- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- American Society of Hematology
- ASH Priorities for Sickle Cell Disease and Sickle Cell Trait

Sickle Cell Research Priorities
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
ASH has developed the following list of sickle cell disease (SCD) research priorities for the next five years. This list includes unaddressed questions and specific research topics that could move the field forward with the hope of curing SCD in the future. The priorities are not listed in rank order.
A. Identify Predictors of Disease Severity
SCD is highly variable clinically, with some patients having a relatively mild course and extended survival and others having frequent and severe complications along with markedly shortened survival. Over the past several decades, researchers have learned about the effects of hemoglobin gene polymorphisms and hemoglobin F levels on the disease course. However, the evidence suggests that information about the hemoglobin genes alone is not sufficient to understand or predict the course of an individual’s disease. Identification of various predictors of disease severity will be vital in the management and treatment of SCD, especially since more recently, several plasma biomarkers and certain genetic polymorphisms have been proposed to influence specific clinical outcomes, including stroke, sickle cell nephropathy, and survival.
Unaddressed Questions:
- Can specific biomarkers and/or genetic polymorphisms identify patients at high risk for clinical events, such as acute chest syndrome, vaso-occlusive episode, or progression of SCD nephropathy?
- Can specific biomarkers and/or genetic polymorphisms identify "responders" vs. "nonresponders" in clinical trials of current and new pharmacologic interventions?
- Can we more precisely define genotype-phenotype relationships?
- What is the role of environmental factors and comorbidities in disease progression?
- What do genetic and biological markers tell us about pathophysiologic mechanisms?
Specific Research Priorities:
- Studies of biomarkers and/or genetic polymorphisms as means of identifying patients at high risk for clinical events, such as acute chest syndrome, stroke, vaso-occlusive episode, or progression of sickle cell nephropathy.
- Study of biomarkers and genetic polymorphisms and their relationships to pathophysiologic mechanisms, including new animal or in vitro models of disease mechanisms.
- Studies of biomarkers or genetic markers in the context of clinical drug trials, in order to determine whether response (or lack thereof) may be predictable, allowing for more personalized therapeutic decisions.
B. Optimize the Use of Existing Therapies
Transfusions and hydroxyurea are the only widely available disease-modifying therapies for SCD, but their effectiveness is currently limited by inadequate utilization, a patient subgroup with less than optimal response, and no FDA-approved adjunct therapies. Evidence-based guidelines are now available for the initiation and use of transfusions and hydroxyurea as well as iron chelation therapy to manage transfusion-acquired hemosiderosis. Emphasis should be placed on improving adherence to these evidence-based therapies and on determining whether these therapies can prevent or even reverse organ dysfunction. In addition, research is needed on new adjunct therapies to blood transfusion and hydroxyurea, as well as disease-specific therapies for co-morbidities such as kidney disease, hypertension, obstructive lung disease and pulmonary hypertension. Further therapeutic trials should include SCD genotypes other than HB SS.
- How to improve adherence to prescribed therapeutic interventions with transfusions, hydroxyurea or chelation?
- How to determine the safety, dosing, and benefits of hydroxyurea for patients with non-HbSS genotypes, especially HbSC?
- How to optimally manage specific SCD-related co-morbidities that include, but are not limited to, kidney disease, hypertension, obstructive lung disease and pulmonary hypertension?
- Prospective clinical trials to determine the efficacy of hydroxyurea in patients with HbSC.
- Longitudinal studies to determine the long-term effects of transfusions and hydroxyurea on preservation or restoration of organ function.
- Clinical trials to modify disease altering co-morbidities, such as kidney disease, hypertension, obstructive lung disease and pulmonary hypertension.
C. Develop Novel Therapies
To date, only one drug has been approved by the FDA to treat SCD, hydroxyurea; moreover, it is only for adults with clinically severe disease. This generally well-tolerated drug has been shown to reduce, but not abolish, the frequent vaso-occlusive episodes and acute chest syndrome characteristic of SCD across a wide age range from infancy to adulthood. In at least some studies, use of hydroxyurea has also been associated with improved survival. While its use was initially promulgated because it increases hemoglobin F levels in many patients, the drug’s mode of action is likely to be much broader, as it also affects leukocyte count, red cell adhesiveness, and other parameters of disease. New therapies addressing diverse pathophysiologic mechanisms of disease are now in various stages of development. However, while many potential therapeutic targets have been identified in animal and in vitro disease models, it is still unknown which targets would be most useful to address in order to reduce the impact of SCD on patients’ lives.
- Which potentially druggable targets are the most critical to address with new pharmacotherapeutic agents?
- Of the new therapeutic agents in development, which might be more useful in treating vaso-occlusive crisis or other acute sequelae of SCD and which would be more useful in a prophylactic setting?
- Are there drugs that would be particularly useful in patients at high risk for specific types of organ damage associated with poor survival ( e.g., stroke, nephropathy or pulmonary hypertension)?
- What new agents could be used in combination, including with hydroxyurea?
- Human and animal studies (not limited to murine models) to identify which druggable targets are the most critical to address with new pharmacotherapeutic agents.
- Phase III and IV studies to develop evidence-based data for efficacy of various treatments (new and old) in acute vaso-occlusion in humans.
- Phase II and III studies to impede the progression of SCD nephropathy or to prevent recurrent stroke.
D. Strengthen Curative Therapies
Therapies that seek to cure SCD through the sustained production of healthy red blood cells are now feasible and must be pursued. Hematopoietic stem cell transplantation (HSCT) and gene therapy are two of the potentially curative therapies for patients with SCD.
- Can HSCT approaches be optimized further for patients with SCD?
- How can HSCT for SCD be made more accessible?
- Is there a role for early HSCT in SCD patients without severe complications?
- Can gene therapy safely provide an adequate and permanent source of adult hemoglobin for patients with SCD?
- How will the use of gene-editing to alter the expression of non-globin genes impact the processes underlying SCD?
- Application of gene correction/repair strategies to SCD
E. Enhance Pain Research
Pain is the most common clinical manifestation of SCD. Patients experience acute, chronic and acute superimposed on chronic pain. The treatment of pain accounts for a large proportion of emergency department visits and hospitalizations of patients with SCD. The prevalence of pain is underestimated by examining healthcare facility utilization, as patients frequently manage painful events at home without seeking medical care. The scope of the problem is far reaching for patients and families, interfering with quality of life, education, and employment.
- What are the mechanisms for pain associated with vaso-occlusion?
- What are the mechanisms for the evolution of chronic pain syndromes in SCD?
- What are the genetic and psychosocial correlates of acute and chronic pain syndromes?
- Basic and clinical studies of neurotransmitters and inflammation in acute and chronic SCD pain.
- Identification of genetic polymorphisms associated with response to pain and opioid medications.
- Application of quantitative sensory testing, biomarkers, and neuroimaging to understanding the mechanisms of different pain syndromes.
- Comparative effectiveness studies of opioids, non-opioids and non-pharmacologic interventions in the management of chronic pain and impact on quality of life.
F. Improve Access to Evidence-Based Care Through Innovative Healthcare Delivery Models
Survival of children with SCD has improved substantially, resulting in an increasing adult population. The majority of adults do not receive medical care at a comprehensive SCD center, and there are an inadequate number of physicians in a community setting with SCD expertise. This disparity results in patients not receiving essential outpatient care and overutilization of emergency department and inpatient services. Preventive interventions are underused and overall health maintenance is compromised.
- What patient-centered healthcare delivery models are effective in providing evidence-based SCD-related interventions?
- What are optimal approaches for providing timely acute and chronic pain management in a community setting?
- Implementation of sustainable patient-centered medical home models to provide comprehensive evidence-based medical care.
- Development of programs to link primary care and hematology/oncology physicians to physicians with SCD expertise to improve access to community-based quality care.
G. Determine the Effects of Quality of Care on Quality of Life
Major randomized controlled trials and high quality observational studies in SCD have had a significant impact on mortality and morbidity. However, there is evidence of inadequate uptake of efficacious treatment modalities in the usual care setting as exemplified by the under use of hydroxyurea in adults with SCD. Furthermore, there is substantial burden on healthcare systems, patients and families in the delivery and receipt of certain interventions such as chronic red blood cell transfusion. There is a need to optimize the use of proven therapies and to measure the impact on the patient’s quality of life.
- What are appropriate quality indicators for medical care delivered outside of a comprehensive SCD center?
- Are outcomes from clinical trials achievable in the community setting?
- Determine the impact of effective therapies on health-related quality of life in usual care settings.
- Define and validate quality of care indicators in children and adults.
- Test interventions to overcome barriers to receiving recommended SCD-specific treatment in a community setting.
H. Investment in Sickle Cell Trait Research
While SCD is a relatively rare condition in the United States, sickle cell trait is not. Millions of Americans of many ethnicities and even greater number of people globally are carriers for the genetic mutation in SCD and therefore have sickle cell trait. The implications of sickle cell trait alone, in combination with other genetic tendencies or in response to certain environmental factors, have been the subject of limited and often inconclusive studies. More rigorous epidemiologic, genetic and clinical research studies are needed in areas such as cardiovascular disease, chronic kidney disease, and exercise physiology to ascertain the contribution of sickle cell trait to outcomes and to provide sound evidence-based clinical guidance for very broad populations.
Unanswered Questions:
- Does sickle cell trait increase the risk for sudden death in connection to vigorous exercise?
- Does sickle cell trait increase the risk of stokes, blood clots, heart or kidney disease?
- Does the inherited gene mutation causing sickle cell trait interact with any other genes or gene products to ameliorate or worsen other conditions?
- Population-based studies of sickle cell trait using existing databases and biorepository samples.
- Prospective studies for exertion-associated genetic associations.
- Cohort studies of athletes with exertion-related symptoms and evidence-based interventions.
Related Item
ASH's research agenda serves as a roadmap for the prioritization of research support across the hematology community and includes recommendations for dedicated resources that will equip researchers to make practice-changing discoveries.
- Sickle cell anemia
On this page
When to see a doctor, risk factors, complications.
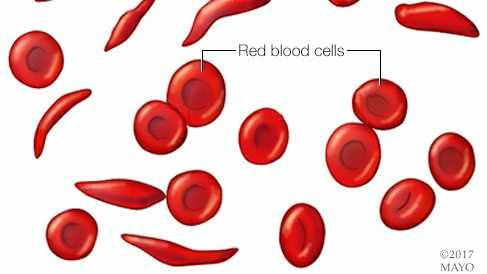
Sickle cell anemia is one of a group of inherited disorders known as sickle cell disease. It affects the shape of red blood cells, which carry oxygen to all parts of the body.
Red blood cells are usually round and flexible, so they move easily through blood vessels. In sickle cell anemia, some red blood cells are shaped like sickles or crescent moons. These sickle cells also become rigid and sticky, which can slow or block blood flow.
The current approach to treatment is to relieve pain and help prevent complications of the disease. However, newer treatments may cure people of the disease.

Red blood cells are usually round and flexible. In sickle cell anemia, some red blood cells look like sickles used to cut wheat. These unusually shaped cells give the disease its name.
Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Symptoms of sickle cell anemia usually appear around 6 months of age. They vary from person to person and may change over time. Symptoms can include:
- Anemia. Sickle cells break apart easily and die. Typical red blood cells usually live for about 120 days before they need to be replaced. But sickle cells usually die in 10 to 20 days, leaving a shortage of red blood cells. This is known as anemia. Without enough red blood cells, the body can't get enough oxygen. This causes fatigue.
Episodes of pain. Periodic episodes of extreme pain, called pain crises, are a major symptom of sickle cell anemia. Pain develops when sickle-shaped red blood cells block blood flow through tiny blood vessels to the chest, abdomen and joints.
The pain varies in intensity and can last for a few hours to a few days. Some people have only a few pain crises a year. Others have a dozen or more a year. A severe pain crisis requires a hospital stay.
Some people with sickle cell anemia also have chronic pain from bone and joint damage, ulcers, and other causes.
- Swelling of hands and feet. Sickle-shaped red blood cells block blood circulation in the hands and feet, which can cause them to swell.
- Frequent infections. The spleen is important for protecting against infections. Sickle cells can damage the spleen, raising the risk of developing infections. Babies and children with sickle cell anemia commonly receive vaccinations and antibiotics to prevent potentially life-threatening infections, such as pneumonia.
- Delayed growth or puberty. Red blood cells provide the body with the oxygen and nutrients needed for growth. A shortage of healthy red blood cells can slow growth in babies and children and delay puberty in teenagers.
- Vision problems. Tiny blood vessels that supply blood to the eyes can become plugged with sickle cells. This can damage the portion of the eye that processes visual images, called the retina, and lead to vision problems.
See your healthcare professional right away if you or your child has symptoms of sickle cell anemia, including fever or stroke.
Infections often start with a fever and can be life-threatening. Because children with sickle cell anemia are prone to infections, seek prompt medical attention for a fever greater than 101.5 degrees Fahrenheit (38.5 degrees Celsius).
Seek emergency care for symptoms of stroke, which include:
- One-sided paralysis or weakness in the face, arms or legs.
- Difficulty walking or talking.
- Sudden vision changes.
- Unexplained numbness.
- Severe headache.
From Mayo Clinic to your inbox
Sickle cell anemia is caused by a change in the gene that tells the body to make hemoglobin. Hemoglobin is the iron-rich compound in red blood cells that allows these cells to carry oxygen from the lungs to the rest of the body. The hemoglobin associated with sickle cell anemia causes red blood cells to become rigid, sticky and misshapen.
For a child to have sickle cell anemia, both parents must carry one copy of the sickle cell gene and pass both copies to the child.
If only one parent passes the sickle cell gene to the child, that child will have the sickle cell trait. With one typical hemoglobin gene and one sickle cell gene, people with the sickle cell trait make both typical hemoglobin and sickle cell hemoglobin.
Their blood might contain some sickle cells, but they generally don't have symptoms. They're carriers of the disease. That means they can pass the gene to their children.
For a baby to have sickle cell anemia, both parents must carry a sickle cell gene. In the United States, sickle cell anemia most commonly affects people of African, Mediterranean and Middle Eastern descent.
Sickle cell anemia can lead to a host of complications, including:
- Stroke. Sickle cells can block blood flow to the brain. Signs of stroke include seizures, weakness or numbness of the arms and legs, sudden speech difficulties, and loss of consciousness. If your child has any of these signs or symptoms, seek medical treatment right away. A stroke can be fatal.
- Acute chest syndrome. A lung infection or sickle cells blocking blood vessels in the lungs can cause this life-threatening complication. Symptoms include chest pain, fever and difficulty breathing. Acute chest syndrome might need emergency medical treatment.
- Avascular necrosis. Sickle cells can block the blood vessels that supply blood to the bones. When the bones don't get enough blood, joints may narrow and bones can die. This can happen anywhere but most often happens in the hip.
- Pulmonary hypertension. People with sickle cell anemia can develop high blood pressure in their lungs. This complication usually affects adults. Shortness of breath and fatigue are common symptoms of this condition, which can be fatal.
- Organ damage. Sickle cells that block blood flow to organs deprive the affected organs of blood and oxygen. In sickle cell anemia, blood also is low in oxygen. This lack of oxygen-rich blood can damage nerves and organs, including the kidneys, liver and spleen, and can be fatal.
- Splenic sequestration. Sickle cells can get trapped in the spleen, causing it to enlarge. This may cause abdominal pain on the left side of the body and can be life-threatening. Parents of children with sickle cell anemia can learn how to locate and feel their child's spleen for enlargement.
- Blindness. Sickle cells can block tiny blood vessels that supply blood to the eyes. Over time, this can lead to blindness.
- Leg ulcers. Sickle cell anemia can cause painful open sores on the legs.
- Gallstones. The breakdown of red blood cells produces a substance called bilirubin. A high level of bilirubin in the body can lead to gallstones.
- Priapism. Sickle cell anemia can cause painful, long-lasting erections, known as priapism. Sickle cells can block the blood vessels in the penis, which can lead to impotence over time.
- Deep vein thrombosis. Sickled red blood cells can cause blood clots, increasing the risk of a clot lodging in a deep vein, known as deep vein thrombosis. It also increases the risk of a blood clot lodging in a lung, known as pulmonary embolism. Either can cause serious illness or even death.
- Pregnancy complications. Sickle cell anemia can increase the risk of high blood pressure and blood clots during pregnancy. It also can increase the risk of miscarriage, premature birth and low birth weight babies.
If you carry the sickle cell trait, it can help to see a genetic counselor before you get pregnant. A counselor can help you understand your risk of having a child with sickle cell anemia. You also can learn about possible treatments, preventive measures and reproductive options.
Dec 22, 2023
- Sickle cell disease. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease. Accessed Aug. 4, 2023.
- Field JJ, et al. Overview of the management and prognosis of sickle cell disease. https://www.uptodate.com/contents/search. Accessed Aug. 4, 2023.
- AskMayoExpert. Sickle cell disease. Mayo Clinic; 2022.
- Sickle cell disease (SCD). Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/sicklecell/index.html. Accessed Aug. 4, 2023.
- Hoffman R, et al. Pain Management and Antiemetic Therapy in Hematologic Disorders. In: Hematology: Basic Principles and Practice. 8th ed. Elsevier; 2023. https://www.clinicalkey.com. Accessed Aug. 4, 2023.
- Ferri FF. Sickle cell disease. In: Ferri's Clinical Advisor 2024. Elsevier; 2024. https://www.clinicalkey.com. Accessed Aug. 4, 2023.
- Lyfgenia (prescribing information). Bluebird Bio; 2023. https://www.fda.gov/vaccines-blood-biologics/lyfgenia. Accessed Dec. 11, 2023.
- Casgevy (prescribing information). Vertex Pharmaceuticals; 2023. https://www.fda.gov/vaccines-blood-biologics/casgevy. Accessed Dec. 11, 2023.
- Diseases & Conditions
- Sickle cell anemia symptoms & causes
News from Mayo Clinic
More Information
Associated procedures.
- Blood transfusion
- Bone marrow transplant
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be part of the cutting-edge research and care that's changing medicine.
- Research article
- Open access
- Published: 27 April 2018
Knowledge, perception and practices towards sickle cell disease: a community survey among adults in Lubaga division, Kampala Uganda
- Sharifu K. Tusuubira ORCID: orcid.org/0000-0001-6280-8871 1 , 2 ,
- Ritah Nakayinga 2 ,
- Bashir Mwambi 2 ,
- John Odda 2 ,
- Sylvia Kiconco 2 &
- Alimah Komuhangi 2
BMC Public Health volume 18 , Article number: 561 ( 2018 ) Cite this article
17k Accesses
17 Citations
12 Altmetric
Metrics details
Worldwide, the burden of Sickle Cell disease (SCD) has not been amply addressed. In Africa, Uganda has the 5th highest burden, a situation aggravated by limited and inaccessible formal social support structures to aid patients and families cope better with the psychosocial burden of SCD. In addition, this has been coupled with stigmatization and discrimination of people living with sickle cell disease causing isolation from family and society.
This cross sectional study therefore set out to determine the attitudes, perception and level of awareness towards Sickle Cell disease in Ugandan communities. The study used an interviewer administered questionnaires to collect the data.
Out of 110 people sampled; 91.2% of the respondents had ever heard of SCD with the highest proportion 38.7% hearing of SCD from friends and family. Close to half of the respondents 48% knew that SCD is inherited, however a large proportion 44.2% did not know the cause of SCD. However, 68.7% of the respondents said they cannot marry a person with SCD.
The study results indicate that more effort needs to be done to promote sickle cell awareness in Uganda communities with emphasis on the inclusion of sickle cell in health education campaigns.
Peer Review reports
Sickle Cell Disease (SCD) is the most common monogenic blood disorder worldwide. It is associated with progressive organ damage coupled with episodes of acute illness [ 1 ]. The episodes of acute illness result from the sticky and stiff red blood cells which clog tiny blood vessels. This often results into various conditions not limited to organ and tissue damage, anemia, increased risk of infection and painful episodes [ 2 ].
Worldwide, SCD contributes a significant burden that is not amply addressed [ 3 ]. It is estimated that 312,000 children will be born worldwide with SCD annually [ 4 ]. With the greatest burden existing in Sub-Saharan Africa, where 75% of the world sickle cell disease occurs [ 5 ]. In Uganda estimates suggest that 15,000 babies are born each year with sickle cell disease [ 6 ]. WHO has indicated the need to improve disease prevention, awareness and early detection in Africa [ 7 , 8 ].
As a result, the Uganda Sickle Cell Rescue Foundation has been actively championing sickle cell disease awareness and prevention since 2013. Following the Ndeezi et al. 2016 study, the Ministry of Health, Uganda has also made significant strides in addressing the sickle cell disease burden by introducing the newborn screening program in selected districts with the highest disease burden. This increased attention is geared to reducing sickle cell related mortality while increasing care and management outcomes. Sickle cell care and management outcomes are complicated by the complex interaction of SCD patients with the socio-ecological system [ 9 , 10 , 11 ]. The dilemma of the person living with sickle cell disease goes beyond grappling with the overwhelming health effects of the disease. The people are often stigmatized and discriminated; this often forces families to hide their sick [ 12 ].This survey therefore set out to determine the prevalence, attitudes, perception and knowledge towards Sickle Cell disease in Ugandan adults. This information is crucial in drawing attention to potential areas for intervention and improvement to foster better outcomes in Sickle cell prevention, awareness and management.
The study was carried out in Lusazze village a peri urban suburb located in Lubaga division, central region of Uganda.
Study design
The study was a cross-sectional survey. Data were collected between October and September 2016 using a structured questionnaire. The Participants completed a demographic questionnaire which contained information about age, gender, marital status, number of children etc. The other questionnaires were related to knowledge, attitudes and perceptions towards sickle cell disease. Sickle cell screening was also done for participants who provided additional consent. Sickle cell screening was carried out using the solubility test [Biolab, India] [ 13 ].
Sample size and sampling technique
The survey considered a sample size of 110 participants which was obtained using the formula by Kish and Leslie (1965) for cross-sectional studies. [ 14 ]. A 95% level of confidence, 50% proportion was estimated and a 5% level of precision were used in the sample size calculation. Convenient sampling was used to select the participants such that all individuals who met the inclusion criteria were included in the survey. Eligible participants were community members who had lived in Lusazze for a period not less than six months. These were interviewed as they came to take part in a sickle cell community outreach.
Sickle cell community outreach
Uganda Sickle Cell Rescue Foundation carries out awareness activities aimed at having communities of sickle cell disease and its social connotations. Before the outreach announcements are made to inform the community about the event. As part of the event, sickle cell information is shared particularly what sickle cell is, how it is caused and how participants can prevent it. In addition, sickle cell screening is offered so as to enable participants know their genotype.
Characteristics of respondents
From Table 1 below, close to two thirds (61.8%) of the respondents are female and in the age group between 19 and 28 years. Slightly more than half (52.9%) of the respondents were not in union (single). The highest proportion of respondents (62.7%) had biological children. More than half (56.9%) of the respondents knew their SCD status.
Knowledge of respondents on sickle cell disease
From Table 2 below, the vast majority (91.2%) of the respondents had ever heard of SCD with the highest proportion (38.7%) of the respondents hearing of SCD from friends and family. Close to half of the respondents (48%) knew that SCD is inherited, however a large proportion (44.2%) did not know the cause of SCD. More than half of the respondents knew some signs and symptoms of SCD. Unfortunately, half of the respondents (50%) did not know how SCD is diagnosed. The highest proportion (45.1%) of the respondents did not know the chance of having a healthy baby when all the parents have SCD. Majority (76.5%) of the respondents noted that conventional medicine was the ideal treatment for SCD.
Respondents’ practice and perception on SCD
From Table 3 below, the vast majority (83.4%) had never tested for SCD, most (90. 2%) of the respondents did not know their partner’s genotype. Most of the respondents (74.3%) reported that they wanted to know their SCD status as their reason to test and the highest proportion (60.8%) noted that knowing their SCD status influenced or can influence their decision to marry while more than two thirds (68.7%,) of the respondents cannot marry a person with SCD. When asked whether people with SCD can work, more than two thirds 70% of the respondents reported that they can work while out of those who reported that they cannot work; half reported that being very weak is the reason as to why they shouldn’t work while the other 50% reported that they are often ill so they do not have to work.
This survey presents one of the first SCD community based findings in Lusaze, a semi-urban area in Lubaga division, central region of Uganda. Most SCD campaigns in Uganda generally focus on creating awareness without collecting baseline data.
This survey targeted female and male adults. This campaign attracted a lower proportion of the males. This may be due the fact that most men were away for work or it may also be due to men’s negative attitude as highlighted by Francis et al. 2008 [ 15 ] . The study results are similar to Musoke et al. 2014 who also highlighted that more women utilize health facilities/ services in Wakiso District [ 16 ]. This may continue to underscore community campaigns since men may be the decision makers in most Ugandan homes.
Most of the participants have heard of SCD which may imply that they know of its existence. However, the fact that only a small proportion obtained such information from a health professional or community meeting may suggest that there is limited effort in health care settings/system to inform the public of SCD. Besides having heard of the disease, a relatively lower proportion had knowledge on the causes, signs and symptoms and prevention and this indicates a much larger problem which may hinder control strategies. Orish et al. 2014 found out that in Ghana schools ranked highest as sources of sickle cell disease knowledge while families ranked highest in our study [ 17 ]. Families are often associated with stigma and discrimination coupled with myths / beliefs about sickle cell disease. One participant said ‘ my parents always cautioned us from marrying from a certain family because they always heard somebody hospitalized ’. Families have sometimes been identified as precipitators of stigma and discrimination [ 18 ]. The results indicate a percentage of people who consider prayer and herbal medicine as a form of therapy for sickle cell disease yet this was a peri urban locality. This is in contrast to another study where rural Ugandans considered prayer as a form of treatment for chronic diseases [ 19 ]. This strengthens the need to promote more health education of sickle cell disease and other chronic illness.
Of note is that a substantial proportion of the participants indicated that they knew their status. When we asked them whether they had tested a greatest proportion had not and indeed the largest proportion had never been screened for SCD. This is because most of them associated sickle cell status with frequent illness which they were free from. This indicates a hefty gap in screening services, yet it may clearly influence family decisions and subsequent control of the disease in the population. Since majority of the participants did not know their partners’ genotype, it may imply that SCD screening before or after marriage is not prioritized yet it may also influence personal or family decisions.
Regarding perception, SCD individuals may be stigmatized and discriminated against since most participants seem to perceive the disease negatively regarding marriage and work. This is similar to what other researchers have found out that individuals with SCD often report SCD related stigma [ 20 , 21 ].
The study limitations include the small sample size from a single area interviewed over a limited period of time. The small sample size affects our ability to generalize the findings. We recommend future research to interview a larger sample size to be able to validate the findings of this study. The study results could also be affected by the recruitment criteria where participants were selected at the sickle cell community campaign. This could be due to their interest or lack of knowledge about sickle cell disease. The sampling approach utilized is prone to bias, given that this was a community event and the participants were largely self-selected.
The results show that the respondents have heard of sickle cell mostly from friends and family. Close to half of the respondents knew that SCD is inherited, however a large proportion did not know the cause of SCD. However, more than two thirds of the respondents said they cannot marry a person with SCD.
Recommendations
There is need for the formulation of strategies to encourage male involvement in SCD campaigns. It is essential for the inclusion of SCD in existing health education programs both at the community and health center settings/levels. The study results indicate that more effort needs to be done to promote sickle cell awareness in Uganda communities.
Abbreviations
Sickle Cell Disease
Weatherall D, Hofman K, Rodgers G, Ruffi NJ, Hrynkow S. A case for developing north-south partnerships for research in sickle cell disease. Blood. 2005;105:921–3.
Article CAS PubMed Google Scholar
Centers for Disease Control and Protection (2015) What is sickle cell disease? Available at: https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease . Accessed 20 July 2016.
Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–6.
Article CAS PubMed PubMed Central Google Scholar
Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–51.
Article PubMed PubMed Central Google Scholar
Piel FB, Hay SI, Gupta S, et al. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484.
Ndeezi G, Kiyaga C, Hernandez AG, Munube D, Howard TA, Ssewanyana I, Nsungwa J, Kiguli S, Ndugwa CM, Ware RE, Jane R. Aceng burden of sickle cell trait and disease in the Uganda sickle surveillance study (US3): a cross-sectional study lancet glob. Health. 2016;4:e195–200.
Google Scholar
WHO. Sickle-cell anaemia. Report A59/9. Geneva: World Health Organization; 2006.
WHO. Sickle-cell disease: a strategy for the WHO African region. Report AFR/RC60/8. Geneva: World Health Organization; 2010.
Haywood C Jr, Lanzkron S, Bediako S, et al. Perceived discrimination, patient trust, and adherence to medical recommendations among persons with sickle cell disease. J Gen Intern Med. 2014;29(12):1657–62. https://doi.org/10.1007/s11606-014-2986-7
Zempsky WT, Loiselle KA, McKay K, Lee BH, Hagstrom JN, Schechter NL. Do children with sickle cell disease receive disparate care for pain in the emergency department? J Emerg Med. 2010;39(5):691–5. https://doi.org/10.1016/j.jemermed.2009.06.003
Article PubMed Google Scholar
Todd KH, Green C, Bonham VL Jr, Haywood C Jr, Ivy E. Sickle cell disease related pain: crisis and conflict. J Pain. 2006;7(7):453–8. https://doi.org/10.1016/j.jpain.2006.05.004
Uganda Sickle Cell Rescue Foundation .Sickle cell stigma Available at: http://www.uscrfuganda.org/ Accessed in 02 Nov 2017.
Nalbandian RM, Nichols BM, Camp FR Jr, Lusher JM, Conte NF, Henry RL, Wolf PL. Dithionite tube test--a rapid, inexpensive technique for the detection of hemoglobin S and non-S sickling hemoglobin. Clin Chem. 1971;17(10):1028–32. PMID: 5095141
CAS PubMed Google Scholar
Kish L. Survey sampling. New York: John Wiley and Sons; 1965.
Bwambale F, Ssali S, Byaruhanga S, Kalyango J, Karamagi C. Voluntary HIV counselling and testing among men in rural western Uganda: implications for HIV prevention. BMC Public Health. 2008;8(1) https://doi.org/10.1186/1471-2458-8-263 .
Musoke D, Boynton P, Butler C, Musoke M. Health seeking behaviour and challenges in utilising health facilities in Wakiso district, Uganda. Afr Health Sci. 2015;14(4):1046. https://doi.org/10.4314/ahs.v14i4.36
Article Google Scholar
Orish V, Onyeabor O, Sanyaolu A, Iriemenam N. Evaluating the knowledge of sickle cell disease and hemoglobin electrophoretic pattern among people living in Sekondi-Takoradi metropolis, Ghana. Journal Of Medicine In The Tropics. 2014;16(2):56. https://doi.org/10.4103/2276-7096.139047
Marsh V, Kamuya D, Molyneux S. ‘All her children are born that way’: gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethnicity & Health. 2011;16(4–5):343–59. https://doi.org/10.1080/13557858.2010.541903
Nnko, S., Bukenya, D., Kavishe, B., Biraro, S., Peck, R., & Kapiga, S. et al. (2015). Chronic diseases in north-West Tanzania and southern Uganda. Public perceptions of terminologies, Aetiologies, symptoms and preferred management. PLOS ONE, 10(11), e0142194. https://doi.org/10.1371/journal.pone.0142194
Adeyemo T, Ojewunmi O, Diaku-Akinwumi I, Ayinde O, Akanmu A. Health related quality of life and perception of stigmatisation in adolescents living with sickle cell disease in Nigeria: a cross sectional study. Pediatr Blood Cancer. 2015;62(7):1245–51.
Bediako S, Lanzkron S, Diener-West M, Onojobi G, Beach M, Haywood C. The measure of sickle cell stigma: initial findings from the improving patient outcomes through respect and trust study. J Health Psychol. 2014;21(5):808–20.
Download references
Acknowledgements
The authors want to thank the Vice Chancellor of Clarke International University (formerly International Health Sciences University) Dr. Rose Clarke Nanyonga for the support. They also want to thank Sam Sendiwala, Hamida Nakintu, Tracy Nagawa and volunteers like Christopher Katende of Lusazze for the contribution. They also extend their utmost thanks to Dr. Bulaimu Muwanga Kibirige, Mr. Erostus Nsubuga, Dr. Sikander Lalani, Dr. Lukiah Mulumba, Dr. Kaggwa Lawrence, Stuart Mwesigwa, Hajj. Haruna Kalule Kibirige and Sis Drolence Namirembe for the support.
Availability of data and materials
The authors confirm that the data and materials from this study are available.
Author information
Authors and affiliations.
Uganda Sickle Cell Rescue Foundation, Plot 4/5 Hotel Close Wampewo Avenue, Clock Tower, P.O. Box 71887, Kampala, Uganda
Sharifu K. Tusuubira
Clarke International University (formerly International Health Sciences University), St. Barnabas Road, Kisugu- Namuwongo, P.O.Box 7782, Kampala, Uganda
Sharifu K. Tusuubira, Ritah Nakayinga, Bashir Mwambi, John Odda, Sylvia Kiconco & Alimah Komuhangi
You can also search for this author in PubMed Google Scholar
Contributions
ST and RN drafted the manuscript. ST, RN, BM, SK and AK participated in the design of the study and data collection. JO participated in the data collection. All authors read and commented on manuscript drafts. All authors approved the final draft. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sharifu K. Tusuubira .
Ethics declarations
Ethics approval and consent to participate.
The ethics and research committee of Clarke International University (formerly International Health Sciences University) approved the study procedures. All participants provided informed written consent for participation after a discussion with a member of the study team.
Competing interests
The authors declare that they have no competing interests where financial or what so ever. .
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Tusuubira, S.K., Nakayinga, R., Mwambi, B. et al. Knowledge, perception and practices towards sickle cell disease: a community survey among adults in Lubaga division, Kampala Uganda. BMC Public Health 18 , 561 (2018). https://doi.org/10.1186/s12889-018-5496-4
Download citation
Received : 26 October 2017
Accepted : 20 April 2018
Published : 27 April 2018
DOI : https://doi.org/10.1186/s12889-018-5496-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sickle cell awareness
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Research in Sickle Cell Disease: From Bedside to Bench to Bedside
Salinas Cisneros, Gabriel 1,2 ; Thein, Swee Lay 1
1 Sickle Cell Branch, National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland, USA
2 Division of Hematology and Oncology, Children’s National Medical Center, Washington, District of Columbia, USA
Received: 13 April 2021 / Accepted: 17 April 2021
Correspondence: Swee Lay Thein ( [email protected] ).
This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND) , where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Sickle cell disease (SCD) is an exemplar of bidirectional translational research, starting with a remarkable astute observation of the abnormally shaped red blood cells that motivated decades of bench research that have now translated into new drugs and genetic therapies. Introduction of hydroxyurea (HU) therapy, the only SCD-modifying treatment for >30 years and now standard care, was initiated through another clinical observation by a pediatrician. While the clinical efficacy of HU is primarily due to its fetal hemoglobin (HbF) induction, the exact mechanism of how it increases HbF remains not fully understood. Unraveling of the molecular mechanism of how HU increases HbF has provided insights on the development of new HbF-reactivating agents in the pipeline. HU has other salutary effects, reduction of cellular adhesion to the vascular endothelium and inflammation, and dissecting these mechanisms has informed bench—both cellular and animal—research for development of the 3 recently approved agents: endari, voxelotor, and crizanlizumab; truly, a bidirectional bench to bedside translation. Decades of research to understand the mechanisms of fetal to adult hemoglobin have also culminated in promising anti-sickling genetic therapies and the first-in-human studies of reactivating an endogenous (γ-globin) gene HBG utilizing innovative genomic approaches.
Introduction
Sickle cell disease (SCD) can trace its first description in the Western literature to a case report in 1910 by Herrick 1 of a young dental male student from Grenada with severe malaise and anemia. Hallmarks of the disease were noted then: “healing ulcers” predominantly on the legs that lasted about a year; anemia with a “hemoglobin (Dare) 40 per cent” and jaundice (“tinge of yellow in the sclerae”), and a disease with “acute exacerbations.” Herrick 1 , 2 also made a remarkable observation that the “red corpuscles varied much in size,” and that “the shape of the reds was very irregular,” but what especially attracted his attention was “the large number of thin, elongated, sickle-shaped and crescent-shaped forms.” He surmised “that some unrecognized change in the composition of the corpuscle itself may be the determining factor” ( Figure 1 ).

It was not until almost 40 years later in 1949 when Pauling and his collaborators 3 discovered that the “…unrecognized change in the composition of the corpuscle” was due to an altered hemoglobin (Hb) structure, thus SCD became the first disease to be understood at a molecular level. The abnormal Hb was later shown to result from the substitution of glutamic acid by valine at position 6 of the β-globin chain of Hb 4 that arose from an A>T base change ( Table 1 ). 5 Genetic simplicity of the sickle mutation in a compact gene encoding an abnormal Hb that was relatively accessible through a simple blood draw has lent SCD to many proof-of-principle and validation experiments for many years. This was facilitated by the globin genes among the first to be cloned and fully analyzed by DNA sequencing. 6 , 7 SCD became a role model for molecular genetics, leading the way in breakthrough discoveries in areas of DNA diagnostics, population and epidemiological genetics, and more recently, genetic therapies. 8 , 9 Certainly for the last century, studies of SCD and genetics of Hb have contributed and benefited other medical conditions more than SCD itself. In the last 10 years, however, we have gained a much better understanding of the sickle pathophysiology. We have also gained incredible insights on the switch from fetal to adult Hb 10 with identification of key regulating factors such as B-cell lymphoma/leukemia 11A (BCL11A) 11 , 12 that together, with major advances in genetic and genomic technologies, 13 , 14 have translated into genetic-based approaches for treating SCD.
Here we take readers through the key discoveries, which showcases the bidirectional bench to bedside research in SCD highlighting the leaps in our understanding that have contributed to new therapeutic options in its management.
The history of SCD pathophysiology—from bench to bedside to bench
After building an electrophoresis machine, Pauling 3 was able to separate normal adult hemoglobin (α2β2, HbA) from abnormal sickle hemoglobin (α2β2 S , HbS) and describe SCD at a molecular level for the first time. But, many questions remained unanswered, such as how HbS lead to the formation of these “thin, elongated sickle-shaped” red cells, the key phenotype in sickle pathophysiology, motivating an enormous amount of basic science studies on the Hb polymer structure, 15 thermodynamics, 16 , 17 and kinetics 18 of HbS polymerization. Since polymerization of HbS can only occur when HbS is deoxygenated, 19 increasing HbS oxygen affinity as a therapeutic approach has been discussed for many years, culminating in the development of oxygen affinity modifying drugs such as voxelotor (also known as Oxbryta or GBT440). Importantly, increasing oxygen binding to HbS could also compromise oxygen delivery, as first discussed by Beutler, 20 an effect that is detrimental in a disease characterized by tissue/organ damage due to oxygen deprivation.
A key bedside observation that fetal Hb (HbF) had beneficial effects was first hypothesized by the pediatrician Watson 21 in 1948, who noted that African American infants with SCD were less prone to have “sickling” events in the first few months of life during which HbF gradually disappears from the blood ( Table 1 ). Since then, multiple observational studies between 1970s and 1990s demonstrating a milder form of SCD in those patients with higher levels of HbF have been published. Clinical and population studies elucidated that the level of HbF in adults is under 2 levels of genetic control. 22 Common genetic variation, historically referred to as heterocellular hereditary persistence of fetal hemoglobin (HPFH), is characterized by modest increases of HbF (1%–4% of total Hb) that are unevenly distributed among the red blood cells (RBCs). Although the HbF increases are modest in healthy adults, co-inheritance of heterocellular HPFH on a background of stress erythropoiesis, such as SCD, leads to increases in HbF levels as high as 25% with immense clinical benefits. Although familial, the inheritance pattern of heterocellular HPFH was not clear until 20 years ago, when genetic studies showed that common HbF variation behaved as a quantitative trait and the levels are predominantly genetically controlled. 23 To date, 3 quantitative trait loci are known: the hemoglobin gene complex ( HBB ) on chromosome 11p ( Xmn 1-Gγ site), the BCL11A gene on chromosome 2, and the HBS1L-MYB intergenic region on chromosome 6q. 24 In contrast, rare variants, historically referred to as pancellular HPFH, are inherited in a Mendelian fashion as alleles of the HBB complex. Carriers for pancellular HPFH have substantial increases in HbF levels of 15% to 30% that are homogeneously distributed among the RBCs. Pancellular HPFH is caused by substantial DNA deletions within the HBB cluster or specific single base changes in the promoters of the γ-globin genes. 25 Persistence of HbF production has no clinical consequences in healthy adults, but ameliorate symptoms of SCD. Indeed, inheritance of a Mendelian form of HPFH in trans to a β S allele (HbS/HPFH) may eliminate clinical consequences of SCD, motivating enormous research on understanding how fetal HbF is repressed in adults. 26
| consists of 2 α-globin and 2 β-globin chains and is the most common human hemoglobin tetramer, accounting for about 97% of the total red blood cell hemoglobin in adulthood |
| consists of 2 α-globin and 2 γ-globin chains. This is the predominant form in the fetus and declines in the first weeks after birth |
| 2): consists of 2 α-globin and 2 mutant β-globin chains. HbS is the most common type of hemoglobin variant and the basis of sickle cell trait and sickle cell anemia |
| Sickle cell disease is caused by the presence of HbS, and includes different sickle genotypes classified according to the hemoglobin abnormality: |
| HbSS: homozygous mutation in β-globin (Glu to Val at position 6) |
| HbSC: compound heterozygotes of HbS (Glu to Val at position 6 and Glu to Lys at position 6) |
| HBS/β thal: compound heterozygotes of HbS with beta thalassemia, the latter can be either beta zero or beta plus, depending on whether beta globin is absent of present but in reduced amounts, respectively |
| Other less common sickle genotypes include compound heterozygotes of HbS with HbD Punjab (HbSD Punjab) and HbS with HbE (HbSE) |
| HbAS refers to heterozygotes or carriers of the HbS mutation: these individuals have HbS of 30%–40% and are asymptomatic. Under extreme conditions, such as physically stressful sports and severe dehydration, HbAS individuals may suffer vaso-occlusive episodes and pain. HbAS individuals are protected against falciparum malaria and can pass the mutant allele to their children |
Translating clinical benefits of hydroxyurea to an improved understanding of sickle pathophysiology
The beneficial effect of HbF led to the first study of hydroxyurea (HU) in 2 patients with the HbSS form of SCD, also referred to as sickle cell anemia (see Table 1 ) in 1984, in which measurable and sustainable increases in HbF could be achieved with minimal toxicity, but no change in clinical course could be observed in the short period of study. 27 Nonetheless, these encouraging preliminary results motivated numerous clinical trials of HU, first in adults 28 and then in pediatric patients with SCD 29 ; its overall safety profile and efficacy led to US Food and Drug Administration (FDA) approval of HU for treatment of SCD in adults in 1998 and in children in 2017.
Our understanding of sickle pathophysiology has also been greatly helped by the use of humanized sickle mouse models, which has provided new insights on adhesion, inflammation, and interactions of the sickled RBCs with their microenvironment—vasculature, neutrophils, monocytes, platelets, and the upregulation of vasculature cyto-adhesion molecules. 30 , 31 Molecules such as P- and E-selectin, fundamental in the adhesion and activation of white blood cells, specially neutrophils, to the vasculature have been found to represent an important component of the pain crisis pathophysiology and have become therapeutic targets. 32
As polymerization of deoxy-HbS is the key event that triggers the downstream consequences of SCD, several therapeutic approaches have focused on mitigation of this root cause, utilizing both genetic and pharmacological anti-sickling strategies. The best-established strategy is induction of HbF synthesis borne out not only by the plentiful clinical and epidemiological studies, but also by the kinetics and thermodynamics of the polymerization process itself. Studies of HbS polymerization kinetics posit that the delay time relative to the transit time through the microcirculation is a major determinant of whether polymerization results in irreversible sickling and hence severity in SCD. The amino acid sequence of γ-globin chain is sufficiently different from β S such that little or no γ-globin takes part in the fiber formation, so the primary effect of HbF (α2γ2) is to simply dilute the intracellular concentration of HbS. 19 Because HbS polymerization is highly sensitive and dependent on intracellular HbS concentration, 33 even a small decrease in HbS concentration is therapeutic because more cells can escape the small vessels before sickling occurs. Strategies that reduce HbS intracellular concentration, such as increasing HbF or the red cell volume (ie, mean corpuscular volume [MCV]), increase the delay time to sickling, while strategies that reduce adherence and shorten transit time should be therapeutic. HU inhibits ribonucleotide reductase causing reversible myelosuppression. Although the exact mechanism of HbF induction is unclear, a primary mechanism relates to the subsequent recovery or “stress erythropoiesis” and release of early erythroid progenitors that synthesize more HbF. This causes the uneven distribution of HbF among the RBCs, 34 one of the reasons proposed for the variable clinical response between SCD patients. 35 , 36 Otherwise, HU-induced HbF increase would be much more effective.
Advances in our understanding of the molecular mechanisms regulating the fetal to adult Hb switch have led to the generation of new agents that do not rely on causing “stress erythropoiesis” and they fall into 2 main groups: those that affect chromatin regulators (such as decitabine on DNA methylation and histone deacetylase [HDAC] inhibitors) and others that affect DNA-binding transcription factors. Contemporaneous genome-wide association studies 11 , 12 identified BCL11A as the first key repressor protein for silencing of the fetal (γ) globin genes joined later by zinc finger and BTB domain-containing protein 7A (ZBTB7A), also known as leukemia related factor (LRF). 37 In 2018, key studies by 2 groups showed that BCL11A and ZBTB7A each bind to a cognate recognition site within the γ-globin promoter. 38 , 39 Besides its role as γ-globin repressor, BCL11A is also essential for B-lymphoid development. 40 Identification of the key erythroid-specific enhancer elements 41 was critical and important in the development of the clinical trials aimed at downregulating BCL11A using 2 different genetic approaches—lentiviral short hairpin RNA (shRNA) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease-9 (Cas-9) editing. 42 , 43 Another genetic approach for reactivating endogenous γ-globin to produce high HbF is to mimic the naturally occurring HPFH variants in the γ-globin promoters by genome-editing to disable binding of BCL11A or ZBTB7A/LRF repressors. 10 , 44 In theory, correcting the sickle mutation ( rs334 ) is the most direct approach, as the same base change is present in all β S alleles, but homology-directed DNA repair is limited by the efficiency at which the correction is achieved and the concomitant generation of insertions/deletions and conversion of the β S gene to a β-thalassemia allele. 45
New therapeutic drug targets that have evolved from molecular dissection of SCD pathophysiology
HU was originally an anti-neoplastic agent in the treatment of patients with myeloproliferative diseases, in whom it has been shown to induce variable moderate increases in HbF and MCVs, 46 but HU is now probably best known as standard therapeutic agent for SCD. 47 , 48 While the clinical efficacy of HU relates predominantly to the level of HbF increase, it also has other salutary therapeutic effects—such as reducing cellular adhesion, hemolysis, and inflammation. 49 Molecular dissection of these mechanisms led to new insights on the pathophysiology of SCD ( Figure 2 ) and new therapeutic targets on vaso-occlusion (endari), HbS polymerization (voxelotor), and vascular adhesion (crizanlizumab) that were approved by the FDA in the last 5 years ( Table 2 ).
| Drug | Mechanism of Action | Phase | Others/ClinicalTrials.gov |
|---|---|---|---|
| Hydroxyurea | Ribonucleotide reductase inhibitor. The exact mechanism of HbF induction remains unknown | FDA approved | |
| Voxelotor | Binds specifically to the N-terminus of the alpha subunit of HbS and stabilizes the oxygenated state of HbS | FDA approved | |
| Panobinostat | HDAC inhibitor: increase levels of γ-globin and inducing production of HbF | Phase 1 | NCT01245179: active, not recruiting |
| Vorinostat | HDAC inhibitor: increase levels of γ-globin and inducing production of HbF | Phase 2 | NCT01000155: terminated early due to poor recruitment |
| IMR-687 | Phosphodiesterase 9 inhibitor: increasing cGMP increasing the production of HbF | Phase 2 | NCT04053803: enrolling by invitation |
| FT-4202 | PK activator: decreasing 2,3-DPG and decreasing the risk of red cell deoxygenation | Phase 2/3 | NCT04624659: recruiting |
| AG-348 (Mitapivat) | PK activator: decreasing 2,3-DPG and decreasing the risk of red cell deoxygenation | Phase 1/2 | NCT04610866: recruiting |
| Crizanlizumab | Monoclonal antibody against P-selectin | FDA approved | |
| Rivipansel | Pan-selectin inhibitor with predilection for E-selectin | Phase 2 | NCT02187003: results recently published at ASH 2020 |
| L-glutamine | Increase NADH and NAD redox potential and decrease endothelial adhesion | FDA approved in the United States | |
| Regadenoson | Adenosine A2A receptor agonist: in vitro studies show decrease iNKT activity | Phase 2 | NCT01788631: completed |
| Canakinumab | IL-1β inhibitor: targeting IL-1β which is an end product of inflammation in SCD | Phase 2 | NCT02961218: completed, results not published |

Endari (L-glutamine)
L-glutamine is an essential amino acid that evolved as an anti-sickle agent through its role as a precursor for the synthesis of glutathione, nicotinamide adenine dinucleotide (NAD), and arginine, all of which protect erythrocytes from oxidative damage and indirectly maintain vascular tone. 50 , 51 Early studies by Nihara et al 52 in 7 SCD patients showed significant increases in nicotinamide adenine dinucleotide - hydrogen (NADH) and NAD redox potential, but no change in Hb concentration. In a follow-up study, erythrocytes from SCD patients who were administered L-glutamine decreased endothelial adhesion in vitro; findings interpreted as glutamine having a role in maintaining RBC membrane integrity and its interaction with the blood vessels and adhesion molecules. 53 In 2017, L-glutamine became the second drug to be licensed by the FDA for patients 5 years or older with SCD ( Table 2 ). The approval was based on a double-blind phase III trial in which 230 children and adults with either HbSS or HbS/β 0 thalassemia were randomized to receive L-glutamine or placebo for 48 weeks. Compared to placebo, L-glutamine was associated with 25% reduction in the number of vaso-occlusive crisis (VOC) events (median 3.0 versus 4.0; P = 0.005), 30% lower hospitalization rates (median 2.0 versus 3.0; P = 0.005), and reduced number of episodes of acute chest syndrome, respectively. Although there were significant increases in NADH and NAD redox potential, and decreased endothelial adhesion of ex vivo treated sickle erythrocytes, there were no changes in Hb or reticulocyte counts. 54 To date, however, L-glutamine has been rejected by the European Medicines Agency because of its relatively small therapeutic effects, and concerns on the high drop-out rate of 36% in the treatment arm, and 24% in the placebo arm.
L-glutamine appears to be reasonably well tolerated, but adherence is poor due to its taste and route of administration (twice daily as oral powder). As it is an amino acid, one should be cautious in its use among SCD patients in whom renal and hepatic dysfunction are not uncommon.
Voxelotor (Oxbryta/GBT440)
Voxelotor (also known as Oxbryta or GBT440) is the second anti-sickling agent that was approved by the FDA in November 2019 for the treatment of SCD in patients aged 12 years and older ( Table 2 ). Voxelotor is anti-sickling because it stabilizes the oxygenated state of Hb through reversible binding to the amino terminus of alpha chain of Hb. 55 The phase III Hemoglobin Oxygen Affinity Modulation to inhibit HbS Polymerization (HOPE) study (ClinicalTrials.gov: NCT03036813) was a randomized, placebo-control study of 274 patients of all SCD genotypes, age 12–65 years, in which voxelotor showed dose-dependent increase in Hb and decrease hemolysis markers, suggestive of decreased sickling. 56 Although these findings did not correlate with a decrease in the number of pain crises in patients with SCD, the promising findings led to FDA approval in November 2019 for patients older than 12 years old with SCD. There is some concern, however, that Hb molecules with the drug bound are in a conformation that delivers very little oxygen, especially detrimental in a disease characterized by decreased oxygen delivery, 57 in which case, the increase in Hb needs to be about the same as the concentration of the drug-bound, nonoxygen delivering Hb. Hopefully, these concerns are addressed in current multicenter phase III clinical studies in both adults (ClinicalTrials.gov: NCT03036813) and children (ClinicalTrials.gov: NCT02850406). In the meanwhile, it remains important to continue to monitor closely the patients while on this medication, particularly in those with prior stroke and silent cerebral infarcts. It should also be noted that HbS-voxelotor complexes, while useful in monitoring voxelotor therapy, causes interference with determination of HbS fraction in routine laboratory techniques—isoelectric-focusing gel, high-performance liquid chromatography, and capillary zone electrophoresis—of Hb fractionation. 58
Crizanlizumab
Adhesion of the sickle erythrocytes and neutrophils with the vascular endothelium leads to upregulation of endothelial adhesion molecules—vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E and P selectins, facilitating vaso-occlusion. Crizanlizumab is a humanized monoclonal antibody that selectively inhibits P-selectin. The study to assess safety and impact of SelG1 with or without hydroxyurea therapy in sickle cell disease patients with pain crises (SUSTAIN) was a phase II multicenter, randomized, placebo-controlled double-blind study in which crizanlizumab was tested in 198 patients with SCD (on or not on HU) for its ability to reduce VOCs over a period of 52 weeks. Results showed a significant reduction of sickle cell-related pain crises per year in the high dose arm (5 mg/kg) as compared to the placebo (1.63 versus 2.98), and a low incidence of adverse events. Patients on the treatment arm also had an increased time-to-first VOC compared with placebo. Although side effects were relatively fewer in patients on crizanlizumab, 1 patient had an intracranial bleed. A phase III is currently ongoing to assess safety and efficacy of crizanlizumab, as this medication may alter platelet function. In November 2019, crizanlizumab (Adakveo) was FDA approved for reduction of VOCs in patients with SCD, 16 years or older ( Table 2 ). 59 , 60 It should be noted that crizanlizumab is a preventive therapy, administered intravenously over 30 minutes on week 0, 2, and every 4 weeks thereafter. There are recent concerns with crizanlizumab due to the increased reports of serious infusion and post-infusion reactions ( https://www.crizanlizumab.info/ ), causing hematologists to discontinue therapy. 61
Promising medications in the pipeline
Rivipansel (also known as GMI1070) is another agent targeting cell adhesion ( Table 2 ), which was developed as a pan-selectin inhibitor, but has greatest activity against E-selectin. A phase II, randomized, placebo-controlled multicenter study in adolescents and adults showed the drug to be safe, and markedly reduced use of opioids during hospitalization (83% reduction compared to placebo) as well as a trend toward a faster resolution of VOC (41 versus 63 h). 62 A phase III study of rivipansel in patients 6 years and older hospitalized for a pain crisis (ClinicalTrials.gov: NCT02187003) was recently completed, and although the drug did not reach its primary or key secondary endpoints, analyses suggested that early administration of rivipansel in vaso-occlusive events may reduce hospital stay and intravenous opioid use in pediatric and adult patients ( https://doi.org/10.1182/blood-2020-134803 ). Although interesting, the clinical impact of rivipansel and its timely use as a preventive medication may be limited for the general SCD population.
All SCD patients have elevated pro-inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor alpha [TNFα], and IL-1β), neutrophils, heme and other molecules with inflammatory potential, referred to as damage-associated molecular patterns. 32 A number of anti-inflammatory agents have been investigated including corticosteroids and regadenoson, an adenosine A 2A receptor agonist. Humanized sickle mouse demonstrated elevated levels of invariant natural killer T cells (iNKT) implicating their role in the pathogenesis of ischemia-reperfusion injury. 63 Reduction of this subset of T cell (iNKT) activity ameliorated the inflammatory injury in the lungs in sickle mice, 64 prompting studies in patients with SCD. 65 , 66 Unfortunately, results showed that low-dose infusion of regadenoson was not sufficient to produce a statistically significant reduction in the activation of iNKT cells or in measures of clinical efficacy. 66 Another study utilized the anti-iNKT cell monoclonal antibody NKTT120. High intravenous doses of NKTT120 were shown to decrease iNKT cells in adults with SCD. It should be noted, however, that the subjects in the study were in steady-state when iNKT cell activation was significantly lower compared to VOC. 65 The implication is that, to be effective in VOC, much higher doses of NKTT120 (NKT Therapeutics, Inc.) may be needed.
IL-1β is a cytokine that is central in the inflammatory response and has also been shown to be elevated in subjects with SCD. 67 , 68 Canakinumab is a humanized monoclonal antibody targeting IL-1β and has been approved by the FDA for treatment of rheumatological disorders in 2009. Its broader role as an inflammatory agent was demonstrated in subjects with previous myocardial infarcts, 69 motivating an ongoing randomized double-blind placebo-controlled phase II study of subcutaneous canakinumab in patients with SCD aged 8–20 years old (ClinicalTrials.gov: NCT02961218) ( Table 2 ). Preliminary results suggest that canakinumab improves pain scores, sleep, and school/work attendance ( https://doi.org/10.1182/blood-2019-123355 ).
Despite high levels of HU-induced HbF, some patients continue to have sickle-related manifestations, which has been attributed to the uneven distribution of HbF among the RBCs. An alternative to increasing HbF synthesis that does not mimic stress erythropoiesis is to increase access of the transcription factors to the γ-globin genes by manipulation of the chromatin regulators (such as decitabine on DNA methylation and HDAC inhibitors). Hypermethylation of the upstream γ-globin promoter sequences is believed to be important in the Hb switch during which the γ genes are silenced by DNA methyltransferase 1 (DNMT1). 70 This led to the use of 5-azacytidine, a first generation DNMT1 inhibitor, but it was quickly abandoned due to its toxicity and carcinogenicity. 70 Decitabine, an analogue of 5-azacytidine, is also a potent DNMT1 inhibitor with a more favorable safety profile, but decitabine is rapidly deaminated and inactivated by cytosine deaminase if taken orally. To overcome this limitation, a clinical study combines decitabine and tetrahydrouridine (THU), a cytosine deaminase inhibitor, as a therapeutic strategy for inducing HbF (ClinicalTrials.gov: NCT01685515). A phase I study showed that decitabine-THU led to the inhibition of DNMT1 protein with induction HbF increase, and more importantly, HbF-enriched RBCs (F cells) increased to 80%. These agents did not induce cytoreduction but increased platelets count, which can be problematic in SCD patient and require further evaluation. 71
HDACs are another group of regulatory molecules involved in epigenetic silencing of the γ-globin genes and have been considered as therapeutic targets for HbF induction ( Table 2 ). Panobinostat is a pan HDAC inhibitor currently being tested in adult patients with SCD as a phase I study (ClinicalTrials.gov: NCT01245179). Increasing cellular cyclic guanosine monophosphate (cGMP) levels has also been proposed as one mechanism of HbF increase by HU. 72 Phosphodiesterase 9 (PDE9) degrades cGMP, and it has been shown to be present in activated RBCs and neutrophils of patients with SCD. PDE9 inhibitors have been studied in clinical trials in patients with SCD with interesting results demonstrating elevation of HbF without deleterious effects in the bone marrow. 73
Exciting drugs in the pipeline with anti-sickling properties have also been derived from a combination of bench and clinical observations. HbS polymerizes only when deoxygenated and its oxygenation is influenced by a few factors. One key factor influencing Hb oxygenation is the concentration of 2,3-diphosphoglycerate (2,3-DPG) in the RBC. Increased intracellular 2,3-DPG decreases oxygen binding and stabilizes the deoxygenated form (T form) of Hb, promoting sickling. 19 It has been noted more than 50 years ago that 2,3-DPG levels in RBCs from SCD patients were significantly higher than that in healthy RBCs, 74 and that adding 2,3-DPG to both healthy and SCD RBCs reduces Hb oxygen affinity. 74 Decreasing 2,3-DPG as a therapeutic target has long been proposed by Poillon et al 75 when they showed that considerable reduction of 2,3-DPG in sickle erythrocytes significantly reduced the sickling tendency. 2,3-DPG is an intermediate substrate in the glycolytic pathway, the only source of ATP production in RBCs. As pyruvate kinase (PK) is a key enzyme in the final step of glycolysis, enhancing its activity in red cells presents a very attractive therapeutic anti-sickling strategy as this leads to a decrease in 2,3-DPG, which increases Hb oxygenation with inhibition of the sickling process. Additionally, the concomitant increase in ATP levels restores ATP depletion in sickled RBCs and improves RBC membrane integrity. Currently, there are 3 ongoing phase I/II clinical studies of PK activation in SCD: 2 studies utilizing Mitapivat/AG-348 in HbSS patients in steady-state (ClinicalTrials.gov: NCT04000165; NCT04610866), and another (FT-4202) in healthy subjects and SCD patients (ClinicalTRials.gov: NCT03815695) ( https://doi.org/10.1182/blood-2020-134269 ). Preliminary data showed that AG-348 data was well-tolerated and safe in subjects with SCD, and support dose-dependent changes in blood glycolytic intermediates consistent with glycolytic pathway activation accompanied by increases in Hb level and decreases in hemolytic markers ( https://doi.org/10.1182/blood-2019-123113 ).
Mitapivat is also currently in phase II/III clinical trials in humans with PK deficiency 76 (ClinicalTrials.gov: NCT02476916, NCT03548220, NCT03559699), as well as in an ongoing phase II study in subjects with nontransfusion-dependent thalassemia (ClinicalTrials.gov: NCT03692052).
Evolution of the curative approaches for SCD
Allogeneic transplantation.
Hemopoietic stem cell transplantation (HSCT) had not been considered as a therapeutic option for SCD until 1984, prompted by the successful reversal of SCD in an 8-year-old SCD child who developed acute myeloid leukemia (AML). 77 The patient received HSCT for the AML from a HLA-matched sister who was a heterozygous carrier for HbS (hemoglobin AS [HbAS]) ( Table 1 ). She was cured of her leukemia and at the same time, her sickle cell complications also resolved. 77 , 78 This successful HSCT demonstrated that reversal of SCD could be achieved without complete reversal of the hematological phenotype to normal hemoglobin genotype (HbAA), and as long as stable mixed hemopoietic chimerism after HSCT can be achieved. 79
The outcomes for both children and adults who receive HLA-matched sibling donor hematopoietic stem cells (HSCs) are now excellent. 80 , 81 Key milestones in making HLA-matched sibling donor HSCT an accepted curative option include: (1) the development of less intense conditioning regimens expanding allogeneic transplantation to adult patients who otherwise would not be able to tolerate the intense myeloablative conditioning 82 and (2) that to reverse the sickle hematology, regardless of whether donors have normal hemoglobin genotype, HbAA, or are carriers for HbS (HbAS), only a minimum of myeloid chimerism of 20% is sufficient. 83 Transplantation of HLA-matched sibling donor HSCs cures SCD, but to date, relatively few (~2000) patients with an average age of 10 years have benefited; the vast majority is excluded due to donor availability, toxicity related to myeloablative conditioning, and graft-versus-host disease (GvHD). 81 , 84 , 85
To enable allogeneic HSCT as a therapeutic option to more patients with SCD, there is a major need to expand alternative donor sources of HSCs that include related haploidentical HSCs, matched unrelated donors, and cord blood. Of these, the most promising is related haploidentical allogeneic HSCT due to donor availability; post-transplantation cyclophosphamide has also improved safety with increased cure rates. 86–88
While the overall survival was 94% in a study of unrelated cord blood transplantation for pediatric patients with SCD and thalassemia, the disease-free survival was not so good at about 50% in the SCD population. 89 Compared to unrelated cord blood transplantation, related cord blood transplantation offers a better probability of success with a 2-year disease-free survival of 90% and a low risk of developing acute GvHD (11%) or chronic GvHD (6%) in pediatric patients with SCD. 90
There are multiple clinical trials ongoing at this point at ClinicalTrials.gov that are assessing different techniques to improve the outcome of patients with SCD undergoing allogeneic HSCT. For more details of the different allogeneic HSCTs, we refer to a recent review. 91
Autologous transplantation and genetic therapies
The genetic simplicity of the sickle mutation affecting an HSC lends itself to genetic therapies, an approach that eliminates the need to find a donor and thus, available to all patients ( Table 3 ). Since these are the patient’s own stem cells, there is no need for immunosuppression, avoiding the risks of GvHD and immune-mediated graft rejection. Following gene modification in vitro, the patient’s own stem cells are reinfused after chemotherapy conditioning. Currently, there are 3 broad approaches: (1) Addition of lentiviral vectors (LVs) that express different versions of non- or anti-sickling genes, or a γ-globin coding sequence in a β-globin gene to increase HbF levels and decrease HbS; (2) addition of a LV that expresses erythroid-specific shRNA for BCL11A to downregulate its expression, thereby increasing γ-globin expression; and (3) editing of the BCL11A gene to delete the regulatory element controlling its expression in erythroid cells.
| Title | ClinicalTrials.gov | Status | Mechanism | Notes |
|---|---|---|---|---|
| Safety and efficacy of LentiGlobin BB305 in β-thalassemia and SCD | NCT02151526 | Completed (March 10, 2020) | Lentiviral β-A-T87Q globin vector | Results published: DOI: 10.1056/NEJMoa1609677 |
| A study evaluating the safety and efficacy of the LentiGlobin BB305 drug product in severe SCD | NCT02140554 | Active, not recruiting | BB305 lentiviral vector encoding the human β-A-T87Q globin gene | NCT03207009 and NCT02906202 related but for patients with β-thalassemia |
| Gene transfer for patients with SCD | NCT02186418 | Active, not recruiting | Autologous CD34+ hematopoietic stem cells transduced ex vivo with gamma-globin lentiviral vector | |
| Safety and feasibility of gene therapy with CSL200 | NCT04091737 | Active, not recruiting | Autologous enriched CD34+ cell fraction that contains CD34+ cells transduced with lentiviral vector encoding human γ-globinG16D and shRNA734 | |
| Stem cell gene therapy for SCD | NCT02247843 | Recruiting | βAS3 lentiviral vector-modified autologous peripheral blood stem cell transplant | |
| Safety and efficacy of gene therapy of the SCD with the lentiviral vector expressing the βAS3 globin gene in patients with SCD | NCT03964792 | Recruiting | Consists of autologous human CD34+ hematopoietic stem and progenitor cells that are enriched in CD34+ cells which have been transduced ex vivo with the lentiviral vector, expressing an βAS3 | |
| Gene transfer for SCD | NCT03282656 | Suspended | Lentiviral anti-BCL11A shRNA | Study paused per DSMB pending investigation of adverse event occurrence in an unrelated gene therapy study involving sickle cell patients (last update February 2021) |
| A study evaluating gene therapy with BB305 lentiviral vector in SCD | NCT04293185 | Suspended | CD34+ hematopoietic stem cells collected by plerixafor mobilization and apheresis, transduced with BB305 lentiviral vector encoding the human β-A-T87Q globin gene | Study suspended due to the occurrence of a suspected unexpected serious adverse reaction (last update March 2021) |
| Transplantation of CRISPR/Cas-9 corrected hematopoietic stem cells (CRISPR_SCD001) in patients with severe SCD | NCT04774536 | Not yet recruiting | Autologous CD34+ cell-enriched population that contains cells modified by the CRISPR/Cas-9 ribonucleoprotein | |
| Safety and efficacy of CRISPR/Cas-9 modified CD34+ hHSPCs | NCT03745287 | Recruiting | Autologous CD34+ hHSPCs modified with CRISPR/Cas-9 at the erythroid lineage-specific enhancer of the BCL11A gene | |
| Safety, tolerability, and efficacy of BIVV003 for autologous hematopoietic stem cell transplantation in patients with severe SCD | NCT03653247 | Recruiting | CD34+ cells transfected ex vivo with zinc finger nuclease messenger ribonucleic acid targeting the BCL11A locus | |
| Safety and efficacy of genome-edited hematopoietic stem and progenitor cells in SCD | NCT04443907 | Recruiting | Genome-edited autologous HSPC investigational drug product. Drugs: OTQ923 and HIX763 | Part C would include pediatric patients that received one of both experimental drugs |
A critical component in autologous HSCT is the amount and quality of CD34 + cells that can be obtained from the patient. Historically, granulocyte colony-stimulating factor (GCS-F) had been used to obtain such cells in non-SCD patients, but the elevated white cell counts from GCS-F mobilization of CD34 + in SCD patients increases the risk of triggering acute severe pain, acute chest syndrome, and even death, and is thus contra-indicated in patients with SCD. Bone marrow harvest is another source, but CD34 + cells obtained from bone marrow harvests are suboptimal in quantity and quality, thus requiring multiple harvests, each harvesting procedure increasing the risk of triggering acute pain crisis. Development of plerixafor as an alternative approach has been crucial in optimization of CD34 + collection in patients with SCD. Plerixafor blocks the binding between chemokine CXC-receptor 4 and the stromal cell triggering mobilization of CD34 + cells into the peripheral blood stream without the uncontrolled increase of total white blood cells. Plerixafor in association with hyper-transfusion therapy has become the preferred way of mobilizing HSCs in patients with SCD. 92–96
Two clinical trials ( Table 3 ) have evolved from preclinical studies in SCD mice that showed that erythroid-specific down regulation of BCL11A is feasible and that it resulted in therapeutic elevation of HbF. One approach utilizes an shRNA embedded in a microRNA contained within a LV to limit knockdown of BCL11A to erythroid precursors. 42 Of 6 patients with a median 18 months (range 7–29 mo) post-therapy, stable HbF induction of 20.4% to 41.15% was observed and the HbF was broadly distributed among the erythrocytes with F cells of 59% to 94%. Sickle complications were reduced or absent in all patients. 42 The other approach utilized CRISPR-Cas editing to disrupt the key erythroid-specific enhancer in BCL11A leading to near normal Hb in 3 patients with HbF of >40% that was distributed pancellularly. 43
Among the ongoing clinical trials on genetic therapy ( Table 3 ), the most promising with the largest clinical experience relies on a lentivirus expressing a mutated β-globin β T87Q (LentiGlobin BB305) with anti-sickling properties. 97 ( https://ash.confex.com/ash/2020/webprogram/Paper134940.html ) At the time of this review, 47 patients with SCD have been treated in 2 related clinical trials (ClinicalTrials.gov: NCT02140554 and NCT04293185). 98 Unfortunately, reports of myelodysplasia and AML in 3 patients led to a temporary pause in enrolment; the clinical trial was allowed to resume when further investigation demonstrated integration of the LV to a nononcogenic gene with no disruption in expression of other genes in the vicinity. The conclusion was that the LV is unlikely to be implicated in cancer development. 98 , 99 Exclusion of busulfan and insertional mutagenesis in these therapy-related leukemias, isolated reports of leukemias in SCD patients, with or without HU, pre-or post-transplantation, 100 suggests that SCD patients may have a relatively increased risk of AML or myelodysplasia due to damage to hemopoietic stem cells related to chronic stress erythropoiesis. If so, it may be prudent to prescreen individuals with SCD for preleukemic progenitor cells as well as somatic mutations in genes involved in epigenetic regulation (DNMT3A, TET2, ASXL1), which are associated with an increased risk of developing blood cancers, referred to as clonal hematopoiesis of indeterminate potential (CHIP) origin. It has also been suggested that curative therapies should be performed in younger patients prior to acquisition of such CHIP variants or all patients should be screened for such variants prior to undergoing marrow conditioning.
Worldwide impact of SCD
SCD may have first appeared in the Western literature in 1910, but the clinical spectrum of SCD has been recognized in West Africa for centuries 101 and probably existed in American slaves during the slavery period before 1910. 102 Due to migration patterns, SCD is now worldwide, affecting millions globally, and the numbers are increasing. 103 , 104 Nevertheless, SCD remains drastically more prevalent in historically malaria-endemic areas, such as sub-Saharan Africa, where carriers (HbAS) for the sickle mutation have a substantial protection against Plasmodium malariae infection. In a recent meta-analysis of SCD prevalence in subjects <5 years old, the birth prevalence of HbAS was estimated at >16,000 per 100,000 live births in Africa; much higher when compared to 800 per 100,000 live births in Europe. 105–107
In 2010, an estimated 300,000 newborns were affected—projected to increase to 400,000 in 2050—of which more than 75% is in Africa. Unfortunately, 50%–80% of the infants born annually with SCD in Africa will not reach their fifth birthday. In the Republic of Congo, almost 12.5% of the pediatric patients hospitalized have SCD and the estimated annual cost of care for each of these patients is above 1000 United States dollars (USD). 108 Trained personnel, access to vaccines, antibiotic prophylaxis, implementation of newborn screening, and blood products—all fundamental for the care and management of patients with SCD—are still limited resources in developing countries. 109 The socioeconomic burden of SCD in Africa, and worldwide, will continue to increase with growth of the world’s population and human migration.
Although groundbreaking research is being performed in developed countries, access to the new medications—L-glutamine, voxelotor, and crizanlizumab—is limited in developing countries. In the meanwhile, studies have shown that HU is safe in malaria-endemic sub-Saharan Africa with no difference in incidence of malaria between children either on or off HU. The overall clinical benefit from HU therapy may even protect the recipients from severe effects of malaria. 110–112 It should be noted, however, that prior to these studies, HU has already been demonstrated to be safe and effective as an alternative to regular blood transfusion therapy for prevention of secondary stroke in children with sickle cell anemia. 113
Conclusions
SCD epitomizes the bidirectional translational research common to many other diseases. An astute observation of “elongated, sickle-shaped and crescent-shaped” RBCs has spurred the way to the uncovering of the first disease at a molecular level. Since then, SCD has been at the forefront of human genetic discovery, which has now translated into the first-in-human studies of reactivating an endogenous (γ-globin) gene utilizing innovative genomic approaches. A cure for this debilitating disease through HSCT and gene therapies is now within reach, but likely to remain available to a minority of the patients for the next few decades. A major unmet need for the vast majority now is a small molecule that targets the root cause of the disease and that can be taken orally. As new drugs and treatments are developed, it is essential that we find ways to make them accessible to all patients in both high- or low-resource countries.
Disclosures
The authors have no conflicts of interest to disclose.
Sources of funding
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute and National Institutes of Health (SLT).
- + Favorites
- View in Gallery
Readers Of this Article Also Read
The eha research roadmap: transfusion medicine, the eha research roadmap: blood coagulation and hemostatic disorders, the eha research roadmap: anemias.
Articles on Sickle cell anemia
Displaying all articles.

Prodigy’s personal mythology: Remembering the ‘fallen angel’ of Mobb Deep
Marcus Evans , McMaster University

New sickle cell disease drug approved for use in England – here’s how voxelotor works
Johan Flygare , Lund University

Anemia afflicts nearly 1 in 4 people worldwide, but there are practical strategies for reducing it
William Gardner , University of Washington ; Nicholas Kassebaum , University of Washington , and Theresa A McHugh , University of Washington
Related Topics
- Iron deficiency
- Red blood cells
- Sickle cell disease
Top contributors
Adjunct Professor in Health Metrics Sciences and Professor of Anesthesiology and Pain Medicine, University of Washington
Researcher and Scientific Writer at the Institute for Health Metrics and Evaluation, University of Washington
Researcher in Neonatal and Child Health at the Institute for Health Metrics and Evaluation, University of Washington
Associate Senior Lecturer, Division of Molecular Medicine and Gene Therapy, Lund University
PhD. Department of Religious Studies, McMaster University
- X (Twitter)
- Unfollow topic Follow topic
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Data and Statistics
- Communication Resources
- Steps to Better Health Toolkit
- What Is Sickle Cell Trait?
- Complications of Sickle Cell Disease
- Stories of Sickle Cell
- Scientific Articles about Sickle Cell Disease
- Sickle Cell Information for Healthcare Providers
- Show All Home
Data and Statistics on Sickle Cell Disease
- Sickle cell disease (SCD) affects about 100,000 people in the United States; more than 90% are non-Hispanic Black or African American, and an estimated 3%–9% are Hispanic or Latino.
- The estimated life expectancy of those with SCD in the United States is more than 20 years shorter than the average expected.
- Many people with SCD do not receive the recommended healthcare screenings and treatments.

Sickle cell disease worldwide
Sickle cell disease (SCD) affects millions of people throughout the world and is particularly common among those whose ancestors came from parts of the world where malaria is or was common:
- Sub-Saharan Africa.
- Spanish-speaking regions in the Western Hemisphere (South America, the Caribbean, and Central America).
- Saudi Arabia.
- Mediterranean countries such as Turkey, Greece, and Italy.
Sickle cell disease in the United States
- The exact number of people living with SCD in the United States is unknown.
- Two studies published in 2010 estimated that SCD affects approximately 100,000 people in the United States.
- More than 90% of people in the United States with SCD are non-Hispanic Black or African American (Black), and an estimated 3%–9% are Hispanic or Latino.
- SCD occurs in about 1 out of every 365 Black or African American births and about 1 out of every 16,300 Hispanic American births.
- About 1 in 13 Black or African American babies is born with sickle cell trait (SCT, inheritance of a sickle cell gene from only one parent).
Complications and Mortality
- In people with SCD, red blood cells become rigid and deform into a crescent or sickle shape. Sickled cells die early and often become lodged in small blood vessels, restricting blood flow, which can lead to serious health problems throughout the body.
- SCD-associated complications include anemia, acute and chronic pain, infections, pneumonia and acute chest syndrome, stroke, and kidney, liver, and heart disease.
- Estimated life expectancy of those with SCD in the United States is more than 20 years shorter than the average expected. Quality-adjusted life expectancy A is more than 30 years shorter.
Healthcare access
- Despite their extensive healthcare needs, many persons with SCD have difficulty accessing appropriate care and report feeling stigmatized and having their symptoms dismissed when they do seek care.
- Given that SCA is a common cause of childhood stroke, the panel recommended that children and adolescents aged 2–16 years with SCA be screened annually with transcranial Doppler (TCD) ultrasound to identify those at increased risk for stroke.
- The panel also recommended that children and adolescents aged 9 months or older with SCA be offered treatment with hydroxyurea, a medication that has been shown to prevent or reduce severe pain episodes, acute chest syndrome, and other SCA-associated complications and to increase patient survival.
- Additionally, less than half of children aged 2–9 years of age were using hydroxyurea and approximately one half of those aged 10–16 years used hydroxyurea.
- These findings highlight the ongoing gaps in health care for people with SCD and speak to the urgent need to address barriers to care.
- Statistical estimate of the average life expectancy for a population of people that considers not only the expected length of life but also the likely quality of life as people age.
- National Academies of Sciences, Engineering, and Medicine. 2020. Addressing sickle cell disease: A strategic plan and blueprint for action. Washington, DC: The National Academies Press.
- Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(Suppl):S512-21.
- Brosseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle cell disease in the United States: national and state estimates. Am J Hematol. 2010 Jan;85(1):77-8.
- Ojodu J, Hulihan MM, Pope SN, Grant AM; Centers for Disease Control and Prevention (CDC). Incidence of sickle cell trait—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014 Dec 12;63(49):1155-8. PMID: 25503918; PMCID: PMC4584538.
- Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2(11):e1915374.
- National Heart Lung and Blood Institute, National Institutes of Health. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014 .
- Schieve LA, Simmons GM, Payne AB, Abe K, Hsu LL, Hulihan M, Pope S, Rhie S, Dupervil B, Hooper WC. Vital Signs: Use of Recommended Health Care Measures to Prevent Selected Complications of Sickle Cell Anemia in Children and Adolescents — Selected U.S. States, 2019. MMWR 2022 Sep 30;71(39):1241-1246.
Sickle Cell Disease (SCD)
Sickle cell disease (SCD) is a group of inherited red blood cell disorders. In SCD, the red blood cells become hard and sticky and look like a C-shaped farm tool called a “sickle.”
For Everyone
Health care providers.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 15 March 2018
- Sickle cell disease
- Gregory J. Kato 1 ,
- Frédéric B. Piel 2 ,
- Clarice D. Reid 3 ,
- Marilyn H. Gaston 4 ,
- Kwaku Ohene-Frempong 5 ,
- Lakshmanan Krishnamurti 6 ,
- Wally R. Smith 7 ,
- Julie A. Panepinto 8 ,
- David J. Weatherall 9 ,
- Fernando F. Costa 10 &
- Elliott P. Vichinsky 11
Nature Reviews Disease Primers volume 4 , Article number: 18010 ( 2018 ) Cite this article
138k Accesses
718 Citations
235 Altmetric
Metrics details
- Genetic predisposition to disease
- Genetic testing
Sickle cell disease (SCD) is a group of inherited disorders caused by mutations in HBB , which encodes haemoglobin subunit β. The incidence is estimated to be between 300,000 and 400,000 neonates globally each year, the majority in sub-Saharan Africa. Haemoglobin molecules that include mutant sickle β-globin subunits can polymerize; erythrocytes that contain mostly haemoglobin polymers assume a sickled form and are prone to haemolysis. Other pathophysiological mechanisms that contribute to the SCD phenotype are vaso-occlusion and activation of the immune system. SCD is characterized by a remarkable phenotypic complexity. Common acute complications are acute pain events, acute chest syndrome and stroke; chronic complications (including chronic kidney disease) can damage all organs. Hydroxycarbamide, blood transfusions and haematopoietic stem cell transplantation can reduce the severity of the disease. Early diagnosis is crucial to improve survival, and universal newborn screening programmes have been implemented in some countries but are challenging in low-income, high-burden settings.
You have full access to this article via your institution.
Similar content being viewed by others
The nephropathy of sickle cell trait and sickle cell disease
Multi-center study on mortality in children, and adults with sickle cell anemia-risk factors and causes of death

Perceptions and preferences for genetic testing for sickle cell disease or trait: a qualitative study in Cameroon, Ghana and Tanzania
Introduction.
Sickle cell disease (SCD) is an umbrella term that defines a group of inherited diseases (including sickle cell anaemia (SCA), HbSC and HbSβ-thalassaemia, see below) characterized by mutations in the gene encoding the haemoglobin subunit β ( HBB ) ( Fig. 1 ). Haemoglobin (Hb) is a tetrameric protein composed of different combinations of globin subunits; each globin subunit is associated with the cofactor haem, which can carry a molecule of oxygen. Hb is expressed by red blood cells, both reticulocytes (immature red blood cells) and erythrocytes (mature red blood cells). Several genes encode different types of globin proteins, and their various tetrameric combinations generate multiple types of Hb, which are normally expressed at different stages of life — embryonic, fetal and adult. Hb A (HbA), the most abundant (>90%) form of adult Hb, comprises two α-globin subunits (encoded by the duplicated HBA1 and HBA2 genes) and two β-globin subunits. A single nucleotide substitution in HBB results in the sickle Hb (HbS) allele β S ; the mutant protein generated from the β S allele is the sickle β-globin subunit and has an amino acid substitution. Under conditions of deoxygenation (that is, when the Hb is not bound to oxygen), Hb tetramers that include two of these mutant sickle β-globin subunits (that is, HbS) can polymerize and cause the erythrocytes to assume a crescent or sickled shape from which the disease takes its name. Hb tetramers with one sickle β-globin subunit can also polymerize, albeit not as efficiently as HbS. Sickle erythrocytes can lead to recurrent vaso-occlusive episodes that are the hallmark of SCD.
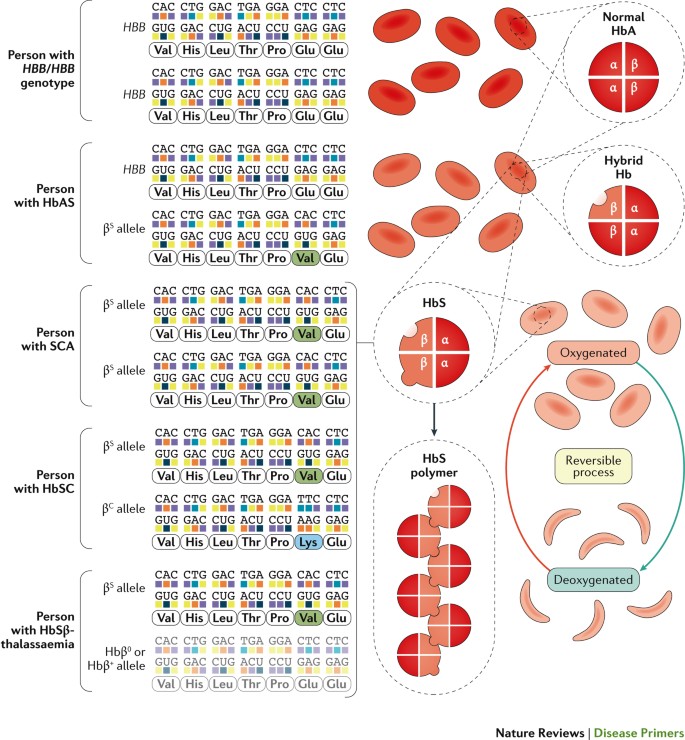
Normal haemoglobin A (HbA) is formed by two α-globin subunits and two β-globin subunits, the latter of which are encoded by HBB . The sickle Hb (HbS) allele, β S , is an HBB allele in which an adenine-to-thymine substitution results in the replacement of glutamic acid with valine at position 6 in the mature β-globin chain. Sickle cell disease (SCD) occurs when both HBB alleles are mutated and at least one of them is the β S allele. Deoxygenated (not bound to oxygen) HbS can polymerize, and HbS polymers can stiffen the erythrocyte. Individuals with one β S allele have the sickle cell trait (HbAS) but not SCD; individuals with sickle cell anaemia (SCA), the most common SCD genotype, have two β S alleles (β S /β S ). Other relatively common SCD genotypes are possible. Individuals with the HbSC genotype have one β S allele and one HBB allele with a different nucleotide substitution ( HBB Glu6Lys, or β C allele) that generates another structural variant of Hb, HbC. The β C allele is mostly prevalent in West Africa or in individuals with ancestry from this region 16 . HbSC disease is a condition with generally milder haemolytic anaemia and less frequent acute and chronic complications than SCA, although retinopathy and osteonecrosis (also known as bone infarction, in which bone tissue is lost owing to interruption of the blood flow) are common occurrences 259 . The β S allele combined with a null HBB allele (Hbβ 0 ) that results in no protein translation causes HbSβ 0 -thalassaemia, a clinical syndrome indistinguishable from SCA except for the presence of microcytosis (a condition in which erythrocytes are abnormally small) 260 . The β S allele combined with a hypomorphic HBB allele (Hbβ + ; with a decreased amount of normal β-globin protein) results in HbSβ + -thalassaemia, a clinical syndrome generally milder than SCA owing to low-level expression of normal HbA. Severe and moderate forms of HbSβ-thalassaemia are most prevalent in the eastern Mediterranean region and parts of India, whereas mild forms are common in populations of African ancestry. Rarely seen compound heterozygous SCD genotypes include HbS combined with HbD, HbE, HbO Arab or Hb Lepore (not shown) 261 .
PowerPoint slide
SCD is inherited as an autosomal codominant trait 1 ; individuals who are heterozygous for the β S allele carry the sickle cell trait (HbAS) but do not have SCD, whereas individuals who are homozygous for the β S allele have SCA. SCA, the most common form of SCD, is a lifelong disease characterized by chronic haemolytic anaemia, unpredictable episodes of pain and widespread organ damage. There is a wide variability in the clinical severity of SCA, as well as in the life expectancy 2 . Genetic and genome-wide association studies have consistently found that high levels of fetal Hb (HbF; the heterodimeric combination of two α-globin proteins and two γ-globin proteins (encoded by HBG1 and HBG2 )) 3 and the co-inheritance of α-thalassaemia (which is caused by mutations in HBA1 and HBA2 ) are associated, on average, with milder SCD phenotypes 2 . However, these two biomarkers explain only a small fraction of the observed phenotypic variability.
Since the 1980s, a rapidly expanding body of knowledge has promoted a better understanding of SCD, particularly in high-income countries 4 , 5 . In the United States, research funding increased exponentially, awareness and education programmes expanded, counselling programmes were improved and universal newborn screening programmes now ensure early diagnosis and intervention. Specific research and training programmes led to a cadre of knowledgeable health professionals working in this field, improved patient management, prevention of complications and extension of life expectancy.
In this Primer, we focus on SCA and aim to balance such remarkable advances with the key major challenges remaining worldwide to improve the prevention and management of this chronic disease and ultimately to discover an affordable cure.
Epidemiology
Natural history.
There is rather little information on the natural history of SCD (which is relevant for SCD prevention and control), especially in areas of high prevalence. The main sources of information are the Jamaican Cohort Study of Sickle Cell Disease, which was initiated in 1973 and followed up all individuals with SCD detected among 100,000 consecutive deliveries in Kingston, Jamaica 6 , and, in the United States, the Cooperative Study of Sickle Cell Disease (CSSCD; 1978–1998), which gathered data on growth and development, disease complications, clinical studies and epidemiology on >3,000 individuals with SCD 7 . Since the discontinuation of the CSSCD, the ongoing natural history of SCD in the United States can be gleaned from a few single-institution ongoing registries, screening populations of clinical trial cohorts and administrative health data sets.
Several cohort studies in high-income and middle-income countries have demonstrated that the clinical course of SCD has substantially changed since the 1970s in both children and adults. Survival similar to that of healthy children has been reported in children with SCA in the United States and the United Kingdom 8 . Adults with SCD in high-income countries can now expect to live well into their sixties, and a median survival of 67 years has been reported for patients with SCD at one London hospital 9 ; nevertheless, survival is still much lower than that of the general population of London. As childhood mortality of SCD has fallen, the transition from paediatric to adult patterns of lifestyle and medical care delivery is increasingly important. For example, in the United States, there is a declining workforce of adult haematologists who are trained specifically in SCD, which means that adults with SCD are treated by primary care physicians or by haematologists–oncologists who are minimally experienced in SCD. There are limited data available about the survival of individuals with SCD in sub-Saharan Africa and India. Data from African studies indicate a childhood SCA mortality (before 5 years of age) of 50–90% 10 .
Distribution
The geographical distribution of the β S allele is mainly driven by two factors: the endemicity of malaria and population movements. The overlap between the geographical distribution of the β S allele and malaria endemicity in sub-Saharan Africa led in the 1950s to the hypothesis that individuals with HbAS might be protected against Plasmodium falciparum malaria 11 . There is now clear evidence that HbAS provides a remarkable protection against severe P. falciparum malaria 12 (in fact, individuals with HbAS are 90% less likely to experience severe malaria than individuals with only normal Hb), which explains the high frequencies of the β S allele observed across sub-Saharan Africa and parts of the Mediterranean, the Middle East and India 13 . Population movements, including the slave trade, have led to a much wider distribution of the β S allele, particularly in North America and Western Europe 14 . Detailed mapping of the β S allele frequency has highlighted that geographical heterogeneities in the prevalence of inherited Hb disorders can occur over short distances 15 .
Prevalence and incidence
The incidence of births with SCA in sub-Saharan Africa was estimated to be ∼ 230,000 in 2010, which corresponds to ∼ 75% of births with SCA worldwide 14 ( Fig. 2 ). In addition, West Africa has the highest incidence of HbSC disease, the second most common type of SCD 16 ( Fig. 1 ). Over the next 40 years, these numbers are predicted to increase, particularly in sub-Saharan Africa 17 . The 2010 estimates reported that there were >3.5 million newborn infants with HbAS in sub-Saharan Africa, who could benefit from a potent protection from severe P. falciparum malaria and its associated mortality 13 . To date, no African country has implemented a national screening programme for SCD 18 . Even in countries where universal screening programmes have been in place for >10 years (for example, the United Kingdom), estimating the prevalence, incidence and burden of disease remains challenging 19 , 20 . In the past 20 years, ∼ 40,000 confirmed cases of SCD were identified in 76 million newborn babies, with >1.1 million newborn babies with the HbAS genotype in the United States 21 . Thus, 1 in every 1,941 neonates had SCD, and 1 in every 67 was heterozygous for the β S allele.
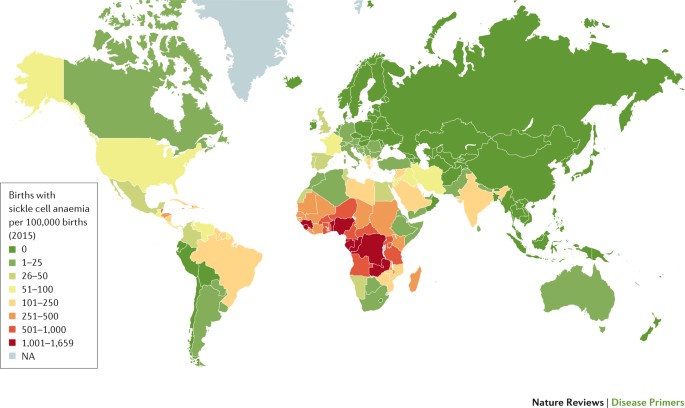
Estimated numbers of births with sickle cell anaemia per 100,000 births per country in 2015. Estimates are derived from prevalence data published in Ref. 14 . Birth data for 2015–2020 were extracted from the 2017 Revision of the United Nations World Population Prospects database . NA, not applicable.
The incidence of SCD varies by state, race and ethnicity 22 , 23 . Among African Americans, ∼ 1 in 360 newborn babies has SCD. Substantial demographical changes have resulted in a more-diverse population at risk and a high prevalence of SCD in immigrant populations. Newborn screening studies for SCD in the state of New York document the marked effect of immigration on the frequency of neonates with SCD 24 , as most of them have foreign-born mothers.
The incidence of SCD in newborn babies varies substantially among the states in Brazil, reflecting the ethnic heterogeneity of the Brazilian population. In 2014, the incidence of SCD was ∼ 1 in 650 newborn babies screened in the state of Bahia, 1 in 1,300 in the state of Rio de Janeiro and 1 in 13,500 in the state of Santa Catarina 25 . Nationwide, in 2016, 1,071 newborn babies had SCD and >60,000 were heterozygous for the β S allele (F.F.C., unpublished observations). There are an estimated 30,000 individuals with SCD in the whole country. The prevalence of the β S allele in Brazil varies from 1.2% to 10.9%, depending on the region, whereas the prevalence of the β C allele is reported to be between 0.15% and 7.4% 25 – 30 . The number of all-age individuals affected by SCA globally is currently unknown and cannot be estimated reliably owing to the paucity of epidemiological data, in particular mortality data, in areas of high prevalence.
Disease severity
The variability in the clinical severity of SCA can partly be explained by genetic modifiers, including factors that affect HbF level and co-inheritance of α-thalassaemia (see below) 31 , 32 . For example, the Arab–India haplotype (a haplotype is a set of DNA polymorphisms that are inherited together), which is found in an area extending from the eastern coast of Saudi Arabia and East Africa to India, is considered to be associated with a phenotype milder than that associated with the four African haplotypes (Benin, Bantu, Cameroon and Senegal haplotypes), and, within India, this phenotype could be milder in the tribal populations than in the non-tribal populations 33 owing to a higher level of HbF 32 . However, evidence suggests that the range of severity of SCD in India is wider than previously thought 34 . Environmental factors (such as the home environment, socio-economic status, nutrition and access to care) also influence the severity of the disease; however, apart from malaria, their role has rarely been investigated 35 , 36 . Although some complications are more frequent in some regions than in others (for example, leg ulcers are common in tropical regions but are relatively rare in temperate climates 37 , whereas priapism (persistent and painful erection) is common in patients of African ancestry but rarer in those of Indian ancestry 38 ), these geographical differences have never been comprehensively and rigorously documented.
Disease burden
It has been estimated that 50–90% of children with SCA who live in sub-Saharan Africa die by 5 years of age 10 . Most of these children die from infections, including invasive pneumococcal disease and malaria 39 , 40 . Owing to the limited data across most areas of high prevalence, it is difficult to precisely assess the future health and economic burden of SCD. As low-income and middle-income countries go through epidemiological transition (that is, changing patterns of population age distributions, mortality, fertility, life expectancy and causes of death, largely driven by public health improvements), which involves substantial reductions in infant mortality that enable SCA diagnoses and treatment, and international migrations contribute to further expand the distribution of the β S allele, the health burden of this disease will increase 41 . Demographical projections estimated that the annual number of newborn babies with SCA worldwide will exceed 400,000 by 2050 (Ref. 17 ).
Mechanisms/pathophysiology
The landmark complication associated with SCA is the vaso-occlusive pain crisis. Although vaso-occlusion is a complex phenomenon, HbS polymerization is the essential pathophysiological occurrence in SCA 42 – 44 . HbS polymerization changes the shape and physical properties of erythrocytes, resulting in haemolytic anaemia and blockage of blood flow, particularly in small (and some large) vessels, which can damage any organ. HbS polymerization can also occur in reticulocytes, which account for ∼ 20% of the red blood cells in individuals with SCA. Direct and indirect consequences of haemolysis play a part in modifying the course and complications of SCD. Furthermore, HbS polymers lead to other abnormalities at the cellular level that contribute to the overall pathophysiological mechanism of SCD. The several variant genotypes of SCD (double heterozygous states or SCA with modifying genes) share a common pathophysiology as described in this section. The variants provide nuanced phenotypic differences or reduced severity ( Fig. 1 ).
Erythrocyte morphology
HbS oxygen affinity and polymerization . HbS has reduced oxygen affinity compared with HbA. Reduced HbS oxygen affinity exacerbates HbS polymerization, which in turn further reduces HbS oxygen affinity 45 ( Fig. 3 ). HbS oxygen affinity is further reduced by 2,3-diphosphoglycerate (2,3-DPG), which is a glycolytic intermediate that is physiologically present at very high levels in sickle erythrocytes and, through interaction with deoxygenated β-globin subunits, reduces Hb oxygen affinity 46 . At any partial pressure of oxygen (pO 2 ), low HbS oxygen affinity kinetically favours an increase in the fraction of deoxygenated HbS (which is the tense conformation (T-state) that readily polymerizes), which in turn promotes HbS polymerization and the formation of sickle erythrocytes. Initial reports indicate that sickle erythrocytes have increased sphingosine kinase activity, leading to high levels of sphingosine-1-phosphate, which also decreases HbS oxygen affinity 47 . Sphingosine kinase is activated by increased levels of plasma adenosine (resulting from the hydrolysis of adenosine nucleotides that are released from erythrocytes during haemolysis) via the erythrocyte adenosine receptor A2b 48 , 49 .
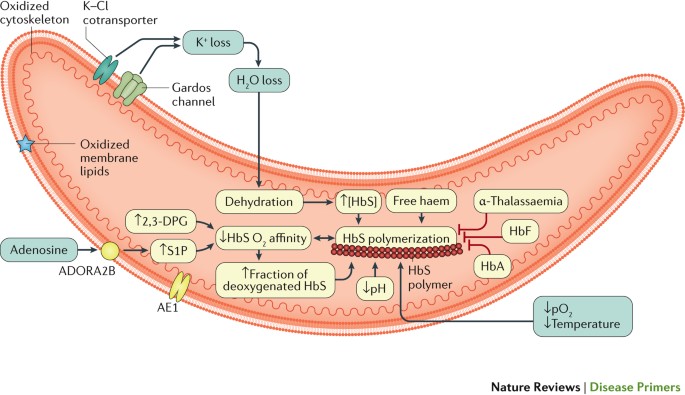
Long polymers of sickle haemoglobin (HbS) align into fibres, which then align into parallel rods. The polymer has a helical structure with 14 HbS molecules in each section 42 , 55 , 262 . The polymerization of HbS depends on many factors, including the HbS concentration, partial pressure of oxygen (pO 2 ), temperature, pH, 2,3-diphosphoglycerate (2,3-DPG) concentration and the presence of different Hb molecules 263 – 265 . The basic concept of HbS polymerization kinetics is the double nucleation mechanism. Before any polymer is detected, there is a latency period (delay time) in which deoxygenated HbS molecules form a small nucleus, which is followed by rapid polymer growth and formation 266 , 267 . Free cytoplasmic haem can increase the attraction of the HbS molecules and the speed of nucleation and polymer formation 268 . Cation homeostasis is abnormal in sickle erythrocytes, leading to the dehydration of cells. Potassium loss occurs via the intermediate conductance calcium-activated potassium channel protein 4 (also known as the putative Gardos channel) and K–Cl cotransporter 1 (KCC1), KCC3 and/or KCC4 (Refs 269 , 270 ). Plasma adenosine can also reprogramme the metabolism of the erythrocyte, altering sphingosine-1-phosphate (S1P). ADORA2B, adenosine receptor A2b; AE1, band 3 anion transport protein; HbA, haemoglobin A; HbF, fetal haemoglobin.
HbS polymerization correlates exponentially with the concentration of HbS within the erythrocyte and also with the composition of other haemoglobins that variably participate in polymers 50 . In α-thalassaemia, reduced production of α-globin subunits favours the formation of unstable β S tetramers (formed by four sickle β-globin subunits), which are proteolyzed, leaving a lower HbS concentration that slows HbS polymerization and haemolysis. Abnormal cation homeostasis (described in the following section) in sickle erythrocytes leads to cell dehydration, which results in increased HbS concentration and polymerization ( Fig. 3 ). As the polymer fibres extend, they deform the erythrocytes and interfere with their flexibility and rheological properties (that is, how they flow), eventually resulting in vaso-occlusion 51 . This impaired blood flow rheology is worsened by erythrocyte aggregation, especially in individuals with SCD and high haematocrit (the percentage of blood volume composed of erythrocytes) 51 . Repeated episodes of HbS polymerization and erythrocyte sickling in conditions of low pO 2 and unsickling in conditions of high pO 2 can lead to severe alterations in the membrane structure and function (see below) and abnormal calcium compartmentalization. Membrane deformation and erythrocyte dehydration eventually result in the formation of an irreversibly sickled cell — a sickle erythrocyte that can no longer revert to its natural shape 52 – 55 .
Altered erythrocyte membrane biology . HbS polymerization directly or indirectly alters the typical lipid bilayer and proteins of the erythrocyte membrane, which leads to reduced cellular hydration, increased haemolysis and abnormal interactions with other blood cells and contributes to early erythrocyte apoptosis 55 – 58 ( Fig. 4 ). Several membrane ion channels are dysfunctional, including the erythroid K–Cl cotransporter 1 (KCC1; encoded by SLC12A4 ), KCC3 (encoded by SLC12A6 ) and KCC4 (encoded by SLC12A7 ), the putative Gardos channel (encoded by KCNN4 ) and P sickle , the polymerization-induced membrane permeability, most likely mediated by piezo-type mechanosensitive ion channel component 1 (PIEZO1), resulting in reduced cellular hydration. In a subpopulation of sickle erythrocytes, phosphatidylserine (which is usually confined to the inner layer of the membrane) is exposed on the erythrocyte surface. Circulating phosphatidylserine-exposing erythrocytes have a role in many important pathophysiological events, including increased haemolysis, endothelial activation, interaction between erythrocytes, white blood cells and platelets and activation of coagulation pathways 59 , 60 . HbS polymers and HbS oxidation (see below) also affect membrane proteins that have structural functions, especially the band 3 anion transport protein, and these changes lead to membrane microvesiculation and the release of erythrocyte microparticles 61 , 62 . These submicron, unilamellar vesicles are shed from the plasma membrane under cellular stress to the membrane and cytoskeleton. In SCD, they are derived in large numbers from erythrocytes 63 but also from platelets, monocytes and endothelial cells. Microvesicles possess cell-surface markers, cytoplasmic proteins and microRNAs derived from their cell of origin and can affect coagulation, adhesion, inflammation and endothelial function 64 , 65 . By contrast, exosomes originate from the endosomal system 66 and have been less studied in SCD.
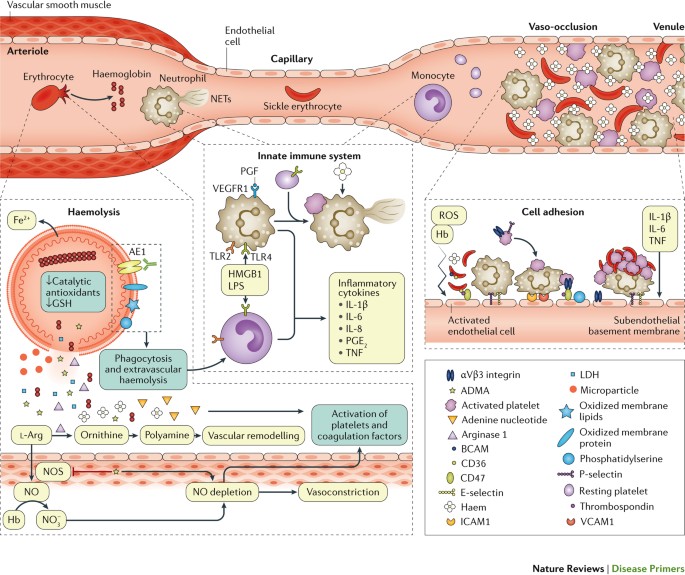
Damage and dysfunction of the erythrocyte membrane caused by sickle haemoglobin (HbS) polymerization lead to haemolysis. Oxidized membrane proteins reveal antigens that bind to existing antibodies, and membranes expose phosphatidylserine; both mechanisms promote phagocytosis of erythrocytes by macrophages, a pathway of extravascular haemolysis. Intravascular haemolysis releases the contents of erythrocytes into the plasma. Hb scavenges nitric oxide (NO), arginase 1 depletes the ʟ-arginine substrate of NO synthase (NOS), and asymmetric dimethylarginine (ADMA) inhibits NOS. Reactive oxygen species (ROS) further deplete NO, leading to vasoconstriction and vascular remodelling, especially in the lung. Adenine nucleotides and NO deficiency promote platelet activation and activation of blood clotting proteins. Haem and other danger-associated molecular pattern (DAMP) molecules activate the innate immune system. Ligand-bound Toll-like receptor 4 (TLR4) and TLR2 activate monocytes and macrophages to release inflammatory cytokines, which promote an inflammatory state and activation of endothelial cells. TLR4 activation on platelets promotes their adhesion to neutrophils, which in turn release DNA to form neutrophil extracellular traps (NETs). Circulating blood cells adhere to each other and to the activated endothelium, contributing and potentially even initiating vaso-occlusion. In postcapillary venules, activated endothelial cells that express P-selectin and E-selectin can bind rolling neutrophils. Activated platelets and adhesive sickle erythrocytes can adhere to circulating or endothelium-bound neutrophils and form aggregates. Sickle erythrocytes might also bind directly to the activated endothelium. The figure shows only some examples of the complex and redundant receptor–ligand interactions involved in the adhesion of circulating cells to the damaged endothelium and exposed subendothelium. AE1, band 3 anion transport protein; BCAM, basal cell adhesion molecule; GSH, glutathione; HMGB1, high mobility group protein B1; ICAM1, intercellular adhesion molecule 1; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; PGE 2 , prostaglandin E2; PGF, placenta growth factor; TNF, tumour necrosis factor; VCAM1, vascular cell adhesion protein 1; VEGFR1, vascular endothelial growth factor receptor 1.
Sickle erythrocytes are highly unstable, with a lifespan that is reduced by ≥75% 65 , 67 . Haemolysis is thought to occur principally via extravascular phagocytosis by macrophages, but a substantial fraction (roughly one-third) occurs through intravascular haemolysis 68 ( Fig. 4 ). It has been hypothesized that the rate of intravascular haemolysis in SCD is insufficient to produce a clinical phenotype, including pulmonary hypertension 69 , the most serious consequence of intravascular haemolysis. However, the epidemiological, biochemical, genetic and physiological data supporting a link between intravascular haemolysis and vasculopathy continue to expand 70 .
Oxidative stress . Haemolysis is both a cause and an effect of oxidative stress. The substantial levels of oxidative stress in sickle erythrocytes enhance HbS auto-oxidation, which could contribute to the damage of the cell membrane, premature erythrocyte ageing and haemolysis 65 . In addition to the accelerated auto-oxidation of HbS, oxygen radicals result from increased expression of oxidases, especially xanthine dehydrogenase and xanthine oxidase, and reduced NADPH oxidase 71 , 72 , extracellular haem and Hb in plasma and probably also from recurrent ischaemia–reperfusion of tissues. Cytoskeletal proteins and membrane lipids become oxidized, and this chronic severe oxidative stress in sickle erythrocytes depletes the levels of catalytic antioxidants 65 such as superoxide dismutase, peroxiredoxin 2 and peroxiredoxin 4 (Refs 46 , 73 ). This issue is worsened by depletion of the endogenous reductant glutathione 46 , 74 ; impaired antioxidant capacity probably contributes to haemolysis.
Free plasma Hb and haem . Extracellular Hb (in plasma or in microparticles 64 , 65 ) and haem in plasma promote severe oxidative stress, especially to blood vessels and blood cells 65 . Continuous auto-oxidation of extracellular Hb produces superoxide, which dismutates into hydrogen peroxide (H 2 O 2 ), a source for additional potent oxidative species, including the ferryl ion, which promotes vasoconstriction 65 . Extracellular Hb scavenges nitric oxide (NO; which is generated by NO synthase (NOS) in endothelial cells and promotes vasodilation) ∼ 1,000-fold more rapidly than cytoplasmic Hb, thereby decreasing NO bioavailability 75 . This decreased bioavailability of NO results in vascular dysfunction, indicated by impaired vasodilatory response to NO donors, activation of endothelial cells (producing cell-surface expression of endothelial adhesion molecules and detected by elaboration of soluble ectodomains of the adhesion molecules into plasma) and haemostatic activation of platelets, indicated by cell-surface expression of P-selectin (which mediates the interaction between activated platelets and leukocytes) and activated αIIbβ3 integrin 70 . Markers of haemolytic severity (such as low Hb or high serum lactate dehydrogenase) predict the clinical risk of developing vascular disease complications (see below).
Disruption of arginine metabolism . Intravascular haemolysis releases two factors that interfere with NOS activity. The enzyme arginase 1 competes with NOS for L -arginine, the substrate required for NO production by NOS 76 . Arginase 1 converts L -arginine into ornithine, which fuels the synthesis of polyamines, which in turn facilitate cell proliferation 77 , potentially of vascular cells, probably promoting vascular remodelling. Asymmetric dimethylarginine (ADMA) is an endogenous NOS inhibitor and a proteolytic product of proteins methylated on arginine; ADMA is abundant in erythrocytes and is also released during haemolysis 78 . Both ADMA and depletion of L -arginine by arginase 1 could contribute to uncoupling of NOS, which then produces reactive oxygen species (ROS) instead of NO 79 , 80 .
Plasma lipids . Individuals with SCA often have a form of dyslipidaemia that is associated with vasculopathy: triglyceride levels are high and correlate with haemolytic severity 81 . Although total cholesterol levels are generally low in individuals with SCA, the levels of apolipoprotein A-I (which promotes hepatic cholesterol catabolism and NOS activity) are particularly low, especially during vaso-occlusive pain crises and in association with markers of pulmonary hypertension and endothelial dysfunction 82 . Genetic variants of apolipoprotein L1 have been associated with renal disease in SCA 83 .
Innate immune system activation
Plasma haem and Hb act as danger-associated molecular patterns (DAMPs) to activate the innate immune system and heighten the adhesiveness of circulating blood cells to each other and to the endothelium, thereby triggering vaso-occlusion 70 ( Fig. 4 ). Haem activates neutrophils to release DNA as neutrophil extracellular traps (NETs) that increase platelet activation and thrombosis and promote pulmonary vaso-occlusion 84 and release of placenta growth factor (PGF) from erythroblasts (nucleated precursors of erythrocytes). PGF is a ligand for vascular endothelial growth factor receptor 1 on endothelial cells and macrophages, promoting release of endothelin 1 (a vasoconstrictor), which contributes to pulmonary hypertension 85 . Toll-like receptor 4 (TLR4) is highly expressed in immune cells in SCD, and tissue damage and platelet activation release high mobility group protein B1 (HMGB1), a high-affinity TLR4 ligand. TLR4 also binds lipopolysaccharide (LPS) derived from Gram-negative bacteria, which could explain why infections promote vaso-occlusive crises in individuals with SCA. TLR4 ligands activate monocytes and macrophages to release inflammatory cytokines, which promote an inflammatory state and activate the adhesiveness of neutrophils, platelets and endothelial cells. Finally, increased intracellular iron from turnover of haemolyzed and transfused erythrocytes is associated with markedly increased expression in the peripheral blood mononuclear cells of several components of the inflammasome pathway 86 .
Cell adhesion and vaso-occlusion
Endothelium activation . Vaso-occlusion in SCA is a complex phenomenon in which interactions between erythrocytes and endothelial cells, leukocytes and platelets play a central part ( Fig. 4 ). Endothelial cells are probably activated by direct contact of sickle erythrocytes, free haem and Hb and hypoxia-induced ROS 87 . Reduced NO bioavailability could induce the expression of adhesion molecules and production of endothelin 1. The increased expression of endothelial adhesion molecules (such as vascular cell adhesion protein 1 (VCAM1) 88 , 89 , intercellular adhesion molecule 1 (ICAM1) 90 , P-selectin, E-selectin, leukocyte surface antigen CD47 and αVβ3 integrin) and exposed heparin sulfate proteoglycans and phosphatidylserine are responsible for erythrocyte and leukocyte adhesion 89 . Activated endothelial cells also produce inflammatory mediators, such as IL-1β, IL-6 and tumour necrosis factor (TNF), which lead to a chronic inflammatory state.
Erythrocytes . Sickle erythrocytes are more adhesive to endothelial cells than normal erythrocytes 87 , 91 . Many adhesion molecules (the most important include α4β1 integrin (also known as very late antigen 4 (VLA4), which is reticulocyte-specific), platelet glycoprotein 4 (also known as CD36) and basal cell adhesion molecule (BCAM)) are overexpressed by sickle red blood cells and mediate the adhesion to the endothelium 92 . Interestingly, reticulocytes and deformable erythrocytes (that is, erythrocytes that have not become permanently sickled) are substantially more adhesive than the irreversible and dense sickle erythrocytes 93 .
Leukocytes . High baseline leukocyte numbers are associated with increased morbidity and mortality in SCA 94 , 95 . Many studies in mouse models of SCA indicate that neutrophils have an important role in vaso-occlusion; neutrophils adhere to the endothelium and sickle erythrocytes could bind to these cells, thereby reducing blood flow and promoting vaso-occlusion 96 . Indeed, neutrophils are in an activated state in SCA and have increased expression of αMβ2 integrin with enhanced adhesion to endothelial and subendothelial proteins (such as fibronectin) 97 . Selectins produced by activated endothelium have an important role in the initial binding of neutrophils to the vascular wall 96 .
Platelets . Platelets play an important part in the pathophysiology of SCA and are in an activated state 96 , with high levels of P-selectin and activated αIIbβ3 integrin. Moreover, several biological markers of activated platelets are increased in SCA (for example, platelet microparticles 64 , thrombospondin 93 , platelet factor 4 (also known as CXC-chemokine ligand 4 (CXCL4)) and β-thromboglobulin). Platelets are found in the circulating heterocellular aggregates of neutrophils and red blood cells (mainly reticulocytes) in the blood from individuals with SCA, and their adhesion to these aggregates is mediated in part through P-selectin 98 . These data strongly suggest that platelets have a role in the formation of these aggregates. Platelets could also act as accessory cells of the innate immune system by releasing cytokines 99 .
Diagnosis, screening and prevention
Diagnostic opportunities.
The goals and methods of diagnosis of SCD vary with the age of the person. In general, there are four overlapping testing periods: preconception, prenatal, neonatal and post-neonatal. Preconception testing is designed to identify asymptomatic potential parents whose offspring would be at risk of SCD. Laboratory techniques used for preconception testing are routine basic methods of protein chemistry that enable separation of Hb species according to their protein structure, including Hb electrophoresis, high-performance liquid chromatography (HPLC) and isoelectric focusing 100 . Prenatal diagnosis is a generally safe but invasive procedure and is offered during early pregnancy to couples who tested positive at preconception screening. It requires fetal DNA samples obtained from chorionic villus analysis performed at 9 weeks of gestation 100 . Non-invasive prenatal diagnosis techniques are being developed but are still investigational. These new techniques can detect fetal DNA in maternal circulation by as early as 4 weeks of gestation. Some couples who test positive at preconception screening might opt for in vitro fertilization with pre-implantation genetic diagnosis, if available, to genetically identify at-risk embryos before embryo transfer occurs 101 .
Newborn screening . Newborn screening for SCD is performed at birth before symptoms occur, using Hb protein analysis methodologies. Two types of newborn screening programmes have been used: selective screening of infants of high-risk parents (targeted screening) and universal screening. Universal screening is generally more cost-effective, identifies more newborn babies with disease and prevents more deaths 17 , 102 . In areas without newborn screening programmes, the initial diagnosis of SCD occurs at approximately 21 months of age 103 . For many individuals with SCD, the initial presentation is a fatal infection or acute splenic sequestration crisis 103 . Early diagnosis accompanied by penicillin prophylaxis and family education reduces the mortality in the first 5 years of life from 25% to <3% 103 , 104 . Similar positive results are found in low-income countries 105 , 106 .
Post-neonatal testing . The requirement of post-neonatal testing for SCD is influenced by several factors that affect the general population's knowledge of their SCD status. These factors include the regional success of neonatal screening programmes, immigration of at-risk patients not previously tested and access to neonatal results in older patients 107 . HbAS is a benign condition and not a disease, but it is also a risk factor for uncommon serious complications 107 . Thus, knowledge of one's own HbAS status is important in the prevention of rare serious complications and in family planning.
HbAS can also be detected by newborn screening programmes; however, HbAS detection is not the primary objective, and many programmes do not provide this information or offer associated counselling. Individuals who wish to have children should be screened to discover heterozygous genotypes that could be important in genetic counselling. HbAS screening enables informed decisions concerning preconception counselling and prenatal diagnosis.
Routine fitness training does not increase the risk of mortality for individuals with HbAS. However, there is a concern of increased risk of rhabdomyolysis (rapid destruction of skeletal muscle) and sudden death during intense, prolonged physical activity; this risk can be mitigated by proper training 108 . In some regions, these observations have resulted in voluntary or mandatory screening of athletes for HbAS 107 . There are rare and specific complications of HbAS that should prompt HbAS testing. These include haematuria (blood in the urine), hyphema (blood inside the eye's anterior chamber) and renal medullary carcinoma, a rare malignancy. HbAS could be a risk factor for chronic kidney disease and pulmonary embolism 109 .
Newborn screening programmes
Newborn screening programmes for SCD are now in place in several European countries, the United States ( Box 1 ), India, Africa ( Box 2 ) and Brazil ( Box 3 ).
Screening in Europe . Newborn screening programmes for SCD in the United Kingdom became universal in 2006 (Ref. 110 ); the primary aim of the programme is to diagnose SCD, but if a baby has HbAS, the parents are provided with specific informational materials. In France, screening for SCD has been in place since 2000 but is restricted to newborn babies whose parents both originate from SCD-endemic regions 111 . In Spain, universal screening has been recommended for regions with a high annual birth rate and SCD prevalence (for example, Catalonia and Madrid), whereas targeted screening is recommended for regions with a low annual birth rate and SCD prevalence 112 . Screening programmes are also present in Italy 113 and Germany 114 .
Screening in the United States . In the United States, statewide newborn screening originated in New York state in 1975 ( Box 1 ), and by 2007, all states had universal screening programmes 21 . In the United States, HPLC and isoelectric focusing are the predominant screening methods 21 , 100 . Confirmation of the diagnosis by DNA analyses to detect Hb variants is commonly used but is not standardized among states. A major gap in these programmes is the lack of follow-up and the variability of statewide education programmes 115 . The identification of substantial clinical morbidity occasionally associated with individuals with HbAS has not yet resulted in routine counselling and genetic testing of family members of newborn babies with HbAS 107 .
Screening in India . The population of India consists of >2,000 different ethnic groups, most of which have practised endogamy (the custom of marrying only within the limits of the local community) over centuries. Thus, although the β S allele has been detected in many ethnic groups, its prevalence has been enriched in some. The at-risk population consists of several hundreds of millions of individuals, predominantly belonging to historically disadvantaged groups 116 . Screening efforts have focused on groups with a high prevalence of the β S allele and areas with large numbers of these at-risk populations. Screening typically consists of an Hb solubility test (a screening test that does not distinguish HbAS from disease) at the point of care with further testing of initial positive samples by HPLC analysis at a reference centre. Screening programmes also include education, testing and genetic counselling. In many hospitals, such services are also offered to the relatives of patients diagnosed with SCD and in the prenatal setting to mothers either previously diagnosed with HbAS or belonging to an at-risk ethnic group. Pilot projects of newborn screening programmes for SCD have been implemented in the states of Gujarat, Maharashtra and Chhattisgarh 105 , 106 , 117 – 120 , which resulted in detailed data on the prevalence of HbAS in various populations, with ranges of 2–40%. There is considerable regional variation in the implementation of follow-up approaches such as comprehensive care, penicillin prophylaxis and immunization against pneumococcus.
Screening in Africa . No country in sub-Saharan Africa has implemented a universal newborn screening programme for any disease 121 . However, a few countries in sub-Saharan Africa have developed pilot newborn screening programmes for SCD. Among these, Ghana's National Newborn Screening Programme for SCD, launched in 2010 following a 15-year pilot study, is the most developed 122 ( Box 2 ). Other countries in Africa where small-scale or pilot newborn screening programmes for SCD have been conducted or are ongoing include Angola 123 , Benin 124 , Burkina Faso 125 , Burundi 126 , Democratic Republic of the Congo 127 , Nigeria 128 , Rwanda 126 , Senegal 129 , Tanzania 130 and Uganda 131 . Screening followed by penicillin prophylaxis can reduce early mortality from pneumococcal bacteraemia 103 , 104 . Nevertheless, current and future numbers of individuals with SCA or HbAS make the scalability of the interventions implemented in high-income, low-burden countries (such as universal newborn screening programmes) in low-resource settings challenging. There is no mandatory or large-scale preconception screening programme for adults who wish to have children in any African country. However, several churches require couples to be screened for SCD-related conditions as a prerequisite for marriage approval. Such screening often involves inexpensive but inconclusive ‘sickling’ and solubility tests, which cannot identify individuals with the β C allele or β-thalassaemia, conditions that, although not characterized by the presence of HbS, are of genetic counselling relevance. There are very few much-needed certified genetic counsellors to support the screening programmes. The Sickle Cell Foundation of Ghana launched the first Sickle Cell Genetic Counsellor Training and Certification Programme in June 2015 ( Box 2 ).
Box 1: Roadmap to screening programmes in the United States
The National Sickle Cell Anemia Control Act (Public Law 92–294) was signed into law in 1972 in response to a presidential initiative and congressional mandate 257 . The act provided for voluntary sickle cell disease (SCD) screening and counselling, education programmes for health professionals and the public and research and training in the diagnosis and treatment of SCD. Because of this legislation, a national broad-based programme of basic and clinical research was established at the NIH and was coordinated across federal agencies. The Comprehensive Sickle Cell Centers were the major component of this programme; ten centres were established in hospitals and universities located in geographical areas with large at-risk populations. These centres provided an integrated programme of research and care for individuals with SCD and emphasized prevention, education, early diagnosis and counselling programmes supported by the NIH. The establishment of treatment guidelines and protocols standardized treatment across the country. The centres gradually shifted towards basic and clinical research, and the NIH Comprehensive Sickle Cell Center programme was disassembled in 2008.
Box 2: Screening in Ghana
The screening programme in Ghana is designed to be universal and includes screening for neonates born at both public and private birth facilities as well as screening at ‘well-baby’, free immunization clinics (that is, public health clinics where babies are brought to receive free immunizations) for babies who were not screened at birth or who were referred from facilities where screening is not available 122 . Babies with possible sickle cell disease (SCD) are referred to a treatment centre, where a second sample is obtained to confirm the initial screening results. Babies with SCD are enrolled in a comprehensive care programme that includes penicillin and antimalarial prophylaxis, folic acid supplementation and parental education about management of SCD. Ghana's National Health Insurance Authority funds newborn screening programmes as part of the mandated free care for children of <5 years of age. By the end of 2015, >400,000 newborn babies were screened for SCD and related conditions. Of the 6,941 newborn babies who were diagnosed with SCD, 80% had been successfully followed up, and 70% of them registered at the Kumasi Centre for SCD, which was established for the pilot screening programme (K.O.-F., unpublished observations). However, follow-up is challenging, as 80% of mothers of babies with SCD initially failed to return for results and had to be reached at their homes, and irregular government funding can cause intermittent shortages of laboratory supplies. Limited funding has stalled the national scale-up of the free screening programme, which currently reaches only 4.2% of the 850,000 annual neonates.
Box 3: Screening in Brazil
The Newborn Screening Program in Brazil was implemented as an official programme of the federal government in 2001, but a few statewide programmes were already in place. As of 2017, the National Program for Newborn Screening (PNTN) is available to all 26 states of the country, although the coverage is highly variable (for example, in 2016, it was ∼ 100% of hospitals in the state of Minas Gerais and ∼ 55% of hospitals in the state of Amapa) (F.F.C., unpublished observations).
The newborn screening programmes enabled the analysis of the survival of children with sickle cell disease (SCD). In the state of Minas Gerais, 3.6 million newborn babies were screened between 1998 and 2012, and 2,500 children were diagnosed with SCD. During the 14-year study period, the mortality was 7.4%. The main causes were infection (45%) and acute splenic sequestration (14%) 258 . In another study in the state of Rio de Janeiro, >1.2 million newborn babies were screened between 2000 and 2010, and 912 had SCD. The mortality was 4.2% during the 10-year period, and the main causes were acute chest syndrome (36.8%), sepsis (31.6%) and splenic sequestration (21.1%) 27 .
Phenotypes in sickle cell disease
There is great phenotypic variability among individuals with SCD. Some variability shows a specific geographical distribution and is associated with known or suspected genetic variants 132 . However, some complications cluster together epidemiologically in subphenotypes, at times united by a common biomarker that suggests a mechanism, such as a particularly low Hb level with a high reticulocyte count or high serum lactate dehydrogenase level, implying more-intense haemolysis. These phenotypes are not mutually exclusive, exist often as a spectrum, can overlap, are probably due to independent genetic modifiers of the underlying mechanisms and might change with ageing.
Vaso-occlusive subphenotype . This SCA subphenotype is characterized by a higher haematocrit than that observed in individuals with other SCA phenotypes; a higher haematocrit promotes higher blood viscosity. Individuals with this phenotype are predisposed to frequent vaso-occlusive pain crises, acute chest syndrome (that is, a vaso-occlusive crisis of the pulmonary vasculature) and osteonecrosis. Co-inheritance of α-thalassaemia reduces haemolysis (by reducing the intracellular concentration of HbS, which slows HbS polymerization and haemolysis) but promotes higher haematocrit 133 .
Haemolysis and vasculopathy subphenotype . This phenotype is characterized by a lower haematocrit than that found in individuals with the vaso-occlusive subphenotype accompanied by higher levels of serum lactate dehydrogenase and bilirubin, which indicate more-severe haemolytic anaemia. Individuals in this group are at risk of ischaemic stroke, pulmonary hypertension, leg ulceration, gallstones, priapism and possibly nephropathy 134 . Decreased NO bioavailability, haem exposure and haem turnover are associated with these vasculopathic complications. The severe anaemia also promotes high cardiac output as a compensatory mechanism, and this excessive blood flow has been suggested to promote vasculopathy in the kidney and potentially in other organs.
High HbF subphenotype . Persistent expression of HbF in the range of 10–25% of total Hb owing to genetic variants generally reduces the clinical severity of SCA 3 , 135 . However, not all individuals with the common, uneven cellular distribution of HbF (heterocellular distribution) have a mild phenotype. Expression levels of 25–50% of HbF in every erythrocyte (pancellular distribution) lead to nearly complete amelioration of SCA, with rare clinical symptoms and no anaemia 136 , a finding that could prompt the development of drugs that can induce ‘globin switching’ (that is, the preferential expression of HBG1 and HBG2 ).
Pain subphenotypes . Individuals with pain-sensitive or pain-protective phenotypes experience pain differently, potentially owing to altered neurophysiology of pain sensation pathways. One example of a genetic modifier of pain is GCH1 , which is associated with pain sensitivity in healthy individuals, and a variant of GCH1 is associated with frequency of severe pain in SCA 137 . Quantitative sensory testing of pain sensitivity is being used to functionally characterize these phenotypes in SCA 138 .
SCD is a complex, multisystem condition characterized by acute and chronic complications ( Fig. 5 ). Advances in general medical care, early diagnosis and comprehensive treatment have led to substantial improvements in the life expectancy of individuals with SCA in high-income countries 8 , 9 as almost all patients survive beyond 18 years of age 139 . However, even with the best of care, life expectancy is still reduced by ∼ 30 years, routine and emergency care for individuals with SCD have great financial costs, the quality of life often deteriorates during adulthood and the social and psychological effects of SCD on affected individuals and their families remain underappreciated 140 . Furthermore, most of these advances have not reached low-income countries 141 .
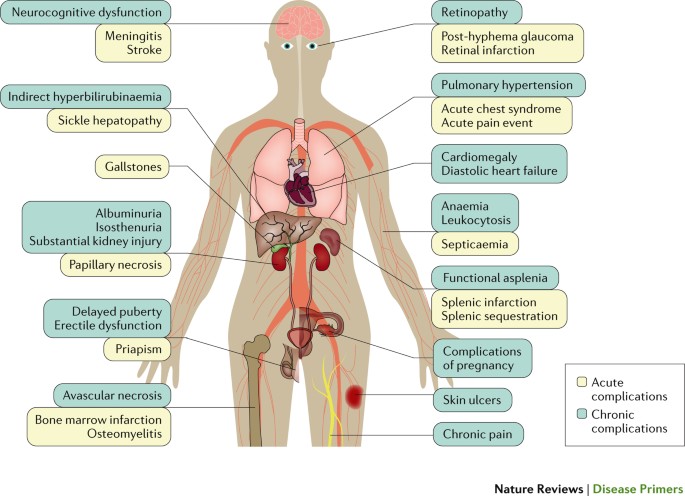
Acute complications bring the individual with sickle cell disease (SCD) to immediate medical attention; pain is the most common acute complication. As individuals with SCD age, chronic complications produce organ dysfunction that can contribute to earlier death. Complications of pregnancy include pre-eclampsia, intrauterine growth restriction, preterm delivery and perinatal mortality.
Three therapies modify the disease course of SCA: hydroxycarbamide, erythrocyte transfusion and haematopoietic stem cell transplantation 142 .
Hydroxycarbamide . Hydroxycarbamide (alternatively known in some countries as hydroxyurea), a ribonucleotide reductase inhibitor, has multiple physiological effects, including increasing HbF expression (in most individuals with SCA 143 ) and decreasing leukocyte count. It was approved by the US FDA in 1998 and by the European Medicines Agency (EMA) in 2007 for the treatment of SCD. The drug significantly reduces the incidence of SCA vaso-occlusive crises, hospitalizations and mortality in high-income countries (with studies ongoing in low-resource countries) with an excellent safety profile 144 , although some patients do not have a beneficial response, usually because of limitations of adherence to treatment 145 but possibly sometimes for pharmacogenomic reasons 146 . Hydroxycarbamide is underutilized because of health-care infrastructure deficiencies in both low-resource and high-resource countries and disproportionate perceptions of carcinogenicity, teratogenicity and reduced fertility, which have not been problems thus far in follow-up studies 143 , 147 , 148 ; however, hydroxycarbamide use is increasing. Snapshots from various cohorts over the years show that in high-resource countries, at specialized SCD clinics, up to 63% of patients with SCA may be on hydroxycarbamide 149 , but the percentage is near zero in most African countries 150 . Because of very favourable clinical trial results in infants and toddlers 151 , hydroxycarbamide is prescribed with increasing frequency to children with SCA — up to 45% in multinational SCD centres 152 . Although there is still limited evidence on whether hydroxycarbamide improves survival and prevents SCD complications in low-income countries 153 , various studies, including the Realizing Effectiveness Across Continents with Hydroxyurea (REACH) trial, are currently underway and should address knowledge gaps about treatment options for SCA in sub-Saharan Africa 150 .
Erythrocyte transfusion . This therapy improves microvascular flow by decreasing the number of circulating sickle erythrocytes and is associated with decreased endothelial injury and inflammatory damage 154 , 155 . Chronic transfusion therapy, prescribed in high-resource countries primarily to the roughly 10% of patients with SCA at high risk of stroke, can ameliorate and prevent stroke and vaso-occlusive crises 156 ; however, several potential adverse effects, including iron overload, alloimmunization (an immune response to foreign antigens that are present in the donor's blood) and haemolytic transfusion reactions, limit its potential benefits. The availability of oral iron-chelating drugs since 2005 has reduced the adverse effects of iron overload. In countries with limited testing of blood products for infectious agents, there are substantial risks of transmission of blood-borne infections, such as hepatitis B, hepatitis C, HIV infection, West Nile virus infection and others. Transfusion protocols with extended erythrocyte matching that includes the erythrocyte antigens Kell, C, E and Jkb and iron-chelation therapy guidelines improve the safety of this therapy 156 . Systematic genotyping of blood groups for the patient has been proposed to reduce alloimmunization 157 .
Haematopoietic stem cell transplantation . Haematopoietic stem cell transplantation in SCA is curative and should be considered in symptomatic patients with a human leukocyte antigen (HLA)-matched family donor. Worldwide, it is estimated that nearly 2,000 individuals with SCA have undergone allogeneic haematopoietic stem cell transplantation; the survival exceeds 90% in US and European studies 158 , 159 . In pooled registry data, the average rate of both acute and chronic graft-versus-host disease has been 14% and is generally lower with newer approaches 158 , and the rate of graft failure has been 2% 159 . Early results with experimental reduced-intensity conditioning regimens (pretransplantation chemotherapy to ablate or suppress the recipient's bone marrow) are very encouraging 160 . However, most patients do not have an HLA-matched related donor. Experimental use of expanded donor pools (haploidentical donors (who share 50% of the HLA antigens with the recipient) and unrelated HLA-matched donors) can increase the probability of cure but also increase the rate of graft rejection and mortality, which seem to improve with ongoing research 161 . Although haematopoietic stem cell transplantation from the bone marrow of a healthy HLA-matched donor can cure SCA, this therapy is limited by the paucity of suitable donors and is available only in high-income countries 162 .
Management of acute complications
The principles of management of acute complications in SCA ( Fig. 5 ) include the need for early diagnosis, consideration of other non-SCD-related causes and rapid initiation of treatment. The use of standardized protocols for common complications improves outcomes.
Acute pain . Acute pain events usually affecting the extremities, chest and back are the most common cause of hospitalization for individuals with SCA. However, the majority of such events are managed at home with NSAIDs or non-prescription oral opioid analgesics without the involvement of the health care provider. The pathophysiology and natural history of acute pain events are complex, and treatment is suboptimal 163 . Individual personalized protocols for outpatient and inpatient pain management improve quality of life and decrease hospital admissions 164 – 166 . The treatment is guided by the severity of pain, which is generally self-reported using pain severity scales. When home management with oral analgesics, hydration and rest is ineffective, rapid triage with timely administration of opioids is recommended. Initial treatment in a day unit compared with an emergency room drastically decreases hospitalization 167 . Initiation of treatment for emergency room patients with SCD is often markedly delayed, with patients with SCD waiting 25–50% longer than patients without SCD with similar pain acuity 168 . In some programmes, innovative emergency room treatment protocols for patients with SCD using standardized time-specific dosing protocols and intranasal fentanyl (an opioid) have substantially reduced time to treatment; similar approaches should be adopted universally 164 , 165 . Once an individual is hospitalized, a standardized protocol using patient-controlled analgesia devices is indicated. These intravenous infusion pumps enable patient self-medication and, in general, result in improved analgesic control and less analgesic use 169 . Incentive spirometry, a simple device that prevents atelectasis (the complete or partial collapse of a lung), with close monitoring of the patient's levels of sedation, hydration and oxygenation improves outcomes. Although intensive analgesia is important for effective medical management of pain in SCD, in some countries, opioids are unavailable owing to resource limitations or are not prescribed or consumed owing to stigma 170 . Vaso-occlusive crises can sometimes result in sudden unexpected death 3 , 171 . The precise aetiology of sudden death in such cases is unclear, although autopsy often shows histopathological evidence of pulmonary arterial hypertension 171 .
Acute chest syndrome . Acute chest syndrome is the second most frequent reason for hospitalization and a leading cause of death in individuals with SCD — it is often linked to and follows an acute pain event 172 . The severity of acute chest syndrome increases with age. In adults, >10% of cases are fatal or complicated by neurological events and multi-organ failure 173 . The initial pulmonary injury is multifactorial, including infection, pulmonary fat embolism, pulmonary infarction and pulmonary embolism 174 . The presence of underlying, often undetected bronchoreactive lung disease can increase the frequency and severity of acute chest syndrome events 175 . Early chest X-ray imaging tests and oxygen monitoring of patients with any pulmonary symptoms are necessary. Hospitalization with broad-spectrum antibiotics, bronchodilators, oxygen supplementation and red cell transfusions is often indicated 176 . Exchange transfusions (in which the patient's blood is replaced by donor blood) and steroids, which decrease acute inflammation, could modify a severe or rapidly deteriorating event 176 , 177 . Exchange transfusion is the most effective method of lowering the level of HbS below 30% of the total Hb without raising the total Hb level above 10 g dl −1 (Ref. 178 ). However, delayed transfusion reactions can complicate transfusion therapy and present as a hyperhaemolytic episode in which the transfused cells and the patient's own red blood cells are destroyed 179 . Steroids often provide benefit but are associated with an ∼ 25% risk of mild or severe complications (in particular, there is a high rate of recurrence of acute chest syndrome once the steroids are stopped); thus, their use is usually limited to life-threatening acute chest syndrome events 180 .
Acute stroke . An acute stroke, including ischaemic and haemorrhagic events, is a medical emergency. Children with SCA have a 300-fold higher risk of acute stroke than other children without SCD, and by 45 years of age, one in four adults with SCA has had a stroke 181 . In the United States, 25% of individuals with SCA develop an overt stroke, and another 35% have non-focal central nervous system injury 181 – 183 . Ischaemic stroke is usually caused by occlusion of a large cerebral artery and can occur as a complication of acute chest syndrome (defined above) or independently, and can manifest with transient ischaemic attack, sudden weakness or loss of consciousness. Prompt evaluation (including MRI of patients with subtler presentations) is indicated. Rapid exchange transfusion is the standard treatment. In addition, chronic transfusion decreases secondary stroke recurrence 178 . The importance of subsequent monthly chronic transfusion to prevent secondary stroke has been re-affirmed by the Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) study 184 .
Intracranial haemorrhage or haemorrhagic stroke account for 3–30% of acute neurological events and have a 25–50% acute mortality 185 . Clinically, these patients present with severe headache or loss of consciousness without hemiparesis. Imaging with angiography could reveal a surgically treatable aneurysm. Individuals with moyamoya vasculopathy, which is a prominent collateral circulation around occluded arteries of the circle of Willis that is frequent in individuals with SCD, are at high risk of intracranial bleeding. When this pathology is electively detected, indirect revascularization using encephaloduroarteriosynangiosis (a surgical procedure that implants the superficial temporal artery to the brain surface, increasing blood flow to the ischaemic area) is often considered to decrease bleeding risk and improve oxygenation 186 , 187 .
Acute anaemic events . Over half of patients with SCD will experience an acute anaemic event, which can be fatal, at some point in their life. The most common types of anaemic events are splenic sequestration crisis, aplastic crisis (temporary absence of erythropoiesis) and hyperhaemolytic crisis. Acute splenic sequestration crisis is characterized by rapid swelling of the spleen and hypovolemia with a sudden fall in Hb levels. As many as 30% of young children experience acute sequestration events, which are a leading cause of infant mortality. Early detection is crucial, and transfusion followed by elective splenectomy is usually required 188 . Nonsurgical supportive care can be successful, and, when necessary, transfusion with extended red blood cell antigen-matched erythrocyte units and selective use of immunosuppressive therapy are indicated.
Cholelithiasis . Cholelithiasis (gallstones) results from the chronic accelerated rate of erythrocyte destruction in individuals with SCD. Haem is metabolized to bilirubin, which in the bile can form insoluble calcium bilirubinate, which in turn precipitates as a pigment and forms gallstones. Of note, a variant of UGT1A1 (which encodes a protein involved in bilirubin processing) increases bilirubin metabolism and, therefore, the formation of gallstones in individuals with SCD 189 . By adulthood ( Fig. 6 ), 20% of patients with SCD have acute complications from gallstones, which can promote cholecystitis (inflammation of the gall bladder) and often necessitate cholecystectomy (surgical removal of the gallbladder) 190 . By contrast, individuals with SCD who also inherit α-thalassemia have reduced haemolysis, bilirubin production and gallstone formation 189 .
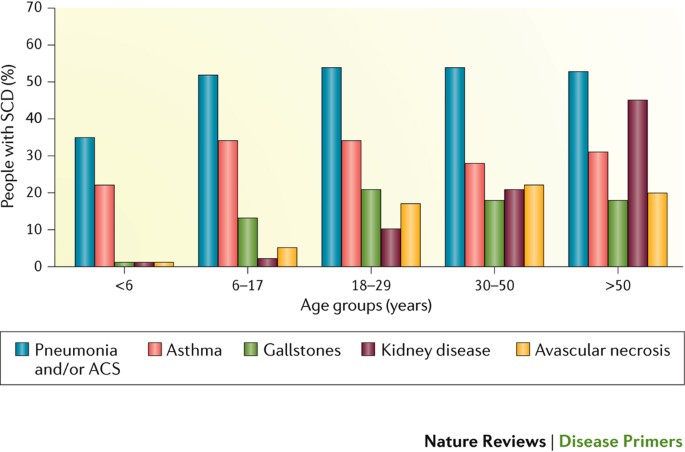
Development of clinical complications in 5,100 individuals with sickle cell disease (SCD) identified in the California Hemoglobinopathy Surveillance Program 271 . ACS, acute chest syndrome.
Long-term management
Improved management of acute complications is associated with a longer survival. As individuals with SCD age, chronic problems resulting from cumulative organ injury can lead to severe morbidity 191 ( Figs 5 , 6 ). Chronic pain is common; the Pain in Sickle Cell Epidemiology Study (PiSCES) found that adults with SCD have pain on 55% of days 192 , and pain, in general, is a poorly managed complication of SCD 193 . Individuals with SCD and recurrent pain have altered brain network connectivity, which affects their response to treatment 194 . Chronic pain requires a multidisciplinary team familiar with neuropathic pain tolerance, withdrawal symptoms and hyperanalgesia syndrome 193 . Hydroxycarbamide, selective use of chronic transfusions in severe cases and long-acting opioids are useful components of a multidisciplinary pain management approach.
Avascular necrosis of the hip is a common cause of chronic pain that eventually develops in many individuals with SCD 195 ; in >20% of hospitalizations, symptoms are related to avascular necrosis. Although core decompression (in which a small core of bone is removed from the damaged area, lowering the bone marrow pressure and stimulating healthy bone regrowth), physiatry (rehabilitation) therapy and analgesics are temporarily helpful, total hip replacement is often required.
Chronic kidney disease is relatively common in older individuals with SCD and is thought to have a poor prognosis in these individuals compared with individuals without SCD 196 . This worse outcome could in part be due to delayed access to dialysis and renal transplant for individuals with SCD, as they might not be considered good candidates for these therapies. Of note, individuals with SCD who receive a timely renal transplantation have an outcome comparable with that of individuals without SCD who receive a transplant 197 , 198 .
Although screening for brain injury with annual transcranial Doppler (TCD) screening and/or MRI and chronic transfusion therapy for high-risk patients decrease the frequency and severity of stroke complications, patients continue to have progressive neurocognitive injury and require close observation and long-term therapy 182 . In addition, implementation of multidisciplinary plans for management of other common chronic complications of SCD (for example, cardiopulmonary dysfunction, priapism and leg ulcers) improves the quality of life of these patients as they age 199 , 200 .
Prevention of complications
Preventive strategies have changed the long-term outcome in SCD more than any other approach. Prevention of life-threatening infections and stroke has drastically reduced childhood mortality in SCD; generalized screening of individuals with SCD for risk factors and early evidence of disease enable the implementation of treatment that can reduce morbidity. Screening for pulmonary, renal and systemic hypertension, retinopathy and damage to other organs is indicated 201 . Detailed generalized screening recommendations for SCD are available 202 , 203 .
Prevention of infection . Until the 1990s, in the United States, up to 30% of young children with SCA died from infections, predominantly due to encapsulated bacteria 104 , owing to a common childhood deficiency of immune response to polysaccharide antigens 204 and exacerbated in SCA by impaired clearance of blood-borne bacteria as a result of functional asplenia 104 . The introduction of prophylactic penicillin treatment decreased the incidence of pneumococcal bacteraemia associated with impaired splenic function by 85% 104 . Prophylactic penicillin has remained safe and beneficial in patients up to at least 5 years of age. The universal use of pneumococcal and other standard vaccinations has further lowered infectious disease mortality. The first conjugated pneumococcal vaccine decreased the rate of pneumococcal bacteraemia in children of <3 years of age by 93.4% and added protection to the large cohort of individuals with SCD who have suboptimal compliance with prophylactic penicillin therapy 205 . Long-term penicillin prophylaxis has raised concerns about the development of penicillin-resistant pneumococcal colonization and disease 206 , especially in low-income countries, although the benefit-to-risk ratio of prophylaxis is still high. The pneumococcal conjugate vaccine PCV13 and pneumococcal polysaccharide vaccine PPSV23 (Ref. 207 ) can prevent infection by most, but not all, serotypes.
Prevention of central nervous system injury . Cerebral vascular injury and neuro-ischaemic damage are a leading cause of death and morbidity in children and adults with SCA. The complications of these events are largely irreversible and mandate universal prevention and screening policies. TCD screening to detect increased vascular velocity can contribute to identifying children at high risk of stroke, which can be largely prevented by initiating transfusion therapy 208 . The landmark Stroke Prevention Trial in Sickle Cell Anaemia (STOP) study demonstrated that neurologically healthy children with elevated TCD measurements (vascular velocity >200 cm s −1 ) are at high risk of stroke, and chronic monthly transfusions reduced the rate of strokes from ∼ 11% to 1% 208 . These findings suggest that all children with SCA should be screened annually with TCD. The STOP II study found that discontinuing these preventive transfusions was not safe and that transfusion therapy for an indefinite period of time might be necessary 209 .
Nevertheless, chronic transfusion therapy for primary stroke prevention is associated with substantial complications and is not available in many low-income countries. Hydroxycarbamide therapy has been associated with decreased TCD vascular velocity 210 . The TCD With Transfusions Changing to Hydroxyurea (TWiTCH) trial determined that hydroxycarbamide therapy at maximum dosing was non-inferior to blood transfusions for primary stroke prevention in children with non-severe vasculopathy on MRI findings and who had been receiving transfusions for ≥1 year 211 . The Stroke Prevention Study in Nigeria (SPIN) provided pilot evidence that TCD screening followed by fixed-dose hydroxycarbamide therapy is feasible and has the potential to prevent strokes in low-resource areas 212 . Global TCD screening of all children with SCA is a major public health priority.
TCD screening does not detect silent infarction involving small-vessel disease, which is a major cause of neurocognitive impairment in SCD. The Silent Cerebral Infarct Transfusion Multi-Centre Clinical Trial (SIT) used MRI to screen children who had normal TCD measurements and no neurological symptoms 213 . Children with small non-focal cerebral infarctions (detected by MRI) were randomly assigned to receive transfusion or observation. Children in the transfusion group had a 59% relative risk reduction for stroke. Whether all children should be screened with MRI remains debated. However, all individuals with soft (subtle) neurological signs or neurocognitive changes (such as sudden unexplained decline in school or work performance) should undergo MRI screening, and those with silent infarction should be offered transfusion therapy. Neurocognitive testing, where available, is a useful tool in identifying individuals who have non-focal ischaemic cerebral injury, which can progress with age and is common in adults with no neurological symptoms 182 .
Prevention of pulmonary complications . Pulmonary disease is a leading cause of morbidity and mortality in individuals with SCD 3 , 191 , 214 . Asthma is an independent predictor of mortality in this population 215 , 216 . Unrecognized bronchoreactive lung disease is common in paediatric patients and increases the severity and frequency of acute chest syndrome events. Many adults have undetected, restrictive chronic lung disease, which is a risk factor of pulmonary failure and myocardial injury 217 . Incorporating respiratory symptom questionnaires and routine spirometry into outpatient management is indicated. Pulmonary hypertension or an elevation in the tricuspid regurgitant jet velocity (TRV), which is a marker of pulmonary hypertension, are also independent predictors of mortality. Individuals with TRV of ≥3 cm s −1 have a tenfold increased mortality compared with individuals with normal TRV 214 . The American Thoracic Society recommends that all adults with SCA undergo serial echocardiography every 1–3 years to detect pulmonary hypertension 203 .
Prevention of renal complications . One-third of individuals with SCA develop chronic kidney disease, and up to 18% of individuals with SCA require dialysis or renal transplantation 218 . Proteinuria is strongly associated with progressive disease; serial urinary screening for proteinuria accompanied with treatment with angiotensin-converting enzyme inhibitors (which correct the proteinuria) could lower the risk of chronic kidney disease 201 . Mild systemic hypertension (120–139/80–90 mmHg) increases the risk of stroke, pulmonary hypertension, nephropathy, mortality and hospitalization in SCD 219 , 220 , and early diagnosis and treatment are beneficial 220 , 221 . Asymptomatic proliferative retinopathy can occur in up to 43% of individuals with HbSC disease and in 14% of individuals with SCA 222 ; if untreated, asymptomatic proliferative retinopathy results in loss of visual acuity 223 .
Comorbidities
Individuals with SCD are prone to other unrelated diseases that can modify each individual's clinical course. Very common (in at least one-third of individuals with SCD) comorbidities identified using screening questionnaires are depression and anxiety 224 , 225 . Depression and anxiety are associated with greater sensitivity to pain 226 and greater health care utilization 227 . Depression is also linked to sleep disturbance 228 and, in general, might be under-recognized and undertreated in individuals with SCD. Asthma is common: it occurs in at least 25% of children with SCD and is associated with an increased incidence of acute pain events, acute chest syndrome and early death 175 , 215 , 216 . Venous thrombosis has been reported in up to 25% of individuals with SCD and could be due to the commonly observed activation of the haemostatic system 229 .

Quality of life
Generic health-related quality-of-life (HRQOL) instruments (for example, the 36-Item Short Form Health Survey (SF-36) for adults and the Pediatric Quality of Life Inventory (PedsQL) for children) 230 , 231 measure physical, emotional and social functioning and enable the comparison of individuals with SCD with healthy individuals. Disease-specific measures, such as the PedsQL Sickle Cell Disease module for children with SCD, have better specificity for detecting differences within a population of individuals with SCD and are designed to detect changes in HRQOL over time 232 .
Both adults and children with SCD have substantially impaired baseline HRQOL 199 , 233 ( Fig. 7 ). Compared with healthy individuals, individuals with SCD have impaired HRQOL in nearly every domain, especially within the areas of pain, fatigue and physical functioning 234 , 235 . Adolescents and adults report poor sleep quality, moderate levels of fatigue and that sleep quality mediates the relationship between pain and fatigue 236 . The baseline physical functioning HRQOL domain of many individuals with SCD is worse than or comparable with that of individuals with other chronic diseases, such as cancer, cystic fibrosis or obesity 237 .
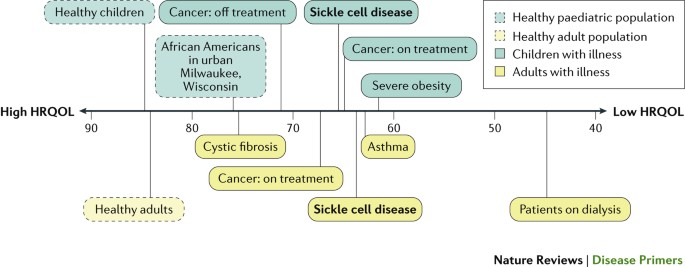
Physical functioning scores measured using the 36-Item Short Form Health Survey (SF-36) and the Pediatric Quality of Life Inventory (PedsQL) generic core scales in healthy individuals and individuals with chronic disease 237 , 272 . Scores range from 100, representing the best health-related quality of life (HRQOL), to 0. Specific areas represented in physical functioning scores include the ability to perform all types of physical activities, such as running, walking for a short distance, lifting heavy objects and bathing without help.
Acute complications, such as an acute vaso-occlusive pain crisis, are significantly associated with worse HRQOL than at baseline 238 . Children with SCD report substantial problems with physical functioning, pain and sleep during and immediately following vaso-occlusive crises 239 . Daily pain can affect the ability to attend school or work 240 , 241 and is predictive of worse HRQOL in adults with SCD 242 . Nearly one-third of adults with SCD report pain almost every day, and over half of the individuals with SCD have pain 50% of the time 241 .
Effect of treatment on health-related quality of life
Adults with SCD who had a favourable response to hydroxycarbamide had better general health and reduced pain than those who received placebo or who had a low response to treatment 243 . Similar results were observed in children with SCD who received hydroxycarbamide 244 or chronic red blood cell transfusion therapy 245 . As more experimental drugs for individuals with SCD are tested in clinical trials, it is imperative to measure the effect of these new therapies on individuals’ HRQOL.
The wide implementation of affordable interventions, including neonatal diagnosis, penicillin prophylaxis and vaccination (which led to substantial reductions in mortality among children with SCA of <5 years of age in high-income countries), could prolong the lives of ∼ 5 million newborn babies with SCA by 2050 (Ref. 17 ). Similarly, large-scale screening and treatment programmes could save the lives of up to 10 million newborn babies with SCA globally, most of them in sub-Saharan Africa 17 , 40 .
Screening for SCD and related conditions is essential in Africa, where the incidence is highest. However, the implementation of universal newborn screening programmes remains a major economical and public health challenge. African communities and governments should also develop culturally acceptable programmes for screening adults for family planning purposes. The development of new, accurate and affordable rapid diagnostic tests would offer a long-awaited point-of-care screening option for low-income and middle-income countries. Clinical validation of such tests showed that they can reliably detect the β S and β C alleles with high specificity and sensitivity 246 . These tests could be used as a large-scale first screening step before confirmation of diagnosis by HPLC or isoelectric focusing, which will be necessary to identify individuals who also have thalassaemia or other Hb variants.
In the short term, the identification of ways to enhance the use of proven therapies, such as hydroxycarbamide and haematopoietic stem cell transplantation, is the quickest route to improve management. Nevertheless, questions remain about the long-term effectiveness of hydroxycarbamide, ways to improve adherence to hydroxycarbamide therapy and possible development of antibacterial resistance in children with SCD under long-term penicillin prophylaxis. Owing to the complexity of SCD and the range of possible complications, a multidrug approach will probably be used by health care providers. However, drug development is a time-consuming process; thus, multidrug treatments will probably be available only in the mid-term or long term. Future work to understand the HRQOL of individuals with SCD over time and outside of the medical system and the effect of therapy on HRQOL is needed to provide tailored care and maximize HRQOL 247 .
Gene therapy has been considered a promising cure for SCD since the mid-1990s. Lentiviral vectors have been developed to insert γ-globin or modified β-globin genes that have been engineered to reduce sickling into haematopoietic stem cells; these vectors are now in clinical trials 248 and have yielded a promising initial result 249 . Newer gene editing approaches based on zinc-finger nucleases and transcription activator-like effector nucleases have been designed and tested for proof of principle in SCD 250 . The development of CRISPR techniques, which enable the precise replacement of a specific region of DNA, is another promising gene therapy approach for SCD, currently tested only in mice 251 and cultured human cells 252 until the multiyear regulatory process is cleared for human trials. However, many ethical issues need to be resolved before these techniques can be used in humans: long-term follow-up trials will be needed to confirm the safety and sustainability of these techniques, and the accessibility of gene therapy in high-burden, low-income areas needs to be addressed. Although some of these current gene therapy strategies are potentially curative, many of them only aim to ameliorate disease severity.
New drugs . In the United States, the decision of the FDA Division of Hematology Products to consider the development of new SCD treatments as a top priority and grant orphan drug status or ‘fast-track’ designation to several drugs and biological products has facilitated investments from pharmaceutical companies. Many products that target one or more of the mechanisms that contribute to the disease process (for example, by boosting HbF levels or countering oxidative stress) are currently in phase II or phase III trials 253 ( Table 1 ). A large clinical trial of an anti-platelet agent, prasugrel, failed to significantly reduce vaso-occlusive crisis episodes in children with SCA 152 , but P-selectin blocking approaches are promising to prevent 149 and to reduce the duration and severity 254 of vaso-occlusive crisis episodes. Enrolment in SCD trials remains challenging: a systematic review of 174 SCD interventional trials closed to enrolment showed that 57% of them terminated owing to low enrolment 255 . However, the recent completion of a series of large, multicentre, multinational clinical trials demonstrates that the community of patients with SCD and health care providers is eager to collaborate with the pharmaceutical industry to find effective new treatments 149 , 150 , 152 , 254 , 256 . The prospects for new treatments in SCD have never looked better.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
How to cite this Primer
Kato, G. J. et al. Sickle cell disease. Nat. Rev. Dis. Primers 4 , 18010 (2018).
Related links
United Nations World Population Prospects database
Neel, J. V. The inheritance of sickle cell anemia. Science 110 , 64–66 (1949).
CAS PubMed Google Scholar
Steinberg, M. H. & Sebastiani, P. Genetic modifiers of sickle cell disease. Am. J. Hematol. 87 , 795–803 (2012).
CAS PubMed PubMed Central Google Scholar
Platt, O. S. et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 330 , 1639–1644 (1994). This landmark natural history study established life expectancy and risk factors for mortality for SCD in the United States.
Piel, F. B., Steinberg, M. H. & Rees, D. C. Sickle cell disease. N. Engl. J. Med. 376 , 1561–1573 (2017).
Ware, R. E., de Montalembert, M., Tshilolo, L. & Abboud, M. R. Sickle cell disease. Lancet 390 , 311–323 (2017).
PubMed Google Scholar
Serjeant, G. R. & Serjeant, B. E. Management of sickle cell disease; lessons from the Jamaican Cohort Study. Blood Rev. 7 , 137–145 (1993).
Bonds, D. R. Three decades of innovation in the management of sickle cell disease: the road to understanding the sickle cell disease clinical phenotype. Blood Rev. 19 , 99–110 (2005).
Quinn, C. T., Rogers, Z. R., McCavit, T. L. & Buchanan, G. R. Improved survival of children and adolescents with sickle cell disease. Blood 115 , 3447–3452 (2010).
Gardner, K. et al. Survival in adults with sickle cell disease in a high-income setting. Blood 128 , 1436–1438 (2016).
Grosse, S. D. et al. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am. J. Prev. Med. 41 , S398–405 (2011).
PubMed PubMed Central Google Scholar
Allison, A. C. Protection afforded by sickle-cell trait against subtertian malarial infection. BMJ 1 , 290–294 (1954).
Luzzatto, L. Sickle cell anaemia and malaria. Mediterr. J. Hematol. Infect. Dis. 4 , e2012065 (2012).
Piel, F. B. et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 1 , 104 (2010).
Piel, F. B. et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet 381 , 142–151 (2013).
Weatherall, D. J. The importance of micromapping the gene frequencies for the common inherited disorders of haemoglobin. Br. J. Haematol. 149 , 635–637 (2010).
Piel, F. B. et al. The distribution of haemoglobin C and its prevalence in newborns in Africa. Sci. Rep. 3 , 1671 (2013).
Piel, F. B., Hay, S. I., Gupta, S., Weatherall, D. J. & Williams, T. N. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 10 , e1001484 (2013). This study places the disease burden of SCA into a global perspective.
Diallo, D. A. & Guindo, A. Sickle cell disease in sub-Saharan Africa: stakes and strategies for control of the disease. Curr. Opin. Hematol. 21 , 210–214 (2014).
Therrell, B. L. Jr., Lloyd-Puryear, M. A., Eckman, J. R. & Mann, M. Y. Newborn screening for sickle cell diseases in the United States: a review of data spanning 2 decades. Semin. Perinatol. 39 , 238–251 (2015).
Charlton, M. NHS Sickle Cell and Thalassaemia Screening Programme Data Report 2015/16: Trends and Performance Analysis (Public Health England, 2017).
Google Scholar
Benson, J. M. & Therrell, B. L. Jr. History and current status of newborn screening for hemoglobinopathies. Semin. Perinatol. 34 , 134–144 (2010).
Feuchtbaum, L., Carter, J., Dowray, S., Currier, R. J. & Lorey, F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 14 , 937–945 (2012).
Ojodu, J. et al. Incidence of sickle cell trait — United States, 2010. MMWR Morb. Mortal. Wkly Rep. 63 , 1155–1158 (2014).
Wang, Y. et al. Sickle cell disease incidence among newborns in New York State by maternal race/ethnicity and nativity. Genet. Med. 15 , 222–228 (2013).
Ministry of Health Brazil. Sickle Cell disease: what you should know about genetic inheritance, 2014 [Portuguese]. Ministério da Saúde http://bvsms.saude.gov.br/bvs/publicacoes/doenca_falciforme_deve_saber_sobre_heranca.pdf (2014).
Silva, W. S. et al. Screening for structural hemoglobin variants in Bahia, Brazil. Int. J. Environ. Res. Publ. Health 13 , 225 (2016).
Lobo, C. L. et al. Newborn screening program for hemoglobinopathies in Rio de Janeiro, Brazil. Pediatr. Blood Cancer 61 , 34–39 (2014).
Braga, J. A., Verissimo, M. P., Saad, S. T., Cancado, R. D. & Loggetto, S. R. Guidelines on neonatal screening and painful vaso-occlusive crisis in sickle cell disease: Associacao Brasileira de Hematologia, Hemoterapia e Terapia Celular: Project guidelines: Associacao Medica Brasileira - 2016. Rev. Bras. Hematol. Hemoter. 38 , 147–157 (2016).
Lervolino, L. G. et al. Prevalence of sickle cell disease and sickle cell trait in national neonatal screening studies. Rev. Bras. Hematol. Hemoter. 33 , 49–54 (2011).
Brandelise, S. et al. Newborn screening for sickle cell disease in Brazil: the Campinas experience. Clin. Lab. Haematol. 26 , 15–19 (2004).
Steinberg, M. H. Predicting clinical severity in sickle cell anaemia. Br. J. Haematol. 129 , 465–481 (2005).
Ngo, D. et al. Fetal hemoglobin in sickle cell anemia: genetic studies of the Arab-Indian haplotype. Blood Cells Mol. Dis. 51 , 22–26 (2013).
Italia, K. et al. Variable phenotypes of sickle cell disease in India with the Arab-Indian haplotype. Br. J. Haematol. 168 , 156–159 (2015).
Mukherjee, M. B. et al. Clinical, hematologic and molecular variability of sickle cell-beta thalassemia in western India. Indian J. Hum. Genet. 16 , 154–158 (2010).
Jones, S. et al. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br. J. Haematol. 131 , 530–533 (2005).
Tewari, S., Brousse, V., Piel, F. B., Menzel, S. & Rees, D. C. Environmental determinants of severity in sickle cell disease. Haematologica 100 , 1108–1116 (2015).
Minniti, C. P., Eckman, J., Sebastiani, P., Steinberg, M. H. & Ballas, S. K. Leg ulcers in sickle cell disease. Am. J. Hematol. 85 , 831–833 (2010).
Dash, B. P. & Kar, B. C. Priapism is rare in sickle cell disease in India. J. Assoc. Physicians India 48 , 255 (2000).
McAuley, C. F. et al. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood 116 , 1663–1668 (2010).
Williams, T. N. et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet 374 , 1364–1370 (2009).
Weatherall, D. J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 115 , 4331–4336 (2010).
Steinberg, M. H. Pathophysiology of sickle cell disease. Baillieres Clin. Haematol. 11 , 163–184 (1998).
Pawliuk, R. et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science 294 , 2368–2371 (2001).
Vekilov, P. G. Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia? Br. J. Haematol. 139 , 173–184 (2007).
Seakins, M., Gibbs, W. N., Milner, P. F. & Bertles, J. F. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J. Clin. Invest. 52 , 422–432 (1973).
Rogers, S. C. et al. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood 121 , 1651–1662 (2013).
Zhang, Y. et al. Elevated sphingosine-1-phosphate promotes sickling and sickle cell disease progression. J. Clin. Invest. 124 , 2750–2761 (2014).
Sun, K. et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 7 , 12086 (2016).
Sun, K. et al. Elevated adenosine signaling via adenosine A2B receptor induces normal and sickle erythrocyte sphingosine kinase 1 activity. Blood 125 , 1643–1652 (2015).
Noguchi, C. T. & Schechter, A. N. Sickle haemoglobin polymerization in solution and in cells. Annu. Rev. Biophys. Biophys. Chem. 14 , 239–263 (1985)
Connes, P. et al. The role of blood rheology in sickle cell disease. Blood Rev. 30 , 111–118 (2016).
Smith, C. M., Krivit, W. & White, J. G. The irreversibly sickled cell. Am. J. Pediatr. Hematol. Oncol. 4 , 307–315 (1982).
Evans, E. A. & Mohandas, N. Membrane-associated sickle hemoglobin: a major determinant of sickle erythrocyte rigidity. Blood 70 , 1443–1449 (1987).
Nash, G. B., Johnson, C. S. & Meiselman, H. J. Rheologic impairment of sickle RBCs induced by repetitive cycles of deoxygenation-reoxygenation. Blood 72 , 539–545 (1988).
Kuypers, F. A. Hemoglobin s polymerization and red cell membrane changes. Hematol. Oncol. Clin. North Am. 28 , 155–179 (2014).
Allan, D. & Raval, P. Some morphological consequences of uncoupling the lipid bilayer from the plasma membrane skeleton in intact erythrocytes. Biomed. Biochim. Acta 42 , S11–16 (1983).
Blumenfeld, N., Zachowski, A., Galacteros, F., Beuzard, Y. & Devaux, P. F. Transmembrane mobility of phospholipids in sickle erythrocytes: effect of deoxygenation on diffusion and asymmetry. Blood 77 , 849–854 (1991).
Fadok, V. A., de Cathelineau, A., Daleke, D. L., Henson, P. M. & Bratton, D. L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276 , 1071–1077 (2001).
Kuypers, F. A. Membrane lipid alterations in hemoglobinopathies. Hematol. Am. Soc. Hematol. Educ. Program 2007 , 68–73 (2007).
Kuypers, F. A. & de Jong, K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell. Mol. Biol. 50 , 147–158 (2004).
Piccin, A., Murphy, W. G. & Smith, O. P. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21 , 157–171 (2007).
Westerman, M. et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br. J. Haematol. 142 , 126–135 (2008).
Westerman, M. & Porter, J. B. Red blood cell-derived microparticles: an overview. Blood Cells Mol. Dis. 59 , 134–139 (2016).
Hebbel, R. P. & Key, N. S. Microparticles in sickle cell anaemia: promise and pitfalls. Br. J. Haematol. 174 , 16–29 (2016).
Alayash, A. I. Oxidative pathways in the sickle cell and beyond. Blood Cells Mol. Dis. https://doi.org/10.1016/j.bcmd.2017.05.009 (2017).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/nrm.2017.125 (2018).
Quinn, C. T. et al. Biochemical surrogate markers of hemolysis do not correlate with directly measured erythrocyte survival in sickle cell anemia. Am. J. Hematol. 91 , 1195–1201 (2016).
Crosby, W. H. The metabolism of hemoglobin and bile pigment in hemolytic disease. Am. J. Med. 18 , 112–122 (1955).
Bunn, H. F. et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 116 , 687–692 (2010).
Kato, G. J., Steinberg, M. H. & Gladwin, M. T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Invest. 127 , 750–760 (2017). This is a comprehensive review of the contribution of haemolysis to SCD pathophysiology.
Wood, K. C. & Granger, D. N. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin. Exp. Pharmacol. Physiol. 34 , 926–932 (2007).
Aslan, M. & Freeman, B. A. Redox-dependent impairment of vascular function in sickle cell disease. Free Radic. Biol. Med. 43 , 1469–1483 (2007).
Cho, C. S. et al. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid. Redox Signal. 13 , 1–11 (2010).
Morris, C. R. et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 111 , 402–410 (2008).
Reiter, C. D. et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8 , 1383–1389 (2002).
Morris, C. R. et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294 , 81–90 (2005).
Miller-Fleming, L., Olin-Sandoval, V., Campbell, K. & Ralser, M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 427 , 3389–3406 (2015).
Landburg, P. P. et al. Plasma asymmetric dimethylarginine concentrations in sickle cell disease are related to the hemolytic phenotype. Blood Cells Mol. Dis. 44 , 229–232 (2010).
Antoniades, C. et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur. Heart J. 30 , 1142–1150 (2009).
Luo, S., Lei, H., Qin, H. & Xia, Y. Molecular mechanisms of endothelial NO synthase uncoupling. Curr. Pharm. Des. 20 , 3548–3553 (2014).
Zorca, S. et al. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. Br. J. Haematol. 149 , 436–445 (2010).
Tumblin, A. et al. Apolipoprotein A-I and serum amyloid A plasma levels are biomarkers of acute painful episodes in patients with sickle cell disease. Haematologica 95 , 1467–1472 (2010).
Saraf, S. L. et al. APOL1, alpha-thalassemia, and BCL11A variants as a genetic risk profile for progression of chronic kidney disease in sickle cell anemia. Haematologica 102 , e1–e6 (2017).
Gladwin, M. T. & Ofori-Acquah, S. F. Erythroid DAMPs drive inflammation in SCD. Blood 123 , 3689–3690 (2014).
Wang, X. et al. Heme-bound iron activates placenta growth factor in erythroid cells via erythroid Kruppel-like factor. Blood 124 , 946–954 (2014).
van Beers, E. J. et al. Iron, inflammation, and early death in adults with sickle cell disease. Circ. Res. 116 , 298–306 (2015).
Hebbel, R. P. Adhesive interactions of sickle erythrocytes with endothelium. J. Clin. Invest. 100 , S83–86 (1997).
Kaul, D. K. et al. Monoclonal antibodies to alphaVbeta3 (7E3 and LM609) inhibit sickle red blood cell-endothelium interactions induced by platelet-activating factor. Blood 95 , 368–374 (2000).
Setty, B. N. & Stuart, M. J. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood 88 , 2311–2320 (1996).
Hines, P. C. et al. Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesion. Blood 101 , 3281–3287 (2003).
Wagner, M. C., Eckman, J. R. & Wick, T. M. Sickle cell adhesion depends on hemodynamics and endothelial activation. J. Lab Clin. Med. 144 , 260–268 (2004).
Murphy, M. M. et al. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood 105 , 3322–3329 (2005).
Sugihara, K., Sugihara, T., Mohandas, N. & Hebbel, R. P. Thrombospondin mediates adherence of CD36+ sickle reticulocytes to endothelial cells. Blood 80 , 2634–2642 (1992).
Miller, S. T. et al. Prediction of adverse outcomes in children with sickle cell disease. N. Engl. J. Med. 342 , 83–89 (2000).
Elmariah, H. et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am. J. Hematol. 89 , 530–535 (2014).
Zhang, D., Xu, C., Manwani, D. & Frenette, P. S. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood 127 , 801–809 (2016). This is an updated review of the principal adhesive pathways involved in SCD vaso-occlusion.
Canalli, A. A. et al. Participation of Mac-1, LFA-1 and VLA-4 integrins in the in vitro adhesion of sickle cell disease neutrophils to endothelial layers, and reversal of adhesion by simvastatin. Haematologica 96 , 526–533 (2011).
Dominical, V. M. et al. Prominent role of platelets in the formation of circulating neutrophil-red cell heterocellular aggregates in sickle cell anemia. Haematologica 99 , e214–e217 (2014).
Davila, J. et al. A novel inflammatory role for platelets in sickle cell disease. Platelets 26 , 726–729 (2015).
Hoppe, C. C. Prenatal and newborn screening for hemoglobinopathies. Int. J. Lab. Hematol. 35 , 297–305 (2013).
Traeger-Synodinos, J. Pre-implantation genetic diagnosis. Best Pract. Res. Clin. Obstet. Gynaecol. 39 , 74–88 (2017).
Robitaille, N., Delvin, E. E. & Hume, H. A. Newborn screening for sickle cell disease: A 1988–2003 Quebec experience. Paediatr. Child Health 11 , 223–227 (2006).
Vichinsky, E., Hurst, D., Earles, A., Kleman, K. & Lubin, B. Newborn screening for sickle cell disease: effect on mortality. Pediatrics 81 , 749–755 (1988).
Gaston, M. H. et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N. Engl. J. Med. 314 , 1593–1599 (1986). This study provides proof that penicillin prophylaxis reduces mortality, which marked a turning point for life expectancy in SCA.
Kate, S. & Lingojwar, D. Epidemiology of sickle cell disorder in the state of Maharashtra. Int. J. Hum. Genet. 2 , 161–167 (2002).
Patra, P. K., Khodiar, P. K., Hambleton, I. R. & Serjeant, G. R. The Chhattisgarh state screening programme for the sickle cell gene: a cost-effective approach to a public health problem. J. Commun. Genet. 6 , 361–368 (2015).
CAS Google Scholar
Naik, R. P. & Haywood, C. Jr. Sickle cell trait diagnosis: clinical and social implications. Hematol. Am. Soc. Hematol. Educ. Program 2015 , 160–167 (2015).
Nelson, D. A. et al. Sickle cell trait, rhabdomyolysis, and mortality among U. S. army soldiers. N. Engl. J. Med. 375 , 435–442 (2016).
Key, N. S., Connes, P. & Derebail, V. K. Negative health implications of sickle cell trait in high income countries: from the football field to the laboratory. Br. J. Haematol. 170 , 5–14 (2015).
Streetly, A., Latinovic, R. & Henthorn, J. Positive screening and carrier results for the England-wide universal newborn sickle cell screening programme by ethnicity and area for 2005–2007. J. Clin. Pathol. 63 , 626–629 (2010).
Thuret, I. et al. Neonatal screening for sickle cell disease in France: evaluation of the selective process. J. Clin. Pathol. 63 , 548–551 (2010).
Manu Pereira, M. & Corrons, J. L. Neonatal haemoglobinopathy screening in Spain. J. Clin. Pathol. 62 , 22–25 (2009).
Colombatti, R. et al. Organizing national responses for rare blood disorders: the Italian experience with sickle cell disease in childhood. Orphanet J. Rare Dis. 8 , 169 (2013).
Kunz, J. B. et al. Significant prevalence of sickle cell disease in Southwest Germany: results from a birth cohort study indicate the necessity for newborn screening. Ann. Hematol. 95 , 397–402 (2016).
Minkovitz, C. S., Grason, H., Ruderman, M. & Casella, J. F. Newborn screening programs and sickle cell disease: a public health services and systems approach. Am. J. Prev. Med. 51 , S39–S47 (2016).
Colah, R. B., Mukherjee, M. B., Martin, S. & Ghosh, K. Sickle cell disease in tribal populations in India. Indian J. Med. Res. 141 , 509–515 (2015).
Chandrashekar, V. & Soni, M. Hemoglobin disorders in South India. ISRN Hematol. 2011 , 748939 (2011).
Italia, K. et al. Hydroxyurea in sickle cell disease — a study of clinico-pharmacological efficacy in the Indian haplotype. Blood Cells Mol. Dis. 42 , 25–31 (2009).
Kamble, M. & Chatruvedi, P. Epidemiology of sickle cell disease in a rural hospital of central India. Indian Pediatr. 37 , 391–396 (2000).
Shukla, R. N. & Solanki, B. R. Sickle-cell trait in Central India. Lancet 1 , 297–298 (1958).
Therrell, B. L. et al. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 39 , 171–187 (2015).
Ohene-Frempong, K., Oduro, J., Tetteh, H. & Nkrumah, F. Screening newborns for sickle cell disease in Ghana. Pediatrics 121 (Suppl. 2), S120–S121(2008).
McGann, P. T. et al. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am. J. Hematol. 88 , 984–989 (2013).
Rahimy, M. C., Gangbo, A., Ahouignan, G. & Alihonou, E. Newborn screening for sickle cell disease in the Republic of Benin. J. Clin. Pathol. 62 , 46–48 (2009).
Kafando, E. et al. Neonatal haemoglobinopathy screening in Burkina Faso. J. Clin. Pathol. 62 , 39–41 (2009).
Mutesa, L. et al. Neonatal screening for sickle cell disease in Central Africa: a study of 1825 newborns with a new enzyme-linked immunosorbent assay test. J. Med. Screen. 14 , 113–116 (2007).
Tshilolo, L. et al. Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: experience from a pioneer project on 31 204 newborns. J. Clin. Pathol. 62 , 35–38 (2009).
Odunvbun, M. E., Okolo, A. A. & Rahimy, C. M. Newborn screening for sickle cell disease in a Nigerian hospital. Publ. Health 122 , 1111–1116 (2008).
Mbodj, M. et al. Sickle cell disease neonatal screening. First evaluation [French]. Dakar Med. 48 , 202–205 (2003).
Makani, J. et al. Health policy for sickle cell disease in Africa: experience from Tanzania on interventions to reduce under-five mortality. Trop. Med. Int. Health 20 , 184–187 (2015).
Ndeezi, G. et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Global Health 4 , e195–e200 (2016).
Serjeant, G. R. The natural history of sickle cell disease. Cold Spring Harb. Perspect. Med. 3 , a011783 (2013).
Fertrin, K. Y. & Costa, F. F. Genomic polymorphisms in sickle cell disease: implications for clinical diversity and treatment. Expert Rev. Hematol. 3 , 443–458 (2010).
Kato, G. J., Gladwin, M. T. & Steinberg, M. H. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 21 , 37–47 (2007).
Lettre, G. & Bauer, D. E. Fetal haemoglobin in sickle-cell disease: from genetic epidemiology to new therapeutic strategies. Lancet 387 , 2554–2564 (2016).
Steinberg, M. H., Chui, D. H., Dover, G. J., Sebastiani, P. & Alsultan, A. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood 123 , 481–485 (2014).
Belfer, I. et al. A GCH1 haplotype confers sex-specific susceptibility to pain crises and altered endothelial function in adults with sickle cell anemia. Am. J. Hematol. 89 , 187–193 (2014).
Brandow, A. M., Stucky, C. L., Hillery, C. A., Hoffmann, R. G. & Panepinto, J. A. Patients with sickle cell disease have increased sensitivity to cold and heat. Am. J. Hematol. 88 , 37–43 (2013).
Quinn, C. T., Rogers, Z. R. & Buchanan, G. R. Survival of children with sickle cell disease. Blood 103 , 4023–4027 (2004).
Anie, K. A. Psychological complications in sickle cell disease. Br. J. Haematol. 129 , 723–729 (2005).
Weatherall, D. J. The challenge of haemoglobinopathies in resource-poor countries. Br. J. Haematol. 154 , 736–744 (2011).
Kassim, A. A. & DeBaun, M. R. The case for and against initiating either hydroxyurea therapy, blood transfusion therapy or hematopoietic stem cell transplant in asymptomatic children with sickle cell disease. Expert Opin. Pharmacother. 15 , 325–336 (2014).
McGann, P. T. & Ware, R. E. Hydroxyurea therapy for sickle cell anemia. Expert Opin. Drug Saf. 14 , 1749–1758 (2015).
Charache, S. et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 332 , 1317–1322 (1995). This landmark trial proved that hydroxycarbamide reduces the frequency of pain episodes in SCA, leading to the approval of this drug.
Walsh, K. E. et al. Medication adherence among pediatric patients with sickle cell disease: a systematic review. Pediatrics 134 , 1175–1183 (2014).
Husain, M., Hartman, A. D. & Desai, P. Pharmacogenomics of sickle cell disease: steps toward personalized medicine. Pharmgenom. Pers. Med. 10 , 261–265 (2017).
Wong, T. E., Brandow, A. M., Lim, W. & Lottenberg, R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood 124 , 3850–3857 (2014).
DeBaun, M. R. Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: a review. Expert Rev. Hematol. 7 , 767–773 (2014).
Ataga, K. I. et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 376 , 429–439 (2017).
McGann, P. T. et al. Hydroxyurea therapy for children with sickle cell anemia in sub-Saharan Africa: rationale and design of the REACH trial. Pediatr. Blood Cancer 63 , 98–104 (2016).
Wang, W. C. et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 377 , 1663–1672 (2011). This article presents evidence that hydroxycarbamide is effective in infants and toddlers with SCA.
Heeney, M. M. et al. A multinational trial of Prasugrel for sickle cell vaso-occlusive events. N. Engl. J. Med. 374 , 625–635 (2016).
Mulaku, M. et al. Evidence review of hydroxyurea for the prevention of sickle cell complications in low-income countries. Arch. Dis. Child. 98 , 908–914 (2013).
Hyacinth, H. I., Adams, R. J., Voeks, J. H., Hibbert, J. M. & Gee, B. E. Frequent red cell transfusions reduced vascular endothelial activation and thrombogenicity in children with sickle cell anemia and high stroke risk. Am. J. Hematol. 89 , 47–51 (2014).
Hyacinth, H. I. et al. Effect of chronic blood transfusion on biomarkers of coagulation activation and thrombin generation in sickle cell patients at risk for stroke. PLoS ONE 10 , e0134193 (2015).
Quirolo, K. & Vichinsky, E. in Rossi's Principles of Transfusion Medicine (eds Simon T. L. et al.) (Wiley-Blackwell, 2016).
Fasano, R. M. & Chou, S. T. Red blood cell antigen genotyping for sickle cell disease, thalassemia, and other transfusion complications. Transfus. Med. Rev. 30 , 197–201 (2016).
Walters, M. C. et al. Indications and results of HLA-identical sibling hematopoietic cell transplantation for sickle cell disease. Biol. Blood Marrow Transplant. 22 , 207–211 (2016).
Gluckman, E. et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood 129 , 1548–1556 (2017).
Hsieh, M. M. et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 312 , 48–56 (2014).
Saraf, S. L. et al. Nonmyeloablative stem cell transplantation with alemtuzumab/low-dose irradiation to cure and improve the quality of life of adults with sickle cell disease. Biol. Blood Marrow Transplant. 22 , 441–448 (2016).
Gluckman, E. Allogeneic transplantation strategies including haploidentical transplantation in sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program 2013 , 370–376 (2013).
Ballas, S. K., Gupta, K. & Adams-Graves, P. Sickle cell pain: a critical reappraisal. Blood 120 , 3647–3656 (2012).
Treadwell, M. J. et al. A quality improvement initiative to improve emergency department care for pediatric patients with sickle cell disease. J. Clin. Outcomes Manag. 21 , 62–70 (2014).
Kavanagh, P. L. et al. Improving the management of vaso-occlusive episodes in the pediatric emergency department. Pediatrics 136 , e1016–1025 (2015).
Tanabe, P. et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am. J. Hematol. 93 , 159–168 (2018).
Lanzkron, S. et al. Impact of a dedicated infusion clinic for acute management of adults with sickle cell pain crisis. Am. J. Hematol. 90 , 376–380 (2015).
Haywood, C. et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am. J. Emerg. Med. 31 , 651–656 (2013).
van Beers, E. J. et al. Patient-controlled analgesia versus continuous infusion of morphine during vaso-occlusive crisis in sickle cell disease, a randomized controlled trial. Am. J. Hematol. 82 , 955–960 (2007).
Makani, J., Ofori-Acquah, S. F., Nnodu, O., Wonkam, A. & Ohene-Frempong, K. Sickle cell disease: new opportunities and challenges in Africa. Sci. World J. 2013 , 193252 (2013).
Manci, E. A. et al. Causes of death in sickle cell disease: an autopsy study. Br. J. Haematol. 123 , 359–365 (2003).
Novelli, E. M. & Gladwin, M. T. Crises in sickle cell disease. Chest 149 , 1082–1093 (2016).
Vichinsky, E. P. et al. Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood 89 , 1787–1792 (1997).
Vichinsky, E. P. et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N. Engl. J. Med. 342 , 1855–1865 (2000). This study comprehensively establishes the causes and outcomes of acute chest syndrome.
DeBaun, M. R. & Strunk, R. C. The intersection between asthma and acute chest syndrome in children with sickle-cell anaemia. Lancet 387 , 2545–2553 (2016).
Howard, J. et al. Guideline on the management of acute chest syndrome in sickle cell disease. Br. J. Haematol. 169 , 492–505 (2015).
Bernini, J. C. et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood 92 , 3082–3089 (1998).
Kassim, A. A., Galadanci, N. A., Pruthi, S. & DeBaun, M. R. How I treat and manage strokes in sickle cell disease. Blood 125 , 3401–3410 (2015).
Gardner, K., Hoppe, C., Mijovic, A. & Thein, S. L. How we treat delayed haemolytic transfusion reactions in patients with sickle cell disease. Br. J. Haematol. 170 , 745–756 (2015).
Ogunlesi, F., Heeney, M. M. & Koumbourlis, A. C. Systemic corticosteroids in acute chest syndrome: friend or foe? Paediatr. Respir. Rev. 15 , 24–27 (2014).
Ohene-Frempong, K. et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 91 , 288–294 (1998).
Vichinsky, E. P. et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA 303 , 1823–1831 (2010).
DeBaun, M. R. et al. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood 119 , 4587–4596 (2012).
Ware, R. E. & Helms, R. W. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood 119 , 3925–3932 (2012).
Strouse, J. J., Hulbert, M. L., DeBaun, M. R., Jordan, L. C. & Casella, J. F. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics 118 , 1916–1924 (2006).
Scott, R. M. & Smith, E. R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 360 , 1226–1237 (2009).
Kennedy, B. C. et al. Pial synangiosis for moyamoya syndrome in children with sickle cell anemia: a comprehensive review of reported cases. Neurosurg. Focus 36 , E12 (2014).
Brousse, V. et al. Acute splenic sequestration crisis in sickle cell disease: cohort study of 190 paediatric patients. Br. J. Haematol. 156 , 643–648 (2012).
Vasavda, N. et al. The linear effects of alpha-thalassaemia, the UGT1A1 and HMOX1 polymorphisms on cholelithiasis in sickle cell disease. Br. J. Haematol. 138 , 263–270 (2007).
Leake, P. A., Reid, M. & Plummer, J. A case series of cholecystectomy in Jamaican sickle cell disease patients — the need for a new strategy. Ann. Med. Surg. 15 , 37–42 (2017).
Powars, D. R., Chan, L. S., Hiti, A., Ramicone, E. & Johnson, C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine 84 , 363–376 (2005).
McClish, D. K. et al. Pain site frequency and location in sickle cell disease: the PiSCES project. Pain 145 , 246–251 (2009).
Ballas, S. K. Update on pain management in sickle cell disease. Hemoglobin 35 , 520–529 (2011).
Darbari, D. S. et al. Frequency of hospitalizations for pain and association with altered brain network connectivity in sickle cell disease. J. Pain 16 , 1077–1086 (2015).
Neumayr, L. D. et al. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. J. Bone Joint Surg. Am. 88 , 2573–2582 (2006).
McClellan, A. C. et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br. J. Haematol. 159 , 360–367 (2012).
Abbott, K. C., Hypolite, I. O. & Agodoa, L. Y. Sickle cell nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin. Nephrol. 58 , 9–15 (2002).
Huang, E. et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol. Dial. Transplant. 28 , 1039–1046 (2013).
Dampier, C. et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am. J. Hematol. 86 , 203–205 (2011).
Chaturvedi, S. & DeBaun, M. R. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: the last 40 years. Am. J. Hematol. 91 , 5–14 (2016).
Yawn, B. P. et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 312 , 1033–1048 (2014).
Buchanan, G. et al. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (National Heart Lung and Blood Institute, 2014). This article presents detailed evidence-based guidelines for the clinical management of individuals with SCD.
Klings, E. S. et al. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am. J. Respir. Crit. Care Med. 189 , 727–740 (2014).
Butler, J. C., Breiman, R. F., Lipman, H. B., Hofmann, J. & Facklam, R. R. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171 , 885–889 (1995).
Halasa, N. B. et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin. Infect. Dis. 44 , 1428–1433 (2007).
Cober, M. P. & Phelps, S. J. Penicillin prophylaxis in children with sickle cell disease. J. Pediatr. Pharmacol. Ther. 15 , 152–159 (2010).
Obaro, S. K. & Iroh Tam, P. Y. Preventing infections in sickle cell disease: the unfinished business. Pediatr. Blood Cancer 63 , 781–785 (2016).
Adams, R. J. et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N. Engl. J. Med. 339 , 5–11 (1998). This study shows that ischaemic stroke can be prevented by chronic transfusion in children identified at high risk by non-invasive ultrasonography screening.
Adams, R. J., Brambilla, D. & Investigators, S. T. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N. Engl. J. Med. 353 , 2769–2778 (2005).
Zimmerman, S. A., Schultz, W. H., Burgett, S., Mortier, N. A. & Ware, R. E. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood 110 , 1043–1047 (2007).
Ware, R. E. et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet 387 , 661–670 (2016).
Galadanci, N. A. et al. Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): challenges of conducting a feasibility trial. Pediatr. Blood Cancer 62 , 395–401 (2015).
DeBaun, M. R. et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N. Engl. J. Med. 371 , 699–710 (2014).
Gladwin, M. T. et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N. Engl. J. Med. 350 , 886–895 (2004).
Boyd, J. H., Macklin, E. A., Strunk, R. C. & DeBaun, M. R. Asthma is associated with Increased mortality in individuals with sickle cell anemia. Haematologica 92 , 1115–1118 (2007).
Glassberg, J. A. et al. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br. J. Haematol. 159 , 472–479 (2012).
Powars, D., Weidman, J. A., Odom-Maryon, T., Niland, J. C. & Johnson, C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine 67 , 66–76 (1988).
Falk, R. J. et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N. Engl. J. Med. 326 , 910–915 (1992).
Gordeuk, V. R. et al. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am. J. Hematol. 83 , 15–18 (2008).
Pegelow, C. H. et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am. J. Med. 102 , 171–177 (1997).
Rodgers, G. P., Walker, E. C. & Podgor, M. J. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am. J. Med. Sci. 305 , 150–156 (1993).
Downes, S. M. et al. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology 112 , 1869–1875 (2005).
Moriarty, B. J., Acheson, R. W., Condon, P. I. & Serjeant, G. R. Patterns of visual loss in untreated sickle cell retinopathy. Eye 2 , 330–335 (1988).
Adam, S. S. et al. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv. 1 , 1983–1992 (2017).
McClish, D. K. et al. Comorbidity, pain, utilization, and psychosocial outcomes in older versus younger sickle cell adults: the PiSCES project. Biomed. Res. Int. 2017 , 4070547 (2017).
Bakshi, N., Lukombo, I., Shnol, H., Belfer, I. & Krishnamurti, L. Psychological characteristics and pain frequency are associated with experimental pain sensitivity in pediatric patients with sickle cell disease. J. Pain 18 , 1216–1228 (2017).
Jonassaint, C. R., Jones, V. L., Leong, S. & Frierson, G. M. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. Br. J. Haematol. 174 , 136–147 (2016).
Wallen, G. R. et al. Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry 14 , 207 (2014).
Noubouossie, D., Key, N. S. & Ataga, K. I. Coagulation abnormalities of sickle cell disease: relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 30 , 245–256 (2016).
Ware, J. E. Jr & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30 , 473–483 (1992).
Varni, J. The PedsQL TM 4.0 Measurement Model for the Pediatric Quality of Life Inventory TM Version 4.0: Administration Guidelines. PedsQL TM http://www.pedsql.org/pedsqladmin.html (2004).
Panepinto, J. A. et al. Determining the longitudinal validity and meaningful differences in HRQL of the PedsQL Sickle Cell Disease Module. Health Qual. Life Outcomes 15 , 124 (2017).
Panepinto, J. A. & Bonner, M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatr. Blood Cancer 59 , 377–385 (2012).
Keller, S. D., Yang, M., Treadwell, M. J., Werner, E. M. & Hassell, K. L. Patient reports of health outcome for adults living with sickle cell disease: development and testing of the ASCQ-Me item banks. Health Qual. Life Outcomes 12 , 125 (2014).
Panepinto, J. A. et al. PedsQL sickle cell disease module: feasibility, reliability, and validity. Pediatr. Blood Cancer 60 , 1338–1344 (2013).
Ameringer, S., Elswick, R. K. Jr & Smith, W. Fatigue in adolescents and young adults with sickle cell disease: biological and behavioral correlates and health-related quality of life. J. Pediatr. Oncol. Nurs. 31 , 6–17 (2014).
McClish, D. K. et al. Health related quality of life in sickle cell patients: the PiSCES project. Health Qual. Life Outcomes 3 , 50 (2005).
Brandow, A. M., Brousseau, D. C., Pajewski, N. M. & Panepinto, J. A. Vaso-occlusive painful events in sickle cell disease: impact on child well-being. Pediatr. Blood Cancer 54 , 92–97 (2010).
Brandow, A. M., Brousseau, D. C. & Panepinto, J. A. Postdischarge pain, functional limitations and impact on caregivers of children with sickle cell disease treated for painful events. Br. J. Haematol. 144 , 782–788 (2009).
Dampier, C., Ely, E., Brodecki, D. & O’Neal, P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J. Pediatr. Hematol. Oncol. 24 , 643–647 (2002).
Smith, W. R. et al. Daily assessment of pain in adults with sickle cell disease. Ann. Intern. Med. 148 , 94–101 (2008).
Smith, W. R. et al. Understanding pain and improving management of sickle cell disease: the PiSCES study. J. Natl Med. Assoc. 97 , 183–193 (2005).
Ballas, S. K. et al. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual. Life Outcomes 4 , 59 (2006).
Thornburg, C. D., Calatroni, A. & Panepinto, J. A. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J. Pediatr. Hematol. Oncol. 33 , 251–254 (2011).
Beverung, L. M. et al. Health-related quality of life in children with sickle cell anemia: impact of blood transfusion therapy. Am. J. Hematol. 90 , 139–143 (2015).
Kanter, J. et al. Validation of a novel point of care testing device for sickle cell disease. BMC Med. 13 , 225 (2015).
Beverung, L. M., Varni, J. W. & Panepinto, J. A. Clinically meaningful interpretation of pediatric health-related quality of life in sickle cell disease. J. Pediatr. Hematol. Oncol. 37 , 128–133 (2015).
Hoban, M. D., Orkin, S. H. & Bauer, D. E. Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease. Blood 127 , 839–848 (2016).
Ribeil, J. A. et al. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 376 , 848–855 (2017).
Tasan, I., Jain, S. & Zhao, H. Use of genome-editing tools to treat sickle cell disease. Hum. Genet. 135 , 1011–1028 (2016).
Traxler, E. A. et al. A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 22 , 987–990 (2016).
Dever, D. P. et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature 539 , 384–389 (2016).
Telen, M. J. Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease. Blood 127 , 810–819 (2016).
Telen, M. J. et al. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood 125 , 2656–2664 (2015).
Lebensburger, J. D. et al. Systematic review of interventional sickle cell trials registered in ClinicalTrials.gov. Clin. Trials 12 , 575–583 (2015).
Niihara, Y. et al. Phase 3 study of L-glutamine therapy in sickle cell anemia and sickle β 0 -thalassemia subgroup analyses show consistent clinical improvement. Blood 128 , 1318–1318 (2016).
Pace, B. Renaissance of Sickle Cell Disease Research in the Genome Era (Imperial College Press, 2007).
Sabarense, A. P., Lima, G. O., Silva, L. M. & Viana, M. B. Survival of children with sickle cell disease in the comprehensive newborn screening programme in Minas Gerais, Brazil. Paediatr. Int. Child Health 35 , 329–332 (2015).
Gualandro, S. F., Fonseca, G. H., Yokomizo, I. K., Gualandro, D. M. & Suganuma, L. M. Cohort study of adult patients with haemoglobin SC disease: clinical characteristics and predictors of mortality. Br. J. Haematol. 171 , 631–637 (2015).
Figueiredo, M. S. The compound state: Hb S/beta-thalassemia. Rev. Bras. Hematol. Hemoter. 37 , 150–152 (2015).
Steinberg, M. H. in Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (eds Steinberg, M. H., Forget, B. G., Higgs, D. R. & Weatherall, D. J. ) 786–810 (Cambridge Univ. Press, 2009).
Harrington, D. J., Adachi, K. & Royer, W. E. Jr. The high resolution crystal structure of deoxyhemoglobin S. J. Mol. Biol. 272 , 398–407 (1997).
Eaton, W. A. & Hofrichter, J. Sickle cell hemoglobin polymerization. Adv. Protein Chem. 40 , 63–279 (1990).
Briehl, R. W. & Ewert, S. Effects of pH, 2,3-diphosphoglycerate and salts on gelation of sickle cell deoxyhemoglobin. J. Mol. Biol. 80 , 445–458 (1973).
Bookchin, R. M., Balazs, T. & Landau, L. C. Determinants of red cell sickling. Effects of varying pH and of increasing intracellular hemoglobin concentration by osmotic shrinkage. J. Lab Clin. Med. 87 , 597–616 (1976).
Eaton, W. A., Hofrichter, J. & Ross, P. D. Editorial: Delay time of gelation: a possible determinant of clinical severity in sickle cell disease. Blood 47 , 621–627 (1976).
Ferrone, F. A. The delay time in sickle cell disease after 40 years: a paradigm assessed. Am. J. Hematol. 90 , 438–445 (2015).
Uzunova, V. V., Pan, W., Galkin, O. & Vekilov, P. G. Free heme and the polymerization of sickle cell hemoglobin. Biophys. J. 99 , 1976–1985 (2010).
Hebbel, R. P., Boogaerts, M. A., Eaton, J. W. & Steinberg, M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N. Engl. J. Med. 302 , 992–995 (1980).
Brugnara, C., de Franceschi, L. & Alper, S. L. Inhibition of Ca 2+ -dependent K + transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Invest. 92 , 520–526 (1993).
Centers for Disease Control and Prevention. Registry and Surveillance System for Hemoglobinopathies (RuSH). CDC https://www.cdc.gov/ncbddd/hemoglobinopathies/rush.html (2017).
Panepinto, J. A., Pajewski, N. M., Foerster, L. M. & Hoffmann, R. G. The performance of the PedsQL generic core scales in children with sickle cell disease. J. Pediatr. Hematol. Oncol. 30 , 666–673 (2008).
[No authors listed.] FDA Briefing Document, Oncologic Drugs Advisory Committee Meeting, NDA 208587, L-glutamine, Applicant: Emmaus Medical, Inc. U.S. Food and Drug Administration https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559734.pdf (2017).
Hoppe, C. C. et al. Design of the DOVE (Determining Effects of Platelet Inhibition on Vaso-Occlusive Events) trial: a global phase 3 double-blind, randomized, placebo-controlled, multicenter study of the efficacy and safety of prasugrel in pediatric patients with sickle cell anemia utilizing a dose titration strategy. Pediatr. Blood Cancer 63 , 299–305 (2016).
Gibbs, W. J. & Hagemann, T. M. Purified poloxamer 188 for sickle cell vaso-occlusive crisis. Ann. Pharmacother. 38 , 320–324 (2004).
Morris, C. R. et al. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 98 , 1375–1382 (2013).
Du, E., Mendelsohn, L., Nichols, J. S., Dao, M. & Kato, G. J. Quantification of anti-sickling effect of Aes-103 in sickle cell disease using an in vitro microfluidic assay. Blood 124 , 2699–2699 (2014).
Sins, J. W. R. et al. Effect of N-acetylcysteine on pain in daily life in patients with sickle cell disease: a randomised clinical trial. Br. J. Haematol. https://doi.org/10.1111/bjh.14809 (2017).
Brousseau, D. C. et al. A multicenter randomized controlled trial of intravenous magnesium for sickle cell pain crisis in children. Blood 126 , 1651–1657 (2015).
Ware, R. E., Helms, R. W. & Investigators, S. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood 119 , 3925–3932 (2012).
Metcalf, B. et al. Discovery of GBT440, an orally bioavailable R-state stabilizer of sickle cell hemoglobin. ACS Med. Chem. Lett. 8 , 321–326 (2017).
Gladwin, M. T. et al. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 305 , 893–902 (2011).
Machado, R. F. et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 118 , 855–864 (2011).
Misra, H. et al. A Phase Ib open label, randomized, safety study of SANGUINATE in patients with sickle cell anemia. Rev. Bras. Hematol. Hemoter 39 , 20–27 (2017).
Telen, M. J. et al. Sevuparin binds to multiple adhesive ligands and reduces sickle red blood cell-induced vaso-occlusion. Br. J. Haematol. 175 , 935–948 (2016).
Moutouh-de Parseval, L. A. et al. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. J. Clin. Invest. 118 , 248–258 (2008).
McArthur, J. G. et al. A novel, highly potent and selective PDE9 inhibitor for the treatment of sickle cell disease. Blood 128 , 268–268 (2016).
Wambebe, C. et al. Double-blind, placebo-controlled, randomised cross-over clinical trial of NIPRISAN in patients with Sickle Cell Disorder. Phytomedicine 8 , 252–261 (2001).
Conran, N. Prospects for early investigational therapies for sickle cell disease. Expert Opin. Investig. Drugs 24 , 595–602 (2015).
Download references
Author information
Authors and affiliations.
Lung and Blood Vascular Medicine Institute and the Division of Hematology–Oncology, Department of Medicine, Heart, University of Pittsburgh, 200 Lothrop Street, Pittsburgh, 15261, PA, USA
Gregory J. Kato
Department of Epidemiology and Biostatistics, MRC-PHE Centre for Environment and Health, School of Public Health, Faculty of Medicine, Imperial College London, London, UK
Frédéric B. Piel
Sickle Cell Disease Branch, National Heart, Lung and Blood Institute, NIH, Bethesda, MD, USA
Clarice D. Reid
The Gaston and Porter Health Improvement Center, Potomac, MD, USA
Marilyn H. Gaston
Sickle Cell Foundation of Ghana, Kumasi, Ghana
Kwaku Ohene-Frempong
Division of Pediatric Hematology–Oncology–BMT, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA
Lakshmanan Krishnamurti
Division of General Internal Medicine, Department of Medicine, Virginia Commonwealth University, Richmond, VA, USA
Wally R. Smith
Department of Pediatrics, Hematology–Oncology–Bone Marrow Transplantation, Medical College of Wisconsin and Children's Hospital of Wisconsin, Milwaukee, WI, USA
Julie A. Panepinto
MRC Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford, UK
David J. Weatherall
INCT de Sangue, Haematology and Haemotherapy Centre, School of Medicine, University of Campinas — UNICAMP, Campinas, São Paulo, Brazil
Fernando F. Costa
Hematology and Oncology, UCSF Benioff Children's Hospital, Oakland, University of California, San Francisco, CA, USA
Elliott P. Vichinsky
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (M.H.G. and C.D.R.); Epidemiology (D.J.W. and F.B.P.); Mechanisms/pathophysiology (G.J.K. and F.F.C.); Diagnosis, screening and prevention (K.O.-F., E.P.V. and L.K.); Management (G.J.K., E.P.V. and F.B.P.); Quality of life (W.R.S. and J.A.P.); Outlook (G.J.K., F.B.P. and E.P.V.); Overview of Primer (G.J.K., F.B.P. and E.P.V.).
Corresponding author
Correspondence to Gregory J. Kato .
Ethics declarations
Competing interests.
G.J.K. is listed as a co-inventor on a patent application by the US NIH for the formulation of topical sodium nitrite (PCT/US2015/060015), receives research support from Bayer Pharmaceuticals and has received research support from AesRx and personal consulting fees (honoraria) from Novartis and Bioverativ outside the submitted work. The University of Pittsburgh received support for G.J.K.'s salary to serve on the steering committee for a clinical trial by Mast Therapeutics. F.B.P. reports personal fees (honoraria) from Novartis outside the submitted work. L.K., W.R.S., J.A.P., D.J.W., F.F.C. and E.V.P. declare no competing interests. Editor's note: all other authors have chosen not to declare any competing interests.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, powerpoint slide for fig. 7, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Kato, G., Piel, F., Reid, C. et al. Sickle cell disease. Nat Rev Dis Primers 4 , 18010 (2018). https://doi.org/10.1038/nrdp.2018.10
Download citation
Published : 15 March 2018
DOI : https://doi.org/10.1038/nrdp.2018.10
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Medical educators’ perceptions of race in clinical practice.
- June Futterman
- Catherine Bi
- Ebiere Okah
BMC Medical Education (2024)
Determination of birth prevalence of sickle cell disease using point of care test HemotypeSC™ at Rundu Hospital, Namibia
- Runyararo Mashingaidze Mano
- Patience Kuona
- Jane Masiiwa Misihairabgwi
BMC Pediatrics (2024)
Biophysical chemistry behind sickle cell anemia and the mechanism of voxelotor action
- Mohd. Suhail
Scientific Reports (2024)
Evaluating sheep hemoglobins with MD simulations as an animal model for sickle cell disease
- Caroline E. Kuczynski
- Christopher D. Porada
- Graça Almeida-Porada
Base Editors-Mediated Gene Therapy in Hematopoietic Stem Cells for Hematologic Diseases
- Chengpeng Zhang
Stem Cell Reviews and Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 15, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Novel gene-editing therapy continues to show positive results in sickle cell patients
by Cleveland Clinic
Researchers have presented the latest findings from a clinical trial aimed at discovering a cure for sickle cell disease, a painful genetic blood disorder with limited treatment options.
Conducted as part of the multicenter RUBY Trial, researchers shared data on the safety and effectiveness of renizgamglogene autogedtemcel (reni-cel, formerly known as EDIT-301), an experimental one-time gene editing cell therapy , among its 18 patients at the European Hematology Association 2024 Hybrid Congress (EHA) in Madrid, Spain.
This innovative treatment modifies a patient's own blood-forming stem cells to correct the mutation responsible for sickle cell disease .
The 18 patients—two of whom were treated at Cleveland Clinic Children's—underwent a procedure where their stem cells were first collected for gene editing.
They then received chemotherapy to clear remaining bone marrow , making room for the repaired cells which were later infused back into their body.
The treatment was well-tolerated with no serious side effects reported. Following treatment, all patients successfully regained their white blood cells and platelets. Importantly, all patients have remained free of painful events since treatment, and those followed for five months or greater have seen their anemia resolve.
"It's encouraging that this gene-editing treatment continues to show promising efficacy for sickle cell patients," said Rabi Hanna, M.D., chairman of the division of pediatric hematology oncology and blood and marrow transplantation at Cleveland Clinic Children's and the RUBY trial's presenting investigator. "These latest results offer hope that this new experimental treatment will continue to show progress and get us closer to a functional cure for this devastating disease."
A significant milestone of the trial is the first use of CRISPR/Cas12a gene-editing technology in a human study to alter the defective gene responsible for sickle cell disease. This precision tool is used to modify blood stem cell genomes to enable robust and healthy blood cell production.
In the United States, between 1 million to 3 million people carry the sickle cell trait , while approximately 100,000 individuals have sickle cell disease. This trait and disease are more common among certain ethnic groups, including African Americans, where approximately 1 in every 365 babies are born with sickle cell disease.
Sickle cell disease is a genetic blood disorder that causes red blood cells to be misshapen like a sickle. Normal red blood cells are round and carry oxygen smoothly through blood vessels. In sickle cell disease, the abnormal cells block blood flow and break apart easily, leading to problems like severe pain, liver and heart issues, and a shorter life span, typically in the mid-40s. Medications can help manage the disease, but a cure is possible only through a blood or marrow transplant, which has risks and often requires a sibling donor.
Explore further
Feedback to editors

LSD use tied to increased distress in unemployed job-seekers

A new 'one-two punch' method for improving checkpoint inhibitor therapy for cancers, including Hodgkin lymphoma

Study identifies mechanism behind glioblastoma's immunotherapy resistance
2 hours ago

Research reports improvements in survival rates in patients with metastatic prostate cancer

Removal of ovaries before menopause associated with reduced white matter in brain
3 hours ago
Novel treatment effectively treats cognitive decline in mice with Alzheimer's disease

Treatment model in France shows better access to methadone in US could save lives
4 hours ago

Chemotherapy before surgery benefits some patients with pancreatic cancer, study finds

New simple test detects rare fatal genetic heart condition
Team discovers why people who lack a specific blood group are genetically predisposed to be overweight or obese
Related stories.

Novel gene therapy shows promise in sickle cell patient clinical trial
Jun 9, 2023
Video: Sickle cell disease explained
Dec 8, 2023
Researchers publish final results of key clinical trial for gene therapy for sickle cell disease
Apr 25, 2024

US approves sickle cell breakthrough with gene editing therapy
New study shows promising evidence for sickle cell gene therapy
Aug 30, 2023
Affordable stroke-risk screening could save the lives of many children with sickle cell disease in sub-Saharan Africa
Apr 3, 2024
Recommended for you

Gut bacteria might discourage binge drinking
5 hours ago

Proof-of-concept study shows sweat health monitor can measure levels of disease markers

Study suggests fewer good gut bacteria increase the risk of serious infection
6 hours ago

Antibiotic resistance: An extremely concerning situation in sub-Saharan African children
Combination targeted treatment produces lasting remissions in people with resistant aggressive B-cell lymphoma
22 hours ago

Scientists reveal how an unstructured protein traps cancer-promoting molecules
Jun 19, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Sickle Cell Disease Update: New Treatments and Challenging Nutritional Interventions
Affiliations.
- 1 Faculty of Pharmacy, University of Coimbra, Pólo das Ciências da Saúde, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal.
- 2 Department of Food Science and Technology, University of the Peloponnese, 24100 Kalamata, Greece.
- 3 Medical School, National and Kapodistrian University of Athens, 11527 Athens, Greece.
- 4 CIISA, Faculty of Veterinary Medicine, University of Lisbon, 1649-004 Lisbon, Portugal.
- PMID: 38257151
- PMCID: PMC10820494
- DOI: 10.3390/nu16020258
Sickle cell disease (SCD), a distinctive and often overlooked illness in the 21st century, is a congenital blood disorder characterized by considerable phenotypic diversity. It comprises a group of disorders, with sickle cell anemia (SCA) being the most prevalent and serious genotype. Although there have been some systematic reviews of global data, worldwide statistics regarding SCD prevalence, morbidity, and mortality remain scarce. In developed countries with a lower number of sickle cell patients, cutting-edge technologies have led to the development of new treatments. However, in developing settings where sickle cell disease (SCD) is more prevalent, medical management, rather than a cure, still relies on the use of hydroxyurea, blood transfusions, and analgesics. This is a disease that affects red blood cells, consequently affecting most organs in diverse manners. We discuss its etiology and the advent of new technologies, but the aim of this study is to understand the various types of nutrition-related studies involving individuals suffering from SCD, particularly in Africa. The interplay of the environment, food, gut microbiota, along with their respective genomes collectively known as the gut microbiome, and host metabolism is responsible for mediating host metabolic phenotypes and modulating gut microbiota. In addition, it serves the purpose of providing essential nutrients. Moreover, it engages in direct interactions with host homeostasis and the immune system, as well as indirect interactions via metabolites. Nutrition interventions and nutritional care are mechanisms for addressing increased nutrient expenditures and are important aspects of supportive management for patients with SCD. Underprivileged areas in Sub-Saharan Africa should be accompanied by efforts to define and promote of the nutritional aspects of SCD. Their importance is key to maintaining well-being and quality of life, especially because new technologies and products remain limited, while the use of native medicinal plant resources is acknowledged.
Keywords: anemia; hemoglobin; microbiota; nutrition; sickle cell; vaso-occlusive crisis.
PubMed Disclaimer
Conflict of interest statement
The authors declare no conflicts of interest.
The most common clinical manifestation…
The most common clinical manifestation of sickle cell disease, a vaso-occlusive crisis (VOC)…
Some of the major complications…
Some of the major complications associated with SCD development.
Some of the tropical plants…
Some of the tropical plants used in SCA in Sub-Saharan Africa.
Similar articles
- Hydroxyurea for children with sickle cell disease in sub-Saharan Africa: A summary of the evidence, opportunities, and challenges. Dexter D, McGann PT. Dexter D, et al. Pharmacotherapy. 2023 May;43(5):430-441. doi: 10.1002/phar.2792. Epub 2023 Mar 26. Pharmacotherapy. 2023. PMID: 36906823 Review.
- Hydroxyurea (hydroxycarbamide) for sickle cell disease. Rankine-Mullings AE, Nevitt SJ. Rankine-Mullings AE, et al. Cochrane Database Syst Rev. 2022 Sep 1;9(9):CD002202. doi: 10.1002/14651858.CD002202.pub3. Cochrane Database Syst Rev. 2022. PMID: 36047926 Free PMC article. Review.
- Preoperative blood transfusions for sickle cell disease. Estcourt LJ, Kimber C, Trivella M, Doree C, Hopewell S. Estcourt LJ, et al. Cochrane Database Syst Rev. 2020 Jul 2;7(7):CD003149. doi: 10.1002/14651858.CD003149.pub4. Cochrane Database Syst Rev. 2020. PMID: 32614473 Free PMC article.
- Pharmacological interventions for painful sickle cell vaso-occlusive crises in adults. Cooper TE, Hambleton IR, Ballas SK, Johnston BA, Wiffen PJ. Cooper TE, et al. Cochrane Database Syst Rev. 2019 Nov 14;2019(11):CD012187. doi: 10.1002/14651858.CD012187.pub2. Cochrane Database Syst Rev. 2019. PMID: 31742673 Free PMC article.
- Sickle Cell Disease. Bender MA, Carlberg K. Bender MA, et al. 2003 Sep 15 [updated 2023 Dec 28]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews ® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. 2003 Sep 15 [updated 2023 Dec 28]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews ® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024. PMID: 20301551 Free Books & Documents. Review.
- Malnutrition in sickle cell anemia: Prevalence, impact, and interventions: A Review. Obeagu EI, Obeagu GU. Obeagu EI, et al. Medicine (Baltimore). 2024 May 17;103(20):e38164. doi: 10.1097/MD.0000000000038164. Medicine (Baltimore). 2024. PMID: 38758879 Free PMC article. Review.
- Folic Acid in the Treatment of Sickle Cell Disease: A Systematic Review. Arrey Agbor DB, Panday P, Ejaz S, Gurugubelli S, Prathi SK, Palou Martinez Y, Nassar ST. Arrey Agbor DB, et al. Cureus. 2024 Apr 10;16(4):e57962. doi: 10.7759/cureus.57962. eCollection 2024 Apr. Cureus. 2024. PMID: 38738102 Free PMC article. Review.
- Elendu C., Amaechi D.C., Alakwe-Ojimba C.E., Elendu T.C., Elendu R.C., Ayabazu C.P., Aina T.O., Aborisade O., Adenikinju J.S. Understanding Sickle Cell Disease: Causes, Symptoms, and Treatment Options. Medicine. 2023;102:E35237. doi: 10.1097/MD.0000000000035237. - DOI - PMC - PubMed
- Tebbi C.K. Sickle Cell Disease, a Review. Hemato. 2022;3:341–366. doi: 10.3390/hemato3020024. - DOI
- Quinn C.T. Minireview: Clinical Severity in Sickle Cell Disease: The Challenges of Definition and Prognostication. Exp. Biol. Med. 2016;241:679–688. doi: 10.1177/1535370216640385. - DOI - PMC - PubMed
- Ershler W.B., De Castro L.M., Pakbaz Z., Moynahan A., Weycker D., Delea T.E., Agodoa I., Cong Z. Hemoglobin and End-Organ Damage in Individuals with Sickle Cell Disease. Curr. Ther. Res. 2023;98:100696. doi: 10.1016/j.curtheres.2023.100696. - DOI - PMC - PubMed
- Inusa B.P.D., Hsu L.L., Kohli N., Patel A., Ominu-Evbota K., Anie K.A., Atoyebi W. Sickle Cell Disease—Genetics, Pathophysiology, Clinical Presentation and Treatment. Int. J. Neonatal Screen. 2019;5:20. doi: 10.3390/ijns5020020. - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding, linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
- Genetic Alliance

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
46 NONPROFIT, COMMUNITY-BASED ORGANIZATIONS AND MEDICAL PROVIDERS IN THE NORTHEAST U.S. COLLABORATE TO 'SHINE THE LIGHT ON SICKLE CELL' ON JUNE 19
News provided by
Jun 13, 2024, 10:24 ET
Share this article
Businesses, Hospitals, Municipal Buildings, Museums, Schools, Landmarks To Illuminate In Red On World Sickle Cell Awareness Day To Show Support of Sickle Cell Warriors, Raise Awareness of Sickle Cell Disease, Advocate For A Universal Cure
Fraternal Organization, Global Healthcare Company, NFL Team, Building Owners Management Association Join Campaign
PHILADELPHIA , June 13, 2024 /PRNewswire/ -- A collaborative of 46 nonprofit, community-based organizations and medical providers in the Northeast U.S. is set to Shine the Light on Sickle Cell on June 19 , World Sickle Cell Awareness Day. This collaborative initiative–which includes the illumination in red of businesses, hospitals, municipal buildings, museums, schools, and other landmarks–aims to unite individuals and communities in support of sickle cell warriors, raise awareness of Sickle Cell Disease (SCD) and advocate for a universal cure. Fraternal organization Phi Beta Sigma Fraternity, Inc., global healthcare company Novo Nordisk Inc., the Baltimore Ravens, and Building Owners Management Association-Philadelphia will also join the campaign that is supported by the federal Health Resources and Services Administration (HRSA) as part of the Sickle Cell Disease Treatment Demonstration Project.
"Now in its sixth year, Shine the Light on Sickle Cell is a powerful testament to what we can achieve when communities, healthcare providers, and advocates unite," said Johns Hopkins University School of Medicine Professor Dr. Rosalyn Stewart . "Our goal is to make Sickle Cell Disease as common a topic as COVID or HIV and to inspire action towards a future where Sickle Cell Disease is better understood, better treated, and ultimately cured."
SCD is a genetic blood disorder that disproportionately affects individuals of African descent yet remains widely misunderstood and underrepresented in public discourse. With over 100,000 people in the U.S. affected by SCD and millions more carrying the Sickle Cell Trait, the need for improved access to care and research advancements is critical. Individuals living with SCD often face numerous health complications, including stroke, acute chest syndrome, and chronic organ damage, leading to a significantly reduced life expectancy compared to the general population and earning them the name of sickle cell warrior. Despite these challenges, there is currently no universal cure for SCD.
"I am deeply committed to shining the light on sickle cell and advocating for better care of people with the disease, a disease that causes severe episodic and chronic pain. Those with sickle cell disease frequently report poor interpersonal treatment within health-care settings," said Dr. Sophie Lanzkron , Director of the Division of Hematology at Thomas Jefferson University . "Eliminating the discrimination in health-care settings is imperative as research has proven its correlation with greater pain severity, stress, depression, and sleep issues."
Shine the Light on Sickle Cell primarily operates in the Northeast region, encompassing New England , the mid- Atlantic, Virginia , West Virginia , the U.S. Virgin Islands , and Puerto Rico . Shine the Light on Sickle Cell's hallmark is the illumination of landmark structures and buildings in red – the emblematic color of the blood cell associated with the genetic disorder and a symbol of the urgency, passion, and unwavering determination in the fight against this disease – in the U.S. and around the world on and around June 19 .
Major landmarks that will Shine the Light on Sickle Cell this year include the Franklin Institute , the Hospital of the University of Pennsylvania , and Boathouse Row ( Philadelphia ); Novo Nordisk headquarters ( Plainsboro Township , New Jersey ); the Baltimore Ravens' M&T Bank Stadium and the Johns Hopkins University Dome ( Baltimore ); the Rhode Island State House ( Providence ); and University of Maryland's Capital Region Medical Center, Bowie Health Center, and Laurel Medical Center ( Washington, D.C. metropolitan area).
Zemoria Brandon is a longtime sickle cell advocate whose husband passed away from the disease in 1998. Now in her role as chair of the Shine the Light on Sickle Cell Steering Committee and administrator/social worker with Sickle Cell Disease Association of America, Philadelphia/Delaware Valley Chapter, Ms. Brandon said: "By illuminating our communities in red, we are symbolizing the urgency and passion needed to address the challenges of Sickle Cell Disease. Although Shine the Light on Sickle Cell began six years ago as an initiative in the Northeastern United States , it grew into a campaign, and now it has become a movement. It is uplifting to have seen participation from 29 states across the country and 21 countries around the world."
Organizations in the collaborative are set to engage their community in various activities, including sickle celebrations, candlelight vigils, blood drives, awareness walks, wearing red, posting photos and videos on social media, and more. Shine the Light on Sickle Cell events (click for details and registration information) include:
- A Juneteenth Blood Drive and marquee illumination hosted by the Sickle Cell Association of Delaware at Cornerstone Fellowship Baptist Church in Wilmington
- A World Sickle Cell Day Conference sponsored by the Sickle Cell Thalassemia Patients Network in New York to discuss National Heart, Lung, and Blood Institute guidelines and American Society of Hematology clinical guidelines
- A Sickle Cellabration hosted by the Sickle Cell Association of New Jersey as part of an Annual Juneteenth Block Party in New Jersey
- A dinner event hosted by the Anemia Falciforme Sickle Cell Disease Association in Puerto Rico
- A Shine the Light on Sickle Cell event hosted by the Sickle Cell Association ( Norfolk ) in conjunction with the Juneteenth United Parade on the Eastern Shore of Virginia
Join the conversation about Shine the Light on Sickle Cell using #shinethelightonsicklecell2024.
About Shine the Light on Sickle Cell
Shine the Light on Sickle Cell is an annual community awareness campaign to celebrate World Sickle Cell Awareness Day on June 19 as proclaimed by the United Nations in 2008 and to advocate for a universal cure. Shine the Light on Sickle Cell is led by a collaborative of 46 nonprofit, community-based organizations and medical providers in the Northeast United States , SiNERGe (Sickle Cell Improvement in the Northeast Region through education), whose aim is to increase awareness of Sickle Cell Disease and advocate for treatments and better outcomes for individuals with the disease. Learn more at Shine the Light on Sickle Cell .
Media Contact ShinePR for Shine the Light on Sickle Cell [email protected]
Participating organizations and provider organizations include (by state):
Connecticut
Citizens for Quality Sickle Cell Care* University of Connecticut
Christiana Care Sickle Cell Association of Delaware
District of Columbia
Faces of Our Children Sickle Cell Association of the National Capital Area Inc.
Maine Medical Center
Adult and Pediatric to Adult Sickle Cell Clinic, Johns Hopkins University Armstead-Barnhill Foundation for Sickle Cell Anemia Association for the Prevention of Sickle Cell Anemia Inc., Harford and Cecil Counties and the Eastern Shore* Christopher Gipson Sickle Cell Moyamoya Foundation Eastern Shore of Maryland Sickle Cell Association Johns Hopkins University Maryland Sickle Cell Disease Association* Project Spirit Sickle Cell Sally's Sunshine Foundation Sickle Cell Coalition of Maryland William E. Proudford Sickle Cell Fund Inc.
Massachusetts
Boston University Massachusetts General Hospital Massachusetts Sickle Cell Disease Association*
New Hampshire
Dartmouth Hitchcock Medical Center
Donna T. Darrien Memorial Foundation for Sickle Cell Newark Beth Israel Medical Center Sickle Cell Association of New Jersey *@
Candice's Sickle Cell Fund^ Children's Hospital of Monefiore New York Sickle Cell Advisory Network NYC Health + Hospitals New York Sickle Cell Advocacy Network (formerly Queens Sickle Cell Anemia Network)* Sickle Cell Advocates of Rochester ^ Sickle Cell Awareness Foundation Corp International Sickle Cell Thalassemia Patients Network*@ Sickle Cell Warriors of Buffalo^ Westchester Sickle Cell Outreach
Pennsylvania
Children's Sickle Cell Foundation Inc.*#@ Crescent Foundation@ Sickle Cell Disease Association of America, Philadelphia/Delaware Valley Chapter*#@ South Central Pennsylvania Sickle Cell Council*# Hospital of the University of Pennsylvania
Puerto Rico
Anemia Falciforme Sickle Cell Disease en Puerto Rico
Rhode Island
Rhode Island Hospital
University of Vermont
Life and Family Foundation Richmond (formerly Living with Sickle Cell RVA) Sickle Cell Association Inc.*
West Virginia
CAMC Institute for Academic Medicine
*Chapters of the Sickle Cell Disease Association of America (SCDAA) #Members of the Pennsylvania Sickle Cell Disease Providers Network (PASCDPN) ^Affiliates of Sickle Cell Thalassemia Patients Network (SCTPN) @HRSA Newborn Screening Grantees
SOURCE Shine the Light on Sickle Cell
Modal title
Repurposed drug may help stabilize vision in rare disease
Clinical trial for RVCL-S patients tests drug already approved as sickle cell disease treatment
Roughly 50 families scattered across the world share ultra-rare variants in a particular gene. Silent for years, the inherited mutations make themselves known when patients reach the fourth decade of life. Changes in vision start a cascade of symptoms. Five to 20 years later, the illness is fatal.
Researchers at Washington University School of Medicine in St. Louis have dedicated many years to understanding the rare condition known as retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations, or RVCL-S, with the aim of developing a treatment for it. In a new study, the team reports that a drug approved by the FDA for another condition may stabilize vision for patients with RVCL-S.
“Fifty percent of family members with these genetic mutations will inherit the disease,” said the study’s co-senior author, Rajendra S. Apte, MD, PhD , the Paul A. Cibis Distinguished Professor of Ophthalmology & Visual Sciences . “These families are devastated by this illness, and we have not been able to offer them much hope. However, our new findings suggest that an already approved medication for a different disease may have the potential to help these patients, although additional studies are needed.”
The study is published June 17 in The Journal of Clinical Investigation.
RVCL-S is marked by progressive vision loss, cognitive decline, dementia and mini strokes, among other neurological manifestations. In the 1980s, two physicians from Washington University initially linked the symptoms to compromised small blood vessels that cause loss of blood flow to retinal, brain, kidney and liver tissues.
“Imagine a traffic jam,” explained first author Wilson Wang, who paused his medical training to pursue independent research in Apte’s laboratory as part of a program at Washington University. “Small blood vessels are the roads feeding into the circulatory highway system. When blood flow trickles, oxygen and nutrients cannot reach the tissue, and damage ensues.”
Other diseases cause similar circulatory system traffic jams. For instance, blockages in small blood vessels are responsible for episodes of excruciating pain in sickle cell disease. Because there is an FDA-approved drug – crizanlizumab – used to help alleviate pain by unclogging congested small blood vessels and preventing blood cells from sticking to the vessel walls in sickle cell anemia patients, co-senior author Andria L. Ford, MD , a professor of neurology and of radiology , launched a clinical trial to test the drug in RVCL-S patients. A collaboration with Apte – an expert in retinal vascular diseases such as diabetic retinopathy and macular degeneration, conditions affecting blood vessels in the retina – focused on patients’ vision, retinal structure and function.
The researchers set out to see if crizanlizumab helps to improve blood flow in the eyes and brain, explained Ford, who is co-director of Washington University’s RVCL Research Center and senior investigator on a parallel study looking at crizanlizumab’s effect on brain lesions in the clinical trial participants.
Eleven RVCL-S patients — who traveled from as far as California despite the challenges of their health and the COVID-19 pandemic — participated in the clinical trial for two years. They received two doses of crizanlizumab in the first month, followed by subsequent monthly infusions.
Images of retinal blood vessels highlighted with fluorescein dye were obtained at baseline and after the first and second years of the study. Wang and Apte, along with colleagues including Dan Spiegelman, a rising third-year medical student, and P. Kumar Rao, MD , a professor of ophthalmology and visual sciences and vice chair of clinical affairs, analyzed the images and compared them with images taken before the patients were treated with the drug. They calculated the amount of space lacking blood flow in a defined region of the retina, a measurement referred to as the nonperfusion index.
After two years on the drug, trial participants saw their nonperfusion indexes plateau, a sign that loss of small blood vessel integrity was slowing, the researchers explained. Routine eye exams revealed that the participants’ vision had stabilized.
Preserving vision in a disease that often ends in blindness has the potential to give patients additional years to read, drive and enjoy activities that bring them happiness. But future studies are needed to test the drug’s ability to slow vision loss in RVCL-S. In a parallel study, the researchers will examine the medication’s impact on brain lesions. If blood vessel blockages in the retina correlate with the brain lesions in these patients, the retina could be used as a biomarker for the systemic disease, Apte explained.
“Until we have a genetic cure, our patients need treatments that halt or slow the progression of the illness,” Ford said. “Washington University is at the forefront of RVCL-S research. Decades of dedication have paved the way for this clinical trial. It represents our commitment to finding treatments that can alter the course of the fatal, rapidly progressing illness.”
Four decades of dedication
Before RVCL-S was identified, patients with the disorder sought answers at Washington University in the 1980s. Seven people, six from one family, were suffering from mysterious neurological symptoms with no known cause. Seven years of inquiry by Washington University physicians led to a published description of the illness, giving their patients a name for their ailments.
Since then, a robust research infrastructure and strong clinical innovation have supported the university’s dedication to understanding and treating the devastating condition.
In 2007, John P. Atkinson, MD , the Samuel B. Grant Professor of Clinical Medicine and one of the key researchers who helped demystify the disease, identified its root cause: mutations in the TREX1 gene. His discovery enabled the development of genetic testing that now gives patients definitive diagnoses. He went on to help establish the university’s RVCL Research Center in 2016, after a $4.1 million donation by Robert Clark and his partners at Clayco – a construction firm in St. Louis – in honor of Clark’s wife who died from the illness at age 50. Two years later, Atkinson led the first clinical trial for RVCL-S .
Although that trial did not show a benefit to patients, the pursuit of treatments for RVCL-S patients has continued.
“We have come a long way with learning about and diagnosing RVCL-S in the last four decades,” Ford said. “But our work doesn’t stop here. More patients have been identified throughout the world, making it possible for other centers to open and bring attention to the rare disease. By working together, we can make a larger impact by increasing the sample size in research studies and finding treatments.”
Wang WX, Spiegelman D, Rao PK, Ford AL, Apte RS. Crizanlizumab for Retinal Vasculopathy with Cerebral Leukoencephalopathy in a Phase 2 Clinical Study. The Journal of Clinical Investigation. June 17, 2024. DOI: 10.1172/JCI180916
The study was supported by The Clayco Foundation; DeNardo Education & Research Foundation Grant; Jeffrey T. Fort Innovation Fund; Siteman Retina Research Fund; a grant from Research to Prevent Blindness Inc. to the Department of Ophthalmology at Washington University; and the National Institutes of Health (NIH), grant numbers NHLBI R01HL129241 and NINDS RF1NS116565. Novartis provided crizanlizumab infusions free for the entire study. The sponsor or funding organization had no role in the design or conduct of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
About Washington University School of Medicine
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,900 faculty. Its National Institutes of Health (NIH) research funding portfolio is the second largest among U.S. medical schools and has grown 56% in the last seven years. Together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,900 faculty physicians practicing at 130 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare . WashU Medicine has a storied history in MD/PhD training, recently dedicated $100 million to scholarships and curriculum renewal for its medical students, and is home to top-notch training programs in every medical subspecialty as well as physical therapy, occupational therapy, and audiology and communications sciences.
Originally published on the School of Medicine website
Comments and respectful dialogue are encouraged, but content will be moderated. Please, no personal attacks, obscenity or profanity, selling of commercial products, or endorsements of political candidates or positions. We reserve the right to remove any inappropriate comments. We also cannot address individual medical concerns or provide medical advice in this forum.
You Might Also Like

Latest from the Newsroom
Recent stories.
Modifying homes for stroke survivors saves lives, extends independence
Book explores consequences of political conversations
New technology allows researchers to precisely, flexibly modulate brain
WashU Experts
Social workers key to psychedelic-assisted therapies
DeFake tool protects voice recordings from cybercriminals
Tremor a reminder that East Coast, Midwest earthquake threat is real
WashU in the News
NFL faces ‘Sunday Ticket’ lawsuit: Here’s what’s at stake for the league
Transcript: Ezra Klein Interviews Yanna Krupnikov
The brain has a waste removal system and scientists are figuring out how it works

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Search Publications
Sickle Cell Disease: Milestones in Research and Clinical Progress

Learn about the history of sickle cell disease in the United States, from its discovery in 1910 to the NHLBI legacy of research that has advanced the understanding of sickle cell disease, improved clinical progress, and paved the way for a cure for all patients.
Web-only Publications

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Medicine (Baltimore)
- v.102(47); 2023 Nov 24
- PMC10681612
Psychosocial challenges of persons with sickle cell anemia: A narrative review
Emmanuel aniekan essien.
a Research and Training Unit, Federal Neuropsychiatric Hospital
Blessing F. Winter-Eteng
Chinyere uchechi onukogu, dominic dennis nkangha, faithful miebaka daniel.
b Community and Clinical Research Division, First On-Call Initiative.
Sickle cell anemia (SCA) is a severe form of sickle cell disease that primarily affects black populations and individuals in tropical countries. This condition causes significant morbidity and mortality and leads to a range of psychosocial challenges. A preliminary search was conducted on Ovid Medline and public databases with a combination of Medical Subject Headings keywords, resulting in 368 articles. The articles were screened based on the selection criteria in a nonsystematic method by 3 researchers, and a narrative synthesis was done to analyze extracted data from selected peer-reviewed article. Mental disorders, sleep disturbances, interpersonal relationship challenges, stigmatization, and workplace discrimination were identified as significant contributors to the psychosocial distress experienced by individuals with SCA and their families. Depression and anxiety were prevalent among individuals with SCA, leading to poor treatment adherence, increased pain, and disruptions in various aspects of life. Sleep disturbances, including sleep-disordered breathing and sleepwalking, were also identified as significant contributors to poor sleep quality in SCA patients. Families of individuals with SCA also face challenges, including psychological stress, financial strain, and social disruption. Stigmatization is common, leading to misconceptions and discrimination. Workplace discrimination is prevalent, with a high unemployment rate among adult SCA patients. Comprehensive care is crucial to address these psychosocial issues. Early identification and intervention, comprehensive support programs, patient and family education, enhanced pain management strategies, and integration of mental health into clinical care are recommended. School-based support, research and advocacy, and community support groups are also important. By addressing these challenges through comprehensive care and support, healthcare professionals, policymakers, and society can reduce psychosocial distress and improve the lives of individuals with SCA.
1. Introduction
Sickle cell disease (SCD) is a group of hematological disorders characterized by abnormal hemoglobin, leading to defective red blood cell morphology and function. [ 1 ] The most severe variant of SCD is sickle cell anemia (SCA), caused by an autosomal recessive mutation in the β-globin hemoglobin gene. [ 1 ] The disease has an uneven distribution documented in literature, and much is known about the prevalence of the disease. However, poor early neonatal screening for hemoglobin genotype hampers reliance on data in resource-poor settings. This global disease affects approximately 300,000 babies annually, with 2-thirds of cases occurring in Africa. [ 1 ] Tropical countries like Nigeria, India, and the Democratic Republic of Congo bear the highest burden of SCA. [ 1 ] The pathological consequences of SCA are extensive and affect various aspects of an individual health and have continued to be the subject of research interest. The hallmark of SCA is the abnormal sickling and loss of pliability of red blood cells, leading to vaso-occlusive crises (VOC), hemolytic crises, chronic anemia, and severe pain episodes. [ 2 ] Additionally, individuals with SCA are prone to strokes, recurrent infections, avascular necrosis, priapism, and growth delay. [ 3 ] While advancements in medical interventions have improved life expectancy for SCA patients in high-income countries, the situation remains challenging in low-resource settings. [ 4 ] The lack of healthcare infrastructure and policies in these regions contributes to poorer outcomes for individuals with SCA. [ 4 ]
In addition to the medical syndromes experienced by individuals with SCA, there is an interplay of psychosocial challenges, including mental disorders, pain crises, sleep disorders, interpersonal relationships, substance abuse, stigma, and workplace discrimination, as suggested by literature. [ 5 – 10 ] However, a wider array of psychosocial challenges impacting the patients, their family and peers, and ignored can affect overall response to management, has been underrepresented in literature. [ 11 ] As a result, SCA patients have reported negative experiences due to practitioners ignoring or undermining the effect of these less overt challenges. Such experiences include stigma produced discrediting pain reports, labeling and stereotyping, blaming patients for not improving their health, discrimination, racism, inadequate pain assessment, and delay in care. [ 12 ] On the other hand, there is evidence on the benefits of social support and programs that lead to overall improvement in health-related quality of life (QOL) and coping. [ 13 – 15 ] However, these programs only exist where there is adequate knowledge of the problem and the necessity. Therefore, it is pertinent to review this subject at a global scale to provide an elaborate perspective of the problem and what policies or intervention strategies have proven to be effective and can be domesticated in other climes. This review aims to summarize findings on the prevalence and impact of mental disorders such as anxiety and depression, pain experiences, interpersonal relationships, workplace discrimination, stigma, sleep disorders, and body dissatisfaction among individuals with SCA. These are important factors to consider in addressing psychosocial challenges and improving management strategies. By recognizing and addressing these issues, healthcare providers can prioritize holistic care for SCA patients and improve the overall management and outcomes for individuals living with SCA.
2. Methodology
To address the aims of this review, we attempted answer the following reviewing questions: How prevalent are mental disorders such as anxiety and depression among individuals with SCA, and what is the impact of these disorders on coping mechanisms, interpersonal relationships with peers and family, and overall psychosocial well-being? What is the prevalence and impact of workplace discrimination, stigma, sleep disorders, and body satisfaction among individuals with SCA, and how do these factors influence mental health, substance use, pain experiences, and overall psychosocial challenges?
2.1. Search strategy
A literature search was conducted to identify relevant articles from electronic databases such as PubMed, Embase, PsycINFO, and Scopus. The search strategy included a combination of Medical Subject Headings keywords on Ovid Medline using the following keywords: “anemia, sickle cell,” “mental health,” “social stigma,” “social support,” “quality of life,” “adaptation, psychological,” “mental disorders,” “sickle cell anemia AND mental disorders,” “sleep,” “substance-related disorders,” “pain,” “family relations,” “body dissatisfaction,” “interpersonal relations,” “psychology,” “pain AND psychology,” “medical psychology,” “social stigma OR social support OR quality of life OR sleep OR substance-related disorders OR Family relations OR body dissatisfaction OR interpersonal relations OR (pain AND psychology),” “sickle cell anemia AND social stigma OR social support OR quality of life OR sleep OR substance-related disorders OR Family relations OR body dissatisfaction OR interpersonal relations OR (pain AND psychology),” “social stigma OR social support or psychological adaptation or substance-related disorders or family relations or interpersonal relations or (pain AND psychology),” “sickle cell anemia AND [social stigma OR social support or psychological adaptation or substance-related disorders or family relations or interpersonal relations or (pain AND psychology)].”
2.2. Study selection criteria
Studies were included if they met the following criteria: were peer-reviewed articles, focused on psychosocial issues in SCA, included participants diagnosed with SCA of any age group, published in the English language, and published between 1989 and 2023. Quantitative and qualitative studies were considered for inclusion, including cross-sectional, longitudinal, and intervention studies (Table (Table1 1 ).
Showing article selection process for reviewing psychosocial issues and sickle cell anemia (SCA).
| Stage | Description | Number of articles |
|---|---|---|
| Initial search | Database search using keywords like “anemia, sickle cell,” “mental health,” “social stigma,” “social support,” “quality of life,” “adaptation, psychological,” “mental disorders,” “sickle cell anemia AND mental disorders,” “sleep,” “substance-related disorders,” “pain,” “family relations,” “body dissatisfaction,” “interpersonal relations,” “psychology,” “pain AND psychology,” “medical psychology,” “social stigma OR social support OR quality of life OR sleep OR substance-related disorders OR Family relations OR body dissatisfaction OR interpersonal relations OR (pain AND psychology),” “sickle cell anemia AND social stigma OR social support OR quality of life OR sleep OR substance-related disorders OR Family relations OR body dissatisfaction OR interpersonal relations OR (pain AND psychology),” “social stigma OR social support or psychological adaptation or substance-related disorders or family relations or interpersonal relations or (pain AND psychology),” “sickle cell anemia AND [social stigma OR social support or psychological adaptation or substance-related disorders or family relations or interpersonal relations or (pain AND psychology)]” | 368 |
| Reference review | Review of reference list of identified articles | +30 |
| Total articles | Total articles acquired from a detailed literature search | 398 |
| Screening | Selection using inclusion criteria | |
| • Language | Published in the English language | 140 |
| • Year of publication | Published between 1989 and 2023 | 149 |
| • Excluding redundant papers | Repetitive or similar articles | 85 |
| • Relevance | Articles Focused on psychosocial issues in Sickle Cell Anemia and included participants diagnosed with SCA of any age group | 98 |
| • Quantitative and qualitative studies, including cross-sectional, longitudinal, and intervention studies, were considered for inclusion. | Articles published in peer-reviewed journals or regulatory repositories | 84 |
| Final selection | Articles meeting all inclusion criteria | 80 |
2.3. Data extraction
A data extraction form was developed to collect relevant information from the selected studies. The extracted data included study characteristics (authors, year of publication, study design), participant characteristics (sample size, age, gender), psychosocial issues examined (including depression, anxiety, stigma, QOL, body dissatisfaction, family and peer support), assessment measures used, and key findings related to psychosocial issues in SCA. All Authors were part of the data extraction. The thematic areas were deduced for most themes based on the original authors’ conclusions in the section on other psychosocial issues. All authors read the entire manuscript and decided on the key takeaway.
2.4. Data analysis
A narrative synthesis approach was employed to analyze the extracted data. The findings from the included studies were organized and categorized based on the specific psychosocial issues investigated. Themes and patterns across the studies were identified, and critical results were summarized to provide a comprehensive overview of the psychosocial challenges in SCA patients.
2.5. Ethical considerations
Ethical approval was not required as this study involved the review of existing literature. However, efforts were made to ensure the confidentiality and anonymity of the participants in the original studies by reporting findings in an aggregated and de-identified manner.
2.6. Limitations
Potential limitations of this review include the exclusion of non-English language articles, the reliance on published literature, which may introduce publication bias, and the possibility of missing relevant studies despite the comprehensive search strategy.
Following this methodology, a nonsystematic and comprehensive evaluation of the psychosocial issues in SCA was conducted. A total of 80 research articles were finally selected for the review (Table (Table1). 1 ). The review findings will contribute to a better understanding of the impact of SCA on individuals’ psychosocial well-being, inform healthcare practices, and highlight areas for further research and intervention development (Table (Table2 2 ).
Table showing psychosocial challenges faced by SCA patients and recommendations.
| Psychosocial issues | Recommendations |
|---|---|
| Poor body image and behavioral/emotional problems | Provide psychological support and counseling services. Encourage self-acceptance and positive body image through education and empowerment. Promote healthy coping strategies and stress management techniques. |
| Stigmatisation | Raise awareness about sickle cell anemia to reduce misconceptions and stereotypes. Implement anti-stigma campaigns in schools, workplaces, and healthcare settings. Train healthcare professionals to provide non-judgmental care and support. Foster a supportive and inclusive environment for individuals with sickle cell anemia. |
| Neurocognitive deficits | Conduct regular neurocognitive assessments to identify specific areas of difficulty. Provide tailored educational interventions and support services. Collaborate with schools to implement appropriate accommodations and Individualized Education Plans (IEPs). Offer cognitive rehabilitation programs to improve cognitive functioning. |
| Workplace discrimination | Advocate for equal employment opportunities and reasonable accommodations. Educate employers about the unique challenges faced by individuals with sickle cell anemia. Establish workplace policies that protect against discrimination. Provide job training and vocational support to enhance employability. |
| Reduced quality of life | Develop comprehensive multidisciplinary care teams to address medical, psychological, and social needs. Offer pain management strategies and access to appropriate healthcare services. Facilitate support groups and peer networks to promote social connections and emotional well-being. Enhance community resources and support systems for individuals with sickle cell anemia. |
SCA = sickle cell anemia.
4. Discussion
4.1. depression and anxiety in sickle cell anemia.
Mental disorders, such as depression and anxiety, are alarmingly common among SCA individuals, profoundly affecting their overall well-being. These disorders have far-reaching consequences, including increased pain, heightened opioid use, poor treatment adherence, interference with professional duties and schooling, and disruptions in family dynamics. [ 16 , 17 ] Depression, in particular, is most prevalent among patients with SCA, [ 18 ] with studies estimating that globally, 21.6% to 44% of adult SCA patients experience depression. [ 19 ] Similar rates have been observed in the United States, [ 20 ] and Africa, [ 17 ] with a local study in Nigeria finding that nearly 50% of participants experienced depressive states. [ 11 , 21 ] The chronic nature of the disease, the severity of symptoms, and the presence of psychosocial stressors contribute to the high prevalence of depression in this population. [ 22 ] Moreover, depression and anxiety have been shown to predict worse mental and physical health outcomes in SCA patients. [ 17 , 23 – 25 ] A cohort study conducted in Holland revealed that patients with anxiety and depressive disorders experienced severe and disabling chronic pain compared to those without these disorders. [ 17 , 23 – 25 ] These mental disorders significantly impact overall health-related QOL more than the genotype. [ 20 ] Recognizing the detrimental effects of mental disorders on individuals with SCA is crucial for providing comprehensive care. By addressing these mental health challenges, healthcare professionals can enhance the overall management and outcomes for individuals living with SCA.
4.2. Pain among patients with sickle cell anemia
Pain is a prominent feature of SCA that can profoundly impact the lives of individuals with the condition. Unlike typical pain experienced in other conditions, the pain in SCA is characterized by its unpredictable nature and the recurrent episodes of acute pain called VOC. [ 26 ] During VOC, individuals with SCA experience varying degrees of neuropathic pain, including hyperalgesia (increased sensitivity to pain) and allodynia (pain from non-painful stimuli). [ 27 ] Unfortunately, the treatment options for chronic pain in SCA are limited, with opioids being the primary choice. [ 28 ] However, the use of opioids comes with its complications, including constipation, mast cell activation, addiction, and respiratory depression. [ 28 ] Additionally, individuals with SCA often require higher doses of opioids compared to those with other acute or chronic diseases, making pain management more challenging. [ 26 ] The limited efficacy of treating neuropathic chronic pain in SCA may be attributed to the diverse underlying pathophysiology that activates nociceptive fibers. [ 29 ] This includes vascular dysfunction, inflammation, ischemia/reperfusion injury, and oxidative stress. [ 29 ] Pain in SCA can be lifelong, influencing cognitive function and contributing to psychological distress. [ 30 ] Pain in SCA can be lifelong, influencing cognitive function and contributing to psychological distress. [ 28 ] Pain also directly affects emotional expression, behavior, and mood. [ 29 , 30 ] Psychological factors, such as catastrophizing, play a role in pain modulation. [ 31 , 32 ] Catastrophizing refers to an exaggerated negative appraisal of pain, and it can significantly impact pain perception during anticipated or actual pain episodes. [ 33 – 35 ] It involves elements of rumination, magnification, and helplessness. [ 35 ] Catastrophizing behavior is positively correlated with the degree of clinical pain. [ 36 ] In children, higher levels of catastrophizing are associated with an increased risk of disability. [ 37 ] Moreover, higher levels of catastrophizing have been linked to more significant depression and poorer QOL in adults with SCA. [ 34 ] Understanding the complex interplay between pain, psychological factors, and SCA is crucial for effective pain management and improving the overall well-being of individuals with the condition.
4.3. Substance abuse among patients with sickle cell anemia
Chronic pain is a prevalent and debilitating symptom experienced by individuals with SCA. To manage this pain, pain medications, including opioids, are commonly used. However, the addictive properties of these medications can lead to further complications in SCA patients. The severity of pain in SCA can be inferred from the increased use of opioids and the incidence of abuse and addiction among SCA patients. [ 38 ] Analgesics that are commonly abused among individuals with SCA include non-steroidal anti-inflammatory drugs, codeine, oxycodone, and more potent opioids like morphine, levorphanol, methadone, pentazocine, oxymorphone, and fentanyl. [ 38 – 40 ] These medications, while effective in managing pain, can have addictive properties.
A comparative study in Nigeria reported a high incidence of pentazocine addiction among SCA patients who used it for VOC. [ 41 ] In this study, 75% of SCA patients using pentazocine for VOC developed addiction, compared to 15% using other analgesics. [ 41 ] Signs of pentazocine dependence, such as intense drug craving, excessive sweating, non-bone body pains, needle marks, excessive spending, begging, stealing, and poor academic performance, were observed. [ 41 ] These findings highlight the severity of pain experienced by individuals with SCA and the need for alternative pain management strategies to minimize the risks associated with opioid use. It is essential to exercise caution when using pentazocine in SCA patients to prevent addiction and its detrimental consequences. [ 41 ]
Exploring non-opioid pain management options and developing tailored strategies for pain control in SCA are crucial. By identifying alternative approaches and implementing comprehensive pain management plans, healthcare providers can mitigate the risks of opioid addiction while effectively addressing the chronic pain experienced by individuals with SCA. This will ultimately enhance the overall QOL for SCA patients.
4.4. Sickle cell disease and sleep
Sleep is vital to overall well-being, particularly in children and teenagers who require proper rest for their growth and development. [ 42 ] However, various factors can contribute to sleep disturbances, including stress from home and school. [ 43 ] Research has shown that sleep problems are associated with increased physical, mental, and environmental health issues. [ 43 ] In the case of individuals with SCA, episodes of acute pain known as VOC and sleep-disordered breathing have been identified as significant contributors to poor sleep. [ 44 ] Children with SCA have a higher prevalence of sleep-disordered breathing than those without the disease. Studies have reported an increased incidence of obstructive sleep apnea syndrome in children with SCA, surpassing even the prevalence in the general pediatric population. [ 44 ]
Sleep-disordered breathing in SCA can lead to behavioral problems, learning difficulties, elevated blood pressure, bed-wetting, and reduced growth. [ 44 ] These findings are consistent with another study that found a high prevalence of snoring and sleep-disordered breathing in children with SCA aged 2–14. [ 45 ] Interestingly, a survey of Saudi children in the same age group showed even higher prevalence rates of obstructive sleep apnea, snoring, and bed-wetting compared to other countries. [ 46 ] Additionally, there is a significant association between sleep-disordered breathing and sleepwalking in children and adults with SCA. [ 47 ] Adults with SCA also experience a higher prevalence of sleep disorders, and there is an inverse relationship between pain and sleep quality in these patients. [ 22 , 48 ]
Understanding the impact of sleep disturbances in individuals with SCA is crucial for their overall well-being. Sleep-disordered breathing and its associated consequences can profoundly affect cognitive function, behavior, and physical health. Healthcare providers should be aware of these issues and consider incorporating sleep assessments and interventions into the comprehensive care of individuals with SCA. By addressing sleep disturbances, healthcare professionals can potentially improve the QOL and overall health outcomes for individuals living with SCA.
4.5. Interpersonal relationship between individuals with sickle cell anemia and family
Family dynamics play a critical role in supporting adolescents with SCD, providing essential comfort, motivation, and overall support that facilitate effective coping mechanisms and positive relationships with family members and peers. [ 49 , 50 ] However, the challenges associated with SCA can significantly impact families, particularly in tropical countries like Nigeria, where the disease burden is substantial. [ 51 , 52 ]
Economic and psychosocial burdens have been identified as significant stressors in these families, including the inability to meet basic needs, loss of income due to caregiving responsibilities, financial strain related to SCA management, disruption of family interactions, increased conflicts, and neglect of other family members. [ 51 , 52 ]
Partners of adult patients also face unique challenges, such as frequent crises or hospitalizations, psychological stress, financial strain, social disruption, and stigmatization. [ 53 – 56 ] Primary caregivers for children and adolescents with SCA often have limited time for socializing within the family, leading to anxiety and frustration for caregivers and patients. [ 57 ] In this context, mothers primarily serve as caregivers and advocates for their children with SCA. [ 58 ] While familial support is crucial, it can generate problems and tensions. Some parents may attempt to shield their children from social realities, leading to conflicts with adolescents who desire independence and autonomy. [ 58 , 59 ] Adolescent patients may also experience feelings of guilt as they are aware of the physical and economic impact of SCA on their families. [ 59 ] Knowledge about SCA has been found to influence psychological functioning and parent-child dynamics within families positively. [ 49 , 60 ] Families with a comprehensive understanding of the disease tend to have better interpersonal relationships and overall adjustment. Effective coping strategies play a significant role in preventing families from becoming overwhelmed. [ 61 ] Factors such as social support, socioeconomic status, family attitudes, the personality and developmental stage of the child, and other variables can influence coping mechanisms.
Public health education focused on the nature of the disease can enhance coping, improve interpersonal relationships, and positively impact families and society. [ 61 , 62 ] Integrating psycho-educational interventions and psychosocial programs into comprehensive clinical management can provide vital support for SCA patients and their families. [ 63 ] This approach can have reciprocal effects, improving coping in spouses and positively influencing the well-being of SCA patients. [ 63 ]
Healthcare professionals can improve the well-being of individuals with SCA and their families by understanding and addressing family dynamics. Further research and evidence-based interventions are needed to alleviate burdens and promote positive outcomes.
4.6. Interpersonal relationship between individuals with sickle cell anemia and peers
The knowledge and understanding of peers regarding SCA significantly influence the relationships with SCA individuals. [ 64 ] This understanding plays a pivotal role in determining the support and acceptance received by individuals with SCA. [ 64 ] Interestingly, patients with fewer hospitalizations tend to have more positive peer relationships. [ 64 ] Conversely, frequent hospitalizations can lead to a reluctance to form connections, resulting in feelings of isolation and potentially triggering mood disorders. [ 62 ] Regrettably, individuals with SCA often face an elevated risk of bullying and problematic peer relationships, including verbal and physical abuse. [ 50 , 65 , 66 ] This risk is particularly pronounced for males with SCA, who may encounter more aggressive behavior from their non-affected peers. [ 50 ] They may become targets due to their smaller size or be unfairly labeled as lazy when experiencing fatigue. These challenging experiences frequently contribute to social isolation and limited interactions with peers. [ 50 ] As a protective meil puttychanism, individuals with SCA may adapt their behavior to avoid confrontation and potential abuse. [ 50 ]
Promoting understanding among peers is crucial to improving the experiences of individuals with SCA. Increasing awareness and empathy can foster positive relationships and reduce the risk of bullying. Providing support and resources can also contribute to their overall well-being. Further research is needed to develop effective strategies for addressing these issues and promoting positive peer relationships.
4.7. Other psychosocial issues
SCA can significantly impact a person self-image, including how they perceive their body and personality. [ 67 ] This can cause poor body image and emotional problems, which can affect academic performance and achievement in children and adolescents with SCA. [ 65 , 66 , 68 ]
Studies have shown that SCA patients experience higher levels of body dissatisfaction, which correlates with increased stress, interpersonal distrust, and feelings of ineffectiveness. [ 69 , 70 ] Stigmatization is also a common experience for individuals with SCA. In England, 75% of respondents reported stigmatizing incidents, such as being labeled lazy when experiencing fatigue. [ 71 , 72 ] Similar findings were observed in a study conducted in South-West Nigeria, where 70% of subjects reported moderate to high perceived stigma. [ 15 ] Stigmatization can arise from various factors, including using opioids for pain relief, race (being Black), delayed growth/puberty, socioeconomic status, and disease severity. [ 71 ] It can come from unexpected sources such as health institutions, healthcare professionals, family, friends, and society. [ 71 ] The experience of stigma in SCA has been associated with impaired sexuality, higher levels of perceived stress and pain, maladaptive coping, more emergency room visits, poor treatment adherence, and depressive symptoms. [ 73 – 77 ]
Functional impairment is another complication in managing SCA. Neurocognitive deficits have been observed in children and adults with SCA, such as lower intelligence quotient, visuomotor and executive dysfunction, poor working memory, attention and planning difficulties, slower processing speed, language impairments, and deficits in prosodic cues. [ 78 – 81 ] Factors contributing to these deficits include stroke or silent infarcts, anemia severity, malnutrition, cerebral ischemia, and psychosocial factors such as low socioeconomic status, frequent hospitalizations, and family stress. [ 79 , 81 ]
Workplace discrimination is a common experience for SCA patients, with more than half of adult patients being unemployed. [ 82 , 83 ] Siblings of SCA patients without the disease have a significantly higher employability rate than those with the disorder. [ 82 , 84 ] Frequent hospitalizations and educational disruptions can lead to academic under-attainment and job under-qualification. [ 83 , 84 ] For those who secure employment, periodic crises and absenteeism can impede job performance and security, and some SCA patients even report being fired due to discrimination by employers. [ 82 , 83 , 85 ]
Overall, SCA patients generally have a lower QOL, with medical complications, stigmatization, and frequent hospitalizations negatively impacting their health-related QOL. [ 15 ] Studies consistently show poorer QOL in SCA patients than the general population, with significant impairments in physical functioning, emotional roles, social functioning, bodily pain, vitality, and public health perception. [ 15 , 86 , 87 ] To improve the QOL for those with SCA, we need targeted interventions that combat stigma, promote positive self-image, enhance cognitive functioning, and facilitate inclusive and supportive workplaces. Further research is required to develop effective policies and interventions to address the multifaceted impact of SCA on individuals’ lives.
5. Conclusion
SCA is a significant burden, particularly among the black population. Efforts to increase awareness and promote premarital screening are ongoing. However, comprehensive management plans that address the psychosocial challenges associated with the disease are needed. This paper highlights the prevalence of depression, anxiety, substance abuse, sleep disturbances, body dissatisfaction, and peer bullying among individuals with SCA. These challenges, including painful crises, stigma, and economic difficulties, significantly impact treatment response, healthcare costs, and overall QOL. Targeted interventions and holistic care are necessary to address the mental health impact and improve well-being (Table (Table2). 2 ). By recognizing and addressing the psychosocial burden of SCA, we can enhance the QOL and provide comprehensive support. This involves implementing interventions for mental well-being, coping strategies, and addressing unique disease challenges. Holistic care can alleviate psychosocial difficulties and improve the QOL for SCA patients.
6. Recommendation
Comprehensive and holistic care for individuals with SCA includes early identification and intervention, comprehensive support programs, patient and family education, enhanced pain management, integration of mental health into clinical care, school-based support, research and advocacy, community support groups, and continuous improvement (Table (Table2). 2 ). Routine mental health screening should be implemented as part of standard care to identify psychosocial issues early. Comprehensive support programs should be integrated into clinical care, including mental health counseling, peer support groups, and educational workshops on coping strategies, pain management, and disease understanding. A multidimensional approach to pain management should be taken, utilizing non-opioid analgesics, physical therapies, and cognitive-behavioral interventions. Collaboration with schools is necessary to support students with SCA, implementing accommodations and fostering an inclusive school environment. Government investment in research is crucial for understanding the psychosocial burden of SCA and identifying effective interventions while advocating for increased funding and resources. Community engagement efforts should focus on reducing stigma and improving support networks, including establishing community-based support groups. Continuous assessment and evaluation and patient and family feedback will improve support services.
Author contributions
Conceptualization: Emmanuel Aniekan Essien, Faithful Miebaka Daniel.
Data curation: Emmanuel Aniekan Essien, Blessing F. Winter-Eteng, Chinyere Uchechi Onukogu, Dominic Dennis Nkangha, Faithful Miebaka Daniel.
Methodology: Emmanuel Aniekan Essien, Faithful Miebaka Daniel.
Project administration: Emmanuel Aniekan Essien, Faithful Miebaka Daniel.
Resources: Emmanuel Aniekan Essien, Blessing F. Winter-Eteng, Faithful Miebaka Daniel.
Supervision: Emmanuel Aniekan Essien, Faithful Miebaka Daniel.
Validation: Emmanuel Aniekan Essien, Faithful Miebaka Daniel.
Visualization: Faithful Miebaka Daniel.
Writing – original draft: Emmanuel Aniekan Essien, Blessing F. Winter-Eteng, Chinyere Uchechi Onukogu, Dominic Dennis Nkangha, Faithful Miebaka Daniel.
Writing – review & editing: Emmanuel Aniekan Essien, Blessing F. Winter-Eteng, Chinyere Uchechi Onukogu, Dominic Dennis Nkangha, Faithful Miebaka Daniel.
Abbreviations:
The authors have no funding and conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
How to cite this article: Essien EA, Winter-Eteng BF, Onukogu CU, Nkangha DD, Daniel FM. Psychosocial challenges of persons with sickle cell anemia: A narrative review. Medicine 2023;102:47 (e36147).
Subscribe Now! Get features like

- Latest News

- Entertainment
- Real Estate
- T20 World Cup 2024
- Weather Today Live
- Crickit Predictor
- World Cup Schedule 2024
- World Cup Most Wickets
- World Cup Points Table
- Budget 2024
- The Interview
- Web Stories
- Virat Kohli
- Mumbai News
- Bengaluru News
- Daily Digest
- Election Schedule 2024

World Sickle Cell Day 2024: Date, theme, history, significance and all you need to know
World sickle cell day is an annual observance held on june 19 to raise awareness about sickle cell disease (scd). from date to theme, check all details inside..
Sickle cell disease (SCD) continues to be a significant yet often overlooked global health crisis, affecting millions worldwide. Annually, countries around the world observe World Sickle Cell Day to raise awareness about this condition. This international awareness day aims to enhance public knowledge and understanding of sickle cell disease , as well as the challenges faced by patients, their families, and caregivers. On this day, various global and local organisations unite to promote awareness campaigns and activities that emphasise the importance of early diagnosis, effective treatment, and preventive measures to manage the disease. From date to history, scroll down to know more about this day. (Also read: Pradosh Vrat in June 2024: Date, shubh muhurat, history, significance and all you need to know )

World Sickle Cell Day 2024: Date and Theme
Every year on June 19, World Sickle Cell Day is observed, and this year it falls on Wednesday. The theme for this year's observance is "Hope Through Progress: Advancing Sickle Cell Care Globally." This theme underscores the importance of unity, collective voices, and heightened awareness to reduce stigma and bring about meaningful change for the millions affected by sickle cell disease.
History of World Sickle Cell Day
Recognised by the United Nations (UN), World Sickle Cell Day aims to raise global awareness of sickle cell disease. A resolution adopted by the United Nations General Assembly on December 22, 2008, recognised sickle cell disease as a public health problem and "one of the world's most important genetic diseases". The resolution urges Member States to promote sickle cell disease awareness nationally and internationally on June 19 each year.
World Sickle Cell Day Significance
World Sickle Cell Day is a significant occasion as it raises awareness by educating the public about sickle cell disease (SCD), its symptoms and the challenges faced by patients, as the disease can be relatively unknown outside affected communities. It also provides a platform for advocacy, enabling SCD organisations and patient advocates to lobby for increased research funding, improved access to healthcare and better treatment options. In addition, it promotes community building by bringing together patients, families, healthcare professionals and researchers to share experiences, support each other and celebrate advances in research and treatment.
What is sickle cell disease?
Sickle cell disease is a genetic blood disorder caused by a mutation in the hemoglobin gene, leading to the production of abnormal hemoglobin S. This results in rigid, crescent-shaped red blood cells that can obstruct blood flow. Symptoms include severe pain episodes (sickle cell crises), anemia, fatigue, swelling in hands and feet, frequent infections, and delayed growth in children. Treatment focuses on managing symptoms and preventing complications, including pain relief medications, blood transfusions, and hydroxyurea to reduce crises. In severe cases, a bone marrow transplant may offer a cure.
Join Hindustan Times
Create free account and unlock exciting features like.

- Terms of use
- Privacy policy
- Weather Today
- HT Newsletters
- Subscription
- Print Ad Rates
- Code of Ethics
- Live Cricket Score
- India Squad
- T20 World Cup Schedule
- Cricket Teams
- Cricket Players
- ICC Rankings
- Cricket Schedule
- Points Table
- T20 World Cup Australia Squad
- Pakistan Squad
- T20 World Cup England Squad
- India T20 World Cup Squad Live
- T20 World Cup Most Wickets
- T20 World Cup New Zealand Squad
- Other Cities
- Income Tax Calculator
- Petrol Prices
- Diesel Prices
- Silver Rate
- Relationships
- Art and Culture
- Taylor Swift: A Primer
- Telugu Cinema
- Tamil Cinema
- Board Exams
- Exam Results
- Competitive Exams
- BBA Colleges
- Engineering Colleges
- Medical Colleges
- BCA Colleges
- Medical Exams
- Engineering Exams
- Horoscope 2024
- Festive Calendar 2024
- Compatibility Calculator
- The Economist Articles
- Lok Sabha States
- Lok Sabha Parties
- Lok Sabha Candidates
- Explainer Video
- On The Record
- Vikram Chandra Daily Wrap
- EPL 2023-24
- ISL 2023-24
- Asian Games 2023
- Public Health
- Economic Policy
- International Affairs
- Climate Change
- Gender Equality
- future tech
- Daily Sudoku
- Daily Crossword
- Daily Word Jumble
- HT Friday Finance
- Explore Hindustan Times
- Privacy Policy
- Terms of Use
- Subscription - Terms of Use

IMAGES
VIDEO
COMMENTS
The National Institutes of Health (NIH) has supported research on sickle cell disease since before the NHLBI was founded in 1948. With each decade that followed, the NHLBI has kept a sustained focus on advancing the understanding of sickle cell disease and improving clinical care. We lead and support research and programs on sickle cell disease ...
Sickle cell disease is an autosomal recessive blood disorder that can lead to anaemia. It is caused by a mutation in the haemoglobin gene, which leads to deformation of red blood cells. Deformed ...
Sickle Cell Research Priorities. ASH has developed the following list of sickle cell disease (SCD) research priorities for the next five years. This list includes unaddressed questions and specific research topics that could move the field forward with the hope of curing SCD in the future. The priorities are not listed in rank order.
A national health education program that aims to bring greater visibility to blood diseases and disorders like anemia, sickle cell disease and others, their diagnosis, treatment and management, and blood safety by translating research for patients and professionals.
Introduction. Sickle cell disease (SCD) is an inherited blood disorder that first appeared in the Western literature in 1910 when Dr. James Herrick described a case of severe malaise and anemia in a 20-year-old dental student from Grenada (Herrick, 1910).On examining his blood smear, he noticed many bizarrely shaped red blood cells, leading him to surmise that "…the cause of the disease ...
Introduction. Sickle cell disease (SCD), a group of inherited hemoglobinopathies characterized by mutations that affect the β-globin chain of hemoglobin, affects approximately 100,000 people in the USA and more than 3 million people worldwide [1, 2].SCD is characterized by chronic hemolytic anemia, severe acute and chronic pain as well as end-organ damage that occurs across the lifespan.
In considering what needs to be achieved to reduce the global burden of sickle cell disease and improve the quality of life of patients, this Commission focuses on five key areas: the epidemiology of sickle cell disease (Section 1); screening and prevention (Section 2); established and emerging treatments for the management of the disease (Section 3); cellular therapies with curative potential ...
Sickle cell anemia is an inherited disorder of the globin chains that causes hemolysis and chronic organ damage. Sickle cell anemia is the most common form of sickle cell disease (SCD), with a lifelong affliction of hemolytic anemia requiring blood transfusions, pain crises, and organ damage. Since the first description of the irregular sickle ...
The NHLBI has researched sickle cell disease since its founding as the National Heart Institute in 1948. Since 1972, when the National Sickle Cell Anemia Control act was passed, the NHLBI has spent more than $1 billion researching the condition. The NHLBI funds basic research and large clinical trials and conducts scientific workshops and ...
Sickle cell anemia is caused by a change in the gene that tells the body to make hemoglobin. Hemoglobin is the iron-rich compound in red blood cells that allows these cells to carry oxygen from the lungs to the rest of the body. The hemoglobin associated with sickle cell anemia causes red blood cells to become rigid, sticky and misshapen.
Sickle cell disease is an increasing global health problem. Estimates suggest that every year approximately 300,000 infants are born with sickle cell anemia, which is defined as homozygosity for ...
Knowledge of respondents on sickle cell disease. From Table 2 below, the vast majority (91.2%) of the respondents had ever heard of SCD with the highest proportion (38.7%) of the respondents hearing of SCD from friends and family. Close to half of the respondents (48%) knew that SCD is inherited, however a large proportion (44.2%) did not know the cause of SCD.
Introduction. Sickle cell disease (SCD) can trace its first description in the Western literature to a case report in 1910 by Herrick 1 of a young dental male student from Grenada with severe malaise and anemia. Hallmarks of the disease were noted then: "healing ulcers" predominantly on the legs that lasted about a year; anemia with a "hemoglobin (Dare) 40 per cent" and jaundice ...
Among young children, adolescents and adult women, anemia strikes 1 in 3 globally. Most cases are driven by dietary iron deficiency, red blood cell disorders and untreated tropical diseases ...
Subjects: Despite a long history of knowing the genetic cause of sickle cell disease (SCD), progress in developing treatments to prevent painful vaso-occlusive crises and the other myriad of associated symptoms has, until recently, been disappointingly slow. As long ago as 1949, Pauling et al described sickle cell anemia as a molecular disease ...
Sickle cell disease (SCD) affects about 100,000 people in the United States; more than 90% are non-Hispanic Black or African American, and an estimated 3%-9% are Hispanic or Latino. The estimated life expectancy of those with SCD in the United States is more than 20 years shorter than the average expected. Many people with SCD do not receive ...
1. Introduction. Sickle cell disease (SCD), an inherited red blood cell disorder, is associated with numerous complications that result in increased morbidity and mortality, and estimated annual medical costs exceeding $1.1 billion. 1-4 Acute vaso-occlusive pain episodes are the main complication of SCD and the most common reason for healthcare encounters. 5 However, some adults with SCD ...
Researchers study a new way to treat sickle cell disease. Activating a protein in red blood cells may improve anemia and alleviate acute episodes of severe pain for people living with sickle cell disease. Swee Lay Thein, M.B., D.Sc., a senior investigator and chief of NHLBI's Sickle Cell Branch, shares insight into a decade-long research ...
Sickle cell disease (SCD) is a group of inherited disorders caused by mutations in HBB, which encodes haemoglobin subunit β. The incidence is estimated to be between 300,000 and 400,000 neonates ...
In low- and middle-income countries, anemia reduction efforts are often touted as a way to improve educational outcomes and reduce poverty. A new study, co-authored by a global health economics ...
In the United States, between 1 million to 3 million people carry the sickle cell trait, while approximately 100,000 individuals have sickle cell disease. This trait and disease are more common ...
Sickle cell disease (SCD), a distinctive and often overlooked illness in the 21st century, is a congenital blood disorder characterized by considerable phenotypic diversity. It comprises a group of disorders, with sickle cell anemia (SCA) being the most prevalent and serious genotype. Although there …
Since 1962, the research and care of sickle cell disease has been a priority for St. Jude: the hospital's first research grant was to study the chronic illness. St. Jude is one of the only institutions equipped to provide care for children with sickle cell disease in the Memphis region.
Over a lifetime, sickle cell disease can harm a patient's spleen, brain, eyes, lungs, liver, heart, kidneys, penis, joints, bones, or skin. Sickle cell disease is a life-long illness, but the severity of the disease varies widely from person to person. In the early 1970s, the average lifespan was only 14 years.
"Our goal is to make Sickle Cell Disease as common a topic as COVID or HIV and to inspire action towards a future where Sickle Cell Disease is better understood, better treated, and ultimately ...
Because there is an FDA-approved drug - crizanlizumab - used to help alleviate pain by unclogging congested small blood vessels and preventing blood cells from sticking to the vessel walls in sickle cell anemia patients, co-senior author Andria L. Ford, MD, a professor of neurology and of radiology, launched a clinical trial to test the ...
Sickle cell disease (SCD) is a group of inherited blood disorders caused by a mutation in the beta subunit of hemoglobin. SCD will hereafter be referred to as Sickle cell anemia (SCA) as this is the term our patients and their families prefer. There are approximately 5000 Canadians living with SCA . The mutated B subunit causes polymerization ...
Learn about the history of sickle cell disease in the United States, from its discovery in 1910 to the NHLBI legacy of research that has advanced the understanding of sickle cell disease, improved clinical progress, and paved the way for a cure for all patients. Publication Date: September 2018. Language: English. Audience: Health Professionals.
Sickle cell anemia (SCA) is a severe form of sickle cell disease that primarily affects black populations and individuals in tropical countries. This condition causes significant morbidity and mortality and leads to a range of psychosocial challenges. A preliminary search was conducted on Ovid Medline and public databases with a combination of ...
Sickle cell disease is a genetic blood disorder caused by a mutation in the hemoglobin gene, leading to the production of abnormal hemoglobin S. This results in rigid, crescent-shaped red blood ...