joann G. Elmore, MD, MPH; Christoph I. Lee, MD, MS
- Screening for Breast Cancer JAMA JAMA Patient Page April 30, 2024 In this JAMA Patient Page, the US Preventive Services Task Force provides a guide to screening for breast cancer. US Preventive Services Task Force
- When Is It Best to Begin Mammograms, and How Often? JAMA Medical News & Perspectives May 3, 2024 This Medical News story discusses new USPSTF recommendations about the timing of screening mammograms. Rita Rubin, MA
- New Recommendations for Breast Cancer Screening—In Pursuit of Health Equity JAMA Network Open Editorial April 30, 2024 Lydia E. Pace, MD, MPH; Nancy L. Keating, MD, MPH
- USPSTF Breast Cancer Screening Guidelines Do Not Go Far Enough JAMA Oncology Editorial April 30, 2024 Wendie A. Berg, MD, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
Henderson JT , Webber EM , Weyrich MS , Miller M , Melnikow J. Screening for Breast Cancer : Evidence Report and Systematic Review for the US Preventive Services Task Force . JAMA. Published online April 30, 2024. doi:10.1001/jama.2023.25844
Manage citations:
© 2024
- Permissions
Screening for Breast Cancer : Evidence Report and Systematic Review for the US Preventive Services Task Force
- 1 Kaiser Permanente Evidence-based Practice Center, Center for Health Research, Portland, Oregon
- 2 University of California Davis Center for Healthcare Policy and Research, Sacramento
- Editorial Toward More Equitable Breast Cancer Outcomes Joann G. Elmore, MD, MPH; Christoph I. Lee, MD, MS JAMA
- Editorial New Recommendations for Breast Cancer Screening—In Pursuit of Health Equity Lydia E. Pace, MD, MPH; Nancy L. Keating, MD, MPH JAMA Network Open
- Editorial USPSTF Breast Cancer Screening Guidelines Do Not Go Far Enough Wendie A. Berg, MD, PhD JAMA Oncology
- US Preventive Services Task Force USPSTF Recommendation: Screening for Breast Cancer US Preventive Services Task Force; Wanda K. Nicholson, MD, MPH, MBA; Michael Silverstein, MD, MPH; John B. Wong, MD; Michael J. Barry, MD; David Chelmow, MD; Tumaini Rucker Coker, MD, MBA; Esa M. Davis, MD, MPH; Carlos Roberto Jaén, MD, PhD, MS; Marie Krousel-Wood, MD, MSPH; Sei Lee, MD, MAS; Li Li, MD, PhD, MPH; Carol M. Mangione, MD, MSPH; Goutham Rao, MD; John M. Ruiz, PhD; James J. Stevermer, MD, MSPH; Joel Tsevat, MD, MPH; Sandra Millon Underwood, PhD, RN; Sarah Wiehe, MD, MPH JAMA
- US Preventive Services Task Force USPSTF Report: Collaborative Modeling to Compare Breast Cancer Screening Strategies Amy Trentham-Dietz, PhD, MS; Christina Hunter Chapman, MD, MS; Jinani Jayasekera, PhD, MS; Kathryn P. Lowry, MD; Brandy M. Heckman-Stoddard, PhD, MPH; John M. Hampton, MS; Jennifer L. Caswell-Jin, MD; Ronald E. Gangnon, PhD; Ying Lu, PhD, MS; Hui Huang, MS; Sarah Stein, PhD; Liyang Sun, MS; Eugenio J. Gil Quessep, MS; Yuanliang Yang, MS; Yifan Lu, BASc; Juhee Song, PhD; Diego F. Muñoz, PhD; Yisheng Li, PhD, MS; Allison W. Kurian, MD, MSc; Karla Kerlikowske, MD; Ellen S. O’Meara, PhD; Brian L. Sprague, PhD; Anna N. A. Tosteson, ScD; Eric J. Feuer, PhD; Donald Berry, PhD; Sylvia K. Plevritis, PhD; Xuelin Huang, PhD; Harry J. de Koning, MD, PhD; Nicolien T. van Ravesteyn, PhD; Sandra J. Lee, ScD; Oguzhan Alagoz, PhD, MS; Clyde B. Schechter, MD, MA; Natasha K. Stout, PhD; Diana L. Miglioretti, PhD, ScM; Jeanne S. Mandelblatt, MD, MPH JAMA
- JAMA Patient Page Screening for Breast Cancer US Preventive Services Task Force JAMA
- Medical News & Perspectives When Is It Best to Begin Mammograms, and How Often? Rita Rubin, MA JAMA
Importance Breast cancer is a leading cause of cancer mortality for US women. Trials have established that screening mammography can reduce mortality risk, but optimal screening ages, intervals, and modalities for population screening guidelines remain unclear.
Objective To review studies comparing different breast cancer screening strategies for the US Preventive Services Task Force.
Data Sources MEDLINE, Cochrane Library through August 22, 2022; literature surveillance through March 2024.
Study Selection English-language publications; randomized clinical trials and nonrandomized studies comparing screening strategies; expanded criteria for screening harms.
Data Extraction and Synthesis Two reviewers independently assessed study eligibility and quality; data extracted from fair- and good-quality studies.
Main Outcomes and Measures Mortality, morbidity, progression to advanced cancer, interval cancers, screening harms.
Results Seven randomized clinical trials and 13 nonrandomized studies were included; 2 nonrandomized studies reported mortality outcomes. A nonrandomized trial emulation study estimated no mortality difference for screening beyond age 74 years (adjusted hazard ratio, 1.00 [95% CI, 0.83 to 1.19]). Advanced cancer detection did not differ following annual or biennial screening intervals in a nonrandomized study. Three trials compared digital breast tomosynthesis (DBT) mammography screening with digital mammography alone. With DBT, more invasive cancers were detected at the first screening round than with digital mammography, but there were no statistically significant differences in interval cancers (pooled relative risk, 0.87 [95% CI, 0.64-1.17]; 3 studies [n = 130 196]; I 2 = 0%). Risk of advanced cancer (stage II or higher) at the subsequent screening round was not statistically significant for DBT vs digital mammography in the individual trials. Limited evidence from trials and nonrandomized studies suggested lower recall rates with DBT. An RCT randomizing individuals with dense breasts to invitations for supplemental screening with magnetic resonance imaging reported reduced interval cancer risk (relative risk, 0.47 [95% CI, 0.29-0.77]) and additional false-positive recalls and biopsy results with the intervention; no longer-term advanced breast cancer incidence or morbidity and mortality outcomes were available. One RCT and 1 nonrandomized study of supplemental ultrasound screening reported additional false-positives and no differences in interval cancers.
Conclusions and Relevance Evidence comparing the effectiveness of different breast cancer screening strategies is inconclusive because key studies have not yet been completed and few studies have reported the stage shift or mortality outcomes necessary to assess relative benefits.
Breast cancer is the second leading cause of cancer mortality for US women, despite a steady overall decline in breast-cancer mortality rates over the past 20 years. 1 The average age-adjusted rate for the years 2016-2020 was 19.6 per 100 000, with an estimated 43 170 deaths in 2023. 1 , 2 The majority of cases occur between the ages of 55 and 74 years, 1 and incidence is highest among women ages 70 to 74 (468.2 per 100 000). 3 Non-Hispanic White women have the highest breast cancer incidence, 4 but mortality is 40% higher for non-Hispanic Black women (27.6 per 100 000) compared with White women (19.7 per 100 000); non-Hispanic Black women experience lower 5-year survival regardless of the cancer subtype or stage at the time of detection. 1 , 5 - 7
Previous reviews of breast cancer screening effectiveness established the benefits and harms of mammography based primarily on large, long-term trials. 8 , 9 In 2016, the US Preventive Services Task Force (USPSTF) recommended screening for breast cancer in women starting at age 50 years every 2 years continuing through age 74 years (B recommendation) and that screening from ages 40 to 49 years should be based on clinical discussions of patient preferences and individual breast cancer risk (C recommendation). 10 This comparative effectiveness systematic review of breast cancer screening strategies was conducted concurrently with a separate decision modeling study. 11 Both informed the USPSTF updated breast cancer screening recommendations. 12
This review addressed 3 key questions (KQs) on the comparative effectiveness and harms of different screening strategies ( Figure 1 ). Methodological details including study selection, a list of excluded studies, detailed study-level results for all outcomes and for specific subpopulations, and contextual observations are available in the full evidence report. 14
Studies included in the 2016 USPSTF reviews 8 , 9 , 15 , 16 were evaluated for inclusion with eligibility criteria for the current review. In addition, database searches for relevant studies published between January 2014 and August 22, 2022, were conducted in MEDLINE, the Cochrane Central Register of Controlled Clinical Trials, and the Cochrane Database of Systematic Reviews (eMethods in the Supplement ). Reference lists of other systematic reviews were searched to identify additional relevant studies. ClinicalTrials.gov was searched for relevant ongoing trials. Ongoing surveillance to identify newly published studies was conducted through March 2024 to identify major studies published in the interim. Two new nonrandomized studies were identified 17 , 18 and are not further discussed, as they would not change interpretation of the review findings or conclusions.
Two independent reviewers screened titles, abstracts, and relevant full-text articles to ensure consistency with a priori inclusion and exclusion criteria (eTable 1 in the Supplement ). We included English-language studies of asymptomatic screening populations not at high risk for breast cancer. The eligible population for this review is adult females (sex assigned at birth). For consistency with the underlying evidence, the term “women” is used throughout this report; however, cancer registries and studies of breast cancer generally infer gender based on physiology and medical history rather than measuring self-reported gender. Included studies compared mammography screening modalities (mammography with or without digital breast tomosynthesis [DBT]), different screening strategies with respect to interval, age to start, age to stop, or supplemental screening strategies using ultrasound or magnetic resonance imaging (MRI) with mammography.
For KQ1, randomized clinical trials (RCTs) or nonrandomized studies of interventions with contemporaneous comparison groups that reported breast cancer morbidity, mortality, all-cause mortality, or quality of life were included. For KQ2, the primary outcome of interest was progression to advanced breast cancer, defined for this review as stage IIB or higher, which encompasses tumors with local lymph node involvement or distant metastases. 19 Study-defined advanced breast cancer outcomes were used when this outcome was not reported (eg, stage II or higher). Invasive breast cancer detection outcomes from multiple screening rounds can indicate whether a screening modality or strategy reduces the risk of advanced cancer by detecting early cancers that would otherwise have progressed (stage shift), thereby potentially reducing breast cancer morbidity and mortality. 20 - 23
For KQ3, RCTs and nonrandomized studies of interventions reporting adverse events, including psychological harms, radiation exposure, and interval invasive cancers (incident or missed due to false-negative screening) were included, regardless of the number of screening rounds reported. False-positive recall, false-positive biopsy recommendation, and false-positive biopsy rates (individuals who underwent a biopsy for a benign lesion) were obtained from included RCTs and from nonrandomized studies reporting cumulative rates of these potential harms of screening.
Two reviewers evaluated all articles that met inclusion criteria using prespecified quality criteria (eTable 2 in the Supplement ). Discordant quality ratings were resolved through discussion and input from a third reviewer. Risk-of-bias assessment was conducted using the USPSTF-specific criteria for randomized trials 13 and an adapted tool from the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I). 24 Studies determined to be at high risk of bias were excluded. One reviewer extracted key elements of included studies into standardized evidence tables in DistillerSR (Evidence Partners) and a second reviewer checked the data for accuracy. Limited evidence on sub-KQs is available in the full report. 14 When available, reported relative risks were provided in the tables, but we calculated and reported crude effect estimates and confidence intervals when studies did not provide them. For KQ2 intermediate detection outcomes, the definition of advanced cancer reported in the studies was used for synthesis; commonly this was stage II or later. Comparisons of prognostic characteristics or markers (eg, grade, tumor size, nodal involvement, receptor status) were included for comparisons as data allowed.
All quantitative analyses were conducted in Stata version 16 (StataCorp). The presence of statistical heterogeneity was assessed among pooled studies using the I 2 statistic. Where effects were sufficiently consistent and clinical and statistical heterogeneity low, random-effects meta-analyses were conducted using the restricted maximum likelihood; all tests were 2-sided, with P < .05 indicating statistical significance.
Aggregate strength of evidence (ie, high, moderate, or low) was assessed for each KQ and comparison using the approach described in the Methods Guide for the Effectiveness and Comparative Effectiveness Reviews, 25 based on consistency, precision, publication bias, and study quality.
Investigators reviewed 10 378 unique citations and 419 full-text articles for all KQs ( Figure 2 ). Twenty studies reported in 45 publications were included. 26 - 45 A full list of included studies by KQ is located in eTable 3 in the Supplement .
Key Question 1. What is the comparative effectiveness of different mammography-based breast cancer screening strategies (eg, by modality, interval, initiation and stopping age, use of supplemental imaging, or personalization based on risk factors) on breast cancer morbidity and mortality?
Two nonrandomized studies reported on the association of different screening programs with breast cancer morbidity and mortality. One study was designed to compare different ages to stop screening 30 and another compared annual and triennial screening intervals. 41
A fair-quality observational study (n = 1 058 013) on age to stop screening used an emulated trial methodology to analyze a random sample of US Medicare A and B claims data for enrollees aged 70 to 84 years (1999 to 2008), eligible for breast cancer screening, and with at least a 10-year estimated life expectancy. The study estimated the effect of stopping screening at ages 70, 75, and 80 years compared with continued annual screening. 30 , 46 Continuation of screening between the ages of 70 and 74 years was associated with reduced mortality risk based on survival analysis (hazard ratio, 0.78 [95% CI, 0.63 to 0.95]), but the absolute difference in the risk of death for the age group was small and the confidence interval included null (1.0 fewer deaths per 1000 screened [95% CI, −2.3 to 0.1]). These results indicate a difference in the cumulative incidence curves that approached a difference in the mortality risk for the age group. Conversely, continued screening vs no screening from ages 75 to 84 years did not result in statistically significant differences in the absolute risk of breast cancer mortality (0.07 fewer deaths per 1000 [95% CI, –0.93 to 1.3]) or the cumulative mortality incidence (hazard ratio, 1.00 [95% CI, 0.83 to 1.19]).
A fair-quality nonrandomized clinical study (n = 14 765) conducted in Finland during the years 1985 to 1995 assigned participants aged 40 to 49 years to annual or triennial screening invitations by alternating birth year. 41 The study reported no difference in breast cancer mortality: 20.3 deaths per 100 000 person-years with annual screening invitations and 17.9 deaths per 100 000 person-years with triennial screening invitations (relative risk [RR], 1.14 [95% CI, 0.59-1.27]).
Key Question 2. What is the comparative effectiveness of different mammography-based breast cancer screening strategies (eg, by modality, interval, initiation and stopping age, use of supplemental imaging, or personalization based on risk factors) on the incidence of and progression to advanced breast cancer?
No eligible studies of age to start or stop screening, supplemental screening, or personalized screening were included, because no RCTs or nonrandomized studies reported more than a single round of screening comparing screening strategies. For screening interval, 1 RCT 26 and 1 nonrandomized study, 41 and for comparisons of different screening modalities (DBT vs digital mammography) 3 RCTs 27 , 33 , 42 and 2 nonrandomized studies, 34 , 44 met eligibility criteria.
Two fair-quality studies addressed the effect of screening interval on the characteristics of detected cancers. A fair-quality United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) RCT comparing screening intervals was conducted as part of the UK National Breast Screening Program. The study randomized participants aged 50 to 62 years to annual (n = 37 530) or triennial (n = 38 492) breast cancer screening during the years 1989 to 1996. 26 After 3 years of screening (1 incidence screen in the triennial screening group), a similar number of cancers (screen-detected and interval) had been diagnosed in the annual and triennial screening groups (6.26 and 5.40 per 1000 screened, respectively; RR, 1.16 [95% CI, 0.96 to 1.40]). No statistically significant differences were found in the cancer characteristics (tumor size, nodal status, histological grade) between groups over the course of the study.
A fair-quality nonrandomized study using Breast Cancer Surveillance Consortium (BCSC) registry data (1996 to 2012) 39 found the relative risk of being diagnosed with a breast cancer with less favorable prognostic characteristics (stage IIB or higher, tumor size >15 mm, or node-positive) was not statistically different for women screened biennially compared with those screened annually for any age category (40-49, 50-59, 60-69, 70-85 years).
Three fair-quality RCTs 27 , 33 , 42 reported cancer detection over 2 rounds of screening, comparing the effects of screening with DBT and digital mammography on the presence of advanced cancer at subsequent screening rounds ( Table 1 ). Participants were randomized to the DBT intervention group or the digital mammography control group at a first round of screening, followed in 2 trials by a second round of screening with digital mammography for all second-round participants (Proteus Donna, 27 RETomo 42 ) and in 1 trial with DBT for all second-round participants (To-Be 33 ). The trials used an identical screening modality for both study groups at the second round because using the same instrument is a stronger design for detection of stage shift.
The RCTs reported increased detection of invasive cancer with DBT at the first round of screening (pooled RR, 1.41 [95% CI, 1.20 to 1.64]; 3 RCTs [n = 129 492]; I 2 = 7.6%) and no statistical difference in invasive cancer at the subsequent screening (pooled RR, 0.87 [95% CI, 0.73 to 1.05]; 3 RCTs [n = 105 064]; I 2 = 0%) (eFigure 1 in the Supplement ). 27 , 33 , 42 There was no statistically significant difference in the incidence of advanced cancers at the subsequent screening round (progression of cancers not found at prior screening that would indicate stage shift) in the individual trials ( Figure 3 ). Results were inconsistent and thus not pooled for the advanced cancer, larger tumor (>20 mm), and node-positive cancer outcomes. The results for histologic grade 3 cancer at the second screening were consistent (pooled RR, 0.97 [95% CI, 0.61-1.55]; 3 RCTs [n = 105 244]; I 2 = 0%) ( Figure 3 ). Due to the small number of cases, it was not possible to assess differences in the detection of cancers lacking hormone or growth factor receptors (ie, triple-negative cancers) that have the worst prognosis among breast cancer subtypes.
Two fair-quality nonrandomized studies of interventions (NRSIs), including a US study using BCSC data, compared breast cancer detection outcomes from screening over multiple rounds (≥2) with either DBT-based mammography or digital mammography alone. 34 , 44 The findings were generally consistent with the trial results for cancer detection and stage shift.
Key Question 3. What are the comparative harms of different mammography-based breast cancer screening strategies (modality, interval, initiation age, use of supplemental imaging, or personalization based on risk factors)?
No eligible studies of age to start screening or personalized screening were identified. For age to stop screening, 1 fair-quality nonrandomized study met eligibility criteria. 30 For comparisons of potential harms associated with different screening intervals, a fair-quality RCT 26 and 2 fair-quality nonrandomized studies 39 , 41 were included. For comparisons of different screening modalities (DBT vs digital mammography), 4 RCTs (3 good- and 1 fair-quality) 27 , 31 , 33 , 42 and 7 fair-quality nonrandomized studies were included. 28 , 32 , 34 - 36 , 43 , 44
In the NRSI using an emulated trial methodology to evaluate the age to stop screening, 30 the 8-year cumulative proportion of participants with a breast cancer diagnosis was higher among those who continued annual screening from ages 70 to 84 years (5.5%) compared with those who discontinued screening (3.9%) at age 70 years. Because fewer cancers were diagnosed among those who discontinued screening, there was a lower risk of undergoing cancer treatment and experiencing related morbidity. Notably, for participants aged 75 to 84 years, screening (and treatment) were not associated with lower breast cancer mortality (see KQ1 results).
The UKCCCR trial included for KQ2 26 reported fewer interval cancers (false-negative and incident cancers) diagnosed in the annual invitation group compared with triennial screening (1.84 vs 2.70 per 1000 women screened, respectively; RR, 0.68 [95% CI, 0.50 to 0.92]). The nonrandomized clinical trial conducted in Finland included for KQ1 41 also reported interval cancers diagnosed with annual vs triennial screening and found no statistical difference in incidence ( P = .22, data not reported). Data from 2 studies from the BCSC registry reported higher probabilities of false-positive recalls and biopsy recommendations with annual screening compared with biennial screening and no statistical difference in interval cancers in adjusted analyses. 32 , 39 , 44
Four RCTs (3 good-quality, 1 fair-quality) 27 , 31 , 33 , 42 and 7 fair-quality nonrandomized studies 28 , 32 , 34 - 36 , 43 , 44 reported outcomes related to potential screening harms associated with DBT-based screening compared with digital mammography–only screening, including interval cancer rates, round-specific and cumulative false-positive recalls and biopsies, and radiation exposure. Meta-analysis of 3 large trials did not show a statistically significant difference in rates of interval cancer after screening with DBT compared with digital mammography (pooled RR, 0.87 [95% CI, 0.64 to 1.17]; 3 RCTs [n = 130 196]; I 2 = 0%) (eFigure 2 in the Supplement ). 27 , 33 , 42
Data on interval cancers were also obtained from 7 nonrandomized studies. 28 , 32 , 34 - 36 , 43 , 44 The most recent BCSC analysis, reporting interval cancer rates across multiple screening rounds with either DBT or digital mammography, did not identify statistically significant differences in invasive or advanced interval cancers. 44
The effects of DBT screening on false-positive recall and false-positive biopsy rates varied across studies 27 , 33 , 42 and by screening round, with small or no statistical differences between study groups, not consistently favoring DBT-based mammography or digital mammography.
Evidence from 2 nonrandomized BCSC studies provided false-positive results across several screening rounds. 32 , 44 In 1 study, rates of false-positive recall and false-positive biopsy rates were lower with DBT in initial screening rounds, but differences were attenuated and not statistically significant compared with digital mammography only after additional rounds of screening ( Table 2 ). 44 The other study reported no statistical difference in 10-year cumulative false-positive biopsy recommendation rates between biennial DBT and digital mammography screening, but false-positive recall was slightly lower with DBT (eFigures 3 and 4 in the Supplement ); no differences by modality were identified for individuals with extremely dense breasts in stratified analyses (eFigure 5 in the Supplement ). 32
Four RCTs 27 , 31 , 33 , 42 and 1 NRSI 35 reported the mean, median, or relative radiation dose received in each study group at a single screening round. The 3 studies using DBT/digital mammography screening reported radiation exposure approximately 2 times higher in the intervention group compared with the digital mammography–only group. 27 , 35 , 42 Differences between study groups in radiation exposure were smaller in studies using DBT with synthetic digital mammography. 33 , 47
The Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, a good-quality RCT conducted in the Netherlands, randomized (1:4) participants aged 50 to 75 years with extremely dense breasts and negative mammography findings (2011-2015) (n = 40 373) to an invitation or no invitation for supplemental MRI screening. 45 (The RCT was not included for KQ2 because second round results in the control group were unavailable). Fifty-nine percent of those randomized to the invitation underwent an MRI examination (n = 4783). In intention-to-treat analysis, 2.2 per 1000 experienced interval breast cancer diagnoses in the supplemental screening invitation group, compared with 4.7 per 1000 screened in the digital mammography control group (RR, 0.47 [95% CI, 0.29 to 0.77]). Adverse events related to the supplemental MRI screening reported in the trial included 5 classified as serious adverse events (2 vasovagal reactions and 3 allergic reactions to the contrast agent) and 2 reports of extravasation (leaking) of the contrast agents and 1 shoulder subluxation. Twenty-seven participants (0.6% of the MRI group) reported a serious adverse event within 30 days of the MRI. Those who underwent supplemental MRI screening also experienced additional recalls (94.9 per 1000 screened), false-positive recalls (80.0 per 1000 screened), and false-positive biopsies (62.7 per 1000 screened).
A fair-quality nonrandomized study used claims data from commercially insured women (MarketScan database) aged 40 to 64 years who had received at least 1 bilateral screening breast MRI (n = 9208) or mammogram (n = 9208) between January 2017 and June 2018. 29 Following propensity score matching, those undergoing screening with MRI were more likely to have additional health care cascade events such as office visits and follow-up tests unrelated to breast conditions (adjusted difference between groups, 19.6 per 100 screened [95% CI, 8.6 to 30.7]) in the subsequent 6 months.
A fair-quality RCT, the Japan Strategic Anti-cancer Randomized Trial, randomly assigned asymptomatic women aged 40 to 49 years (2007-2011) to breast cancer screening with mammography plus handheld ultrasound (digital mammography/ultrasound) (n = 36 859) or mammography only (digital mammography) (n = 36 139). 40 The relative risk of invasive interval cancer was not statistically significantly different for digital mammography/ultrasound vs digital mammography only (RR, 0.58 [95% CI, 0.31 to 1.08]). This result differs from the statistically significant population-average effect reported in the study ( P = .03), which included interval ductal carcinoma in situ (proportion difference, −0.05% [95% CI, −0.09 to 0]). Those undergoing ultrasound in addition to digital mammography experienced 48.0 per 1000 additional false-positive recall results compared with those assigned to digital mammography screening only.
A fair-quality nonrandomized study using data from 2 BCSC registry sites compared screening outcomes for participants receiving ultrasonography on the same day as a screening mammogram (digital mammography/ultrasound) (n = 3386, contributing 6081 screens) compared with those that received only a mammogram (digital mammography) (n = 15 176, contributing 30 062 screens). 37 However, 31% of participants had a first-degree family history of breast cancer or previous breast biopsy. There was no statistical difference in interval cancer risk (adjusted RR, 0.67 [95% CI, 0.33 to 1.37]), and rates of false-positive biopsy were twice as high for the mammography/ultrasound group (adjusted RR, 2.23 [95% CI, 1.03 to 2.58]).
Prior screening effectiveness reviews based on large trials initiated in previous decades established a statistically significant mortality benefit for mammography screening of women aged 50 to 69 years. 8 , 9 , 15 The current review considered comparative effectiveness questions on the relative benefits and harms of different screening start and stop ages, intervals, and modalities for women at average breast cancer risk. Findings are summarized in Table 3 .
The evidence was insufficient for addressing the age to start or end screening. No eligible studies comparing different ages to start screening were identified. Limited evidence from 1 nonrandomized study, using an emulated trial study design, suggested that screening beyond age 74 years may not reduce breast cancer mortality. 30
Evidence was also insufficient for evaluating the effect of screening intervals on breast cancer morbidity and mortality. Two nonrandomized studies found no difference in breast cancer outcomes. 26 , 39 Moderate evidence supported longer screening intervals (eg, biennial) to reduce the cumulative risk of false-positive recall and biopsy. The observational studies of different screening intervals compared individuals who self-selected or were referred for different screening intervals, contributing to risk of bias in the results.
Results from 3 RCTs 27 , 33 , 42 and 2 nonrandomized studies 34 , 44 provided moderate evidence that DBT-based mammography does not reduce the risk of invasive interval cancer or advanced cancer at subsequent screening rounds. Additional rounds of screening and longer follow-up are needed to fully evaluate whether DBT reduces breast cancer morbidity and mortality. Consistent with trial findings, a nonrandomized BCSC study did not find reduced risks of advanced or interval cancers with DBT. 44 Limited evidence from trials on harms of screening with DBT 27 , 33 , 42 indicated similar false-positive recall and biopsy rates. An observational BCSC study did not show differences in the 10-year cumulative false-positive biopsy rates 32 ; lower false-positive recall and biopsy with DBT screening were attenuated after several screening rounds. 44 Additional research is needed to ascertain whether DBT-based screening would reduce false-positives over a lifetime of screening.
The evidence was not adequate to evaluate the benefits and harms of supplemental MRI screening for people with dense breasts. No eligible studies were identified that provide evidence on breast cancer morbidity or mortality outcomes with supplemental MRI screening compared with mammography alone among individuals with dense breasts. The DENSE trial 45 reported fewer interval cancers with 1 round of supplemental MRI screening, but results from a second screening round are not yet published. Evidence of higher advanced cancer incidence in the mammography-only group relative to the MRI group would be needed to anticipate effects on morbidity or mortality. Supplemental MRI led to additional false-positive recalls and biopsies, and uncommon but serious adverse events were observed. 45 Two recent systematic reviews of the test performance literature reported higher cancer detection with supplemental MRI screening along with substantially increased recall and biopsy rates among individuals without cancer. 48 , 49
Lack of a standardized and reliable assessment tool for measuring breast density and density variation across the lifespan pose challenges for research into the optimal screening strategy for persons with dense breasts. 16 Research is also needed to evaluate personalized risk-based screening, based on breast cancer risk factors and personal screening preferences. The ongoing WISDOM trial and My Personalized Breast Screening study (expected completion in 2025) may help to address these research gaps. 50 , 51
Breast cancer is an active area of research, yet few longitudinal RCTs comparing different screening strategies have been conducted following completion of the major trials that established the effectiveness of mammography for reducing breast cancer mortality for women aged 50 to 69 years. This review included 6 new randomized trials, 27 , 31 , 33 , 40 , 42 , 45 4 comparing DBT with digital mammography screening 27 , 31 , 33 , 42 and 2 on supplemental screening compared with mammography alone. 40 , 45 Three of these trials are ongoing 31 , 40 , 45 and have reported preliminary results only. Observational studies were also included, but few studies were available that followed up a screening population over time to compare the health outcomes associated with different screening approaches. These studies, while potentially more representative of a screening population, have higher risk of biased results due to confounding and selection.
Changes in population health, imaging technologies, and available treatments may limit the applicability of previous studies. Recent trials included in this review were conducted outside of the US and enrolled mostly White European populations. No studies evaluated screening outcomes for racial or ethnic groups in the US that experience health inequities and higher rates of breast cancer mortality. Black women are at highest risk of breast cancer mortality, 52 with lower 5-year survival than all other race and ethnicity groups. 7 Breast cancer mortality risk also increases at younger ages for Black women compared with White women. 53 This review did not address additional factors beyond screening that contribute to breast cancer mortality inequities. 54 Rigorous research is essential to understand and identify improvements needed along the pathway from screening to treatment 55 and to address inequities in follow-up time after a positive screening result, time to diagnosis, 56 - 60 and receipt of high-quality treatment and support services. 59 , 61 , 62
Evidence comparing outcomes for different screening intervals and ages to start and stop screening was limited or absent. Trials of personalized screening based on risk and patient preferences are in progress and may address evidence gaps related to optimal screening start ages and intervals. Research is needed to better characterize potential harms of screening, including patient perspectives on experiencing false-positive screening results. Women with false-positive screening results may be less likely to return for their next scheduled mammogram, as reported in a large US health system study. 55 , 63 Rigorous studies that enroll screening populations and report advanced cancer detection, morbidity, and mortality outcomes from multiple rounds of screening are needed to overcome persistent limitations in the evidence on breast cancer screening. Multiple screening rounds are essential to determine whether a screening modality or strategy reduces the risk of advanced cancer by detecting early cancers that would otherwise have progressed (stage shift), potentially reducing breast cancer morbidity and mortality. 20 - 23 , 64
The potential benefits of risk-stratified screening strategies, including the use of supplemental screening with ultrasound or MRI, have not been fully evaluated, although some harms are evident. Longer term follow-up on existing comparative effectiveness trials, complete results from ongoing RCTs of personalized screening programs, 65 , 66 and rigorous new studies are needed to further strengthen the evidence and optimize breast cancer screening strategies.
Evidence comparing the effectiveness of different breast cancer screening strategies is inconclusive because key studies have not yet been completed and few studies have reported the stage shift or mortality outcomes necessary to assess relative benefits.
Accepted for Publication: November 23, 2023.
Published Online: April 30, 2024. doi:10.1001/jama.2023.25844
Corresponding Author: Jillian T. Henderson, PhD, MPH, Kaiser Permanente Evidence-based Practice Center, Center for Health Research, Kaiser Permanente Northwest, 3800 N Interstate Ave, Portland, OR 97227 ( [email protected] ).
Author Contributions: Dr Henderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical review of the manuscript for important intellectual content: Henderson, Weyrich, Miller.
Statistical analysis: Henderson.
Administrative, technical, or material support: Webber, Melnikow.
Supervision: Henderson.
Conflict of Interest Disclosures: None reported.
Funding/Support: This research was funded under contract number 75Q80120D00004, Task Order 75Q80121F32004, from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services.
Role of the Funder/Sponsor: Investigators worked with USPSTF members and AHRQ staff to develop the scope, analytic framework, and key questions for this review. AHRQ had no role in study selection, quality assessment, or synthesis. AHRQ staff provided project oversight, reviewed the report to ensure that the analysis met methodological standards, and distributed the draft for peer review. Otherwise, AHRQ had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript findings.
Disclaimer: The opinions expressed in this document are those of the authors and do not reflect the official position of AHRQ or the US Department of Health and Human Services.
Additional Contributions: The authors gratefully acknowledge the following individuals for their contributions to this project: Howard Tracer, MD (AHRQ); Heidi D. Nelson, MD, MPH, MACP (Kaiser Permanente Bernard J. Tyson School of Medicine); current and former members of the USPSTF who contributed to topic deliberations; and Evidence-based Practice Center staff members Melinda Davies, MA, Jill Pope, and Leslie A. Purdue, MPH, for technical and editorial assistance at the Kaiser Permanente Center for Health Research. USPSTF members, peer reviewers, and federal partner reviewers did not receive financial compensation for their contributions.
Additional Information: A draft version of this evidence report underwent external peer review from 5 content and methods experts (Nehmat Houssami, MBBS, MPH, Med, PhD [University of Sydney-Australia]; Patricia Ganz, MD [UCLA]; Gerald Gartlehner, MD, MPH [Cochrane Austria]; Karla Kerlikowske, MD [UC San Francisco]; Lisa Newman, MD, MPH [New York Presbyterian/Weill Cornell Medical Center]) and 4 scientific representatives from 3 federal partner organizations (Centers for Disease Control and Prevention; Office of Research on Women’s Health; National Institute on Minority Health and Health Disparities). Comments were presented to the USPSTF during its deliberation of the evidence and were considered in preparing the final evidence review.
Editorial Disclaimer: This evidence report is presented as a document in support of the accompanying USPSTF Recommendation Statement. It did not undergo additional peer review after submission to JAMA .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Systematic review
- Open access
- Published: 22 May 2023
Characteristics and impact of interventions to support healthcare providers’ compliance with guideline recommendations for breast cancer: a systematic literature review
- Ignacio Ricci-Cabello 1 , 2 , 3 ,
- Darla Carvallo-Castañeda 4 ,
- Adrián Vásquez-Mejía 4 ,
- Pablo Alonso-Coello 3 , 5 ,
- Zuleika Saz-Parkinson 6 ,
- Elena Parmelli 6 ,
- Gian Paolo Morgano 6 ,
- David Rigau 5 ,
- Ivan Solà 3 , 5 ,
- Luciana Neamtiu 6 &
- Ena Niño-de-Guzmán 5 , 7
Implementation Science volume 18 , Article number: 17 ( 2023 ) Cite this article
2632 Accesses
2 Citations
12 Altmetric
Metrics details
Breast cancer clinical practice guidelines (CPGs) offer evidence-based recommendations to improve quality of healthcare for patients. Suboptimal compliance with breast cancer guideline recommendations remains frequent, and has been associated with a decreased survival. The aim of this systematic review was to characterize and determine the impact of available interventions to support healthcare providers’ compliance with CPGs recommendations in breast cancer healthcare.
We searched for systematic reviews and primary studies in PubMed and Embase (from inception to May 2021). We included experimental and observational studies reporting on the use of interventions to support compliance with breast cancer CPGs. Eligibility assessment, data extraction and critical appraisal was conducted by one reviewer, and cross-checked by a second reviewer. Using the same approach, we synthesized the characteristics and the effects of the interventions by type of intervention (according to the EPOC taxonomy), and applied the GRADE framework to assess the certainty of evidence.
We identified 35 primary studies reporting on 24 different interventions. Most frequently described interventions consisted in computerized decision support systems (12 studies); educational interventions (seven), audit and feedback (two), and multifaceted interventions (nine). There is low quality evidence that educational interventions targeted to healthcare professionals may improve compliance with recommendations concerning breast cancer screening, diagnosis and treatment. There is moderate quality evidence that reminder systems for healthcare professionals improve compliance with recommendations concerning breast cancer screening. There is low quality evidence that multifaceted interventions may improve compliance with recommendations concerning breast cancer screening. The effectiveness of the remaining types of interventions identified have not been evaluated with appropriate study designs for such purpose. There is very limited data on the costs of implementing these interventions.
Conclusions
Different types of interventions to support compliance with breast cancer CPGs recommendations are available, and most of them show positive effects. More robust trials are needed to strengthen the available evidence base concerning their efficacy. Gathering data on the costs of implementing the proposed interventions is needed to inform decisions about their widespread implementation.
Trial registration
CRD42018092884 (PROSPERO)
Peer Review reports
Contributions to the literature
Research has shown that compliance with breast cancer clinical practice guidelines remains suboptimal, leading to increased mortality rates.
Our study is the first systematic review evaluating interventions to support compliance with breast cancer clinical practice guidelines recommendations, and builds upon previous reviews of this topic in more general contexts. We found that a number of different types of interventions have been developed and evaluated, most of them showing beneficial effects.
The quality of the evidence is low for provider educational interventions, moderate for provider reminders, and low for multifaceted interventions. For the rest of the interventions identified, the evidence is uncertain.
This review contributes to recognized gaps in the literature, including ascertaining which types of interventions work best to promote compliance with breast cancer CPGs, as well as identifying new areas for future research.
Findings from this review may help those practitioners and health decision makers interested in improving the quality and safety of breast cancer healthcare provision by enhancing the uptake of clinical practice guidelines.
Introduction
Breast cancer is the most common cancer in women with 2.3 million new cases estimated in 2020, accounting for 11.7% of all cancers [ 1 ]. It is the fifth leading cause of cancer mortality worldwide, with 685,000 deaths [ 1 ]. Breast cancer diagnosis is more frequent in developed countries [ 2 ]. Controlling and preventing breast cancer is an important priority for health policy makers [ 3 ].
Treatment procedures have rapidly evolved over recent years. As new and precise diagnosis strategies emerged, early treatment and prognosis of breast cancer patients have shown great progresses [ 4 ]. Advances in breast cancer screening and treatment have reduced the mortality of breast cancer across the age spectrum in the past decade [ 5 , 6 , 7 ]. Although the use of research evidence can improve professional practice and patient-important outcomes, considering also the huge volume of research evidence available, its translation into daily care routines is generally poor [ 8 , 9 ]. It is estimated that it takes an average of 17 years for only 14% of new scientific discoveries to enter day-to-day clinical practice [ 10 ].
Clinical Practice Guidelines (CPGs) provide recommendations for delivering high quality healthcare [ 11 , 12 ]. However, the impact of CPGs depends not only on their quality, but also on the way and the extent to which they are used by clinicians in routine clinical practice. Large overviews show that approximately 50% of patients receive from general medical practitioners treatments which differ from recommended best practice [ 13 , 14 , 15 , 16 ]. In the area of breast cancer, previous systematic reviews have shown that compliance with breast cancer CPGs [ 17 ], as well as for other types of cancer [ 18 , 19 , 20 ], remains suboptimal. A recent systematic review from our research group [ 21 ] found large variations in providers´ compliance with breast cancer CPGs, with adherence rates ranging from 0 to 84.3%. Sustainable use of CPGs is also notably poor: after 1 year of their implementation, adherence decreases in approximately half of the cases [ 22 ].
Suboptimal compliance with CPGs recommendations could increase healthcare costs if healthcare resources are overused (e.g., overtreatment, overuse of diagnosis or of screening techniques); but also, if they are underused (i.e., increased costs to cover the additional health care needs that people may face with worsening conditions due to under-used resources). Available evidence suggests that outcomes may improve for patients, healthcare professionals and healthcare organizations if decision-makers adhere to evidence-based CPGs [ 23 , 24 ]. This is supported by a recent meta-analysis from our group [ 25 ], which suggests that compliance with CPGs is probably associated with an increase in both, disease-free survival (hazard ratio (HR) = 0.35 (95% CI from 0.15 to 0.82)) and overall survival (HR = 0.67 (95% CI 0.59 to 0.76). Developing interventions to support clinician uptake of breast cancer CPGs is therefore essential for improving healthcare quality and patient important outcomes. Although several interventions to support compliance with breast cancer CPGs have been proposed, no previous study has systematically examined their characteristics and effects.
The aim of this systematic review is to characterize and evaluate the impact of available interventions to support healthcare providers’ compliance with CPGs in breast cancer care.
We conducted a systematic literature review adhering to the PRISMA reporting guidelines [ 26 ] (PRISMA 2020 Checklist available at Additional file 1 ). In this review, we addressed the following two questions: (1) What type of interventions have been used to support healthcare professionals´ compliance with breast cancer CPGs? and; (2) What type of interventions can effectively support healthcare professionals’ compliance with breast cancer CPGs? We registered the protocol in the international prospective register of systematic reviews (PROSPERO registration number CRD42018092884).
We searched for systematic reviews and original studies in MEDLINE (through PubMed) and Embase (through Ovid) using predefined search strategies from inception to May 2021 designed and implemented by an information specialist (IS) from the Iberoamerican Cochrane Centre (IS). The search strategies (available in Additional file 2 ) combined MeSH terms and keywords.
Study selection
We applied the following inclusion criteria:
Population: healthcare professionals providing health services related to the prevention or management of breast cancer. All types of healthcare professionals, and from any setting were included.
Intervention: interventions explicitly aimed at supporting or promoting healthcare professionals’ compliance with available breast cancer CPGs. Such guidelines may address any specific aspect of breast cancer care, including screening, diagnosis, treatment, surveillance or rehabilitation.
Comparator: any comparator, including also studies not using a comparator group.
Outcome: quality of breast cancer care (based on healthcare professionals’ compliance rate with breast cancer CPGs recommendations, but also on their knowledge, attitudes or self-efficacy concerning such recommendations); intervention implementation (fidelity, reach, implementation costs), and; patient health-related outcomes (e.g., survival).
We included experimental (randomized controlled and non-randomized controlled trials), observational (before-after, cohort, case-control, cross-sectional, and case studies), and qualitative or mixed-methods studies. Due to constrained resources, we only included studies published in English. One author (of IRC, DC, APVM) screened the search results based on title and abstract. A second author (ENG, LN, ZSP, EP, DC, APVM, GPM) independently reviewed 20% of all references. Two authors independently assessed eligibility based on the full text of the relevant articles. Disagreements were discussed (involving a third author when needed) until consensus was reached.
Data extraction
One author (ENG, IRC LN, ZSP, EP, DC, APVM, GPM) extracted the following data about the characteristics and results of the included studies using an ad hoc data extraction form which had been piloted in advance: publication year, study design (e.g., randomized controlled trial), study location, setting, number of participants, aim of the study, type of breast cancer guideline (e.g., breast cancer screening), type of intervention (e.g., computerized decision support systems), and outcome(s) assessed (e.g., compliance rate). A second author (ENG, IRC LN, ZSP, EP, DC, APVM, GPM) cross-checked the extracted data for accuracy.
Quality assessment
We used the following tools to determine the risk of bias of the included studies: the Cochrane Collaboration tool for assessing risk of bias in randomized trials (RoB I) [ 27 ], the ROBINS I tool for non-randomized controlled before-after studies [ 28 ], the Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group [ 29 ], the Newcastle-Ottawa scale for cohort studies [ 30 ], the AXIS tool for cross-sectional studies [ 31 ], and the MMAT tool [ 32 ] for mixed methods studies. The specific criteria included by each of these tools are available in Additional file 3 . One author determined the risk of bias of the included studies, and a second author cross-checked the results for accuracy. Disagreements were solved with support from a senior systematic reviewer.
Data synthesis
We described the characteristics and the effects of the interventions narratively and as tabulated summaries. Findings are synthesized by type of intervention. We applied the Cochrane Effective Practice and Organization Care Review Group (EPOC) [ 33 ] taxonomy to classify our findings according to the types of interventions identified. Whereas for the characterization of the interventions we included all the publications identified meeting our eligibility criteria (irrespectively of their design); for the evaluation of the effectiveness of the interventions we focused only on those studies following a suitable design for such purpose [ 34 ]: randomized controlled trials (RCTs), controlled before-after studies, non-randomized controlled trials, and interrupted time series. Although we planned to conduct a meta-analysis on the impact of the interventions on compliance rates, this was finally not feasible due to the inconsistent and poor reporting. Instead, we provide a graphical quantitative description of the compliance rates before and after the implementation of the interventions.
Certainty of the evidence
Following the GRADE approach [ 35 ], we rated the certainty of evidence as high, moderate, low or very low, taking into consideration risk of bias, imprecision, inconsistency, indirectness, and publication bias. This was done by one researcher. and cross-checked by a second reviewer.
Search results
The eligibility process is summarized in a PRISMA flowchart (Fig. 1 ). We retrieved a total of 9065 unique citations from database searches, which were reviewed (through screening by title and abstract) along with 416 additional references identified from the thirteen systematic reviews also identified. We selected 145 references for full text revision, from which 35 primary studies (reporting on 24 different interventions) were finally included in our systematic review [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 ].
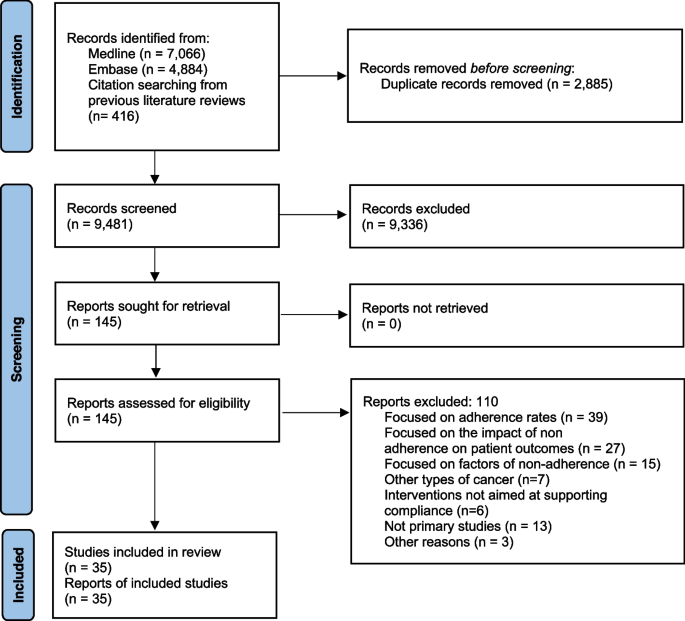
PRISMA flowchart

Characteristics of the included studies
The characteristics of the included studies are summarized in Table 1 and described in detail in Additional file 4 . Most (86%) were published from 2000 onwards. The studies were conducted in six countries: 15 (42%) were conducted in USA [ 37 , 44 , 45 , 46 , 49 , 50 , 51 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 70 ], 12 (34%) in France [ 38 , 39 , 40 , 41 , 42 , 43 , 63 , 64 , 65 , 66 , 67 , 68 ], 3 (9%) in the Netherlands [ 52 , 62 , 69 ], and 3 (9%) in Canada [ 36 , 59 , 61 ]. The remaining two studies were conducted in Australia [ 47 ], and Italy [ 48 ]. Eleven studies described interventions to support compliance with guidelines on diagnosis and treatment [ 41 , 43 , 52 , 56 , 64 , 65 , 66 , 67 , 68 , 69 , 70 ], 9 focused on treatment only [ 38 , 39 , 40 , 42 , 47 , 48 , 49 , 62 , 63 ], 5 on diagnosis only [ 45 , 51 , 58 , 59 , 60 ], and 7 on screening [ 36 , 37 , 46 , 50 , 54 , 57 , 61 ]. Six studies were randomized controlled trials [ 37 , 45 , 50 , 51 , 54 , 60 ], four were non-randomized controlled trials [ 46 , 57 , 58 , 63 ], eight non-controlled before-after studies [ 42 , 49 , 53 , 55 , 59 , 62 , 65 , 69 ], one prospective cohort study , three cross-sectional studies [ 44 , 47 , 56 ], one mixed-methods [ 36 ] and twelve case studies [ 38 , 39 , 40 , 41 , 43 , 48 , 52 , 61 , 64 , 66 , 67 , 68 ].
Thirty of the 35 studies (85%) evaluated the impact of the interventions on compliance rate [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 53 , 54 , 55 , 56 , 58 , 59 , 60 , 62 , 63 , 65 , 66 , 67 , 68 , 69 , 70 ]. Four studies [ 43 , 46 , 50 , 57 ] evaluated the impact on determinants of behavior change related outcomes (providers’ knowledge, attitudes, and self-efficacy about the CPGs recommendations). Two studies evaluated intervention adoption and fidelity [ 36 , 44 ]. No study evaluated the impact of the intervention on patient outcomes, and only one study [ 44 ] evaluated the costs of implementing the interventions.
Characteristics of the interventions to support compliance with breast cancer clinical practice guidelines
Table 2 describes the characteristics of each type of intervention. Twelve studies described two different interventions consisting in the implementation of computerized decision support systems [ 38 , 39 , 40 , 41 , 42 , 43 , 48 , 64 , 65 , 66 , 67 , 68 ], 7 described 6 different educational interventions targeting health care professionals [ 44 , 50 , 55 , 57 , 58 , 59 , 63 ], 9 described 9 multifaceted interventions [ 36 , 37 , 46 , 49 , 51 , 53 , 54 , 60 , 62 ], and two studies described two audit and feedback interventions [ 47 , 69 ]. The rest of the studies described interventions based on: implementation of clinical pathways [ 56 ], integrated knowledge translation systems [ 61 ], medical critiquing system [ 52 ], medical home program [ 70 ], and reminders to providers [ 45 ].
Computerized decision support systems
The use of computerized decision support systems to promote compliance with breast cancer CPGs was described in 12 studies [ 38 , 39 , 40 , 41 , 42 , 43 , 48 , 64 , 65 , 66 , 67 , 68 ]. Eleven of them reported the same intervention, which consisted of a system developed in France called OncoDoc [38, 40–43, 64–68). OncoDoc is a computerized clinical decision support system that provides patient-specific recommendations for breast cancer patients according to CancerEst (local) CPGs [ 71 ]. A study conducted in Italy reported on the development of a similar system, the OncoCure CDSS [ 48 ].
Educational interventions
Seven studies described educational interventions targeting healthcare providers to promote compliance with breast cancer CPGs [ 44 , 50 , 55 , 57 , 58 , 59 , 63 ]. One intervention consisted in the provision of academic detailing on breast cancer screening (based on the American Cancer Society guidelines for the early detection of BC) among primary care physicians in an underserved community in the USA [ 50 ]. An intervention in seven hospitals in France consisted in monthly meetings where local opinion leaders presented the relevant sections of the CPGs, which were subsequently sent to all the participating physicians who were expected to use them in their practice [ 63 ]. Another intervention consisted in a comprehensive continuing medical education package to address pre-identified barriers to guideline adherence. The intervention followed a multimethod approach to physician education including CME conferences, physician newsletters, CBE skills training, BC CME monograph, “question of the month” among hospital staff meetings, primary care office visits, and patient education materials [ 57 , 58 ]. An educational intervention to improve compliance with radiological staging CPGs in early breast cancer patients [ 59 ] consisted of multidisciplinary educational rounds, presenting the Cancer Care Ontario Practice Guidelines [ 72 ]. Another intervention, aimed to support compliance with recommendation against serum tumor marker tests and advanced imaging for BC survivors who are asymptomatic for recurrence, consisted in academic detailing for oncologists at regular meetings [ 55 ]. Another intervention [ 44 ] consisted in an online course to learn to implement and deliver the Strength after Breast Cancer (SABC) guidelines (with recommendations about rehabilitative exercise for breast cancer survivors).
Audit and feedback interventions
We identified two audit and feedback interventions [ 47 , 69 ]. One consisted in sending hospitals a written report with regional benchmark information on nine performance indicators measuring the quality of care based on breast cancer national guidelines [ 69 ]. Healthcare professionals attended sessions twice a year, where an anonymous benchmark was presented for each hospital score compared with the regional mean and the norm scores. Another intervention [ 47 ] audited patients’ medical records according to four agreed indicators. Information from the audit forms was entered into a database, which allowed individualized reports for each participating clinician, providing detailed feedback about their practice, with comparisons across the group and against the agreed criteria.
Other types of single component interventions
Five studies described other strategies to promote compliance with breast cancer CPGs [ 45 , 49 , 52 , 56 , 61 , 70 ]. One intervention consisted on a microcomputer tickler system on the ordering of mammograms [ 45 ]. The system displayed the date of the last mammogram ordered in the “comments” section of the encounter form for each visit. An intervention to support compliance with CPGs follow up recommendations in low-income breast cancer survivors [ 70 ] consisted in the implementation of a medical home program to support primary care case management. Providers and networks participating in this program received a payment per eligible patient per month for care coordination. Another intervention consisted in the implementation of new clinical pathways supplemented by clinical vignettes [ 56 ]. Another intervention consisted in an integrated knowledge translation strategy to be used by guideline developers to improve the uptake of their new CPGs on breast cancer screening [ 61 ]. This integrated knowledge translation strategy was based on the Knowledge to Action Framework [ 73 ], and involved the identification of barriers to knowledge use. An intervention to support compliance with the Dutch breast cancer guideline [ 52 ] consisted of a medical critiquing system (computational method for critiquing clinical actions performed by physicians). The system aimed at providing useful feedback by finding differences between the actual actions and a set of ‘ideal’ actions as described by a CPG.
Multifaceted interventions
We identified nine multifaceted interventions [ 36 , 37 , 46 , 49 , 51 , 53 , 54 , 60 , 62 ]. One intervention to increase compliance with mammography screening [ 37 ] consisted of (i) audit results and a comparison with the network benchmark; (ii) academic detailing of exemplar principles and information from the medical literature; (iii) services of a practice facilitator for 9 months who helped the practitioners design their interventions and facilitate “Plan, Do, Study, Act” processes; and iv) information technology support. In another intervention [ 60 ] to increase screening mammography, primary care providers received (i) a fact sheet providing current information on screening mammography for older women; (ii) telephone follow-up of any questions, and; (iii) copies of a simply written pamphlet on mammography that they could distribute to patients. Another intervention [ 54 ] consisted of biannual feedback to primary care providers regarding compliance with cancer screening CPGs and financial bonuses for “good” performers. Feedback reports documented a site’s scores on each screening measure and a total score across all measures, as well as plan-wide scores for comparison. Another intervention [ 51 ] consisted of an educational intervention accompanied by cue enhancement using mammography chart stickers, and by feedback and token rewards. Another intervention [ 46 ] included (i) use of standardized patients to observe and record healthcare professionals’ performance followed by direct feedback; (ii) newsletters to inform healthcare providers about screening methods; (iii) posters and cards presenting key points about CBE and the importance of routine screening mammograms, and; (iv) patient education materials. An intervention to improve compliance with new CPGs by the American Society for Radiation Oncology (ASTRO) on the proper use of hypofractionation [ 49 ] consisted in implementing five consensus-driven and evidence-based clinical directives to guide adjuvant radiation therapy for breast cancer. Prospective contouring rounds were instituted, wherein the treating physicians presented their directive selection and patient contours for peer-review and consensus opinion. Another intervention combined audit and feedback and education to providers to increase compliance with breast cancer treatment guidelines [ 62 ]. Repeated feedback on the performance of the chemotherapy administration, timing and dosing were given to the participants. The feedback consisted of a demonstration of variation in performance between the different hospitals and the region as a whole. The educational component consisted in four consecutive sessions of discussion about relevant literature that became available in that period regarding chemotherapy dose intensity, sequencing of radiotherapy and the importance of adequate axillary lymph node clearance.
An intervention to promote compliance with new National Comprehensive Cancer Network guidelines from routine testing to omission of ordering complete blood cell count and liver function tests in patients with early breast cancer [ 53 ] involved (i) provision of educational materials; (ii) audit and feedback; (iii) certification; (iv) patient education; (v) financial incentives and (vi) implementation of alerts in the electronic medical records. Another intervention to promote breast cancer screening CPGs [ 36 ] included (i) printed educational materials with the recommendations for breast cancer mammography, (ii) printed educational materials with CPGs recommendations for clinical breast exams and breast self-exams, and (iii) video (12 min) directed at clinicians, exploring strategies for patient discussion around breast cancer screening issues.
Risk of bias
The risk of bias was judged as low in five studies [ 45 , 53 , 54 , 59 , 70 ], moderate in ten [ 36 , 37 , 42 , 44 , 47 , 56 , 57 , 58 , 62 , 63 ], and high in five [ 46 , 49 , 50 , 55 , 65 ]. In four studies [ 51 , 52 , 60 , 69 ] the risk of bias was unclear since there was not enough information available to determine potential biases. We did not assess risk of bias for case studies, due to the lack of appropriate tools available. A detailed description of the risk of bias of the included studies, excluding case studies, is available in Additional file 3 .
Impact of the interventions
Six RCTs [ 37 , 45 , 50 , 51 , 54 , 60 ] and four controlled before-after studies [ 50 , 57 , 58 , 63 ] examined the effectiveness of four provider educational interventions, one intervention based on the use of provider reminders, and five multifaceted interventions. In nine of these interventions (90%), the ultimate goal was to improve compliance with breast cancer screening guidelines. Compliance was uniformly measured in terms of mammography rates (e.g., proportion of eligible women undergoing a mammography screening for breast cancer). Except one multifaceted intervention [ 54 ], the interventions consistently showed relevant beneficial effects (Fig. 2 ).
Compliance rate with guideline recommendations before and after the implementation of the identified interventions
Impact of educational interventions
Four studies evaluated the effectiveness of educational interventions targeted to healthcare providers [ 50 , 57 , 58 , 63 ]. A randomized controlled trial showed that the intervention improved recommendation of mammography (odds ratio (OR) 1.85, 95% CI 1.25–2.74) and clinical breast examination (OR 2.13, 95% CI 1.31–3.46) in female patients aged 40 and over [ 50 ]. One controlled before-after study showed significant ( p < 0.05) improvements in providers’ knowledge, attitudes and self-efficacy towards the new CPG screening recommendations [ 57 ], whereas another controlled before-after study reported a significant improvement in the number of reported mammography referrals of asymptomatic women aged 50 to 75 years in the intervention group but not in the control group [ 58 ]. A controlled before-after study observed an improved compliance to diagnostic and treatment CPG recommendations in the intervention group (from 12% before the intervention to 36% post-intervention; P < 0.001), whereas no significant improvements were observed in the control group [ 63 ].
Impact of provider reminders
A randomized controlled trial [ 45 ] showed that a microcomputer-generated reminder system for ordering mammograms improved compliance with mammography guidelines: 27% (170/639) in the intervention vs 21% (128/623) in the control group (OR = 1.40 (95%CI = 1.01 to 1.82); p = 0.011) after 6 months follow-up.
Impact of multifaceted interventions
Five studies examined the impact of multifaceted interventions. A randomized controlled trial observed that, in comparison with usual care, a multifaceted intervention (including audit and feedback; provider education; information technology support) increased the proportion of women offered a mammogram (38% vs 53%), and the proportion of women with a recorded mammogram (35% vs 52%) [ 37 ]. Another trial observed that a multifaceted intervention (comprising provider education and patient education through pamphlets), did not improve compliance with screening mammography guidelines in the overall sample, but produced significant improvements in specific vulnerable subgroups (elderly, lower educational attainment, black ethnicity and with no private insurance) [ 60 ]. A randomized controlled trial observed that a multifaceted intervention (audit and feedback plus financial incentives) doubled screening rates both in the intervention and control groups, with no statistically significant differences observed between groups [ 54 ]. A trial examining a multifaceted intervention (provider education, cue enhancement plus feedback, and token rewards) observed that mammography compliance rates significantly improved ( p < 0.05) in the intervention (62.8%) in comparison with the control (49.0%) group [ 51 ]. A controlled before-after study observed that a multifaceted intervention (including audit and feedback, patient and professional education) improved the demonstration of breast cancer screening, with significantly more women older than 50 receiving mammograms in the intervention than in the comparison group [ 46 ].
Certainty of evidence
The results from the assessment of the certainty of evidence concerning the impact of the interventions on compliance with breast cancer CPGs is available in Additional file 5 . Based on GRADE criteria, we rated the certainty of evidence as “low” for the four educational interventions targeting healthcare providers. This was due to very serious risk of bias, for which we downgraded the level of evidence two levels. For the only intervention identified consisting in a reminder system for healthcare providers, we rated the certainty of evidence as “moderate” (downgrading one level due to serious indirectness). For the five multifaceted interventions, we rated the evidence as “low”, due to serious risk of bias, and serious inconsistency.
Main findings
In this systematic review, we identified 35 studies describing and evaluating the impact of interventions to support clinician compliance with breast cancer CPGs. We described a range of different types of interventions to support adherence of healthcare professionals to breast cancer CPGs. We observed that there is low quality evidence that educational interventions targeted at healthcare professionals may improve compliance with recommendations concerning breast cancer screening, diagnosis and treatment. There is moderate quality of evidence that reminder systems for healthcare professionals improve compliance with recommendations concerning breast cancer screening. There is low quality of evidence that multifaceted interventions may improve compliance with recommendations concerning breast cancer screening. The effectiveness of the remaining types of interventions identified is uncertain, given the study designs available (e.g., cross-sectional, uncontrolled before-after or case studies). There is very limited data on the costs of implementing these interventions.
Strengths and limitations
The main strength of this systematic review is that it addressed a highly relevant question, and provided much needed evidence to help improve providers’ compliance with breast cancer guidelines globally. An additional strength is that, contrary to previous systematic reviews, ours was not limited to experimental studies. By including observational, and qualitative and mixed-methods studies, we were able to provide a richer characterization of the available interventions.
This review has several limitations. First, we restricted the bibliographic searches to peer-reviewed publications in English language only. This may have resulted in failing to identify additional relevant data that could have further informed our assessments of the available evidence. However, we think that the impact of this limitation is likely to be small, as suggested by a recent meta-epidemiologic study [ 74 ]. Second, the heterogeneity of the reporting of outcome data made meta-analysis not feasible. Third, the heterogeneity in outcomes and the large number of strategies used across studies precluded us to determine the unique influence of each strategy on a given outcome.
Our results in the context of previous research
An important finding of our review is that most of the included studies showed that the interventions were effective in improving compliance to CPGs. This is in line with findings from previous, non-condition-specific reviews, which concluded that guideline dissemination and implementation strategies are likely to be efficient [ 75 , 76 ].
A large proportion of the studies included in our review examined the impact of Computerized Decision Support Systems (CDSS). Previous systematic reviews observed that CDSS significantly improve clinical practice [ 77 , 78 ]. In our review, the evidence about CDSS was only available from observational, uncontrolled studies, and was restricted to two tools in France and Italy in the hospital setting. New studies evaluating other CDSS, and in other settings and countries, are therefore needed.
There is substantial evidence from non-condition specific research that audit and feedback interventions can effectively improve quality of care [ 79 ]. A recent systematic review [ 80 ] examining the effectiveness of cancer (all types) guideline implementation strategies showed that providing feedback on CPG compliance was associated with positive significant changes in patient outcomes. More research is needed about the impact of audit and feedback interventions on the compliance with breast cancer CPGs.
Educational interventions targeted to providers (both in isolation and in combination with other interventions) have shown to improve outcomes in patients with cancer [ 80 ]. Despite the low certainty obtained, the studies in our review consistently showed that educational and multifaceted interventions improve compliance with breast cancer CPGs, supporting also results from previous non-condition specific reviews [ 16 , 81 ], as well as current recommendations from the Institute of Medicine [ 82 ].
In line with our finding concerning electronic reminder interventions, a Cochrane systematic review concluded that computer‐generated reminders to healthcare professionals probably improves compliance with preventive guidelines [ 83 ].
Implications for practice and research
In terms of implications for practice, given that compliance with breast cancer guidelines is associated with better survival outcomes [ 25 ], and that there are still a substantial proportion of breast cancer patients not receiving clinical guidelines recommended care [ 21 ], it is important that the most effective interventions available are implemented to improve breast cancer guideline uptake by healthcare providers.
In terms of implications for research, as in a previous non-condition-specific review [ 76 ], we observed that there is very limited data on the costs of implementing the interventions to support compliance with breast cancer CPGs, as well as a scarcity of studies evaluating the effectiveness of interventions targeting the organization of care (e.g., benchmarking tools). Research in these two areas is urgently needed to allow evidence-based decisions concerning which interventions should be rolled out and implemented widely as part of existing quality improvement programs. Also worth noting is that, up to now, the great majority of the research on this (breast cancer) area has focused on measuring the impact of the interventions on process measures (mostly compliance rates). No study measured the impact on patient outcomes, and only a small minority examined the impact on determinants of compliance behavior (e.g., providers’ knowledge, attitudes, or self-efficacy). Future research would benefit from including a broader range of outcomes (including proximal and distal), as this would help to better measure and understand the extent to which the interventions produce the intended benefits.
Future research is also needed to identify the most effective types of interventions in improving CPGs uptake, as well as the “active ingredients” of multifaceted interventions [ 84 ]. The characteristics of the CPGs intended users, and the context in which the clinical practice occurs are likely to be as important as guideline attributes for promoting adoption of CPG recommendations. Therefore, future research should focus on gaining a deeper understanding about how, when, for whom, and under which circumstances the interventions identified can effectively support guideline adherence. Using a realist evaluation methodology [ 85 ] may prove a valuable strategy in this endeavor. However, as observed in our review, the detailed characteristics of the interventions are very frequently scarcely reported. To allow progress in this area, it is of utmost importance that intervention developers and researchers offer in their published reports a comprehensive characterization of their interventions. The Template for Intervention Description and Replication (TIDieR) guidelines [ 86 ] were specifically designed for this purpose.
Promoting the uptake and use of CPGs at the point of care, represents a final translation step, from scientific findings into practice. In this review we identified a wide range of interventions to support adherence of healthcare professionals to breast cancer CPGs. Most of them are based on computerized decision support systems, provision of education, and audit and feedback, which are delivered either in isolation or in combination with other co-interventions. The certainty of evidence is low for educational interventions. The evidence is moderate for automatic reminder systems, and low for multifaceted interventions. For the rest of the interventions identified, the evidence is uncertain. Future research is very much needed to strengthen the available evidence base, concerning not only their impact on compliance, but also on patient important outcomes, and on their cost-effectiveness.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
American Society for Radiation Oncology
Computerized Decision Support Systems
Clinical Practice Guidelines
Effective Practice and Organization Care
Hazard ratio
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Randomized controlled trial
Strength after Breast Cancer
Template for Intervention Description and Replication
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Bozzi LM, Stuart B, Onukwugha E, Tom SE. Utilization of screening mammograms in the medicare population before and after the affordable care act implementation. J Aging Health. 2020;32(1):25–32.
Sestak I, Cuzick J. Update on breast cancer risk prediction and prevention. Curr Opin Obstet Gynecol. 2015;27(1):92–7.
van Luijt PA, Fracheboud J, Heijnsdijk EA, den Heeten GJ, de Koning HJ. Nation-wide data on screening performance during the transition to digital mammography: observations in 6 million screens. Eur J Cancer (Oxford, England : 1990). 2013;49(16):3517–25.
Article Google Scholar
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. NEngl J Med. 2005;353(17):1784–92.
Article CAS Google Scholar
Gangnon RE, Stout NK, Alagoz O, Hampton JM, Sprague BL, Trentham-Dietz A. Contribution of breast cancer to overall mortality for US women. Med Decis Mak. 2018;38(1_suppl):24s–31s.
Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363(13):1203–10.
Article CAS PubMed Google Scholar
Grol R, Grimshaw J. Evidence-based implementation of evidence-based medicine. Jt Comm J Qual Improve. 1999;25(10):503–13.
CAS Google Scholar
Green LA, Seifert CM. Translation of research into practice: why we can’t “just do it.” J Am Board Fam Pract. 2005;18(6):541–5.
Westfall JM, Mold J, Fagnan L. Practice-Based Research—“Blue Highways” on the NIH Roadmap. JAMA. 2007;297(4):403–6.
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res ed). 2008;336(7650):924–6.
Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317(7156):465–8.
Article CAS PubMed PubMed Central Google Scholar
Flodgren G, O’Brien MA, Parmelli E, Grimshaw JM. Local opinion leaders: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2019;6(6):Cd000125.
PubMed Google Scholar
Grimshaw J, Eccles M, Thomas R, MacLennan G, Ramsay C, Fraser C, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Internal Med. 2006;21 Suppl 2(Suppl 2):S14-20.
Google Scholar
Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 Suppl 2):Ii2-45.
CAS PubMed Google Scholar
Henry NL, Hayes DF, Ramsey SD, Hortobagyi GN, Barlow WE, Gralow JR. Promoting quality and evidence-based care in early-stage breast cancer follow-up. J Natl Cancer Institute. 2014;106(4):dju034.
Keikes L, van Oijen MGH, Lemmens V, Koopman M, Punt CJA. Evaluation of guideline adherence in colorectal cancer treatment in The Netherlands: a survey among medical oncologists by the Dutch Colorectal Cancer Group. Clin Colorectal Cancer. 2018;17(1):58–64.
Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38(5):536–50.
Carpentier MY, Vernon SW, Bartholomew LK, Murphy CC, Bluethmann SM. Receipt of recommended surveillance among colorectal cancer survivors: a systematic review. J Cancer Survivorship. 2013;7(3):464–83.
Niño de Guzmán E, Song Y, Alonso-Coello P, Canelo-Aybar C, Neamtiu L, Parmelli E, et al. Healthcare providers’ adherence to breast cancer guidelines in Europe: a systematic literature review. Breast Cancer Res Treat. 2020;181(3):499–518.
Article PubMed PubMed Central Google Scholar
Ament SM, de Groot JJ, Maessen JM, Dirksen CD, van der Weijden T, Kleijnen J. Sustainability of professionals’ adherence to clinical practice guidelines in medical care: a systematic review. BMJ Open. 2015;5(12): e008073.
Ferron G, Martinez A, Gladieff L, Mery E, David I, Delannes M, et al. Adherence to guidelines in gynecologic cancer surgery. Int J Gynecol Cancer. 2014;24(9):1675–8.
Bahtsevani C, Uden G, Willman A. Outcomes of evidence-based clinical practice guidelines: a systematic review. Int J Technol Assess Health Care. 2004;20(4):427–33.
Ricci-Cabello I, Vásquez-Mejía A, Canelo-Aybar C, Niño de Guzman E, Pérez-Bracchiglione J, Rabassa M, et al. Adherence to breast cancer guidelines is associated with better survival outcomes: a systematic review and meta-analysis of observational studies in EU countries. BMC Health Serv Res. 2020;20(1):920.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
The National Institutes of Health (NIH). Quality assessment tool for before-after (Pre-Post) study with no control group. 2021. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools . Last Accessed 4 Feb 2022 .
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12): e011458.
Pace R, Pluye P, Bartlett G, Macaulay AC, Salsberg J, Jagosh J, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49(1):47–53.
Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy; 2015. epoc.cochrane.org/epoc-taxonomy (Accessed 11 May 2022).
EPOC. Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors (Accessed 9 Feb 2023). 2017.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2.
Armson H, Roder S, Elmslie T, Khan S, Straus SE. How do clinicians use implementation tools to apply breast cancer screening guidelines to practice? Implement Sci. 2018;13(1):79.
Aspy CB, Enright M, Halstead L, Mold JW. Improving mammography screening using best practices and practice enhancement assistants: an Oklahoma Physicians Resource/Research Network (OKPRN) study. J Am Board Fam Med. 2008;21(4):326–33.
Bouaud J, Blaszka-Jaulerry B, Zelek L, Spano JP, Lefranc JP, Cojean-Zelek I, et al. Health information technology: use it well, or don’t! Findings from the use of a decision support system for breast cancer management. AMIA Annu SympProc AMIA Symp. 2014;2014:315–24.
Bouaud J, Pelayo S, Lamy JB, Prebet C, Ngo C, Teixeira L, et al. Implementation of an ontological reasoning to support the guideline-based management of primary breast cancer patients in the DESIREE project. Artificial Intell Med. 2020;108: 101922.
Bouaud J, Seroussi B. Impact of site-specific customizations on physician compliance with guidelines. Stud Health Technol Inform. 2002;90:543–7.
Bouaud J, Seroussi B. Revisiting the EBM decision model to formalize non-compliance with computerized CPGs: results in the management of breast cancer with OncoDoc2. AMIA Annu Symp Proc AMIA Symp. 2011;2011:125–34.
Bouaud J, Seroussi B, Antoine EC, Zelek L, Spielmann M. A before-after study using OncoDoc, a guideline-based decision support-system on breast cancer management: impact upon physician prescribing behaviour. Stud Health Technol Inform. 2001;84(Pt 1):420–4.
Bouaud J, Spano JP, Lefranc JP, Cojean-Zelek I, Blaszka-Jaulerry B, Zelek L, et al. Physicians’ attitudes towards the advice of a guideline-based decision support system: a case study with OncoDoc2 in the management of breast cancer patients. Stud Health Technol Inform. 2015;216:264–9.
Calo WA, Doerksen SE, Spanos K, Pergolotti M, Schmitz KH. Implementing Strength after Breast Cancer (SABC) in outpatient rehabilitation clinics: mapping clinician survey data onto key implementation outcomes. Implement Sci Commun. 2020;1:69.
Chambers CV, Balaban DJ, Carlson BL, Ungemack JA, Grasberger DM. Microcomputer-generated reminders. Improving the compliance of primary care physicians with mammography screening guidelines. J Fam Pract. 1989;29(3):273–80.
Coleman EA, Lord J, Heard J, Coon S, Cantrell M, Mohrmann C, et al. The Delta project: increasing breast cancer screening among rural minority and older women by targeting rural healthcare providers. Oncol Nurs Forum. 2003;30(4):669–77.
Craft PS, Zhang Y, Brogan J, Tait N, Buckingham JM. Implementing clinical practice guidelines: a community-based audit of breast cancer treatment. Med J Aust. 2000;172(5):213–6.
Eccher C, Seyfang A, Ferro A. Implementation and evaluation of an Asbru-based decision support system for adjuvant treatment in breast cancer. Comput Methods Programs Biomed. 2014;117(2):308–21.
Gilbo P, Potters L, Lee L. Implementation and utilization of hypofractionation for breast cancer. Adv Radiation Oncol. 2018;3(3):265–70.
Gorin SS, Ashford AR, Lantigua R, Hossain A, Desai M, Troxel A, et al. Effectiveness of academic detailing on breast cancer screening among primary care physicians in an underserved community. J Am Board Fam Med. 2006;19(2):110–21.
Grady KE, Lemkau JP, Lee NR, Caddell C. Enhancing mammography referral in primary care. Prev Med. 1997;26(6):791–800.
Groot P, Hommersom A, Lucas PJ, Merk RJ, ten Teije A, van Harmelen F, et al. Using model checking for critiquing based on clinical guidelines. Artificial Intell Med. 2009;46(1):19–36.
Hill LA, Vang CA, Kennedy CR, Linebarger JH, Dietrich LL, Parsons BM, et al. A strategy for changing adherence to national guidelines for decreasing laboratory testing for early breast cancer patients. Wisconsin Med J. 2018;117(2):68–72.
Hillman AL, Ripley K, Goldfarb N, Nuamah I, Weiner J, Lusk E. Physician financial incentives and feedback: failure to increase cancer screening in Medicaid managed care. Am J Public Health. 1998;88(11):1699–701.
Kreizenbeck KL, Wong T, Jagels B, Smith JC, Irwin BB, Jensen B, et al. A pilot study to increase adherence to ASCO Choosing Wisely recommendations for breast cancer surveillance at community clinics. J Clin Oncol. 2020;38(29_suppl):18.
Kubal T, Peabody JW, Friedman E, Levine R, Pursell S, Letson DG. Using vignettes to measure and encourage adherence to clinical pathways in a quality-based oncology network: an early report on the moffitt oncology network initiative. Managed Care (Langhorne, Pa). 2015;24(10):56–64.
Lane DS, Messina CR, Grimson R. An educational approach to improving physician breast cancer screening practices and counseling skills. Patient Educ Counsel. 2001;43(3):287–99.
Lane DS, Polednak AP, Burg MA. Effect of continuing medical education and cost reduction on physician compliance with mammography screening guidelines. J Family Pract. 1991;33(4):359–68.
McWhirter E, Yogendran G, Wright F, Pharm GD, Clemons M. Baseline radiological staging in primary breast cancer: impact of educational interventions on adherence to published guidelines. J Eval Clin Pract. 2007;13(4):647–50.
Michielutte R, Sharp PC, Foley KL, Cunningham LE, Spangler JG, Paskett ED, et al. Intervention to increase screening mammography among women 65 and older. Health Educ Res. 2005;20(2):149–62.
Munce S, Kastner M, Cramm H, Lal S, Deschene SM, Auais M, et al. Applying the knowledge to action framework to plan a strategy for implementing breast cancer screening guidelines: an interprofessional perspective. J Cancer Educ. 2013;28(3):481–7.
Ottevanger PB, De Mulder PH, Grol RP, van Lier H, Beex LV. Adherence to the guidelines of the CCCE in the treatment of node-positive breast cancer patients. Eur J Cancer (Oxford, England : 1990). 2004;40(2):198–204.
Ray-Coquard I, Philip T, de Laroche G, Froger X, Suchaud JP, Voloch A, et al. A controlled “before-after” study: impact of a clinical guidelines programme and regional cancer network organization on medical practice. Br J Cancer. 2002;86(3):313–21.
Seroussi B, Bouaud J, Antoine EC. ONCODOC: a successful experiment of computer-supported guideline development and implementation in the treatment of breast cancer. Artificial Intell Med. 2001;22(1):43–64.
Seroussi B, Bouaud J, Gligorov J, Uzan S. Supporting multidisciplinary staff meetings for guideline-based breast cancer management: a study with OncoDoc2. AMIA Annu Symp Proc AMIA Symp. 2007:656-60.
Seroussi B, Laouenan C, Gligorov J, Uzan S, Mentre F, Bouaud J. Which breast cancer decisions remain non-compliant with guidelines despite the use of computerised decision support? Br J Cancer. 2013;109(5):1147–56.
Seroussi B, Soulet A, Messai N, Laouenan C, Mentre F, Bouaud J. Patient clinical profiles associated with physician non-compliance despite the use of a guideline-based decision support system: a case study with OncoDoc2 using data mining techniques. AMIA Annu Symp Proc AMIA Symp. 2012;2012:828–37.
Seroussi B, Soulet A, Spano JP, Lefranc JP, Cojean-Zelek I, Blaszka-Jaulerry B, et al. Which patients may benefit from the use of a decision support system to improve compliance of physician decisions with clinical practice guidelines: a case study with breast cancer involving data mining. Stud Health Technol Inform. 2013;192:534–8.
Veerbeek L, van der Geest L, Wouters M, Guicherit O, Does-den Heijer A, Nortier J, et al. Enhancing the quality of care for patients with breast cancer: seven years of experience with a Dutch auditing system. Eur J Surg Oncol. 2011;37(8):714–8.
Wheeler SB, Kohler RE, Goyal RK, Lich KH, Lin CC, Moore A, et al. Is medical home enrollment associated with receipt of guideline-concordant follow-up care among low-income breast cancer survivors? Med Care. 2013;51(6):494–502.
CancerEst. Principes de prise en charge des cancers du sein en situation non me´tastatique: le re´fe´rentiel CancerEst. 2008.
Nofech-Mozes S, Vella ET, Dhesy-Thind S, Hanna WM. Cancer care Ontario guideline recommendations for hormone receptor testing in breast cancer. Clin Oncol (Royal College of Radiologists (Great Britain)). 2012;24(10):684–96.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Continuing Educ Health Prof. 2006;26(1):13–24.
Nussbaumer-Streit B, Klerings I, Dobrescu AI, Persad E, Stevens A, Garritty C, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42–54.
Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess (Winchester, England). 2004;8(6):iii–iv, 1–72.
Flodgren G, Hall AM, Goulding L, Eccles MP, Grimshaw JM, Leng GC, et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev. 2016;8:Cd010669.
Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765.
Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293(10):1223–38.
Ivers NM, Grimshaw JM, Jamtvedt G, Flottorp S, O’Brien MA, French SD, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Internal Med. 2014;29(11):1534–41.
Tomasone JR, Kauffeldt KD, Chaudhary R, Brouwers MC. Effectiveness of guideline dissemination and implementation strategies on health care professionals’ behaviour and patient outcomes in the cancer care context: a systematic review. Implement Sci. 2020;15(1):41.
Giguère A, Zomahoun HTV, Carmichael PH, Uwizeye CB, Légaré F, Grimshaw JM, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2020;8(8):Cd004398.
Greenfield S, Steinberg E, Auerbach A, Avorn J, Galvin R, Gibbons R. Clinical practice guidelines we can trust. Washington, DC: Institute of Medicine; 2011. p. 2017.
Arditi C, Rège-Walther M, Durieux P, Burnand B. Computer-generated reminders delivered on paper to healthcare professionals: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;7(7):Cd001175.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337: a1655.
Pawson R, Tilley N, Tilley N. Realistic evaluation: sage; 1997.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348: g1687.
Download references
Acknowledgements
Not applicable.
The systematic review was carried out by the Iberoamerican Cochrane Center under Framework contract 443094 for procurement of services between European Commission Joint Research Centre and Asociación Colaboración Cochrane Iberoamericana.
Author information
Authors and affiliations.
Balearic Islands Health Research Institute (IdISBa), Palma, Spain
Ignacio Ricci-Cabello
Primary Care Research Unit of Mallorca, Balearic Islands Health Service, Palma, Spain
CIBER de Epidemiología y Salud Pública (CIBERESP), Madrid, Spain
Ignacio Ricci-Cabello, Pablo Alonso-Coello & Ivan Solà
Facultad de Medicina Humana, Universidad Nacional Mayor de San Marcos, Lima, Peru
Darla Carvallo-Castañeda & Adrián Vásquez-Mejía
Iberoamerican Cochrane Centre-Department of Clinical Epidemiology and Public Health, Biomedical Research Institute Sant Pau (IIB Sant Pau), Barcelona, Spain
Pablo Alonso-Coello, David Rigau, Ivan Solà & Ena Niño-de-Guzmán
European Commission, Joint Research Centre (JRC), Ispra, Italy
Zuleika Saz-Parkinson, Elena Parmelli, Gian Paolo Morgano & Luciana Neamtiu
Cancer Prevention and Control Programme, Catalan Institute of Oncology, IDIBELL, Hospitalet de Llobregat, Barcelona, Spain
Ena Niño-de-Guzmán
You can also search for this author in PubMed Google Scholar
Contributions
EP, ZSP, and LN contributed to conception and design of the study. IS designed the literature search. IRC ENG, LN, ZSP, EP, DC, APVM, and GPM performed the literature screening, data extraction, and quality appraisal of included studies. PAC and IRC evaluated the certainty of evidence. All authors contributed to data interpretation. IRC wrote the first draft of the manuscript. All authors critically reviewed and revised the manuscript and approved the final manuscript.
Corresponding authors
Correspondence to Pablo Alonso-Coello or Elena Parmelli .
Ethics declarations
Ethics approval and consent to participate.
Not applicable (literature review).
Consent for publication
Not applicable (the manuscript does not contain data from any individual person).
Competing interests
EP, LN, and ZSP are or were employees of the Joint Research Centre, European Commission. ENDG, DR, IS, and PAC are employees of the Iberoamerican Cochrane Center.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA 2020 Checklist.
Additional file 2.
Search strategy.
Additional file 3.
Summary of Risk of Bias Assessment.
Additional file 4.
Characteristics and results of the 35 studies included in the review.
Additional file 5.
Evidence Profiles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ricci-Cabello, I., Carvallo-Castañeda, D., Vásquez-Mejía, A. et al. Characteristics and impact of interventions to support healthcare providers’ compliance with guideline recommendations for breast cancer: a systematic literature review. Implementation Sci 18 , 17 (2023). https://doi.org/10.1186/s13012-023-01267-2
Download citation
Received : 17 May 2022
Accepted : 14 March 2023
Published : 22 May 2023
DOI : https://doi.org/10.1186/s13012-023-01267-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Clinical guidelines
- Interventions
- Systematic literature review
Implementation Science
ISSN: 1748-5908
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 14 May 2024
Socio-cultural beliefs and perceptions influencing diagnosis and treatment of breast cancer among women in Ghana: a systematic review
- Agani Afaya 1 , 2 ,
- Emmanuel Anongeba Anaba 3 ,
- Victoria Bam 4 ,
- Richard Adongo Afaya 5 ,
- Ahmed-Rufai Yahaya 6 ,
- Abdul-Aziz Seidu 7 &
- Bright Opoku Ahinkorah 8
BMC Women's Health volume 24 , Article number: 288 ( 2024 ) Cite this article
171 Accesses
Metrics details
Breast cancer is currently the most commonly diagnosed cancer in Ghana and the leading cause of cancer mortality among women. Few published empirical evidence exist on cultural beliefs and perceptions about breast cancer diagnosis and treatment in Ghana. This systematic review sought to map evidence on the socio-cultural beliefs and perceptions influencing the diagnosis and treatment of breast cancer among Ghanaian women.
This review was conducted following the methodological guideline of Joanna Briggs Institute and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses. The literature search was conducted in PubMed, CINAHL via EBSCO host , PsycINFO, Web of Science, and Embase. Studies that were conducted on cultural, religious, and spiritual beliefs were included. The included studies were screened by title, abstract, and full text by three reviewers. Data were charted and results were presented in a narrative synthesis form.
After the title, abstract, and full-text screening, 15 studies were included. Three categories were identified after the synthesis of the charted data. The categories included: cultural, religious and spiritual beliefs and misconceptions about breast cancer. The cultural beliefs included ancestral punishment and curses from the gods for wrongdoing leading to breast cancer. Spiritual beliefs about breast cancer were attributed to spiritual or supernatural forces. People had the religious belief that breast cancer is a test from God and they resorted to prayers for healing. Some women perceived that breast cancer is caused by spider bites, heredity, extreme stress, trauma, infections, diet, or lifestyle.
This study adduces evidence of the socio-cultural beliefs that impact on the diagnosis and treatment of breast cancer among women in Ghana. Taking into consideration the diverse cultural and traditional beliefs about breast cancer diagnosis and treatment, there is a compelling need to intensify nationwide public education on breast cancer to clarify the myths and misconceptions about the disease. We recommend the need to incorporate socio-cultural factors influencing breast cancer diagnosis and treatment into breast cancer awareness programs, education, and interventions in Ghana.
Peer Review reports
Introduction
Breast cancer is a global public health concern due to its increasing incidence coupled with the high mortality rate among women in low- and high-income countries [ 1 ]. In 2020, it was estimated that 2.3 million breast cancer cases were newly diagnosed with approximately 685,000 deaths globally [ 1 ]. In Ghana, breast cancer is the most commonly diagnosed cancer and the leading cause of cancer mortality among women [ 2 ]. In 2020, breast cancer accounted for approximately 31.8% of all cancer cases in Ghana [ 3 ].
Evidence shows that cultural factors such as conceptualizations of health, illness, beliefs, and values influence breast cancer screening among women in certain populations [ 4 , 5 , 6 ]. Breast cancer screening is reported to be relatively low among women living in Ghana. A nationwide study revealed that only 4.5% of Ghanaian women aged 50 years and older had undergone mammography screening [ 7 ]. The low levels of breast cancer screening lead to undetected breast cancer symptoms, contributing to the late-stage diagnosis of breast cancer and subsequent poorer outcomes and mortality [ 8 ]. There have been low levels of awareness and knowledge about breast cancer among women in Ghana [ 9 ]. Also, there is a lack of understanding of the perceptions and beliefs toward breast cancer diagnosis and treatment in Ghana.
Culture is considered a multidimensional set of shared beliefs and socially transmitted ideologies about the world, which are passed on from generation to generation [ 10 , 11 ]. Cultural beliefs within certain communities across the globe are considered a determinant of health risk perceptions and behaviors in promoting or seeking health care in diverse populations [ 12 ]. In traditional Ghanaian communities, good health is recognized as a suitable relationship between the living and the dead and being in harmony with the individuals’ environment. Thus, disease is conceptualized as a malfunctioning of the body system which is probably due to a lack of harmony with supernatural/ancestral forces [ 13 ]. This belief influences how diseases are treated and the steps taken to manage the disease and ultimately how the disease is experienced [ 13 , 14 ]. Cultural beliefs connected to breast cancer are among the key determinants in women’s decision-making regarding breast cancer screening practices in traditional societies [ 14 , 15 ]. In most Ghanaian communities, breast cancer is believed to be associated with supernatural powers, hence, women seek alternative treatments (healing/prayer camps) first and only report to health facilities in advanced stages of breast cancer [ 16 ].
It is therefore important to consider how socio-cultural factors impact breast cancer diagnosis and treatment because these factors influence cancer care in resource-limited settings. To the best of our knowledge, no review has been conducted in Ghana specifically to address the cultural, religious, and spiritual beliefs influencing timely diagnosis and treatment of breast cancer among women. To fill this gap, this systematic review sought to map evidence on the cultural beliefs and perceptions that influence the timely diagnosis and treatment of breast cancer among women.
This systematic review was conducted following the updated methodological guideline of Joanna Briggs Institute (JBI) [ 17 , 18 ] and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement. The updated JBI methodological guidance regarding conducting a mixed methods systematic review recommends that reviewers use a convergent approach to synthesize and integrate both qualitative and quantitative studies [ 18 ]. Therefore, using a mixed methods systematic review involving both quantitative and qualitative studies was deemed the most appropriate study design because this is the first evidence synthesis on the cultural, religious, and spiritual beliefs that influence breast cancer diagnosis and treatment in Ghana.
Inclusion and exclusion criteria
Studies conducted among women and explored the cultural beliefs and perceptions about breast cancer were included.
Studies that were only limited to Ghanaian communities were included.
Empirical studies published in peer-review journals.
Observational studies, using qualitative and/or quantitative methods were also included.
The exclusion criteria involved review studies, conference papers, editorials and abstracts.
Studies published before 2012 were also excluded.
Search strategy
This review adopted the triple-step search strategy proposed by the JBI for all types of reviews [ 19 ]. The first step involved an initial limited search in PubMed for already existing published research articles on sociocultural beliefs and perceptions about breast cancer in Ghana. The initial limited search ensured the identification of relevant keywords used in developing the preliminary search terms. Step two involved a formal search after finalizing and combining the following keywords (‘breast cancer’, ‘cultural beliefs’, ‘religious beliefs’, ‘traditional beliefs’, ‘perception’, and ‘Ghana’) using Boolean operators. A comprehensive search was conducted in PubMed, CINAHL via EBSCO host , PsycINFO, Web of Science, and Embase from 2012–2022. The final step involved manual tracing of the reference list of studies for additional studies. This was done up to the point of saturation where no new information emanated from the subsequent manual search of articles.
Study selection
Following the searches, the identified records were exported into EndNote 2020 reference manager for duplicate removal. After the duplicate removal, the reviewers ensured consistency in screening through the following process: (1) joint screening by two reviewers was conducted until they felt confident to start independent screening, (2) independent blinded screening of titles/abstracts followed by a meeting and discussion of discrepancies and (3) repetition of step 2 until an acceptable agreement was met. Following the screening of the titles/abstracts, full-text review was conducted following a two-step process. The first step involved two reviewers who screened all the articles identified after the title/abstract screening. Thereafter, two independent reviewers assessed the full-text articles for inclusion or exclusion. In the course of the full-text screening, any disagreements that emerged were discussed for consensus. Throughout the screening of the abstracts, full-texts, and data extraction, the reviewers regularly met to discuss and solve emerging issues.
Data extraction
A data extraction form was developed in line with the aim of this review. Two authors independently extracted the relevant information from the included articles. The following information was extracted from the articles: first author’s name, year of publication, study location, study type, aim, study population, and key findings. Disagreements during the data extraction process were resolved by a discussion and where a resolution was not reachable, the last author resolved it through further adjudication. Study selection and data extraction were conducted manually.
Data analysis
A convergent integrated approach [ 20 ] was employed to transform the data into narrative form because the extracted information was from quantitative and qualitative studies. The analysis followed JBI recommendation where we qualitized quantitative data for data transformation because this is less prone to error when codified than when qualitative data is given numerical values. Qualitizing entails taking data from quantitative studies, translating or converting it into textual descriptions so that it can be integrated with qualitative data, and providing a narrative interpretation of the quantitative results [ 18 ]. Following the convergent synthesis of the transformed data, the reviewers undertook repeated, detailed examination of the assembled data to identify categories on the basis of similarity in meaning [ 18 ]. Out of these, three categories were derived from the analysis.
Assessment of methodological quality
Using the Mixed Methods Appraisal Tool (MMAT) version 2018, two researchers (AA and RAA) evaluated each included study’s quality separately [ 21 ]. After discussing disagreements between the two reviewers (AA and RAA), BOA helped to forge a consensus. Methodological quality standards for evaluating research using mixed methodologies, quantitative, and qualitative approaches are included in the MMAT. The MMAT assesses the suitability of the research objective, study design, technique, participant recruitment, data collection, data analysis, results presentation, author comments, and conclusions. Hong et al. [ 21 ] discourages the overall quality scoring of the included studies, therefore, the methodological quality of the studies was evaluated using the recommended guidelines.
Flow Chart of evidence selection
Literature search
Our search yielded a total of 176 records from the electronic databases. After duplicates were automatically removed through the EndNote ( n = 76), 100 records were reviewed independently by two authors based on the title and abstract. Records that did not meet the inclusion ( n = 75) were removed after holding discussions to identify discrepancies in the review process. Thereafter, full texts of the remaining 25 articles were assessed for eligibility. Hand-search of the included study references yielded no results. In total, we included 15 studies [ 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ]. The article selection process is shown in the PRISMA flow diagram (Fig. 1 ).
Characteristics of the included studies and quality
The majority of the studies [ 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ] were conducted in the southern part of Ghana where there are better health infrastructures compared to the northern part of Ghana. Eight of the included studies were qualitative while the rest employed quantitative study designs. The summary of the characteristics of the 15 studies is shown in Table 1 . The appraisal of the included studies was assessed using the MMAT. All the studies were included, and none were excluded due to poor methodological quality. All 15 studies met the screening criteria and provided clear research questions. The studies included clearly stated and described research design, and target population, and used appropriate measurements.
Cultural beliefs
Breast cancer is believed by some sections of Ghanaians to be a curse or a punishment from the lesser gods for sins committed by the individual [ 22 ]. Some women believed that an extra-marital immoral lifestyle provokes God’s retribution for breast cancer development [ 29 ]. Some people believed that it is an ancestral punishment for the woman’s refusal to give birth in order to continue the ancestral lineage [ 23 ] and because of this, they are given spiritual babies to suckle the breast which then causes cancer [ 23 ]. It is also believed some women have been pronounced cursed due to some wrongdoings [ 25 ]. Due to the cultural belief, some women prayed to their ancestors so that traditional medicine will heal them of the breast cancer [ 26 ].
“…when it started, my uncles came to my aid, they took me to the village to see a “Tim Lana” (referring to a traditional healer). He was very good. He told me everything about my problem. So, there was no need for visiting the hospital…” [ 36 ].
Spiritual and religious beliefs
Some studies in Greater Accra, Tamale, and Kumasi indicated that breast cancer was a spiritual attack from humans or family members that sought to kill them while some believe it emanated from evil forces [ 29 , 31 , 36 ]. Participants in some studies indicated that breast cancer is attributed to some spiritual or supernatural forces [ 32 , 33 , 36 ] and can only be cured through spiritual means [ 33 ]. Due to the spiritual beliefs, some women went to traditional healers for treatment [ 26 , 36 ]. A study in the northern part of Ghana revealed that women who suffer from breast cancer are witches and have used their breasts for ritual purposes [ 25 ] while in the southern part of Ghana some participants believed that breast cancer is caused by witches [ 22 ]. For example, a narration from a participant stated:
“I believe my condition is spiritual and I realized it is coming from my mother’s side” [ 31 ].
“The problem is that my disease is a spiritual attack, so it has to be treated spiritually; the hospital drugs cannot get this out of me…” [ 36 ].
Some studies in the southern and northern part of Ghana stated that participants had a religious belief that the disease was a test from God and resulted in prayers for healing [ 31 , 36 ] and also believed that God had the supernatural powers to miraculously melt the breast lump [ 29 , 32 ] and completely cure them [ 32 ]. Some women also believed that it was their fate to get breast cancer [ 36 ]. Due to these religious beliefs some women had to resort to prayer camps for healing which leads to delay in diagnosis and treatment of breast cancer [ 26 ].
Misconceptions about breast cancer
Some women perceived that breast cancer is caused by spider bites [ 24 ], heredity, extreme stress [ 22 , 32 ], trauma, infections [ 22 ], diet, or lifestyle [ 22 , 35 ]. Some perceived risk factors of breast cancer as stated by some women included non-breastfeeding women, obesity, or overweight [ 25 , 30 , 33 ], and contraceptive use [ 30 ]. Some women had the perception that male health practitioners would not be allowed to examine or see their breasts while some preferred male doctors to examine their breasts [ 27 ]. A study in Accra conducted among female nonmedical students revealed that suckling the breast by a male caused breast cancer [ 28 ]. It is also perceived that putting money in the brassieres could be a possible cause of breast cancer among females [ 23 , 35 ]. A study by Iddrisu et al. [ 31 ] and Agbokey [ 23 ] revealed that breast cancer is a disgraceful disease, dangerous, and a fast killer. Some people also believed that breast cancer can be cured [ 27 , 32 ] by herbal treatment or medicine [ 25 ] while some believed that it is not curable [ 27 ]. Some people also believed that breast cancer was contagious and transmissible and avoided sharing equipment with breast cancer survivors [ 31 ]. A breast cancer survivor narrated:
“…my mum believes the disease can be transmitted so she does not allow me to eat with my son. I have separate bowls, spoons, and cups from that of the family…” [ 31 ].
This study reviews the existing literature on socio-cultural beliefs influencing the timely diagnosis and treatment of breast cancer among women, and this revealed diverse cultural, spiritual, and religious beliefs across the regions of Ghana. The current findings emphasize critical issues that lead to misguidance and share ignorance about breast cancer and its treatment among a section of Ghanaian communities which is rooted in their personal beliefs. Cultural beliefs are key in the decision-making process for the treatment of ailments depending on their knowledge level about the condition. This could probably lead to making the right decision or the wrong treatment decision. The diverse cultural, spiritual, and religious beliefs about breast cancer could affect the health seeking behavior of women diagnosed with breast cancer within the Ghanaian communities.
Consistent with a systematic review findings [ 13 ] it is believed that breast cancer emanates as a result of supernatural forces, curses, and punishment from lesser gods/ancestors for wrongdoings. Though not all Africans hold this traditional belief in ancestral spirits, some believe that health and illness are in the hands of a higher power such as God or Allah [ 13 ]. Hence, in most African communities it is common practice to seek traditional medicine for the treatment of diseases which is in line with their beliefs [ 37 ]. Due to the cultural/traditional belief systems and practices, most women report to health facilities with advanced stages of breast cancer which adversely impacts the breast cancer diagnosis and treatment [ 36 ]. Most women resort to traditional or spiritual healing because this method of treatment combines body, soul, and spirit. In some African settings, traditional healers are trusted to treat diseases including cancer because women believe they look for both scientific and metaphysical causes of the disease. It is possible that breast cancer patients who combine both traditional and modern methods of treatment may experience treatment interference. This dual approach can impact treatment effectiveness and lead to adverse effects or complications. The provision of culturally sensitive care by recognizing unique cultural, religious, and social beliefs and practices is of paramount importance for early detection and treatment of breast cancer among women [ 38 , 39 , 40 ]. Globally, women’s cultural beliefs and perceptions towards breast cancer should be examined to optimize timely breast cancer diagnosis and treatment.
Religious fanaticism coupled with lack of knowledge about the disease condition could impede the utilization of medical treatment, especially when religious beliefs impact negatively on people’s health-seeking behaviors [ 36 ]. A study in Nigeria revealed that religious beliefs about breast cancer were observed to be a barrier to breast cancer screening among women [ 41 ]. This review found that some women in the southern part of Ghana believed that breast cancer was a test from God and resorted to prayers because they believed that God had supernatural powers to heal them from the disease. Though religious beliefs are considered to be a source of spiritual strength and help people to cope with the disease, the religious misconceptions, and mistaken beliefs are thought to contribute to delayed heath-seeking attitudes and lack of breast cancer screening among women [ 42 ]. In the current review, it was reported that some women stayed in prayer camps for almost one year seeking healing and later reported to health facilities with advanced breast cancer which has dire consequences on the survival rate of women. Efforts to sensitize women and religious leaders about the early presentation of breast disease to health facilities for diagnosis and treatment would be key to reduce the number of breast cancer cases detained in religious camps. It is also imperative for religious bodies to discuss health related issues including breast cancer to create much awareness about the condition.
This review identified varied perceptions of breast cancer where breast cancer has been attributed to spider bites and putting money in the brassieres among others. Some believed that breast cancer was a contagious and transmissible disease. These findings show poor knowledge level among women concerning breast cancer. Even though in this review most women had heard or were aware of breast cancer, the varied perceptions about breast cancer suggests low knowledge level of breast cancer. The low knowledge level of breast cancer among women have been associated with late presentation of breast cancer to health facilities [ 40 ]. Women presenting to health facilities with advanced stage breast cancer have been associated with low survival rate in the African region as compared to high income countries [ 43 ]. A study conducted in Ghana revealed that the breast cancer survival rate among women was below 50% which was probably due to late presentation and lack of breast cancer screening [ 44 ]. We recommend intensification of public health education campaigns on breast cancer in order to improve women’s knowledge of the disease which will subsequently enhance early presentation, diagnosis, and treatment.
Implication for policy and practice
Metaphors such as spider bites, supernatural forces, witchcraft, and many other beliefs are associated with breast cancer in Ghana which impact the understanding of the disease and whether or not to seek medical treatment. Therefore, culturally sensitive intervention programs targeted at improving breast cancer awareness among women, religious and traditional leaders are imperative. These intervention programs could entail community engagement, workshops, or educational materials tailored to address specific cultural beliefs and misconceptions.
Taking into consideration the diverse cultural beliefs about breast cancer, there is a compelling need for nationwide public education on breast cancer to clarify the myths and misconceptions about the disease. The education program should be culturally tailored to address the myths and misconceptions. It is important that considerations are given to these issues, not only focusing on how these issues affect women’s lives post-treatment but also on how these issues can be resolved to improve diagnosis and treatment of the disease. We recommend that socio-cultural factors influencing breast cancer diagnosis and treatment should be incorporated into breast cancer awareness programs, education, and intervention programs in Ghana. We believe these would help inform women and encourage them to report to health facilities early with breast cancer symptoms to initiate timely diagnosis and treatment to improve the outcomes of the disease in Ghana.
Further research is required to explore appropriate and effective multidimensional culturally sensitive intervention research that integrates cultural beliefs and breast cancer treatment especially, in different Ghanaian communities.
Strengths and limitations of the study
This study has several strengths, one major strength is the extensive and comprehensive search in various electronic databases following the methodological guideline of JBI and reported in accordance with the PRISMA guidelines. Also, the inclusion of both qualitative and quantitative studies, allowed for a more comprehensive understanding of the socio-cultural beliefs influencing breast cancer diagnosis and treatment in Ghana.
The review considered only published studies and possibly may have overlooked unpublished or gray literature that could contribute to a more comprehensive understanding of the subject matter. Most of the studies were concentrated in the southern part of Ghana and therefore the results might not represent all the regions in Ghana.
This study adduces evidence on the socio-cultural beliefs that impact diagnosis and treatment of breast cancer among women in Ghana. As policy makers, clinicians and other stakeholders strive to improve breast cancer diagnosis and treatment, there is a need to address the socio-cultural beliefs to improve breast cancer outcomes in Ghana and potentially reduce breast cancer-related mortality.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Afaya A, Seidu AA, Sang S, Yakong VN, Afaya RA, Shin J, Ahinkorah BO. Mapping evidence on knowledge of breast cancer screening and its uptake among women in Ghana: a scoping review. BMC Health Serv Res. 2022;22(1):526.
Article PubMed PubMed Central Google Scholar
International Agency for Research on Cancer. Global Cancer Observatory. 2021: https://gco.iarc.fr/ .
Donnelly TT, Al Khater A-H, Al-Bader SB, Al Kuwari MG, Al-Meer N, Malik M, Singh R, Chaudhry S, Fung T. Beliefs and attitudes about breast cancer and screening practices among arab women living in Qatar: a cross-sectional study. BMC Womens Health. 2013;13(1):1–16.
Article Google Scholar
Pasick RJ, Burke NJ. A critical review of theory in breast cancer screening promotion across cultures. Annu Rev Public Health. 2008;29:351–68.
Azaiza F, Cohen M. Between traditional and modern perceptions of breast and cervical cancer screenings: a qualitative study of arab women in Israel. Psychooncology. 2008;17(1):34–41.
Agyemang AF, Tei-Muno AN, Dzomeku VM, Nakua EK, Duodu PA, Duah HO, Bentil AB, Agbadi P. The prevalence and predictive factors of breast cancer screening among older Ghanaian women. Heliyon. 2020;6(4):e03838.
Rivera-Franco MM, Leon-Rodriguez E. Delays in breast Cancer detection and treatment in developing countries. Breast Cancer (Auckl). 2018;12:1178223417752677.
PubMed Google Scholar
Boamah Mensah AB, Mensah KB, Aborigo RA, Bangalee V, Oosthuizen F, Kugbey N, Clegg-Lamptey JN, Virnig B, Kulasingam S, Ncama BP. Breast cancer screening pathways in Ghana: applying an exploratory single case study methodology with cross-case analysis. Heliyon. 2022;8(11):e11413.
d’Andrade RG, Strauss C. Human motives and cultural models. Volume 1. Cambridge University Press; 1992.
Koltko-Rivera ME. The psychology of worldviews. Rev Gen Psychol. 2004;8(1):3–58.
Iwelunmor J, Newsome V, Airhihenbuwa CO. Framing the impact of culture on health: a systematic review of the PEN-3 cultural model and its application in public health research and interventions. Ethn Health. 2014;19(1):20–46.
White P. The concept of diseases and health care in African traditional religion in Ghana. HTS: Theological Stud. 2015;71(3):1–7.
Google Scholar
Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub-saharan Africa: implications for diagnosis and management. Womens Health (Lond). 2016;12(1):147–56.
Article CAS PubMed Google Scholar
Kim JG, Hong HC, Lee H, Ferrans CE, Kim EM. Cultural beliefs about breast cancer in Vietnamese women. BMC Womens Health. 2019;19(1):74.
Clegg-Lamptey JN, Dakubo JC, Attobra YN. Psychosocial aspects of breast cancer treatment in Accra, Ghana. East Afr Med J. 2009;86(7):348–53.
CAS PubMed Google Scholar
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26.
Stern C, Lizarondo L, Carrier J, Godfrey C, Rieger K, Salmond S, Apóstolo J, Kirkpatrick P, Loveday H. Methodological guidance for the conduct of mixed methods systematic reviews. JBI Evid Synthesis. 2020;18(10):2108–18.
Peters MD, Godfrey CM, McInerney P, Soares CB, Khalil H, Parker D. The Joanna Briggs Institute reviewers’ manual 2015: methodology for JBI scoping reviews. 2015.
Hong QN, Pluye P, Bujold M, Wassef M. Convergent and sequential synthesis designs: implications for conducting and reporting systematic reviews of qualitative and quantitative evidence. Syst Rev. 2017;6(1):61.
Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon M-P, Griffiths F, Nicolau B. Mixed methods appraisal tool (MMAT), version 2018. Registration Copyr 2018, 1148552(10).
Addae-Korankye A, Abada A, Imoro F. Assessment of breast cancer awareness in Tamale Metropolis in Ghana. World Sci Res. 2014;3(1):1–5.
Agbokey F. Health seeking behaviours for breast cancer among breast cancer patients at the Komfo Anokye Teaching Hospital. Kumasi, Ghana: University of Ghana; 2014.
Agbokey F, Kudzawu E, Dzodzomenyo M, Ae-Ngibise KA, Owusu-Agyei S, Asante KP. Knowledge and Health Seeking Behaviour of Breast Cancer Patients in Ghana. Int J Breast Cancer 2019, 2019:5239840.
Asobayire A, Barley R. Women’s cultural perceptions and attitudes towards breast cancer: Northern Ghana. Health Promot Int. 2015;30(3):647–57.
Asoogo C, Duma SE. Factors contributing to late breast cancer presentation for health care amongst women in Kumasi, Ghana. Curationis 2015, 38(1).
Azumah FD, Onzaberigu NJ. Community knowledge, perception and attitude toward breast cancer in Sekyere East District Ghana. Int J Innov Educ Res. 2017;5(6):221–35.
Boafo IM, Tetteh PM. Self-efficacy and perceived barriers as determinants of breast self-examination among female nonmedical students of the University of Ghana. Int Q Community Health Educ. 2020;40(4):289–97.
Bonsu AB, Ncama BP. Recognizing and appraising symptoms of breast cancer as a reason for delayed presentation in Ghanaian women: a qualitative study. PLoS ONE. 2019;14(1):e0208773.
Article CAS PubMed PubMed Central Google Scholar
Dadzi R, Adam A. Assessment of knowledge and practice of breast self-examination among reproductive age women in Akatsi South district of Volta region of Ghana. PLoS ONE. 2019;14(12):e0226925.
Iddrisu M, Aziato L, Ohene LA. Socioeconomic impact of breast cancer on young women in Ghana: a qualitative study. Nurs Open. 2021;8(1):29–38.
Kugbey N, Oppong Asante K, Meyer-Weitz A. Illness perception and coping among women living with breast cancer in Ghana: an exploratory qualitative study. BMJ Open. 2020;10(7):e033019.
Opoku SY, Benwell M, Yarney J. Knowledge, attitudes, beliefs, behaviour and breast cancer screening practices in Ghana, West Africa. Pan Afr Med J. 2012;11:28.
PubMed PubMed Central Google Scholar
Osei E, Osei Afriyie S, Oppong S, Ampofo E, Amu H. Perceived breast Cancer risk among female undergraduate students in Ghana: a cross-sectional study. J Oncol. 2021;2021:8811353.
Osei-Afriyie S, Addae AK, Oppong S, Amu H, Ampofo E, Osei E. Breast cancer awareness, risk factors and screening practices among future health professionals in Ghana: a cross-sectional study. PLoS ONE. 2021;16(6):e0253373.
Salisu WJ, Mirlashari J, Seylani K, Varaei S, Thorne S. Fatalism, distrust, and breast cancer treatment refusal in Ghana. Can Oncol Nurs J. 2022;32(2):198–205.
Omonzejele PF. African concepts of health, disease, and treatment: an ethical inquiry. Explore (NY). 2008;4(2):120–6.
Wright SV. An investigation into the causes of absconding among black African breast cancer patients. S Afr Med J. 1997;87(11):1540–3.
Benedict AO. The perception of illness in traditional Africa and the development of traditional medical practice. Int J Nurs. 2014;1(1):51–9.
Afaya A, Ramazanu S, Bolarinwa OA, Yakong VN, Afaya RA, Aboagye RG, Daniels-Donkor SS, Yahaya AR, Shin J, Dzomeku VM, et al. Health system barriers influencing timely breast cancer diagnosis and treatment among women in low and middle-income Asian countries: evidence from a mixed-methods systematic review. BMC Health Serv Res. 2022;22(1):1601.
Elewonibi B, BeLue R. The influence of socio-cultural factors on breast cancer screening behaviors in Lagos, Nigeria. Ethn Health. 2019;24(5):544–59.
Saeed S, Asim M, Sohail MM. Fears and barriers: problems in breast cancer diagnosis and treatment in Pakistan. BMC Womens Health. 2021;21(1):151.
Anyigba CA, Awandare GA, Paemka L. Breast cancer in sub-saharan Africa: the current state and uncertain future. Exp Biol Med (Maywood). 2021;246(12):1377–87.
Mensah S, Dogbe J, Kyei I, Addofoh N, Paintsil V, Osei T. Determinants of late presentation and histologic types of breast cancer in women presenting at a Teaching Hospital in Kumasi. Ghana J Cancer Prev Curr Res. 2015;3(4):00089.
Download references
Acknowledgements
The author did not receive any funding for this study.
Author information
Authors and affiliations.
College of Nursing, Yonsei University, 50-1, Yonsei-ro, Seodaemun-gu, Seoul, 03722, South Korea
Agani Afaya
Department of Nursing, School of Nursing and Midwifery, University of Health and Allied Sciences, Ho, Ghana
Department of Population, Family and Reproductive Health, University of Ghana School of Public Health, Accra, Ghana
Emmanuel Anongeba Anaba
Department of Nursing, Faculty of Allied Health Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Victoria Bam
School of Nursing and Midwifery, Griffith University, Queensland, Australia
Richard Adongo Afaya
Department of Internal Medicine, Tamale Teaching Hospital, Tamale, Ghana
Ahmed-Rufai Yahaya
College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Australia
Abdul-Aziz Seidu
School of Clinical Medicine, University of New South Wales, Sydney, Australia
Bright Opoku Ahinkorah
You can also search for this author in PubMed Google Scholar
Contributions
AA, and EAA conceived the study, analyzed and wrote the methods section. AA, VB and RAA conducted the literature search and wrote the background. AA, RAA, and RY screened the included articles and extracted the data. AA, AS and BOA conducted literature search and discussed the results. All the authors reviewed and provided intellectual content and modification. All the authors reviewed and approved the final draft of the manuscript.
Corresponding author
Correspondence to Emmanuel Anongeba Anaba .
Ethics declarations
Ethical approval and consent to participate.
This study did not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Afaya, A., Anaba, E.A., Bam, V. et al. Socio-cultural beliefs and perceptions influencing diagnosis and treatment of breast cancer among women in Ghana: a systematic review. BMC Women's Health 24 , 288 (2024). https://doi.org/10.1186/s12905-024-03106-y
Download citation
Received : 02 March 2023
Accepted : 22 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12905-024-03106-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Religious and spiritual beliefs
- Systematic review
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Comparison efficacy and safety of acupuncture and moxibustion therapies in breast cancer-related lymphedema: A systematic review and network meta-analysis
Contributed equally to this work with: Yawen Xu, Jiangxuan Yu
Roles Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing
Affiliation School of Nursing, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Roles Conceptualization, Data curation, Methodology, Writing – review & editing
Roles Methodology, Writing – review & editing
Roles Conceptualization, Supervision, Writing – review & editing
* E-mail: [email protected]
- Yawen Xu,
- Jiangxuan Yu,
- Rui Shen,
- Xueqi Shan,
- Wenlu Zhou,
- Junjie Wang

- Published: May 14, 2024
- https://doi.org/10.1371/journal.pone.0303513
- Reader Comments
Although several acupuncture and moxibustion therapies have been tested in managing breast cancer-related lymphedema (BCRL), there is little consensus regarding the best options for treating this condition. This systematic review and network meta-analysis compared the efficacy of various acupuncture and/or moxibustion therapies for BCRL.
Seven databases and two clinical registration centers were searched from their inception to December 1 st , 2023. The Cochrane Collaboration risk-of-bias assessment tool evaluated the quality of included RCTs. A pairwise meta-analysis was performed in STATA 16.0, while a network meta-analysis was performed in R 4.2.2.
18 studies were included in this analysis. Our results showed that acupuncture and moxibustion methods had great advantages in improving BCRL of patients with breast cancer. In particular, needle-warming moxibustion (NWM) could be the optimal acupuncture and moxibustion method for improving clinical effectiveness and reducing the degree of swelling of affected limbs.
Our findings suggest that NWM has great potential in treating BCRL. It may reduce arm circumference, lower swelling levels, and improve clinical effectiveness. Nevertheless, more multi-center, high-quality, and large sample RCTs will be needed in the future.
Citation: Xu Y, Yu J, Shen R, Shan X, Zhou W, Wang J (2024) Comparison efficacy and safety of acupuncture and moxibustion therapies in breast cancer-related lymphedema: A systematic review and network meta-analysis. PLoS ONE 19(5): e0303513. https://doi.org/10.1371/journal.pone.0303513
Editor: Ka Ming Chow, Chinese University of Hong Kong, HONG KONG
Received: October 21, 2023; Accepted: April 19, 2024; Published: May 14, 2024
Copyright: © 2024 Xu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: Supported by "The Health Commission of Zhejiang Province [No.2022ZA051]". The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Breast cancer (BC) is a malignancy with the highest incidence in women worldwide. Benefiting from medical advances, postoperative survival is prolonged, and the patient’s quality of life is getting more attention. Breast cancer-related lymphedema (BCRL), usually a temporary or sustainable soft tissue swelling caused by the excessive accumulation of protein-rich lymph in the extracellular spaces of tissue [ 1 ], which manifests as numbness, swelling, pain, dysfunction, and even infection, is a common complication after surgery, radiotherapy, or chemotherapy. The incidence of BCRL in BC survivors ranges from 7% to 58% because of different treatments [ 2 , 3 ]. Although the pathogenesis of BCRL is still incompletely understood, it involves a series of continuous and complex processes including fat deposition, lymphatic fibrosis, and infiltration of inflammatory cells [ 4 ]. As a serious complication, BCRL has not been well recognized and has not been effectively resolved.
Surgical and non-surgical therapies are now available for BCRL. Non-surgical therapies mainly include physical therapy and drugs, but drugs (Diosmin or diuretics) may be limited in clinical practice due to slow reaction times, and adverse effects. Combined decongestive therapy (CDT) is recognized as the recommended physical therapy for lymphedema, which consists of manual lymphatic drainage (MLD), gradient compression bandaging (GCB), functional exercises, and skin care [ 5 ]. However, CDT is an expensive and time-consuming strong treatment method that requires long-term daily one-on-one intervention with a professional therapist, so it brings patients inconvenience and discomfort [ 6 ]. Therefore, considering the current clinical conditions and patient adaptability, for patients with mild to moderate edema, some relatively simple methods such as functional exercise (FE) and pneumatic circulation (PC) are adopted. Whereas for severe cases or those with ineffective CDT, surgery, like lymphatic venous anastomosis (LVA) or vascularized lymph node transfer (VLNT), is the only and final option [ 7 ]. Nevertheless, the results of these methods are very limited and slow, and surgeries may lead to recurrence or infection [ 8 ]. Therefore, it is crucial that there are alternate therapies available.
As complementary or alternative therapies for BCRL, acupuncture and/or moxibustion therapies, using metal fine needles or moxa sticks to stimulate acupoints for therapeutic purposes, have been recommended to relieve the symptoms of BCRL [ 9 ]. According to several meta-analyses, acupuncture and moxibustion therapies show strong potential advantages for the management of BCRL [ 10 , 11 ]. According to a study, local acupuncture might accelerate metabolism, increase blood circulation, and cause accumulation of inflammatory cells in the upper limb edema areas to attempt to mitigate the edema [ 12 ]. Acupuncture’s stimulatory impact leads the damaged or blocked lymphatic vessels to reopen, particularly the micro-vessels that are essential for lymphatic return [ 13 ]. Moxibustion’s warm stimulation could effectively alleviate the pain and promote the healing process [ 14 ].
However, there are various acupuncture and moxibustion techniques, each with varying levels of complexity, costs, as well as safety and efficacy [ 15 , 16 ]. Appropriate acupuncture and moxibustion modality selection can enhance therapeutic outcomes and lower treatment costs, which in turn lowers the strain on clinicians and patients. However, due to insufficient evidence of direct comparisons between acupuncture and moxibustion therapies, clinicians find it difficult to determine which are the most effective for clinical applications [ 17 ]. Network meta-analysis (NMA), in comparison to conventional meta-analysis, may compare different treatments for one health condition and perform quantitative analysis to evaluate and order the efficacy of these therapies [ 18 ]. Therefore, a systematic review and network meta-analysis were used to compare and rank different acupuncture and moxibustion methods, to identify the most effective acupuncture and moxibustion methods and provide a reference for clinical practice.
Our network meta-analysis has been registered on the PROSPERO website (CRD42023392176) and reported following the Preferred Reporting Items for Systematic Reviews and Network Meta-Analysis (PRISMA-NMA) checklist [ 19 ] ( S1 Table ).
Search strategy
We searched PubMed (with MEDLINE), Web of Science, Embase, China National Knowledge Infrastructure (CNKI), Wanfang, China Science and Technology Journal Database (VIP), and SionMed databases to identify relevant English/Chinese medical studies. The search strategy combined MeSH terms with free-text terms. The keywords include “breast cancer”, “breast cancer-related lymphedema”, “lymphedema”, “acupuncture”, and “moxibustion”, as well as their relevant derivatives. Boolean “OR” and “AND” were used to find the intersections of searches for breast cancer with acupuncture and moxibustion. The search period ran from the database’s establishment until December 1 st , 2023. Comprehensive search strategies were provided in the S2 Table .
Additionally, we searched published meta-analyses, ClinicalTrials, Chinese Clinical Trial Registry (ChiCTR), and gray literature. Two researchers examined the findings for accuracy and looked through the included trials’ reference lists.
Inclusion and exclusion criteria
Inclusion criteria..
Studies were deemed qualified if they met all of the specified eligibility criteria (PICOs) described below:
- Type of participants: Women who had lymphedema as a result of surgery, chemotherapy, or radiation for BC. There were no limitations on participants’ lymphedema level, BC stage, age, or nation.
- Type of interventions: The treatment group was given acupuncture and/or moxibustion without the restriction of manipulation techniques, but in the moxibustion measures, only moxa was heated without Traditional Chinese medicines.
- Type of comparators: The control group could receive acupuncture and/or moxibustion (different from the intervention group) or any other treatments (such as oral medication, physical therapy, placebo acupuncture, and so on).
- Type of outcomes:
The primary outcomes were clinical effectiveness rate and extent of lymphedema evaluation using the arm circumference (average arm circumference, circumference of elbow joint). The clinical effectiveness rate was determined based on the effective index (%): Effective index (%) = (pretreatment circumference of the affected arm—posttreatment circumference of the affected arm) / (pretreatment circumference of the affected arm—pretreatment circumference of the unaffected arm). There are two methods for calculating:
- Significantly effective: effective index > 90% or above; effective: 10–90%; ineffective <10%. Clinical effectiveness rate = significantly effective + effective.
- Cure: reduction of 75% or above; significantly effective: reduction of 51–74%; effective: reduction of 25–50%; ineffective: reduction of less than 25%. Clinical effectiveness rate = cure + significantly effective + effective.
The secondary outcomes were composed of the visual analog scale (VAS) score for swelling or pain, and the incidences of adverse events related to acupuncture and moxibustion therapies (such as local skin discomfort, mild scald, vertigo headache, flushing, and itching headaches).
- (5) Type of study: We only considered randomized controlled trials (RCTs).
Exclusion criteria.
The study was excluded if it matched any of the criteria below: (1) duplicate publication, (2) no relevant outcomes, (3) unobtainable original full text, and (4) pretrial (pilot study), animal experiments, letters, reviews, commentaries, protocols, or conference presentations.
Study selection and data extraction
Two researchers independently screened the literature, extracted the data, and conducted cross-checking. If there were any differences, a third researcher was invited to participate in the discussion to conclude. For the included studies, Excel 2021 software was used to extract the following information: basic study features (first author’s name, publication year), information regarding the participants, treatments, and outcomes. If the data were lacking or unclear, the author would be contacted to obtain the necessary information.
Quality assessment
We evaluated the included RCTs’ bias risk through the Cochrane Collaboration risk-of-bias assessment tool [ 20 ] by using the Review Manager (version 5.3) with six assessment aspects. The classifications of "low risk," "unclear risk," and "high risk" were displayed for each study in green, yellow, and red, respectively. If the two reviewers’ assessments disagreed, the entire team voted and discussed in order to reach a conclusion.
Statistical analysis
Firstly, a pairwise meta-analysis of direct comparisons was carried out on trials with clinical homogeneity due to the use of the same type of therapies, participants, and indicator evaluation methods using Stata 16.0 software. For dichotomous outcomes, the effect size was represented using odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was defined as P < 0.05. Weighted mean differences (WMDs) and 95% CIs were estimated for continuous outcomes. To examine the heterogeneity of included studies, we used the I 2 test. If the heterogeneity was non-significant (P value ≧ 0.1 and I 2 ≦ 50%), we used a fixed effect model. Conversely, a random-effect model was adopted, and then the sources of heterogeneity were explored [ 21 ].
Secondly, using the “BUGSnet” and “gemtc” packages in R 4.2.2 software, network meta-analysis was carried out and assessed with Markov Chain Monte Carlo (MCMC) simulation [ 22 , 23 ]. To evaluate model fit, we compared the number of unconstrained data points and the residual deviation. If the quantities were nearly the same, the model fit would be regarded as sufficient. The deviation information criterion (DIC) served as the basis for our decision on whether to use a fixed-effect or a random-effect model. We selected a model with lower DIC values. However, if the DIC values of the two models were close and their difference was within 5, a random-effect model could be chosen. Gelman and Rubin criteria as well as a review of trace plots would be used to assess convergence. The network plot of the outcome was made to visualize multiple comparisons. Consistency was evaluated by node splitting [ 24 ], and inconsistency was defined as P value < 0.05. A heatmap with all potential comparisons was used to display the relative effect estimate from this analysis. For treatment ranking, the surface under the cumulative ranking curve (SUCRA), which ranges from 0% to 100%, was calculated. When comparing the various therapies, the intervention with the highest SUCRA value has the highest possibility that it would be the best one [ 25 ].
In addition, if there were more than 10 studies included in each outcome, comparison-adjusted funnel plots and Egger’s tests were conducted to assess publication bias.
Study selection and study characteristics
The screening process is presented in Fig 1 . In the end, we identified 886 related studies during our initial evaluation. After duplicates were eliminated and titles and abstracts were screened, 73 trials that may meet the criteria were read carefully in full text. Finally, our quantitative synthesis included 18 RCTs in total.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0303513.g001
The studies, involving 1,217 patients and published from 2014 to 2023, included 5 acupuncture and/or moxibustion treatments, namely simple acupuncture (SA), blood-letting and cupping (BLC), electroacupuncture (EA), gentle moxibustion (GM) and needle-warming moxibustion (NWM), as well as 5 comparators consisting of FE, oral medication (OM), PC, placebo acupuncture (PA), and usual care (UC). Table 1 provides further information. The complete relevant data is shown in S1 File .
https://doi.org/10.1371/journal.pone.0303513.t001
NMA was only possible for the outcomes of clinical effectiveness rate and circumference of the elbow joint. However, the transitivity and consistency of the networks for the other outcomes could not be evaluated; hence, pairwise meta-analysis was the only approach employed. The network plots for each intervention that is a part of each NMA are shown in Fig 2 . 9 trials [ 26 – 28 , 32 , 35 , 37 , 39 , 40 , 41 ] with 7 interventions (SA, BLC, GM, NWM, FE, PC, UC) were included in the network plot of the clinical effectiveness rate ( Fig 2(A)) . 10 studies [ 29 – 31 , 34 , 36 – 40 , 43 ] containing 7 therapies (BLC, GM, NWM, FE, OM, PC, UC) were shown in the network plot of the elbow joint circumference ( Fig 2(B)) .
( A ) Clinical effectiveness rate, ( B ) Circumference of the elbow joint. Note: SA = simple acupuncture; BLC = blood-letting and cupping; GM = gentle moxibustion; NWM = needle-warming moxibustion; FE = functional exercises; OM = oral medicine; PC = pneumatic injection; UC = usual care.
https://doi.org/10.1371/journal.pone.0303513.g002
Based on the collected literature, we evaluated the risk of bias. The result is given in Fig 3 . 2 trials did not mention randomness. Due to unclear reporting, 6 trials reported the specific allocation concealment scheme, while the other studies did not. 2 trials were evaluated as having high risk because there was no report of any blind method, but the evaluator judged that it would not affect the measurement of objective outcomes. All the studies fully reported the predetermined outcomes. Most of them did not explain other bias risks and were rated as "unclear risk".
https://doi.org/10.1371/journal.pone.0303513.g003
Meta-analysis
Clinical effectiveness rate (pairwise meta-analysis and nma)..
According to the pairwise meta-analysis, GM (1 study, OR = 7.27, 95%CI: 1.49 to 35.46, P = 0.014), BLC (1 study, OR = 16.43, 95%CI: 3.21 to 84.00, P = 0.001) and SA (2 studies, OR = 6.78, 95%CI: 2.68 to 17.11, P = 0.000) were superior to FE in improving clinical effectiveness rate. However, there was no significant difference when comparing GM with PC (1 study, OR = 3.33, 95%CI: 0.52 to 21.28, P = 0.203). Compared with UC, the clinical effectiveness rate was significantly increased in the GM group (1 study, OR = 5.13, 95%CI: 1.27 to20.81, P = 0.022) and the BLC group (1 study, OR = 2.35, 95%CI: 1.01 to 5.46, P = 0.047). Additionally, it was not found that NWM (1 study, OR = 7.25, 95% CI: 0.82 to 64.46, P = 0.076) and SA (1 study, OR = 4.75, 95%CI: 0.48 to 46.91, P = 0.182) performed better than UC ( S1 Fig ).
The results of the NMA showed that GM, BLC, and SA significantly increased the clinical effectiveness rate compared with FE. In the meanwhile, GM was better than FE ( Fig 4(A)) . Based on the SUCRA of the clinical effectiveness rate, NWM (83.2%) was the optimal intervention, followed by GM (71.8%), BLC (64.7%), and SA (61.2%) ( Fig 4(B)) .
( A ) Heat plot. ( B ) SUCRA plot. Note: ** indicates a significant result.
https://doi.org/10.1371/journal.pone.0303513.g004
Average arm circumference (pairwise meta-analysis only).
6 studies [ 27 , 29 – 31 , 36 , 39 ] with 291 participants explored variations in the average arm circumference. Pairwise meta-analysis showed that NWM (1 study; WMD = -1.00, 95%CI: -1.58 to -0.42, P = 0.001) was more efficacious than OM in reducing average arm circumference. However, there was no significant difference when comparing GM with PC (4 studies, WMD = -0.64, 95%CI: -1.30 to 0.03, P = 0.060, I 2 = 0%). Additionally, it was not found that BLC (1 study, WMD = -0.74, 95% CI: -1.91 to 0.43, P = 0.215) performed better than FE ( S2 Fig ).
Circumference of the elbow joint (pairwise meta-analysis and NMA).
The pairwise meta-analysis revealed no differences between GM and PC (3 studies; WMD = -0.40, 95%CI: -1.27 to 0.47, P = 0.362, I 2 = 0%), BLC and FE (1 study; WMD = -0.90, 95%CI: -2.33 to 0.53, P = 0.216). Compared with UC, NWM (1 study, WMD = -8.06, 95%CI: -11.56 to -4.56, P = 0.000) and BLC (1 study, WMD = -1.70, 95%CI: -2.51 to -0.89, P = 0.000) both showed significant advantages in decreasing the circumference of the elbow joint. Additionally, it was found that BLC (1 study, WMD = -1.49, 95% CI: -2.60 to -0.38, P = 0.009) performed better than OM. We used a random-effect model to compare GM with UC (P = 0.108). Due to limited research and difficulty in conducting sensitivity analysis, high heterogeneity (I 2 = 88.2%) might differ significantly from the severity of lymphedema in the patients included in this study ( S3 Fig ).
The NMA results indicated that no acupuncture and moxibustion therapies could reduce the circumference of the patient’s elbow ( Fig 5(A)) . Furthermore, according to the ranking of the SUCRA plot, the optimal intervention was NWM (82.2%), which was followed by GM (57.8%), and BLC (53.7%) ( Fig 5(B)) .
https://doi.org/10.1371/journal.pone.0303513.g005
VAS score for swelling (pairwise meta-analysis only).
The VAS score for swelling was reported in 6 trials [ 27 , 28 , 30 , 31 , 36 , 42 ]. As the pairwise meta-analysis results indicated, GM (4 studies, WMD = -1.19, 95%CI: -1.52 to -0.87, P = 0.000, I 2 = 21.1%) was superior to PC in decreasing the swelling score. NWM performed greater than UC (1 study, WMD = -0.98, 95%CI: -1.73 to -0.23, P = 0.010), but not greater than PA (1 study, WMD = -0.73, 95%CI: -1.61 to 0.15, P = 0.105) ( S4 Fig ).
VAS score for pain (pairwise meta-analysis only).
3 studies [ 35 , 37 , 39 ] reported VAS scores for pain. Results of a pairwise meta-analysis revealed that when compared to UC, BLC had significant effects on reducing VAS pain score (1 study, WMD = -1.75, 95%CI: -2.24 to -1.26, P = 0.000). When comparing to FE, SA (1 study, WMD = -1.22, 95%CI: -1.71 to -0.73, P = 0.000) performed better in decreasing the VAS score of pain, while BLC (1 study, WMD = -0.42, 95%CI: -1.12 to 0.28, P = 0.242) did not ( S5 Fig ).
Adverse events.
5 studies [ 29 , 30 , 33 , 36 , 42 ] reported that 10 patients had adverse events, 7 of which were related to acupuncture and moxibustion treatments. The specific adverse events included local skin discomfort (2 cases), mild scald caused by positional changes (2 cases), Flushing and itching (1 case), xerostomia (1 case), and swelling (1 case). No patients withdrew from the study due to adverse reactions. We found that the GM method had the highest probability of adverse events.
Assessment of publication bias
In our study, the heterogeneities of most merged results were not high, indicating that our results had a certain degree of stability and reliability. The comparison-adjusted funnel plot of the circumference of the elbow joint lacked visual symmetry. However, Egger’s test showed no statistical difference in both outcomes (P = 0.130), indicating no significant publication bias in this outcome( S6 Fig ). As for other outcomes, publication bias couldn’t be assessed through a funnel plot because their analysis included no more than 10 studies.
Main findings
Patients with breast cancer are more likely to get postoperative lymphedema as a result of surgical techniques, local radiation, injury, and infection, which can lead to limb dysfunction and have a severe long-term impact on the patient’s quality of life. At present, Western medicine mainly uses comprehensive therapies (function exercise, compression, manual massage, and so on) to control swelling, but these methods have limited efficacy, and cost human and financial resources. Practical and effective treatments need to be explored. Acupuncture and moxibustion methods have been widely used in treating BCRL and had good efficacy. However, there might be differences in the efficacy of different acupuncture and moxibustion methods. Therefore, we carried out this network meta-analysis to investigate the efficacy and safety of various acupuncture and moxibustion methods in improving BCRL. Due to the use of different methodologies, scales, and measurement results in the RCTs included in this study, we only conducted both network meta-analysis and pairwise meta-analysis on 2 main outcomes (clinical effectiveness rate and circumference of the elbow joint). In contrast, only a pairwise meta-analysis was conducted on the other outcomes. In terms of the clinical effectiveness rate, the network meta-analysis results were consistent with the pairwise meta-analysis results. GM, BLC, and SA had significant improvements in the clinical effectiveness rate when compared to FE. In reducing the average arm circumference, the pairwise meta-analysis showed that only NWM was superior to OM. In the network meta-analysis of the circumference of the elbow joint, no significant differences were found in the efficacy of different treatments. However, pairwise meta-analysis results indicated that NWM and BLC were both better than UC. and GM has a significant effect in reducing the circumference of the elbow joint. The pairwise meta-analysis provided similar results. In addition, NWM and BLC acupuncture and moxibustion were better than UC in VAS score for swelling and pain, but other acupuncture and moxibustion methods had no obvious effect.
NWM demonstrated the highest likelihood of being ranked as the best method in terms of the clinical effectiveness rate and the circumference of the elbow joint, despite having a relatively small sample size. This is partially consistent with the conclusions of a recently published network meta-analysis [ 44 ]. NWM is a method that combines SA and GM to treat medical conditions [ 45 ]. SA has been widely used to treat cancer, arthritis, and other diseases [ 46 , 47 ]. Some researchers claimed that acupuncture stimulation had the effect of relieving pain and regulating anti-inflammatory indicators (reducing pro-inflammatory cytokines as well as increasing anti-inflammatory cytokines) [ 48 , 49 ]. Meanwhile, GM is second only to NWM in improving clinical effectiveness. The thermal effect of GM can dilate local capillaries, enhancing local lymphatic circulation and distribution [ 50 ], and by providing warm stimulation suitable for the human body, patients maintain a calm emotional, and healthy psychological state [ 51 ]. Multiple studies have shown that NWM relieves symptoms by introducing the thermal stimulation of moxibustion through acupuncture into the deep tissues of the affected limbs [ 52 , 53 ]. The combination of acupuncture and moxibustion may produce a better therapeutic effect than the single use [ 54 ]. Infrared thermography suggests that the higher the body temperature in the deep layer of the affected limb, the more likely the improvement of lymphedema (lymph mainly flows to the deep layer) will be [ 55 ]. This may be one of the reasons why NWM is more effective than SA or GM. Previous studies have also proposed that NWM can probably promote lymphangiogenesis by upregulating the expression level of vascular endothelial growth factor C (VEGF-C), thereby facilitating the absorption of local tissue edema and inflammation [ 26 , 56 ].
BLC refers to the method of using a sterile needle to quickly puncture the skin in the area, followed by cupping to release the appropriate amount of blood [ 57 ]. Pairwise meta-analysis suggested that BLC was superior to usual measures in all outcomes except VAS scores of swelling. This is inconsistent with previous study [ 58 ]. Perhaps due to the limited use of BLC treatment for BCRL in clinical practice, and insufficient sample size to support this [ 59 ]. Notably, EA is a treatment in which a small amount of electricity is applied to the needle after acupuncture is applied to prevent and treat diseases. However, Due to differences in measurement methods and the current situation of combining traditional Chinese medicine decoctions in clinical practice, only one trial using EA was included [ 60 ]. So, the specific efficacy of EA cannot be determined. EA may be used to stimulate the vagus nerve by pulsing radio waves to regulate immune function to promote recovery [ 49 ]. However, we found that in our searches, EA was mainly used in combination with decoctions of traditional Chinese medicine, so the direct effect of EA could not be ascertained.
The mechanism of point combination was relatively complex. In our study, the selection principle appeared to be a combination of local and distal points. Quchi (LI.11) is the most frequently used acupuncture point in the local (upper limb), followed by Ashi points. Quchi (LI.11) is at the bend and depression of the elbow joint, which is a large joint that is easily accessible to the upper limbs in daily life [ 61 ]. Pro-inflammatory gene expression can be reduced by stimulation and associated nerve development can be accelerated and controlled. Ashi points can be considered sensitive in the pathological condition’s hypersensitive state, which is located in the most painful part of the skin [ 62 ]. Zusanli (ST.36) is the most commonly used distal acupuncture point. It is an important health point, thanks to its powerful regulatory effect on various systems in the human body, including nerves, the immune system, and the endocrine system [ 29 ]. Based on the "holistic" concept, the combination of local and distal points can not only directly stimulate the affected area, but also further treat it by regulating the whole body, which is also a major feature of Traditional Chinese Medicine [ 63 ].
Regarding adverse events related to acupuncture and/or moxibustion therapies, we found that local skin discomfort is the most common one. In addition, patients who use the GM method may experience more adverse events, including local skin discomfort, mild scald caused by positional changes, Flushing and itching, xerostomia, and swelling. This may be related to the different feelings of each patient towards thermal stimulation, as well as the impact of moxa smoke [ 64 ]. However, adverse effects are not significant in their intensity. Overall, the side effects of acupuncture and/or moxibustion treatments are mild and acceptable to patients.
A follow-up survey of 3 studies [ 30 , 34 , 42 ] from 1 month to 4 months showed that edema recurred in some patients shortly after acupuncture and/or moxibustion treatment, but all had fewer cases of recurrence compared to control measures (OM and PC). The lack of long-term follow-up studies makes it hard to conclude substantial implications from acupuncture and/or moxibustion therapies in treating BCRL and which acupuncture and/or moxibustion therapy has the best long-term effect.
Strengths and limitations
In our understanding, our research seems to be the latest to explore the most effective and safe acupuncture and/or moxibustion therapy for treating BCRL, which may be beneficial for making reasonable treatment decisions for BCRL because of a shortage of gold-standard treatments and significant medical burden. Secondly, we named the acupuncture and moxibustion methods in this study according to the international standard terminologies on traditional Chinese medicine [ 65 ] issued by the World Health Organization in 2022. Besides, to limit the impact of other measures on the results, we excluded acupuncture and/or moxibustion therapies used in combination with Chinese herbal medicine.
However, it is important to consider the following limitations. Firstly, the number of databases searched was Limited, and we only included 18 trials with 9 treatments. On this account, the majority of comparisons depended on 1–2 studies, which may not be detrimental to the reliability of the primary outcomes. Secondly, the included trials did not perform well in terms of methodological quality, and more than half of the studies had relatively small sample sizes. Therefore, it is possible that the reported effect sizes were exaggerated in these trials. Thirdly, the outcomes have not been standardized either, which has significantly impacted our data consolidation and analysis. This may be due to the lack of standardized treatments for BCRL. Finally, the small number of included studies makes it challenging for us to organize subgroup analysis.
Future perspectives
In our meta-analysis, NMW is currently the best method, but in addition to the selection of methods, the acupoint selection and stimulation are also crucial. And, the selection of acupoints varies depending on the patient’s situation, so future research on specific acupoint combinations and mechanisms is needed. In addition, it is difficult to do a pooled analysis since different studies have employed the circumference of various sections of the affected limb as the outcome. It is advised that the measuring location and calculation method be standardized for future research. BCRL typically has a longer chronic progression, and in addition to the short-term improvement in clinical results, more evidence is needed to confirm whether acupuncture and/or moxibustion therapies have long-term effects. We encourage future researchers to conduct RCTs with high-quality, large sample sizes, and multi-center to validate the available evidence.
Based on our results, acupuncture and moxibustion interventions have great potential to improve the BCRL of patients with breast cancer. Particularly NWM has significant advantages in improving the clinical effectiveness rate of BCRL patients, reducing the arm circumference of affected limbs, and reducing the sense of swelling and pain. NWM combines the advantages of acupuncture and moxibustion, which are simple, economical, safe, and effective. By stimulating fixed acupoints while applying a warming effect to the deep layers of the skin, it can improve the patient’s lymphatic circulation, relieve edema, alleviate pain, and achieve better clinical efficacy.
Supporting information
S1 fig. the pairwise meta-analysis of clinical effectiveness rate..
https://doi.org/10.1371/journal.pone.0303513.s001
S2 Fig. The pairwise meta-analysis of average arm circumference.
https://doi.org/10.1371/journal.pone.0303513.s002
S3 Fig. The pairwise meta-analysis of the circumference of the elbow joint.
https://doi.org/10.1371/journal.pone.0303513.s003
S4 Fig. The pairwise meta-analysis of VAS swelling score.
https://doi.org/10.1371/journal.pone.0303513.s004
S5 Fig. The pairwise meta-analysis of VAS swelling score.
https://doi.org/10.1371/journal.pone.0303513.s005
S6 Fig. The comparison-adjusted funnel plot.
https://doi.org/10.1371/journal.pone.0303513.s006
S1 File. Related data for this study.
https://doi.org/10.1371/journal.pone.0303513.s007
S1 Table. The PRISMA network meta-analysis checklist.
https://doi.org/10.1371/journal.pone.0303513.s008
S2 Table. Comprehensive search strategies.
https://doi.org/10.1371/journal.pone.0303513.s009
S3 Table. The full name of acupoints abbreviations.
https://doi.org/10.1371/journal.pone.0303513.s010
Acknowledgments
The authors would like to thank all the researchers for providing the data for this work.
- View Article
- PubMed/NCBI
- Google Scholar
- 29. Ba T. Clinical observation on warm acupuncture treatment of breast cancer related lymphedema. M.Sc. Thesis, Shanxi University of Chinese Medicine; 2019.
- 30. Shen J. A study based on synodic theory to evaluate the efficacy of drug moxa and clear moxa in the treatment of upper limb lymphedema after breast cancer surgery. M.Sc. Thesis, Beijing University of Chinese Medicine; 2019.
- 33. Huang F. The Clinical Curative Effect Observation and Evaluation of Breast Cancer-related Lymphedema Treated by Joint Needing. M.Sc. Thesis, Shandong University of Traditional Chinese Medicine; 2018.
- 36. Yang M. Clinical efficacy of gentle moxibustion in the treatment of lymphedema associated with breast cancer. M.Sc. Thesis, Beijing University of Chinese Medicine; 2017.
- 39. Zhang YR. Clinical efficacy of blood-letting and cupping in the treatment of upper limb edema after breast cancer surgery. M.Sc. Thesis, Beijing University of Chinese Medicine; 2016.
- 41. Yang SX. A multicenter randomized controlled trial study on the efficacy and safety of warm acupuncture in the treatment of upper limb lymphedema after breast cancer surgery. Thesis, Tianjin University of Traditinial Chinese Medicine; 2023.
- 52. Chen L. Observation on the Effect of Warm Acupuncture on Upper Limb Edema after Breast Cancer Surgery. Thesis, Nanjing University of Chinese Medicine; 2014.
- 54. Zhao Y. Clinical study on electroacupuncture combined with auricular acupuncture in the treatment of upper limb lymphedema after breast cancer surgery of qi deficiency and blood stasis type. Thesis, Yunnan University of Chinese Medicine; 2019.
- 56. Han YJ. The Observation and Mechanism of Warm Acupuncture and Moxibustion for Improving Breast Cancer Related Lymphedema. M.Sc. Thesis, Tianjin University of Traditional Chinese Medicine; 2020.
- 58. Wang BY. Clinical observation on treating 54 cases of upper limb lymphedema after breast cancer operation by pricking and cupping. Thesis, Beijing University of Chinese Medicine; 2016.

IMAGES
VIDEO
COMMENTS
Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality Cancer Treat Rev. 2022 Apr:105:102375. doi: 10.1016 ... For each option, systematic literature searches identified the highest-ranking evidence. For radiotherapy risks, searches for dose-response relationships and modern organ doses were also ...
Background Breast cancer (BC) is the most common cancer among women worldwide. We conducted a systematic review and meta-analysis to cover the existing research gap and contribute to existing knowledge to provide both researchers and clinicians with a better profile on the topic and consequently help improve the quality of life (QoL) of patients with BC. Methods A comprehensive review of ...
For each treatment option, systematic literature searches were conducted to identify the highest-ranking evidence of its effects on mortality. New breast cancer treatments are sometimes recommended in the USA before they are endorsed by European or UK guidelines. Searches were not performed for treatments not yet recommended outside the USA.
Following the PRISMA guidelines, 18 a systematic literature search was conducted from 2000 to 2021 using the following predefined keywords or their combinations: breast cancer, women with breast cancer, young women with breast cancer, breast cancer problems, breast cancer short-term and long-term problems. The search was carried out to identify ...
Breast cancer is one of the most common diseases in women; it can have long-term implications and can even be fatal. However, early detection, achieved through recent advancements in technology, can help reduce mortality. In this paper, different machine intelligence techniques [machine learning (ML), and deep learning (DL)] were analysed in the context of breast cancer. In addition, the ...
This systematic literature review identified the full range of published health utility values relevant to breast cancer from diagnostic or screening, local and systemic therapies, allied health or complementary medicine-related interventions, and treatment-related adverse events.
Preferences of patients with breast cancer for drug therapy play a crucial role in treatment efficacy, satisfaction and adherence. In this systematic review following the PRISMA guidelines, 34 studies were analysed to determine patient preferences at different stages of breast cancer, comparing early stage and metastatic disease.
This review article is an update on the state of surgical options in the treatment of breast cancer. We seek to provide readers with the best practices for oncologically safe and cosmetically superior breast surgery. ... after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann Surg Oncol. 2016 ...
The review identified several significant and interconnected problems in breast cancer patient's treatment and supportive care. The results revealed that these issues are very common among breast cancer patients, and specific attention and serious measures are needed to address these problems.
Study selection process for the systematic literature review and meta-analysis of breast cancer (BC). *Exclusions include not phase II, not phase III or phase II with triple-negative breast cancer (TNBC) focus, phase II not TNBC focus, not phase II or phase III, and not TNBC focus phase II/I. † Exclusions include review articles, other study types, not recurrent/metastatic (R/M) of phase III ...
To determine the impact of breast conservation on quality of life and identify treatment-related and other demographic factors associated with post-breast cancer treatment quality of life. A ...
The mainstay of treatment for female breast cancer is surgical management which has evolved over the last 50 years from radical mastectomy to breast conserving surgeries including oncoplastic techniques and nipple ... a systematic literature review of breast-conservation surgery for male breast cancer. Ann Surg Oncol, 26 (2019), pp. 3939-3944.
Breast cancer is the second leading cause of cancer mortality for US women, despite a steady overall decline in breast-cancer mortality rates over the past 20 years. 1 The average age-adjusted rate for the years 2016-2020 was 19.6 per 100 000, with an estimated 43 170 deaths in 2023. 1,2 The majority of cases occur between the ages of 55 and 74 ...
This systematic literature review aims to further understand the epidemiology and unmet needs of patients with HER2 + breast cancer and BM, and provide a comprehensive overview of the global systemic treatment landscape for this patient population, focusing on differences in clinical trial design and how these differences may impact the ...
Breast cancer is amongst the most common invasive cancers in adults. There are established relationships between anti-cancer treatments for breast cancer and cardiovascular side effects. In recent years, novel anti-cancer treatments have been established, as well as the availability of multi-modal cardiac imaging and the sophistication of treatment for cardiac disease. This review provides an ...
Breast cancer clinical practice guidelines (CPGs) offer evidence-based recommendations to improve quality of healthcare for patients. Suboptimal compliance with breast cancer guideline recommendations remains frequent, and has been associated with a decreased survival. The aim of this systematic review was to characterize and determine the impact of available interventions to support ...
A previous systematic review published in 2015 reported only five studies of signatures evaluated for their ability to predict radiotherapy benefits in clinical cohorts. ... Gene expression signatures have had great success in breast cancer treatment and there is now high-level evidence for their use in ... The literature search revealed four ...
Brain metastases (BMs) occur in 20%-40% of patients with breast cancer during the course of their disease. 1 BMs from solid tumors contribute significantly to morbidity and/or mortality, with about 200,000 patients—10% of all patients with cancer—diagnosed each year in the United States. 2 Notably, Breast Cancer BMs (BCBM) represents second most common (30%) among all cancers. 3 With ...
Breast cancer is currently the most commonly diagnosed cancer in Ghana and the leading cause of cancer mortality among women. Few published empirical evidence exist on cultural beliefs and perceptions about breast cancer diagnosis and treatment in Ghana. This systematic review sought to map evidence on the socio-cultural beliefs and perceptions influencing the diagnosis and treatment of breast ...
Objective Although several acupuncture and moxibustion therapies have been tested in managing breast cancer-related lymphedema (BCRL), there is little consensus regarding the best options for treating this condition. This systematic review and network meta-analysis compared the efficacy of various acupuncture and/or moxibustion therapies for BCRL. Methods Seven databases and two clinical ...
However, there was only one systematic review about snoRNAs in colorectal cancer. 60 Therefore, we conducted a comprehensive analysis of 49 studies to evaluate the expression profiles of snoRNAs as novel biomarkers for the diagnosis and prognosis of cancer. This research had potentially contributed to the development of new diagnostic tools and ...
Abstract. Introduction: Breast cancer is currently the most common malignancy among women, after non-melanoma skin cancer. Upper limb lymphedema is the most feared complication of breast cancer treatment. Currently, the approach described in the literature as the most effective for the treatment of lymphedema is Complex Physical Therapy. In the search for more efficient therapeutic actions ...