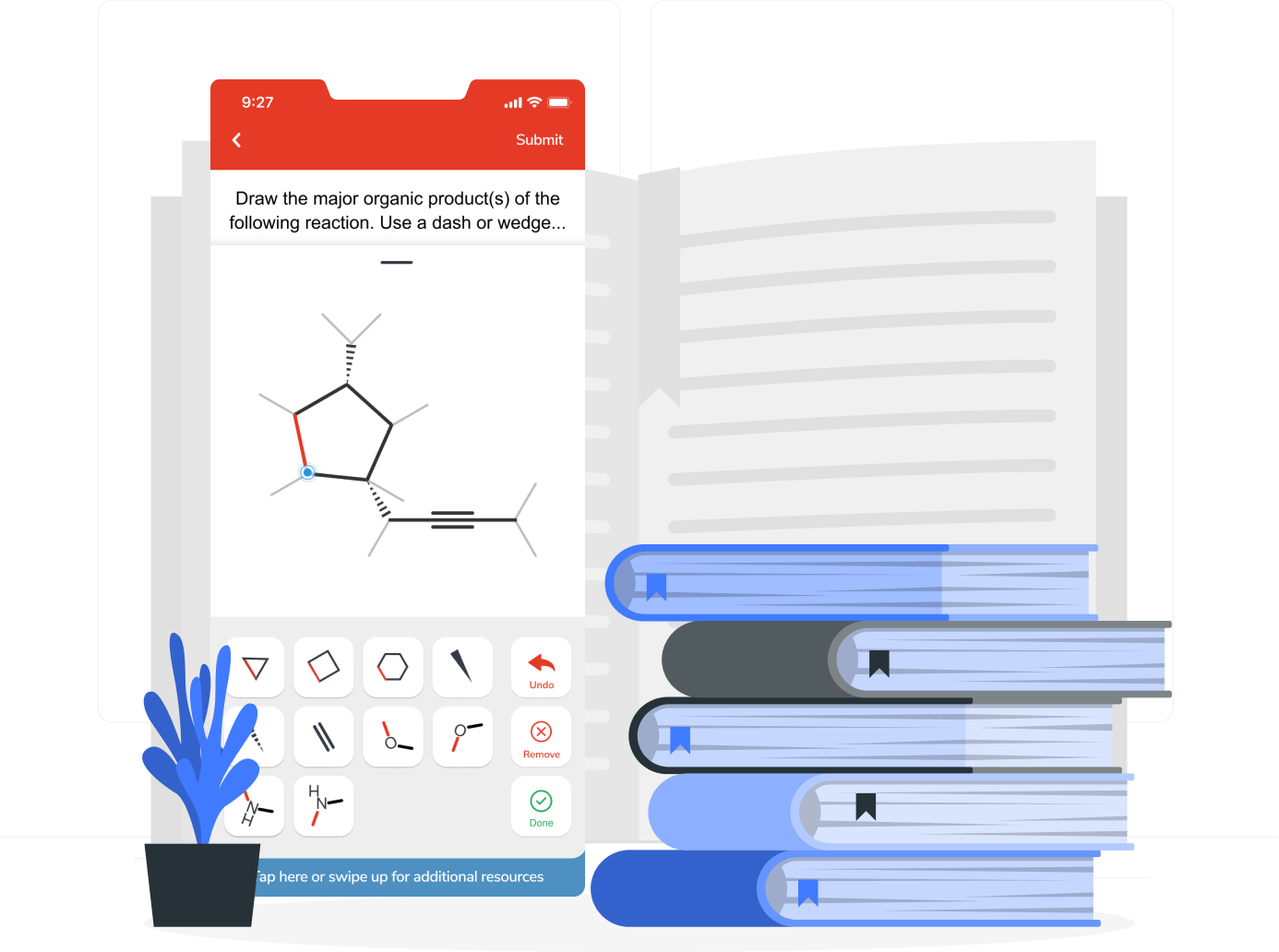
- Book A Demo

Homework & Practice
Aktiv Chemistry is a comprehensive online homework platform that helps students build mastery of chemistry. Take advantage of over 15,000 built-in chemistry problems, scaffolded practice, a powerful instructor dashboard, LMS grade syncing, and much more.

All-in-one platform
Comprehensive learning & assessment.
From in-class active learning, homework assignments, and now secure online quizzes and exams, Aktiv Chemistry’s all-in-one platform provides a comprehensive set of features for formative and summative assessments.
In-class or Synchronous Online
Take attendance, post polls & quizzes, enhance traditional worksheets, or promote think-pair-share activities. Learn More
Assign interactive problem sets with instant feedback and automatic grading that sync with institutional LMS gradebooks.

Timed Online Quizzes & Exams
Create summative assessments with question pools, algorithmic problems, and optional integrated proctoring. Learn More
15,000 PROBLEMS OR WRITE YOUR OWN
Extensive content library.
Building engaging classroom activities has never been easier. Aktiv Chemistry provides an extensive library of questions for chemistry courses such as General Chemistry, Introductory Chemistry, GOB Chemistry, Organic Chemistry. The content library includes discipline-specific problem types such as molecule drawing, chemical equations and naming, dimensional analysis, numerical entry, and more.
Easy and flexible authoring also allows instructors to add their own questions.
Learn More About
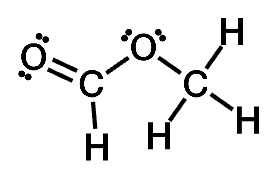
Draw the Lewis structure of (CHO)OCH₃ and then choose the appropriate set of molecular geometries of the three central atoms. Your answer choice is independent of the orientation of your drawn structure.
The solubility of He in water at 25.0 °C is 7.0 × 10⁻⁵ M when the partial pressure of He is 0.20 atm. What is the value of the Henry's law constant for He ?
- monosaccharide
- oligosaccharide
- monopolysaccharide
- heteropolysaccharide
- homopolysaccharide
According to the balanced reaction below, calculate the moles of NH₃ that form when 4.2 mol of N₂H₄ completely reacts 3 N₂H₄(l) → 4 NH₃(g) + N₂(g)
AFFORDABLE & FLEXIBLE
Built to work with any textbook.
Aktiv Chemistry ’s textbook independent approach means instructors have more flexibility for their courses. Build assignments that align to any major publisher textbook or increasingly popular OER options.
For General Chemistry courses, Aktiv Chemistry also offers seamless integration with the OpenStax Chemistry textbooks.
Student Success
More chemistry, less technology.
Drawing chemical structures, entering subscripts and superscripts, and working with significant figures just shouldn’t be hard. That’s why Aktiv Chemistry was designed from the ground up with the student experience in mind. With intuitive user interfaces and scaffolded and visual modules, our goal is to use technology to lower the barrier to chemistry.
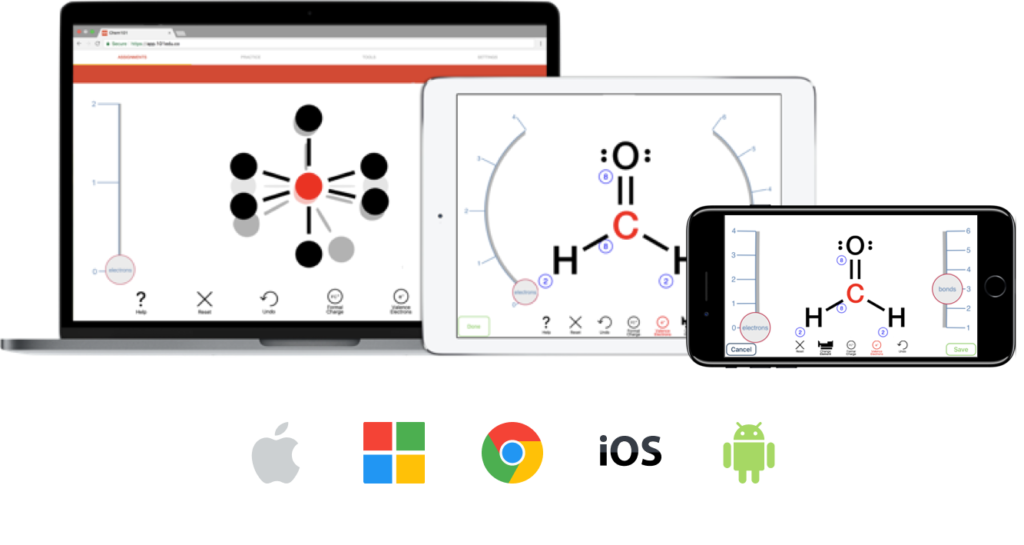
Bring Your Own Device. Any Device.
Students and instructors can access the Aktiv Learning app from any iPhone, iPad, or Android Device. Additionally, the platform is fully accessible on Web with any Mac, PC, or Chromebook.
Aktiv Chemistry ’s mobile-first design ensures that students receive the same experience no matter where they are. Students take advantage of the Aktiv Learning mobile app to work and study on-the-go in places like riding the bus or train, or even on campus when they have downtime.
RANDOMIZATION WITH
Algorithmic problems & question pools.
Aktiv Chemistry offers additional security with both Question Pools and Algorithmic Problems. These features randomize the content that is delivered to each student during homeworks, quizzes, or exams. Instructors can also randomize the order of questions on any assignment.
Built-in variables create thousands of question variants to be delivered that randomize numbers, words, or compounds within the problem statement.
Group together a set of similar questions and deliver a subset of them at random to students.
Problem Pool 1
14 problems in this pool
- Problems to Assign
What is the correct IUPAC name for HBrO(aq)?
What is the correct IUPAC name for Ca(BrO₂)₂?
What is the correct IUPAC name for Ti(ClO₄)₄?
Success Story
Professors from over 700 colleges and universities use Aktiv Chemistry to engage students inside and outside of the classroom. Learn how some of them have transformed their courses.

Helping Students Learn, Interact, and Visualize Chemistry in New Ways

¹ This quote was provided at a time where Aktiv Chemistry was named Chem101. We have replaced the use of Chem101 in any direct quotes with Aktiv Chemistry to minimize confusion.
DESIGNED TO
Support your course, flexible grading policies.
Every Aktiv Chemistry activity has a multitude of grading policies that can be customized depending on the assignment type. Settings include points per problem, participation credit, late submissions with penalties, variable attempts per problem, penalties for incorrect attempts, and more. The Aktiv Chemistry gradebook can be set up to display summary columns or individual columns depending on the assignment type as well.
Industry-leading Support
Any course using Aktiv Chemistry comes with our industry-leading support featuring average response times of 14 minutes and extended hours that include late nights and weekends. Additionally, every Aktiv Chemistry instructor works with an individual member of our Success team that helps with initial course set up, gradebook/LMS integrations, and recommendations for best practices.

LMS Integrated
Aktiv Chemistry’s gradebook can be synced with any popular campus LMS such as Canvas, Blackboard, Moodle, or D2L. The gradebook and associated columns can be customized to separate the various assignment types, to present individual assignments, or display summary columns for each type.
Speak to a Specialist
One of our Learning Specialists will give you a tour of the Aktiv Chemistry or Aktiv Mathematics learning platforms and provide a free instructor playground account with access to the content library.
I Want to Learn More About:

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.E: Homework Chapter 4 Answers
- Last updated
- Save as PDF
- Page ID 208512
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
1. All matter is composed of atoms; atoms of the same element are the same, and atoms of different elements are different; atoms combine in whole-number ratios to form compounds.
3.
a. Sodium---alkali metal
b. Magnesium---Alkaline earth metal
c. Calcium---Alkaline earth metal
d. Chlorine---halogen
e. Potassium---Alkali metal
5. Most of the alpha particles went through the metal sheet because atoms are mostly empty space.
7. Electrons are in orbit about the nucleus.
9. The electron weighs the least. The protons and neutrons are found in the nucleus while electrons are located outside the nucleus.
11. Proton, electron
13. Least, outside
15. a. Z = 8; O; atomic oxygen
b. Z = 27; Co; cobalt
c. Z = 33; As; arsenic
e. Z = 90; Th; thorium
17. a. P b. Mo c. Be
19. a. Ag b. S c. N d. Ne
21. a. 86 b. 74 c. 24 d. 4
23. Column 1 is the alkali metals. Column 2 is the alkaline earth metals. Columns 3 - 12 are the transition metals.
25. The transition metals can exist in more than one charge state. They have variable charge.
27. Calcium and Barium (belong to same group).
29. Magnesium; Beryllium
31. Helium; Xenon; Neon
33. metal; transition metal.
35. a,d
37. a. 1A (1) b. 1A (1) c. 8A (18) d. 6A (16)
39.
41. a and d
43. a and b
45. a. 27 b. 38 c. 54 d. 28
47. a. 10 b. 2 c. 46 d. 30
49. a. Li, Li + , 2nd period, group 1
b. F, F – , 2nd period, group 17
c. Al, Al 3+ , 3nd period, group 13
51. a. 2 b. 3 c. 3 d. 1
53.
a. 1+
b. 2−
c. 2+, 3+
55. SO 3 is sulfur trioxide, while SO 3 2− is the sulfite ion.
57. All isotopes of nitrogen have the same atomic number (Z = 7). Yes, all isotopes of the same element have the same Z.
59. a. Z=1, A=3 b. Z=20, A=42 c. Z=73, A=182 d. Z=24, A=52
61. a. 26 b. 22 c. 2
63. a. b. c. d. e.
65.
a. protons=43; neutrons=54; electrons=43
b. protons=49; neutrons=64; electrons=49
c. protons=28; neutrons=35; electrons=28
d. protons=26; neutrons=29; electrons=26
67.
a. protons=82; neutrons=125; electrons=82
b. protons=8; neutrons=8; electrons=8
c. protons=55; neutrons=82; electrons=55
d. protons=19; neutrons=21; electrons=19
69. This iron atom has 26 protons and 56 − 26 = 30 neutrons.
71. a. He b. B c. Bi & P
73.
75. If "CO" was written as the chemical symbol for cobalt, it would be the same as the chemical formula for carbon monoxide. This ambiguity would create a lot problems such as in labeling and might lead to life-threatening danger. And The chemical symbol is ALWAYS written with the first letter in uppercase and the second letter in lowercase.
77. a) Iron, 26 protons, 24 electrons, and 32 neutrons;
(b) iodine, 53 protons, 54 electrons, and 74 neutrons
79. If the atomic number of uranium is 92, then that is the number of protons in the nucleus. Because the mass number is 235, then the number of neutrons in the nucleus is 235 − 92, or 143.

Chem 101 Homework 9: Rate Laws Question 4
Consider the radioisotope ²²⁶Ra (half life = 1.60 × 10³ years). What is the first order rate constant for ²²⁶Ra?
Answer= ______1/years
1 Expert Answer

J.R. S. answered • 03/11/22
Ph.D. University Professor with 10+ years Tutoring Experience
For a first order reaction, t 1/2 = ln2/k = 0.693/k
1.60x10 3 yrs = 0.693/k
k= 4.3x10 -4 yrs -1
Still looking for help? Get the right answer, fast.
Get a free answer to a quick problem. Most questions answered within 4 hours.
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
RELATED TOPICS
Related questions, how many photons are produced.
Answers · 2
Why does salt crystals dissolve in the water?
Answers · 8
How much copper wire can be made from copper ore?
If a temperature scale were based off benzene..
Answers · 3
why does covalent bonds determine the polarity of water?
Answers · 6
RECOMMENDED TUTORS

find an online tutor
- Chemistry tutors
- Physical Chemistry tutors
- Organic Chemistry tutors
- AP Chemistry tutors
- Biochemistry tutors
- Thermodynamics tutors
- Chemical Engineering tutors
- Heat Transfer tutors
related lessons
- The Words of Chemistry
- Radiation Chemistry
- Introduction to Organic Chemistry
- Drawing Cyclohexane Rings – Organic Chemistry
- Alkanes and Alkenes – Organic Chemistry
- Chemistry Help
- Sachertorte

COMMENTS
CHM 101 Homework Week 4. Course. Fundamentals of Chemistry (CHM101) ... 4-1+Homework - chemistry assignments; Preview text. Ahmed Sohail CHM 101 Professor Marc ... Vessel 1 = equal Vessel 2 = 1 liter 2b. This is due to a change in the enthalpy in vessel 2. 2c. Based on the answer from 2b, the temperature should be relatively the same. 3a. -393 ...
A. Bar has a density of 19.2g/mL. B. Mass of the bar is 11.4kg. C. Volume of the bar is 218L. D. Bar contains 3.49*10^25 atoms. Bar has a density of 19.2g/mL. Why is dissolving of salt in water a chemical change? A. Salt dissociates into ions. B. Water gets saltier. C. Water surrounds the salt compound.
1-2+Homework - chemistry assignments. Assignments None. 14. Chemistry Final Project. Assignments None. 5. ... Chem-101 notes for week 2 lecture; CHM+101L+Distance+Learning+Lab+Safety+Agreement; Unit 01 Rapid Review - Fundamentals of Chemistry Lecture Notes for Chapters 1 through 5 ... Answers. Fundamentals of Chemistry (CHM101) 24 days ago.
Unit 3 CHEM 101 Homework. 85 terms. bugz625. Preview. module three chem 101 problems. 193 terms. Madeline_Vogl. Preview. English. 8 terms. Axel_Benedicto. Preview. Mental Health (Anxiety Level Disorders) 48 terms. Robert_Shaben. Preview. Chem Exam #3 Chem101 Ch 7-02. 15 terms. maddiejb13.
View CHEM_101_Homework_4_Answer_Key.pdf from CHEM 101 at Brigham Young University. Chemistry 101 Written Homework 4 Name Key 1. There are three naturally occurring isotopes of uranium: 238U with an
Chemistry is the study of matter and the changes it undergoes. Here you can browse chemistry videos, articles, and exercises by topic. We keep the library up-to-date, so you may find new or improved material here over time. Unit 1: Atoms, compounds, and ions.
Chemistry. Chemistry questions and answers. Chem 101 Study Guide for Chapter 4 Selected practice problems from the end of each chapter are recommended; these problems are the appropriate section. It's great to do more than those listed. elisted in parentheses i Chapter 4: Types of Chemical Reactions and Solution Stoichiometry (Questions: 2, 3 ...
the charge of the ion. A+BX --> AX+B (ion in solution is replaced through oxidation of an element) displacement reaction. Any metal on the activity series can be (oxidized/reduced) by the ions of elements below it. oxidized. Only metals above _____ in the activity series are able to react with acids to form H2. hydrogen.
Chemistry. Chemistry questions and answers. CHEM 101 Worksheet, Chapter 4: Reactions in Aqueous Solu Multiple choice questions 1-10: circle the correct answer (A-E) Show your work questions 11-14: fill in the answer 2E.CO. 1. Which of the following pairs are both electrolytes? A. NaOH and SO, B.H.SO, and NH.CIC.CH OH and C2H5OHD. HNO2 and H2CO3 2.
Chemistry questions and answers. LAB MANUAL CHEM 101 - GENERAL CHEMISTRY LAB 4 CHEMICAL TESTS FOR ANIONS REVIEW QUESTIONS QI. Specify the acidic and basic radicals of the following salts. Basic Radical (Cation) Acidic Radical (Anion) Salt Sodium carbonate Potassium Sulphate Silver Chloride Q2. Write the symbols of the following anions and ...
View Homework Help - Homework Chapter 4 ANSWERS from CHEM 101 at Qatar University. CHEM 101 Worksheet, Chapter 4: Reactions in Aqueous Solutions I Multiple choice questions 1-10: circle the correct
We've taken extra time to make sure there will absolutely be zero disruption to current Winter and Spring courses. The existing Chem101 mobile and web applications will remain named as Chem101 until May 2022. No URLs or links will change. Starting in May 2022, the Chem101 applications will be renamed to Aktiv Chemistry.
Homework. & Practice. Aktiv Chemistry is a comprehensive online homework platform that helps students build mastery of chemistry. Take advantage of over 15,000 built-in chemistry problems, scaffolded practice, a powerful instructor dashboard, LMS grade syncing, and much more. Book a Demo.
View Chapter 4.4 Homework_ Introduction to Chemistry - CHM101_1004.pdf from CHM 101 at Eastern Gateway Community College. 7/10/2021 Chapter 4.4 Homework: Introduction to Chemistry -
Potassium---Alkali metal. 5. Most of the alpha particles went through the metal sheet because atoms are mostly empty space. 7. Electrons are in orbit about the nucleus. 9. The electron weighs the least. The protons and neutrons are found in the nucleus while electrons are located outside the nucleus. 11.
D. CO2 will be more soluble at lower pressures. water and ethanol. Which of the following liquid pairs would be immiscible? I. carbon tetrachloride (CCl4) and hexane (C6H14) II. water and ethanol (C2H5OH) III. water and carbon tetrachloride (CCl4) solid, liquid and gas. Which of the following phases does a sample of water exist in at its triple ...
Chemistry questions and answers. Chem 101 Study Guide for Chapter 5 Selected practice problems from the end of each chapter are recommended; these problems are listed in parentheses in the appropriate section. It's great to do more than those listed Chapter 5: Gases (Questions: 1, 3, 11, 15, 23, 25, 29, 33, 37, 41, 47, 59, 63, 65, 73, 75, 97 ...
Get the right answer, fast. Ask a question for free. Get a free answer to a quick problem. Most questions answered within 4 hours. OR. Find an Online Tutor Now. Choose an expert and meet online. No packages or subscriptions, pay only for the time you need. Chem 101 Homework 9: Rate Laws Question 4.
Two or more reactants combine to form a new compound. Decomposition Reactions. Single compound decomposes to form two or more products. Displacement Reactions. Cation or anion or both switch. Combustion Reaction. Process of combining with O2. Study with Quizlet and memorize flashcards containing terms like Dissociation, Separated Ions ...
Chemistry with ALEKS 360 access code | 12th Edition. ISBN-13: 9781259974380 ISBN: 1259974383 Authors: Chang, Raymond, Raymond Chang, Raymond Chang, Kenneth Goldsby Rent | Buy. This is an alternate ISBN. View the primary ISBN for: Chemistry 12th Edition Textbook Solutions.
the sum of the kinetic and potential energies of all of the particles that compose a system, is a state functions, ∆E=E (final)-E (initial) ∆E=E (products)-E (reactants) state function. means that something's value depends on the state of the system. q (heat) + system gains thermal energy.
CHEM 101 Recitation Chapter 2 Note: Give your answer with correct number of Significant Figures! 1. Identify each of the following numbers as measured or exact, and write the number of significant figures (SFs) in each of the measured numbers: c) 3.40 × 1. Solutions available. CHEM 101. Grand Canyon University.