- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies

Case Study: Sickle Cell Disease A 25-Year-Old in Transition
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 25-year-old woman with a history of sickle cell disease (SCD) presents to the clinic for follow-up after a hospitalization for a vaso-occlusive pain crisis complicated by influenza A. She has a history of an acute ischemic stroke at age 5 years and has received monthly, simple red cell transfusions since the stroke. Her last transfusion was approximately four months prior. She is taking deferasirox 20 mg/kg daily but occasionally misses doses.
Laboratory results show the following:
| Hemoglobin | 7.5 g/dL |
| Hematocrit | 24% |
| Leukocyte count | 9,300/mm |
| Platelet count | 202,000/m |
| Mean corpuscular volume | 105 fL |
| Hemoglobin electrophoresis | 92% HbS, 6% HbF, 2% HbA2 |
| Aspartate aminotransferase | 24 U/L |
| Alanine aminotransferase | 45 U/L |
| Ferritin | 1,300 ng/mL |
Which of the following is the next best step in diagnosis
- Restart scheduled red blood cell transfusions
- Start prophylactic penicillin
- Discontinue transfusions and start hydroxyurea
- Order transcranial doppler ultrasonography (TCD) to assess risk of stroke
- Increase dose of deferasirox to 25 mg/kg/day
Explanation
The incidence of primary stroke in children with SCD is 0.6 to 0.8 events per 100 patient-years, with a cumulative incidence of 7.8 percent by age 14 years in the Jamaican cohort and 11 percent by age 20 years in the U.S. Cooperative Study of Sickle Cell Disease. Once stroke has occurred, the incidence of recurrent (secondary) stroke ranges from 47 to 93 percent in patients not started on regular transfusions. The Stroke Prevention Trial in SCD (STOP) randomized 130 high-risk children with SCD to either transfusion therapy (to maintain HbS 30%) or observation. These high-risk children had an increased blood flow in the internal carotid or middle cerebral artery by TCD. This study showed a 92 percent reduction in incidence of first stroke in transfused high-risk patients. A follow-up study, STOP2, randomly assigned 72 children whose TCD had normalized after 30 months of transfusion therapy to either ongoing or discontinued transfusions. The study was closed early due to a significant increase in abnormal TCD velocity and stroke risk for those who halted transfusion therapy.
The multicenter phase III TWiTCH trial evaluated children with SCA and abnormal TCD velocities without a history of stroke on chronic transfusions. Data showed that hydroxyurea at maximal tolerated dose was noninferior to chronic transfusions for maintaining TCD velocities as primary stroke prophylaxis (choice C). This patient has a history of ischemic stroke, so the results of TWiTCH do not apply to her.
The Stroke with Transfusions Changing to Hydroxyurea (SWiTCH) study was designed as a phase III multicenter trial to determine the efficacy of hydroxyurea/phlebotomy, compared with transfusions/chelation for children with SCA, stroke, and iron overload in secondary stroke prophylaxis. The primary endpoint was a composite of noninferiority for stroke prevention and superiority for reduction of liver iron content. The trial was terminated at the first scheduled interim analysis for futility for the composite endpoint, which required superiority of phlebotomy over iron chelation for reducing excess iron stores. The incidence of stroke on the hydroxyurea plus phlebotomy arm was higher (7 of 67 patients; 10.4%) than in the transfusion plus chelation arm (1 of 66 patients; 1.5%). These results, though not powered for inferiority, showed a trend towars increased stroke risk with transition to hydroxyurea. In patients with prior stroke, cessation of transfusion therapy is currently not recommended.
Whether chronic transfusion therapy can be stopped after a longer period of transfusions in a patient with a prior stroke remains unclear even though risk of recurrent stroke remains high in adolescence and young adulthood. In patients older than 16 years, TCD velocity criteria to determine stroke risk is not reliable (choice D).
In the Prophylaxis with Oral Penicillin in Children with Sickle Cell Anemia trial, children with SCA were randomly assigned to receive oral prophylactic penicillin or placebo PROPS 1986 ). The trial ended eight months early after the occurrence of 15 cases of pneumococcal sepsis, 13 in the placebo group and two in the penicillin group, showing an 84 percent reduction in pneumococcal sepsis with penicillin prophylaxis. The follow-up study, PROPS II, did not show an increased risk in pneumococcal infections with discontinuation of prophylactic penicillin after age 5 years. Therefore, prophylactic penicillin is not recommended in adults with SCA (choice B).
The trajectory of ferritin in this patient has not been established and an increase in oral iron chelation is not indicated at this time.
Case Study submitted by Marquita Nelson, MD, of University of Chicago, Chicago, IL.
- Hirst C, Owusu-Ofori S Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease . Cochrane Database Syst Rev. 2014 6:CD003427.
- Valadi N, Silva GS, Bowman LS, et al Transcranial Doppler ultrasonography in adults with sickle cell disease . Neurology. 2006 22:572-574.
- Ware RE, Davis BR, Schultz WH, et al Stroke with transfusions changing to hydroxyurea (SWiTCH) . Blood. 2012 119:3925-3932.
- Kumar N, Gross JB Jr, Ahlskog JE TCD with transfusions changing to hydroxyurea (TWiTCH): hydroxyurea therapy as an alternative to transfusions for primary stroke prevention in children with sickle cell anemia . Blood. 2015 126:3.
American Society of Hematology. (1). Case Study: Sickle Cell Disease A 25-Year-Old in Transition. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition .
American Society of Hematology. "Case Study: Sickle Cell Disease A 25-Year-Old in Transition." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition (label-accessed June 15, 2024).
"American Society of Hematology." Case Study: Sickle Cell Disease A 25-Year-Old in Transition, 15 Jun. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/sickle-cell-disease-a-25-year-old-in-transition .
Citation Manager Formats
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Sickle cell anemia
Best practices for patient-centered care.
GENERAL PURPOSE: To provide information on patient-centered care best practices for treating vaso-occlusive crisis due to SCA. LEARNING OBJECTIVES/OUTCOMES: After completing this continuing-education activity, you should be able to: 1 . Describe the prevalence, financial aspects, incidence, and pathophysiology of SCA. 2 . Summarize patient-centered management strategies for patients with vaso-occlusive crisis due to SCA.
- When describing SCA to a patient, the nurse explains that it
- affects more than 1 million people in the US.
- causes RBCs to have longer life spans.
- is an inherited disorder causing a defect of the hemoglobin molecule in RBCs.
- The annual Medicare cost due to patients with SCA is estimated at
- $10 million.
- $100 million.
- $1 billion.
- The US occurrence rate for SCA is
- 1 out of every 365 births.
- 1 out of every 950 births.
- 1 out of every 1,000 births.
- The case study of Mr. E explains his symptoms when arriving at the ED. After an evaluation, the healthcare providers believe that he's experiencing
- drug-seeking behavior.
- a hypertensive crisis.
- a vaso-occlusive crisis.
- In the case study, the healthcare providers for Mr. E feel that his SCA crisis could be related to
- depression.
- dehydration.
- gastroesophageal reflux disease.
- In the case study, the hospitalist reviews Mr. E's existing medications and then addresses management of his present crisis, which includes providing
- esomeprazole.
- lisinopril.
- In adults with SCA, one of the most common symptoms of vaso-occlusive crisis is
- extremity pain.
- Patients with SCA have blood cells that contain an abnormal form of hemoglobin known as
- hemoglobin C.
- hemoglobin S.
- beta-globin hemoglobin.
- Patients with SCA have misshaped RBCs that cause an inadequate blood and oxygen supply by obstructing blood vessels, which leads to
- heavy bleeding.
- various malignancies.
- The patient with SCA begins to show signs of jaundice. This is the result of rupturing sickle-shaped blood cells releasing hemoglobin into the
- gallbladder.
- bloodstream.
- Darbari identified an intensification of stigma toward patients with SCA, including
- “drug seeking.”
- “hypochondriac.”
- “nonadherence.”
- The patient arrives at the hospital experiencing a vaso-occlusive crisis due to SCA. The standard first-line treatment is initiated, which includes
- an RBC transfusion.
- opioid administration.
- comfort measures and relaxation techniques.
- When a patient with SCA is experiencing a crisis, barriers to receiving care for symptom control in the ED include
- overcrowding.
- overtraining of healthcare providers.
- reduced turnover rates of healthcare providers.
- According to the Expert Panel Report released by the National Heart, Lung, and Blood Institute, the best route of medication administration for vaso-occlusive crisis is I.V. or
- subcutaneous.
- The patient with SCA is experiencing vaso-occlusive pain and asks for meperidine. The healthcare provider explains that this medication
- should be avoided.
- needs to be increased by 25% until the pain is controlled.
- is usually combined with another medication to increase effectiveness.
- Within 30 minutes of triage in the ED, safe and effective relief of vaso-occulusive crisis includes
- strict fluid and food restrictions.
- rapid analgesia administration of opioids.
- avoidance of using heat to the affected area.
- Stat labs for vaso-occlusive crisis would most likely include
- drug toxicology.
- sedimentation rate.
- lactate dehydrogenase.
- After the patient with SCA is treated with first-line therapy for severe pain, a CAM therapy is initiated, which includes
- meperidine.
- cold compresses.
- When using morphine for a vaso-occlusive crisis, it acts by increasing
- adverse reactions and shouldn't be used.
- blood flow to areas with less blood flow.
- the stiff sickle shape of RBCs.
- During treatment for SCA, patients are often
- instructed to restrict fluids.
- provided I.V. fluids due to dehydration.
- comforted with cold compresses on painful areas.
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Bullying on the unit, apixaban for a-fib, nurses and smoking cessation: get on the road to success, one hospital's war on diabetes, fine-tuning osteoporosis outcomes.
CLINICAL CASE
Introduction, clinical trial considerations, conclusions, acknowledgments, conflict-of-interest disclosure, off-label drug use, clinical trial considerations in sickle cell disease: patient-reported outcomes, data elements, and the stakeholder engagement framework.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Sherif M. Badawy; Clinical trial considerations in sickle cell disease: patient-reported outcomes, data elements, and the stakeholder engagement framework. Hematology Am Soc Hematol Educ Program 2021; 2021 (1): 196–205. doi: https://doi.org/10.1182/hematology.2021000252
Download citation file:
- Ris (Zotero)
- Reference Manager
Visual Abstract

Patients with sickle cell disease (SCD) have significant impairment in their quality of life across the life span as a consequence of serious disease burden with several SCD-related complications. A number of disease-modifying therapies are currently available, yet long-term clinical benefits in real-world settings remain unclear. Over the past few years, a number of important initiatives have been launched to optimize clinical trials in SCD in different ways, including: (1) established panels through a partnership between the American Society of Hematology (ASH) and the US Food and Drug Administration; (2) the ASH Research Collaborative SCD Clinical Trials Network; (3) the PhenX Toolkit (consensus measures for Phenotypes and eXposures) in SCD; and (4) the Cure Sickle Cell Initiative, led by the National Heart, Lung, and Blood Institute. Electronic patient-reported outcomes assessment is highly recommended, and patient-reported outcomes (PROs) should be evaluated in all SCD trials and reported using Standard Protocol Items Recommendations for Interventional Trials guidelines. Patient-centered outcomes research (PCOR) approaches and meaningful stakeholder engagement throughout the process have the potential to optimize the execution and success of clinical trials in SCD with considerable financial value. This article reviews several clinical trial considerations in SCD related to study design and outcomes assessment as informed by recent initiatives as well as patient-centered research approaches and stakeholder engagement. A proposed hematology stakeholder-engagement framework for clinical trials is also discussed.
Review key considerations for SCD clinical trials related to PROs, medication adherence, developmental issues in children, and the COVID-19 pandemic
Review efforts to optimize clinical trials and outcomes assessment in SCD, such as ASH-FDA panels, the ASH Research Collaborative Clinical Trials Network, the PhenX Toolkit, and the CureSCi
Review the evidence for patient-centered research (PCR) and stakeholder engagement and their potential role in successful trials and cost savings
A 19-year-old man with hemoglobin SS disease presents for his regular clinic visit. He has had no hospitalizations over the past 5 years since he started taking daily hydroxyurea with good adherence. He believes that hydroxyurea helped him a great deal with his quality of life, but he also understands that not every sickle cell patient feels the same. He has learned about other approved and emerging therapies, and he is fascinated by the science behind them. Given his interest in pursuing a career in medicine, he inquires about the possibility of getting involved in sickle cell research to help other sickle cell patients benefit from these therapies.
Sickle cell disease (SCD) is an inherited hemoglobin disorder affecting about 100 000 individuals in the United States and more than 20 million people worldwide, mainly of African descent. 1 , 2 SCD is a chronic, debilitating medical condition that affects patients across their life span and is associated with significant morbidity and early mortality. 2 , 3 SCD patients suffer from a number of acute and chronic complications, including pain episodes, acute chest syndrome, cardiopulmonary disease, kidney damage, liver impairment, splenic sequestration, avascular necrosis, stroke, priapism, and other end organ damage. 2 , 3 These complications lead to significant impairment in patient-reported outcomes (PROs) among children and adults with SCD, especially in the physical and psychosocial domains. 4-6 Patients with SCD have increased health care utilization with frequent hospitalizations and emergency department visits. 7-9 Treatment approaches include preventive strategies (eg, penicillin prophylaxis, transcranial Doppler screening), acute management (eg, opioids), disease-modifying therapies (ie, hydroxyurea, L-glutamine, voxelotor, and crizanlizumab), and curative options (eg, hematopoietic stem cell transplantation, gene therapy, or gene editing). 3
Some earlier clinical trials in SCD faced a number of logistical challenges related to recruitment and retention. 10-12 Barriers to successful trial completion included concerns from parents as to the necessity of the research, patient belief that research was only needed for those with more severe disease, or anxiety related to previous research experience. 11-13 A number of facilitators were also identified from these earlier studies, including educating peers, explaining trial rationales more clearly, improving the readability of consent/assent forms, explaining study protocols using videos and other innovative illustrations, and leveraging patient-centered research (PCR) approaches that involve patients, parents/caregivers and other stakeholders (heretofore referred to as “stakeholders”) across all stages of the research process, from planning a study to the dissemination of findings. 11-13 Despite some initial difficulties in research, several trials in SCD to date have been successfully completed, leading to significant progress in the field of SCD with new therapies now approved by the US Food and Drug Administration (FDA), providing clinical benefits to many SCD patients. Currently, there are several active, ongoing clinical trials with novel disease-modifying therapies and curative approaches, such as gene therapy, gene editing, and hematopoietic stem cell transplantation with various regimens and donors. 14 , 15 Nevertheless, efforts to improve clinical trials, including involving stakeholders in trials, are still ongoing.
Over the past few years, a number of important initiatives have been launched to optimize clinical trials in SCD in different ways. First, consensus recommendations for evidence-based SCD end points have been developed as a result of a collaborative effort from 7 panels of patients, clinicians, and researchers established through a partnership between the American Society of Hematology (ASH) and the FDA. 16 , 17 Second, the ASH Research Collaborative SCD Clinical Trials Network has focused on building partnerships with the SCD community and stakeholders, establishing collaboration across SCD centers, streamlining clinical trial operations with a single institutional review board approval, and facilitating data sharing with a centralized data repository through the ASH Research Collaborative Data Hub. 18 Third, the PhenX (Phenotypes and eXposures) project has been funded by different sources, including the National Human Genome Research Institute, the National Institute on Drug Abuse, the Office of Behavioral and Social Sciences Research, the National Institute of Mental Health, the National Heart, Lung, and Blood Institute (NHLBI), the National Institute on Minority Health and Health Disparities, the National Cancer Institute, and the Tobacco Regulatory Science Program of the National Institutes of Health (NIH). In SCD, PhenX efforts have focused on selecting high-quality SCD-related outcome measures to be included in the Toolkit (consensus measures; www.phenxtoolkit.org ), guided by the Sickle Cell Disease Research and Scientific Panel. 19 Finally, the Cure Sickle Cell Initiative (CureSCi), led by the NHLBI, has centered on innovating genetic therapies, nurturing a collaborative, PCR environment, and establishing data standards for SCD clinical trials. 20 In particular, interest in curative therapies in the SCD community has been growing as evidence has emerged and continues to materialize from ongoing clinical trials.
This article aims to review several clinical trial considerations in SCD related to study design and outcome assessment as informed by recent initiatives, in particular PROs as well as PCR approaches and stakeholder engagement.
PROs and e-PROs
PROs have been defined as “outcomes reported directly by patients themselves and not interpreted by an observer.” 21 Health-related quality of life (HRQOL) is a PRO that is defined as “a multidimensional concept that usually includes self-report of the way in which physical, emotional, social, or other domains of well-being are affected by a disease or its treatment.” 21 A proxy- or parent/caregiver-reported outcome is also commonly used to evaluate PROs and/or HRQOL among pediatric populations. Major regulatory authorities have recognized the value of including PROs evaluation in clinical trials to inform clinical decision-making, pharmaceutical-labeling claims, and product reimbursement. 22 The inclusion of PROs in clinical trial protocols should be well planned in advance and reported according to the Standard Protocol Items Recommendations for Interventional Trials guidelines. 23 Evaluating PROs in clinical trials that involve SCD patients provides an opportunity to measure the impact of a given treatment on their individual functioning and well-being. 5
A number of factors should be considered when assessing PROs in SCD clinical trials, such as eligibility criteria (eg, age), relevant domains of interest, psychometric properties (eg, responsiveness, validity, reliability, and floor/ceiling effects), minimal clinically important differences, generic vs disease-specific approaches, participants' burden (ie, survey length), and mode of administration. 5 Generic measures provide insight into the burden of SCD compared to healthy individuals and those with other chronic medical conditions. 5 On the other hand, disease-specific measures better examine the differences and effects of treatments or interventions across different patient groups and within individual SCD patients. 5 Thus, a combined approach using generic and disease-specific instruments to evaluate SCD patients' PROs is highly recommended, with a preference for patient self-reporting over proxy reporting when possible. 4 , 5 Further, the ASH-FDA panel for PROs has provided detailed guidance and recommendations on PROs selection in SCD clinical trials ( Table 1 ). 16
PROs recommendations in CureSCi CDEs (version 1.0)
| Subdomain . | Population . | Measure . | Classification . |
|---|---|---|---|
| Pain intensity | Adults and children ≥8 years old | NRS | Core |
| VAS | Supplemental | ||
| Pain impact/interference | Adults, SCD-specific | ASCQ-Me Pain Impact | Core |
| Children 5-18 years, SCD-specific | PedsQL Pain Impact SCD module | Core | |
| Children/adults, not SCD-specific | PROMIS Pain Interference | Core | |
| Pain: mixed | Children 5-18 years, SCD-specific | PedsQL Pain and Hurt, SCD modules | Core |
| Painful crises | Adults, SCD-specific | ASCQ-Me Pain Episodes | Core |
| Emotional impact of SCD | Adults, SCD-specific | ASCQ-Me Emotional Impact | Supplemental, highly recommended |
| Children, SCD-specific | PedsQL, SCD Module Emotions | Supplemental, highly recommended | |
| PedsQL, SCD Module Worrying | Supplemental | ||
| Negative affect: mixed | Children, not SCD-specific | PROMIS Physical Stress Experience | Supplemental, highly recommended |
| Low mood | Children/adults, not SCD-specific | PROMIS Emotional Distress: Depression , | Supplemental, highly recommended |
| Anxiety | Children/adults, not SCD-specific | PROMIS Emotional Distress: Anxiety , | Supplemental, highly recommended |
| Fatigue | Children/adults, not SCD-specific | Pediatric/Adult PROMIS Fatigue , | Core |
| Children, SCD-specific | PedsQL Multidimensional Fatigue Scale | Core | |
| Sleep disturbance | Children/adults, not SCD-specific | PROMIS Sleep Disturbance , | Supplemental, highly recommended |
| Adults, SCD-specific | ASCQ-Me Sleep Impact | Supplemental, highly recommended | |
| Adults, not SCD-specific | Pittsburgh Sleep Quality Index | Supplemental | |
| Epworth Sleepiness Scale | Supplemental | ||
| Children, not SCD-specific | Epworth Sleepiness Scale (CHAD) | Supplemental | |
| General function | Adults, not SCD-specific | Canadian Occupational Performance | Supplemental |
| Social function | Adults, SCD-specific | ASCQ-Me Social Functioning Impact | Supplemental |
| Physical function | Adults, SCD-specific | ASCQ-Me Stiffness Impact | Supplemental, highly recommended |
| Adults, not SCD-specific | PROMIS - Physical Function (PF) 12a | Supplemental | |
| Children, not SCD-specific | Pediatric PROMIS - PF Mobility | Supplemental | |
| Pediatric PROMIS - PF Upper Extremity | Supplemental | ||
| Global health/QOL | Adults, not SCD-specific | PROMIS 10 Global Health | Core |
| Children, not SCD-specific | PROMIS 7 + 2 Global Health | Core | |
| Global cognition | Children, 0-3.5 years old | Bayley-III | Supplemental |
| Children, 2.5-7 years old | WPPSI-IV (4th edition) | Supplemental | |
| Children, 6-16 years old | WISC-V (5th edition) | Supplemental | |
| Adults | Wechsler Adult Intelligence Scale | Supplemental | |
| Children and adults | NIH Toolbox | Supplemental, highly recommended | |
| Executive functioning | Children 3-7, 8-11, and ≥12 years | Flanker Inhibitory Control/Attention (NT) | Supplemental, highly recommended |
| Dimensional Change Card Sort Test (NT) | Supplemental, highly recommended | ||
| Children ≥9 years old | Trail Making Test, parts A and B | Supplemental | |
| Children and adults, 8-89 years | Delis-Kaplan Executive Function System | Supplemental | |
| Children and adults, 7-89 years | Wisconsin Card Sort Test | Supplemental | |
| Processing speed | Children ≥7 years old | Pattern Comparison Processing Speed Test | Supplemental, highly recommended |
| Adults | Processing Speed Index | Supplemental | |
| Working memory | Children ≥7 years old | List Sorting Working Memory Test (NT) | Supplemental, highly recommended |
| Subdomain . | Population . | Measure . | Classification . |
|---|---|---|---|
| Pain intensity | Adults and children ≥8 years old | NRS | Core |
| VAS | Supplemental | ||
| Pain impact/interference | Adults, SCD-specific | ASCQ-Me Pain Impact | Core |
| Children 5-18 years, SCD-specific | PedsQL Pain Impact SCD module | Core | |
| Children/adults, not SCD-specific | PROMIS Pain Interference | Core | |
| Pain: mixed | Children 5-18 years, SCD-specific | PedsQL Pain and Hurt, SCD modules | Core |
| Painful crises | Adults, SCD-specific | ASCQ-Me Pain Episodes | Core |
| Emotional impact of SCD | Adults, SCD-specific | ASCQ-Me Emotional Impact | Supplemental, highly recommended |
| Children, SCD-specific | PedsQL, SCD Module Emotions | Supplemental, highly recommended | |
| PedsQL, SCD Module Worrying | Supplemental | ||
| Negative affect: mixed | Children, not SCD-specific | PROMIS Physical Stress Experience | Supplemental, highly recommended |
| Low mood | Children/adults, not SCD-specific | PROMIS Emotional Distress: Depression , | Supplemental, highly recommended |
| Anxiety | Children/adults, not SCD-specific | PROMIS Emotional Distress: Anxiety , | Supplemental, highly recommended |
| Fatigue | Children/adults, not SCD-specific | Pediatric/Adult PROMIS Fatigue , | Core |
| Children, SCD-specific | PedsQL Multidimensional Fatigue Scale | Core | |
| Sleep disturbance | Children/adults, not SCD-specific | PROMIS Sleep Disturbance , | Supplemental, highly recommended |
| Adults, SCD-specific | ASCQ-Me Sleep Impact | Supplemental, highly recommended | |
| Adults, not SCD-specific | Pittsburgh Sleep Quality Index | Supplemental | |
| Epworth Sleepiness Scale | Supplemental | ||
| Children, not SCD-specific | Epworth Sleepiness Scale (CHAD) | Supplemental | |
| General function | Adults, not SCD-specific | Canadian Occupational Performance | Supplemental |
| Social function | Adults, SCD-specific | ASCQ-Me Social Functioning Impact | Supplemental |
| Physical function | Adults, SCD-specific | ASCQ-Me Stiffness Impact | Supplemental, highly recommended |
| Adults, not SCD-specific | PROMIS - Physical Function (PF) 12a | Supplemental | |
| Children, not SCD-specific | Pediatric PROMIS - PF Mobility | Supplemental | |
| Pediatric PROMIS - PF Upper Extremity | Supplemental | ||
| Global health/QOL | Adults, not SCD-specific | PROMIS 10 Global Health | Core |
| Children, not SCD-specific | PROMIS 7 + 2 Global Health | Core | |
| Global cognition | Children, 0-3.5 years old | Bayley-III | Supplemental |
| Children, 2.5-7 years old | WPPSI-IV (4th edition) | Supplemental | |
| Children, 6-16 years old | WISC-V (5th edition) | Supplemental | |
| Adults | Wechsler Adult Intelligence Scale | Supplemental | |
| Children and adults | NIH Toolbox | Supplemental, highly recommended | |
| Executive functioning | Children 3-7, 8-11, and ≥12 years | Flanker Inhibitory Control/Attention (NT) | Supplemental, highly recommended |
| Dimensional Change Card Sort Test (NT) | Supplemental, highly recommended | ||
| Children ≥9 years old | Trail Making Test, parts A and B | Supplemental | |
| Children and adults, 8-89 years | Delis-Kaplan Executive Function System | Supplemental | |
| Children and adults, 7-89 years | Wisconsin Card Sort Test | Supplemental | |
| Processing speed | Children ≥7 years old | Pattern Comparison Processing Speed Test | Supplemental, highly recommended |
| Adults | Processing Speed Index | Supplemental | |
| Working memory | Children ≥7 years old | List Sorting Working Memory Test (NT) | Supplemental, highly recommended |
Pediatric PROMIS measures are available for children self-report ≥8 years old and proxy report.
Adult PROMIS measures are available.
ASCQ-Me, Adult Sickle Cell Quality of Life Measurement Information System; CHAD, children and adolescents; NRS, Numeric Rating Scale; NT, NIH Toolbox; PROMIS, Patient-Reported Outcomes Measurement Information System; VAS, Visual Analog Scale; WISC, Wechsler Intelligence Scale for Children; WPPSI-IV, Wechsler Preschool and Primary Scale of Intelligence.
Publicly available at https://curesickle.org/sites/scdc/files/Doc/SC/Patient_Reported_Outcomes_Recommendations_Summary.pdf .
Finally, the mode of PROs evaluation is another key consideration. The use of electronic approaches or e-PROs has been recommended by the e-PRO consortiums of the International Society for Quality of Life Research, the Professional Society for Health Economics and Outcomes Research, and the regulators (eg, the FDA). 23-25 e-PROs have the following advantages: (1) more precise, complete, timely, and high-quality data, (2) better adherence to study protocol, (3) possible PRO reminders and real-time monitoring, (4) less recall bias, (5) fewer data entry errors, (6) leveraged computerized adaptive testing when needed, (7) integrated skip patterns for relevant questions, (8) lighter workloads for staff, (9) possible cost savings and environmental friendliness with less paper printing, and (10) high acceptability ratings from patients. 5 , 24 , 26 Given the ubiquitous access to smartphones and tablets as well as the growing evidence and acceptability of mobile health interventions among SCD patients, 27 , 28 e-PROs should be strongly considered in SCD clinical trials, whether providing patients with a dedicated device for e-PROs or allowing patients to download an app and use their own phone—an approach we call “Bring your own device,” or BYOD.
Medication adherence
Adherence to any new medication is a critical component of the success of any clinical trial in SCD, yet often little attention is given to ways to monitor and optimize adherence during the course of a study. A number of objective and subjective adherence measures can be considered in the setting of a clinical trial, which might vary based on the study design (eg, randomized controlled trial vs real-world comparative effectiveness trial). Objective measures of medication adherence include biochemical measures (eg, drug levels, biomarkers), electronic monitoring (eg, electronic pill bottles or smartphone app logs), directly observed therapy (ie, in-person or mobile), digital pills (eg, Proteus), pill counts, and pharmacy records (eg, prescription refills). 29 These measures provide a more accurate view of a patient's adherence behavior but require some additional resources. In contrast, subjective measures of medication adherence include a patient's self-reported adherence, using surveys (eg, paper and pencil or electronic) or interviews, and physician assessments. 29 These measures are simple, short, and inexpensive and can provide insight into potential adherence barriers; nevertheless, social desirability and recall bias are important considerations. It is worth noting that recent collaborative, multidisciplinary efforts led to the development of the PROMIS Medication Adherence Scale (PMAS), and its psychometric evaluation is underway in several ongoing trials. 30 PMAS is listed in CureSCi's common data elements (CDEs). Given that both objective and subjective measures of adherence have the potential to capture various aspects of medication-taking behavior, a multimodal strategy is highly recommended. 29 , 31 Further, in randomized controlled trials evaluating the efficacy of a new medication in SCD, it is likely advantageous to use tools that can monitor and enhance adherence behavior to optimize the clinical benefits of a given therapy for study participants. In addition, this can provide a more precise assessment of the differences in study outcomes based on exposure or adherence to either experimental drug vs placebo or active comparator.
CDEs (CureSCi and PhenX Toolkit for SCD)
One of the goals of the NHLBI-led CureSCi is to standardize data collection forms for all clinical research studies in SCD, including those with promising genetic approaches. 20 In 2021 the first set of CDEs were assembled and finalized. These CDEs serve as a critical resource for SCD clinical trials in the effort to improve the efficiency of clinical studies, enhance data quality, enable data sharing, and educate young investigators on various aspects of clinical research methodology. 20 Table 2 includes an overview of the proposed CDEs in CureSCi. In addition, all core data elements that are essential for the initiation of any clinical research study in SCD are included in a Start-Up Resource Listing document. 20 The PhenX Toolkit for SCD is another key NHLBI-funded initiative. The goal of the PhenX Measures for SCD Research project is to help researchers better understand the pathophysiology, natural history, and treatment approaches for SCD. The PhenX Toolkit in SCD is a framework for outcomes assessment and data sharing across various SCD research projects that allows for potential comparisons across studies. 19
NIH-NHLBI CureSCi CDEs (version 1.0)
| Domain . | Subdomain . | Class . | Recommendations . | ||
|---|---|---|---|---|---|
| Participant characteristics | Demographics | C | Demographics | ||
| General health history | C | Baseline abnormal hematopoiesis | Behavioral history short form | ||
| Transfusion history | Medical history | Surgical history | |||
| S | Behavioral history | Medical history supplemental elements | |||
| Hospitalization form | Sleep assessment (ped form) | ||||
| Social history | C | Social status | |||
| S | Education school questionnaire | Social determinants screen | |||
| ACEs screen children (1-17 years) | ACEs screen adults ( ≥18 years) | ||||
| Acute anemia | Chronic anemia | ||||
| Disease and treatment-related events | Asthma | S | Asthma outcomes instrument recommendations (highly recommended) | ||
| Asthma outcomes | Over-read spirometry report form | ||||
| Fertility/bone | C | Endocrine, infertility, and bone health | |||
| Lung | C | 6-minute walk test | Pulmonary function test | ||
| Lung disease assessments guidelines | |||||
| S | PROMIS dyspnea functional limitations | ||||
| Pulmonary hypertension | PROMIS dyspnea severity | ||||
| Pain | C | Acute chest syndrome | SCD-related acute painful episodes | ||
| Priapism | C | Priapism core | |||
| S | Priapism Impact Profile (PIP) | Priapism questionnaire | |||
| Renal | C | Renal function assessments | |||
| Spleen | C | Acute spleen | Chronic spleen | ||
| S | Spleen assessment from the pediatric HU phase 3 clinical trial (BABY HUG) | ||||
| Other | S | Leg ulcers | Retinopathy | Avascular necrosis | |
| Chronic malnutrition | Guidelines malnutrition identification | ||||
| Assessments and examinations | Imaging diagnostics | C | Cardiac MRI | Echocardiogram | Brain MRI |
| S | Functional MRI | Brain MRA | Imaging TCD | ||
| Laboratory tests | C | Genetic diagnostic testing | Hemoglobin variant analysis | ||
| S | Immune function form | Lab assessments-genetics/assays | |||
| Nonimaging | S | Electrocardiogram | |||
| Physical exam | S | Physical exam | NIH Stroke Scale | ||
| Vital signs | C | Vital signs and blood gases | |||
| Treatment and interventions | Drugs | C | Prior and concomitant medications | ||
| S | PROMIS Medical Adherence Scale (PMAS) | Asthma medications list | |||
| Therapies | C | Drug product | Hematopoietic cellular transplant infusion | ||
| Genetics and assays summary of recommendations | |||||
| S | Adhesion and viscosity | Apheresis | Conditioning regimen | ||
| E | Adhesion molecules assay | ||||
| Adverse events and toxicities | C | Cytopenia | Genotoxicity | Iron overload | |
| Infusion-related toxicity | Infection form | ||||
| S | Adverse events | New malignancy | Toxicity form | ||
| Cellular therapy essential data follow-up form | |||||
| Outcomes and end points | PROs | C | See details in | ||
| Mortality | C | Death form | |||
| Domain . | Subdomain . | Class . | Recommendations . | ||
|---|---|---|---|---|---|
| Participant characteristics | Demographics | C | Demographics | ||
| General health history | C | Baseline abnormal hematopoiesis | Behavioral history short form | ||
| Transfusion history | Medical history | Surgical history | |||
| S | Behavioral history | Medical history supplemental elements | |||
| Hospitalization form | Sleep assessment (ped form) | ||||
| Social history | C | Social status | |||
| S | Education school questionnaire | Social determinants screen | |||
| ACEs screen children (1-17 years) | ACEs screen adults ( ≥18 years) | ||||
| Acute anemia | Chronic anemia | ||||
| Disease and treatment-related events | Asthma | S | Asthma outcomes instrument recommendations (highly recommended) | ||
| Asthma outcomes | Over-read spirometry report form | ||||
| Fertility/bone | C | Endocrine, infertility, and bone health | |||
| Lung | C | 6-minute walk test | Pulmonary function test | ||
| Lung disease assessments guidelines | |||||
| S | PROMIS dyspnea functional limitations | ||||
| Pulmonary hypertension | PROMIS dyspnea severity | ||||
| Pain | C | Acute chest syndrome | SCD-related acute painful episodes | ||
| Priapism | C | Priapism core | |||
| S | Priapism Impact Profile (PIP) | Priapism questionnaire | |||
| Renal | C | Renal function assessments | |||
| Spleen | C | Acute spleen | Chronic spleen | ||
| S | Spleen assessment from the pediatric HU phase 3 clinical trial (BABY HUG) | ||||
| Other | S | Leg ulcers | Retinopathy | Avascular necrosis | |
| Chronic malnutrition | Guidelines malnutrition identification | ||||
| Assessments and examinations | Imaging diagnostics | C | Cardiac MRI | Echocardiogram | Brain MRI |
| S | Functional MRI | Brain MRA | Imaging TCD | ||
| Laboratory tests | C | Genetic diagnostic testing | Hemoglobin variant analysis | ||
| S | Immune function form | Lab assessments-genetics/assays | |||
| Nonimaging | S | Electrocardiogram | |||
| Physical exam | S | Physical exam | NIH Stroke Scale | ||
| Vital signs | C | Vital signs and blood gases | |||
| Treatment and interventions | Drugs | C | Prior and concomitant medications | ||
| S | PROMIS Medical Adherence Scale (PMAS) | Asthma medications list | |||
| Therapies | C | Drug product | Hematopoietic cellular transplant infusion | ||
| Genetics and assays summary of recommendations | |||||
| S | Adhesion and viscosity | Apheresis | Conditioning regimen | ||
| E | Adhesion molecules assay | ||||
| Adverse events and toxicities | C | Cytopenia | Genotoxicity | Iron overload | |
| Infusion-related toxicity | Infection form | ||||
| S | Adverse events | New malignancy | Toxicity form | ||
| Cellular therapy essential data follow-up form | |||||
| Outcomes and end points | PROs | C | See details in | ||
| Mortality | C | Death form | |||
ACEs: adverse childhood experiences; Class: classification; C: core; E: exploratory; HU: hydroxyurea; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; Ped: pediatric; S: supplemental; TCD: transcranial Doppler.
Publicly available at https://curesickle.org/system/files/Sickle_Cell_Disease_CDE_Highlight_Summary.pdf .
Developmental issues in pediatric trials
Some issues should be considered when planning outcomes assessment in an SCD clinical trial that involves children and adolescents, such as age-appropriate psychometric properties for PROs, patient or proxy reports or both, and developmental level. 12 Children and adolescents experience many cognitive, psychological, and physical changes over time and show wide variability in their emotional, social, attentional, and intellectual levels of development. 12 These differences have important implications for pediatric clinical trial design and implementation, especially in behavioral and interventional areas, where a one-size-fits-all approach is far from ideal.
Patient-centered research and stakeholder-engagement framework in hematology
Stakeholder involvement in different stages of sickle cell research has been limited, including in development, design, implementation, and dissemination. Most clinical trials in SCD have historically focused on surrogate end points, such as hospitalizations and emergency room visits, as markers of disease activity, with less emphasis on PROs or patient-centered outcomes research (PCOR). The problem with this approach is the possibility of missing what patients and other stakeholders care about the most, making clinical trial findings less relevant to many of them, at least in their view. The Patient-Centered Outcomes Research Institute (PCORI) was established by the US Congress in 2010 to address this issue. Since then, PCORI has funded several SCD projects at different stages and with a wide range of budgets and scopes ( Table 3 ). Other government agencies also support PCOR projects with various levels of expected patient and stakeholder involvement ( Table 4 ). The FDA Patient-Focused Drug Development initiative is another key effort to include patients' perspectives on their medical conditions, the symptoms that have the most impact on their daily lives, and the available therapies and to better understand the factors that drive treatment decisions and a willingness to participate in clinical trials. 25 Moreover, PCOR highlighted important outcomes that were often overlooked by investigators, such as patient- or proxy-reported PROs, treatment satisfaction, caregiver/parent burden, work time off, transportation costs, and out-of-pocket costs.
Examples of SCD projects funded by the PCORI
| Project title . | Project type . | Budget . | Time line . |
|---|---|---|---|
| We'll Take the Village: Engaging the Community to Develop Better Health - Tier I | Pipeline to proposal | $15 000 | 2015 |
| We'll Take the Village: Engaging the Community to Better Health - Tier II | Pipeline to proposal | $25 000 | 2016-2017 |
| OMPASS: COMmunity Participation to Advance the Sickle Cell Story | Pipeline to proposal | $50 000 | 2017-2018 |
| National Sickle Cell Advocate Network (NSCAN) | Engagement award | $249 855 | 2016-2018 |
| Tennessee Sickle Cell Disease Network Project | Engagement award | $249 963 | 2014-2017 |
| Disseminating Results: Missed SCD Clinic Appointments and the Health Belief Model | Engagement award | $417 106 | 2019-2021 |
| Automating Quality and Safety Benchmarking for Children: Meeting the Needs of Health Systems and Patients | PCORnet demonstration | $1 264 641 | 2016-2021 |
| Engaging Parents of Children With SCA and Providers in Shared-Decision Making for HU | Research project | $1 962 454 | 2017-2023 |
| Comparative Effectiveness of a Decision Aid for Therapeutic Options in Sickle Cell Disease | Research project | $2 143 228 | 2013-2018 |
| PATient Navigator to rEduce Readmissions—The PArTNER Study | Research project | $2 054 803 | 2013-2018 |
| Patient-Centered Comprehensive Medication Adherence Management System to Improve Effectiveness of Disease Modifying Therapy With HU in Patients With SCD | Research project | $2 148 331 | 2013-2018 |
| Comparing Two Ways to Help Patients With SCD Manage Pain (CaRISMA) | Research project | $4 343 821 | 2019-2024 |
| Comparing Patient Centered Outcomes in the Management of Pain Between Emergency Departments and Dedicated Acute Care Facilities for Adults With SCD | Research project | $4 358 545 | 2014-2020 |
| National Pediatric Learning Health System (PEDSnet) - phase 1 | PCORnet: CDRN (phase I) | $6 459 893 | 2013-2015 |
| Mid-South Clinical Data Research Network - phase 1 | PCORnet: CDRN (phase 1) | $6 672 017 | 2013-2015 |
| Community Health Workers and Mobile Health for Emerging Adults Transitioning SCD Care (COMETS Trial) | Research project | $8 456 632 | 2017-2024 |
| Research Action for Health Network (REACHnet) | PCORnet: CDRN (phase 2) | $8 641 395 | 2015-2019 |
| Comparative Effectiveness of Peer Mentoring Versus Structured Education-Based Transition Programming for the Management of Care Transitions in Emerging Adults With SCD | Research project | $9 753 462 | 2017-2024 |
| Mid-South Clinical Data Research Network | PCORnet: CDRN (phase 2) | $10 064 128 | 2015-2019 |
| Project title . | Project type . | Budget . | Time line . |
|---|---|---|---|
| We'll Take the Village: Engaging the Community to Develop Better Health - Tier I | Pipeline to proposal | $15 000 | 2015 |
| We'll Take the Village: Engaging the Community to Better Health - Tier II | Pipeline to proposal | $25 000 | 2016-2017 |
| OMPASS: COMmunity Participation to Advance the Sickle Cell Story | Pipeline to proposal | $50 000 | 2017-2018 |
| National Sickle Cell Advocate Network (NSCAN) | Engagement award | $249 855 | 2016-2018 |
| Tennessee Sickle Cell Disease Network Project | Engagement award | $249 963 | 2014-2017 |
| Disseminating Results: Missed SCD Clinic Appointments and the Health Belief Model | Engagement award | $417 106 | 2019-2021 |
| Automating Quality and Safety Benchmarking for Children: Meeting the Needs of Health Systems and Patients | PCORnet demonstration | $1 264 641 | 2016-2021 |
| Engaging Parents of Children With SCA and Providers in Shared-Decision Making for HU | Research project | $1 962 454 | 2017-2023 |
| Comparative Effectiveness of a Decision Aid for Therapeutic Options in Sickle Cell Disease | Research project | $2 143 228 | 2013-2018 |
| PATient Navigator to rEduce Readmissions—The PArTNER Study | Research project | $2 054 803 | 2013-2018 |
| Patient-Centered Comprehensive Medication Adherence Management System to Improve Effectiveness of Disease Modifying Therapy With HU in Patients With SCD | Research project | $2 148 331 | 2013-2018 |
| Comparing Two Ways to Help Patients With SCD Manage Pain (CaRISMA) | Research project | $4 343 821 | 2019-2024 |
| Comparing Patient Centered Outcomes in the Management of Pain Between Emergency Departments and Dedicated Acute Care Facilities for Adults With SCD | Research project | $4 358 545 | 2014-2020 |
| National Pediatric Learning Health System (PEDSnet) - phase 1 | PCORnet: CDRN (phase I) | $6 459 893 | 2013-2015 |
| Mid-South Clinical Data Research Network - phase 1 | PCORnet: CDRN (phase 1) | $6 672 017 | 2013-2015 |
| Community Health Workers and Mobile Health for Emerging Adults Transitioning SCD Care (COMETS Trial) | Research project | $8 456 632 | 2017-2024 |
| Research Action for Health Network (REACHnet) | PCORnet: CDRN (phase 2) | $8 641 395 | 2015-2019 |
| Comparative Effectiveness of Peer Mentoring Versus Structured Education-Based Transition Programming for the Management of Care Transitions in Emerging Adults With SCD | Research project | $9 753 462 | 2017-2024 |
| Mid-South Clinical Data Research Network | PCORnet: CDRN (phase 2) | $10 064 128 | 2015-2019 |
Projects are organized by level of funding support from low to high.
CDRN, clinical data research network; HU, hydroxyurea; PCORnet, National Patient-Centered Clinical Research Network.
Various funding agencies, levels of patients, caregiver and stakeholder engagement, and potential benefits
| Funding agency . | Level of engagement . | Benefits . |
|---|---|---|
| Agency for Healthcare Research and Quality (AHRQ) | Desirable | Possibly beneficial |
| Center for Medicare and Medicaid Services (CMS) Innovation Center | Required | Beneficial |
| National Institutes of Health (NIH) | Potentially advantageous | Possibly beneficial |
| Patient-Centered Outcomes Research Institute (PCORI) | Expected | Beneficial |
| Pharmaceutical companies (industry) | Potentially advantageous | Beneficial |
| Professional societies and organizations | Desirable | Possibly beneficial |
| Funding agency . | Level of engagement . | Benefits . |
|---|---|---|
| Agency for Healthcare Research and Quality (AHRQ) | Desirable | Possibly beneficial |
| Center for Medicare and Medicaid Services (CMS) Innovation Center | Required | Beneficial |
| National Institutes of Health (NIH) | Potentially advantageous | Possibly beneficial |
| Patient-Centered Outcomes Research Institute (PCORI) | Expected | Beneficial |
| Pharmaceutical companies (industry) | Potentially advantageous | Beneficial |
| Professional societies and organizations | Desirable | Possibly beneficial |
Note: Level of engagement is defined as the depth and the extent to which patients, caregivers, and stakeholders are engaged in different stages of a given research project that is proposed for funding by any of the listed agencies.
PCOR projects in SCD most often focus on investigator-initiated comparative effectiveness trials evaluating different established treatment approaches. Involving patients and stakeholders in clinical trial decisions, using measures such as PROs and other outcomes, is critical to ensure the relevance of these assessments to the larger SCD community. Moreover, in 2017 the Clinical Trials Transformation Initiative (CTTI) outlined, by phases of research, different approaches to incorporate patient and stakeholder input across the continuum of a clinical trial. The CTTI also reported some examples of potential benefits for PCOR, such as enhancing the relevance of research questions to patients and stakeholders, choosing the most appropriate primary and secondary study outcomes, improving strategies for engagement, recruitment, and retention, addressing barriers to participation, keeping study burden to a minimum, and optimizing overall clinical trial experience. 32
Involving stakeholders with diverse backgrounds provides the needed insight into personal experiences managing SCD, cultural considerations, adherence barriers, and potential strategies to optimize the uptake of approved therapies as well as the research approach and acceptability of study assessments. 33-35 An important part of stakeholders' engagement is clarifying the scientific rationale for the choice of study design and examining the feasibility of including specific outcomes for a given trial with clear expectations of time lines and levels of involvement. 33-35 Stakeholders may participate in regular study calls (eg, steering committee) and engage in detailed research discussions in which they can offer potential solutions to unexpected challenges and hurdles hindering participants' recruitment, retention, and follow-up. 33-35 Stakeholders may be given the opportunity to contribute to scholarly products from the research project and participate in educational initiatives for the dissemination of research findings.
Figure 1 represents a proposed stakeholder engagement frame- work for clinical trials and research studies in hematology, including SCD. The first layer ( base of the pyramid ) includes the broader SCD community, with online engagement strategies such as polls, surveys, blogs, social media, and discussion boards. The second layer ( middle ) involves more planned stakeholder engagement for in-depth insight into different aspects of the research, with activities such as community studios, focus groups, and/or interviews. This engagement approach is essential to provide a safe environment for stakeholders to give unbiased and critical feedback based on their values, experiences, and backgrounds—especially those who have no internet access, are not active on social media, or have limited health literacy. Finally, the third layer ( top ) represents research partners who are driving the clinical trial or the research study, including investigators, industry partners, and selected, actively engaged patients and stakeholders. This hematology stakeholder-engagement framework ( Figure 1 ) captures various potential stakeholders who either should or could be involved in clinical trials or research studies in hematology, with increasing levels of involvement as we move toward the top of the pyramid. Engagement of all these partners across different levels of the pyramid, including stakeholders and community organizations, is essential to ensure that clinical trials focus on meaningful outcomes for patients. This framework also facilitates collaboration and partnership between researchers and stakeholders while prioritizing outcomes of high value to patients.

Hematology stakeholder-engagement framework in clinical trials and research studies.
Standardized training may be needed to ensure that different stakeholders are equipped with the skill set and adequate preparation needed to be actively involved in the trial or the project as research partners. 33-35 A number of PCOR competencies and engagement principles have been reported in the literature ( Table 5 ). 36 , 37 Furthermore, establishing a detailed engagement plan might be helpful to outline the involvement of stakeholders across different stages of trials or research projects.
PCR competencies and principles
| A. Competencies . | ||
|---|---|---|
| I. Knowledge . | II. Skills . | III. Attitudes . |
| Cultural context | Communication | Community values |
| Knowledge about disease | Conflict management | Emotional intelligence |
| Logistical considerations | Critical thinking | General attitudes toward PCR |
| Participatory approaches | Group participation | Openness and trust |
| Agenda setting | Leadership | Personal attributes |
| Research methodology | Project management | Personal growth |
| Understanding of data | Teamwork | Professional growth |
| Understanding PCR | Prioritization | Self-reflection |
| B. Principles | ||
| Involvement of patients, caregivers, and other stakeholders in all aspects of the research | ||
| Researchers and team members learning about PCR methodology | ||
| Training for patients, caregivers, and other stakeholders on research principles | ||
| Cultural sensitivity and mutual respect | ||
| Fair compensation for effort and time | ||
| Inclusion and diversity for all project-related activities and partnerships | ||
| Planning ahead for meetings, tasks, and milestones with realistic time lines | ||
| Patient, caregivers, and other stakeholders are involved as research partners | ||
| Well-defined roles and strategies informed by collaborative discussions | ||
| Clear and transparent communications | ||
| Shared decision-making process | ||
| Sharing information and data openly | ||
| A. Competencies . | ||
|---|---|---|
| I. Knowledge . | II. Skills . | III. Attitudes . |
| Cultural context | Communication | Community values |
| Knowledge about disease | Conflict management | Emotional intelligence |
| Logistical considerations | Critical thinking | General attitudes toward PCR |
| Participatory approaches | Group participation | Openness and trust |
| Agenda setting | Leadership | Personal attributes |
| Research methodology | Project management | Personal growth |
| Understanding of data | Teamwork | Professional growth |
| Understanding PCR | Prioritization | Self-reflection |
| B. Principles | ||
| Involvement of patients, caregivers, and other stakeholders in all aspects of the research | ||
| Researchers and team members learning about PCR methodology | ||
| Training for patients, caregivers, and other stakeholders on research principles | ||
| Cultural sensitivity and mutual respect | ||
| Fair compensation for effort and time | ||
| Inclusion and diversity for all project-related activities and partnerships | ||
| Planning ahead for meetings, tasks, and milestones with realistic time lines | ||
| Patient, caregivers, and other stakeholders are involved as research partners | ||
| Well-defined roles and strategies informed by collaborative discussions | ||
| Clear and transparent communications | ||
| Shared decision-making process | ||
| Sharing information and data openly | ||
Historically, industry-sponsored clinical trials did not often include significant sponsors of stakeholder engagement beyond small preliminary studies, and this hesitancy may have been due to a lack of familiarity with PCOR methodology and/or the unclear return on investment of study benefits. This has changed over the last decade, and currently, a number of ongoing clinical trials in SCD have established advisory boards with different stakeholders, including patients, caregivers, and advocacy groups. Furthermore, CTTI recently proposed an approach or a conceptual financial model to evaluate the value of stakeholder engagement in clinical trials. 38 CTTI's model is based on an estimated expected net present value (ENPV) incorporating cost, time, revenue, and risk as crucial business drivers. 38 In an example using an oncology development program, the authors reported a potential meaningful impact of stakeholder engagement by avoiding protocol amendments and enhancing enrollment, retention, and completion of study assessments. This positive impact in pre-phase 2 and pre-phase 3 trials was associated with an increase in NPV ($62 million and $65 million, respectively) and ENPV ($35 million and $75 million), adding substantial financial value to these trials. With a hypothetical initial investment of $100 000 dedicated to optimizing stakeholder engagement strategies in a clinical trial, there may be a return on investment, in both NPV and ENPV, that exceeds the investment 500-fold. 38
COVID-19 pandemic and clinical trials
More recently, the COVID-19 pandemic has led to disruptions in our daily routines, personally and professionally, in different ways, including interrupting the execution of clinical trials. 39 , 40 Most institutions stopped new enrollments and allowed the continuation of interventional trials when there were potential clinical benefits for the participants; however, many reported challenges related to delayed and rescheduled study visits, procedures, and assessments and overall difficulty reaching patients. 40 Many of these vulnerable patients were at risk from exposure to COVID-19, and some were intentionally avoiding health care facilities or obeying stay-at-home-orders. 39 This situation highlights the need for considerable adaptability using a hybrid strategy in designing future clinical trials to complete all planned study procedures. 40 Some proposed strategies include (1) prioritizing primary outcomes over exploratory ones; (2) alternating strategies for outcomes assessment; (3) collecting remote data using phone interviews or online tools; (4) obtaining phone numbers and e-mail addresses for patients and 3 family members or friends to ensure maintained contact; (5) using different methods to contact participants, including text messaging, phone calls, e-mail, or social media; (6) employing telemedicine; (7) arranging home visits by health care workers wearing personal protective equipment; (8) allowing study medications to be taken at home; (9) making use of concierge services; (10) using local instead of central lab facilities; and (11) escalating incentives. 12 , 40 , 41 Other approaches should be considered to achieve the highest level of retention and adherence to study interventions, and the statistical analysis plan for primary and secondary outcomes should be revised to reflect any expected meaningful effects or influence relate to the pandemic. 41 For behavioral clinical trials, efforts should be directed to leverage widely available and user-friendly online surveys, databases, and web-based applications to optimize e-consenting and remote enrollment, with completion of all study assessments and delivery of study interventions conducted virtually.
CLINICAL CASE (continued)
The patient has been actively involved as an advisory board member in a few investigator-initiated trials at our institution. He had significant input in these trials, such as feedback on consent and assent forms and the selection of HRQOL domains that are more relevant to SCD patients. In addition, he was able to provide critical insight to inform our efforts to develop and refine a mobile app for adolescents and young adults as a behavioral intervention to monitor and improve medication adherence and HRQOL. His passion for medicine was reinforced, and he is determined to become a hematologist caring for adult SCD patients.
Several clinical trial considerations in SCD are key to success. PROs are significantly impaired among SCD patients and should be included in all clinical trials. A multimodal strategy is highly recommended to assess adherence outcomes. Developmental differences among children and adolescents with SCD should inform the study approach. Engaging patients and stakeholders in SCD clinical trials in meaningful ways is critical to ensure that their voices are heard and that study designs and outcomes are relevant to them, which is essential for future successful dissemination and implementation. This engagement is a dynamic, bidirectional commitment that is mutually beneficial to all involved partners, and it has the potential to improve health outcomes in the larger population of pediatric and adult SCD patients. Stakeholders can play a major role in closing the gap between data-heavy research findings from clinical trials and their implications in clinical practice. It is critical to keep stakeholders engaged and interested throughout the research process, and the sustainability of this partnership is key. Evaluating the financial value of stakeholder engagement is important to estimate the potential cost savings for SCD clinical trials, which might be of considerable financial value. Timely adaptations to address unusual circumstances, such as the COVID-19 pandemic, are often crucial.
This project was supported by a grant (K23HL150232, PI: Badawy) from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the author and does not necessarily represent the views of the National Institutes of Health.
I would like to thank Drs. Robert Liem, Jane Holl, David Cella, Tonya Palermo, and Alexis Thompson for their advice while writing this article.
Sherif M. Badawy: no competing financial interests to declare.
Sherif M. Badawy: nothing to disclose.
- Previous Article
- Next Article
Email alerts
Affiliations, american society of hematology.
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 03 March 2022
Advances in the diagnosis and treatment of sickle cell disease
- A. M. Brandow 1 &
- R. I. Liem ORCID: orcid.org/0000-0003-2057-3749 2
Journal of Hematology & Oncology volume 15 , Article number: 20 ( 2022 ) Cite this article
36k Accesses
58 Citations
2 Altmetric
Metrics details
Sickle cell disease (SCD), which affects approximately 100,000 individuals in the USA and more than 3 million worldwide, is caused by mutations in the βb globin gene that result in sickle hemoglobin production. Sickle hemoglobin polymerization leads to red blood cell sickling, chronic hemolysis and vaso-occlusion. Acute and chronic pain as well as end-organ damage occur throughout the lifespan of individuals living with SCD resulting in significant disease morbidity and a median life expectancy of 43 years in the USA. In this review, we discuss advances in the diagnosis and management of four major complications: acute and chronic pain, cardiopulmonary disease, central nervous system disease and kidney disease. We also discuss advances in disease-modifying and curative therapeutic options for SCD. The recent availability of l -glutamine, crizanlizumab and voxelotor provides an alternative or supplement to hydroxyurea, which remains the mainstay for disease-modifying therapy. Five-year event-free and overall survival rates remain high for individuals with SCD undergoing allogeneic hematopoietic stem cell transplant using matched sibling donors. However, newer approaches to graft-versus-host (GVHD) prophylaxis and the incorporation of post-transplant cyclophosphamide have improved engraftment rates, reduced GVHD and have allowed for alternative donors for individuals without an HLA-matched sibling. Despite progress in the field, additional longitudinal studies, clinical trials as well as dissemination and implementation studies are needed to optimize outcomes in SCD.
Introduction
Sickle cell disease (SCD), a group of inherited hemoglobinopathies characterized by mutations that affect the β-globin chain of hemoglobin, affects approximately 100,000 people in the USA and more than 3 million people worldwide [ 1 , 2 ]. SCD is characterized by chronic hemolytic anemia, severe acute and chronic pain as well as end-organ damage that occurs across the lifespan. SCD is associated with premature mortality with a median age of death of 43 years (IQR 31.5–55 years) [ 3 ]. Treatment requires early diagnosis, prevention of complications and management of end-organ damage. In this review, we discuss recent advances in the diagnosis and management of four major complications in SCD: acute and chronic pain, cardiopulmonary disease, central nervous system disease and kidney disease. Updates in disease-modifying and curative therapies for SCD are also discussed.
Molecular basis and pathophysiology
Hemoglobin S (HbS) results from the replacement of glutamic acid by valine in the sixth position of the β-globin chain of hemoglobin (Fig. 1 ). Severe forms of SCD include hemoglobin SS due to homozygous inheritance of HbS and S/β 0 thalassemia due to co-inheritance of HbS with the β 0 thalassemia mutation. Other forms include co-inheritance of HbS with other β-globin gene mutations such as hemoglobin C, hemoglobin D-Los Angeles/Punjab or β + thalassemia. Hb S has reduced solubility and increased polymerization, which cause red blood cell sickling, hemolysis and vaso-occlusion (Table 1 ) that subsequently lead to pain episodes and end-organ damage such as cardiopulmonary, cerebrovascular and kidney disease (Table 2 ).
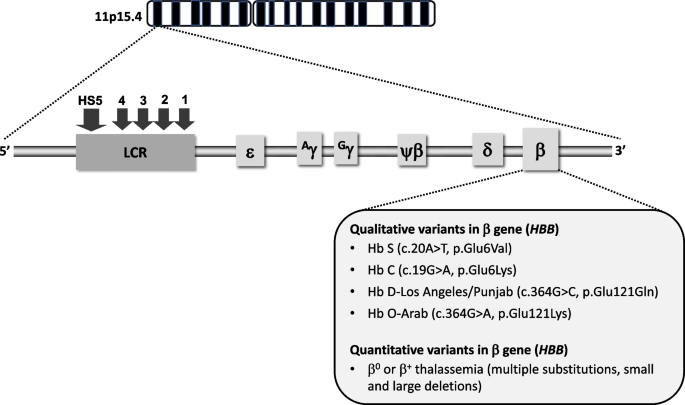
Genetic and molecular basis of sickle cell disease. SCD is caused by mutations in the β globin gene, located on the β globin locus found on the short arm of chromosome 11. The homozygous inheritance of Hb S or co-inheritance of Hb S with the β 0 thalassemia mutation results in the most common forms of severe SCD. Co-inheritance of Hb S with other variants such as Hb C, Hb D-Los Angeles/Punjab, Hb O-Arab or β + thalassemia also leads to clinically significant sickling syndromes (LCR, locus control region; HS, hypersensitivity site)
Acute and chronic pain
Severe intermittent acute pain is the most common SCD complication and accounts for over 70% of acute care visits for individuals with SCD [ 4 ]. Chronic daily pain increases with older age, occurring in 30–40% of adolescents and adults with SCD [ 5 , 6 ]. Acute pain is largely related to vaso-occlusion of sickled red blood cells with ischemia–reperfusion injury and tissue infarction and presents in one isolated anatomic location (e.g., arm, leg, back) or multiple locations. Chronic pain can be caused by sensitization of the central and/or peripheral nervous system and is often diffuse with neuropathic pain features [ 7 , 8 ]. A consensus definition for chronic pain includes “Reports of ongoing pain on most days over the past 6 months either in a single location or multiple locations” [ 9 ]. Disease complications such as avascular necrosis (hip, shoulder) and leg ulcers also cause chronic pain [ 9 ].
Diagnosis of acute and chronic pain
The gold standard for pain assessment and diagnosis is patient self-report. There are no reliable diagnostic tests to confirm the presence of acute or chronic pain in individuals with SCD except when there are identifiable causes like avascular necrosis on imaging or leg ulcers on exam. The effects of pain on individuals’ function are assessed using patient-reported outcome measures (PROs) that determine to what extent pain interferes with individuals’ daily function. Tools shown to be valid, reliable and responsive can be used in clinical practice to track patients’ pain-related function over time to determine additional treatment needs and to compare to population norms [ 10 ]. There are currently no plasma pain biomarkers that improve assessment and management of SCD acute or chronic pain.
Depression and anxiety as co-morbid conditions in SCD can contribute to increased pain, more pain-related distress/interference and poor coping [ 11 ]. The prevalence of depression and anxiety range from 26–33% and 6.5–36%, respectively, in adults with SCD [ 11 , 12 , 13 ]. Adults with SCD have an 11% higher prevalence of depression compared to Black American adults without SCD [ 14 ]. Depression and anxiety can be assessed using self-reported validated screening tools (e.g., Depression: Patient Health Questionnaire (PHQ-9) [ 15 ] for adults, Center for Epidemiologic Studies Depression Scale for Children (CES-DC) [ 16 ], PROMIS assessments for adults and children; Anxiety: Generalized Anxiety Disorder 7-item (GAD-7) scale for adults, State-Trait Anxiety Inventory for Children (STAIC) [ 17 ], PROMIS assessments for adults and children). Individuals who screen positive using these tools should be referred for evaluation by a psychologist/psychiatrist.
Management of acute and chronic pain
The goal of acute pain management is to provide sufficient analgesia to return patients to their usual function, which may mean complete resolution of pain for some or return to baseline chronic pain for others. The goal of chronic pain management is to optimize individuals’ function, which may not mean being pain free. When there is an identifiable cause of chronic pain, treatment of the underlying issue (e.g., joint replacement for avascular necrosis, leg ulcer treatment) is important. Opioids, oral for outpatient management and intravenous for inpatient management, are first line therapy for acute SCD pain. In the acute care setting, analgesics should be initiated within 30–60 min of triage [ 18 ]. Ketamine, a non-opioid analgesic, can be prescribed at sub-anesthetic (analgesic) intravenous doses (0.1–0.3 mg/kg per h, maximum 1 mg/kg per h) as adjuvant treatment for acute SCD pain refractory to opioids [ 18 , 19 ]. In an uncontrolled observational study of 85 patients with SCD receiving ketamine infusions for acute pain, ketamine was associated with a decrease in mean opioid consumption by oral morphine equivalents (3.1 vs. 2.2 mg/kg/day, p < 0.001) and reductions in mean pain scores (0–10 scale) from baseline until discontinuation of the infusion (7.81 vs. 5.44, p < 0.001) [ 20 ]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are routinely used as adjuvant therapy for acute pain treatment [ 18 ]. In a RCT ( n = 20) of hospitalized patients with acute pain, ketorolac was associated with lower total dose of meperidine required (1866.7 ± 12.4 vs. 2804.5 ± 795.1 mg, p < 0.05) and shorter hospitalization (median 3.3 vs. 7.2 days, p = 0.027) [ 21 ]. In a case series of children treated for 70 acute pain events in the ED, 53% of events resolved with ketorolac and hydration alone with reduction in 100 mm visual analog scale (VAS) pain score from 60 to 13 ( p < 0.001) [ 22 ]. Patients at risk for NSAID toxicity (e.g., renal impairment, on anticoagulation) should be identified.
Despite paucity of data, chronic opioid therapy (COT) can be considered after assessing benefits versus harms [ 23 ] and the functional status of patients with SCD who have chronic pain. Harms of COT seen in patient populations other than SCD are dose dependent and include myocardial infarction, bone fracture, increased risk of motor vehicle collisions, sexual dysfunction and mortality [ 23 ]. There are few published studies investigating non-opioid analgesics for chronic SCD pain [ 24 , 25 , 26 ]. In a randomized trial of 39 participants, those who received Vitamin D experienced a range of 6–10 pain days over 24 weeks while those who received placebo experienced 10–16 pain days, which was not significantly different [ 26 ]. In a phase 1, uncontrolled trial of 18 participants taking trifluoperazine, an antipsychotic drug, 8 participants showed a 50% reduction in the VAS (10 cm horizontal line) pain score from baseline on at least 3 assessments over 24 h without severe sedation or supplemental opioid analgesics, 7 participants showed pain reduction on 1 assessment, and the remaining 3 participants showed no reduction [ 24 ]. Although published data are not available for serotonin and norepinephrine reuptake inhibitors (SNRIs), gabapentinoids and tricyclic antidepressants (TCAs) in individuals with SCD, evidence supports their use in fibromyalgia, a chronic pain condition similar to SCD chronic pain in mechanism. A Cochrane Review that included 10 RCTs ( n = 6038) showed that the SNRIs milnacipran and duloxetine, compared to placebo, were associated with a reduction in pain [ 27 ]. A systematic review and meta-analysis of 9 studies ( n = 520) showed the TCA amitriptyline improved pain intensity and function [ 28 ]. Finally, a meta-analysis of 5 RCTs ( n = 1874) of the gabapentinoid pregabalin showed a reduction in pain intensity [ 29 ]. Collectively, the indirect evidence from fibromyalgia supports the conditional recommendation in current SCD practice guidelines to consider these 3 drug classes for chronic SCD pain treatment [ 18 ]. Standard formulary dosing recommendations should be followed and reported adverse effects considered.
Non-pharmacologic therapies (e.g., integrative, psychological-based therapies) are important components of SCD pain treatment. In a case–control study of 101 children with SCD and chronic pain referred for cognitive behavioral therapy (CBT) (57 CBT, 44 no CBT) [ 30 ], CBT was associated with more rapid decrease in pain hospitalizations (estimate − 0.63, p < 0.05) and faster reduction in hospital days over time (estimate − 5.50, p < 0.05). Among 18 children who received CBT and completed PROs pre- and 12 months posttreatment, improvements were seen in mean pain intensity (5.47 vs. 3.76, p = 0.009; 0–10 numeric rating pain scale), functional disability (26.24 vs. 15.18, p < 0.001; 0–60 score range) and pain coping (8.00 vs. 9.65, p = 0.03; 3–15 score range) post treatment [ 30 ]. In 2 uncontrolled clinical trials, acupuncture was associated with a significant reduction in pain scores by 2.1 points (0–10 numeric pain scale) in 24 participants immediately after treatment [ 31 ] or a significant mean difference in pre-post pain scores of 0.9333 (0–10 numeric pain scale) ( p < 0.000) after 33 acupuncture sessions [ 32 ].
Cardiopulmonary disease
Cardiopulmonary disease is associated with increased morbidity and mortality in individuals with SCD. Pulmonary hypertension (PH), most commonly pulmonary arterial hypertension (PAH), is present based on right-heart catheterization in up to 10% of adults with SCD [ 33 ]. Chronic intravascular hemolysis represents the biggest risk factor for development of PAH in SCD and leads to pulmonary arteriole vasoconstriction and smooth muscle proliferation. Based on pulmonary function testing (PFT), obstructive lung disease may be observed in 16% of children and 8% of adults with SCD, while restrictive lung disease may be seen in up to 28% of adults and only 7% of children with SCD [ 34 , 35 ]. Sleep-disordered breathing, which can manifest as obstructive sleep apnea or nocturnal hypoxemia, occurs in up to 42% of children and 46% of adults with SCD [ 36 , 37 ]. Cardiopulmonary disease, including PH or restrictive lung disease, presents with dyspnea with or without exertion, chest pain, hypoxemia or exercise intolerance that is unexplained or increased from baseline. Obstructive lung disease can also present with wheezing.
Diagnosis of cardiopulmonary disease
The confirmation of PH in patients with SCD requires right-heart catheterization. Recently, the mean pulmonary artery pressure threshold used to define PH in the general population was lowered from ≥ 25 to ≥ 20 mm Hg [ 38 ]. Elevated peak tricuspid regurgitant jet velocity (TRJV) ≥ 2.5 m/s on Doppler echocardiogram (ECHO) is associated with early mortality in adults with SCD and may suggest elevated pulmonary artery pressures, especially when other signs of PH (e.g., right-heart strain, septal flattening) or left ventricular diastolic dysfunction, which may contribute to PH, are present [ 39 ]. However, the positive predictive value (PPV) of peak TRJV alone for identifying PH in adults with SCD is only 25% [ 40 ]. Increasing the peak TRJV threshold to at least 2.9 m/s has been shown to increase the PPV to 64%. For a peak TRJV of 2.5–2.8 m/s, an increased N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥ 164.5 pg/mL or a reduced 6-min walk distance (6MWD) < 333 m can also improve the PPV to 62% with a false negative rate of 7% [ 33 , 40 , 41 ].
PFT, which includes spirometry and measurement of lung volumes and diffusion capacity, is standard for diagnosing obstructive and restrictive lung disease in patients with SCD. Emerging modalities include impulse oscillometry, a non-invasive method using forced sound waves to detect changes in lower airway mechanics in individuals unable to perform spirometry [ 42 ], and airway provocation studies using cold air or methacholine to reveal latent airway hyperreactivity [ 43 ]. Formal in-lab, sleep study/polysomnography remains the gold standard to evaluate for sleep-disordered breathing, which may include nocturnal hypoxemia, apnea/hypopnea events and other causes of sleep disruption. Nocturnal hypoxemia may increase red blood cell sickling, cellular adhesion and endothelial dysfunction. In 47 children with SCD, mean overnight oxygen saturation was higher in those with grade 0 compared to grade 2 or 3 cerebral arteriopathy (97 ± 1.6 vs. 93.9 ± 3.7 vs. 93.5 ± 3.0%, p < 0.01) on magnetic resonance angiography and lower overnight oxygen saturation was independently associated with mild, moderate or severe cerebral arteriopathy after adjusting for reticulocytosis (OR 0.50, 95% CI 0.26–0.96, p < 0.05) [ 44 ].
Management of cardiopulmonary disease
Patients with SCD who have symptoms suggestive of cardiopulmonary disease, such as worsening dyspnea, hypoxemia or reduced exercise tolerance, should be evaluated with a diagnostic ECHO and PFT. The presence of snoring, witnessed apnea, respiratory pauses or hypoxemia during sleep, daytime somnolence or nocturnal enuresis in older children and adults is sufficient for a diagnostic sleep study.
Without treatment, the mortality rate in SCD patients with PH is high compared to those without (5-year, all-cause mortality rate of 32 vs. 16%, p < 0.001) [ 33 ]. PAH-targeted therapies should be considered for SCD patients with PAH confirmed by right-heart catheterization. However, the only RCT ( n = 6) in individuals with SCD and PAH confirmed by right-heart catheterization (bosentan versus placebo) was stopped early for poor accrual with no efficacy endpoints analyzed [ 45 ]. In SCD patients with elevated peak TRJV, a randomized controlled trial ( n = 74) of sildenafil, a phosphodiesterase-5 inhibitor, was discontinued early due to increased pain events in the sildenafil versus placebo arm (35 vs. 14%, p = 0.029) with no treatment benefit [ 46 ]. Despite absence of clinical trial data, patients with SCD and confirmed PH should be considered for hydroxyurea or monthly red blood cell transfusions given their disease-modifying benefits. In a retrospective analysis of 13 adults with SCD and PAH, 77% of patients starting at a New York Heart Association (NYHA) functional capacity class III or IV achieved class I/II after a median of 4 exchange transfusions with improvement in median pulmonary vascular resistance (3.7 vs. 2.8 Wood units, p = 0.01) [ 47 ].
Approximately 28% of children with SCD have asthma, which is associated with increased pain episodes that may result from impaired oxygenation leading to sickling and vaso-occlusion as well as with acute chest syndrome and higher mortality [ 48 , 49 , 50 ]. First line therapies include standard beta-adrenergic bronchodilators and supplemental oxygen as needed. When corticosteroids are indicated, courses should be tapered over several days given the risk of rebound SCD pain from abrupt discontinuation. Inhaled corticosteroids such as fluticasone proprionate or beclomethasone diproprionate are reserved for patients with recurrent asthma exacerbations, but their anti-inflammatory effects and impact on preventing pain episodes in patients with SCD who do not have asthma is under investigation [ 51 ]. Finally, management of sleep-disordered breathing is tailored to findings on formal sleep study in consultation with a sleep/pulmonary specialist.
Central nervous system (CNS) complications
CNS complications, such as overt and silent cerebral infarcts, cause significant morbidity in individuals with SCD. Eleven percent of patients with HbSS disease by age 20 years and 24% by age 45 years will have had an overt stroke [ 52 ]. Silent cerebral infarcts occur in 39% by 18 years and in > 50% by 30 years [ 53 , 54 ]. Patients with either type of stroke are at increased risk of recurrent stroke [ 55 ]. Overt stroke involves large-arteries, including middle cerebral arteries and intracranial internal carotid arteries, while silent cerebral infarcts involve penetrating arteries. The pathophysiology of overt stroke includes vasculopathy, increased sickled red blood cell adherence, and hemolysis-induced endothelial activation and altered vasomotor tone [ 56 ]. Overt strokes present as weakness or paresis, dysarthria or aphasia, seizures, sensory deficits, headache or altered level of consciousness, while silent cerebral infarcts are associated with cognitive deficits, including lower IQ and impaired academic performance.
Diagnosis of CNS complications in SCD
Overt stroke is diagnosed by evidence of acute infarct on brain MRI diffusion-weighted imaging and focal deficit on neurologic exam. A silent cerebral infarct is defined by a brain “MRI signal abnormality at least 3 mm in one dimension and visible in 2 planes on fluid-attenuated inversion recovery (FLAIR) T2-weighted images” and no deficit on neurologic exam [ 57 ]. Since silent cerebral infarcts cannot be detected clinically, a screening baseline brain MRI is recommended in school-aged children with SCD [ 58 ]. Recent SCD clinical practice guidelines also suggest a screening brain MRI in adults with SCD to facilitate rehabilitation services, patient and family understanding of cognitive deficits and further needs assessment [ 58 ]. An MRA should be added to screening/diagnostic MRIs to evaluate for cerebral vasculopathy (e.g., moyamoya), which may increase risk for recurrent stroke or hemorrhage [ 59 ].
Annual screening for increased stroke risk by transcranial doppler (TCD) ultrasound is recommended by the American Society of Hematology for children 2–16 years old with HbSS or HbS/β° thalassemia [ 58 ]. Increased stroke risk on non-imaging TCD is indicated by abnormally elevated cerebral blood flow velocity, defined as ≥ 200 cm/s (time-averaged mean of the maximum velocity) on 2 occasions or a single velocity of > 220 cm/s in the distal internal carotid or proximal middle cerebral artery [ 60 ]. Many centers rely on imaging TCD, which results in velocities 10–15% lower than values obtained by non-imaging protocols and therefore, require adjustments to cut-offs for abnormal velocities. Data supporting stroke risk assessment using TCD are lacking for adults with SCD and standard recommendations do not exist.
Neurocognitive deficits occur in over 30% of children and adults with severe SCD [ 61 , 62 ]. These occur as a result of overt and/or silent cerebral infarcts but in some patients, the etiology is unknown. The Bright Futures Guidelines for Health Supervision of Infants, Children and Adolescents or the Cognitive Assessment Toolkit for adults are commonly used tools to screen for developmental delays or neurocognitive impairment [ 58 ]. Abnormal results should prompt referral for formal neuropsychological evaluation, which directs the need for brain imaging to evaluate for silent cerebral infarcts and facilitate educational/vocational accommodations.
Management of CNS complications
Monthly chronic red blood cell transfusions to suppress HbS < 30% are standard of care for primary stroke prevention in children with an abnormal TCD. In an RCT of 130 children, chronic transfusions, compared to no transfusions, were associated with a difference in stroke risk of 92% (1 vs. 10 strokes, p < 0.001) [ 60 ]. However, children with abnormal TCD and no MRI/MRA evidence of cerebral vasculopathy can safely transition to hydroxyurea after 1 year of transfusions [ 63 ]. Lifelong transfusions to maintain HbS < 30% remain standard of care for secondary stroke prevention in individuals with overt stroke [ 64 ]. Chronic monthly red blood cell transfusions should also be considered for children with silent cerebral infarct [ 58 ]. In a randomized controlled trial ( n = 196), monthly transfusions, compared to observation without hydroxyurea, reduced risk of overt stroke, new silent cerebral infarct or enlarging silent cerebral infarct in children with HbSS or HbS/β 0 thalassemia and an existing silent cerebral infarct (2 vs. 4.8 events, incidence rate ratio of 0.41, 95% CI 0.12–0.99, p = 0.04) [ 57 ].
Acute stroke treatment requires transfusion therapy to increase cerebral oxygen delivery. Red blood cell exchange transfusion, defined as replacement of patients’ red blood cells with donor red blood cells, to rapidly reduce HbS to < 30% is the recommended treatment as simple transfusion alone is shown to have a fivefold greater relative risk (57 vs. 21% with recurrent stroke, RR = 5.0; 95% CI 1.3–18.6) of subsequent stroke compared to exchange transfusion [ 65 ]. However, a simple transfusion is often given urgently while preparing for exchange transfusion [ 58 ]. Tissue plasminogen activator (tPA) is not recommended for children with SCD who have an acute stroke since the pathophysiology of SCD stroke is less likely to be thromboembolic in origin and there is risk for harm. Since the benefits and risks of tPA in adults with SCD and overt stroke are not clear, its use depends on co-morbidities, risk factors and stroke protocols but should not delay or replace prompt transfusion therapy.
Data guiding treatment of SCD cerebral vasculopathy (e.g., moyamoya) are limited, and only nonrandomized, low-quality evidence exists for neurosurgical interventions (e.g., encephaloduroarteriosynangiosis) [ 66 ]. Consultation with a neurosurgeon to discuss surgical options in patients with moyamoya and history of stroke or transient ischemic attack should be considered [ 58 ].
Kidney disease
Glomerulopathy, characterized by hyperfiltration leading to albuminuria, is an early asymptomatic manifestation of SCD nephropathy and worsens with age. Hyperfiltration, defined by an absolute increase in glomerular filtration rate, may be seen in 43% of children with SCD [ 67 ]. Albuminuria, defined by the presence of urine albumin ≥ 30 mg/g over 24 h, has been observed in 32% of adults with SCD [ 68 ]. Glomerulopathy results from intravascular hemolysis and endothelial dysfunction in the renal cortex. Medullary hypoperfusion and ischemia also contribute to kidney disease in SCD, causing hematuria, urine concentrating defects and distal tubular dysfunction [ 69 ]. Approximately 20–40% of adults with SCD develop chronic kidney disease (CKD) and are at risk of developing end-stage renal disease (ESRD), with rapid declines in estimated glomerular filtration rate (eGFR) > 3 mL/min/1.73 m 2 associated with increased mortality (HR 2.4, 95% CI 1.31–4.42, p = 0.005) [ 68 ].
Diagnosis of kidney disease in SCD
The diagnosis of sickle cell nephropathy is made by detecting abnormalities such as albuminuria, hematuria or CKD rather than by distinct diagnostic criteria in SCD, which have not been developed. Traditional markers of kidney function such as serum creatinine and eGFR should be interpreted with caution in individuals with SCD because renal hyperfiltration affects their accuracy by increasing both. Practical considerations preclude directly measuring GFR by urine or plasma clearance techniques, which achieves the most accurate results. The accuracy of eGFR, however, may be improved by equations that incorporate serum cystatin C [ 70 ].
Since microalbuminuria/proteinuria precedes CKD in SCD, annual screening for urine microalbumin/protein is recommended beginning at age 10 years [ 71 ]. When evaluating urine for microalbumin concentration, samples from first morning rather than random voids are preferable to exclude orthostatic proteinuria. Recent studies suggest HMOX1 and APOL1 gene variants may be associated with CKD in individuals with SCD [ 72 ]. Potential novel predictors of acute kidney injury in individuals with SCD include urine biomarkers kidney injury molecule 1 (KIM-1) [ 73 ], monocyte chemotactic protein 1 (MCP-1) [ 74 ] and neutrophil gelatinase-associated lipocalin (NGAL) [ 75 ]. Their contribution to chronic kidney disease and interaction with other causes of kidney injury in SCD (e.g., inflammation, hemolysis) are not clear.
Management of kidney disease
Managing kidney complications in SCD should focus on mitigating risk factors for acute and chronic kidney injury such as medication toxicity, reduced kidney perfusion from hypotension and dehydration, and general disease progression, as well as early screening and treatment of microalbuminuria/proteinuria. Acute kidney injury, either an increase in serum creatinine ≥ 0.3 mg/dL or a 50% increase in serum creatinine from baseline, is associated with ketorolac use in children with SCD hospitalized for pain [ 76 ]. Increasing intravenous fluids to maintain urine output > 0.5 to 1 mL/kg/h and limiting NSAIDs and antibiotics associated with nephrotoxicity in this setting are important. Despite absence of controlled clinical trials, hydroxyurea may be associated with improvements in glomerular hyperfiltration and urine concentrating ability in children with SCD [ 77 , 78 ]. Hydroxyurea is also associated with a lower prevalence (34.7 vs. 55.4%, p = 0.01) and likelihood of albuminuria (OR 0.28, 95% CI 0.11–0.75, p = 0.01) in adults with SCD after adjusting for age, angiotensin-converting enzyme inhibitor (ACE-I)/angiotensin receptor blockade (ARB) use and major disease risk factors [ 79 ].
ACE-I or ARB therapy reduces microalbuminuria in patients with SCD. In a phase 2 trial of 36 children and adults, a ≥ 25% reduction in urine albumin-to-creatinine ratio was observed in 83% ( p < 0.0001) and 58% ( p < 0.0001) of patients with macroalbuminuria (> 300 mg/g creatinine) and microalbuminuria (30–300 mg/g creatinine), respectively, after 6 months of treatment with losartan at a dose of 0.7 mg/kg/day (max of 50 mg) in children and 50 mg daily in adults [ 80 ]. However, ACE-I or ARB therapy has not been shown to improve kidney function or prevent CKD. Hemodialysis is associated with a 1-year mortality rate of 26.3% after starting hemodialysis and an increase risk of death in SCD patients with ESRD compared to non-SCD patients with ESRD (44.6 vs. 34.5% deaths, mortality hazard ratio of 2.8, 95% CI 2.31–3.38) [ 81 ]. Renal transplant should be considered for individuals with SCD and ESRD because of recent improvements in renal graft survival and post-transplant mortality [ 82 ].
Disease-modifying therapies in SCD
Since publication of its landmark trial in 1995, hydroxyurea continues to represent a mainstay of disease-modifying therapy for SCD. Hydroxyurea induces fetal hemoglobin production through stress erythropoiesis, reduces inflammation, increases nitric oxide and decreases cell adhesion. The FDA approved hydroxyurea in 1998 for adults with SCD. Subsequently, hydroxyurea was FDA approved for children in 2017 to reduce the frequency pain events and need for blood transfusions in children ≥ 2 years of age [ 63 ]. The landscape of disease-modifying therapies, however, has improved with the recent FDA approval of 3 other treatments— l -glutamine and crizanlizumab for reducing acute complications (e.g., pain), and voxelotor for improving anemia (Table 3 ) [ 83 , 84 , 85 ]. Other therapies in current development focus on inducing fetal hemoglobin, reducing anti-sickling or cellular adhesion, or activating pyruvate kinase-R.
l -glutamine
Glutamine is required for the synthesis of glutathione, nicotinamide adenine dinucleotide and arginine. The essential amino acid protects red blood cells against oxidative damage, which forms the basis for its proposed utility in SCD. The exact mechanism of benefit in SCD, however, remains unclear. In a phase 3 RCT of 230 participants (hemoglobin SS or S/β 0 thalassemia), l -glutamine compared to placebo was associated with fewer pain events (median 3 vs. 4, p = 0.005) and hospitalizations for pain (median 2 vs. 3, p = 0.005) over the 48-week treatment period [ 84 ]. The percentage of patients who had at least 1 episode of acute chest syndrome, defined as presence of chest wall pain with fever and a new pulmonary infiltrate, was lower in the l -glutamine group (8.6 vs. 23.1%, p = 0.003). There were no significant between-group differences in hemoglobin, hematocrit or reticulocyte count. Common side effects of l -glutamine include GI upset (constipation, nausea, vomiting and abdominal pain) and headaches.
Crizanlizumab
P-selectin expression, triggered by inflammation, promotes adhesion of neutrophils, activated platelets and sickle red blood cells to the endothelial surface and to each other, which promotes vaso-occlusion in SCD. Crizanlizumab, given as a monthly intravenous infusion, is a humanized monoclonal antibody that binds P-selectin and blocks the adhesion molecule’s interaction with its ligand, P-selectin glycoprotein ligand 1. FDA approval for crizanlizumab was based on a phase 2 RCT ( n = 198, all genotypes), in which the median rate of pain events (primary endpoint) was lower (1.63 vs. 2.68, p = 0.01) and time to first pain event (secondary endpoint) was longer (4.07 vs. 1.38 months, p = 0.001) for patients on high-dose crizanlizumab (5 mg/kg/dose) compared to placebo treated for 52 weeks (14 doses total) [ 83 ]. In this trial, patients with SCD on chronic transfusion therapy were excluded, but those on stable hydroxyurea dosing were not. Adverse events were uncommon but included headache, back pain, nausea, arthralgia and pain in the extremity.
Polymerization of Hb S in the deoxygenated state represents the initial step in red blood cell sickling, which leads to reduced red blood cell deformability and increased hemolysis. Voxelotor is a first-in-class allosteric modifier of Hb S that increases oxygen affinity. The primary endpoint for the phase 3 RCT of voxelotor ( n = 274, all genotypes) that led to FDA approval was an increase in hemoglobin of at least 1 g/dL after 24 weeks of treatment [ 85 ]. More participants receiving 1500 mg daily of oral voxelotor versus placebo had a hemoglobin response of at least 1 g/dL (51%, 95% CI 41–61 vs. 7%, 95% CI 1–12, p < 0.001). Approximately 2/3 of the participants in these trials were on hydroxyurea, with treatment benefits observed regardless of hydroxyurea status. Despite improvements associated with voxelotor in biomarkers of hemolysis (reticulocyte count, indirect bilirubin and lactate dehydrogenase), annualized incidence rate of vaso-occlusive crisis was not significantly different among treatment groups. Adverse events included headaches, GI symptoms, arthralgia, fatigue and rash.
Curative therapies in SCD
For individuals with SCD undergoing hematopoietic stem cell transplantation (HSCT) using HLA-matched sibling donors and either myeloablative or reduced-intensity conditioning regimens, the five-year event-free and overall survival is high at 91% and 93%, respectively [ 86 ]. Limited availability of HLA-matched sibling donors in this population requires alternative donors or the promise of autologous strategies such as gene-based therapies (i.e. gene addition, transfer or editing) (Table 4 ). Matched unrelated donors have not been used routinely due to increased risk of graft-versus-host disease (GVHD) as high as 19% (95% CI 12–28) in the first 100 days for acute GVHD and 29% (95% CI 21–38) over 3 years for chronic GVHD [ 87 ]. Haplo-identical HSCT, using biological parents or siblings as donors, that incorporate post-transplant cyclophosphamide demonstrates acceptable engraftment rates, transplant-related morbidity and overall mortality [ 88 ]. Regardless of allogeneic HSCT type, older age is associated with lower event-free (102/418 vs. 72/491 events, HR 1.74, 95% CI 1.24–2.45) and overall survival (54/418 vs. 22/491 events, HR 3.15, 95% CI 1.86–5.34) in patients ≥ 13 years old compared to < 12 years old undergoing HSCT [ 87 ].
Advancing research in SCD
Despite progress to date, additional high-quality, longitudinal data are needed to better understand the natural history of the disease and to inform optimal screening for SCD-related complications. In the era of multiple FDA-approved therapies with disease-modifying potential, clinical trials to evaluate additional indications and test them in combination with or compared to each other are needed. Dissemination and implementation studies are also needed to identify barriers and facilitators related to treatment in everyday life, which can be incorporated into decision aids and treatment algorithms for patients and their providers [ 89 ]. Lastly, continued efforts should acknowledge social determinants of health and other factors that affect access and disease-related outcomes such as the role of third-party payers, provider and patient education, health literacy and patient trust. Establishing evidence-derived quality of care metrics can also drive public policy changes required to ensure care optimization for this population.
Conclusions
SCD is associated with complications that include acute and chronic pain as well as end-organ damage such as cardiopulmonary, cerebrovascular and kidney disease that result in increased morbidity and mortality. Several well-designed clinical trials have resulted in key advances in management of SCD in the past decade. Data from these trials have led to FDA approval of 3 new drugs, l -glutamine, crizanlizumab and voxelotor, which prevent acute pain and improve chronic anemia. Moderate to high-quality data support recommendations for managing SCD cerebrovascular disease and early kidney disease. However, further research is needed to determine the best treatment for chronic pain and cardiopulmonary disease in SCD. Comparative effectiveness research, dissemination and implementation studies and a continued focus on social determinants of health are also essential.
Availability of data and materials
Not applicable.
Abbreviations
Six-minute walk distance
Angiotensin-converting enzyme inhibitor
Angiotensin receptor blockade
Cognitive behavioral therapy
Chronic kidney disease
Chronic opioid therapy
Echocardiogram
End stage renal disease
Fluid-attenuated inversion recovery
Glomerular filtration rate
Graft-versus-host disease
Hemoglobin S
Hematopoietic stem cell transplant
Nonsteroidal anti-inflammatory drugs
N-terminal pro-brain natriuretic peptide
New York Heart Association
Pulmonary arterial hypertension
Pulmonary function test
Pulmonary hypertension
Positive predictive value
Patient-reported outcomes
Randomized controlled trial
- Sickle cell disease
Serotonin and norepinephrine reuptake inhibitors
Tricyclic antidepressants
Transcranial Doppler
Tissue plasminogen activator
Tricuspid regurgitant jet velocity
Visual Analog Scale
Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–51.
Article PubMed PubMed Central Google Scholar
Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85(1):77–8.
PubMed Google Scholar
Payne AB, Mehal JM, Chapman C, Haberling DL, Richardson LC, Bean CJ, et al. Trends in sickle cell disease-related mortality in the United States, 1979 to 2017. Ann Emerg Med. 2020;76(3S):S28–36.
Article PubMed Google Scholar
Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–94.
Article CAS PubMed Google Scholar
Sil S, Cohen LL, Dampier C. Psychosocial and functional outcomes in youth with chronic sickle cell pain. Clin J Pain. 2016;32(6):527–33.
Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101.
Ballas SK, Darbari DS. Neuropathy, neuropathic pain, and sickle cell disease. Am J Hematol. 2013;88(11):927–9.
Sharma D, Brandow AM. Neuropathic pain in individuals with sickle cell disease. Neurosci Lett. 2020;714:134445.
Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain. 2017;18(5):490–8.
Darbari DS, Hampson JP, Ichesco E, Kadom N, Vezina G, Evangelou I, et al. Frequency of hospitalizations for pain and association with altered brain network connectivity in sickle cell disease. J Pain. 2015;16(11):1077–86.
Levenson JL, Mcclish DK, Dahman BA, Bovbjerg VE, Penberthy LT, et al. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med. 2008;70(2):192–6.
Treadwell MJBB, Kaur K, Gildengorin G. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:10.
Google Scholar
Jonassaint CR, Jones VL, Leong S, Frierson GM. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. Br J Haematol. 2016;174(1):136–47.
Laurence B, George D, Woods D. Association between elevated depressive symptoms and clinical disease severity in African-American adults with sickle cell disease. J Natl Med Assoc. 2006;98(3):365–9.
PubMed PubMed Central Google Scholar
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Article CAS PubMed PubMed Central Google Scholar
Faulstich ME, Carey MP, Ruggiero L, Enyart P, Gresham F. Assessment of depression in childhood and adolescence: an evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC). Am J Psychiatry. 1986;143(8):1024–7.
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1983.
Brandow AM, Carroll CP, Creary S, Edwards-Elliott R, Glassberg J, Hurley RW, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv. 2020;4(12):2656–701.
Lubega FA, DeSilva MS, Munube D, Nkwine R, Tumukunde J, Agaba PK, et al. Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: a randomized controlled trial. Scand J Pain. 2018;18(1):19–27.
Nobrega R, Sheehy KA, Lippold C, Rice AL, Finkel JC, Quezado ZMN. Patient characteristics affect the response to ketamine and opioids during the treatment of vaso-occlusive episode-related pain in sickle cell disease. Pediatr Res. 2018;83(2):445–54.
Perlin E, Finke H, Castro O, Rana S, Pittman J, Burt R, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43–7.
Beiter JL Jr, Simon HK, Chambliss CR, Adamkiewicz T, Sullivan K. Intravenous ketorolac in the emergency department management of sickle cell pain and predictors of its effectiveness. Arch Pediatr Adolesc Med. 2001;155(4):496–500.
Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–86.
Molokie RE, Wilkie DJ, Wittert H, Suarez ML, Yao Y, Zhao Z, et al. Mechanism-driven phase I translational study of trifluoperazine in adults with sickle cell disease. Eur J Pharmacol. 2014;723:419–24.
Schlaeger JM, Molokie RE, Yao Y, Suarez ML, Golembiewski J, Wilkie DJ, et al. Management of sickle cell pain using pregabalin: a pilot study. Pain Manag Nurs. 2017;18(6):391–400.
Osunkwo I, Ziegler TR, Alvarez J, McCracken C, Cherry K, Osunkwo CE, et al. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. Br J Haematol. 2012;159(2):211–5.
Hauser W, Urrutia G, Tort S, Uceyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. 2013;1:CD010292.
Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26(4):297–307.
Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9:CD011790.
Sil S, Lai K, Lee JL, Gilleland Marchak J, Thompson B, Cohen L, et al. Preliminary evaluation of the clinical implementation of cognitive-behavioral therapy for chronic pain management in pediatric sickle cell disease. Complement Ther Med. 2020;49:102348.
Lu K, Cheng MC, Ge X, Berger A, Xu D, Kato GJ, et al. A retrospective review of acupuncture use for the treatment of pain in sickle cell disease patients: descriptive analysis from a single institution. Clin J Pain. 2014;30(9):825–30.
Mahmood LA, Reece-Stremtan S, Idiokitas R, Martin B, Margulies S, Hardy SJ, et al. Acupuncture for pain management in children with sickle cell disease. Complement Ther Med. 2020;49:102287.
Mehari A, Alam S, Tian X, Cuttica MJ, Barnett CF, Miles G, et al. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187(8):840–7.
Cohen RT, Strunk RC, Rodeghier M, Rosen CL, Kirkham FJ, Kirkby J, et al. Pattern of lung function is not associated with prior or future morbidity in children with sickle cell anemia. Ann Am Thorac Soc. 2016;13(8):1314–23.
Kassim AA, Payne AB, Rodeghier M, Macklin EA, Strunk RC, DeBaun MR. Low forced expiratory volume is associated with earlier death in sickle cell anemia. Blood. 2015;126(13):1544–50.
Sharma S, Efird JT, Knupp C, Kadali R, Liles D, Shiue K, et al. Sleep disorders in adult sickle cell patients. J Clin Sleep Med. 2015;11(3):219–23.
Rosen CL, Debaun MR, Strunk RC, Redline S, Seicean S, Craven DI, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134(2):273–81.
Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1).
Chaturvedi S, Labib Ghafuri D, Kassim A, Rodeghier M, DeBaun MR. Elevated tricuspid regurgitant jet velocity, reduced forced expiratory volume in 1 second, and mortality in adults with sickle cell disease. Am J Hematol. 2017;92(2):125–30.
Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53.
Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296(3):310–8.
Mondal P, Yirinec A, Midya V, Sankoorikal BJ, Smink G, Khokhar A, et al. Diagnostic value of spirometry vs impulse oscillometry: a comparative study in children with sickle cell disease. Pediatr Pulmonol. 2019;54(9):1422–30.
Field JJ, Stocks J, Kirkham FJ, Rosen CL, Dietzen DJ, Semon T, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139(3):563–8.
Dlamini N, Saunders DE, Bynevelt M, Trompeter S, Cox TC, Bucks RS, et al. Nocturnal oxyhemoglobin desaturation and arteriopathy in a pediatric sickle cell disease cohort. Neurology. 2017;89(24):2406–12.
Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149(3):426–35.
Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–64.
Turpin M, Chantalat-Auger C, Parent F, Driss F, Lionnet F, Habibi A, et al. Chronic blood exchange transfusions in the management of pre-capillary pulmonary hypertension complicating sickle cell disease. European Respiratory Journal. 2018;52(4).
Knight-Madden JM, Barton-Gooden A, Weaver SR, Reid M, Greenough A. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 2013;191(1):95–100.
Strunk RC, Cohen RT, Cooper BP, Rodeghier M, Kirkham FJ, Warner JO, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164(4):821-6e1.
Article Google Scholar
Takahashi T, Okubo Y, Handa A. Acute chest syndrome among children hospitalized with vaso-occlusive crisis: a nationwide study in the United States. Pediatr Blood Cancer. 2018;65(3):e26885.
Glassberg J, Minnitti C, Cromwell C, Cytryn L, Kraus T, Skloot GS, et al. Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: a triple-blind, randomized trial. Am J Hematol. 2017;92(7):622–31.
Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–94.
CAS PubMed Google Scholar
Bernaudin F, Verlhac S, Arnaud C, Kamdem A, Vasile M, Kasbi F, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125(10):1653–61.
Kassim AA, Pruthi S, Day M, Rodeghier M, Gindville MC, Brodsky MA, et al. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood. 2016;127(16):2038–40.
Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med. 1978;65(3):461–71.
Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5(6):501–12.
DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710.
DeBaun MR, Jordan LC, King AA, Schatz J, Vichinsky E, Fox CK, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Adv. 2020;4(8):1554–88.
Dobson SR, Holden KR, Nietert PJ, Cure JK, Laver JH, Disco D, et al. Moyamoya syndrome in childhood sickle cell disease: a predictive factor for recurrent cerebrovascular events. Blood. 2002;99(9):3144–50.
Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11.
Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303(18):1823–31.
Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters M, et al. Neurocognitive deficits in children with sickle cell disease: a comprehensive profile. Pediatr Blood Cancer. 2011;56(5):783–8.
Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–70.
Scothorn DJ, Price C, Schwartz D, Terrill C, Buchanan GR, Shurney W, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140(3):348–54.
Hulbert ML, Scothorn DJ, Panepinto JA, Scott JP, Buchanan GR, Sarnaik S, et al. Exchange blood transfusion compared with simple transfusion for first overt stroke is associated with a lower risk of subsequent stroke: a retrospective cohort study of 137 children with sickle cell anemia. J Pediatr. 2006;149(5):710–2.
Hall EM, Leonard J, Smith JL, Guilliams KP, Binkley M, Fallon RJ, et al. Reduction in overt and silent stroke recurrence rate following cerebral revascularization surgery in children with sickle cell disease and severe cerebral vasculopathy. Pediatr Blood Cancer. 2016;63(8):1431–7.
Lebensburger JD, Aban I, Pernell B, Kasztan M, Feig DI, Hilliard LM, et al. Hyperfiltration during early childhood precedes albuminuria in pediatric sickle cell nephropathy. Am J Hematol. 2019;94(4):417–23.
Niss O, Lane A, Asnani MR, Yee ME, Raj A, Creary S, et al. Progression of albuminuria in patients with sickle cell anemia: a multicenter, longitudinal study. Blood Adv. 2020;4(7):1501–11.
Cazenave M, Audard V, Bertocchio JP, Habibi A, Baron S, Prot-Bertoye C, et al. Tubular acidification defect in adults with sickle cell disease. Clin J Am Soc Nephrol. 2020;15(1):16–24.
Yee MEM, Lane PA, Archer DR, Joiner CH, Eckman JR, Guasch A. Estimation of glomerular filtration rate using serum cystatin C and creatinine in adults with sickle cell anemia. Am J Hematol. 2017;92(10):E598–9.
Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–48.
Saraf SL, Zhang X, Shah B, Kanias T, Gudehithlu KP, Kittles R, et al. Genetic variants and cell-free hemoglobin processing in sickle cell nephropathy. Haematologica. 2015;100(10):1275–84.
Hamideh D, Raj V, Harrington T, Li H, Margolles E, Amole F, et al. Albuminuria correlates with hemolysis and NAG and KIM-1 in patients with sickle cell anemia. Pediatr Nephrol. 2014;29(10):1997–2003.
dos Santos TE, Goncalves RP, Barbosa MC, da Silva GB, Jr., Daher Ede F. Monocyte chemoatractant protein-1: a potential biomarker of renal lesion and its relation with oxidative status in sickle cell disease. Blood Cells Mol Dis. 2015;54(3):297–301.
Audard V, Moutereau S, Vandemelebrouck G, Habibi A, Khellaf M, Grimbert P, et al. First evidence of subclinical renal tubular injury during sickle-cell crisis. Orphanet J Rare Dis. 2014;9:67.
Baddam S, Aban I, Hilliard L, Howard T, Askenazi D, Lebensburger JD. Acute kidney injury during a pediatric sickle cell vaso-occlusive pain crisis. Pediatr Nephrol. 2017;32(8):1451–6.
Zahr RS, Hankins JS, Kang G, Li C, Wang WC, Lebensburger J, et al. Hydroxyurea prevents onset and progression of albuminuria in children with sickle cell anemia. Am J Hematol. 2019;94(1):E27–9.
Alvarez O, Miller ST, Wang WC, Luo Z, McCarville MB, Schwartz GJ, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer. 2012;59(4):668–74.
Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant. 2014;29(6):1211–8.
Quinn CT, Saraf SL, Gordeuk VR, Fitzhugh CD, Creary SE, Bodas P, et al. Losartan for the nephropathy of sickle cell anemia: a phase-2, multicenter trial. Am J Hematol. 2017;92(9):E520–8.
McClellan AC, Luthi JC, Lynch JR, Soucie JM, Kulkarni R, Guasch A, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol. 2012;159(3):360–7.
Gérardin C, Moktefi A, Couchoud C, Duquesne A, Ouali N, Gataut P, et al. Survival and specific outcome of sickle cell disease patients after renal transplantation. Br J Haematol. 2019;187(5):676–80.
Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–39.
Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, et al. A phase 3 trial of l -glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226–35.
Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509–19.
Gluckman E, Cappelli B, Bernaudin F, Labopin M, Volt F, Carreras J, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548–56.
Eapen M, Brazauskas R, Walters MC, Bernaudin F, Bo-Subait K, Fitzhugh CD, et al. Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol. 2019;6(11):e585–96.
Bolanos-Meade J, Cooke KR, Gamper CJ, Ali SA, Ambinder RF, Borrello IM, et al. Effect of increased dose of total body irradiation on graft failure associated with HLA-haploidentical transplantation in patients with severe haemoglobinopathies: a prospective clinical trial. Lancet Haematol. 2019;6(4):e183–93.
Krishnamurti L, Ross D, Sinha C, Leong T, Bakshi N, Mittal N, et al. Comparative effectiveness of a web-based patient decision aid for therapeutic options for sickle cell disease: randomized controlled trial. J Med Internet Res. 2019;21(12):e14462.
Download references
Acknowledgements
We would like to acknowledge Lana Mucalo, MD, for supporting data collection for this manuscript.
Author information
Authors and affiliations.
Department of Pediatrics, Section of Pediatric Hematology/Oncology/Bone Marrow Transplantation, Medical College of Wisconsin, Milwaukee, WI, USA
A. M. Brandow
Division of Hematology, Oncology and Stem Cell Transplantation, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
You can also search for this author in PubMed Google Scholar
Contributions
AB and RL contributed equally to the writing and editing of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to R. I. Liem .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors have no competing interests to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Brandow, A.M., Liem, R.I. Advances in the diagnosis and treatment of sickle cell disease. J Hematol Oncol 15 , 20 (2022). https://doi.org/10.1186/s13045-022-01237-z
Download citation
Received : 30 September 2021
Accepted : 15 February 2022
Published : 03 March 2022
DOI : https://doi.org/10.1186/s13045-022-01237-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sickle cell anemia
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
- Case report
- Open access
- Published: 23 August 2013
A 19-year-old man with sickle cell disease presenting with spinal infarction: a case report
- April Edwards 1 ,
- E Leila Jerome Clay 2 , 3 ,
- Valerie Jewells 4 ,
- Stacie Adams 5 ,
- Regina D Crawford 6 &
- Rupa Redding-Lallinger 2
Journal of Medical Case Reports volume 7 , Article number: 210 ( 2013 ) Cite this article
30k Accesses
6 Citations
Metrics details
Introduction
Vasculopathy of the large vessels commonly occurs in sickle cell disease, and as a result cerebral infarction is a well characterized complication of this condition. However, spinal infarction appears to be rare. Spinal infarct is infrequent in the non-sickle cell population as well, and accounts for only about 1 percent of all central nervous system infarcts.
Case presentation
In the present work, we report the case of a 19-year-old African-American man with sickle cell disease who experienced an anterior spinal infarct and subsequent quadriplegia. He was incidentally noted to be a heterozygote for factor V Leiden. We also reviewed the literature and found two previous cases of spinal cord infarction and sickle hemoglobin. Our literature search did not demonstrate that heterozygocity for factor V Leiden plays an important role in spinal cord infarction.
Conclusions
The paucity of cases associated with sickle hemoglobin does not allow us to postulate any particular risk factors with sickle cell disease that might predispose patients to spinal cord infarction. Our patient’s case raises the question as to whether spinal cord infarction is being missed in individuals with sickle cell disease and neurologic symptoms.
Peer Review reports
Cerebral infarction is the most common neurologic complication that occurs with sickle cell disease (SCD); it can be either overt or silent and it can be associated with significant morbidity [ 1 ]. Overt stroke in SCD was first characterized in 1923, and histopathologic studies later revealed large vessel narrowing with superimposed thrombosis as the underlying cause [ 2 , 3 ]. Though cerebral infarction is the most frequent neurological complication, a number of other potentially devastating central nervous system (CNS) sequelae have also been described. These include: intra-cranial hemorrhage, isolated neuropathies, transverse myelitis, auditory and ocular manifestations, and spinal cord involvement [ 1 ]. In the spinal cord there has been a description of cord compression by extramedullary hemopoietic tissue in addition to rare case reports of spinal cord infarction [ 1 , 4 – 6 ].
In the non-sickle cell disease population it appears that spinal infarct is much less frequent than cerebral infarction as well, and accounts for only about 1 percent of all CNS infarcts [ 7 ]. Of those with spinal infarction, most appear to be from traumatic or surgical etiologies than other organic causes [ 7 , 8 ]. Aortic disease is a frequent culprit with many case reports detailing adverse sequelae following surgical repair of aneurysms, but also aortic thrombosis, and aortic dissection [ 8 ]. Other non-traumatic, non-surgical etiologies of spinal cord infarct include: global hypotension and/or arterial insufficiency, often after cardiac arrest; transient ischemic attacks; fibrocartilagenous emboli; arterial vascular malformations; syphilitic arteritis and adjacent spinal disease [ 8 – 10 ]. In a 2006 study, Novy et al . noted that 12 of their 27 patients with spinal infarct had pre-existing spinal disease including compression fractures, spondylolisthesis, chronic arachonoiditis and chronic cervical disk protrusion, and of those 12 patients, 11 had an infarct at the level of their pre-existing disease [ 7 ]. However, the cause of spinal infarct is frequently cryptogenic [ 11 ].
There is considerable evidence that sickle cell disease represents a hypercoagulable state [ 12 – 15 ]. It appears that nearly every component of hemostasis is altered to some degree in SCD [ 15 ]. Studies have indicated that in sickle cell disease there is increased platelet activation and aggregation, increased levels of D-dimer and fibrinogen and fibrin-fibrinogen complex while there are simultaneously decreased factors V, VII, VIIa and proteins C and S [ 13 ]. There is good evidence that there is externalization of phosphatidylserine (a phospholipid normally found in the inner monolayer of red blood cells) in SCD, which is thought to play a significant role in promoting macrophage recognition in erythrophagocytosis and thus triggering a signal for the coagulation process [ 13 , 14 ]. Increased phosphatidylserine exposure is also thought to be associated with increased tissue factor expression [ 14 ]. However, it remains unclear how or to what extent those abnormalities contribute to disease complications such as cerebral and spinal infarcts.
Because cerebral infarcts occur only in a subset of the sickle cell population, it has been postulated that there may be identifiable features of this subgroup that exacerbate the hypercoagulable state of sickle cell disease. In the search for possible characteristics of this subpopulation, some have begun to explore factors that predispose the general population to coagulation abnormalities and thrombophilia. Specifically, there have been case reports of persons with SCD who developed CNS infarcts and were found to have the factor V Leiden, a prothrombin gene variant, a methylenetetrahidrofolate reductase gene mutation, or some combination of those mutations [ 16 – 18 ]. These studies were conducted in Brazil and Israel; notably the prevalence of the factor V Leiden and the prothrombin gene variant are known to be very low in African-Americans [ 19 ]. Also, there have been a few single nucleotide polymorphisms (SNPs) in persons with SCD that have been found to be associated with increased stroke risk: ANXA2, TGFBR3, and TEK were noted in a study including these SNPs [ 20 ]. However, further validation is needed before these can be used to prospectively guide recommendations for molecular genetic testing or treatment [ 20 ]. There is no known identifiable thrombophilic abnormality that predicts cerebral infarction in sickle cell disease.
On the morning of admission, our patient, a 19-year-old African-American man with sickle cell anemia, felt himself to be in his usual state of health, although he had just been discharged the previous day from a hospitalization for acute chest syndrome. He ate breakfast and spent the day watching television. However, at approximately 5:45 p.m. when he used the bathroom, he noticed that he could not pull up his trousers due to weakness in his left arm. As he walked out of the bathroom, he noted that he was having difficulty walking because of weakness in his right leg. As his mother was helping him to his bed, his left leg also became weak. He began experiencing ‘shocking’ pains on both sides of his neck, which were unlike his usual pain, and also noted that he had an erection. These events transpired rapidly, within about six minutes, at which point his family called Emergency Medical Services (EMS) and our patient was transported to our hospital.
On arrival at our hospital, he was alert and oriented and cranial nerves II to XII were intact. He had flaccid paralysis of the bilateral upper extremities and the left lower extremity, and normal tone with 5 out of 5 strength in the right lower extremity. He had areflexia in the biceps, triceps, and brachioradialis bilaterally, hyper-reflexia at the left patella, and sustained clonus at the left Achilles. Sensation was intact throughout. The results of the rest of his physical examination were normal.
Relevant medical history included asthma, recurrent acute chest syndrome (>10 episodes), and intermittent attempts at hydroxyurea treatment with poor compliance over the previous 10 years. Following the identification of silent cerebral infarcts, he was treated for the three years between 2005 and 2008 with exchange transfusions to maintain hemoglobin S < 30 percent; during this time he did very well. At 10 days prior to presentation, he was hospitalized with an acute chest syndrome. During that hospitalization he had an initial PO 2 of 76, a hemoglobin (Hb)/hematocrit (Hct) nadir of 5.8/17, and was found to have a methicillin-resistant Staphylococcus aureus (MRSA) pneumonia. He was treated with antibiotics and a transfusion. His discharge hemoglobin was 6.6 and oxygen saturation 96 percent. He was without symptoms at the time of discharge.
Admission laboratory test data included a white blood cell count of 12 × 10 3 /uL Hb 8.7g/dL, Hct 26 percent, platelets 449 × 10 3 cells/mm 3 with a hemoglobin electrophoresis of HbA 86 percent, HbS 7 percent, and HbC 7 percent. He had a lumbar puncture that demonstrated unremarkable cerebrospinal fluid findings and no evidence of IgG oligoclonal bands. The results of peripheral blood and urine cultures were negative. A chest X-ray showed patchy consolidation in the right upper lobe suspicious for pneumonia. The results of computed tomography (CT) angiography of the head and neck were unremarkable. Given concern for spinal cord involvement, 1.5T T1, T2, and fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) studies of the brain and cervical spine was performed showing an abnormal T2/FLAIR signal in the cervical spinal cord, which was thought at that time likely to be due to artifact. Later the initial MRI was read to also show swelling of the cord in the same area. He was admitted to the neurologic intensive care unit where he received an exchange transfusion with no significant improvement in his symptoms; subsequent hemoglobin electrophoresis showed HbA 85 percent, HbS 9 percent. While in the intensive care unit (ICU) he experienced episodes of hypotension that were initially managed with vasopressors. After his blood pressure stabilized he was transitioned to fludrocortisone and midodrine. He never had respiratory insufficiency. Two days after admission he had a repeat MRI, which showed T2 hyperintense signal extending from C2 through to C7 (Figure 1 A). In addition, diffusion-weighted imaging demonstrated restricted diffusion consistent with a focus of infarction in addition to cord edema and swelling in the gray and white matter of the right side of the cord. There was associated enlargement of the spinal cord consistent with edema from the anterior spinal infarct. A hypercoagulability investigation performed during his hospitalization included a polymerase chain reaction (PCR) study that demonstrated that he was heterozygous for the factor V Leiden 1691 G>A mutation. Other studies performed were for factor VIII, fibrinogen, functional anti-thrombin, lupus anti-coagulant, anti-cardiolipin, all of which were within normal limits. His erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were both elevated, and proteins C and S were found to be low but within the expected range for someone with sickle cell disease. He was anti-coagulated with a heparin drip during his stay in the acute care facility, but this was discontinued on discharge. A monthly exchange transfusion regimen was instituted with the goal of keeping his hemoglobin S level < 30 percent.
A,B T2 hyperintense signal extending from C2 to C7 with edema of the gray and white matter of the cord. The arrows point to the edema. As with all infarcts, the area of infarct is bright on B1000 and dark on apparent diffusion coefficient sequences.
Although initially there was almost complete paralysis of his extremities, over the four days he spent in the neurologic ICU, our patient demonstrated slow but steady progress in regaining some motor function of his affected limbs. He was transferred from the ICU to the wards on day five and began working with physical and occupational therapy. On day 10, he was transferred to a rehabilitation facility, where he made gradual but steady progress in regaining motor function. He was discharged home after three weeks.
Five months after the acute onset of paralysis, he had some residual left arm and leg weakness and spasticity, but was able to walk unassisted and perform most activities of daily living without assistance. A repeat MRI scan showed a persistence of slight T2 signal abnormality in the cervical cord, consistent with previous spinal cord infarction. There was no spinal cord atrophy (Figure 2 ). Our patient continued to make progress, regaining much of his strength and function, and was maintained on a regimen of monthly scheduled exchange transfusions.
A,B Follow-up magnetic resonance imaging study demonstrating no spinal cord atrophy with residual signal from myelomalacia, months after infarct. Arrows point to the decrease in edema.
At 18 months post-infarct he presented with complaints of three hours of generalized weakness, worse in his lower extremities in association with a pain crisis. His symptoms of weakness had largely resolved by the time he arrived at our Emergency Department. On examination he had 4 out of 5 strength in his left lower extremity and 5 out of 5 in right lower extremity, and 3 out of 5 grip strength bilaterally with a slightly unsteady gait; these findings were not substantially different from his post-spinal cord infarction baseline. His hemoglobin S was 51.5 percent at that time. Repeat imaging studies of his brain and spine at that time were unchanged from his prior studies. He was admitted and had an exchange transfusion achieving a post-transfusion HbS of 8.3 percent. He was given daily low-dose (81mg) aspirin. Currently, at 20 months post-spinal cord infarction, his condition is unchanged.
Spinal cord infarct is infrequent compared to cerebral infarction in the general population, and most commonly occurs as a result of a dissecting aortic aneurysm or aortic surgery [ 7 , 8 ]. In persons with sickle hemoglobin, significant spinal cord infarction appears to be an even more rare neurologic complication. To the best of our knowledge, there are only two reported cases of other persons, both now deceased, detailing this pathology [ 4 , 5 ]. Of note, the radiographic findings from our patient have been previously presented in a radiology journal with emphasis on the diffusion-weighted images, but in this report we describe the clinical details and our patient’s subsequent course [ 6 ].
There is a case report from 1970 of a 59-year-old Jamaican woman with presumed sickle cell trait who deteriorated over the course of several years to near complete paraplegia and who was subsequently found to have a slightly swollen spinal cord in the cervical region and atrophic thoracic and lumbar spine cord segments on autopsy [ 4 ]. The authors noted that her vasculature and neural tissue was otherwise without the stigmata of significant atherosclerotic or degenerative disease, and while no thrombosed vessels were found in relation to the areas of necrosis in her spinal cord, there were however, many arteries and veins distended with abnormally shaped sickle red cells [ 4 ]. A 1980 case report describes a 19-year-old African-American man with sickle cell disease who developed sudden-onset quadriplegia and in post-mortem studies was found to have multiple, old, focal and confluent infarcts involving the cortex and subcortical white matter in the brain, and also of the cervical, thoracic, and upper lumbar spinal cord [ 5 ]. There are no data from these case reports in the literature concerning other potential risk factors including any thrombophilic abnormalities, as these were not commonly looked for in 1970 and 1980.
From the available reports that have looked for an association between factor V Leiden and complications of sickle cell disease, there is no evidence of an obvious relationship [ 16 , 21 , 22 ]. Kahn et al. studied a cohort of 82 patients with different sickle cell states, 19 of whom had had a stroke [ 21 ]. Only one of the 82 was heterozygous for factor V Leiden (there were no homozygotes), and this was not a patient who had experienced a stroke, priapism or any other vascular-type disorder [ 21 ]. Andrade et al. similarly examined a cohort of 73 patients with sickle cell disease in Brazil, of whom five had a stroke [ 16 ]. One of the five was a heterozygote for factor V Leiden; of the patients who had not experienced a stroke, none were positive for the factor V Leiden mutation. Interestingly, that patient had a sister who also had sickle cell anemia and stroke, but the sister did not carry the factor V Leiden mutation. We conclude that our patient’s heterozygosity for factor V Leiden did not contribute to the occurrence of the spinal cord infarction.
Our patient has severe sickle cell disease as manifested by multiple bouts of recurrent acute chest syndrome and the presence of a silent cerebral infarction. As a comorbidity which predisposes to more severe disease, he also has asthma. However, he would not be considered to be very unusual in having this degree of illness. Therefore, the question arises as to why he developed the rare complication of spinal cord infarction. It occurred during the recovery from an episode of acute chest syndrome, which is known to be a time period of increased risk for cerebral infarction, but this is clearly not a full explanation given the frequency of acute chest syndrome and the rarity of spinal cord infarction. His hypoxemia had resolved when the spinal cord infarction occurred, and his worsened anemia had been corrected. In addition, his sickle hemoglobin percentage was quite low. Although our review of the literature does not suggest that his infarct can be explained by the factor V Leiden heterozygosity, he was not tested for any of the other genetic variants that have been recently found to be associated with stroke in SCD such as ANXA2, TGFBR3, and TEK. It is possible that a combination of factor V Leiden heterozygosity and another mutation may increase his risk for this complication. However, in order to determine risk factors for this complication, its true incidence in SCD must be known.
It is possible that spinal cord infarction may occur more commonly than previously recognized in sickle cell disease and is missed or misdiagnosed as cerebral infarction. Although in our patient’s case there were clear findings suggestive of spinal cord involvement, some presentations could be more subtle, and many clinicians may not think of the spinal cord when a patient with sickle cell presents with neurologic deficits. We hope that this report may lead others who care for people with sickle cell disease to be vigilant to the possibility of central nervous system infarction involving the spinal cord.
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Sarnaik SA, Lusher JM: Neurological complications of sickle cell anemia. Am J Pediatr Hematol Oncol. 1982, 4: 386-394. 10.1097/00043426-198224000-00006.
Article CAS PubMed Google Scholar
Sydenstricker VP, Mulherin WA, Houseal RN: Sickle cell anemia; report of two cases in children with necropsy in one. Am J Dis Child. 1923, 26: 132-154.
Article Google Scholar
Merkel KH, Ginsberg PL, Parker JC, Post MJ: Cerebrovascular disease in sickle cell anemia: a clinical, pathological and radiological correlation. Stroke. 1978, 9: 45-52. 10.1161/01.STR.9.1.45.
Wolman L, Hardy G: Spinal cord infarction associated with the sickle cell trait. Paraplegia. 1970, 7: 282-291. 10.1038/sc.1969.44.
Rothman SM, Nelson JS: Spinal cord infarction in a patient with sickle cell anemia. Neurology. 1980, 30: 1072-1076. 10.1212/WNL.30.10.1072.
Marquez JC, Granados AM, Castillo M: MRI of cervical spinal cord infarction in a patient with sickle cell disease. Clin Imaging. 2012, 36: 595-598. 10.1016/j.clinimag.2011.12.013.
Article PubMed Google Scholar
Novy J, Carruzzo A, Maeder P, Bogousslavsky J: Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006, 63: 1113-1120. 10.1001/archneur.63.8.1113.
Cheshire WP, Santos CC, Massey EW, Howard JF: Spinal cord infarction: etiology and outcome. Neurology. 1996, 47: 321-330. 10.1212/WNL.47.2.321.
Nance JR, Golomb MR: Ischemic spinal cord infarction in children without vertebral fracture. Pediatr Neurol. 2007, 36: 209-216. 10.1016/j.pediatrneurol.2007.01.006.
Article PubMed PubMed Central Google Scholar
Millichap JJ, Sy BT, Leacock RO: Spinal cord infarction with multiple etiologic factors. J Gen Intern Med. 2007, 22: 151-154. 10.1007/s11606-006-0029-8.
Masson C, Pruvo JP, Meder JF, Cordonnier C, Touzé E, De La Sayette V, Giroud M, Mas JL, Leys D, Study Group on Spinal Cord Infarction of the French Neurovascular Society: Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry. 2004, 75: 1431-1435. 10.1136/jnnp.2003.031724.
Article CAS PubMed PubMed Central Google Scholar
Francis RB: Platelets, coagulation, and fibrinolysis in sickle cell disease: their possible role in vascular occlusion. Blood Coaulation and Fibrinolysis. 1991, 2: 341-353. 10.1097/00001721-199104000-00018.
Ataga KI, Cappellini MD, Rachmilewitz EA: B-Thalassemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol. 2007, 139: 3-13. 10.1111/j.1365-2141.2007.06740.x.
De Franceschi L, Cappellini MD, Olivieri O: Thrombosis and sickle cell disease. Semin Thromb Hemost. 2011, 37: 226-236. 10.1055/s-0031-1273087.
Ataga KI, Orringer EP: Hypercoagulability in sickle cell disease: a curious paradox. Am J Med. 2003, 115: 721-728. 10.1016/j.amjmed.2003.07.011.
Andrade FL, Annichino-Bizzacchi JM, Saad ST, Costa FF, Arruda VR: Prothrombin mutant, factor v leiden, and thermolabile variant of methylenetetrahidrofolate reductase among patients with sickle cell disease in Brazil. Am J Hematol. 1998, 59: 46-50. 10.1002/(SICI)1096-8652(199809)59:1<46::AID-AJH9>3.0.CO;2-#.
Koren A, Zalman L, Levin C, Abu Hana M, Mader R, Shalev S: Venous thromboembolism, factor V Leiden, and methylenetetrahydrofolate reductase in a sickle cell anemia patient. Pediatr Hematol Oncol. 1999, 16: 469-472. 10.1080/088800199277047.
Rahimi Z, Vaisi-Raygani A, Nagel RL, Muniz A: Thrombophilic mutations among Souther Iranian patients with sickle cell disease: high prevalence of factor V Leiden. J Thromb Thrombolysis. 2008, 25: 288-292. 10.1007/s11239-007-0069-x.
Heit JA, Beckman MG, Bockenstedt PL, Grant AM, Key NS, Kulkarni R, Manco-Johnson MJ, Moll S, Ortel TL, Philipp CS, CDC Thrombosis and Hemostasis Centers Research and Prevention Network: Comparison of characteristics from White- and Black-Americans with venous thromboembolism: a cross-sectional study. Am J Hematol. 2010, 85: 467-471. 10.1002/ajh.21735.
Flanagan JM, Frohlich DM, Howard TA, Schultz WH, Driscoll C, Nagasubramanian R, Mortier NA, Kimble AC, Aygun B, Adams RJ, Helms RW, Ware RE: Genetic predictors for stroke in children with sickle cell anemia. Blood. 2011, 117: 6681-6684. 10.1182/blood-2011-01-332205.
Kahn MJ, Scher C, Rozans M, Michaels RK, Leissinger C, Krause J: Factor V Leiden is not responsible for stroke in patients with sickling disorders and is uncommon in African Americans with sickle cell disease. Am J Hematol. 1997, 54: 12-15. 10.1002/(SICI)1096-8652(199701)54:1<12::AID-AJH2>3.0.CO;2-7.
Wright JG, Cooper P, Malia RG, Kulozik AE, Vetter B, Thomas P, Preston FE, Serjeant GR: Activated protein C resistance in homozygous sickle cell disease. Br J Haematol. 1997, 96: 854-856. 10.1046/j.1365-2141.1997.d01-2084.x.
Download references
Acknowledgements
This manuscript was prepared during the corresponding author’s training and was supported by the T32 NIH grant PHS GRANT 5T32 HL 7149–35.

Author information
Authors and affiliations.
Departments of Internal Medicine and Pediatrics, University of North Carolina School of Medicine, 101 Manning Drive, Chapel Hill, NC, 27514, USA
April Edwards
Departments of Pediatrics and Internal Medicine, Division of Hematology and Oncology, University of North Carolina School of Medicine, 170 Manning Drive 1185A, Physician Office Building CB#7236, Chapel Hill, NC, 27599-7236, USA
E Leila Jerome Clay & Rupa Redding-Lallinger
Departments of Pediatrics and Internal Medicine, Division of Hematology and Oncology, Georgia Regents University, 1120 15th Street, BH 2015, Augusta, GA, 30912, USA
E Leila Jerome Clay
Department of Radiology, University of North Carolina School of Medicine, 100 Manning Drive, Radiology CB#7510, Old Clinic Building, Chapel Hill, NC, 27599-7510, USA
Valerie Jewells
Department of Pediatrics, Michigan State University, GRMEP 1000 Monroe Avenue, NW, Grand Rapids, MI, 49503, USA
Stacie Adams
Department of Medicine, Division of Hematology, Duke University Medical Center, 2212 Elba Street DUMC Box 3939, Durham, NC, 27705, USA
Regina D Crawford
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to E Leila Jerome Clay .
Additional information
Competing interests.
The authors declare they have no competing interests.
Authors’ contributions
AE reviewed our patient’s case, data and figures, and was a major contributor in writing the manuscript. ELJC reviewed our patient’s case and data, completed subsequent drafts of the manuscript and was a major contributor in writing the manuscript. VJ provided the radiological findings, figures and interpretations. SA was involved during the initial presentation of our patient’s case. RDC was involved during the initial presentation of our patient’s case. RR-L reviewed our patient’s case, data, co-ordinated the authors and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Edwards, A., Clay, E.L.J., Jewells, V. et al. A 19-year-old man with sickle cell disease presenting with spinal infarction: a case report. J Med Case Reports 7 , 210 (2013). https://doi.org/10.1186/1752-1947-7-210
Download citation
Received : 30 January 2013
Accepted : 27 July 2013
Published : 23 August 2013
DOI : https://doi.org/10.1186/1752-1947-7-210
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sickle Cell Disease
- Cerebral Infarction
- Factor Versus
- Exchange Transfusion
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- Image & Use Policy
- Translations
UC MUSEUM OF PALEONTOLOGY
Understanding Evolution
Your one-stop source for information on evolution
DNA and Mutations
A case study of the effects of mutation: sickle cell anemia.
Sickle cell anemia is a genetic disease with severe symptoms, including pain and anemia. The disease is caused by a mutated version of the gene that helps make hemoglobin — a protein that carries oxygen in red blood cells. People with two copies of the sickle cell gene have the disease. People who carry only one copy of the sickle cell gene do not have the disease, but may pass the gene on to their children.
The mutations that cause sickle cell anemia have been extensively studied and demonstrate how the effects of mutations can be traced from the DNA level up to the level of the whole organism. Consider someone carrying only one copy of the gene. She does not have the disease, but the gene that she carries still affects her, her cells, and her proteins:
Normal hemoglobin (left) and hemoglobin in sickled red blood cells (right) look different; the mutation in the DNA slightly changes the shape of the hemoglobin molecule, allowing it to clump together.
- There are negative effects at the whole organism level Under conditions such as high elevation and intense exercise, a carrier of the sickle cell allele may occasionally show symptoms such as pain and fatigue.
- There are positive effects at the whole organism level Carriers of the sickle cell allele are resistant to malaria, because the parasites that cause this disease are killed inside sickle-shaped blood cells.
This is a chain of causation. What happens at the DNA level propagates up to the level of the complete organism. This example illustrates how a single mutation can have a large effect, in this case, both a positive and a negative one. But in many cases, evolutionary change is based on the accumulation of many mutations, each having a small effect. Whether the mutations are large or small, however, the same chain of causation applies: changes at the DNA level propagate up to the phenotype.
The effects of mutations
Mutations are random
Subscribe to our newsletter
- Teaching resource database
- Correcting misconceptions
- Conceptual framework and NGSS alignment
- Image and use policy
- Evo in the News
- The Tree Room
- Browse learning resources
- Main Navigation
- Account Navigation
- Main Content
JavaScript is disabled on your browser
Please enable JavaScript or upgrade to a JavaScript-capable browser to use the ASH Image Bank.
- ASH Academy

- ABOUT IMAGE BANK
- PERMISSIONS
- COLLECTION Images of peripheral blood and/or bone marrow of blood disorders and normal hematopoiesis.
- ATLAS Normal and abnormal blood cells
- REFERENCE CASES Complete cases of common blood disorders (peripheral blood, bone marrow, and diagnostic studies).
- UPLOAD IMAGES
- Home / Reference Cases
Case history of a child with sickle cell anemia in India
A three years old male child, native of Jharkhand, Central India presented with mild pallor, icterus, and history of on and off abdominal and joint pains. On examination the child had mild splenomegaly. He had history of two prior hospital admissions. First at the age of 1 year, when he was diagnosed to have pneumonia and second, at the age of 3 years (3 months prior to coming to our institution) for fever, anemia and jaundice. He has had three transfusions till now, last transfusion was 3 months back. There is history of sibling death at 5 years of age due to fever and jaundice.
The hemogram showed anemia with leukocytosis. Red cell morphology (Figure 1) revealed severe anisopoikilocytosis with macrocytes, microcytic hypochromic red cells, target cells, many boat cells, sickled RBCs, polychromatic cells and occasional nucleated RBCs. Results of the automated blood cell counts showed Hb 7.7 g/dl, RBC 2.44 x 109/l, MCV 97.1 fl, MCH 31.4 pg, MCHC 32.3 g/dl, RDW 26.6%. There were occasional nucleated red cells and relative neutrophilia. Further to confirm HbS, a sickling test using freshly prepared 2% sodium meta-bisulphite was performed which was positive (Figure 2).
Hemoglobin HPLC on Bio-Rad Variant 2 showed raised fetal hemoglobin (HbF) and a variant peak in S window (71.9%) at retention time of 4.36 mins. Adult Hb (HbA) of 8.5% was noted (Figure 3). Figure 4 shows Cellulose acetate hemoglobin electrophoresis at alkaline pH (8.6), which showed a prominent band in S/D/G region and a faint band in F region. Investigations of the father showed also showed a variant peak in S window (32.9%) at retention time of 4.36 mins along with HbA (57.1%) on HPLC with Bio-Rad Variant II which is diagnostic of Sickle cell trait (Figure 5).
Sickle cell disease (SCD) is the most common symptomatic hemoglobinopathy caused as a result of inheritance of two copies of the sickle β-globin gene variant (βS). A single nucleotide substitution leading to replacement of glutamic acid by valine at position 6 of the β-globin polypeptide chain leads to formation of HbS which is responsible for disease manifestation. SCD has a wide geographical distribution throughout major parts of Africa, the Middle East, India and in some regions of Mediterranean countries. In India, it is mainly concentrated in the central region including parts of Madhya Pradesh, Chattisgarh, Orissa, Maharashtra, Gujrat and Jharkhand. HbS has carrier frequencies varying from 5 to 35% and are especially seen amongst the scheduled tribes, scheduled castes and other backward castes.
Sickle cell mutation is believed to be originated five times in history spontaneously. This can be elucidated by five βS-globin haplotypes. These haplotypes include Senegal (SEN), Benin (BEN), Bantu or the Central African Republic (CAR), Cameroon (CAM) and Arab-Indian (ARAB). They enable us to understand the origin, evolution, migration and natural selection of genetic defects. They can be identified by specific restriction sites within the β-globin gene cluster. Different haplotypes are known to have different HbF levels. Senegal and Arab-Indian haplotypes have higher HbF levels when compared to other haplotypes. However, recently a study has investigated the origins of the sickle mutation by using whole-genome-sequence data to conclude that there might be single origin of sickle allele.
LEARNING POINTS
1. Sickle cell disease (SCD) is the most common symptomatic hemoglobinopathy in the world, largely seen in parts of Africa, the Middle East, India and in some regions of Mediterranean countries.
2. SCA is a monogenic disorder with an autosomal recessive inheritance. The parents are clinically asymptomatic and have normal blood counts. They are usually diagnosed incidentally or as a result of family studies in SCA patients.
3. Neonates are asymptomatic due to high HbF, but symptoms begin to appear by six months of age. Many infants present with lethal complications at first presentation. This emphasizes the importance of newborn screening in these susceptible pre-symptomatic cases in endemic regions.
4. SCA has a variable clinical course amongst different individuals depending upon various genetic determinants like βs haplotype, factors affecting HbF levels and co-inheritance of other disease modifying factors.
5. Diagnosis mainly relies upon identification of HbS (by any of the following HPLC, Hb Electrophoresis, Iso-electric focusing or sickling test). Once HbS is identified, it has to be validated by alternative method.
6. Treatment of sickle cell disease generally aims at relieving symptoms and preventing infections, sickle cell crises and long-term complications. Stem cell transplant is the only potential cure available presently.
HPLC pattern of the index case with sickle cell anemia showing HbS and HbF peaks.
Hemoglobin electrophoresis at alkaline pH. Black arrow shows the index case with HbS and HbF bands.
Loading. Please wait.
Uploading files. please wait..
Creating critical continuity for sickle cell patients

Matthew Harris May 29, 2024
KAMILLE WARE is always prepared.
At just 23 years old, she has a plan for when the throbbing pain strikes in her lower back, when it seeps into her midsection, and moves down her legs.
“I keep cardigans everywhere,” Ware said. “I have one in my car, all over my house.” In her office at Martin University, she keeps a blanket or a heating pad handy. “Heat is my friend.”
These are the simple tricks that Ware uses to manage her sickle cell disease, an inherited condition that affects red blood cells and their ability to carry oxygen.
Ware considers herself fortunate. She grew up on the west side of Indianapolis, only a short drive from expertise at Riley Hospital for Children. She maintained that proximity as she earned degrees at Marian University. And now, her commute to adult care is just five miles from her job as a public health coordinator for the National Center for Racial Equity and Inclusion.
“I've always stayed close by,” Ware said. “All my doctors are here, and I wanted it to stay that way in case something happened.”
Unfortunately, many of the 1,700 Hoosiers diagnosed with sickle cell struggle to strike such a meticulous balancing act. Many rely on care only available at Indiana University School of Medicine but accessing lifespan care means overcoming physical distances from knowledgeable providers as well as entrenched social and economic challenges to care.
Sickle cell mainly afflicts Black individuals, who might be underinsured and who may harbor deep distrust of health care. Their symptoms and unpredictable hospitalizations pose challenges around holding steady work, creating financial stress, housing instability and food insecurity.
Now, though, IU School of Medicine is slowly becoming a haven for care, with a handful of researchers striving to find new treatments. They do this work at a place that ranks among the top public medical schools in research funding and in a city where multiple hospitals combine to serve every patient.

For many sickle cell patients, finding a physician versed in their condition proves the biggest challenge. Surveys of primary care doctors report that most are uncomfortable treating it. And many in private practice don't accept Medicaid, which 80% of sickle cell patients commonly rely on for insurance coverage.
Assuming a patient finds a provider, usually a hematologist, they must face a disease that can affect multiple systems within the body.
Managing care for sickle cell disease becomes an act of deft orchestration involving a neurologist, pulmonologist, and ophthalmologist. All those physicians rarely practice in the same network, much less the same location.
Beyond Indianapolis, IU School of Medicine is piloting telehealth programs in Evansville and Fort Wayne to reduce the need to travel long distances for care. Under this model, a patient would visit a location to join a primary care provider and talk with an expert in Indianapolis. IU is also exploring virtual visits, where patients use a device mailed to them to help with specialist consults.
Even though her office is just 15 minutes away from her providers, Ware's care involves tricky logistics. To check in with her hematologist, Ware visits IU’s Melvin and Bren Simon Comprehensive Cancer Center. To see her pulmonologist, she goes to Sidney and Lois Eskenazi Hospital. To see an ophthalmologist, she must go outside her insurance network.
Despite the complicated logistics, Ware is an outlier. Nearly 70 percent of sickle cell patients don't see any specialist. And for many, that means their care veers off course.

That can be the start of a frustrating cycle. Studies have found almost 60 percent of sickle cell patients are grappling with an immediate financial crisis; one in five confront housing instability. As a result, many individuals wind up with long gaps in care.
When these patients experience severe symptoms, trips to the emergency room become a stopgap. But that means being treated by busy attending physicians who lack a deep knowledge of sickle cell. Often, those physicians assume the worst: A patient is putting on an act to obtain pain medication. Over time, such experiences erode faith in the health care system.
“There's a huge disconnect in how children with sickle cell are viewed and how adults are viewed,” O'Brien said. “When you're consistently mistreated, it creates profound distrust. That's the foundation of what we're trying to undo. We have to demonstrate to them there are ways to help.”
It is frequently the result of what can be a stilted transition in care.

At IU, there's sturdy infrastructure in place to care for children with sickle cell disease. Jacob heads up a nine-person team that includes two nurse educators, a social worker, a transition coordinator, and a psychologist. Collectively, they care for more than 400 patients around the state, handling up to 60 weekly visits.
Around the time a patient enters high school, that team begins preparing them to take control of their care. For example, a nurse educator helps develop vital skills: how to describe symptoms, track medications, ask follow-up questions, and balance appointments. Around the same time, the team starts holding “cross-collaboration conversations” with physicians like O'Brien.
Starting at age 18, a patient like Ware sees an adult and pediatric provider simultaneously. Four years later, the handoff is completed. That sounds fine in theory. “I'd had the same doctors for a good portion of my life,” Ware recalled. “Once you have someone who knows your disease, you don't have to explain things. There's a shorthand. You're just comfortable.”
When a patient does come to O'Brien, the early work is heavily administrative. He fills gaps in medical history, ascertains what medications a patient has tried, and uses that information to start coordinating with peers in different disciplines. And he must do all that while slowly winning over a skeptical patient's confidence.

“Our patients need multiple consults,” O'Brien said. “They need MRIs, urine testing, blood testing, and eye exams. There are multiple appointments, and we ask a lot of them just to keep up with a baseline understanding of their disease. We try to consolidate things as much as we can or bring them together in one place, but it's immensely challenging.”
That’s why O'Brien and Jacob have fleshed out a vision for a sickle cell center, which is not so much a physical space as a robust staff dedicated to integrated care. Ideally, it would wrap in 30 staffers and employ a pharmacist, nutritionist, and physical therapist. Patients would also meet with a “psychosocial team” to keep tabs on a patient's employment and housing.
Addressing mental health would also fill a need often omitted in care plans. Ware considers herself fortunate to have stable family support and skilled providers, but empathy alone can't resolve the frustration and anxiety created by her disease. “A lot of them don't understand what it is to be in your early 20s and have these chronic problems,” Ware added.
Still, establishing a Sickle Cell Center of Excellence would likely require substantial philanthropic support. Doing so would offer benefits beyond lowering barriers to care. For one, it would allow medical students to rotate through during clerkships and gain early exposure to caring for sickle cell patients.
Creating a large pool of patients would also give IU an edge in the competition for national funding that's a fraction of what's needed. Cystic fibrosis—far less common than sickle cell disease—receives 10 times the research funding from the National Institutes of Health. Yet a slice of coveted financing has finally reached the School of Medicine.
In January, Ankit Desai, MD, earned a $3 million grant to test whether an existing drug used for high blood pressure can ease sickle cell symptoms like chest pain, dizziness, and shortness of breath. Propranolol halts the release of stress hormones, slowing a patient's heartbeat and making the organ's lower chambers less likely to ship out blood that lacks enough oxygen.
“Evaluating a therapeutic that has already been consumed by millions for other diseases could help accelerate the potential use in patients with sickle cell more quickly,” said Desai, an associate professor of medicine at the school's Krannert Cardiovascular Research Center.
It would also vital diversity to the treatments O’Brien prescribes, such as hydroxyurea for acute chest syndrome or voxelotor to lower the risk of anemia by preventing sickle cells from clumping together. “None of them are amazing,” O'Brien said. “But they do have a benefit and can improve a patient's life.”
All of those aspirations sound great to Ware, whose early experiences as an adult juggling her aspirations, along with her disease, have only reinforced the crucial role IU plays.
“Having a hub would make that so much easier for us,” Ware said. “It would really help families like mine to have that one-stop shop for all the resources and care they need.”
Tags: Pediatrics
Matthew Harris
Sickle Cell Anemia (SCA) Hesi patient Case Study

Students also viewed

A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Data and Statistics
- Communication Resources
- Steps to Better Health Toolkit
- What Is Sickle Cell Trait?
- Complications of Sickle Cell Disease
- Stories of Sickle Cell
- Scientific Articles about Sickle Cell Disease
- Sickle Cell Information for Healthcare Providers
- Show All Home
About Sickle Cell Disease
- Sickle cell disease (SCD) is a group of inherited blood disorders. Abnormal hemoglobin is produced.
- Red blood cells become hard and sticky and get stuck in small blood vessels, resulting in pain and other serious complications.
- There are several types of SCD, some more severe than others.
- In the United States, SCD is often found at birth through routine newborn screening.

Sickle cell disease (SCD) is a group of inherited red blood cell disorders. Red blood cells contain hemoglobin, a protein that carries oxygen. Healthy red blood cells are round, and they move through small blood vessels to carry oxygen to all parts of the body.
In someone who has SCD, the hemoglobin is abnormal, which causes the red blood cells to become hard and sticky and look like a C-shaped farm tool called a sickle. The sickle cells die early, which causes a constant shortage of red blood cells. Also, when they travel through small blood vessels, sickle cells get stuck and clog the blood flow. This can cause pain and other serious complications (health problems) such as infection, acute chest syndrome, and stroke.
There are several types of SCD. The specific type of SCD a person has depends on the genes they inherited from their parents. People with SCD inherit genes that contain instructions, or code, for abnormal hemoglobin.
Below are the most common types of SCD:
HbSS
People who have this form of SCD inherit two genes, one from each parent, that code for hemoglobin "S." Hemoglobin S is an abnormal form of hemoglobin that causes the red cells to become rigid, and sickle shaped. This is commonly called sickle cell anemia and is usually the most severe form of the disease.
People who have this form of SCD inherit a hemoglobin S gene from one parent and a gene for a different type of abnormal hemoglobin called "C" from the other parent. This is usually a milder form of SCD.
HbS beta thalassemia
People who have this form of SCD inherit a hemoglobin S gene from one parent and a gene for beta thalassemia, another type of hemoglobin abnormality, from the other parent. There are two types of beta thalassemia: "zero" (HbS beta 0 ) and "plus" (HbS beta + ). Those with HbS beta 0 -thalassemia usually have a severe form of SCD. People with HbS beta + -thalassemia tend to have a milder form of SCD.
There also are a few rare types of SCD, such as the following:
HbSD, HbSE, and HbSO
People who have these forms of SCD inherit one hemoglobin S gene and one gene that codes for another abnormal type of hemoglobin ("D," "E," or "O"). The severity of these rarer types of SCD varies.

5 Facts You Should Know About Sickle Cell Disease
Sickle cell trait (SCT)
People who have sickle cell trait (SCT) inherit a hemoglobin S gene from one parent and a normal gene (one that codes for hemoglobin "A") from the other parent. People with SCT usually do not have any of the signs of the disease. However, in rare cases, a person with SCT may develop health problems; this occurs most often when there are other stresses on the body, such as when a person becomes dehydrated or exercises strenuously. Additionally, people who have SCT can pass the abnormal hemoglobin S gene on to their children.
SCD is a genetic condition that is present at birth. It is inherited when a child receives two genes—one from each parent—that code for abnormal hemoglobin.
SCD is diagnosed with a simple blood test. In children born in the United States, it most often is found at birth during routine newborn screening tests at the hospital. In addition, SCD can be diagnosed while the baby is in the womb. Diagnostic tests before the baby is born, such as chorionic villus sampling and amniocentesis , can check for chromosomal or genetic abnormalities in the baby. Chorionic villus sampling tests a tiny piece of the placenta called chorionic villus. Amniocentesis tests a small sample of amniotic fluid surrounding the baby.
Because children with SCD are at an increased risk of infection and other health problems, early diagnosis and treatment are important.
Talk to your doctor to find out how to get tested and to learn about the results after testing.
Complications
People with SCD may start to have signs of the disease during the first year of life, usually around 5 months of age. Symptoms and complications of SCD are different for each person and can range from mild to severe.
Prevention and treatment of SCD complications
Management of SCD is focused on preventing and treating pain episodes and complications. Prevention strategies include lifestyle behaviors such as maintaining adequate fluid intake and avoiding extreme temperatures and medical screenings such as transcranial Doppler (TCD) ultrasound screenings to identify children at increased risk of stroke.
Prevention measures also include medical interventions such as vaccines to prevent infections and blood transfusions to reduce the occurrence of stroke in persons identified to be at risk. When pain crises occur, they can be managed through various clinical strategies include medication and intravenous fluids. Additionally, several medications are available that can be taken regularly to prevent or reduce the occurrence of pain crises and other complications. Bone marrow transplants and newly developed gene therapies are also potential treatment options for some patients.
Below is a list of key organizations of interest to people living with SCD and their families.
American Society of Hematology
- SCD Initiative : An initiative to improve outcomes for people with SCD
- Build Your Own SCD School Binder : Resources to support students with SCD
American College of Emergency Physician's Emergency Department Sickle Cell Care Coalition Information about emergency care for people with SCD
Children's Hospital of Philadelphia Sickle Cell Center Sickle cell school outreach tools and educational resources
ClinicalTrials.gov Up-to-date information on sickle cell disease clinical research trials
Foundation for Women & Girls + with Blood Disorders (FWGBD) An organization dedicated to ensuring all women and girls with blood disorders are correctly diagnosed and optimally treated and managed at every stage of life
National Heart, Lung, and Blood Institute Sickle cell information, tips for healthy living, and resources
National Human Resource Genome Institute Gene therapy education materials for the sickle cell disease community
Sickle Cell Disease Association of America Information, news, research, and resources
Sickle Cell Information Center Information about SCD; resources for patients, families, and healthcare providers; research; clinical trials; news; and books
Sickle Cell Reproductive Health Education Directive Resources on reproductive health care for people living with all types of SCD
- + This includes persons with, or have had, the ability to menstruate.
Sickle Cell Disease (SCD)
Sickle cell disease (SCD) is a group of inherited red blood cell disorders. In SCD, the red blood cells become hard and sticky and look like a C-shaped farm tool called a “sickle.”
For Everyone
Health care providers.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To The Science
Research Making a Difference
- Why It's Important
- Key Accomplishments
- Opportunities & Challenges
Advancing the Research
- Related Reading

WHY IT'S IMPORTANT
NHLBI has several major research goals in blood diseases. One is to ensure the adequacy and safety of the nation’s blood supply. A second is to support efforts to treat and cure blood disorders, such as supporting scientific advances in stem cell biology and new gene and cell-based therapies to repair and regenerate human tissues and organs. By funding and supporting research on the causes, prevention, and treatment of blood disorders, we help researchers make new discoveries, leading to better medical care for the millions of people affected by blood disorders around the world.
KEY ACCOMPLISHMENTS
- The NHLBI-led Cure Sickle Cell Initiative is a patient-focused research effort to develop genetic therapies for use in clinical research.
- NHLBI-sponsored research has helped and continues to help Americans with sickle cell disease live longer and stay well in adulthood.
- NHLBI-supported scientists developed lifesaving treatments for patients with hemophilia and are now working on gene therapy approaches to cure the disease.
- NHLBI-funded research helped develop therapies that increased the survival rates for patients who have aplastic anemia.
OPPORTUNITIES & CHALLENGES
NHLBI’s Strategic Vision guides the Institute’s research activities. Many of the objectives, compelling questions, and critical challenges identified in the plan focus on blood biology, blood disorders, and blood safety. Training the next generation of blood scientists is also a high priority for the NHLBI.
More Information - Blood Disorders and Blood Safety
Researchers are working across scientific disciplines to advance blood disorders research. The NHLBI assists in this effort by enabling early translational research to accelerate the development of new clinical interventions. Currently, we are supporting research resource programs that provide regulatory, pharmacology, toxicology, and manufacturing services to NHLBI investigators who conduct blood science research. The hope is for patients with blood disorders to live long and full lives and for the research to improve blood safety measures both in the United States and around the world.
NHLBI is advancing blood disorders and blood safety research in many ways. Learn more about some of our key efforts related to sickle cell disease, anemia, blood transfusions, and more.
We Perform Research
The NHLBI Division of Intramural Research and its Hematology Branch , Sickle Cell Branch , and Sickle Cell Program include investigators who are actively engaged in research on blood disorders and blood safety.
We Fund Research
The research we fund today will help improve our future health. Our Division of Blood Diseases and Resources is a leader in research on the causes, prevention, and treatment of blood diseases (other than cancers of blood cells). The NHLBI’s Division of Cardiovascular Sciences also supports some blood science–related research.
The Promise of Precision Medicine
Through NHLBI’s Trans-Omics for Precision Medicine (TOPMed) program , researchers will use data from studies focused on heart, lung, blood, and sleep disorders to better predict, prevent, diagnose, and treat blood disorders based on a patient’s unique genes, environment, and molecular signatures. Learn more about NHLBI precision medicine activities .
Funding Global Health Research Efforts
More than 75 percent of infants with sickle cell disease (SCD) are born in sub-Saharan Africa. By funding the SCD in Sub-Saharan Africa (SSA) Network, which includes a Clinical Coordinating Center, a Sickle Africa Data Coordinating Center, and established and new consortium sites, we are building the regional capabilities to research sickle cell disease and monitor patients in Africa. These efforts may ultimately improve treatment and care for Americans who have sickle cell disease.
Improving Blood Safety and Availability
The NHLBI started the Recipient Epidemiology and Donor Evaluation Study (REDS) program in 1989 to protect the nation’s blood supply from threats, improve the benefits of transfusions, and reduce the risks of transfusions. REDS is the largest multicenter research program of its kind in the United States, and after 30 years, it is entering a new, important phase. It now aims to evaluate and improve the safety and effectiveness of transfusion therapies with attention not only to adults but also to understudied populations, including newborns and children.
Addressing Issues in Blood and Marrow Transplantation
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) was established to conduct large, multi-institutional clinical trials to understand the best possible treatment approaches in blood and marrow transplantation. In the United States, nearly 21,000 patients receive blood or marrow transplants annually, mainly for rare blood disorders.
Studying Underlying Factors in Severe Trauma Cases
The Trans-Agency Research Consortium for Trauma-Induced Coagulopathy (TACTIC) research program is no longer active but completed important work. It was established in collaboration with the U.S. Department of Defense to study the disruptions in the normal process of blood clotting that occur in individuals who experience severe physical trauma. Trauma, particularly severe trauma, can disturb the body’s mechanisms for stopping hemorrhage or lead to the overproduction of blood clots, both of which can cause major or even fatal complications.
Supporting scientists performing NHLBI-funded gene therapy research
Research on genetic therapies is part of our broader commitment to advancing scientific discovery aimed at developing safe and effective treatments for heart, lung, and blood disorders and diseases caused by faulty genes. Our National Gene Vector Biorepository and Gene Therapy Resource Program help investigators transform early-stage research into genetic therapies. These programs equip scientists with the tools, resources, safety testing services, and animal models they need to advance genetic therapy research from the laboratory into clinical trials.
Related Health Topics
Related news.

RELATED EVENT

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.188(10); 2016 Jul 12

A 19-year-old woman with sickle cell disease and pain
A 19-year-old woman with homozygous sickle cell disease presents to her family physician’s office with acute generalized bone pain. She reports stress from school deadlines. She prefers to manage her pain at home if possible and not go to the emergency department. She has no comorbidities.
Is this patient having pain from a vasoocclusive crisis?
She reports the character of the pain as being typical for a vasoocclusive crisis (“pain crisis”). She does not have any localized pain that may otherwise suggest complications of sickle cell disease, such as joint osteonecrosis, leg ulceration or cholecystitis. 1 , 2 Because the duration and severity of her pain is short and moderate, she hopes the pain can be controlled with outpatient treatment.
The patient has no worrying features (“red flags”) such as respiratory symptoms, neurologic changes, fever, previous admission to an intensive care unit (ICU) or history of exchange transfusion. 1 Other common triggers of vasoocclusive crisis are excluded (e.g., infection, weather changes, dehydration and menstruation).
A physical examination did not show other causes for the pain, signs of infection, hypoxia or respiratory disease. 3 Although pulse oximetry is not available in the office, the lack of respiratory signs and symptoms, with no history of respiratory concerns, is reassuring. Particularly in children, splenic palpation should be performed to rule out sequestration. 4
What diagnoses should be ruled out before outpatient treatment for vasoocclusive crisis?
Complications from sickle cell disease that require management beyond hydration and analgesia must be treated in hospital. Acute chest syndrome is a leading cause of mortality. It is characterized by respiratory symptoms, hypoxia and/or fever, with an infiltrate on chest radiography. 5 Acute stroke, priapism, splenic sequestration and surgical abdomen all require referral to the emergency department. Patients with febrile illness (body temperature > 38.3°C), particularly in children, should also be referred to the emergency department because of the increased risk of overwhelming postsplenectomy or hyposplenic infection. 6 Medical conditions unrelated to sickle cell disease that may account for the clinical findings should also be considered in these patients.
Are any investigations necessary?
Tests to assess for alternative or coexisting acute diagnoses should be carefully selected based on the likely differential diagnoses and the patient’s presenting symptoms, such as fever, respiratory symptoms, localized pain or palpable splenomegaly. The diagnosis of vasoocclusive crisis is clinical and cannot be made by specific investigation. 2 , 3
The patient’s complete blood cell count results should be interpreted in the context of her steady-state hemoglobin level; this is usually in the range of 60–90 g/L in patients with homozygous sickle cell disease. 1 A reticulocyte count can help to determine whether marked anemia is due to accelerated hemolysis or bone marrow suppression (e.g., viral illness or marrow suppression by hydroxyurea). A 20% drop from the baseline hemoglobin level or a reticulocyte count less than 80 warrants closer monitoring of the hemoglobin level and anemia symptoms. 1
What advice should be offered to the patient to help manage pain at home?
Management of vasoocclusive crisis is symptomatic, and the patient should be advised of the following: administration of rapid, sustained and effective analgesia, which is titrated to an objective pain score, along with hydration ( Box 1 ). Supplemental oxygen has not been proven to be beneficial in the setting of normal blood oxygen level.
Summary of recommendations on managing vasoocclusive pain crises from current clinical guidelines 3 , 7 , 8
Vasoocclusive crises are the most common presentations to health care providers by patients with sickle cell disease. The clinician should:
- Ascertain whether the current pain is typical of previous vasoocclusive crises.
- Evaluate patient for worrying features (“red flags”) on history and physical examination.
- Use the 3Ps of pain management: physical, psychological and pharmacologic. Treat pain with rapid, sustained and effective analgesia, titrated to an objective pain score, and encourage hydration and rest.
- Prescribe acetaminophen, a nonsteroidal anti-inflammatory and an opiate, if no contraindications are present.
- Consider the use of hydroxyurea to reduce the incidence of future pain episodes.
There is a paucity of trial data that supports any one particular analgesia regimen, but the pain literature and expert consensus support the use of multimodal analgesia. 2 , 9 Acetaminophen and a nonsteriodal anti-inflammatory drug should be prescribed with an opiate, unless otherwise contraindicated. 1 – 3 , 7 Patients will often have a preferred opiate that they have found, with experience, to be effective. This patient should be informed about the safe and effective use of analgesia, including initial frequent doses to control pain quickly. Provincial drug databases may provide useful information on prior opiate requirements. She should also be advised to drink maintenance fluids for the duration of the pain episode (e.g., 2.0–2.5 L/day for adults). Transfusions of red blood cells are not recommended for uncomplicated vasoocclusive crisis.
What follow-up can be instituted to prevent future episodes?
Randomized controlled trials and long-term follow-up studies have shown that hydroxyurea reduces the frequency of vasoocclusive crisis and other complications by up to 50%. 10 , 11 Hydroxyurea is now recommended for most patients with sickle cell disease for primary or secondary prevention of organ damage. 1 The patient should be followed, ideally using a shared care model with a primary care physician, by a specialist in sickle cell disease. Steps should be taken to modify the trigger of the vasoocclusive crisis with the aim of reducing future episodes (e.g., in this instance, psychological stress).
Case revisited
Because the patient had no worrying features on history or physical examination of a complicated vasoocclusive crisis, she was prescribed immediate-release hydromorphone in conjunction with acetaminophen and ibuprofen, and was allowed to go home. An opiate contract was put in place. She was excused from school for the rest of the week. She rested at home, kept warm and drank adequate fluids. Her pain resolved, and she was seen in follow-up the next week. She agreed to a hematology referral for counselling about hydroxyurea and was given tips about stress management.
Decisions is a series that focuses on practical evidence-based approaches to common presentations in primary care. The articles address key decisions that a clinician may encounter during initial assessment. The information presented can usually be covered in a typical primary care appointment. Articles should be no longer than 650 words, may include one box, figure or table and should begin with a very brief description (75 words or less) of the clinical situation. The decisions addressed should be presented in the form of questions. A box providing helpful resources for the patient or physician is encouraged.
Competing interests: None declared.
This article has been peer reviewed.
The clinical scenario is fictional.
Contributors: All of the authors contributed substantially to the conception of the article, drafted the work, approved the final version to be published and agreed to act as guarantors of the work.
Type your tag names separated by a space and hit enter

Electrocardiographic Changes and Their Association With Disease Severity in Adults With Sickle Cell Anemia at a Tertiary Care Center: A Cross-Sectional Study. Cureus . 2024 May; 16(5):e60197. C
Introduction Sickle cell anemia (SCA), a severe hematological disorder, is characterized by the presence of sickle-shaped erythrocytes that obstruct capillaries and restrict blood flow. This pathophysiology not only promotes systemic complications but may also influence cardiac function. Cardiac complications are a leading cause of mortality in SCA patients, yet the specific electrocardiographic (ECG) changes associated with disease severity are not thoroughly understood. This cross-sectional study aimed to explore ECG abnormalities in adults with SCA and correlate these findings with disease severity. Methods An observational cross-sectional study was conducted over 18 months, from January 2022 to June 2023, among 140 SCA patients at the Sickle Cell OPD of All India Institute of Medical Sciences, Raipur, Raipur, India. Steady-state SCA (HbS >50%) patients screened by high-performance liquid chromatography were enrolled. A history, physical examination, complete blood count, and ECG were done for all cases. The disease severity score was calculated using the Adegoke and Kuti severity scores, and their association with various ECG changes was studied. The chi-square test (Fisher's exact test, wherever applicable) was used for comparing the proportion. The correlation was done using the Pearson correlation coefficient or Spearman's rho. Results Out of 140 patients, the mean age of the study participants was 26 ± 6 years. More than half of the cases (80; 57%) fall under the 18-27 age group, with a male-to-female ratio of 4:3. A total of 99 (70.7%) of the participants had mild disease, and 41 (29.3%) had moderate disease. The QT interval was significantly higher among patients with mild disease compared to those with moderate disease (p-value: <0.01). QTc dispersion and prolonged QTc interval were significantly higher among patients with moderate disease compared to mild disease (p-value <0.01, 0.04, respectively). Sinus tachycardia and right ventricular hypertrophy with p-pulmonale were significantly higher in moderate severity (p < 0.01). A significant positive correlation was observed between QTc dispersion, P-wave dispersion, and severity (r: 0.19, 0.17; p-value: 0.02, 0.04, respectively). Conclusion As the disease severity progressed, the ECG changes studied had a higher distribution and significance. ECG is a readily and widely accessible investigation that can be used to screen all SCA patients for early recognition of various underlying cardiac complications.
Authors +Show Affiliations
Pub type(s).
- PMC Free PDF
- Kannauje PK

Related Citations
- Relationship Between Disease Severity and Resting Electrocardiograms of Adults With Sickle Cell Anemia in a Tertiary Institution in Southern Nigeria.
- Assessment of Ventricular Repolarization in Sickle Cell Anemia Patients: The Role of QTc Interval, Tp-e Interval and Tp-e/QTc Ratio and Its Gender Implication.
- Electrocardiographic abnormalities and dyslipidaemic syndrome in children with sickle cell anaemia.
- Serum levels of copeptin, C-reactive protein and cortisol in different severity groups of sickle cell anaemia.
- Disease severity and renal function among sickle cell anaemia patients in a tertiary hospital, South-south, Nigeria: a cross sectional study.
- Myocardial injury in patients with sickle cell anaemia and myocardial ischaemia in Calabar, Nigeria.
- Electrocardiographic abnormalities in patients with sickle cell disease: A systematic review and meta-analysis.
- [Electrocardiographic changes in patients with chronic anemia].
- Electrocardiographic Study in Adult Homozygous Sickle Cell Disease Patients in Lagos, Nigeria.
- Relationship between disease severity and folate status of children with sickle cell anaemia in Enugu, South East Nigeria.

IMAGES
VIDEO
COMMENTS
Sickle cell disease (SCD) is a common group of life-threatening, genetic disorders caused by the synthesis of abnormal hemoglobin (sickle hemoglobin), which when deoxygenated, polymerizes and causes sickling of red blood cells. SCD is characterized by chronic hemolytic anemia, vasoocclusion, and progressive vascular injury causing multiorgan ...
Explanation. The incidence of primary stroke in children with SCD is 0.6 to 0.8 events per 100 patient-years, with a cumulative incidence of 7.8 percent by age 14 years in the Jamaican cohort and 11 percent by age 20 years in the U.S. Cooperative Study of Sickle Cell Disease. Once stroke has occurred, the incidence of recurrent (secondary ...
In follow up of Hb electrophoresis, Hb s was 80%, Hb F: 18%, and Hb A2: 2%. Finally in peripheral blood smear, sickling of RBC was detected and the patient was diagnosed with sickle cell anemia and acute splenic sequestration crisis which was associated with acute chest syndrome treated with wide spectrum antibiotic (cefotaxim and erythromycin) and transfusion exchange (Figure 3).
Among patients with sickle cell disease, the lifetime risk of acute chest syndrome is 30 to 50%; it is the second most common cause of hospitalization and a leading cause of death in patients with ...
Additional data on LentiGlobin treatment in sickle cell disease is currently being collected in HGB-206, a multicenter, phase 1/2 clinical study in the United States. 19 Follow-up is more limited ...
Nurse Maggie works in a pediatric hematology unit and is caring for Marcus, a 9-year-old with a history of sickle cell disease who was admitted for a vaso-occlusive crisis, or VOC. After settling Marcus in his room, Nurse Maggie goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Marcus' care by recognizing and analyzing cues, prioritizing ...
Typically, sickle cell disease is diagnosed in infancy or before the age of 3 years with a great number presenting after the age of 6 months. 10 The age at diagnosis is lower in children with HbSS than those with HbAS according to a retrospective study concluded in 2009 with the average age of diagnosis being 2 years old (24-25 months) and ...
A shortage of RBCs due to their sickle shape and short lifespan results in chronic anemia, which leads to fatigue. Patients with SCA have an average hospital length of stay of 5.1 days, with an average cost per patient of over $7,500. In 2016, there were approximately 134,000 inpatient hospitalizations related to SCA.
The case study of Mr. E explains his symptoms when arriving at the ED. After an evaluation, the healthcare providers believe that he's experiencing; drug-seeking behavior. a hypertensive crisis. a vaso-occlusive crisis. In the case study, the healthcare providers for Mr. E feel that his SCA crisis could be related to; depression. dehydration.
Thirty-eight SNPs in 22 genes were genotyped in 130 patients with sickle cell anemia and stroke and in 103 patients who had sickle cell ... results of a case-crossover study. Br J Haematol 2008 ...
Sickle cell disease (SCD) is an inherited hemoglobin disorder affecting about 100 000 individuals in the United States and more than 20 million people worldwide, mainly of African descent. 1,2 SCD is a chronic, debilitating medical condition that affects patients across their life span and is associated with significant morbidity and early ...
This case presents a classic example of an African American individual who is heterozygous for sickle cell disease and who does not manifest any symptoms until he encounters extreme physical conditions. The case explores the initial presentation of sickle cell symptoms in a heterozygote, the assembly of a pedigree and calculation of genetic ...
Sickle cell disease (SCD), a group of inherited hemoglobinopathies characterized by mutations that affect the β-globin chain of hemoglobin, affects approximately 100,000 people in the USA and more than 3 million people worldwide [1, 2].SCD is characterized by chronic hemolytic anemia, severe acute and chronic pain as well as end-organ damage that occurs across the lifespan.
Our patient's case raises the question as to whether spinal cord infarction is being missed in individuals with sickle cell disease and neurologic symptoms. ... Studies have indicated that in sickle cell disease there is increased platelet activation and ... Spinal cord infarction in a patient with sickle cell anemia. Neurology. 1980, 30: ...
DNA and Mutations. A case study of the effects of mutation: Sickle cell anemia. Sickle cell anemia is a genetic disease with severe symptoms, including pain and anemia. The disease is caused by a mutated version of the gene that helps make hemoglobin — a protein that carries oxygen in red blood cells. People with two copies of the sickle cell ...
On examination the child had mild splenomegaly. He had history of two prior hospital admissions. First at the age of 1 year, when he was diagnosed to have pneumonia and second, at the age of 3 years (3 months prior to coming to our institution) for fever, anemia and jaundice. He has had three transfusions till now, last transfusion was 3 months ...
STEM CELL TRANSPLANT REPORT Visit: VISIT NBR EDT STAT seq. e: SEQ_NBR Visit Date: VISIT DT SUBJECT ID Letter Code: LETTER_CD 1 Checked for completeness and accuracy A Certification number B Signature C General Comments Keyer: KEY CERT MOD Status: Mcdified: Edited: EDT DT
Studies have found almost 60 percent of sickle cell patients are grappling with an immediate financial crisis; one in five confront housing instability. As a result, many individuals wind up with long gaps in care. When these patients experience severe symptoms, trips to the emergency room become a stopgap.
SICKLE CELL ANEMIA : A CASE STUDY. January 2017. Journal of Research and Education in Indian Medicine. DOI: 10.5455/JREIM.82-1492076751. Authors: Sagar Bhinde. Institute of Teaching & Research In ...
In myonecrosis, red cells containing sickle hemoglobin become rigid, resulting in reduced blood flow and myonecrosis. Case Report: We present a case study of a patient in sickle cell crisis with an episode of acute pain and swelling to the intrinsic muscles of the foot as a prominent feature of the crises. Although muscle biopsy is considered ...
Created by. bayrodz. Joi Anderson: Joi is an eight-year-old African-American female who arrives at the emergency department with her grandmother, Cheryl, grimacing and crying in excruciating pain. Joi says she hurts all over her body and continues to grimace restlessly as Cheryl tries unsuccessfully to console her.
Sickle cell disease (SCD) is a group of inherited blood disorders. Abnormal hemoglobin is produced. Red blood cells become hard and sticky and get stuck in small blood vessels, resulting in pain and other serious complications. There are several types of SCD, some more severe than others. In the United States, SCD is often found at birth ...
anemia from January 1992 to February 1995. The study ended before the originally planned termination date because of strong evidence for the efficacy of hydroxyurea in the reduction of the frequency of acute vaso-occlusive (painful) crises among adult patients with moderate to severe sickle cell anemia (defined by at least three re ported ...
Anyone who has sickle cell anemia is at risk for stroke, including babies. Approximately 11% of people with sickle cell anemia have strokes by age 20, and 24% have strokes by age 45. Here is information on stroke symptoms: Severe headache. Sudden weakness on one side of your or your child's body. Change in alertness.
Sickle cell disease is a group of inherited red blood cell disorders that affect hemoglobin, the protein that carries oxygen through the body. Normally, red blood cells are disc-shaped and flexible enough to move easily through the blood vessels. In sickle cell disease, red blood cells become crescent- or "sickle"-shaped due to a genetic ...
Anthony is 15 years old and has sickle cell. He was playing sports on a 90F day when he started to get pain in his knees. Eval by camp nurse and brought to ED. Sickle Cell Crisis. Sickle Cell diagnosed at birth. Has frequent pneumonia and many incidents of SC crisis. ##### Continue plan of care with education to help reduce chances of
Chronic hemolytic anemia and vascular occlusion are hallmarks of sickle cell disease (SCD). Blood transfusions are critical for supportive and preventive management of SCD complications. Patients with SCD are at risk for hyperhemolysis syndrome (HHS), a subtype of delayed hemolytic transfusion reactions.
Blood Disorders and Blood Safety. Blood diseases affect millions of people each year. These inherited and acquired diseases, including the anemias, venous thromboembolism, hemophilia, and other bleeding disorders, can affect red and white blood cells, platelets, bone marrow, vascular endothelium, or plasma proteins.
The patient's complete blood cell count results should be interpreted in the context of her steady-state hemoglobin level; this is usually in the range of 60-90 g/L in patients with homozygous sickle cell disease. 1 A reticulocyte count can help to determine whether marked anemia is due to accelerated hemolysis or bone marrow suppression (e ...
Introduction Sickle cell anemia (SCA), a severe hematological disorder, is characterized by the presence of sickle-shaped erythrocytes that obstruct capillaries and restrict blood flow. This pathophysiology not only promotes systemic complications but may also influence cardiac function. Cardiac complications are a leading cause of mortality in SCA patients, yet the specific ...