

How Do You Present a Literature Review in a Conference?
Presenting a literature review at a conference is an art that balances information delivery with engaging storytelling. In the context of “How do you present a literature review in a conference?” it’s essential to transform your extensive review into a concise, impactful presentation.
The presentation involves highlighting key findings, elaborating on methodologies, and illustrating their significance in relation to the conference’s theme. To captivate your audience, incorporate visuals and accurately cite your sources, ensuring your presentation is both informative and visually appealing.
Moreover, an engaging delivery style can substantially impact audience reception. Be prepared to handle questions and encourage discussions, turning your presentation into an interactive learning experience. For more insights and detailed guidance, continue reading our comprehensive article.
What Is the Literature Review?
A literature review is a scholarly endeavor that synthesizes existing research on a specific topic. It’s not merely a summary; it critically analyzes and links various studies. This comprehensive overview helps identify patterns, gaps, and the current state of knowledge.

In undertaking a literature review, the researcher examines relevant publications to establish an understanding of the subject. This process involves evaluating sources’ relevance, credibility, and contributions to the field. The outcome is a cohesive narrative contextualizing the research within its academic landscape, providing a foundation for new inquiries.
Can You Present a Literature Review at A Conference?
Yes, presenting a literature review at a conference is a valuable contribution. It offers insights into existing research and highlights emerging trends in a specific field. This presentation can spark discussions and foster academic collaborations.
When presenting a literature review at an event with multinational participants , the key is to distill complex information into accessible insights. This involves selecting key studies, weaving them into a narrative, and emphasizing their collective significance. Such a presentation can illuminate research gaps, setting the stage for future work.
In doing so, the presenter navigates through various studies, offering a critical analysis and synthesis. This approach educates and engages the audience, inviting them to explore the subject deeper. The literature review thus catalyzes knowledge exchange and scholarly debate at the conference.
Why Should You Present Your Literature Review at A Conference?
Presenting a literature review at a conference is a strategic move for any researcher or academic. It is a platform to share findings, gain feedback, and engage with peers. This opportunity can significantly impact one’s academic journey and research direction.
- Showcasing Expertise : Presenting a review establishes you as a knowledgeable professional. It highlights your ability to analyze and synthesize complex information.
- Networking Opportunities : Conferences attract like-minded professionals, offering a space to build valuable connections. Sharing your review can lead to collaborations and future research opportunities.
- Receiving Constructive Feedback : Peer conference feedback can refine your understanding and approach. This interaction often leads to improvements in your research methodology and perspective.
- Identifying Research Gaps : Discussing your review exposes you to different viewpoints, revealing gaps in current research. This insight can guide your future research endeavors.
Presenting a literature review at a conference is not just about sharing knowledge; it’s a gateway to professional growth, collaboration, and refining your research. It’s an invaluable experience for anyone looking to make a mark in their academic field.
How Do You Present a Literature Review in A Conference?
The process of presenting a literature review at a conference requires careful preparation and strategic execution. It involves a deep understanding of the subject matter and the ability to succinctly and engagingly convey complex ideas. This guide offers a structured approach to ensure your presentation is impactful and memorable.
Step 1: Understand Your Audience
Before you begin, assess who will be attending your session. Tailor your presentation to their knowledge level, interests, and the conference theme.
Step 2: Condense Your Content
Select key findings and essential studies from your review. Focus on presenting these elements clearly and concisely to maintain audience engagement.
Step 3: Create a Compelling Narrative
Weave your selected studies into a story that highlights their relevance and interconnections. This narrative approach makes your presentation more relatable and easier to follow.
Step 4: Utilize Visual Aids
Incorporate visuals like graphs, charts, and infographics to illustrate complex points. These aids can make your presentation more dynamic and understandable.
Step 5: Practice Your Delivery
Rehearse your presentation multiple times. Focus on clarity, pacing, and maintaining a conversational tone to keep your audience engaged.
Step 6: Prepare for Questions
Anticipate potential questions and prepare thoughtful responses. Engaging with your audience in this way can deepen their understanding and interest.
A literature review at a conference offers an ideal platform for showcasing your work and engaging with the academic community. Your presentation will be both informative and engaging if you follow these steps.
Considerations While Presenting Your Paper at A Conference
Presenting a paper at a conference is a crucial moment for any researcher or academic. It’s an opportunity to share your work with peers and experts in your field, receive feedback, and build your professional network. However, several considerations should be taken into account to ensure the presentation is effective and well-received.
- Understand Your Audience : Tailor your presentation to the audience’s expertise and interests. This ensures that your content is relevant and engaging to them.
- Clarity and Conciseness : Be clear and to the point in your delivery. Avoid overloading your presentation with excessive detail or jargon.
- Effective Use of Visuals : Use visuals like charts and slides to complement your speech. Ensure they are clear, relevant, and aid in understanding your points.
- Engaging Delivery : Practice your speech to maintain a natural, confident tone. Avoid monotonous delivery to keep the audience interested.
- Time Management : Adhere strictly to your allotted time slot. Plan your presentation to cover all points without rushing or overextending.
- Prepare for Questions : Anticipate questions and prepare concise, informative answers. This interaction can enhance the audience’s understanding of your work.
A successful conference presentation starts by understanding your audience’s needs and interests. Clear and engaging communication ensures your message resonates, while effective visuals enhance comprehension and retention.
Good time management keeps the presentation focused and allows for interactive discussions or Q&A sessions, fostering deeper engagement with your audience. By considering these factors, you can ensure that your presentation not only conveys your research effectively but also leaves a positive impression on your audience.
Tips to Select the Right Conference for Presenting Your Literature Review
Selecting the right conference to present your research is a critical decision that can significantly impact your academic and professional journey. It’s about finding a platform where your work will be appreciated and can contribute meaningfully to the field. This guide provides strategic tips to help you make an informed choice.

Relevance to Your Field
Choose a conference that aligns closely with your research area. This ensures your work is relevant to the attendees. Look for events where current trends and developments in your field are discussed. A conference with a specific focus can provide a more engaged audience for your topic.
Conference Reputation
Research the conference’s standing in the academic community. Established conferences often attract high-quality research and renowned speakers. Check past conference proceedings to gauge the quality of presentations. A reputable conference can add significant value to your CV and professional profile.
Type of Audience
Consider the typical audience of the conference. Whether it’s more academic or industry-focused can affect the reception of your work. A diverse audience can provide varied perspectives, enriching the discussion around your research. Tailor your presentation to suit the audience for maximum impact.
Networking Opportunities
Evaluate the networking potential of the conference. Conferences are excellent for meeting peers, mentors, and leaders in your field. Look for events that facilitate networking, such as workshops or social gatherings. Networking can open doors to collaborations and future research opportunities.
Publication Opportunities
Some conferences offer publication opportunities in journals or conference proceedings. Choose conferences where your work has the potential to be published. This can provide broader exposure and enhance your research’s credibility. Ensure the publication aligns with reputable and relevant academic journals.

Location and Accessibility
Consider the conference’s location and your ability to attend. The benefits of attending top-notch international conferences are appealing; however, local or regional conferences can also be beneficial. Factor in travel costs, visa requirements, and the conference’s accessibility. Sometimes, a nearby conference can offer more engagement and less logistical stress.
Selecting the right conference requires careful consideration of factors like relevance, reputation, audience type, networking opportunities, publication potential, and location. Making an informed choice can enhance your presentation’s impact, contribute to your professional development, and broaden your academic horizons.
Closing Remarks
In summarizing the key aspects of presenting a literature review at a conference, it’s clear that meticulous preparation and strategic considerations are paramount. From understanding your audience to selecting the right conference, each step is crucial for a successful presentation. “How do you present a literature review in a conference?” becomes a question of not just content, but context and delivery.
Accurate application of these principles ensures that your literature review is not only well-received but also stands out as a significant contribution to your field. Missteps in any of these areas, whether in presentation style or conference selection, can lead to missed opportunities and diminished impact.
Thus, the importance of precision and thoroughness in every aspect of conference presentation cannot be overstated. This holistic approach shapes not only how your work is perceived but also your professional trajectory in the academic community.

Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Don’t miss our future updates! Get subscribed today!
Sign up for email updates and stay in the know about all things Conferences including price changes, early bird discounts, and the latest speakers added to the roster.

Meet and Network With International Delegates from Multidisciplinary Backgrounds.
Useful Links
Quick links, secure payment.

Copyright © Global Conference Alliance Inc 2018 – 2024. All Rights Reserved. Developed by Giant Marketers Inc .
DEAN’S BOOK w/ Prof. CONNIE GRIFFIN
Honors291g-cdg’s blog, literature review/poster presentation guide.
Literature Review & Poster/Visual Presentation Guide GIVING & GETTING EFFECTIVE PRESENTATIONS PRESENTATIONS In many disciplines presentations are given at academic conferences, symposia, and other places where scholars share their work with one another (including the Massachusetts Undergraduate Research Conference). It can be very challenging to display and communicate all of one’s research findings in a synthesized manner and short timeframe. Following are some thoughts about both preparing your presentation and also how to maximize your experience as an audience member. I. PRESENTER’S ROLE: The overall purpose of your presentation is to share your research process and findings with the class. In all cases, whatever topic you choose for your research, the objective is to stimulate in your listeners an understanding of that topic and how you went about developing that understanding for yourself as a researcher. The purpose of your talk is to present your research. Keep that goal in mind as you consider what to include and how to organize it.. In the visual portion of your presentation, be sure to include the following:
1) Title 2) Your research question 3) Examples of what you found (results) including a. Visual and quantitative information b. Important quotes 4) Your conclusion
Remember to keep your presentation (and your visual material) concise. It is very easy to overwhelm an audience with too much text. Also, be sure to use a font size that is large enough to read from several feet away. Presentation considerations. Five minutes go fast! Therefore, stick with the most important points (details can come in the Q&A session), and be sure to organize your presentation logically. Be sure to practice. Nothing will prepare you better than giving your presentation several times to an audience. Speak slowly, clearly, expressively. Make eye contact. Also make sure your visual really does support your oral presentation and aid your audience! Concluding your presentation. End your presentation with a quick summary or suggestion of what’s been gained by your research. Then be prepared for questions. Be ready with a question of your own in case the audience needs prompting. A crucial part of your presentation is thinking about how to engage the audience. Listen closely, be sure you understand each questioner’s intent, and then answer as directly as possible. II. AUDIENCE’S ROLE: Even when not presenting, you play a crucial role in the presentation and determining its quality. As a listener, demonstrate your interest: make eye contact with the presenter as you listen closely, and take notes so you can ask informed, pertinent, and helpful questions during the Q&A period. Putting a presenter at ease can go a long way to ensuring an effective presentation.
The Cersonsky Lab at UW-Madison
The Cersonsky Lab is a research group based at the University of Wisconsin - Madison, Department of Chemical and Biological Engineering
8 Tips for a Literature Review Presentation
by Caleb Youngwerth
Literature reviews for research are very different from any other presentation you may have done before, so prepare to relearn how to present. The goals of research literature reviews are different, the style is different, even the pacing is different. Even if you have previously done a literature review in an academic setting, you will still want to know these tips. I found this out the hard way, so you don’t have to. Also, to clarify, these tips are meant for a literature review of a topic, not a singular study or paper, though many of the tips do apply to both.
1. Highlight current research
The point of a literature review for research is to highlight the current state of research related to your topic, not to simply give background information. Background information is important and should be included, but the focus of the presentation should be showing some current studies that either confirm or challenge the topic you are studying. As much as textbooks from 30 years ago might seem to have all the information you need for your presentation, a research study from this decade does a far better job representing the current state of the topic, which is the end goal of the presentation. Also, since the new research should be the focal point of the presentation, as a general piece of advice, try to give each research study a minimum of one full slide, so you can give a fuller picture of what the study actually concluded and how they reached their conclusion.
2. Alternate old and new
The best way to keep people listening to your presentation is to vary what you include in your presentation. Rather than trying to give all of the background information first and then showcase all the flashy new research, try to use the two interchangeably. Organize the presentation by idea and give all the background needed for the idea, then develop the idea further by using the new research studies to help illustrate your point. By doing this, you not only avoid having to backtrack and reteach the background for each and every new study, but also help keep the presentation interesting for the audience. This method also helps the audience avoid being overwhelmed since only a little bit of new information is introduced at a time. Obviously, you may need to include a brief introductory section that contains nothing but textbook information that is absolutely necessary to understand anything about the topic, but the more varied the presentation, the better.
3. Use complete sentences
Every presentation class up to this point probably has taught you that slides with full sentences are harmful to your presentation because it is distracting to the listener. Unlearn all that information for this style of presentation. Bullet points are still good, but you should have complete ideas (which usually means complete sentences) for every single point. If someone would be able to read your slides and not hear you, and still be able to understand most of your presentation, your literature review is perfect in a research setting. The point of this presentation is to share all the new information you have learned, so hiding it is helping no one. You still do not want to be reading your slides verbatim and can absolutely add information beyond the slides, but all your main ideas should be on the slides.
4. Read smart
I will admit that I stole this tip from Rosy, but it is a very good tip, so I decided to include it. When you read, you want to read as much as you can, but wasting time reading an irrelevant research study is helping no one. When finding a new study, read the abstract, then the conclusion, then the pictures. If it looks like a good study from those three parts, or you personally find it interesting, you then can go over the actual paper and read it, but by reading the less dense parts first, you can get a general idea of the study without actually having to take a lot of time to read the entire paper. Though textbooks and review papers generally are a little more difficult to read using this method, you can still look at the introduction, pictures, and conclusion and save time reading the rest if the source ends up not being interesting or important.
5. Reading is good for you
As much as you want to read smart when you can, the more you read, the more knowledgeable you become. The goal of the presentation is to become an expert on you topic, so the only way you can do that is by reading as much as you can. You should read more information than you present, since many sources you read probably will not fit in a time-constrained presentation. As Rosy likes to say, in anything research, only about 10% of what you know should actually be shared with the world. By reading more, you are better-suited to answer questions, and you also just generally are able to understand what you are studying better because, chances are, the main purpose of this presentation for you is to help you better understand your research. If something looks interesting and is vaguely related to your topic, read it; it will be beneficial to you, even if you do not end up presenting the information.
6. Let pictures talk for you
When reading research papers, the pictures are usually the best part. Your presentation should be the same way. The best way to be able to show the concept you are trying to explain is to literally show it. The best way to show the results of a research study is usually by showing a graph or infographic, so if the paper has a graph that shows the results, you should absolutely use it. Charts, diagrams, and even videos can also help illustrate a piece of background information that might be difficult to put into words. That being said, you should know and be able to explain every single part of the graphic. Otherwise, it loses meaning and makes the audience even more confused. Captions can and should be used to help explain the graphic, not only to remind you, but also let your audience know what the general idea of the graphic is. Since they keep slides interesting, you should probably have some sort of picture on every slide, otherwise the slides will be not only bland, but also likely less informative.
7. Avoid overcrowded slides
Just because you should have a lot of information in your presentation does not mean that your slides need to show that. In fact, a slide with too much information will only harm your presentation since your audience will be distracted trying to read all of a long slide while you are trying to explain it. Doing anything to make slides less dense will help avoid having the audience focused on the slide, so they focus on you more. Transitions that only show one point at a time or wait to reveal an image can be helpful in breaking up an overcrowded slide. Also, simply adding more slides can help since it accomplishes the purpose of putting less information on your slides while still keeping the exact same amount of information. You still want to share as much information as you can with the audience, but overcrowded slides do not accomplish this purpose.
8. Expect questions
Another thing that might be slightly different about a research presentation is questions. Most presentations have the question section after the presenter has finished. Research presentations are different because they allow for questions during the presentation (assuming it is a presentation to a small group). If you get any questions in the middle of the presentation, it is not someone being rude, but simply a fellow researcher who is legitimately curious about your topic. Of course, there will be a question period after the presentation, but you may be asked questions during the presentation. If you read enough information on the topic, you should be able to answer any question easily, but if the question is completely unrelated to anything you read, then it is perfectly reasonable to answer that you did not research the specific area in question. Overall, the questions related to your presentation should not be your biggest worry, but you should definitely be ready.
These are not all the rules for a literature review presentation nor are they set in stone. These are just some tips that I was told or learned that were the most helpful for me, so I hope they will help you too. I had to rewrite my presentation entirely my first literature review because I did not understand some of these differences, so if you give the presentation when you are scheduled to go, you are already better off than I was. Also, do not be afraid to ask anyone in the research group, even Rosy, if you need help. Chances are everyone in the group has given a literature review presentation at some point, so we would be more than happy to help you if you are confused about something. That being said, we are not experts on your topic, so specific questions about organization and content are going to have to be figured out by yourself. Either way, no matter what you do, do not stress out about this presentation. The goal of the presentation is mostly just to help improve your knowledge on a topic, and the presentation is simply to share with the group some of the information you have learned. Best of luck with the presentation, and I hope these tips help clear up what exactly the goal of a literature review presentation in a research setting is.
Jump to navigation

Cochrane Training
Presenting at conferences.

Academic conferences are a useful way to present the results of a Cochrane review to people either through an oral presentation, a poster presentation, or a booth. Conferences also have the additional benefit of networking and an opportunity to promote both Cochrane and the results of your review to peers.
How to present at conferences
Oral presentation.
Good oral presentations should be captivating, get the message across clearly, consider the language and context of the audience, and keep people engaged throughout. Not everyone can be an expert public speaker, and in many ways, it takes practice to become good at delivering engaging oral presentations. Our resources below can help.
The ‘Community Templates’ section on the brand resources page provides templates for PowerPoint presentations that can be used at conferences. There is a video on Creating a PowerPoint Presentation to explain how to use the template.
This video gives some tips for effective presentations at conferences such as:
- Choosing your content
- Using an appropriate structure
- Eliminating jargon
- Creating effective slides
- Finding your passion!
Poster presentation
Make your poster one that people want to stop and look at when you are at a conference. If you are preparing a poster presentation, these resources will help your work stand out in a sea of posters:
- The ‘Community Templates’ on the brand resources page provide pre-branded poster templates. They are very simple to use – you just need to download and add in the content.
- Cochrane officially endorses the #betterposter design. These new templates offer posters with less text and a decluttered design with the main finding in plain English as the highlighted feature. Learn more about the design and watch a quick introduction.
- This information sheet contains useful questions for preparing a poster for a conference.
Conference booth
At some conferences, you may have the opportunity to showcase your work at a booth. If you have multiple dissemination products that you created, you can display them here. You might also want to bring screens or computers to make your booth more interactive. Like posters, you want to make sure your booth is one that people want to visit and interact with.
You can contact Cochrane to discuss your event , get clarification on Cochrane event policies, or help with event branding such as special banners, flyers or branded items to give away.
If you are hosting the symposium or conference, contact Cochrane to have it listed and promoted on our website.
Sharing your presentation
When you know you'll be presenting at a conference, share the details on social media. For more information on social media platforms and how to use them effectively, visit this page. On social media, tell people where you are going, what you'll be presenting, and provide a link to sign up to attend (if possible).
During your presentation, you might want to consider having a colleague or peer live-tweeting. This will give you content to re-tweet later, and give people in the room content to share as well. Others in the room might also be tweeting about your presentation, which you can re-tweet later. You might want to consider live streaming your presentation on YouTube, Facebook or Instagram so your followers who aren’t in attendance can watch you present in real time.
If you don’t have your own social media accounts, we can share a picture of you at a conference on Cochrane’s social media. It is great to get a picture beside your poster, at your booth, or beside something with the conference name. If you are interested, please send the following to Muriah Umoquit at [email protected] : - Your name - Your Instagram/Twitter handle if you want it included - The related Review or Centre group - Title of your poster or presentation - Link to Cochrane Review if appropriate - Title of the conference - Official conference hashtag - A picture
After your presentation, you can distribute materials to your audience so that the information stays with them. This could be copies or recordings of the presentation, or another dissemination product related to what you presented. You can distribute in person at the conference, afterwards if you have the details of who attended your session, or through social media for anyone who may have followed you on a social media platform because of your presentation.
Evaluating the effect of your presentation
Many conferences will do their own evaluation of their conference programming, including oral presentations that were given. They may ask attendees questions about the topic that was presented, the effectiveness of the presenters, and the quality of the presentation. Ask your conference host whether they evaluate presentations. If they do, you can request feedback on your presentation that way.
You can also seek feedback on your own from your audience if you gave a presentation. You can do this through hard copy surveys at tables or chairs that you can collect, through email after your presentation, or you can do live evaluation surveys. These work by surveying people in real time through posing a question you can embed in your presentation, and have audience members provide input on their phones by visiting a link you give them. Sli.do and Menti are popular tools for this.
Examples of presenting at conferences by Cochrane groups
This is a great case study of how Cochrane UK used a booth at a conference – with great tips on what to do before, during, and after the conference.
Back to top

Journal of Education and Research in Nursing

Guidelines for Conducting a Literature Review and Presenting Conference Papers
A literature review provides a solid background for a research paper and reveals a comprehensive knowledge of the literature. Apart from providing you with a useful overview of a particular subject area, a literature review is important for keeping you up to date with what is current in your field. There is no simple recipe for good conference presentations and scholars in different disciplines will take different approaches. Finally, the quality, of the literature review provides an academic with credibility in his or her field. This literature review and conference papers should be prepared very seriously. This paper will outline the nature and purpose of a literature review and present guidelines for how to conduct and write a literature review and also will give practical knowledge about preparing and presenting of good conference.
Derleme Makale Yazımında, Konferans ve Bildiri Sunumu Hazırlamada Pratik Bilgiler
Derleme makaleler belirli bir konunun yararlı bir ‘toparlaması’ olmanın yanı sıra, araştırmacıların uzmanlık alanlarındaki yenilikleri izleyebilmeleri açısından da son derece önemlidir. Bu tip çalışmalar araştırma makalelerine dayanak oluşturdukları gibi kapsamlı bir literatür taramasını da içerir. Başarılı bir konferans ya da bildiri sunumunun basit bir reçetesi yoktur ve farklı disiplinlerde çalışan akademisyenlerin de farklı yaklaşımları söz konusu olmaktadır. Bir akademisyenin kendi alanındaki prestijine katkıda bulunan bu yazıların ve konferans ya da bildiri sunumlarının özenle hazırlanmaları gerekir. Bu yazıda, derleme makalenin temel yapısı ile amaçlarının özetlenmesi, başarılı bir derleme yazı, konferans veya bildiri sunumu hazırlama ve bu bildirilerin sunumuna yönelik pratik bilgilerin iletilmesi amaçlandı.
Journal Citation Indicator: 0.18 CiteScore: 1.1 Source Normalized Impact per Paper: 0.22 SCImago Journal Rank: 0.348

- Abstrating and Indexing
- Aim and Scope
- Editorial Board
- Ethics Policy
- ICMJE Recommendations
- Koç University Semahat Arsel Nursing Education and Research Center
Copyright © 2024 Journal of Education and Research in Nursing

- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to prepare an...
How to prepare an effective research poster
- Related content
- Peer review
- Lucia Hartigan , registrar 1 ,
- Fionnuala Mone , fellow in maternal fetal medicine 1 ,
- Mary Higgins , consultant obstetrician 1 2
- 1 National Maternity Hospital, Dublin
- 2 Obstetrics and Gynaecology, Medicine and Medical Sciences, University College Dublin
- mhiggins{at}nmh.ie
Being asked to give a poster presentation can be exciting, and you need not be daunted by the prospect of working out how to prepare one. As Lucia Hartigan and colleagues explain, many options are available
The long nights are over, the statistics have been run, the abstract has been written, and the email pops into your inbox: “Congratulations! You have been accepted for a poster presentation.”
All that work has been worthwhile. Your consultant congratulates you and your colleagues are envious of your having a legitimate excuse to go away for a couple of days, but now you have to work out how to prepare a poster. Do not despair, for you have many options.
Firstly, take this seriously. A poster is not a consolation prize for not being given an oral presentation. This is your chance to show your work, talk to others in the field, and, if you are lucky, to pick up pointers from experts. Given that just 45% of published abstracts end in a full paper, 1 this may be your only chance to get your work out there, so put some effort into it. If you don’t have access to the services of a graphic designer, then some work will be entailed as it normally takes us a full day to prepare the layout of a poster. If you are lucky enough to have help from a graphic designer, then you will need to check that the data are correct before it is sent to the printer. After all, it will be your name on the poster, not the graphic designer’s.
Secondly, check the details of the requirements. What size poster should you have? If it is too big, it may look arrogant. If it is too small, then it may seem too modest and self effacing. Should it be portrait or landscape? Different meetings have different requirements. Some may stay with traditional paper posters, so you need to factor in printing. Others present them electronically, but may have a deadline by which you need to have uploaded the poster. When planning a meeting the organisers work out how many poster boards there will be and then the numbers, so follow their requirements and read the small print.
Then make a template. It can be tempting to “borrow” a poster template from someone else, and this may buy you some time, but it is important to check what page set-up and size have been selected for the template. If it’s meant for an A2 size and you wish to print your poster on A0 paper, then the stretching may lead to pixillation, which would not look good.
Next, think about your layout. Use text boxes to cover the following areas: title (with authors, institution, and logo), background, methods, results, and conclusions. Check that the text boxes are aligned by using gridlines, and justify your text. Use different colours for titles, and make sure you can read the title from 3 metres away. Some people will put their abstract in a separate box in the top right hand corner underneath the title, and then expand a little in the other areas. That is fine, so long as you follow the golden rule of writing a poster: do not include too much text. One study showed that less than 5% of conference attendees visit posters at meetings and that few ask useful questions. 2 The same research found that, in addition to the scientific content of a poster, the factors that increase visual appeal include pictures, graphs, and a limited use of words. 2 The ideal number of words seems to be between 300 and 400 per square metre.
Now make it look pretty and eye catching, and use lots of graphics. Outline text boxes or fill them with a different colour. If you can present the data using a graph, image, or figures rather than text, then do so, as this will add visual appeal. If you want to put a picture in the background, and it is appropriate to do so, fade the image so that it does not distract from the content.
Fonts are important. Check whether the meeting has set criteria for fonts; if they have, then follow them. You do not want to stand out for the wrong reason. If there are no specified criteria, then the title should be in point size 72-84, depending on the size of the poster. The authors’ names should be either the same size, but in italics, or else a couple of sizes smaller.
If you are including the hospital logo, don’t take a picture that will not size up properly when enlarged. Instead, obtain a proper copy from the hospital administrators.
References can be in small writing. No one is likely to read them, and you are including them only to remind yourself what you learnt in the literature review. One intriguing possibility is the use of a trigger image to link the poster to online content. 3
Finally, there are also things you should not do. Don’t leave your figures unlabelled, include spelling errors, use abbreviations without an explanation, or go outside the boundaries of the poster. Don’t be ashamed that you “only” have a poster. At a good meeting you may find that the comments from passers by are an amazing peer review. We have presented at meetings where world experts have given feedback, and with that feedback we have written the paper on the flight home.
Competing interests: We have read and understood the BMJ Group policy on declaration of interests and have no relevant interests to declare.
- ↵ Scherer RW, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev 2007 ; 2 : MR000005 . OpenUrl PubMed
- ↵ Goodhand JR, Giles CL, Wahed M, Irving PM, Langmead L, Rampton DS. Poster presentations at medical conferences: an effective way of disseminating research? Clin Med 2011 ; 1 : 138 -41. OpenUrl
- ↵ Atherton S, Javed M, Webster S, Hemington-Gorse S. Use of a mobile device app: a potential new tool for poster presentations and surgical education. J Visual Comm Med 2013 ; 36 (1-2): 6 -10. OpenUrl
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Health Serv Res
- v.42(1 Pt 1); 2007 Feb
Preparing and Presenting Effective Research Posters
Associated data.
APPENDIX A.2. Comparison of Research Papers, Presentations, and Posters—Contents.
Posters are a common way to present results of a statistical analysis, program evaluation, or other project at professional conferences. Often, researchers fail to recognize the unique nature of the format, which is a hybrid of a published paper and an oral presentation. This methods note demonstrates how to design research posters to convey study objectives, methods, findings, and implications effectively to varied professional audiences.
A review of existing literature on research communication and poster design is used to identify and demonstrate important considerations for poster content and layout. Guidelines on how to write about statistical methods, results, and statistical significance are illustrated with samples of ineffective writing annotated to point out weaknesses, accompanied by concrete examples and explanations of improved presentation. A comparison of the content and format of papers, speeches, and posters is also provided.
Each component of a research poster about a quantitative analysis should be adapted to the audience and format, with complex statistical results translated into simplified charts, tables, and bulleted text to convey findings as part of a clear, focused story line.
Conclusions
Effective research posters should be designed around two or three key findings with accompanying handouts and narrative description to supply additional technical detail and encourage dialog with poster viewers.
An assortment of posters is a common way to present research results to viewers at a professional conference. Too often, however, researchers treat posters as poor cousins to oral presentations or published papers, failing to recognize the opportunity to convey their findings while interacting with individual viewers. By neglecting to adapt detailed paragraphs and statistical tables into text bullets and charts, they make it harder for their audience to quickly grasp the key points of the poster. By simply posting pages from the paper, they risk having people merely skim their work while standing in the conference hall. By failing to devise narrative descriptions of their poster, they overlook the chance to learn from conversations with their audience.
Even researchers who adapt their paper into a well-designed poster often forget to address the range of substantive and statistical training of their viewers. This step is essential for those presenting to nonresearchers but also pertains when addressing interdisciplinary research audiences. Studies of policymakers ( DiFranza and the Staff of the Advocacy Institute 1996 ; Sorian and Baugh 2002 ) have demonstrated the importance of making it readily apparent how research findings apply to real-world issues rather than imposing on readers to translate statistical findings themselves.
This methods note is intended to help researchers avoid such pitfalls as they create posters for professional conferences. The first section describes objectives of research posters. The second shows how to describe statistical results to viewers with varied levels of statistical training, and the third provides guidelines on the contents and organization of the poster. Later sections address how to prepare a narrative and handouts to accompany a research poster. Because researchers often present the same results as published research papers, spoken conference presentations, and posters, Appendix A compares similarities and differences in the content, format, and audience interaction of these three modes of presenting research results. Although the focus of this note is on presentation of quantitative research results, many of the guidelines about how to prepare and present posters apply equally well to qualitative studies.
WHAT IS A RESEARCH POSTER?
Preparing a poster involves not only creating pages to be mounted in a conference hall, but also writing an associated narrative and handouts, and anticipating the questions you are likely to encounter during the session. Each of these elements should be adapted to the audience, which may include people with different levels of familiarity with your topic and methods ( Nelson et al. 2002 ; Beilenson 2004 ). For example, the annual meeting of the American Public Health Association draws academics who conduct complex statistical analyses along with practitioners, program planners, policymakers, and journalists who typically do not.
Posters are a hybrid form—more detailed than a speech but less than a paper, more interactive than either ( Appendix A ). In a speech, you (the presenter) determine the focus of the presentation, but in a poster session, the viewers drive that focus. Different people will ask about different facets of your research. Some might do policy work or research on a similar topic or with related data or methods. Others will have ideas about how to apply or extend your work, raising new questions or suggesting different contrasts, ways of classifying data, or presenting results. Beilenson (2004) describes the experience of giving a poster as a dialogue between you and your viewers.
By the end of an active poster session, you may have learned as much from your viewers as they have from you, especially if the topic, methods, or audience are new to you. For instance, at David Snowdon's first poster presentation on educational attainment and longevity using data from The Nun Study, another researcher returned several times to talk with Snowdon, eventually suggesting that he extend his research to focus on Alzheimer's disease, which led to an important new direction in his research ( Snowdon 2001 ). In addition, presenting a poster provides excellent practice in explaining quickly and clearly why your project is important and what your findings mean—a useful skill to apply when revising a speech or paper on the same topic.
WRITING FOR A VARIED PROFESSIONAL AUDIENCE
Audiences at professional conferences vary considerably in their substantive and methodological backgrounds. Some will be experts on your topic but not your methods, some will be experts on your methods but not your topic, and most will fall somewhere in between. In addition, advances in research methods imply that even researchers who received cutting-edge methodological training 10 or 20 years ago might not be conversant with the latest approaches. As you design your poster, provide enough background on both the topic and the methods to convey the purpose, findings, and implications of your research to the expected range of readers.
Telling a Simple, Clear Story
Write so your audience can understand why your work is of interest to them, providing them with a clear take-home message that they can grasp in the few minutes they will spend at your poster. Experts in communications and poster design recommend planning your poster around two to three key points that you want your audience to walk away with, then designing the title, charts, and text to emphasize those points ( Briscoe 1996 ; Nelson et al. 2002 ; Beilenson 2004 ). Start by introducing the two or three key questions you have decided will be the focus of your poster, and then provide a brief overview of data and methods before presenting the evidence to answer those questions. Close with a summary of your findings and their implications for research and policy.
A 2001 survey of government policymakers showed that they prefer summaries of research to be written so they can immediately see how the findings relate to issues currently facing their constituencies, without wading through a formal research paper ( Sorian and Baugh 2002 ). Complaints that surfaced about many research reports included that they were “too long, dense, or detailed,” or “too theoretical, technical, or jargony.” On average, respondents said they read only about a quarter of the research material they receive for detail, skim about half of it, and never get to the rest.
To ensure that your poster is one viewers will read, understand, and remember, present your analyses to match the issues and questions of concern to them, rather than making readers translate your statistical results to fit their interests ( DiFranza and the Staff of the Advocacy Institute 1996 ; Nelson et al. 2002 ). Often, their questions will affect how you code your data, specify your model, or design your intervention and evaluation, so plan ahead by familiarizing yourself with your audience's interests and likely applications of your study findings. In an academic journal article, you might report parameter estimates and standard errors for each independent variable in your regression model. In the poster version, emphasize findings for specific program design features, demographic, or geographic groups, using straightforward means of presenting effect size and statistical significance; see “Describing Numeric Patterns and Contrasts” and “Presenting Statistical Test Results” below.
The following sections offer guidelines on how to present statistical findings on posters, accompanied by examples of “poor” and “better” descriptions—samples of ineffective writing annotated to point out weaknesses, accompanied by concrete examples and explanations of improved presentation. These ideas are illustrated with results from a multilevel analysis of disenrollment from the State Children's Health Insurance Program (SCHIP; Phillips et al. 2004 ). I chose that paper to show how to prepare a poster about a sophisticated quantitative analysis of a topic of interest to HSR readers, and because I was a collaborator in that study, which was presented in the three formats compared here—as a paper, a speech, and a poster.
Explaining Statistical Methods
Beilenson (2004) and Briscoe (1996) suggest keeping your description of data and methods brief, providing enough information for viewers to follow the story line and evaluate your approach. Avoid cluttering the poster with too much technical detail or obscuring key findings with excessive jargon. For readers interested in additional methodological information, provide a handout and a citation to the pertinent research paper.
As you write about statistical methods or other technical issues, relate them to the specific concepts you study. Provide synonyms for technical and statistical terminology, remembering that many conferences of interest to policy researchers draw people from a range of disciplines. Even with a quantitatively sophisticated audience, don't assume that people will know the equivalent vocabulary used in other fields. A few years ago, the journal Medical Care published an article whose sole purpose was to compare statistical terminology across various disciplines involved in health services research so that people could understand one another ( Maciejewski et al. 2002 ). After you define the term you plan to use, mention the synonyms from the various fields represented in your audience.
Consider whether acronyms are necessary on your poster. Avoid them if they are not familiar to the field or would be used only once or twice on your poster. If you use acronyms, spell them out at first usage, even those that are common in health services research such as “HEDIS®”(Health Plan Employer Data and Information Set) or “HLM”(hierarchical linear model).
Poor: “We use logistic regression and a discrete-time hazards specification to assess relative hazards of SCHIP disenrollment, with plan level as our key independent variable.” Comment: Terms like “discrete-time hazards specification” may be confusing to readers without training in those methods, which are relatively new on the scene. Also the meaning of “SCHIP” or “plan level” may be unfamiliar to some readers unless defined earlier on the poster.
Better: “Chances of disenrollment from the State Children's Health Insurance Program (SCHIP) vary by amount of time enrolled, so we used hazards models (also known as event history analysis or survival analysis) to correct for those differences when estimating disenrollment patterns for SCHIP plans for different income levels.” Comment: This version clarifies the terms and concepts, naming the statistical method and its synonyms, and providing a sense of why this type of analysis is needed.
To explain a statistical method or assumption, paraphrase technical terms and illustrate how the analytic approach applies to your particular research question and data:
Poor : “The data structure can be formulated as a two-level hierarchical linear model, with families (the level-1 unit of analysis) nested within counties (the level-2 unit of analysis).” Comment: Although this description would be fine for readers used to working with this type of statistical model, those who aren't conversant with those methods may be confused by terminology such as “level-1” and “unit of analysis.”
Better: “The data have a hierarchical (or multilevel) structure, with families clustered within counties.” Comment: By replacing “nested” with the more familiar “clustered,” identifying the specific concepts for the two levels of analysis, and mentioning that “hierarchical” and “multilevel” refer to the same type of analytic structure, this description relates the generic class of statistical model to this particular study.
Presenting Results with Charts
Charts are often the preferred way to convey numeric patterns, quickly revealing the relative sizes of groups, comparative levels of some outcome, or directions of trends ( Briscoe 1996 ; Tufte 2001 ; Nelson et al. 2002 ). As Beilenson puts it, “let your figures do the talking,” reducing the need for long text descriptions or complex tables with lots of tiny numbers. For example, create a pie chart to present sample composition, use a simple bar chart to show how the dependent variable varies across subgroups, or use line charts or clustered bar charts to illustrate the net effects of nonlinear specifications or interactions among independent variables ( Miller 2005 ). Charts that include confidence intervals around point estimates are a quick and effective way to present effect size, direction, and statistical significance. For multivariate analyses, consider presenting only the results for the main variables of interest, listing the other variables in the model in a footnote and including complex statistical tables in a handout.
Provide each chart with a title (in large type) that explains the topic of that chart. A rhetorical question or summary of the main finding can be very effective. Accompany each chart with a few annotations that succinctly describe the patterns in that chart. Although each chart page should be self-explanatory, be judicious: Tufte (2001) cautions against encumbering your charts with too much “nondata ink”—excessive labeling or superfluous features such as arrows and labels on individual data points. Strive for a balance between guiding your readers through the findings and maintaining a clean, uncluttered poster. Use chart types that are familiar to your expected audience. Finally, remember that you can flesh out descriptions of charts and tables in your script rather than including all the details on the poster itself; see “Narrative to Accompany a Poster.”
Describing Numeric Patterns and Contrasts
As you describe patterns or numeric contrasts, whether from simple calculations or complex statistical models, explain both the direction and magnitude of the association. Incorporate the concepts under study and the units of measurement rather than simply reporting coefficients (β's) ( Friedman 1990 ; Miller 2005 ).
Poor: “Number of enrolled children in the family is correlated with disenrollment.” Comment: Neither the direction nor the size of the association is apparent.
Poor [version #2]: “The log-hazard of disenrollment for one-child families was 0.316.” Comment: Most readers find it easier to assess the size and direction from hazards ratios (a form of relative risk) instead of log-hazards (log-relative risks, the β's from a hazards model).
Better: “Families with only one child enrolled in the program were about 1.4 times as likely as larger families to disenroll.” Comment: This version explains the association between number of children and disenrollment without requiring viewers to exponentiate the log-hazard in their heads to assess the size and direction of that association. It also explicitly identifies the group against which one-child families are compared in the model.
Presenting Statistical Test Results
On your poster, use an approach to presenting statistical significance that keeps the focus on your results, not on the arithmetic needed to conduct inferential statistical tests. Replace standard errors or test statistics with confidence intervals, p- values, or symbols, or use formatting such as boldface, italics, or a contrasting color to denote statistically significant findings ( Davis 1997 ; Miller 2005 ). Include the detailed statistical results in handouts for later perusal.
To illustrate these recommendations, Figures 1 and and2 2 demonstrate how to divide results from a complex, multilevel model across several poster pages, using charts and bullets in lieu of the detailed statistical table from the scientific paper ( Table 1 ; Phillips et al. 2004 ). Following experts' advice to focus on one or two key points, these charts emphasize the findings from the final model (Model 5) rather than also discussing each of the fixed- and random-effects specifications from the paper.

Presenting Complex Statistical Results Graphically

Text Summary of Additional Statistical Results
Multilevel Discrete-Time Hazards Models of Disenrollment from SCHIP, New Jersey, January 1998–April 2000
Source : Phillips et al. (2004) .
SCHIP, State Children's Health Insurance Program; LRH, log relative-hazard; SE, standard error.
Figure 1 uses a chart (also from the paper) to present the net effects of a complicated set of interactions between two family-level traits (race and SCHIP plan) and a cross-level interaction between race of the family and county physician racial composition. The title is a rhetorical question that identifies the issue addressed in the chart, and the annotations explain the pattern. The chart version substantially reduces the amount of time viewers need to understand the main take-home point, averting the need to mentally sum and exponentiate several coefficients from the table.
Figure 2 uses bulleted text to summarize other key results from the model, translating log-relative hazards into hazards ratios and interpreting them with minimal reliance on jargon. The results for family race, SCHIP plan, and county physician racial composition are not repeated in Figure 2 , averting the common problem of interpreting main effect coefficients and interaction coefficients without reference to one another.
Alternatively, replace the text summary shown in Figure 2 with Table 2 —a simplified version of Table 1 which presents only the results for Model 5, replaces log-relative hazards with hazards ratios, reports associated confidence intervals in lieu of standard errors, and uses boldface to denote statistical significance. (On a color slide, use a contrasting color in lieu of bold.)
Relative Risks of SCHIP Disenrollment for Other * Family and County Characteristics, New Jersey, January 1998–April 2000
Statistically significant associations are shown in bold.
Based on hierarchical linear model controlling for months enrolled, months-squared, race, SCHIP plan, county physician racial composition, and all variables shown here. Scaled deviance =30,895. Random effects estimate for between-county variance =0.005 (standard error =0.006). SCHIP, State Children's Health Insurance Program; 95% CI, 95% confidence interval.
CONTENTS AND ORGANIZATION OF A POSTER
Research posters are organized like scientific papers, with separate pages devoted to the objectives and background, data and methods, results, and conclusions ( Briscoe 1996 ). Readers view the posters at their own pace and at close range; thus you can include more detail than in slides for a speech (see Appendix A for a detailed comparison of content and format of papers, speeches, and posters). Don't simply post pages from the scientific paper, which are far too text-heavy for a poster. Adapt them, replacing long paragraphs and complex tables with bulleted text, charts, and simple tables ( Briscoe 1996 ; Beilenson 2004 ). Fink (1995) provides useful guidelines for writing text bullets to convey research results. Use presentation software such as PowerPoint to create your pages or adapt them from related slides, facilitating good page layout with generous type size, bullets, and page titles. Such software also makes it easy to create matching handouts (see “Handouts”).
The “W's” (who, what, when, where, why) are an effective way to organize the elements of a poster.
- In the introductory section, describe what you are studying, why it is important, and how your analysis will add to the existing literature in the field.
- In the data and methods section of a statistical analysis, list when, where, who, and how the data were collected, how many cases were involved, and how the data were analyzed. For other types of interventions or program evaluations, list who, when, where, and how many, along with how the project was implemented and assessed.
- In the results section, present what you found.
- In the conclusion, return to what you found and how it can be used to inform programs or policies related to the issue.
Number and Layout of Pages
To determine how many pages you have to work with, find out the dimensions of your assigned space. A 4′ × 8′ bulletin board accommodates the equivalent of about twenty 8.5″ × 11″ pages, but be selective—no poster can capture the full detail of a large series of multivariate models. A trifold presentation board (3′ high by 4′ wide) will hold roughly a dozen pages, organized into three panels ( Appendix B ). Breaking the arrangement into vertical sections allows viewers to read each section standing in one place while following the conventions of reading left-to-right and top-to-bottom ( Briscoe 1996 ).
- At the top of the poster, put an informative title in a large, readable type size. On a 4′ × 8′ bulletin board, there should also be room for an institutional logo.

Suggested Layout for a 4′ × 8′ poster.
- In the left-hand panel, set the stage for the research question, conveying why the topic is of policy interest, summarizing major empirical or theoretical work on related topics, and stating your hypotheses or project aims, and explaining how your work fills in gaps in previous analyses.
- In the middle panel, briefly describe your data source, variables, and methods, then present results in tables or charts accompanied by text annotations. Diagrams, maps, and photographs are very effective for conveying issues difficult to capture succinctly in words ( Miller 2005 ), and to help readers envision the context. A schematic diagram of relationships among variables can be useful for illustrating causal order. Likewise, a diagram can be a succinct way to convey timing of different components of a longitudinal study or the nested structure of a multilevel dataset.
- In the right-hand panel, summarize your findings and relate them back to the research question or project aims, discuss strengths and limitations of your approach, identify research, practice, or policy implications, and suggest directions for future research.
Figure 3 (adapted from Beilenson 2004 ) shows a suggested layout for a 4′ × 8′ bulletin board, designed to be created using software such as Pagemaker that generates a single-sheet presentation; Appendix C shows a complete poster version of the Phillips et al. (2004) multilevel analysis of SCHIP disenrollment. If hardware or budget constraints preclude making a single-sheet poster, a similar configuration can be created using standard 8.5″ × 11″ pages in place of the individual tables, charts, or blocks of text shown in Figure 3 .
Find out well in advance how the posters are to be mounted so you can bring the appropriate supplies. If the room is set up for table-top presentations, tri-fold poster boards are essential because you won't have anything to attach a flat poster board or pages to. If you have been assigned a bulletin board, bring push-pins or a staple gun.
Regardless of whether you will be mounting your poster at the conference or ahead of time, plan how the pages are to be arranged. Experiment with different page arrangements on a table marked with the dimensions of your overall poster. Once you have a final layout, number the backs of the pages or draw a rough sketch to work from as you arrange the pages on the board. If you must pin pages to a bulletin board at the conference venue, allow ample time to make them level and evenly spaced.
Other Design Considerations
A few other issues to keep in mind as you design your poster. Write a short, specific title that fits in large type size on the title banner of your poster. The title will be potential readers' first glimpse of your poster, so make it inviting and easy to read from a distance—at least 40-point type, ideally larger. Beilenson (2004) advises embedding your key finding in the title so viewers don't have to dig through the abstract or concluding page to understand the purpose and conclusions of your work. A caution: If you report a numeric finding in your title, keep in mind that readers may latch onto it as a “factoid” to summarize your conclusions, so select and phrase it carefully ( McDonough 2000 ).
Use at least 14-point type for the body of the poster text. As Briscoe (1996) points out, “many in your audience have reached the bifocal age” and all of them will read your poster while standing, hence long paragraphs in small type will not be appreciated! Make judicious use of color. Use a clear, white, or pastel for the background, with black or another dark color for most text, and a bright, contrasting shade to emphasize key points or to identify statistically significant results ( Davis 1997 ).
NARRATIVE TO ACCOMPANY A POSTER
Prepare a brief oral synopsis of the purpose, findings, and implications of your work to say to interested parties as they pause to read your poster. Keep it short—a few sentences that highlight what you are studying, a couple of key findings, and why they are important. Design your overview as a “sound byte” that captures your main points in a succinct and compelling fashion ( Beilenson 2004 ). After hearing your introduction, listeners will either nod and move along or comment on some aspect of your work that intrigues them. You can then tailor additional discussion to individual listeners, adjusting the focus and amount of detail to suit their interests. Gesture at the relevant pages as you make each point, stating the purpose of each chart or table and explaining its layout before describing the numeric findings; see Miller (2005) for guidelines on how to explain tables and charts to a live audience. Briscoe (1996) points out that these mini-scripts are opportunities for you to fill in details of your story line, allowing you to keep the pages themselves simple and uncluttered.
Prepare short answers to likely questions about various aspects of your work, such as why it is important from a policy or research perspective, or descriptions of data, methods, and specific results. Think of these as little modules from an overall speech—concise descriptions of particular elements of your study that you can choose among in response to questions that arise. Beilenson (2004) also recommends developing a few questions to ask your viewers, inquiring about their reactions to your findings, ideas for additional questions, or names of others working on the topic.
Practice your poster presentation in front of a test audience acquainted with the interests and statistical proficiency of your expected viewers. Ideally, your critic should not be too familiar with your work: A fresh set of eyes and ears is more likely to identify potential points of confusion than someone who is jaded from working closely with the material while writing the paper or drafting the poster ( Beilenson 2004 ). Ask your reviewer to identify elements that are unclear, flag jargon to be paraphrased or defined, and recommend changes to improve clarity ( Miller 2005 ). Have them critique your oral presentation as well as the contents and layout of the poster.
Prepare handouts to distribute to interested viewers. These can be produced from slides created in presentation software, printed several to a page along with a cover page containing the abstract and your contact information. Or package an executive summary or abstract with a few key tables or charts. Handouts provide access to the more detailed literature review, data and methods, full set of results, and citations without requiring viewers to read all of that information from the poster ( Beilenson 2004 ; Miller 2005 ). Although you also can bring copies of the complete paper, it is easier on both you and your viewers if you collect business cards or addresses and mail the paper later.
The quality and effectiveness of research posters at professional conferences is often compromised by authors' failure to take into account the unique nature of such presentations. One common error is posting numerous statistical tables and long paragraphs from a research paper—an approach that overwhelms viewers with too much detail for this type of format and presumes familiarity with advanced statistical techniques. Following recommendations from the literature on research communication and poster design, this paper shows how to focus each poster on a few key points, using charts and text bullets to convey results as part of a clear, straightforward story line, and supplementing with handouts and an oral overview.
Another frequent mistake is treating posters as a one-way means of communication. Unlike published papers, poster sessions are live presentations; unlike speeches, they allow for extended conversation with viewers. This note explains how to create an oral synopsis of the project, short modular descriptions of poster elements, and questions to encourage dialog. By following these guidelines, researchers can substantially improve their conference posters as vehicles to disseminate findings to varied research and policy audiences.
CHECKLIST FOR PREPARING AND PRESENTING AN EFFECTIVE RESEARCH POSTERS
- Design poster to focus on two or three key points.
- Adapt materials to suit expected viewers' knowledge of your topic and methods.
- Design questions to meet their interests and expected applications of your work.
- Paraphrase descriptions of complex statistical methods.
- Spell out acronyms if used.
- Replace large detailed tables with charts or small, simplified tables.
- Accompany tables or charts with bulleted annotations of major findings.
- Describe direction and magnitude of associations.
- Use confidence intervals, p -values, symbols, or formatting to denote statistical significance.
Layout and Format
- Organize the poster into background, data and methods, results, and study implications.
- Divide the material into vertical sections on the poster.
- Use at least 14-point type in the body of your poster, at least 40-point for the title.
Narrative Description
- Rehearse a three to four sentence overview of your research objectives and main findings.
- Summary of key studies and gaps in existing literature
- Data and methods
- Each table, chart, or set of bulleted results
- Research, policy, and practice implications
- Solicit their input on your findings
- Develop additional questions for later analysis
- Identify other researchers in the field
- Prepare handouts to distribute to interested viewers.
- Print slides from presentation software, several to a page.
- Or package an executive summary or abstract with a few key tables or charts.
- Include an abstract and contact information.
Acknowledgments
I would like to thank Ellen Idler, Julie Phillips, Deborah Carr, Diane (Deedee) Davis, and two anonymous reviewers for helpful comments on earlier drafts of this work.
Supplementary Material
The following supplementary material for this article is available online:
APPENDIX A.1. Comparison of Research Papers, Presentations, and Posters—Materials and Audience Interaction.
Suggested Layout for a Tri-Fold Presentation Board.
Example Research Poster of Phillips et al. 2004 Study.
- Beilenson J. Developing Effective Poster Presentations. Gerontology News. 2004; 32 (9):6–9. [ Google Scholar ]
- Briscoe MH. Preparing Scientific Illustrations: A Guide to Better Posters, Presentations, and Publications. 2. New York: Springer-Verlag; 1996. [ Google Scholar ]
- Davis M. Scientific Papers and Presentations. New York: Academic Press; 1997. [ Google Scholar ]
- DiFranza JR. A Researcher's Guide to Effective Dissemination of Policy-Related Research. Princeton, NJ: The Robert Wood Johnson Foundation; 1996. the Staff of the Advocacy Institute, with Assistance from the Center for Strategic Communications. [ Google Scholar ]
- Fink A. How to Report on Surveys. Thousand Oaks, CA: Sage Publications; 1995. [ Google Scholar ]
- Friedman GD. Be Kind to Your Reader. American Journal of Epidemiology. 1990; 132 (4):591–3. [ PubMed ] [ Google Scholar ]
- Maciejewski ML, Diehr P, Smith MA, Hebert P. Common Methodological Terms in Health Services Research and Their Symptoms. Medical Care. 2002; 40 :477–84. [ PubMed ] [ Google Scholar ]
- McDonough J. Experiencing Politics: A Legislator's Stories of Government and Health Care. Berkeley: University of California Press; 2000. [ Google Scholar ]
- Miller JE. The Chicago Guide to Writing about Multivariate Analysis. Chicago Guides to Writing, Editing and Publishing. Chicago: University of Chicago Press; 2005. [ Google Scholar ]
- Nelson DE, Brownson RC, Remington PL, Parvanta C, editors. Communicating Public Health Information Effectively: A Guide for Practitioners. Washington, DC: American Public Health Association; 2002. [ Google Scholar ]
- Phillips JA, Miller JE, Cantor JC, Gaboda D. Context or Composition. What Explains Variation in SCHIP Disenrollment? Health Services Research. 2004; 39 (4, part I):865–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Snowdon D. Aging with Grace: What the Nun Study Teaches Us about Leading Longer, Healthier, and More Meaningful Lives. New York: Bantam Books; 2001. [ Google Scholar ]
- Sorian R, Baugh T. Power of Information Closing the Gap between Research and Policy. Health Affairs. 2002; 21 (2):264–73. [ PubMed ] [ Google Scholar ]
- Tufte ER. The Visual Display of Quantitative Information. 2. Cheshire, CT: Graphics Press; 2001. [ Google Scholar ]
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Conference Presentations

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
This resource provides a detailed overview of the common types of conference papers and sessions graduate students can expect, followed by pointers on presenting conference papers for an audience.
Types of conference papers and sessions
Panel presentations are the most common form of presentation you will encounter in your graduate career. You will be one of three to four participants in a panel or session (the terminology varies depending on the organizers) and be given fifteen to twenty minutes to present your paper. This is often followed by a ten-minute question-and-answer session either immediately after your presentation or after all of the speakers are finished. It is up to the panel organizer to decide upon this framework. In the course of the question-and-answer session, you may also address and query the other panelists if you have questions yourself. Note that you can often propose a conference presentation by yourself and be sorted onto a panel by conference organizers, or you can propose a panel with a group of colleagues. Self-proposed panels typically have more closely related topics than conference-organized panels.
Roundtables feature an average of five to six speakers, each of whom gets the floor for approximately five to ten minutes to speak on their respective topics and/or subtopics. At times, papers from the speakers might be circulated in advance among the roundtable members or even prospective attendees.
Workshops feature one or a few organizers, who usually give a brief presentation but spend the majority of the time for the session facilitating an activity that attendees will do. Some common topics for these sessions typically include learning a technology or generating some content, such as teaching materials.
Lightning talks (or Ignite talks, or Pecha Kucha talks) are very short presentations where presenters' slide decks automatically advance after a few seconds; most individual talks are no longer than 5 minutes, and a lightning talk session typically invites 10 or more presenters to participate over the course of an hour or two rather than limiting the presenters like a panel presentation. A lightning talk session will sometimes be held as a sort of competition where attendees can vote for the best talk.
SIGs (Special Interest Groups) are groups of scholars focused on a particular smaller topic within the purview of the larger conference. The structure of these sessions varies by conference and even by group, but in general they tend to be structured either more like a panel presentation, with presenters and leaders, or more like a roundtable, with several speakers and a particular meeting agenda. These styles resemble, respectively, a miniconference focusing on a particular topic and a committee meeting.
Papers with respondents are structured around a speaker who gives an approximately thirty-minute paper and a respondent who contributes their own thoughts, objections, and further questions in the following fifteen minutes. Finally, the speaker gets that same amount of time to formulate their reply to the respondent.
Poster presentations ask participants to visually display their ideas on a research poster, which is typically displayed with other research posters in a specific area at a conference. The poster needs to be understandable on its own (without the author) as viewers sometimes look through the posters outside the bounds of the poster session, which is a scheduled period of time where poster authors stand with their posters and engage viewers in conversation about the work. Research posters have long tended to follow common templates for design, but in recent years some scholars have begun challenging these templates for improved usability (for example, the Better Poster campaign as described here or the APA template based on the original, here.
You can read more about research posters on our resource here .
Presenting the conference paper
Aim to take less time than you are given! If your presentation slot is 15 minutes, aim for 13 or 14 when you practice. A little leeway and a slightly shorter presentation is a courtesy to your audience and to your fellow presenters, and will not at all imply that you are unprepared or unprofessional — in fact, being able to keep well within your allotted time is the mark of a good presenter.
Make sure you speak slowly and clearly, using accessibility aids if available such as a microphone or closed captioning on a slide deck. Many presenters have begun bringing accessibility copies of their talks, which are printed transcripts of the talk using a larger font for audience members who need them. It is also becoming increasingly common for presenters at conferences to share their slides and copies of their talk via a shortened link or QR code found on the bottom of the slides so that audiences may access them later or even while they are in your session.
The conventions for presentation differ based on field. Some fields tend toward reading papers aloud with very little audiovisual accompaniment; others use slide decks; others speak extemporaneously. You can find out more about typical practices in your field by attending conferences yourself and by asking mentors. Generally, you will be able to improve the accessibility of your presentation if you have a visual accompaniment and prepared remarks.
Even in fields where presenters tend to read papers verbatim, it is rarely a good idea to bring a paper from a class or another research paper you have written without editing it for an oral presentation. Seminar papers tend to be too long to read in 15 minutes, and often lead to graduate students surpassing their time limits. Moreover, research papers are meant to be read — they lack the kinds of repetition and simple sentence structure that are more beneficial to listeners. Finally, conference presentations do not serve the same purposes as most class papers — typically in a class, you're expected to show that you have understood the material, but at a conference, listeners are more interested in hearing what contributions you have that might help them in their own research. It's typical to move the bulk of your literature review to an appendix or another document so that you can discuss other scholarship in the area if it comes up in the Q&A, but during your presentation you're left free to focus on your own methods and findings. (Many presenters will even say: "I'm skipping a lot of [X material] for the sake of time, but I'm happy to discuss it later with anyone who's interested.")
Since you will present your paper orally, you may repeat important points and say more about the structure of the essay than a written submission to a journal (or a paper for your undergraduate or graduate courses) would require. This often means signposting orally when you are moving to a new section of the paper or when you are shifting to a new idea. The thesis of your paper should come early in your presentation to give listeners a clear understanding of what is to follow. At this point, you may also overview or forecast your paper and tell listeners how you will move from one argument to the next. It is generally advised to quickly summarize your important points in a bulleted list at the end of your presentation to remind everyone of the two or three most essential arguments or findings.
If you use a slide presentation, you may want to follow the guidelines presented in the OWL resource, Designing an Effective PowerPoint Presentation .

- Career Advice for Researchers
Q: Can literature review articles be presented at conferences?
I am positive that a journal accepts Literature Review manuscripts. However, I am not so sure about conferences. Can anyone help me?
Asked by Rohan Akut on 25 Jun, 2018
Conferences do not usually accept literature review manuscripts or review articles for publishing in their conference proceedings. However, some conferences do allow publication of review articles as a poster. Alternatively, you can also request for a plenary discussion for your review article at the conference.
It is best to email the conference organizers with your query to get a clarification on their acceptance of review articles.
Further reading:
- 9 Tips for presenting at an academic conference
- How to identify predatory conferences: The Think.Check.Attend checklist
Answered by Editage Insights on 02 Jul, 2018
- Upvote this Answer

This content belongs to the Career Growth Stage
Confirm that you would also like to sign up for free personalized email coaching for this stage.
Trending Searches
- Statement of the problem
- Background of study
- Scope of the study
- Types of qualitative research
- Rationale of the study
- Concept paper
- Literature review
- Introduction in research
- Under "Editor Evaluation"
- Ethics in research
Recent Searches
- Review paper
- Responding to reviewer comments
- Predatory publishers
- Scope and delimitations
- Open access
- Plagiarism in research
- Journal selection tips
- Editor assigned
- Types of articles
- "Reject and Resubmit" status
- Decision in process
- Conflict of interest
- Open access
- Published: 05 June 2019
Good Practice for Conference Abstracts and Presentations: GPCAP
- Cate Foster ORCID: orcid.org/0000-0001-6236-5580 1 ,
- Elizabeth Wager ORCID: orcid.org/0000-0002-4202-7813 2 , 3 ,
- Jackie Marchington ORCID: orcid.org/0000-0001-8482-3028 4 ,
- Mina Patel ORCID: orcid.org/0000-0001-9357-1707 5 ,
- Steve Banner ORCID: orcid.org/0000-0002-7852-9284 6 ,
- Nina C. Kennard ORCID: orcid.org/0000-0002-8480-7033 7 ,
- Antonia Panayi ORCID: orcid.org/0000-0002-1997-3705 8 ,
- Rianne Stacey ORCID: orcid.org/0000-0002-6516-3172 9 &
the GPCAP Working Group
Research Integrity and Peer Review volume 4 , Article number: 11 ( 2019 ) Cite this article
147k Accesses
23 Citations
54 Altmetric
Metrics details
Research that has been sponsored by pharmaceutical, medical device and biotechnology companies is often presented at scientific and medical conferences. However, practices vary between organizations and it can be difficult to follow both individual conference requirements and good publication practice guidelines. Until now, no specific guidelines or recommendations have been available to describe best practice for conference presentations.
This document was developed by a working group of publication professionals and uploaded to PeerJ Preprints for consultation prior to publication; an additional 67 medical societies, medical conference sites and conference companies were also asked to comment. The resulting recommendations aim to complement current good publication practice and authorship guidelines, outline the general principles of best practice for conference presentations and provide recommendations around authorship, contributorship, financial transparency, prior publication and copyright, to conference organizers, authors and industry professionals.
While the authors of this document recognize that individual conference guidelines should be respected, they urge organizers to consider authorship criteria and data transparency when designing submission sites and setting parameters around word/character count and content for abstracts. It is also important to recognize that conference presentations have different limitations to full journal publications, for example, in the case of limited audiences that necessitate refocused abstracts, or where lead authors do not speak the local language, and these have been acknowledged accordingly. The authors also recognize the need for further clarity regarding copyright of previously published abstracts and have made recommendations to assist with best practice.
By following Good Practice for Conference Abstracts and Presentations: GPCAP recommendations, industry professionals, authors and conference organizers will improve consistency, transparency and integrity of publications submitted to conferences worldwide.
Peer Review reports
Note on terminology
Company refers to any medical commercial organization involved with research, such as pharmaceutical or biotechnology companies and medical device manufacturers.
Company-sponsored refers to all types of research (preclinical and clinical, pre- and post-marketing) that is directly sponsored and/or funded by a company. While this classification does not necessarily include research performed under other types of funding arrangement, such as investigator-sponsored or investigator-initiated trials or research (where companies are not involved with conference presentations or publications), those involved in submitting investigator-initiated study material to conferences are encouraged to consider following these recommendations.
Conference is used to refer to meetings, often organized by academic societies, that invite submissions (usually as abstracts) presenting research findings on an aspect of medicine or science. Such conferences have a scientific (or programme) committee that reviews and selects presentations to be given at the meeting from the submitted abstracts.
Abstract refers to those submitted for consideration to scientific and medical conferences (see above).
Presentation refers to posters or slides developed from abstracts accepted for presentation at such conferences.
Lead author refers to the person who normally presents study findings at a conference and is usually listed as the first author. This is often the Principal Investigator.
Society sponsor refers to a member of the society that is holding the conference, who acts as sponsor (or guarantor) of a submitted abstract.
Presenting author refers to the person on the author list who attends the conference and presents the poster or abstract.
Non-author presenter or local presenter refers to a person who presents on behalf of the author group, but who is not listed as an author.
Introduction
Research that has been sponsored (see the ‘Note on terminology’ section for precise definitions of these terms) by commercial organizations (e.g. pharmaceutical, medical device and biotechnology companies) is often presented at scientific and medical conferences. These conferences are pivotal for the presentation of data from ongoing research projects and clinical trials to the relevant audience and are often the first opportunity to disclose and discuss potentially practice-changing data. They facilitate early communication of data long before publication of a full manuscript and also provide the opportunity to present results of additional analyses such as secondary and/or exploratory endpoints and post hoc analyses. However, while abstracts submitted to conferences are reviewed by a scientific committee for suitability and interest to the audience prior to acceptance, it is important to note that they are not considered peer-reviewed as they are not subject to the same rigorous peer-review process as are journal articles. Poster and oral presentations based upon accepted abstracts are rarely, if ever reviewed. Furthermore, a recent systematic review showed that less than 50% of all studies accepted as abstracts went on to be published in full following presentation at a conference [ 1 ]. While it is desirable to strive for full publication after a conference presentation to ensure transparency and allow healthcare professionals to make appropriate informed decisions based on the peer-reviewed literature, this is not always practical and/or achievable. Therefore, it is important that abstracts and conference presentations, particularly for company-sponsored research, are developed with as rigorous a process as that of a full publication, because these may ultimately become the only source for a particular analysis.
While there are recommendations on the preparation of journal articles and qualification for authorship [ 2 ], and guidelines for best practices in the publication of company-sponsored research [ 3 , 4 , 5 ], until now, no specific guidelines have been available to describe good practice and best principles for conference presentations. This has resulted in diverse practices and a lack of standard expectations for transparency and ethical approaches. Although some aspects of good practice in Good Publication Practice (GPP) [ 5 ] and in reporting guidelines such as CONSORT and PRISMA for Abstracts [ 6 , 7 ] can be applied to conference presentations, the most widely cited recommendations on authorship from the International Committee of Medical Journal Editors (ICMJE) relate exclusively to publications in peer-reviewed journals [ 3 ]. These recommendations were not designed for, and therefore are not fully applicable to, abstract submissions and conference presentations and are challenging to implement in practice. Building on the acceptance and recognition of the GPP guidelines (first published as GPP for Pharmaceutical Companies in 2003 [ 3 ], updated in 2010 [ 4 ] and most recently published as GPP3 in 2015 [ 5 ]), this article endeavours to extend their principles and to address challenges relating to the presentation of company-sponsored research at academic meetings. These recommendations, on Good Practice for Conference Abstracts and Presentations (GPCAP), focus on company-sponsored research (see the ‘Note on terminology’ section). However, they do not cover other company activities that may be linked to conferences (e.g. satellite symposia organized alongside scientific conferences, medical education and marketing activities) because these are governed by regional and national legislation or codes (e.g. EFPIA code of practice [ 8 ], FDA regulations [ 9 ]). As with the GPP guidelines, GPCAP focuses on the presentation of all types of company-sponsored research and the specific challenges surrounding this, rather than investigator-sponsored or investigator-initiated trials or research (where companies have no role in their presentation or publication), although many of the principles also apply to the presentation of other types of research at scientific meetings. The aim of GPCAP is therefore to provide guidance on good submission and presentation practice for scientific and medical congresses, specifically addressing certain aspects where current publication guidelines are inadequate.
These recommendations were developed after informal discussions among a group of individuals who have wide experience of working with authors to develop abstracts, posters and slides for oral presentations reporting company-sponsored research. The main impetus for this article arose from a meeting regarding GPP3 updates (with which some of the authors had been involved). Prior to this meeting, two authors had noted that even the revised GPP3 guidelines contained limited advice for conference abstracts and presentations. Meeting participants discussed the requirement for clearer guidance and formed a working group to address this gap. At this point, invitations to join the group were extended to potential authors known to have previously presented relevant research at meetings of the International Society of Medical Publication Professionals (ISMPP) or had a known interest in conference presentations. This also ensured a broader global representation and improved the balance between pharmaceutical and medical communication agency representation. The authors all work or have worked for pharmaceutical companies and/or medical communication agencies (see the ‘Competing interest’ section for specific details). After a search for recommendations and guidelines on this topic revealed nothing specific (either in ICMJE or in a search on EQUATOR), the authors developed an initial outline for this article; individuals worked on pre-agreed sections and then a collective review of the full draft, comprising all sections was completed (see ‘Authors’ contributions’ for specific details). The resulting article was posted as a preprint on PeerJ [ 10 ] on 19 October 2017 for open comment. All comments received (and their responses) can be seen with the preprint on the PeerJ website. These comments were used to revise the recommendations. Some authors invited informal consultation from colleagues, and a courtesy legal review, as appropriate, was completed to ensure compliance with employee company policies. The copyright section was reviewed specifically for appropriate interpretation of copyright law. In addition to the preprint, 65 medical societies and medical conference sites, and two for-profit companies that run conferences on behalf of societies, were contacted for comment via contact emails listed on their websites or via ‘contact us’ options found on their websites. The societies and conferences and conference service companies were selected by recommendation from within the author group, to ensure balance across therapeutic areas, geography and variety of website submission sophistication. Only one of these societies/companies responded. All comments received on the preprint by 10 July 2018 were collated and discussed, and this final version was generated. The preprint was viewed by 2769 unique visitors and downloaded 3300 times between 19 October 2017 and 25 March 2019.
The recommendations are given here by topic, and so there is some overlap by intention, to ensure that all the key elements for any given topic appear together and allow readers to browse by topic.
Recommendations
The following principles aim to cover the key areas relevant for submissions to any research-based conference.
Author listings should reflect those who did the research and can take accountability for its conduct, and for the analysis and interpretation of the findings. Criteria for authorship of conference abstracts and presentations should generally be the same as those for full publications, although there can be occasions where local presenters may be included as authors, for example, where a conference requires a presenter to be listed as an author.
All authors should be involved in the development, and approve the final version, of any abstract, poster or slides that bears their names. For studies involving large numbers of researchers it may be most efficient for a subgroup of those involved in the studies to develop conference abstracts and presentations (similar to the use of a writing group to develop publications from large studies).
Posters and slides should list key contributors and describe their contributions to the research and development of the presentation.
Study registration numbers (e.g. ClinicalTrials.gov , EudraCT, PROSPERO) should be included on abstracts, posters and slides.
All sources of funding for the research and its presentation, and any author conflicts of interest, should be disclosed on posters and slides, on the conference submission site, and if space permits, on abstracts.
Any medical writing support and associated funding should be acknowledged on posters and slides, on the conference submission site, and if space permits, on abstracts.
These recommendations are mapped against the development of an abstract and subsequent conference presentation workflow in Fig. 1 , referenced by section number.
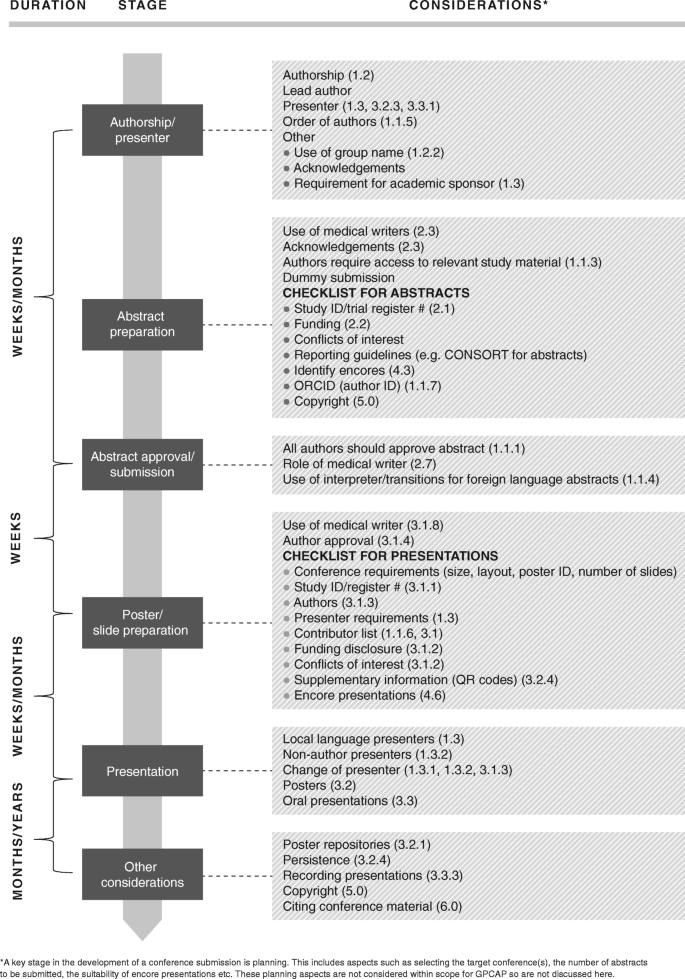
Roadmap of recommendations following abstract and presentation development stages
Recommendations for conference organizers
Conference organizers should:
encourage the inclusion of contributor lists on posters and slides;
include a field for trial registration details on abstract forms (outside the word or character limit) and publish this information with the abstract;
include a field for sponsor information on abstract forms (outside the word or character limit) and publish this information with the abstract;
include a field for disclosing medical writing support on abstract forms (outside the word or character limit) and publish this information with the abstract;
use ORCID identifiers (individual researcher identifiers [ 11 ]) to identify authors and presenters;
not set arbitrary limits on the number of authors, and permit the use of study group names; and
distinguish between authors (meeting the ICMJE criteria) and any additional individuals (who are not authors or contributors) included in the submission, for example, as a result of a requirement for a society member to sponsor submissions. With limited space in any printed book of abstracts, this information might be restricted to appearing with the online version of the abstract.
1.0 Authorship
1.1 authors.
1.1.1 The author listing on conference abstracts and presentations should reflect the people who did the research or contributed substantially to the design of the study or to the interpretation of the results, and who were involved in the development of the presentation and who are willing to take responsibility for the findings. Authorship and author order should be agreed by all authors (see 1.1.5 for factors to consider). While the authorship criteria recommended by the ICMJE are widely used for journal articles [ 2 ], GPP3 recognizes that it may be necessary to adopt slightly different criteria for conference abstracts and presentations [ 5 ]. For example, while all named authors should review (at least once), approve the content of abstracts and presentations and be willing to take responsibility for the findings, it may be impractical to expect all authors to contribute to drafting and critically revising abstracts in the same way as for full manuscripts, because of the abstract brevity, time constraints, etc. There is an argument for limiting the authors to a number that can meaningfully comment and review an abstract (see 1.2.1) and using a study group to identify others involved in the wider study. Our collective past experience indicates that it becomes impractical for everyone to be involved in a group with more than 10 authors, which is also the maximum number suggested by GPP3 [ 5 ].
1.1.2 Authorship criteria for all anticipated journal articles and primary conference presentations should, ideally, be agreed at the start of the research, and author listings for subsequent secondary abstracts and presentations should be finalized well before work starts on the secondary material [ 12 ]. As with journal publications, whatever criteria are used to determine authorship should be applied equally to all authors, regardless of whether they are company employees, contractors, independent clinicians, researchers or consultants.
1.1.3 Authors and contributors should have access to all relevant study materials and data to permit them to understand the research findings. Abstracts may need to be developed soon after results are analysed and before a final clinical study report is available. In such cases, authors should always have access to the protocol, statistical tables and any other information necessary to discuss and develop the planned abstract and presentation.
1.1.4 If individuals are authors on abstracts and presentations written in languages in which they are not proficient, companies should work with them and offer whatever reasonable assistance is required to permit them to discuss and review material effectively (e.g. to provide translations for the authors, or a discussion with an interpreter or local investigator/presenter who can read and explain the text). Authors may also choose not to be listed for such a conference abstract and presentation (see also 1.1.6).
1.1.5 Whatever convention is (or will be) used to determine the order of authors on the related full publications in journals should generally also be used to determine the order of listing on conference abstracts and presentations. The final order should be agreed by all authors; however, conference requirements (e.g. listing the presenting author first) must be respected. In cases where first or last co-authorship is requested, the conference organizers should be contacted for guidance.
1.1.6 While the authorship of conference abstracts and presentations should accurately reflect those who were involved in the research, individuals who meet the ICMJE authorship criteria (and may be listed on a subsequent full publication) may choose not to be listed for a conference abstract and presentation (e.g. if they are unable to review and/or approve the material within the deadline). While this individual choice should be respected, significant contributions to the research should be acknowledged where possible; that is, in a contributor list included on the presentation.
1.1.7 Conference organizers should encourage the use of ORCID identifiers to identify authors on abstracts and presentations, to avoid ambiguity between authors with similar or identical names. (Note: many journals and institutions now require authors to include their ORCID identifier at manuscript submission.)
1.2 Contributors/study groups
1.2.1 We encourage conferences (and company sponsors) not to limit the number of authors (or contributors) who may be listed on an abstract or presentation, because this practice may prevent the author list from accurately reflecting who did the work. However, named authors should be limited to those who have actively participated in the development of the abstract (see 1.1.1). GPP3 recommends an author group of fewer than 10 [ 5 ]; above this number, naming a study group may be a more practical approach. Likewise, if the source data come from a study, and the authors involved in that study meet authorship criteria, then the use of a study group name is strongly recommended.
1.2.2 Study group names may be helpful to acknowledge contributions to projects involving a large number of people, in addition to named authors who have contributed both to the research and to developing the presentation. Inclusion of a study name, either in the title or by including a study group in the author listing, will facilitate linkage of conference abstracts and presentations with journal publications. However, this should not be a substitute for including a unique study identifier such as a registration number for clinical trials (e.g. ClinTrials.gov or EudraCT numbers), which is a more reliable linkage method because these can be used as search terms in relevant databases. Provision should be made for study group membership details to be added during abstract submission and made available via the conference website once an abstract has been accepted.
1.3 Presenters and society sponsors
1.3.1 While the ICMJE criteria are a useful starting point for determining authorship, they were not designed for conference abstracts and presentations. Therefore, in certain circumstances, and if all authors agree, it is permissible for somebody who does not (or will not) meet the ICMJE authorship criteria for a journal article to present findings at a conference. For example, a local presenter may be included (preferably in a contributor list and not as an author) if the authors of the conference presentation will not attend a particular meeting, do not speak the language required or are not members of the academic society hosting the meeting. This local presenter, for example, could be an investigator who recruited patients but did not contribute to the study design or interpretation of data and will not be involved in developing journal articles. In the contributor list, this person should be designated as ‘presenter’ to clarify their role. However, if the conference requires that only authors can present, then the new presenter will need to be added to the author list.
1.3.2 Abstract authors (including company authors) attending a conference should always be preferred as presenters over non-author presenters. In cases where an author is not available to present, and the conference acquiesces to a non-author covering the presentation, the non-author presenter should be familiar with the research design and findings and have a good knowledge of the subject area in order to respond to questions about the presentation even if, unlike the authors, they cannot take direct responsibility for the research. An appropriately qualified individual from the sponsoring company (e.g. Medical Director) could present study findings if authors are not available; however, individuals with a commercial role in the sponsoring company (i.e. sales or marketing) should not act as non-author presenters.
1.3.3 All those listed as authors on an abstract or presentation must be able to take accountability for the research (following the spirit of the ICMJE recommendations). Therefore, if conferences require a society member to sponsor a submission, and none of the authors or study investigators is a member, this sponsorship role should be distinguished from that of the study authors if the sponsor/member was not involved with the research. If an existing author happened to be a society member, then no such distinction would be necessary. If the conference wishes to list the society sponsor, then this role should be indicated on the abstract (e.g. by an asterisk) and in a contributor list (not the author list) on the presentation.
Figure 2 illustrates some scenarios to differentiate between authors and non-author presenters.
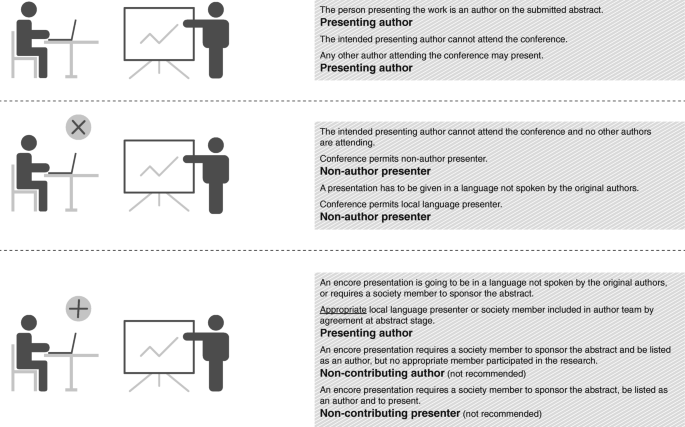
When is a presenter not an author? Different roles possible for authors and presenters of conference presentations
2.0 Conference abstracts
2.1 To facilitate linkage between conference abstracts and presentations, and subsequent publications, abstracts should include a study identifier such as a registration number (for clinical trials), study name, protocol number or grant number. To encourage this, conference organizers should require this information in a specific field on the submission form and publish it with the abstract.
2.2 Abstracts describing company-sponsored research should always name the sponsor and all funding sources (if more than the sponsor).
2.3 Authors or sponsoring companies may involve professional medical writers to support authors in the drafting of abstracts. All authors should agree to these arrangements and work closely with any writers and approve the final version. Space limitations on abstract submission sites usually preclude writing support acknowledgement. Conference organizers should consider requesting this information and publishing it with the abstract.
2.4 We encourage conference organizers to consider the requirements of reporting guidelines when setting limits on the length of abstracts. For example, CONSORT for Abstracts suggests that around 300 words may be needed to adequately report randomized clinical trials [ 7 ].
2.5 We also encourage conference organizers to maximize the available space for content in abstracts by not counting authors, affiliations, trial registration numbers and sponsor acknowledgments towards the word or character limit.
2.6 Most conferences will not consider reports of findings that have already been published in full (i.e. in a peer-reviewed journal). This requirement must be respected and, even if permitted, presenting findings after their full publication should be avoided. However, abstracts presenting findings or novel analyses that are not included in a full publication may be submitted if the conference permits this. In situations where a journal article is in preparation at the same time as abstract submission, subsequent submission of the article may overtake the abstract in acceptance, at which point the conference needs to be advised, and the journal also, to avoid issues of prior data release. It may be necessary to withdraw the abstract, or it might be possible for the journal and conference to come to a mutually acceptable arrangement regarding either delay of the article or amendment to the intended presentation. Posting summary results on a trial register (e.g. ClinicalTrials.gov , EudraCT) or a clinical study report to meet regulatory requirements is not regarded as full publication by the ICMJE [ 2 ] and should not prevent subsequent presentation at conferences.
2.7 As conference submission requirements become more detailed (and therefore labour-intensive), conference organizers should acknowledge that it is acceptable for the abstract submission process to be completed by a third party (e.g. a medical communications company) on behalf of the submitting author, with that author’s permission. Where feasible, the submission might be checked by the submitting author prior to the actual submission; however, there are some sites where submission has to be completed in one sitting, and on other occasions, time differences (and time pressures) may make this impractical.
3.0 Conference presentations (posters and slides)
3.1 general considerations.
3.1.1 Study identifiers (e.g., trial registration numbers) should be included on presentations to improve linkage between conference presentations and subsequent publications (see also Section 4).
3.1.2 All funding sources for the research, any assistance with the presentation (e.g. medical writing support, editorial assistance or design) or support for conference attendance and authors’ conflicts of interest should be clearly disclosed on posters and slides. For posters and slides, such disclosures should be clearly legible (i.e. not significantly smaller or lighter-coloured than the main text).
3.1.3 Author listing and order on posters and slides should be the same as that on the abstract. Authors should not be added to a presentation after the abstract is accepted. However, if an author is unavailable to work on a presentation after abstract acceptance, their name may be removed from the author list but their contribution (to the study and/or publication) should be acknowledged. If an author other than the first-named author is to present, this should be indicated without changing the author order. The principle is to retain the same information about authors as on the abstract for ease of identifying the related presentation. Similarly, the title of the presentation should not be changed after submission; thus, the titles of the abstract and poster or slides should be identical. [If someone not on the author list is to present, and this is known in time for poster preparation, the relevant name could be added as a footnote, or close to the author list thus: (Presenter: J. Doe, ABC Institute, City, Country).]
3.1.4 All named authors should contribute to the development of, and approve, the presentation (see 1.1.1). Authors should be given sufficient time for presentation development and review. Making significant changes to posters or slides after all-author approval should be avoided. If changes must be made after approval, the actual final version must be sent to all authors. As with journal articles, for large studies, it may be most efficient for a subgroup to coordinate the development of a presentation (similar to a writing group for an article). This should be considered when deciding authorship.
3.1.5 Each author’s contributions to the study and to the development of the presentation should be listed.
3.1.6 Conference presentations should include a list of contributors who have made a significant contribution to the research or the presentation, regardless of whether they are listed as authors or attending the meeting. Ideally, permission for such acknowledgment should be sought in writing.
3.1.7 Because abstracts are usually submitted several months before a conference, they may contain interim or preliminary findings. Therefore, by the time of the conference presentation, some details may have changed. If research findings change substantially between abstract submission and conference presentation and affect the conclusions of the research, we recommend that authors alert the conference to this discrepancy. This is particularly pertinent in the case of oral presentations (because abstracts are typically selected for oral presentations based on the impact of the findings). Regardless of whether the new data change the conclusions of the research, we recommend indicating (e.g. in a footnote) any data that are different from those on the accepted abstract.
3.1.8 Authors or sponsoring companies may involve professional medical writers in the production of posters and slides. Authors should agree to these arrangements and work closely with any writers, editors and/or designers throughout the development of the presentation. Such support should be disclosed on the presentation, along with source(s) of funding (see also 3.1.2).
3.2 Posters
3.2.1 While there are platforms where posters can be made permanently available (e.g. on conference websites or platforms such as F1000 Research), some journals regard this as prior publication which may jeopardize full publication. Authors should therefore check the policies of their target journal(s) and of the sponsor or funder before agreeing to a poster being publicly posted.
3.2.2 Posters are not peer-reviewed by conferences and may not describe all aspects of the research. Posters should therefore not be viewed as a substitute for a full article in a peer-reviewed journal. However, if a poster is publicly available (and, ideally, searchable via an indexing system or DOI), it may be cited until the full publication is available, although some journals consider citation of posters as unpublished information rather than full citations. See Section 6 for citation best practice.
3.2.3 The lead author should be given the first option to attend the poster session(s), but this role may be taken by other authors or a local presenter (if no author can attend or if no authors can present in the language of the conference). The poster presenter should ideally be agreed before the abstract is submitted, although it is understood that circumstances may change by the time of the actual conference (see 1.3.1).
3.2.4 While disclosures, funding sources, acknowledgements and contributions should be clearly noted on the main poster, supplementary sources can be used to expand on these if there is not enough room for detailed information, and may be accessed via a QR code (or similar link). Such content should normally be available until the research is published, in full, in a journal (at which point the link should be deactivated). If QR codes (or similar technology) are used to provide copies of the poster or to link to other scientific content, these should only be available to conference attendees, unless the conference elects to make the posters freely available after the conference. Links for the QR codes may be time-limited to close once the conference is finished. Supplementary materials may include translations. Supplementary material should be provided under the same usage conditions as the poster and indicate who is the copyright holder or licensee.
3.3 Slides for oral presentations
3.3.1 While the lead author is normally expected to present study findings at conferences (and is given the first option to do so), this may not be possible due to local language requirements, availability to travel, or personal circumstances, etc. If the lead author chooses not to present study findings, another author may give the oral presentation. If none of the named authors is available or able to give the presentation, a non-author presenter may present the findings if all authors agree to this and the conference permits it (see also 1.3.1 and 1.3.2). The presenter should be agreed before the abstract is submitted (and only changed if that person becomes unavailable). The lead author should discuss the contents of the presentation and the interpretation of the findings with the presenter (and co-authors, if possible) before the conference to ensure the authors’ views are correctly represented.
3.3.2 If a non-author presenter gives a presentation on behalf of the named authors (or study group), this should be indicated at the beginning of the presentation. The presenter’s conflicts of interest should be noted on the disclosure slide.
3.3.3 Recordings of oral presentations may be posted online by conference organizers but, as with posters, care should be taken to ensure this does not jeopardize full publication in a peer-reviewed journal. Slides alone (without the accompanying talk or speaker notes) may be hard to interpret and not provide full context, so care should be taken if these are made publicly available. As with posters (see 3.2.4), online sources may also be considered to host supplementary materials for presentations if they are made available after the presentation. If slides are made publicly available, this should not occur until after the presentation has been given and should only occur with the agreement of all authors and sponsors, who will need to consider any restrictions around the posting of the data and possible ‘prior publication’ concerns for later use (see 6.1.2).
3.3.4 Some scientific meetings offer Continuing Medical Education (CME) credit for attendance at oral presentations. Local regulations and requirements of the accreditation body for this must be respected.
4.0 Encore abstracts and presentations
4.1. It is permissible to present the same research findings at more than one conference if both the first and subsequent conferences allow this. This practice may be referred to as an ‘encore’ (or more specifically an encore abstract or encore presentation). However, presentations of the same findings to the same audience should be avoided.
4.2 Although encore abstracts are not considered to be redundant publications (unlike publication of the same findings in more than one journal), some conferences elect only to accept findings that have not been presented at other conferences, and such requirements must be respected.
4.3 When considering encore abstracts, the authors and sponsoring company should decide whether it is most appropriate to submit identical abstracts to multiple conferences or whether it is better to emphasize different aspects of a trial (e.g. those of interest to different audiences). Use of study identifiers can help identify that multiple conference abstracts and presentations are from a single study. However, to avoid any confusion, we recommend that encores should be specifically identified as such (e.g. by stating that the presentation is an ‘encore’ and listing where previous abstracts of all or some of the findings were presented) (see also 4.4 and 4.6). We also recommend that previous presentations should be listed on the presentation, if accepted.
4.4 Conference organizers should consider including a means of identifying encore abstracts (e.g. including details of prior presentations) on the abstract submission form. This information should not be included in the abstract word or character count.
4.5 Addition of new data to a previously accepted abstract may not necessarily constitute a new abstract: conference guidelines should be consulted to confirm if this is acceptable. If no specific guidelines are provided, then as a general guide, if the new iteration adds any new data other than an update on analyses already contained in a previous abstract, then the new iteration should be regarded as a new abstract.
4.6 Where encore abstracts, or updated abstracts that include previously presented data, are accepted, their presentations should indicate that this is not the first time of presentation, for example, by a statement on the poster or slides such as “Data/some data first presented at [conference name and date]”.
4.7 Encore checklist: When deciding whether to submit an encore abstract to a conference to reach different audiences, authors and study sponsors should consider the following points.
What is the overlap, if any, with the audience of the earlier conference (e.g. in terms of region, specialism or profession)?
Are there any differences in the licensing status of any products mentioned in the presentation between the first and subsequent conference locations? For example, if the first presentation occurred in a region where a product is licensed, but later presentation(s) will take place in a region where it is not yet licensed, this fact may need to be reflected. For international meetings, remember that participants will attend from several regions, so the licensing status in different countries should be clarified.
Presentation at multiple meetings might delay and/or potentially jeopardize the full publication of research in a peer-reviewed journal. Companies should consider whether resources would therefore be better spent on ensuring a timely submission to a journal rather than preparing several encore abstracts and presentations.
5.0 Copyright considerations
5.1 Copyright transfer or publishing licence agreements that are executed during the abstract submission process are common when abstracts are to be formally published (e.g. in a conference-specific journal issue). These agreements relate only to the abstract, not to any subsequent presentation, unless explicitly agreed otherwise.
5.2 Copyright in a presentation is normally held by the authors, unless they have assigned it either to the conference or the sponsoring company. Re-use of a poster (at a subsequent meeting or in another format, such as a poster book or handout) normally requires permission from the copyright holder(s). It may therefore be simplest for authors to assign usage rights to the sponsor company if encore presentations or other types of re-use are planned. If a company author is included, then the copyright for that individual’s contribution rests with the company (not the employee).
5.3 If a conference wishes to acquire usage rights for abstracts, slides, or posters, we recommend that the conference offers an open access option under a Creative Commons (CC) licence. We encourage the use of the least restrictive CC-BY licence, which will allow authors and sponsoring companies the usage rights for subsequent presentations, as well as future publications. If presentations contain third-party material to which the authors do not hold copyright, it should be the responsibility of the conference organizers to clear rights for any further usage. The authors cannot be expected to anticipate the future use of materials by the conference organizers.
5.4 As for any publication, permission must be sought for use of third-party copyrighted material (e.g. a figure) in a presentation (and again for any encore presentations). Material should not be altered simply to avoid having to obtain permission from the copyright holder.
5.5 Peer-to-peer presentation at a scholarly conference by a researcher is generally considered to be fair dealing (UK) [ 13 ] or fair use (USA) [ 14 ], which does not require copyright permission. Any other use of a presentation by a company outside the conference will most likely be considered commercial use, for which permission from the rights holder(s) will be necessary.
6.0 Citing conference material
6.1 References (or citations) in scientific texts provide readers with source or background material and are used to justify or support statements. To be useable, the referenced material must be both permanently accessible and reliable; therefore, citations to full publications in journals that apply rigorous peer review are the ideal. However, if citations are needed for research that has not yet been fully published in a peer-reviewed journal, abstracts that have undergone scientific review (and on the basis of that have been accepted for presentation by a conference) may be cited, especially if they have also been published in a journal and are therefore permanently accessible and discoverable. Abstracts should not be cited after the full (primary) publication has been accepted by a journal.
6.2 Posters and slides are not peer-reviewed by conferences and are often not permanently or widely accessible or discoverable. Citations to posters or slides should therefore be avoided (see 6.1). However, if a poster or slide set is publicly available (and, ideally, discoverable via an indexing system or DOI), it may be cited until the full publication is available (although some journals consider citation of posters or slides as unpublished information rather than full citations). Authors and sponsor companies should ensure that publishing posters or slides online does not jeopardize full publication in a peer-reviewed journal.
6.3 To avoid citing conference posters or slides, companies should consider other dissemination routes such as listing findings as ‘Data on File’ (i.e. an unpublished data package held by the pharmaceutical company, which then should be supplied to anyone requesting those data).
6.4 If specific findings that were presented at a conference are omitted from a journal article (e.g. because of space constraints), they could be made accessible as supplementary material.
These recommendations summarize the authors’ collective experience with a view to outlining the underlying principles for best practice and providing guidance on the practicalities for navigating conference requirements. We did consider whether some of our recommendations could be accomplished by amendments to company–author agreements, but decided that such recommendations for ‘good practice for author agreements’ were beyond the remit and scope of this article and that GPP3 [ 5 ] adequately covers this aspect of author–sponsor relationship. Many of these recommendations are drawn from the working group’s experience across a variety of disease areas and conferences. However, this is also a limitation, in that by the nature of the authors’ work, their experience lies in conferences and conference submission systems with strong industry involvement. We believe that these recommendations could be applied to any type of scientific/medical conference and are as relevant to academic research as to company-sponsored research. Conferences maintain their value to the scientific community by covering the latest research and providing a forum for discussion: this value must not be lost due to lack of transparency or ethics in the preparation and presentation of the new data. By following these recommendations, industry professionals, authors and conference organizers will improve consistency, transparency and integrity of publications submitted to conferences worldwide.
It is earnestly hoped that future input from conference organizers and societies, as well anyone involved in submitting research to conferences, will augment and strengthen these recommendations. We therefore welcome feedback via the website ( https://gpcap.org ).
Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2018;11:MR000005.
Google Scholar
ICMJE Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. Available at www.icmje.org . Accessed 14 May 2019.
Wager E, Field EA, Grossman L. Good publication practice for pharmaceutical companies. Curr Med Res Opinion. 2003;19:149–54.
Article Google Scholar
Graf C, Battisti WP, Bridges D, Bruce-Winkler V, Conaty JM, Ellison JM, Field EA, Gurr JA, Marx ME, Patel M, Sanes-Miller C, Yarker YE. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ. 2009;339:b4330.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, Peña T, Sanes-Miller CH, Veitch K, Woolley KL, Yarker YE. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF, The CONSORT group. CONSORT for reporting randomized controlled trials in journal and conference abstracts. Lancet. 2008;371:281–3.
Beller EM, Glasziou PP, Altman DG, et al. PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10(4):e1001419. https://doi.org/10.1371/journal.pmed.1001419 .
EFPIA Code of Ethics for Pharmaceutical Marketing. http://www.efpia-e4ethics.eu/usd/e4ethics.nsf/_/AFB7FD98CFCD31D5C125806E0043F1FE/%24File/farma_110039.pdf . Accessed 14 May 2019.
US Food & Drug Administration. Laws, regulations, guidances and enforcement actions. Available at https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/DrugMarketingAdvertisingandCommunications/ucm081617.htm . Accessed 14 May 2019.
Foster C, Wager E, Marchington J, Patel M, Banner S, Kennard NC, Panayi A, Stacey R. Good practice for conference abstracts and presentations: GP-CAP. PeerJ Preprints. 2017;5:e3356v1 https://doi.org/10.7287/peerj.preprints.3356v1 .
Open Researcher and Contributor ID (ORCID). Available at https://orcid.org/ . Accessed 14 May 2019.
MPIP Five-step authorship framework. Available at https://www.mpip-initiative.org/5-step-framework.html . Accessed 14 May 2019.
Copyright, Designs and Patents Act 1988, s 29(1); s 30(1). Available at https://www.legislation.gov.uk/ukpga/1988/48/contents . Accessed 14 May 2019.
Copyright Law of the United States | U.S. Copyright Office. Available at https://www.copyright.gov/title17/ . Accessed 14 May 2019.
Download references
Acknowledgements
Special thanks go to Peter Llewellyn of Network Pharma, for hosting the meeting on GPP3 that acted as a catalyst for getting these recommendations underway.
No author has received payment specifically for the development of this article.
Availability of data and materials
Not applicable.
Author information
Authors and affiliations.
Watermeadow Medical, Ashfield Healthcare Communications, Witney, UK
Cate Foster
Sideview, Princes Risborough, UK
Elizabeth Wager
University of Split, Split, Croatia
Caudex, a McCann Health Company, Oxford, UK
Jackie Marchington
Alexion Pharmaceuticals Inc, New Haven, CT, USA
Darwin Grey Communications Ltd, Oxford, UK
Steve Banner
Cello Health MedErgy, a Cello Health PLC Company, Farnham, UK
Nina C. Kennard
Shire International GmbH (now part of Takeda), Global Medical Affairs, Zug, Switzerland
Antonia Panayi
iMed Comms, Ashfield Healthcare Communications, Witney, UK
Rianne Stacey
You can also search for this author in PubMed Google Scholar
Contributions
CF raised the initial suggestion for guidelines, co-developed preliminary sections of text for the initial draft and discussed comments and revisions, reviewed all versions and approved the submitted version. EW drafted the Principles section and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. JM consulted on the initial suggestion for these guidelines, drafted the Copyright section and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. MP co-developed the foundation of the Encore Presentations section, discussed comments and revisions, reviewed all versions and approved the submitted version. SB consulted on the initial suggestion for these guidelines, assisted in the development of the initial draft, reviewed all subsequent drafts and approved the submitted version. NK drafted the abstract and other portions of the text, discussed comments and revisions, reviewed all versions and approved the submitted version. AP developed several sections with the author group, discussed comments and revisions, reviewed all versions and approved the submitted version. RS consulted on the initial suggestion for these guidelines, co-developed preliminary sections of text for the initial draft, discussed comments and revisions, incorporated feedback on the pre-print version, reviewed all versions and approved the submitted version. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Cate Foster .
Ethics declarations
Authors’ information.
Cate Foster, Jackie Marchington, Steve Banner and Nina C Kennard work for medical communication agencies that provide professional medical writing or editing services to not-for-profit and for-profit clients.
Elizabeth Wager is self-employed and provides training, consultancy and editing services on medical publishing and publication ethics for pharmaceutical companies, medical communication agencies, publishers, universities and academic societies.
Mina Patel and Antonia Panayi work in Global Medical Affairs functions within the pharmaceutical industry.
Rianne Stacey worked for a medical communication agency (see above) during the majority of the time the work was done and now works at Jazz Pharmaceuticals, Oxford, UK, in a Global Medical Affairs function within the pharmaceutical industry.
The content and opinions expressed in the article are the authors’ and do not necessarily reflect the policies or practices of their employers.
Ethics approval and consent to participate
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests. Commercial affiliations are noted in the ‘Authors’ information’ section.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Foster, C., Wager, E., Marchington, J. et al. Good Practice for Conference Abstracts and Presentations: GPCAP. Res Integr Peer Rev 4 , 11 (2019). https://doi.org/10.1186/s41073-019-0070-x
Download citation
Received : 27 July 2018
Accepted : 26 April 2019
Published : 05 June 2019
DOI : https://doi.org/10.1186/s41073-019-0070-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Research Integrity and Peer Review
ISSN: 2058-8615
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Customizable, intuitive, and scalable registration forms to sign up attendees with ease.
End-to-end abstract management platform; submission, review, evaluation, program and proceeding export.
Build branded & stunning event websites to engage your attendees.
Personalize your event awarness to expand your reach and drive registrations.
Host interactive virtual events with networking, streaming, engagement tools, and more.
Take a peek at our case studies and other client successes.
Receive guidance, or learn more about troubleshooting.
- Event Registration
- Abstract Management
- Event Webpage
- Event Marketing
- Virtual Conferences
- Case Studies
- Knowledge Center
How to Review Abstracts in an Academic Conference? A Comprehensive Guide for Academic Conference Reviewers

Introduction
Importance of abstract review in academic events.
The abstract review serves as a cornerstone in the organization of academic conferences, ensuring the quality, relevance, and integrity of the event's content. Academic conferences thrive on the dissemination of cutting-edge research and fostering meaningful discussions among scholars, researchers, and practitioners. By meticulously reviewing abstract submissions, conference organizers uphold the standards of academic excellence, curating a program that reflects the latest advancements and insights in various fields of study. Abstract review not only shapes the conference agenda but also plays a crucial role in maintaining the credibility and reputation of the event within the academic community.
The purpose of this blog post is to equip abstract reviewers with the knowledge, tools, and best practices necessary to conduct thorough and effective reviews. As abstract review is a critical aspect of academic event planning and execution, reviewers need to understand their roles, responsibilities, and the criteria by which abstracts should be evaluated. By offering a comprehensive guide to abstract review, this blog post aims to empower reviewers to contribute meaningfully to the selection of high-quality abstracts, thus ensuring the success and impact of academic conferences.
Understanding the Role of Abstract Reviewers

Abstract reviewers play a pivotal role in shaping the content and direction of academic conferences. Understanding their responsibilities and the significance of their expertise is essential for ensuring the success of the review process and, ultimately, the conference itself.
Responsibilities of Abstract Reviewers
Abstract reviewers are tasked with carefully evaluating the submissions received for an academic conference. Their primary responsibilities include:
- Assessing the quality, relevance, and originality of abstracts.
- Providing constructive feedback to authors to help improve their submissions.
- Ensuring adherence to conference guidelines and criteria.
- Selecting abstracts for presentation at the conference based on predetermined criteria.
- Upholding the integrity and standards of the conference by maintaining impartiality and fairness in the review process.
Importance of Expertise in the Subject Area
Expertise in the subject area is paramount for abstract reviewers. Their familiarity with the field allows them to accurately assess the novelty, significance, and methodological rigor of the research presented in the abstracts. Additionally, subject-matter expertise enables reviewers to identify gaps in the literature, assess the potential impact of the research, and provide valuable insights and recommendations to authors.
Role in Ensuring Quality and Relevance of Conference Content
Abstract reviewers play a critical role in ensuring the quality and relevance of the content presented at academic conferences. By carefully evaluating abstract submissions, reviewers help to curate a program that showcases the latest advancements, trends, and innovations in the field. Their thorough and objective assessments contribute to the overall success of the conference by facilitating the dissemination of high-quality research and promoting meaningful dialogue and collaboration among conference attendees.

Criteria for Abstract Evaluation

Evaluating abstracts for academic conferences requires a systematic approach and adherence to specific criteria to ensure consistency, fairness, and quality in the review process. Several key criteria guide abstract evaluation, encompassing various aspects of the submission's content, relevance, and contribution to the field of study.
Writing Rules and Guidelines
Abstracts must adhere to specific writing rules and guidelines outlined by the conference organizers. These guidelines typically include requirements related to word count, formatting, structure, and language usage. Reviewers assess the adherence of abstracts to these rules, ensuring consistency and professionalism in the submissions.
Clarity and Coherence of the Abstract
Clarity and coherence are essential aspects of effective communication in academic writing. Reviewers evaluate the clarity and coherence of abstracts by assessing the organization, flow, and logical structure of the content. Clear and concise abstracts facilitate understanding and engagement with the research presented, enhancing their overall effectiveness.
Relevance to Conference Theme and Topics
Abstracts should demonstrate relevance to the overarching theme and specific topics of the conference. Reviewers evaluate the alignment of abstracts with the conference theme and assess the extent to which the research addresses current issues, challenges, or advancements in the field. Relevance to the conference theme ensures that the program reflects the interests and priorities of conference attendees.
Contribution to the Research Area
The contribution of the research presented in the abstracts to the broader research area is a critical criterion for evaluation. Reviewers assess the novelty, significance, and potential impact of the research findings or insights presented in the abstracts. Abstracts that contribute new knowledge, perspectives, or methodologies to the field are prioritized for inclusion in the conference program.
Appropriateness of Research Methods
Abstracts should provide sufficient information about the research methods employed to conduct the study. Reviewers evaluate the appropriateness and rigor of the research methods used, assessing the validity, reliability, and ethical considerations of the study. Clear descriptions of research methods enable reviewers to assess the validity and credibility of the research findings presented in the abstracts.
Originality and Innovation
Originality and innovation are key criteria for evaluating the quality and significance of abstract submissions. Reviewers assess the extent to which the research presented in the abstract offers novel insights, approaches, or perspectives to the field. Abstracts that demonstrate originality and innovation are more likely to be selected for presentation at the conference, as they contribute to the advancement of knowledge and scholarship in the field.
Scoring and Evaluation Methods for Abstracts

Scoring an abstract involves systematically assessing various aspects of the submission and assigning a numerical rating or score based on predefined evaluation criteria. Abstract reviewers use specific methods and guidelines to evaluate abstracts effectively and fairly, ensuring consistency and objectivity in the review process.
Evaluation Criteria
Abstracts are evaluated based on predetermined criteria that reflect the quality, relevance, and originality of the research presented. Common evaluation criteria include:
- Clarity and coherence of the abstract
- Relevance to the conference theme and topics
- Contribution to the research area
- Appropriateness of research methods
- Originality and innovation
Reviewers assess each criterion individually and assign scores or ratings based on the extent to which the abstract meets or exceeds the established standards.
Rating Scale
Abstract reviewers use a rating scale to assign scores to abstract submissions, typically ranging from a numerical scale (e.g., 1 to 5) or descriptive categories (e.g., excellent, good, fair, poor). The rating scale provides a standardized framework for evaluating abstracts and facilitates consistency and comparability across different reviewers.
Abstract Review Types
Peer Review: In peer review, abstracts are evaluated by experts in the same field or discipline as the author(s) of the submission. Peer review ensures that abstracts are assessed by individuals with the relevant knowledge and expertise to provide informed feedback and evaluations.
Blind Peer Review: In blind peer review, the identities of both the reviewers and the authors are kept anonymous to ensure impartiality and eliminate potential biases. Reviewers assess abstracts based solely on the content and quality of the submission, without being influenced by the identity or affiliation of the authors.
Double-Blind Peer Review: Double-blind peer review extends the principles of blind peer review by anonymizing the identities of both the reviewers and the authors. This ensures maximum impartiality and minimizes potential biases, as neither party is aware of the other's identity during the review process.
Methods for Evaluating Abstracts
Several methods may be used to evaluate abstracts, depending on the preferences of the conference organizers and the complexity of the review process. Common methods include:
- Quantitative Scoring: Reviewers assign numerical scores to abstracts based on predefined evaluation criteria, with higher scores indicating higher quality and relevance.
- Qualitative Assessment: Reviewers provide qualitative feedback and comments on various aspects of the abstract, highlighting strengths, weaknesses, and areas for improvement.
- Comparative Evaluation: Reviewers compare abstracts against each other, identifying standout submissions and selecting the most promising or innovative research for presentation at the conference.
- Consensus Review: Reviewers engage in discussions and deliberations to reach a consensus on the evaluation of abstracts, resolving any discrepancies or disagreements through dialogue and consensus-building.
By employing rigorous evaluation methods and adhering to established criteria and guidelines, abstract reviewers ensure the fairness, objectivity, and integrity of the abstract review process. Their efforts contribute to the selection of high-quality, impactful research for presentation at academic conferences, advancing knowledge and scholarship in their respective fields.
Abstract Review Process and Guidelines

The review process for abstracts in academic conferences involves several steps and adheres to specific guidelines to ensure fairness, consistency, and transparency. Abstract reviewers play a crucial role in this process, following established guidelines and criteria to evaluate submissions effectively.
Assignment of Abstracts to Reviewers
Conference organizers assign abstracts to reviewers based on their expertise and the subject matter of the submissions. Reviewers are selected for their knowledge and experience in the field, ensuring that they are well-equipped to evaluate the content of the abstracts accurately.
Reviewer Guidelines and Instructions
Reviewers receive detailed guidelines and instructions outlining the criteria for evaluation, expectations for feedback, and deadlines for submission. These guidelines provide reviewers with clear guidance on how to assess abstracts and offer constructive feedback to authors.
Abstract Evaluation Criteria and Rating Scale
Reviewers use predetermined evaluation criteria and rating scales to assess the quality, relevance, and originality of abstract submissions. These criteria typically include aspects such as clarity, coherence, relevance to the conference theme, contribution to the research area, appropriateness of research methods, and originality. Reviewers assign scores or ratings to abstracts based on these criteria, helping conference organizers make informed decisions about which submissions to accept for presentation.
Timely Submission of Abstract Reviews
Reviewers are required to submit their evaluations within the specified deadline to ensure timely processing of abstract submissions. Timely submission of reviews is essential for maintaining the efficiency of the review process and meeting the overall timeline for organizing the conference.
Communication with Conference Organizers
Reviewers may communicate with conference organizers as needed to clarify instructions, seek guidance on specific issues, or provide feedback on the review process. Open and transparent communication between reviewers and organizers helps ensure that the review process runs smoothly and that any concerns or questions are addressed promptly.
The review process and guidelines outlined above provide a structured framework for evaluating abstract submissions and selecting high-quality content for presentation at academic conferences. By following these guidelines, reviewers contribute to the success and impact of the conference by ensuring the quality, relevance, and integrity of the content presented.
Handling Conflicts of Interest, Bias, and Plagiarism

Ensuring the integrity and fairness of the abstract review process involves not only addressing conflicts of interest and bias but also conducting plagiarism checks to uphold academic standards and originality in research.
Identifying and Addressing Conflicts of Interest
Reviewers should disclose any potential conflicts of interest that may influence their evaluation of abstract submissions. Conflicts of interest may arise from personal relationships, professional affiliations, financial interests, or other factors that could compromise the reviewer's impartiality. Reviewers should recuse themselves from evaluating abstracts where a conflict of interest exists, and conference organizers should reassign those submissions to alternative reviewers.
Ensuring Fair and Impartial Abstract Reviews
Reviewers must conduct their evaluations objectively and impartially, regardless of personal biases or preferences. They should focus solely on the content and quality of the abstract submissions, applying the established evaluation criteria consistently and fairly to all submissions. Reviewers should avoid favoritism, discrimination, or any other forms of bias that could impact the outcome of the review process.
Confidentiality and Ethical Considerations
Reviewers are entrusted with confidential information during the abstract review process and must maintain the confidentiality of all submissions and reviewer deliberations. They should refrain from discussing or sharing details about abstract submissions with unauthorized individuals and adhere to the confidentiality policies established by the conference organizers. Additionally, reviewers should uphold ethical standards in their interactions with authors and colleagues, treating all participants with respect and professionalism.
Plagiarism Check
Abstract reviewers should also perform plagiarism checks to ensure the originality and authenticity of the submitted research. Plagiarism undermines the integrity of academic discourse and must be identified and addressed promptly. Reviewers should use plagiarism detection tools to compare abstract submissions against existing literature and identify any instances of plagiarism or academic misconduct. Conference organizers should have policies in place to address plagiarism and ensure that all submissions adhere to ethical standards and academic integrity guidelines.
By addressing conflicts of interest, bias, and plagiarism, reviewers uphold the integrity and credibility of the abstract review process, ensuring that only high-quality, original research is selected for presentation at academic conferences.
Providing Constructive Feedback

Offering constructive feedback to authors is a crucial aspect of the abstract review process. Constructive feedback helps authors improve their submissions and enhances the overall quality of the conference program. Reviewers should strive to provide specific, actionable feedback that guides authors in refining their abstracts and advancing their research.
Offering Clear and Specific Feedback
Reviewers should provide clear and specific feedback on various aspects of the abstract, including its clarity, coherence, relevance, methodology, and originality. Feedback should be constructive and focused on areas where the abstract can be strengthened or improved. Reviewers should avoid vague or general comments and instead offer specific suggestions for enhancement.
Suggestions for Improvement
In addition to identifying areas for improvement, reviewers should offer practical suggestions and recommendations to help authors enhance their abstracts. This may include suggestions for clarifying the research objectives, refining the methodology, strengthening the argument or analysis, or expanding on key findings. Reviewers should provide actionable advice that authors can implement to enhance the quality and effectiveness of their submissions.
Encouraging Authors to Address Reviewer Comments
Reviewers should encourage authors to carefully consider and address the feedback provided in their reviews. Authors should view reviewer comments as valuable insights that can help them strengthen their research and improve their abstracts. Reviewers should foster a constructive and supportive dialogue with authors, emphasizing the importance of incorporating reviewer feedback to enhance the overall quality of their submissions.
By providing constructive feedback, reviewers contribute to the professional development and growth of authors, fostering a culture of continuous improvement and excellence in academic research. Constructive feedback helps authors refine their ideas, strengthen their arguments, and ultimately produce higher-quality research that contributes to the advancement of knowledge in their field.
In conclusion, the abstract review process is a critical component of academic conferences, ensuring the quality, relevance, and integrity of the research presented. Abstract reviewers play a vital role in this process, applying rigorous evaluation criteria and providing constructive feedback to authors. By adhering to established guidelines, addressing conflicts of interest and bias, conducting plagiarism checks, and offering constructive feedback, reviewers uphold the integrity and credibility of the review process, contributing to the success and impact of academic conferences.
As abstract reviewers, it is essential to recognize the responsibility and privilege inherent in the role. By approaching the review process with professionalism, objectivity, and a commitment to academic integrity, reviewers can help shape the conference program and facilitate the dissemination of high-quality research. Their contributions play a crucial role in advancing knowledge and scholarship in their respective fields, fostering collaboration, and driving innovation.
If you need to know more about abstract management you can also check the following blog articles:
Abstract Management 101: A Comprehensive Guide for Efficient Abstract Management at Academic Events
Abstract Management 101: A Comprehensive Guide and Checklist for Efficient Abstract Management at Academic Events
How to Streamline and Manage the Workflow of Abstracts for a Conference with Abstract Software like MeetingHand, Oxford Abstracts, Cvent, EasyChair, Exordo, Whova and more?
Mastering the Abstract Management Process in Academic Conferences: From Submission to Publication
If you enjoyed this article, please do not forget to share it with your friends.!
Images by FREEPIK

Researched by Consultants from Top-Tier Management Companies

Powerpoint Templates
Icon Bundle
Kpi Dashboard
Professional
Business Plans
Swot Analysis
Gantt Chart
Business Proposal
Marketing Plan
Project Management
Business Case
Business Model
Cyber Security
Business PPT
Digital Marketing
Digital Transformation
Human Resources
Product Management
Artificial Intelligence
Company Profile
Acknowledgement PPT
PPT Presentation
Reports Brochures
One Page Pitch
Interview PPT
All Categories
10 Best Literature Review Templates for Scholars and Researchers [Free PDF Attached]
Imagine being in a new country and taking a road trip without GPS. You would be so lost. Right? Similarly, think about delving into a topic without having a clue or proper understanding of the reason behind studying it.
That’s when a well-written literature review comes to the rescue. It provides a proper direction to the topic being studied.
The literature review furnishes a descriptive overview of the existing knowledge relevant to the research statement. It is a crucial step in the research process as it enables you to establish the theoretical roots of your field of interest, elucidate your ideas, and develop a suitable methodology. A literature review can include information from various sources, such as journals, books, documents, and other academic materials. This promotes in-depth understanding and analytical thinking, thereby helping in critical evaluation.
Regardless of the type of literature review — evaluative, exploratory, instrumental, systematic, and meta-analysis, a well-written article consists of three basic elements: introduction, body, and conclusion. Also its essence blooms in creating new knowledge through the process of review, critique, and synthesis.
But writing a literature review can be difficult. Right?
Relax, our collection of professionally designed templates will leave no room for mistakes or anxious feelings as they will help you present background information concisely.
10 Designs to Rethink Your Literature Reviews
These designs are fully customizable to help you establish links between your proposition and already existing literature. Our PowerPoint infographics are of the highest quality and contain relevant content. Whether you want to write a short summary or review consisting of several pages, these exclusive layouts will serve the purpose.
Let’s get started.
Template 1: Literature Review PPT Template
This literature review design is a perfect tool for any student looking to present a summary and critique of knowledge on their research statement. Using this layout, you can discuss theoretical and methodological contributions in the related field. You can also talk about past works, books, study materials, etc. The given PPT design is concise, easy to use, and will help develop a strong framework for problem-solving. Download it today.
Download this template
Template 2: Literature Review PowerPoint Slide
Looking to synthesize your latest findings and present them in a persuasive manner? Our literature review theme will help you narrow relevant information and design a framework for rational investigation. The given PPT design will enable you to present your ideas concisely. From summary details to strengths and shortcomings, this template covers it all. Grab it now.

Template 3: Literature Review Template
Craft a literature review that is both informative and persuasive with this amazing PPT slide. This predesigned layout will help you in presenting the summary of information in an engaging manner. Our themes are specifically designed to aid you in demonstrating your critical thinking and objective evaluation. So don't wait any longer – download our literature review template today.

Template 4: Comprehensive Literature Review PPT Slide
Download this tried-and-true literature review template to present a descriptive summary of your research topic statement. The given PPT layout is replete with relevant content to help you strike a balance between supporting and opposing aspects of an argument. This predesigned slide covers components such as strengths, defects, and methodology. It will assist you in cutting the clutter and focus on what's important. Grab it today.

Template 5: Literature Review for Research Project Proposal PPT
Writing a literature review can be overwhelming and time-consuming, but our project proposal PPT slides make the process much easier. This exclusive graphic will help you gather all the information you need by depicting strengths and weaknesses. It will also assist you in identifying and analyzing the most important aspects of your knowledge sources. With our helpful design, writing a literature review is easy and done. Download it now.

Template 6: Literature Review for Research Project Proposal Template
Present a comprehensive and cohesive overview of the information related to your topic with this stunning PPT slide. The given layout will enable you to put forward the facts and logic to develop a new hypothesis for testing. With this high-quality design, you can enumerate different books and study materials taken into consideration. You can also analyze and emphasize the technique opted for inquiry. Get this literature review PowerPoint presentation template now.

Template 7: Literature Review for Research Paper Proposal PowerPoint Slide
Lay a strong foundation for your research topic with this impressive PowerPoint presentation layout. It is easy to use and fully customizable. This design will help you describe the previous research done. Moreover, you can enlist the strengths and weaknesses of the study clearly. Therefore, grab it now.

Template 8: Literature Review for Research Paper Proposal PPT
Download this high-quality PPT template and write a well-formatted literature review. The given layout is professionally designed and easy to follow. It will enable you to emphasize various elements, such as materials referred to, past work, the list of books, approach for analysis, and more. So why wait? Download this PowerPoint design immediately.

Template 9: Literature Review for Academic Student Research Proposal PPT
With this exclusive graphic, you'll have everything you need to create a well-structured and convincing literature review. The given design is well-suited for students and researchers who wish to mention reliable information sources, such as books and journals, and draw inferences from them. You can even focus on the strong points of your study, thereby making an impactful research statement. Therefore, grab this PPT slide today.

Template 10: Literature Review Overview for Research Process PPT
Demonstrate your analytical skills and understanding of the topic with this predesigned PowerPoint graphic. The given research overview PPT theme is perfect for explaining what has been done in the area of your topic of interest. Using this impressive design, you can provide an accurate comparison showcasing the connections between the different works being reviewed. Get it right away.

Creating an effective literature review requires discipline, study, and patience. Our collection of templates will assist you in presenting an extensive and cohesive summary of the relevant works. These PPT layouts are professionally designed, fully editable, and visually appealing. You can modify them and create perfect presentations according to your needs. So download them now!
P.S. Are you looking for a way to communicate your individual story? Save your time with these predesigned book report templates featured in this guide .
Download the free Literature Review Template PDF .
Related posts:
- How to Design the Perfect Service Launch Presentation [Custom Launch Deck Included]
- Quarterly Business Review Presentation: All the Essential Slides You Need in Your Deck
- [Updated 2023] How to Design The Perfect Product Launch Presentation [Best Templates Included]
- 99% of the Pitches Fail! Find Out What Makes Any Startup a Success
Liked this blog? Please recommend us

Top 11 Book Report Templates to Tell Your Inspirational Story [Free PDF Attached]
6 thoughts on “10 best literature review templates for scholars and researchers [free pdf attached]”.
This form is protected by reCAPTCHA - the Google Privacy Policy and Terms of Service apply.

Digital revolution powerpoint presentation slides
Sales funnel results presentation layouts
3d men joinning circular jigsaw puzzles ppt graphics icons

Business Strategic Planning Template For Organizations Powerpoint Presentation Slides
Future plan powerpoint template slide

Project Management Team Powerpoint Presentation Slides

Brand marketing powerpoint presentation slides

Launching a new service powerpoint presentation with slides go to market

Agenda powerpoint slide show
Four key metrics donut chart with percentage
Engineering and technology ppt inspiration example introduction continuous process improvement

Meet our team representing in circular format

- Open access
- Published: 07 November 2019
How should systematic reviewers handle conference abstracts? A view from the trenches
- Roberta W. Scherer ORCID: orcid.org/0000-0003-2493-1769 1 &
- Ian J. Saldanha 2 , 3
Systematic Reviews volume 8 , Article number: 264 ( 2019 ) Cite this article
25k Accesses
155 Citations
43 Altmetric
Metrics details
While identifying and cataloging unpublished studies from conference proceedings is generally recognized as a good practice during systematic reviews, controversy remains whether to include study results that are reported in conference abstracts. Existing guidelines provide conflicting recommendations.
The main argument for including conference abstracts in systematic reviews is that abstracts with positive results are preferentially published, and published sooner, as full-length articles compared with other abstracts. Arguments against including conference abstracts are that (1) searching for abstracts is resource-intensive, (2) abstracts may not contain adequate information, and (3) the information in abstracts may not be dependable. However, studies comparing conference abstracts and fully published articles of the same study find only minor differences, usually with conference abstracts presenting preliminary results. Other studies that have examined differences in treatment estimates of meta-analyses with and without conference abstracts report changes in precision, but usually not in the treatment effect estimate. However, in some cases, including conference abstracts has made a difference in the estimate of the treatment effect, not just its precision. Instead of arbitrarily deciding to include or exclude conference abstracts in systematic reviews, we suggest that systematic reviewers should consider the availability of evidence informing the review. If available evidence is sparse or conflicting, it may be worthwhile to search for conference abstracts. Further, attempts to contact authors of abstracts or search for protocols or trial registers to supplement the information presented in conference abstracts is prudent. If unique information from conference abstracts is included in a meta-analysis, a sensitivity analysis with and without the unique results should be conducted.
Conclusions
Under given circumstances, it is worthwhile to search for and include results from conference abstracts in systematic reviews.
Peer Review reports
Systematic reviewers aim to be comprehensive in summarizing the existing literature addressing specific research questions. This generally involves a thorough search for published studies as well as for ongoing or recently completed studies that are not yet published. Ongoing and recently completed studies are often identified through searches of registries, such as ClinicalTrials.gov, and of conference proceedings. While identifying and cataloging unpublished studies from conference proceedings is generally recognized as a good practice during systematic reviews, controversy remains whether to include study results that are reported in conference abstracts. Current guidelines are conflicting. The United States Agency for Health Care Research and Quality (AHRQ), through its Effective Healthcare Program, recommends that searches for conference abstracts be considered , but Cochrane and the United States National Academy of Sciences (NAS) both recommend always searching for and including conference abstracts in systematic reviews [ 1 , 2 , 3 ]. Our objectives in this commentary are to summarize the existing evidence both for and against the inclusion of conference abstracts in systematic reviews and provide suggestions for systematic reviewers when deciding whether and how to include conference abstracts in systematic reviews.
Arguments for including conference abstracts in systematic reviews
The main argument for including conference abstracts in systematic reviews is that, by doing so, systematic reviewers can be more comprehensive. In our recent Cochrane methodology review, we reported that the proportion of subsequent full publication of studies presented at conferences is low [ 4 ]. We examined 425 biomedical research reports that followed the publication status of 307,028 studies presented as conference abstracts addressing a wide range of medical, allied health, and health policy fields. A meta-analysis of these 425 reports indicated that the overall full publication proportion was only 37% (95% confidence interval [CI], 35 to 39%) for abstracts of all types of studies and only 60% (95% CI, 52 to 67%) for abstracts of randomized controlled trials (RCTs). Through a survival analysis, we found that, among the 181 reports that evaluated time to publication, only 46% of abstracts of all types of studies and 69% of abstracts of RCTs were published, even after 10 years. Thus, at best, approximately 3 in 10 abstracts describing RCTs have never been published in full, implying that the voluntary participation and risk-taking by multitudes of patients have not led to fully realized contributions to science. We and others argue that the failure of trialists to honor their commitment to patients (that patient participation would contribute to science) represents an ethical problem [ 5 , 6 ].
From a systematic reviewer’s perspective, even if the unpublished abstracts were a random 3 in 10 abstracts, restricting a systematic review search to only the published literature would amount to the loss of an immense amount of information and a corresponding loss of precision in meta-analytic estimates of treatment effect. However, publication is not a matter of random chance. Those conducting systematic reviews have long grappled with this problem, known as “publication bias.” Publication bias occurs when either the likelihood of, or the time to, publication of a study is impacted by the direction of the study’s results [ 6 , 7 , 8 , 9 , 10 , 11 , 12 ]. The most frequent scenario for publication bias is when studies with “positive” (or “significant”) results are selectively published, or are published sooner, than studies with either null or negative results.
Publication bias can be conceptualized as occurring in two stages: (I) from a study’s end to presentation of its results at a conference (and publication of an accompanying conference abstract) and (II) from publication of a conference abstract to subsequent “full publication” of the study results, typically in a peer-reviewed journal article [ 13 ]. In the context of publication bias arising during stage II (i.e., if abstracts with positive or significant results are selectively published in full), systematic reviews relying solely on fully published studies can be biased because positive results would be overrepresented. This would lead to a falsely inflated (or biased) estimate of the treatment effect of the intervention being evaluated in the systematic review. Indeed, in our Cochrane methodology review, we found evidence of publication bias in the studies reported in the abstracts [ 4 ]. “Positive” results were associated with full publication, whether “positive” was defined as statistically significant results (risk ratio [RR] = 1.31, 95% CI 1.23 to 1.40) or as results whose direction favored the intervention (RR = 1.17, 95% CI 1.07 to 1.28). Furthermore, abstracts with statistically significant results were published in full sooner than abstracts with non-significant results [ 14 , 15 , 16 ], unearthing another aspect of bias that can arise when a systematic review is performed relatively soon after the completion of a trial(s) testing a new intervention.
Arguments against including conference abstracts in systematic reviews
There are various arguments against including abstracts in systematic reviews. First, identifying relevant conferences, locating their abstracts, and sifting through the often thousands of abstracts can be challenging and resource-intensive. However, EMBASE, a commonly searched database during systematic reviews, now includes conference abstracts from important medical conferences, dating back to 2009 [ 17 ]. Inclusion of conference abstracts in this searchable database means searching for conference abstracts is less resource-intensive than in the past. Second, largely driven by their brevity, abstracts may not contain adequate information for systematic reviewers to appraise the design, methods, risk of bias, outcomes, and results of studies reported in the abstracts [ 18 , 19 , 20 , 21 ]. Third, the dependability of results presented in abstracts also is questionable [ 22 , 23 , 24 ], which occurs at least in part because (1) most abstracts are not peer-reviewed and (2) results reported in abstracts are often preliminary and/or based on limited analyses conducted in a rush to meet conference deadlines. The most frequent types of conflicting information between abstract and full-length journal article have pertained to authors or authorship order, sample size, and estimates of treatment effects (their magnitude or, less frequently, direction) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 ]. Mayo-Wilson and colleagues examined the agreement in reported data across a range of unpublished sources related to the same studies in bipolar depression and neuropathic pain [ 21 , 32 ]. As part of this effort, they compared abstracts with full-length journal articles and clinical study reports and reported that the information presented in abstracts was not dependable either in terms of methods or results.
What are we missing if we do not include conference abstracts in a systematic review?
Various studies have questioned whether the inclusion of “gray” literature or unpublished study results in a systematic review would change the estimates of treatment effect obtained during meta-analyses. Through “meta-epidemiologic” studies, investigators have examined the results of meta-analyses with and without conference abstracts and have reported conflicting, but generally small differences in results [ 21 , 24 , 33 ]. Evidence from a recent systematic review indicates that the inclusion of gray literature (defined more broadly than just conference abstracts) in meta-analyses may change the results from significant to non-significant or from non-significant to significant, or may not change the results [ 24 , 33 ]. We conducted a similar analysis in our Cochrane methodology review [ 4 ]. We were able to do this because some of our included reports that examined full publication of conference abstracts were themselves only available as conference abstracts. Our analysis found that inclusion of reports that were conference abstracts did not change the strength or precision of our meta-analytic results. In our review, it would have been possible to exclude conference abstracts and retain accurate and precise results.
Implications of reasons for non-publication of conference abstracts
The most common reason provided by authors of abstracts for not publishing their study results in full has been reported to simply be “lack of time,” and not because the results were considered unreliable or negative [ 34 ]. This finding suggests that the identification of an abstract without a corresponding full-length journal article should prompt systematic reviewers to search for additional evidence, such as gray literature sources and/or contacting the authors. However, a reasonable argument could be made that, when the same information is available in both a published peer-reviewed article and an abstract for a given study, including the abstract in a systematic review would be superfluous and/or ill-advised because a likely more comprehensive and dependable source of the information, i.e., the peer-reviewed article, is available. Therefore, the presence of a journal article might obviate the need for including a corresponding conference abstract in a systematic review, unless unique outcomes are reported in the abstract.
Considerations when including conference abstracts in systematic reviews
Taken together, the evidence reviewed in this paper (summarized in Table 1 ) suggests that systematic reviewers should take a more nuanced approach to inclusion of conference abstracts. A simple yes or no to the question “Should we include conference abstracts in our systematic review?” is neither sufficient nor appropriate. One aspect to consider is the scope of the review. For example, will the conference abstracts be used to inform policy based on a cadre of systematic reviews or only used within a single review? Benzie and colleagues evaluated the usefulness of including conference abstracts in a “state-of-the-evidence” review and concluded that including conference abstracts validated the results of a search that included only the published literature [ 35 ]. These authors discussed four considerations for basing the decision to include conference abstracts: (1) complexity of the intervention, (2) consensus in the existing literature, (3) importance of context in evaluating the effect of the intervention, and (4) presence of other evidence [ 35 ]. Others who have incorporated conference abstracts for decision-making have noted that the lack of, or conflicting results in, published evidence often requires inclusion of conference abstracts [ 36 ]. In some instances, results in abstracts can confirm the evidence found in fully published studies, but in other instances, abstracts can provide useful additions to the evidence [ 37 ].
When considering the use of conference abstracts in systematic reviews, we largely agree with the recommendations presented in the AHRQ Methods Guide for Comparative Effectiveness Reviews [ 1 ]. Although these recommendations generally do not espouse including conference abstracts in systematic reviews, they provide excellent guidance on when including abstracts should be considered:
• Reviewers should routinely consider conducting a search of conference abstracts and proceedings to identify unpublished or unidentified studies. • Consult the TEP [Technical Expert Panel] for suggestions on particular conferences to search and search those conferences specifically. • Search for conference abstracts of any conference identified by reading the references of key articles. • We do not recommend using conference abstracts for assessing selective outcome reporting and selective analysis reporting, given the variable evidence of concordance between conference abstracts and their subsequent full-text publications [ 1 ].
Our suggestions
Based on the empirical findings summarized in this review and on our experience, we believe that generally relying on conference abstracts is problematic for the various reasons discussed. While meta-epidemiologic studies have shown that inclusion of abstracts does not greatly impact meta-analytic results, it can sometimes make a difference. The dilemma facing a systematic reviewer is to determine when it might. We suggest the following approach (summarized in Fig. 1 ). If the evidence suggests a sizeable effect, or the absence of one (i.e., with the estimate of effect centered at or near the null), with reasonable precision, searching for conference abstracts may be unnecessary. On the other hand, if the evidence does not show a sizeable effect, is imprecise, or is conflicting, then the resources spent finding and including conference abstracts may be worth it. In other words, if only a single study in full-length form is identified, or if the studies identified are few and small, then conference abstracts should probably be searched and included. We refrain from making specific suggestions for what should be construed as a “sizeable” effect. Magnitudes of effect sizes and thresholds for what is considered relevant can vary considerably across outcomes and across fields and disciplines. We also refrain from making specific suggestions for what should be construed as “reasonable precision” because of the various problems inherent in the use of statistical significance (e.g., arbitrariness, dependence on sample size) and the arbitrary thresholds for precision that use of statistical significance can engender [ 38 , 39 , 40 , 41 ].
Flow chart showing our suggestions for how to approach the use of conference abstracts in systematic review
If abstracts are indeed included in a systematic review, the consistent use of CONSORT reporting guidelines for abstracts [ 14 ] would facilitate extraction of information from abstracts. In many cases, however, these reporting guidelines are not followed [ 42 ], so we suggest that diligent attempts be made to contact authors of the abstracts and examine study registers, such as ClinicalTrials.gov, and published protocols to obtain all necessary unreported or unclear information on study methods and results. In addition, to examine the impact of including the abstracts, a sensitivity analysis should always be completed with and without conference abstracts.
Based on the available evidence and on our experience, we suggest that instead of arbitrarily deciding to include conference abstracts or not in a systematic review, systematic reviewers should consider the availability of evidence. If available evidence is sparse or conflicting, it may be worthwhile to include conference abstracts. If results from conference abstracts are included, then it is necessary to make diligent attempts to contact the authors of the abstract and examine study registers and published protocols to obtain further and confirmatory information on methods and results.
Availability of data and materials
Not applicable.
Abbreviations
Agency for Health Care Research and Quality
Confidence interval
United States National Academy of Sciences
Randomized controlled trial
Balshem H, Stevens A, Ansari M, Norris S, Kansagara D, Shamliyan Try, et al. Finding Grey Literature Evidence and Assessing for Outcome and Analysis Reporting Biases When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. Methods Guide for Comparative Effectiveness Reviews [Internet]. Rockville: Agency for Healthcare Research and Quality (US): 2008-AHRQ Methods for Effective Health Care. 2013.
(IOM) IoM. Knowing what works in health care: a roadmap for the nation. Washington, D. C: The National Academies Press; 2008.
Google Scholar
Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011.
Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Syst Rev. 2018;11:Mr000005.
Antes G, Chalmers I. Under-reporting of clinical trials is unethical. Lancet. 2003;361:978–9.
Article Google Scholar
Schmucker C, Schell LK, Portalupi S, Oeller P, Cabrera L, Bassler D, Schwarzer G, Scherer RW, Antes G, von Elm E, Meerpohl JJ. Extent of non-publication in cohorts of studies approved by research ethics committees or included in trial registries. PLoS One. 2014;9:e114023.
Dickersin K, Chan S, Chalmers TC, Sacks HS, Smith H Jr. Publication bias and clinical trials. Control Clin Trials. 1987;8:343–53.
Article CAS Google Scholar
Dickersin K, Min YI, Meinert CL. Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. JAMA. 1992;267:374–8.
Dickersin K, Min YI. NIH clinical trials and publication bias. Online J Curr Clin Trials. 1993.
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72.
Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev. 2009;(1):MR000006. https://doi.org/10.1002/14651858.MR000006.pub3 .
Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol. 1986;4:1529–41.
von Elm E, Costanza MC, Walder B, Tramer MR. More insight into the fate of biomedical meeting abstracts: a systematic review. BMC Med Res Methodol. 2003;3:12.
Hopewell S, Clarke M, Stewart L, Tierney J. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;18(2):MR000011.
Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279:281–6.
Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–5.
EMBASE content: List of conferences covered in Embase. https://www.elsevier.com/solutions/embase-biomedical-research/embase-coverage-and-content . Accessed 26 Marc 2019.
Scherer RW, Sieving PC, Ervin AM, Dickersin K. Can we depend on investigators to identify and register randomized controlled trials? PLoS One. 2012;7:e44183.
Scherer RW, Huynh L, Ervin AM, Taylor J, Dickersin K. ClinicalTrials.gov registration can supplement information in abstracts for systematic reviews: a comparison study. BMC Med Res Methodol. 2013;13:79.
Scherer RW, Huynh L, Ervin AM, Dickersin K. Using ClinicalTrials.gov to supplement information in ophthalmology conference abstracts about trial outcomes: a comparison study. PLoS One. 2015;10:e0130619.
Mayo-Wilson E, Li T, Fusco N, Bertizzolo L, Canner JK, Cowley T, Doshi P, Ehmsen J, Gresham G, Guo N, et al. Cherry-picking by trialists and meta-analysts can drive conclusions about intervention efficacy. J Clin Epidemiol. 2017;91:95–110.
van Driel ML, De Sutter A, De Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. J Clin Epidemiol. 2009;62:838–844.e833.
Hopewell S, Clarke M, Askie L. Reporting of trials presented in conference abstracts needs to be improved. J Clin Epidemiol. 2006;59:681–4.
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Med Res Methodol. 2017;17:64.
Hopewell S. Assessing the impact of abstracts from the Thoracic Society of Australia and New Zealand in Cochrane reviews. Respirology. 2003;8:509–12.
Rosmarakis ES, Soteriades ES, Vergidis PI, Kasiakou SK, Falagas ME. From conference abstract to full paper: differences between data presented in conferences and journals. FASEB J. 2005;19:673–80.
Tam VC, Hotte SJ. Consistency of phase III clinical trial abstracts presented at an annual meeting of the American Society of Clinical Oncology compared with their subsequent full-text publications. J Clin Oncol. 2008;26:2205–11.
Toma M, McAlister FA, Bialy L, Adams D, Vandermeer B, Armstrong PW. Transition from meeting abstract to full-length journal article for randomized controlled trials. JAMA. 2006;295:1281–7.
Saldanha IJ, Scherer RW, Rodriguez-Barraquer I, Jampel HD, Dickersin K. Dependability of results in conference abstracts of randomized controlled trials in ophthalmology and author financial conflicts of interest as a factor associated with full publication. Trials. 2016;17:213.
Weintraub WH. Are published manuscripts representative of the surgical meeting abstracts? An objective appraisal. J Pediatr Surg. 1987;22:11–3.
McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–31.
Mayo-Wilson E, Fusco N, Li T, Hong H, Canner JK, Dickersin K. Multiple outcomes and analyses in clinical trials create challenges for interpretation and research synthesis. J Clin Epidemiol. 2017;86:39–50.
Schmucker CM, Blumle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, von Elm E, Briel M, Meerpohl JJ. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS One. 2017;12:e0176210.
Scherer RW, Ugarte-Gil C, Schmucker C, Meerpohl JJ. Authors report lack of time as main reason for unpublished research presented at biomedical conferences: a systematic review. J Clin Epidemiol. 2015;68:803–10.
Benzies KM, Premji S, Hayden KA, Serrett K. State-of-the-evidence reviews: advantages and challenges of including grey literature. Worldviews Evid-Based Nurs. 2006;3:55–61.
Weizman AV, Griesman J, Bell CM. The use of research abstracts in formulary decision making by the Joint Oncology Drug Review of Canada. Appl Health Econ Health Policy. 2010;8:387–91.
Dundar Y, Dodd S, Williamson P, Dickson R, Walley T. Case study of the comparison of data from conference abstracts and full-text articles in health technology assessment of rapidly evolving technologies: does it make a difference? Int J Technol Assess Health Care. 2006;22:288–94.
Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305–7.
Tong C. Statistical inference enables bad science; statistical thinking enables good science. Am Stat. 2019;73:20–5.
Ioannidis JPA. What have we (not) learnt from millions of scientific papers with P values? Am Stat. 2019;73:20–5.
Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Stat. 2019;73:1–19.
Saric L, Vucic K, Dragicevic K, Vrdoljak M, Jakus D, Vuka I, et al. Comparison of conference abstracts and full-text publications of randomized controlled trials presented at four consecutive World Congresses of Pain: reporting quality and agreement of results. Eur J Pain. 2019;23(1):107–116. https://doi.org/10.1002/ejp.1289 . Epub 2018 Jul 30.
Download references
Acknowledgements
Author information, authors and affiliations.
Center for Clinical Trials and Evidence Synthesis, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe Street, Room E6138, Baltimore, MD, 21205, USA
Roberta W. Scherer
Center for Evidence Synthesis in Health, Department of Health Services, Policy, and Practice (Primary), Brown University School of Public Health, Providence, RI, USA
Ian J. Saldanha
Department of Epidemiology (Joint), Brown University School of Public Health, Providence, RI, USA
You can also search for this author in PubMed Google Scholar
Contributions
RWS conceived the idea for the commentary. IJS developed Fig. 1 , and both authors were involved in contributing to and critically reading the commentary. Both authors read and approved the final manuscript.
Corresponding author
Correspondence to Roberta W. Scherer .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Scherer, R.W., Saldanha, I.J. How should systematic reviewers handle conference abstracts? A view from the trenches. Syst Rev 8 , 264 (2019). https://doi.org/10.1186/s13643-019-1188-0
Download citation
Received : 03 April 2019
Accepted : 05 October 2019
Published : 07 November 2019
DOI : https://doi.org/10.1186/s13643-019-1188-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Systematic Reviews
- Conference Presentations
- BIOMED CENTRAL Proceedings What's included: meeting abstracts published in BioMed Central Journals, usually includes abstracts and conference name. How to search: Browse list of journals indexing conferences.
- BIOSIS Previews This link opens in a new window What's included: Includes citations to individual poster/paper abstracts – rarely with abstract. May include citations to conferences as a whole (without listing individual presentations). How to search: limit search by "Publication Type" to meeting, meeting abstract or meeting paper. more... less... Online BIOSIS information BIOSIS Previews Help
- NLM Meeting Abstracts What's included: collection of abstracts from HIV/AIDS, Health Services Research, and Space Life Sciences meetings. How to search: search, then follow the "Meeting Abstracts" link.
- << Previous: Clinical Trial Registries
- Next: Databases Indexing Grey Literature >>
- Getting Started
- What is a Systematic Review?
- Levels of Evidence
- Locating Systematic Reviews
- Searching Systematically
- Developing Answerable Questions
- Identifying Synonyms & Related Terms
- Using Truncation and Wildcards
- Identifying Search Limits/Exclusion Criteria
- Keyword vs. Subject Searching
- Where to Search
- Search Filters
- Sensitivity vs. Precision
- Core Databases
- Other Databases
- Clinical Trial Registries
- Databases Indexing Grey Literature
- Web Searching
- Handsearching
- Citation Indexes
- Documenting the Search Process
- Managing your Review
Research Support
- Last Updated: May 1, 2024 4:09 PM
- URL: https://guides.library.ucdavis.edu/systematic-reviews

How to present a review paper at a conference?
1 expert answer.

Meilani M. answered • 04/09/19
Entrepreneurs! Marketing Consultant / Computer Tutor / Public Speaking
Hi! If you have some time before your presentation, I highly recommend you consider joining a Toastmasters chapter near you. You will learn the best practices of speaking in public including in situations like yours, AND you will have opportunity to practice your presentation in front of an audience of your fellow toastmasters, and get valuable evaluations with feedback that will help you improve your presentation. You'll also become very comfortable speaking in front of an audience whether it be a boardroom or a large conference hall!
Ok now some other tips - identify your 3 main points only. Don't need to tell about every detail, people have short attentions spans and 3 things they can remember. Write those 3 as bulletpoints in your notes. Then divvy your time up - intro - body - summary
Tell them what you're going to tell them - brief intro
Tell them - 3 main points which you know so well you don't need to read your notes just speak from your heart
Tell them what you told them - summarize the 3 points and close
you can pick 3 of the specific advances to highlight in your presentation and then tell them there are (x) many more in the paper
or you can do overviews for your bullets - that is, one topic is the many advances that were made, such as blah blah blah that did this and blah blah blah that did that. 2nd topic might be why is that important, what are the ramifications, what does it mean? And 3rd topic might be a look to the future.
So you see even within your paper you have a few different ways to approach your presentation. What is it you most want them to know and why, what is the purpose of the conference, to educate, to spur to action, to attract funding, industry symposium, all of the above?
Hope that helps :)
Still looking for help? Get the right answer, fast.
Get a free answer to a quick problem. Most questions answered within 4 hours.
Choose an expert and meet online. No packages or subscriptions, pay only for the time you need.
RELATED TOPICS
Related questions, which statement below accurately illustrates dual coding as it relates to the use of sensory aids.
Answers · 2
Can you give me a powerful hook regarding the topic "Increase in Tuition Fees?
Answers · 3
i need help choosing a topic for my speech
Answers · 7
business speaking
Answers · 1
why don't I have any friends?
Recommended tutors.

find an online tutor
- Speech tutors
- Extemporaneous Speaking tutors
- Public Speaking tutors
- Dissertation Writing tutors
- Language Arts tutors
- Marketing tutors
- Sign Language tutors
- Image Processing tutors
related lessons
- Need help with something else? Try one of our lessons.
- Need help with something else? Try searching for a tutor.

English Majors Present at Seton Hall Literature Conference
English majors Olivia Frew ’24, senior Breanna Guinta, Nicole Mautone ’24, and Hana Vozzo ’24 recently presented their research at Seton Hall University’s “Boundaries and Borders” undergraduate literature conference alongside English majors from Seton Hall, Columbia University, Baruch College, and Keene State University in New Hampshire.
Frew presented on “Myth and Sacrifice: Haitian Womanhood in ‘Breath, Eyes, Memory,’” a 1994 novel by Edwidge Danticat. Guinta presented on her departmental honors thesis research into Nathaniel Parker Willis, a mid-nineteenth-century American author once popular but now largely forgotten. Guinta was much encouraged by the audience response: “I was delighted to receive from my peers and professors at Seton Hall such positive feedback on my presentation. The Seton Hall English department chair especially motivated me to continue my research and gave me some recommendations on other works to check out.”
Mautone’s paper was “Ultimate Weakness to Driving Force: How Religion Empowers the Vampire in ‘Midnight Mass .’ ” Her paper examined the use of religion in Bram Stoker’s 1897 novel “Dracula,” Anne Rice’s 1976 novel “Interview with the Vampire,” and the 2021 Netflix series “Midnight Mass.” Mautone considered herself “lucky to be able to watch Hana Vozzo present and to share a Q&A session with her.” Vozzo’s presentation was on “Symbolism of the Sea: An Exploration of Individual and Cultural Identity in Derek Walcott’s ‘The Schooner Flight.’” Best known for his poetry, in 1992 Walcott was awarded the Nobel Prize in Literature.
All four English majors are members of Monmouth University’s Delta Sigma chapter of Sigma Tau Delta, the International English Honor Society, advised by BethSara Swanson, lecturer in the Department of English. All four students said they enjoyed and benefited from presenting off campus. “I enjoyed seeing the research my peers were working on from other universities,” Guinta said, adding that she was pleased that this “talented group” of English majors represented Monmouth University.
“Conference presentations like this one can be an important step in undergraduate professional development,” said Stanley Blair, Ph.D., associate professor of English. “By presenting on their own time and off campus, these students have demonstrated that they think not just within course requirements, but also beyond them.”


Identifying the practice patterns of optometrists in providing falls prevention management: A mixed-methods systematic review protocol
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Si Ye Lee
- For correspondence: [email protected]
- ORCID record for Khyber Alam
- For correspondence: [email protected]
- ORCID record for Jason Charng
- For correspondence: [email protected]
- ORCID record for Hamed Niyazmand
- For correspondence: [email protected]
- ORCID record for Jingyi Chen
- For correspondence: [email protected]
- ORCID record for Anne-Marie Hill
- For correspondence: [email protected]
- Info/History
- Preview PDF
Objective The objective of this systematic review is to synthesise the best available evidence for optometrists’ practice patterns in providing falls prevention management.
Introduction Falls remain the main cause of injury-related hospitalisation and mortality in Australia and worldwide, significantly affecting older adults. The increased risk of comorbidities, including visual impairment in this cohort is linked to a higher incidence of falls. Despite being primary eye care practitioners, community optometrists may not consistently integrate falls prevention strategies into their practice. Furthermore, the extent to which they adhere to evidence-based recommendations for falls management remains unclear.
Inclusion criteria The review will include optometrists, in regions where optometry is a regulated profession, and report their understanding and practice patterns in delivering falls prevention management to older community-dwelling adults. Qualitative, quantitative, and mixed methods studies will be eligible for inclusion. It is envisioned that most studies will be qualitative. Studies published in English and those published from 1980 onwards will be eligible for inclusion since published evidence for falls prevention began to increase sharply around this time.
Methods The review will follow the JBI guidelines for mixed methods systematic reviews and will be developed and reported in accordance with PRISMA-P guidelines. Databases that will be searched are Excerpta Medica Database (Embase), Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Complete, and OVID MEDLINE. Grey literature will be searched through Conference Proceedings Citation Index (Web of Science), Google Scholar, and ProQuest Dissertations databases. Two reviewers will independently conduct all screening and critical appraisal. The reviewers will screen all articles’ titles and abstracts retrieved from the searches to determine potential eligibility. All full-text articles considered relevant will then be assessed for final eligibility for inclusion. The final included articles will be assessed for methodological rigour using the JBI SUMARI critical appraisal tools, subsequently, all relevant data will be extracted. Discrepancies at any stage of the procedures will be resolved through discussion and consensus with a third senior researcher. A convergent integrated approach to synthesising and integrating the quantitative and qualitative data will be followed.
Review registration CRD42024539668
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
Anne-Marie Hill is supported by a National Health and Medical Research Council (NHMRC) of Australia Investigator (EL2) award (GNT1174179) and the Royal Perth Hospital Research Foundation. Si Ye Lee is conducting this research with the support of an Australian Government Research Training Program Fees Offset scholarship and is a recipient of a Perth Eye Foundation scholarship through the University of Western Australia.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All data produced in the present work are contained in the manuscript.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Public and Global Health
- Addiction Medicine (324)
- Allergy and Immunology (628)
- Anesthesia (165)
- Cardiovascular Medicine (2384)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (380)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (839)
- Epidemiology (11778)
- Forensic Medicine (10)
- Gastroenterology (703)
- Genetic and Genomic Medicine (3752)
- Geriatric Medicine (350)
- Health Economics (635)
- Health Informatics (2401)
- Health Policy (935)
- Health Systems and Quality Improvement (900)
- Hematology (341)
- HIV/AIDS (782)
- Infectious Diseases (except HIV/AIDS) (13324)
- Intensive Care and Critical Care Medicine (769)
- Medical Education (366)
- Medical Ethics (105)
- Nephrology (398)
- Neurology (3514)
- Nursing (198)
- Nutrition (528)
- Obstetrics and Gynecology (675)
- Occupational and Environmental Health (665)
- Oncology (1825)
- Ophthalmology (538)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (234)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1035)
- Pharmacology and Therapeutics (426)
- Primary Care Research (422)
- Psychiatry and Clinical Psychology (3181)
- Public and Global Health (6152)
- Radiology and Imaging (1281)
- Rehabilitation Medicine and Physical Therapy (749)
- Respiratory Medicine (830)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (323)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (146)
- Case Report
- Open access
- Published: 14 May 2024
Motor polyradiculoneuropathy as an unusual presentation of neurobrucellosis: a case report and literature review
- Ahmad Alikhani 1 ,
- Noushin Ahmadi 1 ,
- Mehran Frouzanian 2 &
- Amirsaleh Abdollahi 2
BMC Infectious Diseases volume 24 , Article number: 491 ( 2024 ) Cite this article
144 Accesses
Metrics details
Brucellosis, a zoonotic disease caused by Brucella species, poses a significant global health concern. Among its diverse clinical manifestations, neurobrucellosis remains an infrequent yet debilitating complication. Here, we present a rare case of neurobrucellosis with unusual presentations in a 45-year-old woman. The patient’s clinical course included progressive lower extremity weakness, muscle wasting, and double vision, prompting a comprehensive diagnostic evaluation. Notable findings included polyneuropathy, elevated brucella agglutination titers in both cerebrospinal fluid and blood, abnormal EMG-NCV tests, and resolving symptoms with antibiotic therapy. The clinical presentation, diagnostic challenges, and differentiation from other neurological conditions are discussed. This case underscores the importance of considering neurobrucellosis in regions where brucellosis is prevalent and highlights this rare neurological complication’s distinctive clinical and radiological features. Early recognition and appropriate treatment are crucial to mitigate the significant morbidity associated with neurobrucellosis.
Peer Review reports
Introduction
Brucellosis, caused by Brucella species, is an infectious ailment recognized by various names such as remitting, undulant, Mediterranean, Maltese, Crimean, and goat fever. Humans contract it through the consumption of unpasteurized milk and dairy products, undercooked meat, or skin contact with infected livestock [ 1 , 2 , 3 ]. Various Brucella species, including Brucella melitensis (primarily sourced from sheep and goats), Brucella abortus (found in cattle), Brucella suis (associated with pigs/hogs), and Brucella canis (linked to dogs), can lead to illness in humans [ 3 , 4 , 5 ]. While brucellosis in humans is rarely fatal, it can lead to disability [ 6 ]. Brucellosis ranks among the most prevalent zoonotic diseases, impacting approximately 500,000 individuals yearly [ 7 ]. The combined estimate for the prevalence of brucellosis was 15.53% [ 8 ].
Neurobrucellosis, a rare complication of systemic brucellosis, can occur in adult and pediatric cases [ 9 ], and can manifest at any stage of the disease. They can present in various clinical presentations such as meningitis, encephalitis, meningoencephalitis, myelitis, radiculopathy, polyneuropathy, stroke, cerebral venous thrombosis, and occasionally psychiatric symptoms [ 10 , 11 ]. Although the mortality rate is low, patients often experience persistent neurological issues following neurobrucellosis [ 12 ]. Studies suggest that around 20% of neurobrucellosis cases result in lasting neurological problems [ 13 ]. It is uncommonly considered in cases of meningoencephalitis or polyneuropathy, making it crucial for clinicians to have a high suspicion of it in patients displaying such symptoms, especially in endemic regions, to prevent severe clinical outcomes. In this study, we present a rare case of neurobrucellosis with unusual clinical presentations in a patient admitted to our center.
Case presentation
A 45-year-old female patient, with no prior medical history, presented to our center after enduring distal pain and weakness in her lower extremities for approximately 10 months. Over this period, the muscle weakness progressed, affecting proximal muscles of upper and lower limbs, and leading to a substantial weight loss of 25–30 kg despite maintaining appetite. Initially dismissive of the limb weakness and pain, the patient sought medical attention six months after symptom onset due to the worsening symptoms and gait impairment. Over the subsequent four months, she underwent multiple medical evaluations and tests, including a lumbar X-ray. Following these initial investigations and due to low serum vitamin D levels, vitamin D and calcium supplements were prescribed, and lumbar MRI were requested for further evaluation. (Table 1 )
Upon referral to an infectious disease specialist, the patient’s history of local dairy consumption and positive serologic test for brucellosis prompted treatment with rifampin and doxycycline. However, the patient’s condition deteriorated significantly five days after starting this treatment. She experienced severe gait disorder, lower extremity weakness, diplopia, and blurred vision that had gradually worsened over two weeks. Subsequently, she presented to our center for further assessment.
Upon admission, the patient was unable to stand even with assistance and exhibited diplopia. Cranial nerve examination revealed no abnormalities, except for the II, III, and IV cranial nerves, which could not be thoroughly examined due to the presence of diplopia. The patient tested negative for Kernig and Brudzinski signs. There were no palpable supraclavicular or inguinal lymph nodes. Physical examinations of the breast, axilla, lungs, heart, and abdomen were unremarkable. Muscle strength was reduced in the lower extremities, and deep tendon reflexes of the knee and Achilles were absent. The plantar reflex was non-responsive, and certain reflexes, including biceps, triceps, and brachioradialis, were absent despite normal movement of the upper extremities. Anorectal muscle tone and anal reflex were normal.
Further investigations included normal urinalysis and abdominal and pelvic ultrasound. Chest X-ray and brain CT were also ordered. Due to the patient’s refusal of lumbar puncture, a suspicion of neurobrucellosis led to the initiation of a three-drug regimen (Table 2 ); ceftriaxone 2 g IV twice daily, rifampin 600 mg PO daily, and doxycycline 100 mg PO twice daily. The ophthalmology consultation did not reveal any ocular pathology, and the neurologist ordered brain MRI and EMG-NCV tests. The patient’s brain MRI was unremarkable, but EMG-NCV showed sensory and motor polyneuropathy. Consequently, intravenous immunoglobulin (IVIG) therapy was initiated at a daily dose of 25 g. After five days, the patient consented to lumbar puncture, confirming the diagnosis of brucellosis. Co-trimoxazole 960 mg PO three times daily was added to her treatment regimen, and IVIG therapy continued for seven days. Following a 3-day course of IVIG treatment, the neuropathy symptoms showed significant improvement. By the seventh day, there was a notable enhancement in limb strength, particularly in the upper limbs, reaching a 2-point improvement. After undergoing three weeks of intravenous therapy, the patient transitioned to oral medication. Despite disagreement regarding the necessity of a second CSF examination, the patient was discharged with a prescription for doxycycline, rifampin, and cotrimoxazole. Upon discharge, the patient could walk with the aid of a walker. However, within a month, a slight limp persisted, and by the third-month post-discharge, all symptoms had resolved completely.
Brucellosis is widely spread globally, with more than half a million reported human cases annually [ 14 , 15 ]. Countries like Kenya, Yemen, Syria, Greece, and Eritrea have experienced high rates of brucellosis. The situation of brucellosis has shown signs of improvement in many epidemic regions. However, new areas with high occurrences of this disease continue to emerge, particularly in Africa and the Middle East, where the incidence of the disease varies [ 16 ]. Brucellosis is linked to various neurological complications collectively known as neurobrucellosis, which is an uncommon condition, and only a few cases have been reported globally [ 17 , 18 , 19 , 20 , 21 ]. Our patient exhibited muscle weakness, polyneuropathy, and inability to walk, which are often not regarded as indicative of a brucella infection by many physicians. While the diagnosis of neurobrucellosis can typically be confirmed through classical clinical signs, radiological examinations, and serological tests, patients might not always display typical symptoms, as observed in our case. Hence, in regions where the disease is prevalent, clinicians should maintain a high level of suspicion if patients do not show improvement with standard treatment. Additionally, the lack of awareness among healthcare professionals and limited access to advanced laboratory facilities can lead to misdiagnosis.
The frequent manifestations of neurobrucellosis include meningitis or meningoencephalitis. Typically, it starts with a sudden headache, vomiting, and altered mental state, which can progress to unconsciousness, with or without seizures [ 22 ]. Additionally, brucellosis can lead to several central nervous system issues such as inflammation of cerebral blood vessels, abscesses in the brain or epidural space, strokes, and cerebellar ataxia. Peripheral nerve problems may include nerve damage or radiculopathy, Guillain-Barré syndrome, and a syndrome resembling poliomyelitis [ 13 ]. Nevertheless, the patient exhibited no indications of seizures, brain hemorrhage, stroke, or focal neurological impairments. Instead, the observed symptoms were consistent with radiculopathy and muscular weakness.
In only 7% of neurobrucellosis cases, the peripheral nervous system is affected. Remarkably, our case falls within this rare category, adding to its unique and intriguing nature. Previous case studies have detailed polyradiculoneuropathies, manifesting as acute, subacute, or chronic forms [ 23 ]. Our patient’s condition aligns with chronic motor polyradiculopathy. Interestingly, some of these cases exhibit sensory deficits or resemble Guillain-Barré syndrome [ 23 , 24 ]. In a prior case study conducted by Abuzinadah and colleagues, a comparable case was described as a subacute motor polyradiculopathy. The patient exhibited gradual bilateral lower limb weakness over three weeks, eventually leading to loss of mobility within seven weeks. Brucella was isolated from the cerebrospinal fluid after a two-week incubation period, and high antibody titers were detected in the patient’s serum [ 23 ]. In another study led by Alanazi and colleagues, a 56-year-old man initially diagnosed with Guillain-Barré syndrome experienced worsening symptoms despite appropriate treatment. Following plasma exchange and antibiotics, his condition improved temporarily, only to relapse, raising suspicion of chronic inflammatory demyelinating polyneuropathy, and treatment with IVIG resulted in substantial improvement. Upon further investigation, he was diagnosed with brucellosis [ 24 ]. This highlights the importance of recognizing GBS-like symptoms in regions where brucellosis is prevalent, prompting clinicians to consider the possibility of brucellosis in their diagnosis.
While there are no established criteria for diagnosing neurobrucellosis [ 25 ], certain articles have suggested several methods for its diagnosis. These methods include the presence of symptoms aligning with neurobrucellosis, isolating brucella from cerebrospinal fluid (CSF) or detecting a positive brucella agglutination titer in CSF, observing lymphocytosis, elevated protein, and decreased glucose levels in CSF, or identifying specific diagnostic indicators in cranial imaging such as magnetic resonance imaging or computed tomography (MRI or CT) [ 13 , 26 , 27 , 28 ]. Neurobrucellosis does not present a distinct clinical profile or specific CSF characteristics. Imaging observations of neurobrucellosis fall into four categories: normal, inflammatory (indicated by granulomas and enhanced meninges, perivascular spaces, or lumbar nerve roots), alterations in white matter, and vascular changes [ 29 ]. We suspected neurobrucellosis based on the patient’s clinical symptoms, geographic correlation, high brucella agglutination test titers in both cerebrospinal fluid and blood, symptom resolution following treatment, and the exclusion of other common causes.
In Iran, one differential diagnosis often confused with brucellosis is tuberculosis, as both chronic granulomatous infectious diseases are prevalent here [ 30 , 31 ]. Neurobrucellosis and tuberculosis exhibit significant similarities in clinical symptoms, lab results, and neuroimaging findings. However, deep grey matter involvement and widespread white matter lesions seen in neuroimaging, resembling demyelinating disorders, appear to be distinctive to brucellosis [ 32 ]. There is a noticeable similarity in the clinical symptoms and laboratory findings of brucellosis and tuberculosis [ 33 ]. It is crucial to thoroughly eliminate the possibility of tuberculosis in any suspected or confirmed brucellosis cases before starting antibiotic treatment.
Due to the challenging nature of treating brucellosis and the likelihood of experiencing relapses, it is crucial to provide an extended course of treatment [ 27 ]. This treatment approach should involve a combination of antibiotics that can easily penetrate the cell wall and effectively reach the central nervous system [ 27 , 34 ]. Neurobrucellosis is treated with 3 to 6 months of combination therapy comprising doxycycline, rifampicin, and ceftriaxone or trimethoprim-sulfamethoxazole [ 35 ], similar to the treatment administered to our patient. For patients allergic to cephalosporins, quinolones are recommended, which are considered to be effective in treating brucellosis [ 36 , 37 ]. In complicated situations such as meningitis or endocarditis, streptomycin or gentamicin is administered in the initial 14 days of treatment, in addition to the previously mentioned regimen. Timely and proper treatment results in a positive prognosis, with a less than 1% fatality rate for such complex cases [ 17 , 38 ]. Our patient experienced a highly positive outcome following the prescribed therapy. Initially relying on a walker, a slight limp endured for a month, and by the third month after discharge, all symptoms completely disappeared.
The present study underscores the significance of considering neurobrucellosis as a potential diagnosis when evaluating muscle weakness and radiculopathy, especially in regions where the disease is prevalent. A comprehensive patient history, precise clinical examination, positive serology in blood or cerebrospinal fluid, imaging results, or cerebrospinal fluid analysis can contribute to establishing a conclusive diagnosis.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to our team’s privacy concerns but are available from the corresponding author on reasonable request.
Galińska EM, Zagórski J. Brucellosis in humans–etiology, diagnostics, clinical forms. Ann Agric Environ Med. 2013;20(2):233–8.
PubMed Google Scholar
Głowacka P, Żakowska D, Naylor K, Niemcewicz M, Bielawska-Drózd A. Brucella - Virulence factors, Pathogenesis and treatment. Pol J Microbiol. 2018;67(2):151–61.
Article PubMed PubMed Central Google Scholar
Khurana SK, Sehrawat A, Tiwari R, Prasad M, Gulati B, Shabbir MZ, et al. Bovine brucellosis - a comprehensive review. Vet Q. 2021;41(1):61–88.
Article CAS PubMed PubMed Central Google Scholar
Yagupsky P, Morata P, Colmenero JD. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. 2019;33(1).
Kurmanov B, Zincke D, Su W, Hadfield TL, Aikimbayev A, Karibayev T et al. Assays for identification and differentiation of Brucella species: a review. Microorganisms. 2022;10(8).
Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–86.
Article CAS PubMed Google Scholar
Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol. 2007;25(3):188–202.
Khoshnood S, Pakzad R, Koupaei M, Shirani M, Araghi A, Irani GM, et al. Prevalence, diagnosis, and manifestations of brucellosis: a systematic review and meta-analysis. Front Vet Sci. 2022;9:976215.
Dhar D, Jaipuriar RS, Mondal MS, Shunmugakani SP, Nagarathna S, Kumari P et al. Pediatric neurobrucellosis: a systematic review with case report. J Trop Pediatr. 2022;69(1).
Mahajan SK, Sharma A, Kaushik M, Raina R, Sharma S, Banyal V. Neurobrucellosis: an often forgotten cause of chronic meningitis. Trop Doct. 2016;46(1):54–6.
Article PubMed Google Scholar
Dreshaj S, Shala N, Dreshaj G, Ramadani N, Ponosheci A. Clinical manifestations in 82 neurobrucellosis patients from Kosovo. Mater Sociomed. 2016;28(6):408–11.
Gul HC, Erdem H, Bek S. Overview of neurobrucellosis: a pooled analysis of 187 cases. Int J Infect Dis. 2009;13(6):e339–43.
Guven T, Ugurlu K, Ergonul O, Celikbas AK, Gok SE, Comoglu S, et al. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis. 2013;56(10):1407–12.
Alkahtani AM, Assiry MM, Chandramoorthy HC, Al-Hakami AM, Hamid ME. Sero-prevalence and risk factors of brucellosis among suspected febrile patients attending a referral hospital in southern Saudi Arabia (2014–2018). BMC Infect Dis. 2020;20(1):26.
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–9.
Liu Z, Gao L, Wang M, Yuan M, Li Z. Long ignored but making a comeback: a worldwide epidemiological evolution of human brucellosis. Emerg Microbes Infect. 2024;13(1):2290839.
Naderi H, Sheybani F, Parsa A, Haddad M, Khoroushi F. Neurobrucellosis: report of 54 cases. Trop Med Health. 2022;50(1):77.
Farhan N, Khan EA, Ahmad A, Ahmed KS. Neurobrucellosis: a report of two cases. J Pak Med Assoc. 2017;67(11):1762–3.
Karsen H, Tekin Koruk S, Duygu F, Yapici K, Kati M. Review of 17 cases of neurobrucellosis: clinical manifestations, diagnosis, and management. Arch Iran Med. 2012;15(8):491–4.
Türel O, Sanli K, Hatipoğlu N, Aydoğmuş C, Hatipoğlu H, Siraneci R. Acute meningoencephalitis due to Brucella: case report and review of neurobrucellosis in children. Turk J Pediatr. 2010;52(4):426–9.
Guney F, Gumus H, Ogmegul A, Kandemir B, Emlik D, Arslan U, et al. First case report of neurobrucellosis associated with hydrocephalus. Clin Neurol Neurosurg. 2008;110(7):739–42.
Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3(2):213–21.
Abuzinadah AR, Milyani HA, Alshareef A, Bamaga AK, Alshehri A, Kurdi ME. Brucellosis causing subacute motor polyradiculopathy and the pathological correlation of pseudomyopathic electromyography: a case report. Clin Neurophysiol Pract. 2020;5:130–4.
Alanazi A, Al Najjar S, Madkhali J, Al Malik Y, Al-Khalaf A, Alharbi A. Acute Brucellosis with a Guillain-Barre Syndrome-Like Presentation: a Case Report and Literature Review. Infect Dis Rep. 2021;13(1):1–10.
Raina S, Sharma A, Sharma R, Bhardwaj A, Neurobrucellosis. A Case Report from Himachal Pradesh, India, and review of the literature. Case Rep Infect Dis. 2016;2016:2019535.
PubMed PubMed Central Google Scholar
McLean DR, Russell N, Khan MY. Neurobrucellosis: clinical and therapeutic features. Clin Infect Dis. 1992;15(4):582–90.
Bouferraa Y, Bou Zerdan M, Hamouche R, Azar E, Afif C, Jabbour R. Neurobrucellosis: brief review. Neurologist. 2021;26(6):248–52.
Aygen B, Doğanay M, Sümerkan B, Yildiz O, Kayabaş Ü. Clinical manifestations, complications and treatment of brucellosis: a retrospective evaluation of 480 patients. Méd Mal Infect. 2002;32(9):485–93.
Article Google Scholar
Kizilkilic O, Calli C, Neurobrucellosis. Neuroimaging Clin N Am. 2011;21(4):927–37. ix.
Chalabiani S, Khodadad Nazari M, Razavi Davoodi N, Shabani M, Mardani M, Sarafnejad A, et al. The prevalence of brucellosis in different provinces of Iran during 2013–2015. Iran J Public Health. 2019;48(1):132–8.
Doosti A, Nasehi M, Moradi G, Roshani D, Sharafi S, Ghaderi E. The pattern of tuberculosis in Iran: A National Cross-sectional Study. Iran J Public Health. 2023;52(1):193–200.
Rajan R, Khurana D, Kesav P. Deep gray matter involvement in neurobrucellosis. Neurology. 2013;80(3):e28–9.
Dasari S, Naha K, Prabhu M. Brucellosis and tuberculosis: clinical overlap and pitfalls. Asian Pac J Trop Med. 2013;6(10):823–5.
Ko J, Splitter GA. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin Microbiol Rev. 2003;16(1):65–78.
Zhao S, Cheng Y, Liao Y, Zhang Z, Yin X, Shi S. Treatment efficacy and risk factors of Neurobrucellosis. Med Sci Monit. 2016;22:1005–12.
Hasanain A, Mahdy R, Mohamed A, Ali M. A randomized, comparative study of dual therapy (doxycycline-rifampin) versus triple therapy (doxycycline-rifampin-levofloxacin) for treating acute/subacute brucellosis. Braz J Infect Dis. 2016;20(3):250–4.
Falagas ME, Bliziotis IA. Quinolones for treatment of human brucellosis: critical review of the evidence from microbiological and clinical studies. Antimicrob Agents Chemother. 2006;50(1):22–33.
Budnik I, Fuchs I, Shelef I, Krymko H, Greenberg D. Unusual presentations of pediatric neurobrucellosis. Am J Trop Med Hyg. 2012;86(2):258–60.
Download references
This research did not receive any funding or financial support.
Author information
Authors and affiliations.
Infectious Diseases Department and Antimicrobial Resistance Research Center and Transmissible Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
Ahmad Alikhani & Noushin Ahmadi
Student Research Committee, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
Mehran Frouzanian & Amirsaleh Abdollahi
You can also search for this author in PubMed Google Scholar
Contributions
A.A oversaw and treated the case, including the entire revision process. N.A. contributed to the article’s composition. M.F. authored the discussion section, along with the complete revision. AS.A. played a role in crafting the case report discussion and participated in the entire revision process.
Corresponding author
Correspondence to Amirsaleh Abdollahi .
Ethics declarations
Ethics approval and consent to participate.
In adherence to ethical standards, rigorous protocols were followed to obtain approval from the relevant ethics committee and secure informed consent from all participants involved in the study.
Consent for publication
informed consent was obtained from the patient for both study participation AND publication of identifying information/images in an online open-access publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alikhani, A., Ahmadi, N., Frouzanian, M. et al. Motor polyradiculoneuropathy as an unusual presentation of neurobrucellosis: a case report and literature review. BMC Infect Dis 24 , 491 (2024). https://doi.org/10.1186/s12879-024-09365-2
Download citation
Received : 04 December 2023
Accepted : 29 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12879-024-09365-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neurobrucellosis
- EMG-NCV tests
- Polyradiculoneuropathy
- Antibiotic therapy
- Intravenous immunoglobulin therapy
- Zoonotic disease
- Gait disorder
- Lower extremity weakness
- Blurred vision
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]

COMMENTS
The process of presenting a literature review at a conference requires careful preparation and strategic execution. It involves a deep understanding of the subject matter and the ability to succinctly and engagingly convey complex ideas. This guide offers a structured approach to ensure your presentation is impactful and memorable.
a description of the publication. a summary of the publication's main points. an evaluation of the publication's contribution to the topic. identification of critical gaps, points of disagreement, or potentially flawed methodology or theoretical approaches. indicates potential directions for future research.
3) Examples of what you found (results) including. a. Visual and quantitative information. b. Important quotes. 4) Your conclusion. Remember to keep your presentation (and your visual material) concise. It is very easy to overwhelm an audience with too much text. Also, be sure to use a font size that is large enough to read from several feet away.
In any case, the abstract for a narrative/literature review for a journal article should start with two lines of background information on the topic being discussed in the review. This should be followed by one sentence of the aim of the review. Then, in the next 2-3 sentences, you should describe the methods that you have used - you need to ...
15. I sent an abstract of a review paper for oral presentation to a conference, and it was accepted. The presentation will be about the most important developments and contributions made in the last decade on my specific area of research. Now I am preparing the speech, but I am not sure how to give a 12-minutes speech about all the work that ...
Presenting your Conference Paper 12 Your audience: literature review Your assessor Your supervisor Knows the subject area Knows some of the details Wants to be convinced that youknow Your audience: literature review Your assessor Your supervisor Everyone else Should learn something Your audience: conference Know the subject area Do not know the ...
1. Highlight current research. The point of a literature review for research is to highlight the current state of research related to your topic, not to simply give background information. Background information is important and should be included, but the focus of the presentation should be showing some current studies that either confirm or ...
the ideas and master the techniques and methods inherent in the literature review, this will be helpful to you in your own research. Developing and Presenting 7 Your Literature Review Chapter 7 Objectives Section I: Instruction • Provide an understanding of the function and purpose of a literature review (the "what").
Presenting at Conferences. Academic conferences are a useful way to present the results of a Cochrane review to people either through an oral presentation, a poster presentation, or a booth. Conferences also have the additional benefit of networking and an opportunity to promote both Cochrane and the results of your review to peers.
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Guidelines for Conducting a Literature Review and Presenting Conference Papers Elizabeth A. Herdman. School Of Nursing At Koç University, Istanbul. A literature review provides a solid background for a research paper and reveals a comprehensive knowledge of the literature. Apart from providing you with a useful overview of a particular subject ...
As a first step, we adopt narrative visualization to present literature review as interactive slides. Specifically, we propose a narrative structure with three levels of granularities that the reader can drill down or roll up freely. The logic flow of a slideshow can be organized based on the paper's presentation or citations.
One study showed that less than 5% of conference attendees visit posters at meetings and that few ask useful questions.2 The same research found that, in addition to the scientific content of a poster, the factors that increase visual appeal include pictures, graphs, and a limited use of words.2 The ideal number of words seems to be between 300 ...
Conclusions. Effective research posters should be designed around two or three key findings with accompanying handouts and narrative description to supply additional technical detail and encourage dialog with poster viewers. Keywords: Communication, poster, conference presentation. An assortment of posters is a common way to present research ...
Types of conference papers and sessions. Panel presentations are the most common form of presentation you will encounter in your graduate career. You will be one of three to four participants in a panel or session (the terminology varies depending on the organizers) and be given fifteen to twenty minutes to present your paper.
1 Answer to this question. Answer: Conferences do not usually accept literature review manuscripts or review articles for publishing in their conference proceedings. However, some conferences do allow publication of review articles as a poster. Alternatively, you can also request for a plenary discussion for your review article at the conference.
1.1.6 While the authorship of conference abstracts and presentations should accurately reflect those who were involved in the research, individuals who meet the ICMJE authorship criteria (and may be listed on a subsequent full publication) may choose not to be listed for a conference abstract and presentation (e.g. if they are unable to review ...
The abstract review serves as a cornerstone in the organization of academic conferences, ensuring the quality, relevance, and integrity of the event's content. Academic conferences thrive on the dissemination of cutting-edge research and fostering meaningful discussions among scholars, researchers, and practitioners.
Template 4: Comprehensive Literature Review PPT Slide. Download this tried-and-true literature review template to present a descriptive summary of your research topic statement. The given PPT layout is replete with relevant content to help you strike a balance between supporting and opposing aspects of an argument.
Background While identifying and cataloging unpublished studies from conference proceedings is generally recognized as a good practice during systematic reviews, controversy remains whether to include study results that are reported in conference abstracts. Existing guidelines provide conflicting recommendations. Main body The main argument for including conference abstracts in systematic ...
What's included: almost 800 conferences and more than 260,000 conference abstracts, primarily from journals and journal supplements published from 2009. How to search: limit search by "Publication Type" to conference abstract, conference paper, conference review, or proceeding. more... Embase Video Tutorials.
Then divvy your time up - intro - body - summary. Tell them what you're going to tell them - brief intro. Tell them - 3 main points which you know so well you don't need to read your notes just speak from your heart. Tell them what you told them - summarize the 3 points and close. you can pick 3 of the specific advances to highlight in your ...
WWW '24: Companion Proceedings of the ACM on Web Conference 2024 Linked Open Literature Review using the Neuro-symbolic Open Research Knowledge Graph. ... We present the first steps towards a knowledge ... Read More. Knowledge Extraction for Literature Review. JCDL '16: Proceedings of the 16th ACM/IEEE-CS on Joint Conference on Digital Libraries .
English majors Olivia Frew '24, senior Breanna Guinta, Nicole Mautone '24, and Hana Vozzo '24 recently presented their research at Seton Hall University's "Boundaries and Borders" undergraduate literature conference alongside English majors from Seton Hall, Columbia University, Baruch College, and Keene State University in New Hampshire. Frew presented on "Myth and Sacrifice ...
Objective The objective of this systematic review is to synthesise the best available evidence for optometrists practice patterns in providing falls prevention management. Introduction Falls remain the main cause of injury related hospitalisation and mortality in Australia and worldwide, significantly affecting older adults. The increased risk of comorbidities, including visual impairment in ...
Brucellosis, a zoonotic disease caused by Brucella species, poses a significant global health concern. Among its diverse clinical manifestations, neurobrucellosis remains an infrequent yet debilitating complication. Here, we present a rare case of neurobrucellosis with unusual presentations in a 45-year-old woman. The patient's clinical course included progressive lower extremity weakness ...