

COVID-19 Topics
Featured topics.

The latest on treatments and other therapies for COVID-19
Questions and answers about COVID-19 vaccine guidelines, development, and safety
NIH's role in developing accurate, widely available COVID-19 tests
Clinical Trials
Information about clinical trials on treatments and vaccines for COVID-19
Information about Long COVID symptoms, management, and clinical trials supported by NIH

Mental Health
The latest information on mental health during the COVID-19 pandemic
Search NIH COVID-19 Articles and Resources
Scroll down the page to view all COVID-19 articles, stories, and resources from across NIH. You can also select a topic from the list to view resources on that topic.

Home Test to Treat Program Expands Nationwide
Virtual program provides free COVID-19 and flu testing, telehealth, and at-home treatment to eligible people.

Shining a Light on Long COVID Brain Fog
A new study says the virus seems to be depleting a vital chemical.

Common Cold Virus May Increase Risk for Long COVID
People who were infected by OC43, a common cold virus, may be at higher risk for developing Long COVID.
SARS-CoV-2 Infection May Increase Risk of Heart Disease, Stroke
Research finds that SARS-CoV-2 infects coronary arteries and increases plaque inflammation.

Severe COVID-19 May Cause Long-Term Immune System Changes

SARS-CoV-2 Antibodies From Vaccination During Pregnancy May Transfer to Fetuses
Antibodies against SARS-CoV-2 were found in the blood and cord blood of people vaccinated during pregnancy.

The Long-Term Effects of SARS-CoV-2 on Organs and Energy
By binding to proteins in the mitochondria, the virus may cause lasting damage to cellular energy production.
Understanding Sleep Problems and Long COVID
Researchers explore whether poor sleep is a cause or result of Long COVID
More Evidence That COVID-19 Vaccination While Pregnant Likely Protects Children
Blood and breastmilk from vaccinated women contain antibodies that may protect infants in first months of life

How the Gut Microbiome Could Predict COVID-19 Severity

A Mental Wellness Project Supports Students During COVID-19
Researchers are working with children in Baltimore to learn about student resilience during the pandemic

Measuring the Psychological Distress of COVID-19
People from many racial and ethnic minority groups reported experiencing less distress than White adults
- High Contrast
- Increase Font
- Decrease Font
- Default Font
- Turn Off Animations
Greater Good Science Center • Magazine • In Action • In Education
11 Questions to Ask About COVID-19 Research
Debates have raged on social media, around dinner tables, on TV, and in Congress about the science of COVID-19. Is it really worse than the flu? How necessary are lockdowns? Do masks work to prevent infection? What kinds of masks work best? Is the new vaccine safe?
You might see friends, relatives, and coworkers offer competing answers, often brandishing studies or citing individual doctors and scientists to support their positions. With so much disagreement—and with such high stakes—how can we use science to make the best decisions?
Here at Greater Good , we cover research into social and emotional well-being, and we try to help people apply findings to their personal and professional lives. We are well aware that our business is a tricky one.
Summarizing scientific studies and distilling the key insights that people can apply to their lives isn’t just difficult for the obvious reasons, like understanding and then explaining formal science terms or rigorous empirical and analytic methods to non-specialists. It’s also the case that context gets lost when we translate findings into stories, tips, and tools, especially when we push it all through the nuance-squashing machine of the Internet. Many people rarely read past the headlines, which intrinsically aim to be relatable and provoke interest in as many people as possible. Because our articles can never be as comprehensive as the original studies, they almost always omit some crucial caveats, such as limitations acknowledged by the researchers. To get those, you need access to the studies themselves.
And it’s very common for findings and scientists to seem to contradict each other. For example, there were many contradictory findings and recommendations about the use of masks, especially at the beginning of the pandemic—though as we’ll discuss, it’s important to understand that a scientific consensus did emerge.
Given the complexities and ambiguities of the scientific endeavor, is it possible for a non-scientist to strike a balance between wholesale dismissal and uncritical belief? Are there red flags to look for when you read about a study on a site like Greater Good or hear about one on a Fox News program? If you do read an original source study, how should you, as a non-scientist, gauge its credibility?
Here are 11 questions you might ask when you read about the latest scientific findings about the pandemic, based on our own work here at Greater Good.
1. Did the study appear in a peer-reviewed journal?
In peer review, submitted articles are sent to other experts for detailed critical input that often must be addressed in a revision prior to being accepted and published. This remains one of the best ways we have for ascertaining the rigor of the study and rationale for its conclusions. Many scientists describe peer review as a truly humbling crucible. If a study didn’t go through this process, for whatever reason, it should be taken with a much bigger grain of salt.
“When thinking about the coronavirus studies, it is important to note that things were happening so fast that in the beginning people were releasing non-peer reviewed, observational studies,” says Dr. Leif Hass, a family medicine doctor and hospitalist at Sutter Health’s Alta Bates Summit Medical Center in Oakland, California. “This is what we typically do as hypothesis-generating but given the crisis, we started acting on them.”
In a confusing, time-pressed, fluid situation like the one COVID-19 presented, people without medical training have often been forced to simply defer to expertise in making individual and collective decisions, turning to culturally vetted institutions like the Centers for Disease Control (CDC). Is that wise? Read on.
2. Who conducted the study, and where did it appear?
“I try to listen to the opinion of people who are deep in the field being addressed and assess their response to the study at hand,” says Hass. “With the MRNA coronavirus vaccines, I heard Paul Offit from UPenn at a UCSF Grand Rounds talk about it. He literally wrote the book on vaccines. He reviewed what we know and gave the vaccine a big thumbs up. I was sold.”
From a scientific perspective, individual expertise and accomplishment matters—but so does institutional affiliation.
Why? Because institutions provide a framework for individual accountability as well as safety guidelines. At UC Berkeley, for example , research involving human subjects during COVID-19 must submit a Human Subjects Proposal Supplement Form , and follow a standard protocol and rigorous guidelines . Is this process perfect? No. It’s run by humans and humans are imperfect. However, the conclusions are far more reliable than opinions offered by someone’s favorite YouTuber .
Recommendations coming from institutions like the CDC should not be accepted uncritically. At the same time, however, all of us—including individuals sporting a “Ph.D.” or “M.D.” after their names—must be humble in the face of them. The CDC represents a formidable concentration of scientific talent and knowledge that dwarfs the perspective of any one individual. In a crisis like COVID-19, we need to defer to that expertise, at least conditionally.
“If we look at social media, things could look frightening,” says Hass. When hundreds of millions of people are vaccinated, millions of them will be afflicted anyway, in the course of life, by conditions like strokes, anaphylaxis, and Bell’s palsy. “We have to have faith that people collecting the data will let us know if we are seeing those things above the baseline rate.”
3. Who was studied, and where?
Animal experiments tell scientists a lot, but their applicability to our daily human lives will be limited. Similarly, if researchers only studied men, the conclusions might not be relevant to women, and vice versa.
Many psychology studies rely on WEIRD (Western, educated, industrialized, rich and democratic) participants, mainly college students, which creates an in-built bias in the discipline’s conclusions. Historically, biomedical studies also bias toward gathering measures from white male study participants, which again, limits generalizability of findings. Does that mean you should dismiss Western science? Of course not. It’s just the equivalent of a “Caution,” “Yield,” or “Roadwork Ahead” sign on the road to understanding.
This applies to the coronavirus vaccines now being distributed and administered around the world. The vaccines will have side effects; all medicines do. Those side effects will be worse for some people than others, depending on their genetic inheritance, medical status, age, upbringing, current living conditions, and other factors.
For Hass, it amounts to this question: Will those side effects be worse, on balance, than COVID-19, for most people?
“When I hear that four in 100,000 [of people in the vaccine trials] had Bell’s palsy, I know that it would have been a heck of a lot worse if 100,000 people had COVID. Three hundred people would have died and many others been stuck with chronic health problems.”
4. How big was the sample?
In general, the more participants in a study, the more valid its results. That said, a large sample is sometimes impossible or even undesirable for certain kinds of studies. During COVID-19, limited time has constrained the sample sizes.
However, that acknowledged, it’s still the case that some studies have been much larger than others—and the sample sizes of the vaccine trials can still provide us with enough information to make informed decisions. Doctors and nurses on the front lines of COVID-19—who are now the very first people being injected with the vaccine—think in terms of “biological plausibility,” as Hass says.
Did the admittedly rushed FDA approval of the Pfizer-BioNTech vaccine make sense, given what we already know? Tens of thousands of doctors who have been grappling with COVID-19 are voting with their arms, in effect volunteering to be a sample for their patients. If they didn’t think the vaccine was safe, you can bet they’d resist it. When the vaccine becomes available to ordinary people, we’ll know a lot more about its effects than we do today, thanks to health care providers paving the way.
5. Did the researchers control for key differences, and do those differences apply to you?
Diversity or gender balance aren’t necessarily virtues in experimental research, though ideally a study sample is as representative of the overall population as possible. However, many studies use intentionally homogenous groups, because this allows the researchers to limit the number of different factors that might affect the result.
While good researchers try to compare apples to apples, and control for as many differences as possible in their analyses, running a study always involves trade-offs between what can be accomplished as a function of study design, and how generalizable the findings can be.

The Science of Happiness
What does it take to live a happier life? Learn research-tested strategies that you can put into practice today. Hosted by award-winning psychologist Dacher Keltner. Co-produced by PRX and UC Berkeley’s Greater Good Science Center.
- Apple Podcasts
- Google Podcasts
You also need to ask if the specific population studied even applies to you. For example, when one study found that cloth masks didn’t work in “high-risk situations,” it was sometimes used as evidence against mask mandates.
However, a look beyond the headlines revealed that the study was of health care workers treating COVID-19 patients, which is a vastly more dangerous situation than, say, going to the grocery store. Doctors who must intubate patients can end up being splattered with saliva. In that circumstance, one cloth mask won’t cut it. They also need an N95, a face shield, two layers of gloves, and two layers of gown. For the rest of us in ordinary life, masks do greatly reduce community spread, if as many people as possible are wearing them.
6. Was there a control group?
One of the first things to look for in methodology is whether the population tested was randomly selected, whether there was a control group, and whether people were randomly assigned to either group without knowing which one they were in. This is especially important if a study aims to suggest that a certain experience or treatment might actually cause a specific outcome, rather than just reporting a correlation between two variables (see next point).
For example, were some people randomly assigned a specific meditation practice while others engaged in a comparable activity or exercise? If the sample is large enough, randomized trials can produce solid conclusions. But, sometimes, a study will not have a control group because it’s ethically impossible. We can’t, for example, let sick people go untreated just to see what would happen. Biomedical research often makes use of standard “treatment as usual” or placebos in control groups. They also follow careful ethical guidelines to protect patients from both maltreatment and being deprived necessary treatment. When you’re reading about studies of masks, social distancing, and treatments during the COVID-19, you can partially gauge the reliability and validity of the study by first checking if it had a control group. If it didn’t, the findings should be taken as preliminary.
7. Did the researchers establish causality, correlation, dependence, or some other kind of relationship?
We often hear “Correlation is not causation” shouted as a kind of battle cry, to try to discredit a study. But correlation—the degree to which two or more measurements seem connected—is important, and can be a step toward eventually finding causation—that is, establishing a change in one variable directly triggers a change in another. Until then, however, there is no way to ascertain the direction of a correlational relationship (does A change B, or does B change A), or to eliminate the possibility that a third, unmeasured factor is behind the pattern of both variables without further analysis.
In the end, the important thing is to accurately identify the relationship. This has been crucial in understanding steps to counter the spread of COVID-19 like shelter-in-place orders. Just showing that greater compliance with shelter-in-place mandates was associated with lower hospitalization rates is not as conclusive as showing that one community that enacted shelter-in-place mandates had lower hospitalization rates than a different community of similar size and population density that elected not to do so.
We are not the first people to face an infection without understanding the relationships between factors that would lead to more of it. During the bubonic plague, cities would order rodents killed to control infection. They were onto something: Fleas that lived on rodents were indeed responsible. But then human cases would skyrocket.
Why? Because the fleas would migrate off the rodent corpses onto humans, which would worsen infection. Rodent control only reduces bubonic plague if it’s done proactively; once the outbreak starts, killing rats can actually make it worse. Similarly, we can’t jump to conclusions during the COVID-19 pandemic when we see correlations.
8. Are journalists and politicians, or even scientists, overstating the result?
Language that suggests a fact is “proven” by one study or which promotes one solution for all people is most likely overstating the case. Sweeping generalizations of any kind often indicate a lack of humility that should be a red flag to readers. A study may very well “suggest” a certain conclusion but it rarely, if ever, “proves” it.
This is why we use a lot of cautious, hedging language in Greater Good , like “might” or “implies.” This applies to COVID-19 as well. In fact, this understanding could save your life.
When President Trump touted the advantages of hydroxychloroquine as a way to prevent and treat COVID-19, he was dramatically overstating the results of one observational study. Later studies with control groups showed that it did not work—and, in fact, it didn’t work as a preventative for President Trump and others in the White House who contracted COVID-19. Most survived that outbreak, but hydroxychloroquine was not one of the treatments that saved their lives. This example demonstrates how misleading and even harmful overstated results can be, in a global pandemic.
9. Is there any conflict of interest suggested by the funding or the researchers’ affiliations?
A 2015 study found that you could drink lots of sugary beverages without fear of getting fat, as long as you exercised. The funder? Coca Cola, which eagerly promoted the results. This doesn’t mean the results are wrong. But it does suggest you should seek a second opinion : Has anyone else studied the effects of sugary drinks on obesity? What did they find?
It’s possible to take this insight too far. Conspiracy theorists have suggested that “Big Pharma” invented COVID-19 for the purpose of selling vaccines. Thus, we should not trust their own trials showing that the vaccine is safe and effective.
But, in addition to the fact that there is no compelling investigative evidence that pharmaceutical companies created the virus, we need to bear in mind that their trials didn’t unfold in a vacuum. Clinical trials were rigorously monitored and independently reviewed by third-party entities like the World Health Organization and government organizations around the world, like the FDA in the United States.
Does that completely eliminate any risk? Absolutely not. It does mean, however, that conflicts of interest are being very closely monitored by many, many expert eyes. This greatly reduces the probability and potential corruptive influence of conflicts of interest.
10. Do the authors reference preceding findings and original sources?
The scientific method is based on iterative progress, and grounded in coordinating discoveries over time. Researchers study what others have done and use prior findings to guide their own study approaches; every study builds on generations of precedent, and every scientist expects their own discoveries to be usurped by more sophisticated future work. In the study you are reading, do the researchers adequately describe and acknowledge earlier findings, or other key contributions from other fields or disciplines that inform aspects of the research, or the way that they interpret their results?

Greater Good’s Guide to Well-Being During Coronavirus
Practices, resources, and articles for individuals, parents, and educators facing COVID-19
This was crucial for the debates that have raged around mask mandates and social distancing. We already knew quite a bit about the efficacy of both in preventing infections, informed by centuries of practical experience and research.
When COVID-19 hit American shores, researchers and doctors did not question the necessity of masks in clinical settings. Here’s what we didn’t know: What kinds of masks would work best for the general public, who should wear them, when should we wear them, were there enough masks to go around, and could we get enough people to adopt best mask practices to make a difference in the specific context of COVID-19 ?
Over time, after a period of confusion and contradictory evidence, those questions have been answered . The very few studies that have suggested masks don’t work in stopping COVID-19 have almost all failed to account for other work on preventing the disease, and had results that simply didn’t hold up. Some were even retracted .
So, when someone shares a coronavirus study with you, it’s important to check the date. The implications of studies published early in the pandemic might be more limited and less conclusive than those published later, because the later studies could lean on and learn from previously published work. Which leads us to the next question you should ask in hearing about coronavirus research…
11. Do researchers, journalists, and politicians acknowledge limitations and entertain alternative explanations?
Is the study focused on only one side of the story or one interpretation of the data? Has it failed to consider or refute alternative explanations? Do they demonstrate awareness of which questions are answered and which aren’t by their methods? Do the journalists and politicians communicating the study know and understand these limitations?
When the Annals of Internal Medicine published a Danish study last month on the efficacy of cloth masks, some suggested that it showed masks “make no difference” against COVID-19.
The study was a good one by the standards spelled out in this article. The researchers and the journal were both credible, the study was randomized and controlled, and the sample size (4,862 people) was fairly large. Even better, the scientists went out of their way to acknowledge the limits of their work: “Inconclusive results, missing data, variable adherence, patient-reported findings on home tests, no blinding, and no assessment of whether masks could decrease disease transmission from mask wearers to others.”
Unfortunately, their scientific integrity was not reflected in the ways the study was used by some journalists, politicians, and people on social media. The study did not show that masks were useless. What it did show—and what it was designed to find out—was how much protection masks offered to the wearer under the conditions at the time in Denmark. In fact, the amount of protection for the wearer was not large, but that’s not the whole picture: We don’t wear masks mainly to protect ourselves, but to protect others from infection. Public-health recommendations have stressed that everyone needs to wear a mask to slow the spread of infection.
“We get vaccinated for the greater good, not just to protect ourselves ”
As the authors write in the paper, we need to look to other research to understand the context for their narrow results. In an editorial accompanying the paper in Annals of Internal Medicine , the editors argue that the results, together with existing data in support of masks, “should motivate widespread mask wearing to protect our communities and thereby ourselves.”
Something similar can be said of the new vaccine. “We get vaccinated for the greater good, not just to protect ourselves,” says Hass. “Being vaccinated prevents other people from getting sick. We get vaccinated for the more vulnerable in our community in addition for ourselves.”
Ultimately, the approach we should take to all new studies is a curious but skeptical one. We should take it all seriously and we should take it all with a grain of salt. You can judge a study against your experience, but you need to remember that your experience creates bias. You should try to cultivate humility, doubt, and patience. You might not always succeed; when you fail, try to admit fault and forgive yourself.
Above all, we need to try to remember that science is a process, and that conclusions always raise more questions for us to answer. That doesn’t mean we never have answers; we do. As the pandemic rages and the scientific process unfolds, we as individuals need to make the best decisions we can, with the information we have.
This article was revised and updated from a piece published by Greater Good in 2015, “ 10 Questions to Ask About Scientific Studies .”
About the Authors
Jeremy Adam Smith
Uc berkeley.
Jeremy Adam Smith edits the GGSC’s online magazine, Greater Good . He is also the author or coeditor of five books, including The Daddy Shift , Are We Born Racist? , and (most recently) The Gratitude Project: How the Science of Thankfulness Can Rewire Our Brains for Resilience, Optimism, and the Greater Good . Before joining the GGSC, Jeremy was a John S. Knight Journalism Fellow at Stanford University.
Emiliana R. Simon-Thomas
Emiliana R. Simon-Thomas, Ph.D. , is the science director of the Greater Good Science Center, where she directs the GGSC’s research fellowship program and serves as a co-instructor of its Science of Happiness and Science of Happiness at Work online courses.
You May Also Enjoy

How to Keep the Greater Good in Mind During the Coronavirus Outbreak

How Does COVID-19 Affect Trust in Government?

In a Pandemic, Elbow Touches Might Keep Us Going

How to Form a Pandemic Pod

Why Is COVID-19 Killing So Many Black Americans?

Why Your Sacrifices Matter During the Pandemic
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
17k Accesses
30 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
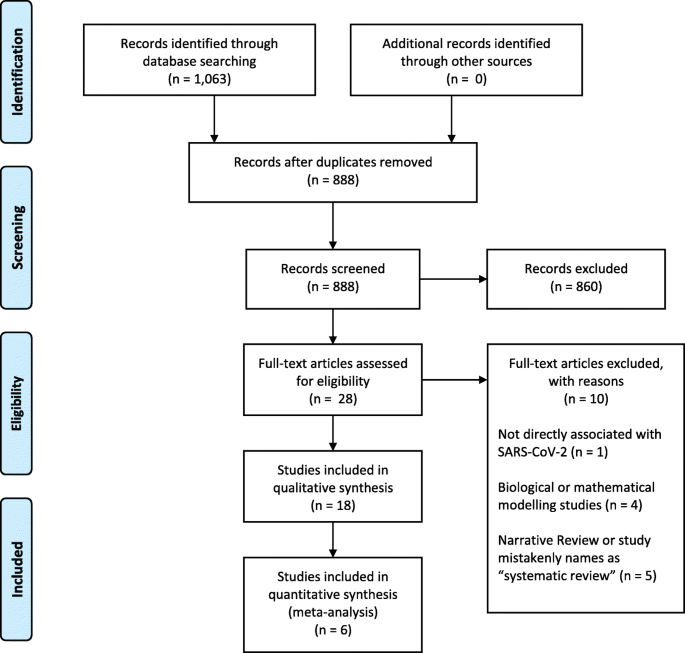
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
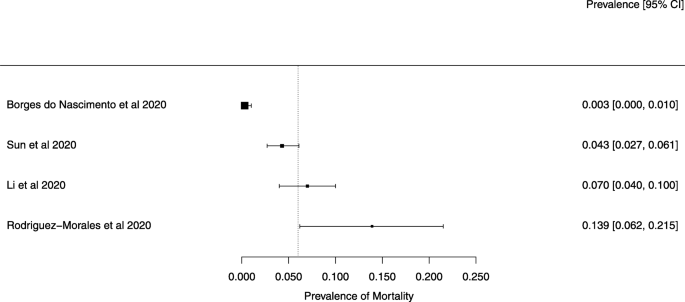
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
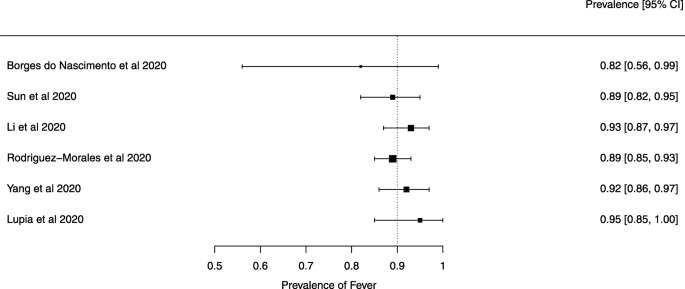
A meta-analysis of the prevalence of fever
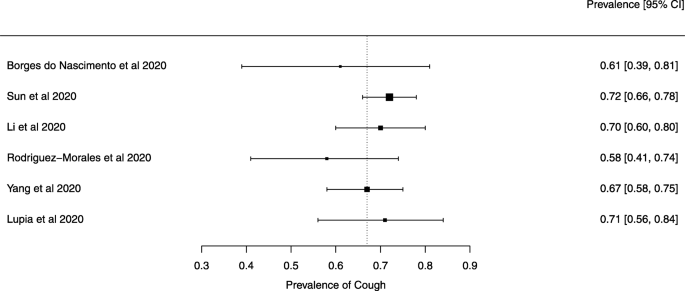
A meta-analysis of the prevalence of cough
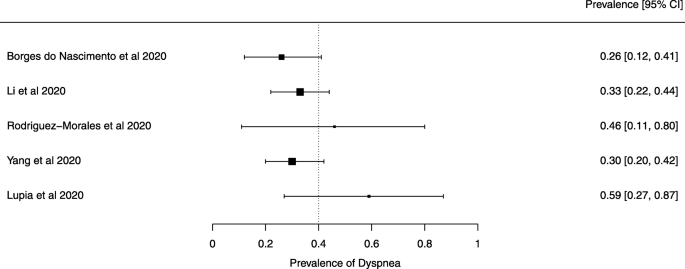
A meta-analysis of the prevalence of dyspnea
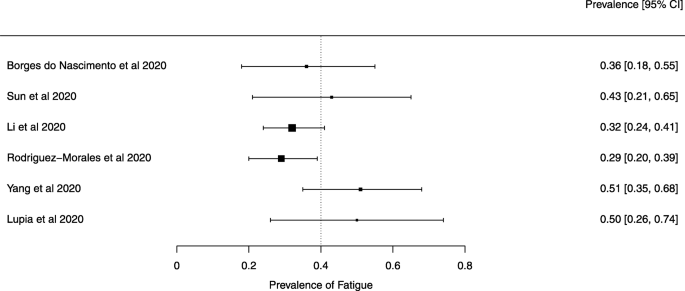
A meta-analysis of the prevalence of fatigue or myalgia
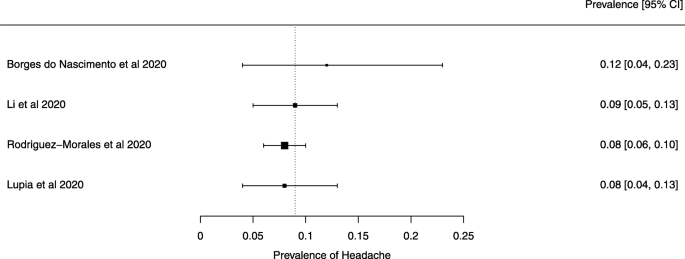
A meta-analysis of the prevalence of headache
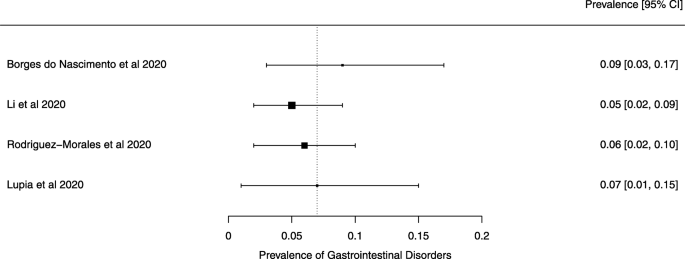
A meta-analysis of the prevalence of gastrointestinal disorders
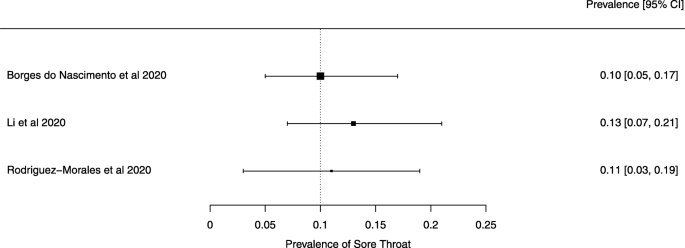
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
SARS-CoV-2 and COVID-19: The most important research questions
Affiliations.
- 1 1School of Biomedical Sciences, The University of Hong Kong, 3/F Laboratory Block, 21 Sassoon Road, Pokfulam, Hong Kong.
- 2 2Department of Microbiology, The University of Hong Kong, Pokfulam, Hong Kong.
- PMID: 32190290
- PMCID: PMC7074995
- DOI: 10.1186/s13578-020-00404-4
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing global health emergency. Here we highlight nine most important research questions concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and viral pathogenesis.
Keywords: 2019 novel coronavirus (2019-nCoV); COVID-19; Novel coronavirus pneumonia (NCP); SARS-CoV-2.
© The Author(s) 2020.
PubMed Disclaimer
Conflict of interest statement
Competing interestsNo potential conflict of interest was reported by the authors.
Similar articles
- Epidemiological feature, viral shedding, and antibody seroconversion among asymptomatic SARS-CoV-2 carriers and symptomatic/presymptomatic COVID-19 patients. Chen Y, Li P, Ding Y, Liu M, Liu L, Yi B, Wu T, Dong H, Lao X, Ding K, Wang H, Zhang D, Tan X, Wang Z, Xu G, Cao G. Chen Y, et al. J Infect Public Health. 2021 Jul;14(7):845-851. doi: 10.1016/j.jiph.2021.05.003. Epub 2021 May 27. J Infect Public Health. 2021. PMID: 34118734 Free PMC article.
- Virus shedding dynamics in asymptomatic and mildly symptomatic patients infected with SARS-CoV-2. Li W, Su YY, Zhi SS, Huang J, Zhuang CL, Bai WZ, Wan Y, Meng XR, Zhang L, Zhou YB, Luo YY, Ge SX, Chen YK, Ma Y. Li W, et al. Clin Microbiol Infect. 2020 Nov;26(11):1556.e1-1556.e6. doi: 10.1016/j.cmi.2020.07.008. Epub 2020 Jul 9. Clin Microbiol Infect. 2020. PMID: 32653662 Free PMC article.
- Human and novel coronavirus infections in children: a review. Rajapakse N, Dixit D. Rajapakse N, et al. Paediatr Int Child Health. 2021 Feb;41(1):36-55. doi: 10.1080/20469047.2020.1781356. Epub 2020 Jun 25. Paediatr Int Child Health. 2021. PMID: 32584199 Review.
- [Diagnosis, treatment, control and prevention of SARS-CoV-2 and coronavirus disease 2019: back to the future]. Ye ZW, Jin DY. Ye ZW, et al. Sheng Wu Gong Cheng Xue Bao. 2020 Apr 25;36(4):571-592. doi: 10.13345/j.cjb.200115. Sheng Wu Gong Cheng Xue Bao. 2020. PMID: 32347053 Review. Chinese.
- A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. Fung SY, et al. Emerg Microbes Infect. 2020 Mar 14;9(1):558-570. doi: 10.1080/22221751.2020.1736644. eCollection 2020. Emerg Microbes Infect. 2020. PMID: 32172672 Free PMC article. Review.
- The Development of Aptamer-Based Gold Nanoparticle Lateral Flow Test Strips for the Detection of SARS-CoV-2 S Proteins on the Surface of Cold-Chain Food Packaging. Li X, Wang J, Yang G, Fang X, Zhao L, Luo Z, Dong Y. Li X, et al. Molecules. 2024 Apr 13;29(8):1776. doi: 10.3390/molecules29081776. Molecules. 2024. PMID: 38675595 Free PMC article.
- Water-sanitation-health nexus in the Indus-Ganga-Brahmaputra River Basin: need for wastewater surveillance of SARS-CoV-2 for preparedness during the future waves of pandemic. Chakraborty P, Vinod PG, Syed JH, Pokhrel B, K Bharat G, Basu AR, Fouzder T, Pasupuleti M, Urbaniak M, Beskoski VP. Chakraborty P, et al. Int J Ecohydrol Hydrobiol. 2022 Apr;22(2):283-294. doi: 10.1016/j.ecohyd.2021.11.001. Epub 2021 Nov 15. Int J Ecohydrol Hydrobiol. 2022. PMID: 38620864 Free PMC article.
- The COVID-19 pandemic: Considerations for the waste and wastewater services sector. Nghiem LD, Morgan B, Donner E, Short MD. Nghiem LD, et al. Case Stud Chem Environ Eng. 2020 May;1:100006. doi: 10.1016/j.cscee.2020.100006. Epub 2020 Apr 30. Case Stud Chem Environ Eng. 2020. PMID: 38620229 Free PMC article.
- Identification of a druggable site on GRP78 at the GRP78-SARS-CoV-2 interface and virtual screening of compounds to disrupt that interface. Lazou M, Hutton JR, Chakravarty A, Joseph-McCarthy D. Lazou M, et al. J Comput Aided Mol Des. 2024 Jan 24;38(1):6. doi: 10.1007/s10822-023-00546-w. J Comput Aided Mol Des. 2024. PMID: 38263499
- Environmental monitoring of SARS-CoV-2 in the metropolitan area of Porto Alegre, Rio Grande do Sul (RS), Brazil. Dutra LB, Stein JF, da Rocha BS, Berger A, de Souza BA, Prandi BA, Mangini AT, Jarenkow A, Campos AAS, Fan FM, de Almeida Silva MC, Lipp-Nissinen KH, Loncan MR, Augusto MR, Franco AC, de Freitas Bueno R, Rigotto C. Dutra LB, et al. Environ Sci Pollut Res Int. 2024 Jan;31(2):2129-2144. doi: 10.1007/s11356-023-31081-8. Epub 2023 Dec 6. Environ Sci Pollut Res Int. 2024. PMID: 38057673 Free PMC article.
- Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, Tsoi HW, Lo SKF, Chan KH, Poon VKM, Chan WM, Ip JD, Cai JP, Cheng VCC, Chen H, Hui CKM, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
- Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.1585. - DOI - PMC - PubMed
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
- Lam TTY, Shum MHH, Zhu HC, Tong YG, Ni XB, Liao YS, Wei W, Cheung WYM, Li WJ, Li LF, Leung GM, Holmes EC, Hu YL, Guan Y. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. BioRxiv. 2020 doi: 10.1101/2020.02.13.945485. - DOI
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. - DOI - PMC - PubMed
LinkOut - more resources
Full text sources.
- BioMed Central
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Fact sheets
- Facts in pictures
Publications
- Questions and answers
- Tools and toolkits
- HIV and AIDS
- Hypertension
- Mental disorders
- Top 10 causes of death
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
There is a current outbreak of Coronavirus (COVID-19) disease Find out more →
- Health topics /
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus.
Most people infected with the virus will experience mild to moderate respiratory illness and recover without requiring special treatment. However, some will become seriously ill and require medical attention. Older people and those with underlying medical conditions like cardiovascular disease, diabetes, chronic respiratory disease, or cancer are more likely to develop serious illness. Anyone can get sick with COVID-19 and become seriously ill or die at any age.
The best way to prevent and slow down transmission is to be well informed about the disease and how the virus spreads. Protect yourself and others from infection by staying at least 1 metre apart from others, wearing a properly fitted mask, and washing your hands or using an alcohol-based rub frequently. Get vaccinated when it’s your turn and follow local guidance.
The virus can spread from an infected person’s mouth or nose in small liquid particles when they cough, sneeze, speak, sing or breathe. These particles range from larger respiratory droplets to smaller aerosols. It is important to practice respiratory etiquette, for example by coughing into a flexed elbow, and to stay home and self-isolate until you recover if you feel unwell.
Stay informed:
- Advice for the public
- Myth busters
- All information on the COVID-19 outbreak
To prevent infection and to slow transmission of COVID-19, do the following:
- Get vaccinated when a vaccine is available to you.
- Stay at least 1 metre apart from others, even if they don’t appear to be sick.
- Wear a properly fitted mask when physical distancing is not possible or when in poorly ventilated settings.
- Choose open, well-ventilated spaces over closed ones. Open a window if indoors.
- Wash your hands regularly with soap and water or clean them with alcohol-based hand rub.
- Cover your mouth and nose when coughing or sneezing.
- If you feel unwell, stay home and self-isolate until you recover.
COVID-19 affects different people in different ways. Most infected people will develop mild to moderate illness and recover without hospitalization.
Most common symptoms:
- loss of taste or smell.
Less common symptoms:
- sore throat
- aches and pains
- a rash on skin, or discolouration of fingers or toes
- red or irritated eyes.
Serious symptoms:
- difficulty breathing or shortness of breath
- loss of speech or mobility, or confusion
- chest pain.
Seek immediate medical attention if you have serious symptoms. Always call before visiting your doctor or health facility.
People with mild symptoms who are otherwise healthy should manage their symptoms at home.
On average it takes 5–6 days from when someone is infected with the virus for symptoms to show, however it can take up to 14 days.
- Q&As on COVID-19 and related health topics
- WHO Coronavirus (COVID-19) Dashboard
- COVID-19 Clinical Care Pathway
- COVID-19 Impact on nutrition analytical framework
- COVID-19 Vaccine delivery toolkit
- Global Clinical Platform for COVID-19
- Coronavirus disease (COVID-19) pandemic
- Access to COVID-19 Tools (ACT) Accelerator
- COVID-19 Technology access pool
- ACT-Accelerator Ethics & Governance Working Group
- Advisory Group on Therapeutics Prioritization for COVID-19
- COVID-19 IHR Emergency Committee
- COVID-19 Infection Prevention and Control Guidance Development Group
- Facilitation Council for the Access to COVID-19 Tools (ACT) Accelerator
- International Travel and Health (ITH) guideline development group (GDG) for COVID-19
- Technical Advisory Group on the COVID-19 Technology Access Pool
- Technical Advisory Group on COVID-19 Vaccine Composition
- Technical Advisory Group on SARS-CoV-2 Virus Evolution
- Working Group on Ethics and COVID-19
- COVID-19 Training
WHO and Switzerland strengthen partnership for global BioHub System
COVID-19 eliminated a decade of progress in global level of life expectancy
Statement on the antigen composition of COVID-19 vaccines
WHO reports widespread overuse of antibiotics in patients hospitalized with COVID-19

WHO SEAR 11th Epidemiological Bulletin 2024
This epidemiological bulletin aims to provide the situation of key infectious diseases in the WHO South-East Asia region to inform risk assessments and...

WHO SEAR 10th Epidemiological Bulletin 2024

WHO SEAR 9th Epidemiological Bulletin 2024

WHO SEAR 8th Epidemiological Bulletin 2024
Who documents.

COVID-19 epidemiological update – 17 June 2024
SARS-CoV-2 PCR percent positivity, as detected in integrated sentinel surveillance as part of the Global Influenza Surveillance and Response System...

COVID-19 epidemiological update – 17 May 2024

Global technical consultation report on proposed terminology for pathogens that transmit through the...
Terminology used to describe the transmission of pathogens through the air varies across scientific disciplines, organizations and the general public....

COVID-19 epidemiological update – 12 April 2024
SARS-CoV-2 PCR percent positivity, as detected in integrated sentinel surveillance as part of the Global Influenza Surveillance and Response System (GISRS)...
Tracking SARS-CoV-2 variants
Promoting a fair and equitable response to the COVID-19 pandemic
Promoting the health of refugees and migrants during COVID-19 pandemic
Preparing and preventing epidemics and pandemics
Development partners making a difference: The European Union supports WHO in eight Asian countries to prepare for the future
Timor-Leste: Saving lives through critical care in remote regions
Infographics

Diagnostic testing for SARS-CoV-2 infection

Why testing is important?

Pandemic preparedness: Introducing WHO's Investigations and Studies (Unity Studies) approach

Nurses Facing COVID - 2023 Health Emergencies "GRAND PRIX" at the 4th Health for All Film Festival

WHO's Science in 5: Older adults and COVID-19 vaccines - 14 October 2022

WHO’s Science in 5 on COVID-19: Genome Sequencing
Tenth meeting of the Intergovernmental Negotiating Body (INB) for a WHO instrument on pandemic prevention, preparedness and response
Strategic Roundtables: Seventy-seventh World Health Assembly
Related links
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The impact of the COVID-19 pandemic on scientific research in the life sciences
Roles Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing
Affiliation AXES, IMT School for Advanced Studies Lucca, Lucca, Italy
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Chair of Systems Design D-MTEC, ETH Zürich, Zurich, Switzerland
- Massimo Riccaboni,
- Luca Verginer

- Published: February 9, 2022
- https://doi.org/10.1371/journal.pone.0263001
- Reader Comments
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on world scientific production in the life sciences and find indications that the usage of medical subject headings (MeSH) has changed following the outbreak. We estimate through a difference-in-differences approach the impact of the start of the COVID-19 pandemic on scientific production using the PubMed database (3.6 Million research papers). We find that COVID-19-related MeSH terms have experienced a 6.5 fold increase in output on average, while publications on unrelated MeSH terms dropped by 10 to 12%. The publication weighted impact has an even more pronounced negative effect (-16% to -19%). Moreover, COVID-19 has displaced clinical trial publications (-24%) and diverted grants from research areas not closely related to COVID-19. Note that since COVID-19 publications may have been fast-tracked, the sudden surge in COVID-19 publications might be driven by editorial policy.
Citation: Riccaboni M, Verginer L (2022) The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE 17(2): e0263001. https://doi.org/10.1371/journal.pone.0263001
Editor: Florian Naudet, University of Rennes 1, FRANCE
Received: April 28, 2021; Accepted: January 10, 2022; Published: February 9, 2022
Copyright: © 2022 Riccaboni, Verginer. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The processed data, instructions on how to process the raw PubMed dataset as well as all code are available via Zenodo at https://doi.org/10.5281/zenodo.5121216 .
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The COVID-19 pandemic has mobilized the world scientific community in 2020, especially in the life sciences [ 1 , 2 ]. In the first three months after the pandemic, the number of scientific papers about COVID-19 was fivefold the number of articles on H1N1 swine influenza [ 3 ]. Similarly, the number of clinical trials related to COVID-19 prophylaxis and treatments skyrocketed [ 4 ]. Thanks to the rapid mobilization of the world scientific community, COVID-19 vaccines have been developed in record time. Despite this undeniable success, there is a rising concern about the negative consequences of COVID-19 on clinical trial research, with many projects being postponed [ 5 – 7 ]. According to Evaluate Pharma, clinical trials were one of the pandemic’s first casualties, with a record number of 160 studies suspended for reasons related to COVID-19 in April 2020 [ 8 , 9 ] reporting a total of 1,200 trials suspended as of July 2020. As a consequence, clinical researchers have been impaired by reduced access to healthcare research infrastructures. Particularly, the COVID-19 outbreak took a tall on women and early-career scientists [ 10 – 13 ]. On a different ground, Shan and colleagues found that non-COVID-19-related articles decreased as COVID-19-related articles increased in top clinical research journals [ 14 ]. Fraser and coworker found that COVID-19 preprints received more attention and citations than non-COVID-19 preprints [ 1 ]. More recently, Hook and Porter have found some early evidence of ‘covidisation’ of academic research, with research grants and output diverted to COVID-19 research in 2020 [ 15 ]. How much should scientists switch their efforts toward SARS-CoV-2 prevention, treatment, or mitigation? There is a growing consensus that the current level of ‘covidisation’ of research can be wasteful [ 4 , 5 , 16 ].
Against this background, in this paper, we investigate if the COVID-19 pandemic has induced a shift in biomedical publications toward COVID-19-related scientific production. The objective of the study is to show that scientific articles listing covid-related Medical Subject Headings (MeSH) when compared against covid-unrelated MeSH have been partially displaced. Specifically, we look at several indicators of scientific production in the life sciences before and after the start of the COVID-19 pandemic: (1) number of papers published, (2) impact factor weighted number of papers, (3) opens access, (4) number of publications related to clinical trials, (5) number of papers listing grants, (6) number of papers listing grants existing before the pandemic. Through a natural experiment approach, we analyze the impact of the pandemic on scientific production in the life sciences. We consider COVID-19 an unexpected and unprecedented exogenous source of variation with heterogeneous effects across biomedical research fields (i.e., MeSH terms).
Based on the difference in difference results, we document the displacement effect that the pandemic has had on several aspects of scientific publishing. The overall picture that emerges from this analysis is that there has been a profound realignment of priorities and research efforts. This shift has displaced biomedical research in fields not related to COVID-19.
The rest of the paper is structured as follows. First, we describe the data and our measure of relatedness to COVID-19. Next, we illustrate the difference-in-differences specification we rely on to identify the impact of the pandemic on scientific output. In the results section, we present the results of the difference-in-differences and network analyses. We document the sudden shift in publications, grants and trials towards COVID-19-related MeSH terms. Finally, we discuss the findings and highlight several policy implications.
Materials and methods
The present analysis is based primarily on PubMed and the Medical Subject Headings (MeSH) terminology. This data is used to estimate the effect of the start of the COVID 19 pandemic via a difference in difference approach. This section is structured as follows. We first introduce the data and then the econometric methodology. This analysis is not based on a pre-registered protocol.
Selection of biomedical publications.
We rely on PubMed, a repository with more than 34 million biomedical citations, for the analysis. Specifically, we analyze the daily updated files up to 31/06/2021, extracting all publications of type ‘Journal Article’. For the principal analysis, we consider 3,638,584 papers published from January 2019 to December 2020. We also analyze 11,122,017 papers published from 2010 onwards to identify the earliest usage of a grant and infer if it was new in 2020. We use the SCImago journal ranking statistics to compute the impact factor weighted number (IFWN) of papers in a given field of research. To assign the publication date, we use the ‘electronically published’ dates and, if missing, the ‘print published’ dates.
Medical subject headings.
We rely on the Medical Subject Headings (MeSH) terminology to approximate narrowly defined biomedical research fields. This terminology is a curated medical vocabulary, which is manually added to papers in the PubMed corpus. The fact that MeSH terms are manually annotated makes this terminology ideal for classification purposes. However, there is a delay between publication and annotation, on the order of several months. To address this delay and have the most recent classification, we search for all 28 425 MeSH terms using PubMed’s ESearch utility and classify paper by the results. The specific API endpoint is https://eutils.ncbi.nlm.nih.gov/entrez/eutils/esearch.fcgi , the relevant scripts are available with the code. For example, we assign the term ‘Ageusia’ (MeSH ID D000370) to all papers listed in the results of the ESearch API. We apply this method to the whole period (January 2019—December 2020) and obtain a mapping from papers to the MeSH terms. For every MeSH term, we keep track of the year they have been established. For instance, COVID-19 terms were established in 2020 (see Table 1 ): in January 2020, the WHO recommended 2019-nCoV and 2019-nCoV acute respiratory disease as provisional names for the virus and disease. The WHO issued the official terms COVID-19 and SARS-CoV-2 at the beginning of February 2020. By manually annotating publications, all publications referring to COVID-19 and SARS-CoV-2 since January 2020 have been labelled with the related MeSH terms. Other MeSH terms related to COVID-19, such as coronavirus, for instance, have been established years before the pandemic (see Table 2 ). We proxy MeSH term usage via search terms using the PubMed EUtilities API; this means that we are not using the hand-labelled MeSH terms but rather the PubMed search results. This means that the accuracy of the MeSH term we assign to a given paper is not perfect. In practice, this means that we have assigned more MeSH terms to a given term than a human annotator would have.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0263001.t001
The list contains only terms with at least 100 publications in 2020.
https://doi.org/10.1371/journal.pone.0263001.t002
Clinical trials and publication types.
We classify publications using PubMed’s ‘PublicationType’ field in the XML baseline files (There are 187 publication types, see https://www.nlm.nih.gov/mesh/pubtypes.html ). We consider a publication to be related to a clinical trial if it lists any of the following descriptors:
- D016430: Clinical Trial
- D017426: Clinical Trial, Phase I
- D017427: Clinical Trial, Phase II
- D017428: Clinical Trial, Phase III
- D017429: Clinical Trial, Phase IV
- D018848: Controlled Clinical Trial
- D065007: Pragmatic Clinical Trial
- D000076362: Adaptive Clinical Trial
- D000077522: Clinical Trial, Veterinary
In our analysis of the impact of COVID-19 on publications related to clinical trials, we only consider MeSH terms that are associated at least once with a clinical trial publication over the two years. We apply this restriction to filter out MeSH terms that are very unlikely to be relevant for clinical trial types of research.
Open access.
We proxy the availability of a journal article to the public, i.e., open access, if it is available from PubMed Central. PubMed Central archives full-text journal articles and provides free access to the public. Note that the copyright license may vary across participating publishers. However, the text of the paper is for all effects and purposes freely available without requiring subscriptions or special affiliation.
We infer if a publication has been funded by checking if it lists any grants. We classify grants as either ‘old’, i.e. existed before 2019, or ‘new’, i.e. first observed afterwards. To do so, we collect all grant IDs for 11,122,017 papers from 2010 on-wards and record their first appearance. This procedure is an indirect inference of the year the grant has been granted. The basic assumption is that if a grant number has not been listed in any publication since 2010, it is very likely a new grant. Specifically, an old grant is a grant listed since 2019 observed at least once from 2010 to 2018.
Note that this procedure is only approximate and has a few shortcomings. Mistyped grant numbers (e.g. ‘1234-M JPN’ and ‘1234-M-JPN’) could appear as new grants, even though they existed before, or new grants might be classified as old grants if they have a common ID (e.g. ‘Grant 1’). Unfortunately, there is no central repository of grant numbers and the associated metadata; however, there are plans to assign DOI numbers to grants to alleviate this problem (See https://gitlab.com/crossref/open_funder_registry for the project).
Impact factor weighted publication numbers (IFWN).
In our analysis, we consider two measures of scientific output. First, we simply count the number of publications by MeSH term. However, since journals vary considerably in terms of impact factor, we also weigh the number of publications by the impact factor of the venue (e.g., journal) where it was published. Specifically, we use the SCImago journal ranking statistics to weigh a paper by the impact factor of the journal it appears in. We use the ‘citation per document in the past two years’ for 45,230 ISSNs. Note that a journal may and often has more than one ISSN, i.e., one for the printed edition and one for the online edition. SCImago applies the same score for a venue across linked ISSNs.
For the impact factor weighted number (IFWN) of publication per MeSH terms, this means that all publications are replaced by the impact score of the journal they appear in and summed up.
COVID-19-relatedness.
To measure how closely related to COVID-19 is a MeSH term, we introduce an index of relatedness to COVID-19. First, we identify the focal COVID-19 terms, which appeared in the literature in 2020 (see Table 1 ). Next, for all other pre-existing MeSH terms, we measure how closely related to COVID-19 they end up being.
Our aim is to show that MeSH terms that existed before and are related have experienced a sudden increase in the number of (impact factor weighted) papers.
Intuitively we can read this measure as: what is the probability in 2020 that a COVID-19 MeSH term is present given that we chose a paper with MeSH term i ? For example, given that in 2020 we choose a paper dealing with “Ageusia” (i.e., Complete or severe loss of the subjective sense of taste), there is a 96% probability that this paper also lists COVID-19, see Table 1 .
Note that a paper listing a related MeSH term does not imply that that paper is doing COVID-19 research, but it implies that one of the MeSH terms listed is often used in COVID-19 research.
In sum, in our analysis, we use the following variables:
- Papers: Number of papers by MeSH term;
- Impact: Impact factor weighted number of papers by MeSH term;
- PMC: Papers listed in PubMed central by MeSH term, as a measure of Open Access publications;
- Trials: number of publications of type “Clinical Trial” by MeSH term;
- Grants: number of papers with at least one grant by MeSH term;
- Old Grants: number of papers listing a grant that has been observed between 2010 and 2018, by MeSH term;
Difference-in-differences
The difference-in-differences (DiD) method is an econometric technique to imitate an experimental research design from observation data, sometimes referred to as a quasi-experimental setup. In a randomized controlled trial, subjects are randomly assigned either to the treated or the control group. Analogously, in this natural experiment, we assume that medical subject headings (MeSH) have been randomly assigned to be either treated (related) or not treated (unrelated) by the pandemic crisis.
Before the COVID, for a future health crisis, the set of potentially impacted medical knowledge was not predictable since it depended on the specifics of the emergency. For instance, ageusia (loss of taste), a medical concept existing since 1991, became known to be a specific symptom of COVID-19 only after the pandemic.
Specifically, we exploit the COVID-19 as an unpredictable and exogenous shock that has deeply affected the publication priorities for biomedical scientific production, as compared to the situation before the pandemic. In this setting, COVID-19 is the treatment, and the identification of this new human coronavirus is the event. We claim that treated MeSH terms, i.e., MeSH terms related to COVID-19, have experienced a sudden increase in terms of scientific production and attention. In contrast, research on untreated MeSH terms, i.e., MeSH terms not related to COVID-19, has been displaced by COVID-19. Our analysis compares the scientific output of COVID-19 related and unrelated MeSH terms before and after January 2020.
In our case, some of the terms turn out to be related to COVID-19 in 2020, whereas most of the MeSH terms are not closely related to COVID-19.
Thus β 1 identifies the overall effect on the control group after the event, β 2 the difference across treated and control groups before the event (i.e. the first difference in DiD) and finally the effect on the treated group after the event, net of the first difference, β 3 . This last parameter identifies the treatment effect on the treated group netting out the pre-treatment difference.
For the DiD to have a causal interpretation, it must be noted that pre-event, the trends of the two groups should be parallel, i.e., the common trend assumption (CTA) must be satisfied. We will show that the CTA holds in the results section.
To specify the DiD model, we need to define a period before and after the event and assign a treatment status or level of exposure to each term.
Before and after.
The pre-treatment period is defined as January 2019 to December 2019. The post-treatment period is defined as the months from January 2020 to December 2020. We argue that the state of biomedical research was similar in those two years, apart from the effect of the pandemic.
Treatment status and exposure.
The treatment is determined by the COVID-19 relatedness index σ i introduced earlier. Specifically, this number indicates the likelihood that COVID-19 will be a listed MeSH term, given that we observe the focal MeSH term i . To show that the effect becomes even stronger the closer related the subject is, and for ease of interpretation, we also discretize the relatedness value into three levels of treatment. Namely, we group MeSH terms with a σ between, 0% to 20%, 20% to 80% and 80% to 100%. The choice of alternative grouping strategies does not significantly affect our results. Results for alternative thresholds of relatedness can be computed using the available source code. We complement the dichotomized analysis by using the treatment intensity (relatedness measure σ ) to show that the result persists.
Panel regression.
In this work, we estimate a random effects panel regression where the units of analysis are 28 318 biomedical research fields (i.e. MeSH terms) observed over time before and after the COVID-19 pandemic. The time resolution is at the monthly level, meaning that for each MeSH term, we have 24 observations from January 2019 to December 2020.
The outcome variable Y it identifies the outcome at time t (i.e., month), for MeSH term i . As before, P t identifies the period with P t = 0 if the month is before January 2020 and P t = 1 if it is on or after this date. In (3) , the treatment level is measure by the relatedness to COVID-19 ( σ i ), where again the γ 1 identifies pre-trend (constant) differences and δ 1 the overall effect.
In total, we estimate six coefficients. As before, the δ l coefficient identifies the DiD effect.
Verifying the Common Trend Assumption (CTA).
We show that the CTA holds for this model by comparing the pre-event trends of the control group to the treated groups (COVID-19 related MeSH terms). Namely, we show that the pre-event trends of the control group are the same as the pre-event trends of the treated group.
Co-occurrence analysis
To investigate if the pandemic has caused a reconfiguration of research priorities, we look at the MeSH term co-occurrence network. Precisely, we extract the co-occurrence network of all 28,318 MeSH terms as they appear in the 3.3 million papers. We considered the co-occurrence networks of 2018, 2019 and 2020. Each node represents a MeSH term in these networks, and a link between them indicates that they have been observed at least once together. The weight of the edge between the MeSH terms is given by the number of times those terms have been jointly observed in the same publications.
Medical language is hugely complicated, and this simple representation does not capture the intricacies, subtle nuances and, in fact, meaning of the terms. Therefore, we do not claim that we can identify how the actual usage of MeSH terms has changed from this object, but rather that it has. Nevertheless, the co-occurrence graph captures rudimentary relations between concepts. We argue that absent a shock to the system, their basic usage patterns, change in importance (within the network) would essentially be the same from year to year. However, if we find that the importance of terms changes more than expected in 2020, it stands to reason that there have been some significant changes.
To show that that MeSH usage has been affected, we compute for each term in the years 2018, 2019 and 2020 their PageRank centrality [ 17 ]. The PageRank centrality tells us how likely a random walker traversing a network would be found at a given node if she follows the weights of the empirical edges (i.e., co-usage probability). Specifically, for the case of the MeSH co-occurrence network, this number represents how often an annotator at the National Library of Medicine would assign that MeSH term following the observed general usage patterns. It is a simplistic measure to capture the complexities of biomedical research. Nevertheless, it captures far-reaching interdependence across MeSH terms as the measure uses the whole network to determine the centrality of every MeSH term. A sudden change in the rankings and thus the position of MeSH terms in this network suggests that a given research subject has risen as it is used more often with other important MeSH terms (or vice versa).
We then compare the growth for each MeSH i term in g i (2019), i.e. before the the COVID-19 pandemic, with the growth after the event ( g i (2020)).
Publication growth
Changes in output and COVID-19 relatedness
Before we show the regression results, we provide descriptive evidence that publications from 2019 to 2020 have drastically increased. By showing that this growth correlates strongly with a MeSH term’s COVID-19 relatedness ( σ ), we demonstrate that (1) σ captures an essential aspect of the growth dynamics and (2) highlight the meteoric rise of highly related terms.
We look at the year over year growth in the number of the impact weighted number of publications per MeSH term from 2018 to 2019 and 2019 to 2020 as defined in the methods section.
Fig 1 shows the yearly growth of the impact weighted number of publications per MeSH term. By comparing the growth of the number of publications from the years 2018, 2019 and 2020, we find that the impact factor weighted number of publications has increased by up to a factor of 100 compared to the previous year for Betacoronavirus, one of the most closely related to COVID-19 MeSH term.
Each dot represents, a MeSH term. The y axis (growth) is in symmetric log scale. The x axis shows the COVID-19 relatedness, σ . Note that the position of the dots on the x-axis is the same in the two plots. Below: MeSH term importance gain (PageRank) and their COVID-19 relatedness.
https://doi.org/10.1371/journal.pone.0263001.g001
Fig 1 , first row, reveals how strongly correlated the growth in the IFWN of publication is to the term’s COVID-19 relatedness. For instance, we see that the term ‘Betacoronavirus’ skyrocketed from 2019 to 2020, which is expected given that SARS-CoV-2 is a species of the genus. Conversely, the term ‘Alphacoronavirus’ has not experienced any growth given that it is twin a genus of the Coronaviridae family, but SARS-CoV-2 is not one of its species. Note also the fast growth in the number of publications dealing with ‘Quarantine’. Moreover, MeSH terms that grew significantly from 2018 to 2019 and were not closely related to COVID-19, like ‘Vaping’, slowed down in 2020. From the graph, the picture emerges that publication growth is correlated with COVID-19 relatedness σ and that the growth for less related terms slowed down.
To show that the usage pattern of MeSH terms has changed following the pandemic, we compute the PageRank centrality using graph-tool [ 18 ] as discussed in the Methods section.
Fig 1 , second row, shows the change in the PageRank centrality of the MeSH terms after the pandemic (2019 to 2020, right plot) and before (2018 to 2019, left plot). If there were no change in the general usage pattern, we would expect the variance in PageRank changes to be narrow across the two periods, see (left plot). However, PageRank scores changed significantly more from 2019 to 2020 than from 2018 to 2019, suggesting that there has been a reconfiguration of the network.
To further support this argument, we carry out a DiD regression analysis.
Common trends assumption
As discussed in the Methods section, we need to show that the CTA assumption holds for the DiD to be defined appropriately. We do this by estimating for each month the number of publications and comparing it across treatment groups. This exercise also serves the purpose of a placebo test. By assuming that each month could have potentially been the event’s timing (i.e., the outbreak), we show that January 2020 is the most likely timing of the event. The regression table, as noted earlier, contains over 70 estimated coefficients, hence for ease of reading, we will only show the predicted outcome per month by group (see Fig 2 ). The full regression table with all coefficients is available in the S1 Table .
The y axis is in log scale. The dashed vertical line identifies January 2020. The dashed horizontal line shows the publications in January 2019 for the 0–20% group before the event. This line highlights that the drop happens after the event. The bands around the lines indicate the 95% confidence interval of the predicted values. The results are the output of the Stata margins command.
https://doi.org/10.1371/journal.pone.0263001.g002
Fig 2 shows the predicted number per outcome variable obtained from the panel regression model. These predictions correspond to the predicted value per relatedness group using the regression parameters estimated via the linear panel regression. The bands around the curves are the 95% confidence intervals.
All outcome measures depict a similar trend per month. Before the event (i.e., January 2020), there is a common trend across all groups. In contrast, after the event, we observe a sudden rise for the outcomes of the COVID-19 related treated groups (green and red lines) and a decline in the outcomes for the unrelated group (blue line). Therefore, we can conclude that the CTA assumption holds.
Regression results
Table 3 shows the DiD regression results (see Eq (3) ) for the selected outcome measures: number of publications (Papers), impact factor weighted number of publications (Impact), open access (OA) publications, clinical trial related publications, and publications with existing grants.
https://doi.org/10.1371/journal.pone.0263001.t003
Table 3 shows results for the discrete treatment level version of the DiD model (see Eq (4) ).
Note that the outcome variable is in natural log scale; hence to get the effect of the independent variable, we need to exponentiate the coefficient. For values close to 0, the effect is well approximated by the percentage change of that magnitude.
In both specifications we see that the least related group, drops in the number of publications between 10% and 13%, respectively (first row of Tables 3 and 4 , exp(−0.102) ≈ 0.87). In line with our expectations, the increase in the number of papers published by MeSH term is positively affected by the relatedness to COVID-19. In the discrete model (row 2), we note that the number of documents with MeSH terms with a COVID-19 relatedness between 20 and 80% grows by 18% and highly related terms by a factor of approximately 6.6 (exp(1.88)). The same general pattern can be observed for the impact weighted publication number, i.e., Model (2). Note, however, that the drop in the impact factor weighted output is more significant, reaching -19% for COVID-19 unrelated publications, and related publications growing by a factor of 8.7. This difference suggests that there might be a bias to publish papers on COVID-19 related subjects in high impact factor journals.
https://doi.org/10.1371/journal.pone.0263001.t004
By looking at the number of open access publications (PMC), we note that the least related group has not been affected negatively by the pandemic. However, the number of COVID-19 related publications has drastically increased for the most COVID-19 related group by a factor of 6.2. Note that the substantial increase in the number of papers available through open access is in large part due to journal and editorial policies to make preferentially COVID research immediately available to the public.
Regarding the number of clinical trial publications, we note that the least related group has been affected negatively, with the number of publications on clinical trials dropping by a staggering 24%. At the same time, publications on clinical trials for COVID-19-related MeSH have increased by a factor of 2.1. Note, however, that the effect on clinical trials is not significant in the continuous regression. The discrepancy across Tables 3 and 4 highlights that, especially for trials, the effect is not linear, where only the publications on clinical trials closely related to COVID-19 experiencing a boost.
It has been reported [ 19 ] that while the number of clinical trials registered to treat or prevent COVID-19 has surged with 179 new registrations in the second week of April 2020 alone. Only a few of these have led to publishable results in the 12 months since [ 20 ]. On the other hand, we find that clinical trial publications, considering related MeSH (but not COVID-19 directly), have had significant growth from the beginning of the pandemic. These results are not contradictory. Indeed counting the number of clinical trial publications listing the exact COVID-19 MeSH term (D000086382), we find 212 publications. While this might seem like a small number, consider that in 2020 only 8,485 publications were classified as clinical trials; thus, targeted trials still made up 2.5% of all clinical trials in 2020 . So while one might doubt the effectiveness of these research efforts, it is still the case that by sheer number, they represent a significant proportion of all publications on clinical trials in 2020. Moreover, COVID-19 specific Clinical trial publications in 2020, being a delayed signal of the actual trials, are a lower bound estimate on the true number of such clinical trials being conducted. This is because COVID-19 studies could only have commenced in 2020, whereas other studies had a head start. Thus our reported estimates are conservative, meaning that the true effect on actual clinical trials is likely larger, not smaller.
Research funding, as proxied by the number of publications with grants, follows a similar pattern, but notably, COVID-19-related MeSH terms list the same proportion of grants established before 2019 as other unrelated MeSH terms, suggesting that grants which were not designated for COVID-19 research have been used to support COVID-19 related research. Overall, the number of publications listing a grant has dropped. Note that this should be because the number of publications overall in the unrelated group has dropped. However, we note that the drop in publications is 10% while the decline in publications with at least one grant is 15%. This difference suggests that publications listing grants, which should have more funding, are disproportionately COVID-19 related papers. To further investigate this aspect, we look at whether the grant was old (pre-2019) or appeared for the first time in or after 2019. It stands to reason that an old grant (pre-2019) would not have been granted for a project dealing with the pandemic. Hence we would expect that COVID-19 related MeSH terms to have a lower proportion of old grants than the unrelated group. In models (6) in Table 4 we show that the number of old grants for the unrelated group drops by 13%. At the same time, the number of papers listing old grants (i.e., pre-2019) among the most related group increased by a factor of 3.1. Overall, these results suggest that COVID-19 related research has been funded largely by pre-existing grants, even though a specific mandate tied to the grants for this use is unlikely.
The scientific community has swiftly reallocated research efforts to cope with the COVID-19 pandemic, mobilizing knowledge across disciplines to find innovative solutions in record time. We document this both in terms of changing trends in the biomedical scientific output and the usage of MeSH terms by the scientific community. The flip side of this sudden and energetic prioritization of effort to fight COVID-19 has been a sudden contraction of scientific production in other relevant research areas. All in all, we find strong support to the hypotheses that the COVID-19 crisis has induced a sudden increase of research output in COVID-19 related areas of biomedical research. Conversely, research in areas not related to COVID-19 has experienced a significant drop in overall publishing rates and funding.
Our paper contributes to the literature on the impact of COVID-19 on scientific research: we corroborate previous findings about the surge of COVID-19 related publications [ 1 – 3 ], partially displacing research in COVID-19 unrelated fields of research [ 4 , 14 ], particularly research related to clinical trials [ 5 – 7 ]. The drop in trial research might have severe consequences for patients affected by life-threatening diseases since it will delay access to new and better treatments. We also confirm the impact of COVID-19 on open access publication output [ 1 ]; also, this is milder than traditional outlets. On top of this, we provide more robust evidence on the impact weighted effect of COVID-19 and grant financed research, highlighting the strong displacement effect of COVID-19 on the allocation of financial resources [ 15 ]. We document a substantial change in the usage patterns of MeSH terms, suggesting that there has been a reconfiguration in the way research terms are being combined. MeSH terms highly related to COVID-19 were peripheral in the MeSH usage networks before the pandemic but have become central since 2020. We conclude that the usage patterns have changed, with COVID-19 related MeSH terms occupying a much more prominent role in 2020 than they did in the previous years.
We also contribute to the literature by estimating the effect of COVID-19 on biomedical research in a natural experiment framework, isolating the specific effects of the COVID-19 pandemic on the biomedical scientific landscape. This is crucial to identify areas of public intervention to sustain areas of biomedical research which have been neglected during the COVID-19 crisis. Moreover, the exploratory analysis on the changes in usage patterns of MeSH terms, points to an increase in the importance of covid-related topics in the broader biomedical research landscape.
Our results provide compelling evidence that research related to COVID-19 has indeed displaced scientific production in other biomedical fields of research not related to COVID-19, with a significant drop in (impact weighted) scientific output related to non-COVID-19 and a marked reduction of financial support for publications not related to COVID-19 [ 4 , 5 , 16 ]. The displacement effect is persistent to the end of 2020. As vaccination progresses, we highlight the urgent need for science policy to re-balance support for research activity that was put on pause because of the COVID-19 pandemic.
We find that COVID-19 dramatically impacted clinical research. Reactivation of clinical trials activities that have been postponed or suspended for reasons related to COVID-19 is a priority that should be considered in the national vaccination plans. Moreover, since grants have been diverted and financial incentives have been targeted to sustain COVID-19 research leading to an excessive entry in COVID-19-related clinical trials and the ‘covidisation’ of research, there is a need to reorient incentives to basic research and otherwise neglected or temporally abandoned areas of biomedical research. Without dedicated support in the recovery plans for neglected research of the COVID-19 era, there is a risk that more medical needs will be unmet in the future, possibly exacerbating the shortage of scientific research for orphan and neglected diseases, which do not belong to COVID-19-related research areas.
Limitations
Our empirical approach has some limits. First, we proxy MeSH term usage via search terms using the PubMed EUtilities API. This means that the accuracy of the MeSH term we assign to a given paper is not fully validated. More time is needed for the completion of manually annotated MeSH terms. Second, the timing of publication is not the moment the research has been carried out. There is a lead time between inception, analysis, write-up, review, revision, and final publication. This delay varies across disciplines. Nevertheless, given that the surge in publications happens around the alleged event date, January 2020, we are confident that the publication date is a reasonable yet imperfect estimate of the timing of the research. Third, several journals have publicly declared to fast-track COVID-19 research. This discrepancy in the speed of publication of COVID-19 related research and other research could affect our results. Specifically, a surge or displacement could be overestimated due to a lag in the publication of COVID-19 unrelated research. We alleviate this bias by estimating the effect considering a considerable time after the event (January 2020 to December 2020). Forth, on the one hand, clinical Trials may lead to multiple publications. Therefore we might overestimate the impact of COVID-19 on the number of clinical trials. On the other hand, COVID-19 publications on clinical trials lag behind, so the number of papers related COVID-19 trials is likely underestimated. Therefore, we note that the focus of this paper is scientific publications on clinical trials rather than on actual clinical trials. Fifth, regarding grants, unfortunately, there is no unique centralized repository mapping grant numbers to years, so we have to proxy old grants with grants that appeared in publications from 2010 to 2018. Besides, grant numbers are free-form entries, meaning that PubMed has no validation step to disambiguate or verify that the grant number has been entered correctly. This has the effect of classifying a grant as new even though it has appeared under a different name. We mitigate this problem by using a long period to collect grant numbers and catch many spellings of the same grant, thereby reducing the likelihood of miss-identifying a grant as new when it existed before. Still, unless unique identifiers are widely used, there is no way to verify this.
So far, there is no conclusive evidence on whether entry into COVID-19 has been excessive. However, there is a growing consensus that COVID-19 has displaced, at least temporally, scientific research in COVID-19 unrelated biomedical research areas. Even though it is certainly expected that more attention will be devoted to the emergency during a pandemic, the displacement of biomedical research in other fields is concerning. Future research is needed to investigate the long-run structural consequences of the COVID-19 crisis on biomedical research.
Supporting information
S1 table. common trend assumption (cta) regression table..
Full regression table with all controls and interactions.
https://doi.org/10.1371/journal.pone.0263001.s001
- View Article
- Google Scholar
- PubMed/NCBI
- 8. Brown A, Edwin E, Fagg J. Evaluate Pharma 2021 Preview; 2020. https://www.evaluate.com/thought-leadership/vantage/evaluate-vantage-2021-preview .
- 15. Hook D, Porter S. The COVID Brain Drain; 2020. https://www.natureindex.com/news-blog/covid-brain-drain-scientific-research .
- 17. Page L, Brin S, Motwani R, Winograd T. The PageRank citation ranking: Bringing order to the web. Stanford InfoLab; 1999.
- Introduction
- Conclusions
- Article Information
Research fields of highly cited studies on COVID-19, including duplicates between each period, are presented.
Publication numbers are given over the entire study period for institutions with the most publications in A, May to June 2020 and B, November to December 2022.
Research fields are presented for the top affiliated institution in A, May to June 2020 (Huazhong University of Science and Technology) and B, November to December 2022 (Harvard University).
eFigure 1. Top Research Fields of Highly Cited Studies on COVID-19
eFigure 2. Top 5 Countries Producing Highly Cited Studies on COVID-19 Using Full Counting Method
eFigure 3. Top 5 Countries of Corresponding Authors Producing Highly Cited Studies on COVID-19
eFigure 4. Top 5 Institutional Affiliations Producing Highly Cited Studies on COVID-19 Using Full Counting Method
eFigure 5. Top 5 Institutional Affiliations of Corresponding Authors Producing Highly Cited Studies on COVID-19
Data Sharing Statement
- Incorrect Institution Name in Author Affiliations JAMA Network Open Correction October 16, 2023
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Funada S , Yoshioka T , Luo Y, et al. Global Trends in Highly Cited Studies in COVID-19 Research. JAMA Netw Open. 2023;6(9):e2332802. doi:10.1001/jamanetworkopen.2023.32802
Manage citations:
© 2024
- Permissions
Global Trends in Highly Cited Studies in COVID-19 Research
- 1 Department of Health Promotion and Human Behavior, School of Public Health, Graduate School of Medicine, Kyoto University, Kyoto, Japan
- 2 Department of Preventive Medicine and Public Health, Keio University School of Medicine, Tokyo, Japan
- 3 Population Health and Policy Research Unit, Medical Education Center, Graduate School of Medicine, Kyoto University, Kyoto, Japan
- 4 Office of Evidence and Analysis, Japan Science and Technology Agency, Tokyo, Japan
- 5 Division of Surveillance and Policy Evaluation, National Cancer Center Institute for Cancer Control, Tokyo, Japan
- Correction Incorrect Institution Name in Author Affiliations JAMA Network Open
Question What is the global trend of highly cited studies investigating COVID-19 since its outbreak?
Findings This cross-sectional study found that the number of highly cited studies peaked at 1292 studies at the end of 2021 and declined to 649 studies at the end of 2022. Highly cited studies from China showed a decreasing trend, while those from the US and UK showed an increasing trend.
Meaning These findings suggest that as the COVID-19 pandemic evolved in the 3 years since its outbreak, there were important shifts in trends of the number and origin of high-profile COVID-19 studies.
Importance Since the onset of the COVID-19 outbreak, an extremely high number of studies have been published worldwide, with variable quality. Research trends of highly cited papers may enable identification of influential research, providing insights for new research ideas; it is therefore important to investigate trends and focus on more influential publications in COVID-19–related studies.
Objective To examine research trends of highly cited studies by conducting a bibliometric analysis of highly cited studies in the previous 2 months about COVID-19.
Design, Setting, and Participants In this cross-sectional study, Essential Science Indicators (ESI) and Web of Science (WOS) Core Collection were used to find studies with a focus on COVID-19 that were identified as highly cited studies from Clarivate Analytics. Highly cited studies were extracted from the ESI database bimonthly between January 2020 and December 2022. Bibliographic details were extracted from WOS and combined with ESI data using unique accession numbers. The number of highly cited studies was counted based on the fractional counting method. Data were analyzed from January through July 2023.
Main Outcomes and Measures The number of publications by research field, country, and institutional affiliation.
Results The number of published COVID-19–related highly cited studies was 14 studies in January to February 2020, peaked at 1292 studies in November to December 2021, and showed a downward trend thereafter, reaching 649 studies in November to December 2022. China had the highest number of highly cited studies per 2-month period until July to August 2020 (138.3 studies vs 103.7 studies for the US, the second highest country), and the US had the greatest number of highly cited studies afterward (159.9 studies vs 157.6 studies for China in September to October 2020). Subsequently, the number of highly cited studies per 2-month period published by China declined (decreasing from 179.7 studies in November to December 2020 to 40.7 studies in September to October 2022), and the UK produced the second largest number of such studies in May to June 2021 (171.3 studies). Similarly, the top 5 institutional affiliations in May to June 2020 by highly cited studies per 2-month period were from China (Huazhong University: 14.7 studies; University of Hong Kong: 6.8 studies; Wuhan University: 4.8 studies; Zhejiang University: 4.5 studies; Fudan University: 4.5 studies), while in November to December 2022, the top 5 institutions were in the US and UK (Harvard University: 15.0 studies; University College London: 11.0 studies; University of Oxford: 10.2 studies; University of London: 9.9 studies; Imperial College London: 5.8 studies).
Conclusions and Relevance This study found that the total number of highly cited studies related to COVID-19 peaked at the end of 2021 and showed a downward trend until the end of 2022, while the origin of these studies shifted from China to the US and UK.
Since the outbreak of COVID-19 in December 2019, numerous studies have been conducted and published worldwide in response to the pandemic. 1 This trend may have been amplified by the use of preprint systems, such as medRxiv 2 and bioRxiv, 3 during the pandemic, as well as by the proliferation of predatory journals. 4 As a result, the total number of COVID-19–related publications, including preprints, has increased dramatically and now exceeds 350 000 studies. 5 The dissemination of COVID-19 research is highly active and constantly evolving. In such an expanding research environment, investigating research trends may help identify knowledge gaps and provide insightful research directions. 6 In addition, comparing trends across countries and institutional affiliations may support scientific policy and research management. 7
However, as previously found in 2020, 8 the increase in COVID-19–related publications has not necessarily been associated with increased high-quality evidence, and this concern has become a reality in 2023. A citation analysis of studies published in predatory journals found that 60% of publications had not attracted any citations and 38% were cited only up to 10 times. 9 The COVID-19 pandemic has seemingly been associated with an exacerbated issue of waste of studies (ie, doing unnecessary or poorly designed studies), 10 making the proper assessment and synthesis of research trends in COVID-19 research challenging. Therefore, some filtering system may be essential to efficiently narrow down desired publications from the vast collection and ensure that relevant and valuable studies are selected.
One way to address this challenge is to analyze highly cited studies, or hot papers, which refers to studies published within the previous 2 years that have received a considerable number of citations in the previous 2 months, placing them in the top 0.1% of studies in the same field. 11 High citation counts indicate that these studies have garnered significant attention from researchers. Furthermore, the list is updated every 2 months, allowing researchers to keep up with the latest trends and analyze them over time to capture shifts in the research landscape. Examining research trends of highly cited studies may allow the identification of influential studies, providing valuable insights for generating new research ideas. Bibliometrics is a scientific domain focused on measuring and quantifying various features in publications by examining the productivity of researchers, affiliations, and countries in specific fields. 12 Therefore, a bibliometric analysis may be appropriate for examining features of highly cited studies in COVID-19 research. To our knowledge, there have been no studies analyzing the trend of COVID-19–related highly cited studies.
This study aimed to investigate research trends of highly cited studies by conducting a bibliometric analysis of these studies on COVID-19 research. Additionally, by presenting these studies in chronological order, we aimed to identify changes in COVID-19 research trends.
This cross-sectional study was a bibliometric analysis of highly cited studies and followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. According to the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan, institutional review board approval and participant consent were not required for this study because it used only published data.
We included all studies with a focus on COVID-19 identified as highly cited studies from Clarivate Analytics. We excluded studies that contained keywords related to COVID-19 in the text but did not investigate COVID-19. There was no restriction on the type of studies included.
This study used the following 5 selection steps for identifying highly cited studies on COVID-19. In step 1, we extracted a total of 18 periods of highly cited studies from the Essential Science Indicators (ESI) (Clarivate Analytics) database bimonthly from January 2020 to December 2022 (January to February 2020 through November to December 2020, January to February 2021 through November to December 2021, and January to February 2022 through November to December 2022). In step 2, based on a unique accession number associated with each highly cited study record in the ESI, we combined bibliographic details, such as abstract, document type, and others, from the Web of Science (WOS) Core Collection (Clarivate Analytics) with ESI data. The accession number is an identification number assigned to each study in WOS, and an individual study is identified by searching with the accession number in WOS. In step 3, we identified highly cited studies with a focus on COVID-19 from their titles and abstracts using the following search terms: “COVID-19” or “2019-nCoV” or “NOVEL 2019” or “CORONAVIRUS DISEASE 2019” or “SARS-COV-2” or “n-COV” or “COVID” or “CORONAVIRUS” or “SARS.” We excluded highly cited studies that did not contain predetermined keywords as non-COVID-19–related highly cited studies. In step 4, a total of 4 researchers (T.I., C.M., N.Y., and H.Y.) making pairs in rotation each independently reviewed titles, abstracts, and full texts and included COVID-19–related highly cited studies that fit eligibility criteria. Any disagreements or ambiguity between pairs were resolved through discussion or cross-check consultation with another researcher if required. In step 5, the same 4 researchers (T.I., C.M., N.Y., and H.Y.) checked how many duplicates were counted as highly cited studies between each period. We conducted this selection step once for each of 18 periods between January 2020 and December 2022.
We collected the following bibliographic information from the WOS database: titles, authors, corresponding authors, affiliations, publication journal, publication date, and research field. Based on this information, we used 3 variables to measure the trend of COVID-19–related research as follows: (1) The research fields variable included 22 ESI categories (agricultural science; biology and biochemistry; chemistry; clinical medicine; computer science; ecology/environment; economics and business; engineering; geosciences; immunology; materials science; mathematics; microbiology; molecular biology and genetics; multidisciplinary; neuroscience and behavior; pharmacology; physics; plant and animal science; psychiatry/psychology; social sciences, general; and space science). 13 Each journal is assigned to 1 field, and the research published in that journal adopts that field assignment. (2) The countries variable included countries of affiliation of all co-authors for each study. (3) The affiliations variable included affiliations of all co-authors for each study.
The bibliometric analysis descriptively summarizes the number of COVID-19–related highly cited studies. We counted the number of highly cited studies based on the fractional counting method. Compared with the full counting method, which counts the full number of each co-author and institutional affiliation, the fractional counting method had a fractional weight of each co-author and institutional affiliation, and each publication had a total weight of 1. 14 Highly cited study counts were compared between research fields, countries, and affiliations. As a sensitivity analysis, we performed the full counting method instead of the fractional counting method. We also performed the fractional counting method on countries and affiliations of corresponding authors as a sensitivity analysis. Data were analyzed using R statistical software version 4.3.1 (R Project for Statistical Computing). Data were analyzed from January through July 2023.
Figure 1 shows the selection step for highly cited studies on COVID-19 research. We identified 73 079 highly cited studies from the ESI database in 18 periods every 2 months between January 2020 and December 2022. From 73 079 highly cited studies, we excluded 57 236 highly cited studies by keyword search and 581 highly cited studies by title, abstract, and full text review. Finally, we identified 15 262 highly cited studies with duplicates and 4131 such studies without duplicates.
Figure 2 shows the number of highly cited studies for COVID-19 research in each period. The total number of highly cited studies exhibited gradual growth, from 3412 studies in January to February 2020 to 4389 studies in November to December 2022. Regarding COVID-19–related highly cited studies, the initial count was 14 studies in January to February 2020, increasing to 1292 studies in November- to December 2021. However, there was a subsequent decline to 649 studies in November to December 2022.
The top 10 research fields of highly cited studies of COVID-19 in each period are given in eFigure 1 in Supplement 1 . Although highly cited studies were predominantly from the clinical medicine field in January to February 2020 (9 of 14 studies [64.3%]), there was a gradual decrease in studies in this field starting in March to April 2022 (427 studies) until November to December 2022 (246 studies). Studies in other fields increased in number over time, with a particular increase in the fields of general social science, psychiatry and psychology, immunology, and molecular biology and genetics. For example, highly cited studies in general social science increased from 0 studies in January to February 2020 to 73 studies in July to August 2022.
Figure 3 shows the top 5 countries with the highest number of highly cited studies. China recorded the highest number of publications per 2-month period from January to February 2020 through July to August 2020 (138.3 studies), with the US following closely behind (103.7 studies during this period) and gradually increasing its output, overtaking China in September to October 2020 (159.9 studies vs 157.6 studies). China’s highly cited study output per 2-month period has been declining since November to December 2020 (decreasing from 179.7 studies in that period to 40.7 studies in September to October 2022), while there has been a steady increase in publications from the UK, increasing from 86.5 studies in November to December 2020 to ultimately overtake China in May to June 2021 (171.3 studies vs 166.6 studies). Starting in March to April 2022 until November to December 2022, the US, UK, and China had substantially reduced numbers of highly cited studies, and the downward trend continued until November to December 2022. The decrease in the number of highly cited studies per 2-month period from March to April 2022 to November to December 2022 was 366.8 studies to 190.6 studies for the US, 243.7 studies to 158.3 studies for the UK, and 107.5 studies to 45.5 studies for China. The trend remained the same using the full counting method (eFigure 2 in Supplement 1 ) and counting corresponding authors’ countries (eFigure 3 in Supplement 1 ) in sensitivity analyses.
Figure 4 shows the distribution of highly cited studies based on institutional affiliation across periods. Figure 4 A and Figure 4 B depict the top 5 facilities in terms of highly cited study publication numbers in May to June 2020 and November to December 2022, respectively. The top 5 institutional affiliations by highly cited studies per 2-month period in May to June 2020 were based in China (Huazhong University: 14.7 studies; University of Hong Kong: 6.8 studies; Wuhan University: 4.8 studies; Zhejiang University: 4.5 studies; Fudan University: 4.5 studies); however, by 2021, they all displayed a decreasing trend ( Figure 4 A). Conversely, in November to December 2022, the top 5 affiliations by highly cited studies per 2-month period were based in the US or the UK (Harvard University: 15.0 studies; University College London: 11.0 studies; University of Oxford: 10.2 studies; University of London: 9.9 studies; Imperial College London: 5.8 studies) ( Figure 4 B). Although there was some turnover, the trend remained the same in sensitivity analyses. There were more facilities in China in May to June 2020 by the full counting method (eFigure 4 in Supplement 1 ) and by affiliations of corresponding authors (eFigure 5 in Supplement 1 ) and more facilities in the US or UK in November to December 2022 by the full counting method (eFigure 4 in Supplement 1 ) and by affiliations of corresponding authors (eFigure 5 in Supplement 1 ).
Figure 5 provides an overview of the research fields of affiliations with the highest number of highly cited studies in May to June 2020 ( Figure 5 A) and November to December 2022 ( Figure 5 B). Huazhong University of Science and Technology published the greatest number of highly cited studies in May to June 2020, with 27 of 34 studies in the clinical medicine field. In contrast, the top highly cited studies in November to December 2022 were from Harvard University, with 73, 13, and 9 highly cited studies in the fields of clinical medicine, molecular biology and genetics, and psychiatry and psychology, respectively.
This cross-sectional study evaluated trends in COVID-19 research by analyzing highly cited studies every 2 months from January 2020 to December 2022. As the pandemic progressed, the number of highly cited studies related to COVID-19 increased sharply. Nevertheless, after reaching a peak at the end of 2021, the number of highly cited studies exhibited a declining trend. In addition, while most highly cited studies were initially from the field of clinical medicine, we observed an increase in the number of publications from other fields through the observational period. Over time, there was a shift in the ranking of countries, with the US overtaking China to produce the highest number of highly cited studies since September to October 2020. The number of highly cited studies from China showed a decreasing trend, while those from the UK exhibited an increasing trend. Institutions that published the greatest number of highly cited studies at the beginning of the pandemic were from China; however, their number of publications gradually decreased, and the top institutions were replaced by those from the US and UK.
To our knowledge, no studies to date have conducted a bibliometric analysis of highly cited studies related to COVID-19 over the past 3 years. However, a bibliometric analysis using the COVID-19 Open Research Dataset (CORD-19) 15 reported that COVID-19 studies, not just highly cited studies, published in 2020 came mostly from the US, China, and UK, which received more than 60% of citations. Similar to our research, that study found that the US steadily increased the number of studies and took the top spot, China had initially led COVID-19 research but experienced a substantial decline in research output over time, and the UK showed the opposite trend, starting with a slow pace of publications and gradually increasing its contributions throughout the year. Another study 16 examined the association of COVID-19–related publications with overall publication rates in high-impact factor journals. They showed that studies related to COVID-19 accounted for approximately 10% to 50% of the total number of publications in each high-impact journal from 2020 to 2021. Additionally, a gradual decline in COVID-19–related publications was observed at the end of 2020. Notably, this declining trend was detected earlier in that study than in our analysis, suggesting a lag in citations given that highly cited studies are determined based on the number of citations after publication.
As reported in a previous study, 17 an increase in COVID-19 cases in a region or country was associated with increased COVID-19–related research activity in that area. This may be associated with 2 factors: the need for data and increased government funding for research to control the pandemic. In addition, our findings of a gradual downward trend in highly cited studies related to COVID-19 may have been associated with a decrease in global attention to COVID-19 research. This trend may also suggest that researchers have gained a better understanding of the etiology and treatment of COVID-19, leading to decreased interest or fatigue with the topic. 18 Changes in distribution, top affiliations, and research fields of highly cited studies suggest a gradual shift in interest in COVID-19 research toward more diversified and broader research areas. Based on results of this investigation, we expect a sustained reduction in the number of highly cited studies on COVID-19. Furthermore, we speculate that the research focus may further diversify, and we intend to examine this hypothesis through ongoing analysis. A bibliometric analysis using highly cited studies may be an appropriate method to capture trends in research by examining high-profile studies every 2 months. Similar methods used in this study may be useful for analyzing research trends in other fields.
This study has several strengths. First, to our knowledge, it is the first bibliometric analysis of highly cited studies related to COVID-19. Investigations of highly cited studies (ie, those with the top 0.1% of citations) may be of greater interest than studies that analyze the total number of COVID-19–related studies. Using this method, we can exclude studies with low scientific impact, such as those published in predatory journals. In addition, highly cited studies are updated every 2 months, allowing the tracking of trends over time. Second, this study used fractional counting rather than full counting. While full counting is widely used in bibliometric analysis, fractional counting allows for field normalization and takes into account effects of aggregating large studies, particularly at the level of countries and research organizations. A comparative study 14 recommended fractional counting in such bibliometric studies.
This study also has several limitations. First, while fractional counting is a strength, it can also be a limitation given that full counting is more widely used, making it challenging to compare our results with those of other studies. However, we also performed full counting as sensitivity analyses and observed no substantial difference in trends compared with fractional counting. This suggests that the difference in counting methods may not be a serious issue. Second, highly cited studies are limited to the top 0.1% of citations and are not representative of all published literature. In addition, the number of citations does not necessarily guarantee the quality of the research. Therefore, it should be noted that this study’s findings represent only trends in influential research. Third, although we used ESI categories to define research fields, these categories do not classify highly cited studies in detail. For example, clinical medicine includes a very broad range of highly cited studies. A more detailed classification may be appropriate for a closer look at research trends. Fourth, although this study identified trends in COVID-19–related highly cited studies, it provides a broad overview rather than a detailed analysis. Several interesting aspects could not be explored in depth in this study, including comparisons with non-COVID-19–related highly cited studies and the evolution of characteristic topics over time, such as lockdown policies and vaccines. To address these gaps, we need further analyses in the future.
In this cross-sectional study, a bibliometric analysis of highly cited studies found that as the COVID-19 pandemic evolved over the 3 years since its outbreak, there was a shift in trends in COVID-19 research. The increase and decrease in the number of highly cited studies related to COVID-19 may suggest shifting interests of researchers. Meanwhile, there was a noticeable increase in the number of topics covered by field, including not only clinical medicine but also a diverse range of topics.
Accepted for Publication: July 31, 2023.
Published: September 8, 2023. doi:10.1001/jamanetworkopen.2023.32802
Correction: This article was corrected on October 16, 2023, to fix the name of an institution in the author affiliations.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Funada S et al. JAMA Network Open .
Corresponding Author: Satoshi Funada, MD, PhD, Department of Health Promotion and Human Behavior, School of Public Health, Graduate School of Medicine, Kyoto University, Yoshida Konoe-cho, Sakyo-ku, Kyoto 606-8501, Japan ( [email protected] ).
Author Contributions: Dr Yoshida had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Funada, Yoshida, Katanoda.
Acquisition, analysis, or interpretation of data: Funada, Yoshioka, Luo, Iwama, Mori, Yamada, Yoshida, Furukawa.
Drafting of the manuscript: Funada, Iwama, Katanoda.
Critical review of the manuscript for important intellectual content: Yoshioka, Luo, Mori, Yamada, Yoshida, Furukawa.
Statistical analysis: Funada, Yoshioka, Mori, Yamada, Yoshida.
Obtained funding: Katanoda.
Administrative, technical, or material support: Mori.
Supervision: Yoshida, Katanoda, Furukawa.
Conflict of Interest Disclosures: Dr Funada reported receiving grants from the Japan Society for the Promotion of Science (JSPS), KDDI Foundation, and Pfizer Health Research Foundation outside the submitted work. Dr Yoshioka reported receiving grants from the JSPS and Japan National Cancer Center outside the submitted work. Dr Luo reported receiving grants from the JSPS outside the submitted work. Dr Furukawa reported receiving personal fees from Boehringer-Ingelheim, DT Axis, Kyoto University Original, Shionogi, and Sony and grants from Shionogi outside the submitted work and having patents pending for 2020-548587, 2022-082495, and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. No other disclosures were reported.
Funding/Support: We acknowledged the support by grant JPMH21HA201 from the Japan Ministry of Health, Labour and Welfare Research Program on Emerging and Reemerging Infectious Diseases and the Japan National Institute of Public Health for language editing and article publishing charges.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We thank Editage for providing English language editing. This company was paid a fee for these services.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Marie Skłodowska-Curie Actions
Developing talents, advancing research
Projects researching COVID–19, SARS-CoV-2 and related topics
The current COVID-19 outbreak has not caught EU-funded research off guard. The Marie Skłodowska-Curie Actions (MSCA) of the European Commission are supporting outstanding researchers in finding solutions to challenges posed by the novel coronavirus disease COVID-19 and other infectious diseases.
This page will be regularly updated with MSCA projects, results and testimonials relevant to COVID-19, SARS-CoV-2 and related topics.
DIAGNOSTICS AND TREATMENTS (including vaccines)
Diabetic nephropathy modelling in hesc-derived 3d kidney organoids.
EPIORGABOLISM is studying how SARS-Co-V2, the coronavirus responsible for the 2019 novel coronavirus disease (COVID-19), interacts with and infects kidney cells. Together with the lung, the kidney is one of the main organs affected by the COVID-19 disease. Dr Carmen Hurtado, researcher of EPIORGABOLISM, is currently generating human kidney organoids from human pluripotent stem cells.
The use of human organoids allows to test treatments against coronavirus in an agile way, dramatically reducing the time human drug trials take. Hurtado is part of international research team has identified a drug capable of blocking the effects of the SARS-CoV-2 virus. The findings have been partially supported by EPIORGABOLISM and published in the journal ‘Cell’.
Find out more
- Trial drug shows promise in fighting coronavirus
- Watch the testimonial of Carmen Hurtado , researcher of the EPIORGABOLISM project.
Host switching pathogens, infectious outbreaks and zoonosis; a Marie Sklodowska-Curie Training Network
HONOURs is teaching 15 talented young researchers, including coronavirologists, to become “preparedness-experts”. The project involves 11 laboratories, all at the forefront of novel virus investigations and characterizations. HONOUR reacted in January 2020, immediately after the emergence of COVID-19, by starting work on SARS-CoV-2. A synthetic biology virus culture system was developed to swiftly evaluate therapy options, next to rapid tests to determine virus shedding on location. The quality of protective immunity was evaluated, and a search started on the most suitable animal model to battle the virus and provide therapy options. HONOURs is devoting its expert knowledge to fight this coronavirus and provide therapy options.
- HONOURs: Virus Outbreak Preparedness and COVID-19
- Visit the HONOURs website
INnate-ImmunomeTabolIsm as Antiviral TargEt
The global COVID-19 pandemic highlights an urgent need for innovation in the development of novel antiviral strategies and therapies. INITIATE has recruited 15 young PhD candidates to become experts in the field of antiviral immunometabolism, with a focus on RNA viruses – including coronaviruses. While it is clear that viral replication, metabolic pathways, and host immune responses are tightly interconnected, the host molecular pathways that impact viral pathogenesis are not well-defined. With the emergence of COVID-19, eight of the INITIATE projects have included SARS-CoV-2 in their research programs to understand coronavirus molecular virology, the role of the host immune response in driving COVID-19 immunopathogenesis and the potential of targeting host metabolism as therapeutic strategies.
Organoids for Virus Research - An innovative training-ETN programme
ORGANOVIR is contributing to COVID-19 research in a variety of ways, and several of its researchers are currently working on the development of new antivirals to combat the disease. Researchers at KU Leuven (Belgium) are studying the way in which coronaviruses evolve, and are searching out possible targets for further remedies. The project also investigating active substances – or a combination of them – in existing medicines that could be effective against SARS-CoV-2. ORGANOVIR is also conducting pre-clinical tests for a vaccine against COVID-19 using a technology based on the yellow fever vaccine.
In parallel, a group of researchers at the Jagiellonian University (Poland) is studying the infection on the single-cell and tissue level in different organs and cell types, working on virus inhibitors and collaborating with companies to create a point of care diagnostics based on different platforms. The group is also studying the course of the pandemic in Poland and monitoring the virus variability in the country.
ORGANOVIR’s coordinators have been intensively working on clinical and diagnostic tasks and set up new COVID-19 research at the Amsterdam UMC (The Netherlands). This has resulted in the launch of COVID-KIDS, a study on immunity in children, and the use of 3D culture models for COVID-19 studies.
- Read about the COVID-19 activities of ORGANOVIR partners
- Read the testimonial of Mariana Guedes , researcher for the OrganoVir project
- Read the testimonial of Thuc Do , researcher for the OrganoVir project
- Air-liquid interface cultures of nasal epithelial cells to investigate factors critical for viral entry into host cells
MECHANISMS OF INFECTION, IMMUNE REACTIONS AND HOST-PATHAGEN INTERACTION
Unravelling species barriers of coronaviruses.
COV RESTRIC targeted the precise mechanisms that allow coronaviruses to jump across species. Dr Stephanie Pfänder, researcher of COV RESTRIC, worked on various virological aspects of emerging viruses – with a focus on emerging coronaviruses. Her work has the potential to lead to novel strategies to protect cells against coronavirus infection. This is crucial to fight the COVID-19 pandemic – and to help insulate society against future coronavirus outbreaks.
- Read the testimonial of Stephanie Pfänder , researcher of the COV RESTRIC project.
- Host proteins involved in species barriers of viral infections
DIGITAL TOOLS, DATA AND MODELLING
Research and innovation staff exchange network of european data scientists.
The NeEDS consortium is currently focusing on the emerging data challenges that come with the COVID-19 pandemic. In Spain, the first cases of the COVID-19 pandemic were confirmed late February 2020 and data started to be collected daily by the different regions. Data and Data Science tools turned out to be crucial to assist decision makers in this highly uncertain context. NeEDS and the scientific collaborations they enjoy were fundamental to create a working group of data scientists from different European universities, which has developed an Artificial Intelligence tool to provide short-term predictions of the pandemic’s evolution. With this novel methodology, NeEDS as contributed to the cooperative efforts coordinated by the Spanish Commission of Mathematics to support data-driven decision making related to the COVID19 pandemic. In a recent interview , Project Coordinator Dolores Romero Morales has reflected on the potential of the NeEDS expertise and the efforts of tackling these data challenges within the team. The consortium is tackling other important Data Science questions, e.g., using spatial data to support COVID19 information apps or addressing the pressing data privacy needs.
- Read about the COVID-19 activities of NeEDS and its partners
- On Sparse Ensemble Methods: An Application to Short-Term Predictions of the Evolution of COVID-19
- Read the testimonials of Remedios Sillero, Cristina Molero and Sandra Benitez , seconded researchers for the NeEDS project.
Pan-genome Graph Algorithms and Data Integration
Researchers involved in PANGAIA are investigating how massive amounts of genome sequence data can be ordered and analysed for their use in biomedicine. Their work has important implications in areas such as bacteria and virus research, investigation of drug resistance mechanisms and vaccine development: big data technology can help to identify the characteristics of new strains of viruses such as SARS-CoV-2 and bacteria by comparing their genomes.
- Identifying large data sets to help coronavirus research
- Identifying pathogenic genes in virus strains at a glance
Modelling Infectious Diseases in Dynamic, relocated, refugee populations
In order to assist policy-makers in mitigating outbreaks, MIDIDP has created realistic models to simulate the spread of infectious diseases in under-vaccinated refugee populations in Europe and neighbouring countries. Dr Hasan Güçlü, researcher of MIDIDP, has created a model that simulates the spread of COVID-19 in populations with variable demographics.
- Read the testimonial of Hasan Güçlü , researcher of the MIDIDP project.
PUBLIC HEALTH, PREPAREDNESS AND RESPONSE
Disability and disease during the 1918 influenza pandemic: implications for preparedness policies.
As the current COVID-19 pandemic shows, people with disabilities are at increased risk for complications and death as they are often neglected in epidemic responses. Dr Jessica Dimka, researcher of DIS2 , is exploring disability as a risk factor in pandemics. Using the 1918 Spanish influenza pandemic as a model, the project seeks to promote more equitable public health plans and interventions. Dimka points out that people with disabilities must be considered in all pandemic strategies: their lives, livelihoods and rights are not expendable.
- Read the testimonial of Jessica Dimka , researcher of the DIS2 project.
MULTIDISCIPLINARY PROJECTS
Protecting human rights and public health in global pandemics.
THEMIS is an interdisciplinary research project that reacts to the increasing occurrence of global pandemics, like the caused by the present COVID-19 disease, and restrictive public health measures taken to respond to these threats. Using a rights-based approach, Dr Patrycja Dąbrowska-Kłosińska, researcher of THEMIS, intends to create a better understanding of how to prepare for, and respond to, global pandemics.
The project seeks to offer a vital reference for policy-making at national, regional and global levels – one that prioritises fair pandemic preparedness to cross-border health threats. The project has offered critical guidance during the current COVID-19 pandemic, which has required a previously unimagined scale of coordinated, public health-control measures as well as consideration of human-rights implications worldwide.
- Read the testimonial of Patrycja Dąbrowska-Kłosińska , researcher of the THEMIS project.
Martí I Franquès COFUND
Since the emergence of COVID-19, several fellows involved in the Martí Franquès Programme (MFP) have been working on solutions to the current crisis. Researchers are developing an epidemiological mathematical model that infers the status of the epidemic, thereby monitoring and estimating the impact of interventions on the spread of COVID-19.
In parallel, another group of researchers is implementing an original virtual screening protocol to reposition approved drugs. This would allow predicting which of them could inhibit the main protease of the virus (M-pro), a key target for antiviral drugs given its essential role in the virus replication.
- Read the testimonial of Benjamin Steinegger , whose research is developing a mathematical framework to monitor and estimate the impact of interventions on the COVID-19 pandemic.

Project outcomes
- Modelling the impact of interventions on the spread of COVID-19
- Prediction of novel inhibitors of the main protease of SARS-CoV-2
- See all the results relevant to COVID-19 produced by MFP fellows
The launch of a new industrial PhD programme at EPFL
Several fellows involved in the EPFLinnovators project are working on solutions to COVID-19 since the start of the crisis. Teams of researchers are developing subunit vaccines against the SARS-CoV-2 virus, investigating the potential use of cyclodextrin derivatives to prevent and treat the infections caused by SARS-CoV-2, and analysing the mechanical aspects of SARS-CoV-2 entry into cells.
- Read the testimonial of Xiaomeng Hu , researcher of the EPFLInnovators project.
- Subunit vaccines against SARS-CoV-2
- Non-toxic cyclodextrin derivative against viruses at micromolar concentration
- Variations in clathrin mediated endocytosis on a mammalian cell membrane
SOCIAL BEHAVIOUR AND IMPACT
Leading fellows.
Over the last decade, the reliance on online products and services has steadily increased, but since the beginning of the COVID-19 pandemic it has escalated to an unprecedented level. Dr Matthew Dennis, researcher of the LEaDing Fellows COFUND project at TU Delft (the Netherlands), examines the ethical implications and value trade-offs as societies attempt to transition across the digital divide. His project highlights that an ethical reflection on this digital transition is urgently needed, as digital solutions to problems generated by COVID-19 may create winners and losers – likely disproportionately affecting vulnerable users. By addressing these issues, the pandemic may foster the kind of social and political interconnectedness that was envisioned at the start of the crisis.
- Read the testimonial of Matthew James Dennis , researcher of the LEaDing Fellows project.
MSCA on social media
The MSCA social media are continuously updated with testimonials of MSCA fellows, supervisors, coordinators and projects working to find solutions to challenges posed by COVID-19 and other infectious diseases.
- MSCA on Twitter
- MSCA Facebook page
Related content
Related information, want to give your feedback about this page, thanks for your feedback.
We are happy to see that your experience was positive. Don't forget to share the pages you like with your friends and colleagues.
If you need to ask a question, please contact Europe direct .
- Frontiers in Surgery
- Thoracic Surgery
- Research Topics
Understanding the Impact of COVID-19 on Cardiothoracic Transplants and Transplant Patients
Total Downloads
Total Views and Downloads
About this Research Topic
The global escalation of post-acute sequelae of COVID-19, known as Long COVID, which affects approximately 65 million people worldwide, underscores the necessity for detailed research into its chronic effects, especially among cardiothoracic transplant recipients. Evidence suggests that long COVID could ...
Keywords : COVID-19, Heart, Lung, Ex Vivo Organ Perfusion, Cardiothoracic Transplantation
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines.
| Manuscript Summary | |
| Manuscript |
Participating Journals
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Medicine (Baltimore)
- v.99(43); 2020 Oct 23
The COVID-19 research landscape
Junhui wang.
a Institute of Medical Information, Chinese Academy of Medical Sciences
b Digital China Health Technologies Co. Ltd., Beijing, China.
Objectives:
The Coronavirus Disease 2019 (COVID-19) caused heavy burdens and brought tremendous challenges to global public health. This study aimed to investigate collaboration relationships, research topics, and research trends on COVID-19 using scientific literature.
COVID-19-related articles published from January 1 to July 1, 2020 were retrieved from PubMed database. A total of 27,370 articles were included. Excel 2010, Medical Text Indexer (MTI), VOSviewer, and D3.js were used to summarize bibliometric features.
The number of the COVID-19 research publications has been continuously increasing after its break. United States was the most productive and active country for COVID-19 research, with the largest number of publications and collaboration relationships. Huazhong University of Science and Technology from China was the most productive institute on the number of publications, and University of Toronto from Canada ranked as Top 1 institute for global research collaboration. Four key research topics were identified, of which the topic of epidemiology and public health interventions has gathered highest attentions. Topic of virus infection and immunity has been more focused during the early stage of COVID-19 outbreak compared with later stage. The topic popularity of clinical symptoms and diagnosis has been steady.
Conclusions:
Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
1. Introduction
A novel coronavirus emerged and caused a rapid spread of phenomena in Wuhan, China, at the end of 2019. In February 11, 2020, the World Health Organization named this disease Coronavirus Disease 2019 (COVID-19). [ 1 ] With the global spread of COVID-19, it threatened human lives, caused heavy burdens, and brought tremendous challenges to social development. To support the public health decision-making and scientific countermeasures implementation, researchers around the world were racing to study on the disease transmission, diagnostic tests, treatments, vaccines, among others. With the joint efforts of researchers and clinicians around the world, more and more COVID-19-related articles have been published and the outputs of scientific research are constantly emerging. As of July 1, 2020, PubMed has included 27,370 published articles on COVID-19.
State of the art literature review about COVID-19 demonstrated that most available literature-based studies could be basically divided into 2 kinds. The first kind is systematic reviews or meta-analyses. Most of them focused on a certain specific subfields of COVID-19 research, such as drug therapy, diagnostic methods, or clinical symptoms. For example, Alzghari et al [ 2 ] performed a systematic review to investigate the effect of Tocilizumab on COVID-19, and Zhu et al [ 3 ] systematically reviewed the CT imaging features of COVID-19 to provide reference for clinical practice. The second kind is the bibliometric analysis which uses quantitative analysis methods to describe literature in a particular research domain. However, some of the bibliometric analysis were targeting at coronavirus, not just severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), for the purpose of providing reference for COVID-19 research, and the time window was usually set for a long retrospective duration. [ 4 – 7 ] For example, Mao et al [ 7 ] analyzed coronavirus articles published from 2003 to 2020. Up to the investigation time of this study, there were limited number of bibliometric studies specific to COVID-19 and most of them were found and implemented at early stage of COVID-19 outbreak. [ 8 , 9 ] For example, Lou et al [ 8 ] executed a query in PubMed using keyword “COVID-19” and analyzed 183 related articles. Most of these previous literature-based studies of COVID-19 provided a specific review for COVID-19 research progresses or clinical observations; however, the description of a whole picture of COVID-19 scientific research using systematical methods was still insufficient.
Therefore, to answer who, what, where, and when questions of COVID-19 studies, we adopted a hybrid method that integrated multi-approaches, including bibliometrics, topic analysis, collaboration analysis, trends analysis, and visualization, to give a timely and systematic review of COVID-19 literatures. The analysis objectives include countries/regions, institutes, collaboration relationships, research topics, and research trends of COVID-19 studies.
2. Materials and methods
2.1. data source.
The data scope of this study is COVID-19-related articles published from January 1, to July 1, 2020. Since PubMed has served as the primary database for retrieving biomedical literature, it was selected as the only data source. [ 10 ] Ethical approval was not required because no human and animal subjects were enrolled.
2.2. Search strategy
The advanced search option was adopted, and the query “((novel coronavirus[Title/Abstract] OR COVID-19[Title/Abstract] OR 2019-nCov[Title/Abstract] OR SARS-Cov-2[Title/Abstract] OR COVID19[Title/Abstract] OR coronavirus disease 2019[Title/Abstract] OR coronavirus disease-19[Title/Abstract]) OR COVID-19[Supplementary Concept]) AND (“2020/01/01”: “2020/07/01”[dp])”was executed on July 1, 2020. In total, 27,370 COVID-19 articles were collected.
2.3. Data collection
All of the retrieved articles were downloaded and saved with PubMed default format. Microsoft Excel 2010 was used to pre-process the data and, in conjunction with Visual Basic for Applications (VBA), to extract analysis objects such as country/region names and institute names. The number of publications of a country is derived by counting the number of publications that contain at least one author's affiliation belongs to this country, and the first affiliation will be selected when an author has more than one affiliations.
2.4. Bibliometric and visualized analysis
MTI (National Library of Medicine, Bethesda, MD), [ 11 ] VOSviewer 1.6.15 (Leiden University, Leiden, Netherlands) [ 12 ] and D3.js (Mike Bostock, Observable, Inc., San Francisco, CA) [ 13 ] were used to carry out bibliometric and visual analysis of the publications. Since Medical Subject Headings (MeSH) represent much richer semantics that author-selected keywords, they were chosen as the object of topic analysis. MTI was used to extract MeSH terms from title and abstract of articles because newly created articles in PubMed will not be indexed with MeSH terms immediately. VOSviewer was used to generate collaborative network of countries/regions/institutes and co-occurrence network of MeSH terms. Finally, D3.js was used to visualize the internal hierarchy and the popularity trend of topics, which identified by MeSH terms co-occurrence clustering.
2.5. Analytical methods
Topic popularity was calculated by proportional frequency equation and tracked in a certain period of time window (10 days window) to identify the research trends. The equation of proportional frequency is as follows:

Where Dpro_t is the proportional frequency of the term in the t time window, D_t is the document frequency of the term, that is, the number of publications containing the term. DAll_t is the total number of publications and DAvg is the average number of publications on each time window. Topic popularity is measured by adding up proportional frequency of all the terms in this topic.
3.1. The Scale of COVID-19 publications
The number of COVID-19 research publications has been continuously increasing after its break. According to the growth trend from the view of global to country level, as shown in Figure Figure1, 1 , United States overtook China Mainland as the largest contributor in publishing COVID-19-related articles in early May 2020. As of July 1, 2020, United States had published 5949 (21.7% of the total) articles, and China Mainland had published 4080 (14.9% of the total) articles in total that are much higher than any of the other countries. The following Italy (10.7%) and UK (8.4%) were also prolific among the top 10 countries (Table (Table1). 1 ). In addition, China Mainland had the highest rate of domestic collaboration (79.4%), whereas Australia had the lowest (34.8%) among the top 10 productive countries.

The growth trend on number of publications about COVID-19 research.
The top 10 productive countries/regions that published COVID-19 research.

3.2. The collaborative network of countries/regions
Collaboration activities on country/region level were measured based on co-author analysis. As shown in Figure Figure2, 2 , there were 76 countries/regions involved in COVID-19 research collaboration which divided into 3 clusters.

The collaboration network on COVID-19 research across countries/regions.
Cluster 1 (blue color) mainly included United States, China Mainland, Canada, and Australia, which were all ranked as Top 10 productive countries. When measuring the collaboration activities, our study further disclosed that United States and China Mainland played the leading role of the COVID-19 research. These two countries had strong internal co-authorship relations, and at the same time had strong external co-authorship relations with other countries/regions. Cluster 2 (green color) was composed with 27 European countries that included UK, Italy, Germany, and France, among others. There were frequent internal collaboration activities among these European countries. In addition, Cluster 3 (red color) included India, Brazil, and other countries of Asia, Africa, and South America with a relatively low frequency of internal collaboration.
Furthermore, total link strength analysis showed that United States was the most active country with the highest number of collaboration relationships with other countries/regions. United States and China Mainland had the largest number of link strength compared with other countries, with a total of 439 collaboration papers. However, Chinese researchers had mostly co-authored with their domestic collaborators, only 20.6% of the studies were collaborated with international researchers outside China Mainland (Table (Table1 1 ).
3.3. The collaborative network of research institutes
The most productive institutes were located at United States, China Mainland, and Europe. There were 307 institutes that had published >10 articles. Table Table2 2 lists the number of publications and internal collaboration publications for top 10 productive institutes. Huazhong University of Science and Technology (523), Wuhan University (340), and University of California (300) were ranked as Top 3 productive institutes by number of publications. Besides, the BMJ editors published 193 latest news and comments about COVID-19 research with the highest rate of internal collaboration of 100%.
The top 10 productive institutes that published COVID-19 research.

Collaboration network among productive institutes was generated based on co-author analysis. Institutes were clearly separated into 5 clusters as shown in Figure Figure3. 3 . Cluster 1 (red color) included 96 institutes which were mostly universities and hospitals of United States, as well as 10 universities from Canada, among which University of Toronto ranked as Top 1 institute for global research collaboration with the largest number of total link strength. Besides, University of California and University of Washington were also the collaboration centers with large number of co-authored articles. The universities, hospitals, and research institutes came from China composed Cluster 2 (blue color), from which Huazhong University of Science and Technology and Wuhan University had the largest number of link strength compared with other institutes, with a total of 60 collaboration papers. Furthermore, >100 institutes from Europe composed Cluster 3 (green color) and Cluster 4 (yellow color), of which universities and hospitals from Italy composed Cluster 4 and the remaining institutes composed Cluster 3. According to co-author analysis on these 2 clusters, University College London and University of Oxford were most active on research collaboration with other institutes. In addition, it was interesting to observe that Cluster 5 (purple color) contributed a relatively small volume of publications but was a self-centered research community mainly composed with 8 universities from Iran.

The collaboration network on COVID-19 research across institutes.
3.4. The identified COVID-19 research topics
To achieve better understanding of what are the researcher's focuses and research progress of COVID-19 with its break timeline, MeSH terms of each article were selected as the observation objects to measure the research topics and topic trends. On the analysis of selected 2000 MeSH terms with their frequency above 10, a MeSH terms co-occurrence network with 584 high-frequency terms were generated, as shown in Figure Figure4. 4 . The network center nodes are COVID-19, severe acute respiratory syndrome coronavirus 2, and Coronavirus Infections. Four topics about COVID-19 research were obviously identified: epidemiology and public health interventions, virus infection and immunity, clinical symptoms and diagnosis, drug treatments, and clinical studies, as shown in Figure Figure5 5 .

The MeSH terms co-occurrence network on COVID-19 research.

The hierarchy of four identified COVID-19 topics.
3.4.1. Topic I: epidemiology and public health interventions
The research topic of epidemiology and public health interventions had gathered great attentions. It contained 281 of the 584 MeSH terms, indicating that the prevention and control of COVID-19 was the most concerned issue at all the stages of disease break. It mainly contained epidemic transmission dynamics, prevention and control measures and effect analysis at different regional levels (global, national, and urban), [ 14 , 15 ] epidemiological investigation, modeling, and trend prediction from the perspective of public health, [ 16 , 17 ] as well as various personal protective measures (Disinfection, Hand Hygiene, Masks, Personal Protective Equipment, Protective Devices), [ 18 , 19 ] and social prevention and control measures (Airway Management, Mass Screening, Social Distance, Social Isolation). [ 20 ] In addition, high attention had been paid to the psychological and mental state (Anxiety, Anxiety Disorders, Depression, Fear, Mental Disorders, Mental Health) of the general public, infected people, and medical workers. [ 21 ]
3.4.2. Topic II: virus infection and immunity
A total of 168 MeSH terms were included in this topic, which was mainly for the molecular biology and immunology studies of SARS-CoV-2 for the purpose of detection and prevention. Three subtopics of Topic II were identified based on content analysis. The first subtopic was the research on the pathogenesis of COVID-19 that included the replication process and infection mechanism of SARS-CoV-2 in human cells, with emphasis on the interaction between SARS-CoV-2 and biological enzymes (RNA-directed DNA polymerase, angiotensin-converting enzyme [ACE2], serine endopeptidases). [ 22 , 23 ] The second subtopic was the studies on the etiological detection methods of SARS-CoV-2 and the most important methods involved were real-time polymerase chain reaction and reverse transcriptase polymerase chain reaction (PCR). [ 24 , 25 ] In addition, COVID-19 vaccine development with the aim of inducing immune response composed the third subtopic. [ 26 , 27 ]
3.4.3. Topic III: clinical symptoms and diagnosis
A total of 111 MeSH terms were included in Topic III, which mainly covered clinical symptoms of COVID-19 patients and various testing methods used for diagnosis. The clinical symptoms (or complications) of COVID-19 mentioned in the literature mainly included: abdominal pain, cough, diarrhea, dyspnea, fatigue, fever, headache, leukopenia, lymphopenia, myalgia, nausea, pharyngitis, pleural effusion, pneumonia, pulmonary embolism, respiratory distress syndrome, respiratory insufficiency, vomiting, among others. [ 28 , 29 ] The diagnostic methods, mostly discussed in the literature, were routine blood tests (alanine transaminase, aspartate aminotransferases, biomarkers, C-reactive protein, leukocyte count, l -lactate dehydrogenase, lymphocyte count, neutrophils, platelet count) and imaging examinations (radiography, tomography, x-rays). [ 30 ]
3.4.4. Topic IV: drug treatments and clinical studies
Topic IV contained 24 MeSH terms, which was the smallest topic. The research content in this topic was mainly in vivo and in vitro trials of multiple drugs and their combinations for the purpose of treating COVID-19. The studied drugs involved antibacterial/antiviral drugs (azithromycin, favipiravir, lopinavir, remdesivir, ribavirin, ritonavir), antimalarials, and rheumatoid arthritis drugs (chloroquine, hydroxychloroquine, tocilizumab) among others. Because of the difference of clinical endpoint and experimental design, the trials results obtained so far are not consistent. For example, some researchers conclude that remdesivir can be used as potent drugs against COVID-19 [ 31 ] ; however, some studies show that remdesivir cannot significantly improve the symptoms of patients with severe COVID-19. [ 32 ] Chloroquine and hydroxychloroquine are in a similar situation to remdesivir. [ 33 , 34 ] Therefore, there is still no widely accepted standard on specific drugs or the best drug treatment options of COVID-19. [ 35 – 37 ]
3.5. Topic popularities and evolvements about COVID-19 research
Topic popularity of the above 4 COVID-19 topics was measured by using proportional frequency equation in Section 2, and the measured results, as shown in Figure Figure6, 6 , were consistent with manually validation results by reviewing literature. According to trend analysis, the topic of epidemiology and public health interventions has gathered great attentions and continuously with high popularity. The characteristics of SARS-CoV-2, such as biological structure, genetic sequence, and infection mechanism, have been well studied, and beyond this, consensus has been reached on COVID-19 clinical symptoms and diagnostic methods.

Trends of topic popularity.
On the topic tracking analysis of epidemiology and public health interventions, we found that most of the early studies and reports were mainly focus on China's epidemic prevention and control. [ 38 , 39 ] By implementing a series of preventive control and medical treatment measures, the pandemic in China had been effectively contained, but the number of confirmed cases outside China continued to increase, as did the corresponding research on epidemiology and public health interventions, which was consistent with the continuously high popularity trending curve of this topic (blue curve), as displayed in Figure Figure6 6 .
For virus infection and immunity study, the topic popularity decreased since early of February 2020. As studying the etiological characteristics of a novel virus, such as biological structure, genetic sequence, and infection mechanism, is the key to pandemic prevention and control, the trend curve of Topic II was in the highest position in the pre-outbreak period (January 2020). With the joint efforts of scientists around the world, substantial progress had been achieved in the understanding of SARS-CoV-2. For example, the genetic sequencing of SARS-CoV-2 was performed by Chinese scientists on January 7, 2020 and the results were timely shared with the WHO on January 12, 2020. Furthermore, the infection mechanism of SARS-CoV-2, especially its relationship with ACE2 was identified, and specific diagnostic PCR tests were produced. [ 40 , 41 ] The above achievements were mainly completed in January and February 2020, starting from February, the trend curve of Topic II gradually declined. However, the curve will remain at a high level because more and more attentions have been paid to vaccine-related research. According to literature reports, there are more than 100 candidate vaccine projects targeting COVID-19 worldwide, and some of them have entered clinical trials. [ 42 , 43 ]
With the continuous increase of confirmed and treated cases, clinicians achieved deeper understanding about COVID-19. Since March 2020, there has been a global consensus on the symptoms and diagnostic criteria for COVID-19. [ 28 , 44 ] In addition, the seventh and final edition of “Diagnosis and Treatment Protocol of COVID-19,” issued by the National Health Commission of the PRC, was also released on March 3, 2020. [ 45 ] As a result, the trend curve of Topic III starts to smooth out since March 2020 (Fig. (Fig.6 6 ).
Although lopinavir/ritonavir was recommended as antiviral drug by the first edition of “Diagnosis and Treatment Protocol of COVID-19” on January 16, 2020 at the beginning of the pandemic, the widespread interest in using antiviral drugs to treat COVID-19 began with a report of the first diagnosed patient who benefit from remdesivir in United States, which was published in NEJM on January 31, 2020. [ 46 ] Therefore, the trend curve of Topic IV in Figure Figure6 6 has risen slightly since February 2020. However, the minimal topic size and low trend curve suggest that drug therapy remains the weak point in the response to COVID-19.
4. Discussion and conclusion
The number of COVID-19 publications has been growing dramatically since March 2020. According to our search strategy, as of the submission of this manuscript (July 13, 2020), the number of COVID-19 publications has exceeded 30,000. Given that COVID-19 pandemic has not been well contained at the global level, relevant research will continue to be carried out and the number of publications will increase accordingly. The methodology in this study can be easily implemented to analyze the future research status of COVID-19, or even applied to other fields.
Although United States and China were the most productive countries, they were not in the identical situation. Since the initial outbreak was in China, Chinese scholars quickly carried out a series of studies and published numerous articles in the early stages of the epidemic. However, Chinese scholars tend to collaborate with domestic scholars rather than aboard. Unlike China, United States has seen a significant increase in the number of publications since April 2020, and has quickly occupied the highest level of participation in global collaboration due to its strong scientific research strength and influence.
Collaboration at the institutional level has obvious geographical characteristics, especially the frequent internal collaborations among institutes located in China, as well as United States. For example, Huazhong University of Science and Technology and Wuhan University, which ranked first and second by the number of publications, co-authored a total of 60 articles, making up the most productive institute pair. Both universities are located in Wuhan and their affiliated hospitals, such as Tongji Hospital, Union Hospital, and Renmin Hospital, are major hospitals for treating COVID-19 patients. The front-line clinical medical workers in those hospitals have conducted a lot of research on virus detection, clinical diagnosis and treatment while fighting against the epidemic.
COVID-19 research topics are continuously evolving with their publication timeline, measuring these changes will help researchers and scientific policy makers understanding the status of COVID-19 research. As indicated by the trend curves of topic popularity, the prevention and control of COVID-19 remains the most important issue at present, and drug therapy remains the weak point in the response to COVID-19. In addition, more support should be given to vaccine research and development, because vaccines are the ultimate solution to the epidemic. [ 5 ]
This study provided an overall investigation of COVID-19 scientific progresses using multiple qualitative and quantitative analysis methods. The collaboration status of COVID-19 research at national and institutional levels was disclosed and 4 topics (epidemiology and public health interventions, virus infection and immunity, clinical symptoms and diagnosis, drug treatments, and clinical studies) were identified and interpreted. Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
4.1. Strengths and limitations
Publications on COVID-19 research were retrieved from PubMed, and the collaboration status and research trends of COVID-19 were measured via bibliometric and visualized analysis, which was considered to be relatively objective and comprehensive. Moreover, well curated MeSH terms were used as the object of topic analysis in this study, compared with author-selected keywords which were usually chosen by existing COVID-19-related bibliometric analysis. [ 4 – 7 ] Due to the limited number and randomness of author-selected keywords, the derived results, especially the co-occurrence analysis results, cannot reflect the real status of the COVID-19 research. Our MeSH terms-based methodology could better disclose the research topics and trends of COVID-19. However, limitations also exist in our research. On the one hand, PubMed was selected as the only data source, so some articles only indexed in other databases such as Web of Science and Scopus might be left out. On the other hand, for the sparisity reason of citation network of published COVID-19 articles, citation analysis has not been adopted in this study. In the future, studies based on citation analysis, such as identification of influential authors and highly-cited articles, will be conducted and included in our further analysis.
Author contributions
Conceptualization, N.H.; Data curation, J.W.; Software, J.W. and N.H.; Visualization, J.W. and N.H.; Writing—original draft, J.W. and N.H.; Writing—review & editing, J.W. and N.H. All authors have read and agreed to the published version of the manuscript.
Conceptualization: Na Hong.
Data curation: Junhui Wang.
Software: Junhui Wang, Na Hong.
Visualization: Junhui Wang, Na Hong.
Writing – original draft: Junhui Wang, Na Hong.
Writing – review & editing: Junhui Wang, Na Hong.
Abbreviations: ACE2 = Angiotensin Converting Enzyme 2, COVID-19 = Coronavirus Disease 2019, MeSH = Medical Subject Headings, MTI = Medical Text Indexer, SARS-COV-2 = severe acute respiratory syndrome coronavirus 2, VBA = Visual Basic for Applications.
How to cite this article: Wang J, Hong N. The COVID-19 research landscape: Measuring topics and collaborations using scientific literature. Medicine . 2020;99:43(e22849).
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE PODCAST
- 17 December 2020
Coronapod: The big COVID research papers of 2020
- Benjamin Thompson ,
- Noah Baker &
- Traci Watson
You can also search for this author in PubMed Google Scholar
Benjamin Thompson, Noah Baker and Traci Watson discuss some of 2020's most significant coronavirus research papers.
In the final Coronapod of 2020, we dive into the scientific literature to reflect on the COVID-19 pandemic. Researchers have discovered so much about SARS-CoV-2 – information that has been vital for public health responses and the rapid development of effective vaccines. But we also look forward to 2021, and the critical questions that remain to be answered about the pandemic.
Papers discussed
A Novel Coronavirus from Patients with Pneumonia in China, 2019 - New England Journal of Medicine, 24 January
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China - The Lancet , 24 January
A pneumonia outbreak associated with a new coronavirus of probable bat origin - Nature , 3 February
A new coronavirus associated with human respiratory disease in China - Nature , 3 February
Temporal dynamics in viral shedding and transmissibility of COVID-19 - Nature Medicine , 15 April
Spread of SARS-CoV-2 in the Icelandic Population - New England Journal of Medicine , 11 June
High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington, March 2020 - Morbidity & Mortality Weekly Report , 15 August
Respiratory virus shedding in exhaled breath and efficacy of face masks - Nature Medicine , 3 April
Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1 - New England Journal of Medicine , 13 April
Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period - Science , 22 May
Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe - Nature, 8 June
The effect of large-scale anti-contagion policies on the COVID-19 pandemic - Nature , 8 June
Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis - The Lancet, 20 June
A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19 - New England Journal of Medicine , 3 June
Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19 - JAMA , 2 September
Immunological memory to SARS-CoV-2 assessed for greater than six months after infection - bioRxiv, 16 November
Coronavirus Disease 2019 (COVID-19) Re-infection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing - Clinical Infectious Diseases , 25 August
Nature’s COVID research updates – summarising key coronavirus papers as they appear
Never miss an episode: Subscribe to the Nature Podcast on Apple Podcasts , Google Podcasts , Spotify or your favourite podcast app. Head here for the Nature Podcast RSS feed .
doi: https://doi.org/10.1038/d41586-020-03609-2
Related Articles
- Public health
First encounter with SARS-CoV-2: immune portraits of COVID susceptibility
News & Views 19 JUN 24
What causes long COVID? Case builds for rogue antibodies
News 13 JUN 24

‘Preposterous’: Anthony Fauci denies cover-up of COVID origins during tense hearing
News 03 JUN 24

Combined COVID-flu vaccines are coming: Moderna jab clears major test
News 28 JUN 24

‘Vindicated’: Embattled misinformation researchers celebrate key US Supreme Court decision
News 26 JUN 24
Establish a global day to tackle postpartum haemorrhage
Correspondence 25 JUN 24
‘Epigenome editor’ silences gene that causes deadly brain disorders
News 27 JUN 24

How blockbuster obesity drugs create a full feeling — even before one bite of food
Ketamine for depression: slow-release pills could make treatment more accessible
PostDoc Researcher, Magnetic Recording Materials Group, National Institute for Materials Science
Starting date would be after January 2025, but it is negotiable.
National Institute for Materials Science
Tenure-Track/Tenured Faculty Positions
Tenure-Track/Tenured Faculty Positions in the fields of energy and resources.
Suzhou, Jiangsu, China
School of Sustainable Energy and Resources at Nanjing University
Postdoctoral Associate- Statistical Genetics
Houston, Texas (US)
Baylor College of Medicine (BCM)
Senior Research Associate (Single Cell/Transcriptomics Senior Bioinformatician)
Metabolic Research Laboratories at the Clinical School, University of Cambridge are recruiting 3 senior bioinformatician specialists to create a dynam
Cambridge, Cambridgeshire (GB)
University of Cambridge
Cancer Biology Postdoctoral Fellow
Tampa, Florida
Moffitt Cancer Center
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
The COVID-19 research landscape
Measuring topics and collaborations using scientific literature.
Editor(s): Mittal., Vinay
a Institute of Medical Information, Chinese Academy of Medical Sciences
b Digital China Health Technologies Co. Ltd., Beijing, China.
∗Correspondence: Na Hong, Digital China Health Technologies Co. Ltd., Beijing 100080, China (e-mail: [email protected] ).
Abbreviations: ACE2 = Angiotensin Converting Enzyme 2, COVID-19 = Coronavirus Disease 2019, MeSH = Medical Subject Headings, MTI = Medical Text Indexer, SARS-COV-2 = severe acute respiratory syndrome coronavirus 2, VBA = Visual Basic for Applications.
How to cite this article: Wang J, Hong N. The COVID-19 research landscape: Measuring topics and collaborations using scientific literature. Medicine . 2020;99:43(e22849).
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial License 4.0 (CCBY-NC), where it is permissible to download, share, remix, transform, and buildup the work provided it is properly cited. The work cannot be used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc/4.0
Objectives:
The Coronavirus Disease 2019 (COVID-19) caused heavy burdens and brought tremendous challenges to global public health. This study aimed to investigate collaboration relationships, research topics, and research trends on COVID-19 using scientific literature.
Method:
COVID-19-related articles published from January 1 to July 1, 2020 were retrieved from PubMed database. A total of 27,370 articles were included. Excel 2010, Medical Text Indexer (MTI), VOSviewer, and D3.js were used to summarize bibliometric features.
Results:
The number of the COVID-19 research publications has been continuously increasing after its break. United States was the most productive and active country for COVID-19 research, with the largest number of publications and collaboration relationships. Huazhong University of Science and Technology from China was the most productive institute on the number of publications, and University of Toronto from Canada ranked as Top 1 institute for global research collaboration. Four key research topics were identified, of which the topic of epidemiology and public health interventions has gathered highest attentions. Topic of virus infection and immunity has been more focused during the early stage of COVID-19 outbreak compared with later stage. The topic popularity of clinical symptoms and diagnosis has been steady.
Conclusions:
Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
1 Introduction
A novel coronavirus emerged and caused a rapid spread of phenomena in Wuhan, China, at the end of 2019. In February 11, 2020, the World Health Organization named this disease Coronavirus Disease 2019 (COVID-19). [1] With the global spread of COVID-19, it threatened human lives, caused heavy burdens, and brought tremendous challenges to social development. To support the public health decision-making and scientific countermeasures implementation, researchers around the world were racing to study on the disease transmission, diagnostic tests, treatments, vaccines, among others. With the joint efforts of researchers and clinicians around the world, more and more COVID-19-related articles have been published and the outputs of scientific research are constantly emerging. As of July 1, 2020, PubMed has included 27,370 published articles on COVID-19.
State of the art literature review about COVID-19 demonstrated that most available literature-based studies could be basically divided into 2 kinds. The first kind is systematic reviews or meta-analyses. Most of them focused on a certain specific subfields of COVID-19 research, such as drug therapy, diagnostic methods, or clinical symptoms. For example, Alzghari et al [2] performed a systematic review to investigate the effect of Tocilizumab on COVID-19, and Zhu et al [3] systematically reviewed the CT imaging features of COVID-19 to provide reference for clinical practice. The second kind is the bibliometric analysis which uses quantitative analysis methods to describe literature in a particular research domain. However, some of the bibliometric analysis were targeting at coronavirus, not just severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), for the purpose of providing reference for COVID-19 research, and the time window was usually set for a long retrospective duration. [4–7] For example, Mao et al [7] analyzed coronavirus articles published from 2003 to 2020. Up to the investigation time of this study, there were limited number of bibliometric studies specific to COVID-19 and most of them were found and implemented at early stage of COVID-19 outbreak. [8,9] For example, Lou et al [8] executed a query in PubMed using keyword “COVID-19” and analyzed 183 related articles. Most of these previous literature-based studies of COVID-19 provided a specific review for COVID-19 research progresses or clinical observations; however, the description of a whole picture of COVID-19 scientific research using systematical methods was still insufficient.
Therefore, to answer who, what, where, and when questions of COVID-19 studies, we adopted a hybrid method that integrated multi-approaches, including bibliometrics, topic analysis, collaboration analysis, trends analysis, and visualization, to give a timely and systematic review of COVID-19 literatures. The analysis objectives include countries/regions, institutes, collaboration relationships, research topics, and research trends of COVID-19 studies.
2 Materials and methods
2.1 data source.
The data scope of this study is COVID-19-related articles published from January 1, to July 1, 2020. Since PubMed has served as the primary database for retrieving biomedical literature, it was selected as the only data source. [10] Ethical approval was not required because no human and animal subjects were enrolled.
2.2 Search strategy
The advanced search option was adopted, and the query “((novel coronavirus[Title/Abstract] OR COVID-19[Title/Abstract] OR 2019-nCov[Title/Abstract] OR SARS-Cov-2[Title/Abstract] OR COVID19[Title/Abstract] OR coronavirus disease 2019[Title/Abstract] OR coronavirus disease-19[Title/Abstract]) OR COVID-19[Supplementary Concept]) AND (“2020/01/01”: “2020/07/01”[dp])”was executed on July 1, 2020. In total, 27,370 COVID-19 articles were collected.
2.3 Data collection
All of the retrieved articles were downloaded and saved with PubMed default format. Microsoft Excel 2010 was used to pre-process the data and, in conjunction with Visual Basic for Applications (VBA), to extract analysis objects such as country/region names and institute names. The number of publications of a country is derived by counting the number of publications that contain at least one author's affiliation belongs to this country, and the first affiliation will be selected when an author has more than one affiliations.
2.4 Bibliometric and visualized analysis
MTI (National Library of Medicine, Bethesda, MD), [11] VOSviewer 1.6.15 (Leiden University, Leiden, Netherlands) [12] and D3.js (Mike Bostock, Observable, Inc., San Francisco, CA) [13] were used to carry out bibliometric and visual analysis of the publications. Since Medical Subject Headings (MeSH) represent much richer semantics that author-selected keywords, they were chosen as the object of topic analysis. MTI was used to extract MeSH terms from title and abstract of articles because newly created articles in PubMed will not be indexed with MeSH terms immediately. VOSviewer was used to generate collaborative network of countries/regions/institutes and co-occurrence network of MeSH terms. Finally, D3.js was used to visualize the internal hierarchy and the popularity trend of topics, which identified by MeSH terms co-occurrence clustering.
2.5 Analytical methods

Where Dpro_t is the proportional frequency of the term in the t time window, D_t is the document frequency of the term, that is, the number of publications containing the term. DAll_t is the total number of publications and DAvg is the average number of publications on each time window. Topic popularity is measured by adding up proportional frequency of all the terms in this topic.
3.1 The Scale of COVID-19 publications
The number of COVID-19 research publications has been continuously increasing after its break. According to the growth trend from the view of global to country level, as shown in Figure 1 , United States overtook China Mainland as the largest contributor in publishing COVID-19-related articles in early May 2020. As of July 1, 2020, United States had published 5949 (21.7% of the total) articles, and China Mainland had published 4080 (14.9% of the total) articles in total that are much higher than any of the other countries. The following Italy (10.7%) and UK (8.4%) were also prolific among the top 10 countries ( Table 1 ). In addition, China Mainland had the highest rate of domestic collaboration (79.4%), whereas Australia had the lowest (34.8%) among the top 10 productive countries.

3.2 The collaborative network of countries/regions
Collaboration activities on country/region level were measured based on co-author analysis. As shown in Figure 2 , there were 76 countries/regions involved in COVID-19 research collaboration which divided into 3 clusters.

Cluster 1 (blue color) mainly included United States, China Mainland, Canada, and Australia, which were all ranked as Top 10 productive countries. When measuring the collaboration activities, our study further disclosed that United States and China Mainland played the leading role of the COVID-19 research. These two countries had strong internal co-authorship relations, and at the same time had strong external co-authorship relations with other countries/regions. Cluster 2 (green color) was composed with 27 European countries that included UK, Italy, Germany, and France, among others. There were frequent internal collaboration activities among these European countries. In addition, Cluster 3 (red color) included India, Brazil, and other countries of Asia, Africa, and South America with a relatively low frequency of internal collaboration.
Furthermore, total link strength analysis showed that United States was the most active country with the highest number of collaboration relationships with other countries/regions. United States and China Mainland had the largest number of link strength compared with other countries, with a total of 439 collaboration papers. However, Chinese researchers had mostly co-authored with their domestic collaborators, only 20.6% of the studies were collaborated with international researchers outside China Mainland ( Table 1 ).
3.3 The collaborative network of research institutes
The most productive institutes were located at United States, China Mainland, and Europe. There were 307 institutes that had published >10 articles. Table 2 lists the number of publications and internal collaboration publications for top 10 productive institutes. Huazhong University of Science and Technology (523), Wuhan University (340), and University of California (300) were ranked as Top 3 productive institutes by number of publications. Besides, the BMJ editors published 193 latest news and comments about COVID-19 research with the highest rate of internal collaboration of 100%.

Collaboration network among productive institutes was generated based on co-author analysis. Institutes were clearly separated into 5 clusters as shown in Figure 3 . Cluster 1 (red color) included 96 institutes which were mostly universities and hospitals of United States, as well as 10 universities from Canada, among which University of Toronto ranked as Top 1 institute for global research collaboration with the largest number of total link strength. Besides, University of California and University of Washington were also the collaboration centers with large number of co-authored articles. The universities, hospitals, and research institutes came from China composed Cluster 2 (blue color), from which Huazhong University of Science and Technology and Wuhan University had the largest number of link strength compared with other institutes, with a total of 60 collaboration papers. Furthermore, >100 institutes from Europe composed Cluster 3 (green color) and Cluster 4 (yellow color), of which universities and hospitals from Italy composed Cluster 4 and the remaining institutes composed Cluster 3. According to co-author analysis on these 2 clusters, University College London and University of Oxford were most active on research collaboration with other institutes. In addition, it was interesting to observe that Cluster 5 (purple color) contributed a relatively small volume of publications but was a self-centered research community mainly composed with 8 universities from Iran.

3.4 The identified COVID-19 research topics
To achieve better understanding of what are the researcher's focuses and research progress of COVID-19 with its break timeline, MeSH terms of each article were selected as the observation objects to measure the research topics and topic trends. On the analysis of selected 2000 MeSH terms with their frequency above 10, a MeSH terms co-occurrence network with 584 high-frequency terms were generated, as shown in Figure 4 . The network center nodes are COVID-19, severe acute respiratory syndrome coronavirus 2, and Coronavirus Infections. Four topics about COVID-19 research were obviously identified: epidemiology and public health interventions, virus infection and immunity, clinical symptoms and diagnosis, drug treatments, and clinical studies, as shown in Figure 5 .

3.4.1 Topic I: epidemiology and public health interventions
The research topic of epidemiology and public health interventions had gathered great attentions. It contained 281 of the 584 MeSH terms, indicating that the prevention and control of COVID-19 was the most concerned issue at all the stages of disease break. It mainly contained epidemic transmission dynamics, prevention and control measures and effect analysis at different regional levels (global, national, and urban), [14,15] epidemiological investigation, modeling, and trend prediction from the perspective of public health, [16,17] as well as various personal protective measures (Disinfection, Hand Hygiene, Masks, Personal Protective Equipment, Protective Devices), [18,19] and social prevention and control measures (Airway Management, Mass Screening, Social Distance, Social Isolation). [20] In addition, high attention had been paid to the psychological and mental state (Anxiety, Anxiety Disorders, Depression, Fear, Mental Disorders, Mental Health) of the general public, infected people, and medical workers. [21]
3.4.2 Topic II: virus infection and immunity
A total of 168 MeSH terms were included in this topic, which was mainly for the molecular biology and immunology studies of SARS-CoV-2 for the purpose of detection and prevention. Three subtopics of Topic II were identified based on content analysis. The first subtopic was the research on the pathogenesis of COVID-19 that included the replication process and infection mechanism of SARS-CoV-2 in human cells, with emphasis on the interaction between SARS-CoV-2 and biological enzymes (RNA-directed DNA polymerase, angiotensin-converting enzyme [ACE2], serine endopeptidases). [22,23] The second subtopic was the studies on the etiological detection methods of SARS-CoV-2 and the most important methods involved were real-time polymerase chain reaction and reverse transcriptase polymerase chain reaction (PCR). [24,25] In addition, COVID-19 vaccine development with the aim of inducing immune response composed the third subtopic. [26,27]
3.4.3 Topic III: clinical symptoms and diagnosis
A total of 111 MeSH terms were included in Topic III, which mainly covered clinical symptoms of COVID-19 patients and various testing methods used for diagnosis. The clinical symptoms (or complications) of COVID-19 mentioned in the literature mainly included: abdominal pain, cough, diarrhea, dyspnea, fatigue, fever, headache, leukopenia, lymphopenia, myalgia, nausea, pharyngitis, pleural effusion, pneumonia, pulmonary embolism, respiratory distress syndrome, respiratory insufficiency, vomiting, among others. [28,29] The diagnostic methods, mostly discussed in the literature, were routine blood tests (alanine transaminase, aspartate aminotransferases, biomarkers, C-reactive protein, leukocyte count, l -lactate dehydrogenase, lymphocyte count, neutrophils, platelet count) and imaging examinations (radiography, tomography, x-rays). [30]
3.4.4 Topic IV: drug treatments and clinical studies
Topic IV contained 24 MeSH terms, which was the smallest topic. The research content in this topic was mainly in vivo and in vitro trials of multiple drugs and their combinations for the purpose of treating COVID-19. The studied drugs involved antibacterial/antiviral drugs (azithromycin, favipiravir, lopinavir, remdesivir, ribavirin, ritonavir), antimalarials, and rheumatoid arthritis drugs (chloroquine, hydroxychloroquine, tocilizumab) among others. Because of the difference of clinical endpoint and experimental design, the trials results obtained so far are not consistent. For example, some researchers conclude that remdesivir can be used as potent drugs against COVID-19 [31] ; however, some studies show that remdesivir cannot significantly improve the symptoms of patients with severe COVID-19. [32] Chloroquine and hydroxychloroquine are in a similar situation to remdesivir. [33,34] Therefore, there is still no widely accepted standard on specific drugs or the best drug treatment options of COVID-19. [35–37]
3.5 Topic popularities and evolvements about COVID-19 research
Topic popularity of the above 4 COVID-19 topics was measured by using proportional frequency equation in Section 2, and the measured results, as shown in Figure 6 , were consistent with manually validation results by reviewing literature. According to trend analysis, the topic of epidemiology and public health interventions has gathered great attentions and continuously with high popularity. The characteristics of SARS-CoV-2, such as biological structure, genetic sequence, and infection mechanism, have been well studied, and beyond this, consensus has been reached on COVID-19 clinical symptoms and diagnostic methods.

On the topic tracking analysis of epidemiology and public health interventions, we found that most of the early studies and reports were mainly focus on China's epidemic prevention and control. [38,39] By implementing a series of preventive control and medical treatment measures, the pandemic in China had been effectively contained, but the number of confirmed cases outside China continued to increase, as did the corresponding research on epidemiology and public health interventions, which was consistent with the continuously high popularity trending curve of this topic (blue curve), as displayed in Figure 6 .
For virus infection and immunity study, the topic popularity decreased since early of February 2020. As studying the etiological characteristics of a novel virus, such as biological structure, genetic sequence, and infection mechanism, is the key to pandemic prevention and control, the trend curve of Topic II was in the highest position in the pre-outbreak period (January 2020). With the joint efforts of scientists around the world, substantial progress had been achieved in the understanding of SARS-CoV-2. For example, the genetic sequencing of SARS-CoV-2 was performed by Chinese scientists on January 7, 2020 and the results were timely shared with the WHO on January 12, 2020. Furthermore, the infection mechanism of SARS-CoV-2, especially its relationship with ACE2 was identified, and specific diagnostic PCR tests were produced. [40,41] The above achievements were mainly completed in January and February 2020, starting from February, the trend curve of Topic II gradually declined. However, the curve will remain at a high level because more and more attentions have been paid to vaccine-related research. According to literature reports, there are more than 100 candidate vaccine projects targeting COVID-19 worldwide, and some of them have entered clinical trials. [42,43]
With the continuous increase of confirmed and treated cases, clinicians achieved deeper understanding about COVID-19. Since March 2020, there has been a global consensus on the symptoms and diagnostic criteria for COVID-19. [28,44] In addition, the seventh and final edition of “Diagnosis and Treatment Protocol of COVID-19,” issued by the National Health Commission of the PRC, was also released on March 3, 2020. [45] As a result, the trend curve of Topic III starts to smooth out since March 2020 ( Fig. 6 ).
Although lopinavir/ritonavir was recommended as antiviral drug by the first edition of “Diagnosis and Treatment Protocol of COVID-19” on January 16, 2020 at the beginning of the pandemic, the widespread interest in using antiviral drugs to treat COVID-19 began with a report of the first diagnosed patient who benefit from remdesivir in United States, which was published in NEJM on January 31, 2020. [46] Therefore, the trend curve of Topic IV in Figure 6 has risen slightly since February 2020. However, the minimal topic size and low trend curve suggest that drug therapy remains the weak point in the response to COVID-19.
4 Discussion and conclusion
The number of COVID-19 publications has been growing dramatically since March 2020. According to our search strategy, as of the submission of this manuscript (July 13, 2020), the number of COVID-19 publications has exceeded 30,000. Given that COVID-19 pandemic has not been well contained at the global level, relevant research will continue to be carried out and the number of publications will increase accordingly. The methodology in this study can be easily implemented to analyze the future research status of COVID-19, or even applied to other fields.
Although United States and China were the most productive countries, they were not in the identical situation. Since the initial outbreak was in China, Chinese scholars quickly carried out a series of studies and published numerous articles in the early stages of the epidemic. However, Chinese scholars tend to collaborate with domestic scholars rather than aboard. Unlike China, United States has seen a significant increase in the number of publications since April 2020, and has quickly occupied the highest level of participation in global collaboration due to its strong scientific research strength and influence.
Collaboration at the institutional level has obvious geographical characteristics, especially the frequent internal collaborations among institutes located in China, as well as United States. For example, Huazhong University of Science and Technology and Wuhan University, which ranked first and second by the number of publications, co-authored a total of 60 articles, making up the most productive institute pair. Both universities are located in Wuhan and their affiliated hospitals, such as Tongji Hospital, Union Hospital, and Renmin Hospital, are major hospitals for treating COVID-19 patients. The front-line clinical medical workers in those hospitals have conducted a lot of research on virus detection, clinical diagnosis and treatment while fighting against the epidemic.
COVID-19 research topics are continuously evolving with their publication timeline, measuring these changes will help researchers and scientific policy makers understanding the status of COVID-19 research. As indicated by the trend curves of topic popularity, the prevention and control of COVID-19 remains the most important issue at present, and drug therapy remains the weak point in the response to COVID-19. In addition, more support should be given to vaccine research and development, because vaccines are the ultimate solution to the epidemic. [5]
This study provided an overall investigation of COVID-19 scientific progresses using multiple qualitative and quantitative analysis methods. The collaboration status of COVID-19 research at national and institutional levels was disclosed and 4 topics (epidemiology and public health interventions, virus infection and immunity, clinical symptoms and diagnosis, drug treatments, and clinical studies) were identified and interpreted. Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
4.1 Strengths and limitations
Publications on COVID-19 research were retrieved from PubMed, and the collaboration status and research trends of COVID-19 were measured via bibliometric and visualized analysis, which was considered to be relatively objective and comprehensive. Moreover, well curated MeSH terms were used as the object of topic analysis in this study, compared with author-selected keywords which were usually chosen by existing COVID-19-related bibliometric analysis. [4–7] Due to the limited number and randomness of author-selected keywords, the derived results, especially the co-occurrence analysis results, cannot reflect the real status of the COVID-19 research. Our MeSH terms-based methodology could better disclose the research topics and trends of COVID-19. However, limitations also exist in our research. On the one hand, PubMed was selected as the only data source, so some articles only indexed in other databases such as Web of Science and Scopus might be left out. On the other hand, for the sparisity reason of citation network of published COVID-19 articles, citation analysis has not been adopted in this study. In the future, studies based on citation analysis, such as identification of influential authors and highly-cited articles, will be conducted and included in our further analysis.
Author contributions
Conceptualization, N.H.; Data curation, J.W.; Software, J.W. and N.H.; Visualization, J.W. and N.H.; Writing—original draft, J.W. and N.H.; Writing—review & editing, J.W. and N.H. All authors have read and agreed to the published version of the manuscript.
Conceptualization: Na Hong.
Data curation: Junhui Wang.
Software: Junhui Wang, Na Hong.
Visualization: Junhui Wang, Na Hong.
Writing – original draft: Junhui Wang, Na Hong.
Writing – review & editing: Junhui Wang, Na Hong.
- Cited Here |
- Google Scholar
bibliometrics; collaboration analysis; COVID-19; topic analysis; trends analysis
- + Favorites
- View in Gallery

The Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine (2021)
Chapter: 8 major findings and research questions, 8 major findings and research questions, introduction.
The COVID-19 pandemic, which began in late 2019, created unprecedented global disruption and infused a significant level of uncertainty into the lives of individuals, both personally and professionally, around the world throughout 2020. The significant effect on vulnerable populations, such as essential workers and the elderly, is well documented, as is the devastating effect the COVID-19 pandemic had on the economy, particularly brick-and-mortar retail and hospitality and food services. Concurrently, the deaths of unarmed Black people at the hands of law enforcement officers created a heightened awareness of the persistence of structural injustices in U.S. society.
Against the backdrop of this public health crisis, economic upheaval, and amplified social consciousness, an ad hoc committee was appointed to review the potential effects of the COVID-19 pandemic on women in academic science, technology, engineering, mathematics, and medicine (STEMM) during 2020. The committee’s work built on the National Academies of Sciences, Engineering, and Medicine report Promising Practices for Addressing the Underrepresentation of Women in Science, Engineering, and Medicine: Opening Doors (the Promising Practices report), which presents evidence-based recommendations to address the well-established structural barriers that impede the advancement of women in STEMM. However, the committee recognized that none of the actions identified in the Promising Practices report were conceived within the context of a pandemic, an economic downturn, or the emergence of national protests against structural racism. The representation and vitality of academic women in STEMM had already warranted national attention prior to these events, and the COVID-19
pandemic appeared to represent an additional risk to the fragile progress that women had made in some STEMM disciplines. Furthermore, the future will almost certainly hold additional, unforeseen disruptions, which underscores the importance of the committee’s work.
In times of stress, there is a risk that the divide will deepen between those who already have advantages and those who do not. In academia, senior and tenured academics are more likely to have an established reputation, a stable salary commitment, and power within the academic system. They are more likely, before the COVID-19 pandemic began, to have established professional networks, generated data that can be used to write papers, and achieved financial and job security. While those who have these advantages may benefit from a level of stability relative to others during stressful times, those who were previously systemically disadvantaged are more likely to experience additional strain and instability.
As this report has documented, during 2020 the COVID-19 pandemic had overall negative effects on women in academic STEMM in areas such productivity, boundary setting and boundary control, networking and community building, burnout rates, and mental well-being. The excessive expectations of caregiving that often fall on the shoulders of women cut across career timeline and rank (e.g., graduate student, postdoctoral scholar, non-tenure-track and other contingent faculty, tenure-track faculty), institution type, and scientific discipline. Although there have been opportunities for innovation and some potential shifts in expectations, increased caregiving demands associated with the COVID-19 pandemic in 2020, such as remote working, school closures, and childcare and eldercare, had disproportionately negative outcomes for women.
The effects of the COVID-19 pandemic on women in STEMM during 2020 are understood better through an intentionally intersectional lens. Productivity, career, boundary setting, mental well-being, and health are all influenced by the ways in which social identities are defined and cultivated within social and power structures. Race and ethnicity, sexual orientation, gender identity, academic career stage, appointment type, institution type, age, and disability status, among many other factors, can amplify or diminish the effects of the COVID-19 pandemic for a given person. For example, non-cisgender women may be forced to return to home environments where their gender identity is not accepted, increasing their stress and isolation, and decreasing their well-being. Women of Color had a higher likelihood of facing a COVID-19–related death in their family compared with their white, non-Hispanic colleagues. The full extent of the effects of the COVID-19 pandemic for women of various social identities was not fully understood at the end of 2020.
Considering the relative paucity of women in many STEMM fields prior to the COVID-19 pandemic, women are more likely to experience academic isolation, including limited access to mentors, sponsors, and role models that share gender, racial, or ethnic identities. Combining this reality with the physical isolation stipulated by public health responses to the COVID-19 pandemic,
women in STEMM were subject to increasing isolation within their fields, networks, and communities. Explicit attention to the early indicators of how the COVID-19 pandemic affected women in academic STEMM careers during 2020, as well as attention to crisis responses throughout history, may provide opportunities to mitigate some of the long-term effects and potentially develop a more resilient and equitable academic STEMM system.
MAJOR FINDINGS
Given the ongoing nature of the COVID-19 pandemic, it was not possible to fully understand the entirety of the short- or long-term implications of this global disruption on the careers of women in academic STEMM. Having gathered preliminary data and evidence available in 2020, the committee found that significant changes to women’s work-life boundaries and divisions of labor, careers, productivity, advancement, mentoring and networking relationships, and mental health and well-being have been observed. The following findings represent those aspects that the committee agreed have been substantiated by the preliminary data, evidence, and information gathered by the end of 2020. They are presented either as Established Research and Experiences from Previous Events or Impacts of the COVID-19 Pandemic during 2020 that parallel the topics as presented in the report.
Established Research and Experiences from Previous Events
| Leading up to the COVID-19 pandemic, the representation of women has slowly increased in STEMM fields, from acquiring Ph.D.s to holding leadership positions, but with caveats to these limited steps of progress; for example, women representation in leadership positions tends to be at institutions with less prestige and fewer resources. While promising and encouraging, such progress is fragile and prone to setbacks especially in times of crisis (see ). | |
| Social crises (e.g., terrorist attacks, natural disasters, racialized violence, and infectious diseases) and COVID-19 pandemic-related disruptions to workload and schedules, added to formerly routine job functions and health risks, have the potential to exacerbate mental health conditions such as insomnia, depression, anxiety, and posttraumatic stress. All of these conditions occur more frequently among women than men. As multiple crises coincided during 2020, there is a greater chance that women will be affected mentally and physically (see and ). |
___________________
1 This finding is primarily based on research on cisgender women and men.
| Structural racism is an omnipresent stressor for Women of Color, who already feel particularly isolated in many fields and disciplines. Attempts to ensure equity for all women may not necessarily create equity for women across various identities if targeted interventions designed to promote gender equity do not account for the racial and ethnic heterogeneity of women in STEMM (see , , and ). |
Impacts of the COVID-19 Pandemic during 2020
| While some research indicates consistency in publications authored by women in specific STEMM disciplines, like Earth and space sciences, during 2020, several other preliminary measures of productivity suggest that COVID-19 disruptions have disproportionately affected women compared with men. Reduced productivity may be compounded by differences in the ways research is conducted, such as whether field research or face-to-face engagement with human subjects is required (see ). | |
| Many administrative decisions regarding institutional supports made during 2020, such as work-from-home provisions and extensions on evaluations or deliverables, are likely to exacerbate underlying gender-based inequalities in academic advancement rather than being gender neutral as assumed. For example, while colleges and universities have offered extensions for those on the tenure track and federal and private funders have offered extensions on funding and grants, these changes do not necessarily align with the needs expressed by women, such as the need for flexibility to contend with limited availability of caregiving and requests for a reduced workload, nor do they generally benefit women faculty who are not on the tenure track. Furthermore, provision of institutional support may be insufficient if it does not account for the challenges faced by those with multiple marginalized identities (see and ). | |
| Organizational-level approaches may be needed to address challenges that have emerged as a result of the COVID-19 pandemic in 2020, as well as those challenges that may have existed before the pandemic but are now more visible and amplified. Reliance on individual coping strategies may be insufficient (see and ). | |
| The COVID-19 pandemic has intensified complications related to worklife boundaries that largely affect women. Preliminary evidence |
| from 2020 suggests women in academic STEMM are experiencing increased workload, decreased productivity, changes in interactions, and difficulties from remote work caused by the COVID-19 pandemic and associated disruptions. Combined with the gendered division of nonemployment labor that affected women before the pandemic, these challenges have been amplified, as demonstrated by a lack of access to childcare, children’s heightened behavioral and academic needs, increased eldercare demands, and personal physical and mental health concerns. These are particularly salient for women who are parents or caregivers (see ). | |
| During the COVID-19 pandemic, technology has allowed for the continuation of information exchange and many collaborations. In some cases technology has facilitated the increased participation of women and underrepresented groups. However, preliminary indicators also show gendered impacts on science and scientific collaborations during 2020. These arise because some collaborations cannot be facilitated online and some collaborations face challenges including finding time in the day to engage synchronously, which presents a larger burden for women who manage the larger share of caregiving and other household duties, especially during the first several months of the COVID-19 pandemic (see ). | |
| During the COVID-19 pandemic in 2020, some professional societies adapted to the needs of members as well as to broader interests of individuals engaged in the disciplines they serve. Transitioning conferences to virtual platforms has produced both positive outcomes, such as lower attendance costs and more open access to content, and negative outcomes, including over-flexibility (e.g., scheduling meetings at non-traditional work hours; last-minute changes) and opportunities for bias in virtual environments (see ). | |
| During the COVID-19 pandemic in 2020, many of the decision-making processes, including financial decisions like lay-offs and furloughs, that were quickly implemented contributed to unilateral decisions that frequently deviated from effective practices in academic governance, such as those in crisis and equity-minded leadership. Fast decisions greatly affected contingent and nontenured faculty members—positions that are more often occupied by women and People of Color. In 2020, these financial decisions already had negative, short-term effects and may portend long-term consequences (see ). | |
| Social support, which is particularly important during stressful situations, is jeopardized by the physical isolation and restricted social interactions that have |
| been imposed during the COVID-19 pandemic. For women who are already isolated within their specific fields or disciplines, additional social isolation may be an important contributor to added stress (see ). | |
| For women in the health professions, major risk factors during the COVID-19 pandemic in 2020 included unpredictability in clinical work, evolving clinical and leadership roles, the psychological demands of unremitting and stressful work, and heightened health risks to family and self (see ). |
RESEARCH QUESTIONS
While this report compiled much of the research, data, and evidence available in 2020 on the effects of the COVID-19 pandemic, future research is still needed to understand all the potential effects, especially any long-term implications. The research questions represent areas the committee identified for future research, rather than specific recommendations. They are presented in six categories that parallel the chapters of the report: Cross-Cutting Themes; Academic Productivity and Institutional Responses; Work-Life Boundaries and Gendered Divisions of Labor; Collaboration, Networking, and Professional Societies; Academic Leadership and Decision-Making; and Mental Health and Well-being. The committee hopes the report will be used as a basis for continued understanding of the impact of the COVID-19 pandemic in its entirety and as a reference for mitigating impacts of future disruptions that affect women in academic STEMM. The committee also hopes that these research questions may enable academic STEMM to emerge from the pandemic era a stronger, more equitable place for women. Therefore, the committee identifies two types of research questions in each category; listed first are those questions aimed at understanding the impacts of the disruptions from the COVID-19 pandemic, followed by those questions exploring the opportunities to help support the full participation of women in the future.
Cross-Cutting Themes
- What are the short- and long-term effects of the COVID-19 pandemic on the career trajectories, job stability, and leadership roles of women, particularly of Black women and other Women of Color? How do these effects vary across institutional characteristics, 2 discipline, and career stage?
2 Institutional characteristics include different institutional types (e.g., research university, liberal arts college, community college), locales (e.g., urban, rural), missions (e.g., Historically Black Colleges and Universities, Hispanic-Serving Institutions, Asian American/Native American/Pacific Islander-Serving Institutions, Tribal Colleges and Universities), and levels of resources.
- How did the confluence of structural racism, economic hardships, and environmental disruptions affect Women of Color during the COVID-19 pandemic? Specifically, how did the murder of George Floyd, Breonna Taylor, and other Black citizens impact Black women academics’ safety, ability to be productive, and mental health?
- How has the inclusion of women in leadership and other roles in the academy influenced the ability of institutions to respond to the confluence of major social crises during the COVID-19 pandemic?
- How can institutions build on the involvement women had across STEMM disciplines during the COVID-19 pandemic to increase the participation of women in STEMM and/or elevate and support women in their current STEMM-related positions?
- How can institutions adapt, leverage, and learn from approaches developed during 2020 to attend to challenges experienced by Women of Color in STEMM in the future?
Academic Productivity and Institutional Responses
- How did the institutional responses (e.g., policies, practices) that were outlined in the Major Findings impact women faculty across institutional characteristics and disciplines?
- What are the short- and long-term effects of faculty evaluation practices and extension policies implemented during the COVID-19 pandemic on the productivity and career trajectories of members of the academic STEMM workforce by gender?
- What adaptations did women use during the transition to online and hybrid teaching modes? How did these techniques and adaptations vary as a function of career stage and institutional characteristics?
- What are examples of institutional changes implemented in response to the COVID-19 pandemic that have the potential to reduce systemic barriers to participation and advancement that have historically been faced by academic women in STEMM, specifically Women of Color and other marginalized women in STEMM? How might positive institutional responses be leveraged to create a more resilient and responsive higher education ecosystem?
- How can or should funding arrangements be altered (e.g., changes in funding for research and/or mentorship programs) to support new ways of interaction for women in STEMM during times of disruption, such as the COVID-19 pandemic?
Work-Life Boundaries and Gendered Divisions of Labor
- How do different social identities (e.g., racial; socioeconomic status; culturally, ethnically, sexually, or gender diverse; immigration status; parents of young children and other caregivers; women without partners) influence the management of work-nonwork boundaries? How did this change during the COVID-19 pandemic?
- How have COVID-19 pandemic-related disruptions affected progress toward reducing the gender gap in academic STEMM labor-force participation? How does this differ for Women of Color or women with caregiving responsibilities?
- How can institutions account for the unique challenges of women faculty with parenthood and caregiving responsibilities when developing effective and equitable policies, practices, or programs?
- How might insights gained about work-life boundaries during the COVID-19 pandemic inform how institutions develop and implement supportive resources (e.g., reductions in workload, on-site childcare, flexible working options)?
Collaboration, Networking, and Professional Societies
- What were the short- and long-term effects of the COVID-19 pandemic-prompted switch from in-person conferences to virtual conferences on conference culture and climate, especially for women in STEMM?
- How will the increase in virtual conferences specifically affect women’s advancement and career trajectories? How will it affect women’s collaborations?
- How has the shift away from attending conferences and in-person networking changed longer-term mentoring and sponsoring relationships, particularly in terms of gender dynamics?
- How can institutions maximize the benefits of digitization and the increased use of technology observed during the COVID-19 pandemic to continue supporting women, especially marginalized women, by increasing accessibility, collaborations, mentorship, and learning?
- How can organizations that support, host, or facilitate online and virtual conferences and networking events (1) ensure open and fair access to participants who face different funding and time constraints; (2) foster virtual connections among peers, mentors, and sponsors; and (3) maintain an inclusive environment to scientists of all backgrounds?
- What policies, practices, or programs can be developed to help women in STEMM maintain a sense of support, structure, and stability during and after periods of disruption?
Academic Leadership and Decision-Making
- What specific interventions did colleges and universities initiate or prioritize to ensure that women were included in decision-making processes during responses to the COVID-19 pandemic?
- How effective were colleges and universities that prioritized equity-minded leadership, shared leadership, and crisis leadership styles at mitigating emerging and potential negative effects of the COVID-19 pandemic on women in their communities?
- What specific aspects of different leadership models translated to more effective strategies to advance women in STEMM, particularly during the COVID-19 pandemic?
- How can examples of intentional inclusion of women in decision-making processes during the COVID-19 pandemic be leveraged to develop the engagement of women as leaders at all levels of academic institutions?
- What are potential “top-down” structural changes in academia that can be implemented to mitigate the adverse effects of the COVID-19 pandemic or other disruptions?
- How can academic leadership, at all levels, more effectively support the mental health needs of women in STEMM?
Mental Health and Well-being
- What is the impact of the COVID-19 pandemic and institutional responses on the mental health and well-being of members of the academic STEMM workforce as a function of gender, race, and career stage?
- How are tools and diagnostic tests to measure aspects of wellbeing, including burnout and insomnia, used in academic settings? How does this change during times of increased stress, such as the COVID-19 pandemic?
- How might insights gained about mental health during the COVID-19 pandemic be used to inform preparedness for future disruptions?
- How can programs that focus on changes in biomarkers of stress and mood dysregulation, such as levels of sleep, activity, and texting patterns, be developed and implemented to better engage women in addressing their mental health?
- What are effective interventions to address the health of women academics in STEMM that specifically account for the effects of stress on women? What are effective interventions to mitigate the excessive levels of stress for Women of Color?
This page intentionally left blank.
The spring of 2020 marked a change in how almost everyone conducted their personal and professional lives, both within science, technology, engineering, mathematics, and medicine (STEMM) and beyond. The COVID-19 pandemic disrupted global scientific conferences and individual laboratories and required people to find space in their homes from which to work. It blurred the boundaries between work and non-work, infusing ambiguity into everyday activities. While adaptations that allowed people to connect became more common, the evidence available at the end of 2020 suggests that the disruptions caused by the COVID-19 pandemic endangered the engagement, experience, and retention of women in academic STEMM, and may roll back some of the achievement gains made by women in the academy to date.
The Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine identifies, names, and documents how the COVID-19 pandemic disrupted the careers of women in academic STEMM during the initial 9-month period since March 2020 and considers how these disruptions - both positive and negative - might shape future progress for women. This publication builds on the 2020 report Promising Practices for Addressing the Underrepresentation of Women in Science, Engineering, and Medicine to develop a comprehensive understanding of the nuanced ways these disruptions have manifested. The Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine will inform the academic community as it emerges from the pandemic to mitigate any long-term negative consequences for the continued advancement of women in the academic STEMM workforce and build on the adaptations and opportunities that have emerged.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- News Releases
- In the News
- News Subscription
- Research Highlights
- Stay Connected
Research on the impacts of a pandemic

- Partnerships
Addressing the needs of land and recreation managers
Americans treasure their national forests. Managing these lands while trying to help keep the public and Forest Service employees safe during a global pandemic has created significant challenges for the agency. In May 2020, the Pacific Northwest Research Station organized a science panel to provide the Pacific Northwest Region (Region 6) with insights, decision support tools, and proposed research relevant to managing the risks and consequences of COVID-19.
Risk assessment helps weigh job hazards
Bruce Marcot, a research wildlife biologist with the station, described the elements and processes necessary for structured decisionmaking, with specific considerations for responding to COVID-19 conditions. He walked through a step-by-step case example of a risk assessment worksheet he helped prepare to mitigate the threat of contagion to field crew workers while they are researching northern spotted owls. “I had been getting calls from ranger districts asking for guidance on how to conduct a risk analysis for COVID-centric job hazards,” Marcot said. He demonstrated how field-going personnel can use the risk assessment worksheet to systematically weigh the potential consequences of conducting field work during the pandemic and for identifying mitigation measures to reduce job hazards.
Economic and social impacts are significant
Turning to the social and economic effects of COVID-19, research social scientists Lee Cerveny and Eric White shared their insights. Cerveny discussed impacts of COVID-19 to cruise ship tourism in southeast Alaska, where 1 in 5 jobs are in the tourism industry. By early May 2020, 70 percent of Alaska cruises for 2020 had been cancelled, with significant repercussions for local economies. She outlined a new collaboration with the University of Alaska to evaluate the effects of COVID-19 on southeast Alaska’s economy and the health and safety of rural communities.
White delivered information on predicted recreation trends and possible economic ripples related to COVID-19 in the Pacific Northwest. Recreation visitor spending is a major contribution to many local economies, as he documented in a 2017 report. Average spending by national forest visitors who stay overnight ranges from $156 for people camping, to more than $800 for those staying in motels. White noted that Forest Service partners in recreation and forestry sectors are often highly leveraged and their bottom line depends on summer revenues. Cerveny and White’s presentation gave Region 6 leadership food for thought as they work to balance the goals of welcoming visitors, supporting economic opportunity, and continuing to protect resident and employee health and safety.
Mapping wildfire smoke exposure and COVID-19
Finally, climate scientist Sim Larkin reviewed the potential implications of wildfire smoke, including the risks to firefighters and downwind communities. Preliminary studies show that the confluence of smoke and COVID-19 this year could significantly affect infections and mortality, both in the firefighting community and in the broader population. Chief Christiansen’s stated intent is to “minimize, to the extent feasible, COVID-19 exposure and transmission and smoke exposure to firefighters and communities.” Larkin shared some of the tools offered by the Interagency Wildland Fire Air Quality Response Program, including a Smoke-COVID-19 dashboard that places COVID-19 infection rates by county alongside current, recent, and projected air quality.
Region 6 Chief of Staff Sally Butts solicited and attended the virtual panel presentation. “Region 6 leadership is really interested in how the research station can help us look at COVID-19 impacts on safety, health, economic, and social issues, and how to evaluate some of the tradeoffs between risks and opportunities in those different sectors,” she said. “The panel provided a lot for our regional leadership team to consider as we look toward the future.”
Forest Service Partners
Interagency Wildland Fire Air Quality Response Program

- June 29, 2024 | Is NASA’s Starliner Ready To Come Home? Find Out What’s Next
- June 29, 2024 | Space Station Crews Wrap Week: Starliner, Spacewalk, and Cargo Updates
- June 28, 2024 | Unlocking the Brain’s GPS: Just Thinking About a Location Activates Mental Maps
- June 28, 2024 | Revolution at Mach 10: NASA-Backed Hypersonic Jets Poised to Transform Space Travel
- June 28, 2024 | Beyond Gravity: UC Berkeley’s Quantum Leap in Dark Energy Research
Religious Faith Linked to Improved Coping in COVID-19 Pandemic, Research Finds
By University of Cambridge June 24, 2024

University of Cambridge research shows that during COVID-19, religious individuals experienced fewer mental health challenges than non-religious ones, benefiting from their faith and religious activities.
Research from the University of Cambridge indicates that during the COVID-19 lockdowns, individuals with religious faith in the UK and US experienced less unhappiness and stress compared to non-religious people.
The studies revealed that strong religious beliefs and practices, including participation in online services, provided significant mental health benefits during the pandemic, with higher religiosity correlating with greater emotional resilience.
Impact of Religion on Mental Health During COVID-19
People of religious faith may have experienced lower levels of stress and unhappiness than secular people during the UK’s COVID-19 lockdowns in 2020 and 2021. This is according to research from the University of Cambridge .
The findings follow a recently published Cambridge-led study suggesting that worsening mental health after experiencing Covid infection – either personally or in those close to you – was also somewhat ameliorated by religious belief. This study looked at the US population during early 2021.
University of Cambridge economists argue that – taken together – these studies show that religion may act as a bulwark against increased distress and reduced well-being during times of crisis, such as a global public health emergency.
Methodology of Studying Religion’s Effects During the Pandemic
“Selection biases make the well-being effects of religion difficult to study,” said Prof Shaun Larcom from Cambridge’s Department of Land Economy, and co-author of the latest study. “People may become religious due to family backgrounds, innate traits, or to cope with new or existing struggles.”
“However, the COVID-19 pandemic was an extraordinary event affecting everyone at around the same time, so we could gauge the impact of a negative shock to well-being right across society. This provided a unique opportunity to measure whether religion was important for how some people deal with a crisis.”
Larcom and his Cambridge colleagues Prof Sriya Iyer and Dr Po-Wen She analyzed survey data collected from 3,884 people in the UK during the first two national lockdowns, and compared it to three waves of data prior to the pandemic.
Findings on Religiosity and Emotional Wellbeing
They found that while lockdowns were associated with a universal uptick in unhappiness, the average increase in feeling miserable was 29% lower for people who described themselves as belonging to a religion. [1]
The researchers also analyzed the data by “religiosity”: the extent of an individual’s commitment to religious beliefs, and how central it is to their life. Those for whom religion makes “some or a great difference” in their lives experienced around half the increase in unhappiness seen in those for whom religion makes little or no difference. [2]
“The study suggests that it is not just being religious, but the intensity of religiosity that is important when coping with a crisis,” said Larcom.
Those self-identifying as religious in the UK are more likely to have certain characteristics, such as being older and female. The research team “controlled” for these statistically to try and isolate the effects caused by faith alone, and still found that the probability of religious people having an increase in depression was around 20% lower than non-religious people.
Comparative Analysis and Additional Insights
There was little overall difference between Christians, Muslims, and Hindus – followers of the three biggest religions in the UK. However, the team did find that well-being among some religious groups appeared to suffer more than others when places of worship were closed during the first lockdown.
“The denial of weekly communal attendance appears to have been particularly affecting for Catholics and Muslims,” said Larcom. The research is published as a working paper by Cambridge’s Faculty of Economics.
For the earlier study, authored by Prof Sriya Iyer, along with colleagues Kishen Shastry, Girish Bahal and Anand Shrivastava from Australia and India, researchers used online surveys to investigate COVID-19 infections among respondents or their immediate family and friends, as well as religious beliefs, and mental health.
The study was conducted during February and March 2021, and involved 5,178 people right across the United States, with findings published in the journal European Economic Review .
Researchers found that almost half of those who reported a COVID-19 infection either in themselves or their immediate social network experienced an associated reduction in well-being.
Where mental health declined, it was around 60% worse on average for the non-religious compared to people of faith with typical levels of “religiosity.” [3]
Interestingly, the positive effects of religion were not found in areas with strictest lockdowns, suggesting access to places of worship might be even more important in a US context. The study also found significant uptake of online religious services, and a 40% lower association between COVID-19 and mental health for those who used them. [4]
“Religious beliefs may be used by some as psychological resources that can shore up self-esteem and add coping skills, combined with practices that provide social support,” said Prof Iyer, from Cambridge’s Faculty of Economics.
“The pandemic presented an opportunity to glean further evidence of this in both the United Kingdom and the United States, two nations characterised by enormous religious diversity.”
Added Larcom: “These studies show a relationship between religion and lower levels of distress during a global crisis. It may be that religious faith builds resilience, and helps people cope with adversity by providing hope, consolation, and meaning in tumultuous times.”
- The increase in the mean measure for unhappiness was 6.1 percent for people who do not identify with a religion during the lockdown, compared to an increase of 4.3 percent for those who do belong to a religion – a difference of 29%.
- For those that religion makes little or no difference, the increase was 6.3 percent. For those for whom religion makes some or a great difference, the increase was around half that, at 3 percent and 3.5 percent respectively.
- This was after controlling for various demographic and environmental traits, including age, race, income, and average mental health rates prior to the pandemic.
- The interpretation is from Column 1 of Table 5: Determinants of mental health, online access to religion. Where the coefficients of Covid {Not accessed online service} is 2.265 and Covid {Accessed online service} is 1.344. Hence the difference is 2.265-1.344 = 0.921 which is 40% of 2.265.
Reference: “Religion, Covid-19 and mental health” by Girish Bahal, Sriya Iyer, Kishen Shastry and Anand Shrivastava, 28 October 2023, European Economic Review . DOI: 10.1016/j.euroecorev.2023.104621
More on SciTechDaily

Critical Leak: How a Spacesuit Malfunction Compromised Spacewalk 90

Research Shows Salt Substitutes Lower Risk of Heart Attack/Stroke and Death
Researchers Link Sleep Quality to Mental Illness Using Wrist Activity Trackers
COVID-19 Pandemic Origins Reconstructed by Genetic Network Analysis
Innovative Cancer Treatment Uses Ultrasound-Activated Drugs for Targeting

Beekeeping Theories Debunked: The Real Buzz on Honeybee Insulation

Ancient Writing Practices Unveiled by Red and Black Ink From Egyptian Papyri

Scientists Reveal How to Make the Healthiest Coffee During COVID-19 Lockdown
3 comments on "religious faith linked to improved coping in covid-19 pandemic, research finds".
So, they’re higher on copium.
Religion is for coping with problems instead of solving them.
That is a very ignorant statement
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 28, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Here are the numbers: COVID-19 is ticking up in some places, but levels remain low
by The Associated Press

Here's a look at the state of COVID-19 in the U.S. as the Centers for Disease Control and Prevention establishes its latest advice on vaccinations .
About 300 COVID-19-associated deaths were occurring weekly in May, according to the most recent provisional CDC data . That's the lowest since the beginning of the pandemic. Nearly 26,000 people died from COVID-19 in the U.S. in the week ending Jan. 9, 2021—the highest weekly toll in the pandemic.
Hospitalizations
The COVID-19 hospitalization rate is 1.5 per 100,000 hospital visits. That's up from about 1.1 in mid-May. It peaked at 35 in early 2022.
Individual COVID-19 cases are no longer tracked, but health officials can analyze wastewater to help them get a big-picture look at where the virus may be spreading. The CDC describes current wastewater levels as "low" nationwide but inching up, with higher levels noted in Florida, Utah, California and Hawaii.
Vaccinations
As of May 11, fewer than one-quarter of U.S. adults had received the latest COVID-19 shot . About 42% of people 75 and older—those most vulnerable to severe disease and death from COVID-19—got the latest shots.
© 2024 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.
Explore further
Feedback to editors

Researchers develop scalable synthesis of cancer-fighting compounds
15 hours ago

New device inspired by python teeth may reduce the risk of rotator cuff re-tearing
18 hours ago

Serotonin 2C receptor regulates memory in mice and humans: Implications for Alzheimer's disease

Fears of attack and no phone signal deter women trail runners, finds study
19 hours ago

Creating supranormal hearing in mice
20 hours ago

Visualizing core pathologies of Parkinson's disease and related disorders in live patients

Novel mechanism for targeting bone marrow adipocytes to prevent bone loss

Breakthrough research makes cancer-fighting viral agent more effective

Crohn's discovery could lead to better treatments for devastating condition
22 hours ago

Chemo drug may cause significant hearing loss in longtime cancer survivors
Related stories.

As COVID-19 ticks up in some places, US health officials recommend a fall vaccination campaign
Jun 28, 2024
New COVID-19 cases, ED visits up for children since July 2021
Sep 8, 2021
Older US adults should get another COVID-19 shot, health officials recommend
Feb 29, 2024

WHO: Weekly COVID cases dip in Europe after weeks of gains
Dec 8, 2021

Report: US death rate declined in 2022, COVID deaths fell by almost half
May 4, 2023

COVID-19 hospitalizations in the US are on the rise again, but not like before
Aug 8, 2023
Recommended for you

Loss of salt and body fluid stimulates kidney regeneration in mice
23 hours ago

Gene therapy halts progression of rare genetic condition in young boy

New predictors of metastasis in patients with early-stage pancreatic cancer

Collaboration develop a potent therapy candidate for fatal prion diseases
Jun 27, 2024

Study reveals how antibody mAb 77 neutralizes measles virus

First specific PET scan for TB could enable more effective treatment
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
COMMENTS
Search NIH COVID-19 Articles and Resources. Scroll down the page to view all COVID-19 articles, stories, and resources from across NIH. You can also select a topic from the list to view resources on that topic. - Any -. Aging.
The 25 most downloaded Nature Communications articles* on COVID-19 published in 2021 illustrate the collaborative efforts of the international community to combat the ongoing pandemic.These papers ...
For example, when one study found that cloth masks didn't work in "high-risk situations," it was sometimes used as evidence against mask mandates. However, a look beyond the headlines revealed that the study was of health care workers treating COVID-19 patients, which is a vastly more dangerous situation than, say, going to the grocery store.
Covid-19 has affected humanity in a major way. An extremely dangerous virus, hitherto unknown to humanity, had to be studied and contained in order to overcome the pandemic. Research on Covid-19 had surged in the early days with an unprecedented surge in the publications on that specific topic.
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
The sixth question concerns how COVID-19 should be treated and what treatment options should be made available. COVID-19 is a self-limiting disease in more than 80% of patients. Severe pneumonia occurred in about 15% of cases as revealed in studies with large cohorts of patients. The gross case fatality is 3.4% worldwide as of February 25, 2020.
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
COVID-19 pandemic has severely impacted the crude, stock market, gold and metals and almost all areas of the global market [ 1 ]. Large research laboratories and corporate houses are working with a high speed to develop medicines and vaccines for the prevention and treatment of this dreaded disease. To deal with these current health management ...
The WHO Covid-19 Research Database was maintained by the WHO Library & Digital Information Networks and was funded by COVID-19 emergency funds. The database was built by BIREME, the Specialized Center of PAHO/AMRO. Its content spanned the time period March 2020 to June 2023. It has now been archived, and no longer searchable since January 2024.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing global health emergency. Here we highlight nine most important research questions concerning virus transmission, asymptomatic and presymptomatic virus shedding, diagnosis, treatment, vaccine development, origin of virus and ...
The ZOE team has now identified an incredible range of symptoms, with over 25 symptoms being recognized for the combination of COVID-19 and long COVID. A big question is how the same virus gives ...
Lower-income nations participate very little in COVID-19 research in 2020. Topic maps of internationally collaborative work show the rise of patient care and public health clusters—two topics that were largely absent from coronavirus research in the two years prior to 2020. ... For example, for an article with authors from the USA, Italy, and ...
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. Most people infected with the virus will experience mild to moderate respiratory illness and recover without requiring special treatment. However, some will become seriously ill and require medical attention. Older people and those with underlying medical ...
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on ...
The outbreak of coronavirus disease 2019 (COVID-19) has created a global health crisis that has had a deep impact on the way we perceive our world and our everyday lives. Not only the rate of contagion and patterns of transmission threatens our sense of agency, but the safety measures put in place to contain the spread of the virus also require social distancing by refraining from doing what ...
This article is part of the Research Topic Coronavirus Disease (COVID-19): The Impact and Role of Mass Media During the Pandemic View all 16 articles. Editorial: Coronavirus Disease (COVID-19): The Impact and Role of Mass Media During the Pandemic ... Dhanani and Franz examined a sample of 1,141 adults (Mage = 44.66; 46.9% female, 74.7% White ...
This trend may also suggest that researchers have gained a better understanding of the etiology and treatment of COVID-19, leading to decreased interest or fatigue with the topic. 18 Changes in distribution, top affiliations, and research fields of highly cited studies suggest a gradual shift in interest in COVID-19 research toward more ...
experienced an average decrease of 11.5 hours of work per week and a 21% decrease in weekly earnings, arnings for 52% of the sample, which again re ects s. variation in the e ects of COVID-19 across students. In terms of labor market expectations, on average, students foresee a 13 percentage points decrease in.
View our collection of covid 19 essays. Find inspiration for topics, titles, outlines, & craft impactful covid 19 papers. ... The study identifies the COVID-19 global pandemic as an example of environmental factors that contribute to or influence teacher burnout. This research was conducted on grounds that teacher well-being remains one of the ...
Government responses to COVID-19 are among the most globally impactful events of the 21st century. ... Multiverse analyses elevate epistemic humility by relaxing the number of subjective choices in the research design process. ... For example, a highly cited study on this topic notes that "Our results show that major non-pharmaceutical ...
THEMIS is an interdisciplinary research project that reacts to the increasing occurrence of global pandemics, like the caused by the present COVID-19 disease, and restrictive public health measures taken to respond to these threats. Using a rights-based approach, Dr Patrycja Dąbrowska-Kłosińska, researcher of THEMIS, intends to create a ...
The global escalation of post-acute sequelae of COVID-19, known as Long COVID, which affects approximately 65 million people worldwide, underscores the necessity for detailed research into its chronic effects, especially among cardiothoracic transplant recipients. Evidence suggests that long COVID could evolve into a chronic illness for those with heart and lung transplants, with debilitating ...
3.5. Topic popularities and evolvements about COVID-19 research. Topic popularity of the above 4 COVID-19 topics was measured by using proportional frequency equation in Section 2, and the measured results, as shown in Figure Figure6, 6, were consistent with manually validation results by reviewing literature. According to trend analysis, the ...
Download MP3. In the final Coronapod of 2020, we dive into the scientific literature to reflect on the COVID-19 pandemic. Researchers have discovered so much about SARS-CoV-2 - information that ...
For example, Mao et al analyzed coronavirus articles published from 2003 to 2020. Up to the investigation time of this study, there were limited number of bibliometric studies specific to COVID-19 and most of them were found and implemented at early stage of COVID-19 outbreak. ... Four topics about COVID-19 research were obviously identified ...
For example, non-cisgender women may be forced to return to home environments where their gender identity is not accepted, increasing their stress and isolation, and decreasing their well-being. ... Research and Experiences from Previous Events or Impacts of the COVID-19 Pandemic during 2020 that parallel the topics as presented in the report ...
He walked through a step-by-step case example of a risk assessment worksheet he helped prepare to mitigate the threat of contagion to field crew workers while they are researching northern spotted owls. ... "Region 6 leadership is really interested in how the research station can help us look at COVID-19 impacts on safety, health, economic ...
Research from the University of Cambridge indicates that during the COVID-19 lockdowns, individuals with religious faith in the UK and US experienced less unhappiness and stress compared to non-religious people.. The studies revealed that strong religious beliefs and practices, including participation in online services, provided significant mental health benefits during the pandemic, with ...
Nearly 26,000 people died from COVID-19 in the U.S. in the week ending Jan. 9, 2021—the highest weekly toll in the pandemic. Hospitalizations The COVID-19 hospitalization rate is 1.5 per 100,000 ...
Given the public health and safety concerns related to COVID-19, the staff is providing the following statement to those affected by COVID-19 regarding Rule 302(b) of Regulation S-T. This staff statement is temporary and remains in effect until the staff provides public notice that it no longer will be in effect; that notice will be published ...