Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 May 2021

Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources
- Manyou Yu 1 ,
- Irene Gouvinhas 1 ,
- João Rocha 2 &
- Ana I. R. N. A. Barros 1 , 3
Scientific Reports volume 11 , Article number: 10041 ( 2021 ) Cite this article
28k Accesses
141 Citations
1 Altmetric
Metrics details
- Biochemistry
Plants with medicinal properties play an increasingly important role in food and pharmaceutical industries for their functions on disease prevention and treatment. This study characterizes the phenolic composition and antioxidant activity of seven medicinal and food plants, including the leaves of Salvia officinalis L., Rosmarinus officinalis L., Olea europaea L., and Punica granatum L., as well as the leaves and young stems of Ruta graveolens L., Mentha piperita L., and Petroselinum crispum , Mill., by using colorimetric, chromatographic, and spectrophotometric assays. Results revealed that the hydro-methanolic leaf extracts of P. granatum (pomegranate) displayed the highest content of total phenols (199.26 mg gallic acid per gram of plant dry weight), ortho -diphenols (391.76 mg gallic acid per gram of plant dry weight), and tannins (99.20 mg epicatechin per gram of plant dry weight), besides a higher content of flavonoids (24 mg catechin per gram of plant dry weight). The highest antioxidant capacity measured by ABTS, DPPH, and FRAP (2.14, 2.27, and 2.33 mM Trolox per gram of plant dry weight, respectively) methods was also obtained in pomegranate leaf extracts, being 4–200 times higher than the other species. Such potent antioxidant activity of pomegranate leaves can be ascribed to the presence of different types of phenolic compounds and the high content in tannins, whilst phenolic acids and flavonoids were found to be the dominant phenolic classes of the other six plants. Consequently, despite the well-known antioxidant properties of these plant species, our study suggests pomegranate leaf can stand out as a relatively more valuable plant source of natural bioactive molecules for developing novel functional food-pharma ingredients, with potential for not only promoting human health but also improving bio-valorization and environment.
Similar content being viewed by others

Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling
Phytochemical composition and in vitro antioxidant and antimicrobial activities of Bersama abyssinica F. seed extracts

New insights of phenolic compounds from optimized fruit extract of Ficus auriculata
Introduction.
The recent development of functional foods and pharmaceutical products based on medicinal and food (namely fruits and vegetables) plants has brought improvements to all aspects of life, including the alleviation of physical disorders, the reduction in the use of synthetic antibiotics, and the increase in life expectancy 1 , 2 . Indeed, these plants have long been used as safe, effective and sustainable sources of natural antioxidants or free radical scavengers, particularly phenolic compounds, such as phenolic acids, flavonoids, tannins, stilbenes, and anthocyanins 2 . Those phenolics are mostly regarded to confer upon the antioxidant activity of medicinal and food plants, making a marked contribution in the fight against many pathological conditions such as cancer, diabetes, aging, cardiovascular, and other degenerative diseases 2 , 3 , 4 , 5 .
Salvia officinalis L., Rosmarinus officinalis L., and Mentha piperita L. commonly named as sage, rosemary, and peppermint, respectively, belongs to the family of Lamiaceae. They are well-known herbs and spices used in foods for flavors and aromas. Infusions, leaves or essential oils of its each species are reported to possess therapeutics in anti-cancer, anti-microbial, anti-diabetes, and gastrointestinal diseases, etc. 3 , 6 , 7 , 8 . Several bioactivities of sage like antinociceptive, hypolipidemic, and memory-enhancing effects have been demonstrated with clinical trials 7 . Rosmarinic acid is abundant both in sage and rosemary, contributing to their anti-inflammatory properties 3 , 6 , 7 . Flavonoids, phenolic lignans and stilbenes, and essential oils are expected to be responsible for the aroma effects of peppermint 8 .
Rue ( Ruta graveolens L.) has been one of the key plants of the European pharmacopoeia since ancient times for the use in tremors, paralysis, nervine disorders, and joint pain 9 . And nowadays, it becomes medicine in Mediterranean region, due to its prominent biological activities, especially neuroprotection 9 , 10 . Rutin, psoralen, limonene, and pinene are reported as main constituents in this plant extracts or rue oils 9 , 10 .
Olive ( Olea europaea L.) oil is one of the major components of the Mediterranean diets. Recently, phenolics present in olive leaves, especially the oleuropein, are reviewed to be potential economic and renewable source of natural by-products, attributed to its antioxidant, antihypertensive, hypoglycemic, hypocholesterolemic and cardioprotective activity 11 , 12 .
Parsley ( Petroselinum crispum Mill.), used as culinary and medicinal herb, is originated from Mediterranean region. Phytochemicals particularly apigenin, coumarins, myristicin, and apiol are active compounds rich in parsley leaves, exhibiting diverse pharmacological properties, such as cyto-, gastro-, brain-, nephron-protective effects, and so on 13 , 14 , 15 .
Pomegranate ( Punica granutum L.) a deciduous shrub in the family of Lythraceae, is one of the oldest known plants. Both the edible (namely fruit juice) and non-edible parts (including seeds, peels, leaves, roots and bark) of this plant have been evidenced to have a wide range of health benefits, largely resulting from its abundant phenolic acid, flavonoids, tannins, amino acids, and alkaloids 16 , 17 . However, the importance of pomegranate leaves, as agricultural and industrial waste, is of great interest and value to be emphasized by means of describing its beneficial effects and studies performed on this field.
Within the frame, materials from the seven medicinal and food plants aforementioned, that is, leaves and young stems (easy for picking) of rue, peppermint, and parsley, as well as the leaves of sage, rosemary, olive, and pomegranate are outstanding for their higher levels of phenolic contents and antioxidant capacities, along with relatively lower (dose-dependent) or inexistent toxicity 6 , 7 , 8 , 9 , 11 , 13 , 15 , 17 . Therefore, in an attempt to explore plant-based alternative solutions in promoting health, as well as paving the way towards our future pre-clinical and clinical studies, we aimed to analyze the phenolic classes (total phenols, ortho -diphenols, flavonoids, and tannins) and antioxidant activities of different plant species under the same evaluation condition. Furthermore, the principal phenolic constituents were chromatographically characterized to investigate the relationship between the phenolic content and antioxidant activity.
Results and discussion
Phenolic content of tested medicinal and food plants.
Results of colorimetric and spectrophotometric analysis of seven medicinal and food plants were showed in Table 1 . In general, the total phenolic content of the selected plant species was found to be at the highest level in pomegranate leaf extracts at 199.26 mg of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW), followed by three Lamiaceae species, including peppermint (70.06 mg GAE g −1 DW), sage (50.89 mg GAE g −1 DW) and rosemary (48.48 mg GAE g −1 DW). On the contrary, parsley displayed the lowest value of total phenols (6.94 mg GAE g −1 DW). The same trend was observed concerning the content of ortho -diphenols and tannins of all investigated samples, reporting the following sequence: pomegranate > peppermint > sage > rosemary > rue > olive > parsley. The ortho -diphenol and tannin content of the methanolic extracts ranged from 26.40 to 391.76 mg GAE g −1 DW, and from 1.33 to 99.20 mg of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW), respectively. Moreover, results on total flavonoids content showed a different pattern compared to other phenolic classes, with peppermint showing maximum values at 70.14 mg of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW), following with rosemary (49.14 mg CATE g −1 ), sage (43.92 mg CATE g −1 ), and pomegranate (24.34 mg CATE g −1 ). Furthermore, the flavonoid content of olive leaf was higher than that of rue, in contrast to the trend of the other phenolic classes. Rosemary and sage had comparatively high levels of flavonoids, while the minimum values were reported for parsley.
Different phenolic contents of different plant samples have been reported in the literature 12 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 . For instance, the total phenol content of sage and peppermint was 27.94 and 45.25 mg GAE 100 g −1 DW, meanwhile the flavonoid content of them was 27.54 and 25.17 mg catechin per 100 g, which were much lower than that of our results 19 . Parsley extracts had 1.583 GAE mL −1 of total phenols, 0.091 mg catechin mL −1 of flavonoids, and 1.167 mg catechin mL −1 of condensed tannins 26 . Salama et al. 12 described significant differences in the amounts of total phenolics, flavonoids, and tannins of olive leaves, under different extraction solvents, ranging from 42.02 to 85.50 mg GAE g −1 , 31.22 to 105.19 mg quercetin g −1 , and 30.92 to 51.03 mg tannic acid g −1 , respectively. The contents of phenolic and flavonoid compounds in rue were 14.1 GAE g −1 and 15.8 mg rutin g −1 of dry extracts 20 . Some studies 27 , 28 , 29 have evidenced considerably high level of phenolics in pomegranate leaf extracts, up to 328 mg GAE g −1 DW. Interestingly, pomegranate leaves are characterized by carbohydrates, reducing sugars, sterols, saponins, flavonoids, ellagitannins, piperidine alkaloids, flavones, glycosidic compounds, which are the richest source of phytochemicals when considering the non-edible parts of this species, some food products (red wine, green tea, etc.), and another 109 medicinal plants 30 , 31 , 32 . Our results disclosed that tannins were the main phenolic compounds of pomegranate leaf extract, which has also been corroborated by other studies 33 .
As shown in data (Table 1 ), significant differences ( p < 0.001) around 29, 15, 92 and 75 times were observable respectively for total phenols, ortho -diphenols, flavonoids and tannins in the seven plant extracts, indicating that each phenolic classes exhibited considerably different content among the studied plants. This result was in agreement with other authors 34 , who found that depending on the plant species and botanical family, strong differences were found among 10 medicinal herbs and 11 spices. Meanwhile, the same authors 34 observed a wide variance of phenolics in different samples of the same species, such as the total phenolic content of nine independent samples of peppermint was from 18.3 to 284.3 mg GAE g −1 . Moreover, contents of total phenolics, flavonoids, and condensed tannins of 13 different provenances of rosemary, collected in different seasons ranged from 22.46 to 44.57 mg GAE g −1 DW, from 1.49 to 5.01 mg quercetin g −1 , and from 0.81 to 1.71 mg CATE g −1 DW, respectively 18 . Our results showed inconsistency with this observation, probably attributed to the varieties, or geographical differences, as well as to the collection time, agroclimatic conditions and other relevant factors 24 , 25 . However, to some extent, pomegranate leaf was supposed to have a relatively higher phenolic content than many other medicinal plants. Therefore, it can be inferred that pomegranate leaf could be an important valuable source of bioactive compounds for medicinal purposes and health care.
In addition, in the current study, the colorimetric analysis of flavonoids varied between pomegranate leaf (orange-yellowish) with other plants (pink) and the standard (catechin, pink) under the same conditions (as below described in the methods). This visual observation may be related to the fact that leaves from pomegranate have different predominant sub-classes of flavonoids, different from that existing in the other studied plants 32 . So, the methodology, especially to normalize the use of standards such as quercetin or rutin 35 should be modified to accurately quantify the amount of flavonoids.
In vitro antioxidant activity
The in vitro antioxidant activity assays were carried out to assess the capacity of plant extracts to scavenge free radicals including 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid radical cation (ABTS +· ) and 2,2‐di(4‐tert‐octylphenyl)‐1‐picrylhydrazyl radical (DPPH·), as well as the ability to reduce ferric (III) iron to ferrous (II) iron. Overall, Table 1 revealed that all the species displayed high antioxidant capacities, although significant differences were observed ( p < 0.001), ranging from 0.01 to 2.14 mM Trolox per gram of plant dry weight (mM Trolox g −1 ) for ABTS, from 0.01 to 2.27 mM Trolox g −1 for DPPH, and from 0.01 to 2.33 mM Trolox g −1 for FRAP (ferric reducing antioxidant power), with large variation over 210-fold. It was found that pomegranate always exhibited the highest antioxidant properties (2.14–2.33 mM Trolox g −1 ) throughout the three measurements, followed by peppermint (0.35–0.50 mM Trolox g −1 ), sage (0.27–0.40 mM Trolox g −1 ), rosemary (0.27–0.42 mM Trolox g −1 ), rue (0.10–0.16 mM Trolox g −1 ), and olive leaf (0.11–0.15 mM Trolox g −1 ). No significant difference was observed between sage and rosemary, and between rue and olive leaf. However, parsley extracts reported the lowest antioxidant potential (0.01 mM Trolox g −1 ).
Previous data regarding the antioxidant capacities of sage, rosemary, rue, olive leaf, peppermint, parsley, and pomegranate leaf have been reported by several authors 12 , 14 , 18 , 22 , 26 , 31 , 36 . The IC 50 values of ABTS and DPPH radical scavenging activity, as well as the EC 50 values of reducing powder regarding olive leaves ranged from 20.13 to 190.95 µg mL −1 , from 17.97 to 41.64 µg mL −1 , and from 90 to 216 µg mL −1 , arising from diverse extraction solvents 12 . Rosemary leaves displayed 75.04 and 9.08 µg mL −1 of IC 50 by ABTS and DPPH assay, along with 4.12 µM by FRAP method 18 . Farnad et al. 22 reported the methanol-ethanol (1:1) extract of peppermint had the best DPPH radical scavenging ability (10.05 mg mL −1 of IC 50 ) and ferric reducing power (184.22 µmol per 100 g powder). The ethanolic extract of parsley displayed 0.34 mg AAE mL −1 (milligrams of ascorbic acid equivalents per milliliter) of DPPH and 0.942 mg AAE mL −1 of FRAP, which was correlated with the anti-glycation activity of this extract 26 . The best antioxidant capacities conducted by DPPH (17.09% of IC 50 ) and FRAP (458.26 mmol Fe II L −1 ) were determined for sage leaves which were collected in May 36 . Cefali et al. 14 stated the rue extracts exhibited antioxidant potential against DPPH (281.02 µg mL −1 of IC 50 ) and ABTS (587.98 µg mL −1 of IC 50 ) radicals, indicating the premature aging protective effect.
Importantly, several studies in vitro and in vivo have recorded the superior antioxidant capacity of pomegranate leaves by contrast with its non-edible parts, of which leaves are as effective as peels in the anti-bacterial, analgesic, acute and chronic anti-inflammatory effects 37 , 38 , while more potent than flowers, stems, and seeds 31 , 39 , 40 , 41 , 42 . Authors proved the potency of pomegranate leaf was higher than that of flower in the prevention of ethylene glycol-induced nephrolithiasis, in the inhibition of DPPH and hydroxyl radicals, and in the reduction of ferric iron 39 , 40 . Data 41 highlighted leaves worked more effectively than stems and led to the most loss of MMP (mitochondrial membrane permeability) potential, consequently suggested as an anti-cancer and anti-proliferative agent. Elfalleh et al. 31 illustrated the highest reducing power (348.68 µg mL −1 of EC 50 ) occurring in the aqueous extract of pomegranate leaf. Furthermore, a higher antioxidant and enzyme inhibitory activity was exposed in two extracts (methanolic and water) of pomegranate leaves among different fruit tree leaves 28 . The ethanolic extracts of pomegranate leaf also exhibited remarkable antioxidant and anti-glycation ability of twenty edible and medicinal plants 29 . The level of anti-radical and ferric reducing properties of pomegranate leaves in our results was similar to some authors 42 . However, comprehensively comparative research involving in the phytochemical and antioxidant properties between pomegranate leaves and other numerous medicinal plants is still scarce; Widely practical application of pomegranate leaf hasn’t come into being, although different biological activities of this material extracts are studied increasingly. Many authors have deeply reviewed for sage, rosemary, peppermint, rue, parsley, and olive leaf. Thus it is worth stressing on the brilliant phenolics and antioxidant property of pomegranate leaves, and developing high added-value products from these materials in the food, pharmaceutical, or even nutraceutical and cosmeceutical industries.
Chromatographic analysis of phenolic compounds
With the development of chromatographic techniques, the phenolic chemistry of many plants has been explored and analyzed to a certain degree, providing us important reference data. To obtain a more complete picture of the quality and quantity of phenolic constituents in the selected plants, 64 phenolic compounds were identified (Table 2 ), of which 59 were quantified with authentic standards relying on RP-HPLC-DAD, as well as by comparison with the literature (retention time, UV/Visible λ max , and spectra). Concentrations of identified phenolics were expressed as milligram per gram dry weight of plant (mg g −1 ).
As shown in Table 2 and Fig. S1 , phenolic profile of diverse plants was significantly different. Leaf extracts of both sage and rosemary were characterized by a high proportion of rosmarinic acid (4.61 mg g −1 or 4.31 mg g −1 , respectively). Rue presented the highest content of rutin (26.10 mg g −1 ), followed by epicatechin gallate (7.82 mg g −1 ). The major phenolic components in olive leaves were oleuropein and its derivatives. Flavanones, especially eriodictyol glycosides, following rutin were found as predominant in the leaf and stem extracts of peppermint. Parsley was described in high amount of apigenin-7- O -apiosylglucoside also called apiin (4.04 mg g −1 ) and epicatechin (3.72 mg g −1 ) in its leaf and stem extracts. The principal phenolic constituents in pomegranate leaves were hydrolyzable tannins, particularly ellagitannin I (56.06 mg g −1 ) and ellagitannin II (45.16 mg g −1 ), ranking the highest concentrations among all identified compounds.
On the other hand, results from Table 2 and Fig. S1 also showed that the most abundant phenolic classes in the tested samples were phenolic acids, flavonoids, tannins, and phenylethanoids. A considerable variation of phenolics was found, ranging, for instance, from 0.03 mg g −1 of 2,3-hydrocybenzoic acid to 56.06 mg g −1 of ellagitannin I. For each identified compound, significant differences were observed ( p < 0.05), such as gallocatechin. The most widespread phenolic acids present in the studied samples included hydroxybenzoic acids (gallic acid and its derivative, vanillic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, and neochlorogenic acid), and their ester derivatives (e.g. rosmarinic acid). Significantly high contents of chlorogenic acid (1.54 mg g −1 ) and neochlorogenic acid (1.96 mg g −1 ), and the presence of coumaric acid were perceptible in rue extracts. Ellagic acid and its derivatives were abundant in pomegranate leaves. Except in sage and rosemary, rosmarinic acid was also found in peppermint (0.23 mg g −1 ), but its concentration was lower than that in literature 43 . The special existence of rosmarinic acid, rosmanol, epirosmanol, carnosol, and carnosic acid in sage and rosemary was consistent with other authors 23 , 25 , 44 , 45 .
Besides flavanols including gallocatechin, catechin and epicatechin gallate, then various flavones (luteolin and apigenin) and flavonols (quercetin and diosmetin), mainly in the forms of their derivatives were widely distributed in the most of the studied species. Among them, the highest content of gallocatechin (2.10 mg g −1 ), catechin (3.61 mg g −1 ) and epicatechin gallate (7.82 mg g −1 ) was detected in parsley, rosemary, and rue, respectively. Epicatechin was only found in parsley with a good quantity (3.72 mg g −1 ). Furthermore, the main flavonoids from our data present in sage, rosemary, rue, peppermint, and parsley were apigenin glycosides, luteolin glycosides, quercetin glycosides, flavanone glycosides, and apigenin glycosides, respectively. In addition, peppermint also had comparative amounts of luteolin and quercetin glycosides. Likewise, pomegranate leaves possessed several apigenin and luteolin glycosides. Particularly, rutin presented the highest proportion (26.10 mg g −1 ) in rue, followed by peppermint (9.90 mg g −1 ), while the lowest (0.86 mg g −1 ) in olive leaf.
An important observation is that pomegranate leaf extracts held the greatest number of hydrolyzable tannins, especially ellagitannins. Nevertheless, no ellagitannins were detected by the HPLC method in the other six plants, while condensed tannins were present by the spectrophotometric approach. This was possibly caused by the lack of authentic standards involving different tannins, which need to be performed in the chromatographic analysis. In practice, certain studies have reported the tannins present in sage, rosemary, peppermint, rue, parsley, and olive leaves 12 , 18 , 19 , 26 , 46 , 47 , 48 , mainly in the form of condensed tannins.
In some cases, phenylethanoids, which are phenethyl alcohol-structured phenolic antioxidants, were abundantly found in olive leaves, including oleuropein and its derivatives, followed by tyrosol and verbascoside. These molecules may conduct to its high antioxidant properties 11 , 49 . Tyrosol existed in highest concentration in rosemary (4.56 mg g −1 ), but in small quantity in olive leaf (0.75 mg g −1 ) and rue (0.19 mg g −1 ).
Many studies have described the domination of rosmarinic acid in sage and rosemary, detected in varied amounts depending on phenophase, genotypes, extraction methods, and geographical conditions 23 , 24 , 25 , 36 , 44 , 45 , 50 . A concentration ranging from 0.27 to 2.49% of rosmarinic acid was determined in rosemary leaf extract, according to regions 44 . Khaleel et al. 45 reported 4.5 µg mL −1 of rosmarinic acid in aqueous extract of rosemary, whereas 17.3 µg mL −1 was measured in our methanolic extract of this plant. Exceptionally high content of rosmarinic acid was found in May extract (19.375 mg L −1 ) of sage leaves described by Generalić et al. 36 , very close to our data (18.653 mg L −1 ). Roby et al. 23 declared that the predominant phenolic compounds in sage methanolic extract were ferulic acid (18%), rosmarinic acid (17%) and apigenin (14%) of the total extracted phenols, while in our results, rosmarinic acid and apigenin glycoside III were primary and accounted for 9% and 13% of the total phenolics of sage. Among more than one-hundred active ingredients of rue, rutin, as one of its major compounds, has been a topic of interest for researchers 9 , 20 . Asgharian et al. 20 detected a high level of rutin (40.15 mg g −1 ) by extraction with 70% ethanol, which was higher than that of our study. Melnyk et al. 46 identified rutin as the highest content of phenolics in the rue methanolic extract, consistent with the present work. Several studies 48 , 49 have reported oleuropein and its derivatives as the dominant phenolics in the olive leaf, according with our results. As shown in data (Table 2 and Fig. S1 ), up to 20 phenolic compounds were identified in methanolic extract of olive leaf, more than those identified in other six plants, evidencing it as a rich source of bioactive compounds. However, the composition of olive leaf shows a remarkable variability due to location, climatic-seasonal factors, and cultivation practices, suggesting a trend to understand the factors that control the composition of olive leaves. This can be worthy for the harvesting and production of suitable extracts to be applied in human health. Kapp et al. 43 demonstrated eriocitrin, as a powerful bioactive compound, was the most abundant phenolics in peppermint, in accord with our records, composed of 38% of its aqueous extract, or reaching from 19.9 to 68.1% in 26 peppermint tea samples, respectively. However, the same authors 43 reported that rosmarinic acid accounted for a highest proportion (54.2%) of phenolics in one peppermint tea sample which was originated from Estonia. Additionally, other authors 22 , 47 , 51 also pointed out different dominant phenolics in peppermint, such as epicatechin, naringenin, caffeic acid, chlorogenic acid, 4-hydroxybenzoic acid, which can be attributed to diverse varieties, growing environment, and extraction conditions. The main finding of the present work performed on parsley corresponded to several studies 15 , 52 that apiin extractability was maximum when the solvent was ethanol, methanol or acetone. Yet Hozayen et al. 53 and Aissani et al. 21 conducted rosmarinic acid and quinic acid as the most abounded constituent in aqueous and methanol extracts of parsley, respectively. Fourteen phenolic constituents (Figure S1 ) of pomegranate leaf extracts were preliminarily identified and quantified by reference to chromatographic parameters and the literature. These results are agreeable to other researchers 33 , 54 , 55 , 56 , highlighting that ellagic acid and its derivatives, ellagitannins (punicalin, granatin A and B, etc.), flavone (apigenin, luteolin) and its glycosides, and flavonol (kaempferol) and its glycosides, are the principal phenolics in pomegranate leaves. In addition, many ellagitannins (such as punicalagins, punicafolin, castalagin, corilagin, strictinin, tercatain, brevifolin), and their galloyl and/or hexahydroxydiphenoyl (HHDP) substitutions, have been isolated from the leaf 57 . Other flavonoid derivatives like kaempferol, gossypin, quercetin, and rutin were also detected as major constituents in hydro-methanolic or hydro-ethanolic leaf extracts of pomegranate leaves 33 , 57 . However, the detailed structures of tannins and flavonoids of pomegranate leaf will require further identification by mass spectrometry and nuclear magnetic resonance spectroscopy.
Correlation analysis
In order to better understand the relationship between the antioxidant activity (by ABTS, DPPH, FRAP assays) and the phenolic composition (total phenols, ortho -diphenols, flavonoids, tannins) of the studied plants, correlation coefficients ( r ) were determined (Fig. 1 ). Strong relationships were characterized between antioxidant capacities with total phenols and ortho -diphenols (Fig. 1 a,b), indicating that phenolic compounds contribute to the inhibition of oxidative processes. The content of tannins was well correlated with antioxidant potential (Fig. 1 d). No correlation of antioxidant activities was found with flavonoid content (Fig. 1 c). However, a better relationship of flavonoids (Fig. 1 e) or tannins (Fig. 1 f) can be obtained with the antioxidant activity if excluding pomegranate or peppermint from the data, respectively. The above analysis demonstrated that the antioxidant potential from different plants was dependent on both the concentrations and the structures of phenolic compounds, in line with Cai et al. 30 . Compared to radical scavenging assays (ABTS and DPPH), the stronger correlation between reducing power and phenolic contents confirmed that FRAP was more closely related to total phenols, ortho -diphenols and tannins, which was also mentioned by Li et al. 1 .
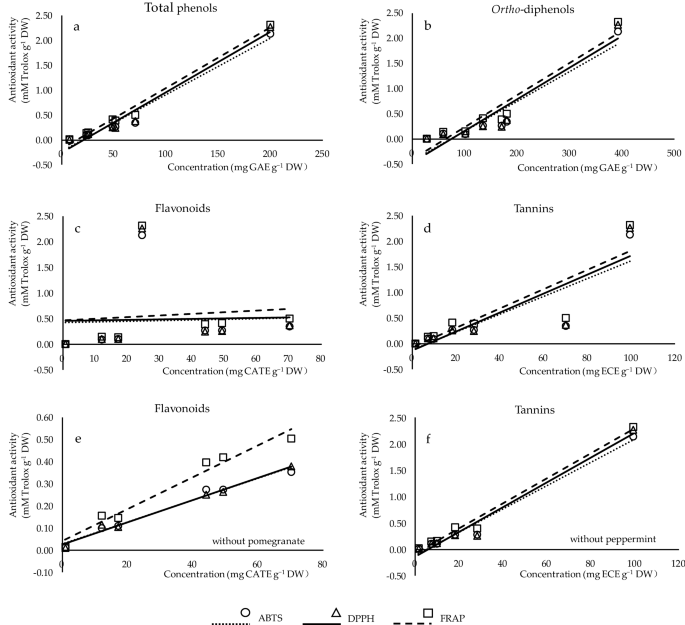
Correlation analysis between the contents of phenolic classes (x-axis) and antioxidant capacities (y-axis) measured by ABTS (circles), DPPH (triangles), and FRAP (squares). ( a – d ) The correlation of total phenols ( r ABTS , DPPH , FRAP = 0.985***, 0.984***, 0.993***), ortho -diphenols ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**), flavonoids ( r ABTS , DPPH , FRAP = 0.038, 0.031, 0.098), and tannins ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**) of the studied plants with their antioxidant activity, respectively. ( e ) The correlation of flavonoids ( r ABTS , DPPH , FRAP = 0.989***, 0.992***, 0.983***) of studied plants excluding pomegranate with their antioxidant activity. ( f ) The correlation of tannins of studied plants excluding peppermint ( r ABTS , DPPH , FRAP = 0.989***, 0.987***, 0.993***) with their antioxidant activity.
There is a highly correlation between the phenolic composition and antioxidant properties of plants. High anti-radical activity of rosemary leaf in summer was strongly related to high amounts of total phenols, total flavonoids, condensed tannins, and carnosic acid 18 . It is suggested that intraperitoneal of hydroalcoholic extract of rue increased serum and brain antioxidant capacity, due to their potent antioxidant activities of total phenolic and flavonoids content, especially rutin, caffeic acid, and apigenin 20 . Parsley methanolic extract inhibited human glioblastima cancer and oxidative stress owing to its antioxidant properties primarily related to phenolic content 21 . Peppermint extracted by various alcoholic solvents are found to have different levels of antioxidant potential, attributed to the presence of vast flavonoids, anthocyanins, and total phenols 22 . The strong reducing power, free radical scavenging capacity, and the inhibition of hydro-peroxide radicals activity of sage leaves can be linked to the high quantity of phenolic acids, especially rosmarinic acid, and certain flavonoids like catechins and flavanols 36 . Makowska-Wąs et al. 49 revealed considerable antioxidant and cytotoxic properties of olive leaf against several human cancers, largely concerned in the existence of phenolic acids, flavonoids, oleuropein, fatty acids, and volatile oils. The high concentration of phenolic components in pomegranate leaf extracts such as tannins, flavonoids, phyto-steroids, terpenoids, and saponins can be responsible for its high antioxidant activity in vitro and in vivo 27 , 28 , 29 , 32 , 58 .
To date, amount of studies have reported the close relationship not only between the phenolic contents but also between the phenolic structures and the antioxidant capacities 28 , 30 , 59 . The level of antioxidant potential of plants mainly depends on the presence and hydroxyl groups of (poly)phenolic compounds. Specifically, the antioxidant ability of phenolic acids is firstly related to the number and position of phenolic hydroxyls, and secondly to the methoxy and carboxylic acid groups 59 . Rosmarinic acid which was mainly detected in sage, rosemary, and peppermint in our work, is an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid, comprising two catechol moieties, thus having two pairs of ortho hydroxyl groups grafted on two phenolic rings 18 . Gallic and chlorogenic acid are well-known antioxidant agents, due to three and two active hydroxyl groups on the aromatic ring, respectively 59 . Moreover, the catechol structure in the B-ring, the 2,3-double bond conjugated to a 4-oxo functionality, and the available of both 3- and 5-hydroxyl groups of flavonoids are essential for assessing their antioxidant properties 28 . Rutin is a rutinoside of quercetin with one of the four hydroxyl groups at position C-3 substituted with glucose and rhamnose sugar groups 20 . Apiin or eriocitrin is a apigenin or eriodictyol glycoside, on which the different glycoside moiety is located at position C-7 via a glycosidic linkage along with two or three residual hydroxyl groups on the phenolic rings 15 , 43 . Furthermore, phenylethanoids are characterized by a phenethyl alcohol (C6–C2) moiety attached to a β-glucopyranose/β-allopyranose via a glycosidic bond. Studies indicated the ortho -dihydroxyphenyl groups were the most significant, and the steric hindrance, the number and the position of phenolic hydroxyls were also thought to play an important role 60 . Oleuropein with two hydroxyl groups is an ester of elenolic acid and hydroxytyrosol, and has a oleosidic skeleton that is common to the secoiridoid glucosides of Oleaceae 49 . The strong correlation of antioxidant property with well-identified phenolic acids, flavonoids, and oleuropein present in sage, rosemary, peppermint, rue, parsley, and olive leaves has been individually demonstrated to explain their diverse biological functions 6 , 7 , 8 , 9 , 11 , 13 . In addition, ellagic acid and tannins, defined as polyphenols, are complex chemical substances, possessing plentiful hydroxyl groups, especially ortho -dihydroxyl or galloyl groups 61 . Bigger tannin molecules appear more galloyl and ortho -dihydroxyl groups, consequently, their activities are stronger 61 . Ellagitannins, ellagic acid, and their metabolites have been reported to exhibit numerous beneficial effects on human health including antioxidant, anti-inflammatory, anti-cancer, prebiotic, and cardio-protective properties 61 . Thus they deserve to be part of a healthy diet as functional foods.
The researches on the structure–activity relationship between phenolics and their antioxidant activities have focused on phenolic acids and flavonoids, as well as oleuropein and its derivatives owing to their partially acknowledged health-promoting effects 2 , 30 . However, the benefits of medicinal and food plants may arise from the action of some less well-studied antioxidant molecules or from a synergy of certain antioxidants 30 . Cai et al. 30 found some anticancer-related medicinal plants contained higher quantities and more sorts of tannins, quinones, phenolic terpenoids and special phenolic glycosides than that of phenolic acids and flavonoids. Regarding pomegranate leaves, some authors detected kaempferol 54 or kaempferol 3- O -glycoside 33 as the main compound in ethanolic extracts, while others found as ellagic acid 55 . The principal ellagitannins of pomegranate leaves also differed from one another, considered as granatin B 56 , or castalagin derivative 33 , or undefined galloy-HHDP derivatives 55 . This difference may be induced by varieties, phenology, and growing conditions. In our study, the potent antioxidant capacity of pomegranate leaves was highly correlated with the content of tannins, which can be considered as the key antioxidant contributors of this plant material. However, the chemical structures of the tentatively identified ellagitannins were not determined, and studies on these constituents are also incomplete. Therefore, it is important to note although this is a preliminary study to provide a baseline of data for future investigations, a major limitation is that identified phyto-constituents were neither isolated, nor separately analyzed for their bioactivities. Moreover, the association between these compounds and antioxidant effect of pomegranate leaf is yet to be well understood. In this regard, it is necessary to further characterize the structure of these less-exploited phenolics (tannins) and their associated biological properties within pomegranate leaf. Hence, the results presented in our study confirm pomegranate leaf as a promising natural alternative in the development of antioxidant products, thereby assisting in the prevention and treatment of some diseases.
Conclusions
The level of different phenolic classes, antioxidant capacities and the phenolic profiles of seven medicinal and food plants were evaluated and correlated, including the leaves of sage, rosemary, olive, and pomegranate, as well as the leaves and young stems of rue, peppermint, and parsley. This study compared and demonstrated these plant extracts as valuable sources of bioactive compounds, likely for preparing novel functional products in various industries. High correlations of phenolic composition with antioxidant potential were investigated in our analysis. Different kinds of phenolic acids and flavonoids along with their derivatives were found widespread in the studied plant materials. Phenylethanoids especially oleuropein and its derivatives were characterized as the most abundant constituents of olive leaf extracts, probably contributing to its beneficial biological properties. While tannins particularly ellagitannins were supposed to be the main contributor to the features of pomegranate leaf. Interestingly, our results highlighted that the hydro-methanolic extracts of Punica granatum L. (pomegranate) leaves displayed the greatest levels of free radical scavenging capacity and ferric reducing antioxidant power, as well as the highest contents of total phenols, ortho -diphenols and tannins; a relatively high content of flavonoids was also found. Studies have increasingly evidenced the close association of tannins and less-studied compounds with antioxidant activity in medicinal and food plants 12 , 18 , 19 , 26 , 48 . Thus it is expected that richer phenolic types, namely tannins and phenolic glycosides, and their higher concentrations, are maintained in pomegranate leaves, making it possible to explore active ingredients and bioavailable products in the food-pharm, nutraceutical or cosmeceutical industries.
Moreover, only a limited number of researches have pointed out the comparison of biological activities and phenolic components of the tested plant organs, which belong to tree plants or shrub plants with large or small leaves. Many authors have stated the importance of vegetables, fruits, medicinal and aromatic plants in the current dietary patterns 2 , 3 , 4 , 5 , 29 , 30 , 50 . However, it doesn’t mean the agricultural and industrial waste like the tree leaves are useless for application. Extracts of olive leaves have attracted more attention recently, being reviewed as promising cheap, renewable and plenty source of bio-phenols for by-products. Some articles proved pomegranate leaf as a safe substrate due to its lower or inexistent toxicity 17 , 35 . In addition, ellagitannins as effective ingredients in teas are considered to be more abundant in the large-leaf tree than those from the small-leaf tree 61 , 62 . Therefore, as per olive leaf, research into finding new uses for by-products of pomegranate leaf may be proved as a strong argument for not only promoting human health but also improving bio-valorization and environment. However, samples of pomegranate leaves were not collected from different varieties or different seasons. Hence, studies on these issues would be of much interest in the future, in order to select the most promising matrix of the wasted bio-phenol materials.
Materials and methods
Chemicals and standards.
Compounds: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS ·+ ), (±)-6-hydroxy-2,5,7,8-tetramethylchromone-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhidrazyl radical (DPPH · ), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), sodium carbonate, sodium molybdate, potassium persulfate, and hydrochloric acid, all extra pure (> 99%) were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Reagents: ferric chloride, methanol, aluminum chloride, sodium nitrite, all extra pure (> 99%), and methyl cellulose (1500 centipoises viscosity at 2%) were acquired from Merck (Merck, Darmstadt, Germany). Sodium hydroxide, ammonium sulfate, Folin-Ciocalteu’s reagent and acetic acid, all extra pure (> 99%) were purchased from Panreac (Panreac Química S.L.U., Barcelona, Spain). Authentic standards of phenolic compounds used in the chromatographic analysis, including that protocatechuic acid (> 97%), p -hydroxybenzoic acid (> 99%), benzoic acid (> 99.5%) were obtained from Fluka (Fluka Chemika, Neu-Ulm, Switzerland), and caffeic acid (> 98%) was from Panreac (Panreac Química S.L.U., Barcelona, Spain). Standards: neochlorogenic acid (> 95%), chlorogenic acid (> 99%), vanillic acid (> 97%), syringic acid (≥ 99%), myricitin-3- O -glucoside (≥ 99%), p -coumaric acid (> 99%), rutin (quercetin-3-rutinoside) (≥ 94%), ellagic acid (≥ 95%), ferulic acid (> 99%), apigenin-7- O -glucoside (≥ 95%), rosmarinic acid (≥ 98%), luteolin (≥ 98%), quercetin (> 95%), trans -cinnamic acid (> 95%), and kaempferol (> 90%) were purchased from Chem-Lab (Chem-Lab N.V., Zedelgem, Belgium). Gallic acid (> 97.5%), tyrosol (> 98%), caftaric acid (≥ 97%), catechin (≥ 98%), gentisic acid (≥ 98%), epicatechin (≥ 98%), 4-hydrocinnamic acid (> 95%), luteolin-7- O -glucoside (≥ 98%), isorhamnetina-3- O -glucoside (> 95%), oleuropein (> 98%), resveratrol (≥ 99%), and trans -stilben (> 96%) were acquired from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Chromatography solvents were of RP-HPLC-DAD grade according to the analysis performed. Ultrapure water was obtained using a Water Purification System (Arioso Power, Human Corporation, Seoul, Korea).
Plant materials
From about one-hundred common medicinal and food plants reported in literature references, we have selected seven medicinal and food plants (Table S1 ) in this study according to following criteria: (1) higher phenolic content and antioxidant capacity, (2) lower or inexistent toxicity. Plant species were botanically authenticated by Prof. António Crespí (Department of Biology and Environment, University of Trás-os-Montes e Alto Douro, UTAD, Portugal) and Dr. João Rocha (Chemistry Centre-Vila Real, UTAD, Portugal). Samples of each species were hand-picked randomly from a pool of individual specimens (n > 10) that are naturally growing in the Botanical Garden of UTAD (Vila Real, Portugal), which belongs to the international network of botanical gardens. Sage, rosemary, rue, peppermint, and parsley are present in the Aromatic and Medicinal Plants collection; olive is present in the Mediterranean Calcareous collection; pomegranate is present in the Garden Fruits collection (more detailed information of each plant species can be checked at http://jb.utad.pt/ ). Thus, a mixture sample for each species was obtained and used for the subsequent analysis. The collected samples were immediately dried at 40 ℃ (Drying Cabinet, LEEC, Nottingham, UK) for 72 h, before being ground into a fine powder with a blender (MB 800, KINEMATICA AG, Malters, Switzerland), and hermetically stored in the dark, at room temperature (RT) until analysis. Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material have complied with relevant institutional, national, and international guidelines and legislation.
Preparation of plant phenolic extracts
The sample powder of each species was weighed and extracted in triplicate with 40 mg of dry weight (DW). The extraction was performed by agitating (30 min, 200 rpm, RT) the mixture of the powder and 1.5 mL of a hydro-methanolic solution (methanol:H 2 O, 70:30, v/v) in an orbital shaker (GFL 3005, GEMINI, Apeldoorn, Netherlands). Afterwards, the suspensions were centrifuged (10,000 rpm, 4 ℃) for 15 min (Sigma 2-16KL Refrigerated Centrifuges, Sigma Laborzentrifugen, Berlin, Germany). The supernatants were collected in a 5 mL volumetric flask, and the solid residues were then extracted twice via the same procedure. All the three supernatants from successive extractions were kept together and the final volume came to 5 mL with the above-mentioned extraction solvent.
Content of different phenolic classes
The content of total phenols, ortho -diphenols, and flavonoids was determined by colorimetric and spectrophotometric approaches according to the literature 63 . The content of tannins was evaluated by the methyl cellulose (MC) methodology previously reported by Dambergs et al. 64 .
For the determination of total phenol content, 20 μL of diluted sample, 100 μL of diluted Folin-Ciocalteu reagent (90%, v/v), and 80 μL aqueous sodium carbonate (7.5%, w/v) were mixed in sequence. The mixture was incubated for 30 min at 42 ℃ in the dark and measured at 750 nm, using gallic acid as standard. Results were expressed in milligrams of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW).
For the assessment of ortho -diphenols content, 40 μL of sodium molybdate solution (5%, w/v) prepared with hydro-methanol (50%, v/v) was added to 160 μL of diluted extract. The mixture was stood for 15 min at RT, protected from light, before the absorbance at 375 nm was read. The content was quantified using gallic acid as standard. Results were defined in mg GAE g −1 DW.
For the quantification of total flavonoids content, 24 μL of diluted extract and 28 μL of sodium nitrite (5%, w/v) were mixed. After 5 min at RT, 28 μL of a 10% (w/v) aluminum chloride solution was added in the mixture and reacted for 6 min. Afterwards, 120 μL of sodium hydroxide (1 M) was added and the final mixture was read at 520 nm after agitation for 30 s in a microplate reader. The results were expressed in milligrams of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW).
The above-mentioned assays were undertaken with a microplate reader (Multiskan FC Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland) in 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) with a final volume of 200 µL.
The content of tannins was evaluated both in treatment and control groups simultaneously, by adding 600 μL of methyl cellulose (MC) solution (treatment) or water (control) to 200 μL of sample in a 2 mL Eppendorf. The mixture was stirred manually for 2–3 min at RT. Four hundred μL of saturated ammonium sulfate and 800 μL of water were added successively both in the treatment and control groups until 2 mL of total volume was reached. The final mixture was vortexed and kept for 10 min. After centrifugation (10,000 rpm, 16 ℃, 5 min), the absorbance was read at 280 nm, by using a conventional spectrophotometer (Helios Gamma UV Spectrophotometer, Thermo Electron Corporation, Warwickshire, UK). The absorbance of tannins was obtained by subtracting the treatment absorbance from the value registered from the control, using epicatechin as standard. The results were described in milligrams of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW).
Evaluation of in vitro antioxidant activity
The antioxidant activity of sample extracts was determined by ABTS, DPPH and FRAP (ferric reducing antioxidant power) spectrophotometric methods, reported by Mena et al. 65 , with some modifications.
The ABTS + radicals were produced by mixing 5 mL of ABTS stock solution (7.0 mM) with 88 μL of potassium persulfate (148 mM), and diluted to a working solution with sodium acetate buffer (20 mM, pH 4.5), showing an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 188 μL of ABTS working solution and 12 μL of sample dilutions (water used as blank) were mixed and reacted for 30 min at RT, and then the absorbance was read at 734 nm.
The DPPH radicals (8.87 mM) were formed with methanol (99.9%) and diluted in a working solution with hydro-methanol (70%, v/v), achieving an absorbance of 1000 at 520 nm. A mixture of 190 μL of DPPH working solution and 10 μL of sample dilutions (70% hydro-methanol used as blank) was incubated for 15 min at RT, reading the absorbance at 520 nm.
The FRAP working solution was prepared by mixing 10-volume acetate buffer (300 mM, pH 3.6), 1-volume TPTZ (10 mM dissolved in hydrochloric acid), and 1-volume ferric chloride (20 mM in water). The mixture was maintained at 37 ℃ for 10 min before use. The reaction of FRAP working solution (180 μL) and sample dilutions (20 μL) was kept at 37 ℃ for 30 min and the absorbance read at 593 nm.
The three antioxidant assays were adapted to microscale using 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) and microplate readers (Multiskan GO Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland), using Trolox as standard. All the results were expressed in millimoles of Trolox per gram of plant dry weight (mM Trolox g −1 DW).
Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) system (Thermo Finnigan, San Diego, CA, USA) was carried out to determine the (poly)phenolic profile of each plant extract, as previously described 63 . The analysis equipment is composed of three parts, including LC pump (Surveyor), autosampler (Surveyor), and PDA detector (Surveyor). Sample extracts, in triplicate, and 31 pure standard compounds (all in HPLC grade), including 17 phenolic acids, 10 flavonoids, 2 phenylethanoids and 2 stilbenoids, were prepared and filtered through 0.45 μm PVDF filters (Millex-HV Syringe Filter Unit, Merck Millipore, Bedford, MA, USA) and injected into a C18 column (250 × 4.6 mm, 5 μm particle size; ACE, Aberdeen, Scotland), using a mobile phase composed of water/formic acid (99.9:0.1, v/v) (solvent A) and acetonitrile/formic acid (99.9:0.1, v/v) (solvent B). The linear gradient program (t in min and %B) was: t = 0–0%; t = 5–0%; t = 20–20%; t = 35–50%; t = 40–100%; t = 45–0%; and t = 65–0%. The injection volume was 20 μL and the flow rate was kept at 1.0 mL min −1 . UV/Vis detection was recorded from 200 to 600 nm range. Peaks were monitored at 280 and 330 nm, and identified by congruent retention time compared with standards. Data acquisition, peak integration and analysis were performed using Chromeleon software (Version 7.1; Thermo Scientific, Dionex, USA). The three extracts of each medicinal plant were chromatographed and results were expressed in milligram per liter of sample extracts (mg L −1 ).
Data and statistical analysis
All the measurements of phenolic phytochemicals and antioxidant activity of the plant extracts were conducted in triplicate. The results of phenolic content and antioxidant activity are presented as mean ± standard deviation (SD). Concentrations of individual identified phenolic compounds are presented as mean (n = 3) with the determination of the Least Significant Difference (LSD) for a p value < 0.05. The obtained data were subjected to analysis of variance (ANOVA) and a multiple range test (Tukey’s test) with IBM SPSS statistics 21.0 software (SPSS Inc., Chicago, USA). Pearson ( r ) analysis was carried out to establish correlations between phenolic chemical classes and antioxidant activity.
Li, Q. et al. Cholinesterase, β -amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 108 , 512–519. https://doi.org/10.1016/j.indcrop.2017.07.001 (2017).
Article CAS Google Scholar
Nollet, L. M. & Gutierrez-Uribe, J. A. Phenolic Compounds in Food: Characterization and Analysis 1st edn, 167 (CRC Press, 2018).
Book Google Scholar
Uritu, C. M. et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018 , 7801543. https://doi.org/10.1155/2018/7801543 (2018).
Article PubMed PubMed Central Google Scholar
Etkin, N. L. Plants and Indigenous Medicine and Diet: Biobehavioral Approaches eBook, 336 (Taylor & Francis, 2019).
Watson, R. R. & Preedy, V. R. Fruits, Vegetables, and Herbs: Bioactive Foods in Health Promotion 1st edn. (Elsevier Academic Press, 2016).
Google Scholar
Alavi, M. S., Fanoudi, S., Ghasemzadeh Rahbardar, M., Mehri, S. & Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. https://doi.org/10.1002/ptr.6894 (2020).
Article PubMed Google Scholar
Ghorbani, A. & Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 7 , 433–440. https://doi.org/10.1016/j.jtcme.2016.12.014 (2017).
Mahendran, G. & Rahman, L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint ( Mentha × piperita L.)—a review. Phytother. Res. 34 , 2088–2139. https://doi.org/10.1002/ptr.6664 (2020).
Shamal Badhusha, P. A. et al. Traditional uses, phytochemistry and ethanopharmacology of Ruta graveolens Linn: A review. Int. J. Pharm. Drug Anal. 20 , 1–4 (2020).
Colucci-D’Amato, L. & Cimaglia, G. Ruta graveolens as a potential source of neuroactive compounds to promote and restore neural functions. J. Tradit. Complement. Med. 10 , 309–314. https://doi.org/10.1016/j.jtcme.2020.05.002 (2020).
Şahin, S. & Bilgin, M. Olive tree ( Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 98 , 1271–1279. https://doi.org/10.1002/jsfa.8619 (2018).
Article CAS PubMed Google Scholar
Salama, Z. A. et al. In-vitro antioxidant, antimicrobial and anticancer activities of banana leaves ( Musa acuminata ) and olive leaves ( Olea europaea L.) as by-products. Res. J. Pharm. Technol. 13 , 687–696. https://doi.org/10.5958/0974-360X.2020.00132.8 (2020).
Article Google Scholar
Farzaei, M. H., Abbasabadi, Z., Ardekani, M. R. S., Rahimi, R. & Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 33 , 815–826. https://doi.org/10.1016/S0254-6272(14)60018-2 (2013).
Cefali, L. C. et al. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinífera L.. Plants 8 , 453. https://doi.org/10.3390/plants8110453 (2019).
Article CAS PubMed Central Google Scholar
Mara de Menezes Epifanio, N. et al. Chemical characterization and in vivo antioxidant activity of parsley ( Petroselinum crispum ) aqueous extract. Food Funct. 11 , 5346–5356. https://doi.org/10.1039/D0FO00484G (2020).
Vučić, V., Grabež, M., Trchounian, A. & Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 25 , 1817–1827. https://doi.org/10.2174/1381612825666190708183941 (2019).
Viswanatha, G. L., Venkataranganna, M. V., Prasad, N. B. L. & Ashok, G. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J. Intercult. Ethnopharmacol. 5 , 415–421. https://doi.org/10.5455/jice.20160904102857 (2016).
Article CAS PubMed PubMed Central Google Scholar
Yeddes, W., Chalghoum, A., Aidi-Wannes, W., Ksouri, R. & Saidani Tounsi, M. Effect of bioclimatic area and season on phenolics and antioxidant activities of rosemary ( Rosmarinus officinalis L.) leaves. J. Essential Oil Res. 31 , 432–443. https://doi.org/10.1080/10412905.2019.1577305 (2019).
Christova-Bagdassarian, V. L., Bagdassarian, K. S., Atanassova, M. S. & Ahmad, M. A. Comparative analysis of total phenolic and total flavonoid contents, rutin, tannins and antioxidant capacity in Apiaceae and Lamiaceae families. Indian Hortic. J. 4 , 131–140 (2014). https://www.academia.edu/15503276/Comparative_Analysis_of_Total_Phenolic_and_Total_Flavonoid_Contents_Rutin_Tannins_and_Antioxidant_Capacity_in_Apiaceae_and_Lamiaceae_families?source=swp_share
Asgharian, S. et al. Ruta graveolens and rutin, as its major compound: Investigating their effect on spatial memory and passive avoidance memory in rats. Pharm. Biol. 58 , 447–453. https://doi.org/10.1080/13880209.2020.1762669 (2020).
Aissani, N., Albouchi, F. & Sebai, H. Anticancer effect in human glioblastoma and antioxidant activity of Petroselinum crispum L. methanol extract. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1842894 (2020).
Farnad, N., Heidari, R. & Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint ( Mentha piperita ). J. Food Meas. Charact. 8 , 113–121. https://doi.org/10.1007/s11694-014-9171-x (2014).
Roby, M. H. H., Sarhan, M. A., Selim, K.A.-H. & Khalel, K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme ( Thymus vulgaris L.), sage ( Salvia officinalis L.), and marjoram ( Origanum majorana L.) extracts. Ind. Crops Prod. 43 , 827–831. https://doi.org/10.1016/j.indcrop.2012.08.029 (2013).
Dent, M., Dragović-Uzelac, V., Penić, M., Bosiljkov, T. & Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage ( Salvia officinalis L.) extracts. Food Technol. Biotech. 51 , 84–91 (2013).
CAS Google Scholar
Mulinacci, N. et al. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 85 , 167–176. https://doi.org/10.1016/j.talanta.2011.03.050 (2011).
Ramkissoon, J. S., Mahomoodally, M. F., Ahmed, N. & Subratty, A. H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 6 , 561–569. https://doi.org/10.1016/S1995-7645(13)60097-8 (2013).
Mestry, S. N., Dhodi, J. B., Kumbhar, S. B. & Juvekar, A. R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 7 , 273–280. https://doi.org/10.1016/j.jtcme.2016.06.008 (2017).
Uysal, S., Zengin, G., Aktumsek, A. & Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 22 , 518–532. https://doi.org/10.1016/j.jff.2016.02.006 (2016).
Kaewnarin, K., Niamsup, H., Shank, L. & Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci. 41 , 105–116 (2014).
Cai, Y., Luo, Q., Sun, M. & Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74 , 2157–2184. https://doi.org/10.1016/j.lfs.2003.09.047 (2004).
Elfalleh, W. et al. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6 , 4724–4730 (2012).
Fellah, B. et al. Untargeted metabolomics reveals changes in phenolic profile following in vitro large intestine fermentation of non-edible parts of Punica granatum L.. Food Res. Int. 128 , 108807. https://doi.org/10.1016/j.foodres.2019.108807 (2020).
Pinheiro, A. J. M. C. R. et al. Punica granatum L. leaf extract attenuates lung inflammation in mice with acute lung injury. J. Immunol. Res. 2018 , 1–11. https://doi.org/10.1155/2018/6879183 (2018).
Ulewicz-Magulska, B. & Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Food Hum. Nutr. 74 , 61–67. https://doi.org/10.1007/s11130-018-0699-5 (2019).
Ankita, P., Deepti, B. & Nilam, M. Flavonoid rich fraction of Punica granatum improves early diabetic nephropathy by ameliorating proteinuria and disturbed glucose homeostasis in experimental animals. Pharm. Biol. 53 , 61–71. https://doi.org/10.3109/13880209.2014.910533 (2015).
Generalić, I. et al. Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem. 127 , 427–433. https://doi.org/10.1016/j.foodchem.2011.01.013 (2011).
Salwe, K. J. & Sachdev, D. Evaluation of antinociceptive and anti-inflammatory effect of the hydroalcoholic extracts of leaves and fruit peel of P. granatum in experimental animals. Asian J. Pharm. Clin. Res. 7 , 137–141 (2014).
AlFadel, F., Al Laham, S. & Alkhatib, R. The anti-bacterial activity of various parts of Punica granatum on antibiotics resistance Escherichia coli . Seeds 21 , 76 (2014).
Gheith, I. & El-Mahmoudy, A. Potent anti-oxidant and anti-inflammatory potentials of Punica granatum leaf and flower hydromethanolic extracts in vitro. Biosci. J. https://doi.org/10.14393/BJ-v33n2-33736 (2017).
Pararin, S., Rouhi, L. & Ghasemi Pirbalouti, A. The beneficial effect of hydro-alcoholic extract of Punica granatum L. leaves and flower on ethylene glycol-induced kidney calculi in RATS. J. Herb. Drugs (Int. J. Med. Herbs) 7 , 59–64 (2016).
Kiraz, Y., Neergheen-Bhujun, V. S., Rummun, N. & Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 37 , 1803–1815. https://doi.org/10.1007/s13277-015-3962-5 (2016).
Rummun, N., Somanah, J., Ramsaha, S., Bahorun, T. & Neergheen-Bhujun, V. S. Bioactivity of nonedible parts of Punica granatum L.: A potential source of functional ingredients. Int. J. Food Sci. 2013 , 602312. https://doi.org/10.1155/2013/602312 (2013).
Kapp, K. et al. Commercial peppermint ( Mentha × piperita L) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 53 , 758–766. https://doi.org/10.1016/j.foodres.2013.02.015 (2013).
Meziane-Assami, D., Tomao, V., Ruiz, K., Meklati, B. Y. & Chemat, F. Geographical differentiation of rosemary based on GC/MS and fast HPLC analyses. Food Anal. Method 6 , 282–288. https://doi.org/10.1007/s12161-012-9430-6 (2013).
Khaleel, I. R., Kadhim, M. I. & Subhi, J. H. Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from rosemary ( Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (Hplc). Al-Nahrain J. Sci. 14 , 20 (2018).
Melnyk, M., Vodoslavskyi, V. & Obodianskyi, M. Research of phenolic compounds of ruta graveolens l and stellaria media (l.) vill. Asian J. Pharm. Clin. Res. 11 , 152–156 (2018).
Figueroa-Pérez, M. G. et al. Diabetic nephropathy is ameliorated with peppermint ( Mentha piperita ) infusions prepared from salicylic acid-elicited plants. J. Funct. Foods 43 , 55–61. https://doi.org/10.1016/j.jff.2018.01.029 (2018).
Dekanski, D. et al. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serb. Chem. Soc. 74 , 367–377. https://doi.org/10.2298/JSC0904367D (2009).
Makowska-Wąs, J. et al. Identification of predominant phytochemical compounds and cytotoxic activity of wild olive leaves ( Olea europaea L. ssp. sylvestris) harvested in South Portugal. Chem. Biodiv. 14 , e1600331. https://doi.org/10.1002/cbdv.201600331 (2017).
Skotti, E. et al. Biological activity of selected Greek medicinal and aromatic plants extracts on Alternaria alternata. Emirates J. Food Agric. 28 , 796–804. https://doi.org/10.9755/ejfa.2016-06-618 (2016).
Arruda, M. O. et al. The hydroalcoholic extract obtained from Mentha piperita L. leaves attenuates oxidative stress and improves survival in lipopolysaccharide-treated macrophages. J. Immunol. Res. 2017 , 2078794. https://doi.org/10.1155/2017/2078794 (2017).
Luthria, D. L., Mukhopadhyay, S. & Kwansa, A. L. A systematic approach for extraction of phenolic compounds using parsley ( Petroselinum crispum ) flakes as a model substrate. J. Sci. Food Agric. 86 , 1350–1358. https://doi.org/10.1002/jsfa.2521 (2006).
Hozayen, W. G., El-Desouky, M. A., Soliman, H. A., Ahmed, R. R. & Khaliefa, A. K. Antiosteoporotic effect of Petroselinum crispum , Ocimum basilicum and Cichorium intybus L. in glucocorticoid-induced osteoporosis in rats. Bmc. Complement. Altern. Med. 16 , 165. https://doi.org/10.1186/s12906-016-1140-y (2016).
Marques, L. C. et al. Anti-inflammatory effects of a pomegranate leaf extract in LPS-induced peritonitis. Planta Med. 82 , 1463–1467. https://doi.org/10.1055/s-0042-108856 (2016).
Swilam, N. & Nematallah, K. A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 10 , 14851. https://doi.org/10.1038/s41598-020-71847-5 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Akkawi, M., Abu-Lafi, S. & Abu-Remeleh, Q. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 7 , 193–200 (2019).
Pinheiro, A. C. et al. Galloyl-hexahydroxydiphenoyl (HHDP)-glucose isolated from Punica granatum L. leaves protects against lipopolysaccharide (LPS)-induced acute lung injury in BALB/c mice. Front. Immunol. 10 , 1978. https://doi.org/10.3389/fimmu.2019.01978 (2019).
Lakshminarayanashastry Viswanatha, G., Venkatanarasappa Venkataranganna, M. & Lingeswara Prasad, N. B. Methanolic leaf extract of Punica granatum attenuates ischemia-reperfusion brain injury in Wistar rats: Potential antioxidant and anti-inflammatory mechanisms. Iran. J. Basic Med. Sci. 22 , 187–196. https://doi.org/10.22038/ijbms.2018.30660.7389 (2019).
Chen, J. et al. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 10 , 2611. https://doi.org/10.1038/s41598-020-59451-z (2020).
Xue, Z. & Yang, B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules (Basel, Switzerland) 21 , 991. https://doi.org/10.3390/molecules21080991 (2016).
Fraga-Corral, M. et al. Technological application of tannin-based extracts. Molecules 25 , 614. https://doi.org/10.3390/molecules25030614 (2020).
Yang, X. & Tomás-Barberán, F. A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 67 , 5394–5404. https://doi.org/10.1021/acs.jafc.8b05010 (2019).
Gouvinhas, I. et al. Monitoring the antioxidant and antimicrobial power of grape ( Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 126 , 83–91. https://doi.org/10.1016/j.indcrop.2018.10.006 (2018).
Dambergs, R. G., Mercurio, M. D., Kassara, S., Cozzolino, D. & Smith, P. A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 66 , 656–664. https://doi.org/10.1366/11-06516 (2012).
Article ADS CAS PubMed Google Scholar
Mena, P. et al. Phytochemical characterisation for industrial use of pomegranate ( Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 91 , 1893–1906. https://doi.org/10.1002/jsfa.4411 (2011).
Download references
Acknowledgements
The experiments were approved by the FCT-Portuguese Foundation for Science and Technology (PD/BD/135333/2017), under the Doctoral Programme “Agricultural Production Chains-from fork to farm” (PD/00122/2012).
This research was funded by the FCT (Portuguese Foundation for Science and Technology) Grant number UIDB/04033/2020.
Author information
Authors and affiliations.
Centre for the Research and Technology of Agro-Environmental and Biological Sciences, CITAB, University de Trás-os-Montes e Alto Douro, UTAD, 5000-801, Vila Real, Portugal
Manyou Yu, Irene Gouvinhas & Ana I. R. N. A. Barros
Chemistry Centre-Vila Real, CQ-VR, UTAD, 5000-801, Vila Real, Portugal
Department of Chemistry, School of Life Sciences and Environment, UTAD, Quinta de Prados, 5001-801, Vila Real, Portugal
Ana I. R. N. A. Barros
You can also search for this author in PubMed Google Scholar
Contributions
M.Y. carried out data analysis, wrote the manuscript, and participated in all experimental measurements. I.G. developed and performed the chromatographic analysis. J.R. supervised botanical identification and sample collection. A.I.R.N.A.B. conceived all experiments, performed theoretical calculations, and supervised data analysis and interpretation. All authors reviewed the manuscript and participated in editing the manuscript.
Corresponding authors
Correspondence to Manyou Yu or Ana I. R. N. A. Barros .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, M., Gouvinhas, I., Rocha, J. et al. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11 , 10041 (2021). https://doi.org/10.1038/s41598-021-89437-4
Download citation
Received : 04 January 2021
Accepted : 27 April 2021
Published : 11 May 2021
DOI : https://doi.org/10.1038/s41598-021-89437-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Insight into antioxidant-like activity and computational exploration of identified bioactive compounds in talinum triangulare (jacq.) aqueous extract as potential cholinesterase inhibitors.
- Olakunle Bamikole Afolabi
- Oluwaseun Ruth Olasehinde
- Oghenerobor Benjamin Akpor
BMC Complementary Medicine and Therapies (2024)
Integrated metabolomic and transcriptomic dynamic profiles of endopleura coloration during fruit maturation in three walnut cultivars
- Hengzhao Liu
- Huijuan Zhou
BMC Plant Biology (2024)
Chemoprofiling and medicinal potential of underutilized leaves of Cyperus scariosus
- Yashika Gandhi
- Vijay Kumar
- Thomas J. Webster
Scientific Reports (2024)
Rheological characteristics of wheat flour fortified with Musaceae flours and physicochemical properties of layered flat-bread (Parotta)
- K. Ashwath Kumar
- Crassina Kasar
- P. Prabhasankar
Journal of Food Measurement and Characterization (2024)
Revisiting the antioxidant-prooxidant conundrum in cancer research
- Mohd Farhan
Medical Oncology (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Pharmacognosy - Medicinal Plants
Medicinal Plants for Treatment of Prevalent Diseases
Submitted: 20 July 2018 Reviewed: 15 October 2018 Published: 17 January 2019
DOI: 10.5772/intechopen.82049
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Pharmacognosy - Medicinal Plants
Edited by Shagufta Perveen and Areej Al-Taweel
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
5,679 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Overall attention for this chapters
This chapter focuses on reviewing publications on medicinal plants used in the treatment of common diseases such as malaria, cholera, pneumonia, tuberculosis and asthma. Traditional medicine is still recognized as the preferred primary health care system in many rural communities, due to a number of reasons including affordability and effectiveness. The review concentrated on current literature on medicinal plants, highlighting on information about ethnobotany, phytochemistry and pharmacology. The search for publications on medicinal plants with scientifically proven efficacy was carried out using electronic databases such as Science Direct, Google Scholar, SciFinder and PubMed. In all, about 46 species of different families with potent biological and pharmacological activities were reviewed. All the plants reviewed exhibited potent activity confirming their various traditional uses and their ability to treat prevalent diseases.
- medicinal plants
- tuberculosis
Author Information
Susana oteng mintah *.
- Department of Microbiology, Center for Plant Medicine Research, Ghana
Tonny Asafo-Agyei
- Plant Development Department, Center for Plant Medicine Research, Ghana
Mary-Ann Archer
- Department of Pharmaceutics, Center for Plant Medicine Research, Ghana
Peter Atta-Adjei Junior
Daniel boamah, doris kumadoh.
- Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Ghana
Alfred Appiah
- Center for Plant Medicine Research, Ghana
Augustine Ocloo
Yaw duah boakye.
- Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Ghana
Christian Agyare
*Address all correspondence to: [email protected]
1. Introduction
Traditional medicine is still recognized as the preferred primary health care system in many communities, with over 60% of the world’s population and about 80% in developing countries depending directly on medicinal plants for their medical purposes [ 1 ]. This is due to a number of reasons including affordability, accessibility and low cost [ 2 ].
The use of plants to cure several kinds of human diseases has a long history. Various parts of plants such as leaf, stem, bark, root, etc. are being used to prevent, allay symptoms or revert abnormalities back to normal. Since the practice of “herbal remedies” does not adhere strictly to facts accrued using scientific approaches, orthodox medicine sees “herbal medicines” as an alternative medicine. However, most of the pharmaceutical products currently dispensed by physicians have a long history of use as herbal remedies, including opium, aspirin, digitalis and quinine. Modern medicine today utilizes active compounds isolated from higher plants, and about 80% of these active ingredients indicate a positive correlation between their modern therapeutic use and the traditional uses [ 3 ].
The search for, and use of drugs and dietary supplements obtained from plants have increased in recent years. Scientist such as pharmacologists, microbiologists, botanists, and phytochemists are combing the Earth for phytochemicals and clues that could be developed into medicines for various diseases treatment. This study therefore reviewed electronic database (Google Scholar, SciFinder, PubMed, etc.) for medicinal plants that have potent activity in treating some prevalent and common ailments like malaria, diarrhea, tuberculosis, pneumonia and asthma.
2. Medicinal plants with demonstrated anti-malarial activity
Malaria is one of the world’s most important parasitic disease and a leading cause of death especially in developing countries [ 4 ]. It is endemic in about 100 developing countries, leading to about 1.2 million estimated deaths each year in Africa [ 5 ], with pregnant women and children below 5 years being mostly affected [ 6 ]. A wide range of medicinal plants is employed for the treatment of malaria, since majority of the people who get infected cannot afford the existing expensive orthodox medicines [ 7 ]. The problem of resistance to existing antimalarial agents by parasite has necessitated the search for new and potent agents, and the focus of researchers is on natural products especially medicinal plants since active compounds like quinine and artemisinin were isolated from plants and have been lead compounds for antimalarial drug development [ 8 , 9 ]. Various medicinal plants have been investigated for their anti-malarial activity and some with demonstrated potent in vitro activity have been reviewed below.
2.1 Cryptolepis sanguinolenta
C. sanguinolenta (Lindl.) Schlechter (Apocynaceae) is known by Ghanaians as ‘Ghana quinine’ and specifically by the Asantes and Ewes as ‘Nibima’ and ‘Kadze,’ respectively [ 10 ]. It is a twining and scrambling thin-stemmed shrub, indigenous to Africa, with much ethno-medicinal importance and interest in the West African sub-region [ 11 ]. It is used traditionally for the treatment of malaria, upper respiratory and urinary tract infections, diarrhea, hypertension and as cicatrizant of wounds [ 12 , 13 ]. The ethanolic and aqueous extracts of C. sanguinolenta exhibited an in vitro antiplasmodial activity against multi-drug resistance Plasmodium falciparum (K1) strain, with all the extracts inhibiting 90% of parasite growth at concentrations below 23 μg/mL. The ethanolic roots and leaves extracts showed potent activity with IC 50 of 0.895 ± 0.02 and 3.01 ± 0.02 μg/mL, respectively. While the aqueous roots and leaves extracts had IC 50 of 2.32 ± 0.3 and 13.5 ± 0.7 μg/mL, respectively [ 14 ]. Evaluating the clinical efficacy of a tea bag formulation of the root of C. sanguinolenta in patients with uncomplicated malaria showed that within 72 h, Fifty percent (50%) of the patients had their P. falciparum parasitaemia cleared, and all patients, by Day 7. By Day 3, all presenting symptoms such as fever, chills, nausea and vomiting were completely no more. The overall cure rate when one tea bag of C. sanguinolenta was taken three times a day for 5 days was 93.5%, due to two cases of recrudescence on Days 21 and 28 [ 15 ].
2.2 Terminalia ivorensis
T. ivorensis A. Chev. belongs to the family Combretaceae and is commonly known as ‘black afara’ and by the Asantes as ‘amire.’ It is a large deciduous forest tree of 15–46 m high, normally grown as timber plantation in many tropical countries [ 16 ]. In traditional medicine, various parts of the plant is used to treat malaria, yellow fever, pile, stomach ulcer, wounds and other infections [ 17 , 18 ]. A study by Komlaga et al. [ 19 ] revealed an active in vitro antiplasmodial activity of T. ivorensis aqueous leaf extract, against P. falciparum chloroquine-sensitive (3D7) and chloroquine resistant (W2) strains with IC 50 of 0.64 ± 0.14 and 10.52 ± 3.55 μg/mL, respectively. The ethanolic stem bark extract also showed an in vitro antimalarial activity against chloroquine-resistant strains of P. falciparum with an IC 50 of 6.949 μg/mL [ 20 ].
2.3 Elaeis guineensis
E. guineensis Jacq (Arecaceae), popularly known as oil palm is a monocotyledonous plant which belongs to the coccoid group of palms. It grows up to 15 m high with a lifetime of over 100 years and occurs throughout the tropical rainforest belt of West Africa [ 21 ]. E. guineensis is commonly used for treating gonorrhea, rheumatism, headache, wounds [ 22 ]. An in vitro anti-plasmodial assay revealed that, the ethanolic extract of E. guineensis leaves has potent antimalarial activity with IC 50 of 1.195 μg/mL, against chloroquine-resistant P. falciparum [ 20 ].
2.4 Phyllanthus emblica
P. emblica L. of the family Euphorbiaceae is a deciduous medium-sized plant (10–18 m high), native to tropical south eastern Asia and widely distributed in most subtropical and tropical countries. It is commonly known as Indian gooseberry, rich in vitamin C, minerals and amino acids which helps to build up lost vitality and vigor [ 23 , 24 ]. Various parts of the plant is used traditionally for the treatment of diarrhea, inflammation, diabetes, jaundice, cough, asthma, peptic ulcer, skin diseases, leprosy, intermittent fevers, headache, anemia, dizziness, snakebite and scorpion-sting [ 25 ]. In an SYBR green I-based fluorescence assay to assess the anti-plasmodial potential of P. emblica , the methanol leaf extract exhibited potent activity against CQ-sensitive (3D7) and CQ-resistant (Dd2 and INDO) strains of P. falciparum with IC 50 of 3.125, 4.8 and 5 μg/mL, respectively. Also the ethyl acetate leaf extract showed activity with IC 50 of 7.25, 15 and 9 μg/mL against 3D7, Dd2 and INDO P. falciparum strains, respectively [ 26 ].
2.5 Syzygium aromaticum
S. aromaticum (L.) Merril. & Perry, syn. Eugenia caryophyllata , an ancient and valuable spice is a member of the family Myrtaceae and is commonly known as clove. It is mostly used as a spice to flavor all kinds of foods and has other medicinal values including anthelmintic, anti-asthma and other allergic disorders, anti-inflammatory, antioxidant, antiviral and anti-parasitic properties [ 27 ]. A study by Bagavan et al. [ 26 ], revealed the antimalarial activity of methanol extract of S. aromaticum flower buds with IC 50 of 6.25, 9.5 and 10 μg/mL against P. falciparum CQ-sensitive (3D7) and CQ-resistant (Dd2 and INDO) strains, respectively.
2.6 Goniothalamus marcanii
G. tamirensis Pierre ex Finet & Gagnep is an accepted synonym for the species and is from the family Annonaceae. It occurs naturally in tropical and subtropical parts of Southeast Asia. 80%-EtOH extracts showed an in vitro antimalarial activity (IC 50 = 6.3 μg/mL) against the drug resistant K1 strain of P. falciparum [ 28 ].
2.7 Casearia sylvestris
C. sylvestris var. lingua (Cambess.) Eichler, (Salicaceae) is an evergreen shrub or small tree with long, slender branches and a very dense globose crown. Usually 4–6 m tall, but can grow up to 20 m high, with wide distribution throughout South America. It has been employed in traditional medicine for treating snake bites, wounds, inflammation, fevers, gastric ulcers and diarrhea [ 29 ]. The hexane extracts of C. sylvestris stem wood, stem bark, root bark, leaf and root wood as well as ethanol extract of the root bark, exhibited potent in vitro antiplasmodial activity against chloroquine-resistance FcB1/Colombia P. falciparum strain with IC 50 values of 0.9 ± 0.2, 1.0 ± 0.4, 1.2 ± 0.4, 1.3 ± 0.1, 2.3 ± 0.5 and 7.7 ± 1.1 μg/mL, respectively [ 30 ].
2.8 Cupania vernalis
C. vernalis Cambess. (Sapindaceae) is a semi-deciduous tree with elongated and dense crown, which can grow up to 10–22 m tall. It can be found in almost all forest formations in Brazil, South America, Argentina, Uruguay, Paraguay and Bolivia. The tree serves as source of tannins and wood locally, and in traditional medicine as diuretic, stimulant, expectorant, natural surfactant, sedative and for treating stomach-ache and dermatitis [ 31 ]. The hexane and ethanol leaf extracts showed active antimalarial activity against chloroquine-resistance (FcB1/Colombia) P. falciparum with IC 50 of 0.9 ± 0.3 and 6.6 ± 0.2 μg/mL, respectively [ 30 ].
2.9 Xylopia emarginata
X. emarginata Mart. is a species of plant in the Annonaceae family. It is native to Cerrado vegetation in Brazil. It is an evergreen tree with a very narrow, almost columnar crown which can grow up to 10–20 m tall and 30–40 cm in diameter. It usually grows in large clusters, forming a homogeneous mass. It is a species characteristic of swamp forest, and does not grow in the driest places. It is used as a condiment in food, a carminative and aphrodisiac in traditional medicine [ 32 ]. X. emarginata hexane root bark and stem bark extracts were able to inhibit P. falciparum (chloroquine-resistance FcB1/Colombia strains) with IC 50 of 4.9 ± 0.2 and 5.2 ± 0.4 μg/mL, respectively [ 30 ].
2.10 Xylopia aromatic
X. aromatica (Lam.) Mart. belongs to the family Annonaceae and the accepted name is X. xylopioides . It is a tree native to Cerrado grassland vegetation, particularly in the states of Goiás and Minas Gerais, in eastern Brazil. It is a medium-sized tree with long, hanging branches that can make the crown look like a Christmas tree. Leaves are alternate, narrow, pointed, in a flat plane and arranged regularly along the branches. It is a common roadside and farmland species of the Pacific slope, not in the forest [ 33 ]. The root wood and root bark hexane extracts demonstrated an in vitro antimalarial activity against chloroquine-resistance (FcB1/Colombia) strains of P. falciparum with IC 50 of 4.7 ± 0.9 and 6.8 ± 0.6 μg/mL, respectively [ 30 ].
2.11 Aspidosperma macrocarpon
A. macrocarpon Mart. (Apocynaceae) is a deciduous tree with an open crown growing up to 3–25 m tall and 25-35 cm in diameter. It is a timber tree, native to Brazil, Venezuela, Bolivia, Paraguay and Peru. Traditionally, it is employed in the treatment of fever [ 33 ]. The in vitro antiplasmodial study of the ethanol extract revealed an effective activity against P. falciparum (chloroquine-resistance FcB1/Colombia) with an IC 50 of 4.9 ± 1.1 μg/mL [ 30 ].
2.12 Azadirachta indica
A. indica A. Juss is commonly known as neem tree or Indian lilac and belongs to the mahogany family Meliaceae. It is an evergreen, fast-growing tree that can reach a height of 15–20 m with few of them growing up to 35–40 m, but in severe drought it may shed most of its leaves or nearly all leaves. It is typically grown in tropical and semi-tropical regions. Neem is effective against certain fungi that infect humans and hence used to treat skin diseases like eczema, psoriasis [ 34 ]. The 80% methanol leaf extract showed in vitro anti-plasmodial activity against chloroquine and pyrimethamine sensitive, 3D7 strain, and chloroquine resistant and pyrimethamine sensitive, Dd2 strain, with IC 50 of 5.8 and 1.7 μg/mL, respectively [ 35 ].
2.13 Harrisonia abyssinica
H. abyssinica Oliv. of the family Rutaceae, is a spiny, evergreen shrub that branches from the base and can become a spreading or much-branched tree. It usually grows up to 6–13 m tall and commonly found in Tropical Africa, in the areas of Sierra Leone, Cameroon, Sudan, Ethiopia, Uganda, Kenya, Angola, Zambia and Mozambique [ 33 ]. The methanolic stem bark extract inhibited chloroquine resistant P. falciparum strain Dd2, with IC 50 value of 4.7 ± 0.113 while in chloroquine sensitive P. falciparum strain 3D7, the IC 50 value was 10 ± 0.114 μg/mL [ 35 ].
2.14 Maytenus senegalensis
M. senegalensis Lam. Exell which belongs to the family Celastraceae is an African shrubs or trees widely distributed throughout Central and South America, Southeast Asia, Micronesia and Australasia, the Indian Ocean and Africa, growing up to 15 m high with spines up to 7 cm long. Traditionally, it is an anti-inflammatory herbal drug and is useful in treating toothaches [ 36 ]. The stem bark methanol extract showed anti-plasmodial activity with IC 50 of 3.9 and 10 μg/mL when treated in vitro on chloroquine sensitive, 3D7 and chloroquine resistant, Dd2 strains, respectively [ 35 ].
3. Medicinal plants with demonstrated activity against Vibrio cholera
Cholera is an acute intestinal disease caused by a facultative anaerobic, Gram-negative, comma-shaped rod bacterium, known as V. cholerae . Cholera is a life threatening disease transmitted by the fecal-oral route. The organisms adhere to and colonize the small bowel within a short incubation period, where they secrete cholera enterotoxin leading to severe and watery diarrhea accompanied with vomiting, dehydration and eventually death if not treated promptly [ 37 ]. Various antibiotics have been effective for the treatment of cholera; however, the worldwide problem of microbial resistance to existing antimicrobial medicines has led to most antibiotic failure. Researchers are therefore shifting their focus to natural products, especially medicinal plant, with effective antimicrobial properties. Some medicinal plants with potent anti-cholera activity are reviewed below.
3.1 Terminalia chebula
T. chebula Retz. (Combretaceae) commonly known as black or chebulic myrobalan is a medium to large deciduous tree growing up to 30 m tall, with a trunk of 1 m in diameter. It leaves are oval, alternate to subopposite in arrangement and is a native to South Asia, from India and Nepal east to southwest China, Sri Lanka, Malaysia and Vietnam. Traditionally, it has been used for treatment of indigestion, diarrhea and diabetes [ 38 ]. The plant extract used to treat Cholera worked effectively against the strains of V. cholera the causative agent. The methanol fruit extract of T. chebula had strong bactericidal activity with MIC ranging from 0.125 to 1.5 mg/mL and MBC ranging from 0.25 to 2 mg/mL, against multi-drug resistance strains of V. cholerae (serotypes O1, O139, and non-O1, non-O139) [ 39 ].
3.2 Syzygium cumini
S. cumini (L.) Skeels (Myrtaceae), known as Jam is an evergreen tropical tree, native to the Indian Subcontinent, adjoining regions of Southeast Asia, China and Queensland. It Grows up to 30 m and can live more than 100 years, with a dense foliage which provides shade and is grown just for its ornamental value. The leaves are pinkish when young, and changes to dark green with a yellow midrib as they mature [ 40 ]. The seeds have traditionally been used to treat diarrhea, dysentery, piles, indigestion and diabetes. S. cumini methanol seed extract exhibited a bactericidal anti-cholera activity against multi-drug resistance strains of V. cholerae (serotypes O1, O139, and non-O1, non-O139), with MICs and MBCs ranging from 1.25–3 mg/mL [ 39 ]. Also Sharma et al. [ 41 ] reported the in vitro anti-vibrio activity of the ethanolic stem bark extract against different strains of V. cholera with MICS ranging from 2.5 to 20 mg/mL.
3.3 Saraca indica
S. indica auct. L. commonly known as Asoka-tree or Ashok is a plant belonging to the Detarioideae subfamily of the Fabaceae family. Asoka tree is an evergreen tree with a spreading crown which can grow up to 24 m tall and 34 cm in diameter. The original plant specimen came from Java. Some traditional uses of the plant include treatment of dyspepsia, fever, burning sensation, colic, ulcers, menorrhagia, leucorrhoea, pimples [ 42 ]. S. indica evoked strong bactericidal activity against different strains of multi-drug resistance V. cholera , with MBCs ranging from 1 to 4 mg/mL [ 39 ]. A study by Sharma et al. [ 41 ] also showed the anti-vibrio potential of the ethanolic stem bark extract, with MICs range of 2.5–10 mg/mL against 13 strains of V. cholera .
3.4 Butea monosperma
B. monosperma (Lam.) Taub. (Papilionaceae) is a native to tropical and sub-tropical parts of the Indian Subcontinent and Southeast Asia, ranging across India, Bangladesh, Nepal, Sri Lanka, Myanmar, Thailand, Laos, Cambodia, Vietnam, Malaysia and western Indonesia. Common names include flame-of-the-forest and bastard teak. It is a medium-sized dry season-deciduous tree, growing to 15 m tall. Leaves are pinnate, with (8–16 cm) petiole and three leaflets of 10–20 cm long. Its flowers are used in traditional medicine for the treatment of ulcer, inflammation, hepatic disorder and eye diseases [ 43 ]. The methanol flower extract showed anti-cholera activity with MIC and MBC ranging from 1.75 to 5 mg/mL against different strains of multi-drug resistance V. cholera [ 39 ].
3.5 Euphorbia serpens
E. serpens Kunth is a member of the Euphorbiaceae family. It is native to South America but it can be found on most continents as an introduced species and often a weed. This is an annual herb forming a mat of prostrate stems [ 44 ]. Purified bioactive fraction of aqueous extract of E. serpens exhibited an anti-Vibrio activity at a Minimum Inhibitory Concentration of 3.92 mg/mL [ 45 ].
3.6 Acacia farnesiana
Vachellia farnesiana , also known as A. farnesiana (L.) Willd, commonly known as sweet acacia or needle bush, is a species of shrub or small tree in the legume family, Fabaceae. The species grows to a height of 4.6–9.1 m and grows multiple trunks. V. farnesiana has been used in Colombia to treat malaria, in the Philippines the leaves are traditionally rubbed on the skin to treat skin diseases in livestock. In Malaysia, an infusion of the plant’s flowers and leaves is mixed with turmeric for post-partum treatment [ 46 ]. The bark methanolic extract revealed a potent bactericidal activity against two strains of V. cholera , O139 (AI-1837) and O1 (569-B) with MBCs of 0.5 ± 0.1 and 0.9 ± 0.1, respectively [ 47 ].
3.7 Artemisia ludoviciana
A. ludoviciana (Nutt.) White sagebrush of the family Asteraceae is native to North America where it is widespread across most of the United States, Canada and Mexico. It is a rhizomatous perennial plant growing to height of 0.33–1 m. Medicinally, it is used for dermatological purposes and for treating cold [ 48 ]. The anti-cholera activity of the methanol whole plant extract was effective and bactericidal against O139 (AI-1837) and O1 (569-B) V. cholera strains. The minimum bactericidal concentrations against the two strains were 0.7 ± 0.2 and 1 ± 0.3, respectively [ 47 ].
3.8 Ocimum basilicum
O. basilicum (L.) Basil (Lamiaceae) can be found in Tropical Asia. It is a perennial growing up to 0.5 m tall and by 0.3 m in diameter. Medicinally it is used for the treatment of fever, colds, influenza, poor digestion, nausea, abdominal cramps, gastro-enteritis, migraine, insomnia, depression and exhaustion [ 49 ]. The methanol whole plant extract exhibited a bactericidal activity against V. cholera O139 (AI-1837) and O1 (569-B) strains with MBCs of 2 ± 0.6 and 3 ± 0.5, respectively [ 47 ].
3.9 Opuntia ficus
O. ficus-indica (L.) of the family Cactaceae is species of cactus that has long been domesticated. It is commonly known as prickly pear or Nopal cactus. It originated from Mexico and cultivated in other parts of the world including Mediterranean Basin, Middle East and northern Africa [ 50 ]. A study by Sánchez et al. [ 47 ], revealed the anti-cholera activity of the methanol cladode extract of O. ficus , with minimum bactericidal concentrations against O139 (AI-1837) and O1 (569-B) V. cholera strains to be 3 ± 0.05 and 3 ± 0.1, respectively.
3.10 Lawsonia inermis
L. inermis Linn. (Apocynaceae) commonly known in India as Henna is a flowering plant and the sole species of the genus Lawsonia. It is a tall shrub or small tree, standing 1.8–7.6 m tall, glabrous and multi-branched, with spine-tipped branchlets. The henna plant is native to northern Africa, western and southern Asia, northern Australia, and thrives well in semi-arid zones and tropical areas. It is useful medicinally for burning sensation, leprosy, skin diseases, amenorrhoea, and dysmenorrhea and as abortifacient [ 51 ]. The ethanolic leaf extract exhibited an in vitro anti-vibrio activity with MICs ranging from 2.5 to 10 mg/mL against 13 strains of V. cholera [ 41 ].
4. Medicinal plants with demonstrated anti-tuberculosis activity
Tuberculosis (TB) is an airborne infectious disease which does not only affect the lungs but also other parts of the body such as the brain and spine [ 52 ]. The main cause of TB is Mycobacterium tuberculosis . Other M. tuberculosis complex that causes TB include M. bovis , M. africanum , M. canetti and M. microti [ 53 ]. The predominant symptoms of active TB are fever, night sweat, weight loss and chronic cough with blood containing sputum. However, most TB infections are latent which may progress into active disease if left untreated [ 52 ]. Treatment of TB is very tedious and requires a long course with multiple antibiotics involved. However, this fastidious bacteria have become resistant to most antibiotics, and hence researchers are working tirelessly to come up with new and effective products especially from natural products such as medicinal plant. Some medicinal plants that have been investigated to possess active anti-tuberculosis activity are reviewed below.
4.1 Anogeissus leiocarpa
A. leiocarpa (Combretaceae) commonly called African birch is a tall deciduous tree which is indigenous to the savannas of tropical Africa. Traditionally, its stem and root barks are used to treat gonorrhea, worm infestation, cough, asthma and tuberculosis [ 54 ]. The susceptibility of clinical isolates of M. tuberculosis to the methanolic extract of A. leiocarpa was investigated using the broth dilution method. The results demonstrated anti-mycobacterial property (MIC 78 μg/mL). A. leiocarpa fraction showed an increased anti-mycobacterial activity (MIC 7.8 μg/mL) [ 55 ].
4.2 Terminalia avicennioides
T. avicennioides (Combretaceae) is a tree commonly found in West Africa. Its root bark, fruit and mistletoes are used traditionally to treat diarrhea, hemoptysis, sore throat, TB, asthma and cough [ 54 ]. The in vitro antibacterial studies using broth dilution method of methanolic extract of T. avicennioides showed a significant anti-mycobacterial activity (MIC 78 μg/mL) against clinical isolates of M. tuberculosis. The n-hexane and ethyl acetate fractions obtained from the crude methanol extract of T. avicennioides showed inhibitory activity (MIC 200 and 625 μg/mL, respectively) against attenuated strains of M. bovis . A further study of T. avicennioides fraction obtained demonstrated anti-mycobacterial activity (MIC 4.7 μg/mL) [ 55 ].
4.3 Capparis brassii
C. brassii (Capparidaceae), the narrow-leaf caper bush is distributed in the coastal forest and mixed woodland from tropical West Africa to South-East Africa. The root bark is used to treat TB in folk medicine [ 54 ]. The methanol extract of C. brassii has demonstrated some level of anti-mycobacterial activity (MIC 1.25 mg/mL) against clinically isolated strains of M. tuberculosis [ 55 ].
4.4 Combretum spp.
Combretum (Combretaceae) commonly called the bush willows has about 370 species of shrubs and trees, predominant in southern and tropical Africa, Madagascar, Asia and tropical America. Traditionally, its root and stem barks are used to treat cough, bronchitis and TB [ 54 ]. The methanol extract exhibited anti-mycobacterial activity (MIC 1.25 mg/mL) against M. tuberculosis clinical isolates when evaluated in vitro using the broth microdilution method [ 55 ].
4.5 Solanum torvum
S. torvum (Solanaceae) also called turkey berry is an upright bushy and spiny perennial plant which is native to the Caribbean, southern Mexico, tropical and central America. However, it is also widely naturalized in the warmer and coastal regions of New South Wales, northern and eastern Australia, tropical Africa, Asia, Papua New Guinea, South-Eastern USA and on several pacific islands. The juice from this plant is used for the treatment of fever, sore throat, dropsy, rheumatism, gonorrhea, stomach ache, chest ailment, and asthma, while leaves and fruits can also be used to control a wide range of microbial activity [ 56 ]. The crude leave extract of S. torvum has demonstrated a significant inhibitory activity against two stains of M. tuberculosis (H37Ra and H37Rv) with MIC of 156.3 and 1250 μg/mL, respectively [ 57 ].
4.6 Galenia africana
G. africana (Aizoaceae) is an upright green to yellow-green aromatic woody perennial shrublet commonly found on the western and southern edges of Karoo [ 16 ]. The ethanolic extract of G. africana demonstrated anti-mycobacterial activity (MIC 1.2 mg/mL) against M. tuberculosis. A further study of flavone, 5,7,2′-trihydroxyflavone which was isolated from G. africana showed an increased activity (MIC 0.1 mg/mL) against M. tuberculosis [ 58 ].
4.7 Allium sativum
A. sativum (Amaryllidaceae) popularly called garlic is a bulbous plant, native to northern and eastern Iran and Central Asia [ 59 ], however, garlic can grow in the wild and in places where it has become naturalized. During World War I and II, garlic was used as an antiseptic to prevent gangrene [ 60 ]. Aside its reported nutritional value, garlic can demonstrate antimicrobial effect at temperature as high as 120°C. The aqueous and ethanolic extracts of A. sativum has shown anti-tuberculosis activity (MIC 0.05 and 0.1 mg/mL, respectively) against M. tuberculosis , H37Ra via the use of Microplate Alamar Blue Assay (MABA) [ 61 ]. A study by Gupta et al. [ 62 ] also showed the inhibitory activity of A. sativum against multidrug resistant isolates DKU-156 and JAL-1236, as well as sensitive M. tuberculosis H37Ra with percentage inhibition of 72, 72 and 63%, respectively.
4.8 Allium cepa
A. cepa commonly called onions is from the family Liliaceae. Onions have several pharmacological activity such as antidiabetic, antioxidant, anticancer, cardiovascular, antimicrobial and others [ 63 ]. The minimum inhibitory concentration by which the ethanolic and aqueous extracts of the tissue of A. cepa inhibited the growth of M. tuberculosis H37Ra was recorded to be 0.1 mg/mL for both extracts [ 61 ]. Another in vitro study showed a 79% proportion of inhibition of aqueous extract of the bulb of A. cepa against MDR isolate JAL-1236 [ 62 ].
4.9 Cinnamomum verum
C. verum, (formerly C. zeylanicum ) of the family Lauraceae, commonly known as cinnamon tree is an evergreen small tropical plant native to Sri Lanka, it is also cultivated in Madagascar and Seychelles on commercial scale [ 33 ]. Its anti-tuberculosis activity reported by Sivakumar and Jayaraman, [ 61 ] revealed that, the aqueous and ethanolic extracts of the bark of C. verum exhibited anti-mycobacterial activity (MIC 0.1 and 0.2 mg/mL, respectively) against M. tuberculosis H37Ra.
4.10 Acalypha indica
A. indica popularly known as Indian nettle is from the family Euphorbiaceae. In Africa, it is distributed in Nigeria, from eastern part of Sudan to Somalia and south through DR Congo and East Africa to Southern Africa. It also occurs in South-East Asia, India, Oceania and widely in the Indian Ocean islands. Traditionally, it is used as an antifungal and antibacterial agent for both human and plant pathogens. It is also used as an expectorant to treat pneumonia and asthma [ 33 ]. The in-vitro study of the aqueous leave extract of A. indica against MDR isolate DKU-156, JAL-1236 and sensitive M. tuberculosis H37Rv, demonstrated 95, 68 and 68% inhibition, respectively [ 62 ].
5. Medicinal plants with demonstrated activity against pneumonia
Pneumonia is a respiratory tract infection characterized by the inflammation of one or both lungs as a results of the accumulation of pus in the alveoli. Pneumonia which can be caused by bacteria, viruses or fungi can be mild, severe or life threatening. Bacterial pneumonia can be caused by Streptococcus pneumoniae which is the commonest cause, Staphylococcus aureus , Moraxella catarrhalis , Klebsiella pneumoniae , Haemophilus influenza , Chlamydophila pneumonia and Legionella pneumophila . Pneumocystis jirovecii pneumonia (PCP) is a fungal pneumonia commonly found in immunocompromised patients. Viral pneumonia can also be caused by adenovirus, Varicella zoster, Influenza virus and respiratory syncytial virus [ 64 , 65 ]. Traditionally, medicinal plants have been employed for treating pneumonia and hence the need to prove, scientifically, their folkloric uses. Researchers have investigated such plant, and below is a review on some of the reported plants with demonstrated activity.
5.1 Echinops adenocaulos
In Ethiopian herbal medicine, members of the genus Echinops from family Asteraceae are used for the treatment of diarrhea, intestinal worm infestation, hemorrhoids, migraine and different forms of infections [ 66 ]. Zamzam water extract of E. adenocaulos demonstrated an antibacterial activity against multidrug resistance S. pneumoniae with a minimum inhibitory concentration (MIC) of 0.781 mg/mL [ 67 ].

5.2 Verbascum fruticulosum
Various species of Verbascum , of the family Scrophulariaceae, have been used to treat pulmonary diseases in traditional medicine as a results of its antibacterial activity against Klebsiella pneumonia and Staphylococcus aureus [ 68 ]. The in vitro antimicrobial activity of aqueous extract of V. fruticulosum against multidrug resistant clinical isolate of S. pneumoniae showed a high antibacterial activity with MIC value of 0.195 mg/mL [ 67 ].
5.3 Parietaria judaica
P. judaica commonly known as pellitory of wall from family Urticaceae has been valued for its use as a diuretic, balm for wounds and burns and also as a soother for chronic cough in herbal medicine [ 69 ]. The micro-broth dilution method was used to study the inhibitory activity of aqueous extract of P. Judaica. The extract was able to inhibit multidrug resistant S. pneumonia at an MIC value of 3.125 mg/mL [ 67 ].
5.4 Urtica urens
U. urens commonly known as dwarf nettle or annual nettle from family Urticaceae is used medicinally for the treatment of pulmonary diseases [ 70 ]. A study by Saleh Fares et al. [ 67 ] on the inhibitory activity of the aqueous extract of this plant against multidrug resistant clinical isolates of S. pneumoniae , using micro-broth dilution method, gave an MIC of 6.25 mg/mL. This illustrates its potential to be used as medicine in the treatment pneumonia caused by multidrug resistant S. pneumoniae.
5.5 Beta vulgaris
B. vulgaris popularly known as sugar beet from family Amaranthaceae is a sugar producing plant. Sugar-producing plants contain bioactive compounds, which are active against microbes and hence are able to protect the sugar from fermenting or from undergoing any alteration [ 71 ]. The study of the antimicrobial activity of the crude ethanolic leaf (lamina and midrib) extracts as well as fractions (n-hexane and chloroform) against K. pneumonia, showed zones of growth inhibition at different concentrations tested. At 1 mg/12 μL, the lamina and midrib crude extracts recorded 19 and 9 mm inhibition zone. The chloroform lamina and midrib fraction recorded 12 and 14 mm at concentration 1 mg/6 μL, while at concentration 1 mg/12 μL, their inhibition zones were 15 and 20 mm, respectively. Also the n-hexane lamina and midrib fractions had 20 and 16 mm inhibition zones (1 mg/6 μL),while 36 and 32 mm zones of inhibition (1 mg/12 μL) were recorded, respectively [ 72 ].
6. Medicinal plants with demonstrated anti-asthmatic activity
Asthma is a complex inflammatory disease and congestive respiratory disorder brought about by airway narrowing. It symptoms may include episodic wheezing, cough and chest tightness resulting in airflow block. It leads to changes in the levels of eosinophils, mast cells, lymphocytes, cytokines and other inflammatory cell products. There is increased prevalence worldwide especially in industrialized countries and among children with increased morbidity and mortality rate [ 73 , 74 ]. Medicinal plants have been screened for properties that enhance their activity as anti-asthmatic agents, since current medications have adverse side effects. Few of such plants with demonstrated activity are reviewed below.
6.1 Curcuma longa
C. longa L. is a rhizomatous herbaceous perennial flowering plant of the ginger family, Zingiberaceae. It is native to the Indian subcontinent and Southeast Asia, and requires temperatures between 20 and 30°C and a considerable amount of annual rainfall to thrive. Methanolic extracts (curcumin-II at 200 mg/kg and curcumin-I at 100 mg/kg) of the finger rhizomes of C. longa reduced significantly (P < 0.01) estimated white blood cells count in ovalbumin (OVA) sensitized Wistar rat models for both long and short term. At a higher dosage, curcumin-II (200 mg/kg) tends to protect intact mast cells from degranulation [ 3 ]. This suggests that curcumin can be used as complementary medicine in the treatment of Asthma.
6.2 Aerva lanata
A. lanata (L.) A. L. Juss. ex Schult (Amaranthaceae) is a perennial herb, frequently becoming more or less woody at the base. The stems can be erect to prostrate, sometimes scrambling or climbing into other plants for support. It is widespread in the tropics and subtropics of Africa through Asia to the Philippines and New Guinea. It is used traditionally for treating cough, sore throat, indigestion, wounds, and diabetics and as a vermifuge for children [ 75 ]. The ethanol extract of aerial parts of A. lanata at 100 μg/mL significantly (***p < 0.01) exhibited percentage decreased contraction in the isolated goat tracheal chain preparation model. Also in clonidine induced mast cell degranulation, the extract at 30 and 60 mg/kg administered orally, showed percentage protection of 64.2 and 68.9%, respectively [ 76 ].
6.3 Cynodon dactylon
C. dactylon (L.) Pers, of the family Poaceae is a short-lived, prostrate, perennial grass. It is widely naturalized in the temperate to tropical zones of Europe, Africa, Asia, the Pacific and the Americas. Its habitat is along roadsides and in exposed rocky or sandy sites. It use in traditional medicine to stop bleeding in minor injuries, for weak vision and eye disorders, piles, asthma, tumors among others [ 77 ]. The findings of Savali et al. [ 78 ], indicated that isolated C. dactylon compound was potent and has significant (p < 0.01 and p < 0.001) inhibitory effect on compound 48/80 induced anaphylactic reaction and mast cell activation. Also, compound 48/80 induced increased level of nitric oxide in rat serum and rat peritoneal mast cells were significantly inhibited.
6.4 Piper betle
P. betle L. (Piperaceae) commonly referred to as Betel pepper, is an evergreen climbing shrub producing woody stems, 5–20 m long, and distributed in Southeast Asia—probably originally from Malaysia. It is traditionally used to cure cough, cold, pruritis, asthma and rheumatism [ 79 ]. Ethanol and aqueous extract of leaves at doses 100 and 200 mg/kg possesses anti-asthmatic activity on histamine induced bronchoconstriction in guinea pig and histamine induced dose dependent contraction of guinea pig tracheal chain [ 80 ].
6.5 Lepidium sativum
L. sativum L . (Brassicaceae) also referred to as Garden cress is a profusely-branched, erect, annual plant growing up to 80 cm tall [ 81 ]. It commonly grown in many regions of Saudi Arabia and the Eastern Province. The seeds are used to cure bronchitis, asthma, cough, and useful as abortifacient, antibacterial, aphrodisiac, diuretic, expectorant, gastrointestinal stimulant, gastroprotective, laxative and stomachic [ 82 ]. The bronchodilatory effect of ethanolic seed extract and ethyl acetate, n-butanol and methanol fractions, against histamine and acetylcholine induced acute bronchospasm in guinea pigs, exhibited significant inhibition of bronchospasm, with n-butanol fraction showing a significant (p < 0.001) protection comparable to the reference standards used in the study [ 83 ]. Rehman et al. [ 84 ] also confirmed the bronchodilatory effect of L. sativum crude extract by investigating the various pathways for its activity in airway disorders. It was revealed that, the extract’s activity was mediated through a combination of anticholinergic, Ca ++ antagonist and phosphodiesterase inhibitory pathways.
6.6 Curculigo orchioides
C. orchioides Gaertn. (Hypoxidaceae) is a stemless evergreen perennial herb producing a cluster of leaves from the roots and spreading to form a clump. It grows up to 50 cm tall. It ranges from East Asia—South China, Japan, Indian subcontinent, Myanmar, Thailand, Cambodia, Laos, Vietnam, Malaysia, Indonesia, Philippines, New Guinea, W. Pacific. Alcoholic extract of C. orchioides rhizomes at doses (100–400 mg/kg) shows mast cell stabilizing and antihistaminic activity on Compound 48/80-induced mast cell degranulation and systemic anaphylaxis [ 85 ]. Also Pandit et al. [ 86 ] established the usefulness of the ethanol extract in treating asthma, as it was reported to exhibit significant relaxant effect (p < 0.01) at concentrations 100 and 25 μg/mL in isolated goat tracheal chain and isolated guinea pig ileum preparations respectively. In an in vivo study using histamine induced bronchoconstriction in guinea pigs, egg albumin induced passive paw anaphylaxis in rats and haloperidol-induced catalepsy in mice, there was significant (p < 0.01) protection at lower doses. Again, maximum increase in leucocytes and lymphocytes (99%) and maximum decrease in eosinophils up to 0% at dose 375 mg/kg p.o. was reported in milk-induced total leukocytes and differential leukocyte counts.
6.7 Casuarina equisetifolia
C. equisetifolia L. (Casuarinaceae) also commonly known as Common Ru, is an evergreen tree with a finely branched, feathery crown usually growing from 6 to 35 m and 20–100 cm in diameter. The tree is widely planted throughout the tropics, and ranges from East Asia to Bangladesh, Myanmar, Thailand, Vietnam, Malaysia, Indonesia, Philippines, Australia and the Pacific [ 87 ]. The methanol extract of wood and bark (10–80 mcg/mL) exhibited a significant dose dependent (p < 0.05) antihistaminic activity by inhibiting the histamine induced contraction of trachea. The wood extract (100 mg/kg, i.p.) significantly reduced clonidine induced catalepsy (p < 0.05) and mast cell degranulation (p < 0.001) [ 88 ].
7. Conclusion
All the plants reviewed exhibited potent activity confirming their various traditional uses and their ability to treat prevalent diseases. There is therefore the need to subject these plants to further studies, by isolating active compounds which can be processed into new and potent medicines and the need to study their mechanisms of action.
- 1. Shrestha PM, Dhillion SS. Medicinal plant diversity and use in the highlands of Dolakha district, Nepal. Journal of Ethnopharmacology. 2003; 86 (1):81-96
- 2. Asase A, Kokubun T, Grayer RJ, Kite G, Simmonds MSJ, Oteng-Yeboah AA, et al. Chemical constituents and antimicrobial activity of medicinal plants from Ghana: Cassia sieberiana , Haematostaphis barteri , Mitragyna inermis and Pseudocedrela kotschyi . Phytotherapy Research. 2008; 22 (8):1013-1016
- 3. Sarkar S, Zaidi S, Chaturvedi AK, Srivastava R, Dwivedi PK, Shukla R. Search for a herbal medicine: Anti-asthmatic activity of methanolic extract of Curcuma longa . Journal of Pharmacognosy and Phytochemistry. 2015; 3 :59-72
- 4. Fischer PR, Bialek R. Prevention of malaria in children. Clinical Infectious Diseases. 2002; 34 (4):493-498
- 5. WHO. World Malaria Report 2014. Washington, DC: World Health Organization; 2015
- 6. Tabuti JRS. Herbal medicines used in the treatment of malaria in Budiope county, Uganda. Journal of Ethnopharmacology. 2008; 116 (1):33-42
- 7. Zirihi GN, Mambu L, Guédé-Guina F, Bodo B, Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. Journal of Ethnopharmacology. 2005; 98 (3):281-285
- 8. Basco LK, Mitaku S, Skaltsounis A-L, Ravelomanantsoa N, Tillequin F, Koch M, et al. In vitro activities of furoquinoline and acridone alkaloids against Plasmodium falciparum . Antimicrobial Agents and Chemotherapy. 1994; 38 (5):1169-1171
- 9. Chiyaka C, Garira W, Dube S. Effects of treatment and drug resistance on the transmission dynamics of malaria in endemic areas. Theoretical Population Biology. 2009; 75 (1):14-29
- 10. Ameyaw Y. Morpho-histological characters for the identification of Cryptolepis sanguinolenta (Lindl.) Schtr. International Journal ofScience and Nature. 2012; 3 (2):331-339
- 11. Irvine FR. Woody plants of Ghana. In: Woody Plants of Ghana. England, UK: Oxford University Press; 1961
- 12. Boye GL, Ampofo O. Proceedings of the First International Symposium on Cryptolepine. Kumasi, Ghana: University of Science and Technology; 1983
- 13. Wright CW, Phillipson JD, Awe SO, Kirby GC, Warhurst DC, Quetin-Leclercq J, et al. Antimalarial activity of cryptolepine and some other anhydronium bases. Phytotherapy Research. 1996; 10 (4):361-363
- 14. Paulo A, Gomes ET, Steele J, Warhurst DC, Houghton PJ. Antiplasmodial activity of Cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Medica. 2000; 66 (01):30-34
- 15. Bugyei KA, Boye GL, Addy ME. Clinical efficacy of a tea-bag formulation of Cryptolepis sanguinolenta root in the treatment of acute uncomplicated falciparum malaria. Ghana Medical Journal. 2010; 44 (1):3-9
- 16. Burkill HM. The Useful Plants of West Tropical Africa. 2nd ed. Vol. 1. Kew: Royal Botanic Gardens; 1985
- 17. Oliver-Bever BEP. Medicinal Plants in Tropical West Africa. England: Cambridge University Press; 1986
- 18. Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. Journal of Ethnopharmacology. 2009; 125 (3):393-403
- 19. Komlaga G, Cojean S, Dickson RA, Beniddir MA, Suyyagh-Albouz S, Mensah MLK, et al. Antiplasmodial activity of selected medicinal plants used to treat malaria in Ghana. Parasitology Research. 2016; 115 (8):3185-3195
- 20. Annan K, Sarpong K, Asare C, Dickson R, Amponsah KI, Gyan B, et al. In vitro anti-plasmodial activity of three herbal remedies for malaria in Ghana: Adenia cissampeloides (Planch.) Harms., Termina liaivorensis A. Chev, and Elaeis guineensis Jacq. Pharmacognosy Research. 2012; 4 (4):225
- 21. Henson IE. A brief history of the oil palm. In: Palm Oil. Amsterdam, Netherlands: Elsevier; 2012. pp. 1-29
- 22. Mshana NR. Traditional Medicine and Pharmacopoeia: Contribution to the Revision of Ethnobotanical and Floristic Studies in Ghana. Accra, Ghana: Organization of African Unity/Scientific, Technical & Research Commission; 2000. 920 p
- 23. Calixto JB, Santos ARS, Filho VC, Yunes RA. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Medicinal Research Reviews. 1998; 18 (4):225-258
- 24. Gaire BP, Subedi L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chinese Journal of Integrative Medicine. 2014:1-8. DOI: 10.1007/s11655-014-1984-2
- 25. Mirunalini S, Krishnaveni M. Therapeutic potential of Phyllanthus emblica (amla): The ayurvedic wonder. Journal of Basic and Clinical Physiology and Pharmacology. 2010; 21 (1):93-105
- 26. Bagavan A, Rahuman AA, Kaushik NK, Sahal D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum . Parasitology Research. 2011; 108 (1):15-22
- 27. Mittal M, Gupta N, Parashar P, Mehra V, Khatri M. Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: A comprehensive review. International Journal of Pharmacy and Pharmaceutical Sciences. 2014; 6 (8):67-72
- 28. Ichino C, Soonthornchareonnon N, Chuakul W, Kiyohara H, Ishiyama A, Sekiguchi H, et al. Screening of Thai medicinal plant extracts and their active constituents for in vitro antimalarial activity. Phytotherapy Research. 2006; 20 (4):307-309
- 29. Sleumer HO. Flora neotropica: Monograph number 22. Flacourtiaceae. New York New York Bot Gard Organ Flora Neotrop 499p Illus, maps, keys. Icones, Maps Geog. 1980;4
- 30. De Mesquita ML, Grellier P, Mambu L, De Paula JE, Espindola LS. In vitro antiplasmodial activity of Brazilian Cerrado plants used as traditional remedies. Journal of Ethnopharmacology. 2007; 110 (1):165-170
- 31. Lorenzi H. Brazilian trees. Vol. 2. Brazil: Instituto plantarum de estudos da flora; 2002
- 32. Luchi AE. Fibre pits in wood of Xylopia emarginata Mart.(Annonaceae), Reserva Biológica e Estação Ecológica de Mogi-Guaçu, São Paulo State, Brazil. Hoehnea. 2016; 43 (3):517-520
- 33. Fern K, Fern A, Morris R. Useful tropical plants database. Recuper http//tropical theferns info . 2014
- 34. Porter AH. Neem: India’s Tree of Life. BBC News; 2006. p. 17
- 35. El Tahir A, Satti GMH, Khalid SA. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Maytenus senegalensis (Lam.) Exell. Journal of Ethnopharmacology. 1999; 64 (3):227-233
- 36. Da Silva G, Serrano R, Silva O. Maytenus heterophylla and Maytenus senegalensis , two traditional herbal medicines. Journal of Natural Science, Biology and Medicine. 2011; 2 (1):59
- 37. Finkelstein RA. Cholera, Vibrio Cholerae O1 and O139, and Other Pathogenic Vibrios, Chapter 24. Univ Texas Med Branch; 1996
- 38. Wu Z, Raven PH, Hong D. Flora of China. Volume 5: Ulmaceae through Basellaceae. Beijing, and Missouri Botanical Garden Press, St. Louis: Science Press; 2003
- 39. Acharyya S, Patra A, Bag PK. Evaluation of the antimicrobial activity of some medicinal plants against enteric bacteria with particular reference to multi-drug resistant Vibrio cholerae. Tropical Journal of Pharmaceutical Research. 2009; 8 (3):231-237
- 40. Govaerts RHA, Faden RB. World Checklist of Selected Plant Families. Kew: Royal Botanic Gardens; 2013
- 41. Sharma A, Patel VK, Chaturvedi AN. Vibriocidal activity of certain medicinal plants used in Indian folklore medicine by tribals of Mahakoshal region of Central India. Indian Journal of Pharmacology. 2009; 41 (3):129
- 42. Srivastava GN, Bagchi GD, Srivastava AK. Pharmacognosy of Ashoka stem bark and its adulterants. International Journal of Crude Drug Research. 1988; 26 (2):65-72
- 43. Muthuswamy R, Senthamarai R. Anatomical investigation of flower of Butea monosperma lam. Ancient Science of Life. 2014; 34 (2):73
- 44. Hyde MA, Wursten BT, Ballings P, Palgrave CM. Flora of Zimbabwe: Species information. Cardiospermum halicacabum . 2011
- 45. Payne A, Mukhopadhyay AK, Deka S, Saikia L, Nandi SP. Anti-vibrio and antioxidant properties of two weeds: Euphorbia serpens and Amaranthus viridis . Research Journal of Medicinal Plant. 2015; 9 :170-178
- 46. US Department of Agriculture NRCS. The Plants Database. Greensboro, NC, USA: National Plant Data Team; 2012
- 47. Sánchez E, García S, Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae . Applied and Environmental Microbiology. 2010; 76 (20):6888-6894
- 48. Turner BL. The comps of Mexico: A systematic account of the family Asteraceae (chapter 8: Liabeae and Vernonieae). Phytologia Memoirs. 2007; 12 :1-144
- 49. Chevallier A. The Encyclopaedia of Medicinal Plants—A Practical Reference Guide to over 550 Key Herbs and their Medicinal Uses. London: DK Publishing; 1996
- 50. Wiersema JH. Taxonomic information on cultivated plants in the USDA/ARS germplasm resources information network (GRIN). In: II International Symposium on Taxonomy of Cultivated Plants 413. 1994. pp. 109-116
- 51. Warrier PK, Nambiar VPK. Indian Medicinal Plants: A Compendium of 500 Species. Vol. 5. Telangana, India: Orient Blackswan; 1993
- 52. WHO. Tuberculosis Fact sheet N 104. 2015. Avaialble at: http://www.who.int/mediacentre/factsheets/fs104/en. 2015
- 53. Van Soolingen D, Hoogenboezem T, De Haas PEW, Hermans PWM, Koedam MA, Teppema KS, et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: Characterization of an exceptional isolate from Africa. International Journal of Systematic and Evolutionary Microbiology. 1997; 47 (4):1236-1245
- 54. Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K. An ethnobotanical survey of indigenous flora for treating tuberculosis and other respiratory diseases in Niger State, Nigeria. Journal of Phytomedicine and Therapeutics. 2007; 12 (1):1-21
- 55. Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K, Oladosu P, et al. Evaluation of in vitro antimycobacterial activity of Nigerian plants used for treatment of respiratory diseases. African Journal of Biotechnology. 2008; 7 (11):1630-1636
- 56. Manandhar NP. Plants and People of Nepal. Portland, Oregon: Timber Press; 2002
- 57. Nguta JM, Appiah-Opong R, Nyarko AK, Yeboah-Manu D, Addo PGA, Otchere I, et al. Antimycobacterial and cytotoxic activity of selected medicinal plant extracts. Journal of Ethnopharmacology. 2016; 182 :10-15
- 58. Mativandlela SPN, Meyer JJM, Hussein AA, Houghton PJ, Hamilton CJ, Lall N. Activity against Mycobacterium smegmatis and M. tuberculosis by extract of South African medicinal plants. Phytotherapy Research. 2008; 22 (6):841-845
- 59. Block E. Garlic and other alliums: The lore and the science. Cambridge: Royal Society of Chemistry; 2010. pp. 454
- 60. Tattelman E. Health effects of garlic. American Family Physician. 2005; 72 (1):103-106
- 61. Sivakumar A, Jayaraman G. Anti-tuberculosis activity of commonly used medicinal plants of South India. Journal of Medicinal Plants Research. 2011; 5 (31):6881-6884
- 62. Gupta R, Thakur B, Singh P, Singh HB, Sharma VD, Katoch VM, et al. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. The Indian Journal of Medical Research. 2010; 131 (6):809
- 63. Kuete V. Other health benefits of African medicinal spices and vegetables. In: Medicinal Spices and Vegetables from Africa. England: Elsevier; 2017. pp. 329-349
- 64. van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009; 374 (9700):1543-1556
- 65. File TM Jr. Community-acquired pneumonia. Lancet. 2003; 362 (9400):1991-2001
- 66. Hymete A, Iversen T-H, Rohloff J, Erko B. Screening of Echinops ellenbeckii and Echinops longisetus for biological activities and chemical constituents. Phytomedicine. 2005; 12 (9):675-679
- 67. Saleh Fares GO, Abdallah L, Almasri M, Slaileh A, Zurba Z. Antibacterial activity of selected Palestinian wild plant extracts against multidrug-resistant clinical isolate of Streptococcus pneumoniae. Journal of Pharmacy Research. 2013; 1 (10):963-969
- 68. Turker AU, Camper ND. Biological activity of common mullein, a medicinal plant. Journal of Ethnopharmacology. 2002; 82 (2-3):117-125
- 69. Giachetti D, Taddei E, Taddei I. Diuretic and uricosuric activity of Parietaria judaica L. Bollettino della Società Italiana di Biologia Sperimentale. 1986; 62 (2):197
- 70. Ozkarsli M, Sevim H, Sen A. In vivo effects of Urtica urens (dwarf nettle) on the expression of CYP1A in control and 3-methylcholanthrene-exposed rats. Xenobiotica. 2008; 38 (1):48-61
- 71. Srivastava R, Kulshreshtha DK. Bioactive polysaccharides from plants. Phytochemistry. 1989; 28 (11):2877-2883
- 72. Hussain Z, Mohammad P, Sadozai SK, Khan KM, Nawaz Y, Perveen S. Extraction of anti-pneumonia fractions from the leaves of sugar beets Beta vulgaris . Journal of Pharmacy Research. 2011; 4 (12):4783
- 73. Masoli M, Fabian D, Holt S, Beasley R. Program GI for A (GINA). The global burden of asthma: Executive summary of the GINA dissemination committee report. Allergy. 2004; 59 (5):469-478
- 74. Braman SS. The global burden of asthma. Chest. 2006; 130 (1):4S-12S
- 75. Umaramani M, Sivakanesan R. Vitamin C content of commonly eaten green leafy vegetables in fresh and under different storage conditions. Tropical Plant Research. 2015; 2 (3):240-245
- 76. Kumar D, Prasad DN, Parkash J, Bhatnagar SP, Kumar D. Antiasthmatic activity of ethanolic extract of Aerva lanata Linn. Pharmacology Online. 2009; 2 :1075-1081
- 77. Nandkarni AK. Indian Materia Medica. Vol I & II (Reprinted). Bombay: Popular Prakashan; 1982
- 78. Savali AS, Biradar PR, Jirankali MC. Antianaphylactic and mast cell stabilization activity of Cynodon dactylon . International Journal of Pharmacy and Pharmaceutical Sciences. 2010; 2 (2):69-73
- 79. Pradhan D, Suri KA, Pradhan DK, Biswasroy P. Golden heart of the nature: Piper betle L. Journal of Pharmacognosy and Phytochemistry. 2013; 1 (6):147-167
- 80. Jawale NM, Shewale AB, Nerkar GS, Patil VR. Evalution of antihistaminic activity of leaves of Piper betel Linn. Pharmacology. 2009; 3 :966-977
- 81. Facciola S. Cornucopia: A Source Book of Edible Plants. Vista, California: Kampong Publications; 1990
- 82. Duke JA. Handbook of Medicinal Herbs. Boca Raton, Florida: CRC Press; 2002
- 83. Mali R, Mahajan S, Mehta A. Studies on bronchodilatory effect of Lepidium sativum against allergen induced bronchospasm in Guinea pigs. Pharmacognosy Magazine. 2008; 4 (15):189
- 84. Rehman N, Khan A, Alkharfy KM, Gilani A-H. Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. Evidence-Based Complementary and Alternative Medicine. 2012; 2012 :8
- 85. Venkatesh P, Mukherjee PK, Nema NK, Bandyopadhyay A, Fukui H, Mizuguchi H. Mast cell stabilization and antihistaminic potentials of Curculigo orchioides rhizomes. Journal of Ethnopharmacology. 2009; 126 (3):434-436
- 86. Pandit P, Singh A, Bafna AR, Kadam PV, Patil MJ. Evaluation of antiasthmatic activity of Curculigo orchioides Gaertn. Rhizomes. Indian Journal of Pharmaceutical Sciences. 2008; 70 (4):440
- 87. Jensen M. Trees Commonly Cultivated in Southeast Asia: An Illustrated Field Guide. Vol. 38. Bangkok, Thailand: RAP Publ.; 1995. p. 93
- 88. Aher AN, Pal SC, Patil UK, Yadav SK, Bhattacharya S. Evaluation of anthistaminic activity of Casuarina equisetifolia frost (Casuarinaceae). Pharmacology. 2009; 1 :1144-1149
© 2019 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Pharmacognosy.
Edited by Shagufta Perveen
Published: 19 June 2019
By Carlos Alberto Méndez-Cuesta, Ana Laura Esquivel C...
1593 downloads
By Pranati Srivastava and Syed Abul Kalam
1695 downloads
By Mauricio Salinas-Santander, Patricia Alvarez-Ortiz...
1303 downloads

Medicinal and Aromatic Plants
Current Research Status, Value-Addition to Their Waste, and Agro-Industrial Potential (Vol I)
- © 2024
- Lakhan Kumar 0 ,
- Navneeta Bharadvaja 1 ,
- Ram Singh 2 ,
- Raksha Anand 3
Department of Biotechnology, Delhi Technological University, New Delhi, India
You can also search for this editor in PubMed Google Scholar
Department of Applied Chemistry, Delhi Technological University, New Delhi, India
- Compiles knowledge on recent progress, challenges, and way forward in medicinal plant research
- Presents qualitative and quantitative analysis of phytocompounds of industrial importance and their extraction
- Addresses the issue of management of waste plant parts post extraction as well post-harvest zero waste approach
Part of the book series: Sustainable Landscape Planning and Natural Resources Management (SLPNRM)
203 Accesses
14 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
Due to complex phytochemical components and associated beneficial properties, numerous medicinal and aromatic plants, in whole or parts, have been used for nutritional purposes or the treatment of various diseases and disorders in humans and animals. Essential oils from medicinal and aromatic plants (MAPs) have been exploited for product formulations of pharmaceuticals, cosmetics, food and beverage, colorants, biopesticides, and several other utility chemicals of industrial importance. There is scientific evidence of many medicinal plant extracts possessing immunomodulatory, immunostimulatory, antidiabetic, anticarcinogenic, antimicrobial, and antioxidant properties, thus demonstrating their traditional use in popular medicine. With the advent of modern technology, the exploitation of natural resources has exponentially increased in order to fulfill the demand of an increased human population with improved quality of life. The traditional agriculture and production-based supply of commodities is inadequate to meet the current demand. Biotechnological approaches are gaining importance to bridge the gaps in demand and supply. In the proposed book, medicinal and aromatic plant-based secondary metabolites have been discussed in terms of their therapeutic potential and industrial relevance. To discuss the qualitative and quantitative analysis of a range of medicinal and aromatic plants-based secondary metabolites (SMs), bioprocess development for their extraction and bioseparation, a brief overview of their industrial relevance, various tissue culturing strategies, biotechnological approaches to enhance production, scale-up strategies, management of residual biomass post extraction of target SMs is central to the idea of the proposed book. A section will explore the verticals mentioned above. In the next section, the book addresses the approaches for conserving and improving medicinal and aromatic plant genetic resources. In the third section, approaches to managing the post-harvest crop residue and secondary metabolites extracted plant biomass will be thoroughly discussed. The recent integration of artificial intelligence to improve medicinal and aromatic plant research at several levels, including the development and employment of computational approaches to enhance secondary metabolite production, tissue culture, drug design and discovery, and disease treatment, will be included in the fourth section. The book summarizes current research status, gaps in knowledge, agro-industrial potential, waste or residual plant biomass management, conservation strategies, and computational approaches in the area of medicinal and aromatic plants with an aim to translate biotechnological interventions into reality.
- Biotechnological approaches
- Bioprocess development
- Computational approaches on MAPs
- Conservation of genetic resources
- Medicinal and aromatic plants
- Secondary metabolites
- Translational research
- Zero-waste approach
Table of contents (15 chapters)
Front matter, biologically active compounds from medicinal and aromatic plants for industrial applications.
- Sevinç Yeşilyurt, Muazzez Gürgan, Mehmet Sertkahya
In-vitro Propagation to Conserve Medicinally Important Plants: Insight, Procedures, and Opportunities
- V Samridha, Saket Chandra
Harnessing In-Vitro Propagation for the Sustainable Conservation of Medicinal Plants: Challenges and Prospects
- Yogesh K. Ahlawat, Kushi Yadav, Maryam Samani, Darshana Chaudhary
Response of Cultivated Industrial Crops to Abiotic Stress: Strategies to Enhance Target Metabolite Productivity
- Rakesh Chandra Nainwal, Shweta Singh, Devendra Singh, Shri Krishna Tewari
Clove: Tiny Buds with Global Fame
- Leila Mohtashami, Shokoufeh Aalinezhad, Zahra Boghrati, Royanama Rahimi, Seyed Ahmad Emami
Konkan’s Curcuma : Insights Into Morphological and Genetic Diversity, Phytochemical Treasures, and In-Vitro Micropropagation
- Hafsa Shaikh, Pallavi Vyas, Penna Suprasanna
Recent Advances in Extraction, Analysis, Value Addition, and Applications of Essential Oils
- Munmun Kumar Singh, Swati Singh, Suyashi Mishra, Uma Shankar, Aransha Maurya, Ram Swaroop Verma
Green Techniques for the Extraction of Bioactives from Withania Somnifera for Agro-Industrial Potential
- Arti Shukla, Kapil Dev
Phytochemical Importance of Medicinal Plants as Potential Sources Against Neurodegenerative Diseases
- Vibha Pandey, Debasis Chakrabarty
Exploring Therapeutic Potential of Indian Ayurvedic Plants for Parkinson’s Disease Treatment
- Philip Thomas, Ravishankar Patil
Computational Strategies for Maximizing Biomass and Metabolite Yields for Bioproduction
- Yogesh K. Ahlawat, Vanshika Srivastava, Maryam Samani, Sarahani Harun, Vinothienii Rajuloo, Darshna Chaudhary
Plant Essential Oils as Multifunctional Biomolecules for Applications in Therapeutics, Food and Other Industries
- Irshika Divanji, Ravishankar Patil, Penna Suprasanna
Phytotherapy: An Alternative Approach to Treat Glioblastoma
- Pratibha Kumari, Priti Giri, Prem Lal Uniyal
Gene-Based Management of Alzheimer’s Disease: Role of Coumarins of Ferulago Genus
- Farid Dabaghian, Seyede Reyhane Abbasi Husseini Niaraki, Niloufar Azargashb, Shokoufeh Aalinezhad, Mohammad Sharifzadeh, Mohammad-Reza Delnavazi et al.
Harnessing the Power of Aromatic and Medicinal Plants for Natural Product Innovation
- Shiuly Bhowmick, Tanya Singh, Puneet Singh Chauhan
Editors and Affiliations
Lakhan Kumar, Navneeta Bharadvaja, Raksha Anand
About the editors
Lakhan Kumar works toward Environmental Sustainability. He completed his B.Tech. in Biotechnology from the National Institute of Technology, Jalandhar, and his M.Tech. in Industrial Biotechnology from Delhi Technological University, Delhi. He obtained his Ph.D. in Biotechnology from Delhi Technological University, Delhi, India. His areas of interest include bioenergy, bioprocess engineering, algal biorefinery, plant biotechnology, and remediation of environmental pollutants.
Navneeta Bharadvaja is working as an Assistant Professor at the Department of Biotechnology, Delhi Technological University, Delhi, India-110042. She is an accomplished plant biotechnologist. She has more than 16 years of Research and Teaching experience. She has guided 5 Ph.D. students and more than 100 B.Tech./M.Tech./M.Sc Students. She has published more than 60 peer-reviewed scientific articles in the fields of Medicinal and Aromatic Plants, Algal Biotechnology, Bioremediation, and Biofuels.
Ram Singh is currently working as a Professor at the Department of Applied Chemistry, Delhi Technological University, Delhi, India-110042. He has extensive experience in organic synthesis, plants, natural product chemistry, biomimetic chemistry, and chemical biology. He has published over 100 research papers in peer-reviewed journals, authored eight books, 20 book chapters, and 31 Modules for ePG-Pathshala, and contributed to more than 100 conferences. He has supervised 6 Ph.D. and 10 M.Tech students. His research has been funded by DST, CSIR, and DRDO, and he has carried out several projects in the area of natural product chemistry. He is on the Editorial Advisory Board of various journals of repute and is a Life Member of various societies.
Raksha Anand works on the development of algal nutraceuticals and waste and biomass valorization. She completed her B.Sc. (Hons.) degree in Biotechnology from the School of Basic Sciences and Research (SBSR), Sharda University, and her Master’s degree in Biotechnology from Delhi Technological University, Delhi. Her areas of interest include Nutraceuticals and Lifestyle Disease Management, Algal Biorefinery, Plant Biotechnology, and Environmental remediation. She has published several peer-reviewed articles and book chapters majorly in Nutraceuticals, Wastewater Treatment, Microbial Fuel Cells, and Bioremediation. She is editing a contributed book on algal-derived nutraceuticals.
Bibliographic Information
Book Title : Medicinal and Aromatic Plants
Book Subtitle : Current Research Status, Value-Addition to Their Waste, and Agro-Industrial Potential (Vol I)
Editors : Lakhan Kumar, Navneeta Bharadvaja, Ram Singh, Raksha Anand
Series Title : Sustainable Landscape Planning and Natural Resources Management
DOI : https://doi.org/10.1007/978-3-031-60117-0
Publisher : Springer Cham
eBook Packages : Earth and Environmental Science , Earth and Environmental Science (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2024
Hardcover ISBN : 978-3-031-60116-3 Published: 30 June 2024
Softcover ISBN : 978-3-031-60119-4 Due: 31 July 2024
eBook ISBN : 978-3-031-60117-0 Published: 29 June 2024
Series ISSN : 2948-1910
Series E-ISSN : 2948-1929
Edition Number : 1
Number of Pages : VI, 222
Number of Illustrations : 94 b/w illustrations, 23 illustrations in colour
Topics : Waste Management/Waste Technology , Plant Genetics and Genomics , Biotechnology , Plant Breeding/Biotechnology
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Applications of some advanced sequencing, analytical, and computational approaches in medicinal plant research: a review
Affiliations.
- 1 Department of Biosciences, Integral University, Lucknow, Uttar Pradesh, 226026, India.
- 2 Institute of Biosciences and Technology, Shri Ramswaroop Memorial University, Barabanki, Uttar Pradesh, 225003, India.
- 3 College of Horticulture and Forestry Thunag, Dr. Y. S. Parmar University of Horticulture and Forestry, Nauni, Solan, Himachal Pradesh, 173230, India.
- 4 Department of Bioengineering, Integral University, Lucknow, Uttar Pradesh, 226026, India. [email protected].
- PMID: 38117315
- DOI: 10.1007/s11033-023-09057-1
The potential active chemicals found in medicinal plants, which have long been employed as natural medicines, are abundant. Exploring the genes responsible for producing these compounds has given new insights into medicinal plant research. Previously, the authentication of medicinal plants was done via DNA marker sequencing. With the advancement of sequencing technology, several new techniques like next-generation sequencing, single molecule sequencing, and fourth-generation sequencing have emerged. These techniques enshrined the role of molecular approaches for medicinal plants because all the genes involved in the biosynthesis of medicinal compound(s) could be identified through RNA-seq analysis. In several research insights, transcriptome data have also been used for the identification of biosynthesis pathways. miRNAs in several medicinal plants and their role in the biosynthesis pathway as well as regulation of the disease-causing genes were also identified. In several research articles, an in silico study was also found to be effective in identifying the inhibitory effect of medicinal plant-based compounds against virus' gene(s). The use of advanced analytical methods like spectroscopy and chromatography in metabolite proofing of secondary metabolites has also been reported in several recent research findings. Furthermore, advancement in molecular and analytic methods will give new insight into studying the traditionally important medicinal plants that are still unexplored.
Keywords: DNA markers; Functional genomics; Medicinal plants; Metabolite profiling; Natural compound; Next generation sequencing.
© 2023. The Author(s), under exclusive licence to Springer Nature B.V.
PubMed Disclaimer
Similar articles
- The Current Developments in Medicinal Plant Genomics Enabled the Diversification of Secondary Metabolites' Biosynthesis. Alami MM, Ouyang Z, Zhang Y, Shu S, Yang G, Mei Z, Wang X. Alami MM, et al. Int J Mol Sci. 2022 Dec 14;23(24):15932. doi: 10.3390/ijms232415932. Int J Mol Sci. 2022. PMID: 36555572 Free PMC article. Review.
- Next-generation sequencing in the biodiversity conservation of endangered medicinal plants. Sharma R, Patil C, Majeed J, Kumar S, Aggarwal G. Sharma R, et al. Environ Sci Pollut Res Int. 2022 Oct;29(49):73795-73808. doi: 10.1007/s11356-022-22842-y. Epub 2022 Sep 13. Environ Sci Pollut Res Int. 2022. PMID: 36098925 Review.
- The Integration of Metabolomics and Next-Generation Sequencing Data to Elucidate the Pathways of Natural Product Metabolism in Medicinal Plants. Scossa F, Benina M, Alseekh S, Zhang Y, Fernie AR. Scossa F, et al. Planta Med. 2018 Aug;84(12-13):855-873. doi: 10.1055/a-0630-1899. Epub 2018 May 29. Planta Med. 2018. PMID: 29843183 Review.
- De Novo Deep Transcriptome Analysis of Medicinal Plants for Gene Discovery in Biosynthesis of Plant Natural Products. Han R, Rai A, Nakamura M, Suzuki H, Takahashi H, Yamazaki M, Saito K. Han R, et al. Methods Enzymol. 2016;576:19-45. doi: 10.1016/bs.mie.2016.03.001. Epub 2016 Apr 4. Methods Enzymol. 2016. PMID: 27480681 Review.
- Renaissance in phytomedicines: promising implications of NGS technologies. Sharma S, Shrivastava N. Sharma S, et al. Planta. 2016 Jul;244(1):19-38. doi: 10.1007/s00425-016-2492-8. Epub 2016 Mar 22. Planta. 2016. PMID: 27002972 Review.
- Veeresham C (2012) Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res 3:200 - PubMed - PMC - DOI
- D’Agostino A, Di Marco G, Rubini M, Marvelli S, Rizzoli E, Canini A, Gismondi A (2021) Environmental implications and evidence of natural products from dental calculi of a Neolithic-Chalcolithic community (central Italy). Sci Rep 11:10665 - PubMed - PMC - DOI
- Hardy K, Buckley S, Collins MJ, Estalrrich A, Brothwell D, Copeland L, García-Tabernero A, García-Vargas S, De La Rasilla M, Lalueza-Fox C (2012) Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften 99:617–626 - PubMed - DOI
- Rasool Hassan B (2012) Medicinal plants (importance and uses). Pharmaceut Anal Acta 3:2153–2435 - DOI
- Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R (2015) The application of medicinal plants in traditional and modern medicine: a review of Thymus vulgaris. Int J Clin Med 6:635 - DOI
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Study shows wild chimpanzees seek out medicinal plants to treat illness and injuries
Chimpanzees appear to consume plants with medicinal properties to treat their ailments, according to a new study led by the University of Oxford, published this week in the journal PLOS ONE .

To investigate this, a team of researchers combined behavioral observations of wild chimpanzees ( Pan troglodytes ) with pharmacological testing of the potentially medicinal plants they eat. They monitored the behavior and health of 51 chimpanzees from two communities in the Budongo Central Forest Reserve in Uganda, who were habituated to the presence of humans.
Next, they collected plant extracts from 13 species of trees and herbs in the reserve that they suspected the chimpanzees might be using to self-medicate. These included plants that they observed sick or injured chimpanzees eating, but were not part of their normal diet, and plants that previous research has suggested chimpanzees might consume for their medicinal properties.
The extracts were then tested for their anti-inflammatory and antibiotic properties at Neubrandenburg University of Applied Sciences, led by Dr Fabien Schultz.
The researchers found that 88% of the plant extracts inhibited bacterial growth, while 33% had anti-inflammatory properties. Dead wood from a tree in the Dogbane family ( Alstonia boonei ) showed the strongest antibacterial activity and also had anti-inflammatory properties, suggesting that the chimpanzees may consume it to treat wounds. Interestingly, Alstonia boonei is also used as a medicinal plant in East African communities to treat a variety of conditions, including bacterial infections, gastro-intestinal issues, snake bites, and asthma.

The results provide compelling evidence that chimpanzees seek out specific plants for their medicinal effects. The study is the most in-depth analysis to date that combines both behavioral and pharmacological evidence of the medicinal benefits to wild chimpanzees of feeding on bark and dead wood.
Lead author Dr Elodie Freymann , from the University of Oxford’s School of Anthropology & Museum Ethnography, said: ‘To study wild chimpanzee self-medication you have to act like a detective—gathering multidisciplinary evidence to piece together a case. After spending months in the field collecting behavioral clues that led us to specific plant species, it was thrilling to analyze the pharmacological results and discover that many of these plants exhibited high levels of bioactivity.’
With both antibiotic-resistant bacteria and chronic inflammatory diseases becoming urgent global health challenges, the researchers note that the medicinal plants growing in Budongo Central Forest Reserve could aid the development of valuable new drugs.
Dr Freymann added: ‘Our study highlights the medicinal knowledge that can be gained from observing other species in the wild and underscores the urgent need to preserve these forest pharmacies for future generations.’
The study ‘Pharmacological and behavioral investigation of putative self-medicative plants in Budongo Chimpanzee diets’ has been published in PLOS ONE .
Based on a press release by PLOS
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Worldwide research trends on medicinal plants.

1. Introduction
2. materials and methods, 3.1. global evolution trend, 3.2. global subject category, 3.3. distribution of publications by countries, 3.4. institutions (affiliations), 3.5. authors, 3.6. keywords, 3.6.1. global perspective, 3.6.2. keywords related to plants, 3.7. clusters, 3.8. collaboration network of countries, 4. conclusions, author contributions, acknowledgments, conflicts of interest.
- Fonnegra, F.G. Plantas Medicinales Aprobadas en Colombia ; University of Antioquia: Antioquia, Colombia, 2007. [ Google Scholar ]
- Joppa, L.N.; Roberts, D.L.; Myers, N.; Pimm, S.L. Biodiversity hotspots house most undiscovered plant species. Proc. Natl. Acad. Sci. USA 2011 , 108 , 13171–13176. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014 , 344 , 1246752. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002 , 81 , 81–100. [ Google Scholar ] [ CrossRef ]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines-A review. Int. J. Biodivers. Conserv. 2012 , 4 , 101–112. [ Google Scholar ] [ CrossRef ]
- WHO. World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. 2000. Available online: https://apps.who.int/iris/bitstream/handle/10665/66783/WHO_EDM_TRM_2000.1.pdf (accessed on 12 May 2020).
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006 , 27 , 1–93. [ Google Scholar ] [ CrossRef ]
- Houghton, P.J. The role of plants in traditional medicine and current therapy. J. Altern. Complementary Med. 1995 , 1 , 131–143. [ Google Scholar ] [ CrossRef ]
- Kinghorn, A.D.; Seo, E.K. Plants as Sources of Drugs. ACS Symposium Series, Vol. 647. Agricultural Materials as Renewable Resources, Chapter 12, pp. 179–193. Available online: https://pubs.acs.org/doi/abs/10.1021/bk-1996-0647.ch012 (accessed on 12 May 2020).
- Jones, W.P.; Chin, Y.W.; Kinghorn, A.D. The role of pharmacognosy in modern medicine and pharmacy. Curr. Drug Targets 2006 , 7 , 247–264. [ Google Scholar ] [ CrossRef ]
- Faridi, P.; Zarshenas, M.M.; Abolhassanzadeh, Z.; Mohagheghzadeh, A. Collection and storage of medicinal plants in The Canon of Medicine. Pharmacogn. J. 2010 , 2 , 216–218. [ Google Scholar ] [ CrossRef ]
- Koh, G. The Canon of Medicine (Al-Qanun fi’l-tibb) By Ibn Sina (Avicenna) 11th century. BMJ 2009 , 339 , b5358. [ Google Scholar ] [ CrossRef ]
- Reeds, K.M. Renaissance humanism and botany. Ann. Sci. 1976 , 33 , 519–542. [ Google Scholar ] [ CrossRef ]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Rollinger, J.M. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015 , 33 , 1582–1614. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Gertsch, J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharmacol. 2009 , 122 , 177–183. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arceusz, A.; Radecka, I.; Wesolowski, M. Identification of diversity in elements content in medicinal plants belonging to different plant families. Food Chem. 2010 , 120 , 52–58. [ Google Scholar ] [ CrossRef ]
- Salmerón-Manzano, E.; Manzano-Agugliaro, F. Worldwide Research on Low Cost Technologies through Bibliometric Analysis. Inventions 2020 , 5 , 9. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Chan, K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 2003 , 52 , 1361–1371. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Adoum, O.A. Determination of toxicity levels of some savannah plants using brine shrimp test (BST). Bayero. J. Pure Appl. Sci. 2009 , 2 , 135–138. [ Google Scholar ]
- Li, S.Y.; Zhang, B.L.; Tan, F.P. Glycopenia coma caused by taking both dimethylbiguanide and Momordica charantia buccal tablet in one patient. Chin. J. New Drugs Clin. Remedies 2004 , 23 , 189–190. [ Google Scholar ]
- Basch, E.; Gabardi, S.; Ulbricht, C. Bitter melon (Momordica charantia): A review of efficacy and safety. Am. J. Health Syst. Pharm. 2003 , 60 , 356–359. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Street, R.A.; Stirk, W.A.; Van Staden, J. South African traditional medicinal plant trade—challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008 , 119 , 705–710. [ Google Scholar ] [ CrossRef ]
- Masondo, N.A.; Makunga, N.P. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. S. Afr. J. Bot. 2019 , 126 , 40–57. [ Google Scholar ] [ CrossRef ]
- Viljoen, A.; Sandasi, M.; Vermaak, I. The role of the South African Journal of Botany as a vehicle to promote medicinal plant research–A bibliometric appraisal. S. Afr. J. Bot. 2019 , 122 , 3–10. [ Google Scholar ] [ CrossRef ]
- Masondo, N.A.; Stafford, G.I.; Aremu, A.O.; Makunga, N.P. Acetylcholinesterase inhibitors from southern African plants: An overview of ethnobotanical, pharmacological potential and phytochemical research including and beyond Alzheimer’s disease treatment. S. Afr. J. Bot. 2019 , 120 , 39–64. [ Google Scholar ] [ CrossRef ]
- TchuifonTchuifon, D.; Fu, H.Z.; Ho, Y.S. Cameroon publications in the Science Citation Index Expanded: Bibliometric analysis. Revista De Biología Trop. 2017 , 65 , 1582–1591. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Dandara, C. Pharmacogenomics implications of using herbal medicinal plants on African populations in health transition. Pharmaceuticals 2015 , 8 , 637–663. [ Google Scholar ] [ CrossRef ]
- Fernández, B.E.; Armas, R.C. Cuban scientific production about medicinal plants and natural products from PlantMedCUBA database, 1967–2010. Revista Cubana De Plantas Medicinales 2013 , 18 , 348–360. [ Google Scholar ]
- Zhang, G.; Si, J.; Zhu, Y. Bibliometrics of woody medical plants in China. China J. Chin. Mater. Med. 2010 , 35 , 654–657. [ Google Scholar ]
- Xu, W.; Zou, Z.; Pei, J.; Huang, L. Longitudinal trend of global artemisinin research in chemistry subject areas (1983–2017). Bioorg. Med. Chem. 2018 , 26 , 5379–5387. [ Google Scholar ] [ CrossRef ]
- Gupta, B.M.; Ahmed, K.M.; Dhawan, S.M.; Gupta, R. Aloe Vera (Medicinal Plant) research: A scientometric assessment of global publications output during 2007-16. Pharmacogn. J. 2018 . [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Xu, W.; Choi, H.K.; Huang, L. State of Panax ginseng research: A global analysis. Molecules 2017 , 22 , 1518. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Al-Qallaf, C.L. A bibliometric analysis of the Punica grantum L. literature. Malays. J. Libr. Inf. Sci. 2017 , 14 , 83–103. [ Google Scholar ]
- Ram, S. A bibliometric assessment of apocynin (Apocynum cannabinum) research. Ann. Libr. Inf. Stud. (ALIS) 2013 , 60 , 149–158. [ Google Scholar ]
- Gupta, B.M.; Ahmed, K.M.; Bansal, J.; Bansal, M. Andrographis paniculata Global Publications Output: A Bibliometric Assessment during 2003-18. Int. J. Pharm. Investig. 2019 , 9 , 101–108. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ortega-Cuadros, M.; Tofiño-Rivera, A.P. Exploratory review of the antibacterial and antifungal activity of Lippia alba (Mill.) N. E. Br (bushy matgrass). Revista Cubana De Plantas Medicinales . 2019, 24. Available online: http://www.revplantasmedicinales.sld.cu/index.php/pla/article/view/771/361 (accessed on 12 May 2020).
- Ahmed, K.M.; Gupta, B.M. India’s contribution on antioxidants: A bibliometric analysis, 2001–2010. Scientometrics 2013 , 94 , 741–754. [ Google Scholar ] [ CrossRef ]
- Basu, T.; Mallik, A.; Mandal, N. Evolving importance of anticancer research using herbal medicine: A scientometric analysis. Scientometrics 2017 , 110 , 1375–1396. [ Google Scholar ] [ CrossRef ]
- Yeung, A.W.K.; El-Demerdash, A.; Berindan-Neagoe, I.; Atanasov, A.G.; Ho, Y.S. Molecular responses of cancers by natural products: Modifications of autophagy revealed by literature analysis. Crit. Rev. ™ Oncog. 2018 , 23 , 5–6. [ Google Scholar ] [ CrossRef ]
- Du, J.; Tang, X.L. Natural products against cancer: A comprehensive bibliometric study of the research projects, publications, patents and drugs. J. Cancer Res. Ther. 2014 , 10 , 27. [ Google Scholar ]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018 , 10 , 391. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Montoya, F.G.; Alcayde, A.; Baños, R.; Manzano-Agugliaro, F. A fast method for identifying worldwide scientific collaborations using the Scopus database. Telemat. Inform. 2018 , 35 , 168–185. [ Google Scholar ] [ CrossRef ]
- Novas, N.; Alcayde, A.; El Khaled, D.; Manzano-Agugliaro, F. Coatings in Photovoltaic Solar Energy Worldwide Research. Coatings 2019 , 9 , 797. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- la Cruz-Lovera, D.; Perea-Moreno, A.J.; la Cruz-Fernández, D.; Alvarez-Bermejo, J.A.; Manzano-Agugliaro, F. Worldwide research on energy efficiency and sustainability in public buildings. Sustainability 2017 , 9 , 1294. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004 , 74 , 2157–2184. [ Google Scholar ] [ CrossRef ]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010 , 4 , 118. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004 , 52 , 4. [ Google Scholar ]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001 , 49 , 5165–5170. [ Google Scholar ] [ CrossRef ]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999 , 86 , 985–990. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Comparative anti-inflammatory effects of plant-and marine-derived omega-3 fatty acids explored in an endothelial cell line. Mol. Cell Biol. Lipids 2020 , 1865 , 158662. [ Google Scholar ] [ CrossRef ]
- Rocha, F.G.; de Mello Brandenburg, M.; Pawloski, P.L.; da Silva Soley, B.; Costa, S.C.A.; Meinerz, C.C.; Cabrini, D.A. Preclinical study of the topical anti-inflammatory activity of Cyperus rotundus L. extract (Cyperaceae) in models of skin inflammation. J. Ethnopharmacol. 2020 , 254 , 112709. [ Google Scholar ] [ CrossRef ]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010 , 101 , 4676–4689. [ Google Scholar ] [ CrossRef ]
- Gullett, N.P.; Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010 , 37 , 258–281. [ Google Scholar ] [ CrossRef ]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012 , 2 , 320–330. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA) 2013 , 1830 , 3670–3695. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009 , 57 , 5987–6000. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012 , 143 , 656–663. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Babu, N.P.; Pandikumar, P.; Ignacimuthu, S. Anti-inflammatory activity of Albizia lebbeck Benth, an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol. 2009 , 125 , 356–360. [ Google Scholar ] [ CrossRef ]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009 , 9 , 31–59. [ Google Scholar ] [ CrossRef ]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013 , 162750. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010 , 128 , 279–313. [ Google Scholar ] [ CrossRef ]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011 , 134 , 519–541. [ Google Scholar ] [ CrossRef ]
- Toyang, N.J.; Verpoorte, R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013 , 146 , 681–723. [ Google Scholar ] [ CrossRef ]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009 , 14 , 2167–2180. [ Google Scholar ] [ CrossRef ]
- Sarkhail, P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 2014 , 156 , 235–270. [ Google Scholar ] [ CrossRef ]
- Saini, N.; Singh, D.; Sandhir, R. Neuroprotective effects of Bacopa monnieri in experimental model of dementia. Neurochem. Res. 2012 , 37 , 1928–1937. [ Google Scholar ] [ CrossRef ]
- Garrido-Cardenas, J.A.; González-Cerón, L.; Manzano-Agugliaro, F.; Mesa-Valle, C. Plasmodium genomics: An approach for learning about and ending human malaria. Parasitol. Res. 2019 , 118 , 1–27. [ Google Scholar ] [ CrossRef ]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; González-Cerón, L.; Gil-Montoya, F.; Alcayde-Garcia, A.; Novas, N.; Mesa-Valle, C. The Identification of Scientific Communities and Their Approach to Worldwide Malaria Research. Int. J. Environ. Res. Public Health 2018 , 15 , 2703. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Garrido-Cardenas, J.A.; Cebrián-Carmona, J.; González-Cerón, L.; Manzano-Agugliaro, F.; Mesa-Valle, C. Analysis of Global Research on Malaria and Plasmodium vivax. Int. J. Environ. Res. Public Health 2019 , 16 , 1928. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro, F. Genetic approach towards a vaccine against malaria. Eur. J. Clin. Microbiol. Infect. Dis. 2018 , 37 , 1829–1839. [ Google Scholar ] [ CrossRef ]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.Y.; Zhou, J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016 , 2 , 382–392. [ Google Scholar ] [ CrossRef ]
- Broekaert, W.F.; Cammue, B.P.; De Bolle, M.F.; Thevissen, K.; De Samblanx, G.W.; Osborn, R.W.; Nielson, K. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997 , 16 , 297–323. [ Google Scholar ] [ CrossRef ]
- Sarrafchi, A.; Bahmani, M.; Shirzad, H.; Rafieian-Kopaei, M. Oxidative stress and Parkinson’s disease: New hopes in treatment with herbal antioxidants. Curr. Pharm. Des. 2016 , 22 , 238–246. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Castro-Aceituno, V.; Ahn, S.; Simu, S.Y.; Singh, P.; Mathiyalagan, R.; Lee, H.A.; Yang, D.C. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed. Pharmacother. 2016 , 84 , 158–165. [ Google Scholar ] [ CrossRef ]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015 , 81 , 975–994. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018 , 130 , 451–465. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016 , 21 , 264. [ Google Scholar ] [ CrossRef ]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Fernandes, E. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015 , 116 , 398–413. [ Google Scholar ] [ CrossRef ]
- Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro, F. Human parasitology worldwide research. Parasitology 2018 , 145 , 699–712. [ Google Scholar ] [ CrossRef ]
- Kamte, S.L.N.; Ranjbarian, F.; Campagnaro, G.D.; Nya, P.C.B.; Mbuntcha, H.; Woguem, V.; Benelli, G. Trypanosoma brucei inhibition by essential oils from medicinal and aromatic plants traditionally used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus). Int. J. Environ. Res. Public Health 2017 , 14 , 737. [ Google Scholar ] [ CrossRef ]
- Njume, C.; Goduka, N.I. Treatment of diarrhoea in rural African communities: An overview of measures to maximise the medicinal potentials of indigenous plants. Int. J. Environ. Res. Public Health 2012 , 9 , 3911–3933. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Garrido-Cardenas, J.A.; de Lamo-Sevilla, C.; Cabezas-Fernández, M.T.; Manzano-Agugliaro, F.; Martínez-Lirola, M. Global tuberculosis research and its future prospects. Tuberculosis 2020 , 121 , 101917. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Institution | Country | N | Keyword | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Chinese Academy of Sciences | China | 2322 | Unclassified Drug | Drug Isolation | Drug Structure |
| Chinese Academy of Medical Sciences | China | 1432 | Chemistry | Unclassified Drug | Isolation And Purification |
| Peking Union Medical College Hospital | China | 1200 | Chemistry | Unclassified Drug | Isolation And Purification |
| Ministry of Education China | China | 1192 | Unclassified Drug | Controlled Study | Chemistry |
| China Pharmaceutical University | China | 851 | Unclassified Drug | Chemistry | Plant Extract |
| Kunming Institute of Botany Chinese Academy of Sciences | China | 694 | Unclassified Drug | Drug Isolation | Drug Structure |
| China Academy of Chinese Medical Sciences | China | 650 | Unclassified Drug | Chemistry | Drugs, Chinese Herbal |
| Universidade de Sao Paulo—USP | Brazil | 626 | Unclassified Drug | Plant Extract | Controlled Study |
| Shenyang Pharmaceutical University | China | 598 | Unclassified Drug | Chemistry | Drug Isolation |
| University of Chinese Academy of Sciences | China | 549 | Unclassified Drug | Controlled Study | Drug Isolation |
| UNESP-Universidade Estadual Paulista | Brazil | 534 | Unclassified Drug | Plant Extract | Controlled Study |
| Kyung Hee University | South Korea | 533 | Unclassified Drug | Controlled Study | Plant Extract |
| King Saud University | Saudi Arabia | 533 | Unclassified Drug | Plant Extract | Controlled Study |
| Beijing University of Chinese Medicine | China | 533 | Chemistry | Drugs, Chinese Herbal | Herbaceous Agent |
| University of Karachi | Pakistan | 520 | Unclassified Drug | Plant Extract | Drug Isolation |
| Zhejiang University | China | 497 | Unclassified Drug | Chemistry | Controlled Study |
| Seoul National University | South Korea | 496 | Unclassified Drug | Controlled Study | Plant Extract |
| Tehran University of Medical Sciences | Iran | 461 | Unclassified Drug | Plant Extract | Controlled Study |
| Universidad Nacional Autónoma de México | Mexico | 453 | Unclassified Drug | Plant Extract | Controlled Study |
| Université de Yaoundé I | Cameroon | 451 | Unclassified Drug | Plant Extract | Controlled Study |
| Peking University | China | 434 | Unclassified Drug | Chemistry | Isolation And Purification |
| Second Military Medical University | China | 425 | Unclassified Drug | Plant Extract | Controlled Study |
| Universidade Federal do Rio de Janeiro | Brazil | 414 | Unclassified Drug | Plant Extract | Controlled Study |
| CNRS Centre National de la Recherche Scientifique | France | 410 | Unclassified Drug | Plant Extract | Controlled Study |
| Universiti Putra Malaysia | Malaysia | 406 | Unclassified Drug | Plant Extract | Controlled Study |
| Author | Scopus Author ID | N | Affiliation, Country | h-Index | |
|---|---|---|---|---|---|
| 1 | Van Staden, J. | 7201832631 | 238 | University of KwaZulu-Natal, South Africa | 69 |
| 2 | Rahmatullah, M. | 6701489271 | 175 | University of Dhaka, Bangladesh | 38 |
| 3 | Huang, L.Q. | 56156528000 | 150 | China Academy of Chinese Medical Sciences, China | 36 |
| 4 | Choudhary, M.I. | 35228815600 | 142 | University of Karachi, Pakistan | 53 |
| 5 | Afolayan, A.J. | 7003478648 | 137 | University of Fort Hare, South Africa | 41 |
| 6 | Heinrich, M. | 16156235300 | 124 | UCL, London, United Kingdom | 54 |
| 7 | Khan, I.A. | 26643155300 | 124 | University of Mississippi, United States | 54 |
| 8 | Efferth, T. | 7005243974 | 122 | Johannes Gutenberg Universität Mainz, Germany | 70 |
| 9 | Farnsworth, N.R. | 35392089500 | 118 | University of Illinois at Chicago, United States | 63 |
| 10 | Rafieian-Kopaei, M. | 6506929448 | 115 | Shahrekord University of Medical Sciences, Iran | 60 |
| 11 | Kuete, V. | 15757756200 | 114 | University of Dschang, Cameroon | 38 |
| 12 | Xiao, P.G. | 7103088959 | 113 | Ministry of Education China, China | 37 |
| 13 | Vilegas, W. | 7004140097 | 107 | UNESP-Universidade Estadual Paulista, Brazil | 36 |
| 14 | Hao, X.J. | 7202000647 | 105 | Chinese Academy of Sciences, China | 38 |
| 15 | Sun, H.D. | 7404828012 | 105 | Kunming Institute of Botany Chinese Academy of Sciences, China | 47 |
| 16 | Li, P. | 56381767900 | 101 | China Pharmaceutical University, China | 51 |
| Rank | Country | N | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| 1 | China | 19,846 | Unclassified Drug | Chemistry | Controlled Study | Plant Extract |
| 2 | India | 16,372 | Unclassified Drug | Plant Extract | Controlled Study | Animal Experiment |
| 3 | USA | 7339 | Unclassified Drug | Plant Extract | Controlled Study | Chemistry |
| 4 | Brazil | 5993 | Unclassified Drug | Plant Extract | Controlled Study | Animal Experiment |
| 5 | Japan | 4557 | Unclassified Drug | Plant Extract | Drug Isolation | Controlled Study |
| 6 | South Korea | 4131 | Unclassified Drug | Controlled Study | Plant Extract | Animals |
| 7 | Germany | 3867 | Unclassified Drug | Plant Extract | Controlled Study | Chemistry |
| 8 | Iran | 3771 | Unclassified Drug | Plant Extract | Controlled Study | Essential Oil |
| 9 | United Kingdom | 2377 | Unclassified Drug | Plant Extract | Controlled Study | Chemistry |
| 10 | Pakistan | 2220 | Unclassified Drug | Plant Extract | Controlled Study | Chemistry |
| 11 | Italy | 2135 | Unclassified Drug | Plant Extract | Controlled Study | Chemistry |
| 12 | France | 2031 | Unclassified Drug | Plant Extract | Controlled Study | Drug Isolation |
| Part of the Plant | Documents | Main Family Studied | Keyword | N |
|---|---|---|---|---|
| Leaf-Leaves | 14652 | Asteraceae, Fabaceae, Lamiaceae | Plant Leaf | 12,009 |
| Plant Leaves | 4664 | |||
| Root-Roots | 9581 | Asteraceae, Fabaceae | Plant Root | 7695 |
| Plant Roots | 3920 | |||
| Seed | 5204 | Fabaceae, Asteraceae | Plant Seed | 3789 |
| Seeds | 2149 | |||
| Stem | 4480 | Fabaceae, Asteraceae, Apocynaceae | Plant Stem | 3561 |
| Plant Stems | 1462 | |||
| Fruit | 4357 | Fabaceae, Asteraceae, | Fruit | 3423 |
| Fruits | 259 | |||
| Bark | 3358 | Fabaceae, Meliaceae, Euphorbiaceae, Apocynaceae, Asteraceae | Bark | 3146 |
| Plant Bark | 1171 | |||
| Flower | 2615 | Asteraceae, Lamiaceae, Fabaceae | Flower | 2081 |
| Flowers | 804 | |||
| Rhizome | 2519 | Zingiberaceae, Asteraceae | Rhizome | 1969 |
| Rank | Plant Family | Documents | Main Country | Main Affiliation (Country) |
|---|---|---|---|---|
| 1 | Fabaceae | 4492 | USA | Universidade de Sao Paulo – USP (Brazil) |
| 2 | Leguminosae | 3255 | USA | Wageningen University and Research Centre (Netherlands) |
| 3 | Asteraceae | 2743 | China | Chinese Academy of Sciences (China) |
| 4 | Lamiaceae | 1825 | China | Chinese Academy of Sciences (China) |
| 5 | Apocynaceae | 962 | India | Chinese Academy of Sciences (China) |
| 6 | Angiosperm | 914 | China | Chinese Academy of Medical Sciences & Peking Union Medical College (China) |
| 7 | Euphorbiaceae | 898 | India | Chinese Academy of Sciences (China) |
| 8 | Apiaceae (Umbelliferae) | 884(135) | China | Tehran University of Medical Sciences (Iran) |
| 9 | Rubiaceae | 814 | India | Chinese Academy of Sciences (China) |
| 10 | Rutaceae | 732 | India | CNRS Centre National de la Recherche Scientifique (France) |
| 11 | Solanaceae | 539 | India | University of Development Alternative (Bangladesh) |
| 12 | Rosaceae | 582 | China | Chinese Academy of Sciences (China) |
| 13 | Compositae | 352 | China | Lanzhou University (China) |
| Cluster | Color | Main Keywords | Topic |
|---|---|---|---|
| 1-1 | Red | Human, Phytotherapy, herbaceous agent, traditional medicine, ethnobotany, diabetes mellitus | Traditional medicine |
| 1-2 | Green | Drug isolation, drug structure, chemistry, drug determination, molecular structure | Drug determination |
| 1-3 | Purple | Animal, mouse, mice, animal cell, apoptosis, anti-inflammatory effect, protein expression | Animals-in vivo study |
| 1-4 | Yellow | Unclassified drug, drug screening, flavonoid, phytochemistry, plant leaf | Unclassified drug |
| 1-5 | Blue | Drug efficacy, animal experiment, dose response, oxidative stress, histopathology | Drug efficacy |
| 1-6 | Cian | Solvent, ethanol, neuroprotection, acetic acid, sodium chloride | Effect of extraction solvent |
| 1-7 | Orange | antimalarial activity, antimalarials, Plasmodium berghei, Plasmodium falciparum | Malaria |
| Cluster | Color | Main Keywords | Topic |
|---|---|---|---|
| 2-1 | Red | Unclassified drug, chemistry, plant extract, phytochemistry, flavonoid | Unclassified drug |
| 2-2 | Green | Traditional medicine, herbaceous agent, phytotherapy, ethnopharmacology, drug efficacy | Traditional medicine |
| 2-3 | Blue | In vitro study, human cell, antineoplastic agent, cytotoxicity, apoptosis | Cancer |
| 2-4 | Cyan | In vivo study, male, oxidative stress, animal tissue, rat, antidiabetic activity, liver protection | In vivo study- antidiabetic activity |
| 2-5 | Purple | Metabolism, animal, anti-inflammatory activity, mouse, dose response | Animals- Anti-inflammatory activity |
| Cluster | Color | Main Countries | Number of Countries | Leader |
|---|---|---|---|---|
| 1 | Green | Brazil, Italy, Turkey, Spain | 16 | Brazil |
| 2 | Grey | South Africa, Belgium, France, Morocco | 14 | South Africa |
| 3 | Blue | India, Iran, Iraq, Chile | 12 | India |
| 4 | Yellow | Germany, Pakistan, Saudi Arabia, Egypt | 12 | Pakistan |
| 5 | Purple | Indonesia, Malaysia, Thailand, Australia | 10 | Indonesia |
| 6 | Cian | USA, UK, Japan, Canada, South Korea | 8 | USA |
| 7 | Orange | Cameroon, Kenya, Denmark, Nepal | 5 | Cameroon |
| 8 | Red | China, Taiwan, Singapore, Hong Kong | 5 | China |
Share and Cite
Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020 , 17 , 3376. https://doi.org/10.3390/ijerph17103376
Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide Research Trends on Medicinal Plants. International Journal of Environmental Research and Public Health . 2020; 17(10):3376. https://doi.org/10.3390/ijerph17103376
Salmerón-Manzano, Esther, Jose Antonio Garrido-Cardenas, and Francisco Manzano-Agugliaro. 2020. "Worldwide Research Trends on Medicinal Plants" International Journal of Environmental Research and Public Health 17, no. 10: 3376. https://doi.org/10.3390/ijerph17103376
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Chimpanzees seen self-medicating with healing plants when sick or injured
The chimps sought out unappetizing plants with medicinal but little nutritional value, scientists said. The findings could be a pathway to novel human medicines.

The chimpanzee was sick. It had diarrhea and tapeworms — not unusual for a wild chimpanzee in the Budongo Forest of Uganda. What intrigued the watching research team was what the ape did about it.
Soon after its symptoms developed, the male traveled with two others away from the community’s home to a site in the forest with a particular type of tree. It collected some dead wood from the Alstonia boonei and chewed it . The plant has long been used in traditional medicine , and when the scientists tested it, they confirmed it had high antibacterial and anti-inflammatory properties. The chimp made a full recovery.
The chimp’s behavior was one of many instances observed over eight months that suggest chimpanzees could be using the forest as a natural drugstore. The study, published Thursday in the journal PLOS One, was carried out by a team led by Elodie Freymann of the University of Oxford and Fabien Schultz of Neubrandenburg University of Applied Sciences in Germany, which found that chimpanzees were consuming a variety of plants with medicinal effects but little other nutritional value, often when they had a health issue such as an injury or a parasite.
The findings offered strong support for “novel self-medicative behaviors in wild chimpanzees,” the researchers wrote, adding that further study of the animals’ behavior could “benefit our own species, potentially leading to the discovery of novel human medicines.”
The next area of investigation will be the “most interesting plant extracts” consumed by the chimpanzees, Schultz said in an email. There are “lots of ‘ifs,’” he said, but theoretically, “one day the knowledge of chimpanzees could save human lives.”
He was particularly interested in the potential application of the chimps’ go-to plants in addressing antibiotic-resistant bacteria and chronic inflammatory diseases — though he cautioned that there is a long road between this study and any possible drug breakthroughs.
The team observed two chimpanzee communities in the Budongo Forest for four months each. They tracked what the great apes ate and analyzed components of 13 plant species that seemed wholly unappetizing to a chimpanzee, such as bark and resin, to determine whether the materials had healing effects.
“Pharmacological results suggest that Budongo chimpanzees consume several species with potent medicinal properties,” the authors wrote.
Those struggling the most with parasites — something the scientists ascertained through testing their feces — had eaten plant material with the strongest antibacterial properties. An injured chimpanzee had eaten a fern with anti-inflammatory effects that was otherwise rarely consumed by the groups. All plant species, when tested in a laboratory, inhibited bacterial growth of E. coli, and some had been found in previous studies to have cancer-fighting or analgesic properties.
The authors noted that 11 of the 13 plant species had recorded uses in traditional medicine.
The researchers were surprised at the range of the ailments the chimps turned to plants for — and by the plants’ potency. “Maybe it shouldn’t have been as much of a surprise,” Freymann said in an email, “because the chimpanzees are incredibly smart and it makes perfect sense they would have figured out by now which plants can help them when ill or injured.”
She said the research showed it was “highly unlikely” the chimpanzees were eating the medicinal plants coincidentally as part of their diet. “In many of these cases, the ill or injured chimps sought out these resources when no other member of their group did,” she said.
The study adds to a body of research that suggests some animals may use plants or insects to self-medicate. Our closest cousins, the apes, have often played a starring role in this field, called zoopharmacognosy.
Last month, scientists published their observation in the journal Scientific Reports of an orangutan in Indonesia applying the juice and chewed-up leaves of a plant known for its medicinal effects to an injury on its face — which then healed without signs of infection. Two years ago, a different study of chimpanzees, in the Loango National Park in Gabon, said the animals had been seen repeatedly applying insects to wounds .
Isabelle Laumer, a primatologist and cognitive biologist at the Max Planck Institute of Animal Behavior in Germany who was the orangutan report’s lead author but was not involved in the PLOS One study, said in an interview that the new study has contributed “really important findings” that opened up avenues for further research.
“It’s always very fascinating to find out that our closest relatives are showing behaviors that we humans also show,” she said. “I think this study, again, points towards the similarities that we share.”
The authors of the PLOS One study called for strong conservation efforts to allow the continuation of such research, and to explore its potential benefits to humans in finding plants with medicinal properties. “It is imperative that we urgently prioritize the preservation of our wild forest pharmacies as well as our primate cousins who inhabit them,” they said.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Plants (Basel)

Medicinal Plants and Their Traditional Uses in Local Communities around Cherangani Hills, Western Kenya
Yuvenalis m. mbuni.
1 Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, China; moc.oohay@araromevuy (Y.M.M.); nc.sacgbw@iewgnehsgnaw (S.W.); moc.liamg@26egorojnnairb (B.N.M.); [email protected] (N.J.M.); nc.sacgbw@uhnawgnaug (G.H.)
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 National Museums of Kenya, East African Herbarium, P. O. Box 45166, Nairobi 00100, Kenya; ek.ro.smuesum@ukutump (P.M.M.); moc.oohay@olomaynouw (N.O.W.)
Shengwei Wang
4 Sino-Africa Joint Research Center (SAJOREC), Chinese Academy of Sciences, Wuhan 430074, China
Brian N. Mwangi
Ndungu j. mbari, paul m. musili, nyamolo o. walter, guangwan hu.
5 Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Wuhan 430074, China
Yadong Zhou
Qingfeng wang, associated data.
Medicinal plants are vital sources of easily accessible remedy used in the countryside healthcare system. This study aimed to find and make record of plants that are used for medicinal therapy by three communities living in Cherangani Hills. So far no single study has documented medicinal plants as a whole in the area. Ethnobotanical data were obtained through interviewing informants using semi-structured questionnaires and extracting information from journals and books. Descriptive statistical analysis was applied to describe the data. Overall 296 plant species from 80 families and 191 genera were identified. Asteraceae family was the most dominant, representing 10.7% of the total plant species recorded. Roots (35.9%) represented the most commonly used parts of the plant. The commonly used method of preparation was decoction (54.9%). The reported diseases were classified into 14 diverse ailment groups out of the 81 health conditions on their underlying user reports. Rural communities in Cherangani Hills are rich sources of plants with medicinal properties. Therapeutic uses of the compiled plants provide basic information that can aid scientists to conduct additional research dedicated to conservation of species and pharmacological studies of species with the greatest significance.
1. Introduction
Medicinal plants have been a vital source of both curative and preventive medical therapy preparations for human beings, which also has been used for the extraction of important bioactive compounds [ 1 , 2 , 3 ]. It is estimated that almost 80% of the world’s total population, regularly, depends on traditional medicine and products for its healthcare needs especially in third world countries. Many sick people in the developing regions combine the conventional medicine with traditional medicine [ 4 , 5 , 6 ]. Traditional medicines are usually cheaper than modern medicines, and probably the only natural remedies available and accessible in the remote rural communities in developing countries [ 7 ]. Rural dwellers prefer traditional medicines because of their close proximity to the traditional healers and the fact that the healers understand their culture and environment as well as their patients. In rural areas, access to western healthcare is a problem especially in the Sub-Saharan countries, because conventional healthcare is concentrated in towns [ 8 ]. Plant medicine has continuously been practiced for a long period, especially in some African tribes with a long history [ 9 ]. The Kenyan diversified flora with over 7000 plant species is one of the richest in East Africa [ 10 ]. Consequently, the higher number of plant species have led to discovery of many medicinal plants in the region. In Kenya, more than 70% of the people use local home-made remedies as their first source of medicine, while more than 90% use plant related remedies at one time or another [ 11 ]. Phytotherapy is another fundamental part of the native communities of Kenya who have vital indigenous knowledge acquired through generations. However, this practice is often less transferred owing to industrialization and adoption of western life style. Traditional knowledge in many Kenyan ethnic tribes remain untapped since the medicinal plants have not been fully documented as the information is passed orally from one generation to the other posing danger of its loss [ 8 , 10 ].
Indiscriminate trade of plant resources, uncontrolled collecting methods, habitat change, overexploitation, and climate change pose great threats to availability of plant medicine in most third world countries, thus, creating a pressing need for better methods of conservation and viable use of priority plant resources [ 12 ]. In Kenya, research on ethnobotany has been on going after independence and several publication of guides and books have been published [ 13 , 14 , 15 , 16 ]. Recording and preserving the traditional knowledge on medicinal plants has become very important practice in recent times [ 17 ]. Several ethnobotanical and ethnopharmacological research studies have been published documenting Kenya’s medicinal plant knowledge and use: Marakwet county [ 11 , 18 ], Northern Kenya [ 19 ], Siaya county [ 20 , 21 ], Tugen [ 22 ], Machakos county [ 23 , 24 ], Samburu county [ 25 , 26 , 27 ], Sekanani Valley, Maasai Mara [ 28 ], Kajiado county [ 29 , 30 , 31 , 32 , 33 ], Embu and Mbeere county [ 34 ], Makueni county [ 35 ], Mount Elgon [ 36 ], Nakuru county [ 37 ], Nandi county [ 38 , 39 , 40 , 41 ], Tharaka Nithi county [ 42 ], Kakamega county [ 43 , 44 , 45 , 46 , 47 ], Kitui county [ 48 ], Elgeyo Marakwet county [ 49 ], Kericho county [ 50 ], Machakos county [ 51 ], Narok county [ 52 , 53 , 54 ], Trans-Mara county [ 55 ], Kilifi county [ 56 ]. However, in Kenya, many areas and ethnic societies are yet to be ethno botanically surveyed.
This study focused on three communities in Cherangani Hills and the medicinal plants used to treat different ailments. The documentation of the natural resources is key as it will assist in the conservation of residual and remaining forests [ 38 ]. The databases obtained in this research forms a foundation for potential development of new medicines [ 10 ]. Ethnobotanical investigations are vital in preserving traditional medicine through suitable documentation of plants, which also assist in its sustainability [ 7 ]. Previous studies have been carried out in sections of Cherangani hills hence this research aimed to cover medicinal plants in the entire study region.
2. Material and Methods
2.1. study area.
This study covered the human settlement areas around and adjacent to the Cherangani Hills forest ecosystem found in the western side of Kenya ( Figure 1 ). Cherangani Hills reserve (35°26′ E, 1°16′ N), cuts across three counties, namely Trans Nzoia (1551 Ha), Elgeyo-Marakwet (74,249 Ha), and West Pokot (34,380 Ha), totaling 110,181 Ha, and is occupied by three ethnic groups comprising Luhya, Marakwet, and Pokot people respectively. The hills comprise 12 forest blocks where medicinal plant resources were collected and include Kipkunurr, Kapolet, Sogotio, Chemurkoi, Kaisungor, Cheboyit, Embobut, Kererr, Kiptaberr, Kapkanyar, Toropket, and Lelan [ 57 ].

Cherangani hills forest Ecosystem. ( a ) Map of Kenya ( b ) the distribution of Cherangani hills.
2.2. Selection of Respondents
Purposive sampling was applied in the field investigation, where traditional therapists and elders helped to pin point medicinal plant practitioners and emphasis was laid on both women and men [ 58 , 59 ]. Seventy-eight practitioners (38 women and 40 men) were sampled near each of the 12 forest block locations. Selected group of respondents were distinguished in the region because of their long tradition in providing services allied to traditional health remedies. Fifty-one practitioners were traditional healers and the remaining number were village elders who had acquired familiarity on medicinal healing skills of plants from their parents and close relatives.
2.3. Ethnobotanical Data Collection and Plant Identification
Ethnobotanical information were gathered between September 2017 and January 2019 by interviewing, using methodological ways designed in ethnopharmacological in field data collection. The local chiefs were informed afore about the initiation of the survey and permission was allowed. Interviews, discussions, formal and informal conversations, as well as field visits were conducted [ 60 ]. More information was sourced from literature studies including journal articles and books [ 61 ]. Botanical names, local names, diseases treated, method of preparation, dosage, and modes of administration were recorded. An interview was carried in the local dialect and translated to English. Data on habit, habitat, and plant parts used were recorded. For each described plant species, a specimen was taken and preliminary identification was performed in the field. The specimens were pressed, dried, and the identification results were confirmed at the East African herbarium. A specimen voucher number was given and prepared for each collected herbarium specimen and deposited in the East African Herbarium ( Supplementary material Table S1 ). Authentication of identified plant specimens was verified using the Flora of East African by comparisons with authenticated specimens at the East African Herbarium (EA), Nairobi, Kenya. The scientific names indicated in Supplementary material 1 , in this research work are the recognized names according to “The plant List” database.
2.4. Data Analysis
2.4.1. informant consensus factor.
Informant consensus factor (ICF) was computed using a mathematical expression: ICF = (N ur − N t )/(N ur − 1), where N ur refers to the summed up number of citations for each disease group and N t is the number of plant species used in that category [ 62 , 63 ]. The lowest ICF value is 0.00 and the highest is 1.00. Low ICF values indicates that informants do not agree on which plant medicine to use in a particular ailment, while high ICF values indicate that a limited number of plant species are known to be administered by a large number of informants to treat a specific disease. High ICF values can further be investigated and used to find species of important bioactive compounds [ 64 ].
2.4.2. Fidelity Level (FL)
Fidelity level (FL) is the total number of informants who referenced the consumption of some medicinal plants to treat a specific disease in the region and is calculated by the following formula: FL = Np/N × 100, where Np represents total number of informants citing the use of the plant to be administered to a particular disease and N denotes the total number of informants who utilized the plants as a medicine group [ 63 , 65 ]. Plant species with a higher percentage of FL shows the frequency and high usage in healing a specific disease by the informants in the community and vice versa when the percentage is low.
2.4.3. Jaccard’s Coefficient of Similarity (JCS)
Jaccard’s coefficient of similarity (JCS) was computed to compare the medicinal plant composition and their similarity with other counties in Kenya. Similarity values were computed between other areas already studied by other researchers in different regions in comparison with the present study area. JCS, was calculated as: JCS = c/(a + b + c), a representing the total number of medicinal plant species obtained in area A, b is the total number of medicinal plant species discovered only in area B, and c is the total number of common plant species occurring in areas A and B [ 66 ].
3.1. Demographic Profile of Respondents
A total of 40 (51.2%) males and 38 females (48.7%) were interviewed. The results between male and female informants were almost equal. The lowest age of informants was 15 and the highest 85 years, with the highest modal class being (66–75) years, representing 30.8%. The frequency of other age class include, 15–25 (1.3%), 26–35 (3.9%), 36–45 (6.4%), 46–55 (12.8%), 56–65 (19.2%), 66–75 (30.8%), >76–85 (25.6%) ( Table 1 ). Illiterate (42.1%), Primary (33.3%), Secondary (21.8%), Tertiary (2.7%) ( Table 1 ).
Demographic data of the informants around Cherangani Hills.
| Count | % | Expected Mean Observation | Statistics | |
|---|---|---|---|---|
| Gender | value 0.820847 | |||
| Male | 40 | 51.28 | 39 | |
| Female | 38 | 48.72 | 39 | |
| Age * | % | value < 0.001 | ||
| 15–25 | 1 | 1.28 | 13 | |
| 26–30 | 3 | 3.85 | 13 | |
| 36–45 | 5 | 6.41 | 13 | |
| 46–55 | 10 | 12.82 | 13 | |
| 56–65 | 15 | 19.23 | 13 | |
| 66–75 | 24 | 30.77 | 13 | |
| 76–85 | 20 | 25.64 | 13 | |
| Educational status * | % | value < 0.001 | ||
| illiterate | 33 | 42.31 | 19.5 | |
| primary | 26 | 33.33 | 19.5 | |
| secondary | 17 | 21.79 | 19.5 | |
| tertiary | 2 | 2.7 | 19.5 |
* Significant difference ( p < 0.05) between the averages of paired categories.
3.2. Diversity of Medicinal Plant Use
This study compiled 296 medicinal plants traditionally managing various human diseases ( Supplementary material Table S1 ) resulting to 80 families and 191 genera. The largest percentage of medicinal plants obtained belonged to the family Asteraceae (32 species), followed by Leguminosae (28), Lamiaceae (18), Rubiaceae (14), Euphorbiaceae (12), Apocynaceae (10), Malvaceae (10), and Anacardiaceae (8). The result revealed that species in Leguminosae family contained the highest percentage (8.7%) in treating different ailments. This was followed by Asteraceae (7.7%), Lamiaceae (6.1%), Rutaceae, Anacardiaceae (4.6% each), and with the rest of the families treated less than 4.2% of the ailments ( Table 2 ).
Highest families and genera of medicinal plants.
| Family | Species | Genera | Genera | Family | Species |
|---|---|---|---|---|---|
| Asteraceae | 32 | 21 | Acacia | Leguminosae | 7 |
| Leguminosae | 28 | 15 | Vernonia | Vernonia | 6 |
| Lamiaceae | 18 | 11 | Crotalaria | Leguminosae | 5 |
| Rubiaceae | 14 | 9 | Rhus | Anacardiaceae | 5 |
| Euphorbiaceae | 12 | 8 | Maytenus | Celastraceae | 5 |
| Apocynaceae | 10 | 8 | Solanum | Solanaceae | 5 |
| Malvaceae | 10 | 6 | Helichrysum | Asteraceae | 4 |
| Anacardiaceae | 8 | 4 | Dombeya | Malvaceae | 4 |
| Amaranthaceae | 6 | 4 | Ficus | Moraceae | 4 |
| Celastraceae | 6 | 2 | Polygonum | Polygonaceae | 4 |
| Solanaceae | 6 | 2 |
3.3. The Habitat for Medicinal Plants
The most common plant habitat identified was bushland 20.0%, followed by escarpment 17.9%, highland forest 14.8%, grassland forest 13.8%, woodland 9.5%, riverine 7.4%, valley 6.2%, cultivated 4.8%, wooded grassland 2.9% and forest margins at 2%. Figure 2 constitutes the habitats of medicinal plant species, of Cherangani hills, consequently a high plant diversity for the production of roots, bark, leaves, fruits, and flowers as medicinal resources.

Plant habitats for medicinal plants of Cherangani Hills.
3.4. Habit, Parts Used for Medicine and Methods of Preparation
Growth habit of shrubs have the highest percentage of 35.1% of the total medicinal plants in this study. Total of 27.5% of trees are represented by the total number plant species ( Figure 3 a), followed by herbs (26.5%), climbers (10%), epiphytes, and parasites with 0.3% each. The plant parts used include roots (35.9%), leaves (34.9%), bark (15.0%), fruits (5.2%), branches (5.0%), whole plant (1.9%), flowers (1.1%), seeds (0.2%), and barks of roots (0.2%) ( Figure 3 b). People living in the study area use different methods to prepare different medicines for treatment of different ailments. Decoction (boiling) proved to be used more commonly as the mode of preparation (53.3%), followed by pounding/crushing (24.5%), and chewing (9.3%). Other preparation methods represented less than 5% ( Figure 4 ).

( a ) Medicinal plant habit, ( b ) plant parts for herbal preparation around Cherangani Hills.

Preparation methods for medicinal plants.
3.5. Informant Consensus Factor (ICF)
To obtain the accurate ICF, the reported diseases were grouped into 14 different ailment groups out of the 81 health conditions based on their use reports ( Table 3 ). The results of the reported ailments are as follows; digestive system disorders (25.2%), respiratory tract infections (18.3%), parasitic diseases (17.9%) ( Table 3 ). Stomachache (8.9%), malaria (6.7%), aphrodisiac (6%), coughing (4.8%), and abdominal pains (3.4%) were the most common disease mentioned. Within the three major disease groups, digestive system disorders had 139 use-reports, followed by respiratory tract infections (101) and parasitic diseases and other infections (99) use-reports. The greatest ICF (0.79) was mostly for metabolic disorders, followed by gynecological issues (0.76). Respiratory tract infections, erectile dysfunctions, and impotence were less frequently and had the lowest IFC of 0.27 and 0.18 respectively.
Informant consensus factors for categorized ailments.
| Ailment Categories | Specific Conditions | Number of Used Reports | % of Total Species | No. of Taxa | ICF |
|---|---|---|---|---|---|
| Digestive system disorders | Ulcers, diarrhea, stomachache, dysentery, constipation, low appetite, nausea, purgative, intestinal worms, gastrointestinal disorders, amoeba. | 139 | 25.18 | 108 | 0.22 |
| Respiratory tract infections | Cold, cough, respiratory infections, asthma, bronchitis, flue, sore throat, tuberculosis | 101 | 18.30 | 74 | 0.27 |
| Parasitic diseases and other infections | Malaria, fever, measles, headache, yellow fever, ear, conjunctivitis, toothache, mouth blisters eye infections | 99 | 17.93 | 61 | 0.62 |
| Erectile dysfunctions and importance | Male sexual vitality, aphrodisiac | 12 | 7.07 | 10 | 0.18 |
| Gynecological issues | Fertility enhancer, heavy menstrual flows, uterine cleansing, weakness during pregnancy, induction of labor, sterility in women, induce pregnancy, removing placenta, regulation of monthly periods, abortion, after birth pains, menstrual pains | 31 | 5.98 | 8 | 0.76 |
| Skin infections | Wounds, burns, smallpox, ringworms, warts, skin rashes, leprosy, astringent, boils | 33 | 5.62 | 9 | 0.75 |
| Circulatory system diseases | Hypertension, anemia, cuts, hemorrhoids, blood cleanser, hemorrhage, heart attack reduce bleeding, edema. | 22 | 3.99 | 8 | 0.67 |
| Blood and Urinary system disorders | Urinary infections, kidney inflammations. | 11 | 3.8 | 4 | 0.70 |
| Poisonous and animal bites | Snake, centipede and insect bites | 16 | 2.89 | 8 | 0.53 |
| Muscular-skeletal problems inflammation | Backache, joint pains, rheumatism, fractures, joints inflammation, swollen body parts. | 21 | 2.72 | 10 | 0.50 |
| Neurological and nervous System disorders | Convulsions, epilepsy, memory and neurological disorders, madness reduction. | 5 | 2.17 | 4 | 0.50 |
| Genital apparatus diseases | Genital organs infection, sterility, infertility, prostate infections, syphilis, and gonorrhea | 39 | 1.99 | 12 | 0.71 |
| Metabolic disorders | Liver diseases, hepatic. | 15 | 1.45 | 6 | 0.79 |
| Cancers | Breast cancer, prostate cancer, skin cancer | 8 | 0.91 | 4 | 0.57 |
3.6. Fidelity Level (FL)
The calculated fidelity level (FL) of 18 important plant species varied from 36.2 to 90.9% ( Table 4 ). Carissa spinarum L. and Warburgia ugandensis Sprague depicted 90.5% and 90.9% FL respectively against malaria and respiratory disorders as the most utilized plants. Asparagus racemosus Willd. and Tragia brevipes Pax. registered FL of (36.2%) and (38.5%) treating kidney diseases and rheumatism respectively. Clausena anisata (Willd.) Hook.f. ex Benth. at 60% FL proved to treat heart diseases according to user reports in the study area. Respondents also preferred using Basella alba L. as a vegetable and in the study area it stood at 85.7% FL.
Medicinal plants highly utilized in Cherangani.
| Frequently Used Species | Local Name | Part Used | Popular Use | N | N | FL% | References |
|---|---|---|---|---|---|---|---|
| (A.Rich.) Hochst. | Arolwa (M), Roluwo (P) | R, B | Enlarged spleen and liver | 32 | 59 | 54.3 | [ ] |
| L. | Loketetwo (P) Eshikata (L) | R, L | Malaria | 38 | 42 | 90.5 | [ , , , ] |
| (Hochst.) R.Br. ex Vatke | Chebobet (M), Shikuma (L) | R | Chest pains | 16 | 25 | 64.0 | [ , , , , , , ] |
| Engl. | Cherotwo (M), Tolkos (P), Linakha (L) | L | Pneumonia | 24 | 27 | 88.9 | [ , , , , , , ] |
| (Hook.f.) Skeels | Mukombelo (L) | R | Aphrodisiac | 24 | 36 | 66.7 | [ , , ] |
| (L.) Lam. | Kipkeres (M), Katamwa (P) | R, Fr, B | Coughs, Colds | 35 | 47 | 74.5 | [ , , , , , , , , , ] |
| (Willd.) DC. | Lamaiwo (M), Cheptimanwa (P) | B, Fr | Abdominal pains | 13 | 17 | 76.5 | [ , , , , ] |
| L. | Kimonwo (M), Pondon (P) Libono (L) | R, L | Diarrhea | 20 | 28 | 71.4 | [ , , , , , , , , ] |
| DC. | Gorgorwa (P), Korkorwo (M) Omurembe (L) | B, L | Indigestion | 23 | 34 | 67.6 | [ , , , , , , , , , , ] |
| (Hook.f.) Kalkman | Tendwo (M) | B, L | Prostate cancer | 9 | 14 | 64.3 | [ , , , , , , , , , ] |
| Willd. | Kabungai (M) | R | Kidney diseases | 17 | 47 | 36.2 | [ , , , , ] |
| Sprague | Sokwo (M) | B, L | Respiratory disorders | 20 | 22 | 90.9 | [ , ] |
| (L.) Dunal | Tarkukai (M), Akakagh (P) | R, L | Relives labor pains | 21 | 29 | 72.4 | [ , , , , , , , ] |
| L. | Inderema (L), | L | Regulates monthly periods | 24 | 28 | 85.7 | [ , , , , , , , ] |
| (Willd.) Hook.f. ex Benth. | Cheboinoiywa (M), Kisimbari (L) | R, B, Br | Heart diseases | 6 | 10 | 60.0 | [ , , , , , ] |
| Quart.-Dill. and A.Rich. | Sinindet (M), Sinendet (P) | Br, L | Syphilis and Gonorrhea | 32 | 43 | 74.4 | [ , , , , , ] |
| Mildbr. | Kimelei (M) | R, L | Ulcers | 27 | 31 | 87.0 | [ , , , ] |
| Pax | Kimelei (M), Chemelei (P) | Br, L, R | Rheumatism | 5 | 13 | 38.5 | [ , , , , , , , ] |
Key: Local name: Marakwet = (M), Pokot = (P), Luhya = (L); Parts used (PU): L—leaves, R—roots, B—bark, Fr—fruit, Br—branches; N P = represents the number of people mentioning a particular disease treated by a particular plant; N = represents the informants who used the local plants as a medicine group; FL = fidelity level.
3.7. Jaccard’s Coefficient of Similarity
This study represents the first scientific documentation of ethnobotanical uses of 296 medicinal plant used by the three communities in Cherangani hills. The current report on the ethnomedicinal uses of plants was compared to those of previous studies done in other regions of Kenya ( Table 5 ). It was found that Marakwet (18%), Sungurur (16%), and Keiyo (14%) had the highest Jaccard’s coefficient of similarity in the makeup of medicinal plant species whereas the degree of similarity was lower in areas like Nandi (0.05%) and Kitui (0.06%) ( Table 5 ).
A comparison of medicinal plants within the study area and those in other extents.
| Study Area (County) | Year of Study | Species No. (x and y) | Common Species (z) | Jaccard’s Coefficient | % Similarity | References |
|---|---|---|---|---|---|---|
| Cherangani | 2019 | 286 | This review | |||
| Machakos | 2018 | 51 | 23 | 0.06 | 6 | [ ] |
| Kakamega | 2018 | 250 | 66 | 0.13 | 13 | [ ] |
| Kakamega | 2018 | 94 | 54 | 0.12 | 12 | [ ] |
| Sungurur | 2017 | 99 | 72 | 0.16 | 16 | [ ] |
| Makueni | 2017 | 42 | 21 | 0.06 | 6 | [ ] |
| Nandi | 2015 | 56 | 34 | 0.09 | 9 | [ ] |
| Tharaka Nithi | 2015 | 72 | 21 | 0.06 | 6 | [ ] |
| Marakwet | 1978 | 111 | 86 | 0.18 | 18 | [ ] |
| Kakamega | 2014 | 65 | 25 | 0.07 | 7 | [ ] |
| Keiyo | 2014 | 73 | 59 | 0.14 | 14 | [ ] |
| Mt. Elgon | 2010 | 107 | 52 | 0.12 | 12 | [ ] |
| Nandi | 2008 | 40 | 19 | 0.05 | 5 | [ ] |
| Embu and Mbeere | 2007 | 86 | 45 | 0.11 | 11 | [ ] |
3.8. Threats to Medicinal Plants
Informants’ responses showed that many factors have contributed to the threats faced by plants of medicinal importance in the study area. ( Table 6 ). Agricultural expansion (38.5%) was the main threat to important medicinal species, followed by overgrazing (20.5%), overharvesting (17.9%), firewood and Charcoal production (10.3%), environmental degradation (7.7%). Some respondents pointed out that other threats exist within the study area that are a result of deforestation and loss of habitat (5.1%).
Threats to medicinal plants in Cherangani Hills.
| Threats | Frequency (N = 78) | Percentage (%) |
|---|---|---|
| Agricultural expansion | 30 | 38.5 |
| Overgrazing | 16 | 20.5 |
| Overharvesting | 14 | 17.9 |
| Firewood and Charcoal production | 8 | 10.3 |
| Environmental degradation | 6 | 7.7 |
| Others | 4 | 5.1 |
4. Discussion
The communities around Cherangani hills forest reserve use a large diversity of flora in the treatment of a myriad of diseases and the native people have a broad traditional knowledge on plants of medicinal importance. The higher percentage of people that rely on medicinal plants could be attributed to the high cost of western medicine and inaccessibility of government medical facilities [ 33 , 74 ]. There was an insignificant difference between men and women in the knowledge of medicinal plants. Comparing with other study area in Kenya [ 51 ], there was no gender preference in the passing of medicinal plants knowledge from the parents to their offspring across local communities around Cherangani Hills. Informants in the age group above 45 years appeared to know more medicinal plants perhaps as a result of having more experience interacting with medicinal plants in their ecosystem. Additionally, fewer medicinal plants were known to those who attended tertiary levels of education compared to illiterate informants. The insignificant use of the plants of medicinal importance by the literates in the community can be attributed to lack of general preparation procedures and scientific information on their efficiency as well as their toxicity levels. Additionally, the collection as well as storage methods were identified as essential considerations by the literate members in the community. This indicates that there exists a generational disjunction in the passage of traditional medicinal plant knowledge. This can be linked to the influence of formal education as it was observed that illiterate informants had an upper hand in medicinal plants knowledge as compared to their tertiary level counterparts. Exposure of younger people to modern education and lifestyle has led them to prefer western medical treatment over traditional medicine hence despising medicinal plant treatments compared to those unexposed and uneducated [ 70 ].
Out of the 296 medicinal plants recorded, shrubs are commonly used because of their relatively higher resistance to drought, hence preferred as they are available for harvesting all year round [ 75 ]. Roots are most preferred compared to other parts of the plant as they are traditionally considered to have a higher strength of medicine and are readily available in all the seasons of the year [ 33 , 76 , 77 , 78 ]. Leaves are also highly utilized because they are obtained easily in large quantities in contrast to other plant parts. Moreover, a majority of traditional healers prefer to use leaves as they are considered to accumulate active ingredients by photosynthetic pigments such as alkaloids and tannins [ 79 , 80 ].
A myriad of methods of preparation are used within the three communities of Cherangani Hills and Kenya as a whole. In the study area it was uncovered that decoction was the most widely used method of preparation mainly because of the ease of using water to prepare them. Such a large variety of preparation methods that have been studied has been highlighted in some parts of Kenya and in other countries [ 36 , 48 , 51 , 61 , 76 , 81 ]. It has been established that more than one method is used in preparing many of the medicinal plants studied. However, the type of plant species, condition of ailment being treated, and plant parts used determined the method of preparation. Within the Marakwet, Luhya, and Pokot communities, the common way of administration of the prepared medicine was through drinking, which is in line with many other studies [ 36 , 48 , 51 ].
Gastrointestinal ailments were the most frequently treated using medicinal plant followed by sensory-neuron diseases. In similar fashion, disorders of the gastrointestinal system and parasitic infections were the commonly treated ailments and similar results have been reported in Kenya and Zegie peninsula [ 33 , 77 , 82 ]. Stomachache and diseases related to digestive system could be attributed to poor sanitation as a result of high levels of poverty within the study region as in the case cited by other studies [ 47 , 77 , 78 , 83 ]. High FL of a species in the scope of this study shows the extensive use of a particular plant species to treat certain diseases by the inhabitants because of its ease of accessibility and its effectiveness to treat the diseases. Such information may lead to the efficacy of these plants and their chemical and pharmacological components of the reported activity against various diseases. For example, Carissa spinarum L. with FL = 90.5 is good for treating malaria. The plant contains important bioactive constituents including glycosides, acids, saponins, tannins, terpenoids, and alkaloids which have medicinal value [ 79 , 84 ]. Within the area studied, Carissa spinarum L. is mainly used in treating malaria, chest pains, epilepsy, diarrhea, coughs, breast cancer, arthritis, and gonorrhea.
Different ecological climatic conditions have been characterized with different plant diversity, hence pointing to some of the probable reasons for similarities and differences of plants of medicinal value found in our study area and other extents. Data collected and analyzed from the region of study reveal remarkable differences in parts of the plant used, preparation mode of herbal medicine and their use as has been documented in other regions. However, 82% of medicinal applications were new and unique to the study at hand.
The use of medicinal plant species recorded in the same study area had 14%, 16%, and 18% JCS respectively and the neighboring areas like Kakamega [ 44 , 46 , 47 ] showed remarkable similarity at, 12%, 12%, and 13% JCS respectively. There was close resemblance in terms of the usage of medicinal plants because of close proximity to the research area.
Threatened medicinal plant species recorded in this study include Warburgia ugandensis Sprague. (VU) and Ansellia africana Lindl. (VU). The rest of the medicinal plants are either data deficient (DD), not evaluated (NE), or of least concern (LC), by IUCN. Many factors have been associated with the dangers faced by the medicinal plants in the study region. Informants’ insights show that the main threats to plants of medicinal value were forest encroachment for agricultural expansion, overharvesting, overgrazing, and environmental degradation ( Table 6 ). Majority of the respondents indicated that agricultural activity (38.5%) was a significant danger to the existence of medicinal plants and their conservation because of an increase in human population. Some respondents pointed out that medicinal plants within the area of study had other threats as a result of deforestation and loss of habitat. A study by Mutwiwa [ 51 ] also listed overgrazing, charcoal burning, and environmental degradation as some of the threats faced by the plants of medicinal value in Machakos County, Mwala sub-county, Kenya. From our assessment in the course the field investigations, it was further noted that none of the listed medicinal plants were cultivated by the communities.
5. Conclusions
Planting fast-growing plant species for the production of charcoal would greatly help in lowering the harvesting of medicinal plants and enhance the conservation of vulnerable plant species. Proper grazing management of domestic animals should be enforced by the authorities in the forest reserves to reduce overgrazing especially in the areas of the forest that are more susceptible to overgrazing like the forest periphery. Proper harvesting regulations should be implemented and followed to reduce overharvesting and overexploitation of medicinal plants especially those species that are used more frequently to treat common ailments. This study provides a detailed report and an appreciation of medicinal knowledge among the three communities around the Cherangani Hills. The awareness of the importance of medicinal plants in human healthcare is important as scientific evaluation promises their future use in the development of new drugs for emerging diseases. The information on medicinal plants, dosages, and the ailments treated might be heavily eroded in the days to come because of the observed poor record keeping and the increasing use of western medication. This inventory therefore can be used as a source of information for the conservation agencies to enable proper management of plant biodiversity and its resources.
Acknowledgments
We are in gratitude to the Kenya Forest Service and National Environment and Management Authority for enabling the access to permits that empowered us to take on botanical investigations in the Cherangani Hills Forest. Our appreciation also goes to the research interviewees for generously sharing the information with us. We are indebted to the participating students and staff of University of Chinese Academy of Sciences and talented staff of the East African Herbarium, botany department, Nairobi, who helped in the data collection and identification.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/3/331/s1 . Table S1: Medicinal plants of Cherangani hills, their habit, habitat, part used, method of preparation and administration, and references. Abbreviations for voucher specimens: FOKP- Flora of Kenya Project; SAJIT- Sino Africa Joint Investigation Team; YMM = Yuvenalis Morara Mbuni; IR = Interview results [ 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 ].
Author Contributions
Y.M.M. designed and conceptualized the study. Y.M.M., S.W., B.N.M., N.O.W., N.J.M., P.M.M. and G.H. carried out the ethno-botanical survey, investigation, data curation. Y.M.M. and S.W. drafted the manuscript and analyzed the data. G.H., Y.Z. and Q.W. administered the project, supervised and reviewed the analyzed data, and gave constructive comments. All authors have read and agree to the published version of the manuscript.
This work obtained funding through grants from Sino-Africa Joint Research Center, CAS, China (Y323771W07 and SAJC201322), and National Natural Science Foundation of China (31800176).
Conflicts of Interest
No conflict of interest.

IMAGES
VIDEO
COMMENTS
Figure 5. Temporal evolution on medical plants publications for Top 12 countries. The first group is the leaders of this research, China and India, with between 800 and 1100 publications per year. China led the research from 1996 to 2010, and from this year to 2016, the leader was India, after which it returned to China.
Based on current research and financial investments, medicinal plants will, seemingly, continue to play an important role as a health aid (Hoareau and DaSilva, 1999). The use of traditional medicine and medicinal plants in most developing countries, as a normative basis for the maintenance of good health, has been widely observed ( UNESCO, 1996 ).
Medicinal plants represent the most ancient form of medication, used for thousands of years in traditional medicine in many countries around the world. The empirical knowledge about their beneficial effects was transmitted over the centuries within human communities [ 1 ]. Natural products play a pivotal role as a source of drug compounds and ...
The use of medicinal plants has been done since ancient times and may even be considered the origin of modern medicine. Compounds of plant origin have been and still are an important source of compounds for drugs. In this study a bibliometric study of all the works indexed in the Scopus database until 2019 has been carried out, analyzing more ...
Abstract. This comprehensive review aims to provide an in-depth analysis of the therapeutic properties of medicinal plants. The paper begins with an introduction to medicinal plants, providing an ...
Integrative research on medicinal plants should lead to a greater focus on conserving genetic, phenotypic, chemical, and biocultural diversity, especially near their centers of origin, where such diversity is likely to be high, and on revealing and preserving the complicated history and relationships between humans and medicinal plants.
The Divine Farmer's Materia Medica, the earliest medical text in traditional Chinese medicine, written around ad 200, describes the medicinal and toxicological properties of 365 entries, most of ...
Traditional medicinal plants are an important element of indigenous medical systems in China and rest of the world. 4 Traditional medicine refers to any ancient and culturally based health care practice differing from scientific medicine and is largely transmitted orally by communities of different cultures. 5 Traditional system of medicine is one of the centuries-old practice and long-serving ...
In 1998, Simmonds helped raise funds to create a 7,000-sample traditional Chinese medicine plant collection at Kew, and she currently leads a 300-strong research team focused on unlocking ...
Medicinal Plant. Herbalism or herbal medicine is the study of medicinal herbs and plants, their botanical properties, their uses, and their applications for welfare of mankind. ... This chapter presents research advances done for the search of suitable drugs from medicinal plants against viruses with special consideration of severe acute ...
Phenolic content of tested medicinal and food plants. Results of colorimetric and spectrophotometric analysis of seven medicinal and food plants were showed in Table 1.In general, the total ...
This chapter focuses on reviewing publications on medicinal plants used in the treatment of common diseases such as malaria, cholera, pneumonia, tuberculosis and asthma. Traditional medicine is still recognized as the preferred primary health care system in many rural communities, due to a number of reasons including affordability and effectiveness. The review concentrated on current ...
Medicinal plants and their traditional uses in different locations. Juan José Maldonado Miranda, in Phytomedicine, 2021. 4 Medicinal plants. The term medicinal plants was first used in 1967 in the context of the study of hallucinogenic plants. A medicinal plant is that species of the plant kingdom, whose parts (flowers, leaves, roots, stems, fruits, or seeds) are directly used or used in some ...
Briefly, the research was done using medicinal plants in "article title, abstract, keywords" and then 175,818 documents were retrieved. The second step was the limitation of the results to ...
Recently, we proposed an in vitro model based on the interaction between the medicinal plant E. purpurea and its microbiota to connect fundamental and applied research on plant secondary metabolite modulation . We are confident that this model and other ones developed in the same way can represent a valuable tool to discover new strategies in ...
Accordingly, medicinal-plant-based drug discovery still remains an important area, hitherto unexplored, where a systematic search may definitely provide important leads against various pharmacological targets.Ironically, the potential benefits of plant-based medicines have led to unscientific exploitation of the natural resources, a phenomenon ...
The book summarizes current research status, gaps in knowledge, agro-industrial potential, waste or residual plant biomass management, conservation strategies, and computational approaches in the area of medicinal and aromatic plants with an aim to translate biotechnological interventions into reality.
Keywords: medicinal plants; drugs; worldwide research; bibliometrics; traditional medicine. 1. Introduction. T en percent of all vascular plants are used as medicinal plants [1], and there are ...
The potential active chemicals found in medicinal plants, which have long been employed as natural medicines, are abundant. Exploring the genes responsible for producing these compounds has given new insights into medicinal plant research. Previously, the authentication of medicinal plants was done via DNA marker sequencing.
2. Antimicrobial Activity of Medicinal Plant Extracts. Extracts isolated from medicinal plants have been reported exhibit various biological activities such as antimicrobial, anti-inflammatory, and antioxidant activities [].The antimicrobial compounds from medicinal plants may inhibit the growth of bacteria, fungi, viruses, and protozoa by different mechanisms than those of presently used ...
The results provide compelling evidence that chimpanzees seek out specific plants for their medicinal effects. The study is the most in-depth analysis to date that combines both behavioral and pharmacological evidence of the medicinal benefits to wild chimpanzees of feeding on bark and dead wood.
Many plants produce compounds that have medicinal effects on humans and other animals. Wild chimpanzees eat a variety of plant matter, including some that is nutritionally poor but may treat or ...
The use of medicinal plants has been done since ancient times and may even be considered the origin of modern medicine. Compounds of plant origin have been and still are an important source of compounds for drugs. In this study a bibliometric study of all the works indexed in the Scopus database until 2019 has been carried out, analyzing more than 100,000 publications. On the one hand, the ...
The plant preparations were given to the livestock orally for respiratory ailments and topically for dermatological disorders. The most commonly used plant parts were leaves (91%), bark (25%) and roots (29%), and in many cases more than one plant part was used to prepare the remedies.
A medicinal plant is any plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes, or which are precursors for chemo-pharmaceutical semi-synthesis.
Introduction. Medicinal plants (MPs) are the main source of natural metabolites such as pigments, condiments, insecticides and medicines. MPs have been used to treat diverse diseases in China, India and Egypt for 5000 years and are still used today, despite the availability of pharmaceuticals [].Plant-derived monomers (morphine, artemisinin, taxol, digitali, vinblastine, etc.) are essential ...
2) In 2021, researchers looked at how well an ointment formulation containing Lamium album extract healed wounds; the results were published in the Journal of Medicinal Plants. When tested on rats ...
She said the research showed it was "highly unlikely" the chimpanzees were eating the medicinal plants coincidentally as part of their diet. "In many of these cases, the ill or injured ...
those reported by other researches on sh fed with medicinal plant extracts, including rainbow trout [54], Nile tilapia [55], gold sh [ 56 ] and striped cat sh [ 57 ].
1. Introduction. Medicinal plants have been a vital source of both curative and preventive medical therapy preparations for human beings, which also has been used for the extraction of important bioactive compounds [1,2,3].It is estimated that almost 80% of the world's total population, regularly, depends on traditional medicine and products for its healthcare needs especially in third world ...