Engaging Low-Income Parents in Childhood Obesity Prevention from Start to Finish: A Case Study
- Original Paper
- Open access
- Published: 20 June 2012
- Volume 38 , pages 1–11, ( 2013 )

Cite this article
You have full access to this open access article

- Janine M. Jurkowski 1 ,
- Lisa L. Green Mills 1 ,
- Hal A. Lawson 2 ,
- Mary C. Bovenzi 1 ,
- Ronald Quartimon 3 &
- Kirsten K. Davison 4
17k Accesses
31 Citations
1 Altmetric
Explore all metrics
Prevention of childhood obesity is a national priority. Parents influence young children’s healthy lifestyles, so it is paradoxical that obesity interventions focus primarily on children. Evidence and theory suggest that including parents in interventions offers promise for effective childhood obesity prevention. This case study engaged parents’ as co-researchers in the design, implementation and evaluation of an intervention for low-income families with a child enrolled in Head Start. Parent engagement mechanisms include: (1) targeted partnership development (2) operationalizing a Community Advisory Board (CAB) that was the key decision making body; (3) a majority of CAB members were parents who were positioned as experts, and (4) addressing structural barriers to parent participation. Lessons learned are provided for future research, and practice.
Similar content being viewed by others

Using the RE-AIM framework to evaluate the feasibility of a parent-focused intervention targeting childhood obesity
A narrative account of implementation lessons learnt from the dissemination of an up-scaled state-wide child obesity management program in australia: peach™ (parenting, eating and activity for child health) queensland.

A Qualitative Approach: Evaluating the Childhood Health and Obesity Initiative Communities Empowered for Success (CHOICES) Pilot Study
Avoid common mistakes on your manuscript.
Introduction
Preventing childhood obesity is a national priority for health professionals and policy makers. Consistent with a general call for researchers to engage parents in child health research [ 1 ], parental involvement specifically in childhood obesity programs and prevention efforts has been stressed [ 2 – 4 ]. This case study responds to the need for parent engagement as experts throughout the entire research process and, using the example of a childhood obesity prevention initiative, illustrates strategies to engage parents in program development, implementation and evaluation. Parent participation in obesity prevention is increasingly emphasized given links between parents’ attitudes, knowledge, and behavior and children’s dietary, physical activity, and screen-based behavioral factors associated with childhood obesity [ 5 ]. Parents are the most knowledgeable about their family’s needs, motivations, and resources for behavioral change, and they understand family dynamics and ecological factors that influence daily living [ 1 ]. Parents also have insight regarding program relevance and feasibility. As such, parents active family engagement is crucial for the success of preventive interventions [ 6 , 7 ].
A growing body of research and relevant theory emphasizes the importance of utilizing parents as change agents in childhood obesity prevention [ 2 , 8 ]. Although parents have been targeted for studies on treatment of childhood obesity [ 4 , 9 ], parents are less frequently the direct targets for the prevention of childhood obesity. What is more, the evidence for effective involvement of parents in obesity prevention such as dietary [ 10 ] and physical activity [ 11 ] interventions is weak. Evidence of program effectiveness among low-income and ethnic minority children who disproportionately experience childhood obesity is also minimal [ 12 ]. Parent engagement in research is challenged by low participation rates and high attrition [ 13 ]. New approaches are needed to ensure successful engagement of parents in prevention efforts.
One approach is to engage parents in the development, implementation and evaluation of childhood obesity prevention interventions to better integrate parent’s sociocultural context in order to improve program acceptance, cultural relevance and participation. A strategy for operationalizing the level of participation is to utilize the Ladder of Citizen Participation [ 14 ], with slight modifications to emphasize the role of parents in health promotion. The Ladder of Parent Participation provides a useful framework for describing the characteristics and extent of parent participation and therefore, the application of CBPR in the literature (See Fig. 1 ). The ladder has eight rungs representing progressively increasing levels of community engagement. In the case of childhood obesity prevention, high levels of parent participation, in which parents have more contribution to the research process, may improve parent buy-in, participation and program sustainability.
Ladder of Parent Participation. Modified from Sherry Arnstein’s 1969 Ladder of Citizen Participation [ 14 ]
Community-Based Participatory Research (CBPR) is an approach that can be used during the research process to increase the level of parent participation to achieve higher rungs on the Ladder of Participation. CBPR involves community members actively and equitably in decisions throughout the research process, which is often guided by participatory principles [ 15 ]. The use of CBPR in childhood obesity research is increasing, but parents, as key stakeholders, are still infrequently engaged. Many CBPR intervention studies to address childhood obesity have primarily engaged community representatives who are in a profession that serves the target population or who have expertise in some area of childhood obesity. Such stakeholders typically include school administrators, teachers, cooks, providers and other community-based professionals [ 16 ]. Studies that engage parents, most often fall between Rung 3 and 5 of the Ladder of Participation in which parents provide input and are informed of study processes, often during formative stages of the study, but do not have decision making power. Although other studies have involved parents, there are no known examples in which parents are engaged throughout the entire research process. Given the history of hierarchical relationships between low-income families and service or health professionals [ 17 ], engaging parents throughout the research process may serve to open communication, break down hierarchical relationships and build trust.
Case Study Overview
This manuscript describes a parent-centered CBPR case study that expands upon the CBPR literature on childhood obesity prevention by engaging parents directly throughout the entire research process with the goal of fostering parent empowerment and encouraging co-learning across all stakeholders [ 18 ]. Low-income parents are engaged as equal partners, providing unique expertise during the development, implementation and evaluation of a childhood obesity prevention initiative. The case study of Communities for Healthy Living (CHL) , so named by the partnership, is intended to provide a starting point from which dialogue around engaging parents throughout the research process can begin, propelling the identification of effective engagement strategies that can be tested alongside gains in program effectiveness and sustainability. To this end, we discuss (a) the process of partnership development (Phase 1 of the study), (b) the operation of the advisory board as an effective decision making body, and (c) the provision of structural supports to foster active and equal parent involvement. The conclusion outlines the benefits and challenges of using the CBPR approach to engage parents and lessons learned along the way.
Research Setting
The Communities for Healthy Living case study takes place within the context of a study funded by the National Institute of Minority Health and Health Disparities of NIH, which funded 6 research studies utilizing CBPR in the development of interventions addressing health disparities. Because the studies were funded under the American Recovery and Reinvestment Act of 2009, each was constrained to a rapid 2-year timeline to develop and pilot test the intervention. The goal of this study was to develop and pilot test a childhood obesity intervention for low-income families using a CBPR approach to actively engage parents across three phases, Phase 1: Partnership development, Phase 2: Community assessment and intervention development, and Phase 3: Intervention implementation and evaluation. The family-centered intervention targeted parent/caregivers with children participating in Head Start programs in Rensselaer County, NY (about 500 children ages 6 weeks–5 years old) for childhood obesity prevention. Rensselaer County, in Upstate New York, has areas designated as Medically Underserved Areas [ 19 ], and 28 % of all families with children under age 5 living below the poverty level [ 20 ].
Partnership Development
Formation of the decision making body.
A partnership with the community-based organization (CBO) administering Head Start in the county was developed concurrently with proposal development. The CBO director and the Head Start Policy Council, consisting of parents and community members, provided a written commitment to the partnership, feedback on the grant idea, and recommendations for potential community partners. Potential partners were interviewed to determine their interest in the study purpose and their agreement with partner responsibilities. A local reverend of a church serving the neighborhoods where Head Start families reside and a nurse from a local pediatric clinic serving over 60 % of the Head Start families were invited to be partners during this process and became the first members of the planned CHL Community Advisory Board (CAB).
Upon receipt of funding, the Family and Communities Partnership manager for Head Start and program development staff of the CBO were also invited to join the CAB. Candidates for the project coordinator position were jointly interviewed by CBO staff and the research team. Through a subcontract with the CBO, the agreed upon project coordinator was hired as a staff member of the organization. Formally placing the project coordinator within the organizational structure of the CBO was intended to create project visibility at the organization, build relationships with organizational staff and parents, and facilitate organizational cultural exchange. The project coordinator hired had experience working in the community served by the CBO and was responsible for organizing and supporting the CAB, including recruiting additional members to the CAB, particularly parents. It was critical to engage parents early in the process to build trust and foster sustained participation by including them in project decision making as early in the research process as possible with the intent of engaging parents at the highest levels of the Ladder of Participation [ 14 ].
CBO staff members on the CAB, who worked directly with Head Start parents, recruited parents of children who currently attended one of the five Head Start Centers and who also exhibited commitment to other Head Start activities. The project coordinator met with the parents to begin the relationship with the project. Additional parents joined after participating in the research or hearing about the project through other parents. Community members recruited to the CAB included a representative from a local cooperative extension, a CBO board member and other community agency representatives who lived within the community and were familiar with community resources. Throughout the first 2 years of the CAB, the board was comprised of 10 parents and 7 community representatives who consistently attended meetings, with several other parents and community representatives attending less frequently. Having parents serve as the majority of decision makers was important for maintaining a high level of parent participation [ 14 ]. See Table 1 for composition of the CAB.
Partnership Principles and Operating Guidelines
Many CBPR projects develop principles to help clarify the terms of partnerships, codify expectations between partners and serve as guiding values for the partnership and research process [ 21 , 22 ]. CHL CAB members reviewed various other CBPR projects’ partnership principles before beginning the process of developing their own during Phase 1 of the project (Partnership Development). The partnership principles were developed during an 8 month period and approved shortly before the end of year 1, although they served to guide CAB activities even prior to final approval. The CAB also decided to create operating guidelines to sustain active involvement, in response to the inconsistent participation of some members. Several CAB members expressed frustration about time spent ‘updating those who do not show up’. A sample of operating guidelines was obtained from a previous participatory project and refined to meet the needs of the CAB. The guidelines were developed, revised and approved by unanimous vote over a 3 month period. The partnership principles and an outline of the operating guidelines are presented in “Appendix A and B ”.
Operation of the Community Advisory Board
Cab meeting structure.
Due to the rapid timeline of CHL, CAB meetings were held twice a month for the first 6 months and then once a month for the remainder of the grant. In total, 25 meetings, including Workgroup meetings were conducted during the study. These meetings were held in one of the CBO’s buildings housing a Head Start center. Agenda items for the meetings were created with input from the academic staff, CBO staff, the project coordinator and CAB members. The meeting structure varied depending on agenda items, and included a combination of small group and whole group discussions. Meetings were primarily run by the project coordinator, with the researchers facilitating when there was discussion and interpretation of data, and CAB members leading discussion of specific agenda items. Although efforts were made by the project coordinator to have a formal leadership structure within the CAB, none of the CAB members wanted to be an officer.
Small Work Group Meetings
Full CAB meetings were supplemented throughout the 2-year project with small Work Group meetings held at the CBO and at the university. During the first 3 months, smaller parent only meetings were held prior to full CAB meetings to foster social connections among parents. Discussions in these groups during Phase 1 focused on encouraging parents to think critically about factors that influence children’s risk for obesity and to participate as experts and co-researchers. These meetings provided time for parents to talk openly about their experiences as parents and to ask questions without CAB professionals present. After three of these meetings, parents felt comfortable being vocal in the larger CAB. By the fourth month of CAB meetings, parents had a strong presence at meetings and were active participants in the research process.
The full CAB was also split into four small Workgroups to focus on multiple aspects of the research simultaneously. Most of the CAB participated in at least one group but some CAB members chose to participate in multiple groups. An Ethics Workgroup focused on the participatory process. A Data Workgroup helped guide the community assessment by developing the focus groups’ topic and interview guide, conducting data analysis and interpreting findings. An Education workgroup guided the development of materials for the Parents Connect for Healthy Families curriculum. A Social Marketing Workgroup developed the Communities for Healthy Living logo, mission, project pamphlet and childhood obesity awareness poster campaign. All of these features were important for branding and were included in communications, and CHL sponsored events.
Fostering Active Participation of the Community Advisory Board
Active engagement throughout the research process.
Although it is not unusual to have advisory boards on which community members provide input but do not share decision making power, this study’s aim was to involve CAB parents at rung 6 or 7 of the levels of the Ladder of Participation (Fig. 1 ); therefore, there was a need to foster CAB involvement outside of CAB meetings. A project policy was to include CAB members in as many activities as they were willing to participate. In addition to participating in CAB meetings, parents participated in day to day research activities alongside academic partners as equal partners. Their expertise was highly valued and included when decisions were made for the research activities. Figure 2 presents a summary of CAB activities and decisions, which varied across the three phases of the project. During Phase 1 of the project, the main focus was partnership development. In Phase 2, the CAB fully participated in a thorough community assessment and the design of the Communities for Healthy Living intervention. In Phase 3, the CAB focused its efforts on program implementation and evaluation.
Community Advisory Board Parent involvement in communities for Healthy Living activities and decisions throughout the 3 phases of the project
The first CAB meeting during Phase 1 was essential for setting the participatory tone and describing the purpose of the funded research. Academic staff described the specific aims of the project including the CBPR approach, the role of parents as experts, the responsibility to the funder and what is known about childhood obesity and its risk factors with parents. At that point, the project coordinator engaged parents and community members in a discussion to obtain preliminary perspectives on childhood obesity. During the second and third meetings, the CAB worked in small groups with a flip chart and a set of questions to discuss. They were asked to prioritize the essential barriers and facilitators to child health, family health, and parents’ ability to take care of their children’s health. Benefits of this process include, (1) increasing critical consciousness (a component of empowerment) of childhood obesity among CAB members, (2) identifying social determinants of childhood obesity and other child health issues that were relevant to their community, (3) building relationships between CAB members and the CHL academic staff, and (4) operationalizing the expertise of parents by documenting their contribution to these discussions. During these meetings, CAB members were also trained in research ethics and received IRB certification. Phase 1 of Fig. 2 outlines the specific activities in which CAB members participated and the decisions in which they were actively involved.
During Phase 2 of the project (Community assessment and Program Development), CAB parents participated in the design and implementation of the mixed-method community assessment, the dissemination of the results, the development and implementation of the intervention and its evaluation. CAB member participation in research team meetings during Phase 2 facilitated their participation in decision making on a continual basis equal to that of research team members. They suggested that certain discussions needed to be brought to the entire CAB and they were involved in project problem solving and data collection planning. Research team members, parents, and other CAB members worked together to develop research questions and develop and revise data collection instruments. Several parents also recruited and administered assessment tools.
Also during Phase 2, some CAB parents spent their summer integrally involved in intervention development (see Phase 2 Decisions in Fig. 2 ). In addition to being involved in the step by step design of the social marketing campaign and other educational material targeting parents, they also helped develop a 6-week parent program, Parents Connect for Healthy Families, and an intensive 4-day train-the-trainer session for parent facilitators. The program focused on increasing awareness of childhood obesity and its risk behaviors and providing communication, conflict resolution, stress management, and social networking skills, including how to leverage community resources.
During Phase 3 of the project (Program Implementation and Evaluation), four of the CAB parents participated as program facilitators. These Head Start parents participated in a 4-day training seminar along with other parents and then facilitated the administration of the Parents Connect for Healthy Families curriculum to their peers in the Head Start community. Engaging parents in both the design and leadership of the program ensured its relevance, and was an important part of the participatory process. Other parents who joined the project as program facilitators subsequently joined the CAB after their experience working with the program.
Structural Support for Parent Engagement
Several structural supports were put in place to encourage consistent parent engagement. With the exception of data analysis and research meetings, CAB and most Workgroup meetings were held at a Head Start center immediately after the end of the school day. Parents were able to pick their children up and attend meetings in the same building. Childcare was provided onsite by Head Start teachers. Dinner was also provided to CAB members and their children at the beginning of CAB meetings, which allowed time for free conversation. This opportunity for community representatives, parents and university staff to interact helped build relationships. CAB members networked with each other and the academic staff, which led to tangible benefits for many members. Examples include a parent talking to a nurse about her interest in becoming a nurse, and another talking to the researchers about programs offered at the university.
Finally, parent engagement was encouraged by the provision of gift cards. Members of the research team were compensated by the grant. To reinforce the stated value of equality, CAB members were offered $25 gift cards to acknowledge the time and expertise they contributed. CHL also offered gift cards for parents who volunteered in activities such as recruitment, data interpretation, and intervention development and facilitation. While they were warmly received by the parents, several parents expressed that although the cards were helpful, they would still attend meetings if they were not offered because they are committed to the project.
Although a core group of CAB parents and community members participated across all project phases, CAB attendance decreased over time as the project had fewer decisions to make. This is obvious in Fig. 3 showing meeting attendance throughout the project phases. During Phase 3 the focus shifted to program implementation and the majority of CAB parent involvement shifted towards participating as a parent facilitator or by helping the project coordinator administer the parent program or social marketing campaign. After the completion of the pilot intervention, the project focused on the evaluation, including data entry and analysis. Fewer parents attended meetings as there was less to do until the data was ready to present. However, four to five parents participated in data entry and other research activities during this time. One parent attended two conferences and presented on CHL alongside researchers. Also, parents continue to participate in the development of abstracts, posters and presentations for dissemination of the results. They are also actively involved in the development of additional research grant proposals.
Community Advisory Board attendance throughout the 3 phases of the communities for Healthy Living Project
Summary of Parent Participation
The research team employed various innovative strategies and structural accommodations which successfully fostered parents’ continuous involvement in decision making and day to day activities throughout all phases of the research process as ‘experts’, hence engaging CAB parents at the highest rungs of the Ladder of Participation. Parents were equal to the researchers and community representatives, whose roles on the CAB were related to their professions. Parents engaged in co-learning with community members on the CAB and academic staff, sharing their expertise, a necessity in child health research [ 1 ]. Most previous childhood obesity interventions [ 16 , 23 ] involved parents or caregivers at the level of informed consultant (the fifth rung of the Ladder of Participation) which involves community members as advisors, whose input may or may not influence decisions [ 14 ]. These studies, [ 16 , 23 ] advanced the field in that parents were involved in the intervention development process, during which parents gave input and advice and were informed how their input influenced the subsequent intervention. However, CHL is the only known study that achieved the highest rungs of the Ladder of Participation in which parents participated throughout the entire research process.
There were many intended and unintended benefits gained as a result of this study’s CBPR approach. Parents displayed strong buy-into CHL’s messages and activities and on their own accord, promoted the CHL intervention to other Head Start parents and organization staff. These strategies resulted in sustained active participation of parents that led to additional trained, committed co-researchers that (a) contributed unique and valuable expertise to the project and (b) resulted in a more salient, culturally-responsive and sustainable intervention.
Although the purpose of this paper is to describe rather than evaluate the participatory process (evaluation presented elsewhere) [ 24 ], the benefits to parents were identified anecdotally and through CAB evaluation surveys and in-depth interviews. Briefly, parents expressed that they built supportive relationships with each other. The co-learning among parents and between parents, academic staff and community organizations influenced parents’ knowledge of resources as well as their confidence to access and utilize those resources. For example, at least two of the ten CAB parents decided to pursue an academic degree after speaking with other parents with young children who recently completed programs. One parent who completed college while her child was in the Head Start program mentored another parent to help her learn study skills. Many parents reported adding skills they learned through CHL to their resume. One reported at a CAB meeting that adding the skill of interviewing helped her get a new job. Analysis of in-depth CAB member interviews found that parents described an increase in knowledge and confidence about their ability to advocate and disseminate their knowledge within their community. The evaluation of the participatory process will be presented in a separate paper.
The level of engagement for parents resulted in some repercussions. First, some of the leadership of the partner organization were concerned that empowering parents through active engagement may create activist parents who would become vocal with local politicians using the community organization’s name. They feared the potential creation of rifts that the organization could not afford. The researchers responded by promising to appropriately train parents if they decided to advocate outside the organization and reminding parents that there is a protocol to follow for speaking on behalf of an organization.
Some community/organizational representatives felt unclear about their role on the CAB because of the focus on engaging parents. This resulted in inconsistent participation among some. Regardless, a core group of community representatives participated regularly and gave positive feedback on the role of parents and the benefits of participation. In addition, non-parent CAB members tended to re-engage during the second year of the study as the intervention began. Of the organizational representatives who participated in the first CAB meeting, all but one were still involved in the project and attending CAB meetings in the second year of the study.
Additionally, there was the perception of the development of a hierarchy among some parents, during Phase 2, the implementation of the childhood obesity intervention targeting all Head Start families. It was expressed that parents who were parent program facilitators developed stronger relationships with each other and the academic staff as a result of their greater level of participation. It is notable that the parents who felt this hierarchy felt comfortable expressing their feelings to the project coordinator. The coordinator made extra effort to reconnect with parents whose participation dropped off in response. Another challenge was the level of parent expectation of what CHL staff would actually be able to do for them. At times, CHL staff may have been perceived as service providers similar to staff at the CBO. While CHL staff were supportive of parents, there were limitations to how CHL staff could assist parents. Some parents initially expressed frustration, but through on-going discussions and role clarification, they became comfortable with the level of support provided.
Finally, formalizing and sustaining the 17 member CAB was a challenge during Phase 3 and the no cost extension of the grant. After two attempts to have an election of CAB officers, the idea of creating a formal CAB with officers never came to fruition. CAB members had multiple competing priorities and although they were actively involved in CHL, they did not want to commit to running meetings or potentially delay activities and decisions if enough of the officers did not attend a particular meeting. Further, during Phase 3, CAB member participation dropped to a core group of nine members and during the no cost phase of CHL, CAB meetings had an average of four members. Although these members are active as described by their participation in dissemination and grant proposal development, maintaining the CAB without an active intervention research agenda poses a challenge.
Lessons Learned
This case study identified specific strategies to foster parent engagement. Structured by a commitment to engage parents as true experts and equal partners, the participatory process was careful to build skills and facilitate consistent and active participation so that parents were able to be equal partners in the research process. The use of small groups helped foster confidence among parents as well as allowed CHL staff to emphasize their commitment to parents being considered valuable experts. The implementation of planned, focused activities and designated networking time over meals fostered interaction above and beyond project conversations and fostered trust, which was important for relationship building and a positive work environment in the CAB. The development of operational guidelines and partnership principles set the tone for the level of commitment needed, created a mission for the CAB, and maintained the infrastructure of parent involvement. Placing the project coordinator at the community organization and hiring one that was familiar with the neighborhoods served by the Head Start Centers was essential for cutting across the community and academic cultures and also represented a commitment to the community and community outreach. The structural support of meals, incentives, child care and convenient meeting locations not only demonstrated the commitment to parent involvement but also facilitated parent involvement as shown by the level of participation (see Fig. 3 ). All of the aforementioned encouraged involvement of parents throughout the entire research process.
CHL’s successful engagement of parents in the design, implementation and evaluation of an intervention to address childhood obesity adds to the childhood obesity intervention literature. The outlined CBPR strategies to facilitate parent engagement were designed to avoid tokenism [ 15 ]. CHL’s innovative design of engaging parents as “experts” successfully bridged the cultural, socio-economic, and interpersonal divides between parents and the professionals which resulted in a true participatory process. Leveling the playing field in research with low-income parents is more challenging than doing so with community organization representatives because of the lack of education or traditional forms of expertise defined by employment or a profession. CHL brought together people with different levels of privilege to work as equals on a research project. The challenges of treating and engaging parents typically known as “clients” and “the target population” as equal members on the CAB should not be under-estimated. CHL was designed to address this challenge, and the strategies used in CHL can inform other CBPR studies.
To advance the field and improve child health, it is essential to work with parents in the research process. By documenting CHL’s participatory process and concrete strategies for engaging parents, other child health researchers should be encouraged and empowered to actively engage parents and other caregivers in their research, which will in turn benefit the health of children and families. The strategies described in this case study are examples of strategies that other researchers can use to engage parents in the research process. All of CHL’s strategies have a fundamental underlying point of view: parents can be engaged as experts in child health research and their expertise is valuable and essential. From this vantage point, other researchers can also employ these strategies, all for the benefit of (1) childhood obesity research and (2) most importantly, the “target population”, families who have children at risk for or who experience this growing public health problem.
Morabia, A., & Costanza, M. C. (2010). Engaging parents and children in designing child health research. Preventive Medicine, 51 (2), 101–102.
Article PubMed Google Scholar
Golan, M. (2006). Parents as agents of change in childhood obesity-from research to practice. International Journal of Pediatric Obesity, 1 (2), 66–76.
Vaughn, K., & Waldrop, J. (2007). Childhood obesity. Part II. Parent education key to beating early childhood obesity. Nurse Pract, 32 (3), 36–41; quiz 41–33.
Google Scholar
Epstein, L. H., Valoski, A., Wing, R. R., & McCurley, J. (1994). 10-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychology, 13 (5), 373–383.
Article PubMed CAS Google Scholar
Lindsay, A. C., Sussner, K. M., Kim, J., & Gortmaker, S. (2006). The role of parents in preventing childhood obesity. Future of Children, 16 (1), 169–186.
Becker, D., Hogue, A., & Liddle, H. (2002). Methods of engagement in family-based preventive intervention. Child and Adolescent Social Work Journal, 19 (2), 163–179.
Article Google Scholar
Dietz, W., & Gortmaker, S. (2001). Preventing obesity and children and adolescents. Annual Review of Public Health, 22 , 337–353.
Davison, K., Lawson, H., & Coatsworth, J. (in press). The Family-center Action Model of Intervention Layout and Implementation (FAMILI): The example of childhood obesity. Health Promot Pract .
Golan, M., Kaufman, V., & Shahar, D. R. (2006). Childhood obesity treatment: Targeting parents exclusively v. parents and children. British Journal of Nutrition, 95 (5), 1008–1015.
Hingle, M. D., O’Connor, T. M., Dave, J. M., & Baranowski, T. (2010). Parental involvement in interventions to improve child dietary intake: A systematic review. Preventive Medicine, 51 (2), 103–111.
O’Connor, T. M., Jago, R., & Baranowski, T. (2009). Engaging parents to increase youth physical activity a systematic review. American Journal of Preventive Medicine, 37 (2), 141–149.
Zhang, Q., & Wang, Y. (2004). Socioeconomic inequality of obesity in the United States: Do gender, age, and ethnicity matter? Social Science and Medicine, 58 (6), 1171–1180.
Prinz, R. J., Smith, E. P., Dumas, J. E., Laughlin, J. E., White, D. W., & Barron, R. (2001). Recruitment and retention of participants in prevention trials involving family-based interventions. American Journal of Preventive Medicine, 20 (1 Suppl), 31–37.
Arnstein, S. (1969). A ladder of citizen participation. Journal of the American Institute of Planners, 35 (4), 216–224.
Israel, B., Eng, E., Schulz, A., & Parker, E. (2005). Methods in community-based participatory research for health . San Francisco, CA: Wiley.
Economos, C. D., Hyatt, R. R., Goldberg, J. P., Must, A., Naumova, E. N., Collins, J. J., et al. (2007). A community intervention reduces BMI z-score in children: Shape up Somerville first year results. Obesity (Silver Spring), 15 (5), 1325–1336.
Berge, J. M., Mendenhall, T. J., & Doherty, W. J. (2009). Using Community-based Participatory Research (CBPR) to target health disparities in families. Family Relations, 58 (4), 475–488.
Stuttaford, M., & Coe, C. (2007). The “learning” component of participatory learning and action in health research: Reflections from a local sure start evaluation. Qualitative Health Research, 17 (10), 1351–1360.
Health Resources and Services Administration. (2012). Medically underserved areas/populations by state and county. retrieved April 26, 2012, from http://muafind.hrsa.gov/index.aspx .
US Census Bureau. (2010). American FactFinder fact sheet: Rensselaer County, NY. Retrieved April 26, 2012, from http://factfinder2.census.gov .
Community-Campus Partnerships for Health. (2012). CCPH Principles of Partnership. Retrieved 12/5/2011, from http://www.ccph.info/ .
Levy, S. R., Baldyga, W., & Jurkowski, J. M. (2003). Developing community health promotion interventions: Selecting partners and fostering collaboration. Health Promotion Practice, 4 (3), 314–322.
De Bock, F., Fischer, J. E., Hoffmann, K., & Renz-Polster, H. (2010). A participatory parent-focused intervention promoting physical activity in preschools: Design of a cluster-randomized trial. BMC Public Health, 10 , 49.
Jurkowski, J., Li, K., Deane, G., Lawson, H., & Davison, K. (2011). Measuring changes in empowerment among low - income parents participating in a CBPR project. Paper presented at the American Public Health Association 139th Annual Meeting, Washington, DC.
Download references
Acknowledgments
The authors would like to especially thank Kara Gilmore, BA, who provided input on this paper. The authors would also like to thank the parents, grandparents and community partners of Communities for Healthy Living and the staff of the Commission on Economic Opportunities for the Greater Capital District for their time and commitment to CHL. This research was supported by the National Center on Minority Health and Health Disparities, National Institutes of Health (grant number R24MD001120) through the American Recovery and Reinvestment Act. This research was affiliated with the Center for the Elimination of Minority Health Disparities (grant number 1 P20 MD003373). All procedures were approved by the Institutional Review Board at the University at Albany. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center on Minority Health and Health Disparities or the National Institutes of Health.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and affiliations.
Department of Health Policy, Management and Behavior, School of Public Health, University at Albany, State University of New York, 1 University Place, Rensselaer, NY, 12144, USA
Janine M. Jurkowski, Lisa L. Green Mills & Mary C. Bovenzi
School of Social Welfare and Department of Educational Administration and Policy Studies, University at Albany, State University of New York, Albany, NY, USA
Hal A. Lawson
Commission on Economic Opportunity, Troy, NY, USA
Ronald Quartimon
Department of Nutrition, Harvard School of Public Health, Harvard University, Boston, MA, USA
Kirsten K. Davison
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Janine M. Jurkowski .
See Table 2 .
See Table 3 .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Jurkowski, J.M., Green Mills, L.L., Lawson, H.A. et al. Engaging Low-Income Parents in Childhood Obesity Prevention from Start to Finish: A Case Study. J Community Health 38 , 1–11 (2013). https://doi.org/10.1007/s10900-012-9573-9
Download citation
Published : 20 June 2012
Issue Date : February 2013
DOI : https://doi.org/10.1007/s10900-012-9573-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Community based participatory research
- Childhood obesity
- Parent engagement
- Health promotion
- Find a journal
- Publish with us
- Track your research
- Funding Opportunities
Measures Registry User Guides
Case Studies
Want to know more?
Stay up to date
The Measures Registry User Guides include several examples or case studies for each of the four domains. These case studies are listed in the table below. For ease of use, we have grouped the project designs for the case studies into three categories—intervention, epidemiology, and surveillance. However, these types of projects can be characterized in other ways, as well, as detailed in the framework for individual physical activity .
The user guide authors described various approaches in their case studies. For consistency across the four domains, we applied three major steps to these approaches—background, considerations, and measure selection.
Readers will note that the case studies differ in one important aspect. The food environment and physical activity environment user guides mention specific measures that were considered and then selected to meet the needs of the specific projects described in the case studies. In contrast, the case studies for the individual diet and individual physical activity domains describe measures in a more generic way.
Never miss a newsletter
We are social.
Check us out on Facebook, LinkedIn, Twitter and YouTube
- Open access
- Published: 24 August 2017
Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis
- Tayla Ash ORCID: orcid.org/0000-0001-7621-3545 1 , 2 ,
- Alen Agaronov 1 ,
- Ta’Loria Young 3 ,
- Alyssa Aftosmes-Tobio 2 &
- Kirsten K. Davison 1 , 2
International Journal of Behavioral Nutrition and Physical Activity volume 14 , Article number: 113 ( 2017 ) Cite this article
44k Accesses
163 Citations
9 Altmetric
Metrics details
A wide range of interventions has been implemented and tested to prevent obesity in children. Given parents’ influence and control over children’s energy-balance behaviors, including diet, physical activity, media use, and sleep, family interventions are a key strategy in this effort. The objective of this study was to profile the field of recent family-based childhood obesity prevention interventions by employing systematic review and quantitative content analysis methods to identify gaps in the knowledge base.
Using a comprehensive search strategy, we searched the PubMed, PsycIFO, and CINAHL databases to identify eligible interventions aimed at preventing childhood obesity with an active family component published between 2008 and 2015. Characteristics of study design, behavioral domains targeted, and sample demographics were extracted from eligible articles using a comprehensive codebook.
More than 90% of the 119 eligible interventions were based in the United States, Europe, or Australia. Most interventions targeted children 2–5 years of age (43%) or 6–10 years of age (35%), with few studies targeting the prenatal period (8%) or children 14–17 years of age (7%). The home (28%), primary health care (27%), and community (33%) were the most common intervention settings. Diet (90%) and physical activity (82%) were more frequently targeted in interventions than media use (55%) and sleep (20%). Only 16% of interventions targeted all four behavioral domains. In addition to studies in developing countries, racial minorities and non-traditional families were also underrepresented. Hispanic/Latino and families of low socioeconomic status were highly represented.
Conclusions
The limited number of interventions targeting diverse populations and obesity risk behaviors beyond diet and physical activity inhibit the development of comprehensive, tailored interventions. To ensure a broad evidence base, more interventions implemented in developing countries and targeting racial minorities, children at both ends of the age spectrum, and media and sleep behaviors would be beneficial. This study can help inform future decision-making around the design and funding of family-based interventions to prevent childhood obesity.
Childhood obesity continues to be a pervasive global public health issue as children worldwide are significantly heavier than prior generations [ 1 ]. Over the past few decades, the prevalence of obesity among children and adolescents has risen by 47% [ 2 ]. Increases have been seen in both developed and developing countries, with recent prevalence estimates of 23 and 13%, respectively [ 2 ]. Despite evidence of a plateau in the rates of obesity, at least among young children in developed countries, current levels are still too high, posing short- and long-term impacts on children’s physical, psychological, social, and economic well-being [ 2 , 3 , 4 , 5 ]. Of equal, if not greater concern, racial/ethnic and socioeconomic disparities appear to be widening in some countries [ 5 , 6 , 7 , 8 ]. Given the extensive disease burden, treatment resistance of obesity, and lack of signs of attenuation for rates in the developing world, scientists, clinicians, and practitioners are working hard to devise and test interventions to prevent childhood obesity and reduce associated disparities [ 2 , 9 ].
One category of interventions to prevent childhood obesity that has grown considerably in recent years is family-based interventions. This was in part due to a number of key reports published in 2007, including an Institute of Medicine (IOM) report on the recent progress of childhood obesity prevention [ 10 ] and a report from a committee of experts representing 15 professional organizations appointed to make evidence-based recommendations for the prevention, assessment, and treatment of childhood obesity [ 11 , 12 ]. In both reports, parents are described as integral targets in interventions, given their highly influential role in supporting and managing the four behaviors that affect children’s energy balance (diet, physical activity, media use, and sleep) [ 13 , 14 , 15 ]. This includes not only parenting practices and rules, but also the environments to which children are exposed, and the adoption of parents’ own behavioral habits by children [ 15 , 16 , 17 , 18 , 19 ].
Since the release of these reports, there has been a proliferation of family-based interventions to prevent and treat childhood obesity as documented in at least five published reviews of this literature in the past decade [ 20 , 21 , 22 , 23 , 24 ]. While these reviews convey extensive information around intervention effectiveness, they cannot reveal gaps in the knowledge base. Quantitative content analysis [ 25 , 26 , 27 ] can be used to code intervention and participant characteristics, and a review of the resulting data can reveal areas and populations receiving a great deal of attention, as well as those where few or no studies exist, thereby highlighting knowledge gaps. With a focus on childhood obesity interventions, pertinent questions to address include: whether interventions have continued to focus primarily on diet and physical activity, neglecting the more recently established predictors of media use and sleep [ 28 , 29 , 30 ]; whether some behaviors are more likely to be targeted among certain age groups or settings than others; and whether there are gaps with regard to the populations targeted by interventions to date, in particular, the representation of vulnerable populations (e.g. families living in developing countries, those of low socioeconomic status, racial and ethnic minorities, immigrants, and non-traditional families) [ 2 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ]. In addition to ethical reasons, from a pragmatic viewpoint, it is difficult to identify best practices to prevent childhood obesity in vulnerable populations when few interventions have focused on that population [ 38 , 39 ].
The goal of this study is to profile family-based interventions to prevent childhood obesity published since 2008 to identify gaps in intervention design and methodology. In particular, we use quantitative content analysis to systematically document intervention and sample characteristics with the goal of directing future research to address the identified knowledge gaps.
We used a multistage process informed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify family-based childhood obesity prevention interventions that were written in English and published between January 1, 2008 and December 31, 2015 [ 40 ]. Using an a priori defined protocol, we identified relevant articles and systematically screened articles against inclusion and exclusion criteria. The systematic review protocol was registered in the PROSPERO database (CRD42016042009).
Following the identification of eligible studies, we conducted a quantitative content analysis to profile recent interventions for childhood obesity prevention. Content analysis, originally used in communication sciences but increasingly utilized in public health, is a research method used to generate objective, systematic, and quantitative descriptions of a topic of interest [ 25 , 26 , 27 ]. Our research team has previously employed this technique to survey observational studies on parenting and childhood obesity published between 2009 and 2015 [ 41 , 42 ].
Search strategy and initial screening
With the help of a research librarian, two authors (TA, AA) searched three databases (PubMed, PsycINFO, and CINAHL) using individually tailored search strategies most appropriate for each database. The selected databases are the three most common databases used in recent systematic reviews. Our search strategy consisted of search strings composed of terms targeting four concepts: (1) family (e.g. family, mother, father, home), (2) intervention (e.g. prevention, promotion), (3) children (e.g. child, infant, youth), and (4) obesity (e.g. overweight, body mass) (see Additional file 1 for full search strategy for one database). We searched title, abstract, and medical subject headings (MeSH) or descriptor subjects (DE) term fields. Animal studies (e.g. rats), non-original research articles (e.g. commentaries, editorials, case reports), studies written in languages other than English and studies focused on populations older than 18 years were excluded using search limits and NOT terms. We restricted the search to articles published since January 1, 2008, to capture interventions implemented after the release of the IOM and expert committee reports. Furthermore, a start point of January 2008 ensured the feasibility of this study given the labor and time intensive process to screen and code studies. In a recent systematic review of family-based interventions for the treatment and prevention of childhood obesity, more than 80% of eligible studies were published since 2008 [ 43 ]. Thus, a start date of 2008 appropriately balances feasibility of implementation and the validity of the resulting information. The search end date was December 31, 2015.
The search yielded 12,274 hits, representing 9152 unique articles after removing duplicates (see Fig. 1 ). Following a review of titles by three authors (TA, AA, TY) and one research assistant, 7451 articles were removed based on exclusion criteria, resulting in 1701 articles that proceeded to abstract review. Articles were removed during title review if they were not written in English or published in the designated time frame, were not original research articles, did not include human subjects, did not target children, were observational studies, were not relevant to the topic of childhood obesity (e.g. papers about Anorexia Nervosa), or included special clinical populations.
PRISMA flow diagram for identifying and screening eligible family-based childhood obesity prevention interventions
Application of eligibility criteria
Three authors (TA, AA, TY) and one research assistant screened articles against the eligibility criteria during abstract review, while two authors (TA, AA) screened during full-text review, applying the aforementioned exclusion criteria. Eligible studies included family-based interventions for childhood obesity prevention published since 2008. We defined family-based interventions as those involving active and repeated involvement in intervention activities from at least one parent or guardian [ 19 ]. Examples of intervention activities that qualify as active parent involvement include workshops and counseling. Examples of passive involvement, which were excluded, include sending home brochures for parents, or simply inviting parents to a single event, but not involving them in the intervention in an integral way. We defined obesity interventions as those that reported at least one weight-related outcome (weight, body mass index, etc.) or which self-identified as an obesity intervention. We defined interventions as preventive if they did not explicitly focus on weight loss or management, or if they did not recruit only children with obesity. The final inclusion criterion was that the intervention was designed with the intent of benefiting children (child being defined as <18 years of age), excluded interventions in which the objective was to better parent health outcomes.
Of the 1701 articles screened at the abstract level, 329 proceeded to full-text screening, of which 159 articles met the eligibility criteria and were included in the final pool of eligible papers (see Additional file 2 for a list of eligible articles). We examined intervention name, trial number, the last name of the first author, and the last name of the last author to identify articles that originated from the same intervention. After collating, 119 unique interventions were identified, which included interventions with published outcome data, and interventions for which only a protocol was published. Percent agreement for all screening criteria ranged between 86 and 98%. Discrepancies were discussed and resolved.
To ensure a fully inclusive search strategy, we also reviewed the references of a random subset of the articles meeting the inclusion criteria. A subset of 5% was chosen given the large sample size. No additional studies meeting the eligibility criteria were identified in the process, suggesting that the employed search was exhaustive.
Data extraction
For all eligible articles, we used conventional content analysis methodology [ 25 , 26 , 27 ] to extract and analyze article, intervention, and participant characteristics. We developed a comprehensive codebook to standardize the coding process. Multiple authors (TA, AA, AA-T) tested the codebook by coding five articles not included in the final pool of studies. An additional round of testing included 10 randomly selected articles from the study pool. After pilot testing the codebook and establishing reliability (see intercoder reliability), two trained coders (TA, AA) each coded half of the 159 eligible articles.
Article characteristics
We coded publication year, journal, funding sources, and type of paper. All specific funding sources for a given intervention were extracted and classified after web-based searching. Funding sources were categorized as federal, foundation, corporate, or university, and then further coded based on the specific federal, foundation or corporate agency. For type of paper, articles were coded as an intervention protocol or outcome evaluation. Articles that reported any intervention outcomes were coded as outcome evaluations; interventions that only described the intervention (or provided only baseline data) were coded as protocols. Because a seemingly large number of protocols were discovered among the final pool of articles, we elected to include them in the study. Interventions in which only a protocol has been published tend to represent the next generation of intervention studies and thus lend to a better understanding of the field’s trajectory.
Intervention characteristics
We coded a wide range of intervention characteristics including geographic region of the study, age of target child, intervention setting, length of intervention, delivery mode, evaluation design, intervention recipient, behavioral domains targeted, and theory used. Age of the target child at baseline was coded as prenatal (i.e., the intervention started before birth), 0–1 years, 2–5 years, 6–10 years, 11–13 years, and 14–17 years. If the age range fell predominantly into one category, any subsequent categories were only coded affirmative if the ages of participants crossed at least 2 years into a given range. Intervention setting was coded as home, primary care or health clinic, community-based, school, and childcare/preschool. Community-based interventions included those taking place in community gardens, parks, or recreational facilities. Interventions taking place at universities were also coded as community-based. In cases where intervention setting was ambiguous, or the intervention was not setting specific, we coded the intervention setting as unclear.
Intervention length was coded as less than 13 weeks (3 months), 13–51 weeks (3–11.9 months), or 52 weeks (12 months) or more. Two different types of intervention delivery modes were coded: in-person and technology-based. Technology-based approaches included those using computers, social media, text messages, or anything else involving the Internet. Evaluation design was coded as either randomized-controlled trial or quasi-experimental trial. We also extracted data on intervention recipients (i.e. those who directly received the intervention program or materials). This was coded as adults, children, or both. Behavioral domains targeted included diet, physical activity, media use, and sleep. Finally, we coded use of theory. Theories were specified using the following categories: social cognitive theory, parenting styles, ecological frameworks, transtheoretical model of behavior change, health belief model, theory of planned behavior, or other. For age category, intervention setting, delivery mode, intervention recipients, and theory, multiple categories could be selected.
Sample characteristics
Sample characteristics were coded for the inclusion of participants from underserved populations and non-traditional families, and racial/ethnic composition of the sample. We coded sample characteristics for outcome evaluations only ( n = 84 studies) because intervention protocols generally do not include this information. We coded whether the intervention included any participants from the following underserved or non-traditional groups: low socioeconomic status (SES), racial/ethnic minorities (i.e., Black/African American, Hispanic/Latino, Indigenous), immigrant families, single parents, non-biological parents, and non-residential parents. Low SES was defined as either low income (self-identified by the study) or low education (high school diploma or less). Families participating in low-income qualifying programs (Women, Infants, and Children services, Supplemental Nutrition Assistance Program, free or reduced school lunch, Head Start, etc.) were considered low SES. We coded parents as single if they self-identified as such, were not cohabitating, or were widowed or divorced. In studies where limited information was provided and marital status was simply dichotomized as married or not married, not married was used as a proxy for single. Finally, we coded whether the sample included participants from each racial/ethnic group (i.e. White, Black/African American, Hispanic/Latino, Asian, Indigenous, and multiracial/other). For all sample characteristics, in addition to coding whether families belonging to each of the groups were included, we also coded whether they made up at least 50% of the sample, as well as 90% of the sample. The purpose of these categories was to distinguish between studies that included only a few families from a given category and those in which at least half the sample belonged to the category. If at least 90% of the families included in a sample belonged to a given category, the sample was considered to be predominantly that category (e.g. predominantly-Hispanic). Samples coded affirmative for 90% criteria were also coded affirmative for the 50% criteria.
Inter-rater reliability
Both coders coded randomly selected articles from the final study pool until reliability was sufficiently established. Ultimately, this included four rounds of coding a total of 55 articles. We computed Cohen’s kappa as a measure of agreement between the coders, using weighted kappas for ordinal variables [ 44 ]. The final average kappa across all variables was 0.87, and the average percent agreement was 92%. Three variables had kappas below 0.70, the conservative threshold for adequate inter-rater reliability [ 45 ]. These variables included the following: inclusion of children 11–13 years old (kappa 0.36), inclusion of children 14–17 years old (kappa 0.65), and childcare/preschool setting (kappa 0.46). Because percent agreement for each of these variables was high (>89%), and given that kappa coefficients are difficult to interpret when variability is low [ 45 , 46 ], which would result from a category (e.g. inclusion of children 14–17 years) being infrequently coded or endorsed, they were retained in the analyses. Coders were retrained on the three variables prior to coding the remainder of the articles.
Data synthesis and analysis
Both inter-rater reliability and all other analyses were conducted in STATA 13 [StataCorp LP, College Station, TX, USA]. One coder (TA) cleaned the data. The majority of missing data was not reported (i.e., were missing by design) and therefore coded as ‘0’ (no/not sure). Where data were missing, one of the coders (TA) returned to the full-text article to confirm and correct any errors.
For article characteristics (e.g. publication year, journal), the unit of analysis is article, with a denominator of 159 articles. For intervention and sample characteristics, which are presented in Tables 1 - 3 , the unit of analysis is intervention. In instances where multiple studies were published on the same intervention, the data extracted from each study were synthesized into a single entry [ 47 ]. For example, if both a protocol and outcome evaluation were published for an intervention, the intervention was marked as having an outcome evaluation. As a result, a denominator of 119 interventions was used to assess intervention characteristics. Interventions with a protocol only were not included in the assessment of sample characteristics because sample information is infrequently reported in such papers. Thus the denominator for sample characteristics was 85 interventions with published outcome data.
We also examined article and intervention characteristics separately for protocols and outcome evaluations. Given that few differences were identified, this information is presented in Additional file 3 : Table S1 to streamline the presentation of results.
The number of eligible articles published each year was as follows: 2008 = 6 (4%), 2009 = 5 (3%), 2010 = 14 (9%), 2011 = 15 (9%), 2012 = 33 (21%), 2013 = 35 (22%), 2014 = 23 (14%), and 2015 = 28 (18%). The predominant journals in which articles were published included BioMed Central Public Health ( n = 28, 18%), Contemporary Clinical Trials ( n = 12, 8%), Childhood Obesity ( n = 9, 6%), Pediatrics ( n = 7, 4%), Pediatric Obesity ( n = 6, 4%), and Preventive Medicine ( n = 6, 4%).
Eligible articles described 119 unique interventions. Table 1 summarizes additional intervention characteristics for eligible interventions. For more than a fourth of these interventions ( n = 34, 29%), only an intervention protocol was identified (i.e., no published outcomes were available). More than half ( n = 66, 56%) of the interventions were based in the U.S. Studies based in Europe/United Kingdom ( n = 30, 25%), Australia/New Zealand ( n = 10, 8%), and Canada ( n = 6, 5%) comprised 38%. Few interventions were conducted in countries in Central America, South America, Asia, Africa, the Middle East, or the Caribbean.
Less than a third of interventions were implemented for a year or more ( n = 33, 28%). Interventions that were implemented in-person ( n = 101, 85%) were more common than those delivered using technology ( n = 27, 23%). Fourteen (12%) of interventions had both in-person and technology components. Five interventions (4%) had neither an in-person nor a technology component; these interventions consisted of printed materials and phone calls. Nearly three out of four interventions utilized a randomized controlled trial design ( n = 87, 73%). Because active parent engagement was a requirement for eligibility in this review, parents were intervention recipients in all interventions. Children were also intervention recipients in approximately half of the interventions ( n = 65, 55%).
A slight majority of interventions were federally funded ( n = 75, 63%). Of these, about half ( n = 34, 29% of the 119 eligible interventions) received funding from the National Institutes of Health, with the National Institute of Diabetes and Digestive and Kidney Diseases ( n = 14, 12%) and the National Heart, Lung, and Blood Institute ( n = 7, 6%) being the two leading funding institutes (data not shown). The United States Department of Agriculture funded 10 (8%) interventions. Twenty-three (19%) interventions received federal funding from countries other than the United States, with Australia funding the most ( n = 6, 5%). Of the 50 (42%) interventions funded by foundations, the Robert Woods Johnson Foundation was the leading funder ( n = 5, 4%). A similar proportion of interventions received corporate ( n = 21, 18%) or university funding ( n = 23, 19%). Many interventions ( n = 46, 39%) received multiple types of funding, and funding source was not listed in 8 (7%) of interventions.
A majority of interventions mentioned theory ( n = 85, 71%), with many ( n = 34, 29%) using multiple theories. However, interventions varied greatly with respect to how heavily theory was emphasized. Social cognitive theory was the most widely noted theory ( n = 49, 41%).
Approximately 40% of interventions targeted families with children ages 2–5 years ( n = 51, 43%) or 6–10 years ( n = 42, 35%), whereas fewer than 10% of interventions targeted families during the prenatal period ( n = 10, 8%) or families of children with 14–17-year-olds ( n = 8, 7%). One in three interventions were implemented in a home setting ( n = 33, 28%), a primary care/health clinic ( n = 32, 27%) or in the community ( n = 39, 33%), and one in five ( n = 24) were implemented in multiple settings. Finally, just over half ( n = 69, 58%) of studies targeted a behavioral domain beyond diet and physical activity (i.e., they targeted media use and/or sleep in addition to diet and physical activity), and only a few ( n = 3, 3%) interventions did not target either diet or physical activity.
Table 2 provides a cross tabulation of age of target child, setting, and behavioral domains. A number of patterns are apparent. First, interventions that targeted children in the earlier years of life (prenatal to age 5 years) tended to be focused in the home ( n = 28, 31%) and primary care settings ( n = 30, 33%), whereas interventions that targeted older children occurred most frequently in community ( n = 40, 53%) and school ( n = 20, 27%) settings. Second, media use was least frequently included in school-based interventions ( n = 9, 43%). Physical activity was most frequently targeted in a school setting ( n = 21, 100%), and least likely to be targeted in homes ( n = 23, 70%). Sleep was most often included in home-based ( n = 8, 24%), health-based ( n = 8, 25%), and childcare-based ( n = 3, 27%) interventions; it was seldom targeted in families with school-age children ( n = 4, 10%) and has not been targeted in families with children older than 10 years of age.
Sample characteristics are summarized in Table 3 . Underserved families appeared well-represented, particularly low SES families ( n = 62, 73%). A slight majority of samples included at least some racial or ethnic minority families ( n = 46, 54%), and just over a quarter included immigrant families ( n = 24, 28%). Ethnic minorities (i.e., Hispanics) were better represented than racial minorities. About half of the interventions included families identifying as Hispanic/Latino ( n = 40, 47%).
The most frequently represented racial group was White ( n = 30, 35%), followed by Black/African American ( n = 26, 31%), Asian ( n = 20, 24%), and then Indigenous ( n = 12, 14%). Notably, many interventions ( n = 29, 34%) did not specify the racial/ethnic background of families. Fig. 2 provides a more detailed assessment of the racial/ethnic composition of U.S.-based interventions (non-U.S. interventions infrequently reported participant race or ethnicity and were therefore not included). In 42% ( n = 21) of U.S.-based interventions, Hispanic/Latino families made up at least half of the sample, and in 30% ( n = 15) of interventions they made up at least 90% of the sample. Again, families identifying as White were the most represented racial group ( n = 24, 48%). Less than 20% of studies included a sample that was at least half Black/African American ( n = 5, 10%), Asian ( n = 2, 4%), or Indigenous ( n = 1, 2%).
Inclusion and representation for racial/ethnic groups in U.S. family-based childhood obesity prevention interventions ( n = 50)
Few studies included non-traditional families; less than a third of interventions included any single parent households ( n = 23, 27%) and less than 5% included non-biological parents ( n = 2, 2%) or non-residential parents ( n = 0, 0%).
Comparing protocols to outcome evaluations
When comparing interventions with evaluations to those with protocols only, a proxy for more recent interventions, interventions with protocols targeted more domains than those with evaluations. The proportion of evaluation and protocols that targeted just one behavioral domain was 20 and 12%, respectively, while the proportion targeting all four behavioral domains was 13 and 24%, respectively. Other notable differences were that interventions with protocols only were more likely to be of longer duration, utilize technology, adopt a randomized controlled trial design, target parents exclusively, receive federal funding, and use theory (see Additional file 3 : Table S1).
Parents are important agents of change in the childhood obesity epidemic [ 20 , 22 , 48 , 49 ]. This study used rigorous systematic methods to conduct a quantitative content analysis of family-based interventions to prevent childhood published between 2008 and 2015 to profile the field of recent family-based childhood obesity prevention interventions and identify knowledge gaps. We identified gaps in both intervention content and sample demographics. Key research gaps include studies in low-income countries, interventions for children on both the lower and higher ends of the age spectrum, and interventions targeting media use and sleep. Racial minorities and children from non-traditional families have also been underrepresented.
Intervention gaps and implications
The vast majority of studies were conducted in developed, or high-income, countries. Given the rapid increase of obesity as a significant public health burden in developing countries, this study demonstrates a need for further intervention efforts in low- and middle-income countries [ 50 , 51 ]. Although obesity rates are lower in low- and middle-income countries than developed countries, two-thirds of people with obesity worldwide live in developing countries where rates of obesity are increasing [ 2 ]. The small number of studies in these geographic regions limits the development of locally relevant programs and policies aiming to address the growing problem of obesity in these regions.
Non-traditional families were underrepresented in interventions. This is concerning given that children from non-traditional families have an elevated risk for obesity [ 31 , 32 , 33 , 34 , 35 , 36 ]. The changing nature of family structures, including the increasing number of single-parent households over time, [ 52 ] calls for a more inclusive approach to defining what is considered a family in research. Like non-traditional families, Black/African American, Asian, and Indigenous families have been underrepresented. Racial and ethnic minorities are vulnerable populations who experience elevated risk for obesity [ 33 , 34 ]. Initiatives to fund interventions specifically targeted at racial and ethnic minorities may have increased the number of interventions targeting Hispanics, but not racial minorities. Thus, more efforts are needed that specifically target families identifying as races other than White. The lack of studies including adequate representation of these groups limits the scientific community’s understanding of effective strategies in high-risk communities and fails to fully address noted health disparities.
Family-based childhood obesity prevention interventions have focused heavily on children 2–10 years of age, despite the robust evidence demonstrating the importance of prevention efforts as early as infancy and the prenatal period [ 53 , 54 ]. Establishing healthy habits early in life is critical given the difficulty of changing energy-balance behaviors later on. While it has been established that prenatal life influences childhood obesity risk, the low number of interventions beginning in the prenatal period, in particular, may be due to a general lack of understanding of the mechanisms responsible for this association, and general debate in the field about how early intervention efforts should begin [ 55 , 56 ].
This study also revealed gaps in behavioral domains targeted, as interventions have not adequately targeted media use and sleep. Moreover, only 16% of interventions targeted all four behavioral domains. The emphasis of interventions on diet and physical activity may reflect their relative contribution to obesity risk. However, behavioral risk factors for obesity are interconnected, and thus may be better addressed by considering complimentary and supplementary behaviors [ 57 , 58 , 59 ]. While it can be argued that targeted messages may have a greater impact, the research gaps identified in this study (e.g. the lack of interventions targeting sleep among older children) highlight areas of needed research in the field. It is worth acknowledging how varied intervention length was across studies, with about a third of interventions being less than 3 months long. This is important given the difficulty in making and sustaining lifestyle changes.
Comparisons with observational studies
The results of this study are consistent with findings from a content analysis by Gicevic et al. on observational research on parenting and childhood obesity published over a similar time frame [ 41 ]. The majority of studies were conducted in developed countries; diet and physical activity were the most heavily targeted behavioral domains; most studies targeted children ages 2–10; and there was a low representation, or at least specification, of non-traditional families. Also consistent with Gicevic et al., non-U.S. studies seldom reported the racial/ethnic composition of the sample [ 41 ].
Limitations
There are several limitations to this study that are worth noting. First, this study focused on articles published over a relatively narrow time-period. Given the immense number of records initially identified, we needed to consider the feasibility of screening and then thoroughly coding eligible articles. Thus we decided to focus on recent literature. Additionally, it was not a focus of this study to look at time trends. Future studies that wish to see how the field is changing should do time-trend analyses, ideally taking into account a longer period of time. Another limitation of this study is that we did not assess intervention effectiveness or quality. While this may limit the potential utility of this review, we chose to focus on the results of the content analysis and not include this information because it is included in prior reviews of family-based interventions for childhood obesity prevention published in the past 10 years [ 20 , 21 , 22 , 23 , 24 , 60 ]. Although systematic reviews can identify effective intervention strategies, they cannot identify the absence of information or gaps in the literature. This study explicitly addressed this shortfall in prior reviews. Lastly, the results of this study may be influenced by the number and choice of databases searched, and may be subject to publication bias. Given the large volume of studies (~7000) obtained by searching PubMed, and the considerable overlap with other databases (i.e. the number of duplicates), we limited our search to the three most commonly searched databases in previous reviews [ 20 , 21 , 22 , 23 , 24 , 41 , 60 ]. By limiting our search, it is possible that a few otherwise eligible studies were missed. It is also possible that including other databases (e.g. EMBASE, Dissertation Abstracts International) would have slightly increased the proportion of non-U.S. based interventions.
Despite limitations, this study used a novel approach to synthesize and profile the recent literature on family-based childhood obesity prevention interventions. Results demonstrate the current emphasis in interventions, and lack of adequate representation of various groups. More interventions that recruit diverse populations, and target behaviors beyond diet and physical activity, are needed to better understand the influence of these characteristics when designing and implementing family-based childhood obesity prevention interventions. The results of this study can be used to inform decision-making around intervention design and funding aimed at filling gaps in the knowledge base. Filling these gaps will lead to a better understanding of how best to target a wide range of behaviors in diverse populations.
Abbreviations
Institute of Medicine
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Socioeconomic status
United States
Lobstein T, Jackson-Leach R, Moodie ML, Hall KD, Gortmaker SL, Swinburn BA, et al. Child and adolescent obesity: part of a bigger picture. Lancet. 2015;385(9986):2510–20.
Article PubMed PubMed Central Google Scholar
Ng M, Fleming T, Robinson M, Thomas B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–81.
Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13(1):6–13.
Article PubMed Google Scholar
Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–52.
Article CAS PubMed PubMed Central Google Scholar
Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011;6(5–6):342–60.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14.
Wang YC, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976-2006. Int J Pediatr Obes. 2011;6(1):12–20.
Article Google Scholar
Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes. 2009;34:41–7.
Battle EK, Brownell KD. Confronting a rising tide of eating disorders and obesity: treatment vs. prevention and policy. Addict Behav. 1996;21(6):755–65.
Article CAS PubMed Google Scholar
Koplan JP, Liverman CT, Kraak VI, Wisham SL. Progress in preventing childhood obesity: how do we measure up? Washington: National Academy Press, Institute of Medicine; 2007.
Google Scholar
Barlow SE. Expert committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92.
Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics. 2007 Dec;120(Suppl 4):S229–53.
Birch LL, Davison KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin N Am. 2001;48(4):893–907.
Article CAS Google Scholar
Jago R, Edwards MJ, Urbanski CR, Sebire SJ. General and specific approaches to media parenting: a systematic review of current measures, associations with screen-viewing, and measurement implications. Child Obes. 2013;9(Suppl):S51–72.
Loprinzi PD, Trost SG. Parental influences on physical activity behavior in preschool children. Prev Med. 2010;50(3):129–33.
Pearson N, Biddle SJ, Gorely T. Family correlates of fruit and vegetable consumption in children and adolescents: a systematic review. Public Health Nutr. 2009;12(2):267–83.
Jago R, Sebire S, Lucas PJ, Turner KM, Bentley GF, Goodred JK, Steqart-Brown S, Fox KR. Parental modeling, media equipment and screen-viewing among young children: cross-sectional study. BMJ Open. 2013;3(4). doi: 10.1136/bmjopen-2013-002593 .
Nepper MJ, Chai W. Associations of the home food environment with eating behaviors and weight status among children and adolescents. J Nutr Food Sci. 2015;S12(004). doi: 10.4172/2155-9600.S12-004 .
Janz KF, Dawson JD, Mahoney LT. Tracking physical fitness and physical activity from childhood to adolescence: the muscatine study. Med Sci Sports Exerc. 2000;32(7):1250–7.
Hingle MD, O’Connor TM, Dave JM, Baranowski T. Parental involvement in interventions to improve child dietary intake: a systematic review. Prev Med. 2010;51(2):103–11.
Kitzmann KM, Beech BM. Family-based interventions for pediatric obesity: methodological and conceptual challenges from family psychology. J Fam Psychol. 2006;20(2):175–89.
Golan M. Parents as agents of change in childhood obesity—from research to practice. Int J Pediatr Obes. 2006;1(2):66–76.
Knowlden AP, Sharma M. Systematic review of family and home-based interventions targeting paediatric overweight and obesity. Obes Rev. 2012;13(6):499–508.
Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: a systematic review of randomized controlled trials. Obes Rev. 2013;14(4):265–78.
Berelson B. Content analysis in communication research. New York: Free Press Content analysis in communication research; 1952. p. 220.
Manganello J, Blake N. A study of quantitative content analysis of health messages in U.S. Media from 1985 to 2005. Health Commun. 2010;25(5):387–96.
Krippendorff K. Content analysis: an introduction to its methodology. Beverly Hills: Sage Publications; 1980.
Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986-1990. Arch Pediatr Adolesc Med. 1996;150:356–62.
Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A Systematic Review and Meta-analysis. Obesity. 2008;16(2):265–74.
Li L, Zhang S, Huang Y, Chen K. Sleep duration and obesity in children: a systematic review and meta-analysis of prospective cohort studies. J Paediatr Child Health. 2017;53(4):378–85.
Chen AY, Escarce JJ. Family structure and childhood obesity, early childhood longitudinal study—kindergarten cohort. Prev Chronic Dis. 2010;7(3):A50.
PubMed PubMed Central Google Scholar
Gibson LY, Byrne SM, Davis EA, Blair E, Jacoby P, Zubrick SR. The role of family and maternal factors in childhood obesity. Med J Aust. 2007;186(11):591–5.
PubMed Google Scholar
Egeland GM, Harrison GG. Health disparities: promoting indigenous peoples’ health through traditional food systems and self-determination. In: indigenous peoples’ food systems & well-being. Rome: FAO; 2013.
Felgal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults 1999-2010. JAMA. 2014;307(5):491–7.
Health, United States. 2002 with Chartbook on trends in the health of Americans. Hyattsville: National Center for Health Statistics; 2002.
Kington RS, Nickens HW. Racial and ethnic differences in health: recent trends, current patterns, future directions. In: Smelser NJ, Wilson WJ, Mitchell F, editors. America becoming: racial trends and their consequences. Washington: National Academy Press; 2001.
Kaushal N. Adversities of acculturation? Prevalence of obesity among immigrants. Health Econ. 2009;18(3):291–303.
Dumbka L, Garza C, Roosa M, Stoerzinger H. Recruitment and retention of high-risk families into a preventative parent training intervention. J Primary Prevention. 1997;18(1):25–39.
Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28.
Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;2009, 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 .
Gicevic S, Aftosmes-Tobio A, Manganello JA, Ganter C, Simon CL, Newlan S, Davison KK. Parenting and childhood obesity research: a quantitative content analysis of published research 2009-2015. Obes Rev. 2016;17(8):724–34.
Davison KK, Gicevic S, Aftosmes-Tobio A, Ganter C, Simon CL, Newlan S, Manganello JA. Fathers’representation in observational studies on parenting and childhood obesity: a systematic review and content analysis. Am J Public Health. 2016;106(11):1980.
Morgan PJ, Young MD, Lloyd AB, Wang ML, Eather N, Miller A, et al. Involvement of fathers in pediatric obesity treatment and prevention trials: a systematic review. Pediatrics. 2017;139(2):e20162635.
Hallgren KA. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34.
Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–9.
Lantz CA, Nebenzahl E. Behavior and interpretation of the kappa statistic: resolution of the two paradoxes. J Clin Epidemiol. 1996;49(4):431–4.
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011. Version 5.1.0. Available at: http://handbook.cochrane.org . Accessed 8 Nov 2016.
Jelalian E, Saelens BE. Empirically supported treatments in pediatric psychology: pediatric obesity. J Pediatric Psychol. 1999;24(3):223–48.
Campbell K, Hesketh K. Strategies which aim to positively impact on weight, physical activity, diet and sedentary behaviours in children from zero to five years. A systematic review of the literature. Obes Rev. 2007;8:327–38.
Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131(3):871S–3S.
CAS PubMed Google Scholar
Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35(1):93–9.
McLanahan S, Percheski C. Family structure and the reproduction of inequalities. Annu Rev Sociol. 2008;34:257–76.
Baird J, Fisher D, Lucas P, Kleijnen. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929.
Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev . 2005;6(2):143–54.
Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin N Am. 2009;36(2):361–77.
Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr. 1998;132(5):768–76.
Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev Med. 2009;46(3):181–8.
Driskell M-M, Dyment S, Mauriello L, Castle P, Sherman K. Relationships among multiple behaviors for childhood and adolescent obesity prevention. Prev Med. 2008;46(30):209–15.
Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity in children and adolescents. Med Sci Sports Exerc. 2000;32(5):963–75.
Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132(5):667–91.
Download references
Acknowledgments
We would like to acknowledge Carol Mita and Selma Gicevic for their assistance in constructing the search strategy. We would also like to acknowledge Martina Sepulveda for assisting with screening.
The authors received no funding for this study and have no relevant financial relationships to disclose.
Availability of data and materials
The data extracted in the current study is available from the corresponding author on reasonable request.
Author information
Authors and affiliations.
Harvard T.H. Chan School of Public Health, Department of Social and Behavioral Sciences, SPH-2 655 Huntington Avenue, Boston, 02115, USA
Tayla Ash, Alen Agaronov & Kirsten K. Davison
Harvard T.H. Chan School of Public Health, Department of Nutrition, Kresge Building 677 Huntington Avenue, Boston, 02115, USA
Tayla Ash, Alyssa Aftosmes-Tobio & Kirsten K. Davison
Harvard T.H. Chan School of Public Health, University of Texas at Austin, 110 Inner Campus Drive, Austin, 78705, USA
Ta’Loria Young
You can also search for this author in PubMed Google Scholar
Contributions
TA and AA developed the search strategy, performed the literature search, conducted article screening, and data extraction, and drafted the manuscript. In addition, TA cleaned the data, ran the analyses, and generated the Tables. TY assisted with article screening and drafted a portion of the manuscript. AAT created the codebook, assisted with screening and coding training, provided input on result interpretation, and edited the manuscript. KKD conceptualized the study, supervised the systematic review process, provided input on coding categories, helped generate the tables, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tayla Ash .
Ethics declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Full search strategy for PubMed database to identify eligible family-based childhood obesity prevention interventions published between 2008 and 2015. (DOCX 135 kb)
Additional file 2:
List of eligible articles published between 2008 and 2015 detailing a family-based childhood obesity prevention intervention. (DOCX 210 kb)
Additional file 3: Table S1.
Intervention characteristics of family-based childhood obesity prevention interventions separating studies with evaluations from protocols. (DOCX 116 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Ash, T., Agaronov, A., Young, T. et al. Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis. Int J Behav Nutr Phys Act 14 , 113 (2017). https://doi.org/10.1186/s12966-017-0571-2
Download citation
Received : 25 January 2017
Accepted : 16 August 2017
Published : 24 August 2017
DOI : https://doi.org/10.1186/s12966-017-0571-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Childhood obesity
- Physical activity
- Sedentary behavior
- Family-based
International Journal of Behavioral Nutrition and Physical Activity
ISSN: 1479-5868
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Case Studies
Tackling childhood obesity with advocacy.
Obesity is a complex public health problem that requires multi-faceted solutions, including the need to influence policy. Pediatricians have been an untapped resource in the effort to change local policies to better support the fight against childhood obesity.
Our Approach
NICHQ trained multidisciplinary teams in healthcare organizations in Alabama, Arkansas, Kentucky, Mississippi, New Mexico, North Carolina and Texas to establish baseline advocacy skills and develop implementation plans for spreading the learnings in their communities. After testing the work in these seven sites, we established a robust, interactive, online training module to scale the ability to train healthcare professionals nationwide.
The Results
More than 350 participants have participated in advocacy trainings and have gone on to work with legislators, school boards, town and city officials, and other leaders to change the policies that affect communities. Some pressed for better nutrition or physical education standards in schools. Others worked with community coalitions to facilitate changes that will make it easier for kids to be active. Our work has led to:
- children having daily access to fresh fruits and vegetables
- new trails where families can walk and bike together
- community gardens where children proudly take home vegetables they’ve helped to grow
- town-sponsored shuttle buses that help residents get to their local farmers market
Take the advocacy course >

With comprehensive curriculum and training development to catalyze change, healthcare professionals are helping their communities grow healthy and active children today, tomorrow and for generations yet to come.
Looking for a change agent to enhance your initiative?
Contact us to get started!
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Effectiveness of a...
Effectiveness of a childhood obesity prevention programme delivered through schools, targeting 6 and 7 year olds: cluster randomised controlled trial (WAVES study)
- Related content
- Peer review
This article has a correction. Please see:
- Effectiveness of a childhood obesity prevention programme delivered through schools, targeting 6 and 7 year olds: cluster randomised controlled trial (WAVES study) - May 02, 2018
- Peymane Adab , professor of public health and chief investigator 1 ,
- Miranda J Pallan , senior clinical lecturer 1 ,
- Emma R Lancashire , Senior Research Fellow and trial co-ordinator 1 ,
- Karla Hemming , senior lecturer in medical statistics and trial statistician 1 ,
- Emma Frew , reader in health economics 1 ,
- Tim Barrett , professor of paediatrics 2 ,
- Raj Bhopal , Bruce and John Usher chair in public health 3 ,
- Janet E Cade , professor of nutritional epidemiology and public health 4 ,
- Alastair Canaway , Research fellow 5 ,
- Joanne L Clarke , PhD student 1 ,
- Amanda Daley , reader in behavioural medicine 1 ,
- Jonathan J Deeks , professor of biostatistics 1 ,
- Joan L Duda , professor of sport and exercise psychology 6 ,
- Ulf Ekelund , professor of physical activity epidemiology and public health, and senior investigator scientist 7 8 ,
- Paramjit Gill , clinical reader in primary care research 1 ,
- Tania Griffin , research fellow 1 ,
- Eleanor McGee , public health nutrition lead 9 ,
- Kiya Hurley , PhD student 1 ,
- James Martin , PhD student 1 ,
- Jayne Parry , professor of policy and public health 1 ,
- Sandra Passmore , health education consultant and education advisor 10 ,
- K K Cheng , professor of public health and primary care 1
- 1 Institute of Applied Health Research, University of Birmingham, Birmingham, UK
- 2 School of Clinical and Experimental Medicine, University of Birmingham; Birmingham, UK
- 3 Edinburgh Migration, Ethnicity and Health Research Group, Usher Institute of Population Health Sciences and Informatics, The University of Edinburgh, UK
- 4 Nutritional Epidemiology Group, School of Food Science and Nutrition, University of Leeds, Leeds, UK
- 5 Warwick CTU, University of Warwick, Warwick, UK
- 6 School of Sport, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham, UK
- 7 Cambridge MRC Epidemiology Unit, Cambridge, UK
- 8 Department of Sports Medicine, Norwegian School of Sport Sciences, Oslo, Norway
- 9 Birmingham Community Healthcare NHS Trust, Birmingham, UK
- 10 Services for Education, Birmingham, UK
- Correspondence to: P Adab, Public Institute of Applied Health Research, Murray Learning Centre, University of Birmingham, B15 2TT, UK p.adab{at}bham.ac.uk
- Accepted 19 December 2017
Objective To assess the effectiveness of a school and family based healthy lifestyle programme (WAVES intervention) compared with usual practice, in preventing childhood obesity.
Design Cluster randomised controlled trial.
Setting UK primary schools from the West Midlands.
Participants 200 schools were randomly selected from all state run primary schools within 35 miles of the study centre (n=980), oversampling those with high minority ethnic populations. These schools were randomly ordered and sequentially invited to participate. 144 eligible schools were approached to achieve the target recruitment of 54 schools. After baseline measurements 1467 year 1 pupils aged 5 and 6 years (control: 28 schools, 778 pupils) were randomised, using a blocked balancing algorithm. 53 schools remained in the trial and data on 1287 (87.7%) and 1169 (79.7%) pupils were available at first follow-up (15 month) and second follow-up (30 month), respectively.
Interventions The 12 month intervention encouraged healthy eating and physical activity, including a daily additional 30 minute school time physical activity opportunity, a six week interactive skill based programme in conjunction with Aston Villa football club, signposting of local family physical activity opportunities through mail-outs every six months, and termly school led family workshops on healthy cooking skills.
Main outcome measures The protocol defined primary outcomes, assessed blind to allocation, were between arm difference in body mass index (BMI) z score at 15 and 30 months. Secondary outcomes were further anthropometric, dietary, physical activity, and psychological measurements, and difference in BMI z score at 39 months in a subset.
Results Data for primary outcome analyses were: baseline, 54 schools: 1392 pupils (732 controls); first follow-up (15 months post-baseline), 53 schools: 1249 pupils (675 controls); second follow-up (30 months post-baseline), 53 schools: 1145 pupils (621 controls). The mean BMI z score was non-significantly lower in the intervention arm compared with the control arm at 15 months (mean difference −0.075 (95% confidence interval −0.183 to 0.033, P=0.18) in the baseline adjusted models. At 30 months the mean difference was −0.027 (−0.137 to 0.083, P=0.63). There was no statistically significant difference between groups for other anthropometric, dietary, physical activity, or psychological measurements (including assessment of harm).
Conclusions The primary analyses suggest that this experiential focused intervention had no statistically significant effect on BMI z score or on preventing childhood obesity. Schools are unlikely to impact on the childhood obesity epidemic by incorporating such interventions without wider support across multiple sectors and environments.
Trial registration Current Controlled Trials ISRCTN97000586 .
Introduction
Excess weight in childhood is a global problem, affecting around 41 million children under the age of 5 years. 1 In addition to physical and psychosocial health consequences in these early years, childhood excess weight is an important predictor of obesity in adulthood, 2 with additional adverse health and economic 3 effects. In the UK around a quarter of children have excess weight at school entry (age 4 or 5 years). 4 The proportion of very overweight children doubles during the subsequent six years (from approximately 9% to 19%), 4 highlighting this period as critical for preventive action.
Systematic reviews of childhood obesity prevention studies suggest that school based interventions may be effective in reducing the proportion of children with excess weight. 5 6 Heterogeneity of study design and interventions precludes conclusions about which combination of components are likely to be most effective. Nevertheless, overall, longer duration, multicomponent interventions, targeting school curriculums and food and physical activity environments, providing teacher support, and extending activities to the home and community were more likely to be associated with positive results. However, trials to date have had several methodological weaknesses that limit recommendations for widespread implementation. 5 In particular, few previous trials reported longer term outcomes, subgroup effects, or cost effectiveness.
We report the results of the West Midlands ActiVe lifestyle and healthy Eating in School children (WAVES) study; a cluster randomised controlled trial evaluating an intervention that aims to prevent excess weight in primary school children. The trial dealt with the main limitations identified in previous research: use of the Medical Research Council framework for complex intervention development and evaluation 7 ; a sample size large enough to detect clinically significant differences in adiposity; a comprehensive process evaluation; assessment of longer term effects, using a range of adiposity and psychosocial measures; and an objective measure of physical activity.
Trial design and eligibility
This was a school based, cluster randomised, controlled trial evaluating the effectiveness of a complex obesity prevention intervention on primary school children’s body mass index (BMI) z scores at 15 and 30 months after baseline measurements (3 and 18 months post-intervention completion). 8
Primary schools in the West Midlands, UK, within 35 miles of the study centre were eligible for inclusion (n=980). The region includes a multiethnic population from diverse socioeconomic backgrounds living in rural and urban areas. We excluded schools with fewer than 17 year 1 (aged 5 and 6 years) pupils (minimum cluster size) or schools in “special measures” (unlikely to have capacity to contribute to study). Within participating schools, all children in year 1 at recruitment were eligible for inclusion.
Interventions and intervention development
Irrespective of whether children participated in measurements, intervention delivery was at school class level to all eligible children and their families.
The development process of the WAVES study intervention commenced in 2005. We summarised intervention components incorporated in previous childhood obesity prevention trials (70 included studies within eight systematic reviews) in relation to setting, target behaviour, and type of activity. To help prioritise intervention components we then conducted focus groups with parents, school staff, and local health, government, and community members. The discussions considered the perceived importance and feasibility of implementation of techniques (eg, reward behaviours, role model, exposure to opportunities for physical activity), activities (eg, education materials, cooking workshops), and particular settings (eg, school curriculum, community setting). We checked prioritised ideas against available local resources, and the intervention package was formed with input from an expert group of professionals. Thus we balanced the prioritised intervention components (eg, role models to influence behaviour, or family campaigns) with the resources that were readily available in our setting (eg, the Villa Vitality programme described later). This intervention comprised activities within two broad aims: increasing children’s physical activity levels through school and home and supporting the development of health behaviour skills in families through activity based learning. 9 The intervention was further refined following a feasibility study. 10 That study showed that the proposed measurements could be completed successfully (measurements obtained for 574 out of 606 children with consent (95%) at baseline) and that loss to follow-up two years after baseline was at an acceptable level (follow-up measurements obtained for 83% and 86% of children in control and intervention schools, respectively). The feasibility study was not powered to investigate intervention outcomes, but the direction of effect was in favour of the intervention for most outcomes. In particular, children in the intervention arm compared with control arm had significantly lower adjusted BMI z scores at follow-up (−0.15 kg/m 2 , 95% confidence interval −0.27 to −0.03). Table 1 provides details of the finalised intervention.
Summary of WAVES study intervention programme
- View inline
WAVES study intervention and its delivery
The intervention components, delivered over 12 months, targeted the home and school environment. The target group, based on findings from the feasibility study, was year 2 children (aged 6 or 7 years) and their families. Several behaviour change strategies were employed to encourage increased physical activity and improved diet quality. School staff were provided with training and resources for intervention delivery. A termly family newsletter reinforced messages delivered through the various intervention components. The intervention programme (summarised in table 1 ) comprised four overlapping components:
(1) Thirty minutes of additional moderate to vigorous physical activity on each school day—at least 15 minutes to be outside of break times, although class teachers customised timing of delivery and exact activities undertaken according to their class circumstances, supported by resources supplied as part of the study. Class teachers selected two preferred resources out of four offered and were taken through each selected resource and its detailed delivery materials by a researcher
(2) Termly cooking workshops during school time, which parents were invited to attend to participate in with their child and that were preceded by short classroom sessions for the children. School staff responsible for implementation (with the exception of two schools where equivalent training was delivered in school by the same researcher) attended a one day training session. To minimise teacher preparation time and ensure delivery of consistent nutritional messages, the presentation and interactive activity materials, together with take home information sheets and suggested lesson and workshop plans were provided, but timing of sessions and how parents were involved was left to the discretion of teachers
(3) A six week programme (Villa Vitality) developed to encourage healthy eating and increase physical activity and delivered by staff from an iconic sporting institution. School classes spent two days undertaking activities (indoor based movement routines, using dance mats, ball skills session, interactive nutritional sessions, and an opportunity to practise cooking skills) at an English premier league football club, separated by a six week period during which teachers were asked to spend curriculum time working on a class project and involving children and their parents with weekly health challenges. The teacher customised the elements undertaken in school supported by a school visit from a member of staff from Villa Vitality
(4) Information sheets signposting children and their families on ways to be active over the summer (identical for all schools) and physical activity opportunities in their local area (school specific sheets produced by the study team and checked before distribution by the school).
Comparator intervention
Schools allocated to the comparator arm continued with ongoing year 2 health related activities. In addition, we provided citizenship education resources, excluding topics related to healthy eating and physical activity.
The primary outcome for clinical effectiveness specified in our analysis plan and trial protocol was the difference in BMI z scores between arms at 15 and 30 months. Table 2 summarises the trial protocol prespecified secondary outcome measures. At trial registration, the secondary outcomes of waist circumference, sum of four skinfolds, and body fat percentage were included as primary outcomes. All outcomes were assessed at 15 and 30 months post-baseline measures (3 and 18 months post-intervention). Further details on the methods, including standardised operating procedures for all primary and secondary outcome measurements, are available in the final report of the WAVES study, available through the National Institute for Health Research website ( www.journalslibrary.nihr.ac.uk ).
Summary of measurements undertaken within WAVES study and their associated outcome variables
Implementation
The trial statistician (KH) undertook sampling and subsequent randomisation, and the trial coordinator (ERL) recruited schools. To enable subgroup analysis we stratified schools by ethnic mix of pupils, and we used a weighted random sampling strategy to increase the selection likelihood (3:1) of schools with a higher minority ethnic population. Using this method, we selected 200 schools, which were ordered using a random number generator and sequentially invited to participate. To allow measurement of a large number of children in a limited timeframe within study resources, we recruited and randomised the schools into two groups (27 schools in each group), one year apart. Parental informed consent was sought and verbal assent from the children was obtained for all measurements undertaken.
Participant assessment and data collection procedures
Baseline assessment took place when children were at the end of year 1 (aged 5 or 6 years). Outcome assessments using identical procedures were undertaken at 15 months (first follow-up) and 30 months (second follow-up) post-baseline (aged 7 or 8, and 8 or 9 years, respectively). In schools recruited in the first year (group 1), we further assessed at 39 months (third follow-up) post-baseline (aged 9 or 10 years), but this was not possible for schools recruited in the second year (group 2) within the trial timetable. We collected data from school records, direct assessment of participating children in school, and parent questionnaires distributed at the time of pupil measurements. Trained research staff undertook assessments using standardised protocols and validated instruments, as detailed in the protocol 8 and summarised in table 2 .
Sample size
Sample size was based on the primary outcome (BMI z score), taking into account repeated measures (estimated correlation between measures=0.9), varying cluster size (assuming mean 25 (SD 23) cluster size), and likely estimates of the intraclass correlation coefficient (0 to 0.04). To detect a clinically meaningful difference of 0.25 BMI z score 11 between intervention and comparator groups with 90% power, a two sided α of 0.05, and estimated pupil dropout rate of 20%, we needed a follow-up sample of 1000 children from 50 schools. Allowing for school drop-out of 8%, we recruited 54 schools. This sample size also provides more than 80% power to detect a 0.125 difference in BMI z score (clinically important difference for prevention 12 ) and an approximately 7% difference in the change of proportion of children who are overweight or obese from baseline to follow-up in control compared with intervention schools.
Randomisation
A blocked balancing algorithm was used to randomise participating schools to intervention or comparator arms. Schools were randomly allocated according to a randomisation scheme, which minimised imbalance 13 on several characteristics: percentage of pupils eligible for free school meals (measure of deprivation), proportion of pupils from South Asian, black African-Caribbean, white, or other ethnic groups, and school size. We randomised the first 27 schools (group 1) within the first block. A year later we randomly allocated the remaining 27 schools (group 2) in a similar way, but conditioning on the allocations that had already been made in group 1.
To ensure concealment of allocation we carried out randomisation after baseline measurements. Sessional researchers blind to arm allocation mainly undertook further data collection. Supplementary figure 1 summarises the timeline for trial processes.
Statistical analysis
Analyses of all outcomes were by intention to treat and are reported at 15, 30, and 39 months after baseline (3, 18, and 27 months after the end of the intervention). For the primary analyses (complete case analysis), we used mixed linear regression models for all continuous outcomes (eg, BMI z score) and Poisson mixed regression for binary outcomes to allow estimation of adjusted risk differences consistent with CONSORT guidelines. To accommodate any non-normality of the outcomes, we transformed data when necessary and when such transformation improved the model. The baseline adjusted model included the baseline measurement and treatment arm as the independent variables, and to account for the clustered nature of the sample, school as the random effect. We also report models further adjusted for prespecified baseline school and child level covariates. Planned subgroup analyses, using interaction tests, examined whether any intervention effects differed by ethnicity, sex, socioeconomic status, baseline weight status, and fidelity of implementation.
Sensitivity analyses included using multiple imputation (using chained equations) for missing values for each outcome, exploring cluster heterogeneity by period (group 1 versus group 2 schools), and methods of adjusting for missing baseline variables to maximise use of available data and heterogeneity of the intraclass correlation coefficient in intervention and control arms. Additional details on the statistical methods are available in the final report, available through the National Institute for Health Research website ( www.journalslibrary.nihr.ac.uk ).
We set the level of statistical significance at 0.05 (two sided) for the primary outcomes (see table 2 ) and at 0.01 for all other outcomes. Analyses were carried out in Stata 13 14 and REALCOM-impute 15 software.
Because of the timelines of recruitment and outcome assessments, there was no opportunity for interim analyses. The trial steering committee maintained assessment of data quality and completion.
Process evaluation
We used a variety of methods for assessment of intervention delivery and process, including interviews with teachers; parent and child focus groups; head teacher, class teacher, and parental questionnaires; teacher logbooks; and direct observation of sessions by researchers. 16 With the exception of the signposting sheets for which there was no variation in implementation between schools, we used a consensus method for each of the other three intervention components to allocate schools a score on a 5 point Likert scale for each dimension of the process evaluation (fidelity and adherence; reach, dose, and exposure; recruitment, quality, and participant responsiveness). Context and information on programme differentiation influence all of these and were therefore also considered throughout this score allocation process. We then ranked schools by total score, and grouped the schools to reflect low, medium, or high intervention implementation. A detailed report on the method used to synthesise the process evaluation data is published elsewhere. 17
Changes to methods from trial registration stage
The trial registration was submitted before the practical planning for the trial had started. Some aspects were subsequently altered in the development of the trial protocol, but the trial registration was not updated and therefore does not incorporate these changes. Supplementary table 1 summarises all changes between trial registration and trial protocol. In particular, at the early planning stages for the trial (and before the start of baseline measurements) the investigator team modified the primary outcomes from those specified at trial registration. To increase power to detect change and for consistency and comparability with previous trials, we changed the primary outcome for clinical effectiveness from the binary variable specified in the registry (of difference in proportion of children categorised as overweight or obese between arms) to the continuous outcome specified in the protocol of difference in BMI z scores between arms. Concurrently this binary variable and the additional anthropometric measurements included as primary outcomes at trial registration were specified as secondary outcomes. The reporting of the trial is in keeping with the published protocol, 8 which was submitted before the start of data analysis, but any differences between what is reported and the trial registration information are specified in both the text and the tables.
Patient and public involvement
Public involvement was a key feature of the early phases of trial development and feasibility testing before this main trial. Intervention development was informed by detailed consultation with parents, teachers, and other school staff. The intervention was further refined and the process for measuring outcomes tested and adapted by asking the children, parents, and teachers about their experiences during the feasibility study. Measures of wellbeing and body dissatisfaction were included as outcomes based on their perceived importance among school staff. Our research team includes an education advisor at the Health Education Service, who has regular contact with schools and advised on school and participant recruitment. No patients were involved in this trial.
Figure 1 shows the flow of schools and pupils during the trial. Among 2462 eligible pupils from 54 participating schools at baseline, parental consent for baseline measurements was obtained from 1467 (59.6%). Recruitment took place between April and May 2011 (group 1 schools and pupils) and from January to May 2012 (group 2 schools and pupils). Table 3 summarises the baseline characteristics. Although school characteristics were balanced between the two groups, there was baseline imbalance at the pupil level, with children in the control arm compared with intervention arm more likely to be male (52.7% v 49.2%), from generally less deprived households (mean index of multiple deprivation score 37.6 v 39.8), less likely to be overweight (mean BMI z score 0.15 v 0.23), more likely to consume five portions of fruit and vegetables daily (64.8% v 59.8%), and more likely to achieve at least 60 minutes of moderate to vigorous physical activity daily (49.6% v 46.4%).
Flow of school recruitment and trial arm allocation
- Download figure
- Open in new tab
- Download powerpoint
Baseline characteristics of school pupils participating in the WAVES study overall and by trial arm
Primary outcomes
The primary outcomes are also reported in the trial protocol ( table 4 ). At 15 months the mean BMI z score was non-significantly lower in the intervention arm compared with control arm: mean difference −0.075 (95% confidence interval −0.183 to 0.033, P=0.18) in baseline adjusted models (n=1197, 86% of those with baseline BMI z score available) and −0.077 (−0.191 to 0.037, P=0.19) in further adjusted (n=837, 60% of those with baseline BMI z score available) models. At 30 months the mean difference was smaller and remained non-significant (−0.027, −0.137 to 0.083; P=0.63) in the baseline adjusted model (n=1094, 79% of those with baseline BMI z score available).
Adjusted differences for body mass index (BMI) z score between control and intervention groups at first, second, and third follow-up
Secondary outcomes
The secondary outcomes are as reported in the trial protocol and trial registration information, unless stated otherwise ( table 5 ).
Adjusted differences for secondary outcomes (anthropometric, diet, physical activity, and psychosocial) between control and intervention arm at first and second follow-up
Anthropometric measurements —these are included as primary outcomes in trial registration information (see “Changes to methods from trial registration stage”). In the intervention arm compared with control arm, the baseline adjusted risk difference in the proportion of children who were overweight or obese was −0.013 (99% confidence interval −0.075 to 0.071, P=0.66) and 0.002 (−0.068 to 0.093, P=0.95) at 15 and 30 months, respectively. The mean difference in sum of skinfolds, waist circumference z score, and body fat percentage were all non-significant, but slightly favoured the control group.
Diet, physical activity, and blood pressure —the mean differences in total daily energy intake, physical activity energy expenditure, and systolic and diastolic blood pressures between groups were inconsistent in direction and statistically non-significant at both follow-ups.
Longer term clinical effectiveness —among group 1 school participants who were followed up at 39 months (488 pupils (246 controls), 27 schools (14 controls)), the mean BMI z score was lower in the intervention arm compared with control arm in the baseline adjusted model (mean difference −0.20, 99% confidence interval −0.46 to 0.05, P=0.04) and further adjusted models (−0.18; −0.39 to 0.03, P=0.03). We were not aware of any contextual or intervention delivery aspects that differed between the groups. To investigate why the intervention appeared more effective at this later time point, we undertook post hoc analysis to consider whether schools recruited in group 1 differed from those in group 2, both in characteristics (see appendix, table A1) and in outcomes at earlier time points (see appendix, table A2). This showed a noticeable imbalance in baseline adiposity between arms in group 2 schools and baseline differences in ethnicity, deprivation, and adiposity between group 1 and group 2 schools. There was a significant interaction in the effect of the intervention on the primary outcome between groups (P=0.001 (first follow-up) and P=0.02 (second follow-up) in the partially adjusted model). Analysis of outcomes by school group showed a statistically significantly lower BMI z score in the intervention arm compared with control arm at first follow-up in group 1 schools (mean difference –0.23, 95% confidence interval −0.35 to −0.12, P<0.01 for baseline adjusted model), which was maintained through to the third follow-up (although no longer statistically significant at the 1% level). In contrast there was no significant difference between arms at any time point in group 2 schools (see appendix, table A2).
Harms —quality of life, as total score or subdomains, social acceptance, or dissatisfaction with body image did not differ significantly between arms at any time. Thus we found no evidence of harm from the intervention.
Subgroup and sensitivity analyses —all subgroup analyses (by ethnicity, sex, socioeconomic or weight status, and fidelity of implementation) and sensitivity analyses were consistent with the main analyses and did not change any conclusions (results not shown).
Process evaluation —detailed results from the process evaluation are reported separately. 17 18 19 Briefly, the intervention was generally well implemented, although no school delivered all components completely as intended. The scores developed to represent overall fidelity of programme implementation show that just under half the schools (12/26) achieved at least 75% of the maximum possible score and only five schools failed to achieve at least 65% of that maximum. Teachers found the daily physical activity intervention component the most challenging to deliver, with only four of 26 schools (17%) achieving high implementation fidelity for that component and 58% of schools (15/26) allocated to the low implementation fidelity group. In contrast, 42% (11/26) and 65% (17/26) of schools achieved high implementation fidelity for the cooking workshop and Villa Vitality components, respectively, with classification to the low implementation fidelity group for 42% (11/26) (cooking workshop) and 27% (7/26) (Villa Vitality). However, despite some challenges to implementation, the interviews and focus groups indicated that the programme was often well received both by teachers 19 and by parents and children (see box). 20
Quotes about the programme
It was fantastic and combining the sport and the nutrition was brilliant (teacher)
There’s no doubt about it they’ve loved it, yeah . . . so it’s been really good for them and that’s what it’s all about really isn’t it (teacher)
It’s good to have it reinforced I think from somebody other than your parents, sometimes if your teacher says it, it’s true! (parent)
She’s willing to try more fruits and vegetables, that’s what I’m pleased with probably more, before she was quite picky with what she’d have, but now she is willing to try new things (parent)
I teached my mum how to cook it when we cooked in Aston Villa. And I chop a bit at home because I learned how to chop at Aston Villa (child)
Because I’ve done my exercise I can think harder and try (child)
We found no overall evidence of improvement in the primary outcomes of reduction in body mass index (BMI) z scores at 15 and 30 months after a childhood obesity prevention programme delivered through schools and targeting 6 and 7 year olds. However, confidence intervals did not exclude between arm differences in BMI z score of 0.125, thought to be clinically important for prevention. The intervention did not have any effects on secondary anthropometric, behavioural, or clinical outcomes, and there were no differential effects in prespecified subgroups. A clinically significant difference in BMI z score in favour of the intervention was seen in the first cohort of schools recruited that had data available at 39 months. Subsequent post hoc analysis suggests this may have been a cohort effect, with evidence of effectiveness in group 1 schools at all time points but no effect seen in group 2 schools at any time point. The outcomes used to assess harm did not differ between the groups.
Strengths and weaknesses of this study
The WAVES study is a large childhood obesity prevention trial within a socioeconomically and ethnically diverse population, with sufficient sample size to assess the primary outcome. Phased development of the 12 month multicomponent intervention was guided by the Medical Research Council framework for complex interventions, 9 21 including a successful feasibility trial. 10 The intervention comprised elements identified as promising in systematic reviews 5 6 and incorporated a range of behaviour change techniques, including those associated with positive outcomes in previous childhood obesity prevention trials. 22 Outcomes were assessed with mainly objective measurements, using validated instruments and standardised protocols. Loss to follow-up was relatively small, with 80% of pupils retained to the second follow-up, and loss of one school. A prespecified analysis plan took account of clustering, and the findings were robust to a range of sensitivity analyses. This was also one of few trials that undertook longer term follow-up (39 months) to assess sustainability of intervention effects. Comprehensive process evaluation (described in more detail elsewhere 16 ) helped to contextualise the findings and to interpret the results. 17
Nevertheless, there were also several limitations. Parental consent for study measurements being obtained for only 60% of eligible children could introduce selection bias; however, a pupil level comparison of demographic characteristics (sex, ethnicity, deprivation) between those with and those without consent did not show any major differences. The balancing algorithm to allocate schools was based on whole school (cluster) level data. However, within clusters, only children from one year group were included, and just over half of those consented to study measurements. There was notable baseline imbalance between arms in the group 2 cohort (with the intervention arm having greater adiposity than the control arm), which, despite the use of adjustment methods, may have attenuated the main results. Statistical adjustment assumes a common linear relation between covariates and outcome in all clusters, and misspecification of the model may lead to both under-adjustment and over-adjustment. Baseline imbalance is a known limitation of cluster trials and can best be overcome with recruitment of larger numbers of clusters. Although follow-up to 30 months was in all groups, longer term follow-up (to 39 months) was limited to a subset of participating schools. The Child And Diet Evaluation Tool (CADET) provided a quick, practical dietary assessment tool with relatively low respondent burden, 24 25 resulting in useable data from approximately 85% of children at baseline (81% first follow-up, 82% second follow-up). However, estimates of dietary intake may not reflect habitual intake, there was a risk of misreporting, 26 and there may have been seasonal variation 27 between data collection periods. Usable data on physical activity were available for 76% of children at baseline (60% first follow-up, 52% second follow-up). These are similar to the rates achieved in other such studies. 28
Comparison with other studies
Our results build on the findings of previous reviews and address limitations in previous childhood obesity prevention trials. Two systematic reviews suggested that there was moderate 6 to strong 5 evidence of effectiveness of school based interventions in preventing childhood obesity, although heterogeneity of interventions, variable design quality, and lack of longer term follow-up limit interpretation. A meta-analysis showed that the summary magnitude of effect on BMI z score compared with the control was −0.15 units, 5 which is smaller than the effect size used for estimating sample size in our trial. Nevertheless, the WAVES study was larger than the 21 previous obesity prevention trials with low risk of bias included in the meta-analysis (n=9 to 574). Since the publication of the reviews, findings from another UK cluster randomised controlled trial, the Active for Life Year 5 (AFLY5) including more than 2000 children from 60 schools are available. 28 The trial primarily attempted to influence activity levels and fruit and vegetable consumption, although it also reported on adiposity outcomes. The intervention was curriculum based, focusing on educational approaches rather than the more experiential skills based intervention in the WAVES study. In contrast with our trial, the target population was children at the end of the primary school years, when rates of obesity have already increased substantially, and included few children from minority ethnic groups and more deprived areas. Nevertheless, similar to our findings, there was no evidence of an intervention effect on behavioural or weight outcomes at 12 months.
Interpretation of the findings
The balance of components, intensity, and behaviour change strategies used to deliver the intervention may have contributed to the absence of evidence of effect on the primary outcomes in WAVES and other trials. Although fidelity of implementation for the WAVES study intervention programme was reasonably high overall, no school delivered all components completely per protocol, and a few schools failed to deliver some or all of the components. This may have attenuated any effect. 29 In addition, owing to competing demands on teachers, components that required greater teacher input tended to be less well implemented and this was the main explanation for differences in fidelity between components. This suggests that delivery of a more intensive teacher led intervention in a school setting would not be feasible without additional resources. Educational and experiential interventions of longer duration that are embedded within a whole school setting are likely to be prohibitively costly and complex to evaluate using clinical trial methods. The intervention was developed on the basis of promising strategies in trials published before the feasibility study (about 10 years before the definitive trial). As a result, strategies such as those based on behavioural economics aimed at altering the social and physical environment were not included as part of the intervention. Although the findings from the feasibility study suggested the WAVES intervention was promising, intervention delivery for the trial and subsequent follow-up measurements took place some years later, during which time wider environmental changes might have diluted any effects. Researcher contact with schools during the feasibility study was also much greater, but this was not replicable in the definitive trial with a larger number of schools and would not be implementable outside of a trial setting. Methodological limitations with baseline imbalance may have also contributed to the observed findings with heterogeneity of effect between schools. However, even the cohort effect observed in group 1 schools was small, suggesting that childhood obesity prevention is unlikely to be achieved by schools alone. While school is an important setting for influencing children’s health behaviour, and delivery of knowledge and skills to support healthy lifestyles is one of its mandatory functions, wider influences from the family, community, media, and the food industry must also be considered. The qualitative data from teachers 19 and parents, 20 collected as part of our process evaluation, support the possibility that these wider influences have a greater effect than any school based intervention. A metasynthesis of qualitative studies exploring the role of primary schools in preventing childhood obesity highlighted the need for schools, parents, and government to work together to promote healthy lifestyles in children and to support activities in the school setting. 30
Conclusions
The multicomponent WAVES study intervention, which was feasible to deliver and for which there was no evidence of harm, did not result in a statistically significant difference in BMI z score overall, and there was no evidence of effect on measured diet or physical activity levels in children. Although wider implementation of this intervention cannot be recommended for obesity prevention, the lower cost components could be considered by schools to fulfil their mandated responsibilities for education on health and wellbeing. Within the context of the wider evidence, it is likely that any effect of school based educational, motivational, and skill centred interventions on obesity prevention is small. Several community based interventions targeting wider environments have also been evaluated recently, using non-randomised experimental designs. Although a few of these have shown evidence of small effects and lower weight gain in children from intervention communities, 31 32 the findings are not consistent 33 and need further evaluation. Interventions based on behavioural economics such as nudge theory 34 also merit further investigation. Even marginal effects may be important within a wider systems approach to obesity prevention, which incorporates multiple agencies and widespread policy change to support healthy behaviours.

What is already known on this topic
Comprehensive systematic reviews have suggested that school based interventions could be effective in preventing childhood obesity in high income countries
Heterogeneity in intervention components and outcomes limit practical recommendations
Furthermore, inconsistent findings in relation to differential effects on subgroups, and impact on inequalities, limited data on potential harms, process measures, and long term effects, as well as lack of data on cost effectiveness, restrict interpretation and wider applicability
What this study adds
The WAVES study evaluated a theoretically informed, skills based intervention targeting children’s diet and physical activity behaviours through schools and families
It did not result in any meaningful effect on adiposity, dietary intake, or physical activity after 15 or 30 months
Although such interventions can fulfil the responsibility of schools for wider education, without upstream support they are unlikely to halt the childhood obesity epidemic
Acknowledgments
The order of authorship is based on relative contributions for the first five authors (PA to EF) and last author (KKC). The remaining authors (TB to SP) are listed alphabetically.
We thank the children, school staff, and parents who participated in the trial; the children, teachers, and parents who took part in interviews and focus groups as part of the process evaluation; the support of staff at Aston Villa football club in delivering the Villa Vitality programme; the study team, including Behnoush Mohammadpoor Ahranjani and Emma Popo who helped in overseeing the study measurements and data collection; the administrative team who facilitated the running of the project; the research staff who undertook the study measurements; and Robert Lancashire who developed the trial database and oversaw data management.
Trial monitoring and data monitoring
The trial steering committee met annually and included: chair/statistician: Kelvin Jordan, Keele University; subject expert: Peter Whincup, St George’s, University of London; health economist: Louise Longworth, Brunel University; public representative: John Bennett, PHSE advisor; and investigators: Peymane Adab and Miranda Pallan, University of Birmingham.
Owing to the timelines of recruitment and outcome assessments, there was no opportunity for interim analyses, and the trial steering committee undertook the role of assessment of data quality and completion.
Contributors: All WAVES study trial co-investigators contributed to the development of the design for the WAVES study trial and had contributed to the intervention development as part of the BEACHeS Study. PA wrote the first draft of the paper and all authors contributed to critical revisions. PA, MP, and KKC planned the overall design of the trial. ERL coordinated all aspects of the trial, oversaw data collection, collation, and cleaning, and contributed to data analysis. She also contributed to the first draft of the manuscript. EF contributed to the design of the trial and analysis plan, and AC advised on study instruments and undertook some of the analysis. JJD and KaH contributed to sampling, sample size estimation, and the statistical analysis plan. JM undertook data analysis, supervised by KaH and with support from ERL. UE advised on physical activity measurements and related methods and oversaw the preparation of the physical activity data. JEC advised on dietary assessment and related methods and oversaw the CADET data preparation. KiH led data collection and analysis of dietary data. JLD advised on some of the psychosocial assessment methods. TB, PG, and RB advised on clinical measurement processes. RB and PG advised on aspects related to ethnicity. AD advised on the physical activity intervention component. SP advised on school recruitment and approaches to keeping schools engaged. EM advised on the dietary intervention components. JP advised on process evaluation, and TG designed the detailed methods for this. TG and JLC contributed to data collection and undertook analysis and interpretation of the process evaluation. The final manuscript was read and approved by all authors. PA, ERL, and MP are guarantors.
Funding: This study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project reference No 06/85/11). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The University of Birmingham holds the relevant insurance policy for this study and acted as the main sponsor. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Technology Assessment, NIHR, National Health Service, or the Department of Health. The funders have played no role in the design, collection, analysis, and interpretation of data, nor in the writing of the manuscript and in the decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The trial was approved by NHS Research Ethics Service Committee West Midlands, The Black Country (NHS REC No 10/H1202/69).
Data sharing: Requests for access to data from the WAVES study should be addressed to the corresponding author at p.adab{at}bham.ac.uk . All the individual participant data collected during the trial (including the data dictionary) will be available, after deidentification, immediately after publication with no end date. The study protocol has been published. All proposals requesting data access will need to specify how it is planned to use the data, and all proposals will need approval of the trial co-investigator team before data release.
Transparency: The guarantors (PA, ERL, and MP) affirm that the manuscript is an honest, accurate, and transparent account of the study bring reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/ .
- ↵ World Health Organization. Commission on Ending Childhood Obesity. Report of the Commission on Ending Childhood Obesity. Geneva, 2016.
- Serdula MK ,
- Coates RJ ,
- Freedman DS ,
- Williamson DF ,
- Withrow D ,
- ↵ Lifestyles Statistics Team HaSCIC. National Child Measurement Programme: England, 2014/15 school year, 2015.
- de Silva-Sanigorski A ,
- Wilson RF ,
- Campbell M ,
- Fitzpatrick R ,
- Pallan MJ ,
- Lancashire ER ,
- Kolsgaard MLP ,
- Brunborg C ,
- Anderssen SA ,
- Tonstad S ,
- Andersen LF
- ↵ Stata statistical software: Release 13. [ program ] StataCorp LP , 2013 .
- Carpenter JR ,
- Goldstein H ,
- Griffin TL ,
- Clarke JL ,
- WAVES study trial investigators
- Macintyre S ,
- Nazareth I ,
- Petticrew M ,
- Medical Research Council Guidance
- Lorencatto F
- Greenwood DC
- Christian MS ,
- Nykjaer C ,
- Hancock N ,
- Macdiarmid J ,
- Zaborskis A ,
- Moceviciene R ,
- Iannotti RJ
- Kipping RR ,
- Naylor P-J ,
- Nettlefold L ,
- Fletcher B ,
- Lancashire E ,
- Roillet M ,
- Swinburn B ,
- Malakellis M ,
- De Henauw S ,
- Huybrechts I ,
- De Bourdeaudhuij I ,
- IDEFICS consortium
- Freeman JV ,
- Jackson LV ,
- Thalange NKS ,
BRIEF RESEARCH REPORT article
Listening to the community: identifying obesity prevention strategies for rural preschool-aged children.

- 1 Department of Applied Health Science, Indiana University Bloomington, Bloomington, IN, United States
- 2 Department of Health Behavior, North Carolina Translational and Clinical Sciences Institute, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3 Granville Vance Public Health Department, Henderson, NC, United States
- 4 Department of International Health, Johns Hopkins University, Baltimore, MD, United States
- 5 Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 6 Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, United States
Multi-level interventions promoting healthy weight in rural preschool children aged 2–5 years are limited. With the goal of developing a community-informed obesity prevention intervention for rural preschool-aged children, the purpose of this descriptive study was to identify: (1) community settings and intervention strategies to prioritize for an intervention; (2) potential implementation challenges and solutions; and (3) immediate interventions the study team and community partners could collaboratively implement. Workshops occurred in two rural communities in Indiana (2 workshops) and North Carolina (2 workshops), with high obesity rates. A guide was developed to moderate discussions and participants voted to rank community settings and intervention strategies. There were 9–15 participants per workshop, including parents, childcare providers, and representatives of community organizations. Community settings identified as priorities for child obesity prevention included the home, educational settings (preschools), food outlets, recreational facilities, and social media. Priority intervention strategies included providing nutrition and physical activity education, increasing access to healthy foods and physical activity in the built environment, and enhancing food security. Potential intervention implementation challenges centered on poor parental engagement; using personalized invitations and providing transportation support to families were proffered solutions. Immediate interventions to collaboratively implement focused on making playgrounds esthetically pleasing for physical activity using game stencils, and nutrition education for families via quarterly newsletters. This participatory approach with community partners provided insight into two rural communities’ needs for child obesity prevention, community assets (settings) to leverage, and potential intervention strategies to prioritize. Findings will guide the development of a multi-level community-based intervention.
1 Introduction
Childhood obesity rates in the United States (U.S.) are high. From 2010 to 2020, obesity prevalence in children aged 2–5 years increased from 10 to 13% ( 1 ). This public health concern is more acute in rural communities, with studies reporting 26% higher odds of obesity in rural versus urban children ( 2 ). Obesity prevention is preferrable to treatment in rural children ( 3 ), but often difficult to achieve because of multiple risk factors ( 4 , 5 ) occurring at the child (e.g., child diet/physical activity [PA]), family (e.g., socioeconomics), organizational (out-of-home care settings), community (e.g., built environment), and policy levels ( 6 , 7 ). Interventions targeting a single level of influence demonstrate mixed results in terms of effects on child weight ( 8 ). To effectively address child obesity in rural areas requires that interventions simultaneously target multi-level influences. The Socioecological Model, which posits that child obesity is influenced by factors at multiple levels of influence, including individual, interpersonal (family), organizational, community, and policy levels ( 9 ), provides a framework for understanding the critical need for multi-level child obesity prevention interventions.
Multi-level community-based interventions (e.g., Shape Up Somerville, Romp & Chomp) have been shown to promote sustainable improvements in child weight ( 10 – 16 ). This type of intervention exposes entire communities to obesity prevention efforts and simultaneously targets change at multiple levels that influence child obesity (e.g., child and family) ( 16 , 17 ). Applying this intervention approach requires that researchers engage with persons having first-hand knowledge about communities to ensure applicability, effectiveness, and sustainability of an intervention ( 18 , 19 ). To the authors’ knowledge, there is one multi-level, childhood obesity prevention intervention that has targeted rural U.S. communities, with results unpublished ( 20 , 21 ), but no such studies have targeted rural children aged 2–5 years.
Although rural communities have strengths, including the tightknit social ties among residents, strong cultural traditions, and proximity to natural landscapes that offer opportunities for outdoor activities ( 22 – 25 ), lack of access to resources that support wellbeing can make it difficult to implement and sustain interventions in rural communities. From June 2019 to July 2021, the current study team conducted formative research in two rural communities in Indiana (IN) and North Carolina (NC) to identify barriers, facilitators, and opportunities to address obesity in preschool children aged 2–5 years (published elsewhere) ( 26 ). Guided by the formative research, with the goal of developing a community-based intervention for preschool-aged children, the study team conducted workshops to engage with partners from the two rural communities (e.g., parents, representatives of community organizations) in the identification of: (1) community settings to prioritize for a child obesity prevention intervention; (2) intervention strategies at multiple levels of influence (e.g., child, family) to prioritize; (3) challenges that might be encountered while implementing an intervention, with potential strategies for navigating challenges; and (4) immediate interventions the study team and community partners could begin to implement collaboratively with little or no funding. This paper describes results from the workshops.
2.1 Study setting and participants
This descriptive study occurred in spring of 2022 in two rural counties (“communities” hereon) in IN and NC. Rurality was defined using U.S. Department of Agriculture’s Rural–Urban Commuting Area Codes ( 27 ). Both communities are considered high-need, with child poverty levels (18–32%) ( 28 , 29 ) that exceed the national poverty average (16%) ( 28 , 30 ), and high child and/or adult obesity (20–39%). Both communities differ in racial/ethnic make-up; the IN community is predominantly (96%) non-Hispanic White ( 31 ), while the NC community is diverse, with Black/African-Americans comprising 52% and Hispanic/Latino, 9% ( 32 ). Study participants included parents of children aged 2–5 years, childcare providers, representatives of community organizations serving children/families, and community residents interested in improving child health.
To recruit participants, two study team members (KP, TE) participated in a meeting for an existing coalition of community leaders in each community. At each meeting, the study team shared initial results from the formative research conducted to learn about barriers, facilitators and opportunities to promote healthy weight in children aged 2–5 years in both communities ( 26 ). The study team invited coalition members to participate in community workshops, sought insight from coalition members about how to structure the workshops (e.g., where/when to host workshops, incentives to offer), and enlisted their assistance with participant recruitment. Thereafter, personalized invitations were sent to coalition members, other community leaders who were not members of the coalitions (e.g., librarians, faith-based leaders), and persons from the formative research ( 26 ). Coalition members and other community leaders received several copies of the invitation to distribute to community residents in their network. Persons interested in participating in the workshops were instructed to notify the study team by telephone/email.
Overall, 110 invitation cards (65 in IN, 45 in NC) were mailed, with the goal to recruit up to 15 participants per community, a threshold that would allow for robust discussions among participants based on the study team’s prior experiences with conducting community workshops ( 33 , 34 ). The study team aimed to recruit a diverse representation of participants, including parents, childcare providers, and representatives of community organizations that serve families, but there were no set quota requirements. Two workshops were held per community. In IN, 15 persons participated in the first workshop, while 11 participated in the second workshop. In NC, there were 9 persons in the first workshop, and 13 in the second workshop. Study procedures were approved by the Institutional Review Board at Indiana University Bloomington. Written informed consent was obtained from participants before each workshop.
2.2 Overview of the community workshops
Community workshops occurred on Saturday mornings at publicly accessible community facilities. Short breaks were incorporated, refreshments were provided, and participants received a thank-you gift. The first workshop was 3 h and participants received $75 upon completion, versus $50 for the second workshop lasting 2 h. The study team developed a discussion guide ( Supplementary Table 1 ) for the workshops that was informed by the formative research in the two communities ( 26 ) and similar studies that used community workshops to design community-based interventions ( 18 , 33 , 34 ). The Socioecological Model ( 9 ) and previous multi-level obesity prevention studies ( 10 , 11 , 13 , 18 , 35 , 36 ) provided a theoretical framework to understand influencing factors, and prioritized community settings and strategies to promote healthy dietary intake and PA at the child, family/peer, organizational (e.g., childcare settings), community (e.g., built environment), and policy levels. The workshops were intended to be interactive and participatory. Each workshop began with a description of the purpose of the workshop, completion of informed consent and a demographic survey by participants, and an ice-breaker activity. Facilitation of each workshop was led by the same study team member (TE), with assistance from another team member (KP/AL). Flip charts displayed in the meeting room were used to record participants’ responses, and discussions were audio-taped.
2.3 Data collection at the community workshops
Guided by prior childhood obesity prevention studies ( 11 – 15 ), data collection for this study focused on two behavioral targets, to: (i) promote healthy dietary intake (specifically, increase fruits and vegetables; reduce fast food; reduce sweet/salty snacks; reduce sugar-sweetened beverages; and promote water consumption) and (ii) promote PA ( Figure 1 ). Similar contents were covered at each workshop across both communities using the discussion guide, however, where necessary, the facilitator combined behavioral targets for discussions because of time constraints. Data collection began with a discussion among participants about factors influencing the choice to engage in the target dietary and PA behaviors in families with children aged 2–5 years in various settings (home, childcare). While related information was collected in the formative research, discussions around influencing factors that impact child healthy weight behaviors helped to set the stage for ensuing discussions about settings and strategies to prioritize in an intervention. Participants’ responses were recorded on flip charts and then reflected back at the end of the discussion.
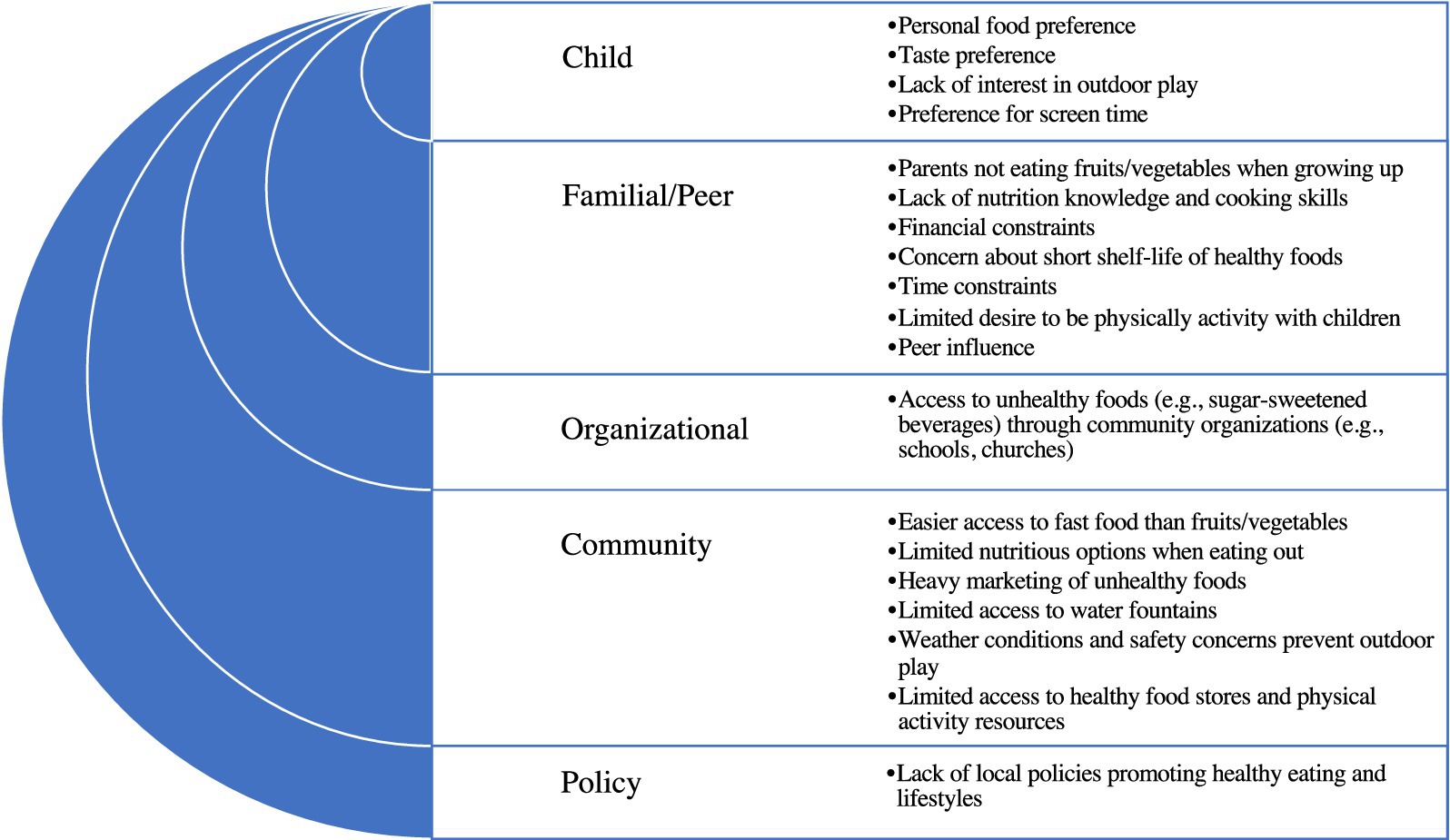
Figure 1 . Factors influencing the consumption of unhealthy foods and physical inactivity among families with children aged 2–5 years in two rural communities in Indiana and North Carolina: summary of results from community workshops.
Next, participants were asked to specify community settings in which to intervene to promote each behavioral target. After this discussion was exhausted, four sticky dots were provided to each participant so they could vote on settings they thought should be prioritized in an intervention. Votes were tallied and reported back to participants. During the next phase of discussions, participants were asked to specify potential intervention strategies for each behavioral target. Given time constraints, some behavioral targets were combined for discussion (e.g., strategies to reduce sugar-sweetened beverages combined with strategies to promote water consumption). After the discussion was exhausted, participants received 4 to 6 sticky dots to vote on intervention strategies that they thought should be prioritized for each behavioral target. Votes were tallied and restated to participants. The moderators then asked participants to specify challenges they thought the study team might encounter in implementing a potential intervention and strategies that might help to navigate challenges. Given time constraints in IN, the discussion about challenges occurred only in NC, and participants’ responses were captured on flip charts.
Final discussions centered on identifying immediate interventions from the priority list that the study team and community partners could begin to work on with little or no funding, and how the community and academic partners could begin to work together collaboratively to develop a multi-level obesity prevention intervention for children aged 2–5 years and families in their community. The study team shared workshop summaries with participants by email about 10 days after each workshop and also provided a printed copy of the summary from the first workshop at the second workshop. Email and in-person communications included requests for participants to check that the summaries accurately reflected discussions held, and report related concerns.
2.4 Data analysis
Audio-taped recordings from each workshop were transcribed without identifiers and were reviewed for accuracy and completeness. Data coding and content analysis were conducted by study team members trained in qualitative analysis (TE, KP) using the transcripts, supplemented with flipchart notes. Differences in the application of codes and content analyses were discussed by the coders and resolved by consensus. Notably, participants’ responses about factors that influence the choice to engage in the target dietary and PA behaviors were coded into five descriptive categories, guided by the Socioecological Model ( 9 ) and prior multilevel child obesity prevention research ( 10 , 11 , 13 , 18 , 35 , 36 ): child; family; organizational; community; and policy. Community settings with the four highest votes for each behavioral target were coded into descriptive categories based on similarities in function (e.g., educational, recreational settings) by state. A similar process was used to code and summarize responses about potential intervention strategies to prioritize. Responses about challenges the study team might encounter in implementing a potential intervention, strategies for navigating challenges, and immediate interventions to begin to implement were described. Demographic characteristics of participants were summarized using frequencies and percentages in R (version 4.2.1, Vienna, Austria), a software for quantitative analyses.
Demographic characteristics of participants are shown in Table 1 . There were 9–15 participants per workshop. The second workshop included some of the persons who had participated in the first workshop (5 in IN, 7 in NC) and new participants (5 in IN, 6 in NC). Participants described themselves as parents, grandparents, child-care providers, representatives of community organizations (e.g., healthcare, business, government, youth service), or a combination of those roles. Participants were predominantly female, with more racial diversity in NC compared to IN.
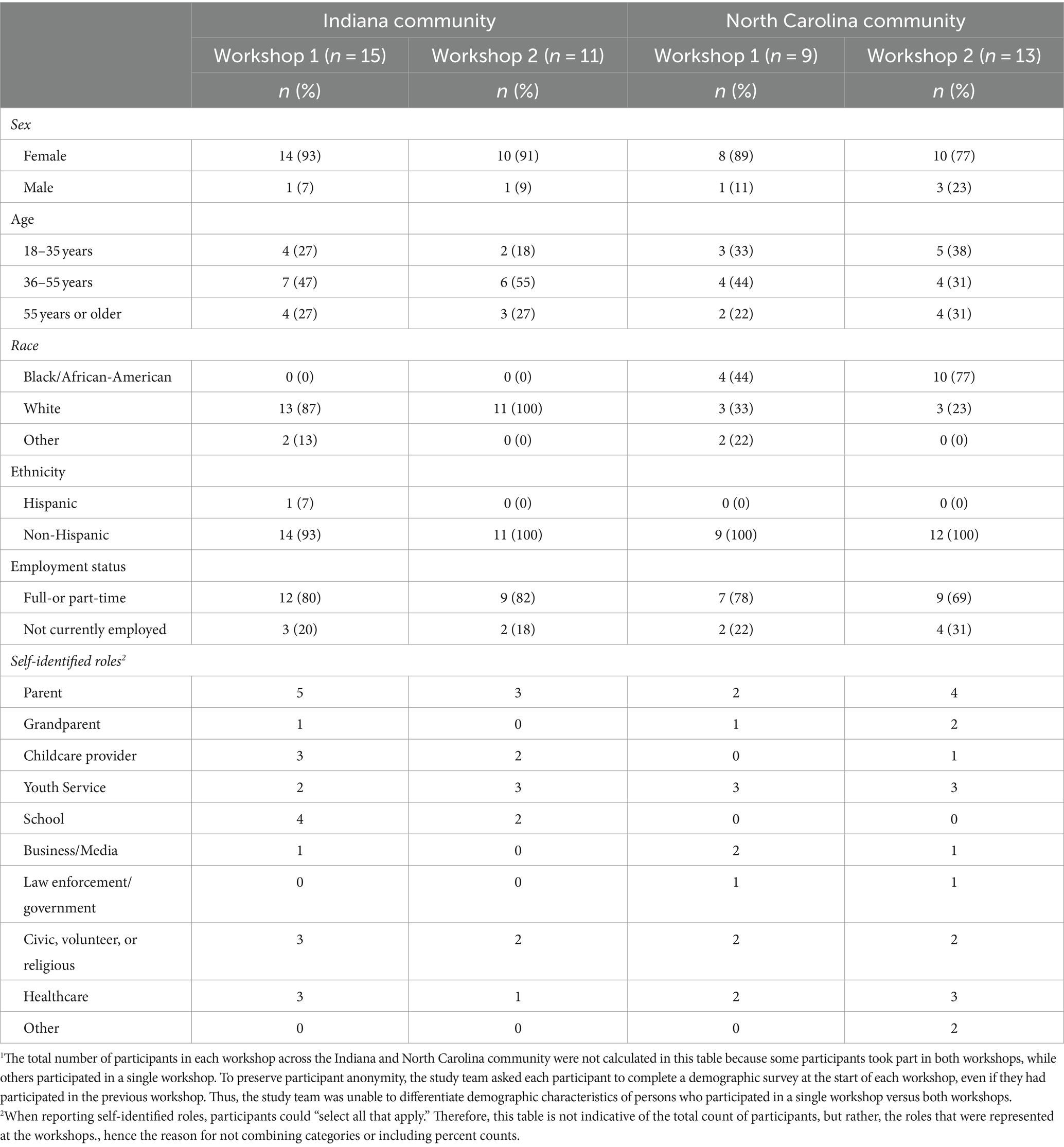
Table 1 . Demographic characteristics of participants in community workshops conducted in two rural communities in Indiana and North Carolina 1 .
Participants listed examples of factors that influence the choice to engage in the target dietary and PA behaviors but did not prioritize or rank factors in order of importance ( Figure 1 ). At the child level, these included child preference for unhealthy foods, peer influence, lack of interest in outdoor play, and preference for electronic media. A participant said: “ I really want her [child] to eat healthy, but at the same time, I want her to eat. She’s literally… her food choices, she will eat chicken nuggets. She will eat French fries. She will eat ramen, carrots, and grapes. That’s it.” Familial and peer influences included parental perception that healthy foods are expensive with short shelf-life, limited knowledge about how to obtain, prepare or preserve fruits/vegetables, and lack of time to prepare nutritious meals and/or be active with children. A participant described: “I think it’s the balance, but I’m really blessed to have the life where I can do this. We are very intentional when we eat at home. Everything is healthy at home…when we go to grandparents, that’s kind of the time for the treat… Not everyone has that option.” Organizational factors centered around perceptions that unhealthy foods were easily accessible through community organizations (e.g., schools, churches).
Community influences included lack of access to outlets that carry healthy foods (supermarkets) and resources that promote PA (parks), marketing of unhealthy foods to children, outdoor weather, and concern about child safety while playing outdoors. Describing the lack of access to PA resources, a participant said: “ One really sad thing that happened with the little kid basketball program was they used to have a preschool and kindergarten little boys’ basketball, biddy ball. Then this year, because it had to get serious, you had to try out as a first grader. Kindergarten and preschool was dropped.” Additionally, lack of policy to support healthy eating and PA was cited as a challenge. Describing this, a participant said: I do think there’s a role for the community as a whole. And that would be the government to make the rules of how we are exposed, to make our decisions… If we recognize there’s a problem, there’s only one way that we can step forward as a community to do that: to set some principles and rules that guide us in that.”
Community settings that participants ranked highest as the top places to promote healthy dietary intake and PA in children are described in Table 2 . For the promotion of healthy dietary intake, settings that overlapped between the IN and NC communities included educational settings (e.g., childcare centers), food outlets (e.g., grocery stores), youth sports, community gathering places (e.g., churches), and social media. Also, participants prioritized the home (NC), recreation facilities (IN), and other locations (e.g., community events) (NC) as settings to promote healthy dietary intake in their communities. In terms of promoting PA, settings that overlapped across both communities were educational and recreation facilities (e.g., parks, trails). Additional settings for promoting PA that participants prioritized were the home (NC), social media (IN), and community gathering places (IN).
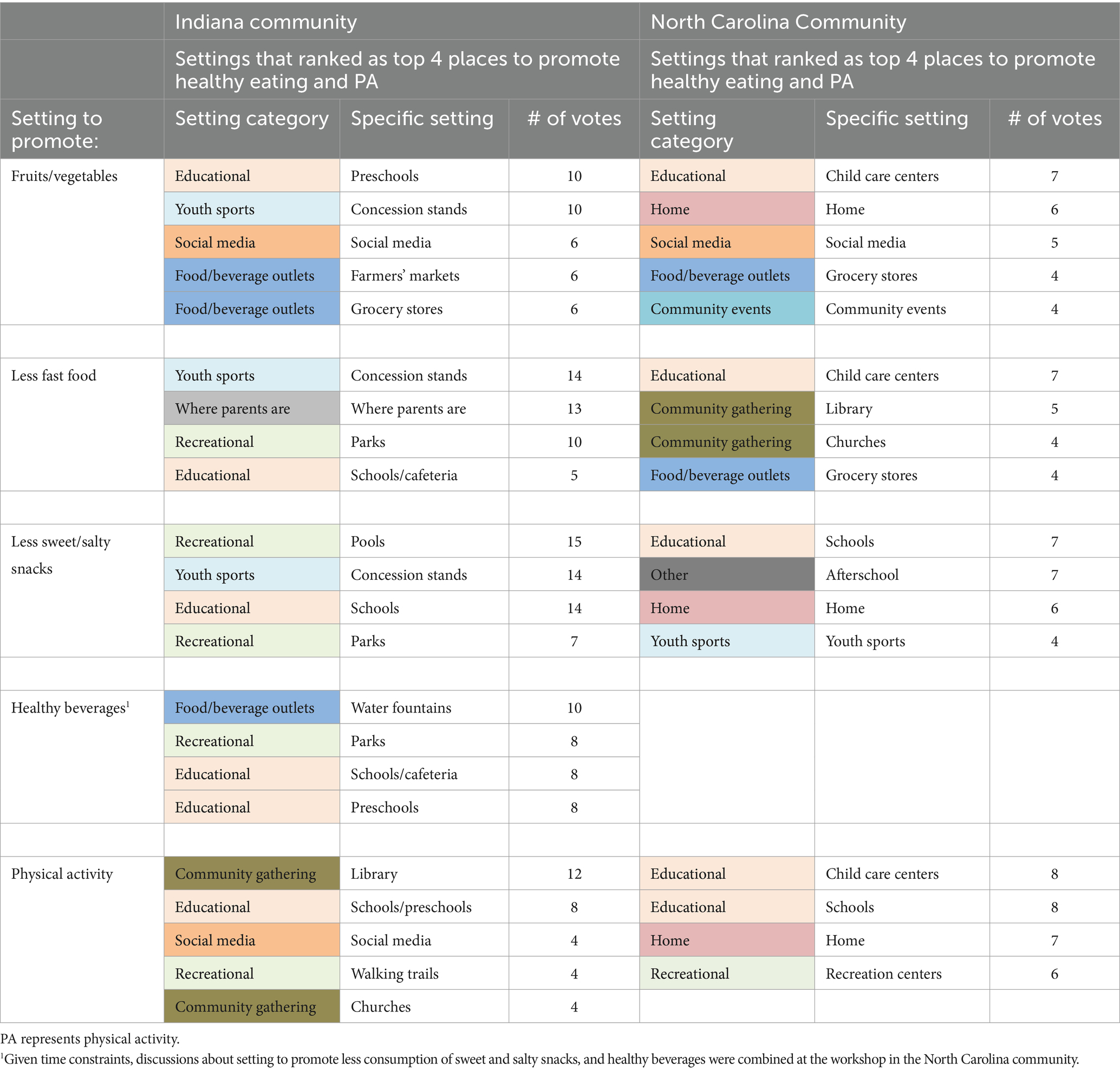
Table 2 . Community settings that workshop participants in the two rural communities in Indiana and North Carolina ranked highest for the promotion of healthy dietary intake and physical activity in children aged 2–5 years.
Intervention strategies that participants ranked highest are shown in Table 3 . Strategies for promoting healthy dietary intake overlapped between the two communities, focusing on: providing nutrition education opportunities (e.g., nutrition education for parents, fruit and vegetable gardening with children at preschools); enhancing access to healthy foods in the built environment (e.g., via community gardens); and enhancing food security through access to food programs (e.g., backpack buddy programs at childcare settings to provide children from food-insecure households with take-home meals). Partnerships with community organizations to increase healthy food offerings in childcare settings were also recommended (NC). For PA promotion, an intervention strategy that overlapped between both communities centered on providing PA education opportunities for children/families (e.g., PA lessons at childcare, organized community events that promote PA). Additional strategies that participants prioritized included: providing enhanced access to PA-promoting resources in the built environment (e.g., adding game stencils to playgrounds) (IN); offering incentives (e.g., free passes to bounce houses) (IN); and leveraging community facilities and local organizations to offer PA to families (NC).
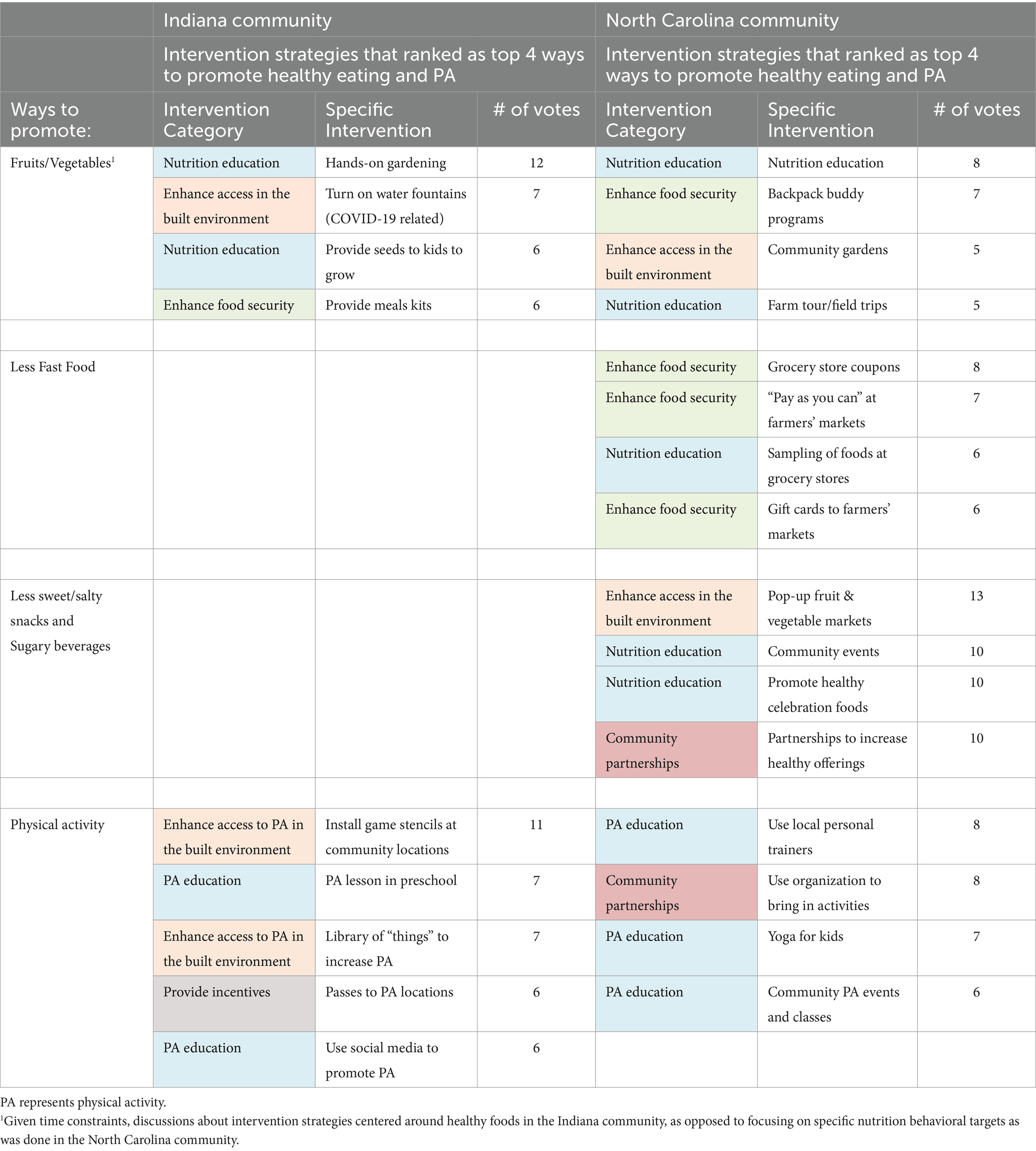
Table 3 . Intervention strategies that workshop participants in the two rural communities in Indiana and North Carolina ranked highest for the promotion of healthy dietary intake and physical activity in children aged 2–5 years.
Due to time constraints, discussions about challenges the study team might encounter in implementing an intervention and potential navigation strategies occurred only in NC. The major challenge that was discussed centered around low parental engagement in an intervention that might occur because of parents’ busy schedules (lack of time) and limitations with transportation given the community’s lack of a public transit system. Participants suggested using personalized invitations to enhance parental engagement. Organizing intervention activities to occur at community settings where parents typically spend time with children (e.g., childcare centers, parks) was also suggested. Another concern that was discussed centered on the transience of community partners and health initiatives that made it difficult to create sustainable health promotion programs, but no solutions were proffered.
Participants identified immediate interventions they could begin to implement with the study team with limited funding. In IN, the immediate intervention was to install game stencils at public playgrounds/parks to promote PA in children, whereas in NC it was to create a quarterly newsletter about healthy lifestyles to disseminate to families. Participants shared examples of local agencies [e.g., REMC Electric Company (IN), Triangle North Healthcare Foundation (NC)] from which grant funding could be sought to support the immediate interventions. Participants indicated willingness to continue to engage with the study team via quarterly meetings to advance the obesity prevention efforts identified from this study.
4 Discussion
This paper describes results from four workshops with community partners to guide the development of a rural multi-level community-based intervention to promote healthy weight in children aged 2–5 years. In the current study, participants described factors influencing the choice to engage in healthy weight behaviors in their community. They cited several factors at the child (e.g., child preference), familial/peer (e.g., financial and time constraints), organizational (e.g., limited access to healthy foods and PA opportunities through organizations), community (e.g., food deserts), and policy levels (lack of nutrition and PA-promoting policies). Participants’ responses about factors that influence the choice to eat healthy and be physically active were consistent with the initial formative research conducted by the current study team in both communities ( 26 ) and other studies of rural communities ( 4 , 7 , 37 ).
Discussions at the workshops were used to identify community settings to prioritize in a rural, obesity prevention intervention for children aged 2–5 years. Rural areas vary widely with regards to the availability of resources that can support healthy lifestyles ( 37 ) (e.g., supermarkets, recreation centers), but existing community-identified settings can serve as trusted, anchor organizations that can be leveraged in the implementation of community-based child obesity prevention interventions ( 37 , 38 ). Community settings that participants identified align with studies of children and adults that report social media, rural social networks (e.g., social or family gatherings) ( 10 , 11 , 18 , 37 ), food outlets ( 10 , 11 , 18 , 35 – 37 , 39 ), and shared community spaces (e.g., schools, faith-based/civic organizations) ( 10 , 11 , 13 , 18 , 37 , 40 ) as natural settings to reach and engage with rural children/families. Notably, representatives of healthcare organizations were present at the workshops and discussions about services/programs available at healthcare settings in the community occurred, however, participants did not prioritize healthcare settings as places to reach or intervene with families.
Intervention strategies identified by study participants can be implemented across several of the settings they prioritized. Providing nutrition and PA education opportunities to children/families and offering incentives to promote healthy lifestyles were recommended by participants. Given the paucity of nutrition and PA resources in most rural areas ( 26 ), it not a surprise that participants recommended the need to increase access to healthy foods and PA-promoting resources in their community’s built environment. With many rural areas’ high levels of food insecurity ( 41 ), it is also not a surprise that participants recommended enhancing food security in their community through access to food programs. Going forward, the goal of the study team is to work collaboratively with community partners to develop a multi-level intervention that incorporates the community-identified priorities for obesity prevention for children aged 2–5 years, and then seek grant funding to pilot-test the intervention.
Using a community-engaged approach, as was done in the current study, helps researchers build trust with partners in rural communities ( 18 , 37 , 42 , 43 ) and allows researchers and community partners to work together in a collaborative manner to design child obesity prevention interventions that are culturally-appropriate, relevant, and acceptable to communities ( 18 , 34 , 42 ). This community-engaged approach is crucial for creating community-based interventions that are likely to be impactful and sustainable in the long-term ( 18 , 38 , 44 ).
At the workshops, participants discussed the installation of game stencils at public playgrounds/parks to promote PA in children (IN) and the dissemination of a quarterly newsletter about healthy lifestyles to families (NC) as immediate interventions that could be implemented with limited funding. To implement these, in IN, the study team collaborated with a community partner (Greene County Foundation, IN) to apply for two small grants that were awarded in the fall of 2022 by the South Center Indiana REMC and the Bloomington Board of Realtors. Using the grant funds, the study team and community partners have painted playground stencils for use by children at three public libraries and two childcare centers. For NC, the study team is working with community partners to develop a series of electronic newsletters, the first of which was shared with community partners in the spring of 2023 to distribute to families served through their respective organizations’ communication channels.
This study has some limitations. Because rural areas differ with regards to resources available to promote healthy weight behaviors, the findings of this study may not be generalizable to all rural communities. While the study team spread the word about the workshops throughout the communities, it is possible that the sample was biased toward persons most interested/passionate in promoting health in their community. Additionally, workshops occurred in the main townships of both counties, thus, excluding participation by interested community members without access to a means of transportation. Childcare support was not provided at the workshops, limiting attendance by parents who could not afford or find childcare. Nevertheless, a strength of this study is the sizable number of participants ( 9 – 15 ) with varied demographic characteristics that allowed for the inclusion of diverse perspectives at the workshops. Additionally, the use of a participatory approach in which community partners and the study team collaboratively identified community priorities for preventing obesity in children aged 2–5 years is a strength.
Results from each workshop were summarized and shared with participants and other community partners via a factsheet. The study team will use the results to work collaboratively with community partners to develop a rural multi-level community-based obesity prevention intervention for children aged 2–5 years.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Human Research Protection Program (HRPP) Office for Research Compliance Indiana University Bloomington. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KP: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. AL: Writing – original draft, Writing – review & editing, Data curation, Conceptualization. LH: Writing – review & editing, Writing – original draft, Conceptualization. DG: Writing – review & editing, Writing – original draft, Conceptualization. JG: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. DW: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. TH: Writing – review & editing, Writing – original draft. TE: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by funds from the National Institutes of Health (R03HD097393), Indiana University Bloomington School of Public Health, and North Carolina Translational and Clinical Sciences Institute (NC TraCS) at the University of North Carolina at Chapel Hill.
Acknowledgments
The authors thank: community partners in Indiana and North Carolina who helped to facilitate the successful completion of this study; and study participants for the valuable insight shared.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1372890/full#supplementary-material
1. Hu, K, and Staiano, AE. Trends in obesity prevalence among children and adolescents aged 2 to 19 years in the US from 2011 to 2020. JAMA Pediatr . (2022) 176:1037–9. doi: 10.1001/jamapediatrics.2022.2052
PubMed Abstract | Crossref Full Text | Google Scholar
2. Johnson, JA 3rd, and Johnson, AM. Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Child Obes . (2015) 11:233–41. doi: 10.1089/chi.2014.0085
3. Pandita, A, Sharma, D, Pandita, D, Pawar, S, Tariq, M, and Kaul, A. Childhood obesity: prevention is better than cure. Diab Metab Syndr Obes . (2016) 9:83–9. doi: 10.2147/DMSO.S90783
4. Kumanyika, S, and Grier, S. Targeting interventions for ethnic minority and low-income populations. Futur Child . (2006) 16:187–07. doi: 10.1353/foc.2006.0005
Crossref Full Text | Google Scholar
5. Trivedi, T, Liu, J, Probst, J, Merchant, A, Jhones, S, and Martin, AB. Obesity and obesity-related behaviors among rural and urban adults in the USA. Rural Remote Health . (2015) 15:3267. doi: 10.22605/RRH3267
6. Sahoo, K, Sahoo, B, Choudhury, AK, Sofi, NY, Kumar, R, and Bhadoria, AS. Childhood obesity: causes and consequences. J Family Med Prim Care . (2015) 4:187–92. doi: 10.4103/2249-4863.154628
7. Scaglioni, S, Arrizza, C, Vecchi, F, and Tedeschi, S. Determinants of children’s eating behavior. Am J Clin Nutr . (2011) 94:S2006–11. doi: 10.3945/ajcn.110.001685
8. Ling, J, Robbins, LB, and Wen, F. Interventions to prevent and manage overweight or obesity in preschool children: a systematic review. Int J Nurs Stud . (2016) 53:270–89. doi: 10.1016/j.ijnurstu.2015.10.017
9. Glanz, K, Rimer, BK, and Viswanath, K. Health behavior and health education: Theory, research, and practice. San Francisco, CA: John Wiley & Sons (2008).
Google Scholar
10. Novotny, R, Davis, J, Butel, J, Boushey, CJ, Fialkowski, MK, Nigg, CR, et al. Effect of the Children's healthy living program on young child overweight, obesity, and acanthosis Nigricans in the US-Affiliated Pacific region: A randomized clinical trial. JAMA Netw Open . (2018) 1:e183896. doi: 10.1001/jamanetworkopen.2018.3896
11. Economos, CD, Hyatt, RR, Must, A, Goldberg, JP, Kuder, J, Naumova, EN, et al. Shape up Somerville two-year results: a community-based environmental change intervention sustains weight reduction in children. Prev Med . (2013) 57:322–7. doi: 10.1016/j.ypmed.2013.06.001
12. Woo Baidal, JA, Nelson, CC, Perkins, M, Colchamiro, R, Leung-Strle, P, Kwass, JA, et al. Childhood obesity prevention in the women, infants, and children program: outcomes of the MA-CORD study. Obesity (Silver Spring) . (2017) 25:1167–74. doi: 10.1002/oby.21865
13. Chomitz, VR, McGowan, RJ, Wendel, JM, Williams, SA, Cabral, HJ, King, SE, et al. Healthy living Cambridge kids: a community-based participatory effort to promote healthy weight and fitness. Obesity (Silver Spring) . (2010) 18:S45–53.
14. Novotny, R, Yamanaka, AB, Butel, J, Boushey, CJ, Dela Cruz, R, Aflague, T, et al. Maintenance outcomes of the Children's healthy living program on overweight, obesity, and acanthosis Nigricans among young children in the US-Affiliated Pacific region: A randomized clinical trial. JAMA Netw Open . (2022) 5:e2214802. doi: 10.1001/jamanetworkopen.2022.14802
15. de Silva-Sanigorski, AM, Bell, AC, Kremer, P, Nichols, M, Crellin, M, Smith, M, et al. Reducing obesity in early childhood: results from Romp & Chomp, an Australian community-wide intervention program. Am J Clin Nutr . (2010) 91:831–40. doi: 10.3945/ajcn.2009.28826
16. Wolfenden, L, Wyse, R, Nichols, M, Allender, S, Millar, L, and McElduff, P. A systematic review and meta-analysis of whole of community interventions to prevent excessive population weight gain. Prev Med . (2014) 62:193–00. doi: 10.1016/j.ypmed.2014.01.031
17. Ewart-Pierce, E, Mejia Ruiz, MJ, and Gittelsohn, J. "whole-of-community" obesity prevention: A review of challenges and opportunities in multilevel multicomponent interventions. Curr Obes Rep . (2016) 5:361–74. doi: 10.1007/s13679-016-0226-7
18. Gittelsohn, J, Novotny, R, Trude, ACB, Butel, J, and Mikkelsen, BE. Challenges and lessons learned from multi-level multi-component interventions to prevent and reduce childhood obesity. Int J Environ Res Public Health . (2018) 16. doi: 10.3390/ijerph16010030
19. Trickett, EJ, Beehler, S, Deutsch, C, Green, LW, Hawe, P, McLeroy, K, et al. Advancing the science of community-level interventions. Am J Public Health . (2011) 101:1410–9. doi: 10.2105/AJPH.2010.300113
20. Ko, LK, Rillamas-Sun, E, Bishop, S, Cisneros, O, Holte, S, and Thompson, B. Together we STRIDE: A quasi-experimental trial testing the effectiveness of a multi-level obesity intervention for Hispanic children in rural communities. Contemp Clin Trials . (2018) 67:81–6. doi: 10.1016/j.cct.2018.02.013
21. Rillamas-Sun, E, Bishop, S, Cisneros, O, Mendoza, JA, Kratz, M, and Ko, LK. Psychosocial factors of diet and physical activity among rural, Hispanic children: findings from a multilevel health intervention study. J Racial Ethn Health Disparities . (2019) 6:1218–27. doi: 10.1007/s40615-019-00623-7
22. Curtin, L, and Crohn, TJ. Overview of rural communities: contexts, challenges and resilience (2015). Available at: https://www.apa.org/pi/aids/resources/exchange/2015/01/rural-communities (Accessed January 4, 2022).
23. Cromartie, J, von Reichert, C, and Arthun, R. Why some return home to rural American and why it matters (2015). Available at: https://www.ers.usda.gov/amber-waves/2015/july/why-some-return-home-to-rural-america-and-why-it-matters/ (Accessed January 4, 2022).
24. Meit, M, and Knudson, A. Leveraging rural strengths to overcome population health challenges. Am J Public Health . (2020) 110:1281–2. doi: 10.2105/AJPH.2020.305641
25. Louison, L, and Fleming, O. Context matters: Recommendations for Funders & Program Developers Supporting Implementation in rural communities . Chapel Hill: Frank Porter Graham Child Development Institute, University of North Carolina Chapel Hill (2016).
26. Pope, KJ, Whitcomb, C, Vu, M, Harrison, LM, Gittelsohn, J, Ward, D, et al. Barriers, facilitators, and opportunities to promote healthy weight behaviors among preschool-aged children in two rural US communities. BMC Public Health . (2023) 23:53. doi: 10.1186/s12889-022-14770-w
27. U.S. Department of Agriculture. (2024). Rural-Urban Continuum Codes, January.
28. University of Wisconsin Population Health Institute. County health rankings and roadmaps . Indiana: Robert Wood Johnson Foundation (2024).
29. University of Wisconsin Population Health Institute. County health rankings and roadmaps . North Carolina: Robert Wood Johnson Foundation (2024).
30. Granville Vance Public Health. 2018 Community health assessment (2018).
31. U.S. Census Bureau. Greene County, Indiana Quickfacts (2021).
32. U.S. Census Bureau. Vance County, North Carolina Quickfacts (2021). Available at: https://www.census.gov/quickfacts/fact/table/vancecountynorthcarolina/POP060210 (Accessed 2022 September 2019).
33. Vastine, A, Gittelsohn, J, Ethelbah, B, Anliker, J, and Caballero, B. Formative research and stakeholder participation in intervention development. Am J Health Behav . (2005) 29:57–69. doi: 10.5993/AJHB.29.1.5
34. Gittelsohn, J, Roache, C, Kratzmann, M, Reid, R, Ogina, J, and Sharma, S. Participatory research for chronic disease prevention in Inuit communities. Am J Health Behav . (2010) 34:453–64. doi: 10.5993/AJHB.34.4.7
35. Taveras, EM, Perkins, M, Anand, S, Woo Baidal, JA, Nelson, CC, Kamdar, N, et al. Clinical effectiveness of the Massachusetts childhood obesity research demonstration initiative among low-income children. Obesity (Silver Spring) . (2017) 25:1159–66. doi: 10.1002/oby.21866
36. French, SA, Sherwood, NE, Veblen-Mortenson, S, Crain, AL, JaKa, MM, Mitchell, NR, et al. Multicomponent obesity prevention intervention in low-income preschoolers: primary and subgroup analyses of the NET-works randomized clinical trial, 2012-2017. Am J Public Health . (2018) 108:1695–06. doi: 10.2105/AJPH.2018.304696
37. Walsh Center for Rural Health Analysis. Exploring strategies to improve health and equity in rural communities . Bethesda, Maryland: University of Chicago (2018).
38. Center for Community Health and Development. Chapter 3, section 8: Identifying community assets and resources (n.d.). Available at: https://ctb.ku.edu/en/table-of-contents/assessment/assessing-community-needs-and-resources/identify-community-assets/main .
39. Robinson, TN, Matheson, D, Desai, M, Wilson, DM, Weintraub, DL, Haskell, WL, et al. Family, community and clinic collaboration to treat overweight and obese children: Stanford GOALS-A randomized controlled trial of a three-year, multi-component, multi-level, multi-setting intervention. Contemp Clin Trials . (2013) 36:421–35. doi: 10.1016/j.cct.2013.09.001
40. Umstattd Meyer, MR, Perry, CK, Sumrall, JC, Patterson, MS, Walsh, SM, Clendennen, SC, et al. Physical activity-related policy and environmental strategies to prevent obesity in rural communities: A systematic review of the literature, 2002-2013. Prev Chronic Dis . (2016) 13:E03.
41. Coleman-Jensen, A, Rabbitt, M, Gregory, C, and Singh, A. Household food security in the United States in 2015 . (2016).
42. Summers, A, Confair, AR, Flamm, L, Goheer, A, Graham, K, Muindi, M, et al. Designing the healthy bodies, healthy souls church-based diabetes prevention program through a participatory process. Am J Health Educ . (2013) 44:53–66. doi: 10.1080/19325037.2013.764245
43. Bolton, KA, Fraser, P, Lowe, J, Moodie, M, Bell, C, Strugnell, C, et al. Generating change through collective impact and systems science for childhood obesity prevention: the GenR8 change case study. PLoS One . (2022) 17:e0266654. doi: 10.1371/journal.pone.0266654
44. Mikkelsen, BE, Novotny, R, and Gittelsohn, J. Multi-level, multi-component approaches to community based interventions for healthy living-A three case comparison. Int J Environ Res Public Health . (2016) 13. doi: 10.3390/ijerph13101023
Keywords: childhood obesity, multi-level interventions, community engagement, rural, preschool-aged children
Citation: Pope KJ, Lightfoot AF, Harrison LM, Getz D, Gittelsohn J, Ward D, Hannon TS and Erinosho T (2024) Listening to the community: identifying obesity prevention strategies for rural preschool-aged children. Front. Public Health . 12:1372890. doi: 10.3389/fpubh.2024.1372890
Received: 18 January 2024; Accepted: 13 May 2024; Published: 31 May 2024.
Reviewed by:
Copyright © 2024 Pope, Lightfoot, Harrison, Getz, Gittelsohn, Ward, Hannon and Erinosho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Temitope Erinosho, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Community-level interventions: case studies
Resources Policy Dossiers Community-level interventions Community-level interventions: case studies
- Sugar-Sweetened Beverage Tax
- Digital Marketing
- School-based interventions
- Community-level interventions
- Pregnancy & Obesity
- Childhood Obesity Treatment
- Front-of-pack nutrition labelling
- Obesity & COVID-19
- Physical Activity
- Food Systems
- Weight Stigma
Tackling obesity: What the UK can learn from other countries
This report reviews a number of policy measures and programmes implemented by national and local governments outside of the UK to address the rising prevalence of overweight and obesity.
Developing a framework for estimating the potential impact of obesity interventions in a European city
This article tries to identify the potential comparative cost-effective of interventions at a city-level to reduce BMI and the amount of investment in different types of interventions.
Obesity prevention in a City State: Lessons from New York City during the Bloomberg Administration
This paper examines the experiences of many of the key decision makers and others involved in undertaking this work in New York City to identify the key components required to introduce and sustain the government’s reforms to address the obesogenic environment.
What can be learned from the Amsterdam Healthy Weight programme to inform the policy response to obesity in England?
This report is the response to a request made to OPRU on 16 November 2017 by the Department of Health to answer the question: "What can be learned from the Amsterdam Healthy Weight programme to inform the policy response to obesity in England?"
EPODE approach for childhood obesity prevention: methods, progress and international development
The aim of this paper is to provide a detailed description of Ensemble Prévenons l’Obsésité des Enfants (EPODE) methodology.
Community-based interventions to reduce overweight and obesity in China: a systematic review of the Chinese and English literature
This systematic review describes existing literature in Chinese journals and identifies effective components from interventions with anthropometric measures as outcomes.
A Statewide Strategy to Battle Child Obesity in Delaware
This paper aims to provide an overview of the 5-2-1-Almost None prevention initiative developed by Nemours and implemented in Delaware.
Improving weight status in childhood: results from the eat well be active Community Programs
The primary objective of this study is to evaluate the effectiveness of eat well be active intervention, programmes which aim to contribute to the healthy weight of children and young people and their families in chosen communities.
Shape Up Somerville two-year results: A community-based environmental change intervention sustains weight reduction in children
This paper looks at the two-year results of the Shape Up Somerville intervention when the implementation and responsibility were institutionalised into the community.
Shaping Up Somerville: A community initiative in Massachusetts
This short piece presents the Shape Up Somerville intervention and identifies some of the critical factors that led to its success.
Economic Evaluation of a Community-based Obesity Prevention Program in Children: The APPLE Project
The aim of this study was to assess the costs of implementing APPLE in relation to effects on weight, including any benefits in health-related quality of life terms.
Obesity Prevention: A Systematic Review of Setting-Based Interventions from Nordic Countries and the Netherlands
The aim of this review was to identify, synthesise and evaluate the quality of interventions aimed at preventing obesity in different settings from Nordic countries and the Netherlands.
Community-based pilot intervention to tackle childhood obesity: a whole system approach
The aim of this paper is to describe the methodology and the practical steps in developing the Go-Golborne programme.
Process evaluation of an up-scaled community based child obesity treatment program: NSW Go4Fun®
This paper “describes the up-scaling of Go4Fun in New South Wales and the characteristics of the population it has reached and retained since inception in 2009, including characteristics of children who completed and did not complete the programme.”
Assessing the short-term outcomes of a community-based intervention for overweight and obese children: the MEND 5-7 programme
The aim of this study was "to report outcomes from the UK service level delivery of MEND 5-7."
Putting Health into Place: Introducing NHS England's Healthy New Towns programme
Established by NHS England, the Healthy New Towns is "a three-year programme, to look at how health and wellbeing can be planned and designed into new places."
Pop up Parks
Pop up Parks is "a global movement that supports, shares and implements projects that invite people to be more playful and creative in urban environments."
The intervention of childhood obesity and adolescents in Shantou city
In this study, the effects of a multicomponent, school-based intervention for childhood obesity in Shantou city was analysed.
Reducing unhealthy weight gain in Fijian adolescents: results of the Healthy Youth Healthy Communities study
The Healthy Youth Healthy Communities study (HYHC) study, which is part of the Pacific Obesity Prevention in Communities project, is a three-year obesity prevention intervention on Fijian children and adolescents.
A Community Intervention Reduces BMI z-score in children: Shape Up Somerville First Year Results
Shape up Somerville (SUS): Eat Smart, Play Hard, is 'one of the first collaborative community-based participatory research initiatives designed to change the environment to prevent obesity in early elementary school-age children'.
The Impact of a Multi-Level Multi-Component Childhood Obesity Prevention Intervention on Healthy Food Availability, Sales and Purchasing in a Low-Income Urban Area
In this community-based intervention for childhood overweight and obesity prevention, multilevel multicomponent strategies were implemented to slow weight gain in children living in a low-income food desert.
Effective multi-level, multi-sector, school-based obesity prevention programming improves the weight, blood pressure, and academic performance especially among low-income, minority children
In order to address the ongoing childhood obesity epidemic seen across communities worldwide, researchers instituted the Healthier Options for Public Schoolchildren (HOPS)/The OrganWise Guys (OWG) study.
Interventions for preventing obesity in children
The goal of this article was to 'update the evidence base for children given the exponential growth of studies in this field [and] ensure that the review remains current and practice ]relevant, with particular regard for health equity'.
E ffect of a Culturally Adapted Behavioral Intervention for Latino Adults on Weight Loss Over 2 Years
One way to intervene in the Latino community to reduce levels of obesity is technology-mediated weight-loss strategies. This two-year behavioural intervention used technology to reach a wide range of the Latino community.
Tackling childhood obesity in the community using a participatory action research project with local children and young people.
Health professionals in White City (London, UK) 'hypothesised that a programme to signpost activities in the area and motivate individuals to take these up would improve behaviour and self-esteem'.
The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease, or diabetes
In this obesity intervention, the effectiveness of a community-based WFPB dietary programme was measured in a population of New Zealanders.
Downward trends in the prevalence of childhood overweight in the setting of 12-year school-and community-based programmes
In 1992, a nutritional programme was instituted in schools in two towns in northern France, with other community-based interventions after. This study reviews the outcomes of the study following the children for the next 12 years, compared to children in two similar non-intervention schools.
Relationship between primary school healthy eating and physical activity promoting environments and children's dietary intake, physical activity and weight status: a longitudinal study in the West Midlands UK
State primary schools in the West Midlands (UK) implemented a childhood obesity prevention trial from 2011-2015. WAVES included children from diverse ethnic backgrounds within the participating schools.
A Cooking Intervention to Increase Vegetable Consumption by Parents With Children Enrolled in an Early Head Start Home Visiting Program: A Pilot Study in Portland, Oregon, 2013-2014
In Portland, Oregon, The Eart Head Start Home Visiting program was implemented to improve 'confidence in cooking vegetables among low-income parents with children aged 0-3 years' and increase vegetable consumption.
Testing the feasibility of a sustainable preschool obesity prevention approach: a mixed-methods service evaluation of a volunteer-led HENRY programme
The UK charity HENRY has been providing evidence-based behaviour change programmes to parents across the UK to reduce childhood obesity. They intervene at a parental level to help parents achieve healthier outcomes for themselves and their children.
Reducing obesity in early childhood: results from Romp & Chomp, an Australian community-wide intervention program
The multi-setting, multi-strategy intervention in the city of Geelong took place from 2004 to 2008 and used community capacity building and environmental changes to 'increase healthy eating and active play in early childhood care and educational settings'.
Randomised Controlled Trial of the MEND Program: A Family-based Community Intervention for Childhood Obesity
The physical and physiological impacts of obesity on a person are well documented. In order to address paediatric obesity in London, The Mind, Exercise, Nutrition, Do It (MEND) community-based programme was implemented on a group of 54 8-12-year-olds.

Systematic reviews

WOF introduction
Share this page.
Training & Events
SCOPE E-Learning
We offer the only internationally recognised course on obesity management. Read more here.
Global Obesity Observatory
We offer various statistics, maps and key data around the topic of obesity. You can find all that and more here.
Policy & Advocacy
Our Policy Priorities
We have developed five key areas of policy that are a priority to us. Want to know more? Check them out here!
- Our Members
- Member Benefits
- Membership Application Form
- Partnerships
- Global Obesity Forum
- Patient Portal
- Finance Committee
- Annual Report and Financials
- Prevalence of Obesity
- Causes of Obesity
- Obesity Classification
- Prevention of Obesity
- Obesity as a disease
- Commercial determinants of obesity
- Childhood Obesity
- Obesity in Universal Health Coverage
- The ROOTS of Obesity
- World Obesity Day
- Healthy Venues
- Reinventing the Food System: A Report
- The Spotlight Project
- SCOPE Examination
- Guide to SCOPE Certification
- SCOPE Fellows
- Leadership Programme
- Accreditation
- SCOPE Schools
- SCOPE Pricing
- SCOPE Sessions
- SCOPE Accredited Events
- Event Archive
- International Congress on Obesity
Sign up for notifications
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Glob Pediatr Health
Childhood and Adolescent Obesity in the United States: A Public Health Concern
Adekunle sanyaolu.
1 Federal Ministry of Health, Abuja, Nigeria
Chuku Okorie
2 Essex County College, Newark, NJ, USA
3 Saint James School of Medicine, Anguilla, British West Indies
Jennifer Locke
Saif rehman.
Childhood and adolescent obesity have reached epidemic levels in the United States. Currently, about 17% of US children are presenting with obesity. Obesity can affect all aspects of the children including their psychological as well as cardiovascular health; also, their overall physical health is affected. The association between obesity and other conditions makes it a public health concern for children and adolescents. Due to the increase in the prevalence of obesity among children, a variety of research studies have been conducted to discover what associations and risk factors increase the probability that a child will present with obesity. While a complete picture of all the risk factors associated with obesity remains elusive, the combination of diet, exercise, physiological factors, and psychological factors is important in the control and prevention of childhood obesity; thus, all researchers agree that prevention is the key strategy for controlling the current problem. Primary prevention methods are aimed at educating the child and family, as well as encouraging appropriate diet and exercise from a young age through adulthood, while secondary prevention is targeted at lessening the effect of childhood obesity to prevent the child from continuing the unhealthy habits and obesity into adulthood. A combination of both primary and secondary prevention is necessary to achieve the best results. This review article highlights the health implications including physiological and psychological factors comorbidities, as well as the epidemiology, risk factors, prevention, and control of childhood and adolescent obesity in the United States.
Introduction
Childhood and adolescent obesity have reached epidemic levels in the United States, affecting the lives of millions of people. In the past 3 decades, the prevalence of childhood obesity has more than doubled in children and tripled in adolescents. 1 The latest data from the National Health and Nutrition Examination Survey show that the prevalence of obesity among US children and adolescents was 18.5% in 2015-2016. Overall, the prevalence of obesity among adolescents (12-19 years; 20.6%) and school-aged children (6-11 years; 18.4%) was higher than among preschool-aged children (2-5 years; 13.9%). School-aged boys (20.4%) had a higher prevalence of obesity than preschool-aged boys (14.3%). Adolescent girls (20.9%) had a higher prevalence of obesity than preschool-aged girls (13.5%; Figure 1 ). 1 Moreover, the rates of obesity have been steadily rising from 1999-2000 through 2015-2016 ( Figure 2 ). 1 According to Ahmad et al, 80% of adolescents aged 10 to 14 years, 25% of children younger than the age of 5 years, and 50% of children aged 6 to 9 years with obesity are at risk of remaining adults with obesity. 2

Prevalence of obesity among children and adolescents aged 2 to 19 years, by sex and age: the United States, 2015-2016.

Trends in obesity prevalence among children and adolescents aged 2 to 19 years: the United States, 1999-2000 through 2015-2016.
Obesity can affect all aspects of children and adolescents including but not limited to their psychological health and cardiovascular health and also their overall physical health. 3 The association between obesity and morbid outcomes makes it a public health concern for children and adolescents. 4 Obesity has an enormous impact on both physical and psychological health. Consequently, it is associated with several comorbidity conditions such as hypertension, hyperlipidemia, diabetes, sleep apnea, poor self-esteem, and even serious forms of depression. 5 In addition, children with obesity who were followed-up to adulthood were much more likely to suffer from cardiovascular and digestive diseases. 3 The increase in body fat also exposes the children to increase in the risk of numerous forms of cancers, such as breast, colon, esophageal, kidney, and pancreatic cancers. 6
Due to its public health significance, the increasing trend in childhood obesity needs to be closely monitored. 7 However, these trends have proved to be challenging to quantify and compare. While there are many factors and areas to consider when discussing obesity in children and adolescents, there are a few trends that are evident in recent studies. For example, the prevalence of obesity varies among ethnic groups, age, sex, education levels, and socioeconomic status. A report published by the National Center for Health Statistics using data from the National Health and Nutrition Examination Survey provides the most recent national estimates from 2015 to 2016 on obesity prevalence by sex, age, race, and overall estimates from 1999-2000 through 2015-2016. 1 Prevalence of obesity among non-Hispanic black (22.0%) and Hispanic (25.8%) children and adolescents aged 2 to 19 years was higher than among both non-Hispanic white (14.1%) and non-Hispanic Asian (11.0%) children and adolescents. There were no significant differences in the prevalence of obesity between non-Hispanic white and non-Hispanic Asian children and adolescents or between non-Hispanic black and Hispanic children and adolescents. The pattern among girls was similar to the pattern in all children and adolescents. The prevalence of obesity was 25.1% in non-Hispanic black, 23.6% in Hispanic, 13.5% in non-Hispanic white, and 10.1% in non-Hispanic Asian girls. The pattern among boys was similar to the pattern in all children and adolescents except that Hispanic boys (28.0%) had a higher prevalence of obesity than non-Hispanic black boys (19.0%; Figure 3 ). 1 This review article is aimed at studying the health implications including physical and psychological factors and comorbidities, as well as the epidemiology, risk factors, prevention, and control of childhood and adolescent obesity in the United States.

Prevalence of obesity among children and adolescents aged 2 to 19 years, by sex and race and Hispanic origin: the United States, 2015-2016.
Methodology
We performed a literature search using online electronic databases (PubMed, MedlinePlus, Mendeley, Google Scholar, Research Gate, Global Health, and Scopus) using the keywords “childhood,” “adolescents,” “obesity,” “BMI,” and “overweight.” Articles were retrieved and selected based on relevance to the research question.
Ethical Approval and Informed Consent
Ethics approval and informed consent were not required for this narrative review.
Definition of Childhood Obesity
Defining obesity requires a suitable measurement of body fat and an appropriate cutoff range. 8 Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared, rounded to 1 decimal place. Obesity in children and adolescents was defined as a BMI of greater than or equal to the age- and sex-specific 95th percentile and overweight with a BMI between the 85th and 95th percentiles of the 2000 Centers for Disease Control and Prevention (CDC) growth charts. 9
However, the use of the BMI percentile according to the age/sex of the CDC growth charts for very high BMIs can result in estimates that differ substantially from those that are observed, 10 , 11 and this constrains the maximum BMI that is attainable at given sex and age. 12 , 13 These limitations have resulted in the classification of severe obesity as a BMI ≥120% of the 95th percentile rather than a percentile greater than the 95th percentile. 11 , 14 A BMI of 120% of the 95th percentile corresponds to a BMI of ~35 among 16 to 18 year olds.
Physiology of Energy Regulation and Obesity
Obesity is a chronic multifactorial disease, characterized by an excessive accumulation of adipose tissue, commonly as a result of excessive food intake and/or low energy expenditure. Obesity can be triggered by genetic, psychological, lifestyle, nutritional, environmental, and hormonal factors. 15
Obesity is found in individuals that are susceptible genetically and involves the biological defense of an elevated body fat mass, the mechanism of which could be explained in part by interactions between brain reward and homeostatic circuits, inflammatory signaling, accumulation of lipid metabolites, or other mechanisms that impair hypothalamic neurons. 16
Normal energy regulation physiology is under tight neurohormonal control. The neurohormonal control is performed in the central nervous system through neuroendocrine connections, in which circulating peripheral hormones, such as leptin and insulin, provide signals to specialized neurons of the hypothalamus reflecting body fat stores and induces appropriate responses to maintain the stability of these stores. The hypothalamic region is where the center of the regulation of hunger and satiety is located. Some of them target the activity of endogenous peptides, such as ghrelin, pancreatic polypeptide, 17 peptide YY, and neuropeptide Y, 18 as well as their receptors.
The physiology of energy regulation may result in obesity in susceptible people when it goes awry from genetic and environmental modulators. There is strong evidence of the majority of obesity cases that are associated with central resistance to both leptin and insulin actions. 19 , 20 The environmental modulators equally play critical roles in obesity. Changes in the circadian clock are associated with temporal alterations in feeding behavior and increased weight gain. 21 Stress interferes with cognitive processes such as executive function and self-regulation. Second, stress can affect behavior by inducing overeating and consumption of foods that are high in calories, fat, or sugar; by decreasing physical activity; and by shortening sleep. Third, stress triggers physiological changes in the hypothalamic-pituitary-adrenal axis, reward processing in the brain, and possibly the gut microbiome. Finally, stress can stimulate the production of biochemical hormones and peptides such as leptin, ghrelin, and neuropeptide Y. 17
The lateral hypothalamus (LH) plays a fundamental role in regulating feeding and reward-related behaviors; however, the contributions of neuronal subpopulations in the LH are yet to be identified thoroughly. 22 The LH has also been associated with other aspects of body weight regulation, such as physical activity and thermogenesis. 23 The LH contains a heterogeneous assembly of neuronal cell populations, in which γ-aminobutyric acid (GABA) neurons predominate. 23 LH GABA neurons are known to mediate multiple behaviors important for body weight regulation, thus altering energy expenditure. 23
Etiology and Risk Factors
Excess body fat is a major health concern in childhood and adolescent populations. The dramatic increase in childhood obesity foreshadows the serious health consequences of their adult life. As obesity begins from childhood and spans through adult life, it becomes increasingly more difficult to treat successfully. Being able to identify the risk factors and potential causes of childhood obesity is one of the best strategies for preventing the epidemic. 24
According to the Morbidity and Mortality Weekly Report released in 2011, there is an acceptance that there is no single cause of childhood obesity and that energy imbalance is just a part of the numerous factors. 25 Many children have a discrepancy between what is taken in and what is expended. 26 For example, children with obesity consume approximately 1000 calories more than what is necessary for their body to function healthily and to be able to participate in regular physical activities. Over 10 years, there will be an excess of 57 pounds of unnecessary weight. With excessive caloric intake, as well as sedentary lifestyles, childhood obesity will continue to rise if no changes are implemented. Adding daily physical activity, better sleep patterns, as well as dietary changes can help decrease the number of excess calories and help with obesity-related problems in the future.
Also, during childhood, excess fat accumulates when the increase in caloric intake exceeds the total energy expenditure. 26 Furthermore, children living in the United States today compared with children living in the 1900s are participating in more than 6 hours per day activities on social media. This includes but is not limited to traditional television, video gaming, and blogging/Facebook activities. An additional economic rationalization for the increase in childhood obesity is technology. In other words, Americans can now eat more in less time.
In a study, Cutler et al found that an increase in consumption of food tends to be related to technology innovation in food production and transportation. Technology has thus made it increasingly possible for firms to mass prepare food and ship to consumers for ready consumption, thereby taking advantage of scale economies in food preparation. The result of this change has been a significant reduction in the time costs for food production. These lower time costs have led to increased food consumption and, ultimately, increased weights. 27 Eliminating the time cost of food preparation disproportionately increases consumption for hyperbolic discounters because time delay is a particularly important mechanism for discouraging those individuals from consuming. 27 Society today prefers immediate satisfaction with regard to food and convenience over the long-term goals of living a long, healthy life. The availability of high-caloric, less-expensive food coupled with the extensive advertisement and easy accessibility of these foods has contributed immensely to the rising trend of obesity. 28 For example, there have been reductions in the price of McDonalds and Coca-Cola (5.44% and 34.89%, respectively) between 1990 and 2007, while there was about a 17% increase in the price of fruits and vegetables between 1997 and 2003. 29
Likewise, only 16% of children walk or bike to school today as compared with 42% in the late 1960s. However, the distance, convenience, weather, scanty sidewalks, and anxiety about crimes against children could all contribute to this difference. Furthermore, with elementary, middle, and high school combined, only 13.8% of these schools provide adequate daily physical education classes for at least 4 hours a week. 30
Some other potential risk factors have been reported through research studies that involve issues that affect the child in utero and childhood. Table 1 represents potential risk factors and confounders of childhood obesity. 31
Potential Risk Factors of Childhood Obesity.
Abbreviations: BMI, body mass index; SES, socioeconomic status.
Catalano et al argues that maternal BMI before conception, independent of maternal glucose status or birth weight, is a strong predictor of childhood obesity. 32 Infants at the highest quarter for weight at 8 and 18 months are more likely to become children with obesity at age 7, than children in the lower quarters. Certain behaviors have been linked to childhood obesity and overweight; these are a lack of physical activity and unhealthy eating patterns (eating more food away from home, drinking more sugar-sweetened drinks, and snacking more frequently), resulting in excess energy intake. 22 , 31 In addition, when one parent presents with obesity, there is an increased potential for the child to become obese over the years. Naturally, the risk is higher for the children when both parents present with obesity. Furthermore, a study that followed children over time observed that children who got less sleep <10.5 hours at age 3 were 45% more likely to be children with obesity at the age of 7, than children who got greater than 12 hours of sleep during their first 3 years of life. 33 , 34
While all the above-mentioned factors are informative, there is still the need for further research concerning childhood and adolescent obesity and obesity in general. Risk factors for obesity in childhood are still somewhat uncertain, and evidence-based research for preventative strategies is lacking. Moreover, effective action to prevent the childhood obesity epidemic requires evidence-based on early life risk factors, and this evidence, unfortunately, is still incomplete. Furthermore, a research study has attempted to capture the complete picture of childhood obesity early life course risk factors. In the study, they identified that parental BMI and gestational weight gain among other factors should be considered in prevention programs. 35
Health Effects of Childhood Obesity
Childhood obesity is known to have a significant impact on both physical and psychological health. Sahoo et al stated that “childhood obesity can profoundly affect children’s physical health, social and emotional well-being, as well as self-esteem.” They associated poor academic performance and a lower quality of life experienced by the child with childhood obesity. They also stated that “metabolic, cardiovascular, orthopedic, neurological, hepatic, pulmonary, and menstrual disorders among others are consequences of childhood obesity.” 36 There are many health consequences of childhood obesity, and three of the more common ones are sleep apnea, diabetes, and cardiovascular diseases. 36
Psychological Consequences of Obesity
Several studies related to childhood and adolescent obesity have focused primarily on physiological consequences. Other studies have been conducted regarding the association between psychiatric disorders and obesity; these have resulted in conflict due to obesity being found to be an insignificant factor for psychopathology. However, a comparative study by Britz et al found that high rates of mood, anxiety, somatoform, and eating disorders were detected among children with obesity. The study also observed that most psychiatric disorders began after the onset of obesity. In this large population-based study, it was found that a staggering 60% of females and 35% of males reported that they have engaged in binge eating and expressed a lack of control over their diet. 37
Goldfield et al conducted a study among 1400 adolescents with obesity, overweight, and normal weight in grades 7 to 12. Their BMIs, as determined by the International Obesity Task Force, were the criteria used to define each group. Each participant completed a questionnaire on body images, eating behaviors, and moods. Adolescents with obesity reported significantly higher body dissatisfaction, social isolation, depression symptoms, anhedonia, and negative self-esteem than those of normal weight. 38 There is widespread stigmatization of people with obesity that causes harm rather than the intention to motivate people to lose weight. Stigma contributes to behaviors such as binge eating, social isolation, avoidance of health care services, decreased physical activity, and increased weight gain, which worsens obesity and creates additional barriers to healthy behavior change. 39 Weight-based bullying in youth is considered a common, serious problem in many countries. 40 In a study conducted by O’Brien et al, to test whether the association between weight stigma experiences and disordered eating behaviors, that is, emotional eating, uncontrolled eating, and loss-of-control eating, are mediated by weight bias internalization and psychological distress among 634 undergraduate university students, and results of statistical analyses showed that weight stigma was significantly associated with all measures of disordered eating, and with weight bias internalization and psychological distress. 41
Asthma and Obesity
There is mounting evidence that childhood obesity is a risk factor for the development of asthma. 42 A research study was conducted by Belamarich et al to investigate 1322 children aged 4 to 9 years with asthma. Obesity, as defined by the CDC, is the BMI, with weight and height being greater than the 95th percentile. This was the criteria used to identify the 249 children with obesity, while the BMI between the 5th and 95th percentile identified the children who were not obese. After a baseline assessment was done, the 9-month study found that the children with obesity had a higher number of days of wheezing over 2 weeks (4.0 vs 3.4) and as well had more unscheduled emergency hospital visits (39% vs 31%). 42
Obesity directly correlates with the severity of asthma, as well as poor response to corticosteroids. 43 In fact, children with obesity who also have a history of asthma are more challenging to control and linked to worse quality of life. 44 A prospective trial found that weight loss in patients with obesity and a history of asthma can significantly aid them to control the asthma attacks. 43
Chronic Inflammation and Childhood Obesity
Lumeng and Saltiel reported that obesity in children affects multiple organ systems and predisposes them to diseases. The effect of obesity on the tissue can manifest in the development of insulin-resistant type 2 diabetes, the risk of cancer, and pulmonary diseases. 45
The inflammatory response to obesity triggers pathogens, systematic increases in circulatory inflammatory cytokines, and acute-phase reactants (eg, C-reactive proteins), which inflames the tissues. This is often caused by the activation of tissue leukocytes. Chronic inflammation in children with obesity can induce meta-inflammation that is unique when compared with other inflammatory paradigms (eg, infection, autoimmune diseases). 45 Researchers have reported that children with obesity are at risk of lifelong meta-inflammation. In these children, the inflammatory markers are elevated as early as in the third year of life. 45 , 46 This has been linked to heart disease later in life. 19 The long-term consequences of such findings can cause cumulative vascular damage that correlates with the increased weight status. 47
The short-term and long-term effects of obesity on the health of children is a significant concern because of the negative psychological and health consequences. 46 The potential negative psychological outcomes are depressive symptoms, poor body image, low self-esteem, a risk for eating disorders, and behavior and learning problems. Additional negative health consequences include insulin resistance, type 2 diabetes, asthma, hypertension, high total, and low-density lipoprotein cholesterol and triglyceride levels in the blood, low high-density lipoprotein cholesterol levels in the blood, sleep apnea, early puberty, orthopedic problems, and nonalcoholic steatohepatitis 46 , 47 ( Figure 4 ). Children with obesity are more likely to become adults with obesity, thus increasing their risk for several diseases before they even reach their teen years. 48

Comorbidities and potential health consequences of childhood obesity. 47
Prevention and Control
There are two primary components to the prevention and control of childhood obesity.
The first is to educate parents on proper nutritional requirements for their children and the second is to implement the learned information. Educating parents on proper nutrition and dietary caloric intake requirements for their children is at the forefront for the prevention of obesity; however, the way the information is disseminated may affect the usefulness of the information. For example, one of the main limitations to the education of parents about childhood obesity is that typically written information is used as the conduit to health information and disease prevention. 49 The Growing Right Onto Wellness (GROW) trial used a systematic assessment of patient education material that was used for the prevention of childhood obesity in the low health literate population. 49 Results suggest that the average readability is of grade 6 level (SMOG [Simple Measure of Gobbledygook] Index 5.63 ± 0.76 and Fry graph 6.0 ± 0.85) and that adjustment of education material must be done for low health literate populations to adequately comprehend educational material and maintain motivation on the prevention of childhood obesity. 49 A similar study was conducted to further support this improvement when using color-coordinated diagrams to help parents visualize instead of trying to comprehend with numbers and words. It proved to be successful as parents were able to see where they were going wrong and make the necessary changes in their children’s diet. 49
Similarly, the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development conducted a study on 744 adolescents and parents, and analyzed data to determine if parental (maternal and paternal, individually) reactions to children’s behavior was related to childhood obesity. 50 The study concluded that informing parents that their attitude toward their children’s behaviors will play a prominent role in preventing childhood obesity. 50 Parental education on nutrition, health, and the involvement of politicians, physicians, and school personnel are key for the prevention of childhood obesity. However, community and educational institutions have begun legislating and incorporating programs such as providing healthy foods at schools and also health information sessions directed toward young individuals, aimed at preventing childhood obesity in the United States and Canada. 51
Another effective prevention measure against childhood obesity is the awareness of parents on the meal and snack portion sizes. In a systematic review conducted on the effects of portion size manipulation with children and portion education/training interventions on dietary intake with parents, it was determined that the ability of adults to accurately estimate portion size improved following education/training. 52 Education of parents and children on diet requirements has its limitations in that the information must be easy to understand and be easily accessible in order to be practical. Making the available education materials easier to understand from just tables and numbers to more relatable aspects such as colors or figures, parents were able to visualize the changes they need to make whether it is with regard to portion sizes or even seeing how much childhood obesity is present in their family. Although much of the literature provided to parents is targeted to help those with lower numeracy skills, many parents benefited from the information being comparative from right/wrong and good/bad with regard to dieting. 49
The study recommended that proper educational materials, including useful and understandable literature, be used to control meal portion sizes and to help parents identify when children are at risk of obesity. Similarly, healthy eating practices should be taught by schools as a mandatory and essential method in the prevention of childhood obesity. 52
The implementation of healthy eating practices and adequate exercise regimes are essential in the prevention and control of childhood obesity. For example, information from systematic reviews, randomized controlled trials, and well-designed observational studies indicate that evidence-based prevention and control of childhood obesity can be accomplished with the collaboration of community/school, primary health care, and home-based/family-based interventions that involve both physical activity and dietary component. 53 In particular, the control of children with obesity is of significant value, as is the prevention of obesity. Two randomized control trials of 182 families were conducted from November 2005 to September 2007, and they studied the efficacy of US pediatric obesity treatment guidelines in children aged 4 to 9 years with a standardized BMI (ZBMI) greater than the 85 percentile. 54 Briefly, Trial 1 studied the impact on ZBMI by reducing snack foods and sugar-sweetened beverages and increasing fruits, vegetables, and low-fat dairy. 54 Trial 2 studied the impact on ZBMI by decreasing sugar-sweetened beverages and increasing physical activity and increasing low-fat milk consumption and reducing television watching. In Trial 1, the resulting ZBMI reduced within 6 months, and this was maintained through to the 12th month (ΔZBMI 0-12 months = −0.12 ± 0.22). 53 In Trial 2, the resulting ZBMI reduced within 6 months and continued to improve till the 12 months (ΔZBMI 0-12 months = −0.16 ± 0.31). 50
A similar cluster-randomized trial in England studied the effects of the reduction of carbonated beverages on the number of children with obesity in 29 classes (644 children). 51 Results indicate that a decrease of 0.6 glasses of carbonated drinks (250 mL) over three days per week decreased the number of children with obesity by 0.2%, while the control group increased by 7.5% (mean difference = 7.7%, 2.2% to 13.1%) at 12 months. However, diet control is only one component of the control and prevention of childhood obesity, while adequate exercise is another. 55
A systematic review and meta-analyses of the impact of diet and exercise programs (single or combined) was done on their effects on metabolic risk reduction in the pediatric population. 56 Analyses indicated that the addition of exercise to dietary intervention led to greater improvements in the levels of high-density lipoprotein cholesterol (3.86 mg/dL; 95% confidence interval [CI] = 2.70 to 4.63), fasting glucose (−2.16 mg/dL; 95% CI = −3.78 to −0.72), and fasting insulin (−2.75 µIU/mL; 95% CI = −4.50 to −1.00) over 6 months. 56 Diet and exercise are both important factors in the control and prevention of childhood obesity. It is our recommendation that parents and community (teachers and doctors) should be involved in identifying children at risk based on their BMI and participate in implementing practices such as good diet control through the reduction of sugary drinks, fatty foods, and also encouraging safe exercise programs to prevent and control childhood obesity in the society. 56
While all of the previous data express the more obvious prevention methods with regard to childhood obesity, it is imperative to note that ensuring that the whole family is involved in the intervention will yield the greatest results. 2 All current studies indicate that families must be included in childhood treatment of obesity. However, for the success of the child’s weight loss program, it is vital that the parents understand that the causes of obesity are often a mixture of four factors: genetic causes, parental habits, overeating, and poor exercise habits. Thus, instilling some responsibility on the parents and informing them that controlled food preparation, diet control, and family participation in physical activities will all assist in the treatment and control of obesity in their children. 2
Childhood obesity has increased significantly in recent decades and has quickly become a public health crisis in the United States and all over the world. Its increase in prevalence has provoked widespread research efforts to identify the factors that contributed to these changes. 57 Obesity starts with an imbalance between caloric intake and caloric expenditure. 58 Children with obesity are at greater risk of adult obesity; therefore, if we can educate and improve the health habits of families even before they start having children, this can help reduce the increasing rate of childhood obesity in the United States. Parents and caregivers with proper education on the causes and consequences of childhood obesity can help prevent childhood obesity by providing healthy meals and snacks, daily physical activity, and nutrition education to their family members. 59 Families need to take the approach of not adapting to their family being on a diet but more of a healthy lifestyle. A family’s home environment can influence children at a young age; therefore, making changes starting in the household early can educate and influence them to grow up healthy. Although prevention programs may be more expensive in the short term, the long-term benefits acquired through prevention are much more likely to save an even greater amount of health care costs. Not only will the children have a better childhood and self-esteem, but prevention programs can also decrease the incidence of cardiovascular diseases, diabetes, stroke, and possibly cancers in adulthood. 60 The overall need to decrease the obesity rate will help children and their families in the generations to come by building a healthy lifestyle and environment. In order to tackle the climbing obesity rate, overall health and lifestyle needs to be a priority as they balance one with the other. 49 While effective interventions to thwart childhood obesity still remain elusive, the sustainability of the interventions already in place will enable children and their families to adopt these important health behaviors as lifelong practices and improve their health. 58
Treatment of Obesity and the Physiology of Energy Regulation
As discussed previously, a variety of mechanisms participate in weight regulation and the development of obesity in children, including genetics, developmental influences (“metabolic programming” or epigenetics), individual and family health behaviors, and environmental factors. Among these potential mechanisms, only environmental factors are potentially modifiable during childhood and adolescence.
Unfortunately, despite intensive lifestyle modifications and support for healthy practices within the children’s environment, some children will continue to struggle with extreme excess weight and associated comorbidities. 61 , 62 Therefore, a combination of pharmacotherapy and lifestyle modification can be considered. 61 Overweight children should not be treated with medications unless significant, severe comorbidities persist despite lifestyle modification. The use of pharmacotherapy should also be considered in overweight children with a strong family history of type 2 diabetes or cardiovascular risk factors. Constant bidirectional communication between the brain and the gastrointestinal tract, as well as the brain and other relevant tissues (ie, adipose tissue, pancreas, and liver), ensures that the brain constantly perceives and responds accordingly to the energy status/needs of the body. This elegant biological system is subject to disruption by a toxic obesogenic environment, leading to syndromes such as leptin and insulin resistance, and ultimately further exposing individuals who are obese to further weight gain and type 2 diabetes mellitus. Currently, the only Food and Drug Administration–approved prescription drug indicated for the treatment of pediatric obesity is orlistat (Xenical; Genentech USA, Inc, South San Francisco, CA). 63 Orlistat works by inhibiting gastric and pancreatic lipases, the enzymes that break down triglycerides in the intestine. Moreover, imaging studies in humans are beginning to examine the influence that higher- order/hedonic brain regions have on homeostatic areas, as well as their responsiveness to homeostatic peripheral signals. With a greater understanding of these mechanisms, the field moves closer to understanding and eventually treating the casualties of obesity.
The number of children with obesity in the United States has increased substantially over the years; due to its public health significance, the increasing trends need to be closely monitored. While a complete picture of all the risk factors associated with obesity remains elusive, many of the studies agreed that prevention is the key strategy for controlling the current problem. Since the combination of diet, exercise, and physiological and psychological factors are all important factors in the control and prevention of childhood obesity, primary prevention methods should be aimed at educating the child and family and encouraging appropriate diet and exercise from a young age through adulthood while secondary prevention should be targeted at lessening the effect of childhood obesity by preventing the child from continuing unhealthy habits and obesity into adulthood. A combination of primary and secondary prevention is necessary to achieve the best results. Thus, a combined implementation of both types of preventions can significantly help lower the current prevalence of childhood and adolescent obesity in the United States. Failure to take appropriate actions could lead to serious public health consequences.
Author Contributions: AS: Contributed to conception and design; drafted manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
XQ: Contributed to the acquisition, analysis, and interpretation.
JL: Contributed to the acquisition, analysis, and interpretation.
SR: Contributed to the acquisition, analysis, and interpretation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.

You are using an outdated browser. This page doesn't support Internet Explorer 6, 7 and 8. Please upgrade your browser or activate Google Chrome Frame to improve your experience.
MINI REVIEW
Genetics, pharmacotherapy, and dietary interventions in childhood obesity.

- School of Food Science and Biotechnology, Research Institute of Tailored Food Technology, Kyungpook National University, Daegu, Republic of Korea
Childhood obesity has emerged as a major global health issue, contributing to the increased prevalence of chronic conditions and adversely affecting the quality of life and future prospects of affected individuals, thereby presenting a substantial societal challenge. This complex condition, influenced by the interplay of genetic predispositions and environmental factors, is characterized by excessive energy intake due to uncontrolled appetite regulation and a Westernized diet. Managing obesity in childhood requires specific considerations compared with adulthood, given the vulnerability of the critical juvenile–adolescent period to toxicity and developmental defects. Consequently, common treatment options for adult obesity may not directly apply to younger populations. Therefore, research on childhood obesity has focused on genetic defects in regulating energy intake, alongside pharmacotherapy and dietary interventions as management approaches, with an emphasis on safety concerns. This review aims to summarize canonical knowledge and recent findings on genetic factors contributing to childhood obesity. Additionally, it assesses the efficacy and safety of existing pharmacotherapies and dietary interventions and suggests future research directions. By providing a comprehensive understanding of the complex dynamics of childhood obesity, this review aims to offer insights into more targeted and effective strategies for addressing this condition, including personalized healthcare solutions.
Introduction
Childhood obesity has emerged as a critical global health concern, notably in developed countries where obesity rates among children and adolescents have nearly tripled in the last 30 years. Projections by the World Obesity Federation anticipate that by 2030, approximately 250 million children worldwide will be obese [ 1 – 3 ]. This condition markedly increases the risk of chronic diseases, including fatty liver and type 2 diabetes [ 1 , 4 – 8 ]. The persistent transition from obesity in childhood to adulthood is especially concerning, with >80% of affected adolescents expected to remain obese as adults [ 9 ]. Beyond impacting physical health, this trend affects self-esteem, social relationships, and future economic prospects, underscoring the urgent need for action [ 1 , 10 , 11 ].
The etiology of childhood obesity is multifaceted, involving a complex interplay of genetic and environmental factors [ 1 ]. Advances in genetic research have illuminated the role of specific genetic factors influencing energy homeostasis, particularly appetite regulation [ 12 , 13 ]. Studies on the genetics of obesity have identified key genes as pivotal contributors to the condition, enhancing our understanding of its biological mechanisms and opening new avenues for preventive and therapeutic strategies.
In addition to deepening our understanding of the mechanisms involved in obesity, genetic insights also drive the development of pharmacotherapies targeting specific metabolic pathways. Such treatments have shown promise in adults, signaling a potential shift toward more effective obesity management. However, their use in children and adolescents remains limited, being primarily reserved for cases of severe obesity and diabetes in adolescents, where treatment benefits are considered to outweigh the risks [ 14 ]. Alongside this cautious approach, new medications are under development, emphasizing improved efficacy and safety, accompanied by more rigorous clinical validation.
Environmental factors, particularly diet and lifestyle with reduced physical activity, also play a crucial role in childhood obesity development [ 15 ]. Modern dietary patterns, often labeled the Western diet, are mainly characterized by a high caloric intake of saturated fats and refined carbohydrates and frequent consumption of sugar-sweetened beverages, which closely correlate with rising childhood obesity rates [ 16 – 20 ]. Consequently, management strategies for childhood obesity are increasingly focused on dietary interventions, such as ketogenic diets, fasting-based interventions, and dietary supplements. Ongoing research explores the effectiveness and safety of these dietary interventions in preventing and treating childhood obesity.
This review aims to summarize current knowledge on genetic factors contributing to childhood obesity, evaluate the efficacy and safety of existing pharmacotherapies and dietary interventions (outlined in Figure 1 ), and suggest directions for future research. By presenting a comprehensive understanding of the complex dynamics involved in childhood obesity, this review highlights potential approaches for more effective and safe treatment strategies, ultimately providing foundations for tailored interventions addressing genetic predispositions and environmental influences.
Figure 1 . Schematic summary of the multifaceted etiology and management approaches in childhood obesity. This figure illustrates the complex interplay between genetic and environmental factors influencing the development of childhood obesity. It also delineates primary management strategies, including pharmacotherapy and dietary interventions. The schematic was created using illustrations from https://biorender.com .
Genetic factors in childhood obesity
Twin studies have highlighted the heritability of obesity, with estimates for body mass index (BMI) heritability reaching up to 70% [ 21 , 22 ]. The genetic landscape of childhood obesity has been extensively explored, revealing multiple genetic factors contributing to its development ( Figure 1 ). Childhood obesity is predominantly polygenic, involving multiple genes, each contributing modestly but collectively exerting a substantial impact [ 23 , 24 ]. In contrast to monogenic forms of obesity, resulting from single genetic defects with pivotal effects [ 25 ], the genetic predisposition to childhood obesity in the broader population is shaped by numerous common genetic variants, collectively exerting a substantial impact on the obesity phenotype.
The advent of genome-wide association studies (GWAS) has markedly advanced our understanding of the genetic basis of obesity. A landmark discovery involved identifying variants in the fat mass and obesity-associated ( FTO ) gene as a major risk factor for obesity in the general population and severe childhood obesity. The strongest association was noted for single-nucleotide polymorphisms in the first intron of FTO . The influence of FTO gene variants on energy homeostasis is mediated through their impact on appetite regulation, with certain variants linked to increased energy intake and high-calorie food preference [ 26 – 29 ].
Monogenic obesity, although rare, is predominantly identified in patient cohorts with severe and early-onset obesity, highlighting its strong correlation with severe childhood obesity. Monogenic obesity is mainly attributed to genetic mutations associated with the central regulation of energy homeostasis, particularly appetite control driven by the leptin–melanocortin signaling pathway [ 21 ]. Genes implicated in monogenic obesity include Lep ( leptin ), LEPR ( leptin receptor ), POMC ( pro-opiomelanocortin ), AGRP ( agouti-related protein ), MC4R ( melanocortin 4 receptor ), PCSK1 ( proprotein convertase subtilisin/kexin type 1 ), SH2B1 ( SH2B adaptor protein 1 ), PHIP ( proline-rich protein 5 ), MRAP2 ( melanocortin 2 receptor accessory protein 2 ), and SIM1 ( single-minded 1 ) [ 30 – 42 ]. In most monogenic obesity cases, genetic mutations drive abnormal feeding behavior, resulting in early-onset, severe hyperphagic obesity.
Recently, the Iroquois homeodomain transcription factor genes IRX3 and IRX5 have emerged as novel genetic determinants in human obesity, revealing the complex genetic interactions underlying this condition. Known for their similar expression patterns and cooperative roles during mammalian development, the IRX3 and IRX5 genes have been implicated in obesity through interactions with intronic FTO locus variants. Chromatin conformation capture techniques revealed that these variants physically interact with IRX3 and IRX5 promoter regions, serving as enhancers that increase IRX3 / IRX5 expression levels in the hypothalamus and adipose tissue [ 43 – 45 ]. This upregulation influences crucial physiological processes, including feeding control, thermogenesis, and adipogenesis, positioning IRX3 / IRX5 as central mediators of FTO variant–associated obesity effects [ 46 – 48 ]. Notably, Sim1 interacts with IRX3 / IRX5 . Specifically, loss-of-function mutations in SIM1 are linked to hyperphagic childhood obesity, and Sim1 haploinsufficiency leads to ectopic expression of IRX3 / IRX5 in the hypothalamus in mice, causing neurodevelopmental defects and contributing to appetite dysregulation and hyperphagic obesity [ 49 ]. Further research is warranted to explore the mechanistic evidence for the tissue- or cell-type-specific roles of IRX3 / IRX5 , particularly their involvement in regulating energy homeostasis. This evolving genetic narrative emphasizes the need to elucidate these pathways for further advancements in childhood obesity prevention and treatment.
Pharmacotherapy in childhood obesity
Managing childhood obesity often involves pharmacological intervention, especially in cases where a child presents with a severe obesity phenotype and critical health issues. The cautious application of pharmacotherapy in young patients with obesity stems from concerns regarding potential long-term impacts on growth and overall development. Current pharmacotherapy options are predominantly limited to adolescents, particularly in cases of severe obesity with accompanying comorbid conditions. Pharmacotherapies currently used in childhood obesity cases are summarized in Figure 1 .
Classical pharmacotherapy: orlistat and phentermine
Among the drugs approved for adults, only a few have received approval for childhood–adolescent obesity treatment. Until the early 2020s, orlistat and phentermine were the sole U.S. Food and Drug Administration (FDA)-approved medications for this purpose [ 50 , 51 ]. Orlistat, a lipase inhibitor, reduces the hydrolysis of ingested triglycerides, decreasing gastrointestinal fat absorption. Clinical trials have demonstrated its efficacy in BMI reduction compared with placebo groups [ 52 ], leading to its approval for use in adolescents aged ≥12; however, orlistat has potential side effects, including diarrhea and hepatic injury, resulting in dropout rates of around 35%–75% within 3 months [ 52 – 54 ]. Long-term use of orlistat may disrupt the absorption of fat-soluble vitamins and minerals, negatively impacting growth or pubertal development [ 55 , 56 ].
Phentermine, a sympathomimetic amine anorectic, is FDA-approved for monotherapy in adolescents aged ≥16 with severe obesity and additional related health complications. A recent clinical advancement involved the FDA approving the phentermine–topiramate combination for weight loss in obese individuals aged ≥12. Topiramate, originally an antiepileptic agent, contributes to weight loss by inhibiting carbonic anhydrase and increasing γ-aminobutyric acid (GABA) activity, suppressing appetite [ 57 – 59 ]. This combination, leveraging distinct mechanisms, offers a more effective weight loss solution than either drug alone, allowing for lower doses of each medication and enhancing overall treatment efficacy and safety profile. Phentermine/topiramate may pose safety concerns such as mood disorders, cognitive impairment, nephrolithiasis, cardiac risks, and teratogenic effects [ 60 ].
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist approved by the FDA in 2020, offers a targeted pharmacological approach for managing monogenic obesity linked specifically to POMC , PCSK1 , or LEPR genetic deficiencies [ 61 , 62 ]. These genetic variants can disrupt signaling through the MC4R pathway, leading to hyperphagia and severe early-onset obesity [ 21 , 63 ]. MC4R agonist serves as an alternative activator of the MC4R pathway in patients who have POMC deficiencies due to mutations in either POMC or PCSK1 and in those with LEPR deficiencies caused by mutations in LEPR , which is crucial for POMC function. Hence, the MC4R agonist effectively reduces hyperphagia and promotes weight loss for treating severe obesity linked to these specific genetic disorders [ 62 , 64 , 65 ]. While its effectiveness in clinical trials is notable, its application is limited to these particular genetic disorders and not applicable to general childhood obesity. Setmelanotide therapy is associated with potential side effects, including skin hyperpigmentation, sexual dysfunction, depression, and suicidal ideation [ 66 ].
Innovative pharmacotherapy: GLP-1 receptor agonists
Glucagon-like peptide-1 receptor agonists (GLP1RAs), such as liraglutide and semaglutide, have become pivotal pharmacological agents for managing obesity. Originally developed to treat type 2 diabetes, GLP1RAs unexpectedly induce weight loss. Studies have indicated that GLP1RAs primarily act on the central nervous system to reduce appetite, delay gastric emptying to prolong satiety and alter brain pathways that decrease reward-driven eating behaviors. Ultimately, these actions lead to decreased energy intake and promote weight loss in general obesity and syndromic monogenic forms of obesity, including Prader-Willi syndrome and MC4R mutations [ 67 – 74 ]. Having successfully promoted weight loss in adults, GLP1RAs have received FDA approval, which was extended to adolescents. Specifically, liraglutide treatment has resulted in notable BMI reductions without negative impacts on pubertal development or growth, making it an appropriate option for adolescents aged ≥12 [ 75 , 76 ]. Recent preliminary investigations into the safety and effectiveness of liraglutide in the 6–12 age group resulted in the initiation of the SCALE KIDS clinical trial, a study assessing its viability as a childhood anti-obesity treatment [ 77 ]. Additionally, semaglutide also received FDA approval for weight management in adolescents aged ≥12 with severe obesity in 2022 [ 78 , 79 ]. This represents a major advancement in expanding therapeutic options for childhood obesity management.
Tirzepatide, recently approved for chronic weight management in adults, is a dual agonist targeting GLP1R and glucose-dependent insulinotropic polypeptide receptor (GIPR), offering a novel approach to obesity treatment by simultaneously enhancing glucose regulation and reducing appetite [ 80 , 81 ]. It has been shown that GLP1R–GIPR dual agonist is superior in weight reduction to GLP1RAs and offers additional benefits, including improved insulin sensitivity, lipid profiles, and blood pressure [ 82 , 83 ]. This demonstrates groundbreaking potential in the pharmacology of obesity and related metabolic diseases. Building on its success in adults, tirzepatide is currently in phase 1 clinical trials for children and adolescents aged 6–11 and 12–17 to assess its safety and efficacy. This expansion into pediatric studies reflects a proactive step towards addressing childhood obesity, providing hope for a new, effective treatment option that could mitigate the long-term health consequences associated with early-onset obesity. In the future, the development of novel and effective drugs with favorable safety profiles is expected to revolutionize the approach to treating childhood obesity treatment, even within younger populations. Potential side effects of GLP1RAs or GLP1R–GIPR dual agonists include gastrointestinal symptoms, such as nausea, vomiting, diarrhea, cardiovascular conduction abnormalities, and sinus tachycardia [ 84 , 85 ].
Off-label medications
Off-label medication refers to the use of pharmaceutical drugs for an unapproved age group, dosage, or condition. In the context of childhood obesity, metformin is a common example of an off-label medication. Metformin is a well-established, approved option for managing type 2 diabetes in adults and adolescents [ 86 , 87 ]. Some research suggests that metformin may be effective for weight loss [ 88 – 91 ]; however, due to its modest and inconsistent weight-loss effects, the FDA has yet to approve metformin as a weight-loss agent. Consequently, its use in treating obesity in children has also not received official approval. Nonetheless, multiple lines of evidence demonstrate metformin’s favorable effects on weight management in children and adolescents with obesity, along with a safe profile. This makes metformin a viable and accessible option for off-label use in combating childhood obesity [ 92 , 93 ]. Although metformin’s efficacy and safety profile are established for children, its off-label use still introduces potential risks. The lack of comprehensive clinical data specifically for treating childhood obesity means that the potential benefits must be cautiously weighed against risks that are not fully understood or might be underestimated. Consequently, the use of off-label medications such as metformin in treating childhood obesity requires careful consideration and underscores the necessity for more rigorous research to confirm their safety and effectiveness in these younger patients.
Dietary interventions in childhood obesity
Dietary interventions play a pivotal role as an alternative strategy for addressing childhood obesity, particularly as pharmacotherapy is often reserved for severe cases accompanied by additional metabolic complications [ 94 ]. These interventions, focusing on altering dietary habits and behaviors, aim to cultivate healthy eating practices conducive to long-term weight management and overall health enhancement. Emerging dietary strategies, including ketogenic diets, fasting-based interventions, and dietary supplements, are gaining attention for their potential in combating childhood obesity ( Figure 1 ).
Ketogenic diet
The ketogenic diet, characterized by high fat and low carbohydrate intake, prompts the body to convert fats and ketone bodies for energy, entering ketosis [ 95 ]. This metabolic shift makes the diet a popular non-pharmacological option for obesity management, given its potential to promote weight loss through enhanced lipolysis and reduced insulin levels [ 96 – 98 ]. Although the ketogenic diet is considered beneficial for obesity-related metabolic and cardiovascular risk factors in adults [ 99 , 100 ], its role in childhood weight management is still being explored. Clinical trials and animal studies have shown the diet’s effectiveness in promoting weight loss and addressing metabolic issues caused by obesity [ 101 ]. However, the long-term safety and efficacy of ketogenic diets in the pediatric population require further investigation. Specifically, maintaining a ketogenic diet for extended periods may lead to elevated levels of circulating triglycerides, lipoproteins, and increased lipolysis, potentially increasing the risk of cardiovascular disease [ 102 – 104 ]. Challenges, such as limited food variety and maintaining long-term adherence, present additional considerations for young patients with obesity. Although ketogenic diets offer potential benefits, the associated risks, such as nutrient deficiencies, growth and developmental impacts, and metabolic complications, necessitate careful monitoring to ensure these diets are applied safely in children [ 105 , 106 ].
Fasting-based interventions
Eating pattern-based dietary interventions, including intermittent fasting, time-restricted feeding, and the fasting-mimicking diet, are gaining attention for their potential metabolic benefits. Intermittent fasting (IF) involves alternating cycles of fasting and eating; time-restricted feeding (TRF) restricts daily food intake to a specific time window, typically 6–8 h, promoting a consistent daily fasting period; the fasting-mimicking diet (FMD) entails consuming an extremely low-calorie diet mimicking the physiological effects of fasting, achieving the advantages of fasting without complete food abstention. These approaches are being explored for adaptability and potential health benefits in animal experiments and clinical settings to trigger beneficial metabolic changes that aid weight management and improve overall health by leveraging the body’s natural responses to fasting periods, including improved lipolysis and thermogenesis and glucose management [ 107 – 112 ]. Despite their simplicity and departure from traditional calorie counting, implementing fasting-based strategies in pediatric populations warrants careful evaluation owing to the critical nutritional needs of growing children and adolescents and the potential impact on their physical and cognitive development [ 113 , 114 ]. Although these fasting methods offer a fresh perspective on dietary management with demonstrated feasibility and positive outcomes [ 115 – 118 ], the evidence supporting their utility as acceptable therapeutic approaches, particularly for younger demographics, is still emerging. Comprehensive research is needed to establish their efficacy and safety and develop age-appropriate guidelines for children and adolescents.
Dietary supplements
Dietary supplements, including vitamins, nutrients, probiotics, plant extracts, and polyphenols, are increasingly recognized for their potential role in managing childhood obesity [ 119 ]. Omega-3 polyunsaturated fatty acids and vitamin D have been extensively researched in pediatric populations. Despite growing interest, their use in children is marked by controversy, largely due to inconsistent clinical outcomes that raise questions regarding treatment efficacy and reliability. For example, some studies have associated omega-3 supplementation with improvements in insulin resistance and fatty liver disease, as well as weight reduction in patients with obesity. In contrast, other studies have suggested no significant effect on body weight, indicating unclear impacts on anthropometric indices [ 120 – 122 ]. This emphasizes the need for larger pediatric studies to ascertain the effectiveness of omega-3.
The focus on probiotics, driven by insights into the role of the human microbiome in health, signals a shift in our understanding of the causes of obesity [ 123 ]. Probiotics, specifically Lactobacillus and Bifidobacterium species, show promise in reducing BMI and improving metabolic parameters, indicating their potential as an intervention for children with metabolic issues. However, cautious use of dietary supplements is recommended owing to limited evidence regarding their safety and effectiveness in children, potential interactions with medications, and unknown long-term health consequences [ 124 – 126 ]. This situation highlights the urgent need for comprehensive clinical trials to verify the safety and benefits of dietary supplement use in childhood obesity treatment.
Other approaches
Lifestyle interventions are foundational in managing childhood obesity, particularly through increased physical activity and exercise. These approaches are the first line of defense, especially in a preventive and managing manner. Encouraging a healthy diet and regular physical activity are essential, as these modifications can significantly impact overall health and prevent the progression of obesity [ 127 ]. Hence, lifestyle interventions are usually combined with pharmacological or dietary interventions to enhance the efficacy of these treatments [ 1 ]. This integrative approach is especially crucial when obesity reaches severe levels, as lifestyle changes alone often become insufficient [ 128 – 130 ]. Thus, most clinical treatment approaches for childhood obesity include combined treatment with lifestyle interventions as an effective integrative approach.
Metabolic and bariatric surgery (MBS), including procedures such as sleeve gastrectomy, gastric bypass, and gastric banding, is recognized as the most effective treatment for adolescents with severe obesity, notably reducing appetite and facilitating substantial weight loss alongside improvements in comorbidities and overall quality of life [ 131 , 132 ]. These surgeries are considered for adolescents under stringent criteria, typically for those with a BMI ≥35 who also have severe comorbidities or a BMI ≥40. Despite the significant benefits, MBS carries potential risks, including nutritional deficiencies, the need for reoperations, and other surgical complications [ 131 ]. However, a recent large study indicated that MBS is effective across younger pediatric age groups without affecting vertical growth [ 133 ], affirming its utility as a crucial intervention in severe cases of childhood obesity and associated comorbidities. Consequently, this method is increasingly regarded as a viable final option for managing severe childhood obesity, prompting discussions about lowering the stringent criteria for surgery eligibility in younger patients [ 134 ].
Managing childhood obesity necessitates a comprehensive approach, incorporating tailored pharmacological and dietary interventions to meet each child’s unique requirements. While appropriate for severe cases, pharmacotherapy must be applied judiciously to prevent adverse impacts on childhood growth and development. Although dietary interventions aiming to alter immediate eating habits and establish long-term nutritional practices for prevention and treatment are perceived as safe, their safety warrants further investigation. The potential synergy between pharmacotherapy and dietary intervention is gaining recognition, showing promise in effectively managing childhood obesity while balancing metabolic control with overall health, as demonstrated in other diseases [ 129 , 135 , 136 ].
The undeniable role of genetics in obesity influences predisposition to the condition and impacts responses to various treatment options. As treatment options progress, obesity management is increasingly likely to prioritize personalized medicine and nutrition, advocating for interventions and dietary plans tailored to each individual’s genetic makeup. The polygenic risk score (PRS) is a tool that estimates an individual’s genetic liability to a trait or disease based on their genotype profile and data from relevant GWAS. Regarding childhood obesity, PRS can be crucial in predicting obesity susceptibility and informing personalized intervention strategies [ 137 ]. Several studies have already constructed PRSs specifically for childhood obesity [ 23 , 24 , 138 , 139 ], illustrating the potential of genetic insights to guide more effective prevention and treatment approaches.
This personalized approach requires a comprehensive understanding of the genetic factors contributing to obesity and how these interact with various treatment and dietary strategies. It is also essential to refine diagnostic measures to better and, more specifically, diagnose childhood obesity, given the limitations of using BMI as the sole parameter for assessing childhood obesity [ 140 , 141 ]. Identifying genetic predispositions and tailoring treatments aims to enhance efficacy and minimize adverse effects. Ultimately, this approach would lead to safer and more effective management of childhood obesity, ensuring that interventions are as individualized as the genetic profiles they aim to accommodate.
Future research should explore the molecular mechanisms underlying the interactions among pharmacotherapies, dietary interventions, and genetic factors. In addition to genetic predispositions, understanding the role of gene–environmental interactions is becoming increasingly crucial. Epigenetics—modifications that change gene expression without altering the DNA sequence—mediates the effects of environmental variables on the expression of genes. These modifications include DNA methylation, histone alterations, and microRNA (miRNA) regulation. By affecting how genes are expressed in response to environmental cues, epigenetic mechanisms can contribute to the complexity of obesity pathogenesis and its related metabolic disorders [ 142 – 144 ]. Recognizing these interactions provides valuable insights into how personalized interventions can be tailored to individuals based on genetic makeup, environmental exposures, and lifestyle choices. This integrated approach emphasizes the necessity of advancing our understanding of epigenetics to develop more precise and effective strategies for preventing and managing childhood obesity. Advancements in computational technologies, such as artificial intelligence and high-throughput genomic analysis, promise increased accessibility to personalized treatments in the near future, marking a major step toward more effectively addressing childhood obesity.
Author contributions
JS conceived, designed, and wrote the manuscript.
The author declares that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Kyungpook National University Research Fund, 2023.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, body mass index; GWAS, genome-wide association studies; FTO, fat mass and obesity-associated protein; IRX3/5, Iroquois homeobox 3 and 5; SIM1, single-minded family BHLH transcription factor 1; POMC, pro-opiomelanocortin; PCSK1, proprotein convertase subtilisin/Kexin type 1; LEPR, leptin receptor; AGRP, agouti-related protein; MC4R, melanocortin 4 receptor; SH2B1, SH2B adaptor protein 1; MRAP2, melanocortin 2 receptor accessory protein 2; PHIP, Pleckstrin homology domain-interacting protein; FDA, U.S. Food and Drug Administration; GLP1RAs, glucagon-like peptide-1 receptor agonists; GABA, γ-aminobutyric acid; GIPR, glucose-dependent insulinotropic polypeptide receptor; IF, intermittent fasting; TRF, time-restricted feeding; FMD, fasting-mimicking diet; MBS, metabolic and bariatric surgery.
1. Lister, NB, Baur, LA, Felix, JF, Hill, AJ, Marcus, C, Reinehr, T, et al. Child and adolescent obesity. Nat Rev Dis Primers (2023) 9(1):24. doi:10.1038/s41572-023-00435-4
PubMed Abstract | CrossRef Full Text | Google Scholar
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (2017) 390(10113):2627–42. doi:10.1016/S0140-6736(17)32129-3
3. Atlas of Childhood Obesity. Atlas of childhood obesity . World Obesity Federation (2019). Available from: https://www.worldobesity.org/membersarea/global-atlas-on-childhood-obesity . (Accessed May 02, 2024)
Google Scholar
4. Diabetes Canada Clinical Practice Guidelines Expert Committee, Wharton, S, Pedersen, SD, Lau, DC, Sharma, AM, and Sharma, AM. Weight management in diabetes. Can J Diabetes (2018) 42(Suppl. 1):S124–S129. doi:10.1016/j.jcjd.2017.10.015
5. Abbasi, A, Juszczyk, D, van Jaarsveld, CHM, and Gulliford, MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc (2017) 1(5):524–37. doi:10.1210/js.2017-00044
6. Anderson, EL, Howe, LD, Fraser, A, Callaway, MP, Sattar, N, Day, C, et al. Weight trajectories through infancy and childhood and risk of non-alcoholic fatty liver disease in adolescence: the ALSPAC study. J Hepatol (2014) 61(3):626–32. doi:10.1016/j.jhep.2014.04.018
7. Caprio, S, Santoro, N, and Weiss, R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab (2020) 2(3):223–32. doi:10.1038/s42255-020-0183-z
8. Weiss, R, Dziura, J, Burgert, TS, Tamborlane, WV, Taksali, SE, Yeckel, CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med (2004) 350(23):2362–74. doi:10.1056/NEJMoa031049
9. Simmonds, M, Llewellyn, A, Owen, CG, and Woolacott, N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev (2016) 17(2):95–107. doi:10.1111/obr.12334
10. Segal, AB, Huerta, MC, Aurino, E, and Sassi, F. The impact of childhood obesity on human capital in high-income countries: a systematic review. Obes Rev (2021) 22(1):e13104. doi:10.1111/obr.13104
11. Ding, W, Lehrer, SF, Rosenquist, JN, and Audrain-McGovern, J. The impact of poor health on academic performance: new evidence using genetic markers. J Health Econ (2009) 28(3):578–97. doi:10.1016/j.jhealeco.2008.11.006
12. Littleton, SH, Berkowitz, RI, and Grant, SFA. Genetic determinants of childhood obesity. Mol Diagn Ther (2020) 24(6):653–63. doi:10.1007/s40291-020-00496-1
13. Vourdoumpa, A, Paltoglou, G, and Charmandari, E. The genetic basis of childhood obesity: a systematic review. Nutrients (2023) 15(6):1416. doi:10.3390/nu15061416
14. Rajjo, T, Mohammed, K, Alsawas, M, Ahmed, AT, Farah, W, Asi, N, et al. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab (2017) 102(3):763–75. doi:10.1210/jc.2016-2574
15. Jia, P, Shi, Y, Jiang, Q, Dai, S, Yu, B, Yang, S, et al. Environmental determinants of childhood obesity: a meta-analysis. Lancet Glob Health (2023) 11(Suppl. 1):S7. doi:10.1016/S2214-109X(23)00092-X
16. Keller, A, and Bucher Della Torre, S. Sugar-sweetened beverages and obesity among children and adolescents: a review of systematic literature reviews. Child Obes (2015) 11(4):338–46. doi:10.1089/chi.2014.0117
17. Kim, J, and Lim, H. Nutritional management in childhood obesity. J Obes Metab Syndr (2019) 28(4):225–35. doi:10.7570/jomes.2019.28.4.225
18. Qahwaji, DM. Impact of dietary intake and physical activity on metabolic syndrome in Saudi adults: an exploratory pilot study. Prev Nutr Food Sci (2022) 27(1):45–9. doi:10.3746/pnf.2022.27.1.45
19. Datar, A, and Nicosia, N. Junk food in schools and childhood obesity. J Pol Anal Manage. (2012) 31(2):312–37. doi:10.1002/pam.21602
CrossRef Full Text | Google Scholar
20. Bradley, P. Refined carbohydrates, phenotypic plasticity and the obesity epidemic. Med Hypotheses (2019) 131:109317. doi:10.1016/j.mehy.2019.109317
21. Loos, RJF, and Yeo, GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet (2022) 23(2):120–33. doi:10.1038/s41576-021-00414-z
22. Wardle, J, Carnell, S, Haworth, CM, and Plomin, R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr (2008) 87(2):398–404. doi:10.1093/ajcn/87.2.398
23. Khera, AV, Chaffin, M, Wade, KH, Zahid, S, Brancale, J, Xia, R, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell (2019) 177(3):587–96.e9. doi:10.1016/j.cell.2019.03.028
24. Helgeland, O, Vaudel, M, Sole-Navais, P, Flatley, C, Juodakis, J, Bacelis, J, et al. Characterization of the genetic architecture of infant and early childhood body mass index. Nat Metab (2022) 4(3):344–58. doi:10.1038/s42255-022-00549-1
25. Huvenne, H, Dubern, B, Clement, K, and Poitou, C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts (2016) 9(3):158–73. doi:10.1159/000445061
26. Dina, C, Meyre, D, Gallina, S, Durand, E, Korner, A, Jacobson, P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet (2007) 39(6):724–6. doi:10.1038/ng2048
27. Wardle, J, Llewellyn, C, Sanderson, S, and Plomin, R. The FTO gene and measured food intake in children. Int J Obes (Lond) (2009) 33(1):42–5. doi:10.1038/ijo.2008.174
28. Frayling, TM, Timpson, NJ, Weedon, MN, Zeggini, E, Freathy, RM, Lindgren, CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science (2007) 316(5826):889–94. doi:10.1126/science.1141634
29. Cecil, JE, Tavendale, R, Watt, P, Hetherington, MM, and Palmer, CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med (2008) 359(24):2558–66. doi:10.1056/NEJMoa0803839
30. Friedman, JM. Leptin and the endocrine control of energy balance. Nat Metab (2019) 1(8):754–64. doi:10.1038/s42255-019-0095-y
31. Krude, H, Biebermann, H, Luck, W, Horn, R, Brabant, G, and Gruters, A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet (1998) 19(2):155–7. doi:10.1038/509
32. Montague, CT, Farooqi, IS, Whitehead, JP, Soos, MA, Rau, H, Wareham, NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature (1997) 387(6636):903–8. doi:10.1038/43185
33. Clement, K, Vaisse, C, Lahlou, N, Cabrol, S, Pelloux, V, Cassuto, D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature (1998) 392(6674):398–401. doi:10.1038/32911
34. Yeo, GS, Farooqi, IS, Aminian, S, Halsall, DJ, Stanhope, RG, and O'Rahilly, S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet (1998) 20(2):111–2. doi:10.1038/2404
35. Vaisse, C, Clement, K, Guy-Grand, B, and Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet (1998) 20(2):113–4. doi:10.1038/2407
36. Nead, KT, Li, A, Wehner, MR, Neupane, B, Gustafsson, S, Butterworth, A, et al. Contribution of common non-synonymous variants in PCSK1 to body mass index variation and risk of obesity: a systematic review and meta-analysis with evidence from up to 331 175 individuals. Hum Mol Genet (2015) 24(12):3582–94. doi:10.1093/hmg/ddv097
37. Stijnen, P, Tuand, K, Varga, TV, Franks, PW, Aertgeerts, B, and Creemers, JW. The association of common variants in PCSK1 with obesity: a HuGE review and meta-analysis. Am J Epidemiol (2014) 180(11):1051–65. doi:10.1093/aje/kwu237
38. Doche, ME, Bochukova, EG, Su, HW, Pearce, LR, Keogh, JM, Henning, E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest (2012) 122(12):4732–6. doi:10.1172/JCI62696
39. Marenne, G, Hendricks, AE, Perdikari, A, Bounds, R, Payne, F, Keogh, JM, et al. Exome sequencing identifies genes and gene sets contributing to severe childhood obesity, linking PHIP variants to repressed POMC transcription. Cel Metab (2020) 31(6):1107–19.e12. doi:10.1016/j.cmet.2020.05.007
40. Asai, M, Ramachandrappa, S, Joachim, M, Shen, Y, Zhang, R, Nuthalapati, N, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science (2013) 341(6143):275–8. doi:10.1126/science.1233000
41. Bonnefond, A, Raimondo, A, Stutzmann, F, Ghoussaini, M, Ramachandrappa, S, Bersten, DC, et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi-like features. J Clin Invest (2013) 123(7):3037–41. doi:10.1172/JCI68035
42. Jr, JL, Butte, NF, and Zinn, AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet (2000) 9(1):101–8. doi:10.1093/hmg/9.1.101
43. Smemo, S, Tena, JJ, Kim, KH, Gamazon, ER, Sakabe, NJ, Gomez-Marin, C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature (2014) 507(7492):371–5. doi:10.1038/nature13138
44. Claussnitzer, M, Dankel, SN, Kim, KH, Quon, G, Meuleman, W, Haugen, C, et al. FTO obesity variant circuitry and adipocyte browning in humans. N Engl J Med (2015) 373(10):895–907. doi:10.1056/NEJMoa1502214
45. Sobreira, DR, Joslin, AC, Zhang, Q, Williamson, I, Hansen, GT, Farris, KM, et al. Extensive pleiotropism and allelic heterogeneity mediate metabolic effects of IRX3 and IRX5. Science (2021) 372(6546):1085–91. doi:10.1126/science.abf1008
46. Son, JE, Dou, Z, Kim, KH, Wanggou, S, Cha, VSB, Mo, R, et al. Irx3 and Irx5 in Ins2-Cre(+) cells regulate hypothalamic postnatal neurogenesis and leptin response. Nat Metab (2021) 3(5):701–13. doi:10.1038/s42255-021-00382-y
47. Dou, Z, Son, JE, and Hui, CC. Irx3 and Irx5 - novel regulatory factors of postnatal hypothalamic neurogenesis. Front Neurosci (2021) 15:763856. doi:10.3389/fnins.2021.763856
48. Son, JE, Dou, Z, Kim, KH, and Hui, CC. Deficiency of Irx5 protects mice from obesity and associated metabolic abnormalities. Int J Obes (Lond) (2022) 46(11):2029–39. doi:10.1038/s41366-022-01221-0
49. Son, JE, Dou, Z, Wanggou, S, Chan, J, Mo, R, Li, X, et al. Ectopic expression of Irx3 and Irx5 in the paraventricular nucleus of the hypothalamus contributes to defects in Sim1 haploinsufficiency. Sci Adv (2021) 7(44):eabh4503. doi:10.1126/sciadv.abh4503
50. Chao, AM, Wadden, TA, and Berkowitz, RI. The safety of pharmacologic treatment for pediatric obesity. Expert Opin Drug Saf (2018) 17(4):379–85. doi:10.1080/14740338.2018.1437143
51. Cardel, MI, Jastreboff, AM, and Kelly, AS. Treatment of adolescent obesity in 2020. JAMA (2019) 322(17):1707–8. doi:10.1001/jama.2019.14725
52. Chanoine, JP, Hampl, S, Jensen, C, Boldrin, M, and Hauptman, J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA (2005) 293(23):2873–83. doi:10.1001/jama.293.23.2873
53. Viner, RM, Hsia, Y, Neubert, A, and Wong, IC. Rise in antiobesity drug prescribing for children and adolescents in the UK: a population-based study. Br J Clin Pharmacol (2009) 68(6):844–51. doi:10.1111/j.1365-2125.2009.03528.x
54. Willemen, MJ, Mantel-Teeuwisse, AK, M Straus, SMJ, Leufkens, HG, Egberts, AC, and M Sturkenboom, MCJ. Cardiovascular and psychiatric risk profile and patterns of use in patients starting anti-obesity drugs. Pharmacoepidemiol Drug Saf (2009) 18(7):631–8. doi:10.1002/pds.1759
55. McDuffie, JR, Calis, KA, Booth, SL, Uwaifo, GI, and Yanovski, JA. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy (2002) 22(7):814–22. doi:10.1592/phco.22.11.814.33627
56. Freemark, M. Pharmacotherapy of childhood obesity: an evidence-based, conceptual approach. Diabetes Care (2007) 30(2):395–402. doi:10.2337/dc06-1569
57. Kelly, AS, Bensignor, MO, Hsia, DS, Shoemaker, AH, Shih, W, Peterson, C, et al. Phentermine/topiramate for the treatment of adolescent obesity. NEJM Evid (2022) 1(6). doi:10.1056/evidoa2200014
58. Gadde, KM, Allison, DB, Ryan, DH, Peterson, CA, Troupin, B, Schwiers, ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. The Lancet (2011) 377(9774):1341–52. doi:10.1016/S0140-6736(11)60205-5
59. Kelly, AS. Current and future pharmacotherapies for obesity in children and adolescents. Nat Rev Endocrinol (2023) 19(9):534–41. doi:10.1038/s41574-023-00858-9
60. Johnson, DB, and Quick, J Topiramate and phentermine . Treasure Island (FL): StatPearls (2024).
61. Wabitsch, M, Farooqi, S, Fluck, CE, Bratina, N, Mallya, UG, Stewart, M, et al. Natural history of obesity due to POMC, PCSK1, and LEPR deficiency and the impact of setmelanotide. J Endocr Soc (2022) 6(6):bvac057. doi:10.1210/jendso/bvac057
62. Markham, A. Setmelanotide: first approval. Drugs (2021) 81(3):397–403. doi:10.1007/s40265-021-01470-9
63. Clement, K, Mosbah, H, and Poitou, C. Rare genetic forms of obesity: from gene to therapy. Physiol Behav (2020) 227:113134. doi:10.1016/j.physbeh.2020.113134
64. Kuhnen, P, Clement, K, Wiegand, S, Blankenstein, O, Gottesdiener, K, Martini, LL, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med (2016) 375(3):240–6. doi:10.1056/NEJMoa1512693
65. Clement, K, van den Akker, E, Argente, J, Bahm, A, Chung, WK, Connors, H, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol (2020) 8(12):960–70. doi:10.1016/S2213-8587(20)30364-8
66. Hussain, A, and Farzam, K Setmelanotide . Treasure Island (FL): StatPearls (2024).
67. Drucker, DJ. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab (2022) 57:101351. doi:10.1016/j.molmet.2021.101351
68. Secher, A, Jelsing, J, Baquero, AF, Hecksher-Sorensen, J, Cowley, MA, Dalboge, LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest (2014) 124(10):4473–88. doi:10.1172/JCI75276
69. Drucker, DJ, Habener, JF, and Holst, JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest (2017) 127(12):4217–27. doi:10.1172/JCI97233
70. Gribble, FM, and Reimann, F. Metabolic Messengers: glucagon-like peptide 1. Nat Metab (2021) 3(2):142–8. doi:10.1038/s42255-020-00327-x
71. Iepsen, EW, Have, CT, Veedfald, S, Madsbad, S, Holst, JJ, Grarup, N, et al. GLP-1 receptor agonist treatment in morbid obesity and type 2 diabetes due to pathogenic homozygous melanocortin-4 receptor mutation: a case report. Cel Rep Med (2020) 1(1):100006. doi:10.1016/j.xcrm.2020.100006
72. Iepsen, EW, Zhang, J, Thomsen, HS, Hansen, EL, Hollensted, M, Madsbad, S, et al. Patients with obesity caused by melanocortin-4 receptor mutations can Be treated with a glucagon-like peptide-1 receptor agonist. Cel Metab (2018) 28(1):23–32 e3. doi:10.1016/j.cmet.2018.05.008
73. Wilding, JPH, Batterham, RL, Calanna, S, Davies, M, Van Gaal, LF, Lingvay, I, et al. Once-Weekly semaglutide in adults with overweight or obesity. N Engl J Med (2021) 384(11):989–1002. doi:10.1056/NEJMoa2032183
74. Pi-Sunyer, X, Astrup, A, Fujioka, K, Greenway, F, Halpern, A, Krempf, M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med (2015) 373(1):11–22. doi:10.1056/NEJMoa1411892
75. Kelly, AS, Auerbach, P, Barrientos-Perez, M, Gies, I, Hale, PM, Marcus, C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med (2020) 382(22):2117–28. doi:10.1056/NEJMoa1916038
76. Danne, T, Biester, T, Kapitzke, K, Jacobsen, SH, Jacobsen, LV, Petri, KCC, et al. Liraglutide in an adolescent population with obesity: a randomized, double-blind, placebo-controlled 5-week trial to assess safety, tolerability, and pharmacokinetics of liraglutide in adolescents aged 12-17 years. J Pediatr (2017) 181:146–53.e3. doi:10.1016/j.jpeds.2016.10.076
77. Mastrandrea, LD, Witten, L, Carlsson Petri, KC, Hale, PM, Hedman, HK, and Riesenberg, RA. Liraglutide effects in a paediatric (7-11 y) population with obesity: a randomized, double-blind, placebo-controlled, short-term trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics. Pediatr Obes (2019) 14(5):e12495. doi:10.1111/ijpo.12495
78. Alorfi, NM, and Alshehri, FS. Usage of glucagon-like peptide-1 for obesity in children; updated review of Clinicaltrials.gov. J Multidisciplinary Healthc (2023) 16:2179–87. doi:10.2147/JMDH.S419245
79. Weghuber, D, Barrett, T, Barrientos-Perez, M, Gies, I, Hesse, D, Jeppesen, OK, et al. Once-Weekly semaglutide in adolescents with obesity. N Engl J Med (2022) 387(24):2245–57. doi:10.1056/NEJMoa2208601
80. Garvey, WT, Frias, JP, Jastreboff, AM, le Roux, CW, Sattar, N, Aizenberg, D, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet (2023) 402(10402):613–26. doi:10.1016/S0140-6736(23)01200-X
81. Jastreboff, AM, Aronne, LJ, Ahmad, NN, Wharton, S, Connery, L, Alves, B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med (2022) 387(3):205–16. doi:10.1056/NEJMoa2206038
82. Frias, JP, Davies, MJ, Rosenstock, J, Perez Manghi, FC, Fernandez Lando, L, Bergman, BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med (2021) 385(6):503–15. doi:10.1056/NEJMoa2107519
83. Azuri, J, Hammerman, A, Aboalhasan, E, Sluckis, B, and Arbel, R. Tirzepatide versus semaglutide for weight loss in patients with type 2 diabetes mellitus: a value for money analysis. Diabetes Obes Metab (2023) 25(4):961–4. doi:10.1111/dom.14940
84. Latif, W, Lambrinos, KJ, and Rodriguez, R Compare and contrast the glucagon-like peptide-1 receptor agonists (GLP1RAs) . Treasure Island (FL): StatPearls (2024).
85. Farzam, K, and Patel, P Tirzepatide . Treasure Island (FL): StatPearls (2024).
86. Foretz, M, Guigas, B, and Viollet, B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol (2023) 19(8):460–76. doi:10.1038/s41574-023-00833-4
87. TODAY Study Group, Zeitler, P, Hirst, K, Pyle, L, Linder, B, Copeland, K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med (2012) 366(24):2247–56. doi:10.1056/NEJMoa1109333
88. Stumvoll, M, Nurjhan, N, Perriello, G, Dailey, G, and Gerich, JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med (1995) 333(9):550–4. doi:10.1056/NEJM199508313330903
89. DeFronzo, RA, and Goodman, AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med (1995) 333(9):541–9. doi:10.1056/NEJM199508313330902
90. Seifarth, C, Schehler, B, and Schneider, HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes (2012) 121(1):27–31. doi:10.1055/s-0032-1327734
91. The Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care (2012) 35(4):731–7. doi:10.2337/dc11-1299
92. Bouza, C, Lopez-Cuadrado, T, Gutierrez-Torres, LF, and Amate, J. Efficacy and safety of metformin for treatment of overweight and obesity in adolescents: an updated systematic review and meta-analysis. Obes Facts (2012) 5(5):753–65. doi:10.1159/000345023
93. Masarwa, R, Brunetti, VC, Aloe, S, Henderson, M, Platt, RW, and Filion, KB. Efficacy and safety of metformin for obesity: a systematic review. Pediatrics (2021) 147(3):e20201610. doi:10.1542/peds.2020-1610
94. Verduci, E, Bronsky, J, Embleton, N, Gerasimidis, K, Indrio, F, Koglmeier, J, et al. Role of dietary factors, food habits, and lifestyle in childhood obesity development: a position paper from the European society for paediatric gastroenterology, hepatology and nutrition committee on nutrition. J Pediatr Gastroenterol Nutr (2021) 72(5):769–83. doi:10.1097/MPG.0000000000003075
95. Paoli, A, Bosco, G, Camporesi, EM, and Mangar, D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol (2015) 6:27. doi:10.3389/fpsyg.2015.00027
96. Ludwig, DS, and Ebbeling, CB. The carbohydrate-insulin model of obesity: beyond "calories in, calories out. JAMA Intern Med (2018) 178(8):1098–103. doi:10.1001/jamainternmed.2018.2933
97. Deemer, SE, Plaisance, EP, and Martins, C. Impact of ketosis on appetite regulation-a review. Nutr Res (2020) 77:1–11. doi:10.1016/j.nutres.2020.02.010
98. Nelson, AB, Queathem, ED, Puchalska, P, and Crawford, PA. Metabolic Messengers: ketone bodies. Nat Metab (2023) 5(12):2062–74. doi:10.1038/s42255-023-00935-3
99. Crosby, L, Davis, B, Joshi, S, Jardine, M, Paul, J, Neola, M, et al. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr (2021) 8:702802. doi:10.3389/fnut.2021.702802
100. Soni, S, Tabatabaei Dakhili, SA, Ussher, JR, and Dyck, JRB. The therapeutic potential of ketones in cardiometabolic disease: impact on heart and skeletal muscle. Am J Physiology-Cell Physiol (2024) 326(2):C551–C566. doi:10.1152/ajpcell.00501.2023
101. Favret, J, Wood, CT, and Maradiaga Panayotti, GM. Ketogenic diet as an advanced option for the management of pediatric obesity. Curr Opin Endocrinol Diabetes Obes (2021) 28(5):488–95. doi:10.1097/MED.0000000000000661
102. Kwiterovich Jr, PO, Vining, EP, Pyzik, P, Skolasky, R, and Freeman, JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA (2003) 290(7):912–20. doi:10.1001/jama.290.7.912
103. Chawla, S, Tessarolo Silva, F, Amaral Medeiros, S, Mekary, RA, and Radenkovic, D. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients (2020) 12(12):3774. doi:10.3390/nu12123774
104. Leow, ZZX, Guelfi, KJ, Davis, EA, Jones, TW, and Fournier, PA. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med (2018) 35:1258–63. doi:10.1111/dme.13663
105. Cai, QY, Zhou, ZJ, Luo, R, Gan, J, Li, SP, Mu, DZ, et al. Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J Pediatr (2017) 13(6):528–36. doi:10.1007/s12519-017-0053-2
106. Wells, J, Swaminathan, A, Paseka, J, and Hanson, C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-A review. Nutrients (2020) 12(6):1809. doi:10.3390/nu12061809
107. Lee, JH, Verma, N, Thakkar, N, Yeung, C, and Sung, HK. Intermittent fasting: physiological implications on outcomes in mice and men. Physiology (Bethesda) (2020) 35(3):185–95. doi:10.1152/physiol.00030.2019
108. Manoogian, ENC, Chow, LS, Taub, PR, Laferrere, B, and Panda, S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr Rev (2022) 43(2):405–36. doi:10.1210/endrev/bnab027
109. Mishra, A, Mirzaei, H, Guidi, N, Vinciguerra, M, Mouton, A, Linardic, M, et al. Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat Metab (2021) 3(10):1342–56. doi:10.1038/s42255-021-00469-6
110. Kim, KH, Kim, YH, Son, JE, Lee, JH, Kim, S, Choe, MS, et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res (2017) 27(11):1309–26. doi:10.1038/cr.2017.126
111. Longo, VD, and Mattson, MP. Fasting: molecular mechanisms and clinical applications. Cel Metab (2014) 19(2):181–92. doi:10.1016/j.cmet.2013.12.008
112. Wilkinson, MJ, Manoogian, ENC, Zadourian, A, Lo, H, Fakhouri, S, Shoghi, A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cel Metab (2020) 31(1):92–104 e5. doi:10.1016/j.cmet.2019.11.004
113. Liu, K, Liu, B, Wittert, GA, Thompson, CH, Hutchison, AT, and Heilbronn, LK. Intermittent fasting increases growth differentiation factor 15 in females with overweight or obesity but not associated with food intake. Obes Res Clin Pract (2023) 17(1):91–3. doi:10.1016/j.orcp.2022.12.001
114. Varady, KA, Cienfuegos, S, Ezpeleta, M, and Gabel, K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol (2022) 18(5):309–21. doi:10.1038/s41574-022-00638-x
115. Tucker, JM, Siegel, R, Murray, PJ, Han, JC, Boyer, K, Reed, N, et al. Acceptability of time-limited eating in pediatric weight management. Front Endocrinol (Lausanne) (2022) 13:811489. doi:10.3389/fendo.2022.811489
116. Vidmar, AP, Goran, MI, and Raymond, JK. Time-limited eating in pediatric patients with obesity: a case series. J Food Sci Nutr Res (2019) 2(3):236–44. doi:10.26502/jfsnr.2642-11000022
117. Vidmar, AP, Naguib, M, Raymond, JK, Salvy, SJ, Hegedus, E, Wee, CP, et al. Time-limited eating and continuous glucose monitoring in adolescents with obesity: a pilot study. Nutrients (2021) 13(11):3697. doi:10.3390/nu13113697
118. Jebeile, H, Gow, ML, Lister, NB, Mosalman Haghighi, M, Ayer, J, Cowell, CT, et al. Intermittent energy restriction is a feasible, effective, and acceptable intervention to treat adolescents with obesity. J Nutr (2019) 149(7):1189–97. doi:10.1093/jn/nxz049
119. Fiore, G, Pascuzzi, MC, Di Profio, E, Corsello, A, Agostinelli, M, La Mendola, A, et al. Bioactive compounds in childhood obesity and associated metabolic complications: current evidence, controversies and perspectives. Pharmacol Res (2023) 187:106599. doi:10.1016/j.phrs.2022.106599
120. Delpino, FM, Figueiredo, LM, and da Silva, BGC. Effects of omega-3 supplementation on body weight and body fat mass: a systematic review. Clin Nutr ESPEN (2021) 44:122–9. doi:10.1016/j.clnesp.2021.04.023
121. Lopez-Alarcon, M, Martinez-Coronado, A, Velarde-Castro, O, Rendon-Macias, E, and Fernandez, J. Supplementation of n3 long-chain polyunsaturated fatty acid synergistically decreases insulin resistance with weight loss of obese prepubertal and pubertal children. Arch Med Res (2011) 42(6):502–8. doi:10.1016/j.arcmed.2011.06.010
122. Svensson, V, Johansson, E, Fischer, M, Deng, SL, Hagstromer, M, and Danielsson, P. Omega-3 fatty acids does not affect physical activity and body weight in primary school children - a double-blind randomized placebo-controlled trial. Sci Rep (2018) 8(1):12725. doi:10.1038/s41598-018-31229-4
123. Fan, Y, and Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol (2021) 19(1):55–71. doi:10.1038/s41579-020-0433-9
124. Wang, W, Yan, Y, Yu, F, Zhang, W, and Su, S. Role of oral and gut microbiota in childhood obesity. Folia Microbiol (Praha) (2023) 68(2):197–206. doi:10.1007/s12223-023-01033-3
125. Pihl, AF, Fonvig, CE, Stjernholm, T, Hansen, T, Pedersen, O, and Holm, JC. The role of the gut microbiota in childhood obesity. Child Obes (2016) 12(4):292–9. doi:10.1089/chi.2015.0220
126. Sanchez, M, Panahi, S, and Tremblay, A. Childhood obesity: a role for gut microbiota? Int J Environ Res Public Health (2014) 12(1):162–75. doi:10.3390/ijerph120100162
127. Salam, RA, Padhani, ZA, Das, JK, Shaikh, AY, Hoodbhoy, Z, Jeelani, SM, et al. Effects of lifestyle modification interventions to prevent and manage child and adolescent obesity: a systematic review and meta-analysis. Nutrients (2020) 12(8):2208. doi:10.3390/nu12082208
128. Bulbul, S. Exercise in the treatment of childhood obesity. Turk Pediatri Ars (2020) 55(1):2–10. doi:10.14744/TurkPediatriArs.2019.60430
129. Herouvi, D, Paltoglou, G, Soldatou, A, Kalpia, C, Karanasios, S, and Karavanaki, K. Lifestyle and pharmacological interventions and treatment indications for the management of obesity in children and adolescents. Children (Basel) (2023) 10(7):1230. doi:10.3390/children10071230
130. Headid III, RJ, and Park, SY. The impacts of exercise on pediatric obesity. Clin Exp Pediatr (2021) 64(5):196–207. doi:10.3345/cep.2020.00997
131. Ahn, SM. Current issues in bariatric surgery for adolescents with severe obesity: durability, complications, and timing of intervention. J Obes Metab Syndr (2020) 29(1):4–11. doi:10.7570/jomes19073
132. Fox, CK, Kelly, AS, Reilly, JL, Theis-Mahon, N, and Raatz, SJ. Current and future state of pharmacological management of pediatric obesity. Int J Obes (Lond) (2024). doi:10.1038/s41366-024-01465-y
133. Alqahtani, AR, Elahmedi, M, Abdurabu, HY, and Alqahtani, S. Ten-year outcomes of children and adolescents who underwent sleeve gastrectomy: weight loss, comorbidity resolution, adverse events, and growth velocity. J Am Coll Surgeons (2021) 233(6):657–64. doi:10.1016/j.jamcollsurg.2021.08.678
134. Elkhoury, D, Elkhoury, C, and Gorantla, VR. Improving access to child and adolescent weight loss surgery: a review of updated national and international practice guidelines. Cureus (2023) 15(4):e38117. doi:10.7759/cureus.38117
135. Pietrocola, F, Pol, J, Vacchelli, E, Rao, S, Enot, DP, Baracco, EE, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell (2016) 30(1):147–60. doi:10.1016/j.ccell.2016.05.016
136. Czepiel, KS, Perez, NP, Campoverde Reyes, KJ, Sabharwal, S, and Stanford, FC. Pharmacotherapy for the treatment of overweight and obesity in children, adolescents, and young adults in a large health system in the US. Front Endocrinol (Lausanne) (2020) 11:290. doi:10.3389/fendo.2020.00290
137. Choi, SW, Mak, TS, and O'Reilly, PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc (2020) 15(9):2759–72. doi:10.1038/s41596-020-0353-1
138. Torkamani, A, Wineinger, NE, and Topol, EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet (2018) 19(9):581–90. doi:10.1038/s41576-018-0018-x
139. Torkamani, A, and Topol, E. Polygenic risk scores expand to obesity. Cell (2019) 177(3):518–20. doi:10.1016/j.cell.2019.03.051
140. Markovic-Jovanovic, SR, Stolic, RV, and Jovanovic, AN. The reliability of body mass index in the diagnosis of obesity and metabolic risk in children. J Pediatr Endocrinol Metab : JPEM (2015) 28(5-6):515–23. doi:10.1515/jpem-2014-0389
141. Vanderwall, C, Randall Clark, R, Eickhoff, J, and Carrel, AL. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatr (2017) 17(1):135. doi:10.1186/s12887-017-0891-z
142. Alfano, R, Robinson, O, Handakas, E, Nawrot, TS, Vineis, P, and Plusquin, M. Perspectives and challenges of epigenetic determinants of childhood obesity: a systematic review. Obes Rev (2022) 23(Suppl. 1):e13389. doi:10.1111/obr.13389
143. Wu, FY, and Yin, RX. Recent progress in epigenetics of obesity. Diabetol Metab Syndr (2022) 14(1):171. doi:10.1186/s13098-022-00947-1
144. Matsumura, Y, Wei, FY, and Sakai, J. Epitranscriptomics in metabolic disease. Nat Metab (2023) 5(3):370–84. doi:10.1038/s42255-023-00764-4
Keywords: childhood obesity, genetics, pharmacotherapy, dietary intervention, personalized therapy
Citation: Son JE (2024) Genetics, pharmacotherapy, and dietary interventions in childhood obesity. J. Pharm. Pharm. Sci 27:12861. doi: 10.3389/jpps.2024.12861
Received: 18 February 2024; Accepted: 16 May 2024; Published: 28 May 2024.
Copyright © 2024 Son. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joe Eun Son, [email protected]
THIS ARTICLE IS PART OF THE SPECIAL ISSUE View all 3 Articles
Pharmacotherapy of Energy Metabolism in Obesity
ORIGINAL ARTICLE
People also looked at.
Translating an early childhood obesity prevention program for local community implementation: a case study of the Melbourne InFANT Program
Affiliations.
- 1 Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Science, Deakin University, Geelong, VIC, Australia. [email protected].
- 2 Centre for Obesity Management and Prevention Research Excellence in Primary Health Care (COMPaRE-PHC), Sydney, Australia. [email protected].
- 3 Centre of Research Excellence in Early Prevention of Obesity in Childhood, Sydney, Australia. [email protected].
- 4 Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Science, Deakin University, Geelong, VIC, Australia.
- 5 Centre of Research Excellence in Early Prevention of Obesity in Childhood, Sydney, Australia.
- 6 Centre for Obesity Management and Prevention Research Excellence in Primary Health Care (COMPaRE-PHC), Sydney, Australia.
- 7 Prevention and Population Health, Department of Health and Human Services, Melbourne, Australia.
- PMID: 27502184
- PMCID: PMC4977772
- DOI: 10.1186/s12889-016-3361-x
Background: While there is a growing interest in the field of research translation, there are few published examples of public health interventions that have been effectively scaled up and implemented in the community. This paper provides a case study of the community-wide implementation of the Melbourne Infant, Feeding, Activity and Nutrition Trial (InFANT), an obesity prevention program for parents with infants aged 3-18 months. The study explored key factors influencing the translation of the Program into routine practice and the respective role of policy makers, researchers and implementers.
Methods: Case studies were conducted of five of the eight prevention areas in Victoria, Australia who implemented the Program. Cases were selected on the basis of having implemented the Program for 6 months or more. Data were collected from January to June 2015 and included 18 individual interviews, one focus group and observation of two meetings. A total of 28 individuals, including research staff (n = 4), policy makers (n = 2) and implementers (n = 22), contributed to the data collected. Thematic analysis was conducted using cross case comparisons and key themes were verified through member checking.
Results: Key facilitators of implementation included availability of a pre-packaged evidence based program addressing a community need, along with support and training provided by research staff to local implementers. Partnerships between researchers and policy makers facilitated initial program adoption, while local partnerships supported community implementation. Community partnerships were facilitated by local coordinators through alignment of program goals with existing policies and services. Workforce capacity for program delivery and administration was a challenge, largely overcome by embedding the Program into existing roles. Adapting the Program to fit local circumstance was critical for feasible and sustainable delivery, however balancing this with program fidelity was a critical issue. The lack of ongoing funding to support translation activities was a barrier for researchers continued involvement in community implementation.
Conclusion: Policy makers, researchers and practitioners have important and complementary roles to play in supporting the translation of effective research interventions into practice. New avenues need to be explored to strengthen partnerships between researchers and end users to support the integration of effective public health research interventions into practice.
Keywords: Children; Dissemination; Implementation; Infants; Obesity prevention; Research translation.
Publication types
- Randomized Controlled Trial
- Cluster Analysis
- Community Health Services / methods*
- Focus Groups
- Pediatric Obesity / prevention & control*
- Program Evaluation / statistics & numerical data*
- Open access
- Published: 27 May 2024
Predicting higher child BMI z-score and obesity incidence in Malaysia: a longitudinal analysis of a dynamic cohort study
- Ruth Salway 1 , 2 ,
- Miranda Armstrong 1 ,
- Jeevitha Mariapun 3 ,
- Daniel D Reidpath 4 ,
- Sophia Brady 1 ,
- Mohamed Shajahan Yasin 3 ,
- Tin Tin Su 5 na1 &
- Laura Johnson 2 na1
BMC Public Health volume 24 , Article number: 1408 ( 2024 ) Cite this article
217 Accesses
Metrics details
To target public health obesity prevention, we need to predict who might become obese i.e. predictors of increasing Body Mass Index (BMI) or obesity incidence. Predictors of incidence may be distinct from more well-studied predictors of prevalence, therefore we explored parent, child and sociodemographic predictors of child/adolescent BMI z-score and obesity incidence over 5 years in Malaysia.
The South East Asia Community Observatory in Segamat, Malaysia, provided longitudinal data on children and their parents ( n = 1767). Children were aged 6–14 years at baseline (2013-14) and followed up 5 years later. Linear multilevel models estimated associations with child BMI z-score at follow-up, adjusting for baseline BMI z-score and potential confounders. Predictors included parent cardiometabolic health (overweight/obesity, central obesity, hypertension, hyperglycaemia), and socio-demographics (ethnicity, employment, education). Logistic multilevel models explored predictors of obesity incidence.
Higher baseline BMI z-score predicted higher follow-up BMI z-score both in childhood to late adolescence (0.60; 95% CI: 0.55, 0.65) and early to late adolescence (0.76; 95% CI: 0.70, 0.82). There was inconsistent evidence of association between child BMI z-score at follow-up with parent cardiometabolic risk factors independent of baseline child BMI z-score. For example, maternal obesity, but not overweight, predicted a higher BMI z-score in childhood to early adolescence (overweight: 0.16; 95% CI: -0.03, 0.36, obesity: 0.41; 95% CI: 0.20, 0.61), and paternal overweight, but not obesity, predicted a higher BMI z-score in early to late adolescence (overweight: 0.22; 95% CI: 0.01, 0.43, obesity: 0.16; 95% CI: -0.10, 0.41). Parental obesity consistently predicted five-year obesity incidence in early to late adolescence, but not childhood to early adolescence. An adolescent without obesity at baseline with parents with obesity, had 3–4 times greater odds of developing obesity during follow-up (incidence OR = 3.38 (95% CI: 1.14–9.98, mother) and OR = 4.37 (95% CI 1.34–14.27, father) respectively).
Conclusions
Having a higher BMI z-score at baseline was a stronger predictor of a higher BMI z-score at follow-up than any parental or sociodemographic factor. Targeting prevention efforts based on parent or sociodemographic factors is unwarranted but early childhood remains a key period for universal obesity prevention.
Peer Review reports
Obesity is a major public health concern, which increases the risk of developing non-communicable diseases (NCDs) such as diabetes, stroke and cardiovascular disease [ 1 ]. Children and adolescents with obesity are five times more likely to become adults with obesity, with approximately 80% of adolescents with obesity remaining so in adulthood [ 2 ]. Obesity in childhood and adolescence is independently associated with the development of NCDs later in life [ 3 ]. To date, no childhood obesity treatments show long-term success [ 4 , 5 ], so preventing new incidence of obesity in childhood and adolescence is vital for long-term NCD prevention. Prevention is particularly important in low-and-middle-income countries (LMICs) where the prevalence of NCDs is lower but increasing rapidly [ 6 ]. In Malaysia, NCDs are the most common cause of death [ 7 ], and obesity prevalence in children and adolescents has more than doubled between 2011 and 2019 (6% and 15% respectively) [ 8 , 9 ], pointing to a need to understand new incidence of obesity. Obesity rates differ by ethnicity, with higher rates among Malay and Indian and lower among Chinese ethnicities [ 9 , 10 ]. Increasing obesity prevalence in Asian LMICs is generally attributed to changes in dietary and physical activity patterns caused by economic factors and urbanisation [ 11 ]. These include shifts towards more calorie-dense westernized foods and an increase in sedentary, indoor behaviours driven by a lack of open spaces or neighbourhood safety [ 12 , 13 ]. Universal prevention efforts to improve eating and activity behaviours are generally ineffective, often because long-term behaviour change requires intensive and sustained interventions. In resource poor settings, targeting prevention strategies at populations subgroups most likely to develop obesity could be more cost-effective. Thus understanding predictors of obesity incidence as well as prevalence is essential to identifying such subgroups before they develop obesity.
Parent obesity is consistently associated with child obesity prevalence, but less is known about associations with incidence. Two meta-analyses estimated the odds of childhood obesity for a child with parents with overweight/obesity to be double that of a child with parents of healthy weight [ 14 , 15 ]. Cross-sectional associations are also seen between parent and child body mass index (BMI), with stronger associations for those children with higher BMIs, suggesting an intergenerational transmission of risk for a high BMI [ 16 , 17 ]. Maternal pre-pregnancy BMI is prospectively associated with offspring BMI in both childhood and adulthood [ 18 ], but it is not clear whether associations are gender-specific, with some studies showing a stronger maternal association [ 17 , 19 ] while others show no differences between mothers and fathers [ 14 , 20 , 21 ]. Most studies of obesity prevalence are in high income countries, and systematic reviews suggest weaker associations in LMICs [ 14 , 15 ]. In Malaysia, a previous cross-sectional analysis found a two-fold higher obesity prevalence among children with one or more parents with obesity or central obesity [ 22 ], and the prevalence of overweight mother-child pairs increased from 15 to 22% between 2006 and 2015 [ 23 ]. While prevalence studies identify groups in need of treatment, understanding predictors of greater BMI gain over time and obesity incidence could identify targets for prevention. Annual obesity incidence estimates decrease with age, from 3.2% in 5–13 year-olds to 1.8% in 13–18 year-olds in the US [ 24 ] with similar patterns in other high-income countries [ 25 , 26 ]. However, obesity incidence is often lower in LMICs [ 27 ], and less is known about how incidence is related to parental risk factors in these countries. Identifying those children who may be at risk of developing obesity in future, based on current parental risk factors may allow intervention before obesity develops and thus reduce new incidence and future prevalence.
Few studies have explored the relationship between broader parental cardiometabolic risk factors, such as hypertension and hyperglycaemia, with child obesity although associations have been reported with parent cardiovascular health [ 28 ]and diabetes in parents [ 29 , 30 ]. Intergenerational transmission of risk of NCDs could be via genetic or lifestyle mechanisms, and parents with hypertension or hyperglycaemia may have poorer diets and lower physical activity that may be shared with their children [ 31 , 32 ]. To our knowledge, no studies have described differences in BMI or obesity incidence among children in Malaysia and little is known about the factors associated with higher gains in BMI and the development of obesity in this population. Therefore, we aimed to explore how parental weight status and cardiometabolic risk factors are longitudinally associated with child BMI z-score (follow-up adjusted for baseline) and obesity incidence.
Analysis is reported following STROBE guidelines (Supplement). Data are from two health surveys from the South East Asia Community Observatory (SEACO) health and demographic surveillance system cohort in Malaysia [ 33 ], which undertakes annual enumeration of households within five of 11 sub-districts of the Segamat district. Population-wide individual level health surveys of participants aged 5 years and above were undertaken in 2013–14 and 2018-19, and collected questionnaire and biophysical measurement data on around 25,000 participants in each round (55–56% response rate of the total SEACO population), with around 10,000 participating at both timepoints.
We analysed individual data on children aged 6–14 years at baseline (11–19 years at follow-up) and their parents from the SEACO health surveys, using household structure information taken from the enumeration in 2013-14 and 2018-19 to match parents and children [ 33 ]. Participants and households were linked across surveys using a unique SEACO participant ID. The analysis sample consisted of all children who had data at both timepoints and were matched to at least one parent with data. Fig. 1 shows the flow of participants from baseline to main analysis. We treated data for 59 mothers who were pregnant as missing and excluded one pregnant adolescent. Of the 4,388 children available at baseline, 1,855 (42%) had data at follow-up, with 1,768 (95%; 40% of the baseline cohort) matched to at least one parent, and 1,341 (72%; 31% of the baseline) having two parents identified. The final analysis sample size after multiple imputation (see below) was 1,768 (40% of baseline cohort).
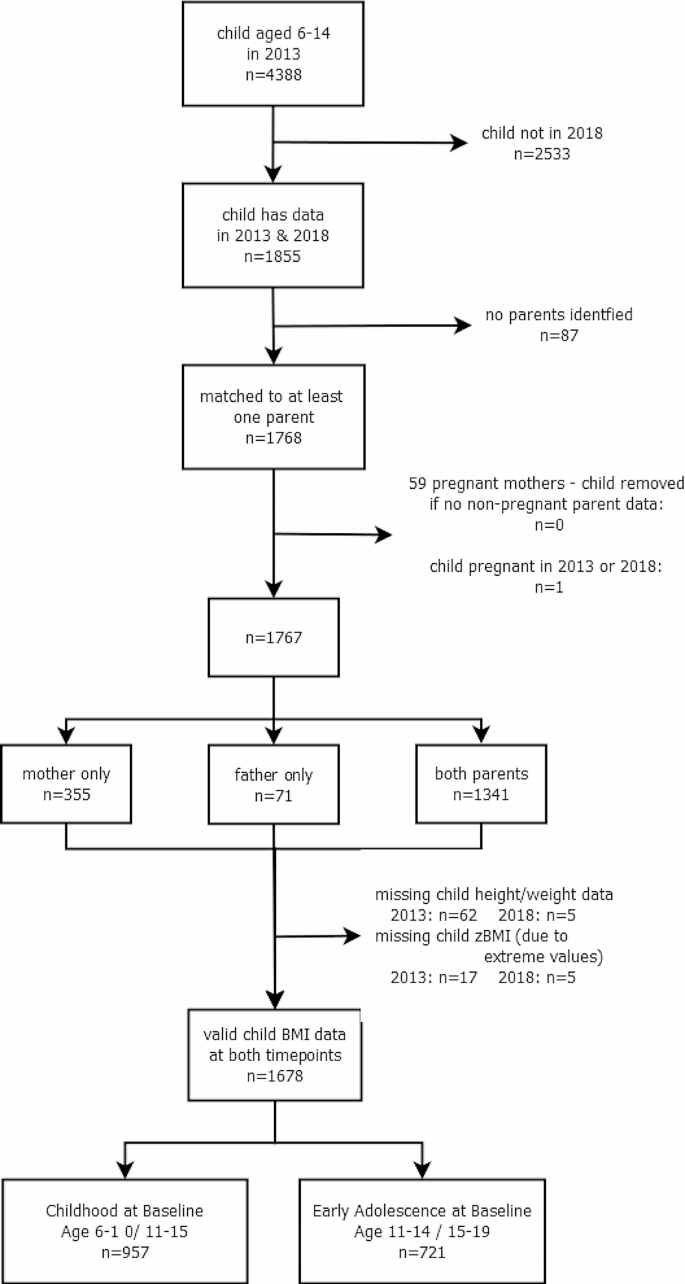
Flow diagram of participants
Ethical considerations
Ethics approval for both surveys were obtained through the Monash University Human Research Ethics Committee: MUHREC (3837) for the Health Round Survey 2013 and MUHREC (13,242) for the Health Round Survey 2018. All participants gave informed consent which allows for secondary analysis without additional consent, and data was provided in anonymised form. As part of the SEACO Health Round surveys, adult participants received free health screenings (blood pressure, blood glucose, BMI and waist circumference), with referral letters provided to high-risk respondents for future health check-ups at local clinics.
Height and weight were measured without footwear or head gear (except a light scarf or veil) using a Transtek digital weighing scale with height gauge, (model GBS-721) by trained data collectors, with one measurement taken following the SEACO Standard Operating Protocol (SOP). Child BMI was calculated from weight and height and converted to age-adjusted standardised z-scores (BMI z-score) using the sex-specific World Health Organization (WHO) 2007 BMI reference for children aged 5–19. Children were classified with thinness, overweight or obesity according to WHO definitions [ 34 ] if the standardised BMI z-score was <-2 standard deviations (SD), >1SD and > 2SD, respectively, with all remaining children classified as healthy weight.
Parent risk factors
Anthropometric measurements of all participating parents (mothers and fathers) were taken by trained data collectors in the participant’s home, with parent height and weight measured in the same way as for children, following the SEACO SOP. Waist circumference was measured using an AccuFitness Myotape, with the measurement taken at the midpoint between the lower margin of the tip of the rib and the upper point of the iliac crest (hip bone), following the WHO STEPS protocol [ 35 ]. Blood pressure was recorded after participants had been sitting for at least 15 min. Three blood pressure measurements were taken using an Omron automated blood pressure monitor (HEM-7203) with 30–60 s between subsequent measurements, and the mean of the final two readings used, according to the SEACO SOP. Participants were asked how long since they had taken food or drink (other than water), and random non-fasting blood glucose was measured using a finger-prick Omron blood glucose monitoring system, (HGM-111). BMI (kg/m 2 ) was calculated from height and weight and classified with thinness (< 18 kg/m 2 ), overweight (> 25 kg/m 2 and < 30 kg/m 2 ), obesity (≥ 30 kg/m 2 ), and of healthy weight otherwise [ 36 ], in line with previous analyses of the SEACO data [ 22 ]. Central obesity was defined using International Diabetes Federation (IDF) recommendations for Asian populations [ 37 ], as waist circumference ≥ 90 cm in men and ≥ 80 cm in women. Hypertension was defined as either systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg [ 38 ]. Hyperglycaemia was defined as random non-fasting blood glucose ≥ 11.1 mmol/l [ 39 ].
Confounders
Parent and child age, gender and ethnicity were self-reported in the main SEACO census. Inconsistencies between the two timepoints were checked using date of birth and date of data collection with precedence given to age at baseline in 2013. We grouped children into two age groups, corresponding to Malaysian primary and secondary school ages: childhood at baseline (aged 6–10 years at baseline, aged 11–15 at follow-up) and adolescence at baseline (aged 11–14 years at baseline, aged 15–19 at follow-up)). Ethnicity was recoded into four groups: Malay, Chinese, Indian and Other (comprising indigenous (Orang Asli) and Other, grouped due to low numbers). Missing child ethnicity was derived from parent ethnicity, with preference given to 2013 data. Parental working status was classified as Working (part-time, full-time, casual) or Not Working (unemployed, housewife, student, retired). Highest parental education (up to primary, secondary, tertiary) was recorded for each parent.
Statistical analysis
Baseline sample characteristics (child: age, gender, ethnicity, BMI z-score and weight category, and parent: age, gender, highest education, employment status, weight category, central obesity, hypertension and hyperglycaemia) were described using means and SDs, or percentages, as appropriate, by child gender and age group (results provided in main document). Child and Head of Household characteristics were compared for baseline and analysis samples, and for children with zero, one and two identified parents (see Appendix). Five-year incidence rate of obesity was calculated as the percentage without obesity at baseline with obesity at follow-up. Similarly, five-year remission rates were calculated as the percentage of those with obesity who were without obesity at follow-up, and five-year persistence rate as the percentage who remained with obesity (main document).
To investigate associations between parental risk factors (overweight/obesity, central obesity, hypertension and hyperglycaemia) and change in child BMI z-score, we modelled child BMI z-score in 2018 adjusted for baseline [ 40 ]. While concerns have been raised around the use of BMI z-scores for assessing change in child BMI for children with extreme BMIs, in particular those with severe obesity [ 41 ], this study focuses on estimating associations for the general population where BMI z-score has been found to be suitable for assessing change [ 42 , 43 ] and so distortion in the extremes will have less of an impact, although associations will be underestimated for children with very high BMI. We fitted a linear multilevel model for children nested within parents, with child BMI z-score at follow-up as the outcome, adjusted for time to follow-up and baseline child BMI z-score; this assumes a linear change between baseline and follow-up. We first fitted models for each parental risk factor separately. We then fitted adjusted models, controlling for child characteristics (gender, age, ethnicity) and parent sociodemographic characteristics (age, working status and education Associations with parent hypertension and hyperglycaemia models were additionally adjusted for parent BMI category. The adjusted estimates are illustrated in Fig. 3 in the main document; estimates for unadjusted and adjusted models are given in the Appendix. We also fit models for the association between follow-up BMI z-score adjusted for baseline BMI z-score, and sociodemographic variables, specifically ethnicity, parental age, education and employment status. In a post-hoc analysis, we investigated associations between parental risk factors and obesity incidence, which focuses on children with higher BMI moving to a weight status of higher risk, and does not suffer from the same issues as using BMI z-score. We used logistic multilevel models, with a binary outcome for child with obesity at follow-up, restricted to those who were without obesity at baseline, and adjusted for time to follow-up, child characteristics, parent sociodemographic characteristics, and other parental risk factors as described above (main document). All analyses were stratified by age group, with separate models for mothers and fathers. A test for gender interactions did not support the need for stratification by gender, therefore results are reported for the whole sample combined. Adjusted models excluded child ethnicity ’Other’ due to small numbers. Descriptive summaries were performed in Stata v17 [ 44 ] and multilevel models were run in MLwiN v3.05 via the Stata command runmlwin, using restrictive iterative generalised least squares. Sensitivity analyses compared adjusted model estimates using imputed data with complete case analyses, and we repeated the analysis, first using Asian population BMI cut-offs for parental overweight (> 23 kg/m 2 and < 25 kg/m 2 ) and obesity (> 25 kg/m 2 ) and second excluding children with thinness, as for these children an increase in BMI might be considered beneficial thus potentially violating the linearity assumption (Appendix).
Missing data
We restricted analysis to children with BMI data at both timepoints who could be matched to at least one parent ( n = 1678; 38% of baseline). Missing data on gender, age and ethnicity for children and parents was taken from the other timepoint where available. Multiple imputation was used for parent risk factors to maximise the information used in the study and increase the precision of estimates [ 45 ]. We imputed missing parent data if they were included for at least one timepoint; note we did not impute child data at missing timepoints or BMI z-score.
Missing data was imputed using the jomo package in R (v4.0.2) via a simulation-based approach with a multivariate normal model to account for the multilevel structure. All analysis variables were included, and variables associated with missingness: total size of household, number of children and household structure (children/ parents, children/grandparents, and multi-generational/complex households). Continuous parental risk factors were imputed on a log scale, and parent BMI, central obesity, hypertension and hyperglycaemia were calculated from these. Twenty imputation datasets were created for each age group separately, using a burn-in of 500 iterations and 500 iterations between imputation datasets, selected by visual inspection. Model estimates were combined across the imputation datasets using Rubin’s rules [ 46 ].
There were 1,678 children included at both timepoints, with valid child BMI data, were not pregnant and were matched to at least one parent (Fig. 1 ). At baseline, 957 were aged 6–10 years (childhood at baseline) and 721 aged 11–14 (adolescence at baseline). Of these, 68% were Malay, 16% Chinese, 13% Indian and 2% Other, and 56% were female (Table 1 ). At baseline, 16% had obesity, 16% overweight and 16% thinness, with similar prevalences at follow-up. Compared to those excluded owing to missing child BMI data at a single timepoint, the children in the longitudinal analysis sample were a year younger, had lower baseline BMI z-score, were less likely to be male or Chinese, and came from households where the Head of Household had higher education and was less likely to be Indian (Table S1 ). Children with matched parents had higher BMI z-scores, were more likely to be male and were less likely to be Chinese (Table S2 ). Missing data varied between 8 and 27% for maternal and 20–36% for paternal variables (Table S3 ), primarily due to missing hypertension and hyperglycaemia. We imputed data on 9% of mothers and 24% of fathers; baseline characteristics were broadly similar between collected data and imputed data (Table S3 ). The average age of mothers was 41 years, with father slightly older at 45 years. Most parents (70%) were educated to secondary level, with most fathers in employment (91%) while mothers less likely to be employed (31%).
BMI z-score was higher at follow-up compared to baseline for girls, the childhood age-group at baseline. Whereas BMI z-score was lower at follow-up compared to baseline for the adolescent age-group at baseline (Table 1 ). Obesity prevalence was 16–18%, with movement between weight categories over time (Fig. 2 ). Five-year obesity incidence was higher in childhood (at baseline) than adolescence (at baseline) (10.8% and 6.1% respectively; Table 2 ). Five-year obesity remission rates of 42% were observed (Table 2 ). Of those participants with a healthy weight at baseline, 19% developed overweight or obesity at follow-up, compared to 7% developing thinness.
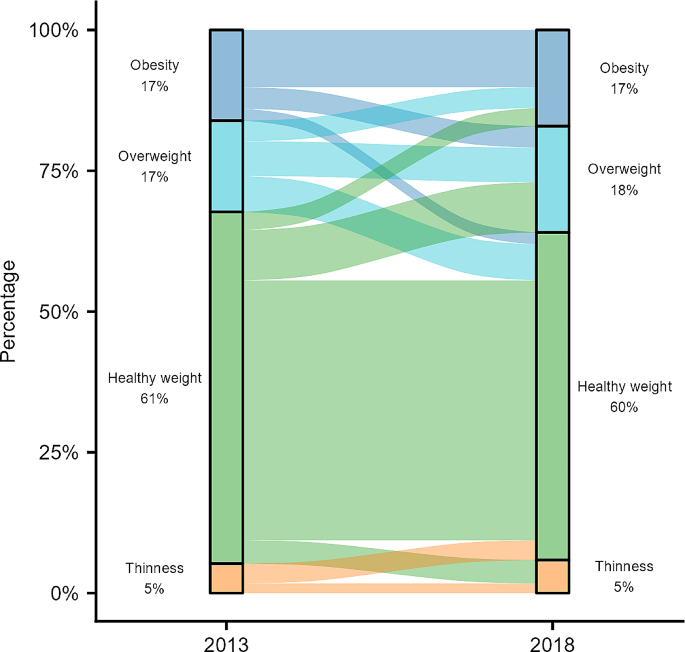
Change in child BMI category between 2013 and 2018
Fig. 3 (and Table S4 & S5 ) shows modelled associations between parent cardiometabolic health and child BMI z-score at follow-up. For all models, higher child BMI z-score at baseline was the strongest predictor of a higher child BMI z-score at follow-up. Each unit of baseline BMI z-score was associated with a 0.60 (95% CI: 0.55, 0.65) higher follow-up BMI z-score for childhood to early adolescence and 0.76 (95% CI: 0.70, 0.82) higher for early to late adolescence. Associations with parental cardiometabolic risk factors were much smaller with weak or no evidence of association. In childhood to early adolescence, compared to healthy maternal weight, obesity (B = 0.41 (95% CI: 0.20, 0.61)), but not overweight (B = 0.16 (95% CI: -0.03, 0.36)) was associated with an increase in child BMI z-score of at follow-up (Table S4 ). While not associated with higher BMI z-score among younger participants (childhood to early adolescence), compared to a healthy paternal weight, overweight (B = 0.22 (95% CI: 0.01, 0.43)) but not obesity (B = 0.16 (95% CI: -0.10, 0.41)) showed small associations with a higher BMI z-score in older participants (early to late adolescence) (Table S4 ). Parental BMI and waist circumference were strongly correlated (0.73–0.75) and so associations with central obesity followed the same patterns as parental overweight/obesity. Associations between child BMI z-score at follow-up and baseline parent hypertension and hyperglycaemia (Table S4 ), ethnicity, parent employment status or educational attainment (Table S5 ) were small with weak or no evidence of association.
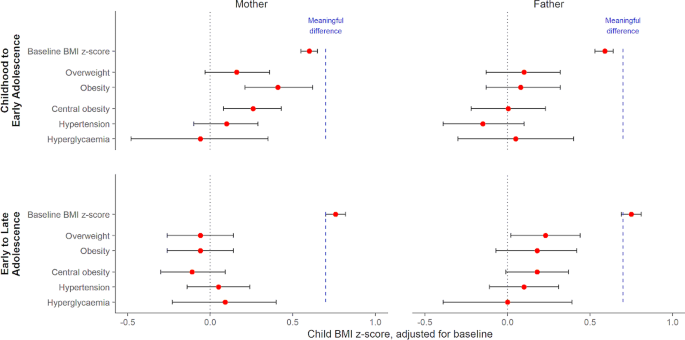
Association between parental risk factors and baseline-adjusted child BMI z-score at follow-up
Model estimates and 95% confidence intervals by age strata and parent (see also Table S4 ). Models adjusted for baseline BMI z-score, time interval, child (gender, age, ethnicity) and relevant parent characteristics (age, education, other cardiometabolic risk factors). The dashed blue line represents the minimum change in BMI z-score for a meaningful clinical impact on child and adolescent lipids and blood pressure, based on a recent meta-analysis (El-Medany et al., 2020).
Childhood to early adolescence group were aged 6–10 at baseline (11–15 at follow-up) and early to late adolescence group aged 11–14 at baseline (16–19 at follow-up).
We saw similar patterns for obesity incidence, with stronger maternal associations in childhood to early adolescence, and paternal associations in early to late adolescence (Table 3 ). In childhood to early adolescence, the odds of obesity incidence were similar among those with a parent with overweight vs. a healthy weight parent (paternal OR = 0.67 (95% CI 0.33–1.37); maternal OR = 1.33 (95% CI: 0.71–2.48)). Obesity incidence odds were higher among the older age group, especially for those with a parent with obesity at 3–4 times higher (paternal OR = 4.37 (95% CI 1.34–14.27); maternal OR = 3.38 (95% CI: 1.14–9.98)). Associations with parental central obesity followed similar patterns, especially for maternal central obesity, where odds were higher than for BMI-based overweight/obesity (Table 3 ). There were no marked associations with parent hypertension or hyperglycaemia (ORs between 0.5 and 1.5 with wide confidence intervals).
Complete case analysis showed similar associations (Table S6 ), with slightly stronger associations for mothers with overweight. Sensitivity analyses were run for the association between child BMI z-score at follow-up and parental weight category using the overweight/obesity definition for Asian populations, which has lower BMI thresholds (Table S7 ). We saw similar patterns of associations to before, but with parental obesity rather than parental overweight; specifically associations with maternal obesity for those in childhood to early adolescence, and associations with paternal obesity for those in early to late adolescence. As the linearity assumption between baseline and follow-up BMI z-score did not hold for low values of baseline BMI z-score (Figure S1 )., we repeated the analysis excluding children with thinness (Table S8 ) but found no difference in the reported associations between child BMI z-score at follow-up and parental cardiometabolic risk factors.
We have reported how parental cardiometabolic factors (overweight, obesity, central obesity, hypertension and hyperglycaemia) are associated with child BMI z-score at five-year follow-up, and the development of obesity in Malaysian children. Five-year increases in BMI z-score depended on child age at baseline, with larger increases between childhood and early adolescence compared to early and late adolescence. Previous cross-sectional analyses highlight associations between parental weight status and sociodemographic factors and child BMI z-score at a single timepoint [ 21 , 22 ]. We found parental obesity was weakly associated with child BMI z-score differences, below values considered clinically meaningful, but associated with high odds of developing obesity, suggesting that targeted childhood obesity prevention strategies may need to focus on children of parents with obesity who may not be immediately at risk. For example, existing adult weight management programmes, such as ‘My body is fit and fabulous’ in Malaysia aimed at housewives [ 47 ], could also include advice and support aimed at the whole family to target children in childhood and early adolescence before they develop obesity.
In Segamat, obesity prevalence was estimated at 16% in 6–10 year olds, 18% in 10–14 year olds and 16% in those aged 15 or more, with overweight prevalence at 15%, 20% and 15% respectively. This is consistent with overweight and obesity prevalences of 15% across ages 5–18 reported for Malaysian children in the National Health and Morbidity Survey 2019 and elsewhere [ 9 , 21 , 23 ], but highlights variation with age, with higher rates during the early adolescent period. While obesity prevalence was relatively stable, this masked substantial change. Over five years, new obesity incidence was 8.8%, while 42% of those with obesity at baseline were without obesity at follow-up. Both the incidence and remission rates are slightly lower than those reported in high-income countries [ 24 , 25 ] and we did not observe higher remission rates among younger children as other studies have reported [ 48 , 49 , 50 ]. In general, the majority of children who shift between BMI categories tend to do so at the boundaries. While some of the remission may be attributable to measurement error or regression to the mean, it may also be due to individual behaviour change, timing of maturation [ 48 ], or lower odds of remission associated with low birthweight [ 50 ].
Malaysia has a double burden of both thinness and overweight/obesity but we found more children with healthy weight at baseline developing overweight/obesity than thinness at follow-up (19% and 7% respectively). Our findings may reflect differences in LMICs where obesity rates are still increasing, with higher incidence in childhood not balanced by higher remission rates, leading to an increasing obesity prevalence over time, although conclusions are limited by having only two time points.
In childhood to early adolescence, maternal overweight/obesity were associated with higher mean BMI z-score at follow-up compared to mothers of healthy weight, whilst in early to late adolescence the associations of higher offspring BMI z-score were strongest with paternal overweight and obesity. Note that we have used WHO international BMI thresholds for parental overweight/obesity, but we found similar maternal and paternal patterns by child age group when using lower risk thresholds suggested for Asian populations, with the key difference being associations with parental obesity but not overweight, reflecting the shift in the thresholds. We observed similar patterns for central obesity, which is in line with the strong correlation between BMI and waist circumference. A recent meta-analysis [ 51 ] indicates a minimum mean increase in BMI z-score for a clinical impact on lipid profiles and blood pressure in 4–19 year olds is 0.7 z-scores. The associations we observed are over five years, and estimates (and confidence interval bounds) are much smaller than 0.7 and thus do not suggest associations are substantial enough to alter metabolic health in a clinically-relevant way. While larger cross-sectional associations between parental overweight/obesity and children’s BMI z-score [ 14 , 15 , 22 ] may be due to genetic predisposition to obesity, common obesogenic lifestyles, including diet and physical activity, due to shared living environment and/or behavioural factors passed down from parent to child [ 14 ], our analysis suggests these factors have a far smaller impact on the five-year difference in BMI z-score. We note, however, that our results may underestimate the association for those children with very high BMIs, and so further research on associations for children with severe obesity may be warranted.
There were no marked associations with parental hypertension, hyperglycaemia, or socioeconomic status, thus our study suggests that childhood obesity prevention strategies may be best targeted at those who have parents with overweight or obesity. The strongest associations observed were with BMI z-score at baseline, as children with higher initial BMI experienced larger five-year increases than expected compared to WHO references.
While increasing BMI z-scores can indicate growth in excess of normal expectations for a given age and sex, higher-than-average BMI is not in itself a cause for concern if children remain on the same trajectories into adolescence, because they will stay within the healthy BMI range. Both BMI z-score and overweight/obesity definitions in children are based on comparison to a historic cross-sectional reference population (in this study, the WHO reference population) and thus differences we observe may be a result of different underlying characteristics of populations, such as the timing of pubertal growth spurts, rather than necessarily cause for concern. Figure S2 (see Appendix) shows that median BMI was higher than the WHO growth reference at all ages for boys and girls, with the rate of change in BMI temporally shifted, so the fastest rate of increase occurred 1–2 years younger. The strong associations with baseline BMI z-score suggest that excessive growth trajectories are initiated earlier in childhood and are perpetuated into adolescence [ 52 , 53 , 54 ]. Thus, the small associations between parental risk factors and offspring BMI z-score may be because those children predisposed to obesity, having already lived with overweight or obesity from a young age. However, these difficulties in assessing change in BMI in childhood make it challenging to interpret results.
Five-year obesity incidence was higher between childhood and early adolescence than between early and late adolescence (10.8% and 6.1% respectively), consistent with patterns elsewhere of higher incidence at younger ages [ 24 , 25 ], and in line with the theory that excessive growth tends to occur earlier in childhood. Associations between parental overweight/obesity and child obesity incidence were larger in early to late adolescence than in childhood to early adolescence with parental overweight associated with a doubling in odds of five-year obesity incidence and parental obesity associated with a 3–4 times higher odds. Note that BMI changes considerably during puberty with a growth spurt and the adiposity rebound, which will occur predominantly within the childhood to early adolescence group, and may account for the weaker associations within this group. In contrast, the stronger associations in the early to late adolescence group, once puberty is more established, highlights that a child with one or both parents with obesity may not develop obesity until late adolescence, and so may not have been identified as at risk when younger. This is of concern because associations with obesity in adulthood are stronger for those with obesity in adolescence than in childhood [ 2 ], and thus indicate that this group of children may require targeting at a younger age even if they do not yet have obesity. Associations between parental obesity and adolescent obesity incidence but not BMI z-score may indicate a differential importance of parent weight for child BMI across the distribution of child BMI [ 16 , 17 ]. For example, children with higher baseline BMI z-scores would need a smaller increase in BMI z-score (such as is associated with having a parent with obesity) to move into the overweight or obesity category. Thus, parental obesity may have a stronger association for children closer to the cut-off for obesity at baseline, especially in early adolescence.
We saw some evidence of different associations by parent gender, with maternal weight dominating in childhood to early adolescence and paternal weight in early to late adolescence. Maternal obesity before and during pregnancy (a key developmental period) is associated with offspring BMI in childhood, potentially driven by intrauterine, genetic or lifestyle factors such as smoking [ 53 , 55 ]. Some cross-sectional studies have found stronger maternal associations [ 17 , 19 ] and our results suggest that associations may continue into childhood, possibly reflecting the common role of the mother as primary caregiver in younger children, especially in more traditional communities, with more influence over lifestyle factors such as diet and physical activity. However, we found this was replaced by a weaker paternal association in early to late adolescence. Thus maternal associations in childhood may thus result in earlier puberty [ 56 ], and an earlier adolescent increase in BMI, while paternal weight is associated with higher BMI at the end of adolescence. However, all these associations were small and other evidence is inconclusive, especially for fathers. While much of the current evidence focuses on maternal associations, fathers are under-represented, and more research is needed to determine the paternal role throughout childhood and adolescence. Understanding further our findings of a stronger paternal association in early to late adolescence is of particular importance as this is a critical time for BMI trajectories into adulthood.
Strengths and limitations
This study uses prospective data with a robust sampling design in the SEACO cohort, resulting in high levels of parental data, especially for fathers who are typically under-represented, with most studies focusing on mothers [ 23 ]. Parental cardiometabolic risk factors were measured objectively rather than using self-report measures. However, because data were collected at household level, matching children to parents was difficult especially with multiple families in a household and we were unable to distinguish between biological and non-biological parents. We also excluded a substantial number of children due to missing data at follow-up, and did not have data on lifestyle factors such as diet or physical activity. While multiple imputation maximises the available information in the data, this is under a missing at random assumption. Exploring changes in BMI in this age group is challenging due to puberty and childhood growth patterns, and so care should be taken in interpreting results. Furthermore, Segamat is a semi-rural region so is not generalisable to the wider Malaysian population, although overweight/obesity estimates are similar [ 23 ]. Finally, our analysis focuses on the general population; estimates of associations may be underestimated for those children with severe obesity.
In this Malaysian cohort, child/adolescent obesity prevalence was stable at 16–18%, with a five-year incidence of obesity at 8.8%. Parental overweight/obesity was prospectively associated with slightly higher child BMI z-score after adjusting for baseline, but the largest follow-up BMI z-scores were among children with a higher baseline BMI z-score. These findings support the importance of childhood as a key period for obesity prevention, rather than later intervention based on parent cardiometabolic risk. However, those in early adolescence with higher BMI z-score and at least one parent with obesity may be at an increased risk of becoming obese during late adolescence.
Data availability
Data are from an ongoing prospective cohort study and are available from SEACO by completion of a data application form to: https://www.monash.edu.my/seaco/research-and-training/how-to-collaborate-with-seaco.
Abbreviations
Body mass index
International Diabetes Federation
Low-and-middle-income country
Non-communicable disease
South East Asia Community Observatory
World Health Organization
World Health Organization. Global Health Risks: Mortality and burden of disease attributable to selected major risks. In. Geneva: World Health Organization; 2009.
Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17(2):95–107.
Article CAS PubMed Google Scholar
Lin X, Xu Y, Xu J, Pan X, Song X, Shan L, Zhao Y, Shan PF. Global burden of noncommunicable disease attributable to high body mass index in 195 countries and territories, 1990–2017. Endocrine. 2020;69(2):310–20.
Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, Olajide J, Mainardi GM, Corpeleijn E, O’Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6:CD012651.
PubMed Google Scholar
Al-Khudairy L, Loveman E, Colquitt JL, Mead E, Johnson RE, Fraser H, Olajide J, Murphy M, Velho RM, O’Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017;6:CD012691.
Miranda JJ, Barrientos-Gutierrez T, Corvalan C, Hyder AA, Lazo-Porras M, Oni T, Wells JCK. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat Med. 2019;25(11):1667–79.
Low WY, Lee YK, Samy AL. Non-communicable diseases in the Asia-Pacific region: prevalence, risk factors and community-based prevention. Int J Occup Med Environ Health. 2015;28(1):20–6.
Institute for Public Health (IPH). National Health and Morbidity Survey 2015 (NHMS 2015). Vol. II: Non-Communicable Diseases, Risk Factors & Other Health Problems. In. Malaysia; 2015.
Institute for Public Health: National Health and Morbidity Survey (NHMS). 2019: Vol. I: NCDs – Non-Communicable Diseases: Risk Factors and other Health Problems. In. Malaysia: Institute for Public Health, National Institutes of Health, Ministry of Health; 2020.
Rampal L, Rampal S, Khor GL, Zain AM, Ooyub SB, Rahmat RB, FGhani S, N, Krishnan J. A national study on the prevalence of obesity among 16,127 malaysians. Asia Pac J Clin Nutr. 2007;16(3):561–6.
Li M, Dibley M, Baur L, Twigg S, Magnusson R. Child and adolescent obesity in Asia. A modern epidemic: Expert perspectives on obesity and diabetes. Sydney, NSW: Sydney University; 2012.
Google Scholar
Gupta N, Goel K, Shah P, Misra A. Childhood obesity in developing countries: epidemiology, determinants, and prevention. Endocr Rev. 2012;33(1):48–70.
Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S9–30.
Wang Y, Min J, Khuri J, Li M. A systematic examination of the Association between parental and child obesity across Countries. Adv Nutr. 2017;8(3):436–48.
Article PubMed PubMed Central Google Scholar
Lee JS, Jin MH, Lee HJ. Global relationship between parent and child obesity: a systematic review and meta-analysis. Clin Exp Pediatr. 2022;65(1):35–46.
Article PubMed Google Scholar
Dolton P, Xiao M. The intergenerational transmission of body mass index across countries. Econ Hum Biol. 2017;24:140–52.
Johnson PCD, Logue J, McConnachie A, Abu-Rmeileh NME, Hart C, Upton MN, Lean M, Sattar N, Watt G. Intergenerational change and familial aggregation of body mass index. Eur J Epidemiol. 2011;27(1):53–61.
Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, Pemu A, Rankin J. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med. 2019;16(6):e1002817.
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in Young Adulthood from Childhood and parental obesity. New Eng J Med 1997(13):869–73.
Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of parents and children (ALSPAC). Arch Dis Child. 2007;92(10):876–80.
Ahmad A, Zulaily N, Shahril MR, Wafa SW, Mohd Amin R, Piernas C, Ahmed A. Obesity determinants among Malaysian 12-year old school adolescents: findings from the HAT study. BMC Pediatr. 2021;21(1):418.
Partap U, Young EH, Allotey P, Sandhu MS, Reidpath DD. Anthropometric and cardiometabolic risk factors in parents and child obesity in Segamat, Malaysia. Int J Epidemiol. 2017;46(5):1523–32.
Mohamed NN, Rohana AJ, Hamid NAA, Hu FB, Malik VS, Mohd Yusoff MF, Aris T, The Global N. Epidemiologic transition Initiative G: Intergenerational Transmission of Obesity from mothers to their offspring: Trends and Associated factors derived from the Malaysian National Health and Morbidity Survey (NHMS). Nutrients 2022, 14(11).
Cheung PC, Cunningham SA, Narayan KM, Kramer MR. Childhood Obesity Incidence in the United States: a systematic review. Child Obes. 2016;12(1):1–11.
Hughes AR, Sherriff A, Lawlor DA, Ness AR, Reilly JJ. Incidence of obesity during childhood and adolescence in a large contemporary cohort. Prev Med. 2011;52(5):300–4.
Ikeda N, Nishi N. First incidence and associated factors of overweight and obesity from preschool to primary school: longitudinal analysis of a national cohort in Japan. Int J Obes (Lond). 2019;43(4):751–60.
Do LM, Tran TK, Eriksson B, Petzold M, Ascher H. Prevalence and incidence of overweight and obesity among Vietnamese preschool children: a longitudinal cohort study. BMC Pediatr. 2017;17(1):150.
Muchira JM, Gona PN, Mogos MF, Stuart-Shor E, Leveille SG, Piano MR, Hayman LL. Temporal trends and Familial Clustering of Ideal Cardiovascular Health in parents and offspring over the Life Course: An Investigation using the Framingham Heart Study. J Am Heart Assoc. 2020;9(12):e016292.
Article CAS PubMed PubMed Central Google Scholar
Linares Segovia B, Gutierrez Tinoco M, Izquierdo Arrizon A, Guizar Mendoza JM, Amador Licona N. Long-term consequences for offspring of paternal diabetes and metabolic syndrome. Exp Diabetes Res. 2012;2012:684562.
Kawasaki M, Arata N, Miyazaki C, Mori R, Kikuchi T, Ogawa Y, Ota E. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: a systematic review and meta-analysis. PLoS ONE. 2018;13(1):e0190676.
Niermann CYN, Spengler S, Gubbels JS. Physical activity, screen time, and dietary intake in families: a cluster-analysis with Mother-Father-Child triads. Front Public Health. 2018;6:276.
Bogl LH, Silventoinen K, Hebestreit A, Intemann T, Williams G, Michels N, Molnar D, Page AS, Pala V, Papoutsou S et al. Familial resemblance in Dietary intakes of children, adolescents, and parents: does Dietary Quality play a role? Nutrients 2017, 9(8).
Partap U, Young EH, Allotey P, Soyiri IN, Jahan N, Komahan K, Devarajan N, Sandhu MS, Reidpath DD. HDSS Profile: the South East Asia Community Observatory Health and Demographic Surveillance System (SEACO HDSS). Int J Epidemiol. 2017;46(5):1370–g1371.
WHO. Growth Reference 5–19 Years - Application Tools. In. Geneva: World Health Organization; 2014.
STEPwise approach to NCD risk factor surveillance (STEPS). https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps.
World Health Organization. Obesity and overweight. In. Geneva: World Health Organization; 2015.
IDF. The IDF Consensus on the Definition of the Metabolic Syndrome in Children and Adolescents. In. Brussels: International Diabetes Federation; 2007.
Whitworth J. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertensb. 2003;21:1983–92.
Article Google Scholar
WHO, IDF. Definition and diagnosis of diabetes Mellitus and Intermediate Hyperglycemia. In. Geneva: World Health Organization; 2006.
Tennant PWG, Arnold KF, Ellison GTH, Gilthorpe MS. Analyses of ‘change scores’ do not estimate causal effects in observational data. Int J Epidemiol. 2022;51(5):1604–15.
Freedman DS, Berenson GS. Tracking of BMI z scores for severe obesity. Pediatrics. 2017;140(3):e20171072.
Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99(11):1020–4.
Inokuchi M, Matsuo N, Takayama JI, Hasegawa T. BMI z-score is the optimal measure of annual adiposity change in elementary school children. Ann Hum Biol. 2011;38(6):747–51.
StataCorp.: Stata Statistical Software: Release 17. In. College Station, TX: StataCorp LLC; 2021.
Dong Y, Peng C-Y. Principled missing data methods for researchers. SpringerPlus. 2013;2:222.
Rubin D. Multiple imputation after 18 years. J Am Stat Assoc. 1996;91(434):473–89.
Ambak R, Mohamad Nor NS, Puteh N, Mohd Tamil A, Omar MA, Shahar S, Ahmad NA, Aris T. The effect of weight loss intervention programme on health-related quality of life among low income overweight and obese housewives in the MyBFF@home study. BMC Womens Health. 2018;18(Suppl 1):111.
Kim J, Must A, Fitzmaurice GM, Gillman MW, Chomitz V, Kramer E, McGowan R, Peterson KE. Incidence and remission rates of overweight among children aged 5 to 13 years in a district-wide school surveillance system. Am J Public Health. 2005;95(9):1588–94.
von Kries R, Beyerlein A, Muller MJ, Heinrich J, Landsberg B, Bolte G, Chmitorz A, Plachta-Danielzik S. Different age-specific incidence and remission rates in pre-school and primary school suggest need for targeted obesity prevention in childhood. Int J Obes (Lond). 2012;36(4):505–10.
Luan D, Mezuk B, Bauer KW. Remission of obesity among a nationally representative sample of US children. Pediatr Obes 2019, 14(1).
El-Medany A, Birch L, Hunt L, Matson R, Chong A, Beynon R, Hamilton-Shield J, Perry R. What change in body Mass Index is required to Improve Cardiovascular outcomes in Childhood and adolescent obesity through Lifestyle interventions: a Meta-regression. Child Obes. 2020;16(7):449–78.
Dos Santos CS, Picoito J, Nunes C, Loureiro I. Early individual and Family Predictors of Weight Trajectories from Early Childhood to adolescence: results from the Millennium Cohort Study. Front Pediatr. 2020;8:417.
Nedelec R, Miettunen J, Mannikko M, Jarvelin MR, Sebert S. Maternal and infant prediction of the child BMI trajectories; studies across two generations of Northern Finland birth cohorts. Int J Obes (Lond). 2021;45(2):404–14.
Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes. 2003;27(4):505–13.
Article CAS Google Scholar
Bond TA, Karhunen V, Wielscher M, Auvinen J, Mannikko M, Keinanen-Kiukaanniemi S, Gunter MJ, Felix JF, Prokopenko I, Yang J, et al. Exploring the role of genetic confounding in the association between maternal and offspring body mass index: evidence from three birth cohorts. Int J Epidemiol. 2020;49(1):233–43.
Chen YC, Fan HY, Yang C, Hsieh RH, Pan WH, Lee YL. Assessing causality between childhood adiposity and early puberty: a bidirectional mendelian randomization and longitudinal study. Metabolism. 2019;100:153961.
Download references
Acknowledgements
The authors would like to express their appreciation to the SEACO Field Teams and survey participants. The research described in this paper was supported by the South East Asia Community Observatory (SEACO, https://www.monash.edu.my/seaco ). The views, however, are those of the authors and there is no real or implied endorsement by SEACO.
This work was supported by funding from UK Medical Research Council and the Malaysian Ministry of Higher Education/UK-MY Joint Partnership on Non-Communicable Diseases 2019/MR/T018984/1. Monash University funds the SEACO health and demographic surveillance system. Co-authors of this study are also supported by the National Institute for Health and Care Research Bristol Biomedical Research Centre (MA). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Tin Tin Su and Laura Johnson share the joint senior authorship.
Authors and Affiliations
Centre for Exercise, Nutrition & Health Sciences, School for Policy Studies, University of Bristol, 8 Priory Road, Bristol, BS8 1TZ, UK
Ruth Salway, Miranda Armstrong & Sophia Brady
Population Health Sciences, Bristol Medical School, University of Bristol, Canynge Hall, Bristol, BS8 2PN, UK
Ruth Salway & Laura Johnson
Clinical School Johor Bahru, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Subang Jaya, Malaysia
Jeevitha Mariapun & Mohamed Shajahan Yasin
Institute for Global Health and Development, Queen Margaret University, Edinburgh, EH21 6UU, Scotland
Daniel D Reidpath
South East Asia Community Observatory (SEACO), and Global Public Health, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Subang Jaya, Malaysia
You can also search for this author in PubMed Google Scholar
Contributions
The study was conceived by LJ and TTS, and funding was obtained by LJ, TTS, MA, DR and MY. The analyses were planned by LJ and RS, who wrote the analysis plan with input from TTS, JM, DR, MY and MA. LJ supervised the analysis and drafting and RS undertook the analysis and wrote the first draft of the article. All authors critically reviewed the article and approved the final manuscript.
Corresponding author
Correspondence to Ruth Salway .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval for both surveys were obtained through the Monash University Human Research Ethics Committee: MUHREC (3837) for the Health Round Survey 2013 and MUHREC (13242) for the Health Round Survey 2018. All participants gave informed consent which allows for secondary analysis without additional consent, and data was provided in anonymised form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. For transparency: LJ has received, for research unrelated to the current paper, institutional funding from UKRI, World Cancer Research Fund, National Institute for Health Research UK, Joint Programs Initiative EU FP7, Alpro foundation, Danone Baby Nutrition, Kellogg Europe, and the Wellcome Trust. MA has received, for research unrelated to the current paper, institutional funding from the Centre for Aging Better, NIHR and Cancer Research UK.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Salway, R., Armstrong, M., Mariapun, J. et al. Predicting higher child BMI z-score and obesity incidence in Malaysia: a longitudinal analysis of a dynamic cohort study. BMC Public Health 24 , 1408 (2024). https://doi.org/10.1186/s12889-024-18917-9
Download citation
Received : 20 November 2023
Accepted : 21 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12889-024-18917-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adolescents
- Intergenerational obesity
- Cardiometabolic risk factors
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Society in general and primary care providers in particular are often highly biased toward people with obesity. 24 A study with obese children aged 9 to 11 years found implicit bias to be around 5%. 25 Rather than ignoring or denying bias, primary care providers must instead develop the self-awareness to recognize and manage it, realize that it ...
The HOME Plus study used a randomized controlled trial design to test the effects of a community-based, family-focused childhood obesity prevention program. A description of the methodology can be found elsewhere ( Fulkerson et al., 2014 ). Primary meal preparing parents (n=160) and one 8-12 year old child (n=160) per family were recruited ...
This updated synthesis of obesity prevention interventions for children aged 6-18 years, found a small beneficial impact on child BMI for school-based obesity prevention interventions. A more comprehensive assessment of interventions is required to identify mechanisms of effective interventions to inform future obesity prevention public health policy, which may be particularly salient in for ...
Objective: To evaluate the efficacy of childhood obesity interventions and conduct a taxonomy of intervention components that are most effective in changing obesity-related health outcomes in children 2-5 years of age. Methods: Comprehensive searches located 51 studies from 18,335 unique records. Eligible studies: (1) assessed children aged 2-5, living in the United States; (2) evaluated ...
Evidence and theory suggest that including parents in interventions offers promise for effective childhood obesity prevention. This case study engaged parents' as co-researchers in the design, implementation and evaluation of an intervention for low-income families with a child enrolled in Head Start. Parent engagement mechanisms include: (1 ...
To improve data capacity for childhood obesity research, program evaluation, and surveillance, the US Centers for Disease Control and Prevention (CDC) is leading the Childhood Obesity Data Initiative (CODI). 11 On the basis of a participatory framework of local and national clinical, community, and public health representatives, CODI ...
Preventing childhood obesity is a national priority for health professionals and policy makers. Consistent with a general call for researchers to engage parents in child health research [], parental involvement specifically in childhood obesity programs and prevention efforts has been stressed [2-4].This case study responds to the need for parent engagement as experts throughout the entire ...
Launched in 2009, the National Collaborative on Childhood Obesity Research (NCCOR) brings together four of the nation's leading research funders — the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), the Robert Wood Johnson Foundation (RWJF), and the U.S. Department of Agriculture (USDA) — to accelerate progress in reducing childhood obesity in ...
To improve data capacity for childhood obesity research, program evaluation, and surveillance, the US Centers for Disease Control and Prevention (CDC) is leading the Childhood Obesity Data Initiative (CODI).11 On the basis of a participatory framework of local and national clinical, community, and public health representatives, CODI ...
This case study engaged parents' as co-researchers in the design, implementation and evaluation of an intervention for low-income families with a child enrolled in Head Start. Prevention of childhood obesity is a national priority. Parents influence young children's healthy lifestyles, so it is paradoxical that obesity interventions focus primarily on children. Evidence and theory suggest ...
This paper aims to describe differences between an urban and rural program delivery of a family-focused, community-based intervention program to prevent and reduce obesity among children. This paper uses a case study format to provide a descriptive analysis of similar obesity prevention programs, designed by the same research team, implemented ...
A wide range of interventions has been implemented and tested to prevent obesity in children. Given parents' influence and control over children's energy-balance behaviors, including diet, physical activity, media use, and sleep, family interventions are a key strategy in this effort. The objective of this study was to profile the field of recent family-based childhood obesity prevention ...
Tackling Childhood Obesity with Advocacy. The Robert Wood Johnson Foundation and the American Academy of Pediatrics turned to NICHQ to develop national resources and a training curriculum to engage and train healthcare professionals to become advocates for obesity prevention within their communities. Obesity is a complex public health problem ...
The Childhood Obesity Data Initiative: A Case Study in Implementing Clinical-Community Infrastructure Enhancements to Support Health Services Research and Public Health J Public Health Manag Pract . 2022 Mar-Apr;28(2):E430-E440. doi: 10.1097/PHH.0000000000001419.
The objective of this study was to profile the field of recent family-based childhood obesity prevention interventions by employing systematic review and quantitative content analysis methods to identify gaps in the knowledge base. ... children at both ends of the age spectrum, and media and sleep behaviors would be beneficial. This study can ...
Objective To assess the effectiveness of a school and family based healthy lifestyle programme (WAVES intervention) compared with usual practice, in preventing childhood obesity. Design Cluster randomised controlled trial. Setting UK primary schools from the West Midlands. Participants 200 schools were randomly selected from all state run primary schools within 35 miles of the study centre (n ...
Many unhealthy dietary and physical activity habits that foster the development of obesity are established by the age of five. Presently, approximately 70 percent of children in the United States are currently enrolled in early childcare facilities, making this an ideal setting to implement and evaluate childhood obesity prevention efforts. We describe here the methods for conducting an ...
1. Introduction. Treatment of obese children involves family-wide change to increase physical activity and improve dietary habits; yet, such treatment has limited success [1,2].There is an ever growing appreciation that social determinants of health (neighborhood and built environment, economic stability, education, social and community context, and health and healthcare) can greatly impact ...
1 Introduction. Childhood obesity rates in the United States (U.S.) are high. From 2010 to 2020, obesity prevalence in children aged 2-5 years increased from 10 to 13% ().This public health concern is more acute in rural communities, with studies reporting 26% higher odds of obesity in rural versus urban children ().Obesity prevention is preferrable to treatment in rural children (), but ...
Purpose: Despite public health efforts to reduce childhood obesity, there remains an unequal distribution of obesity among rural and urban children, with higher rates in rural areas. However, few studies have compared differences in program delivery. This paper aims to describe differences between an urban and rural program delivery of a family-focused, community-based intervention program to ...
Resources Policy Dossiers Childhood Obesity Treatment Childhood obesity treatment: case studies. In this section. We offer the only internationally recognised course on obesity management. Read more here. Read More. We offer various statistics, maps and key data around the topic of obesity. You can find all that and more here.
Testing the feasibility of a sustainable preschool obesity prevention approach: a mixed-methods service evaluation of a volunteer-led HENRY programme. The UK charity HENRY has been providing evidence-based behaviour change programmes to parents across the UK to reduce childhood obesity.
Introduction. Childhood and adolescent obesity have reached epidemic levels in the United States, affecting the lives of millions of people. In the past 3 decades, the prevalence of childhood obesity has more than doubled in children and tripled in adolescents. 1 The latest data from the National Health and Nutrition Examination Survey show that the prevalence of obesity among US children and ...
Obesity can also fuel heart disease risk factors such as high blood pressure (hypertension) and high cholesterol. Early obesity increases the risk of heart issues later in life. A 2023 study found ...
Genetic factors in childhood obesity. Twin studies have highlighted the ... Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care (2012 ... Time-limited eating in pediatric patients with obesity: a case series. J Food Sci Nutr Res (2019) 2(3):236-44 ...
Background: While there is a growing interest in the field of research translation, there are few published examples of public health interventions that have been effectively scaled up and implemented in the community. This paper provides a case study of the community-wide implementation of the Melbourne Infant, Feeding, Activity and Nutrition Trial (InFANT), an obesity prevention program for ...
To target public health obesity prevention, we need to predict who might become obese i.e. predictors of increasing Body Mass Index (BMI) or obesity incidence. Predictors of incidence may be distinct from more well-studied predictors of prevalence, therefore we explored parent, child and sociodemographic predictors of child/adolescent BMI z-score and obesity incidence over 5 years in Malaysia.