Search form
Roots of major depression revealed in all their genetic complexity.

(© stock.adobe.com)
A massive genome-wide association study (GWAS) of genetic and health records of 1.2 million people from four separate data banks has identified 178 gene variants linked to major depression, a disorder that will affect as many as one in every five people during their lifetimes.
The results of the study, led by the U.S. Department of Veterans Affairs (V.A.) researchers at Yale University School of Medicine and University of California-San Diego (UCSD), may one day help identify people most at risk of depression and related psychiatric disorders and help doctors prescribe drugs best suited to treat the disorder.
The study was published May 27 in the journal Nature Neuroscience .
For the study, the research team analyzed medical records and genomes collected from more than 300,000 participants in the V.A.’s Million Veteran Program (MVP), one of the largest and most diverse databanks of genetic and medical information in the world.
These new data were combined in a meta-analysis with genetic and health records from the UK Biobank, FinnGen (a Finland-based biobank), and results from the consumer genetics company 23andMe. This part of the study included 1.2 million participants. The researchers crosschecked their findings from that analysis with an entirely separate sample of 1.3 million volunteers from 23andMe customers.
When the two sets of data from the different sources were compared, genetic variants linked to depression replicated with statistical significance for most of the markers tested.
“ What is most heartening is we could replicate our findings in independent data sets,” said Daniel Levey , an associate research scientist in the Yale Department of Psychiatry and co-lead author. “Replication is a hallmark of good science, and this paper points to just how reliable and stable results from GWAS studies are becoming.”
Like many mental health disorders, depression is genetically complex and is characterized by combinations of many different genetic variants, the researchers say.
“ That’s why we weren’t surprised by how many variants we found,” said Joel Gelernter , the Foundations Fund Professor of Psychiatry at Yale, professor of genetics and of neuroscience, and co-senior author of the study. “And we don’t know how many more there are left to discover — hundreds? Maybe even thousands?”
The size of the new GWAS study will help clinicians to develop polygenic risk scores to pinpoint those most at risk of developing major depression and other related psychiatric disorders such as anxiety or post-traumatic stress disorder, the authors say.
The study also provides deep insights into the underlying biology of genetic disorders. For instance, one gene variant implicated in depression, NEGR1 , is a neural growth regulator active in the hypothalamus, an area of the brain previously linked to depression. That confirms research done by the late Yale neuroscientist Ronald Duman on the role of neurotrophic factors in depression, Levey said.
“ It’s really striking when completely different kinds of research converge on similar biology, and that’s what’s happening here,” he said.
Insights into the functions of the variants can also help identify many drugs that hold promise in the treatment of depression, the researchers say. For instance, the drug riluzole, which is approved for the treatment of amyotrophic lateral sclerosis (ALS), modulates glutamate transmission in brain. Several gene variants linked by the new study to depression affect the glutamate system, which is actively being studied for depression treatments.
“ One of the real goals of the research is bringing forward new ways to treat people suffering from depression,” added co-senior author Dr. Murray Stein, staff psychiatrist at the V.A. San Diego Healthcare System and Distinguished Professor of Psychiatry and Public Health at UCSD.
Research was primarily funded by the U.S. Department of Veterans Affairs, including the Million Veteran Program and the Cooperative Studies Program. Levey also received support from a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation.
- Study of veterans details genetic basis for anxiety, links anxiety and depression
- Immune system may have another job — combatting depression
Health & Medicine

Yale’s 323rd Commencement events to be held May 19 and 20

Visit the Yale 2024 website

In Farr Lecture, Yale’s Peter Aronson reflects on serendipity

Cynthia Zarin’s new novel weaves memories of winter
- Show More Articles
Major Depression and Genetics
How common is major depression? At least 10% of people in the U.S. will experience major depressive disorder at some point in their lives. Two times as many women as men experience major depression.
How do we know that genes play a role in causing depression? Scientists look at patterns of illness in families to estimate their “heritability,” or roughly what percentage of their cause is due to genes. To do this we find people with the disease who have a twin, and then find out whether the twin is also ill. Identical (monozygotic) twins share 100% of their genes, while non-identical (“fraternal” or dizygotic) twins share 50% of their genes. If genes are part of the cause, we expect a patient’s identical twin to have a much higher risk of disease than a patient’s non-identical twin. That is the case for major depression. Heritability is probably 40-50%, and might be higher for severe depression.
This could mean that in most cases of depression, around 50% of the cause is genetic, and around 50% is unrelated to genes (psychological or physical factors). Or it could mean that in some cases, the tendency to become depressed is almost completely genetic, and in other cases it is not really genetic at all. We don’t know the answer yet.
We can also look at adoption studies, to see whether an adopted person’s risk of depression is greater if a biological parent had depression. This also seems to be the case.
What about non-genetic factors? There are probably many non-genetic factors that increase risk of depression, many of which are probably not yet known. Severe childhood physical or sexual abuse, childhood emotional and physical neglect, and severe life stress are probably all risk factors. Losing a parent early in life probably also increases risk to some extent.
If someone has a family history of depression, are they at very high risk? If someone has a parent or sibling with major depression, that person probably has a 2 or 3 times greater risk of developing depression compared with the average person (or around 20-30% instead of 10%).
The situation is a little different if the parent or sibling has had depression more than once (“recurrent depression”), and if the depression started relatively early in life (childhood, teens or twenties). This form of depression is less common – the exact percentage of the population is not known, but is probably around 3-5%. But the siblings and children of people with this form of depression probably develop it at a rate that is 4 or 5 times greater than the average person.
Is there a “depression gene”? Some diseases are caused by a single defective gene. Cystic fibrosis, several kinds of muscular dystrophy, and Huntington’s disease are examples. These are usually rare diseases. But many common disorders like depression, diabetes and high blood pressure are also influenced by genes. In these disorders, there seem to be combinations of genetic changes that predispose some people to become ill. We don’t yet know how many genes are involved in depression, but it is very doubtful that any one gene causes depression in any large number of people.
So no one simply “inherits” depression from their mother or father. Each person inherits a unique combination of genes from their mother and father, and certain combinations can predispose to a particular illness.
How are major depression and bipolar disorder related? Most people who suffer from depression do not have episodes of mania. We use the term major depression for depression without mania. Most people who experience mania also have major depression. We use the term bipolar disorder (or manic-depression) for this pattern. Major depressive disorder and bipolar disorder are the two “major mood disorders.” For more information on the symptoms of mania abd bipolar disorder, see the links at the bottom of this page. Most people with major depression do not have close relatives with bipolar disorder, but the relatives of people with bipolar disorder are at increased risk of both major depression and bipolar disorder.
What about major depression and anxiety disorders? There are probably genetic changes that can increase the predisposition to both major depression and to certain anxiety disorders including generalized anxiety disorder, panic disorder and social phobia. Also, some people have a more general lifelong tendency to experience unpleasant emotions and anxiety in response to stress. Psychologists use terms like “neuroticism” and “negative affectivity” to refer to this tendency, and people who have it are also more likely to experience major depression.
However, many people who develop major depression did not have this type of personality before their depression started.
Genetic Architectures of Adolescent Depression Trajectories in 2 Longitudinal Population Cohorts
Affiliations.
- 1 Division of Psychiatry, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom.
- 2 Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
- 3 School of Health and Wellbeing, University of Glasgow, Glasgow, United Kingdom.
- 4 School of Medical Sciences, Örebro University, Örebro, Sweden.
- 5 Generation Scotland, Centre for Genomic and Experimental Medicine, Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, United Kingdom.
- 6 MRC Integrative Epidemiology Unit, University of Bristol, Bristol, United Kingdom.
- PMID: 38748406
- PMCID: PMC11097103 (available on 2025-05-15 )
- DOI: 10.1001/jamapsychiatry.2024.0983
Importance: Adolescent depression is characterized by diverse symptom trajectories over time and has a strong genetic influence. Research has determined genetic overlap between depression and other psychiatric conditions; investigating the shared genetic architecture of heterogeneous depression trajectories is crucial for understanding disease etiology, prediction, and early intervention.
Objective: To investigate univariate and multivariate genetic risk for adolescent depression trajectories and assess generalizability across ancestries.
Design, setting, and participants: This cohort study entailed longitudinal growth modeling followed by polygenic risk score (PRS) association testing for individual and multitrait genetic models. Two longitudinal cohorts from the US and UK were used: the Adolescent Brain and Cognitive Development (ABCD; N = 11 876) study and the Avon Longitudinal Study of Parents and Children (ALSPAC; N = 8787) study. Included were adolescents with genetic information and depression measures at up to 8 and 4 occasions, respectively. Study data were analyzed January to July 2023.
Main outcomes and measures: Trajectories were derived from growth mixture modeling of longitudinal depression symptoms. PRSs were computed for depression, anxiety, neuroticism, bipolar disorder, schizophrenia, attention-deficit/hyperactivity disorder, and autism in European ancestry. Genomic structural equation modeling was used to build multitrait genetic models of psychopathology followed by multitrait PRS. Depression PRSs were computed in African, East Asian, and Hispanic ancestries in the ABCD cohort only. Association testing was performed between all PRSs and trajectories for both cohorts.
Results: A total sample size of 14 112 adolescents (at baseline: mean [SD] age, 10.5 [0.5] years; 7269 male sex [52%]) from both cohorts were included in this analysis. Distinct depression trajectories (stable low, adolescent persistent, increasing, and decreasing) were replicated in the ALSPAC cohort (6096 participants; 3091 female [51%]) and ABCD cohort (8016 participants; 4274 male [53%]) between ages 10 and 17 years. Most univariate PRSs showed significant uniform associations with persistent trajectories, but fewer were significantly associated with intermediate (increasing and decreasing) trajectories. Multitrait PRSs-derived from a hierarchical factor model-showed the strongest associations for persistent trajectories (ABCD cohort: OR, 1.46; 95% CI, 1.26-1.68; ALSPAC cohort: OR, 1.34; 95% CI, 1.20-1.49), surpassing the effect size of univariate PRS in both cohorts. Multitrait PRSs were associated with intermediate trajectories but to a lesser extent (ABCD cohort: hierarchical increasing, OR, 1.27; 95% CI, 1.13-1.43; decreasing, OR, 1.23; 95% CI, 1.09-1.40; ALSPAC cohort: hierarchical increasing, OR, 1.16; 95% CI, 1.04-1.28; decreasing, OR, 1.32; 95% CI, 1.18-1.47). Transancestral genetic risk for depression showed no evidence for association with trajectories.
Conclusions and relevance: Results of this cohort study revealed a high multitrait genetic loading of persistent symptom trajectories, consistent across traits and cohorts. Variability in univariate genetic association with intermediate trajectories may stem from environmental factors. Multitrait genetics may strengthen depression prediction models, but more diverse data are needed for generalizability.
Grants and funding
- WT_/Wellcome Trust/United Kingdom

Can Genetic Testing Reveal the Right Antidepressant?
Precision medicine: how your dna can determine the best antidepressant..
Updated April 27, 2024 | Reviewed by Lybi Ma
- Find a therapist to overcome depression or anxiety
- Pharmacogenetic testing helps identify the best medications for patients based on their unique genetic makeup.
- This testing reduces trial and error with psychiatric medications
- Testing can lead to reduced side effects and a faster response in depression treatment.
By Joshua Plutchik and Lori Plutchik, M.D.
The treatment of clinical depression presents unique challenges, with many patients voicing concerns that echo a disheartening struggle: "I have tried every medication for depression, and nothing works." Or, "I cannot tolerate the side effects of any antidepressants ." Such sentiments underscore the acute need for more tailored therapeutic strategies in mental health care.
Depression is a deeply debilitating disorder that affects over 21 million American adults annually. The repercussions are severe, leading to an astonishing loss of approximately 200 million workdays and inflicting an economic burden of approximately $20 billion each year. Beyond these stark statistics, the human cost is even more alarming. Depression profoundly erodes the quality of life, severely strains personal relationships, and, in severe cases, can culminate in suicide .
Regarding the need for personalized treatment approaches in mental health, consider a parallel with another medical specialty. Imagine seeking treatment for a swollen, painful knee. How would an orthopedist ensure that treatment effectively addresses the underlying cause? Typically, before suggesting surgery, they would perform a comprehensive assessment, including a physical exam, blood tests, and imaging. Why should psychiatric treatment be any different? Just as we would not rush to surgery for a swollen knee without thorough diagnostics, prescribing antidepressants without a comprehensive understanding of a patient’s genetic profile seems increasingly outdated. Pharmacogenetic testing offers critical data, guiding the selection of treatment strategies with precision.
The last decade has seen transformative advancements in psychiatric pharmacogenetics, thus potentially revolutionizing psychiatric care. Dr. Seema Patel, PharmD, BCPP, a medical science liaison at Genomind, elucidates the profound effect of this testing: "The way that pharmacogenetic testing can help you with your medications is being able to identify based on your unique genetics how you might respond to certain medications and what the risk of side effects would be with certain medications" (S. Patel, personal communication, March 24, 2024).
The Mechanics and Merits of Pharmacogenetic Testing
Pharmacogenetic testing is easily administered through a cheek swab in a clinical setting or at home. Many commercial insurance plans and Medicare now cover this. The concept is both simple and profoundly effective: by examining how individual genetic profiles influence drug metabolism and response, this testing pinpoints which class of antidepressants, such as SSRIs or SNRIs, are likely to be most effective for an individual. In addition, it can determine which specific medications are least likely to cause side effects.
Pharmacogenetic testing is also insightful about how non-pharmaceutical interventions, such as dietary supplements like l-methylfolate or magnesium, might benefit patients. The test even offers personalized insight into the benefits of exercise for mental health based on patients' genetic variants.
Scientific Validation of Pharmacogenetic Testing
The promise of pharmacogenetic testing extends beyond the theoretical realm, with robust research substantiating its efficacy. Dr. Patel cited a meta-analysis by Bousman and colleagues (2019) that indicated patients receiving pharmacogenetic-guided treatment exhibited a 70 percent higher probability of achieving remission when compared with those treated under standard care protocols. This supports earlier findings by Rosenblat and colleagues (2018), whose research demonstrated significantly enhanced response and remission rates in the treatment of depression when treatment was informed by genetic testing.
Further, Dr. Patel referenced a study by Swen and colleagues (2023), which found that pharmacogenetic-guided care reduced the risk of adverse drug reactions by 30 percent compared to traditional methods. Another compelling study by David and colleagues (2021) indicated that pharmacogenetic-guided patients were 50 percent less likely to be hospitalized than those under standard care.
The Future of Pharmacogenetic Testing in Psychiatry: A Holistic and Personalized Approach
It is crucial to note that while pharmacogenetic testing is a powerful tool, it should not be the sole basis for psychiatric treatment decisions. It is a component of a multifaceted approach that includes comprehensive clinical evaluations and ongoing patient monitoring. As research continues to advance and more psychiatrists adopt this technology, the hope is that fewer patients will have to endure the often debilitating journey through ineffective treatments. Pharmacogenetic testing promises a deeper understanding of individual responses to psychiatric medications but also paves the way for more personalized, effective mental health treatment.
Bousman, C. A., Arandjelovic, K., Mancuso, S. G., Eyre, H. A., & Dunlop, B. W. (2019). Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics, 20(1), 37–47. https://doi.org/10.2217/pgs-2018-0142
David, V., Fylan, B., Bryant, E., Smith, H., Sagoo, G. S., & Rattray, M. (2021). An Analysis of Pharmacogenomic-Guided Pathways and Their Effect on Medication Changes and Hospital Admissions: A Systematic Review and Meta-Analysis. Frontiers in Genetics, 12. https://doi.org/10.3389/fgene.2021.698148
Rosenblat, J. D., Lee, Y., & McIntyre, R. S. (2018). The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis. Journal of Affective Disorders, 241, 484–491. https://doi.org/10.1016/j.jad.2018.08.056
Swen, J. J., van der Wouden, C. H., Manson, L. E., Abdullah-Koolmees, H., Blagec, K., Blagus, T., Böhringer, S., Cambon-Thomsen, A., Cecchin, E., Cheung, K.-C., Deneer, V. H., Dupui, M., Ingelman-Sundberg, M., Jonsson, S., Joefield-Roka, C., Just, K. S., Karlsson, M. O., Konta, L., Koopmann, R., & Kriek, M. (2023). A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet (London, England), 401(10374), 347–356. https://doi.org/10.1016/S0140-6736(22)01841-4

Lori Plutchik, M.D., is a distinguished board-certified psychiatrist in New York City, who has been in practice for over 25 years.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Best Family Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
Is Mental Illness Genetic? What the Research Says
Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
:max_bytes(150000):strip_icc():format(webp)/IMG_9791-89504ab694d54b66bbd72cb84ffb860e.jpg)
Daniel B. Block, MD, is an award-winning, board-certified psychiatrist who operates a private practice in Pennsylvania.
:max_bytes(150000):strip_icc():format(webp)/block-8924ca72ff94426d940e8f7e639e3942.jpg)
Adene Sanchez / Getty Images
Is Mental Illness Genetic?
- What the Research Says
- Family History
- Estimating Your Risk
- Reducing Your Risk
Mental illness affects how people behave, think, and feel, which can impact many areas of a person's life, including their ability to work, cope with challenges, and relate to others. If you have a family member with a mental illness, you may wonder whether mental illness is genetic and about your own risk of developing a mental health condition.
Genetics Can Play a Role But Not Always
While researchers have long recognized that mental illnesses tend to run in families, having a family member with a mental disorder is no guarantee that you will have the same condition.
This article explores whether mental illness is genetic and the different factors that affect your overall risk of developing a mental disorder.
The exact causes of mental illness are not fully understood, but genetics appear to be one piece of the puzzle. Researchers have long noted that certain conditions tend to run families, partly because of genetics, but also because of environmental factors such as shared upbringing.
Certain mental health conditions appear to be more closely tied to genetics, and research suggests that there are shared genetic factors that appear to play a part in causing these disorders.
However, genes alone are not responsible for causing mental illness. And no single gene variant could determine with certainty that a person will have a mental illness.
In other words, just because you have family members with a mental disorder does not mean you will develop it.
According to the Centers for Disease Control and Prevention (CDC), mental illness does not have a single cause. Instead, it is often influenced by several factors, including:
- Alcohol or drug use
- Biological factors and abnormalities in the brain
- Experiencing chronic medical conditions
- Social isolation and loneliness
- Traumatic or adverse life experiences
Research on the Genetics of Mental Illness
In one study published in The Lancet , scientists found that certain genetic glitches were associated with five disorders, suggesting a shared underlying genetic vulnerability.
The five disorders are:
- Attention deficit hyperactivity disorder (ADHD)
- Bipolar disorder
- Major depression
- Schizophrenia
These conditions share variations in two genes that influence the development of the cellular structure responsible for regulating the calcium flow in neurons. This calcium flow plays an essential role in neurotransmission.
This part of the brain's circuitry is linked to several essential mental functions, such as attention, thinking, memory , and emotion. Disrupted neurotransmission can result in problems often associated with different mental health conditions.
While such findings are significant, researchers caution that these genetic variations account for only a small portion of the potential risk for mental illness. Other factors also play a part. Environmental factors often interact with genetic predispositions to increase a person's risk.
Such findings may play an essential role in the future treatment of mental health conditions. Rather than looking primarily at symptoms to diagnose a condition, mental health professionals may one day be able to look at the underlying biology of the condition. This may lead to the development of new treatments that are based on a disease's biology and not the symptoms that they have.
Impact of a Family History of Mental Illness
Currently, no genetic tests can determine if you have specific genes or gene combinations that might make you more vulnerable to a specific mental disorder. Instead, looking at your family history may offer clues about your possible risk.
Some mental disorders tend to run in families, so if you have a close relative with a condition, it might mean that your risk is higher.
However, having a family member with a disorder doesn't necessarily mean that you will also develop the condition.
But, understanding the risk may help you be more alert to early symptoms. Recognizing symptoms can lead to earlier treatment and better outcomes.
What's Your Risk of Inheriting a Mental Illness?
Determining your own specific risk is complicated, and estimates vary for different conditions.
Schizophrenia and bipolar disorder are two conditions that are strongly linked to genetics, and one study found that the heritability was 64% for schizophrenia and 59% for bipolar disorder. There was also a significant risk of comorbidity for the two conditions due to the shared genetic effects.
Studies that estimate the relative risk of developing different conditions suggest the following:
Schizophrenia Risk
Below lists the following circumstances and the percent risk (the likelihood of you developing the condition if your circumstance matches what's listed):
- If one of your parents has schizophrenia : 6%
- If both of your parents have schizophrenia : 45%
- If your sibling has schizophrenia : 9%
- If your identical twin has schizophrenia : 40% to 50%
- If your non-identical twin has schizophrenia : 17%
- If an aunt, uncle, or grandparent has schizophrenia : 3%
Risk for Bipolar Disorder
- If one of your parents has bipolar disorder : 5%
- If both of your parents have bipolar disorder : 40%
- If your sibling has bipolar disorder : 5%
- If your identical twin has bipolar disorder : 40% to 70%
- If your non-identical twin has bipolar disorder : 20%
- If an aunt, uncle, or grandparent has bipolar disorder : 5%
However, these are just estimates. More research is needed to better understand genetic risks and other factors that might play a role. The lifetime risk of bipolar disorder or schizophrenia is 1 in 100 (or 1%).
Other conditions, including anxiety and depression , are also tied to genetics, but the inheritance patterns are less clear. Some estimates suggest that if you have a first-degree relative with depression, your risk of developing the condition is around two to three times higher.
Looking at your family may have some predictive value in determining your possible risk for mental illness. However, it is important to note that many people who develop mental health conditions do not have any significant family history of mental illness.
How to Reduce Your Risk of Mental Illness
While there is nothing you can do to change non-modifiable risk factors such as genetics, you can take steps to care for your mental health. Even if you are at a higher risk of developing mental illness, taking care of your well-being, watching for early symptoms, and seeking help when you need it can ensure that you have the care and resources to live your best life.
Protect Your Sleep
Sleep and mental health have a complex relationship. Many mental health conditions can cause problems with sleep, but it is also believed that poor sleep can contribute to the onset of mental illness.
Research has also shown that people with mental health conditions such as schizophrenia, bipolar disorder, and depression experience sleep disturbances at a higher rate than people without these conditions.
Getting enough sleep can benefit your mental health and may help reduce the severity of your symptoms.
Stay Physically Active
Exercise has well-documented physical health benefits, but it can also improve mental health. People who exercise regularly report feeling more energetic, happier, and less anxious than those who don't. Some evidence also suggests that exercise can play an important role in the prevention and treatment of certain mental health conditions, including anxiety and depression.
There are a number of ways that exercise can improve mental health, including by:
- Reducing stress and anxiety
- Boosting mood and self-esteem
- Improving sleep
- Increasing social interaction and feelings of connectedness
- Enhancing cognitive function
You don't have to run a marathon or lift weights to reap the benefits of exercise—even moderate activity, such as walking, can have positive effects.
Eat a Healthy Diet
What you eat can affect your mental health. A healthy diet includes plenty of fruits, vegetables, and whole grains, and limits processed foods, saturated fats, and refined sugars. It also includes whole food sources of proteins, such as legumes, nuts and seeds, grass-fed, pasture-raised organic beef (if any red meat all) and dairy, and pasture-raised organic poultry and eggs.
There is some evidence that certain nutrients, such as omega-3 fatty acids, may be particularly beneficial for mental health. Omega-3 fatty acids are found in fish such as salmon and tuna, as well as in nuts and seeds.
Eating a healthy diet is one way to help protect mental health. While more research is needed, following a diet rich in whole grains, legumes, fruits, and vegetables has been shown to help lower a person's risk for depression.
Cultivate a Strong Support System
Having a strong social support system of family and friends is crucial for maintaining good mental health. A supportive network can provide essential emotional and practical assistance, and can help you cope with life's stresses and challenges.
One way to build a robust support system is to get involved in your community. Connecting with others with similar interests or experiences can help you feel less alone and more connected. There are many ways to get involved in your community, such as through volunteering, joining a club or organization or participating in local events.
You can also stay connected to friends and family members who live far away by using technology such as social media, video chat, and email.
Manage Your Stress
Stress is a part of life. However, it can sometimes become overwhelming or chronic. When stress is constant or severe, it can take a toll on mental and physical health.
There are many ways to manage stress, such as exercise, relaxation techniques, and positive thinking. Finding what works for you is important—what works for one person may not work for another.
Some stress management techniques that may help include:
- Deep breathing
- Getting enough sleep
- Spending time in nature
- Connecting with friends and family
- Hobbies and activities that bring joy
Limit Alcohol and Avoid Substance Use
Drinking too much alcohol or using drugs can worsen mental health problems and make them harder to treat. If you are struggling with a mental health problem, avoiding drugs and alcohol is important for protecting your mental well-being.
If you drink alcohol, it is essential to do so in moderation. For healthy adults, that means up to one drink a day for women and two drinks a day for men.
If you are taking medication for a mental health condition, alcohol can interfere with its effectiveness and may cause negative side effects. It's important to talk to your doctor about your concerns about drinking alcohol while taking medication.
Get Help If You Need It
If you are struggling with a mental health problem, don't hesitate to seek professional help. A mental health professional can provide support, guidance, and treatment. They can diagnose your condition, recommend treatments, and help you develop coping skills that will help you better manage your condition.
While you can't change your genetics, you can take steps to protect your mental health. Adequate sleep, a healthy diet, regular exercise, social support, and stress management are good places to start.
A Word From Verywell
While genetics do appear to influence the risk of developing mental illness, the causes of mental health conditions are complex. Genes account for some risk, but factors such as adverse life events, stress, substance use, chronic medical conditions, and biological factors also play a significant role.
Combinations of genetic factors may elevate risk, and inherited characteristics may also influence how a person responds to different environmental stressors. Recognizing your risk, watching for signs of problems, protecting your mental health, and getting help when you need it can help ensure your well-being and improve outcomes.
Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis . Lancet . 2013;381(9875):1371-1379. doi:10.1016/S0140-6736(12)62129-1
Centers for Disease Control and Prevention. About mental health .
National Institute of Mental Health. Five mental disorders share some of the same genes .
Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study . Lancet . 2009;373(9659):234-239. doi:10.1016/S0140-6736(09)60072-6
Rethink Mental Illness. Does mental illness run in families ?
Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder . Schizophr Bull . 2014;40(3):504-515. doi:10.1093/schbul/sbu016
Shadrina M, Bondarenko EA, Slominsky PA. Genetics factors in major depression disease . Front Psychiatry . 2018;9:334. doi:10.3389/fpsyt.2018.00334
Scott AJ, Webb TL, Rowse G. Does improving sleep lead to better mental health?. A protocol for a meta-analytic review of randomised controlled trials . BMJ Open . 2017;7(9):e016873. doi:10.1136/bmjopen-2017-016873
Hacimusalar Y, Karaaslan O, Misir E, Amuk OC, Hacimusalar G. Sleep quality impairments in schizophrenia and bipolar affective disorder patients continue during periods of remission: a case-controlled study . Sleep Sci . 2022;15(1):47-54. doi:10.5935/1984-0063.20210036
Hu MX, Turner D, Generaal E, et al. Exercise interventions for the prevention of depression: a systematic review of meta-analyses. BMC Public Health . 2020;20(1):1255. doi:10.1186/s12889-020-09323-y
Reimers A, Ljung H. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders . Ther Adv Psychopharmacol . 2019;9:2045125319858901. doi:10.1177/2045125319858901
Nicolaou M, Colpo M, Vermeulen E, et al. Association of a priori dietary patterns with depressive symptoms: a harmonised meta-analysis of observational studies . Psychol Med . 2020;50(11):1872-1883. doi:10.1017/S0033291719001958
National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined .
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 08 June 2021
Gene expression studies in Depression development and treatment: an overview of the underlying molecular mechanisms and biological processes to identify biomarkers
- Nicole Mariani ORCID: orcid.org/0000-0001-7918-3492 1 ,
- Nadia Cattane 2 ,
- Carmine Pariante ORCID: orcid.org/0000-0002-9132-5091 1 &
- Annamaria Cattaneo ORCID: orcid.org/0000-0002-9963-848X 2 , 3
Translational Psychiatry volume 11 , Article number: 354 ( 2021 ) Cite this article
11k Accesses
37 Citations
1 Altmetric
Metrics details
A combination of different risk factors, such as genetic, environmental and psychological factors, together with immune system, stress response, brain neuroplasticity and the regulation of neurotransmitters, is thought to lead to the development of major depressive disorder (MDD). A growing number of studies have tried to investigate the underlying mechanisms of MDD by analysing the expression levels of genes involved in such biological processes. These studies have shown that MDD is not just a brain disorder, but also a body disorder, and this is mainly due to the interplay between the periphery and the Central Nervous System (CNS). To this purpose, most of the studies conducted so far have mainly dedicated to the analysis of the gene expression levels using postmortem brain tissue as well as peripheral blood samples of MDD patients. In this paper, we reviewed the current literature on candidate gene expression alterations and the few existing transcriptomics studies in MDD focusing on inflammation, neuroplasticity, neurotransmitters and stress-related genes. Moreover, we focused our attention on studies, which have investigated mRNA levels as biomarkers to predict therapy outcomes. This is important as many patients do not respond to antidepressant medication or could experience adverse side effects, leading to the interruption of treatment. Unfortunately, the right choice of antidepressant for each individual still remains largely a matter of taking an educated guess.
Similar content being viewed by others

Major depressive disorder: hypothesis, mechanism, prevention and treatment

The serotonin theory of depression: a systematic umbrella review of the evidence

Cell subtype-specific effects of genetic variation in the Alzheimer’s disease brain
Introduction.
Major depressive disorder (MDD) is a complex psychiatric disorder characterized by low mood, anhedonia, feelings of guilt or low self-worth, disturbed sleep or appetite, low energy and suicidal ideation 1 , 2 . It is one of the main causes of disability worldwide and is a major contributor to the overall global burden of disease 3 .
The combination of genetic, environmental and psychological factors is believed to be the cause of MDD. In fact, for instance, environmental factors, such as stressful and traumatic events, can affect not only biological systems restricted to the brain, but also pathophysiological pathways within the entire body 4 , 5 . Well-established evidence suggests deregulation in the inflammatory response, in the hypothalamus-pituitary-adrenal (HPA) axis and in several neuronal systems in the pathogenesis of MDD 6 , 7 . As such, acute and chronic stress have been proposed to trigger the dysregulation of these systems and to lead to the development of MDD 8 . Hence, biological systems such as immune system, stress response, brain neuroplasticity and the regulation of neurotransmitters seem to be the ones more involved in MDD.
To date, different approaches have been used to understand the molecular mechanisms underlying MDD. Among the others, gene expression is being used in a large number of studies to analyse the expression of dozens of genes in MDD.
To this purpose, most of the studies conducted so far have mainly investigated the gene expression levels using postmortem brain tissue 9 as well as peripheral blood samples of MDD patients. While the use of brain tissue is limited and has several limitations due to the influence of agonal and postmortem factors on gene expression levels 10 , the use of peripheral blood samples seems to have multiple advantages. Indeed, peripheral blood samples allow to collect large sample sizes, to obtain a fast RNA stabilization, as well as the isolation of specific cell subtypes, such as peripheral blood mononuclear cells (PBMCs) or leukocytes and to monitor the patients’ well-being.
The association between the brain and the periphery has been demonstrated several years ago by Sullivan and colleagues 11 , who have shown genes shared among whole blood and 16 brain tissues, where 60% of transcripts were expressed in the whole blood and in at least one tissue of the central nervous system (CNS). In detail, both whole blood and brain tissues have similar expression of genes relevant to MDD such as genes encoding for neurotransmitter receptors and transporters, growth factors, hormones and cytokines. In addition to these data, transcriptional profiling in peripheral blood has allowed the discovery of possible biomarkers for patients with psychiatric and neurological disorders including patients affected by MDD 12 , 13 , 14 .
Based on this, we reviewed the current literature on candidate gene expression in MDD, mainly focusing on genes related to inflammation, neuroplasticity, neurotransmitters, stress response and treatment outcomes. We have also included a few existing transcriptomics studies, which identified changes in gene expression levels by using a hypothesis-free approach.
Blood gene expression alterations in MDD have been already reviewed in 2013 15 by our group. Although in the paper by Hepgul et al. we focused on inflammation, GR functionality and neuroplasticity, we did not report gene expression studies in relation to treatment outcome. Since in these last years a large body of studies has investigated gene expression alterations in association with MDD from 2013 to date, also including treatment outcomes, we have seen the need for a more up-to-date review.
Inflammation-related genes
In recent years, several studies have suggested an increased inflammatory response in MDD, indicated by altered levels of pro- and anti-inflammatory cytokines 16 , 17 . Furthermore, other studies have linked several autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, multiple sclerosis and inflammatory bowel diseases, with MDD, suggesting a very strong relationship between inflammation and MDD 18 , 19 . However, although it is well known that depression can influence immune responses and vice versa, the underlying molecular mechanisms are still unclear.
Among all the molecules involved in the immune response, cytokines, known as chemical messengers between immune cells, represent the most important key players in mediating depressive symptoms. They include various groups of molecules produced, upon stimulation by pathogens or dysfunctional cells, by immune cells of the periphery as well as cells of the central nervous system such as microglia, astrocytes, oligodendrocytes. Moreover, also neurons can release cytokines and chemokines as well as respond to them through cytokine and chemokine receptors 20 .
For this reason, also taking into account that cytokines can cross the blood–brain barrier 21 , they may represent a potentially useful biomarker resource relating to mood disorders.
Several components of the immune system, including the Toll-like receptors (TLRs), their intracellular signaling molecules and their related pro-inflammatory transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and interferon regulatory transcription factor 3 (IRF3) play crucial roles in the production of pro-inflammatory cytokines, including Interleukin (IL)-1b and IL-18 22 .
To investigate the role of inflammation in MDD, several studies available so far have measured the mRNA levels of genes involved in inflammation in the peripheral blood and postmortem brain tissues of patients with MDD (see Table 1 ). For example, the study conducted by Rizavi et al. in 2016 23 indicated an increased expression of pro-inflammatory cytokines and their receptors in the lymphocytes of depressed patients as compared to control subjects, proposing an abnormal expression not only of genes encoding for pro-inflammatory cytokines, but also of genes encoding for their membrane-bound receptors in MDD. Moreover, Momeni et al. 24 showed higher mRNA levels of an adaptor protein (ASC), correlated with absent in melanoma 2 (AIM2) gene, in peripheral blood of depressed patients. AIM2 is a component of inflammasomes, which can trigger caspase-1 via ASC following a pathogen-associated molecular pattern (PAMP) or danger-associated molecular pattern (DAMP) recognition. Therefore, the activation of caspase-1 can trigger the induction of IL-1 and IL-18, two important pro-inflammatory cytokines. Similarly, the Genome-Based Therapeutic Drugs for Depression (GENDEP) project showed that the mRNA expression of inflammation-related genes, such as IL-1b, macrophage inhibiting factor (MIF) and tumor necrosis factor (TNF) are higher in non-responders depressed patients before treatment 25 .
In contrast, Spindola et al. 26 have investigated MDD in childhood and adolescence, analysing the mRNA expression of 12 genes including some inflammation-related genes. Interestingly, TNF, TNFR1 and IL-1b were expressed at significantly lower levels in the MDD group when compared with healthy controls suggesting that the regulation of inflammatory response might play a key role in early MDD pathophysiology. However, it has been proposed that findings in adults can differ from those in children 27 . In fact, factors such as traumatic events, abuse of alcohol and smoking identified in adulthood but not in childhood could affect MDD in adults.
Of course, the activation of the immune system observed in patients with MDD is not limited to changes in cytokines production. In fact, it has been postulated that oxidative stress, a trigger of inflammation, has an important role in the pathogenesis and neuroprogression of MDD 28 . In physiological conditions, multiple defence systems are involved in protecting cells from damage by reactive oxygen species (ROS). The main antioxidative enzymes (AOEs) include copper-zinc and manganese superoxide dismutase (CuZnSOD and MnSOD, respectively), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GLR) 29 , 30 . Antioxidant protection is tightly regulated by redox-sensitive transcriptional factors such as the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) 31 , 32 and NF-κB 33 , 34 . In this regard, Lukic et al. 35 provided evidence that MDD is characterized by an upregulation of redox-sensitive transcriptional factors (Nrf2 and NF-κB) and AOEs (MnSOD, CuZnSOD and CAT), indicating a pro-oxidative state in the PBMC of MDD patients. Specifically, they found higher mRNA levels of Nrf2 and its regulator Keap1, as well as NF-κB in the cytoplasm of PBMC of depressed patients as compared to controls. This state was further reflected by increased levels of MnSOD, CuZnSOD and CAT proteins and by the lack of correlation between MnSOD and CAT, which, according to the authors’ hypothesis, could indicate impaired oxidative detoxification capacity in MDD patients. Moreover, the authors found a positive correlation between increased levels of MnSOD, CuZnSOD and CAT in MDD patients and the levels of Nrf2, while increased levels of SODs were also positively related to NF-κB. These findings suggest that alterations in antioxidative defence systems lead to an alteration in the pro-inflammatory signalling found in MDD.
Recently, it has also been reported that the neurotransmitter serotonin (5-HT) can regulate the immune system. Peripheral 5-HT is a potent immune modulator and affects immune cells via its receptors and the recently identified process of serotonylation, an independent mechanism by which serotonin leads to the activation of intracellular processes 36 . Based on this, Amidfar et al. 37 measured the relative expression levels of 5-HT2A and 5-HT3A receptors in PBMCs of patients with MDD, and found that depressed patients have higher 5-HTR2A mRNA levels than healthy subjects.
Finally, in the Biodep study 38 we have recently shown that drug-free and treatment-resistant depressed patients not only have higher pro-inflammatory cytokines/chemokines, but we have also shown an increased expression of the P2X purinoceptor 7 (P2RX7). P2RX7 has a crucial role in the activation of the inflammatory processes and it is ubiquitously expressed among cells of the immune system, including microglia cells 39 . Additionally, its expression has been identified in neuronal cells, where it can regulate the function of different neurotransmitters relevant to MDD 40 .
Overall, these studies have shown a positive correlation between an upregulated expression of pro-inflammatory molecules and MDD, suggesting that inflammation is one of the key factors involved in the pathogenesis and progression of MDD. Moreover, these studies suggest the utility of inflammation-related gene expression levels as biomarkers for MDD treatment response.
Neuroplasticity
In addition to increased inflammatory levels, to date, several studies have demonstrated an impairment of neuroplasticity in MDD 41 , 42 . For example, alterations in synaptic and morphological plasticity have been reported in patients with MDD 43 , 44 , 45 . Numerous studies have also tried to understand the intracellular mechanisms underlying these alterations and their role in MDD (see Table 2 ). Evidence indicates that multiple neurotrophic/growth factors, such as brain-derived neurotrophic factor (BDNF) and glial cell-line-derived neurotrophic factor (GDNF) play a key role in neural plasticity 46 , 47 . BDNF is, in fact, involved in proliferation, migration, differentiation and survival of neurons in humans 48 . This finding has been confirmed by Hong et al. 49 , who examined the mRNA levels of BDNF and the mitogen-activated protein kinase 1/2 (MEK1/2), an immediate activator of the MEK–ERK pathway mediated by BDNF, in the leukocytes of MDD patients and healthy controls. Interestingly, the authors have shown decreased mRNA levels of BDNF and MEK1 in depressed patients as compared with controls, supporting the involvement of BDNF and MEK1 in the pathogenesis of MDD.
Furthermore, vascular endothelial growth factor (VEGF), a neurotrophic and an angiogenic growth factor, has been implicated in different physiological processes such as angiogenesis, neuroprotection, neuronal survival, regeneration, growth, differentiation and axonal outgrowth 50 , 51 , 52 , 53 . Different studies have proposed that changes in VEGF expression levels can be linked to mood disorders, including MDD 54 , 55 .
A well-known oxygen-sensitive transcriptional activator of VEGF, the hypoxia inducible factor-1 (HIF-1), is induced by hypoxia, ischemia and by the activation of the expression of different genes such as VEGF, erythropoietin (EPO), glucose transporter-1,3 (GLUT1,3), lactate dehydrogenase-A (LDHA), phosphoglycerate kinase 1 (PGK1), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-3 (PFKFB3), insulin-like growth factor-2 (IGF-2) and BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNip3). Moreover, it contributes to angiogenesis, erythropoiesis, glucose metabolism, cell proliferation/survival and apoptosis 56 , 57 . According to this background, Shibata et al. 58 investigated the mRNA expression levels of HIF-1 (α and β) and its target genes (VEGF, GLUT1, PGK1, PFKFB3 and LDHA) in peripheral white blood cells of patients affected by MDD and bipolar disorder (BPD). The authors found increased expression levels of HIF-1, VEGF, PFKFB3, GLUT1, PGK1 and LDHA in MDD subjects as compared to the control group.
Moreover, the neuronal membrane glycoprotein M6a (GPM6A), a member of the myelin proteolipid protein (PLP/DM20) family, plays an important role in stress response in different animal models 59 , 60 . Based on this notion, Fuchsova et al. 61 hypothesized that alterations in the expression of the stress responsive neuroplasticity-related genes, such as the members of the PLP family, could be involved in the aetiology of MDD. They demonstrated that, GPM6A mRNA levels were significantly reduced in the hippocampus of depressed suicides. Conversely, GPM6B, but not PLP1, was downregulated. All these findings suggest that changes in the balance between mRNA levels of all the studied genes could lead to significant alterations in the neuronal connectivity causing pathological behaviours. According to the authors, these findings suggest that reduced GPM6B expression could contribute to oligodendrocyte misfunction linked with MDD.
Several studies have also suggested that the Transcription factor 4 (TCF4) gene is involved in the early differentiation of neurons, is related to memory efficiency 62 , and affects the immune response of the brain 63 , 64 . Mossakowska-Wójcik et al. 65 analysed the mRNA and protein levels of TCF4 in blood of MDD patients and healthy subjects. TCF4 expression at both the mRNA and protein level was decreased in patients with MDD when compared with controls, suggesting that reduced mRNA and protein levels of the TCF4 gene might result in the worsening of cognitive functions, which could alter the development or course of MDD.
Furthermore, in 2012 Ziemiańska et al. showed that neuropsin (NP), a kallikrein gene-related endoprotease, has an important role in neuroplasticity processes, including intracellular signal cascades and regulation of gene expression that are involved in long-term synaptic plasticity 66 . In this regard, Bobińska et al. 67 have compared the gene expression levels of NP gene in peripheral blood samples of a group of MDD patients and healthy subjects, showing that the expression levels of the human NP gene were significantly higher in MDD patients than in controls. According to the authors, a possible explanation of these results could be the young age of the examined individuals in both groups, in fact other studies have shown that NP expression levels gradually decrease in the cerebral cortex during ageing 68 .
Altogether, the presented studies have shown that a dysregulation of neurotrophic/growth factor systems such as BDNF and VEGF as well as of other genes involved in the regulation of neuroplasticity can underlie the development of cognitive impairment, often observed in MDD.
Neurotransmitters
Research over the years has attempted to define the relationships between specific neurotransmitters in the brain and specific symptoms of MDD. Indeed, it has been proposed that different neurotransmitters may regulate different brain functions, neurochemical mechanisms, and subsequently, specific antidepressant drugs could target symptom-specific neurotransmitters 69 . MDD has been widely linked to imbalances in the brain with regard to the neurotransmitters serotonin, norepinephrine and dopamine and, recently, another neurotransmitter, glutamate, has been also implicated in MDD.
Interestingly, it has been shown that the release of some neurotransmitters, including the release of noradrenaline, serotonin, GABA, glutamate, and dopamine, is facilitated by the activated a-7 nicotinic acetylcholine receptor (a7 nAChR) via the increased permeability to cations, including Ca(2+) 70 . A7 nAChR is coded by the Cholinergic Receptor Nicotinic Alpha 7 Subunit (CHRNA7) gene, which is partially duplicated by a chimeric gene, CHRFAM7A. On these bases, Kunii et al. 71 (Table 3 ) have investigated the expression of CHRNA7 and CHRFAM7A in the dorsolateral prefrontal cortex in a large cohort of patients with schizophrenia, BPD and MDD. They found that the expression levels of CHRNA7 were significantly increased in MDD patients as compared with all other groups. Similarly, the expression of CHRFAM7A was significantly elevated in all diagnostic groups, especially in the MDD group, as compared with the healthy group and the ratio of CHRFAM7A/CHRNA7 levels was significantly different between the diagnostic groups, suggesting an aberrant function of nAChRs in mental illnesses.
Moreover, norepinephrine has a role in the recognition and response to stressful situations, and it has been suggested that an aberrant norepinephrinergic system could lead to an increased vulnerability to MDD 72 . Dopamine plays an important role in regulating our drive to seek out rewards, as well as our ability to obtain a sense of pleasure. Low dopamine levels could help to explain why people suffering from MDD do not show the same sense of pleasure 73 . 5-HT is a monoamine involved in a number of physiological processes, and MDD appears, in part, to be a result of diminished activity of the serotonin system 69 . 5-HT is both a neurotransmitter and a neuromodulator that regulates different pathophysiological aspects of MDD, including mood, sleep, energy balance and immunity 74 , 75 , 76 .
While the role of these three neurotransmitters (norepinephrine, dopamine and 5-HT) in MDD has been studying for many years 77 , the implication of glutamate in this psychiatric disorder has been recently discovered. Indeed, a growing body of data shows that abnormalities of the glutamate system lead to altered behaviours that correlate with psychiatric disorders, including MDD 78 . Glutamate is an excitatory neurotransmitter that is widely distributed in the brain, exerting its effects through the stimulation of several glutamate receptor (GluR) subtypes. These include the 2-amino-3-(3-hydroxy-5-methyl-iso- xazol-4-yl) propanoic acid (AMPA), N-methyl-D-aspartate (NMDA), kainate (KAR) and metabotropic (mGluR) receptors 79 . Four studies have mainly analysed mRNA expression in postmortem brain tissues of glutamate receptors and transporters (Table 3 ).
For instance, Gray et al. 80 have tested the hypothesis that GluR gene expression is altered in the dorsolateral prefrontal cortex (DLPFC) in MDD in a large cohort of postmortem subjects from three diagnostic groups: MDD suicide, MDD non-suicide and a group of controls with no history of psychiatric disorders. They have reported higher expression levels of a number of GluR genes in the DLPFC of MDD patients as compared to controls. In particular, they have found higher expression levels of GRIN1, GRIN2A-D, GRIA2-4, GRIK1-2, GRM1, GRM4, GRM5 and GRM7 in female patients as compared to male patients with MDD. In contrast, GRM5 expression levels were lower in male MDD patients than in male controls and, finally, in all sample (both male and female) when MDD suicides were compared with MDD non-suicides, GRIN2B, GRIK3 and GRM2 were expressed at higher levels in the suicide subjects. Taken together, these data indicate that a disruption of the glutamate system occurs in the DLPFC of patients with MDD, above all in those who completed suicide. According to the authors, this disruption may be more severe in female patients.
In addition, because of several studies indicate that the locus coeruleus (LC) has a major role in the origin of clinical MDD and possibly suicide, Chandley et al. 81 examined the gene expression levels of glutamate receptors, NMDA and AMPA in postmortem noradrenergic LC neurons from subjects with MDD (most of which died by suicide) and matched to healthy controls. They evaluated the expression of all NMDA receptor subunit genes in the LC and for the remaining glutamate receptor genes, including the AMPA, kainate and metabotropic glutamate receptors, examining only those that demonstrated measurable gene expression in the mouse LC, according to the Allen Brain Atlas, an online publicly available resource that integrates gene expression and connectivity data with neuroanatomical information for the mouse, human and non-human primate 82 , 83 . They found elevated expression levels of genes encoding specific ionotropic NMDA receptor subunits and specific metabotropic receptors in both MDD and control subjects. Specifically, the authors found highly expressed GRIN1 subunit, moderate gene expression levels of GRIN2A, GRIN2B, GRIN2D subunits and lower levels of GRIN2C and GRIN3A subunits. The functional NMDA receptor complex is made of a glycine binding NR1 subunit combined with at least one of the other glutamate binding NR2 or NR3 subunits. Although the NMDA receptor complex is permeable to both potassium and calcium, calcium is essential in activating the PI3K and CREB cell-signalling pathways that distinguish the NMDA family of receptor signalling from the other ionotropic glutamate receptors 84 , 85 . This is particularly intriguing since, in the same work, Chandley and colleagues 81 observed elevations in NMDA receptor subunit gene expression in MDD patients when compared to controls, but no expression changes in the moderately expressed GluA1 receptor (GRIA1) or the highly expressed GluA2 (GRIA2) and GluA4 (GRIA4) of the AMPA ionotropic family, nor in any of the receptor subunits (GRIK1, GRIK3 and GRIK5) from the kainate ionotropic class of receptors. Moreover, they have found an increase in expression levels of two metabotropic glutamate receptor genes (GRM5, GRM4) in LC neurons from MDD subjects in comparison to normal control subjects.
Earlier, in 2013, the same authors 86 examined the expression of three glutamate-related genes (two glutamate transporters, SLC1A3 and SLC1A2, and an encoding glutamine synthase GLUL) concentrated in glia, and of a glia gene (GFAP) in postmortem tissues from men with MDD and from matched healthy controls. They found evidence of astrocyte dysfunctions in the LC region in individuals with MDD, which included reduced expression levels of SLC1A3, SLC1A2 and GFAP, together with lower GFAP protein levels, and reduced density of GFAP-positive astrocytes. This study provided a direct evidence of astrocyte pathology in LC, indicating that glia cell abnormalities reported in more superior/rostral brain regions 43 , 87 extend to the brainstem and may contribute to the pathology of the monoamine systems in MDD. Similarly, Oh et al. 88 studied the role of the glutamate transporters (SLC1A2 and SLC1A3) in the dorsolateral prefrontal cortex of MDD subjects. Using data from the Stanley neuropathology consortium integrative database (SNCID 89 ), they analysed the mRNA levels of the gamma-aminobutyric acid-synthesizing enzyme (GAD1) and investigated a possible linkage between changes in SLC1A2 and GAD1 expression levels. They observed that the expression levels of GAD1 and SLC1A2 were lower in the DLPFC of subjects with MDD as compared to controls and, that GAD1 mRNA levels were significantly associated with SLC1A2 mRNA expression levels in the same area in the group of MDD patients.
All the above-mentioned studies have demonstrated the involvement of several neurotransmitters in the pathogenesis of MDD. Particularly, they not only have consolidated the role of serotonin, dopamine and norepinephrine, but also shown abnormalities of the glutamate system. In fact, these studies have observed that the pathophysiology of MDD is associated with dysfunctions in the glutamatergic system, and with alterations in the mechanisms regulating the clearance and metabolism of glutamate in brain areas mediating cognitive–emotional behaviours.
Stress-related mechanisms
Stress and/or trauma are associated with dramatic increases in the risk of developing depressive disorders 90 . The stress response system is linking the CNS and the endocrine system and it allows responding to short-term and long-term stressors. The key neuroendocrine component of this response to stress is the HPA axis, which acts as an interface between cognitive and non-cognitive stressors processed in the CNS and in the peripheral endocrine response system 91 . To understand the mechanisms of stress response, several studies have assessed the mRNA levels of genes involved in the stress response in patients with MDD (see Table 4 ). It is well known that the glucocorticoid receptor (GR) plays a crucial role in mediating the negative feedback regulation of the HPA axis 92 , 93 and, recently, several studies have investigated the GR expression levels and functionality in patients with MDD. To this purpose, Roy et al. 94 have studied the mRNA levels of stress-related genes, such as BDNF, Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1 or GR), FK506 Binding Protein 5 (FKBP5), Corticotropin Releasing Hormone Binding Protein (CRHBP), and Corticotropin Releasing Hormone Receptor 1 (CRHR1) in PBMC of MDD patients and their matched controls. NR3C1 encodes the GR, which can function both as a transcription factor that binds to glucocorticoid responsive elements (GRE) in the promoters of glucocorticoid responsive genes by activating their transcription, and as a regulator of other transcription factors. FKBP5 is a co-chaperone of hsp90, which regulates GR’s sensitivity, whereas BDNF expression is regulated by GR. The authors have found a reduction in the expression levels of most of the analysed genes, including BDNF, FKBP5 and NR3C1 in MDD patients as compared to controls, confirming that lower expression levels of these transcripts may induce a maladaptive response toward stressful stimuli, increasing the risk for MDD.
Similarly, Iacob et al. 95 analysed the expression levels of glucocorticoid and mineralocorticoid receptors, respectively, NR3C1 and NR3C2, and also genes related to the glucocorticoid pathway as oxytocin prepropeptide encoding gene (OXT) and oxytocin receptor (OXTR). They observed that MDD patients showed increased expression levels of OXTR and confirmed deregulation in the oxytocinergic signalling, referring to signalling pathway proteins including oxytocin, oxytocin receptors and related regulatory factors.
Another important gene involved in the mediation of the glucocorticoid effects on brain function is a serine/threonine kinase (Serum/Glucocorticoid Regulated Kinase 1 (SGK1)), which plays a key role in the cellular response and neuronal functions, including adult hippocampal neurogenesis 96 . In fact, Anacker et al. 97 found an increase in the SGK1 gene expression levels in the peripheral blood of drug-free depressed patients, identifying SGK1 as a key gene involved in the GR activation, which may be of particular relevance for stress-induced mental disorders, such as MDD.
To assess the hypothesis that stress is associated with MDD, Teyssier et al. 98 measured the expression of a set of candidate biomarkers in peripheral blood leukocytes. These genes are FOS and DUSP1 (involved in the cell-signalling response to biopsychological stress), TERT, STMN1 and p16INK4a (biomarkers of telomere dysfunction and cellular senescence), and OGG1 (which catalyses the repair of oxidized 8-oxoguanine DNA base and is a sensible marker of oxidative stress). The OGG1, p16INK4a and STMN1 genes were significantly upregulated in the leucocytes of MDD patients when compared to controls, indicating an association between the upregulation of these transcripts and the increased risk of developing MDD.
Although overall it has been shown that depressed patients show altered expression levels of stress-related genes in peripheral blood samples, some of the previously mentioned studies highlighted also the presence of contrasting results that could be due to the patients’ pharmacological treatment. However, this should be better investigated in further studies.
Antidepressant therapy is an essential treatment for MDD, however, a substantial group of treated patients do not respond to the therapy, or suffer from severe side effects, such as gastrointestinal (GI) disturbances, anxiety, agitation and insomnia 99 . To date, different studies have been carried out to identify and validate biomarkers involved in the antidepressant treatment response (Table 5 ). This might open the door to personalized medication and, thus, might improve treatment efficacy and reduce side effects.
In order to provide evidence supporting a personalized medicine approach for the treatment of MDD, Cattaneo et al. 25 analysed the blood mRNA expression levels of 15 candidate genes across three biological systems, such as the GR complex, inflammation and neuroplasticity that have been more consistently described as abnormal in MDD 100 . To this purpose, they examined a well-characterized group of MDD patients from the GENDEP study 101 , 102 , before and after 8 weeks of treatment with one of two pharmacologically different antidepressants: the selective serotonin reuptake inhibitor, escitalopram and the tricyclic noradrenaline reuptake inhibitor, nortryptline. Cattaneo and her team measured the transcriptional levels of the following genes: FKBP-4, FKBP5 and GR, for the GR complex; IL-1a, IL-1b, IL-4, IL-6, IL-7, IL-8, IL-10, MIF and TNF-a, for the inflammatory system; BDNF, p11 and VGF (non-acronymic), for neuroplasticity. Data showed a dissociation between genes that predict treatment response (‘predictors’) and genes that change longitudinally in patients who respond (‘targets’) to antidepressant treatment. Specifically, among the 15 genes, only higher levels of three inflammation-related genes, IL-1b, MIF and TNF-a, predict a lack of response to antidepressants, even if a successful antidepressant response is not associated with a reduction in the levels of these genes. In contrast, a successful antidepressant response is associated with a reduction in the levels of the inflammation-related gene, IL-6, and of the GR-associated gene, FKBP5, as well as with an increase in the neuroplasticity-associated genes, VGF and BDNF.
Following this study, our group has carried out the largest non-interventional study so far investigating candidate mRNA gene expression in depressed patients characterised by their current depressive symptoms and by their response to antidepressant treatment 38 . As previously mentioned, we have found that treatment-resistant and drug-free depressed patients have an increased inflammasome activation (higher pro-inflammatory cytokines/chemokines and P2RX7 mRNAs expression) and glucocorticoid resistance (lower GR and higher FKBP5 mRNAs expression); whereas responsive patients were alike controls except for having lower CXCL12.
According to the neurotrophic hypothesis of MDD, an association between effects on neuroplasticity and clinical response to antidepressant drug therapy has been suggested by several studies. For example, Breitfeld et al. 103 have tried to identify a possible association between functional biomarkers related to neuroplasticity effects of antidepressants with treatment response and resistance in patient-derived lymphoblastoid cell lines (LCLs) from the STAR*D study. Specifically, they identified five potential biomarkers that have been associated with cell proliferative effects of antidepressants (ex vivo) or with LCL donor’s clinical response/remission in antidepressant drug therapy: transcription factor 7-like 2 (TCF7L2), frizzled class receptor 7 (FZD7), wingless-type MMTV integration site family member 2B (WNT2B), p-glycoprotein (ABCB1) and sulfotransferase 4A1 (SULT4A1). Interestingly, the most notable differences in the expression levels between responder- and treatment resistance-derived LCLs were observed for WNT2B, FZD7 and ABCB1. ABCB1 is the most studied member of the ATP-binding cassette (ABC) transporter family and it plays a key role in cellular detoxification and transmembrane transport across the blood–brain barrier. The allocrite spectrum includes neurotoxic agents (such as glucocorticoids, drugs and xenobiotics) and hence, ABCB1 has neuroprotective effects resulting in a possible increased response to antidepressants. WNT2B and FZD7 are elements of the canonical WNT signalling pathway regulating neurogenesis, synaptic plasticity and dendritic arborization 104 . While FZD7 inhibits the WNT signalling, WNT2B and chronic antidepressant treatment activate this pathway resulting in increased neurogenesis. Altogether these effects might be responsible for enhanced neuronal plasticity and likely for remission from MDD.
Moreover, the serotonin transporter has been linked to MDD in candidate gene studies and in gene-to-environment interaction studies, hence it plays a key role in MDD pathophysiology 105 , 106 . The serotonin transporter protein (SLC6A4) is the main target of many antidepressants, although the relationship between pathophysiology and therapeutic effects of antidepressants is still not clear 107 , 108 . Based on previous studies on SLC6A4 mRNA gene expression variation in peripheral tissues, Belzeaux et al. 109 explored whether SLC6A4 mRNA could be a target biomarker of antidepressant treatment during a major depressive episode that varies between the baseline and the 30-week follow-up period in responder patients. Interestingly, decreased expression levels of SLC6A4 were observed in responder patients across a 30-week follow-up, whereas non-responder subjects showed increased mRNA levels of SLC6A4. Conversely, healthy controls exhibited a stable pattern of SLC6A4 mRNA expression across the 30-week follow-up period. These data support that the serotonin transporter protein, the main target of many antidepressants, could be a valid target biomarker in MDD patients for a personalized medicine approach.
As suggested by our results in responder and non-responder patients, gene expression variation of selected genes, monitored across a long period of time, could be informative of clinical evolution and potential relapses or recurrences.
Another interesting hypothesis of MDD suggests that the inflammasome is a central mediator by which psychological and physical stressors could contribute to the development of the disorder 110 . In this regard, the study performed by Alcocer-Gómez et al. 111 examined this hypothesis to determine whether NLRP3 inflammasome could be activated in PBMC from MDD patients and to shed light on the implication of mitochondrial oxidative stress. Furthermore, they studied the effects of amitriptyline, a tricyclic antidepressant drug, on NLRP3 inflammasome activation. The authors found that MDD patients showed reduced serum levels of IL-1b and IL-18, and a significant reduction in NLRP3 and caspase-1 activation. Moreover, they observed that the association between the Beck’s Depression Inventory (BDI) scores and IL-1b and IL-18 serum levels was reduced when controlling for antidepressant treatment, suggesting that antidepressants can modulate the inflammation levels.
Another promising candidate in the field of pharmacological treatment options regarding MDD is represented by the mitochondrial translocator protein (TSPO), a 5-helical transmembrane protein located in the outer mitochondrial membrane 112 . It plays an important role in neurosteroid synthesis and in systemic endocrine regulation, with implications in the pathophysiology of immune, inflammatory, neurodegenerative, neoplastic and psychiatric diseases 113 . Interestingly, Sarubin et al. 114 investigated the effects of antidepressant treatment on TSPO expression levels in platelets obtained from 37 patients suffering from MDD, analysing TSPO levels in depressed patients before and after 6 weeks of antidepressant treatment. A significant change in TSPO levels over 6 weeks of treatment was observed within the complete sample of MDD patients. Interestingly, responders showed a greater reduction in TSPO levels as compared to non-responders. These results are in contrast with the hypothesis of the authors, expecting to find increased TSPO levels during antidepressant therapy along with a decrease in depressive symptoms. Therefore, they concluded that TSPO expression in platelets cannot be considered an appropriate biomarker for the analysis on the course of MDD.
Overall, the above studies have shown that patients who responded to the antidepressant therapy had restored levels of inflammation-related genes, such as IL-6 and IL-1b, of stress-related genes, including FKBP5, as well as of neuroplasticity-associated genes, such as VGF and BDNF.
Whole-genome transcriptome assays
High-throughput technologies such as microarrays allow to explore the expression levels of the whole genome and the identification of changes in gene expression by using a hypothesis-free approach. In the last decade, several studies have used these technologies to identify gene expression differences related to MDD (Table 6 ). Together with the hypothesis-driven approach, mainly based on the analysis of candidate genes expression levels, transcriptomics studies can allow the identification of new biomarkers associated with MDD that can help the development of novel intervention strategies and the introduction of personalized medicine.
Recently, Hepgul et al. 115 investigated whether gene expression changes in peripheral blood of patients with Hepatitis C at the baseline are associated with the future development of IFN-α-induced MDD (before IFN-α administration) and identified longitudinal changes in gene expression from baseline to treatment week (TW) 4 and TW24 following IFN-α treatment, in those subjects who did or did not develop MDD. Specifically, at the baseline, 73 genes were differentially expressed between patients who later developed MDD and those who did not. At TW4, 592 genes, primarily IFN-α-responsive genes, were significantly modulated in the whole sample; most of these genes were modulated only in patients who developed MDD, with an enhancement in inflammation-, neuroplasticity- and oxidative stress-related genes. Similar results were observed at TW24. These data clearly indicate that patients who develop IFN-α-induced MDD have an augmented biological sensitivity to IFN-α. Beyond the IFN-α treatment, the identified transcriptomics signature could be used as a biomarker for the early identification of individuals at high risk of developing MDD or to generate molecular targets for the discovery of new therapeutic strategies in MDD.
Another microarray study carried by Hennings et al. 116 , performed on peripheral blood samples collected at the admission and after 2 and 5 weeks of treatment from MDD male patients remitters and non-responders, identified 127 transcripts significantly associated with the treatment response. The authors also analysed these transcripts in an independent replication sample of 142 depressed in patients confirming that lower expression of retinoid-related orphan receptor alpha (RORα), germinal centre expressed transcript 2 (GCET2) and chitinase 3-like protein 2 (CHI3L2) on admission was associated with beneficial treatment response. In addition, leukocyte-specific protein 1 (LSP1) significantly decreased after 5 weeks of treatment in MDD responder patients.
Furthermore, in another interesting study, Duric et al. 117 provided new evidence that disruption of synaptic and glutamatergic signalling pathways contributes to the pathophysiology of MDD by examining the genetic profile of micro-dissected subfields of postmortem hippocampus from MDD subjects. The authors found a significant dysregulation of synaptic function/structure related genes Synaptosome Associated Protein 25 (SNAP25), Disks large homolog 2 (DLG2), Microtubule-associated protein 1A (MAP1A) and 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid receptor subunit genes GLUR1 and GLUR3.
Finally, a recent study 118 has performed genome-wide gene expression analyses in depressed patients prospectively divided in responders and non-responders to an 8-week trial of escitalopram treatment. The authors have found two genes exhibiting an increase in their mRNA expression levels in the non-responders group: CHN2 and JAK2. Specifically, CHN2 could alter the hippocampal neurogenesis, whereas JAK2 activates both innate and adaptive immunity, indicating that these genes could be possible candidate predictors of the treatment response.
The above studies not only have confirmed previous findings (such as an association between abnormalities in the immune and stress response as well as in neuroplasticity and neurotransmitters pathways and MDD), but they have also shown the huge advantage of performing whole-genome transcriptome assays to identify pathways and molecular mechanisms that are altered in MDD by using a hypothesis-free approach.
Conclusions
In this review we have presented several studies which have investigated the expression levels of different genes in MDD patients, mostly obtained from whole blood but also from isolated mononuclear cells, isolated monocytes and postmortem brain tissues. Altogether these studies have identified a pattern of altered expression in several genes belonging to different biological systems such as inflammation, neurotransmission, HPA axis and neuroplasticity supporting data shown in our previous review published in 2013 by Hepgul et al. 15 .
In addition, to provide evidence supporting a personalized medicine approach to the treatment of MDD, we have reviewed studies that have analysed changes in gene expression levels associated with the treatment response. This association suggests that the gene expression approach, both hypothesis-driven and hypothesis-free, is particularly relevant from a clinical point of view as it allows to identify biomarkers that can help in the personalization of therapy and in the future development of novel intervention and treatment strategies.
Several studies have suggested that changes in gene expression measured in the blood mirror gene expression alterations occurring in the brain 11 , 119 and a recent review of transcriptomic studies suggests that between 35% and 80% of known transcripts are present in both brain and blood tissue samples 119 . Moreover, Yan and colleagues have demonstrated the presence of an extracellular RNA at similar level both in the brain as well as in plasma samples 120 .
Another important aspect to consider is that peripheral blood biomarkers should be considered also in a different way. Indeed, in the clinical setting it is more important the clinical predictive value of a molecule instead of demonstrating the presence of its levels in a similar way both in the periphery and in the brain. In this context, inflammatory mediators, especially when measured in terms of mRNA levels, are a great example of how they can be used as predictors of treatment response in patients with MDD without being worried about the presence of similar levels in the brain. Hence, blood could serve diagnostic/prognostic purposes for MDD through profiling peripheral gene expression levels in blood cells 11 , 121 .
On the other hand, it is important to consider the difficulties in finding appropriate biomarkers, considering the heterogeneity of MDD. Indeed, some of the alterations associated with the disease might be influenced by several factors including childhood trauma, sex differences, lifestyle and demographic variables. For instance, it has been shown that trauma in childhood can lead to long-lasting effects on peripheral inflammation later in life, such as increased pro-inflammatory cytokine levels 122 , 123 . Moreover, it has been shown that, although depressive symptoms are associated with inflammation, this association is highly influenced by race and gender 124 . Therefore, large cohorts characterized by all these factors could allow the identification of peripheral biomarkers associated with specific endophenotypes of depression and associated with specific clinical variables known to influence also treatment response.
Altogether, these data have shown that the measurement of gene expression levels can be particularly helpful in the clinical setting, for an early prediction of treatment response in MDD patients. Indeed, as widely discussed in this review, mRNA biomarkers can predict the antidepressant response when measured at baseline or they can be useful in monitoring the efficacy of the treatment when measured during the therapy. This could lead to an improvement in the antidepressant response not only with a benefit for depressed patients, but also with a reduction of the associated health care costs.
However, to our knowledge, no gene expression biomarkers have been translated into the clinical practice yet, since most of the available studies have often used assays that are laboratory-specific and that are mainly based on a relative rather than absolute quantification.
Briefly, the relative quantification method compares the expression levels of a target gene in one group to those in an another group, for example patients to controls, using internal controls (housekeeping genes) for normalization. However, this relative gene expression based approach, although helpful in the identification and prioritization of novel biomarkers associated with antidepressant treatment, cannot be reflected as a routine into the clinical practice.
Conversely, an absolute gene expression quantification based approach could represent the best one to be implemented in the clinical setting, as it may help clinicians to predict and monitor the antidepressant response in a shorter time. Indeed, the absolute quantification is based on a standard curve, which is prepared from samples of known template concentration. Then, the concentration of any unknown sample can be determined by simple interpolation of its signal into this standard curve. Interestingly, because of these standard parameters, absolute mRNA values allow to establish given thresholds that are more likely to be individually measured and that are more comparable across different laboratories. For example, in a possible clinical setting scenario, the quantification of an absolute expression of certain biomarkers that, like cytokines, can predict the treatment response, can provide real-time information on the status of those biological factors that can influence treatment response. For example patients whose absolute mRNA values of pro-inflammatory cytokines are below the suggested cut-off could receive standard care treatment with conventional antidepressant drugs, whereas patients with absolute mRNA values higher than the suggested cut-off could be early directed toward more assertive antidepressant strategies, with patients receiving from the beginning a combination of antidepressant drugs, or adjuvant therapies such as anti-inflammatory drugs to pushdown the inflammatory status making the antidepressant therapies more efficacius.
Thus, an absolute quantification of gene expression biomarkers could avoid exposing depressed patients to unnecessary pharmacological strategies based on a try-and-error approach.
In conclusion, the absolute quantification of gene expression biomarkers represent the best approach to focus on in the next few years, to implement gene expression measurement in the clinical setting.
Regier, D. A., Kuhl, E. A. & Kupfer, D. J. The DSM-5: Classification and criteria changes. World Psychiatry 12 , 92–98 (2013).
Bernstein, D. P. et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151 , 1132–1136 (1994).
Article CAS PubMed Google Scholar
James, S. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1789–1858 (2018).
Article Google Scholar
Penninx, B. W., Milaneschi, Y., Lamers, F. & Vogelzangs, N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 11 , 129 (2013).
Article PubMed PubMed Central Google Scholar
Belmaker, R. H. & Agam, G. Major depressive disorder. N. Engl. J. Med . 358 , 55–68 (2008).
Heim, C., Newport, D. J., Mletzko, T., Miller, A. H. & Nemeroff, C. B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33 , 693–710 (2008).
Danese, A. et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 65 , 409–415 (2008).
Mazure, C. M., Bruce, M. L., Maciejewski, P. K. & Jacobs, S. C. Adverse life events and cognitive-personality characteristics in the prediction of major depression and antidepressant response. Am. J. Psychiatry 157 , 896–903 (2000).
Mehta, D., Menke, A. & Binder, E. B. Gene expression studies in major depression. Curr. Psychiatry Rep. 12 , 135–144 (2010).
Tomita, H. et al. Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol. Psychiatry 55 , 346–352 (2004).
Article CAS PubMed PubMed Central Google Scholar
Sullivan, P. F., Fan, C. & Perou, C. M. Evaluating the comparability of gene expression in blood and brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141b , 261–268 (2006).
Article PubMed Google Scholar
Spijker, S. et al. Stimulated gene expression profiles as a blood marker of major depressive disorder. Biol. Psychiatry 68 , 179–186 (2010).
Segman, R. H. et al. Blood mononuclear cell gene expression signature of postpartum depression. Mol. Psychiatry 15 , 93–100 (2010).
Menke, A. et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology 37 , 1455–1464 (2012).
Hepgul, N., Cattaneo, A., Zunszain, P. A. & Pariante, C. M. Depression pathogenesis and treatment: what can we learn from blood mRNA expression?. BMC Med. 11 , 28 (2013).
Howren, M. B., Lamkin, D. M. & Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71 , 171–186 (2009).
Eyre, H. A. et al. A meta-analysis of chemokines in major depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 68 , 1–8 (2016).
Article CAS Google Scholar
Vattakatuchery, J. J., Rickards, H. & Cavanna, A. E. Pathogenic mechanisms of depression in multiple sclerosis. J. Neuropsychiatry Clin. Neurosci. 23 , 261–276 (2011).
Euesden, J., Euesden, J., Danese, A., Lewis, C. M. & Maughan, B. A bidirectional relationship between depression and the autoimmune disorders—-new perspectives from the National Child Development Study. PLoS ONE 12 , e0173015 (2017).
Article PubMed PubMed Central CAS Google Scholar
Benveniste, E. N. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am. J. Physiol. 263 , C1–16 (1992).
Banks, W. A., Kastin, A. J. & Broadwell, R. D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 2 , 241–248 (1995).
Hajebrahimi, B. et al. The adapter proteins of TLRs, TRIF and MYD88, are upregulated in depressed individuals. Int. J. Psychiatry Clin. Pract. 18 , 41–44 (2014).
Rizavi, H. S. et al. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res. 240 , 314–320 (2016).
Momeni, M. et al. ASC provides a potential link between depression and inflammatory disorders: a clinical study of depressed Iranian medical students. Nord. J. Psychiatry 70 , 280–284 (2016).
Cattaneo, A. et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology 38 , 377–385 (2013).
Spindola, L. M. et al. Gene expression in blood of children and adolescents: mediation between childhood maltreatment and major depressive disorder. J. Psychiatr. Res. 92 , 24–30 (2017).
Lilic, D., Cant, A. J., Abinun, M., Calvert, J. E. & Spickett, G. P. Cytokine production differs in children and adults. Pediatr. Res. 42 , 237–240 (1997).
Ng, F., Berk, M., Dean, O. & Bush, A. I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11 , 851–876 (2008).
Matés, J. M., Pérez-Gómez, C. & de Castro, I. N. Antioxidant enzymes and human diseases. Clin. Biochem. 32 , 595–603 (1999).
Maes, M., Galecki, P., Chang, Y. S. & Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 35 , 676–692 (2011).
Surh, Y. J., Kundu, J. K. & Na, H. K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 74 , 1526–1539 (2008).
Lee, J. S. & Surh, Y. J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 224 , 171–184 (2005).
Schreck, R., Albermann, K. & Baeuerle, P. A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic. Res. Commun. 17 , 221–237 (1992).
Baeuerle, P. A. & Baltimore, D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell 53 , 211–217 (1988).
Lukic, I. et al. Lymphocyte levels of redox-sensitive transcription factors and antioxidative enzymes as indicators of pro-oxidative state in depressive patients. Neuropsychobiology 70 , 1–9 (2014).
Shajib, M. S. & Khan, W. I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. (Oxf.) 213 , 561–574 (2015).
Amidfar, M. et al. Increased levels of 5HT2A receptor mRNA expression in peripheral blood mononuclear cells of patients with major depression: correlations with severity and duration of illness. Nord. J. Psychiatry 71 , 282–288 (2017).
Cattaneo, A. et al. Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl. Psychiatry 10 , 232 (2020).
Adinolfi, E. et al. The P2X7 receptor: a main player in inflammation. Biochem. Pharmacol. 151 , 234–244 (2018).
Ribeiro, D. E. et al. P2X7 receptor signaling in stress and depression. Int. J. Mol. Sci. 20 , 11 (2019).
Pittenger, C. & Duman, R. S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33 , 88–109 (2008).
Christoffel, D. J., Golden, S. A. & Russo, S. J. Structural and synaptic plasticity in stress-related disorders. Rev. Neurosci. 22 , 535–549 (2011).
Rajkowska, G. & Miguel-Hidalgo, J. J. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 6 , 219–233 (2007).
Kang, H. J. et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 18 , 1413–1417 (2012).
Drevets, W. C., Price, J. L. & Furey, M. L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 213 , 93–118 (2008).
Takebayashi, M. et al. Decreased levels of whole blood glial cell line-derived neurotrophic factor (GDNF) in remitted patients with mood disorders. Int. J. Neuropsychopharmacol. 9 , 607–612 (2006).
Castrén, E. & Rantamäki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 70 , 289–297 (2010).
Article PubMed CAS Google Scholar
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24 , 677–736 (2001).
Hong, W. et al. Significantly decreased mRNA levels of BDNF and MEK1 genes in treatment-resistant depression. Neuroreport 25 , 753–755 (2014).
Warner-Schmidt, J. L. & Duman, R. S. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl Acad. Sci. USA 104 , 4647–4652 (2007).
Sun, Y. et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest . 111 , 1843–1851 (2003).
Nowacka, M. M. & Obuchowicz, E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 46 , 1–10 (2012).
Fournier, N. M., Lee, B., Banasr, M., Elsayed, M. & Duman, R. S. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology 63 , 642–652 (2012).
Takebayashi, M., Hashimoto, R., Hisaoka, K., Tsuchioka, M. & Kunugi, H. Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J. Neural Transm. (Vienna) 117 , 1119–1122 (2010).
Lee, B. H. & Kim, Y. K. Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J. Affect Disord. 136 , 181–184 (2012).
Fan, X., Heijnen, C. J., van der Kooij, M. A., Groenendaal, F. & van Bel, F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res. Rev. 62 , 99–108 (2009).
Ke, Q. & Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 70 , 1469–1480 (2006).
Shibata, T. et al. The alteration of hypoxia inducible factor-1 (HIF-1) and its target genes in mood disorder patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 43 , 222–229 (2013).
Monteleone, M. C. et al. Prenatal stress changes the glycoprotein GPM6A gene expression and induces epigenetic changes in rat offspring brain. Epigenetics 9 , 152–160 (2014).
Cooper, B., Fuchs, E. & Flügge, G. Expression of the axonal membrane glycoprotein M6a is regulated by chronic stress. PLoS ONE 4 , e3659 (2009).
Fuchsova, B., Alvarez Juliá, A., Rizavi, H. S., Frasch, A. C. & Pandey, G. N. Altered expression of neuroplasticity-related genes in the brain of depressed suicides. Neuroscience 299 , 1–17 (2015).
Stefansson, H. et al. Common variants conferring risk of schizophrenia. Nature 460 , 744–747 (2009).
Talarowska, M., Szemraj, J. & Gałecki, P. The role of interleukin genes in the course of depression. Open Med. (Wars., Pol.) 11 , 41–48 (2016).
Hoyo-Becerra, C., Schlaak, J. F. & Hermann, D. M. Insights from interferon-α-related depression for the pathogenesis of depression associated with inflammation. Brain Behav. Immun. 42 , 222–231 (2014).
Mossakowska-Wójcik, J., Orzechowska, A., Talarowska, M., Szemraj, J. & Gałecki, P. The importance of TCF4 gene in the etiology of recurrent depressive disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 80 , 304–308 (2018).
Ziemiańska, K., Konopka, A. & Wilczyński, G. M. The role of extracellular proteolysis in synaptic plasticity of the central nervous system. Postepy Hig. Med. Dosw. (Online) 66 , 959–975 (2012).
Bobińska, K. et al. Human neuropsin gene in depression. Psychiatr. Danub 29 , 195–200 (2017).
Konar, A. & Thakur, M. K. Neuropsin expression correlates with dendritic marker MAP2c level in different brain regions of aging mice. Mol. Neurobiol. 51 , 1130–1138 (2015).
Veenstra-VanderWeele, J., Anderson, G. M. & Cook, E. H. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur. J. Pharmacol. 410 , 165–181 (2000).
Albuquerque, E. X., Pereira, E. F. R., Alkondon, M. & Rogers, S. W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89 , 73–120 (2009).
Kunii, Y. et al. CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am. J. Psychiatry 172 , 1122–1130 (2015).
Moret, C. & Briley, M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 7 , 9–13 (2011).
CAS PubMed PubMed Central Google Scholar
Dailly, E., Chenu, F., Renard, C. E. & Bourin, M. Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 18 , 601–607 (2004).
Meneses, A. 5-HT system and cognition. Neurosci. Biobehav. Rev. 23 , 1111–1125 (1999).
Fajardo, O., Galeno, J., Urbina, M., Carreira, I. & Lima, L. Serotonin, serotonin 5-HT(1A) receptors and dopamine in blood peripheral lymphocytes of major depression patients. Int. Immunopharmacol. 3 , 1345–1352 (2003).
Baganz, N. L. & Blakely, R. D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 4 , 48–63 (2013).
Ruhé, H. G., Mason, N. S. & Schene, A. H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry 12 , 331–359 (2007).
Sanacora, G., Treccani, G. & Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62 , 63–77 (2012).
Lodge, D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology 56 , 6–21 (2009).
Gray, A. L., Hyde, T. M., Deep-Soboslay, A., Kleinman, J. E. & Sodhi, M. S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 20 , 1057–1068 (2015).
Chandley, M. J. et al. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int. J. Neuropsychopharmacol. 17 , 1569–1578 (2014).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445 , 168–176 (2007).
Pajvani, U. B. & Accili, D. The new biology of diabetes. Diabetologia 58 , 2459–2468 (2015).
Lonze, B. E. & Ginty, D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35 , 605–623 (2002).
Papadia, S., Stevenson, P., Hardingham, N. R., Bading, H. & Hardingham, G. E. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J. Neurosci. 25 , 4279–4287 (2005).
Chandley, M. J. et al. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J. Psychiatry Neurosci. 38 , 276–284 (2013).
Altshuler, L. L. et al. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 12 , 541–549 (2010).
Oh, D. H. et al. An association between the reduced levels of SLC1A2 and GAD1 in the dorsolateral prefrontal cortex in major depressive disorder: possible involvement of an attenuated RAF/MEK/ERK signaling pathway. J. Neural Transm. (Vienna) 121 , 783–792 (2014).
Gehringer, F. et al. FOXO1 confers maintenance of the dark zone proliferation and survival program and can be pharmacologically targeted in burkitt lymphoma. Cancers (Basel) 11 , 10 (2019).
Heim, C. & Binder, E. B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 233 , 102–111 (2012).
Smith, S. M. & Vale, W. W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 8 , 383–395 (2006).
Pariante, C. M. & Miller, A. H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry 49 , 391–404 (2001).
de Kloet, E. R., Sibug, R. M., Helmerhorst, F. M. & Schmidt, M. V. Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev. 29 , 271–281 (2005).
Roy, B., Shelton, R. C. & Dwivedi, Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J. Psychiatr. Res. 89 , 115–124 (2017).
Iacob, E. et al. Dysregulation of leukocyte gene expression in women with medication-refractory depression versus healthy non-depressed controls. BMC Psychiatry 13 , 273 (2013).
Lang, F., Strutz-Seebohm, N., Seebohm, G. & Lang, U. E. Significance of SGK1 in the regulation of neuronal function. J. Physiol. 588 , 3349–3354 (2010).
Anacker, C. et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl Acad. Sci. USA 110 , 8708–8713 (2013).
Teyssier, J. R., Rey, R., Ragot, S., Chauvet-Gelinier, J. C. & Bonin, B. Correlative gene expression pattern linking RNF123 to cellular stress-senescence genes in patients with depressive disorder: implication of DRD1 in the cerebral cortex. J. Affect Disord. 151 , 432–438 (2013).
Ferguson, J. M. SSRI antidepressant medications: adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry 3 , 22–27 (2001).
Chopra, K., Kumar, B. & Kuhad, A. Pathobiological targets of depression. Expert Opin. Ther. Targets 15 , 379–400 (2011).
Uher, R. et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am. J. Psychiatry 167 , 555–564 (2010).
Uher, R. et al. Genetic predictors of response to antidepressants in the GENDEP project. Pharmacogenomics J. 9 , 225–233 (2009).
Breitfeld, J., Scholl, C., Steffens, M., Laje, G. & Stingl, J. C. Gene expression and proliferation biomarkers for antidepressant treatment resistance. Transl. Psychiatry 7 , e1061 (2017).
Inestrosa, N. C. & Arenas, E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 11 , 77–86 (2010).
Karg, K., Burmeister, M., Shedden, K. & Sen, S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry 68 , 444–454 (2011).
Clarke, H., Flint, J., Attwood, A. S. & Munafò, M. R. Association of the 5- HTTLPR genotype and unipolar depression: a meta-analysis. Psychol. Med. 40 , 1767–1778 (2010).
Sibille, E. & Lewis, D. A. SERT-ainly involved in depression, but when?. Am. J. Psychiatry 163 , 8–11 (2006).
Lesch, K. P. Serotonergic gene expression and depression: implications for developing novel antidepressants. J. Affect. Disord. 62 , 57–76 (2001).
Belzeaux, R., Loundou, A., Azorin, J.-M., Naudin, J. & Ibrahim, E. C. Longitudinal monitoring of the serotonin transporter gene expression to assess major depressive episode evolution. Neuropsychobiology 70 , 220–227 (2014).
Iwata, M., Ota, K. T. & Duman, R. S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 31 , 105–114 (2013).
Alcocer-Gómez, E. et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 36 , 111–117 (2014).
Rupprecht, R. et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 9 , 971–988 (2010).
Gut, P., Zweckstetter, M. & Banati, R. B. Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol. Metab. 26 , 349–356 (2015).
Sarubin, N. et al. Translocator protein (TSPO) expression in platelets of depressed patients decreases during antidepressant therapy. Pharmacopsychiatry 49 , 204–209 (2016).
Hepgul, N. et al. Transcriptomics in interferon-α-treated patients identifies inflammation-, neuroplasticity- and oxidative stress-related signatures as predictors and correlates of depression. Neuropsychopharmacology 41 , 2502–2511 (2016).
Hennings, J. M. et al. RNA expression profiling in depressed patients suggests retinoid-related orphan receptor alpha as a biomarker for antidepressant response. Transl. Psychiatry 5 , e538 (2015).
Duric, V. et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 16 , 69–82 (2013).
Ju, C. et al. Integrated genome-wide methylation and expression analyses reveal functional predictors of response to antidepressants. Transl. Psychiatry 9 , 254 (2019).
Tylee, D. S., Kawaguchi, D. M. & Glatt, S. J. On the outside, looking in: a review and evaluation of the comparability of blood and brain “‐omes”. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 162 , 595–603 (2013).
Yan, Z. et al. Presymptomatic increase of an extracellular RNA in blood plasma associates with the development of Alzheimer’s disease. Curr. Biol. 30 , 1771 (2020).
Liew, C. C., Ma, J., Tang, H. C., Zheng, R. & Dempsey, A. A. The peripheral blood transcriptome dynamically reflects system wide biology: a potential diagnostic tool. J. Lab Clin. Med. 147 , 126–132 (2006).
Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M. & Mondelli, V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 21 , 642–649 (2016).
Coelho, R., Viola, T. W., Walss‐Bass, C., Brietzke, E. & Grassi‐Oliveira, R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr. Scandinavica 129 , 180–192 (2014).
Morris, A. A. et al. Association between depression and inflammation-differences by race and sex: the META-Health study. Psychosom. Med. 73 , 462–468 (2011).
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163 , 1905–1917 (2006).
Gelenberg, A. et al. American Psychiatric Association practice guidelines for the treatment of patients with major depressive disorder. Am. J. Psychiatry 167 , 9–118 (2010).
Google Scholar
Trivedi, M. H. et al. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med . 354 , 1243–1252 (2006).
Rush, A. J. et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N. Engl. J. Med . 354 , 1231–1242 (2006).
Trivedi, M. H. Right patient, right treatment, right time: biosignatures and precision medicine in depression. World Psychiatry 15 , 237–238 (2016).
Download references
Acknowledgements
A.C. and N.C. received funding from Ricerca Corrente (Italian Ministry of Health). A.C. was also supported by the PSR (Piano Sostegno Ricerca) from the University of Milan.).
Author information
Authors and affiliations.
Stress, Psychiatry and Immunology Laboratory, Department of Psychological Medicine, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK
Nicole Mariani & Carmine Pariante
IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Biological Psychiatry Laboratory, Brescia, Italy
Nadia Cattane & Annamaria Cattaneo
Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy
- Annamaria Cattaneo
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Annamaria Cattaneo .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mariani, N., Cattane, N., Pariante, C. et al. Gene expression studies in Depression development and treatment: an overview of the underlying molecular mechanisms and biological processes to identify biomarkers. Transl Psychiatry 11 , 354 (2021). https://doi.org/10.1038/s41398-021-01469-6
Download citation
Received : 23 October 2020
Revised : 29 April 2021
Accepted : 06 May 2021
Published : 08 June 2021
DOI : https://doi.org/10.1038/s41398-021-01469-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genetic factors associated with suicidal behaviors and alcohol use disorders in an american indian population.
- David A. Gilder
- Cindy L. Ehlers
Molecular Psychiatry (2024)
Long-term consequences of COVID-19 on mental health and the impact of a physically active lifestyle: a narrative review
- Leonardo Roever
- Bruno Raphael Ribeiro Cavalcante
- Alex Cleber Improta-Caria
Annals of General Psychiatry (2023)
Astrocyte regulation of synaptic signaling in psychiatric disorders
- Anna Kruyer
- Peter W. Kalivas
- Michael D. Scofield
Neuropsychopharmacology (2023)
Integrating genetics and transcriptomics to study major depressive disorder: a conceptual framework, bioinformatic approaches, and recent findings
- Emily M. Hicks
- Carina Seah
- Laura M. Huckins
Translational Psychiatry (2023)
Higher immune-related gene expression in major depression is independent of CRP levels: results from the BIODEP study
- Luca Sforzini
- Carmine M. Pariante
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Memorial Day Sale! Save 25%️
Is depression genetic what you need to know.
- Matt Sommers, PharmD
- May 17, 2024 — 13 min read
Key Highlights
- Depression has a genetic component, with research suggesting that around 40% of people with the condition can link it back to genetic factors.
- Having a family history of depression, such as a parent or sibling with the condition, increases the risk of developing depression.
- Environmental factors, such as childhood trauma or severe life stress, can also contribute to the development of depression.
- Treatment options for depression include antidepressant medications and therapy, such as cognitive behavioral therapy.
- Prevention strategies for depression involve early detection, intervention, and support for at-risk individuals.
- Lifestyle changes, such as maintaining a healthy diet, regular exercise, and managing stress, can help reduce the risk of depression.
Introduction
Depression is a common mental health disorder that affects millions of people worldwide. It is characterized by persistent feelings of sadness, emptiness, and a loss of interest in activities. While the exact cause of depression is not fully understood, research suggests that there is a genetic component to the condition. In other words, some individuals may have a higher risk of developing depression due to their genetic makeup.
Having a family history of depression can increase the likelihood of developing the condition. Studies have shown that individuals with a first-degree relative, such as a parent or sibling, with depression may have a two to four times greater risk of experiencing depression themselves. This suggests that genetic factors play a role in the development of depression.
However, it's important to note that genetics is not the sole determinant of depression. Environmental factors, such as traumatic life events, chronic stress, and certain medical conditions, can also contribute to the onset of depression. Understanding the interplay between genetics and environmental factors is crucial in order to effectively prevent, diagnose, and treat depression.
In this blog, we will explore the genetic and environmental factors that contribute to depression, the role of genetics in the development of the condition, treatment options, and prevention strategies. By understanding the underlying causes of depression, we can better support individuals who are at risk and provide effective interventions to improve mental health outcomes.
Understanding Depression
Depression, also known as major depressive disorder, is a common mental health condition that affects a person's mood, thoughts, and overall well-being. It is characterized by persistent feelings of sadness, hopelessness, and a loss of interest or pleasure in activities.
Depression is a serious condition that can significantly impact a person's daily functioning and quality of life. It is classified as a mood disorder, along with other conditions such as bipolar disorder. While everyone experiences feelings of sadness or grief at times, depression is different in that it involves prolonged periods of intense sadness and a lack of enjoyment in life. This can lead to social withdrawal and can impact important responsibilities like work or school.
Depression is a complex condition with various factors that contribute to its development. It can be caused by a combination of genetic, environmental, and psychological factors. Understanding the different aspects of depression is crucial in order to provide effective treatment and support for individuals experiencing this condition.

Defining Depression: More Than Just Sadness
Depression, or major depressive disorder, is a mental illness that goes beyond temporary feelings of sadness. It is characterized by persistent and intense feelings of sadness, hopelessness, and a lack of interest or pleasure in activities that were once enjoyable.
The symptoms of depression can vary from person to person, but common signs include changes in appetite and sleep patterns, low energy levels, difficulty concentrating, feelings of guilt or worthlessness, and can include thoughts of death or suicide. These symptoms often interfere with daily activities and can significantly impact a person's quality of life.
Depression is a serious mental illness that requires professional treatment and support. It is not a sign of weakness or a character flaw but rather a medical condition that can be effectively managed with the right interventions. If you or someone you know is experiencing symptoms of depression, it is important to seek help from a mental health professional.
Symptoms and Diagnosis: Recognizing Depression in Yourself and Others
Recognizing the symptoms of depression is essential for early diagnosis and intervention. While everyone experiences sadness or low moods at times, it is important to differentiate between temporary feelings and the persistent symptoms of depression.
Common depressive symptoms include a persistent sad or irritable mood, loss of interest or pleasure in activities, changes in appetite or weight, sleep disturbances, fatigue or loss of energy, feelings of worthlessness or guilt, difficulty concentrating, and recurring thoughts of death or suicide.
A diagnosis of depression is typically made by a mental health professional, such as a psychiatrist or psychologist. They will evaluate the presence and severity of symptoms, as well as the duration of symptoms, in order to determine if it meets the criteria for major depressive disorder.
It is important to remember that depression is a treatable condition, and seeking professional help is crucial for effective management. If you or someone you know is experiencing symptoms of depression, it is important to reach out to a healthcare professional for support and guidance.
The Genetics of Depression
The role of genetics in the development of depression has been the focus of extensive research. While it is clear that there is a genetic component to the condition, the exact genes and mechanisms involved are still being explored.
Studies have shown that individuals with a family history of depression, such as a parent or sibling with the condition, have a higher risk of developing depression themselves. This suggests that there are specific gene variants that may contribute to the development of the condition.
However, it is important to note that genetics is not the sole determinant of depression. Environmental factors, such as stressful life events and other social and psychological factors, can also play a significant role in the development of the condition. The interplay between genetics and the environment is complex and requires further study to fully understand its impact on depression.
What Research Says About Depression and Genetics
Twin studies have provided valuable insights into the genetic link to depression. These studies involve comparing the rates of depression between identical twins, who share 100% of their genes, and fraternal twins, who share approximately 50% of their genes.
Research has consistently shown that there is a higher concordance rate for depression in identical twins compared to fraternal twins. This suggests that genetic factors play a significant role in the risk of developing depression.
However, it is important to note that genetics is not the sole determinant of depression. Environmental factors, such as stressful life events, can also contribute to the development of the condition, even in individuals with a genetic predisposition.
How Genes Influence Depression: A Closer Look
Genes play a crucial role in the development of depression, but it is important to understand that there is no single "depression gene." Instead, depression is influenced by multiple genetic factors.
Research suggests that different genes are involved in the development of depression, each with a small effect. These genes contribute to the overall genetic component of depression.
It is also important to note that genes do not act in isolation. They interact with environmental factors to influence the risk of developing depression. This complex gene-environment interaction requires further study to fully understand its impact on the development of the condition.
Environmental Factors and Depression
While genetics play a significant role in the development of depression, environmental factors also contribute to the risk of developing the condition. Stressful life events, such as the loss of a loved one, relationship problems, financial difficulties, or exposure to trauma, can trigger or exacerbate depressive symptoms.
Environmental factors can interact with genetic factors to increase the risk of developing depression. For example, individuals with a genetic predisposition to depression may be more vulnerable to the effects of stressful life events.
Understanding the interplay between genetics and the environment is crucial in order to effectively prevent and treat depression. By addressing both genetic and environmental factors, healthcare professionals can provide a comprehensive approach to the management of depression.
Life Events and Stress: How They Trigger Depression
Traumatic events and chronic stress can have a significant impact on mental health, including the development of depression. Exposure to traumatic events, such as physical or sexual abuse, accidents, or natural disasters, can trigger the onset of depressive symptoms.
Chronic stress, such as ongoing financial difficulties, work-related stress, or relationship problems, can also contribute to the development of depression. Prolonged exposure to stress can disrupt the body's stress response systems and increase the risk of developing both mental and physical health conditions. With increased stress comes increased cortisol levels. When cortisol remains elevated long-term, the effects can be far-reaching, including higher rates of heart disease, more susceptibility to infection, and an increased risk of mental health disorders like depression.
These environmental factors can interact with genetic factors to increase the risk of depression. Individuals with a genetic predisposition to depression may be more vulnerable to the effects of traumatic events or chronic stress.
Understanding the role of environmental factors in the development of depression is essential in order to effectively prevent and treat the condition. Stressors are not always avoidable, so it is crucial to identify and learn to manage them effectively. By addressing both genetic and environmental factors, healthcare professionals can provide comprehensive care for individuals with depression.
Interplay Between Genetics and Environment
While genetic factors play a significant role in predisposing individuals to depression, the environment also contributes considerably to its development. The interplay between genetics and environmental influences is complex.
Stressful life events, childhood trauma, and socio-economic factors can interact with genetic predispositions to increase the risk of depression. Understanding how these factors interact can provide valuable insights into personalized treatment approaches that consider both genetic susceptibilities and environmental triggers. This intricate relationship highlights the importance of a holistic approach to addressing depression.
Depression Across the Lifespan
Depression can affect individuals of all ages, from children to older adults. The symptoms and manifestations of depression can vary depending on the age group.
Childhood and adolescent depression may present with symptoms such as irritability, social withdrawal, changes in appetite or sleep, academic difficulties, physical complaints, or thoughts of suicide. It is important to recognize the early signs of depression in children and provide appropriate support and intervention.
Adults and elderly individuals may experience symptoms such as persistent sadness, a loss of interest in activities, changes in appetite or sleep, fatigue, difficulty concentrating, or thoughts of death or suicide.
Understanding the unique aspects of depression across the lifespan is crucial in order to provide targeted interventions and support for individuals of all ages.
Childhood and Adolescent Depression: Early Signs
Recognizing the early signs of depression in children and adolescents is essential for early intervention and support. Some common early signs of childhood depression include persistent sadness, irritability, social withdrawal, changes in appetite or sleep patterns, academic difficulties, or physical complaints such as headaches or stomach aches.
It is important to note that these symptoms can be indicative of other issues as well, and a thorough evaluation by a mental health professional is necessary for an accurate diagnosis. Early intervention is crucial for preventing the long-term consequences of childhood depression.
Childhood depression can have a significant impact on brain development and overall well-being. By identifying and addressing depressive symptoms early on, healthcare professionals can provide appropriate support and intervention to promote healthy development and well-being in children and adolescents.
Depression in Adults and the Elderly: Differences in Manifestation
Depression in adults and the elderly may present with similar symptoms to childhood and adolescent depression, but there are also some unique aspects to consider.
In adults, symptoms of depression can include persistent sadness, a loss of interest in activities, changes in appetite or sleep, feelings of guilt or worthlessness, fatigue, difficulty concentrating, or recurring thoughts of death or suicide.
Elderly individuals may experience similar symptoms, but depression in older adults can often be masked by other physical health conditions or attributed to normal aging. It is important to recognize the signs of depression in the elderly and provide appropriate support and intervention.
Understanding the differences in the manifestation of depression across different age groups is crucial in order to provide targeted interventions and support for individuals of all ages.
Treatment Options for Depression
Depression is a treatable condition, and there are various treatment options available. The most common treatments for depression include medication and therapy.
Antidepressant medications can help relieve the symptoms of depression by balancing the levels of chemicals in the brain that are associated with mood regulation. There are different types of antidepressant medications available, such as selective serotonin reuptake inhibitors (SSRIs) , serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs). SSRIs like escitalopram, fluoxetine, and others have become some of the most commonly prescribed medications for depression early in therapy. Working with a healthcare professional is important to find the right medication and dosage for your individual needs.
Therapy, such as cognitive behavioral therapy (CBT) , can also be an effective treatment for depression. CBT focuses on identifying and changing negative thought patterns and behaviors that contribute to depressive symptoms. Other forms of therapy, such as interpersonal therapy (IPT) and psychodynamic therapy, may also be helpful in addressing underlying issues and promoting emotional well-being.
Combining medication and therapy often provides the best outcomes for individuals with depression. Working with a healthcare professional to develop a personalized treatment plan that addresses your specific needs and goals is important.
Medication and Genetics: Finding the Right Match
Genetic factors can significantly impact how effective antidepressant medications can be and how likely they are to cause side effects. Tailoring medication to the individual's genetic makeup can optimize outcomes. Based on genetic testing, personalized medicine helps identify which medications are most suitable for a patient. Understanding how genes influence the body's response to antidepressants is key to finding the right match. By considering genetic factors, healthcare providers can navigate the complex landscape of depression treatment more effectively. Matching medication to genetics holds promise for improving patient care and outcomes.
Therapy and Lifestyle Changes: Non-Genetic Interventions
In addition to medication, therapy, and other treatments, lifestyle changes can also play a significant role in the management of depression. While genetics may contribute to the risk of developing depression, lifestyle factors can influence its severity and course.
Cognitive behavioral therapy (CBT) is a type of therapy that focuses on identifying and changing negative thought patterns and behaviors that contribute to depressive symptoms. It can help individuals develop healthier coping mechanisms and improve their overall well-being.
Lifestyle changes, such as maintaining a healthy diet, regular exercise, getting enough sleep, managing stress, and engaging in pleasurable activities, can also support mental health and reduce symptoms of depression. These non-genetic interventions can provide additional support and improve overall well-being.
Working with a healthcare professional to develop a comprehensive treatment plan that addresses genetic and non-genetic factors is important. By combining medication, therapy, and lifestyle changes, individuals can effectively manage their depression and improve their quality of life.
Preventing Depression
While it may not be possible to completely prevent depression, there are strategies that can help reduce the risk and promote mental well-being. Prevention strategies focus on early detection, intervention, and support for individuals who are at risk.
Identifying at-risk individuals, such as those with a family history of depression or individuals who have experienced traumatic events, is an important step in prevention. Providing support and resources to these individuals can help them manage stress and build resilience.
Early detection and intervention are crucial in preventing the onset and progression of depression. Healthcare professionals, family, and friends can all play a key role in identifying symptoms and providing appropriate support and treatment.
Prevention strategies also involve promoting mental health and well-being through education, awareness campaigns, and providing resources and support. By addressing risk factors and promoting mental well-being, we can reduce the impact of depression on individuals and communities.
Strategies for At-Risk Individuals
For individuals at risk of depression due to genetic factors or family history, early interventions and lifestyle adjustments can be pivotal. Regular exercise, proper nutrition, adequate sleep, and stress management techniques can help mitigate the likelihood of developing major depression. For help with stress management, meeting with a therapist to help define sources of stress and personalized strategies to better manage life’s stressors can be very helpful.
Engaging in supportive social activities, seeking therapy, and building resilience through mindfulness practices are also beneficial strategies. Monitoring symptoms closely and seeking professional help when needed are crucial steps in the prevention of depression in at-risk populations.
Importance of Early Detection and Intervention
Early detection and intervention are crucial in effectively managing depression and reducing its impact. Recognizing the signs and symptoms of depression early on can lead to prompt intervention and improved outcomes.
Healthcare professionals play a key role in early detection. By conducting thorough assessments and evaluations, they can identify symptoms of depression and provide appropriate support and treatment. Family, friends, and community members can also help by fostering a non-judgmental, supportive environment.
Early intervention involves providing the necessary resources and interventions to individuals experiencing depressive symptoms. This may include therapy, medication, lifestyle changes, and other supportive measures. The goal is to address symptoms early on and prevent the escalation of the condition.
Access to mental health services is essential in supporting early detection and intervention. By providing accessible and affordable mental health services, individuals can receive the support they need at the earliest stages of depression.
By prioritizing early detection and intervention, healthcare professionals can provide timely and effective support for individuals with depression, leading to improved outcomes and better overall mental health.
In conclusion, understanding the interplay between genetics and environmental factors is crucial in comprehending the complexities of depression. While genetic predispositions may play a role, environmental triggers can also significantly impact its onset and severity. Recognizing the symptoms, seeking appropriate treatment, and creating a supportive environment are key steps in managing depression effectively. Remember, you are not alone; seeking help and addressing concerns is a sign of strength, not weakness. If you have concerns about depression, reach out to mental health professionals for guidance and support. Your well-being matters.
When considering treatment options your genetics can also play a vital role in determining which medications will be best suited for you. A simple test can help reduce the trial and error process associated with finding the right medication. Find out more by visiting www.clarityxdna.com
Frequently Asked Questions
Can depression be completely cured.
Depression is a treatable condition, but it may not be completely cured for everyone. The goal of treatment is to manage symptoms and improve quality of life. The duration of treatment may vary depending on the individual and the type and severity of depression.
How Can I Tell If My Depression Is Genetic?
Identifying if your depression is genetic involves looking at your family history. If you have a first-degree relative, such as a parent or sibling, with depression, you may have a higher risk of developing the condition. Genetic testing can also provide more information about your individual genetic risk factors.
What Are the Chances of Inheriting Depression?
The chances of inheriting depression can vary depending on various factors. Research suggests that individuals with a family history of depression are more likely to develop the condition. Studies with twins have shown that there is a higher concordance rate for depression in identical twins compared to fraternal twins, indicating a genetic predisposition.
Can Lifestyle Changes Override Genetic Predisposition to Depression?
While genetic predisposition can increase the risk of developing depression, lifestyle changes can play a significant role in managing the condition. Engaging in regular exercise, maintaining a healthy diet, managing stress, and seeking support can help reduce the impact of genetic predisposition and promote mental well-being.
Where to Find Support and Resources
Finding support and resources is crucial for individuals with depression and their loved ones. Support groups, mental health resources, and healthcare professionals can provide valuable guidance and assistance. Various organizations and online platforms offer support and information for individuals with depression and their families.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3077049/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6065213/
https://www.ncbi.nlm.nih.gov/books/NBK559078/
https://www.apa.org/ptsd-guideline/patients-and-families/cognitive-behavioral
https://www.ncbi.nlm.nih.gov/books/NBK554406/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3897684/
https://my.clevelandclinic.org/health/diseases/14938-depression-in-children
Take the first step with an easy in-home saliva test

Recent Posts

Is Schizophrenia Genetic? What you need to know

Is Bipolar Disorder Genetic? What you need to know

Trazodone® for Sleep: A Comprehensive Guide Key Highlights

Get 25% Off
plus unlock free shipping!
A New Study Is Finally Attempting to Pin Down The Root Causes of Depression
Most experts agree that depression is not one thing.

The core experiences of depression — changes in energy, activity, thinking, and mood — have been described for more than 10,000 years. The word “depression” has been used for about 350 years.
Given this long history, it may surprise you that experts don’t agree about what depression is, how to define it, or what causes it.
However, many experts do agree that depression is not one thing . It’s a large family of illnesses with different causes and mechanisms. This makes choosing the best treatment for each person challenging.
Reactive vs endogenous depression
One strategy is to search for sub-types of depression and see whether they might do better with different kinds of treatments. One example is contrasting “reactive” depression with “endogenous” depression.
Reactive depression (also thought of as social or psychological depression) is presented as being triggered by exposure to stressful life events. These might be being assaulted or losing a loved one — an understandable reaction to an outside trigger.
Endogenous depression (also thought of as biological or genetic depression) is proposed to be caused by something inside , such as genes or brain chemistry.
Many people working clinically in mental health accept this sub-typing. You might have read about this online .
But we think this approach is way too simple.
While stressful life events and genes may, individually, contribute to causing depression, they also interact to increase the risk of someone developing depression. And evidence shows that there is a genetic component to being exposed to stressors. Some genes affect things such as personality. Some affect how we interact with our environments.

What we did and what we found
Our team set out to look at the role of genes and stressors to see if classifying depression as reactive or endogenous was valid.
In the Australian Genetics of Depression Study , people with depression answered surveys about exposure to stressful life events. We analyzed DNA from their saliva samples to calculate their genetic risk for mental disorders.
Our question was simple. Does genetic risk for depression, bipolar disorder, schizophrenia, ADHD, anxiety, and neuroticism (a personality trait) influence people’s reported exposure to stressful life events?
You may be wondering why we bothered calculating the genetic risk for mental disorders in people who already have depression. Every person has genetic variants linked to mental disorders. Some people have more, some less. Even people who already have depression might have a low genetic risk for it. These people may have developed their particular depression from some other constellation of causes.
We looked at the genetic risk of conditions other than depression for a couple of reasons. First, genetic variants linked to depression overlap with those linked to other mental disorders. Second, two people with depression may have completely different genetic variants. So, we wanted to cast a wide net to look at a wider spectrum of genetic variants linked to mental disorders.
If reactive and endogenous depression sub-types are valid, we’d expect people with a lower genetic component to their depression (the reactive group) to report more stressful life events. And we’d expect those with a higher genetic component (the endogenous group) would report fewer stressful life events.
But after studying more than 14,000 people with depression, we found the opposite.
We found people at higher genetic risk for depression, anxiety, ADHD, or schizophrenia say they’ve been exposed to more stressors .
Assault with a weapon, sexual assault, accidents, legal and financial troubles, and childhood abuse and neglect were all more common in people with a higher genetic risk of depression, anxiety, ADHD, or schizophrenia.
These associations were not strongly influenced by people’s age, sex, or relationships with family. We didn’t look at other factors that may influence these associations, such as socioeconomic status. We also relied on people’s memory of past events, which may not be accurate.
How do genes play a role?
Genetic risk for mental disorders changes people’s sensitivity to the environment.
Imagine two people, one with a high genetic risk for depression and one with a low risk. They both lose their jobs. The genetically vulnerable person experiences the job loss as a threat to their self-worth and social status. There is a sense of shame and despair. They can’t bring themselves to look for another job for fear of losing it, too. For the other, the job loss feels less about them and more about the company. These two people internalize the event differently and remember it differently.
Genetic risk for mental disorders also might make it more likely people find themselves in environments where bad things happen. For example, a higher genetic risk for depression might affect self-worth, making people more likely to get into dysfunctional relationships, which then go badly.
What does our study mean for depression?
First, it confirms genes and environments are not independent. Genes influence the environments we end up in and what then happens. Genes also influence how we react to those events.
Second, our study doesn’t support a distinction between reactive and endogenous depression. Genes and environments have a complex interplay. Most cases of depression are a mix of genetics, biology, and stressors.
Third, people with depression who appear to have a stronger genetic component to their depression report their lives are punctuated by more serious stressors.
So, clinically, people with higher genetic vulnerability might benefit from learning specific techniques to manage their stress. This might help some people reduce their chance of developing depression in the first place. It might also help some people with depression reduce their ongoing exposure to stressors.
This article was originally published on The Conversation by Jacob Crouse and Ian Hickie at the University of Sydney . Read the original article here .
- Mental Health
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Treatment-resistant depression linked to body mass index: Study
by Vanderbilt University Medical Center

Genetic factors are a small but significant contributor to severe depression that does not respond to standard therapy, according to researchers at Vanderbilt University Medical Center and Massachusetts General Hospital.
The heritability of treatment-resistant depression (TRD) was found to have significant genetic overlap with schizophrenia, attention deficit disorder , cognitive, alcohol and smoking traits, and body mass index (BMI), suggesting a shared biology and, potentially, new treatment avenues.
The report, published May 15 in The American Journal of Psychiatry , provides insights into the genetics and biology underlying TRD, supports the utility of estimating disease probabilities from clinical data for genomic investigations, and "lays the groundwork for future efforts to apply genomic data for biomarker and drug development."
"Despite the large proportion of patients with TRD, the biology has remained poorly understood. Our work here provides genetic support for new biological directions to explore in addressing this gap," said Douglas Ruderfer, Ph.D., associate professor of Medicine (Genetic Medicine), Psychiatry, and Biomedical Informatics.
"This work finally gives us some new leads, rather than reinventing the same antidepressants over and over again, for a condition that is extremely common," said Roy Perlis, MD, professor of Psychiatry at Harvard Medical School and director of the MGH Center for Experimental Drugs and Diagnostics.
Nearly 2 out of every 10 people in the United States experience severe depression , and roughly a third of them do not respond to antidepressant treatments and therapies. TRD is associated with a significantly increased risk for suicide.
Despite evidence that treatment resistance may be a heritable trait, the "genetic architecture" of this condition remains unclear, largely because of the lack of a consistent and rigorous definition of treatment resistance, and the challenge of recruiting enough research subjects to study.
To overcome these barriers, the researchers selected a surrogate for the condition—whether an individual diagnosed with major depressive disorder had received electroconvulsive therapy (ECT).
ECT applies a low voltage applied to the head to induce a generalized seizure without muscle convulsions. Approximately half of patients with TRD respond to ECT, which is thought to improve symptoms by stimulating "rewiring" of brain circuits after they are disrupted by the electrical current.
To ensure the study was sufficiently "powered," or had enough patients from which valid results could be obtained, the researchers developed a machine-learning model to predict, from clinical information recorded in the electronic health record (EHR), which patients were most likely to receive ECT.
The researchers applied this model to EHRs and biobanks from Mass General Brigham and VUMC and validated the results by comparing the predicted cases to actual ECT cases identified through the Geisinger Health System in Pennsylvania, and the Million Veteran Program of the U.S. Department of Veterans Affairs.
More than 154,000 patients from the four health systems with medical records and genotypes, or sequences of their DNA samples, were included in a genome-wide association study , which can identify genetic associations with health conditions (in this case, a marker for TRD).
The study identified genes clustered in two locations, or loci, on different chromosomes that correlated significantly the likelihood of ECT predicted by the model. The first locus overlapped with a previously reported chromosomal location associated with body mass index (BMI).
The ECT-BMI relationship was inverse—patients with lower body weight tended to be at higher risk for treatment resistance.
This finding is supported by studies that found patients with anorexia nervosa, an eating disorder characterized by extremely low body weight, are more likely than those with a higher BMI to be resistant to treatment of coinciding depression.
The other locus associated with ECT points to a gene that is highly expressed in brain regions that regulate body weight and appetite. Recently this gene also has been implicated in bipolar disorder, a major psychiatric illness.
Large studies are currently underway to collect tens of thousands of ECT cases for a case-control study.
Confirmation of the link between the ECT marker for TRD and the complex metabolic pathways underlying food intake, maintenance of body weight, and energy balance could open the door to new, more effective treatments for treatment of major depressive disorder, the researchers said.
Co-authors from VUMC were JooEun Kang, MD, Ph.D., Michael Ripperger, Drew Wilimitis, Theodore Morley, Lide Han, Ph.D., Stephan Heckers, MD, MSc, and Colin G. Walsh, MD, MA; at MGH they included Thomas McCoy, MD, and Victor Castro.
Explore further
Feedback to editors
New analysis estimates the effects of race-neutral lung function testing on patients, hospitals, and beyond
3 hours ago

World-first trial shows benefits of finding and treating undiagnosed asthma and COPD
7 hours ago

Acetaminophen shows promise in warding off acute respiratory distress syndrome, organ injury in patients with sepsis
10 hours ago

Modular communicative leadless ICD found to be safe and exceeds performance expectations
May 18, 2024

Creativity and humor shown to promote well-being in older adults via similar mechanisms

Sweet taste receptor affects how glucose is handled metabolically by humans

Better medical record-keeping needed to fight antibiotic overuse, studies suggest

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses

A long-term ketogenic diet accumulates aged cells in normal tissues, new study shows
May 17, 2024

Gut bacteria enhance cancer immunotherapy in mouse study
Related stories.

Researchers confirm postpartum depression heritability, home in on treatment mechanism
Oct 19, 2023

New clues to the mechanism behind treatment-resistant depression
Nov 2, 2023

Study shows link between genetics and response to electroconvulsive therapy
Oct 31, 2022

Study reveals new genetic link between anorexia nervosa and being an early riser
Jan 4, 2024

Study explores genetic risk for suicide attempt
Feb 1, 2019

Shared genetics may shape treatment options for certain brain disorders
Jun 20, 2018
Recommended for you
The neural signature of subjective disgust could apply to both sensory and socio-moral experiences
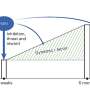
Study uncovers key factors for resilience after trauma
Under stress, study finds an observer is more likely to help the victim than to punish the perpetrator
May 16, 2024
Genetics, environment and health disparities linked to increased stress and mental health challenges during adolescence

Why do we overindulge? Study explores how distraction affects 'hedonic consumption'

Scientists want to know how the smells of nature benefit our health
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Genetics Basics
- Family Health History
- About Cascade Testing
Genetic Disorders
What to know.
Genetic disorders are health problems that happen because of some type of abnormality in a person's genetic material. There are several types of genetic disorders. Some disorders are caused by a genetic change (mutation) in a single gene; some are caused by an abnormality in one of the chromosomes; and some are complex, involving numerous genes and influences from environmental factors.

Genetic disorders are health problems that happen because of some type of abnormality in a person's genetic material. There are several types of genetic disorders. In some cases, a genetic change in a single gene can cause someone to have a disease or condition. In other cases, the gene does not have a genetic change, but a person has more or fewer copies of the gene than most people, and this causes a disease or condition. Some diseases or conditions occur when a person does not have the same number of chromosomes as most people or has part of a chromosome that is missing, extra, or not in the right place.
Most genetic disorders happen due to the combination of many genetic changes acting together with a person's behaviors and environment. These are sometimes called complex conditions.
A detailed description of the basic concepts of genetics can be found here .
Single gene disorders
DNA contains the instructions for making your body work. DNA is made up of two strands that wind around each other. Each DNA strand includes chemicals called nitrogen bases—T (thymine), A (adenine), C (cytosine), and G (guanine)—that make up the DNA code. Genes are specific sections of DNA that have instructions for making proteins. Proteins make up most of the parts of your body and make your body work the right way.
Some diseases and conditions happen when a person has a genetic change (sometimes called a mutation) in one of their genes. These types of diseases are called single gene disorders. Sometimes, what happens is that one of the DNA bases is changed. For example, part of a gene that usually has the sequence TAC is changed to the sequence TTC. This can change the way the gene works, for example, by changing the protein that is made. In other cases, one or more of the bases in the DNA sequence are missing altogether, or there are extra bases.
Genetic changes can be passed down to a child from their parents. When this happens, the disease or condition is called hereditary or inherited. Or the changes can happen for the first time in the process of making the sperm or egg or early in development, so the child will have the genetic change but the parents will not.
DNA, genes, and chromosomes
Single gene disorders that affect a gene on one of the 22 autosomal chromosome pairs are called autosomal disorders. Disorders that affect the sex chromosome are called X-linked disorders. Disorders are further described according to whether the affected genetic change is dominant or recessive.
For some diseases and conditions, everyone who inherits the genetic change will have the disease or condition, but how serious it is can vary from person to person. In other cases, people who have the genetic change will be more likely to develop the disease or condition, but some of them will never develop it.
Autosomal dominant
With autosomal dominant diseases or conditions, a person only needs a genetic change in one copy of the gene to have the disease. If one parent has an autosomal dominant disease or condition, each child has a 50% (1 in 2) chance of inheriting the genetic change that causes the condition.
Examples of autosomal dominant conditions include hereditary breast and ovarian cancer caused by genetic changes (mutations) to the BRCA1 and BRCA2 genes ; Lynch syndrome ; and familial hypercholesterolemia .
Autosomal recessive
With autosomal recessive diseases or conditions, a person needs a genetic change in both copies of the gene to have the disease or condition. While a person with a genetic change in only one copy of the gene will not have the disease or condition, they can still pass the genetic change down to their children. These parents are sometimes called "carriers" of the disease because they "carry" the genetic change that causes the disease or condition but do not have the disease themselves.
A parent who is a carrier of a disease has a 50% (1 in 2) chance of passing the gene with the genetic change on to each of their children. If both parents are carriers of the disease, each child has a 25% (1 in 4) chance of inheriting two genes with the genetic change and thus of having the disease. Carrier screening looks for autosomal recessive genetic changes in parents to see if they could have a child with the disease or condition.
Examples of autosomal recessive disorders are sickle cell disease and cystic fibrosis .
Females have two X chromosomes, and males have one X chromosome and one Y chromosome. Each daughter gets an X from her mother and an X from her father. Each son gets an X from his mother and a Y from his father.
Some diseases or conditions happen when a gene on the X chromosome has a genetic change. Because males only have one copy of all the genes on the X chromosome, they are much more likely to be affected by X-linked genetic disorders than females. A female with a genetic change on only one of her two X chromosomes may not have the disease or condition at all. However, in some cases, females with the genetic change on one of their X chromosomes can have the disease or condition, but it is often a milder form of the disease than usually occurs in males.
Because males inherit an X chromosome from their mother, a female with a genetic change on one copy of the gene has a 50% (1 in 2) chance of passing the genetic change on to each of her sons. Her sons could have the disease or condition even though she does not.
Examples of X-linked conditions include fragile X syndrome , Duchenne muscular dystrophy , and hereditary hemophilia .
Chromosomal abnormalities
Different number of chromosomes.
People usually have 23 pairs of chromosomes. But sometimes a person is born with a different number. Having an extra chromosome is called trisomy. Missing a chromosome is called monosomy.
For example, people with Down syndrome have an extra copy of chromosome 21. This extra copy changes the body's and brain's normal development and causes intellectual and physical problems for the person. Some disorders are caused by having a different number of sex chromosomes. For example, people with Turner syndrome usually have only one sex chromosome, an X. Women with Turner syndrome can have problems with growth and heart defects.
Changes in chromosomes
Sometimes chromosomes are incomplete or shaped differently than usual. Missing a small part of a chromosome is called a deletion. A translocation is when part of one chromosome has moved to another chromosome. An inversion is when part of a chromosome has been flipped over.
For example, people with Williams syndrome are missing a small part of chromosome 7. This deletion can result in intellectual disability and a distinctive facial appearance and personality.
Complex conditions
Complex disorders are caused by genetic changes in many different genes working together with environmental factors. Environmental factors include exposures and behaviors such as air pollution, smoking, alcohol use, the amount of exercise a person gets, or the foods they eat. Having a family health history of a complex condition can make you more likely to have that condition yourself. However, genetic testing would not be recommended because there is not a single genetic change causing the condition that could be found by genetic testing.
Most chronic diseases, such as most cases of heart disease , cancer , diabetes , osteoporosis , and asthma , are complex disorders. So are most cases of developmental disabilities, such as autism spectrum disorder and attention deficit / hyperactivity disorder (ADHD) , and mental health conditions, such as depression and schizophrenia .
The vast amount of genetic information available has allowed researchers to develop methods to study which types of genetic changes are found more often in people with a given disease or condition. This allows researchers to estimate a person's risk for a particular disorder based on which genetic changes they have. This estimate is known as the polygenic risk score.
Some important issues need to be considered before polygenic risk scores can be routinely used in health care and public health. Studies are looking at how useful polygenic risk scores are in real-life clinical practice. Information on how each gene change affects disease risk comes from population-level genetic studies. Addressing diversity in development of polygenic risk scores is important, because polygenic risk scores developed from studies in one population (for example, people of Northern European ancestry) might not work as well for other populations (for example, people of West African ancestry). Also, how each gene change affects the polygenic risk score varies from study to study.
Once polygenic risk scores are ready to be used routinely in clinical practice, public health efforts will be needed to address issues such as access, insurance coverage, and sharing of results across health systems.
Genomics and Your Health
Learn more about genomics and its importance for your health
share this!
May 16, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
The genetic basis and process of inbreeding depression in an elite hybrid rice
by Science China Press
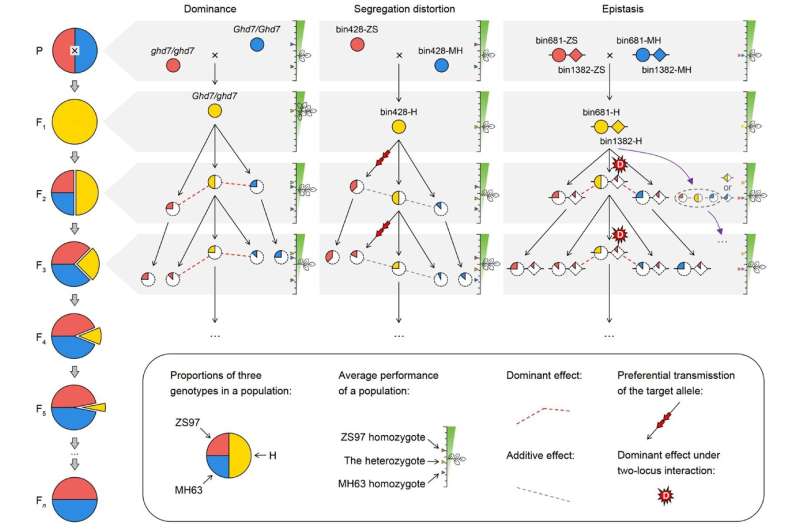
Inbreeding depression is defined as reduced fitness or performance arising from increasing homozygosity of progenies due to successive inbreeding, whereas heterosis refers to the superiority of a hybrid over its parent resulting from the increase in heterozygosity. These two closely related phenomena are of fundamental importance to crop breeding and evolutionary biology.
Despite a comprehensive understanding of heterosis, the genetic basis of inbreeding depression has been debated for a long time, especially in self-pollinating species such as rice. Therefore, Xiaodong Xu and his colleagues in Zhang and Ouyang' group performed a complete dissection of the genetic basis of inbreeding depression, using a continuous inbreeding population derived from an elite hybrid rice.
The findings are published in the journal Science China Life Sciences .
Variable degrees of inbreeding depressions were detected for all traits except heading date, showing continuous and reduced declines from F 1 to F 5 generation due to the continuous halving of heterozygosity among generations.
Using three panicle traits as models, it was found that increased homozygosity for alleles at quantitative trait loci (QTLs) with positive dominant effects, which contributed to heterosis of the hybrid relative to its parents, can lower the performance of the offspring and explain a large portion of inbreeding depression. These loci with dominant effects constitute the main correlation between heterosis and inbreeding depression.
However, distinct from heterosis, a biased transmission ratio of alleles for QTLs with either dominant or additive effects in the segregation distortion region would also change the expected homozygosity and thus lead to slight depression effects. When an allele with low performance was preferentially transferred in the offspring due to segregation distortion, the extent of depression will increase.
In addition, two-locus interactions may change the extent and direction of the depression effects of the target loci, and overall interactions would promote inbreeding depression among generations. Moreover, the actual inbreeding depression was evaluated between generations considering the heterozygosity decay in the background after inbreeding.
The researchers found inconsistent or various degrees of background depression from the F 2 to F 3 generation assuming different genotypes of the target locus, which may affect the actual depression effect of the locus due to epistasis.
Taken together, the results suggest that the genetic architecture of inbreeding depression and heterosis is closely linked but also differs in their intrinsic mechanisms, which expands understanding of the whole-genome architecture of inbreeding depression. It will help breeders to integrate high-efficiency QTLs and heterotic heterozygotes to develop high-yield crops in the future.
This study was reported by Qifa Zhang and Yidan Ouyang' group from the National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan, China.
Journal information: Science China Life Sciences
Provided by Science China Press
Explore further
Feedback to editors

Blue Origin flies thrill seekers to space, including oldest astronaut
3 hours ago
Composition of gut microbiota could influence decision-making
May 18, 2024
Researchers realize multiphoton electron emission with non-classical light

Saturday Citations: Mediterranean diet racks up more points; persistent quantum coherence; vegan dogs

Physicists propose path to faster, more flexible robots

Scientists develop new geochemical 'fingerprint' to trace contaminants in fertilizer
May 17, 2024
Study reveals how a sugar-sensing protein acts as a 'machine' to switch plant growth—and oil production—on and off

Researchers develop world's smallest quantum light detector on a silicon chip

How heat waves are affecting Arctic phytoplankton

Horse remains show Pagan-Christian trade networks supplied horses from overseas for the last horse sacrifices in Europe
Relevant physicsforums posts, dna-maternity test - could you see other relationship than mother, and now, here comes covid-19 version ba.2, ba.4, ba.5,..., is it usual for vaccine injection site to hurt again during infection, a brief biography of dr virgina apgar, creator of the baby apgar test.
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
May 9, 2024
The Cass Report (UK)
May 1, 2024
More from Biology and Medical
Related Stories

Study shows inbreeding reduces cooperation in banded mongooses
Aug 11, 2020

Genomics of Isle Royale wolves reveal impacts of inbreeding
May 29, 2019

Scandinavian wolves found to carry many harmful mutations
Dec 14, 2022

Inbreeding detrimental for survival
Jul 30, 2020

Genome sequencing reveals extensive inbreeding in Scandinavian wolves
Nov 20, 2017

Higher levels of inbreeding significantly lower chances horses will race, new study finds
Jul 13, 2022
Recommended for you

A new 'rule of biology' may have come to light, expanding insight into evolution and aging

New research shows the true cost of reproduction across the animal kingdom

Researchers in Portugal develop an image analysis AI platform to boost worldwide research
Researchers use machine-learning modeling tools to improve zinc-finger nuclease editing technology
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
In A New Study, Researchers Discovered Inflammatory Pathways That Link Coronary Artery Disease And Major Depression
There are various factors and conditions that can cause cardiomyopathy, including both genetic predispositions and lifestyle factors.
Earlier research has demonstrated a notable intersection between coronary artery disease and major depression, indicating that as many as 44% of individuals with coronary artery disease also experience major depression. However, despite these correlations, the fundamental biological pathways linking these conditions have remained unknown.
Inflammation is one shared element in both conditions. Patients afflicted with depression and those with coronary artery disease both exhibit heightened levels of inflammatory markers, suggesting a common biological pathway.
So, this observation prompted the researchers to delve deeper into whether these connections are rooted in genetics.
They used transcriptome-wide association scans, an advanced method aiding in the detection of genetic variations impacting gene expression linked to both major depression and coronary artery disease. Through this approach, the team pinpointed 185 genes significantly associated with both conditions.
The genes were significantly engaged in biological processes associated with inflammation. This convergence implies a potential shared genetic susceptibility between these conditions, influencing their progression via inflammatory pathways.
“This work suggests that chronic low-level inflammation may be a significant contributor to both major depression and cardiovascular disease,” said Lea Davis, a corresponding author of the study.
Additionally, the research team delved into the practical implications of these genetic discoveries in clinical settings by scrutinizing extensive electronic health records from Vanderbilt University Medical Center, Massachusetts General Hospital, and the National Institutes of Health’s All of Us Research Program.
Most notably, they observed that the occurrence of cardiomyopathy in patients exhibiting genetic markers for both coronary artery disease and major depression was actually lower than anticipated. This was especially true in contrast to individuals solely affected by coronary artery disease.
These findings pushed the researchers to suggest that perhaps conventional treatments for major depression and coronary artery disease, including statins and antidepressants, could potentially offer a protective effect against cardiomyopathy by mitigating inflammation.
This idea suggests that both major depression and coronary artery disease cause chronic inflammation, and if we can control this inflammation, it may be key for treating or stopping other conditions, such as cardiomyopathy.
© 2023 Chip Chick Media Inc. All rights reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Genetics of Depression: Progress at Last
Niamh mullins.
1 MRC Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, SE5 8AF UK
Cathryn M. Lewis
2 Division of Genetics and Molecular Medicine, King’s College London, London, SE1 9RT UK
Purpose of Review
We will describe the success of recent genome-wide association studies that identify genetic variants associated with depression and outline the strategies used to reduce heterogeneity and increase sample size.
Recent Findings
The CONVERGE consortium identified two genetic associations by focusing on a sample of Chinese women with recurrent severe depression. Three other loci have been found in Europeans by combining cohorts with clinical diagnosis and measures of depressive symptoms to increase sample size. 23andMe identified 15 loci associated with depression using self-report of clinical diagnosis in a study of over 300,000 individuals.
The first genetic associations with depression have been identified, and this number is now expected to increase linearly with sample size, as seen in other polygenic disorders. These loci provide invaluable insights into the biology of depression and exciting opportunities to develop new biomarkers and therapeutic targets.
Introduction
Major depressive disorder (MDD) is a common psychiatric illness and global public health problem [ 1 ]. It is the third leading cause of years lived with disability worldwide and a major contributor to early mortality from suicide [ 2 ]. Alleviating the burden of this costly disease is an important priority; however, limited understanding of the biological basis of depression has hindered the development of novel treatments and interventions.
Depression is a complex disorder with a heritability of 37% estimated from twin studies [ 3 ]. Despite robust evidence for a genetic component, identifying the specific genetic variants involved in the disorder has been a major challenge. Genome-wide association studies (GWAS) test differences in allele frequencies between disease and control groups at millions of common single nucleotide polymorphisms (SNPs) across the genome. These differences may be functionally relevant to the disease or may represent loci which are transmitted in linkage disequilibrium with a causative polymorphism. Early GWAS studies of MDD were not promising, despite having sample sizes similar to successful studies for other common diseases and traits, including psychiatric disorders. In a GWAS of over 9000 clinically ascertained MDD cases and 9000 healthy controls conducted by the Psychiatric Genomics Consortium (PGC), no SNPs reached the genome-wide significance threshold [ 4 ]. The CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) Consortium conducted a GWAS of depressive symptoms in over 30,000 individuals which also failed to identify any genetic associations [ 5 ].
Challenges of Depression Genetics
There are several reasons why identifying risk loci for MDD has proven difficult. First, like most complex diseases, depression is a polygenic disorder arising from the combined effect of many genetic variants with individually small effect sizes [ 6 ]. Several sources provide evidence for the polygenic architecture of depression, despite a lack of genome-wide significant loci. Polygenic risk scoring uses association statistics from a discovery GWAS to weight the genotypes of individuals in an independent test sample and sums these effects across multiple SNPs into a polygenic risk score (PRS) [ 7 ]. Differences in PRS between cases and controls in the independent sample show that the PRS is capturing genetic susceptibility that is predictive of disease status. PRS for MDD generated from results of the PGC GWAS showed modest, although significant prediction for depression in independent samples ( R 2 = 0.6%, P < 10 −6 ), consistent with the presence of small genetic effects that the original GWAS was underpowered to detect at genome-wide significance [ 4 ]. SNP heritability ( h SNP 2 of MDD in the PGC GWAS was 0.21 (s.e. 0.021) [ 4 , 9 ], again confirming the polygenic etiology of MDD. Large sample sizes are essential to detect small individual genetic effects, and pooling samples within research consortia has been key to the success of GWAS on many human traits.
The second characteristic of MDD which poses challenges to genetic analysis is its high lifetime prevalence of ~15% [ 1 ]. For a common disorder, the mean difference in phenotypic liability between case and control groups is smaller, for both unscreened and screened controls, and thus power to detect allele frequency differences between them is reduced. Power calculations show that samples 2.4-fold larger are needed for GWAS of MDD compared with schizophrenia (prevalence 1%), to identify a variant that explains the same proportion of risk [ 10 ]. Third, the heritability of MDD is modest, at 37%, compared with other psychiatric disorders, meaning that risk alleles are likely to have smaller effect sizes [ 3 , 11 ]. To account for this lower heritability, samples 4–5 times larger would be required for MDD than schizophrenia to capture an equal amount of genetic variance [ 10 ].
Finally, depression is a particularly heterogeneous disorder. Some genetic heterogeneity is inherent to polygenicity; affected individuals may have different combinations of risk alleles and unaffected individuals will also carry many of these variants. But subphenotyping of the nine core symptoms of MDD indicates that almost 1500 symptom combinations can fulfill the diagnostic criteria and that two patients with a diagnosis of MDD may not have a single symptom in common [ 12 ]. Subtypes of depression such as recurrence or early-onset may be more heritable [ 3 , 13 ]. Another striking example of heterogeneity is sex differences, with depression twice as prevalent among women than men and twin studies indicating that ~45% of the genetic liability to MDD is not shared between sexes [ 14 – 16 ]. Polygenic risk scoring methods also enable us to look for genetic similarities across traits and suggest that postpartum depression may be more genetically similar to bipolar disorder, that typical depression shows more pleiotropy with schizophrenia, and that atypical depression, characterized by increased appetite and weight, additionally shares genetic effects with BMI [ 17 , 18 ]. These findings together provide compelling evidence that depression is likely composed of subtypes with differences in biological etiology and a heterogeneous genetic architecture. Therefore, the successful identification of genetic associations with MDD requires either increased sample sizes or empirically driven efforts to reduce heterogeneity. This review will outline recent genetic studies on depression which have adopted such strategies. Studies are described in detail, showing how each has advanced our understanding of the genetic underpinnings of depression, with summary information presented in Table Table1 1 .
Recent genome-wide association studies on depression
MDD major depressive disorder
a On the liability scale, given a prevalence of 15%
b In the discovery cohort, on the liability scale, given a prevalence of 25%
CONVERGE Consortium
The CONVERGE ( C hina, O xford and V irginia Commonwealth University E xperimental R esearch on G enetic E pidemiology) Consortium has collected a large depression cohort with detailed clinical, genetic and environmental data that is a powerful resource to dissect the etiology of depression [ 19 , 20 •]. The study aimed to ascertain a more homogeneous sample by restricting the phenotype to recurrent severe depression in women. Using low-coverage sequencing of 5303 Han Chinese MDD cases and 5337 controls screened to exclude MDD, two SNPs on chromosome 10 showed evidence of association: one near the SIRT1 gene and the other in an intron of LHPP [ 20 •]. Both loci replicated in an independent Chinese sample and the genetic signal at the SIRT1 locus increased when further restricting the sample to melancholia, a more severe subtype of MDD [ 20 •]. This study demonstrates the value of focusing on a homogeneous phenotype where genetic effects should be larger and easier to detect, even at the expense of a smaller sample size. SIRT1 is involved in the biogenesis of mitochondria, which are the cell’s energy-producing organelles. Supporting the genetic association, the CONVERGE consortium report increased mitochondrial DNA in MDD cases versus controls, with the amount of increase positively correlated with stressors such as childhood sexual abuse and lifetime adverse events [ 21 ].
Although these genetic associations are a considerable step forward, the variants identified in individuals of East Asian ancestry have low frequencies in populations of European ancestry, and therefore no replication in the PGC depression samples or other studies has been achieved [ 20 •, 22 •]. The trans-ancestry genetic correlation between the PGC and CONVERGE GWAS results is ~0.3, indicating there are likely population differences in the genetic etiology of MDD, a finding with important implications for future studies [ 23 ]. Further comparison of the studies using genetic correlation and polygenic risk scoring weakly supports an overlap of SNP effects between the studies and strengthens when focusing on female only and recurrent MDD cases from the PGC [ 23 ]. This indicates that some of the genetic differences between the PGC and CONVERGE results may be due to differences in the specific MDD phenotype studied.
Social Science Genetic Association Consortium
The Social Science Genetic Association Consortium (SSGAC) has pursued the alternate strategy of increasing sample size, by analyzing multiple cohorts with heterogeneous measures of depression [ 24 •]. They utilized data from two case-control studies of MDD: summary statistics from the PGC GWAS (9240 MDD cases, 9519 healthy controls) and dbGaP-accessible genotypes from the GERA (Resource for Genetic Epidemiology Research on Adult Health and Aging) study (7231 MDD cases, 49,316 controls) [ 4 , 25 ]. These clinical samples were meta-analyzed with a GWAS on a measure of depressive symptoms in the UK Biobank, where adults in the general population were asked two questions about feelings of unenthusiasm or disinterest and depression or hopelessness in the past 2 weeks [ 26 ]. Combining these datasets resulted in a sample of 180,866 individuals and found two genome-wide significant associations with “depressive symptoms” which replicated on look up in an independent depression GWAS by 23andMe [ 24 •]. One SNP is in the KSR2 (kinase suppressor of ras 2) gene and the other is in the DCC gene, which encodes a transmembrane receptor involved in axon guidance. The h SNP 2 for depressive symptoms from the total sample was 0.04 (s.e. 0.004), which is considerably lower than the estimates from clinically ascertained MDD samples (~0.2 in both the PGC and CONVERGE studies) [ 9 , 27 ]. This may result from mixing heterogeneous measures of depression which are influenced by different combinations of genetic variants and the weak information on depression symptoms from just two questions. Nevertheless, the SSGAC attributes the success of their study to exploiting the genetic correlation between clinical depression and depressive symptoms to combine studies and increase sample size [ 24 •]. While such a strategy may increase power for individual SNPs which influence both clinical depression and depressive symptoms, it may dilute associations for SNPs which only play a role in one phenotype and this has implications for replicating specific associations in different samples.
The direct-to-consumer genetic testing company 23andMe (Mountain View, CA) also took the approach of increasing sample size. They used self-report data from consumers who participated in their research initiative and ascertained 75,607 individuals reporting previous clinical diagnosis or treatment for major depression and 231,747 individuals reporting no history of depression [ 22 •]. They carried out meta-analysis of these results with the PGC GWAS results and then analyzed a replication sample of an additional 45,773 cases and 106,354 controls from 23andMe. A total of 17 independent SNPs from 15 regions reached genome-wide significance after joint analysis over all three data sets (Table (Table1) 1 ) [ 22 •]. Two of the loci were significant in both the meta-analysis and independent replication sample. In a locus spanning MEF2C (myocyte enhancer factor 2C) and TMEM161B (transmembrane protein 161B), two independent SNPs were significant. MEF2C is a transcription factor which plays a role in synaptic learning and memory and variants in the gene have been implicated in epilepsy, mental retardation, and schizophrenia [ 28 – 30 ]. The other locus encompasses the NEGR1 gene, encoding neuronal growth regulator 1, which is involved in neurite outgrowth [ 31 ].
The strategy of less intensive phenotyping used in this study is a novel approach in psychiatric research, as cases have traditionally been ascertained using structured clinical interviews. To demonstrate the validity of the self-report measure, the authors calculated the genetic correlation between the results from the 23andMe study and those from the PGC GWAS. There was a high positive correlation of 0.72 (s.e. 0.09) between the results indicating common variant genetic overlap [ 22 •]. However the h SNP 2 from the meta-analysis of the 23andMe discovery cohort and the PGC GWAS was 0.06, showing a substantially lower genetic component than the PGC h SNP 2 estimate of 0.21 [ 9 , 22 •]. This indicates that while the phenotypes are genetically correlated, the genetic signal in the 23andMe sample is likely weaker than in the PGC, which could reasonably be due to some diagnostic misclassification. The success of this 23andMe study in identifying genetic variants at genome-wide significance shows that large sample size can outweigh any reduction in power from additional heterogeneity or limited clinical information. Genotyping is now inexpensive compared with conducting detailed clinical interviews and 23andMe’s light-phenotyping approach may be more likely to attract the large number of participants required in the absence of high-quality phenotype information.
CHARGE Consortium and PGC
Depression can be conceptualized along a spectrum of severity from subthreshold or minor depression to MDD of varying severity (e.g., mild, moderate, severe). Using a continuum approach to depression may augment statistical power because sample size can be increased substantially and individuals who fall anywhere along the phenotypic spectrum can be included. This was the rationale for combining the results of the CHARGE consortium GWAS of depressive symptoms and the PGC GWAS on MDD [ 32 •]. Depressive symptoms were evaluated in individuals over 40 years old using validated questionnaires (mostly using the Center for Epidemiological Studies Depression Scale CES-D), which focused on depressive symptoms in the previous weeks rather than lifetime. This meta-analysis of a broad depression phenotype identified one genome-wide significant SNP, which replicated in an independent sample comprising newly ascertained MDD cases from the PGC and individuals assessed for depressive symptoms from the Health and Retirement Study [ 32 •]. The SNP is located in an intron of FHIT , which is expressed in several brain regions and encodes a tumor suppressor protein also involved in oxidative stress and the circadian clock [ 32 •].
In this study, the genetic correlation ( r g ) between depressive symptoms and MDD was 1.00 (s.e. 0.2) which supports the concept of a depression continuum capturing similar genetic underpinnings to a study of depression cases and controls. Notably the h SNP 2 of the broad depression phenotype was 0.3 (s.e. 0.04), which was greater than the h SNP 2 of depressive symptoms or MDD separately (0.04 (s.e. 0.01) and 0.21 (s.e. 0.02), respectively) [ 32 •]. Testing the genetic correlation between different phenotypic measures before combining them can be informative about heritability in the subsequent sample and can be used to assess whether the sample size achieved will be sufficient to outweigh any heterogeneity introduced.
Power and Study Design
The power of these studies to identify MDD-associated variants differs considerably by sample size and design. We calculated the genotype relative risk (GRR) which the study had 50% power to identify (Table (Table1), 1 ), assuming a multiplicative model, allele frequency of 0.3, MDD prevalence of 15%, and fully screened controls [ 33 ]. The power of 50% was chosen to reflect the polygenic architecture of MDD, where many SNPs of modest effect sizes contribute, and each study has low power to detect a specific variant, but higher power to detect a subset of SNPs having a pre-specified GRR. Using standard power calculations, the 23andMe study would have 50% power to detect a variant with GRR 1.024, but the PGC MDD study could only detect a GRR of 1.11. However, such power calculations make simplistic assumptions about study design, for example that selected participants are divided into MDD cases and controls (defined as non-cases), with cases generally being over-sampled from the population. In practice, studies such as CONVERGE and some PGC MDD cohorts select severe, recurrent cases of MDD and exclude any individuals with mild to moderate depression. This selection of severe cases and healthier controls with no history of depression increases the power of the study by inducing a larger difference in allele frequency between cases and controls. In contrast, study power will be reduced by any misclassification of cases and controls, which may be more likely in studies based on self-report or limited phenotypic information at a single time point.
Two of the studies listed in Table Table1 1 use a quantitative phenotype of the number of depressive symptoms (SSGAC, CHARGE). The CHARGE study of 51,258 participants would have 50% power to detect a variant accounting for 0.0058% of trait variance. A study of 180,000 participants, similar to SSGAC, could detect a variant accounting for 0.017% of trait variance (with 50% power), but the SSGAC study used only two questions on depressive symptoms, reducing its power from this theoretical value.
The studies described here illustrate two approaches to dissect the genetic contribution to depression: through a case-control study of lifetime diagnosis of depression or using a continuous measure of the count of depressive symptoms, usually covering the previous 2 weeks. Although the time scales for these measures differ, the genetic correlation between these measures is high, for example r g = 1 between CHARGE and the PGC MDD study [ 32 •]. The relationship between the power of a case-control and continuous phenotype was derived by Yang et al. [ 34 ] and shows that a cohort study with a continuous phenotype on N individuals has lower power than a case-control study with N /2 cases and N /2 controls when the disease prevalence is below 10%. This validates the design of studies such as CONVERGE, ascertaining recurrent cases of MDD where the population prevalence in China is already low at 3.6% [ 35 ]. In Western countries where MDD prevalence is 15–20%, studies based on an underlying quantitative trait may have higher power than an equivalently sized case-control study.
Studies must balance the trade-off between gains in power from increased sample size or reduced heterogeneity. As the results of CONVERGE and the 23andMe studies show, both approaches can be successful in identifying genetic variants for depression, and researchers need to decide which strategy maximizes the use of their resources. Since depression is a common disorder, large sample sizes can be accrued through consortia and inventive new methods such as leveraging electronic medical records, population biobanks, and online recruitment. One limitation of mixing heterogeneous measures of depression or less intensive phenotyping is that any associations discovered may be more difficult to interpret. But the approach of increasing sample size can be used to find loci whose role in MDD can then be dissected in follow-up samples with more detailed phenotypic data, even if these have smaller sample size. Large samples with different depression phenotypes will help to disentangle the genetic background of different forms of depression.
Environment
While the focus of this review is on genetics, the role of the environment in depression cannot be ignored, with twin studies showing that it accounts for 63% of the variance [ 3 ]. In contrast to genetic associations, the environmental risk factors are well-established and include social isolation, unemployment, and relationship stressors [ 36 ]. Childhood abuse or neglect is one of the strongest environmental risk factors, more than doubling the risk for depression in adult life [ 37 ]. Gene-by-environment interactions (G×E) whereby genetic effects are moderated by specific environmental factors have long been postulated to play a role in depression. Most G×E research has focused on candidate genes such as the serotonin transporter promoter polymorphism ( 5-HTTLPR ) interacting with stressful life events or childhood trauma. Over a decade’s worth of studies on this interaction has produced inconsistent results, and recently, an extensive, pre-registered meta-analysis concluded a lack of evidence for the 5-HTTLPR interaction with environmental adversity [ 38 •].
Since the genetic liability for depression is known to be polygenic, studies have begun to test for interactions between environmental factors and polygenic risk scores, which capture the cumulative effect of many common variants in a single measure. To date, two studies have reported no interaction between PRS for MDD and adult stressful life events in the etiology of depression [ 39 , 40 ]. Two studies have found significant interactions between PRS for MDD and childhood trauma, albeit in opposing directions [ 39 , 41 ]. The reason for these discrepant results is unclear but further research is warranted as the detection of G×E has implications for future research strategies to identify genetic associations. In the Netherlands Study of Depression and Anxiety (NESDA), PRS had a stronger effect on MDD in individuals exposed to childhood trauma, which suggests that focusing on exposed individuals could render genetic effects larger, more homogeneous and easier to detect [ 41 ]. However, in the RADIANT UK study, the effect of PRS on MDD risk was stronger in those unexposed to childhood trauma, suggesting that more power could be leveraged from GWAS by focusing only on individuals not exposed to trauma, as these MDD cases may have a stronger genetic predisposition. In summary, the analysis of cohorts with heterogeneous environmental exposures may also contribute to the difficulty in identifying genetic associations with MDD. Thus far, SNPs have been analyzed across average environmental backgrounds in GWAS but reducing environmental heterogeneity could be a valuable strategy to increase genetic effect sizes. There is a need for depression samples with good quality environmental data, which now can be more expensive and difficult to attain than genotype data.
Conclusions
The first progress has been made towards identifying genetic variants involved in MDD with studies amassing the critical sample size necessary to reach an inflection point beyond which the number of genetic associations is expected to increase linearly with sample size [ 42 •]. The critical goal of GWAS is to identify the biological pathways underpinning depression and even risk alleles with small effects could yield enormous insights. As sample sizes continue to increase, MDD GWAS will uncover more and more of the genetic architecture of this debilitating disorder, as we have seen in GWAS studies on schizophrenia [ 30 ]. The next challenge is to establish the molecular mechanisms by which GWAS loci mediate their effects and translate these into much-needed new biomarkers and therapeutic targets. We have turned the corner in identifying genetic variants for depression, and the next few years will bring exciting opportunities to turn biological findings into clinical tools.
Acknowledgements
This paper represents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Compliance with Ethical Standards
Conflict of interest.
Niamh Mullins and Cathryn Lewis each declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Genetic Disorders
Papers of particular interest, published recently, have been highlighted as: • Of importance
- Share full article
Advertisement
Supported by
Study Suggests Genetics as a Cause, Not Just a Risk, for Some Alzheimer’s
People with two copies of the gene variant APOE4 are almost certain to get Alzheimer’s, say researchers, who proposed a framework under which such patients could be diagnosed years before symptoms.

By Pam Belluck
Scientists are proposing a new way of understanding the genetics of Alzheimer’s that would mean that up to a fifth of patients would be considered to have a genetically caused form of the disease.
Currently, the vast majority of Alzheimer’s cases do not have a clearly identified cause. The new designation, proposed in a study published Monday, could broaden the scope of efforts to develop treatments, including gene therapy, and affect the design of clinical trials.
It could also mean that hundreds of thousands of people in the United States alone could, if they chose, receive a diagnosis of Alzheimer’s before developing any symptoms of cognitive decline, although there currently are no treatments for people at that stage.
The new classification would make this type of Alzheimer’s one of the most common genetic disorders in the world, medical experts said.
“This reconceptualization that we’re proposing affects not a small minority of people,” said Dr. Juan Fortea, an author of the study and the director of the Sant Pau Memory Unit in Barcelona, Spain. “Sometimes we say that we don’t know the cause of Alzheimer’s disease,” but, he said, this would mean that about 15 to 20 percent of cases “can be tracked back to a cause, and the cause is in the genes.”
The idea involves a gene variant called APOE4. Scientists have long known that inheriting one copy of the variant increases the risk of developing Alzheimer’s, and that people with two copies, inherited from each parent, have vastly increased risk.
The new study , published in the journal Nature Medicine, analyzed data from over 500 people with two copies of APOE4, a significantly larger pool than in previous studies. The researchers found that almost all of those patients developed the biological pathology of Alzheimer’s, and the authors say that two copies of APOE4 should now be considered a cause of Alzheimer’s — not simply a risk factor.
The patients also developed Alzheimer’s pathology relatively young, the study found. By age 55, over 95 percent had biological markers associated with the disease. By 65, almost all had abnormal levels of a protein called amyloid that forms plaques in the brain, a hallmark of Alzheimer’s. And many started developing symptoms of cognitive decline at age 65, younger than most people without the APOE4 variant.
“The critical thing is that these individuals are often symptomatic 10 years earlier than other forms of Alzheimer’s disease,” said Dr. Reisa Sperling, a neurologist at Mass General Brigham in Boston and an author of the study.
She added, “By the time they are picked up and clinically diagnosed, because they’re often younger, they have more pathology.”
People with two copies, known as APOE4 homozygotes, make up 2 to 3 percent of the general population, but are an estimated 15 to 20 percent of people with Alzheimer’s dementia, experts said. People with one copy make up about 15 to 25 percent of the general population, and about 50 percent of Alzheimer’s dementia patients.
The most common variant is called APOE3, which seems to have a neutral effect on Alzheimer’s risk. About 75 percent of the general population has one copy of APOE3, and more than half of the general population has two copies.
Alzheimer’s experts not involved in the study said classifying the two-copy condition as genetically determined Alzheimer’s could have significant implications, including encouraging drug development beyond the field’s recent major focus on treatments that target and reduce amyloid.
Dr. Samuel Gandy, an Alzheimer’s researcher at Mount Sinai in New York, who was not involved in the study, said that patients with two copies of APOE4 faced much higher safety risks from anti-amyloid drugs.
When the Food and Drug Administration approved the anti-amyloid drug Leqembi last year, it required a black-box warning on the label saying that the medication can cause “serious and life-threatening events” such as swelling and bleeding in the brain, especially for people with two copies of APOE4. Some treatment centers decided not to offer Leqembi, an intravenous infusion, to such patients.
Dr. Gandy and other experts said that classifying these patients as having a distinct genetic form of Alzheimer’s would galvanize interest in developing drugs that are safe and effective for them and add urgency to current efforts to prevent cognitive decline in people who do not yet have symptoms.
“Rather than say we have nothing for you, let’s look for a trial,” Dr. Gandy said, adding that such patients should be included in trials at younger ages, given how early their pathology starts.
Besides trying to develop drugs, some researchers are exploring gene editing to transform APOE4 into a variant called APOE2, which appears to protect against Alzheimer’s. Another gene-therapy approach being studied involves injecting APOE2 into patients’ brains.
The new study had some limitations, including a lack of diversity that might make the findings less generalizable. Most patients in the study had European ancestry. While two copies of APOE4 also greatly increase Alzheimer’s risk in other ethnicities, the risk levels differ, said Dr. Michael Greicius, a neurologist at Stanford University School of Medicine who was not involved in the research.
“One important argument against their interpretation is that the risk of Alzheimer’s disease in APOE4 homozygotes varies substantially across different genetic ancestries,” said Dr. Greicius, who cowrote a study that found that white people with two copies of APOE4 had 13 times the risk of white people with two copies of APOE3, while Black people with two copies of APOE4 had 6.5 times the risk of Black people with two copies of APOE3.
“This has critical implications when counseling patients about their ancestry-informed genetic risk for Alzheimer’s disease,” he said, “and it also speaks to some yet-to-be-discovered genetics and biology that presumably drive this massive difference in risk.”
Under the current genetic understanding of Alzheimer’s, less than 2 percent of cases are considered genetically caused. Some of those patients inherited a mutation in one of three genes and can develop symptoms as early as their 30s or 40s. Others are people with Down syndrome, who have three copies of a chromosome containing a protein that often leads to what is called Down syndrome-associated Alzheimer’s disease .
Dr. Sperling said the genetic alterations in those cases are believed to fuel buildup of amyloid, while APOE4 is believed to interfere with clearing amyloid buildup.
Under the researchers’ proposal, having one copy of APOE4 would continue to be considered a risk factor, not enough to cause Alzheimer’s, Dr. Fortea said. It is unusual for diseases to follow that genetic pattern, called “semidominance,” with two copies of a variant causing the disease, but one copy only increasing risk, experts said.
The new recommendation will prompt questions about whether people should get tested to determine if they have the APOE4 variant.
Dr. Greicius said that until there were treatments for people with two copies of APOE4 or trials of therapies to prevent them from developing dementia, “My recommendation is if you don’t have symptoms, you should definitely not figure out your APOE status.”
He added, “It will only cause grief at this point.”
Finding ways to help these patients cannot come soon enough, Dr. Sperling said, adding, “These individuals are desperate, they’ve seen it in both of their parents often and really need therapies.”
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
The Fight Against Alzheimer’s Disease
Alzheimer’s is the most common form of dementia, but much remains unknown about this daunting disease..
How is Alzheimer’s diagnosed? What causes Alzheimer’s? We answered some common questions .
A study suggests that genetics can be a cause of Alzheimer’s , not just a risk, raising the prospect of diagnosis years before symptoms appear.
Determining whether someone has Alzheimer’s usually requires an extended diagnostic process . But new criteria could lead to a diagnosis on the basis of a simple blood test .
The F.D.A. has given full approval to the Alzheimer’s drug Leqembi. Here is what to know about i t.
Alzheimer’s can make communicating difficult. We asked experts for tips on how to talk to someone with the disease .

COMMENTS
The genetic dissection of major depressive disorder (MDD) ranks as one of the success stories of psychiatric genetics, with genome-wide association studies (GWAS) identifying 178 genetic risk loci ...
Depression is a complex psychiatric disorder involving several environmental, genetic, and epigenetic factors. About 30-40% of all types of depression are genetic (Edvardsen et al., 2009). Until now, genetic, and non-genetic studies are still on the way to understanding the complex mechanism of this disease, and there are still many questions ...
Depression. Depression (lat. depressio—gloominess, oppression) is a psychiatric disorder characterized by a pathologically low mood (hypothymia) and negative esteem about oneself, one's status in the real world, and one's future ().In other words, the major depressive disorder is a complex and heterogeneous illness with an etiopathogenesis that is based upon multiple factors that may act at ...
Depression is known to run in families; people with major depressive disorder are three times more likely than those without the disorder to have a first degree relative who also has depression. 23 Twin studies, which allow for simultaneous quantification of genetic and environmental influences, suggest that depression is moderately heritable.
Major depressive disorder (MDD) is a common, debilitating, phenotypically heterogeneous disorder with heritability ranges from 30% to 50%. Compared to other psychiatric disorders, its high prevalence, moderate heritability, and strong polygenicity have posed major challenges for gene-mapping in MDD. ….
By Bill Hathaway. May 27, 2021. (© stock.adobe.com) A massive genome-wide association study (GWAS) of genetic and health records of 1.2 million people from four separate data banks has identified 178 gene variants linked to major depression, a disorder that will affect as many as one in every five people during their lifetimes.
In this article we review recent findings from genetic association studies and G×E studies related to depression, and outline areas for future research. Several excellent reviews of this literature aimed at the genetic research community have already been published (see, e.g., references 28-33 ). We aim to provide a review for a broad ...
Genome-wide association studies find that a large number of genetic variants jointly influence the risk of depression, which is summarized by polygenic indices (PGIs) of depressive symptoms and major depression. But PGIs by design remain agnostic about the causal mechanisms linking genes to depression. Meanwhile, the role of adverse life ...
If genes are part of the cause, we expect a patient's identical twin to have a much higher risk of disease than a patient's non-identical twin. That is the case for major depression. Heritability is probably 40-50%, and might be higher for severe depression. This could mean that in most cases of depression, around 50% of the cause is ...
Major depressive disorder and the neuroticism personality trait have overlapping genetic susceptibilities. Most genetic studies of MDD have considered a small set of functional polymorphisms relevant to monoaminergic neurotransmission. Meta-analyses suggest small positive associations between the polymorphism in the serotonin transporter ...
Candidate gene studies focused on genes involved in the pathophysiology of depression led to conflicting results and unpowered associations, primarily due to the small sample sizes and different races and populations. 14 The development of new technologies and bioinformatic methodologies, as well as the formation of consortiums that allow ...
Importance: Adolescent depression is characterized by diverse symptom trajectories over time and has a strong genetic influence. Research has determined genetic overlap between depression and other psychiatric conditions; investigating the shared genetic architecture of heterogeneous depression trajectories is crucial for understanding disease etiology, prediction, and early intervention.
The interaction of both genetic and environmental factor can play a role in the development of depression [4]. Gender, age, socioeconomic status, stressful life events, childhood adversity, and co ...
Depression is a deeply debilitating disorder that affects over 21 million American adults annually. The repercussions are severe, leading to an astonishing loss of approximately 200 million ...
Research on the Genetics of Mental Illness. In one study published in The Lancet, scientists found that certain genetic glitches were associated with five disorders, suggesting a shared underlying genetic vulnerability. The five disorders are: Autism. Attention deficit hyperactivity disorder (ADHD) Bipolar disorder.
Heim, C. & Binder, E. B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 233 ...
Evidence suggests that such approaches are not without problems and may produce genetic signals that are less specific as they likely confound the loci that predispose to clinical depression with those that impact on risk for mild dysphoria. 13 Nonetheless, replicated genetic loci for depressive phenotypes account for less than 5% of the ...
Research suggests that individuals with a family history of depression are more likely to develop the condition. Studies with twins have shown that there is a higher concordance rate for depression in identical twins compared to fraternal twins, indicating a genetic predisposition.
Rather, it has been suggested that there are over a hundred different genetic variants that are weakly involved in depression, none of which are necessary or sufficient for the development of depression and many of which are not specific to depression (Howard et al., Citation 2019). Furthermore, such research in molecular genetics has yielded a ...
Genetic risk for mental disorders changes people's sensitivity to the environment. Imagine two people, one with a high genetic risk for depression and one with a low risk. They both lose their jobs.
The heritability of treatment-resistant depression (TRD) was found to have significant genetic overlap with schizophrenia, attention deficit disorder, cognitive, alcohol and smoking traits, and ...
There are several types of genetic disorders. In some cases, a genetic change in a single gene can cause someone to have a disease or condition. In other cases, the gene does not have a genetic change, but a person has more or fewer copies of the gene than most people, and this causes a disease or condition.
The researchers found inconsistent or various degrees of background depression from the F 2 to F 3 generation assuming different genotypes of the target locus, which may affect the actual ...
Earlier research has demonstrated a notable intersection between coronary artery disease and major depression, indicating that as many as 44% of individuals with coronary artery disease also ...
New research suggests people tend to be lonelier in young adulthood and late life. But experts say it doesn't have to be that way. By Christina Caron When Surgeon General Vivek Murthy went on a ...
Depression is a complex disorder with a heritability of 37% estimated from twin studies [ 3 ]. Despite robust evidence for a genetic component, identifying the specific genetic variants involved in the disorder has been a major challenge. Genome-wide association studies (GWAS) test differences in allele frequencies between disease and control ...
A study suggests that genetics can be a cause of Alzheimer's, not just a risk, raising the prospect of diagnosis years before symptoms appear. Determining whether someone has Alzheimer's ...